User login
When does shyness become a disorder?
Social phobia was accorded official psychiatric diagnostic status in the United States less than 20 years ago, but has been described in the medical literature for centuries. Hippocrates described such a patient: “He dare not come in company for fear he should be misused, disgraced, overshoot himself in gestures or speeches or be sick; he thinks every man observes him.”1
This observation was made more than 2,000 years ago. Yet social anxiety disorder (SAD) was left largely unstudied until the mid-1980s.2 An estimated 20 million people in the U.S. suffer from this disorder.
What causes some people to break into a cold sweat at the thought of the most casual encounter with a checkout clerk, a coworker, or an acquaintance? Limited evidence points to underlying biological abnormalities in SAD, but there have been no conclusive findings.
Two main subtypes of SAD exist (Box 1). Roughly 25% of sufferers have discrete or nongeneralized SAD, that is, circumscribed social fears limited to one or two situations, such as speaking in public or performing before an audience. The remaining 75% suffer from generalized SAD, the more severe subtype in which all or nearly all interpersonal interactions are difficult.
Generalized SAD often begins early in life, with a mean onset at about age 15, but 35% of the time SAD occurs in individuals before age 10.3 This subtype appears to run in families, while the nongeneralized subtype does not, suggesting that a genetic inheritance is possible. From an etiological perspective, the possible effects of parenting styles of socially anxious parents, or acquisition of social anxiety conditioned by experiencing extreme embarrassment, may also contribute to the development of SAD in some people. Approximately twice as many females as males are affected, and almost all are affected before age 25.3,4 When social fears interfere with social, occupational, or family life, the affected individual is not suffering from normal "shyness," but rather a treatable anxiety disorder.
–Toastmasters slogan
Generalized
- Most social interactions
- Early onset
- Social skills deficit
- High comorbidity
- Lower achievement
- Remission rare
Nongeneralized
- Limited fears
- Later onset
- Social skills normal
- Less comorbidity
- Less Impairment
- Remits often
The National Comorbidity Survey (NCS) estimated lifetime prevalence of SAD at 13.3% and 12-month prevalence at 7.6%, making it the third most common psychiatric disorder, following only major depression and alcohol abuse/dependence.5 Despite this high rate, SAD remains woefully underdiagnosed.
Anyone who has had to speak in public, play a musical instrument at a recital, or perform in some way under the watchful expectation of an audience has experienced anxiety as he or she anticipates the "big moment” (Box 2). Once the performance is under way, the anxiety usually lessens to a more manageable level for most people. In fact, nearly one in three Americans will admit to moderate or great fear of speaking in public.
Mr. L, a 40-year-old eighth-grade teacher, consulted a psychiatrist because he was scheduled to be evaluated by a state education accreditation committee while teaching class. Though he had always passed these before, he had been worried sick for weeks and was experiencing panic attacks each time he thought about the accreditation visit.
He lived with his mother, had never dated, and had few friends. He was extremely inhibited outside the classroom, brought cash to stores to avoid being observed while writing a check or signing credit card slips, and avoided social gatherings outside of his church, which he attended with his mother and tolerated with distress.
Further history revealed that he had quit medical school during his third year because he had so much difficulty presenting cases to the attending on ward rounds that he chose to leave the profession in order to avoid feeling sick each morning and afternoon.
Enough people encounter the fear of public speaking to support the weekly Toastmasters meetings in most U.S. cities. Many people overcome their social anxiety about public speaking or performing with continued practice. However, those with nongeneralized SAD, who are among the most severely affected, may remain so fearful of speaking or performing under scrutiny that they avoid it at any cost—even if it means passing up a job or promotion or even choosing to change professions.
The majority (75%) of those with SAD—representing approximately 15 million individuals in the U.S.—suffer from generalized SAD, a much more severe, potentially disabling subtype. These unfortunate individuals fear and avoid most or all social interactions outside their home except those with family or close friends. When they encounter or even anticipate entering feared social situations, individuals with generalized SAD experience severe anxiety. Blushing, tremulousness, and sweating can be noticed by others, and thus are particularly distressing to those with SAD.
Recovery without treatment is rare. The typically early age at onset of generalized SAD3,4 imposes greater limitations on development of social competence than on those who develop more discrete fear of public speaking or performing later in life—after socialization skills have already developed.
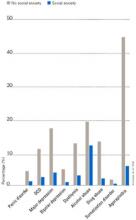
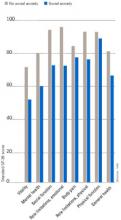
Individuals with SAD frequently suffer from comorbid psychiatric disorders, mostly depression and/or other anxiety disorders.6Figure 1 shows that individuals with SAD are at significantly increased risk for depression, other anxiety disorders, and alcohol and drug abuse. Since generalized SAD usually appears at an earlier age than other anxiety disorders, it represents a risk factor for subsequent depression. The level of functional impairment caused by SAD is similar to that caused by major depression7 (Figure 2).
As more comorbid psychiatric disorders accrue, impairment and increased risk for additional disorders may occur. Further, the risk of suicide is increased in those with comorbid SAD vs. those with SAD only. The findings suggest that if social anxiety were detected and treated effectively at an early age, it might be possible to prevent other psychiatric disorders—particularly depression—as well as the predictable morbidity and mortality that accompanies untreated SAD. Given the estimated $44 billion annual cost of anxiety disorders in the U.S.,8 research targeted at testing this hypothesis would appear to be a good investment.
Figure 1 HOW PREVALENT IS LIFETIME COMORBIDITY IN SAD?
- Inherent avoidance of scrutiny, (e.g., evaluation)
- Uncertain diagnostic threshold
- Acceptance of pathological shyness as ‘just my personality’
- Lack of understanding by professionals, family, friends
- Coping strategies that mask disability
- Comorbid psychiatric disorders that mask SAD
Figure 2 QUALITY OF LIFE IN PATIENTS WITH SOCIAL ANXIETY DISORDER
Seeing the unseen: making the diagnosis quickly
Although SAD is extremely common, a variety of factors may contribute to the low rate of recognition of the disorder (Box 3). Because of their intense discomfort toward scrutiny by authority figures such as their physician, individuals with SAD may not be willing to discuss their fears. Studies estimating the prevalence in primary care suggest that these individuals visit their referring physicians at about the same rate as the general population.6,9-11 Affected individuals are unlikely to seek psychiatric treatment unless they have a comorbid depression or anxiety.6,7
Table 1
DIFFERENTIAL DIAGNOSIS FOR SOCIAL ANXIETY DISORDER
| Condition | Diagnostic features |
|---|---|
| Posttraumatic stress disorder | Temporally follows traumatic event; cues related to trauma, not exclusively to social situations |
| Panic disorder | Unexpected panic attacks, not exclusively socially mediated anxiety |
| Agoraphobia | Fearful avoidance of situations in which panic attacks may occur, not limited to social situations |
| Major depression or atypical depression | Social withdrawal temporally related to mood disturbance, not to fear of humiliation or embarrassment; atypical depression with rejection sensitivity associated with other symptoms (e.g., hypersomnia, hyperphagia, anergy, mood reactivity) |
| Generalized anxiety disorder | Focus of worry not limited to social situations; social discomfort or avoidance not a key feature |
| Body dysmorphic disorder | Avoidance of social activity focused on concern over perceived ugliness |
| Avoidant personality disorder | Often present in generalized social anxiety disorder; may represent more severe end of social anxiety disorder spectrum; social activity desired but avoided |
| Schizotypal/schizoid personality disorders | Avoidance of social situations is preferred by individual and is not due to fear of embarrassment or humiliation |
| Normal shyness | Minimal or no interference with social, occupational, or family functioning |
| Adapted from Lydiard RB. Social anxiety disorder: comorbidity and its implications. J Clin Psychiatry.2001;62(suppl 1):17-23. | |
SAD can be difficult to tease apart from other coexisting conditions. Individuals who present for treatment of other anxiety disorders, depression, or substance abuse disorders should be considered at risk for current but undetected SAD. Many of the symptoms overlap (Table 1). The key diagnostic feature, which leads to the diagnosis of SAD, is that the fear and avoidance specifically are related to being in or anticipating a feared social situation (Box 4).
Social
- Attending parties, weddings etc.
- Conversing in a group
- Speaking on the telephone
- Interacting with authority figure (e.g., teacher or boss)
- Making eye contact
- Ordering in a restaurant
Performance
- Public speaking
- Eating in public
- Writing a check
- Using a public toilet
- Taking a test
- Trying on clothes in a store
- Speaking up at a meeting
Many clinicians mistake social anxiety for panic disorder, since panic attacks in people with SAD are often cued by social situations. There can be up to a 40% overlap of SAD with panic disorder.4 Without probing carefully into the focus on fear and avoidance, SAD can be easily overlooked. Individuals with panic disorder experience unexpected attacks and are terrified at the prospect of additional attacks, while those with SAD experience attacks linked to social situations and fear scrutiny and embarrassment more than the attacks themselves.
SAD and major depression frequently coexist,4,11,12 challenging clinicians to distinguish social reticence and withdrawal accompanying depression from the fearful avoidance that typifies SAD. SAD usually precedes depression. Asking if the patient experienced social anxiety prior to the onset of depression can help identify SAD with comorbid depression.
Alcohol-related disorders are twice as likely to occur in those affected by SAD as in those not affected. The risk for females increases more than it does for males.2-4 SAD most often precedes alcohol abuse. Studies show that about 30% of patients receiving treatment for alcohol abuse/dependence have SAD. If it remains undetected, the risk of rapid relapse is high. That’s because patients are highly unlikely to participate in psychosocial treatments that help sustain post-treatment abstinence, such as the Alcoholics Anonymous 12-step program. A recent study found that both social anxiety and alcohol abuse disorders improve when SAD in alcoholics is treated.13
A substantially higher percentage of adults with SAD, especially women, have histories of prior childhood sexual and/or physical abuse than the general population.14 Recent studies both in women following rape and in combat veterans with posttraumatic stress disorder (PTSD) suggest that those with perceived life-threatening events are at higher risk for developing secondary SAD than are individuals who experience less severe trauma.15,16 We do not yet know if secondary SAD in trauma victims is different in character or response to treatment.
Individuals with certain medical conditions can develop symptoms resembling SAD. These include stuttering, benign essential tremor,17 Parkinson’s disease, disfiguring burn injuries, and possibly irritable bowel syndrome. Such patients are technically excluded from being diagnosed with SAD, though they would meet criteria if the diagnostic rules were suspended. A limited body of literature and clinical experience suggests that symptoms secondary to physical conditions may respond to the same treatment as SAD in medically healthy persons. A treatment trial for selected patients with SAD symptoms associated with medical conditions may provide significant benefits. Clearly more research is needed in this area.
- “Being embarrassed or looking stupid are among my worst fears.”
- “Fear of embarrassment causes me to avoid doing things or speaking to people.”
- “I avoid activities in which I am the center of attention.”
Connor et al, Depress Anxiety 2001;14:137
A recently developed self-rating scale, the Social Phobia Inventory (SPIN), assesses the spectrum of cognitive, behavioral, and physiological symptoms associated with SAD.18 Three of the 17 SPIN items have been found to identify generalized SAD with a high degree of sensitivity (Box 5).
The 3 main goals of SAD treatment are
- Acute reduction and control of pathological anxiety and related phobic avoidance;
- Adequate treatment of depression or other comorbid conditions;
- Long-term management of the social phobia.
Significant advances in treatment have emerged over the past 2 decades. We now know that cognitive behavioral therapy (CBT), medication, and their combination are efficacious.
Social situations involving speaking or performance are usually predictable, and nongeneralized SAD is thus amenable to use of a beta-blocker or benzodiazepine (Box 6). Beta-blockers are often adequate for control of tremor and increased heart rate. Some patients may also benefit more from judicious use of a benzodiazepine prior to the event.
In contrast, generalized SAD is less predictable, and continual treatment is recommended. Ideally, a medication regimen would be easily tolerated long-term, and would have antidepressant effects and a broad spectrum of efficacy against commonly coexisting disorders. Because of the significant risk for depression in individuals with SAD, first-line antidepressant treatment is preferred when possible over other medications.
The selective serotonin reuptake inhibitors (SSRIs) are now considered the first-line pharmacological treatment for social phobia. Paroxetine was the first to receive FDA approval for generalized SAD.19 Large multicenter studies supporting the efficacy of two other SSRIs—sertraline and fluvoxamine—have been reported.12 The SSRIs also appear to work against the other psychiatric disorders with which SAD commonly co-occurs, such as panic disorder, major depression, generalized anxiety disorder, and PTSD.
The empirical database is very limited, but it appears that SSRI treatment for a significant percentage of patients with SAD may require higher doses (up to twice the amount) than are usually needed for depression.10 Approximately 50% to 65% of patients with generalized SAD respond to any given SSRI. In our experience, failure to respond to one SSRI does not preclude response to a second SSRI.
The irreversible monoamine oxidase (MAO) inhibitor phenelzine was the first antidepressant shown to be useful for SAD. Tranylcypromine is less well studied, but also appears to be effective. The significant side effects (weight gain, orthostatic hypotension, insomnia) and inconvenience of administration have reduced use of these agents.
Nongeneralized
- PRN treatment
- Beta-blockers
- Benzodiazepines
Generalized
- Continuous treatment
- Broad-spectrum antidepressants
- Benzodiazepines
- MAOIs
- Antiepileptic agents
The tricyclic antidepressants are probably not effective, with the exception of clomipramine (also a potent inhibitor of serotonin reuptake).12 Clomipramine, while an effective anxiolytic and antidepressant, causes prohibitive side effects in many patients (e.g., sexual dysfunction and weight gain).
Key elements for individual or group setting
- Cognitive “restructuring”
- Social skills enhancement
The newer antidepressants venlafaxine and nefazodone are less well studied than the SSRIs, but show promise as potential broad-spectrum agents. Bupropion, a novel antidepressant, and the azapirone anxiolytic buspirone do not appear to work against SAD.
The main role of the benzodiazepines in SAD treatment is adjunctive to antidepressants or in some patients intolerant of, or unresponsive to, other treatments. Clonazepam, alprazolam, and probably others are effective for SAD, but they may not effectively treat or prevent depression or other commonly associated disorders.
The anticonvulsant gabapentin has been shown in one controlled study to be effective in treating SAD.20 This agent may be particularly useful for complicated patients such as those with a history of alcohol-related disorders, bipolar-spectrum disorder, or intolerance to SSRIs.
In parallel with the development of effective psychopharmacological treatments, several types of behavioral and cognitive behavioral treatments have been investigated, including imaginal flooding, graduated exposure, social skills training, cognitive-behavior approaches, and combined cognitive restructuring and graduated exposure.21 These treatments involve similar elements targeted at the cognitive distortions and avoidance behaviors, which represent core features of SAD (Box 7).
Many clinicians believe that combined pharmacotherapy and CBT treatment are superior to either modality alone for treating SAD. The little empirical information available indicates that acute treatment differences between drug alone and drug in combination with CBT are not impressive. However, there appears to be a lower rate of relapse following CBT than after medication discontinuation.
Despite our ability to treat this disorder, only a small fraction of sufferers get treatment. If untreated, the risk of comorbidity is extremely high. Routine screening for SAD, especially in younger individuals, could provide for early detection and treatment. Psychiatrists can play an important role in early detection and treatment by educating consumers, teachers, school nurses, psychologists, and pediatricians.
Related resources
- Lydiard, R.B. Social anxiety disorder comorbidity and its implications J. Clin. Psychiatry. 2001;62(suppl):17-23.
- American Psychiatric Association, http://www.psych.org
- American Psychological Association, http://www.apa.org
- National Institute for Mental Health: Anxiety Disorders, http://www.nimh.nih.gov/anxiety/
- Anxiety Disorders Associations of America, http://www.adaa.org/
Drug brand names
- Alprazolam • Xanax
- Atenolol • Tenormin
- Bupropion • Wellbutrin
- Buspirone • Buspar
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Gabapentin • Neurontin
- Lorazepam • Ativan
- Nefazodone • Serzone
- Paroxetine • Paxil
- Phenelzine • Nardil
- Propranolol • Inderal
- Sertraline • Zoloft
- Tranylcypromine • Parnate
- Venlafaxine • Effexor
Disclosure
The author reports that he has received grant/research support and has served as a consultant to and on the speaker's bureau of Bristol-Myers Squibb Co., GlaxoSmithKline, Pfizer Inc., Eli Lilly and Co., Parke-Davis, and Solvay Pharmaceuticals. He also has received grant/research support and served as a consultant for Forest Pharmaceuticals, Wyeth-Ayerst Pharmaceuticals, and Roche; received grant/research support from Sanofi-Synthelabo; and has served as consultant for Dupont Pharmaceuticals and AstraZeneca.
1. Burton R. The Anatomy of Melancholy, vol. 1, 11th ed. London, England. Thomas Tegg, Cheapside; 1845.
2. Liebowitz MR, Gorman JM, Fyer AJ, Klein DF. Social phobia: review of a neglected anxiety disorder. Arch Gen Psychiatry. 1985;42:729-736.
3. Magee WJ, Eaton WW, Wittchen HU, et al. Agoraphobia, simple phobia, and social phobia in the National Comorbidity Survey. Arch Gen Psychiatry. 1996;53:159-168.
4. Schneier FR, Johnson J, et al. Social phobia: comorbidity and morbidity in an epidemiologic sample. Arch Gen Psychiatry. 1992;49:282-288.
5. Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8-19.
6. Goldenberg IM, et al. The infrequency of “pure culture” diagnosis among the anxiety disorders. J Clin Psychiatry. 1996;57:528-533.
7. Wittchen HU, Beloch E. The impact of social phobia on quality of life. Int Clin Psychopharmacol. 1996;11(suppl):15-23.
8. Greenberg PE, Sisitsky T, Kessler RC, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60:427-435.
9. Weiller E, Bisserbe JC, Boyer P, et al. Social phobia in general health care. Br J Psychiatry. 1996;168:169-174.
10. Katzelnick DJ, Greist JH. Social anxiety disorder: an unrecognized problem in primary care. J Clin Psychiatry. 2001;62:11.-
11. Davidson J. Social anxiety disorder under scrutiny. Depress Anxiety. 2000;11:93-98.
12. Lydiard RB. Social anxiety disorder: treatment role of the SSRIs. In Montgomery SA, den Boer JA (eds). SSRIs in Depression and Anxiety Perspectives in Psychiatry ,vol 8. NY: Wiley, Chichester, 129-150, 2001.
13. Randall CL, et al. Paroxetine improves both social anxiety and alcohol use in dually-diagnosed patients at the American College of Neuropsychopharmacology. San Juan, Puerto Rico. Dec 10-15, 2000.
14. Stein MB, Walker JR, Anderson G, et al. Childhood physical and sexual abuse in patients with anxiety disorders and in a community sample. Am J Psychiatry. 1996;153:275-277.
15. Boudreaux E, Kilpatrick DG, Resnick HS, et al. Criminal victimization, posttraumatic stress disorder, and comorbid psychopathology among a community sample of women. J Trauma Stress. 1998;11:665-678.
16. Orsillo SM, Heimberg RG, Juster HR, Garrett J. Social phobia and PTSD in Vietnam veterans. J Trauma Stress. 1996;9:235-252.
17. George MS, Lydiard RB. Social phobia secondary to physical disability: a review of benign essential tremor (BET) and stuttering. Psychosomatics. 1994;35:520-523.
18. Connor KM, et al. Mini-SPIN: A brief screening assessment for generalized social anxiety disorder. Depress Anxiety. 2001;14:137-140.
19. Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280:708-713.
20. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19:341-348.
21. Heimberg RG. Specific issues in the cognitive behavioral treatment of social phobia. J Clin Psychiatry. 1993;54(suppl 12):36-45.
Social phobia was accorded official psychiatric diagnostic status in the United States less than 20 years ago, but has been described in the medical literature for centuries. Hippocrates described such a patient: “He dare not come in company for fear he should be misused, disgraced, overshoot himself in gestures or speeches or be sick; he thinks every man observes him.”1
This observation was made more than 2,000 years ago. Yet social anxiety disorder (SAD) was left largely unstudied until the mid-1980s.2 An estimated 20 million people in the U.S. suffer from this disorder.
What causes some people to break into a cold sweat at the thought of the most casual encounter with a checkout clerk, a coworker, or an acquaintance? Limited evidence points to underlying biological abnormalities in SAD, but there have been no conclusive findings.
Two main subtypes of SAD exist (Box 1). Roughly 25% of sufferers have discrete or nongeneralized SAD, that is, circumscribed social fears limited to one or two situations, such as speaking in public or performing before an audience. The remaining 75% suffer from generalized SAD, the more severe subtype in which all or nearly all interpersonal interactions are difficult.
Generalized SAD often begins early in life, with a mean onset at about age 15, but 35% of the time SAD occurs in individuals before age 10.3 This subtype appears to run in families, while the nongeneralized subtype does not, suggesting that a genetic inheritance is possible. From an etiological perspective, the possible effects of parenting styles of socially anxious parents, or acquisition of social anxiety conditioned by experiencing extreme embarrassment, may also contribute to the development of SAD in some people. Approximately twice as many females as males are affected, and almost all are affected before age 25.3,4 When social fears interfere with social, occupational, or family life, the affected individual is not suffering from normal "shyness," but rather a treatable anxiety disorder.
–Toastmasters slogan
Generalized
- Most social interactions
- Early onset
- Social skills deficit
- High comorbidity
- Lower achievement
- Remission rare
Nongeneralized
- Limited fears
- Later onset
- Social skills normal
- Less comorbidity
- Less Impairment
- Remits often
The National Comorbidity Survey (NCS) estimated lifetime prevalence of SAD at 13.3% and 12-month prevalence at 7.6%, making it the third most common psychiatric disorder, following only major depression and alcohol abuse/dependence.5 Despite this high rate, SAD remains woefully underdiagnosed.
Anyone who has had to speak in public, play a musical instrument at a recital, or perform in some way under the watchful expectation of an audience has experienced anxiety as he or she anticipates the "big moment” (Box 2). Once the performance is under way, the anxiety usually lessens to a more manageable level for most people. In fact, nearly one in three Americans will admit to moderate or great fear of speaking in public.
Mr. L, a 40-year-old eighth-grade teacher, consulted a psychiatrist because he was scheduled to be evaluated by a state education accreditation committee while teaching class. Though he had always passed these before, he had been worried sick for weeks and was experiencing panic attacks each time he thought about the accreditation visit.
He lived with his mother, had never dated, and had few friends. He was extremely inhibited outside the classroom, brought cash to stores to avoid being observed while writing a check or signing credit card slips, and avoided social gatherings outside of his church, which he attended with his mother and tolerated with distress.
Further history revealed that he had quit medical school during his third year because he had so much difficulty presenting cases to the attending on ward rounds that he chose to leave the profession in order to avoid feeling sick each morning and afternoon.
Enough people encounter the fear of public speaking to support the weekly Toastmasters meetings in most U.S. cities. Many people overcome their social anxiety about public speaking or performing with continued practice. However, those with nongeneralized SAD, who are among the most severely affected, may remain so fearful of speaking or performing under scrutiny that they avoid it at any cost—even if it means passing up a job or promotion or even choosing to change professions.
The majority (75%) of those with SAD—representing approximately 15 million individuals in the U.S.—suffer from generalized SAD, a much more severe, potentially disabling subtype. These unfortunate individuals fear and avoid most or all social interactions outside their home except those with family or close friends. When they encounter or even anticipate entering feared social situations, individuals with generalized SAD experience severe anxiety. Blushing, tremulousness, and sweating can be noticed by others, and thus are particularly distressing to those with SAD.
Recovery without treatment is rare. The typically early age at onset of generalized SAD3,4 imposes greater limitations on development of social competence than on those who develop more discrete fear of public speaking or performing later in life—after socialization skills have already developed.
Individuals with SAD frequently suffer from comorbid psychiatric disorders, mostly depression and/or other anxiety disorders.6Figure 1 shows that individuals with SAD are at significantly increased risk for depression, other anxiety disorders, and alcohol and drug abuse. Since generalized SAD usually appears at an earlier age than other anxiety disorders, it represents a risk factor for subsequent depression. The level of functional impairment caused by SAD is similar to that caused by major depression7 (Figure 2).
As more comorbid psychiatric disorders accrue, impairment and increased risk for additional disorders may occur. Further, the risk of suicide is increased in those with comorbid SAD vs. those with SAD only. The findings suggest that if social anxiety were detected and treated effectively at an early age, it might be possible to prevent other psychiatric disorders—particularly depression—as well as the predictable morbidity and mortality that accompanies untreated SAD. Given the estimated $44 billion annual cost of anxiety disorders in the U.S.,8 research targeted at testing this hypothesis would appear to be a good investment.
Figure 1 HOW PREVALENT IS LIFETIME COMORBIDITY IN SAD?
- Inherent avoidance of scrutiny, (e.g., evaluation)
- Uncertain diagnostic threshold
- Acceptance of pathological shyness as ‘just my personality’
- Lack of understanding by professionals, family, friends
- Coping strategies that mask disability
- Comorbid psychiatric disorders that mask SAD
Figure 2 QUALITY OF LIFE IN PATIENTS WITH SOCIAL ANXIETY DISORDER
Seeing the unseen: making the diagnosis quickly
Although SAD is extremely common, a variety of factors may contribute to the low rate of recognition of the disorder (Box 3). Because of their intense discomfort toward scrutiny by authority figures such as their physician, individuals with SAD may not be willing to discuss their fears. Studies estimating the prevalence in primary care suggest that these individuals visit their referring physicians at about the same rate as the general population.6,9-11 Affected individuals are unlikely to seek psychiatric treatment unless they have a comorbid depression or anxiety.6,7
Table 1
DIFFERENTIAL DIAGNOSIS FOR SOCIAL ANXIETY DISORDER
| Condition | Diagnostic features |
|---|---|
| Posttraumatic stress disorder | Temporally follows traumatic event; cues related to trauma, not exclusively to social situations |
| Panic disorder | Unexpected panic attacks, not exclusively socially mediated anxiety |
| Agoraphobia | Fearful avoidance of situations in which panic attacks may occur, not limited to social situations |
| Major depression or atypical depression | Social withdrawal temporally related to mood disturbance, not to fear of humiliation or embarrassment; atypical depression with rejection sensitivity associated with other symptoms (e.g., hypersomnia, hyperphagia, anergy, mood reactivity) |
| Generalized anxiety disorder | Focus of worry not limited to social situations; social discomfort or avoidance not a key feature |
| Body dysmorphic disorder | Avoidance of social activity focused on concern over perceived ugliness |
| Avoidant personality disorder | Often present in generalized social anxiety disorder; may represent more severe end of social anxiety disorder spectrum; social activity desired but avoided |
| Schizotypal/schizoid personality disorders | Avoidance of social situations is preferred by individual and is not due to fear of embarrassment or humiliation |
| Normal shyness | Minimal or no interference with social, occupational, or family functioning |
| Adapted from Lydiard RB. Social anxiety disorder: comorbidity and its implications. J Clin Psychiatry.2001;62(suppl 1):17-23. | |
SAD can be difficult to tease apart from other coexisting conditions. Individuals who present for treatment of other anxiety disorders, depression, or substance abuse disorders should be considered at risk for current but undetected SAD. Many of the symptoms overlap (Table 1). The key diagnostic feature, which leads to the diagnosis of SAD, is that the fear and avoidance specifically are related to being in or anticipating a feared social situation (Box 4).
Social
- Attending parties, weddings etc.
- Conversing in a group
- Speaking on the telephone
- Interacting with authority figure (e.g., teacher or boss)
- Making eye contact
- Ordering in a restaurant
Performance
- Public speaking
- Eating in public
- Writing a check
- Using a public toilet
- Taking a test
- Trying on clothes in a store
- Speaking up at a meeting
Many clinicians mistake social anxiety for panic disorder, since panic attacks in people with SAD are often cued by social situations. There can be up to a 40% overlap of SAD with panic disorder.4 Without probing carefully into the focus on fear and avoidance, SAD can be easily overlooked. Individuals with panic disorder experience unexpected attacks and are terrified at the prospect of additional attacks, while those with SAD experience attacks linked to social situations and fear scrutiny and embarrassment more than the attacks themselves.
SAD and major depression frequently coexist,4,11,12 challenging clinicians to distinguish social reticence and withdrawal accompanying depression from the fearful avoidance that typifies SAD. SAD usually precedes depression. Asking if the patient experienced social anxiety prior to the onset of depression can help identify SAD with comorbid depression.
Alcohol-related disorders are twice as likely to occur in those affected by SAD as in those not affected. The risk for females increases more than it does for males.2-4 SAD most often precedes alcohol abuse. Studies show that about 30% of patients receiving treatment for alcohol abuse/dependence have SAD. If it remains undetected, the risk of rapid relapse is high. That’s because patients are highly unlikely to participate in psychosocial treatments that help sustain post-treatment abstinence, such as the Alcoholics Anonymous 12-step program. A recent study found that both social anxiety and alcohol abuse disorders improve when SAD in alcoholics is treated.13
A substantially higher percentage of adults with SAD, especially women, have histories of prior childhood sexual and/or physical abuse than the general population.14 Recent studies both in women following rape and in combat veterans with posttraumatic stress disorder (PTSD) suggest that those with perceived life-threatening events are at higher risk for developing secondary SAD than are individuals who experience less severe trauma.15,16 We do not yet know if secondary SAD in trauma victims is different in character or response to treatment.
Individuals with certain medical conditions can develop symptoms resembling SAD. These include stuttering, benign essential tremor,17 Parkinson’s disease, disfiguring burn injuries, and possibly irritable bowel syndrome. Such patients are technically excluded from being diagnosed with SAD, though they would meet criteria if the diagnostic rules were suspended. A limited body of literature and clinical experience suggests that symptoms secondary to physical conditions may respond to the same treatment as SAD in medically healthy persons. A treatment trial for selected patients with SAD symptoms associated with medical conditions may provide significant benefits. Clearly more research is needed in this area.
- “Being embarrassed or looking stupid are among my worst fears.”
- “Fear of embarrassment causes me to avoid doing things or speaking to people.”
- “I avoid activities in which I am the center of attention.”
Connor et al, Depress Anxiety 2001;14:137
A recently developed self-rating scale, the Social Phobia Inventory (SPIN), assesses the spectrum of cognitive, behavioral, and physiological symptoms associated with SAD.18 Three of the 17 SPIN items have been found to identify generalized SAD with a high degree of sensitivity (Box 5).
The 3 main goals of SAD treatment are
- Acute reduction and control of pathological anxiety and related phobic avoidance;
- Adequate treatment of depression or other comorbid conditions;
- Long-term management of the social phobia.
Significant advances in treatment have emerged over the past 2 decades. We now know that cognitive behavioral therapy (CBT), medication, and their combination are efficacious.
Social situations involving speaking or performance are usually predictable, and nongeneralized SAD is thus amenable to use of a beta-blocker or benzodiazepine (Box 6). Beta-blockers are often adequate for control of tremor and increased heart rate. Some patients may also benefit more from judicious use of a benzodiazepine prior to the event.
In contrast, generalized SAD is less predictable, and continual treatment is recommended. Ideally, a medication regimen would be easily tolerated long-term, and would have antidepressant effects and a broad spectrum of efficacy against commonly coexisting disorders. Because of the significant risk for depression in individuals with SAD, first-line antidepressant treatment is preferred when possible over other medications.
The selective serotonin reuptake inhibitors (SSRIs) are now considered the first-line pharmacological treatment for social phobia. Paroxetine was the first to receive FDA approval for generalized SAD.19 Large multicenter studies supporting the efficacy of two other SSRIs—sertraline and fluvoxamine—have been reported.12 The SSRIs also appear to work against the other psychiatric disorders with which SAD commonly co-occurs, such as panic disorder, major depression, generalized anxiety disorder, and PTSD.
The empirical database is very limited, but it appears that SSRI treatment for a significant percentage of patients with SAD may require higher doses (up to twice the amount) than are usually needed for depression.10 Approximately 50% to 65% of patients with generalized SAD respond to any given SSRI. In our experience, failure to respond to one SSRI does not preclude response to a second SSRI.
The irreversible monoamine oxidase (MAO) inhibitor phenelzine was the first antidepressant shown to be useful for SAD. Tranylcypromine is less well studied, but also appears to be effective. The significant side effects (weight gain, orthostatic hypotension, insomnia) and inconvenience of administration have reduced use of these agents.
Nongeneralized
- PRN treatment
- Beta-blockers
- Benzodiazepines
Generalized
- Continuous treatment
- Broad-spectrum antidepressants
- Benzodiazepines
- MAOIs
- Antiepileptic agents
The tricyclic antidepressants are probably not effective, with the exception of clomipramine (also a potent inhibitor of serotonin reuptake).12 Clomipramine, while an effective anxiolytic and antidepressant, causes prohibitive side effects in many patients (e.g., sexual dysfunction and weight gain).
Key elements for individual or group setting
- Cognitive “restructuring”
- Social skills enhancement
The newer antidepressants venlafaxine and nefazodone are less well studied than the SSRIs, but show promise as potential broad-spectrum agents. Bupropion, a novel antidepressant, and the azapirone anxiolytic buspirone do not appear to work against SAD.
The main role of the benzodiazepines in SAD treatment is adjunctive to antidepressants or in some patients intolerant of, or unresponsive to, other treatments. Clonazepam, alprazolam, and probably others are effective for SAD, but they may not effectively treat or prevent depression or other commonly associated disorders.
The anticonvulsant gabapentin has been shown in one controlled study to be effective in treating SAD.20 This agent may be particularly useful for complicated patients such as those with a history of alcohol-related disorders, bipolar-spectrum disorder, or intolerance to SSRIs.
In parallel with the development of effective psychopharmacological treatments, several types of behavioral and cognitive behavioral treatments have been investigated, including imaginal flooding, graduated exposure, social skills training, cognitive-behavior approaches, and combined cognitive restructuring and graduated exposure.21 These treatments involve similar elements targeted at the cognitive distortions and avoidance behaviors, which represent core features of SAD (Box 7).
Many clinicians believe that combined pharmacotherapy and CBT treatment are superior to either modality alone for treating SAD. The little empirical information available indicates that acute treatment differences between drug alone and drug in combination with CBT are not impressive. However, there appears to be a lower rate of relapse following CBT than after medication discontinuation.
Despite our ability to treat this disorder, only a small fraction of sufferers get treatment. If untreated, the risk of comorbidity is extremely high. Routine screening for SAD, especially in younger individuals, could provide for early detection and treatment. Psychiatrists can play an important role in early detection and treatment by educating consumers, teachers, school nurses, psychologists, and pediatricians.
Related resources
- Lydiard, R.B. Social anxiety disorder comorbidity and its implications J. Clin. Psychiatry. 2001;62(suppl):17-23.
- American Psychiatric Association, http://www.psych.org
- American Psychological Association, http://www.apa.org
- National Institute for Mental Health: Anxiety Disorders, http://www.nimh.nih.gov/anxiety/
- Anxiety Disorders Associations of America, http://www.adaa.org/
Drug brand names
- Alprazolam • Xanax
- Atenolol • Tenormin
- Bupropion • Wellbutrin
- Buspirone • Buspar
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Gabapentin • Neurontin
- Lorazepam • Ativan
- Nefazodone • Serzone
- Paroxetine • Paxil
- Phenelzine • Nardil
- Propranolol • Inderal
- Sertraline • Zoloft
- Tranylcypromine • Parnate
- Venlafaxine • Effexor
Disclosure
The author reports that he has received grant/research support and has served as a consultant to and on the speaker's bureau of Bristol-Myers Squibb Co., GlaxoSmithKline, Pfizer Inc., Eli Lilly and Co., Parke-Davis, and Solvay Pharmaceuticals. He also has received grant/research support and served as a consultant for Forest Pharmaceuticals, Wyeth-Ayerst Pharmaceuticals, and Roche; received grant/research support from Sanofi-Synthelabo; and has served as consultant for Dupont Pharmaceuticals and AstraZeneca.
Social phobia was accorded official psychiatric diagnostic status in the United States less than 20 years ago, but has been described in the medical literature for centuries. Hippocrates described such a patient: “He dare not come in company for fear he should be misused, disgraced, overshoot himself in gestures or speeches or be sick; he thinks every man observes him.”1
This observation was made more than 2,000 years ago. Yet social anxiety disorder (SAD) was left largely unstudied until the mid-1980s.2 An estimated 20 million people in the U.S. suffer from this disorder.
What causes some people to break into a cold sweat at the thought of the most casual encounter with a checkout clerk, a coworker, or an acquaintance? Limited evidence points to underlying biological abnormalities in SAD, but there have been no conclusive findings.
Two main subtypes of SAD exist (Box 1). Roughly 25% of sufferers have discrete or nongeneralized SAD, that is, circumscribed social fears limited to one or two situations, such as speaking in public or performing before an audience. The remaining 75% suffer from generalized SAD, the more severe subtype in which all or nearly all interpersonal interactions are difficult.
Generalized SAD often begins early in life, with a mean onset at about age 15, but 35% of the time SAD occurs in individuals before age 10.3 This subtype appears to run in families, while the nongeneralized subtype does not, suggesting that a genetic inheritance is possible. From an etiological perspective, the possible effects of parenting styles of socially anxious parents, or acquisition of social anxiety conditioned by experiencing extreme embarrassment, may also contribute to the development of SAD in some people. Approximately twice as many females as males are affected, and almost all are affected before age 25.3,4 When social fears interfere with social, occupational, or family life, the affected individual is not suffering from normal "shyness," but rather a treatable anxiety disorder.
–Toastmasters slogan
Generalized
- Most social interactions
- Early onset
- Social skills deficit
- High comorbidity
- Lower achievement
- Remission rare
Nongeneralized
- Limited fears
- Later onset
- Social skills normal
- Less comorbidity
- Less Impairment
- Remits often
The National Comorbidity Survey (NCS) estimated lifetime prevalence of SAD at 13.3% and 12-month prevalence at 7.6%, making it the third most common psychiatric disorder, following only major depression and alcohol abuse/dependence.5 Despite this high rate, SAD remains woefully underdiagnosed.
Anyone who has had to speak in public, play a musical instrument at a recital, or perform in some way under the watchful expectation of an audience has experienced anxiety as he or she anticipates the "big moment” (Box 2). Once the performance is under way, the anxiety usually lessens to a more manageable level for most people. In fact, nearly one in three Americans will admit to moderate or great fear of speaking in public.
Mr. L, a 40-year-old eighth-grade teacher, consulted a psychiatrist because he was scheduled to be evaluated by a state education accreditation committee while teaching class. Though he had always passed these before, he had been worried sick for weeks and was experiencing panic attacks each time he thought about the accreditation visit.
He lived with his mother, had never dated, and had few friends. He was extremely inhibited outside the classroom, brought cash to stores to avoid being observed while writing a check or signing credit card slips, and avoided social gatherings outside of his church, which he attended with his mother and tolerated with distress.
Further history revealed that he had quit medical school during his third year because he had so much difficulty presenting cases to the attending on ward rounds that he chose to leave the profession in order to avoid feeling sick each morning and afternoon.
Enough people encounter the fear of public speaking to support the weekly Toastmasters meetings in most U.S. cities. Many people overcome their social anxiety about public speaking or performing with continued practice. However, those with nongeneralized SAD, who are among the most severely affected, may remain so fearful of speaking or performing under scrutiny that they avoid it at any cost—even if it means passing up a job or promotion or even choosing to change professions.
The majority (75%) of those with SAD—representing approximately 15 million individuals in the U.S.—suffer from generalized SAD, a much more severe, potentially disabling subtype. These unfortunate individuals fear and avoid most or all social interactions outside their home except those with family or close friends. When they encounter or even anticipate entering feared social situations, individuals with generalized SAD experience severe anxiety. Blushing, tremulousness, and sweating can be noticed by others, and thus are particularly distressing to those with SAD.
Recovery without treatment is rare. The typically early age at onset of generalized SAD3,4 imposes greater limitations on development of social competence than on those who develop more discrete fear of public speaking or performing later in life—after socialization skills have already developed.
Individuals with SAD frequently suffer from comorbid psychiatric disorders, mostly depression and/or other anxiety disorders.6Figure 1 shows that individuals with SAD are at significantly increased risk for depression, other anxiety disorders, and alcohol and drug abuse. Since generalized SAD usually appears at an earlier age than other anxiety disorders, it represents a risk factor for subsequent depression. The level of functional impairment caused by SAD is similar to that caused by major depression7 (Figure 2).
As more comorbid psychiatric disorders accrue, impairment and increased risk for additional disorders may occur. Further, the risk of suicide is increased in those with comorbid SAD vs. those with SAD only. The findings suggest that if social anxiety were detected and treated effectively at an early age, it might be possible to prevent other psychiatric disorders—particularly depression—as well as the predictable morbidity and mortality that accompanies untreated SAD. Given the estimated $44 billion annual cost of anxiety disorders in the U.S.,8 research targeted at testing this hypothesis would appear to be a good investment.
Figure 1 HOW PREVALENT IS LIFETIME COMORBIDITY IN SAD?
- Inherent avoidance of scrutiny, (e.g., evaluation)
- Uncertain diagnostic threshold
- Acceptance of pathological shyness as ‘just my personality’
- Lack of understanding by professionals, family, friends
- Coping strategies that mask disability
- Comorbid psychiatric disorders that mask SAD
Figure 2 QUALITY OF LIFE IN PATIENTS WITH SOCIAL ANXIETY DISORDER
Seeing the unseen: making the diagnosis quickly
Although SAD is extremely common, a variety of factors may contribute to the low rate of recognition of the disorder (Box 3). Because of their intense discomfort toward scrutiny by authority figures such as their physician, individuals with SAD may not be willing to discuss their fears. Studies estimating the prevalence in primary care suggest that these individuals visit their referring physicians at about the same rate as the general population.6,9-11 Affected individuals are unlikely to seek psychiatric treatment unless they have a comorbid depression or anxiety.6,7
Table 1
DIFFERENTIAL DIAGNOSIS FOR SOCIAL ANXIETY DISORDER
| Condition | Diagnostic features |
|---|---|
| Posttraumatic stress disorder | Temporally follows traumatic event; cues related to trauma, not exclusively to social situations |
| Panic disorder | Unexpected panic attacks, not exclusively socially mediated anxiety |
| Agoraphobia | Fearful avoidance of situations in which panic attacks may occur, not limited to social situations |
| Major depression or atypical depression | Social withdrawal temporally related to mood disturbance, not to fear of humiliation or embarrassment; atypical depression with rejection sensitivity associated with other symptoms (e.g., hypersomnia, hyperphagia, anergy, mood reactivity) |
| Generalized anxiety disorder | Focus of worry not limited to social situations; social discomfort or avoidance not a key feature |
| Body dysmorphic disorder | Avoidance of social activity focused on concern over perceived ugliness |
| Avoidant personality disorder | Often present in generalized social anxiety disorder; may represent more severe end of social anxiety disorder spectrum; social activity desired but avoided |
| Schizotypal/schizoid personality disorders | Avoidance of social situations is preferred by individual and is not due to fear of embarrassment or humiliation |
| Normal shyness | Minimal or no interference with social, occupational, or family functioning |
| Adapted from Lydiard RB. Social anxiety disorder: comorbidity and its implications. J Clin Psychiatry.2001;62(suppl 1):17-23. | |
SAD can be difficult to tease apart from other coexisting conditions. Individuals who present for treatment of other anxiety disorders, depression, or substance abuse disorders should be considered at risk for current but undetected SAD. Many of the symptoms overlap (Table 1). The key diagnostic feature, which leads to the diagnosis of SAD, is that the fear and avoidance specifically are related to being in or anticipating a feared social situation (Box 4).
Social
- Attending parties, weddings etc.
- Conversing in a group
- Speaking on the telephone
- Interacting with authority figure (e.g., teacher or boss)
- Making eye contact
- Ordering in a restaurant
Performance
- Public speaking
- Eating in public
- Writing a check
- Using a public toilet
- Taking a test
- Trying on clothes in a store
- Speaking up at a meeting
Many clinicians mistake social anxiety for panic disorder, since panic attacks in people with SAD are often cued by social situations. There can be up to a 40% overlap of SAD with panic disorder.4 Without probing carefully into the focus on fear and avoidance, SAD can be easily overlooked. Individuals with panic disorder experience unexpected attacks and are terrified at the prospect of additional attacks, while those with SAD experience attacks linked to social situations and fear scrutiny and embarrassment more than the attacks themselves.
SAD and major depression frequently coexist,4,11,12 challenging clinicians to distinguish social reticence and withdrawal accompanying depression from the fearful avoidance that typifies SAD. SAD usually precedes depression. Asking if the patient experienced social anxiety prior to the onset of depression can help identify SAD with comorbid depression.
Alcohol-related disorders are twice as likely to occur in those affected by SAD as in those not affected. The risk for females increases more than it does for males.2-4 SAD most often precedes alcohol abuse. Studies show that about 30% of patients receiving treatment for alcohol abuse/dependence have SAD. If it remains undetected, the risk of rapid relapse is high. That’s because patients are highly unlikely to participate in psychosocial treatments that help sustain post-treatment abstinence, such as the Alcoholics Anonymous 12-step program. A recent study found that both social anxiety and alcohol abuse disorders improve when SAD in alcoholics is treated.13
A substantially higher percentage of adults with SAD, especially women, have histories of prior childhood sexual and/or physical abuse than the general population.14 Recent studies both in women following rape and in combat veterans with posttraumatic stress disorder (PTSD) suggest that those with perceived life-threatening events are at higher risk for developing secondary SAD than are individuals who experience less severe trauma.15,16 We do not yet know if secondary SAD in trauma victims is different in character or response to treatment.
Individuals with certain medical conditions can develop symptoms resembling SAD. These include stuttering, benign essential tremor,17 Parkinson’s disease, disfiguring burn injuries, and possibly irritable bowel syndrome. Such patients are technically excluded from being diagnosed with SAD, though they would meet criteria if the diagnostic rules were suspended. A limited body of literature and clinical experience suggests that symptoms secondary to physical conditions may respond to the same treatment as SAD in medically healthy persons. A treatment trial for selected patients with SAD symptoms associated with medical conditions may provide significant benefits. Clearly more research is needed in this area.
- “Being embarrassed or looking stupid are among my worst fears.”
- “Fear of embarrassment causes me to avoid doing things or speaking to people.”
- “I avoid activities in which I am the center of attention.”
Connor et al, Depress Anxiety 2001;14:137
A recently developed self-rating scale, the Social Phobia Inventory (SPIN), assesses the spectrum of cognitive, behavioral, and physiological symptoms associated with SAD.18 Three of the 17 SPIN items have been found to identify generalized SAD with a high degree of sensitivity (Box 5).
The 3 main goals of SAD treatment are
- Acute reduction and control of pathological anxiety and related phobic avoidance;
- Adequate treatment of depression or other comorbid conditions;
- Long-term management of the social phobia.
Significant advances in treatment have emerged over the past 2 decades. We now know that cognitive behavioral therapy (CBT), medication, and their combination are efficacious.
Social situations involving speaking or performance are usually predictable, and nongeneralized SAD is thus amenable to use of a beta-blocker or benzodiazepine (Box 6). Beta-blockers are often adequate for control of tremor and increased heart rate. Some patients may also benefit more from judicious use of a benzodiazepine prior to the event.
In contrast, generalized SAD is less predictable, and continual treatment is recommended. Ideally, a medication regimen would be easily tolerated long-term, and would have antidepressant effects and a broad spectrum of efficacy against commonly coexisting disorders. Because of the significant risk for depression in individuals with SAD, first-line antidepressant treatment is preferred when possible over other medications.
The selective serotonin reuptake inhibitors (SSRIs) are now considered the first-line pharmacological treatment for social phobia. Paroxetine was the first to receive FDA approval for generalized SAD.19 Large multicenter studies supporting the efficacy of two other SSRIs—sertraline and fluvoxamine—have been reported.12 The SSRIs also appear to work against the other psychiatric disorders with which SAD commonly co-occurs, such as panic disorder, major depression, generalized anxiety disorder, and PTSD.
The empirical database is very limited, but it appears that SSRI treatment for a significant percentage of patients with SAD may require higher doses (up to twice the amount) than are usually needed for depression.10 Approximately 50% to 65% of patients with generalized SAD respond to any given SSRI. In our experience, failure to respond to one SSRI does not preclude response to a second SSRI.
The irreversible monoamine oxidase (MAO) inhibitor phenelzine was the first antidepressant shown to be useful for SAD. Tranylcypromine is less well studied, but also appears to be effective. The significant side effects (weight gain, orthostatic hypotension, insomnia) and inconvenience of administration have reduced use of these agents.
Nongeneralized
- PRN treatment
- Beta-blockers
- Benzodiazepines
Generalized
- Continuous treatment
- Broad-spectrum antidepressants
- Benzodiazepines
- MAOIs
- Antiepileptic agents
The tricyclic antidepressants are probably not effective, with the exception of clomipramine (also a potent inhibitor of serotonin reuptake).12 Clomipramine, while an effective anxiolytic and antidepressant, causes prohibitive side effects in many patients (e.g., sexual dysfunction and weight gain).
Key elements for individual or group setting
- Cognitive “restructuring”
- Social skills enhancement
The newer antidepressants venlafaxine and nefazodone are less well studied than the SSRIs, but show promise as potential broad-spectrum agents. Bupropion, a novel antidepressant, and the azapirone anxiolytic buspirone do not appear to work against SAD.
The main role of the benzodiazepines in SAD treatment is adjunctive to antidepressants or in some patients intolerant of, or unresponsive to, other treatments. Clonazepam, alprazolam, and probably others are effective for SAD, but they may not effectively treat or prevent depression or other commonly associated disorders.
The anticonvulsant gabapentin has been shown in one controlled study to be effective in treating SAD.20 This agent may be particularly useful for complicated patients such as those with a history of alcohol-related disorders, bipolar-spectrum disorder, or intolerance to SSRIs.
In parallel with the development of effective psychopharmacological treatments, several types of behavioral and cognitive behavioral treatments have been investigated, including imaginal flooding, graduated exposure, social skills training, cognitive-behavior approaches, and combined cognitive restructuring and graduated exposure.21 These treatments involve similar elements targeted at the cognitive distortions and avoidance behaviors, which represent core features of SAD (Box 7).
Many clinicians believe that combined pharmacotherapy and CBT treatment are superior to either modality alone for treating SAD. The little empirical information available indicates that acute treatment differences between drug alone and drug in combination with CBT are not impressive. However, there appears to be a lower rate of relapse following CBT than after medication discontinuation.
Despite our ability to treat this disorder, only a small fraction of sufferers get treatment. If untreated, the risk of comorbidity is extremely high. Routine screening for SAD, especially in younger individuals, could provide for early detection and treatment. Psychiatrists can play an important role in early detection and treatment by educating consumers, teachers, school nurses, psychologists, and pediatricians.
Related resources
- Lydiard, R.B. Social anxiety disorder comorbidity and its implications J. Clin. Psychiatry. 2001;62(suppl):17-23.
- American Psychiatric Association, http://www.psych.org
- American Psychological Association, http://www.apa.org
- National Institute for Mental Health: Anxiety Disorders, http://www.nimh.nih.gov/anxiety/
- Anxiety Disorders Associations of America, http://www.adaa.org/
Drug brand names
- Alprazolam • Xanax
- Atenolol • Tenormin
- Bupropion • Wellbutrin
- Buspirone • Buspar
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Gabapentin • Neurontin
- Lorazepam • Ativan
- Nefazodone • Serzone
- Paroxetine • Paxil
- Phenelzine • Nardil
- Propranolol • Inderal
- Sertraline • Zoloft
- Tranylcypromine • Parnate
- Venlafaxine • Effexor
Disclosure
The author reports that he has received grant/research support and has served as a consultant to and on the speaker's bureau of Bristol-Myers Squibb Co., GlaxoSmithKline, Pfizer Inc., Eli Lilly and Co., Parke-Davis, and Solvay Pharmaceuticals. He also has received grant/research support and served as a consultant for Forest Pharmaceuticals, Wyeth-Ayerst Pharmaceuticals, and Roche; received grant/research support from Sanofi-Synthelabo; and has served as consultant for Dupont Pharmaceuticals and AstraZeneca.
1. Burton R. The Anatomy of Melancholy, vol. 1, 11th ed. London, England. Thomas Tegg, Cheapside; 1845.
2. Liebowitz MR, Gorman JM, Fyer AJ, Klein DF. Social phobia: review of a neglected anxiety disorder. Arch Gen Psychiatry. 1985;42:729-736.
3. Magee WJ, Eaton WW, Wittchen HU, et al. Agoraphobia, simple phobia, and social phobia in the National Comorbidity Survey. Arch Gen Psychiatry. 1996;53:159-168.
4. Schneier FR, Johnson J, et al. Social phobia: comorbidity and morbidity in an epidemiologic sample. Arch Gen Psychiatry. 1992;49:282-288.
5. Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8-19.
6. Goldenberg IM, et al. The infrequency of “pure culture” diagnosis among the anxiety disorders. J Clin Psychiatry. 1996;57:528-533.
7. Wittchen HU, Beloch E. The impact of social phobia on quality of life. Int Clin Psychopharmacol. 1996;11(suppl):15-23.
8. Greenberg PE, Sisitsky T, Kessler RC, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60:427-435.
9. Weiller E, Bisserbe JC, Boyer P, et al. Social phobia in general health care. Br J Psychiatry. 1996;168:169-174.
10. Katzelnick DJ, Greist JH. Social anxiety disorder: an unrecognized problem in primary care. J Clin Psychiatry. 2001;62:11.-
11. Davidson J. Social anxiety disorder under scrutiny. Depress Anxiety. 2000;11:93-98.
12. Lydiard RB. Social anxiety disorder: treatment role of the SSRIs. In Montgomery SA, den Boer JA (eds). SSRIs in Depression and Anxiety Perspectives in Psychiatry ,vol 8. NY: Wiley, Chichester, 129-150, 2001.
13. Randall CL, et al. Paroxetine improves both social anxiety and alcohol use in dually-diagnosed patients at the American College of Neuropsychopharmacology. San Juan, Puerto Rico. Dec 10-15, 2000.
14. Stein MB, Walker JR, Anderson G, et al. Childhood physical and sexual abuse in patients with anxiety disorders and in a community sample. Am J Psychiatry. 1996;153:275-277.
15. Boudreaux E, Kilpatrick DG, Resnick HS, et al. Criminal victimization, posttraumatic stress disorder, and comorbid psychopathology among a community sample of women. J Trauma Stress. 1998;11:665-678.
16. Orsillo SM, Heimberg RG, Juster HR, Garrett J. Social phobia and PTSD in Vietnam veterans. J Trauma Stress. 1996;9:235-252.
17. George MS, Lydiard RB. Social phobia secondary to physical disability: a review of benign essential tremor (BET) and stuttering. Psychosomatics. 1994;35:520-523.
18. Connor KM, et al. Mini-SPIN: A brief screening assessment for generalized social anxiety disorder. Depress Anxiety. 2001;14:137-140.
19. Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280:708-713.
20. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19:341-348.
21. Heimberg RG. Specific issues in the cognitive behavioral treatment of social phobia. J Clin Psychiatry. 1993;54(suppl 12):36-45.
1. Burton R. The Anatomy of Melancholy, vol. 1, 11th ed. London, England. Thomas Tegg, Cheapside; 1845.
2. Liebowitz MR, Gorman JM, Fyer AJ, Klein DF. Social phobia: review of a neglected anxiety disorder. Arch Gen Psychiatry. 1985;42:729-736.
3. Magee WJ, Eaton WW, Wittchen HU, et al. Agoraphobia, simple phobia, and social phobia in the National Comorbidity Survey. Arch Gen Psychiatry. 1996;53:159-168.
4. Schneier FR, Johnson J, et al. Social phobia: comorbidity and morbidity in an epidemiologic sample. Arch Gen Psychiatry. 1992;49:282-288.
5. Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8-19.
6. Goldenberg IM, et al. The infrequency of “pure culture” diagnosis among the anxiety disorders. J Clin Psychiatry. 1996;57:528-533.
7. Wittchen HU, Beloch E. The impact of social phobia on quality of life. Int Clin Psychopharmacol. 1996;11(suppl):15-23.
8. Greenberg PE, Sisitsky T, Kessler RC, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60:427-435.
9. Weiller E, Bisserbe JC, Boyer P, et al. Social phobia in general health care. Br J Psychiatry. 1996;168:169-174.
10. Katzelnick DJ, Greist JH. Social anxiety disorder: an unrecognized problem in primary care. J Clin Psychiatry. 2001;62:11.-
11. Davidson J. Social anxiety disorder under scrutiny. Depress Anxiety. 2000;11:93-98.
12. Lydiard RB. Social anxiety disorder: treatment role of the SSRIs. In Montgomery SA, den Boer JA (eds). SSRIs in Depression and Anxiety Perspectives in Psychiatry ,vol 8. NY: Wiley, Chichester, 129-150, 2001.
13. Randall CL, et al. Paroxetine improves both social anxiety and alcohol use in dually-diagnosed patients at the American College of Neuropsychopharmacology. San Juan, Puerto Rico. Dec 10-15, 2000.
14. Stein MB, Walker JR, Anderson G, et al. Childhood physical and sexual abuse in patients with anxiety disorders and in a community sample. Am J Psychiatry. 1996;153:275-277.
15. Boudreaux E, Kilpatrick DG, Resnick HS, et al. Criminal victimization, posttraumatic stress disorder, and comorbid psychopathology among a community sample of women. J Trauma Stress. 1998;11:665-678.
16. Orsillo SM, Heimberg RG, Juster HR, Garrett J. Social phobia and PTSD in Vietnam veterans. J Trauma Stress. 1996;9:235-252.
17. George MS, Lydiard RB. Social phobia secondary to physical disability: a review of benign essential tremor (BET) and stuttering. Psychosomatics. 1994;35:520-523.
18. Connor KM, et al. Mini-SPIN: A brief screening assessment for generalized social anxiety disorder. Depress Anxiety. 2001;14:137-140.
19. Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA. 1998;280:708-713.
20. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19:341-348.
21. Heimberg RG. Specific issues in the cognitive behavioral treatment of social phobia. J Clin Psychiatry. 1993;54(suppl 12):36-45.
Rapid-cycling bipolar disorder: Which therapies are most effective?
Patients with rapid-cycling bipolar disorder (RCBD) can be frustrating to treat. Despite growing research and data, knowledge and effective therapies remain limited. How do you manage patients with rapid cycling who do not respond robustly to lithium, divalproex, or carbamazepine monotherapy? Are combination therapies likely to be more effective? Where does lamotrigine fit in? Is there a role for conventional antidepressants?
We’ll explore these and related questions—but the final answers are not yet in. Recognition of RCBD is important because it presents such difficult treatment challenges. Available evidence does suggest that rapid cycling as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (Box 1), describes a clinically specific course of illness that may require treatments different from currently used traditional drug therapies for nonrapid cycling bipolar disorder, particularly as no one agent appears to provide ideal bimodal treatment and prophylaxis of this bipolar disorder variant.
Rapid cycling is a specifier of the longitudinal course of illness presentation that is seen almost exclusively in bipolar disorder and is associated with a greater morbidity. Dunner and Fieve1 originally coined the term when evaluating clinical factors associated with lithium prophylaxis failure. Since that time the validity of rapid cycling as a distinct course modifier for bipolar disorder has been supported by multiple studies, leading to its inclusion in the fourth edition of the Diagnostic and Statistical Manual of the APA (1994).
According to DSM-IV, the course specifier of rapid cycling applies to “at least 4 episodes of a mood disturbance in the previous 12 months that meet criteria for a manic episode, a hypomanic episode, or a major depressive episode.” The episodes must be demarcated by a full or partial remission lasting at least 2 months or by a switch to a mood state of opposite polarity.
Early reports noted that patients suffering from RCBD did not respond adequately when treated with lithium.1 Other observations indicated that divalproex was more effective in this patient population, particularly for the illness’ hypomanic or manic phases.2 We hope that the following evaluation of these and other drug therapies will prove helpful.
Watch out for antidepressants
Most concerning has been the frequency and severity of treatment-refractory depressive phases of RCBD that may be exacerbated by antidepressant use (cycle induction or acceleration). Indeed, the frequent recurrence of refractory depression has been described as the hallmark of this bipolar disorder variant.3
Lithium: the scale weighs against it
Although an excellent mood stabilizer for most patients with bipolar disorder, lithium monotherapy is less than ideal for patients with the rapid-cycling variant, particularly in treatment or prevention of depressive or mixed episodes. The efficacy of lithium is likely decreased by the concurrent administration of antidepressant medication and increased when administered with other mood stabilizers.
The landmark article by Dunner and Fieve,1 which described a placebo-controlled, double-blind maintenance study in a general cohort of 55 patients, tried to clarify factors associated with the failure of lithium prophylaxis in bipolar disorder. Rapid cyclers comprised 20% of the subjects and 80% were nonrapid cyclers. Rapid cyclers were disproportionately represented in the lithium failure group. Lithium failures included 82% (9 of 11) of rapid cyclers compared to 41% (18 of 44) of nonrapid cyclers. Lithium failure was defined as (1) hospitalization for, or (2) treatment of, mania or (3) depression during lithium therapy, or as mood symptoms that, as documented by rating scales, were sufficient to warrant a diagnosis of mild depression, hypomania, or mania persisting for at least 2 weeks.
Kukopulos et al4 replicated the findings of Dunner and Fieve in a study of the longitudinal clinical course of 434 bipolar patients. Of these patients, 50 were rapid cyclers and had received continuous lithium therapy for more than a year, with good to partial prophylaxis in only 28%. Maj and colleagues5 published a 5-year prospective study of lithium therapy in 402 patients with bipolar disorder and noted the absence of rapid cycling in good responders to lithium but an incidence rate of 26% in nonresponders to lithium.
Other investigators have reported better response in RCBD. In a select cohort of lithium-responsive bipolar I and II patients, Tondo et al6 concluded that lithium maintenance yields striking long-term reductions in depressive and manic morbidity, more so in rapid cycling type II patients. This study, however, was in a cohort of lithium responders and excluded patients who had been exposed to antipsychotic or antidepressant medications for more than 3 months, those on chronic anticonvulsant therapy, and those with substance abuse disorders.
Although most studies do report poor response to lithium therapy in RCBD, Wehr and colleagues7 suggest that in some patients with rapid cycling, the discontinuation of antidepressant drugs may allow lithium to act as a more effective anticycling mood-stabilizing agent.
Divalproex: effective in manic phase
In contrast to lithium, an open trial of a homogenous cohort of patients with RCBD by Calabrese and colleagues3,8 found divalproex to possess moderate to marked acute and prophylactic antimanic properties with only modest antidepressant effects (Table 1). Data from 6 open studies involving 147 patients with rapid cycling suggest that divalproex possesses moderate to marked efficacy in the manic phase, but poor to moderate efficacy in the depressed phase. Positive outcome predictors were bipolar II and mixed states, no prior lithium therapy, and a positive family history of affective disorder. Predictors of negative response included increase in frequency and severity of mania, and borderline personality disorder.
Divalproex therapy in combination with lithium may improve response rates.9 Calabrese and colleagues, however, have examined large cohorts of patients, including those comorbid with alcohol, cocaine, and/or cannabis abuse, treated with a lithium-divalproex combination over 6-month study periods. The researchers found that only 25% to 50% of patients stabilized, and that of those not exhibiting a response, the majority (75%) did not respond because of treatment-refractory depression in the context of RCBD.3
Although experts believe divalproex to be more effective than lithium in preventing episodes associated with RCBD, such a conclusion awaits confirmation with the near completion of a double-blind, 20-month maintenance trial sponsored by the National Institute of Mental Health (NIMH).
Carbamazepine’s role in combination therapy
Early reports by Post and colleagues in 1987 suggested that rapid cycling predicted positive response to carbamazepine, but later findings by Okuma in 1993 refuted this. Other collective open and controlled studies suggest that this anticonvulsant possesses moderate to marked efficacy in the manic phase, and poor to moderate efficacy in the depressed phase of RCBD. Again, combination therapy with lithium may offer greater efficacy. Of significance, carbamazepine treatment outcomes have not been prospectively evaluated in a homogeneous cohort of rapid cyclers.
The limitations of carbamazepine therapy are well known and available evidence also does not seem to support monotherapy with this agent as being useful in RCBD, especially in the treatment and prophylaxis of depressive or mixed phases of the disorder. Thus, further controlled studies are needed to examine the agent’s potential role and safety in combination therapies for RCBD.
Table 1
SPECTRUM OF ACUTE AND PROPHYLACTIC EFFICACY OF DIVALPROEX IN RAPID-CYCLING BIPOLAR DISORDER
| Spectrum of marked responses to divalproex in bipolar rapid cycling | ||
|---|---|---|
| Acute | Prophylactic | |
| Dysphoric hypomania/mania | 87% | 89% |
| Elated hypomania/mania | 64% | 77% |
| Depression (n = 101, mean follow-up 15 months) | 21% | 38% |
| Adapted from Calabrese and Delucchi. Am J Psychiatry. 1990;147:431-434 and Calabrese et al. J Clin Psychopharmacol. 1993;13:280-283. | ||
Lamotrigine: best hope for monotherapy
Lamotrigine monotherapy has been reported to be effective in some RCBD cases. The data suggest that it possesses both antidepressant and mood-stabilizing properties.10
An open, naturalistic study of 5 women with treatment-refractory rapid cycling by Fatemi et al11 demonstrated both mood-stabilizing and antidepressant effects from lamotrigine monotherapy or augmentation at a mean dose of 185 ±33.5 mg/d. In 14 clinical reports involving 207 patients with bipolar disorder, 66 of whom had rapid cycling, lamotrigine was observed to possess moderate to marked efficacy in depression and hypomania, but only moderate efficacy in mania.
An open, prospective study compared the efficacy of lamotrigine add-on or monotherapy in 41 rapid cyclers to 34 nonrapid cyclers across 48 weeks. Improvement from baseline to last visit was significant between both subgroups for depressive and hypomanic symptoms. Patients presenting with more severe manic symptoms did less well.13
In the first double-blind, placebo-controlled study of lamotrigine in RCBD,12 182 of 324 patients with rapid cycling responded to treatment with open-label lamotrigine and were then randomized to the study’s double-blind phase. Forty-one percent of lamotrigine-treated vs. 26% of placebo-treated patients were stable without relapse during 6 months of monotherapy. Patients with rapid-cycling bipolar II disorder consistently experienced more improvement than their bipolar I counterparts (Figure 1). The results of this only prospective, placebo-controlled, acute-treatment study of rapid-cycling bipolar patients to date indicate that lamotrigine monotherapy is useful for some patients with RCBD, particularly those with bipolar II.
Frye et al14 conducted a double-blind, placebo-controlled study of 23 patients with rapid cycling utilizing a crossover series of three 6-week monotherapy evaluations of lamotrigine, gabapentin, and placebo. Marked antidepressant response on lamotrigine was seen in 45% of the participants compared with 19% of patients on placebo and a similar response rate among those on gabapentin.
A study evaluating the safety and efficacy of 2 dosages of lamotrigine (50 mg/d or 200 mg/d) compared with placebo in the treatment of a major depressive episode in patients with bipolar I disorder over 7 weeks demonstrated significant antidepressant efficacy.15 These bipolar outpatients displayed clinical improvement as early as the third week of treatment, and switch rates for both dosages did not exceed that of placebo. Patients with RCBD, however, were excluded from this initial trial. Subsequent studies have demonstrated similar magnitudes of efficacy in patients with RCBD, primarily in the prevention of depressive episodes, including the 6-month RCBD maintenance study10 and 2 recently completed 18-month maintenance studies of patients with bipolar I disorder, either recently manic or recently depressed.
Lamotrigine thus may have a special role in RCBD treatment. Its most significant side effect in bipolar disorder is benign rash, which has occurred in 9.0% (108 of 1,198) of patients randomized to lamotrigine vs. 7.6% (80 of 1,056) of those randomized to placebo in pivotal multicenter, double-blind, placebo-controlled bipolar trials.
In mood disorder trials conducted to date, the rate of serious rash, defined as requiring both drug discontinuation and hospitalization, has been 0.06% (2 of 3,153) on lamotrigine and 0.09% (1 out of 1,053) on placebo. No cases of Steven’s Johnson syndrome or toxic epidermal necrolysis were observed.16 A low starting dose and gradual titration of lamotrigine (Table 2) appear to minimize the risk of serious rash.
Levothyroxine: possible add-on therapy
Levothyroxine should be considered as add-on therapy in patients with known hypothyroidism, borderline hypothyroidism, or otherwise treatment-refractory cases.
The strategy of thyroid supplementation is derived from Gjessing’s 1936 report of success in administering hypermetabolic doses of thyroid hormone to patients with periodic catatonia. Stancer and Persad17 initially reported the potential efficacy of this therapeutic maneuver in rapid cyclers; remissions were induced by hypermetabolic thyroid doses in 5 of 7 patients with treatment-refractory bipolar disorder. No data currently support any prophylactic efficacy of thyroid supplementation monotherapy for RCBD treatment.
Bauer and Whybrow18 suggest that thyroid supplementation with T4 added to mood stabilizers augments efficacy independent of pre-existing thyroid function. The potential side effects of long-term levothryoxine administration, namely osteoporosis and cardiac arrhythmias, limit the usefulness of thyroid augmentation in RCBD.
Atypical antipsychotics: perhaps in combination
Coadministering atypical antipsychotics in other mood stabilizers may help rapid cyclers with current or past psychotic symptoms during their mood episodes, but further study is clearly needed.
Atypical antipsychotic medications may have specific mood-stabilizing properties, particularly in the management of mixed and manic states. In the first prospective trial of clozapine monotherapy in bipolar disorder, Calabrese and colleagues in 1994 reported that rapid cycling did not appear to predict nonresponse to treatment.
In the first randomized, controlled trial involving clozapine in bipolar disorder, Suppes et al19 noted significant improvement for symptoms of mania, psychosis, and global improvement. Subjects with nonpsychotic bipolar disorder showed a degree of improvement similar to that seen in the entire clozapine-treated group. These results support a mood-stabilizing role for clozapine.
Preliminary studies of risperidone and olanzapine further suggest therapeutic utility for atypical antipsychotics.
Figure 1 LAMOTRIGINE VS. PLACEBO IN RAPID-CYCLING BIPOLAR DISORDER
Table 2
DOSAGE PLAN FOR LAMOTRIGINE IN MONOTHERAPY AND COMBINATION
| Treatment period | Type of therapy | Daily dosage |
|---|---|---|
| Weeks 1-2 | Monotherapy With divalproex With carbamazepine | 25 mg 12.5 mg 50 mg |
| Weeks 3-4 | Monotherapy With divalproex With carbamazepine | 50 mg 25 mg 100 mg |
| Week 5 | Monotherapy With divalproex With carbamazepine | 100 mg 50 mg 200 mg |
| Thereafter | Monotherapy With divalproex With carbamazepine | 200 mg 100 mg 400 mg |
Topiramate: in patients with weight problems
Evidence from 12 open studies of 223 patients with bipolar disorder suggest that this anticonvulsant may possess mood-stabilizing properties while sparing patients the weight gain commonly seen with other pharmacotherapies.
Two studies of topiramate as an add-on therapy come to mind. Marcotte in 1998 retrospectively studied 44 patients with RCBD, 23 (52%) of whom demonstrated moderate or marked improvement on a mean topiramate dosage of 200 mg/d.
Sachs also reported that symptoms for some patients with a treatment-refractory bipolar disorder could be improved when topiramate was added to their medication regimen. Available data encourage further controlled studies of topiramate add-on therapy for RCBD, especially in obese patients.
Gabapentin: contradictory reports
Preliminary data from 14 open-label studies of gabapentin in 302 bipolar patients reported a response rate of around 67% when used as an add-on therapy, usually in mania or mixed states. A moderate antimanic effect was observed among a total of 23 rapid cyclers in 9 of the studies.
The reports are contradictory, however, and available data do not firmly support the agent’s efficacy in RCBD treatment.
Other potential uses for gabapentin are being investigated, such as antidepressant augmentation, anxiety, and chronic pain. Thus, patients with RCBD and a comorbid illness may benefit from add-on gabapentin.
ECT: Some limited success
Electroconvulsive therapy (ECT) has been implicated less frequently than antidepressants in the induction of rapid cycling, and when this does occur it is usually in the context of combined ECT/antidepressant therapies.
Berma and Wolpert in 1987 reported a case of successful ECT treatment of rapid cycling in an adolescent who had been treated with trimipramine for depression. Vanelle et al in 1994 suggested that maintenance ECT works well in 22 treatment-resistant bipolar patients, including 4 with rapid cycling, over an 18-month treatment period.
Behavioral intervention: changing sleep routines
NIMH research of 15 rapid cyclers who were studied for 3 months looked at behavioral interventions and their effect on switching (Feldman-Naim 1997). This study suggested that patients were more likely to switch from depression into hypomania/mania during daytime hours and from mania/hypomania into depression during nighttime.
The use of light therapy or activity and exercise during depression and the use of induced sleep or exposure to darkness during mania/hypomania may be therapeutic. Wehr and colleagues supported this in a 1998 report of one patient studied over several years, comparing this rapid-cycling patient’s regular sleep routine with prolonged (10 to 14 hours per night) and enforced bed rest in the dark.7 The promotion of sleep by scheduling regular nighttime periods of enforced bed rest in the dark may help prevent mania and stabilize mood in rapid cyclers.
Other add-on possibilities
Haykal in 1990 reported bupropion to be an effective add-on treatment in 5 of 6 patients with refractory, rapid-cycling bipolar II disorder. Benefit has also been reported from clorgyline, clonidine, magnesium, primidone, and acetazolamide.
Calcium-channel blockers may also offer clinical utility, although supportive evidence is limited. Nimodipine was evaluated for efficacy in 30 patients with treatment-refractory affective illness by the NIMH and Passaglia et al in 1998. Patients who improved on this agent had ultradian rapid cycling, defined in the study as those with affective episodes lasting as short as a week.
A recommended treatment strategy
Based on the available data in bipolar I rapid cycling, we recommend initial treatment with divalproex followed by augmentation with lithium if hypomanic or manic episodes persist, lamotrigine if breakthrough episodes are predominantly depressive, and atypical antipsychotics if psychotic symptoms or true mixed states remain.
For patients presenting with bipolar II rapid cycling, we recommend starting with lamotrigine, then augmenting with divalproex or lithium for breakthrough episodes. Lamotrigine shows more promise because of its reportedly greater antidepressant properties and lack of cycle induction or switching, but offers only modest antimanic therapy.
Pending further investigations, current application of the data suggests that when treating patients with RCBD, conventional antidepressants should be avoided and, if first-line therapies are not effective, the clinician should consider moving to combination drug therapy with 2 or more agents.
Related resources
- Bauer MS, Calabrese JR, Dunner DL, et al. Multi-site data reanalysis: validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151:506-515.
- Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine in treatment refractory mania. Am J Psychiatry. 1996;153(6):759-764.
- Calabrese JR, Bowden CL, McElroy SL, et al. Spectrum of activity of lamotrigine in treatment refractory bipolar disorder. Am J Psychiatry. 1999;156(7):1019-1023.
- Sachs GS. Printz DJ. Kahn DA. Carpenter D. Docherty JP. The expert consensus guideline series: medication treatment of bipolar disorder 2000. Postgraduate Medicine. April 2000; Spec No:1-104.
Drug brand names
- Buproprion • Wellbutrin
- Carbamazepine • Tegretol
- Clonidine • Catapres
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levothyroxine • Synthroid, Levothroid, Levoxyl
- Olanzapine • Zyprexa
- Nimodipine • Nimotop
- Primidonem • Mysoline
- Topiramate • Topamax
Disclosure
Dr. Muzina reports that he is a member of the Eli Lilly and Co. speaker’s bureau.
Dr. Calabrese reports that he receives research/grant support from Abbott Laboratories, GlaxoSmithKline, and Wyeth-Ayerst Pharmaceuticals, and serves as a consultant to Abbott Pharmaceuticals, Eli Lilly and Co., GlaxoSmithKline, Novartis Pharmaceuticals Corp., and Parke Davis/Warner Lambert.
1. Dunner DL, Fieve RR. Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry. 1974;20:229-233.
2. Calabrese JR, Delucchi GA. Spectrum of efficacy of valproate in 55 rapid-cycling manic depressives. Am J Psychiatry. 1990;147(4):431-434.
3. Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: Focus on depression as its hallmark. J Clin Psychiatry. 2001;62(Suppl 14):34-41.
4. Kukopulos A, Reginaldi D, Laddomada P, et al. Course of the manic depressive cycle and changes caused by treatment. Pharmacopsychiatry. 1980;13:156-167.
5. Maj M, et al. Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry. 1998;155:30-35.
6. Tondo L, Baldessarini RJ, et al. Lithium maintenance treatment of depression and mania in bipolar I and bipolar II disorders. Am J Psychiatry. 1998;155:638-645.
7. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145:179-84.
8. Calabrese JR, Woyshville MJ, Kimmel SE. Rapport DJ: Predictors of valproate response in bipolar rapid cycling. J Clin Psychopharmacology. 1993;13(4):280-283.
9. Sharma V, Persad E, et al. Treatment of rapid cycling bipolar disorder with combination therapy of valproate and lithium. Can J Psychiatry. 1993;38:137-139.
10. Calabrese JR, Suppes T, Bowden CL, et al. for the Lamictal 614 Study Group. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry. 2000;61(11):841-850.
11. Fatemi SH, Rapport DJ, Calabrese JR, et al. Lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry 1997;58:522-527.
12. Calabrese JR, Bowden CL, et al. for the Lamictal 602 Study Group. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60(2):79-88.
13. Bowden CL, Calabrese JR, McElroy SL, et al. Comparison of the efficacy of lamotrigine in rapid cycling and non-rapid cycling bipolar disorder. Biol Psychiatry. 1999;45(8):953-958.
14. Frye MA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607-614.
15. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999;60(2):79-88.
16. Calabrese JR, Bowden CL, DeVeaugh-Geiss J, et al. Lamotrigine demonstrates long term mood stabilization in recently manic patients. Annual meeting of the American Psychiatric Association, New Orleans, 2001.
17. Stancer HC, Persad E. Treatment of intractable rapid-cycling manic-depressive disorder with levothyroxine. Clinical observations. Arch Gen Psychiatry. 1982;39(3):311-312.
18. Bauer MS, Whybrow PC. Rapid cycling bipolar affective disorder. II. Treatment of refractory rapid cycling with high-dose levothyroxine: a preliminary study.. Arch Gen Psychiatry. 1990;47(5):435-40.
19. Suppes T, Webb A, Paul B, Carmody T, et al:. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156:1164-1169.
Patients with rapid-cycling bipolar disorder (RCBD) can be frustrating to treat. Despite growing research and data, knowledge and effective therapies remain limited. How do you manage patients with rapid cycling who do not respond robustly to lithium, divalproex, or carbamazepine monotherapy? Are combination therapies likely to be more effective? Where does lamotrigine fit in? Is there a role for conventional antidepressants?
We’ll explore these and related questions—but the final answers are not yet in. Recognition of RCBD is important because it presents such difficult treatment challenges. Available evidence does suggest that rapid cycling as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (Box 1), describes a clinically specific course of illness that may require treatments different from currently used traditional drug therapies for nonrapid cycling bipolar disorder, particularly as no one agent appears to provide ideal bimodal treatment and prophylaxis of this bipolar disorder variant.
Rapid cycling is a specifier of the longitudinal course of illness presentation that is seen almost exclusively in bipolar disorder and is associated with a greater morbidity. Dunner and Fieve1 originally coined the term when evaluating clinical factors associated with lithium prophylaxis failure. Since that time the validity of rapid cycling as a distinct course modifier for bipolar disorder has been supported by multiple studies, leading to its inclusion in the fourth edition of the Diagnostic and Statistical Manual of the APA (1994).
According to DSM-IV, the course specifier of rapid cycling applies to “at least 4 episodes of a mood disturbance in the previous 12 months that meet criteria for a manic episode, a hypomanic episode, or a major depressive episode.” The episodes must be demarcated by a full or partial remission lasting at least 2 months or by a switch to a mood state of opposite polarity.
Early reports noted that patients suffering from RCBD did not respond adequately when treated with lithium.1 Other observations indicated that divalproex was more effective in this patient population, particularly for the illness’ hypomanic or manic phases.2 We hope that the following evaluation of these and other drug therapies will prove helpful.
Watch out for antidepressants
Most concerning has been the frequency and severity of treatment-refractory depressive phases of RCBD that may be exacerbated by antidepressant use (cycle induction or acceleration). Indeed, the frequent recurrence of refractory depression has been described as the hallmark of this bipolar disorder variant.3
Lithium: the scale weighs against it
Although an excellent mood stabilizer for most patients with bipolar disorder, lithium monotherapy is less than ideal for patients with the rapid-cycling variant, particularly in treatment or prevention of depressive or mixed episodes. The efficacy of lithium is likely decreased by the concurrent administration of antidepressant medication and increased when administered with other mood stabilizers.
The landmark article by Dunner and Fieve,1 which described a placebo-controlled, double-blind maintenance study in a general cohort of 55 patients, tried to clarify factors associated with the failure of lithium prophylaxis in bipolar disorder. Rapid cyclers comprised 20% of the subjects and 80% were nonrapid cyclers. Rapid cyclers were disproportionately represented in the lithium failure group. Lithium failures included 82% (9 of 11) of rapid cyclers compared to 41% (18 of 44) of nonrapid cyclers. Lithium failure was defined as (1) hospitalization for, or (2) treatment of, mania or (3) depression during lithium therapy, or as mood symptoms that, as documented by rating scales, were sufficient to warrant a diagnosis of mild depression, hypomania, or mania persisting for at least 2 weeks.
Kukopulos et al4 replicated the findings of Dunner and Fieve in a study of the longitudinal clinical course of 434 bipolar patients. Of these patients, 50 were rapid cyclers and had received continuous lithium therapy for more than a year, with good to partial prophylaxis in only 28%. Maj and colleagues5 published a 5-year prospective study of lithium therapy in 402 patients with bipolar disorder and noted the absence of rapid cycling in good responders to lithium but an incidence rate of 26% in nonresponders to lithium.
Other investigators have reported better response in RCBD. In a select cohort of lithium-responsive bipolar I and II patients, Tondo et al6 concluded that lithium maintenance yields striking long-term reductions in depressive and manic morbidity, more so in rapid cycling type II patients. This study, however, was in a cohort of lithium responders and excluded patients who had been exposed to antipsychotic or antidepressant medications for more than 3 months, those on chronic anticonvulsant therapy, and those with substance abuse disorders.
Although most studies do report poor response to lithium therapy in RCBD, Wehr and colleagues7 suggest that in some patients with rapid cycling, the discontinuation of antidepressant drugs may allow lithium to act as a more effective anticycling mood-stabilizing agent.
Divalproex: effective in manic phase
In contrast to lithium, an open trial of a homogenous cohort of patients with RCBD by Calabrese and colleagues3,8 found divalproex to possess moderate to marked acute and prophylactic antimanic properties with only modest antidepressant effects (Table 1). Data from 6 open studies involving 147 patients with rapid cycling suggest that divalproex possesses moderate to marked efficacy in the manic phase, but poor to moderate efficacy in the depressed phase. Positive outcome predictors were bipolar II and mixed states, no prior lithium therapy, and a positive family history of affective disorder. Predictors of negative response included increase in frequency and severity of mania, and borderline personality disorder.
Divalproex therapy in combination with lithium may improve response rates.9 Calabrese and colleagues, however, have examined large cohorts of patients, including those comorbid with alcohol, cocaine, and/or cannabis abuse, treated with a lithium-divalproex combination over 6-month study periods. The researchers found that only 25% to 50% of patients stabilized, and that of those not exhibiting a response, the majority (75%) did not respond because of treatment-refractory depression in the context of RCBD.3
Although experts believe divalproex to be more effective than lithium in preventing episodes associated with RCBD, such a conclusion awaits confirmation with the near completion of a double-blind, 20-month maintenance trial sponsored by the National Institute of Mental Health (NIMH).
Carbamazepine’s role in combination therapy
Early reports by Post and colleagues in 1987 suggested that rapid cycling predicted positive response to carbamazepine, but later findings by Okuma in 1993 refuted this. Other collective open and controlled studies suggest that this anticonvulsant possesses moderate to marked efficacy in the manic phase, and poor to moderate efficacy in the depressed phase of RCBD. Again, combination therapy with lithium may offer greater efficacy. Of significance, carbamazepine treatment outcomes have not been prospectively evaluated in a homogeneous cohort of rapid cyclers.
The limitations of carbamazepine therapy are well known and available evidence also does not seem to support monotherapy with this agent as being useful in RCBD, especially in the treatment and prophylaxis of depressive or mixed phases of the disorder. Thus, further controlled studies are needed to examine the agent’s potential role and safety in combination therapies for RCBD.
Table 1
SPECTRUM OF ACUTE AND PROPHYLACTIC EFFICACY OF DIVALPROEX IN RAPID-CYCLING BIPOLAR DISORDER
| Spectrum of marked responses to divalproex in bipolar rapid cycling | ||
|---|---|---|
| Acute | Prophylactic | |
| Dysphoric hypomania/mania | 87% | 89% |
| Elated hypomania/mania | 64% | 77% |
| Depression (n = 101, mean follow-up 15 months) | 21% | 38% |
| Adapted from Calabrese and Delucchi. Am J Psychiatry. 1990;147:431-434 and Calabrese et al. J Clin Psychopharmacol. 1993;13:280-283. | ||
Lamotrigine: best hope for monotherapy
Lamotrigine monotherapy has been reported to be effective in some RCBD cases. The data suggest that it possesses both antidepressant and mood-stabilizing properties.10
An open, naturalistic study of 5 women with treatment-refractory rapid cycling by Fatemi et al11 demonstrated both mood-stabilizing and antidepressant effects from lamotrigine monotherapy or augmentation at a mean dose of 185 ±33.5 mg/d. In 14 clinical reports involving 207 patients with bipolar disorder, 66 of whom had rapid cycling, lamotrigine was observed to possess moderate to marked efficacy in depression and hypomania, but only moderate efficacy in mania.
An open, prospective study compared the efficacy of lamotrigine add-on or monotherapy in 41 rapid cyclers to 34 nonrapid cyclers across 48 weeks. Improvement from baseline to last visit was significant between both subgroups for depressive and hypomanic symptoms. Patients presenting with more severe manic symptoms did less well.13
In the first double-blind, placebo-controlled study of lamotrigine in RCBD,12 182 of 324 patients with rapid cycling responded to treatment with open-label lamotrigine and were then randomized to the study’s double-blind phase. Forty-one percent of lamotrigine-treated vs. 26% of placebo-treated patients were stable without relapse during 6 months of monotherapy. Patients with rapid-cycling bipolar II disorder consistently experienced more improvement than their bipolar I counterparts (Figure 1). The results of this only prospective, placebo-controlled, acute-treatment study of rapid-cycling bipolar patients to date indicate that lamotrigine monotherapy is useful for some patients with RCBD, particularly those with bipolar II.
Frye et al14 conducted a double-blind, placebo-controlled study of 23 patients with rapid cycling utilizing a crossover series of three 6-week monotherapy evaluations of lamotrigine, gabapentin, and placebo. Marked antidepressant response on lamotrigine was seen in 45% of the participants compared with 19% of patients on placebo and a similar response rate among those on gabapentin.
A study evaluating the safety and efficacy of 2 dosages of lamotrigine (50 mg/d or 200 mg/d) compared with placebo in the treatment of a major depressive episode in patients with bipolar I disorder over 7 weeks demonstrated significant antidepressant efficacy.15 These bipolar outpatients displayed clinical improvement as early as the third week of treatment, and switch rates for both dosages did not exceed that of placebo. Patients with RCBD, however, were excluded from this initial trial. Subsequent studies have demonstrated similar magnitudes of efficacy in patients with RCBD, primarily in the prevention of depressive episodes, including the 6-month RCBD maintenance study10 and 2 recently completed 18-month maintenance studies of patients with bipolar I disorder, either recently manic or recently depressed.
Lamotrigine thus may have a special role in RCBD treatment. Its most significant side effect in bipolar disorder is benign rash, which has occurred in 9.0% (108 of 1,198) of patients randomized to lamotrigine vs. 7.6% (80 of 1,056) of those randomized to placebo in pivotal multicenter, double-blind, placebo-controlled bipolar trials.
In mood disorder trials conducted to date, the rate of serious rash, defined as requiring both drug discontinuation and hospitalization, has been 0.06% (2 of 3,153) on lamotrigine and 0.09% (1 out of 1,053) on placebo. No cases of Steven’s Johnson syndrome or toxic epidermal necrolysis were observed.16 A low starting dose and gradual titration of lamotrigine (Table 2) appear to minimize the risk of serious rash.
Levothyroxine: possible add-on therapy
Levothyroxine should be considered as add-on therapy in patients with known hypothyroidism, borderline hypothyroidism, or otherwise treatment-refractory cases.
The strategy of thyroid supplementation is derived from Gjessing’s 1936 report of success in administering hypermetabolic doses of thyroid hormone to patients with periodic catatonia. Stancer and Persad17 initially reported the potential efficacy of this therapeutic maneuver in rapid cyclers; remissions were induced by hypermetabolic thyroid doses in 5 of 7 patients with treatment-refractory bipolar disorder. No data currently support any prophylactic efficacy of thyroid supplementation monotherapy for RCBD treatment.
Bauer and Whybrow18 suggest that thyroid supplementation with T4 added to mood stabilizers augments efficacy independent of pre-existing thyroid function. The potential side effects of long-term levothryoxine administration, namely osteoporosis and cardiac arrhythmias, limit the usefulness of thyroid augmentation in RCBD.
Atypical antipsychotics: perhaps in combination
Coadministering atypical antipsychotics in other mood stabilizers may help rapid cyclers with current or past psychotic symptoms during their mood episodes, but further study is clearly needed.
Atypical antipsychotic medications may have specific mood-stabilizing properties, particularly in the management of mixed and manic states. In the first prospective trial of clozapine monotherapy in bipolar disorder, Calabrese and colleagues in 1994 reported that rapid cycling did not appear to predict nonresponse to treatment.
In the first randomized, controlled trial involving clozapine in bipolar disorder, Suppes et al19 noted significant improvement for symptoms of mania, psychosis, and global improvement. Subjects with nonpsychotic bipolar disorder showed a degree of improvement similar to that seen in the entire clozapine-treated group. These results support a mood-stabilizing role for clozapine.
Preliminary studies of risperidone and olanzapine further suggest therapeutic utility for atypical antipsychotics.
Figure 1 LAMOTRIGINE VS. PLACEBO IN RAPID-CYCLING BIPOLAR DISORDER
Table 2
DOSAGE PLAN FOR LAMOTRIGINE IN MONOTHERAPY AND COMBINATION
| Treatment period | Type of therapy | Daily dosage |
|---|---|---|
| Weeks 1-2 | Monotherapy With divalproex With carbamazepine | 25 mg 12.5 mg 50 mg |
| Weeks 3-4 | Monotherapy With divalproex With carbamazepine | 50 mg 25 mg 100 mg |
| Week 5 | Monotherapy With divalproex With carbamazepine | 100 mg 50 mg 200 mg |
| Thereafter | Monotherapy With divalproex With carbamazepine | 200 mg 100 mg 400 mg |
Topiramate: in patients with weight problems
Evidence from 12 open studies of 223 patients with bipolar disorder suggest that this anticonvulsant may possess mood-stabilizing properties while sparing patients the weight gain commonly seen with other pharmacotherapies.
Two studies of topiramate as an add-on therapy come to mind. Marcotte in 1998 retrospectively studied 44 patients with RCBD, 23 (52%) of whom demonstrated moderate or marked improvement on a mean topiramate dosage of 200 mg/d.
Sachs also reported that symptoms for some patients with a treatment-refractory bipolar disorder could be improved when topiramate was added to their medication regimen. Available data encourage further controlled studies of topiramate add-on therapy for RCBD, especially in obese patients.
Gabapentin: contradictory reports
Preliminary data from 14 open-label studies of gabapentin in 302 bipolar patients reported a response rate of around 67% when used as an add-on therapy, usually in mania or mixed states. A moderate antimanic effect was observed among a total of 23 rapid cyclers in 9 of the studies.
The reports are contradictory, however, and available data do not firmly support the agent’s efficacy in RCBD treatment.
Other potential uses for gabapentin are being investigated, such as antidepressant augmentation, anxiety, and chronic pain. Thus, patients with RCBD and a comorbid illness may benefit from add-on gabapentin.
ECT: Some limited success
Electroconvulsive therapy (ECT) has been implicated less frequently than antidepressants in the induction of rapid cycling, and when this does occur it is usually in the context of combined ECT/antidepressant therapies.
Berma and Wolpert in 1987 reported a case of successful ECT treatment of rapid cycling in an adolescent who had been treated with trimipramine for depression. Vanelle et al in 1994 suggested that maintenance ECT works well in 22 treatment-resistant bipolar patients, including 4 with rapid cycling, over an 18-month treatment period.
Behavioral intervention: changing sleep routines
NIMH research of 15 rapid cyclers who were studied for 3 months looked at behavioral interventions and their effect on switching (Feldman-Naim 1997). This study suggested that patients were more likely to switch from depression into hypomania/mania during daytime hours and from mania/hypomania into depression during nighttime.
The use of light therapy or activity and exercise during depression and the use of induced sleep or exposure to darkness during mania/hypomania may be therapeutic. Wehr and colleagues supported this in a 1998 report of one patient studied over several years, comparing this rapid-cycling patient’s regular sleep routine with prolonged (10 to 14 hours per night) and enforced bed rest in the dark.7 The promotion of sleep by scheduling regular nighttime periods of enforced bed rest in the dark may help prevent mania and stabilize mood in rapid cyclers.
Other add-on possibilities
Haykal in 1990 reported bupropion to be an effective add-on treatment in 5 of 6 patients with refractory, rapid-cycling bipolar II disorder. Benefit has also been reported from clorgyline, clonidine, magnesium, primidone, and acetazolamide.
Calcium-channel blockers may also offer clinical utility, although supportive evidence is limited. Nimodipine was evaluated for efficacy in 30 patients with treatment-refractory affective illness by the NIMH and Passaglia et al in 1998. Patients who improved on this agent had ultradian rapid cycling, defined in the study as those with affective episodes lasting as short as a week.
A recommended treatment strategy
Based on the available data in bipolar I rapid cycling, we recommend initial treatment with divalproex followed by augmentation with lithium if hypomanic or manic episodes persist, lamotrigine if breakthrough episodes are predominantly depressive, and atypical antipsychotics if psychotic symptoms or true mixed states remain.
For patients presenting with bipolar II rapid cycling, we recommend starting with lamotrigine, then augmenting with divalproex or lithium for breakthrough episodes. Lamotrigine shows more promise because of its reportedly greater antidepressant properties and lack of cycle induction or switching, but offers only modest antimanic therapy.
Pending further investigations, current application of the data suggests that when treating patients with RCBD, conventional antidepressants should be avoided and, if first-line therapies are not effective, the clinician should consider moving to combination drug therapy with 2 or more agents.
Related resources
- Bauer MS, Calabrese JR, Dunner DL, et al. Multi-site data reanalysis: validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151:506-515.
- Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine in treatment refractory mania. Am J Psychiatry. 1996;153(6):759-764.
- Calabrese JR, Bowden CL, McElroy SL, et al. Spectrum of activity of lamotrigine in treatment refractory bipolar disorder. Am J Psychiatry. 1999;156(7):1019-1023.
- Sachs GS. Printz DJ. Kahn DA. Carpenter D. Docherty JP. The expert consensus guideline series: medication treatment of bipolar disorder 2000. Postgraduate Medicine. April 2000; Spec No:1-104.
Drug brand names
- Buproprion • Wellbutrin
- Carbamazepine • Tegretol
- Clonidine • Catapres
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levothyroxine • Synthroid, Levothroid, Levoxyl
- Olanzapine • Zyprexa
- Nimodipine • Nimotop
- Primidonem • Mysoline
- Topiramate • Topamax
Disclosure
Dr. Muzina reports that he is a member of the Eli Lilly and Co. speaker’s bureau.
Dr. Calabrese reports that he receives research/grant support from Abbott Laboratories, GlaxoSmithKline, and Wyeth-Ayerst Pharmaceuticals, and serves as a consultant to Abbott Pharmaceuticals, Eli Lilly and Co., GlaxoSmithKline, Novartis Pharmaceuticals Corp., and Parke Davis/Warner Lambert.
Patients with rapid-cycling bipolar disorder (RCBD) can be frustrating to treat. Despite growing research and data, knowledge and effective therapies remain limited. How do you manage patients with rapid cycling who do not respond robustly to lithium, divalproex, or carbamazepine monotherapy? Are combination therapies likely to be more effective? Where does lamotrigine fit in? Is there a role for conventional antidepressants?
We’ll explore these and related questions—but the final answers are not yet in. Recognition of RCBD is important because it presents such difficult treatment challenges. Available evidence does suggest that rapid cycling as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (Box 1), describes a clinically specific course of illness that may require treatments different from currently used traditional drug therapies for nonrapid cycling bipolar disorder, particularly as no one agent appears to provide ideal bimodal treatment and prophylaxis of this bipolar disorder variant.
Rapid cycling is a specifier of the longitudinal course of illness presentation that is seen almost exclusively in bipolar disorder and is associated with a greater morbidity. Dunner and Fieve1 originally coined the term when evaluating clinical factors associated with lithium prophylaxis failure. Since that time the validity of rapid cycling as a distinct course modifier for bipolar disorder has been supported by multiple studies, leading to its inclusion in the fourth edition of the Diagnostic and Statistical Manual of the APA (1994).
According to DSM-IV, the course specifier of rapid cycling applies to “at least 4 episodes of a mood disturbance in the previous 12 months that meet criteria for a manic episode, a hypomanic episode, or a major depressive episode.” The episodes must be demarcated by a full or partial remission lasting at least 2 months or by a switch to a mood state of opposite polarity.
Early reports noted that patients suffering from RCBD did not respond adequately when treated with lithium.1 Other observations indicated that divalproex was more effective in this patient population, particularly for the illness’ hypomanic or manic phases.2 We hope that the following evaluation of these and other drug therapies will prove helpful.
Watch out for antidepressants
Most concerning has been the frequency and severity of treatment-refractory depressive phases of RCBD that may be exacerbated by antidepressant use (cycle induction or acceleration). Indeed, the frequent recurrence of refractory depression has been described as the hallmark of this bipolar disorder variant.3
Lithium: the scale weighs against it
Although an excellent mood stabilizer for most patients with bipolar disorder, lithium monotherapy is less than ideal for patients with the rapid-cycling variant, particularly in treatment or prevention of depressive or mixed episodes. The efficacy of lithium is likely decreased by the concurrent administration of antidepressant medication and increased when administered with other mood stabilizers.
The landmark article by Dunner and Fieve,1 which described a placebo-controlled, double-blind maintenance study in a general cohort of 55 patients, tried to clarify factors associated with the failure of lithium prophylaxis in bipolar disorder. Rapid cyclers comprised 20% of the subjects and 80% were nonrapid cyclers. Rapid cyclers were disproportionately represented in the lithium failure group. Lithium failures included 82% (9 of 11) of rapid cyclers compared to 41% (18 of 44) of nonrapid cyclers. Lithium failure was defined as (1) hospitalization for, or (2) treatment of, mania or (3) depression during lithium therapy, or as mood symptoms that, as documented by rating scales, were sufficient to warrant a diagnosis of mild depression, hypomania, or mania persisting for at least 2 weeks.
Kukopulos et al4 replicated the findings of Dunner and Fieve in a study of the longitudinal clinical course of 434 bipolar patients. Of these patients, 50 were rapid cyclers and had received continuous lithium therapy for more than a year, with good to partial prophylaxis in only 28%. Maj and colleagues5 published a 5-year prospective study of lithium therapy in 402 patients with bipolar disorder and noted the absence of rapid cycling in good responders to lithium but an incidence rate of 26% in nonresponders to lithium.
Other investigators have reported better response in RCBD. In a select cohort of lithium-responsive bipolar I and II patients, Tondo et al6 concluded that lithium maintenance yields striking long-term reductions in depressive and manic morbidity, more so in rapid cycling type II patients. This study, however, was in a cohort of lithium responders and excluded patients who had been exposed to antipsychotic or antidepressant medications for more than 3 months, those on chronic anticonvulsant therapy, and those with substance abuse disorders.
Although most studies do report poor response to lithium therapy in RCBD, Wehr and colleagues7 suggest that in some patients with rapid cycling, the discontinuation of antidepressant drugs may allow lithium to act as a more effective anticycling mood-stabilizing agent.
Divalproex: effective in manic phase
In contrast to lithium, an open trial of a homogenous cohort of patients with RCBD by Calabrese and colleagues3,8 found divalproex to possess moderate to marked acute and prophylactic antimanic properties with only modest antidepressant effects (Table 1). Data from 6 open studies involving 147 patients with rapid cycling suggest that divalproex possesses moderate to marked efficacy in the manic phase, but poor to moderate efficacy in the depressed phase. Positive outcome predictors were bipolar II and mixed states, no prior lithium therapy, and a positive family history of affective disorder. Predictors of negative response included increase in frequency and severity of mania, and borderline personality disorder.
Divalproex therapy in combination with lithium may improve response rates.9 Calabrese and colleagues, however, have examined large cohorts of patients, including those comorbid with alcohol, cocaine, and/or cannabis abuse, treated with a lithium-divalproex combination over 6-month study periods. The researchers found that only 25% to 50% of patients stabilized, and that of those not exhibiting a response, the majority (75%) did not respond because of treatment-refractory depression in the context of RCBD.3
Although experts believe divalproex to be more effective than lithium in preventing episodes associated with RCBD, such a conclusion awaits confirmation with the near completion of a double-blind, 20-month maintenance trial sponsored by the National Institute of Mental Health (NIMH).
Carbamazepine’s role in combination therapy
Early reports by Post and colleagues in 1987 suggested that rapid cycling predicted positive response to carbamazepine, but later findings by Okuma in 1993 refuted this. Other collective open and controlled studies suggest that this anticonvulsant possesses moderate to marked efficacy in the manic phase, and poor to moderate efficacy in the depressed phase of RCBD. Again, combination therapy with lithium may offer greater efficacy. Of significance, carbamazepine treatment outcomes have not been prospectively evaluated in a homogeneous cohort of rapid cyclers.
The limitations of carbamazepine therapy are well known and available evidence also does not seem to support monotherapy with this agent as being useful in RCBD, especially in the treatment and prophylaxis of depressive or mixed phases of the disorder. Thus, further controlled studies are needed to examine the agent’s potential role and safety in combination therapies for RCBD.
Table 1
SPECTRUM OF ACUTE AND PROPHYLACTIC EFFICACY OF DIVALPROEX IN RAPID-CYCLING BIPOLAR DISORDER
| Spectrum of marked responses to divalproex in bipolar rapid cycling | ||
|---|---|---|
| Acute | Prophylactic | |
| Dysphoric hypomania/mania | 87% | 89% |
| Elated hypomania/mania | 64% | 77% |
| Depression (n = 101, mean follow-up 15 months) | 21% | 38% |
| Adapted from Calabrese and Delucchi. Am J Psychiatry. 1990;147:431-434 and Calabrese et al. J Clin Psychopharmacol. 1993;13:280-283. | ||
Lamotrigine: best hope for monotherapy
Lamotrigine monotherapy has been reported to be effective in some RCBD cases. The data suggest that it possesses both antidepressant and mood-stabilizing properties.10
An open, naturalistic study of 5 women with treatment-refractory rapid cycling by Fatemi et al11 demonstrated both mood-stabilizing and antidepressant effects from lamotrigine monotherapy or augmentation at a mean dose of 185 ±33.5 mg/d. In 14 clinical reports involving 207 patients with bipolar disorder, 66 of whom had rapid cycling, lamotrigine was observed to possess moderate to marked efficacy in depression and hypomania, but only moderate efficacy in mania.
An open, prospective study compared the efficacy of lamotrigine add-on or monotherapy in 41 rapid cyclers to 34 nonrapid cyclers across 48 weeks. Improvement from baseline to last visit was significant between both subgroups for depressive and hypomanic symptoms. Patients presenting with more severe manic symptoms did less well.13
In the first double-blind, placebo-controlled study of lamotrigine in RCBD,12 182 of 324 patients with rapid cycling responded to treatment with open-label lamotrigine and were then randomized to the study’s double-blind phase. Forty-one percent of lamotrigine-treated vs. 26% of placebo-treated patients were stable without relapse during 6 months of monotherapy. Patients with rapid-cycling bipolar II disorder consistently experienced more improvement than their bipolar I counterparts (Figure 1). The results of this only prospective, placebo-controlled, acute-treatment study of rapid-cycling bipolar patients to date indicate that lamotrigine monotherapy is useful for some patients with RCBD, particularly those with bipolar II.
Frye et al14 conducted a double-blind, placebo-controlled study of 23 patients with rapid cycling utilizing a crossover series of three 6-week monotherapy evaluations of lamotrigine, gabapentin, and placebo. Marked antidepressant response on lamotrigine was seen in 45% of the participants compared with 19% of patients on placebo and a similar response rate among those on gabapentin.
A study evaluating the safety and efficacy of 2 dosages of lamotrigine (50 mg/d or 200 mg/d) compared with placebo in the treatment of a major depressive episode in patients with bipolar I disorder over 7 weeks demonstrated significant antidepressant efficacy.15 These bipolar outpatients displayed clinical improvement as early as the third week of treatment, and switch rates for both dosages did not exceed that of placebo. Patients with RCBD, however, were excluded from this initial trial. Subsequent studies have demonstrated similar magnitudes of efficacy in patients with RCBD, primarily in the prevention of depressive episodes, including the 6-month RCBD maintenance study10 and 2 recently completed 18-month maintenance studies of patients with bipolar I disorder, either recently manic or recently depressed.
Lamotrigine thus may have a special role in RCBD treatment. Its most significant side effect in bipolar disorder is benign rash, which has occurred in 9.0% (108 of 1,198) of patients randomized to lamotrigine vs. 7.6% (80 of 1,056) of those randomized to placebo in pivotal multicenter, double-blind, placebo-controlled bipolar trials.
In mood disorder trials conducted to date, the rate of serious rash, defined as requiring both drug discontinuation and hospitalization, has been 0.06% (2 of 3,153) on lamotrigine and 0.09% (1 out of 1,053) on placebo. No cases of Steven’s Johnson syndrome or toxic epidermal necrolysis were observed.16 A low starting dose and gradual titration of lamotrigine (Table 2) appear to minimize the risk of serious rash.
Levothyroxine: possible add-on therapy
Levothyroxine should be considered as add-on therapy in patients with known hypothyroidism, borderline hypothyroidism, or otherwise treatment-refractory cases.
The strategy of thyroid supplementation is derived from Gjessing’s 1936 report of success in administering hypermetabolic doses of thyroid hormone to patients with periodic catatonia. Stancer and Persad17 initially reported the potential efficacy of this therapeutic maneuver in rapid cyclers; remissions were induced by hypermetabolic thyroid doses in 5 of 7 patients with treatment-refractory bipolar disorder. No data currently support any prophylactic efficacy of thyroid supplementation monotherapy for RCBD treatment.
Bauer and Whybrow18 suggest that thyroid supplementation with T4 added to mood stabilizers augments efficacy independent of pre-existing thyroid function. The potential side effects of long-term levothryoxine administration, namely osteoporosis and cardiac arrhythmias, limit the usefulness of thyroid augmentation in RCBD.
Atypical antipsychotics: perhaps in combination
Coadministering atypical antipsychotics in other mood stabilizers may help rapid cyclers with current or past psychotic symptoms during their mood episodes, but further study is clearly needed.
Atypical antipsychotic medications may have specific mood-stabilizing properties, particularly in the management of mixed and manic states. In the first prospective trial of clozapine monotherapy in bipolar disorder, Calabrese and colleagues in 1994 reported that rapid cycling did not appear to predict nonresponse to treatment.
In the first randomized, controlled trial involving clozapine in bipolar disorder, Suppes et al19 noted significant improvement for symptoms of mania, psychosis, and global improvement. Subjects with nonpsychotic bipolar disorder showed a degree of improvement similar to that seen in the entire clozapine-treated group. These results support a mood-stabilizing role for clozapine.
Preliminary studies of risperidone and olanzapine further suggest therapeutic utility for atypical antipsychotics.
Figure 1 LAMOTRIGINE VS. PLACEBO IN RAPID-CYCLING BIPOLAR DISORDER
Table 2
DOSAGE PLAN FOR LAMOTRIGINE IN MONOTHERAPY AND COMBINATION
| Treatment period | Type of therapy | Daily dosage |
|---|---|---|
| Weeks 1-2 | Monotherapy With divalproex With carbamazepine | 25 mg 12.5 mg 50 mg |
| Weeks 3-4 | Monotherapy With divalproex With carbamazepine | 50 mg 25 mg 100 mg |
| Week 5 | Monotherapy With divalproex With carbamazepine | 100 mg 50 mg 200 mg |
| Thereafter | Monotherapy With divalproex With carbamazepine | 200 mg 100 mg 400 mg |
Topiramate: in patients with weight problems
Evidence from 12 open studies of 223 patients with bipolar disorder suggest that this anticonvulsant may possess mood-stabilizing properties while sparing patients the weight gain commonly seen with other pharmacotherapies.
Two studies of topiramate as an add-on therapy come to mind. Marcotte in 1998 retrospectively studied 44 patients with RCBD, 23 (52%) of whom demonstrated moderate or marked improvement on a mean topiramate dosage of 200 mg/d.
Sachs also reported that symptoms for some patients with a treatment-refractory bipolar disorder could be improved when topiramate was added to their medication regimen. Available data encourage further controlled studies of topiramate add-on therapy for RCBD, especially in obese patients.
Gabapentin: contradictory reports
Preliminary data from 14 open-label studies of gabapentin in 302 bipolar patients reported a response rate of around 67% when used as an add-on therapy, usually in mania or mixed states. A moderate antimanic effect was observed among a total of 23 rapid cyclers in 9 of the studies.
The reports are contradictory, however, and available data do not firmly support the agent’s efficacy in RCBD treatment.
Other potential uses for gabapentin are being investigated, such as antidepressant augmentation, anxiety, and chronic pain. Thus, patients with RCBD and a comorbid illness may benefit from add-on gabapentin.
ECT: Some limited success
Electroconvulsive therapy (ECT) has been implicated less frequently than antidepressants in the induction of rapid cycling, and when this does occur it is usually in the context of combined ECT/antidepressant therapies.
Berma and Wolpert in 1987 reported a case of successful ECT treatment of rapid cycling in an adolescent who had been treated with trimipramine for depression. Vanelle et al in 1994 suggested that maintenance ECT works well in 22 treatment-resistant bipolar patients, including 4 with rapid cycling, over an 18-month treatment period.
Behavioral intervention: changing sleep routines
NIMH research of 15 rapid cyclers who were studied for 3 months looked at behavioral interventions and their effect on switching (Feldman-Naim 1997). This study suggested that patients were more likely to switch from depression into hypomania/mania during daytime hours and from mania/hypomania into depression during nighttime.
The use of light therapy or activity and exercise during depression and the use of induced sleep or exposure to darkness during mania/hypomania may be therapeutic. Wehr and colleagues supported this in a 1998 report of one patient studied over several years, comparing this rapid-cycling patient’s regular sleep routine with prolonged (10 to 14 hours per night) and enforced bed rest in the dark.7 The promotion of sleep by scheduling regular nighttime periods of enforced bed rest in the dark may help prevent mania and stabilize mood in rapid cyclers.
Other add-on possibilities
Haykal in 1990 reported bupropion to be an effective add-on treatment in 5 of 6 patients with refractory, rapid-cycling bipolar II disorder. Benefit has also been reported from clorgyline, clonidine, magnesium, primidone, and acetazolamide.
Calcium-channel blockers may also offer clinical utility, although supportive evidence is limited. Nimodipine was evaluated for efficacy in 30 patients with treatment-refractory affective illness by the NIMH and Passaglia et al in 1998. Patients who improved on this agent had ultradian rapid cycling, defined in the study as those with affective episodes lasting as short as a week.
A recommended treatment strategy
Based on the available data in bipolar I rapid cycling, we recommend initial treatment with divalproex followed by augmentation with lithium if hypomanic or manic episodes persist, lamotrigine if breakthrough episodes are predominantly depressive, and atypical antipsychotics if psychotic symptoms or true mixed states remain.
For patients presenting with bipolar II rapid cycling, we recommend starting with lamotrigine, then augmenting with divalproex or lithium for breakthrough episodes. Lamotrigine shows more promise because of its reportedly greater antidepressant properties and lack of cycle induction or switching, but offers only modest antimanic therapy.
Pending further investigations, current application of the data suggests that when treating patients with RCBD, conventional antidepressants should be avoided and, if first-line therapies are not effective, the clinician should consider moving to combination drug therapy with 2 or more agents.
Related resources
- Bauer MS, Calabrese JR, Dunner DL, et al. Multi-site data reanalysis: validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151:506-515.
- Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine in treatment refractory mania. Am J Psychiatry. 1996;153(6):759-764.
- Calabrese JR, Bowden CL, McElroy SL, et al. Spectrum of activity of lamotrigine in treatment refractory bipolar disorder. Am J Psychiatry. 1999;156(7):1019-1023.
- Sachs GS. Printz DJ. Kahn DA. Carpenter D. Docherty JP. The expert consensus guideline series: medication treatment of bipolar disorder 2000. Postgraduate Medicine. April 2000; Spec No:1-104.
Drug brand names
- Buproprion • Wellbutrin
- Carbamazepine • Tegretol
- Clonidine • Catapres
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levothyroxine • Synthroid, Levothroid, Levoxyl
- Olanzapine • Zyprexa
- Nimodipine • Nimotop
- Primidonem • Mysoline
- Topiramate • Topamax
Disclosure
Dr. Muzina reports that he is a member of the Eli Lilly and Co. speaker’s bureau.
Dr. Calabrese reports that he receives research/grant support from Abbott Laboratories, GlaxoSmithKline, and Wyeth-Ayerst Pharmaceuticals, and serves as a consultant to Abbott Pharmaceuticals, Eli Lilly and Co., GlaxoSmithKline, Novartis Pharmaceuticals Corp., and Parke Davis/Warner Lambert.
1. Dunner DL, Fieve RR. Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry. 1974;20:229-233.
2. Calabrese JR, Delucchi GA. Spectrum of efficacy of valproate in 55 rapid-cycling manic depressives. Am J Psychiatry. 1990;147(4):431-434.
3. Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: Focus on depression as its hallmark. J Clin Psychiatry. 2001;62(Suppl 14):34-41.
4. Kukopulos A, Reginaldi D, Laddomada P, et al. Course of the manic depressive cycle and changes caused by treatment. Pharmacopsychiatry. 1980;13:156-167.
5. Maj M, et al. Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry. 1998;155:30-35.
6. Tondo L, Baldessarini RJ, et al. Lithium maintenance treatment of depression and mania in bipolar I and bipolar II disorders. Am J Psychiatry. 1998;155:638-645.
7. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145:179-84.
8. Calabrese JR, Woyshville MJ, Kimmel SE. Rapport DJ: Predictors of valproate response in bipolar rapid cycling. J Clin Psychopharmacology. 1993;13(4):280-283.
9. Sharma V, Persad E, et al. Treatment of rapid cycling bipolar disorder with combination therapy of valproate and lithium. Can J Psychiatry. 1993;38:137-139.
10. Calabrese JR, Suppes T, Bowden CL, et al. for the Lamictal 614 Study Group. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry. 2000;61(11):841-850.
11. Fatemi SH, Rapport DJ, Calabrese JR, et al. Lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry 1997;58:522-527.
12. Calabrese JR, Bowden CL, et al. for the Lamictal 602 Study Group. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60(2):79-88.
13. Bowden CL, Calabrese JR, McElroy SL, et al. Comparison of the efficacy of lamotrigine in rapid cycling and non-rapid cycling bipolar disorder. Biol Psychiatry. 1999;45(8):953-958.
14. Frye MA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607-614.
15. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999;60(2):79-88.
16. Calabrese JR, Bowden CL, DeVeaugh-Geiss J, et al. Lamotrigine demonstrates long term mood stabilization in recently manic patients. Annual meeting of the American Psychiatric Association, New Orleans, 2001.
17. Stancer HC, Persad E. Treatment of intractable rapid-cycling manic-depressive disorder with levothyroxine. Clinical observations. Arch Gen Psychiatry. 1982;39(3):311-312.
18. Bauer MS, Whybrow PC. Rapid cycling bipolar affective disorder. II. Treatment of refractory rapid cycling with high-dose levothyroxine: a preliminary study.. Arch Gen Psychiatry. 1990;47(5):435-40.
19. Suppes T, Webb A, Paul B, Carmody T, et al:. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156:1164-1169.
1. Dunner DL, Fieve RR. Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry. 1974;20:229-233.
2. Calabrese JR, Delucchi GA. Spectrum of efficacy of valproate in 55 rapid-cycling manic depressives. Am J Psychiatry. 1990;147(4):431-434.
3. Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: Focus on depression as its hallmark. J Clin Psychiatry. 2001;62(Suppl 14):34-41.
4. Kukopulos A, Reginaldi D, Laddomada P, et al. Course of the manic depressive cycle and changes caused by treatment. Pharmacopsychiatry. 1980;13:156-167.
5. Maj M, et al. Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry. 1998;155:30-35.
6. Tondo L, Baldessarini RJ, et al. Lithium maintenance treatment of depression and mania in bipolar I and bipolar II disorders. Am J Psychiatry. 1998;155:638-645.
7. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145:179-84.
8. Calabrese JR, Woyshville MJ, Kimmel SE. Rapport DJ: Predictors of valproate response in bipolar rapid cycling. J Clin Psychopharmacology. 1993;13(4):280-283.
9. Sharma V, Persad E, et al. Treatment of rapid cycling bipolar disorder with combination therapy of valproate and lithium. Can J Psychiatry. 1993;38:137-139.
10. Calabrese JR, Suppes T, Bowden CL, et al. for the Lamictal 614 Study Group. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry. 2000;61(11):841-850.
11. Fatemi SH, Rapport DJ, Calabrese JR, et al. Lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry 1997;58:522-527.
12. Calabrese JR, Bowden CL, et al. for the Lamictal 602 Study Group. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60(2):79-88.
13. Bowden CL, Calabrese JR, McElroy SL, et al. Comparison of the efficacy of lamotrigine in rapid cycling and non-rapid cycling bipolar disorder. Biol Psychiatry. 1999;45(8):953-958.
14. Frye MA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607-614.
15. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999;60(2):79-88.
16. Calabrese JR, Bowden CL, DeVeaugh-Geiss J, et al. Lamotrigine demonstrates long term mood stabilization in recently manic patients. Annual meeting of the American Psychiatric Association, New Orleans, 2001.
17. Stancer HC, Persad E. Treatment of intractable rapid-cycling manic-depressive disorder with levothyroxine. Clinical observations. Arch Gen Psychiatry. 1982;39(3):311-312.
18. Bauer MS, Whybrow PC. Rapid cycling bipolar affective disorder. II. Treatment of refractory rapid cycling with high-dose levothyroxine: a preliminary study.. Arch Gen Psychiatry. 1990;47(5):435-40.
19. Suppes T, Webb A, Paul B, Carmody T, et al:. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156:1164-1169.
Rapid-cycling bipolar disorder: Which therapies are most effective?
Patients with rapid-cycling bipolar disorder (RCBD) can be frustrating to treat. Despite growing research and data, knowledge and effective therapies remain limited. How do you manage patients with rapid cycling who do not respond robustly to lithium, divalproex, or carbamazepine monotherapy? Are combination therapies likely to be more effective? Where does lamotrigine fit in? Is there a role for conventional antidepressants?
We’ll explore these and related questions—but the final answers are not yet in. Recognition of RCBD is important because it presents such difficult treatment challenges. Available evidence does suggest that rapid cycling as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (Box 1), describes a clinically specific course of illness that may require treatments different from currently used traditional drug therapies for nonrapid cycling bipolar disorder, particularly as no one agent appears to provide ideal bimodal treatment and prophylaxis of this bipolar disorder variant.
Rapid cycling is a specifier of the longitudinal course of illness presentation that is seen almost exclusively in bipolar disorder and is associated with a greater morbidity. Dunner and Fieve1 originally coined the term when evaluating clinical factors associated with lithium prophylaxis failure. Since that time the validity of rapid cycling as a distinct course modifier for bipolar disorder has been supported by multiple studies, leading to its inclusion in the fourth edition of the Diagnostic and Statistical Manual of the APA (1994).
According to DSM-IV, the course specifier of rapid cycling applies to “at least 4 episodes of a mood disturbance in the previous 12 months that meet criteria for a manic episode, a hypomanic episode, or a major depressive episode.” The episodes must be demarcated by a full or partial remission lasting at least 2 months or by a switch to a mood state of opposite polarity.
Early reports noted that patients suffering from RCBD did not respond adequately when treated with lithium.1 Other observations indicated that divalproex was more effective in this patient population, particularly for the illness’ hypomanic or manic phases.2 We hope that the following evaluation of these and other drug therapies will prove helpful.
Watch out for antidepressants
Most concerning has been the frequency and severity of treatment-refractory depressive phases of RCBD that may be exacerbated by antidepressant use (cycle induction or acceleration). Indeed, the frequent recurrence of refractory depression has been described as the hallmark of this bipolar disorder variant.3
Lithium: the scale weighs against it
Although an excellent mood stabilizer for most patients with bipolar disorder, lithium monotherapy is less than ideal for patients with the rapid-cycling variant, particularly in treatment or prevention of depressive or mixed episodes. The efficacy of lithium is likely decreased by the concurrent administration of antidepressant medication and increased when administered with other mood stabilizers.
The landmark article by Dunner and Fieve,1 which described a placebo-controlled, double-blind maintenance study in a general cohort of 55 patients, tried to clarify factors associated with the failure of lithium prophylaxis in bipolar disorder. Rapid cyclers comprised 20% of the subjects and 80% were nonrapid cyclers. Rapid cyclers were disproportionately represented in the lithium failure group. Lithium failures included 82% (9 of 11) of rapid cyclers compared to 41% (18 of 44) of nonrapid cyclers. Lithium failure was defined as (1) hospitalization for, or (2) treatment of, mania or (3) depression during lithium therapy, or as mood symptoms that, as documented by rating scales, were sufficient to warrant a diagnosis of mild depression, hypomania, or mania persisting for at least 2 weeks.
Kukopulos et al4 replicated the findings of Dunner and Fieve in a study of the longitudinal clinical course of 434 bipolar patients. Of these patients, 50 were rapid cyclers and had received continuous lithium therapy for more than a year, with good to partial prophylaxis in only 28%. Maj and colleagues5 published a 5-year prospective study of lithium therapy in 402 patients with bipolar disorder and noted the absence of rapid cycling in good responders to lithium but an incidence rate of 26% in nonresponders to lithium.
Other investigators have reported better response in RCBD. In a select cohort of lithium-responsive bipolar I and II patients, Tondo et al6 concluded that lithium maintenance yields striking long-term reductions in depressive and manic morbidity, more so in rapid cycling type II patients. This study, however, was in a cohort of lithium responders and excluded patients who had been exposed to antipsychotic or antidepressant medications for more than 3 months, those on chronic anticonvulsant therapy, and those with substance abuse disorders.
Although most studies do report poor response to lithium therapy in RCBD, Wehr and colleagues7 suggest that in some patients with rapid cycling, the discontinuation of antidepressant drugs may allow lithium to act as a more effective anticycling mood-stabilizing agent.
Divalproex: effective in manic phase
In contrast to lithium, an open trial of a homogenous cohort of patients with RCBD by Calabrese and colleagues3,8 found divalproex to possess moderate to marked acute and prophylactic antimanic properties with only modest antidepressant effects (Table 1). Data from 6 open studies involving 147 patients with rapid cycling suggest that divalproex possesses moderate to marked efficacy in the manic phase, but poor to moderate efficacy in the depressed phase. Positive outcome predictors were bipolar II and mixed states, no prior lithium therapy, and a positive family history of affective disorder. Predictors of negative response included increase in frequency and severity of mania, and borderline personality disorder.
Divalproex therapy in combination with lithium may improve response rates.9 Calabrese and colleagues, however, have examined large cohorts of patients, including those comorbid with alcohol, cocaine, and/or cannabis abuse, treated with a lithium-divalproex combination over 6-month study periods. The researchers found that only 25% to 50% of patients stabilized, and that of those not exhibiting a response, the majority (75%) did not respond because of treatment-refractory depression in the context of RCBD.3
Although experts believe divalproex to be more effective than lithium in preventing episodes associated with RCBD, such a conclusion awaits confirmation with the near completion of a double-blind, 20-month maintenance trial sponsored by the National Institute of Mental Health (NIMH).
Carbamazepine’s role in combination therapy
Early reports by Post and colleagues in 1987 suggested that rapid cycling predicted positive response to carbamazepine, but later findings by Okuma in 1993 refuted this. Other collective open and controlled studies suggest that this anticonvulsant possesses moderate to marked efficacy in the manic phase, and poor to moderate efficacy in the depressed phase of RCBD. Again, combination therapy with lithium may offer greater efficacy. Of significance, carbamazepine treatment outcomes have not been prospectively evaluated in a homogeneous cohort of rapid cyclers.
The limitations of carbamazepine therapy are well known and available evidence also does not seem to support monotherapy with this agent as being useful in RCBD, especially in the treatment and prophylaxis of depressive or mixed phases of the disorder. Thus, further controlled studies are needed to examine the agent’s potential role and safety in combination therapies for RCBD.
Table 1
SPECTRUM OF ACUTE AND PROPHYLACTIC EFFICACY OF DIVALPROEX IN RAPID-CYCLING BIPOLAR DISORDER
| Spectrum of marked responses to divalproex in bipolar rapid cycling | ||
|---|---|---|
| Acute | Prophylactic | |
| Dysphoric hypomania/mania | 87% | 89% |
| Elated hypomania/mania | 64% | 77% |
| Depression (n = 101, mean follow-up 15 months) | 21% | 38% |
| Adapted from Calabrese and Delucchi. Am J Psychiatry. 1990;147:431-434 and Calabrese et al. J Clin Psychopharmacol. 1993;13:280-283. | ||
Lamotrigine: best hope for monotherapy
Lamotrigine monotherapy has been reported to be effective in some RCBD cases. The data suggest that it possesses both antidepressant and mood-stabilizing properties.10
An open, naturalistic study of 5 women with treatment-refractory rapid cycling by Fatemi et al11 demonstrated both mood-stabilizing and antidepressant effects from lamotrigine monotherapy or augmentation at a mean dose of 185 ±33.5 mg/d. In 14 clinical reports involving 207 patients with bipolar disorder, 66 of whom had rapid cycling, lamotrigine was observed to possess moderate to marked efficacy in depression and hypomania, but only moderate efficacy in mania.
An open, prospective study compared the efficacy of lamotrigine add-on or monotherapy in 41 rapid cyclers to 34 nonrapid cyclers across 48 weeks. Improvement from baseline to last visit was significant between both subgroups for depressive and hypomanic symptoms. Patients presenting with more severe manic symptoms did less well.13
In the first double-blind, placebo-controlled study of lamotrigine in RCBD,12 182 of 324 patients with rapid cycling responded to treatment with open-label lamotrigine and were then randomized to the study’s double-blind phase. Forty-one percent of lamotrigine-treated vs. 26% of placebo-treated patients were stable without relapse during 6 months of monotherapy. Patients with rapid-cycling bipolar II disorder consistently experienced more improvement than their bipolar I counterparts (Figure 1). The results of this only prospective, placebo-controlled, acute-treatment study of rapid-cycling bipolar patients to date indicate that lamotrigine monotherapy is useful for some patients with RCBD, particularly those with bipolar II.
Frye et al14 conducted a double-blind, placebo-controlled study of 23 patients with rapid cycling utilizing a crossover series of three 6-week monotherapy evaluations of lamotrigine, gabapentin, and placebo. Marked antidepressant response on lamotrigine was seen in 45% of the participants compared with 19% of patients on placebo and a similar response rate among those on gabapentin.
A study evaluating the safety and efficacy of 2 dosages of lamotrigine (50 mg/d or 200 mg/d) compared with placebo in the treatment of a major depressive episode in patients with bipolar I disorder over 7 weeks demonstrated significant antidepressant efficacy.15 These bipolar outpatients displayed clinical improvement as early as the third week of treatment, and switch rates for both dosages did not exceed that of placebo. Patients with RCBD, however, were excluded from this initial trial. Subsequent studies have demonstrated similar magnitudes of efficacy in patients with RCBD, primarily in the prevention of depressive episodes, including the 6-month RCBD maintenance study10 and 2 recently completed 18-month maintenance studies of patients with bipolar I disorder, either recently manic or recently depressed.
Lamotrigine thus may have a special role in RCBD treatment. Its most significant side effect in bipolar disorder is benign rash, which has occurred in 9.0% (108 of 1,198) of patients randomized to lamotrigine vs. 7.6% (80 of 1,056) of those randomized to placebo in pivotal multicenter, double-blind, placebo-controlled bipolar trials.
In mood disorder trials conducted to date, the rate of serious rash, defined as requiring both drug discontinuation and hospitalization, has been 0.06% (2 of 3,153) on lamotrigine and 0.09% (1 out of 1,053) on placebo. No cases of Steven’s Johnson syndrome or toxic epidermal necrolysis were observed.16 A low starting dose and gradual titration of lamotrigine (Table 2) appear to minimize the risk of serious rash.
Levothyroxine: possible add-on therapy
Levothyroxine should be considered as add-on therapy in patients with known hypothyroidism, borderline hypothyroidism, or otherwise treatment-refractory cases.
The strategy of thyroid supplementation is derived from Gjessing’s 1936 report of success in administering hypermetabolic doses of thyroid hormone to patients with periodic catatonia. Stancer and Persad17 initially reported the potential efficacy of this therapeutic maneuver in rapid cyclers; remissions were induced by hypermetabolic thyroid doses in 5 of 7 patients with treatment-refractory bipolar disorder. No data currently support any prophylactic efficacy of thyroid supplementation monotherapy for RCBD treatment.
Bauer and Whybrow18 suggest that thyroid supplementation with T4 added to mood stabilizers augments efficacy independent of pre-existing thyroid function. The potential side effects of long-term levothryoxine administration, namely osteoporosis and cardiac arrhythmias, limit the usefulness of thyroid augmentation in RCBD.
Atypical antipsychotics: perhaps in combination
Coadministering atypical antipsychotics in other mood stabilizers may help rapid cyclers with current or past psychotic symptoms during their mood episodes, but further study is clearly needed.
Atypical antipsychotic medications may have specific mood-stabilizing properties, particularly in the management of mixed and manic states. In the first prospective trial of clozapine monotherapy in bipolar disorder, Calabrese and colleagues in 1994 reported that rapid cycling did not appear to predict nonresponse to treatment.
In the first randomized, controlled trial involving clozapine in bipolar disorder, Suppes et al19 noted significant improvement for symptoms of mania, psychosis, and global improvement. Subjects with nonpsychotic bipolar disorder showed a degree of improvement similar to that seen in the entire clozapine-treated group. These results support a mood-stabilizing role for clozapine.
Preliminary studies of risperidone and olanzapine further suggest therapeutic utility for atypical antipsychotics.
Figure 1 LAMOTRIGINE VS. PLACEBO IN RAPID-CYCLING BIPOLAR DISORDER
Table 2
DOSAGE PLAN FOR LAMOTRIGINE IN MONOTHERAPY AND COMBINATION
| Treatment period | Type of therapy | Daily dosage |
|---|---|---|
| Weeks 1-2 | Monotherapy With divalproex With carbamazepine | 25 mg 12.5 mg 50 mg |
| Weeks 3-4 | Monotherapy With divalproex With carbamazepine | 50 mg 25 mg 100 mg |
| Week 5 | Monotherapy With divalproex With carbamazepine | 100 mg 50 mg 200 mg |
| Thereafter | Monotherapy With divalproex With carbamazepine | 200 mg 100 mg 400 mg |
Topiramate: in patients with weight problems
Evidence from 12 open studies of 223 patients with bipolar disorder suggest that this anticonvulsant may possess mood-stabilizing properties while sparing patients the weight gain commonly seen with other pharmacotherapies.
Two studies of topiramate as an add-on therapy come to mind. Marcotte in 1998 retrospectively studied 44 patients with RCBD, 23 (52%) of whom demonstrated moderate or marked improvement on a mean topiramate dosage of 200 mg/d.
Sachs also reported that symptoms for some patients with a treatment-refractory bipolar disorder could be improved when topiramate was added to their medication regimen. Available data encourage further controlled studies of topiramate add-on therapy for RCBD, especially in obese patients.
Gabapentin: contradictory reports
Preliminary data from 14 open-label studies of gabapentin in 302 bipolar patients reported a response rate of around 67% when used as an add-on therapy, usually in mania or mixed states. A moderate antimanic effect was observed among a total of 23 rapid cyclers in 9 of the studies.
The reports are contradictory, however, and available data do not firmly support the agent’s efficacy in RCBD treatment.
Other potential uses for gabapentin are being investigated, such as antidepressant augmentation, anxiety, and chronic pain. Thus, patients with RCBD and a comorbid illness may benefit from add-on gabapentin.
ECT: Some limited success
Electroconvulsive therapy (ECT) has been implicated less frequently than antidepressants in the induction of rapid cycling, and when this does occur it is usually in the context of combined ECT/antidepressant therapies.
Berma and Wolpert in 1987 reported a case of successful ECT treatment of rapid cycling in an adolescent who had been treated with trimipramine for depression. Vanelle et al in 1994 suggested that maintenance ECT works well in 22 treatment-resistant bipolar patients, including 4 with rapid cycling, over an 18-month treatment period.
Behavioral intervention: changing sleep routines
NIMH research of 15 rapid cyclers who were studied for 3 months looked at behavioral interventions and their effect on switching (Feldman-Naim 1997). This study suggested that patients were more likely to switch from depression into hypomania/mania during daytime hours and from mania/hypomania into depression during nighttime.
The use of light therapy or activity and exercise during depression and the use of induced sleep or exposure to darkness during mania/hypomania may be therapeutic. Wehr and colleagues supported this in a 1998 report of one patient studied over several years, comparing this rapid-cycling patient’s regular sleep routine with prolonged (10 to 14 hours per night) and enforced bed rest in the dark.7 The promotion of sleep by scheduling regular nighttime periods of enforced bed rest in the dark may help prevent mania and stabilize mood in rapid cyclers.
Other add-on possibilities
Haykal in 1990 reported bupropion to be an effective add-on treatment in 5 of 6 patients with refractory, rapid-cycling bipolar II disorder. Benefit has also been reported from clorgyline, clonidine, magnesium, primidone, and acetazolamide.
Calcium-channel blockers may also offer clinical utility, although supportive evidence is limited. Nimodipine was evaluated for efficacy in 30 patients with treatment-refractory affective illness by the NIMH and Passaglia et al in 1998. Patients who improved on this agent had ultradian rapid cycling, defined in the study as those with affective episodes lasting as short as a week.
A recommended treatment strategy
Based on the available data in bipolar I rapid cycling, we recommend initial treatment with divalproex followed by augmentation with lithium if hypomanic or manic episodes persist, lamotrigine if breakthrough episodes are predominantly depressive, and atypical antipsychotics if psychotic symptoms or true mixed states remain.
For patients presenting with bipolar II rapid cycling, we recommend starting with lamotrigine, then augmenting with divalproex or lithium for breakthrough episodes. Lamotrigine shows more promise because of its reportedly greater antidepressant properties and lack of cycle induction or switching, but offers only modest antimanic therapy.
Pending further investigations, current application of the data suggests that when treating patients with RCBD, conventional antidepressants should be avoided and, if first-line therapies are not effective, the clinician should consider moving to combination drug therapy with 2 or more agents.
Related resources
- Bauer MS, Calabrese JR, Dunner DL, et al. Multi-site data reanalysis: validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151:506-515.
- Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine in treatment refractory mania. Am J Psychiatry. 1996;153(6):759-764.
- Calabrese JR, Bowden CL, McElroy SL, et al. Spectrum of activity of lamotrigine in treatment refractory bipolar disorder. Am J Psychiatry. 1999;156(7):1019-1023.
- Sachs GS. Printz DJ. Kahn DA. Carpenter D. Docherty JP. The expert consensus guideline series: medication treatment of bipolar disorder 2000. Postgraduate Medicine. April 2000; Spec No:1-104.
Drug brand names
- Buproprion • Wellbutrin
- Carbamazepine • Tegretol
- Clonidine • Catapres
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levothyroxine • Synthroid, Levothroid, Levoxyl
- Olanzapine • Zyprexa
- Nimodipine • Nimotop
- Primidonem • Mysoline
- Topiramate • Topamax
1. Dunner DL, Fieve RR. Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry. 1974;20:229-233.
2. Calabrese JR, Delucchi GA. Spectrum of efficacy of valproate in 55 rapid-cycling manic depressives. Am J Psychiatry. 1990;147(4):431-434.
3. Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: Focus on depression as its hallmark. J Clin Psychiatry. 2001;62(Suppl 14):34-41.
4. Kukopulos A, Reginaldi D, Laddomada P, et al. Course of the manic depressive cycle and changes caused by treatment. Pharmacopsychiatry. 1980;13:156-167.
5. Maj M, et al. Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry. 1998;155:30-35.
6. Tondo L, Baldessarini RJ, et al. Lithium maintenance treatment of depression and mania in bipolar I and bipolar II disorders. Am J Psychiatry. 1998;155:638-645.
7. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145:179-84.
8. Calabrese JR, Woyshville MJ, Kimmel SE. Rapport DJ: Predictors of valproate response in bipolar rapid cycling. J Clin Psychopharmacology. 1993;13(4):280-283.
9. Sharma V, Persad E, et al. Treatment of rapid cycling bipolar disorder with combination therapy of valproate and lithium. Can J Psychiatry. 1993;38:137-139.
10. Calabrese JR, Suppes T, Bowden CL, et al. for the Lamictal 614 Study Group. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry. 2000;61(11):841-850.
11. Fatemi SH, Rapport DJ, Calabrese JR, et al. Lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry 1997;58:522-527.
12. Calabrese JR, Bowden CL, et al. for the Lamictal 602 Study Group. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60(2):79-88.
13. Bowden CL, Calabrese JR, McElroy SL, et al. Comparison of the efficacy of lamotrigine in rapid cycling and non-rapid cycling bipolar disorder. Biol Psychiatry. 1999;45(8):953-958.
14. Frye MA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607-614.
15. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999;60(2):79-88.
16. Calabrese JR, Bowden CL, DeVeaugh-Geiss J, et al. Lamotrigine demonstrates long term mood stabilization in recently manic patients. Annual meeting of the American Psychiatric Association, New Orleans, 2001.
17. Stancer HC, Persad E. Treatment of intractable rapid-cycling manic-depressive disorder with levothyroxine. Clinical observations. Arch Gen Psychiatry. 1982;39(3):311-312.
18. Bauer MS, Whybrow PC. Rapid cycling bipolar affective disorder. II. Treatment of refractory rapid cycling with high-dose levothyroxine: a preliminary study.. Arch Gen Psychiatry. 1990;47(5):435-40.
19. Suppes T, Webb A, Paul B, Carmody T, et al:. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156:1164-1169.
Patients with rapid-cycling bipolar disorder (RCBD) can be frustrating to treat. Despite growing research and data, knowledge and effective therapies remain limited. How do you manage patients with rapid cycling who do not respond robustly to lithium, divalproex, or carbamazepine monotherapy? Are combination therapies likely to be more effective? Where does lamotrigine fit in? Is there a role for conventional antidepressants?
We’ll explore these and related questions—but the final answers are not yet in. Recognition of RCBD is important because it presents such difficult treatment challenges. Available evidence does suggest that rapid cycling as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (Box 1), describes a clinically specific course of illness that may require treatments different from currently used traditional drug therapies for nonrapid cycling bipolar disorder, particularly as no one agent appears to provide ideal bimodal treatment and prophylaxis of this bipolar disorder variant.
Rapid cycling is a specifier of the longitudinal course of illness presentation that is seen almost exclusively in bipolar disorder and is associated with a greater morbidity. Dunner and Fieve1 originally coined the term when evaluating clinical factors associated with lithium prophylaxis failure. Since that time the validity of rapid cycling as a distinct course modifier for bipolar disorder has been supported by multiple studies, leading to its inclusion in the fourth edition of the Diagnostic and Statistical Manual of the APA (1994).
According to DSM-IV, the course specifier of rapid cycling applies to “at least 4 episodes of a mood disturbance in the previous 12 months that meet criteria for a manic episode, a hypomanic episode, or a major depressive episode.” The episodes must be demarcated by a full or partial remission lasting at least 2 months or by a switch to a mood state of opposite polarity.
Early reports noted that patients suffering from RCBD did not respond adequately when treated with lithium.1 Other observations indicated that divalproex was more effective in this patient population, particularly for the illness’ hypomanic or manic phases.2 We hope that the following evaluation of these and other drug therapies will prove helpful.
Watch out for antidepressants
Most concerning has been the frequency and severity of treatment-refractory depressive phases of RCBD that may be exacerbated by antidepressant use (cycle induction or acceleration). Indeed, the frequent recurrence of refractory depression has been described as the hallmark of this bipolar disorder variant.3
Lithium: the scale weighs against it
Although an excellent mood stabilizer for most patients with bipolar disorder, lithium monotherapy is less than ideal for patients with the rapid-cycling variant, particularly in treatment or prevention of depressive or mixed episodes. The efficacy of lithium is likely decreased by the concurrent administration of antidepressant medication and increased when administered with other mood stabilizers.
The landmark article by Dunner and Fieve,1 which described a placebo-controlled, double-blind maintenance study in a general cohort of 55 patients, tried to clarify factors associated with the failure of lithium prophylaxis in bipolar disorder. Rapid cyclers comprised 20% of the subjects and 80% were nonrapid cyclers. Rapid cyclers were disproportionately represented in the lithium failure group. Lithium failures included 82% (9 of 11) of rapid cyclers compared to 41% (18 of 44) of nonrapid cyclers. Lithium failure was defined as (1) hospitalization for, or (2) treatment of, mania or (3) depression during lithium therapy, or as mood symptoms that, as documented by rating scales, were sufficient to warrant a diagnosis of mild depression, hypomania, or mania persisting for at least 2 weeks.
Kukopulos et al4 replicated the findings of Dunner and Fieve in a study of the longitudinal clinical course of 434 bipolar patients. Of these patients, 50 were rapid cyclers and had received continuous lithium therapy for more than a year, with good to partial prophylaxis in only 28%. Maj and colleagues5 published a 5-year prospective study of lithium therapy in 402 patients with bipolar disorder and noted the absence of rapid cycling in good responders to lithium but an incidence rate of 26% in nonresponders to lithium.
Other investigators have reported better response in RCBD. In a select cohort of lithium-responsive bipolar I and II patients, Tondo et al6 concluded that lithium maintenance yields striking long-term reductions in depressive and manic morbidity, more so in rapid cycling type II patients. This study, however, was in a cohort of lithium responders and excluded patients who had been exposed to antipsychotic or antidepressant medications for more than 3 months, those on chronic anticonvulsant therapy, and those with substance abuse disorders.
Although most studies do report poor response to lithium therapy in RCBD, Wehr and colleagues7 suggest that in some patients with rapid cycling, the discontinuation of antidepressant drugs may allow lithium to act as a more effective anticycling mood-stabilizing agent.
Divalproex: effective in manic phase
In contrast to lithium, an open trial of a homogenous cohort of patients with RCBD by Calabrese and colleagues3,8 found divalproex to possess moderate to marked acute and prophylactic antimanic properties with only modest antidepressant effects (Table 1). Data from 6 open studies involving 147 patients with rapid cycling suggest that divalproex possesses moderate to marked efficacy in the manic phase, but poor to moderate efficacy in the depressed phase. Positive outcome predictors were bipolar II and mixed states, no prior lithium therapy, and a positive family history of affective disorder. Predictors of negative response included increase in frequency and severity of mania, and borderline personality disorder.
Divalproex therapy in combination with lithium may improve response rates.9 Calabrese and colleagues, however, have examined large cohorts of patients, including those comorbid with alcohol, cocaine, and/or cannabis abuse, treated with a lithium-divalproex combination over 6-month study periods. The researchers found that only 25% to 50% of patients stabilized, and that of those not exhibiting a response, the majority (75%) did not respond because of treatment-refractory depression in the context of RCBD.3
Although experts believe divalproex to be more effective than lithium in preventing episodes associated with RCBD, such a conclusion awaits confirmation with the near completion of a double-blind, 20-month maintenance trial sponsored by the National Institute of Mental Health (NIMH).
Carbamazepine’s role in combination therapy
Early reports by Post and colleagues in 1987 suggested that rapid cycling predicted positive response to carbamazepine, but later findings by Okuma in 1993 refuted this. Other collective open and controlled studies suggest that this anticonvulsant possesses moderate to marked efficacy in the manic phase, and poor to moderate efficacy in the depressed phase of RCBD. Again, combination therapy with lithium may offer greater efficacy. Of significance, carbamazepine treatment outcomes have not been prospectively evaluated in a homogeneous cohort of rapid cyclers.
The limitations of carbamazepine therapy are well known and available evidence also does not seem to support monotherapy with this agent as being useful in RCBD, especially in the treatment and prophylaxis of depressive or mixed phases of the disorder. Thus, further controlled studies are needed to examine the agent’s potential role and safety in combination therapies for RCBD.
Table 1
SPECTRUM OF ACUTE AND PROPHYLACTIC EFFICACY OF DIVALPROEX IN RAPID-CYCLING BIPOLAR DISORDER
| Spectrum of marked responses to divalproex in bipolar rapid cycling | ||
|---|---|---|
| Acute | Prophylactic | |
| Dysphoric hypomania/mania | 87% | 89% |
| Elated hypomania/mania | 64% | 77% |
| Depression (n = 101, mean follow-up 15 months) | 21% | 38% |
| Adapted from Calabrese and Delucchi. Am J Psychiatry. 1990;147:431-434 and Calabrese et al. J Clin Psychopharmacol. 1993;13:280-283. | ||
Lamotrigine: best hope for monotherapy
Lamotrigine monotherapy has been reported to be effective in some RCBD cases. The data suggest that it possesses both antidepressant and mood-stabilizing properties.10
An open, naturalistic study of 5 women with treatment-refractory rapid cycling by Fatemi et al11 demonstrated both mood-stabilizing and antidepressant effects from lamotrigine monotherapy or augmentation at a mean dose of 185 ±33.5 mg/d. In 14 clinical reports involving 207 patients with bipolar disorder, 66 of whom had rapid cycling, lamotrigine was observed to possess moderate to marked efficacy in depression and hypomania, but only moderate efficacy in mania.
An open, prospective study compared the efficacy of lamotrigine add-on or monotherapy in 41 rapid cyclers to 34 nonrapid cyclers across 48 weeks. Improvement from baseline to last visit was significant between both subgroups for depressive and hypomanic symptoms. Patients presenting with more severe manic symptoms did less well.13
In the first double-blind, placebo-controlled study of lamotrigine in RCBD,12 182 of 324 patients with rapid cycling responded to treatment with open-label lamotrigine and were then randomized to the study’s double-blind phase. Forty-one percent of lamotrigine-treated vs. 26% of placebo-treated patients were stable without relapse during 6 months of monotherapy. Patients with rapid-cycling bipolar II disorder consistently experienced more improvement than their bipolar I counterparts (Figure 1). The results of this only prospective, placebo-controlled, acute-treatment study of rapid-cycling bipolar patients to date indicate that lamotrigine monotherapy is useful for some patients with RCBD, particularly those with bipolar II.
Frye et al14 conducted a double-blind, placebo-controlled study of 23 patients with rapid cycling utilizing a crossover series of three 6-week monotherapy evaluations of lamotrigine, gabapentin, and placebo. Marked antidepressant response on lamotrigine was seen in 45% of the participants compared with 19% of patients on placebo and a similar response rate among those on gabapentin.
A study evaluating the safety and efficacy of 2 dosages of lamotrigine (50 mg/d or 200 mg/d) compared with placebo in the treatment of a major depressive episode in patients with bipolar I disorder over 7 weeks demonstrated significant antidepressant efficacy.15 These bipolar outpatients displayed clinical improvement as early as the third week of treatment, and switch rates for both dosages did not exceed that of placebo. Patients with RCBD, however, were excluded from this initial trial. Subsequent studies have demonstrated similar magnitudes of efficacy in patients with RCBD, primarily in the prevention of depressive episodes, including the 6-month RCBD maintenance study10 and 2 recently completed 18-month maintenance studies of patients with bipolar I disorder, either recently manic or recently depressed.
Lamotrigine thus may have a special role in RCBD treatment. Its most significant side effect in bipolar disorder is benign rash, which has occurred in 9.0% (108 of 1,198) of patients randomized to lamotrigine vs. 7.6% (80 of 1,056) of those randomized to placebo in pivotal multicenter, double-blind, placebo-controlled bipolar trials.
In mood disorder trials conducted to date, the rate of serious rash, defined as requiring both drug discontinuation and hospitalization, has been 0.06% (2 of 3,153) on lamotrigine and 0.09% (1 out of 1,053) on placebo. No cases of Steven’s Johnson syndrome or toxic epidermal necrolysis were observed.16 A low starting dose and gradual titration of lamotrigine (Table 2) appear to minimize the risk of serious rash.
Levothyroxine: possible add-on therapy
Levothyroxine should be considered as add-on therapy in patients with known hypothyroidism, borderline hypothyroidism, or otherwise treatment-refractory cases.
The strategy of thyroid supplementation is derived from Gjessing’s 1936 report of success in administering hypermetabolic doses of thyroid hormone to patients with periodic catatonia. Stancer and Persad17 initially reported the potential efficacy of this therapeutic maneuver in rapid cyclers; remissions were induced by hypermetabolic thyroid doses in 5 of 7 patients with treatment-refractory bipolar disorder. No data currently support any prophylactic efficacy of thyroid supplementation monotherapy for RCBD treatment.
Bauer and Whybrow18 suggest that thyroid supplementation with T4 added to mood stabilizers augments efficacy independent of pre-existing thyroid function. The potential side effects of long-term levothryoxine administration, namely osteoporosis and cardiac arrhythmias, limit the usefulness of thyroid augmentation in RCBD.
Atypical antipsychotics: perhaps in combination
Coadministering atypical antipsychotics in other mood stabilizers may help rapid cyclers with current or past psychotic symptoms during their mood episodes, but further study is clearly needed.
Atypical antipsychotic medications may have specific mood-stabilizing properties, particularly in the management of mixed and manic states. In the first prospective trial of clozapine monotherapy in bipolar disorder, Calabrese and colleagues in 1994 reported that rapid cycling did not appear to predict nonresponse to treatment.
In the first randomized, controlled trial involving clozapine in bipolar disorder, Suppes et al19 noted significant improvement for symptoms of mania, psychosis, and global improvement. Subjects with nonpsychotic bipolar disorder showed a degree of improvement similar to that seen in the entire clozapine-treated group. These results support a mood-stabilizing role for clozapine.
Preliminary studies of risperidone and olanzapine further suggest therapeutic utility for atypical antipsychotics.
Figure 1 LAMOTRIGINE VS. PLACEBO IN RAPID-CYCLING BIPOLAR DISORDER
Table 2
DOSAGE PLAN FOR LAMOTRIGINE IN MONOTHERAPY AND COMBINATION
| Treatment period | Type of therapy | Daily dosage |
|---|---|---|
| Weeks 1-2 | Monotherapy With divalproex With carbamazepine | 25 mg 12.5 mg 50 mg |
| Weeks 3-4 | Monotherapy With divalproex With carbamazepine | 50 mg 25 mg 100 mg |
| Week 5 | Monotherapy With divalproex With carbamazepine | 100 mg 50 mg 200 mg |
| Thereafter | Monotherapy With divalproex With carbamazepine | 200 mg 100 mg 400 mg |
Topiramate: in patients with weight problems
Evidence from 12 open studies of 223 patients with bipolar disorder suggest that this anticonvulsant may possess mood-stabilizing properties while sparing patients the weight gain commonly seen with other pharmacotherapies.
Two studies of topiramate as an add-on therapy come to mind. Marcotte in 1998 retrospectively studied 44 patients with RCBD, 23 (52%) of whom demonstrated moderate or marked improvement on a mean topiramate dosage of 200 mg/d.
Sachs also reported that symptoms for some patients with a treatment-refractory bipolar disorder could be improved when topiramate was added to their medication regimen. Available data encourage further controlled studies of topiramate add-on therapy for RCBD, especially in obese patients.
Gabapentin: contradictory reports
Preliminary data from 14 open-label studies of gabapentin in 302 bipolar patients reported a response rate of around 67% when used as an add-on therapy, usually in mania or mixed states. A moderate antimanic effect was observed among a total of 23 rapid cyclers in 9 of the studies.
The reports are contradictory, however, and available data do not firmly support the agent’s efficacy in RCBD treatment.
Other potential uses for gabapentin are being investigated, such as antidepressant augmentation, anxiety, and chronic pain. Thus, patients with RCBD and a comorbid illness may benefit from add-on gabapentin.
ECT: Some limited success
Electroconvulsive therapy (ECT) has been implicated less frequently than antidepressants in the induction of rapid cycling, and when this does occur it is usually in the context of combined ECT/antidepressant therapies.
Berma and Wolpert in 1987 reported a case of successful ECT treatment of rapid cycling in an adolescent who had been treated with trimipramine for depression. Vanelle et al in 1994 suggested that maintenance ECT works well in 22 treatment-resistant bipolar patients, including 4 with rapid cycling, over an 18-month treatment period.
Behavioral intervention: changing sleep routines
NIMH research of 15 rapid cyclers who were studied for 3 months looked at behavioral interventions and their effect on switching (Feldman-Naim 1997). This study suggested that patients were more likely to switch from depression into hypomania/mania during daytime hours and from mania/hypomania into depression during nighttime.
The use of light therapy or activity and exercise during depression and the use of induced sleep or exposure to darkness during mania/hypomania may be therapeutic. Wehr and colleagues supported this in a 1998 report of one patient studied over several years, comparing this rapid-cycling patient’s regular sleep routine with prolonged (10 to 14 hours per night) and enforced bed rest in the dark.7 The promotion of sleep by scheduling regular nighttime periods of enforced bed rest in the dark may help prevent mania and stabilize mood in rapid cyclers.
Other add-on possibilities
Haykal in 1990 reported bupropion to be an effective add-on treatment in 5 of 6 patients with refractory, rapid-cycling bipolar II disorder. Benefit has also been reported from clorgyline, clonidine, magnesium, primidone, and acetazolamide.
Calcium-channel blockers may also offer clinical utility, although supportive evidence is limited. Nimodipine was evaluated for efficacy in 30 patients with treatment-refractory affective illness by the NIMH and Passaglia et al in 1998. Patients who improved on this agent had ultradian rapid cycling, defined in the study as those with affective episodes lasting as short as a week.
A recommended treatment strategy
Based on the available data in bipolar I rapid cycling, we recommend initial treatment with divalproex followed by augmentation with lithium if hypomanic or manic episodes persist, lamotrigine if breakthrough episodes are predominantly depressive, and atypical antipsychotics if psychotic symptoms or true mixed states remain.
For patients presenting with bipolar II rapid cycling, we recommend starting with lamotrigine, then augmenting with divalproex or lithium for breakthrough episodes. Lamotrigine shows more promise because of its reportedly greater antidepressant properties and lack of cycle induction or switching, but offers only modest antimanic therapy.
Pending further investigations, current application of the data suggests that when treating patients with RCBD, conventional antidepressants should be avoided and, if first-line therapies are not effective, the clinician should consider moving to combination drug therapy with 2 or more agents.
Related resources
- Bauer MS, Calabrese JR, Dunner DL, et al. Multi-site data reanalysis: validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151:506-515.
- Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine in treatment refractory mania. Am J Psychiatry. 1996;153(6):759-764.
- Calabrese JR, Bowden CL, McElroy SL, et al. Spectrum of activity of lamotrigine in treatment refractory bipolar disorder. Am J Psychiatry. 1999;156(7):1019-1023.
- Sachs GS. Printz DJ. Kahn DA. Carpenter D. Docherty JP. The expert consensus guideline series: medication treatment of bipolar disorder 2000. Postgraduate Medicine. April 2000; Spec No:1-104.
Drug brand names
- Buproprion • Wellbutrin
- Carbamazepine • Tegretol
- Clonidine • Catapres
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levothyroxine • Synthroid, Levothroid, Levoxyl
- Olanzapine • Zyprexa
- Nimodipine • Nimotop
- Primidonem • Mysoline
- Topiramate • Topamax
Patients with rapid-cycling bipolar disorder (RCBD) can be frustrating to treat. Despite growing research and data, knowledge and effective therapies remain limited. How do you manage patients with rapid cycling who do not respond robustly to lithium, divalproex, or carbamazepine monotherapy? Are combination therapies likely to be more effective? Where does lamotrigine fit in? Is there a role for conventional antidepressants?
We’ll explore these and related questions—but the final answers are not yet in. Recognition of RCBD is important because it presents such difficult treatment challenges. Available evidence does suggest that rapid cycling as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (Box 1), describes a clinically specific course of illness that may require treatments different from currently used traditional drug therapies for nonrapid cycling bipolar disorder, particularly as no one agent appears to provide ideal bimodal treatment and prophylaxis of this bipolar disorder variant.
Rapid cycling is a specifier of the longitudinal course of illness presentation that is seen almost exclusively in bipolar disorder and is associated with a greater morbidity. Dunner and Fieve1 originally coined the term when evaluating clinical factors associated with lithium prophylaxis failure. Since that time the validity of rapid cycling as a distinct course modifier for bipolar disorder has been supported by multiple studies, leading to its inclusion in the fourth edition of the Diagnostic and Statistical Manual of the APA (1994).
According to DSM-IV, the course specifier of rapid cycling applies to “at least 4 episodes of a mood disturbance in the previous 12 months that meet criteria for a manic episode, a hypomanic episode, or a major depressive episode.” The episodes must be demarcated by a full or partial remission lasting at least 2 months or by a switch to a mood state of opposite polarity.
Early reports noted that patients suffering from RCBD did not respond adequately when treated with lithium.1 Other observations indicated that divalproex was more effective in this patient population, particularly for the illness’ hypomanic or manic phases.2 We hope that the following evaluation of these and other drug therapies will prove helpful.
Watch out for antidepressants
Most concerning has been the frequency and severity of treatment-refractory depressive phases of RCBD that may be exacerbated by antidepressant use (cycle induction or acceleration). Indeed, the frequent recurrence of refractory depression has been described as the hallmark of this bipolar disorder variant.3
Lithium: the scale weighs against it
Although an excellent mood stabilizer for most patients with bipolar disorder, lithium monotherapy is less than ideal for patients with the rapid-cycling variant, particularly in treatment or prevention of depressive or mixed episodes. The efficacy of lithium is likely decreased by the concurrent administration of antidepressant medication and increased when administered with other mood stabilizers.
The landmark article by Dunner and Fieve,1 which described a placebo-controlled, double-blind maintenance study in a general cohort of 55 patients, tried to clarify factors associated with the failure of lithium prophylaxis in bipolar disorder. Rapid cyclers comprised 20% of the subjects and 80% were nonrapid cyclers. Rapid cyclers were disproportionately represented in the lithium failure group. Lithium failures included 82% (9 of 11) of rapid cyclers compared to 41% (18 of 44) of nonrapid cyclers. Lithium failure was defined as (1) hospitalization for, or (2) treatment of, mania or (3) depression during lithium therapy, or as mood symptoms that, as documented by rating scales, were sufficient to warrant a diagnosis of mild depression, hypomania, or mania persisting for at least 2 weeks.
Kukopulos et al4 replicated the findings of Dunner and Fieve in a study of the longitudinal clinical course of 434 bipolar patients. Of these patients, 50 were rapid cyclers and had received continuous lithium therapy for more than a year, with good to partial prophylaxis in only 28%. Maj and colleagues5 published a 5-year prospective study of lithium therapy in 402 patients with bipolar disorder and noted the absence of rapid cycling in good responders to lithium but an incidence rate of 26% in nonresponders to lithium.
Other investigators have reported better response in RCBD. In a select cohort of lithium-responsive bipolar I and II patients, Tondo et al6 concluded that lithium maintenance yields striking long-term reductions in depressive and manic morbidity, more so in rapid cycling type II patients. This study, however, was in a cohort of lithium responders and excluded patients who had been exposed to antipsychotic or antidepressant medications for more than 3 months, those on chronic anticonvulsant therapy, and those with substance abuse disorders.
Although most studies do report poor response to lithium therapy in RCBD, Wehr and colleagues7 suggest that in some patients with rapid cycling, the discontinuation of antidepressant drugs may allow lithium to act as a more effective anticycling mood-stabilizing agent.
Divalproex: effective in manic phase
In contrast to lithium, an open trial of a homogenous cohort of patients with RCBD by Calabrese and colleagues3,8 found divalproex to possess moderate to marked acute and prophylactic antimanic properties with only modest antidepressant effects (Table 1). Data from 6 open studies involving 147 patients with rapid cycling suggest that divalproex possesses moderate to marked efficacy in the manic phase, but poor to moderate efficacy in the depressed phase. Positive outcome predictors were bipolar II and mixed states, no prior lithium therapy, and a positive family history of affective disorder. Predictors of negative response included increase in frequency and severity of mania, and borderline personality disorder.
Divalproex therapy in combination with lithium may improve response rates.9 Calabrese and colleagues, however, have examined large cohorts of patients, including those comorbid with alcohol, cocaine, and/or cannabis abuse, treated with a lithium-divalproex combination over 6-month study periods. The researchers found that only 25% to 50% of patients stabilized, and that of those not exhibiting a response, the majority (75%) did not respond because of treatment-refractory depression in the context of RCBD.3
Although experts believe divalproex to be more effective than lithium in preventing episodes associated with RCBD, such a conclusion awaits confirmation with the near completion of a double-blind, 20-month maintenance trial sponsored by the National Institute of Mental Health (NIMH).
Carbamazepine’s role in combination therapy
Early reports by Post and colleagues in 1987 suggested that rapid cycling predicted positive response to carbamazepine, but later findings by Okuma in 1993 refuted this. Other collective open and controlled studies suggest that this anticonvulsant possesses moderate to marked efficacy in the manic phase, and poor to moderate efficacy in the depressed phase of RCBD. Again, combination therapy with lithium may offer greater efficacy. Of significance, carbamazepine treatment outcomes have not been prospectively evaluated in a homogeneous cohort of rapid cyclers.
The limitations of carbamazepine therapy are well known and available evidence also does not seem to support monotherapy with this agent as being useful in RCBD, especially in the treatment and prophylaxis of depressive or mixed phases of the disorder. Thus, further controlled studies are needed to examine the agent’s potential role and safety in combination therapies for RCBD.
Table 1
SPECTRUM OF ACUTE AND PROPHYLACTIC EFFICACY OF DIVALPROEX IN RAPID-CYCLING BIPOLAR DISORDER
| Spectrum of marked responses to divalproex in bipolar rapid cycling | ||
|---|---|---|
| Acute | Prophylactic | |
| Dysphoric hypomania/mania | 87% | 89% |
| Elated hypomania/mania | 64% | 77% |
| Depression (n = 101, mean follow-up 15 months) | 21% | 38% |
| Adapted from Calabrese and Delucchi. Am J Psychiatry. 1990;147:431-434 and Calabrese et al. J Clin Psychopharmacol. 1993;13:280-283. | ||
Lamotrigine: best hope for monotherapy
Lamotrigine monotherapy has been reported to be effective in some RCBD cases. The data suggest that it possesses both antidepressant and mood-stabilizing properties.10
An open, naturalistic study of 5 women with treatment-refractory rapid cycling by Fatemi et al11 demonstrated both mood-stabilizing and antidepressant effects from lamotrigine monotherapy or augmentation at a mean dose of 185 ±33.5 mg/d. In 14 clinical reports involving 207 patients with bipolar disorder, 66 of whom had rapid cycling, lamotrigine was observed to possess moderate to marked efficacy in depression and hypomania, but only moderate efficacy in mania.
An open, prospective study compared the efficacy of lamotrigine add-on or monotherapy in 41 rapid cyclers to 34 nonrapid cyclers across 48 weeks. Improvement from baseline to last visit was significant between both subgroups for depressive and hypomanic symptoms. Patients presenting with more severe manic symptoms did less well.13
In the first double-blind, placebo-controlled study of lamotrigine in RCBD,12 182 of 324 patients with rapid cycling responded to treatment with open-label lamotrigine and were then randomized to the study’s double-blind phase. Forty-one percent of lamotrigine-treated vs. 26% of placebo-treated patients were stable without relapse during 6 months of monotherapy. Patients with rapid-cycling bipolar II disorder consistently experienced more improvement than their bipolar I counterparts (Figure 1). The results of this only prospective, placebo-controlled, acute-treatment study of rapid-cycling bipolar patients to date indicate that lamotrigine monotherapy is useful for some patients with RCBD, particularly those with bipolar II.
Frye et al14 conducted a double-blind, placebo-controlled study of 23 patients with rapid cycling utilizing a crossover series of three 6-week monotherapy evaluations of lamotrigine, gabapentin, and placebo. Marked antidepressant response on lamotrigine was seen in 45% of the participants compared with 19% of patients on placebo and a similar response rate among those on gabapentin.
A study evaluating the safety and efficacy of 2 dosages of lamotrigine (50 mg/d or 200 mg/d) compared with placebo in the treatment of a major depressive episode in patients with bipolar I disorder over 7 weeks demonstrated significant antidepressant efficacy.15 These bipolar outpatients displayed clinical improvement as early as the third week of treatment, and switch rates for both dosages did not exceed that of placebo. Patients with RCBD, however, were excluded from this initial trial. Subsequent studies have demonstrated similar magnitudes of efficacy in patients with RCBD, primarily in the prevention of depressive episodes, including the 6-month RCBD maintenance study10 and 2 recently completed 18-month maintenance studies of patients with bipolar I disorder, either recently manic or recently depressed.
Lamotrigine thus may have a special role in RCBD treatment. Its most significant side effect in bipolar disorder is benign rash, which has occurred in 9.0% (108 of 1,198) of patients randomized to lamotrigine vs. 7.6% (80 of 1,056) of those randomized to placebo in pivotal multicenter, double-blind, placebo-controlled bipolar trials.
In mood disorder trials conducted to date, the rate of serious rash, defined as requiring both drug discontinuation and hospitalization, has been 0.06% (2 of 3,153) on lamotrigine and 0.09% (1 out of 1,053) on placebo. No cases of Steven’s Johnson syndrome or toxic epidermal necrolysis were observed.16 A low starting dose and gradual titration of lamotrigine (Table 2) appear to minimize the risk of serious rash.
Levothyroxine: possible add-on therapy
Levothyroxine should be considered as add-on therapy in patients with known hypothyroidism, borderline hypothyroidism, or otherwise treatment-refractory cases.
The strategy of thyroid supplementation is derived from Gjessing’s 1936 report of success in administering hypermetabolic doses of thyroid hormone to patients with periodic catatonia. Stancer and Persad17 initially reported the potential efficacy of this therapeutic maneuver in rapid cyclers; remissions were induced by hypermetabolic thyroid doses in 5 of 7 patients with treatment-refractory bipolar disorder. No data currently support any prophylactic efficacy of thyroid supplementation monotherapy for RCBD treatment.
Bauer and Whybrow18 suggest that thyroid supplementation with T4 added to mood stabilizers augments efficacy independent of pre-existing thyroid function. The potential side effects of long-term levothryoxine administration, namely osteoporosis and cardiac arrhythmias, limit the usefulness of thyroid augmentation in RCBD.
Atypical antipsychotics: perhaps in combination
Coadministering atypical antipsychotics in other mood stabilizers may help rapid cyclers with current or past psychotic symptoms during their mood episodes, but further study is clearly needed.
Atypical antipsychotic medications may have specific mood-stabilizing properties, particularly in the management of mixed and manic states. In the first prospective trial of clozapine monotherapy in bipolar disorder, Calabrese and colleagues in 1994 reported that rapid cycling did not appear to predict nonresponse to treatment.
In the first randomized, controlled trial involving clozapine in bipolar disorder, Suppes et al19 noted significant improvement for symptoms of mania, psychosis, and global improvement. Subjects with nonpsychotic bipolar disorder showed a degree of improvement similar to that seen in the entire clozapine-treated group. These results support a mood-stabilizing role for clozapine.
Preliminary studies of risperidone and olanzapine further suggest therapeutic utility for atypical antipsychotics.
Figure 1 LAMOTRIGINE VS. PLACEBO IN RAPID-CYCLING BIPOLAR DISORDER
Table 2
DOSAGE PLAN FOR LAMOTRIGINE IN MONOTHERAPY AND COMBINATION
| Treatment period | Type of therapy | Daily dosage |
|---|---|---|
| Weeks 1-2 | Monotherapy With divalproex With carbamazepine | 25 mg 12.5 mg 50 mg |
| Weeks 3-4 | Monotherapy With divalproex With carbamazepine | 50 mg 25 mg 100 mg |
| Week 5 | Monotherapy With divalproex With carbamazepine | 100 mg 50 mg 200 mg |
| Thereafter | Monotherapy With divalproex With carbamazepine | 200 mg 100 mg 400 mg |
Topiramate: in patients with weight problems
Evidence from 12 open studies of 223 patients with bipolar disorder suggest that this anticonvulsant may possess mood-stabilizing properties while sparing patients the weight gain commonly seen with other pharmacotherapies.
Two studies of topiramate as an add-on therapy come to mind. Marcotte in 1998 retrospectively studied 44 patients with RCBD, 23 (52%) of whom demonstrated moderate or marked improvement on a mean topiramate dosage of 200 mg/d.
Sachs also reported that symptoms for some patients with a treatment-refractory bipolar disorder could be improved when topiramate was added to their medication regimen. Available data encourage further controlled studies of topiramate add-on therapy for RCBD, especially in obese patients.
Gabapentin: contradictory reports
Preliminary data from 14 open-label studies of gabapentin in 302 bipolar patients reported a response rate of around 67% when used as an add-on therapy, usually in mania or mixed states. A moderate antimanic effect was observed among a total of 23 rapid cyclers in 9 of the studies.
The reports are contradictory, however, and available data do not firmly support the agent’s efficacy in RCBD treatment.
Other potential uses for gabapentin are being investigated, such as antidepressant augmentation, anxiety, and chronic pain. Thus, patients with RCBD and a comorbid illness may benefit from add-on gabapentin.
ECT: Some limited success
Electroconvulsive therapy (ECT) has been implicated less frequently than antidepressants in the induction of rapid cycling, and when this does occur it is usually in the context of combined ECT/antidepressant therapies.
Berma and Wolpert in 1987 reported a case of successful ECT treatment of rapid cycling in an adolescent who had been treated with trimipramine for depression. Vanelle et al in 1994 suggested that maintenance ECT works well in 22 treatment-resistant bipolar patients, including 4 with rapid cycling, over an 18-month treatment period.
Behavioral intervention: changing sleep routines
NIMH research of 15 rapid cyclers who were studied for 3 months looked at behavioral interventions and their effect on switching (Feldman-Naim 1997). This study suggested that patients were more likely to switch from depression into hypomania/mania during daytime hours and from mania/hypomania into depression during nighttime.
The use of light therapy or activity and exercise during depression and the use of induced sleep or exposure to darkness during mania/hypomania may be therapeutic. Wehr and colleagues supported this in a 1998 report of one patient studied over several years, comparing this rapid-cycling patient’s regular sleep routine with prolonged (10 to 14 hours per night) and enforced bed rest in the dark.7 The promotion of sleep by scheduling regular nighttime periods of enforced bed rest in the dark may help prevent mania and stabilize mood in rapid cyclers.
Other add-on possibilities
Haykal in 1990 reported bupropion to be an effective add-on treatment in 5 of 6 patients with refractory, rapid-cycling bipolar II disorder. Benefit has also been reported from clorgyline, clonidine, magnesium, primidone, and acetazolamide.
Calcium-channel blockers may also offer clinical utility, although supportive evidence is limited. Nimodipine was evaluated for efficacy in 30 patients with treatment-refractory affective illness by the NIMH and Passaglia et al in 1998. Patients who improved on this agent had ultradian rapid cycling, defined in the study as those with affective episodes lasting as short as a week.
A recommended treatment strategy
Based on the available data in bipolar I rapid cycling, we recommend initial treatment with divalproex followed by augmentation with lithium if hypomanic or manic episodes persist, lamotrigine if breakthrough episodes are predominantly depressive, and atypical antipsychotics if psychotic symptoms or true mixed states remain.
For patients presenting with bipolar II rapid cycling, we recommend starting with lamotrigine, then augmenting with divalproex or lithium for breakthrough episodes. Lamotrigine shows more promise because of its reportedly greater antidepressant properties and lack of cycle induction or switching, but offers only modest antimanic therapy.
Pending further investigations, current application of the data suggests that when treating patients with RCBD, conventional antidepressants should be avoided and, if first-line therapies are not effective, the clinician should consider moving to combination drug therapy with 2 or more agents.
Related resources
- Bauer MS, Calabrese JR, Dunner DL, et al. Multi-site data reanalysis: validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151:506-515.
- Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine in treatment refractory mania. Am J Psychiatry. 1996;153(6):759-764.
- Calabrese JR, Bowden CL, McElroy SL, et al. Spectrum of activity of lamotrigine in treatment refractory bipolar disorder. Am J Psychiatry. 1999;156(7):1019-1023.
- Sachs GS. Printz DJ. Kahn DA. Carpenter D. Docherty JP. The expert consensus guideline series: medication treatment of bipolar disorder 2000. Postgraduate Medicine. April 2000; Spec No:1-104.
Drug brand names
- Buproprion • Wellbutrin
- Carbamazepine • Tegretol
- Clonidine • Catapres
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levothyroxine • Synthroid, Levothroid, Levoxyl
- Olanzapine • Zyprexa
- Nimodipine • Nimotop
- Primidonem • Mysoline
- Topiramate • Topamax
1. Dunner DL, Fieve RR. Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry. 1974;20:229-233.
2. Calabrese JR, Delucchi GA. Spectrum of efficacy of valproate in 55 rapid-cycling manic depressives. Am J Psychiatry. 1990;147(4):431-434.
3. Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: Focus on depression as its hallmark. J Clin Psychiatry. 2001;62(Suppl 14):34-41.
4. Kukopulos A, Reginaldi D, Laddomada P, et al. Course of the manic depressive cycle and changes caused by treatment. Pharmacopsychiatry. 1980;13:156-167.
5. Maj M, et al. Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry. 1998;155:30-35.
6. Tondo L, Baldessarini RJ, et al. Lithium maintenance treatment of depression and mania in bipolar I and bipolar II disorders. Am J Psychiatry. 1998;155:638-645.
7. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145:179-84.
8. Calabrese JR, Woyshville MJ, Kimmel SE. Rapport DJ: Predictors of valproate response in bipolar rapid cycling. J Clin Psychopharmacology. 1993;13(4):280-283.
9. Sharma V, Persad E, et al. Treatment of rapid cycling bipolar disorder with combination therapy of valproate and lithium. Can J Psychiatry. 1993;38:137-139.
10. Calabrese JR, Suppes T, Bowden CL, et al. for the Lamictal 614 Study Group. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry. 2000;61(11):841-850.
11. Fatemi SH, Rapport DJ, Calabrese JR, et al. Lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry 1997;58:522-527.
12. Calabrese JR, Bowden CL, et al. for the Lamictal 602 Study Group. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60(2):79-88.
13. Bowden CL, Calabrese JR, McElroy SL, et al. Comparison of the efficacy of lamotrigine in rapid cycling and non-rapid cycling bipolar disorder. Biol Psychiatry. 1999;45(8):953-958.
14. Frye MA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607-614.
15. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999;60(2):79-88.
16. Calabrese JR, Bowden CL, DeVeaugh-Geiss J, et al. Lamotrigine demonstrates long term mood stabilization in recently manic patients. Annual meeting of the American Psychiatric Association, New Orleans, 2001.
17. Stancer HC, Persad E. Treatment of intractable rapid-cycling manic-depressive disorder with levothyroxine. Clinical observations. Arch Gen Psychiatry. 1982;39(3):311-312.
18. Bauer MS, Whybrow PC. Rapid cycling bipolar affective disorder. II. Treatment of refractory rapid cycling with high-dose levothyroxine: a preliminary study.. Arch Gen Psychiatry. 1990;47(5):435-40.
19. Suppes T, Webb A, Paul B, Carmody T, et al:. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156:1164-1169.
1. Dunner DL, Fieve RR. Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry. 1974;20:229-233.
2. Calabrese JR, Delucchi GA. Spectrum of efficacy of valproate in 55 rapid-cycling manic depressives. Am J Psychiatry. 1990;147(4):431-434.
3. Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: Focus on depression as its hallmark. J Clin Psychiatry. 2001;62(Suppl 14):34-41.
4. Kukopulos A, Reginaldi D, Laddomada P, et al. Course of the manic depressive cycle and changes caused by treatment. Pharmacopsychiatry. 1980;13:156-167.
5. Maj M, et al. Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry. 1998;155:30-35.
6. Tondo L, Baldessarini RJ, et al. Lithium maintenance treatment of depression and mania in bipolar I and bipolar II disorders. Am J Psychiatry. 1998;155:638-645.
7. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145:179-84.
8. Calabrese JR, Woyshville MJ, Kimmel SE. Rapport DJ: Predictors of valproate response in bipolar rapid cycling. J Clin Psychopharmacology. 1993;13(4):280-283.
9. Sharma V, Persad E, et al. Treatment of rapid cycling bipolar disorder with combination therapy of valproate and lithium. Can J Psychiatry. 1993;38:137-139.
10. Calabrese JR, Suppes T, Bowden CL, et al. for the Lamictal 614 Study Group. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry. 2000;61(11):841-850.
11. Fatemi SH, Rapport DJ, Calabrese JR, et al. Lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry 1997;58:522-527.
12. Calabrese JR, Bowden CL, et al. for the Lamictal 602 Study Group. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60(2):79-88.
13. Bowden CL, Calabrese JR, McElroy SL, et al. Comparison of the efficacy of lamotrigine in rapid cycling and non-rapid cycling bipolar disorder. Biol Psychiatry. 1999;45(8):953-958.
14. Frye MA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607-614.
15. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999;60(2):79-88.
16. Calabrese JR, Bowden CL, DeVeaugh-Geiss J, et al. Lamotrigine demonstrates long term mood stabilization in recently manic patients. Annual meeting of the American Psychiatric Association, New Orleans, 2001.
17. Stancer HC, Persad E. Treatment of intractable rapid-cycling manic-depressive disorder with levothyroxine. Clinical observations. Arch Gen Psychiatry. 1982;39(3):311-312.
18. Bauer MS, Whybrow PC. Rapid cycling bipolar affective disorder. II. Treatment of refractory rapid cycling with high-dose levothyroxine: a preliminary study.. Arch Gen Psychiatry. 1990;47(5):435-40.
19. Suppes T, Webb A, Paul B, Carmody T, et al:. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156:1164-1169.
A diagnosis that’s yours to make: Accidental hypothermia in the elderly
You may well be the first specialist to evaluate an elderly patient with accidental hypothermia, a severe medical illness, because patients with this condition may present initially with cognitive impairment and disruptive behavior. This problem is particularly evident when evaluating elderly patients. Accidental hypothermia commonly mimics major mental illness, may be induced or exacerbated by psychotropic medications, is commonly fatal, and may remain unrecognized without a high index of suspicion.
Hypothermia is defined as a fall in body temperature below 95°F or 35°C (Box 1). Clinical mercury thermometers commonly range between 96°F and 106°F. Thus, the family member or clinician may not suspect hypothermia after the initial temperature measurement.
The diagnosis of accidental hypothermia is straightforward if there is a history of environmental exposure, but such evidence is often lacking in urban settings and among the elderly. Also, particularly in the elderly, hypothermia may occur at room temperature, secondary to diseases that strike the hypothalamic thermoregulatory center.
Subjects with core body temperatures dropping from 95°F to 90°F develop amnesia, dysarthria, confusion, and disruptive behavior.1 Further cooling as the body temperature falls to 82.4°F yields stupor, paradoxical undressing, and hallucinations. These characteristics are illustrated in the accompanying vignette of Ms. B.
![]()
The body’s thermoregulatory center located in the hypothalamus normally maintains core body temperature between 97.5°F (36.5°C) and 99.5°F (37.5°C). When body temperature declines, heat production increases by shivering, and heat loss is reduced by decreasing cutaneous blood flow.
Accidental hypothermia is defined as an unintentional fall in body temperature below 95°F (35°C). The coordinated systems responsible for thermoregulation start to fail. Heat loss through radiation, conduction, convection, respiration, and evaporation occurs because compensatory physiologic mechanisms are both limited and impaired.
Schizophrenia and the hypothalamus
Over the course of 6 months before her death, Ms. B. showed evidence of both thermoregulatory dysfunction and autonomic nervous system instability. We do not know if these hypothalamic problems were separate from, or intrinsic parts of, her schizophrenia.
The hypothalamus regulates autonomic, endocrine, and visceral function. Hypothalamic dysfunction may be an intrinsic part of schizophrenia. Such dysfunction occurs most commonly in the periventricular and supraoptic nuclei of the hypothalamus.2 These areas are adjacent to hypothalamic areas regulating body temperature.3
Lesions in anterior parts of the hypothalamus (temperaturesensitive neurons in the preoptic nuclei—located close to nuclei controlling thirst and osmotic regulation) may induce hyperthermia, impairing heat-dissipating mechanisms including vasodilatation and sweating. Lesions in posterior parts of the hypothalamus may impair heat conservation and heat production mechanisms and induce hypothermia.4
Associated medical problems
Independent of drug treatment, metabolic and cardiovascular problems occur more frequently in patients suffering from schizophrenia than they do in the general population.5 Ms. B. developed hypertension, diabetes mellitus, dyslipidemia, and coronary artery disease.
Diabetes mellitus in particular is a risk factor for hypothermia and may be found in more than 10 percent of elderly patients who suffered thermoregulatory failure before dying.6 Diabetes may impair autonomic system vasomotor stability and the body’s ability to vasoconstrict to preserve body heat.
Dementia and hypothermia
Cognitive impairment is a core feature of schizophrenia,7 and dementia is a common outcome among elderly patients suffering with the disorder.8 We don’t know whether Ms. B.’s progressive cognitive deterioration derived from dementia associated with schizophrenia or from a separate process such as Alzheimer’s disease.
Alzheimer’s disease may limit behavioral responses to cooling or even recognition that the body temperature is dropping.9 This disease is associated with weight loss (and attendant loss of body fat that acts, in part, as insulation), hypothalamic pathologic changes, and decreased serotonin activity in the hypothalamus. The processes leading to Ms. B.’s progressive cognitive impairment most likely contributed to hypothalamic dysregulation and subsequent accidental hypothermia.
Ms. B.’s repeated disrobing during her stay at the adult care facility was ascribed to dementia. Serial body temperature measurements were not available, so we do not know the extent to which the disrobing may have been paradoxical—that is, undressing when cold rather than dressing more warmly. Paradoxical undressing is found during moderate (82.4°F to 90°F) hypothermia.1
Medications and hypothermia
Normally, mild hypothermia induces vasoconstriction and initial increases in heart rate and cardiac output. (The latter increase is principally driven by the accelerated heart rate rather than increased stroke volume.) These changes tend to protect the patient from further lowering of body temperature. But Ms. B.’s medications included the vasodilator, isosorbide dinitrate; the beta-blocker, metoprolol; and the angiotensin-converting enzyme (ACE) inhibitor, lisinopril. All these agents impaired her capacities to vasoconstrict and to increase cardiac output, thereby reducing her ability to conserve body heat.
Over the course of several months, Ms. B., a woman in her mid-70s, manifested features of accidental hypothermia, which went undiagnosed amid a backdrop of a long history of schizophrenia and a more recent history of dementia.
In 1996, almost 5 years before developing accidental hypothermia, Ms. B. sought care for paranoia, nervousness, and dysphoria. The records showed a history of cigarette abuse, diet-controlled type 2 diabetes mellitus of more than 20 years duration, and kidney surgery. She was cognitively intact and had received doses of up to 3 mg/bid of risperidone and desipramine. A few months later, temazepam was added for insomnia. Still later, following the death of her husband, lorazepam was added.
Until late 1999, Ms. B. remained psychiatrically stable. Then she became more anxious and her lorazepam dosage was increased. But in June 2000, she was admitted to a local hospital following a month of confusion, weakness, and slurred speech. The precipitating event was a fall. A head CT scan showed brain atrophy and white-matter disease. Extensive condylomata led to a partial vulvectomy. Her lowest recorded oral temperature was 95.6°F.
Ms. B. returned to a residential home briefly but was readmitted when she was found unresponsive; hypotension and bradycardia were detected. Cardiac catheterization showed normal left ventricular function and severe 3-vessel coronary artery disease with a 50% obstruction of the left main coronary artery. This procedure was complicated by severe agitation, confusion, and a large post-catheterization hematoma requiring blood transfusions.
Following discussions with the cardiac surgeons, the family considered Ms. B. too ill to undergo coronary artery bypass surgery. The lowest recorded oral temperature was 94°F.
Ms. B. returned to the residential home—but not for long. In August 2000, she was again taken to the hospital. She was confused, threatening to harm herself with a knife, and eating “hair grease.” Her medications now included temazepam, lorazepam, risperidone, paroxetine, and desipramine—plus aspirin, verapamil, lisinopril, metoprolol, amlodipine, and isosorbide dinitrate for coronary heart disease and hypertension. The admission database included a temperature of 96.2°F. She received a Global Assessment of Functioning score of 20 contrasted with a high score of 70 the preceding year.
Ms. B.’s hospital stay lasted 2 months. Confusion and disorientation persisted one month after admission while still undergoing psychiatric care. Midway during her hospitalization, she underwent a cholecystectomy.
When she was discharged to an assisted living facility, Ms. B. required assistance with self-care and restraint with a posey vest. Dementia was considered the major psychiatric problem. Medications now included amlodipine, aspirin, famotidine, isosorbide dinitrate, lisinopril, metoprolol, oxybutynin, metoclopramide, lorazepam 0.5 mg 3 times a day, and risperidone 1 mg twice daily.
Two weeks later, Ms. B. was still confused and disoriented. Risperidone was increased to 1 mg 3 times daily and lorazepam was increased to 0.5 mg 4 times daily. A week later, the nursing staff noted further deterioration. She would wander, on occasion even into the street. Subsequently, she began disrobing for no apparent reason, 3 to 4 times a week.
In early December 2000, nurses called an ambulance because Ms. B. was “lethargic, unresponsive to name call.” The ambulance crew noted she was “foaming at the mouth,” lying "naked" in bed, and very “cold” to the touch. At the hospital, hypothermia was documented with a body temperature of 84°F rectally. (Of note, the patient’s roommate manifested a normal body temperature, was cognitively intact, and did not complain that their room was cold.) Medications at the time of admission included lisinopril 10 mg/d, aspirin 325 mg/d, amlodipine 10 mg/d, oxybutynin 5 mg twice daily, lorazepam 0.5 mg 3 times daily, metoprolol 50 mg twice daily, famotidine 20 mg twice daily, isosorbide dinitrate 10 mg 3 times daily, metoclopramide 10 mg 4 times daily, and risperidone 1 mg twice daily.
Initially, Ms. B. manifested bradycardia requiring temporary pacing, and hemoconcentration without explanation for the low body temperature. Despite return to normal body temperature within 24 hours, vasomotor instability, body temperatures ranging between 95.9°F and 100.1°F, encephalopathy, and general organ failure persisted. Ms. B. was pronounced dead on the 18th hospital day. An autopsy was not performed.
Amlodipine, a calcium channel blocker, enhances vasodilatation and may also have limited Ms. B.’s capacity to vasoconstrict. Calcium channel blockers may have variable effects on intraoperative core body temperature in humans.10
Phenothiazines, particularly the low-potency agents in this class, are the antipsychotic drugs most commonly associated with drug-induced hypothermia.6,9,11 Phenothiazines seem to have a direct effect on hypothalamic thermoregulation. About a month before developing moderate hypothermia, Ms. B. received an increase in her risperidone dosage from 1 mg twice daily to 1 mg 3 times daily because of agitation. The package insert for risperidone states:
- Medical conditions
- Hypoglycemia
- Hypothyroidism
- Adrenal insufficiency
- Hypopituitarism
- Stroke
- Malnutrition
- Shock
- Sepsis
- Hepatic or renal failure
- Burns
- Exfoliative dermatitis
- Immobility or debilitation
- Hypothalamic disorders
- Parkinson’s disease
- Spinal cord injury
- Diabetic ketoacidosis
- Psychiatric conditions
- Alzheimer’s disease
- Schizophrenia
- Medications
- Ethanol
- Phenothiazines
- Barbiturates
- Anesthetics
- Neuromuscular blockers
*Adapted from Danzl DF. Hypothermia. Harrison’s 15th Ed., Principles of Internal Medicine, New York: McGraw-Hill, 2001, p. 107.
“Disruption of body temperature regulation has been attributed to antipsychotic agents. Both hyperthermia and hypothermia have been reported in association with Risperdal use. Caution is advised when prescribing for patients who will be exposed to temperature extremes.”12
Lorazepam very rarely may be associated with hypothermia. In animal studies, zolpidem, diazepam, and lorazepam produced comparable dose-dependent hypothermia.13 Ms. B. had her dosage of lorazepam increased from 0.5 mg 3 times daily to 0.5 mg 4 times daily because of increasing agitation and wandering. About 10 days before developing moderate hypothermia, she became more lethargic and the nursing staff was directed to withhold lorazepam if she appeared unduly sedated. At this point, Ms. B. may have had a drug-induced delirium superimposed upon dementia or a toxic-metabolic encephalopathy superimposed upon dementia. In her case, we do not know if druginduced or metabolic-induced changes (or a combination of the two) best explained her change in mental status.
Once accidental hypothermia sets in
During the days before Ms. B. developed moderate hypothermia, the temperature outside the assisted living facility ranged from 25°F to 40°F. When she was found by the nursing staff to be unusually unresponsive, she was wearing her nightgown under bed sheets. Even if her room temperature had been at 70°F, an almost 30°F gradient would exist between that and normal body temperature (98.6°F). In complete thermodysregulation, her body temperature of 84°F could have been reached within 5 to 8 hours. The colder the room, the faster her body would cool in the presence of thermodysregulation.
Although sepsis and adverse environmental exposure are the most common conditions leading to hypothermia, up to onethird of cases of accidental hypothermia in the elderly occur during the warmer months, with one-half of these cases found in the hospital.6 In cases of accidental hypothermia occurring during the winter, one-half occur in a normal room temperature setting.9
In a United Kingdom study, about 25% of elderly patients with hypothermia died.9 Still, the severity of underlying disease is more predictive of mortality than is the degree of hypothermia.14 Ms. B.’s fatal clinical course was that of multiple organ failure complicated by hypothermia. No mention was made in the hospital records of her vulnerability to hypothermia. This vulnerability placed significant burden on the assisted living facility staff.
Hypothermia should be considered in the differential diagnosis of confusion and disruptive behavior in the elderly patient. In Ms. B.’s case, an early diagnosis of accidental hypothermia by a psychiatrist could have made a difference.
Related resources Oriented to mental health issues
- Kramer MR, Vandijk J, Rosin AJ. Mortality in elderly patients with thermoregulatory failure. Arch Intern Med. 1989;149:1521-1523.
- Murphy PJ. Hypothermia. In Oxford Textbook of Geriatric Medicine. Evans JG, Williams TF, Beattie BL, Michel J-P, Wilcock GK, eds. New York: Oxford University Press, 2000:857-863.
- Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992; 10:311-327.
- Fischbeck KH, Simon RP. Neurological manifestations of accidental hypothermia. Ann Neurol. 1981; 10:384-387.
Drug brand names
- Amlodipine • Norvasc
- Famotidine • Pepcid
- Isosorbide dinitrate • Isordil
- Lisinopril • Prinivil
- Metoclopramide • Reglan
- Metoprolol • Lopressor
- Oxybutynin • Ditropan
- Paroxetine • Paxil
- Risperidone • Risperdal
- Zolpidem • Ambien
Disclosure
The author reports that he is on the speakers’ bureau of Janssen Pharmaceutica, Eli Lilly and Co., Pfizer Inc., Wyeth-Ayerst Pharmaceuticals, Forest Pharmaceuticals, and GlaxoSmithKline.
1. Danzl DF, Pozos RS. Accidental hypothermia. N Engl J Med. 1994;331:1756-1760.
2. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs. 1997;7:121-138.
3. Grossman SP. Physiology of thirst. In: Schnur DB, Kirch DG, eds. Water balance in schizophrenia. Washington, DC: American Psychiatric Press, Inc., 1996;53-87.
4. Guyton AC, Hall JE. Behavioral and Motivational Mechanisms of the Brain—The Limbic System and the Hypothalamus. Textbook of Medical Physiology. Philadelphia: W.B. Saunders, 1996;749-760.
5. Fontaine KR, Heo M, Harrigan EP, Shear CL, et al. Estimating the consequences of antipsychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101:277-288.
6. Kramer MR, Vandijk J, Rosin AJ. Mortality in elderly patients with thermoregulatory failure. Arch Intern Med. 1989;149:1521-1523.
7. Mohamed S, Paulsen JS, O’Leary D, Arndt S, Andreasen N. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch Gen Psychiatry. 1999;56:749-754.
8. Vieweg V, Tucker R, Talbot PC, Blair CE, Lewis R. Mini-Mental State Examination scores of subjects with nondementing diagnoses on admission to a geropsychiatric hospital. Med Psychiatry. 2001;4:19-22.
9. Murphy PJ. Hypothermia. In: Evans JG, Williams TF, Beattie BL, Michel J-P, Wilcock GK, eds. Oxford Textbook of Geriatric Medicine. New York: Oxford University Press, 2000;857-863.
10. Vassilieff N, Rosencher N, Sessler DL, Conseiller C, Lienhart A. Nifedipine and intraoperative core body temperature in humans. Anesthesiology. 1994;80:123-128.
11. Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992;10:311-327.
12. Physicians’ Desk Reference. 54th ed. Montvale, NJ: Medical Economics Company, Inc., 2000.
13. Elliott EE, White JM. The acute effects of zolpidem compared to diazepam and lorazepam using radiotelemetry. Neuropharmacology. 2001;40:717-721.
14. Fischbeck KH, Simon RP. Neurological manifestations of accidental hypothermia. Ann Neurol. 1981;10:384-387.
You may well be the first specialist to evaluate an elderly patient with accidental hypothermia, a severe medical illness, because patients with this condition may present initially with cognitive impairment and disruptive behavior. This problem is particularly evident when evaluating elderly patients. Accidental hypothermia commonly mimics major mental illness, may be induced or exacerbated by psychotropic medications, is commonly fatal, and may remain unrecognized without a high index of suspicion.
Hypothermia is defined as a fall in body temperature below 95°F or 35°C (Box 1). Clinical mercury thermometers commonly range between 96°F and 106°F. Thus, the family member or clinician may not suspect hypothermia after the initial temperature measurement.
The diagnosis of accidental hypothermia is straightforward if there is a history of environmental exposure, but such evidence is often lacking in urban settings and among the elderly. Also, particularly in the elderly, hypothermia may occur at room temperature, secondary to diseases that strike the hypothalamic thermoregulatory center.
Subjects with core body temperatures dropping from 95°F to 90°F develop amnesia, dysarthria, confusion, and disruptive behavior.1 Further cooling as the body temperature falls to 82.4°F yields stupor, paradoxical undressing, and hallucinations. These characteristics are illustrated in the accompanying vignette of Ms. B.
![]()
The body’s thermoregulatory center located in the hypothalamus normally maintains core body temperature between 97.5°F (36.5°C) and 99.5°F (37.5°C). When body temperature declines, heat production increases by shivering, and heat loss is reduced by decreasing cutaneous blood flow.
Accidental hypothermia is defined as an unintentional fall in body temperature below 95°F (35°C). The coordinated systems responsible for thermoregulation start to fail. Heat loss through radiation, conduction, convection, respiration, and evaporation occurs because compensatory physiologic mechanisms are both limited and impaired.
Schizophrenia and the hypothalamus
Over the course of 6 months before her death, Ms. B. showed evidence of both thermoregulatory dysfunction and autonomic nervous system instability. We do not know if these hypothalamic problems were separate from, or intrinsic parts of, her schizophrenia.
The hypothalamus regulates autonomic, endocrine, and visceral function. Hypothalamic dysfunction may be an intrinsic part of schizophrenia. Such dysfunction occurs most commonly in the periventricular and supraoptic nuclei of the hypothalamus.2 These areas are adjacent to hypothalamic areas regulating body temperature.3
Lesions in anterior parts of the hypothalamus (temperaturesensitive neurons in the preoptic nuclei—located close to nuclei controlling thirst and osmotic regulation) may induce hyperthermia, impairing heat-dissipating mechanisms including vasodilatation and sweating. Lesions in posterior parts of the hypothalamus may impair heat conservation and heat production mechanisms and induce hypothermia.4
Associated medical problems
Independent of drug treatment, metabolic and cardiovascular problems occur more frequently in patients suffering from schizophrenia than they do in the general population.5 Ms. B. developed hypertension, diabetes mellitus, dyslipidemia, and coronary artery disease.
Diabetes mellitus in particular is a risk factor for hypothermia and may be found in more than 10 percent of elderly patients who suffered thermoregulatory failure before dying.6 Diabetes may impair autonomic system vasomotor stability and the body’s ability to vasoconstrict to preserve body heat.
Dementia and hypothermia
Cognitive impairment is a core feature of schizophrenia,7 and dementia is a common outcome among elderly patients suffering with the disorder.8 We don’t know whether Ms. B.’s progressive cognitive deterioration derived from dementia associated with schizophrenia or from a separate process such as Alzheimer’s disease.
Alzheimer’s disease may limit behavioral responses to cooling or even recognition that the body temperature is dropping.9 This disease is associated with weight loss (and attendant loss of body fat that acts, in part, as insulation), hypothalamic pathologic changes, and decreased serotonin activity in the hypothalamus. The processes leading to Ms. B.’s progressive cognitive impairment most likely contributed to hypothalamic dysregulation and subsequent accidental hypothermia.
Ms. B.’s repeated disrobing during her stay at the adult care facility was ascribed to dementia. Serial body temperature measurements were not available, so we do not know the extent to which the disrobing may have been paradoxical—that is, undressing when cold rather than dressing more warmly. Paradoxical undressing is found during moderate (82.4°F to 90°F) hypothermia.1
Medications and hypothermia
Normally, mild hypothermia induces vasoconstriction and initial increases in heart rate and cardiac output. (The latter increase is principally driven by the accelerated heart rate rather than increased stroke volume.) These changes tend to protect the patient from further lowering of body temperature. But Ms. B.’s medications included the vasodilator, isosorbide dinitrate; the beta-blocker, metoprolol; and the angiotensin-converting enzyme (ACE) inhibitor, lisinopril. All these agents impaired her capacities to vasoconstrict and to increase cardiac output, thereby reducing her ability to conserve body heat.
Over the course of several months, Ms. B., a woman in her mid-70s, manifested features of accidental hypothermia, which went undiagnosed amid a backdrop of a long history of schizophrenia and a more recent history of dementia.
In 1996, almost 5 years before developing accidental hypothermia, Ms. B. sought care for paranoia, nervousness, and dysphoria. The records showed a history of cigarette abuse, diet-controlled type 2 diabetes mellitus of more than 20 years duration, and kidney surgery. She was cognitively intact and had received doses of up to 3 mg/bid of risperidone and desipramine. A few months later, temazepam was added for insomnia. Still later, following the death of her husband, lorazepam was added.
Until late 1999, Ms. B. remained psychiatrically stable. Then she became more anxious and her lorazepam dosage was increased. But in June 2000, she was admitted to a local hospital following a month of confusion, weakness, and slurred speech. The precipitating event was a fall. A head CT scan showed brain atrophy and white-matter disease. Extensive condylomata led to a partial vulvectomy. Her lowest recorded oral temperature was 95.6°F.
Ms. B. returned to a residential home briefly but was readmitted when she was found unresponsive; hypotension and bradycardia were detected. Cardiac catheterization showed normal left ventricular function and severe 3-vessel coronary artery disease with a 50% obstruction of the left main coronary artery. This procedure was complicated by severe agitation, confusion, and a large post-catheterization hematoma requiring blood transfusions.
Following discussions with the cardiac surgeons, the family considered Ms. B. too ill to undergo coronary artery bypass surgery. The lowest recorded oral temperature was 94°F.
Ms. B. returned to the residential home—but not for long. In August 2000, she was again taken to the hospital. She was confused, threatening to harm herself with a knife, and eating “hair grease.” Her medications now included temazepam, lorazepam, risperidone, paroxetine, and desipramine—plus aspirin, verapamil, lisinopril, metoprolol, amlodipine, and isosorbide dinitrate for coronary heart disease and hypertension. The admission database included a temperature of 96.2°F. She received a Global Assessment of Functioning score of 20 contrasted with a high score of 70 the preceding year.
Ms. B.’s hospital stay lasted 2 months. Confusion and disorientation persisted one month after admission while still undergoing psychiatric care. Midway during her hospitalization, she underwent a cholecystectomy.
When she was discharged to an assisted living facility, Ms. B. required assistance with self-care and restraint with a posey vest. Dementia was considered the major psychiatric problem. Medications now included amlodipine, aspirin, famotidine, isosorbide dinitrate, lisinopril, metoprolol, oxybutynin, metoclopramide, lorazepam 0.5 mg 3 times a day, and risperidone 1 mg twice daily.
Two weeks later, Ms. B. was still confused and disoriented. Risperidone was increased to 1 mg 3 times daily and lorazepam was increased to 0.5 mg 4 times daily. A week later, the nursing staff noted further deterioration. She would wander, on occasion even into the street. Subsequently, she began disrobing for no apparent reason, 3 to 4 times a week.
In early December 2000, nurses called an ambulance because Ms. B. was “lethargic, unresponsive to name call.” The ambulance crew noted she was “foaming at the mouth,” lying "naked" in bed, and very “cold” to the touch. At the hospital, hypothermia was documented with a body temperature of 84°F rectally. (Of note, the patient’s roommate manifested a normal body temperature, was cognitively intact, and did not complain that their room was cold.) Medications at the time of admission included lisinopril 10 mg/d, aspirin 325 mg/d, amlodipine 10 mg/d, oxybutynin 5 mg twice daily, lorazepam 0.5 mg 3 times daily, metoprolol 50 mg twice daily, famotidine 20 mg twice daily, isosorbide dinitrate 10 mg 3 times daily, metoclopramide 10 mg 4 times daily, and risperidone 1 mg twice daily.
Initially, Ms. B. manifested bradycardia requiring temporary pacing, and hemoconcentration without explanation for the low body temperature. Despite return to normal body temperature within 24 hours, vasomotor instability, body temperatures ranging between 95.9°F and 100.1°F, encephalopathy, and general organ failure persisted. Ms. B. was pronounced dead on the 18th hospital day. An autopsy was not performed.
Amlodipine, a calcium channel blocker, enhances vasodilatation and may also have limited Ms. B.’s capacity to vasoconstrict. Calcium channel blockers may have variable effects on intraoperative core body temperature in humans.10
Phenothiazines, particularly the low-potency agents in this class, are the antipsychotic drugs most commonly associated with drug-induced hypothermia.6,9,11 Phenothiazines seem to have a direct effect on hypothalamic thermoregulation. About a month before developing moderate hypothermia, Ms. B. received an increase in her risperidone dosage from 1 mg twice daily to 1 mg 3 times daily because of agitation. The package insert for risperidone states:
- Medical conditions
- Hypoglycemia
- Hypothyroidism
- Adrenal insufficiency
- Hypopituitarism
- Stroke
- Malnutrition
- Shock
- Sepsis
- Hepatic or renal failure
- Burns
- Exfoliative dermatitis
- Immobility or debilitation
- Hypothalamic disorders
- Parkinson’s disease
- Spinal cord injury
- Diabetic ketoacidosis
- Psychiatric conditions
- Alzheimer’s disease
- Schizophrenia
- Medications
- Ethanol
- Phenothiazines
- Barbiturates
- Anesthetics
- Neuromuscular blockers
*Adapted from Danzl DF. Hypothermia. Harrison’s 15th Ed., Principles of Internal Medicine, New York: McGraw-Hill, 2001, p. 107.
“Disruption of body temperature regulation has been attributed to antipsychotic agents. Both hyperthermia and hypothermia have been reported in association with Risperdal use. Caution is advised when prescribing for patients who will be exposed to temperature extremes.”12
Lorazepam very rarely may be associated with hypothermia. In animal studies, zolpidem, diazepam, and lorazepam produced comparable dose-dependent hypothermia.13 Ms. B. had her dosage of lorazepam increased from 0.5 mg 3 times daily to 0.5 mg 4 times daily because of increasing agitation and wandering. About 10 days before developing moderate hypothermia, she became more lethargic and the nursing staff was directed to withhold lorazepam if she appeared unduly sedated. At this point, Ms. B. may have had a drug-induced delirium superimposed upon dementia or a toxic-metabolic encephalopathy superimposed upon dementia. In her case, we do not know if druginduced or metabolic-induced changes (or a combination of the two) best explained her change in mental status.
Once accidental hypothermia sets in
During the days before Ms. B. developed moderate hypothermia, the temperature outside the assisted living facility ranged from 25°F to 40°F. When she was found by the nursing staff to be unusually unresponsive, she was wearing her nightgown under bed sheets. Even if her room temperature had been at 70°F, an almost 30°F gradient would exist between that and normal body temperature (98.6°F). In complete thermodysregulation, her body temperature of 84°F could have been reached within 5 to 8 hours. The colder the room, the faster her body would cool in the presence of thermodysregulation.
Although sepsis and adverse environmental exposure are the most common conditions leading to hypothermia, up to onethird of cases of accidental hypothermia in the elderly occur during the warmer months, with one-half of these cases found in the hospital.6 In cases of accidental hypothermia occurring during the winter, one-half occur in a normal room temperature setting.9
In a United Kingdom study, about 25% of elderly patients with hypothermia died.9 Still, the severity of underlying disease is more predictive of mortality than is the degree of hypothermia.14 Ms. B.’s fatal clinical course was that of multiple organ failure complicated by hypothermia. No mention was made in the hospital records of her vulnerability to hypothermia. This vulnerability placed significant burden on the assisted living facility staff.
Hypothermia should be considered in the differential diagnosis of confusion and disruptive behavior in the elderly patient. In Ms. B.’s case, an early diagnosis of accidental hypothermia by a psychiatrist could have made a difference.
Related resources Oriented to mental health issues
- Kramer MR, Vandijk J, Rosin AJ. Mortality in elderly patients with thermoregulatory failure. Arch Intern Med. 1989;149:1521-1523.
- Murphy PJ. Hypothermia. In Oxford Textbook of Geriatric Medicine. Evans JG, Williams TF, Beattie BL, Michel J-P, Wilcock GK, eds. New York: Oxford University Press, 2000:857-863.
- Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992; 10:311-327.
- Fischbeck KH, Simon RP. Neurological manifestations of accidental hypothermia. Ann Neurol. 1981; 10:384-387.
Drug brand names
- Amlodipine • Norvasc
- Famotidine • Pepcid
- Isosorbide dinitrate • Isordil
- Lisinopril • Prinivil
- Metoclopramide • Reglan
- Metoprolol • Lopressor
- Oxybutynin • Ditropan
- Paroxetine • Paxil
- Risperidone • Risperdal
- Zolpidem • Ambien
Disclosure
The author reports that he is on the speakers’ bureau of Janssen Pharmaceutica, Eli Lilly and Co., Pfizer Inc., Wyeth-Ayerst Pharmaceuticals, Forest Pharmaceuticals, and GlaxoSmithKline.
You may well be the first specialist to evaluate an elderly patient with accidental hypothermia, a severe medical illness, because patients with this condition may present initially with cognitive impairment and disruptive behavior. This problem is particularly evident when evaluating elderly patients. Accidental hypothermia commonly mimics major mental illness, may be induced or exacerbated by psychotropic medications, is commonly fatal, and may remain unrecognized without a high index of suspicion.
Hypothermia is defined as a fall in body temperature below 95°F or 35°C (Box 1). Clinical mercury thermometers commonly range between 96°F and 106°F. Thus, the family member or clinician may not suspect hypothermia after the initial temperature measurement.
The diagnosis of accidental hypothermia is straightforward if there is a history of environmental exposure, but such evidence is often lacking in urban settings and among the elderly. Also, particularly in the elderly, hypothermia may occur at room temperature, secondary to diseases that strike the hypothalamic thermoregulatory center.
Subjects with core body temperatures dropping from 95°F to 90°F develop amnesia, dysarthria, confusion, and disruptive behavior.1 Further cooling as the body temperature falls to 82.4°F yields stupor, paradoxical undressing, and hallucinations. These characteristics are illustrated in the accompanying vignette of Ms. B.
![]()
The body’s thermoregulatory center located in the hypothalamus normally maintains core body temperature between 97.5°F (36.5°C) and 99.5°F (37.5°C). When body temperature declines, heat production increases by shivering, and heat loss is reduced by decreasing cutaneous blood flow.
Accidental hypothermia is defined as an unintentional fall in body temperature below 95°F (35°C). The coordinated systems responsible for thermoregulation start to fail. Heat loss through radiation, conduction, convection, respiration, and evaporation occurs because compensatory physiologic mechanisms are both limited and impaired.
Schizophrenia and the hypothalamus
Over the course of 6 months before her death, Ms. B. showed evidence of both thermoregulatory dysfunction and autonomic nervous system instability. We do not know if these hypothalamic problems were separate from, or intrinsic parts of, her schizophrenia.
The hypothalamus regulates autonomic, endocrine, and visceral function. Hypothalamic dysfunction may be an intrinsic part of schizophrenia. Such dysfunction occurs most commonly in the periventricular and supraoptic nuclei of the hypothalamus.2 These areas are adjacent to hypothalamic areas regulating body temperature.3
Lesions in anterior parts of the hypothalamus (temperaturesensitive neurons in the preoptic nuclei—located close to nuclei controlling thirst and osmotic regulation) may induce hyperthermia, impairing heat-dissipating mechanisms including vasodilatation and sweating. Lesions in posterior parts of the hypothalamus may impair heat conservation and heat production mechanisms and induce hypothermia.4
Associated medical problems
Independent of drug treatment, metabolic and cardiovascular problems occur more frequently in patients suffering from schizophrenia than they do in the general population.5 Ms. B. developed hypertension, diabetes mellitus, dyslipidemia, and coronary artery disease.
Diabetes mellitus in particular is a risk factor for hypothermia and may be found in more than 10 percent of elderly patients who suffered thermoregulatory failure before dying.6 Diabetes may impair autonomic system vasomotor stability and the body’s ability to vasoconstrict to preserve body heat.
Dementia and hypothermia
Cognitive impairment is a core feature of schizophrenia,7 and dementia is a common outcome among elderly patients suffering with the disorder.8 We don’t know whether Ms. B.’s progressive cognitive deterioration derived from dementia associated with schizophrenia or from a separate process such as Alzheimer’s disease.
Alzheimer’s disease may limit behavioral responses to cooling or even recognition that the body temperature is dropping.9 This disease is associated with weight loss (and attendant loss of body fat that acts, in part, as insulation), hypothalamic pathologic changes, and decreased serotonin activity in the hypothalamus. The processes leading to Ms. B.’s progressive cognitive impairment most likely contributed to hypothalamic dysregulation and subsequent accidental hypothermia.
Ms. B.’s repeated disrobing during her stay at the adult care facility was ascribed to dementia. Serial body temperature measurements were not available, so we do not know the extent to which the disrobing may have been paradoxical—that is, undressing when cold rather than dressing more warmly. Paradoxical undressing is found during moderate (82.4°F to 90°F) hypothermia.1
Medications and hypothermia
Normally, mild hypothermia induces vasoconstriction and initial increases in heart rate and cardiac output. (The latter increase is principally driven by the accelerated heart rate rather than increased stroke volume.) These changes tend to protect the patient from further lowering of body temperature. But Ms. B.’s medications included the vasodilator, isosorbide dinitrate; the beta-blocker, metoprolol; and the angiotensin-converting enzyme (ACE) inhibitor, lisinopril. All these agents impaired her capacities to vasoconstrict and to increase cardiac output, thereby reducing her ability to conserve body heat.
Over the course of several months, Ms. B., a woman in her mid-70s, manifested features of accidental hypothermia, which went undiagnosed amid a backdrop of a long history of schizophrenia and a more recent history of dementia.
In 1996, almost 5 years before developing accidental hypothermia, Ms. B. sought care for paranoia, nervousness, and dysphoria. The records showed a history of cigarette abuse, diet-controlled type 2 diabetes mellitus of more than 20 years duration, and kidney surgery. She was cognitively intact and had received doses of up to 3 mg/bid of risperidone and desipramine. A few months later, temazepam was added for insomnia. Still later, following the death of her husband, lorazepam was added.
Until late 1999, Ms. B. remained psychiatrically stable. Then she became more anxious and her lorazepam dosage was increased. But in June 2000, she was admitted to a local hospital following a month of confusion, weakness, and slurred speech. The precipitating event was a fall. A head CT scan showed brain atrophy and white-matter disease. Extensive condylomata led to a partial vulvectomy. Her lowest recorded oral temperature was 95.6°F.
Ms. B. returned to a residential home briefly but was readmitted when she was found unresponsive; hypotension and bradycardia were detected. Cardiac catheterization showed normal left ventricular function and severe 3-vessel coronary artery disease with a 50% obstruction of the left main coronary artery. This procedure was complicated by severe agitation, confusion, and a large post-catheterization hematoma requiring blood transfusions.
Following discussions with the cardiac surgeons, the family considered Ms. B. too ill to undergo coronary artery bypass surgery. The lowest recorded oral temperature was 94°F.
Ms. B. returned to the residential home—but not for long. In August 2000, she was again taken to the hospital. She was confused, threatening to harm herself with a knife, and eating “hair grease.” Her medications now included temazepam, lorazepam, risperidone, paroxetine, and desipramine—plus aspirin, verapamil, lisinopril, metoprolol, amlodipine, and isosorbide dinitrate for coronary heart disease and hypertension. The admission database included a temperature of 96.2°F. She received a Global Assessment of Functioning score of 20 contrasted with a high score of 70 the preceding year.
Ms. B.’s hospital stay lasted 2 months. Confusion and disorientation persisted one month after admission while still undergoing psychiatric care. Midway during her hospitalization, she underwent a cholecystectomy.
When she was discharged to an assisted living facility, Ms. B. required assistance with self-care and restraint with a posey vest. Dementia was considered the major psychiatric problem. Medications now included amlodipine, aspirin, famotidine, isosorbide dinitrate, lisinopril, metoprolol, oxybutynin, metoclopramide, lorazepam 0.5 mg 3 times a day, and risperidone 1 mg twice daily.
Two weeks later, Ms. B. was still confused and disoriented. Risperidone was increased to 1 mg 3 times daily and lorazepam was increased to 0.5 mg 4 times daily. A week later, the nursing staff noted further deterioration. She would wander, on occasion even into the street. Subsequently, she began disrobing for no apparent reason, 3 to 4 times a week.
In early December 2000, nurses called an ambulance because Ms. B. was “lethargic, unresponsive to name call.” The ambulance crew noted she was “foaming at the mouth,” lying "naked" in bed, and very “cold” to the touch. At the hospital, hypothermia was documented with a body temperature of 84°F rectally. (Of note, the patient’s roommate manifested a normal body temperature, was cognitively intact, and did not complain that their room was cold.) Medications at the time of admission included lisinopril 10 mg/d, aspirin 325 mg/d, amlodipine 10 mg/d, oxybutynin 5 mg twice daily, lorazepam 0.5 mg 3 times daily, metoprolol 50 mg twice daily, famotidine 20 mg twice daily, isosorbide dinitrate 10 mg 3 times daily, metoclopramide 10 mg 4 times daily, and risperidone 1 mg twice daily.
Initially, Ms. B. manifested bradycardia requiring temporary pacing, and hemoconcentration without explanation for the low body temperature. Despite return to normal body temperature within 24 hours, vasomotor instability, body temperatures ranging between 95.9°F and 100.1°F, encephalopathy, and general organ failure persisted. Ms. B. was pronounced dead on the 18th hospital day. An autopsy was not performed.
Amlodipine, a calcium channel blocker, enhances vasodilatation and may also have limited Ms. B.’s capacity to vasoconstrict. Calcium channel blockers may have variable effects on intraoperative core body temperature in humans.10
Phenothiazines, particularly the low-potency agents in this class, are the antipsychotic drugs most commonly associated with drug-induced hypothermia.6,9,11 Phenothiazines seem to have a direct effect on hypothalamic thermoregulation. About a month before developing moderate hypothermia, Ms. B. received an increase in her risperidone dosage from 1 mg twice daily to 1 mg 3 times daily because of agitation. The package insert for risperidone states:
- Medical conditions
- Hypoglycemia
- Hypothyroidism
- Adrenal insufficiency
- Hypopituitarism
- Stroke
- Malnutrition
- Shock
- Sepsis
- Hepatic or renal failure
- Burns
- Exfoliative dermatitis
- Immobility or debilitation
- Hypothalamic disorders
- Parkinson’s disease
- Spinal cord injury
- Diabetic ketoacidosis
- Psychiatric conditions
- Alzheimer’s disease
- Schizophrenia
- Medications
- Ethanol
- Phenothiazines
- Barbiturates
- Anesthetics
- Neuromuscular blockers
*Adapted from Danzl DF. Hypothermia. Harrison’s 15th Ed., Principles of Internal Medicine, New York: McGraw-Hill, 2001, p. 107.
“Disruption of body temperature regulation has been attributed to antipsychotic agents. Both hyperthermia and hypothermia have been reported in association with Risperdal use. Caution is advised when prescribing for patients who will be exposed to temperature extremes.”12
Lorazepam very rarely may be associated with hypothermia. In animal studies, zolpidem, diazepam, and lorazepam produced comparable dose-dependent hypothermia.13 Ms. B. had her dosage of lorazepam increased from 0.5 mg 3 times daily to 0.5 mg 4 times daily because of increasing agitation and wandering. About 10 days before developing moderate hypothermia, she became more lethargic and the nursing staff was directed to withhold lorazepam if she appeared unduly sedated. At this point, Ms. B. may have had a drug-induced delirium superimposed upon dementia or a toxic-metabolic encephalopathy superimposed upon dementia. In her case, we do not know if druginduced or metabolic-induced changes (or a combination of the two) best explained her change in mental status.
Once accidental hypothermia sets in
During the days before Ms. B. developed moderate hypothermia, the temperature outside the assisted living facility ranged from 25°F to 40°F. When she was found by the nursing staff to be unusually unresponsive, she was wearing her nightgown under bed sheets. Even if her room temperature had been at 70°F, an almost 30°F gradient would exist between that and normal body temperature (98.6°F). In complete thermodysregulation, her body temperature of 84°F could have been reached within 5 to 8 hours. The colder the room, the faster her body would cool in the presence of thermodysregulation.
Although sepsis and adverse environmental exposure are the most common conditions leading to hypothermia, up to onethird of cases of accidental hypothermia in the elderly occur during the warmer months, with one-half of these cases found in the hospital.6 In cases of accidental hypothermia occurring during the winter, one-half occur in a normal room temperature setting.9
In a United Kingdom study, about 25% of elderly patients with hypothermia died.9 Still, the severity of underlying disease is more predictive of mortality than is the degree of hypothermia.14 Ms. B.’s fatal clinical course was that of multiple organ failure complicated by hypothermia. No mention was made in the hospital records of her vulnerability to hypothermia. This vulnerability placed significant burden on the assisted living facility staff.
Hypothermia should be considered in the differential diagnosis of confusion and disruptive behavior in the elderly patient. In Ms. B.’s case, an early diagnosis of accidental hypothermia by a psychiatrist could have made a difference.
Related resources Oriented to mental health issues
- Kramer MR, Vandijk J, Rosin AJ. Mortality in elderly patients with thermoregulatory failure. Arch Intern Med. 1989;149:1521-1523.
- Murphy PJ. Hypothermia. In Oxford Textbook of Geriatric Medicine. Evans JG, Williams TF, Beattie BL, Michel J-P, Wilcock GK, eds. New York: Oxford University Press, 2000:857-863.
- Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992; 10:311-327.
- Fischbeck KH, Simon RP. Neurological manifestations of accidental hypothermia. Ann Neurol. 1981; 10:384-387.
Drug brand names
- Amlodipine • Norvasc
- Famotidine • Pepcid
- Isosorbide dinitrate • Isordil
- Lisinopril • Prinivil
- Metoclopramide • Reglan
- Metoprolol • Lopressor
- Oxybutynin • Ditropan
- Paroxetine • Paxil
- Risperidone • Risperdal
- Zolpidem • Ambien
Disclosure
The author reports that he is on the speakers’ bureau of Janssen Pharmaceutica, Eli Lilly and Co., Pfizer Inc., Wyeth-Ayerst Pharmaceuticals, Forest Pharmaceuticals, and GlaxoSmithKline.
1. Danzl DF, Pozos RS. Accidental hypothermia. N Engl J Med. 1994;331:1756-1760.
2. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs. 1997;7:121-138.
3. Grossman SP. Physiology of thirst. In: Schnur DB, Kirch DG, eds. Water balance in schizophrenia. Washington, DC: American Psychiatric Press, Inc., 1996;53-87.
4. Guyton AC, Hall JE. Behavioral and Motivational Mechanisms of the Brain—The Limbic System and the Hypothalamus. Textbook of Medical Physiology. Philadelphia: W.B. Saunders, 1996;749-760.
5. Fontaine KR, Heo M, Harrigan EP, Shear CL, et al. Estimating the consequences of antipsychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101:277-288.
6. Kramer MR, Vandijk J, Rosin AJ. Mortality in elderly patients with thermoregulatory failure. Arch Intern Med. 1989;149:1521-1523.
7. Mohamed S, Paulsen JS, O’Leary D, Arndt S, Andreasen N. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch Gen Psychiatry. 1999;56:749-754.
8. Vieweg V, Tucker R, Talbot PC, Blair CE, Lewis R. Mini-Mental State Examination scores of subjects with nondementing diagnoses on admission to a geropsychiatric hospital. Med Psychiatry. 2001;4:19-22.
9. Murphy PJ. Hypothermia. In: Evans JG, Williams TF, Beattie BL, Michel J-P, Wilcock GK, eds. Oxford Textbook of Geriatric Medicine. New York: Oxford University Press, 2000;857-863.
10. Vassilieff N, Rosencher N, Sessler DL, Conseiller C, Lienhart A. Nifedipine and intraoperative core body temperature in humans. Anesthesiology. 1994;80:123-128.
11. Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992;10:311-327.
12. Physicians’ Desk Reference. 54th ed. Montvale, NJ: Medical Economics Company, Inc., 2000.
13. Elliott EE, White JM. The acute effects of zolpidem compared to diazepam and lorazepam using radiotelemetry. Neuropharmacology. 2001;40:717-721.
14. Fischbeck KH, Simon RP. Neurological manifestations of accidental hypothermia. Ann Neurol. 1981;10:384-387.
1. Danzl DF, Pozos RS. Accidental hypothermia. N Engl J Med. 1994;331:1756-1760.
2. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs. 1997;7:121-138.
3. Grossman SP. Physiology of thirst. In: Schnur DB, Kirch DG, eds. Water balance in schizophrenia. Washington, DC: American Psychiatric Press, Inc., 1996;53-87.
4. Guyton AC, Hall JE. Behavioral and Motivational Mechanisms of the Brain—The Limbic System and the Hypothalamus. Textbook of Medical Physiology. Philadelphia: W.B. Saunders, 1996;749-760.
5. Fontaine KR, Heo M, Harrigan EP, Shear CL, et al. Estimating the consequences of antipsychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101:277-288.
6. Kramer MR, Vandijk J, Rosin AJ. Mortality in elderly patients with thermoregulatory failure. Arch Intern Med. 1989;149:1521-1523.
7. Mohamed S, Paulsen JS, O’Leary D, Arndt S, Andreasen N. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch Gen Psychiatry. 1999;56:749-754.
8. Vieweg V, Tucker R, Talbot PC, Blair CE, Lewis R. Mini-Mental State Examination scores of subjects with nondementing diagnoses on admission to a geropsychiatric hospital. Med Psychiatry. 2001;4:19-22.
9. Murphy PJ. Hypothermia. In: Evans JG, Williams TF, Beattie BL, Michel J-P, Wilcock GK, eds. Oxford Textbook of Geriatric Medicine. New York: Oxford University Press, 2000;857-863.
10. Vassilieff N, Rosencher N, Sessler DL, Conseiller C, Lienhart A. Nifedipine and intraoperative core body temperature in humans. Anesthesiology. 1994;80:123-128.
11. Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992;10:311-327.
12. Physicians’ Desk Reference. 54th ed. Montvale, NJ: Medical Economics Company, Inc., 2000.
13. Elliott EE, White JM. The acute effects of zolpidem compared to diazepam and lorazepam using radiotelemetry. Neuropharmacology. 2001;40:717-721.
14. Fischbeck KH, Simon RP. Neurological manifestations of accidental hypothermia. Ann Neurol. 1981;10:384-387.
Cognitive enhancers for dementia: Do they work?
No psychiatrist likes to see the month-by-month deterioration in an Alzheimer’s patient—the losses in cognition, the declining ability to function, the behavioral aberrations that upset family and friends.
The problem will accelerate in the decades ahead as the proportion of elderly in the population increase. More than 4 million people in the United States are afflicted with this disorder. Prevalence rates as high as 10% have been estimated for individuals older than 65. Patients with the disease have estimated direct costs of $20,000 to $61,000 per year if the duration lasts 7 to 8 years.1
Although behavioral and functional deficits account for the high costs associated with Alzheimer’s disease (AD), the disorder is defined by cognitive criteria (Box 1). The majority of medication trials have been aimed at symptomatic treatment. More recently, studies have been designed to prevent or delay the onset of AD. Early on, initial therapies directed toward AD were aimed at reversing the cholinergic deficit (Box 2). Clinical trials utilizing lecithin (25-100 g/d) and choline (<16 g/d) as precursors of acetylcholine did not lead to significant benefit.6 Augmenting central cholinergic levels with acetylcholinesterase (AChE) inhibitors has consistently detected symptomatic improvement.
In recent years, the Food and Drug Administration has approved 4 AChE inhibitors—tacrine, donepezil, rivastigmine, and galantamine—for the treatment of AD. I will discuss only the latter 3, since tacrine, the first to Nshow benefit, has a high rate of adverse effects and is of limited use.7 The AChE inhibitors may improve cognition and behavioral symptoms and delay progression of the illness. They can also have beneficial effects on activities of daily living (ADL) and can reduce costs and improve caregiver burden.8
- The development of multiple cognitive deficits manifested by both:
- Memory impairment (impaired ability to learn new information or to recall previously learned information) and
- One (or more) of the following cognitive disturbances:
- Aphasia (language disturbance)
- Apraxia (impaired ability to carry out motor activities despite intact motor function)
- Agnosia (failure to recognize or identify objects despite intact sensory function)
- Disturbance in executive functioning (i.e., planning, organization, sequencing, abstracting)
- The cognitive deficits in Criteria A1 and A2 each cause significant impairment in social or occupational functioning and represent a significant decline from a previous level of functioning.
- The course is characterized by gradual onset and continuing cognitive decline.
Source: American Psychatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington DC: American Psychiatric Press., 1994.
The three AChE inhibitors have unique basic properties (Box 3). In order to maximize and prolong positive drug effects, it is important to start early and adjust dosage during the treatment9 (Table 1). Side effects are tolerable; the most common include nausea, vomiting, and diarrhea. Titrating the dosage slowly can reduce these. The cholinergic quality of these medications dictate that they be prescribed with caution in patients with bradycardic arrhythmias such as sick sinus syndrome, asthma, or chronic obstructive pulmonary disease.10
Effects on cognition and global assessments
Numerous efficacy studies examining cognition and global assessments in AD patients have been performed with the AChE inhibitors. Their major therapeutic effect is to maintain cognitive function at a constant level during a 6- to 12-month period of treatment, as compared to placebo. Comparison of clinical effects of all 3 agents demonstrates a similar magnitude of improvement. For some drugs, this may represent an upper limit, whereas for others it may still be possible to further increase the benefit.
The pathophysiologic processes implicated in Alzheimer’s disease (AD)include amyloid precursor protein metabolism, tau phosphorylation, apolipoprotein E, inflammation, oxidative stress, and apoptosis. Neuropathological features include amyloid plaques, neurofibrillary tangles, neuronal and synaptic loss, microgliosis, and astrocytosis. The resulting clinical syndrome of dementia is associated with neurotransmitter deficits and intracortical disconnection.
The central cholinergic neurotransmitter system is impaired in AD. This system is involved in learning and memory. The limbic system and neocortex receive projections from the cholinergic system in the septal nuclei and substantia innominata. This includes the medial septal nucleus, diagonal band, and nucleus basalis.2 Literature on animals has demonstrated that basal forebrain lesions impair learning and memory. Cholinergic agonists can improve this. Furthermore, cholinergic antagonists such as scopolamine and atropine can impair learning and memory in humans and animals.3 In AD, a 58% to 93% reduction in choline acetyltransferase levels (and other cholinergic markers) in the cortex and hippocampus can be observed and this correlates with dementia severity.4 Early AD is characterized by neuronal loss and tangles in the cholinergic nucleus basalis of Meynert.5
Results of 4 double-blind, placebo-controlled clinical trials of donepezil, involving more than 1,900 individuals with mild to moderate AD, have been published recently. In all, significant improvements in cognition were observed consistently for both therapeutic doses of donepezil (5 mg/d and 10 mg/d), relative to placebo. Similar benefits were reported for global functioning.
The long-term clinical efficacy and safety of donepezil versus placebo across 1 year in patients with mild to moderate AD was investigated.14 The Gottfries-Brane-Steen global assessment for rating dementia symptoms demonstrated the benefit of donepezil over placebo at weeks 24, 36, and 52, and at the study end point. Advantages of donepezil were also observed in cognition and ADL.
Donepezil also appears to work for patients with moderate to severe AD. In a recent 24-week study,15 patients receiving donepezil showed benefits on the Clinician’s Interview-Based Impression of Change with Caregiver Input (CIBIC+), compared with placebo, at all visits up to week 24 and at the study’s end point. All other secondary measures showed significant differences between the groups in favor of donepezil at the end of the study. These data suggest that donepezil’s benefits extend into more advanced stages of AD than those previously investigated, with good tolerability.
Clinical trials of rivastigmine (1.5-6 mg twice daily PO) have also demonstrated benefits on cognitive and global measures.16 The efficacy of rivastigmine tartrate (ENA 713) in patients with mild to moderately severe Alzheimer’s disease was evaluated in a 26-week open-label extension of a 26-week, double-blind, placebo-controlled study. By 52 weeks, patients originally treated with rivastigmine 6 to 12 mg/d had significantly better cognitive function than did patients originally treated with placebo.17- 19
Donepezil is a second-generation, piperidine-class, selective and reversible acetylcholinesterase (AChE) inhibitor. It is structurally dissimilar from other established AChE inhibitors.
Experimentally, donepezil inhibits AChE activity in human erythrocytes and increases extracellular acetylcholine levels in the cerebral cortex and the hippocampus of the rat. Pharmacologically, donepezil has a half-life of approximately 70h, lending itself to once-daily administration.11
Rivastigmine (ENA 713, or carbamoylatine) is an AChE inhibitor with brain-region selectivity and a long duration of action. Both preclinical studies and studies in human volunteers have shown that rivastigmine induces substantially greater inhibition of AChE in the central nervous system compartment than it does in the periphery (40% inhibition of central AChE compared with 10% inhibition of plasma butylcholinesterase in healthy volunteers). Rivastigmine also preferentially inhibits the G1 enzymatic form of AChE, which predominates in the brains of patients with Alzheimer’s disease (AD).
Evidence from animal studies also suggests that rivastigmine is a more potent inhibitor of AChE in the cortex and hippocampus, the brain regions most affected by AD. The principal metabolite of rivastigmine has at least 10-fold lower activity against AChE compared with the parent drug.
Rivastigmine is completely metabolized; the major route of elimination of the metabolites is renal. Rivastigmine is inactivated during the process of interacting with and inhibiting AChE, and, in contrast to other AChE inhibitors, the hepatic cytochrome P-450 (CYP-450) system is not involved in the metabolism of rivastigmine.12
Galantamine is an allosterically potentiating ligand that modulates nicotinic cholinergic receptors (nAChR) to increase acetylcholine release as well as acting as an AChE inhibitor. In preclinical experiments, the drug significantly improves learning, reduces AChE levels, and increases nicotinic receptor binding. Action of galantamine is on the most abundant nAChR in the human brain, the alpha4/beta2subtype.13
Clinical trials of galantamine (4-12 mg/bid PO) have demonstrated similar benefits.20 Following a 4-week placebo run-in, patients were randomized to 1 of 4 treatment arms: placebo or galantamine escalated to final maintenance dosages of 8, 16, or 24 mg/d for a 5-month treatment phase. At study’s end, the galantamine-place-bo differences on the cognitive subscale of the AD Assessment Scale were 3.3 points for the 16 mg/d group and 3.6 points for the 24 mg/d group. Treatment discontinuations due to adverse events were low in all galantamine groups (6% to 10%) and comparable with that in the placebo group (7%). The incidence of adverse events in the galantamine groups, notably gastrointestinal symptoms, was low and most adverse events were mild.
Other studies examining galantamine have demonstrated similar clinical benefits.8, 21
When using AChE inhibitors, the slope of cognitive decline is similar in treated and untreated patients after the initial improvement. These drugs essentially do not reverse the disorder’s course but shift upward the curve describing the time course of cognitive decline. This applies also to behavioral and functional benefits. Thus the benefit obtained is symptomatic and not neuroprotective, and is lost after discontinuing the medications.
Effects on functioning and behavior
In a 24-week multinational clinical trial, patients receiving donepezil (10 mg/d) were more able than placebo-treated patients to perform complex daily functioning tasks.22 Similar effects have been observed with rivastigmine and galantamine.16, 20
All 3 AChE inhibitors have demonstrated improvements in the behavioral changes associated with AD. Cummings et al23 tested the hypothesis that behavioral disturbances are reported at significantly lower rates by caregivers of AD patients receiving donepezil, compared with a group of patients not receiving a drug for treatment of dementia. Donepezil patients were described as significantly less likely to be threatening, destroy property, and talk loudly, and significantly fewer were treated with sedatives.
Table 1
COMPARING THEAChE INHIBITORS
| Donepezil | Rivastigmine | Galantamine | |
|---|---|---|---|
| Chemical class | Piperidine | Carbamate | Phenanthrene alkaloid |
| AChE inhibitor | Yes | Yes | Yes |
| BuChE inhibitor | Small | Yes | Small |
| Nicotinic modulation | No | No | Yes |
| Elimination half-life | 50-70 h | 0.6-2 h | 5-7 h |
| Administration | Once daily | Twice daily | Twice daily |
| Starting dosage | 5 mg/d | 1.5 mg bid | 4 mg bid |
| Total recommended dosage | 5-10 mg/d | 6-12 mg/d | 16-24 mg/d |
| Adapted from Conn DK. Cholinesterase inhibitors: Comparing the options for mild-to-moderate dementia. Geriatrics56: 56-57, 2001. | |||
An open-label study by Weiner et al24 examined the effects of donepezil on emotional and behavioral symptoms using the CERAD Behavior Rating Scale for Dementia and its subscales. In a group of 25 AD patients treated with donepezil, scores returned to baseline levels at 12 months. In contrast, the scores of the reference group worsened minimally.
Galantamine has also proved effective in treating behavioral symptoms associated with AD. In the Tariot et al20 study, galantamine at 16 mg/d and 24 mg/d had a significantly better outcome on CIBIC+, ADL, and behavioral symptoms versus placebo.
Rosler et al25 assessed the ability of rivastigmine to improve behavioral symptoms in AD. Using the behavioral component of the CIBIC+, results showed that long-term treatment with rivastigmine could slow the progression of symptoms. Symptoms showing stabilization included aggressiveness, activity disturbances, hallucinations, and paranoid features. The results also suggest that patients treated earlier with rivastigmine may attain a greater benefit than those whose treatment is delayed 6 months.
Rivastigmine also has significant effects on controlling behavioral symptoms in patients with Lewy body dementia.26 A placebo-controlled, double-blind, multicenter study was performed in 120 patients with Lewy body dementia. Individuals were given up to 12 mg/d rivastigmine or placebo for 20 weeks, followed by 3 weeks rest. Assessment by neuropsychiatric inventory was made at baseline, and again at weeks 12, 20, and 23.
Patients taking rivastigmine were significantly less apathetic and anxious than those on placebo, and had fewer delusions and hallucinations. Almost twice as many patients on rivastigmine as on placebo (37, 63% versus 18, 30%) showed at least a 30% improvement from baseline. In a computerized cognitive assessment system and neuropsychological tests, patients were significantly faster and better than those on placebo, particularly when performing tasks with a substantial attentional component.
Cost effectiveness
Numerous studies have demonstrated that AChE inhibitors are cost savers in AD treatment. Fillit et al27 examined the impact of donepezil in a multisite managed care organization for 2 years using claims data for 70 individuals with AD and related dementias. The median per diem medical costs were $1.22 lower post treatment than they were in the pretreatment phase. Moreover, posttreatment costs were reduced in 6 of 7 service settings, with median per diem savings of $0.77 in outpatient care and $0.65 in office visits.
Neumann et al28 utilized cost-effectiveness analysis to predict that for mild AD, donepezil would pay for itself in cost offsets if the drug’s effect exceeds 2 years. Donepezil costs were partially offset by a reduction in the costs of care due to enhanced cognitive functioning and the delay in placing the patient in more costly disease stages and settings.
One study used the disease-progression model to estimate potential per-patient savings resulting from the treatment of AD in Canada.29 Rivastigmine was estimated to delay the transition to more severe stages of AD by up to 188 days for patients with mild AD after 2 years of treatment. For patients with mild-to-moderate and moderate disease, this delay was estimated to be 106 and 44 days, respectively.
The Assessment of Health Economics in Alzheimer’s Disease model uses algorithms to predict the time until patients with AD require full-time care. A study, performed in Canada, compared treatment with galantamine to treatment without pharmacological interventions.30 Galantamine was predicted to reduce the duration of full-time care by almost 10%. Approximately 5.6 patients with mild-to-moderate disease must be placed on treatment to avoid 1 year of full-time care, resulting in savings averaging $528 per patient. For patients with moderate disease, 3.9 patients must be placed on treatment to avoid 1 year of full-time care, with savings predicted at $2,533 per patient.
Caregiving burden
Fillit et al addressed caregiver well-being in a self-administered, nationwide survey of AD caregivers. Caregivers of patients treated with donepezil (n = 274) were compared with caregivers of patients not treated with donepezil (n = 274).31 The Caregiver Burden Scale measured time demands and distress linked to commonly performed caregiving tasks. Donepezil caregivers reported significantly lower scores on difficulty of caregiving. Similar findings have been observed with galantamine.32
Related resources
- Bullock R. New drugs for Alzheimer’s disease and other dementias. Br J Psychiatry. 2002;180(2):135-139.
- Brodaty H, Ames D, et al. Pharmacological treatment of cognitive deficits in Alzheimer’s disease. Med J Aust. 2001;175(6):324-329.
- Ahmed MB. Alzheimer’s disease: recent advances in etiology, diagnosis, and management. Tex Med. 2001;97(12):50-58.
- Frisoni GB. Treatment of Alzheimer’s disease with acetyl-cholinesterase inhibitors: bridging the gap between evidence and practice. J Neurol. 2001;248(7):551-7.
Drug brand names
- Tacrine • Cognex
- Donepezil • Aricept
- Rivastigmine • Exelon
- Galantamine • Reminyl
Disclosure
The author reports that he receives grant/research support from, serves as a consultant to, and is on the speaker’s bureau of Janssen Pharmaceutica and Pfizer Inc., and serves as consultant to and is on the speaker’s bureau of Novartis Pharmaceuticals Corp.
1. Irizarry M, Hyman B. Alzheimer’s disease. In: Principles of neuroepidemiology. Batchelor T, Cudkowicz M, eds. Boston: Butterworth-Heinemann, 2001:69-98.
2. Mesulam MM, Mufson EJ, Levey AI, Warner BH. Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983a;214:170-197.
3. Drachman DA, Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30:113-121.
4. Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403.-
5. Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122-126.
6. Higgins JPT, Flicker L. Lecithin and cognitive impairment. The Cochrane Database of Systematic Reviews. 2001.;
7. Knapp MJ, Knopman DS, Solomon PR, Pendlebury WW, Davis CS, Gracon SI. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer’s disease. The Tacrine Study Group. JAMA. 1994;271:985-991.
8. Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000;54:2262-2268.
9. Giacobini E. Cholinesterase inhibitors stabilize Alzheimer’s disease. Methods Find Exp Clin Pharmacol. 2000;22:609-613.
10. Irizarry MC, Hyman BT. Alzhemier Disease Therapeutics. J Neuropathology Exp Neurology. 2001;60:923-928.
11. Wilkinson DG. The pharmacology of donepezil: a new treatment of Alzheimer’s disease. Expert Opin Pharmacother. 1999;1:121-135.
12. Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetyl-cholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin Ther. 1998;20:634-647.
13. Samochocki M, Zerlin M, Jostock R, et al. Galantamine is an allosterically potentiating ligand of the human alpha4/beta2 nAChR. Acta Neurol Scand. 2000(Suppl);;176:68-73.
14. Winblad B, Engedal K, Soininen H, Verhey F, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489-495.
15. Feldman H, Gauthier S, Hecker J, et al. Donepezil MSAD Study Investigators Group.A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease. Neurology. 2001;57:613-620.
16. Farlow M, Anand R, Messina J, Jr, Hartman R, Veach J. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s disease. Eur Neurol. 2000;44:236-241.
17. Rogers S, Friedhoff L. Donepezil Study Group.The efficacy and safety of donepezil in patients with Alzhemier’s disease. Dementia. 1996;7:293-303.
18. Rogers S, Farlow M, Doody R, Morris R, Friedhoff L. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50:136-145.
19. Rosler M, Anand R, Cicin-Sain A, Gauthier S, Agid Y, DalBianco P, Stahelin HB, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: International randomized controlled trial. BMJ. 1999;318:633-638.
20. Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C. A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology. 2000;54:2269-2276.
21. Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: Multicentre randomized controlled trial. Galantamine International - 1 Study Group. BMJ. 2000;321:1445-1449.
22. Knopman DS. Management of cognition and function: new results from the clinical trials programme of Aricept (R) (donepezil Hcl). Int J Neuropsychopharmacol. 2000;3:13-20.
23. Cummings JL, Donohue JA, Brooks RL. The relationship between donepezil and behavioral disturbances in patients with Alzheimer’s disease. Am J Geriatr Psychiatry. 2000;8:134-140.
24. Weiner MF, Martin-Cook K, Foster BM, Saine K, et al. Effects of donepezil on emotional/behavioral symptoms in Alzheimer’s disease patients. J Clin Psychiatry. 2000;487-492.
25. Rosler M, Retz W, Retz-Junginger P, Dennler HJ. Effects of two-year treatment with the cholinesterase inhibitor rivastigmine on behavioural symptoms in Alzheimer’s disease. Behav Neurol. 1998;11:211-216.
26. McKeith I, Del Ser T, Spano P, Emre M, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
27. Fillit H, Gutterman EM, Lewis B. Donepezil use in managed Medicare: effect on health care costs and utilization. Clin Ther. 1999;21:2173-2185.
28. Neumann PJ, Hermann RC, Kuntz KM, Araki SS, Duff SB, Leon J, Berenbaum PA, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer’s disease. Neurology. 1999;52:1115-1116.
29. Hauber AB, Gnanasakthy A, Mauskopf JA. Savings in the cost of caring for patients with Alzheimer’s disease in Canada: an analysis of treatment with rivastigmine. Clin Ther. 2000;22:439-451.
30. Getsios D, Caro JJ, Caro G, Ishak K. The AHEAD Study Group. Assessment of health economics in Alzheimer’s disease (AHEAD): galantamine treatment in Canada. Neurology. 2001;57:972-978.
31. Fillit HM, Gutterman EM, Brooks RL. Impact of donepezil on caregiving burden for patients with Alzheimer’s disease. Int Psychogeriatr. 2000;12(3):389-401.
32. Blesa R. Galantamine: therapeutic effects beyond cognition. Dement Geriatr Cogn Disord. 2000;11(Suppl)1:28-34.
No psychiatrist likes to see the month-by-month deterioration in an Alzheimer’s patient—the losses in cognition, the declining ability to function, the behavioral aberrations that upset family and friends.
The problem will accelerate in the decades ahead as the proportion of elderly in the population increase. More than 4 million people in the United States are afflicted with this disorder. Prevalence rates as high as 10% have been estimated for individuals older than 65. Patients with the disease have estimated direct costs of $20,000 to $61,000 per year if the duration lasts 7 to 8 years.1
Although behavioral and functional deficits account for the high costs associated with Alzheimer’s disease (AD), the disorder is defined by cognitive criteria (Box 1). The majority of medication trials have been aimed at symptomatic treatment. More recently, studies have been designed to prevent or delay the onset of AD. Early on, initial therapies directed toward AD were aimed at reversing the cholinergic deficit (Box 2). Clinical trials utilizing lecithin (25-100 g/d) and choline (<16 g/d) as precursors of acetylcholine did not lead to significant benefit.6 Augmenting central cholinergic levels with acetylcholinesterase (AChE) inhibitors has consistently detected symptomatic improvement.
In recent years, the Food and Drug Administration has approved 4 AChE inhibitors—tacrine, donepezil, rivastigmine, and galantamine—for the treatment of AD. I will discuss only the latter 3, since tacrine, the first to Nshow benefit, has a high rate of adverse effects and is of limited use.7 The AChE inhibitors may improve cognition and behavioral symptoms and delay progression of the illness. They can also have beneficial effects on activities of daily living (ADL) and can reduce costs and improve caregiver burden.8
- The development of multiple cognitive deficits manifested by both:
- Memory impairment (impaired ability to learn new information or to recall previously learned information) and
- One (or more) of the following cognitive disturbances:
- Aphasia (language disturbance)
- Apraxia (impaired ability to carry out motor activities despite intact motor function)
- Agnosia (failure to recognize or identify objects despite intact sensory function)
- Disturbance in executive functioning (i.e., planning, organization, sequencing, abstracting)
- The cognitive deficits in Criteria A1 and A2 each cause significant impairment in social or occupational functioning and represent a significant decline from a previous level of functioning.
- The course is characterized by gradual onset and continuing cognitive decline.
Source: American Psychatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington DC: American Psychiatric Press., 1994.
The three AChE inhibitors have unique basic properties (Box 3). In order to maximize and prolong positive drug effects, it is important to start early and adjust dosage during the treatment9 (Table 1). Side effects are tolerable; the most common include nausea, vomiting, and diarrhea. Titrating the dosage slowly can reduce these. The cholinergic quality of these medications dictate that they be prescribed with caution in patients with bradycardic arrhythmias such as sick sinus syndrome, asthma, or chronic obstructive pulmonary disease.10
Effects on cognition and global assessments
Numerous efficacy studies examining cognition and global assessments in AD patients have been performed with the AChE inhibitors. Their major therapeutic effect is to maintain cognitive function at a constant level during a 6- to 12-month period of treatment, as compared to placebo. Comparison of clinical effects of all 3 agents demonstrates a similar magnitude of improvement. For some drugs, this may represent an upper limit, whereas for others it may still be possible to further increase the benefit.
The pathophysiologic processes implicated in Alzheimer’s disease (AD)include amyloid precursor protein metabolism, tau phosphorylation, apolipoprotein E, inflammation, oxidative stress, and apoptosis. Neuropathological features include amyloid plaques, neurofibrillary tangles, neuronal and synaptic loss, microgliosis, and astrocytosis. The resulting clinical syndrome of dementia is associated with neurotransmitter deficits and intracortical disconnection.
The central cholinergic neurotransmitter system is impaired in AD. This system is involved in learning and memory. The limbic system and neocortex receive projections from the cholinergic system in the septal nuclei and substantia innominata. This includes the medial septal nucleus, diagonal band, and nucleus basalis.2 Literature on animals has demonstrated that basal forebrain lesions impair learning and memory. Cholinergic agonists can improve this. Furthermore, cholinergic antagonists such as scopolamine and atropine can impair learning and memory in humans and animals.3 In AD, a 58% to 93% reduction in choline acetyltransferase levels (and other cholinergic markers) in the cortex and hippocampus can be observed and this correlates with dementia severity.4 Early AD is characterized by neuronal loss and tangles in the cholinergic nucleus basalis of Meynert.5
Results of 4 double-blind, placebo-controlled clinical trials of donepezil, involving more than 1,900 individuals with mild to moderate AD, have been published recently. In all, significant improvements in cognition were observed consistently for both therapeutic doses of donepezil (5 mg/d and 10 mg/d), relative to placebo. Similar benefits were reported for global functioning.
The long-term clinical efficacy and safety of donepezil versus placebo across 1 year in patients with mild to moderate AD was investigated.14 The Gottfries-Brane-Steen global assessment for rating dementia symptoms demonstrated the benefit of donepezil over placebo at weeks 24, 36, and 52, and at the study end point. Advantages of donepezil were also observed in cognition and ADL.
Donepezil also appears to work for patients with moderate to severe AD. In a recent 24-week study,15 patients receiving donepezil showed benefits on the Clinician’s Interview-Based Impression of Change with Caregiver Input (CIBIC+), compared with placebo, at all visits up to week 24 and at the study’s end point. All other secondary measures showed significant differences between the groups in favor of donepezil at the end of the study. These data suggest that donepezil’s benefits extend into more advanced stages of AD than those previously investigated, with good tolerability.
Clinical trials of rivastigmine (1.5-6 mg twice daily PO) have also demonstrated benefits on cognitive and global measures.16 The efficacy of rivastigmine tartrate (ENA 713) in patients with mild to moderately severe Alzheimer’s disease was evaluated in a 26-week open-label extension of a 26-week, double-blind, placebo-controlled study. By 52 weeks, patients originally treated with rivastigmine 6 to 12 mg/d had significantly better cognitive function than did patients originally treated with placebo.17- 19
Donepezil is a second-generation, piperidine-class, selective and reversible acetylcholinesterase (AChE) inhibitor. It is structurally dissimilar from other established AChE inhibitors.
Experimentally, donepezil inhibits AChE activity in human erythrocytes and increases extracellular acetylcholine levels in the cerebral cortex and the hippocampus of the rat. Pharmacologically, donepezil has a half-life of approximately 70h, lending itself to once-daily administration.11
Rivastigmine (ENA 713, or carbamoylatine) is an AChE inhibitor with brain-region selectivity and a long duration of action. Both preclinical studies and studies in human volunteers have shown that rivastigmine induces substantially greater inhibition of AChE in the central nervous system compartment than it does in the periphery (40% inhibition of central AChE compared with 10% inhibition of plasma butylcholinesterase in healthy volunteers). Rivastigmine also preferentially inhibits the G1 enzymatic form of AChE, which predominates in the brains of patients with Alzheimer’s disease (AD).
Evidence from animal studies also suggests that rivastigmine is a more potent inhibitor of AChE in the cortex and hippocampus, the brain regions most affected by AD. The principal metabolite of rivastigmine has at least 10-fold lower activity against AChE compared with the parent drug.
Rivastigmine is completely metabolized; the major route of elimination of the metabolites is renal. Rivastigmine is inactivated during the process of interacting with and inhibiting AChE, and, in contrast to other AChE inhibitors, the hepatic cytochrome P-450 (CYP-450) system is not involved in the metabolism of rivastigmine.12
Galantamine is an allosterically potentiating ligand that modulates nicotinic cholinergic receptors (nAChR) to increase acetylcholine release as well as acting as an AChE inhibitor. In preclinical experiments, the drug significantly improves learning, reduces AChE levels, and increases nicotinic receptor binding. Action of galantamine is on the most abundant nAChR in the human brain, the alpha4/beta2subtype.13
Clinical trials of galantamine (4-12 mg/bid PO) have demonstrated similar benefits.20 Following a 4-week placebo run-in, patients were randomized to 1 of 4 treatment arms: placebo or galantamine escalated to final maintenance dosages of 8, 16, or 24 mg/d for a 5-month treatment phase. At study’s end, the galantamine-place-bo differences on the cognitive subscale of the AD Assessment Scale were 3.3 points for the 16 mg/d group and 3.6 points for the 24 mg/d group. Treatment discontinuations due to adverse events were low in all galantamine groups (6% to 10%) and comparable with that in the placebo group (7%). The incidence of adverse events in the galantamine groups, notably gastrointestinal symptoms, was low and most adverse events were mild.
Other studies examining galantamine have demonstrated similar clinical benefits.8, 21
When using AChE inhibitors, the slope of cognitive decline is similar in treated and untreated patients after the initial improvement. These drugs essentially do not reverse the disorder’s course but shift upward the curve describing the time course of cognitive decline. This applies also to behavioral and functional benefits. Thus the benefit obtained is symptomatic and not neuroprotective, and is lost after discontinuing the medications.
Effects on functioning and behavior
In a 24-week multinational clinical trial, patients receiving donepezil (10 mg/d) were more able than placebo-treated patients to perform complex daily functioning tasks.22 Similar effects have been observed with rivastigmine and galantamine.16, 20
All 3 AChE inhibitors have demonstrated improvements in the behavioral changes associated with AD. Cummings et al23 tested the hypothesis that behavioral disturbances are reported at significantly lower rates by caregivers of AD patients receiving donepezil, compared with a group of patients not receiving a drug for treatment of dementia. Donepezil patients were described as significantly less likely to be threatening, destroy property, and talk loudly, and significantly fewer were treated with sedatives.
Table 1
COMPARING THEAChE INHIBITORS
| Donepezil | Rivastigmine | Galantamine | |
|---|---|---|---|
| Chemical class | Piperidine | Carbamate | Phenanthrene alkaloid |
| AChE inhibitor | Yes | Yes | Yes |
| BuChE inhibitor | Small | Yes | Small |
| Nicotinic modulation | No | No | Yes |
| Elimination half-life | 50-70 h | 0.6-2 h | 5-7 h |
| Administration | Once daily | Twice daily | Twice daily |
| Starting dosage | 5 mg/d | 1.5 mg bid | 4 mg bid |
| Total recommended dosage | 5-10 mg/d | 6-12 mg/d | 16-24 mg/d |
| Adapted from Conn DK. Cholinesterase inhibitors: Comparing the options for mild-to-moderate dementia. Geriatrics56: 56-57, 2001. | |||
An open-label study by Weiner et al24 examined the effects of donepezil on emotional and behavioral symptoms using the CERAD Behavior Rating Scale for Dementia and its subscales. In a group of 25 AD patients treated with donepezil, scores returned to baseline levels at 12 months. In contrast, the scores of the reference group worsened minimally.
Galantamine has also proved effective in treating behavioral symptoms associated with AD. In the Tariot et al20 study, galantamine at 16 mg/d and 24 mg/d had a significantly better outcome on CIBIC+, ADL, and behavioral symptoms versus placebo.
Rosler et al25 assessed the ability of rivastigmine to improve behavioral symptoms in AD. Using the behavioral component of the CIBIC+, results showed that long-term treatment with rivastigmine could slow the progression of symptoms. Symptoms showing stabilization included aggressiveness, activity disturbances, hallucinations, and paranoid features. The results also suggest that patients treated earlier with rivastigmine may attain a greater benefit than those whose treatment is delayed 6 months.
Rivastigmine also has significant effects on controlling behavioral symptoms in patients with Lewy body dementia.26 A placebo-controlled, double-blind, multicenter study was performed in 120 patients with Lewy body dementia. Individuals were given up to 12 mg/d rivastigmine or placebo for 20 weeks, followed by 3 weeks rest. Assessment by neuropsychiatric inventory was made at baseline, and again at weeks 12, 20, and 23.
Patients taking rivastigmine were significantly less apathetic and anxious than those on placebo, and had fewer delusions and hallucinations. Almost twice as many patients on rivastigmine as on placebo (37, 63% versus 18, 30%) showed at least a 30% improvement from baseline. In a computerized cognitive assessment system and neuropsychological tests, patients were significantly faster and better than those on placebo, particularly when performing tasks with a substantial attentional component.
Cost effectiveness
Numerous studies have demonstrated that AChE inhibitors are cost savers in AD treatment. Fillit et al27 examined the impact of donepezil in a multisite managed care organization for 2 years using claims data for 70 individuals with AD and related dementias. The median per diem medical costs were $1.22 lower post treatment than they were in the pretreatment phase. Moreover, posttreatment costs were reduced in 6 of 7 service settings, with median per diem savings of $0.77 in outpatient care and $0.65 in office visits.
Neumann et al28 utilized cost-effectiveness analysis to predict that for mild AD, donepezil would pay for itself in cost offsets if the drug’s effect exceeds 2 years. Donepezil costs were partially offset by a reduction in the costs of care due to enhanced cognitive functioning and the delay in placing the patient in more costly disease stages and settings.
One study used the disease-progression model to estimate potential per-patient savings resulting from the treatment of AD in Canada.29 Rivastigmine was estimated to delay the transition to more severe stages of AD by up to 188 days for patients with mild AD after 2 years of treatment. For patients with mild-to-moderate and moderate disease, this delay was estimated to be 106 and 44 days, respectively.
The Assessment of Health Economics in Alzheimer’s Disease model uses algorithms to predict the time until patients with AD require full-time care. A study, performed in Canada, compared treatment with galantamine to treatment without pharmacological interventions.30 Galantamine was predicted to reduce the duration of full-time care by almost 10%. Approximately 5.6 patients with mild-to-moderate disease must be placed on treatment to avoid 1 year of full-time care, resulting in savings averaging $528 per patient. For patients with moderate disease, 3.9 patients must be placed on treatment to avoid 1 year of full-time care, with savings predicted at $2,533 per patient.
Caregiving burden
Fillit et al addressed caregiver well-being in a self-administered, nationwide survey of AD caregivers. Caregivers of patients treated with donepezil (n = 274) were compared with caregivers of patients not treated with donepezil (n = 274).31 The Caregiver Burden Scale measured time demands and distress linked to commonly performed caregiving tasks. Donepezil caregivers reported significantly lower scores on difficulty of caregiving. Similar findings have been observed with galantamine.32
Related resources
- Bullock R. New drugs for Alzheimer’s disease and other dementias. Br J Psychiatry. 2002;180(2):135-139.
- Brodaty H, Ames D, et al. Pharmacological treatment of cognitive deficits in Alzheimer’s disease. Med J Aust. 2001;175(6):324-329.
- Ahmed MB. Alzheimer’s disease: recent advances in etiology, diagnosis, and management. Tex Med. 2001;97(12):50-58.
- Frisoni GB. Treatment of Alzheimer’s disease with acetyl-cholinesterase inhibitors: bridging the gap between evidence and practice. J Neurol. 2001;248(7):551-7.
Drug brand names
- Tacrine • Cognex
- Donepezil • Aricept
- Rivastigmine • Exelon
- Galantamine • Reminyl
Disclosure
The author reports that he receives grant/research support from, serves as a consultant to, and is on the speaker’s bureau of Janssen Pharmaceutica and Pfizer Inc., and serves as consultant to and is on the speaker’s bureau of Novartis Pharmaceuticals Corp.
No psychiatrist likes to see the month-by-month deterioration in an Alzheimer’s patient—the losses in cognition, the declining ability to function, the behavioral aberrations that upset family and friends.
The problem will accelerate in the decades ahead as the proportion of elderly in the population increase. More than 4 million people in the United States are afflicted with this disorder. Prevalence rates as high as 10% have been estimated for individuals older than 65. Patients with the disease have estimated direct costs of $20,000 to $61,000 per year if the duration lasts 7 to 8 years.1
Although behavioral and functional deficits account for the high costs associated with Alzheimer’s disease (AD), the disorder is defined by cognitive criteria (Box 1). The majority of medication trials have been aimed at symptomatic treatment. More recently, studies have been designed to prevent or delay the onset of AD. Early on, initial therapies directed toward AD were aimed at reversing the cholinergic deficit (Box 2). Clinical trials utilizing lecithin (25-100 g/d) and choline (<16 g/d) as precursors of acetylcholine did not lead to significant benefit.6 Augmenting central cholinergic levels with acetylcholinesterase (AChE) inhibitors has consistently detected symptomatic improvement.
In recent years, the Food and Drug Administration has approved 4 AChE inhibitors—tacrine, donepezil, rivastigmine, and galantamine—for the treatment of AD. I will discuss only the latter 3, since tacrine, the first to Nshow benefit, has a high rate of adverse effects and is of limited use.7 The AChE inhibitors may improve cognition and behavioral symptoms and delay progression of the illness. They can also have beneficial effects on activities of daily living (ADL) and can reduce costs and improve caregiver burden.8
- The development of multiple cognitive deficits manifested by both:
- Memory impairment (impaired ability to learn new information or to recall previously learned information) and
- One (or more) of the following cognitive disturbances:
- Aphasia (language disturbance)
- Apraxia (impaired ability to carry out motor activities despite intact motor function)
- Agnosia (failure to recognize or identify objects despite intact sensory function)
- Disturbance in executive functioning (i.e., planning, organization, sequencing, abstracting)
- The cognitive deficits in Criteria A1 and A2 each cause significant impairment in social or occupational functioning and represent a significant decline from a previous level of functioning.
- The course is characterized by gradual onset and continuing cognitive decline.
Source: American Psychatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington DC: American Psychiatric Press., 1994.
The three AChE inhibitors have unique basic properties (Box 3). In order to maximize and prolong positive drug effects, it is important to start early and adjust dosage during the treatment9 (Table 1). Side effects are tolerable; the most common include nausea, vomiting, and diarrhea. Titrating the dosage slowly can reduce these. The cholinergic quality of these medications dictate that they be prescribed with caution in patients with bradycardic arrhythmias such as sick sinus syndrome, asthma, or chronic obstructive pulmonary disease.10
Effects on cognition and global assessments
Numerous efficacy studies examining cognition and global assessments in AD patients have been performed with the AChE inhibitors. Their major therapeutic effect is to maintain cognitive function at a constant level during a 6- to 12-month period of treatment, as compared to placebo. Comparison of clinical effects of all 3 agents demonstrates a similar magnitude of improvement. For some drugs, this may represent an upper limit, whereas for others it may still be possible to further increase the benefit.
The pathophysiologic processes implicated in Alzheimer’s disease (AD)include amyloid precursor protein metabolism, tau phosphorylation, apolipoprotein E, inflammation, oxidative stress, and apoptosis. Neuropathological features include amyloid plaques, neurofibrillary tangles, neuronal and synaptic loss, microgliosis, and astrocytosis. The resulting clinical syndrome of dementia is associated with neurotransmitter deficits and intracortical disconnection.
The central cholinergic neurotransmitter system is impaired in AD. This system is involved in learning and memory. The limbic system and neocortex receive projections from the cholinergic system in the septal nuclei and substantia innominata. This includes the medial septal nucleus, diagonal band, and nucleus basalis.2 Literature on animals has demonstrated that basal forebrain lesions impair learning and memory. Cholinergic agonists can improve this. Furthermore, cholinergic antagonists such as scopolamine and atropine can impair learning and memory in humans and animals.3 In AD, a 58% to 93% reduction in choline acetyltransferase levels (and other cholinergic markers) in the cortex and hippocampus can be observed and this correlates with dementia severity.4 Early AD is characterized by neuronal loss and tangles in the cholinergic nucleus basalis of Meynert.5
Results of 4 double-blind, placebo-controlled clinical trials of donepezil, involving more than 1,900 individuals with mild to moderate AD, have been published recently. In all, significant improvements in cognition were observed consistently for both therapeutic doses of donepezil (5 mg/d and 10 mg/d), relative to placebo. Similar benefits were reported for global functioning.
The long-term clinical efficacy and safety of donepezil versus placebo across 1 year in patients with mild to moderate AD was investigated.14 The Gottfries-Brane-Steen global assessment for rating dementia symptoms demonstrated the benefit of donepezil over placebo at weeks 24, 36, and 52, and at the study end point. Advantages of donepezil were also observed in cognition and ADL.
Donepezil also appears to work for patients with moderate to severe AD. In a recent 24-week study,15 patients receiving donepezil showed benefits on the Clinician’s Interview-Based Impression of Change with Caregiver Input (CIBIC+), compared with placebo, at all visits up to week 24 and at the study’s end point. All other secondary measures showed significant differences between the groups in favor of donepezil at the end of the study. These data suggest that donepezil’s benefits extend into more advanced stages of AD than those previously investigated, with good tolerability.
Clinical trials of rivastigmine (1.5-6 mg twice daily PO) have also demonstrated benefits on cognitive and global measures.16 The efficacy of rivastigmine tartrate (ENA 713) in patients with mild to moderately severe Alzheimer’s disease was evaluated in a 26-week open-label extension of a 26-week, double-blind, placebo-controlled study. By 52 weeks, patients originally treated with rivastigmine 6 to 12 mg/d had significantly better cognitive function than did patients originally treated with placebo.17- 19
Donepezil is a second-generation, piperidine-class, selective and reversible acetylcholinesterase (AChE) inhibitor. It is structurally dissimilar from other established AChE inhibitors.
Experimentally, donepezil inhibits AChE activity in human erythrocytes and increases extracellular acetylcholine levels in the cerebral cortex and the hippocampus of the rat. Pharmacologically, donepezil has a half-life of approximately 70h, lending itself to once-daily administration.11
Rivastigmine (ENA 713, or carbamoylatine) is an AChE inhibitor with brain-region selectivity and a long duration of action. Both preclinical studies and studies in human volunteers have shown that rivastigmine induces substantially greater inhibition of AChE in the central nervous system compartment than it does in the periphery (40% inhibition of central AChE compared with 10% inhibition of plasma butylcholinesterase in healthy volunteers). Rivastigmine also preferentially inhibits the G1 enzymatic form of AChE, which predominates in the brains of patients with Alzheimer’s disease (AD).
Evidence from animal studies also suggests that rivastigmine is a more potent inhibitor of AChE in the cortex and hippocampus, the brain regions most affected by AD. The principal metabolite of rivastigmine has at least 10-fold lower activity against AChE compared with the parent drug.
Rivastigmine is completely metabolized; the major route of elimination of the metabolites is renal. Rivastigmine is inactivated during the process of interacting with and inhibiting AChE, and, in contrast to other AChE inhibitors, the hepatic cytochrome P-450 (CYP-450) system is not involved in the metabolism of rivastigmine.12
Galantamine is an allosterically potentiating ligand that modulates nicotinic cholinergic receptors (nAChR) to increase acetylcholine release as well as acting as an AChE inhibitor. In preclinical experiments, the drug significantly improves learning, reduces AChE levels, and increases nicotinic receptor binding. Action of galantamine is on the most abundant nAChR in the human brain, the alpha4/beta2subtype.13
Clinical trials of galantamine (4-12 mg/bid PO) have demonstrated similar benefits.20 Following a 4-week placebo run-in, patients were randomized to 1 of 4 treatment arms: placebo or galantamine escalated to final maintenance dosages of 8, 16, or 24 mg/d for a 5-month treatment phase. At study’s end, the galantamine-place-bo differences on the cognitive subscale of the AD Assessment Scale were 3.3 points for the 16 mg/d group and 3.6 points for the 24 mg/d group. Treatment discontinuations due to adverse events were low in all galantamine groups (6% to 10%) and comparable with that in the placebo group (7%). The incidence of adverse events in the galantamine groups, notably gastrointestinal symptoms, was low and most adverse events were mild.
Other studies examining galantamine have demonstrated similar clinical benefits.8, 21
When using AChE inhibitors, the slope of cognitive decline is similar in treated and untreated patients after the initial improvement. These drugs essentially do not reverse the disorder’s course but shift upward the curve describing the time course of cognitive decline. This applies also to behavioral and functional benefits. Thus the benefit obtained is symptomatic and not neuroprotective, and is lost after discontinuing the medications.
Effects on functioning and behavior
In a 24-week multinational clinical trial, patients receiving donepezil (10 mg/d) were more able than placebo-treated patients to perform complex daily functioning tasks.22 Similar effects have been observed with rivastigmine and galantamine.16, 20
All 3 AChE inhibitors have demonstrated improvements in the behavioral changes associated with AD. Cummings et al23 tested the hypothesis that behavioral disturbances are reported at significantly lower rates by caregivers of AD patients receiving donepezil, compared with a group of patients not receiving a drug for treatment of dementia. Donepezil patients were described as significantly less likely to be threatening, destroy property, and talk loudly, and significantly fewer were treated with sedatives.
Table 1
COMPARING THEAChE INHIBITORS
| Donepezil | Rivastigmine | Galantamine | |
|---|---|---|---|
| Chemical class | Piperidine | Carbamate | Phenanthrene alkaloid |
| AChE inhibitor | Yes | Yes | Yes |
| BuChE inhibitor | Small | Yes | Small |
| Nicotinic modulation | No | No | Yes |
| Elimination half-life | 50-70 h | 0.6-2 h | 5-7 h |
| Administration | Once daily | Twice daily | Twice daily |
| Starting dosage | 5 mg/d | 1.5 mg bid | 4 mg bid |
| Total recommended dosage | 5-10 mg/d | 6-12 mg/d | 16-24 mg/d |
| Adapted from Conn DK. Cholinesterase inhibitors: Comparing the options for mild-to-moderate dementia. Geriatrics56: 56-57, 2001. | |||
An open-label study by Weiner et al24 examined the effects of donepezil on emotional and behavioral symptoms using the CERAD Behavior Rating Scale for Dementia and its subscales. In a group of 25 AD patients treated with donepezil, scores returned to baseline levels at 12 months. In contrast, the scores of the reference group worsened minimally.
Galantamine has also proved effective in treating behavioral symptoms associated with AD. In the Tariot et al20 study, galantamine at 16 mg/d and 24 mg/d had a significantly better outcome on CIBIC+, ADL, and behavioral symptoms versus placebo.
Rosler et al25 assessed the ability of rivastigmine to improve behavioral symptoms in AD. Using the behavioral component of the CIBIC+, results showed that long-term treatment with rivastigmine could slow the progression of symptoms. Symptoms showing stabilization included aggressiveness, activity disturbances, hallucinations, and paranoid features. The results also suggest that patients treated earlier with rivastigmine may attain a greater benefit than those whose treatment is delayed 6 months.
Rivastigmine also has significant effects on controlling behavioral symptoms in patients with Lewy body dementia.26 A placebo-controlled, double-blind, multicenter study was performed in 120 patients with Lewy body dementia. Individuals were given up to 12 mg/d rivastigmine or placebo for 20 weeks, followed by 3 weeks rest. Assessment by neuropsychiatric inventory was made at baseline, and again at weeks 12, 20, and 23.
Patients taking rivastigmine were significantly less apathetic and anxious than those on placebo, and had fewer delusions and hallucinations. Almost twice as many patients on rivastigmine as on placebo (37, 63% versus 18, 30%) showed at least a 30% improvement from baseline. In a computerized cognitive assessment system and neuropsychological tests, patients were significantly faster and better than those on placebo, particularly when performing tasks with a substantial attentional component.
Cost effectiveness
Numerous studies have demonstrated that AChE inhibitors are cost savers in AD treatment. Fillit et al27 examined the impact of donepezil in a multisite managed care organization for 2 years using claims data for 70 individuals with AD and related dementias. The median per diem medical costs were $1.22 lower post treatment than they were in the pretreatment phase. Moreover, posttreatment costs were reduced in 6 of 7 service settings, with median per diem savings of $0.77 in outpatient care and $0.65 in office visits.
Neumann et al28 utilized cost-effectiveness analysis to predict that for mild AD, donepezil would pay for itself in cost offsets if the drug’s effect exceeds 2 years. Donepezil costs were partially offset by a reduction in the costs of care due to enhanced cognitive functioning and the delay in placing the patient in more costly disease stages and settings.
One study used the disease-progression model to estimate potential per-patient savings resulting from the treatment of AD in Canada.29 Rivastigmine was estimated to delay the transition to more severe stages of AD by up to 188 days for patients with mild AD after 2 years of treatment. For patients with mild-to-moderate and moderate disease, this delay was estimated to be 106 and 44 days, respectively.
The Assessment of Health Economics in Alzheimer’s Disease model uses algorithms to predict the time until patients with AD require full-time care. A study, performed in Canada, compared treatment with galantamine to treatment without pharmacological interventions.30 Galantamine was predicted to reduce the duration of full-time care by almost 10%. Approximately 5.6 patients with mild-to-moderate disease must be placed on treatment to avoid 1 year of full-time care, resulting in savings averaging $528 per patient. For patients with moderate disease, 3.9 patients must be placed on treatment to avoid 1 year of full-time care, with savings predicted at $2,533 per patient.
Caregiving burden
Fillit et al addressed caregiver well-being in a self-administered, nationwide survey of AD caregivers. Caregivers of patients treated with donepezil (n = 274) were compared with caregivers of patients not treated with donepezil (n = 274).31 The Caregiver Burden Scale measured time demands and distress linked to commonly performed caregiving tasks. Donepezil caregivers reported significantly lower scores on difficulty of caregiving. Similar findings have been observed with galantamine.32
Related resources
- Bullock R. New drugs for Alzheimer’s disease and other dementias. Br J Psychiatry. 2002;180(2):135-139.
- Brodaty H, Ames D, et al. Pharmacological treatment of cognitive deficits in Alzheimer’s disease. Med J Aust. 2001;175(6):324-329.
- Ahmed MB. Alzheimer’s disease: recent advances in etiology, diagnosis, and management. Tex Med. 2001;97(12):50-58.
- Frisoni GB. Treatment of Alzheimer’s disease with acetyl-cholinesterase inhibitors: bridging the gap between evidence and practice. J Neurol. 2001;248(7):551-7.
Drug brand names
- Tacrine • Cognex
- Donepezil • Aricept
- Rivastigmine • Exelon
- Galantamine • Reminyl
Disclosure
The author reports that he receives grant/research support from, serves as a consultant to, and is on the speaker’s bureau of Janssen Pharmaceutica and Pfizer Inc., and serves as consultant to and is on the speaker’s bureau of Novartis Pharmaceuticals Corp.
1. Irizarry M, Hyman B. Alzheimer’s disease. In: Principles of neuroepidemiology. Batchelor T, Cudkowicz M, eds. Boston: Butterworth-Heinemann, 2001:69-98.
2. Mesulam MM, Mufson EJ, Levey AI, Warner BH. Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983a;214:170-197.
3. Drachman DA, Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30:113-121.
4. Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403.-
5. Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122-126.
6. Higgins JPT, Flicker L. Lecithin and cognitive impairment. The Cochrane Database of Systematic Reviews. 2001.;
7. Knapp MJ, Knopman DS, Solomon PR, Pendlebury WW, Davis CS, Gracon SI. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer’s disease. The Tacrine Study Group. JAMA. 1994;271:985-991.
8. Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000;54:2262-2268.
9. Giacobini E. Cholinesterase inhibitors stabilize Alzheimer’s disease. Methods Find Exp Clin Pharmacol. 2000;22:609-613.
10. Irizarry MC, Hyman BT. Alzhemier Disease Therapeutics. J Neuropathology Exp Neurology. 2001;60:923-928.
11. Wilkinson DG. The pharmacology of donepezil: a new treatment of Alzheimer’s disease. Expert Opin Pharmacother. 1999;1:121-135.
12. Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetyl-cholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin Ther. 1998;20:634-647.
13. Samochocki M, Zerlin M, Jostock R, et al. Galantamine is an allosterically potentiating ligand of the human alpha4/beta2 nAChR. Acta Neurol Scand. 2000(Suppl);;176:68-73.
14. Winblad B, Engedal K, Soininen H, Verhey F, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489-495.
15. Feldman H, Gauthier S, Hecker J, et al. Donepezil MSAD Study Investigators Group.A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease. Neurology. 2001;57:613-620.
16. Farlow M, Anand R, Messina J, Jr, Hartman R, Veach J. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s disease. Eur Neurol. 2000;44:236-241.
17. Rogers S, Friedhoff L. Donepezil Study Group.The efficacy and safety of donepezil in patients with Alzhemier’s disease. Dementia. 1996;7:293-303.
18. Rogers S, Farlow M, Doody R, Morris R, Friedhoff L. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50:136-145.
19. Rosler M, Anand R, Cicin-Sain A, Gauthier S, Agid Y, DalBianco P, Stahelin HB, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: International randomized controlled trial. BMJ. 1999;318:633-638.
20. Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C. A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology. 2000;54:2269-2276.
21. Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: Multicentre randomized controlled trial. Galantamine International - 1 Study Group. BMJ. 2000;321:1445-1449.
22. Knopman DS. Management of cognition and function: new results from the clinical trials programme of Aricept (R) (donepezil Hcl). Int J Neuropsychopharmacol. 2000;3:13-20.
23. Cummings JL, Donohue JA, Brooks RL. The relationship between donepezil and behavioral disturbances in patients with Alzheimer’s disease. Am J Geriatr Psychiatry. 2000;8:134-140.
24. Weiner MF, Martin-Cook K, Foster BM, Saine K, et al. Effects of donepezil on emotional/behavioral symptoms in Alzheimer’s disease patients. J Clin Psychiatry. 2000;487-492.
25. Rosler M, Retz W, Retz-Junginger P, Dennler HJ. Effects of two-year treatment with the cholinesterase inhibitor rivastigmine on behavioural symptoms in Alzheimer’s disease. Behav Neurol. 1998;11:211-216.
26. McKeith I, Del Ser T, Spano P, Emre M, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
27. Fillit H, Gutterman EM, Lewis B. Donepezil use in managed Medicare: effect on health care costs and utilization. Clin Ther. 1999;21:2173-2185.
28. Neumann PJ, Hermann RC, Kuntz KM, Araki SS, Duff SB, Leon J, Berenbaum PA, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer’s disease. Neurology. 1999;52:1115-1116.
29. Hauber AB, Gnanasakthy A, Mauskopf JA. Savings in the cost of caring for patients with Alzheimer’s disease in Canada: an analysis of treatment with rivastigmine. Clin Ther. 2000;22:439-451.
30. Getsios D, Caro JJ, Caro G, Ishak K. The AHEAD Study Group. Assessment of health economics in Alzheimer’s disease (AHEAD): galantamine treatment in Canada. Neurology. 2001;57:972-978.
31. Fillit HM, Gutterman EM, Brooks RL. Impact of donepezil on caregiving burden for patients with Alzheimer’s disease. Int Psychogeriatr. 2000;12(3):389-401.
32. Blesa R. Galantamine: therapeutic effects beyond cognition. Dement Geriatr Cogn Disord. 2000;11(Suppl)1:28-34.
1. Irizarry M, Hyman B. Alzheimer’s disease. In: Principles of neuroepidemiology. Batchelor T, Cudkowicz M, eds. Boston: Butterworth-Heinemann, 2001:69-98.
2. Mesulam MM, Mufson EJ, Levey AI, Warner BH. Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983a;214:170-197.
3. Drachman DA, Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30:113-121.
4. Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403.-
5. Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122-126.
6. Higgins JPT, Flicker L. Lecithin and cognitive impairment. The Cochrane Database of Systematic Reviews. 2001.;
7. Knapp MJ, Knopman DS, Solomon PR, Pendlebury WW, Davis CS, Gracon SI. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer’s disease. The Tacrine Study Group. JAMA. 1994;271:985-991.
8. Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000;54:2262-2268.
9. Giacobini E. Cholinesterase inhibitors stabilize Alzheimer’s disease. Methods Find Exp Clin Pharmacol. 2000;22:609-613.
10. Irizarry MC, Hyman BT. Alzhemier Disease Therapeutics. J Neuropathology Exp Neurology. 2001;60:923-928.
11. Wilkinson DG. The pharmacology of donepezil: a new treatment of Alzheimer’s disease. Expert Opin Pharmacother. 1999;1:121-135.
12. Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetyl-cholinesterase inhibitor for the treatment of Alzheimer’s disease. Clin Ther. 1998;20:634-647.
13. Samochocki M, Zerlin M, Jostock R, et al. Galantamine is an allosterically potentiating ligand of the human alpha4/beta2 nAChR. Acta Neurol Scand. 2000(Suppl);;176:68-73.
14. Winblad B, Engedal K, Soininen H, Verhey F, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489-495.
15. Feldman H, Gauthier S, Hecker J, et al. Donepezil MSAD Study Investigators Group.A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease. Neurology. 2001;57:613-620.
16. Farlow M, Anand R, Messina J, Jr, Hartman R, Veach J. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s disease. Eur Neurol. 2000;44:236-241.
17. Rogers S, Friedhoff L. Donepezil Study Group.The efficacy and safety of donepezil in patients with Alzhemier’s disease. Dementia. 1996;7:293-303.
18. Rogers S, Farlow M, Doody R, Morris R, Friedhoff L. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50:136-145.
19. Rosler M, Anand R, Cicin-Sain A, Gauthier S, Agid Y, DalBianco P, Stahelin HB, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: International randomized controlled trial. BMJ. 1999;318:633-638.
20. Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C. A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology. 2000;54:2269-2276.
21. Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: Multicentre randomized controlled trial. Galantamine International - 1 Study Group. BMJ. 2000;321:1445-1449.
22. Knopman DS. Management of cognition and function: new results from the clinical trials programme of Aricept (R) (donepezil Hcl). Int J Neuropsychopharmacol. 2000;3:13-20.
23. Cummings JL, Donohue JA, Brooks RL. The relationship between donepezil and behavioral disturbances in patients with Alzheimer’s disease. Am J Geriatr Psychiatry. 2000;8:134-140.
24. Weiner MF, Martin-Cook K, Foster BM, Saine K, et al. Effects of donepezil on emotional/behavioral symptoms in Alzheimer’s disease patients. J Clin Psychiatry. 2000;487-492.
25. Rosler M, Retz W, Retz-Junginger P, Dennler HJ. Effects of two-year treatment with the cholinesterase inhibitor rivastigmine on behavioural symptoms in Alzheimer’s disease. Behav Neurol. 1998;11:211-216.
26. McKeith I, Del Ser T, Spano P, Emre M, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036.
27. Fillit H, Gutterman EM, Lewis B. Donepezil use in managed Medicare: effect on health care costs and utilization. Clin Ther. 1999;21:2173-2185.
28. Neumann PJ, Hermann RC, Kuntz KM, Araki SS, Duff SB, Leon J, Berenbaum PA, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer’s disease. Neurology. 1999;52:1115-1116.
29. Hauber AB, Gnanasakthy A, Mauskopf JA. Savings in the cost of caring for patients with Alzheimer’s disease in Canada: an analysis of treatment with rivastigmine. Clin Ther. 2000;22:439-451.
30. Getsios D, Caro JJ, Caro G, Ishak K. The AHEAD Study Group. Assessment of health economics in Alzheimer’s disease (AHEAD): galantamine treatment in Canada. Neurology. 2001;57:972-978.
31. Fillit HM, Gutterman EM, Brooks RL. Impact of donepezil on caregiving burden for patients with Alzheimer’s disease. Int Psychogeriatr. 2000;12(3):389-401.
32. Blesa R. Galantamine: therapeutic effects beyond cognition. Dement Geriatr Cogn Disord. 2000;11(Suppl)1:28-34.
Treatment-resistant depression: Newer alternatives
As many as 50% of depressed patients do not achieve a 50% or greater reduction in severity of symptoms after an adequate antidepressant trial.1 Moreover, among those who do respond to acute treatment, longer-term residual depressive symptoms are quite common. Persistent subsyndromal depressive symptoms are associated with impaired psychosocial functioning and increased risk of relapse. This possibility has reinforced an evolving consensus that full depressive remission rather than response is the proper goal of treatment.2
When your patient’s depressive symptoms fail to remit following an initial pharmacotherapy course, you must decide which of numerous possible next steps to pursue, in what combinations, and following what sequence. The range of strategies include the following:
- Pursuing an extended trial with the initial agent using higher than usual dosages (e.g., fluoxetine 40 to 80 mg/qd);
- Augmenting an antidepressant with an agent that offers no established antidepressant efficacy on its own, such as buspirone, lithium, thyroid, or estrogen;
- Combining an antidepressant with another antidepressant, with another somatic therapy such as electroconvulsive treatment or phototherapy, or with some form of psychotherapy;
- Switching to an antidepressant within the same class (e.g., from one selective serotonin reuptake inhibitor [SSRI] to another), or outside of class (from an SSRI to an atypical antidepressant), or switching to non-pharmacological somatic therapy or psychotherapy.3
A predictable lag exists between innovative clinical applications and the randomized, controlled trials (RCTs) designed to evaluate them. The result: Approaches that are thoroughly described in the literature involve medication regimens that are no longer first-line, such as lithium or thyroid augmentation of tricyclic antidepressants (TCAs), or the combination of TCAs with SSRIs. Conversely, approaches most widely used in current psychiatric practice (e.g., addition of bupropion to SSRIs) have received relatively little systematic attention.4
Further, while predictors of initial antidepressant response have been hard to come by, even less is known about predictors of antidepressant response after lack of response to previous antidepressants. The working hypotheses that clinicians use to decide which strategies to pursue, though plausible, are largely untested, whether based on characteristics of a patient’s initial response (e.g., non-response or partial response)5 or on side effects or comorbid diagnoses.
This review will describe new research and emerging strategies that address the common clinical problem of unremitted depression despite one or more adequate courses of antidepressant treatment.
A look at new augmentations and combinations
Adding a second agent is an appealing strategy when patients have tolerated an initial antidepressant without troublesome side effects or have shown partial response (≥ 25% and <50% symptom reduction) and/or when a second agent may serve an important additional goal such as treating a comorbid condition—attention-deficit disorder or smoking, for example—or a side effect (e.g., nausea or insomnia).
Evidence supporting a relation between folate and depression has accrued over decades. Low folate levels may be associated with poorer response to fluoxetine.
Coppen and Bailey25 found that depressed patients had higher initial response rates to fluoxetine when combined with supplemental folic acid (500 mcg) than they did with placebo. The effect was significant only among female subjects. Study participants were naïve to fluoxetine and did not have established treatment resistance.
Still, the safety, tolerability, cost-effectiveness, and high patient acceptability of folic acid should stimulate further study of augmentation with folic acid supplementation and other naturally occurring agents related to folate in the one-carbon cycle, including S-adenosyl-methionine (SAMe).
The primary drawbacks to augmentation/combination strategies are an increased risk of drug interactions, cost, and potential decrement in adherence to treatment as the regimen’s complexity increases.
Thyroid augmentation Although thyroid augmentation of TCAs has been the subject of numerous RCTs, no controlled trials of thyroid augmentation of SSRIs or other newer-generation antidepressants are available.
Lithium augmentation Studies of lithium augmentation of SSRIs have yielded generally modest response rates with questionable durability.6,7
Noradrenergic and/or dopaminergic agonist agents Open trials and case series in the treatment of patients with unremitted depression have supported combining SSRIs and venlafaxine with agents that possess primarily noradrenergic and/or dopaminergic agonist properties. These include bupropion,8 psychostimulants,9 and direct dopamine agonists, including pergolide and pramipexole.10
These combinations have the advantage of potentially ameliorating several common SSRI side effects, particularly sedation, sexual dysfunction, and putative SSRI-related apathy. In the case of psychostimulants and/or bupropion, the combinations may treat such common comorbidities as attention-deficit/hyperactivity disorder and smoking.
Modafinil The combination of the antinarcoleptic modafinil with SSRIs and other newer-generation antidepressants has also attracted interest. Its efficacy as an antidepressant adjunct and as a remedy for side effects must still be established, however.11
Buspirone augmentation The augmentation of SSRIs and other antidepressants with the antianxiety azaperone buspirone is supported by impressive response rates in several open trials. But the only placebo-controlled trial evaluating buspirone augmentation in resistant depression failed to find a significant drug:placebo difference.12 Still, its tolerability and anxiolytic efficacy, and potential for ameliorating sexual dysfunction in some patients, support its judicious use as an augmenting agent pending further study.
Pindolol Studies of this beta-agonist, 5HT-1A antagonist, as an antidepressant-augmenting combination have yielded promising results in some studies, including a negative 10-day, placebo-controlled trial.13 Some patients experience jitteriness or irritability on pindolol. Its role in treating resistant depression remains to be established.
Noradrenergic TCAs The combination of SSRIs with these agents is a good example of a strategy based on pairing complimentary antidepressant mechanisms. Double-blind, controlled trials of fluoxetine plus desipramine6,7 have tempered the enthusiasm generated by earlier open studies, however. Response rates in these trials range from 25% to 30%, no higher than lithium augmentation and slightly lower than higher-dose fluoxetine.
Mirtazapine A more recent combination is mirtazapine with SSRIs or with high-dose venlafaxine.14 In some patients, this combination may attenuate sexual dysfunction, insomnia, and gastrointestinal side effects of SSRIs and venlafaxine by virtue of a 5-HT2, 5-HT3, and histamine receptor blockade by mirtazapine. This combination may also capitalize on the combined antidepressant effects of direct norepinephrine (NE) and serotonin (5-HT) reuptake inhibition as well as an alpha2 adrenergic auto- and hetero-receptor blockade—facilitating presynaptic NE and 5HT release—and 5-HT2 receptor antagonism.
Antipsychotics The use of antipsychotics for nonpsychotic unipolar depression has been controversial. However, in the context of lower apparent risks of tardive dyskinesia with the newer, atypical antipsychotics, their use as antidepressant augmenting agents has been revisited. In one double-blind, placebo-controlled trial, the combination of fluoxetine plus olanzapine was more effective in a well-defined refractory depressed population than was either medication alone.15 (See Box 1 for a discussion of fluoxetine and folic acid.)
Ostroff and Nelson16 reported an extremely rapid (1 week) response after the addition of risperidone among 8 depressed individuals who had not responded to fluoxetine or paroxetine alone. The activating properties of ziprasadone for some patients, combined with its lower propensity for producing weight gain than other atypical antipsychotics, make it another potentially attractive candidate for antidepressant augmentation, though one that requires further study.
Anticonvulsant augmentation While controlled studies of anticonvulsant augmentation of newer-generation antidepressants are lacking, the efficacy of lamotrigine for treating bipolar depression17 has encouraged clinicians to combine the agent with antidepressants in unipolar depression. The potential sedative/anxiolytic effects of other anticonvulsants, including gabapentin and valproate, have also supported their use in resistant depression complicated by anxiety or irritability. Omega-3-fatty acids may work as mood-stabilizing substances when used for antidepressant augmentation.
Antidepressant switches: In class or out?
Switching from TCAs to SSRIs or vice versa following nonresponse has yielded generally high (50% to 60%) response rates.3 But the more common question now is whether to switch from an initial SSRI to another SSRI, to an SSRI-like agent with an additional noradrenergic mechanism (such as venlafaxine), or to an atypical antidepressant such as bupropion or mirtazapine.
Traditional teaching and regular psychiatric practice5 have favored a switch outside of class. More recently, however, a number of uncontrolled studies looking at response rates to a second SSRI following inadequate response to and/or tolerance of an initial SSRI have shown response rates of 40% to 75%.18 This coincides with response rates seen in studies involving crossover to antidepressants with differing mechanisms. Inferences from these studies are limited by, first, determining nonresponse retrospectively and, second, lumping together patients who did not remain on the first SSRI because of nonresponse with others who curtailed treatment because of intolerance.
Because of its dual 5-HT/NE reuptake inhibitory activity, particularly at higher doses, venlafaxine is a popular choice following failure of an initial SSRI. One-third of subjects with extremely resistant depression (3 or more trials of antidepressants) responded to venlafaxine.19 DeMontigny et al20 also demonstrated its efficacy (with a 58% response rate) as a switching agent among resistant, depressed patients.
But comparative response rates for switching to venlafaxine from an SSRI vs. from other antidepressant classes have not been well delineated, nor have response rates for switching from SSRIs and other antidepressants to venlafaxine vs. to other non-SSRI agents.
Switches from SSRIs to nefazodone and mirtazapine or the selective NE reuptake inhibitor reboxetine have shown reasonably high response rates.3 Studies comparing these various strategies head to head are lacking. In the absence of data, reasonable guides for switching agents include projected tolerability and existence of comorbid conditions that may respond well to one agent over another.
Another issue is whether to cross-taper, that is, to lower the dose of the first agent while titrating up the next, or to discontinue the first before the second. When catastrophic drug interactions would result from overlap, such as the switch from an SSRI to a monoamine oxidase (MAO) inhibitor, a suitable wash-out period is necessary. This can also occur if the first antidepressant is believed to cause intolerable side effects.
In most other instances where no absolute contraindications exist, cross-tapering may be worthwhile despite theoretical risks such as serotonin syndrome when overlapping an SSRI and venlafaxine. Cross-tapering helps reduce the risk of side effects associated with abrupt discontinuation including nausea, myalgias, headache, and dysphoria, and may minimize the loss of antidepressant benefit from the initial agent before the impact of the second agent is realized.
Great hope for new knowledge with STAR*D
In recognition of the substantial direct and indirect costs, the morbidity and mortality associated with unremitted depression, and the considerable gaps in knowledge about optimal management at key decision points, the National Institute of Mental Health (NIMH) has launched the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. This is a multisite, multistep, prospective, randomized clinical trial of nonpsychotic major depressive disorder.21
STAR*D represents an initiative unique in the history of depression trials. Its novel “equipoise stratified” design26 is a hybrid between strict randomization to treatments without input from patients or clinicians (completely randomized designs) and a form of randomization in which subjects are assigned only to overarching strategies within which the particular treatments are determined by the clinician (clinician’s choice designs).
In STAR*D, a patient is asked at each level to define with his or her clinician all treatments that would be equally acceptable, based on a preference for certain potential side effects over others, interest in psychotherapy, or desire to retain a medication to which the patient has responded at least partially.
To be eligible to continue, however, the patient must agree to be randomized across at least some of the options testing some of the key hypotheses. For example, a participant at Level 2 who specifically wished to pursue cognitive therapy could not do that alone, but could agree to randomization to cognitive therapy with or without citalopram.
The study seeks to balance the goal of recruiting and retaining a broadly representative cross-section of patients—many of whom would reasonably expect to have some say about their treatment with their prescribing clinician—with the goal of providing statistically rigorous, randomization-based inferences upon which meaningful treatment guidelines can be based.
Approximately 4,000 adult depressed outpatients without an established history of antidepressant resistance will be recruited across the country. In contrast to efficacy trials, this effectiveness trial is meant to more closely parallel real-world practice (Box 2).
Patients will be treated initially with citalopram for 12 weeks (Level 1), with general guidelines for titration based upon response and tolerability. It is anticipated that about 2,000 individuals will exhibit unremitting depression despite SSRI treatment and/or intolerance to the SSRI and will be eligible for randomization to subsequent “switching” or “augmentation” strategies; this forms the core of the study across 3 successive levels.
Level 2 randomized treatments include four distinctive switching options—sertraline, bupropion, venlafaxine, or cognitive therapy—as well as 3 augmentation options—citalopram plus bupropion, buspirone, or cognitive therapy.
Those whose depression fails to remit will be eligible for randomization at Level 3 to different switching options—mirtazapine vs. nortripyline, or augmentation options—lithium or thyroid—added to the primary Level 2 antidepressant.
Finally, at Level 4, patients who continue to have unremitted depression despite successive treatments will be eligible for random assignment to 1 of 2 switching options—tranylcypromine vs. the combination of mirtazapine and venlafaxine.
Two new somatic treatments
Repetitive Transcranial Magnetic Stimulation (rTMS) rTMS is used to induce electrical current in the brain without causing seizures. Using a rapidly alternating current with a hand-held electromagnet that generates about 2 tesla will, in turn, induce or interrupt current (depolarization) in cortical interneurons about 2 cm below the surface of the skull. rTMS delivers energy without the impedance of the skull and is thought to be excitatory at high frequencies (20 Hz) and inhibitory at lower frequencies (5 to 10 Hz). This theory remains to be rigorously tested.22
Overall, the clinical effects reported in early studies on rTMS have been modest at best, while later studies have found either no difference from placebo or a robust effect. rTMS is a potential therapeutic tool not yet ready for widespread clinical use.22
Vagus Nerve Stimulation The NeuroCybernetic Prosthesis System (Cyberonics, Inc., Houston, Tex) stimulates the left cervical vagus nerve for treatment of resistant partial-onset epileptic seizures.
An open treatment trial of vagus nerve stimulation (VNS) for treatment-resistant depression was done based on mood improvement observed in patients treated for seizure disorders. Positron-emission tomographic studies revealed activation of limbic structures and neurochemical and neuronal pathway findings.23 The investigators reported improvement in a sample of 30 patients with major depressive disorder. Definite conclusions await controlled clinical trials and more work is needed to determine the ultimate mechanisms that might be antidepressant.23,24
Related resources
- Amsterdam JD, Hornig M, Nierenberg AA. Treatment-resistant mood disorders. Cambridge, UK: Cambridge University Press; 2001.
- Stahl SM. Essential Psychopharmacology. Cambridge, UK: Cambridge University Press; 1996.
- STAR*D Web site: http://www.edc.gsph.pitt.edu/stard
Drug brand names
- Bupropion • Wellbutrin, Wellbutrin SR, Zybar
- Buspirone • BuSpar, BuSpar DIVIDOSE
- Citalopram • Celexa
- Fluoxetine • Prozac, Prozac Weekly
- Gabapentin • Neurontin
- Haloperidol • Haldol, Haldol Decanoate
- Lamotrigine • Lamictal
- Mirtazapine • Remeron, Remeron Soltab
- Modafinil • Provigil
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Pergolide • Permax
- Pramipexole • Mirapex
- Reboxetine • Edronax
- Risperidone • Risperidal
- S-adenosyl-methionine • SAM-e
- Sertaline • Zoloft
- Tranycypromine • Nardil, Parnate, Marplan
- Valproate • Epival, Deprox, Alti-Valproic
- Venlafaxine • Effexor, Effexor X12
- Ziprasadone • Geodon
Disclosure
Dr. Alpert receives research funding, speaking honoraria or consultation fees from Organon, Forest Pharmaceuticals, GlaxoSmithKline, Pfizer Inc., and Pharmavite.
Dr. Nierenberg receives research funding, speaking honoraria, or consultation fees from Eli Lilly and Co., Wyeth-Ayerst Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Innapharma, BMS, Cyberonics, Lichtner, and Pfizer Inc.
1. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. The Psychiatric Clinics of North America: Treatment Resistant Depression. 1997;19(2):179-200.
2. Nierenberg AA, Wright EC. Evolution of remission as the new standard in the treatment of depression. J Clin Psychiatry. 1999;60(22):7-11.
3. Rosenbaum JF, Fava M, Nierenberg AA, Sachs GS. Treatment-resistant mood disorders. In Gabbard GO, ed. Treatments of Psychiatric Disorders. 3rd ed. Washington, DC: American Psychiatric Press, Inc.; 2001;1307-1384.
4. Mischoulon D, Fava M, Rosenbaum JF. Strategies for augmentation of SSRI treatment: A survey of an academic psychopharmacology practice Harvard Review of Psychiatry. 1999;6:322-326.
5. Fredman SJ, Fava M, Kienke AS, et al. Partial response, non-response, and relapse on SSRIs in major depression: A survey of current “next-step” practices. J Clin Psychiatry. 2000;61:403-407.
6. Fava M, Rosenbaum JF, McGrath PJ, et al. A double-blind, controlled study of lithium and tricyclic augmentation of fluoxetine in treatment resistant depression. Am J Psychiatry. 1994;151:1372-1374.
7. Fava M, Alpert JE, Nierenberg AA, Worthington JJ, III, Rosenbaum JF. Double-blind study of high-dose fluoxetine versus lithium or desipramine augmentation of fluoxetine in partial and nonresponders to fluoxetine. 153rd Annual Meeting of the American Psychiatric Association. Chicago, Ill, 2000.
8. Bodkin JA, Lasser RA, Wines JD, Jr, et al. Combining serotonin reuptake inhibitors and bupropion in partial responders to antidepressant monotherapy. J Clin Psychiatry. 1997;58:137-145.
9. Stoll AL, Pillay SS, et al. Methylphenidate augmentation of serotonin selective reuptake inhibitors: a case series. J Clin Psychiatry. 1996;57(8):356-359.
10. Sporn J, Ghaemi SN, Sambur MR, Rankin MA, Recht J, Sachs GS, Rosenbaum JF, Fava M. Pramipexole augmentation in the treatment of unipolar and bipolar depression: A retrospective chart review. Ann Clin Psychiatry. 2000;12:137-140.
11. Menza MA, Kaufman KR, Castellanos A. Modafinil augmentation of antidepressant treatment in depression. J Clin Psychiatry. 2000;61(5):378-81.
12. Landen M, Bjorling G, Agren H, Fahlen T. A randomized double-blind placebo-controlled trial of buspirone in combination with an SSRI in patients with treatment-refractory depression. J Clin Psychiatry. 1998;59:664-668.
13. Perez V, Soler J, Puigdemont D, et al. A double-blind, randomized, placebo-controlled trial of pindolol augmentation in depressive patients resistant to serotonin reuptake inhibitors. Arch Gen Psychiatry. 1999;56:375-379.
14. Carpenter LL, Jocic Z, Hall JM, Rasmussen SA, Price LH. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry. 1999;60(1):45-49.
15. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry. 2001;158:131-134.
16. Ostroff RB, Nelson JC. Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry. 1999;60:256-259.
17. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry. 1999;60:79-88.
18. Thase ME, Blomgren SL, Birkett MA, et al. Fluoxetine treatment of patients with major depressive disorder who failed initial treatment with sertraline. J Clin Psychiatry 1997;58:16-21.
19. Nierenberg AA, Feighner JP, Rudolph R, et al. Venlafaxine for treatment-resistant unipolar depression. J Clin Psychopharmacol. 1994;14:419-423.
20. de Montigny C, Silverstone PH, et al. Venlafaxine in treatment-resistant major depression: a Canadian multicenter, open-label trial. J Clin Psychopharmacol. 1999;19(5):401-406.
21. Rush JA, Fava M, et al. Sequenced treatment alternatives to relieve depression (STAR*D): Rationale and design. Controlled Clinical Trials. 2001 (in press).
22. Sackeim H. Repetitive transcranial magnetic stimulation: What are the next steps? Biol Psychiatry. 2000;48:959-961.
23. Rush AJ, George MS, Sackeim HA, et al. Vagus Nerve Stimulation (VNSTM) for treatment-resistant depressions: A multicenter study. Biol Psychiatry 2000;47.-
24. Rosenbaum JF, Heninger G. Vagus nerve stimulation for treatment-resistant depression. BiolPsychiatry. 2000;47(4):273-275.
25. Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affective Disord. 2000;60:121-130.
26. Lavori PW, Rush JA, Wisniewski SR, et al. Strengthening clinical effectiveness trials: Equipose-stratified randomization. Biol Psychiatry. 2001;50(10):792-801.
As many as 50% of depressed patients do not achieve a 50% or greater reduction in severity of symptoms after an adequate antidepressant trial.1 Moreover, among those who do respond to acute treatment, longer-term residual depressive symptoms are quite common. Persistent subsyndromal depressive symptoms are associated with impaired psychosocial functioning and increased risk of relapse. This possibility has reinforced an evolving consensus that full depressive remission rather than response is the proper goal of treatment.2
When your patient’s depressive symptoms fail to remit following an initial pharmacotherapy course, you must decide which of numerous possible next steps to pursue, in what combinations, and following what sequence. The range of strategies include the following:
- Pursuing an extended trial with the initial agent using higher than usual dosages (e.g., fluoxetine 40 to 80 mg/qd);
- Augmenting an antidepressant with an agent that offers no established antidepressant efficacy on its own, such as buspirone, lithium, thyroid, or estrogen;
- Combining an antidepressant with another antidepressant, with another somatic therapy such as electroconvulsive treatment or phototherapy, or with some form of psychotherapy;
- Switching to an antidepressant within the same class (e.g., from one selective serotonin reuptake inhibitor [SSRI] to another), or outside of class (from an SSRI to an atypical antidepressant), or switching to non-pharmacological somatic therapy or psychotherapy.3
A predictable lag exists between innovative clinical applications and the randomized, controlled trials (RCTs) designed to evaluate them. The result: Approaches that are thoroughly described in the literature involve medication regimens that are no longer first-line, such as lithium or thyroid augmentation of tricyclic antidepressants (TCAs), or the combination of TCAs with SSRIs. Conversely, approaches most widely used in current psychiatric practice (e.g., addition of bupropion to SSRIs) have received relatively little systematic attention.4
Further, while predictors of initial antidepressant response have been hard to come by, even less is known about predictors of antidepressant response after lack of response to previous antidepressants. The working hypotheses that clinicians use to decide which strategies to pursue, though plausible, are largely untested, whether based on characteristics of a patient’s initial response (e.g., non-response or partial response)5 or on side effects or comorbid diagnoses.
This review will describe new research and emerging strategies that address the common clinical problem of unremitted depression despite one or more adequate courses of antidepressant treatment.
A look at new augmentations and combinations
Adding a second agent is an appealing strategy when patients have tolerated an initial antidepressant without troublesome side effects or have shown partial response (≥ 25% and <50% symptom reduction) and/or when a second agent may serve an important additional goal such as treating a comorbid condition—attention-deficit disorder or smoking, for example—or a side effect (e.g., nausea or insomnia).
Evidence supporting a relation between folate and depression has accrued over decades. Low folate levels may be associated with poorer response to fluoxetine.
Coppen and Bailey25 found that depressed patients had higher initial response rates to fluoxetine when combined with supplemental folic acid (500 mcg) than they did with placebo. The effect was significant only among female subjects. Study participants were naïve to fluoxetine and did not have established treatment resistance.
Still, the safety, tolerability, cost-effectiveness, and high patient acceptability of folic acid should stimulate further study of augmentation with folic acid supplementation and other naturally occurring agents related to folate in the one-carbon cycle, including S-adenosyl-methionine (SAMe).
The primary drawbacks to augmentation/combination strategies are an increased risk of drug interactions, cost, and potential decrement in adherence to treatment as the regimen’s complexity increases.
Thyroid augmentation Although thyroid augmentation of TCAs has been the subject of numerous RCTs, no controlled trials of thyroid augmentation of SSRIs or other newer-generation antidepressants are available.
Lithium augmentation Studies of lithium augmentation of SSRIs have yielded generally modest response rates with questionable durability.6,7
Noradrenergic and/or dopaminergic agonist agents Open trials and case series in the treatment of patients with unremitted depression have supported combining SSRIs and venlafaxine with agents that possess primarily noradrenergic and/or dopaminergic agonist properties. These include bupropion,8 psychostimulants,9 and direct dopamine agonists, including pergolide and pramipexole.10
These combinations have the advantage of potentially ameliorating several common SSRI side effects, particularly sedation, sexual dysfunction, and putative SSRI-related apathy. In the case of psychostimulants and/or bupropion, the combinations may treat such common comorbidities as attention-deficit/hyperactivity disorder and smoking.
Modafinil The combination of the antinarcoleptic modafinil with SSRIs and other newer-generation antidepressants has also attracted interest. Its efficacy as an antidepressant adjunct and as a remedy for side effects must still be established, however.11
Buspirone augmentation The augmentation of SSRIs and other antidepressants with the antianxiety azaperone buspirone is supported by impressive response rates in several open trials. But the only placebo-controlled trial evaluating buspirone augmentation in resistant depression failed to find a significant drug:placebo difference.12 Still, its tolerability and anxiolytic efficacy, and potential for ameliorating sexual dysfunction in some patients, support its judicious use as an augmenting agent pending further study.
Pindolol Studies of this beta-agonist, 5HT-1A antagonist, as an antidepressant-augmenting combination have yielded promising results in some studies, including a negative 10-day, placebo-controlled trial.13 Some patients experience jitteriness or irritability on pindolol. Its role in treating resistant depression remains to be established.
Noradrenergic TCAs The combination of SSRIs with these agents is a good example of a strategy based on pairing complimentary antidepressant mechanisms. Double-blind, controlled trials of fluoxetine plus desipramine6,7 have tempered the enthusiasm generated by earlier open studies, however. Response rates in these trials range from 25% to 30%, no higher than lithium augmentation and slightly lower than higher-dose fluoxetine.
Mirtazapine A more recent combination is mirtazapine with SSRIs or with high-dose venlafaxine.14 In some patients, this combination may attenuate sexual dysfunction, insomnia, and gastrointestinal side effects of SSRIs and venlafaxine by virtue of a 5-HT2, 5-HT3, and histamine receptor blockade by mirtazapine. This combination may also capitalize on the combined antidepressant effects of direct norepinephrine (NE) and serotonin (5-HT) reuptake inhibition as well as an alpha2 adrenergic auto- and hetero-receptor blockade—facilitating presynaptic NE and 5HT release—and 5-HT2 receptor antagonism.
Antipsychotics The use of antipsychotics for nonpsychotic unipolar depression has been controversial. However, in the context of lower apparent risks of tardive dyskinesia with the newer, atypical antipsychotics, their use as antidepressant augmenting agents has been revisited. In one double-blind, placebo-controlled trial, the combination of fluoxetine plus olanzapine was more effective in a well-defined refractory depressed population than was either medication alone.15 (See Box 1 for a discussion of fluoxetine and folic acid.)
Ostroff and Nelson16 reported an extremely rapid (1 week) response after the addition of risperidone among 8 depressed individuals who had not responded to fluoxetine or paroxetine alone. The activating properties of ziprasadone for some patients, combined with its lower propensity for producing weight gain than other atypical antipsychotics, make it another potentially attractive candidate for antidepressant augmentation, though one that requires further study.
Anticonvulsant augmentation While controlled studies of anticonvulsant augmentation of newer-generation antidepressants are lacking, the efficacy of lamotrigine for treating bipolar depression17 has encouraged clinicians to combine the agent with antidepressants in unipolar depression. The potential sedative/anxiolytic effects of other anticonvulsants, including gabapentin and valproate, have also supported their use in resistant depression complicated by anxiety or irritability. Omega-3-fatty acids may work as mood-stabilizing substances when used for antidepressant augmentation.
Antidepressant switches: In class or out?
Switching from TCAs to SSRIs or vice versa following nonresponse has yielded generally high (50% to 60%) response rates.3 But the more common question now is whether to switch from an initial SSRI to another SSRI, to an SSRI-like agent with an additional noradrenergic mechanism (such as venlafaxine), or to an atypical antidepressant such as bupropion or mirtazapine.
Traditional teaching and regular psychiatric practice5 have favored a switch outside of class. More recently, however, a number of uncontrolled studies looking at response rates to a second SSRI following inadequate response to and/or tolerance of an initial SSRI have shown response rates of 40% to 75%.18 This coincides with response rates seen in studies involving crossover to antidepressants with differing mechanisms. Inferences from these studies are limited by, first, determining nonresponse retrospectively and, second, lumping together patients who did not remain on the first SSRI because of nonresponse with others who curtailed treatment because of intolerance.
Because of its dual 5-HT/NE reuptake inhibitory activity, particularly at higher doses, venlafaxine is a popular choice following failure of an initial SSRI. One-third of subjects with extremely resistant depression (3 or more trials of antidepressants) responded to venlafaxine.19 DeMontigny et al20 also demonstrated its efficacy (with a 58% response rate) as a switching agent among resistant, depressed patients.
But comparative response rates for switching to venlafaxine from an SSRI vs. from other antidepressant classes have not been well delineated, nor have response rates for switching from SSRIs and other antidepressants to venlafaxine vs. to other non-SSRI agents.
Switches from SSRIs to nefazodone and mirtazapine or the selective NE reuptake inhibitor reboxetine have shown reasonably high response rates.3 Studies comparing these various strategies head to head are lacking. In the absence of data, reasonable guides for switching agents include projected tolerability and existence of comorbid conditions that may respond well to one agent over another.
Another issue is whether to cross-taper, that is, to lower the dose of the first agent while titrating up the next, or to discontinue the first before the second. When catastrophic drug interactions would result from overlap, such as the switch from an SSRI to a monoamine oxidase (MAO) inhibitor, a suitable wash-out period is necessary. This can also occur if the first antidepressant is believed to cause intolerable side effects.
In most other instances where no absolute contraindications exist, cross-tapering may be worthwhile despite theoretical risks such as serotonin syndrome when overlapping an SSRI and venlafaxine. Cross-tapering helps reduce the risk of side effects associated with abrupt discontinuation including nausea, myalgias, headache, and dysphoria, and may minimize the loss of antidepressant benefit from the initial agent before the impact of the second agent is realized.
Great hope for new knowledge with STAR*D
In recognition of the substantial direct and indirect costs, the morbidity and mortality associated with unremitted depression, and the considerable gaps in knowledge about optimal management at key decision points, the National Institute of Mental Health (NIMH) has launched the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. This is a multisite, multistep, prospective, randomized clinical trial of nonpsychotic major depressive disorder.21
STAR*D represents an initiative unique in the history of depression trials. Its novel “equipoise stratified” design26 is a hybrid between strict randomization to treatments without input from patients or clinicians (completely randomized designs) and a form of randomization in which subjects are assigned only to overarching strategies within which the particular treatments are determined by the clinician (clinician’s choice designs).
In STAR*D, a patient is asked at each level to define with his or her clinician all treatments that would be equally acceptable, based on a preference for certain potential side effects over others, interest in psychotherapy, or desire to retain a medication to which the patient has responded at least partially.
To be eligible to continue, however, the patient must agree to be randomized across at least some of the options testing some of the key hypotheses. For example, a participant at Level 2 who specifically wished to pursue cognitive therapy could not do that alone, but could agree to randomization to cognitive therapy with or without citalopram.
The study seeks to balance the goal of recruiting and retaining a broadly representative cross-section of patients—many of whom would reasonably expect to have some say about their treatment with their prescribing clinician—with the goal of providing statistically rigorous, randomization-based inferences upon which meaningful treatment guidelines can be based.
Approximately 4,000 adult depressed outpatients without an established history of antidepressant resistance will be recruited across the country. In contrast to efficacy trials, this effectiveness trial is meant to more closely parallel real-world practice (Box 2).
Patients will be treated initially with citalopram for 12 weeks (Level 1), with general guidelines for titration based upon response and tolerability. It is anticipated that about 2,000 individuals will exhibit unremitting depression despite SSRI treatment and/or intolerance to the SSRI and will be eligible for randomization to subsequent “switching” or “augmentation” strategies; this forms the core of the study across 3 successive levels.
Level 2 randomized treatments include four distinctive switching options—sertraline, bupropion, venlafaxine, or cognitive therapy—as well as 3 augmentation options—citalopram plus bupropion, buspirone, or cognitive therapy.
Those whose depression fails to remit will be eligible for randomization at Level 3 to different switching options—mirtazapine vs. nortripyline, or augmentation options—lithium or thyroid—added to the primary Level 2 antidepressant.
Finally, at Level 4, patients who continue to have unremitted depression despite successive treatments will be eligible for random assignment to 1 of 2 switching options—tranylcypromine vs. the combination of mirtazapine and venlafaxine.
Two new somatic treatments
Repetitive Transcranial Magnetic Stimulation (rTMS) rTMS is used to induce electrical current in the brain without causing seizures. Using a rapidly alternating current with a hand-held electromagnet that generates about 2 tesla will, in turn, induce or interrupt current (depolarization) in cortical interneurons about 2 cm below the surface of the skull. rTMS delivers energy without the impedance of the skull and is thought to be excitatory at high frequencies (20 Hz) and inhibitory at lower frequencies (5 to 10 Hz). This theory remains to be rigorously tested.22
Overall, the clinical effects reported in early studies on rTMS have been modest at best, while later studies have found either no difference from placebo or a robust effect. rTMS is a potential therapeutic tool not yet ready for widespread clinical use.22
Vagus Nerve Stimulation The NeuroCybernetic Prosthesis System (Cyberonics, Inc., Houston, Tex) stimulates the left cervical vagus nerve for treatment of resistant partial-onset epileptic seizures.
An open treatment trial of vagus nerve stimulation (VNS) for treatment-resistant depression was done based on mood improvement observed in patients treated for seizure disorders. Positron-emission tomographic studies revealed activation of limbic structures and neurochemical and neuronal pathway findings.23 The investigators reported improvement in a sample of 30 patients with major depressive disorder. Definite conclusions await controlled clinical trials and more work is needed to determine the ultimate mechanisms that might be antidepressant.23,24
Related resources
- Amsterdam JD, Hornig M, Nierenberg AA. Treatment-resistant mood disorders. Cambridge, UK: Cambridge University Press; 2001.
- Stahl SM. Essential Psychopharmacology. Cambridge, UK: Cambridge University Press; 1996.
- STAR*D Web site: http://www.edc.gsph.pitt.edu/stard
Drug brand names
- Bupropion • Wellbutrin, Wellbutrin SR, Zybar
- Buspirone • BuSpar, BuSpar DIVIDOSE
- Citalopram • Celexa
- Fluoxetine • Prozac, Prozac Weekly
- Gabapentin • Neurontin
- Haloperidol • Haldol, Haldol Decanoate
- Lamotrigine • Lamictal
- Mirtazapine • Remeron, Remeron Soltab
- Modafinil • Provigil
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Pergolide • Permax
- Pramipexole • Mirapex
- Reboxetine • Edronax
- Risperidone • Risperidal
- S-adenosyl-methionine • SAM-e
- Sertaline • Zoloft
- Tranycypromine • Nardil, Parnate, Marplan
- Valproate • Epival, Deprox, Alti-Valproic
- Venlafaxine • Effexor, Effexor X12
- Ziprasadone • Geodon
Disclosure
Dr. Alpert receives research funding, speaking honoraria or consultation fees from Organon, Forest Pharmaceuticals, GlaxoSmithKline, Pfizer Inc., and Pharmavite.
Dr. Nierenberg receives research funding, speaking honoraria, or consultation fees from Eli Lilly and Co., Wyeth-Ayerst Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Innapharma, BMS, Cyberonics, Lichtner, and Pfizer Inc.
As many as 50% of depressed patients do not achieve a 50% or greater reduction in severity of symptoms after an adequate antidepressant trial.1 Moreover, among those who do respond to acute treatment, longer-term residual depressive symptoms are quite common. Persistent subsyndromal depressive symptoms are associated with impaired psychosocial functioning and increased risk of relapse. This possibility has reinforced an evolving consensus that full depressive remission rather than response is the proper goal of treatment.2
When your patient’s depressive symptoms fail to remit following an initial pharmacotherapy course, you must decide which of numerous possible next steps to pursue, in what combinations, and following what sequence. The range of strategies include the following:
- Pursuing an extended trial with the initial agent using higher than usual dosages (e.g., fluoxetine 40 to 80 mg/qd);
- Augmenting an antidepressant with an agent that offers no established antidepressant efficacy on its own, such as buspirone, lithium, thyroid, or estrogen;
- Combining an antidepressant with another antidepressant, with another somatic therapy such as electroconvulsive treatment or phototherapy, or with some form of psychotherapy;
- Switching to an antidepressant within the same class (e.g., from one selective serotonin reuptake inhibitor [SSRI] to another), or outside of class (from an SSRI to an atypical antidepressant), or switching to non-pharmacological somatic therapy or psychotherapy.3
A predictable lag exists between innovative clinical applications and the randomized, controlled trials (RCTs) designed to evaluate them. The result: Approaches that are thoroughly described in the literature involve medication regimens that are no longer first-line, such as lithium or thyroid augmentation of tricyclic antidepressants (TCAs), or the combination of TCAs with SSRIs. Conversely, approaches most widely used in current psychiatric practice (e.g., addition of bupropion to SSRIs) have received relatively little systematic attention.4
Further, while predictors of initial antidepressant response have been hard to come by, even less is known about predictors of antidepressant response after lack of response to previous antidepressants. The working hypotheses that clinicians use to decide which strategies to pursue, though plausible, are largely untested, whether based on characteristics of a patient’s initial response (e.g., non-response or partial response)5 or on side effects or comorbid diagnoses.
This review will describe new research and emerging strategies that address the common clinical problem of unremitted depression despite one or more adequate courses of antidepressant treatment.
A look at new augmentations and combinations
Adding a second agent is an appealing strategy when patients have tolerated an initial antidepressant without troublesome side effects or have shown partial response (≥ 25% and <50% symptom reduction) and/or when a second agent may serve an important additional goal such as treating a comorbid condition—attention-deficit disorder or smoking, for example—or a side effect (e.g., nausea or insomnia).
Evidence supporting a relation between folate and depression has accrued over decades. Low folate levels may be associated with poorer response to fluoxetine.
Coppen and Bailey25 found that depressed patients had higher initial response rates to fluoxetine when combined with supplemental folic acid (500 mcg) than they did with placebo. The effect was significant only among female subjects. Study participants were naïve to fluoxetine and did not have established treatment resistance.
Still, the safety, tolerability, cost-effectiveness, and high patient acceptability of folic acid should stimulate further study of augmentation with folic acid supplementation and other naturally occurring agents related to folate in the one-carbon cycle, including S-adenosyl-methionine (SAMe).
The primary drawbacks to augmentation/combination strategies are an increased risk of drug interactions, cost, and potential decrement in adherence to treatment as the regimen’s complexity increases.
Thyroid augmentation Although thyroid augmentation of TCAs has been the subject of numerous RCTs, no controlled trials of thyroid augmentation of SSRIs or other newer-generation antidepressants are available.
Lithium augmentation Studies of lithium augmentation of SSRIs have yielded generally modest response rates with questionable durability.6,7
Noradrenergic and/or dopaminergic agonist agents Open trials and case series in the treatment of patients with unremitted depression have supported combining SSRIs and venlafaxine with agents that possess primarily noradrenergic and/or dopaminergic agonist properties. These include bupropion,8 psychostimulants,9 and direct dopamine agonists, including pergolide and pramipexole.10
These combinations have the advantage of potentially ameliorating several common SSRI side effects, particularly sedation, sexual dysfunction, and putative SSRI-related apathy. In the case of psychostimulants and/or bupropion, the combinations may treat such common comorbidities as attention-deficit/hyperactivity disorder and smoking.
Modafinil The combination of the antinarcoleptic modafinil with SSRIs and other newer-generation antidepressants has also attracted interest. Its efficacy as an antidepressant adjunct and as a remedy for side effects must still be established, however.11
Buspirone augmentation The augmentation of SSRIs and other antidepressants with the antianxiety azaperone buspirone is supported by impressive response rates in several open trials. But the only placebo-controlled trial evaluating buspirone augmentation in resistant depression failed to find a significant drug:placebo difference.12 Still, its tolerability and anxiolytic efficacy, and potential for ameliorating sexual dysfunction in some patients, support its judicious use as an augmenting agent pending further study.
Pindolol Studies of this beta-agonist, 5HT-1A antagonist, as an antidepressant-augmenting combination have yielded promising results in some studies, including a negative 10-day, placebo-controlled trial.13 Some patients experience jitteriness or irritability on pindolol. Its role in treating resistant depression remains to be established.
Noradrenergic TCAs The combination of SSRIs with these agents is a good example of a strategy based on pairing complimentary antidepressant mechanisms. Double-blind, controlled trials of fluoxetine plus desipramine6,7 have tempered the enthusiasm generated by earlier open studies, however. Response rates in these trials range from 25% to 30%, no higher than lithium augmentation and slightly lower than higher-dose fluoxetine.
Mirtazapine A more recent combination is mirtazapine with SSRIs or with high-dose venlafaxine.14 In some patients, this combination may attenuate sexual dysfunction, insomnia, and gastrointestinal side effects of SSRIs and venlafaxine by virtue of a 5-HT2, 5-HT3, and histamine receptor blockade by mirtazapine. This combination may also capitalize on the combined antidepressant effects of direct norepinephrine (NE) and serotonin (5-HT) reuptake inhibition as well as an alpha2 adrenergic auto- and hetero-receptor blockade—facilitating presynaptic NE and 5HT release—and 5-HT2 receptor antagonism.
Antipsychotics The use of antipsychotics for nonpsychotic unipolar depression has been controversial. However, in the context of lower apparent risks of tardive dyskinesia with the newer, atypical antipsychotics, their use as antidepressant augmenting agents has been revisited. In one double-blind, placebo-controlled trial, the combination of fluoxetine plus olanzapine was more effective in a well-defined refractory depressed population than was either medication alone.15 (See Box 1 for a discussion of fluoxetine and folic acid.)
Ostroff and Nelson16 reported an extremely rapid (1 week) response after the addition of risperidone among 8 depressed individuals who had not responded to fluoxetine or paroxetine alone. The activating properties of ziprasadone for some patients, combined with its lower propensity for producing weight gain than other atypical antipsychotics, make it another potentially attractive candidate for antidepressant augmentation, though one that requires further study.
Anticonvulsant augmentation While controlled studies of anticonvulsant augmentation of newer-generation antidepressants are lacking, the efficacy of lamotrigine for treating bipolar depression17 has encouraged clinicians to combine the agent with antidepressants in unipolar depression. The potential sedative/anxiolytic effects of other anticonvulsants, including gabapentin and valproate, have also supported their use in resistant depression complicated by anxiety or irritability. Omega-3-fatty acids may work as mood-stabilizing substances when used for antidepressant augmentation.
Antidepressant switches: In class or out?
Switching from TCAs to SSRIs or vice versa following nonresponse has yielded generally high (50% to 60%) response rates.3 But the more common question now is whether to switch from an initial SSRI to another SSRI, to an SSRI-like agent with an additional noradrenergic mechanism (such as venlafaxine), or to an atypical antidepressant such as bupropion or mirtazapine.
Traditional teaching and regular psychiatric practice5 have favored a switch outside of class. More recently, however, a number of uncontrolled studies looking at response rates to a second SSRI following inadequate response to and/or tolerance of an initial SSRI have shown response rates of 40% to 75%.18 This coincides with response rates seen in studies involving crossover to antidepressants with differing mechanisms. Inferences from these studies are limited by, first, determining nonresponse retrospectively and, second, lumping together patients who did not remain on the first SSRI because of nonresponse with others who curtailed treatment because of intolerance.
Because of its dual 5-HT/NE reuptake inhibitory activity, particularly at higher doses, venlafaxine is a popular choice following failure of an initial SSRI. One-third of subjects with extremely resistant depression (3 or more trials of antidepressants) responded to venlafaxine.19 DeMontigny et al20 also demonstrated its efficacy (with a 58% response rate) as a switching agent among resistant, depressed patients.
But comparative response rates for switching to venlafaxine from an SSRI vs. from other antidepressant classes have not been well delineated, nor have response rates for switching from SSRIs and other antidepressants to venlafaxine vs. to other non-SSRI agents.
Switches from SSRIs to nefazodone and mirtazapine or the selective NE reuptake inhibitor reboxetine have shown reasonably high response rates.3 Studies comparing these various strategies head to head are lacking. In the absence of data, reasonable guides for switching agents include projected tolerability and existence of comorbid conditions that may respond well to one agent over another.
Another issue is whether to cross-taper, that is, to lower the dose of the first agent while titrating up the next, or to discontinue the first before the second. When catastrophic drug interactions would result from overlap, such as the switch from an SSRI to a monoamine oxidase (MAO) inhibitor, a suitable wash-out period is necessary. This can also occur if the first antidepressant is believed to cause intolerable side effects.
In most other instances where no absolute contraindications exist, cross-tapering may be worthwhile despite theoretical risks such as serotonin syndrome when overlapping an SSRI and venlafaxine. Cross-tapering helps reduce the risk of side effects associated with abrupt discontinuation including nausea, myalgias, headache, and dysphoria, and may minimize the loss of antidepressant benefit from the initial agent before the impact of the second agent is realized.
Great hope for new knowledge with STAR*D
In recognition of the substantial direct and indirect costs, the morbidity and mortality associated with unremitted depression, and the considerable gaps in knowledge about optimal management at key decision points, the National Institute of Mental Health (NIMH) has launched the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. This is a multisite, multistep, prospective, randomized clinical trial of nonpsychotic major depressive disorder.21
STAR*D represents an initiative unique in the history of depression trials. Its novel “equipoise stratified” design26 is a hybrid between strict randomization to treatments without input from patients or clinicians (completely randomized designs) and a form of randomization in which subjects are assigned only to overarching strategies within which the particular treatments are determined by the clinician (clinician’s choice designs).
In STAR*D, a patient is asked at each level to define with his or her clinician all treatments that would be equally acceptable, based on a preference for certain potential side effects over others, interest in psychotherapy, or desire to retain a medication to which the patient has responded at least partially.
To be eligible to continue, however, the patient must agree to be randomized across at least some of the options testing some of the key hypotheses. For example, a participant at Level 2 who specifically wished to pursue cognitive therapy could not do that alone, but could agree to randomization to cognitive therapy with or without citalopram.
The study seeks to balance the goal of recruiting and retaining a broadly representative cross-section of patients—many of whom would reasonably expect to have some say about their treatment with their prescribing clinician—with the goal of providing statistically rigorous, randomization-based inferences upon which meaningful treatment guidelines can be based.
Approximately 4,000 adult depressed outpatients without an established history of antidepressant resistance will be recruited across the country. In contrast to efficacy trials, this effectiveness trial is meant to more closely parallel real-world practice (Box 2).
Patients will be treated initially with citalopram for 12 weeks (Level 1), with general guidelines for titration based upon response and tolerability. It is anticipated that about 2,000 individuals will exhibit unremitting depression despite SSRI treatment and/or intolerance to the SSRI and will be eligible for randomization to subsequent “switching” or “augmentation” strategies; this forms the core of the study across 3 successive levels.
Level 2 randomized treatments include four distinctive switching options—sertraline, bupropion, venlafaxine, or cognitive therapy—as well as 3 augmentation options—citalopram plus bupropion, buspirone, or cognitive therapy.
Those whose depression fails to remit will be eligible for randomization at Level 3 to different switching options—mirtazapine vs. nortripyline, or augmentation options—lithium or thyroid—added to the primary Level 2 antidepressant.
Finally, at Level 4, patients who continue to have unremitted depression despite successive treatments will be eligible for random assignment to 1 of 2 switching options—tranylcypromine vs. the combination of mirtazapine and venlafaxine.
Two new somatic treatments
Repetitive Transcranial Magnetic Stimulation (rTMS) rTMS is used to induce electrical current in the brain without causing seizures. Using a rapidly alternating current with a hand-held electromagnet that generates about 2 tesla will, in turn, induce or interrupt current (depolarization) in cortical interneurons about 2 cm below the surface of the skull. rTMS delivers energy without the impedance of the skull and is thought to be excitatory at high frequencies (20 Hz) and inhibitory at lower frequencies (5 to 10 Hz). This theory remains to be rigorously tested.22
Overall, the clinical effects reported in early studies on rTMS have been modest at best, while later studies have found either no difference from placebo or a robust effect. rTMS is a potential therapeutic tool not yet ready for widespread clinical use.22
Vagus Nerve Stimulation The NeuroCybernetic Prosthesis System (Cyberonics, Inc., Houston, Tex) stimulates the left cervical vagus nerve for treatment of resistant partial-onset epileptic seizures.
An open treatment trial of vagus nerve stimulation (VNS) for treatment-resistant depression was done based on mood improvement observed in patients treated for seizure disorders. Positron-emission tomographic studies revealed activation of limbic structures and neurochemical and neuronal pathway findings.23 The investigators reported improvement in a sample of 30 patients with major depressive disorder. Definite conclusions await controlled clinical trials and more work is needed to determine the ultimate mechanisms that might be antidepressant.23,24
Related resources
- Amsterdam JD, Hornig M, Nierenberg AA. Treatment-resistant mood disorders. Cambridge, UK: Cambridge University Press; 2001.
- Stahl SM. Essential Psychopharmacology. Cambridge, UK: Cambridge University Press; 1996.
- STAR*D Web site: http://www.edc.gsph.pitt.edu/stard
Drug brand names
- Bupropion • Wellbutrin, Wellbutrin SR, Zybar
- Buspirone • BuSpar, BuSpar DIVIDOSE
- Citalopram • Celexa
- Fluoxetine • Prozac, Prozac Weekly
- Gabapentin • Neurontin
- Haloperidol • Haldol, Haldol Decanoate
- Lamotrigine • Lamictal
- Mirtazapine • Remeron, Remeron Soltab
- Modafinil • Provigil
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Pergolide • Permax
- Pramipexole • Mirapex
- Reboxetine • Edronax
- Risperidone • Risperidal
- S-adenosyl-methionine • SAM-e
- Sertaline • Zoloft
- Tranycypromine • Nardil, Parnate, Marplan
- Valproate • Epival, Deprox, Alti-Valproic
- Venlafaxine • Effexor, Effexor X12
- Ziprasadone • Geodon
Disclosure
Dr. Alpert receives research funding, speaking honoraria or consultation fees from Organon, Forest Pharmaceuticals, GlaxoSmithKline, Pfizer Inc., and Pharmavite.
Dr. Nierenberg receives research funding, speaking honoraria, or consultation fees from Eli Lilly and Co., Wyeth-Ayerst Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Innapharma, BMS, Cyberonics, Lichtner, and Pfizer Inc.
1. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. The Psychiatric Clinics of North America: Treatment Resistant Depression. 1997;19(2):179-200.
2. Nierenberg AA, Wright EC. Evolution of remission as the new standard in the treatment of depression. J Clin Psychiatry. 1999;60(22):7-11.
3. Rosenbaum JF, Fava M, Nierenberg AA, Sachs GS. Treatment-resistant mood disorders. In Gabbard GO, ed. Treatments of Psychiatric Disorders. 3rd ed. Washington, DC: American Psychiatric Press, Inc.; 2001;1307-1384.
4. Mischoulon D, Fava M, Rosenbaum JF. Strategies for augmentation of SSRI treatment: A survey of an academic psychopharmacology practice Harvard Review of Psychiatry. 1999;6:322-326.
5. Fredman SJ, Fava M, Kienke AS, et al. Partial response, non-response, and relapse on SSRIs in major depression: A survey of current “next-step” practices. J Clin Psychiatry. 2000;61:403-407.
6. Fava M, Rosenbaum JF, McGrath PJ, et al. A double-blind, controlled study of lithium and tricyclic augmentation of fluoxetine in treatment resistant depression. Am J Psychiatry. 1994;151:1372-1374.
7. Fava M, Alpert JE, Nierenberg AA, Worthington JJ, III, Rosenbaum JF. Double-blind study of high-dose fluoxetine versus lithium or desipramine augmentation of fluoxetine in partial and nonresponders to fluoxetine. 153rd Annual Meeting of the American Psychiatric Association. Chicago, Ill, 2000.
8. Bodkin JA, Lasser RA, Wines JD, Jr, et al. Combining serotonin reuptake inhibitors and bupropion in partial responders to antidepressant monotherapy. J Clin Psychiatry. 1997;58:137-145.
9. Stoll AL, Pillay SS, et al. Methylphenidate augmentation of serotonin selective reuptake inhibitors: a case series. J Clin Psychiatry. 1996;57(8):356-359.
10. Sporn J, Ghaemi SN, Sambur MR, Rankin MA, Recht J, Sachs GS, Rosenbaum JF, Fava M. Pramipexole augmentation in the treatment of unipolar and bipolar depression: A retrospective chart review. Ann Clin Psychiatry. 2000;12:137-140.
11. Menza MA, Kaufman KR, Castellanos A. Modafinil augmentation of antidepressant treatment in depression. J Clin Psychiatry. 2000;61(5):378-81.
12. Landen M, Bjorling G, Agren H, Fahlen T. A randomized double-blind placebo-controlled trial of buspirone in combination with an SSRI in patients with treatment-refractory depression. J Clin Psychiatry. 1998;59:664-668.
13. Perez V, Soler J, Puigdemont D, et al. A double-blind, randomized, placebo-controlled trial of pindolol augmentation in depressive patients resistant to serotonin reuptake inhibitors. Arch Gen Psychiatry. 1999;56:375-379.
14. Carpenter LL, Jocic Z, Hall JM, Rasmussen SA, Price LH. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry. 1999;60(1):45-49.
15. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry. 2001;158:131-134.
16. Ostroff RB, Nelson JC. Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry. 1999;60:256-259.
17. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry. 1999;60:79-88.
18. Thase ME, Blomgren SL, Birkett MA, et al. Fluoxetine treatment of patients with major depressive disorder who failed initial treatment with sertraline. J Clin Psychiatry 1997;58:16-21.
19. Nierenberg AA, Feighner JP, Rudolph R, et al. Venlafaxine for treatment-resistant unipolar depression. J Clin Psychopharmacol. 1994;14:419-423.
20. de Montigny C, Silverstone PH, et al. Venlafaxine in treatment-resistant major depression: a Canadian multicenter, open-label trial. J Clin Psychopharmacol. 1999;19(5):401-406.
21. Rush JA, Fava M, et al. Sequenced treatment alternatives to relieve depression (STAR*D): Rationale and design. Controlled Clinical Trials. 2001 (in press).
22. Sackeim H. Repetitive transcranial magnetic stimulation: What are the next steps? Biol Psychiatry. 2000;48:959-961.
23. Rush AJ, George MS, Sackeim HA, et al. Vagus Nerve Stimulation (VNSTM) for treatment-resistant depressions: A multicenter study. Biol Psychiatry 2000;47.-
24. Rosenbaum JF, Heninger G. Vagus nerve stimulation for treatment-resistant depression. BiolPsychiatry. 2000;47(4):273-275.
25. Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affective Disord. 2000;60:121-130.
26. Lavori PW, Rush JA, Wisniewski SR, et al. Strengthening clinical effectiveness trials: Equipose-stratified randomization. Biol Psychiatry. 2001;50(10):792-801.
1. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. The Psychiatric Clinics of North America: Treatment Resistant Depression. 1997;19(2):179-200.
2. Nierenberg AA, Wright EC. Evolution of remission as the new standard in the treatment of depression. J Clin Psychiatry. 1999;60(22):7-11.
3. Rosenbaum JF, Fava M, Nierenberg AA, Sachs GS. Treatment-resistant mood disorders. In Gabbard GO, ed. Treatments of Psychiatric Disorders. 3rd ed. Washington, DC: American Psychiatric Press, Inc.; 2001;1307-1384.
4. Mischoulon D, Fava M, Rosenbaum JF. Strategies for augmentation of SSRI treatment: A survey of an academic psychopharmacology practice Harvard Review of Psychiatry. 1999;6:322-326.
5. Fredman SJ, Fava M, Kienke AS, et al. Partial response, non-response, and relapse on SSRIs in major depression: A survey of current “next-step” practices. J Clin Psychiatry. 2000;61:403-407.
6. Fava M, Rosenbaum JF, McGrath PJ, et al. A double-blind, controlled study of lithium and tricyclic augmentation of fluoxetine in treatment resistant depression. Am J Psychiatry. 1994;151:1372-1374.
7. Fava M, Alpert JE, Nierenberg AA, Worthington JJ, III, Rosenbaum JF. Double-blind study of high-dose fluoxetine versus lithium or desipramine augmentation of fluoxetine in partial and nonresponders to fluoxetine. 153rd Annual Meeting of the American Psychiatric Association. Chicago, Ill, 2000.
8. Bodkin JA, Lasser RA, Wines JD, Jr, et al. Combining serotonin reuptake inhibitors and bupropion in partial responders to antidepressant monotherapy. J Clin Psychiatry. 1997;58:137-145.
9. Stoll AL, Pillay SS, et al. Methylphenidate augmentation of serotonin selective reuptake inhibitors: a case series. J Clin Psychiatry. 1996;57(8):356-359.
10. Sporn J, Ghaemi SN, Sambur MR, Rankin MA, Recht J, Sachs GS, Rosenbaum JF, Fava M. Pramipexole augmentation in the treatment of unipolar and bipolar depression: A retrospective chart review. Ann Clin Psychiatry. 2000;12:137-140.
11. Menza MA, Kaufman KR, Castellanos A. Modafinil augmentation of antidepressant treatment in depression. J Clin Psychiatry. 2000;61(5):378-81.
12. Landen M, Bjorling G, Agren H, Fahlen T. A randomized double-blind placebo-controlled trial of buspirone in combination with an SSRI in patients with treatment-refractory depression. J Clin Psychiatry. 1998;59:664-668.
13. Perez V, Soler J, Puigdemont D, et al. A double-blind, randomized, placebo-controlled trial of pindolol augmentation in depressive patients resistant to serotonin reuptake inhibitors. Arch Gen Psychiatry. 1999;56:375-379.
14. Carpenter LL, Jocic Z, Hall JM, Rasmussen SA, Price LH. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry. 1999;60(1):45-49.
15. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry. 2001;158:131-134.
16. Ostroff RB, Nelson JC. Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry. 1999;60:256-259.
17. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry. 1999;60:79-88.
18. Thase ME, Blomgren SL, Birkett MA, et al. Fluoxetine treatment of patients with major depressive disorder who failed initial treatment with sertraline. J Clin Psychiatry 1997;58:16-21.
19. Nierenberg AA, Feighner JP, Rudolph R, et al. Venlafaxine for treatment-resistant unipolar depression. J Clin Psychopharmacol. 1994;14:419-423.
20. de Montigny C, Silverstone PH, et al. Venlafaxine in treatment-resistant major depression: a Canadian multicenter, open-label trial. J Clin Psychopharmacol. 1999;19(5):401-406.
21. Rush JA, Fava M, et al. Sequenced treatment alternatives to relieve depression (STAR*D): Rationale and design. Controlled Clinical Trials. 2001 (in press).
22. Sackeim H. Repetitive transcranial magnetic stimulation: What are the next steps? Biol Psychiatry. 2000;48:959-961.
23. Rush AJ, George MS, Sackeim HA, et al. Vagus Nerve Stimulation (VNSTM) for treatment-resistant depressions: A multicenter study. Biol Psychiatry 2000;47.-
24. Rosenbaum JF, Heninger G. Vagus nerve stimulation for treatment-resistant depression. BiolPsychiatry. 2000;47(4):273-275.
25. Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affective Disord. 2000;60:121-130.
26. Lavori PW, Rush JA, Wisniewski SR, et al. Strengthening clinical effectiveness trials: Equipose-stratified randomization. Biol Psychiatry. 2001;50(10):792-801.
Treatment-resistant depression: Newer alternatives
As many as 50% of depressed patients do not achieve a 50% or greater reduction in severity of symptoms after an adequate antidepressant trial.1 Moreover, among those who do respond to acute treatment, longer-term residual depressive symptoms are quite common. Persistent subsyndromal depressive symptoms are associated with impaired psychosocial functioning and increased risk of relapse. This possibility has reinforced an evolving consensus that full depressive remission rather than response is the proper goal of treatment.2
When your patient’s depressive symptoms fail to remit following an initial pharmacotherapy course, you must decide which of numerous possible next steps to pursue, in what combinations, and following what sequence. The range of strategies include the following:
- Pursuing an extended trial with the initial agent using higher than usual dosages (e.g., fluoxetine 40 to 80 mg/qd);
- Augmenting an antidepressant with an agent that offers no established antidepressant efficacy on its own, such as buspirone, lithium, thyroid, or estrogen;
- Combining an antidepressant with another antidepressant, with another somatic therapy such as electroconvulsive treatment or phototherapy, or with some form of psychotherapy;
- Switching to an antidepressant within the same class (e.g., from one selective serotonin reuptake inhibitor [SSRI] to another), or outside of class (from an SSRI to an atypical antidepressant), or switching to non-pharmacological somatic therapy or psychotherapy.3
A predictable lag exists between innovative clinical applications and the randomized, controlled trials (RCTs) designed to evaluate them. The result: Approaches that are thoroughly described in the literature involve medication regimens that are no longer first-line, such as lithium or thyroid augmentation of tricyclic antidepressants (TCAs), or the combination of TCAs with SSRIs. Conversely, approaches most widely used in current psychiatric practice (e.g., addition of bupropion to SSRIs) have received relatively little systematic attention.4
Further, while predictors of initial antidepressant response have been hard to come by, even less is known about predictors of antidepressant response after lack of response to previous antidepressants. The working hypotheses that clinicians use to decide which strategies to pursue, though plausible, are largely untested, whether based on characteristics of a patient’s initial response (e.g., non-response or partial response)5 or on side effects or comorbid diagnoses.
This review will describe new research and emerging strategies that address the common clinical problem of unremitted depression despite one or more adequate courses of antidepressant treatment.
A look at new augmentations and combinations
Adding a second agent is an appealing strategy when patients have tolerated an initial antidepressant without troublesome side effects or have shown partial response (≥ 25% and <50% symptom reduction) and/or when a second agent may serve an important additional goal such as treating a comorbid condition—attention-deficit disorder or smoking, for example—or a side effect (e.g., nausea or insomnia).
Evidence supporting a relation between folate and depression has accrued over decades. Low folate levels may be associated with poorer response to fluoxetine.
Coppen and Bailey25 found that depressed patients had higher initial response rates to fluoxetine when combined with supplemental folic acid (500 mcg) than they did with placebo. The effect was significant only among female subjects. Study participants were naïve to fluoxetine and did not have established treatment resistance.
Still, the safety, tolerability, cost-effectiveness, and high patient acceptability of folic acid should stimulate further study of augmentation with folic acid supplementation and other naturally occurring agents related to folate in the one-carbon cycle, including S-adenosyl-methionine (SAMe).
The primary drawbacks to augmentation/combination strategies are an increased risk of drug interactions, cost, and potential decrement in adherence to treatment as the regimen’s complexity increases.
Thyroid augmentation Although thyroid augmentation of TCAs has been the subject of numerous RCTs, no controlled trials of thyroid augmentation of SSRIs or other newer-generation antidepressants are available.
Lithium augmentation Studies of lithium augmentation of SSRIs have yielded generally modest response rates with questionable durability.6,7
Noradrenergic and/or dopaminergic agonist agents Open trials and case series in the treatment of patients with unremitted depression have supported combining SSRIs and venlafaxine with agents that possess primarily noradrenergic and/or dopaminergic agonist properties. These include bupropion,8 psychostimulants,9 and direct dopamine agonists, including pergolide and pramipexole.10
These combinations have the advantage of potentially ameliorating several common SSRI side effects, particularly sedation, sexual dysfunction, and putative SSRI-related apathy. In the case of psychostimulants and/or bupropion, the combinations may treat such common comorbidities as attention-deficit/hyperactivity disorder and smoking.
Modafinil The combination of the antinarcoleptic modafinil with SSRIs and other newer-generation antidepressants has also attracted interest. Its efficacy as an antidepressant adjunct and as a remedy for side effects must still be established, however.11
Buspirone augmentation The augmentation of SSRIs and other antidepressants with the antianxiety azaperone buspirone is supported by impressive response rates in several open trials. But the only placebo-controlled trial evaluating buspirone augmentation in resistant depression failed to find a significant drug:placebo difference.12 Still, its tolerability and anxiolytic efficacy, and potential for ameliorating sexual dysfunction in some patients, support its judicious use as an augmenting agent pending further study.
Pindolol Studies of this beta-agonist, 5HT-1A antagonist, as an antidepressant-augmenting combination have yielded promising results in some studies, including a negative 10-day, placebo-controlled trial.13 Some patients experience jitteriness or irritability on pindolol. Its role in treating resistant depression remains to be established.
Noradrenergic TCAs The combination of SSRIs with these agents is a good example of a strategy based on pairing complimentary antidepressant mechanisms. Double-blind, controlled trials of fluoxetine plus desipramine6,7 have tempered the enthusiasm generated by earlier open studies, however. Response rates in these trials range from 25% to 30%, no higher than lithium augmentation and slightly lower than higher-dose fluoxetine.
Mirtazapine A more recent combination is mirtazapine with SSRIs or with high-dose venlafaxine.14 In some patients, this combination may attenuate sexual dysfunction, insomnia, and gastrointestinal side effects of SSRIs and venlafaxine by virtue of a 5-HT2, 5-HT3, and histamine receptor blockade by mirtazapine. This combination may also capitalize on the combined antidepressant effects of direct norepinephrine (NE) and serotonin (5-HT) reuptake inhibition as well as an alpha2 adrenergic auto- and hetero-receptor blockade—facilitating presynaptic NE and 5HT release—and 5-HT2 receptor antagonism.
Antipsychotics The use of antipsychotics for nonpsychotic unipolar depression has been controversial. However, in the context of lower apparent risks of tardive dyskinesia with the newer, atypical antipsychotics, their use as antidepressant augmenting agents has been revisited. In one double-blind, placebo-controlled trial, the combination of fluoxetine plus olanzapine was more effective in a well-defined refractory depressed population than was either medication alone.15 (See Box 1 for a discussion of fluoxetine and folic acid.)
Ostroff and Nelson16 reported an extremely rapid (1 week) response after the addition of risperidone among 8 depressed individuals who had not responded to fluoxetine or paroxetine alone. The activating properties of ziprasadone for some patients, combined with its lower propensity for producing weight gain than other atypical antipsychotics, make it another potentially attractive candidate for antidepressant augmentation, though one that requires further study.
Anticonvulsant augmentation While controlled studies of anticonvulsant augmentation of newer-generation antidepressants are lacking, the efficacy of lamotrigine for treating bipolar depression17 has encouraged clinicians to combine the agent with antidepressants in unipolar depression. The potential sedative/anxiolytic effects of other anticonvulsants, including gabapentin and valproate, have also supported their use in resistant depression complicated by anxiety or irritability. Omega-3-fatty acids may work as mood-stabilizing substances when used for antidepressant augmentation.
Antidepressant switches: In class or out?
Switching from TCAs to SSRIs or vice versa following nonresponse has yielded generally high (50% to 60%) response rates.3 But the more common question now is whether to switch from an initial SSRI to another SSRI, to an SSRI-like agent with an additional noradrenergic mechanism (such as venlafaxine), or to an atypical antidepressant such as bupropion or mirtazapine.
Traditional teaching and regular psychiatric practice5 have favored a switch outside of class. More recently, however, a number of uncontrolled studies looking at response rates to a second SSRI following inadequate response to and/or tolerance of an initial SSRI have shown response rates of 40% to 75%.18 This coincides with response rates seen in studies involving crossover to antidepressants with differing mechanisms. Inferences from these studies are limited by, first, determining nonresponse retrospectively and, second, lumping together patients who did not remain on the first SSRI because of nonresponse with others who curtailed treatment because of intolerance.
Because of its dual 5-HT/NE reuptake inhibitory activity, particularly at higher doses, venlafaxine is a popular choice following failure of an initial SSRI. One-third of subjects with extremely resistant depression (3 or more trials of antidepressants) responded to venlafaxine.19 DeMontigny et al20 also demonstrated its efficacy (with a 58% response rate) as a switching agent among resistant, depressed patients.
But comparative response rates for switching to venlafaxine from an SSRI vs. from other antidepressant classes have not been well delineated, nor have response rates for switching from SSRIs and other antidepressants to venlafaxine vs. to other non-SSRI agents.
Switches from SSRIs to nefazodone and mirtazapine or the selective NE reuptake inhibitor reboxetine have shown reasonably high response rates.3 Studies comparing these various strategies head to head are lacking. In the absence of data, reasonable guides for switching agents include projected tolerability and existence of comorbid conditions that may respond well to one agent over another.
Another issue is whether to cross-taper, that is, to lower the dose of the first agent while titrating up the next, or to discontinue the first before the second. When catastrophic drug interactions would result from overlap, such as the switch from an SSRI to a monoamine oxidase (MAO) inhibitor, a suitable wash-out period is necessary. This can also occur if the first antidepressant is believed to cause intolerable side effects.
In most other instances where no absolute contraindications exist, cross-tapering may be worthwhile despite theoretical risks such as serotonin syndrome when overlapping an SSRI and venlafaxine. Cross-tapering helps reduce the risk of side effects associated with abrupt discontinuation including nausea, myalgias, headache, and dysphoria, and may minimize the loss of antidepressant benefit from the initial agent before the impact of the second agent is realized.
Great hope for new knowledge with STAR*D
In recognition of the substantial direct and indirect costs, the morbidity and mortality associated with unremitted depression, and the considerable gaps in knowledge about optimal management at key decision points, the National Institute of Mental Health (NIMH) has launched the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. This is a multisite, multistep, prospective, randomized clinical trial of nonpsychotic major depressive disorder.21
STAR*D represents an initiative unique in the history of depression trials. Its novel “equipoise stratified” design26 is a hybrid between strict randomization to treatments without input from patients or clinicians (completely randomized designs) and a form of randomization in which subjects are assigned only to overarching strategies within which the particular treatments are determined by the clinician (clinician’s choice designs).
In STAR*D, a patient is asked at each level to define with his or her clinician all treatments that would be equally acceptable, based on a preference for certain potential side effects over others, interest in psychotherapy, or desire to retain a medication to which the patient has responded at least partially.
To be eligible to continue, however, the patient must agree to be randomized across at least some of the options testing some of the key hypotheses. For example, a participant at Level 2 who specifically wished to pursue cognitive therapy could not do that alone, but could agree to randomization to cognitive therapy with or without citalopram.
The study seeks to balance the goal of recruiting and retaining a broadly representative cross-section of patients—many of whom would reasonably expect to have some say about their treatment with their prescribing clinician—with the goal of providing statistically rigorous, randomization-based inferences upon which meaningful treatment guidelines can be based.
Approximately 4,000 adult depressed outpatients without an established history of antidepressant resistance will be recruited across the country. In contrast to efficacy trials, this effectiveness trial is meant to more closely parallel real-world practice (Box 2).
Patients will be treated initially with citalopram for 12 weeks (Level 1), with general guidelines for titration based upon response and tolerability. It is anticipated that about 2,000 individuals will exhibit unremitting depression despite SSRI treatment and/or intolerance to the SSRI and will be eligible for randomization to subsequent “switching” or “augmentation” strategies; this forms the core of the study across 3 successive levels.
Level 2 randomized treatments include four distinctive switching options—sertraline, bupropion, venlafaxine, or cognitive therapy—as well as 3 augmentation options—citalopram plus bupropion, buspirone, or cognitive therapy.
Those whose depression fails to remit will be eligible for randomization at Level 3 to different switching options—mirtazapine vs. nortripyline, or augmentation options—lithium or thyroid—added to the primary Level 2 antidepressant.
Finally, at Level 4, patients who continue to have unremitted depression despite successive treatments will be eligible for random assignment to 1 of 2 switching options—tranylcypromine vs. the combination of mirtazapine and venlafaxine.
Two new somatic treatments
Repetitive Transcranial Magnetic Stimulation (rTMS) rTMS is used to induce electrical current in the brain without causing seizures. Using a rapidly alternating current with a hand-held electromagnet that generates about 2 tesla will, in turn, induce or interrupt current (depolarization) in cortical interneurons about 2 cm below the surface of the skull. rTMS delivers energy without the impedance of the skull and is thought to be excitatory at high frequencies (20 Hz) and inhibitory at lower frequencies (5 to 10 Hz). This theory remains to be rigorously tested.22
Overall, the clinical effects reported in early studies on rTMS have been modest at best, while later studies have found either no difference from placebo or a robust effect. rTMS is a potential therapeutic tool not yet ready for widespread clinical use.22
Vagus Nerve Stimulation The NeuroCybernetic Prosthesis System (Cyberonics, Inc., Houston, Tex) stimulates the left cervical vagus nerve for treatment of resistant partial-onset epileptic seizures.
An open treatment trial of vagus nerve stimulation (VNS) for treatment-resistant depression was done based on mood improvement observed in patients treated for seizure disorders. Positron-emission tomographic studies revealed activation of limbic structures and neurochemical and neuronal pathway findings.23 The investigators reported improvement in a sample of 30 patients with major depressive disorder. Definite conclusions await controlled clinical trials and more work is needed to determine the ultimate mechanisms that might be antidepressant.23,24
Related resources
- Amsterdam JD, Hornig M, Nierenberg AA. Treatment-resistant mood disorders. Cambridge, UK: Cambridge University Press; 2001.
- Stahl SM. Essential Psychopharmacology. Cambridge, UK: Cambridge University Press; 1996.
- STAR*D Web site: http://www.edc.gsph.pitt.edu/stard
Drug brand names
- Bupropion • Wellbutrin, Wellbutrin SR, Zybar
- Buspirone • BuSpar, BuSpar DIVIDOSE
- Citalopram • Celexa
- Fluoxetine • Prozac, Prozac Weekly
- Gabapentin • Neurontin
- Haloperidol • Haldol, Haldol Decanoate
- Lamotrigine • Lamictal
- Mirtazapine • Remeron, Remeron Soltab
- Modafinil • Provigil
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Pergolide • Permax
- Pramipexole • Mirapex
- Reboxetine • Edronax
- Risperidone • Risperidal
- S-adenosyl-methionine • SAM-e
- Sertaline • Zoloft
- Tranycypromine • Nardil, Parnate, Marplan
- Valproate • Epival, Deprox, Alti-Valproic
- Venlafaxine • Effexor, Effexor X12
- Ziprasadone • Geodon
Disclosure
Dr. Alpert receives research funding, speaking honoraria or consultation fees from Organon, Forest Pharmaceuticals, GlaxoSmithKline, Pfizer Inc., and Pharmavite.
Dr. Nierenberg receives research funding, speaking honoraria, or consultation fees from Eli Lilly and Co., Wyeth-Ayerst Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Innapharma, BMS, Cyberonics, Lichtner, and Pfizer Inc.
1. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. The Psychiatric Clinics of North America: Treatment Resistant Depression. 1997;19(2):179-200.
2. Nierenberg AA, Wright EC. Evolution of remission as the new standard in the treatment of depression. J Clin Psychiatry. 1999;60(22):7-11.
3. Rosenbaum JF, Fava M, Nierenberg AA, Sachs GS. Treatment-resistant mood disorders. In Gabbard GO, ed. Treatments of Psychiatric Disorders. 3rd ed. Washington, DC: American Psychiatric Press, Inc.; 2001;1307-1384.
4. Mischoulon D, Fava M, Rosenbaum JF. Strategies for augmentation of SSRI treatment: A survey of an academic psychopharmacology practice Harvard Review of Psychiatry. 1999;6:322-326.
5. Fredman SJ, Fava M, Kienke AS, et al. Partial response, non-response, and relapse on SSRIs in major depression: A survey of current “next-step” practices. J Clin Psychiatry. 2000;61:403-407.
6. Fava M, Rosenbaum JF, McGrath PJ, et al. A double-blind, controlled study of lithium and tricyclic augmentation of fluoxetine in treatment resistant depression. Am J Psychiatry. 1994;151:1372-1374.
7. Fava M, Alpert JE, Nierenberg AA, Worthington JJ, III, Rosenbaum JF. Double-blind study of high-dose fluoxetine versus lithium or desipramine augmentation of fluoxetine in partial and nonresponders to fluoxetine. 153rd Annual Meeting of the American Psychiatric Association. Chicago, Ill, 2000.
8. Bodkin JA, Lasser RA, Wines JD, Jr, et al. Combining serotonin reuptake inhibitors and bupropion in partial responders to antidepressant monotherapy. J Clin Psychiatry. 1997;58:137-145.
9. Stoll AL, Pillay SS, et al. Methylphenidate augmentation of serotonin selective reuptake inhibitors: a case series. J Clin Psychiatry. 1996;57(8):356-359.
10. Sporn J, Ghaemi SN, Sambur MR, Rankin MA, Recht J, Sachs GS, Rosenbaum JF, Fava M. Pramipexole augmentation in the treatment of unipolar and bipolar depression: A retrospective chart review. Ann Clin Psychiatry. 2000;12:137-140.
11. Menza MA, Kaufman KR, Castellanos A. Modafinil augmentation of antidepressant treatment in depression. J Clin Psychiatry. 2000;61(5):378-81.
12. Landen M, Bjorling G, Agren H, Fahlen T. A randomized double-blind placebo-controlled trial of buspirone in combination with an SSRI in patients with treatment-refractory depression. J Clin Psychiatry. 1998;59:664-668.
13. Perez V, Soler J, Puigdemont D, et al. A double-blind, randomized, placebo-controlled trial of pindolol augmentation in depressive patients resistant to serotonin reuptake inhibitors. Arch Gen Psychiatry. 1999;56:375-379.
14. Carpenter LL, Jocic Z, Hall JM, Rasmussen SA, Price LH. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry. 1999;60(1):45-49.
15. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry. 2001;158:131-134.
16. Ostroff RB, Nelson JC. Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry. 1999;60:256-259.
17. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry. 1999;60:79-88.
18. Thase ME, Blomgren SL, Birkett MA, et al. Fluoxetine treatment of patients with major depressive disorder who failed initial treatment with sertraline. J Clin Psychiatry 1997;58:16-21.
19. Nierenberg AA, Feighner JP, Rudolph R, et al. Venlafaxine for treatment-resistant unipolar depression. J Clin Psychopharmacol. 1994;14:419-423.
20. de Montigny C, Silverstone PH, et al. Venlafaxine in treatment-resistant major depression: a Canadian multicenter, open-label trial. J Clin Psychopharmacol. 1999;19(5):401-406.
21. Rush JA, Fava M, et al. Sequenced treatment alternatives to relieve depression (STAR*D): Rationale and design. Controlled Clinical Trials. 2001 (in press).
22. Sackeim H. Repetitive transcranial magnetic stimulation: What are the next steps? Biol Psychiatry. 2000;48:959-961.
23. Rush AJ, George MS, Sackeim HA, et al. Vagus Nerve Stimulation (VNSTM) for treatment-resistant depressions: A multicenter study. Biol Psychiatry 2000;47.-
24. Rosenbaum JF, Heninger G. Vagus nerve stimulation for treatment-resistant depression. BiolPsychiatry. 2000;47(4):273-275.
25. Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affective Disord. 2000;60:121-130.
26. Lavori PW, Rush JA, Wisniewski SR, et al. Strengthening clinical effectiveness trials: Equipose-stratified randomization. Biol Psychiatry. 2001;50(10):792-801.
As many as 50% of depressed patients do not achieve a 50% or greater reduction in severity of symptoms after an adequate antidepressant trial.1 Moreover, among those who do respond to acute treatment, longer-term residual depressive symptoms are quite common. Persistent subsyndromal depressive symptoms are associated with impaired psychosocial functioning and increased risk of relapse. This possibility has reinforced an evolving consensus that full depressive remission rather than response is the proper goal of treatment.2
When your patient’s depressive symptoms fail to remit following an initial pharmacotherapy course, you must decide which of numerous possible next steps to pursue, in what combinations, and following what sequence. The range of strategies include the following:
- Pursuing an extended trial with the initial agent using higher than usual dosages (e.g., fluoxetine 40 to 80 mg/qd);
- Augmenting an antidepressant with an agent that offers no established antidepressant efficacy on its own, such as buspirone, lithium, thyroid, or estrogen;
- Combining an antidepressant with another antidepressant, with another somatic therapy such as electroconvulsive treatment or phototherapy, or with some form of psychotherapy;
- Switching to an antidepressant within the same class (e.g., from one selective serotonin reuptake inhibitor [SSRI] to another), or outside of class (from an SSRI to an atypical antidepressant), or switching to non-pharmacological somatic therapy or psychotherapy.3
A predictable lag exists between innovative clinical applications and the randomized, controlled trials (RCTs) designed to evaluate them. The result: Approaches that are thoroughly described in the literature involve medication regimens that are no longer first-line, such as lithium or thyroid augmentation of tricyclic antidepressants (TCAs), or the combination of TCAs with SSRIs. Conversely, approaches most widely used in current psychiatric practice (e.g., addition of bupropion to SSRIs) have received relatively little systematic attention.4
Further, while predictors of initial antidepressant response have been hard to come by, even less is known about predictors of antidepressant response after lack of response to previous antidepressants. The working hypotheses that clinicians use to decide which strategies to pursue, though plausible, are largely untested, whether based on characteristics of a patient’s initial response (e.g., non-response or partial response)5 or on side effects or comorbid diagnoses.
This review will describe new research and emerging strategies that address the common clinical problem of unremitted depression despite one or more adequate courses of antidepressant treatment.
A look at new augmentations and combinations
Adding a second agent is an appealing strategy when patients have tolerated an initial antidepressant without troublesome side effects or have shown partial response (≥ 25% and <50% symptom reduction) and/or when a second agent may serve an important additional goal such as treating a comorbid condition—attention-deficit disorder or smoking, for example—or a side effect (e.g., nausea or insomnia).
Evidence supporting a relation between folate and depression has accrued over decades. Low folate levels may be associated with poorer response to fluoxetine.
Coppen and Bailey25 found that depressed patients had higher initial response rates to fluoxetine when combined with supplemental folic acid (500 mcg) than they did with placebo. The effect was significant only among female subjects. Study participants were naïve to fluoxetine and did not have established treatment resistance.
Still, the safety, tolerability, cost-effectiveness, and high patient acceptability of folic acid should stimulate further study of augmentation with folic acid supplementation and other naturally occurring agents related to folate in the one-carbon cycle, including S-adenosyl-methionine (SAMe).
The primary drawbacks to augmentation/combination strategies are an increased risk of drug interactions, cost, and potential decrement in adherence to treatment as the regimen’s complexity increases.
Thyroid augmentation Although thyroid augmentation of TCAs has been the subject of numerous RCTs, no controlled trials of thyroid augmentation of SSRIs or other newer-generation antidepressants are available.
Lithium augmentation Studies of lithium augmentation of SSRIs have yielded generally modest response rates with questionable durability.6,7
Noradrenergic and/or dopaminergic agonist agents Open trials and case series in the treatment of patients with unremitted depression have supported combining SSRIs and venlafaxine with agents that possess primarily noradrenergic and/or dopaminergic agonist properties. These include bupropion,8 psychostimulants,9 and direct dopamine agonists, including pergolide and pramipexole.10
These combinations have the advantage of potentially ameliorating several common SSRI side effects, particularly sedation, sexual dysfunction, and putative SSRI-related apathy. In the case of psychostimulants and/or bupropion, the combinations may treat such common comorbidities as attention-deficit/hyperactivity disorder and smoking.
Modafinil The combination of the antinarcoleptic modafinil with SSRIs and other newer-generation antidepressants has also attracted interest. Its efficacy as an antidepressant adjunct and as a remedy for side effects must still be established, however.11
Buspirone augmentation The augmentation of SSRIs and other antidepressants with the antianxiety azaperone buspirone is supported by impressive response rates in several open trials. But the only placebo-controlled trial evaluating buspirone augmentation in resistant depression failed to find a significant drug:placebo difference.12 Still, its tolerability and anxiolytic efficacy, and potential for ameliorating sexual dysfunction in some patients, support its judicious use as an augmenting agent pending further study.
Pindolol Studies of this beta-agonist, 5HT-1A antagonist, as an antidepressant-augmenting combination have yielded promising results in some studies, including a negative 10-day, placebo-controlled trial.13 Some patients experience jitteriness or irritability on pindolol. Its role in treating resistant depression remains to be established.
Noradrenergic TCAs The combination of SSRIs with these agents is a good example of a strategy based on pairing complimentary antidepressant mechanisms. Double-blind, controlled trials of fluoxetine plus desipramine6,7 have tempered the enthusiasm generated by earlier open studies, however. Response rates in these trials range from 25% to 30%, no higher than lithium augmentation and slightly lower than higher-dose fluoxetine.
Mirtazapine A more recent combination is mirtazapine with SSRIs or with high-dose venlafaxine.14 In some patients, this combination may attenuate sexual dysfunction, insomnia, and gastrointestinal side effects of SSRIs and venlafaxine by virtue of a 5-HT2, 5-HT3, and histamine receptor blockade by mirtazapine. This combination may also capitalize on the combined antidepressant effects of direct norepinephrine (NE) and serotonin (5-HT) reuptake inhibition as well as an alpha2 adrenergic auto- and hetero-receptor blockade—facilitating presynaptic NE and 5HT release—and 5-HT2 receptor antagonism.
Antipsychotics The use of antipsychotics for nonpsychotic unipolar depression has been controversial. However, in the context of lower apparent risks of tardive dyskinesia with the newer, atypical antipsychotics, their use as antidepressant augmenting agents has been revisited. In one double-blind, placebo-controlled trial, the combination of fluoxetine plus olanzapine was more effective in a well-defined refractory depressed population than was either medication alone.15 (See Box 1 for a discussion of fluoxetine and folic acid.)
Ostroff and Nelson16 reported an extremely rapid (1 week) response after the addition of risperidone among 8 depressed individuals who had not responded to fluoxetine or paroxetine alone. The activating properties of ziprasadone for some patients, combined with its lower propensity for producing weight gain than other atypical antipsychotics, make it another potentially attractive candidate for antidepressant augmentation, though one that requires further study.
Anticonvulsant augmentation While controlled studies of anticonvulsant augmentation of newer-generation antidepressants are lacking, the efficacy of lamotrigine for treating bipolar depression17 has encouraged clinicians to combine the agent with antidepressants in unipolar depression. The potential sedative/anxiolytic effects of other anticonvulsants, including gabapentin and valproate, have also supported their use in resistant depression complicated by anxiety or irritability. Omega-3-fatty acids may work as mood-stabilizing substances when used for antidepressant augmentation.
Antidepressant switches: In class or out?
Switching from TCAs to SSRIs or vice versa following nonresponse has yielded generally high (50% to 60%) response rates.3 But the more common question now is whether to switch from an initial SSRI to another SSRI, to an SSRI-like agent with an additional noradrenergic mechanism (such as venlafaxine), or to an atypical antidepressant such as bupropion or mirtazapine.
Traditional teaching and regular psychiatric practice5 have favored a switch outside of class. More recently, however, a number of uncontrolled studies looking at response rates to a second SSRI following inadequate response to and/or tolerance of an initial SSRI have shown response rates of 40% to 75%.18 This coincides with response rates seen in studies involving crossover to antidepressants with differing mechanisms. Inferences from these studies are limited by, first, determining nonresponse retrospectively and, second, lumping together patients who did not remain on the first SSRI because of nonresponse with others who curtailed treatment because of intolerance.
Because of its dual 5-HT/NE reuptake inhibitory activity, particularly at higher doses, venlafaxine is a popular choice following failure of an initial SSRI. One-third of subjects with extremely resistant depression (3 or more trials of antidepressants) responded to venlafaxine.19 DeMontigny et al20 also demonstrated its efficacy (with a 58% response rate) as a switching agent among resistant, depressed patients.
But comparative response rates for switching to venlafaxine from an SSRI vs. from other antidepressant classes have not been well delineated, nor have response rates for switching from SSRIs and other antidepressants to venlafaxine vs. to other non-SSRI agents.
Switches from SSRIs to nefazodone and mirtazapine or the selective NE reuptake inhibitor reboxetine have shown reasonably high response rates.3 Studies comparing these various strategies head to head are lacking. In the absence of data, reasonable guides for switching agents include projected tolerability and existence of comorbid conditions that may respond well to one agent over another.
Another issue is whether to cross-taper, that is, to lower the dose of the first agent while titrating up the next, or to discontinue the first before the second. When catastrophic drug interactions would result from overlap, such as the switch from an SSRI to a monoamine oxidase (MAO) inhibitor, a suitable wash-out period is necessary. This can also occur if the first antidepressant is believed to cause intolerable side effects.
In most other instances where no absolute contraindications exist, cross-tapering may be worthwhile despite theoretical risks such as serotonin syndrome when overlapping an SSRI and venlafaxine. Cross-tapering helps reduce the risk of side effects associated with abrupt discontinuation including nausea, myalgias, headache, and dysphoria, and may minimize the loss of antidepressant benefit from the initial agent before the impact of the second agent is realized.
Great hope for new knowledge with STAR*D
In recognition of the substantial direct and indirect costs, the morbidity and mortality associated with unremitted depression, and the considerable gaps in knowledge about optimal management at key decision points, the National Institute of Mental Health (NIMH) has launched the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. This is a multisite, multistep, prospective, randomized clinical trial of nonpsychotic major depressive disorder.21
STAR*D represents an initiative unique in the history of depression trials. Its novel “equipoise stratified” design26 is a hybrid between strict randomization to treatments without input from patients or clinicians (completely randomized designs) and a form of randomization in which subjects are assigned only to overarching strategies within which the particular treatments are determined by the clinician (clinician’s choice designs).
In STAR*D, a patient is asked at each level to define with his or her clinician all treatments that would be equally acceptable, based on a preference for certain potential side effects over others, interest in psychotherapy, or desire to retain a medication to which the patient has responded at least partially.
To be eligible to continue, however, the patient must agree to be randomized across at least some of the options testing some of the key hypotheses. For example, a participant at Level 2 who specifically wished to pursue cognitive therapy could not do that alone, but could agree to randomization to cognitive therapy with or without citalopram.
The study seeks to balance the goal of recruiting and retaining a broadly representative cross-section of patients—many of whom would reasonably expect to have some say about their treatment with their prescribing clinician—with the goal of providing statistically rigorous, randomization-based inferences upon which meaningful treatment guidelines can be based.
Approximately 4,000 adult depressed outpatients without an established history of antidepressant resistance will be recruited across the country. In contrast to efficacy trials, this effectiveness trial is meant to more closely parallel real-world practice (Box 2).
Patients will be treated initially with citalopram for 12 weeks (Level 1), with general guidelines for titration based upon response and tolerability. It is anticipated that about 2,000 individuals will exhibit unremitting depression despite SSRI treatment and/or intolerance to the SSRI and will be eligible for randomization to subsequent “switching” or “augmentation” strategies; this forms the core of the study across 3 successive levels.
Level 2 randomized treatments include four distinctive switching options—sertraline, bupropion, venlafaxine, or cognitive therapy—as well as 3 augmentation options—citalopram plus bupropion, buspirone, or cognitive therapy.
Those whose depression fails to remit will be eligible for randomization at Level 3 to different switching options—mirtazapine vs. nortripyline, or augmentation options—lithium or thyroid—added to the primary Level 2 antidepressant.
Finally, at Level 4, patients who continue to have unremitted depression despite successive treatments will be eligible for random assignment to 1 of 2 switching options—tranylcypromine vs. the combination of mirtazapine and venlafaxine.
Two new somatic treatments
Repetitive Transcranial Magnetic Stimulation (rTMS) rTMS is used to induce electrical current in the brain without causing seizures. Using a rapidly alternating current with a hand-held electromagnet that generates about 2 tesla will, in turn, induce or interrupt current (depolarization) in cortical interneurons about 2 cm below the surface of the skull. rTMS delivers energy without the impedance of the skull and is thought to be excitatory at high frequencies (20 Hz) and inhibitory at lower frequencies (5 to 10 Hz). This theory remains to be rigorously tested.22
Overall, the clinical effects reported in early studies on rTMS have been modest at best, while later studies have found either no difference from placebo or a robust effect. rTMS is a potential therapeutic tool not yet ready for widespread clinical use.22
Vagus Nerve Stimulation The NeuroCybernetic Prosthesis System (Cyberonics, Inc., Houston, Tex) stimulates the left cervical vagus nerve for treatment of resistant partial-onset epileptic seizures.
An open treatment trial of vagus nerve stimulation (VNS) for treatment-resistant depression was done based on mood improvement observed in patients treated for seizure disorders. Positron-emission tomographic studies revealed activation of limbic structures and neurochemical and neuronal pathway findings.23 The investigators reported improvement in a sample of 30 patients with major depressive disorder. Definite conclusions await controlled clinical trials and more work is needed to determine the ultimate mechanisms that might be antidepressant.23,24
Related resources
- Amsterdam JD, Hornig M, Nierenberg AA. Treatment-resistant mood disorders. Cambridge, UK: Cambridge University Press; 2001.
- Stahl SM. Essential Psychopharmacology. Cambridge, UK: Cambridge University Press; 1996.
- STAR*D Web site: http://www.edc.gsph.pitt.edu/stard
Drug brand names
- Bupropion • Wellbutrin, Wellbutrin SR, Zybar
- Buspirone • BuSpar, BuSpar DIVIDOSE
- Citalopram • Celexa
- Fluoxetine • Prozac, Prozac Weekly
- Gabapentin • Neurontin
- Haloperidol • Haldol, Haldol Decanoate
- Lamotrigine • Lamictal
- Mirtazapine • Remeron, Remeron Soltab
- Modafinil • Provigil
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Pergolide • Permax
- Pramipexole • Mirapex
- Reboxetine • Edronax
- Risperidone • Risperidal
- S-adenosyl-methionine • SAM-e
- Sertaline • Zoloft
- Tranycypromine • Nardil, Parnate, Marplan
- Valproate • Epival, Deprox, Alti-Valproic
- Venlafaxine • Effexor, Effexor X12
- Ziprasadone • Geodon
Disclosure
Dr. Alpert receives research funding, speaking honoraria or consultation fees from Organon, Forest Pharmaceuticals, GlaxoSmithKline, Pfizer Inc., and Pharmavite.
Dr. Nierenberg receives research funding, speaking honoraria, or consultation fees from Eli Lilly and Co., Wyeth-Ayerst Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Innapharma, BMS, Cyberonics, Lichtner, and Pfizer Inc.
As many as 50% of depressed patients do not achieve a 50% or greater reduction in severity of symptoms after an adequate antidepressant trial.1 Moreover, among those who do respond to acute treatment, longer-term residual depressive symptoms are quite common. Persistent subsyndromal depressive symptoms are associated with impaired psychosocial functioning and increased risk of relapse. This possibility has reinforced an evolving consensus that full depressive remission rather than response is the proper goal of treatment.2
When your patient’s depressive symptoms fail to remit following an initial pharmacotherapy course, you must decide which of numerous possible next steps to pursue, in what combinations, and following what sequence. The range of strategies include the following:
- Pursuing an extended trial with the initial agent using higher than usual dosages (e.g., fluoxetine 40 to 80 mg/qd);
- Augmenting an antidepressant with an agent that offers no established antidepressant efficacy on its own, such as buspirone, lithium, thyroid, or estrogen;
- Combining an antidepressant with another antidepressant, with another somatic therapy such as electroconvulsive treatment or phototherapy, or with some form of psychotherapy;
- Switching to an antidepressant within the same class (e.g., from one selective serotonin reuptake inhibitor [SSRI] to another), or outside of class (from an SSRI to an atypical antidepressant), or switching to non-pharmacological somatic therapy or psychotherapy.3
A predictable lag exists between innovative clinical applications and the randomized, controlled trials (RCTs) designed to evaluate them. The result: Approaches that are thoroughly described in the literature involve medication regimens that are no longer first-line, such as lithium or thyroid augmentation of tricyclic antidepressants (TCAs), or the combination of TCAs with SSRIs. Conversely, approaches most widely used in current psychiatric practice (e.g., addition of bupropion to SSRIs) have received relatively little systematic attention.4
Further, while predictors of initial antidepressant response have been hard to come by, even less is known about predictors of antidepressant response after lack of response to previous antidepressants. The working hypotheses that clinicians use to decide which strategies to pursue, though plausible, are largely untested, whether based on characteristics of a patient’s initial response (e.g., non-response or partial response)5 or on side effects or comorbid diagnoses.
This review will describe new research and emerging strategies that address the common clinical problem of unremitted depression despite one or more adequate courses of antidepressant treatment.
A look at new augmentations and combinations
Adding a second agent is an appealing strategy when patients have tolerated an initial antidepressant without troublesome side effects or have shown partial response (≥ 25% and <50% symptom reduction) and/or when a second agent may serve an important additional goal such as treating a comorbid condition—attention-deficit disorder or smoking, for example—or a side effect (e.g., nausea or insomnia).
Evidence supporting a relation between folate and depression has accrued over decades. Low folate levels may be associated with poorer response to fluoxetine.
Coppen and Bailey25 found that depressed patients had higher initial response rates to fluoxetine when combined with supplemental folic acid (500 mcg) than they did with placebo. The effect was significant only among female subjects. Study participants were naïve to fluoxetine and did not have established treatment resistance.
Still, the safety, tolerability, cost-effectiveness, and high patient acceptability of folic acid should stimulate further study of augmentation with folic acid supplementation and other naturally occurring agents related to folate in the one-carbon cycle, including S-adenosyl-methionine (SAMe).
The primary drawbacks to augmentation/combination strategies are an increased risk of drug interactions, cost, and potential decrement in adherence to treatment as the regimen’s complexity increases.
Thyroid augmentation Although thyroid augmentation of TCAs has been the subject of numerous RCTs, no controlled trials of thyroid augmentation of SSRIs or other newer-generation antidepressants are available.
Lithium augmentation Studies of lithium augmentation of SSRIs have yielded generally modest response rates with questionable durability.6,7
Noradrenergic and/or dopaminergic agonist agents Open trials and case series in the treatment of patients with unremitted depression have supported combining SSRIs and venlafaxine with agents that possess primarily noradrenergic and/or dopaminergic agonist properties. These include bupropion,8 psychostimulants,9 and direct dopamine agonists, including pergolide and pramipexole.10
These combinations have the advantage of potentially ameliorating several common SSRI side effects, particularly sedation, sexual dysfunction, and putative SSRI-related apathy. In the case of psychostimulants and/or bupropion, the combinations may treat such common comorbidities as attention-deficit/hyperactivity disorder and smoking.
Modafinil The combination of the antinarcoleptic modafinil with SSRIs and other newer-generation antidepressants has also attracted interest. Its efficacy as an antidepressant adjunct and as a remedy for side effects must still be established, however.11
Buspirone augmentation The augmentation of SSRIs and other antidepressants with the antianxiety azaperone buspirone is supported by impressive response rates in several open trials. But the only placebo-controlled trial evaluating buspirone augmentation in resistant depression failed to find a significant drug:placebo difference.12 Still, its tolerability and anxiolytic efficacy, and potential for ameliorating sexual dysfunction in some patients, support its judicious use as an augmenting agent pending further study.
Pindolol Studies of this beta-agonist, 5HT-1A antagonist, as an antidepressant-augmenting combination have yielded promising results in some studies, including a negative 10-day, placebo-controlled trial.13 Some patients experience jitteriness or irritability on pindolol. Its role in treating resistant depression remains to be established.
Noradrenergic TCAs The combination of SSRIs with these agents is a good example of a strategy based on pairing complimentary antidepressant mechanisms. Double-blind, controlled trials of fluoxetine plus desipramine6,7 have tempered the enthusiasm generated by earlier open studies, however. Response rates in these trials range from 25% to 30%, no higher than lithium augmentation and slightly lower than higher-dose fluoxetine.
Mirtazapine A more recent combination is mirtazapine with SSRIs or with high-dose venlafaxine.14 In some patients, this combination may attenuate sexual dysfunction, insomnia, and gastrointestinal side effects of SSRIs and venlafaxine by virtue of a 5-HT2, 5-HT3, and histamine receptor blockade by mirtazapine. This combination may also capitalize on the combined antidepressant effects of direct norepinephrine (NE) and serotonin (5-HT) reuptake inhibition as well as an alpha2 adrenergic auto- and hetero-receptor blockade—facilitating presynaptic NE and 5HT release—and 5-HT2 receptor antagonism.
Antipsychotics The use of antipsychotics for nonpsychotic unipolar depression has been controversial. However, in the context of lower apparent risks of tardive dyskinesia with the newer, atypical antipsychotics, their use as antidepressant augmenting agents has been revisited. In one double-blind, placebo-controlled trial, the combination of fluoxetine plus olanzapine was more effective in a well-defined refractory depressed population than was either medication alone.15 (See Box 1 for a discussion of fluoxetine and folic acid.)
Ostroff and Nelson16 reported an extremely rapid (1 week) response after the addition of risperidone among 8 depressed individuals who had not responded to fluoxetine or paroxetine alone. The activating properties of ziprasadone for some patients, combined with its lower propensity for producing weight gain than other atypical antipsychotics, make it another potentially attractive candidate for antidepressant augmentation, though one that requires further study.
Anticonvulsant augmentation While controlled studies of anticonvulsant augmentation of newer-generation antidepressants are lacking, the efficacy of lamotrigine for treating bipolar depression17 has encouraged clinicians to combine the agent with antidepressants in unipolar depression. The potential sedative/anxiolytic effects of other anticonvulsants, including gabapentin and valproate, have also supported their use in resistant depression complicated by anxiety or irritability. Omega-3-fatty acids may work as mood-stabilizing substances when used for antidepressant augmentation.
Antidepressant switches: In class or out?
Switching from TCAs to SSRIs or vice versa following nonresponse has yielded generally high (50% to 60%) response rates.3 But the more common question now is whether to switch from an initial SSRI to another SSRI, to an SSRI-like agent with an additional noradrenergic mechanism (such as venlafaxine), or to an atypical antidepressant such as bupropion or mirtazapine.
Traditional teaching and regular psychiatric practice5 have favored a switch outside of class. More recently, however, a number of uncontrolled studies looking at response rates to a second SSRI following inadequate response to and/or tolerance of an initial SSRI have shown response rates of 40% to 75%.18 This coincides with response rates seen in studies involving crossover to antidepressants with differing mechanisms. Inferences from these studies are limited by, first, determining nonresponse retrospectively and, second, lumping together patients who did not remain on the first SSRI because of nonresponse with others who curtailed treatment because of intolerance.
Because of its dual 5-HT/NE reuptake inhibitory activity, particularly at higher doses, venlafaxine is a popular choice following failure of an initial SSRI. One-third of subjects with extremely resistant depression (3 or more trials of antidepressants) responded to venlafaxine.19 DeMontigny et al20 also demonstrated its efficacy (with a 58% response rate) as a switching agent among resistant, depressed patients.
But comparative response rates for switching to venlafaxine from an SSRI vs. from other antidepressant classes have not been well delineated, nor have response rates for switching from SSRIs and other antidepressants to venlafaxine vs. to other non-SSRI agents.
Switches from SSRIs to nefazodone and mirtazapine or the selective NE reuptake inhibitor reboxetine have shown reasonably high response rates.3 Studies comparing these various strategies head to head are lacking. In the absence of data, reasonable guides for switching agents include projected tolerability and existence of comorbid conditions that may respond well to one agent over another.
Another issue is whether to cross-taper, that is, to lower the dose of the first agent while titrating up the next, or to discontinue the first before the second. When catastrophic drug interactions would result from overlap, such as the switch from an SSRI to a monoamine oxidase (MAO) inhibitor, a suitable wash-out period is necessary. This can also occur if the first antidepressant is believed to cause intolerable side effects.
In most other instances where no absolute contraindications exist, cross-tapering may be worthwhile despite theoretical risks such as serotonin syndrome when overlapping an SSRI and venlafaxine. Cross-tapering helps reduce the risk of side effects associated with abrupt discontinuation including nausea, myalgias, headache, and dysphoria, and may minimize the loss of antidepressant benefit from the initial agent before the impact of the second agent is realized.
Great hope for new knowledge with STAR*D
In recognition of the substantial direct and indirect costs, the morbidity and mortality associated with unremitted depression, and the considerable gaps in knowledge about optimal management at key decision points, the National Institute of Mental Health (NIMH) has launched the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. This is a multisite, multistep, prospective, randomized clinical trial of nonpsychotic major depressive disorder.21
STAR*D represents an initiative unique in the history of depression trials. Its novel “equipoise stratified” design26 is a hybrid between strict randomization to treatments without input from patients or clinicians (completely randomized designs) and a form of randomization in which subjects are assigned only to overarching strategies within which the particular treatments are determined by the clinician (clinician’s choice designs).
In STAR*D, a patient is asked at each level to define with his or her clinician all treatments that would be equally acceptable, based on a preference for certain potential side effects over others, interest in psychotherapy, or desire to retain a medication to which the patient has responded at least partially.
To be eligible to continue, however, the patient must agree to be randomized across at least some of the options testing some of the key hypotheses. For example, a participant at Level 2 who specifically wished to pursue cognitive therapy could not do that alone, but could agree to randomization to cognitive therapy with or without citalopram.
The study seeks to balance the goal of recruiting and retaining a broadly representative cross-section of patients—many of whom would reasonably expect to have some say about their treatment with their prescribing clinician—with the goal of providing statistically rigorous, randomization-based inferences upon which meaningful treatment guidelines can be based.
Approximately 4,000 adult depressed outpatients without an established history of antidepressant resistance will be recruited across the country. In contrast to efficacy trials, this effectiveness trial is meant to more closely parallel real-world practice (Box 2).
Patients will be treated initially with citalopram for 12 weeks (Level 1), with general guidelines for titration based upon response and tolerability. It is anticipated that about 2,000 individuals will exhibit unremitting depression despite SSRI treatment and/or intolerance to the SSRI and will be eligible for randomization to subsequent “switching” or “augmentation” strategies; this forms the core of the study across 3 successive levels.
Level 2 randomized treatments include four distinctive switching options—sertraline, bupropion, venlafaxine, or cognitive therapy—as well as 3 augmentation options—citalopram plus bupropion, buspirone, or cognitive therapy.
Those whose depression fails to remit will be eligible for randomization at Level 3 to different switching options—mirtazapine vs. nortripyline, or augmentation options—lithium or thyroid—added to the primary Level 2 antidepressant.
Finally, at Level 4, patients who continue to have unremitted depression despite successive treatments will be eligible for random assignment to 1 of 2 switching options—tranylcypromine vs. the combination of mirtazapine and venlafaxine.
Two new somatic treatments
Repetitive Transcranial Magnetic Stimulation (rTMS) rTMS is used to induce electrical current in the brain without causing seizures. Using a rapidly alternating current with a hand-held electromagnet that generates about 2 tesla will, in turn, induce or interrupt current (depolarization) in cortical interneurons about 2 cm below the surface of the skull. rTMS delivers energy without the impedance of the skull and is thought to be excitatory at high frequencies (20 Hz) and inhibitory at lower frequencies (5 to 10 Hz). This theory remains to be rigorously tested.22
Overall, the clinical effects reported in early studies on rTMS have been modest at best, while later studies have found either no difference from placebo or a robust effect. rTMS is a potential therapeutic tool not yet ready for widespread clinical use.22
Vagus Nerve Stimulation The NeuroCybernetic Prosthesis System (Cyberonics, Inc., Houston, Tex) stimulates the left cervical vagus nerve for treatment of resistant partial-onset epileptic seizures.
An open treatment trial of vagus nerve stimulation (VNS) for treatment-resistant depression was done based on mood improvement observed in patients treated for seizure disorders. Positron-emission tomographic studies revealed activation of limbic structures and neurochemical and neuronal pathway findings.23 The investigators reported improvement in a sample of 30 patients with major depressive disorder. Definite conclusions await controlled clinical trials and more work is needed to determine the ultimate mechanisms that might be antidepressant.23,24
Related resources
- Amsterdam JD, Hornig M, Nierenberg AA. Treatment-resistant mood disorders. Cambridge, UK: Cambridge University Press; 2001.
- Stahl SM. Essential Psychopharmacology. Cambridge, UK: Cambridge University Press; 1996.
- STAR*D Web site: http://www.edc.gsph.pitt.edu/stard
Drug brand names
- Bupropion • Wellbutrin, Wellbutrin SR, Zybar
- Buspirone • BuSpar, BuSpar DIVIDOSE
- Citalopram • Celexa
- Fluoxetine • Prozac, Prozac Weekly
- Gabapentin • Neurontin
- Haloperidol • Haldol, Haldol Decanoate
- Lamotrigine • Lamictal
- Mirtazapine • Remeron, Remeron Soltab
- Modafinil • Provigil
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Pergolide • Permax
- Pramipexole • Mirapex
- Reboxetine • Edronax
- Risperidone • Risperidal
- S-adenosyl-methionine • SAM-e
- Sertaline • Zoloft
- Tranycypromine • Nardil, Parnate, Marplan
- Valproate • Epival, Deprox, Alti-Valproic
- Venlafaxine • Effexor, Effexor X12
- Ziprasadone • Geodon
Disclosure
Dr. Alpert receives research funding, speaking honoraria or consultation fees from Organon, Forest Pharmaceuticals, GlaxoSmithKline, Pfizer Inc., and Pharmavite.
Dr. Nierenberg receives research funding, speaking honoraria, or consultation fees from Eli Lilly and Co., Wyeth-Ayerst Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Innapharma, BMS, Cyberonics, Lichtner, and Pfizer Inc.
1. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. The Psychiatric Clinics of North America: Treatment Resistant Depression. 1997;19(2):179-200.
2. Nierenberg AA, Wright EC. Evolution of remission as the new standard in the treatment of depression. J Clin Psychiatry. 1999;60(22):7-11.
3. Rosenbaum JF, Fava M, Nierenberg AA, Sachs GS. Treatment-resistant mood disorders. In Gabbard GO, ed. Treatments of Psychiatric Disorders. 3rd ed. Washington, DC: American Psychiatric Press, Inc.; 2001;1307-1384.
4. Mischoulon D, Fava M, Rosenbaum JF. Strategies for augmentation of SSRI treatment: A survey of an academic psychopharmacology practice Harvard Review of Psychiatry. 1999;6:322-326.
5. Fredman SJ, Fava M, Kienke AS, et al. Partial response, non-response, and relapse on SSRIs in major depression: A survey of current “next-step” practices. J Clin Psychiatry. 2000;61:403-407.
6. Fava M, Rosenbaum JF, McGrath PJ, et al. A double-blind, controlled study of lithium and tricyclic augmentation of fluoxetine in treatment resistant depression. Am J Psychiatry. 1994;151:1372-1374.
7. Fava M, Alpert JE, Nierenberg AA, Worthington JJ, III, Rosenbaum JF. Double-blind study of high-dose fluoxetine versus lithium or desipramine augmentation of fluoxetine in partial and nonresponders to fluoxetine. 153rd Annual Meeting of the American Psychiatric Association. Chicago, Ill, 2000.
8. Bodkin JA, Lasser RA, Wines JD, Jr, et al. Combining serotonin reuptake inhibitors and bupropion in partial responders to antidepressant monotherapy. J Clin Psychiatry. 1997;58:137-145.
9. Stoll AL, Pillay SS, et al. Methylphenidate augmentation of serotonin selective reuptake inhibitors: a case series. J Clin Psychiatry. 1996;57(8):356-359.
10. Sporn J, Ghaemi SN, Sambur MR, Rankin MA, Recht J, Sachs GS, Rosenbaum JF, Fava M. Pramipexole augmentation in the treatment of unipolar and bipolar depression: A retrospective chart review. Ann Clin Psychiatry. 2000;12:137-140.
11. Menza MA, Kaufman KR, Castellanos A. Modafinil augmentation of antidepressant treatment in depression. J Clin Psychiatry. 2000;61(5):378-81.
12. Landen M, Bjorling G, Agren H, Fahlen T. A randomized double-blind placebo-controlled trial of buspirone in combination with an SSRI in patients with treatment-refractory depression. J Clin Psychiatry. 1998;59:664-668.
13. Perez V, Soler J, Puigdemont D, et al. A double-blind, randomized, placebo-controlled trial of pindolol augmentation in depressive patients resistant to serotonin reuptake inhibitors. Arch Gen Psychiatry. 1999;56:375-379.
14. Carpenter LL, Jocic Z, Hall JM, Rasmussen SA, Price LH. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry. 1999;60(1):45-49.
15. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry. 2001;158:131-134.
16. Ostroff RB, Nelson JC. Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry. 1999;60:256-259.
17. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry. 1999;60:79-88.
18. Thase ME, Blomgren SL, Birkett MA, et al. Fluoxetine treatment of patients with major depressive disorder who failed initial treatment with sertraline. J Clin Psychiatry 1997;58:16-21.
19. Nierenberg AA, Feighner JP, Rudolph R, et al. Venlafaxine for treatment-resistant unipolar depression. J Clin Psychopharmacol. 1994;14:419-423.
20. de Montigny C, Silverstone PH, et al. Venlafaxine in treatment-resistant major depression: a Canadian multicenter, open-label trial. J Clin Psychopharmacol. 1999;19(5):401-406.
21. Rush JA, Fava M, et al. Sequenced treatment alternatives to relieve depression (STAR*D): Rationale and design. Controlled Clinical Trials. 2001 (in press).
22. Sackeim H. Repetitive transcranial magnetic stimulation: What are the next steps? Biol Psychiatry. 2000;48:959-961.
23. Rush AJ, George MS, Sackeim HA, et al. Vagus Nerve Stimulation (VNSTM) for treatment-resistant depressions: A multicenter study. Biol Psychiatry 2000;47.-
24. Rosenbaum JF, Heninger G. Vagus nerve stimulation for treatment-resistant depression. BiolPsychiatry. 2000;47(4):273-275.
25. Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affective Disord. 2000;60:121-130.
26. Lavori PW, Rush JA, Wisniewski SR, et al. Strengthening clinical effectiveness trials: Equipose-stratified randomization. Biol Psychiatry. 2001;50(10):792-801.
1. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. The Psychiatric Clinics of North America: Treatment Resistant Depression. 1997;19(2):179-200.
2. Nierenberg AA, Wright EC. Evolution of remission as the new standard in the treatment of depression. J Clin Psychiatry. 1999;60(22):7-11.
3. Rosenbaum JF, Fava M, Nierenberg AA, Sachs GS. Treatment-resistant mood disorders. In Gabbard GO, ed. Treatments of Psychiatric Disorders. 3rd ed. Washington, DC: American Psychiatric Press, Inc.; 2001;1307-1384.
4. Mischoulon D, Fava M, Rosenbaum JF. Strategies for augmentation of SSRI treatment: A survey of an academic psychopharmacology practice Harvard Review of Psychiatry. 1999;6:322-326.
5. Fredman SJ, Fava M, Kienke AS, et al. Partial response, non-response, and relapse on SSRIs in major depression: A survey of current “next-step” practices. J Clin Psychiatry. 2000;61:403-407.
6. Fava M, Rosenbaum JF, McGrath PJ, et al. A double-blind, controlled study of lithium and tricyclic augmentation of fluoxetine in treatment resistant depression. Am J Psychiatry. 1994;151:1372-1374.
7. Fava M, Alpert JE, Nierenberg AA, Worthington JJ, III, Rosenbaum JF. Double-blind study of high-dose fluoxetine versus lithium or desipramine augmentation of fluoxetine in partial and nonresponders to fluoxetine. 153rd Annual Meeting of the American Psychiatric Association. Chicago, Ill, 2000.
8. Bodkin JA, Lasser RA, Wines JD, Jr, et al. Combining serotonin reuptake inhibitors and bupropion in partial responders to antidepressant monotherapy. J Clin Psychiatry. 1997;58:137-145.
9. Stoll AL, Pillay SS, et al. Methylphenidate augmentation of serotonin selective reuptake inhibitors: a case series. J Clin Psychiatry. 1996;57(8):356-359.
10. Sporn J, Ghaemi SN, Sambur MR, Rankin MA, Recht J, Sachs GS, Rosenbaum JF, Fava M. Pramipexole augmentation in the treatment of unipolar and bipolar depression: A retrospective chart review. Ann Clin Psychiatry. 2000;12:137-140.
11. Menza MA, Kaufman KR, Castellanos A. Modafinil augmentation of antidepressant treatment in depression. J Clin Psychiatry. 2000;61(5):378-81.
12. Landen M, Bjorling G, Agren H, Fahlen T. A randomized double-blind placebo-controlled trial of buspirone in combination with an SSRI in patients with treatment-refractory depression. J Clin Psychiatry. 1998;59:664-668.
13. Perez V, Soler J, Puigdemont D, et al. A double-blind, randomized, placebo-controlled trial of pindolol augmentation in depressive patients resistant to serotonin reuptake inhibitors. Arch Gen Psychiatry. 1999;56:375-379.
14. Carpenter LL, Jocic Z, Hall JM, Rasmussen SA, Price LH. Mirtazapine augmentation in the treatment of refractory depression. J Clin Psychiatry. 1999;60(1):45-49.
15. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry. 2001;158:131-134.
16. Ostroff RB, Nelson JC. Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry. 1999;60:256-259.
17. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry. 1999;60:79-88.
18. Thase ME, Blomgren SL, Birkett MA, et al. Fluoxetine treatment of patients with major depressive disorder who failed initial treatment with sertraline. J Clin Psychiatry 1997;58:16-21.
19. Nierenberg AA, Feighner JP, Rudolph R, et al. Venlafaxine for treatment-resistant unipolar depression. J Clin Psychopharmacol. 1994;14:419-423.
20. de Montigny C, Silverstone PH, et al. Venlafaxine in treatment-resistant major depression: a Canadian multicenter, open-label trial. J Clin Psychopharmacol. 1999;19(5):401-406.
21. Rush JA, Fava M, et al. Sequenced treatment alternatives to relieve depression (STAR*D): Rationale and design. Controlled Clinical Trials. 2001 (in press).
22. Sackeim H. Repetitive transcranial magnetic stimulation: What are the next steps? Biol Psychiatry. 2000;48:959-961.
23. Rush AJ, George MS, Sackeim HA, et al. Vagus Nerve Stimulation (VNSTM) for treatment-resistant depressions: A multicenter study. Biol Psychiatry 2000;47.-
24. Rosenbaum JF, Heninger G. Vagus nerve stimulation for treatment-resistant depression. BiolPsychiatry. 2000;47(4):273-275.
25. Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affective Disord. 2000;60:121-130.
26. Lavori PW, Rush JA, Wisniewski SR, et al. Strengthening clinical effectiveness trials: Equipose-stratified randomization. Biol Psychiatry. 2001;50(10):792-801.
Innovative and practical treatments for obsessive-compulsive disorder
When you suspect a patient has obsessive-compulsive disorder (OCD) (Box 1), how can you differentiate OCD from psychosis? Once you have made the diagnosis, what critical factors suggest treatment will be successful—or unsuccessful? Is behavioral therapy more effective than medication? Which medications are most likely to be effective? The answers to these questions can help you improve the well-being of your patients with OCD.
Differential diagnosis
Unfortunately, many patients with severe OCD are misdiagnosed with psychosis or schizophrenia spectrum disorder and spend many years suffering without proper treatment.3 Despite many similarities between patients with severe OCD and psychosis—including rigid belief systems, unrealistic concerns, magical thinking, and odd behaviors—patients with OCD can recognize the irrational nature of their beliefs when they are not in the throes of anxiety.
Patients with OCD also will usually respond to behavioral interventions within a few weeks while patients who are psychotic usually get progressively worse. Treatment must be given time as both cohorts will get anxious or increase their negative symptoms initially, but patients with OCD should soon habituate and find symptom relief.
Some patients have OCD with psychotic features and tend to have more difficulty responding to behavior therapy without medication. Patients with both OCD and schizotypal personalities respond poorly to both behavior therapy and psychotropic medications.4
Obsessions are intrusive and unwanted thoughts, images, or impulses that produce anxiety. They commonly consist of obsessive fears involving causing harm to others, contamination, safety, religiosity, incompletion, pathological doubt, magical thinking, and the need for certainty, and symmetry.
Usually, obsessions will be accompanied by compulsions, which are behaviors or thoughts performed to reduce the anxiety caused by the obsessions. Compulsions typically consist of excessive washing, checking until it “feels right,” and mental retracing. In rare cases, patients present with only obsessions, which are more difficult to treat than compulsions. Most patients will have several types of symptoms.
To meet the criteria for OCD, patients must be preoccupied by obsessive thoughts and engage in compulsions, which will be frequent, intense, of long duration (more than 2 hours/day), and interfere with the individual’s ability to function. The Yale-Brown Obsessive Compulsive Symptoms Checklist and Scale1 are reliable assessment tools to identify types of symptoms and degree of severity.
Anne is a 53-year-old widow whose OCD symptoms consisted of not letting anything pass her lips that she considered contaminated, lest she become ill with cancer. Her symptoms became so severe that she restricted her diet to a specific brand of peanut butter and milk. The manner in which she ate the peanut butter was rife with checking rituals. If she thought that there might be something wrong with the jar, she threw it away. If she thought the jar was “safe,” she poured the peanut butter directly into her mouth, avoiding the risk of dirty utensils. She drank milk out of the carton. By the time she began treatment, she was malnourished and slightly dehydrated.
Anne’s restrictive diet was also a product of obsessive label checking. Her label reading inevitably resulted in her seeing ordinary household items that she considered risky and would then avoid. Other avoidance behaviors included spitting out saliva and not licking her lips due to fear of what might be ingested, and avoidance of medication, toothpaste, eye drops, skin lotion, and food she feared others had touched.
The good intentions of people in Anne’s community had the effect of enabling her OCD. For example, the local grocer made sure to keep a few cases of Anne's preferred brand of peanut butter in stock for when she needed it. She bought in bulk, but returned unopened jars that she thought were contaminated. As is common with obsessions, no real evidence is needed to legitimize avoidance.
To help Anne break the OCD cycle of avoidance, a meal plan was devised. Although she looked anorexic, but was not, this approach succeeded because she greatly missed the experience of eating and tasting a variety of foods. She also agreed to drink daily nutritional supplements until her diet was more enriching, and had weekly weigh-ins to track her weight gain.
Anne also began a regimen of fluoxetine, which ultimately improved her ability to use the behavior therapy techniques. She was started at 5 mg/d in liquid form. The dosage was increased to 40 mg/d across 1 month, then changed to pill form and titrated to 80 mg/d, which was maintained at discharge.
Exposure and response prevention therapy (ERP) was also administered in twice-daily, 2-hour sessions for about 3 months. Exposure therapy consisted of accompanying Anne to the local supermarket and having her purchase any kind of food that she wanted, regardless of its nutritional value. Her initial purchases consisted of cheesecake, doughnuts, juice, herbal tea, canned ravioli, cereal, lasagna, and snacks.
For response prevention related to food purchases, Anne was prevented from reading labels and examining individual items for imperfections. She was encouraged to buy the first item on the shelf and put it in her basket.
The next step in exposure therapy was to supervise her eating habits. While she looked forward to tasting the food she bought, she was apprehensive because of the obsessive doubt about their purity. Firm but kind encouragement helped her take one bite after another, and this success built on itself. She was excited to be finally confronting her obsessive fears, tasting the foods she restricted herself from for so long, and taking better care of herself. Her complexion improved, and her weight increased.
At times she was highly anxious and looked for ways to avoid the exposure, but with redirection was able to stay on track. She eventually was able to eat community food, eat at a restaurant, use beauty and hygiene products, and have contact with artificial or chemical substances.
Ironically, Anne’s vocational interest was in cooking and after discharge from the program, she investigated employment in hotel/restaurant work and studies at culinary school.
Predictors for successful treatment
Insight Researchers3 found that about 52% of patients with insight into the reasonableness of their obsessions responded to medications, while none who lacked insight responded. Therefore, it pays to assess patients’ insight and ability to recognize the long-term consequences of OCD to themselves and those around them.
Some patients who have suffered with treatment-refractory OCD for most of their lives lack a premorbid high level of functioning to serve as a reference for normalized behavior. Educating these patients to see the advantages of living without certain negative behaviors improves their receptivity to treatment.
Patients who lack insight often refuse to acknowledge that many of their behaviors are manifestations of OCD. Such patients, however, are usually more amenable to giving up or modifying their dysfunctional behaviors—and the clinician more likely to avoid confrontations—if they are shown how certain behaviors undermine their goals.
Cost-benefit analysis Because of the aversive nature of exposure and response prevention therapy (ERP) and the negative side effects of many medications, some patients may find it easier to live with their symptoms, as painful as they are, rather than undergo the discomfort of behavior therapy. Because the prognosis is poor in such cases, patients need to be convinced that the discomfort of treatment is merely short-term, while the discomfort of the illness could last forever if left untreated.
Motivation In our experience, motivation has played a crucial role in determining treatment outcome for severe refractory OCD. And regardless of the severity of their symptoms, patients who are fed up with their symptoms, or are tired of living a life controlled by their obsessions, usually are excellent candidates for treatment.
Conversely, those who enter treatment as a result of external pressure from spouses or family face a less positive prognosis. High emotional expressiveness, overinvolvement, and hostility by relatives is related to higher attrition rates in treatment.3 Because ERP is so aversive, these patients will find ways to dilute the treatment’s effectiveness. In many cases, they do the minimal amount of work required to stay in treatment to avoid whatever consequences their families would impose for not adhering to treatment.
One marker to assess compliance is whether the clinician feels he or she is investing more time and effort into the patient’s treatment than the patient is. If so, this should be addressed in a timely manner. Also, sporadic attendance at sessions and noncompliance with medications, homework, and behavior therapy assignments may also portend a poor outcome. Remember, though, that noncompliance and lack of motivation are fluid states; many previously noncompliant patients later return to treatment better motivated and more compliant.
Predictors for a lower success rate
Secondary gain Researchers4 found that patients who were enabled by their families had more severe symptoms than those who were not. These relational and environmental factors should be discussed openly. If the patient finds that many of his or her life needs are being met secondary to the illness, that patient might not agree to an aversive treatment.
To overcome this, urge family members or other individuals who provide dysfunctional reinforcers to remove them from the environment. Meet with the patient and family/friends and frankly point out dysfunctional gains and the ways in which family members unknowingly allow the gains to continue (e.g., giving the patient more money after he or she overspent his or her allowance). A family behavioral contract should be devised to address how these gains will be reasonably eliminated.
Recognize, too, that a patient may find it difficult to give up the secondary gains, detrimental as they may be, without adequate skills or coping mechanisms to fill the void. So in some cases, it is best not to remove all the secondary gains at once; this can cause many patients to terminate treatment prematurely.
Trauma or abuse history Many patients with treatment refractory OCD have trauma histories and cannot habituate to the behavioral tasks because of dissociation, emotional numbing, or some form of distraction that mediates their anxiety and prevents proper habituation. If the patient is adequately complying with the exposures, yet still is unable to confront every feared stimulus, inquire about a trauma or abuse history (Box 2).
Substance abuse The stress that is inherent to ERP can cause many patients to relapse or abuse illicit substances to manage their anxiety. Therefore, patients with severe substance abuse problems often have great difficulty handling ERP, as they are asked to experience the very discomfort that initially caused them to abuse drugs and alcohol.
Exposure and response prevention therapy (ERP) may be contraindicated for OCD patients with comorbid posttraumatic stress disorder (PTSD). Patients with trauma histories, especially those for whom the trauma precipitated the onset of OCD symptoms, should receive trauma treatment before or in conjunction with ERP in order to be effective.
Patients with OCD and PTSD should receive adjunctive cognitive behavioral therapy (CBT) for their PTSD. Skills training modules, such as dialectical behavior therapy (DBT) and other CBT treatments, often provide the patient with the necessary skills to regulate the trauma-related stressors that are triggered during ERP and can cause premature termination of treatment.
If habituation is not occurring in the absence of trauma, ask whether the patient is dissociating, daydreaming, numbing, or distracting, as these avoidances will jeopardize his or her ability to benefit from ERP. Teaching the patient grounding techniques and alternate coping mechanisms, such as those found in the mindfulness and distress tolerance module of DBT, can help some patients tolerate their anxiety.
For trauma patients whose dissociation, numbing, or distraction is severe, home-based or residential treatment may be required. There, they can be coached during ERP to bring their attention back to the feared stimuli and deal with the negative fallout of their trauma..
In such cases, a patient cannot realistically be asked to give up a coping mechanism, faulty as it may be, until a more functional reinforcer takes its place. Hence, skills training is a crucial part of treatment for this group.
Residential treatment for OCD patients with comorbid substance abuse in remission may be necessary to ensure a positive outcome. Patients should continue recovery work concurrent to behavior therapy to prevent relapse.
High-risk OCD symptoms Patients who have more traditional OCD symptoms usually have a good prognosis. Unfortunately some symptoms do not respond to ERP treatment. These include:
- Repeating, hoarding, and symmetry. Though evidence suggests that hoarding is predictive of poor outcomes,5 treatment carried out in the home can be effective over a 24-week trial.6
- Incompletion, or the need for things to feel right.
- Rigid and overvalued belief systems.
- Sexual and religious obsessions. These appear to be more resistant to behavior therapy and selective serotonin reuptake inhibitors (SSRIs).7
More research needs to be conducted to offer patients with these symptoms better respite.
Researchers also found that patients with childhood and adolescent onset of symptoms, tics, history of hospitalization, and terminated treatment against medical advice are more likely than other OCD patients to develop more severe symptoms in adulthood.8 Patients with OCD who also suffer from generalized anxiety disorders are more likely than those without GAD to drop out of treatment.9
Behavior therapy: first choice
ERP is considered the premier treatment for OCD and is suitable for both adults and children.10 Exposure forces patients to confront their feared stimuli. Response prevention blocks patients from engaging in compulsions or avoidance behaviors intended to reduce their discomfort. Patients are asked to identify situations that trigger their obsession and compulsions and rank them along a fear hierarchy. Patients confront a moderately rated situation and, once they become habituated to it, move up the fear hierarchy to the next situation.
ERP has been proven effective for OCD not only as an individual behavior treatment, but also when done in a group setting11 or when delivered online or by telephone.12
Table 1
Dosage levels for SRIs in OCD
| Clomipramine | 150-200 mg/d |
| Fluoxetine | 40-80 mg/d |
| Sertraline | 50-200 mg/d |
| Fluvoxamine | 200-300 mg/d |
| Paroxetine | 40-60 mg/d |
| Citalopram | 40-60 mg/d |
| The higher end of the dosage ranges shown above is preferred if tolerated. All clinical trials with SRIs for OCD should last at least 10 weeks. | |
Some clinicians prefer cognitive behavioral therapy (CBT) to ERP because it is less aversive. Researchers found that patients who were treated with either CBT or ERP improved. Patients treated with ERP, however, were more likely to maintain their gains in recovery 3 months after treatment concluded.13 Evidence suggests that ERP or CBT when implemented alone, or when applied in conjunction with fluvoxamine,14 are equally effective.
ERP should be managed only by clinicians specially trained in this modality. Several treatment centers across the country provide specialized care for OCD patients. For the nearest treatment center in your community that accepts referrals for ERP, contact the OC Foundation in North Branford, Conn. (See Related Resources.)
Medication for OCD: SRIs as first-line therapy
Experts agree that first-line somatic treatments for OCD include not only behavior therapy but also serotonin reuptake inhibitors (SRIs),15 that is, clomipramine or selective serotonin reuptake inhibitors, (SSRIs) (Table 1).
Caution: Many patients who “respond” to treatment in clinical studies remain symptomatic and meaningfully affected by their residual illness. Therefore, it is critical that you inform patients at the outset that 100% reduction in symptoms is rare.
SRIs Overwhelming evidence from multiple randomized, double-blind, placebo-controlled studies support the efficacy of SRIs. In adults, well-designed and controlled trials have demonstrated the relative efficacy of clomipramine, fluoxetine, sertraline, paroxetine, and fluvoxamine vs. placebo.
SRIs also have been shown to be significantly more effective than tricyclic antidepressants (TCAs) in both placebo-controlled and non-placebo-controlled studies.
Despite initial reports that clomipramine may be more effective than SSRIs, a growing number of studies and a recent comprehensive literature review suggest that the SRIs all have comparable efficacy.16 Because clomipramine has significantly more anticholinergic- and antiadrenergic-mediated side effects than the SSRIs, however, many clinicians choose SSRIs as the initial agent.
When using SRIs, remember that response is typically delayed; an adequate trial requires at least 10 weeks. Indeed, a meaningful proportion of responders continue to emerge past the 8-week mark. Experts suggest that optimal dosages of SRIs for OCD may exceed those typically used for major depression. Guidelines for SRI dosage ranges for OCD appear in (Table 1).
Data regarding treatment duration also suggest that discontinuation of SRIs results in a high relapse rate, though the use of lower maintenance dosages of SRIs is still debated.
Table 2
Ratings of SRI-augmenting agents for OCD treatment
| Likely effective ♦♦ | Possibly effective (insufficient data for adequate assessment of efficacy) ♦ |
|---|---|
| Neuroleptics | Clonidine |
| Busipirone | Fenfluramine |
| Clonazepam | Nortriptyline |
| Lithium | Pindolol |
| Trazodone | |
| Tryptophan | |
| Dosage for these agents has not been adequately studied for augmentation of SRIs. Clinical trial length should be for 2 to 8 weeks. | |
Augmentation of SRIs When first-line interventions fail, second-line pharmacological approaches include augmentation of SRIs with additional medications (Table 2). Numerous agents have been tried for patients who were unresponsive or only partially responsive to SRIs alone.17 Few controlled trials of such strategies have been conducted, however. The most impressive data document the benefits of adding low doses of dopamine antagonists (both conventional and atypical neuroleptics).
Table 3
Using alternative monotherapies
| Drug | Dosage | Duration | Comments |
|---|---|---|---|
| Clonazepam | 0.5-5 mg/d | ≥ 4 weeks | extrapolated from experience with benzodiazepines for other anxiety disorders and a few reports in OCD |
| MAO inhibitor | 60-90 mg/d | ≥ 10 weeks | extrapolated from clinical practice with MAO inhibitors for major depression, panic disorder; tyramine diet must be adhered to; adequate washout of most antidepressants is required before initiating |
| Buspirone | up to 60 mg/d | ≥ 6 weeks | reflecting protocols adopted in clinical trials for OCD |
Recent uncontrolled studies of augmentation with atypical neuroleptics have yielded encouraging preliminary results, as has one controlled trial of augmentation of an SRI with risperidone. Other data suggest that lithium, buspirone, and clonazepam may also be effective.
Numerous other agents have been tried in combination with SRIs, including clonidine, tryptophan, fenfluramine, pindolol, trazodone, nortriptyline, and other antidepressants. The small number of subjects, lack of sufficient controls, and mixed results preclude drawing even preliminary conclusions as to the potential efficacy of such strategies.
Alternative Monotherapies For patients who do not respond satisfactorily to trials of SRIs alone or to augmentation strategies, consider alternative monotherapies in place of SRIs (Table 3). In addition to uncontrolled data, positive controlled studies lend some support for trials of clonazepam, monoamine oxidase (MAO) inhibitors, and buspirone.
Pertinent negative findings are worthy of mention. In contrast to promising results with risperidone as an augmenter, an open trial of the atypical antipsychotic clozapine suggests inefficacy as a monotherapy. Several case reports suggest that clozapine can actually precipitate obsessive-compulsive symptoms in patients with psychotic disorders.18 Controlled trials have not demonstrated the efficacy of trazodone, clonidine, and diphenhydramine as monotherapies.
Pharmacotherapy + or vs. behavioral therapy
Only a few studies directly comparing behavior therapy vs. medication have been reported. In practice, the two are routinely used in concert. Experts have long recommended this treatment approach. Two recent studies19,20 have demonstrated that the combination is more effective than either treatment alone.
In another study, behavior therapy significantly outperformed clomipramine; no significant incremental benefit was seen from the two treatments in combination.21 However, the dosages of clomipramine were relatively low (mean=164 mg/d and maximum=225 mg/d) and of inadequate duration (6 weeks). Still another older head-to-head comparison of behavior therapy and clomipramine showed that medication was better for reducing obsessional doubt, whereas behavior therapy more effectively reduced compulsive rituals.
Third-line treatments may include the unproven augmentation therapies described above, or intravenous clomipramine if available.22
Treatments of last resort
Finally, other nonpharmacologic treatments, including neurosurgery and electroconvulsive therapy (ECT), have remained controversial and are reserved for particular clinical situations or as treatments of last resort.
Despite a large body of uncontrolled data reporting antiobsessional benefits from a variety of neurosurgical procedures, ethical considerations and technical limitations have precluded the performance of sham-controlled studies to definitively establish the efficacy of these strategies.
Neurosurgical treatment of OCD is reserved for patients with severe and debilitating illness who have failed an exhaustive array of other available treatment options and who provide informed consent or assent. Currently, the most commonly employed neurosurgical treatments for OCD include anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leukotomy. In recent prospective trials of cingulotomy and capsulotomy, approximately 45% of patients experienced a 35% or more symptom reduction.
With the advent of innovative surgical devices that allow functional neurosurgery without craniotomy (e.g., by gamma knife), the performance of ethical, double-blind, sham-controlled trials of neurosurgery for OCD is now feasible. A team of investigators from Brown University and Massachusetts General Hospital is conducting one such study that tests the efficacy of anterior capsulotomy.
There are no controlled data regarding the efficacy of ECT for OCD. Given the high comorbidity of major affective illness in OCD and the well-established efficacy of ECT for major depression, it is not surprising that some patients with OCD have reportedly shown clinical improvement after ECT. Several limited case series and anecdotal reports suggest that ECT may help in some circumstances, and such intervention would seem prudent in some cases where severe, comorbid affective illness is present.23
- Jenike MA, Baer, L, Minichiello WE, eds. Obsessive Compulsive Disorders: Practical Management. 3rd ed. Boston: Mosby, 1998.
- Jenike MA. An update on obsessive-compulsive disorder. Bulletin of the Menninger Clinic. 2001;65:4-25.
- Obsessive-Compulsive Foundation, (203) 315-2190, www.ocfoundation.org
Drug brand names
- Buspirone • BuSpar, BuSpar DIVIDOSE
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Ativar, Diastat, Halcion
- Clonidine • Catapres, Catapres TTS-1
- Clozapine • Clozaril
- Fenfluramine • Pondimin
- Fluoxetine • Prozac, Prozac Weekly
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Phenelzine • Nardil, Parnate
- Pindolol • Inderol, Corgard, Betaloc
- Risperidone • Risperidal
- Sertaline • Zoloft
- Trazodone • Desyrel
- Tryptophan* • L-Tryptophan, Alti-trytophan
Disclosure
Dr. Boxill and Ms. Shapiro report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
Dr. Dougherty reports conflicts of interest with Pfizer Inc., Forest Pharmaceuticals, and Solvay Pharmaceuticals.
1. Goodman Wk, Price LH, et al. The Yale Brown Obsessive Compulsive Scale:1. Development, Use and Reliability. Arch Gen Psychiatry. 1989;46:1012-1016.
2. Catapano F, Sperandeo R, Perris F, Lanzaro M, Maj M. Insight and resistance in patients with obsessive compulsive disorder. Psychopathol. 2001;34(2):62-68.
3. Amir N, Freshman M, Foa EB. Family distress and involvement in relatives of obsessive-compulsive disorder patients. J Anxiety Disord. 2000;14(3):209-217.
4. Chambless DL, Steketee G. Expressed emotion and behavior therapy outcome: a prospective study with obsessive compulsive and agrophobic outpatients. J Consult Clin Psychol. 1999;67(5):658-665.
5. Black DW, Monahan P, Gable J, et al. Hoarding and treatment in 38 nondepressed subjects with OCD. J Clin Psychiatry. 1998;59(8):420-425.
6. Rosqvist J, Egan D, Manzo P, et al. Home-based behavior therapy for obsessive compulsive disorder: A case series with data. J Anxiety Disord. 2001;15(5):395-400.
7. Alonso P, Menchon JM, Pifarre J, et al. Long term follow up and predictors of clinical outcome in obsessive compulsive patients treated with serotonin reuptake inhibitors and behavioral therapy. J Clin Psychiatry. 2001;62(7):535-540.
8. Wewetzer C, Jans T, Muller B, et al. Long term outcome and prognosis of obsessive compulsive disorder with onset in childhood or adolescence. Eur Child Adoles Psychiatry. 2001;10(1):37-46.
9. Steketee G, Chambless DL, Tran GQ. Effects of axis I and axis II comorbidity on behavior therapy outcome for obsessive-compulsive disorder and agrophobia. Compr Psychiatry. 2001;42(1):76-86.
10. Piacentini J. Cognitive behavioral therapy in childhood OCD. Child Adolesc Psychiatr Clin N Am. 1999;8(3):599-616.
11. Himle JA, Rassi S, et al. Group behavioral therapy of obsessive Compulsive disorder: seven vs. twelve-week outcomes. Depress Anxiety. 2001;13(4):161-165.
12. Nakagawa A, Marks IM, Park JM, Bachofen M, Baer L, Dottl SL, Greist JH. Self treatment of obsessive compulsive disorder guided by manual and computer conducted telephone interview. J Telemed Telecare. 2001;6(1):22-26.
13. McLean PD, Whittal ML, et al. Cognitive verses behavior therapy in the group treatment of obsessive compulsive disorder. J Consult Clin Psychol. 2001;69(2):205-214.
14. van Balkom AJ, de Haan E, van Oppen P, et al. Cognitive and behavioral therapies alone versus in combination with fluvoxamine in the treatment of obsessive-compulsive disorder. J Nerv Ment Dis 1998;186:492-499.
15. Dougherty D, Rauch SL. Serotonin-reuptake inhibitors in the treatment of OCD. In: Obsessive-Compulsive Disorders: Diagnosis’Etiology’Treatment. Hollander E, Stein DJ, eds. New York: Marcel Dekker, 1997;145-160.
16. Pigott TA. Seay SM: A review of the efficacy of selective serotonin reuptake inhibitors in obsessive-compulsive disorders. J Clin Psychiatry. 1999;60:101-106.
17. McDougle CJ, Goodman WK. Combination pharmacological treatment strategies. In: Obsessive-Compulsive Disorders: Diagnosis’Etiology’Treatment. Hollander E, Stein DJ, eds. New York: Marcel Dekker, 1997;203-223.
18. McDougle CJ, Barr LC, et al. Lack of efficacy of clozapine monotherapy in refractory obsessive-compulsive disorder. Am J Psychiatry. 1995;152(12):1812-1814.
19. Honagen F, et al. Combination of behaviour therapy with fluvoxamine in comparison with behaviour therapy and placebo. Br J Psychiatry. 1998;173(suppl 35):71-78.
20. O’Connor K, Todorov C, Robillard S, et al. Cognitive-behaviour therapy and medication in the treatment of obsessive-compulsive disorder: a controlled study. Can J Psychiatry. 1999;44:64-71.
21. Rachman S, Cobb J, et al. The behavioural treatment of obsessional-compulsive disorders, with and without clomipramine. Behav Res Ther. 1979;17(5):467-478.
22. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
23. Jenike MA, Rauch SL. Managing the patient with treatment resistant obsessive compulsive disorder: current strategies. J Clin Psychiatry. 1994;55:3(suppl):11-17.
When you suspect a patient has obsessive-compulsive disorder (OCD) (Box 1), how can you differentiate OCD from psychosis? Once you have made the diagnosis, what critical factors suggest treatment will be successful—or unsuccessful? Is behavioral therapy more effective than medication? Which medications are most likely to be effective? The answers to these questions can help you improve the well-being of your patients with OCD.
Differential diagnosis
Unfortunately, many patients with severe OCD are misdiagnosed with psychosis or schizophrenia spectrum disorder and spend many years suffering without proper treatment.3 Despite many similarities between patients with severe OCD and psychosis—including rigid belief systems, unrealistic concerns, magical thinking, and odd behaviors—patients with OCD can recognize the irrational nature of their beliefs when they are not in the throes of anxiety.
Patients with OCD also will usually respond to behavioral interventions within a few weeks while patients who are psychotic usually get progressively worse. Treatment must be given time as both cohorts will get anxious or increase their negative symptoms initially, but patients with OCD should soon habituate and find symptom relief.
Some patients have OCD with psychotic features and tend to have more difficulty responding to behavior therapy without medication. Patients with both OCD and schizotypal personalities respond poorly to both behavior therapy and psychotropic medications.4
Obsessions are intrusive and unwanted thoughts, images, or impulses that produce anxiety. They commonly consist of obsessive fears involving causing harm to others, contamination, safety, religiosity, incompletion, pathological doubt, magical thinking, and the need for certainty, and symmetry.
Usually, obsessions will be accompanied by compulsions, which are behaviors or thoughts performed to reduce the anxiety caused by the obsessions. Compulsions typically consist of excessive washing, checking until it “feels right,” and mental retracing. In rare cases, patients present with only obsessions, which are more difficult to treat than compulsions. Most patients will have several types of symptoms.
To meet the criteria for OCD, patients must be preoccupied by obsessive thoughts and engage in compulsions, which will be frequent, intense, of long duration (more than 2 hours/day), and interfere with the individual’s ability to function. The Yale-Brown Obsessive Compulsive Symptoms Checklist and Scale1 are reliable assessment tools to identify types of symptoms and degree of severity.
Anne is a 53-year-old widow whose OCD symptoms consisted of not letting anything pass her lips that she considered contaminated, lest she become ill with cancer. Her symptoms became so severe that she restricted her diet to a specific brand of peanut butter and milk. The manner in which she ate the peanut butter was rife with checking rituals. If she thought that there might be something wrong with the jar, she threw it away. If she thought the jar was “safe,” she poured the peanut butter directly into her mouth, avoiding the risk of dirty utensils. She drank milk out of the carton. By the time she began treatment, she was malnourished and slightly dehydrated.
Anne’s restrictive diet was also a product of obsessive label checking. Her label reading inevitably resulted in her seeing ordinary household items that she considered risky and would then avoid. Other avoidance behaviors included spitting out saliva and not licking her lips due to fear of what might be ingested, and avoidance of medication, toothpaste, eye drops, skin lotion, and food she feared others had touched.
The good intentions of people in Anne’s community had the effect of enabling her OCD. For example, the local grocer made sure to keep a few cases of Anne's preferred brand of peanut butter in stock for when she needed it. She bought in bulk, but returned unopened jars that she thought were contaminated. As is common with obsessions, no real evidence is needed to legitimize avoidance.
To help Anne break the OCD cycle of avoidance, a meal plan was devised. Although she looked anorexic, but was not, this approach succeeded because she greatly missed the experience of eating and tasting a variety of foods. She also agreed to drink daily nutritional supplements until her diet was more enriching, and had weekly weigh-ins to track her weight gain.
Anne also began a regimen of fluoxetine, which ultimately improved her ability to use the behavior therapy techniques. She was started at 5 mg/d in liquid form. The dosage was increased to 40 mg/d across 1 month, then changed to pill form and titrated to 80 mg/d, which was maintained at discharge.
Exposure and response prevention therapy (ERP) was also administered in twice-daily, 2-hour sessions for about 3 months. Exposure therapy consisted of accompanying Anne to the local supermarket and having her purchase any kind of food that she wanted, regardless of its nutritional value. Her initial purchases consisted of cheesecake, doughnuts, juice, herbal tea, canned ravioli, cereal, lasagna, and snacks.
For response prevention related to food purchases, Anne was prevented from reading labels and examining individual items for imperfections. She was encouraged to buy the first item on the shelf and put it in her basket.
The next step in exposure therapy was to supervise her eating habits. While she looked forward to tasting the food she bought, she was apprehensive because of the obsessive doubt about their purity. Firm but kind encouragement helped her take one bite after another, and this success built on itself. She was excited to be finally confronting her obsessive fears, tasting the foods she restricted herself from for so long, and taking better care of herself. Her complexion improved, and her weight increased.
At times she was highly anxious and looked for ways to avoid the exposure, but with redirection was able to stay on track. She eventually was able to eat community food, eat at a restaurant, use beauty and hygiene products, and have contact with artificial or chemical substances.
Ironically, Anne’s vocational interest was in cooking and after discharge from the program, she investigated employment in hotel/restaurant work and studies at culinary school.
Predictors for successful treatment
Insight Researchers3 found that about 52% of patients with insight into the reasonableness of their obsessions responded to medications, while none who lacked insight responded. Therefore, it pays to assess patients’ insight and ability to recognize the long-term consequences of OCD to themselves and those around them.
Some patients who have suffered with treatment-refractory OCD for most of their lives lack a premorbid high level of functioning to serve as a reference for normalized behavior. Educating these patients to see the advantages of living without certain negative behaviors improves their receptivity to treatment.
Patients who lack insight often refuse to acknowledge that many of their behaviors are manifestations of OCD. Such patients, however, are usually more amenable to giving up or modifying their dysfunctional behaviors—and the clinician more likely to avoid confrontations—if they are shown how certain behaviors undermine their goals.
Cost-benefit analysis Because of the aversive nature of exposure and response prevention therapy (ERP) and the negative side effects of many medications, some patients may find it easier to live with their symptoms, as painful as they are, rather than undergo the discomfort of behavior therapy. Because the prognosis is poor in such cases, patients need to be convinced that the discomfort of treatment is merely short-term, while the discomfort of the illness could last forever if left untreated.
Motivation In our experience, motivation has played a crucial role in determining treatment outcome for severe refractory OCD. And regardless of the severity of their symptoms, patients who are fed up with their symptoms, or are tired of living a life controlled by their obsessions, usually are excellent candidates for treatment.
Conversely, those who enter treatment as a result of external pressure from spouses or family face a less positive prognosis. High emotional expressiveness, overinvolvement, and hostility by relatives is related to higher attrition rates in treatment.3 Because ERP is so aversive, these patients will find ways to dilute the treatment’s effectiveness. In many cases, they do the minimal amount of work required to stay in treatment to avoid whatever consequences their families would impose for not adhering to treatment.
One marker to assess compliance is whether the clinician feels he or she is investing more time and effort into the patient’s treatment than the patient is. If so, this should be addressed in a timely manner. Also, sporadic attendance at sessions and noncompliance with medications, homework, and behavior therapy assignments may also portend a poor outcome. Remember, though, that noncompliance and lack of motivation are fluid states; many previously noncompliant patients later return to treatment better motivated and more compliant.
Predictors for a lower success rate
Secondary gain Researchers4 found that patients who were enabled by their families had more severe symptoms than those who were not. These relational and environmental factors should be discussed openly. If the patient finds that many of his or her life needs are being met secondary to the illness, that patient might not agree to an aversive treatment.
To overcome this, urge family members or other individuals who provide dysfunctional reinforcers to remove them from the environment. Meet with the patient and family/friends and frankly point out dysfunctional gains and the ways in which family members unknowingly allow the gains to continue (e.g., giving the patient more money after he or she overspent his or her allowance). A family behavioral contract should be devised to address how these gains will be reasonably eliminated.
Recognize, too, that a patient may find it difficult to give up the secondary gains, detrimental as they may be, without adequate skills or coping mechanisms to fill the void. So in some cases, it is best not to remove all the secondary gains at once; this can cause many patients to terminate treatment prematurely.
Trauma or abuse history Many patients with treatment refractory OCD have trauma histories and cannot habituate to the behavioral tasks because of dissociation, emotional numbing, or some form of distraction that mediates their anxiety and prevents proper habituation. If the patient is adequately complying with the exposures, yet still is unable to confront every feared stimulus, inquire about a trauma or abuse history (Box 2).
Substance abuse The stress that is inherent to ERP can cause many patients to relapse or abuse illicit substances to manage their anxiety. Therefore, patients with severe substance abuse problems often have great difficulty handling ERP, as they are asked to experience the very discomfort that initially caused them to abuse drugs and alcohol.
Exposure and response prevention therapy (ERP) may be contraindicated for OCD patients with comorbid posttraumatic stress disorder (PTSD). Patients with trauma histories, especially those for whom the trauma precipitated the onset of OCD symptoms, should receive trauma treatment before or in conjunction with ERP in order to be effective.
Patients with OCD and PTSD should receive adjunctive cognitive behavioral therapy (CBT) for their PTSD. Skills training modules, such as dialectical behavior therapy (DBT) and other CBT treatments, often provide the patient with the necessary skills to regulate the trauma-related stressors that are triggered during ERP and can cause premature termination of treatment.
If habituation is not occurring in the absence of trauma, ask whether the patient is dissociating, daydreaming, numbing, or distracting, as these avoidances will jeopardize his or her ability to benefit from ERP. Teaching the patient grounding techniques and alternate coping mechanisms, such as those found in the mindfulness and distress tolerance module of DBT, can help some patients tolerate their anxiety.
For trauma patients whose dissociation, numbing, or distraction is severe, home-based or residential treatment may be required. There, they can be coached during ERP to bring their attention back to the feared stimuli and deal with the negative fallout of their trauma..
In such cases, a patient cannot realistically be asked to give up a coping mechanism, faulty as it may be, until a more functional reinforcer takes its place. Hence, skills training is a crucial part of treatment for this group.
Residential treatment for OCD patients with comorbid substance abuse in remission may be necessary to ensure a positive outcome. Patients should continue recovery work concurrent to behavior therapy to prevent relapse.
High-risk OCD symptoms Patients who have more traditional OCD symptoms usually have a good prognosis. Unfortunately some symptoms do not respond to ERP treatment. These include:
- Repeating, hoarding, and symmetry. Though evidence suggests that hoarding is predictive of poor outcomes,5 treatment carried out in the home can be effective over a 24-week trial.6
- Incompletion, or the need for things to feel right.
- Rigid and overvalued belief systems.
- Sexual and religious obsessions. These appear to be more resistant to behavior therapy and selective serotonin reuptake inhibitors (SSRIs).7
More research needs to be conducted to offer patients with these symptoms better respite.
Researchers also found that patients with childhood and adolescent onset of symptoms, tics, history of hospitalization, and terminated treatment against medical advice are more likely than other OCD patients to develop more severe symptoms in adulthood.8 Patients with OCD who also suffer from generalized anxiety disorders are more likely than those without GAD to drop out of treatment.9
Behavior therapy: first choice
ERP is considered the premier treatment for OCD and is suitable for both adults and children.10 Exposure forces patients to confront their feared stimuli. Response prevention blocks patients from engaging in compulsions or avoidance behaviors intended to reduce their discomfort. Patients are asked to identify situations that trigger their obsession and compulsions and rank them along a fear hierarchy. Patients confront a moderately rated situation and, once they become habituated to it, move up the fear hierarchy to the next situation.
ERP has been proven effective for OCD not only as an individual behavior treatment, but also when done in a group setting11 or when delivered online or by telephone.12
Table 1
Dosage levels for SRIs in OCD
| Clomipramine | 150-200 mg/d |
| Fluoxetine | 40-80 mg/d |
| Sertraline | 50-200 mg/d |
| Fluvoxamine | 200-300 mg/d |
| Paroxetine | 40-60 mg/d |
| Citalopram | 40-60 mg/d |
| The higher end of the dosage ranges shown above is preferred if tolerated. All clinical trials with SRIs for OCD should last at least 10 weeks. | |
Some clinicians prefer cognitive behavioral therapy (CBT) to ERP because it is less aversive. Researchers found that patients who were treated with either CBT or ERP improved. Patients treated with ERP, however, were more likely to maintain their gains in recovery 3 months after treatment concluded.13 Evidence suggests that ERP or CBT when implemented alone, or when applied in conjunction with fluvoxamine,14 are equally effective.
ERP should be managed only by clinicians specially trained in this modality. Several treatment centers across the country provide specialized care for OCD patients. For the nearest treatment center in your community that accepts referrals for ERP, contact the OC Foundation in North Branford, Conn. (See Related Resources.)
Medication for OCD: SRIs as first-line therapy
Experts agree that first-line somatic treatments for OCD include not only behavior therapy but also serotonin reuptake inhibitors (SRIs),15 that is, clomipramine or selective serotonin reuptake inhibitors, (SSRIs) (Table 1).
Caution: Many patients who “respond” to treatment in clinical studies remain symptomatic and meaningfully affected by their residual illness. Therefore, it is critical that you inform patients at the outset that 100% reduction in symptoms is rare.
SRIs Overwhelming evidence from multiple randomized, double-blind, placebo-controlled studies support the efficacy of SRIs. In adults, well-designed and controlled trials have demonstrated the relative efficacy of clomipramine, fluoxetine, sertraline, paroxetine, and fluvoxamine vs. placebo.
SRIs also have been shown to be significantly more effective than tricyclic antidepressants (TCAs) in both placebo-controlled and non-placebo-controlled studies.
Despite initial reports that clomipramine may be more effective than SSRIs, a growing number of studies and a recent comprehensive literature review suggest that the SRIs all have comparable efficacy.16 Because clomipramine has significantly more anticholinergic- and antiadrenergic-mediated side effects than the SSRIs, however, many clinicians choose SSRIs as the initial agent.
When using SRIs, remember that response is typically delayed; an adequate trial requires at least 10 weeks. Indeed, a meaningful proportion of responders continue to emerge past the 8-week mark. Experts suggest that optimal dosages of SRIs for OCD may exceed those typically used for major depression. Guidelines for SRI dosage ranges for OCD appear in (Table 1).
Data regarding treatment duration also suggest that discontinuation of SRIs results in a high relapse rate, though the use of lower maintenance dosages of SRIs is still debated.
Table 2
Ratings of SRI-augmenting agents for OCD treatment
| Likely effective ♦♦ | Possibly effective (insufficient data for adequate assessment of efficacy) ♦ |
|---|---|
| Neuroleptics | Clonidine |
| Busipirone | Fenfluramine |
| Clonazepam | Nortriptyline |
| Lithium | Pindolol |
| Trazodone | |
| Tryptophan | |
| Dosage for these agents has not been adequately studied for augmentation of SRIs. Clinical trial length should be for 2 to 8 weeks. | |
Augmentation of SRIs When first-line interventions fail, second-line pharmacological approaches include augmentation of SRIs with additional medications (Table 2). Numerous agents have been tried for patients who were unresponsive or only partially responsive to SRIs alone.17 Few controlled trials of such strategies have been conducted, however. The most impressive data document the benefits of adding low doses of dopamine antagonists (both conventional and atypical neuroleptics).
Table 3
Using alternative monotherapies
| Drug | Dosage | Duration | Comments |
|---|---|---|---|
| Clonazepam | 0.5-5 mg/d | ≥ 4 weeks | extrapolated from experience with benzodiazepines for other anxiety disorders and a few reports in OCD |
| MAO inhibitor | 60-90 mg/d | ≥ 10 weeks | extrapolated from clinical practice with MAO inhibitors for major depression, panic disorder; tyramine diet must be adhered to; adequate washout of most antidepressants is required before initiating |
| Buspirone | up to 60 mg/d | ≥ 6 weeks | reflecting protocols adopted in clinical trials for OCD |
Recent uncontrolled studies of augmentation with atypical neuroleptics have yielded encouraging preliminary results, as has one controlled trial of augmentation of an SRI with risperidone. Other data suggest that lithium, buspirone, and clonazepam may also be effective.
Numerous other agents have been tried in combination with SRIs, including clonidine, tryptophan, fenfluramine, pindolol, trazodone, nortriptyline, and other antidepressants. The small number of subjects, lack of sufficient controls, and mixed results preclude drawing even preliminary conclusions as to the potential efficacy of such strategies.
Alternative Monotherapies For patients who do not respond satisfactorily to trials of SRIs alone or to augmentation strategies, consider alternative monotherapies in place of SRIs (Table 3). In addition to uncontrolled data, positive controlled studies lend some support for trials of clonazepam, monoamine oxidase (MAO) inhibitors, and buspirone.
Pertinent negative findings are worthy of mention. In contrast to promising results with risperidone as an augmenter, an open trial of the atypical antipsychotic clozapine suggests inefficacy as a monotherapy. Several case reports suggest that clozapine can actually precipitate obsessive-compulsive symptoms in patients with psychotic disorders.18 Controlled trials have not demonstrated the efficacy of trazodone, clonidine, and diphenhydramine as monotherapies.
Pharmacotherapy + or vs. behavioral therapy
Only a few studies directly comparing behavior therapy vs. medication have been reported. In practice, the two are routinely used in concert. Experts have long recommended this treatment approach. Two recent studies19,20 have demonstrated that the combination is more effective than either treatment alone.
In another study, behavior therapy significantly outperformed clomipramine; no significant incremental benefit was seen from the two treatments in combination.21 However, the dosages of clomipramine were relatively low (mean=164 mg/d and maximum=225 mg/d) and of inadequate duration (6 weeks). Still another older head-to-head comparison of behavior therapy and clomipramine showed that medication was better for reducing obsessional doubt, whereas behavior therapy more effectively reduced compulsive rituals.
Third-line treatments may include the unproven augmentation therapies described above, or intravenous clomipramine if available.22
Treatments of last resort
Finally, other nonpharmacologic treatments, including neurosurgery and electroconvulsive therapy (ECT), have remained controversial and are reserved for particular clinical situations or as treatments of last resort.
Despite a large body of uncontrolled data reporting antiobsessional benefits from a variety of neurosurgical procedures, ethical considerations and technical limitations have precluded the performance of sham-controlled studies to definitively establish the efficacy of these strategies.
Neurosurgical treatment of OCD is reserved for patients with severe and debilitating illness who have failed an exhaustive array of other available treatment options and who provide informed consent or assent. Currently, the most commonly employed neurosurgical treatments for OCD include anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leukotomy. In recent prospective trials of cingulotomy and capsulotomy, approximately 45% of patients experienced a 35% or more symptom reduction.
With the advent of innovative surgical devices that allow functional neurosurgery without craniotomy (e.g., by gamma knife), the performance of ethical, double-blind, sham-controlled trials of neurosurgery for OCD is now feasible. A team of investigators from Brown University and Massachusetts General Hospital is conducting one such study that tests the efficacy of anterior capsulotomy.
There are no controlled data regarding the efficacy of ECT for OCD. Given the high comorbidity of major affective illness in OCD and the well-established efficacy of ECT for major depression, it is not surprising that some patients with OCD have reportedly shown clinical improvement after ECT. Several limited case series and anecdotal reports suggest that ECT may help in some circumstances, and such intervention would seem prudent in some cases where severe, comorbid affective illness is present.23
- Jenike MA, Baer, L, Minichiello WE, eds. Obsessive Compulsive Disorders: Practical Management. 3rd ed. Boston: Mosby, 1998.
- Jenike MA. An update on obsessive-compulsive disorder. Bulletin of the Menninger Clinic. 2001;65:4-25.
- Obsessive-Compulsive Foundation, (203) 315-2190, www.ocfoundation.org
Drug brand names
- Buspirone • BuSpar, BuSpar DIVIDOSE
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Ativar, Diastat, Halcion
- Clonidine • Catapres, Catapres TTS-1
- Clozapine • Clozaril
- Fenfluramine • Pondimin
- Fluoxetine • Prozac, Prozac Weekly
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Phenelzine • Nardil, Parnate
- Pindolol • Inderol, Corgard, Betaloc
- Risperidone • Risperidal
- Sertaline • Zoloft
- Trazodone • Desyrel
- Tryptophan* • L-Tryptophan, Alti-trytophan
Disclosure
Dr. Boxill and Ms. Shapiro report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
Dr. Dougherty reports conflicts of interest with Pfizer Inc., Forest Pharmaceuticals, and Solvay Pharmaceuticals.
When you suspect a patient has obsessive-compulsive disorder (OCD) (Box 1), how can you differentiate OCD from psychosis? Once you have made the diagnosis, what critical factors suggest treatment will be successful—or unsuccessful? Is behavioral therapy more effective than medication? Which medications are most likely to be effective? The answers to these questions can help you improve the well-being of your patients with OCD.
Differential diagnosis
Unfortunately, many patients with severe OCD are misdiagnosed with psychosis or schizophrenia spectrum disorder and spend many years suffering without proper treatment.3 Despite many similarities between patients with severe OCD and psychosis—including rigid belief systems, unrealistic concerns, magical thinking, and odd behaviors—patients with OCD can recognize the irrational nature of their beliefs when they are not in the throes of anxiety.
Patients with OCD also will usually respond to behavioral interventions within a few weeks while patients who are psychotic usually get progressively worse. Treatment must be given time as both cohorts will get anxious or increase their negative symptoms initially, but patients with OCD should soon habituate and find symptom relief.
Some patients have OCD with psychotic features and tend to have more difficulty responding to behavior therapy without medication. Patients with both OCD and schizotypal personalities respond poorly to both behavior therapy and psychotropic medications.4
Obsessions are intrusive and unwanted thoughts, images, or impulses that produce anxiety. They commonly consist of obsessive fears involving causing harm to others, contamination, safety, religiosity, incompletion, pathological doubt, magical thinking, and the need for certainty, and symmetry.
Usually, obsessions will be accompanied by compulsions, which are behaviors or thoughts performed to reduce the anxiety caused by the obsessions. Compulsions typically consist of excessive washing, checking until it “feels right,” and mental retracing. In rare cases, patients present with only obsessions, which are more difficult to treat than compulsions. Most patients will have several types of symptoms.
To meet the criteria for OCD, patients must be preoccupied by obsessive thoughts and engage in compulsions, which will be frequent, intense, of long duration (more than 2 hours/day), and interfere with the individual’s ability to function. The Yale-Brown Obsessive Compulsive Symptoms Checklist and Scale1 are reliable assessment tools to identify types of symptoms and degree of severity.
Anne is a 53-year-old widow whose OCD symptoms consisted of not letting anything pass her lips that she considered contaminated, lest she become ill with cancer. Her symptoms became so severe that she restricted her diet to a specific brand of peanut butter and milk. The manner in which she ate the peanut butter was rife with checking rituals. If she thought that there might be something wrong with the jar, she threw it away. If she thought the jar was “safe,” she poured the peanut butter directly into her mouth, avoiding the risk of dirty utensils. She drank milk out of the carton. By the time she began treatment, she was malnourished and slightly dehydrated.
Anne’s restrictive diet was also a product of obsessive label checking. Her label reading inevitably resulted in her seeing ordinary household items that she considered risky and would then avoid. Other avoidance behaviors included spitting out saliva and not licking her lips due to fear of what might be ingested, and avoidance of medication, toothpaste, eye drops, skin lotion, and food she feared others had touched.
The good intentions of people in Anne’s community had the effect of enabling her OCD. For example, the local grocer made sure to keep a few cases of Anne's preferred brand of peanut butter in stock for when she needed it. She bought in bulk, but returned unopened jars that she thought were contaminated. As is common with obsessions, no real evidence is needed to legitimize avoidance.
To help Anne break the OCD cycle of avoidance, a meal plan was devised. Although she looked anorexic, but was not, this approach succeeded because she greatly missed the experience of eating and tasting a variety of foods. She also agreed to drink daily nutritional supplements until her diet was more enriching, and had weekly weigh-ins to track her weight gain.
Anne also began a regimen of fluoxetine, which ultimately improved her ability to use the behavior therapy techniques. She was started at 5 mg/d in liquid form. The dosage was increased to 40 mg/d across 1 month, then changed to pill form and titrated to 80 mg/d, which was maintained at discharge.
Exposure and response prevention therapy (ERP) was also administered in twice-daily, 2-hour sessions for about 3 months. Exposure therapy consisted of accompanying Anne to the local supermarket and having her purchase any kind of food that she wanted, regardless of its nutritional value. Her initial purchases consisted of cheesecake, doughnuts, juice, herbal tea, canned ravioli, cereal, lasagna, and snacks.
For response prevention related to food purchases, Anne was prevented from reading labels and examining individual items for imperfections. She was encouraged to buy the first item on the shelf and put it in her basket.
The next step in exposure therapy was to supervise her eating habits. While she looked forward to tasting the food she bought, she was apprehensive because of the obsessive doubt about their purity. Firm but kind encouragement helped her take one bite after another, and this success built on itself. She was excited to be finally confronting her obsessive fears, tasting the foods she restricted herself from for so long, and taking better care of herself. Her complexion improved, and her weight increased.
At times she was highly anxious and looked for ways to avoid the exposure, but with redirection was able to stay on track. She eventually was able to eat community food, eat at a restaurant, use beauty and hygiene products, and have contact with artificial or chemical substances.
Ironically, Anne’s vocational interest was in cooking and after discharge from the program, she investigated employment in hotel/restaurant work and studies at culinary school.
Predictors for successful treatment
Insight Researchers3 found that about 52% of patients with insight into the reasonableness of their obsessions responded to medications, while none who lacked insight responded. Therefore, it pays to assess patients’ insight and ability to recognize the long-term consequences of OCD to themselves and those around them.
Some patients who have suffered with treatment-refractory OCD for most of their lives lack a premorbid high level of functioning to serve as a reference for normalized behavior. Educating these patients to see the advantages of living without certain negative behaviors improves their receptivity to treatment.
Patients who lack insight often refuse to acknowledge that many of their behaviors are manifestations of OCD. Such patients, however, are usually more amenable to giving up or modifying their dysfunctional behaviors—and the clinician more likely to avoid confrontations—if they are shown how certain behaviors undermine their goals.
Cost-benefit analysis Because of the aversive nature of exposure and response prevention therapy (ERP) and the negative side effects of many medications, some patients may find it easier to live with their symptoms, as painful as they are, rather than undergo the discomfort of behavior therapy. Because the prognosis is poor in such cases, patients need to be convinced that the discomfort of treatment is merely short-term, while the discomfort of the illness could last forever if left untreated.
Motivation In our experience, motivation has played a crucial role in determining treatment outcome for severe refractory OCD. And regardless of the severity of their symptoms, patients who are fed up with their symptoms, or are tired of living a life controlled by their obsessions, usually are excellent candidates for treatment.
Conversely, those who enter treatment as a result of external pressure from spouses or family face a less positive prognosis. High emotional expressiveness, overinvolvement, and hostility by relatives is related to higher attrition rates in treatment.3 Because ERP is so aversive, these patients will find ways to dilute the treatment’s effectiveness. In many cases, they do the minimal amount of work required to stay in treatment to avoid whatever consequences their families would impose for not adhering to treatment.
One marker to assess compliance is whether the clinician feels he or she is investing more time and effort into the patient’s treatment than the patient is. If so, this should be addressed in a timely manner. Also, sporadic attendance at sessions and noncompliance with medications, homework, and behavior therapy assignments may also portend a poor outcome. Remember, though, that noncompliance and lack of motivation are fluid states; many previously noncompliant patients later return to treatment better motivated and more compliant.
Predictors for a lower success rate
Secondary gain Researchers4 found that patients who were enabled by their families had more severe symptoms than those who were not. These relational and environmental factors should be discussed openly. If the patient finds that many of his or her life needs are being met secondary to the illness, that patient might not agree to an aversive treatment.
To overcome this, urge family members or other individuals who provide dysfunctional reinforcers to remove them from the environment. Meet with the patient and family/friends and frankly point out dysfunctional gains and the ways in which family members unknowingly allow the gains to continue (e.g., giving the patient more money after he or she overspent his or her allowance). A family behavioral contract should be devised to address how these gains will be reasonably eliminated.
Recognize, too, that a patient may find it difficult to give up the secondary gains, detrimental as they may be, without adequate skills or coping mechanisms to fill the void. So in some cases, it is best not to remove all the secondary gains at once; this can cause many patients to terminate treatment prematurely.
Trauma or abuse history Many patients with treatment refractory OCD have trauma histories and cannot habituate to the behavioral tasks because of dissociation, emotional numbing, or some form of distraction that mediates their anxiety and prevents proper habituation. If the patient is adequately complying with the exposures, yet still is unable to confront every feared stimulus, inquire about a trauma or abuse history (Box 2).
Substance abuse The stress that is inherent to ERP can cause many patients to relapse or abuse illicit substances to manage their anxiety. Therefore, patients with severe substance abuse problems often have great difficulty handling ERP, as they are asked to experience the very discomfort that initially caused them to abuse drugs and alcohol.
Exposure and response prevention therapy (ERP) may be contraindicated for OCD patients with comorbid posttraumatic stress disorder (PTSD). Patients with trauma histories, especially those for whom the trauma precipitated the onset of OCD symptoms, should receive trauma treatment before or in conjunction with ERP in order to be effective.
Patients with OCD and PTSD should receive adjunctive cognitive behavioral therapy (CBT) for their PTSD. Skills training modules, such as dialectical behavior therapy (DBT) and other CBT treatments, often provide the patient with the necessary skills to regulate the trauma-related stressors that are triggered during ERP and can cause premature termination of treatment.
If habituation is not occurring in the absence of trauma, ask whether the patient is dissociating, daydreaming, numbing, or distracting, as these avoidances will jeopardize his or her ability to benefit from ERP. Teaching the patient grounding techniques and alternate coping mechanisms, such as those found in the mindfulness and distress tolerance module of DBT, can help some patients tolerate their anxiety.
For trauma patients whose dissociation, numbing, or distraction is severe, home-based or residential treatment may be required. There, they can be coached during ERP to bring their attention back to the feared stimuli and deal with the negative fallout of their trauma..
In such cases, a patient cannot realistically be asked to give up a coping mechanism, faulty as it may be, until a more functional reinforcer takes its place. Hence, skills training is a crucial part of treatment for this group.
Residential treatment for OCD patients with comorbid substance abuse in remission may be necessary to ensure a positive outcome. Patients should continue recovery work concurrent to behavior therapy to prevent relapse.
High-risk OCD symptoms Patients who have more traditional OCD symptoms usually have a good prognosis. Unfortunately some symptoms do not respond to ERP treatment. These include:
- Repeating, hoarding, and symmetry. Though evidence suggests that hoarding is predictive of poor outcomes,5 treatment carried out in the home can be effective over a 24-week trial.6
- Incompletion, or the need for things to feel right.
- Rigid and overvalued belief systems.
- Sexual and religious obsessions. These appear to be more resistant to behavior therapy and selective serotonin reuptake inhibitors (SSRIs).7
More research needs to be conducted to offer patients with these symptoms better respite.
Researchers also found that patients with childhood and adolescent onset of symptoms, tics, history of hospitalization, and terminated treatment against medical advice are more likely than other OCD patients to develop more severe symptoms in adulthood.8 Patients with OCD who also suffer from generalized anxiety disorders are more likely than those without GAD to drop out of treatment.9
Behavior therapy: first choice
ERP is considered the premier treatment for OCD and is suitable for both adults and children.10 Exposure forces patients to confront their feared stimuli. Response prevention blocks patients from engaging in compulsions or avoidance behaviors intended to reduce their discomfort. Patients are asked to identify situations that trigger their obsession and compulsions and rank them along a fear hierarchy. Patients confront a moderately rated situation and, once they become habituated to it, move up the fear hierarchy to the next situation.
ERP has been proven effective for OCD not only as an individual behavior treatment, but also when done in a group setting11 or when delivered online or by telephone.12
Table 1
Dosage levels for SRIs in OCD
| Clomipramine | 150-200 mg/d |
| Fluoxetine | 40-80 mg/d |
| Sertraline | 50-200 mg/d |
| Fluvoxamine | 200-300 mg/d |
| Paroxetine | 40-60 mg/d |
| Citalopram | 40-60 mg/d |
| The higher end of the dosage ranges shown above is preferred if tolerated. All clinical trials with SRIs for OCD should last at least 10 weeks. | |
Some clinicians prefer cognitive behavioral therapy (CBT) to ERP because it is less aversive. Researchers found that patients who were treated with either CBT or ERP improved. Patients treated with ERP, however, were more likely to maintain their gains in recovery 3 months after treatment concluded.13 Evidence suggests that ERP or CBT when implemented alone, or when applied in conjunction with fluvoxamine,14 are equally effective.
ERP should be managed only by clinicians specially trained in this modality. Several treatment centers across the country provide specialized care for OCD patients. For the nearest treatment center in your community that accepts referrals for ERP, contact the OC Foundation in North Branford, Conn. (See Related Resources.)
Medication for OCD: SRIs as first-line therapy
Experts agree that first-line somatic treatments for OCD include not only behavior therapy but also serotonin reuptake inhibitors (SRIs),15 that is, clomipramine or selective serotonin reuptake inhibitors, (SSRIs) (Table 1).
Caution: Many patients who “respond” to treatment in clinical studies remain symptomatic and meaningfully affected by their residual illness. Therefore, it is critical that you inform patients at the outset that 100% reduction in symptoms is rare.
SRIs Overwhelming evidence from multiple randomized, double-blind, placebo-controlled studies support the efficacy of SRIs. In adults, well-designed and controlled trials have demonstrated the relative efficacy of clomipramine, fluoxetine, sertraline, paroxetine, and fluvoxamine vs. placebo.
SRIs also have been shown to be significantly more effective than tricyclic antidepressants (TCAs) in both placebo-controlled and non-placebo-controlled studies.
Despite initial reports that clomipramine may be more effective than SSRIs, a growing number of studies and a recent comprehensive literature review suggest that the SRIs all have comparable efficacy.16 Because clomipramine has significantly more anticholinergic- and antiadrenergic-mediated side effects than the SSRIs, however, many clinicians choose SSRIs as the initial agent.
When using SRIs, remember that response is typically delayed; an adequate trial requires at least 10 weeks. Indeed, a meaningful proportion of responders continue to emerge past the 8-week mark. Experts suggest that optimal dosages of SRIs for OCD may exceed those typically used for major depression. Guidelines for SRI dosage ranges for OCD appear in (Table 1).
Data regarding treatment duration also suggest that discontinuation of SRIs results in a high relapse rate, though the use of lower maintenance dosages of SRIs is still debated.
Table 2
Ratings of SRI-augmenting agents for OCD treatment
| Likely effective ♦♦ | Possibly effective (insufficient data for adequate assessment of efficacy) ♦ |
|---|---|
| Neuroleptics | Clonidine |
| Busipirone | Fenfluramine |
| Clonazepam | Nortriptyline |
| Lithium | Pindolol |
| Trazodone | |
| Tryptophan | |
| Dosage for these agents has not been adequately studied for augmentation of SRIs. Clinical trial length should be for 2 to 8 weeks. | |
Augmentation of SRIs When first-line interventions fail, second-line pharmacological approaches include augmentation of SRIs with additional medications (Table 2). Numerous agents have been tried for patients who were unresponsive or only partially responsive to SRIs alone.17 Few controlled trials of such strategies have been conducted, however. The most impressive data document the benefits of adding low doses of dopamine antagonists (both conventional and atypical neuroleptics).
Table 3
Using alternative monotherapies
| Drug | Dosage | Duration | Comments |
|---|---|---|---|
| Clonazepam | 0.5-5 mg/d | ≥ 4 weeks | extrapolated from experience with benzodiazepines for other anxiety disorders and a few reports in OCD |
| MAO inhibitor | 60-90 mg/d | ≥ 10 weeks | extrapolated from clinical practice with MAO inhibitors for major depression, panic disorder; tyramine diet must be adhered to; adequate washout of most antidepressants is required before initiating |
| Buspirone | up to 60 mg/d | ≥ 6 weeks | reflecting protocols adopted in clinical trials for OCD |
Recent uncontrolled studies of augmentation with atypical neuroleptics have yielded encouraging preliminary results, as has one controlled trial of augmentation of an SRI with risperidone. Other data suggest that lithium, buspirone, and clonazepam may also be effective.
Numerous other agents have been tried in combination with SRIs, including clonidine, tryptophan, fenfluramine, pindolol, trazodone, nortriptyline, and other antidepressants. The small number of subjects, lack of sufficient controls, and mixed results preclude drawing even preliminary conclusions as to the potential efficacy of such strategies.
Alternative Monotherapies For patients who do not respond satisfactorily to trials of SRIs alone or to augmentation strategies, consider alternative monotherapies in place of SRIs (Table 3). In addition to uncontrolled data, positive controlled studies lend some support for trials of clonazepam, monoamine oxidase (MAO) inhibitors, and buspirone.
Pertinent negative findings are worthy of mention. In contrast to promising results with risperidone as an augmenter, an open trial of the atypical antipsychotic clozapine suggests inefficacy as a monotherapy. Several case reports suggest that clozapine can actually precipitate obsessive-compulsive symptoms in patients with psychotic disorders.18 Controlled trials have not demonstrated the efficacy of trazodone, clonidine, and diphenhydramine as monotherapies.
Pharmacotherapy + or vs. behavioral therapy
Only a few studies directly comparing behavior therapy vs. medication have been reported. In practice, the two are routinely used in concert. Experts have long recommended this treatment approach. Two recent studies19,20 have demonstrated that the combination is more effective than either treatment alone.
In another study, behavior therapy significantly outperformed clomipramine; no significant incremental benefit was seen from the two treatments in combination.21 However, the dosages of clomipramine were relatively low (mean=164 mg/d and maximum=225 mg/d) and of inadequate duration (6 weeks). Still another older head-to-head comparison of behavior therapy and clomipramine showed that medication was better for reducing obsessional doubt, whereas behavior therapy more effectively reduced compulsive rituals.
Third-line treatments may include the unproven augmentation therapies described above, or intravenous clomipramine if available.22
Treatments of last resort
Finally, other nonpharmacologic treatments, including neurosurgery and electroconvulsive therapy (ECT), have remained controversial and are reserved for particular clinical situations or as treatments of last resort.
Despite a large body of uncontrolled data reporting antiobsessional benefits from a variety of neurosurgical procedures, ethical considerations and technical limitations have precluded the performance of sham-controlled studies to definitively establish the efficacy of these strategies.
Neurosurgical treatment of OCD is reserved for patients with severe and debilitating illness who have failed an exhaustive array of other available treatment options and who provide informed consent or assent. Currently, the most commonly employed neurosurgical treatments for OCD include anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leukotomy. In recent prospective trials of cingulotomy and capsulotomy, approximately 45% of patients experienced a 35% or more symptom reduction.
With the advent of innovative surgical devices that allow functional neurosurgery without craniotomy (e.g., by gamma knife), the performance of ethical, double-blind, sham-controlled trials of neurosurgery for OCD is now feasible. A team of investigators from Brown University and Massachusetts General Hospital is conducting one such study that tests the efficacy of anterior capsulotomy.
There are no controlled data regarding the efficacy of ECT for OCD. Given the high comorbidity of major affective illness in OCD and the well-established efficacy of ECT for major depression, it is not surprising that some patients with OCD have reportedly shown clinical improvement after ECT. Several limited case series and anecdotal reports suggest that ECT may help in some circumstances, and such intervention would seem prudent in some cases where severe, comorbid affective illness is present.23
- Jenike MA, Baer, L, Minichiello WE, eds. Obsessive Compulsive Disorders: Practical Management. 3rd ed. Boston: Mosby, 1998.
- Jenike MA. An update on obsessive-compulsive disorder. Bulletin of the Menninger Clinic. 2001;65:4-25.
- Obsessive-Compulsive Foundation, (203) 315-2190, www.ocfoundation.org
Drug brand names
- Buspirone • BuSpar, BuSpar DIVIDOSE
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Ativar, Diastat, Halcion
- Clonidine • Catapres, Catapres TTS-1
- Clozapine • Clozaril
- Fenfluramine • Pondimin
- Fluoxetine • Prozac, Prozac Weekly
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Phenelzine • Nardil, Parnate
- Pindolol • Inderol, Corgard, Betaloc
- Risperidone • Risperidal
- Sertaline • Zoloft
- Trazodone • Desyrel
- Tryptophan* • L-Tryptophan, Alti-trytophan
Disclosure
Dr. Boxill and Ms. Shapiro report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
Dr. Dougherty reports conflicts of interest with Pfizer Inc., Forest Pharmaceuticals, and Solvay Pharmaceuticals.
1. Goodman Wk, Price LH, et al. The Yale Brown Obsessive Compulsive Scale:1. Development, Use and Reliability. Arch Gen Psychiatry. 1989;46:1012-1016.
2. Catapano F, Sperandeo R, Perris F, Lanzaro M, Maj M. Insight and resistance in patients with obsessive compulsive disorder. Psychopathol. 2001;34(2):62-68.
3. Amir N, Freshman M, Foa EB. Family distress and involvement in relatives of obsessive-compulsive disorder patients. J Anxiety Disord. 2000;14(3):209-217.
4. Chambless DL, Steketee G. Expressed emotion and behavior therapy outcome: a prospective study with obsessive compulsive and agrophobic outpatients. J Consult Clin Psychol. 1999;67(5):658-665.
5. Black DW, Monahan P, Gable J, et al. Hoarding and treatment in 38 nondepressed subjects with OCD. J Clin Psychiatry. 1998;59(8):420-425.
6. Rosqvist J, Egan D, Manzo P, et al. Home-based behavior therapy for obsessive compulsive disorder: A case series with data. J Anxiety Disord. 2001;15(5):395-400.
7. Alonso P, Menchon JM, Pifarre J, et al. Long term follow up and predictors of clinical outcome in obsessive compulsive patients treated with serotonin reuptake inhibitors and behavioral therapy. J Clin Psychiatry. 2001;62(7):535-540.
8. Wewetzer C, Jans T, Muller B, et al. Long term outcome and prognosis of obsessive compulsive disorder with onset in childhood or adolescence. Eur Child Adoles Psychiatry. 2001;10(1):37-46.
9. Steketee G, Chambless DL, Tran GQ. Effects of axis I and axis II comorbidity on behavior therapy outcome for obsessive-compulsive disorder and agrophobia. Compr Psychiatry. 2001;42(1):76-86.
10. Piacentini J. Cognitive behavioral therapy in childhood OCD. Child Adolesc Psychiatr Clin N Am. 1999;8(3):599-616.
11. Himle JA, Rassi S, et al. Group behavioral therapy of obsessive Compulsive disorder: seven vs. twelve-week outcomes. Depress Anxiety. 2001;13(4):161-165.
12. Nakagawa A, Marks IM, Park JM, Bachofen M, Baer L, Dottl SL, Greist JH. Self treatment of obsessive compulsive disorder guided by manual and computer conducted telephone interview. J Telemed Telecare. 2001;6(1):22-26.
13. McLean PD, Whittal ML, et al. Cognitive verses behavior therapy in the group treatment of obsessive compulsive disorder. J Consult Clin Psychol. 2001;69(2):205-214.
14. van Balkom AJ, de Haan E, van Oppen P, et al. Cognitive and behavioral therapies alone versus in combination with fluvoxamine in the treatment of obsessive-compulsive disorder. J Nerv Ment Dis 1998;186:492-499.
15. Dougherty D, Rauch SL. Serotonin-reuptake inhibitors in the treatment of OCD. In: Obsessive-Compulsive Disorders: Diagnosis’Etiology’Treatment. Hollander E, Stein DJ, eds. New York: Marcel Dekker, 1997;145-160.
16. Pigott TA. Seay SM: A review of the efficacy of selective serotonin reuptake inhibitors in obsessive-compulsive disorders. J Clin Psychiatry. 1999;60:101-106.
17. McDougle CJ, Goodman WK. Combination pharmacological treatment strategies. In: Obsessive-Compulsive Disorders: Diagnosis’Etiology’Treatment. Hollander E, Stein DJ, eds. New York: Marcel Dekker, 1997;203-223.
18. McDougle CJ, Barr LC, et al. Lack of efficacy of clozapine monotherapy in refractory obsessive-compulsive disorder. Am J Psychiatry. 1995;152(12):1812-1814.
19. Honagen F, et al. Combination of behaviour therapy with fluvoxamine in comparison with behaviour therapy and placebo. Br J Psychiatry. 1998;173(suppl 35):71-78.
20. O’Connor K, Todorov C, Robillard S, et al. Cognitive-behaviour therapy and medication in the treatment of obsessive-compulsive disorder: a controlled study. Can J Psychiatry. 1999;44:64-71.
21. Rachman S, Cobb J, et al. The behavioural treatment of obsessional-compulsive disorders, with and without clomipramine. Behav Res Ther. 1979;17(5):467-478.
22. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
23. Jenike MA, Rauch SL. Managing the patient with treatment resistant obsessive compulsive disorder: current strategies. J Clin Psychiatry. 1994;55:3(suppl):11-17.
1. Goodman Wk, Price LH, et al. The Yale Brown Obsessive Compulsive Scale:1. Development, Use and Reliability. Arch Gen Psychiatry. 1989;46:1012-1016.
2. Catapano F, Sperandeo R, Perris F, Lanzaro M, Maj M. Insight and resistance in patients with obsessive compulsive disorder. Psychopathol. 2001;34(2):62-68.
3. Amir N, Freshman M, Foa EB. Family distress and involvement in relatives of obsessive-compulsive disorder patients. J Anxiety Disord. 2000;14(3):209-217.
4. Chambless DL, Steketee G. Expressed emotion and behavior therapy outcome: a prospective study with obsessive compulsive and agrophobic outpatients. J Consult Clin Psychol. 1999;67(5):658-665.
5. Black DW, Monahan P, Gable J, et al. Hoarding and treatment in 38 nondepressed subjects with OCD. J Clin Psychiatry. 1998;59(8):420-425.
6. Rosqvist J, Egan D, Manzo P, et al. Home-based behavior therapy for obsessive compulsive disorder: A case series with data. J Anxiety Disord. 2001;15(5):395-400.
7. Alonso P, Menchon JM, Pifarre J, et al. Long term follow up and predictors of clinical outcome in obsessive compulsive patients treated with serotonin reuptake inhibitors and behavioral therapy. J Clin Psychiatry. 2001;62(7):535-540.
8. Wewetzer C, Jans T, Muller B, et al. Long term outcome and prognosis of obsessive compulsive disorder with onset in childhood or adolescence. Eur Child Adoles Psychiatry. 2001;10(1):37-46.
9. Steketee G, Chambless DL, Tran GQ. Effects of axis I and axis II comorbidity on behavior therapy outcome for obsessive-compulsive disorder and agrophobia. Compr Psychiatry. 2001;42(1):76-86.
10. Piacentini J. Cognitive behavioral therapy in childhood OCD. Child Adolesc Psychiatr Clin N Am. 1999;8(3):599-616.
11. Himle JA, Rassi S, et al. Group behavioral therapy of obsessive Compulsive disorder: seven vs. twelve-week outcomes. Depress Anxiety. 2001;13(4):161-165.
12. Nakagawa A, Marks IM, Park JM, Bachofen M, Baer L, Dottl SL, Greist JH. Self treatment of obsessive compulsive disorder guided by manual and computer conducted telephone interview. J Telemed Telecare. 2001;6(1):22-26.
13. McLean PD, Whittal ML, et al. Cognitive verses behavior therapy in the group treatment of obsessive compulsive disorder. J Consult Clin Psychol. 2001;69(2):205-214.
14. van Balkom AJ, de Haan E, van Oppen P, et al. Cognitive and behavioral therapies alone versus in combination with fluvoxamine in the treatment of obsessive-compulsive disorder. J Nerv Ment Dis 1998;186:492-499.
15. Dougherty D, Rauch SL. Serotonin-reuptake inhibitors in the treatment of OCD. In: Obsessive-Compulsive Disorders: Diagnosis’Etiology’Treatment. Hollander E, Stein DJ, eds. New York: Marcel Dekker, 1997;145-160.
16. Pigott TA. Seay SM: A review of the efficacy of selective serotonin reuptake inhibitors in obsessive-compulsive disorders. J Clin Psychiatry. 1999;60:101-106.
17. McDougle CJ, Goodman WK. Combination pharmacological treatment strategies. In: Obsessive-Compulsive Disorders: Diagnosis’Etiology’Treatment. Hollander E, Stein DJ, eds. New York: Marcel Dekker, 1997;203-223.
18. McDougle CJ, Barr LC, et al. Lack of efficacy of clozapine monotherapy in refractory obsessive-compulsive disorder. Am J Psychiatry. 1995;152(12):1812-1814.
19. Honagen F, et al. Combination of behaviour therapy with fluvoxamine in comparison with behaviour therapy and placebo. Br J Psychiatry. 1998;173(suppl 35):71-78.
20. O’Connor K, Todorov C, Robillard S, et al. Cognitive-behaviour therapy and medication in the treatment of obsessive-compulsive disorder: a controlled study. Can J Psychiatry. 1999;44:64-71.
21. Rachman S, Cobb J, et al. The behavioural treatment of obsessional-compulsive disorders, with and without clomipramine. Behav Res Ther. 1979;17(5):467-478.
22. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
23. Jenike MA, Rauch SL. Managing the patient with treatment resistant obsessive compulsive disorder: current strategies. J Clin Psychiatry. 1994;55:3(suppl):11-17.
Innovative and practical treatments for obsessive-compulsive disorder
When you suspect a patient has obsessive-compulsive disorder (OCD) (Box 1), how can you differentiate OCD from psychosis? Once you have made the diagnosis, what critical factors suggest treatment will be successful—or unsuccessful? Is behavioral therapy more effective than medication? Which medications are most likely to be effective? The answers to these questions can help you improve the well-being of your patients with OCD.
Differential diagnosis
Unfortunately, many patients with severe OCD are misdiagnosed with psychosis or schizophrenia spectrum disorder and spend many years suffering without proper treatment.3 Despite many similarities between patients with severe OCD and psychosis—including rigid belief systems, unrealistic concerns, magical thinking, and odd behaviors—patients with OCD can recognize the irrational nature of their beliefs when they are not in the throes of anxiety.
Patients with OCD also will usually respond to behavioral interventions within a few weeks while patients who are psychotic usually get progressively worse. Treatment must be given time as both cohorts will get anxious or increase their negative symptoms initially, but patients with OCD should soon habituate and find symptom relief.
Some patients have OCD with psychotic features and tend to have more difficulty responding to behavior therapy without medication. Patients with both OCD and schizotypal personalities respond poorly to both behavior therapy and psychotropic medications.4
Obsessions are intrusive and unwanted thoughts, images, or impulses that produce anxiety. They commonly consist of obsessive fears involving causing harm to others, contamination, safety, religiosity, incompletion, pathological doubt, magical thinking, and the need for certainty, and symmetry.
Usually, obsessions will be accompanied by compulsions, which are behaviors or thoughts performed to reduce the anxiety caused by the obsessions. Compulsions typically consist of excessive washing, checking until it “feels right,” and mental retracing. In rare cases, patients present with only obsessions, which are more difficult to treat than compulsions. Most patients will have several types of symptoms.
To meet the criteria for OCD, patients must be preoccupied by obsessive thoughts and engage in compulsions, which will be frequent, intense, of long duration (more than 2 hours/day), and interfere with the individual’s ability to function. The Yale-Brown Obsessive Compulsive Symptoms Checklist and Scale1 are reliable assessment tools to identify types of symptoms and degree of severity.
Anne is a 53-year-old widow whose OCD symptoms consisted of not letting anything pass her lips that she considered contaminated, lest she become ill with cancer. Her symptoms became so severe that she restricted her diet to a specific brand of peanut butter and milk. The manner in which she ate the peanut butter was rife with checking rituals. If she thought that there might be something wrong with the jar, she threw it away. If she thought the jar was “safe,” she poured the peanut butter directly into her mouth, avoiding the risk of dirty utensils. She drank milk out of the carton. By the time she began treatment, she was malnourished and slightly dehydrated.
Anne’s restrictive diet was also a product of obsessive label checking. Her label reading inevitably resulted in her seeing ordinary household items that she considered risky and would then avoid. Other avoidance behaviors included spitting out saliva and not licking her lips due to fear of what might be ingested, and avoidance of medication, toothpaste, eye drops, skin lotion, and food she feared others had touched.
The good intentions of people in Anne’s community had the effect of enabling her OCD. For example, the local grocer made sure to keep a few cases of Anne's preferred brand of peanut butter in stock for when she needed it. She bought in bulk, but returned unopened jars that she thought were contaminated. As is common with obsessions, no real evidence is needed to legitimize avoidance.
To help Anne break the OCD cycle of avoidance, a meal plan was devised. Although she looked anorexic, but was not, this approach succeeded because she greatly missed the experience of eating and tasting a variety of foods. She also agreed to drink daily nutritional supplements until her diet was more enriching, and had weekly weigh-ins to track her weight gain.
Anne also began a regimen of fluoxetine, which ultimately improved her ability to use the behavior therapy techniques. She was started at 5 mg/d in liquid form. The dosage was increased to 40 mg/d across 1 month, then changed to pill form and titrated to 80 mg/d, which was maintained at discharge.
Exposure and response prevention therapy (ERP) was also administered in twice-daily, 2-hour sessions for about 3 months. Exposure therapy consisted of accompanying Anne to the local supermarket and having her purchase any kind of food that she wanted, regardless of its nutritional value. Her initial purchases consisted of cheesecake, doughnuts, juice, herbal tea, canned ravioli, cereal, lasagna, and snacks.
For response prevention related to food purchases, Anne was prevented from reading labels and examining individual items for imperfections. She was encouraged to buy the first item on the shelf and put it in her basket.
The next step in exposure therapy was to supervise her eating habits. While she looked forward to tasting the food she bought, she was apprehensive because of the obsessive doubt about their purity. Firm but kind encouragement helped her take one bite after another, and this success built on itself. She was excited to be finally confronting her obsessive fears, tasting the foods she restricted herself from for so long, and taking better care of herself. Her complexion improved, and her weight increased.
At times she was highly anxious and looked for ways to avoid the exposure, but with redirection was able to stay on track. She eventually was able to eat community food, eat at a restaurant, use beauty and hygiene products, and have contact with artificial or chemical substances.
Ironically, Anne’s vocational interest was in cooking and after discharge from the program, she investigated employment in hotel/restaurant work and studies at culinary school.
Predictors for successful treatment
Insight Researchers3 found that about 52% of patients with insight into the reasonableness of their obsessions responded to medications, while none who lacked insight responded. Therefore, it pays to assess patients’ insight and ability to recognize the long-term consequences of OCD to themselves and those around them.
Some patients who have suffered with treatment-refractory OCD for most of their lives lack a premorbid high level of functioning to serve as a reference for normalized behavior. Educating these patients to see the advantages of living without certain negative behaviors improves their receptivity to treatment.
Patients who lack insight often refuse to acknowledge that many of their behaviors are manifestations of OCD. Such patients, however, are usually more amenable to giving up or modifying their dysfunctional behaviors—and the clinician more likely to avoid confrontations—if they are shown how certain behaviors undermine their goals.
Cost-benefit analysis Because of the aversive nature of exposure and response prevention therapy (ERP) and the negative side effects of many medications, some patients may find it easier to live with their symptoms, as painful as they are, rather than undergo the discomfort of behavior therapy. Because the prognosis is poor in such cases, patients need to be convinced that the discomfort of treatment is merely short-term, while the discomfort of the illness could last forever if left untreated.
Motivation In our experience, motivation has played a crucial role in determining treatment outcome for severe refractory OCD. And regardless of the severity of their symptoms, patients who are fed up with their symptoms, or are tired of living a life controlled by their obsessions, usually are excellent candidates for treatment.
Conversely, those who enter treatment as a result of external pressure from spouses or family face a less positive prognosis. High emotional expressiveness, overinvolvement, and hostility by relatives is related to higher attrition rates in treatment.3 Because ERP is so aversive, these patients will find ways to dilute the treatment’s effectiveness. In many cases, they do the minimal amount of work required to stay in treatment to avoid whatever consequences their families would impose for not adhering to treatment.
One marker to assess compliance is whether the clinician feels he or she is investing more time and effort into the patient’s treatment than the patient is. If so, this should be addressed in a timely manner. Also, sporadic attendance at sessions and noncompliance with medications, homework, and behavior therapy assignments may also portend a poor outcome. Remember, though, that noncompliance and lack of motivation are fluid states; many previously noncompliant patients later return to treatment better motivated and more compliant.
Predictors for a lower success rate
Secondary gain Researchers4 found that patients who were enabled by their families had more severe symptoms than those who were not. These relational and environmental factors should be discussed openly. If the patient finds that many of his or her life needs are being met secondary to the illness, that patient might not agree to an aversive treatment.
To overcome this, urge family members or other individuals who provide dysfunctional reinforcers to remove them from the environment. Meet with the patient and family/friends and frankly point out dysfunctional gains and the ways in which family members unknowingly allow the gains to continue (e.g., giving the patient more money after he or she overspent his or her allowance). A family behavioral contract should be devised to address how these gains will be reasonably eliminated.
Recognize, too, that a patient may find it difficult to give up the secondary gains, detrimental as they may be, without adequate skills or coping mechanisms to fill the void. So in some cases, it is best not to remove all the secondary gains at once; this can cause many patients to terminate treatment prematurely.
Trauma or abuse history Many patients with treatment refractory OCD have trauma histories and cannot habituate to the behavioral tasks because of dissociation, emotional numbing, or some form of distraction that mediates their anxiety and prevents proper habituation. If the patient is adequately complying with the exposures, yet still is unable to confront every feared stimulus, inquire about a trauma or abuse history (Box 2).
Substance abuse The stress that is inherent to ERP can cause many patients to relapse or abuse illicit substances to manage their anxiety. Therefore, patients with severe substance abuse problems often have great difficulty handling ERP, as they are asked to experience the very discomfort that initially caused them to abuse drugs and alcohol.
Exposure and response prevention therapy (ERP) may be contraindicated for OCD patients with comorbid posttraumatic stress disorder (PTSD). Patients with trauma histories, especially those for whom the trauma precipitated the onset of OCD symptoms, should receive trauma treatment before or in conjunction with ERP in order to be effective.
Patients with OCD and PTSD should receive adjunctive cognitive behavioral therapy (CBT) for their PTSD. Skills training modules, such as dialectical behavior therapy (DBT) and other CBT treatments, often provide the patient with the necessary skills to regulate the trauma-related stressors that are triggered during ERP and can cause premature termination of treatment.
If habituation is not occurring in the absence of trauma, ask whether the patient is dissociating, daydreaming, numbing, or distracting, as these avoidances will jeopardize his or her ability to benefit from ERP. Teaching the patient grounding techniques and alternate coping mechanisms, such as those found in the mindfulness and distress tolerance module of DBT, can help some patients tolerate their anxiety.
For trauma patients whose dissociation, numbing, or distraction is severe, home-based or residential treatment may be required. There, they can be coached during ERP to bring their attention back to the feared stimuli and deal with the negative fallout of their trauma..
In such cases, a patient cannot realistically be asked to give up a coping mechanism, faulty as it may be, until a more functional reinforcer takes its place. Hence, skills training is a crucial part of treatment for this group.
Residential treatment for OCD patients with comorbid substance abuse in remission may be necessary to ensure a positive outcome. Patients should continue recovery work concurrent to behavior therapy to prevent relapse.
High-risk OCD symptoms Patients who have more traditional OCD symptoms usually have a good prognosis. Unfortunately some symptoms do not respond to ERP treatment. These include:
- Repeating, hoarding, and symmetry. Though evidence suggests that hoarding is predictive of poor outcomes,5 treatment carried out in the home can be effective over a 24-week trial.6
- Incompletion, or the need for things to feel right.
- Rigid and overvalued belief systems.
- Sexual and religious obsessions. These appear to be more resistant to behavior therapy and selective serotonin reuptake inhibitors (SSRIs).7
More research needs to be conducted to offer patients with these symptoms better respite.
Researchers also found that patients with childhood and adolescent onset of symptoms, tics, history of hospitalization, and terminated treatment against medical advice are more likely than other OCD patients to develop more severe symptoms in adulthood.8 Patients with OCD who also suffer from generalized anxiety disorders are more likely than those without GAD to drop out of treatment.9
Behavior therapy: first choice
ERP is considered the premier treatment for OCD and is suitable for both adults and children.10 Exposure forces patients to confront their feared stimuli. Response prevention blocks patients from engaging in compulsions or avoidance behaviors intended to reduce their discomfort. Patients are asked to identify situations that trigger their obsession and compulsions and rank them along a fear hierarchy. Patients confront a moderately rated situation and, once they become habituated to it, move up the fear hierarchy to the next situation.
ERP has been proven effective for OCD not only as an individual behavior treatment, but also when done in a group setting11 or when delivered online or by telephone.12
Table 1
Dosage levels for SRIs in OCD
| Clomipramine | 150-200 mg/d |
| Fluoxetine | 40-80 mg/d |
| Sertraline | 50-200 mg/d |
| Fluvoxamine | 200-300 mg/d |
| Paroxetine | 40-60 mg/d |
| Citalopram | 40-60 mg/d |
| The higher end of the dosage ranges shown above is preferred if tolerated. All clinical trials with SRIs for OCD should last at least 10 weeks. | |
Some clinicians prefer cognitive behavioral therapy (CBT) to ERP because it is less aversive. Researchers found that patients who were treated with either CBT or ERP improved. Patients treated with ERP, however, were more likely to maintain their gains in recovery 3 months after treatment concluded.13 Evidence suggests that ERP or CBT when implemented alone, or when applied in conjunction with fluvoxamine,14 are equally effective.
ERP should be managed only by clinicians specially trained in this modality. Several treatment centers across the country provide specialized care for OCD patients. For the nearest treatment center in your community that accepts referrals for ERP, contact the OC Foundation in North Branford, Conn. (See Related Resources.)
Medication for OCD: SRIs as first-line therapy
Experts agree that first-line somatic treatments for OCD include not only behavior therapy but also serotonin reuptake inhibitors (SRIs),15 that is, clomipramine or selective serotonin reuptake inhibitors, (SSRIs) (Table 1).
Caution: Many patients who “respond” to treatment in clinical studies remain symptomatic and meaningfully affected by their residual illness. Therefore, it is critical that you inform patients at the outset that 100% reduction in symptoms is rare.
SRIs Overwhelming evidence from multiple randomized, double-blind, placebo-controlled studies support the efficacy of SRIs. In adults, well-designed and controlled trials have demonstrated the relative efficacy of clomipramine, fluoxetine, sertraline, paroxetine, and fluvoxamine vs. placebo.
SRIs also have been shown to be significantly more effective than tricyclic antidepressants (TCAs) in both placebo-controlled and non-placebo-controlled studies.
Despite initial reports that clomipramine may be more effective than SSRIs, a growing number of studies and a recent comprehensive literature review suggest that the SRIs all have comparable efficacy.16 Because clomipramine has significantly more anticholinergic- and antiadrenergic-mediated side effects than the SSRIs, however, many clinicians choose SSRIs as the initial agent.
When using SRIs, remember that response is typically delayed; an adequate trial requires at least 10 weeks. Indeed, a meaningful proportion of responders continue to emerge past the 8-week mark. Experts suggest that optimal dosages of SRIs for OCD may exceed those typically used for major depression. Guidelines for SRI dosage ranges for OCD appear in (Table 1).
Data regarding treatment duration also suggest that discontinuation of SRIs results in a high relapse rate, though the use of lower maintenance dosages of SRIs is still debated.
Table 2
Ratings of SRI-augmenting agents for OCD treatment
| Likely effective ♦♦ | Possibly effective (insufficient data for adequate assessment of efficacy) ♦ |
|---|---|
| Neuroleptics | Clonidine |
| Busipirone | Fenfluramine |
| Clonazepam | Nortriptyline |
| Lithium | Pindolol |
| Trazodone | |
| Tryptophan | |
| Dosage for these agents has not been adequately studied for augmentation of SRIs. Clinical trial length should be for 2 to 8 weeks. | |
Augmentation of SRIs When first-line interventions fail, second-line pharmacological approaches include augmentation of SRIs with additional medications (Table 2). Numerous agents have been tried for patients who were unresponsive or only partially responsive to SRIs alone.17 Few controlled trials of such strategies have been conducted, however. The most impressive data document the benefits of adding low doses of dopamine antagonists (both conventional and atypical neuroleptics).
Table 3
Using alternative monotherapies
| Drug | Dosage | Duration | Comments |
|---|---|---|---|
| Clonazepam | 0.5-5 mg/d | ≥ 4 weeks | extrapolated from experience with benzodiazepines for other anxiety disorders and a few reports in OCD |
| MAO inhibitor | 60-90 mg/d | ≥ 10 weeks | extrapolated from clinical practice with MAO inhibitors for major depression, panic disorder; tyramine diet must be adhered to; adequate washout of most antidepressants is required before initiating |
| Buspirone | up to 60 mg/d | ≥ 6 weeks | reflecting protocols adopted in clinical trials for OCD |
Recent uncontrolled studies of augmentation with atypical neuroleptics have yielded encouraging preliminary results, as has one controlled trial of augmentation of an SRI with risperidone. Other data suggest that lithium, buspirone, and clonazepam may also be effective.
Numerous other agents have been tried in combination with SRIs, including clonidine, tryptophan, fenfluramine, pindolol, trazodone, nortriptyline, and other antidepressants. The small number of subjects, lack of sufficient controls, and mixed results preclude drawing even preliminary conclusions as to the potential efficacy of such strategies.
Alternative Monotherapies For patients who do not respond satisfactorily to trials of SRIs alone or to augmentation strategies, consider alternative monotherapies in place of SRIs (Table 3). In addition to uncontrolled data, positive controlled studies lend some support for trials of clonazepam, monoamine oxidase (MAO) inhibitors, and buspirone.
Pertinent negative findings are worthy of mention. In contrast to promising results with risperidone as an augmenter, an open trial of the atypical antipsychotic clozapine suggests inefficacy as a monotherapy. Several case reports suggest that clozapine can actually precipitate obsessive-compulsive symptoms in patients with psychotic disorders.18 Controlled trials have not demonstrated the efficacy of trazodone, clonidine, and diphenhydramine as monotherapies.
Pharmacotherapy + or vs. behavioral therapy
Only a few studies directly comparing behavior therapy vs. medication have been reported. In practice, the two are routinely used in concert. Experts have long recommended this treatment approach. Two recent studies19,20 have demonstrated that the combination is more effective than either treatment alone.
In another study, behavior therapy significantly outperformed clomipramine; no significant incremental benefit was seen from the two treatments in combination.21 However, the dosages of clomipramine were relatively low (mean=164 mg/d and maximum=225 mg/d) and of inadequate duration (6 weeks). Still another older head-to-head comparison of behavior therapy and clomipramine showed that medication was better for reducing obsessional doubt, whereas behavior therapy more effectively reduced compulsive rituals.
Third-line treatments may include the unproven augmentation therapies described above, or intravenous clomipramine if available.22
Treatments of last resort
Finally, other nonpharmacologic treatments, including neurosurgery and electroconvulsive therapy (ECT), have remained controversial and are reserved for particular clinical situations or as treatments of last resort.
Despite a large body of uncontrolled data reporting antiobsessional benefits from a variety of neurosurgical procedures, ethical considerations and technical limitations have precluded the performance of sham-controlled studies to definitively establish the efficacy of these strategies.
Neurosurgical treatment of OCD is reserved for patients with severe and debilitating illness who have failed an exhaustive array of other available treatment options and who provide informed consent or assent. Currently, the most commonly employed neurosurgical treatments for OCD include anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leukotomy. In recent prospective trials of cingulotomy and capsulotomy, approximately 45% of patients experienced a 35% or more symptom reduction.
With the advent of innovative surgical devices that allow functional neurosurgery without craniotomy (e.g., by gamma knife), the performance of ethical, double-blind, sham-controlled trials of neurosurgery for OCD is now feasible. A team of investigators from Brown University and Massachusetts General Hospital is conducting one such study that tests the efficacy of anterior capsulotomy.
There are no controlled data regarding the efficacy of ECT for OCD. Given the high comorbidity of major affective illness in OCD and the well-established efficacy of ECT for major depression, it is not surprising that some patients with OCD have reportedly shown clinical improvement after ECT. Several limited case series and anecdotal reports suggest that ECT may help in some circumstances, and such intervention would seem prudent in some cases where severe, comorbid affective illness is present.23
- Jenike MA, Baer, L, Minichiello WE, eds. Obsessive Compulsive Disorders: Practical Management. 3rd ed. Boston: Mosby, 1998.
- Jenike MA. An update on obsessive-compulsive disorder. Bulletin of the Menninger Clinic. 2001;65:4-25.
- Obsessive-Compulsive Foundation, (203) 315-2190, www.ocfoundation.org
Drug brand names
- Buspirone • BuSpar, BuSpar DIVIDOSE
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Ativar, Diastat, Halcion
- Clonidine • Catapres, Catapres TTS-1
- Clozapine • Clozaril
- Fenfluramine • Pondimin
- Fluoxetine • Prozac, Prozac Weekly
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Phenelzine • Nardil, Parnate
- Pindolol • Inderol, Corgard, Betaloc
- Risperidone • Risperidal
- Sertaline • Zoloft
- Trazodone • Desyrel
- Tryptophan* • L-Tryptophan, Alti-trytophan
Disclosure
Dr. Boxill and Ms. Shapiro report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
Dr. Dougherty reports conflicts of interest with Pfizer Inc., Forest Pharmaceuticals, and Solvay Pharmaceuticals.
1. Goodman Wk, Price LH, et al. The Yale Brown Obsessive Compulsive Scale:1. Development, Use and Reliability. Arch Gen Psychiatry. 1989;46:1012-1016.
2. Catapano F, Sperandeo R, Perris F, Lanzaro M, Maj M. Insight and resistance in patients with obsessive compulsive disorder. Psychopathol. 2001;34(2):62-68.
3. Amir N, Freshman M, Foa EB. Family distress and involvement in relatives of obsessive-compulsive disorder patients. J Anxiety Disord. 2000;14(3):209-217.
4. Chambless DL, Steketee G. Expressed emotion and behavior therapy outcome: a prospective study with obsessive compulsive and agrophobic outpatients. J Consult Clin Psychol. 1999;67(5):658-665.
5. Black DW, Monahan P, Gable J, et al. Hoarding and treatment in 38 nondepressed subjects with OCD. J Clin Psychiatry. 1998;59(8):420-425.
6. Rosqvist J, Egan D, Manzo P, et al. Home-based behavior therapy for obsessive compulsive disorder: A case series with data. J Anxiety Disord. 2001;15(5):395-400.
7. Alonso P, Menchon JM, Pifarre J, et al. Long term follow up and predictors of clinical outcome in obsessive compulsive patients treated with serotonin reuptake inhibitors and behavioral therapy. J Clin Psychiatry. 2001;62(7):535-540.
8. Wewetzer C, Jans T, Muller B, et al. Long term outcome and prognosis of obsessive compulsive disorder with onset in childhood or adolescence. Eur Child Adoles Psychiatry. 2001;10(1):37-46.
9. Steketee G, Chambless DL, Tran GQ. Effects of axis I and axis II comorbidity on behavior therapy outcome for obsessive-compulsive disorder and agrophobia. Compr Psychiatry. 2001;42(1):76-86.
10. Piacentini J. Cognitive behavioral therapy in childhood OCD. Child Adolesc Psychiatr Clin N Am. 1999;8(3):599-616.
11. Himle JA, Rassi S, et al. Group behavioral therapy of obsessive Compulsive disorder: seven vs. twelve-week outcomes. Depress Anxiety. 2001;13(4):161-165.
12. Nakagawa A, Marks IM, Park JM, Bachofen M, Baer L, Dottl SL, Greist JH. Self treatment of obsessive compulsive disorder guided by manual and computer conducted telephone interview. J Telemed Telecare. 2001;6(1):22-26.
13. McLean PD, Whittal ML, et al. Cognitive verses behavior therapy in the group treatment of obsessive compulsive disorder. J Consult Clin Psychol. 2001;69(2):205-214.
14. van Balkom AJ, de Haan E, van Oppen P, et al. Cognitive and behavioral therapies alone versus in combination with fluvoxamine in the treatment of obsessive-compulsive disorder. J Nerv Ment Dis 1998;186:492-499.
15. Dougherty D, Rauch SL. Serotonin-reuptake inhibitors in the treatment of OCD. In: Obsessive-Compulsive Disorders: Diagnosis’Etiology’Treatment. Hollander E, Stein DJ, eds. New York: Marcel Dekker, 1997;145-160.
16. Pigott TA. Seay SM: A review of the efficacy of selective serotonin reuptake inhibitors in obsessive-compulsive disorders. J Clin Psychiatry. 1999;60:101-106.
17. McDougle CJ, Goodman WK. Combination pharmacological treatment strategies. In: Obsessive-Compulsive Disorders: Diagnosis’Etiology’Treatment. Hollander E, Stein DJ, eds. New York: Marcel Dekker, 1997;203-223.
18. McDougle CJ, Barr LC, et al. Lack of efficacy of clozapine monotherapy in refractory obsessive-compulsive disorder. Am J Psychiatry. 1995;152(12):1812-1814.
19. Honagen F, et al. Combination of behaviour therapy with fluvoxamine in comparison with behaviour therapy and placebo. Br J Psychiatry. 1998;173(suppl 35):71-78.
20. O’Connor K, Todorov C, Robillard S, et al. Cognitive-behaviour therapy and medication in the treatment of obsessive-compulsive disorder: a controlled study. Can J Psychiatry. 1999;44:64-71.
21. Rachman S, Cobb J, et al. The behavioural treatment of obsessional-compulsive disorders, with and without clomipramine. Behav Res Ther. 1979;17(5):467-478.
22. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
23. Jenike MA, Rauch SL. Managing the patient with treatment resistant obsessive compulsive disorder: current strategies. J Clin Psychiatry. 1994;55:3(suppl):11-17.
When you suspect a patient has obsessive-compulsive disorder (OCD) (Box 1), how can you differentiate OCD from psychosis? Once you have made the diagnosis, what critical factors suggest treatment will be successful—or unsuccessful? Is behavioral therapy more effective than medication? Which medications are most likely to be effective? The answers to these questions can help you improve the well-being of your patients with OCD.
Differential diagnosis
Unfortunately, many patients with severe OCD are misdiagnosed with psychosis or schizophrenia spectrum disorder and spend many years suffering without proper treatment.3 Despite many similarities between patients with severe OCD and psychosis—including rigid belief systems, unrealistic concerns, magical thinking, and odd behaviors—patients with OCD can recognize the irrational nature of their beliefs when they are not in the throes of anxiety.
Patients with OCD also will usually respond to behavioral interventions within a few weeks while patients who are psychotic usually get progressively worse. Treatment must be given time as both cohorts will get anxious or increase their negative symptoms initially, but patients with OCD should soon habituate and find symptom relief.
Some patients have OCD with psychotic features and tend to have more difficulty responding to behavior therapy without medication. Patients with both OCD and schizotypal personalities respond poorly to both behavior therapy and psychotropic medications.4
Obsessions are intrusive and unwanted thoughts, images, or impulses that produce anxiety. They commonly consist of obsessive fears involving causing harm to others, contamination, safety, religiosity, incompletion, pathological doubt, magical thinking, and the need for certainty, and symmetry.
Usually, obsessions will be accompanied by compulsions, which are behaviors or thoughts performed to reduce the anxiety caused by the obsessions. Compulsions typically consist of excessive washing, checking until it “feels right,” and mental retracing. In rare cases, patients present with only obsessions, which are more difficult to treat than compulsions. Most patients will have several types of symptoms.
To meet the criteria for OCD, patients must be preoccupied by obsessive thoughts and engage in compulsions, which will be frequent, intense, of long duration (more than 2 hours/day), and interfere with the individual’s ability to function. The Yale-Brown Obsessive Compulsive Symptoms Checklist and Scale1 are reliable assessment tools to identify types of symptoms and degree of severity.
Anne is a 53-year-old widow whose OCD symptoms consisted of not letting anything pass her lips that she considered contaminated, lest she become ill with cancer. Her symptoms became so severe that she restricted her diet to a specific brand of peanut butter and milk. The manner in which she ate the peanut butter was rife with checking rituals. If she thought that there might be something wrong with the jar, she threw it away. If she thought the jar was “safe,” she poured the peanut butter directly into her mouth, avoiding the risk of dirty utensils. She drank milk out of the carton. By the time she began treatment, she was malnourished and slightly dehydrated.
Anne’s restrictive diet was also a product of obsessive label checking. Her label reading inevitably resulted in her seeing ordinary household items that she considered risky and would then avoid. Other avoidance behaviors included spitting out saliva and not licking her lips due to fear of what might be ingested, and avoidance of medication, toothpaste, eye drops, skin lotion, and food she feared others had touched.
The good intentions of people in Anne’s community had the effect of enabling her OCD. For example, the local grocer made sure to keep a few cases of Anne's preferred brand of peanut butter in stock for when she needed it. She bought in bulk, but returned unopened jars that she thought were contaminated. As is common with obsessions, no real evidence is needed to legitimize avoidance.
To help Anne break the OCD cycle of avoidance, a meal plan was devised. Although she looked anorexic, but was not, this approach succeeded because she greatly missed the experience of eating and tasting a variety of foods. She also agreed to drink daily nutritional supplements until her diet was more enriching, and had weekly weigh-ins to track her weight gain.
Anne also began a regimen of fluoxetine, which ultimately improved her ability to use the behavior therapy techniques. She was started at 5 mg/d in liquid form. The dosage was increased to 40 mg/d across 1 month, then changed to pill form and titrated to 80 mg/d, which was maintained at discharge.
Exposure and response prevention therapy (ERP) was also administered in twice-daily, 2-hour sessions for about 3 months. Exposure therapy consisted of accompanying Anne to the local supermarket and having her purchase any kind of food that she wanted, regardless of its nutritional value. Her initial purchases consisted of cheesecake, doughnuts, juice, herbal tea, canned ravioli, cereal, lasagna, and snacks.
For response prevention related to food purchases, Anne was prevented from reading labels and examining individual items for imperfections. She was encouraged to buy the first item on the shelf and put it in her basket.
The next step in exposure therapy was to supervise her eating habits. While she looked forward to tasting the food she bought, she was apprehensive because of the obsessive doubt about their purity. Firm but kind encouragement helped her take one bite after another, and this success built on itself. She was excited to be finally confronting her obsessive fears, tasting the foods she restricted herself from for so long, and taking better care of herself. Her complexion improved, and her weight increased.
At times she was highly anxious and looked for ways to avoid the exposure, but with redirection was able to stay on track. She eventually was able to eat community food, eat at a restaurant, use beauty and hygiene products, and have contact with artificial or chemical substances.
Ironically, Anne’s vocational interest was in cooking and after discharge from the program, she investigated employment in hotel/restaurant work and studies at culinary school.
Predictors for successful treatment
Insight Researchers3 found that about 52% of patients with insight into the reasonableness of their obsessions responded to medications, while none who lacked insight responded. Therefore, it pays to assess patients’ insight and ability to recognize the long-term consequences of OCD to themselves and those around them.
Some patients who have suffered with treatment-refractory OCD for most of their lives lack a premorbid high level of functioning to serve as a reference for normalized behavior. Educating these patients to see the advantages of living without certain negative behaviors improves their receptivity to treatment.
Patients who lack insight often refuse to acknowledge that many of their behaviors are manifestations of OCD. Such patients, however, are usually more amenable to giving up or modifying their dysfunctional behaviors—and the clinician more likely to avoid confrontations—if they are shown how certain behaviors undermine their goals.
Cost-benefit analysis Because of the aversive nature of exposure and response prevention therapy (ERP) and the negative side effects of many medications, some patients may find it easier to live with their symptoms, as painful as they are, rather than undergo the discomfort of behavior therapy. Because the prognosis is poor in such cases, patients need to be convinced that the discomfort of treatment is merely short-term, while the discomfort of the illness could last forever if left untreated.
Motivation In our experience, motivation has played a crucial role in determining treatment outcome for severe refractory OCD. And regardless of the severity of their symptoms, patients who are fed up with their symptoms, or are tired of living a life controlled by their obsessions, usually are excellent candidates for treatment.
Conversely, those who enter treatment as a result of external pressure from spouses or family face a less positive prognosis. High emotional expressiveness, overinvolvement, and hostility by relatives is related to higher attrition rates in treatment.3 Because ERP is so aversive, these patients will find ways to dilute the treatment’s effectiveness. In many cases, they do the minimal amount of work required to stay in treatment to avoid whatever consequences their families would impose for not adhering to treatment.
One marker to assess compliance is whether the clinician feels he or she is investing more time and effort into the patient’s treatment than the patient is. If so, this should be addressed in a timely manner. Also, sporadic attendance at sessions and noncompliance with medications, homework, and behavior therapy assignments may also portend a poor outcome. Remember, though, that noncompliance and lack of motivation are fluid states; many previously noncompliant patients later return to treatment better motivated and more compliant.
Predictors for a lower success rate
Secondary gain Researchers4 found that patients who were enabled by their families had more severe symptoms than those who were not. These relational and environmental factors should be discussed openly. If the patient finds that many of his or her life needs are being met secondary to the illness, that patient might not agree to an aversive treatment.
To overcome this, urge family members or other individuals who provide dysfunctional reinforcers to remove them from the environment. Meet with the patient and family/friends and frankly point out dysfunctional gains and the ways in which family members unknowingly allow the gains to continue (e.g., giving the patient more money after he or she overspent his or her allowance). A family behavioral contract should be devised to address how these gains will be reasonably eliminated.
Recognize, too, that a patient may find it difficult to give up the secondary gains, detrimental as they may be, without adequate skills or coping mechanisms to fill the void. So in some cases, it is best not to remove all the secondary gains at once; this can cause many patients to terminate treatment prematurely.
Trauma or abuse history Many patients with treatment refractory OCD have trauma histories and cannot habituate to the behavioral tasks because of dissociation, emotional numbing, or some form of distraction that mediates their anxiety and prevents proper habituation. If the patient is adequately complying with the exposures, yet still is unable to confront every feared stimulus, inquire about a trauma or abuse history (Box 2).
Substance abuse The stress that is inherent to ERP can cause many patients to relapse or abuse illicit substances to manage their anxiety. Therefore, patients with severe substance abuse problems often have great difficulty handling ERP, as they are asked to experience the very discomfort that initially caused them to abuse drugs and alcohol.
Exposure and response prevention therapy (ERP) may be contraindicated for OCD patients with comorbid posttraumatic stress disorder (PTSD). Patients with trauma histories, especially those for whom the trauma precipitated the onset of OCD symptoms, should receive trauma treatment before or in conjunction with ERP in order to be effective.
Patients with OCD and PTSD should receive adjunctive cognitive behavioral therapy (CBT) for their PTSD. Skills training modules, such as dialectical behavior therapy (DBT) and other CBT treatments, often provide the patient with the necessary skills to regulate the trauma-related stressors that are triggered during ERP and can cause premature termination of treatment.
If habituation is not occurring in the absence of trauma, ask whether the patient is dissociating, daydreaming, numbing, or distracting, as these avoidances will jeopardize his or her ability to benefit from ERP. Teaching the patient grounding techniques and alternate coping mechanisms, such as those found in the mindfulness and distress tolerance module of DBT, can help some patients tolerate their anxiety.
For trauma patients whose dissociation, numbing, or distraction is severe, home-based or residential treatment may be required. There, they can be coached during ERP to bring their attention back to the feared stimuli and deal with the negative fallout of their trauma..
In such cases, a patient cannot realistically be asked to give up a coping mechanism, faulty as it may be, until a more functional reinforcer takes its place. Hence, skills training is a crucial part of treatment for this group.
Residential treatment for OCD patients with comorbid substance abuse in remission may be necessary to ensure a positive outcome. Patients should continue recovery work concurrent to behavior therapy to prevent relapse.
High-risk OCD symptoms Patients who have more traditional OCD symptoms usually have a good prognosis. Unfortunately some symptoms do not respond to ERP treatment. These include:
- Repeating, hoarding, and symmetry. Though evidence suggests that hoarding is predictive of poor outcomes,5 treatment carried out in the home can be effective over a 24-week trial.6
- Incompletion, or the need for things to feel right.
- Rigid and overvalued belief systems.
- Sexual and religious obsessions. These appear to be more resistant to behavior therapy and selective serotonin reuptake inhibitors (SSRIs).7
More research needs to be conducted to offer patients with these symptoms better respite.
Researchers also found that patients with childhood and adolescent onset of symptoms, tics, history of hospitalization, and terminated treatment against medical advice are more likely than other OCD patients to develop more severe symptoms in adulthood.8 Patients with OCD who also suffer from generalized anxiety disorders are more likely than those without GAD to drop out of treatment.9
Behavior therapy: first choice
ERP is considered the premier treatment for OCD and is suitable for both adults and children.10 Exposure forces patients to confront their feared stimuli. Response prevention blocks patients from engaging in compulsions or avoidance behaviors intended to reduce their discomfort. Patients are asked to identify situations that trigger their obsession and compulsions and rank them along a fear hierarchy. Patients confront a moderately rated situation and, once they become habituated to it, move up the fear hierarchy to the next situation.
ERP has been proven effective for OCD not only as an individual behavior treatment, but also when done in a group setting11 or when delivered online or by telephone.12
Table 1
Dosage levels for SRIs in OCD
| Clomipramine | 150-200 mg/d |
| Fluoxetine | 40-80 mg/d |
| Sertraline | 50-200 mg/d |
| Fluvoxamine | 200-300 mg/d |
| Paroxetine | 40-60 mg/d |
| Citalopram | 40-60 mg/d |
| The higher end of the dosage ranges shown above is preferred if tolerated. All clinical trials with SRIs for OCD should last at least 10 weeks. | |
Some clinicians prefer cognitive behavioral therapy (CBT) to ERP because it is less aversive. Researchers found that patients who were treated with either CBT or ERP improved. Patients treated with ERP, however, were more likely to maintain their gains in recovery 3 months after treatment concluded.13 Evidence suggests that ERP or CBT when implemented alone, or when applied in conjunction with fluvoxamine,14 are equally effective.
ERP should be managed only by clinicians specially trained in this modality. Several treatment centers across the country provide specialized care for OCD patients. For the nearest treatment center in your community that accepts referrals for ERP, contact the OC Foundation in North Branford, Conn. (See Related Resources.)
Medication for OCD: SRIs as first-line therapy
Experts agree that first-line somatic treatments for OCD include not only behavior therapy but also serotonin reuptake inhibitors (SRIs),15 that is, clomipramine or selective serotonin reuptake inhibitors, (SSRIs) (Table 1).
Caution: Many patients who “respond” to treatment in clinical studies remain symptomatic and meaningfully affected by their residual illness. Therefore, it is critical that you inform patients at the outset that 100% reduction in symptoms is rare.
SRIs Overwhelming evidence from multiple randomized, double-blind, placebo-controlled studies support the efficacy of SRIs. In adults, well-designed and controlled trials have demonstrated the relative efficacy of clomipramine, fluoxetine, sertraline, paroxetine, and fluvoxamine vs. placebo.
SRIs also have been shown to be significantly more effective than tricyclic antidepressants (TCAs) in both placebo-controlled and non-placebo-controlled studies.
Despite initial reports that clomipramine may be more effective than SSRIs, a growing number of studies and a recent comprehensive literature review suggest that the SRIs all have comparable efficacy.16 Because clomipramine has significantly more anticholinergic- and antiadrenergic-mediated side effects than the SSRIs, however, many clinicians choose SSRIs as the initial agent.
When using SRIs, remember that response is typically delayed; an adequate trial requires at least 10 weeks. Indeed, a meaningful proportion of responders continue to emerge past the 8-week mark. Experts suggest that optimal dosages of SRIs for OCD may exceed those typically used for major depression. Guidelines for SRI dosage ranges for OCD appear in (Table 1).
Data regarding treatment duration also suggest that discontinuation of SRIs results in a high relapse rate, though the use of lower maintenance dosages of SRIs is still debated.
Table 2
Ratings of SRI-augmenting agents for OCD treatment
| Likely effective ♦♦ | Possibly effective (insufficient data for adequate assessment of efficacy) ♦ |
|---|---|
| Neuroleptics | Clonidine |
| Busipirone | Fenfluramine |
| Clonazepam | Nortriptyline |
| Lithium | Pindolol |
| Trazodone | |
| Tryptophan | |
| Dosage for these agents has not been adequately studied for augmentation of SRIs. Clinical trial length should be for 2 to 8 weeks. | |
Augmentation of SRIs When first-line interventions fail, second-line pharmacological approaches include augmentation of SRIs with additional medications (Table 2). Numerous agents have been tried for patients who were unresponsive or only partially responsive to SRIs alone.17 Few controlled trials of such strategies have been conducted, however. The most impressive data document the benefits of adding low doses of dopamine antagonists (both conventional and atypical neuroleptics).
Table 3
Using alternative monotherapies
| Drug | Dosage | Duration | Comments |
|---|---|---|---|
| Clonazepam | 0.5-5 mg/d | ≥ 4 weeks | extrapolated from experience with benzodiazepines for other anxiety disorders and a few reports in OCD |
| MAO inhibitor | 60-90 mg/d | ≥ 10 weeks | extrapolated from clinical practice with MAO inhibitors for major depression, panic disorder; tyramine diet must be adhered to; adequate washout of most antidepressants is required before initiating |
| Buspirone | up to 60 mg/d | ≥ 6 weeks | reflecting protocols adopted in clinical trials for OCD |
Recent uncontrolled studies of augmentation with atypical neuroleptics have yielded encouraging preliminary results, as has one controlled trial of augmentation of an SRI with risperidone. Other data suggest that lithium, buspirone, and clonazepam may also be effective.
Numerous other agents have been tried in combination with SRIs, including clonidine, tryptophan, fenfluramine, pindolol, trazodone, nortriptyline, and other antidepressants. The small number of subjects, lack of sufficient controls, and mixed results preclude drawing even preliminary conclusions as to the potential efficacy of such strategies.
Alternative Monotherapies For patients who do not respond satisfactorily to trials of SRIs alone or to augmentation strategies, consider alternative monotherapies in place of SRIs (Table 3). In addition to uncontrolled data, positive controlled studies lend some support for trials of clonazepam, monoamine oxidase (MAO) inhibitors, and buspirone.
Pertinent negative findings are worthy of mention. In contrast to promising results with risperidone as an augmenter, an open trial of the atypical antipsychotic clozapine suggests inefficacy as a monotherapy. Several case reports suggest that clozapine can actually precipitate obsessive-compulsive symptoms in patients with psychotic disorders.18 Controlled trials have not demonstrated the efficacy of trazodone, clonidine, and diphenhydramine as monotherapies.
Pharmacotherapy + or vs. behavioral therapy
Only a few studies directly comparing behavior therapy vs. medication have been reported. In practice, the two are routinely used in concert. Experts have long recommended this treatment approach. Two recent studies19,20 have demonstrated that the combination is more effective than either treatment alone.
In another study, behavior therapy significantly outperformed clomipramine; no significant incremental benefit was seen from the two treatments in combination.21 However, the dosages of clomipramine were relatively low (mean=164 mg/d and maximum=225 mg/d) and of inadequate duration (6 weeks). Still another older head-to-head comparison of behavior therapy and clomipramine showed that medication was better for reducing obsessional doubt, whereas behavior therapy more effectively reduced compulsive rituals.
Third-line treatments may include the unproven augmentation therapies described above, or intravenous clomipramine if available.22
Treatments of last resort
Finally, other nonpharmacologic treatments, including neurosurgery and electroconvulsive therapy (ECT), have remained controversial and are reserved for particular clinical situations or as treatments of last resort.
Despite a large body of uncontrolled data reporting antiobsessional benefits from a variety of neurosurgical procedures, ethical considerations and technical limitations have precluded the performance of sham-controlled studies to definitively establish the efficacy of these strategies.
Neurosurgical treatment of OCD is reserved for patients with severe and debilitating illness who have failed an exhaustive array of other available treatment options and who provide informed consent or assent. Currently, the most commonly employed neurosurgical treatments for OCD include anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leukotomy. In recent prospective trials of cingulotomy and capsulotomy, approximately 45% of patients experienced a 35% or more symptom reduction.
With the advent of innovative surgical devices that allow functional neurosurgery without craniotomy (e.g., by gamma knife), the performance of ethical, double-blind, sham-controlled trials of neurosurgery for OCD is now feasible. A team of investigators from Brown University and Massachusetts General Hospital is conducting one such study that tests the efficacy of anterior capsulotomy.
There are no controlled data regarding the efficacy of ECT for OCD. Given the high comorbidity of major affective illness in OCD and the well-established efficacy of ECT for major depression, it is not surprising that some patients with OCD have reportedly shown clinical improvement after ECT. Several limited case series and anecdotal reports suggest that ECT may help in some circumstances, and such intervention would seem prudent in some cases where severe, comorbid affective illness is present.23
- Jenike MA, Baer, L, Minichiello WE, eds. Obsessive Compulsive Disorders: Practical Management. 3rd ed. Boston: Mosby, 1998.
- Jenike MA. An update on obsessive-compulsive disorder. Bulletin of the Menninger Clinic. 2001;65:4-25.
- Obsessive-Compulsive Foundation, (203) 315-2190, www.ocfoundation.org
Drug brand names
- Buspirone • BuSpar, BuSpar DIVIDOSE
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Ativar, Diastat, Halcion
- Clonidine • Catapres, Catapres TTS-1
- Clozapine • Clozaril
- Fenfluramine • Pondimin
- Fluoxetine • Prozac, Prozac Weekly
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Phenelzine • Nardil, Parnate
- Pindolol • Inderol, Corgard, Betaloc
- Risperidone • Risperidal
- Sertaline • Zoloft
- Trazodone • Desyrel
- Tryptophan* • L-Tryptophan, Alti-trytophan
Disclosure
Dr. Boxill and Ms. Shapiro report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
Dr. Dougherty reports conflicts of interest with Pfizer Inc., Forest Pharmaceuticals, and Solvay Pharmaceuticals.
When you suspect a patient has obsessive-compulsive disorder (OCD) (Box 1), how can you differentiate OCD from psychosis? Once you have made the diagnosis, what critical factors suggest treatment will be successful—or unsuccessful? Is behavioral therapy more effective than medication? Which medications are most likely to be effective? The answers to these questions can help you improve the well-being of your patients with OCD.
Differential diagnosis
Unfortunately, many patients with severe OCD are misdiagnosed with psychosis or schizophrenia spectrum disorder and spend many years suffering without proper treatment.3 Despite many similarities between patients with severe OCD and psychosis—including rigid belief systems, unrealistic concerns, magical thinking, and odd behaviors—patients with OCD can recognize the irrational nature of their beliefs when they are not in the throes of anxiety.
Patients with OCD also will usually respond to behavioral interventions within a few weeks while patients who are psychotic usually get progressively worse. Treatment must be given time as both cohorts will get anxious or increase their negative symptoms initially, but patients with OCD should soon habituate and find symptom relief.
Some patients have OCD with psychotic features and tend to have more difficulty responding to behavior therapy without medication. Patients with both OCD and schizotypal personalities respond poorly to both behavior therapy and psychotropic medications.4
Obsessions are intrusive and unwanted thoughts, images, or impulses that produce anxiety. They commonly consist of obsessive fears involving causing harm to others, contamination, safety, religiosity, incompletion, pathological doubt, magical thinking, and the need for certainty, and symmetry.
Usually, obsessions will be accompanied by compulsions, which are behaviors or thoughts performed to reduce the anxiety caused by the obsessions. Compulsions typically consist of excessive washing, checking until it “feels right,” and mental retracing. In rare cases, patients present with only obsessions, which are more difficult to treat than compulsions. Most patients will have several types of symptoms.
To meet the criteria for OCD, patients must be preoccupied by obsessive thoughts and engage in compulsions, which will be frequent, intense, of long duration (more than 2 hours/day), and interfere with the individual’s ability to function. The Yale-Brown Obsessive Compulsive Symptoms Checklist and Scale1 are reliable assessment tools to identify types of symptoms and degree of severity.
Anne is a 53-year-old widow whose OCD symptoms consisted of not letting anything pass her lips that she considered contaminated, lest she become ill with cancer. Her symptoms became so severe that she restricted her diet to a specific brand of peanut butter and milk. The manner in which she ate the peanut butter was rife with checking rituals. If she thought that there might be something wrong with the jar, she threw it away. If she thought the jar was “safe,” she poured the peanut butter directly into her mouth, avoiding the risk of dirty utensils. She drank milk out of the carton. By the time she began treatment, she was malnourished and slightly dehydrated.
Anne’s restrictive diet was also a product of obsessive label checking. Her label reading inevitably resulted in her seeing ordinary household items that she considered risky and would then avoid. Other avoidance behaviors included spitting out saliva and not licking her lips due to fear of what might be ingested, and avoidance of medication, toothpaste, eye drops, skin lotion, and food she feared others had touched.
The good intentions of people in Anne’s community had the effect of enabling her OCD. For example, the local grocer made sure to keep a few cases of Anne's preferred brand of peanut butter in stock for when she needed it. She bought in bulk, but returned unopened jars that she thought were contaminated. As is common with obsessions, no real evidence is needed to legitimize avoidance.
To help Anne break the OCD cycle of avoidance, a meal plan was devised. Although she looked anorexic, but was not, this approach succeeded because she greatly missed the experience of eating and tasting a variety of foods. She also agreed to drink daily nutritional supplements until her diet was more enriching, and had weekly weigh-ins to track her weight gain.
Anne also began a regimen of fluoxetine, which ultimately improved her ability to use the behavior therapy techniques. She was started at 5 mg/d in liquid form. The dosage was increased to 40 mg/d across 1 month, then changed to pill form and titrated to 80 mg/d, which was maintained at discharge.
Exposure and response prevention therapy (ERP) was also administered in twice-daily, 2-hour sessions for about 3 months. Exposure therapy consisted of accompanying Anne to the local supermarket and having her purchase any kind of food that she wanted, regardless of its nutritional value. Her initial purchases consisted of cheesecake, doughnuts, juice, herbal tea, canned ravioli, cereal, lasagna, and snacks.
For response prevention related to food purchases, Anne was prevented from reading labels and examining individual items for imperfections. She was encouraged to buy the first item on the shelf and put it in her basket.
The next step in exposure therapy was to supervise her eating habits. While she looked forward to tasting the food she bought, she was apprehensive because of the obsessive doubt about their purity. Firm but kind encouragement helped her take one bite after another, and this success built on itself. She was excited to be finally confronting her obsessive fears, tasting the foods she restricted herself from for so long, and taking better care of herself. Her complexion improved, and her weight increased.
At times she was highly anxious and looked for ways to avoid the exposure, but with redirection was able to stay on track. She eventually was able to eat community food, eat at a restaurant, use beauty and hygiene products, and have contact with artificial or chemical substances.
Ironically, Anne’s vocational interest was in cooking and after discharge from the program, she investigated employment in hotel/restaurant work and studies at culinary school.
Predictors for successful treatment
Insight Researchers3 found that about 52% of patients with insight into the reasonableness of their obsessions responded to medications, while none who lacked insight responded. Therefore, it pays to assess patients’ insight and ability to recognize the long-term consequences of OCD to themselves and those around them.
Some patients who have suffered with treatment-refractory OCD for most of their lives lack a premorbid high level of functioning to serve as a reference for normalized behavior. Educating these patients to see the advantages of living without certain negative behaviors improves their receptivity to treatment.
Patients who lack insight often refuse to acknowledge that many of their behaviors are manifestations of OCD. Such patients, however, are usually more amenable to giving up or modifying their dysfunctional behaviors—and the clinician more likely to avoid confrontations—if they are shown how certain behaviors undermine their goals.
Cost-benefit analysis Because of the aversive nature of exposure and response prevention therapy (ERP) and the negative side effects of many medications, some patients may find it easier to live with their symptoms, as painful as they are, rather than undergo the discomfort of behavior therapy. Because the prognosis is poor in such cases, patients need to be convinced that the discomfort of treatment is merely short-term, while the discomfort of the illness could last forever if left untreated.
Motivation In our experience, motivation has played a crucial role in determining treatment outcome for severe refractory OCD. And regardless of the severity of their symptoms, patients who are fed up with their symptoms, or are tired of living a life controlled by their obsessions, usually are excellent candidates for treatment.
Conversely, those who enter treatment as a result of external pressure from spouses or family face a less positive prognosis. High emotional expressiveness, overinvolvement, and hostility by relatives is related to higher attrition rates in treatment.3 Because ERP is so aversive, these patients will find ways to dilute the treatment’s effectiveness. In many cases, they do the minimal amount of work required to stay in treatment to avoid whatever consequences their families would impose for not adhering to treatment.
One marker to assess compliance is whether the clinician feels he or she is investing more time and effort into the patient’s treatment than the patient is. If so, this should be addressed in a timely manner. Also, sporadic attendance at sessions and noncompliance with medications, homework, and behavior therapy assignments may also portend a poor outcome. Remember, though, that noncompliance and lack of motivation are fluid states; many previously noncompliant patients later return to treatment better motivated and more compliant.
Predictors for a lower success rate
Secondary gain Researchers4 found that patients who were enabled by their families had more severe symptoms than those who were not. These relational and environmental factors should be discussed openly. If the patient finds that many of his or her life needs are being met secondary to the illness, that patient might not agree to an aversive treatment.
To overcome this, urge family members or other individuals who provide dysfunctional reinforcers to remove them from the environment. Meet with the patient and family/friends and frankly point out dysfunctional gains and the ways in which family members unknowingly allow the gains to continue (e.g., giving the patient more money after he or she overspent his or her allowance). A family behavioral contract should be devised to address how these gains will be reasonably eliminated.
Recognize, too, that a patient may find it difficult to give up the secondary gains, detrimental as they may be, without adequate skills or coping mechanisms to fill the void. So in some cases, it is best not to remove all the secondary gains at once; this can cause many patients to terminate treatment prematurely.
Trauma or abuse history Many patients with treatment refractory OCD have trauma histories and cannot habituate to the behavioral tasks because of dissociation, emotional numbing, or some form of distraction that mediates their anxiety and prevents proper habituation. If the patient is adequately complying with the exposures, yet still is unable to confront every feared stimulus, inquire about a trauma or abuse history (Box 2).
Substance abuse The stress that is inherent to ERP can cause many patients to relapse or abuse illicit substances to manage their anxiety. Therefore, patients with severe substance abuse problems often have great difficulty handling ERP, as they are asked to experience the very discomfort that initially caused them to abuse drugs and alcohol.
Exposure and response prevention therapy (ERP) may be contraindicated for OCD patients with comorbid posttraumatic stress disorder (PTSD). Patients with trauma histories, especially those for whom the trauma precipitated the onset of OCD symptoms, should receive trauma treatment before or in conjunction with ERP in order to be effective.
Patients with OCD and PTSD should receive adjunctive cognitive behavioral therapy (CBT) for their PTSD. Skills training modules, such as dialectical behavior therapy (DBT) and other CBT treatments, often provide the patient with the necessary skills to regulate the trauma-related stressors that are triggered during ERP and can cause premature termination of treatment.
If habituation is not occurring in the absence of trauma, ask whether the patient is dissociating, daydreaming, numbing, or distracting, as these avoidances will jeopardize his or her ability to benefit from ERP. Teaching the patient grounding techniques and alternate coping mechanisms, such as those found in the mindfulness and distress tolerance module of DBT, can help some patients tolerate their anxiety.
For trauma patients whose dissociation, numbing, or distraction is severe, home-based or residential treatment may be required. There, they can be coached during ERP to bring their attention back to the feared stimuli and deal with the negative fallout of their trauma..
In such cases, a patient cannot realistically be asked to give up a coping mechanism, faulty as it may be, until a more functional reinforcer takes its place. Hence, skills training is a crucial part of treatment for this group.
Residential treatment for OCD patients with comorbid substance abuse in remission may be necessary to ensure a positive outcome. Patients should continue recovery work concurrent to behavior therapy to prevent relapse.
High-risk OCD symptoms Patients who have more traditional OCD symptoms usually have a good prognosis. Unfortunately some symptoms do not respond to ERP treatment. These include:
- Repeating, hoarding, and symmetry. Though evidence suggests that hoarding is predictive of poor outcomes,5 treatment carried out in the home can be effective over a 24-week trial.6
- Incompletion, or the need for things to feel right.
- Rigid and overvalued belief systems.
- Sexual and religious obsessions. These appear to be more resistant to behavior therapy and selective serotonin reuptake inhibitors (SSRIs).7
More research needs to be conducted to offer patients with these symptoms better respite.
Researchers also found that patients with childhood and adolescent onset of symptoms, tics, history of hospitalization, and terminated treatment against medical advice are more likely than other OCD patients to develop more severe symptoms in adulthood.8 Patients with OCD who also suffer from generalized anxiety disorders are more likely than those without GAD to drop out of treatment.9
Behavior therapy: first choice
ERP is considered the premier treatment for OCD and is suitable for both adults and children.10 Exposure forces patients to confront their feared stimuli. Response prevention blocks patients from engaging in compulsions or avoidance behaviors intended to reduce their discomfort. Patients are asked to identify situations that trigger their obsession and compulsions and rank them along a fear hierarchy. Patients confront a moderately rated situation and, once they become habituated to it, move up the fear hierarchy to the next situation.
ERP has been proven effective for OCD not only as an individual behavior treatment, but also when done in a group setting11 or when delivered online or by telephone.12
Table 1
Dosage levels for SRIs in OCD
| Clomipramine | 150-200 mg/d |
| Fluoxetine | 40-80 mg/d |
| Sertraline | 50-200 mg/d |
| Fluvoxamine | 200-300 mg/d |
| Paroxetine | 40-60 mg/d |
| Citalopram | 40-60 mg/d |
| The higher end of the dosage ranges shown above is preferred if tolerated. All clinical trials with SRIs for OCD should last at least 10 weeks. | |
Some clinicians prefer cognitive behavioral therapy (CBT) to ERP because it is less aversive. Researchers found that patients who were treated with either CBT or ERP improved. Patients treated with ERP, however, were more likely to maintain their gains in recovery 3 months after treatment concluded.13 Evidence suggests that ERP or CBT when implemented alone, or when applied in conjunction with fluvoxamine,14 are equally effective.
ERP should be managed only by clinicians specially trained in this modality. Several treatment centers across the country provide specialized care for OCD patients. For the nearest treatment center in your community that accepts referrals for ERP, contact the OC Foundation in North Branford, Conn. (See Related Resources.)
Medication for OCD: SRIs as first-line therapy
Experts agree that first-line somatic treatments for OCD include not only behavior therapy but also serotonin reuptake inhibitors (SRIs),15 that is, clomipramine or selective serotonin reuptake inhibitors, (SSRIs) (Table 1).
Caution: Many patients who “respond” to treatment in clinical studies remain symptomatic and meaningfully affected by their residual illness. Therefore, it is critical that you inform patients at the outset that 100% reduction in symptoms is rare.
SRIs Overwhelming evidence from multiple randomized, double-blind, placebo-controlled studies support the efficacy of SRIs. In adults, well-designed and controlled trials have demonstrated the relative efficacy of clomipramine, fluoxetine, sertraline, paroxetine, and fluvoxamine vs. placebo.
SRIs also have been shown to be significantly more effective than tricyclic antidepressants (TCAs) in both placebo-controlled and non-placebo-controlled studies.
Despite initial reports that clomipramine may be more effective than SSRIs, a growing number of studies and a recent comprehensive literature review suggest that the SRIs all have comparable efficacy.16 Because clomipramine has significantly more anticholinergic- and antiadrenergic-mediated side effects than the SSRIs, however, many clinicians choose SSRIs as the initial agent.
When using SRIs, remember that response is typically delayed; an adequate trial requires at least 10 weeks. Indeed, a meaningful proportion of responders continue to emerge past the 8-week mark. Experts suggest that optimal dosages of SRIs for OCD may exceed those typically used for major depression. Guidelines for SRI dosage ranges for OCD appear in (Table 1).
Data regarding treatment duration also suggest that discontinuation of SRIs results in a high relapse rate, though the use of lower maintenance dosages of SRIs is still debated.
Table 2
Ratings of SRI-augmenting agents for OCD treatment
| Likely effective ♦♦ | Possibly effective (insufficient data for adequate assessment of efficacy) ♦ |
|---|---|
| Neuroleptics | Clonidine |
| Busipirone | Fenfluramine |
| Clonazepam | Nortriptyline |
| Lithium | Pindolol |
| Trazodone | |
| Tryptophan | |
| Dosage for these agents has not been adequately studied for augmentation of SRIs. Clinical trial length should be for 2 to 8 weeks. | |
Augmentation of SRIs When first-line interventions fail, second-line pharmacological approaches include augmentation of SRIs with additional medications (Table 2). Numerous agents have been tried for patients who were unresponsive or only partially responsive to SRIs alone.17 Few controlled trials of such strategies have been conducted, however. The most impressive data document the benefits of adding low doses of dopamine antagonists (both conventional and atypical neuroleptics).
Table 3
Using alternative monotherapies
| Drug | Dosage | Duration | Comments |
|---|---|---|---|
| Clonazepam | 0.5-5 mg/d | ≥ 4 weeks | extrapolated from experience with benzodiazepines for other anxiety disorders and a few reports in OCD |
| MAO inhibitor | 60-90 mg/d | ≥ 10 weeks | extrapolated from clinical practice with MAO inhibitors for major depression, panic disorder; tyramine diet must be adhered to; adequate washout of most antidepressants is required before initiating |
| Buspirone | up to 60 mg/d | ≥ 6 weeks | reflecting protocols adopted in clinical trials for OCD |
Recent uncontrolled studies of augmentation with atypical neuroleptics have yielded encouraging preliminary results, as has one controlled trial of augmentation of an SRI with risperidone. Other data suggest that lithium, buspirone, and clonazepam may also be effective.
Numerous other agents have been tried in combination with SRIs, including clonidine, tryptophan, fenfluramine, pindolol, trazodone, nortriptyline, and other antidepressants. The small number of subjects, lack of sufficient controls, and mixed results preclude drawing even preliminary conclusions as to the potential efficacy of such strategies.
Alternative Monotherapies For patients who do not respond satisfactorily to trials of SRIs alone or to augmentation strategies, consider alternative monotherapies in place of SRIs (Table 3). In addition to uncontrolled data, positive controlled studies lend some support for trials of clonazepam, monoamine oxidase (MAO) inhibitors, and buspirone.
Pertinent negative findings are worthy of mention. In contrast to promising results with risperidone as an augmenter, an open trial of the atypical antipsychotic clozapine suggests inefficacy as a monotherapy. Several case reports suggest that clozapine can actually precipitate obsessive-compulsive symptoms in patients with psychotic disorders.18 Controlled trials have not demonstrated the efficacy of trazodone, clonidine, and diphenhydramine as monotherapies.
Pharmacotherapy + or vs. behavioral therapy
Only a few studies directly comparing behavior therapy vs. medication have been reported. In practice, the two are routinely used in concert. Experts have long recommended this treatment approach. Two recent studies19,20 have demonstrated that the combination is more effective than either treatment alone.
In another study, behavior therapy significantly outperformed clomipramine; no significant incremental benefit was seen from the two treatments in combination.21 However, the dosages of clomipramine were relatively low (mean=164 mg/d and maximum=225 mg/d) and of inadequate duration (6 weeks). Still another older head-to-head comparison of behavior therapy and clomipramine showed that medication was better for reducing obsessional doubt, whereas behavior therapy more effectively reduced compulsive rituals.
Third-line treatments may include the unproven augmentation therapies described above, or intravenous clomipramine if available.22
Treatments of last resort
Finally, other nonpharmacologic treatments, including neurosurgery and electroconvulsive therapy (ECT), have remained controversial and are reserved for particular clinical situations or as treatments of last resort.
Despite a large body of uncontrolled data reporting antiobsessional benefits from a variety of neurosurgical procedures, ethical considerations and technical limitations have precluded the performance of sham-controlled studies to definitively establish the efficacy of these strategies.
Neurosurgical treatment of OCD is reserved for patients with severe and debilitating illness who have failed an exhaustive array of other available treatment options and who provide informed consent or assent. Currently, the most commonly employed neurosurgical treatments for OCD include anterior cingulotomy, anterior capsulotomy, subcaudate tractotomy, and limbic leukotomy. In recent prospective trials of cingulotomy and capsulotomy, approximately 45% of patients experienced a 35% or more symptom reduction.
With the advent of innovative surgical devices that allow functional neurosurgery without craniotomy (e.g., by gamma knife), the performance of ethical, double-blind, sham-controlled trials of neurosurgery for OCD is now feasible. A team of investigators from Brown University and Massachusetts General Hospital is conducting one such study that tests the efficacy of anterior capsulotomy.
There are no controlled data regarding the efficacy of ECT for OCD. Given the high comorbidity of major affective illness in OCD and the well-established efficacy of ECT for major depression, it is not surprising that some patients with OCD have reportedly shown clinical improvement after ECT. Several limited case series and anecdotal reports suggest that ECT may help in some circumstances, and such intervention would seem prudent in some cases where severe, comorbid affective illness is present.23
- Jenike MA, Baer, L, Minichiello WE, eds. Obsessive Compulsive Disorders: Practical Management. 3rd ed. Boston: Mosby, 1998.
- Jenike MA. An update on obsessive-compulsive disorder. Bulletin of the Menninger Clinic. 2001;65:4-25.
- Obsessive-Compulsive Foundation, (203) 315-2190, www.ocfoundation.org
Drug brand names
- Buspirone • BuSpar, BuSpar DIVIDOSE
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Ativar, Diastat, Halcion
- Clonidine • Catapres, Catapres TTS-1
- Clozapine • Clozaril
- Fenfluramine • Pondimin
- Fluoxetine • Prozac, Prozac Weekly
- Fluvoxamine • Luvox
- Paroxetine • Paxil
- Phenelzine • Nardil, Parnate
- Pindolol • Inderol, Corgard, Betaloc
- Risperidone • Risperidal
- Sertaline • Zoloft
- Trazodone • Desyrel
- Tryptophan* • L-Tryptophan, Alti-trytophan
Disclosure
Dr. Boxill and Ms. Shapiro report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
Dr. Dougherty reports conflicts of interest with Pfizer Inc., Forest Pharmaceuticals, and Solvay Pharmaceuticals.
1. Goodman Wk, Price LH, et al. The Yale Brown Obsessive Compulsive Scale:1. Development, Use and Reliability. Arch Gen Psychiatry. 1989;46:1012-1016.
2. Catapano F, Sperandeo R, Perris F, Lanzaro M, Maj M. Insight and resistance in patients with obsessive compulsive disorder. Psychopathol. 2001;34(2):62-68.
3. Amir N, Freshman M, Foa EB. Family distress and involvement in relatives of obsessive-compulsive disorder patients. J Anxiety Disord. 2000;14(3):209-217.
4. Chambless DL, Steketee G. Expressed emotion and behavior therapy outcome: a prospective study with obsessive compulsive and agrophobic outpatients. J Consult Clin Psychol. 1999;67(5):658-665.
5. Black DW, Monahan P, Gable J, et al. Hoarding and treatment in 38 nondepressed subjects with OCD. J Clin Psychiatry. 1998;59(8):420-425.
6. Rosqvist J, Egan D, Manzo P, et al. Home-based behavior therapy for obsessive compulsive disorder: A case series with data. J Anxiety Disord. 2001;15(5):395-400.
7. Alonso P, Menchon JM, Pifarre J, et al. Long term follow up and predictors of clinical outcome in obsessive compulsive patients treated with serotonin reuptake inhibitors and behavioral therapy. J Clin Psychiatry. 2001;62(7):535-540.
8. Wewetzer C, Jans T, Muller B, et al. Long term outcome and prognosis of obsessive compulsive disorder with onset in childhood or adolescence. Eur Child Adoles Psychiatry. 2001;10(1):37-46.
9. Steketee G, Chambless DL, Tran GQ. Effects of axis I and axis II comorbidity on behavior therapy outcome for obsessive-compulsive disorder and agrophobia. Compr Psychiatry. 2001;42(1):76-86.
10. Piacentini J. Cognitive behavioral therapy in childhood OCD. Child Adolesc Psychiatr Clin N Am. 1999;8(3):599-616.
11. Himle JA, Rassi S, et al. Group behavioral therapy of obsessive Compulsive disorder: seven vs. twelve-week outcomes. Depress Anxiety. 2001;13(4):161-165.
12. Nakagawa A, Marks IM, Park JM, Bachofen M, Baer L, Dottl SL, Greist JH. Self treatment of obsessive compulsive disorder guided by manual and computer conducted telephone interview. J Telemed Telecare. 2001;6(1):22-26.
13. McLean PD, Whittal ML, et al. Cognitive verses behavior therapy in the group treatment of obsessive compulsive disorder. J Consult Clin Psychol. 2001;69(2):205-214.
14. van Balkom AJ, de Haan E, van Oppen P, et al. Cognitive and behavioral therapies alone versus in combination with fluvoxamine in the treatment of obsessive-compulsive disorder. J Nerv Ment Dis 1998;186:492-499.
15. Dougherty D, Rauch SL. Serotonin-reuptake inhibitors in the treatment of OCD. In: Obsessive-Compulsive Disorders: Diagnosis’Etiology’Treatment. Hollander E, Stein DJ, eds. New York: Marcel Dekker, 1997;145-160.
16. Pigott TA. Seay SM: A review of the efficacy of selective serotonin reuptake inhibitors in obsessive-compulsive disorders. J Clin Psychiatry. 1999;60:101-106.
17. McDougle CJ, Goodman WK. Combination pharmacological treatment strategies. In: Obsessive-Compulsive Disorders: Diagnosis’Etiology’Treatment. Hollander E, Stein DJ, eds. New York: Marcel Dekker, 1997;203-223.
18. McDougle CJ, Barr LC, et al. Lack of efficacy of clozapine monotherapy in refractory obsessive-compulsive disorder. Am J Psychiatry. 1995;152(12):1812-1814.
19. Honagen F, et al. Combination of behaviour therapy with fluvoxamine in comparison with behaviour therapy and placebo. Br J Psychiatry. 1998;173(suppl 35):71-78.
20. O’Connor K, Todorov C, Robillard S, et al. Cognitive-behaviour therapy and medication in the treatment of obsessive-compulsive disorder: a controlled study. Can J Psychiatry. 1999;44:64-71.
21. Rachman S, Cobb J, et al. The behavioural treatment of obsessional-compulsive disorders, with and without clomipramine. Behav Res Ther. 1979;17(5):467-478.
22. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
23. Jenike MA, Rauch SL. Managing the patient with treatment resistant obsessive compulsive disorder: current strategies. J Clin Psychiatry. 1994;55:3(suppl):11-17.
1. Goodman Wk, Price LH, et al. The Yale Brown Obsessive Compulsive Scale:1. Development, Use and Reliability. Arch Gen Psychiatry. 1989;46:1012-1016.
2. Catapano F, Sperandeo R, Perris F, Lanzaro M, Maj M. Insight and resistance in patients with obsessive compulsive disorder. Psychopathol. 2001;34(2):62-68.
3. Amir N, Freshman M, Foa EB. Family distress and involvement in relatives of obsessive-compulsive disorder patients. J Anxiety Disord. 2000;14(3):209-217.
4. Chambless DL, Steketee G. Expressed emotion and behavior therapy outcome: a prospective study with obsessive compulsive and agrophobic outpatients. J Consult Clin Psychol. 1999;67(5):658-665.
5. Black DW, Monahan P, Gable J, et al. Hoarding and treatment in 38 nondepressed subjects with OCD. J Clin Psychiatry. 1998;59(8):420-425.
6. Rosqvist J, Egan D, Manzo P, et al. Home-based behavior therapy for obsessive compulsive disorder: A case series with data. J Anxiety Disord. 2001;15(5):395-400.
7. Alonso P, Menchon JM, Pifarre J, et al. Long term follow up and predictors of clinical outcome in obsessive compulsive patients treated with serotonin reuptake inhibitors and behavioral therapy. J Clin Psychiatry. 2001;62(7):535-540.
8. Wewetzer C, Jans T, Muller B, et al. Long term outcome and prognosis of obsessive compulsive disorder with onset in childhood or adolescence. Eur Child Adoles Psychiatry. 2001;10(1):37-46.
9. Steketee G, Chambless DL, Tran GQ. Effects of axis I and axis II comorbidity on behavior therapy outcome for obsessive-compulsive disorder and agrophobia. Compr Psychiatry. 2001;42(1):76-86.
10. Piacentini J. Cognitive behavioral therapy in childhood OCD. Child Adolesc Psychiatr Clin N Am. 1999;8(3):599-616.
11. Himle JA, Rassi S, et al. Group behavioral therapy of obsessive Compulsive disorder: seven vs. twelve-week outcomes. Depress Anxiety. 2001;13(4):161-165.
12. Nakagawa A, Marks IM, Park JM, Bachofen M, Baer L, Dottl SL, Greist JH. Self treatment of obsessive compulsive disorder guided by manual and computer conducted telephone interview. J Telemed Telecare. 2001;6(1):22-26.
13. McLean PD, Whittal ML, et al. Cognitive verses behavior therapy in the group treatment of obsessive compulsive disorder. J Consult Clin Psychol. 2001;69(2):205-214.
14. van Balkom AJ, de Haan E, van Oppen P, et al. Cognitive and behavioral therapies alone versus in combination with fluvoxamine in the treatment of obsessive-compulsive disorder. J Nerv Ment Dis 1998;186:492-499.
15. Dougherty D, Rauch SL. Serotonin-reuptake inhibitors in the treatment of OCD. In: Obsessive-Compulsive Disorders: Diagnosis’Etiology’Treatment. Hollander E, Stein DJ, eds. New York: Marcel Dekker, 1997;145-160.
16. Pigott TA. Seay SM: A review of the efficacy of selective serotonin reuptake inhibitors in obsessive-compulsive disorders. J Clin Psychiatry. 1999;60:101-106.
17. McDougle CJ, Goodman WK. Combination pharmacological treatment strategies. In: Obsessive-Compulsive Disorders: Diagnosis’Etiology’Treatment. Hollander E, Stein DJ, eds. New York: Marcel Dekker, 1997;203-223.
18. McDougle CJ, Barr LC, et al. Lack of efficacy of clozapine monotherapy in refractory obsessive-compulsive disorder. Am J Psychiatry. 1995;152(12):1812-1814.
19. Honagen F, et al. Combination of behaviour therapy with fluvoxamine in comparison with behaviour therapy and placebo. Br J Psychiatry. 1998;173(suppl 35):71-78.
20. O’Connor K, Todorov C, Robillard S, et al. Cognitive-behaviour therapy and medication in the treatment of obsessive-compulsive disorder: a controlled study. Can J Psychiatry. 1999;44:64-71.
21. Rachman S, Cobb J, et al. The behavioural treatment of obsessional-compulsive disorders, with and without clomipramine. Behav Res Ther. 1979;17(5):467-478.
22. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
23. Jenike MA, Rauch SL. Managing the patient with treatment resistant obsessive compulsive disorder: current strategies. J Clin Psychiatry. 1994;55:3(suppl):11-17.
How to recognize and treat the pathological gambler
Mr. R., 37, started gambling during college. He often bet on sporting events with friends. The gambling was sporadic and apparently did not cause any problems in his life.
Over the next 3 or 4 years, he started visiting the local casino every couple of months with friends and playing blackjack. He occasionally won, but even if he lost he was able to return home without being preoccupied by the experience.
During the past year the frequency with which he gambled had increased. Prior to treatment, he was going to the casino 2 or 3 nights per week. Although he intended to gamble only for a few hours and spend no more than $100 each visit, Mr. R. usually spent most of the evening at the casino and squandered $500 to $1,000 per night. Mr. R. reported daily—and severe—urges to gamble. He would often see a billboard for the casino while driving home from his job as an accountant. The advertisement triggered his urges; instead of going home, he would drive to the casino.
While at work, Mr. R. spent so much time thinking of ways to win at gambling—which tables to play, how to lay the bets, which “lucky” shirt to wear—that he could not complete his assignments in a timely manner. He found it difficult to stay at work, even when he had meetings. Instead, when he felt urges to gamble, he would leave early.
Mr. R. felt ashamed of his gambling and kept it a secret from his wife. If unable to gamble when he had urges, he felt anxious and irritable. Because of his gambling, he missed family functions and lied to his wife and family.
Although married for only 3 years, his wife had already talked about divorce. Mr. R. had significant credit card debt and had to file for bankruptcy. Only then did he tell his wife about his gambling problem.
After telling his primary physician he was depressed, Mr. R. was treated with citalopram 20mg/d. The medication helped his mood but did not decrease his urges to gamble or alter his gambling behavior.
When he presented to our clinic, Mr. R. was off medication and still gambling 2 or 3 times per week. He was then treated with naltrexone 25mg/d for 2 days, then 50mg/d. Because of a possible drug-drug interaction with naltrexone, he agreed to stop taking over-the-counter nonsteroidal analgesics. After 2 weeks, the dosage was increased to 100mg/d taken in the morning with food. Mr. R. reported that his urges to gamble, although still present, were significantly reduced.
After another 2 weeks, the dosage was increased to 150mg/d. Mr. R. reported that his urges to gamble were gone. Without the urges, he was able to stop gambling. Liver function tests were performed every 2 weeks for the first 2 months of treatment and every month thereafter for 3 months.
This case illustrates many of the clinical features of pathological gambling, defined by persistent and recurrent maladaptive patterns of gambling behavior. The disorder often goes undiagnosed and untreated, though preliminary data suggest that it may be relatively common. The lifetime prevalence of pathological gambling is 1.6% among adults, and 3.9% among those younger than 18.1 Pathological gamblers usually experience painful financial losses and perhaps as a consequence have high rates of bankruptcy, divorce, and criminal behavior.2 They also often suffer from comorbid mood, anxiety, and alcohol use disorders.3 In fact, the suicide rate in cities with established, legal casinos is 2 to 4 times higher than in cities without.4
How to identify the pathological gambler
Pathological gambling is often a secret disorder. If left untreated, it frequently becomes a chronic condition. To make the diagnosis, use the simple screening instrument shown in (Table 1).5 The patient is likely to be suffering from pathological gambling disorder if he or she answered five or more of the questions “yes.” The last two questions are useful when trying to determine if someone has a gambling problem that is not readily apparent. Some people gamble in binges—for example, they may only gamble when they have available funds. These people may, however, be significantly impaired by the cravings or urges to gamble even though they may not have gambled for weeks.
Data suggest that the male-to-female ratio in pathological gambling is approximately 2:1. Gambling usually begins in early adulthood, with males tending to start at an earlier age. Although many pathological gamblers take several years to develop a problem, almost one-half report feeling “addicted” within a year after starting to gamble.2 Females appear to develop pathological gambling disorder in a shorter time.
Most pathological gamblers are fairly specific about their choice of gambling activity. Women tend to play slot machines and bingo, whereas men choose sporting events, blackjack, and cards. Both cite advertisements as a common trigger of their urges to gamble, although females are more likely to report that feeling bored or lonely may also trigger these urges.
Despite their preoccupation with gambling, many pathological gamblers function quite well, although often below their capacity. But others are severely debilitated—unable to function socially or occupationally.
In a series of 131 patients with pathological gambling disorder, 44% had lost all their savings, 24% had filed for bankruptcy, 23% had lost their homes or cars, and 15% had significant marital problems because of their gambling.2 Financial concerns may become so distressing that many pathological gamblers engage in illegal behavior, such as stealing, embezzlement, and writing bad checks.2
Table 1
Questions that can help diagnose pathological gambling
| Yes | No | Questions | |
|---|---|---|---|
| ○ | ○ | 1. Are you preoccupied with previous or future gambling experiences? That is, do you think about them a lot and wish you could think about them less? | |
| ○ | ○ | 2. Have you been unable to stop gambling or decrease the amount you gamble? | |
| ○ | ○ | 3. When you tried to stop or cut down, did you feel more irritable or anxious? | |
| ○ | ○ | 4. Has the amount you gamble increased to get the same sort of “high” or excitement? | |
| ○ | ○ | 5. Do you gamble as a way of escaping feelings of depression? | |
| ○ | ○ | 6. After you lose, do you go back in the next couple of days to try and win back the money? | |
| What effect has your gambling had on your life? | |||
| ○ | ○ | 7. Have you lied to others about your gambling? | |
| ○ | ○ | 8. Have you committed illegal acts as a result of gambling or lack of money? | |
| ○ | ○ | 9. Has gambling significantly interfered with school, job, or relationships? | |
| ○ | ○ | 10. Have you needed to borrow money because of gambling? | |
| ○ | ○ | 11. When you aren’t gambling, do you have urges to gamble? | |
| ○ | ○ | 12. If you have urges to gamble, do they preoccupy you or interfere with school, job, or relationships? | |
| adapted from DSM-IV criteria5 | |||
Table 2 Medical options for pathological gambling
Treatment response findings appear promising
Only a few controlled treatment studies of pathological gambling have been done, but the findings on the response to treatment appear promising. Thus far, the uses of serotonin reuptake inhibitors (SRIs), either clomipramine or selective serotonin reuptake inhibitors (SSRIs), the opioid antagonist naltrexone, mood stabilizers, and atypical neuroleptics have met with varying degrees of success. Additional strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive behavioral therapies) may represent important adjunctive components.6
Because no medication is currently approved by the Food and Drug Administration (FDA) for treating pathological gambling, it is important to inform your patients of any “off-label” use of medications for this disorder, as well as the empirical basis for considering the medication.
The role of SRIs Response to SRIs usually means decreased thoughts about gambling, decreased gambling behavior, and improved social and occupational functioning. Patients may initially report feeling both less preoccupied with gambling and less anxious about having thoughts of gambling. For people who gamble because they are depressed and trying to escape loneliness or depressed feelings, SRIs are a reasonable first-line medication (Table 2).
As in the treatment of obsessive-compulsive disorder (OCD), dosages of SRIs required to treat pathological gambling symptoms appear to be higher than the average dosages required to treat depressive disorders. Some studies suggest that a significant initial response may be largely placebo. This means that improvement should be monitored for several months and that patients and clinicians need to be cautious about early improvement. An SRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dose tolerated or recommended by the manufacturer has been reached.
The following SRIs have been used with varying degrees of success:
- Clomipramine has been shown effective in reducing gambling behavior with dosages between 125mg/d to 175mg/d.7
- Fluvoxamine has demonstrated mixed results in 3 studies of pathological gambling. Two studies supported its efficacy at an average dosage of 195mg/d to 207mg/d,8-9 but a third found that time spent gambling did not decrease when subjects took 200mg/d.10
- Citalopram has also shown some benefit as a possible treatment option in a single study.11
- Paroxetine, at dosages between 20mg/d and 60mg/d, was found to decrease thoughts of gambling and gambling behavior after approximately 6 to 8 weeks of treatment.12
- Fluoxetine (20mg/d) plus monthly supportive psychotherapy was found to improve gambling symptoms more than did supportive therapy alone.13
When naltrexone is indicated Naltrexone appears to be a reasonable first-line agent for patients who report intense urges to gamble (Table 2). Many patients who report “obsessions” with gambling may suffer from uncontrollable urges that interfere with daily functioning. By eliminating or reducing the urges, the preoccupation often disappears. Patients taking naltrexone often report less-intense urges. The urges may not go away completely; instead, they are often reduced enough for the patient to resist them more easily. Patients also report enjoying the gambling experience less when taking naltrexone; the “high” associated with gambling is reduced.
Naltrexone has been tested in psychiatric conditions in which urges are a dominant symptom.14 The greatest amount of evidence supports the agent’s use in treating alcohol dependence (see related article on page 55) and opiate dependence, both of which are FDA-approved indications.
In the case of pathological gambling, a small body of literature suggests that naltrexone is effective. One case report describes a patient suffering from both pathological gambling and alcohol dependence who responded to naltrexone 50mg/d.15 The first study using naltrexone in pathological gambling showed a significant decline in the intensity of urges to gamble, gambling thoughts, and gambling behavior when using 157 mg/d on average.16 This was followed by a larger study in which an average naltrexone dosage of 188mg/d resulted in improvement in gambling urges, thoughts, and behavior.14
Clinically, a patient will usually respond to a particular dose of naltrexone within 2 weeks. After that, an adjustment in dose is usually necessary. Patients often report nausea and diarrhea. Dizziness, sedation, and headaches occur less commonly. The side effects are usually mild and go away within the first week. Nausea, however, may be moderate to severe in some patients, so patients should be started on 25mg/d for the first 3 or 4 days to reduce that possibility. Ondansetron 4 mg/d is often given adjunctively for the same period to prevent the nausea.
Given the risk of associated hepatic transaminase elevations, liver function tests should be monitored in all patients taking naltrexone.14,16 A boxed warning refers to the potential hepatotoxicity of naltrexone at 50 mg/d, the dosage recommended for treating alcohol or opioid dependence. The warning also states that naltrexone use is contraindicated in acute hepatitis or liver failure, and its use in individuals with active liver disease must be carefully considered.
Initial liver function tests should be evaluated prior to naltrexone administration and again 3 to 4 weeks after starting the drug. Repeat testing should be performed at 2- to 4-week intervals for the next 2 months, a potential high-risk period. Thereafter, tests should be done approximately once a month for the following 3 months. After 6 months, liver enzyme elevations appear to occur rarely and testing 3 to 4 times a year should suffice unless an undue risk arises, e.g., excessive alcohol consumption. If elevated, the enzymes return to normal levels after discontinuing the naltrexone.
Research in this area is still in an early stage and clinicians prescribing the drug for pathological gambling should take extra caution in administering naltrexone at high doses and monitor for potential adverse consequences. Nonsteroidal analgesics should not be used in conjunction with high-dose naltrexone,14 as their concurrent use seems to cause a higher risk of hepatic transaminase elevation.
Mood stabilizers Successful responses to lithium and carbamazepine were described in 2 early case reports. Three subjects who were treated with lithium 1,800mg/d reported cessation of gambling.17 An early case report also found that carbamazepine resulted in improvement in pathological gambling disorder.18 Preliminary studies of lithium and valproate further support the notion that mood stabilizers may be useful. The benefit from carbamazepine, lithium, and valproate may be attributed to their efficacy in treating bipolar disorder and to the existence of features shared by pathological gambling and bipolar disorder (e.g., impulsivity).
Atypical antipsychotics Although there is little evidence that atypical antipsychotics work against pathological gambling when used alone, clinically atypical neuroleptic augmentation of SSRIs may be beneficial.
Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including OCD. A recent trial of olanzapine in the treatment of pathological video poker gamblers showed no difference in outcomes between the patients on medication and those on placebo.19
Cognitive behavioral therapy There is also mounting evidence that cognitive behavioral treatments are effective for pathological gambling.6,20-21 Combined pharmacological and behavioral therapy is considered the optimal treatment strategy for many psychiatric disorders, including substance dependence.
In our clinical experience, patients who only partially respond or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive behavioral therapies. Future studies should explore directly how pharmacological and behavioral therapy contribute to clinical improvement as part of combination treatment strategies for pathological gambling.
Ways to enhance compliance
Pathological gamblers, like those with bipolar disorder or substance dependence, often fail to comply with treatment.
Patients suffering from mania may not adhere to treatment with mood stabilizers in part because drug treatment may reduce positive or euphoric experiences. Similarly, the “high” associated with drug use often makes patients ambivalent about taking medications to remain abstinent.
In treating opioid dependence with naltrexone, a reward system has been incorporated in a contingency management fashion to substitute for drug-related reward and to enhance compliance with the medication.22 Recruitment of friends or family has also been used to enhance compliance with naltrexone in treating opioid dependence.22
In the case of pathological gamblers, a self-rewarding system for each day of nongambling can be used (e.g., a favorite meal or a movie).6 Family members can help by increasing social or other activities that can take the patient’s mind off gambling, particularly at high-risk times (weekends and payday).6
How long should you treat?
No guidelines exist for recommended adequate treatment trials for pathological gambling. Available data, however, suggest that an adequate medication trial may require a relatively long duration (4 months or longer).
Many pathological gamblers have never discussed their difficulties, and this unquantifiable therapeutic response may resemble a medication response. Clinicians must monitor symptoms long enough to assess the difference between response to placebo and to medication.
The optimal duration of treatment and rates of relapse associated with discontinuation are not known. Many patients relapse upon discontinuation of medication, and long-term treatment, perhaps 2 to 3 years, may be warranted.
Treatment-resistant pathological gambling
It is not unusual to encounter patients who have had no response or only a partial response to treatment. Several approaches appear promising (Table 2):
- If a patient has had an adequate trial of either an SRI or naltrexone as monotherapy, adding another agent appears to result in additional clinical improvement.
- A patient who has failed to respond to either an SRI or naltrexone may be effectively treated with a mood stabilizer, either as monotherapy or as augmentation. Some patients with pathological gambling disorder, although not screening positive for a manic episode, exhibit symptoms consistent with cyclothymia or other subclinical cycling mood disorder. For such patients lithium, valproate, or another drug with putative mood stabilizing properties may represent an appropriate option.
- The possibility of adding atypical antipsychotics to SRIs in the SRI-refractory pathological gambler warrants consideration.
- Patients who only partially respond or fail to respond to medication alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies, since this is considered the optimal treatment strategy for many psychiatric disorders.
Related resources
- Gamblers Anonymous International Service Office. Los Angeles, Calif. http://www.gamblersanonymous.org.
- National Research Council. Pathological Gambling: A Critical Review. Washington, DC: National Academy Press, 1990.
- James K. National Gambling Impact Study Commission: Final Report to Congress 1999. http://www.ngisc.gov/reports/finrpt.html
Drug brand names
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clomipramine • Anafranil
- Fluoxetine• Prozac, Prozac Weekly
- Fluvoxamine • Luvox
- Naltrexone • ReVia
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Valproic acid •Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
1. Shaffer HJ, Hall MN, Vander Bilt J. Estimating the prevalence of disordered gambling behavior in the United States and Canada: a research synthesis. Am J Public Health. 1999;89(9):1369-1376.
2. Grant JE, Kim SW. Demographic and clinical features of 131 adult pathological gamblers. J Clin Psychiatry. 2001;62(12):957-962.
3. Black DW, Moyer TM. Clinical features and psychiatric comorbidity of subjects with pathological gambling behavior. Psychiatric Serv. 1998;49(11):1434-1439.
4. Phillips DP, Welty WR, Smith MM. Elevated suicide levels associated with legalized gambling. Suicide Life Threat Behav. 1997;27(4):373-378.
5. American Psychiatric Association Committee on Nomenclature and Statistics. Diagnostic and Statistical Manual of Mental Disorders. 4th ed., text revision. Washington, DC: American Psychiatric Association, 2000.
6. Petry NM, Roll JM. A behavioral approach to understanding and treating pathological gambling. Semin Clin Neuropsychiatry. 2001;6(3):177-183.
7. Hollander E, Frenkel M, DeCaria C, et al. Treatment of pathological gambling with clomipramine. Am J Psychiatry. 1992;149(5):710-711.
8. Hollander E, DeCaria CM, Mari E, et al. Short-term single-blind fluvoxamine treatment of pathological gambling. Am J Psychiatry. 1998;155(12):1781-1783.
9. Hollander E, DeCaria CM, et al. A randomized double-blind fluvoxamine/placebo crossover trial in pathological gambling. Biol Psychiatry. 2000;47(9):813-817.
10. Blanco-Jerez C. A long-term, double-blind, placebo-controlled study of fluvoxamine for pathological gambling. Presented at the 152nd Annual Meeting of the American Psychiatric Association, Washington, DC, May 16-21, 1999 [abstract].
11. Zimmerman M, Breen R. An open-label study of citalopram in the treatment of pathological gambling. Presented at the 11th International Conference on Gambling and Risk Taking, Las Vegas, Nev, June 17-21, 2000 [abstract].
12. Kim SW, Grant JE, Shin YC, Toth JA, et al. A double-blind, placebo-controlled study of the efficacy and safety of paroxetine in the treatment of pathological gambling disorder. J Clin Psychiatry. 2002 (in press).
13. De la Gandara JJ. Fluoxetine: open-trial in pathological gambling. Presented at the 152 Annual Meeting of the American Psychiatric Association, Washington, DC, May 16-21, 1999 [abstract].
14. Kim SW, Grant JE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921.
15. Crockford DN, el-Guebaly N. Naltrexone in the treatment of pathological gambling and alcohol dependence. Can J Psychiatry. 1998;43(1):86.-
16. Kim SW, Grant JE. An open naltrexone treatment study of pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289.
17. Moskowitz JA. Lithium and lady luck: use of lithium carbonate in compulsive gambling. NY State J Med. 1980;80(5):785-788.
18. Haller R, Hinterhuber H. Treatment of pathological gambling with carbamazepine. Pharmacopsychiatry. 1994;27:129.-
19. Rugle L. The use of olanzapine in the treatment of video poker pathological gamblers. Presented at the conference titled, “The Comorbidity of Pathological Gambling: A Current Research Synthesis,” Dec 3 5, 2000, Las Vegas, Nev [abstract].
20. Ladoceur R, Sylvain C, Boutin C, et al. Cognitive treatment of pathological gambling. J Nerv Ment Dis. 2001;189(11):774-780.
21. Sylvain C, Ladouceur R, Boisvert JM. Cognitive and behavioral treatment of pathological gambling: a controlled study. J Consult Clin Psychol. 1997;65(5):727-732.
22. Carroll KM, Ball SA, Nich C, et al. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence. Arch Gen Psychiatry. 2001;58(8):755-761.
Mr. R., 37, started gambling during college. He often bet on sporting events with friends. The gambling was sporadic and apparently did not cause any problems in his life.
Over the next 3 or 4 years, he started visiting the local casino every couple of months with friends and playing blackjack. He occasionally won, but even if he lost he was able to return home without being preoccupied by the experience.
During the past year the frequency with which he gambled had increased. Prior to treatment, he was going to the casino 2 or 3 nights per week. Although he intended to gamble only for a few hours and spend no more than $100 each visit, Mr. R. usually spent most of the evening at the casino and squandered $500 to $1,000 per night. Mr. R. reported daily—and severe—urges to gamble. He would often see a billboard for the casino while driving home from his job as an accountant. The advertisement triggered his urges; instead of going home, he would drive to the casino.
While at work, Mr. R. spent so much time thinking of ways to win at gambling—which tables to play, how to lay the bets, which “lucky” shirt to wear—that he could not complete his assignments in a timely manner. He found it difficult to stay at work, even when he had meetings. Instead, when he felt urges to gamble, he would leave early.
Mr. R. felt ashamed of his gambling and kept it a secret from his wife. If unable to gamble when he had urges, he felt anxious and irritable. Because of his gambling, he missed family functions and lied to his wife and family.
Although married for only 3 years, his wife had already talked about divorce. Mr. R. had significant credit card debt and had to file for bankruptcy. Only then did he tell his wife about his gambling problem.
After telling his primary physician he was depressed, Mr. R. was treated with citalopram 20mg/d. The medication helped his mood but did not decrease his urges to gamble or alter his gambling behavior.
When he presented to our clinic, Mr. R. was off medication and still gambling 2 or 3 times per week. He was then treated with naltrexone 25mg/d for 2 days, then 50mg/d. Because of a possible drug-drug interaction with naltrexone, he agreed to stop taking over-the-counter nonsteroidal analgesics. After 2 weeks, the dosage was increased to 100mg/d taken in the morning with food. Mr. R. reported that his urges to gamble, although still present, were significantly reduced.
After another 2 weeks, the dosage was increased to 150mg/d. Mr. R. reported that his urges to gamble were gone. Without the urges, he was able to stop gambling. Liver function tests were performed every 2 weeks for the first 2 months of treatment and every month thereafter for 3 months.
This case illustrates many of the clinical features of pathological gambling, defined by persistent and recurrent maladaptive patterns of gambling behavior. The disorder often goes undiagnosed and untreated, though preliminary data suggest that it may be relatively common. The lifetime prevalence of pathological gambling is 1.6% among adults, and 3.9% among those younger than 18.1 Pathological gamblers usually experience painful financial losses and perhaps as a consequence have high rates of bankruptcy, divorce, and criminal behavior.2 They also often suffer from comorbid mood, anxiety, and alcohol use disorders.3 In fact, the suicide rate in cities with established, legal casinos is 2 to 4 times higher than in cities without.4
How to identify the pathological gambler
Pathological gambling is often a secret disorder. If left untreated, it frequently becomes a chronic condition. To make the diagnosis, use the simple screening instrument shown in (Table 1).5 The patient is likely to be suffering from pathological gambling disorder if he or she answered five or more of the questions “yes.” The last two questions are useful when trying to determine if someone has a gambling problem that is not readily apparent. Some people gamble in binges—for example, they may only gamble when they have available funds. These people may, however, be significantly impaired by the cravings or urges to gamble even though they may not have gambled for weeks.
Data suggest that the male-to-female ratio in pathological gambling is approximately 2:1. Gambling usually begins in early adulthood, with males tending to start at an earlier age. Although many pathological gamblers take several years to develop a problem, almost one-half report feeling “addicted” within a year after starting to gamble.2 Females appear to develop pathological gambling disorder in a shorter time.
Most pathological gamblers are fairly specific about their choice of gambling activity. Women tend to play slot machines and bingo, whereas men choose sporting events, blackjack, and cards. Both cite advertisements as a common trigger of their urges to gamble, although females are more likely to report that feeling bored or lonely may also trigger these urges.
Despite their preoccupation with gambling, many pathological gamblers function quite well, although often below their capacity. But others are severely debilitated—unable to function socially or occupationally.
In a series of 131 patients with pathological gambling disorder, 44% had lost all their savings, 24% had filed for bankruptcy, 23% had lost their homes or cars, and 15% had significant marital problems because of their gambling.2 Financial concerns may become so distressing that many pathological gamblers engage in illegal behavior, such as stealing, embezzlement, and writing bad checks.2
Table 1
Questions that can help diagnose pathological gambling
| Yes | No | Questions | |
|---|---|---|---|
| ○ | ○ | 1. Are you preoccupied with previous or future gambling experiences? That is, do you think about them a lot and wish you could think about them less? | |
| ○ | ○ | 2. Have you been unable to stop gambling or decrease the amount you gamble? | |
| ○ | ○ | 3. When you tried to stop or cut down, did you feel more irritable or anxious? | |
| ○ | ○ | 4. Has the amount you gamble increased to get the same sort of “high” or excitement? | |
| ○ | ○ | 5. Do you gamble as a way of escaping feelings of depression? | |
| ○ | ○ | 6. After you lose, do you go back in the next couple of days to try and win back the money? | |
| What effect has your gambling had on your life? | |||
| ○ | ○ | 7. Have you lied to others about your gambling? | |
| ○ | ○ | 8. Have you committed illegal acts as a result of gambling or lack of money? | |
| ○ | ○ | 9. Has gambling significantly interfered with school, job, or relationships? | |
| ○ | ○ | 10. Have you needed to borrow money because of gambling? | |
| ○ | ○ | 11. When you aren’t gambling, do you have urges to gamble? | |
| ○ | ○ | 12. If you have urges to gamble, do they preoccupy you or interfere with school, job, or relationships? | |
| adapted from DSM-IV criteria5 | |||
Table 2 Medical options for pathological gambling
Treatment response findings appear promising
Only a few controlled treatment studies of pathological gambling have been done, but the findings on the response to treatment appear promising. Thus far, the uses of serotonin reuptake inhibitors (SRIs), either clomipramine or selective serotonin reuptake inhibitors (SSRIs), the opioid antagonist naltrexone, mood stabilizers, and atypical neuroleptics have met with varying degrees of success. Additional strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive behavioral therapies) may represent important adjunctive components.6
Because no medication is currently approved by the Food and Drug Administration (FDA) for treating pathological gambling, it is important to inform your patients of any “off-label” use of medications for this disorder, as well as the empirical basis for considering the medication.
The role of SRIs Response to SRIs usually means decreased thoughts about gambling, decreased gambling behavior, and improved social and occupational functioning. Patients may initially report feeling both less preoccupied with gambling and less anxious about having thoughts of gambling. For people who gamble because they are depressed and trying to escape loneliness or depressed feelings, SRIs are a reasonable first-line medication (Table 2).
As in the treatment of obsessive-compulsive disorder (OCD), dosages of SRIs required to treat pathological gambling symptoms appear to be higher than the average dosages required to treat depressive disorders. Some studies suggest that a significant initial response may be largely placebo. This means that improvement should be monitored for several months and that patients and clinicians need to be cautious about early improvement. An SRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dose tolerated or recommended by the manufacturer has been reached.
The following SRIs have been used with varying degrees of success:
- Clomipramine has been shown effective in reducing gambling behavior with dosages between 125mg/d to 175mg/d.7
- Fluvoxamine has demonstrated mixed results in 3 studies of pathological gambling. Two studies supported its efficacy at an average dosage of 195mg/d to 207mg/d,8-9 but a third found that time spent gambling did not decrease when subjects took 200mg/d.10
- Citalopram has also shown some benefit as a possible treatment option in a single study.11
- Paroxetine, at dosages between 20mg/d and 60mg/d, was found to decrease thoughts of gambling and gambling behavior after approximately 6 to 8 weeks of treatment.12
- Fluoxetine (20mg/d) plus monthly supportive psychotherapy was found to improve gambling symptoms more than did supportive therapy alone.13
When naltrexone is indicated Naltrexone appears to be a reasonable first-line agent for patients who report intense urges to gamble (Table 2). Many patients who report “obsessions” with gambling may suffer from uncontrollable urges that interfere with daily functioning. By eliminating or reducing the urges, the preoccupation often disappears. Patients taking naltrexone often report less-intense urges. The urges may not go away completely; instead, they are often reduced enough for the patient to resist them more easily. Patients also report enjoying the gambling experience less when taking naltrexone; the “high” associated with gambling is reduced.
Naltrexone has been tested in psychiatric conditions in which urges are a dominant symptom.14 The greatest amount of evidence supports the agent’s use in treating alcohol dependence (see related article on page 55) and opiate dependence, both of which are FDA-approved indications.
In the case of pathological gambling, a small body of literature suggests that naltrexone is effective. One case report describes a patient suffering from both pathological gambling and alcohol dependence who responded to naltrexone 50mg/d.15 The first study using naltrexone in pathological gambling showed a significant decline in the intensity of urges to gamble, gambling thoughts, and gambling behavior when using 157 mg/d on average.16 This was followed by a larger study in which an average naltrexone dosage of 188mg/d resulted in improvement in gambling urges, thoughts, and behavior.14
Clinically, a patient will usually respond to a particular dose of naltrexone within 2 weeks. After that, an adjustment in dose is usually necessary. Patients often report nausea and diarrhea. Dizziness, sedation, and headaches occur less commonly. The side effects are usually mild and go away within the first week. Nausea, however, may be moderate to severe in some patients, so patients should be started on 25mg/d for the first 3 or 4 days to reduce that possibility. Ondansetron 4 mg/d is often given adjunctively for the same period to prevent the nausea.
Given the risk of associated hepatic transaminase elevations, liver function tests should be monitored in all patients taking naltrexone.14,16 A boxed warning refers to the potential hepatotoxicity of naltrexone at 50 mg/d, the dosage recommended for treating alcohol or opioid dependence. The warning also states that naltrexone use is contraindicated in acute hepatitis or liver failure, and its use in individuals with active liver disease must be carefully considered.
Initial liver function tests should be evaluated prior to naltrexone administration and again 3 to 4 weeks after starting the drug. Repeat testing should be performed at 2- to 4-week intervals for the next 2 months, a potential high-risk period. Thereafter, tests should be done approximately once a month for the following 3 months. After 6 months, liver enzyme elevations appear to occur rarely and testing 3 to 4 times a year should suffice unless an undue risk arises, e.g., excessive alcohol consumption. If elevated, the enzymes return to normal levels after discontinuing the naltrexone.
Research in this area is still in an early stage and clinicians prescribing the drug for pathological gambling should take extra caution in administering naltrexone at high doses and monitor for potential adverse consequences. Nonsteroidal analgesics should not be used in conjunction with high-dose naltrexone,14 as their concurrent use seems to cause a higher risk of hepatic transaminase elevation.
Mood stabilizers Successful responses to lithium and carbamazepine were described in 2 early case reports. Three subjects who were treated with lithium 1,800mg/d reported cessation of gambling.17 An early case report also found that carbamazepine resulted in improvement in pathological gambling disorder.18 Preliminary studies of lithium and valproate further support the notion that mood stabilizers may be useful. The benefit from carbamazepine, lithium, and valproate may be attributed to their efficacy in treating bipolar disorder and to the existence of features shared by pathological gambling and bipolar disorder (e.g., impulsivity).
Atypical antipsychotics Although there is little evidence that atypical antipsychotics work against pathological gambling when used alone, clinically atypical neuroleptic augmentation of SSRIs may be beneficial.
Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including OCD. A recent trial of olanzapine in the treatment of pathological video poker gamblers showed no difference in outcomes between the patients on medication and those on placebo.19
Cognitive behavioral therapy There is also mounting evidence that cognitive behavioral treatments are effective for pathological gambling.6,20-21 Combined pharmacological and behavioral therapy is considered the optimal treatment strategy for many psychiatric disorders, including substance dependence.
In our clinical experience, patients who only partially respond or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive behavioral therapies. Future studies should explore directly how pharmacological and behavioral therapy contribute to clinical improvement as part of combination treatment strategies for pathological gambling.
Ways to enhance compliance
Pathological gamblers, like those with bipolar disorder or substance dependence, often fail to comply with treatment.
Patients suffering from mania may not adhere to treatment with mood stabilizers in part because drug treatment may reduce positive or euphoric experiences. Similarly, the “high” associated with drug use often makes patients ambivalent about taking medications to remain abstinent.
In treating opioid dependence with naltrexone, a reward system has been incorporated in a contingency management fashion to substitute for drug-related reward and to enhance compliance with the medication.22 Recruitment of friends or family has also been used to enhance compliance with naltrexone in treating opioid dependence.22
In the case of pathological gamblers, a self-rewarding system for each day of nongambling can be used (e.g., a favorite meal or a movie).6 Family members can help by increasing social or other activities that can take the patient’s mind off gambling, particularly at high-risk times (weekends and payday).6
How long should you treat?
No guidelines exist for recommended adequate treatment trials for pathological gambling. Available data, however, suggest that an adequate medication trial may require a relatively long duration (4 months or longer).
Many pathological gamblers have never discussed their difficulties, and this unquantifiable therapeutic response may resemble a medication response. Clinicians must monitor symptoms long enough to assess the difference between response to placebo and to medication.
The optimal duration of treatment and rates of relapse associated with discontinuation are not known. Many patients relapse upon discontinuation of medication, and long-term treatment, perhaps 2 to 3 years, may be warranted.
Treatment-resistant pathological gambling
It is not unusual to encounter patients who have had no response or only a partial response to treatment. Several approaches appear promising (Table 2):
- If a patient has had an adequate trial of either an SRI or naltrexone as monotherapy, adding another agent appears to result in additional clinical improvement.
- A patient who has failed to respond to either an SRI or naltrexone may be effectively treated with a mood stabilizer, either as monotherapy or as augmentation. Some patients with pathological gambling disorder, although not screening positive for a manic episode, exhibit symptoms consistent with cyclothymia or other subclinical cycling mood disorder. For such patients lithium, valproate, or another drug with putative mood stabilizing properties may represent an appropriate option.
- The possibility of adding atypical antipsychotics to SRIs in the SRI-refractory pathological gambler warrants consideration.
- Patients who only partially respond or fail to respond to medication alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies, since this is considered the optimal treatment strategy for many psychiatric disorders.
Related resources
- Gamblers Anonymous International Service Office. Los Angeles, Calif. http://www.gamblersanonymous.org.
- National Research Council. Pathological Gambling: A Critical Review. Washington, DC: National Academy Press, 1990.
- James K. National Gambling Impact Study Commission: Final Report to Congress 1999. http://www.ngisc.gov/reports/finrpt.html
Drug brand names
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clomipramine • Anafranil
- Fluoxetine• Prozac, Prozac Weekly
- Fluvoxamine • Luvox
- Naltrexone • ReVia
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Valproic acid •Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
Mr. R., 37, started gambling during college. He often bet on sporting events with friends. The gambling was sporadic and apparently did not cause any problems in his life.
Over the next 3 or 4 years, he started visiting the local casino every couple of months with friends and playing blackjack. He occasionally won, but even if he lost he was able to return home without being preoccupied by the experience.
During the past year the frequency with which he gambled had increased. Prior to treatment, he was going to the casino 2 or 3 nights per week. Although he intended to gamble only for a few hours and spend no more than $100 each visit, Mr. R. usually spent most of the evening at the casino and squandered $500 to $1,000 per night. Mr. R. reported daily—and severe—urges to gamble. He would often see a billboard for the casino while driving home from his job as an accountant. The advertisement triggered his urges; instead of going home, he would drive to the casino.
While at work, Mr. R. spent so much time thinking of ways to win at gambling—which tables to play, how to lay the bets, which “lucky” shirt to wear—that he could not complete his assignments in a timely manner. He found it difficult to stay at work, even when he had meetings. Instead, when he felt urges to gamble, he would leave early.
Mr. R. felt ashamed of his gambling and kept it a secret from his wife. If unable to gamble when he had urges, he felt anxious and irritable. Because of his gambling, he missed family functions and lied to his wife and family.
Although married for only 3 years, his wife had already talked about divorce. Mr. R. had significant credit card debt and had to file for bankruptcy. Only then did he tell his wife about his gambling problem.
After telling his primary physician he was depressed, Mr. R. was treated with citalopram 20mg/d. The medication helped his mood but did not decrease his urges to gamble or alter his gambling behavior.
When he presented to our clinic, Mr. R. was off medication and still gambling 2 or 3 times per week. He was then treated with naltrexone 25mg/d for 2 days, then 50mg/d. Because of a possible drug-drug interaction with naltrexone, he agreed to stop taking over-the-counter nonsteroidal analgesics. After 2 weeks, the dosage was increased to 100mg/d taken in the morning with food. Mr. R. reported that his urges to gamble, although still present, were significantly reduced.
After another 2 weeks, the dosage was increased to 150mg/d. Mr. R. reported that his urges to gamble were gone. Without the urges, he was able to stop gambling. Liver function tests were performed every 2 weeks for the first 2 months of treatment and every month thereafter for 3 months.
This case illustrates many of the clinical features of pathological gambling, defined by persistent and recurrent maladaptive patterns of gambling behavior. The disorder often goes undiagnosed and untreated, though preliminary data suggest that it may be relatively common. The lifetime prevalence of pathological gambling is 1.6% among adults, and 3.9% among those younger than 18.1 Pathological gamblers usually experience painful financial losses and perhaps as a consequence have high rates of bankruptcy, divorce, and criminal behavior.2 They also often suffer from comorbid mood, anxiety, and alcohol use disorders.3 In fact, the suicide rate in cities with established, legal casinos is 2 to 4 times higher than in cities without.4
How to identify the pathological gambler
Pathological gambling is often a secret disorder. If left untreated, it frequently becomes a chronic condition. To make the diagnosis, use the simple screening instrument shown in (Table 1).5 The patient is likely to be suffering from pathological gambling disorder if he or she answered five or more of the questions “yes.” The last two questions are useful when trying to determine if someone has a gambling problem that is not readily apparent. Some people gamble in binges—for example, they may only gamble when they have available funds. These people may, however, be significantly impaired by the cravings or urges to gamble even though they may not have gambled for weeks.
Data suggest that the male-to-female ratio in pathological gambling is approximately 2:1. Gambling usually begins in early adulthood, with males tending to start at an earlier age. Although many pathological gamblers take several years to develop a problem, almost one-half report feeling “addicted” within a year after starting to gamble.2 Females appear to develop pathological gambling disorder in a shorter time.
Most pathological gamblers are fairly specific about their choice of gambling activity. Women tend to play slot machines and bingo, whereas men choose sporting events, blackjack, and cards. Both cite advertisements as a common trigger of their urges to gamble, although females are more likely to report that feeling bored or lonely may also trigger these urges.
Despite their preoccupation with gambling, many pathological gamblers function quite well, although often below their capacity. But others are severely debilitated—unable to function socially or occupationally.
In a series of 131 patients with pathological gambling disorder, 44% had lost all their savings, 24% had filed for bankruptcy, 23% had lost their homes or cars, and 15% had significant marital problems because of their gambling.2 Financial concerns may become so distressing that many pathological gamblers engage in illegal behavior, such as stealing, embezzlement, and writing bad checks.2
Table 1
Questions that can help diagnose pathological gambling
| Yes | No | Questions | |
|---|---|---|---|
| ○ | ○ | 1. Are you preoccupied with previous or future gambling experiences? That is, do you think about them a lot and wish you could think about them less? | |
| ○ | ○ | 2. Have you been unable to stop gambling or decrease the amount you gamble? | |
| ○ | ○ | 3. When you tried to stop or cut down, did you feel more irritable or anxious? | |
| ○ | ○ | 4. Has the amount you gamble increased to get the same sort of “high” or excitement? | |
| ○ | ○ | 5. Do you gamble as a way of escaping feelings of depression? | |
| ○ | ○ | 6. After you lose, do you go back in the next couple of days to try and win back the money? | |
| What effect has your gambling had on your life? | |||
| ○ | ○ | 7. Have you lied to others about your gambling? | |
| ○ | ○ | 8. Have you committed illegal acts as a result of gambling or lack of money? | |
| ○ | ○ | 9. Has gambling significantly interfered with school, job, or relationships? | |
| ○ | ○ | 10. Have you needed to borrow money because of gambling? | |
| ○ | ○ | 11. When you aren’t gambling, do you have urges to gamble? | |
| ○ | ○ | 12. If you have urges to gamble, do they preoccupy you or interfere with school, job, or relationships? | |
| adapted from DSM-IV criteria5 | |||
Table 2 Medical options for pathological gambling
Treatment response findings appear promising
Only a few controlled treatment studies of pathological gambling have been done, but the findings on the response to treatment appear promising. Thus far, the uses of serotonin reuptake inhibitors (SRIs), either clomipramine or selective serotonin reuptake inhibitors (SSRIs), the opioid antagonist naltrexone, mood stabilizers, and atypical neuroleptics have met with varying degrees of success. Additional strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive behavioral therapies) may represent important adjunctive components.6
Because no medication is currently approved by the Food and Drug Administration (FDA) for treating pathological gambling, it is important to inform your patients of any “off-label” use of medications for this disorder, as well as the empirical basis for considering the medication.
The role of SRIs Response to SRIs usually means decreased thoughts about gambling, decreased gambling behavior, and improved social and occupational functioning. Patients may initially report feeling both less preoccupied with gambling and less anxious about having thoughts of gambling. For people who gamble because they are depressed and trying to escape loneliness or depressed feelings, SRIs are a reasonable first-line medication (Table 2).
As in the treatment of obsessive-compulsive disorder (OCD), dosages of SRIs required to treat pathological gambling symptoms appear to be higher than the average dosages required to treat depressive disorders. Some studies suggest that a significant initial response may be largely placebo. This means that improvement should be monitored for several months and that patients and clinicians need to be cautious about early improvement. An SRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dose tolerated or recommended by the manufacturer has been reached.
The following SRIs have been used with varying degrees of success:
- Clomipramine has been shown effective in reducing gambling behavior with dosages between 125mg/d to 175mg/d.7
- Fluvoxamine has demonstrated mixed results in 3 studies of pathological gambling. Two studies supported its efficacy at an average dosage of 195mg/d to 207mg/d,8-9 but a third found that time spent gambling did not decrease when subjects took 200mg/d.10
- Citalopram has also shown some benefit as a possible treatment option in a single study.11
- Paroxetine, at dosages between 20mg/d and 60mg/d, was found to decrease thoughts of gambling and gambling behavior after approximately 6 to 8 weeks of treatment.12
- Fluoxetine (20mg/d) plus monthly supportive psychotherapy was found to improve gambling symptoms more than did supportive therapy alone.13
When naltrexone is indicated Naltrexone appears to be a reasonable first-line agent for patients who report intense urges to gamble (Table 2). Many patients who report “obsessions” with gambling may suffer from uncontrollable urges that interfere with daily functioning. By eliminating or reducing the urges, the preoccupation often disappears. Patients taking naltrexone often report less-intense urges. The urges may not go away completely; instead, they are often reduced enough for the patient to resist them more easily. Patients also report enjoying the gambling experience less when taking naltrexone; the “high” associated with gambling is reduced.
Naltrexone has been tested in psychiatric conditions in which urges are a dominant symptom.14 The greatest amount of evidence supports the agent’s use in treating alcohol dependence (see related article on page 55) and opiate dependence, both of which are FDA-approved indications.
In the case of pathological gambling, a small body of literature suggests that naltrexone is effective. One case report describes a patient suffering from both pathological gambling and alcohol dependence who responded to naltrexone 50mg/d.15 The first study using naltrexone in pathological gambling showed a significant decline in the intensity of urges to gamble, gambling thoughts, and gambling behavior when using 157 mg/d on average.16 This was followed by a larger study in which an average naltrexone dosage of 188mg/d resulted in improvement in gambling urges, thoughts, and behavior.14
Clinically, a patient will usually respond to a particular dose of naltrexone within 2 weeks. After that, an adjustment in dose is usually necessary. Patients often report nausea and diarrhea. Dizziness, sedation, and headaches occur less commonly. The side effects are usually mild and go away within the first week. Nausea, however, may be moderate to severe in some patients, so patients should be started on 25mg/d for the first 3 or 4 days to reduce that possibility. Ondansetron 4 mg/d is often given adjunctively for the same period to prevent the nausea.
Given the risk of associated hepatic transaminase elevations, liver function tests should be monitored in all patients taking naltrexone.14,16 A boxed warning refers to the potential hepatotoxicity of naltrexone at 50 mg/d, the dosage recommended for treating alcohol or opioid dependence. The warning also states that naltrexone use is contraindicated in acute hepatitis or liver failure, and its use in individuals with active liver disease must be carefully considered.
Initial liver function tests should be evaluated prior to naltrexone administration and again 3 to 4 weeks after starting the drug. Repeat testing should be performed at 2- to 4-week intervals for the next 2 months, a potential high-risk period. Thereafter, tests should be done approximately once a month for the following 3 months. After 6 months, liver enzyme elevations appear to occur rarely and testing 3 to 4 times a year should suffice unless an undue risk arises, e.g., excessive alcohol consumption. If elevated, the enzymes return to normal levels after discontinuing the naltrexone.
Research in this area is still in an early stage and clinicians prescribing the drug for pathological gambling should take extra caution in administering naltrexone at high doses and monitor for potential adverse consequences. Nonsteroidal analgesics should not be used in conjunction with high-dose naltrexone,14 as their concurrent use seems to cause a higher risk of hepatic transaminase elevation.
Mood stabilizers Successful responses to lithium and carbamazepine were described in 2 early case reports. Three subjects who were treated with lithium 1,800mg/d reported cessation of gambling.17 An early case report also found that carbamazepine resulted in improvement in pathological gambling disorder.18 Preliminary studies of lithium and valproate further support the notion that mood stabilizers may be useful. The benefit from carbamazepine, lithium, and valproate may be attributed to their efficacy in treating bipolar disorder and to the existence of features shared by pathological gambling and bipolar disorder (e.g., impulsivity).
Atypical antipsychotics Although there is little evidence that atypical antipsychotics work against pathological gambling when used alone, clinically atypical neuroleptic augmentation of SSRIs may be beneficial.
Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including OCD. A recent trial of olanzapine in the treatment of pathological video poker gamblers showed no difference in outcomes between the patients on medication and those on placebo.19
Cognitive behavioral therapy There is also mounting evidence that cognitive behavioral treatments are effective for pathological gambling.6,20-21 Combined pharmacological and behavioral therapy is considered the optimal treatment strategy for many psychiatric disorders, including substance dependence.
In our clinical experience, patients who only partially respond or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive behavioral therapies. Future studies should explore directly how pharmacological and behavioral therapy contribute to clinical improvement as part of combination treatment strategies for pathological gambling.
Ways to enhance compliance
Pathological gamblers, like those with bipolar disorder or substance dependence, often fail to comply with treatment.
Patients suffering from mania may not adhere to treatment with mood stabilizers in part because drug treatment may reduce positive or euphoric experiences. Similarly, the “high” associated with drug use often makes patients ambivalent about taking medications to remain abstinent.
In treating opioid dependence with naltrexone, a reward system has been incorporated in a contingency management fashion to substitute for drug-related reward and to enhance compliance with the medication.22 Recruitment of friends or family has also been used to enhance compliance with naltrexone in treating opioid dependence.22
In the case of pathological gamblers, a self-rewarding system for each day of nongambling can be used (e.g., a favorite meal or a movie).6 Family members can help by increasing social or other activities that can take the patient’s mind off gambling, particularly at high-risk times (weekends and payday).6
How long should you treat?
No guidelines exist for recommended adequate treatment trials for pathological gambling. Available data, however, suggest that an adequate medication trial may require a relatively long duration (4 months or longer).
Many pathological gamblers have never discussed their difficulties, and this unquantifiable therapeutic response may resemble a medication response. Clinicians must monitor symptoms long enough to assess the difference between response to placebo and to medication.
The optimal duration of treatment and rates of relapse associated with discontinuation are not known. Many patients relapse upon discontinuation of medication, and long-term treatment, perhaps 2 to 3 years, may be warranted.
Treatment-resistant pathological gambling
It is not unusual to encounter patients who have had no response or only a partial response to treatment. Several approaches appear promising (Table 2):
- If a patient has had an adequate trial of either an SRI or naltrexone as monotherapy, adding another agent appears to result in additional clinical improvement.
- A patient who has failed to respond to either an SRI or naltrexone may be effectively treated with a mood stabilizer, either as monotherapy or as augmentation. Some patients with pathological gambling disorder, although not screening positive for a manic episode, exhibit symptoms consistent with cyclothymia or other subclinical cycling mood disorder. For such patients lithium, valproate, or another drug with putative mood stabilizing properties may represent an appropriate option.
- The possibility of adding atypical antipsychotics to SRIs in the SRI-refractory pathological gambler warrants consideration.
- Patients who only partially respond or fail to respond to medication alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies, since this is considered the optimal treatment strategy for many psychiatric disorders.
Related resources
- Gamblers Anonymous International Service Office. Los Angeles, Calif. http://www.gamblersanonymous.org.
- National Research Council. Pathological Gambling: A Critical Review. Washington, DC: National Academy Press, 1990.
- James K. National Gambling Impact Study Commission: Final Report to Congress 1999. http://www.ngisc.gov/reports/finrpt.html
Drug brand names
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clomipramine • Anafranil
- Fluoxetine• Prozac, Prozac Weekly
- Fluvoxamine • Luvox
- Naltrexone • ReVia
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Valproic acid •Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
1. Shaffer HJ, Hall MN, Vander Bilt J. Estimating the prevalence of disordered gambling behavior in the United States and Canada: a research synthesis. Am J Public Health. 1999;89(9):1369-1376.
2. Grant JE, Kim SW. Demographic and clinical features of 131 adult pathological gamblers. J Clin Psychiatry. 2001;62(12):957-962.
3. Black DW, Moyer TM. Clinical features and psychiatric comorbidity of subjects with pathological gambling behavior. Psychiatric Serv. 1998;49(11):1434-1439.
4. Phillips DP, Welty WR, Smith MM. Elevated suicide levels associated with legalized gambling. Suicide Life Threat Behav. 1997;27(4):373-378.
5. American Psychiatric Association Committee on Nomenclature and Statistics. Diagnostic and Statistical Manual of Mental Disorders. 4th ed., text revision. Washington, DC: American Psychiatric Association, 2000.
6. Petry NM, Roll JM. A behavioral approach to understanding and treating pathological gambling. Semin Clin Neuropsychiatry. 2001;6(3):177-183.
7. Hollander E, Frenkel M, DeCaria C, et al. Treatment of pathological gambling with clomipramine. Am J Psychiatry. 1992;149(5):710-711.
8. Hollander E, DeCaria CM, Mari E, et al. Short-term single-blind fluvoxamine treatment of pathological gambling. Am J Psychiatry. 1998;155(12):1781-1783.
9. Hollander E, DeCaria CM, et al. A randomized double-blind fluvoxamine/placebo crossover trial in pathological gambling. Biol Psychiatry. 2000;47(9):813-817.
10. Blanco-Jerez C. A long-term, double-blind, placebo-controlled study of fluvoxamine for pathological gambling. Presented at the 152nd Annual Meeting of the American Psychiatric Association, Washington, DC, May 16-21, 1999 [abstract].
11. Zimmerman M, Breen R. An open-label study of citalopram in the treatment of pathological gambling. Presented at the 11th International Conference on Gambling and Risk Taking, Las Vegas, Nev, June 17-21, 2000 [abstract].
12. Kim SW, Grant JE, Shin YC, Toth JA, et al. A double-blind, placebo-controlled study of the efficacy and safety of paroxetine in the treatment of pathological gambling disorder. J Clin Psychiatry. 2002 (in press).
13. De la Gandara JJ. Fluoxetine: open-trial in pathological gambling. Presented at the 152 Annual Meeting of the American Psychiatric Association, Washington, DC, May 16-21, 1999 [abstract].
14. Kim SW, Grant JE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921.
15. Crockford DN, el-Guebaly N. Naltrexone in the treatment of pathological gambling and alcohol dependence. Can J Psychiatry. 1998;43(1):86.-
16. Kim SW, Grant JE. An open naltrexone treatment study of pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289.
17. Moskowitz JA. Lithium and lady luck: use of lithium carbonate in compulsive gambling. NY State J Med. 1980;80(5):785-788.
18. Haller R, Hinterhuber H. Treatment of pathological gambling with carbamazepine. Pharmacopsychiatry. 1994;27:129.-
19. Rugle L. The use of olanzapine in the treatment of video poker pathological gamblers. Presented at the conference titled, “The Comorbidity of Pathological Gambling: A Current Research Synthesis,” Dec 3 5, 2000, Las Vegas, Nev [abstract].
20. Ladoceur R, Sylvain C, Boutin C, et al. Cognitive treatment of pathological gambling. J Nerv Ment Dis. 2001;189(11):774-780.
21. Sylvain C, Ladouceur R, Boisvert JM. Cognitive and behavioral treatment of pathological gambling: a controlled study. J Consult Clin Psychol. 1997;65(5):727-732.
22. Carroll KM, Ball SA, Nich C, et al. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence. Arch Gen Psychiatry. 2001;58(8):755-761.
1. Shaffer HJ, Hall MN, Vander Bilt J. Estimating the prevalence of disordered gambling behavior in the United States and Canada: a research synthesis. Am J Public Health. 1999;89(9):1369-1376.
2. Grant JE, Kim SW. Demographic and clinical features of 131 adult pathological gamblers. J Clin Psychiatry. 2001;62(12):957-962.
3. Black DW, Moyer TM. Clinical features and psychiatric comorbidity of subjects with pathological gambling behavior. Psychiatric Serv. 1998;49(11):1434-1439.
4. Phillips DP, Welty WR, Smith MM. Elevated suicide levels associated with legalized gambling. Suicide Life Threat Behav. 1997;27(4):373-378.
5. American Psychiatric Association Committee on Nomenclature and Statistics. Diagnostic and Statistical Manual of Mental Disorders. 4th ed., text revision. Washington, DC: American Psychiatric Association, 2000.
6. Petry NM, Roll JM. A behavioral approach to understanding and treating pathological gambling. Semin Clin Neuropsychiatry. 2001;6(3):177-183.
7. Hollander E, Frenkel M, DeCaria C, et al. Treatment of pathological gambling with clomipramine. Am J Psychiatry. 1992;149(5):710-711.
8. Hollander E, DeCaria CM, Mari E, et al. Short-term single-blind fluvoxamine treatment of pathological gambling. Am J Psychiatry. 1998;155(12):1781-1783.
9. Hollander E, DeCaria CM, et al. A randomized double-blind fluvoxamine/placebo crossover trial in pathological gambling. Biol Psychiatry. 2000;47(9):813-817.
10. Blanco-Jerez C. A long-term, double-blind, placebo-controlled study of fluvoxamine for pathological gambling. Presented at the 152nd Annual Meeting of the American Psychiatric Association, Washington, DC, May 16-21, 1999 [abstract].
11. Zimmerman M, Breen R. An open-label study of citalopram in the treatment of pathological gambling. Presented at the 11th International Conference on Gambling and Risk Taking, Las Vegas, Nev, June 17-21, 2000 [abstract].
12. Kim SW, Grant JE, Shin YC, Toth JA, et al. A double-blind, placebo-controlled study of the efficacy and safety of paroxetine in the treatment of pathological gambling disorder. J Clin Psychiatry. 2002 (in press).
13. De la Gandara JJ. Fluoxetine: open-trial in pathological gambling. Presented at the 152 Annual Meeting of the American Psychiatric Association, Washington, DC, May 16-21, 1999 [abstract].
14. Kim SW, Grant JE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921.
15. Crockford DN, el-Guebaly N. Naltrexone in the treatment of pathological gambling and alcohol dependence. Can J Psychiatry. 1998;43(1):86.-
16. Kim SW, Grant JE. An open naltrexone treatment study of pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289.
17. Moskowitz JA. Lithium and lady luck: use of lithium carbonate in compulsive gambling. NY State J Med. 1980;80(5):785-788.
18. Haller R, Hinterhuber H. Treatment of pathological gambling with carbamazepine. Pharmacopsychiatry. 1994;27:129.-
19. Rugle L. The use of olanzapine in the treatment of video poker pathological gamblers. Presented at the conference titled, “The Comorbidity of Pathological Gambling: A Current Research Synthesis,” Dec 3 5, 2000, Las Vegas, Nev [abstract].
20. Ladoceur R, Sylvain C, Boutin C, et al. Cognitive treatment of pathological gambling. J Nerv Ment Dis. 2001;189(11):774-780.
21. Sylvain C, Ladouceur R, Boisvert JM. Cognitive and behavioral treatment of pathological gambling: a controlled study. J Consult Clin Psychol. 1997;65(5):727-732.
22. Carroll KM, Ball SA, Nich C, et al. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence. Arch Gen Psychiatry. 2001;58(8):755-761.