User login
Thoracic Oncology & Chest Procedures Network
Ultrasound and Chest Imaging Section
Advanced critical care echocardiography: A noninvasive tool for hemodynamic assessment in critically ill patients
Hemodynamic assessments in critically ill patients are important to guide accurate management; however, traditional invasive methods of measuring cardiac output have significant limitations, including risks of infection and bleeding. ACCE can provide a multitude of hemodynamic measurements from cardiac output (CO) to right ventricular systolic pressure (RVSP) and left atrial pressure (LAP). Combinations of left ventricular function parameters, along with estimation of filling pressures, can help distinguish between types of shock. Schmidt and colleagues (Sci Rep. 2022;12[1]:7187) demonstrated that measurement of these indices in the majority of patients helped elucidate the cause for hemodynamic compromise. They found presence of a cardiac index (CI) < 2.5/min.m2 was associated with a doubling of ICU mortality as compared with predictions based on severity of illness scores in otherwise hemodynamically stable patients. Hollenberg and colleagues (Am J Cardiol. 2021;153:135-39) demonstrated the feasibility of a simpler stratification using the left ventricular ejection fraction (LVEF) and CI in coronavirus disease 2019 patients with shock, where low CI despite having a preserved LVEF was associated with worse outcomes.
Quick, reliable data are an intensivist’s friend. Utilizing ACCE at the bedside adds another tool in our arsenal to provide real-time hemodynamic data that can be used to manage patients in the ICU. ACCE also allows repeated measurements to determine changes based on therapeutic interventions initiated.
In recognition of the importance of ACCE as a tool for intensivists, the National Board of Echocardiography (NBE) now offers a pathway toward board certification with the Examination of Special Competence in Critical Care Echocardiography (CCEeXAM). CHEST continues to offer cutting-edge courses in ACCE, as well as a board review course for learners interested in sitting for the CCEeXAM.
Amik Sodhi, MD, FCCP
Gul Zaidi, MD, FCCP
Members-at-Large
Ultrasound and Chest Imaging Section
Advanced critical care echocardiography: A noninvasive tool for hemodynamic assessment in critically ill patients
Hemodynamic assessments in critically ill patients are important to guide accurate management; however, traditional invasive methods of measuring cardiac output have significant limitations, including risks of infection and bleeding. ACCE can provide a multitude of hemodynamic measurements from cardiac output (CO) to right ventricular systolic pressure (RVSP) and left atrial pressure (LAP). Combinations of left ventricular function parameters, along with estimation of filling pressures, can help distinguish between types of shock. Schmidt and colleagues (Sci Rep. 2022;12[1]:7187) demonstrated that measurement of these indices in the majority of patients helped elucidate the cause for hemodynamic compromise. They found presence of a cardiac index (CI) < 2.5/min.m2 was associated with a doubling of ICU mortality as compared with predictions based on severity of illness scores in otherwise hemodynamically stable patients. Hollenberg and colleagues (Am J Cardiol. 2021;153:135-39) demonstrated the feasibility of a simpler stratification using the left ventricular ejection fraction (LVEF) and CI in coronavirus disease 2019 patients with shock, where low CI despite having a preserved LVEF was associated with worse outcomes.
Quick, reliable data are an intensivist’s friend. Utilizing ACCE at the bedside adds another tool in our arsenal to provide real-time hemodynamic data that can be used to manage patients in the ICU. ACCE also allows repeated measurements to determine changes based on therapeutic interventions initiated.
In recognition of the importance of ACCE as a tool for intensivists, the National Board of Echocardiography (NBE) now offers a pathway toward board certification with the Examination of Special Competence in Critical Care Echocardiography (CCEeXAM). CHEST continues to offer cutting-edge courses in ACCE, as well as a board review course for learners interested in sitting for the CCEeXAM.
Amik Sodhi, MD, FCCP
Gul Zaidi, MD, FCCP
Members-at-Large
Ultrasound and Chest Imaging Section
Advanced critical care echocardiography: A noninvasive tool for hemodynamic assessment in critically ill patients
Hemodynamic assessments in critically ill patients are important to guide accurate management; however, traditional invasive methods of measuring cardiac output have significant limitations, including risks of infection and bleeding. ACCE can provide a multitude of hemodynamic measurements from cardiac output (CO) to right ventricular systolic pressure (RVSP) and left atrial pressure (LAP). Combinations of left ventricular function parameters, along with estimation of filling pressures, can help distinguish between types of shock. Schmidt and colleagues (Sci Rep. 2022;12[1]:7187) demonstrated that measurement of these indices in the majority of patients helped elucidate the cause for hemodynamic compromise. They found presence of a cardiac index (CI) < 2.5/min.m2 was associated with a doubling of ICU mortality as compared with predictions based on severity of illness scores in otherwise hemodynamically stable patients. Hollenberg and colleagues (Am J Cardiol. 2021;153:135-39) demonstrated the feasibility of a simpler stratification using the left ventricular ejection fraction (LVEF) and CI in coronavirus disease 2019 patients with shock, where low CI despite having a preserved LVEF was associated with worse outcomes.
Quick, reliable data are an intensivist’s friend. Utilizing ACCE at the bedside adds another tool in our arsenal to provide real-time hemodynamic data that can be used to manage patients in the ICU. ACCE also allows repeated measurements to determine changes based on therapeutic interventions initiated.
In recognition of the importance of ACCE as a tool for intensivists, the National Board of Echocardiography (NBE) now offers a pathway toward board certification with the Examination of Special Competence in Critical Care Echocardiography (CCEeXAM). CHEST continues to offer cutting-edge courses in ACCE, as well as a board review course for learners interested in sitting for the CCEeXAM.
Amik Sodhi, MD, FCCP
Gul Zaidi, MD, FCCP
Members-at-Large
Thoracic Oncology & Chest Procedures Network
Interventional Procedures Section
Mind the gap: Improving adherence to lung cancer screening follow-up
The gap in adherence rates between a disciplined clinical trial and the heterogenous patchwork of U.S. health care is hardly unusual, but as lung cancer remains the number one cancer killer both worldwide and in the United States, one such disparity bears closer scrutiny.
In 2011, the National Lung Screening Trial (NLST) demonstrated a 20% reduction in lung cancer mortality with the implementation of low dose CT scan screening with 95% adherence to CT scan follow-up within 15 months of initial screening imaging (Aberle, et al. N Engl J Med. 2011;365[5]:395-409). Unfortunately, estimates of real-world adherence to lung cancer screening (LCS) follow-up fall to 51% even within an extended 18-month window (Hirsch, et al. Ann Am Thorac Soc. 2019;16[10]:1329-32).
Recent studies compared adherence to LCS follow-up between centralized and decentralized screening programs. Centralized programs used dedicated program coordinators and a tracking system, while decentralized programs relied on primary care providers.(Sakoda, et al. JAMA Network Open. 2021;4[4]:e218559). A subsequent study demonstrated adherence of 70% vs 41% among patients in centralized vs decentralized programs, respectively (Smith, et al. Chest. 2022;161[3]:818-25).
This gap is even more pronounced in majority-Black populations. Kunitomo and colleagues showed 33% lower odds of adherence to LCS follow-up compared with White patients (Kunitomo, et al. Chest. 2022;161[1]:266-75). Another study in a diverse, majority-Black patient population showed only 31% adherence to LCS follow-up at 1 year (Erkmen, et al. Cancer Causes Control. 2021;32[3]:291-8).
How could we close this gap? Centralized LCS programs show promise of increasing adherence to LCS follow-up. Heightened awareness of and targeted investment to mitigate racial inequities in LCS is imperative.
Jose De Cardenas MD
John Howe, MD
Members-at-Large
Interventional Procedures Section
Mind the gap: Improving adherence to lung cancer screening follow-up
The gap in adherence rates between a disciplined clinical trial and the heterogenous patchwork of U.S. health care is hardly unusual, but as lung cancer remains the number one cancer killer both worldwide and in the United States, one such disparity bears closer scrutiny.
In 2011, the National Lung Screening Trial (NLST) demonstrated a 20% reduction in lung cancer mortality with the implementation of low dose CT scan screening with 95% adherence to CT scan follow-up within 15 months of initial screening imaging (Aberle, et al. N Engl J Med. 2011;365[5]:395-409). Unfortunately, estimates of real-world adherence to lung cancer screening (LCS) follow-up fall to 51% even within an extended 18-month window (Hirsch, et al. Ann Am Thorac Soc. 2019;16[10]:1329-32).
Recent studies compared adherence to LCS follow-up between centralized and decentralized screening programs. Centralized programs used dedicated program coordinators and a tracking system, while decentralized programs relied on primary care providers.(Sakoda, et al. JAMA Network Open. 2021;4[4]:e218559). A subsequent study demonstrated adherence of 70% vs 41% among patients in centralized vs decentralized programs, respectively (Smith, et al. Chest. 2022;161[3]:818-25).
This gap is even more pronounced in majority-Black populations. Kunitomo and colleagues showed 33% lower odds of adherence to LCS follow-up compared with White patients (Kunitomo, et al. Chest. 2022;161[1]:266-75). Another study in a diverse, majority-Black patient population showed only 31% adherence to LCS follow-up at 1 year (Erkmen, et al. Cancer Causes Control. 2021;32[3]:291-8).
How could we close this gap? Centralized LCS programs show promise of increasing adherence to LCS follow-up. Heightened awareness of and targeted investment to mitigate racial inequities in LCS is imperative.
Jose De Cardenas MD
John Howe, MD
Members-at-Large
Interventional Procedures Section
Mind the gap: Improving adherence to lung cancer screening follow-up
The gap in adherence rates between a disciplined clinical trial and the heterogenous patchwork of U.S. health care is hardly unusual, but as lung cancer remains the number one cancer killer both worldwide and in the United States, one such disparity bears closer scrutiny.
In 2011, the National Lung Screening Trial (NLST) demonstrated a 20% reduction in lung cancer mortality with the implementation of low dose CT scan screening with 95% adherence to CT scan follow-up within 15 months of initial screening imaging (Aberle, et al. N Engl J Med. 2011;365[5]:395-409). Unfortunately, estimates of real-world adherence to lung cancer screening (LCS) follow-up fall to 51% even within an extended 18-month window (Hirsch, et al. Ann Am Thorac Soc. 2019;16[10]:1329-32).
Recent studies compared adherence to LCS follow-up between centralized and decentralized screening programs. Centralized programs used dedicated program coordinators and a tracking system, while decentralized programs relied on primary care providers.(Sakoda, et al. JAMA Network Open. 2021;4[4]:e218559). A subsequent study demonstrated adherence of 70% vs 41% among patients in centralized vs decentralized programs, respectively (Smith, et al. Chest. 2022;161[3]:818-25).
This gap is even more pronounced in majority-Black populations. Kunitomo and colleagues showed 33% lower odds of adherence to LCS follow-up compared with White patients (Kunitomo, et al. Chest. 2022;161[1]:266-75). Another study in a diverse, majority-Black patient population showed only 31% adherence to LCS follow-up at 1 year (Erkmen, et al. Cancer Causes Control. 2021;32[3]:291-8).
How could we close this gap? Centralized LCS programs show promise of increasing adherence to LCS follow-up. Heightened awareness of and targeted investment to mitigate racial inequities in LCS is imperative.
Jose De Cardenas MD
John Howe, MD
Members-at-Large
Sleep Medicine Network
Home-based Mechanical Ventilation and Neuromuscular Disease Section
Navigating the latest device supply chain challenge: Mechanical airway clearance
(distal airways). Cough augmentation techniques provide lung volume recruitment on the insufflation phase, in addition to mobilization of secretions with augmentation of the peak expiratory flow rate to >160 L/min on the exhalation phase.
A mechanical insufflation-exsufflation (MI-E) device (T70 Cough Assist - Phillips) is now on indefinite backorder. This creates a dangerous situation for our patients requiring cough augmentation for survival. Alternative options that provide both MI-E and high frequency oscillation include two systems (Synclara Cough System – Hill-rom and the Biwaze Cough System-ABM Respiratory Care).
The Synclara can only be obtained in a direct-to-patient model, contracting with individual respiratory therapists, outside of the standard durable medical equipment model. The final MI-E model option is the VOCSYN multifunctional ventilator (ventilator, cough assist, nebulizer, oxygen concentrator, suction). This multifunction ventilator has had variable acceptance with HCPCS code E0467. If the VOCSYN is chosen, the patient cannot have been issued any component devices or have reached the 36-month cap for oxygen equipment (CR 10854 special payment rule, 42 CFR414.222).
As the supply of devices is exhausted, we will need to shift to evidence-based manual options. Manual cough augmentation can be done effectively with a bag-valve mask, using breath stacking to achieve maximal lung insufflation, optimizing the length tension relationship of elastic recoil on exhalation to increase peak cough flow (PCF).
This can be done alone but is more effective when combined with manually assisted cough (Bach JR. Chest. 1993;104[5]:1553-62). These interventions require training of the caregivers, using resources such as those found at www.canventottawa.ca.
With continued supply chain instability, manual airway clearance techniques should be considered in patients with less advanced cough impairment (PCF 160-270 L/min), to save the remaining devices for those with PCF of <160 L/min.
Jeanette Brown, MD, PhD
Karin Provost, DO, PhD
Members-at-Large
Home-based Mechanical Ventilation and Neuromuscular Disease Section
Navigating the latest device supply chain challenge: Mechanical airway clearance
(distal airways). Cough augmentation techniques provide lung volume recruitment on the insufflation phase, in addition to mobilization of secretions with augmentation of the peak expiratory flow rate to >160 L/min on the exhalation phase.
A mechanical insufflation-exsufflation (MI-E) device (T70 Cough Assist - Phillips) is now on indefinite backorder. This creates a dangerous situation for our patients requiring cough augmentation for survival. Alternative options that provide both MI-E and high frequency oscillation include two systems (Synclara Cough System – Hill-rom and the Biwaze Cough System-ABM Respiratory Care).
The Synclara can only be obtained in a direct-to-patient model, contracting with individual respiratory therapists, outside of the standard durable medical equipment model. The final MI-E model option is the VOCSYN multifunctional ventilator (ventilator, cough assist, nebulizer, oxygen concentrator, suction). This multifunction ventilator has had variable acceptance with HCPCS code E0467. If the VOCSYN is chosen, the patient cannot have been issued any component devices or have reached the 36-month cap for oxygen equipment (CR 10854 special payment rule, 42 CFR414.222).
As the supply of devices is exhausted, we will need to shift to evidence-based manual options. Manual cough augmentation can be done effectively with a bag-valve mask, using breath stacking to achieve maximal lung insufflation, optimizing the length tension relationship of elastic recoil on exhalation to increase peak cough flow (PCF).
This can be done alone but is more effective when combined with manually assisted cough (Bach JR. Chest. 1993;104[5]:1553-62). These interventions require training of the caregivers, using resources such as those found at www.canventottawa.ca.
With continued supply chain instability, manual airway clearance techniques should be considered in patients with less advanced cough impairment (PCF 160-270 L/min), to save the remaining devices for those with PCF of <160 L/min.
Jeanette Brown, MD, PhD
Karin Provost, DO, PhD
Members-at-Large
Home-based Mechanical Ventilation and Neuromuscular Disease Section
Navigating the latest device supply chain challenge: Mechanical airway clearance
(distal airways). Cough augmentation techniques provide lung volume recruitment on the insufflation phase, in addition to mobilization of secretions with augmentation of the peak expiratory flow rate to >160 L/min on the exhalation phase.
A mechanical insufflation-exsufflation (MI-E) device (T70 Cough Assist - Phillips) is now on indefinite backorder. This creates a dangerous situation for our patients requiring cough augmentation for survival. Alternative options that provide both MI-E and high frequency oscillation include two systems (Synclara Cough System – Hill-rom and the Biwaze Cough System-ABM Respiratory Care).
The Synclara can only be obtained in a direct-to-patient model, contracting with individual respiratory therapists, outside of the standard durable medical equipment model. The final MI-E model option is the VOCSYN multifunctional ventilator (ventilator, cough assist, nebulizer, oxygen concentrator, suction). This multifunction ventilator has had variable acceptance with HCPCS code E0467. If the VOCSYN is chosen, the patient cannot have been issued any component devices or have reached the 36-month cap for oxygen equipment (CR 10854 special payment rule, 42 CFR414.222).
As the supply of devices is exhausted, we will need to shift to evidence-based manual options. Manual cough augmentation can be done effectively with a bag-valve mask, using breath stacking to achieve maximal lung insufflation, optimizing the length tension relationship of elastic recoil on exhalation to increase peak cough flow (PCF).
This can be done alone but is more effective when combined with manually assisted cough (Bach JR. Chest. 1993;104[5]:1553-62). These interventions require training of the caregivers, using resources such as those found at www.canventottawa.ca.
With continued supply chain instability, manual airway clearance techniques should be considered in patients with less advanced cough impairment (PCF 160-270 L/min), to save the remaining devices for those with PCF of <160 L/min.
Jeanette Brown, MD, PhD
Karin Provost, DO, PhD
Members-at-Large
Airways Disorders Network
Asthma and COPD Section
Go TEAM! Shared decision-making tool for patient-clinician collaboration in severe asthma
Shared decision-making is associated with improved medication adherence in adults (Wilson, et al. Am J Respir Crit Care Med. 2010;181[6]:566-77) and quality of life and asthma control in children (Taylor, et al. J Asthma. 2018;55[6]:675-83). The Global Initiative for Asthma committee recommends a patient-clinician partnership. Activated and engaged patients play a major role in their asthma management (https://ginasthma.org/gina-reports). Shared decision-making discussions should include potential benefits and harms of the therapeutic options, patient’s values and lifestyle preferences, and addressing concerns.
The CHEST Foundation, the Allergy and Asthma Network, and the American College of Allergy, Asthma, and Immunology developed an online shared decision- making tool for severe asthma (https://asthma.chestnet.org/sdm-tool).
This tool utilizes patient’s values, specifics about triggers, asthma control, medication side effects, and lifestyle preferences to identify personalized management options. The tool provides information about recommended therapeutic options in simple terms, including potential benefits, possible side effects, expected treatment frequency and duration, and financial aid information. The treatment options currently explained in this tool include anti-immunoglobulin E, anti-interleukin-5, anti-interleukin-4/13, bronchial thermoplasty, long-acting muscarinic antagonist, macrolides, oral corticosteroids, and standard of care.
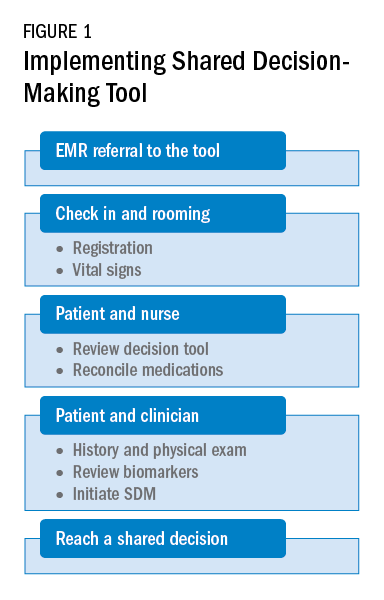
As a team, the patient and the health care professional can use this tool during office visits to help guide management. Figure 1 shows a suggested workflow to utilize the tool in clinical practice.
Potential barriers include excess time and increased human resources. Barrier mitigation may include reviewing the tool and reconciling the medications before the clinician enters the room. With these interventions, many clinician encounters may be completed in 10 to 15 minutes.
Farrukh Abbas, MBBS
Fellow-in-Training
Asthma and COPD Section
Go TEAM! Shared decision-making tool for patient-clinician collaboration in severe asthma
Shared decision-making is associated with improved medication adherence in adults (Wilson, et al. Am J Respir Crit Care Med. 2010;181[6]:566-77) and quality of life and asthma control in children (Taylor, et al. J Asthma. 2018;55[6]:675-83). The Global Initiative for Asthma committee recommends a patient-clinician partnership. Activated and engaged patients play a major role in their asthma management (https://ginasthma.org/gina-reports). Shared decision-making discussions should include potential benefits and harms of the therapeutic options, patient’s values and lifestyle preferences, and addressing concerns.
The CHEST Foundation, the Allergy and Asthma Network, and the American College of Allergy, Asthma, and Immunology developed an online shared decision- making tool for severe asthma (https://asthma.chestnet.org/sdm-tool).
This tool utilizes patient’s values, specifics about triggers, asthma control, medication side effects, and lifestyle preferences to identify personalized management options. The tool provides information about recommended therapeutic options in simple terms, including potential benefits, possible side effects, expected treatment frequency and duration, and financial aid information. The treatment options currently explained in this tool include anti-immunoglobulin E, anti-interleukin-5, anti-interleukin-4/13, bronchial thermoplasty, long-acting muscarinic antagonist, macrolides, oral corticosteroids, and standard of care.
As a team, the patient and the health care professional can use this tool during office visits to help guide management. Figure 1 shows a suggested workflow to utilize the tool in clinical practice.
Potential barriers include excess time and increased human resources. Barrier mitigation may include reviewing the tool and reconciling the medications before the clinician enters the room. With these interventions, many clinician encounters may be completed in 10 to 15 minutes.

Farrukh Abbas, MBBS
Fellow-in-Training
Asthma and COPD Section
Go TEAM! Shared decision-making tool for patient-clinician collaboration in severe asthma
Shared decision-making is associated with improved medication adherence in adults (Wilson, et al. Am J Respir Crit Care Med. 2010;181[6]:566-77) and quality of life and asthma control in children (Taylor, et al. J Asthma. 2018;55[6]:675-83). The Global Initiative for Asthma committee recommends a patient-clinician partnership. Activated and engaged patients play a major role in their asthma management (https://ginasthma.org/gina-reports). Shared decision-making discussions should include potential benefits and harms of the therapeutic options, patient’s values and lifestyle preferences, and addressing concerns.
The CHEST Foundation, the Allergy and Asthma Network, and the American College of Allergy, Asthma, and Immunology developed an online shared decision- making tool for severe asthma (https://asthma.chestnet.org/sdm-tool).
This tool utilizes patient’s values, specifics about triggers, asthma control, medication side effects, and lifestyle preferences to identify personalized management options. The tool provides information about recommended therapeutic options in simple terms, including potential benefits, possible side effects, expected treatment frequency and duration, and financial aid information. The treatment options currently explained in this tool include anti-immunoglobulin E, anti-interleukin-5, anti-interleukin-4/13, bronchial thermoplasty, long-acting muscarinic antagonist, macrolides, oral corticosteroids, and standard of care.
As a team, the patient and the health care professional can use this tool during office visits to help guide management. Figure 1 shows a suggested workflow to utilize the tool in clinical practice.
Potential barriers include excess time and increased human resources. Barrier mitigation may include reviewing the tool and reconciling the medications before the clinician enters the room. With these interventions, many clinician encounters may be completed in 10 to 15 minutes.

Farrukh Abbas, MBBS
Fellow-in-Training
Diffuse Lung Disease & Transplant Network
Pulmonary Physiology & Rehabilitation Section
Interpretive strategies for routine lung function tests
In December 2021, the European Respiratory Journal published the, ERS/ATS technical standard on interpretive strategies for routine lung function tests (Stanojevic S, et al. Eur Respir J. 2021 Dec 23;2101499). Briefly, a few of the updated recommendations are discussed here.
First, the task force recommends the use of Global Lung Initiative (GLI) reference values for spirometry, lung volumes, and diffusing capacity of carbon monoxide. GLI equations were derived from the largest sample of healthy individuals to date and provide an internal consistency across all ages.
Second, it is now recommended that z-scores are used as opposed to percent predicted in grading severity of impairment. Z-scores, which refer to the number of standard deviations a measurement is positioned from the predicted value, centered at zero, account better for age, sex, and height biases compared with percent predicted, and is simplified into mild (z-score -1.65 to -2.5), moderate (-2.51 to -4), and severe (< -4) categories.
A bronchodilator response is now defined as a > 10% change from the predicted value in FEV1 or FVC while the concept of a conditional change score in children and FEV1Q in adults has been introduced to describe lung function change.
The recommendations reflect and reiterate a shift in reporting a range of values, rather than using absolute threshold values, with an emphasis on the classification of physiologic impairments. The uncertainty present as lung function approaches the lower limit of normal is acknowledged, emphasizing the importance of pretest probability in making a clinical diagnosis and/or clinical decision. We encourage all pulmonary clinicians to review this important paper for more detailed information on these changes.
Tom DeCato, MD, Vice-Chair
Gina Lee, MD, Member-at-Large
Pulmonary Physiology & Rehabilitation Section
Interpretive strategies for routine lung function tests
In December 2021, the European Respiratory Journal published the, ERS/ATS technical standard on interpretive strategies for routine lung function tests (Stanojevic S, et al. Eur Respir J. 2021 Dec 23;2101499). Briefly, a few of the updated recommendations are discussed here.
First, the task force recommends the use of Global Lung Initiative (GLI) reference values for spirometry, lung volumes, and diffusing capacity of carbon monoxide. GLI equations were derived from the largest sample of healthy individuals to date and provide an internal consistency across all ages.
Second, it is now recommended that z-scores are used as opposed to percent predicted in grading severity of impairment. Z-scores, which refer to the number of standard deviations a measurement is positioned from the predicted value, centered at zero, account better for age, sex, and height biases compared with percent predicted, and is simplified into mild (z-score -1.65 to -2.5), moderate (-2.51 to -4), and severe (< -4) categories.
A bronchodilator response is now defined as a > 10% change from the predicted value in FEV1 or FVC while the concept of a conditional change score in children and FEV1Q in adults has been introduced to describe lung function change.
The recommendations reflect and reiterate a shift in reporting a range of values, rather than using absolute threshold values, with an emphasis on the classification of physiologic impairments. The uncertainty present as lung function approaches the lower limit of normal is acknowledged, emphasizing the importance of pretest probability in making a clinical diagnosis and/or clinical decision. We encourage all pulmonary clinicians to review this important paper for more detailed information on these changes.
Tom DeCato, MD, Vice-Chair
Gina Lee, MD, Member-at-Large
Pulmonary Physiology & Rehabilitation Section
Interpretive strategies for routine lung function tests
In December 2021, the European Respiratory Journal published the, ERS/ATS technical standard on interpretive strategies for routine lung function tests (Stanojevic S, et al. Eur Respir J. 2021 Dec 23;2101499). Briefly, a few of the updated recommendations are discussed here.
First, the task force recommends the use of Global Lung Initiative (GLI) reference values for spirometry, lung volumes, and diffusing capacity of carbon monoxide. GLI equations were derived from the largest sample of healthy individuals to date and provide an internal consistency across all ages.
Second, it is now recommended that z-scores are used as opposed to percent predicted in grading severity of impairment. Z-scores, which refer to the number of standard deviations a measurement is positioned from the predicted value, centered at zero, account better for age, sex, and height biases compared with percent predicted, and is simplified into mild (z-score -1.65 to -2.5), moderate (-2.51 to -4), and severe (< -4) categories.
A bronchodilator response is now defined as a > 10% change from the predicted value in FEV1 or FVC while the concept of a conditional change score in children and FEV1Q in adults has been introduced to describe lung function change.
The recommendations reflect and reiterate a shift in reporting a range of values, rather than using absolute threshold values, with an emphasis on the classification of physiologic impairments. The uncertainty present as lung function approaches the lower limit of normal is acknowledged, emphasizing the importance of pretest probability in making a clinical diagnosis and/or clinical decision. We encourage all pulmonary clinicians to review this important paper for more detailed information on these changes.
Tom DeCato, MD, Vice-Chair
Gina Lee, MD, Member-at-Large
Airways Disorders Network
Pediatric Chest Medicine Section
Hope is on the horizon—new RSV protection for all infants
as available preventive therapies are limited and currently reserved for former preterm infants and those with certain underlying medical conditions (Brady MT, et al. Pediatrics. 2014;134[2]:415). Globally, RSV is a significant cause of lower respiratory tract infection impacting all age groups, yet, in infants and young children, the first infection may cause severe bronchiolitis that can be fatal (Li Y, et al. Lancet. 2022;399:2047).
There are currently three approaches for protection at various stages of clinical development. The first is direct administration of antibodies to the infant. Two potent, longer-lasting, single-dose monoclonal antibody products, including nirsevimab which is a monoclonal antibody to the RSV fusion protein that has an extended half-life, for the general infant population are in phase 3 trials (Hammitt LL, et al. N Engl J Med. 2022;386:837; Griffin PM, et al. N Engl J Med. 2020;383:415).
Passive antibody acquired from maternal vaccination in pregnancy is a second approach. Notably, a recent phase 3 trial that evaluated maternal vaccination did not show significance with respect to the primary end point of medically significant RSV-associated lower respiratory tract infection in infants up to 90 days of life (Madhi SA. N Engl J Med. 2020;383:426).
The third type of protection is active vaccination. Increased understanding of the biology of RSV and related technological advances have resulted in the entry of multiple vaccines into clinical development for pediatrics and adults, some of which may receive regulatory approval in the near future (Munoz FM, et al. Vaccine. 2021;39[22]:3053).
The burden of RSV is tremendous, yet the future of RSV protection looks promising.
Anne C. Coates, MD, FCCP, Member-at-Large
Mary Cataletto, MD, FCCP, Member-at-Large
Pediatric Chest Medicine Section
Hope is on the horizon—new RSV protection for all infants
as available preventive therapies are limited and currently reserved for former preterm infants and those with certain underlying medical conditions (Brady MT, et al. Pediatrics. 2014;134[2]:415). Globally, RSV is a significant cause of lower respiratory tract infection impacting all age groups, yet, in infants and young children, the first infection may cause severe bronchiolitis that can be fatal (Li Y, et al. Lancet. 2022;399:2047).
There are currently three approaches for protection at various stages of clinical development. The first is direct administration of antibodies to the infant. Two potent, longer-lasting, single-dose monoclonal antibody products, including nirsevimab which is a monoclonal antibody to the RSV fusion protein that has an extended half-life, for the general infant population are in phase 3 trials (Hammitt LL, et al. N Engl J Med. 2022;386:837; Griffin PM, et al. N Engl J Med. 2020;383:415).
Passive antibody acquired from maternal vaccination in pregnancy is a second approach. Notably, a recent phase 3 trial that evaluated maternal vaccination did not show significance with respect to the primary end point of medically significant RSV-associated lower respiratory tract infection in infants up to 90 days of life (Madhi SA. N Engl J Med. 2020;383:426).
The third type of protection is active vaccination. Increased understanding of the biology of RSV and related technological advances have resulted in the entry of multiple vaccines into clinical development for pediatrics and adults, some of which may receive regulatory approval in the near future (Munoz FM, et al. Vaccine. 2021;39[22]:3053).
The burden of RSV is tremendous, yet the future of RSV protection looks promising.
Anne C. Coates, MD, FCCP, Member-at-Large
Mary Cataletto, MD, FCCP, Member-at-Large
Pediatric Chest Medicine Section
Hope is on the horizon—new RSV protection for all infants
as available preventive therapies are limited and currently reserved for former preterm infants and those with certain underlying medical conditions (Brady MT, et al. Pediatrics. 2014;134[2]:415). Globally, RSV is a significant cause of lower respiratory tract infection impacting all age groups, yet, in infants and young children, the first infection may cause severe bronchiolitis that can be fatal (Li Y, et al. Lancet. 2022;399:2047).
There are currently three approaches for protection at various stages of clinical development. The first is direct administration of antibodies to the infant. Two potent, longer-lasting, single-dose monoclonal antibody products, including nirsevimab which is a monoclonal antibody to the RSV fusion protein that has an extended half-life, for the general infant population are in phase 3 trials (Hammitt LL, et al. N Engl J Med. 2022;386:837; Griffin PM, et al. N Engl J Med. 2020;383:415).
Passive antibody acquired from maternal vaccination in pregnancy is a second approach. Notably, a recent phase 3 trial that evaluated maternal vaccination did not show significance with respect to the primary end point of medically significant RSV-associated lower respiratory tract infection in infants up to 90 days of life (Madhi SA. N Engl J Med. 2020;383:426).
The third type of protection is active vaccination. Increased understanding of the biology of RSV and related technological advances have resulted in the entry of multiple vaccines into clinical development for pediatrics and adults, some of which may receive regulatory approval in the near future (Munoz FM, et al. Vaccine. 2021;39[22]:3053).
The burden of RSV is tremendous, yet the future of RSV protection looks promising.
Anne C. Coates, MD, FCCP, Member-at-Large
Mary Cataletto, MD, FCCP, Member-at-Large
Critical Care Network
Sepsis and Shock Section
SEP-1 measure saves lives, let’s not debate!
On December 21, 2021, the National Quality Form (NQF) re-endorsed Measure 0500 Severe Sepsis and Septic Shock: Management Bundle, which CMS adopts as the SEP-1 core measure. The decision was initially met by a request for appeal. On April 29, 2022, the appeals board met to adjudicate the appeal and voted unanimously to uphold the Standards Approval Committee (CSAC) decision to endorse the measure once again (https://tinyurl.com/yc4tjxbz).
The appeals board voted 5-0 on whether procedural errors reasonably affected the outcome of the original endorsement and whether there was new information or evidence unavailable at the time of the CSAC endorsement decision that would reasonably affect the outcome of the original endorsement decision.
even though the results of this bundled approach support an opportunity to save lives. SEP-1 compliance is associated with a lower 30-day mortality, and rendering this care saves lives.
In the Townsend, et al cohort study (Chest. 2022 Feb;161[2]:392) examining patient level Medicare data from October 2015 – March 2017, there was an absolute risk reduction of 5.67% in a standard propensity matched comparison of SEP-1 compliant vs noncompliant care. With a more stringent match, the absolute risk reduction was 4.06%. That’s an outcome that our patients likely appreciate the most…lives saved.
As former CHEST President, Dr. Steven Simpson highlighted in his April 2022 commentary (CHEST Physician. 2022 April;17[4]:15), “Success is not dependent only on what we do but on when we do it.” Let’s not debate any further.
Namita Jayaprakash, MBBCh
Member-at-Large
Sepsis and Shock Section
SEP-1 measure saves lives, let’s not debate!
On December 21, 2021, the National Quality Form (NQF) re-endorsed Measure 0500 Severe Sepsis and Septic Shock: Management Bundle, which CMS adopts as the SEP-1 core measure. The decision was initially met by a request for appeal. On April 29, 2022, the appeals board met to adjudicate the appeal and voted unanimously to uphold the Standards Approval Committee (CSAC) decision to endorse the measure once again (https://tinyurl.com/yc4tjxbz).
The appeals board voted 5-0 on whether procedural errors reasonably affected the outcome of the original endorsement and whether there was new information or evidence unavailable at the time of the CSAC endorsement decision that would reasonably affect the outcome of the original endorsement decision.
even though the results of this bundled approach support an opportunity to save lives. SEP-1 compliance is associated with a lower 30-day mortality, and rendering this care saves lives.
In the Townsend, et al cohort study (Chest. 2022 Feb;161[2]:392) examining patient level Medicare data from October 2015 – March 2017, there was an absolute risk reduction of 5.67% in a standard propensity matched comparison of SEP-1 compliant vs noncompliant care. With a more stringent match, the absolute risk reduction was 4.06%. That’s an outcome that our patients likely appreciate the most…lives saved.
As former CHEST President, Dr. Steven Simpson highlighted in his April 2022 commentary (CHEST Physician. 2022 April;17[4]:15), “Success is not dependent only on what we do but on when we do it.” Let’s not debate any further.
Namita Jayaprakash, MBBCh
Member-at-Large
Sepsis and Shock Section
SEP-1 measure saves lives, let’s not debate!
On December 21, 2021, the National Quality Form (NQF) re-endorsed Measure 0500 Severe Sepsis and Septic Shock: Management Bundle, which CMS adopts as the SEP-1 core measure. The decision was initially met by a request for appeal. On April 29, 2022, the appeals board met to adjudicate the appeal and voted unanimously to uphold the Standards Approval Committee (CSAC) decision to endorse the measure once again (https://tinyurl.com/yc4tjxbz).
The appeals board voted 5-0 on whether procedural errors reasonably affected the outcome of the original endorsement and whether there was new information or evidence unavailable at the time of the CSAC endorsement decision that would reasonably affect the outcome of the original endorsement decision.
even though the results of this bundled approach support an opportunity to save lives. SEP-1 compliance is associated with a lower 30-day mortality, and rendering this care saves lives.
In the Townsend, et al cohort study (Chest. 2022 Feb;161[2]:392) examining patient level Medicare data from October 2015 – March 2017, there was an absolute risk reduction of 5.67% in a standard propensity matched comparison of SEP-1 compliant vs noncompliant care. With a more stringent match, the absolute risk reduction was 4.06%. That’s an outcome that our patients likely appreciate the most…lives saved.
As former CHEST President, Dr. Steven Simpson highlighted in his April 2022 commentary (CHEST Physician. 2022 April;17[4]:15), “Success is not dependent only on what we do but on when we do it.” Let’s not debate any further.
Namita Jayaprakash, MBBCh
Member-at-Large
Sleep Medicine Network
Respiratory-Related Sleep Disorders Section
Reducing racial disparities in sleep apnea
For example, a growing body of research has shown that black race is associated with underdiagnosis of OSA, greater disease severity at time of diagnosis and reduced PAP adherence (Hsu N, et al. J Clin Sleep Med. 2020;16[8]:1249; Thornton JD, et al. Ann Am Thorac Soc. 2022;19[2]:272).
A recent article (Billings ME, et al. Chest. 2021;159[3]:1232) offered potential strategies to mitigate racial disparities in sleep apnea management. To expand access to care, they advocate embracing telemedicine for those who may have difficulty coming to clinic – due to transportation issues, arranging sufficient time off work, or residing in remote locations. On the other hand, an over-reliance on telemedicine has the potential to worsen disparities in populations whose access to technology is limited.
The authors also recommend inpatient screening of high-risk patient populations to detect disease earlier and to help facilitate referrals to a sleep center. They propose the idea of “peer buddies” of similar racial and socioeconomic backgrounds to provide support and counseling, while cautioning against overburden these populations. Finally, they propose broadening the sleep provider workforce by training primary care providers to manage OSA.
The higher proportion of nonwhite providers in these groups as compared with sleep specialists may improve care, since concordant race provision has been associated with better communication. Underlying these interventions is the need to diversify representation within the medical field at large.
Swetha Gogineni, MD, Vice-Chair
Lauren Tobias, MD, Member-at-Large
Respiratory-Related Sleep Disorders Section
Reducing racial disparities in sleep apnea
For example, a growing body of research has shown that black race is associated with underdiagnosis of OSA, greater disease severity at time of diagnosis and reduced PAP adherence (Hsu N, et al. J Clin Sleep Med. 2020;16[8]:1249; Thornton JD, et al. Ann Am Thorac Soc. 2022;19[2]:272).
A recent article (Billings ME, et al. Chest. 2021;159[3]:1232) offered potential strategies to mitigate racial disparities in sleep apnea management. To expand access to care, they advocate embracing telemedicine for those who may have difficulty coming to clinic – due to transportation issues, arranging sufficient time off work, or residing in remote locations. On the other hand, an over-reliance on telemedicine has the potential to worsen disparities in populations whose access to technology is limited.
The authors also recommend inpatient screening of high-risk patient populations to detect disease earlier and to help facilitate referrals to a sleep center. They propose the idea of “peer buddies” of similar racial and socioeconomic backgrounds to provide support and counseling, while cautioning against overburden these populations. Finally, they propose broadening the sleep provider workforce by training primary care providers to manage OSA.
The higher proportion of nonwhite providers in these groups as compared with sleep specialists may improve care, since concordant race provision has been associated with better communication. Underlying these interventions is the need to diversify representation within the medical field at large.
Swetha Gogineni, MD, Vice-Chair
Lauren Tobias, MD, Member-at-Large
Respiratory-Related Sleep Disorders Section
Reducing racial disparities in sleep apnea
For example, a growing body of research has shown that black race is associated with underdiagnosis of OSA, greater disease severity at time of diagnosis and reduced PAP adherence (Hsu N, et al. J Clin Sleep Med. 2020;16[8]:1249; Thornton JD, et al. Ann Am Thorac Soc. 2022;19[2]:272).
A recent article (Billings ME, et al. Chest. 2021;159[3]:1232) offered potential strategies to mitigate racial disparities in sleep apnea management. To expand access to care, they advocate embracing telemedicine for those who may have difficulty coming to clinic – due to transportation issues, arranging sufficient time off work, or residing in remote locations. On the other hand, an over-reliance on telemedicine has the potential to worsen disparities in populations whose access to technology is limited.
The authors also recommend inpatient screening of high-risk patient populations to detect disease earlier and to help facilitate referrals to a sleep center. They propose the idea of “peer buddies” of similar racial and socioeconomic backgrounds to provide support and counseling, while cautioning against overburden these populations. Finally, they propose broadening the sleep provider workforce by training primary care providers to manage OSA.
The higher proportion of nonwhite providers in these groups as compared with sleep specialists may improve care, since concordant race provision has been associated with better communication. Underlying these interventions is the need to diversify representation within the medical field at large.
Swetha Gogineni, MD, Vice-Chair
Lauren Tobias, MD, Member-at-Large
Pulmonary Vascular & Cardiovascular Network
Pulmonary Vascular Disease Section
Restoration of RV function in PAH: Is it the holy grail to improve mortality and long-term outcomes?
Despite several therapeutic advances, PAH continues to be associated with high mortality. Even mild increases in mean pulmonary arterial pressure (mPAP) have been shown to directly impact outcomes (Maron BA, et al. Circulation. 2016 Mar 29;133[13]:1240), leading to a change in the hemodynamic definition of PAH (mPAP > 20 mm Hg) at the 2018 World Symposium on Pulmonary Hypertension (WSPH) (Galiè N, et al. Eur Respir J. 2019;53[1]:1801889). The WSPH also recommended a more aggressive and proactive approach to move patients to “low-risk” status.
Elevated mPAP results in increased RV afterload with subsequent RV dysfunction and consequent abnormal remodeling, which is associated with poor outcomes. Reversal of RV remodeling has been demonstrated in patients after PEA for CTEPH and/or lung transplantation for PAH (D’Armini AM, et al. J Thorac Cardiovasc Surg. 2007;133:162).
Aggressive mPAP reduction facilitates RV recovery, which may alter the course of PAH in the form of improved survival. RV dysfunction is mainly attributed to afterload mismatch and uncoupling of the RV. Although oral therapies have shown significant improvements in symptoms, functional class, and delaying clinical worsening, normalization of RV size and function is often not achieved. More aggressive reduction of mPAP with a combination of parenteral and oral therapies has been shown to be more effective in restoring RV function (Vizza CD, et al. Am J Respir Crit Care Med. 2022;205) with the ultimate goal of improving quality and quantity of life in those affected by PAH.
Vijay Balasubramanian, MD, FCCP, Chair
Jean M. Elwing, MD, FCCP, Ex-Officio
Pulmonary Vascular Disease Section
Restoration of RV function in PAH: Is it the holy grail to improve mortality and long-term outcomes?
Despite several therapeutic advances, PAH continues to be associated with high mortality. Even mild increases in mean pulmonary arterial pressure (mPAP) have been shown to directly impact outcomes (Maron BA, et al. Circulation. 2016 Mar 29;133[13]:1240), leading to a change in the hemodynamic definition of PAH (mPAP > 20 mm Hg) at the 2018 World Symposium on Pulmonary Hypertension (WSPH) (Galiè N, et al. Eur Respir J. 2019;53[1]:1801889). The WSPH also recommended a more aggressive and proactive approach to move patients to “low-risk” status.
Elevated mPAP results in increased RV afterload with subsequent RV dysfunction and consequent abnormal remodeling, which is associated with poor outcomes. Reversal of RV remodeling has been demonstrated in patients after PEA for CTEPH and/or lung transplantation for PAH (D’Armini AM, et al. J Thorac Cardiovasc Surg. 2007;133:162).
Aggressive mPAP reduction facilitates RV recovery, which may alter the course of PAH in the form of improved survival. RV dysfunction is mainly attributed to afterload mismatch and uncoupling of the RV. Although oral therapies have shown significant improvements in symptoms, functional class, and delaying clinical worsening, normalization of RV size and function is often not achieved. More aggressive reduction of mPAP with a combination of parenteral and oral therapies has been shown to be more effective in restoring RV function (Vizza CD, et al. Am J Respir Crit Care Med. 2022;205) with the ultimate goal of improving quality and quantity of life in those affected by PAH.
Vijay Balasubramanian, MD, FCCP, Chair
Jean M. Elwing, MD, FCCP, Ex-Officio
Pulmonary Vascular Disease Section
Restoration of RV function in PAH: Is it the holy grail to improve mortality and long-term outcomes?
Despite several therapeutic advances, PAH continues to be associated with high mortality. Even mild increases in mean pulmonary arterial pressure (mPAP) have been shown to directly impact outcomes (Maron BA, et al. Circulation. 2016 Mar 29;133[13]:1240), leading to a change in the hemodynamic definition of PAH (mPAP > 20 mm Hg) at the 2018 World Symposium on Pulmonary Hypertension (WSPH) (Galiè N, et al. Eur Respir J. 2019;53[1]:1801889). The WSPH also recommended a more aggressive and proactive approach to move patients to “low-risk” status.
Elevated mPAP results in increased RV afterload with subsequent RV dysfunction and consequent abnormal remodeling, which is associated with poor outcomes. Reversal of RV remodeling has been demonstrated in patients after PEA for CTEPH and/or lung transplantation for PAH (D’Armini AM, et al. J Thorac Cardiovasc Surg. 2007;133:162).
Aggressive mPAP reduction facilitates RV recovery, which may alter the course of PAH in the form of improved survival. RV dysfunction is mainly attributed to afterload mismatch and uncoupling of the RV. Although oral therapies have shown significant improvements in symptoms, functional class, and delaying clinical worsening, normalization of RV size and function is often not achieved. More aggressive reduction of mPAP with a combination of parenteral and oral therapies has been shown to be more effective in restoring RV function (Vizza CD, et al. Am J Respir Crit Care Med. 2022;205) with the ultimate goal of improving quality and quantity of life in those affected by PAH.
Vijay Balasubramanian, MD, FCCP, Chair
Jean M. Elwing, MD, FCCP, Ex-Officio
Thoracic Oncology & Chest Procedures Network
Interventional Procedures Section
Lung cancer is the leading cause of cancer-related deaths worldwide and forms of a large burden of cancer-related mortality in the United States. With the rapid advent of new disease-directed therapy, including molecular and targeted therapies, the outlook for management of lung cancer has changed dramatically over the last decade. The choice of therapy, as well as prognosis, is dependent on the stage at diagnosis. It is thus imperative that we accurately differentiate between stages I, II, and III disease by assessment of hilar and mediastinal lymph nodes.
Traditionally, CT and PET/CT scans have been the mainstay to assess stage, with patients with abnormal lymph nodes or high risk of nodal metastasis (≥ T2 disease or “central” location) undergoing invasive mediastinal evaluation (Silvestri, et al. Chest. 2013 May;143(5 Suppl):e211S). The decision to perform invasive mediastinal staging for T1 tumors remains a matter of discussion. DuComb and colleagues, in their study demonstrated a high rate of N2 metastasis (8.1%) even amongst those with T1 tumors, which was independent of tumor location (DuComb, et al. Chest. 2020 Nov;158[5]:2192). This rate is consistent with previous reported rates ranging from 6.9% to 13% of N2 disease in patients with no radiographic evidence of lymph node metastasis (Gonzalez-Stawinski, et al. J Thorac Cardiovasc Surg. 2003 Dec;126[6]:1900; Bao, et al. J Thorac Dis. 2014;6[12]:1697; Shin, et al. Eur Respir J. 2019;53[3]:1801508). The above indicates a possible role of invasive mediastinal staging using EBUS-TBNA in patients with T1 disease to accurately stage the disease prior to curative intent treatment.
While the role of EBUS-TBNA in diagnosis and staging has been a role of ongoing research, data are limited on prognostic implications of EBUS-guided staging in patients with NSCLC. In a recently published paper in Chest, Hwangbo and colleagues assessed the prognostic impact of staging via EBUS in these patients (Hwangbo et al. Chest 2022 May;161[5]:1382). In the 1,089 patients who underwent EBUS-TBNA, they observed a significant difference in survival based on the staging established via EBUS-TBNA, highlighting the importance of EBUS-TBNA in staging for NSCLC. Also of note, patients with false-negative EBUS results had favorable survival that was similar to patients with pathologic N1 disease. While the exact reason for this is unclear and may be related to disease burden, the authors postulated that this may provide a rationale to performing surgery after negative EBUS-TBNA results.
Abhinav Agrawal, MD, FCCP, Member-at-Large
Ellen Volker, MD, MSPH, FCCP, Member-at-Large
Interventional Procedures Section
Lung cancer is the leading cause of cancer-related deaths worldwide and forms of a large burden of cancer-related mortality in the United States. With the rapid advent of new disease-directed therapy, including molecular and targeted therapies, the outlook for management of lung cancer has changed dramatically over the last decade. The choice of therapy, as well as prognosis, is dependent on the stage at diagnosis. It is thus imperative that we accurately differentiate between stages I, II, and III disease by assessment of hilar and mediastinal lymph nodes.
Traditionally, CT and PET/CT scans have been the mainstay to assess stage, with patients with abnormal lymph nodes or high risk of nodal metastasis (≥ T2 disease or “central” location) undergoing invasive mediastinal evaluation (Silvestri, et al. Chest. 2013 May;143(5 Suppl):e211S). The decision to perform invasive mediastinal staging for T1 tumors remains a matter of discussion. DuComb and colleagues, in their study demonstrated a high rate of N2 metastasis (8.1%) even amongst those with T1 tumors, which was independent of tumor location (DuComb, et al. Chest. 2020 Nov;158[5]:2192). This rate is consistent with previous reported rates ranging from 6.9% to 13% of N2 disease in patients with no radiographic evidence of lymph node metastasis (Gonzalez-Stawinski, et al. J Thorac Cardiovasc Surg. 2003 Dec;126[6]:1900; Bao, et al. J Thorac Dis. 2014;6[12]:1697; Shin, et al. Eur Respir J. 2019;53[3]:1801508). The above indicates a possible role of invasive mediastinal staging using EBUS-TBNA in patients with T1 disease to accurately stage the disease prior to curative intent treatment.
While the role of EBUS-TBNA in diagnosis and staging has been a role of ongoing research, data are limited on prognostic implications of EBUS-guided staging in patients with NSCLC. In a recently published paper in Chest, Hwangbo and colleagues assessed the prognostic impact of staging via EBUS in these patients (Hwangbo et al. Chest 2022 May;161[5]:1382). In the 1,089 patients who underwent EBUS-TBNA, they observed a significant difference in survival based on the staging established via EBUS-TBNA, highlighting the importance of EBUS-TBNA in staging for NSCLC. Also of note, patients with false-negative EBUS results had favorable survival that was similar to patients with pathologic N1 disease. While the exact reason for this is unclear and may be related to disease burden, the authors postulated that this may provide a rationale to performing surgery after negative EBUS-TBNA results.
Abhinav Agrawal, MD, FCCP, Member-at-Large
Ellen Volker, MD, MSPH, FCCP, Member-at-Large
Interventional Procedures Section
Lung cancer is the leading cause of cancer-related deaths worldwide and forms of a large burden of cancer-related mortality in the United States. With the rapid advent of new disease-directed therapy, including molecular and targeted therapies, the outlook for management of lung cancer has changed dramatically over the last decade. The choice of therapy, as well as prognosis, is dependent on the stage at diagnosis. It is thus imperative that we accurately differentiate between stages I, II, and III disease by assessment of hilar and mediastinal lymph nodes.
Traditionally, CT and PET/CT scans have been the mainstay to assess stage, with patients with abnormal lymph nodes or high risk of nodal metastasis (≥ T2 disease or “central” location) undergoing invasive mediastinal evaluation (Silvestri, et al. Chest. 2013 May;143(5 Suppl):e211S). The decision to perform invasive mediastinal staging for T1 tumors remains a matter of discussion. DuComb and colleagues, in their study demonstrated a high rate of N2 metastasis (8.1%) even amongst those with T1 tumors, which was independent of tumor location (DuComb, et al. Chest. 2020 Nov;158[5]:2192). This rate is consistent with previous reported rates ranging from 6.9% to 13% of N2 disease in patients with no radiographic evidence of lymph node metastasis (Gonzalez-Stawinski, et al. J Thorac Cardiovasc Surg. 2003 Dec;126[6]:1900; Bao, et al. J Thorac Dis. 2014;6[12]:1697; Shin, et al. Eur Respir J. 2019;53[3]:1801508). The above indicates a possible role of invasive mediastinal staging using EBUS-TBNA in patients with T1 disease to accurately stage the disease prior to curative intent treatment.
While the role of EBUS-TBNA in diagnosis and staging has been a role of ongoing research, data are limited on prognostic implications of EBUS-guided staging in patients with NSCLC. In a recently published paper in Chest, Hwangbo and colleagues assessed the prognostic impact of staging via EBUS in these patients (Hwangbo et al. Chest 2022 May;161[5]:1382). In the 1,089 patients who underwent EBUS-TBNA, they observed a significant difference in survival based on the staging established via EBUS-TBNA, highlighting the importance of EBUS-TBNA in staging for NSCLC. Also of note, patients with false-negative EBUS results had favorable survival that was similar to patients with pathologic N1 disease. While the exact reason for this is unclear and may be related to disease burden, the authors postulated that this may provide a rationale to performing surgery after negative EBUS-TBNA results.
Abhinav Agrawal, MD, FCCP, Member-at-Large
Ellen Volker, MD, MSPH, FCCP, Member-at-Large




















