User login
Are you ready for CHEST 2023 in Hawai’i?
Just a few weeks ahead of CHEST 2023, we’re sharing the can’t-miss opportunities available on site at the meeting.
Beyond the top-tier education, CHEST 2023 has a lot to offer attendees in the way of networking, development, and unique experiences that will all make for a memorable meeting.
We’re sharing a preview of the many opportunities that will be available over the 4 days of the meeting. For more specifics on these events, including locations, visit the CHEST 2023 website. You can also download the CHEST 2023 mobile app, which will be available in mid-September.
Networking and development
• For those who want to get more involved with the CHEST community, the Networks Mixer (Monday, October 9, 4 PM HST) is open to all who’d like to learn more about the seven CHEST Networks and the 21 clinically-focused Sections within them.
• The annual Women in Chest Medicine Luncheon (Monday, October 9, 12:45 PM HST) will feature a panel of three women speaking about their experiences, their advice, how to support other women in the field, and more. This event is free, but preregistration is required. Scan the QR code to sign up.
• The first-ever Ohana Mixer (Tuesday, October 10, 6 PM HST) is an opportunity for CHEST attendees to celebrate the spirit of community that unites us across our differences. Attendees can network with each other, meet the members of our newly formed Interest Groups – including the leaders of our Women in Chest Medicine Interest Group and Respiratory Care Interest Group – and socialize with presenters from our three local CHEST Community Connections organizations.
• The Trainee Lounge will feature activities like speed mentoring, a lunch and learn with the Keynote Speaker, Dr. Cedric “Jamie” Rutland, financial wellness presentations, and more.
CHEST experiences
• The Opening Session (Sunday, October 8, 3:15 PM HST) will showcase traditional Hawaiian performances and the Keynote Address from Dr. Rutland. Immediately following, the CHEST Welcome Reception will feature live music and a traditional Hawaiian luau.
• For the second year, CHEST After Hours (Monday, October 9, 3 PM HST) will feature clinicians sharing stories of their personal triumphs, tribulations, and more experiences within medicine.
• Each year, the CHEST Challenge Championship (Tuesday, October 10, 7 PM HST) gives pulmonary and critical care medicine fellows-in-training an opportunity to compete in a live Jeopardy-style game – with bragging rights and cash prizes on the line.
• The Wellness Zone has a packed schedule of events, including beachy workouts, food demonstrations, meditation, and more.
Exhibit hall activities
• Opportunities to network with and hear presentations from local Hawaiian organizations, such as the Waianae Coast Comprehensive Health Center
• Hands-on, experiential education escape rooms
• Live educational games, including Hocus POCUS Diagnosis, PulmMemory, Peer Pressure, and more
• Simulation experiences, including Aspirated and Need for Speed – Airway Bleed
Mark your calendars now to participate in all that CHEST 2023 has to offer. We’ll see you in Hawai’i!
Just a few weeks ahead of CHEST 2023, we’re sharing the can’t-miss opportunities available on site at the meeting.
Beyond the top-tier education, CHEST 2023 has a lot to offer attendees in the way of networking, development, and unique experiences that will all make for a memorable meeting.
We’re sharing a preview of the many opportunities that will be available over the 4 days of the meeting. For more specifics on these events, including locations, visit the CHEST 2023 website. You can also download the CHEST 2023 mobile app, which will be available in mid-September.
Networking and development
• For those who want to get more involved with the CHEST community, the Networks Mixer (Monday, October 9, 4 PM HST) is open to all who’d like to learn more about the seven CHEST Networks and the 21 clinically-focused Sections within them.
• The annual Women in Chest Medicine Luncheon (Monday, October 9, 12:45 PM HST) will feature a panel of three women speaking about their experiences, their advice, how to support other women in the field, and more. This event is free, but preregistration is required. Scan the QR code to sign up.
• The first-ever Ohana Mixer (Tuesday, October 10, 6 PM HST) is an opportunity for CHEST attendees to celebrate the spirit of community that unites us across our differences. Attendees can network with each other, meet the members of our newly formed Interest Groups – including the leaders of our Women in Chest Medicine Interest Group and Respiratory Care Interest Group – and socialize with presenters from our three local CHEST Community Connections organizations.
• The Trainee Lounge will feature activities like speed mentoring, a lunch and learn with the Keynote Speaker, Dr. Cedric “Jamie” Rutland, financial wellness presentations, and more.
CHEST experiences
• The Opening Session (Sunday, October 8, 3:15 PM HST) will showcase traditional Hawaiian performances and the Keynote Address from Dr. Rutland. Immediately following, the CHEST Welcome Reception will feature live music and a traditional Hawaiian luau.
• For the second year, CHEST After Hours (Monday, October 9, 3 PM HST) will feature clinicians sharing stories of their personal triumphs, tribulations, and more experiences within medicine.
• Each year, the CHEST Challenge Championship (Tuesday, October 10, 7 PM HST) gives pulmonary and critical care medicine fellows-in-training an opportunity to compete in a live Jeopardy-style game – with bragging rights and cash prizes on the line.
• The Wellness Zone has a packed schedule of events, including beachy workouts, food demonstrations, meditation, and more.
Exhibit hall activities
• Opportunities to network with and hear presentations from local Hawaiian organizations, such as the Waianae Coast Comprehensive Health Center
• Hands-on, experiential education escape rooms
• Live educational games, including Hocus POCUS Diagnosis, PulmMemory, Peer Pressure, and more
• Simulation experiences, including Aspirated and Need for Speed – Airway Bleed
Mark your calendars now to participate in all that CHEST 2023 has to offer. We’ll see you in Hawai’i!
Just a few weeks ahead of CHEST 2023, we’re sharing the can’t-miss opportunities available on site at the meeting.
Beyond the top-tier education, CHEST 2023 has a lot to offer attendees in the way of networking, development, and unique experiences that will all make for a memorable meeting.
We’re sharing a preview of the many opportunities that will be available over the 4 days of the meeting. For more specifics on these events, including locations, visit the CHEST 2023 website. You can also download the CHEST 2023 mobile app, which will be available in mid-September.
Networking and development
• For those who want to get more involved with the CHEST community, the Networks Mixer (Monday, October 9, 4 PM HST) is open to all who’d like to learn more about the seven CHEST Networks and the 21 clinically-focused Sections within them.
• The annual Women in Chest Medicine Luncheon (Monday, October 9, 12:45 PM HST) will feature a panel of three women speaking about their experiences, their advice, how to support other women in the field, and more. This event is free, but preregistration is required. Scan the QR code to sign up.
• The first-ever Ohana Mixer (Tuesday, October 10, 6 PM HST) is an opportunity for CHEST attendees to celebrate the spirit of community that unites us across our differences. Attendees can network with each other, meet the members of our newly formed Interest Groups – including the leaders of our Women in Chest Medicine Interest Group and Respiratory Care Interest Group – and socialize with presenters from our three local CHEST Community Connections organizations.
• The Trainee Lounge will feature activities like speed mentoring, a lunch and learn with the Keynote Speaker, Dr. Cedric “Jamie” Rutland, financial wellness presentations, and more.
CHEST experiences
• The Opening Session (Sunday, October 8, 3:15 PM HST) will showcase traditional Hawaiian performances and the Keynote Address from Dr. Rutland. Immediately following, the CHEST Welcome Reception will feature live music and a traditional Hawaiian luau.
• For the second year, CHEST After Hours (Monday, October 9, 3 PM HST) will feature clinicians sharing stories of their personal triumphs, tribulations, and more experiences within medicine.
• Each year, the CHEST Challenge Championship (Tuesday, October 10, 7 PM HST) gives pulmonary and critical care medicine fellows-in-training an opportunity to compete in a live Jeopardy-style game – with bragging rights and cash prizes on the line.
• The Wellness Zone has a packed schedule of events, including beachy workouts, food demonstrations, meditation, and more.
Exhibit hall activities
• Opportunities to network with and hear presentations from local Hawaiian organizations, such as the Waianae Coast Comprehensive Health Center
• Hands-on, experiential education escape rooms
• Live educational games, including Hocus POCUS Diagnosis, PulmMemory, Peer Pressure, and more
• Simulation experiences, including Aspirated and Need for Speed – Airway Bleed
Mark your calendars now to participate in all that CHEST 2023 has to offer. We’ll see you in Hawai’i!
Upper airway ultrasound: Easy to learn, facile to use!
Thoracic Oncology & Chest Procedures Network
Ultrasound & Chest Imaging Section
Point-of-care ultrasound (POCUS) is integral to the delivery of high-quality patient care. The benefits of POCUS for timely diagnosis and procedural assistance are well documented. With continued innovation, its novel benefits can extend to the upper airway evaluation in both inpatient and outpatient settings.
Adi et al notes that POCUS can serve as an adjunct to traditional airway checklists and help intensivists/anesthesiologists identify potentially difficult laryngoscopies, choose the correct endotracheal tube size to reduce the risk of subglottic stenosis, and help confirm appropriate endotracheal tube placement (Adi, et al. J Emerg Crit Care Med. 2019;3:31).
The prediction of a difficult airway is a potentially lifesaving use for this technology. The authors note that smaller studies demonstrate promising results in four techniques: the inability to visualize the hyoid bone using the sublingual approach, a shorter hyomental distance in morbidly obese patients, anterior neck thickness at different anatomical levels (vocal cords, hyoid bone, and thyroid membrane), and a tongue thickness of more than 6.1 cm from the submental approach were all capable of predicting difficult tracheal intubation with varying degrees of sensitivity and specificity.
In the outpatient setting, an understanding of the upper airway anatomy can help with sleep apnea screenings. Korotun, et al. demonstrated in a small sample that ultrasound evaluation of hyoid bone excursion during hypoglossal nerve stimulation may be a useful tool to predict response to therapy and guide hypoglossal nerve stimulator settings (Korotun, et al. Sleep. 2020;43[Suppl_1]:A247-A248).Upper airway ultrasound is easy to learn. The anatomical landmarks are similar in most patients. This convenient tool can be added to your patient care repertoire in a variety of clinical settings.
Sameer Khanijo, MD, FCCP
Section Member-at-Large
Navitha Ramesh, MD, FCCP
Section Vice-Chair
Thoracic Oncology & Chest Procedures Network
Ultrasound & Chest Imaging Section
Point-of-care ultrasound (POCUS) is integral to the delivery of high-quality patient care. The benefits of POCUS for timely diagnosis and procedural assistance are well documented. With continued innovation, its novel benefits can extend to the upper airway evaluation in both inpatient and outpatient settings.
Adi et al notes that POCUS can serve as an adjunct to traditional airway checklists and help intensivists/anesthesiologists identify potentially difficult laryngoscopies, choose the correct endotracheal tube size to reduce the risk of subglottic stenosis, and help confirm appropriate endotracheal tube placement (Adi, et al. J Emerg Crit Care Med. 2019;3:31).
The prediction of a difficult airway is a potentially lifesaving use for this technology. The authors note that smaller studies demonstrate promising results in four techniques: the inability to visualize the hyoid bone using the sublingual approach, a shorter hyomental distance in morbidly obese patients, anterior neck thickness at different anatomical levels (vocal cords, hyoid bone, and thyroid membrane), and a tongue thickness of more than 6.1 cm from the submental approach were all capable of predicting difficult tracheal intubation with varying degrees of sensitivity and specificity.
In the outpatient setting, an understanding of the upper airway anatomy can help with sleep apnea screenings. Korotun, et al. demonstrated in a small sample that ultrasound evaluation of hyoid bone excursion during hypoglossal nerve stimulation may be a useful tool to predict response to therapy and guide hypoglossal nerve stimulator settings (Korotun, et al. Sleep. 2020;43[Suppl_1]:A247-A248).Upper airway ultrasound is easy to learn. The anatomical landmarks are similar in most patients. This convenient tool can be added to your patient care repertoire in a variety of clinical settings.
Sameer Khanijo, MD, FCCP
Section Member-at-Large
Navitha Ramesh, MD, FCCP
Section Vice-Chair
Thoracic Oncology & Chest Procedures Network
Ultrasound & Chest Imaging Section
Point-of-care ultrasound (POCUS) is integral to the delivery of high-quality patient care. The benefits of POCUS for timely diagnosis and procedural assistance are well documented. With continued innovation, its novel benefits can extend to the upper airway evaluation in both inpatient and outpatient settings.
Adi et al notes that POCUS can serve as an adjunct to traditional airway checklists and help intensivists/anesthesiologists identify potentially difficult laryngoscopies, choose the correct endotracheal tube size to reduce the risk of subglottic stenosis, and help confirm appropriate endotracheal tube placement (Adi, et al. J Emerg Crit Care Med. 2019;3:31).
The prediction of a difficult airway is a potentially lifesaving use for this technology. The authors note that smaller studies demonstrate promising results in four techniques: the inability to visualize the hyoid bone using the sublingual approach, a shorter hyomental distance in morbidly obese patients, anterior neck thickness at different anatomical levels (vocal cords, hyoid bone, and thyroid membrane), and a tongue thickness of more than 6.1 cm from the submental approach were all capable of predicting difficult tracheal intubation with varying degrees of sensitivity and specificity.
In the outpatient setting, an understanding of the upper airway anatomy can help with sleep apnea screenings. Korotun, et al. demonstrated in a small sample that ultrasound evaluation of hyoid bone excursion during hypoglossal nerve stimulation may be a useful tool to predict response to therapy and guide hypoglossal nerve stimulator settings (Korotun, et al. Sleep. 2020;43[Suppl_1]:A247-A248).Upper airway ultrasound is easy to learn. The anatomical landmarks are similar in most patients. This convenient tool can be added to your patient care repertoire in a variety of clinical settings.
Sameer Khanijo, MD, FCCP
Section Member-at-Large
Navitha Ramesh, MD, FCCP
Section Vice-Chair
Addressing disparities in goals-of-care conversations
Critical Care Network
Nonrespiratory Critical Care Section
Goals-of-care discussions are essential to management of the intensive care unit (ICU) patient. Racial inequities in end-of-life decision making have been documented for many years, with literature demonstrating that marginalized populations are less likely to have EHR-documented goals-of-care discussions and more likely to have concerns regarding clinician communication.
A recently published randomized control trial in JAMA highlights an intervention that offers promise in addressing disparities in goals-of-care conversations. Curtis, et al. studied whether priming physicians with a communication guide advising on discussion prompts and language for goals-of-care could improve the rate of documented goals-of-care discussions among hospitalized older adults with serious illness. The study found that for patients in the intervention arm, there was a significant increase in proportion of goals-of-care discussions within 30 days. Notably, the difference in documented goals-of-care discussions between arms was greater in the subgroup of patients from underserved groups (Curtis JR, et al. JAMA. 2023;329[23]:2028-37).
Nevertheless, while interventions may help increase the rate of goals-of-care discussions, it is also important to address the content of discussions themselves. You and colleagues recently published a mixed-methods study assessing the impact of race on shared decision-making behaviors during family/caregiver meetings. The authors found that while ICU physicians approached shared decision making with White and Black families similarly, Black families felt their physicians provided less validation of the family role in decision making than White families did (You H, et al. Ann Am Thorac Soc. 2023 May;20[5]:759-62). These findings highlight the importance of ongoing work that focuses not only on quantity but also on quality of communication regarding goals-of-care for patients from diverse backgrounds.
Divya Shankar MD
Section Fellow-in-Training
Muhammad Hayat-Syed MD
Section Vice Chair
Critical Care Network
Nonrespiratory Critical Care Section
Goals-of-care discussions are essential to management of the intensive care unit (ICU) patient. Racial inequities in end-of-life decision making have been documented for many years, with literature demonstrating that marginalized populations are less likely to have EHR-documented goals-of-care discussions and more likely to have concerns regarding clinician communication.
A recently published randomized control trial in JAMA highlights an intervention that offers promise in addressing disparities in goals-of-care conversations. Curtis, et al. studied whether priming physicians with a communication guide advising on discussion prompts and language for goals-of-care could improve the rate of documented goals-of-care discussions among hospitalized older adults with serious illness. The study found that for patients in the intervention arm, there was a significant increase in proportion of goals-of-care discussions within 30 days. Notably, the difference in documented goals-of-care discussions between arms was greater in the subgroup of patients from underserved groups (Curtis JR, et al. JAMA. 2023;329[23]:2028-37).
Nevertheless, while interventions may help increase the rate of goals-of-care discussions, it is also important to address the content of discussions themselves. You and colleagues recently published a mixed-methods study assessing the impact of race on shared decision-making behaviors during family/caregiver meetings. The authors found that while ICU physicians approached shared decision making with White and Black families similarly, Black families felt their physicians provided less validation of the family role in decision making than White families did (You H, et al. Ann Am Thorac Soc. 2023 May;20[5]:759-62). These findings highlight the importance of ongoing work that focuses not only on quantity but also on quality of communication regarding goals-of-care for patients from diverse backgrounds.
Divya Shankar MD
Section Fellow-in-Training
Muhammad Hayat-Syed MD
Section Vice Chair
Critical Care Network
Nonrespiratory Critical Care Section
Goals-of-care discussions are essential to management of the intensive care unit (ICU) patient. Racial inequities in end-of-life decision making have been documented for many years, with literature demonstrating that marginalized populations are less likely to have EHR-documented goals-of-care discussions and more likely to have concerns regarding clinician communication.
A recently published randomized control trial in JAMA highlights an intervention that offers promise in addressing disparities in goals-of-care conversations. Curtis, et al. studied whether priming physicians with a communication guide advising on discussion prompts and language for goals-of-care could improve the rate of documented goals-of-care discussions among hospitalized older adults with serious illness. The study found that for patients in the intervention arm, there was a significant increase in proportion of goals-of-care discussions within 30 days. Notably, the difference in documented goals-of-care discussions between arms was greater in the subgroup of patients from underserved groups (Curtis JR, et al. JAMA. 2023;329[23]:2028-37).
Nevertheless, while interventions may help increase the rate of goals-of-care discussions, it is also important to address the content of discussions themselves. You and colleagues recently published a mixed-methods study assessing the impact of race on shared decision-making behaviors during family/caregiver meetings. The authors found that while ICU physicians approached shared decision making with White and Black families similarly, Black families felt their physicians provided less validation of the family role in decision making than White families did (You H, et al. Ann Am Thorac Soc. 2023 May;20[5]:759-62). These findings highlight the importance of ongoing work that focuses not only on quantity but also on quality of communication regarding goals-of-care for patients from diverse backgrounds.
Divya Shankar MD
Section Fellow-in-Training
Muhammad Hayat-Syed MD
Section Vice Chair
Use of frailty assessment in lung transplant evaluation
Diffuse Lung Disease & Transplant Network
Lung Transplant Section
Frailty, a concept that originated in the geriatric population, is a state of vulnerability resulting from a decline in reserve and function across physiological systems. While it is more commonly observed in older adults, some aging-associated syndromes, such as sarcopenia, impaired cognition, inflammation, and malnutrition, may be present in younger patients with end-stage organ disease. These syndromes can be associated with biological age, as opposed to chronological age, which explains why younger patients with end-stage organ disease can develop frailty (Schaenman JM, et al. Am J Transplant. 2021 Jun;21[6]:2018-24). Frailty in the lung transplant population is associated with increased morbidity and mortality while on the waitlist and post-transplant (Montgomery E, et al. J Transplant. 2020 Aug 7:3239495). In 2021, the International Society of Heart and Lung Transplantation recommended including a frailty assessment to complete a patient’s transplant evaluation. The committee cautioned using current assessment tools, as they are not yet accepted as the standard of care (Leard, et al. J Heart Lung Transplant. 2021 Nov;40[11]:1349-79). Existing tools being used evolved from studies of community-dwelling older adults with no predilection for distinct organ disease, which include the Fried Physical Frailty Phenotype (FPFP) and the Short Physical Performance Battery (SPPB). Along with physical limitations, frail patients tend to have abnormal biomarkers including higher inflammatory cytokines, such as plasma IL-6 and tumor necrosis factor receptor 1, and lower insulin-like growth factor I and leptin (Singer JP, et al. Am J Respir Crit Care Med. 2015;192[11]1325-34). The concept of a lung-focused approach to frailty, which considers biomarkers and body composition, is currently being researched (Singer JP, et al. J Heart Lung Transplant. 2023;S1053-S2498[23]00049-9). This disease-specific frailty scale would identify lung transplant candidates who may benefit from targeted interventions, and such frailty would also be expected to improve after transplant.
Erin Meier, MD
Section Fellow-in-Training
Anupam Kumar, MD, FCCP
Section Member-at-Large
Diffuse Lung Disease & Transplant Network
Lung Transplant Section
Frailty, a concept that originated in the geriatric population, is a state of vulnerability resulting from a decline in reserve and function across physiological systems. While it is more commonly observed in older adults, some aging-associated syndromes, such as sarcopenia, impaired cognition, inflammation, and malnutrition, may be present in younger patients with end-stage organ disease. These syndromes can be associated with biological age, as opposed to chronological age, which explains why younger patients with end-stage organ disease can develop frailty (Schaenman JM, et al. Am J Transplant. 2021 Jun;21[6]:2018-24). Frailty in the lung transplant population is associated with increased morbidity and mortality while on the waitlist and post-transplant (Montgomery E, et al. J Transplant. 2020 Aug 7:3239495). In 2021, the International Society of Heart and Lung Transplantation recommended including a frailty assessment to complete a patient’s transplant evaluation. The committee cautioned using current assessment tools, as they are not yet accepted as the standard of care (Leard, et al. J Heart Lung Transplant. 2021 Nov;40[11]:1349-79). Existing tools being used evolved from studies of community-dwelling older adults with no predilection for distinct organ disease, which include the Fried Physical Frailty Phenotype (FPFP) and the Short Physical Performance Battery (SPPB). Along with physical limitations, frail patients tend to have abnormal biomarkers including higher inflammatory cytokines, such as plasma IL-6 and tumor necrosis factor receptor 1, and lower insulin-like growth factor I and leptin (Singer JP, et al. Am J Respir Crit Care Med. 2015;192[11]1325-34). The concept of a lung-focused approach to frailty, which considers biomarkers and body composition, is currently being researched (Singer JP, et al. J Heart Lung Transplant. 2023;S1053-S2498[23]00049-9). This disease-specific frailty scale would identify lung transplant candidates who may benefit from targeted interventions, and such frailty would also be expected to improve after transplant.
Erin Meier, MD
Section Fellow-in-Training
Anupam Kumar, MD, FCCP
Section Member-at-Large
Diffuse Lung Disease & Transplant Network
Lung Transplant Section
Frailty, a concept that originated in the geriatric population, is a state of vulnerability resulting from a decline in reserve and function across physiological systems. While it is more commonly observed in older adults, some aging-associated syndromes, such as sarcopenia, impaired cognition, inflammation, and malnutrition, may be present in younger patients with end-stage organ disease. These syndromes can be associated with biological age, as opposed to chronological age, which explains why younger patients with end-stage organ disease can develop frailty (Schaenman JM, et al. Am J Transplant. 2021 Jun;21[6]:2018-24). Frailty in the lung transplant population is associated with increased morbidity and mortality while on the waitlist and post-transplant (Montgomery E, et al. J Transplant. 2020 Aug 7:3239495). In 2021, the International Society of Heart and Lung Transplantation recommended including a frailty assessment to complete a patient’s transplant evaluation. The committee cautioned using current assessment tools, as they are not yet accepted as the standard of care (Leard, et al. J Heart Lung Transplant. 2021 Nov;40[11]:1349-79). Existing tools being used evolved from studies of community-dwelling older adults with no predilection for distinct organ disease, which include the Fried Physical Frailty Phenotype (FPFP) and the Short Physical Performance Battery (SPPB). Along with physical limitations, frail patients tend to have abnormal biomarkers including higher inflammatory cytokines, such as plasma IL-6 and tumor necrosis factor receptor 1, and lower insulin-like growth factor I and leptin (Singer JP, et al. Am J Respir Crit Care Med. 2015;192[11]1325-34). The concept of a lung-focused approach to frailty, which considers biomarkers and body composition, is currently being researched (Singer JP, et al. J Heart Lung Transplant. 2023;S1053-S2498[23]00049-9). This disease-specific frailty scale would identify lung transplant candidates who may benefit from targeted interventions, and such frailty would also be expected to improve after transplant.
Erin Meier, MD
Section Fellow-in-Training
Anupam Kumar, MD, FCCP
Section Member-at-Large
DPP1 a promising target for bronchiectasis
Airway Disorders Network
Bronchiectasis Section
and persistent inflammation. In bronchiectasis, excessive neutrophil accumulation in the airways leads to release of neutrophil serine proteases (NSPs), which contributes to tissue damage and perpetuates the inflammatory process in the lungs. The three main NSPs include neutrophil elastase (NE), proteinase3, and cathepsin G. Elevations in NE activity in sputum in NCFBE are associated with increased exacerbations and declines in lung function. Dipeptidyl peptidase 1 (DPP1), an enzyme primarily found in neutrophils, is responsible for activating NSPs during neutrophil maturation. In bronchiectasis, increased DPP1 activity results in an augmented production of active NSPs, exacerbating lung damage and inflammation.
Brensocatib, an oral, reversible inhibitor of DPP1 is currently being developed as a novel approach to managing bronchiectasis. Brensocatib was evaluated in a phase 2 clinical trial (WILLOW), a randomized, double-blind, placebo-controlled trial involving adults with non–cystic fibrosis bronchiectasis (NCFBE). Treatment with brensocatib for 24 weeks significantly prolonged the time to the first exacerbation at both the 10 mg and 25 mg doses and lowered the risk of exacerbation by 40% relative to placebo. The treatment was well tolerated, with no significant safety concerns. Results of a recent post hoc analysis from the WILLOW study show that brensocatib effectively reduces exacerbations and slows lung function decline across different severities of bronchiectasis. These findings suggest that brensocatib holds potential as the 1st new therapeutic option for patients with NCFBE, with currently no FDA-approved drugs. Results of a larger-scale phase 3 trial are awaited later this year, which will hopefully confirm these results and ascertain the long-term safety.
Shyamsunder Subramanian, MD, MBBS, FCCP
Section Chair
Airway Disorders Network
Bronchiectasis Section
and persistent inflammation. In bronchiectasis, excessive neutrophil accumulation in the airways leads to release of neutrophil serine proteases (NSPs), which contributes to tissue damage and perpetuates the inflammatory process in the lungs. The three main NSPs include neutrophil elastase (NE), proteinase3, and cathepsin G. Elevations in NE activity in sputum in NCFBE are associated with increased exacerbations and declines in lung function. Dipeptidyl peptidase 1 (DPP1), an enzyme primarily found in neutrophils, is responsible for activating NSPs during neutrophil maturation. In bronchiectasis, increased DPP1 activity results in an augmented production of active NSPs, exacerbating lung damage and inflammation.
Brensocatib, an oral, reversible inhibitor of DPP1 is currently being developed as a novel approach to managing bronchiectasis. Brensocatib was evaluated in a phase 2 clinical trial (WILLOW), a randomized, double-blind, placebo-controlled trial involving adults with non–cystic fibrosis bronchiectasis (NCFBE). Treatment with brensocatib for 24 weeks significantly prolonged the time to the first exacerbation at both the 10 mg and 25 mg doses and lowered the risk of exacerbation by 40% relative to placebo. The treatment was well tolerated, with no significant safety concerns. Results of a recent post hoc analysis from the WILLOW study show that brensocatib effectively reduces exacerbations and slows lung function decline across different severities of bronchiectasis. These findings suggest that brensocatib holds potential as the 1st new therapeutic option for patients with NCFBE, with currently no FDA-approved drugs. Results of a larger-scale phase 3 trial are awaited later this year, which will hopefully confirm these results and ascertain the long-term safety.
Shyamsunder Subramanian, MD, MBBS, FCCP
Section Chair
Airway Disorders Network
Bronchiectasis Section
and persistent inflammation. In bronchiectasis, excessive neutrophil accumulation in the airways leads to release of neutrophil serine proteases (NSPs), which contributes to tissue damage and perpetuates the inflammatory process in the lungs. The three main NSPs include neutrophil elastase (NE), proteinase3, and cathepsin G. Elevations in NE activity in sputum in NCFBE are associated with increased exacerbations and declines in lung function. Dipeptidyl peptidase 1 (DPP1), an enzyme primarily found in neutrophils, is responsible for activating NSPs during neutrophil maturation. In bronchiectasis, increased DPP1 activity results in an augmented production of active NSPs, exacerbating lung damage and inflammation.
Brensocatib, an oral, reversible inhibitor of DPP1 is currently being developed as a novel approach to managing bronchiectasis. Brensocatib was evaluated in a phase 2 clinical trial (WILLOW), a randomized, double-blind, placebo-controlled trial involving adults with non–cystic fibrosis bronchiectasis (NCFBE). Treatment with brensocatib for 24 weeks significantly prolonged the time to the first exacerbation at both the 10 mg and 25 mg doses and lowered the risk of exacerbation by 40% relative to placebo. The treatment was well tolerated, with no significant safety concerns. Results of a recent post hoc analysis from the WILLOW study show that brensocatib effectively reduces exacerbations and slows lung function decline across different severities of bronchiectasis. These findings suggest that brensocatib holds potential as the 1st new therapeutic option for patients with NCFBE, with currently no FDA-approved drugs. Results of a larger-scale phase 3 trial are awaited later this year, which will hopefully confirm these results and ascertain the long-term safety.
Shyamsunder Subramanian, MD, MBBS, FCCP
Section Chair
Add hands-on and interactive learning opportunities to your CHEST 2023 schedule
Explore the many ticketed sessions, and sign up early in case they sell out.
Simulation sessions
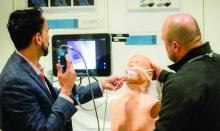
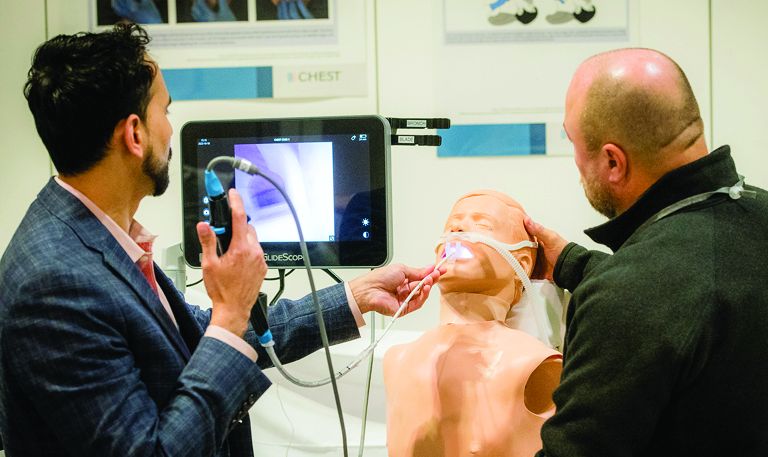
If you’re looking to gain hands-on exposure to equipment and tools that may not be available at your home institution, look no further than these simulation sessions. Choose from 25 different sessions offering firsthand experience with procedures relevant to your clinical practice.
“It’s a great opportunity to teach higher stakes procedures in a very low stakes environment where everybody’s comfortable and everybody’s learning from each other,” said Live Learning Subcommittee Chair, Nicholas Pastis, MD, FCCP.
CHEST 2023 simulation sessions will address clinical topics, including endobronchial ultrasound, cardiopulmonary exercise testing (CPET), intubation and cricothyrotomy, bronchoscopy management, and more. These sessions are taught by experts who use these real-world strategies in their daily practice.
CHEST 2022 attendee, Weston Bowker, MD, found value in the simulation courses he was able to attend in Nashville.
“It’s fantastic just to work with some of the leading experts in the field, especially from an interventional pulmonology standpoint. And, you truly get a different experience than maybe what your home institution offers,” he said.
Problem-based learning sessions
Exercise your critical thinking skills by working to resolve real-world clinical problems during these small group sessions. Refine your expertise on topics like lung cancer screening and staging, biologics in asthma, pneumonia, and more.
“Problem-based learning courses take a clinical problem or case study that is somewhat controversial to create a learning environment where the problem itself drives the learning with participants,” said CHEST 2023 Scientific Program Committee Chair, Aneesa Das, MD, FCCP. “These are small group sessions where learners can actively participate and collaborate to discuss various perspectives on the issue and work toward potential solutions.”
This year’s problem-based learning courses were chosen based on common controversies in chest medicine and current hot topics in medicine.
Dr. Das is excited for the Using CPET to Solve Your Difficult Cases course. “Cardiopulmonary exercise tests can sometimes be difficult even for seasoned physicians. This is always an amazing problem-based learning topic,” she added.
Meet the Professor sessions
Connect with leading chest medicine experts during these limited-capacity discussions capped at 24 registrants per session. Meet the Professor attendees will have the opportunity to engage in stimulating conversations on bronchiectasis, central airway obstructions, obesity hypoventilation, and sublobar resection.
“Meet the Professor sessions are a unique opportunity to interact and learn from a leader in the field in a very small group setting on a high-yield topic,” said Dr. Das. “These sessions allow for a learning environment that is personalized and intimate.”
Explore the many ticketed sessions, and sign up early in case they sell out.
Simulation sessions
If you’re looking to gain hands-on exposure to equipment and tools that may not be available at your home institution, look no further than these simulation sessions. Choose from 25 different sessions offering firsthand experience with procedures relevant to your clinical practice.
“It’s a great opportunity to teach higher stakes procedures in a very low stakes environment where everybody’s comfortable and everybody’s learning from each other,” said Live Learning Subcommittee Chair, Nicholas Pastis, MD, FCCP.
CHEST 2023 simulation sessions will address clinical topics, including endobronchial ultrasound, cardiopulmonary exercise testing (CPET), intubation and cricothyrotomy, bronchoscopy management, and more. These sessions are taught by experts who use these real-world strategies in their daily practice.
CHEST 2022 attendee, Weston Bowker, MD, found value in the simulation courses he was able to attend in Nashville.
“It’s fantastic just to work with some of the leading experts in the field, especially from an interventional pulmonology standpoint. And, you truly get a different experience than maybe what your home institution offers,” he said.
Problem-based learning sessions
Exercise your critical thinking skills by working to resolve real-world clinical problems during these small group sessions. Refine your expertise on topics like lung cancer screening and staging, biologics in asthma, pneumonia, and more.
“Problem-based learning courses take a clinical problem or case study that is somewhat controversial to create a learning environment where the problem itself drives the learning with participants,” said CHEST 2023 Scientific Program Committee Chair, Aneesa Das, MD, FCCP. “These are small group sessions where learners can actively participate and collaborate to discuss various perspectives on the issue and work toward potential solutions.”
This year’s problem-based learning courses were chosen based on common controversies in chest medicine and current hot topics in medicine.
Dr. Das is excited for the Using CPET to Solve Your Difficult Cases course. “Cardiopulmonary exercise tests can sometimes be difficult even for seasoned physicians. This is always an amazing problem-based learning topic,” she added.
Meet the Professor sessions
Connect with leading chest medicine experts during these limited-capacity discussions capped at 24 registrants per session. Meet the Professor attendees will have the opportunity to engage in stimulating conversations on bronchiectasis, central airway obstructions, obesity hypoventilation, and sublobar resection.
“Meet the Professor sessions are a unique opportunity to interact and learn from a leader in the field in a very small group setting on a high-yield topic,” said Dr. Das. “These sessions allow for a learning environment that is personalized and intimate.”
Explore the many ticketed sessions, and sign up early in case they sell out.
Simulation sessions
If you’re looking to gain hands-on exposure to equipment and tools that may not be available at your home institution, look no further than these simulation sessions. Choose from 25 different sessions offering firsthand experience with procedures relevant to your clinical practice.
“It’s a great opportunity to teach higher stakes procedures in a very low stakes environment where everybody’s comfortable and everybody’s learning from each other,” said Live Learning Subcommittee Chair, Nicholas Pastis, MD, FCCP.
CHEST 2023 simulation sessions will address clinical topics, including endobronchial ultrasound, cardiopulmonary exercise testing (CPET), intubation and cricothyrotomy, bronchoscopy management, and more. These sessions are taught by experts who use these real-world strategies in their daily practice.
CHEST 2022 attendee, Weston Bowker, MD, found value in the simulation courses he was able to attend in Nashville.
“It’s fantastic just to work with some of the leading experts in the field, especially from an interventional pulmonology standpoint. And, you truly get a different experience than maybe what your home institution offers,” he said.
Problem-based learning sessions
Exercise your critical thinking skills by working to resolve real-world clinical problems during these small group sessions. Refine your expertise on topics like lung cancer screening and staging, biologics in asthma, pneumonia, and more.
“Problem-based learning courses take a clinical problem or case study that is somewhat controversial to create a learning environment where the problem itself drives the learning with participants,” said CHEST 2023 Scientific Program Committee Chair, Aneesa Das, MD, FCCP. “These are small group sessions where learners can actively participate and collaborate to discuss various perspectives on the issue and work toward potential solutions.”
This year’s problem-based learning courses were chosen based on common controversies in chest medicine and current hot topics in medicine.
Dr. Das is excited for the Using CPET to Solve Your Difficult Cases course. “Cardiopulmonary exercise tests can sometimes be difficult even for seasoned physicians. This is always an amazing problem-based learning topic,” she added.
Meet the Professor sessions
Connect with leading chest medicine experts during these limited-capacity discussions capped at 24 registrants per session. Meet the Professor attendees will have the opportunity to engage in stimulating conversations on bronchiectasis, central airway obstructions, obesity hypoventilation, and sublobar resection.
“Meet the Professor sessions are a unique opportunity to interact and learn from a leader in the field in a very small group setting on a high-yield topic,” said Dr. Das. “These sessions allow for a learning environment that is personalized and intimate.”
RAPID updates in pleural infection
Thoracic Oncology & Chest Imaging Network
Interventional Procedures Section
The MIST-2 trial (Rahman, et al. N Engl J Med. 2011;365:518), the first randomized trial to show the benefit of intrapleural enzyme therapy (IET) with tissue plasminogen activator and deoxyribonuclease for the treatment of complicated pleural infection (cPI) is the foundational study for the use of IET. It was from this cohort that the first prospectively validated mortality prediction score for cPI was developed – the RAPID score (Rahman, et al. Chest. 2014;145[4]:848).
The RAPID score, comprised of Renal, Age, Purulence, Infection source, and Dietary factors (albumin) divides patients with cPI into three 3-month mortality groups: low (1.5%), medium (17.8%), and high (47.8%). The score was externally validated in the PILOT trial (Corcoran, et al. Eur Respir J. 2020;56[5]:2000130). Mortality outcomes were separately assessed in 1-, 3-, and 5-year follow-up by White, et al (Ann Am Thorac Soc. 2015;12[9]:1310) and found to bear out with an increased OR for mortality of 14.3 and 53.3 in the medium and high risk groups, respectively. Of note, there was a surgical referral rate of only 4% to16% in the study cohort, and the original study did not distinguish between IET use or surgery.
To look at RAPID in a purely surgical cohort, Stüben, et al (Sci Rep. 2023;13[1]:3206) applied the RAPID score to a cohort of patients with empyema all treated with initial surgical drainage. They found the RAPID score to be an accurate predictor of 90-day mortality and improved with the addition of diabetes and renal replacement therapy. Liou, et al (J Thorac Dis. 2023;15[3]:985) showed that patients with a low RAPID score who were taken to surgery early had improved length of stay and organ failure and mortality rates compared with those taken later.
Can the RAPID score differentiate between those who need IET alone, early surgery, or late surgery? Not yet, but several prospective studies are underway to help improve outcomes in this ancient disease. Until then, the RAPID score remains a useful risk-stratification tool for an increasingly broad population of patients with pleural infection.
Max Diddams, MD
Section Fellow-in-Training
Thoracic Oncology & Chest Imaging Network
Interventional Procedures Section
The MIST-2 trial (Rahman, et al. N Engl J Med. 2011;365:518), the first randomized trial to show the benefit of intrapleural enzyme therapy (IET) with tissue plasminogen activator and deoxyribonuclease for the treatment of complicated pleural infection (cPI) is the foundational study for the use of IET. It was from this cohort that the first prospectively validated mortality prediction score for cPI was developed – the RAPID score (Rahman, et al. Chest. 2014;145[4]:848).
The RAPID score, comprised of Renal, Age, Purulence, Infection source, and Dietary factors (albumin) divides patients with cPI into three 3-month mortality groups: low (1.5%), medium (17.8%), and high (47.8%). The score was externally validated in the PILOT trial (Corcoran, et al. Eur Respir J. 2020;56[5]:2000130). Mortality outcomes were separately assessed in 1-, 3-, and 5-year follow-up by White, et al (Ann Am Thorac Soc. 2015;12[9]:1310) and found to bear out with an increased OR for mortality of 14.3 and 53.3 in the medium and high risk groups, respectively. Of note, there was a surgical referral rate of only 4% to16% in the study cohort, and the original study did not distinguish between IET use or surgery.
To look at RAPID in a purely surgical cohort, Stüben, et al (Sci Rep. 2023;13[1]:3206) applied the RAPID score to a cohort of patients with empyema all treated with initial surgical drainage. They found the RAPID score to be an accurate predictor of 90-day mortality and improved with the addition of diabetes and renal replacement therapy. Liou, et al (J Thorac Dis. 2023;15[3]:985) showed that patients with a low RAPID score who were taken to surgery early had improved length of stay and organ failure and mortality rates compared with those taken later.
Can the RAPID score differentiate between those who need IET alone, early surgery, or late surgery? Not yet, but several prospective studies are underway to help improve outcomes in this ancient disease. Until then, the RAPID score remains a useful risk-stratification tool for an increasingly broad population of patients with pleural infection.
Max Diddams, MD
Section Fellow-in-Training
Thoracic Oncology & Chest Imaging Network
Interventional Procedures Section
The MIST-2 trial (Rahman, et al. N Engl J Med. 2011;365:518), the first randomized trial to show the benefit of intrapleural enzyme therapy (IET) with tissue plasminogen activator and deoxyribonuclease for the treatment of complicated pleural infection (cPI) is the foundational study for the use of IET. It was from this cohort that the first prospectively validated mortality prediction score for cPI was developed – the RAPID score (Rahman, et al. Chest. 2014;145[4]:848).
The RAPID score, comprised of Renal, Age, Purulence, Infection source, and Dietary factors (albumin) divides patients with cPI into three 3-month mortality groups: low (1.5%), medium (17.8%), and high (47.8%). The score was externally validated in the PILOT trial (Corcoran, et al. Eur Respir J. 2020;56[5]:2000130). Mortality outcomes were separately assessed in 1-, 3-, and 5-year follow-up by White, et al (Ann Am Thorac Soc. 2015;12[9]:1310) and found to bear out with an increased OR for mortality of 14.3 and 53.3 in the medium and high risk groups, respectively. Of note, there was a surgical referral rate of only 4% to16% in the study cohort, and the original study did not distinguish between IET use or surgery.
To look at RAPID in a purely surgical cohort, Stüben, et al (Sci Rep. 2023;13[1]:3206) applied the RAPID score to a cohort of patients with empyema all treated with initial surgical drainage. They found the RAPID score to be an accurate predictor of 90-day mortality and improved with the addition of diabetes and renal replacement therapy. Liou, et al (J Thorac Dis. 2023;15[3]:985) showed that patients with a low RAPID score who were taken to surgery early had improved length of stay and organ failure and mortality rates compared with those taken later.
Can the RAPID score differentiate between those who need IET alone, early surgery, or late surgery? Not yet, but several prospective studies are underway to help improve outcomes in this ancient disease. Until then, the RAPID score remains a useful risk-stratification tool for an increasingly broad population of patients with pleural infection.
Max Diddams, MD
Section Fellow-in-Training
Noninvasive mechanical ventilation in unilateral diaphragm paralysis
Sleep Medicine Network
Home-Based Mechanical Ventilation & Neuromuscular Disease Section
The diaphragm plays a key a role in respiratory mechanics, particularly during the inspiratory cycle. Unilateral diaphragm paralysis (UDP) from traumatic, compressive, inflammatory, neuropathic, or iatrogenic phrenic nerve injury presents with exertional dyspnea or orthopnea, though more severe cases may present with hypoventilation, hypercapnia, and daytime fatigue. Diagnostic workup requires evaluation beyond radiography to determine if diaphragm elevation indicates paralysis with or without paradox. Severity of symptoms and degree of impairment do not consistently correlate with fluoroscopic/ultrasound findings during sniff maneuver, degree of restriction by spirometry, or supine forces. Compensatory accessory muscle use during daytime breathing can mask symptoms, and there can be severe nocturnal hypoventilation related to UDP.
For symptomatic patients, treatment recommendations require understanding of the etiology and the likelihood of resolution vs progression, or association with progressive systemic conditions. Nighttime noninvasive ventilation (NIV) is considered useful since diaphragmatic weakness worsens in supine position, and hypoventilation during REM sleep without accessory muscle support is exacerbated (Steier J, et al. Eur Respir J. 2008;32[6]:1479). However, evidence for NIV in UDP remains low quality. NIV has been proposed for ventilatory support particularly when hypercapnia is present (Wiebel M, et al. Med Klin. 1995;90[1 Suppl 1]:20). For patients with progressive neuromuscular conditions, NIV with a backup rate is strongly recommended (Steindor M, et al. Respir Care. 2021;66[3]:410; Benditt JO. Respir Care. 2019;64[6]:679), but access to respiratory assist devices is limited for isolated UDP under current reimbursement algorithms without demonstrable hypercapnia or significant restrictive spirometry. The recent ONMAP recommendations calling for use of symptom severity to support initiating NIV if FVC>80% have not yet been adopted (Morgenthaler TI, et al. Chest. 2021;160[5]:e419). Without marked spirometric restriction or hypercapnia, most patients must fail conservative PAP therapy prior to escalation to NIV, and initiation of a backup rate remains debated. Nevertheless, the only large case series evaluating the predominant features of polysomnography in UDP suggests high incidence of central apneas, suggesting a backup rate may indeed be required independent of the need to support neuromuscular function (Singh M, et al. Can J Anesthesiology. 2021;68[7]:1064). Further assessment of the features, needs, and understanding of the natural trajectory is essential to guide approach to sleep-related hypoventilation in UDP.
Landy V. Luna Diaz
Section Fellow-in-Training
Bethany L. Lussier, MD, FCCP
Section Member-at-Large
Sleep Medicine Network
Home-Based Mechanical Ventilation & Neuromuscular Disease Section
The diaphragm plays a key a role in respiratory mechanics, particularly during the inspiratory cycle. Unilateral diaphragm paralysis (UDP) from traumatic, compressive, inflammatory, neuropathic, or iatrogenic phrenic nerve injury presents with exertional dyspnea or orthopnea, though more severe cases may present with hypoventilation, hypercapnia, and daytime fatigue. Diagnostic workup requires evaluation beyond radiography to determine if diaphragm elevation indicates paralysis with or without paradox. Severity of symptoms and degree of impairment do not consistently correlate with fluoroscopic/ultrasound findings during sniff maneuver, degree of restriction by spirometry, or supine forces. Compensatory accessory muscle use during daytime breathing can mask symptoms, and there can be severe nocturnal hypoventilation related to UDP.
For symptomatic patients, treatment recommendations require understanding of the etiology and the likelihood of resolution vs progression, or association with progressive systemic conditions. Nighttime noninvasive ventilation (NIV) is considered useful since diaphragmatic weakness worsens in supine position, and hypoventilation during REM sleep without accessory muscle support is exacerbated (Steier J, et al. Eur Respir J. 2008;32[6]:1479). However, evidence for NIV in UDP remains low quality. NIV has been proposed for ventilatory support particularly when hypercapnia is present (Wiebel M, et al. Med Klin. 1995;90[1 Suppl 1]:20). For patients with progressive neuromuscular conditions, NIV with a backup rate is strongly recommended (Steindor M, et al. Respir Care. 2021;66[3]:410; Benditt JO. Respir Care. 2019;64[6]:679), but access to respiratory assist devices is limited for isolated UDP under current reimbursement algorithms without demonstrable hypercapnia or significant restrictive spirometry. The recent ONMAP recommendations calling for use of symptom severity to support initiating NIV if FVC>80% have not yet been adopted (Morgenthaler TI, et al. Chest. 2021;160[5]:e419). Without marked spirometric restriction or hypercapnia, most patients must fail conservative PAP therapy prior to escalation to NIV, and initiation of a backup rate remains debated. Nevertheless, the only large case series evaluating the predominant features of polysomnography in UDP suggests high incidence of central apneas, suggesting a backup rate may indeed be required independent of the need to support neuromuscular function (Singh M, et al. Can J Anesthesiology. 2021;68[7]:1064). Further assessment of the features, needs, and understanding of the natural trajectory is essential to guide approach to sleep-related hypoventilation in UDP.
Landy V. Luna Diaz
Section Fellow-in-Training
Bethany L. Lussier, MD, FCCP
Section Member-at-Large
Sleep Medicine Network
Home-Based Mechanical Ventilation & Neuromuscular Disease Section
The diaphragm plays a key a role in respiratory mechanics, particularly during the inspiratory cycle. Unilateral diaphragm paralysis (UDP) from traumatic, compressive, inflammatory, neuropathic, or iatrogenic phrenic nerve injury presents with exertional dyspnea or orthopnea, though more severe cases may present with hypoventilation, hypercapnia, and daytime fatigue. Diagnostic workup requires evaluation beyond radiography to determine if diaphragm elevation indicates paralysis with or without paradox. Severity of symptoms and degree of impairment do not consistently correlate with fluoroscopic/ultrasound findings during sniff maneuver, degree of restriction by spirometry, or supine forces. Compensatory accessory muscle use during daytime breathing can mask symptoms, and there can be severe nocturnal hypoventilation related to UDP.
For symptomatic patients, treatment recommendations require understanding of the etiology and the likelihood of resolution vs progression, or association with progressive systemic conditions. Nighttime noninvasive ventilation (NIV) is considered useful since diaphragmatic weakness worsens in supine position, and hypoventilation during REM sleep without accessory muscle support is exacerbated (Steier J, et al. Eur Respir J. 2008;32[6]:1479). However, evidence for NIV in UDP remains low quality. NIV has been proposed for ventilatory support particularly when hypercapnia is present (Wiebel M, et al. Med Klin. 1995;90[1 Suppl 1]:20). For patients with progressive neuromuscular conditions, NIV with a backup rate is strongly recommended (Steindor M, et al. Respir Care. 2021;66[3]:410; Benditt JO. Respir Care. 2019;64[6]:679), but access to respiratory assist devices is limited for isolated UDP under current reimbursement algorithms without demonstrable hypercapnia or significant restrictive spirometry. The recent ONMAP recommendations calling for use of symptom severity to support initiating NIV if FVC>80% have not yet been adopted (Morgenthaler TI, et al. Chest. 2021;160[5]:e419). Without marked spirometric restriction or hypercapnia, most patients must fail conservative PAP therapy prior to escalation to NIV, and initiation of a backup rate remains debated. Nevertheless, the only large case series evaluating the predominant features of polysomnography in UDP suggests high incidence of central apneas, suggesting a backup rate may indeed be required independent of the need to support neuromuscular function (Singh M, et al. Can J Anesthesiology. 2021;68[7]:1064). Further assessment of the features, needs, and understanding of the natural trajectory is essential to guide approach to sleep-related hypoventilation in UDP.
Landy V. Luna Diaz
Section Fellow-in-Training
Bethany L. Lussier, MD, FCCP
Section Member-at-Large
Challenges in developing effective treatments for idiopathic pulmonary fibrosis: Lessons from the ISABELA trials
Diffuse Lung Disease & Lung Transplant Network
Interstitial Lung Disease Section
Idiopathic pulmonary fibrosis (IPF) is a fatal lung disease that affects an estimated 100,000 people in the United States alone. Despite the availability of two approved antifibrotic drugs, nintedanib and pirfenidone, there is still a need for effective treatments to improve patient outcomes.
The ISABELA 1 and 2 trials were two Phase III clinical trials designed to evaluate ziritaxestat, a novel autotaxin inhibitor, in patients with IPF. Unfortunately, both trials were terminated early after an interim analysis revealed a lack of efficacy and safety concerns. Specifically, neither dose of ziritaxestat showed any benefit on the rate of decline for FVC over 52 weeks. Moreover, the treatment with ziritaxestat showed no benefit on the reported secondary outcomes. Patients in the ziritaxestat groups experienced worse outcomes in terms of time to first respiratory- related hospitalization, respiratory- related mortality, and first acute IPF exacerbation. Pooled data for both trials showed higher all-cause mortality for the ziritaxestat groups in relation to placebo (Maher T, et al. JAMA. 2023;329[18]:1567).
These disappointing results highlight the challenges of developing effective treatments for IPF. The complexity of IPF as a disease, with multiple pathways contributing to its pathogenesis, makes it difficult to identify effective therapeutic targets. In addition, clinical trials for new treatments must also account for the availability of approved antifibrotic therapies, which creates an added challenge for clinical trial design.
Matthew Huang, MD
Section Fellow-in-Training
Brad Bemiss, MD
Section Member-at-Large
Diffuse Lung Disease & Lung Transplant Network
Interstitial Lung Disease Section
Idiopathic pulmonary fibrosis (IPF) is a fatal lung disease that affects an estimated 100,000 people in the United States alone. Despite the availability of two approved antifibrotic drugs, nintedanib and pirfenidone, there is still a need for effective treatments to improve patient outcomes.
The ISABELA 1 and 2 trials were two Phase III clinical trials designed to evaluate ziritaxestat, a novel autotaxin inhibitor, in patients with IPF. Unfortunately, both trials were terminated early after an interim analysis revealed a lack of efficacy and safety concerns. Specifically, neither dose of ziritaxestat showed any benefit on the rate of decline for FVC over 52 weeks. Moreover, the treatment with ziritaxestat showed no benefit on the reported secondary outcomes. Patients in the ziritaxestat groups experienced worse outcomes in terms of time to first respiratory- related hospitalization, respiratory- related mortality, and first acute IPF exacerbation. Pooled data for both trials showed higher all-cause mortality for the ziritaxestat groups in relation to placebo (Maher T, et al. JAMA. 2023;329[18]:1567).
These disappointing results highlight the challenges of developing effective treatments for IPF. The complexity of IPF as a disease, with multiple pathways contributing to its pathogenesis, makes it difficult to identify effective therapeutic targets. In addition, clinical trials for new treatments must also account for the availability of approved antifibrotic therapies, which creates an added challenge for clinical trial design.
Matthew Huang, MD
Section Fellow-in-Training
Brad Bemiss, MD
Section Member-at-Large
Diffuse Lung Disease & Lung Transplant Network
Interstitial Lung Disease Section
Idiopathic pulmonary fibrosis (IPF) is a fatal lung disease that affects an estimated 100,000 people in the United States alone. Despite the availability of two approved antifibrotic drugs, nintedanib and pirfenidone, there is still a need for effective treatments to improve patient outcomes.
The ISABELA 1 and 2 trials were two Phase III clinical trials designed to evaluate ziritaxestat, a novel autotaxin inhibitor, in patients with IPF. Unfortunately, both trials were terminated early after an interim analysis revealed a lack of efficacy and safety concerns. Specifically, neither dose of ziritaxestat showed any benefit on the rate of decline for FVC over 52 weeks. Moreover, the treatment with ziritaxestat showed no benefit on the reported secondary outcomes. Patients in the ziritaxestat groups experienced worse outcomes in terms of time to first respiratory- related hospitalization, respiratory- related mortality, and first acute IPF exacerbation. Pooled data for both trials showed higher all-cause mortality for the ziritaxestat groups in relation to placebo (Maher T, et al. JAMA. 2023;329[18]:1567).
These disappointing results highlight the challenges of developing effective treatments for IPF. The complexity of IPF as a disease, with multiple pathways contributing to its pathogenesis, makes it difficult to identify effective therapeutic targets. In addition, clinical trials for new treatments must also account for the availability of approved antifibrotic therapies, which creates an added challenge for clinical trial design.
Matthew Huang, MD
Section Fellow-in-Training
Brad Bemiss, MD
Section Member-at-Large
Transitioning from pediatric to adult care
Airways Disorders Network
Pediatric Chest Medicine Section
For young adults with chronic health conditions, the process of transitioning to adult health care is complicated, resulting in frustration for patients and families, and clinicians, as well as increased morbidity and mortality (Varty et al. J Pediatr Nurs. 2020;55:201). As such, there have been efforts to determine practices that can minimize risk and improve satisfaction with the transition process.
The National Alliance to Advance Adolescent Health developed the “Got Transition” program with input from pediatric and adult clinicians, as well as patient advocates (White, et al. Six Core Elements of Health Care Transition™ 3.0. Washington, DC: Got Transition, The National Alliance to Advance Adolescent Health, July 2020). CF R.I.S.E is a similar program aimed specifically at improving the transition to adult care among patients with cystic fibrosis (www.cfrise.com). Got Transition provides the following recommendations pertinent to both pediatric and adult providers.
Pediatric clinics should start to assess transition readiness in early adolescence, and provide training pertinent to any skill gaps identified. This may include knowledge about condition-specific self-care skills, as well as navigation of the health care system. An individualized plan can then be developed, including timing of transition and identification of an appropriate adult provider.
The transfer should include communication between the pediatric and adult care providers prior to and, if needed, after the patient’s first appointment with the adult provider. Adult clinics can enhance the transition process by establishing a method to welcome transitioning young adult patients and orient them to the practice, addressing patient concerns regarding the transition, and assessing the patients’ self-management skills with resources provided, as needed.
Both pediatric and adult providers have a role in helping patients transition safely and smoothly from pediatric to adult care.
Sarah Cohen, MD
Section Fellow-in-Training
Airways Disorders Network
Pediatric Chest Medicine Section
For young adults with chronic health conditions, the process of transitioning to adult health care is complicated, resulting in frustration for patients and families, and clinicians, as well as increased morbidity and mortality (Varty et al. J Pediatr Nurs. 2020;55:201). As such, there have been efforts to determine practices that can minimize risk and improve satisfaction with the transition process.
The National Alliance to Advance Adolescent Health developed the “Got Transition” program with input from pediatric and adult clinicians, as well as patient advocates (White, et al. Six Core Elements of Health Care Transition™ 3.0. Washington, DC: Got Transition, The National Alliance to Advance Adolescent Health, July 2020). CF R.I.S.E is a similar program aimed specifically at improving the transition to adult care among patients with cystic fibrosis (www.cfrise.com). Got Transition provides the following recommendations pertinent to both pediatric and adult providers.
Pediatric clinics should start to assess transition readiness in early adolescence, and provide training pertinent to any skill gaps identified. This may include knowledge about condition-specific self-care skills, as well as navigation of the health care system. An individualized plan can then be developed, including timing of transition and identification of an appropriate adult provider.
The transfer should include communication between the pediatric and adult care providers prior to and, if needed, after the patient’s first appointment with the adult provider. Adult clinics can enhance the transition process by establishing a method to welcome transitioning young adult patients and orient them to the practice, addressing patient concerns regarding the transition, and assessing the patients’ self-management skills with resources provided, as needed.
Both pediatric and adult providers have a role in helping patients transition safely and smoothly from pediatric to adult care.
Sarah Cohen, MD
Section Fellow-in-Training
Airways Disorders Network
Pediatric Chest Medicine Section
For young adults with chronic health conditions, the process of transitioning to adult health care is complicated, resulting in frustration for patients and families, and clinicians, as well as increased morbidity and mortality (Varty et al. J Pediatr Nurs. 2020;55:201). As such, there have been efforts to determine practices that can minimize risk and improve satisfaction with the transition process.
The National Alliance to Advance Adolescent Health developed the “Got Transition” program with input from pediatric and adult clinicians, as well as patient advocates (White, et al. Six Core Elements of Health Care Transition™ 3.0. Washington, DC: Got Transition, The National Alliance to Advance Adolescent Health, July 2020). CF R.I.S.E is a similar program aimed specifically at improving the transition to adult care among patients with cystic fibrosis (www.cfrise.com). Got Transition provides the following recommendations pertinent to both pediatric and adult providers.
Pediatric clinics should start to assess transition readiness in early adolescence, and provide training pertinent to any skill gaps identified. This may include knowledge about condition-specific self-care skills, as well as navigation of the health care system. An individualized plan can then be developed, including timing of transition and identification of an appropriate adult provider.
The transfer should include communication between the pediatric and adult care providers prior to and, if needed, after the patient’s first appointment with the adult provider. Adult clinics can enhance the transition process by establishing a method to welcome transitioning young adult patients and orient them to the practice, addressing patient concerns regarding the transition, and assessing the patients’ self-management skills with resources provided, as needed.
Both pediatric and adult providers have a role in helping patients transition safely and smoothly from pediatric to adult care.
Sarah Cohen, MD
Section Fellow-in-Training