User login
The triple overlap: COPD-OSA-OHS. Is it time for new definitions?
In our current society, it is likely that the “skinny patient with COPD” who walks into your clinic is less and less your “traditional” patient with COPD. We are seeing in our health care systems more of the “blue bloaters” – patients with COPD and significant obesity. This phenotype is representing what we are seeing worldwide as a consequence of the rising obesity prevalence. In the United States, the prepandemic (2017-2020) estimated percentage of adults over the age of 40 with obesity, defined as a body mass index (BMI) of at least 30 kg/m2, was over 40%. Moreover, the estimated percentage of adults with morbid obesity (BMI at least 40 kg/m2) is close to 10% (Akinbami, LJ et al. Vital Health Stat. 2022:190:1-36) and trending up. These patients with the “triple overlap” of morbid obesity, COPD, and awake daytime hypercapnia are being seen in clinics and in-hospital settings with increasing frequency, often presenting with complicating comorbidities such as acute respiratory failure, acute heart failure, kidney disease, or pulmonary hypertension. We are now faced with managing these patients with complex disease.
The obesity paradox does not seem applicable in the triple overlap phenotype. Patients with COPD who are overweight, defined as “mild obesity,” have lower mortality when compared with normal weight and underweight patients with COPD; however, this effect diminishes when BMI increases beyond 32 kg/m2. With increasing obesity severity and aging, the risk of both obstructive sleep apnea (OSA) and hypoventilation increases. It is well documented that COPD-OSA overlap is linked to worse outcomes and that continuous positive airway pressure (CPAP) as first-line therapy decreases readmission rates and mortality. The pathophysiology of hypoventilation in obesity is complex and multifactorial, and, although significant overlaps likely exist with comorbid COPD, by current definitions, to establish a diagnosis of obesity hypoventilation syndrome (OHS), one must have excluded other causes of hypoventilation, such as COPD.
These patients with the triple overlap of morbid obesity, awake daytime hypercapnia, and COPD are the subset of patients that providers struggle to fit in a diagnosis or in clinical research trials.
The triple overlap is a distinct syndrome
Different labels have been used in the medical literature: hypercapnic OSA-COPD overlap, morbid obesity and OSA-COPD overlap, hypercapnic morbidly obese COPD and OHS-COPD overlap. A better characterization of this distinctive phenotype is much needed. Patients with OSA-COPD overlap, for example, have an increased propensity to develop hypercapnia at higher FEV1 when compared with COPD without OSA – but this is thought to be a consequence of prolonged and frequent apneas and hypopneas compounded with obesity-related central hypoventilation. We found that morbidly obese patients with OSA-COPD overlap have a higher hypoxia burden, more severe OSA, and are frequently prescribed noninvasive ventilation after a failed titration polysomnogram (Htun ZM, et al. Am J Respir Crit Care Med. 2019;199:A1382), perhaps signaling a distinctive phenotype with worse outcomes, but the study had the inherent limitations of a single-center, retrospective design lacking data on awake hypercapnia. On the other side, the term OHS-COPD is contradictory and confusing based on current OHS diagnostic criteria.
In standardizing diagnostic criteria for patients with this triple overlap syndrome, challenges remain: would the patient with a BMI of 70 kg/m2 and fixed chronic airflow obstruction with FEV1 72% fall under the category of hypercapnic COPD vs OHS? Do these patients have worse outcomes regardless of their predominant feature? Would outcomes change if the apnea hypopnea index (AHI) is 10/h vs 65/h? More importantly, do patients with the triple overlap of COPD, morbid obesity, and daytime hypercapnia have worse outcomes when compared with hypercapnic COPD, or OHS with/without OSA? These questions can be better addressed once we agree on a definition. The patients with triple overlap syndrome have been traditionally excluded from clinical trials: the patient with morbid obesity has been excluded from chronic hypercapnic COPD clinical trials, and the patient with COPD has been excluded from OHS trials.
There are no specific clinical guidelines for this triple overlap phenotype. Positive airway pressure is the mainstay of treatment. CPAP is recommended as first-line therapy for patients with OSA-COPD overlap syndrome, while noninvasive ventilation (NIV) with bilevel positive airway pressure (BPAP) is recommended as first-line for the stable ambulatory hypercapnic patient with COPD. It is unclear if NIV is superior to CPAP in patients with triple overlap syndrome, although recently published data showed greater efficacy in reducing carbon dioxide (PaCO2) and improving quality of life in a small group of subjects (Zheng et al. J Clin Sleep Med. 2022;18[1]:99-107). To take a step further, the subtleties of NIV set up, such as rise time and minimum inspiratory time, are contradictory: the goal in ventilating patients with COPD is to shorten inspiratory time, prolonging expiratory time, therefore allowing a shortened inspiratory cycle. In obesity, ventilation strategies aim to prolong and sustain inspiratory time to improve ventilation and dependent atelectasis. Another area of uncertainty is device selection. Should we aim to provide a respiratory assist device (RAD): the traditional, rent to own bilevel PAP without auto-expiratory positive airway pressure (EPAP) capabilities and lower maximum inspiratory pressure delivery capacity, vs a home mechanical ventilator at a higher expense, life-time rental, and one-way only data monitoring, which limits remote prescription adjustments, but allow auto-EPAP settings for patients with comorbid OSA? More importantly, how do we get these patients, who do not fit in any of the specified insurance criteria for PAP therapy approved for treatment?
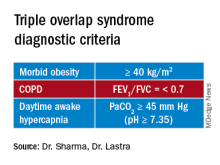
A uniform diagnostic definition and clear taxonomy allows for resource allocation, from government funded grants for clinical trials to a better-informed distribution of health care systems resources and support health care policy changes to improve patient-centric outcomes. Here, we propose that the morbidly obese patient (BMI >40 kg/m2) with chronic airflow obstruction and a forced expiratory ratio (FEV1/FVC) <0.7 with awake daytime hypercapnia (PaCO2 > 45 mm Hg) represents a different entity/phenotype and fits best under the triple overlap syndrome taxonomy.
We suspect that these patients have worse outcomes, including comorbidity burden, quality of life, exacerbation rates, longer hospital length-of-stay, and respiratory and all-cause mortality. Large, multicenter, controlled trials comparing the long-term effectiveness of NIV and CPAP: measurements of respiratory function, gas exchange, blood pressure, and health related quality of life are needed. This is a group of patients that may specifically benefit from volume-targeted pressure support mode ventilation with auto-EPAP capabilities upon discharge from the hospital after an acute exacerbation.
Inpatient (sleep medicine) and outpatient transitions
In patients hospitalized with the triple overlap syndrome, there are certain considerations that are of special interest. Given comorbid hypercapnia and limited data on NIV superiority over CPAP, a sleep study should not be needed for NIV qualification. In addition, the medical team may consider the following (Figure 1):
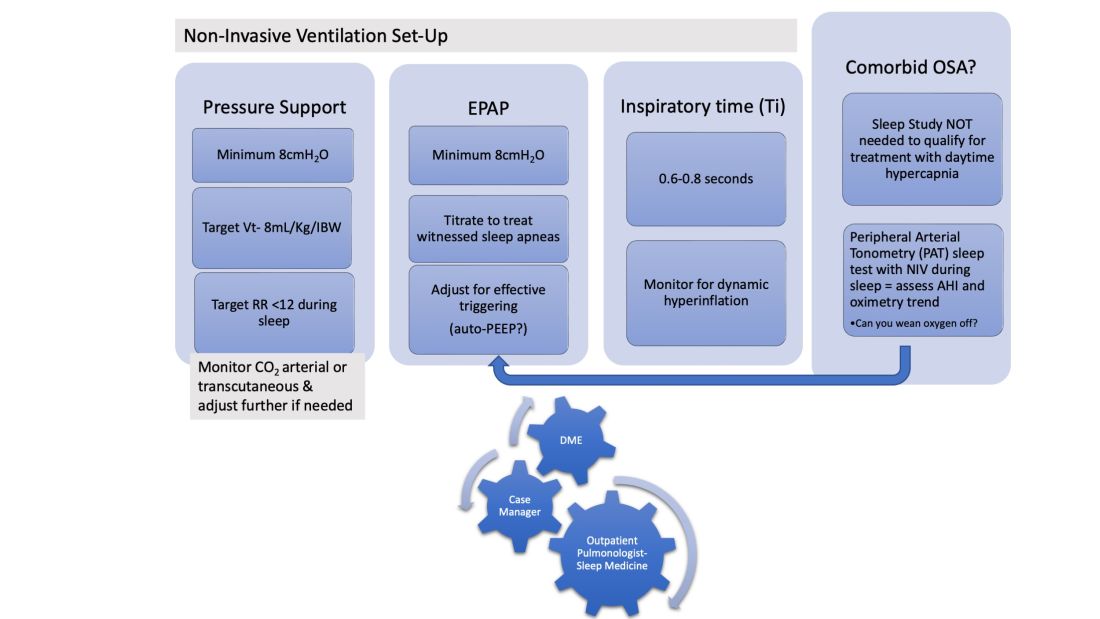
1. Noninvasive Ventilation:
a. Maintaining a high-pressure support differential between inspiratory positive airway pressure (IPAP) and EPAP. This can usually be achieved at 8-10 cm H2O, further adjusting to target a tidal volume (Vt) of 8 mL/kg of ideal body weight (IBW).
b. Higher EPAP: To overcome dependent atelectasis, improve ventilation-perfusion (VQ) matching, and better treat upper airway resistance both during wakefulness and sleep. Also, adjustments of EPAP at bedside should be considered to counteract auto-PEEP-related ineffective triggering if observed.
c. OSA screening and EPAP adjustment: for high residual obstructive apneas or hypopneas if data are available on the NIV device, or with the use of peripheral arterial tonometry sleep testing devices with NIV on overnight before discharge.
d. Does the patient meet criteria for oxygen supplementation at home? Wean oxygen off, if possible.
2. Case-managers can help establish services with a durable medical equipment provider with expertise in advanced PAP devices.3. Obesity management, Consider referral to an obesity management program for lifestyle/dietary modifications along with pharmacotherapy or bariatric surgery interventions.
4. Close follow-up, track exacerbations. Device download data are crucial to monitor adherence/tolerance and treatment effectiveness with particular interest in AHI, oximetry, and CO2 trends monitoring. Some patients may need dedicated titration polysomnograms to adjust ventilation settings, for optimization of residual OSA or for oxygen addition or discontinuation.
Conclusion
Patients with the triple overlap phenotype have not been systematically defined, studied, or included in clinical trials. We anticipate that these patients have worse outcomes: quality of life, symptom and comorbidity burden, exacerbation rates, in-hospital mortality, longer hospital stay and ICU stay, and respiratory and all-cause mortality. This is a group of patients that may specifically benefit from domiciliary NIV set-up upon discharge from the hospital with close follow-up. Properly identifying these patients will help pulmonologists and health care systems direct resources to optimally manage this complex group of patients. Funding of research trials to support clinical guidelines development should be prioritized. Triple overlap syndrome is different from COPD-OSA overlap, OHS with moderate to severe OSA, or OHS without significant OSA.
In our current society, it is likely that the “skinny patient with COPD” who walks into your clinic is less and less your “traditional” patient with COPD. We are seeing in our health care systems more of the “blue bloaters” – patients with COPD and significant obesity. This phenotype is representing what we are seeing worldwide as a consequence of the rising obesity prevalence. In the United States, the prepandemic (2017-2020) estimated percentage of adults over the age of 40 with obesity, defined as a body mass index (BMI) of at least 30 kg/m2, was over 40%. Moreover, the estimated percentage of adults with morbid obesity (BMI at least 40 kg/m2) is close to 10% (Akinbami, LJ et al. Vital Health Stat. 2022:190:1-36) and trending up. These patients with the “triple overlap” of morbid obesity, COPD, and awake daytime hypercapnia are being seen in clinics and in-hospital settings with increasing frequency, often presenting with complicating comorbidities such as acute respiratory failure, acute heart failure, kidney disease, or pulmonary hypertension. We are now faced with managing these patients with complex disease.
The obesity paradox does not seem applicable in the triple overlap phenotype. Patients with COPD who are overweight, defined as “mild obesity,” have lower mortality when compared with normal weight and underweight patients with COPD; however, this effect diminishes when BMI increases beyond 32 kg/m2. With increasing obesity severity and aging, the risk of both obstructive sleep apnea (OSA) and hypoventilation increases. It is well documented that COPD-OSA overlap is linked to worse outcomes and that continuous positive airway pressure (CPAP) as first-line therapy decreases readmission rates and mortality. The pathophysiology of hypoventilation in obesity is complex and multifactorial, and, although significant overlaps likely exist with comorbid COPD, by current definitions, to establish a diagnosis of obesity hypoventilation syndrome (OHS), one must have excluded other causes of hypoventilation, such as COPD.
These patients with the triple overlap of morbid obesity, awake daytime hypercapnia, and COPD are the subset of patients that providers struggle to fit in a diagnosis or in clinical research trials.
The triple overlap is a distinct syndrome
Different labels have been used in the medical literature: hypercapnic OSA-COPD overlap, morbid obesity and OSA-COPD overlap, hypercapnic morbidly obese COPD and OHS-COPD overlap. A better characterization of this distinctive phenotype is much needed. Patients with OSA-COPD overlap, for example, have an increased propensity to develop hypercapnia at higher FEV1 when compared with COPD without OSA – but this is thought to be a consequence of prolonged and frequent apneas and hypopneas compounded with obesity-related central hypoventilation. We found that morbidly obese patients with OSA-COPD overlap have a higher hypoxia burden, more severe OSA, and are frequently prescribed noninvasive ventilation after a failed titration polysomnogram (Htun ZM, et al. Am J Respir Crit Care Med. 2019;199:A1382), perhaps signaling a distinctive phenotype with worse outcomes, but the study had the inherent limitations of a single-center, retrospective design lacking data on awake hypercapnia. On the other side, the term OHS-COPD is contradictory and confusing based on current OHS diagnostic criteria.
In standardizing diagnostic criteria for patients with this triple overlap syndrome, challenges remain: would the patient with a BMI of 70 kg/m2 and fixed chronic airflow obstruction with FEV1 72% fall under the category of hypercapnic COPD vs OHS? Do these patients have worse outcomes regardless of their predominant feature? Would outcomes change if the apnea hypopnea index (AHI) is 10/h vs 65/h? More importantly, do patients with the triple overlap of COPD, morbid obesity, and daytime hypercapnia have worse outcomes when compared with hypercapnic COPD, or OHS with/without OSA? These questions can be better addressed once we agree on a definition. The patients with triple overlap syndrome have been traditionally excluded from clinical trials: the patient with morbid obesity has been excluded from chronic hypercapnic COPD clinical trials, and the patient with COPD has been excluded from OHS trials.
There are no specific clinical guidelines for this triple overlap phenotype. Positive airway pressure is the mainstay of treatment. CPAP is recommended as first-line therapy for patients with OSA-COPD overlap syndrome, while noninvasive ventilation (NIV) with bilevel positive airway pressure (BPAP) is recommended as first-line for the stable ambulatory hypercapnic patient with COPD. It is unclear if NIV is superior to CPAP in patients with triple overlap syndrome, although recently published data showed greater efficacy in reducing carbon dioxide (PaCO2) and improving quality of life in a small group of subjects (Zheng et al. J Clin Sleep Med. 2022;18[1]:99-107). To take a step further, the subtleties of NIV set up, such as rise time and minimum inspiratory time, are contradictory: the goal in ventilating patients with COPD is to shorten inspiratory time, prolonging expiratory time, therefore allowing a shortened inspiratory cycle. In obesity, ventilation strategies aim to prolong and sustain inspiratory time to improve ventilation and dependent atelectasis. Another area of uncertainty is device selection. Should we aim to provide a respiratory assist device (RAD): the traditional, rent to own bilevel PAP without auto-expiratory positive airway pressure (EPAP) capabilities and lower maximum inspiratory pressure delivery capacity, vs a home mechanical ventilator at a higher expense, life-time rental, and one-way only data monitoring, which limits remote prescription adjustments, but allow auto-EPAP settings for patients with comorbid OSA? More importantly, how do we get these patients, who do not fit in any of the specified insurance criteria for PAP therapy approved for treatment?
A uniform diagnostic definition and clear taxonomy allows for resource allocation, from government funded grants for clinical trials to a better-informed distribution of health care systems resources and support health care policy changes to improve patient-centric outcomes. Here, we propose that the morbidly obese patient (BMI >40 kg/m2) with chronic airflow obstruction and a forced expiratory ratio (FEV1/FVC) <0.7 with awake daytime hypercapnia (PaCO2 > 45 mm Hg) represents a different entity/phenotype and fits best under the triple overlap syndrome taxonomy.
We suspect that these patients have worse outcomes, including comorbidity burden, quality of life, exacerbation rates, longer hospital length-of-stay, and respiratory and all-cause mortality. Large, multicenter, controlled trials comparing the long-term effectiveness of NIV and CPAP: measurements of respiratory function, gas exchange, blood pressure, and health related quality of life are needed. This is a group of patients that may specifically benefit from volume-targeted pressure support mode ventilation with auto-EPAP capabilities upon discharge from the hospital after an acute exacerbation.
Inpatient (sleep medicine) and outpatient transitions
In patients hospitalized with the triple overlap syndrome, there are certain considerations that are of special interest. Given comorbid hypercapnia and limited data on NIV superiority over CPAP, a sleep study should not be needed for NIV qualification. In addition, the medical team may consider the following (Figure 1):

1. Noninvasive Ventilation:
a. Maintaining a high-pressure support differential between inspiratory positive airway pressure (IPAP) and EPAP. This can usually be achieved at 8-10 cm H2O, further adjusting to target a tidal volume (Vt) of 8 mL/kg of ideal body weight (IBW).
b. Higher EPAP: To overcome dependent atelectasis, improve ventilation-perfusion (VQ) matching, and better treat upper airway resistance both during wakefulness and sleep. Also, adjustments of EPAP at bedside should be considered to counteract auto-PEEP-related ineffective triggering if observed.
c. OSA screening and EPAP adjustment: for high residual obstructive apneas or hypopneas if data are available on the NIV device, or with the use of peripheral arterial tonometry sleep testing devices with NIV on overnight before discharge.
d. Does the patient meet criteria for oxygen supplementation at home? Wean oxygen off, if possible.
2. Case-managers can help establish services with a durable medical equipment provider with expertise in advanced PAP devices.3. Obesity management, Consider referral to an obesity management program for lifestyle/dietary modifications along with pharmacotherapy or bariatric surgery interventions.
4. Close follow-up, track exacerbations. Device download data are crucial to monitor adherence/tolerance and treatment effectiveness with particular interest in AHI, oximetry, and CO2 trends monitoring. Some patients may need dedicated titration polysomnograms to adjust ventilation settings, for optimization of residual OSA or for oxygen addition or discontinuation.
Conclusion
Patients with the triple overlap phenotype have not been systematically defined, studied, or included in clinical trials. We anticipate that these patients have worse outcomes: quality of life, symptom and comorbidity burden, exacerbation rates, in-hospital mortality, longer hospital stay and ICU stay, and respiratory and all-cause mortality. This is a group of patients that may specifically benefit from domiciliary NIV set-up upon discharge from the hospital with close follow-up. Properly identifying these patients will help pulmonologists and health care systems direct resources to optimally manage this complex group of patients. Funding of research trials to support clinical guidelines development should be prioritized. Triple overlap syndrome is different from COPD-OSA overlap, OHS with moderate to severe OSA, or OHS without significant OSA.
In our current society, it is likely that the “skinny patient with COPD” who walks into your clinic is less and less your “traditional” patient with COPD. We are seeing in our health care systems more of the “blue bloaters” – patients with COPD and significant obesity. This phenotype is representing what we are seeing worldwide as a consequence of the rising obesity prevalence. In the United States, the prepandemic (2017-2020) estimated percentage of adults over the age of 40 with obesity, defined as a body mass index (BMI) of at least 30 kg/m2, was over 40%. Moreover, the estimated percentage of adults with morbid obesity (BMI at least 40 kg/m2) is close to 10% (Akinbami, LJ et al. Vital Health Stat. 2022:190:1-36) and trending up. These patients with the “triple overlap” of morbid obesity, COPD, and awake daytime hypercapnia are being seen in clinics and in-hospital settings with increasing frequency, often presenting with complicating comorbidities such as acute respiratory failure, acute heart failure, kidney disease, or pulmonary hypertension. We are now faced with managing these patients with complex disease.
The obesity paradox does not seem applicable in the triple overlap phenotype. Patients with COPD who are overweight, defined as “mild obesity,” have lower mortality when compared with normal weight and underweight patients with COPD; however, this effect diminishes when BMI increases beyond 32 kg/m2. With increasing obesity severity and aging, the risk of both obstructive sleep apnea (OSA) and hypoventilation increases. It is well documented that COPD-OSA overlap is linked to worse outcomes and that continuous positive airway pressure (CPAP) as first-line therapy decreases readmission rates and mortality. The pathophysiology of hypoventilation in obesity is complex and multifactorial, and, although significant overlaps likely exist with comorbid COPD, by current definitions, to establish a diagnosis of obesity hypoventilation syndrome (OHS), one must have excluded other causes of hypoventilation, such as COPD.
These patients with the triple overlap of morbid obesity, awake daytime hypercapnia, and COPD are the subset of patients that providers struggle to fit in a diagnosis or in clinical research trials.
The triple overlap is a distinct syndrome
Different labels have been used in the medical literature: hypercapnic OSA-COPD overlap, morbid obesity and OSA-COPD overlap, hypercapnic morbidly obese COPD and OHS-COPD overlap. A better characterization of this distinctive phenotype is much needed. Patients with OSA-COPD overlap, for example, have an increased propensity to develop hypercapnia at higher FEV1 when compared with COPD without OSA – but this is thought to be a consequence of prolonged and frequent apneas and hypopneas compounded with obesity-related central hypoventilation. We found that morbidly obese patients with OSA-COPD overlap have a higher hypoxia burden, more severe OSA, and are frequently prescribed noninvasive ventilation after a failed titration polysomnogram (Htun ZM, et al. Am J Respir Crit Care Med. 2019;199:A1382), perhaps signaling a distinctive phenotype with worse outcomes, but the study had the inherent limitations of a single-center, retrospective design lacking data on awake hypercapnia. On the other side, the term OHS-COPD is contradictory and confusing based on current OHS diagnostic criteria.
In standardizing diagnostic criteria for patients with this triple overlap syndrome, challenges remain: would the patient with a BMI of 70 kg/m2 and fixed chronic airflow obstruction with FEV1 72% fall under the category of hypercapnic COPD vs OHS? Do these patients have worse outcomes regardless of their predominant feature? Would outcomes change if the apnea hypopnea index (AHI) is 10/h vs 65/h? More importantly, do patients with the triple overlap of COPD, morbid obesity, and daytime hypercapnia have worse outcomes when compared with hypercapnic COPD, or OHS with/without OSA? These questions can be better addressed once we agree on a definition. The patients with triple overlap syndrome have been traditionally excluded from clinical trials: the patient with morbid obesity has been excluded from chronic hypercapnic COPD clinical trials, and the patient with COPD has been excluded from OHS trials.
There are no specific clinical guidelines for this triple overlap phenotype. Positive airway pressure is the mainstay of treatment. CPAP is recommended as first-line therapy for patients with OSA-COPD overlap syndrome, while noninvasive ventilation (NIV) with bilevel positive airway pressure (BPAP) is recommended as first-line for the stable ambulatory hypercapnic patient with COPD. It is unclear if NIV is superior to CPAP in patients with triple overlap syndrome, although recently published data showed greater efficacy in reducing carbon dioxide (PaCO2) and improving quality of life in a small group of subjects (Zheng et al. J Clin Sleep Med. 2022;18[1]:99-107). To take a step further, the subtleties of NIV set up, such as rise time and minimum inspiratory time, are contradictory: the goal in ventilating patients with COPD is to shorten inspiratory time, prolonging expiratory time, therefore allowing a shortened inspiratory cycle. In obesity, ventilation strategies aim to prolong and sustain inspiratory time to improve ventilation and dependent atelectasis. Another area of uncertainty is device selection. Should we aim to provide a respiratory assist device (RAD): the traditional, rent to own bilevel PAP without auto-expiratory positive airway pressure (EPAP) capabilities and lower maximum inspiratory pressure delivery capacity, vs a home mechanical ventilator at a higher expense, life-time rental, and one-way only data monitoring, which limits remote prescription adjustments, but allow auto-EPAP settings for patients with comorbid OSA? More importantly, how do we get these patients, who do not fit in any of the specified insurance criteria for PAP therapy approved for treatment?
A uniform diagnostic definition and clear taxonomy allows for resource allocation, from government funded grants for clinical trials to a better-informed distribution of health care systems resources and support health care policy changes to improve patient-centric outcomes. Here, we propose that the morbidly obese patient (BMI >40 kg/m2) with chronic airflow obstruction and a forced expiratory ratio (FEV1/FVC) <0.7 with awake daytime hypercapnia (PaCO2 > 45 mm Hg) represents a different entity/phenotype and fits best under the triple overlap syndrome taxonomy.
We suspect that these patients have worse outcomes, including comorbidity burden, quality of life, exacerbation rates, longer hospital length-of-stay, and respiratory and all-cause mortality. Large, multicenter, controlled trials comparing the long-term effectiveness of NIV and CPAP: measurements of respiratory function, gas exchange, blood pressure, and health related quality of life are needed. This is a group of patients that may specifically benefit from volume-targeted pressure support mode ventilation with auto-EPAP capabilities upon discharge from the hospital after an acute exacerbation.
Inpatient (sleep medicine) and outpatient transitions
In patients hospitalized with the triple overlap syndrome, there are certain considerations that are of special interest. Given comorbid hypercapnia and limited data on NIV superiority over CPAP, a sleep study should not be needed for NIV qualification. In addition, the medical team may consider the following (Figure 1):

1. Noninvasive Ventilation:
a. Maintaining a high-pressure support differential between inspiratory positive airway pressure (IPAP) and EPAP. This can usually be achieved at 8-10 cm H2O, further adjusting to target a tidal volume (Vt) of 8 mL/kg of ideal body weight (IBW).
b. Higher EPAP: To overcome dependent atelectasis, improve ventilation-perfusion (VQ) matching, and better treat upper airway resistance both during wakefulness and sleep. Also, adjustments of EPAP at bedside should be considered to counteract auto-PEEP-related ineffective triggering if observed.
c. OSA screening and EPAP adjustment: for high residual obstructive apneas or hypopneas if data are available on the NIV device, or with the use of peripheral arterial tonometry sleep testing devices with NIV on overnight before discharge.
d. Does the patient meet criteria for oxygen supplementation at home? Wean oxygen off, if possible.
2. Case-managers can help establish services with a durable medical equipment provider with expertise in advanced PAP devices.3. Obesity management, Consider referral to an obesity management program for lifestyle/dietary modifications along with pharmacotherapy or bariatric surgery interventions.
4. Close follow-up, track exacerbations. Device download data are crucial to monitor adherence/tolerance and treatment effectiveness with particular interest in AHI, oximetry, and CO2 trends monitoring. Some patients may need dedicated titration polysomnograms to adjust ventilation settings, for optimization of residual OSA or for oxygen addition or discontinuation.
Conclusion
Patients with the triple overlap phenotype have not been systematically defined, studied, or included in clinical trials. We anticipate that these patients have worse outcomes: quality of life, symptom and comorbidity burden, exacerbation rates, in-hospital mortality, longer hospital stay and ICU stay, and respiratory and all-cause mortality. This is a group of patients that may specifically benefit from domiciliary NIV set-up upon discharge from the hospital with close follow-up. Properly identifying these patients will help pulmonologists and health care systems direct resources to optimally manage this complex group of patients. Funding of research trials to support clinical guidelines development should be prioritized. Triple overlap syndrome is different from COPD-OSA overlap, OHS with moderate to severe OSA, or OHS without significant OSA.
Introducing CHEST President-Designate John A. Howington, MD, MBA, FCCP
John A. Howington, MD, MBA, FCCP, is a cardiothoracic surgeon currently serving as Chief of Oncology Services and Chair of Thoracic Surgery at Ascension Saint Thomas Health and a professor at the University of Tennessee Health Sciences Center in Nashville, Tennessee.
Dr. Howington received his undergraduate degree from Tennessee Technological University and medical degree from the University of Tennessee. He completed his general surgery residency at the University of Missouri, Kansas City and thoracic surgery residency at Vanderbilt University Medical Center.
Most recently, he received his Physician Executive MBA from the University of Tennessee.
As a passionate thoracic surgeon, he has lent his knowledge to the extensive CHEST lung cancer guideline portfolio for more than a decade. He offers regular leadership in multidisciplinary and executive forums and has spearheaded a series of quality improvement initiatives at Ascension. He has served in a variety of leadership roles with CHEST and with other national thoracic surgery societies.
Dr. Howington began his CHEST leadership journey with the Networks, as a member of the Interventional Chest Medicine Steering Committee and then as the Thoracic Oncology Network Chair (2008-2010).
Other leadership positions include serving as the President of the CHEST Foundation (2014-2016), member of the Scientific Program Committee and Membership Committee, and, recently, as the Chair of the Finance Committee from 2018-2021.
Since 2017, he has served on the Board of Regents as a Member at Large. Dr. Howington will serve as the 87th CHEST President in 2025.
John A. Howington, MD, MBA, FCCP, is a cardiothoracic surgeon currently serving as Chief of Oncology Services and Chair of Thoracic Surgery at Ascension Saint Thomas Health and a professor at the University of Tennessee Health Sciences Center in Nashville, Tennessee.
Dr. Howington received his undergraduate degree from Tennessee Technological University and medical degree from the University of Tennessee. He completed his general surgery residency at the University of Missouri, Kansas City and thoracic surgery residency at Vanderbilt University Medical Center.
Most recently, he received his Physician Executive MBA from the University of Tennessee.
As a passionate thoracic surgeon, he has lent his knowledge to the extensive CHEST lung cancer guideline portfolio for more than a decade. He offers regular leadership in multidisciplinary and executive forums and has spearheaded a series of quality improvement initiatives at Ascension. He has served in a variety of leadership roles with CHEST and with other national thoracic surgery societies.
Dr. Howington began his CHEST leadership journey with the Networks, as a member of the Interventional Chest Medicine Steering Committee and then as the Thoracic Oncology Network Chair (2008-2010).
Other leadership positions include serving as the President of the CHEST Foundation (2014-2016), member of the Scientific Program Committee and Membership Committee, and, recently, as the Chair of the Finance Committee from 2018-2021.
Since 2017, he has served on the Board of Regents as a Member at Large. Dr. Howington will serve as the 87th CHEST President in 2025.
John A. Howington, MD, MBA, FCCP, is a cardiothoracic surgeon currently serving as Chief of Oncology Services and Chair of Thoracic Surgery at Ascension Saint Thomas Health and a professor at the University of Tennessee Health Sciences Center in Nashville, Tennessee.
Dr. Howington received his undergraduate degree from Tennessee Technological University and medical degree from the University of Tennessee. He completed his general surgery residency at the University of Missouri, Kansas City and thoracic surgery residency at Vanderbilt University Medical Center.
Most recently, he received his Physician Executive MBA from the University of Tennessee.
As a passionate thoracic surgeon, he has lent his knowledge to the extensive CHEST lung cancer guideline portfolio for more than a decade. He offers regular leadership in multidisciplinary and executive forums and has spearheaded a series of quality improvement initiatives at Ascension. He has served in a variety of leadership roles with CHEST and with other national thoracic surgery societies.
Dr. Howington began his CHEST leadership journey with the Networks, as a member of the Interventional Chest Medicine Steering Committee and then as the Thoracic Oncology Network Chair (2008-2010).
Other leadership positions include serving as the President of the CHEST Foundation (2014-2016), member of the Scientific Program Committee and Membership Committee, and, recently, as the Chair of the Finance Committee from 2018-2021.
Since 2017, he has served on the Board of Regents as a Member at Large. Dr. Howington will serve as the 87th CHEST President in 2025.
Critical Care Network
Mechanical Ventilation and Airways Section
Noninvasive ventilation
Noninvasive ventilation (NIV) is a ventilation modality that supports breathing by using mechanically assisted breaths without the need for intubation or a surgical airway. NIV is divided into two main types, negative-pressure ventilation (NPV) and noninvasive positive-pressure ventilation (NIPPV).
NPV
NPV periodically generates a negative (subatmospheric) pressure on the thorax wall, reflecting the natural breathing mechanism. As this negative pressure is transmitted into the thorax, normal atmospheric pressure air outside the thorax is pulled in for inhalation. Initiated by the negative pressure generator switching off, exhalation is passive due to elastic recoil of the lung and chest wall. The iron lung was a neck-to-toe horizontal cylinder used for NPV during the polio epidemic. New NPV devices are designed to fit the thorax only, using a cuirass (a torso-covering body armor molded shell).
For years, NPV use declined as NIPPV use increased. However, during the shortage of NIPPV devices during COVID and a recent recall of certain CPAP devices, NPV use has increased. NPV is an excellent alternative for those who cannot tolerate a facial mask due to facial deformity, claustrophobia, or excessive airway secretion (Corrado A et al. European Resp J. 2002;20[1]:187).
NIPPV
NIPPV is divided into several subtypes, including continuous positive airway pressure (CPAP), bilevel positive airway pressure (BPAP or BiPAP), and average volume-assured pressure support (AVAPS or VAPS). CPAP is defined as a single pressure delivered in inhalation (Pi) and exhalation (Pe). The increased mean airway pressure provides improved oxygenation (O2) but not ventilation (CO2). BPAP uses dual pressures with Pi higher than Pe. The increased mean airway pressure provides improved O2 while the difference between Pi minus Pe increases ventilation and decreases CO2.
AVAPS is a form of BPAP where Pi varies in an automated range to achieve the ordered tidal volume. In AVAPS, the generator adjusts Pi based on the average delivered tidal volume. If the average delivered tidal volume is less than the set tidal volume, Pi gradually increases while not exceeding Pi Max. Patients notice improved comfort of AVAPS with a variable Pi vs. BPAP with a fixed Pi (Frank A et al. Chest. 2018;154[4]:1060A).
Samantha Tauscher, DO, Resident-in-Training
Herbert Patrick, MD, MSEE, FCCP , Member-at-Large
Mechanical Ventilation and Airways Section
Noninvasive ventilation
Noninvasive ventilation (NIV) is a ventilation modality that supports breathing by using mechanically assisted breaths without the need for intubation or a surgical airway. NIV is divided into two main types, negative-pressure ventilation (NPV) and noninvasive positive-pressure ventilation (NIPPV).
NPV
NPV periodically generates a negative (subatmospheric) pressure on the thorax wall, reflecting the natural breathing mechanism. As this negative pressure is transmitted into the thorax, normal atmospheric pressure air outside the thorax is pulled in for inhalation. Initiated by the negative pressure generator switching off, exhalation is passive due to elastic recoil of the lung and chest wall. The iron lung was a neck-to-toe horizontal cylinder used for NPV during the polio epidemic. New NPV devices are designed to fit the thorax only, using a cuirass (a torso-covering body armor molded shell).
For years, NPV use declined as NIPPV use increased. However, during the shortage of NIPPV devices during COVID and a recent recall of certain CPAP devices, NPV use has increased. NPV is an excellent alternative for those who cannot tolerate a facial mask due to facial deformity, claustrophobia, or excessive airway secretion (Corrado A et al. European Resp J. 2002;20[1]:187).
NIPPV
NIPPV is divided into several subtypes, including continuous positive airway pressure (CPAP), bilevel positive airway pressure (BPAP or BiPAP), and average volume-assured pressure support (AVAPS or VAPS). CPAP is defined as a single pressure delivered in inhalation (Pi) and exhalation (Pe). The increased mean airway pressure provides improved oxygenation (O2) but not ventilation (CO2). BPAP uses dual pressures with Pi higher than Pe. The increased mean airway pressure provides improved O2 while the difference between Pi minus Pe increases ventilation and decreases CO2.
AVAPS is a form of BPAP where Pi varies in an automated range to achieve the ordered tidal volume. In AVAPS, the generator adjusts Pi based on the average delivered tidal volume. If the average delivered tidal volume is less than the set tidal volume, Pi gradually increases while not exceeding Pi Max. Patients notice improved comfort of AVAPS with a variable Pi vs. BPAP with a fixed Pi (Frank A et al. Chest. 2018;154[4]:1060A).
Samantha Tauscher, DO, Resident-in-Training
Herbert Patrick, MD, MSEE, FCCP , Member-at-Large
Mechanical Ventilation and Airways Section
Noninvasive ventilation
Noninvasive ventilation (NIV) is a ventilation modality that supports breathing by using mechanically assisted breaths without the need for intubation or a surgical airway. NIV is divided into two main types, negative-pressure ventilation (NPV) and noninvasive positive-pressure ventilation (NIPPV).
NPV
NPV periodically generates a negative (subatmospheric) pressure on the thorax wall, reflecting the natural breathing mechanism. As this negative pressure is transmitted into the thorax, normal atmospheric pressure air outside the thorax is pulled in for inhalation. Initiated by the negative pressure generator switching off, exhalation is passive due to elastic recoil of the lung and chest wall. The iron lung was a neck-to-toe horizontal cylinder used for NPV during the polio epidemic. New NPV devices are designed to fit the thorax only, using a cuirass (a torso-covering body armor molded shell).
For years, NPV use declined as NIPPV use increased. However, during the shortage of NIPPV devices during COVID and a recent recall of certain CPAP devices, NPV use has increased. NPV is an excellent alternative for those who cannot tolerate a facial mask due to facial deformity, claustrophobia, or excessive airway secretion (Corrado A et al. European Resp J. 2002;20[1]:187).
NIPPV
NIPPV is divided into several subtypes, including continuous positive airway pressure (CPAP), bilevel positive airway pressure (BPAP or BiPAP), and average volume-assured pressure support (AVAPS or VAPS). CPAP is defined as a single pressure delivered in inhalation (Pi) and exhalation (Pe). The increased mean airway pressure provides improved oxygenation (O2) but not ventilation (CO2). BPAP uses dual pressures with Pi higher than Pe. The increased mean airway pressure provides improved O2 while the difference between Pi minus Pe increases ventilation and decreases CO2.
AVAPS is a form of BPAP where Pi varies in an automated range to achieve the ordered tidal volume. In AVAPS, the generator adjusts Pi based on the average delivered tidal volume. If the average delivered tidal volume is less than the set tidal volume, Pi gradually increases while not exceeding Pi Max. Patients notice improved comfort of AVAPS with a variable Pi vs. BPAP with a fixed Pi (Frank A et al. Chest. 2018;154[4]:1060A).
Samantha Tauscher, DO, Resident-in-Training
Herbert Patrick, MD, MSEE, FCCP , Member-at-Large
Delays in diagnosing IPF. Noninvasive ventilation. BPA and CTEPH.
Diffuse Lung Disease & Transplant Network
Interstitial Lung Disease Section
Delay in diagnosis of IPF: How bad is the problem?
Idiopathic pulmonary fibrosis (IPF) is a devastating disease with a poor prognosis. Antifibrotic therapies for IPF are only capable of slowing disease progression without reversing established fibrosis. As such, the therapeutic efficacy of antifibrotic therapy may be reduced in patients whose diagnosis is delayed.
Unfortunately, diagnostic delay is common in IPF. Studies demonstrate that IPF diagnosis is delayed by more than a year after symptom onset in 43% of subjects, and more than 3 years in 19% of subjects (Cosgrove GP et al. BMC Pulm Med. 2018;18[9]). Approximately one-third of patients with IPF have undergone chest CT imaging more than 3 years prior to diagnosis, and around the same proportion has seen a pulmonologist within the same time span (Mooney J, et al. Ann Am Thorac Soc. 2019;16[3]:393). A median delay to IPF diagnosis of 2.2 years was noted in patients presenting to a tertiary academic medical center and was associated with an increased risk of death independent of age, sex, and forced vital capacity (adjusted hazard ratio per doubling of delay was 1.3) (Lamas DJ et al. Am J Respir Crit Care Med. 2011;184:842).
Robust improvements are clearly required for identifying patients with IPF earlier in their disease course. The Bridging Specialties Initiative from CHEST and the Three Lakes Foundation is one resource designed to improve the timely diagnosis of ILD (ILD Clinician Toolkit available at https://www.chestnet.org/Guidelines-and-Topic-Collections/Bridging-Specialties/Timely-Diagnosis-for-ILD-Patients/Clinician-Toolkit). This, and other initiatives will hopefully reduce delays in diagnosing IPF, allowing for optimal patient care.
Adrian Shifren, MBBCh, FCCP, Member-at-Large
Saniya Khan, MD, MBBS, Member-at-Large
Robert Case Jr., MD, Pulmonary & Critical Care Fellow
Critical Care Network
Mechanical Ventilation and Airways Section
Noninvasive ventilation
Noninvasive ventilation (NIV) is a ventilation modality that supports breathing by using mechanically assisted breaths without the need for intubation or a surgical airway. NIV is divided into two main types, negative-pressure ventilation (NPV) and noninvasive positive-pressure ventilation (NIPPV).
NPV
NPV periodically generates a negative (subatmospheric) pressure on the thorax wall, reflecting the natural breathing mechanism. As this negative pressure is transmitted into the thorax, normal atmospheric pressure air outside the thorax is pulled in for inhalation. Initiated by the negative pressure generator switching off, exhalation is passive due to elastic recoil of the lung and chest wall. The iron lung was a neck-to-toe horizontal cylinder used for NPV during the polio epidemic. New NPV devices are designed to fit the thorax only, using a cuirass (a torso-covering body armor molded shell).
For years, NPV use declined as NIPPV use increased. However, during the shortage of NIPPV devices during COVID and a recent recall of certain CPAP devices, NPV use has increased. NPV is an excellent alternative for those who cannot tolerate a facial mask due to facial deformity, claustrophobia, or excessive airway secretion (Corrado A et al. European Resp J. 2002;20[1]:187).
NIPPV
NIPPV is divided into several subtypes, including continuous positive airway pressure (CPAP), bilevel positive airway pressure (BPAP or BiPAP), and average volume-assured pressure support (AVAPS or VAPS). CPAP is defined as a single pressure delivered in inhalation (Pi) and exhalation (Pe). The increased mean airway pressure provides improved oxygenation (O2) but not ventilation (CO2). BPAP uses dual pressures with Pi higher than Pe. The increased mean airway pressure provides improved O2 while the difference between Pi minus Pe increases ventilation and decreases CO2.
AVAPS is a form of BPAP where Pi varies in an automated range to achieve the ordered tidal volume. In AVAPS, the generator adjusts Pi based on the average delivered tidal volume. If the average delivered tidal volume is less than the set tidal volume, Pi gradually increases while not exceeding Pi Max. Patients notice improved comfort of AVAPS with a variable Pi vs. BPAP with a fixed Pi (Frank A et al. Chest. 2018;154[4]:1060A).
Samantha Tauscher, DO, Resident-in-Training
Herbert Patrick, MD, MSEE, FCCP , Member-at-Large
Pulmonary Vascular & Cardiovascular Disease Network
Pulmonary Vascular Disease Section
A RACE to the finish: Revisiting the role of BPA in the management of CTEPH
Pulmonary thromboendarterectomy (PTE) is the treatment of choice for patients with CTEPH (Kim NH et al. Eur Respir J. 2019;53:1801915). However, this leaves about 40% of CTEPH patients who are not operative candidates due to inaccessible distal clot burden or significant comorbidities (Pepke-Zaba J et al. Circulation 2011;124:1973). For these inoperable situations, riociguat is the only FDA-approved medical therapy (Delcroix M et al. Eur Respir J. 2021;57:2002828). Balloon pulmonary angioplasty (BPA) became a treatment option for these patients in the last 2 decades. As technique refined, BPA demonstrated improved safety data along with improved hemodynamics and increased exercise capacity (Kataoka M et al. Circ Cardiovasc Interv. 2012;5:756).
A recently published crossover study, the RACE trial, compared riociguat with BPA in treating inoperable CTEPH (Jaïs X et al. Lancet Respir Med. 2022;10[10]:961). Patients were randomly assigned to either riociguat or BPA for 26 weeks. At 26 weeks, patients with pulmonary vascular resistance (PVR) more than 4 Woods Units (WU) were crossed over to receive either BPA or riociguat therapy. At 26 weeks, the BPA arm showed a greater reduction in PVR but more complications, including lung injury and hemoptysis. After a 26-week crossover period, the reduction in PVR was similar in both arms. The complication rate in the BPA arm was lower when preceded by riociguat.
In patients with inoperable CTEPH, BPA has emerged as an attractive management option in addition to the medical therapy with riociguat. However, BPA should be performed at expert centers with experience. Further studies are needed to strengthen the role and optimal timing of BPA in management of post PTE patients with residual PH.
Samantha Pettigrew, MD, Fellow-in-Training
Janine Vintich, MD, FCCP, Member-at-Large
Diffuse Lung Disease & Transplant Network
Interstitial Lung Disease Section
Delay in diagnosis of IPF: How bad is the problem?
Idiopathic pulmonary fibrosis (IPF) is a devastating disease with a poor prognosis. Antifibrotic therapies for IPF are only capable of slowing disease progression without reversing established fibrosis. As such, the therapeutic efficacy of antifibrotic therapy may be reduced in patients whose diagnosis is delayed.
Unfortunately, diagnostic delay is common in IPF. Studies demonstrate that IPF diagnosis is delayed by more than a year after symptom onset in 43% of subjects, and more than 3 years in 19% of subjects (Cosgrove GP et al. BMC Pulm Med. 2018;18[9]). Approximately one-third of patients with IPF have undergone chest CT imaging more than 3 years prior to diagnosis, and around the same proportion has seen a pulmonologist within the same time span (Mooney J, et al. Ann Am Thorac Soc. 2019;16[3]:393). A median delay to IPF diagnosis of 2.2 years was noted in patients presenting to a tertiary academic medical center and was associated with an increased risk of death independent of age, sex, and forced vital capacity (adjusted hazard ratio per doubling of delay was 1.3) (Lamas DJ et al. Am J Respir Crit Care Med. 2011;184:842).
Robust improvements are clearly required for identifying patients with IPF earlier in their disease course. The Bridging Specialties Initiative from CHEST and the Three Lakes Foundation is one resource designed to improve the timely diagnosis of ILD (ILD Clinician Toolkit available at https://www.chestnet.org/Guidelines-and-Topic-Collections/Bridging-Specialties/Timely-Diagnosis-for-ILD-Patients/Clinician-Toolkit). This, and other initiatives will hopefully reduce delays in diagnosing IPF, allowing for optimal patient care.
Adrian Shifren, MBBCh, FCCP, Member-at-Large
Saniya Khan, MD, MBBS, Member-at-Large
Robert Case Jr., MD, Pulmonary & Critical Care Fellow
Critical Care Network
Mechanical Ventilation and Airways Section
Noninvasive ventilation
Noninvasive ventilation (NIV) is a ventilation modality that supports breathing by using mechanically assisted breaths without the need for intubation or a surgical airway. NIV is divided into two main types, negative-pressure ventilation (NPV) and noninvasive positive-pressure ventilation (NIPPV).
NPV
NPV periodically generates a negative (subatmospheric) pressure on the thorax wall, reflecting the natural breathing mechanism. As this negative pressure is transmitted into the thorax, normal atmospheric pressure air outside the thorax is pulled in for inhalation. Initiated by the negative pressure generator switching off, exhalation is passive due to elastic recoil of the lung and chest wall. The iron lung was a neck-to-toe horizontal cylinder used for NPV during the polio epidemic. New NPV devices are designed to fit the thorax only, using a cuirass (a torso-covering body armor molded shell).
For years, NPV use declined as NIPPV use increased. However, during the shortage of NIPPV devices during COVID and a recent recall of certain CPAP devices, NPV use has increased. NPV is an excellent alternative for those who cannot tolerate a facial mask due to facial deformity, claustrophobia, or excessive airway secretion (Corrado A et al. European Resp J. 2002;20[1]:187).
NIPPV
NIPPV is divided into several subtypes, including continuous positive airway pressure (CPAP), bilevel positive airway pressure (BPAP or BiPAP), and average volume-assured pressure support (AVAPS or VAPS). CPAP is defined as a single pressure delivered in inhalation (Pi) and exhalation (Pe). The increased mean airway pressure provides improved oxygenation (O2) but not ventilation (CO2). BPAP uses dual pressures with Pi higher than Pe. The increased mean airway pressure provides improved O2 while the difference between Pi minus Pe increases ventilation and decreases CO2.
AVAPS is a form of BPAP where Pi varies in an automated range to achieve the ordered tidal volume. In AVAPS, the generator adjusts Pi based on the average delivered tidal volume. If the average delivered tidal volume is less than the set tidal volume, Pi gradually increases while not exceeding Pi Max. Patients notice improved comfort of AVAPS with a variable Pi vs. BPAP with a fixed Pi (Frank A et al. Chest. 2018;154[4]:1060A).
Samantha Tauscher, DO, Resident-in-Training
Herbert Patrick, MD, MSEE, FCCP , Member-at-Large
Pulmonary Vascular & Cardiovascular Disease Network
Pulmonary Vascular Disease Section
A RACE to the finish: Revisiting the role of BPA in the management of CTEPH
Pulmonary thromboendarterectomy (PTE) is the treatment of choice for patients with CTEPH (Kim NH et al. Eur Respir J. 2019;53:1801915). However, this leaves about 40% of CTEPH patients who are not operative candidates due to inaccessible distal clot burden or significant comorbidities (Pepke-Zaba J et al. Circulation 2011;124:1973). For these inoperable situations, riociguat is the only FDA-approved medical therapy (Delcroix M et al. Eur Respir J. 2021;57:2002828). Balloon pulmonary angioplasty (BPA) became a treatment option for these patients in the last 2 decades. As technique refined, BPA demonstrated improved safety data along with improved hemodynamics and increased exercise capacity (Kataoka M et al. Circ Cardiovasc Interv. 2012;5:756).
A recently published crossover study, the RACE trial, compared riociguat with BPA in treating inoperable CTEPH (Jaïs X et al. Lancet Respir Med. 2022;10[10]:961). Patients were randomly assigned to either riociguat or BPA for 26 weeks. At 26 weeks, patients with pulmonary vascular resistance (PVR) more than 4 Woods Units (WU) were crossed over to receive either BPA or riociguat therapy. At 26 weeks, the BPA arm showed a greater reduction in PVR but more complications, including lung injury and hemoptysis. After a 26-week crossover period, the reduction in PVR was similar in both arms. The complication rate in the BPA arm was lower when preceded by riociguat.
In patients with inoperable CTEPH, BPA has emerged as an attractive management option in addition to the medical therapy with riociguat. However, BPA should be performed at expert centers with experience. Further studies are needed to strengthen the role and optimal timing of BPA in management of post PTE patients with residual PH.
Samantha Pettigrew, MD, Fellow-in-Training
Janine Vintich, MD, FCCP, Member-at-Large
Diffuse Lung Disease & Transplant Network
Interstitial Lung Disease Section
Delay in diagnosis of IPF: How bad is the problem?
Idiopathic pulmonary fibrosis (IPF) is a devastating disease with a poor prognosis. Antifibrotic therapies for IPF are only capable of slowing disease progression without reversing established fibrosis. As such, the therapeutic efficacy of antifibrotic therapy may be reduced in patients whose diagnosis is delayed.
Unfortunately, diagnostic delay is common in IPF. Studies demonstrate that IPF diagnosis is delayed by more than a year after symptom onset in 43% of subjects, and more than 3 years in 19% of subjects (Cosgrove GP et al. BMC Pulm Med. 2018;18[9]). Approximately one-third of patients with IPF have undergone chest CT imaging more than 3 years prior to diagnosis, and around the same proportion has seen a pulmonologist within the same time span (Mooney J, et al. Ann Am Thorac Soc. 2019;16[3]:393). A median delay to IPF diagnosis of 2.2 years was noted in patients presenting to a tertiary academic medical center and was associated with an increased risk of death independent of age, sex, and forced vital capacity (adjusted hazard ratio per doubling of delay was 1.3) (Lamas DJ et al. Am J Respir Crit Care Med. 2011;184:842).
Robust improvements are clearly required for identifying patients with IPF earlier in their disease course. The Bridging Specialties Initiative from CHEST and the Three Lakes Foundation is one resource designed to improve the timely diagnosis of ILD (ILD Clinician Toolkit available at https://www.chestnet.org/Guidelines-and-Topic-Collections/Bridging-Specialties/Timely-Diagnosis-for-ILD-Patients/Clinician-Toolkit). This, and other initiatives will hopefully reduce delays in diagnosing IPF, allowing for optimal patient care.
Adrian Shifren, MBBCh, FCCP, Member-at-Large
Saniya Khan, MD, MBBS, Member-at-Large
Robert Case Jr., MD, Pulmonary & Critical Care Fellow
Critical Care Network
Mechanical Ventilation and Airways Section
Noninvasive ventilation
Noninvasive ventilation (NIV) is a ventilation modality that supports breathing by using mechanically assisted breaths without the need for intubation or a surgical airway. NIV is divided into two main types, negative-pressure ventilation (NPV) and noninvasive positive-pressure ventilation (NIPPV).
NPV
NPV periodically generates a negative (subatmospheric) pressure on the thorax wall, reflecting the natural breathing mechanism. As this negative pressure is transmitted into the thorax, normal atmospheric pressure air outside the thorax is pulled in for inhalation. Initiated by the negative pressure generator switching off, exhalation is passive due to elastic recoil of the lung and chest wall. The iron lung was a neck-to-toe horizontal cylinder used for NPV during the polio epidemic. New NPV devices are designed to fit the thorax only, using a cuirass (a torso-covering body armor molded shell).
For years, NPV use declined as NIPPV use increased. However, during the shortage of NIPPV devices during COVID and a recent recall of certain CPAP devices, NPV use has increased. NPV is an excellent alternative for those who cannot tolerate a facial mask due to facial deformity, claustrophobia, or excessive airway secretion (Corrado A et al. European Resp J. 2002;20[1]:187).
NIPPV
NIPPV is divided into several subtypes, including continuous positive airway pressure (CPAP), bilevel positive airway pressure (BPAP or BiPAP), and average volume-assured pressure support (AVAPS or VAPS). CPAP is defined as a single pressure delivered in inhalation (Pi) and exhalation (Pe). The increased mean airway pressure provides improved oxygenation (O2) but not ventilation (CO2). BPAP uses dual pressures with Pi higher than Pe. The increased mean airway pressure provides improved O2 while the difference between Pi minus Pe increases ventilation and decreases CO2.
AVAPS is a form of BPAP where Pi varies in an automated range to achieve the ordered tidal volume. In AVAPS, the generator adjusts Pi based on the average delivered tidal volume. If the average delivered tidal volume is less than the set tidal volume, Pi gradually increases while not exceeding Pi Max. Patients notice improved comfort of AVAPS with a variable Pi vs. BPAP with a fixed Pi (Frank A et al. Chest. 2018;154[4]:1060A).
Samantha Tauscher, DO, Resident-in-Training
Herbert Patrick, MD, MSEE, FCCP , Member-at-Large
Pulmonary Vascular & Cardiovascular Disease Network
Pulmonary Vascular Disease Section
A RACE to the finish: Revisiting the role of BPA in the management of CTEPH
Pulmonary thromboendarterectomy (PTE) is the treatment of choice for patients with CTEPH (Kim NH et al. Eur Respir J. 2019;53:1801915). However, this leaves about 40% of CTEPH patients who are not operative candidates due to inaccessible distal clot burden or significant comorbidities (Pepke-Zaba J et al. Circulation 2011;124:1973). For these inoperable situations, riociguat is the only FDA-approved medical therapy (Delcroix M et al. Eur Respir J. 2021;57:2002828). Balloon pulmonary angioplasty (BPA) became a treatment option for these patients in the last 2 decades. As technique refined, BPA demonstrated improved safety data along with improved hemodynamics and increased exercise capacity (Kataoka M et al. Circ Cardiovasc Interv. 2012;5:756).
A recently published crossover study, the RACE trial, compared riociguat with BPA in treating inoperable CTEPH (Jaïs X et al. Lancet Respir Med. 2022;10[10]:961). Patients were randomly assigned to either riociguat or BPA for 26 weeks. At 26 weeks, patients with pulmonary vascular resistance (PVR) more than 4 Woods Units (WU) were crossed over to receive either BPA or riociguat therapy. At 26 weeks, the BPA arm showed a greater reduction in PVR but more complications, including lung injury and hemoptysis. After a 26-week crossover period, the reduction in PVR was similar in both arms. The complication rate in the BPA arm was lower when preceded by riociguat.
In patients with inoperable CTEPH, BPA has emerged as an attractive management option in addition to the medical therapy with riociguat. However, BPA should be performed at expert centers with experience. Further studies are needed to strengthen the role and optimal timing of BPA in management of post PTE patients with residual PH.
Samantha Pettigrew, MD, Fellow-in-Training
Janine Vintich, MD, FCCP, Member-at-Large
Continuing our list of CHEST 2022 Winners
CHEST FOUNDATION GRANT AWARDS
CHEST Foundation Research Grant in Women’s Lung Health Disparities
Laura Sanapo, MD, The Miriam Hospital, Providence, R.I.
This grant is jointly supported by the CHEST Foundation and the Respiratory Health Association.
CHEST Foundation Research Grant in Chronic Obstructive Pulmonary Disease
Benjamin Wu, MD, New York University
This grant is supported by AstraZeneca.
CHEST Foundation Research Grant in Chronic Obstructive Pulmonary Disease
Richard Zou, MD, University of Pittsburgh Medical Center
This grant is supported by the CHEST Foundation.
CHEST Foundation and AASM Foundation Research Grant in Sleep Medicine
Gonzalo Labarca, MD, Universidad San Sebastian, Concepción, Chile
This grant is jointly supported by the CHEST Foundation and AASM Foundation.
CHEST Foundation and American Academy of Dental Sleep Medicine Research Grant in Sleep Apnea
Sherri Katz, MD, FCCP, Children’s Hospital of Eastern Ontario, Ottawa
This grant is supported by the CHEST Foundation and American Academy of Dental Sleep Medicine.
CHEST Foundation Research Grant in Sleep Medicine
Nancy Stewart, DO, University of Kansas Medical Center, Kansas City
This grant is supported by Jazz Pharmaceuticals.
CHEST Foundation Research Grant in Severe Asthma
Gareth Walters, MD, University Hospitals Birmingham (England)
This grant is supported by AstraZeneca.
CHEST Foundation Research Grant in Severe Asthma
Andréanne Côté, MD, Institut Universitaire de Cardiologie et de Pneumologie de Québec, Quebec
This grant is supported by AstraZeneca.
CHEST Foundation and APCCMPD Research Grant in Medical Education
Christopher Leba, MD, MPH, University of California, San Francisco
This grant is jointly supported by the CHEST Foundation and APCCMPD.
CHEST Foundation Research Grant in COVID-19
Clea Barnett, MD, New York University
This grant is supported by the CHEST Foundation.
CHEST Foundation Research Grant in Critical Care
Katherine Walker, MD, Brigham and Women’s Hospital, Harvard Medical School, Boston
This grant is supported by the CHEST Foundation.
CHEST Foundation Research Grant in Venous Thromboembolism
Daniel Lachant, DO, University of Rochester (N.Y.) Medical Center/Strong Memorial Hospital
This grant is supported by the CHEST Foundation.
CHEST Foundation Research Grant in Pulmonary Hypertension
Christina Thornton, MD, PhD, University of Calgary (Alta.)
This grant is supported by the CHEST Foundation.
CHEST Foundation Research Grant in Pulmonary Fibrosis
Christina Eckhardt, MD, Columbia University, New York
This grant is supported by an independent grant from Boehringer Ingelheim Pharmaceuticals and Genentech.
CHEST Foundation Research Grant in Pulmonary Fibrosis
John Kim, MD, University of Virginia, Charlottesville
This grant is supported by an independent grant from Boehringer Ingelheim Pharmaceuticals and Genentech.
John R. Addrizzo, MD, FCCP Research Grant in Sarcoidosis
Kerry Hena, MD, New York University
This grant is in honor of John R. Addrizzo, MD, FCCP and is jointly supported by the Addrizzo family and the CHEST Foundation.
CHEST Foundation Research Grant in Pediatric Lung Health
Adam Shapiro, MD, McGill University Health Centre, Montreal
This grant is supported by the CHEST Foundation.
CHEST Foundation Young Investigator Grant
Sameer Avasarala, MD, Case Western Reserve University, Cleveland
This grant is supported by the CHEST Foundation.
CHEST/ALA/ATS Respiratory Health Equity Research Award
Matthew Triplette, MD, Fred Hutchinson Cancer Research Center, Seattle
The Respiratory Health Equity Research Award is jointly supported by the American Lung Association, the American Thoracic Society, and the CHEST Foundation.
CHEST/ALA/ATS Respiratory Health Equity Research Award
Ayobami Akenroye, MD, MPH, Brigham and Women’s Hospital, Boston
The Respiratory Health Equity Research Award is jointly supported by the American Lung Association, the American Thoracic Society, and the CHEST Foundation.
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Lorriane Odhiambo, PhD, Augusta (Ga.) University
This grant is supported by the CHEST Foundation.
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Katie Stevens, Team Telomere, New York
This grant is supported by the CHEST Foundation.
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Matthew Sharpe, MD, The University of Kansas Medical Center, Kansas City
This grant is supported by the CHEST Foundation.
SCIENTIFIC ABSTRACT AWARDS
Alfred Soffer Research Awards
Presented abstracts will be judged by session moderators, and award recipients will be selected for their outstanding original scientific research. Finalists will be evaluated on the basis of their written abstract and the quality of their oral presentation. This award is named in honor of Alfred Soffer, MD, Master FCCP, who was Editor in Chief of the journal CHEST® from 1968 to 1993, and Executive Director of CHEST from 1969 to 1992.
Young Investigator Awards
Investigators who are enrolled in a training or fellowship program or who have completed a fellowship program within 5 years prior to CHEST 2022 are eligible for Young Investigator Awards.
Presenters will be evaluated on the basis of their written abstract and presentation. Recipients will be selected by judges from the Scientific Presentations and Awards Committee for their outstanding original scientific research.
Top Rapid Fire Abstract Award
Awards are granted to two presenters from all the rapid fire sessions at the CHEST Annual Meeting for outstanding original scientific research and presentation
Top Case Report Award
Awards are granted to one presenter in each oral case report session at the CHEST Annual Meeting for outstanding original scientific research and presentation
Top Rapid Fire Case Report Award
Awards are granted to one presenter in each rapid fire oral case report session at the CHEST Annual Meeting for outstanding original scientific research and presentation
ALFRED SOFFER RESEARCH AWARD WINNERS
Palak Rath, MD
A Sense Of Urgency: Boarding Of Critical Care Medicine Patients In The ED
Syed Nazeer Mahmood, MD
Quantifying The Risk For Overtreatment And Undertreatment Of Severe Community Onset Pneumonia
YOUNG INVESTIGATOR AWARD WINNERS
Anusha Devarajan, MD, MBBS
Pneumomediastinum And Pneumothorax In COVID-19 Pneumonia: A Matched Case-Control Study
Marjan Islam, MD
Thoracic Ultrasound In COVID-19: Use Of Lung And Diaphragm Ultrasound In Evaluating Dyspnea In Survivors Of Critical Illness From COVID-19 Pneumonia In A Post-ICU Clinic
Aaron St Laurent, MD
Duchenne Muscular Dystrophy Respiratory Profiles From Real-World Registry Data: A Retrospective Longitudinal Study
ABSTRACT RAPID FIRE WINNERS
Andrew J.O. Davis, MD
Early Gas Exchange Parameters Not Associated With Survival In COVID-19-Associated ARDS Patients Requiring Prolonged Venovenous Extracorporeal Membrane Oxygenation
Benjamin Emmanuel
Clinical Outcomes In Patients With Severe Asthma Who Had Or Had Not Initiated Biologic Therapy: Results From The CLEAR Study
CASE REPORT SESSION WINNERS
Sathya Alekhya Bukkuri
Smarca4-Deficient Undifferentiated Tumor: A Rare Thoracic Malignancy
Zachary A. Banbury, MD
Fungal Aortitis In A Patient For Whom Blood Transfusion Is Not An Option: A Rare But Potentially Fatal Complication Of Aortic Valve Replacement
Harinivaas Shanmugavel Geetha, MD
Respiratory Distress After Potentially Fatal Aspirin Overdose: When To Intubate?
Lisa Hayes
Systemic Epstein-Barr Virus-Related T-Cell Lymphoproliferative Disorder: A Rare Cause Of Dyspnea And Pulmonary Infiltrates In An Immunocompetent Adult
Mohammed Alsaggaf, MBBS
Calcium Oxalate Deposition In Pulmonary Aspergillosis
Cheyenne Snavely
Traffic Jam In The Vasculature: A Case Of Pulmonary Leukostasis
Clarissa Smith, MD
Talcoma In Lung Cancer Screening: A Rare Benign Cause Of PET Scan Avidity
Nitin Gupta, MD
The Clue Is In The Blood Gas: A Rare Manifestation Of Lactic Acidosis
Moses Hayrabedian, MD
A Century-Old Infection Mimicking Malignancy: A Case Of Diffuse Histoplasmosis
Gabriel R. Schroeder, MD
A Case Of Wind-Instrument Associated Hypersensitivity Pneumonitis
Fizza Sajid, DO
Leaping From Lush Tropical Environments To The L-Train: A Case Of Severe Leptospirosis In New York City
Krista R. Dollar, MD
Looking Past The Ground Glass: It Was Only Skin Deep
Konstantin Golubykh, MD
Point-Of-Care Ultrasound In The Timely Diagnosis Of Colonic Necrosis
Arsal Tharwani
Abdominal Compression In End-Stage Fibrotic Interstitial Lung Disease (ILD) Improves Respiratory Compliance
Ryan Kozloski
When Asthma Isn’t: Multispecialty Approach To Fibrosing Mediastinitis
Zach S. Jarrett, DO
Vanishing Cancer: A Case Of Smoking-Related Organizing Pneumonia
Stephen Simeone
Intravascular Papillary Endothelial Hyperplasia Presenting As Thrombus In Transit With Acute Pulmonary Embolism
David Gruen, MD
Tackling Posterior Reversible Encephalopathy Syndrome (PRES): A Rare Case Of Subtherapeutic Tacrolimus Causing PRES In Steroid-Resistant Nephropathy
Nicholas Kunce, MD
An Unusual Case Of Subacute Bacterial Endocarditis Presenting With Catastrophic Subarachnoid Hemorrhage
Phillip J. Gary, MD
Sarcoid-Like Reaction After Treatment With Pembrolizumab
Shreya Podder, MD
Endobronchial Valves For Treatment Of Persistent Air Leak After Secondary Spontaneous Pneumothorax In Patients With Cystic Fibrosis
Alina Aw Wasim, MD, MBBS
Chest-Wall Castleman Disease Mimicking Thymoma Drop Metastasis
Ndausung Udongwo
The ‘Rat Bite Sign” On Cardiac MRI: Left Dominant Arrhythmogenic Cardiomyopathy As An Atypical Etiology Of Sudden Cardiac Arrest
Grant Senyei, MD
Management Of Ventriculopleural Shunt-Associated Pleural Effusion
Garima Singh, MD
COVID-19-Associated Thrombotic Thrombocytopenia Purpura (TTP)
CASE REPORT RAPID FIRE WINNERS
Sandeep Patri
Hyperammonemia Postlung Transplantation: An Uncommon But Life-Threatening Complication
Trung Nguyen
Dyspnea During Pregnancy Revealing Multiple Pulmonary Arteriovenous Malformations And A New Diagnosis Of Hereditary Hemorrhagic Telangiectasia
Pedro J. Baez, MD
Adenoid Cystic Adenocarcinoma: A Rare Esophageal Malignancy Misdiagnosed As COPD
Brette Guckian, DO
Management Of Pulmonary Cement Emboli After Kyphoplasty
Brinn Demars, DO
Tumor Emboli In The Pulmonary Artery Secondary To Chondrosarcoma: A Rare Presentation Mimicking Pulmonary Thromboembolism
Aakriti Arora
A Case Of Pulmonary Hypertension As A Possible Extracranial Manifestation Of Moyamoya Disease
Racine Elaine Reinoso
Clot In Transit: The Role Of Point-Of-Care Ultrasound In Early Diagnosis And Improved Outcomes
Qiraat Azeem, MD
A Case Of Autosomal-Dominant Hyper-IgE Syndrome Masquerading As Cystic Fibrosis
Jason R. Ballengee, DO
Third-Trimester Pregnancy Complicated By Non-Small Cell Lung Cancer Initially Presenting With Central Airway Obstruction And Stenosis
Sam Shafer
Caught In The Fray: Neurosarcoidosis Presenting As Chronic Respiratory Failure
Takkin Lo, MD, MPH
China White In Asthmatic Recreational Drug Users: Does It Contribute To Pneumatocele Development?
Sanjeev Shrestha, MD
Successful Treatment Of Microscopic Polyangiitis Using Novel Steroid-Sparing Agent Avacopan
Kristina Menchaca, MD
Cardiac Tamponade Without The Beck Triad: A Complication Of Severe Hypothyroidism
Olivia Millay, BS
Spontaneous Coronary Artery Dissection Of Left Anterior Descending Artery Complicated By Ventricular Septal Rupture
Akruti P. Prabhakar, DO
Delayed Lead Perforation Of The Right Atrium In The Presence Of Persistent Left Superior Vena Cava: A Rare Coincidence
Kevin Hsu, MD
A Modified Valsalva Maneuver For Ventilated And Sedated Patients With Unstable Supraventricular Tachycardia
Nang San Hti Lar Seng
Cardiovascular Manifestations Of Paraaortic Paragangliomas
Rocio Castillo-Larios
Membranous Dehiscence After Tracheal Resection And Reconstruction Healed Spontaneously With Conservative Treatment
Fizza Sajid, DO
A Young Broken Heart, Reversed
Janeen Grant-Sittol, MD
Inhaled Tranexamic Acid Use For Massive Hemoptysis In Vasculitis-Induced Bronchoalveolar Hemorrhage
Raman G. Kutty, Md, PhD
Progressive Lung Infiltrates In Patient With Acquired Immunodeficiency: A Rare Case Of GLILD
Tanwe Shende
Mycobacterium Shimoidei: A Rare Nontuberculous Infection In A U.S. Patient
Sarah M. Upson, MD
Not Your Typical Lactic Acidosis
Prachi Saluja, MD
Late-Onset Immune Thrombotic Thrombocytopenic Purpura (TTP) After Asymptomatic COVID-19 Infection
Steven S. Wu, MD
Type 1 Multiple Endocrine Neoplasia-Associated Tracheobronchial Tumors Managed By Rigid Bronchoscopy-Directed Multimodal Tumor Destruction
Konstantin Golubykh, MD
The Reversal That Helped: Role Of Bedside Echocardiography In Takotsubo Cardiomyopathy
Eric Salomon, MD
Obstructive Tracheobronchial Pulmonary Aspergillosis Managed With Local Bronchoscopic Intervention Alone
Daniel Hoesterey, MD
A Rare Case Of Critical Illness Due To Eczema Herpeticum With Disseminated Herpes Simplex Virus Infection
Awab U. Khan, DO
Severe Colchicine Toxicity In A Suicide Attempt Causing Multiorgan Failure: A Survival Story
Jacob Cebulko
Disseminated Strongyloidiasis In A Patient With Acute Lymphocytic Leukemia
Hasan Baher, MD
Hiding In Plain Sight: Disseminated Pulmonary TB
Navneet Ramesh
Multimodal Management Of Gastric Variceal Bleeding In Hemorrhagic Shock
Jason L. Peng, MD
Improving Compliance With Continuous Anterior Chest Compression In ARDS Caused By COVID-19: A Case Series
Sushan Gupta, MD
Complete Resolution Of Vasoreactive Pulmonary Artery Hypertension In Chronic Hypersensitive Pneumonitis
Mamta S. Chhabria, MD
A Fistulous Issue: Gastropleural Fistula As A Complication Of Gastrectomy
Anita Singh, DO, MBA
Identifying A Novel Surfactant Protein Mutation In A Family With Pulmonary Fibrosis
Rana Prathap Padappayil, MBBS
Delayed Cerebral Venous Sinus Thrombosis (CSVT) After An Invasive Meningioma Resection: An Uncommon Presentation Of A Common Complication
Rubabin Tooba, MD
The Morphing Cavity: An Image Series Of A Patient’s Pulmonary Infarction Over Time
Sally Ziatabar, DO
A Rare Case Of Disseminated Blastomycosis
Sumukh Arun Kumar
Incidental Pulmonary Cavitary Lesions As An Uncommon Presentation Of Lemierre Syndrome
Sophia Emetu
Pet Peeve: Dyspnea From Undiagnosed Pasteurella Multocida Empyema
Chidambaram Ramasamy, MD
A Case Of Diffuse Alveolar-Septal Pulmonary Amyloidosis And Cardiomyopathy
Rachel Swier
Acid-Fast Bacteria In Bronchiectasis: If The Glass Slipper Does Not Fit, Non-TB Mycobacteria, Consider Tsukamurella
Catherine Durant, MD
Idiopathic Multicentric Castleman Disease With Tafro Syndrome And Sjögren Syndrome
Ali Al-Hilli, MD, MSc
Sarcoidosis-Like Reaction During Treatment For Metastatic Breast Cancer With CDK 4/6 Inhibitors: Just An Epiphenomenon Or A Causal Relationship?
Scott Slusarenko, DO
Rapidly Progressive Perimyocarditis In SARS-CoV-2 Infection
Agatha M. Formoso, MD
Two Infants Presenting With Polymicrobial Pneumonia And Hypoxemic Respiratory Failure Associated With Heterozygous Variants In Carmil2 And Itk
Juan Adams-Chahin
The Silence Of “Lam”: A Case Of Tuberous Sclerosis Complex Associated With Lymphangioleiomyomatosis (Lam)
Kathleen Capaccione, MD
Lung Cancer Is Not Always The Answer: Exploring The Differential Diagnosis Of Thoracic Masses
Joann Wongvravit, DO
West Nile-Induced Myasthenia Gravis Crisis: An Unexpected Case Of Respiratory Failure
Ethan Karle, Do
A Rare Cause Of Community-Acquired Bacterial Pneumonia In A Patient With Poorly Controlled Diabetes
Taylor C. Becker, MD
Calcified Cavitary Conundrum: Delayed Diagnosis Of Histoplasmosis
Anneka Hutton, MD
Disseminated Listeriosis: A Deadly Triplicate
Omar Kandah, DO
COVID-19 Cardiac Tamponade With Cardiogenic Shock In A Previously Vaccinated Young Adult: A Case Report
Cihan Caglayan, MD
Partial Anomalous Pulmonary Venous Connection Diagnosed After Central Venous Catheter Placement
Michelle Jones, DO
Delayed Hemophagocytic Lymphohistiocytosis (HLH) Diagnosis In A Patient With Pulmonary Sarcoidosis And Newly Diagnosed T-Cell Lymphoma: A Case Report
Mariah Evarts, MD
A Normotensive Woman With Profound Lactic Acidosis And Stress-Induced Cardiomyopathy
Rachel V. Tan, MD
A Four-Boding Future: Polyviral Infection With SARS-CoV-2, Parainfluenza Virus Type 3, Influenza A, And Adenovirus
Thanh Hoang
Recurrent Syncope From Intermittent Torsades In Loperamide Abuse
Alissa Ali, MD
Ground Glass Opacities In A Patient Receiving Treatment With All-Trans Retinoic Acid And Arsenic Trioxide
Sean M. Masi, DO, MBA
Ferritin-Guided Therapeutic Plasma Exchange (TPE) Administration In COVID-19-Induced Cytokine Storm Syndrome: A Case Series
Anjali Sachdeva
Successful Biopsy Of Aortopulmonary Window Lymph Node With Robotic-Assisted Bronchoscopy
Rehan Saeed, MD
Multiple Sclerosis After COVID-19: A Sign Of Things To Come?
Harshitha Mergey Devender
Invasive Pulmonary Aspergillosis Associated With Nonspecific Interstitial Pneumonia Causing Recurrent Respiratory Failure
Be sure to check out the other award winners on page 20 in the January issue of CHEST Physician: https://tinyurl.com/2bcdcbj3 .
CHEST FOUNDATION GRANT AWARDS
CHEST Foundation Research Grant in Women’s Lung Health Disparities
Laura Sanapo, MD, The Miriam Hospital, Providence, R.I.
This grant is jointly supported by the CHEST Foundation and the Respiratory Health Association.
CHEST Foundation Research Grant in Chronic Obstructive Pulmonary Disease
Benjamin Wu, MD, New York University
This grant is supported by AstraZeneca.
CHEST Foundation Research Grant in Chronic Obstructive Pulmonary Disease
Richard Zou, MD, University of Pittsburgh Medical Center
This grant is supported by the CHEST Foundation.
CHEST Foundation and AASM Foundation Research Grant in Sleep Medicine
Gonzalo Labarca, MD, Universidad San Sebastian, Concepción, Chile
This grant is jointly supported by the CHEST Foundation and AASM Foundation.
CHEST Foundation and American Academy of Dental Sleep Medicine Research Grant in Sleep Apnea
Sherri Katz, MD, FCCP, Children’s Hospital of Eastern Ontario, Ottawa
This grant is supported by the CHEST Foundation and American Academy of Dental Sleep Medicine.
CHEST Foundation Research Grant in Sleep Medicine
Nancy Stewart, DO, University of Kansas Medical Center, Kansas City
This grant is supported by Jazz Pharmaceuticals.
CHEST Foundation Research Grant in Severe Asthma
Gareth Walters, MD, University Hospitals Birmingham (England)
This grant is supported by AstraZeneca.
CHEST Foundation Research Grant in Severe Asthma
Andréanne Côté, MD, Institut Universitaire de Cardiologie et de Pneumologie de Québec, Quebec
This grant is supported by AstraZeneca.
CHEST Foundation and APCCMPD Research Grant in Medical Education
Christopher Leba, MD, MPH, University of California, San Francisco
This grant is jointly supported by the CHEST Foundation and APCCMPD.
CHEST Foundation Research Grant in COVID-19
Clea Barnett, MD, New York University
This grant is supported by the CHEST Foundation.
CHEST Foundation Research Grant in Critical Care
Katherine Walker, MD, Brigham and Women’s Hospital, Harvard Medical School, Boston
This grant is supported by the CHEST Foundation.
CHEST Foundation Research Grant in Venous Thromboembolism
Daniel Lachant, DO, University of Rochester (N.Y.) Medical Center/Strong Memorial Hospital
This grant is supported by the CHEST Foundation.
CHEST Foundation Research Grant in Pulmonary Hypertension
Christina Thornton, MD, PhD, University of Calgary (Alta.)
This grant is supported by the CHEST Foundation.
CHEST Foundation Research Grant in Pulmonary Fibrosis
Christina Eckhardt, MD, Columbia University, New York
This grant is supported by an independent grant from Boehringer Ingelheim Pharmaceuticals and Genentech.
CHEST Foundation Research Grant in Pulmonary Fibrosis
John Kim, MD, University of Virginia, Charlottesville
This grant is supported by an independent grant from Boehringer Ingelheim Pharmaceuticals and Genentech.
John R. Addrizzo, MD, FCCP Research Grant in Sarcoidosis
Kerry Hena, MD, New York University
This grant is in honor of John R. Addrizzo, MD, FCCP and is jointly supported by the Addrizzo family and the CHEST Foundation.
CHEST Foundation Research Grant in Pediatric Lung Health
Adam Shapiro, MD, McGill University Health Centre, Montreal
This grant is supported by the CHEST Foundation.
CHEST Foundation Young Investigator Grant
Sameer Avasarala, MD, Case Western Reserve University, Cleveland
This grant is supported by the CHEST Foundation.
CHEST/ALA/ATS Respiratory Health Equity Research Award
Matthew Triplette, MD, Fred Hutchinson Cancer Research Center, Seattle
The Respiratory Health Equity Research Award is jointly supported by the American Lung Association, the American Thoracic Society, and the CHEST Foundation.
CHEST/ALA/ATS Respiratory Health Equity Research Award
Ayobami Akenroye, MD, MPH, Brigham and Women’s Hospital, Boston
The Respiratory Health Equity Research Award is jointly supported by the American Lung Association, the American Thoracic Society, and the CHEST Foundation.
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Lorriane Odhiambo, PhD, Augusta (Ga.) University
This grant is supported by the CHEST Foundation.
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Katie Stevens, Team Telomere, New York
This grant is supported by the CHEST Foundation.
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Matthew Sharpe, MD, The University of Kansas Medical Center, Kansas City
This grant is supported by the CHEST Foundation.
SCIENTIFIC ABSTRACT AWARDS
Alfred Soffer Research Awards
Presented abstracts will be judged by session moderators, and award recipients will be selected for their outstanding original scientific research. Finalists will be evaluated on the basis of their written abstract and the quality of their oral presentation. This award is named in honor of Alfred Soffer, MD, Master FCCP, who was Editor in Chief of the journal CHEST® from 1968 to 1993, and Executive Director of CHEST from 1969 to 1992.
Young Investigator Awards
Investigators who are enrolled in a training or fellowship program or who have completed a fellowship program within 5 years prior to CHEST 2022 are eligible for Young Investigator Awards.
Presenters will be evaluated on the basis of their written abstract and presentation. Recipients will be selected by judges from the Scientific Presentations and Awards Committee for their outstanding original scientific research.
Top Rapid Fire Abstract Award
Awards are granted to two presenters from all the rapid fire sessions at the CHEST Annual Meeting for outstanding original scientific research and presentation
Top Case Report Award
Awards are granted to one presenter in each oral case report session at the CHEST Annual Meeting for outstanding original scientific research and presentation
Top Rapid Fire Case Report Award
Awards are granted to one presenter in each rapid fire oral case report session at the CHEST Annual Meeting for outstanding original scientific research and presentation
ALFRED SOFFER RESEARCH AWARD WINNERS
Palak Rath, MD
A Sense Of Urgency: Boarding Of Critical Care Medicine Patients In The ED
Syed Nazeer Mahmood, MD
Quantifying The Risk For Overtreatment And Undertreatment Of Severe Community Onset Pneumonia
YOUNG INVESTIGATOR AWARD WINNERS
Anusha Devarajan, MD, MBBS
Pneumomediastinum And Pneumothorax In COVID-19 Pneumonia: A Matched Case-Control Study
Marjan Islam, MD
Thoracic Ultrasound In COVID-19: Use Of Lung And Diaphragm Ultrasound In Evaluating Dyspnea In Survivors Of Critical Illness From COVID-19 Pneumonia In A Post-ICU Clinic
Aaron St Laurent, MD
Duchenne Muscular Dystrophy Respiratory Profiles From Real-World Registry Data: A Retrospective Longitudinal Study
ABSTRACT RAPID FIRE WINNERS
Andrew J.O. Davis, MD
Early Gas Exchange Parameters Not Associated With Survival In COVID-19-Associated ARDS Patients Requiring Prolonged Venovenous Extracorporeal Membrane Oxygenation
Benjamin Emmanuel
Clinical Outcomes In Patients With Severe Asthma Who Had Or Had Not Initiated Biologic Therapy: Results From The CLEAR Study
CASE REPORT SESSION WINNERS
Sathya Alekhya Bukkuri
Smarca4-Deficient Undifferentiated Tumor: A Rare Thoracic Malignancy
Zachary A. Banbury, MD
Fungal Aortitis In A Patient For Whom Blood Transfusion Is Not An Option: A Rare But Potentially Fatal Complication Of Aortic Valve Replacement
Harinivaas Shanmugavel Geetha, MD
Respiratory Distress After Potentially Fatal Aspirin Overdose: When To Intubate?
Lisa Hayes
Systemic Epstein-Barr Virus-Related T-Cell Lymphoproliferative Disorder: A Rare Cause Of Dyspnea And Pulmonary Infiltrates In An Immunocompetent Adult
Mohammed Alsaggaf, MBBS
Calcium Oxalate Deposition In Pulmonary Aspergillosis
Cheyenne Snavely
Traffic Jam In The Vasculature: A Case Of Pulmonary Leukostasis
Clarissa Smith, MD
Talcoma In Lung Cancer Screening: A Rare Benign Cause Of PET Scan Avidity
Nitin Gupta, MD
The Clue Is In The Blood Gas: A Rare Manifestation Of Lactic Acidosis
Moses Hayrabedian, MD
A Century-Old Infection Mimicking Malignancy: A Case Of Diffuse Histoplasmosis
Gabriel R. Schroeder, MD
A Case Of Wind-Instrument Associated Hypersensitivity Pneumonitis
Fizza Sajid, DO
Leaping From Lush Tropical Environments To The L-Train: A Case Of Severe Leptospirosis In New York City
Krista R. Dollar, MD
Looking Past The Ground Glass: It Was Only Skin Deep
Konstantin Golubykh, MD
Point-Of-Care Ultrasound In The Timely Diagnosis Of Colonic Necrosis
Arsal Tharwani
Abdominal Compression In End-Stage Fibrotic Interstitial Lung Disease (ILD) Improves Respiratory Compliance
Ryan Kozloski
When Asthma Isn’t: Multispecialty Approach To Fibrosing Mediastinitis
Zach S. Jarrett, DO
Vanishing Cancer: A Case Of Smoking-Related Organizing Pneumonia
Stephen Simeone
Intravascular Papillary Endothelial Hyperplasia Presenting As Thrombus In Transit With Acute Pulmonary Embolism
David Gruen, MD
Tackling Posterior Reversible Encephalopathy Syndrome (PRES): A Rare Case Of Subtherapeutic Tacrolimus Causing PRES In Steroid-Resistant Nephropathy
Nicholas Kunce, MD
An Unusual Case Of Subacute Bacterial Endocarditis Presenting With Catastrophic Subarachnoid Hemorrhage
Phillip J. Gary, MD
Sarcoid-Like Reaction After Treatment With Pembrolizumab
Shreya Podder, MD
Endobronchial Valves For Treatment Of Persistent Air Leak After Secondary Spontaneous Pneumothorax In Patients With Cystic Fibrosis
Alina Aw Wasim, MD, MBBS
Chest-Wall Castleman Disease Mimicking Thymoma Drop Metastasis
Ndausung Udongwo
The ‘Rat Bite Sign” On Cardiac MRI: Left Dominant Arrhythmogenic Cardiomyopathy As An Atypical Etiology Of Sudden Cardiac Arrest
Grant Senyei, MD
Management Of Ventriculopleural Shunt-Associated Pleural Effusion
Garima Singh, MD
COVID-19-Associated Thrombotic Thrombocytopenia Purpura (TTP)
CASE REPORT RAPID FIRE WINNERS
Sandeep Patri
Hyperammonemia Postlung Transplantation: An Uncommon But Life-Threatening Complication
Trung Nguyen
Dyspnea During Pregnancy Revealing Multiple Pulmonary Arteriovenous Malformations And A New Diagnosis Of Hereditary Hemorrhagic Telangiectasia
Pedro J. Baez, MD
Adenoid Cystic Adenocarcinoma: A Rare Esophageal Malignancy Misdiagnosed As COPD
Brette Guckian, DO
Management Of Pulmonary Cement Emboli After Kyphoplasty
Brinn Demars, DO
Tumor Emboli In The Pulmonary Artery Secondary To Chondrosarcoma: A Rare Presentation Mimicking Pulmonary Thromboembolism
Aakriti Arora
A Case Of Pulmonary Hypertension As A Possible Extracranial Manifestation Of Moyamoya Disease
Racine Elaine Reinoso
Clot In Transit: The Role Of Point-Of-Care Ultrasound In Early Diagnosis And Improved Outcomes
Qiraat Azeem, MD
A Case Of Autosomal-Dominant Hyper-IgE Syndrome Masquerading As Cystic Fibrosis
Jason R. Ballengee, DO
Third-Trimester Pregnancy Complicated By Non-Small Cell Lung Cancer Initially Presenting With Central Airway Obstruction And Stenosis
Sam Shafer
Caught In The Fray: Neurosarcoidosis Presenting As Chronic Respiratory Failure
Takkin Lo, MD, MPH
China White In Asthmatic Recreational Drug Users: Does It Contribute To Pneumatocele Development?
Sanjeev Shrestha, MD
Successful Treatment Of Microscopic Polyangiitis Using Novel Steroid-Sparing Agent Avacopan
Kristina Menchaca, MD
Cardiac Tamponade Without The Beck Triad: A Complication Of Severe Hypothyroidism
Olivia Millay, BS
Spontaneous Coronary Artery Dissection Of Left Anterior Descending Artery Complicated By Ventricular Septal Rupture
Akruti P. Prabhakar, DO
Delayed Lead Perforation Of The Right Atrium In The Presence Of Persistent Left Superior Vena Cava: A Rare Coincidence
Kevin Hsu, MD
A Modified Valsalva Maneuver For Ventilated And Sedated Patients With Unstable Supraventricular Tachycardia
Nang San Hti Lar Seng
Cardiovascular Manifestations Of Paraaortic Paragangliomas
Rocio Castillo-Larios
Membranous Dehiscence After Tracheal Resection And Reconstruction Healed Spontaneously With Conservative Treatment
Fizza Sajid, DO
A Young Broken Heart, Reversed
Janeen Grant-Sittol, MD
Inhaled Tranexamic Acid Use For Massive Hemoptysis In Vasculitis-Induced Bronchoalveolar Hemorrhage
Raman G. Kutty, Md, PhD
Progressive Lung Infiltrates In Patient With Acquired Immunodeficiency: A Rare Case Of GLILD
Tanwe Shende
Mycobacterium Shimoidei: A Rare Nontuberculous Infection In A U.S. Patient
Sarah M. Upson, MD
Not Your Typical Lactic Acidosis
Prachi Saluja, MD
Late-Onset Immune Thrombotic Thrombocytopenic Purpura (TTP) After Asymptomatic COVID-19 Infection
Steven S. Wu, MD
Type 1 Multiple Endocrine Neoplasia-Associated Tracheobronchial Tumors Managed By Rigid Bronchoscopy-Directed Multimodal Tumor Destruction
Konstantin Golubykh, MD
The Reversal That Helped: Role Of Bedside Echocardiography In Takotsubo Cardiomyopathy
Eric Salomon, MD
Obstructive Tracheobronchial Pulmonary Aspergillosis Managed With Local Bronchoscopic Intervention Alone
Daniel Hoesterey, MD
A Rare Case Of Critical Illness Due To Eczema Herpeticum With Disseminated Herpes Simplex Virus Infection
Awab U. Khan, DO
Severe Colchicine Toxicity In A Suicide Attempt Causing Multiorgan Failure: A Survival Story
Jacob Cebulko
Disseminated Strongyloidiasis In A Patient With Acute Lymphocytic Leukemia
Hasan Baher, MD
Hiding In Plain Sight: Disseminated Pulmonary TB
Navneet Ramesh
Multimodal Management Of Gastric Variceal Bleeding In Hemorrhagic Shock
Jason L. Peng, MD
Improving Compliance With Continuous Anterior Chest Compression In ARDS Caused By COVID-19: A Case Series
Sushan Gupta, MD
Complete Resolution Of Vasoreactive Pulmonary Artery Hypertension In Chronic Hypersensitive Pneumonitis
Mamta S. Chhabria, MD
A Fistulous Issue: Gastropleural Fistula As A Complication Of Gastrectomy
Anita Singh, DO, MBA
Identifying A Novel Surfactant Protein Mutation In A Family With Pulmonary Fibrosis
Rana Prathap Padappayil, MBBS
Delayed Cerebral Venous Sinus Thrombosis (CSVT) After An Invasive Meningioma Resection: An Uncommon Presentation Of A Common Complication
Rubabin Tooba, MD
The Morphing Cavity: An Image Series Of A Patient’s Pulmonary Infarction Over Time
Sally Ziatabar, DO
A Rare Case Of Disseminated Blastomycosis
Sumukh Arun Kumar
Incidental Pulmonary Cavitary Lesions As An Uncommon Presentation Of Lemierre Syndrome
Sophia Emetu
Pet Peeve: Dyspnea From Undiagnosed Pasteurella Multocida Empyema
Chidambaram Ramasamy, MD
A Case Of Diffuse Alveolar-Septal Pulmonary Amyloidosis And Cardiomyopathy
Rachel Swier
Acid-Fast Bacteria In Bronchiectasis: If The Glass Slipper Does Not Fit, Non-TB Mycobacteria, Consider Tsukamurella
Catherine Durant, MD
Idiopathic Multicentric Castleman Disease With Tafro Syndrome And Sjögren Syndrome
Ali Al-Hilli, MD, MSc
Sarcoidosis-Like Reaction During Treatment For Metastatic Breast Cancer With CDK 4/6 Inhibitors: Just An Epiphenomenon Or A Causal Relationship?
Scott Slusarenko, DO
Rapidly Progressive Perimyocarditis In SARS-CoV-2 Infection
Agatha M. Formoso, MD
Two Infants Presenting With Polymicrobial Pneumonia And Hypoxemic Respiratory Failure Associated With Heterozygous Variants In Carmil2 And Itk
Juan Adams-Chahin
The Silence Of “Lam”: A Case Of Tuberous Sclerosis Complex Associated With Lymphangioleiomyomatosis (Lam)
Kathleen Capaccione, MD
Lung Cancer Is Not Always The Answer: Exploring The Differential Diagnosis Of Thoracic Masses
Joann Wongvravit, DO
West Nile-Induced Myasthenia Gravis Crisis: An Unexpected Case Of Respiratory Failure
Ethan Karle, Do
A Rare Cause Of Community-Acquired Bacterial Pneumonia In A Patient With Poorly Controlled Diabetes
Taylor C. Becker, MD
Calcified Cavitary Conundrum: Delayed Diagnosis Of Histoplasmosis
Anneka Hutton, MD
Disseminated Listeriosis: A Deadly Triplicate
Omar Kandah, DO
COVID-19 Cardiac Tamponade With Cardiogenic Shock In A Previously Vaccinated Young Adult: A Case Report
Cihan Caglayan, MD
Partial Anomalous Pulmonary Venous Connection Diagnosed After Central Venous Catheter Placement
Michelle Jones, DO
Delayed Hemophagocytic Lymphohistiocytosis (HLH) Diagnosis In A Patient With Pulmonary Sarcoidosis And Newly Diagnosed T-Cell Lymphoma: A Case Report
Mariah Evarts, MD
A Normotensive Woman With Profound Lactic Acidosis And Stress-Induced Cardiomyopathy
Rachel V. Tan, MD
A Four-Boding Future: Polyviral Infection With SARS-CoV-2, Parainfluenza Virus Type 3, Influenza A, And Adenovirus
Thanh Hoang
Recurrent Syncope From Intermittent Torsades In Loperamide Abuse
Alissa Ali, MD
Ground Glass Opacities In A Patient Receiving Treatment With All-Trans Retinoic Acid And Arsenic Trioxide
Sean M. Masi, DO, MBA
Ferritin-Guided Therapeutic Plasma Exchange (TPE) Administration In COVID-19-Induced Cytokine Storm Syndrome: A Case Series
Anjali Sachdeva
Successful Biopsy Of Aortopulmonary Window Lymph Node With Robotic-Assisted Bronchoscopy
Rehan Saeed, MD
Multiple Sclerosis After COVID-19: A Sign Of Things To Come?
Harshitha Mergey Devender
Invasive Pulmonary Aspergillosis Associated With Nonspecific Interstitial Pneumonia Causing Recurrent Respiratory Failure
Be sure to check out the other award winners on page 20 in the January issue of CHEST Physician: https://tinyurl.com/2bcdcbj3 .
CHEST FOUNDATION GRANT AWARDS
CHEST Foundation Research Grant in Women’s Lung Health Disparities
Laura Sanapo, MD, The Miriam Hospital, Providence, R.I.
This grant is jointly supported by the CHEST Foundation and the Respiratory Health Association.
CHEST Foundation Research Grant in Chronic Obstructive Pulmonary Disease
Benjamin Wu, MD, New York University
This grant is supported by AstraZeneca.
CHEST Foundation Research Grant in Chronic Obstructive Pulmonary Disease
Richard Zou, MD, University of Pittsburgh Medical Center
This grant is supported by the CHEST Foundation.
CHEST Foundation and AASM Foundation Research Grant in Sleep Medicine
Gonzalo Labarca, MD, Universidad San Sebastian, Concepción, Chile
This grant is jointly supported by the CHEST Foundation and AASM Foundation.
CHEST Foundation and American Academy of Dental Sleep Medicine Research Grant in Sleep Apnea
Sherri Katz, MD, FCCP, Children’s Hospital of Eastern Ontario, Ottawa
This grant is supported by the CHEST Foundation and American Academy of Dental Sleep Medicine.
CHEST Foundation Research Grant in Sleep Medicine
Nancy Stewart, DO, University of Kansas Medical Center, Kansas City
This grant is supported by Jazz Pharmaceuticals.
CHEST Foundation Research Grant in Severe Asthma
Gareth Walters, MD, University Hospitals Birmingham (England)
This grant is supported by AstraZeneca.
CHEST Foundation Research Grant in Severe Asthma
Andréanne Côté, MD, Institut Universitaire de Cardiologie et de Pneumologie de Québec, Quebec
This grant is supported by AstraZeneca.
CHEST Foundation and APCCMPD Research Grant in Medical Education
Christopher Leba, MD, MPH, University of California, San Francisco
This grant is jointly supported by the CHEST Foundation and APCCMPD.
CHEST Foundation Research Grant in COVID-19
Clea Barnett, MD, New York University
This grant is supported by the CHEST Foundation.
CHEST Foundation Research Grant in Critical Care
Katherine Walker, MD, Brigham and Women’s Hospital, Harvard Medical School, Boston
This grant is supported by the CHEST Foundation.
CHEST Foundation Research Grant in Venous Thromboembolism
Daniel Lachant, DO, University of Rochester (N.Y.) Medical Center/Strong Memorial Hospital
This grant is supported by the CHEST Foundation.
CHEST Foundation Research Grant in Pulmonary Hypertension
Christina Thornton, MD, PhD, University of Calgary (Alta.)
This grant is supported by the CHEST Foundation.
CHEST Foundation Research Grant in Pulmonary Fibrosis
Christina Eckhardt, MD, Columbia University, New York
This grant is supported by an independent grant from Boehringer Ingelheim Pharmaceuticals and Genentech.
CHEST Foundation Research Grant in Pulmonary Fibrosis
John Kim, MD, University of Virginia, Charlottesville
This grant is supported by an independent grant from Boehringer Ingelheim Pharmaceuticals and Genentech.
John R. Addrizzo, MD, FCCP Research Grant in Sarcoidosis
Kerry Hena, MD, New York University
This grant is in honor of John R. Addrizzo, MD, FCCP and is jointly supported by the Addrizzo family and the CHEST Foundation.
CHEST Foundation Research Grant in Pediatric Lung Health
Adam Shapiro, MD, McGill University Health Centre, Montreal
This grant is supported by the CHEST Foundation.
CHEST Foundation Young Investigator Grant
Sameer Avasarala, MD, Case Western Reserve University, Cleveland
This grant is supported by the CHEST Foundation.
CHEST/ALA/ATS Respiratory Health Equity Research Award
Matthew Triplette, MD, Fred Hutchinson Cancer Research Center, Seattle
The Respiratory Health Equity Research Award is jointly supported by the American Lung Association, the American Thoracic Society, and the CHEST Foundation.
CHEST/ALA/ATS Respiratory Health Equity Research Award
Ayobami Akenroye, MD, MPH, Brigham and Women’s Hospital, Boston
The Respiratory Health Equity Research Award is jointly supported by the American Lung Association, the American Thoracic Society, and the CHEST Foundation.
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Lorriane Odhiambo, PhD, Augusta (Ga.) University
This grant is supported by the CHEST Foundation.
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Katie Stevens, Team Telomere, New York
This grant is supported by the CHEST Foundation.
CHEST Foundation Community Service Grant Honoring D. Robert McCaffree, MD, Master FCCP
Matthew Sharpe, MD, The University of Kansas Medical Center, Kansas City
This grant is supported by the CHEST Foundation.
SCIENTIFIC ABSTRACT AWARDS
Alfred Soffer Research Awards
Presented abstracts will be judged by session moderators, and award recipients will be selected for their outstanding original scientific research. Finalists will be evaluated on the basis of their written abstract and the quality of their oral presentation. This award is named in honor of Alfred Soffer, MD, Master FCCP, who was Editor in Chief of the journal CHEST® from 1968 to 1993, and Executive Director of CHEST from 1969 to 1992.
Young Investigator Awards
Investigators who are enrolled in a training or fellowship program or who have completed a fellowship program within 5 years prior to CHEST 2022 are eligible for Young Investigator Awards.
Presenters will be evaluated on the basis of their written abstract and presentation. Recipients will be selected by judges from the Scientific Presentations and Awards Committee for their outstanding original scientific research.
Top Rapid Fire Abstract Award
Awards are granted to two presenters from all the rapid fire sessions at the CHEST Annual Meeting for outstanding original scientific research and presentation
Top Case Report Award
Awards are granted to one presenter in each oral case report session at the CHEST Annual Meeting for outstanding original scientific research and presentation
Top Rapid Fire Case Report Award
Awards are granted to one presenter in each rapid fire oral case report session at the CHEST Annual Meeting for outstanding original scientific research and presentation
ALFRED SOFFER RESEARCH AWARD WINNERS
Palak Rath, MD
A Sense Of Urgency: Boarding Of Critical Care Medicine Patients In The ED
Syed Nazeer Mahmood, MD
Quantifying The Risk For Overtreatment And Undertreatment Of Severe Community Onset Pneumonia
YOUNG INVESTIGATOR AWARD WINNERS
Anusha Devarajan, MD, MBBS
Pneumomediastinum And Pneumothorax In COVID-19 Pneumonia: A Matched Case-Control Study
Marjan Islam, MD
Thoracic Ultrasound In COVID-19: Use Of Lung And Diaphragm Ultrasound In Evaluating Dyspnea In Survivors Of Critical Illness From COVID-19 Pneumonia In A Post-ICU Clinic
Aaron St Laurent, MD
Duchenne Muscular Dystrophy Respiratory Profiles From Real-World Registry Data: A Retrospective Longitudinal Study
ABSTRACT RAPID FIRE WINNERS
Andrew J.O. Davis, MD
Early Gas Exchange Parameters Not Associated With Survival In COVID-19-Associated ARDS Patients Requiring Prolonged Venovenous Extracorporeal Membrane Oxygenation
Benjamin Emmanuel
Clinical Outcomes In Patients With Severe Asthma Who Had Or Had Not Initiated Biologic Therapy: Results From The CLEAR Study
CASE REPORT SESSION WINNERS
Sathya Alekhya Bukkuri
Smarca4-Deficient Undifferentiated Tumor: A Rare Thoracic Malignancy
Zachary A. Banbury, MD
Fungal Aortitis In A Patient For Whom Blood Transfusion Is Not An Option: A Rare But Potentially Fatal Complication Of Aortic Valve Replacement
Harinivaas Shanmugavel Geetha, MD
Respiratory Distress After Potentially Fatal Aspirin Overdose: When To Intubate?
Lisa Hayes
Systemic Epstein-Barr Virus-Related T-Cell Lymphoproliferative Disorder: A Rare Cause Of Dyspnea And Pulmonary Infiltrates In An Immunocompetent Adult
Mohammed Alsaggaf, MBBS
Calcium Oxalate Deposition In Pulmonary Aspergillosis
Cheyenne Snavely
Traffic Jam In The Vasculature: A Case Of Pulmonary Leukostasis
Clarissa Smith, MD
Talcoma In Lung Cancer Screening: A Rare Benign Cause Of PET Scan Avidity
Nitin Gupta, MD
The Clue Is In The Blood Gas: A Rare Manifestation Of Lactic Acidosis
Moses Hayrabedian, MD
A Century-Old Infection Mimicking Malignancy: A Case Of Diffuse Histoplasmosis
Gabriel R. Schroeder, MD
A Case Of Wind-Instrument Associated Hypersensitivity Pneumonitis
Fizza Sajid, DO
Leaping From Lush Tropical Environments To The L-Train: A Case Of Severe Leptospirosis In New York City
Krista R. Dollar, MD
Looking Past The Ground Glass: It Was Only Skin Deep
Konstantin Golubykh, MD
Point-Of-Care Ultrasound In The Timely Diagnosis Of Colonic Necrosis
Arsal Tharwani
Abdominal Compression In End-Stage Fibrotic Interstitial Lung Disease (ILD) Improves Respiratory Compliance
Ryan Kozloski
When Asthma Isn’t: Multispecialty Approach To Fibrosing Mediastinitis
Zach S. Jarrett, DO
Vanishing Cancer: A Case Of Smoking-Related Organizing Pneumonia
Stephen Simeone
Intravascular Papillary Endothelial Hyperplasia Presenting As Thrombus In Transit With Acute Pulmonary Embolism
David Gruen, MD
Tackling Posterior Reversible Encephalopathy Syndrome (PRES): A Rare Case Of Subtherapeutic Tacrolimus Causing PRES In Steroid-Resistant Nephropathy
Nicholas Kunce, MD
An Unusual Case Of Subacute Bacterial Endocarditis Presenting With Catastrophic Subarachnoid Hemorrhage
Phillip J. Gary, MD
Sarcoid-Like Reaction After Treatment With Pembrolizumab
Shreya Podder, MD
Endobronchial Valves For Treatment Of Persistent Air Leak After Secondary Spontaneous Pneumothorax In Patients With Cystic Fibrosis
Alina Aw Wasim, MD, MBBS
Chest-Wall Castleman Disease Mimicking Thymoma Drop Metastasis
Ndausung Udongwo
The ‘Rat Bite Sign” On Cardiac MRI: Left Dominant Arrhythmogenic Cardiomyopathy As An Atypical Etiology Of Sudden Cardiac Arrest
Grant Senyei, MD
Management Of Ventriculopleural Shunt-Associated Pleural Effusion
Garima Singh, MD
COVID-19-Associated Thrombotic Thrombocytopenia Purpura (TTP)
CASE REPORT RAPID FIRE WINNERS
Sandeep Patri
Hyperammonemia Postlung Transplantation: An Uncommon But Life-Threatening Complication
Trung Nguyen
Dyspnea During Pregnancy Revealing Multiple Pulmonary Arteriovenous Malformations And A New Diagnosis Of Hereditary Hemorrhagic Telangiectasia
Pedro J. Baez, MD
Adenoid Cystic Adenocarcinoma: A Rare Esophageal Malignancy Misdiagnosed As COPD
Brette Guckian, DO
Management Of Pulmonary Cement Emboli After Kyphoplasty
Brinn Demars, DO
Tumor Emboli In The Pulmonary Artery Secondary To Chondrosarcoma: A Rare Presentation Mimicking Pulmonary Thromboembolism
Aakriti Arora
A Case Of Pulmonary Hypertension As A Possible Extracranial Manifestation Of Moyamoya Disease
Racine Elaine Reinoso
Clot In Transit: The Role Of Point-Of-Care Ultrasound In Early Diagnosis And Improved Outcomes
Qiraat Azeem, MD
A Case Of Autosomal-Dominant Hyper-IgE Syndrome Masquerading As Cystic Fibrosis
Jason R. Ballengee, DO
Third-Trimester Pregnancy Complicated By Non-Small Cell Lung Cancer Initially Presenting With Central Airway Obstruction And Stenosis
Sam Shafer
Caught In The Fray: Neurosarcoidosis Presenting As Chronic Respiratory Failure
Takkin Lo, MD, MPH
China White In Asthmatic Recreational Drug Users: Does It Contribute To Pneumatocele Development?
Sanjeev Shrestha, MD
Successful Treatment Of Microscopic Polyangiitis Using Novel Steroid-Sparing Agent Avacopan
Kristina Menchaca, MD
Cardiac Tamponade Without The Beck Triad: A Complication Of Severe Hypothyroidism
Olivia Millay, BS
Spontaneous Coronary Artery Dissection Of Left Anterior Descending Artery Complicated By Ventricular Septal Rupture
Akruti P. Prabhakar, DO
Delayed Lead Perforation Of The Right Atrium In The Presence Of Persistent Left Superior Vena Cava: A Rare Coincidence
Kevin Hsu, MD
A Modified Valsalva Maneuver For Ventilated And Sedated Patients With Unstable Supraventricular Tachycardia
Nang San Hti Lar Seng
Cardiovascular Manifestations Of Paraaortic Paragangliomas
Rocio Castillo-Larios
Membranous Dehiscence After Tracheal Resection And Reconstruction Healed Spontaneously With Conservative Treatment
Fizza Sajid, DO
A Young Broken Heart, Reversed
Janeen Grant-Sittol, MD
Inhaled Tranexamic Acid Use For Massive Hemoptysis In Vasculitis-Induced Bronchoalveolar Hemorrhage
Raman G. Kutty, Md, PhD
Progressive Lung Infiltrates In Patient With Acquired Immunodeficiency: A Rare Case Of GLILD
Tanwe Shende
Mycobacterium Shimoidei: A Rare Nontuberculous Infection In A U.S. Patient
Sarah M. Upson, MD
Not Your Typical Lactic Acidosis
Prachi Saluja, MD
Late-Onset Immune Thrombotic Thrombocytopenic Purpura (TTP) After Asymptomatic COVID-19 Infection
Steven S. Wu, MD
Type 1 Multiple Endocrine Neoplasia-Associated Tracheobronchial Tumors Managed By Rigid Bronchoscopy-Directed Multimodal Tumor Destruction
Konstantin Golubykh, MD
The Reversal That Helped: Role Of Bedside Echocardiography In Takotsubo Cardiomyopathy
Eric Salomon, MD
Obstructive Tracheobronchial Pulmonary Aspergillosis Managed With Local Bronchoscopic Intervention Alone
Daniel Hoesterey, MD
A Rare Case Of Critical Illness Due To Eczema Herpeticum With Disseminated Herpes Simplex Virus Infection
Awab U. Khan, DO
Severe Colchicine Toxicity In A Suicide Attempt Causing Multiorgan Failure: A Survival Story
Jacob Cebulko
Disseminated Strongyloidiasis In A Patient With Acute Lymphocytic Leukemia
Hasan Baher, MD
Hiding In Plain Sight: Disseminated Pulmonary TB
Navneet Ramesh
Multimodal Management Of Gastric Variceal Bleeding In Hemorrhagic Shock
Jason L. Peng, MD
Improving Compliance With Continuous Anterior Chest Compression In ARDS Caused By COVID-19: A Case Series
Sushan Gupta, MD
Complete Resolution Of Vasoreactive Pulmonary Artery Hypertension In Chronic Hypersensitive Pneumonitis
Mamta S. Chhabria, MD
A Fistulous Issue: Gastropleural Fistula As A Complication Of Gastrectomy
Anita Singh, DO, MBA
Identifying A Novel Surfactant Protein Mutation In A Family With Pulmonary Fibrosis
Rana Prathap Padappayil, MBBS
Delayed Cerebral Venous Sinus Thrombosis (CSVT) After An Invasive Meningioma Resection: An Uncommon Presentation Of A Common Complication
Rubabin Tooba, MD
The Morphing Cavity: An Image Series Of A Patient’s Pulmonary Infarction Over Time
Sally Ziatabar, DO
A Rare Case Of Disseminated Blastomycosis
Sumukh Arun Kumar
Incidental Pulmonary Cavitary Lesions As An Uncommon Presentation Of Lemierre Syndrome
Sophia Emetu
Pet Peeve: Dyspnea From Undiagnosed Pasteurella Multocida Empyema
Chidambaram Ramasamy, MD
A Case Of Diffuse Alveolar-Septal Pulmonary Amyloidosis And Cardiomyopathy
Rachel Swier
Acid-Fast Bacteria In Bronchiectasis: If The Glass Slipper Does Not Fit, Non-TB Mycobacteria, Consider Tsukamurella
Catherine Durant, MD
Idiopathic Multicentric Castleman Disease With Tafro Syndrome And Sjögren Syndrome
Ali Al-Hilli, MD, MSc
Sarcoidosis-Like Reaction During Treatment For Metastatic Breast Cancer With CDK 4/6 Inhibitors: Just An Epiphenomenon Or A Causal Relationship?
Scott Slusarenko, DO
Rapidly Progressive Perimyocarditis In SARS-CoV-2 Infection
Agatha M. Formoso, MD
Two Infants Presenting With Polymicrobial Pneumonia And Hypoxemic Respiratory Failure Associated With Heterozygous Variants In Carmil2 And Itk
Juan Adams-Chahin
The Silence Of “Lam”: A Case Of Tuberous Sclerosis Complex Associated With Lymphangioleiomyomatosis (Lam)
Kathleen Capaccione, MD
Lung Cancer Is Not Always The Answer: Exploring The Differential Diagnosis Of Thoracic Masses
Joann Wongvravit, DO
West Nile-Induced Myasthenia Gravis Crisis: An Unexpected Case Of Respiratory Failure
Ethan Karle, Do
A Rare Cause Of Community-Acquired Bacterial Pneumonia In A Patient With Poorly Controlled Diabetes
Taylor C. Becker, MD
Calcified Cavitary Conundrum: Delayed Diagnosis Of Histoplasmosis
Anneka Hutton, MD
Disseminated Listeriosis: A Deadly Triplicate
Omar Kandah, DO
COVID-19 Cardiac Tamponade With Cardiogenic Shock In A Previously Vaccinated Young Adult: A Case Report
Cihan Caglayan, MD
Partial Anomalous Pulmonary Venous Connection Diagnosed After Central Venous Catheter Placement
Michelle Jones, DO
Delayed Hemophagocytic Lymphohistiocytosis (HLH) Diagnosis In A Patient With Pulmonary Sarcoidosis And Newly Diagnosed T-Cell Lymphoma: A Case Report
Mariah Evarts, MD
A Normotensive Woman With Profound Lactic Acidosis And Stress-Induced Cardiomyopathy
Rachel V. Tan, MD
A Four-Boding Future: Polyviral Infection With SARS-CoV-2, Parainfluenza Virus Type 3, Influenza A, And Adenovirus
Thanh Hoang
Recurrent Syncope From Intermittent Torsades In Loperamide Abuse
Alissa Ali, MD
Ground Glass Opacities In A Patient Receiving Treatment With All-Trans Retinoic Acid And Arsenic Trioxide
Sean M. Masi, DO, MBA
Ferritin-Guided Therapeutic Plasma Exchange (TPE) Administration In COVID-19-Induced Cytokine Storm Syndrome: A Case Series
Anjali Sachdeva
Successful Biopsy Of Aortopulmonary Window Lymph Node With Robotic-Assisted Bronchoscopy
Rehan Saeed, MD
Multiple Sclerosis After COVID-19: A Sign Of Things To Come?
Harshitha Mergey Devender
Invasive Pulmonary Aspergillosis Associated With Nonspecific Interstitial Pneumonia Causing Recurrent Respiratory Failure
Be sure to check out the other award winners on page 20 in the January issue of CHEST Physician: https://tinyurl.com/2bcdcbj3 .
President's Report
Here we are, 1 month into the new year, and it already feels like my time as President of the American College of Chest Physicians will pass too quickly. One of my goals is to share some thoughts on issues important to our profession by contributing quarterly to CHEST Physician. CHEST has always been like an extended family to me, and I look forward to having this regular touchpoint with all of you.
For my first written contribution, I want to focus on the future of medicine through medical education and involvement in professional associations because I am, at heart, a medical educator.
During my address at the CHEST Annual Meeting 2022, I spoke on how CHEST provided me with networking, mentoring, and volunteer opportunities that were critical in advancing my career. Those same opportunities should be extended to everyone in pulmonary, critical care, and sleep medicine – whether a current member or prospective member.
Lighting a fire
Attending my first CHEST Annual Meeting was possible due to my nomination for a leadership development course. The connections I made during the meeting really lit a fire within me. We need to engage with early career clinicians and provide them the same exposure and encouragement that I received.
To instill this fire in the next generation, I encourage each of our established members, years (or decades) into their careers, to pass along their expertise to someone who is just starting out, whether it be a trainee or a junior faculty member. If this applies to you: encourage a new attending who has never been to a CHEST event to attend with you; invite a fellow or resident to submit an abstract or case report to the journal CHEST® with your oversight; or simply volunteer to speak at your medical school or residency program about why you chose PCCM and the career it has given you.
Think back to when you were embarking on your journey toward where you are now – what would it have meant to be able to get career advice or even just a friendly conversation started with someone at your current level?
CHEST offerings and accreditations
Beyond bringing someone to a CHEST Annual Meeting – which you should definitely do – work with your learners at medical schools and residency programs to expose them to CHEST much earlier in their careers. The Trainings and Transitions Committee is an excellent resource to guide newer clinicians and can provide a vital source of encouragement and support. If your institution doesn’t have a simulation learning center or if it has limited offerings, the hands-on learning opportunities offered at CHEST headquarters may be a fit. Accredited by the Society for Simulation in Healthcare (SSH) and the Accreditation Council for Continuing Medical Education (ACCME), CHEST currently offers 24 courses with four new courses planned for 2023 in a wide variety of areas, including courses on ultrasound and bronchoscopy.
There are so many ways to introduce early career clinicians to CHEST, and it can begin with one personal outreach. If you are working on a project for CHEST right now, consider inviting an early career clinician to join you on it – this may be the opportunity that will change their career. It did for me.
As medical professionals, each of us plays an important role in the future of medicine, and the CHEST organization can bring us together to strengthen our impact.
If you are interested in brainstorming ideas for how to engage your medical students, residents, or fellows, please feel free to contact me or anyone at CHEST to help create a plan.
I look forward to the next time we connect.
Doreen J. Addrizzo-Harris, MD, FCCP
CHEST President
Here we are, 1 month into the new year, and it already feels like my time as President of the American College of Chest Physicians will pass too quickly. One of my goals is to share some thoughts on issues important to our profession by contributing quarterly to CHEST Physician. CHEST has always been like an extended family to me, and I look forward to having this regular touchpoint with all of you.
For my first written contribution, I want to focus on the future of medicine through medical education and involvement in professional associations because I am, at heart, a medical educator.
During my address at the CHEST Annual Meeting 2022, I spoke on how CHEST provided me with networking, mentoring, and volunteer opportunities that were critical in advancing my career. Those same opportunities should be extended to everyone in pulmonary, critical care, and sleep medicine – whether a current member or prospective member.
Lighting a fire
Attending my first CHEST Annual Meeting was possible due to my nomination for a leadership development course. The connections I made during the meeting really lit a fire within me. We need to engage with early career clinicians and provide them the same exposure and encouragement that I received.
To instill this fire in the next generation, I encourage each of our established members, years (or decades) into their careers, to pass along their expertise to someone who is just starting out, whether it be a trainee or a junior faculty member. If this applies to you: encourage a new attending who has never been to a CHEST event to attend with you; invite a fellow or resident to submit an abstract or case report to the journal CHEST® with your oversight; or simply volunteer to speak at your medical school or residency program about why you chose PCCM and the career it has given you.
Think back to when you were embarking on your journey toward where you are now – what would it have meant to be able to get career advice or even just a friendly conversation started with someone at your current level?
CHEST offerings and accreditations
Beyond bringing someone to a CHEST Annual Meeting – which you should definitely do – work with your learners at medical schools and residency programs to expose them to CHEST much earlier in their careers. The Trainings and Transitions Committee is an excellent resource to guide newer clinicians and can provide a vital source of encouragement and support. If your institution doesn’t have a simulation learning center or if it has limited offerings, the hands-on learning opportunities offered at CHEST headquarters may be a fit. Accredited by the Society for Simulation in Healthcare (SSH) and the Accreditation Council for Continuing Medical Education (ACCME), CHEST currently offers 24 courses with four new courses planned for 2023 in a wide variety of areas, including courses on ultrasound and bronchoscopy.
There are so many ways to introduce early career clinicians to CHEST, and it can begin with one personal outreach. If you are working on a project for CHEST right now, consider inviting an early career clinician to join you on it – this may be the opportunity that will change their career. It did for me.
As medical professionals, each of us plays an important role in the future of medicine, and the CHEST organization can bring us together to strengthen our impact.
If you are interested in brainstorming ideas for how to engage your medical students, residents, or fellows, please feel free to contact me or anyone at CHEST to help create a plan.
I look forward to the next time we connect.
Doreen J. Addrizzo-Harris, MD, FCCP
CHEST President
Here we are, 1 month into the new year, and it already feels like my time as President of the American College of Chest Physicians will pass too quickly. One of my goals is to share some thoughts on issues important to our profession by contributing quarterly to CHEST Physician. CHEST has always been like an extended family to me, and I look forward to having this regular touchpoint with all of you.
For my first written contribution, I want to focus on the future of medicine through medical education and involvement in professional associations because I am, at heart, a medical educator.
During my address at the CHEST Annual Meeting 2022, I spoke on how CHEST provided me with networking, mentoring, and volunteer opportunities that were critical in advancing my career. Those same opportunities should be extended to everyone in pulmonary, critical care, and sleep medicine – whether a current member or prospective member.
Lighting a fire
Attending my first CHEST Annual Meeting was possible due to my nomination for a leadership development course. The connections I made during the meeting really lit a fire within me. We need to engage with early career clinicians and provide them the same exposure and encouragement that I received.
To instill this fire in the next generation, I encourage each of our established members, years (or decades) into their careers, to pass along their expertise to someone who is just starting out, whether it be a trainee or a junior faculty member. If this applies to you: encourage a new attending who has never been to a CHEST event to attend with you; invite a fellow or resident to submit an abstract or case report to the journal CHEST® with your oversight; or simply volunteer to speak at your medical school or residency program about why you chose PCCM and the career it has given you.
Think back to when you were embarking on your journey toward where you are now – what would it have meant to be able to get career advice or even just a friendly conversation started with someone at your current level?
CHEST offerings and accreditations
Beyond bringing someone to a CHEST Annual Meeting – which you should definitely do – work with your learners at medical schools and residency programs to expose them to CHEST much earlier in their careers. The Trainings and Transitions Committee is an excellent resource to guide newer clinicians and can provide a vital source of encouragement and support. If your institution doesn’t have a simulation learning center or if it has limited offerings, the hands-on learning opportunities offered at CHEST headquarters may be a fit. Accredited by the Society for Simulation in Healthcare (SSH) and the Accreditation Council for Continuing Medical Education (ACCME), CHEST currently offers 24 courses with four new courses planned for 2023 in a wide variety of areas, including courses on ultrasound and bronchoscopy.
There are so many ways to introduce early career clinicians to CHEST, and it can begin with one personal outreach. If you are working on a project for CHEST right now, consider inviting an early career clinician to join you on it – this may be the opportunity that will change their career. It did for me.
As medical professionals, each of us plays an important role in the future of medicine, and the CHEST organization can bring us together to strengthen our impact.
If you are interested in brainstorming ideas for how to engage your medical students, residents, or fellows, please feel free to contact me or anyone at CHEST to help create a plan.
I look forward to the next time we connect.
Doreen J. Addrizzo-Harris, MD, FCCP
CHEST President
CHEST simulation courses support learning for every career stage
One mark of an excellent clinician is their commitment to lifelong learning, and CHEST’s hands-on simulation courses offer the chance for practitioners of all experience levels to enhance their knowledge.
A variety of interactive courses are offered at CHEST’s state-of-the-art Innovation, Simulation, and Training Center in Glenview, Illinois, covering topics like ultrasonography, bronchoscopy, and mechanical ventilation. And this year, our simulation schedule will offer several new sessions on advances in invasive and noninvasive ventilation, critical care transesophageal echocardiography, master-level EBUS practice, and mechanical circulatory support.
Each course is led by expert instructors and includes attendees from a full range of career stages, from trainees and mid-career clinicians to long-time CHEST faculty members.
At a fall 2022 session of the Ultrasonography: Essentials in Critical Care course, Adil Ahmed, MD, an intern at the University of Texas Health Science Center at San Antonio, shared his perspective attending as a resident.
“CHEST has lots of specialized resources and renowned faculty members, and they’re doing an exceptional job,” he said. “A lot of the things I’ve learned in the first workshop alone are completely brand new to me. I think more programs should start sending residents to these courses.”
Trainees don’t just attend simulation courses – they teach them, too. Carmen Mei, MD, a pulmonary and critical care fellow at Rutgers University, was a faculty member at the recent ultrasound course. She taught attendees representing a wide array of ages.
“It’s a learning environment. Everybody’s very engaged, no matter where they are in their career,” she said.
As a mid-career clinician, Yonatan Y. Greenstein, MD, FCCP – who serves as a co-chair of the ultrasonography course – appreciates the diversity of experiences among attendees.
“Over the years, we’ve found that the wide breadth enhances the course because learners appreciate the questions that are brought up from different angles,” he said.
For experienced clinicians like CHEST Immediate Past President David Schulman, MD, MPH, FCCP, the interactive courses provide an opportunity to continue expanding his expertise. At the ultrasound course, Dr. Schulman said he enjoyed the chance to extend and refine his skillset alongside clinicians with a broad range of experience levels.
“Ultrasound is one of those skills that many clinicians, even in their forties and older, have never trained in. It’s great to see how the more junior learners approach this with a very excited mindset, and they’re learning right beside mid-career faculty who didn’t have the exposure to ultrasound when they were young,” he said.
To find the simulation course that’s the best fit for your practice, visit chestnet.org/simulation.
One mark of an excellent clinician is their commitment to lifelong learning, and CHEST’s hands-on simulation courses offer the chance for practitioners of all experience levels to enhance their knowledge.
A variety of interactive courses are offered at CHEST’s state-of-the-art Innovation, Simulation, and Training Center in Glenview, Illinois, covering topics like ultrasonography, bronchoscopy, and mechanical ventilation. And this year, our simulation schedule will offer several new sessions on advances in invasive and noninvasive ventilation, critical care transesophageal echocardiography, master-level EBUS practice, and mechanical circulatory support.
Each course is led by expert instructors and includes attendees from a full range of career stages, from trainees and mid-career clinicians to long-time CHEST faculty members.
At a fall 2022 session of the Ultrasonography: Essentials in Critical Care course, Adil Ahmed, MD, an intern at the University of Texas Health Science Center at San Antonio, shared his perspective attending as a resident.
“CHEST has lots of specialized resources and renowned faculty members, and they’re doing an exceptional job,” he said. “A lot of the things I’ve learned in the first workshop alone are completely brand new to me. I think more programs should start sending residents to these courses.”
Trainees don’t just attend simulation courses – they teach them, too. Carmen Mei, MD, a pulmonary and critical care fellow at Rutgers University, was a faculty member at the recent ultrasound course. She taught attendees representing a wide array of ages.
“It’s a learning environment. Everybody’s very engaged, no matter where they are in their career,” she said.
As a mid-career clinician, Yonatan Y. Greenstein, MD, FCCP – who serves as a co-chair of the ultrasonography course – appreciates the diversity of experiences among attendees.
“Over the years, we’ve found that the wide breadth enhances the course because learners appreciate the questions that are brought up from different angles,” he said.
For experienced clinicians like CHEST Immediate Past President David Schulman, MD, MPH, FCCP, the interactive courses provide an opportunity to continue expanding his expertise. At the ultrasound course, Dr. Schulman said he enjoyed the chance to extend and refine his skillset alongside clinicians with a broad range of experience levels.
“Ultrasound is one of those skills that many clinicians, even in their forties and older, have never trained in. It’s great to see how the more junior learners approach this with a very excited mindset, and they’re learning right beside mid-career faculty who didn’t have the exposure to ultrasound when they were young,” he said.
To find the simulation course that’s the best fit for your practice, visit chestnet.org/simulation.
One mark of an excellent clinician is their commitment to lifelong learning, and CHEST’s hands-on simulation courses offer the chance for practitioners of all experience levels to enhance their knowledge.
A variety of interactive courses are offered at CHEST’s state-of-the-art Innovation, Simulation, and Training Center in Glenview, Illinois, covering topics like ultrasonography, bronchoscopy, and mechanical ventilation. And this year, our simulation schedule will offer several new sessions on advances in invasive and noninvasive ventilation, critical care transesophageal echocardiography, master-level EBUS practice, and mechanical circulatory support.
Each course is led by expert instructors and includes attendees from a full range of career stages, from trainees and mid-career clinicians to long-time CHEST faculty members.
At a fall 2022 session of the Ultrasonography: Essentials in Critical Care course, Adil Ahmed, MD, an intern at the University of Texas Health Science Center at San Antonio, shared his perspective attending as a resident.
“CHEST has lots of specialized resources and renowned faculty members, and they’re doing an exceptional job,” he said. “A lot of the things I’ve learned in the first workshop alone are completely brand new to me. I think more programs should start sending residents to these courses.”
Trainees don’t just attend simulation courses – they teach them, too. Carmen Mei, MD, a pulmonary and critical care fellow at Rutgers University, was a faculty member at the recent ultrasound course. She taught attendees representing a wide array of ages.
“It’s a learning environment. Everybody’s very engaged, no matter where they are in their career,” she said.
As a mid-career clinician, Yonatan Y. Greenstein, MD, FCCP – who serves as a co-chair of the ultrasonography course – appreciates the diversity of experiences among attendees.
“Over the years, we’ve found that the wide breadth enhances the course because learners appreciate the questions that are brought up from different angles,” he said.
For experienced clinicians like CHEST Immediate Past President David Schulman, MD, MPH, FCCP, the interactive courses provide an opportunity to continue expanding his expertise. At the ultrasound course, Dr. Schulman said he enjoyed the chance to extend and refine his skillset alongside clinicians with a broad range of experience levels.
“Ultrasound is one of those skills that many clinicians, even in their forties and older, have never trained in. It’s great to see how the more junior learners approach this with a very excited mindset, and they’re learning right beside mid-career faculty who didn’t have the exposure to ultrasound when they were young,” he said.
To find the simulation course that’s the best fit for your practice, visit chestnet.org/simulation.
CHEST President shares inside look at priorities, plans for 2023
Attendees at the CHEST 2022 Opening Session on October 16 got a sneak peek into plans and priorities for CHEST President Doreen J. Addrizzo-Harris, MD, FCCP, in 2023 – and some insights into her own path to the role.
A longtime leader at CHEST, she shared how members’ pandemic response reminded her of the great impact the organization can have. In March 2020, Dr. Addrizzo-Harris was overseeing ICU staffing at NYU Langone Health’s Bellevue Hospital Center and organizing dozens of volunteer physicians to help meet the pandemic care burden.
“I knew all too quickly that we wouldn’t have enough intensivists,” said Dr. Addrizzo-Harris. “It was a quick call very late one night, probably around 1 am, that I made to CHEST CEO, Bob Musacchio, that helped materialize a monumental effort ... many of these physicians were CHEST members themselves. They were fearless and unselfish, and they came to help us in our time of need.”
She saw this same spirit of dedication and drive in CHEST’s leadership and staff, she said – one she will continue and expand upon during her presidency.
“I’ve watched our last three presidents lead by great example ... with innovation and nimbleness, in a time when we were so isolated from each other and so tired from the long hours that we worked each day,” she said. “They, along with the Board of Regents, the CEO, and our phenomenal staff, were able to keep CHEST amazingly alive and vibrant and more connected than ever. They are truly inspiring. For 2023, I hope to take this incredible energy to the next level.”
As CHEST president, Dr. in the United States and advancing international outreach initiatives launched by CHEST Past President David Schulman, MD, MPH, FCCP. This also includes supporting and building upon CHEST’s ongoing commitment to diversity, equity, and inclusion initiatives to encourage greater representation in the field and improve patient care.
“Whether it’s through supporting our clinical research grants, expanding patient education and advocacy, or programs like the First 5 Minutes™ and the Harold Amos scholarship program, we want to train our leaders for the future,” she said.
Revisit the September issue of CHEST Physician, and watch future issues to learn more about Dr. Addrizzo-Harris and her plans for the presidency.
Attendees at the CHEST 2022 Opening Session on October 16 got a sneak peek into plans and priorities for CHEST President Doreen J. Addrizzo-Harris, MD, FCCP, in 2023 – and some insights into her own path to the role.
A longtime leader at CHEST, she shared how members’ pandemic response reminded her of the great impact the organization can have. In March 2020, Dr. Addrizzo-Harris was overseeing ICU staffing at NYU Langone Health’s Bellevue Hospital Center and organizing dozens of volunteer physicians to help meet the pandemic care burden.
“I knew all too quickly that we wouldn’t have enough intensivists,” said Dr. Addrizzo-Harris. “It was a quick call very late one night, probably around 1 am, that I made to CHEST CEO, Bob Musacchio, that helped materialize a monumental effort ... many of these physicians were CHEST members themselves. They were fearless and unselfish, and they came to help us in our time of need.”
She saw this same spirit of dedication and drive in CHEST’s leadership and staff, she said – one she will continue and expand upon during her presidency.
“I’ve watched our last three presidents lead by great example ... with innovation and nimbleness, in a time when we were so isolated from each other and so tired from the long hours that we worked each day,” she said. “They, along with the Board of Regents, the CEO, and our phenomenal staff, were able to keep CHEST amazingly alive and vibrant and more connected than ever. They are truly inspiring. For 2023, I hope to take this incredible energy to the next level.”
As CHEST president, Dr. in the United States and advancing international outreach initiatives launched by CHEST Past President David Schulman, MD, MPH, FCCP. This also includes supporting and building upon CHEST’s ongoing commitment to diversity, equity, and inclusion initiatives to encourage greater representation in the field and improve patient care.
“Whether it’s through supporting our clinical research grants, expanding patient education and advocacy, or programs like the First 5 Minutes™ and the Harold Amos scholarship program, we want to train our leaders for the future,” she said.
Revisit the September issue of CHEST Physician, and watch future issues to learn more about Dr. Addrizzo-Harris and her plans for the presidency.
Attendees at the CHEST 2022 Opening Session on October 16 got a sneak peek into plans and priorities for CHEST President Doreen J. Addrizzo-Harris, MD, FCCP, in 2023 – and some insights into her own path to the role.
A longtime leader at CHEST, she shared how members’ pandemic response reminded her of the great impact the organization can have. In March 2020, Dr. Addrizzo-Harris was overseeing ICU staffing at NYU Langone Health’s Bellevue Hospital Center and organizing dozens of volunteer physicians to help meet the pandemic care burden.
“I knew all too quickly that we wouldn’t have enough intensivists,” said Dr. Addrizzo-Harris. “It was a quick call very late one night, probably around 1 am, that I made to CHEST CEO, Bob Musacchio, that helped materialize a monumental effort ... many of these physicians were CHEST members themselves. They were fearless and unselfish, and they came to help us in our time of need.”
She saw this same spirit of dedication and drive in CHEST’s leadership and staff, she said – one she will continue and expand upon during her presidency.
“I’ve watched our last three presidents lead by great example ... with innovation and nimbleness, in a time when we were so isolated from each other and so tired from the long hours that we worked each day,” she said. “They, along with the Board of Regents, the CEO, and our phenomenal staff, were able to keep CHEST amazingly alive and vibrant and more connected than ever. They are truly inspiring. For 2023, I hope to take this incredible energy to the next level.”
As CHEST president, Dr. in the United States and advancing international outreach initiatives launched by CHEST Past President David Schulman, MD, MPH, FCCP. This also includes supporting and building upon CHEST’s ongoing commitment to diversity, equity, and inclusion initiatives to encourage greater representation in the field and improve patient care.
“Whether it’s through supporting our clinical research grants, expanding patient education and advocacy, or programs like the First 5 Minutes™ and the Harold Amos scholarship program, we want to train our leaders for the future,” she said.
Revisit the September issue of CHEST Physician, and watch future issues to learn more about Dr. Addrizzo-Harris and her plans for the presidency.
CHEST 2022 award winners More award winners
Each year,
MASTER FELLOW AWARD
Gerard A. Silvestri, MD, MS, Master FCCP
DISTINGUISHED SERVICE AWARD
Aneesa M. Das, MD, FCCP
COLLEGE MEDALIST AWARD
William R. Auger, MD, FCCP
ALFRED SOFFER AWARD FOR EDITORIAL EXCELLENCE
Todd W. Rice, MD, FCCP
EARLY CAREER CLINICIAN EDUCATOR AWARD
Mauricio Danckers, MD, FCCP
MASTER CLINICIAN EDUCATOR AWARD
Neil R. MacIntyre, MD, FCCP
PRESIDENTIAL CITATION
CHEST Staff
EDWARD C. ROSENOW III, MD, MASTER FCCP/MASTER TEACHER ENDOWED HONOR LECTURE
Alexander S. Niven, MD, FCCP
THOMAS L. PETTY, MD, MASTER FCCP MEMORIAL LECTURE
Sandra G. Adams, MD, FCCP
2021 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Kenneth I. Berger, MD, FCCP
PRESIDENTIAL HONOR LECTURE
Jack D. Buckley, MD, MPH, FCCP
PASQUALE CIAGLIA MEMORIAL LECTURE IN INTERVENTIONAL MEDICINE
Nicholas J. Pastis, MD, FCCP
ROGER C. BONE MEMORIAL LECTURE IN CRITICAL CARE
E. Wesley Ely, MD, MPH, FCCP
MURRAY KORNFELD MEMORIAL FOUNDERS AWARD
Marin H. Kollef, MD, FCCP
OM P. SHARMA, MD, MASTER FCCP MEMORIAL LECTURE
Daniel A. Culver, DO, FCCP
RICHARD S. IRWIN, MD, MASTER FCCP HONOR LECTURE
Nneka O. Sederstrom, PhD, MS, MA, FCCP
2022 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Martin J. Tobin, MBBCh, FCCP
MARK J. ROSEN, MD, MASTER FCCP ENDOWED MEMORIAL LECTURE
Stephanie M. Levine, MD, FCCP
MARGARET PFROMMER ENDOWED MEMORIAL LECTURE IN HOME-BASED MECHANICAL VENTILATION
Lisa Wolfe, MD, FCCP
CHEST CHALLENGE FINALISTS
1st Place – Mayo Clinic
Amjad Kanj, MD
Paige Marty, MD
Zhenmei Zhang, MD
Program Director: Darlene Nelson, MD, FCCP
2nd Place – Brooke Army Medical Center
Joshua Boster, MD
Tyler Campbell, DO
Daniel Foster, MD
Program Director: Robert Walter, MD, PhD
3rd Place – NewYork-Presbyterian Brooklyn Methodist
Albina Guri, DO
Jahrul Islam, MD
Sylvana Salama, MD
Program Director: Anthony Saleh, MD, FCCP
Please Note: Award winners from the following categories will be listed in the February issue of CHEST Physician.
CHEST Foundation Grant Awards
Scientific Abstract Awards
Alfred Soffer Research Award Winners
Young Investigator Award Winners
Abstract Rapid Fire Winners
Case Report Session Winners
Case Report Rapid Fire Winners
Each year,
MASTER FELLOW AWARD
Gerard A. Silvestri, MD, MS, Master FCCP
DISTINGUISHED SERVICE AWARD
Aneesa M. Das, MD, FCCP
COLLEGE MEDALIST AWARD
William R. Auger, MD, FCCP
ALFRED SOFFER AWARD FOR EDITORIAL EXCELLENCE
Todd W. Rice, MD, FCCP
EARLY CAREER CLINICIAN EDUCATOR AWARD
Mauricio Danckers, MD, FCCP
MASTER CLINICIAN EDUCATOR AWARD
Neil R. MacIntyre, MD, FCCP
PRESIDENTIAL CITATION
CHEST Staff
EDWARD C. ROSENOW III, MD, MASTER FCCP/MASTER TEACHER ENDOWED HONOR LECTURE
Alexander S. Niven, MD, FCCP
THOMAS L. PETTY, MD, MASTER FCCP MEMORIAL LECTURE
Sandra G. Adams, MD, FCCP
2021 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Kenneth I. Berger, MD, FCCP
PRESIDENTIAL HONOR LECTURE
Jack D. Buckley, MD, MPH, FCCP
PASQUALE CIAGLIA MEMORIAL LECTURE IN INTERVENTIONAL MEDICINE
Nicholas J. Pastis, MD, FCCP
ROGER C. BONE MEMORIAL LECTURE IN CRITICAL CARE
E. Wesley Ely, MD, MPH, FCCP
MURRAY KORNFELD MEMORIAL FOUNDERS AWARD
Marin H. Kollef, MD, FCCP
OM P. SHARMA, MD, MASTER FCCP MEMORIAL LECTURE
Daniel A. Culver, DO, FCCP
RICHARD S. IRWIN, MD, MASTER FCCP HONOR LECTURE
Nneka O. Sederstrom, PhD, MS, MA, FCCP
2022 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Martin J. Tobin, MBBCh, FCCP
MARK J. ROSEN, MD, MASTER FCCP ENDOWED MEMORIAL LECTURE
Stephanie M. Levine, MD, FCCP
MARGARET PFROMMER ENDOWED MEMORIAL LECTURE IN HOME-BASED MECHANICAL VENTILATION
Lisa Wolfe, MD, FCCP
CHEST CHALLENGE FINALISTS
1st Place – Mayo Clinic
Amjad Kanj, MD
Paige Marty, MD
Zhenmei Zhang, MD
Program Director: Darlene Nelson, MD, FCCP
2nd Place – Brooke Army Medical Center
Joshua Boster, MD
Tyler Campbell, DO
Daniel Foster, MD
Program Director: Robert Walter, MD, PhD
3rd Place – NewYork-Presbyterian Brooklyn Methodist
Albina Guri, DO
Jahrul Islam, MD
Sylvana Salama, MD
Program Director: Anthony Saleh, MD, FCCP
Please Note: Award winners from the following categories will be listed in the February issue of CHEST Physician.
CHEST Foundation Grant Awards
Scientific Abstract Awards
Alfred Soffer Research Award Winners
Young Investigator Award Winners
Abstract Rapid Fire Winners
Case Report Session Winners
Case Report Rapid Fire Winners
Each year,
MASTER FELLOW AWARD
Gerard A. Silvestri, MD, MS, Master FCCP
DISTINGUISHED SERVICE AWARD
Aneesa M. Das, MD, FCCP
COLLEGE MEDALIST AWARD
William R. Auger, MD, FCCP
ALFRED SOFFER AWARD FOR EDITORIAL EXCELLENCE
Todd W. Rice, MD, FCCP
EARLY CAREER CLINICIAN EDUCATOR AWARD
Mauricio Danckers, MD, FCCP
MASTER CLINICIAN EDUCATOR AWARD
Neil R. MacIntyre, MD, FCCP
PRESIDENTIAL CITATION
CHEST Staff
EDWARD C. ROSENOW III, MD, MASTER FCCP/MASTER TEACHER ENDOWED HONOR LECTURE
Alexander S. Niven, MD, FCCP
THOMAS L. PETTY, MD, MASTER FCCP MEMORIAL LECTURE
Sandra G. Adams, MD, FCCP
2021 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Kenneth I. Berger, MD, FCCP
PRESIDENTIAL HONOR LECTURE
Jack D. Buckley, MD, MPH, FCCP
PASQUALE CIAGLIA MEMORIAL LECTURE IN INTERVENTIONAL MEDICINE
Nicholas J. Pastis, MD, FCCP
ROGER C. BONE MEMORIAL LECTURE IN CRITICAL CARE
E. Wesley Ely, MD, MPH, FCCP
MURRAY KORNFELD MEMORIAL FOUNDERS AWARD
Marin H. Kollef, MD, FCCP
OM P. SHARMA, MD, MASTER FCCP MEMORIAL LECTURE
Daniel A. Culver, DO, FCCP
RICHARD S. IRWIN, MD, MASTER FCCP HONOR LECTURE
Nneka O. Sederstrom, PhD, MS, MA, FCCP
2022 DISTINGUISHED SCIENTIST HONOR LECTURE IN CARDIOPULMONARY PHYSIOLOGY
Martin J. Tobin, MBBCh, FCCP
MARK J. ROSEN, MD, MASTER FCCP ENDOWED MEMORIAL LECTURE
Stephanie M. Levine, MD, FCCP
MARGARET PFROMMER ENDOWED MEMORIAL LECTURE IN HOME-BASED MECHANICAL VENTILATION
Lisa Wolfe, MD, FCCP
CHEST CHALLENGE FINALISTS
1st Place – Mayo Clinic
Amjad Kanj, MD
Paige Marty, MD
Zhenmei Zhang, MD
Program Director: Darlene Nelson, MD, FCCP
2nd Place – Brooke Army Medical Center
Joshua Boster, MD
Tyler Campbell, DO
Daniel Foster, MD
Program Director: Robert Walter, MD, PhD
3rd Place – NewYork-Presbyterian Brooklyn Methodist
Albina Guri, DO
Jahrul Islam, MD
Sylvana Salama, MD
Program Director: Anthony Saleh, MD, FCCP
Please Note: Award winners from the following categories will be listed in the February issue of CHEST Physician.
CHEST Foundation Grant Awards
Scientific Abstract Awards
Alfred Soffer Research Award Winners
Young Investigator Award Winners
Abstract Rapid Fire Winners
Case Report Session Winners
Case Report Rapid Fire Winners
CHEST Challenge returned to the stage in the Music City
After several years of virtual competitions, the CHEST Challenge Championship returned to the stage at CHEST 2022 in Nashville, where outstanding fellows from Brooke Army Medical Center, Mayo Clinic, and NewYork-Presbyterian Brooklyn Methodist battled to compete in unconventional skills challenges and clinical trivia.
After an excellent showing from all three institutions, (not to mention, the ultimate bragging rights and the chance to raise the coveted Rosen Cup). Runner-up Brooke Army Medical Center received $3,000, and NewYork-Presbyterian Brooklyn Methodist received $1,000.
This year’s Jeopardy-style championship included a variety of category types, including everything from straightforward clinical answers in “Asthmalogic” about asthma-related issues and “Under a Microscope” for topics related to histopathology, to brain-boggling alternate options, such as “Rhyme Time,” which twisted answers in rhyming phrases.
The competition also included timed skills challenges that tested the competitors physically – and presented some very special guests.
In “Bugs and Drugs,” Team Methodist sprinted to grab and then matched unlabeled pathogen photographs with their appropriate therapeutic agents in less than 35 seconds. In another, Team Brooke aced the challenge of performing timed procedures on three different body parts in Dr. Frankenstein’s laboratory, while the monster himself (played by Board of Regents member Victor J. Test, MD, FCCP) worked to distract them.
Mayo Clinic was already in the lead by the time the Final Challenge wager was presented by William Kelly, MD, FCCP, so the team responded to the answer “This disease is inherited as an autosomal recessive trait and is a variant in the SCL34A2 gene” with their own unique reply: “Thank you, CHEST,” and a symbolic wager of $22.
Drs. Kanj, Marty, and Zhang credited their success to their training program back home, as well as the support of friends and colleagues on-site, including Program Director, Darlene Nelson, MD, FCCP. The team also prepared with mock sessions days before the championship and had a strong fan base cheering them on in the audience.
Want to join rising stars in pulmonary, critical care, and sleep medicine for next year’s championship in Hawai’i? Watch CHEST’s social media in the spring for the first phase of CHEST Challenge.
After several years of virtual competitions, the CHEST Challenge Championship returned to the stage at CHEST 2022 in Nashville, where outstanding fellows from Brooke Army Medical Center, Mayo Clinic, and NewYork-Presbyterian Brooklyn Methodist battled to compete in unconventional skills challenges and clinical trivia.
After an excellent showing from all three institutions, (not to mention, the ultimate bragging rights and the chance to raise the coveted Rosen Cup). Runner-up Brooke Army Medical Center received $3,000, and NewYork-Presbyterian Brooklyn Methodist received $1,000.
This year’s Jeopardy-style championship included a variety of category types, including everything from straightforward clinical answers in “Asthmalogic” about asthma-related issues and “Under a Microscope” for topics related to histopathology, to brain-boggling alternate options, such as “Rhyme Time,” which twisted answers in rhyming phrases.
The competition also included timed skills challenges that tested the competitors physically – and presented some very special guests.
In “Bugs and Drugs,” Team Methodist sprinted to grab and then matched unlabeled pathogen photographs with their appropriate therapeutic agents in less than 35 seconds. In another, Team Brooke aced the challenge of performing timed procedures on three different body parts in Dr. Frankenstein’s laboratory, while the monster himself (played by Board of Regents member Victor J. Test, MD, FCCP) worked to distract them.
Mayo Clinic was already in the lead by the time the Final Challenge wager was presented by William Kelly, MD, FCCP, so the team responded to the answer “This disease is inherited as an autosomal recessive trait and is a variant in the SCL34A2 gene” with their own unique reply: “Thank you, CHEST,” and a symbolic wager of $22.
Drs. Kanj, Marty, and Zhang credited their success to their training program back home, as well as the support of friends and colleagues on-site, including Program Director, Darlene Nelson, MD, FCCP. The team also prepared with mock sessions days before the championship and had a strong fan base cheering them on in the audience.
Want to join rising stars in pulmonary, critical care, and sleep medicine for next year’s championship in Hawai’i? Watch CHEST’s social media in the spring for the first phase of CHEST Challenge.
After several years of virtual competitions, the CHEST Challenge Championship returned to the stage at CHEST 2022 in Nashville, where outstanding fellows from Brooke Army Medical Center, Mayo Clinic, and NewYork-Presbyterian Brooklyn Methodist battled to compete in unconventional skills challenges and clinical trivia.
After an excellent showing from all three institutions, (not to mention, the ultimate bragging rights and the chance to raise the coveted Rosen Cup). Runner-up Brooke Army Medical Center received $3,000, and NewYork-Presbyterian Brooklyn Methodist received $1,000.
This year’s Jeopardy-style championship included a variety of category types, including everything from straightforward clinical answers in “Asthmalogic” about asthma-related issues and “Under a Microscope” for topics related to histopathology, to brain-boggling alternate options, such as “Rhyme Time,” which twisted answers in rhyming phrases.
The competition also included timed skills challenges that tested the competitors physically – and presented some very special guests.
In “Bugs and Drugs,” Team Methodist sprinted to grab and then matched unlabeled pathogen photographs with their appropriate therapeutic agents in less than 35 seconds. In another, Team Brooke aced the challenge of performing timed procedures on three different body parts in Dr. Frankenstein’s laboratory, while the monster himself (played by Board of Regents member Victor J. Test, MD, FCCP) worked to distract them.
Mayo Clinic was already in the lead by the time the Final Challenge wager was presented by William Kelly, MD, FCCP, so the team responded to the answer “This disease is inherited as an autosomal recessive trait and is a variant in the SCL34A2 gene” with their own unique reply: “Thank you, CHEST,” and a symbolic wager of $22.
Drs. Kanj, Marty, and Zhang credited their success to their training program back home, as well as the support of friends and colleagues on-site, including Program Director, Darlene Nelson, MD, FCCP. The team also prepared with mock sessions days before the championship and had a strong fan base cheering them on in the audience.
Want to join rising stars in pulmonary, critical care, and sleep medicine for next year’s championship in Hawai’i? Watch CHEST’s social media in the spring for the first phase of CHEST Challenge.