User login
An update on the pharmacologic treatment of hypersomnia
The hypersomnias are an etiologically diverse group of disorders of wakefulness and sleep, characterized principally by excessive daytime sleepiness (EDS), often despite sufficient or even long total sleep durations. Hypersomnolence may be severely disabling and isolating for patients, is associated with decreased quality of life and economic disadvantage, and, in some cases, may pose a personal and public health danger through drowsy driving. Though historically, management of these patients has been principally supportive and aimed at reducing daytime functional impairment, new and evolving treatments are quickly changing management paradigms in this population. This brief review highlights some of the newest pharmacotherapeutic advances in this dynamic field.
Hypersomnolence is a common presenting concern primary care and sleep clinics, with an estimated prevalence of EDS in the general adult population of as high as 6%.1 The initial diagnosis of hypersomnia is, broadly, a clinical one, with careful consideration to the patient’s report of daytime sleepiness and functional impairment, sleep/wake cycle, and any medical comorbidities. The primary hypersomnias include narcolepsy type 1 (narcolepsy with cataplexy, NT1) and narcolepsy type 2 (without cataplexy, NT2), Kleine-Levin Syndrome (KLS), and idiopathic hypersomnia. Secondary hypersomnia disorders are more commonly encountered in clinical practice and include hypersomnia attributable to another medical condition (including psychiatric and neurologic disorders), hypersomnia related to medication effects, and EDS related to behaviorally insufficient sleep. Distinguishing primary and secondary etiologies, when possible, is important as treatment pathways may vary considerably between hypersomnias.
Generally, overnight in-lab polysomnography is warranted to exclude untreated or sub-optimally treated sleep-disordered breathing or movement disorders which may undermine sleep quality. In the absence of any such findings, this is usually followed by daytime multiple sleep latency testing (MSLT). The MSLT is comprised of four to five scheduled daytime naps in the sleep lab and is designed to quantify a patient’s propensity to sleep during the day and to identify architectural sleep abnormalities which indicate narcolepsy. Specifically, narcolepsy is identified by MSLT when a patient exhibits a sleep onset latency of ≤ 8 minutes and at least two sleep-onset REM periods (SOREMPs), or, one SOREMP on MSLT with a second noted on the preceding night’s PSG. Actigraphy or sleep logs may be helpful in quantifying a patient’s total sleep time in their home environment. Adjunctive laboratory tests for narcolepsy including HLA typing and CSF hypocretin testing may sometimes be indicated.
General hypersomnia management usually consists of the use of wakefulness promoting agents, such as stimulants (eg, dexmethylphenidate) and dopamine-modulating agents (eg, modafinil, armodafinil), and adjunctive supportive strategies, including planned daytime naps and elimination of modifiable secondary causes. In those patients with hypersomnolence associated with depression or anxiety, the use of antidepressants, including SSRI, SNRI, and DNRIs, is often effective, and these medications can also improve cataplexy symptoms in narcoleptics. KLS may respond to treatment with lithium, shortening the duration of the striking hypersomnolent episodes characteristic of this rare syndrome, and there is some indication that ketamine may also be a helpful adjunctive in some cases. In treatment-refractory cases of hypersomnolence associated with GABA-A receptor potentiation, drugs such as flumazenil and clarithromycin appear to improve subjective measures of hypersomnolence.2,3 In patients with narcolepsy, sodium oxybate (available as Xyrem and, more recently, as a generic medication) has proven to be clinically very useful, reducing EDS and the frequency and severity of cataplexy and sleep disturbance associated with this condition. In July 2020, the FDA approved a new, low-sodium formulation of sodium oxybate (Xywav) for patients 7 years of age and older with a diagnosis of narcolepsy, a helpful option in those patients with cardiovascular and renal disease.
Despite this broadening armamentarium, in many cases daytime sleepiness and functional impairment is refractory to typical pharmacotherapy. In this context, we would like to highlight two newer pharmacotherapeutic options, solriamfetol and pitolisant.
Solriamfetol
Solriamfetol (Sunosi) is a Schedule IV FDA-approved medication indicated for treatment of EDS in adults with narcolepsy or obstructive sleep apnea. The precise mechanism of action is unknown, but this medication is believed to inhibit both dopamine and norepinerphrine reuptake in the brain, similar to the widely-prescribed NDRI buproprion. In a 12-week RCT study on its effects on narcolepsy in adults, solriamfetol improved important measures of wakefulness and sleepiness, without associated polysomnographic evidence of significant sleep disruption.4 In another 12-week RCT study of solriamfetol in adult patients with EDS related to OSA, there was a dose-dependent improvement in measures of wakefulness.5 Some notable side-effects seen with this medication include anxiety and elevated mood, as well as increases in blood pressure. A subsequent study of this medication found that it was efficacious at maintenance of improvements at 6 months.6 Given the theorized mechanism of action as an NDRI, future observation and studies could provide insights on its effect on depression, as well.
Pitolisant
Histaminergic neurons within the CNS play an important role in the promotion of wakefulness. Pitolisant (Wakix) is an interesting wakefulness-promoting agent for adult patients with narcolepsy. It acts as an inverse agonist and antagonist of histamine H3 receptors, resulting in a reduction of the usual feedback inhibition effected through the H3 receptor, thereby enhancing CNS release of histamine and other neurotransmitters. This medication was approved by the FDA in August 2019 and is currently indicated for adult patients with narcolepsy. The HARMONY I trial comparing pitolisant with both placebo and modafinil in adults with narcolepsy and EDS demonstrated improvement in measures of sleepiness and maintenance of wakefulness over placebo, and noninferiority to modafinil.7 In addition, pitolisant had a favorable side-effect profile compared with modafinil. Subsequent studies have reaffirmed the safety profile of pitolisant, including its minimal abuse potential. In one recent placebo-controlled trial of the use of pitolisant in a population of 268 adults with positive airway pressure (PAP) non-adherence, pitolisant was found to improve measures of EDS and related patient-reported measurements in patients with OSA who were CPAP nonadherent.8 Though generally well-tolerated by patients, in initial clinical trials pitolisant was associated with increased headache, insomnia, and nausea relative to placebo, among other less commonly reported adverse effects. Pitolisant is QT interval-prolonging, so caution must be taken when using this medication in combination other medications which may induce QT interval prolongation, including SSRIs.
Future directions
Greater awareness of the hypersomnias and their management has led to improved outcomes and access to care for these patients, yet these disorders remain burdensome and the treatments imperfect. Looking forward, novel pharmacotherapies that target underlying mechanisms rather than symptom palliation will allow for more precise treatments. Ongoing investigations of hypocretin receptor agonists seek to target one critical central mediator of wakefulness. Recent studies have highlighted the association of dysautonomia with hypersomnia, offering interesting insight into possible future targets to improve the function and quality of life of these patients.9 Similarly, understanding of the interplay between psychiatric disorders and primary and secondary hypersomnias may offer new therapeutic pathways.
As treatment plans targeting hypersomnia become more comprehensive and holistic, with an increased emphasis on self-care, sleep hygiene, and mental health awareness, in addition to mechanism-specific treatments, we hope they will ultimately provide improved symptom and burden relief for our patients.
Dr. Shih Yee-Marie Tan Gipson is a psychiatrist and Dr. Kevin Gipson is a sleep medicine specialist, both with Massachusetts General Hospital, Boston.
References
1 Dauvilliers, et al. Hypersomnia. Dialogues Clin Neurosci. 2005;7(4):347-356.
2 Trotti, et al. Clarithromycin in gamma-aminobutyric acid-related hypersomnolence: A randomized, crossover trial. Ann Neurol. 2015;78(3):454-465. doi: 10.1002/ana.24459.
3 Trotti, et al. Flumazenil for the treatment of refractory hypersomnolence: Clinical experience with 153 patients. J Clin Sleep Med. 2016;12(10):1389-1394. doi: 10.5664/jcsm.6196.
4 Thorpy, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019; 85(3):359-370. doi: 10.1002/ana.25423.
5 Schweitzer, et al. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): A randomized controlled trial. Am J Respir Crit Care Med. 2019;199(11):1421-1431. doi: 10.1164/rccm.201806-1100OC.
6 Malhotra, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020; 43(2): doi: 10.1093/sleep/zsz220.
7 Dauvilliers, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068-1075. doi: 10.1016/S1474-4422(13)70225-4.
8 Dauvilliers, et al. Pitolisant for daytime sleepiness in obstructive sleep apnea patients refusing CPAP: A randomized trial. Am J Respir Crit Care Med. 2020. doi: 10.1164/rccm.201907-1284OC.
9 Miglis, et al. Frequency and severity of autonomic symptoms in idiopathic hypersomnia. J Clin Sleep Med. 2020; 16(5):749-756. doi: 10.5664/jcsm.8344.
The hypersomnias are an etiologically diverse group of disorders of wakefulness and sleep, characterized principally by excessive daytime sleepiness (EDS), often despite sufficient or even long total sleep durations. Hypersomnolence may be severely disabling and isolating for patients, is associated with decreased quality of life and economic disadvantage, and, in some cases, may pose a personal and public health danger through drowsy driving. Though historically, management of these patients has been principally supportive and aimed at reducing daytime functional impairment, new and evolving treatments are quickly changing management paradigms in this population. This brief review highlights some of the newest pharmacotherapeutic advances in this dynamic field.
Hypersomnolence is a common presenting concern primary care and sleep clinics, with an estimated prevalence of EDS in the general adult population of as high as 6%.1 The initial diagnosis of hypersomnia is, broadly, a clinical one, with careful consideration to the patient’s report of daytime sleepiness and functional impairment, sleep/wake cycle, and any medical comorbidities. The primary hypersomnias include narcolepsy type 1 (narcolepsy with cataplexy, NT1) and narcolepsy type 2 (without cataplexy, NT2), Kleine-Levin Syndrome (KLS), and idiopathic hypersomnia. Secondary hypersomnia disorders are more commonly encountered in clinical practice and include hypersomnia attributable to another medical condition (including psychiatric and neurologic disorders), hypersomnia related to medication effects, and EDS related to behaviorally insufficient sleep. Distinguishing primary and secondary etiologies, when possible, is important as treatment pathways may vary considerably between hypersomnias.
Generally, overnight in-lab polysomnography is warranted to exclude untreated or sub-optimally treated sleep-disordered breathing or movement disorders which may undermine sleep quality. In the absence of any such findings, this is usually followed by daytime multiple sleep latency testing (MSLT). The MSLT is comprised of four to five scheduled daytime naps in the sleep lab and is designed to quantify a patient’s propensity to sleep during the day and to identify architectural sleep abnormalities which indicate narcolepsy. Specifically, narcolepsy is identified by MSLT when a patient exhibits a sleep onset latency of ≤ 8 minutes and at least two sleep-onset REM periods (SOREMPs), or, one SOREMP on MSLT with a second noted on the preceding night’s PSG. Actigraphy or sleep logs may be helpful in quantifying a patient’s total sleep time in their home environment. Adjunctive laboratory tests for narcolepsy including HLA typing and CSF hypocretin testing may sometimes be indicated.
General hypersomnia management usually consists of the use of wakefulness promoting agents, such as stimulants (eg, dexmethylphenidate) and dopamine-modulating agents (eg, modafinil, armodafinil), and adjunctive supportive strategies, including planned daytime naps and elimination of modifiable secondary causes. In those patients with hypersomnolence associated with depression or anxiety, the use of antidepressants, including SSRI, SNRI, and DNRIs, is often effective, and these medications can also improve cataplexy symptoms in narcoleptics. KLS may respond to treatment with lithium, shortening the duration of the striking hypersomnolent episodes characteristic of this rare syndrome, and there is some indication that ketamine may also be a helpful adjunctive in some cases. In treatment-refractory cases of hypersomnolence associated with GABA-A receptor potentiation, drugs such as flumazenil and clarithromycin appear to improve subjective measures of hypersomnolence.2,3 In patients with narcolepsy, sodium oxybate (available as Xyrem and, more recently, as a generic medication) has proven to be clinically very useful, reducing EDS and the frequency and severity of cataplexy and sleep disturbance associated with this condition. In July 2020, the FDA approved a new, low-sodium formulation of sodium oxybate (Xywav) for patients 7 years of age and older with a diagnosis of narcolepsy, a helpful option in those patients with cardiovascular and renal disease.
Despite this broadening armamentarium, in many cases daytime sleepiness and functional impairment is refractory to typical pharmacotherapy. In this context, we would like to highlight two newer pharmacotherapeutic options, solriamfetol and pitolisant.
Solriamfetol
Solriamfetol (Sunosi) is a Schedule IV FDA-approved medication indicated for treatment of EDS in adults with narcolepsy or obstructive sleep apnea. The precise mechanism of action is unknown, but this medication is believed to inhibit both dopamine and norepinerphrine reuptake in the brain, similar to the widely-prescribed NDRI buproprion. In a 12-week RCT study on its effects on narcolepsy in adults, solriamfetol improved important measures of wakefulness and sleepiness, without associated polysomnographic evidence of significant sleep disruption.4 In another 12-week RCT study of solriamfetol in adult patients with EDS related to OSA, there was a dose-dependent improvement in measures of wakefulness.5 Some notable side-effects seen with this medication include anxiety and elevated mood, as well as increases in blood pressure. A subsequent study of this medication found that it was efficacious at maintenance of improvements at 6 months.6 Given the theorized mechanism of action as an NDRI, future observation and studies could provide insights on its effect on depression, as well.
Pitolisant
Histaminergic neurons within the CNS play an important role in the promotion of wakefulness. Pitolisant (Wakix) is an interesting wakefulness-promoting agent for adult patients with narcolepsy. It acts as an inverse agonist and antagonist of histamine H3 receptors, resulting in a reduction of the usual feedback inhibition effected through the H3 receptor, thereby enhancing CNS release of histamine and other neurotransmitters. This medication was approved by the FDA in August 2019 and is currently indicated for adult patients with narcolepsy. The HARMONY I trial comparing pitolisant with both placebo and modafinil in adults with narcolepsy and EDS demonstrated improvement in measures of sleepiness and maintenance of wakefulness over placebo, and noninferiority to modafinil.7 In addition, pitolisant had a favorable side-effect profile compared with modafinil. Subsequent studies have reaffirmed the safety profile of pitolisant, including its minimal abuse potential. In one recent placebo-controlled trial of the use of pitolisant in a population of 268 adults with positive airway pressure (PAP) non-adherence, pitolisant was found to improve measures of EDS and related patient-reported measurements in patients with OSA who were CPAP nonadherent.8 Though generally well-tolerated by patients, in initial clinical trials pitolisant was associated with increased headache, insomnia, and nausea relative to placebo, among other less commonly reported adverse effects. Pitolisant is QT interval-prolonging, so caution must be taken when using this medication in combination other medications which may induce QT interval prolongation, including SSRIs.
Future directions
Greater awareness of the hypersomnias and their management has led to improved outcomes and access to care for these patients, yet these disorders remain burdensome and the treatments imperfect. Looking forward, novel pharmacotherapies that target underlying mechanisms rather than symptom palliation will allow for more precise treatments. Ongoing investigations of hypocretin receptor agonists seek to target one critical central mediator of wakefulness. Recent studies have highlighted the association of dysautonomia with hypersomnia, offering interesting insight into possible future targets to improve the function and quality of life of these patients.9 Similarly, understanding of the interplay between psychiatric disorders and primary and secondary hypersomnias may offer new therapeutic pathways.
As treatment plans targeting hypersomnia become more comprehensive and holistic, with an increased emphasis on self-care, sleep hygiene, and mental health awareness, in addition to mechanism-specific treatments, we hope they will ultimately provide improved symptom and burden relief for our patients.
Dr. Shih Yee-Marie Tan Gipson is a psychiatrist and Dr. Kevin Gipson is a sleep medicine specialist, both with Massachusetts General Hospital, Boston.
References
1 Dauvilliers, et al. Hypersomnia. Dialogues Clin Neurosci. 2005;7(4):347-356.
2 Trotti, et al. Clarithromycin in gamma-aminobutyric acid-related hypersomnolence: A randomized, crossover trial. Ann Neurol. 2015;78(3):454-465. doi: 10.1002/ana.24459.
3 Trotti, et al. Flumazenil for the treatment of refractory hypersomnolence: Clinical experience with 153 patients. J Clin Sleep Med. 2016;12(10):1389-1394. doi: 10.5664/jcsm.6196.
4 Thorpy, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019; 85(3):359-370. doi: 10.1002/ana.25423.
5 Schweitzer, et al. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): A randomized controlled trial. Am J Respir Crit Care Med. 2019;199(11):1421-1431. doi: 10.1164/rccm.201806-1100OC.
6 Malhotra, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020; 43(2): doi: 10.1093/sleep/zsz220.
7 Dauvilliers, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068-1075. doi: 10.1016/S1474-4422(13)70225-4.
8 Dauvilliers, et al. Pitolisant for daytime sleepiness in obstructive sleep apnea patients refusing CPAP: A randomized trial. Am J Respir Crit Care Med. 2020. doi: 10.1164/rccm.201907-1284OC.
9 Miglis, et al. Frequency and severity of autonomic symptoms in idiopathic hypersomnia. J Clin Sleep Med. 2020; 16(5):749-756. doi: 10.5664/jcsm.8344.
The hypersomnias are an etiologically diverse group of disorders of wakefulness and sleep, characterized principally by excessive daytime sleepiness (EDS), often despite sufficient or even long total sleep durations. Hypersomnolence may be severely disabling and isolating for patients, is associated with decreased quality of life and economic disadvantage, and, in some cases, may pose a personal and public health danger through drowsy driving. Though historically, management of these patients has been principally supportive and aimed at reducing daytime functional impairment, new and evolving treatments are quickly changing management paradigms in this population. This brief review highlights some of the newest pharmacotherapeutic advances in this dynamic field.
Hypersomnolence is a common presenting concern primary care and sleep clinics, with an estimated prevalence of EDS in the general adult population of as high as 6%.1 The initial diagnosis of hypersomnia is, broadly, a clinical one, with careful consideration to the patient’s report of daytime sleepiness and functional impairment, sleep/wake cycle, and any medical comorbidities. The primary hypersomnias include narcolepsy type 1 (narcolepsy with cataplexy, NT1) and narcolepsy type 2 (without cataplexy, NT2), Kleine-Levin Syndrome (KLS), and idiopathic hypersomnia. Secondary hypersomnia disorders are more commonly encountered in clinical practice and include hypersomnia attributable to another medical condition (including psychiatric and neurologic disorders), hypersomnia related to medication effects, and EDS related to behaviorally insufficient sleep. Distinguishing primary and secondary etiologies, when possible, is important as treatment pathways may vary considerably between hypersomnias.
Generally, overnight in-lab polysomnography is warranted to exclude untreated or sub-optimally treated sleep-disordered breathing or movement disorders which may undermine sleep quality. In the absence of any such findings, this is usually followed by daytime multiple sleep latency testing (MSLT). The MSLT is comprised of four to five scheduled daytime naps in the sleep lab and is designed to quantify a patient’s propensity to sleep during the day and to identify architectural sleep abnormalities which indicate narcolepsy. Specifically, narcolepsy is identified by MSLT when a patient exhibits a sleep onset latency of ≤ 8 minutes and at least two sleep-onset REM periods (SOREMPs), or, one SOREMP on MSLT with a second noted on the preceding night’s PSG. Actigraphy or sleep logs may be helpful in quantifying a patient’s total sleep time in their home environment. Adjunctive laboratory tests for narcolepsy including HLA typing and CSF hypocretin testing may sometimes be indicated.
General hypersomnia management usually consists of the use of wakefulness promoting agents, such as stimulants (eg, dexmethylphenidate) and dopamine-modulating agents (eg, modafinil, armodafinil), and adjunctive supportive strategies, including planned daytime naps and elimination of modifiable secondary causes. In those patients with hypersomnolence associated with depression or anxiety, the use of antidepressants, including SSRI, SNRI, and DNRIs, is often effective, and these medications can also improve cataplexy symptoms in narcoleptics. KLS may respond to treatment with lithium, shortening the duration of the striking hypersomnolent episodes characteristic of this rare syndrome, and there is some indication that ketamine may also be a helpful adjunctive in some cases. In treatment-refractory cases of hypersomnolence associated with GABA-A receptor potentiation, drugs such as flumazenil and clarithromycin appear to improve subjective measures of hypersomnolence.2,3 In patients with narcolepsy, sodium oxybate (available as Xyrem and, more recently, as a generic medication) has proven to be clinically very useful, reducing EDS and the frequency and severity of cataplexy and sleep disturbance associated with this condition. In July 2020, the FDA approved a new, low-sodium formulation of sodium oxybate (Xywav) for patients 7 years of age and older with a diagnosis of narcolepsy, a helpful option in those patients with cardiovascular and renal disease.
Despite this broadening armamentarium, in many cases daytime sleepiness and functional impairment is refractory to typical pharmacotherapy. In this context, we would like to highlight two newer pharmacotherapeutic options, solriamfetol and pitolisant.
Solriamfetol
Solriamfetol (Sunosi) is a Schedule IV FDA-approved medication indicated for treatment of EDS in adults with narcolepsy or obstructive sleep apnea. The precise mechanism of action is unknown, but this medication is believed to inhibit both dopamine and norepinerphrine reuptake in the brain, similar to the widely-prescribed NDRI buproprion. In a 12-week RCT study on its effects on narcolepsy in adults, solriamfetol improved important measures of wakefulness and sleepiness, without associated polysomnographic evidence of significant sleep disruption.4 In another 12-week RCT study of solriamfetol in adult patients with EDS related to OSA, there was a dose-dependent improvement in measures of wakefulness.5 Some notable side-effects seen with this medication include anxiety and elevated mood, as well as increases in blood pressure. A subsequent study of this medication found that it was efficacious at maintenance of improvements at 6 months.6 Given the theorized mechanism of action as an NDRI, future observation and studies could provide insights on its effect on depression, as well.
Pitolisant
Histaminergic neurons within the CNS play an important role in the promotion of wakefulness. Pitolisant (Wakix) is an interesting wakefulness-promoting agent for adult patients with narcolepsy. It acts as an inverse agonist and antagonist of histamine H3 receptors, resulting in a reduction of the usual feedback inhibition effected through the H3 receptor, thereby enhancing CNS release of histamine and other neurotransmitters. This medication was approved by the FDA in August 2019 and is currently indicated for adult patients with narcolepsy. The HARMONY I trial comparing pitolisant with both placebo and modafinil in adults with narcolepsy and EDS demonstrated improvement in measures of sleepiness and maintenance of wakefulness over placebo, and noninferiority to modafinil.7 In addition, pitolisant had a favorable side-effect profile compared with modafinil. Subsequent studies have reaffirmed the safety profile of pitolisant, including its minimal abuse potential. In one recent placebo-controlled trial of the use of pitolisant in a population of 268 adults with positive airway pressure (PAP) non-adherence, pitolisant was found to improve measures of EDS and related patient-reported measurements in patients with OSA who were CPAP nonadherent.8 Though generally well-tolerated by patients, in initial clinical trials pitolisant was associated with increased headache, insomnia, and nausea relative to placebo, among other less commonly reported adverse effects. Pitolisant is QT interval-prolonging, so caution must be taken when using this medication in combination other medications which may induce QT interval prolongation, including SSRIs.
Future directions
Greater awareness of the hypersomnias and their management has led to improved outcomes and access to care for these patients, yet these disorders remain burdensome and the treatments imperfect. Looking forward, novel pharmacotherapies that target underlying mechanisms rather than symptom palliation will allow for more precise treatments. Ongoing investigations of hypocretin receptor agonists seek to target one critical central mediator of wakefulness. Recent studies have highlighted the association of dysautonomia with hypersomnia, offering interesting insight into possible future targets to improve the function and quality of life of these patients.9 Similarly, understanding of the interplay between psychiatric disorders and primary and secondary hypersomnias may offer new therapeutic pathways.
As treatment plans targeting hypersomnia become more comprehensive and holistic, with an increased emphasis on self-care, sleep hygiene, and mental health awareness, in addition to mechanism-specific treatments, we hope they will ultimately provide improved symptom and burden relief for our patients.
Dr. Shih Yee-Marie Tan Gipson is a psychiatrist and Dr. Kevin Gipson is a sleep medicine specialist, both with Massachusetts General Hospital, Boston.
References
1 Dauvilliers, et al. Hypersomnia. Dialogues Clin Neurosci. 2005;7(4):347-356.
2 Trotti, et al. Clarithromycin in gamma-aminobutyric acid-related hypersomnolence: A randomized, crossover trial. Ann Neurol. 2015;78(3):454-465. doi: 10.1002/ana.24459.
3 Trotti, et al. Flumazenil for the treatment of refractory hypersomnolence: Clinical experience with 153 patients. J Clin Sleep Med. 2016;12(10):1389-1394. doi: 10.5664/jcsm.6196.
4 Thorpy, et al. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019; 85(3):359-370. doi: 10.1002/ana.25423.
5 Schweitzer, et al. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): A randomized controlled trial. Am J Respir Crit Care Med. 2019;199(11):1421-1431. doi: 10.1164/rccm.201806-1100OC.
6 Malhotra, et al. Long-term study of the safety and maintenance of efficacy of solriamfetol (JZP-110) in the treatment of excessive sleepiness in participants with narcolepsy or obstructive sleep apnea. Sleep. 2020; 43(2): doi: 10.1093/sleep/zsz220.
7 Dauvilliers, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068-1075. doi: 10.1016/S1474-4422(13)70225-4.
8 Dauvilliers, et al. Pitolisant for daytime sleepiness in obstructive sleep apnea patients refusing CPAP: A randomized trial. Am J Respir Crit Care Med. 2020. doi: 10.1164/rccm.201907-1284OC.
9 Miglis, et al. Frequency and severity of autonomic symptoms in idiopathic hypersomnia. J Clin Sleep Med. 2020; 16(5):749-756. doi: 10.5664/jcsm.8344.
Management of EVALI in the ICU
Since 2019, more than 2,700 individuals have been hospitalized with electronic cigarette- (e-cigarette), or vaping-associated lung injury (EVALI). This entity first reached clinical attention after a series of otherwise healthy young adults presented with dyspnea, severe hypoxia, and diffuse pulmonary infiltrates in the Midwest (Layden J, et al. N Engl J Med. 2020;382[10]:903). Investigation of these cases revealed an association with the use of e-cigarettes, or vaping. As cases continued to mount, the link between vaping and acute lung injury became increasingly apparent.
How it presents
EVALI can present in variable ways, ranging from mild cough or dyspnea without hypoxia to severe acute respiratory distress syndrome (ARDS), requiring advanced life support. Although challenging in the ICU setting, obtaining a detailed history of vaping is crucial to make the diagnosis. Collateral history can be helpful, but if unrevealing, it should not be considered sufficient to exclude vaping as potential etiology, particularly in adolescent e-cigarette users, where parental awareness of substance use history may be limited. If a vaping history is obtained, it is important to assess the substance(s) vaped, how these substances were obtained, and methods of inhalation. While e-cigarettes are the most commonly recognized method of vaping, alternate methods such as “dabbing” and “dripping,” are increasingly popular among vape users, often utilizing modified e-liquid components that may not be reported by patients unless specifically queried.
About 82% of patients hospitalized with EVALI reported vaping tetrahydrocannabinol- (THC) containing fluid. This is important because, unlike nicotine based e-liquids that are primarily purchased over the counter, more than 70% of THC-containing e-liquids are reportedly obtained through informal sources, including illegal distributors. In contrast, only 14% of patients hospitalized with EVALI reported vaping of commercial nicotine products alone. Nicotine-based e-liquids can also be modified, and informal purchasing sources remain a concern, particularly among younger users.
The onset of respiratory symptoms in EVALI is often preceded by several days of a systemic prodrome, including low-grade fevers, myalgia, gastrointestinal complaints, and fatigue (MacMurdo M, et al. Chest. 2020;157[6]:e181). The diagnosis of EVALI is made clinically, and alternative etiologies of lung injury (eg, infections) should be excluded. As there is significant overlap between the presenting symptoms of EVALI and COVID-19 infection, patients should be tested for COVID-19 before a diagnosis of EVALI can be made.
Imaging patterns of EVALI include diffuse alveolar damage (the most common), comprising of diffuse ground-glass opacities, septal thickening, and heterogeneous consolidation (MacMurdo M, et al. Chest. 2020;157[6]:e181). Bilateral ground glass opacities suggestive of organizing pneumonia have also been described. Atypical patterns of nodularity suggestive of hypersensitivity pneumonitis are significantly less common. Given the variety of imaging patterns, EVALI should be considered as a differential diagnosis in all patients presenting with new bilateral pulmonary infiltrates and severe hypoxia.
Early evaluation of these patients revealed lipid-laden macrophages in the bronchoalveolar lavage (BAL) fluid of these patients, raising concern for exogenous lipid inhalation resulting in the development of lipoid pneumonia (Maddock SD, et al. N Engl J Med. 2019;381[15]:1488). Analysis of BAL fluid revealed the presence of vitamin E acetate, a diluent utilized to cut, or dilute, e-liquid (Blount BC, et al. MMWR. 2019;68[45]:1040). This supported the hypothesis that the outbreak of EVALI was being driven, at least in part, by contaminated or self-modified e-liquid. Evaluation of lung biopsies revealed different pathologic patterns of acute lung injury, including diffuse alveolar damage and organizing pneumonia. Importantly, while lipid-laden macrophages were detected, other characteristics of lipoid pneumonia were absent (Mukhopadhyay S, et al. Am J Clin Path. 2019;153[1]30).
How to manage EVALI
Approximately half of patients hospitalized with EVALI required ICU admission. However, there is likely a substantial portion of patients with mild disease who may not be represented in the current registry since they did not require hospitalization. The management is primarily supportive and, in patients who require mechanical ventilation, following lung-protective ventilator strategies is of paramount importance. Steroids have been used in some case series, particularly for patients presenting with more severe disease, but data on benefit, optimal dose, and duration are limited.
Vaping cessation is crucial and should be aggressively encouraged. Newer generations of e-cigarettes contain comparatively higher nicotine concentrations, and likely have high potential for nicotine addiction. Treatment for nicotine dependence, including pharmacologic therapy, needs to be considered in all patients following recovery from EVALI.
With supportive care and removal of ongoing exposure, recovery is anticipated in most patients. Long-term outcomes in patients who develop EVALI remain unclear. Although early fibrosis was present in some patients who had transbronchial biopsies, the long-term effects on pulmonary function that may be seen in patients with a history of EVALI are yet to be determined.
What about policy?
New regulations related to e-cigarette use have been proposed in response to the increasing prevalence of vaping and the EVALI outbreak. These regulations center primarily on limiting adolescent e-cigarette usage. Tobacco 21, federal legislation passed in 2019, makes it illegal to sell tobacco products to those under the age of 21. The FDA also issued an enforcement policy on unauthorized flavored e-cigarette products. However, this has been criticized for not being comprehensive enough. For example, tobacco and menthol flavors were not included in the ban. Furthermore, THC-containing e-liquid remains largely unregulated at the federal level, and state-level regulation varies significantly by marijuana legalization status.
Policy initiatives that restrict sales without also addressing drivers of e-cigarette use, such as nicotine dependence and aggressive marketing campaigns, are of particular concern and are likely to disproportionately impact younger users. Another unintended effect of e-cigarette sales restrictions may result in a new wave of illegal product distribution and e-liquid modification. Supporting this hypothesis was the finding that the risk of EVALI was higher in states without legalized recreational marijuana, suggesting that users who obtained e-liquid through these informal sources were at greater risk of exposure to contaminated product (Wing C, et al. JAMA Netw Open. 2020;3[4]:e202187). While the CDC is no longer actively tracking EVALI cases, they continue to be reported, and vape use remains common (Armatas C, et al. MMWR. 69[25]:801). As long as e-cigarettes remain in use, another EVALI outbreak remains possible.
It remains important for the intensivist to be familiar with the full spectrum of vaping methods, and to report suspected cases when they arise. While treatable, much remains unknown about the long-term effects on this patient population. Further research is needed to better understand the long-term outcomes in patients with EVALI, in addition to the treatment of nicotine dependence and substance use associated with vaping. Finally, comprehensive regulation to curb e-cigarette usage is needed, particularly among adolescents. However, legislation that is too narrow in scope runs the risk of channeling adolescent e-cigarette users to obtain product through informal sources, further increasing their risk for EVALI. As clinicians, we cannot afford to drop our guard!
Dr. Macmurdo and Dr. Choi are with Cleveland Clinic, Respiratory Institute, Cleveland, Ohio.
Since 2019, more than 2,700 individuals have been hospitalized with electronic cigarette- (e-cigarette), or vaping-associated lung injury (EVALI). This entity first reached clinical attention after a series of otherwise healthy young adults presented with dyspnea, severe hypoxia, and diffuse pulmonary infiltrates in the Midwest (Layden J, et al. N Engl J Med. 2020;382[10]:903). Investigation of these cases revealed an association with the use of e-cigarettes, or vaping. As cases continued to mount, the link between vaping and acute lung injury became increasingly apparent.
How it presents
EVALI can present in variable ways, ranging from mild cough or dyspnea without hypoxia to severe acute respiratory distress syndrome (ARDS), requiring advanced life support. Although challenging in the ICU setting, obtaining a detailed history of vaping is crucial to make the diagnosis. Collateral history can be helpful, but if unrevealing, it should not be considered sufficient to exclude vaping as potential etiology, particularly in adolescent e-cigarette users, where parental awareness of substance use history may be limited. If a vaping history is obtained, it is important to assess the substance(s) vaped, how these substances were obtained, and methods of inhalation. While e-cigarettes are the most commonly recognized method of vaping, alternate methods such as “dabbing” and “dripping,” are increasingly popular among vape users, often utilizing modified e-liquid components that may not be reported by patients unless specifically queried.
About 82% of patients hospitalized with EVALI reported vaping tetrahydrocannabinol- (THC) containing fluid. This is important because, unlike nicotine based e-liquids that are primarily purchased over the counter, more than 70% of THC-containing e-liquids are reportedly obtained through informal sources, including illegal distributors. In contrast, only 14% of patients hospitalized with EVALI reported vaping of commercial nicotine products alone. Nicotine-based e-liquids can also be modified, and informal purchasing sources remain a concern, particularly among younger users.
The onset of respiratory symptoms in EVALI is often preceded by several days of a systemic prodrome, including low-grade fevers, myalgia, gastrointestinal complaints, and fatigue (MacMurdo M, et al. Chest. 2020;157[6]:e181). The diagnosis of EVALI is made clinically, and alternative etiologies of lung injury (eg, infections) should be excluded. As there is significant overlap between the presenting symptoms of EVALI and COVID-19 infection, patients should be tested for COVID-19 before a diagnosis of EVALI can be made.
Imaging patterns of EVALI include diffuse alveolar damage (the most common), comprising of diffuse ground-glass opacities, septal thickening, and heterogeneous consolidation (MacMurdo M, et al. Chest. 2020;157[6]:e181). Bilateral ground glass opacities suggestive of organizing pneumonia have also been described. Atypical patterns of nodularity suggestive of hypersensitivity pneumonitis are significantly less common. Given the variety of imaging patterns, EVALI should be considered as a differential diagnosis in all patients presenting with new bilateral pulmonary infiltrates and severe hypoxia.
Early evaluation of these patients revealed lipid-laden macrophages in the bronchoalveolar lavage (BAL) fluid of these patients, raising concern for exogenous lipid inhalation resulting in the development of lipoid pneumonia (Maddock SD, et al. N Engl J Med. 2019;381[15]:1488). Analysis of BAL fluid revealed the presence of vitamin E acetate, a diluent utilized to cut, or dilute, e-liquid (Blount BC, et al. MMWR. 2019;68[45]:1040). This supported the hypothesis that the outbreak of EVALI was being driven, at least in part, by contaminated or self-modified e-liquid. Evaluation of lung biopsies revealed different pathologic patterns of acute lung injury, including diffuse alveolar damage and organizing pneumonia. Importantly, while lipid-laden macrophages were detected, other characteristics of lipoid pneumonia were absent (Mukhopadhyay S, et al. Am J Clin Path. 2019;153[1]30).
How to manage EVALI
Approximately half of patients hospitalized with EVALI required ICU admission. However, there is likely a substantial portion of patients with mild disease who may not be represented in the current registry since they did not require hospitalization. The management is primarily supportive and, in patients who require mechanical ventilation, following lung-protective ventilator strategies is of paramount importance. Steroids have been used in some case series, particularly for patients presenting with more severe disease, but data on benefit, optimal dose, and duration are limited.
Vaping cessation is crucial and should be aggressively encouraged. Newer generations of e-cigarettes contain comparatively higher nicotine concentrations, and likely have high potential for nicotine addiction. Treatment for nicotine dependence, including pharmacologic therapy, needs to be considered in all patients following recovery from EVALI.
With supportive care and removal of ongoing exposure, recovery is anticipated in most patients. Long-term outcomes in patients who develop EVALI remain unclear. Although early fibrosis was present in some patients who had transbronchial biopsies, the long-term effects on pulmonary function that may be seen in patients with a history of EVALI are yet to be determined.
What about policy?
New regulations related to e-cigarette use have been proposed in response to the increasing prevalence of vaping and the EVALI outbreak. These regulations center primarily on limiting adolescent e-cigarette usage. Tobacco 21, federal legislation passed in 2019, makes it illegal to sell tobacco products to those under the age of 21. The FDA also issued an enforcement policy on unauthorized flavored e-cigarette products. However, this has been criticized for not being comprehensive enough. For example, tobacco and menthol flavors were not included in the ban. Furthermore, THC-containing e-liquid remains largely unregulated at the federal level, and state-level regulation varies significantly by marijuana legalization status.
Policy initiatives that restrict sales without also addressing drivers of e-cigarette use, such as nicotine dependence and aggressive marketing campaigns, are of particular concern and are likely to disproportionately impact younger users. Another unintended effect of e-cigarette sales restrictions may result in a new wave of illegal product distribution and e-liquid modification. Supporting this hypothesis was the finding that the risk of EVALI was higher in states without legalized recreational marijuana, suggesting that users who obtained e-liquid through these informal sources were at greater risk of exposure to contaminated product (Wing C, et al. JAMA Netw Open. 2020;3[4]:e202187). While the CDC is no longer actively tracking EVALI cases, they continue to be reported, and vape use remains common (Armatas C, et al. MMWR. 69[25]:801). As long as e-cigarettes remain in use, another EVALI outbreak remains possible.
It remains important for the intensivist to be familiar with the full spectrum of vaping methods, and to report suspected cases when they arise. While treatable, much remains unknown about the long-term effects on this patient population. Further research is needed to better understand the long-term outcomes in patients with EVALI, in addition to the treatment of nicotine dependence and substance use associated with vaping. Finally, comprehensive regulation to curb e-cigarette usage is needed, particularly among adolescents. However, legislation that is too narrow in scope runs the risk of channeling adolescent e-cigarette users to obtain product through informal sources, further increasing their risk for EVALI. As clinicians, we cannot afford to drop our guard!
Dr. Macmurdo and Dr. Choi are with Cleveland Clinic, Respiratory Institute, Cleveland, Ohio.
Since 2019, more than 2,700 individuals have been hospitalized with electronic cigarette- (e-cigarette), or vaping-associated lung injury (EVALI). This entity first reached clinical attention after a series of otherwise healthy young adults presented with dyspnea, severe hypoxia, and diffuse pulmonary infiltrates in the Midwest (Layden J, et al. N Engl J Med. 2020;382[10]:903). Investigation of these cases revealed an association with the use of e-cigarettes, or vaping. As cases continued to mount, the link between vaping and acute lung injury became increasingly apparent.
How it presents
EVALI can present in variable ways, ranging from mild cough or dyspnea without hypoxia to severe acute respiratory distress syndrome (ARDS), requiring advanced life support. Although challenging in the ICU setting, obtaining a detailed history of vaping is crucial to make the diagnosis. Collateral history can be helpful, but if unrevealing, it should not be considered sufficient to exclude vaping as potential etiology, particularly in adolescent e-cigarette users, where parental awareness of substance use history may be limited. If a vaping history is obtained, it is important to assess the substance(s) vaped, how these substances were obtained, and methods of inhalation. While e-cigarettes are the most commonly recognized method of vaping, alternate methods such as “dabbing” and “dripping,” are increasingly popular among vape users, often utilizing modified e-liquid components that may not be reported by patients unless specifically queried.
About 82% of patients hospitalized with EVALI reported vaping tetrahydrocannabinol- (THC) containing fluid. This is important because, unlike nicotine based e-liquids that are primarily purchased over the counter, more than 70% of THC-containing e-liquids are reportedly obtained through informal sources, including illegal distributors. In contrast, only 14% of patients hospitalized with EVALI reported vaping of commercial nicotine products alone. Nicotine-based e-liquids can also be modified, and informal purchasing sources remain a concern, particularly among younger users.
The onset of respiratory symptoms in EVALI is often preceded by several days of a systemic prodrome, including low-grade fevers, myalgia, gastrointestinal complaints, and fatigue (MacMurdo M, et al. Chest. 2020;157[6]:e181). The diagnosis of EVALI is made clinically, and alternative etiologies of lung injury (eg, infections) should be excluded. As there is significant overlap between the presenting symptoms of EVALI and COVID-19 infection, patients should be tested for COVID-19 before a diagnosis of EVALI can be made.
Imaging patterns of EVALI include diffuse alveolar damage (the most common), comprising of diffuse ground-glass opacities, septal thickening, and heterogeneous consolidation (MacMurdo M, et al. Chest. 2020;157[6]:e181). Bilateral ground glass opacities suggestive of organizing pneumonia have also been described. Atypical patterns of nodularity suggestive of hypersensitivity pneumonitis are significantly less common. Given the variety of imaging patterns, EVALI should be considered as a differential diagnosis in all patients presenting with new bilateral pulmonary infiltrates and severe hypoxia.
Early evaluation of these patients revealed lipid-laden macrophages in the bronchoalveolar lavage (BAL) fluid of these patients, raising concern for exogenous lipid inhalation resulting in the development of lipoid pneumonia (Maddock SD, et al. N Engl J Med. 2019;381[15]:1488). Analysis of BAL fluid revealed the presence of vitamin E acetate, a diluent utilized to cut, or dilute, e-liquid (Blount BC, et al. MMWR. 2019;68[45]:1040). This supported the hypothesis that the outbreak of EVALI was being driven, at least in part, by contaminated or self-modified e-liquid. Evaluation of lung biopsies revealed different pathologic patterns of acute lung injury, including diffuse alveolar damage and organizing pneumonia. Importantly, while lipid-laden macrophages were detected, other characteristics of lipoid pneumonia were absent (Mukhopadhyay S, et al. Am J Clin Path. 2019;153[1]30).
How to manage EVALI
Approximately half of patients hospitalized with EVALI required ICU admission. However, there is likely a substantial portion of patients with mild disease who may not be represented in the current registry since they did not require hospitalization. The management is primarily supportive and, in patients who require mechanical ventilation, following lung-protective ventilator strategies is of paramount importance. Steroids have been used in some case series, particularly for patients presenting with more severe disease, but data on benefit, optimal dose, and duration are limited.
Vaping cessation is crucial and should be aggressively encouraged. Newer generations of e-cigarettes contain comparatively higher nicotine concentrations, and likely have high potential for nicotine addiction. Treatment for nicotine dependence, including pharmacologic therapy, needs to be considered in all patients following recovery from EVALI.
With supportive care and removal of ongoing exposure, recovery is anticipated in most patients. Long-term outcomes in patients who develop EVALI remain unclear. Although early fibrosis was present in some patients who had transbronchial biopsies, the long-term effects on pulmonary function that may be seen in patients with a history of EVALI are yet to be determined.
What about policy?
New regulations related to e-cigarette use have been proposed in response to the increasing prevalence of vaping and the EVALI outbreak. These regulations center primarily on limiting adolescent e-cigarette usage. Tobacco 21, federal legislation passed in 2019, makes it illegal to sell tobacco products to those under the age of 21. The FDA also issued an enforcement policy on unauthorized flavored e-cigarette products. However, this has been criticized for not being comprehensive enough. For example, tobacco and menthol flavors were not included in the ban. Furthermore, THC-containing e-liquid remains largely unregulated at the federal level, and state-level regulation varies significantly by marijuana legalization status.
Policy initiatives that restrict sales without also addressing drivers of e-cigarette use, such as nicotine dependence and aggressive marketing campaigns, are of particular concern and are likely to disproportionately impact younger users. Another unintended effect of e-cigarette sales restrictions may result in a new wave of illegal product distribution and e-liquid modification. Supporting this hypothesis was the finding that the risk of EVALI was higher in states without legalized recreational marijuana, suggesting that users who obtained e-liquid through these informal sources were at greater risk of exposure to contaminated product (Wing C, et al. JAMA Netw Open. 2020;3[4]:e202187). While the CDC is no longer actively tracking EVALI cases, they continue to be reported, and vape use remains common (Armatas C, et al. MMWR. 69[25]:801). As long as e-cigarettes remain in use, another EVALI outbreak remains possible.
It remains important for the intensivist to be familiar with the full spectrum of vaping methods, and to report suspected cases when they arise. While treatable, much remains unknown about the long-term effects on this patient population. Further research is needed to better understand the long-term outcomes in patients with EVALI, in addition to the treatment of nicotine dependence and substance use associated with vaping. Finally, comprehensive regulation to curb e-cigarette usage is needed, particularly among adolescents. However, legislation that is too narrow in scope runs the risk of channeling adolescent e-cigarette users to obtain product through informal sources, further increasing their risk for EVALI. As clinicians, we cannot afford to drop our guard!
Dr. Macmurdo and Dr. Choi are with Cleveland Clinic, Respiratory Institute, Cleveland, Ohio.
COVID-19 and the future of telehealth for sleep medicine
On March 18, 2020, the doors to our sleep center were physically closed. Two potential exposures to COVID-19 within a few hours, the palpable anxiety of our team, and a poor grasp of the virus and the growing pandemic moved us to make this decision. Up to that point, we could not help but feel we were playing “catch up” with our evolving set of safety measures to the escalating risk. Like so many other sleep centers around the country, a complete transition to virtual care was needed to ensure the safety of our patients and our team. It was perhaps that moment that we felt the emotional impact that our world had changed, altering both our personal lives and sleep medicine practice as we knew it. This event, while unfortunate, also provided a transformative opportunity to reimagine our identity, accelerating the efforts to bring the future of sleep medicine into the present.
Our team’s clinical evolution and innovation efforts have been guided by efforts to reconsider sleep medicine paradigms. Innovation progress was deliberate with incremental implementations that typically required repeat business cases with multiple approving parties and budgetary access. Those barriers largely dissolved once COVID-19 intensified, and a large portion of the strategies on our roadmap were put into production. In a matter of a couple weeks, our services completely transitioned to remote and virtual care, while most of the team of 55 persons were moved to “work-from-home.” A suite of technologies (automated questionnaires, automated and two-way text messaging templates, consumer wearable technologies, and population management dashboards) were put on the table (Somnoware, Inc.), and each of our longitudinal care teams (eg, adult obstructive sleep apnea, pediatrics, chronic respiratory failure, commercial driver, insomnia programs, etc) worked to embed them into new care pathways. This effort further consolidated technology as the backbone of our work and the enabler of remote virtual collaboration between sleep center personnel (respiratory case managers, medical assistants and nursing team, and physician and leadership personnel) to enhance our team-based approach. Moreover, we felt this point in time was ripe to swallow the proverbial “red pill” and approach patient care with shifted paradigms. We discuss three areas of active effort to leverage technology in this COVID-19 environment to accelerate a transition toward how we envision the future of sleep medicine.
Reimagined sleep diagnostics
Our virtual obstructive sleep apnea (OSA) diagnostic process includes utilizing a disposable home sleep apnea test (HSAT) device with wireless data transfer (WatchPAT ONE, Itamar Medical) while HSAT and PAP (positive airway pressure) setups are supported by information sheets, online videos (YouTube), automated interactive platforms (Emmi Solutions; Hwang D. Am J Respir Crit Care Med. 2018 Jan 1;197[1]:117), and synchronous provider video visits. Our more radical shift, however, is in approaching OSA diagnosis based principally on symptoms and secondarily supported by physiologic measurements and response to therapy. This “clinical diagnosis” approach reduces our reliance on traditional sleep testing and allows patient wearables to provide supportive physiologic data (eg, oximetry) to help determine OSA severity and phenotype. Its immediate impact is in limiting the need to send and retrieve potentially contaminated equipment. Broader clinical advantages include overcoming the imprecise nature of the apnea-hypopnea index (which often has dramatic night-to-night variability) through data collection over extended durations, improving disease assessment due to availability of complementary sleep/activity data in the person’s usual setting, and tracking changes after therapy initiation.
Our post-COVID-19 re-opening of polysomnography (PSG) services, after a temporary shutdown, introduces home PSG (Type II) for approximately half our patients without suspected complex breathing conditions while reserving attended PSG (Type I) for those who may require noninvasive ventilation. The immediate incentive is in reducing viral exposure by limiting patient traffic and risk of PAP trial aerosolization while also improving access to accommodate the backlog of patients requiring PSG. This approach furthers the paradigm shift to emphasizing care in the home setting. Testing in the patient’s usual environment and enabling multiple night/day testing may be clinically advantageous.
Shift in emphasis to care management
The emphasis of sleep medicine has traditionally focused on diagnostics through performing PSG and HSAT. Our field has invested tremendous effort in developing guidelines for processing sleep studies, but the scoring and interpretation of those studies is extremely labor intensive. Reimagining the diagnostic approach reduces the need to manually process studies—wearable data are produced automatically, HSAT can be auto-scored, and artificial intelligence platforms can score PSGs (Goldstein CA. J Clin Sleep Med. 2020 Apr 15;16[4]:609), which allows a shift in resources and emphasis to follow-up care. A comprehensive discussion of technology-based tools to enhance care management is beyond the purview of this editorial. However, an overview of our current efforts includes: (1) utilizing population management dashboards to automatically risk stratify different cohorts of patients (eg, adult OSA, pediatrics, commercial drivers, chronic respiratory failure, etc) to identify patients “at-risk” (eg, based on OSA severity, symptoms, co-morbidities, and PAP adherence); (2) applying enhanced patient-provider interchange tools that include automated and “intelligent” electronic questionnaires, automated personalized text messaging/emails, and two-way messaging to deliver care; (3) utilizing remote patient monitoring to enhance holistic, personalized management, such as with remote activity/sleep trackers, blood pressure monitors, glucometers, and weight scales. We are engaged with efforts to validate the impact of these data to provide more personalized feedback, directly impact clinical outcomes, facilitate interdisciplinary collaboration, and identify acutely ill patients. Furthermore, a holistic approach beyond a narrow focus on PAP may create a positive collateral effect on adherence by targeting engagement with broader areas of health; and (4) implementing machine learning tools to directly support providers and patients (examples discussed in the next section.) Each of our teams has created workflows embedding these strategies throughout new care pathways. 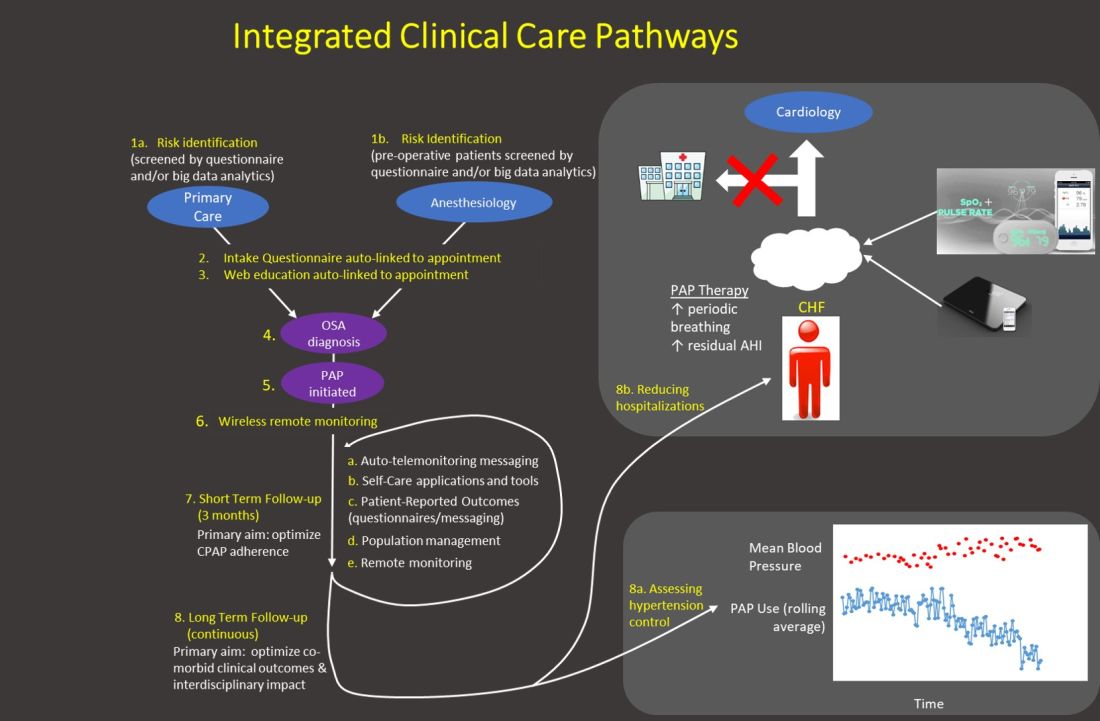
Generally, our emphasis during the first 3 months after PAP initiation focuses on achieving therapy adherence, and the post-3-month period broadens the efforts to target clinical outcomes. Recent trials with low PAP usage that failed to confirm the benefit of PAP on cardiovascular outcomes (McEvoy DR, et al. N Engl J Med. 2016;375:919) strongly suggest greater investment in cost-effective long-term strategies is imperative to increase our field’s relevance.
Application of artificial intelligence
We describe current efforts to apply artificial intelligence (AI) into clinical care: (1) We are implementing machine learning (ML) PSG scoring, which can potentially improve both the consistency and efficiency of scoring, further enabling greater investment in follow-up care. The future of sleep study processing, however, will likely depend on computer vision to “view” details inaccessible to the human eye and produce novel metrics that better inform clinical phenotypes (eg, cardiovascular risk, response to alternative therapies, etc). For example, “brain age” has been derived from EEG tracings that could reflect the degree of impact of sleep disorders on neurocognitive function (Fernandez C, unpublished data); (2) Machine learning clinical decision tools are in development to predict PAP adherence and timing of discontinuation, predict timing of cardiovascular disease onset and hospitalization, personalizing adherence targets, automating triaging of patients to home or PSG testing, and innumerable other predictions at clinical decision inflection points. Prediction outputs may be presented as risk profiles embedded in each patient’s “chart,” as personalized alerts, and in gamification strategies. For example, machine learning personalized cardiovascular risk scores can be regularly updated based on degree of PAP use to incentivize adherence; (3) Artificial providers may provide consistent, personalized, and holistic supplementary care. Many people rely on AI-bots for social support and cognitive-behavioral therapy (CBT) for depression. A sleep wellness bot, currently in planning stages, is intended to be the primary interface for many of the strategies described above that enhance engagement with PAP and therapies for comorbid conditions, provide CBT and lifestyle accountability, and collect patient reported data. This artificial provider would be a constant companion providing interactive, personalized, and continuous management to complement traditional intermittent live-person care.
The current health-care environment embodies the principle to “never let a serious crisis go to waste.” COVID-19 has accelerated the progression into the future by fostering an opening to embrace novel application of technologies to support changes in paradigms. Furthermore, health-care infrastructures that typically progress deliberately changed seemingly in a single moment. The Center for Medicare Services issued broad authorization to reimburse for telemedicine in response to COVID-19. Continued evolution in infrastructures will dictate progress with innovation, and a greater transition to outcomes-based incentives may be necessary to accommodate many of the strategies described above that rely on nonsynchronous care. But, we may be experiencing the moment when health care starts to catch up with the world in its embrace of technology. Sleep and pulmonary medicine can be a leader by providing a successful template for other specialties in optimizing chronic disease management.
Dr. Hwang is Medical Director, Kaiser Permanente SBC Sleep Center, and co-chair, Sleep Medicine, Kaiser Permanente Southern California.
On March 18, 2020, the doors to our sleep center were physically closed. Two potential exposures to COVID-19 within a few hours, the palpable anxiety of our team, and a poor grasp of the virus and the growing pandemic moved us to make this decision. Up to that point, we could not help but feel we were playing “catch up” with our evolving set of safety measures to the escalating risk. Like so many other sleep centers around the country, a complete transition to virtual care was needed to ensure the safety of our patients and our team. It was perhaps that moment that we felt the emotional impact that our world had changed, altering both our personal lives and sleep medicine practice as we knew it. This event, while unfortunate, also provided a transformative opportunity to reimagine our identity, accelerating the efforts to bring the future of sleep medicine into the present.
Our team’s clinical evolution and innovation efforts have been guided by efforts to reconsider sleep medicine paradigms. Innovation progress was deliberate with incremental implementations that typically required repeat business cases with multiple approving parties and budgetary access. Those barriers largely dissolved once COVID-19 intensified, and a large portion of the strategies on our roadmap were put into production. In a matter of a couple weeks, our services completely transitioned to remote and virtual care, while most of the team of 55 persons were moved to “work-from-home.” A suite of technologies (automated questionnaires, automated and two-way text messaging templates, consumer wearable technologies, and population management dashboards) were put on the table (Somnoware, Inc.), and each of our longitudinal care teams (eg, adult obstructive sleep apnea, pediatrics, chronic respiratory failure, commercial driver, insomnia programs, etc) worked to embed them into new care pathways. This effort further consolidated technology as the backbone of our work and the enabler of remote virtual collaboration between sleep center personnel (respiratory case managers, medical assistants and nursing team, and physician and leadership personnel) to enhance our team-based approach. Moreover, we felt this point in time was ripe to swallow the proverbial “red pill” and approach patient care with shifted paradigms. We discuss three areas of active effort to leverage technology in this COVID-19 environment to accelerate a transition toward how we envision the future of sleep medicine.
Reimagined sleep diagnostics
Our virtual obstructive sleep apnea (OSA) diagnostic process includes utilizing a disposable home sleep apnea test (HSAT) device with wireless data transfer (WatchPAT ONE, Itamar Medical) while HSAT and PAP (positive airway pressure) setups are supported by information sheets, online videos (YouTube), automated interactive platforms (Emmi Solutions; Hwang D. Am J Respir Crit Care Med. 2018 Jan 1;197[1]:117), and synchronous provider video visits. Our more radical shift, however, is in approaching OSA diagnosis based principally on symptoms and secondarily supported by physiologic measurements and response to therapy. This “clinical diagnosis” approach reduces our reliance on traditional sleep testing and allows patient wearables to provide supportive physiologic data (eg, oximetry) to help determine OSA severity and phenotype. Its immediate impact is in limiting the need to send and retrieve potentially contaminated equipment. Broader clinical advantages include overcoming the imprecise nature of the apnea-hypopnea index (which often has dramatic night-to-night variability) through data collection over extended durations, improving disease assessment due to availability of complementary sleep/activity data in the person’s usual setting, and tracking changes after therapy initiation.
Our post-COVID-19 re-opening of polysomnography (PSG) services, after a temporary shutdown, introduces home PSG (Type II) for approximately half our patients without suspected complex breathing conditions while reserving attended PSG (Type I) for those who may require noninvasive ventilation. The immediate incentive is in reducing viral exposure by limiting patient traffic and risk of PAP trial aerosolization while also improving access to accommodate the backlog of patients requiring PSG. This approach furthers the paradigm shift to emphasizing care in the home setting. Testing in the patient’s usual environment and enabling multiple night/day testing may be clinically advantageous.
Shift in emphasis to care management
The emphasis of sleep medicine has traditionally focused on diagnostics through performing PSG and HSAT. Our field has invested tremendous effort in developing guidelines for processing sleep studies, but the scoring and interpretation of those studies is extremely labor intensive. Reimagining the diagnostic approach reduces the need to manually process studies—wearable data are produced automatically, HSAT can be auto-scored, and artificial intelligence platforms can score PSGs (Goldstein CA. J Clin Sleep Med. 2020 Apr 15;16[4]:609), which allows a shift in resources and emphasis to follow-up care. A comprehensive discussion of technology-based tools to enhance care management is beyond the purview of this editorial. However, an overview of our current efforts includes: (1) utilizing population management dashboards to automatically risk stratify different cohorts of patients (eg, adult OSA, pediatrics, commercial drivers, chronic respiratory failure, etc) to identify patients “at-risk” (eg, based on OSA severity, symptoms, co-morbidities, and PAP adherence); (2) applying enhanced patient-provider interchange tools that include automated and “intelligent” electronic questionnaires, automated personalized text messaging/emails, and two-way messaging to deliver care; (3) utilizing remote patient monitoring to enhance holistic, personalized management, such as with remote activity/sleep trackers, blood pressure monitors, glucometers, and weight scales. We are engaged with efforts to validate the impact of these data to provide more personalized feedback, directly impact clinical outcomes, facilitate interdisciplinary collaboration, and identify acutely ill patients. Furthermore, a holistic approach beyond a narrow focus on PAP may create a positive collateral effect on adherence by targeting engagement with broader areas of health; and (4) implementing machine learning tools to directly support providers and patients (examples discussed in the next section.) Each of our teams has created workflows embedding these strategies throughout new care pathways. 
Generally, our emphasis during the first 3 months after PAP initiation focuses on achieving therapy adherence, and the post-3-month period broadens the efforts to target clinical outcomes. Recent trials with low PAP usage that failed to confirm the benefit of PAP on cardiovascular outcomes (McEvoy DR, et al. N Engl J Med. 2016;375:919) strongly suggest greater investment in cost-effective long-term strategies is imperative to increase our field’s relevance.
Application of artificial intelligence
We describe current efforts to apply artificial intelligence (AI) into clinical care: (1) We are implementing machine learning (ML) PSG scoring, which can potentially improve both the consistency and efficiency of scoring, further enabling greater investment in follow-up care. The future of sleep study processing, however, will likely depend on computer vision to “view” details inaccessible to the human eye and produce novel metrics that better inform clinical phenotypes (eg, cardiovascular risk, response to alternative therapies, etc). For example, “brain age” has been derived from EEG tracings that could reflect the degree of impact of sleep disorders on neurocognitive function (Fernandez C, unpublished data); (2) Machine learning clinical decision tools are in development to predict PAP adherence and timing of discontinuation, predict timing of cardiovascular disease onset and hospitalization, personalizing adherence targets, automating triaging of patients to home or PSG testing, and innumerable other predictions at clinical decision inflection points. Prediction outputs may be presented as risk profiles embedded in each patient’s “chart,” as personalized alerts, and in gamification strategies. For example, machine learning personalized cardiovascular risk scores can be regularly updated based on degree of PAP use to incentivize adherence; (3) Artificial providers may provide consistent, personalized, and holistic supplementary care. Many people rely on AI-bots for social support and cognitive-behavioral therapy (CBT) for depression. A sleep wellness bot, currently in planning stages, is intended to be the primary interface for many of the strategies described above that enhance engagement with PAP and therapies for comorbid conditions, provide CBT and lifestyle accountability, and collect patient reported data. This artificial provider would be a constant companion providing interactive, personalized, and continuous management to complement traditional intermittent live-person care.
The current health-care environment embodies the principle to “never let a serious crisis go to waste.” COVID-19 has accelerated the progression into the future by fostering an opening to embrace novel application of technologies to support changes in paradigms. Furthermore, health-care infrastructures that typically progress deliberately changed seemingly in a single moment. The Center for Medicare Services issued broad authorization to reimburse for telemedicine in response to COVID-19. Continued evolution in infrastructures will dictate progress with innovation, and a greater transition to outcomes-based incentives may be necessary to accommodate many of the strategies described above that rely on nonsynchronous care. But, we may be experiencing the moment when health care starts to catch up with the world in its embrace of technology. Sleep and pulmonary medicine can be a leader by providing a successful template for other specialties in optimizing chronic disease management.
Dr. Hwang is Medical Director, Kaiser Permanente SBC Sleep Center, and co-chair, Sleep Medicine, Kaiser Permanente Southern California.
On March 18, 2020, the doors to our sleep center were physically closed. Two potential exposures to COVID-19 within a few hours, the palpable anxiety of our team, and a poor grasp of the virus and the growing pandemic moved us to make this decision. Up to that point, we could not help but feel we were playing “catch up” with our evolving set of safety measures to the escalating risk. Like so many other sleep centers around the country, a complete transition to virtual care was needed to ensure the safety of our patients and our team. It was perhaps that moment that we felt the emotional impact that our world had changed, altering both our personal lives and sleep medicine practice as we knew it. This event, while unfortunate, also provided a transformative opportunity to reimagine our identity, accelerating the efforts to bring the future of sleep medicine into the present.
Our team’s clinical evolution and innovation efforts have been guided by efforts to reconsider sleep medicine paradigms. Innovation progress was deliberate with incremental implementations that typically required repeat business cases with multiple approving parties and budgetary access. Those barriers largely dissolved once COVID-19 intensified, and a large portion of the strategies on our roadmap were put into production. In a matter of a couple weeks, our services completely transitioned to remote and virtual care, while most of the team of 55 persons were moved to “work-from-home.” A suite of technologies (automated questionnaires, automated and two-way text messaging templates, consumer wearable technologies, and population management dashboards) were put on the table (Somnoware, Inc.), and each of our longitudinal care teams (eg, adult obstructive sleep apnea, pediatrics, chronic respiratory failure, commercial driver, insomnia programs, etc) worked to embed them into new care pathways. This effort further consolidated technology as the backbone of our work and the enabler of remote virtual collaboration between sleep center personnel (respiratory case managers, medical assistants and nursing team, and physician and leadership personnel) to enhance our team-based approach. Moreover, we felt this point in time was ripe to swallow the proverbial “red pill” and approach patient care with shifted paradigms. We discuss three areas of active effort to leverage technology in this COVID-19 environment to accelerate a transition toward how we envision the future of sleep medicine.
Reimagined sleep diagnostics
Our virtual obstructive sleep apnea (OSA) diagnostic process includes utilizing a disposable home sleep apnea test (HSAT) device with wireless data transfer (WatchPAT ONE, Itamar Medical) while HSAT and PAP (positive airway pressure) setups are supported by information sheets, online videos (YouTube), automated interactive platforms (Emmi Solutions; Hwang D. Am J Respir Crit Care Med. 2018 Jan 1;197[1]:117), and synchronous provider video visits. Our more radical shift, however, is in approaching OSA diagnosis based principally on symptoms and secondarily supported by physiologic measurements and response to therapy. This “clinical diagnosis” approach reduces our reliance on traditional sleep testing and allows patient wearables to provide supportive physiologic data (eg, oximetry) to help determine OSA severity and phenotype. Its immediate impact is in limiting the need to send and retrieve potentially contaminated equipment. Broader clinical advantages include overcoming the imprecise nature of the apnea-hypopnea index (which often has dramatic night-to-night variability) through data collection over extended durations, improving disease assessment due to availability of complementary sleep/activity data in the person’s usual setting, and tracking changes after therapy initiation.
Our post-COVID-19 re-opening of polysomnography (PSG) services, after a temporary shutdown, introduces home PSG (Type II) for approximately half our patients without suspected complex breathing conditions while reserving attended PSG (Type I) for those who may require noninvasive ventilation. The immediate incentive is in reducing viral exposure by limiting patient traffic and risk of PAP trial aerosolization while also improving access to accommodate the backlog of patients requiring PSG. This approach furthers the paradigm shift to emphasizing care in the home setting. Testing in the patient’s usual environment and enabling multiple night/day testing may be clinically advantageous.
Shift in emphasis to care management
The emphasis of sleep medicine has traditionally focused on diagnostics through performing PSG and HSAT. Our field has invested tremendous effort in developing guidelines for processing sleep studies, but the scoring and interpretation of those studies is extremely labor intensive. Reimagining the diagnostic approach reduces the need to manually process studies—wearable data are produced automatically, HSAT can be auto-scored, and artificial intelligence platforms can score PSGs (Goldstein CA. J Clin Sleep Med. 2020 Apr 15;16[4]:609), which allows a shift in resources and emphasis to follow-up care. A comprehensive discussion of technology-based tools to enhance care management is beyond the purview of this editorial. However, an overview of our current efforts includes: (1) utilizing population management dashboards to automatically risk stratify different cohorts of patients (eg, adult OSA, pediatrics, commercial drivers, chronic respiratory failure, etc) to identify patients “at-risk” (eg, based on OSA severity, symptoms, co-morbidities, and PAP adherence); (2) applying enhanced patient-provider interchange tools that include automated and “intelligent” electronic questionnaires, automated personalized text messaging/emails, and two-way messaging to deliver care; (3) utilizing remote patient monitoring to enhance holistic, personalized management, such as with remote activity/sleep trackers, blood pressure monitors, glucometers, and weight scales. We are engaged with efforts to validate the impact of these data to provide more personalized feedback, directly impact clinical outcomes, facilitate interdisciplinary collaboration, and identify acutely ill patients. Furthermore, a holistic approach beyond a narrow focus on PAP may create a positive collateral effect on adherence by targeting engagement with broader areas of health; and (4) implementing machine learning tools to directly support providers and patients (examples discussed in the next section.) Each of our teams has created workflows embedding these strategies throughout new care pathways. 
Generally, our emphasis during the first 3 months after PAP initiation focuses on achieving therapy adherence, and the post-3-month period broadens the efforts to target clinical outcomes. Recent trials with low PAP usage that failed to confirm the benefit of PAP on cardiovascular outcomes (McEvoy DR, et al. N Engl J Med. 2016;375:919) strongly suggest greater investment in cost-effective long-term strategies is imperative to increase our field’s relevance.
Application of artificial intelligence
We describe current efforts to apply artificial intelligence (AI) into clinical care: (1) We are implementing machine learning (ML) PSG scoring, which can potentially improve both the consistency and efficiency of scoring, further enabling greater investment in follow-up care. The future of sleep study processing, however, will likely depend on computer vision to “view” details inaccessible to the human eye and produce novel metrics that better inform clinical phenotypes (eg, cardiovascular risk, response to alternative therapies, etc). For example, “brain age” has been derived from EEG tracings that could reflect the degree of impact of sleep disorders on neurocognitive function (Fernandez C, unpublished data); (2) Machine learning clinical decision tools are in development to predict PAP adherence and timing of discontinuation, predict timing of cardiovascular disease onset and hospitalization, personalizing adherence targets, automating triaging of patients to home or PSG testing, and innumerable other predictions at clinical decision inflection points. Prediction outputs may be presented as risk profiles embedded in each patient’s “chart,” as personalized alerts, and in gamification strategies. For example, machine learning personalized cardiovascular risk scores can be regularly updated based on degree of PAP use to incentivize adherence; (3) Artificial providers may provide consistent, personalized, and holistic supplementary care. Many people rely on AI-bots for social support and cognitive-behavioral therapy (CBT) for depression. A sleep wellness bot, currently in planning stages, is intended to be the primary interface for many of the strategies described above that enhance engagement with PAP and therapies for comorbid conditions, provide CBT and lifestyle accountability, and collect patient reported data. This artificial provider would be a constant companion providing interactive, personalized, and continuous management to complement traditional intermittent live-person care.
The current health-care environment embodies the principle to “never let a serious crisis go to waste.” COVID-19 has accelerated the progression into the future by fostering an opening to embrace novel application of technologies to support changes in paradigms. Furthermore, health-care infrastructures that typically progress deliberately changed seemingly in a single moment. The Center for Medicare Services issued broad authorization to reimburse for telemedicine in response to COVID-19. Continued evolution in infrastructures will dictate progress with innovation, and a greater transition to outcomes-based incentives may be necessary to accommodate many of the strategies described above that rely on nonsynchronous care. But, we may be experiencing the moment when health care starts to catch up with the world in its embrace of technology. Sleep and pulmonary medicine can be a leader by providing a successful template for other specialties in optimizing chronic disease management.
Dr. Hwang is Medical Director, Kaiser Permanente SBC Sleep Center, and co-chair, Sleep Medicine, Kaiser Permanente Southern California.
COVID-19: Just a virus, right?
My first exposure to the notion of scarce resources was in medical school. I had to discuss the ethical principles behind the allocation of organs for transplantation, specifically livers and the required abstinence from alcohol ... but this was just an exercise, right?
A few years later, during residency, I heard the anecdotes from one of my internal medicine attendings about the time he spent in Europe as a visiting geriatrics fellow in the 1970s. The health-care districts in the region would be allotted an annual budget, and it was up to those districts how to best allocate those resources to meet, to the best of their abilities, the health-care needs of their population. He vividly recalled that a patient he cared for, an individual over 65 in need of renal replacement therapy for a reversible condition, who was not offered such therapy despite the clear benefit. There was a finite amount of resources, and those resources were thought to be better spent on public health measures like vaccination ... but that was on another continent and in another era, right?
I remember when I first heard of an outbreak of viral pneumonia in China in January of this year. As someone prone to anxiety, my first strategy was to put my head in the sand and wait it out. This strategy didn’t last very long – within a couple of weeks, there were confirmed cases in the United States. It was now apparent that this virus was not going to be contained. In an impressively short amount of time, SARS-CoV 2 has infected over 3.5 million individuals and killed almost a quarter million people worldwide. In the United States, we have seen almost 1.2 million cases and lost over 68 thousand lives. This pandemic has managed to devastate multiple countries, health care systems, and economies. It has also challenged every physician’s ideas of beneficence and justice ... but it’s just a virus, right?
Beneficence, the principle of medical ethics regarding acting in the patient’s best interest, had always seemed to me to be a no-brainer. Not like autonomy, which can get sticky, or justice, which I really had not had to consider much prior to 2020. Of course, I would always do what was best for my patient, I thought, why wouldn’t I?
Justice, the principle that deals with the distribution of scarce health-care resources, is the wrench that has been thrown into the beneficence works in the age of COVID-19. In a country and an era in which I had not dreamed we would ever have to think about how to support multiple people with one ventilator, we have had to do just that (“Joint Statement on Multiple Patients per Ventilator,” CHEST News, Mar 27, 2020). Things that I have taken for granted through all of my training are now worth their weight in gold—from sedative drips and inhalers down to videolaryngoscopy blades and face masks. I can’t just do what is best for my patient because sometimes what is best for my patient is not what is best for my next patient, what is best for my team, or even what is best for me and for my family. COVID-19 has reminded us of the uncomfortable truth that when contemplating justice, the patient in front of us is not the only person we have to consider.
Early on, before things in the United States had surged, I asked the twitter community what I thought would be a hypothetical question: “An employee needs to urgently help a COVID-19 patient. There is no appropriate PPE available due to shortage. What should happen?”
Like the idea of splitting ventilators, it was a thought I had never considered pre-COVID-19. Our instinct as physicians, especially as critical care physicians, is to intervene in emergency situations as quickly as possible. The extensive PPE required to manage COVID-19 patients has slowed that process, but, as many institutions are reaching the ends of their PPE stores, our safety is now placed at odds with that of our patient’s. To stay back violates what we feel is our duty to our patients, to go in violates our duty to ourselves, to our families, and to the rest of our patients. To care adequately for your patient is to put yourself at risk (and vice-versa), and this is a problem that I don’t think we have an answer for.
COVID-19 threatens many good and noble things, and what is worse, it directly puts them at odds with one another. They are paired sliding scales, where more of one means less of the other. If I have enough masks, it means my colleague probably doesn’t. If we have enough ventilators, it means another city doesn’t. If I get a break to be with my family, it means someone else is having to leave theirs to tend to patients who are sicker, lonelier, and more numerous than in any other time in recent memory.
And if these situations and resource limitations don’t provide enough moral injury for health-care workers, there are some specifics of humanity’s response to the pandemic that are exceptionally hurtful.
We as a country had notice, which was squandered. Instead of caution and preparation, we saw the powers that be make light of the serious situation most scientists and clinicians warned was coming. Instead of efforts to find or create PPE, we saw accusations against us of misuse and waste (“Trump comments about hospital mask thefts spark backlash from doctors,” Newsweek, March 30, 2020). Instead of support, we saw our altruism taken advantage of and used against us in unsafe and unfair situations. We have seen physicians in training and full-fledged attendings alike treated unfairly by their supervisors, instead of protected. Every instance of anti-science opinion or action from our friends and families that we once tolerated now feels like a personal affront, as these directly increase our risk and our immediate family’s risk of contracting the illness. We are being touted as heroes and angels, but really, we’re afraid—afraid of our patients, afraid of illness, afraid for our families, and afraid of jobs that we used to love. We don’t want to be praised; we just want to work our regular jobs safely and with adequate support.
I don’t know what health care looks like at the end of all of this. Relationships between physicians and health-care administrations were strained before the pandemic, to say the least. How can health-care workers just go back to business as usual, working for entities that were so ill-prepared, and, in many cases, calloused toward the concerns of their employees?
COVID-19 has revealed the fragility of our health-care system, our public health capabilities, and our economy. The pandemic has forced us to finally acknowledge something that has been true all along—our resources are finite, and tension exists between what is right and what is profitable, and between what is just and what is easy.
But it’s just a virus, right?
Dr. Fridenmaker is a Pulmonary and Critical Care Fellow at the University of Kentucky, Lexington.
My first exposure to the notion of scarce resources was in medical school. I had to discuss the ethical principles behind the allocation of organs for transplantation, specifically livers and the required abstinence from alcohol ... but this was just an exercise, right?
A few years later, during residency, I heard the anecdotes from one of my internal medicine attendings about the time he spent in Europe as a visiting geriatrics fellow in the 1970s. The health-care districts in the region would be allotted an annual budget, and it was up to those districts how to best allocate those resources to meet, to the best of their abilities, the health-care needs of their population. He vividly recalled that a patient he cared for, an individual over 65 in need of renal replacement therapy for a reversible condition, who was not offered such therapy despite the clear benefit. There was a finite amount of resources, and those resources were thought to be better spent on public health measures like vaccination ... but that was on another continent and in another era, right?
I remember when I first heard of an outbreak of viral pneumonia in China in January of this year. As someone prone to anxiety, my first strategy was to put my head in the sand and wait it out. This strategy didn’t last very long – within a couple of weeks, there were confirmed cases in the United States. It was now apparent that this virus was not going to be contained. In an impressively short amount of time, SARS-CoV 2 has infected over 3.5 million individuals and killed almost a quarter million people worldwide. In the United States, we have seen almost 1.2 million cases and lost over 68 thousand lives. This pandemic has managed to devastate multiple countries, health care systems, and economies. It has also challenged every physician’s ideas of beneficence and justice ... but it’s just a virus, right?
Beneficence, the principle of medical ethics regarding acting in the patient’s best interest, had always seemed to me to be a no-brainer. Not like autonomy, which can get sticky, or justice, which I really had not had to consider much prior to 2020. Of course, I would always do what was best for my patient, I thought, why wouldn’t I?
Justice, the principle that deals with the distribution of scarce health-care resources, is the wrench that has been thrown into the beneficence works in the age of COVID-19. In a country and an era in which I had not dreamed we would ever have to think about how to support multiple people with one ventilator, we have had to do just that (“Joint Statement on Multiple Patients per Ventilator,” CHEST News, Mar 27, 2020). Things that I have taken for granted through all of my training are now worth their weight in gold—from sedative drips and inhalers down to videolaryngoscopy blades and face masks. I can’t just do what is best for my patient because sometimes what is best for my patient is not what is best for my next patient, what is best for my team, or even what is best for me and for my family. COVID-19 has reminded us of the uncomfortable truth that when contemplating justice, the patient in front of us is not the only person we have to consider.
Early on, before things in the United States had surged, I asked the twitter community what I thought would be a hypothetical question: “An employee needs to urgently help a COVID-19 patient. There is no appropriate PPE available due to shortage. What should happen?”
Like the idea of splitting ventilators, it was a thought I had never considered pre-COVID-19. Our instinct as physicians, especially as critical care physicians, is to intervene in emergency situations as quickly as possible. The extensive PPE required to manage COVID-19 patients has slowed that process, but, as many institutions are reaching the ends of their PPE stores, our safety is now placed at odds with that of our patient’s. To stay back violates what we feel is our duty to our patients, to go in violates our duty to ourselves, to our families, and to the rest of our patients. To care adequately for your patient is to put yourself at risk (and vice-versa), and this is a problem that I don’t think we have an answer for.
COVID-19 threatens many good and noble things, and what is worse, it directly puts them at odds with one another. They are paired sliding scales, where more of one means less of the other. If I have enough masks, it means my colleague probably doesn’t. If we have enough ventilators, it means another city doesn’t. If I get a break to be with my family, it means someone else is having to leave theirs to tend to patients who are sicker, lonelier, and more numerous than in any other time in recent memory.
And if these situations and resource limitations don’t provide enough moral injury for health-care workers, there are some specifics of humanity’s response to the pandemic that are exceptionally hurtful.
We as a country had notice, which was squandered. Instead of caution and preparation, we saw the powers that be make light of the serious situation most scientists and clinicians warned was coming. Instead of efforts to find or create PPE, we saw accusations against us of misuse and waste (“Trump comments about hospital mask thefts spark backlash from doctors,” Newsweek, March 30, 2020). Instead of support, we saw our altruism taken advantage of and used against us in unsafe and unfair situations. We have seen physicians in training and full-fledged attendings alike treated unfairly by their supervisors, instead of protected. Every instance of anti-science opinion or action from our friends and families that we once tolerated now feels like a personal affront, as these directly increase our risk and our immediate family’s risk of contracting the illness. We are being touted as heroes and angels, but really, we’re afraid—afraid of our patients, afraid of illness, afraid for our families, and afraid of jobs that we used to love. We don’t want to be praised; we just want to work our regular jobs safely and with adequate support.
I don’t know what health care looks like at the end of all of this. Relationships between physicians and health-care administrations were strained before the pandemic, to say the least. How can health-care workers just go back to business as usual, working for entities that were so ill-prepared, and, in many cases, calloused toward the concerns of their employees?
COVID-19 has revealed the fragility of our health-care system, our public health capabilities, and our economy. The pandemic has forced us to finally acknowledge something that has been true all along—our resources are finite, and tension exists between what is right and what is profitable, and between what is just and what is easy.
But it’s just a virus, right?
Dr. Fridenmaker is a Pulmonary and Critical Care Fellow at the University of Kentucky, Lexington.
My first exposure to the notion of scarce resources was in medical school. I had to discuss the ethical principles behind the allocation of organs for transplantation, specifically livers and the required abstinence from alcohol ... but this was just an exercise, right?
A few years later, during residency, I heard the anecdotes from one of my internal medicine attendings about the time he spent in Europe as a visiting geriatrics fellow in the 1970s. The health-care districts in the region would be allotted an annual budget, and it was up to those districts how to best allocate those resources to meet, to the best of their abilities, the health-care needs of their population. He vividly recalled that a patient he cared for, an individual over 65 in need of renal replacement therapy for a reversible condition, who was not offered such therapy despite the clear benefit. There was a finite amount of resources, and those resources were thought to be better spent on public health measures like vaccination ... but that was on another continent and in another era, right?
I remember when I first heard of an outbreak of viral pneumonia in China in January of this year. As someone prone to anxiety, my first strategy was to put my head in the sand and wait it out. This strategy didn’t last very long – within a couple of weeks, there were confirmed cases in the United States. It was now apparent that this virus was not going to be contained. In an impressively short amount of time, SARS-CoV 2 has infected over 3.5 million individuals and killed almost a quarter million people worldwide. In the United States, we have seen almost 1.2 million cases and lost over 68 thousand lives. This pandemic has managed to devastate multiple countries, health care systems, and economies. It has also challenged every physician’s ideas of beneficence and justice ... but it’s just a virus, right?
Beneficence, the principle of medical ethics regarding acting in the patient’s best interest, had always seemed to me to be a no-brainer. Not like autonomy, which can get sticky, or justice, which I really had not had to consider much prior to 2020. Of course, I would always do what was best for my patient, I thought, why wouldn’t I?
Justice, the principle that deals with the distribution of scarce health-care resources, is the wrench that has been thrown into the beneficence works in the age of COVID-19. In a country and an era in which I had not dreamed we would ever have to think about how to support multiple people with one ventilator, we have had to do just that (“Joint Statement on Multiple Patients per Ventilator,” CHEST News, Mar 27, 2020). Things that I have taken for granted through all of my training are now worth their weight in gold—from sedative drips and inhalers down to videolaryngoscopy blades and face masks. I can’t just do what is best for my patient because sometimes what is best for my patient is not what is best for my next patient, what is best for my team, or even what is best for me and for my family. COVID-19 has reminded us of the uncomfortable truth that when contemplating justice, the patient in front of us is not the only person we have to consider.
Early on, before things in the United States had surged, I asked the twitter community what I thought would be a hypothetical question: “An employee needs to urgently help a COVID-19 patient. There is no appropriate PPE available due to shortage. What should happen?”
Like the idea of splitting ventilators, it was a thought I had never considered pre-COVID-19. Our instinct as physicians, especially as critical care physicians, is to intervene in emergency situations as quickly as possible. The extensive PPE required to manage COVID-19 patients has slowed that process, but, as many institutions are reaching the ends of their PPE stores, our safety is now placed at odds with that of our patient’s. To stay back violates what we feel is our duty to our patients, to go in violates our duty to ourselves, to our families, and to the rest of our patients. To care adequately for your patient is to put yourself at risk (and vice-versa), and this is a problem that I don’t think we have an answer for.
COVID-19 threatens many good and noble things, and what is worse, it directly puts them at odds with one another. They are paired sliding scales, where more of one means less of the other. If I have enough masks, it means my colleague probably doesn’t. If we have enough ventilators, it means another city doesn’t. If I get a break to be with my family, it means someone else is having to leave theirs to tend to patients who are sicker, lonelier, and more numerous than in any other time in recent memory.
And if these situations and resource limitations don’t provide enough moral injury for health-care workers, there are some specifics of humanity’s response to the pandemic that are exceptionally hurtful.
We as a country had notice, which was squandered. Instead of caution and preparation, we saw the powers that be make light of the serious situation most scientists and clinicians warned was coming. Instead of efforts to find or create PPE, we saw accusations against us of misuse and waste (“Trump comments about hospital mask thefts spark backlash from doctors,” Newsweek, March 30, 2020). Instead of support, we saw our altruism taken advantage of and used against us in unsafe and unfair situations. We have seen physicians in training and full-fledged attendings alike treated unfairly by their supervisors, instead of protected. Every instance of anti-science opinion or action from our friends and families that we once tolerated now feels like a personal affront, as these directly increase our risk and our immediate family’s risk of contracting the illness. We are being touted as heroes and angels, but really, we’re afraid—afraid of our patients, afraid of illness, afraid for our families, and afraid of jobs that we used to love. We don’t want to be praised; we just want to work our regular jobs safely and with adequate support.
I don’t know what health care looks like at the end of all of this. Relationships between physicians and health-care administrations were strained before the pandemic, to say the least. How can health-care workers just go back to business as usual, working for entities that were so ill-prepared, and, in many cases, calloused toward the concerns of their employees?
COVID-19 has revealed the fragility of our health-care system, our public health capabilities, and our economy. The pandemic has forced us to finally acknowledge something that has been true all along—our resources are finite, and tension exists between what is right and what is profitable, and between what is just and what is easy.
But it’s just a virus, right?
Dr. Fridenmaker is a Pulmonary and Critical Care Fellow at the University of Kentucky, Lexington.
Evolving treatment of cystic fibrosis: Path toward a normal lifespan
Cystic fibrosis (CF) is an autosomal recessive disorder affecting thousands of people worldwide. When this genetic disease was first discovered in the first half of the 20th century, the median survival was approximately 5 years of age. Since then, median survival for patients with CF has steadily improved. Today, it is 47.4 years based on Cystic Fibrosis Foundation registry data from 2018. Patients with CF are living longer and staying healthier; the discussion to follow is how patients, researchers, and the CF Foundation reached this point.
In 1938, pediatrician and pathologist Dorothy Anderson observed on the autopsies of children thought to have celiac disease pancreatic lesions she termed “cystic fibrosis of the pancreas.” In addition to the abnormal pancreas, she noted abnormal lungs filled with mucus that obstructed the airways.
Paul Di Sant’Agnese recognized during a heatwave in late 1948 that children with CF were routinely being diagnosed with heatstroke and dehydration. This helped lead to the discovery that these children had elevated salt content in their sweat, paving the way for the development of the sweat chloride test in 1959 by Gibson and Cooke. Not only did Dr. Di Sant’Agnese recognize excess salt in the sweat of patients with CF, but with the help of several concerned parents of children with CF, he established the Cystic Fibrosis Foundation in 1955. The Foundation helped organize the care center model over the next decades, increasing from 30 care centers in 1962 to over 100 in 1978. The care center model also developed a patient registry to track patient care longitudinally.
In June 1989, Francis Collins and Lap-Chee Tsui discovered the location of the CF transmembrane conductance regulator (CFTR) protein using a novel technique called chromosome jumping (Rommens JM, et al. Science. 1989;245[4922]1059). The discovery was a breakthrough in basic science research, but it would take 3 more decades before this discovery could be translated into a medication that could be used by most patients for everyday care.
In the early 1990s, when median survival for patients with CF was 29 years of age, the CF Foundation and Genentech, Inc., coordinated a 24-week multicenter double-blind randomized control trial (RCT) for a new inhaled medication that digested the extracellular DNA from the neutrophils that accumulated in the airways of patients with CF. Inhaled recombinant human DNase in these patients reduced the risk of pulmonary exacerbations and also had a small improvement in pulmonary function in the group compared with the placebo group (Fuchs H, et al. N Engl J Med. 1994;331:637). Five years later, another double-blind RCT demonstrated that inhaled tobramycin in patients with CF whose disease was colonized with Pseudomonas aeruginosa improved pulmonary function and reduced the risk of hospitalizations (Ramsey B, et al. N Engl J Med. 1999;340:23). In 2006, the use of hypertonic saline solution in patients with CF decreased the overall pulmonary exacerbation rate (Elkins MR, et al. N Engl J Med. 2006;354:229). The combination of these inhaled medications, along with inhaled aztreonam, formed the backbone of inhalation therapy for CF care today.
In 1998, even with the ongoing development and approval of new CF medications by the pharmaceutical industry, Robert Beall, CEO of the CF Foundation, realized that he needed to challenge the current drug development paradigm. Instead of trying to convince companies to develop CF medications, he started a concept called venture philanthropy. This concept entailed the CF Foundation financially investing in pharmaceutical companies’ development of new medications. The Foundation first invested in a small company named Aurora Biosciences (known today as Vertex Pharmaceuticals) in 2000. Aurora Biosciences specialized in high throughput screening. This process uses a unique technology allowing one to test the therapeutic reaction of airway cells to thousands of chemical compounds in a single day, instead of using the traditional process of tediously pipetting compounds one by one. Today, the CF Foundation has invested millions of dollars into bioscience research to advance CF care.
In 2011, the results of a study were published in which a small molecule altered defective CFTR protein in patients with CF with the CFTR mutation G551D, thus improving chloride transport at the airway surface. In the original study, after 24 weeks of therapy receiving the medication known as ivacaftor, predicted FEV1 in patients with CF improved 10.6%, and the patients were 55% less likely to have a pulmonary exacerbation compared with those receiving a placebo. This breakthrough provided patients with CF the first medication that could correct the CFTR at the source of the problem (Ramsey BW, et al. N Engl J Med. 2011;365:1663). Ivacaftor was approved by the US FDA in 2012.
Ivacaftor provided proof of concept that using small molecules could improve CFTR function. Ivacaftor was only beneficial to a small percentage of patients and was not effective in patients with CF who had either 1 or 2 F508del CFTR mutations. In 2015, patients with CF with F508del homozygous treated with a combination therapy of lumacaftor/ivacaftor had predicted FEV1% improved 2.6% to 4.0%. More importantly, there was a significant reduction in the number of pulmonary exacerbations per year compared with placebo. Unexpectedly, some of the patients experienced bronchoconstriction while receiving lumacaftor/ivacaftor (Wainwright CE, et al. N Engl J Med. 2015; 373:220). The problem was recognized, and a new small molecule to improve the processing and trafficking of CFTR called tezacaftor was developed. The combination of tezacaftor/ivacaftor in patients with CF who were F508del homozygous demonstrated a similar reduction in pulmonary exacerbations, an absolute improvement of predicted FEV1 of 4%, and no increased respiratory symptoms compared with the placebo arm (Taylor-Cousar JL, et al. N Engl J Med. 2017;377[21]2013).
CFTR modulators were a major breakthrough for patients with CF, but the efficacy of these therapies was dependent on the patients’ genotype and ranged from mildly to moderately effective. Unfortunately, these therapies were ineffective for the patients who were delta 508 heterozygotes. Starting in the summer of 2018, VX 445-tezacaftor-ivacaftor (ETI) was compared with placebo in patients with CF who were 1 copy of F508del and a second CFTR mutation that has minimal function. The study found an absolute improvement in predicted FEV1 of 14.3% and a 63% reduction in exacerbations at 24 weeks compared with placebo (Middleton PG, et al. N Engl J Med. 2019;381:1809). In late 2019, based on these data, ETI was approved by the FDA for all patients with CF who were F508del heterozygous. This innovation provided effective therapy to 90% of the CF population.
With the discovery of many highly effective therapies beneficial in most patients, the CF Foundation started a program called Path to a Cure to find therapies for the 10% of patients with CF who were not candidates for ETI or other CFTR modulators. This program looks to develop novel methods to restore CFTR protein function and repair or replace the CFTR protein via gene editing or gene transfer. This process creates many challenges that are quite complex, but patients, researchers, physicians, and CF Foundation will not stop working until CF stands for CURE FOUND.
Today, patients with CF are living longer, and many are eligible or have already started ETI therapy. This medication and the many others being developed will hopefully lead to patients with CF living a normal lifespan in the near future.
Dr. Finklea is Assistant Professor of Medicine, Division of Pulmonary and Critical Care, University of Texas Southwestern, Dallas, Texas. Dr. Finklea receives grant support from the Cystic Fibrosis Foundation.
Cystic fibrosis (CF) is an autosomal recessive disorder affecting thousands of people worldwide. When this genetic disease was first discovered in the first half of the 20th century, the median survival was approximately 5 years of age. Since then, median survival for patients with CF has steadily improved. Today, it is 47.4 years based on Cystic Fibrosis Foundation registry data from 2018. Patients with CF are living longer and staying healthier; the discussion to follow is how patients, researchers, and the CF Foundation reached this point.
In 1938, pediatrician and pathologist Dorothy Anderson observed on the autopsies of children thought to have celiac disease pancreatic lesions she termed “cystic fibrosis of the pancreas.” In addition to the abnormal pancreas, she noted abnormal lungs filled with mucus that obstructed the airways.
Paul Di Sant’Agnese recognized during a heatwave in late 1948 that children with CF were routinely being diagnosed with heatstroke and dehydration. This helped lead to the discovery that these children had elevated salt content in their sweat, paving the way for the development of the sweat chloride test in 1959 by Gibson and Cooke. Not only did Dr. Di Sant’Agnese recognize excess salt in the sweat of patients with CF, but with the help of several concerned parents of children with CF, he established the Cystic Fibrosis Foundation in 1955. The Foundation helped organize the care center model over the next decades, increasing from 30 care centers in 1962 to over 100 in 1978. The care center model also developed a patient registry to track patient care longitudinally.
In June 1989, Francis Collins and Lap-Chee Tsui discovered the location of the CF transmembrane conductance regulator (CFTR) protein using a novel technique called chromosome jumping (Rommens JM, et al. Science. 1989;245[4922]1059). The discovery was a breakthrough in basic science research, but it would take 3 more decades before this discovery could be translated into a medication that could be used by most patients for everyday care.
In the early 1990s, when median survival for patients with CF was 29 years of age, the CF Foundation and Genentech, Inc., coordinated a 24-week multicenter double-blind randomized control trial (RCT) for a new inhaled medication that digested the extracellular DNA from the neutrophils that accumulated in the airways of patients with CF. Inhaled recombinant human DNase in these patients reduced the risk of pulmonary exacerbations and also had a small improvement in pulmonary function in the group compared with the placebo group (Fuchs H, et al. N Engl J Med. 1994;331:637). Five years later, another double-blind RCT demonstrated that inhaled tobramycin in patients with CF whose disease was colonized with Pseudomonas aeruginosa improved pulmonary function and reduced the risk of hospitalizations (Ramsey B, et al. N Engl J Med. 1999;340:23). In 2006, the use of hypertonic saline solution in patients with CF decreased the overall pulmonary exacerbation rate (Elkins MR, et al. N Engl J Med. 2006;354:229). The combination of these inhaled medications, along with inhaled aztreonam, formed the backbone of inhalation therapy for CF care today.
In 1998, even with the ongoing development and approval of new CF medications by the pharmaceutical industry, Robert Beall, CEO of the CF Foundation, realized that he needed to challenge the current drug development paradigm. Instead of trying to convince companies to develop CF medications, he started a concept called venture philanthropy. This concept entailed the CF Foundation financially investing in pharmaceutical companies’ development of new medications. The Foundation first invested in a small company named Aurora Biosciences (known today as Vertex Pharmaceuticals) in 2000. Aurora Biosciences specialized in high throughput screening. This process uses a unique technology allowing one to test the therapeutic reaction of airway cells to thousands of chemical compounds in a single day, instead of using the traditional process of tediously pipetting compounds one by one. Today, the CF Foundation has invested millions of dollars into bioscience research to advance CF care.
In 2011, the results of a study were published in which a small molecule altered defective CFTR protein in patients with CF with the CFTR mutation G551D, thus improving chloride transport at the airway surface. In the original study, after 24 weeks of therapy receiving the medication known as ivacaftor, predicted FEV1 in patients with CF improved 10.6%, and the patients were 55% less likely to have a pulmonary exacerbation compared with those receiving a placebo. This breakthrough provided patients with CF the first medication that could correct the CFTR at the source of the problem (Ramsey BW, et al. N Engl J Med. 2011;365:1663). Ivacaftor was approved by the US FDA in 2012.
Ivacaftor provided proof of concept that using small molecules could improve CFTR function. Ivacaftor was only beneficial to a small percentage of patients and was not effective in patients with CF who had either 1 or 2 F508del CFTR mutations. In 2015, patients with CF with F508del homozygous treated with a combination therapy of lumacaftor/ivacaftor had predicted FEV1% improved 2.6% to 4.0%. More importantly, there was a significant reduction in the number of pulmonary exacerbations per year compared with placebo. Unexpectedly, some of the patients experienced bronchoconstriction while receiving lumacaftor/ivacaftor (Wainwright CE, et al. N Engl J Med. 2015; 373:220). The problem was recognized, and a new small molecule to improve the processing and trafficking of CFTR called tezacaftor was developed. The combination of tezacaftor/ivacaftor in patients with CF who were F508del homozygous demonstrated a similar reduction in pulmonary exacerbations, an absolute improvement of predicted FEV1 of 4%, and no increased respiratory symptoms compared with the placebo arm (Taylor-Cousar JL, et al. N Engl J Med. 2017;377[21]2013).
CFTR modulators were a major breakthrough for patients with CF, but the efficacy of these therapies was dependent on the patients’ genotype and ranged from mildly to moderately effective. Unfortunately, these therapies were ineffective for the patients who were delta 508 heterozygotes. Starting in the summer of 2018, VX 445-tezacaftor-ivacaftor (ETI) was compared with placebo in patients with CF who were 1 copy of F508del and a second CFTR mutation that has minimal function. The study found an absolute improvement in predicted FEV1 of 14.3% and a 63% reduction in exacerbations at 24 weeks compared with placebo (Middleton PG, et al. N Engl J Med. 2019;381:1809). In late 2019, based on these data, ETI was approved by the FDA for all patients with CF who were F508del heterozygous. This innovation provided effective therapy to 90% of the CF population.
With the discovery of many highly effective therapies beneficial in most patients, the CF Foundation started a program called Path to a Cure to find therapies for the 10% of patients with CF who were not candidates for ETI or other CFTR modulators. This program looks to develop novel methods to restore CFTR protein function and repair or replace the CFTR protein via gene editing or gene transfer. This process creates many challenges that are quite complex, but patients, researchers, physicians, and CF Foundation will not stop working until CF stands for CURE FOUND.
Today, patients with CF are living longer, and many are eligible or have already started ETI therapy. This medication and the many others being developed will hopefully lead to patients with CF living a normal lifespan in the near future.
Dr. Finklea is Assistant Professor of Medicine, Division of Pulmonary and Critical Care, University of Texas Southwestern, Dallas, Texas. Dr. Finklea receives grant support from the Cystic Fibrosis Foundation.
Cystic fibrosis (CF) is an autosomal recessive disorder affecting thousands of people worldwide. When this genetic disease was first discovered in the first half of the 20th century, the median survival was approximately 5 years of age. Since then, median survival for patients with CF has steadily improved. Today, it is 47.4 years based on Cystic Fibrosis Foundation registry data from 2018. Patients with CF are living longer and staying healthier; the discussion to follow is how patients, researchers, and the CF Foundation reached this point.
In 1938, pediatrician and pathologist Dorothy Anderson observed on the autopsies of children thought to have celiac disease pancreatic lesions she termed “cystic fibrosis of the pancreas.” In addition to the abnormal pancreas, she noted abnormal lungs filled with mucus that obstructed the airways.
Paul Di Sant’Agnese recognized during a heatwave in late 1948 that children with CF were routinely being diagnosed with heatstroke and dehydration. This helped lead to the discovery that these children had elevated salt content in their sweat, paving the way for the development of the sweat chloride test in 1959 by Gibson and Cooke. Not only did Dr. Di Sant’Agnese recognize excess salt in the sweat of patients with CF, but with the help of several concerned parents of children with CF, he established the Cystic Fibrosis Foundation in 1955. The Foundation helped organize the care center model over the next decades, increasing from 30 care centers in 1962 to over 100 in 1978. The care center model also developed a patient registry to track patient care longitudinally.
In June 1989, Francis Collins and Lap-Chee Tsui discovered the location of the CF transmembrane conductance regulator (CFTR) protein using a novel technique called chromosome jumping (Rommens JM, et al. Science. 1989;245[4922]1059). The discovery was a breakthrough in basic science research, but it would take 3 more decades before this discovery could be translated into a medication that could be used by most patients for everyday care.
In the early 1990s, when median survival for patients with CF was 29 years of age, the CF Foundation and Genentech, Inc., coordinated a 24-week multicenter double-blind randomized control trial (RCT) for a new inhaled medication that digested the extracellular DNA from the neutrophils that accumulated in the airways of patients with CF. Inhaled recombinant human DNase in these patients reduced the risk of pulmonary exacerbations and also had a small improvement in pulmonary function in the group compared with the placebo group (Fuchs H, et al. N Engl J Med. 1994;331:637). Five years later, another double-blind RCT demonstrated that inhaled tobramycin in patients with CF whose disease was colonized with Pseudomonas aeruginosa improved pulmonary function and reduced the risk of hospitalizations (Ramsey B, et al. N Engl J Med. 1999;340:23). In 2006, the use of hypertonic saline solution in patients with CF decreased the overall pulmonary exacerbation rate (Elkins MR, et al. N Engl J Med. 2006;354:229). The combination of these inhaled medications, along with inhaled aztreonam, formed the backbone of inhalation therapy for CF care today.
In 1998, even with the ongoing development and approval of new CF medications by the pharmaceutical industry, Robert Beall, CEO of the CF Foundation, realized that he needed to challenge the current drug development paradigm. Instead of trying to convince companies to develop CF medications, he started a concept called venture philanthropy. This concept entailed the CF Foundation financially investing in pharmaceutical companies’ development of new medications. The Foundation first invested in a small company named Aurora Biosciences (known today as Vertex Pharmaceuticals) in 2000. Aurora Biosciences specialized in high throughput screening. This process uses a unique technology allowing one to test the therapeutic reaction of airway cells to thousands of chemical compounds in a single day, instead of using the traditional process of tediously pipetting compounds one by one. Today, the CF Foundation has invested millions of dollars into bioscience research to advance CF care.
In 2011, the results of a study were published in which a small molecule altered defective CFTR protein in patients with CF with the CFTR mutation G551D, thus improving chloride transport at the airway surface. In the original study, after 24 weeks of therapy receiving the medication known as ivacaftor, predicted FEV1 in patients with CF improved 10.6%, and the patients were 55% less likely to have a pulmonary exacerbation compared with those receiving a placebo. This breakthrough provided patients with CF the first medication that could correct the CFTR at the source of the problem (Ramsey BW, et al. N Engl J Med. 2011;365:1663). Ivacaftor was approved by the US FDA in 2012.
Ivacaftor provided proof of concept that using small molecules could improve CFTR function. Ivacaftor was only beneficial to a small percentage of patients and was not effective in patients with CF who had either 1 or 2 F508del CFTR mutations. In 2015, patients with CF with F508del homozygous treated with a combination therapy of lumacaftor/ivacaftor had predicted FEV1% improved 2.6% to 4.0%. More importantly, there was a significant reduction in the number of pulmonary exacerbations per year compared with placebo. Unexpectedly, some of the patients experienced bronchoconstriction while receiving lumacaftor/ivacaftor (Wainwright CE, et al. N Engl J Med. 2015; 373:220). The problem was recognized, and a new small molecule to improve the processing and trafficking of CFTR called tezacaftor was developed. The combination of tezacaftor/ivacaftor in patients with CF who were F508del homozygous demonstrated a similar reduction in pulmonary exacerbations, an absolute improvement of predicted FEV1 of 4%, and no increased respiratory symptoms compared with the placebo arm (Taylor-Cousar JL, et al. N Engl J Med. 2017;377[21]2013).
CFTR modulators were a major breakthrough for patients with CF, but the efficacy of these therapies was dependent on the patients’ genotype and ranged from mildly to moderately effective. Unfortunately, these therapies were ineffective for the patients who were delta 508 heterozygotes. Starting in the summer of 2018, VX 445-tezacaftor-ivacaftor (ETI) was compared with placebo in patients with CF who were 1 copy of F508del and a second CFTR mutation that has minimal function. The study found an absolute improvement in predicted FEV1 of 14.3% and a 63% reduction in exacerbations at 24 weeks compared with placebo (Middleton PG, et al. N Engl J Med. 2019;381:1809). In late 2019, based on these data, ETI was approved by the FDA for all patients with CF who were F508del heterozygous. This innovation provided effective therapy to 90% of the CF population.
With the discovery of many highly effective therapies beneficial in most patients, the CF Foundation started a program called Path to a Cure to find therapies for the 10% of patients with CF who were not candidates for ETI or other CFTR modulators. This program looks to develop novel methods to restore CFTR protein function and repair or replace the CFTR protein via gene editing or gene transfer. This process creates many challenges that are quite complex, but patients, researchers, physicians, and CF Foundation will not stop working until CF stands for CURE FOUND.
Today, patients with CF are living longer, and many are eligible or have already started ETI therapy. This medication and the many others being developed will hopefully lead to patients with CF living a normal lifespan in the near future.
Dr. Finklea is Assistant Professor of Medicine, Division of Pulmonary and Critical Care, University of Texas Southwestern, Dallas, Texas. Dr. Finklea receives grant support from the Cystic Fibrosis Foundation.
COVID-19 and impact on sleep medicine practices
Introduction
Since reported in late 2019 in Wuhan China, the disease named “novel coronavirus disease 2019” (COVID-19), caused by the virus referred to as Severe Acute Respiratory Syndrome-causing Coronavirus-2 (SARS-CoV-2) has spread widely to many parts of the world. As of April 13, 2020, a total of 210 countries reported more than 1.9 million cases, resulting in more than 119,000 deaths.1 All 50 states have reported cases of COVID-19 to the Centers for Disease Control and Prevention (CDC), and most US states are reporting community spread. While levels of COVID-19 activity vary by region, the CDC has reported that the US remains in the acceleration phase of the pandemic, and that widespread transmission is expected.
On March 18, the Centers for Medicare & Medicaid Services (CMS) advised2 that all elective surgeries and nonessential medical, surgical, and dental procedures should be delayed to promote physical distancing, preserve personal protective equipment (PPE), and enable health-care workers (HCW) to redirect work to high-need areas. California was the first to issue a statewide shelter-in-place order on March 19, and by April, leaders in 42 states, the District of Columbia, and Puerto Rico issued similar stay-at-home orders. The White House has announced that physical distancing should continue until at least April 30. With the potential for an explosion of new cases that could overwhelm health-care resources, “business as usual” ceased to exist practically overnight.
The speed with which these events transpired, the demand to tailor response within days or even hours, the lack of robust data to support decision-making, the possibility of spread by asymptomatic carriers, and the potential risk for airborne, as well as droplet and fecal-oral spread, caused sleep medicine clinicians to rely on expert consensus and clinical judgment. The goal of such guidance has been to optimize care to patients with sleep disorders, while protecting the health and safety of all. Sleep medicine practices have had to balance efforts to reduce viral exposure and transmission, the need to triage health-care resources and personnel, and maintain access to care.
General clinical measures
From the outset, in areas of community spread, sleep medicine practices were called to adapt to now-standard measures, such as provider self-quarantine if ill or exposed, in-person clinic triage strategies for patients and staff prior to entrance to facilities to rapidly identify people with respiratory illness (eg, temperature monitoring), elimination of nonessential visitors, and infection control measures such as vigilant cleaning and appropriate use of personal protective equipment (PPE) during patient interactions. Typical issues facing sleep medicine practices include the need to prioritize urgent or emergency care, track canceled or postponed visits, and maintain access to communication with patients, the health-care team, payors, and employers.
Infection mitigation recommendations: sleep laboratories and ambulatory practices
Diagnostic testing
By mid-March, relatively early in the course of the outbreak in the US, the American Academy of Sleep Medicine (AASM) released recommendations for sleep clinics and laboratories regarding continuation of in-lab diagnostic, split-night, and titration studies, as well as clinical interactions and telemedicine, taking into account the CDC mitigation strategies3 which vary according to level of community transmission or impact of COVID-19.
This advisory was updated repeatedly over the ensuing weeks, most recently on April 8, as community-based spread increased. The AASM now strongly urges all sleep clinicians to postpone in-laboratory polysomnography (PSG) for adults and children, both diagnostic and positive airway pressure (PAP) titrations, except in emergencies. Data regarding adherence with these recommendations are lacking; anecdotal reports suggest that sleep medicine communities most heavily affected by the community spread are indeed following this practice.
The AASM guidance also advises use of home sleep apnea testing (HSAT) with consideration of single-use components or devices, use of mail-in recorders, and/or removal of reusable devices from service for 72 hours between patients.
Positive airway pressure (PAP) therapy
The potential for PAP devices to promote the aerosolization of viral particles, which could increase transmission to others on shared ventilation networks in homes and health-care settings, requires careful attention.
Generally, exhaled particle size depends on multiple characteristics, including the force and pressure at generation and environmental conditions (eg, temperature, relative humidity, and air flow). Large-size particles remain suspended in the air only briefly and settle within 1 meter from the source; these are usually mediated by breathing zones of individuals.4 However, smaller particles can travel farther, with distance governed by airflow that is driven by many variables, including ventilation, human movement, and temperature gradients. While droplets tend to evaporate rapidly, dry residues can remain suspended in the air.5 Infectious respiratory aerosols can occur as droplets >5 mcm diameter, or droplet nuclei (<5 mcm diameter).6 Present evidence indicates that SARS-CoV-2 transmission occurs primarily through droplet spread in settings with normal breathing. However, the World Health Organization (WHO) advises more stringent, airborne precautions for aerosol-generating procedures with COVID-19. Such procedures include intubation, extubation, noninvasive ventilation, high-flow nasal cannula, and cardiopulmonary resuscitation before intubation.7 Some evidence indicates that SARS-CoV-2 can linger in aerosol form for hours,8 and aerosol transmission is therefore plausible. Non-peer reviewed data in real-world settings indicates the presence of SARS-CoV-2 in air samples from hallways outside and in rooms adjacent to COVID-19-containing patients.9
These findings raised some concerns about use of PAP in medical and home environments, leading to the recommendation that the decision to continue or withhold PAP temporarily be made based on a risk-benefit evaluation. Scant data hint that PAP therapy may be safe to use in rooms that support appropriate ventilation (eg, negative pressure rooms). Regarding mask type, recently, a group reported the possibility that oronasal masks have a better aerosol dispersal profile.5 However, this conclusion was based on a single study of a specific model of oronasal mask, which demonstrated an absence of ability to measure a dispersion air jet, because the exhalation ports on the mask caused diffuse rather than directed dispersion of air.10 The same study found, that when the jet could be measured (with nasal pillows or with leak from any interface), greater dispersion was indeed evident. While anecdotal practical methods to filter exhaled air from PAP devices to reduce aerosol transmission have been proposed, data regarding successful reduction in transmission are still lacking, and such methods are not endorsed by mask manufacturers.
Ambulatory clinics: role of telemedicine
As the spread of COVID-19 disease accelerated, the AASM recommended that sleep medicine practices postpone and reschedule all nonemergency, in-person appointments, and conduct as many visits as possible by telemedicine.
This rapid transition posed many layers of logistical complexity, including how to quickly initiate or scale up an often fledging telemedicine presence; scheduling and instructing patients for telemedicine encounters; problem-solving in situations with limited device and Internet availability; triaging patients based on risk; and tracking postponed appointments. Administrators, medical assistants, nurses, advanced practitioners, respiratory therapists, technologists, and physicians have learned new ways of doing things, and laboratory personnel have undergone training and transitioned to new roles and responsibilities during postponement of lab studies. Training programs, in particular, have had to be nimble in finding ways to meet the educational needs of sleep medicine fellows that leveraged telemedicine opportunities.
Economic implications of transformed sleep medicine practices
While deploying such systematic change costs both time and money, sleep practices are also confronted with questions around lost revenue from drops in laboratory and clinic volumes. Many additional questions around reimbursement and revenue shortfalls are present, and short-term, furloughed employees may not be able to sustain income loss, which could result in difficulty in resuming services when the COVID-19 threat has been reduced.
Helpfully, during this public health emergency, CMS has expanded coverage for telemedicine services and waived requirements for face-to-face or in-person encounters,11 and some private payers have followed. Additionally, for the duration of the public health emergency, Medicare will cover PAP devices based on the clinician’s assessment of the patient without requiring PSG or a home sleep apnea test (HSAT). However, CMS has not clarified what follow-up testing, if any, may be required after this public health emergency is over. The duration of these new payment models remains uncertain.
Recommendations for PAP users
Patients and families, practitioners, and group living facilities have all expressed concerns about use of PAP during the epidemic given presumed increased risk of viral spread. In many hospital protocols, the use of PAP is restricted or disallowed for patients with suspected or confirmed COVID-19. Guidance regarding out-of-hospital use of PAP has been sparse.
AASM has recommended avoidance of PAP or noninvasive ventilation (NIV) for those with presumed or confirmed COVID-19 who cannot self-isolate according to CDC guidance. Risk-benefit assessment is recommended for those who perform safety-sensitive activities or have higher-risk medical conditions. During the period that PAP is withheld, alternative or modifying therapies can be considered, such as positional therapy or oral appliance.
Cleaning device components and washing and replacing filters as recommended by the manufacturer, as well as simple but important interventions like handwashing before and after touching the face or airway gear is thought to be especially important during this time.
Conclusions
The COVID-19 pandemic has fueled unprecedented, rapid changes in the way sleep medicine practices deliver care to millions of patients. These changes have been propelled by practitioners and staff who have embraced adaptability, creativity, resourcefulness, and attention to safety and effectiveness. Widespread use of telemedicine services, greater reliance on ambulatory testing, ongoing risk-benefit stratification, leveraging technology and teamwork, and sharing knowledge as it becomes available has resulted in care that is more accessible and convenient for some vulnerable patients, and, yet, challenges persist in accessing needed care. Necessity has been the mother of invention, and we expect the field will need to continue to rebalance as the situation evolves. The ultimate test of these rapid innovations will be how sleep medicine patients fare in the long run, in terms of their health, safety, mortality, and overall quality of life. Future research must address these questions, and the resulting information may yet inform the way sleep medicine is practiced in the years to come.
Dr. Shannon is Medical Director, EVAL Research Institute, Palo Alto, CA; Dr. Gurubhagavatula is Associate Professor, Perelman School of Medicine, University of Pennsylvania, and with Crescenz VA Medical Center, Philadelphia, PA.
1. Worldometer. COVID-19 coronavirus pandemic.
2. Centers for Medicare & Medicaid Services. CMS releases recommendations on adult elective surgeries, non-essential medical, surgical, and dental procedures during COVID-19 response. 2020 Mar 18.
3. Centers for Medicare & Medicaid Services. Implementation of mitigation strategies for communities with local COVID-19 transmission.
4. Tang JW et al. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100-14.
5. Martina Ferioli et al. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. European Respiratory Review 2020;29:200068. doi: 10.1183/16000617.0068-2020.
6. World Health Organization. 2014 Apr. Infection prevention and control of epidemic and pandemic-prone acute respiratory infections in health care.
7. World Health Organization. 2020 Feb 27. Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19) Interim guidance.
8. Van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7. doi: 10.1056/NEJMc2004973.
9. Joshua L Santarpia et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. 2020 Mar 26. doi: 10.1101/2020.03.23.20039446.
10. David S. Hui et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019 Apr 11.53(4):pii: 1802339. doi: 10.1183/13993003.02339-2018.
11. Worldometer. COVID-19 coronavirus pandemic.
Introduction
Since reported in late 2019 in Wuhan China, the disease named “novel coronavirus disease 2019” (COVID-19), caused by the virus referred to as Severe Acute Respiratory Syndrome-causing Coronavirus-2 (SARS-CoV-2) has spread widely to many parts of the world. As of April 13, 2020, a total of 210 countries reported more than 1.9 million cases, resulting in more than 119,000 deaths.1 All 50 states have reported cases of COVID-19 to the Centers for Disease Control and Prevention (CDC), and most US states are reporting community spread. While levels of COVID-19 activity vary by region, the CDC has reported that the US remains in the acceleration phase of the pandemic, and that widespread transmission is expected.
On March 18, the Centers for Medicare & Medicaid Services (CMS) advised2 that all elective surgeries and nonessential medical, surgical, and dental procedures should be delayed to promote physical distancing, preserve personal protective equipment (PPE), and enable health-care workers (HCW) to redirect work to high-need areas. California was the first to issue a statewide shelter-in-place order on March 19, and by April, leaders in 42 states, the District of Columbia, and Puerto Rico issued similar stay-at-home orders. The White House has announced that physical distancing should continue until at least April 30. With the potential for an explosion of new cases that could overwhelm health-care resources, “business as usual” ceased to exist practically overnight.
The speed with which these events transpired, the demand to tailor response within days or even hours, the lack of robust data to support decision-making, the possibility of spread by asymptomatic carriers, and the potential risk for airborne, as well as droplet and fecal-oral spread, caused sleep medicine clinicians to rely on expert consensus and clinical judgment. The goal of such guidance has been to optimize care to patients with sleep disorders, while protecting the health and safety of all. Sleep medicine practices have had to balance efforts to reduce viral exposure and transmission, the need to triage health-care resources and personnel, and maintain access to care.
General clinical measures
From the outset, in areas of community spread, sleep medicine practices were called to adapt to now-standard measures, such as provider self-quarantine if ill or exposed, in-person clinic triage strategies for patients and staff prior to entrance to facilities to rapidly identify people with respiratory illness (eg, temperature monitoring), elimination of nonessential visitors, and infection control measures such as vigilant cleaning and appropriate use of personal protective equipment (PPE) during patient interactions. Typical issues facing sleep medicine practices include the need to prioritize urgent or emergency care, track canceled or postponed visits, and maintain access to communication with patients, the health-care team, payors, and employers.
Infection mitigation recommendations: sleep laboratories and ambulatory practices
Diagnostic testing
By mid-March, relatively early in the course of the outbreak in the US, the American Academy of Sleep Medicine (AASM) released recommendations for sleep clinics and laboratories regarding continuation of in-lab diagnostic, split-night, and titration studies, as well as clinical interactions and telemedicine, taking into account the CDC mitigation strategies3 which vary according to level of community transmission or impact of COVID-19.
This advisory was updated repeatedly over the ensuing weeks, most recently on April 8, as community-based spread increased. The AASM now strongly urges all sleep clinicians to postpone in-laboratory polysomnography (PSG) for adults and children, both diagnostic and positive airway pressure (PAP) titrations, except in emergencies. Data regarding adherence with these recommendations are lacking; anecdotal reports suggest that sleep medicine communities most heavily affected by the community spread are indeed following this practice.
The AASM guidance also advises use of home sleep apnea testing (HSAT) with consideration of single-use components or devices, use of mail-in recorders, and/or removal of reusable devices from service for 72 hours between patients.
Positive airway pressure (PAP) therapy
The potential for PAP devices to promote the aerosolization of viral particles, which could increase transmission to others on shared ventilation networks in homes and health-care settings, requires careful attention.
Generally, exhaled particle size depends on multiple characteristics, including the force and pressure at generation and environmental conditions (eg, temperature, relative humidity, and air flow). Large-size particles remain suspended in the air only briefly and settle within 1 meter from the source; these are usually mediated by breathing zones of individuals.4 However, smaller particles can travel farther, with distance governed by airflow that is driven by many variables, including ventilation, human movement, and temperature gradients. While droplets tend to evaporate rapidly, dry residues can remain suspended in the air.5 Infectious respiratory aerosols can occur as droplets >5 mcm diameter, or droplet nuclei (<5 mcm diameter).6 Present evidence indicates that SARS-CoV-2 transmission occurs primarily through droplet spread in settings with normal breathing. However, the World Health Organization (WHO) advises more stringent, airborne precautions for aerosol-generating procedures with COVID-19. Such procedures include intubation, extubation, noninvasive ventilation, high-flow nasal cannula, and cardiopulmonary resuscitation before intubation.7 Some evidence indicates that SARS-CoV-2 can linger in aerosol form for hours,8 and aerosol transmission is therefore plausible. Non-peer reviewed data in real-world settings indicates the presence of SARS-CoV-2 in air samples from hallways outside and in rooms adjacent to COVID-19-containing patients.9
These findings raised some concerns about use of PAP in medical and home environments, leading to the recommendation that the decision to continue or withhold PAP temporarily be made based on a risk-benefit evaluation. Scant data hint that PAP therapy may be safe to use in rooms that support appropriate ventilation (eg, negative pressure rooms). Regarding mask type, recently, a group reported the possibility that oronasal masks have a better aerosol dispersal profile.5 However, this conclusion was based on a single study of a specific model of oronasal mask, which demonstrated an absence of ability to measure a dispersion air jet, because the exhalation ports on the mask caused diffuse rather than directed dispersion of air.10 The same study found, that when the jet could be measured (with nasal pillows or with leak from any interface), greater dispersion was indeed evident. While anecdotal practical methods to filter exhaled air from PAP devices to reduce aerosol transmission have been proposed, data regarding successful reduction in transmission are still lacking, and such methods are not endorsed by mask manufacturers.
Ambulatory clinics: role of telemedicine
As the spread of COVID-19 disease accelerated, the AASM recommended that sleep medicine practices postpone and reschedule all nonemergency, in-person appointments, and conduct as many visits as possible by telemedicine.
This rapid transition posed many layers of logistical complexity, including how to quickly initiate or scale up an often fledging telemedicine presence; scheduling and instructing patients for telemedicine encounters; problem-solving in situations with limited device and Internet availability; triaging patients based on risk; and tracking postponed appointments. Administrators, medical assistants, nurses, advanced practitioners, respiratory therapists, technologists, and physicians have learned new ways of doing things, and laboratory personnel have undergone training and transitioned to new roles and responsibilities during postponement of lab studies. Training programs, in particular, have had to be nimble in finding ways to meet the educational needs of sleep medicine fellows that leveraged telemedicine opportunities.
Economic implications of transformed sleep medicine practices
While deploying such systematic change costs both time and money, sleep practices are also confronted with questions around lost revenue from drops in laboratory and clinic volumes. Many additional questions around reimbursement and revenue shortfalls are present, and short-term, furloughed employees may not be able to sustain income loss, which could result in difficulty in resuming services when the COVID-19 threat has been reduced.
Helpfully, during this public health emergency, CMS has expanded coverage for telemedicine services and waived requirements for face-to-face or in-person encounters,11 and some private payers have followed. Additionally, for the duration of the public health emergency, Medicare will cover PAP devices based on the clinician’s assessment of the patient without requiring PSG or a home sleep apnea test (HSAT). However, CMS has not clarified what follow-up testing, if any, may be required after this public health emergency is over. The duration of these new payment models remains uncertain.
Recommendations for PAP users
Patients and families, practitioners, and group living facilities have all expressed concerns about use of PAP during the epidemic given presumed increased risk of viral spread. In many hospital protocols, the use of PAP is restricted or disallowed for patients with suspected or confirmed COVID-19. Guidance regarding out-of-hospital use of PAP has been sparse.
AASM has recommended avoidance of PAP or noninvasive ventilation (NIV) for those with presumed or confirmed COVID-19 who cannot self-isolate according to CDC guidance. Risk-benefit assessment is recommended for those who perform safety-sensitive activities or have higher-risk medical conditions. During the period that PAP is withheld, alternative or modifying therapies can be considered, such as positional therapy or oral appliance.
Cleaning device components and washing and replacing filters as recommended by the manufacturer, as well as simple but important interventions like handwashing before and after touching the face or airway gear is thought to be especially important during this time.
Conclusions
The COVID-19 pandemic has fueled unprecedented, rapid changes in the way sleep medicine practices deliver care to millions of patients. These changes have been propelled by practitioners and staff who have embraced adaptability, creativity, resourcefulness, and attention to safety and effectiveness. Widespread use of telemedicine services, greater reliance on ambulatory testing, ongoing risk-benefit stratification, leveraging technology and teamwork, and sharing knowledge as it becomes available has resulted in care that is more accessible and convenient for some vulnerable patients, and, yet, challenges persist in accessing needed care. Necessity has been the mother of invention, and we expect the field will need to continue to rebalance as the situation evolves. The ultimate test of these rapid innovations will be how sleep medicine patients fare in the long run, in terms of their health, safety, mortality, and overall quality of life. Future research must address these questions, and the resulting information may yet inform the way sleep medicine is practiced in the years to come.
Dr. Shannon is Medical Director, EVAL Research Institute, Palo Alto, CA; Dr. Gurubhagavatula is Associate Professor, Perelman School of Medicine, University of Pennsylvania, and with Crescenz VA Medical Center, Philadelphia, PA.
1. Worldometer. COVID-19 coronavirus pandemic.
2. Centers for Medicare & Medicaid Services. CMS releases recommendations on adult elective surgeries, non-essential medical, surgical, and dental procedures during COVID-19 response. 2020 Mar 18.
3. Centers for Medicare & Medicaid Services. Implementation of mitigation strategies for communities with local COVID-19 transmission.
4. Tang JW et al. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100-14.
5. Martina Ferioli et al. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. European Respiratory Review 2020;29:200068. doi: 10.1183/16000617.0068-2020.
6. World Health Organization. 2014 Apr. Infection prevention and control of epidemic and pandemic-prone acute respiratory infections in health care.
7. World Health Organization. 2020 Feb 27. Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19) Interim guidance.
8. Van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7. doi: 10.1056/NEJMc2004973.
9. Joshua L Santarpia et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. 2020 Mar 26. doi: 10.1101/2020.03.23.20039446.
10. David S. Hui et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019 Apr 11.53(4):pii: 1802339. doi: 10.1183/13993003.02339-2018.
11. Worldometer. COVID-19 coronavirus pandemic.
Introduction
Since reported in late 2019 in Wuhan China, the disease named “novel coronavirus disease 2019” (COVID-19), caused by the virus referred to as Severe Acute Respiratory Syndrome-causing Coronavirus-2 (SARS-CoV-2) has spread widely to many parts of the world. As of April 13, 2020, a total of 210 countries reported more than 1.9 million cases, resulting in more than 119,000 deaths.1 All 50 states have reported cases of COVID-19 to the Centers for Disease Control and Prevention (CDC), and most US states are reporting community spread. While levels of COVID-19 activity vary by region, the CDC has reported that the US remains in the acceleration phase of the pandemic, and that widespread transmission is expected.
On March 18, the Centers for Medicare & Medicaid Services (CMS) advised2 that all elective surgeries and nonessential medical, surgical, and dental procedures should be delayed to promote physical distancing, preserve personal protective equipment (PPE), and enable health-care workers (HCW) to redirect work to high-need areas. California was the first to issue a statewide shelter-in-place order on March 19, and by April, leaders in 42 states, the District of Columbia, and Puerto Rico issued similar stay-at-home orders. The White House has announced that physical distancing should continue until at least April 30. With the potential for an explosion of new cases that could overwhelm health-care resources, “business as usual” ceased to exist practically overnight.
The speed with which these events transpired, the demand to tailor response within days or even hours, the lack of robust data to support decision-making, the possibility of spread by asymptomatic carriers, and the potential risk for airborne, as well as droplet and fecal-oral spread, caused sleep medicine clinicians to rely on expert consensus and clinical judgment. The goal of such guidance has been to optimize care to patients with sleep disorders, while protecting the health and safety of all. Sleep medicine practices have had to balance efforts to reduce viral exposure and transmission, the need to triage health-care resources and personnel, and maintain access to care.
General clinical measures
From the outset, in areas of community spread, sleep medicine practices were called to adapt to now-standard measures, such as provider self-quarantine if ill or exposed, in-person clinic triage strategies for patients and staff prior to entrance to facilities to rapidly identify people with respiratory illness (eg, temperature monitoring), elimination of nonessential visitors, and infection control measures such as vigilant cleaning and appropriate use of personal protective equipment (PPE) during patient interactions. Typical issues facing sleep medicine practices include the need to prioritize urgent or emergency care, track canceled or postponed visits, and maintain access to communication with patients, the health-care team, payors, and employers.
Infection mitigation recommendations: sleep laboratories and ambulatory practices
Diagnostic testing
By mid-March, relatively early in the course of the outbreak in the US, the American Academy of Sleep Medicine (AASM) released recommendations for sleep clinics and laboratories regarding continuation of in-lab diagnostic, split-night, and titration studies, as well as clinical interactions and telemedicine, taking into account the CDC mitigation strategies3 which vary according to level of community transmission or impact of COVID-19.
This advisory was updated repeatedly over the ensuing weeks, most recently on April 8, as community-based spread increased. The AASM now strongly urges all sleep clinicians to postpone in-laboratory polysomnography (PSG) for adults and children, both diagnostic and positive airway pressure (PAP) titrations, except in emergencies. Data regarding adherence with these recommendations are lacking; anecdotal reports suggest that sleep medicine communities most heavily affected by the community spread are indeed following this practice.
The AASM guidance also advises use of home sleep apnea testing (HSAT) with consideration of single-use components or devices, use of mail-in recorders, and/or removal of reusable devices from service for 72 hours between patients.
Positive airway pressure (PAP) therapy
The potential for PAP devices to promote the aerosolization of viral particles, which could increase transmission to others on shared ventilation networks in homes and health-care settings, requires careful attention.
Generally, exhaled particle size depends on multiple characteristics, including the force and pressure at generation and environmental conditions (eg, temperature, relative humidity, and air flow). Large-size particles remain suspended in the air only briefly and settle within 1 meter from the source; these are usually mediated by breathing zones of individuals.4 However, smaller particles can travel farther, with distance governed by airflow that is driven by many variables, including ventilation, human movement, and temperature gradients. While droplets tend to evaporate rapidly, dry residues can remain suspended in the air.5 Infectious respiratory aerosols can occur as droplets >5 mcm diameter, or droplet nuclei (<5 mcm diameter).6 Present evidence indicates that SARS-CoV-2 transmission occurs primarily through droplet spread in settings with normal breathing. However, the World Health Organization (WHO) advises more stringent, airborne precautions for aerosol-generating procedures with COVID-19. Such procedures include intubation, extubation, noninvasive ventilation, high-flow nasal cannula, and cardiopulmonary resuscitation before intubation.7 Some evidence indicates that SARS-CoV-2 can linger in aerosol form for hours,8 and aerosol transmission is therefore plausible. Non-peer reviewed data in real-world settings indicates the presence of SARS-CoV-2 in air samples from hallways outside and in rooms adjacent to COVID-19-containing patients.9
These findings raised some concerns about use of PAP in medical and home environments, leading to the recommendation that the decision to continue or withhold PAP temporarily be made based on a risk-benefit evaluation. Scant data hint that PAP therapy may be safe to use in rooms that support appropriate ventilation (eg, negative pressure rooms). Regarding mask type, recently, a group reported the possibility that oronasal masks have a better aerosol dispersal profile.5 However, this conclusion was based on a single study of a specific model of oronasal mask, which demonstrated an absence of ability to measure a dispersion air jet, because the exhalation ports on the mask caused diffuse rather than directed dispersion of air.10 The same study found, that when the jet could be measured (with nasal pillows or with leak from any interface), greater dispersion was indeed evident. While anecdotal practical methods to filter exhaled air from PAP devices to reduce aerosol transmission have been proposed, data regarding successful reduction in transmission are still lacking, and such methods are not endorsed by mask manufacturers.
Ambulatory clinics: role of telemedicine
As the spread of COVID-19 disease accelerated, the AASM recommended that sleep medicine practices postpone and reschedule all nonemergency, in-person appointments, and conduct as many visits as possible by telemedicine.
This rapid transition posed many layers of logistical complexity, including how to quickly initiate or scale up an often fledging telemedicine presence; scheduling and instructing patients for telemedicine encounters; problem-solving in situations with limited device and Internet availability; triaging patients based on risk; and tracking postponed appointments. Administrators, medical assistants, nurses, advanced practitioners, respiratory therapists, technologists, and physicians have learned new ways of doing things, and laboratory personnel have undergone training and transitioned to new roles and responsibilities during postponement of lab studies. Training programs, in particular, have had to be nimble in finding ways to meet the educational needs of sleep medicine fellows that leveraged telemedicine opportunities.
Economic implications of transformed sleep medicine practices
While deploying such systematic change costs both time and money, sleep practices are also confronted with questions around lost revenue from drops in laboratory and clinic volumes. Many additional questions around reimbursement and revenue shortfalls are present, and short-term, furloughed employees may not be able to sustain income loss, which could result in difficulty in resuming services when the COVID-19 threat has been reduced.
Helpfully, during this public health emergency, CMS has expanded coverage for telemedicine services and waived requirements for face-to-face or in-person encounters,11 and some private payers have followed. Additionally, for the duration of the public health emergency, Medicare will cover PAP devices based on the clinician’s assessment of the patient without requiring PSG or a home sleep apnea test (HSAT). However, CMS has not clarified what follow-up testing, if any, may be required after this public health emergency is over. The duration of these new payment models remains uncertain.
Recommendations for PAP users
Patients and families, practitioners, and group living facilities have all expressed concerns about use of PAP during the epidemic given presumed increased risk of viral spread. In many hospital protocols, the use of PAP is restricted or disallowed for patients with suspected or confirmed COVID-19. Guidance regarding out-of-hospital use of PAP has been sparse.
AASM has recommended avoidance of PAP or noninvasive ventilation (NIV) for those with presumed or confirmed COVID-19 who cannot self-isolate according to CDC guidance. Risk-benefit assessment is recommended for those who perform safety-sensitive activities or have higher-risk medical conditions. During the period that PAP is withheld, alternative or modifying therapies can be considered, such as positional therapy or oral appliance.
Cleaning device components and washing and replacing filters as recommended by the manufacturer, as well as simple but important interventions like handwashing before and after touching the face or airway gear is thought to be especially important during this time.
Conclusions
The COVID-19 pandemic has fueled unprecedented, rapid changes in the way sleep medicine practices deliver care to millions of patients. These changes have been propelled by practitioners and staff who have embraced adaptability, creativity, resourcefulness, and attention to safety and effectiveness. Widespread use of telemedicine services, greater reliance on ambulatory testing, ongoing risk-benefit stratification, leveraging technology and teamwork, and sharing knowledge as it becomes available has resulted in care that is more accessible and convenient for some vulnerable patients, and, yet, challenges persist in accessing needed care. Necessity has been the mother of invention, and we expect the field will need to continue to rebalance as the situation evolves. The ultimate test of these rapid innovations will be how sleep medicine patients fare in the long run, in terms of their health, safety, mortality, and overall quality of life. Future research must address these questions, and the resulting information may yet inform the way sleep medicine is practiced in the years to come.
Dr. Shannon is Medical Director, EVAL Research Institute, Palo Alto, CA; Dr. Gurubhagavatula is Associate Professor, Perelman School of Medicine, University of Pennsylvania, and with Crescenz VA Medical Center, Philadelphia, PA.
1. Worldometer. COVID-19 coronavirus pandemic.
2. Centers for Medicare & Medicaid Services. CMS releases recommendations on adult elective surgeries, non-essential medical, surgical, and dental procedures during COVID-19 response. 2020 Mar 18.
3. Centers for Medicare & Medicaid Services. Implementation of mitigation strategies for communities with local COVID-19 transmission.
4. Tang JW et al. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100-14.
5. Martina Ferioli et al. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. European Respiratory Review 2020;29:200068. doi: 10.1183/16000617.0068-2020.
6. World Health Organization. 2014 Apr. Infection prevention and control of epidemic and pandemic-prone acute respiratory infections in health care.
7. World Health Organization. 2020 Feb 27. Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19) Interim guidance.
8. Van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7. doi: 10.1056/NEJMc2004973.
9. Joshua L Santarpia et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. 2020 Mar 26. doi: 10.1101/2020.03.23.20039446.
10. David S. Hui et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019 Apr 11.53(4):pii: 1802339. doi: 10.1183/13993003.02339-2018.
11. Worldometer. COVID-19 coronavirus pandemic.
Hyperoxia in the ICU: Is less more?
“All things are poison and nothing is without poison, only the dose permits something not to be poisonous.” Paracelsus once said.
A bit of history
Oxygen was discovered in 1775 and was since noted to be both vital and poisonous. It was much later in 1899 that it was demonstrated that partial pressures of oxygen up to 75% led to both severe lung injury and death as compared with levels of 40% to 50%. While the administration of oxygen in hypoxic patients is beneficial, this intervention in healthy subjects leads to a reduction in heart rate, cardiac index, and an increase in mean arterial pressure, systemic vascular resistance, and large artery stiffness.
While oxygen itself is not toxic, the reactive oxygen species that form as a result of oxygen metabolism are. A study showed that supplementation of oxygen in patients with COPD, or in women undergoing C-section with the use of spinal anesthesia, leads to an increase in reactive oxygen species (Winslow RM. Transfusion. 2013;53[2]:424).
Hyperoxia has multiple clinical effects on lung physiology and gas exchange that include worsening hypoxemia secondary to absorptive atelectasis and damage to the airways and lung parenchyma (Sackner MA, et al. Ann Intern Med. 1975;82[1]:40).
High levels of inspired oxygen could also lead to accentuation of hypercapnia as explained by the Haldane effect; a reduction of the affinity for carbon dioxide leading to an increase in PaC02. High oxygen levels can also decrease the hypoxic drive for ventilation leading to worsening hypercapnia.
Hyperoxia is a situation routinely encountered in clinical practice, as well, often resulting from an overzealous attempt to prevent or reverse hypoxia. ICU physicians, though aware of potential threats of hyperoxia, often fail to translate such concerns in their clinical practice (Helmerhorst HJ, et al. Ann Intensive Care. 2014;4:23).
Effects of hyperoxia in CNS and cardiovascular disease
The last 2 decades have seen several studies looking into the effects of hyperoxia in specific clinical scenarios. Arterial hyperoxia was found to be independently associated with in-hospital death in ventilated stroke patients in the ICU, as compared with either arterial normoxia or hypoxia (Rincon F, et al. Crit Care Med. 2014;42[2]:387). The AVOID trial showed that supplemental oxygen therapy in patients with ST-elevation myocardial infarction, but without hypoxia, increased early myocardial injury with risk of larger myocardial infarct size at 6 months. (Stub D, et al. Circulation. 2015;131[24]:2143).
Hyperoxia in the ICU
Although the potential risks of hyperoxia in conditions such as stroke and cardiac arrest had been observed, the jury was still out on its effects on a critically ill, mixed population, as routinely encountered in the ICU. Oxygen-ICU, a single center trial published in 2016, was one of the first looking at a mixed ICU population, while assessing the effects of a conservative oxygen delivery strategy against a conventional one (Girardis M, et al. JAMA. 2016;316[15]:1583). The researchers noted a significant mortality difference favoring conservative oxygen therapy, particularly in intubated patients. The IOTA group’s systematic review and meta-analysis of 16,000 patients, showed an increased relative risk of death in-hospital with hyperoxia, that persisted over a prolonged period while conferring no obvious advantages (Chu DK, et al. Lancet. 2018;391[10131]:1693).
With the growing body of evidence, the need of the hour was an ICU-based randomized trial that may settle the debate. The 21 center, 1,000 patient ICU-ROX trial promised to deliver on that (Mackle D, et al. N Engl J Med. 2019 Oct 14. doi: 10.1056/NEJMoa1903297). The study design was more reflective of real-life clinical scenarios than some of its predecessors, with the control group exposed to usual-oxygen therapy instead of liberal hyperoxia. Both groups had a lower saturation threshold of 91% while the conservative-oxygen group had an upper limit of 97% along with a conscious effort made to drop the FIO2 to 21%. Though both groups had similar median PaO2 levels, the conservative group spent much greater time (median 29 hours) at 21% FIO2 than the usual group (median 1 hour). SpO2 targets also allowed frequent changes to oxygen delivery without the need for blood gases.
Presuming the primary effect of oxygen toxicity would be on the lungs, the study was powered for a primary outcome of ventilator-free-days, which showed no significant difference among the groups. No significant differences in mortality or other secondary outcomes were observed.
The ICU-ROX trial leaves us with a few questions, the most important are:
Are the detrimental effects of hyperoxia limited to certain disease-specific groups or generally applicable?
The evidence is substantial inpatients with cardiac arrest/myocardial injury. A prespecified subgroup analysis in ICU-ROX indicated a higher number of ventilator-free days with conservative oxygen therapy in patients with hypoxic ischemic encephalopathy. When asked, Dr. Paul Young, one of the investigators of the ICU-ROX group, states, “These are actually pretty small subgroups, and the number of mortality events is quite small. My belief is that these data are best viewed as hypothesis generating rather than practice changing”
Where do we stand?
While we look for further answers regarding the consequences of hyperoxia, it is established that conservative oxygen therapy aimed at reducing delivered FIO2 is a safe practice without any adverse outcomes. The conservative oxygen group in ICU-ROX allowed SpO2 levels as low as 91% with no serious hypoxic events. On the other hand, the IOTA group in their data analysis suggested a possible increase in mortality risk, which was dose-dependent on the magnitude of increase in SpO2, in the range of 94% to 96%. Based on the available evidence, it is reasonable to encourage targeting lowest FIO2 values needed to maintain SpO2 between 91% and 96% in our ICU patients. There would always be a small fraction of patients, such as those with ARDS or severe hypoxic respiratory failure, in whom this may not be achievable given fluctuating and unreliable SpO2 levels in the setting of profound hypoxia.
What lies ahead?
As the debate rages on, in an effort to answer this question for once and for all, the researchers of ICU-ROX are planning to conduct a multinational, multicenter RCT, the MEGA-ROX. An ICU trial of this size has not been attempted before and, given the sample size, Dr. Young feels the MEGA-ROX will be powered to detect an absolute mortality difference as low as 1.5%, if it does exist. There is a distinct possibility that conservative oxygen therapy will be best for patients with some diagnoses while liberal oxygen will be best for patients with other diagnoses. “We are conducting a number of parallel nested trials within the overall 40,000 participant trial sample. Each of these nested trials will evaluate a prespecified hypothesis in a specific cohort of critically ill patients and is accompanied by an appropriate power calculation. This will be able to address any heterogeneity of treatment effect among the different subgroups,” he concluded. As we eagerly await the results of MEGA-ROX, there may be a growing belief among intensivists that when it comes to oxygen in the ICU, less may be truly more.
Dr. Chaaban and Dr. Sen are with the University of Kentucky College of Medicine, Lexington, Kentucky.
Correction, 4/10/20: An earlier version of this article misstated Dr. Sen's name
“All things are poison and nothing is without poison, only the dose permits something not to be poisonous.” Paracelsus once said.
A bit of history
Oxygen was discovered in 1775 and was since noted to be both vital and poisonous. It was much later in 1899 that it was demonstrated that partial pressures of oxygen up to 75% led to both severe lung injury and death as compared with levels of 40% to 50%. While the administration of oxygen in hypoxic patients is beneficial, this intervention in healthy subjects leads to a reduction in heart rate, cardiac index, and an increase in mean arterial pressure, systemic vascular resistance, and large artery stiffness.
While oxygen itself is not toxic, the reactive oxygen species that form as a result of oxygen metabolism are. A study showed that supplementation of oxygen in patients with COPD, or in women undergoing C-section with the use of spinal anesthesia, leads to an increase in reactive oxygen species (Winslow RM. Transfusion. 2013;53[2]:424).
Hyperoxia has multiple clinical effects on lung physiology and gas exchange that include worsening hypoxemia secondary to absorptive atelectasis and damage to the airways and lung parenchyma (Sackner MA, et al. Ann Intern Med. 1975;82[1]:40).
High levels of inspired oxygen could also lead to accentuation of hypercapnia as explained by the Haldane effect; a reduction of the affinity for carbon dioxide leading to an increase in PaC02. High oxygen levels can also decrease the hypoxic drive for ventilation leading to worsening hypercapnia.
Hyperoxia is a situation routinely encountered in clinical practice, as well, often resulting from an overzealous attempt to prevent or reverse hypoxia. ICU physicians, though aware of potential threats of hyperoxia, often fail to translate such concerns in their clinical practice (Helmerhorst HJ, et al. Ann Intensive Care. 2014;4:23).
Effects of hyperoxia in CNS and cardiovascular disease
The last 2 decades have seen several studies looking into the effects of hyperoxia in specific clinical scenarios. Arterial hyperoxia was found to be independently associated with in-hospital death in ventilated stroke patients in the ICU, as compared with either arterial normoxia or hypoxia (Rincon F, et al. Crit Care Med. 2014;42[2]:387). The AVOID trial showed that supplemental oxygen therapy in patients with ST-elevation myocardial infarction, but without hypoxia, increased early myocardial injury with risk of larger myocardial infarct size at 6 months. (Stub D, et al. Circulation. 2015;131[24]:2143).
Hyperoxia in the ICU
Although the potential risks of hyperoxia in conditions such as stroke and cardiac arrest had been observed, the jury was still out on its effects on a critically ill, mixed population, as routinely encountered in the ICU. Oxygen-ICU, a single center trial published in 2016, was one of the first looking at a mixed ICU population, while assessing the effects of a conservative oxygen delivery strategy against a conventional one (Girardis M, et al. JAMA. 2016;316[15]:1583). The researchers noted a significant mortality difference favoring conservative oxygen therapy, particularly in intubated patients. The IOTA group’s systematic review and meta-analysis of 16,000 patients, showed an increased relative risk of death in-hospital with hyperoxia, that persisted over a prolonged period while conferring no obvious advantages (Chu DK, et al. Lancet. 2018;391[10131]:1693).
With the growing body of evidence, the need of the hour was an ICU-based randomized trial that may settle the debate. The 21 center, 1,000 patient ICU-ROX trial promised to deliver on that (Mackle D, et al. N Engl J Med. 2019 Oct 14. doi: 10.1056/NEJMoa1903297). The study design was more reflective of real-life clinical scenarios than some of its predecessors, with the control group exposed to usual-oxygen therapy instead of liberal hyperoxia. Both groups had a lower saturation threshold of 91% while the conservative-oxygen group had an upper limit of 97% along with a conscious effort made to drop the FIO2 to 21%. Though both groups had similar median PaO2 levels, the conservative group spent much greater time (median 29 hours) at 21% FIO2 than the usual group (median 1 hour). SpO2 targets also allowed frequent changes to oxygen delivery without the need for blood gases.
Presuming the primary effect of oxygen toxicity would be on the lungs, the study was powered for a primary outcome of ventilator-free-days, which showed no significant difference among the groups. No significant differences in mortality or other secondary outcomes were observed.
The ICU-ROX trial leaves us with a few questions, the most important are:
Are the detrimental effects of hyperoxia limited to certain disease-specific groups or generally applicable?
The evidence is substantial inpatients with cardiac arrest/myocardial injury. A prespecified subgroup analysis in ICU-ROX indicated a higher number of ventilator-free days with conservative oxygen therapy in patients with hypoxic ischemic encephalopathy. When asked, Dr. Paul Young, one of the investigators of the ICU-ROX group, states, “These are actually pretty small subgroups, and the number of mortality events is quite small. My belief is that these data are best viewed as hypothesis generating rather than practice changing”
Where do we stand?
While we look for further answers regarding the consequences of hyperoxia, it is established that conservative oxygen therapy aimed at reducing delivered FIO2 is a safe practice without any adverse outcomes. The conservative oxygen group in ICU-ROX allowed SpO2 levels as low as 91% with no serious hypoxic events. On the other hand, the IOTA group in their data analysis suggested a possible increase in mortality risk, which was dose-dependent on the magnitude of increase in SpO2, in the range of 94% to 96%. Based on the available evidence, it is reasonable to encourage targeting lowest FIO2 values needed to maintain SpO2 between 91% and 96% in our ICU patients. There would always be a small fraction of patients, such as those with ARDS or severe hypoxic respiratory failure, in whom this may not be achievable given fluctuating and unreliable SpO2 levels in the setting of profound hypoxia.
What lies ahead?
As the debate rages on, in an effort to answer this question for once and for all, the researchers of ICU-ROX are planning to conduct a multinational, multicenter RCT, the MEGA-ROX. An ICU trial of this size has not been attempted before and, given the sample size, Dr. Young feels the MEGA-ROX will be powered to detect an absolute mortality difference as low as 1.5%, if it does exist. There is a distinct possibility that conservative oxygen therapy will be best for patients with some diagnoses while liberal oxygen will be best for patients with other diagnoses. “We are conducting a number of parallel nested trials within the overall 40,000 participant trial sample. Each of these nested trials will evaluate a prespecified hypothesis in a specific cohort of critically ill patients and is accompanied by an appropriate power calculation. This will be able to address any heterogeneity of treatment effect among the different subgroups,” he concluded. As we eagerly await the results of MEGA-ROX, there may be a growing belief among intensivists that when it comes to oxygen in the ICU, less may be truly more.
Dr. Chaaban and Dr. Sen are with the University of Kentucky College of Medicine, Lexington, Kentucky.
Correction, 4/10/20: An earlier version of this article misstated Dr. Sen's name
“All things are poison and nothing is without poison, only the dose permits something not to be poisonous.” Paracelsus once said.
A bit of history
Oxygen was discovered in 1775 and was since noted to be both vital and poisonous. It was much later in 1899 that it was demonstrated that partial pressures of oxygen up to 75% led to both severe lung injury and death as compared with levels of 40% to 50%. While the administration of oxygen in hypoxic patients is beneficial, this intervention in healthy subjects leads to a reduction in heart rate, cardiac index, and an increase in mean arterial pressure, systemic vascular resistance, and large artery stiffness.
While oxygen itself is not toxic, the reactive oxygen species that form as a result of oxygen metabolism are. A study showed that supplementation of oxygen in patients with COPD, or in women undergoing C-section with the use of spinal anesthesia, leads to an increase in reactive oxygen species (Winslow RM. Transfusion. 2013;53[2]:424).
Hyperoxia has multiple clinical effects on lung physiology and gas exchange that include worsening hypoxemia secondary to absorptive atelectasis and damage to the airways and lung parenchyma (Sackner MA, et al. Ann Intern Med. 1975;82[1]:40).
High levels of inspired oxygen could also lead to accentuation of hypercapnia as explained by the Haldane effect; a reduction of the affinity for carbon dioxide leading to an increase in PaC02. High oxygen levels can also decrease the hypoxic drive for ventilation leading to worsening hypercapnia.
Hyperoxia is a situation routinely encountered in clinical practice, as well, often resulting from an overzealous attempt to prevent or reverse hypoxia. ICU physicians, though aware of potential threats of hyperoxia, often fail to translate such concerns in their clinical practice (Helmerhorst HJ, et al. Ann Intensive Care. 2014;4:23).
Effects of hyperoxia in CNS and cardiovascular disease
The last 2 decades have seen several studies looking into the effects of hyperoxia in specific clinical scenarios. Arterial hyperoxia was found to be independently associated with in-hospital death in ventilated stroke patients in the ICU, as compared with either arterial normoxia or hypoxia (Rincon F, et al. Crit Care Med. 2014;42[2]:387). The AVOID trial showed that supplemental oxygen therapy in patients with ST-elevation myocardial infarction, but without hypoxia, increased early myocardial injury with risk of larger myocardial infarct size at 6 months. (Stub D, et al. Circulation. 2015;131[24]:2143).
Hyperoxia in the ICU
Although the potential risks of hyperoxia in conditions such as stroke and cardiac arrest had been observed, the jury was still out on its effects on a critically ill, mixed population, as routinely encountered in the ICU. Oxygen-ICU, a single center trial published in 2016, was one of the first looking at a mixed ICU population, while assessing the effects of a conservative oxygen delivery strategy against a conventional one (Girardis M, et al. JAMA. 2016;316[15]:1583). The researchers noted a significant mortality difference favoring conservative oxygen therapy, particularly in intubated patients. The IOTA group’s systematic review and meta-analysis of 16,000 patients, showed an increased relative risk of death in-hospital with hyperoxia, that persisted over a prolonged period while conferring no obvious advantages (Chu DK, et al. Lancet. 2018;391[10131]:1693).
With the growing body of evidence, the need of the hour was an ICU-based randomized trial that may settle the debate. The 21 center, 1,000 patient ICU-ROX trial promised to deliver on that (Mackle D, et al. N Engl J Med. 2019 Oct 14. doi: 10.1056/NEJMoa1903297). The study design was more reflective of real-life clinical scenarios than some of its predecessors, with the control group exposed to usual-oxygen therapy instead of liberal hyperoxia. Both groups had a lower saturation threshold of 91% while the conservative-oxygen group had an upper limit of 97% along with a conscious effort made to drop the FIO2 to 21%. Though both groups had similar median PaO2 levels, the conservative group spent much greater time (median 29 hours) at 21% FIO2 than the usual group (median 1 hour). SpO2 targets also allowed frequent changes to oxygen delivery without the need for blood gases.
Presuming the primary effect of oxygen toxicity would be on the lungs, the study was powered for a primary outcome of ventilator-free-days, which showed no significant difference among the groups. No significant differences in mortality or other secondary outcomes were observed.
The ICU-ROX trial leaves us with a few questions, the most important are:
Are the detrimental effects of hyperoxia limited to certain disease-specific groups or generally applicable?
The evidence is substantial inpatients with cardiac arrest/myocardial injury. A prespecified subgroup analysis in ICU-ROX indicated a higher number of ventilator-free days with conservative oxygen therapy in patients with hypoxic ischemic encephalopathy. When asked, Dr. Paul Young, one of the investigators of the ICU-ROX group, states, “These are actually pretty small subgroups, and the number of mortality events is quite small. My belief is that these data are best viewed as hypothesis generating rather than practice changing”
Where do we stand?
While we look for further answers regarding the consequences of hyperoxia, it is established that conservative oxygen therapy aimed at reducing delivered FIO2 is a safe practice without any adverse outcomes. The conservative oxygen group in ICU-ROX allowed SpO2 levels as low as 91% with no serious hypoxic events. On the other hand, the IOTA group in their data analysis suggested a possible increase in mortality risk, which was dose-dependent on the magnitude of increase in SpO2, in the range of 94% to 96%. Based on the available evidence, it is reasonable to encourage targeting lowest FIO2 values needed to maintain SpO2 between 91% and 96% in our ICU patients. There would always be a small fraction of patients, such as those with ARDS or severe hypoxic respiratory failure, in whom this may not be achievable given fluctuating and unreliable SpO2 levels in the setting of profound hypoxia.
What lies ahead?
As the debate rages on, in an effort to answer this question for once and for all, the researchers of ICU-ROX are planning to conduct a multinational, multicenter RCT, the MEGA-ROX. An ICU trial of this size has not been attempted before and, given the sample size, Dr. Young feels the MEGA-ROX will be powered to detect an absolute mortality difference as low as 1.5%, if it does exist. There is a distinct possibility that conservative oxygen therapy will be best for patients with some diagnoses while liberal oxygen will be best for patients with other diagnoses. “We are conducting a number of parallel nested trials within the overall 40,000 participant trial sample. Each of these nested trials will evaluate a prespecified hypothesis in a specific cohort of critically ill patients and is accompanied by an appropriate power calculation. This will be able to address any heterogeneity of treatment effect among the different subgroups,” he concluded. As we eagerly await the results of MEGA-ROX, there may be a growing belief among intensivists that when it comes to oxygen in the ICU, less may be truly more.
Dr. Chaaban and Dr. Sen are with the University of Kentucky College of Medicine, Lexington, Kentucky.
Correction, 4/10/20: An earlier version of this article misstated Dr. Sen's name
Expansion of the donor pool in lung transplantation
Lung transplants are increasing, with 2,562 performed in the United States in 2018 – a 31% increase over the preceding 5 years. With this increased demand for donor lungs, waitlist mortality in the United States is 9.4 deaths per 100 waitlist-years for obstructive lung diseases and as high as 29.7 deaths per 100 waitlist-years for restrictive lung diseases (Valapour M, et al. Lung. Am J Transplant. 2020;20[suppl s1]:427). Conversely, lungs are utilized from eligible multiorgan donors only 15% to 20% of the time, usually due to concerns over donor history or organ quality (Young KA, et al. Chest. 2019;155[3]:465). In light of this imbalance of supply and demand, lung transplant specialists are making significant efforts to expand the donor pool of available organs. Three of these strategies include: (1) applications of ex-vivo lung perfusion (EVLP) technology; (2) use of lungs from hepatitis C-positive donors for hep-C negative recipients; and (3) increasing utilization of donation after cardiac death.
Normothermic ex-vivo lung perfusion is a technology which allows donor lungs to be perfused and ventilated after removal from the donor but before transplant into the recipient. This is in contrast to the traditional method of cold static preservation. The proposed advantage of using this technology is to allow time for a more thorough assessment of graft quality and to improve function of grafts not meeting established criteria for transplant, all-the-while decreasing organ ischemia despite an increased cross-clamp time. There are currently four commercial systems available capable of EVLP. Broadly speaking, three EVLP management protocols exist (Toronto, Lund, and OCS), which differ in perfusate composition, target flow, pulmonary arterial pressure, left atrial pressure, and ventilatory settings. Notably, the Toronto protocol uses a closed left atrium, whereas the Lund and OCS protocol use an open left atrium. There are excellent published reviews of the different systems (Possoz J, et al. J Thorac Dis. 2019;11[4]:1635). EVLP has now been studied for two different goals: (1) to allow an extended evaluation of lungs of questionable quality before transplant; or (2) for routine use in all lung transplantations in place of cold static preservation.
In most studies concerning the use of EVLP for reconditioning of donor lungs, “high risk” or “extended criteria” refers to one or more of the following: P/F ratios < 300 on arterial blood gas, macroscopic abnormalities (eg, pulmonary edema, poor lung compliance), donation after circulatory death, or high-risk history (eg, aspiration). The largest cohort with the longest follow-up addressing the role of EVLP for donation of lungs with extended criteria was published from the Toronto Lung Transplant Group. Their results have demonstrated equivalent graft survival and rates of chronic lung allograft dysfunction (CLAD) up to 9 years posttransplant compared with standard criteria donor lungs, despite utilizing lower quality lungs and having a longer median preservation (Divithotawela C, et al. JAMA Surg. 2019;154[12]:1143). The group’s subsequent lung transplant rates have increased over the past decade.
A separate study addressed the same question but differed in that it was a single-arm, multicenter, international trial that tracked the outcomes of 93 extended criteria lungs placed on EVLP (including a large proportion acquired via donation after circulatory death) (Loor G, et al. Lancet Respir Med. 2019;7[11]:975). Among these, 87% of eligible lungs were transplanted, and outcomes were excellent, albeit shorter in follow-up compared with the Toronto cohort (eg, primary graft dysfunction grade 3 (PGD3) within 72 hours was 44% and 1-year survival was 91%). Based on these trials and many other retrospective reports, it has been concluded by many experts in the field that EVLP-treated extended criteria donor lungs perform equally well to standard criteria donor lungs.
Two RCTs have been conducted to evaluate whether EVLP is noninferior to static cold storage with donor lungs meeting “standard criteria” for transplant. The first was a single center study at the Medical University of Vienna, that looked at 80 recipient/donor pairs. Lungs in the EVLP arm underwent 4 hours of perfusion with frequent reassessment of quality before transplant, whereas the lungs in the control arm went directly to transplant. This study met noninferiority criteria looking at primary outcomes of PGD grade >1 and 30-day survival (Slama A, et al. J Heart Lung Transplant. 2017;36[7]:744). The second study was a phase 3, multicenter, international trial that included 320 recipient/donor pairs randomized to either EVLP (without a prespecified time on the EVLP system) or static cold storage. This trial met noninferiority for safety endpoints (lung graft-related adverse events within 30 days) and a composite primary outcome of PGD grade 3 incidence within 72 hours and 30-day survival (Warnecke G, et al. Lancet Respir Med. 2018;6[5]:357). The authors also tested and found superiority of EVLP in lower PGD grade 3 frequency compared with control. While these RCTs may suggest a role for EVLP in the procurement process of standard criteria organs in addition to extended criteria organs in the future, major criticisms for these trials include the lack of a demonstrable clinical benefit over cold storage beyond the lower PGD3 rates.
In the era of direct-acting antiviral agents available to treat HCV infection, there has been efforts to study the early use of anti-HCV medications to prevent infection as a result of heart or lung transplant from HCV viremic donors to HCV-negative recipients. In one major trial on efficacy, it was found that 4 weeks of sofosbuvir and velpatasvir, when started within a few hours of transplant, was sufficient to achieve a sustained (undetectable) virologic response at 12 weeks after completion of the antiviral regimen (Woolley AE, et al. N Engl J Med. 2019;380[17]:1606). Therefore, many transplant centers have adopted protocols to increase the donor pool (by CDC estimates about 4% of solid organ donors are HCV-positive) by accepting HCV nucleic acid amplification test (NAT)-positive donors for HCV-negative recipients, after appropriate informed consent.
Donation after cardiac death (DCD), which is alternatively known as donation after circulatory determination of death (DCDD), generally refers to organ procurement taking place after cessation of circulation, often after inpatient withdrawal of support. This is in contrast to the much more common practice of donation after brain death (DBD). Addressing concerns over the quality of lungs donated in the context of DCD compared with DBD, analyses of ISHLT registry data have demonstrated no differences in hospital length of stay or survival at 1 or 5 years (Van Raemdonck D, et al. J Heart Lung Transplant. 2019;38[12]:1235). Outcomes comparing specific mechanisms of donor death in DCD remain relatively unknown, such as outcomes from donors withdrawn from life support vs donors who had an uncontrolled cardiac death.
These methods for expanding the donor pool are not mutually exclusive, and, in fact, application of EVLP for lungs obtained in the context of DCD seems to be increasingly common. Optimization of protocols with collaboration between lung transplant centers will be paramount as we move forward in advancing this field. As we do so, efforts to successfully increase the donor pool will serve to provide a life-saving therapy to an ever-growing number of patients with end-stage lung disease.
Dr. Sala and Dr. Tomic are with the Division of Pulmonary and Critical Care Medicine, Northwestern University, Chicago, Illinois.
Lung transplants are increasing, with 2,562 performed in the United States in 2018 – a 31% increase over the preceding 5 years. With this increased demand for donor lungs, waitlist mortality in the United States is 9.4 deaths per 100 waitlist-years for obstructive lung diseases and as high as 29.7 deaths per 100 waitlist-years for restrictive lung diseases (Valapour M, et al. Lung. Am J Transplant. 2020;20[suppl s1]:427). Conversely, lungs are utilized from eligible multiorgan donors only 15% to 20% of the time, usually due to concerns over donor history or organ quality (Young KA, et al. Chest. 2019;155[3]:465). In light of this imbalance of supply and demand, lung transplant specialists are making significant efforts to expand the donor pool of available organs. Three of these strategies include: (1) applications of ex-vivo lung perfusion (EVLP) technology; (2) use of lungs from hepatitis C-positive donors for hep-C negative recipients; and (3) increasing utilization of donation after cardiac death.
Normothermic ex-vivo lung perfusion is a technology which allows donor lungs to be perfused and ventilated after removal from the donor but before transplant into the recipient. This is in contrast to the traditional method of cold static preservation. The proposed advantage of using this technology is to allow time for a more thorough assessment of graft quality and to improve function of grafts not meeting established criteria for transplant, all-the-while decreasing organ ischemia despite an increased cross-clamp time. There are currently four commercial systems available capable of EVLP. Broadly speaking, three EVLP management protocols exist (Toronto, Lund, and OCS), which differ in perfusate composition, target flow, pulmonary arterial pressure, left atrial pressure, and ventilatory settings. Notably, the Toronto protocol uses a closed left atrium, whereas the Lund and OCS protocol use an open left atrium. There are excellent published reviews of the different systems (Possoz J, et al. J Thorac Dis. 2019;11[4]:1635). EVLP has now been studied for two different goals: (1) to allow an extended evaluation of lungs of questionable quality before transplant; or (2) for routine use in all lung transplantations in place of cold static preservation.
In most studies concerning the use of EVLP for reconditioning of donor lungs, “high risk” or “extended criteria” refers to one or more of the following: P/F ratios < 300 on arterial blood gas, macroscopic abnormalities (eg, pulmonary edema, poor lung compliance), donation after circulatory death, or high-risk history (eg, aspiration). The largest cohort with the longest follow-up addressing the role of EVLP for donation of lungs with extended criteria was published from the Toronto Lung Transplant Group. Their results have demonstrated equivalent graft survival and rates of chronic lung allograft dysfunction (CLAD) up to 9 years posttransplant compared with standard criteria donor lungs, despite utilizing lower quality lungs and having a longer median preservation (Divithotawela C, et al. JAMA Surg. 2019;154[12]:1143). The group’s subsequent lung transplant rates have increased over the past decade.
A separate study addressed the same question but differed in that it was a single-arm, multicenter, international trial that tracked the outcomes of 93 extended criteria lungs placed on EVLP (including a large proportion acquired via donation after circulatory death) (Loor G, et al. Lancet Respir Med. 2019;7[11]:975). Among these, 87% of eligible lungs were transplanted, and outcomes were excellent, albeit shorter in follow-up compared with the Toronto cohort (eg, primary graft dysfunction grade 3 (PGD3) within 72 hours was 44% and 1-year survival was 91%). Based on these trials and many other retrospective reports, it has been concluded by many experts in the field that EVLP-treated extended criteria donor lungs perform equally well to standard criteria donor lungs.
Two RCTs have been conducted to evaluate whether EVLP is noninferior to static cold storage with donor lungs meeting “standard criteria” for transplant. The first was a single center study at the Medical University of Vienna, that looked at 80 recipient/donor pairs. Lungs in the EVLP arm underwent 4 hours of perfusion with frequent reassessment of quality before transplant, whereas the lungs in the control arm went directly to transplant. This study met noninferiority criteria looking at primary outcomes of PGD grade >1 and 30-day survival (Slama A, et al. J Heart Lung Transplant. 2017;36[7]:744). The second study was a phase 3, multicenter, international trial that included 320 recipient/donor pairs randomized to either EVLP (without a prespecified time on the EVLP system) or static cold storage. This trial met noninferiority for safety endpoints (lung graft-related adverse events within 30 days) and a composite primary outcome of PGD grade 3 incidence within 72 hours and 30-day survival (Warnecke G, et al. Lancet Respir Med. 2018;6[5]:357). The authors also tested and found superiority of EVLP in lower PGD grade 3 frequency compared with control. While these RCTs may suggest a role for EVLP in the procurement process of standard criteria organs in addition to extended criteria organs in the future, major criticisms for these trials include the lack of a demonstrable clinical benefit over cold storage beyond the lower PGD3 rates.
In the era of direct-acting antiviral agents available to treat HCV infection, there has been efforts to study the early use of anti-HCV medications to prevent infection as a result of heart or lung transplant from HCV viremic donors to HCV-negative recipients. In one major trial on efficacy, it was found that 4 weeks of sofosbuvir and velpatasvir, when started within a few hours of transplant, was sufficient to achieve a sustained (undetectable) virologic response at 12 weeks after completion of the antiviral regimen (Woolley AE, et al. N Engl J Med. 2019;380[17]:1606). Therefore, many transplant centers have adopted protocols to increase the donor pool (by CDC estimates about 4% of solid organ donors are HCV-positive) by accepting HCV nucleic acid amplification test (NAT)-positive donors for HCV-negative recipients, after appropriate informed consent.
Donation after cardiac death (DCD), which is alternatively known as donation after circulatory determination of death (DCDD), generally refers to organ procurement taking place after cessation of circulation, often after inpatient withdrawal of support. This is in contrast to the much more common practice of donation after brain death (DBD). Addressing concerns over the quality of lungs donated in the context of DCD compared with DBD, analyses of ISHLT registry data have demonstrated no differences in hospital length of stay or survival at 1 or 5 years (Van Raemdonck D, et al. J Heart Lung Transplant. 2019;38[12]:1235). Outcomes comparing specific mechanisms of donor death in DCD remain relatively unknown, such as outcomes from donors withdrawn from life support vs donors who had an uncontrolled cardiac death.
These methods for expanding the donor pool are not mutually exclusive, and, in fact, application of EVLP for lungs obtained in the context of DCD seems to be increasingly common. Optimization of protocols with collaboration between lung transplant centers will be paramount as we move forward in advancing this field. As we do so, efforts to successfully increase the donor pool will serve to provide a life-saving therapy to an ever-growing number of patients with end-stage lung disease.
Dr. Sala and Dr. Tomic are with the Division of Pulmonary and Critical Care Medicine, Northwestern University, Chicago, Illinois.
Lung transplants are increasing, with 2,562 performed in the United States in 2018 – a 31% increase over the preceding 5 years. With this increased demand for donor lungs, waitlist mortality in the United States is 9.4 deaths per 100 waitlist-years for obstructive lung diseases and as high as 29.7 deaths per 100 waitlist-years for restrictive lung diseases (Valapour M, et al. Lung. Am J Transplant. 2020;20[suppl s1]:427). Conversely, lungs are utilized from eligible multiorgan donors only 15% to 20% of the time, usually due to concerns over donor history or organ quality (Young KA, et al. Chest. 2019;155[3]:465). In light of this imbalance of supply and demand, lung transplant specialists are making significant efforts to expand the donor pool of available organs. Three of these strategies include: (1) applications of ex-vivo lung perfusion (EVLP) technology; (2) use of lungs from hepatitis C-positive donors for hep-C negative recipients; and (3) increasing utilization of donation after cardiac death.
Normothermic ex-vivo lung perfusion is a technology which allows donor lungs to be perfused and ventilated after removal from the donor but before transplant into the recipient. This is in contrast to the traditional method of cold static preservation. The proposed advantage of using this technology is to allow time for a more thorough assessment of graft quality and to improve function of grafts not meeting established criteria for transplant, all-the-while decreasing organ ischemia despite an increased cross-clamp time. There are currently four commercial systems available capable of EVLP. Broadly speaking, three EVLP management protocols exist (Toronto, Lund, and OCS), which differ in perfusate composition, target flow, pulmonary arterial pressure, left atrial pressure, and ventilatory settings. Notably, the Toronto protocol uses a closed left atrium, whereas the Lund and OCS protocol use an open left atrium. There are excellent published reviews of the different systems (Possoz J, et al. J Thorac Dis. 2019;11[4]:1635). EVLP has now been studied for two different goals: (1) to allow an extended evaluation of lungs of questionable quality before transplant; or (2) for routine use in all lung transplantations in place of cold static preservation.
In most studies concerning the use of EVLP for reconditioning of donor lungs, “high risk” or “extended criteria” refers to one or more of the following: P/F ratios < 300 on arterial blood gas, macroscopic abnormalities (eg, pulmonary edema, poor lung compliance), donation after circulatory death, or high-risk history (eg, aspiration). The largest cohort with the longest follow-up addressing the role of EVLP for donation of lungs with extended criteria was published from the Toronto Lung Transplant Group. Their results have demonstrated equivalent graft survival and rates of chronic lung allograft dysfunction (CLAD) up to 9 years posttransplant compared with standard criteria donor lungs, despite utilizing lower quality lungs and having a longer median preservation (Divithotawela C, et al. JAMA Surg. 2019;154[12]:1143). The group’s subsequent lung transplant rates have increased over the past decade.
A separate study addressed the same question but differed in that it was a single-arm, multicenter, international trial that tracked the outcomes of 93 extended criteria lungs placed on EVLP (including a large proportion acquired via donation after circulatory death) (Loor G, et al. Lancet Respir Med. 2019;7[11]:975). Among these, 87% of eligible lungs were transplanted, and outcomes were excellent, albeit shorter in follow-up compared with the Toronto cohort (eg, primary graft dysfunction grade 3 (PGD3) within 72 hours was 44% and 1-year survival was 91%). Based on these trials and many other retrospective reports, it has been concluded by many experts in the field that EVLP-treated extended criteria donor lungs perform equally well to standard criteria donor lungs.
Two RCTs have been conducted to evaluate whether EVLP is noninferior to static cold storage with donor lungs meeting “standard criteria” for transplant. The first was a single center study at the Medical University of Vienna, that looked at 80 recipient/donor pairs. Lungs in the EVLP arm underwent 4 hours of perfusion with frequent reassessment of quality before transplant, whereas the lungs in the control arm went directly to transplant. This study met noninferiority criteria looking at primary outcomes of PGD grade >1 and 30-day survival (Slama A, et al. J Heart Lung Transplant. 2017;36[7]:744). The second study was a phase 3, multicenter, international trial that included 320 recipient/donor pairs randomized to either EVLP (without a prespecified time on the EVLP system) or static cold storage. This trial met noninferiority for safety endpoints (lung graft-related adverse events within 30 days) and a composite primary outcome of PGD grade 3 incidence within 72 hours and 30-day survival (Warnecke G, et al. Lancet Respir Med. 2018;6[5]:357). The authors also tested and found superiority of EVLP in lower PGD grade 3 frequency compared with control. While these RCTs may suggest a role for EVLP in the procurement process of standard criteria organs in addition to extended criteria organs in the future, major criticisms for these trials include the lack of a demonstrable clinical benefit over cold storage beyond the lower PGD3 rates.
In the era of direct-acting antiviral agents available to treat HCV infection, there has been efforts to study the early use of anti-HCV medications to prevent infection as a result of heart or lung transplant from HCV viremic donors to HCV-negative recipients. In one major trial on efficacy, it was found that 4 weeks of sofosbuvir and velpatasvir, when started within a few hours of transplant, was sufficient to achieve a sustained (undetectable) virologic response at 12 weeks after completion of the antiviral regimen (Woolley AE, et al. N Engl J Med. 2019;380[17]:1606). Therefore, many transplant centers have adopted protocols to increase the donor pool (by CDC estimates about 4% of solid organ donors are HCV-positive) by accepting HCV nucleic acid amplification test (NAT)-positive donors for HCV-negative recipients, after appropriate informed consent.
Donation after cardiac death (DCD), which is alternatively known as donation after circulatory determination of death (DCDD), generally refers to organ procurement taking place after cessation of circulation, often after inpatient withdrawal of support. This is in contrast to the much more common practice of donation after brain death (DBD). Addressing concerns over the quality of lungs donated in the context of DCD compared with DBD, analyses of ISHLT registry data have demonstrated no differences in hospital length of stay or survival at 1 or 5 years (Van Raemdonck D, et al. J Heart Lung Transplant. 2019;38[12]:1235). Outcomes comparing specific mechanisms of donor death in DCD remain relatively unknown, such as outcomes from donors withdrawn from life support vs donors who had an uncontrolled cardiac death.
These methods for expanding the donor pool are not mutually exclusive, and, in fact, application of EVLP for lungs obtained in the context of DCD seems to be increasingly common. Optimization of protocols with collaboration between lung transplant centers will be paramount as we move forward in advancing this field. As we do so, efforts to successfully increase the donor pool will serve to provide a life-saving therapy to an ever-growing number of patients with end-stage lung disease.
Dr. Sala and Dr. Tomic are with the Division of Pulmonary and Critical Care Medicine, Northwestern University, Chicago, Illinois.
Stimulation to titration: An update on hypoglossal nerve stimulation for OSA
Clinical significance
Continuous positive airway pressure remains the gold standard and first-line treatment for moderate to severe OSA. When CPAP and other medical therapies fail or are poorly adopted, surgical solutions - either standalone or in unison - can be directed to target precision therapy.
The newest of these techniques is neuromodulation of the lingual musculature, particularly by way of selective stimulation of the hypoglossal nerve, which first demonstrated success in human clinical trials in 1996.1 Upper airway stimulation (UAS) was formally FDA-approved in 2014 (Inspire Medical Systems, Inc). UAS is designed to eliminate clinically significant OSA through stimulation of the anteriorly directed branches of the hypoglossal nerve, increasing the posterior airway space in a multilevel fashion.2 Since this time, over 7,500 patients have been treated with Inspire in nine countries (United States, Germany, The Netherlands, Switzerland, Belgium, Spain, France, Italy, and Finland). Prospective, international multicenter trials have demonstrated 68% to 96% clinical efficacy in well selected individuals. This is defined as a ≥ 50% reduction in the apnea hypopnea index (AHI) to an overall AHI of ≤ 20/hour.3,4 Additionally, post-UAS analysis demonstrates subjective reduction in daytime sleepiness as reported by Epworth sleepiness scores, with improvements in sleep-related quality of life. Further, UAS reduces socially disruptive snoring with 85% of bedpartners reporting soft to no snoring at 48-month follow-up.5 The procedure has also demonstrated long-term cost benefit in the US health-care system.6
Background and pathophysiology
Oliven and colleagues7 first observed the critical finding that selective intra-muscular stimulation of the genioglossus muscle lowered airway critical closing pressure (PCrit), thereby stabilizing the pharyngeal airway. Conversely, activation of the “retrusor” musculature, namely the hyoglossus and styloglossus muscles, increased Pcrit, increasing collapsibility of the pharyngeal airway.
Therapeutic implantation requires three incisions directed to the neck, chest, and right rib space (between the 4th to 6th intercostal spaces), with an operative time of 90 minutes or less in experienced hands. The majority of patients are discharged on the day of the procedure. Morbidity remains low with minimal pain reported during recovery. The most common complication is that of temporary tongue weakness, which typically resolves within 2 to 3 weeks. While very infrequent, patients should be counseled on the risk of postoperative hematoma, which can precipitate infection and subsequent explant of the device. Average recovery time spans between 3 and 7 days with activation of the device 4 weeks after surgical implantation to allow for appropriate tissue healing and reduce the risk of dislodgement of the implanted components. In contrast to other surgical treatment options, UAS is also reversible with no underlying alteration to existing pharyngeal anatomy apart from external incisions created during the procedure.
Stimulation to titration
As the need for a multidisciplinary approach to salvage of patients failing first-line therapy for OSA continues to grow, UAS with its multilevel impact continues to be of key interest. In similar fashion to established medical therapies such as PAP and oral appliance therapy (OAT), close observation between sleep medicine specialists and the implanting surgeon during the adaptation period with attention paid to titration parameters such as stimulation duration, pulse width, amplitude, and polarity, allow optimization of response outcome.
The stimulation electrode, which is designed in the form of a cuff to envelope the anterior (protrusor) branches of the hypoglossal nerve receives electrical stimulation from the implanted pulse generator, implanted above the pectoralis muscle of the chest wall. This design allows for collaborative awake and overnight titration of the device as directed by a sleep medicine physician. Attention is paid not only to the voltage “strength” administered with each pulse but also the degree of synchronization between respiration and stimulation, as well as pattern of pulse administration. Our experience remains that true success and adaptation to therapy requires not just meticulous surgical technique but a diligent approach to postoperative therapeutic titration to achieve a comfortable, yet effective, voltage for maintaining airway patency. Thus, akin to initiation of CPAP, UAS requires regular follow-up and device fine-tuning with patient comfort taken into consideration to achieve optimal results, and patient expectation should be aligned with this process.
Current indications
Success in UAS relies heavily on appropriate presurgical evaluation and clinical phenotyping. The following surgical indications have been demonstrated in the Stimulation Therapy for Apnea Reduction (STAR) trial and subsequent 3-year clinical follow-up: AHI between 15 and 80 events/hour (with ≤ 25% central apneas) and a BMI ≤ 32.8
As OSA often results from multi-level airway collapse, UAS targets an increase not only in the diameter of the retropalatal/oropharyngeal airway space but also the antero-posterior hypopharyngeal airway. Original criteria for implantation excluded patients with a pattern of complete circumferential collapse (CCC) noted on dynamic airway evaluation during pre-implant drug-induced sleep endoscopy (DISE). DISE aims to precisely target dynamic airway collapse patterns during simulated (propofol or midazolom induced) sleep.
Future directions
The effects of UAS are dependent on upper-airway cross-sectional area, particularly diameter. In patients who demonstrate CCC, the anteroposterior direction of activation derived from the UAS stimulus is unable to overcome CCC. In a recent prospective study, our group demonstrated that CCC can be converted to an airway collapse pattern compatible with UAS implantation, using a modified palatopharyngoplasty prior to UAS implantation. By stabilizing the lateral walls of the oropharyngeal airway with pre-implant palatal surgery, UAS is able to successfully direct widening of the airway cross-sectional area in an antero-posterior fashion. This exciting finding potentially allows for expansion of current indications, thus opening treatment to a wider patient population.9 Still, UAS remains highly studied in a relatively uniform patient population with data in more diverse subsets requiring further directed attention to expand and better define optimal patient populations for treatment.
From the perspective of improving patient adaptation and tolerance in UAS, a well-established concept in the CPAP literature can be applied, as explained by the Starling resistor model. The starling resistor is comprised of two rigid tubes connected by a collapsible segment in between. In parallel, the pharynx is a collapsible muscular tube connected on either end by the nose/nasal cavity and the trachea – both of which are bony/cartilaginous, noncollapsible structures. As has been shown in the use of CPAP, the same pressure required to maintain stability of the collapsible muscular pharynx via nasal breathing may lead to pharyngeal collapse when applied orally.10 This concept has also been directed towards UAS with our clinical experience demonstrating that oro or oronasal breathers tend to require a higher amplitude to maintain airway patency versus nasal breathers. This is an important area for future-directed study as medically/surgically improving nasal breathing in UAS subjects may subsequently lower amplitude requirements and improve patient tolerance.
Future direction to allow for improvement in the technology for application in a broader populational segment, external or alternative device powering mechanisms, along with MRI Compatibility and reducing the number of required external incisions will continue to broaden the patient selection criteria. As we move from a “stimulation” to a precision-tailored “stimulation and titration” approach, the mid to long term data supporting UAS remains very promising with 5-year follow up demonstrating sustained polysomnographic and subjective reported outcomes in well selected patients.
Dr. Awad is Assistant Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Northwestern University, Chicago, Illinois. Dr. Capasso is Associate Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Stanford Hospital and Clinics, Stanford, California.
References
1. Schwartz AR et al. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol. 1996;81(2):643-52. doi: 10.1152/jappl.1996.81.2.643.
2. Ong AA et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg. 2016;154(5):970-7. doi: 10.1177/0194599816636835.
3. Woodson BT et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: The STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-8. doi: 10.1177/0194599815616618.
4. Heiser C et al. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter german postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-84. doi: 10.1177/0194599816683378.
5. Gillespie MB et al. Upper airway stimulation for obstructive sleep apnea: Patient-reported outcomes after 48 months of follow-up. Otolaryngol Head Neck Surg. 2017;156(4):765-71. doi: 10.1177/0194599817691491.
6. Pietzsch JB et al. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: A model-based projection based on the star trial. Sleep. 2015;38(5):735-44. doi: 10.5665/sleep.4666.
7. Oliven A et al. Improved upper airway patency elicited by electrical stimulation of the hypoglossus nerves. Respiration. 1996;63(4):213-16. doi: 10.1159/000196547.
8. Strollo PJ et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-49. doi: 10.1056/NEJMoa1308659.
9. Liu YC et al. Palatopharyngoplasty resolves concentric collapse in patients ineligible for upper airway stimulation. Laryngoscope. Forthcoming.
10. De Andrade RGS et al. Impact of the type of mask on the effectiveness of and adherence to continuous positive airway pressure treatment for obstructive sleep apnea. J Bras Pneumol. 2014;40(6):658-68. doi: 10.1590/S1806-37132014000600010
Clinical significance
Continuous positive airway pressure remains the gold standard and first-line treatment for moderate to severe OSA. When CPAP and other medical therapies fail or are poorly adopted, surgical solutions - either standalone or in unison - can be directed to target precision therapy.
The newest of these techniques is neuromodulation of the lingual musculature, particularly by way of selective stimulation of the hypoglossal nerve, which first demonstrated success in human clinical trials in 1996.1 Upper airway stimulation (UAS) was formally FDA-approved in 2014 (Inspire Medical Systems, Inc). UAS is designed to eliminate clinically significant OSA through stimulation of the anteriorly directed branches of the hypoglossal nerve, increasing the posterior airway space in a multilevel fashion.2 Since this time, over 7,500 patients have been treated with Inspire in nine countries (United States, Germany, The Netherlands, Switzerland, Belgium, Spain, France, Italy, and Finland). Prospective, international multicenter trials have demonstrated 68% to 96% clinical efficacy in well selected individuals. This is defined as a ≥ 50% reduction in the apnea hypopnea index (AHI) to an overall AHI of ≤ 20/hour.3,4 Additionally, post-UAS analysis demonstrates subjective reduction in daytime sleepiness as reported by Epworth sleepiness scores, with improvements in sleep-related quality of life. Further, UAS reduces socially disruptive snoring with 85% of bedpartners reporting soft to no snoring at 48-month follow-up.5 The procedure has also demonstrated long-term cost benefit in the US health-care system.6
Background and pathophysiology
Oliven and colleagues7 first observed the critical finding that selective intra-muscular stimulation of the genioglossus muscle lowered airway critical closing pressure (PCrit), thereby stabilizing the pharyngeal airway. Conversely, activation of the “retrusor” musculature, namely the hyoglossus and styloglossus muscles, increased Pcrit, increasing collapsibility of the pharyngeal airway.
Therapeutic implantation requires three incisions directed to the neck, chest, and right rib space (between the 4th to 6th intercostal spaces), with an operative time of 90 minutes or less in experienced hands. The majority of patients are discharged on the day of the procedure. Morbidity remains low with minimal pain reported during recovery. The most common complication is that of temporary tongue weakness, which typically resolves within 2 to 3 weeks. While very infrequent, patients should be counseled on the risk of postoperative hematoma, which can precipitate infection and subsequent explant of the device. Average recovery time spans between 3 and 7 days with activation of the device 4 weeks after surgical implantation to allow for appropriate tissue healing and reduce the risk of dislodgement of the implanted components. In contrast to other surgical treatment options, UAS is also reversible with no underlying alteration to existing pharyngeal anatomy apart from external incisions created during the procedure.
Stimulation to titration
As the need for a multidisciplinary approach to salvage of patients failing first-line therapy for OSA continues to grow, UAS with its multilevel impact continues to be of key interest. In similar fashion to established medical therapies such as PAP and oral appliance therapy (OAT), close observation between sleep medicine specialists and the implanting surgeon during the adaptation period with attention paid to titration parameters such as stimulation duration, pulse width, amplitude, and polarity, allow optimization of response outcome.
The stimulation electrode, which is designed in the form of a cuff to envelope the anterior (protrusor) branches of the hypoglossal nerve receives electrical stimulation from the implanted pulse generator, implanted above the pectoralis muscle of the chest wall. This design allows for collaborative awake and overnight titration of the device as directed by a sleep medicine physician. Attention is paid not only to the voltage “strength” administered with each pulse but also the degree of synchronization between respiration and stimulation, as well as pattern of pulse administration. Our experience remains that true success and adaptation to therapy requires not just meticulous surgical technique but a diligent approach to postoperative therapeutic titration to achieve a comfortable, yet effective, voltage for maintaining airway patency. Thus, akin to initiation of CPAP, UAS requires regular follow-up and device fine-tuning with patient comfort taken into consideration to achieve optimal results, and patient expectation should be aligned with this process.
Current indications
Success in UAS relies heavily on appropriate presurgical evaluation and clinical phenotyping. The following surgical indications have been demonstrated in the Stimulation Therapy for Apnea Reduction (STAR) trial and subsequent 3-year clinical follow-up: AHI between 15 and 80 events/hour (with ≤ 25% central apneas) and a BMI ≤ 32.8
As OSA often results from multi-level airway collapse, UAS targets an increase not only in the diameter of the retropalatal/oropharyngeal airway space but also the antero-posterior hypopharyngeal airway. Original criteria for implantation excluded patients with a pattern of complete circumferential collapse (CCC) noted on dynamic airway evaluation during pre-implant drug-induced sleep endoscopy (DISE). DISE aims to precisely target dynamic airway collapse patterns during simulated (propofol or midazolom induced) sleep.
Future directions
The effects of UAS are dependent on upper-airway cross-sectional area, particularly diameter. In patients who demonstrate CCC, the anteroposterior direction of activation derived from the UAS stimulus is unable to overcome CCC. In a recent prospective study, our group demonstrated that CCC can be converted to an airway collapse pattern compatible with UAS implantation, using a modified palatopharyngoplasty prior to UAS implantation. By stabilizing the lateral walls of the oropharyngeal airway with pre-implant palatal surgery, UAS is able to successfully direct widening of the airway cross-sectional area in an antero-posterior fashion. This exciting finding potentially allows for expansion of current indications, thus opening treatment to a wider patient population.9 Still, UAS remains highly studied in a relatively uniform patient population with data in more diverse subsets requiring further directed attention to expand and better define optimal patient populations for treatment.
From the perspective of improving patient adaptation and tolerance in UAS, a well-established concept in the CPAP literature can be applied, as explained by the Starling resistor model. The starling resistor is comprised of two rigid tubes connected by a collapsible segment in between. In parallel, the pharynx is a collapsible muscular tube connected on either end by the nose/nasal cavity and the trachea – both of which are bony/cartilaginous, noncollapsible structures. As has been shown in the use of CPAP, the same pressure required to maintain stability of the collapsible muscular pharynx via nasal breathing may lead to pharyngeal collapse when applied orally.10 This concept has also been directed towards UAS with our clinical experience demonstrating that oro or oronasal breathers tend to require a higher amplitude to maintain airway patency versus nasal breathers. This is an important area for future-directed study as medically/surgically improving nasal breathing in UAS subjects may subsequently lower amplitude requirements and improve patient tolerance.
Future direction to allow for improvement in the technology for application in a broader populational segment, external or alternative device powering mechanisms, along with MRI Compatibility and reducing the number of required external incisions will continue to broaden the patient selection criteria. As we move from a “stimulation” to a precision-tailored “stimulation and titration” approach, the mid to long term data supporting UAS remains very promising with 5-year follow up demonstrating sustained polysomnographic and subjective reported outcomes in well selected patients.
Dr. Awad is Assistant Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Northwestern University, Chicago, Illinois. Dr. Capasso is Associate Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Stanford Hospital and Clinics, Stanford, California.
References
1. Schwartz AR et al. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol. 1996;81(2):643-52. doi: 10.1152/jappl.1996.81.2.643.
2. Ong AA et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg. 2016;154(5):970-7. doi: 10.1177/0194599816636835.
3. Woodson BT et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: The STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-8. doi: 10.1177/0194599815616618.
4. Heiser C et al. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter german postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-84. doi: 10.1177/0194599816683378.
5. Gillespie MB et al. Upper airway stimulation for obstructive sleep apnea: Patient-reported outcomes after 48 months of follow-up. Otolaryngol Head Neck Surg. 2017;156(4):765-71. doi: 10.1177/0194599817691491.
6. Pietzsch JB et al. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: A model-based projection based on the star trial. Sleep. 2015;38(5):735-44. doi: 10.5665/sleep.4666.
7. Oliven A et al. Improved upper airway patency elicited by electrical stimulation of the hypoglossus nerves. Respiration. 1996;63(4):213-16. doi: 10.1159/000196547.
8. Strollo PJ et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-49. doi: 10.1056/NEJMoa1308659.
9. Liu YC et al. Palatopharyngoplasty resolves concentric collapse in patients ineligible for upper airway stimulation. Laryngoscope. Forthcoming.
10. De Andrade RGS et al. Impact of the type of mask on the effectiveness of and adherence to continuous positive airway pressure treatment for obstructive sleep apnea. J Bras Pneumol. 2014;40(6):658-68. doi: 10.1590/S1806-37132014000600010
Clinical significance
Continuous positive airway pressure remains the gold standard and first-line treatment for moderate to severe OSA. When CPAP and other medical therapies fail or are poorly adopted, surgical solutions - either standalone or in unison - can be directed to target precision therapy.
The newest of these techniques is neuromodulation of the lingual musculature, particularly by way of selective stimulation of the hypoglossal nerve, which first demonstrated success in human clinical trials in 1996.1 Upper airway stimulation (UAS) was formally FDA-approved in 2014 (Inspire Medical Systems, Inc). UAS is designed to eliminate clinically significant OSA through stimulation of the anteriorly directed branches of the hypoglossal nerve, increasing the posterior airway space in a multilevel fashion.2 Since this time, over 7,500 patients have been treated with Inspire in nine countries (United States, Germany, The Netherlands, Switzerland, Belgium, Spain, France, Italy, and Finland). Prospective, international multicenter trials have demonstrated 68% to 96% clinical efficacy in well selected individuals. This is defined as a ≥ 50% reduction in the apnea hypopnea index (AHI) to an overall AHI of ≤ 20/hour.3,4 Additionally, post-UAS analysis demonstrates subjective reduction in daytime sleepiness as reported by Epworth sleepiness scores, with improvements in sleep-related quality of life. Further, UAS reduces socially disruptive snoring with 85% of bedpartners reporting soft to no snoring at 48-month follow-up.5 The procedure has also demonstrated long-term cost benefit in the US health-care system.6
Background and pathophysiology
Oliven and colleagues7 first observed the critical finding that selective intra-muscular stimulation of the genioglossus muscle lowered airway critical closing pressure (PCrit), thereby stabilizing the pharyngeal airway. Conversely, activation of the “retrusor” musculature, namely the hyoglossus and styloglossus muscles, increased Pcrit, increasing collapsibility of the pharyngeal airway.
Therapeutic implantation requires three incisions directed to the neck, chest, and right rib space (between the 4th to 6th intercostal spaces), with an operative time of 90 minutes or less in experienced hands. The majority of patients are discharged on the day of the procedure. Morbidity remains low with minimal pain reported during recovery. The most common complication is that of temporary tongue weakness, which typically resolves within 2 to 3 weeks. While very infrequent, patients should be counseled on the risk of postoperative hematoma, which can precipitate infection and subsequent explant of the device. Average recovery time spans between 3 and 7 days with activation of the device 4 weeks after surgical implantation to allow for appropriate tissue healing and reduce the risk of dislodgement of the implanted components. In contrast to other surgical treatment options, UAS is also reversible with no underlying alteration to existing pharyngeal anatomy apart from external incisions created during the procedure.
Stimulation to titration
As the need for a multidisciplinary approach to salvage of patients failing first-line therapy for OSA continues to grow, UAS with its multilevel impact continues to be of key interest. In similar fashion to established medical therapies such as PAP and oral appliance therapy (OAT), close observation between sleep medicine specialists and the implanting surgeon during the adaptation period with attention paid to titration parameters such as stimulation duration, pulse width, amplitude, and polarity, allow optimization of response outcome.
The stimulation electrode, which is designed in the form of a cuff to envelope the anterior (protrusor) branches of the hypoglossal nerve receives electrical stimulation from the implanted pulse generator, implanted above the pectoralis muscle of the chest wall. This design allows for collaborative awake and overnight titration of the device as directed by a sleep medicine physician. Attention is paid not only to the voltage “strength” administered with each pulse but also the degree of synchronization between respiration and stimulation, as well as pattern of pulse administration. Our experience remains that true success and adaptation to therapy requires not just meticulous surgical technique but a diligent approach to postoperative therapeutic titration to achieve a comfortable, yet effective, voltage for maintaining airway patency. Thus, akin to initiation of CPAP, UAS requires regular follow-up and device fine-tuning with patient comfort taken into consideration to achieve optimal results, and patient expectation should be aligned with this process.
Current indications
Success in UAS relies heavily on appropriate presurgical evaluation and clinical phenotyping. The following surgical indications have been demonstrated in the Stimulation Therapy for Apnea Reduction (STAR) trial and subsequent 3-year clinical follow-up: AHI between 15 and 80 events/hour (with ≤ 25% central apneas) and a BMI ≤ 32.8
As OSA often results from multi-level airway collapse, UAS targets an increase not only in the diameter of the retropalatal/oropharyngeal airway space but also the antero-posterior hypopharyngeal airway. Original criteria for implantation excluded patients with a pattern of complete circumferential collapse (CCC) noted on dynamic airway evaluation during pre-implant drug-induced sleep endoscopy (DISE). DISE aims to precisely target dynamic airway collapse patterns during simulated (propofol or midazolom induced) sleep.
Future directions
The effects of UAS are dependent on upper-airway cross-sectional area, particularly diameter. In patients who demonstrate CCC, the anteroposterior direction of activation derived from the UAS stimulus is unable to overcome CCC. In a recent prospective study, our group demonstrated that CCC can be converted to an airway collapse pattern compatible with UAS implantation, using a modified palatopharyngoplasty prior to UAS implantation. By stabilizing the lateral walls of the oropharyngeal airway with pre-implant palatal surgery, UAS is able to successfully direct widening of the airway cross-sectional area in an antero-posterior fashion. This exciting finding potentially allows for expansion of current indications, thus opening treatment to a wider patient population.9 Still, UAS remains highly studied in a relatively uniform patient population with data in more diverse subsets requiring further directed attention to expand and better define optimal patient populations for treatment.
From the perspective of improving patient adaptation and tolerance in UAS, a well-established concept in the CPAP literature can be applied, as explained by the Starling resistor model. The starling resistor is comprised of two rigid tubes connected by a collapsible segment in between. In parallel, the pharynx is a collapsible muscular tube connected on either end by the nose/nasal cavity and the trachea – both of which are bony/cartilaginous, noncollapsible structures. As has been shown in the use of CPAP, the same pressure required to maintain stability of the collapsible muscular pharynx via nasal breathing may lead to pharyngeal collapse when applied orally.10 This concept has also been directed towards UAS with our clinical experience demonstrating that oro or oronasal breathers tend to require a higher amplitude to maintain airway patency versus nasal breathers. This is an important area for future-directed study as medically/surgically improving nasal breathing in UAS subjects may subsequently lower amplitude requirements and improve patient tolerance.
Future direction to allow for improvement in the technology for application in a broader populational segment, external or alternative device powering mechanisms, along with MRI Compatibility and reducing the number of required external incisions will continue to broaden the patient selection criteria. As we move from a “stimulation” to a precision-tailored “stimulation and titration” approach, the mid to long term data supporting UAS remains very promising with 5-year follow up demonstrating sustained polysomnographic and subjective reported outcomes in well selected patients.
Dr. Awad is Assistant Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Northwestern University, Chicago, Illinois. Dr. Capasso is Associate Professor – Department of Otolaryngology/Head & Neck Surgery, and Chief – Division of Sleep Surgery; Stanford Hospital and Clinics, Stanford, California.
References
1. Schwartz AR et al. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol. 1996;81(2):643-52. doi: 10.1152/jappl.1996.81.2.643.
2. Ong AA et al. Efficacy of upper airway stimulation on collapse patterns observed during drug-induced sedation endoscopy. Otolaryngol Head Neck Surg. 2016;154(5):970-7. doi: 10.1177/0194599816636835.
3. Woodson BT et al. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: The STAR trial. Otolaryngol Head Neck Surg. 2016;154(1):181-8. doi: 10.1177/0194599815616618.
4. Heiser C et al. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter german postmarket study. Otolaryngol Head Neck Surg. 2017;156(2):378-84. doi: 10.1177/0194599816683378.
5. Gillespie MB et al. Upper airway stimulation for obstructive sleep apnea: Patient-reported outcomes after 48 months of follow-up. Otolaryngol Head Neck Surg. 2017;156(4):765-71. doi: 10.1177/0194599817691491.
6. Pietzsch JB et al. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: A model-based projection based on the star trial. Sleep. 2015;38(5):735-44. doi: 10.5665/sleep.4666.
7. Oliven A et al. Improved upper airway patency elicited by electrical stimulation of the hypoglossus nerves. Respiration. 1996;63(4):213-16. doi: 10.1159/000196547.
8. Strollo PJ et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-49. doi: 10.1056/NEJMoa1308659.
9. Liu YC et al. Palatopharyngoplasty resolves concentric collapse in patients ineligible for upper airway stimulation. Laryngoscope. Forthcoming.
10. De Andrade RGS et al. Impact of the type of mask on the effectiveness of and adherence to continuous positive airway pressure treatment for obstructive sleep apnea. J Bras Pneumol. 2014;40(6):658-68. doi: 10.1590/S1806-37132014000600010
Resurgence of black lung among U.S. coal miners
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Advances in technology over the last century, as well as the exportation of many high exposure jobs, nearly eliminated lung diseases caused by occupational exposure to respirable dust (the pneumoconioses) in the United States. One such example of this near elimination is black lung, or coal workers' pneumoconiosis (CWP), following the 1969 Federal Coal Mine Health and Safety Act. The Act established permissible exposure limits to respirable dust, designed to prevent the most severe forms of CWP from occurring, and a national respiratory health screening program for underground coal miners. Between 1970 and the mid-1990s, disease prevalence plummeted from nearly 35% to less than 5% prevalence among longer tenured miners, and from 3% to less than 1% in miners with less than 10 years of mining tenure (Hall NB, et al. Curr Environ Health Rep. 2019;6[3]:137).
Many assumed that this was the last we'd hear of black lung - that the cases of disease existing in the 1990s were likely caused by exposures that occurred prior to the 1969 Act, and within a few years, no further cases would be detected. This appeared to be an entirely reasonable assumption in the 1990s given the 30 years of declining prevalence and the continuous technological advances designed to continue reductions in dust exposures. In fact, the precipitous decline in black lung was briefly viewed as a public health triumph, as the most severe forms appeared to be near eradication in the United States just 2 decades ago (Attfield MD, et al. Am J Public Health. 1992;82[7]:971; Attfield MD, et al. Am J Public Health. 1992;82[7]:964). However, what has since been observed is a strong and ongoing resurgence of the potentially deadly fibrotic interstitial disease starting in the early 2000s (Figure 1), with the most striking increase observed in the Central Appalachian states of Kentucky, Virginia, and West Virginia (Blackley DJ, et al. Am J Respir Crit Care Med. 2014;190[6]:708; Blackley DJ, et al. Am J Public Health. 2018;108[9]:1220).
Of great concern is the resurgence of complicated Black Lung (progressive massive fibrosis [PMF]), which is completely disabling and leads to premature mortality. The prevalence of PMF is higher today than when NIOSH started formally tracking the disease in the 1970s, especially among specific populations.
Since the mid-2000s, NIOSH and others have described the following(Hall NB, et al. Curr Environ Health Rep. 2019;6[3]:137):
· Increasing prevalence and severity of CWP both nationwide and specifically in Central Appalachia.
· Rapid progression of CWP.
· Increases in the frequency of lung transplantation for CWP.
· Severe disease among surface coal miners with no underground mining tenure.
· Increased severity of disease among former and retired miners.
· Hundreds of cases of PMF among coal miners seeking care at clinics in eastern Kentucky and southwestern Virginia.
· Increasing numbers of miners with PMF filing for federal black lung compensation.
· Radiologic and pathologic indications of increased respirable silica exposure among coal miners.
· Premature mortality in miners diagnosed with CWP.
· Underutilization of a secondary prevention worker removal program designed to reduce the exposure of miners with disease.
· Former miners with severe disease describing extreme pressure to operate. outside of applicable protective federal standards in order to increase productivity
In our surveillance work, we have talked to many miners who, after having months or years' worth of extensive workups for pneumonia, sarcoidosis, lung cancer, and/or diseases other than the pneumoconioses, have eventually learned that they actually had dust-induced lung disease attributable to their work. Additionally, through our evaluation of the transplantation data, it has become clear that dust-related lung disease is likely underreported or underrecognized among those receiving lung transplants. Finally, through analysis of mortality data, it is apparent that CWP is also underreported as a cause of death among miners with black lung. We mention these points to emphasize how important it is to document a full occupational history for proper diagnoses, early intervention, and improved public health information to inform primary and secondary disease prevention efforts.
Resources for clinicians
CWP is most commonly identified using plain posterior-anterior chest radiography and presence/severity of fibrotic change is described using an international standard established by the International Labour Office (International Labour Office. Guidelines for the use of the ILO international classification of radiographs of pneumoconioses. Geneva: International Labour Office; 2011). In the United States, NIOSH operates the B Reader Training and Certification Program, which offers a free self-study syllabus, https://www.cdc.gov/niosh/topics/chestradiography/breader.html, and in-person training courses on occasion, to assist physicians in learning and demonstrating continuous competency in classifying chest radiographs of dust-exposed workers according to the ILO Standards (Halldin CN, et al. J Occup Environ Med. 2019;61[12]:1045). The B Reader Program and ILO Standards are currently undergoing a decade-long revision process where both will feature digitally acquired chest radiograph images. This process should be fully complete in the following months.
To educate miners, mine operators, and others about the risks of respirable dust, NIOSH produced an educational video, Faces of Black Lung, in 2008 that featured two miners in their 50s and 60s who had complicated Black Lung. Because of the resurgence of disease and particularly severe cases being identified among much younger miners, NIOSH recently released an updated version of the video, Faces of Black Lung II, where three Kentucky underground miners, ages 39, 42, and 48, describe the incredible disability and quality of life lost due to a disease caused by gross overexposure of respirable coal mine dust.
Unfortunately, the 42-year-old miner died from complications stemming from Black Lung less than a year after filming his part in the video, and the other two miners have been advised to be evaluated for lung transplantation. We hope that these men's stories will help younger miners relate to the risks of respirable coal mine dust and help others understand the severity of disease as all three of these men struggled to breathe just describing their day to day tasks.
Parting message
No one should ever have to consider a lung transplant at the age of 40 because they went to work attempting to provide for their family. No one should ever be faced with end-of-life planning while their kids are in grade school because of a disease they acquired at work. Respirable coal mine dust is the only cause of black lung, and the coal mining industry has the necessary technology and tools to prevent harmful exposures to respirable dust, and, together with miners, must successfully and consistently implement dust suppression controls. There is no cure for black lung; it's irreversible and can be first recognized and continue to progress even after a miner has left exposure. However, early identification and appropriate intervention can prevent progression to the most disabling manifestations. The role of the clinician is to be part of the early identification of black lung through including CWP in the differential diagnosis for unusual or unexpected respiratory illness in otherwise healthy primarily working aged miners. The public health community must continue to monitor disease prevalence in working populations and implement policies and recommendations to support the efforts of those on the frontline - the miners, industry, and health-care workers.
The Energy Information Agency projects that coal will continue to be a substantial source of U.S. energy production and consumption well into the mid- to late-century. Unfortunately, Black Lung has made a resurgence and is killing miners, and each of us has a role to play in eliminating it once and for all. We will continue to carry out our mandate to screen working coal miners for respiratory disease; however, given the continued contraction of the coal mining industry, it's much more likely for cases of disease to be recognized in the clinic setting. Therefore, we reiterate our previous plea to clinicians: when identifying an individual with interstitial fibrosis consider their full occupational history.
Dr. Halldin and Dr. Laney are from the Surveillance Branch, Respiratory Health Division, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Advances in technology over the last century, as well as the exportation of many high exposure jobs, nearly eliminated lung diseases caused by occupational exposure to respirable dust (the pneumoconioses) in the United States. One such example of this near elimination is black lung, or coal workers' pneumoconiosis (CWP), following the 1969 Federal Coal Mine Health and Safety Act. The Act established permissible exposure limits to respirable dust, designed to prevent the most severe forms of CWP from occurring, and a national respiratory health screening program for underground coal miners. Between 1970 and the mid-1990s, disease prevalence plummeted from nearly 35% to less than 5% prevalence among longer tenured miners, and from 3% to less than 1% in miners with less than 10 years of mining tenure (Hall NB, et al. Curr Environ Health Rep. 2019;6[3]:137).
Many assumed that this was the last we'd hear of black lung - that the cases of disease existing in the 1990s were likely caused by exposures that occurred prior to the 1969 Act, and within a few years, no further cases would be detected. This appeared to be an entirely reasonable assumption in the 1990s given the 30 years of declining prevalence and the continuous technological advances designed to continue reductions in dust exposures. In fact, the precipitous decline in black lung was briefly viewed as a public health triumph, as the most severe forms appeared to be near eradication in the United States just 2 decades ago (Attfield MD, et al. Am J Public Health. 1992;82[7]:971; Attfield MD, et al. Am J Public Health. 1992;82[7]:964). However, what has since been observed is a strong and ongoing resurgence of the potentially deadly fibrotic interstitial disease starting in the early 2000s (Figure 1), with the most striking increase observed in the Central Appalachian states of Kentucky, Virginia, and West Virginia (Blackley DJ, et al. Am J Respir Crit Care Med. 2014;190[6]:708; Blackley DJ, et al. Am J Public Health. 2018;108[9]:1220).
Of great concern is the resurgence of complicated Black Lung (progressive massive fibrosis [PMF]), which is completely disabling and leads to premature mortality. The prevalence of PMF is higher today than when NIOSH started formally tracking the disease in the 1970s, especially among specific populations.
Since the mid-2000s, NIOSH and others have described the following(Hall NB, et al. Curr Environ Health Rep. 2019;6[3]:137):
· Increasing prevalence and severity of CWP both nationwide and specifically in Central Appalachia.
· Rapid progression of CWP.
· Increases in the frequency of lung transplantation for CWP.
· Severe disease among surface coal miners with no underground mining tenure.
· Increased severity of disease among former and retired miners.
· Hundreds of cases of PMF among coal miners seeking care at clinics in eastern Kentucky and southwestern Virginia.
· Increasing numbers of miners with PMF filing for federal black lung compensation.
· Radiologic and pathologic indications of increased respirable silica exposure among coal miners.
· Premature mortality in miners diagnosed with CWP.
· Underutilization of a secondary prevention worker removal program designed to reduce the exposure of miners with disease.
· Former miners with severe disease describing extreme pressure to operate. outside of applicable protective federal standards in order to increase productivity
In our surveillance work, we have talked to many miners who, after having months or years' worth of extensive workups for pneumonia, sarcoidosis, lung cancer, and/or diseases other than the pneumoconioses, have eventually learned that they actually had dust-induced lung disease attributable to their work. Additionally, through our evaluation of the transplantation data, it has become clear that dust-related lung disease is likely underreported or underrecognized among those receiving lung transplants. Finally, through analysis of mortality data, it is apparent that CWP is also underreported as a cause of death among miners with black lung. We mention these points to emphasize how important it is to document a full occupational history for proper diagnoses, early intervention, and improved public health information to inform primary and secondary disease prevention efforts.
Resources for clinicians
CWP is most commonly identified using plain posterior-anterior chest radiography and presence/severity of fibrotic change is described using an international standard established by the International Labour Office (International Labour Office. Guidelines for the use of the ILO international classification of radiographs of pneumoconioses. Geneva: International Labour Office; 2011). In the United States, NIOSH operates the B Reader Training and Certification Program, which offers a free self-study syllabus, https://www.cdc.gov/niosh/topics/chestradiography/breader.html, and in-person training courses on occasion, to assist physicians in learning and demonstrating continuous competency in classifying chest radiographs of dust-exposed workers according to the ILO Standards (Halldin CN, et al. J Occup Environ Med. 2019;61[12]:1045). The B Reader Program and ILO Standards are currently undergoing a decade-long revision process where both will feature digitally acquired chest radiograph images. This process should be fully complete in the following months.
To educate miners, mine operators, and others about the risks of respirable dust, NIOSH produced an educational video, Faces of Black Lung, in 2008 that featured two miners in their 50s and 60s who had complicated Black Lung. Because of the resurgence of disease and particularly severe cases being identified among much younger miners, NIOSH recently released an updated version of the video, Faces of Black Lung II, where three Kentucky underground miners, ages 39, 42, and 48, describe the incredible disability and quality of life lost due to a disease caused by gross overexposure of respirable coal mine dust.
Unfortunately, the 42-year-old miner died from complications stemming from Black Lung less than a year after filming his part in the video, and the other two miners have been advised to be evaluated for lung transplantation. We hope that these men's stories will help younger miners relate to the risks of respirable coal mine dust and help others understand the severity of disease as all three of these men struggled to breathe just describing their day to day tasks.
Parting message
No one should ever have to consider a lung transplant at the age of 40 because they went to work attempting to provide for their family. No one should ever be faced with end-of-life planning while their kids are in grade school because of a disease they acquired at work. Respirable coal mine dust is the only cause of black lung, and the coal mining industry has the necessary technology and tools to prevent harmful exposures to respirable dust, and, together with miners, must successfully and consistently implement dust suppression controls. There is no cure for black lung; it's irreversible and can be first recognized and continue to progress even after a miner has left exposure. However, early identification and appropriate intervention can prevent progression to the most disabling manifestations. The role of the clinician is to be part of the early identification of black lung through including CWP in the differential diagnosis for unusual or unexpected respiratory illness in otherwise healthy primarily working aged miners. The public health community must continue to monitor disease prevalence in working populations and implement policies and recommendations to support the efforts of those on the frontline - the miners, industry, and health-care workers.
The Energy Information Agency projects that coal will continue to be a substantial source of U.S. energy production and consumption well into the mid- to late-century. Unfortunately, Black Lung has made a resurgence and is killing miners, and each of us has a role to play in eliminating it once and for all. We will continue to carry out our mandate to screen working coal miners for respiratory disease; however, given the continued contraction of the coal mining industry, it's much more likely for cases of disease to be recognized in the clinic setting. Therefore, we reiterate our previous plea to clinicians: when identifying an individual with interstitial fibrosis consider their full occupational history.
Dr. Halldin and Dr. Laney are from the Surveillance Branch, Respiratory Health Division, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Advances in technology over the last century, as well as the exportation of many high exposure jobs, nearly eliminated lung diseases caused by occupational exposure to respirable dust (the pneumoconioses) in the United States. One such example of this near elimination is black lung, or coal workers' pneumoconiosis (CWP), following the 1969 Federal Coal Mine Health and Safety Act. The Act established permissible exposure limits to respirable dust, designed to prevent the most severe forms of CWP from occurring, and a national respiratory health screening program for underground coal miners. Between 1970 and the mid-1990s, disease prevalence plummeted from nearly 35% to less than 5% prevalence among longer tenured miners, and from 3% to less than 1% in miners with less than 10 years of mining tenure (Hall NB, et al. Curr Environ Health Rep. 2019;6[3]:137).
Many assumed that this was the last we'd hear of black lung - that the cases of disease existing in the 1990s were likely caused by exposures that occurred prior to the 1969 Act, and within a few years, no further cases would be detected. This appeared to be an entirely reasonable assumption in the 1990s given the 30 years of declining prevalence and the continuous technological advances designed to continue reductions in dust exposures. In fact, the precipitous decline in black lung was briefly viewed as a public health triumph, as the most severe forms appeared to be near eradication in the United States just 2 decades ago (Attfield MD, et al. Am J Public Health. 1992;82[7]:971; Attfield MD, et al. Am J Public Health. 1992;82[7]:964). However, what has since been observed is a strong and ongoing resurgence of the potentially deadly fibrotic interstitial disease starting in the early 2000s (Figure 1), with the most striking increase observed in the Central Appalachian states of Kentucky, Virginia, and West Virginia (Blackley DJ, et al. Am J Respir Crit Care Med. 2014;190[6]:708; Blackley DJ, et al. Am J Public Health. 2018;108[9]:1220).
Of great concern is the resurgence of complicated Black Lung (progressive massive fibrosis [PMF]), which is completely disabling and leads to premature mortality. The prevalence of PMF is higher today than when NIOSH started formally tracking the disease in the 1970s, especially among specific populations.
Since the mid-2000s, NIOSH and others have described the following(Hall NB, et al. Curr Environ Health Rep. 2019;6[3]:137):
· Increasing prevalence and severity of CWP both nationwide and specifically in Central Appalachia.
· Rapid progression of CWP.
· Increases in the frequency of lung transplantation for CWP.
· Severe disease among surface coal miners with no underground mining tenure.
· Increased severity of disease among former and retired miners.
· Hundreds of cases of PMF among coal miners seeking care at clinics in eastern Kentucky and southwestern Virginia.
· Increasing numbers of miners with PMF filing for federal black lung compensation.
· Radiologic and pathologic indications of increased respirable silica exposure among coal miners.
· Premature mortality in miners diagnosed with CWP.
· Underutilization of a secondary prevention worker removal program designed to reduce the exposure of miners with disease.
· Former miners with severe disease describing extreme pressure to operate. outside of applicable protective federal standards in order to increase productivity
In our surveillance work, we have talked to many miners who, after having months or years' worth of extensive workups for pneumonia, sarcoidosis, lung cancer, and/or diseases other than the pneumoconioses, have eventually learned that they actually had dust-induced lung disease attributable to their work. Additionally, through our evaluation of the transplantation data, it has become clear that dust-related lung disease is likely underreported or underrecognized among those receiving lung transplants. Finally, through analysis of mortality data, it is apparent that CWP is also underreported as a cause of death among miners with black lung. We mention these points to emphasize how important it is to document a full occupational history for proper diagnoses, early intervention, and improved public health information to inform primary and secondary disease prevention efforts.
Resources for clinicians
CWP is most commonly identified using plain posterior-anterior chest radiography and presence/severity of fibrotic change is described using an international standard established by the International Labour Office (International Labour Office. Guidelines for the use of the ILO international classification of radiographs of pneumoconioses. Geneva: International Labour Office; 2011). In the United States, NIOSH operates the B Reader Training and Certification Program, which offers a free self-study syllabus, https://www.cdc.gov/niosh/topics/chestradiography/breader.html, and in-person training courses on occasion, to assist physicians in learning and demonstrating continuous competency in classifying chest radiographs of dust-exposed workers according to the ILO Standards (Halldin CN, et al. J Occup Environ Med. 2019;61[12]:1045). The B Reader Program and ILO Standards are currently undergoing a decade-long revision process where both will feature digitally acquired chest radiograph images. This process should be fully complete in the following months.
To educate miners, mine operators, and others about the risks of respirable dust, NIOSH produced an educational video, Faces of Black Lung, in 2008 that featured two miners in their 50s and 60s who had complicated Black Lung. Because of the resurgence of disease and particularly severe cases being identified among much younger miners, NIOSH recently released an updated version of the video, Faces of Black Lung II, where three Kentucky underground miners, ages 39, 42, and 48, describe the incredible disability and quality of life lost due to a disease caused by gross overexposure of respirable coal mine dust.
Unfortunately, the 42-year-old miner died from complications stemming from Black Lung less than a year after filming his part in the video, and the other two miners have been advised to be evaluated for lung transplantation. We hope that these men's stories will help younger miners relate to the risks of respirable coal mine dust and help others understand the severity of disease as all three of these men struggled to breathe just describing their day to day tasks.
Parting message
No one should ever have to consider a lung transplant at the age of 40 because they went to work attempting to provide for their family. No one should ever be faced with end-of-life planning while their kids are in grade school because of a disease they acquired at work. Respirable coal mine dust is the only cause of black lung, and the coal mining industry has the necessary technology and tools to prevent harmful exposures to respirable dust, and, together with miners, must successfully and consistently implement dust suppression controls. There is no cure for black lung; it's irreversible and can be first recognized and continue to progress even after a miner has left exposure. However, early identification and appropriate intervention can prevent progression to the most disabling manifestations. The role of the clinician is to be part of the early identification of black lung through including CWP in the differential diagnosis for unusual or unexpected respiratory illness in otherwise healthy primarily working aged miners. The public health community must continue to monitor disease prevalence in working populations and implement policies and recommendations to support the efforts of those on the frontline - the miners, industry, and health-care workers.
The Energy Information Agency projects that coal will continue to be a substantial source of U.S. energy production and consumption well into the mid- to late-century. Unfortunately, Black Lung has made a resurgence and is killing miners, and each of us has a role to play in eliminating it once and for all. We will continue to carry out our mandate to screen working coal miners for respiratory disease; however, given the continued contraction of the coal mining industry, it's much more likely for cases of disease to be recognized in the clinic setting. Therefore, we reiterate our previous plea to clinicians: when identifying an individual with interstitial fibrosis consider their full occupational history.
Dr. Halldin and Dr. Laney are from the Surveillance Branch, Respiratory Health Division, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV.




















