User login
Hospitals Teaching CT Surgery Excel At Lung Resection
SAN FRANCISCO - Pulmonary resection outcomes vary with respect to the kind of hospital in which the procedure is performed, particularly whether it is a teaching hospital, according to Dr. Castigliano M. Bhamidipati, of the University of Virginia Medical School, Charlottesville.
"In comparison to other hospitals, including general surgery teaching hospitals, cardiothoracic surgery teaching hospitals have lower morbidity and mortality," Dr. Bhamidipati said at the AATS annual meeting.
He and his colleagues examined the results of pulmonary resections performed at cardiothoracic (CT) teaching, general surgery (GS) teaching, nonsurgical (NS) teaching, and nonteaching (NT) hospitals.
The researchers evaluated the discharge records of nearly 500,000 adults who underwent either pneumonectomy, segmentectomy, lobar resections, or nonanatomic resections in an all-payer inpatient database for the time period between 2003 and 2009, according to Dr. Bhamidipati.
The hospital's teaching status as used in the study was determined by a linkage to the Association of American Medical Colleges’ Graduate Medical Education Tracking System.
The researchers examined patient demographics, risk factors, and hospital characteristics, and used multiple logistic regression models to examine in-hospital mortality, occurrence of any complications, and failure to rescue (death following a complication).
The mean annual percent of pulmonary resection volume among hospitals was CT (16%), GS (17%), NS (28%), and NT (39%).
The average age of pulmonary resection recipients among hospitals was similar, as were their mean number of comorbidities. The CT hospitals treated the smallest number of Medicare patients and the highest number of patients with Medicaid, a significant difference, said Dr. Bhamidipati.
He reported that the unadjusted mortality for all procedures was significantly lowest at the CT hospitals (CT: 2.6%; GS: 2.8%; NS: 3.4%; NT 3.6%).
Similarly, any complication was also least likely to occur at a CT hospital (CT: 20.5%, GS: 23.5%, NS: 24.6%, NT: 24.9%), he added.
Unadjusted procedural complications were found to be similar across hospitals, although pulmonary complications were significantly less likely to occur at CT hospitals compared with the others, according to Dr. Bhamidipati.
Following case-mix adjustment, the risk of having any complication after segmentectomy or nonanatomic resection was found to be significantly lower at CT hospitals than at the GS hospitals, he said.
In addition, among the pneumonectomy recipients, CT teaching status independently and significantly reduced the adjusted odds ratio (AOR) of failure to rescue by more than 25% compared with NS (AOR 0.34 vs. AOR 0.62).
Similarly, for the pneumonectomy patients, performance of the surgery at CT centers significantly lowered the AOR of death by more than 30% compared with GS hospitals (AOR 0.33 vs. AOR 0.69).
"These results support using CT hospital teaching status as an independent prognosticator of outcomes in pulmonary resections," concluded Dr. Bhamidipati.
Dr. Bhamidipati reported that he and his colleagues had no relevant disclosures.
SAN FRANCISCO - Pulmonary resection outcomes vary with respect to the kind of hospital in which the procedure is performed, particularly whether it is a teaching hospital, according to Dr. Castigliano M. Bhamidipati, of the University of Virginia Medical School, Charlottesville.
"In comparison to other hospitals, including general surgery teaching hospitals, cardiothoracic surgery teaching hospitals have lower morbidity and mortality," Dr. Bhamidipati said at the AATS annual meeting.
He and his colleagues examined the results of pulmonary resections performed at cardiothoracic (CT) teaching, general surgery (GS) teaching, nonsurgical (NS) teaching, and nonteaching (NT) hospitals.
The researchers evaluated the discharge records of nearly 500,000 adults who underwent either pneumonectomy, segmentectomy, lobar resections, or nonanatomic resections in an all-payer inpatient database for the time period between 2003 and 2009, according to Dr. Bhamidipati.
The hospital's teaching status as used in the study was determined by a linkage to the Association of American Medical Colleges’ Graduate Medical Education Tracking System.
The researchers examined patient demographics, risk factors, and hospital characteristics, and used multiple logistic regression models to examine in-hospital mortality, occurrence of any complications, and failure to rescue (death following a complication).
The mean annual percent of pulmonary resection volume among hospitals was CT (16%), GS (17%), NS (28%), and NT (39%).
The average age of pulmonary resection recipients among hospitals was similar, as were their mean number of comorbidities. The CT hospitals treated the smallest number of Medicare patients and the highest number of patients with Medicaid, a significant difference, said Dr. Bhamidipati.
He reported that the unadjusted mortality for all procedures was significantly lowest at the CT hospitals (CT: 2.6%; GS: 2.8%; NS: 3.4%; NT 3.6%).
Similarly, any complication was also least likely to occur at a CT hospital (CT: 20.5%, GS: 23.5%, NS: 24.6%, NT: 24.9%), he added.
Unadjusted procedural complications were found to be similar across hospitals, although pulmonary complications were significantly less likely to occur at CT hospitals compared with the others, according to Dr. Bhamidipati.
Following case-mix adjustment, the risk of having any complication after segmentectomy or nonanatomic resection was found to be significantly lower at CT hospitals than at the GS hospitals, he said.
In addition, among the pneumonectomy recipients, CT teaching status independently and significantly reduced the adjusted odds ratio (AOR) of failure to rescue by more than 25% compared with NS (AOR 0.34 vs. AOR 0.62).
Similarly, for the pneumonectomy patients, performance of the surgery at CT centers significantly lowered the AOR of death by more than 30% compared with GS hospitals (AOR 0.33 vs. AOR 0.69).
"These results support using CT hospital teaching status as an independent prognosticator of outcomes in pulmonary resections," concluded Dr. Bhamidipati.
Dr. Bhamidipati reported that he and his colleagues had no relevant disclosures.
SAN FRANCISCO - Pulmonary resection outcomes vary with respect to the kind of hospital in which the procedure is performed, particularly whether it is a teaching hospital, according to Dr. Castigliano M. Bhamidipati, of the University of Virginia Medical School, Charlottesville.
"In comparison to other hospitals, including general surgery teaching hospitals, cardiothoracic surgery teaching hospitals have lower morbidity and mortality," Dr. Bhamidipati said at the AATS annual meeting.
He and his colleagues examined the results of pulmonary resections performed at cardiothoracic (CT) teaching, general surgery (GS) teaching, nonsurgical (NS) teaching, and nonteaching (NT) hospitals.
The researchers evaluated the discharge records of nearly 500,000 adults who underwent either pneumonectomy, segmentectomy, lobar resections, or nonanatomic resections in an all-payer inpatient database for the time period between 2003 and 2009, according to Dr. Bhamidipati.
The hospital's teaching status as used in the study was determined by a linkage to the Association of American Medical Colleges’ Graduate Medical Education Tracking System.
The researchers examined patient demographics, risk factors, and hospital characteristics, and used multiple logistic regression models to examine in-hospital mortality, occurrence of any complications, and failure to rescue (death following a complication).
The mean annual percent of pulmonary resection volume among hospitals was CT (16%), GS (17%), NS (28%), and NT (39%).
The average age of pulmonary resection recipients among hospitals was similar, as were their mean number of comorbidities. The CT hospitals treated the smallest number of Medicare patients and the highest number of patients with Medicaid, a significant difference, said Dr. Bhamidipati.
He reported that the unadjusted mortality for all procedures was significantly lowest at the CT hospitals (CT: 2.6%; GS: 2.8%; NS: 3.4%; NT 3.6%).
Similarly, any complication was also least likely to occur at a CT hospital (CT: 20.5%, GS: 23.5%, NS: 24.6%, NT: 24.9%), he added.
Unadjusted procedural complications were found to be similar across hospitals, although pulmonary complications were significantly less likely to occur at CT hospitals compared with the others, according to Dr. Bhamidipati.
Following case-mix adjustment, the risk of having any complication after segmentectomy or nonanatomic resection was found to be significantly lower at CT hospitals than at the GS hospitals, he said.
In addition, among the pneumonectomy recipients, CT teaching status independently and significantly reduced the adjusted odds ratio (AOR) of failure to rescue by more than 25% compared with NS (AOR 0.34 vs. AOR 0.62).
Similarly, for the pneumonectomy patients, performance of the surgery at CT centers significantly lowered the AOR of death by more than 30% compared with GS hospitals (AOR 0.33 vs. AOR 0.69).
"These results support using CT hospital teaching status as an independent prognosticator of outcomes in pulmonary resections," concluded Dr. Bhamidipati.
Dr. Bhamidipati reported that he and his colleagues had no relevant disclosures.
NCCN Affirms CT Scans For Heavy Smokers
The benefits of routine lung cancer screening in high-risk individuals outweigh the potential risks, according to members of a National Comprehensive Cancer Network guidelines panel that recommended low-dose helical CT screening of two high-risk groups.
Mary E. Reid, Ph.D., of the Roswell Park Cancer Institute in Buffalo, N.Y., acknowledged the burdens – in particular, the cost and requisite resource utilization – associated with following all high-risk patients who screen positive. But, she said, "the evidence [in favor of] the recommendations is really strong and supports their implementation."
Lung cancer, she noted, is the only one of the top four deadliest cancers (lung, prostate, breast, and colorectal) that is not currently subject to routine screening.
Dr. Reid and colleagues on the National Comprehensive Cancer Network (NCCN) Guidelines Panel for Lung Cancer Screening presented the update at the NCCN annual conference March 14-18 in Hollywood, Fla. It had been issued in October 2011 and followed a New England Journal of Medicine report that low-dose CT screening of heavy smokers reduced lung cancer mortality by 20%, compared with annual chest x-rays, in the National Lung Screening Trial (NLST).
The revised guidelines recommend annual low-dose helical CT screening for the following two groups of high-risk individuals:
• Those aged 55-74 years with a minimum smoking history of 30 pack-years who either are current smokers or quit within the past 15 years.
• Those aged 50 years or older with a minimum smoking history of 20 pack-years plus one additional lung cancer risk factor, excluding secondhand exposure.
Evidence from the randomized, controlled NLST suggests that early detection via screening reduced lung-cancer specific mortality in the former risk group, which characterizes the NLST patient population. Specifically, 1 in 100 high-risk individuals who were enrolled in the study screened positive on their first low-dose CT exam, and one life was saved for every 320 high-risk individuals screened over 2 years (three screens) (N. Engl. J. Med. 2011;365:395-409). The NCCN recommendation for this group is category 1, the highest level.
The recommendation for annual screening in the second high-risk group is based on less-robust evidence and a nonuniform consensus of the NCCN panel members, Dr. Reid said. As such, it is a less-emphatic category 2B recommendation.
The NCCN screening recommendations have been deemed by some experts to be premature in the absence of cost-efficacy analysis, particularly because of the high false-positive rates observed in both the CT group (96.4%) and the radiography group (94.5%), as well as the potentially harmful effects of radiation exposure associated with low-dose CT screening.
Despite the favorable outcome of their study, the NLST authors stressed the need for rigorous cost-effectiveness analyses before the crafting of public policy recommendations. "The reductions in lung-cancer mortality must be weighed against the harms from positive screening results and overdiagnosis, as well as the costs," they wrote. "The cost component of low-dose CT screening includes not only the screening examination itself but also the diagnostic follow-up and treatment."
In addition to recommending appropriate candidates for routine screening and the proposed frequency of the scans, the new NCCN guidelines outline lung cancer risk factors, address the risks and benefits of screening as well as screening accuracy, and offer an algorithm for the evaluation and follow-up of positive screens. Specifically, the guidelines recommend the following:
• Basing the frequency of low-dose CT in high-risk patients on the size and status (solid, nonsolid, part-solid, ground-glass, ground-glass opacity) of the nodule on baseline CT.
• Excising all nodules that increase in size or become solid or part-solid during follow-up.
• Considering PET with CT for nodules 8 mm or larger at baseline.
• Performing biopsy or excision of nodules that are suspicious for lung cancer, based on PET with CT findings.
• Reexamining within 1 month solid endobronchial nodules with low-dose CT immediately after vigorous coughing.
• Counseling smokers to quit.
The NCCN is the first professional organization to recommend routine low-dose CT screening for individuals who are considered to be at high-risk for lung cancer, according to Dr. Reid. Last summer, the International Association for the Study of Lung Cancer issued a call for physicians to discuss lung cancer screening with patients who match the high-risk smoking history of those enrolled in the NSLT.
Dr. Reid reported having no financial conflicts of interest.
The benefits of routine lung cancer screening in high-risk individuals outweigh the potential risks, according to members of a National Comprehensive Cancer Network guidelines panel that recommended low-dose helical CT screening of two high-risk groups.
Mary E. Reid, Ph.D., of the Roswell Park Cancer Institute in Buffalo, N.Y., acknowledged the burdens – in particular, the cost and requisite resource utilization – associated with following all high-risk patients who screen positive. But, she said, "the evidence [in favor of] the recommendations is really strong and supports their implementation."
Lung cancer, she noted, is the only one of the top four deadliest cancers (lung, prostate, breast, and colorectal) that is not currently subject to routine screening.
Dr. Reid and colleagues on the National Comprehensive Cancer Network (NCCN) Guidelines Panel for Lung Cancer Screening presented the update at the NCCN annual conference March 14-18 in Hollywood, Fla. It had been issued in October 2011 and followed a New England Journal of Medicine report that low-dose CT screening of heavy smokers reduced lung cancer mortality by 20%, compared with annual chest x-rays, in the National Lung Screening Trial (NLST).
The revised guidelines recommend annual low-dose helical CT screening for the following two groups of high-risk individuals:
• Those aged 55-74 years with a minimum smoking history of 30 pack-years who either are current smokers or quit within the past 15 years.
• Those aged 50 years or older with a minimum smoking history of 20 pack-years plus one additional lung cancer risk factor, excluding secondhand exposure.
Evidence from the randomized, controlled NLST suggests that early detection via screening reduced lung-cancer specific mortality in the former risk group, which characterizes the NLST patient population. Specifically, 1 in 100 high-risk individuals who were enrolled in the study screened positive on their first low-dose CT exam, and one life was saved for every 320 high-risk individuals screened over 2 years (three screens) (N. Engl. J. Med. 2011;365:395-409). The NCCN recommendation for this group is category 1, the highest level.
The recommendation for annual screening in the second high-risk group is based on less-robust evidence and a nonuniform consensus of the NCCN panel members, Dr. Reid said. As such, it is a less-emphatic category 2B recommendation.
The NCCN screening recommendations have been deemed by some experts to be premature in the absence of cost-efficacy analysis, particularly because of the high false-positive rates observed in both the CT group (96.4%) and the radiography group (94.5%), as well as the potentially harmful effects of radiation exposure associated with low-dose CT screening.
Despite the favorable outcome of their study, the NLST authors stressed the need for rigorous cost-effectiveness analyses before the crafting of public policy recommendations. "The reductions in lung-cancer mortality must be weighed against the harms from positive screening results and overdiagnosis, as well as the costs," they wrote. "The cost component of low-dose CT screening includes not only the screening examination itself but also the diagnostic follow-up and treatment."
In addition to recommending appropriate candidates for routine screening and the proposed frequency of the scans, the new NCCN guidelines outline lung cancer risk factors, address the risks and benefits of screening as well as screening accuracy, and offer an algorithm for the evaluation and follow-up of positive screens. Specifically, the guidelines recommend the following:
• Basing the frequency of low-dose CT in high-risk patients on the size and status (solid, nonsolid, part-solid, ground-glass, ground-glass opacity) of the nodule on baseline CT.
• Excising all nodules that increase in size or become solid or part-solid during follow-up.
• Considering PET with CT for nodules 8 mm or larger at baseline.
• Performing biopsy or excision of nodules that are suspicious for lung cancer, based on PET with CT findings.
• Reexamining within 1 month solid endobronchial nodules with low-dose CT immediately after vigorous coughing.
• Counseling smokers to quit.
The NCCN is the first professional organization to recommend routine low-dose CT screening for individuals who are considered to be at high-risk for lung cancer, according to Dr. Reid. Last summer, the International Association for the Study of Lung Cancer issued a call for physicians to discuss lung cancer screening with patients who match the high-risk smoking history of those enrolled in the NSLT.
Dr. Reid reported having no financial conflicts of interest.
The benefits of routine lung cancer screening in high-risk individuals outweigh the potential risks, according to members of a National Comprehensive Cancer Network guidelines panel that recommended low-dose helical CT screening of two high-risk groups.
Mary E. Reid, Ph.D., of the Roswell Park Cancer Institute in Buffalo, N.Y., acknowledged the burdens – in particular, the cost and requisite resource utilization – associated with following all high-risk patients who screen positive. But, she said, "the evidence [in favor of] the recommendations is really strong and supports their implementation."
Lung cancer, she noted, is the only one of the top four deadliest cancers (lung, prostate, breast, and colorectal) that is not currently subject to routine screening.
Dr. Reid and colleagues on the National Comprehensive Cancer Network (NCCN) Guidelines Panel for Lung Cancer Screening presented the update at the NCCN annual conference March 14-18 in Hollywood, Fla. It had been issued in October 2011 and followed a New England Journal of Medicine report that low-dose CT screening of heavy smokers reduced lung cancer mortality by 20%, compared with annual chest x-rays, in the National Lung Screening Trial (NLST).
The revised guidelines recommend annual low-dose helical CT screening for the following two groups of high-risk individuals:
• Those aged 55-74 years with a minimum smoking history of 30 pack-years who either are current smokers or quit within the past 15 years.
• Those aged 50 years or older with a minimum smoking history of 20 pack-years plus one additional lung cancer risk factor, excluding secondhand exposure.
Evidence from the randomized, controlled NLST suggests that early detection via screening reduced lung-cancer specific mortality in the former risk group, which characterizes the NLST patient population. Specifically, 1 in 100 high-risk individuals who were enrolled in the study screened positive on their first low-dose CT exam, and one life was saved for every 320 high-risk individuals screened over 2 years (three screens) (N. Engl. J. Med. 2011;365:395-409). The NCCN recommendation for this group is category 1, the highest level.
The recommendation for annual screening in the second high-risk group is based on less-robust evidence and a nonuniform consensus of the NCCN panel members, Dr. Reid said. As such, it is a less-emphatic category 2B recommendation.
The NCCN screening recommendations have been deemed by some experts to be premature in the absence of cost-efficacy analysis, particularly because of the high false-positive rates observed in both the CT group (96.4%) and the radiography group (94.5%), as well as the potentially harmful effects of radiation exposure associated with low-dose CT screening.
Despite the favorable outcome of their study, the NLST authors stressed the need for rigorous cost-effectiveness analyses before the crafting of public policy recommendations. "The reductions in lung-cancer mortality must be weighed against the harms from positive screening results and overdiagnosis, as well as the costs," they wrote. "The cost component of low-dose CT screening includes not only the screening examination itself but also the diagnostic follow-up and treatment."
In addition to recommending appropriate candidates for routine screening and the proposed frequency of the scans, the new NCCN guidelines outline lung cancer risk factors, address the risks and benefits of screening as well as screening accuracy, and offer an algorithm for the evaluation and follow-up of positive screens. Specifically, the guidelines recommend the following:
• Basing the frequency of low-dose CT in high-risk patients on the size and status (solid, nonsolid, part-solid, ground-glass, ground-glass opacity) of the nodule on baseline CT.
• Excising all nodules that increase in size or become solid or part-solid during follow-up.
• Considering PET with CT for nodules 8 mm or larger at baseline.
• Performing biopsy or excision of nodules that are suspicious for lung cancer, based on PET with CT findings.
• Reexamining within 1 month solid endobronchial nodules with low-dose CT immediately after vigorous coughing.
• Counseling smokers to quit.
The NCCN is the first professional organization to recommend routine low-dose CT screening for individuals who are considered to be at high-risk for lung cancer, according to Dr. Reid. Last summer, the International Association for the Study of Lung Cancer issued a call for physicians to discuss lung cancer screening with patients who match the high-risk smoking history of those enrolled in the NSLT.
Dr. Reid reported having no financial conflicts of interest.
NCCN Affirms CT Scans For Heavy Smokers
The benefits of routine lung cancer screening in high-risk individuals outweigh the potential risks, according to members of a National Comprehensive Cancer Network guidelines panel that recommended low-dose helical CT screening of two high-risk groups.
Mary E. Reid, Ph.D., of the Roswell Park Cancer Institute in Buffalo, N.Y., acknowledged the burdens – in particular, the cost and requisite resource utilization – associated with following all high-risk patients who screen positive. But, she said, "the evidence [in favor of] the recommendations is really strong and supports their implementation."
Lung cancer, she noted, is the only one of the top four deadliest cancers (lung, prostate, breast, and colorectal) that is not currently subject to routine screening.
Dr. Reid and colleagues on the National Comprehensive Cancer Network (NCCN) Guidelines Panel for Lung Cancer Screening presented the update at the NCCN annual conference March 14-18 in Hollywood, Fla. It had been issued in October 2011 and followed a New England Journal of Medicine report that low-dose CT screening of heavy smokers reduced lung cancer mortality by 20%, compared with annual chest x-rays, in the National Lung Screening Trial (NLST).
The revised guidelines recommend annual low-dose helical CT screening for the following two groups of high-risk individuals:
• Those aged 55-74 years with a minimum smoking history of 30 pack-years who either are current smokers or quit within the past 15 years.
• Those aged 50 years or older with a minimum smoking history of 20 pack-years plus one additional lung cancer risk factor, excluding secondhand exposure.
Evidence from the randomized, controlled NLST suggests that early detection via screening reduced lung-cancer specific mortality in the former risk group, which characterizes the NLST patient population. Specifically, 1 in 100 high-risk individuals who were enrolled in the study screened positive on their first low-dose CT exam, and one life was saved for every 320 high-risk individuals screened over 2 years (three screens) (N. Engl. J. Med. 2011;365:395-409). The NCCN recommendation for this group is category 1, the highest level.
The recommendation for annual screening in the second high-risk group is based on less-robust evidence and a nonuniform consensus of the NCCN panel members, Dr. Reid said. As such, it is a less-emphatic category 2B recommendation.
The NCCN screening recommendations have been deemed by some experts to be premature in the absence of cost-efficacy analysis, particularly because of the high false-positive rates observed in both the CT group (96.4%) and the radiography group (94.5%), as well as the potentially harmful effects of radiation exposure associated with low-dose CT screening.
Despite the favorable outcome of their study, the NLST authors stressed the need for rigorous cost-effectiveness analyses before the crafting of public policy recommendations. "The reductions in lung-cancer mortality must be weighed against the harms from positive screening results and overdiagnosis, as well as the costs," they wrote. "The cost component of low-dose CT screening includes not only the screening examination itself but also the diagnostic follow-up and treatment."
In addition to recommending appropriate candidates for routine screening and the proposed frequency of the scans, the new NCCN guidelines outline lung cancer risk factors, address the risks and benefits of screening as well as screening accuracy, and offer an algorithm for the evaluation and follow-up of positive screens. Specifically, the guidelines recommend the following:
• Basing the frequency of low-dose CT in high-risk patients on the size and status (solid, nonsolid, part-solid, ground-glass, ground-glass opacity) of the nodule on baseline CT.
• Excising all nodules that increase in size or become solid or part-solid during follow-up.
• Considering PET with CT for nodules 8 mm or larger at baseline.
• Performing biopsy or excision of nodules that are suspicious for lung cancer, based on PET with CT findings.
• Reexamining within 1 month solid endobronchial nodules with low-dose CT immediately after vigorous coughing.
• Counseling smokers to quit.
The NCCN is the first professional organization to recommend routine low-dose CT screening for individuals who are considered to be at high-risk for lung cancer, according to Dr. Reid. Last summer, the International Association for the Study of Lung Cancer issued a call for physicians to discuss lung cancer screening with patients who match the high-risk smoking history of those enrolled in the NSLT.
Dr. Reid reported having no financial conflicts of interest.
The benefits of routine lung cancer screening in high-risk individuals outweigh the potential risks, according to members of a National Comprehensive Cancer Network guidelines panel that recommended low-dose helical CT screening of two high-risk groups.
Mary E. Reid, Ph.D., of the Roswell Park Cancer Institute in Buffalo, N.Y., acknowledged the burdens – in particular, the cost and requisite resource utilization – associated with following all high-risk patients who screen positive. But, she said, "the evidence [in favor of] the recommendations is really strong and supports their implementation."
Lung cancer, she noted, is the only one of the top four deadliest cancers (lung, prostate, breast, and colorectal) that is not currently subject to routine screening.
Dr. Reid and colleagues on the National Comprehensive Cancer Network (NCCN) Guidelines Panel for Lung Cancer Screening presented the update at the NCCN annual conference March 14-18 in Hollywood, Fla. It had been issued in October 2011 and followed a New England Journal of Medicine report that low-dose CT screening of heavy smokers reduced lung cancer mortality by 20%, compared with annual chest x-rays, in the National Lung Screening Trial (NLST).
The revised guidelines recommend annual low-dose helical CT screening for the following two groups of high-risk individuals:
• Those aged 55-74 years with a minimum smoking history of 30 pack-years who either are current smokers or quit within the past 15 years.
• Those aged 50 years or older with a minimum smoking history of 20 pack-years plus one additional lung cancer risk factor, excluding secondhand exposure.
Evidence from the randomized, controlled NLST suggests that early detection via screening reduced lung-cancer specific mortality in the former risk group, which characterizes the NLST patient population. Specifically, 1 in 100 high-risk individuals who were enrolled in the study screened positive on their first low-dose CT exam, and one life was saved for every 320 high-risk individuals screened over 2 years (three screens) (N. Engl. J. Med. 2011;365:395-409). The NCCN recommendation for this group is category 1, the highest level.
The recommendation for annual screening in the second high-risk group is based on less-robust evidence and a nonuniform consensus of the NCCN panel members, Dr. Reid said. As such, it is a less-emphatic category 2B recommendation.
The NCCN screening recommendations have been deemed by some experts to be premature in the absence of cost-efficacy analysis, particularly because of the high false-positive rates observed in both the CT group (96.4%) and the radiography group (94.5%), as well as the potentially harmful effects of radiation exposure associated with low-dose CT screening.
Despite the favorable outcome of their study, the NLST authors stressed the need for rigorous cost-effectiveness analyses before the crafting of public policy recommendations. "The reductions in lung-cancer mortality must be weighed against the harms from positive screening results and overdiagnosis, as well as the costs," they wrote. "The cost component of low-dose CT screening includes not only the screening examination itself but also the diagnostic follow-up and treatment."
In addition to recommending appropriate candidates for routine screening and the proposed frequency of the scans, the new NCCN guidelines outline lung cancer risk factors, address the risks and benefits of screening as well as screening accuracy, and offer an algorithm for the evaluation and follow-up of positive screens. Specifically, the guidelines recommend the following:
• Basing the frequency of low-dose CT in high-risk patients on the size and status (solid, nonsolid, part-solid, ground-glass, ground-glass opacity) of the nodule on baseline CT.
• Excising all nodules that increase in size or become solid or part-solid during follow-up.
• Considering PET with CT for nodules 8 mm or larger at baseline.
• Performing biopsy or excision of nodules that are suspicious for lung cancer, based on PET with CT findings.
• Reexamining within 1 month solid endobronchial nodules with low-dose CT immediately after vigorous coughing.
• Counseling smokers to quit.
The NCCN is the first professional organization to recommend routine low-dose CT screening for individuals who are considered to be at high-risk for lung cancer, according to Dr. Reid. Last summer, the International Association for the Study of Lung Cancer issued a call for physicians to discuss lung cancer screening with patients who match the high-risk smoking history of those enrolled in the NSLT.
Dr. Reid reported having no financial conflicts of interest.
The benefits of routine lung cancer screening in high-risk individuals outweigh the potential risks, according to members of a National Comprehensive Cancer Network guidelines panel that recommended low-dose helical CT screening of two high-risk groups.
Mary E. Reid, Ph.D., of the Roswell Park Cancer Institute in Buffalo, N.Y., acknowledged the burdens – in particular, the cost and requisite resource utilization – associated with following all high-risk patients who screen positive. But, she said, "the evidence [in favor of] the recommendations is really strong and supports their implementation."
Lung cancer, she noted, is the only one of the top four deadliest cancers (lung, prostate, breast, and colorectal) that is not currently subject to routine screening.
Dr. Reid and colleagues on the National Comprehensive Cancer Network (NCCN) Guidelines Panel for Lung Cancer Screening presented the update at the NCCN annual conference March 14-18 in Hollywood, Fla. It had been issued in October 2011 and followed a New England Journal of Medicine report that low-dose CT screening of heavy smokers reduced lung cancer mortality by 20%, compared with annual chest x-rays, in the National Lung Screening Trial (NLST).
The revised guidelines recommend annual low-dose helical CT screening for the following two groups of high-risk individuals:
• Those aged 55-74 years with a minimum smoking history of 30 pack-years who either are current smokers or quit within the past 15 years.
• Those aged 50 years or older with a minimum smoking history of 20 pack-years plus one additional lung cancer risk factor, excluding secondhand exposure.
Evidence from the randomized, controlled NLST suggests that early detection via screening reduced lung-cancer specific mortality in the former risk group, which characterizes the NLST patient population. Specifically, 1 in 100 high-risk individuals who were enrolled in the study screened positive on their first low-dose CT exam, and one life was saved for every 320 high-risk individuals screened over 2 years (three screens) (N. Engl. J. Med. 2011;365:395-409). The NCCN recommendation for this group is category 1, the highest level.
The recommendation for annual screening in the second high-risk group is based on less-robust evidence and a nonuniform consensus of the NCCN panel members, Dr. Reid said. As such, it is a less-emphatic category 2B recommendation.
The NCCN screening recommendations have been deemed by some experts to be premature in the absence of cost-efficacy analysis, particularly because of the high false-positive rates observed in both the CT group (96.4%) and the radiography group (94.5%), as well as the potentially harmful effects of radiation exposure associated with low-dose CT screening.
Despite the favorable outcome of their study, the NLST authors stressed the need for rigorous cost-effectiveness analyses before the crafting of public policy recommendations. "The reductions in lung-cancer mortality must be weighed against the harms from positive screening results and overdiagnosis, as well as the costs," they wrote. "The cost component of low-dose CT screening includes not only the screening examination itself but also the diagnostic follow-up and treatment."
In addition to recommending appropriate candidates for routine screening and the proposed frequency of the scans, the new NCCN guidelines outline lung cancer risk factors, address the risks and benefits of screening as well as screening accuracy, and offer an algorithm for the evaluation and follow-up of positive screens. Specifically, the guidelines recommend the following:
• Basing the frequency of low-dose CT in high-risk patients on the size and status (solid, nonsolid, part-solid, ground-glass, ground-glass opacity) of the nodule on baseline CT.
• Excising all nodules that increase in size or become solid or part-solid during follow-up.
• Considering PET with CT for nodules 8 mm or larger at baseline.
• Performing biopsy or excision of nodules that are suspicious for lung cancer, based on PET with CT findings.
• Reexamining within 1 month solid endobronchial nodules with low-dose CT immediately after vigorous coughing.
• Counseling smokers to quit.
The NCCN is the first professional organization to recommend routine low-dose CT screening for individuals who are considered to be at high-risk for lung cancer, according to Dr. Reid. Last summer, the International Association for the Study of Lung Cancer issued a call for physicians to discuss lung cancer screening with patients who match the high-risk smoking history of those enrolled in the NSLT.
Dr. Reid reported having no financial conflicts of interest.
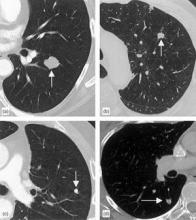
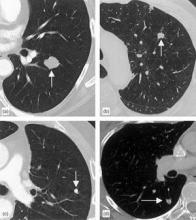
10 Biomarkers May Aid Lung Cancer Detection
A panel of 10 serum biomarkers for lung cancer could offer more accurate interpretation of nodules detected on computed tomography, avoiding invasive biopsies and radiographic follow-up.
"CT-screening detection of an indeterminate pulmonary nodule, a nonspecific but frequent finding in high-risk subjects with a smoking history, creates a diagnostic dilemma," wrote investigator William L. Bigbee, Ph.D., and his colleagues in the April issue of the Journal of Thoracic Oncology.
"Although the biomarker model we described could not detect every lung cancer, it offers a significant clinical improvement over CT imaging alone.... Also, patients with nodules not identified as cancer by the model would continue to receive follow-up clinical monitoring and would be biopsied if the nodules grew in size, which is the current standard of care," the researchers said (J. Thorac. Oncol. 2012;7:698-708).
Dr. Bigbee of the University of Pittsburgh and his colleagues cite results of the National Lung Screening Trial (NLST), published in June 2011, which showed for the first time that low-dose CT screening of heavy smokers could reduce lung cancer mortality by 20%. But, as Dr. Bigbee et al. note in the current study, the "vast majority" of positive results in the NLST program turned out to be false after diagnostic evaluation. Moreover, smaller nodules are least likely to be malignant and least likely to be considered for biopsy or surgery.
For the current study, the researchers initially looked at a "training" set of 56 patients with non–small cell lung cancer in the University of Pittsburgh Cancer Institute Georgia Cooper Lung Research Registry. These cases were matched with 56 controls from the Pittsburgh Lung Screening Study (PLuSS), a volunteer cohort at high risk for lung cancer. All controls were known to be cancer free. The authors then analyzed serum samples from both groups for the presence of 70 potential cancer-associated biomarkers.
"Together, these biomarkers incorporate a wide range of host and tumor derived factors that allow a broad analysis of the lung cancer/host interaction, and includes a number of previously described epithelial cell cancer-associated serological markers," wrote the investigators. "The initial goal of this discovery study was to identify the most robust subset of these biomarkers to discriminate lung cancer and matched control samples."
Using a rule-learning algorithm, they whittled the field of potential biomarkers down to eight: prolactin, transthyretin, E-selectin, C-C motif, thrombospondin-1, chemokine 5, macrophage migration inhibitory factor, plasminogen activator inhibitor 1, and receptor tyrosine-protein kinase erbB-2.
"This rule model distinguished the lung cancer case samples from the control samples in the training set with a sensitivity of 92.9% and specificity of 87.5%," they reported.
Ultimately, two additional biomarkers were added to the panel – cytokeratin fragment 19-9 and serum amyloid A protein – and an additional set of cases and controls, 30 in each cohort, was assessed, in a blinded "verification" set.
In this set, the authors calculated an overall classification performance of 73.3% sensitivity and 93.3% specificity. Only 10 misclassifications occurred among 60 predictions made. Moreover, when looking at accuracy according to patient demographic factors, the researchers found that the 10-biomarker panel was equally good at distinguishing males and females as either cases or controls and that neither current smoking status nor airway obstruction skewed the results.
Nor did the presence of nodules visible on CT scan confound the biomarkers’ predictive value. "In fact, those PLuSS subjects with a suspicious nodule were more often correctly classified as controls than those with no nodule or a benign nodule," wrote the authors.
They add that all nodules found in controls remained clinically noncancerous at least 3 years after initial detection, with either resolution or no further growth on subsequent CT scans.
Finally, Dr. Bigbee assessed the model’s accuracy with early- vs. late-stage tumors.
"Among stage I/II lung tumors, the 10-biomarker panel misclassified 15% of stage I/II tumors in the verification set, compared to 50% of the stage III/IV tumors, suggesting the model performs well in discriminating early-stage lung cancer," he wrote. "With a specificity of 93.3%, the 10-biomarker model [balanced accuracy] was 89.2% in stage I/II disease."
The authors conceded that the biomarker panel presented here would not suffice for general population screening. However, in a clinical context, among high-risk patients, the model "may provide clinical utility in guiding interpretation of screening CT scans, even in tobacco-exposed persons with COPD or emphysema," they wrote.
"Formal validation in larger patient cohorts will be needed to confirm these initial findings."
The authors disclosed that funding for this study was supplied by grants from the National Cancer Institute. Dr. Bigbee stated that there were no personal disclosures.
A panel of 10 serum biomarkers for lung cancer could offer more accurate interpretation of nodules detected on computed tomography, avoiding invasive biopsies and radiographic follow-up.
"CT-screening detection of an indeterminate pulmonary nodule, a nonspecific but frequent finding in high-risk subjects with a smoking history, creates a diagnostic dilemma," wrote investigator William L. Bigbee, Ph.D., and his colleagues in the April issue of the Journal of Thoracic Oncology.
"Although the biomarker model we described could not detect every lung cancer, it offers a significant clinical improvement over CT imaging alone.... Also, patients with nodules not identified as cancer by the model would continue to receive follow-up clinical monitoring and would be biopsied if the nodules grew in size, which is the current standard of care," the researchers said (J. Thorac. Oncol. 2012;7:698-708).
Dr. Bigbee of the University of Pittsburgh and his colleagues cite results of the National Lung Screening Trial (NLST), published in June 2011, which showed for the first time that low-dose CT screening of heavy smokers could reduce lung cancer mortality by 20%. But, as Dr. Bigbee et al. note in the current study, the "vast majority" of positive results in the NLST program turned out to be false after diagnostic evaluation. Moreover, smaller nodules are least likely to be malignant and least likely to be considered for biopsy or surgery.
For the current study, the researchers initially looked at a "training" set of 56 patients with non–small cell lung cancer in the University of Pittsburgh Cancer Institute Georgia Cooper Lung Research Registry. These cases were matched with 56 controls from the Pittsburgh Lung Screening Study (PLuSS), a volunteer cohort at high risk for lung cancer. All controls were known to be cancer free. The authors then analyzed serum samples from both groups for the presence of 70 potential cancer-associated biomarkers.
"Together, these biomarkers incorporate a wide range of host and tumor derived factors that allow a broad analysis of the lung cancer/host interaction, and includes a number of previously described epithelial cell cancer-associated serological markers," wrote the investigators. "The initial goal of this discovery study was to identify the most robust subset of these biomarkers to discriminate lung cancer and matched control samples."
Using a rule-learning algorithm, they whittled the field of potential biomarkers down to eight: prolactin, transthyretin, E-selectin, C-C motif, thrombospondin-1, chemokine 5, macrophage migration inhibitory factor, plasminogen activator inhibitor 1, and receptor tyrosine-protein kinase erbB-2.
"This rule model distinguished the lung cancer case samples from the control samples in the training set with a sensitivity of 92.9% and specificity of 87.5%," they reported.
Ultimately, two additional biomarkers were added to the panel – cytokeratin fragment 19-9 and serum amyloid A protein – and an additional set of cases and controls, 30 in each cohort, was assessed, in a blinded "verification" set.
In this set, the authors calculated an overall classification performance of 73.3% sensitivity and 93.3% specificity. Only 10 misclassifications occurred among 60 predictions made. Moreover, when looking at accuracy according to patient demographic factors, the researchers found that the 10-biomarker panel was equally good at distinguishing males and females as either cases or controls and that neither current smoking status nor airway obstruction skewed the results.
Nor did the presence of nodules visible on CT scan confound the biomarkers’ predictive value. "In fact, those PLuSS subjects with a suspicious nodule were more often correctly classified as controls than those with no nodule or a benign nodule," wrote the authors.
They add that all nodules found in controls remained clinically noncancerous at least 3 years after initial detection, with either resolution or no further growth on subsequent CT scans.
Finally, Dr. Bigbee assessed the model’s accuracy with early- vs. late-stage tumors.
"Among stage I/II lung tumors, the 10-biomarker panel misclassified 15% of stage I/II tumors in the verification set, compared to 50% of the stage III/IV tumors, suggesting the model performs well in discriminating early-stage lung cancer," he wrote. "With a specificity of 93.3%, the 10-biomarker model [balanced accuracy] was 89.2% in stage I/II disease."
The authors conceded that the biomarker panel presented here would not suffice for general population screening. However, in a clinical context, among high-risk patients, the model "may provide clinical utility in guiding interpretation of screening CT scans, even in tobacco-exposed persons with COPD or emphysema," they wrote.
"Formal validation in larger patient cohorts will be needed to confirm these initial findings."
The authors disclosed that funding for this study was supplied by grants from the National Cancer Institute. Dr. Bigbee stated that there were no personal disclosures.
A panel of 10 serum biomarkers for lung cancer could offer more accurate interpretation of nodules detected on computed tomography, avoiding invasive biopsies and radiographic follow-up.
"CT-screening detection of an indeterminate pulmonary nodule, a nonspecific but frequent finding in high-risk subjects with a smoking history, creates a diagnostic dilemma," wrote investigator William L. Bigbee, Ph.D., and his colleagues in the April issue of the Journal of Thoracic Oncology.
"Although the biomarker model we described could not detect every lung cancer, it offers a significant clinical improvement over CT imaging alone.... Also, patients with nodules not identified as cancer by the model would continue to receive follow-up clinical monitoring and would be biopsied if the nodules grew in size, which is the current standard of care," the researchers said (J. Thorac. Oncol. 2012;7:698-708).
Dr. Bigbee of the University of Pittsburgh and his colleagues cite results of the National Lung Screening Trial (NLST), published in June 2011, which showed for the first time that low-dose CT screening of heavy smokers could reduce lung cancer mortality by 20%. But, as Dr. Bigbee et al. note in the current study, the "vast majority" of positive results in the NLST program turned out to be false after diagnostic evaluation. Moreover, smaller nodules are least likely to be malignant and least likely to be considered for biopsy or surgery.
For the current study, the researchers initially looked at a "training" set of 56 patients with non–small cell lung cancer in the University of Pittsburgh Cancer Institute Georgia Cooper Lung Research Registry. These cases were matched with 56 controls from the Pittsburgh Lung Screening Study (PLuSS), a volunteer cohort at high risk for lung cancer. All controls were known to be cancer free. The authors then analyzed serum samples from both groups for the presence of 70 potential cancer-associated biomarkers.
"Together, these biomarkers incorporate a wide range of host and tumor derived factors that allow a broad analysis of the lung cancer/host interaction, and includes a number of previously described epithelial cell cancer-associated serological markers," wrote the investigators. "The initial goal of this discovery study was to identify the most robust subset of these biomarkers to discriminate lung cancer and matched control samples."
Using a rule-learning algorithm, they whittled the field of potential biomarkers down to eight: prolactin, transthyretin, E-selectin, C-C motif, thrombospondin-1, chemokine 5, macrophage migration inhibitory factor, plasminogen activator inhibitor 1, and receptor tyrosine-protein kinase erbB-2.
"This rule model distinguished the lung cancer case samples from the control samples in the training set with a sensitivity of 92.9% and specificity of 87.5%," they reported.
Ultimately, two additional biomarkers were added to the panel – cytokeratin fragment 19-9 and serum amyloid A protein – and an additional set of cases and controls, 30 in each cohort, was assessed, in a blinded "verification" set.
In this set, the authors calculated an overall classification performance of 73.3% sensitivity and 93.3% specificity. Only 10 misclassifications occurred among 60 predictions made. Moreover, when looking at accuracy according to patient demographic factors, the researchers found that the 10-biomarker panel was equally good at distinguishing males and females as either cases or controls and that neither current smoking status nor airway obstruction skewed the results.
Nor did the presence of nodules visible on CT scan confound the biomarkers’ predictive value. "In fact, those PLuSS subjects with a suspicious nodule were more often correctly classified as controls than those with no nodule or a benign nodule," wrote the authors.
They add that all nodules found in controls remained clinically noncancerous at least 3 years after initial detection, with either resolution or no further growth on subsequent CT scans.
Finally, Dr. Bigbee assessed the model’s accuracy with early- vs. late-stage tumors.
"Among stage I/II lung tumors, the 10-biomarker panel misclassified 15% of stage I/II tumors in the verification set, compared to 50% of the stage III/IV tumors, suggesting the model performs well in discriminating early-stage lung cancer," he wrote. "With a specificity of 93.3%, the 10-biomarker model [balanced accuracy] was 89.2% in stage I/II disease."
The authors conceded that the biomarker panel presented here would not suffice for general population screening. However, in a clinical context, among high-risk patients, the model "may provide clinical utility in guiding interpretation of screening CT scans, even in tobacco-exposed persons with COPD or emphysema," they wrote.
"Formal validation in larger patient cohorts will be needed to confirm these initial findings."
The authors disclosed that funding for this study was supplied by grants from the National Cancer Institute. Dr. Bigbee stated that there were no personal disclosures.
Major Finding: A panel of 10 serum biomarkers for lung cancer had a 73.3% sensitivity and 93.3% specificity in a blinded verification set, with the best performance in early, stage I/II cases.
Data Source: The study compared the panel in cases from the University of Pittsburgh Cancer Institute Georgia Cooper Lung Research Registry and controls from the Pittsburgh Lung Screening Study.
Disclosures: The authors disclosed that funding for this study was supplied by grants from the National Cancer Institute. Dr. Bigbee stated that there were no personal disclosures.
10 Biomarkers May Aid Lung Cancer Detection
A panel of 10 serum biomarkers for lung cancer could offer more accurate interpretation of nodules detected on computed tomography, avoiding invasive biopsies and radiographic follow-up.
"CT-screening detection of an indeterminate pulmonary nodule, a nonspecific but frequent finding in high-risk subjects with a smoking history, creates a diagnostic dilemma," wrote investigator William L. Bigbee, Ph.D., and his colleagues in the April issue of the Journal of Thoracic Oncology.
"Although the biomarker model we described could not detect every lung cancer, it offers a significant clinical improvement over CT imaging alone.... Also, patients with nodules not identified as cancer by the model would continue to receive follow-up clinical monitoring and would be biopsied if the nodules grew in size, which is the current standard of care," the researchers said (J. Thorac. Oncol. 2012;7:698-708).
Dr. Bigbee of the University of Pittsburgh and his colleagues cite results of the National Lung Screening Trial (NLST), published in June 2011, which showed for the first time that low-dose CT screening of heavy smokers could reduce lung cancer mortality by 20%. But, as Dr. Bigbee et al. note in the current study, the "vast majority" of positive results in the NLST program turned out to be false after diagnostic evaluation. Moreover, smaller nodules are least likely to be malignant and least likely to be considered for biopsy or surgery.
For the current study, the researchers initially looked at a "training" set of 56 patients with non–small cell lung cancer in the University of Pittsburgh Cancer Institute Georgia Cooper Lung Research Registry. These cases were matched with 56 controls from the Pittsburgh Lung Screening Study (PLuSS), a volunteer cohort at high risk for lung cancer. All controls were known to be cancer free. The authors then analyzed serum samples from both groups for the presence of 70 potential cancer-associated biomarkers.
"Together, these biomarkers incorporate a wide range of host and tumor derived factors that allow a broad analysis of the lung cancer/host interaction, and includes a number of previously described epithelial cell cancer-associated serological markers," wrote the investigators. "The initial goal of this discovery study was to identify the most robust subset of these biomarkers to discriminate lung cancer and matched control samples."
Using a rule-learning algorithm, they whittled the field of potential biomarkers down to eight: prolactin, transthyretin, E-selectin, C-C motif, thrombospondin-1, chemokine 5, macrophage migration inhibitory factor, plasminogen activator inhibitor 1, and receptor tyrosine-protein kinase erbB-2.
"This rule model distinguished the lung cancer case samples from the control samples in the training set with a sensitivity of 92.9% and specificity of 87.5%," they reported.
Ultimately, two additional biomarkers were added to the panel – cytokeratin fragment 19-9 and serum amyloid A protein – and an additional set of cases and controls, 30 in each cohort, was assessed, in a blinded "verification" set.
In this set, the authors calculated an overall classification performance of 73.3% sensitivity and 93.3% specificity. Only 10 misclassifications occurred among 60 predictions made. Moreover, when looking at accuracy according to patient demographic factors, the researchers found that the 10-biomarker panel was equally good at distinguishing males and females as either cases or controls and that neither current smoking status nor airway obstruction skewed the results.
Nor did the presence of nodules visible on CT scan confound the biomarkers’ predictive value. "In fact, those PLuSS subjects with a suspicious nodule were more often correctly classified as controls than those with no nodule or a benign nodule," wrote the authors.
They add that all nodules found in controls remained clinically noncancerous at least 3 years after initial detection, with either resolution or no further growth on subsequent CT scans.
Finally, Dr. Bigbee assessed the model’s accuracy with early- vs. late-stage tumors.
"Among stage I/II lung tumors, the 10-biomarker panel misclassified 15% of stage I/II tumors in the verification set, compared to 50% of the stage III/IV tumors, suggesting the model performs well in discriminating early-stage lung cancer," he wrote. "With a specificity of 93.3%, the 10-biomarker model [balanced accuracy] was 89.2% in stage I/II disease."
The authors conceded that the biomarker panel presented here would not suffice for general population screening. However, in a clinical context, among high-risk patients, the model "may provide clinical utility in guiding interpretation of screening CT scans, even in tobacco-exposed persons with COPD or emphysema," they wrote.
"Formal validation in larger patient cohorts will be needed to confirm these initial findings."
The authors disclosed that funding for this study was supplied by grants from the National Cancer Institute. Dr. Bigbee stated that there were no personal disclosures.
A panel of 10 serum biomarkers for lung cancer could offer more accurate interpretation of nodules detected on computed tomography, avoiding invasive biopsies and radiographic follow-up.
"CT-screening detection of an indeterminate pulmonary nodule, a nonspecific but frequent finding in high-risk subjects with a smoking history, creates a diagnostic dilemma," wrote investigator William L. Bigbee, Ph.D., and his colleagues in the April issue of the Journal of Thoracic Oncology.
"Although the biomarker model we described could not detect every lung cancer, it offers a significant clinical improvement over CT imaging alone.... Also, patients with nodules not identified as cancer by the model would continue to receive follow-up clinical monitoring and would be biopsied if the nodules grew in size, which is the current standard of care," the researchers said (J. Thorac. Oncol. 2012;7:698-708).
Dr. Bigbee of the University of Pittsburgh and his colleagues cite results of the National Lung Screening Trial (NLST), published in June 2011, which showed for the first time that low-dose CT screening of heavy smokers could reduce lung cancer mortality by 20%. But, as Dr. Bigbee et al. note in the current study, the "vast majority" of positive results in the NLST program turned out to be false after diagnostic evaluation. Moreover, smaller nodules are least likely to be malignant and least likely to be considered for biopsy or surgery.
For the current study, the researchers initially looked at a "training" set of 56 patients with non–small cell lung cancer in the University of Pittsburgh Cancer Institute Georgia Cooper Lung Research Registry. These cases were matched with 56 controls from the Pittsburgh Lung Screening Study (PLuSS), a volunteer cohort at high risk for lung cancer. All controls were known to be cancer free. The authors then analyzed serum samples from both groups for the presence of 70 potential cancer-associated biomarkers.
"Together, these biomarkers incorporate a wide range of host and tumor derived factors that allow a broad analysis of the lung cancer/host interaction, and includes a number of previously described epithelial cell cancer-associated serological markers," wrote the investigators. "The initial goal of this discovery study was to identify the most robust subset of these biomarkers to discriminate lung cancer and matched control samples."
Using a rule-learning algorithm, they whittled the field of potential biomarkers down to eight: prolactin, transthyretin, E-selectin, C-C motif, thrombospondin-1, chemokine 5, macrophage migration inhibitory factor, plasminogen activator inhibitor 1, and receptor tyrosine-protein kinase erbB-2.
"This rule model distinguished the lung cancer case samples from the control samples in the training set with a sensitivity of 92.9% and specificity of 87.5%," they reported.
Ultimately, two additional biomarkers were added to the panel – cytokeratin fragment 19-9 and serum amyloid A protein – and an additional set of cases and controls, 30 in each cohort, was assessed, in a blinded "verification" set.
In this set, the authors calculated an overall classification performance of 73.3% sensitivity and 93.3% specificity. Only 10 misclassifications occurred among 60 predictions made. Moreover, when looking at accuracy according to patient demographic factors, the researchers found that the 10-biomarker panel was equally good at distinguishing males and females as either cases or controls and that neither current smoking status nor airway obstruction skewed the results.
Nor did the presence of nodules visible on CT scan confound the biomarkers’ predictive value. "In fact, those PLuSS subjects with a suspicious nodule were more often correctly classified as controls than those with no nodule or a benign nodule," wrote the authors.
They add that all nodules found in controls remained clinically noncancerous at least 3 years after initial detection, with either resolution or no further growth on subsequent CT scans.
Finally, Dr. Bigbee assessed the model’s accuracy with early- vs. late-stage tumors.
"Among stage I/II lung tumors, the 10-biomarker panel misclassified 15% of stage I/II tumors in the verification set, compared to 50% of the stage III/IV tumors, suggesting the model performs well in discriminating early-stage lung cancer," he wrote. "With a specificity of 93.3%, the 10-biomarker model [balanced accuracy] was 89.2% in stage I/II disease."
The authors conceded that the biomarker panel presented here would not suffice for general population screening. However, in a clinical context, among high-risk patients, the model "may provide clinical utility in guiding interpretation of screening CT scans, even in tobacco-exposed persons with COPD or emphysema," they wrote.
"Formal validation in larger patient cohorts will be needed to confirm these initial findings."
The authors disclosed that funding for this study was supplied by grants from the National Cancer Institute. Dr. Bigbee stated that there were no personal disclosures.
A panel of 10 serum biomarkers for lung cancer could offer more accurate interpretation of nodules detected on computed tomography, avoiding invasive biopsies and radiographic follow-up.
"CT-screening detection of an indeterminate pulmonary nodule, a nonspecific but frequent finding in high-risk subjects with a smoking history, creates a diagnostic dilemma," wrote investigator William L. Bigbee, Ph.D., and his colleagues in the April issue of the Journal of Thoracic Oncology.
"Although the biomarker model we described could not detect every lung cancer, it offers a significant clinical improvement over CT imaging alone.... Also, patients with nodules not identified as cancer by the model would continue to receive follow-up clinical monitoring and would be biopsied if the nodules grew in size, which is the current standard of care," the researchers said (J. Thorac. Oncol. 2012;7:698-708).
Dr. Bigbee of the University of Pittsburgh and his colleagues cite results of the National Lung Screening Trial (NLST), published in June 2011, which showed for the first time that low-dose CT screening of heavy smokers could reduce lung cancer mortality by 20%. But, as Dr. Bigbee et al. note in the current study, the "vast majority" of positive results in the NLST program turned out to be false after diagnostic evaluation. Moreover, smaller nodules are least likely to be malignant and least likely to be considered for biopsy or surgery.
For the current study, the researchers initially looked at a "training" set of 56 patients with non–small cell lung cancer in the University of Pittsburgh Cancer Institute Georgia Cooper Lung Research Registry. These cases were matched with 56 controls from the Pittsburgh Lung Screening Study (PLuSS), a volunteer cohort at high risk for lung cancer. All controls were known to be cancer free. The authors then analyzed serum samples from both groups for the presence of 70 potential cancer-associated biomarkers.
"Together, these biomarkers incorporate a wide range of host and tumor derived factors that allow a broad analysis of the lung cancer/host interaction, and includes a number of previously described epithelial cell cancer-associated serological markers," wrote the investigators. "The initial goal of this discovery study was to identify the most robust subset of these biomarkers to discriminate lung cancer and matched control samples."
Using a rule-learning algorithm, they whittled the field of potential biomarkers down to eight: prolactin, transthyretin, E-selectin, C-C motif, thrombospondin-1, chemokine 5, macrophage migration inhibitory factor, plasminogen activator inhibitor 1, and receptor tyrosine-protein kinase erbB-2.
"This rule model distinguished the lung cancer case samples from the control samples in the training set with a sensitivity of 92.9% and specificity of 87.5%," they reported.
Ultimately, two additional biomarkers were added to the panel – cytokeratin fragment 19-9 and serum amyloid A protein – and an additional set of cases and controls, 30 in each cohort, was assessed, in a blinded "verification" set.
In this set, the authors calculated an overall classification performance of 73.3% sensitivity and 93.3% specificity. Only 10 misclassifications occurred among 60 predictions made. Moreover, when looking at accuracy according to patient demographic factors, the researchers found that the 10-biomarker panel was equally good at distinguishing males and females as either cases or controls and that neither current smoking status nor airway obstruction skewed the results.
Nor did the presence of nodules visible on CT scan confound the biomarkers’ predictive value. "In fact, those PLuSS subjects with a suspicious nodule were more often correctly classified as controls than those with no nodule or a benign nodule," wrote the authors.
They add that all nodules found in controls remained clinically noncancerous at least 3 years after initial detection, with either resolution or no further growth on subsequent CT scans.
Finally, Dr. Bigbee assessed the model’s accuracy with early- vs. late-stage tumors.
"Among stage I/II lung tumors, the 10-biomarker panel misclassified 15% of stage I/II tumors in the verification set, compared to 50% of the stage III/IV tumors, suggesting the model performs well in discriminating early-stage lung cancer," he wrote. "With a specificity of 93.3%, the 10-biomarker model [balanced accuracy] was 89.2% in stage I/II disease."
The authors conceded that the biomarker panel presented here would not suffice for general population screening. However, in a clinical context, among high-risk patients, the model "may provide clinical utility in guiding interpretation of screening CT scans, even in tobacco-exposed persons with COPD or emphysema," they wrote.
"Formal validation in larger patient cohorts will be needed to confirm these initial findings."
The authors disclosed that funding for this study was supplied by grants from the National Cancer Institute. Dr. Bigbee stated that there were no personal disclosures.
Major Finding: A panel of 10 serum biomarkers for lung cancer had a 73.3% sensitivity and 93.3% specificity in a blinded verification set, with the best performance in early, stage I/II cases.
Data Source: The study compared the panel in cases from the University of Pittsburgh Cancer Institute Georgia Cooper Lung Research Registry and controls from the Pittsburgh Lung Screening Study.
Disclosures: The authors disclosed that funding for this study was supplied by grants from the National Cancer Institute. Dr. Bigbee stated that there were no personal disclosures.
Diagnostic Concerns in Staging With EBUS-FNA
FT. LAUDERDALE, FLA. – Appropriate staging of non–small cell lung cancer (NSCLC) is critical to patient treatment and prognosis. Endobronchial ultrasound–guided fine-needle aspiration (EBUS-FNA) has gradually gained acceptance as a diagnostic tool to pathologically stage the mediastinum in patients with NSCLC, according to Dr. Bryan A. Whitson at the annual meeting of the Society of Thoracic Surgeons.
Because EBUS-FNA is a test to detect cancer, it is critical that it have few false-negative results, i.e., have a high negative predictive value (NPV). However, by virtue of the size and volume of mediastinal lymph node FNA samples, nondiagnostic results occur with some frequency.
Nondiagnostic samples decrease a test’s diagnostic performance, since they cannot help guide a clinical decision, and a portion of these samples may actually be false negatives.
The standard calculation of NPV does not factor in nondiagnostic samples. According to a study by the researchers in the Division of Thoracic and Foregut Surgery at the University of Minnesota, which Dr. Whitson presented, one must take nondiagnostic samples into consideration to determine the true diagnostic performance of EBUS-FNA.
To conservatively calculate NPV, the researchers modified the standard definition (true negatives/[true negatives plus false negatives]), creating an alternative NPV definition (true negatives/[true negatives plus false negatives plus nondiagnostic]). This definition takes into account the possibility that these nondiagnostic samples may be false negatives, which assumes the worst-case scenario.
The thoracic team at the University of Minnesota then compared the results of these two definitions of NPV in a retrospective analysis of their prospective database of patients with NSCLC who had EBUS-FNA between January 2007 and July 2011.
Dr. Bryan Whitson, who is a surgeon at the University of Minnesota, presented the team’s results.
A total of 120 patients with NSCLC who underwent EBUS-FNA were included in the analsyis. EBUS-FNAs were assessed using rapid on-site cytologic evaluation (ROSE) and a false-negative definition consisting of negative FNAs coupled to NSCLC-positive surgical biopsy of the same site. Diagnostic performance was evaluated comparing results of the inclusion or exclusion of nondiagnostic samples in the calculation of NPV.
Seven (5.8%) of the 120 patients had nondiagnostic results. One patient had a false negative. When the seven nondiagnostic procedures were excluded, the NPV was 96.3%; when they were included, the NPV dropped to 76.5%.
Both sensitivity and accuracy also dropped with the inclusion of the nondiagnostic procedures. Sensitivity dropped from 98.8% to 91% and overall accuracy from 98.2% to 92.2% when nondiagnostic procedures were included.
"Our data indicate that a conservative calculation of NPV that includes nondiagnostic samples should be used to assess the real diagnostic accuracy of EBUS-FNA in patients with NSCLC. Otherwise, decisions based on these results could be flawed and patients inappropriately staged. In our experience, and using this assessment, EBUS-FNA shows a true NPV that is less than most clinicians assume," Dr. Whitson said.
Ultimately, the thoracic surgeons at the University of Minnesota believe that "EBUS-FNA should be the primary method to stage the mediastinum in patients with NSCLC, since it enables pathologic staging without the need for an incision, with preservation of tissue planes, and with minimal complications."
However, in view of the limitations of EBUS-FNA, "we perform an immediate surgical biopsy when in doubt of the reliability of an EBUS-FNA result. A thoracic surgeon facile in EBUS can offer most patients minimally invasive mediastinal staging, with selective surgical biopsy in the same anesthetic setting, which can ensure accuracy and streamline patient care," according to Dr. Whitson and his colleagues.
FT. LAUDERDALE, FLA. – Appropriate staging of non–small cell lung cancer (NSCLC) is critical to patient treatment and prognosis. Endobronchial ultrasound–guided fine-needle aspiration (EBUS-FNA) has gradually gained acceptance as a diagnostic tool to pathologically stage the mediastinum in patients with NSCLC, according to Dr. Bryan A. Whitson at the annual meeting of the Society of Thoracic Surgeons.
Because EBUS-FNA is a test to detect cancer, it is critical that it have few false-negative results, i.e., have a high negative predictive value (NPV). However, by virtue of the size and volume of mediastinal lymph node FNA samples, nondiagnostic results occur with some frequency.
Nondiagnostic samples decrease a test’s diagnostic performance, since they cannot help guide a clinical decision, and a portion of these samples may actually be false negatives.
The standard calculation of NPV does not factor in nondiagnostic samples. According to a study by the researchers in the Division of Thoracic and Foregut Surgery at the University of Minnesota, which Dr. Whitson presented, one must take nondiagnostic samples into consideration to determine the true diagnostic performance of EBUS-FNA.
To conservatively calculate NPV, the researchers modified the standard definition (true negatives/[true negatives plus false negatives]), creating an alternative NPV definition (true negatives/[true negatives plus false negatives plus nondiagnostic]). This definition takes into account the possibility that these nondiagnostic samples may be false negatives, which assumes the worst-case scenario.
The thoracic team at the University of Minnesota then compared the results of these two definitions of NPV in a retrospective analysis of their prospective database of patients with NSCLC who had EBUS-FNA between January 2007 and July 2011.
Dr. Bryan Whitson, who is a surgeon at the University of Minnesota, presented the team’s results.
A total of 120 patients with NSCLC who underwent EBUS-FNA were included in the analsyis. EBUS-FNAs were assessed using rapid on-site cytologic evaluation (ROSE) and a false-negative definition consisting of negative FNAs coupled to NSCLC-positive surgical biopsy of the same site. Diagnostic performance was evaluated comparing results of the inclusion or exclusion of nondiagnostic samples in the calculation of NPV.
Seven (5.8%) of the 120 patients had nondiagnostic results. One patient had a false negative. When the seven nondiagnostic procedures were excluded, the NPV was 96.3%; when they were included, the NPV dropped to 76.5%.
Both sensitivity and accuracy also dropped with the inclusion of the nondiagnostic procedures. Sensitivity dropped from 98.8% to 91% and overall accuracy from 98.2% to 92.2% when nondiagnostic procedures were included.
"Our data indicate that a conservative calculation of NPV that includes nondiagnostic samples should be used to assess the real diagnostic accuracy of EBUS-FNA in patients with NSCLC. Otherwise, decisions based on these results could be flawed and patients inappropriately staged. In our experience, and using this assessment, EBUS-FNA shows a true NPV that is less than most clinicians assume," Dr. Whitson said.
Ultimately, the thoracic surgeons at the University of Minnesota believe that "EBUS-FNA should be the primary method to stage the mediastinum in patients with NSCLC, since it enables pathologic staging without the need for an incision, with preservation of tissue planes, and with minimal complications."
However, in view of the limitations of EBUS-FNA, "we perform an immediate surgical biopsy when in doubt of the reliability of an EBUS-FNA result. A thoracic surgeon facile in EBUS can offer most patients minimally invasive mediastinal staging, with selective surgical biopsy in the same anesthetic setting, which can ensure accuracy and streamline patient care," according to Dr. Whitson and his colleagues.
FT. LAUDERDALE, FLA. – Appropriate staging of non–small cell lung cancer (NSCLC) is critical to patient treatment and prognosis. Endobronchial ultrasound–guided fine-needle aspiration (EBUS-FNA) has gradually gained acceptance as a diagnostic tool to pathologically stage the mediastinum in patients with NSCLC, according to Dr. Bryan A. Whitson at the annual meeting of the Society of Thoracic Surgeons.
Because EBUS-FNA is a test to detect cancer, it is critical that it have few false-negative results, i.e., have a high negative predictive value (NPV). However, by virtue of the size and volume of mediastinal lymph node FNA samples, nondiagnostic results occur with some frequency.
Nondiagnostic samples decrease a test’s diagnostic performance, since they cannot help guide a clinical decision, and a portion of these samples may actually be false negatives.
The standard calculation of NPV does not factor in nondiagnostic samples. According to a study by the researchers in the Division of Thoracic and Foregut Surgery at the University of Minnesota, which Dr. Whitson presented, one must take nondiagnostic samples into consideration to determine the true diagnostic performance of EBUS-FNA.
To conservatively calculate NPV, the researchers modified the standard definition (true negatives/[true negatives plus false negatives]), creating an alternative NPV definition (true negatives/[true negatives plus false negatives plus nondiagnostic]). This definition takes into account the possibility that these nondiagnostic samples may be false negatives, which assumes the worst-case scenario.
The thoracic team at the University of Minnesota then compared the results of these two definitions of NPV in a retrospective analysis of their prospective database of patients with NSCLC who had EBUS-FNA between January 2007 and July 2011.
Dr. Bryan Whitson, who is a surgeon at the University of Minnesota, presented the team’s results.
A total of 120 patients with NSCLC who underwent EBUS-FNA were included in the analsyis. EBUS-FNAs were assessed using rapid on-site cytologic evaluation (ROSE) and a false-negative definition consisting of negative FNAs coupled to NSCLC-positive surgical biopsy of the same site. Diagnostic performance was evaluated comparing results of the inclusion or exclusion of nondiagnostic samples in the calculation of NPV.
Seven (5.8%) of the 120 patients had nondiagnostic results. One patient had a false negative. When the seven nondiagnostic procedures were excluded, the NPV was 96.3%; when they were included, the NPV dropped to 76.5%.
Both sensitivity and accuracy also dropped with the inclusion of the nondiagnostic procedures. Sensitivity dropped from 98.8% to 91% and overall accuracy from 98.2% to 92.2% when nondiagnostic procedures were included.
"Our data indicate that a conservative calculation of NPV that includes nondiagnostic samples should be used to assess the real diagnostic accuracy of EBUS-FNA in patients with NSCLC. Otherwise, decisions based on these results could be flawed and patients inappropriately staged. In our experience, and using this assessment, EBUS-FNA shows a true NPV that is less than most clinicians assume," Dr. Whitson said.
Ultimately, the thoracic surgeons at the University of Minnesota believe that "EBUS-FNA should be the primary method to stage the mediastinum in patients with NSCLC, since it enables pathologic staging without the need for an incision, with preservation of tissue planes, and with minimal complications."
However, in view of the limitations of EBUS-FNA, "we perform an immediate surgical biopsy when in doubt of the reliability of an EBUS-FNA result. A thoracic surgeon facile in EBUS can offer most patients minimally invasive mediastinal staging, with selective surgical biopsy in the same anesthetic setting, which can ensure accuracy and streamline patient care," according to Dr. Whitson and his colleagues.
Diagnostic Concerns in Staging With EBUS-FNA
FT. LAUDERDALE, FLA. – Appropriate staging of non–small cell lung cancer (NSCLC) is critical to patient treatment and prognosis. Endobronchial ultrasound–guided fine-needle aspiration (EBUS-FNA) has gradually gained acceptance as a diagnostic tool to pathologically stage the mediastinum in patients with NSCLC, according to Dr. Bryan A. Whitson at the annual meeting of the Society of Thoracic Surgeons.
Because EBUS-FNA is a test to detect cancer, it is critical that it have few false-negative results, i.e., have a high negative predictive value (NPV). However, by virtue of the size and volume of mediastinal lymph node FNA samples, nondiagnostic results occur with some frequency.
Nondiagnostic samples decrease a test’s diagnostic performance, since they cannot help guide a clinical decision, and a portion of these samples may actually be false negatives.
The standard calculation of NPV does not factor in nondiagnostic samples. According to a study by the researchers in the Division of Thoracic and Foregut Surgery at the University of Minnesota, which Dr. Whitson presented, one must take nondiagnostic samples into consideration to determine the true diagnostic performance of EBUS-FNA.
To conservatively calculate NPV, the researchers modified the standard definition (true negatives/[true negatives plus false negatives]), creating an alternative NPV definition (true negatives/[true negatives plus false negatives plus nondiagnostic]). This definition takes into account the possibility that these nondiagnostic samples may be false negatives, which assumes the worst-case scenario.
The thoracic team at the University of Minnesota then compared the results of these two definitions of NPV in a retrospective analysis of their prospective database of patients with NSCLC who had EBUS-FNA between January 2007 and July 2011.
Dr. Bryan Whitson, who is a surgeon at the University of Minnesota, presented the team’s results.
A total of 120 patients with NSCLC who underwent EBUS-FNA were included in the analsyis. EBUS-FNAs were assessed using rapid on-site cytologic evaluation (ROSE) and a false-negative definition consisting of negative FNAs coupled to NSCLC-positive surgical biopsy of the same site. Diagnostic performance was evaluated comparing results of the inclusion or exclusion of nondiagnostic samples in the calculation of NPV.
Seven (5.8%) of the 120 patients had nondiagnostic results. One patient had a false negative. When the seven nondiagnostic procedures were excluded, the NPV was 96.3%; when they were included, the NPV dropped to 76.5%.
Both sensitivity and accuracy also dropped with the inclusion of the nondiagnostic procedures. Sensitivity dropped from 98.8% to 91% and overall accuracy from 98.2% to 92.2% when nondiagnostic procedures were included.
"Our data indicate that a conservative calculation of NPV that includes nondiagnostic samples should be used to assess the real diagnostic accuracy of EBUS-FNA in patients with NSCLC. Otherwise, decisions based on these results could be flawed and patients inappropriately staged. In our experience, and using this assessment, EBUS-FNA shows a true NPV that is less than most clinicians assume," Dr. Whitson said.
Ultimately, the thoracic surgeons at the University of Minnesota believe that "EBUS-FNA should be the primary method to stage the mediastinum in patients with NSCLC, since it enables pathologic staging without the need for an incision, with preservation of tissue planes, and with minimal complications."
However, in view of the limitations of EBUS-FNA, "we perform an immediate surgical biopsy when in doubt of the reliability of an EBUS-FNA result. A thoracic surgeon facile in EBUS can offer most patients minimally invasive mediastinal staging, with selective surgical biopsy in the same anesthetic setting, which can ensure accuracy and streamline patient care," according to Dr. Whitson and his colleagues.
FT. LAUDERDALE, FLA. – Appropriate staging of non–small cell lung cancer (NSCLC) is critical to patient treatment and prognosis. Endobronchial ultrasound–guided fine-needle aspiration (EBUS-FNA) has gradually gained acceptance as a diagnostic tool to pathologically stage the mediastinum in patients with NSCLC, according to Dr. Bryan A. Whitson at the annual meeting of the Society of Thoracic Surgeons.
Because EBUS-FNA is a test to detect cancer, it is critical that it have few false-negative results, i.e., have a high negative predictive value (NPV). However, by virtue of the size and volume of mediastinal lymph node FNA samples, nondiagnostic results occur with some frequency.
Nondiagnostic samples decrease a test’s diagnostic performance, since they cannot help guide a clinical decision, and a portion of these samples may actually be false negatives.
The standard calculation of NPV does not factor in nondiagnostic samples. According to a study by the researchers in the Division of Thoracic and Foregut Surgery at the University of Minnesota, which Dr. Whitson presented, one must take nondiagnostic samples into consideration to determine the true diagnostic performance of EBUS-FNA.
To conservatively calculate NPV, the researchers modified the standard definition (true negatives/[true negatives plus false negatives]), creating an alternative NPV definition (true negatives/[true negatives plus false negatives plus nondiagnostic]). This definition takes into account the possibility that these nondiagnostic samples may be false negatives, which assumes the worst-case scenario.
The thoracic team at the University of Minnesota then compared the results of these two definitions of NPV in a retrospective analysis of their prospective database of patients with NSCLC who had EBUS-FNA between January 2007 and July 2011.
Dr. Bryan Whitson, who is a surgeon at the University of Minnesota, presented the team’s results.
A total of 120 patients with NSCLC who underwent EBUS-FNA were included in the analsyis. EBUS-FNAs were assessed using rapid on-site cytologic evaluation (ROSE) and a false-negative definition consisting of negative FNAs coupled to NSCLC-positive surgical biopsy of the same site. Diagnostic performance was evaluated comparing results of the inclusion or exclusion of nondiagnostic samples in the calculation of NPV.
Seven (5.8%) of the 120 patients had nondiagnostic results. One patient had a false negative. When the seven nondiagnostic procedures were excluded, the NPV was 96.3%; when they were included, the NPV dropped to 76.5%.
Both sensitivity and accuracy also dropped with the inclusion of the nondiagnostic procedures. Sensitivity dropped from 98.8% to 91% and overall accuracy from 98.2% to 92.2% when nondiagnostic procedures were included.
"Our data indicate that a conservative calculation of NPV that includes nondiagnostic samples should be used to assess the real diagnostic accuracy of EBUS-FNA in patients with NSCLC. Otherwise, decisions based on these results could be flawed and patients inappropriately staged. In our experience, and using this assessment, EBUS-FNA shows a true NPV that is less than most clinicians assume," Dr. Whitson said.
Ultimately, the thoracic surgeons at the University of Minnesota believe that "EBUS-FNA should be the primary method to stage the mediastinum in patients with NSCLC, since it enables pathologic staging without the need for an incision, with preservation of tissue planes, and with minimal complications."
However, in view of the limitations of EBUS-FNA, "we perform an immediate surgical biopsy when in doubt of the reliability of an EBUS-FNA result. A thoracic surgeon facile in EBUS can offer most patients minimally invasive mediastinal staging, with selective surgical biopsy in the same anesthetic setting, which can ensure accuracy and streamline patient care," according to Dr. Whitson and his colleagues.
FT. LAUDERDALE, FLA. – Appropriate staging of non–small cell lung cancer (NSCLC) is critical to patient treatment and prognosis. Endobronchial ultrasound–guided fine-needle aspiration (EBUS-FNA) has gradually gained acceptance as a diagnostic tool to pathologically stage the mediastinum in patients with NSCLC, according to Dr. Bryan A. Whitson at the annual meeting of the Society of Thoracic Surgeons.
Because EBUS-FNA is a test to detect cancer, it is critical that it have few false-negative results, i.e., have a high negative predictive value (NPV). However, by virtue of the size and volume of mediastinal lymph node FNA samples, nondiagnostic results occur with some frequency.
Nondiagnostic samples decrease a test’s diagnostic performance, since they cannot help guide a clinical decision, and a portion of these samples may actually be false negatives.
The standard calculation of NPV does not factor in nondiagnostic samples. According to a study by the researchers in the Division of Thoracic and Foregut Surgery at the University of Minnesota, which Dr. Whitson presented, one must take nondiagnostic samples into consideration to determine the true diagnostic performance of EBUS-FNA.
To conservatively calculate NPV, the researchers modified the standard definition (true negatives/[true negatives plus false negatives]), creating an alternative NPV definition (true negatives/[true negatives plus false negatives plus nondiagnostic]). This definition takes into account the possibility that these nondiagnostic samples may be false negatives, which assumes the worst-case scenario.
The thoracic team at the University of Minnesota then compared the results of these two definitions of NPV in a retrospective analysis of their prospective database of patients with NSCLC who had EBUS-FNA between January 2007 and July 2011.
Dr. Bryan Whitson, who is a surgeon at the University of Minnesota, presented the team’s results.
A total of 120 patients with NSCLC who underwent EBUS-FNA were included in the analsyis. EBUS-FNAs were assessed using rapid on-site cytologic evaluation (ROSE) and a false-negative definition consisting of negative FNAs coupled to NSCLC-positive surgical biopsy of the same site. Diagnostic performance was evaluated comparing results of the inclusion or exclusion of nondiagnostic samples in the calculation of NPV.
Seven (5.8%) of the 120 patients had nondiagnostic results. One patient had a false negative. When the seven nondiagnostic procedures were excluded, the NPV was 96.3%; when they were included, the NPV dropped to 76.5%.
Both sensitivity and accuracy also dropped with the inclusion of the nondiagnostic procedures. Sensitivity dropped from 98.8% to 91% and overall accuracy from 98.2% to 92.2% when nondiagnostic procedures were included.
"Our data indicate that a conservative calculation of NPV that includes nondiagnostic samples should be used to assess the real diagnostic accuracy of EBUS-FNA in patients with NSCLC. Otherwise, decisions based on these results could be flawed and patients inappropriately staged. In our experience, and using this assessment, EBUS-FNA shows a true NPV that is less than most clinicians assume," Dr. Whitson said.
Ultimately, the thoracic surgeons at the University of Minnesota believe that "EBUS-FNA should be the primary method to stage the mediastinum in patients with NSCLC, since it enables pathologic staging without the need for an incision, with preservation of tissue planes, and with minimal complications."
However, in view of the limitations of EBUS-FNA, "we perform an immediate surgical biopsy when in doubt of the reliability of an EBUS-FNA result. A thoracic surgeon facile in EBUS can offer most patients minimally invasive mediastinal staging, with selective surgical biopsy in the same anesthetic setting, which can ensure accuracy and streamline patient care," according to Dr. Whitson and his colleagues.
More NSCLC Upstaging Seen With Thoracotomy
FT. LAUDERDALE, FLA. – Pretreatment staging of presumed N0 non–small cell lung cancer (NSCLC) misses unsuspected lymph node metastases that are subsequently discovered during surgical specimen evaluation in 10%-25% of cases. Thus it is critically important that surgical node dissection be done sufficiently completely to capture these misses, providing the appropriate upstaging for treatment decisions.
With the increasing movement to video-assisted thoracic surgery (VATS) approaches to lobectomy and segmentectomy, it is important to evaluate the comparative efficiency of VATS vs. thoracotomy (Open) in capturing this upstaging.
To this end, an examination of the Society of Thoracic Surgeons (STS) General Thoracic Database was performed to determine the frequency of nodal metastases identified in clinically node-negative tumors by Open and VATS approaches to compare completeness of surgical nodal dissections, according to Dr. Daniel J. Boffa.
A total of 11,531 clinical stage I primary lung cancers resected from 2001 to 2010 were analyzed from the STS General Thoracic Database. These comprised 7,137 Open and 4,394 VATS procedures.
The researchers found significantly greater nodal upstaging in the Open groups (14.3%) as compared with the VATS group (11.6%).
Dr. Boffa stated that this was primarily due to the significantly greater upstaging from N0 to N1 that was seen in the Open (9.8%) versus the VATS group (7.0%). In contrast, upstaging from N0 to N2 was found to be similar in both groups (5.5% Open vs. 5.2% VATS, a non-significant difference).
Dr. Boffa presented this research at the annual meeting of the Society of Thoracic Surgeons.
When a multivariate analysis controlling for T status, laterality, body mass index, age, and sex was performed, N0 to N1 upstaging remained significantly less common with VATS than with Open surgery.
In a separate analysis, pathologic confirmation of clinical N1 also occurred significantly less often in the VATS group (42%) as compared with the Open group (54%), according to Dr. Boffa, who is an assistant professor of surgery at the Yale School of Medicine, New Haven, Conn.
"Mediastinal nodal evaluation by VATS and thoracotomy results in equivalent upstaging. However, lower rates of N1 upstaging and stage confirmation in the VATS group may indicate variability in the completeness of the peribronchial and hilar lymph node evaluation," according to Dr. Boffa.
"Systematic hilar dissection is encouraged, particularly as more surgeons adopt the VATS approach," he concluded, reiterating the importance of appropriate staging in the planning of patient treatment.
Dr. Boffa presented his research as one of the Richard E. Clark database papers during the STS annual meeting.
Dr. Boffa reported that he had no relevant conflicts of interest.
FT. LAUDERDALE, FLA. – Pretreatment staging of presumed N0 non–small cell lung cancer (NSCLC) misses unsuspected lymph node metastases that are subsequently discovered during surgical specimen evaluation in 10%-25% of cases. Thus it is critically important that surgical node dissection be done sufficiently completely to capture these misses, providing the appropriate upstaging for treatment decisions.
With the increasing movement to video-assisted thoracic surgery (VATS) approaches to lobectomy and segmentectomy, it is important to evaluate the comparative efficiency of VATS vs. thoracotomy (Open) in capturing this upstaging.
To this end, an examination of the Society of Thoracic Surgeons (STS) General Thoracic Database was performed to determine the frequency of nodal metastases identified in clinically node-negative tumors by Open and VATS approaches to compare completeness of surgical nodal dissections, according to Dr. Daniel J. Boffa.
A total of 11,531 clinical stage I primary lung cancers resected from 2001 to 2010 were analyzed from the STS General Thoracic Database. These comprised 7,137 Open and 4,394 VATS procedures.
The researchers found significantly greater nodal upstaging in the Open groups (14.3%) as compared with the VATS group (11.6%).
Dr. Boffa stated that this was primarily due to the significantly greater upstaging from N0 to N1 that was seen in the Open (9.8%) versus the VATS group (7.0%). In contrast, upstaging from N0 to N2 was found to be similar in both groups (5.5% Open vs. 5.2% VATS, a non-significant difference).
Dr. Boffa presented this research at the annual meeting of the Society of Thoracic Surgeons.
When a multivariate analysis controlling for T status, laterality, body mass index, age, and sex was performed, N0 to N1 upstaging remained significantly less common with VATS than with Open surgery.
In a separate analysis, pathologic confirmation of clinical N1 also occurred significantly less often in the VATS group (42%) as compared with the Open group (54%), according to Dr. Boffa, who is an assistant professor of surgery at the Yale School of Medicine, New Haven, Conn.
"Mediastinal nodal evaluation by VATS and thoracotomy results in equivalent upstaging. However, lower rates of N1 upstaging and stage confirmation in the VATS group may indicate variability in the completeness of the peribronchial and hilar lymph node evaluation," according to Dr. Boffa.
"Systematic hilar dissection is encouraged, particularly as more surgeons adopt the VATS approach," he concluded, reiterating the importance of appropriate staging in the planning of patient treatment.
Dr. Boffa presented his research as one of the Richard E. Clark database papers during the STS annual meeting.
Dr. Boffa reported that he had no relevant conflicts of interest.
FT. LAUDERDALE, FLA. – Pretreatment staging of presumed N0 non–small cell lung cancer (NSCLC) misses unsuspected lymph node metastases that are subsequently discovered during surgical specimen evaluation in 10%-25% of cases. Thus it is critically important that surgical node dissection be done sufficiently completely to capture these misses, providing the appropriate upstaging for treatment decisions.
With the increasing movement to video-assisted thoracic surgery (VATS) approaches to lobectomy and segmentectomy, it is important to evaluate the comparative efficiency of VATS vs. thoracotomy (Open) in capturing this upstaging.
To this end, an examination of the Society of Thoracic Surgeons (STS) General Thoracic Database was performed to determine the frequency of nodal metastases identified in clinically node-negative tumors by Open and VATS approaches to compare completeness of surgical nodal dissections, according to Dr. Daniel J. Boffa.
A total of 11,531 clinical stage I primary lung cancers resected from 2001 to 2010 were analyzed from the STS General Thoracic Database. These comprised 7,137 Open and 4,394 VATS procedures.
The researchers found significantly greater nodal upstaging in the Open groups (14.3%) as compared with the VATS group (11.6%).
Dr. Boffa stated that this was primarily due to the significantly greater upstaging from N0 to N1 that was seen in the Open (9.8%) versus the VATS group (7.0%). In contrast, upstaging from N0 to N2 was found to be similar in both groups (5.5% Open vs. 5.2% VATS, a non-significant difference).
Dr. Boffa presented this research at the annual meeting of the Society of Thoracic Surgeons.
When a multivariate analysis controlling for T status, laterality, body mass index, age, and sex was performed, N0 to N1 upstaging remained significantly less common with VATS than with Open surgery.
In a separate analysis, pathologic confirmation of clinical N1 also occurred significantly less often in the VATS group (42%) as compared with the Open group (54%), according to Dr. Boffa, who is an assistant professor of surgery at the Yale School of Medicine, New Haven, Conn.
"Mediastinal nodal evaluation by VATS and thoracotomy results in equivalent upstaging. However, lower rates of N1 upstaging and stage confirmation in the VATS group may indicate variability in the completeness of the peribronchial and hilar lymph node evaluation," according to Dr. Boffa.
"Systematic hilar dissection is encouraged, particularly as more surgeons adopt the VATS approach," he concluded, reiterating the importance of appropriate staging in the planning of patient treatment.
Dr. Boffa presented his research as one of the Richard E. Clark database papers during the STS annual meeting.
Dr. Boffa reported that he had no relevant conflicts of interest.
More NSCLC Upstaging Seen With Thoracotomy
FT. LAUDERDALE, FLA. – Pretreatment staging of presumed N0 non–small cell lung cancer (NSCLC) misses unsuspected lymph node metastases that are subsequently discovered during surgical specimen evaluation in 10%-25% of cases. Thus it is critically important that surgical node dissection be done sufficiently completely to capture these misses, providing the appropriate upstaging for treatment decisions.
With the increasing movement to video-assisted thoracic surgery (VATS) approaches to lobectomy and segmentectomy, it is important to evaluate the comparative efficiency of VATS vs. thoracotomy (Open) in capturing this upstaging.
To this end, an examination of the Society of Thoracic Surgeons (STS) General Thoracic Database was performed to determine the frequency of nodal metastases identified in clinically node-negative tumors by Open and VATS approaches to compare completeness of surgical nodal dissections, according to Dr. Daniel J. Boffa.
A total of 11,531 clinical stage I primary lung cancers resected from 2001 to 2010 were analyzed from the STS General Thoracic Database. These comprised 7,137 Open and 4,394 VATS procedures.
The researchers found significantly greater nodal upstaging in the Open groups (14.3%) as compared with the VATS group (11.6%).
Dr. Boffa stated that this was primarily due to the significantly greater upstaging from N0 to N1 that was seen in the Open (9.8%) versus the VATS group (7.0%). In contrast, upstaging from N0 to N2 was found to be similar in both groups (5.5% Open vs. 5.2% VATS, a non-significant difference).
Dr. Boffa presented this research at the annual meeting of the Society of Thoracic Surgeons.
When a multivariate analysis controlling for T status, laterality, body mass index, age, and sex was performed, N0 to N1 upstaging remained significantly less common with VATS than with Open surgery.
In a separate analysis, pathologic confirmation of clinical N1 also occurred significantly less often in the VATS group (42%) as compared with the Open group (54%), according to Dr. Boffa, who is an assistant professor of surgery at the Yale School of Medicine, New Haven, Conn.
"Mediastinal nodal evaluation by VATS and thoracotomy results in equivalent upstaging. However, lower rates of N1 upstaging and stage confirmation in the VATS group may indicate variability in the completeness of the peribronchial and hilar lymph node evaluation," according to Dr. Boffa.
"Systematic hilar dissection is encouraged, particularly as more surgeons adopt the VATS approach," he concluded, reiterating the importance of appropriate staging in the planning of patient treatment.
Dr. Boffa presented his research as one of the Richard E. Clark database papers during the STS annual meeting.
Dr. Boffa reported that he had no relevant conflicts of interest.
FT. LAUDERDALE, FLA. – Pretreatment staging of presumed N0 non–small cell lung cancer (NSCLC) misses unsuspected lymph node metastases that are subsequently discovered during surgical specimen evaluation in 10%-25% of cases. Thus it is critically important that surgical node dissection be done sufficiently completely to capture these misses, providing the appropriate upstaging for treatment decisions.
With the increasing movement to video-assisted thoracic surgery (VATS) approaches to lobectomy and segmentectomy, it is important to evaluate the comparative efficiency of VATS vs. thoracotomy (Open) in capturing this upstaging.
To this end, an examination of the Society of Thoracic Surgeons (STS) General Thoracic Database was performed to determine the frequency of nodal metastases identified in clinically node-negative tumors by Open and VATS approaches to compare completeness of surgical nodal dissections, according to Dr. Daniel J. Boffa.
A total of 11,531 clinical stage I primary lung cancers resected from 2001 to 2010 were analyzed from the STS General Thoracic Database. These comprised 7,137 Open and 4,394 VATS procedures.
The researchers found significantly greater nodal upstaging in the Open groups (14.3%) as compared with the VATS group (11.6%).
Dr. Boffa stated that this was primarily due to the significantly greater upstaging from N0 to N1 that was seen in the Open (9.8%) versus the VATS group (7.0%). In contrast, upstaging from N0 to N2 was found to be similar in both groups (5.5% Open vs. 5.2% VATS, a non-significant difference).
Dr. Boffa presented this research at the annual meeting of the Society of Thoracic Surgeons.
When a multivariate analysis controlling for T status, laterality, body mass index, age, and sex was performed, N0 to N1 upstaging remained significantly less common with VATS than with Open surgery.
In a separate analysis, pathologic confirmation of clinical N1 also occurred significantly less often in the VATS group (42%) as compared with the Open group (54%), according to Dr. Boffa, who is an assistant professor of surgery at the Yale School of Medicine, New Haven, Conn.
"Mediastinal nodal evaluation by VATS and thoracotomy results in equivalent upstaging. However, lower rates of N1 upstaging and stage confirmation in the VATS group may indicate variability in the completeness of the peribronchial and hilar lymph node evaluation," according to Dr. Boffa.
"Systematic hilar dissection is encouraged, particularly as more surgeons adopt the VATS approach," he concluded, reiterating the importance of appropriate staging in the planning of patient treatment.
Dr. Boffa presented his research as one of the Richard E. Clark database papers during the STS annual meeting.
Dr. Boffa reported that he had no relevant conflicts of interest.
FT. LAUDERDALE, FLA. – Pretreatment staging of presumed N0 non–small cell lung cancer (NSCLC) misses unsuspected lymph node metastases that are subsequently discovered during surgical specimen evaluation in 10%-25% of cases. Thus it is critically important that surgical node dissection be done sufficiently completely to capture these misses, providing the appropriate upstaging for treatment decisions.
With the increasing movement to video-assisted thoracic surgery (VATS) approaches to lobectomy and segmentectomy, it is important to evaluate the comparative efficiency of VATS vs. thoracotomy (Open) in capturing this upstaging.
To this end, an examination of the Society of Thoracic Surgeons (STS) General Thoracic Database was performed to determine the frequency of nodal metastases identified in clinically node-negative tumors by Open and VATS approaches to compare completeness of surgical nodal dissections, according to Dr. Daniel J. Boffa.
A total of 11,531 clinical stage I primary lung cancers resected from 2001 to 2010 were analyzed from the STS General Thoracic Database. These comprised 7,137 Open and 4,394 VATS procedures.
The researchers found significantly greater nodal upstaging in the Open groups (14.3%) as compared with the VATS group (11.6%).
Dr. Boffa stated that this was primarily due to the significantly greater upstaging from N0 to N1 that was seen in the Open (9.8%) versus the VATS group (7.0%). In contrast, upstaging from N0 to N2 was found to be similar in both groups (5.5% Open vs. 5.2% VATS, a non-significant difference).
Dr. Boffa presented this research at the annual meeting of the Society of Thoracic Surgeons.
When a multivariate analysis controlling for T status, laterality, body mass index, age, and sex was performed, N0 to N1 upstaging remained significantly less common with VATS than with Open surgery.
In a separate analysis, pathologic confirmation of clinical N1 also occurred significantly less often in the VATS group (42%) as compared with the Open group (54%), according to Dr. Boffa, who is an assistant professor of surgery at the Yale School of Medicine, New Haven, Conn.
"Mediastinal nodal evaluation by VATS and thoracotomy results in equivalent upstaging. However, lower rates of N1 upstaging and stage confirmation in the VATS group may indicate variability in the completeness of the peribronchial and hilar lymph node evaluation," according to Dr. Boffa.
"Systematic hilar dissection is encouraged, particularly as more surgeons adopt the VATS approach," he concluded, reiterating the importance of appropriate staging in the planning of patient treatment.
Dr. Boffa presented his research as one of the Richard E. Clark database papers during the STS annual meeting.
Dr. Boffa reported that he had no relevant conflicts of interest.
Survey: Women CT Surgeons Report Job Satisfaction
Women represent a minority of cardiothoracic surgeons in the United States, with roughly equal numbers being found in academic and private practice, according to a recent survey presented at the annual meeting of the Society for Thoracic Surgeons.
Overall, these women reported a high level of job satisfaction and the vast majority of them were still in practice.
Although there were comparatively few women in senior staff and faculty positions, in part because over 50% of women currently in practice entered the profession since the year 2000, "this exponential increase in the number of women in the field over the past ten years provides optimism for a continued recruitment," according to the report by Dr. Jessica S. Donington, assistant professor of cardiothoracic surgery at New York University, New York, N.Y.,and her colleagues on behalf of The Women in Thoracic Surgery (WTS).
The WTS was established as a professional organization in 1986, with the designated mission of providing mutual support and facilitating the professional advancement of women in cardiothoracic surgery.
With 2011 being the 50th anniversary of the first certification of a woman by the American Board of Thoracic Surgery (ABTS) and the year in which the 200th female was certified by the ABTS, the WTS deemed it important to survey the status of female cardiothoracic surgeons in the United States.
The WTS survey was designed to measure career progression and to provide insights in order to improve recruitment. All ABTS-certified women were surveyed anonymously in December 2010 using tools from surveymonkey.com, according to Dr. Donington.
Contact information was obtained from CTSNet, Google, and institutions of known employment.
Of the 204 living women with ABTS certification, 190 were contacted. Of these, 64% responded to the survey. The questions comprised five areas: demographics, training, practice activities, activities of non-practicing CT surgeons, and career satisfaction.
The researchers grouped the respondents by year of certification: Group 1 (1961-1999) and Group 2 (2000-2010). They used the STS/AATS 2009 practice survey of the entire thoracic surgery workforce in order to make broad-based comparisons.
The mean age of the women respondents was 48 years, with the majority being white (nearly 78%) and urban dwellers (61%).
Half of the women were certified within the past 10 years. Overall, the respondents had been practicing for a mean of 8 years, worked in groups of 2-10 surgeons, and were the only female surgeon in their group. The respondents had a mean of 9.1 years of training with 56% reporting non-Accreditation Council for Graduate Medical Education (ACGME) training time.
There was a significant increase in the duration of training and in the resultant debt over time. Group 1 respondents reported training for 8.5 years vs. 9.5 years in Group 2, with a doubling of graduates with educational debt greater than $100,000 in the later era.
Overall, the distribution of 118 respondents who answered the subspecialty question was 28% adult cardiac; 8.5% congenital cardiac; slightly less than 1% cardiopulmonary transplant; and 13.2% mixed subspecialties.
The 35.6% percent of women who identified themselves as general thoracic surgeons is higher than the 18% reported by the entire thoracic surgery workforce, with the percentage of women who identified themselves as general thoracic surgeons more than doubling in the second cohort of diplomates as compared with the first.
Slightly over 10% of respondents were no longer in CT surgery practice. All of these were certified prior to 2000, and the majority left practice due to retirement, health issues, or career advancement, according to their responses.
There was a large shift from the mixed category, which was cited by nearly 28% of the 51 women certified prior to 2000, compared with 3% of the 67 women certified after 2000.
This could be accounted for primarily by large increases in the adult cardiac (from nearly 20% prior to 2000 compared with 34% after 2000) and the general thoracic (from around 22% to 46%) subspecialty categories, reflecting the increase in subspecialization of our field.
Half of the women who responded had academic appointments, with 52% of these at the assistant professor level and 20% at the professor level. A minority of the women (20%) had protected research time, and 30% had secured research funding.
Overall, 64% of respondents reported that they were always or almost always satisfied with their profession.
The greatest source of dissatisfaction reported by both groups was demands on time, with workplace politics being a key concern expressed by Group 1 respondents, and lack of support a key concern expressed by Group 2.
Eighty percent of those polled stated that, if given the opportunity to choose a profession based on what they knew now, they would pursue cardiothoracic surgery again.
"Women still represent a minority of cardiothoracic surgeons in the United States, and over half are still very junior, having entered the profession since the year 2000.
Although the majority report being satisfied with their careers, and important academic milestones have been reached by some, there remains a scarcity of women in senior leadership positions.
"Since mentors and positive role models are consistently reported as significant factors in specialty decisions by surgical residents, this may still be the largest hurdle to recruiting women into cardiothoracic surgery," concluded Dr. Donington, in her presentation of the report.
Dr. Donington reported that she had no relevant financial disclosures.☐
Body text starts here....
Second and subsequent grafs
Doctor?s Name and Bio
Body text starts here....
Second and subsequent grafs
Doctor?s Name and Bio
Body text starts here....
Second and subsequent grafs
Doctor?s Name and Bio
Women represent a minority of cardiothoracic surgeons in the United States, with roughly equal numbers being found in academic and private practice, according to a recent survey presented at the annual meeting of the Society for Thoracic Surgeons.
Overall, these women reported a high level of job satisfaction and the vast majority of them were still in practice.
Although there were comparatively few women in senior staff and faculty positions, in part because over 50% of women currently in practice entered the profession since the year 2000, "this exponential increase in the number of women in the field over the past ten years provides optimism for a continued recruitment," according to the report by Dr. Jessica S. Donington, assistant professor of cardiothoracic surgery at New York University, New York, N.Y.,and her colleagues on behalf of The Women in Thoracic Surgery (WTS).
The WTS was established as a professional organization in 1986, with the designated mission of providing mutual support and facilitating the professional advancement of women in cardiothoracic surgery.
With 2011 being the 50th anniversary of the first certification of a woman by the American Board of Thoracic Surgery (ABTS) and the year in which the 200th female was certified by the ABTS, the WTS deemed it important to survey the status of female cardiothoracic surgeons in the United States.
The WTS survey was designed to measure career progression and to provide insights in order to improve recruitment. All ABTS-certified women were surveyed anonymously in December 2010 using tools from surveymonkey.com, according to Dr. Donington.
Contact information was obtained from CTSNet, Google, and institutions of known employment.
Of the 204 living women with ABTS certification, 190 were contacted. Of these, 64% responded to the survey. The questions comprised five areas: demographics, training, practice activities, activities of non-practicing CT surgeons, and career satisfaction.
The researchers grouped the respondents by year of certification: Group 1 (1961-1999) and Group 2 (2000-2010). They used the STS/AATS 2009 practice survey of the entire thoracic surgery workforce in order to make broad-based comparisons.
The mean age of the women respondents was 48 years, with the majority being white (nearly 78%) and urban dwellers (61%).
Half of the women were certified within the past 10 years. Overall, the respondents had been practicing for a mean of 8 years, worked in groups of 2-10 surgeons, and were the only female surgeon in their group. The respondents had a mean of 9.1 years of training with 56% reporting non-Accreditation Council for Graduate Medical Education (ACGME) training time.
There was a significant increase in the duration of training and in the resultant debt over time. Group 1 respondents reported training for 8.5 years vs. 9.5 years in Group 2, with a doubling of graduates with educational debt greater than $100,000 in the later era.
Overall, the distribution of 118 respondents who answered the subspecialty question was 28% adult cardiac; 8.5% congenital cardiac; slightly less than 1% cardiopulmonary transplant; and 13.2% mixed subspecialties.
The 35.6% percent of women who identified themselves as general thoracic surgeons is higher than the 18% reported by the entire thoracic surgery workforce, with the percentage of women who identified themselves as general thoracic surgeons more than doubling in the second cohort of diplomates as compared with the first.
Slightly over 10% of respondents were no longer in CT surgery practice. All of these were certified prior to 2000, and the majority left practice due to retirement, health issues, or career advancement, according to their responses.
There was a large shift from the mixed category, which was cited by nearly 28% of the 51 women certified prior to 2000, compared with 3% of the 67 women certified after 2000.
This could be accounted for primarily by large increases in the adult cardiac (from nearly 20% prior to 2000 compared with 34% after 2000) and the general thoracic (from around 22% to 46%) subspecialty categories, reflecting the increase in subspecialization of our field.
Half of the women who responded had academic appointments, with 52% of these at the assistant professor level and 20% at the professor level. A minority of the women (20%) had protected research time, and 30% had secured research funding.
Overall, 64% of respondents reported that they were always or almost always satisfied with their profession.
The greatest source of dissatisfaction reported by both groups was demands on time, with workplace politics being a key concern expressed by Group 1 respondents, and lack of support a key concern expressed by Group 2.
Eighty percent of those polled stated that, if given the opportunity to choose a profession based on what they knew now, they would pursue cardiothoracic surgery again.
"Women still represent a minority of cardiothoracic surgeons in the United States, and over half are still very junior, having entered the profession since the year 2000.
Although the majority report being satisfied with their careers, and important academic milestones have been reached by some, there remains a scarcity of women in senior leadership positions.
"Since mentors and positive role models are consistently reported as significant factors in specialty decisions by surgical residents, this may still be the largest hurdle to recruiting women into cardiothoracic surgery," concluded Dr. Donington, in her presentation of the report.
Dr. Donington reported that she had no relevant financial disclosures.☐
Women represent a minority of cardiothoracic surgeons in the United States, with roughly equal numbers being found in academic and private practice, according to a recent survey presented at the annual meeting of the Society for Thoracic Surgeons.
Overall, these women reported a high level of job satisfaction and the vast majority of them were still in practice.
Although there were comparatively few women in senior staff and faculty positions, in part because over 50% of women currently in practice entered the profession since the year 2000, "this exponential increase in the number of women in the field over the past ten years provides optimism for a continued recruitment," according to the report by Dr. Jessica S. Donington, assistant professor of cardiothoracic surgery at New York University, New York, N.Y.,and her colleagues on behalf of The Women in Thoracic Surgery (WTS).
The WTS was established as a professional organization in 1986, with the designated mission of providing mutual support and facilitating the professional advancement of women in cardiothoracic surgery.
With 2011 being the 50th anniversary of the first certification of a woman by the American Board of Thoracic Surgery (ABTS) and the year in which the 200th female was certified by the ABTS, the WTS deemed it important to survey the status of female cardiothoracic surgeons in the United States.
The WTS survey was designed to measure career progression and to provide insights in order to improve recruitment. All ABTS-certified women were surveyed anonymously in December 2010 using tools from surveymonkey.com, according to Dr. Donington.
Contact information was obtained from CTSNet, Google, and institutions of known employment.
Of the 204 living women with ABTS certification, 190 were contacted. Of these, 64% responded to the survey. The questions comprised five areas: demographics, training, practice activities, activities of non-practicing CT surgeons, and career satisfaction.
The researchers grouped the respondents by year of certification: Group 1 (1961-1999) and Group 2 (2000-2010). They used the STS/AATS 2009 practice survey of the entire thoracic surgery workforce in order to make broad-based comparisons.
The mean age of the women respondents was 48 years, with the majority being white (nearly 78%) and urban dwellers (61%).
Half of the women were certified within the past 10 years. Overall, the respondents had been practicing for a mean of 8 years, worked in groups of 2-10 surgeons, and were the only female surgeon in their group. The respondents had a mean of 9.1 years of training with 56% reporting non-Accreditation Council for Graduate Medical Education (ACGME) training time.
There was a significant increase in the duration of training and in the resultant debt over time. Group 1 respondents reported training for 8.5 years vs. 9.5 years in Group 2, with a doubling of graduates with educational debt greater than $100,000 in the later era.
Overall, the distribution of 118 respondents who answered the subspecialty question was 28% adult cardiac; 8.5% congenital cardiac; slightly less than 1% cardiopulmonary transplant; and 13.2% mixed subspecialties.
The 35.6% percent of women who identified themselves as general thoracic surgeons is higher than the 18% reported by the entire thoracic surgery workforce, with the percentage of women who identified themselves as general thoracic surgeons more than doubling in the second cohort of diplomates as compared with the first.
Slightly over 10% of respondents were no longer in CT surgery practice. All of these were certified prior to 2000, and the majority left practice due to retirement, health issues, or career advancement, according to their responses.
There was a large shift from the mixed category, which was cited by nearly 28% of the 51 women certified prior to 2000, compared with 3% of the 67 women certified after 2000.
This could be accounted for primarily by large increases in the adult cardiac (from nearly 20% prior to 2000 compared with 34% after 2000) and the general thoracic (from around 22% to 46%) subspecialty categories, reflecting the increase in subspecialization of our field.
Half of the women who responded had academic appointments, with 52% of these at the assistant professor level and 20% at the professor level. A minority of the women (20%) had protected research time, and 30% had secured research funding.
Overall, 64% of respondents reported that they were always or almost always satisfied with their profession.
The greatest source of dissatisfaction reported by both groups was demands on time, with workplace politics being a key concern expressed by Group 1 respondents, and lack of support a key concern expressed by Group 2.
Eighty percent of those polled stated that, if given the opportunity to choose a profession based on what they knew now, they would pursue cardiothoracic surgery again.
"Women still represent a minority of cardiothoracic surgeons in the United States, and over half are still very junior, having entered the profession since the year 2000.
Although the majority report being satisfied with their careers, and important academic milestones have been reached by some, there remains a scarcity of women in senior leadership positions.
"Since mentors and positive role models are consistently reported as significant factors in specialty decisions by surgical residents, this may still be the largest hurdle to recruiting women into cardiothoracic surgery," concluded Dr. Donington, in her presentation of the report.
Dr. Donington reported that she had no relevant financial disclosures.☐
Major Finding: Key numerical finding (e.g., number needed to treat to prevent one death/event; number lived or died as result of intervention). Maximum 20 words/2 sentences.
Data Source: : Include type of study (e.g., randomized, placebo controlled trial; retrospective case-control study). Include number in the study. Written in sentence form..
Disclosures: Sponsor of study, funding source, relevant disclosures. If author has no relevant disclosures, "Dr. X reported having no financial disclosures." If necessary, "Meeting Y did not require reports of financial disclosures." Check meeting website because many list disclosures. Written in sentence form.