User login
Lung Resection Mortality Rate Varies by Surgeon Specialty
SAN DIEGO - General surgeons perform the majority of lung resections for cancer in the United States, yet lung cancer resections performed by thoracic surgeons had significantly lower in-hospital mortality rates than did those performed by general surgeons and cardiac surgeons, according to results of a large analysis of national hospital data.
When performing a lung cancer resection, thoracic surgeons performed lymphadenectomy significantly more often than did general surgeons and cardiac surgeons.
"Lymph node status in lung cancer is the main determinant of stage, prognosis, and need for further therapy," Dr. Michelle Ellis said at the annual meeting of the Society of Thoracic Surgeons.
"The performance of lymphadenectomy at the time of lung cancer resection can be considered a process measure of quality."
Previously published studies have demonstrated that general surgeons perform the majority of thoracic cases in the United States, while surgeons who specialize in thoracic surgery have lower perioperative morbidity and mortality.
"Furthermore, patients who have their lung resection performed by a board-certified cardiothoracic surgeon specializing in general thoracic surgery have longer overall and cancer-specific survival," said Dr. Ellis of Oregon Health and Science University, Portland.
"We hypothesized that the completeness of intraoperative oncologic staging at the time of primary lung cancer resection varies by surgeon specialty, and may explain the observed differences in outcome."
To test the hypothesis, Dr. Ellis, with the assistance of Dr. Paul H. Schipper and Dr. John T. Vetto, reviewed 222,233 primary lung cancer cases from the Nationwide Inpatient Sample from 1998 to 2007 who were treated surgically with limited lung resection, lobectomy, or pneumonectomy.
The main outcome measure was the presence of lymphadenectomy or mediastinoscopy performed during the same admission.
The researchers divided the surgeons into three main groups based on their case mix of thoracic, cardiac, or other types of surgery. A thoracic surgeon was defined as someone who performed greater than 75% general thoracic surgery operations and less than 10% cardiac operations; a general surgeon was defined as someone who performed fewer than 75% thoracic operations and fewer than 10% cardiac operations, and a cardiac surgeon was defined as someone who performed greater than 10% cardiac operations.
Dr. Ellis reported that lung cancer resections were performed by general surgeons in 62% of cases, by cardiac surgeons in 35% of cases, and by thoracic surgeons in 3% of cases.
The median annual case volume was 21 for thoracic surgeons, 23 for cardiac surgeons, and 8 for general surgeons.
In-hospital mortality rates for thoracic, cardiac, and general surgeons were 2.3%, 3.4%, and 4.0%, respectively. This translated into an odds ratio for in-hospital mortality of 1.33 for cases performed by cardiac surgeons and 1.55 for those performed by general surgeons.
Thoracic surgeons performed lymphadenectomy significantly more often than did their counterparts (73% vs. 55% for both cardiac and general surgeons). Thoracic surgeons also performed mediastinoscopy significantly more often (16% vs. 10% by cardiac surgeons and 11% by general surgeons).
Multivariate analysis revealed that patients were significantly less likely to undergo lymphadenectomy if they were in the lowest two quartiles of household income (odds ratio, 0.74); insured by Medicare (OR, 0.93); received their care at a rural hospital (OR, 0.60) or at an urban nonteaching hospital (OR, 0.74); or had their resection performed by a general surgeon (OR, 0.47) or by a cardiac surgeon (OR, 0.47).
"A patient was more than twice as likely to have a lymphadenectomy performed if the lung cancer resection was performed by a thoracic surgeon," Dr. Ellis said.
Next, the researchers assessed the impact of case volume on their multivariate model. They determined that for every doubling of thoracic surgery case volume, there was a significant increase in the likelihood that a lymphadenectomy would be performed (OR, 1.28).
On the other hand, for every doubling of general surgery case volume, there was a significant decrease in lymphadenectomy rates (OR, 0.95). Doubling of cardiac surgery case volume did not affect lymphadenectomy rates.
"Lymphadenectomy rates for all surgeon groups did improve over the study period," Dr. Ellis said.
"However, despite these improvements, cardiac and general surgeons still have lymphadenectomy rates significantly lower than [those of] cardiac surgeons. The next step is to ensure that all patients receive adequate staging of the mediastinum, possibly through disseminating knowledge, creating centers of excellence, or providing opportunities to learn the skills necessary to perform adequate lung cancer surgery."
She acknowledged certain limitations of the study, including the fact that it contains only single-admission information. "It also has limited cancer-specific data such as stage, and has no mechanism for long-term follow-up," she said. In addition, surgeons are anonymous in the database, so board certification could not be determined.
Dr. Ellis said that she had no relevant financial disclosures to make.
SAN DIEGO - General surgeons perform the majority of lung resections for cancer in the United States, yet lung cancer resections performed by thoracic surgeons had significantly lower in-hospital mortality rates than did those performed by general surgeons and cardiac surgeons, according to results of a large analysis of national hospital data.
When performing a lung cancer resection, thoracic surgeons performed lymphadenectomy significantly more often than did general surgeons and cardiac surgeons.
"Lymph node status in lung cancer is the main determinant of stage, prognosis, and need for further therapy," Dr. Michelle Ellis said at the annual meeting of the Society of Thoracic Surgeons.
"The performance of lymphadenectomy at the time of lung cancer resection can be considered a process measure of quality."
Previously published studies have demonstrated that general surgeons perform the majority of thoracic cases in the United States, while surgeons who specialize in thoracic surgery have lower perioperative morbidity and mortality.
"Furthermore, patients who have their lung resection performed by a board-certified cardiothoracic surgeon specializing in general thoracic surgery have longer overall and cancer-specific survival," said Dr. Ellis of Oregon Health and Science University, Portland.
"We hypothesized that the completeness of intraoperative oncologic staging at the time of primary lung cancer resection varies by surgeon specialty, and may explain the observed differences in outcome."
To test the hypothesis, Dr. Ellis, with the assistance of Dr. Paul H. Schipper and Dr. John T. Vetto, reviewed 222,233 primary lung cancer cases from the Nationwide Inpatient Sample from 1998 to 2007 who were treated surgically with limited lung resection, lobectomy, or pneumonectomy.
The main outcome measure was the presence of lymphadenectomy or mediastinoscopy performed during the same admission.
The researchers divided the surgeons into three main groups based on their case mix of thoracic, cardiac, or other types of surgery. A thoracic surgeon was defined as someone who performed greater than 75% general thoracic surgery operations and less than 10% cardiac operations; a general surgeon was defined as someone who performed fewer than 75% thoracic operations and fewer than 10% cardiac operations, and a cardiac surgeon was defined as someone who performed greater than 10% cardiac operations.
Dr. Ellis reported that lung cancer resections were performed by general surgeons in 62% of cases, by cardiac surgeons in 35% of cases, and by thoracic surgeons in 3% of cases.
The median annual case volume was 21 for thoracic surgeons, 23 for cardiac surgeons, and 8 for general surgeons.
In-hospital mortality rates for thoracic, cardiac, and general surgeons were 2.3%, 3.4%, and 4.0%, respectively. This translated into an odds ratio for in-hospital mortality of 1.33 for cases performed by cardiac surgeons and 1.55 for those performed by general surgeons.
Thoracic surgeons performed lymphadenectomy significantly more often than did their counterparts (73% vs. 55% for both cardiac and general surgeons). Thoracic surgeons also performed mediastinoscopy significantly more often (16% vs. 10% by cardiac surgeons and 11% by general surgeons).
Multivariate analysis revealed that patients were significantly less likely to undergo lymphadenectomy if they were in the lowest two quartiles of household income (odds ratio, 0.74); insured by Medicare (OR, 0.93); received their care at a rural hospital (OR, 0.60) or at an urban nonteaching hospital (OR, 0.74); or had their resection performed by a general surgeon (OR, 0.47) or by a cardiac surgeon (OR, 0.47).
"A patient was more than twice as likely to have a lymphadenectomy performed if the lung cancer resection was performed by a thoracic surgeon," Dr. Ellis said.
Next, the researchers assessed the impact of case volume on their multivariate model. They determined that for every doubling of thoracic surgery case volume, there was a significant increase in the likelihood that a lymphadenectomy would be performed (OR, 1.28).
On the other hand, for every doubling of general surgery case volume, there was a significant decrease in lymphadenectomy rates (OR, 0.95). Doubling of cardiac surgery case volume did not affect lymphadenectomy rates.
"Lymphadenectomy rates for all surgeon groups did improve over the study period," Dr. Ellis said.
"However, despite these improvements, cardiac and general surgeons still have lymphadenectomy rates significantly lower than [those of] cardiac surgeons. The next step is to ensure that all patients receive adequate staging of the mediastinum, possibly through disseminating knowledge, creating centers of excellence, or providing opportunities to learn the skills necessary to perform adequate lung cancer surgery."
She acknowledged certain limitations of the study, including the fact that it contains only single-admission information. "It also has limited cancer-specific data such as stage, and has no mechanism for long-term follow-up," she said. In addition, surgeons are anonymous in the database, so board certification could not be determined.
Dr. Ellis said that she had no relevant financial disclosures to make.
SAN DIEGO - General surgeons perform the majority of lung resections for cancer in the United States, yet lung cancer resections performed by thoracic surgeons had significantly lower in-hospital mortality rates than did those performed by general surgeons and cardiac surgeons, according to results of a large analysis of national hospital data.
When performing a lung cancer resection, thoracic surgeons performed lymphadenectomy significantly more often than did general surgeons and cardiac surgeons.
"Lymph node status in lung cancer is the main determinant of stage, prognosis, and need for further therapy," Dr. Michelle Ellis said at the annual meeting of the Society of Thoracic Surgeons.
"The performance of lymphadenectomy at the time of lung cancer resection can be considered a process measure of quality."
Previously published studies have demonstrated that general surgeons perform the majority of thoracic cases in the United States, while surgeons who specialize in thoracic surgery have lower perioperative morbidity and mortality.
"Furthermore, patients who have their lung resection performed by a board-certified cardiothoracic surgeon specializing in general thoracic surgery have longer overall and cancer-specific survival," said Dr. Ellis of Oregon Health and Science University, Portland.
"We hypothesized that the completeness of intraoperative oncologic staging at the time of primary lung cancer resection varies by surgeon specialty, and may explain the observed differences in outcome."
To test the hypothesis, Dr. Ellis, with the assistance of Dr. Paul H. Schipper and Dr. John T. Vetto, reviewed 222,233 primary lung cancer cases from the Nationwide Inpatient Sample from 1998 to 2007 who were treated surgically with limited lung resection, lobectomy, or pneumonectomy.
The main outcome measure was the presence of lymphadenectomy or mediastinoscopy performed during the same admission.
The researchers divided the surgeons into three main groups based on their case mix of thoracic, cardiac, or other types of surgery. A thoracic surgeon was defined as someone who performed greater than 75% general thoracic surgery operations and less than 10% cardiac operations; a general surgeon was defined as someone who performed fewer than 75% thoracic operations and fewer than 10% cardiac operations, and a cardiac surgeon was defined as someone who performed greater than 10% cardiac operations.
Dr. Ellis reported that lung cancer resections were performed by general surgeons in 62% of cases, by cardiac surgeons in 35% of cases, and by thoracic surgeons in 3% of cases.
The median annual case volume was 21 for thoracic surgeons, 23 for cardiac surgeons, and 8 for general surgeons.
In-hospital mortality rates for thoracic, cardiac, and general surgeons were 2.3%, 3.4%, and 4.0%, respectively. This translated into an odds ratio for in-hospital mortality of 1.33 for cases performed by cardiac surgeons and 1.55 for those performed by general surgeons.
Thoracic surgeons performed lymphadenectomy significantly more often than did their counterparts (73% vs. 55% for both cardiac and general surgeons). Thoracic surgeons also performed mediastinoscopy significantly more often (16% vs. 10% by cardiac surgeons and 11% by general surgeons).
Multivariate analysis revealed that patients were significantly less likely to undergo lymphadenectomy if they were in the lowest two quartiles of household income (odds ratio, 0.74); insured by Medicare (OR, 0.93); received their care at a rural hospital (OR, 0.60) or at an urban nonteaching hospital (OR, 0.74); or had their resection performed by a general surgeon (OR, 0.47) or by a cardiac surgeon (OR, 0.47).
"A patient was more than twice as likely to have a lymphadenectomy performed if the lung cancer resection was performed by a thoracic surgeon," Dr. Ellis said.
Next, the researchers assessed the impact of case volume on their multivariate model. They determined that for every doubling of thoracic surgery case volume, there was a significant increase in the likelihood that a lymphadenectomy would be performed (OR, 1.28).
On the other hand, for every doubling of general surgery case volume, there was a significant decrease in lymphadenectomy rates (OR, 0.95). Doubling of cardiac surgery case volume did not affect lymphadenectomy rates.
"Lymphadenectomy rates for all surgeon groups did improve over the study period," Dr. Ellis said.
"However, despite these improvements, cardiac and general surgeons still have lymphadenectomy rates significantly lower than [those of] cardiac surgeons. The next step is to ensure that all patients receive adequate staging of the mediastinum, possibly through disseminating knowledge, creating centers of excellence, or providing opportunities to learn the skills necessary to perform adequate lung cancer surgery."
She acknowledged certain limitations of the study, including the fact that it contains only single-admission information. "It also has limited cancer-specific data such as stage, and has no mechanism for long-term follow-up," she said. In addition, surgeons are anonymous in the database, so board certification could not be determined.
Dr. Ellis said that she had no relevant financial disclosures to make.
FDA Clears Imaging App for iPhone/iPad
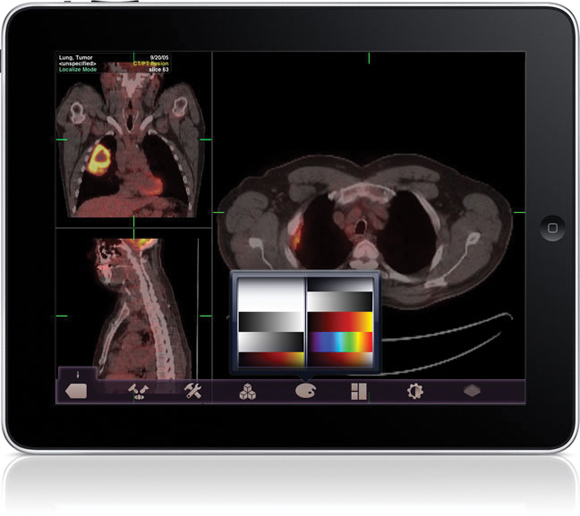
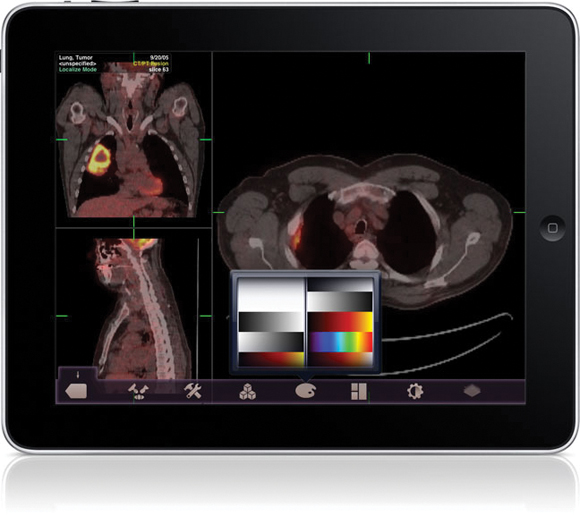
The Food and Drug Administration gave its first clearance to an application that will allow physicians to review radiology images on Apple's iPad and iPhone in the absence of a standard workstation.
The FDA cleared the Mobile MIM app for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and positron emission tomography. The agency cautioned that it is not intended to replace standard workstations, and should only be used when one is not available.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image's luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine if he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site Mobile MIM is now available in Apple's App Store.
The Food and Drug Administration gave its first clearance to an application that will allow physicians to review radiology images on Apple's iPad and iPhone in the absence of a standard workstation.
The FDA cleared the Mobile MIM app for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and positron emission tomography. The agency cautioned that it is not intended to replace standard workstations, and should only be used when one is not available.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image's luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine if he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site Mobile MIM is now available in Apple's App Store.
The Food and Drug Administration gave its first clearance to an application that will allow physicians to review radiology images on Apple's iPad and iPhone in the absence of a standard workstation.
The FDA cleared the Mobile MIM app for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and positron emission tomography. The agency cautioned that it is not intended to replace standard workstations, and should only be used when one is not available.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image's luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine if he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site Mobile MIM is now available in Apple's App Store.
FDA Clears Imaging App for iPhone/iPad
The Food and Drug Administration gave its first clearance to an application that will allow physicians to review radiology images on Apple's iPad and iPhone in the absence of a standard workstation.
The FDA cleared the Mobile MIM app for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and positron emission tomography. The agency cautioned that it is not intended to replace standard workstations, and should only be used when one is not available.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image's luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine if he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site Mobile MIM is now available in Apple's App Store.
The Food and Drug Administration gave its first clearance to an application that will allow physicians to review radiology images on Apple's iPad and iPhone in the absence of a standard workstation.
The FDA cleared the Mobile MIM app for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and positron emission tomography. The agency cautioned that it is not intended to replace standard workstations, and should only be used when one is not available.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image's luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine if he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site Mobile MIM is now available in Apple's App Store.
The Food and Drug Administration gave its first clearance to an application that will allow physicians to review radiology images on Apple's iPad and iPhone in the absence of a standard workstation.
The FDA cleared the Mobile MIM app for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and positron emission tomography. The agency cautioned that it is not intended to replace standard workstations, and should only be used when one is not available.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image's luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine if he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site Mobile MIM is now available in Apple's App Store.
FDA Clears Imaging App for iPhone/iPad
The Food and Drug Administration gave its first clearance to an application that will allow physicians to review radiology images on Apple's iPad and iPhone in the absence of a standard workstation.
The FDA cleared the Mobile MIM app for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and positron emission tomography. The agency cautioned that it is not intended to replace standard workstations, and should only be used when one is not available.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image's luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine if he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site Mobile MIM is now available in Apple's App Store.
The Food and Drug Administration gave its first clearance to an application that will allow physicians to review radiology images on Apple's iPad and iPhone in the absence of a standard workstation.
The FDA cleared the Mobile MIM app for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and positron emission tomography. The agency cautioned that it is not intended to replace standard workstations, and should only be used when one is not available.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image's luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine if he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site Mobile MIM is now available in Apple's App Store.
The Food and Drug Administration gave its first clearance to an application that will allow physicians to review radiology images on Apple's iPad and iPhone in the absence of a standard workstation.
The FDA cleared the Mobile MIM app for viewing images and making diagnoses using computed tomography, magnetic resonance imaging, and positron emission tomography. The agency cautioned that it is not intended to replace standard workstations, and should only be used when one is not available.
The app can measure distance on the image as well as image intensity; it can also display measurement lines, regions of interest, and annotations.
The FDA noted that the luminance displayed by a mobile device can vary greatly, even among identical models. The image's luminance also can vary based on ambient lighting. The app includes an interactive contrast test that will allow a user to determine if he or she can properly distinguish subtle differences in contrast.
The Mobile MIM app was created by Cleveland-based MIM Software Inc. The company said on its Web site Mobile MIM is now available in Apple's App Store.
First-Line Tarceva May Benefit NSCLC With EGFR Mutation
Genentech Inc. and partner OSI Pharmaceuticals Inc. are set to pursue a broader label for Tarceva (erlotinib) in the United States as a first-line treatment of advanced non-small cell lung cancer with epidermal growth factor receptor mutations, after reporting positive top-line results in that setting from a phase III European study.
Genentech announced that compared with platinum-based chemo-therapy, Tarceva, an EGFR inhibitor, improved progression-free survival in an interim analysis of the EURTAC study of 178 newly-diagnosed advanced NSCLC patients who had tested positive for the mutations. Safety was in line with Tarceva's profile. In light of the efficacy and safety results, the trial was halted early on the recommendation of its independent data monitoring committee.
Tarceva is currently approved in the United States and Europe as a maintenance and second-line treatment for advanced or metastatic NSCLC with and without EGFR activating mutations. An estimated 10% of NSCLC carries the EGFR mutations and according to Genentech a first-line indication would mean Tarceva could emerge as the first-choice for that sliver of the patient population, ahead of chemotherapy and other drugs approved for first-line NSCLC.
Genentech's parent company, Roche, had already submitted a bid to expand the drug's label to the European Medicines Agency in June 2010.
Then, in November 2010, Roche announced that it was sublicensing a diagnostic assay for EGFR mutations from Genzyme Corporation and collaborating with OSI on the development of a PCR- based companion diagnostic test to identify people with non-small cell lung cancer that harbors EGFR activating mutations.
Genentech and OSI plan to talk to the Food and Drug Administration about possibilities for a first-line indication in NSCLC and also for the companion diagnostic test in development, but timing on these discussions has not yet been decided.
It's unclear whether the drug would be submitted to the FDA simultaneously with a diagnostic test, which was the case in a recent approval of a new, narrow indication for Herceptin in a particular type of gastric cancer.
The test in development by Roche and OSI was not the same diagnostic used in the EURTAC study, which was designed and sponsored by the Spanish Lung Cancer Group. Genentech said it still needs to validate the test used in the EURTAC study using samples from the trial, prior to talks with FDA. It's also unclear at this time whether another study beyond EURTAC would be needed to expand the U.S. label.
Genentech did not disclose the magnitude of the benefit for progression-free survival - the primary end point - in the EURTAC trial. Secondary end points include overall survival, 1-year survival, objective response rate, and safety profile.
In the SATURN trial of Tarceva as a maintenance therapy for NSCLC, the drug showed only a modest PFS benefit for NSCLC patients overall (12.3 weeks for the drug versus 11.1 weeks for placebo). Its use as a maintenance treatment has proven controversial since the FDA approved the indication despite a negative vote by an advisory committee.
However, SATURN showed dramatically better results for patients who had EGFR mutations. In this subgroup, which accounted for 11% of the total population, PFS was 44.6 weeks for the treated group versus the 11 weeks for placebo. Based on the data, some physician surveys have suggested more willingness to use Tarceva as a maintenance treatment in the case of EGFR mutations.
Elsevier Global Medical News and "The Pink Sheet" are published by Elsevier.
Genentech Inc. and partner OSI Pharmaceuticals Inc. are set to pursue a broader label for Tarceva (erlotinib) in the United States as a first-line treatment of advanced non-small cell lung cancer with epidermal growth factor receptor mutations, after reporting positive top-line results in that setting from a phase III European study.
Genentech announced that compared with platinum-based chemo-therapy, Tarceva, an EGFR inhibitor, improved progression-free survival in an interim analysis of the EURTAC study of 178 newly-diagnosed advanced NSCLC patients who had tested positive for the mutations. Safety was in line with Tarceva's profile. In light of the efficacy and safety results, the trial was halted early on the recommendation of its independent data monitoring committee.
Tarceva is currently approved in the United States and Europe as a maintenance and second-line treatment for advanced or metastatic NSCLC with and without EGFR activating mutations. An estimated 10% of NSCLC carries the EGFR mutations and according to Genentech a first-line indication would mean Tarceva could emerge as the first-choice for that sliver of the patient population, ahead of chemotherapy and other drugs approved for first-line NSCLC.
Genentech's parent company, Roche, had already submitted a bid to expand the drug's label to the European Medicines Agency in June 2010.
Then, in November 2010, Roche announced that it was sublicensing a diagnostic assay for EGFR mutations from Genzyme Corporation and collaborating with OSI on the development of a PCR- based companion diagnostic test to identify people with non-small cell lung cancer that harbors EGFR activating mutations.
Genentech and OSI plan to talk to the Food and Drug Administration about possibilities for a first-line indication in NSCLC and also for the companion diagnostic test in development, but timing on these discussions has not yet been decided.
It's unclear whether the drug would be submitted to the FDA simultaneously with a diagnostic test, which was the case in a recent approval of a new, narrow indication for Herceptin in a particular type of gastric cancer.
The test in development by Roche and OSI was not the same diagnostic used in the EURTAC study, which was designed and sponsored by the Spanish Lung Cancer Group. Genentech said it still needs to validate the test used in the EURTAC study using samples from the trial, prior to talks with FDA. It's also unclear at this time whether another study beyond EURTAC would be needed to expand the U.S. label.
Genentech did not disclose the magnitude of the benefit for progression-free survival - the primary end point - in the EURTAC trial. Secondary end points include overall survival, 1-year survival, objective response rate, and safety profile.
In the SATURN trial of Tarceva as a maintenance therapy for NSCLC, the drug showed only a modest PFS benefit for NSCLC patients overall (12.3 weeks for the drug versus 11.1 weeks for placebo). Its use as a maintenance treatment has proven controversial since the FDA approved the indication despite a negative vote by an advisory committee.
However, SATURN showed dramatically better results for patients who had EGFR mutations. In this subgroup, which accounted for 11% of the total population, PFS was 44.6 weeks for the treated group versus the 11 weeks for placebo. Based on the data, some physician surveys have suggested more willingness to use Tarceva as a maintenance treatment in the case of EGFR mutations.
Elsevier Global Medical News and "The Pink Sheet" are published by Elsevier.
Genentech Inc. and partner OSI Pharmaceuticals Inc. are set to pursue a broader label for Tarceva (erlotinib) in the United States as a first-line treatment of advanced non-small cell lung cancer with epidermal growth factor receptor mutations, after reporting positive top-line results in that setting from a phase III European study.
Genentech announced that compared with platinum-based chemo-therapy, Tarceva, an EGFR inhibitor, improved progression-free survival in an interim analysis of the EURTAC study of 178 newly-diagnosed advanced NSCLC patients who had tested positive for the mutations. Safety was in line with Tarceva's profile. In light of the efficacy and safety results, the trial was halted early on the recommendation of its independent data monitoring committee.
Tarceva is currently approved in the United States and Europe as a maintenance and second-line treatment for advanced or metastatic NSCLC with and without EGFR activating mutations. An estimated 10% of NSCLC carries the EGFR mutations and according to Genentech a first-line indication would mean Tarceva could emerge as the first-choice for that sliver of the patient population, ahead of chemotherapy and other drugs approved for first-line NSCLC.
Genentech's parent company, Roche, had already submitted a bid to expand the drug's label to the European Medicines Agency in June 2010.
Then, in November 2010, Roche announced that it was sublicensing a diagnostic assay for EGFR mutations from Genzyme Corporation and collaborating with OSI on the development of a PCR- based companion diagnostic test to identify people with non-small cell lung cancer that harbors EGFR activating mutations.
Genentech and OSI plan to talk to the Food and Drug Administration about possibilities for a first-line indication in NSCLC and also for the companion diagnostic test in development, but timing on these discussions has not yet been decided.
It's unclear whether the drug would be submitted to the FDA simultaneously with a diagnostic test, which was the case in a recent approval of a new, narrow indication for Herceptin in a particular type of gastric cancer.
The test in development by Roche and OSI was not the same diagnostic used in the EURTAC study, which was designed and sponsored by the Spanish Lung Cancer Group. Genentech said it still needs to validate the test used in the EURTAC study using samples from the trial, prior to talks with FDA. It's also unclear at this time whether another study beyond EURTAC would be needed to expand the U.S. label.
Genentech did not disclose the magnitude of the benefit for progression-free survival - the primary end point - in the EURTAC trial. Secondary end points include overall survival, 1-year survival, objective response rate, and safety profile.
In the SATURN trial of Tarceva as a maintenance therapy for NSCLC, the drug showed only a modest PFS benefit for NSCLC patients overall (12.3 weeks for the drug versus 11.1 weeks for placebo). Its use as a maintenance treatment has proven controversial since the FDA approved the indication despite a negative vote by an advisory committee.
However, SATURN showed dramatically better results for patients who had EGFR mutations. In this subgroup, which accounted for 11% of the total population, PFS was 44.6 weeks for the treated group versus the 11 weeks for placebo. Based on the data, some physician surveys have suggested more willingness to use Tarceva as a maintenance treatment in the case of EGFR mutations.
Elsevier Global Medical News and "The Pink Sheet" are published by Elsevier.
Lung Debris May Help Identify Surgical Margins
CHICAGO - A novel technique utilizing stapled lung debris could help determine adequate and inadequate surgical margins in resected non-small cell lung cancer, results of a prospective study suggest.
Researchers at Albany (N.Y.) Medical College and the Hospital of St. Raphael in New Haven, Conn., are using cytology to analyze lung tissue taken from spent staple cartridges used during sublobar resection. The staple cartridge is simply mixed with 30 cc of normal saline and serves as the cytologic margin, Dr. Thomas Fabian explained at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
"People have [observed] that certain staples used through cancers can potentially contaminate new tissue planes, so that is how the idea was born," he said in an interview.
Dr. Fabian and his colleagues prospectively compared staple-line cytology with traditional histopathologic evaluation of surgical specimens taken from 97 patients undergoing diagnostic sublobar wedge resection between November 2007 and September 2009. Of the 98 specimens retrieved, 30 were benign and 68 were malignant.
Staple-line cytology was 100% accurate in the evaluation of benign lesions when compared with histology, he said.
In the 68 malignant nodules, initial blinded cytologic evaluation was positive in 7, surgical pathology was positive in 6, and both were positive in 4.
Subsequent unblinded review of both specimens changed the final pathologic interpretation in 4 (6%) of the 68 cases, said Dr. Fabian, chief of thoracic surgery at the Albany Medical Center. The interpretation changed from a negative margin to a positive margin in 3 surgical specimens (7%) and in 1 staple-line cytology specimen (2%).
According to analysis of the unblinded data, staple-line cytology demonstrated an overall accuracy of 96%, with 88% sensitivity, 97% specificity, 70% positive-predictive value, and 99% negative-predictive value.
Dr. Fabian described staple-line cytology as a simple technique that could serve as an adjunct to the gold standard of histopathology, which he said is prone to inaccuracies including both false positives and false negatives.
"We need to reevaluate the techniques that allow us to accurately assess surgical margins - particularly in the setting of sublobar resections, given the growing interest in this technique," according to Dr. Fabian.
"The cytologic technique appears to be sensitive, specific, and accurate, but it does need to be validated at other institutions and with additional studies," he added.
Dr. Fabian acknowledged that by design the study lacked clinical outcome data and said further evaluation is ongoing. The next step is to evaluate the technique in patients undergoing sublobar resection with curative intent.
Of the 68 malignant samples, 43 were diagnosed as adenocarcinoma, 7 as squamous cell carcinoma, 3 as large cell, 1 as small cell, 5 as carcinoid, and 9 as other histologies.
Dr. Fabian disclosed serving as a speaker for, and receiving research funding and honoraria from, Covidien. His coauthors reported no conflicts.
CHICAGO - A novel technique utilizing stapled lung debris could help determine adequate and inadequate surgical margins in resected non-small cell lung cancer, results of a prospective study suggest.
Researchers at Albany (N.Y.) Medical College and the Hospital of St. Raphael in New Haven, Conn., are using cytology to analyze lung tissue taken from spent staple cartridges used during sublobar resection. The staple cartridge is simply mixed with 30 cc of normal saline and serves as the cytologic margin, Dr. Thomas Fabian explained at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
"People have [observed] that certain staples used through cancers can potentially contaminate new tissue planes, so that is how the idea was born," he said in an interview.
Dr. Fabian and his colleagues prospectively compared staple-line cytology with traditional histopathologic evaluation of surgical specimens taken from 97 patients undergoing diagnostic sublobar wedge resection between November 2007 and September 2009. Of the 98 specimens retrieved, 30 were benign and 68 were malignant.
Staple-line cytology was 100% accurate in the evaluation of benign lesions when compared with histology, he said.
In the 68 malignant nodules, initial blinded cytologic evaluation was positive in 7, surgical pathology was positive in 6, and both were positive in 4.
Subsequent unblinded review of both specimens changed the final pathologic interpretation in 4 (6%) of the 68 cases, said Dr. Fabian, chief of thoracic surgery at the Albany Medical Center. The interpretation changed from a negative margin to a positive margin in 3 surgical specimens (7%) and in 1 staple-line cytology specimen (2%).
According to analysis of the unblinded data, staple-line cytology demonstrated an overall accuracy of 96%, with 88% sensitivity, 97% specificity, 70% positive-predictive value, and 99% negative-predictive value.
Dr. Fabian described staple-line cytology as a simple technique that could serve as an adjunct to the gold standard of histopathology, which he said is prone to inaccuracies including both false positives and false negatives.
"We need to reevaluate the techniques that allow us to accurately assess surgical margins - particularly in the setting of sublobar resections, given the growing interest in this technique," according to Dr. Fabian.
"The cytologic technique appears to be sensitive, specific, and accurate, but it does need to be validated at other institutions and with additional studies," he added.
Dr. Fabian acknowledged that by design the study lacked clinical outcome data and said further evaluation is ongoing. The next step is to evaluate the technique in patients undergoing sublobar resection with curative intent.
Of the 68 malignant samples, 43 were diagnosed as adenocarcinoma, 7 as squamous cell carcinoma, 3 as large cell, 1 as small cell, 5 as carcinoid, and 9 as other histologies.
Dr. Fabian disclosed serving as a speaker for, and receiving research funding and honoraria from, Covidien. His coauthors reported no conflicts.
CHICAGO - A novel technique utilizing stapled lung debris could help determine adequate and inadequate surgical margins in resected non-small cell lung cancer, results of a prospective study suggest.
Researchers at Albany (N.Y.) Medical College and the Hospital of St. Raphael in New Haven, Conn., are using cytology to analyze lung tissue taken from spent staple cartridges used during sublobar resection. The staple cartridge is simply mixed with 30 cc of normal saline and serves as the cytologic margin, Dr. Thomas Fabian explained at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
"People have [observed] that certain staples used through cancers can potentially contaminate new tissue planes, so that is how the idea was born," he said in an interview.
Dr. Fabian and his colleagues prospectively compared staple-line cytology with traditional histopathologic evaluation of surgical specimens taken from 97 patients undergoing diagnostic sublobar wedge resection between November 2007 and September 2009. Of the 98 specimens retrieved, 30 were benign and 68 were malignant.
Staple-line cytology was 100% accurate in the evaluation of benign lesions when compared with histology, he said.
In the 68 malignant nodules, initial blinded cytologic evaluation was positive in 7, surgical pathology was positive in 6, and both were positive in 4.
Subsequent unblinded review of both specimens changed the final pathologic interpretation in 4 (6%) of the 68 cases, said Dr. Fabian, chief of thoracic surgery at the Albany Medical Center. The interpretation changed from a negative margin to a positive margin in 3 surgical specimens (7%) and in 1 staple-line cytology specimen (2%).
According to analysis of the unblinded data, staple-line cytology demonstrated an overall accuracy of 96%, with 88% sensitivity, 97% specificity, 70% positive-predictive value, and 99% negative-predictive value.
Dr. Fabian described staple-line cytology as a simple technique that could serve as an adjunct to the gold standard of histopathology, which he said is prone to inaccuracies including both false positives and false negatives.
"We need to reevaluate the techniques that allow us to accurately assess surgical margins - particularly in the setting of sublobar resections, given the growing interest in this technique," according to Dr. Fabian.
"The cytologic technique appears to be sensitive, specific, and accurate, but it does need to be validated at other institutions and with additional studies," he added.
Dr. Fabian acknowledged that by design the study lacked clinical outcome data and said further evaluation is ongoing. The next step is to evaluate the technique in patients undergoing sublobar resection with curative intent.
Of the 68 malignant samples, 43 were diagnosed as adenocarcinoma, 7 as squamous cell carcinoma, 3 as large cell, 1 as small cell, 5 as carcinoid, and 9 as other histologies.
Dr. Fabian disclosed serving as a speaker for, and receiving research funding and honoraria from, Covidien. His coauthors reported no conflicts.
Microscopic Vascular Invasion Emerging as a Powerful Prognosticator in Early Lung Cancer
CHICAGO - New data suggest that microscopic vascular invasion may be a more powerful prognosticator in early lung cancer than are the tumor size-based categories suggested in the new TNM staging system.
Italian researchers used histologic and immunohistochemical techniques to identify microscopic vascular invasion (MVI), or the presence of neoplastic structures inside the lumen of a vessel, in one-third (154) of 512 patients with resected, pathologically staged T1a to T3 node-negative non-small cell lung cancer (NSCLC). The 2009 edition of the tumor, node, metastasis (TNM) staging system for lung tumors was used.
MVI was significantly correlated with the presence of tumor-infiltrating lymphocytes (odds ratio 1.65, P value = .03), adenocarcinoma histology (OR 1.32, P = .003), and increased tumor size (OR 1.13, P = .009).
Five-year overall survival was significantly lower for patients with MVI at 50% vs. those without MVI at 66% (P = .001), Dr. Enrico Ruffini said at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
The difference in survival remained significant even in those with squamous cell carcinoma (45% vs. 61%, P = .05), although it was more pronounced in those with adenocarcinoma (56% vs. 70%, P = .03).
"Microscopic vascular invasion is a significant independent negative prognostic factor," he said.
When patients with pT1a-T2b tumors were stratified by T-size category, the presence of MVI resulted in a one-category upstaging for each T category, said Dr. Ruffini of the division of thoracic surgery at the University of Torino (Italy). For example, T1a patients with MVI had a prognosis similar to that of patients with T1b tumors without MVI. The number of T3 cases was too small to stratify.
T size was prognostic of survival in the MVI-negative patients (P = .03) but was not a statistically significant factor in MVI-positive patients (P = .9), indicating that MVI is indeed a more powerful prognosticator, he said.
The 2009 TNM stresses the importance of tumor size as a major prognostic factor, but no TNM edition has so far included MVI as a major determinant in the staging of NSCLC.
In a multivariate survival analysis that included age, sex, histology, grading, T-size determinant, MVI, perineural invasion, and tumor-infiltrating lymphocytes, MVI was a stronger prognostic indicator (hazard ratio 1.43, P = .02) than T-size determinant (HR 1.06, P = .06), Dr. Ruffini said.
"The use of adjuvant chemotherapy in NSCLC patients with MVI may be considered," he said.
Invited discussant Dr. Mark Socinski pointed out that 88% of patients in the analysis had 5 cm or smaller tumors, a category of patients in which the role of adjuvant therapy has been discouraged. He highlighted the recent LACE meta-analysis of 4,584 NSCLC patients in five cisplatin-based adjuvant chemotherapy trials that showed an overall significant survival benefit of 4% at 5 years, but also a potentially negative effect in resected stage 1A (Ann. Oncol. 2010 Oct;21 Suppl. 7:vii196-vii198).
"We need to make sure [MVI] is easily reproducible amongst pathologists, and we also clearly need to demonstrate that adjuvant therapy can overcome the biologic impact of this histopathologic finding," said Dr. Socinski of the Lineberger Comprehensive Cancer Center at the University of North Carolina, Chapel Hill.
Dr. Ruffini acknowledged that bias could have been introduced into the study through its retrospective design, use of overall survival rather than disease-free survival as an outcome measure, and the long study period of January 1998 to August 2008. Prospective validation of MVI is underway using the prospective International Association for the Study of Lung Cancer database, he said.
The median tumor size among the 512 patients was 3.4 cm, with 164 classified as having T1a (less than 2 cm) tumors, 123 T1b (2-3 cm), 164 T2a (3-5 cm), 50 T2b (5-7 cm), and 11 T3 (greater than 7 cm) tumors.
The researchers and Dr. Socinski disclosed no relevant conflicts.
Subtle histologic markers have long been championed as a potential means to this ends, but historically gain little traction because essentially all are trumped by the presence of either metastic disease or regional lymph node involvement as important risks for recurrence. Consequently, the use of more sophisticated, but perhaps less reproduceable, pathologic markers is retricted to node-negative cancers, where T (of TNM) descriptors are important. This represents only about one-quarter of all lung cancers detected.
The authors have proposed microscopic vascular invasion (MVI) as an important factor that might be a reasonable addition to the T aspect of the new staging system. Their data demonstrate that MVI (found in a relatively small cohort of all node-negative patients in their study) appears to be an important risk for mortality. However, the road to the perfect staging system is paved with new histopathologic markers, and few are adopted because another one soon emerges and it is difficult for pathologists to keep up.
I think that molecular and radiologic characterization will eventually supplant all such subjective histopathologic markers and, within the next few years, will make the microscope something we'll be telling our grandkids about.
Subtle histologic markers have long been championed as a potential means to this ends, but historically gain little traction because essentially all are trumped by the presence of either metastic disease or regional lymph node involvement as important risks for recurrence. Consequently, the use of more sophisticated, but perhaps less reproduceable, pathologic markers is retricted to node-negative cancers, where T (of TNM) descriptors are important. This represents only about one-quarter of all lung cancers detected.
The authors have proposed microscopic vascular invasion (MVI) as an important factor that might be a reasonable addition to the T aspect of the new staging system. Their data demonstrate that MVI (found in a relatively small cohort of all node-negative patients in their study) appears to be an important risk for mortality. However, the road to the perfect staging system is paved with new histopathologic markers, and few are adopted because another one soon emerges and it is difficult for pathologists to keep up.
I think that molecular and radiologic characterization will eventually supplant all such subjective histopathologic markers and, within the next few years, will make the microscope something we'll be telling our grandkids about.
Subtle histologic markers have long been championed as a potential means to this ends, but historically gain little traction because essentially all are trumped by the presence of either metastic disease or regional lymph node involvement as important risks for recurrence. Consequently, the use of more sophisticated, but perhaps less reproduceable, pathologic markers is retricted to node-negative cancers, where T (of TNM) descriptors are important. This represents only about one-quarter of all lung cancers detected.
The authors have proposed microscopic vascular invasion (MVI) as an important factor that might be a reasonable addition to the T aspect of the new staging system. Their data demonstrate that MVI (found in a relatively small cohort of all node-negative patients in their study) appears to be an important risk for mortality. However, the road to the perfect staging system is paved with new histopathologic markers, and few are adopted because another one soon emerges and it is difficult for pathologists to keep up.
I think that molecular and radiologic characterization will eventually supplant all such subjective histopathologic markers and, within the next few years, will make the microscope something we'll be telling our grandkids about.
CHICAGO - New data suggest that microscopic vascular invasion may be a more powerful prognosticator in early lung cancer than are the tumor size-based categories suggested in the new TNM staging system.
Italian researchers used histologic and immunohistochemical techniques to identify microscopic vascular invasion (MVI), or the presence of neoplastic structures inside the lumen of a vessel, in one-third (154) of 512 patients with resected, pathologically staged T1a to T3 node-negative non-small cell lung cancer (NSCLC). The 2009 edition of the tumor, node, metastasis (TNM) staging system for lung tumors was used.
MVI was significantly correlated with the presence of tumor-infiltrating lymphocytes (odds ratio 1.65, P value = .03), adenocarcinoma histology (OR 1.32, P = .003), and increased tumor size (OR 1.13, P = .009).
Five-year overall survival was significantly lower for patients with MVI at 50% vs. those without MVI at 66% (P = .001), Dr. Enrico Ruffini said at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
The difference in survival remained significant even in those with squamous cell carcinoma (45% vs. 61%, P = .05), although it was more pronounced in those with adenocarcinoma (56% vs. 70%, P = .03).
"Microscopic vascular invasion is a significant independent negative prognostic factor," he said.
When patients with pT1a-T2b tumors were stratified by T-size category, the presence of MVI resulted in a one-category upstaging for each T category, said Dr. Ruffini of the division of thoracic surgery at the University of Torino (Italy). For example, T1a patients with MVI had a prognosis similar to that of patients with T1b tumors without MVI. The number of T3 cases was too small to stratify.
T size was prognostic of survival in the MVI-negative patients (P = .03) but was not a statistically significant factor in MVI-positive patients (P = .9), indicating that MVI is indeed a more powerful prognosticator, he said.
The 2009 TNM stresses the importance of tumor size as a major prognostic factor, but no TNM edition has so far included MVI as a major determinant in the staging of NSCLC.
In a multivariate survival analysis that included age, sex, histology, grading, T-size determinant, MVI, perineural invasion, and tumor-infiltrating lymphocytes, MVI was a stronger prognostic indicator (hazard ratio 1.43, P = .02) than T-size determinant (HR 1.06, P = .06), Dr. Ruffini said.
"The use of adjuvant chemotherapy in NSCLC patients with MVI may be considered," he said.
Invited discussant Dr. Mark Socinski pointed out that 88% of patients in the analysis had 5 cm or smaller tumors, a category of patients in which the role of adjuvant therapy has been discouraged. He highlighted the recent LACE meta-analysis of 4,584 NSCLC patients in five cisplatin-based adjuvant chemotherapy trials that showed an overall significant survival benefit of 4% at 5 years, but also a potentially negative effect in resected stage 1A (Ann. Oncol. 2010 Oct;21 Suppl. 7:vii196-vii198).
"We need to make sure [MVI] is easily reproducible amongst pathologists, and we also clearly need to demonstrate that adjuvant therapy can overcome the biologic impact of this histopathologic finding," said Dr. Socinski of the Lineberger Comprehensive Cancer Center at the University of North Carolina, Chapel Hill.
Dr. Ruffini acknowledged that bias could have been introduced into the study through its retrospective design, use of overall survival rather than disease-free survival as an outcome measure, and the long study period of January 1998 to August 2008. Prospective validation of MVI is underway using the prospective International Association for the Study of Lung Cancer database, he said.
The median tumor size among the 512 patients was 3.4 cm, with 164 classified as having T1a (less than 2 cm) tumors, 123 T1b (2-3 cm), 164 T2a (3-5 cm), 50 T2b (5-7 cm), and 11 T3 (greater than 7 cm) tumors.
The researchers and Dr. Socinski disclosed no relevant conflicts.
CHICAGO - New data suggest that microscopic vascular invasion may be a more powerful prognosticator in early lung cancer than are the tumor size-based categories suggested in the new TNM staging system.
Italian researchers used histologic and immunohistochemical techniques to identify microscopic vascular invasion (MVI), or the presence of neoplastic structures inside the lumen of a vessel, in one-third (154) of 512 patients with resected, pathologically staged T1a to T3 node-negative non-small cell lung cancer (NSCLC). The 2009 edition of the tumor, node, metastasis (TNM) staging system for lung tumors was used.
MVI was significantly correlated with the presence of tumor-infiltrating lymphocytes (odds ratio 1.65, P value = .03), adenocarcinoma histology (OR 1.32, P = .003), and increased tumor size (OR 1.13, P = .009).
Five-year overall survival was significantly lower for patients with MVI at 50% vs. those without MVI at 66% (P = .001), Dr. Enrico Ruffini said at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
The difference in survival remained significant even in those with squamous cell carcinoma (45% vs. 61%, P = .05), although it was more pronounced in those with adenocarcinoma (56% vs. 70%, P = .03).
"Microscopic vascular invasion is a significant independent negative prognostic factor," he said.
When patients with pT1a-T2b tumors were stratified by T-size category, the presence of MVI resulted in a one-category upstaging for each T category, said Dr. Ruffini of the division of thoracic surgery at the University of Torino (Italy). For example, T1a patients with MVI had a prognosis similar to that of patients with T1b tumors without MVI. The number of T3 cases was too small to stratify.
T size was prognostic of survival in the MVI-negative patients (P = .03) but was not a statistically significant factor in MVI-positive patients (P = .9), indicating that MVI is indeed a more powerful prognosticator, he said.
The 2009 TNM stresses the importance of tumor size as a major prognostic factor, but no TNM edition has so far included MVI as a major determinant in the staging of NSCLC.
In a multivariate survival analysis that included age, sex, histology, grading, T-size determinant, MVI, perineural invasion, and tumor-infiltrating lymphocytes, MVI was a stronger prognostic indicator (hazard ratio 1.43, P = .02) than T-size determinant (HR 1.06, P = .06), Dr. Ruffini said.
"The use of adjuvant chemotherapy in NSCLC patients with MVI may be considered," he said.
Invited discussant Dr. Mark Socinski pointed out that 88% of patients in the analysis had 5 cm or smaller tumors, a category of patients in which the role of adjuvant therapy has been discouraged. He highlighted the recent LACE meta-analysis of 4,584 NSCLC patients in five cisplatin-based adjuvant chemotherapy trials that showed an overall significant survival benefit of 4% at 5 years, but also a potentially negative effect in resected stage 1A (Ann. Oncol. 2010 Oct;21 Suppl. 7:vii196-vii198).
"We need to make sure [MVI] is easily reproducible amongst pathologists, and we also clearly need to demonstrate that adjuvant therapy can overcome the biologic impact of this histopathologic finding," said Dr. Socinski of the Lineberger Comprehensive Cancer Center at the University of North Carolina, Chapel Hill.
Dr. Ruffini acknowledged that bias could have been introduced into the study through its retrospective design, use of overall survival rather than disease-free survival as an outcome measure, and the long study period of January 1998 to August 2008. Prospective validation of MVI is underway using the prospective International Association for the Study of Lung Cancer database, he said.
The median tumor size among the 512 patients was 3.4 cm, with 164 classified as having T1a (less than 2 cm) tumors, 123 T1b (2-3 cm), 164 T2a (3-5 cm), 50 T2b (5-7 cm), and 11 T3 (greater than 7 cm) tumors.
The researchers and Dr. Socinski disclosed no relevant conflicts.
Low-Dose Aspirin Cut Cancer Death Rates 30%-40%
LONDON - The daily, long-term use of low-dose aspirin cuts the risk of death from several types of cancer, according to a large meta-analysis.
In a meta-analysis of eight randomized clinical trials involving 25,570 patients, low-dose aspirin taken for 5 years or longer reduced mortality from esophageal, pancreatic, brain, stomach, colorectal, prostate, and even lung cancer, with doses as low as 75 mg/day having an effect.
This is the first time that low-dose aspirin has been linked to a reduction in cancer mortality other than colorectal cancer, said Dr. Peter M. Rothwell, who conceived and coordinated the research.
Dr. Rothwell of the John Radcliffe Hospital and the University of Oxford, United Kingdom, and his associates in October 2010 showed that low-dose aspirin reduced the 20-year risk of new colon cancer cases by approximately one-quarter and deaths by a third (Lancet 2010;376:1741-50).
The current study looked at all deaths from cancer that occurred during or after completion of eight randomized clinical trials that had been performed to look at the effects of daily aspirin vs. control for the primary or secondary prevention of vascular events (Lancet 2010 [doi:10.1016/S0140-6736(10)62110-1]).
Across all eight trials, 674 cancer deaths occurred in 25,570 patients, with aspirin treatment significantly reducing the risk of death, compared with no aspirin treatment (pooled odds ratio [OR] 0.79, 95% confidence interval [CI] 0.68-0.92, P = .003).
Using individual patient data available for seven of the trials and in which 657 cancer deaths occurred in 23,535 patients, the benefit of aspirin therapy was apparent only after 5 years or more of follow-up. The hazard ratio (HR) for death from all types of cancer was 0.66 (95% CI 0.50-0.87, P =.003), with a greater effect seen in patients with gastrointestinal tumors (HR 0.46, 95% CI 0.27-0.77, P =.003).
"We found that within the trials, while people were still on aspirin vs. no aspirin, the aspirin group had about a 30%-40% reduction in cancer deaths between year 5 and the end of the trial," Dr. Rothwell said at a press briefing.
To determine the longer-term effects of aspirin on cancer mortality, the team looked more closely at data from three of the trials. These had all been conducted in the United Kingdom and continued to collect information on cancer deaths via national death certification and cancer registration systems long after the trials had concluded.
In all, individual patient data were obtained on 1,634 cancer deaths that had occurred in 12,659 patients. Aspirin was found to reduce the 20-year risk of death from all solid cancers by 20% (HR 0.80, 95% CI 0.72-0.88, P less than .0001). Again, the effect on gastrointestinal cancer was greater (HR 0.65, 95% CI 0.54-0.78, P less than .0001), but there was no effect on hematologic malignancies.
At least 5 years of therapy were needed to reduce the risk of death from esophageal, pancreatic, brain, or lung cancer, with 10 years or more treatment required to see an effect on stomach and colorectal cancer death rates, and 15 years or more for prostate cancer. With regard to both lung and esophageal cancer, the effect of aspirin was limited to adenocarcinomas.
While the findings do not mean that everyone over the age of 40 years should now start taking daily aspirin to prevent cancer, given the increased risk of bleeding in some individuals, "We should probably stop taking people off aspirin unless they've got side effects," Dr. Rothwell said in an interview, adding "We probably shouldn't discourage those who want to take aspirin as actively as we have been doing."
"There is a fundamental difference between the treatment and the prevention of a disease," said Dr. Peter Elwood, professor of epidemiology at Cardiff University, Wales. Dr. Elwood suggested that deciding to take a daily dose of aspirin to prevent cancer could be another choice patients make once they have all the relevant facts, much as lifestyle changes are advised but not prescribed for cardiovascular disease prevention.
Dr. Rothwell has received honoraria from pharmaceutical companies with an interest in antiplatelet therapy, including AstraZeneca and Bayer.
LONDON - The daily, long-term use of low-dose aspirin cuts the risk of death from several types of cancer, according to a large meta-analysis.
In a meta-analysis of eight randomized clinical trials involving 25,570 patients, low-dose aspirin taken for 5 years or longer reduced mortality from esophageal, pancreatic, brain, stomach, colorectal, prostate, and even lung cancer, with doses as low as 75 mg/day having an effect.
This is the first time that low-dose aspirin has been linked to a reduction in cancer mortality other than colorectal cancer, said Dr. Peter M. Rothwell, who conceived and coordinated the research.
Dr. Rothwell of the John Radcliffe Hospital and the University of Oxford, United Kingdom, and his associates in October 2010 showed that low-dose aspirin reduced the 20-year risk of new colon cancer cases by approximately one-quarter and deaths by a third (Lancet 2010;376:1741-50).
The current study looked at all deaths from cancer that occurred during or after completion of eight randomized clinical trials that had been performed to look at the effects of daily aspirin vs. control for the primary or secondary prevention of vascular events (Lancet 2010 [doi:10.1016/S0140-6736(10)62110-1]).
Across all eight trials, 674 cancer deaths occurred in 25,570 patients, with aspirin treatment significantly reducing the risk of death, compared with no aspirin treatment (pooled odds ratio [OR] 0.79, 95% confidence interval [CI] 0.68-0.92, P = .003).
Using individual patient data available for seven of the trials and in which 657 cancer deaths occurred in 23,535 patients, the benefit of aspirin therapy was apparent only after 5 years or more of follow-up. The hazard ratio (HR) for death from all types of cancer was 0.66 (95% CI 0.50-0.87, P =.003), with a greater effect seen in patients with gastrointestinal tumors (HR 0.46, 95% CI 0.27-0.77, P =.003).
"We found that within the trials, while people were still on aspirin vs. no aspirin, the aspirin group had about a 30%-40% reduction in cancer deaths between year 5 and the end of the trial," Dr. Rothwell said at a press briefing.
To determine the longer-term effects of aspirin on cancer mortality, the team looked more closely at data from three of the trials. These had all been conducted in the United Kingdom and continued to collect information on cancer deaths via national death certification and cancer registration systems long after the trials had concluded.
In all, individual patient data were obtained on 1,634 cancer deaths that had occurred in 12,659 patients. Aspirin was found to reduce the 20-year risk of death from all solid cancers by 20% (HR 0.80, 95% CI 0.72-0.88, P less than .0001). Again, the effect on gastrointestinal cancer was greater (HR 0.65, 95% CI 0.54-0.78, P less than .0001), but there was no effect on hematologic malignancies.
At least 5 years of therapy were needed to reduce the risk of death from esophageal, pancreatic, brain, or lung cancer, with 10 years or more treatment required to see an effect on stomach and colorectal cancer death rates, and 15 years or more for prostate cancer. With regard to both lung and esophageal cancer, the effect of aspirin was limited to adenocarcinomas.
While the findings do not mean that everyone over the age of 40 years should now start taking daily aspirin to prevent cancer, given the increased risk of bleeding in some individuals, "We should probably stop taking people off aspirin unless they've got side effects," Dr. Rothwell said in an interview, adding "We probably shouldn't discourage those who want to take aspirin as actively as we have been doing."
"There is a fundamental difference between the treatment and the prevention of a disease," said Dr. Peter Elwood, professor of epidemiology at Cardiff University, Wales. Dr. Elwood suggested that deciding to take a daily dose of aspirin to prevent cancer could be another choice patients make once they have all the relevant facts, much as lifestyle changes are advised but not prescribed for cardiovascular disease prevention.
Dr. Rothwell has received honoraria from pharmaceutical companies with an interest in antiplatelet therapy, including AstraZeneca and Bayer.
LONDON - The daily, long-term use of low-dose aspirin cuts the risk of death from several types of cancer, according to a large meta-analysis.
In a meta-analysis of eight randomized clinical trials involving 25,570 patients, low-dose aspirin taken for 5 years or longer reduced mortality from esophageal, pancreatic, brain, stomach, colorectal, prostate, and even lung cancer, with doses as low as 75 mg/day having an effect.
This is the first time that low-dose aspirin has been linked to a reduction in cancer mortality other than colorectal cancer, said Dr. Peter M. Rothwell, who conceived and coordinated the research.
Dr. Rothwell of the John Radcliffe Hospital and the University of Oxford, United Kingdom, and his associates in October 2010 showed that low-dose aspirin reduced the 20-year risk of new colon cancer cases by approximately one-quarter and deaths by a third (Lancet 2010;376:1741-50).
The current study looked at all deaths from cancer that occurred during or after completion of eight randomized clinical trials that had been performed to look at the effects of daily aspirin vs. control for the primary or secondary prevention of vascular events (Lancet 2010 [doi:10.1016/S0140-6736(10)62110-1]).
Across all eight trials, 674 cancer deaths occurred in 25,570 patients, with aspirin treatment significantly reducing the risk of death, compared with no aspirin treatment (pooled odds ratio [OR] 0.79, 95% confidence interval [CI] 0.68-0.92, P = .003).
Using individual patient data available for seven of the trials and in which 657 cancer deaths occurred in 23,535 patients, the benefit of aspirin therapy was apparent only after 5 years or more of follow-up. The hazard ratio (HR) for death from all types of cancer was 0.66 (95% CI 0.50-0.87, P =.003), with a greater effect seen in patients with gastrointestinal tumors (HR 0.46, 95% CI 0.27-0.77, P =.003).
"We found that within the trials, while people were still on aspirin vs. no aspirin, the aspirin group had about a 30%-40% reduction in cancer deaths between year 5 and the end of the trial," Dr. Rothwell said at a press briefing.
To determine the longer-term effects of aspirin on cancer mortality, the team looked more closely at data from three of the trials. These had all been conducted in the United Kingdom and continued to collect information on cancer deaths via national death certification and cancer registration systems long after the trials had concluded.
In all, individual patient data were obtained on 1,634 cancer deaths that had occurred in 12,659 patients. Aspirin was found to reduce the 20-year risk of death from all solid cancers by 20% (HR 0.80, 95% CI 0.72-0.88, P less than .0001). Again, the effect on gastrointestinal cancer was greater (HR 0.65, 95% CI 0.54-0.78, P less than .0001), but there was no effect on hematologic malignancies.
At least 5 years of therapy were needed to reduce the risk of death from esophageal, pancreatic, brain, or lung cancer, with 10 years or more treatment required to see an effect on stomach and colorectal cancer death rates, and 15 years or more for prostate cancer. With regard to both lung and esophageal cancer, the effect of aspirin was limited to adenocarcinomas.
While the findings do not mean that everyone over the age of 40 years should now start taking daily aspirin to prevent cancer, given the increased risk of bleeding in some individuals, "We should probably stop taking people off aspirin unless they've got side effects," Dr. Rothwell said in an interview, adding "We probably shouldn't discourage those who want to take aspirin as actively as we have been doing."
"There is a fundamental difference between the treatment and the prevention of a disease," said Dr. Peter Elwood, professor of epidemiology at Cardiff University, Wales. Dr. Elwood suggested that deciding to take a daily dose of aspirin to prevent cancer could be another choice patients make once they have all the relevant facts, much as lifestyle changes are advised but not prescribed for cardiovascular disease prevention.
Dr. Rothwell has received honoraria from pharmaceutical companies with an interest in antiplatelet therapy, including AstraZeneca and Bayer.
First-Line Tarceva May Benefit NSCLC With EGFR Mutation
Genentech Inc. and partner OSI Pharmaceuticals Inc. are set to pursue a broader label for Tarceva (erlotinib) in the United States as a first-line treatment of advanced non-small cell lung cancer with epidermal growth factor receptor mutations, after reporting positive top-line results in that setting from a phase III European study.
Genentech announced that compared with platinum-based chemo-therapy, Tarceva, an EGFR inhibitor, improved progression-free survival in an interim analysis of the EURTAC study of 178 newly-diagnosed advanced NSCLC patients who had tested positive for the mutations. Safety was in line with Tarceva's profile. In light of the efficacy and safety results, the trial was halted early on the recommendation of its independent data monitoring committee.
Tarceva is currently approved in the United States and Europe as a maintenance and second-line treatment for advanced or metastatic NSCLC with and without EGFR activating mutations. An estimated 10% of NSCLC carries the EGFR mutations and according to Genentech a first-line indication would mean Tarceva could emerge as the first-choice for that sliver of the patient population, ahead of chemotherapy and other drugs approved for first-line NSCLC.
Genentech's parent company, Roche, had already submitted a bid to expand the drug's label to the European Medicines Agency in June 2010.
Then, in November 2010, Roche announced that it was sublicensing a diagnostic assay for EGFR mutations from Genzyme Corporation and collaborating with OSI on the development of a PCR- based companion diagnostic test to identify people with non-small cell lung cancer that harbors EGFR activating mutations.
Genentech and OSI plan to talk to the Food and Drug Administration about possibilities for a first-line indication in NSCLC and also for the companion diagnostic test in development, but timing on these discussions has not yet been decided.
It's unclear whether the drug would be submitted to the FDA simultaneously with a diagnostic test, which was the case in a recent approval of a new, narrow indication for Herceptin in a particular type of gastric cancer.
The test in development by Roche and OSI was not the same diagnostic used in the EURTAC study, which was designed and sponsored by the Spanish Lung Cancer Group. Genentech said it still needs to validate the test used in the EURTAC study using samples from the trial, prior to talks with FDA. It's also unclear at this time whether another study beyond EURTAC would be needed to expand the U.S. label.
Genentech did not disclose the magnitude of the benefit for progression-free survival - the primary end point - in the EURTAC trial. Secondary end points include overall survival, 1-year survival, objective response rate, and safety profile.
In the SATURN trial of Tarceva as a maintenance therapy for NSCLC, the drug showed only a modest PFS benefit for NSCLC patients overall (12.3 weeks for the drug versus 11.1 weeks for placebo). Its use as a maintenance treatment has proven controversial since the FDA approved the indication despite a negative vote by an advisory committee.
However, SATURN showed dramatically better results for patients who had EGFR mutations. In this subgroup, which accounted for 11% of the total population, PFS was 44.6 weeks for the treated group versus the 11 weeks for placebo. Based on the data, some physician surveys have suggested more willingness to use Tarceva as a maintenance treatment in the case of EGFR mutations.
Elsevier Global Medical News and "The Pink Sheet" are published by Elsevier.
Genentech Inc. and partner OSI Pharmaceuticals Inc. are set to pursue a broader label for Tarceva (erlotinib) in the United States as a first-line treatment of advanced non-small cell lung cancer with epidermal growth factor receptor mutations, after reporting positive top-line results in that setting from a phase III European study.
Genentech announced that compared with platinum-based chemo-therapy, Tarceva, an EGFR inhibitor, improved progression-free survival in an interim analysis of the EURTAC study of 178 newly-diagnosed advanced NSCLC patients who had tested positive for the mutations. Safety was in line with Tarceva's profile. In light of the efficacy and safety results, the trial was halted early on the recommendation of its independent data monitoring committee.
Tarceva is currently approved in the United States and Europe as a maintenance and second-line treatment for advanced or metastatic NSCLC with and without EGFR activating mutations. An estimated 10% of NSCLC carries the EGFR mutations and according to Genentech a first-line indication would mean Tarceva could emerge as the first-choice for that sliver of the patient population, ahead of chemotherapy and other drugs approved for first-line NSCLC.
Genentech's parent company, Roche, had already submitted a bid to expand the drug's label to the European Medicines Agency in June 2010.
Then, in November 2010, Roche announced that it was sublicensing a diagnostic assay for EGFR mutations from Genzyme Corporation and collaborating with OSI on the development of a PCR- based companion diagnostic test to identify people with non-small cell lung cancer that harbors EGFR activating mutations.
Genentech and OSI plan to talk to the Food and Drug Administration about possibilities for a first-line indication in NSCLC and also for the companion diagnostic test in development, but timing on these discussions has not yet been decided.
It's unclear whether the drug would be submitted to the FDA simultaneously with a diagnostic test, which was the case in a recent approval of a new, narrow indication for Herceptin in a particular type of gastric cancer.
The test in development by Roche and OSI was not the same diagnostic used in the EURTAC study, which was designed and sponsored by the Spanish Lung Cancer Group. Genentech said it still needs to validate the test used in the EURTAC study using samples from the trial, prior to talks with FDA. It's also unclear at this time whether another study beyond EURTAC would be needed to expand the U.S. label.
Genentech did not disclose the magnitude of the benefit for progression-free survival - the primary end point - in the EURTAC trial. Secondary end points include overall survival, 1-year survival, objective response rate, and safety profile.
In the SATURN trial of Tarceva as a maintenance therapy for NSCLC, the drug showed only a modest PFS benefit for NSCLC patients overall (12.3 weeks for the drug versus 11.1 weeks for placebo). Its use as a maintenance treatment has proven controversial since the FDA approved the indication despite a negative vote by an advisory committee.
However, SATURN showed dramatically better results for patients who had EGFR mutations. In this subgroup, which accounted for 11% of the total population, PFS was 44.6 weeks for the treated group versus the 11 weeks for placebo. Based on the data, some physician surveys have suggested more willingness to use Tarceva as a maintenance treatment in the case of EGFR mutations.
Elsevier Global Medical News and "The Pink Sheet" are published by Elsevier.
Genentech Inc. and partner OSI Pharmaceuticals Inc. are set to pursue a broader label for Tarceva (erlotinib) in the United States as a first-line treatment of advanced non-small cell lung cancer with epidermal growth factor receptor mutations, after reporting positive top-line results in that setting from a phase III European study.
Genentech announced that compared with platinum-based chemo-therapy, Tarceva, an EGFR inhibitor, improved progression-free survival in an interim analysis of the EURTAC study of 178 newly-diagnosed advanced NSCLC patients who had tested positive for the mutations. Safety was in line with Tarceva's profile. In light of the efficacy and safety results, the trial was halted early on the recommendation of its independent data monitoring committee.
Tarceva is currently approved in the United States and Europe as a maintenance and second-line treatment for advanced or metastatic NSCLC with and without EGFR activating mutations. An estimated 10% of NSCLC carries the EGFR mutations and according to Genentech a first-line indication would mean Tarceva could emerge as the first-choice for that sliver of the patient population, ahead of chemotherapy and other drugs approved for first-line NSCLC.
Genentech's parent company, Roche, had already submitted a bid to expand the drug's label to the European Medicines Agency in June 2010.
Then, in November 2010, Roche announced that it was sublicensing a diagnostic assay for EGFR mutations from Genzyme Corporation and collaborating with OSI on the development of a PCR- based companion diagnostic test to identify people with non-small cell lung cancer that harbors EGFR activating mutations.
Genentech and OSI plan to talk to the Food and Drug Administration about possibilities for a first-line indication in NSCLC and also for the companion diagnostic test in development, but timing on these discussions has not yet been decided.
It's unclear whether the drug would be submitted to the FDA simultaneously with a diagnostic test, which was the case in a recent approval of a new, narrow indication for Herceptin in a particular type of gastric cancer.
The test in development by Roche and OSI was not the same diagnostic used in the EURTAC study, which was designed and sponsored by the Spanish Lung Cancer Group. Genentech said it still needs to validate the test used in the EURTAC study using samples from the trial, prior to talks with FDA. It's also unclear at this time whether another study beyond EURTAC would be needed to expand the U.S. label.
Genentech did not disclose the magnitude of the benefit for progression-free survival - the primary end point - in the EURTAC trial. Secondary end points include overall survival, 1-year survival, objective response rate, and safety profile.
In the SATURN trial of Tarceva as a maintenance therapy for NSCLC, the drug showed only a modest PFS benefit for NSCLC patients overall (12.3 weeks for the drug versus 11.1 weeks for placebo). Its use as a maintenance treatment has proven controversial since the FDA approved the indication despite a negative vote by an advisory committee.
However, SATURN showed dramatically better results for patients who had EGFR mutations. In this subgroup, which accounted for 11% of the total population, PFS was 44.6 weeks for the treated group versus the 11 weeks for placebo. Based on the data, some physician surveys have suggested more willingness to use Tarceva as a maintenance treatment in the case of EGFR mutations.
Elsevier Global Medical News and "The Pink Sheet" are published by Elsevier.
Lung Debris May Help Identify Surgical Margins
CHICAGO - A novel technique utilizing stapled lung debris could help determine adequate and inadequate surgical margins in resected non-small cell lung cancer, results of a prospective study suggest.
Researchers at Albany (N.Y.) Medical College and the Hospital of St. Raphael in New Haven, Conn., are using cytology to analyze lung tissue taken from spent staple cartridges used during sublobar resection. The staple cartridge is simply mixed with 30 cc of normal saline and serves as the cytologic margin, Dr. Thomas Fabian explained at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
"People have [observed] that certain staples used through cancers can potentially contaminate new tissue planes, so that is how the idea was born," he said in an interview.
Dr. Fabian and his colleagues prospectively compared staple-line cytology with traditional histopathologic evaluation of surgical specimens taken from 97 patients undergoing diagnostic sublobar wedge resection between November 2007 and September 2009. Of the 98 specimens retrieved, 30 were benign and 68 were malignant.
Staple-line cytology was 100% accurate in the evaluation of benign lesions when compared with histology, he said.
In the 68 malignant nodules, initial blinded cytologic evaluation was positive in 7, surgical pathology was positive in 6, and both were positive in 4.
Subsequent unblinded review of both specimens changed the final pathologic interpretation in 4 (6%) of the 68 cases, said Dr. Fabian, chief of thoracic surgery at the Albany Medical Center. The interpretation changed from a negative margin to a positive margin in 3 surgical specimens (7%) and in 1 staple-line cytology specimen (2%).
According to analysis of the unblinded data, staple-line cytology demonstrated an overall accuracy of 96%, with 88% sensitivity, 97% specificity, 70% positive-predictive value, and 99% negative-predictive value.
Dr. Fabian described staple-line cytology as a simple technique that could serve as an adjunct to the gold standard of histopathology, which he said is prone to inaccuracies including both false positives and false negatives.
"We need to reevaluate the techniques that allow us to accurately assess surgical margins - particularly in the setting of sublobar resections, given the growing interest in this technique," according to Dr. Fabian.
"The cytologic technique appears to be sensitive, specific, and accurate, but it does need to be validated at other institutions and with additional studies," he added.
Dr. Fabian acknowledged that by design the study lacked clinical outcome data and said further evaluation is ongoing. The next step is to evaluate the technique in patients undergoing sublobar resection with curative intent.
Of the 68 malignant samples, 43 were diagnosed as adenocarcinoma, 7 as squamous cell carcinoma, 3 as large cell, 1 as small cell, 5 as carcinoid, and 9 as other histologies.
Dr. Fabian disclosed serving as a speaker for, and receiving research funding and honoraria from, Covidien. His coauthors reported no conflicts.
CHICAGO - A novel technique utilizing stapled lung debris could help determine adequate and inadequate surgical margins in resected non-small cell lung cancer, results of a prospective study suggest.
Researchers at Albany (N.Y.) Medical College and the Hospital of St. Raphael in New Haven, Conn., are using cytology to analyze lung tissue taken from spent staple cartridges used during sublobar resection. The staple cartridge is simply mixed with 30 cc of normal saline and serves as the cytologic margin, Dr. Thomas Fabian explained at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
"People have [observed] that certain staples used through cancers can potentially contaminate new tissue planes, so that is how the idea was born," he said in an interview.
Dr. Fabian and his colleagues prospectively compared staple-line cytology with traditional histopathologic evaluation of surgical specimens taken from 97 patients undergoing diagnostic sublobar wedge resection between November 2007 and September 2009. Of the 98 specimens retrieved, 30 were benign and 68 were malignant.
Staple-line cytology was 100% accurate in the evaluation of benign lesions when compared with histology, he said.
In the 68 malignant nodules, initial blinded cytologic evaluation was positive in 7, surgical pathology was positive in 6, and both were positive in 4.
Subsequent unblinded review of both specimens changed the final pathologic interpretation in 4 (6%) of the 68 cases, said Dr. Fabian, chief of thoracic surgery at the Albany Medical Center. The interpretation changed from a negative margin to a positive margin in 3 surgical specimens (7%) and in 1 staple-line cytology specimen (2%).
According to analysis of the unblinded data, staple-line cytology demonstrated an overall accuracy of 96%, with 88% sensitivity, 97% specificity, 70% positive-predictive value, and 99% negative-predictive value.
Dr. Fabian described staple-line cytology as a simple technique that could serve as an adjunct to the gold standard of histopathology, which he said is prone to inaccuracies including both false positives and false negatives.
"We need to reevaluate the techniques that allow us to accurately assess surgical margins - particularly in the setting of sublobar resections, given the growing interest in this technique," according to Dr. Fabian.
"The cytologic technique appears to be sensitive, specific, and accurate, but it does need to be validated at other institutions and with additional studies," he added.
Dr. Fabian acknowledged that by design the study lacked clinical outcome data and said further evaluation is ongoing. The next step is to evaluate the technique in patients undergoing sublobar resection with curative intent.
Of the 68 malignant samples, 43 were diagnosed as adenocarcinoma, 7 as squamous cell carcinoma, 3 as large cell, 1 as small cell, 5 as carcinoid, and 9 as other histologies.
Dr. Fabian disclosed serving as a speaker for, and receiving research funding and honoraria from, Covidien. His coauthors reported no conflicts.
CHICAGO - A novel technique utilizing stapled lung debris could help determine adequate and inadequate surgical margins in resected non-small cell lung cancer, results of a prospective study suggest.
Researchers at Albany (N.Y.) Medical College and the Hospital of St. Raphael in New Haven, Conn., are using cytology to analyze lung tissue taken from spent staple cartridges used during sublobar resection. The staple cartridge is simply mixed with 30 cc of normal saline and serves as the cytologic margin, Dr. Thomas Fabian explained at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
"People have [observed] that certain staples used through cancers can potentially contaminate new tissue planes, so that is how the idea was born," he said in an interview.
Dr. Fabian and his colleagues prospectively compared staple-line cytology with traditional histopathologic evaluation of surgical specimens taken from 97 patients undergoing diagnostic sublobar wedge resection between November 2007 and September 2009. Of the 98 specimens retrieved, 30 were benign and 68 were malignant.
Staple-line cytology was 100% accurate in the evaluation of benign lesions when compared with histology, he said.
In the 68 malignant nodules, initial blinded cytologic evaluation was positive in 7, surgical pathology was positive in 6, and both were positive in 4.
Subsequent unblinded review of both specimens changed the final pathologic interpretation in 4 (6%) of the 68 cases, said Dr. Fabian, chief of thoracic surgery at the Albany Medical Center. The interpretation changed from a negative margin to a positive margin in 3 surgical specimens (7%) and in 1 staple-line cytology specimen (2%).
According to analysis of the unblinded data, staple-line cytology demonstrated an overall accuracy of 96%, with 88% sensitivity, 97% specificity, 70% positive-predictive value, and 99% negative-predictive value.
Dr. Fabian described staple-line cytology as a simple technique that could serve as an adjunct to the gold standard of histopathology, which he said is prone to inaccuracies including both false positives and false negatives.
"We need to reevaluate the techniques that allow us to accurately assess surgical margins - particularly in the setting of sublobar resections, given the growing interest in this technique," according to Dr. Fabian.
"The cytologic technique appears to be sensitive, specific, and accurate, but it does need to be validated at other institutions and with additional studies," he added.
Dr. Fabian acknowledged that by design the study lacked clinical outcome data and said further evaluation is ongoing. The next step is to evaluate the technique in patients undergoing sublobar resection with curative intent.
Of the 68 malignant samples, 43 were diagnosed as adenocarcinoma, 7 as squamous cell carcinoma, 3 as large cell, 1 as small cell, 5 as carcinoid, and 9 as other histologies.
Dr. Fabian disclosed serving as a speaker for, and receiving research funding and honoraria from, Covidien. His coauthors reported no conflicts.