User login
How to improve outcomes in gestational diabetes— for mother and baby
- Are oral hypoglycemic agents equivalent to insulin in the treatment of gestational diabetes?
Aaron B. Caughey, MD, PhD; (Examining the Evidence, March 2011)
Gestational diabetes mellitus (GDM) was once thought to be a mild condition that had few lasting consequences. Now, we know that it carries significant short- and long-term implications for women and their offspring. A growing body of evidence suggests that early detection and aggressive monitoring and management of GDM can greatly improve outcomes for pregnant women and their babies. This article outlines the parameters of this approach.
GDM increases maternal risks even after pregnancy
Even mild degrees of hyperglycemia during pregnancy can harm mother and baby. Hyperglycemia is associated with an elevated risk of hypertensive disorders during pregnancy, as well as preterm labor, cesarean delivery, and later metabolic disorders—but there is no obvious threshold of hyperglycemia at which these risks increase.1
GDM is a strong predictor that a woman will later develop type 2 diabetes.2 One study found that GDM increases that risk as much as sevenfold over a woman’s lifetime.3 GDM is also associated with an elevated risk of cardiovascular disease, particularly if the woman has a family history of type 2 diabetes.4
Obesity appears to worsen the consequences of GDM for women.5 A recent literature review found that the risk of GDM is positively associated with the prepregnancy body mass index (BMI).6
One of the most common and serious types of morbidity affecting infants born to women who have GDM is large size for gestational age, which imparts a significantly elevated risk of injury at the time of vaginal birth and increases the risk of trauma to the mother during cesarean delivery.
GDM is not benign in the fetus, either
Evidence is increasing that GDM raises the risk of adverse clinical consequences in the fetus. The two most frequent and serious types of morbidity affecting infants born to mothers who have GDM are:
- large size for gestational age
- respiratory distress syndrome.7
Infants who are large for gestational age (LGA) face a significantly elevated risk of injury at the time of vaginal birth, such as shoulder dystocia and newborn asphyxia.8 Cesarean delivery is the preferred route for the LGA infant, but it often increases the risk of trauma to the mother, compared with the vaginal route.8
Respiratory distress syndrome, common among premature infants, also affects many infants born to women who have GDM— even near-term infants—because hyperglycemia appears to delay fetal lung maturity.9
Recent studies indicate that exposure to maternal hyperglycemia also increases a child’s risk of long-term complications. Children born to mothers who have GDM have nearly twice the risk of childhood obesity and metabolic syndrome, compared with children born to mothers who do not have GDM.10 In addition, several studies have found that children born to obese mothers who have GDM are more likely to develop type 2 diabetes than are children of non-obese mothers without GDM.3,11
Occasionally, infants of women who have GDM are born with hypoglycemia; this condition arises from an insulin surge in response to maternal hyperglycemia. In an infant, hypoglycemia can lead to seizures and death, and maternal hypoglycemia can cause neuro-psychological deficits in the infant.12
Other health problems related to GDM include jaundice and developmental delays in walking and other motor skills.13
The two-step, 100-g, 3-hour oral glucose tolerance test (OGTT) has been the gold standard for diagnosis of GDM in the United States for many years. However, this approach is expensive—rendering it impractical in some settings. Moreover, reproducibility is only approximately 78%.14
The World Health Organization recently reviewed evidence underlying various diagnostic techniques and recommended a one-step, 2-hour, 75-g OGTT for GDM.14 Another recent review of the literature on the various screening protocols underscores the validity of this approach.15
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study of more than 23,000 nondiabetic women incorporated the 2-hour, 75-g OGTT.16 Investigators found that elevated glucose levels on this test are highly predictive of birth weight above the 90th percentile and a cord-blood serum C-peptide level above the 90th percentile. However, the test has weaker predictive value for primary cesarean delivery and clinical neonatal hypoglycemia.
Based on the work of HAPO, the American Diabetes Association (ADA) revised its guidelines for diabetes assessment and now recommends that physicians perform a 75-g OGTT at 24 to 28 weeks’ gestation, with plasma glucose measurement in the fasting state and at 1 and 2 hours. A single abnormal level merits a diagnosis of GDM in women not previously diagnosed with overt diabetes.17
Any diagnosis of GDM warrants aggressive treatment
Perhaps the single greatest controversy in the field of diabetes centers on the level of hyperglycemia at which aggressive treatment of GDM should begin. Traditionally, aggressive therapy (i.e., insulin) was not initiated until the fasting plasma glucose level reached 95 mg/dL or higher or the 1-hour glucose level reached 130 mg/dL or higher (ALGORITHM). However, recent studies suggest that aggressive treatment should be administered for any diagnosis of GDM.
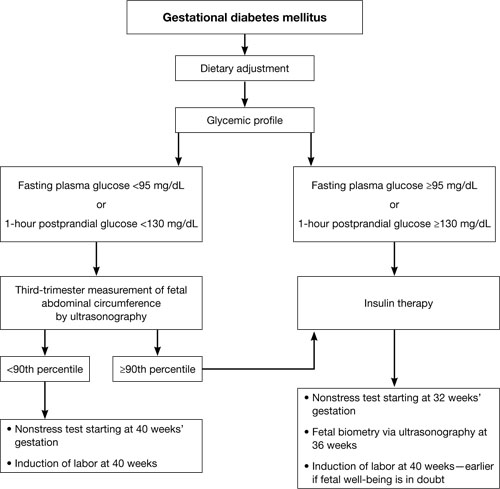
Typical management plan for gestational diabetesFor example, the HAPO study was designed to determine the level of glucose intolerance during pregnancy, short of diabetes, associated with adverse outcomes.16 It found that even mild hyperglycemia is associated with adverse fetal outcomes and that diagnostic criteria for GDM cannot easily be based on any particular level of hyperglycemia.
Several other studies have demonstrated that aggressive treatment of mild GDM can ameliorate many of its negative effects. In 2005, for instance, Bonomo and coworkers explored the effect on newborns of treating a very mild level of gestational glucose intolerance among 300 women.18 The randomized trial involved three groups:
- Group A – standard management, which entailed no special care, diet, or pharmacotherapy
- Group B – dietary treatment and regular monitoring
- Group C – randomly selected pregnant women who were matched by BMI and age and who had normal screening test results.
The women in Group B experienced significant improvements in fasting and 2-hour postprandial glucose levels. In addition, the fasting glucose level at delivery was significantly lower in Group B, compared with the other two groups. More important, fewer LGA infants were born to the women in Group B (6.0%) than in Group A (14.0%) and Group C (9.1%).
Landon and colleagues obtained similar findings when they randomized almost 1,000 pregnant women who had mild GDM to 1) usual prenatal care or 2) dietary intervention, self-monitoring of blood glucose, and, if necessary, insulin therapy.19
Insulin analogs have joined the treatment options
Standard treatment for GDM involves diet and nutritional therapy and, when needed, insulin. A diet that limits carbohydrate in-take can significantly reduce glycemia after meals in women who have GDM.20
For years, human insulin was the only option for treating diabetes that cannot be controlled by diet and lifestyle modifications alone. Recently, however, several insulin analogs have come on the market. Only two of them have been well studied in pregnancy:
- 28B-L-lysine-29B-L-proline insulin (lispro)
- 28B-aspartic acid insulin (aspart).
These two analogs have been tested primarily in the setting of type 1 diabetes, but both improve postprandial glucose excursions, compared with human regular insulin, and both may be associated with a lower risk of delayed postprandial hypoglycemia.21,22
Some oral agents appear to be safe
Several oral antihyperglycemic agents are available for the management of diabetes (TABLE). However, in the past, oral agents were not used in pregnant women out of concern over reports of fetal anomalies and other adverse outcomes in animal studies and some human cases. More recent evidence suggests that glyburide and metformin are safe and effective for use in GDM.23-25
Oral antihyperglycemic agents and their potential side effects
| Class | Agents | Effects |
|---|---|---|
| Insulin secretagogue | Sulfonylureas and meglitinides such as glyburide, glipizide, glimepiride, repaglinide, nateglinide | Hypoglycemia if caloric intake is reduced Some are long-acting (increasing risk of prolonged hypoglycemia) |
| Biguanide | Metformin | Risk of lactic acidosis when used in the setting of renal dysfunction, circulatory compromise, or hypoxemia Relatively slow onset of action GI complications: nausea, diarrhea |
| Thiazoladinedione | Rosiglitazone, pioglitazone | Long delay to onset of action (2–3 weeks) Associated with fluid retention (particularly when used with insulin) and increased risk of congestive heart failure Use contraindicated in presence of liver disease or elevated transaminases |
| Alpha-glucosidase inhibitor | Acarbose, miglitol | Prandial/meal agent (no effect in the fasting patient) Abdominal bloating and flatus Pure dextrose is required to treat hypoglycemia that occurs in the setting of these agents |
| Glucagon-like peptide–1 mimetic | Exenatide | Newer agents with limited inpatient experience Abdominal bloating and nausea secondary to delayed gastric emptying |
| Dipeptidyl peptidase IV inhibitor | Sitagliptin | Newer agent with limited inpatient experience |
Langer and coworkers compared glyburide with insulin in the management of GDM and found the agents to be equally effective, with comparable levels of risk of large size for gestational age, macrosomia, hypoglycemia (in infants), NICU admission, and fetal anomaly.23 Subsequent studies have confirmed these findings, although at least one suggests that women who have a high fasting plasma glucose level may not respond adequately to glyburide.26 None of these studies has been large enough or long enough to truly assess whether these oral medications are equivalent to insulin in the management of GDM without posing significant long-term complications for mothers or babies, or both.
For more on the use of oral agents in GDM, see Dr. Aaron B. Caughey’s commentary on the subject of this issue.
Continuous monitoring may detect occult hyperglycemia and hypoglycemia
The traditional method of monitoring the blood glucose level is to stick a finger to obtain a blood sample and use a test strip and a meter to measure the concentration of glucose in the sample. Most meters on the market are reasonably accurate. However, research has demonstrated that they are least accurate during episodes of hypoglycemia.27
Automated continuous glucose-monitoring systems are less intrusive than the traditional method, but they are usually reserved for people who have type 1 diabetes requiring intensive insulin therapy. However, because data suggest that even short periods of hyperglycemia or hypoglycemia can be detrimental to a developing fetus, there is increasing interest in utilizing continuous glucose monitoring for GDM.
Several research groups have compared continuous glucose monitoring with finger-stick monitoring and found that women randomized to continuous monitoring have lower mean hemoglobin A1c levels from 32 to 36 weeks’ gestation.28,29 (See “Exploring the value of continuous glucose monitoring in gestational diabetes?”) Women undergoing continuous monitoring also have:
- lower mean birth-weight standard- deviation scores
- lower median customized birth-weight centiles
- a reduced risk of macrosomia.
One study found that information gleaned from continuous glucose monitoring provided additional information that altered clinical management in 42 of 68 (62%) cases. These additional data included evidence of undetected and potentially dangerous postprandial hyperglycemia and overnight hypoglycemia.29
Yogev and colleagues found that continuous glucose monitoring is significantly more sensitive than traditional methods in detecting periods of hypoglycemia in women who have GDM. They also found that asymptomatic hypoglycemic events are common during pharmacotherapy in gestations affected by GDM.30 The same group used continuous glucose monitoring at night in obese, nondiabetic women to identify previously undetected:
- high postprandial glucose peak values
- increased 1- and 2-hour postprandial glucose levels
- increased time to the glucose peak
- significantly lower mean blood glucose levels.31
Insurers were reluctant to cover continuous glucose monitoring devices when they first became available. Since then, however, much progress has been made. Nevertheless, inadequate reimbursement for the time it takes a clinician to change a patient’s treatment regimen and her subsequent management remains a significant barrier to adoption of these systems.32 The key to success with continuous glucose monitoring is to train the patient to use it properly.
Exploring the value of continuous glucose monitoring in gestational diabetes
Tanenberg R, Bode B, Lane W, et al. Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc. 2004;79(12):1521–1526.
The American Diabetes association recommends that patients on insulin self-monitor blood glucose three or four times daily to guide adjustments in therapy and ensure a stable and optimal hemoglobin A1c level. “however, adherence to frequent blood-glucose monitoring is low, and less than 54% of patients with insulin-treated diabetes are reported to self-monitor their blood glucose at least three times each day,” say tanenberg and coworkers.
To determine whether use of a continuous glucose-monitoring system improves metabolic control, the investigators randomized 109 patients who had insulin-treated diabetes to continuous monitoring or frequent self-monitoring. at enrollment, all patients had insulin-treated diabetes and inadequate metabolic control. at the end of the study, both groups used continuous monitoring for 3 days; these values were used to calculate measures of hypoglycemia.
In the study, the women in the self-monitoring group were counseled to measure capillary blood glucose a minimum of four times daily, as well as when they experienced symptoms of hypoglycemia, which was defined as a blood glucose measurement of 60 mg/dL or lower. any hypoglycemic event was considered to be over when the measurement exceeded 60 mg/dL for at least 30 minutes.
Findings
Hemoglobin A1c levels were similar between groups at baseline, and both groups showed significant (P < .001) and similar (P=.95) improvement in these levels after 12 weeks of study. however, the continuous-monitoring group had a significantly shorter duration of hypoglycemic events than the self-monitoring group at week 12 (49.4±40.8 minutes vs 81.0±61.1 minutes per event, respectively; P=.009).
Tanenberg and coworkers hypothesize that the improvement in hemoglobin A1c in the self-monitoring group was a result of monitoring that was more frequent (7 times a day) than is typical. they concluded that use of continuous monitoring to guide therapy adjustments in patients who use insulin significantly reduces the duration of hypoglycemia, compared with adjustments guided by self-monitoring values alone.
We want to hear from you! Tell us what you think.
1. Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the United States: determinants outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003;188(5):1372-1378.
2. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668-1669.
3. Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med. 2000;9(1):83-88.
4. Gunderson EP, Jacobs DR, Chiang V, et al. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am J Obstet Gynecol. 2009;201(2):177.e1-9.
5. van Hoorn J, Dekker G, Jeffries B. Gestational diabetes versus obesity as risk factors for pregnancy-induced hypertensive disorders and fetal macrosomia. Aust N Z J Obstet Gynaecol. 2002;42(1):29-34.
6. Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194-203.
7. Johns K, Olynik C, Mase R, Kreisman S, Tildesley H. Gestational diabetes mellitus outcome in 394 patients. J Obstet Gynaecol Can. 2006;28(2):122-127.
8. Jones CW. Gestational diabetes and its impact on the neonate. Neonatal Netw. 2001;20(6):17-23
9. De Luca AK, Nakazawa CY, Azevedo BC, Rudge MV, De Araujo Costa RA, Calderon IM. Influence of glycemic control on fetal lung maturity in gestations affected by diabetes or mild hyperglycemia. Acta Obstet Gynecol Scand. 2009;88(9):1036-1040.
10. Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200(6):672.-e1–4.
11. Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med. 2008;21(3):149-157
12. ter Braak EW, Evers IM, Willem Erkelens D, Visser GH. Maternal hypoglycemia during pregnancy in type 1 diabetes: maternal and fetal consequences. Diabetes Metab Res Rev. 2002;18(2):96-105.
13. Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2010;23(3):199-203.
14. Harlass FE, Brady K, Read JA. Reproducibility of the oral glucose tolerance test in pregnancy. Am J Obstet Gynecol. 1991;164(2):564-568.
15. Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care. 2008;31(8):1650-1655.
16. Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. New Engl J Med. 2008;358(19):1991-2002.
17. American Diabetes Association. Executive summary: standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S4-10.
18. Bonomo M, Corica D, Mion E, et al. Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: a randomized clinical trial. Diabet Med. 2005;22(11):1536-1541.
19. Landon MB, Spong CY, Thom E, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348.
20. 20Cypryk K, Kaminska P, Kosinski M, Pertynska-Marczewska M, Lewinski A. A comparison of the effectiveness tolerability and safety of high and low carbohydrate diets in women with gestational diabetes. Endokrynol Pol. 2007;58(4):314-319.
21. Lapolla A, Dalfrà MG, Spezia R, et al. Outcome of pregnancy in type 1 diabetic patients treated with insulin lispro or regular insulin: an Italian experience. Acta Diabetol. 2008;45(1):61-66.
22. Hod M, Damm P, Kaaja R, et al. Insulin Aspart Pregnancy Study Group. Fetal and perinatal outcomes in type 1 diabetes pregnancy: a randomized study comparing insulin aspart with human insulin in 322 subjects. Am J Obstet Gynecol. 2008;198(2):186-187.
23. Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzles O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343(16):1134-1138.
24. Ho FL, Liew CF, Cunanan EC, Lee KO. Oral hypoglycaemic agents for diabetes in pregnancy—an appraisal of the current evidence for oral anti-diabetic drug use in pregnancy. Ann Acad Med Singapore. 2007;36(8):672-678.
25. Nicholson W, Bolen S, Witkop CT, et al. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113(1):193-205.
26. Conway DL, Gonzales O, Skiver D. Use of glyburide for the treatment of gestational diabetes: the San Antonio experience. Matern Fetal Neonatal Med. 2004;15(1):51-55.
27. Carr S, Coustan DR, Martelly P, et al. Precision of reflectance meters in screening for gestational diabetes. Obstet Gynecol. 1989;73(5 Pt 1):727-731.
28. Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ. 2008;337:a1680.-doi: 10.1136/bmj.a1680.
29. McLachlan K, Jenkins A, O’Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Aust N Z J Obstet Gynaecol. 2007;47(3):186-190.
30. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Undiagnosed asymptomatic hypoglycemia: diet insulin, and glyburide for gestational diabetic pregnancy. Obstet Gynecol. 2004;104(1):88-93.
31. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191(3):949-953.
32. Bartelme A, Bridger P. The role of reimbursement in the adoption of continuous glucose monitors. J Diabetes Sci Technol. 2009;3(4):992-995.
- Are oral hypoglycemic agents equivalent to insulin in the treatment of gestational diabetes?
Aaron B. Caughey, MD, PhD; (Examining the Evidence, March 2011)
Gestational diabetes mellitus (GDM) was once thought to be a mild condition that had few lasting consequences. Now, we know that it carries significant short- and long-term implications for women and their offspring. A growing body of evidence suggests that early detection and aggressive monitoring and management of GDM can greatly improve outcomes for pregnant women and their babies. This article outlines the parameters of this approach.
GDM increases maternal risks even after pregnancy
Even mild degrees of hyperglycemia during pregnancy can harm mother and baby. Hyperglycemia is associated with an elevated risk of hypertensive disorders during pregnancy, as well as preterm labor, cesarean delivery, and later metabolic disorders—but there is no obvious threshold of hyperglycemia at which these risks increase.1
GDM is a strong predictor that a woman will later develop type 2 diabetes.2 One study found that GDM increases that risk as much as sevenfold over a woman’s lifetime.3 GDM is also associated with an elevated risk of cardiovascular disease, particularly if the woman has a family history of type 2 diabetes.4
Obesity appears to worsen the consequences of GDM for women.5 A recent literature review found that the risk of GDM is positively associated with the prepregnancy body mass index (BMI).6

One of the most common and serious types of morbidity affecting infants born to women who have GDM is large size for gestational age, which imparts a significantly elevated risk of injury at the time of vaginal birth and increases the risk of trauma to the mother during cesarean delivery.
GDM is not benign in the fetus, either
Evidence is increasing that GDM raises the risk of adverse clinical consequences in the fetus. The two most frequent and serious types of morbidity affecting infants born to mothers who have GDM are:
- large size for gestational age
- respiratory distress syndrome.7
Infants who are large for gestational age (LGA) face a significantly elevated risk of injury at the time of vaginal birth, such as shoulder dystocia and newborn asphyxia.8 Cesarean delivery is the preferred route for the LGA infant, but it often increases the risk of trauma to the mother, compared with the vaginal route.8
Respiratory distress syndrome, common among premature infants, also affects many infants born to women who have GDM— even near-term infants—because hyperglycemia appears to delay fetal lung maturity.9
Recent studies indicate that exposure to maternal hyperglycemia also increases a child’s risk of long-term complications. Children born to mothers who have GDM have nearly twice the risk of childhood obesity and metabolic syndrome, compared with children born to mothers who do not have GDM.10 In addition, several studies have found that children born to obese mothers who have GDM are more likely to develop type 2 diabetes than are children of non-obese mothers without GDM.3,11
Occasionally, infants of women who have GDM are born with hypoglycemia; this condition arises from an insulin surge in response to maternal hyperglycemia. In an infant, hypoglycemia can lead to seizures and death, and maternal hypoglycemia can cause neuro-psychological deficits in the infant.12
Other health problems related to GDM include jaundice and developmental delays in walking and other motor skills.13
The two-step, 100-g, 3-hour oral glucose tolerance test (OGTT) has been the gold standard for diagnosis of GDM in the United States for many years. However, this approach is expensive—rendering it impractical in some settings. Moreover, reproducibility is only approximately 78%.14
The World Health Organization recently reviewed evidence underlying various diagnostic techniques and recommended a one-step, 2-hour, 75-g OGTT for GDM.14 Another recent review of the literature on the various screening protocols underscores the validity of this approach.15
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study of more than 23,000 nondiabetic women incorporated the 2-hour, 75-g OGTT.16 Investigators found that elevated glucose levels on this test are highly predictive of birth weight above the 90th percentile and a cord-blood serum C-peptide level above the 90th percentile. However, the test has weaker predictive value for primary cesarean delivery and clinical neonatal hypoglycemia.
Based on the work of HAPO, the American Diabetes Association (ADA) revised its guidelines for diabetes assessment and now recommends that physicians perform a 75-g OGTT at 24 to 28 weeks’ gestation, with plasma glucose measurement in the fasting state and at 1 and 2 hours. A single abnormal level merits a diagnosis of GDM in women not previously diagnosed with overt diabetes.17
Any diagnosis of GDM warrants aggressive treatment
Perhaps the single greatest controversy in the field of diabetes centers on the level of hyperglycemia at which aggressive treatment of GDM should begin. Traditionally, aggressive therapy (i.e., insulin) was not initiated until the fasting plasma glucose level reached 95 mg/dL or higher or the 1-hour glucose level reached 130 mg/dL or higher (ALGORITHM). However, recent studies suggest that aggressive treatment should be administered for any diagnosis of GDM.

Typical management plan for gestational diabetesFor example, the HAPO study was designed to determine the level of glucose intolerance during pregnancy, short of diabetes, associated with adverse outcomes.16 It found that even mild hyperglycemia is associated with adverse fetal outcomes and that diagnostic criteria for GDM cannot easily be based on any particular level of hyperglycemia.
Several other studies have demonstrated that aggressive treatment of mild GDM can ameliorate many of its negative effects. In 2005, for instance, Bonomo and coworkers explored the effect on newborns of treating a very mild level of gestational glucose intolerance among 300 women.18 The randomized trial involved three groups:
- Group A – standard management, which entailed no special care, diet, or pharmacotherapy
- Group B – dietary treatment and regular monitoring
- Group C – randomly selected pregnant women who were matched by BMI and age and who had normal screening test results.
The women in Group B experienced significant improvements in fasting and 2-hour postprandial glucose levels. In addition, the fasting glucose level at delivery was significantly lower in Group B, compared with the other two groups. More important, fewer LGA infants were born to the women in Group B (6.0%) than in Group A (14.0%) and Group C (9.1%).
Landon and colleagues obtained similar findings when they randomized almost 1,000 pregnant women who had mild GDM to 1) usual prenatal care or 2) dietary intervention, self-monitoring of blood glucose, and, if necessary, insulin therapy.19
Insulin analogs have joined the treatment options
Standard treatment for GDM involves diet and nutritional therapy and, when needed, insulin. A diet that limits carbohydrate in-take can significantly reduce glycemia after meals in women who have GDM.20
For years, human insulin was the only option for treating diabetes that cannot be controlled by diet and lifestyle modifications alone. Recently, however, several insulin analogs have come on the market. Only two of them have been well studied in pregnancy:
- 28B-L-lysine-29B-L-proline insulin (lispro)
- 28B-aspartic acid insulin (aspart).
These two analogs have been tested primarily in the setting of type 1 diabetes, but both improve postprandial glucose excursions, compared with human regular insulin, and both may be associated with a lower risk of delayed postprandial hypoglycemia.21,22
Some oral agents appear to be safe
Several oral antihyperglycemic agents are available for the management of diabetes (TABLE). However, in the past, oral agents were not used in pregnant women out of concern over reports of fetal anomalies and other adverse outcomes in animal studies and some human cases. More recent evidence suggests that glyburide and metformin are safe and effective for use in GDM.23-25
Oral antihyperglycemic agents and their potential side effects
| Class | Agents | Effects |
|---|---|---|
| Insulin secretagogue | Sulfonylureas and meglitinides such as glyburide, glipizide, glimepiride, repaglinide, nateglinide | Hypoglycemia if caloric intake is reduced Some are long-acting (increasing risk of prolonged hypoglycemia) |
| Biguanide | Metformin | Risk of lactic acidosis when used in the setting of renal dysfunction, circulatory compromise, or hypoxemia Relatively slow onset of action GI complications: nausea, diarrhea |
| Thiazoladinedione | Rosiglitazone, pioglitazone | Long delay to onset of action (2–3 weeks) Associated with fluid retention (particularly when used with insulin) and increased risk of congestive heart failure Use contraindicated in presence of liver disease or elevated transaminases |
| Alpha-glucosidase inhibitor | Acarbose, miglitol | Prandial/meal agent (no effect in the fasting patient) Abdominal bloating and flatus Pure dextrose is required to treat hypoglycemia that occurs in the setting of these agents |
| Glucagon-like peptide–1 mimetic | Exenatide | Newer agents with limited inpatient experience Abdominal bloating and nausea secondary to delayed gastric emptying |
| Dipeptidyl peptidase IV inhibitor | Sitagliptin | Newer agent with limited inpatient experience |
Langer and coworkers compared glyburide with insulin in the management of GDM and found the agents to be equally effective, with comparable levels of risk of large size for gestational age, macrosomia, hypoglycemia (in infants), NICU admission, and fetal anomaly.23 Subsequent studies have confirmed these findings, although at least one suggests that women who have a high fasting plasma glucose level may not respond adequately to glyburide.26 None of these studies has been large enough or long enough to truly assess whether these oral medications are equivalent to insulin in the management of GDM without posing significant long-term complications for mothers or babies, or both.
For more on the use of oral agents in GDM, see Dr. Aaron B. Caughey’s commentary on the subject of this issue.
Continuous monitoring may detect occult hyperglycemia and hypoglycemia
The traditional method of monitoring the blood glucose level is to stick a finger to obtain a blood sample and use a test strip and a meter to measure the concentration of glucose in the sample. Most meters on the market are reasonably accurate. However, research has demonstrated that they are least accurate during episodes of hypoglycemia.27
Automated continuous glucose-monitoring systems are less intrusive than the traditional method, but they are usually reserved for people who have type 1 diabetes requiring intensive insulin therapy. However, because data suggest that even short periods of hyperglycemia or hypoglycemia can be detrimental to a developing fetus, there is increasing interest in utilizing continuous glucose monitoring for GDM.
Several research groups have compared continuous glucose monitoring with finger-stick monitoring and found that women randomized to continuous monitoring have lower mean hemoglobin A1c levels from 32 to 36 weeks’ gestation.28,29 (See “Exploring the value of continuous glucose monitoring in gestational diabetes?”) Women undergoing continuous monitoring also have:
- lower mean birth-weight standard- deviation scores
- lower median customized birth-weight centiles
- a reduced risk of macrosomia.
One study found that information gleaned from continuous glucose monitoring provided additional information that altered clinical management in 42 of 68 (62%) cases. These additional data included evidence of undetected and potentially dangerous postprandial hyperglycemia and overnight hypoglycemia.29
Yogev and colleagues found that continuous glucose monitoring is significantly more sensitive than traditional methods in detecting periods of hypoglycemia in women who have GDM. They also found that asymptomatic hypoglycemic events are common during pharmacotherapy in gestations affected by GDM.30 The same group used continuous glucose monitoring at night in obese, nondiabetic women to identify previously undetected:
- high postprandial glucose peak values
- increased 1- and 2-hour postprandial glucose levels
- increased time to the glucose peak
- significantly lower mean blood glucose levels.31
Insurers were reluctant to cover continuous glucose monitoring devices when they first became available. Since then, however, much progress has been made. Nevertheless, inadequate reimbursement for the time it takes a clinician to change a patient’s treatment regimen and her subsequent management remains a significant barrier to adoption of these systems.32 The key to success with continuous glucose monitoring is to train the patient to use it properly.
Exploring the value of continuous glucose monitoring in gestational diabetes
Tanenberg R, Bode B, Lane W, et al. Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc. 2004;79(12):1521–1526.
The American Diabetes association recommends that patients on insulin self-monitor blood glucose three or four times daily to guide adjustments in therapy and ensure a stable and optimal hemoglobin A1c level. “however, adherence to frequent blood-glucose monitoring is low, and less than 54% of patients with insulin-treated diabetes are reported to self-monitor their blood glucose at least three times each day,” say tanenberg and coworkers.
To determine whether use of a continuous glucose-monitoring system improves metabolic control, the investigators randomized 109 patients who had insulin-treated diabetes to continuous monitoring or frequent self-monitoring. at enrollment, all patients had insulin-treated diabetes and inadequate metabolic control. at the end of the study, both groups used continuous monitoring for 3 days; these values were used to calculate measures of hypoglycemia.
In the study, the women in the self-monitoring group were counseled to measure capillary blood glucose a minimum of four times daily, as well as when they experienced symptoms of hypoglycemia, which was defined as a blood glucose measurement of 60 mg/dL or lower. any hypoglycemic event was considered to be over when the measurement exceeded 60 mg/dL for at least 30 minutes.
Findings
Hemoglobin A1c levels were similar between groups at baseline, and both groups showed significant (P < .001) and similar (P=.95) improvement in these levels after 12 weeks of study. however, the continuous-monitoring group had a significantly shorter duration of hypoglycemic events than the self-monitoring group at week 12 (49.4±40.8 minutes vs 81.0±61.1 minutes per event, respectively; P=.009).
Tanenberg and coworkers hypothesize that the improvement in hemoglobin A1c in the self-monitoring group was a result of monitoring that was more frequent (7 times a day) than is typical. they concluded that use of continuous monitoring to guide therapy adjustments in patients who use insulin significantly reduces the duration of hypoglycemia, compared with adjustments guided by self-monitoring values alone.
We want to hear from you! Tell us what you think.
- Are oral hypoglycemic agents equivalent to insulin in the treatment of gestational diabetes?
Aaron B. Caughey, MD, PhD; (Examining the Evidence, March 2011)
Gestational diabetes mellitus (GDM) was once thought to be a mild condition that had few lasting consequences. Now, we know that it carries significant short- and long-term implications for women and their offspring. A growing body of evidence suggests that early detection and aggressive monitoring and management of GDM can greatly improve outcomes for pregnant women and their babies. This article outlines the parameters of this approach.
GDM increases maternal risks even after pregnancy
Even mild degrees of hyperglycemia during pregnancy can harm mother and baby. Hyperglycemia is associated with an elevated risk of hypertensive disorders during pregnancy, as well as preterm labor, cesarean delivery, and later metabolic disorders—but there is no obvious threshold of hyperglycemia at which these risks increase.1
GDM is a strong predictor that a woman will later develop type 2 diabetes.2 One study found that GDM increases that risk as much as sevenfold over a woman’s lifetime.3 GDM is also associated with an elevated risk of cardiovascular disease, particularly if the woman has a family history of type 2 diabetes.4
Obesity appears to worsen the consequences of GDM for women.5 A recent literature review found that the risk of GDM is positively associated with the prepregnancy body mass index (BMI).6

One of the most common and serious types of morbidity affecting infants born to women who have GDM is large size for gestational age, which imparts a significantly elevated risk of injury at the time of vaginal birth and increases the risk of trauma to the mother during cesarean delivery.
GDM is not benign in the fetus, either
Evidence is increasing that GDM raises the risk of adverse clinical consequences in the fetus. The two most frequent and serious types of morbidity affecting infants born to mothers who have GDM are:
- large size for gestational age
- respiratory distress syndrome.7
Infants who are large for gestational age (LGA) face a significantly elevated risk of injury at the time of vaginal birth, such as shoulder dystocia and newborn asphyxia.8 Cesarean delivery is the preferred route for the LGA infant, but it often increases the risk of trauma to the mother, compared with the vaginal route.8
Respiratory distress syndrome, common among premature infants, also affects many infants born to women who have GDM— even near-term infants—because hyperglycemia appears to delay fetal lung maturity.9
Recent studies indicate that exposure to maternal hyperglycemia also increases a child’s risk of long-term complications. Children born to mothers who have GDM have nearly twice the risk of childhood obesity and metabolic syndrome, compared with children born to mothers who do not have GDM.10 In addition, several studies have found that children born to obese mothers who have GDM are more likely to develop type 2 diabetes than are children of non-obese mothers without GDM.3,11
Occasionally, infants of women who have GDM are born with hypoglycemia; this condition arises from an insulin surge in response to maternal hyperglycemia. In an infant, hypoglycemia can lead to seizures and death, and maternal hypoglycemia can cause neuro-psychological deficits in the infant.12
Other health problems related to GDM include jaundice and developmental delays in walking and other motor skills.13
The two-step, 100-g, 3-hour oral glucose tolerance test (OGTT) has been the gold standard for diagnosis of GDM in the United States for many years. However, this approach is expensive—rendering it impractical in some settings. Moreover, reproducibility is only approximately 78%.14
The World Health Organization recently reviewed evidence underlying various diagnostic techniques and recommended a one-step, 2-hour, 75-g OGTT for GDM.14 Another recent review of the literature on the various screening protocols underscores the validity of this approach.15
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study of more than 23,000 nondiabetic women incorporated the 2-hour, 75-g OGTT.16 Investigators found that elevated glucose levels on this test are highly predictive of birth weight above the 90th percentile and a cord-blood serum C-peptide level above the 90th percentile. However, the test has weaker predictive value for primary cesarean delivery and clinical neonatal hypoglycemia.
Based on the work of HAPO, the American Diabetes Association (ADA) revised its guidelines for diabetes assessment and now recommends that physicians perform a 75-g OGTT at 24 to 28 weeks’ gestation, with plasma glucose measurement in the fasting state and at 1 and 2 hours. A single abnormal level merits a diagnosis of GDM in women not previously diagnosed with overt diabetes.17
Any diagnosis of GDM warrants aggressive treatment
Perhaps the single greatest controversy in the field of diabetes centers on the level of hyperglycemia at which aggressive treatment of GDM should begin. Traditionally, aggressive therapy (i.e., insulin) was not initiated until the fasting plasma glucose level reached 95 mg/dL or higher or the 1-hour glucose level reached 130 mg/dL or higher (ALGORITHM). However, recent studies suggest that aggressive treatment should be administered for any diagnosis of GDM.

Typical management plan for gestational diabetesFor example, the HAPO study was designed to determine the level of glucose intolerance during pregnancy, short of diabetes, associated with adverse outcomes.16 It found that even mild hyperglycemia is associated with adverse fetal outcomes and that diagnostic criteria for GDM cannot easily be based on any particular level of hyperglycemia.
Several other studies have demonstrated that aggressive treatment of mild GDM can ameliorate many of its negative effects. In 2005, for instance, Bonomo and coworkers explored the effect on newborns of treating a very mild level of gestational glucose intolerance among 300 women.18 The randomized trial involved three groups:
- Group A – standard management, which entailed no special care, diet, or pharmacotherapy
- Group B – dietary treatment and regular monitoring
- Group C – randomly selected pregnant women who were matched by BMI and age and who had normal screening test results.
The women in Group B experienced significant improvements in fasting and 2-hour postprandial glucose levels. In addition, the fasting glucose level at delivery was significantly lower in Group B, compared with the other two groups. More important, fewer LGA infants were born to the women in Group B (6.0%) than in Group A (14.0%) and Group C (9.1%).
Landon and colleagues obtained similar findings when they randomized almost 1,000 pregnant women who had mild GDM to 1) usual prenatal care or 2) dietary intervention, self-monitoring of blood glucose, and, if necessary, insulin therapy.19
Insulin analogs have joined the treatment options
Standard treatment for GDM involves diet and nutritional therapy and, when needed, insulin. A diet that limits carbohydrate in-take can significantly reduce glycemia after meals in women who have GDM.20
For years, human insulin was the only option for treating diabetes that cannot be controlled by diet and lifestyle modifications alone. Recently, however, several insulin analogs have come on the market. Only two of them have been well studied in pregnancy:
- 28B-L-lysine-29B-L-proline insulin (lispro)
- 28B-aspartic acid insulin (aspart).
These two analogs have been tested primarily in the setting of type 1 diabetes, but both improve postprandial glucose excursions, compared with human regular insulin, and both may be associated with a lower risk of delayed postprandial hypoglycemia.21,22
Some oral agents appear to be safe
Several oral antihyperglycemic agents are available for the management of diabetes (TABLE). However, in the past, oral agents were not used in pregnant women out of concern over reports of fetal anomalies and other adverse outcomes in animal studies and some human cases. More recent evidence suggests that glyburide and metformin are safe and effective for use in GDM.23-25
Oral antihyperglycemic agents and their potential side effects
| Class | Agents | Effects |
|---|---|---|
| Insulin secretagogue | Sulfonylureas and meglitinides such as glyburide, glipizide, glimepiride, repaglinide, nateglinide | Hypoglycemia if caloric intake is reduced Some are long-acting (increasing risk of prolonged hypoglycemia) |
| Biguanide | Metformin | Risk of lactic acidosis when used in the setting of renal dysfunction, circulatory compromise, or hypoxemia Relatively slow onset of action GI complications: nausea, diarrhea |
| Thiazoladinedione | Rosiglitazone, pioglitazone | Long delay to onset of action (2–3 weeks) Associated with fluid retention (particularly when used with insulin) and increased risk of congestive heart failure Use contraindicated in presence of liver disease or elevated transaminases |
| Alpha-glucosidase inhibitor | Acarbose, miglitol | Prandial/meal agent (no effect in the fasting patient) Abdominal bloating and flatus Pure dextrose is required to treat hypoglycemia that occurs in the setting of these agents |
| Glucagon-like peptide–1 mimetic | Exenatide | Newer agents with limited inpatient experience Abdominal bloating and nausea secondary to delayed gastric emptying |
| Dipeptidyl peptidase IV inhibitor | Sitagliptin | Newer agent with limited inpatient experience |
Langer and coworkers compared glyburide with insulin in the management of GDM and found the agents to be equally effective, with comparable levels of risk of large size for gestational age, macrosomia, hypoglycemia (in infants), NICU admission, and fetal anomaly.23 Subsequent studies have confirmed these findings, although at least one suggests that women who have a high fasting plasma glucose level may not respond adequately to glyburide.26 None of these studies has been large enough or long enough to truly assess whether these oral medications are equivalent to insulin in the management of GDM without posing significant long-term complications for mothers or babies, or both.
For more on the use of oral agents in GDM, see Dr. Aaron B. Caughey’s commentary on the subject of this issue.
Continuous monitoring may detect occult hyperglycemia and hypoglycemia
The traditional method of monitoring the blood glucose level is to stick a finger to obtain a blood sample and use a test strip and a meter to measure the concentration of glucose in the sample. Most meters on the market are reasonably accurate. However, research has demonstrated that they are least accurate during episodes of hypoglycemia.27
Automated continuous glucose-monitoring systems are less intrusive than the traditional method, but they are usually reserved for people who have type 1 diabetes requiring intensive insulin therapy. However, because data suggest that even short periods of hyperglycemia or hypoglycemia can be detrimental to a developing fetus, there is increasing interest in utilizing continuous glucose monitoring for GDM.
Several research groups have compared continuous glucose monitoring with finger-stick monitoring and found that women randomized to continuous monitoring have lower mean hemoglobin A1c levels from 32 to 36 weeks’ gestation.28,29 (See “Exploring the value of continuous glucose monitoring in gestational diabetes?”) Women undergoing continuous monitoring also have:
- lower mean birth-weight standard- deviation scores
- lower median customized birth-weight centiles
- a reduced risk of macrosomia.
One study found that information gleaned from continuous glucose monitoring provided additional information that altered clinical management in 42 of 68 (62%) cases. These additional data included evidence of undetected and potentially dangerous postprandial hyperglycemia and overnight hypoglycemia.29
Yogev and colleagues found that continuous glucose monitoring is significantly more sensitive than traditional methods in detecting periods of hypoglycemia in women who have GDM. They also found that asymptomatic hypoglycemic events are common during pharmacotherapy in gestations affected by GDM.30 The same group used continuous glucose monitoring at night in obese, nondiabetic women to identify previously undetected:
- high postprandial glucose peak values
- increased 1- and 2-hour postprandial glucose levels
- increased time to the glucose peak
- significantly lower mean blood glucose levels.31
Insurers were reluctant to cover continuous glucose monitoring devices when they first became available. Since then, however, much progress has been made. Nevertheless, inadequate reimbursement for the time it takes a clinician to change a patient’s treatment regimen and her subsequent management remains a significant barrier to adoption of these systems.32 The key to success with continuous glucose monitoring is to train the patient to use it properly.
Exploring the value of continuous glucose monitoring in gestational diabetes
Tanenberg R, Bode B, Lane W, et al. Use of the Continuous Glucose Monitoring System to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc. 2004;79(12):1521–1526.
The American Diabetes association recommends that patients on insulin self-monitor blood glucose three or four times daily to guide adjustments in therapy and ensure a stable and optimal hemoglobin A1c level. “however, adherence to frequent blood-glucose monitoring is low, and less than 54% of patients with insulin-treated diabetes are reported to self-monitor their blood glucose at least three times each day,” say tanenberg and coworkers.
To determine whether use of a continuous glucose-monitoring system improves metabolic control, the investigators randomized 109 patients who had insulin-treated diabetes to continuous monitoring or frequent self-monitoring. at enrollment, all patients had insulin-treated diabetes and inadequate metabolic control. at the end of the study, both groups used continuous monitoring for 3 days; these values were used to calculate measures of hypoglycemia.
In the study, the women in the self-monitoring group were counseled to measure capillary blood glucose a minimum of four times daily, as well as when they experienced symptoms of hypoglycemia, which was defined as a blood glucose measurement of 60 mg/dL or lower. any hypoglycemic event was considered to be over when the measurement exceeded 60 mg/dL for at least 30 minutes.
Findings
Hemoglobin A1c levels were similar between groups at baseline, and both groups showed significant (P < .001) and similar (P=.95) improvement in these levels after 12 weeks of study. however, the continuous-monitoring group had a significantly shorter duration of hypoglycemic events than the self-monitoring group at week 12 (49.4±40.8 minutes vs 81.0±61.1 minutes per event, respectively; P=.009).
Tanenberg and coworkers hypothesize that the improvement in hemoglobin A1c in the self-monitoring group was a result of monitoring that was more frequent (7 times a day) than is typical. they concluded that use of continuous monitoring to guide therapy adjustments in patients who use insulin significantly reduces the duration of hypoglycemia, compared with adjustments guided by self-monitoring values alone.
We want to hear from you! Tell us what you think.
1. Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the United States: determinants outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003;188(5):1372-1378.
2. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668-1669.
3. Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med. 2000;9(1):83-88.
4. Gunderson EP, Jacobs DR, Chiang V, et al. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am J Obstet Gynecol. 2009;201(2):177.e1-9.
5. van Hoorn J, Dekker G, Jeffries B. Gestational diabetes versus obesity as risk factors for pregnancy-induced hypertensive disorders and fetal macrosomia. Aust N Z J Obstet Gynaecol. 2002;42(1):29-34.
6. Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194-203.
7. Johns K, Olynik C, Mase R, Kreisman S, Tildesley H. Gestational diabetes mellitus outcome in 394 patients. J Obstet Gynaecol Can. 2006;28(2):122-127.
8. Jones CW. Gestational diabetes and its impact on the neonate. Neonatal Netw. 2001;20(6):17-23
9. De Luca AK, Nakazawa CY, Azevedo BC, Rudge MV, De Araujo Costa RA, Calderon IM. Influence of glycemic control on fetal lung maturity in gestations affected by diabetes or mild hyperglycemia. Acta Obstet Gynecol Scand. 2009;88(9):1036-1040.
10. Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200(6):672.-e1–4.
11. Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med. 2008;21(3):149-157
12. ter Braak EW, Evers IM, Willem Erkelens D, Visser GH. Maternal hypoglycemia during pregnancy in type 1 diabetes: maternal and fetal consequences. Diabetes Metab Res Rev. 2002;18(2):96-105.
13. Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2010;23(3):199-203.
14. Harlass FE, Brady K, Read JA. Reproducibility of the oral glucose tolerance test in pregnancy. Am J Obstet Gynecol. 1991;164(2):564-568.
15. Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care. 2008;31(8):1650-1655.
16. Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. New Engl J Med. 2008;358(19):1991-2002.
17. American Diabetes Association. Executive summary: standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S4-10.
18. Bonomo M, Corica D, Mion E, et al. Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: a randomized clinical trial. Diabet Med. 2005;22(11):1536-1541.
19. Landon MB, Spong CY, Thom E, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348.
20. 20Cypryk K, Kaminska P, Kosinski M, Pertynska-Marczewska M, Lewinski A. A comparison of the effectiveness tolerability and safety of high and low carbohydrate diets in women with gestational diabetes. Endokrynol Pol. 2007;58(4):314-319.
21. Lapolla A, Dalfrà MG, Spezia R, et al. Outcome of pregnancy in type 1 diabetic patients treated with insulin lispro or regular insulin: an Italian experience. Acta Diabetol. 2008;45(1):61-66.
22. Hod M, Damm P, Kaaja R, et al. Insulin Aspart Pregnancy Study Group. Fetal and perinatal outcomes in type 1 diabetes pregnancy: a randomized study comparing insulin aspart with human insulin in 322 subjects. Am J Obstet Gynecol. 2008;198(2):186-187.
23. Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzles O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343(16):1134-1138.
24. Ho FL, Liew CF, Cunanan EC, Lee KO. Oral hypoglycaemic agents for diabetes in pregnancy—an appraisal of the current evidence for oral anti-diabetic drug use in pregnancy. Ann Acad Med Singapore. 2007;36(8):672-678.
25. Nicholson W, Bolen S, Witkop CT, et al. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113(1):193-205.
26. Conway DL, Gonzales O, Skiver D. Use of glyburide for the treatment of gestational diabetes: the San Antonio experience. Matern Fetal Neonatal Med. 2004;15(1):51-55.
27. Carr S, Coustan DR, Martelly P, et al. Precision of reflectance meters in screening for gestational diabetes. Obstet Gynecol. 1989;73(5 Pt 1):727-731.
28. Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ. 2008;337:a1680.-doi: 10.1136/bmj.a1680.
29. McLachlan K, Jenkins A, O’Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Aust N Z J Obstet Gynaecol. 2007;47(3):186-190.
30. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Undiagnosed asymptomatic hypoglycemia: diet insulin, and glyburide for gestational diabetic pregnancy. Obstet Gynecol. 2004;104(1):88-93.
31. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191(3):949-953.
32. Bartelme A, Bridger P. The role of reimbursement in the adoption of continuous glucose monitors. J Diabetes Sci Technol. 2009;3(4):992-995.
1. Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the United States: determinants outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003;188(5):1372-1378.
2. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668-1669.
3. Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med. 2000;9(1):83-88.
4. Gunderson EP, Jacobs DR, Chiang V, et al. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am J Obstet Gynecol. 2009;201(2):177.e1-9.
5. van Hoorn J, Dekker G, Jeffries B. Gestational diabetes versus obesity as risk factors for pregnancy-induced hypertensive disorders and fetal macrosomia. Aust N Z J Obstet Gynaecol. 2002;42(1):29-34.
6. Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev. 2009;10(2):194-203.
7. Johns K, Olynik C, Mase R, Kreisman S, Tildesley H. Gestational diabetes mellitus outcome in 394 patients. J Obstet Gynaecol Can. 2006;28(2):122-127.
8. Jones CW. Gestational diabetes and its impact on the neonate. Neonatal Netw. 2001;20(6):17-23
9. De Luca AK, Nakazawa CY, Azevedo BC, Rudge MV, De Araujo Costa RA, Calderon IM. Influence of glycemic control on fetal lung maturity in gestations affected by diabetes or mild hyperglycemia. Acta Obstet Gynecol Scand. 2009;88(9):1036-1040.
10. Esakoff TF, Cheng YW, Sparks TN, Caughey AB. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200(6):672.-e1–4.
11. Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med. 2008;21(3):149-157
12. ter Braak EW, Evers IM, Willem Erkelens D, Visser GH. Maternal hypoglycemia during pregnancy in type 1 diabetes: maternal and fetal consequences. Diabetes Metab Res Rev. 2002;18(2):96-105.
13. Reece EA. The fetal and maternal consequences of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2010;23(3):199-203.
14. Harlass FE, Brady K, Read JA. Reproducibility of the oral glucose tolerance test in pregnancy. Am J Obstet Gynecol. 1991;164(2):564-568.
15. Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care. 2008;31(8):1650-1655.
16. Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. New Engl J Med. 2008;358(19):1991-2002.
17. American Diabetes Association. Executive summary: standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S4-10.
18. Bonomo M, Corica D, Mion E, et al. Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: a randomized clinical trial. Diabet Med. 2005;22(11):1536-1541.
19. Landon MB, Spong CY, Thom E, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. A multicenter randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339-1348.
20. 20Cypryk K, Kaminska P, Kosinski M, Pertynska-Marczewska M, Lewinski A. A comparison of the effectiveness tolerability and safety of high and low carbohydrate diets in women with gestational diabetes. Endokrynol Pol. 2007;58(4):314-319.
21. Lapolla A, Dalfrà MG, Spezia R, et al. Outcome of pregnancy in type 1 diabetic patients treated with insulin lispro or regular insulin: an Italian experience. Acta Diabetol. 2008;45(1):61-66.
22. Hod M, Damm P, Kaaja R, et al. Insulin Aspart Pregnancy Study Group. Fetal and perinatal outcomes in type 1 diabetes pregnancy: a randomized study comparing insulin aspart with human insulin in 322 subjects. Am J Obstet Gynecol. 2008;198(2):186-187.
23. Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzles O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343(16):1134-1138.
24. Ho FL, Liew CF, Cunanan EC, Lee KO. Oral hypoglycaemic agents for diabetes in pregnancy—an appraisal of the current evidence for oral anti-diabetic drug use in pregnancy. Ann Acad Med Singapore. 2007;36(8):672-678.
25. Nicholson W, Bolen S, Witkop CT, et al. Benefits and risks of oral diabetes agents compared with insulin in women with gestational diabetes: a systematic review. Obstet Gynecol. 2009;113(1):193-205.
26. Conway DL, Gonzales O, Skiver D. Use of glyburide for the treatment of gestational diabetes: the San Antonio experience. Matern Fetal Neonatal Med. 2004;15(1):51-55.
27. Carr S, Coustan DR, Martelly P, et al. Precision of reflectance meters in screening for gestational diabetes. Obstet Gynecol. 1989;73(5 Pt 1):727-731.
28. Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ. 2008;337:a1680.-doi: 10.1136/bmj.a1680.
29. McLachlan K, Jenkins A, O’Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Aust N Z J Obstet Gynaecol. 2007;47(3):186-190.
30. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Undiagnosed asymptomatic hypoglycemia: diet insulin, and glyburide for gestational diabetic pregnancy. Obstet Gynecol. 2004;104(1):88-93.
31. Yogev Y, Ben-Haroush A, Chen R, Rosenn B, Hod M, Langer O. Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol. 2004;191(3):949-953.
32. Bartelme A, Bridger P. The role of reimbursement in the adoption of continuous glucose monitors. J Diabetes Sci Technol. 2009;3(4):992-995.
New group B strep guidelines clarify management of key groups
- Neonatal death from group B strep
(Medical Verdicts, March 2011)
Before widespread intrapartum prophylaxis against group B Streptococcus (GBS) was initiated in the late 1990s, roughly 7,500 newborns developed invasive GBS disease every year in the United States, and the case-fatality rate reached an astonishing—and disheartening—50%.1 Now that all pregnant women undergo culture-based screening at 35 to 37 weeks’ gestation, the incidence of early-onset neonatal GBS disease has declined precipitously.
According to a report issued late last year by the Centers for Disease Control and Prevention (CDC), GBS now causes roughly 1,200 cases of early-onset invasive disease every year, approximately 70% of them among infants born at or after 37 weeks’ gestation, and the case-fatality rate is 4% to 6%.2 Mortality is higher among preterm infants, with a case-fatality rate of 20% to 30% for infants born at or before 33 weeks’ gestation, compared with 2% to 3% for full-term infants.2
Despite progress, GBS remains the leading cause of early-onset neonatal sepsis in the United States. In November 2010, to spur further improvement, the CDC updated its guidelines on prevention of perinatal GBS, and ACOG and other professional organizations endorsed the new recommendations. This article highlights changes to the guidelines—the first since 2002—in four critical areas:
- clarification of who should receive GBS prophylaxis, and when
- updated algorithms for screening and intrapartum prophylaxis for women who experience preterm labor or pre-term premature rupture of membranes (pPROM)
- new recommended dosage of penicillin G for prophylaxis
- updated regimens for prophylaxis among women who are allergic to penicillin.2
When is intrapartum antibiotic prophylaxis indicated? When is it not?
| Indicated | Not indicated |
|---|---|
Previous infant with invasive GBS disease GBS bacteriuria during any trimester of the current pregnancy* Positive GBS vaginal-rectal screening culture in late gestation† during current pregnancy* Unknown GBS status at the onset of labor (culture not done, incomplete, or results unknown) and any of the following:
| Colonization with GBS during a previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) GBS bacteriuria during previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) Negative vaginal and rectal GBS screening culture in late gestation† during the current pregnancy, regardless of intrapartum risk factors Cesarean delivery performed before onset of labor on a woman who has intact amniotic membranes, regardless of GBS colonization status or gestational age |
| SOURCE: CDC2 * Intrapartum antibiotic prophylaxis is not indicated in this circumstance if a cesarean delivery is performed before onset of labor on a woman who has intact amniotic membranes. † Optimal timing for prenatal GBS screening is at 35–37 weeks’ gestation. § Recommendations for the use of intrapartum antibiotics for prevention of early-onset GBS disease in the setting of threatened preterm delivery are presented in FIGURES 1 and 2. ¶ If amnionitis is suspected, broad-spectrum antibiotic therapy that includes an agent known to be active against GBS should replace GBS prophylaxis. ** NAAT testing for GBS is optional and might not be available in all settings. If intrapartum NAAT is negative for GBS but any other intrapartum risk factor (delivery at <37 weeks’ gestation, amniotic membrane rupture at ≥18 hours, or temperature ≥100.4°F [≥38.0°C]) is present, then intrapartum antibiotic prophylaxis is indicated. | |
Who should receive prophylaxis?
In its report, the CDC reiterated the indications and “nonindications” for intrapartum prophylaxis (TABLE). Among the clarifications:
- Women who have GBS isolated from the urine at any time during pregnancy should undergo intrapartum prophylaxis. They do not need third-trimester screening for GBS.
- Women who had a previous infant with invasive GBS disease should also undergo intrapartum prophylaxis, with no need for third-trimester screening
- All other pregnant women should undergo screening at 35 to 37 weeks’ gestation. If results are positive, intrapartum prophylaxis is indicated.
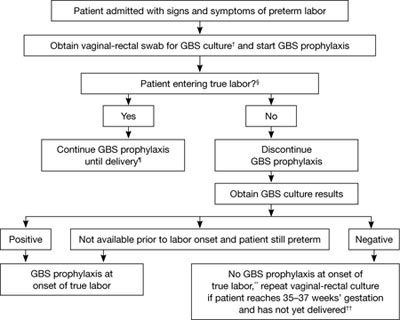
FIGURE 1 Recommended management when a patient experiences preterm labor*
SOURCE: CDC2
*At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Patient should be regularly assessed for progression to true labor; if the patient is considered not to be in true labor, discontinue GBS prophylaxis.
¶ If GBS culture results become available prior to delivery and are negative, discontinue GBS prophylaxis.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with a history of preterm labor is readmitted with signs and symptoms of preterm labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
CDC now offers distinct algorithms for preterm labor and pPROM
To clarify the management of two distinct groups of women, the CDC developed separate algorithms for GBS prophylaxis in the setting of threatened preterm delivery—one for spontaneous preterm labor (FIGURE 1) and another for pPROM (FIGURE 2). In addition, it now recommends:
- When GBS prophylaxis is given to a woman who has signs and symptoms of preterm labor, it should be discontinued if it is later determined that she is not in true labor
- If antibiotics given to prolong latency for pPROM include adequate coverage for GBS (i.e., 2 g intravenous [IV] ampicillin followed by 1 g IV ampicillin every 6 hours for 48 hours), no additional prophylaxis for GBS is necessary, provided delivery occurs during administration of that antibiotic regimen. Oral antibiotics alone are not adequate for GBS prophylaxis.
- When a woman who has pPROM is not in labor and is receiving antibiotics with adequate GBS coverage to prolong latency, she should be managed according to the standard of care for pPROM. GBS testing results should not affect the duration of antibiotics.
- When a woman who has pPROM is not in labor and is not receiving antibiotics to prolong latency (or is receiving antibiotics that do not have adequate GBS coverage), she should undergo GBS prophylaxis for 48 hours unless a GBS screen performed within 5 weeks was negative.
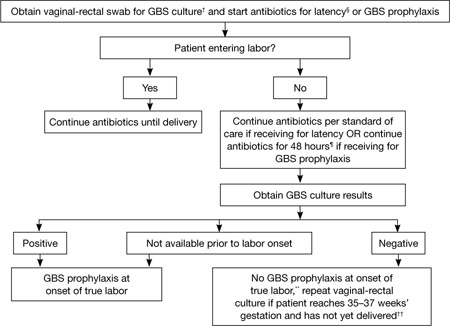
FIGURE 2 GBS screening and prophylaxis for preterm premature rupture of membranes (pPROM)*
SOURCE: CDC2
* At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Antibiotics given for latency in the setting of pPROM that include ampicillin 2 g IV once, followed by 1 g IV every 6 hours for at least 48 hours are adequate for GBS prophylaxis. If other regimens are used, GBS prophylaxis should be initiated in addition.
¶ GBS prophylaxis should be discontinued at 48 hours for women with pPROM who are not in labor. If results from a GBS screen performed at admission become available during the 48-hour period and are negative, GBS prophylaxis should be discontinued at that time.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with pPROM is entering labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
New dosage allows room for flexibility
The CDC now recommends a dosage of 5 million units of IV penicillin G for GBS prophylaxis, followed by 2.5 to 3.0 million units IV every 4 hours. The range of 2.5 to 3.0 million units is recommended to ensure that the drug reaches an adequate concentration in the fetal circulation and amniotic fluid without being neurotoxic. The choice of dosage within that range should be guided by which formulations of penicillin G are readily available, says the CDC.
Penicillin remains the agent of choice for intrapartum prophylaxis, but ampicillin is an acceptable alternative.
If a woman is allergic to penicillin but has no history of anaphylaxis, angioedema, respiratory distress, or urticaria following administration of a penicillin or cephalosporin, she should be given 2 g IV cefazolin, followed by 1 g IV cefazolin every 8 hours until delivery. If she does have a history of anaphylaxis or is at high risk for anaphylaxis, ask the laboratory for antimicrobial susceptibility testing on the antenatal GBS culture. If the isolate is susceptible to clindamycin, give her 900 mg IV clindamycin every 8 hours until delivery. If it is not susceptible to clindamycin, give her 1 g IV vancomycin every 12 hours until the time of delivery.
The CDC no longer considers erythromycin to be an acceptable alternative for intrapartum GBS prophylaxis for penicillin-allergic women at high risk of anaphylaxis.
Where we go from here
Although early-onset GBS disease has become relatively uncommon, the rate of maternal GBS colonization remains unchanged since the 1970s. Therefore, it is important to continue efforts to sustain and improve on the progress that has been made. There is also a need to monitor for potential adverse consequences of intrapartum antibiotic prophylaxis, such as emergence of bacterial antimicrobial resistance or an increased incidence or severity of nonGBS neonatal pathogens, the CDC observes. “In the absence of a licensed GBS vaccine, universal screening and intrapartum antibiotic prophylaxis continue to be the cornerstones of early-onset GBS disease prevention."
We want to hear from you! Tell us what you think.
1. Baker CJ, Barrett FF. Group B streptococcal infections in infants. The importance of the various serotypes. JAMA. 1974;230(8):1158-1160.
2. Verani JR, McGee L, Schrag SJ. Centers for Disease Control and Prevention. Prevention of Perinatal Group B Streptococcal Disease: Revised Guidelines from CDC, 2010. MMWR. 2010;59(RR-10):1-36.
- Neonatal death from group B strep
(Medical Verdicts, March 2011)
Before widespread intrapartum prophylaxis against group B Streptococcus (GBS) was initiated in the late 1990s, roughly 7,500 newborns developed invasive GBS disease every year in the United States, and the case-fatality rate reached an astonishing—and disheartening—50%.1 Now that all pregnant women undergo culture-based screening at 35 to 37 weeks’ gestation, the incidence of early-onset neonatal GBS disease has declined precipitously.
According to a report issued late last year by the Centers for Disease Control and Prevention (CDC), GBS now causes roughly 1,200 cases of early-onset invasive disease every year, approximately 70% of them among infants born at or after 37 weeks’ gestation, and the case-fatality rate is 4% to 6%.2 Mortality is higher among preterm infants, with a case-fatality rate of 20% to 30% for infants born at or before 33 weeks’ gestation, compared with 2% to 3% for full-term infants.2
Despite progress, GBS remains the leading cause of early-onset neonatal sepsis in the United States. In November 2010, to spur further improvement, the CDC updated its guidelines on prevention of perinatal GBS, and ACOG and other professional organizations endorsed the new recommendations. This article highlights changes to the guidelines—the first since 2002—in four critical areas:
- clarification of who should receive GBS prophylaxis, and when
- updated algorithms for screening and intrapartum prophylaxis for women who experience preterm labor or pre-term premature rupture of membranes (pPROM)
- new recommended dosage of penicillin G for prophylaxis
- updated regimens for prophylaxis among women who are allergic to penicillin.2
When is intrapartum antibiotic prophylaxis indicated? When is it not?
| Indicated | Not indicated |
|---|---|
Previous infant with invasive GBS disease GBS bacteriuria during any trimester of the current pregnancy* Positive GBS vaginal-rectal screening culture in late gestation† during current pregnancy* Unknown GBS status at the onset of labor (culture not done, incomplete, or results unknown) and any of the following:
| Colonization with GBS during a previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) GBS bacteriuria during previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) Negative vaginal and rectal GBS screening culture in late gestation† during the current pregnancy, regardless of intrapartum risk factors Cesarean delivery performed before onset of labor on a woman who has intact amniotic membranes, regardless of GBS colonization status or gestational age |
| SOURCE: CDC2 * Intrapartum antibiotic prophylaxis is not indicated in this circumstance if a cesarean delivery is performed before onset of labor on a woman who has intact amniotic membranes. † Optimal timing for prenatal GBS screening is at 35–37 weeks’ gestation. § Recommendations for the use of intrapartum antibiotics for prevention of early-onset GBS disease in the setting of threatened preterm delivery are presented in FIGURES 1 and 2. ¶ If amnionitis is suspected, broad-spectrum antibiotic therapy that includes an agent known to be active against GBS should replace GBS prophylaxis. ** NAAT testing for GBS is optional and might not be available in all settings. If intrapartum NAAT is negative for GBS but any other intrapartum risk factor (delivery at <37 weeks’ gestation, amniotic membrane rupture at ≥18 hours, or temperature ≥100.4°F [≥38.0°C]) is present, then intrapartum antibiotic prophylaxis is indicated. | |
Who should receive prophylaxis?
In its report, the CDC reiterated the indications and “nonindications” for intrapartum prophylaxis (TABLE). Among the clarifications:
- Women who have GBS isolated from the urine at any time during pregnancy should undergo intrapartum prophylaxis. They do not need third-trimester screening for GBS.
- Women who had a previous infant with invasive GBS disease should also undergo intrapartum prophylaxis, with no need for third-trimester screening
- All other pregnant women should undergo screening at 35 to 37 weeks’ gestation. If results are positive, intrapartum prophylaxis is indicated.

FIGURE 1 Recommended management when a patient experiences preterm labor*
SOURCE: CDC2
*At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Patient should be regularly assessed for progression to true labor; if the patient is considered not to be in true labor, discontinue GBS prophylaxis.
¶ If GBS culture results become available prior to delivery and are negative, discontinue GBS prophylaxis.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with a history of preterm labor is readmitted with signs and symptoms of preterm labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
CDC now offers distinct algorithms for preterm labor and pPROM
To clarify the management of two distinct groups of women, the CDC developed separate algorithms for GBS prophylaxis in the setting of threatened preterm delivery—one for spontaneous preterm labor (FIGURE 1) and another for pPROM (FIGURE 2). In addition, it now recommends:
- When GBS prophylaxis is given to a woman who has signs and symptoms of preterm labor, it should be discontinued if it is later determined that she is not in true labor
- If antibiotics given to prolong latency for pPROM include adequate coverage for GBS (i.e., 2 g intravenous [IV] ampicillin followed by 1 g IV ampicillin every 6 hours for 48 hours), no additional prophylaxis for GBS is necessary, provided delivery occurs during administration of that antibiotic regimen. Oral antibiotics alone are not adequate for GBS prophylaxis.
- When a woman who has pPROM is not in labor and is receiving antibiotics with adequate GBS coverage to prolong latency, she should be managed according to the standard of care for pPROM. GBS testing results should not affect the duration of antibiotics.
- When a woman who has pPROM is not in labor and is not receiving antibiotics to prolong latency (or is receiving antibiotics that do not have adequate GBS coverage), she should undergo GBS prophylaxis for 48 hours unless a GBS screen performed within 5 weeks was negative.

FIGURE 2 GBS screening and prophylaxis for preterm premature rupture of membranes (pPROM)*
SOURCE: CDC2
* At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Antibiotics given for latency in the setting of pPROM that include ampicillin 2 g IV once, followed by 1 g IV every 6 hours for at least 48 hours are adequate for GBS prophylaxis. If other regimens are used, GBS prophylaxis should be initiated in addition.
¶ GBS prophylaxis should be discontinued at 48 hours for women with pPROM who are not in labor. If results from a GBS screen performed at admission become available during the 48-hour period and are negative, GBS prophylaxis should be discontinued at that time.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with pPROM is entering labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
New dosage allows room for flexibility
The CDC now recommends a dosage of 5 million units of IV penicillin G for GBS prophylaxis, followed by 2.5 to 3.0 million units IV every 4 hours. The range of 2.5 to 3.0 million units is recommended to ensure that the drug reaches an adequate concentration in the fetal circulation and amniotic fluid without being neurotoxic. The choice of dosage within that range should be guided by which formulations of penicillin G are readily available, says the CDC.
Penicillin remains the agent of choice for intrapartum prophylaxis, but ampicillin is an acceptable alternative.
If a woman is allergic to penicillin but has no history of anaphylaxis, angioedema, respiratory distress, or urticaria following administration of a penicillin or cephalosporin, she should be given 2 g IV cefazolin, followed by 1 g IV cefazolin every 8 hours until delivery. If she does have a history of anaphylaxis or is at high risk for anaphylaxis, ask the laboratory for antimicrobial susceptibility testing on the antenatal GBS culture. If the isolate is susceptible to clindamycin, give her 900 mg IV clindamycin every 8 hours until delivery. If it is not susceptible to clindamycin, give her 1 g IV vancomycin every 12 hours until the time of delivery.
The CDC no longer considers erythromycin to be an acceptable alternative for intrapartum GBS prophylaxis for penicillin-allergic women at high risk of anaphylaxis.
Where we go from here
Although early-onset GBS disease has become relatively uncommon, the rate of maternal GBS colonization remains unchanged since the 1970s. Therefore, it is important to continue efforts to sustain and improve on the progress that has been made. There is also a need to monitor for potential adverse consequences of intrapartum antibiotic prophylaxis, such as emergence of bacterial antimicrobial resistance or an increased incidence or severity of nonGBS neonatal pathogens, the CDC observes. “In the absence of a licensed GBS vaccine, universal screening and intrapartum antibiotic prophylaxis continue to be the cornerstones of early-onset GBS disease prevention."
We want to hear from you! Tell us what you think.
- Neonatal death from group B strep
(Medical Verdicts, March 2011)
Before widespread intrapartum prophylaxis against group B Streptococcus (GBS) was initiated in the late 1990s, roughly 7,500 newborns developed invasive GBS disease every year in the United States, and the case-fatality rate reached an astonishing—and disheartening—50%.1 Now that all pregnant women undergo culture-based screening at 35 to 37 weeks’ gestation, the incidence of early-onset neonatal GBS disease has declined precipitously.
According to a report issued late last year by the Centers for Disease Control and Prevention (CDC), GBS now causes roughly 1,200 cases of early-onset invasive disease every year, approximately 70% of them among infants born at or after 37 weeks’ gestation, and the case-fatality rate is 4% to 6%.2 Mortality is higher among preterm infants, with a case-fatality rate of 20% to 30% for infants born at or before 33 weeks’ gestation, compared with 2% to 3% for full-term infants.2
Despite progress, GBS remains the leading cause of early-onset neonatal sepsis in the United States. In November 2010, to spur further improvement, the CDC updated its guidelines on prevention of perinatal GBS, and ACOG and other professional organizations endorsed the new recommendations. This article highlights changes to the guidelines—the first since 2002—in four critical areas:
- clarification of who should receive GBS prophylaxis, and when
- updated algorithms for screening and intrapartum prophylaxis for women who experience preterm labor or pre-term premature rupture of membranes (pPROM)
- new recommended dosage of penicillin G for prophylaxis
- updated regimens for prophylaxis among women who are allergic to penicillin.2
When is intrapartum antibiotic prophylaxis indicated? When is it not?
| Indicated | Not indicated |
|---|---|
Previous infant with invasive GBS disease GBS bacteriuria during any trimester of the current pregnancy* Positive GBS vaginal-rectal screening culture in late gestation† during current pregnancy* Unknown GBS status at the onset of labor (culture not done, incomplete, or results unknown) and any of the following:
| Colonization with GBS during a previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) GBS bacteriuria during previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) Negative vaginal and rectal GBS screening culture in late gestation† during the current pregnancy, regardless of intrapartum risk factors Cesarean delivery performed before onset of labor on a woman who has intact amniotic membranes, regardless of GBS colonization status or gestational age |
| SOURCE: CDC2 * Intrapartum antibiotic prophylaxis is not indicated in this circumstance if a cesarean delivery is performed before onset of labor on a woman who has intact amniotic membranes. † Optimal timing for prenatal GBS screening is at 35–37 weeks’ gestation. § Recommendations for the use of intrapartum antibiotics for prevention of early-onset GBS disease in the setting of threatened preterm delivery are presented in FIGURES 1 and 2. ¶ If amnionitis is suspected, broad-spectrum antibiotic therapy that includes an agent known to be active against GBS should replace GBS prophylaxis. ** NAAT testing for GBS is optional and might not be available in all settings. If intrapartum NAAT is negative for GBS but any other intrapartum risk factor (delivery at <37 weeks’ gestation, amniotic membrane rupture at ≥18 hours, or temperature ≥100.4°F [≥38.0°C]) is present, then intrapartum antibiotic prophylaxis is indicated. | |
Who should receive prophylaxis?
In its report, the CDC reiterated the indications and “nonindications” for intrapartum prophylaxis (TABLE). Among the clarifications:
- Women who have GBS isolated from the urine at any time during pregnancy should undergo intrapartum prophylaxis. They do not need third-trimester screening for GBS.
- Women who had a previous infant with invasive GBS disease should also undergo intrapartum prophylaxis, with no need for third-trimester screening
- All other pregnant women should undergo screening at 35 to 37 weeks’ gestation. If results are positive, intrapartum prophylaxis is indicated.

FIGURE 1 Recommended management when a patient experiences preterm labor*
SOURCE: CDC2
*At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Patient should be regularly assessed for progression to true labor; if the patient is considered not to be in true labor, discontinue GBS prophylaxis.
¶ If GBS culture results become available prior to delivery and are negative, discontinue GBS prophylaxis.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with a history of preterm labor is readmitted with signs and symptoms of preterm labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
CDC now offers distinct algorithms for preterm labor and pPROM
To clarify the management of two distinct groups of women, the CDC developed separate algorithms for GBS prophylaxis in the setting of threatened preterm delivery—one for spontaneous preterm labor (FIGURE 1) and another for pPROM (FIGURE 2). In addition, it now recommends:
- When GBS prophylaxis is given to a woman who has signs and symptoms of preterm labor, it should be discontinued if it is later determined that she is not in true labor
- If antibiotics given to prolong latency for pPROM include adequate coverage for GBS (i.e., 2 g intravenous [IV] ampicillin followed by 1 g IV ampicillin every 6 hours for 48 hours), no additional prophylaxis for GBS is necessary, provided delivery occurs during administration of that antibiotic regimen. Oral antibiotics alone are not adequate for GBS prophylaxis.
- When a woman who has pPROM is not in labor and is receiving antibiotics with adequate GBS coverage to prolong latency, she should be managed according to the standard of care for pPROM. GBS testing results should not affect the duration of antibiotics.
- When a woman who has pPROM is not in labor and is not receiving antibiotics to prolong latency (or is receiving antibiotics that do not have adequate GBS coverage), she should undergo GBS prophylaxis for 48 hours unless a GBS screen performed within 5 weeks was negative.

FIGURE 2 GBS screening and prophylaxis for preterm premature rupture of membranes (pPROM)*
SOURCE: CDC2
* At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Antibiotics given for latency in the setting of pPROM that include ampicillin 2 g IV once, followed by 1 g IV every 6 hours for at least 48 hours are adequate for GBS prophylaxis. If other regimens are used, GBS prophylaxis should be initiated in addition.
¶ GBS prophylaxis should be discontinued at 48 hours for women with pPROM who are not in labor. If results from a GBS screen performed at admission become available during the 48-hour period and are negative, GBS prophylaxis should be discontinued at that time.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with pPROM is entering labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
New dosage allows room for flexibility
The CDC now recommends a dosage of 5 million units of IV penicillin G for GBS prophylaxis, followed by 2.5 to 3.0 million units IV every 4 hours. The range of 2.5 to 3.0 million units is recommended to ensure that the drug reaches an adequate concentration in the fetal circulation and amniotic fluid without being neurotoxic. The choice of dosage within that range should be guided by which formulations of penicillin G are readily available, says the CDC.
Penicillin remains the agent of choice for intrapartum prophylaxis, but ampicillin is an acceptable alternative.
If a woman is allergic to penicillin but has no history of anaphylaxis, angioedema, respiratory distress, or urticaria following administration of a penicillin or cephalosporin, she should be given 2 g IV cefazolin, followed by 1 g IV cefazolin every 8 hours until delivery. If she does have a history of anaphylaxis or is at high risk for anaphylaxis, ask the laboratory for antimicrobial susceptibility testing on the antenatal GBS culture. If the isolate is susceptible to clindamycin, give her 900 mg IV clindamycin every 8 hours until delivery. If it is not susceptible to clindamycin, give her 1 g IV vancomycin every 12 hours until the time of delivery.
The CDC no longer considers erythromycin to be an acceptable alternative for intrapartum GBS prophylaxis for penicillin-allergic women at high risk of anaphylaxis.
Where we go from here
Although early-onset GBS disease has become relatively uncommon, the rate of maternal GBS colonization remains unchanged since the 1970s. Therefore, it is important to continue efforts to sustain and improve on the progress that has been made. There is also a need to monitor for potential adverse consequences of intrapartum antibiotic prophylaxis, such as emergence of bacterial antimicrobial resistance or an increased incidence or severity of nonGBS neonatal pathogens, the CDC observes. “In the absence of a licensed GBS vaccine, universal screening and intrapartum antibiotic prophylaxis continue to be the cornerstones of early-onset GBS disease prevention."
We want to hear from you! Tell us what you think.
1. Baker CJ, Barrett FF. Group B streptococcal infections in infants. The importance of the various serotypes. JAMA. 1974;230(8):1158-1160.
2. Verani JR, McGee L, Schrag SJ. Centers for Disease Control and Prevention. Prevention of Perinatal Group B Streptococcal Disease: Revised Guidelines from CDC, 2010. MMWR. 2010;59(RR-10):1-36.
1. Baker CJ, Barrett FF. Group B streptococcal infections in infants. The importance of the various serotypes. JAMA. 1974;230(8):1158-1160.
2. Verani JR, McGee L, Schrag SJ. Centers for Disease Control and Prevention. Prevention of Perinatal Group B Streptococcal Disease: Revised Guidelines from CDC, 2010. MMWR. 2010;59(RR-10):1-36.
FDA Warning: CV Deaths, Risks With Obstetric Terbutaline
The Food and Drug Administration has issued a warning advising against the use of injectable terbutaline for preventing and for prolonged treatment of preterm labor and against any use of oral terbutaline in this setting, prompted by postmarketing reports of deaths and other serious cardiovascular events associated with the use of the drug in this setting.
“Death and serious adverse reactions, including increased heart rate, transient hyperglycemia, hypokalemia, cardiac arrhythmias, pulmonary edema, and myocardial ischemia have been reported after prolonged administration of oral or injectable terbutaline to pregnant women,” according to an FDA statement.
Based on these reports and a review of the medical literature, the agency “has concluded that the risk of serious adverse events outweighs any potential benefit to pregnant women receiving prolonged treatment with terbutaline injection (beyond 48–72 hours), or acute or prolonged treatment with oral terbutaline,” the FDA said.
This is not the first FDA warning issued about the obstetric-related risks of terbutaline, an FDA-approved treatment for bronchospasm that has been used off label to treat and prevent preterm labor and to treat uterine hyperstimulation. In 1997, the agency notified health care professionals in a letter about concerns over the safety of long-term administration of subcutaneous terbutaline and revised the drug's labeling about the risk of serious cardiovascular adverse events associated with this use. But despite studies reporting a lack of safety and efficacy of terbutaline for the treatment of recurrent preterm labor and professional association recommendations, “prolonged use of terbutaline continues, with serious and sometimes fatal consequences,” the FDA statement said.
Between 1976, when terbutaline was first marketed, and 2009, 16 maternal deaths associated with the obstetric use of terbutaline were reported to the FDA's Adverse Event Reporting System (AERS). Between January 1998, soon after the FDA issued the warning letter, and July 2009, 12 maternal cases of serious cardiovascular events associated with the use of terbutaline were reported.
While there are “certain obstetrical conditions” in which health care professionals “may decide that the benefit of terbutaline injection for an individual patient in a hospital setting clearly outweighs the risk,” the FDA statement said that terbutaline administered by injection or continuous infusion pump should not be used for more than 48–72 hours, and injectable terbutaline should not be used in outpatient settings. These warnings are being added to a new boxed warning in terbutaline labels.
In an interview, Dr. Washington Hill of Sarasota (Fla.) Memorial Hospital, welcomed the FDA warnings. Still, he cautioned that subcutaneous terbutaline should not be entirely abandoned because there are some obstetric situations where its use is beneficial, when used in the hospital setting and not in a pump. These include reducing contractions in a woman with tachysystole of the uterus due to pitocin or for intrauterine resuscitation, adjunct to external cephalic version, relaxing an inverted uterus or as an adjunct to managing inversion of the uterus, transporting a patient with contractions from one location to another, and rarely when delivering a second twin when the uterus needs to be relaxed.
Dr. Hill had no relevant financial disclosures.
The Food and Drug Administration has issued a warning advising against the use of injectable terbutaline for preventing and for prolonged treatment of preterm labor and against any use of oral terbutaline in this setting, prompted by postmarketing reports of deaths and other serious cardiovascular events associated with the use of the drug in this setting.
“Death and serious adverse reactions, including increased heart rate, transient hyperglycemia, hypokalemia, cardiac arrhythmias, pulmonary edema, and myocardial ischemia have been reported after prolonged administration of oral or injectable terbutaline to pregnant women,” according to an FDA statement.
Based on these reports and a review of the medical literature, the agency “has concluded that the risk of serious adverse events outweighs any potential benefit to pregnant women receiving prolonged treatment with terbutaline injection (beyond 48–72 hours), or acute or prolonged treatment with oral terbutaline,” the FDA said.
This is not the first FDA warning issued about the obstetric-related risks of terbutaline, an FDA-approved treatment for bronchospasm that has been used off label to treat and prevent preterm labor and to treat uterine hyperstimulation. In 1997, the agency notified health care professionals in a letter about concerns over the safety of long-term administration of subcutaneous terbutaline and revised the drug's labeling about the risk of serious cardiovascular adverse events associated with this use. But despite studies reporting a lack of safety and efficacy of terbutaline for the treatment of recurrent preterm labor and professional association recommendations, “prolonged use of terbutaline continues, with serious and sometimes fatal consequences,” the FDA statement said.
Between 1976, when terbutaline was first marketed, and 2009, 16 maternal deaths associated with the obstetric use of terbutaline were reported to the FDA's Adverse Event Reporting System (AERS). Between January 1998, soon after the FDA issued the warning letter, and July 2009, 12 maternal cases of serious cardiovascular events associated with the use of terbutaline were reported.
While there are “certain obstetrical conditions” in which health care professionals “may decide that the benefit of terbutaline injection for an individual patient in a hospital setting clearly outweighs the risk,” the FDA statement said that terbutaline administered by injection or continuous infusion pump should not be used for more than 48–72 hours, and injectable terbutaline should not be used in outpatient settings. These warnings are being added to a new boxed warning in terbutaline labels.
In an interview, Dr. Washington Hill of Sarasota (Fla.) Memorial Hospital, welcomed the FDA warnings. Still, he cautioned that subcutaneous terbutaline should not be entirely abandoned because there are some obstetric situations where its use is beneficial, when used in the hospital setting and not in a pump. These include reducing contractions in a woman with tachysystole of the uterus due to pitocin or for intrauterine resuscitation, adjunct to external cephalic version, relaxing an inverted uterus or as an adjunct to managing inversion of the uterus, transporting a patient with contractions from one location to another, and rarely when delivering a second twin when the uterus needs to be relaxed.
Dr. Hill had no relevant financial disclosures.
The Food and Drug Administration has issued a warning advising against the use of injectable terbutaline for preventing and for prolonged treatment of preterm labor and against any use of oral terbutaline in this setting, prompted by postmarketing reports of deaths and other serious cardiovascular events associated with the use of the drug in this setting.
“Death and serious adverse reactions, including increased heart rate, transient hyperglycemia, hypokalemia, cardiac arrhythmias, pulmonary edema, and myocardial ischemia have been reported after prolonged administration of oral or injectable terbutaline to pregnant women,” according to an FDA statement.
Based on these reports and a review of the medical literature, the agency “has concluded that the risk of serious adverse events outweighs any potential benefit to pregnant women receiving prolonged treatment with terbutaline injection (beyond 48–72 hours), or acute or prolonged treatment with oral terbutaline,” the FDA said.
This is not the first FDA warning issued about the obstetric-related risks of terbutaline, an FDA-approved treatment for bronchospasm that has been used off label to treat and prevent preterm labor and to treat uterine hyperstimulation. In 1997, the agency notified health care professionals in a letter about concerns over the safety of long-term administration of subcutaneous terbutaline and revised the drug's labeling about the risk of serious cardiovascular adverse events associated with this use. But despite studies reporting a lack of safety and efficacy of terbutaline for the treatment of recurrent preterm labor and professional association recommendations, “prolonged use of terbutaline continues, with serious and sometimes fatal consequences,” the FDA statement said.
Between 1976, when terbutaline was first marketed, and 2009, 16 maternal deaths associated with the obstetric use of terbutaline were reported to the FDA's Adverse Event Reporting System (AERS). Between January 1998, soon after the FDA issued the warning letter, and July 2009, 12 maternal cases of serious cardiovascular events associated with the use of terbutaline were reported.
While there are “certain obstetrical conditions” in which health care professionals “may decide that the benefit of terbutaline injection for an individual patient in a hospital setting clearly outweighs the risk,” the FDA statement said that terbutaline administered by injection or continuous infusion pump should not be used for more than 48–72 hours, and injectable terbutaline should not be used in outpatient settings. These warnings are being added to a new boxed warning in terbutaline labels.
In an interview, Dr. Washington Hill of Sarasota (Fla.) Memorial Hospital, welcomed the FDA warnings. Still, he cautioned that subcutaneous terbutaline should not be entirely abandoned because there are some obstetric situations where its use is beneficial, when used in the hospital setting and not in a pump. These include reducing contractions in a woman with tachysystole of the uterus due to pitocin or for intrauterine resuscitation, adjunct to external cephalic version, relaxing an inverted uterus or as an adjunct to managing inversion of the uterus, transporting a patient with contractions from one location to another, and rarely when delivering a second twin when the uterus needs to be relaxed.
Dr. Hill had no relevant financial disclosures.
Don't Delay Suturing for Postpartum Hemorrhage
Major Finding: A 2- to 6-hour delay in suture placement was found to be independently associated with a fourfold increase in the odds of hysterectomy.
Data Source: A prospective population-based study of 1.2 million women, 210 of whom were treated with a uterine compression suture to control postpartum hemorrhage.
Disclosures: This study was funded by the charity Wellbeing of Women. Dr. Kayem disclosed that he is the beneficiary of a postdoctoral grant from the AXA Research Fund. Another study author, Marian Knight, is funded by a personal fellowship from the National Coordinating Centre for Research Capacity Development of the National Institute for Health Research. This was an independent study from UKOSS, which is partially funded by the Policy Research Programme in the Department of Health.
Uterine compression sutures for postpartum hemorrhage are more likely to fail when there is a delay of 2–6 hours between delivery and placement of the sutures, according to a large prospective population-based study.
Of 1.2 million women who delivered in the United Kingdom between September 2007 and March 2009, 210 who were treated with a uterine compression suture to control postpartum hemorrhage had adequate information for analysis. Of those, 25% continued to bleed and underwent hysterectomy, Dr. Gilles Kayem of the University of Oxford (England) and his colleagues reported.
Suture failure occurred in 42% of those with a 2- to 6-hour delay in suture placement, compared with only 16% of those with earlier suture placement. After adjustment for numerous socioeconomic, maternal, and medical factors, a 2- to 6-hour delay in suture placement was found to be independently associated with a fourfold increase in the odds of hysterectomy, the investigators found (Obstet. Gynecol. 2011;117:14-20).
“One possible explanation may be that unrecognized bleeding that prolongs the delay between the delivery and the treatment increases the risk of hysterectomy,” they wrote, explaining that “a higher blood loss and disseminated intravascular coagulation would lead to clinical conditions that render hysterectomy almost inevitable.”
Failure in this study was also more likely in women older than age 35 years, compared with younger women (adjusted odds ratio, 2.77); those who were multiparous, compared with nulliparous women (AOR, 2.83); those who were unemployed or employed in routine or manual occupations, compared with those in managerial positions (AOR, 3.54); and those who had a vaginal delivery, compared with those who had a cesarean delivery (AOR, 6.08), the researchers found.
It is interesting, they noted, that vaginal delivery was the factor associated with the highest odds of hysterectomy in this study.
“It is possible that the obstetrician is more reluctant to perform a laparotomy to insert a compression suture after excessive bleeding after a vaginal delivery than after a cesarean delivery and that, therefore, only the women with the most severe hemorrhage were selected by the obstetrician to have a uterine compression suture after a vaginal delivery,” they speculated.
Another possible explanation is that other methods – such as intrauterine balloon or uterine packing – were used successfully in some cases of hemorrhage after vaginal delivery, and thus were not identified in this study, suggesting that cases involving uterine compression sutures after a vaginal delivery may be the most serious, and no other treatment modalities were available to treat the affected patients, they noted.
No differences in failure rates were seen among suture types (B-Lynch, modified B-Lynch, and 32 other techniques such as figure-of-eight, multiple compression, or square sutures).
However, because this was not a randomized study, comparisons among the suture methods were limited, as the baseline populations treated may have differed.
In all, 129 women (61%) had a hemorrhage resulting from atony. Hysterectomy rates according to the different types of uterine compression suture also were not significantly different. The hysterectomy rate was 26% in cases with atony and 23% in cases with other causes, such as placenta accreta, placenta previa, and uterine tear. After adjustment for a number of variables, the risk of hysterectomy was no different in women with atony compared with other causes of hemorrhage.
Patients included in the study were women identified via the U.K. Obstetric Surveillance System (UKOSS). Case patients were those giving birth who were treated with a uterine compression suture to treat postpartum hemorrhage.
Strengths of the study include the collection of comprehensive population-based national information about women who were treated with compression sutures for postpartum hemorrhage, they said. The findings emphasize the need for careful evaluation of blood loss following delivery so that delays in recognizing and managing hemorrhage can be avoided, they concluded.
View on The News
Technique Often Useful
Dr. Carolyn Zelop said that this study has important implications for practice. “I think the take-home message … is that [the use of compression sutures] is a very reliable technique, but that it's probably less useful in the setting of placenta accreta,” explaining that uterine atony appears to be the indication that leads to the most success with this technique.
Another important point made by the authors is that in the setting of vaginal delivery that is complicated by postpartum hemorrhage, it is important to “be on the clock and ready to move to the next intervention,” since a delay of 2–6 hours in suture placement was associated with increased risk of hysterectomy.
Although it seems logical that a clinician might be reluctant to proceed with laparotomy after vaginal delivery, a prolonged delay could predispose a patient to unrecognized blood loss, and increase the risk of compression suture failure. If mechanical tamponade techniques fail to control hemorrhaging, the clinician should proceed with laparotomy and uterine compression suture placement, she advised.
Vitals
DR. ZELOP is director of maternal-fetal medicine at Beth Israel Deaconess Medical Center in Boston. She said she had no relevant financial disclosures.
Major Finding: A 2- to 6-hour delay in suture placement was found to be independently associated with a fourfold increase in the odds of hysterectomy.
Data Source: A prospective population-based study of 1.2 million women, 210 of whom were treated with a uterine compression suture to control postpartum hemorrhage.
Disclosures: This study was funded by the charity Wellbeing of Women. Dr. Kayem disclosed that he is the beneficiary of a postdoctoral grant from the AXA Research Fund. Another study author, Marian Knight, is funded by a personal fellowship from the National Coordinating Centre for Research Capacity Development of the National Institute for Health Research. This was an independent study from UKOSS, which is partially funded by the Policy Research Programme in the Department of Health.
Uterine compression sutures for postpartum hemorrhage are more likely to fail when there is a delay of 2–6 hours between delivery and placement of the sutures, according to a large prospective population-based study.
Of 1.2 million women who delivered in the United Kingdom between September 2007 and March 2009, 210 who were treated with a uterine compression suture to control postpartum hemorrhage had adequate information for analysis. Of those, 25% continued to bleed and underwent hysterectomy, Dr. Gilles Kayem of the University of Oxford (England) and his colleagues reported.
Suture failure occurred in 42% of those with a 2- to 6-hour delay in suture placement, compared with only 16% of those with earlier suture placement. After adjustment for numerous socioeconomic, maternal, and medical factors, a 2- to 6-hour delay in suture placement was found to be independently associated with a fourfold increase in the odds of hysterectomy, the investigators found (Obstet. Gynecol. 2011;117:14-20).
“One possible explanation may be that unrecognized bleeding that prolongs the delay between the delivery and the treatment increases the risk of hysterectomy,” they wrote, explaining that “a higher blood loss and disseminated intravascular coagulation would lead to clinical conditions that render hysterectomy almost inevitable.”
Failure in this study was also more likely in women older than age 35 years, compared with younger women (adjusted odds ratio, 2.77); those who were multiparous, compared with nulliparous women (AOR, 2.83); those who were unemployed or employed in routine or manual occupations, compared with those in managerial positions (AOR, 3.54); and those who had a vaginal delivery, compared with those who had a cesarean delivery (AOR, 6.08), the researchers found.
It is interesting, they noted, that vaginal delivery was the factor associated with the highest odds of hysterectomy in this study.
“It is possible that the obstetrician is more reluctant to perform a laparotomy to insert a compression suture after excessive bleeding after a vaginal delivery than after a cesarean delivery and that, therefore, only the women with the most severe hemorrhage were selected by the obstetrician to have a uterine compression suture after a vaginal delivery,” they speculated.
Another possible explanation is that other methods – such as intrauterine balloon or uterine packing – were used successfully in some cases of hemorrhage after vaginal delivery, and thus were not identified in this study, suggesting that cases involving uterine compression sutures after a vaginal delivery may be the most serious, and no other treatment modalities were available to treat the affected patients, they noted.
No differences in failure rates were seen among suture types (B-Lynch, modified B-Lynch, and 32 other techniques such as figure-of-eight, multiple compression, or square sutures).
However, because this was not a randomized study, comparisons among the suture methods were limited, as the baseline populations treated may have differed.
In all, 129 women (61%) had a hemorrhage resulting from atony. Hysterectomy rates according to the different types of uterine compression suture also were not significantly different. The hysterectomy rate was 26% in cases with atony and 23% in cases with other causes, such as placenta accreta, placenta previa, and uterine tear. After adjustment for a number of variables, the risk of hysterectomy was no different in women with atony compared with other causes of hemorrhage.
Patients included in the study were women identified via the U.K. Obstetric Surveillance System (UKOSS). Case patients were those giving birth who were treated with a uterine compression suture to treat postpartum hemorrhage.
Strengths of the study include the collection of comprehensive population-based national information about women who were treated with compression sutures for postpartum hemorrhage, they said. The findings emphasize the need for careful evaluation of blood loss following delivery so that delays in recognizing and managing hemorrhage can be avoided, they concluded.
View on The News
Technique Often Useful
Dr. Carolyn Zelop said that this study has important implications for practice. “I think the take-home message … is that [the use of compression sutures] is a very reliable technique, but that it's probably less useful in the setting of placenta accreta,” explaining that uterine atony appears to be the indication that leads to the most success with this technique.
Another important point made by the authors is that in the setting of vaginal delivery that is complicated by postpartum hemorrhage, it is important to “be on the clock and ready to move to the next intervention,” since a delay of 2–6 hours in suture placement was associated with increased risk of hysterectomy.
Although it seems logical that a clinician might be reluctant to proceed with laparotomy after vaginal delivery, a prolonged delay could predispose a patient to unrecognized blood loss, and increase the risk of compression suture failure. If mechanical tamponade techniques fail to control hemorrhaging, the clinician should proceed with laparotomy and uterine compression suture placement, she advised.
Vitals
DR. ZELOP is director of maternal-fetal medicine at Beth Israel Deaconess Medical Center in Boston. She said she had no relevant financial disclosures.
Major Finding: A 2- to 6-hour delay in suture placement was found to be independently associated with a fourfold increase in the odds of hysterectomy.
Data Source: A prospective population-based study of 1.2 million women, 210 of whom were treated with a uterine compression suture to control postpartum hemorrhage.
Disclosures: This study was funded by the charity Wellbeing of Women. Dr. Kayem disclosed that he is the beneficiary of a postdoctoral grant from the AXA Research Fund. Another study author, Marian Knight, is funded by a personal fellowship from the National Coordinating Centre for Research Capacity Development of the National Institute for Health Research. This was an independent study from UKOSS, which is partially funded by the Policy Research Programme in the Department of Health.
Uterine compression sutures for postpartum hemorrhage are more likely to fail when there is a delay of 2–6 hours between delivery and placement of the sutures, according to a large prospective population-based study.
Of 1.2 million women who delivered in the United Kingdom between September 2007 and March 2009, 210 who were treated with a uterine compression suture to control postpartum hemorrhage had adequate information for analysis. Of those, 25% continued to bleed and underwent hysterectomy, Dr. Gilles Kayem of the University of Oxford (England) and his colleagues reported.
Suture failure occurred in 42% of those with a 2- to 6-hour delay in suture placement, compared with only 16% of those with earlier suture placement. After adjustment for numerous socioeconomic, maternal, and medical factors, a 2- to 6-hour delay in suture placement was found to be independently associated with a fourfold increase in the odds of hysterectomy, the investigators found (Obstet. Gynecol. 2011;117:14-20).
“One possible explanation may be that unrecognized bleeding that prolongs the delay between the delivery and the treatment increases the risk of hysterectomy,” they wrote, explaining that “a higher blood loss and disseminated intravascular coagulation would lead to clinical conditions that render hysterectomy almost inevitable.”
Failure in this study was also more likely in women older than age 35 years, compared with younger women (adjusted odds ratio, 2.77); those who were multiparous, compared with nulliparous women (AOR, 2.83); those who were unemployed or employed in routine or manual occupations, compared with those in managerial positions (AOR, 3.54); and those who had a vaginal delivery, compared with those who had a cesarean delivery (AOR, 6.08), the researchers found.
It is interesting, they noted, that vaginal delivery was the factor associated with the highest odds of hysterectomy in this study.
“It is possible that the obstetrician is more reluctant to perform a laparotomy to insert a compression suture after excessive bleeding after a vaginal delivery than after a cesarean delivery and that, therefore, only the women with the most severe hemorrhage were selected by the obstetrician to have a uterine compression suture after a vaginal delivery,” they speculated.
Another possible explanation is that other methods – such as intrauterine balloon or uterine packing – were used successfully in some cases of hemorrhage after vaginal delivery, and thus were not identified in this study, suggesting that cases involving uterine compression sutures after a vaginal delivery may be the most serious, and no other treatment modalities were available to treat the affected patients, they noted.
No differences in failure rates were seen among suture types (B-Lynch, modified B-Lynch, and 32 other techniques such as figure-of-eight, multiple compression, or square sutures).
However, because this was not a randomized study, comparisons among the suture methods were limited, as the baseline populations treated may have differed.
In all, 129 women (61%) had a hemorrhage resulting from atony. Hysterectomy rates according to the different types of uterine compression suture also were not significantly different. The hysterectomy rate was 26% in cases with atony and 23% in cases with other causes, such as placenta accreta, placenta previa, and uterine tear. After adjustment for a number of variables, the risk of hysterectomy was no different in women with atony compared with other causes of hemorrhage.
Patients included in the study were women identified via the U.K. Obstetric Surveillance System (UKOSS). Case patients were those giving birth who were treated with a uterine compression suture to treat postpartum hemorrhage.
Strengths of the study include the collection of comprehensive population-based national information about women who were treated with compression sutures for postpartum hemorrhage, they said. The findings emphasize the need for careful evaluation of blood loss following delivery so that delays in recognizing and managing hemorrhage can be avoided, they concluded.
View on The News
Technique Often Useful
Dr. Carolyn Zelop said that this study has important implications for practice. “I think the take-home message … is that [the use of compression sutures] is a very reliable technique, but that it's probably less useful in the setting of placenta accreta,” explaining that uterine atony appears to be the indication that leads to the most success with this technique.
Another important point made by the authors is that in the setting of vaginal delivery that is complicated by postpartum hemorrhage, it is important to “be on the clock and ready to move to the next intervention,” since a delay of 2–6 hours in suture placement was associated with increased risk of hysterectomy.
Although it seems logical that a clinician might be reluctant to proceed with laparotomy after vaginal delivery, a prolonged delay could predispose a patient to unrecognized blood loss, and increase the risk of compression suture failure. If mechanical tamponade techniques fail to control hemorrhaging, the clinician should proceed with laparotomy and uterine compression suture placement, she advised.
Vitals
DR. ZELOP is director of maternal-fetal medicine at Beth Israel Deaconess Medical Center in Boston. She said she had no relevant financial disclosures.
From Obstetrics & Gynecology
Found: Most Cost-Effective Down Syndrome Test
Major Finding: Overall, the contingent screening test showed a cost-effectiveness ratio of $26,833 Canadian dollars per case of DS. The next most cost-effective test was the serum integrated screening test, but the incremental cost-effectiveness ratio (ICER) between the serum integrated screening and contingent screening tests was $3,815 Canadian dollars for every DS birth detected.
Data Source: A cost-effective study of Down syndrome screening tests based on a computer model including 110,948 pregnancies.
Disclosures: The journal did not provide any disclosure information.
Contingent screening for prenatal Down syndrome was the most cost-effective stratagem, compared with other screening protocols, based on data from a computer simulation study of 110,948 pregnancies.
Several screening options are available in Canada and the United States, but data to help clinicians choose among the tests are limited, wrote Dr. Jean Gekas of the Centre Hospitalier de l'Université Laval in Quebec City, and colleagues.
The researchers used a computer model to analyze a virtual population of 110,948 pregnant women based on the demographic, genetic, and Down syndrome (DS) phenotype characteristics of the local population in Quebec. The tests included were the combined test, triple test, quadruple test, integrated test, serum integrated test, sequential screening test, contingent screening test, and amniocentesis for women aged 35 years and older (Am. J. Obstet. Gynecol. 2011;204:175.e1-8).
“The contingent strategy seems to be the most cost effective and is associated with an attractive rate of procedure-related euploid miscarriages and unnecessary terminations,” they wrote. “Moreover, this screening option provides a majority of women with reassurance early in gestation and may minimize costs by limiting retesting.”
Overall, the contingent screening test showed a cost-effectiveness ratio of $26,833 Canadian dollars per case of DS. The next most cost-effective test was the serum integrated screening test, but the incremental cost-effectiveness ratio (ICER) between the serum integrated screening and contingent screening tests was $3,815 Canadian dollars for every DS birth detected.
The combined test, currently popular in the United States and Canada, had a slightly higher cost-effectiveness ratio of $47,358 Canadian dollars than does the contingent test, but it allowed 91% of women to be reassured in the first trimester, they said.
Similar results on major outcomes including false positives, unnecessary terminations, and DS pregnancies were observed for contingent tests and several other screening tests. But the contingent screening test was associated with one of the lowest rates of procedure-related miscarriages (10). The contingent test also allowed 78% of patients to be reassured in the first trimester.
The combined test was associated with the highest rate of DS pregnancies in the first trimester (90%), and the highest rates of procedure-related euploid miscarriages (71) and unnecessary terminations (24). The combined test was the most expensive of the tests that followed screening strategies, most likely due to the requirement of a nuchal fold transparency test, and the high rate of false positives and subsequent unnecessary terminations associated with it, Dr. Gekas and associates said.
The computer model showed that the integrated test had the lowest rate of procedure-related miscarriages. However, widespread use of the integrated test would prevent women from being reassured in the first trimester, they noted.
The study was limited by the lack of prospective data and the potential inability to generalize the results across countries, they said. However, “it is unlikely that a large-scale prospective clinical trial comparing these eight screening approaches could rapidly be organized across North America.”
Major Finding: Overall, the contingent screening test showed a cost-effectiveness ratio of $26,833 Canadian dollars per case of DS. The next most cost-effective test was the serum integrated screening test, but the incremental cost-effectiveness ratio (ICER) between the serum integrated screening and contingent screening tests was $3,815 Canadian dollars for every DS birth detected.
Data Source: A cost-effective study of Down syndrome screening tests based on a computer model including 110,948 pregnancies.
Disclosures: The journal did not provide any disclosure information.
Contingent screening for prenatal Down syndrome was the most cost-effective stratagem, compared with other screening protocols, based on data from a computer simulation study of 110,948 pregnancies.
Several screening options are available in Canada and the United States, but data to help clinicians choose among the tests are limited, wrote Dr. Jean Gekas of the Centre Hospitalier de l'Université Laval in Quebec City, and colleagues.
The researchers used a computer model to analyze a virtual population of 110,948 pregnant women based on the demographic, genetic, and Down syndrome (DS) phenotype characteristics of the local population in Quebec. The tests included were the combined test, triple test, quadruple test, integrated test, serum integrated test, sequential screening test, contingent screening test, and amniocentesis for women aged 35 years and older (Am. J. Obstet. Gynecol. 2011;204:175.e1-8).
“The contingent strategy seems to be the most cost effective and is associated with an attractive rate of procedure-related euploid miscarriages and unnecessary terminations,” they wrote. “Moreover, this screening option provides a majority of women with reassurance early in gestation and may minimize costs by limiting retesting.”
Overall, the contingent screening test showed a cost-effectiveness ratio of $26,833 Canadian dollars per case of DS. The next most cost-effective test was the serum integrated screening test, but the incremental cost-effectiveness ratio (ICER) between the serum integrated screening and contingent screening tests was $3,815 Canadian dollars for every DS birth detected.
The combined test, currently popular in the United States and Canada, had a slightly higher cost-effectiveness ratio of $47,358 Canadian dollars than does the contingent test, but it allowed 91% of women to be reassured in the first trimester, they said.
Similar results on major outcomes including false positives, unnecessary terminations, and DS pregnancies were observed for contingent tests and several other screening tests. But the contingent screening test was associated with one of the lowest rates of procedure-related miscarriages (10). The contingent test also allowed 78% of patients to be reassured in the first trimester.
The combined test was associated with the highest rate of DS pregnancies in the first trimester (90%), and the highest rates of procedure-related euploid miscarriages (71) and unnecessary terminations (24). The combined test was the most expensive of the tests that followed screening strategies, most likely due to the requirement of a nuchal fold transparency test, and the high rate of false positives and subsequent unnecessary terminations associated with it, Dr. Gekas and associates said.
The computer model showed that the integrated test had the lowest rate of procedure-related miscarriages. However, widespread use of the integrated test would prevent women from being reassured in the first trimester, they noted.
The study was limited by the lack of prospective data and the potential inability to generalize the results across countries, they said. However, “it is unlikely that a large-scale prospective clinical trial comparing these eight screening approaches could rapidly be organized across North America.”
Major Finding: Overall, the contingent screening test showed a cost-effectiveness ratio of $26,833 Canadian dollars per case of DS. The next most cost-effective test was the serum integrated screening test, but the incremental cost-effectiveness ratio (ICER) between the serum integrated screening and contingent screening tests was $3,815 Canadian dollars for every DS birth detected.
Data Source: A cost-effective study of Down syndrome screening tests based on a computer model including 110,948 pregnancies.
Disclosures: The journal did not provide any disclosure information.
Contingent screening for prenatal Down syndrome was the most cost-effective stratagem, compared with other screening protocols, based on data from a computer simulation study of 110,948 pregnancies.
Several screening options are available in Canada and the United States, but data to help clinicians choose among the tests are limited, wrote Dr. Jean Gekas of the Centre Hospitalier de l'Université Laval in Quebec City, and colleagues.
The researchers used a computer model to analyze a virtual population of 110,948 pregnant women based on the demographic, genetic, and Down syndrome (DS) phenotype characteristics of the local population in Quebec. The tests included were the combined test, triple test, quadruple test, integrated test, serum integrated test, sequential screening test, contingent screening test, and amniocentesis for women aged 35 years and older (Am. J. Obstet. Gynecol. 2011;204:175.e1-8).
“The contingent strategy seems to be the most cost effective and is associated with an attractive rate of procedure-related euploid miscarriages and unnecessary terminations,” they wrote. “Moreover, this screening option provides a majority of women with reassurance early in gestation and may minimize costs by limiting retesting.”
Overall, the contingent screening test showed a cost-effectiveness ratio of $26,833 Canadian dollars per case of DS. The next most cost-effective test was the serum integrated screening test, but the incremental cost-effectiveness ratio (ICER) between the serum integrated screening and contingent screening tests was $3,815 Canadian dollars for every DS birth detected.
The combined test, currently popular in the United States and Canada, had a slightly higher cost-effectiveness ratio of $47,358 Canadian dollars than does the contingent test, but it allowed 91% of women to be reassured in the first trimester, they said.
Similar results on major outcomes including false positives, unnecessary terminations, and DS pregnancies were observed for contingent tests and several other screening tests. But the contingent screening test was associated with one of the lowest rates of procedure-related miscarriages (10). The contingent test also allowed 78% of patients to be reassured in the first trimester.
The combined test was associated with the highest rate of DS pregnancies in the first trimester (90%), and the highest rates of procedure-related euploid miscarriages (71) and unnecessary terminations (24). The combined test was the most expensive of the tests that followed screening strategies, most likely due to the requirement of a nuchal fold transparency test, and the high rate of false positives and subsequent unnecessary terminations associated with it, Dr. Gekas and associates said.
The computer model showed that the integrated test had the lowest rate of procedure-related miscarriages. However, widespread use of the integrated test would prevent women from being reassured in the first trimester, they noted.
The study was limited by the lack of prospective data and the potential inability to generalize the results across countries, they said. However, “it is unlikely that a large-scale prospective clinical trial comparing these eight screening approaches could rapidly be organized across North America.”
From the American Journal Of Obstetrics & Gynecology
Universal MRSA Screening at L&D: Little Benefit
BOSTON – Active surveillance testing for methicillin-resistant Staphylococcus aureus colonization of pregnant women who were admitted to labor and delivery units costs a lot of bucks for only a little bang.
Over a 20-month period, a universal methicillin-resistant S. aureus (MRSA) screening program, required by Illinois law, cost $90,950 but had no apparent impact either on MRSA disease in the postpartum period or on nosocomial MRSA infections in a postpartum ward and newborn nursery, said Naseem Helo, a fourth-year medical student at Loyola University Medical Center in Maywood, Ill.
Among 2,254 pregnant women who were admitted to the labor and delivery unit, 1,819 (81%) received a nasal MRSA test at a cost of $50 each and 39 women (2%) screened positive, for a cost of more than $2,300 per positive screen, Mr. Helo said at the meeting, which was sponsored by the American Society for Microbiology.
Of the 39 MRSA-colonized women, 13 went on to have a cesarean section, 21 had vaginal delivery, 2 had miscarriages, and 3 were lost to follow-up because they did not deliver at the center.
When investigators looked at the effect of the positive results on practice, they found that although 9 of 13 (69%) women who had cesareans had positive test results available before the surgery, only 3 of the 9 (33%) received vancomycin prophylaxis.
“During the newborn stay, no newborns had complications of MRSA disease, and there were no nosocomial infections in our labor and delivery service, postpartum ward, and newborn nursery during the 20-month study period or 2 years prior to the study,” Mr. Helo said.
The investigators suggested that the decision to implement universal MRSA surveillance should be driven by MRSA colonization rates in specific geographic populations.
BOSTON – Active surveillance testing for methicillin-resistant Staphylococcus aureus colonization of pregnant women who were admitted to labor and delivery units costs a lot of bucks for only a little bang.
Over a 20-month period, a universal methicillin-resistant S. aureus (MRSA) screening program, required by Illinois law, cost $90,950 but had no apparent impact either on MRSA disease in the postpartum period or on nosocomial MRSA infections in a postpartum ward and newborn nursery, said Naseem Helo, a fourth-year medical student at Loyola University Medical Center in Maywood, Ill.
Among 2,254 pregnant women who were admitted to the labor and delivery unit, 1,819 (81%) received a nasal MRSA test at a cost of $50 each and 39 women (2%) screened positive, for a cost of more than $2,300 per positive screen, Mr. Helo said at the meeting, which was sponsored by the American Society for Microbiology.
Of the 39 MRSA-colonized women, 13 went on to have a cesarean section, 21 had vaginal delivery, 2 had miscarriages, and 3 were lost to follow-up because they did not deliver at the center.
When investigators looked at the effect of the positive results on practice, they found that although 9 of 13 (69%) women who had cesareans had positive test results available before the surgery, only 3 of the 9 (33%) received vancomycin prophylaxis.
“During the newborn stay, no newborns had complications of MRSA disease, and there were no nosocomial infections in our labor and delivery service, postpartum ward, and newborn nursery during the 20-month study period or 2 years prior to the study,” Mr. Helo said.
The investigators suggested that the decision to implement universal MRSA surveillance should be driven by MRSA colonization rates in specific geographic populations.
BOSTON – Active surveillance testing for methicillin-resistant Staphylococcus aureus colonization of pregnant women who were admitted to labor and delivery units costs a lot of bucks for only a little bang.
Over a 20-month period, a universal methicillin-resistant S. aureus (MRSA) screening program, required by Illinois law, cost $90,950 but had no apparent impact either on MRSA disease in the postpartum period or on nosocomial MRSA infections in a postpartum ward and newborn nursery, said Naseem Helo, a fourth-year medical student at Loyola University Medical Center in Maywood, Ill.
Among 2,254 pregnant women who were admitted to the labor and delivery unit, 1,819 (81%) received a nasal MRSA test at a cost of $50 each and 39 women (2%) screened positive, for a cost of more than $2,300 per positive screen, Mr. Helo said at the meeting, which was sponsored by the American Society for Microbiology.
Of the 39 MRSA-colonized women, 13 went on to have a cesarean section, 21 had vaginal delivery, 2 had miscarriages, and 3 were lost to follow-up because they did not deliver at the center.
When investigators looked at the effect of the positive results on practice, they found that although 9 of 13 (69%) women who had cesareans had positive test results available before the surgery, only 3 of the 9 (33%) received vancomycin prophylaxis.
“During the newborn stay, no newborns had complications of MRSA disease, and there were no nosocomial infections in our labor and delivery service, postpartum ward, and newborn nursery during the 20-month study period or 2 years prior to the study,” Mr. Helo said.
The investigators suggested that the decision to implement universal MRSA surveillance should be driven by MRSA colonization rates in specific geographic populations.
From the Interscience Conference On Antimicrobial Agents And Chemotherapy
Early Amniotomy Shortens Labor in Nulliparas
SAN FRANCISCO – Early amniotomy appears safe and efficacious for shortening labor at term in nulliparous women having an indication for labor induction, according to results of a randomized controlled trial.
Among the 585 women studied, the average time from induction to delivery was 2.3 hours, or 11% shorter with early amniotomy vs. standard care, investigators reported at the meeting This benefit was achieved without an increase in rates of maternal or neonatal infections.
“Based on this clinical trial, it would seem that early amniotomy may be a useful adjunct for nulliparous labor inductions,” said Dr. George A. Macones, the Mitchell and Elaine Yanow Professor and chair of the department of ob.gyn. at Washington University in St. Louis.
Many studies have evaluated different methods of labor induction, he noted. “However, surprisingly, there are very few data on the timing of amniotomy in labor induction and how this may improve or worsen outcomes.”
Amniotomy is easy and inexpensive and may shorten labor, according to Dr. Macones. But it also may be associated with rare complications such as umbilical cord prolapse, and possibly with an increased infection risk resulting from a longer duration of ruptured membranes.
Women were eligible for the trial if they were nulliparous, had a singleton pregnancy, were at term (37 weeks' gestation or later), and needed induction as determined by their treating physician. They were excluded if they were HIV positive or had cervical dilatation exceeding 4 cm at the time of admission to labor and delivery.
The women were randomly assigned in a 1:1 ratio to nonblinded management with either early amniotomy (defined as artificial rupture of membranes performed when cervical dilatation was equal to or less than 4 cm) or standard care (defined as artificial rupture of membranes performed when cervical dilatation was greater than 4 cm). The primary method of induction (misoprostol, cervical Foley catheter, oxytocin, and/or prostaglandin gel) was left to the treating physician's discretion. “Just to be clear, we did not study the timing of amniotomy as a primary method of induction, but rather as an adjunct to other methods,” Dr. Macones noted.
All other decisions about intrapartum and postpartum care were similarly left up to the treating physicians. The 585 women randomized were 23 years old on average, and the majority (70%) was black. Almost a third had gestational hypertension or preeclampsia, and another third were positive for group B streptococcus. The mean gestational age was about 39.5 weeks, and the mean cervical dilatation was 1.1 cm on admission. The leading indications for induction were a gestation past 40 weeks (39%) and gestational hypertension or preeclampsia (28%).
The primary methods of induction used were similar across groups. In nearly three-fourths of women, the treating physicians used multiple methods.
Most women received epidural analgesia, with no difference between groups, according to Dr. Macones.
Median cervical dilatation at the time of rupture of membranes was 4 cm less in the early amniotomy group, compared with the standard care group (3.0 vs. 7.0 cm; P = .001). In intent-to-treat analyses, the time from induction to delivery was 2.3 hours shorter with early amniotomy (19.0 vs. 21.3 hours; P = .004). “This difference in the length of labor occurred mainly and not surprisingly in the first stage of labor, but not in the second stage,” Dr. Macones noted. In addition, these women were more likely to deliver within 24 hours of labor induction (68% vs. 56%; P = .002).
The early amniotomy group did not differ significantly from the standard care group with respect to rates of cesarean delivery (41% vs. 40%), cord prolapse (0.7% vs. 0%), and abruption (0.4% vs. 0.6%).
Fetal heart rate data were not analyzed, but rates of amnioinfusion (a “reasonable proxy” for variable decelerations) were similar, according to Dr. Macones.
The two groups also had statistically indistinguishable rates of infectious outcomes, including chorioamnionitis (11.5% vs. 8.5%) postpartum fever (10.4% vs. 9.4%) in the mother, and NICU admission (13.6% vs. 15.0%) and suspected or confirmed sepsis (9.7% vs. 11.1%) in the neonate.
In questions posed after the presentation, one attendee asked how the 4-cm threshold was selected for early amniotomy, and whether the findings would be similar with, say, a 2-cm threshold instead. “We chose 4 cm based on some earlier work in spontaneous labor with rupturing membranes,” Dr. Macones explained. “I agree that we could dial that down a bit.” However, within the early amniotomy group, the efficacy and safety findings appeared similar regardless of the timing of the procedure, he said.
When asked if the study was mixing cervical ripening with labor induction, Dr. Macones said, “I think the lines between ripening and induction are actually quite gray.” He contended that the study's aim was to assess the impact of amniotomy when the intention was to perform it as early as possible.
An alternative approach would be to look at women once their cervix is ripened and then ask what the role of amniotomy is, he acknowledged. “But I think that's a little bit different question than we actually had.”
Dr. Macones did not report any relevant financial disclosures.
'This difference in length of labor occurred mainly and not surprisingly in the first stage of labor.'
Source DR. MACONES
SAN FRANCISCO – Early amniotomy appears safe and efficacious for shortening labor at term in nulliparous women having an indication for labor induction, according to results of a randomized controlled trial.
Among the 585 women studied, the average time from induction to delivery was 2.3 hours, or 11% shorter with early amniotomy vs. standard care, investigators reported at the meeting This benefit was achieved without an increase in rates of maternal or neonatal infections.
“Based on this clinical trial, it would seem that early amniotomy may be a useful adjunct for nulliparous labor inductions,” said Dr. George A. Macones, the Mitchell and Elaine Yanow Professor and chair of the department of ob.gyn. at Washington University in St. Louis.
Many studies have evaluated different methods of labor induction, he noted. “However, surprisingly, there are very few data on the timing of amniotomy in labor induction and how this may improve or worsen outcomes.”
Amniotomy is easy and inexpensive and may shorten labor, according to Dr. Macones. But it also may be associated with rare complications such as umbilical cord prolapse, and possibly with an increased infection risk resulting from a longer duration of ruptured membranes.
Women were eligible for the trial if they were nulliparous, had a singleton pregnancy, were at term (37 weeks' gestation or later), and needed induction as determined by their treating physician. They were excluded if they were HIV positive or had cervical dilatation exceeding 4 cm at the time of admission to labor and delivery.
The women were randomly assigned in a 1:1 ratio to nonblinded management with either early amniotomy (defined as artificial rupture of membranes performed when cervical dilatation was equal to or less than 4 cm) or standard care (defined as artificial rupture of membranes performed when cervical dilatation was greater than 4 cm). The primary method of induction (misoprostol, cervical Foley catheter, oxytocin, and/or prostaglandin gel) was left to the treating physician's discretion. “Just to be clear, we did not study the timing of amniotomy as a primary method of induction, but rather as an adjunct to other methods,” Dr. Macones noted.
All other decisions about intrapartum and postpartum care were similarly left up to the treating physicians. The 585 women randomized were 23 years old on average, and the majority (70%) was black. Almost a third had gestational hypertension or preeclampsia, and another third were positive for group B streptococcus. The mean gestational age was about 39.5 weeks, and the mean cervical dilatation was 1.1 cm on admission. The leading indications for induction were a gestation past 40 weeks (39%) and gestational hypertension or preeclampsia (28%).
The primary methods of induction used were similar across groups. In nearly three-fourths of women, the treating physicians used multiple methods.
Most women received epidural analgesia, with no difference between groups, according to Dr. Macones.
Median cervical dilatation at the time of rupture of membranes was 4 cm less in the early amniotomy group, compared with the standard care group (3.0 vs. 7.0 cm; P = .001). In intent-to-treat analyses, the time from induction to delivery was 2.3 hours shorter with early amniotomy (19.0 vs. 21.3 hours; P = .004). “This difference in the length of labor occurred mainly and not surprisingly in the first stage of labor, but not in the second stage,” Dr. Macones noted. In addition, these women were more likely to deliver within 24 hours of labor induction (68% vs. 56%; P = .002).
The early amniotomy group did not differ significantly from the standard care group with respect to rates of cesarean delivery (41% vs. 40%), cord prolapse (0.7% vs. 0%), and abruption (0.4% vs. 0.6%).
Fetal heart rate data were not analyzed, but rates of amnioinfusion (a “reasonable proxy” for variable decelerations) were similar, according to Dr. Macones.
The two groups also had statistically indistinguishable rates of infectious outcomes, including chorioamnionitis (11.5% vs. 8.5%) postpartum fever (10.4% vs. 9.4%) in the mother, and NICU admission (13.6% vs. 15.0%) and suspected or confirmed sepsis (9.7% vs. 11.1%) in the neonate.
In questions posed after the presentation, one attendee asked how the 4-cm threshold was selected for early amniotomy, and whether the findings would be similar with, say, a 2-cm threshold instead. “We chose 4 cm based on some earlier work in spontaneous labor with rupturing membranes,” Dr. Macones explained. “I agree that we could dial that down a bit.” However, within the early amniotomy group, the efficacy and safety findings appeared similar regardless of the timing of the procedure, he said.
When asked if the study was mixing cervical ripening with labor induction, Dr. Macones said, “I think the lines between ripening and induction are actually quite gray.” He contended that the study's aim was to assess the impact of amniotomy when the intention was to perform it as early as possible.
An alternative approach would be to look at women once their cervix is ripened and then ask what the role of amniotomy is, he acknowledged. “But I think that's a little bit different question than we actually had.”
Dr. Macones did not report any relevant financial disclosures.
'This difference in length of labor occurred mainly and not surprisingly in the first stage of labor.'
Source DR. MACONES
SAN FRANCISCO – Early amniotomy appears safe and efficacious for shortening labor at term in nulliparous women having an indication for labor induction, according to results of a randomized controlled trial.
Among the 585 women studied, the average time from induction to delivery was 2.3 hours, or 11% shorter with early amniotomy vs. standard care, investigators reported at the meeting This benefit was achieved without an increase in rates of maternal or neonatal infections.
“Based on this clinical trial, it would seem that early amniotomy may be a useful adjunct for nulliparous labor inductions,” said Dr. George A. Macones, the Mitchell and Elaine Yanow Professor and chair of the department of ob.gyn. at Washington University in St. Louis.
Many studies have evaluated different methods of labor induction, he noted. “However, surprisingly, there are very few data on the timing of amniotomy in labor induction and how this may improve or worsen outcomes.”
Amniotomy is easy and inexpensive and may shorten labor, according to Dr. Macones. But it also may be associated with rare complications such as umbilical cord prolapse, and possibly with an increased infection risk resulting from a longer duration of ruptured membranes.
Women were eligible for the trial if they were nulliparous, had a singleton pregnancy, were at term (37 weeks' gestation or later), and needed induction as determined by their treating physician. They were excluded if they were HIV positive or had cervical dilatation exceeding 4 cm at the time of admission to labor and delivery.
The women were randomly assigned in a 1:1 ratio to nonblinded management with either early amniotomy (defined as artificial rupture of membranes performed when cervical dilatation was equal to or less than 4 cm) or standard care (defined as artificial rupture of membranes performed when cervical dilatation was greater than 4 cm). The primary method of induction (misoprostol, cervical Foley catheter, oxytocin, and/or prostaglandin gel) was left to the treating physician's discretion. “Just to be clear, we did not study the timing of amniotomy as a primary method of induction, but rather as an adjunct to other methods,” Dr. Macones noted.
All other decisions about intrapartum and postpartum care were similarly left up to the treating physicians. The 585 women randomized were 23 years old on average, and the majority (70%) was black. Almost a third had gestational hypertension or preeclampsia, and another third were positive for group B streptococcus. The mean gestational age was about 39.5 weeks, and the mean cervical dilatation was 1.1 cm on admission. The leading indications for induction were a gestation past 40 weeks (39%) and gestational hypertension or preeclampsia (28%).
The primary methods of induction used were similar across groups. In nearly three-fourths of women, the treating physicians used multiple methods.
Most women received epidural analgesia, with no difference between groups, according to Dr. Macones.
Median cervical dilatation at the time of rupture of membranes was 4 cm less in the early amniotomy group, compared with the standard care group (3.0 vs. 7.0 cm; P = .001). In intent-to-treat analyses, the time from induction to delivery was 2.3 hours shorter with early amniotomy (19.0 vs. 21.3 hours; P = .004). “This difference in the length of labor occurred mainly and not surprisingly in the first stage of labor, but not in the second stage,” Dr. Macones noted. In addition, these women were more likely to deliver within 24 hours of labor induction (68% vs. 56%; P = .002).
The early amniotomy group did not differ significantly from the standard care group with respect to rates of cesarean delivery (41% vs. 40%), cord prolapse (0.7% vs. 0%), and abruption (0.4% vs. 0.6%).
Fetal heart rate data were not analyzed, but rates of amnioinfusion (a “reasonable proxy” for variable decelerations) were similar, according to Dr. Macones.
The two groups also had statistically indistinguishable rates of infectious outcomes, including chorioamnionitis (11.5% vs. 8.5%) postpartum fever (10.4% vs. 9.4%) in the mother, and NICU admission (13.6% vs. 15.0%) and suspected or confirmed sepsis (9.7% vs. 11.1%) in the neonate.
In questions posed after the presentation, one attendee asked how the 4-cm threshold was selected for early amniotomy, and whether the findings would be similar with, say, a 2-cm threshold instead. “We chose 4 cm based on some earlier work in spontaneous labor with rupturing membranes,” Dr. Macones explained. “I agree that we could dial that down a bit.” However, within the early amniotomy group, the efficacy and safety findings appeared similar regardless of the timing of the procedure, he said.
When asked if the study was mixing cervical ripening with labor induction, Dr. Macones said, “I think the lines between ripening and induction are actually quite gray.” He contended that the study's aim was to assess the impact of amniotomy when the intention was to perform it as early as possible.
An alternative approach would be to look at women once their cervix is ripened and then ask what the role of amniotomy is, he acknowledged. “But I think that's a little bit different question than we actually had.”
Dr. Macones did not report any relevant financial disclosures.
'This difference in length of labor occurred mainly and not surprisingly in the first stage of labor.'
Source DR. MACONES
From the Annual Meeting of the Society For Maternal-Fetal Medicine
NAAT Outperforms Antepartum GBS Culture
Major Finding: The rapid intrapartum GBS test, compared with antepartum culture, had a significantly better sensitivity (91% vs. 69%) and negative predictive value (97% vs. 91%). The specificity and positive predictive value were similarly high.
Data Source: A prospective study of 559 women in labor who had documented antepartum culture results and had not received intrapartum antibiotics.
Disclosures: Dr. Gupta did not report any relevant financial disclosures. Cepheid loaned the investigators the system used for rapid testing.
SAN FRANCISCO – A rapid intrapartum test for group B streptococcus may improve identification of colonized pregnant women, according to study results reported at the meeting
When the rapid test – a nucleic acid amplification test (NAAT) that yields results within an hour – was compared with a conventional antepartum bacterial culture, the rapid test was superior in identifying which of the 559 laboring women in the study had group B streptococcus (GBS) colonization (sensitivity, 91% vs. 69%). The rapid test also had a significantly better negative predictive value, and its specificity and positive predictive value were similarly high.
“In our population, intrapartum NAAT appeared to have superior test characteristics to antepartum culture for predicting intrapartum GBS culture status,” said Dr. Munish Gupta, an associate director of the neonatal intensive care unit at the Beth Israel Deaconess Medical Center in Boston.
“NAAT may be able to help identify women who are positive for GBS by intrapartum culture but negative by antepartum culture, a group of women that would be at particularly high risk for GBS transmission to their newborns by current standard management,” he commented.
In fact, about 60% of cases of neonatal early-onset GBS disease occur in infants born to mothers who screened negative by conventional means during their pregnancy, according to Dr. Gupta. “In addition, it has been well documented that results of antepartum GBS screening cultures do not always accurately predict intrapartum GBS status.”
The investigators prospectively studied pregnant women who were admitted to the labor and delivery department, had documented results of an antepartum GBS culture performed at 35–37 weeks' gestation, and had not received intrapartum antibiotics.
Two intrapartum rectovaginal samples were obtained according to Centers for Disease Control and Prevention guidelines.
One sample was sent for GBS culture by the hospital laboratory, and the result of this intrapartum culture served as the reference standard for the study, Dr. Gupta explained.
The other sample was used for rapid testing with the Xpert GBS test, performed in the department 24/7 on a system loaned to the investigators by the manufacturer, Cepheid.
“Of note, the intrapartum culture and NAAT results were not used for clinical management,” he pointed out.
Study results were based on 559 women who were 32 years old, on average. About 61% were white, 14% were Asian or Pacific Islander, 13% were black, 10% were Hispanic, and the rest were of other races or ethnicities.
The women had a mean gestational age of 39.4 weeks, and 99% had singleton pregnancies. Nearly three-fourths delivered vaginally.
Overall, 24% of the women had a positive result on the intrapartum GBS culture, according to Dr. Gupta.
The rapid test, compared with antepartum culture, had a significantly better sensitivity (91% vs. 69%) and negative predictive value (97% vs. 91%), and a similarly high specificity (98% vs. 96%) and positive predictive value (92% vs. 84%).
The results of antepartum and intrapartum culture were discordant in 10% of women overall. But the rate of discordance varied by race/ethnicity (P = .006), ranging from 4% in Asian women to 18% and 19% in their black and Hispanic counterparts, respectively.
Among women with a negative antepartum culture, 9% had a positive intrapartum culture, Dr. Gupta reported. Most of this subset did not receive intrapartum antibiotics (98%) and had infants who did not receive a sepsis evaluation (78%). Fully 81% of the subset had a positive rapid test result.
Previous studies of NAAT testing have raised concerns about the frequency of indeterminate test results in clinical practice, he noted. The results of the rapid test were indeterminate on first testing in 13% of the women studied, but they were indeterminate on repeated testing in merely 2%. The sample preparation time for the rapid test was 5 minutes, and the median processing time was 48 minutes, with 99.6% of samples processed in 50 minutes or less.
“Future work is needed to continue to explore the role of intrapartum GBS NAAT in clinical practice,” Dr. Gupta asserted. “It may be that the NAAT will prove to be a useful adjunct in certain populations in which the antepartum culture may not be a sufficient determinant of intrapartum GBS risk, such as [women who] are GBS negative on antepartum culture, black and Hispanic women, and preterm deliveries.”
The study did not have adequate power to assess the potential impact of the rapid test on neonatal outcomes, but they should be a focus in future studies, he recommended.
When asked by an attendee if he would argue with new guidelines that recommend prophylaxis in women with risk factors even if they have a negative NAAT test result, Dr. Gupta emphatically said he would not argue with them.
“I don't think any of us … involved in this study would look at the NAAT as a replacement for antepartum screening. Antepartum screening clearly has been incredibly effective and very important for reducing the burden of GBS disease, so I think the idea would be that NAAT might be a supplement in certain populations who are currently being missed by the antepartum culture,” he commented. “So I agree with current treatment for either antepartum culture–positive moms or moms with other risk factors. This [test] doesn't really seem to replace that.”
Major Finding: The rapid intrapartum GBS test, compared with antepartum culture, had a significantly better sensitivity (91% vs. 69%) and negative predictive value (97% vs. 91%). The specificity and positive predictive value were similarly high.
Data Source: A prospective study of 559 women in labor who had documented antepartum culture results and had not received intrapartum antibiotics.
Disclosures: Dr. Gupta did not report any relevant financial disclosures. Cepheid loaned the investigators the system used for rapid testing.
SAN FRANCISCO – A rapid intrapartum test for group B streptococcus may improve identification of colonized pregnant women, according to study results reported at the meeting
When the rapid test – a nucleic acid amplification test (NAAT) that yields results within an hour – was compared with a conventional antepartum bacterial culture, the rapid test was superior in identifying which of the 559 laboring women in the study had group B streptococcus (GBS) colonization (sensitivity, 91% vs. 69%). The rapid test also had a significantly better negative predictive value, and its specificity and positive predictive value were similarly high.
“In our population, intrapartum NAAT appeared to have superior test characteristics to antepartum culture for predicting intrapartum GBS culture status,” said Dr. Munish Gupta, an associate director of the neonatal intensive care unit at the Beth Israel Deaconess Medical Center in Boston.
“NAAT may be able to help identify women who are positive for GBS by intrapartum culture but negative by antepartum culture, a group of women that would be at particularly high risk for GBS transmission to their newborns by current standard management,” he commented.
In fact, about 60% of cases of neonatal early-onset GBS disease occur in infants born to mothers who screened negative by conventional means during their pregnancy, according to Dr. Gupta. “In addition, it has been well documented that results of antepartum GBS screening cultures do not always accurately predict intrapartum GBS status.”
The investigators prospectively studied pregnant women who were admitted to the labor and delivery department, had documented results of an antepartum GBS culture performed at 35–37 weeks' gestation, and had not received intrapartum antibiotics.
Two intrapartum rectovaginal samples were obtained according to Centers for Disease Control and Prevention guidelines.
One sample was sent for GBS culture by the hospital laboratory, and the result of this intrapartum culture served as the reference standard for the study, Dr. Gupta explained.
The other sample was used for rapid testing with the Xpert GBS test, performed in the department 24/7 on a system loaned to the investigators by the manufacturer, Cepheid.
“Of note, the intrapartum culture and NAAT results were not used for clinical management,” he pointed out.
Study results were based on 559 women who were 32 years old, on average. About 61% were white, 14% were Asian or Pacific Islander, 13% were black, 10% were Hispanic, and the rest were of other races or ethnicities.
The women had a mean gestational age of 39.4 weeks, and 99% had singleton pregnancies. Nearly three-fourths delivered vaginally.
Overall, 24% of the women had a positive result on the intrapartum GBS culture, according to Dr. Gupta.
The rapid test, compared with antepartum culture, had a significantly better sensitivity (91% vs. 69%) and negative predictive value (97% vs. 91%), and a similarly high specificity (98% vs. 96%) and positive predictive value (92% vs. 84%).
The results of antepartum and intrapartum culture were discordant in 10% of women overall. But the rate of discordance varied by race/ethnicity (P = .006), ranging from 4% in Asian women to 18% and 19% in their black and Hispanic counterparts, respectively.
Among women with a negative antepartum culture, 9% had a positive intrapartum culture, Dr. Gupta reported. Most of this subset did not receive intrapartum antibiotics (98%) and had infants who did not receive a sepsis evaluation (78%). Fully 81% of the subset had a positive rapid test result.
Previous studies of NAAT testing have raised concerns about the frequency of indeterminate test results in clinical practice, he noted. The results of the rapid test were indeterminate on first testing in 13% of the women studied, but they were indeterminate on repeated testing in merely 2%. The sample preparation time for the rapid test was 5 minutes, and the median processing time was 48 minutes, with 99.6% of samples processed in 50 minutes or less.
“Future work is needed to continue to explore the role of intrapartum GBS NAAT in clinical practice,” Dr. Gupta asserted. “It may be that the NAAT will prove to be a useful adjunct in certain populations in which the antepartum culture may not be a sufficient determinant of intrapartum GBS risk, such as [women who] are GBS negative on antepartum culture, black and Hispanic women, and preterm deliveries.”
The study did not have adequate power to assess the potential impact of the rapid test on neonatal outcomes, but they should be a focus in future studies, he recommended.
When asked by an attendee if he would argue with new guidelines that recommend prophylaxis in women with risk factors even if they have a negative NAAT test result, Dr. Gupta emphatically said he would not argue with them.
“I don't think any of us … involved in this study would look at the NAAT as a replacement for antepartum screening. Antepartum screening clearly has been incredibly effective and very important for reducing the burden of GBS disease, so I think the idea would be that NAAT might be a supplement in certain populations who are currently being missed by the antepartum culture,” he commented. “So I agree with current treatment for either antepartum culture–positive moms or moms with other risk factors. This [test] doesn't really seem to replace that.”
Major Finding: The rapid intrapartum GBS test, compared with antepartum culture, had a significantly better sensitivity (91% vs. 69%) and negative predictive value (97% vs. 91%). The specificity and positive predictive value were similarly high.
Data Source: A prospective study of 559 women in labor who had documented antepartum culture results and had not received intrapartum antibiotics.
Disclosures: Dr. Gupta did not report any relevant financial disclosures. Cepheid loaned the investigators the system used for rapid testing.
SAN FRANCISCO – A rapid intrapartum test for group B streptococcus may improve identification of colonized pregnant women, according to study results reported at the meeting
When the rapid test – a nucleic acid amplification test (NAAT) that yields results within an hour – was compared with a conventional antepartum bacterial culture, the rapid test was superior in identifying which of the 559 laboring women in the study had group B streptococcus (GBS) colonization (sensitivity, 91% vs. 69%). The rapid test also had a significantly better negative predictive value, and its specificity and positive predictive value were similarly high.
“In our population, intrapartum NAAT appeared to have superior test characteristics to antepartum culture for predicting intrapartum GBS culture status,” said Dr. Munish Gupta, an associate director of the neonatal intensive care unit at the Beth Israel Deaconess Medical Center in Boston.
“NAAT may be able to help identify women who are positive for GBS by intrapartum culture but negative by antepartum culture, a group of women that would be at particularly high risk for GBS transmission to their newborns by current standard management,” he commented.
In fact, about 60% of cases of neonatal early-onset GBS disease occur in infants born to mothers who screened negative by conventional means during their pregnancy, according to Dr. Gupta. “In addition, it has been well documented that results of antepartum GBS screening cultures do not always accurately predict intrapartum GBS status.”
The investigators prospectively studied pregnant women who were admitted to the labor and delivery department, had documented results of an antepartum GBS culture performed at 35–37 weeks' gestation, and had not received intrapartum antibiotics.
Two intrapartum rectovaginal samples were obtained according to Centers for Disease Control and Prevention guidelines.
One sample was sent for GBS culture by the hospital laboratory, and the result of this intrapartum culture served as the reference standard for the study, Dr. Gupta explained.
The other sample was used for rapid testing with the Xpert GBS test, performed in the department 24/7 on a system loaned to the investigators by the manufacturer, Cepheid.
“Of note, the intrapartum culture and NAAT results were not used for clinical management,” he pointed out.
Study results were based on 559 women who were 32 years old, on average. About 61% were white, 14% were Asian or Pacific Islander, 13% were black, 10% were Hispanic, and the rest were of other races or ethnicities.
The women had a mean gestational age of 39.4 weeks, and 99% had singleton pregnancies. Nearly three-fourths delivered vaginally.
Overall, 24% of the women had a positive result on the intrapartum GBS culture, according to Dr. Gupta.
The rapid test, compared with antepartum culture, had a significantly better sensitivity (91% vs. 69%) and negative predictive value (97% vs. 91%), and a similarly high specificity (98% vs. 96%) and positive predictive value (92% vs. 84%).
The results of antepartum and intrapartum culture were discordant in 10% of women overall. But the rate of discordance varied by race/ethnicity (P = .006), ranging from 4% in Asian women to 18% and 19% in their black and Hispanic counterparts, respectively.
Among women with a negative antepartum culture, 9% had a positive intrapartum culture, Dr. Gupta reported. Most of this subset did not receive intrapartum antibiotics (98%) and had infants who did not receive a sepsis evaluation (78%). Fully 81% of the subset had a positive rapid test result.
Previous studies of NAAT testing have raised concerns about the frequency of indeterminate test results in clinical practice, he noted. The results of the rapid test were indeterminate on first testing in 13% of the women studied, but they were indeterminate on repeated testing in merely 2%. The sample preparation time for the rapid test was 5 minutes, and the median processing time was 48 minutes, with 99.6% of samples processed in 50 minutes or less.
“Future work is needed to continue to explore the role of intrapartum GBS NAAT in clinical practice,” Dr. Gupta asserted. “It may be that the NAAT will prove to be a useful adjunct in certain populations in which the antepartum culture may not be a sufficient determinant of intrapartum GBS risk, such as [women who] are GBS negative on antepartum culture, black and Hispanic women, and preterm deliveries.”
The study did not have adequate power to assess the potential impact of the rapid test on neonatal outcomes, but they should be a focus in future studies, he recommended.
When asked by an attendee if he would argue with new guidelines that recommend prophylaxis in women with risk factors even if they have a negative NAAT test result, Dr. Gupta emphatically said he would not argue with them.
“I don't think any of us … involved in this study would look at the NAAT as a replacement for antepartum screening. Antepartum screening clearly has been incredibly effective and very important for reducing the burden of GBS disease, so I think the idea would be that NAAT might be a supplement in certain populations who are currently being missed by the antepartum culture,” he commented. “So I agree with current treatment for either antepartum culture–positive moms or moms with other risk factors. This [test] doesn't really seem to replace that.”
From the Annual Meeting of the Society For Maternal-Fetal Medicine
Cytokines, Fetal Growth, and RA
Major Finding: IL-10 in the first trimester seems to protect against low birth weight, while IL-6 seems to increase the risk. TNF-alpha exerts its influence in the third trimester, when higher levels also seem to protect against lower birth weights.
Data Source: A study of 302 pregnant women with rheumatoid arthritis and 33 controls.
Disclosures: The study was funded by the Dutch Arthritis Association. Dr. Dolhain did not disclose any pertinent financial relationships.
Circulating cytokines appear to influence fetal growth in pregnant women who have rheumatoid arthritis, results of a Dutch study suggest.
High levels of interleukin-10, IL-6, and TNF-alpha might all play a role, each acting independently – and at different stages of pregnancy – to increase the risk of low birth weight among infants born to these mothers, Dr. Radboud Dolhain and colleagues wrote (J. Reprod. Immunol. 2010 [doi: 10.1016/j.jri.2010.08.010]).
Dr. Dolhain of Erasmus University Medical Centre, Rotterdam, the Netherlands, and his coinvestigators examined circulating cytokines in 134 pregnant patients with rheumatoid arthritis during their first trimester, 168 in their third trimester, and 33 healthy controls. Birth weights were analyzed using standard deviation scores.
Disease activity in the women was based on the disease activity score for 28 joints (DAS28); the scale runs from 0–10, with higher numbers indicating greater disease activity.
Since IL-10, IL-6 and TNF-alpha generally decrease during pregnancy, the investigators sought to determine if any increasing gestational levels correlated with birth weight. Among the first trimester patients, 12 had detectable IL-10; all of these women had a higher disease activity score than did those with no IL-10 (mean DAS28 4.4 vs. 3.6).
Birth weights were compared between the two groups, which were matched with regard to disease activity, parity, and prednisone use.
The mean birth weight standard deviation was significantly greater in the IL-10 positive group (0.92) than in the matched negative group (0.15), Dr. Dolhain and his associates reported.
This association with IL-10 was not seen in the third trimester pregnancies.
The investigators then examined the effect of IL-6 by stratifying IL-6 levels and disease activity scores in the first and third trimester.
In the two groups with high disease activity (DAS28 3.8 or higher), birth weight standard deviation was significantly lower in mothers with high IL-6.
In the high IL-6 group, the birth weight standard deviation was −0.19, compared with 0.36 in the low IL-6 group.
Again, the authors found no such association in the third trimester.
“In the first trimester, elevated IL-10 seems to protect against the negative influence of RA disease activity on birth weight, [while] IL-6 seems to amplify this negative influence,” the investigators wrote.
“Both cytokines create a birth weight standard deviation of more than 0.50, which is considered clinically relevant. In the third trimester, there is no influence, suggesting an early critical window.”
TNF-alpha, however, did exert an influence in the third trimester of pregnancy, Dr. Dolhain and his colleagues noted. Stratifying TNF-alpha in the same way, they concluded that the birth weight standard deviation was lower in the group with low TNF-alpha (0.05) than in the group with high TNF-alpha (0.52).
This association was not present in the first trimester.
The finding that increased TNF-alpha is associated with better birth weights may require a rethinking of anti–TNF-alpha therapy for pregnant women, they suggested.
This implies that “TNF blockers, which are more and more prescribed during pregnancy to treat rheumatoid arthritis, should be used with caution,” Dr. Dolhain and his associates said.
Major Finding: IL-10 in the first trimester seems to protect against low birth weight, while IL-6 seems to increase the risk. TNF-alpha exerts its influence in the third trimester, when higher levels also seem to protect against lower birth weights.
Data Source: A study of 302 pregnant women with rheumatoid arthritis and 33 controls.
Disclosures: The study was funded by the Dutch Arthritis Association. Dr. Dolhain did not disclose any pertinent financial relationships.
Circulating cytokines appear to influence fetal growth in pregnant women who have rheumatoid arthritis, results of a Dutch study suggest.
High levels of interleukin-10, IL-6, and TNF-alpha might all play a role, each acting independently – and at different stages of pregnancy – to increase the risk of low birth weight among infants born to these mothers, Dr. Radboud Dolhain and colleagues wrote (J. Reprod. Immunol. 2010 [doi: 10.1016/j.jri.2010.08.010]).
Dr. Dolhain of Erasmus University Medical Centre, Rotterdam, the Netherlands, and his coinvestigators examined circulating cytokines in 134 pregnant patients with rheumatoid arthritis during their first trimester, 168 in their third trimester, and 33 healthy controls. Birth weights were analyzed using standard deviation scores.
Disease activity in the women was based on the disease activity score for 28 joints (DAS28); the scale runs from 0–10, with higher numbers indicating greater disease activity.
Since IL-10, IL-6 and TNF-alpha generally decrease during pregnancy, the investigators sought to determine if any increasing gestational levels correlated with birth weight. Among the first trimester patients, 12 had detectable IL-10; all of these women had a higher disease activity score than did those with no IL-10 (mean DAS28 4.4 vs. 3.6).
Birth weights were compared between the two groups, which were matched with regard to disease activity, parity, and prednisone use.
The mean birth weight standard deviation was significantly greater in the IL-10 positive group (0.92) than in the matched negative group (0.15), Dr. Dolhain and his associates reported.
This association with IL-10 was not seen in the third trimester pregnancies.
The investigators then examined the effect of IL-6 by stratifying IL-6 levels and disease activity scores in the first and third trimester.
In the two groups with high disease activity (DAS28 3.8 or higher), birth weight standard deviation was significantly lower in mothers with high IL-6.
In the high IL-6 group, the birth weight standard deviation was −0.19, compared with 0.36 in the low IL-6 group.
Again, the authors found no such association in the third trimester.
“In the first trimester, elevated IL-10 seems to protect against the negative influence of RA disease activity on birth weight, [while] IL-6 seems to amplify this negative influence,” the investigators wrote.
“Both cytokines create a birth weight standard deviation of more than 0.50, which is considered clinically relevant. In the third trimester, there is no influence, suggesting an early critical window.”
TNF-alpha, however, did exert an influence in the third trimester of pregnancy, Dr. Dolhain and his colleagues noted. Stratifying TNF-alpha in the same way, they concluded that the birth weight standard deviation was lower in the group with low TNF-alpha (0.05) than in the group with high TNF-alpha (0.52).
This association was not present in the first trimester.
The finding that increased TNF-alpha is associated with better birth weights may require a rethinking of anti–TNF-alpha therapy for pregnant women, they suggested.
This implies that “TNF blockers, which are more and more prescribed during pregnancy to treat rheumatoid arthritis, should be used with caution,” Dr. Dolhain and his associates said.
Major Finding: IL-10 in the first trimester seems to protect against low birth weight, while IL-6 seems to increase the risk. TNF-alpha exerts its influence in the third trimester, when higher levels also seem to protect against lower birth weights.
Data Source: A study of 302 pregnant women with rheumatoid arthritis and 33 controls.
Disclosures: The study was funded by the Dutch Arthritis Association. Dr. Dolhain did not disclose any pertinent financial relationships.
Circulating cytokines appear to influence fetal growth in pregnant women who have rheumatoid arthritis, results of a Dutch study suggest.
High levels of interleukin-10, IL-6, and TNF-alpha might all play a role, each acting independently – and at different stages of pregnancy – to increase the risk of low birth weight among infants born to these mothers, Dr. Radboud Dolhain and colleagues wrote (J. Reprod. Immunol. 2010 [doi: 10.1016/j.jri.2010.08.010]).
Dr. Dolhain of Erasmus University Medical Centre, Rotterdam, the Netherlands, and his coinvestigators examined circulating cytokines in 134 pregnant patients with rheumatoid arthritis during their first trimester, 168 in their third trimester, and 33 healthy controls. Birth weights were analyzed using standard deviation scores.
Disease activity in the women was based on the disease activity score for 28 joints (DAS28); the scale runs from 0–10, with higher numbers indicating greater disease activity.
Since IL-10, IL-6 and TNF-alpha generally decrease during pregnancy, the investigators sought to determine if any increasing gestational levels correlated with birth weight. Among the first trimester patients, 12 had detectable IL-10; all of these women had a higher disease activity score than did those with no IL-10 (mean DAS28 4.4 vs. 3.6).
Birth weights were compared between the two groups, which were matched with regard to disease activity, parity, and prednisone use.
The mean birth weight standard deviation was significantly greater in the IL-10 positive group (0.92) than in the matched negative group (0.15), Dr. Dolhain and his associates reported.
This association with IL-10 was not seen in the third trimester pregnancies.
The investigators then examined the effect of IL-6 by stratifying IL-6 levels and disease activity scores in the first and third trimester.
In the two groups with high disease activity (DAS28 3.8 or higher), birth weight standard deviation was significantly lower in mothers with high IL-6.
In the high IL-6 group, the birth weight standard deviation was −0.19, compared with 0.36 in the low IL-6 group.
Again, the authors found no such association in the third trimester.
“In the first trimester, elevated IL-10 seems to protect against the negative influence of RA disease activity on birth weight, [while] IL-6 seems to amplify this negative influence,” the investigators wrote.
“Both cytokines create a birth weight standard deviation of more than 0.50, which is considered clinically relevant. In the third trimester, there is no influence, suggesting an early critical window.”
TNF-alpha, however, did exert an influence in the third trimester of pregnancy, Dr. Dolhain and his colleagues noted. Stratifying TNF-alpha in the same way, they concluded that the birth weight standard deviation was lower in the group with low TNF-alpha (0.05) than in the group with high TNF-alpha (0.52).
This association was not present in the first trimester.
The finding that increased TNF-alpha is associated with better birth weights may require a rethinking of anti–TNF-alpha therapy for pregnant women, they suggested.
This implies that “TNF blockers, which are more and more prescribed during pregnancy to treat rheumatoid arthritis, should be used with caution,” Dr. Dolhain and his associates said.
From the Journal Of Reproductive Immunology
'Know the Label' Campaign
The Doctors Company and the PDR Network have launched a national campaign to “educate physicians and improve their knowledge of ever-changing FDA-approved medication labeling.”
The “Know the Label” program will allow physicians to earn free Continuing Medical Education credit for reading full Food and Drug Administration-approved medication labeling online at www.PDR.net
Last year, more than one-quarter of drugs had “a material labeling change,” the statement said. For more information, visit www.pdrnetwork.comwww.thedoctors.com
The Doctors Company and the PDR Network have launched a national campaign to “educate physicians and improve their knowledge of ever-changing FDA-approved medication labeling.”
The “Know the Label” program will allow physicians to earn free Continuing Medical Education credit for reading full Food and Drug Administration-approved medication labeling online at www.PDR.net
Last year, more than one-quarter of drugs had “a material labeling change,” the statement said. For more information, visit www.pdrnetwork.comwww.thedoctors.com
The Doctors Company and the PDR Network have launched a national campaign to “educate physicians and improve their knowledge of ever-changing FDA-approved medication labeling.”
The “Know the Label” program will allow physicians to earn free Continuing Medical Education credit for reading full Food and Drug Administration-approved medication labeling online at www.PDR.net
Last year, more than one-quarter of drugs had “a material labeling change,” the statement said. For more information, visit www.pdrnetwork.comwww.thedoctors.com
