User login
Postcesarean Oxytocin Boluses of Low Benefit
BANFF, ALTA. — The routine practice of giving oxytocin boluses to reduce the risk of postpartum hemorrhage appears to be of limited benefit even in high-risk patients after cesarean section, as long as an appropriate oxytocin infusion is given, according to the first randomized, placebo-controlled trial of the practice, said Dr. Kylie King from Maitland (Australia) Hospital.
The practice of administering oxytocin boluses has recently come under scrutiny. “Although the adverse hemodynamic effects [of oxytocin boluses] are well documented, one recently reported death associated with a 10-U bolus in the U.K. has prompted a change in dose from 10 to 5 units given slowly,” she said at the annual meeting of the Society for Obstetric Anesthesia and Perinatology. “This begs the question: Is a bolus necessary? Is 5 U the right dose? How slowly should it be given? And might an infusion be sufficient?”
Her study, which was conducted at British Columbia Women's Hospital in Vancouver, compared 143 subjects: 70 received an intravenous 5-U bolus of oxytocin, and 73 received normal saline, given over 30 seconds following cesarean section and cord clamping.
Both groups also received an identical infusion of 40 U of oxytocin in 500 mL of normal saline over 30 minutes, followed by 20 U of oxytocin in 1 L of saline over the next 8 hours.
“Our hypothesis was that the bolus, given in addition to the infusion, would reduce the need for additional drugs to contract the uterus,” said Dr. King. Because previous studies have suggested that oxytocin may have little or no effect in a low-risk population, study subjects were specifically selected as being high risk for postpartum hemorrhage. “Multiple gestations and macrosomia were the most common risk factors.”
Overall, 53% of the cesarean sections were elective, with 47% classified as emergency procedures. The need for additional uterotonics was high—between 30% and 40% overall—confirming that the population was indeed high risk, but need for more uterotonics was similar in both groups as assessed by a surgeon who was blinded to the patients' randomization. In addition, there was no difference between groups in the secondary outcomes of estimated blood loss, need for blood transfusion or hypotension.
“Even in a high-risk group, a 5-U bolus is of limited additional benefit provided that an adequate infusion is given,” concluded Dr. King. “Getting a stronger initial contraction at 1 minute doesn't reduce the need for additional uterotonics over the next 24 hours.”
BANFF, ALTA. — The routine practice of giving oxytocin boluses to reduce the risk of postpartum hemorrhage appears to be of limited benefit even in high-risk patients after cesarean section, as long as an appropriate oxytocin infusion is given, according to the first randomized, placebo-controlled trial of the practice, said Dr. Kylie King from Maitland (Australia) Hospital.
The practice of administering oxytocin boluses has recently come under scrutiny. “Although the adverse hemodynamic effects [of oxytocin boluses] are well documented, one recently reported death associated with a 10-U bolus in the U.K. has prompted a change in dose from 10 to 5 units given slowly,” she said at the annual meeting of the Society for Obstetric Anesthesia and Perinatology. “This begs the question: Is a bolus necessary? Is 5 U the right dose? How slowly should it be given? And might an infusion be sufficient?”
Her study, which was conducted at British Columbia Women's Hospital in Vancouver, compared 143 subjects: 70 received an intravenous 5-U bolus of oxytocin, and 73 received normal saline, given over 30 seconds following cesarean section and cord clamping.
Both groups also received an identical infusion of 40 U of oxytocin in 500 mL of normal saline over 30 minutes, followed by 20 U of oxytocin in 1 L of saline over the next 8 hours.
“Our hypothesis was that the bolus, given in addition to the infusion, would reduce the need for additional drugs to contract the uterus,” said Dr. King. Because previous studies have suggested that oxytocin may have little or no effect in a low-risk population, study subjects were specifically selected as being high risk for postpartum hemorrhage. “Multiple gestations and macrosomia were the most common risk factors.”
Overall, 53% of the cesarean sections were elective, with 47% classified as emergency procedures. The need for additional uterotonics was high—between 30% and 40% overall—confirming that the population was indeed high risk, but need for more uterotonics was similar in both groups as assessed by a surgeon who was blinded to the patients' randomization. In addition, there was no difference between groups in the secondary outcomes of estimated blood loss, need for blood transfusion or hypotension.
“Even in a high-risk group, a 5-U bolus is of limited additional benefit provided that an adequate infusion is given,” concluded Dr. King. “Getting a stronger initial contraction at 1 minute doesn't reduce the need for additional uterotonics over the next 24 hours.”
BANFF, ALTA. — The routine practice of giving oxytocin boluses to reduce the risk of postpartum hemorrhage appears to be of limited benefit even in high-risk patients after cesarean section, as long as an appropriate oxytocin infusion is given, according to the first randomized, placebo-controlled trial of the practice, said Dr. Kylie King from Maitland (Australia) Hospital.
The practice of administering oxytocin boluses has recently come under scrutiny. “Although the adverse hemodynamic effects [of oxytocin boluses] are well documented, one recently reported death associated with a 10-U bolus in the U.K. has prompted a change in dose from 10 to 5 units given slowly,” she said at the annual meeting of the Society for Obstetric Anesthesia and Perinatology. “This begs the question: Is a bolus necessary? Is 5 U the right dose? How slowly should it be given? And might an infusion be sufficient?”
Her study, which was conducted at British Columbia Women's Hospital in Vancouver, compared 143 subjects: 70 received an intravenous 5-U bolus of oxytocin, and 73 received normal saline, given over 30 seconds following cesarean section and cord clamping.
Both groups also received an identical infusion of 40 U of oxytocin in 500 mL of normal saline over 30 minutes, followed by 20 U of oxytocin in 1 L of saline over the next 8 hours.
“Our hypothesis was that the bolus, given in addition to the infusion, would reduce the need for additional drugs to contract the uterus,” said Dr. King. Because previous studies have suggested that oxytocin may have little or no effect in a low-risk population, study subjects were specifically selected as being high risk for postpartum hemorrhage. “Multiple gestations and macrosomia were the most common risk factors.”
Overall, 53% of the cesarean sections were elective, with 47% classified as emergency procedures. The need for additional uterotonics was high—between 30% and 40% overall—confirming that the population was indeed high risk, but need for more uterotonics was similar in both groups as assessed by a surgeon who was blinded to the patients' randomization. In addition, there was no difference between groups in the secondary outcomes of estimated blood loss, need for blood transfusion or hypotension.
“Even in a high-risk group, a 5-U bolus is of limited additional benefit provided that an adequate infusion is given,” concluded Dr. King. “Getting a stronger initial contraction at 1 minute doesn't reduce the need for additional uterotonics over the next 24 hours.”
Expertise Is Vital in Management of Antepartum Hemorrhage
MIAMI BEACH — Clinical acumen is crucial to diagnosis and management of antepartum hemorrhage of both known and unknown origin, according to a presentation at an ob.gyn. conference sponsored by the University of Miami.
Placenta previa and abruption are more common presentations, whereas vasa previa occurs less frequently.
However, vasa previa—where blood vessels cross the cervical opening—can cause massive hemorrhaging.
Prenatal diagnosis via ultrasound improves survival, compared with diagnosis at delivery.
There are fewer data regarding hemorrhage of unknown origin, but pooled findings from several studies suggest a threefold increased risk of premature birth and twofold increased risk of stillbirth, Dr. Amanda Cotter said.
There is an increased risk of mortality with hemorrhage, Dr. Cotter said. In one study of 108 obstetric maternal deaths in North Carolina, cardiomyopathy was the leading cause of mortality, responsible for 21% (Obstet. Gynecol. 2005;106:1228–34). Hemorrhage was the second leading cause, a culprit in 14% of deaths.
Placenta previa causes approximately 20% of all cases of antepartum hemorrhage, Dr. Cotter said. It has an incidence of 1 in 200 live births. Sentinel bleed at about 30 weeks' gestation is another indicator of placenta previa, Dr. Cotter said. About 10% of women with this condition present without bleeding or pain, making it an ultrasound diagnosis. Another 20% of patients experience contractions with bleeding. The remaining 70% of women with placenta previa present with painless bleeding.
“Some women report standing up and blood runs down their leg and puddles on the floor,” said Dr. Cotter, a faculty member in the division of maternal fetal medicine, University of Miami.
“We must use ultrasound to do this diagnosis—but is it transabdominal or transvaginal?” Dr. Cotter asked. Only an experienced operator should perform transvaginal ultrasound in a bleeding patient, she said. “You have to prevent the probe from entering the cervix and causing any fetal insult.” Therefore, transabdominal is the preferred approach to ultrasound in these patients.
“Rule out placental separation, even with previa, to improve our diagnostic accuracy,” Dr. Cotter said.
Risk for placenta accreta—an abnormally firm attachment of the placenta to the uterine wall—varies depending on a patient's history. For example, a woman with no history of previa or cesarean section has a 5% risk, Dr. Cotter said. If she had a previous previa and one cesarean, the risk is approximately 24%.
The risk increases to 67% for a woman with a previous previa and a history of multiple cesarean deliveries. “This also applies to patients with multiple, previous D&Cs,” she added.
Ultrasound will show placenta previa or low-lying placenta. Dr. Cotter cited the case of a 35-year-old woman who presented for a routine pregnancy exam. She had blood vessels formed across the myometrium from her uterus to her bladder.
“I knew this was a placenta accreta—I left the placenta in without touching it at all—and did a breach delivery via C-section,” Dr. Cotter said. “She did very well, had no bleeding in postpartum period, and had normal resumption of her menses. I performed a tubal ligation with her permission at the same time so she should not be back in the same situation.”
Placental abruption is often associated with substance abuse during pregnancy, particularly cocaine. Ultrasound might show a retroplacental or preplacental hematoma, and increased placental thickness and echogenicity, Dr. Cotter said. “You can also sometimes see a subchorionic collection.”
Vasa previa occurs with an incidence of 1 in 2,500 pregnancies. “We don't see this as often,” Dr. Cotter said. It occurs when blood vessels transverse the internal cervical os.
“Once these membranes rupture, you will have massive bleeding,” she noted.
Early detection is important. In a study of 155 pregnancies complicated by vasa previa, 39% were diagnosed prenatally and the fetal survival rate was 97% (Obstet. Gynecol. 2004;103:937–42). In contrast, in the 61% diagnosed at delivery, survival dropped to 44%.
“Use color flow Doppler to confirm the diagnosis,” Dr. Cotter recommended.
The etiology of antepartum hemorrhage is unknown in 2%–3% of cases, Dr. Cotter said. Bleeding in these cases is associated with adverse outcomes.
“The most likely reason these people are bleeding is they have very tiny abruptions. So we have to monitor for preterm delivery and closely monitor the fetus up to delivery,” she added.
A meeting attendee asked if a physical examination of the cervix should be the first step when a patient presents with bleeding and does not have a diagnosis. “No, I would do an ultrasound first before I do an exam,” Dr. Cotter replied.
There is a paucity of data in the literature regarding this presentation, Dr. Cotter said. Pooled data from four studies suggest a threefold increase in risk of premature birth and twofold increase in risk of stillbirth with bleeding of unknown origin, she said. “It is important to counsel our patients with bleeding of unknown origin that they are at increased risk. I hope you will leave here and have a heightened awareness about increased risk of preterm delivery.”
Another meeting attendee asked about a connection between bleeding and risk of mental retardation. “I always counsel patients with recurrent bleeding that there is an elevated risk of cerebral palsy,” Dr. Cotter said.
MIAMI BEACH — Clinical acumen is crucial to diagnosis and management of antepartum hemorrhage of both known and unknown origin, according to a presentation at an ob.gyn. conference sponsored by the University of Miami.
Placenta previa and abruption are more common presentations, whereas vasa previa occurs less frequently.
However, vasa previa—where blood vessels cross the cervical opening—can cause massive hemorrhaging.
Prenatal diagnosis via ultrasound improves survival, compared with diagnosis at delivery.
There are fewer data regarding hemorrhage of unknown origin, but pooled findings from several studies suggest a threefold increased risk of premature birth and twofold increased risk of stillbirth, Dr. Amanda Cotter said.
There is an increased risk of mortality with hemorrhage, Dr. Cotter said. In one study of 108 obstetric maternal deaths in North Carolina, cardiomyopathy was the leading cause of mortality, responsible for 21% (Obstet. Gynecol. 2005;106:1228–34). Hemorrhage was the second leading cause, a culprit in 14% of deaths.
Placenta previa causes approximately 20% of all cases of antepartum hemorrhage, Dr. Cotter said. It has an incidence of 1 in 200 live births. Sentinel bleed at about 30 weeks' gestation is another indicator of placenta previa, Dr. Cotter said. About 10% of women with this condition present without bleeding or pain, making it an ultrasound diagnosis. Another 20% of patients experience contractions with bleeding. The remaining 70% of women with placenta previa present with painless bleeding.
“Some women report standing up and blood runs down their leg and puddles on the floor,” said Dr. Cotter, a faculty member in the division of maternal fetal medicine, University of Miami.
“We must use ultrasound to do this diagnosis—but is it transabdominal or transvaginal?” Dr. Cotter asked. Only an experienced operator should perform transvaginal ultrasound in a bleeding patient, she said. “You have to prevent the probe from entering the cervix and causing any fetal insult.” Therefore, transabdominal is the preferred approach to ultrasound in these patients.
“Rule out placental separation, even with previa, to improve our diagnostic accuracy,” Dr. Cotter said.
Risk for placenta accreta—an abnormally firm attachment of the placenta to the uterine wall—varies depending on a patient's history. For example, a woman with no history of previa or cesarean section has a 5% risk, Dr. Cotter said. If she had a previous previa and one cesarean, the risk is approximately 24%.
The risk increases to 67% for a woman with a previous previa and a history of multiple cesarean deliveries. “This also applies to patients with multiple, previous D&Cs,” she added.
Ultrasound will show placenta previa or low-lying placenta. Dr. Cotter cited the case of a 35-year-old woman who presented for a routine pregnancy exam. She had blood vessels formed across the myometrium from her uterus to her bladder.
“I knew this was a placenta accreta—I left the placenta in without touching it at all—and did a breach delivery via C-section,” Dr. Cotter said. “She did very well, had no bleeding in postpartum period, and had normal resumption of her menses. I performed a tubal ligation with her permission at the same time so she should not be back in the same situation.”
Placental abruption is often associated with substance abuse during pregnancy, particularly cocaine. Ultrasound might show a retroplacental or preplacental hematoma, and increased placental thickness and echogenicity, Dr. Cotter said. “You can also sometimes see a subchorionic collection.”
Vasa previa occurs with an incidence of 1 in 2,500 pregnancies. “We don't see this as often,” Dr. Cotter said. It occurs when blood vessels transverse the internal cervical os.
“Once these membranes rupture, you will have massive bleeding,” she noted.
Early detection is important. In a study of 155 pregnancies complicated by vasa previa, 39% were diagnosed prenatally and the fetal survival rate was 97% (Obstet. Gynecol. 2004;103:937–42). In contrast, in the 61% diagnosed at delivery, survival dropped to 44%.
“Use color flow Doppler to confirm the diagnosis,” Dr. Cotter recommended.
The etiology of antepartum hemorrhage is unknown in 2%–3% of cases, Dr. Cotter said. Bleeding in these cases is associated with adverse outcomes.
“The most likely reason these people are bleeding is they have very tiny abruptions. So we have to monitor for preterm delivery and closely monitor the fetus up to delivery,” she added.
A meeting attendee asked if a physical examination of the cervix should be the first step when a patient presents with bleeding and does not have a diagnosis. “No, I would do an ultrasound first before I do an exam,” Dr. Cotter replied.
There is a paucity of data in the literature regarding this presentation, Dr. Cotter said. Pooled data from four studies suggest a threefold increase in risk of premature birth and twofold increase in risk of stillbirth with bleeding of unknown origin, she said. “It is important to counsel our patients with bleeding of unknown origin that they are at increased risk. I hope you will leave here and have a heightened awareness about increased risk of preterm delivery.”
Another meeting attendee asked about a connection between bleeding and risk of mental retardation. “I always counsel patients with recurrent bleeding that there is an elevated risk of cerebral palsy,” Dr. Cotter said.
MIAMI BEACH — Clinical acumen is crucial to diagnosis and management of antepartum hemorrhage of both known and unknown origin, according to a presentation at an ob.gyn. conference sponsored by the University of Miami.
Placenta previa and abruption are more common presentations, whereas vasa previa occurs less frequently.
However, vasa previa—where blood vessels cross the cervical opening—can cause massive hemorrhaging.
Prenatal diagnosis via ultrasound improves survival, compared with diagnosis at delivery.
There are fewer data regarding hemorrhage of unknown origin, but pooled findings from several studies suggest a threefold increased risk of premature birth and twofold increased risk of stillbirth, Dr. Amanda Cotter said.
There is an increased risk of mortality with hemorrhage, Dr. Cotter said. In one study of 108 obstetric maternal deaths in North Carolina, cardiomyopathy was the leading cause of mortality, responsible for 21% (Obstet. Gynecol. 2005;106:1228–34). Hemorrhage was the second leading cause, a culprit in 14% of deaths.
Placenta previa causes approximately 20% of all cases of antepartum hemorrhage, Dr. Cotter said. It has an incidence of 1 in 200 live births. Sentinel bleed at about 30 weeks' gestation is another indicator of placenta previa, Dr. Cotter said. About 10% of women with this condition present without bleeding or pain, making it an ultrasound diagnosis. Another 20% of patients experience contractions with bleeding. The remaining 70% of women with placenta previa present with painless bleeding.
“Some women report standing up and blood runs down their leg and puddles on the floor,” said Dr. Cotter, a faculty member in the division of maternal fetal medicine, University of Miami.
“We must use ultrasound to do this diagnosis—but is it transabdominal or transvaginal?” Dr. Cotter asked. Only an experienced operator should perform transvaginal ultrasound in a bleeding patient, she said. “You have to prevent the probe from entering the cervix and causing any fetal insult.” Therefore, transabdominal is the preferred approach to ultrasound in these patients.
“Rule out placental separation, even with previa, to improve our diagnostic accuracy,” Dr. Cotter said.
Risk for placenta accreta—an abnormally firm attachment of the placenta to the uterine wall—varies depending on a patient's history. For example, a woman with no history of previa or cesarean section has a 5% risk, Dr. Cotter said. If she had a previous previa and one cesarean, the risk is approximately 24%.
The risk increases to 67% for a woman with a previous previa and a history of multiple cesarean deliveries. “This also applies to patients with multiple, previous D&Cs,” she added.
Ultrasound will show placenta previa or low-lying placenta. Dr. Cotter cited the case of a 35-year-old woman who presented for a routine pregnancy exam. She had blood vessels formed across the myometrium from her uterus to her bladder.
“I knew this was a placenta accreta—I left the placenta in without touching it at all—and did a breach delivery via C-section,” Dr. Cotter said. “She did very well, had no bleeding in postpartum period, and had normal resumption of her menses. I performed a tubal ligation with her permission at the same time so she should not be back in the same situation.”
Placental abruption is often associated with substance abuse during pregnancy, particularly cocaine. Ultrasound might show a retroplacental or preplacental hematoma, and increased placental thickness and echogenicity, Dr. Cotter said. “You can also sometimes see a subchorionic collection.”
Vasa previa occurs with an incidence of 1 in 2,500 pregnancies. “We don't see this as often,” Dr. Cotter said. It occurs when blood vessels transverse the internal cervical os.
“Once these membranes rupture, you will have massive bleeding,” she noted.
Early detection is important. In a study of 155 pregnancies complicated by vasa previa, 39% were diagnosed prenatally and the fetal survival rate was 97% (Obstet. Gynecol. 2004;103:937–42). In contrast, in the 61% diagnosed at delivery, survival dropped to 44%.
“Use color flow Doppler to confirm the diagnosis,” Dr. Cotter recommended.
The etiology of antepartum hemorrhage is unknown in 2%–3% of cases, Dr. Cotter said. Bleeding in these cases is associated with adverse outcomes.
“The most likely reason these people are bleeding is they have very tiny abruptions. So we have to monitor for preterm delivery and closely monitor the fetus up to delivery,” she added.
A meeting attendee asked if a physical examination of the cervix should be the first step when a patient presents with bleeding and does not have a diagnosis. “No, I would do an ultrasound first before I do an exam,” Dr. Cotter replied.
There is a paucity of data in the literature regarding this presentation, Dr. Cotter said. Pooled data from four studies suggest a threefold increase in risk of premature birth and twofold increase in risk of stillbirth with bleeding of unknown origin, she said. “It is important to counsel our patients with bleeding of unknown origin that they are at increased risk. I hope you will leave here and have a heightened awareness about increased risk of preterm delivery.”
Another meeting attendee asked about a connection between bleeding and risk of mental retardation. “I always counsel patients with recurrent bleeding that there is an elevated risk of cerebral palsy,” Dr. Cotter said.
A Higher Baseline Body Temperature May Be Key to Labor-Associated Fever
BANFF, ALTA. — Some women who develop fever during labor may have a predisposition to labor-associated fever and may be identifiable by their higher baseline body temperatures, Tiffany Gelfand, D.O., said at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
And among women predisposed to labor-associated fever, epidural analgesia may have an additive effect, suggested Dr. Gelfand of Brigham and Women's Hospital, Boston.
“Maternal fever is not a benign thing; it's been related to neonatal cognitive deficits and cerebral encephalopathy, and even cognitive deficits 6 years out,” she said in an interview. “We don't really know what the interaction is between the epidural and the patients who are predisposed to have a fever, but we really need to find those risk factors before we go ahead and say that certain patients shouldn't have an epidural.”
Her prospective study followed 107 women in labor, at term, with maternal temperature measured every hour from admittance until delivery. It found that among 86 women who received epidural, and 21 who received either opioids or no analgesia, 35% developed a temperature above 99.5° F, and 17% had a temperature above 100.4° F. Fever was much more common in women who received epidural, compared with those who did not (37% vs. 14%), she said. But a common characteristic of all women who became febrile was a higher baseline body temperature.
“What was so interesting about this study was that these patients were already different the second they walked through the door. Even before they had an epidural, their baseline temperature, although afebrile, was significantly different. We don't know why they are different, and we need to figure that out.”
The study shows that the gradual, steady rise in temperature that has been previously reported with epidural analgesia is actually an artifact, due to averaging patients who develop clinical fever and those who do not, explained Dr. Gelfand. When women who became febrile were removed from the analysis, the rest demonstrated consistently low temperatures throughout labor, she said. “We think it might be some of type of inflammatory cause—perhaps an undisclosed infectious source,” she speculated, and noted that the nature of the fever was also different in women who received epidural, compared with those who did not. “Among women undergoing natural childbirth, we saw fever that responded well to acetaminophen, whereas in women who received the epidural, their fever did not respond to acetaminophen. We think it might be a different mechanism.”
A further analysis of the study, presented as a poster, looked at only the 86 women who received analgesia and found that, compared with the afebrile women, women who became febrile had some known obstetric risk factors such as longer time from rupture of membranes and a higher number of vaginal exams.
BANFF, ALTA. — Some women who develop fever during labor may have a predisposition to labor-associated fever and may be identifiable by their higher baseline body temperatures, Tiffany Gelfand, D.O., said at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
And among women predisposed to labor-associated fever, epidural analgesia may have an additive effect, suggested Dr. Gelfand of Brigham and Women's Hospital, Boston.
“Maternal fever is not a benign thing; it's been related to neonatal cognitive deficits and cerebral encephalopathy, and even cognitive deficits 6 years out,” she said in an interview. “We don't really know what the interaction is between the epidural and the patients who are predisposed to have a fever, but we really need to find those risk factors before we go ahead and say that certain patients shouldn't have an epidural.”
Her prospective study followed 107 women in labor, at term, with maternal temperature measured every hour from admittance until delivery. It found that among 86 women who received epidural, and 21 who received either opioids or no analgesia, 35% developed a temperature above 99.5° F, and 17% had a temperature above 100.4° F. Fever was much more common in women who received epidural, compared with those who did not (37% vs. 14%), she said. But a common characteristic of all women who became febrile was a higher baseline body temperature.
“What was so interesting about this study was that these patients were already different the second they walked through the door. Even before they had an epidural, their baseline temperature, although afebrile, was significantly different. We don't know why they are different, and we need to figure that out.”
The study shows that the gradual, steady rise in temperature that has been previously reported with epidural analgesia is actually an artifact, due to averaging patients who develop clinical fever and those who do not, explained Dr. Gelfand. When women who became febrile were removed from the analysis, the rest demonstrated consistently low temperatures throughout labor, she said. “We think it might be some of type of inflammatory cause—perhaps an undisclosed infectious source,” she speculated, and noted that the nature of the fever was also different in women who received epidural, compared with those who did not. “Among women undergoing natural childbirth, we saw fever that responded well to acetaminophen, whereas in women who received the epidural, their fever did not respond to acetaminophen. We think it might be a different mechanism.”
A further analysis of the study, presented as a poster, looked at only the 86 women who received analgesia and found that, compared with the afebrile women, women who became febrile had some known obstetric risk factors such as longer time from rupture of membranes and a higher number of vaginal exams.
BANFF, ALTA. — Some women who develop fever during labor may have a predisposition to labor-associated fever and may be identifiable by their higher baseline body temperatures, Tiffany Gelfand, D.O., said at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
And among women predisposed to labor-associated fever, epidural analgesia may have an additive effect, suggested Dr. Gelfand of Brigham and Women's Hospital, Boston.
“Maternal fever is not a benign thing; it's been related to neonatal cognitive deficits and cerebral encephalopathy, and even cognitive deficits 6 years out,” she said in an interview. “We don't really know what the interaction is between the epidural and the patients who are predisposed to have a fever, but we really need to find those risk factors before we go ahead and say that certain patients shouldn't have an epidural.”
Her prospective study followed 107 women in labor, at term, with maternal temperature measured every hour from admittance until delivery. It found that among 86 women who received epidural, and 21 who received either opioids or no analgesia, 35% developed a temperature above 99.5° F, and 17% had a temperature above 100.4° F. Fever was much more common in women who received epidural, compared with those who did not (37% vs. 14%), she said. But a common characteristic of all women who became febrile was a higher baseline body temperature.
“What was so interesting about this study was that these patients were already different the second they walked through the door. Even before they had an epidural, their baseline temperature, although afebrile, was significantly different. We don't know why they are different, and we need to figure that out.”
The study shows that the gradual, steady rise in temperature that has been previously reported with epidural analgesia is actually an artifact, due to averaging patients who develop clinical fever and those who do not, explained Dr. Gelfand. When women who became febrile were removed from the analysis, the rest demonstrated consistently low temperatures throughout labor, she said. “We think it might be some of type of inflammatory cause—perhaps an undisclosed infectious source,” she speculated, and noted that the nature of the fever was also different in women who received epidural, compared with those who did not. “Among women undergoing natural childbirth, we saw fever that responded well to acetaminophen, whereas in women who received the epidural, their fever did not respond to acetaminophen. We think it might be a different mechanism.”
A further analysis of the study, presented as a poster, looked at only the 86 women who received analgesia and found that, compared with the afebrile women, women who became febrile had some known obstetric risk factors such as longer time from rupture of membranes and a higher number of vaginal exams.
Maternal Emotions Can Affect Neonatal Physiology
BOSTON — Maternal cortisol levels during pregnancy are highly correlated with newborn cortisol levels in the first days of life, a study has shown. Additionally, both prenatal anxiety and postpartum depression independently predict newborn cortisol levels, according to Raquel Costa, a doctoral candidate at the University of Minho in Braga, Portugal.
These findings from a prospective investigation of 56 mother-child pairs support the hypothesis that a woman's emotional state both during pregnancy and after childbirth can have a significant effect on newborn physiology, Ms. Costa said in a poster presentation at a meeting of the Society for Research in Child Development.
All of the women in the current study were enrolled during their third trimester of pregnancy, and none had multiple gestations. Upon enrollment through 48 hours after childbirth, each woman provided 24 urine samples to measure cortisol levels, as well as blood samples to measure oxytocin levels. For assessment of depression and anxiety at both time points, each woman completed the 10-item Edinburgh Postnatal Depression Scale (EPDS) and the State-Trait Anxiety Inventory (STAI). Newborn cortisol levels were measured via urine sample within 2 days of birth.
Approximately 30% of the mothers screened positive for depression during pregnancy, and 17% screened positive after childbirth, Ms. Costa said. With respect to anxiety, positive screens were observed in 29% of the women prior to giving birth and 20% after childbirth, she said.
Measurement of maternal cortisol showed that approximately 53% of the women had levels higher than the normative range of 20–90 mcg/24 hours during pregnancy, and 17% had levels higher than the normative range after childbirth, Ms. Costa reported.
Regarding oxytocin measurements, prior to childbirth 52% of the women had levels higher than the group's mean. Following childbirth, 45% of the mothers had oxytocin levels higher than the group's postpartum mean.
In a t test for independent samples to analyze differences in newborns' cortisol levels according to mothers' depressive mood, anxiety, oxytocin, and cortisol levels before and after childbirth, “there were no significant differences in newborn cortisol levels according to mother's psychopathology or hormone levels,” Ms. Costa stated.
However, linear regression analysis on the neonatal cortisol levels with the maternal depressive mood, anxiety, oxytocin, and cortisol levels both during pregnancy and post partum “suggested that maternal anxiety during pregnancy and depression after childbirth were predictive of neonatal cortisol levels,” said Ms. Costa. Neither maternal cortisol nor oxytocin levels were predictive of newborn cortisol levels in the regression analyses, she noted.
Together with the results of earlier studies linking elevated prenatal cortisol levels in mothers to short gestation and low birth weight in newborns, and those associating prenatal stress with behavioral problems and motor/cognitive deficits in newborns, the findings of the current study highlight the importance of assessing maternal emotional status and stress hormone levels during pregnancy, and implementing early intervention strategies when warranted, Ms. Costa said.
BOSTON — Maternal cortisol levels during pregnancy are highly correlated with newborn cortisol levels in the first days of life, a study has shown. Additionally, both prenatal anxiety and postpartum depression independently predict newborn cortisol levels, according to Raquel Costa, a doctoral candidate at the University of Minho in Braga, Portugal.
These findings from a prospective investigation of 56 mother-child pairs support the hypothesis that a woman's emotional state both during pregnancy and after childbirth can have a significant effect on newborn physiology, Ms. Costa said in a poster presentation at a meeting of the Society for Research in Child Development.
All of the women in the current study were enrolled during their third trimester of pregnancy, and none had multiple gestations. Upon enrollment through 48 hours after childbirth, each woman provided 24 urine samples to measure cortisol levels, as well as blood samples to measure oxytocin levels. For assessment of depression and anxiety at both time points, each woman completed the 10-item Edinburgh Postnatal Depression Scale (EPDS) and the State-Trait Anxiety Inventory (STAI). Newborn cortisol levels were measured via urine sample within 2 days of birth.
Approximately 30% of the mothers screened positive for depression during pregnancy, and 17% screened positive after childbirth, Ms. Costa said. With respect to anxiety, positive screens were observed in 29% of the women prior to giving birth and 20% after childbirth, she said.
Measurement of maternal cortisol showed that approximately 53% of the women had levels higher than the normative range of 20–90 mcg/24 hours during pregnancy, and 17% had levels higher than the normative range after childbirth, Ms. Costa reported.
Regarding oxytocin measurements, prior to childbirth 52% of the women had levels higher than the group's mean. Following childbirth, 45% of the mothers had oxytocin levels higher than the group's postpartum mean.
In a t test for independent samples to analyze differences in newborns' cortisol levels according to mothers' depressive mood, anxiety, oxytocin, and cortisol levels before and after childbirth, “there were no significant differences in newborn cortisol levels according to mother's psychopathology or hormone levels,” Ms. Costa stated.
However, linear regression analysis on the neonatal cortisol levels with the maternal depressive mood, anxiety, oxytocin, and cortisol levels both during pregnancy and post partum “suggested that maternal anxiety during pregnancy and depression after childbirth were predictive of neonatal cortisol levels,” said Ms. Costa. Neither maternal cortisol nor oxytocin levels were predictive of newborn cortisol levels in the regression analyses, she noted.
Together with the results of earlier studies linking elevated prenatal cortisol levels in mothers to short gestation and low birth weight in newborns, and those associating prenatal stress with behavioral problems and motor/cognitive deficits in newborns, the findings of the current study highlight the importance of assessing maternal emotional status and stress hormone levels during pregnancy, and implementing early intervention strategies when warranted, Ms. Costa said.
BOSTON — Maternal cortisol levels during pregnancy are highly correlated with newborn cortisol levels in the first days of life, a study has shown. Additionally, both prenatal anxiety and postpartum depression independently predict newborn cortisol levels, according to Raquel Costa, a doctoral candidate at the University of Minho in Braga, Portugal.
These findings from a prospective investigation of 56 mother-child pairs support the hypothesis that a woman's emotional state both during pregnancy and after childbirth can have a significant effect on newborn physiology, Ms. Costa said in a poster presentation at a meeting of the Society for Research in Child Development.
All of the women in the current study were enrolled during their third trimester of pregnancy, and none had multiple gestations. Upon enrollment through 48 hours after childbirth, each woman provided 24 urine samples to measure cortisol levels, as well as blood samples to measure oxytocin levels. For assessment of depression and anxiety at both time points, each woman completed the 10-item Edinburgh Postnatal Depression Scale (EPDS) and the State-Trait Anxiety Inventory (STAI). Newborn cortisol levels were measured via urine sample within 2 days of birth.
Approximately 30% of the mothers screened positive for depression during pregnancy, and 17% screened positive after childbirth, Ms. Costa said. With respect to anxiety, positive screens were observed in 29% of the women prior to giving birth and 20% after childbirth, she said.
Measurement of maternal cortisol showed that approximately 53% of the women had levels higher than the normative range of 20–90 mcg/24 hours during pregnancy, and 17% had levels higher than the normative range after childbirth, Ms. Costa reported.
Regarding oxytocin measurements, prior to childbirth 52% of the women had levels higher than the group's mean. Following childbirth, 45% of the mothers had oxytocin levels higher than the group's postpartum mean.
In a t test for independent samples to analyze differences in newborns' cortisol levels according to mothers' depressive mood, anxiety, oxytocin, and cortisol levels before and after childbirth, “there were no significant differences in newborn cortisol levels according to mother's psychopathology or hormone levels,” Ms. Costa stated.
However, linear regression analysis on the neonatal cortisol levels with the maternal depressive mood, anxiety, oxytocin, and cortisol levels both during pregnancy and post partum “suggested that maternal anxiety during pregnancy and depression after childbirth were predictive of neonatal cortisol levels,” said Ms. Costa. Neither maternal cortisol nor oxytocin levels were predictive of newborn cortisol levels in the regression analyses, she noted.
Together with the results of earlier studies linking elevated prenatal cortisol levels in mothers to short gestation and low birth weight in newborns, and those associating prenatal stress with behavioral problems and motor/cognitive deficits in newborns, the findings of the current study highlight the importance of assessing maternal emotional status and stress hormone levels during pregnancy, and implementing early intervention strategies when warranted, Ms. Costa said.
Case Focuses Anesthesiologists on Infection Control
BANFF, ALTA. — The much publicized death from spinal meningitis of Washington-area resident Julie LeMoult within hours of labor epidural and delivery has focused the attention of many obstetric anesthesiologists on the issue of infection control, according to Dr. Samuel Hughes, professor of anesthesia at the University of California, San Francisco, and director of obstetric anesthesia at San Francisco General Hospital.
“This tragic case was certainly disconcerting and brought many of our patients in to us with questions,” said Dr. Hughes, speaking at the annual meeting of the Society for Obstetric Anesthesia and Perinatology. He stressed that the courts have not yet ruled on the source of the infection.
Potential guidelines or a practice advisory regarding neuraxial infection control techniques have been under discussion within the American Society of Anesthesiologists (ASA) since 2005, said Dr. Hughes, who is former chair of the ASA's obstetric anesthesia committee. However, currently, there remains a void, with no standard of care in this area, he said. “We must institute uniform sterile safety practices. I think coming to some sort of broad guidelines would be a purposeful thing,” he said
But it is unlikely that studies will ever be able to show that more rigid, uniform infection control procedures will reduce the risk of neuraxial infection—because the incidence of such infections is so low, he said. The two most serious complications resulting from neuraxial anesthesia—epidural abscess and meningitis—are both rare and underreported, making determination of their true incidence difficult. The best literature review identified 15 cases of bacterial meningitis and 8 cases of epidural abscess associated with obstetrical regional anesthesia over a 32-year period (1966–1998), with a further five abscesses attributed to spontaneous infection, he said. And a more recent meta-analysis covering 1966–2005 suggested an incidence of deep epidural infection of 7 per million (Anesthesiology 2006;105:394–9).
“We must be cautious in quoting the incidence of such extremely rare events because, in fact, we just do not know and likely will not—not without an extremely large survey with mandatory reporting, even with as many as 2.4 million women per year in the United States having a regional anesthetic!” he wrote in the ASA Newsletter (2007 Feb;71:No 2).
While academics and medical societies debate the intricacies of future infection control guidelines, Dr. Hughes urged clinicians not to forget common sense, and the most basic of infection control practices, hand-washing. Thorough hand-washing with an alcohol-based antiseptic solution is the first recommendation from the American Society of Regional Anesthesia and Pain Medicine (ASRA), which released recommendations on infection control last year (Reg. Anesth. Pain Med. 2006;31:311–323), although not specifically for obstetric anesthesia, he said. The second and third recommendations are the removal of jewelry and the use of sterile gloves (after, not instead of, hand-washing). “Now that the ASRA recommendations are out there, they may slowly become the standard of care if nothing else supersedes them,” he suggested.
Perhaps the most controversial ASRA recommendation is for the wearing of masks—something Dr. Hughes strongly agrees with. While studies suggest that surgical wound infection is not reduced by mask-wearing, “more than 50% of the cases of meningitis related to anesthesia are caused by streptococcal organisms which live in the upper airway,” he said. “There have been case reports linking practitioners' specific upper airway infections to infections in patients—so anything that reduce s bacterial dispersion is a good idea. Certainly masks are a way to protect the practitioner and the patient at very little cost.”
Surveys in the United States and around the world underscore the fact that sterile technique varies widely among providers of regional anesthesia, said Dr. Hughes.
BANFF, ALTA. — The much publicized death from spinal meningitis of Washington-area resident Julie LeMoult within hours of labor epidural and delivery has focused the attention of many obstetric anesthesiologists on the issue of infection control, according to Dr. Samuel Hughes, professor of anesthesia at the University of California, San Francisco, and director of obstetric anesthesia at San Francisco General Hospital.
“This tragic case was certainly disconcerting and brought many of our patients in to us with questions,” said Dr. Hughes, speaking at the annual meeting of the Society for Obstetric Anesthesia and Perinatology. He stressed that the courts have not yet ruled on the source of the infection.
Potential guidelines or a practice advisory regarding neuraxial infection control techniques have been under discussion within the American Society of Anesthesiologists (ASA) since 2005, said Dr. Hughes, who is former chair of the ASA's obstetric anesthesia committee. However, currently, there remains a void, with no standard of care in this area, he said. “We must institute uniform sterile safety practices. I think coming to some sort of broad guidelines would be a purposeful thing,” he said
But it is unlikely that studies will ever be able to show that more rigid, uniform infection control procedures will reduce the risk of neuraxial infection—because the incidence of such infections is so low, he said. The two most serious complications resulting from neuraxial anesthesia—epidural abscess and meningitis—are both rare and underreported, making determination of their true incidence difficult. The best literature review identified 15 cases of bacterial meningitis and 8 cases of epidural abscess associated with obstetrical regional anesthesia over a 32-year period (1966–1998), with a further five abscesses attributed to spontaneous infection, he said. And a more recent meta-analysis covering 1966–2005 suggested an incidence of deep epidural infection of 7 per million (Anesthesiology 2006;105:394–9).
“We must be cautious in quoting the incidence of such extremely rare events because, in fact, we just do not know and likely will not—not without an extremely large survey with mandatory reporting, even with as many as 2.4 million women per year in the United States having a regional anesthetic!” he wrote in the ASA Newsletter (2007 Feb;71:No 2).
While academics and medical societies debate the intricacies of future infection control guidelines, Dr. Hughes urged clinicians not to forget common sense, and the most basic of infection control practices, hand-washing. Thorough hand-washing with an alcohol-based antiseptic solution is the first recommendation from the American Society of Regional Anesthesia and Pain Medicine (ASRA), which released recommendations on infection control last year (Reg. Anesth. Pain Med. 2006;31:311–323), although not specifically for obstetric anesthesia, he said. The second and third recommendations are the removal of jewelry and the use of sterile gloves (after, not instead of, hand-washing). “Now that the ASRA recommendations are out there, they may slowly become the standard of care if nothing else supersedes them,” he suggested.
Perhaps the most controversial ASRA recommendation is for the wearing of masks—something Dr. Hughes strongly agrees with. While studies suggest that surgical wound infection is not reduced by mask-wearing, “more than 50% of the cases of meningitis related to anesthesia are caused by streptococcal organisms which live in the upper airway,” he said. “There have been case reports linking practitioners' specific upper airway infections to infections in patients—so anything that reduce s bacterial dispersion is a good idea. Certainly masks are a way to protect the practitioner and the patient at very little cost.”
Surveys in the United States and around the world underscore the fact that sterile technique varies widely among providers of regional anesthesia, said Dr. Hughes.
BANFF, ALTA. — The much publicized death from spinal meningitis of Washington-area resident Julie LeMoult within hours of labor epidural and delivery has focused the attention of many obstetric anesthesiologists on the issue of infection control, according to Dr. Samuel Hughes, professor of anesthesia at the University of California, San Francisco, and director of obstetric anesthesia at San Francisco General Hospital.
“This tragic case was certainly disconcerting and brought many of our patients in to us with questions,” said Dr. Hughes, speaking at the annual meeting of the Society for Obstetric Anesthesia and Perinatology. He stressed that the courts have not yet ruled on the source of the infection.
Potential guidelines or a practice advisory regarding neuraxial infection control techniques have been under discussion within the American Society of Anesthesiologists (ASA) since 2005, said Dr. Hughes, who is former chair of the ASA's obstetric anesthesia committee. However, currently, there remains a void, with no standard of care in this area, he said. “We must institute uniform sterile safety practices. I think coming to some sort of broad guidelines would be a purposeful thing,” he said
But it is unlikely that studies will ever be able to show that more rigid, uniform infection control procedures will reduce the risk of neuraxial infection—because the incidence of such infections is so low, he said. The two most serious complications resulting from neuraxial anesthesia—epidural abscess and meningitis—are both rare and underreported, making determination of their true incidence difficult. The best literature review identified 15 cases of bacterial meningitis and 8 cases of epidural abscess associated with obstetrical regional anesthesia over a 32-year period (1966–1998), with a further five abscesses attributed to spontaneous infection, he said. And a more recent meta-analysis covering 1966–2005 suggested an incidence of deep epidural infection of 7 per million (Anesthesiology 2006;105:394–9).
“We must be cautious in quoting the incidence of such extremely rare events because, in fact, we just do not know and likely will not—not without an extremely large survey with mandatory reporting, even with as many as 2.4 million women per year in the United States having a regional anesthetic!” he wrote in the ASA Newsletter (2007 Feb;71:No 2).
While academics and medical societies debate the intricacies of future infection control guidelines, Dr. Hughes urged clinicians not to forget common sense, and the most basic of infection control practices, hand-washing. Thorough hand-washing with an alcohol-based antiseptic solution is the first recommendation from the American Society of Regional Anesthesia and Pain Medicine (ASRA), which released recommendations on infection control last year (Reg. Anesth. Pain Med. 2006;31:311–323), although not specifically for obstetric anesthesia, he said. The second and third recommendations are the removal of jewelry and the use of sterile gloves (after, not instead of, hand-washing). “Now that the ASRA recommendations are out there, they may slowly become the standard of care if nothing else supersedes them,” he suggested.
Perhaps the most controversial ASRA recommendation is for the wearing of masks—something Dr. Hughes strongly agrees with. While studies suggest that surgical wound infection is not reduced by mask-wearing, “more than 50% of the cases of meningitis related to anesthesia are caused by streptococcal organisms which live in the upper airway,” he said. “There have been case reports linking practitioners' specific upper airway infections to infections in patients—so anything that reduce s bacterial dispersion is a good idea. Certainly masks are a way to protect the practitioner and the patient at very little cost.”
Surveys in the United States and around the world underscore the fact that sterile technique varies widely among providers of regional anesthesia, said Dr. Hughes.
New Data Reinforce Valproate–Birth Defects Link
BOSTON — Two new data sets reinforce the recommendation to avoid valproate as a first-line therapy for any indication in women of childbearing years.
The findings, presented at the annual meeting of the American Academy of Neurology, strengthen evidence of a link between valproate use and an increased risk of major congenital malformations as well as impaired cognitive development of children exposed in utero.
“Not only our study, but nine other studies on valproate's anatomical and behavioral effects, have shown similar signals of poor outcome with this drug,” Dr. Kimford J Meador commented. “The drug should not be a first-line therapy for any indication in women of childbearing age. At the very minimum, women need to be aware of these risks if they are going to take this drug. We need to remember that half of U.S. pregnancies are unplanned.”
Dr. Meador, the Melvin Greer Professor of Neurology and director of the epilepsy program at the University of Florida, Gainesville, presented interim data from the ongoing Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study for which investigators enrolled 185 children whose mothers took carbamazepine (48), lamotrigine (66), phenytoin (42), or valproate (29) for epilepsy during pregnancy. Data were given on the children's mental development at 2 years; the prospective study will follow the cohort to age 6.
Mean IQ scores based on the Mental Development Index (MDI) from the Bayley Scale were lowest for children in the valproate group (81), Dr. Meador said. A score below 85 is considered to be below normal limits. Mean scores in the other groups were 94 for lamotrigine, 95 for phenytoin, and 96 for carbamazepine.
In addition, he said, the percentage of children in the valproate group with an MDI of less than 70 (correlating with mental retardation) was 24%, about double that seen in any of the other groups (carbamazepine, 13%; lamotrigine, 11%; and phenytoin, 12%).
The study also found an inverse relationship between maternal valproate blood levels during pregnancy and MDI scores in the children, Dr. Meador noted. All of the valproate relationships remained constant even after maternal IQ—an important driver of childhood IQ, maternal epilepsy type, and past medical history were controlled for.
The mechanism of brain injury in the valproate group is probably third-trimester neuronal apoptosis, Dr. Meador said in an interview. “We think it's similar to what's seen in fetal alcohol syndrome, and the apoptosis appears to occur at a relatively lower dose than it would with other drugs, such as phenytoin. I think that's why we see such an increased signal.”
NEAD only includes the children of women with epilepsy—the group that accounts for the smallest proportion of valproate scrips, he added. “Most of the prescriptions are written for other things, including psychotropic and pain indications. [Fewer] than half are for epilepsy.”
In the third quarter of 2006, about 16% of women of childbearing age with epilepsy were taking the drug, making it the fourth leading antiepileptic in the United States for this group. Valproate sales jumped 28% last year, an increase “that has to include some portion of women of childbearing age,” Dr. Meador said.
The second study, GlaxoSmithKline's lamotrigine pregnancy registry, found that valproate in conjunction with lamotrigine significantly increases the risk of a major birth defect. Lamotrigine is a GSK product.
The registry, now in its 14th year, has prospectively enrolled 2,400 pregnancies occurring in 32 countries. There are known outcome data on 1,539 pregnancies, said Marianne Cunnington, Ph.D., of GSK.
The risk of a major birth defect in the 908 first-trimester exposures to lamotrigine only was 2.9%, similar to the background population risk of 2%–3%. The risk associated with nonvalproate polytherapy was 2.6%. However, when lamotrigine polytherapy included valproate, the risk of a major congenital malformation jumped to more than 11%.
“We have a signal for an increased risk for polytherapy including valproate,” she said, although “it's unclear whether valproate is responsible for this increased risk.”
Dr. Meador noted, however, that six other studies have concluded that valproate significantly increases the risk of birth defects.
BOSTON — Two new data sets reinforce the recommendation to avoid valproate as a first-line therapy for any indication in women of childbearing years.
The findings, presented at the annual meeting of the American Academy of Neurology, strengthen evidence of a link between valproate use and an increased risk of major congenital malformations as well as impaired cognitive development of children exposed in utero.
“Not only our study, but nine other studies on valproate's anatomical and behavioral effects, have shown similar signals of poor outcome with this drug,” Dr. Kimford J Meador commented. “The drug should not be a first-line therapy for any indication in women of childbearing age. At the very minimum, women need to be aware of these risks if they are going to take this drug. We need to remember that half of U.S. pregnancies are unplanned.”
Dr. Meador, the Melvin Greer Professor of Neurology and director of the epilepsy program at the University of Florida, Gainesville, presented interim data from the ongoing Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study for which investigators enrolled 185 children whose mothers took carbamazepine (48), lamotrigine (66), phenytoin (42), or valproate (29) for epilepsy during pregnancy. Data were given on the children's mental development at 2 years; the prospective study will follow the cohort to age 6.
Mean IQ scores based on the Mental Development Index (MDI) from the Bayley Scale were lowest for children in the valproate group (81), Dr. Meador said. A score below 85 is considered to be below normal limits. Mean scores in the other groups were 94 for lamotrigine, 95 for phenytoin, and 96 for carbamazepine.
In addition, he said, the percentage of children in the valproate group with an MDI of less than 70 (correlating with mental retardation) was 24%, about double that seen in any of the other groups (carbamazepine, 13%; lamotrigine, 11%; and phenytoin, 12%).
The study also found an inverse relationship between maternal valproate blood levels during pregnancy and MDI scores in the children, Dr. Meador noted. All of the valproate relationships remained constant even after maternal IQ—an important driver of childhood IQ, maternal epilepsy type, and past medical history were controlled for.
The mechanism of brain injury in the valproate group is probably third-trimester neuronal apoptosis, Dr. Meador said in an interview. “We think it's similar to what's seen in fetal alcohol syndrome, and the apoptosis appears to occur at a relatively lower dose than it would with other drugs, such as phenytoin. I think that's why we see such an increased signal.”
NEAD only includes the children of women with epilepsy—the group that accounts for the smallest proportion of valproate scrips, he added. “Most of the prescriptions are written for other things, including psychotropic and pain indications. [Fewer] than half are for epilepsy.”
In the third quarter of 2006, about 16% of women of childbearing age with epilepsy were taking the drug, making it the fourth leading antiepileptic in the United States for this group. Valproate sales jumped 28% last year, an increase “that has to include some portion of women of childbearing age,” Dr. Meador said.
The second study, GlaxoSmithKline's lamotrigine pregnancy registry, found that valproate in conjunction with lamotrigine significantly increases the risk of a major birth defect. Lamotrigine is a GSK product.
The registry, now in its 14th year, has prospectively enrolled 2,400 pregnancies occurring in 32 countries. There are known outcome data on 1,539 pregnancies, said Marianne Cunnington, Ph.D., of GSK.
The risk of a major birth defect in the 908 first-trimester exposures to lamotrigine only was 2.9%, similar to the background population risk of 2%–3%. The risk associated with nonvalproate polytherapy was 2.6%. However, when lamotrigine polytherapy included valproate, the risk of a major congenital malformation jumped to more than 11%.
“We have a signal for an increased risk for polytherapy including valproate,” she said, although “it's unclear whether valproate is responsible for this increased risk.”
Dr. Meador noted, however, that six other studies have concluded that valproate significantly increases the risk of birth defects.
BOSTON — Two new data sets reinforce the recommendation to avoid valproate as a first-line therapy for any indication in women of childbearing years.
The findings, presented at the annual meeting of the American Academy of Neurology, strengthen evidence of a link between valproate use and an increased risk of major congenital malformations as well as impaired cognitive development of children exposed in utero.
“Not only our study, but nine other studies on valproate's anatomical and behavioral effects, have shown similar signals of poor outcome with this drug,” Dr. Kimford J Meador commented. “The drug should not be a first-line therapy for any indication in women of childbearing age. At the very minimum, women need to be aware of these risks if they are going to take this drug. We need to remember that half of U.S. pregnancies are unplanned.”
Dr. Meador, the Melvin Greer Professor of Neurology and director of the epilepsy program at the University of Florida, Gainesville, presented interim data from the ongoing Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study for which investigators enrolled 185 children whose mothers took carbamazepine (48), lamotrigine (66), phenytoin (42), or valproate (29) for epilepsy during pregnancy. Data were given on the children's mental development at 2 years; the prospective study will follow the cohort to age 6.
Mean IQ scores based on the Mental Development Index (MDI) from the Bayley Scale were lowest for children in the valproate group (81), Dr. Meador said. A score below 85 is considered to be below normal limits. Mean scores in the other groups were 94 for lamotrigine, 95 for phenytoin, and 96 for carbamazepine.
In addition, he said, the percentage of children in the valproate group with an MDI of less than 70 (correlating with mental retardation) was 24%, about double that seen in any of the other groups (carbamazepine, 13%; lamotrigine, 11%; and phenytoin, 12%).
The study also found an inverse relationship between maternal valproate blood levels during pregnancy and MDI scores in the children, Dr. Meador noted. All of the valproate relationships remained constant even after maternal IQ—an important driver of childhood IQ, maternal epilepsy type, and past medical history were controlled for.
The mechanism of brain injury in the valproate group is probably third-trimester neuronal apoptosis, Dr. Meador said in an interview. “We think it's similar to what's seen in fetal alcohol syndrome, and the apoptosis appears to occur at a relatively lower dose than it would with other drugs, such as phenytoin. I think that's why we see such an increased signal.”
NEAD only includes the children of women with epilepsy—the group that accounts for the smallest proportion of valproate scrips, he added. “Most of the prescriptions are written for other things, including psychotropic and pain indications. [Fewer] than half are for epilepsy.”
In the third quarter of 2006, about 16% of women of childbearing age with epilepsy were taking the drug, making it the fourth leading antiepileptic in the United States for this group. Valproate sales jumped 28% last year, an increase “that has to include some portion of women of childbearing age,” Dr. Meador said.
The second study, GlaxoSmithKline's lamotrigine pregnancy registry, found that valproate in conjunction with lamotrigine significantly increases the risk of a major birth defect. Lamotrigine is a GSK product.
The registry, now in its 14th year, has prospectively enrolled 2,400 pregnancies occurring in 32 countries. There are known outcome data on 1,539 pregnancies, said Marianne Cunnington, Ph.D., of GSK.
The risk of a major birth defect in the 908 first-trimester exposures to lamotrigine only was 2.9%, similar to the background population risk of 2%–3%. The risk associated with nonvalproate polytherapy was 2.6%. However, when lamotrigine polytherapy included valproate, the risk of a major congenital malformation jumped to more than 11%.
“We have a signal for an increased risk for polytherapy including valproate,” she said, although “it's unclear whether valproate is responsible for this increased risk.”
Dr. Meador noted, however, that six other studies have concluded that valproate significantly increases the risk of birth defects.
Reducing the medicolegal risk of vacuum extraction
CASE Three hours of pushing
C.A., age 29 years, is 40 weeks’ pregnant with her first child. After an unremarkable pregnancy, she arrives at the hospital for cervical ripening and induction of labor. Oxytocin is given, and labor progresses uneventfully. When C.A.’s cervix is dilated 8 cm, however, labor stalls. The physician orders placement of a pressure catheter and increases the dosage of oxytocin, and the cervix dilates fully. Although C.A. pushes well, the vertex descends only from +1 to +2 station (of 5 stations) after 3 hours.
How would you manage this delivery?
One option in C.A.’s case is operative vaginal delivery using the vacuum extractor, which has replaced the forceps as the most commonly used approach for operative vaginal delivery. Like the forceps, the vacuum extractor has vociferous detractors as well as supporters. Liberal use of cesarean section and questions regarding the safety of operative vaginal delivery vis-à-vis cesarean section have fueled the debate over its role in obstetric practice.
Among the benefits of vacuum extraction are its cost-effectiveness and shorter hospital stay (TABLE 1). It also obviates the need for cesarean section, including repeat cesarean. Risks include an increased incidence of genital tract trauma and a greater risk of fetal subgaleal hemorrhage.
We review 4 critical spheres of concern in regard to vacuum extraction:
- Patient selection
- Informed consent
- Technique
- Documentation
Increased understanding of these aspects of vacuum extraction will improve outcomes for the patient and limit medicolegal risk.
In the case of C.A., the physician offered 3 options:
- Continue maternal expulsive efforts to allow descent
- Attempt delivery by vacuum extraction
- Proceed to cesarean section on the basis of protracted descent.
Risks and benefits were reviewed with the patient, who chose to deliver by cesarean section. A 3,780-g infant in occiput posterior position was delivered safely.
TABLE 1
Delicate balance: Risks and benefits of operative vaginal delivery
| WHO? | BENEFIT | RISK |
|---|---|---|
| Mother | Cost-effective Less blood loss Lower risk of febrile morbidity Maternal preference No need for cesarean section or repeat cesarean Shorter hospitalization and convalescence | Increased incidence of genital tract trauma Possible damage to pelvic floor, with urinary and anal incontinence |
| Fetus | Fewer respiratory difficulties at birth | Increased risk of subgaleal hemorrhage Association with shoulder dystocia |
1. Patient selection: Maternal and fetal indications
Vacuum extraction may be justified for maternal or fetal indications.1,2 Maternal indications include prolongation or arrest of the second stage of labor, or the need to shorten the second stage, for reasons such as maternal cardiac disease, complex congenital cardiovascular disorders, and maternal exhaustion.
No definitive time limit for the second stage of labor
There is more flexibility today than in the past about what constitutes a “safe” length of the second stage. Recommendations concerning when the mother should begin pushing—and for how long—have evolved from a strict time limit to a focus on progression. If the fetal heart rate (FHR) tracing is reassuring, the second stage no longer needs to be limited to 2 or 3 hours. On the contrary, if the patient is still able and willing to push, changes in positioning and further expectant management remain acceptable in contemporary practice.3 Otherwise, a trial of vacuum extraction may be appropriate.
Vacuum extraction is particularly useful when the mother has difficulty pushing because of exhaustion and the fetal head has descended enough that it distends the labia between contractions, as in outlet deliveries.
Fetal indications
Fetal indications for operative vaginal delivery include distress, jeopardy, or a “nonreassuring” FHR tracing. Such a tracing may include late and prolonged decelerations, baseline bradycardia or tachycardia with or without variable decelerations, or, occasionally, a normal baseline rate with diminished variability.
Use vacuum or forceps?
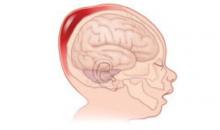
The choice depends on which device would achieve delivery in the safest manner with the lowest risk of fetal injury. With the vacuum, force is exerted directly on the fetal scalp and only secondarily on the fetal skull. This puts fetal vessels that traverse the subgaleal space at risk for injury (FIGURE). With forceps, force is exerted directly on the fetal skull and mitigated by the petrous bone. Little or no force is exerted on the fetal scalp, lessening the risk of traumatic injury such as potentially fatal subgaleal hemorrhage.
Indications and contraindications for vacuum extraction are similar, but not identical, to those for forceps delivery (TABLE 2).2,3 The most important determinant for either device is the experience of the operator. You must be familiar with the instrument and technique before making any attempt to assist delivery. An inability to accurately assess fetal position or station, fetopelvic proportion, adequacy of labor, engagement of the fetal head, or any degree of malpresentation (including minor degrees of deflexion) is a contraindication to a trial of operative vaginal delivery.
Vacuum extraction should be reserved for fetuses at more than 34 weeks’ gestation because of the increased risk of intracranial hemorrhage associated with prematurity.
All decisions involving vacuum extraction should be made with caution. The adequacy of the pelvis, estimated fetal size, and any suggestions of fetopelvic disproportion are of particular significance.3
FIGURE
Subgaleal hemorrhage, a deadly complication
Blood can accumulate in a large potential space between the galea aponeurotica and the periosteum of the cranial bones after vacuum extraction. An infant with subgaleal hemorrhage will exhibit a boggy scalp, with swelling that crosses the suture lines and expands head circumferenceTABLE 2
Factors that predict success—or failure—of vacuum extraction
| When a woman fits overlapping categories, the decision to use vacuum extraction—or not—may be a judgment call* |
| GOOD CANDIDACY |
| Multiparous |
| Term pregnancy |
| Occiput anterior position, well-flexed |
| Wide subpubic arch |
| Compliant |
| MARGINAL CANDIDACY |
| Primiparous |
| Post-term |
| Occiput posterior position |
| Average subpubic arch |
| Gestational diabetes |
| Arrest disorders in second stage |
| POOR CANDIDACY |
| Protraction disorders in second stage |
| Narrow subpubic arch |
| Uncertain position of fetal head |
| Deflexion or asynclitism |
| Anticipated large-for-gestational-age infant |
| Poor maternal compliance |
| * When faced with a good indication in a marginal candidate, we recommend delivery in a “double setup” situation in which preparations are made for both vacuum extraction and cesarean section. If the vacuum can be properly applied, the first application of traction is crucial. We will only proceed if significant descent is achieved. If the fetal head (not the scalp) can be advanced a full station, then we proceed cautiously. If not, ready access to cesarean section allows for completion of the delivery in a timely manner. |
2. Informed consent: Elicit the patient’s desires
Thorough discussion with the patient and her family—to explain the reasoning behind the clinical decision to use the vacuum extractor and delineate the alternatives—is paramount. Moreover, the patient should be encouraged to actively participate in this discussion.
Among the alternatives to vacuum extraction are expectant observation and expedited delivery by cesarean section. Because patients increasingly are requesting elective cesarean section in the absence of obvious obstetric indications, this option should receive extra attention.
Most women still consider vaginal delivery an important milestone of female adulthood. When safety concerns arise and the situation makes vaginal delivery unwise, many women experience disappointment and postpartum depression over their “failed” attempt at vaginal delivery. These perceptions need to be addressed in discussions with the patient.
The risk–benefit equation
Vacuum extraction lessens the risk of maternal lacerations, either of the lower genital tract in the case of obstetric forceps, or of the cervix and lower uterine segment in the case of cesarean section. In addition, vacuum extraction can be performed comfortably in the absence of regional anesthesia.
Avoiding cesarean section can produce multiple benefits
Another maternal benefit of vacuum extraction is the decreased need for cesarean section. A reduction in the primary cesarean rate also lowers the need for repeat cesarean section, which can be more technically challenging than primary C-section due to the presence of dense scar tissue and intra-abdominal adhesions. Cesarean section also increases the risk of placenta accreta, increta, or percreta in subsequent pregnancies. These complications increase the likelihood of emergency hysterectomy, massive blood loss, and serious maternal morbidity and mortality.
Even in the absence of placenta accreta, both primary and repeat cesarean sections raise the risk of hemorrhage and febrile morbidity, prolong convalescence, and increase cost, compared with vaginal delivery. For these reasons, avoiding primary cesarean section can obviate the need for multiple surgical procedures and their attendant risks. The degree to which these factors favor vaginal delivery over cesarean section is subject to debate.
Maternal risks include pelvic floor trauma
Both vacuum extraction and forceps delivery increase the risk of anal sphincter injury and can impair fecal continence.4 Both methods also appear to increase trauma to the genital tract in comparison with spontaneous delivery and may predispose the woman to pelvic floor dysfunction, including urinary and anal incontinence.5-10 However, anal sphincter trauma was less frequent after vacuum extraction than after forceps delivery.1
Other maternal injuries associated with vacuum extraction include perineal lacerations and injuries to the vulva, vagina, and cervix. Vacuum extraction also has been implicated as a significant risk factor for postpartum hemorrhage11 and genital-tract infection.1
Fewer neonatal respiratory problems with vaginal delivery
Compared with cesarean section, vaginal delivery is thought to diminish the risk of intrapartum aspiration and respiratory problems in the newborn. It also may facilitate the transition from fetal to neonatal circulation and reduce the need for immediate resuscitation at birth.
Neonatal risks include soft-tissue injury and potential hemorrhage
Infants delivered by vacuum extraction have a significantly higher rate of intracranial hemorrhage, brachial plexus injuries, convulsions, central nervous system depression, and the need for mechanical ventilation, compared with spontaneously delivered infants (TABLE 3).12,13
Although vacuum extraction is associated with a wide range of soft tissue injuries, they are often less serious than the fetal scalp injuries associated with obstetric forceps. Cup marks, bruising, and minor lacerations of the scalp and caput succedaneum are common fetal injuries, although the majority resolve without apparent sequelae.14
Subgaleal hemorrhage is the most serious neonatal complication of vacuum extraction, occurring in 1% to 3.8% of vacuum extractions (FIGURE).15 It coexists with neonatal coagulopathy in 19% to 29% of newborns16 and increases the risk of progression to hemorrhagic shock and death. Subgaleal hemorrhage has a mortality rate ranging from 2.7% to 22.8%.15-17
Cephalhematoma is another complication associated with vacuum extraction. It involves an accumulation of blood beneath the periosteum of a cranial bone (usually the parietal bone), and it almost always resolves spontaneously. The incidence of cephalhematoma varies. It is significantly more common in deliveries involving vacuum extraction (9.8%) than in forceps deliveries (4.1%).18 Its incidence increases with the length of time the vacuum cup is applied and with paramedian application.18
Intracranial hemorrhage occurs in 1 of 860 vacuum extractions, 1 of 664 forceps deliveries, 1 of 954 cesarean deliveries, and 1 of 1,900 spontaneous deliveries.12 Subdural hemorrhage is the most common form of intracranial hemorrhage and is almost invariably the result of birth trauma. However, asymptomatic subdural hematoma occurs in up to 6.1% of uncomplicated vaginal deliveries.19
Other, less common types of intracranial hemorrhage, such as subarachnoid, intraventricular, and intraparenchymal hemorrhage, have a more complex etiology, which includes birth asphyxia, hemorrhagic diathesis, infection, and vascular abnormalities.20
Retinal hemorrhage also may occur after vacuum extraction, with an incidence of 49% to 77.8%, compared with 30.3% after forceps delivery, 30.4% after normal vaginal delivery, and 8.3% after cesarean delivery.21 It generally resolves spontaneously without any permanent damage.22
TABLE 3
Vacuum extraction can injure the fetus
| DIRECT INJURY |
| Cephalhematoma |
| Intracranial hemorrhage (parenchymal, subdural, intraventricular, subarachnoid) |
| Nerve injury |
| Scalp laceration, abrasion, ecchymoses, necrosis |
| Skull fracture |
| Subgaleal hemorrhage |
| INDIRECT INJURY |
| Anemia, hyperbilirubinemia |
| Brachial plexus injury |
| Scalp infection or abscess |
| SOURCE: O’Grady et al31 |
Shoulder dystocia and brachial plexus palsy
Vacuum extraction also is associated with shoulder dystocia and brachial plexus palsy, although the primary risk factor for these complications is thought to be increased fetal size.23-25 The incidence of shoulder dystocia with vacuum extraction is 3.5%, compared with 1.5% for forceps delivery.25
The risk of brachial plexus palsy also increases with vacuum extraction, especially as the duration of the procedure increases.25
Less common complications associated with vacuum extraction are skull fractures, fetal hemorrhage from bleeding at the site of scalp electrodes, sepsis originating from infected scalp trauma, and corneal injury.
No long-term impairment
Long-term outcome studies of children delivered by vacuum extraction show no differences in physical or cognitive functioning or intelligence scores, compared with other modes of delivery.26
3. Technique: Create conditions that ensure success
Certain prerequisites to vacuum extraction can assure successful application and strict adherence to protocol. These prerequisites include having an appropriate indication, thorough informed consent, proper maternal positioning, adequate anesthesia, and knowledge of fetal position and station (TABLE 4).1 These objectives can be accomplished in the following steps:
- After an informed consent discussion, assess maternal positioning and repeat the pelvic exam. Also ascertain the adequacy of anesthesia. Insert a bladder catheter.
- Perform a “ghost” trial of vacuum extraction to visualize the procedure before the actual attempt.
- Test the function of the vacuum.
- Lubricate the vacuum cup with surgical soap or gel, insert it into the vagina, and maneuver it onto the fetal head. Place the vacuum extractor over the sagittal suture about 6 cm distal to the anterior fontanel and 2 cm proximal to the posterior fontanel. (The illustration on page 74 demonstrates positioning.) Apply a small degree of vacuum (approximately 20 mm Hg). Double-check application.
- Gradually apply full vacuum (550–600 mm Hg, depending on cup size), allowing the scalp to mold to the extractor cup.
- Apply 2-handed traction in concert with uterine contractions and supplemented by maternal pushing. Assuming there is no loss of vacuum (“pop-off” of the cup), the initial traction effort should produce a gain in station. If a “pop-off” occurs, a single additional attempt at delivery may be warranted.
- As the head crowns, perform episiotomy as needed and slowly deliver the fetal head. Remove the vacuum cup.
- After delivery of the placenta, inspect the vagina, cervix, and perineum closely.
- Dictate a full operative note and annotate the delivery in the chart. See the section on documentation, below.
Vacuum extraction may fail for a number of reasons (TABLE 5).
TABLE 4
Perform these predelivery checks before applying traction
| Is anesthesia adequate? Is maternal positioning correct? |
| Is the bladder empty? |
| Is the fetus in the proper attitude (flexion)? |
| Is fetal status reassuring? |
Is the vacuum properly applied?
|
| Has the patient been instructed on when and how long to push? |
| Are the proposed maneuvers appropriate? |
TABLE 5
Why might vacuum extraction fail?
| INSTRUMENT-RELATED |
| Pump failure |
| Vacuum leak |
| TECHNIQUE-RELATED |
| Failure to encourage maternal valsalva with traction efforts |
| Inappropriate intensity of traction |
| Incorrect axis of traction |
| Maternal tissue trapped beneath vacuum cup |
| Poor cup position |
| OBSTETRIC CONDITIONS |
Congenital anomaly
|
| Fetal macrosomia |
| Incomplete cervical dilation |
Position and attitude problems
|
| Unappreciated cephalopelvic disproportion |
| SOURCE: Modified from Plauche et al32 |
Most important variable: Cup placement
The single most critical step in vacuum extraction is placement of the cup. It should be applied at the point of maximum fetal cranial flexion, which is proximal to the leading edge of the posterior fontanel.
Once full vacuum is achieved, encourage the mother to push with the next contraction, and apply steady traction in concert with her efforts.
The initial application of traction should be directed to maintain proper flexion of the fetal head, and should bring about descent of the fetal head. If there is no descent with the first application of traction, and correct technique and cup placement have been applied, abandon operative vaginal delivery (TABLE 6).
Do not make a further attempt to deliver the child using forceps, as the risk of intracranial hemorrhage appears to be highest in infants delivered using a combination of vacuum extraction and forceps.
TABLE 6
Repeat traction efforts reap a diminishing return
| NUMBER OF TRACTION EFFORTS | SUCCESS RATE | |
|---|---|---|
| VACUUM EXTRACTION (N=433) | FORCEPS (N=555) | |
| 1 or 2 | 68.4% | 38.4% |
| 3 or 4 | 24.9% | 48.6% |
| 5 or more | 6.7% | 12.9% |
| Adapted from Sjostedt33 | ||
4. Documentation: The chart is the most important witness
The value of complete and contemporaneous notation cannot be overstated. The patient’s chart is the permanent repository of the record of delivery. It is without doubt the most important witness to the event and should be treated as such. Include a dictated operative note as well as notation in the chart itself. Notes should be legible and properly dated, with the time of day indicated.
When operative vaginal delivery is performed, record the following:
- indication for the procedure
- course of labor
- anesthesia
- personnel present
- instruments used
- position and station of the fetal head
- force and duration of traction
- complications, including how they were recognized and managed
- immediate condition of the newborn and all steps taken in resuscitation.
Operative vaginal delivery is no newcomer to obstetrics. Hindu writings from about 1000 BC, and Hippocrates’ own musings from the fifth century BC, describe instruments and techniques to combat arrested labor and salvage the lives of both mother and child.27 Crude forceps were described by the Muslim physician Albucasis in the 11th century.27
Before the advent of safe cesarean section, many maternal lives were no doubt saved by these instruments and techniques. Unfortunately, destruction of the fetus and maternal death were frequent outcomes of operative vaginal delivery by forceps before the 20th century.28
As for vacuum extraction in particular, the idea of attaching a device to the fetal head to aid in delivery is credited to Arnett, a 19th century surgeon and inventor, who envisioned the “pneumatic tractor.”29
In 1957, Malmstrom reintroduced the vacuum as an aid in delivery, designing a rigid cup that was connected by rubber tubing to a vacuum source.30 This allowed the separation of the pump mechanism from the cup and made for easier application.
Most recently, Kobayashi developed the soft-cup design, a low-cost flexible plastic alternative that allows for a disposable instrument.31
Minimizing medicolegal risk
The best way to prevent an accusation of medical malpractice is to develop strong clinical and interpersonal skills. These simple, intuitive suggestions may help:
- Understand the role of operative vaginal delivery in current practice.
- Develop a simple and interactive discussion model for use in labor and delivery with the patient and her family.
- Consider a woman’s preferences for delivery.
- Know the indications and contraindications for vacuum extraction.
- Use the checks and safeguards listed under 3. Technique: Create conditions that ensure success.
- Perform vacuum extraction in the cesarean section room. Stop the procedure at once if any problem arises, and proceed to cesarean delivery.
- Make all chart notations completely legible, and add dictated notes.
If you are a new physician or lack significant experience with vacuum extraction, ask for input, supervision, and education from more experienced clinicians. Also make it a point to ask about department guidelines and review the credentialing process. Once you become adept at vacuum extraction, mentor more junior colleagues.
Two critical concerns
When contemplating vacuum-assisted delivery, 2 risks are paramount:
- failure of the vacuum extractor to achieve delivery
- the potential for fetal and maternal injury.
Training must ensure appropriate case selection and technique. Vacuum extraction must be performed with the same precision and care used with forceps. If application of the device is incorrect, or if there is a wrong direction of traction, excessive traction, or traction in the presence of disproportion, the cup will slip or pop off, and vacuum delivery will fail, with the potential for traumatic fetal injury.
All risks must be discussed with the patient to fulfill informed consent, and the risks and benefits of alternative treatments should be part of the discussion. Active participation, in considering how best to approach delivery, is required of all parties concerned.
The vacuum extractor can be a useful adjunct in certain circumstances, and its use has become widespread in American delivery suites. As with the obstetric forceps, which largely antedated its use, the vacuum extractor can lessen the overall risks of childbirth for both mother and infant.
The authors report no financial relationships relevant to this article.
1. O’Grady JP, Gimovsky ML, McIlhargie CJ, eds. Operative Obstetrics. Pearl River, NY: Parthenon Publishing; 1995.
2. Johanson RB, Menon BKV. Vacuum extraction versus forceps for assisted vaginal delivery. Cochrane Database Syst Rev. 2000;(2):CD000224.-
3. Bloom SL, Casey BM, Schaffer JL, et al. Pushing in the second stage of labor. Am J Obstet Gynecol 2006;194:10-13.
4. Operative vaginal delivery. ACOG Practice Bulletin #17. Washington, DC: American College of Obstetricians and Gynecologists; June 2000.
5. Power D, Fitzpatrick M, O’Herlihy C. Obstetric anal sphincter injury: how to avoid, how to repair: a literature review. J Fam Pract 2006;55:193-200.
6. Chaliha C, Kalia V, Stanton S, et al. Antenatal prediction of postpartum urinary and fecal incontinence. Obstet Gynecol 1999;94:689-694.
7. Bofill JA, Rust OA, Schorr SJ, et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. Am J Obstet Gynecol 1996;175:1325-1330.
8. Salamalekis E, Loghis C, Pyrgiotis E, et al. Soft cup vacuum extractor versus forceps delivery. J Obstet Gynecol. 1995;15:245-246.
9. Zetterstrom JP, Lopez A, Anzen B, et al. Anal incontinence after vaginal delivery: a prospective study in primiparous women. Br J Obstet Gynaecol. 1999;106:324-330.
10. Johanson RB, Heycock E, Carter J, et al. Maternal and child health after assisted vaginal delivery: five-year follow up of a randomized controlled study comparing forceps and ventouse. Br J Obstet Gynaecol. 1999;106:544-549
11. Faltin DL, Otero M, Petignat P, et al. Women’s health 18 years after rupture of the anal sphincter during childbirth: I. Fecal incontinence. Am J Obstet Gynecol. 2006;194:1255-1259.
12. Plauche WC. Fetal cranial injuries related to delivery with the Malmsträm vacuum extractor. Obstet Gynecol. 1979;53:750-757.
13. Towner D, Castro MA, Eby-Wilkens E, et al. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
14. Sheiner E, Sarid L, Levy A, et al. Obstetric risk factors and outcome of pregnancies complicated with early postpartum hemorrhage: a population-based study. J Matern Fetal Neonatal Med. 2005;18:149-154.
15. Johanson R. Choice of instrument for vaginal delivery. Curr Opin Obstet Gynecol. 1997;9:361-365.
16. Chadwick LM, Pemberton PJ, Kurinczuk JJ. Neonatal subgaleal hematoma: associated risk factors, complications, and outcome. J Pediatr Child Health. 1996;32:228-232.
17. Ng PC, Siu YK, Lewindon PJ. Subaponeurotic hemorrhage in the 1990s: a 3-year surveillance. Acta Pediatr. 1995;84:1065-1069
18. Bofill JA, Rust OA, Devidas M, et al. Neonatal cephalohematoma from vacuum extraction. J Reprod Med. 1997;42:565-569.
19. Doumouchtsis SK, Arulkumaran S. Head injuries after instrumental vaginal deliveries. Curr Opin Obstet Gynecol. 2006;18:129-134.
20. Govaert P. Cranial Hemorrhage in the Term Newborn Infant. London: Mac Keith Press; 1993.
21. Hughes LA, May K, Talbot JF, Parsons MA. Incidence, distribution, and duration of birth-related retinal hemorrhages: a prospective study. J AAPOS. 2006;10:102-106.
22. Sheiner E, Levy A, Hershkovitz R, et al. Determining factors associated with shoulder dystocia: a population-based study. Eur J Obstet Gynecol Reprod Biol. 2006;126:11-15.
23. Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:15-18.
24. Mollberg M, Hagerg H, Bager B, et al. Risk factors for obstetric brachial plexus palsy among neonates delivered by vacuum extraction. Obstet Gynecol. 2005;106:913-918.
25. Caughey AB, Sandberg PL, Alantnik MG, et al. Forceps compared with vacuum: rates of neonatal and maternal morbidity. Obstet Gynecol. 2005;106:908-912.
26. Ngan HYS, Miu P, Ko L, et al. Long-term neurological sequelae following vacuum extractor delivery. Aust NZ J Obstet Gynecol. 1990;30:111-114.
27. Lyons AS, Petrucelli RJ. Medicine: An Illustrated History. New York: Harry N Abrams; 1978.
28. Speert H. Obstetric and Gynecologic Milestones Illustrated. Pearl River, NY: Parthenon Publishing; 1996.
29. Arnett N. Elements of Physics or Natural Philosophy, General and Medical, Explained Independently of Technical Mathematics and Containing New Disquisitions and Practical Suggestions. 2nd ed. Philadelphia: Carney and Lea; 1831.
30. Malmstrom T. The vacuum extractor, an obstetrical instrument. I. Acta Obstet Gynecol Scand. 1957;36(suppl 3):5-50.
31. O’Grady JP, Gimovsky ML, McIlhargie CJ. Vacuum Extraction in Modern Obstetric Practice. Pearl River, NY: Parthenon Publishing; 1995.
32. Plauche WC, Morrison JC, O’Sullivan MJ. Surgical Obstetrics. Philadelphia: WB Saunders; 1992.
33. Sjostedt JE. The vacuum extractor and forceps in obstetrics: a clinical study. Acta Obstet Gynecol Scand. 1967;48:638-639.
CASE Three hours of pushing
C.A., age 29 years, is 40 weeks’ pregnant with her first child. After an unremarkable pregnancy, she arrives at the hospital for cervical ripening and induction of labor. Oxytocin is given, and labor progresses uneventfully. When C.A.’s cervix is dilated 8 cm, however, labor stalls. The physician orders placement of a pressure catheter and increases the dosage of oxytocin, and the cervix dilates fully. Although C.A. pushes well, the vertex descends only from +1 to +2 station (of 5 stations) after 3 hours.
How would you manage this delivery?
One option in C.A.’s case is operative vaginal delivery using the vacuum extractor, which has replaced the forceps as the most commonly used approach for operative vaginal delivery. Like the forceps, the vacuum extractor has vociferous detractors as well as supporters. Liberal use of cesarean section and questions regarding the safety of operative vaginal delivery vis-à-vis cesarean section have fueled the debate over its role in obstetric practice.
Among the benefits of vacuum extraction are its cost-effectiveness and shorter hospital stay (TABLE 1). It also obviates the need for cesarean section, including repeat cesarean. Risks include an increased incidence of genital tract trauma and a greater risk of fetal subgaleal hemorrhage.
We review 4 critical spheres of concern in regard to vacuum extraction:
- Patient selection
- Informed consent
- Technique
- Documentation
Increased understanding of these aspects of vacuum extraction will improve outcomes for the patient and limit medicolegal risk.
In the case of C.A., the physician offered 3 options:
- Continue maternal expulsive efforts to allow descent
- Attempt delivery by vacuum extraction
- Proceed to cesarean section on the basis of protracted descent.
Risks and benefits were reviewed with the patient, who chose to deliver by cesarean section. A 3,780-g infant in occiput posterior position was delivered safely.
TABLE 1
Delicate balance: Risks and benefits of operative vaginal delivery
| WHO? | BENEFIT | RISK |
|---|---|---|
| Mother | Cost-effective Less blood loss Lower risk of febrile morbidity Maternal preference No need for cesarean section or repeat cesarean Shorter hospitalization and convalescence | Increased incidence of genital tract trauma Possible damage to pelvic floor, with urinary and anal incontinence |
| Fetus | Fewer respiratory difficulties at birth | Increased risk of subgaleal hemorrhage Association with shoulder dystocia |
1. Patient selection: Maternal and fetal indications
Vacuum extraction may be justified for maternal or fetal indications.1,2 Maternal indications include prolongation or arrest of the second stage of labor, or the need to shorten the second stage, for reasons such as maternal cardiac disease, complex congenital cardiovascular disorders, and maternal exhaustion.
No definitive time limit for the second stage of labor
There is more flexibility today than in the past about what constitutes a “safe” length of the second stage. Recommendations concerning when the mother should begin pushing—and for how long—have evolved from a strict time limit to a focus on progression. If the fetal heart rate (FHR) tracing is reassuring, the second stage no longer needs to be limited to 2 or 3 hours. On the contrary, if the patient is still able and willing to push, changes in positioning and further expectant management remain acceptable in contemporary practice.3 Otherwise, a trial of vacuum extraction may be appropriate.
Vacuum extraction is particularly useful when the mother has difficulty pushing because of exhaustion and the fetal head has descended enough that it distends the labia between contractions, as in outlet deliveries.
Fetal indications
Fetal indications for operative vaginal delivery include distress, jeopardy, or a “nonreassuring” FHR tracing. Such a tracing may include late and prolonged decelerations, baseline bradycardia or tachycardia with or without variable decelerations, or, occasionally, a normal baseline rate with diminished variability.
Use vacuum or forceps?
The choice depends on which device would achieve delivery in the safest manner with the lowest risk of fetal injury. With the vacuum, force is exerted directly on the fetal scalp and only secondarily on the fetal skull. This puts fetal vessels that traverse the subgaleal space at risk for injury (FIGURE). With forceps, force is exerted directly on the fetal skull and mitigated by the petrous bone. Little or no force is exerted on the fetal scalp, lessening the risk of traumatic injury such as potentially fatal subgaleal hemorrhage.
Indications and contraindications for vacuum extraction are similar, but not identical, to those for forceps delivery (TABLE 2).2,3 The most important determinant for either device is the experience of the operator. You must be familiar with the instrument and technique before making any attempt to assist delivery. An inability to accurately assess fetal position or station, fetopelvic proportion, adequacy of labor, engagement of the fetal head, or any degree of malpresentation (including minor degrees of deflexion) is a contraindication to a trial of operative vaginal delivery.
Vacuum extraction should be reserved for fetuses at more than 34 weeks’ gestation because of the increased risk of intracranial hemorrhage associated with prematurity.
All decisions involving vacuum extraction should be made with caution. The adequacy of the pelvis, estimated fetal size, and any suggestions of fetopelvic disproportion are of particular significance.3
FIGURE
Subgaleal hemorrhage, a deadly complication
Blood can accumulate in a large potential space between the galea aponeurotica and the periosteum of the cranial bones after vacuum extraction. An infant with subgaleal hemorrhage will exhibit a boggy scalp, with swelling that crosses the suture lines and expands head circumferenceTABLE 2
Factors that predict success—or failure—of vacuum extraction
| When a woman fits overlapping categories, the decision to use vacuum extraction—or not—may be a judgment call* |
| GOOD CANDIDACY |
| Multiparous |
| Term pregnancy |
| Occiput anterior position, well-flexed |
| Wide subpubic arch |
| Compliant |
| MARGINAL CANDIDACY |
| Primiparous |
| Post-term |
| Occiput posterior position |
| Average subpubic arch |
| Gestational diabetes |
| Arrest disorders in second stage |
| POOR CANDIDACY |
| Protraction disorders in second stage |
| Narrow subpubic arch |
| Uncertain position of fetal head |
| Deflexion or asynclitism |
| Anticipated large-for-gestational-age infant |
| Poor maternal compliance |
| * When faced with a good indication in a marginal candidate, we recommend delivery in a “double setup” situation in which preparations are made for both vacuum extraction and cesarean section. If the vacuum can be properly applied, the first application of traction is crucial. We will only proceed if significant descent is achieved. If the fetal head (not the scalp) can be advanced a full station, then we proceed cautiously. If not, ready access to cesarean section allows for completion of the delivery in a timely manner. |
2. Informed consent: Elicit the patient’s desires
Thorough discussion with the patient and her family—to explain the reasoning behind the clinical decision to use the vacuum extractor and delineate the alternatives—is paramount. Moreover, the patient should be encouraged to actively participate in this discussion.
Among the alternatives to vacuum extraction are expectant observation and expedited delivery by cesarean section. Because patients increasingly are requesting elective cesarean section in the absence of obvious obstetric indications, this option should receive extra attention.
Most women still consider vaginal delivery an important milestone of female adulthood. When safety concerns arise and the situation makes vaginal delivery unwise, many women experience disappointment and postpartum depression over their “failed” attempt at vaginal delivery. These perceptions need to be addressed in discussions with the patient.
The risk–benefit equation
Vacuum extraction lessens the risk of maternal lacerations, either of the lower genital tract in the case of obstetric forceps, or of the cervix and lower uterine segment in the case of cesarean section. In addition, vacuum extraction can be performed comfortably in the absence of regional anesthesia.
Avoiding cesarean section can produce multiple benefits
Another maternal benefit of vacuum extraction is the decreased need for cesarean section. A reduction in the primary cesarean rate also lowers the need for repeat cesarean section, which can be more technically challenging than primary C-section due to the presence of dense scar tissue and intra-abdominal adhesions. Cesarean section also increases the risk of placenta accreta, increta, or percreta in subsequent pregnancies. These complications increase the likelihood of emergency hysterectomy, massive blood loss, and serious maternal morbidity and mortality.
Even in the absence of placenta accreta, both primary and repeat cesarean sections raise the risk of hemorrhage and febrile morbidity, prolong convalescence, and increase cost, compared with vaginal delivery. For these reasons, avoiding primary cesarean section can obviate the need for multiple surgical procedures and their attendant risks. The degree to which these factors favor vaginal delivery over cesarean section is subject to debate.
Maternal risks include pelvic floor trauma
Both vacuum extraction and forceps delivery increase the risk of anal sphincter injury and can impair fecal continence.4 Both methods also appear to increase trauma to the genital tract in comparison with spontaneous delivery and may predispose the woman to pelvic floor dysfunction, including urinary and anal incontinence.5-10 However, anal sphincter trauma was less frequent after vacuum extraction than after forceps delivery.1
Other maternal injuries associated with vacuum extraction include perineal lacerations and injuries to the vulva, vagina, and cervix. Vacuum extraction also has been implicated as a significant risk factor for postpartum hemorrhage11 and genital-tract infection.1
Fewer neonatal respiratory problems with vaginal delivery
Compared with cesarean section, vaginal delivery is thought to diminish the risk of intrapartum aspiration and respiratory problems in the newborn. It also may facilitate the transition from fetal to neonatal circulation and reduce the need for immediate resuscitation at birth.
Neonatal risks include soft-tissue injury and potential hemorrhage
Infants delivered by vacuum extraction have a significantly higher rate of intracranial hemorrhage, brachial plexus injuries, convulsions, central nervous system depression, and the need for mechanical ventilation, compared with spontaneously delivered infants (TABLE 3).12,13
Although vacuum extraction is associated with a wide range of soft tissue injuries, they are often less serious than the fetal scalp injuries associated with obstetric forceps. Cup marks, bruising, and minor lacerations of the scalp and caput succedaneum are common fetal injuries, although the majority resolve without apparent sequelae.14
Subgaleal hemorrhage is the most serious neonatal complication of vacuum extraction, occurring in 1% to 3.8% of vacuum extractions (FIGURE).15 It coexists with neonatal coagulopathy in 19% to 29% of newborns16 and increases the risk of progression to hemorrhagic shock and death. Subgaleal hemorrhage has a mortality rate ranging from 2.7% to 22.8%.15-17
Cephalhematoma is another complication associated with vacuum extraction. It involves an accumulation of blood beneath the periosteum of a cranial bone (usually the parietal bone), and it almost always resolves spontaneously. The incidence of cephalhematoma varies. It is significantly more common in deliveries involving vacuum extraction (9.8%) than in forceps deliveries (4.1%).18 Its incidence increases with the length of time the vacuum cup is applied and with paramedian application.18
Intracranial hemorrhage occurs in 1 of 860 vacuum extractions, 1 of 664 forceps deliveries, 1 of 954 cesarean deliveries, and 1 of 1,900 spontaneous deliveries.12 Subdural hemorrhage is the most common form of intracranial hemorrhage and is almost invariably the result of birth trauma. However, asymptomatic subdural hematoma occurs in up to 6.1% of uncomplicated vaginal deliveries.19
Other, less common types of intracranial hemorrhage, such as subarachnoid, intraventricular, and intraparenchymal hemorrhage, have a more complex etiology, which includes birth asphyxia, hemorrhagic diathesis, infection, and vascular abnormalities.20
Retinal hemorrhage also may occur after vacuum extraction, with an incidence of 49% to 77.8%, compared with 30.3% after forceps delivery, 30.4% after normal vaginal delivery, and 8.3% after cesarean delivery.21 It generally resolves spontaneously without any permanent damage.22
TABLE 3
Vacuum extraction can injure the fetus
| DIRECT INJURY |
| Cephalhematoma |
| Intracranial hemorrhage (parenchymal, subdural, intraventricular, subarachnoid) |
| Nerve injury |
| Scalp laceration, abrasion, ecchymoses, necrosis |
| Skull fracture |
| Subgaleal hemorrhage |
| INDIRECT INJURY |
| Anemia, hyperbilirubinemia |
| Brachial plexus injury |
| Scalp infection or abscess |
| SOURCE: O’Grady et al31 |
Shoulder dystocia and brachial plexus palsy
Vacuum extraction also is associated with shoulder dystocia and brachial plexus palsy, although the primary risk factor for these complications is thought to be increased fetal size.23-25 The incidence of shoulder dystocia with vacuum extraction is 3.5%, compared with 1.5% for forceps delivery.25
The risk of brachial plexus palsy also increases with vacuum extraction, especially as the duration of the procedure increases.25
Less common complications associated with vacuum extraction are skull fractures, fetal hemorrhage from bleeding at the site of scalp electrodes, sepsis originating from infected scalp trauma, and corneal injury.
No long-term impairment
Long-term outcome studies of children delivered by vacuum extraction show no differences in physical or cognitive functioning or intelligence scores, compared with other modes of delivery.26
3. Technique: Create conditions that ensure success
Certain prerequisites to vacuum extraction can assure successful application and strict adherence to protocol. These prerequisites include having an appropriate indication, thorough informed consent, proper maternal positioning, adequate anesthesia, and knowledge of fetal position and station (TABLE 4).1 These objectives can be accomplished in the following steps:
- After an informed consent discussion, assess maternal positioning and repeat the pelvic exam. Also ascertain the adequacy of anesthesia. Insert a bladder catheter.
- Perform a “ghost” trial of vacuum extraction to visualize the procedure before the actual attempt.
- Test the function of the vacuum.
- Lubricate the vacuum cup with surgical soap or gel, insert it into the vagina, and maneuver it onto the fetal head. Place the vacuum extractor over the sagittal suture about 6 cm distal to the anterior fontanel and 2 cm proximal to the posterior fontanel. (The illustration on page 74 demonstrates positioning.) Apply a small degree of vacuum (approximately 20 mm Hg). Double-check application.
- Gradually apply full vacuum (550–600 mm Hg, depending on cup size), allowing the scalp to mold to the extractor cup.
- Apply 2-handed traction in concert with uterine contractions and supplemented by maternal pushing. Assuming there is no loss of vacuum (“pop-off” of the cup), the initial traction effort should produce a gain in station. If a “pop-off” occurs, a single additional attempt at delivery may be warranted.
- As the head crowns, perform episiotomy as needed and slowly deliver the fetal head. Remove the vacuum cup.
- After delivery of the placenta, inspect the vagina, cervix, and perineum closely.
- Dictate a full operative note and annotate the delivery in the chart. See the section on documentation, below.
Vacuum extraction may fail for a number of reasons (TABLE 5).
TABLE 4
Perform these predelivery checks before applying traction
| Is anesthesia adequate? Is maternal positioning correct? |
| Is the bladder empty? |
| Is the fetus in the proper attitude (flexion)? |
| Is fetal status reassuring? |
Is the vacuum properly applied?
|
| Has the patient been instructed on when and how long to push? |
| Are the proposed maneuvers appropriate? |
TABLE 5
Why might vacuum extraction fail?
| INSTRUMENT-RELATED |
| Pump failure |
| Vacuum leak |
| TECHNIQUE-RELATED |
| Failure to encourage maternal valsalva with traction efforts |
| Inappropriate intensity of traction |
| Incorrect axis of traction |
| Maternal tissue trapped beneath vacuum cup |
| Poor cup position |
| OBSTETRIC CONDITIONS |
Congenital anomaly
|
| Fetal macrosomia |
| Incomplete cervical dilation |
Position and attitude problems
|
| Unappreciated cephalopelvic disproportion |
| SOURCE: Modified from Plauche et al32 |
Most important variable: Cup placement
The single most critical step in vacuum extraction is placement of the cup. It should be applied at the point of maximum fetal cranial flexion, which is proximal to the leading edge of the posterior fontanel.
Once full vacuum is achieved, encourage the mother to push with the next contraction, and apply steady traction in concert with her efforts.
The initial application of traction should be directed to maintain proper flexion of the fetal head, and should bring about descent of the fetal head. If there is no descent with the first application of traction, and correct technique and cup placement have been applied, abandon operative vaginal delivery (TABLE 6).
Do not make a further attempt to deliver the child using forceps, as the risk of intracranial hemorrhage appears to be highest in infants delivered using a combination of vacuum extraction and forceps.
TABLE 6
Repeat traction efforts reap a diminishing return
| NUMBER OF TRACTION EFFORTS | SUCCESS RATE | |
|---|---|---|
| VACUUM EXTRACTION (N=433) | FORCEPS (N=555) | |
| 1 or 2 | 68.4% | 38.4% |
| 3 or 4 | 24.9% | 48.6% |
| 5 or more | 6.7% | 12.9% |
| Adapted from Sjostedt33 | ||
4. Documentation: The chart is the most important witness
The value of complete and contemporaneous notation cannot be overstated. The patient’s chart is the permanent repository of the record of delivery. It is without doubt the most important witness to the event and should be treated as such. Include a dictated operative note as well as notation in the chart itself. Notes should be legible and properly dated, with the time of day indicated.
When operative vaginal delivery is performed, record the following:
- indication for the procedure
- course of labor
- anesthesia
- personnel present
- instruments used
- position and station of the fetal head
- force and duration of traction
- complications, including how they were recognized and managed
- immediate condition of the newborn and all steps taken in resuscitation.
Operative vaginal delivery is no newcomer to obstetrics. Hindu writings from about 1000 BC, and Hippocrates’ own musings from the fifth century BC, describe instruments and techniques to combat arrested labor and salvage the lives of both mother and child.27 Crude forceps were described by the Muslim physician Albucasis in the 11th century.27
Before the advent of safe cesarean section, many maternal lives were no doubt saved by these instruments and techniques. Unfortunately, destruction of the fetus and maternal death were frequent outcomes of operative vaginal delivery by forceps before the 20th century.28
As for vacuum extraction in particular, the idea of attaching a device to the fetal head to aid in delivery is credited to Arnett, a 19th century surgeon and inventor, who envisioned the “pneumatic tractor.”29
In 1957, Malmstrom reintroduced the vacuum as an aid in delivery, designing a rigid cup that was connected by rubber tubing to a vacuum source.30 This allowed the separation of the pump mechanism from the cup and made for easier application.
Most recently, Kobayashi developed the soft-cup design, a low-cost flexible plastic alternative that allows for a disposable instrument.31
Minimizing medicolegal risk
The best way to prevent an accusation of medical malpractice is to develop strong clinical and interpersonal skills. These simple, intuitive suggestions may help:
- Understand the role of operative vaginal delivery in current practice.
- Develop a simple and interactive discussion model for use in labor and delivery with the patient and her family.
- Consider a woman’s preferences for delivery.
- Know the indications and contraindications for vacuum extraction.
- Use the checks and safeguards listed under 3. Technique: Create conditions that ensure success.
- Perform vacuum extraction in the cesarean section room. Stop the procedure at once if any problem arises, and proceed to cesarean delivery.
- Make all chart notations completely legible, and add dictated notes.
If you are a new physician or lack significant experience with vacuum extraction, ask for input, supervision, and education from more experienced clinicians. Also make it a point to ask about department guidelines and review the credentialing process. Once you become adept at vacuum extraction, mentor more junior colleagues.
Two critical concerns
When contemplating vacuum-assisted delivery, 2 risks are paramount:
- failure of the vacuum extractor to achieve delivery
- the potential for fetal and maternal injury.
Training must ensure appropriate case selection and technique. Vacuum extraction must be performed with the same precision and care used with forceps. If application of the device is incorrect, or if there is a wrong direction of traction, excessive traction, or traction in the presence of disproportion, the cup will slip or pop off, and vacuum delivery will fail, with the potential for traumatic fetal injury.
All risks must be discussed with the patient to fulfill informed consent, and the risks and benefits of alternative treatments should be part of the discussion. Active participation, in considering how best to approach delivery, is required of all parties concerned.
The vacuum extractor can be a useful adjunct in certain circumstances, and its use has become widespread in American delivery suites. As with the obstetric forceps, which largely antedated its use, the vacuum extractor can lessen the overall risks of childbirth for both mother and infant.
The authors report no financial relationships relevant to this article.
CASE Three hours of pushing
C.A., age 29 years, is 40 weeks’ pregnant with her first child. After an unremarkable pregnancy, she arrives at the hospital for cervical ripening and induction of labor. Oxytocin is given, and labor progresses uneventfully. When C.A.’s cervix is dilated 8 cm, however, labor stalls. The physician orders placement of a pressure catheter and increases the dosage of oxytocin, and the cervix dilates fully. Although C.A. pushes well, the vertex descends only from +1 to +2 station (of 5 stations) after 3 hours.
How would you manage this delivery?
One option in C.A.’s case is operative vaginal delivery using the vacuum extractor, which has replaced the forceps as the most commonly used approach for operative vaginal delivery. Like the forceps, the vacuum extractor has vociferous detractors as well as supporters. Liberal use of cesarean section and questions regarding the safety of operative vaginal delivery vis-à-vis cesarean section have fueled the debate over its role in obstetric practice.
Among the benefits of vacuum extraction are its cost-effectiveness and shorter hospital stay (TABLE 1). It also obviates the need for cesarean section, including repeat cesarean. Risks include an increased incidence of genital tract trauma and a greater risk of fetal subgaleal hemorrhage.
We review 4 critical spheres of concern in regard to vacuum extraction:
- Patient selection
- Informed consent
- Technique
- Documentation
Increased understanding of these aspects of vacuum extraction will improve outcomes for the patient and limit medicolegal risk.
In the case of C.A., the physician offered 3 options:
- Continue maternal expulsive efforts to allow descent
- Attempt delivery by vacuum extraction
- Proceed to cesarean section on the basis of protracted descent.
Risks and benefits were reviewed with the patient, who chose to deliver by cesarean section. A 3,780-g infant in occiput posterior position was delivered safely.
TABLE 1
Delicate balance: Risks and benefits of operative vaginal delivery
| WHO? | BENEFIT | RISK |
|---|---|---|
| Mother | Cost-effective Less blood loss Lower risk of febrile morbidity Maternal preference No need for cesarean section or repeat cesarean Shorter hospitalization and convalescence | Increased incidence of genital tract trauma Possible damage to pelvic floor, with urinary and anal incontinence |
| Fetus | Fewer respiratory difficulties at birth | Increased risk of subgaleal hemorrhage Association with shoulder dystocia |
1. Patient selection: Maternal and fetal indications
Vacuum extraction may be justified for maternal or fetal indications.1,2 Maternal indications include prolongation or arrest of the second stage of labor, or the need to shorten the second stage, for reasons such as maternal cardiac disease, complex congenital cardiovascular disorders, and maternal exhaustion.
No definitive time limit for the second stage of labor
There is more flexibility today than in the past about what constitutes a “safe” length of the second stage. Recommendations concerning when the mother should begin pushing—and for how long—have evolved from a strict time limit to a focus on progression. If the fetal heart rate (FHR) tracing is reassuring, the second stage no longer needs to be limited to 2 or 3 hours. On the contrary, if the patient is still able and willing to push, changes in positioning and further expectant management remain acceptable in contemporary practice.3 Otherwise, a trial of vacuum extraction may be appropriate.
Vacuum extraction is particularly useful when the mother has difficulty pushing because of exhaustion and the fetal head has descended enough that it distends the labia between contractions, as in outlet deliveries.
Fetal indications
Fetal indications for operative vaginal delivery include distress, jeopardy, or a “nonreassuring” FHR tracing. Such a tracing may include late and prolonged decelerations, baseline bradycardia or tachycardia with or without variable decelerations, or, occasionally, a normal baseline rate with diminished variability.
Use vacuum or forceps?
The choice depends on which device would achieve delivery in the safest manner with the lowest risk of fetal injury. With the vacuum, force is exerted directly on the fetal scalp and only secondarily on the fetal skull. This puts fetal vessels that traverse the subgaleal space at risk for injury (FIGURE). With forceps, force is exerted directly on the fetal skull and mitigated by the petrous bone. Little or no force is exerted on the fetal scalp, lessening the risk of traumatic injury such as potentially fatal subgaleal hemorrhage.
Indications and contraindications for vacuum extraction are similar, but not identical, to those for forceps delivery (TABLE 2).2,3 The most important determinant for either device is the experience of the operator. You must be familiar with the instrument and technique before making any attempt to assist delivery. An inability to accurately assess fetal position or station, fetopelvic proportion, adequacy of labor, engagement of the fetal head, or any degree of malpresentation (including minor degrees of deflexion) is a contraindication to a trial of operative vaginal delivery.
Vacuum extraction should be reserved for fetuses at more than 34 weeks’ gestation because of the increased risk of intracranial hemorrhage associated with prematurity.
All decisions involving vacuum extraction should be made with caution. The adequacy of the pelvis, estimated fetal size, and any suggestions of fetopelvic disproportion are of particular significance.3
FIGURE
Subgaleal hemorrhage, a deadly complication
Blood can accumulate in a large potential space between the galea aponeurotica and the periosteum of the cranial bones after vacuum extraction. An infant with subgaleal hemorrhage will exhibit a boggy scalp, with swelling that crosses the suture lines and expands head circumferenceTABLE 2
Factors that predict success—or failure—of vacuum extraction
| When a woman fits overlapping categories, the decision to use vacuum extraction—or not—may be a judgment call* |
| GOOD CANDIDACY |
| Multiparous |
| Term pregnancy |
| Occiput anterior position, well-flexed |
| Wide subpubic arch |
| Compliant |
| MARGINAL CANDIDACY |
| Primiparous |
| Post-term |
| Occiput posterior position |
| Average subpubic arch |
| Gestational diabetes |
| Arrest disorders in second stage |
| POOR CANDIDACY |
| Protraction disorders in second stage |
| Narrow subpubic arch |
| Uncertain position of fetal head |
| Deflexion or asynclitism |
| Anticipated large-for-gestational-age infant |
| Poor maternal compliance |
| * When faced with a good indication in a marginal candidate, we recommend delivery in a “double setup” situation in which preparations are made for both vacuum extraction and cesarean section. If the vacuum can be properly applied, the first application of traction is crucial. We will only proceed if significant descent is achieved. If the fetal head (not the scalp) can be advanced a full station, then we proceed cautiously. If not, ready access to cesarean section allows for completion of the delivery in a timely manner. |
2. Informed consent: Elicit the patient’s desires
Thorough discussion with the patient and her family—to explain the reasoning behind the clinical decision to use the vacuum extractor and delineate the alternatives—is paramount. Moreover, the patient should be encouraged to actively participate in this discussion.
Among the alternatives to vacuum extraction are expectant observation and expedited delivery by cesarean section. Because patients increasingly are requesting elective cesarean section in the absence of obvious obstetric indications, this option should receive extra attention.
Most women still consider vaginal delivery an important milestone of female adulthood. When safety concerns arise and the situation makes vaginal delivery unwise, many women experience disappointment and postpartum depression over their “failed” attempt at vaginal delivery. These perceptions need to be addressed in discussions with the patient.
The risk–benefit equation
Vacuum extraction lessens the risk of maternal lacerations, either of the lower genital tract in the case of obstetric forceps, or of the cervix and lower uterine segment in the case of cesarean section. In addition, vacuum extraction can be performed comfortably in the absence of regional anesthesia.
Avoiding cesarean section can produce multiple benefits
Another maternal benefit of vacuum extraction is the decreased need for cesarean section. A reduction in the primary cesarean rate also lowers the need for repeat cesarean section, which can be more technically challenging than primary C-section due to the presence of dense scar tissue and intra-abdominal adhesions. Cesarean section also increases the risk of placenta accreta, increta, or percreta in subsequent pregnancies. These complications increase the likelihood of emergency hysterectomy, massive blood loss, and serious maternal morbidity and mortality.
Even in the absence of placenta accreta, both primary and repeat cesarean sections raise the risk of hemorrhage and febrile morbidity, prolong convalescence, and increase cost, compared with vaginal delivery. For these reasons, avoiding primary cesarean section can obviate the need for multiple surgical procedures and their attendant risks. The degree to which these factors favor vaginal delivery over cesarean section is subject to debate.
Maternal risks include pelvic floor trauma
Both vacuum extraction and forceps delivery increase the risk of anal sphincter injury and can impair fecal continence.4 Both methods also appear to increase trauma to the genital tract in comparison with spontaneous delivery and may predispose the woman to pelvic floor dysfunction, including urinary and anal incontinence.5-10 However, anal sphincter trauma was less frequent after vacuum extraction than after forceps delivery.1
Other maternal injuries associated with vacuum extraction include perineal lacerations and injuries to the vulva, vagina, and cervix. Vacuum extraction also has been implicated as a significant risk factor for postpartum hemorrhage11 and genital-tract infection.1
Fewer neonatal respiratory problems with vaginal delivery
Compared with cesarean section, vaginal delivery is thought to diminish the risk of intrapartum aspiration and respiratory problems in the newborn. It also may facilitate the transition from fetal to neonatal circulation and reduce the need for immediate resuscitation at birth.
Neonatal risks include soft-tissue injury and potential hemorrhage
Infants delivered by vacuum extraction have a significantly higher rate of intracranial hemorrhage, brachial plexus injuries, convulsions, central nervous system depression, and the need for mechanical ventilation, compared with spontaneously delivered infants (TABLE 3).12,13
Although vacuum extraction is associated with a wide range of soft tissue injuries, they are often less serious than the fetal scalp injuries associated with obstetric forceps. Cup marks, bruising, and minor lacerations of the scalp and caput succedaneum are common fetal injuries, although the majority resolve without apparent sequelae.14
Subgaleal hemorrhage is the most serious neonatal complication of vacuum extraction, occurring in 1% to 3.8% of vacuum extractions (FIGURE).15 It coexists with neonatal coagulopathy in 19% to 29% of newborns16 and increases the risk of progression to hemorrhagic shock and death. Subgaleal hemorrhage has a mortality rate ranging from 2.7% to 22.8%.15-17
Cephalhematoma is another complication associated with vacuum extraction. It involves an accumulation of blood beneath the periosteum of a cranial bone (usually the parietal bone), and it almost always resolves spontaneously. The incidence of cephalhematoma varies. It is significantly more common in deliveries involving vacuum extraction (9.8%) than in forceps deliveries (4.1%).18 Its incidence increases with the length of time the vacuum cup is applied and with paramedian application.18
Intracranial hemorrhage occurs in 1 of 860 vacuum extractions, 1 of 664 forceps deliveries, 1 of 954 cesarean deliveries, and 1 of 1,900 spontaneous deliveries.12 Subdural hemorrhage is the most common form of intracranial hemorrhage and is almost invariably the result of birth trauma. However, asymptomatic subdural hematoma occurs in up to 6.1% of uncomplicated vaginal deliveries.19
Other, less common types of intracranial hemorrhage, such as subarachnoid, intraventricular, and intraparenchymal hemorrhage, have a more complex etiology, which includes birth asphyxia, hemorrhagic diathesis, infection, and vascular abnormalities.20
Retinal hemorrhage also may occur after vacuum extraction, with an incidence of 49% to 77.8%, compared with 30.3% after forceps delivery, 30.4% after normal vaginal delivery, and 8.3% after cesarean delivery.21 It generally resolves spontaneously without any permanent damage.22
TABLE 3
Vacuum extraction can injure the fetus
| DIRECT INJURY |
| Cephalhematoma |
| Intracranial hemorrhage (parenchymal, subdural, intraventricular, subarachnoid) |
| Nerve injury |
| Scalp laceration, abrasion, ecchymoses, necrosis |
| Skull fracture |
| Subgaleal hemorrhage |
| INDIRECT INJURY |
| Anemia, hyperbilirubinemia |
| Brachial plexus injury |
| Scalp infection or abscess |
| SOURCE: O’Grady et al31 |
Shoulder dystocia and brachial plexus palsy
Vacuum extraction also is associated with shoulder dystocia and brachial plexus palsy, although the primary risk factor for these complications is thought to be increased fetal size.23-25 The incidence of shoulder dystocia with vacuum extraction is 3.5%, compared with 1.5% for forceps delivery.25
The risk of brachial plexus palsy also increases with vacuum extraction, especially as the duration of the procedure increases.25
Less common complications associated with vacuum extraction are skull fractures, fetal hemorrhage from bleeding at the site of scalp electrodes, sepsis originating from infected scalp trauma, and corneal injury.
No long-term impairment
Long-term outcome studies of children delivered by vacuum extraction show no differences in physical or cognitive functioning or intelligence scores, compared with other modes of delivery.26
3. Technique: Create conditions that ensure success
Certain prerequisites to vacuum extraction can assure successful application and strict adherence to protocol. These prerequisites include having an appropriate indication, thorough informed consent, proper maternal positioning, adequate anesthesia, and knowledge of fetal position and station (TABLE 4).1 These objectives can be accomplished in the following steps:
- After an informed consent discussion, assess maternal positioning and repeat the pelvic exam. Also ascertain the adequacy of anesthesia. Insert a bladder catheter.
- Perform a “ghost” trial of vacuum extraction to visualize the procedure before the actual attempt.
- Test the function of the vacuum.
- Lubricate the vacuum cup with surgical soap or gel, insert it into the vagina, and maneuver it onto the fetal head. Place the vacuum extractor over the sagittal suture about 6 cm distal to the anterior fontanel and 2 cm proximal to the posterior fontanel. (The illustration on page 74 demonstrates positioning.) Apply a small degree of vacuum (approximately 20 mm Hg). Double-check application.
- Gradually apply full vacuum (550–600 mm Hg, depending on cup size), allowing the scalp to mold to the extractor cup.
- Apply 2-handed traction in concert with uterine contractions and supplemented by maternal pushing. Assuming there is no loss of vacuum (“pop-off” of the cup), the initial traction effort should produce a gain in station. If a “pop-off” occurs, a single additional attempt at delivery may be warranted.
- As the head crowns, perform episiotomy as needed and slowly deliver the fetal head. Remove the vacuum cup.
- After delivery of the placenta, inspect the vagina, cervix, and perineum closely.
- Dictate a full operative note and annotate the delivery in the chart. See the section on documentation, below.
Vacuum extraction may fail for a number of reasons (TABLE 5).
TABLE 4
Perform these predelivery checks before applying traction
| Is anesthesia adequate? Is maternal positioning correct? |
| Is the bladder empty? |
| Is the fetus in the proper attitude (flexion)? |
| Is fetal status reassuring? |
Is the vacuum properly applied?
|
| Has the patient been instructed on when and how long to push? |
| Are the proposed maneuvers appropriate? |
TABLE 5
Why might vacuum extraction fail?
| INSTRUMENT-RELATED |
| Pump failure |
| Vacuum leak |
| TECHNIQUE-RELATED |
| Failure to encourage maternal valsalva with traction efforts |
| Inappropriate intensity of traction |
| Incorrect axis of traction |
| Maternal tissue trapped beneath vacuum cup |
| Poor cup position |
| OBSTETRIC CONDITIONS |
Congenital anomaly
|
| Fetal macrosomia |
| Incomplete cervical dilation |
Position and attitude problems
|
| Unappreciated cephalopelvic disproportion |
| SOURCE: Modified from Plauche et al32 |
Most important variable: Cup placement
The single most critical step in vacuum extraction is placement of the cup. It should be applied at the point of maximum fetal cranial flexion, which is proximal to the leading edge of the posterior fontanel.
Once full vacuum is achieved, encourage the mother to push with the next contraction, and apply steady traction in concert with her efforts.
The initial application of traction should be directed to maintain proper flexion of the fetal head, and should bring about descent of the fetal head. If there is no descent with the first application of traction, and correct technique and cup placement have been applied, abandon operative vaginal delivery (TABLE 6).
Do not make a further attempt to deliver the child using forceps, as the risk of intracranial hemorrhage appears to be highest in infants delivered using a combination of vacuum extraction and forceps.
TABLE 6
Repeat traction efforts reap a diminishing return
| NUMBER OF TRACTION EFFORTS | SUCCESS RATE | |
|---|---|---|
| VACUUM EXTRACTION (N=433) | FORCEPS (N=555) | |
| 1 or 2 | 68.4% | 38.4% |
| 3 or 4 | 24.9% | 48.6% |
| 5 or more | 6.7% | 12.9% |
| Adapted from Sjostedt33 | ||
4. Documentation: The chart is the most important witness
The value of complete and contemporaneous notation cannot be overstated. The patient’s chart is the permanent repository of the record of delivery. It is without doubt the most important witness to the event and should be treated as such. Include a dictated operative note as well as notation in the chart itself. Notes should be legible and properly dated, with the time of day indicated.
When operative vaginal delivery is performed, record the following:
- indication for the procedure
- course of labor
- anesthesia
- personnel present
- instruments used
- position and station of the fetal head
- force and duration of traction
- complications, including how they were recognized and managed
- immediate condition of the newborn and all steps taken in resuscitation.
Operative vaginal delivery is no newcomer to obstetrics. Hindu writings from about 1000 BC, and Hippocrates’ own musings from the fifth century BC, describe instruments and techniques to combat arrested labor and salvage the lives of both mother and child.27 Crude forceps were described by the Muslim physician Albucasis in the 11th century.27
Before the advent of safe cesarean section, many maternal lives were no doubt saved by these instruments and techniques. Unfortunately, destruction of the fetus and maternal death were frequent outcomes of operative vaginal delivery by forceps before the 20th century.28
As for vacuum extraction in particular, the idea of attaching a device to the fetal head to aid in delivery is credited to Arnett, a 19th century surgeon and inventor, who envisioned the “pneumatic tractor.”29
In 1957, Malmstrom reintroduced the vacuum as an aid in delivery, designing a rigid cup that was connected by rubber tubing to a vacuum source.30 This allowed the separation of the pump mechanism from the cup and made for easier application.
Most recently, Kobayashi developed the soft-cup design, a low-cost flexible plastic alternative that allows for a disposable instrument.31
Minimizing medicolegal risk
The best way to prevent an accusation of medical malpractice is to develop strong clinical and interpersonal skills. These simple, intuitive suggestions may help:
- Understand the role of operative vaginal delivery in current practice.
- Develop a simple and interactive discussion model for use in labor and delivery with the patient and her family.
- Consider a woman’s preferences for delivery.
- Know the indications and contraindications for vacuum extraction.
- Use the checks and safeguards listed under 3. Technique: Create conditions that ensure success.
- Perform vacuum extraction in the cesarean section room. Stop the procedure at once if any problem arises, and proceed to cesarean delivery.
- Make all chart notations completely legible, and add dictated notes.
If you are a new physician or lack significant experience with vacuum extraction, ask for input, supervision, and education from more experienced clinicians. Also make it a point to ask about department guidelines and review the credentialing process. Once you become adept at vacuum extraction, mentor more junior colleagues.
Two critical concerns
When contemplating vacuum-assisted delivery, 2 risks are paramount:
- failure of the vacuum extractor to achieve delivery
- the potential for fetal and maternal injury.
Training must ensure appropriate case selection and technique. Vacuum extraction must be performed with the same precision and care used with forceps. If application of the device is incorrect, or if there is a wrong direction of traction, excessive traction, or traction in the presence of disproportion, the cup will slip or pop off, and vacuum delivery will fail, with the potential for traumatic fetal injury.
All risks must be discussed with the patient to fulfill informed consent, and the risks and benefits of alternative treatments should be part of the discussion. Active participation, in considering how best to approach delivery, is required of all parties concerned.
The vacuum extractor can be a useful adjunct in certain circumstances, and its use has become widespread in American delivery suites. As with the obstetric forceps, which largely antedated its use, the vacuum extractor can lessen the overall risks of childbirth for both mother and infant.
The authors report no financial relationships relevant to this article.
1. O’Grady JP, Gimovsky ML, McIlhargie CJ, eds. Operative Obstetrics. Pearl River, NY: Parthenon Publishing; 1995.
2. Johanson RB, Menon BKV. Vacuum extraction versus forceps for assisted vaginal delivery. Cochrane Database Syst Rev. 2000;(2):CD000224.-
3. Bloom SL, Casey BM, Schaffer JL, et al. Pushing in the second stage of labor. Am J Obstet Gynecol 2006;194:10-13.
4. Operative vaginal delivery. ACOG Practice Bulletin #17. Washington, DC: American College of Obstetricians and Gynecologists; June 2000.
5. Power D, Fitzpatrick M, O’Herlihy C. Obstetric anal sphincter injury: how to avoid, how to repair: a literature review. J Fam Pract 2006;55:193-200.
6. Chaliha C, Kalia V, Stanton S, et al. Antenatal prediction of postpartum urinary and fecal incontinence. Obstet Gynecol 1999;94:689-694.
7. Bofill JA, Rust OA, Schorr SJ, et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. Am J Obstet Gynecol 1996;175:1325-1330.
8. Salamalekis E, Loghis C, Pyrgiotis E, et al. Soft cup vacuum extractor versus forceps delivery. J Obstet Gynecol. 1995;15:245-246.
9. Zetterstrom JP, Lopez A, Anzen B, et al. Anal incontinence after vaginal delivery: a prospective study in primiparous women. Br J Obstet Gynaecol. 1999;106:324-330.
10. Johanson RB, Heycock E, Carter J, et al. Maternal and child health after assisted vaginal delivery: five-year follow up of a randomized controlled study comparing forceps and ventouse. Br J Obstet Gynaecol. 1999;106:544-549
11. Faltin DL, Otero M, Petignat P, et al. Women’s health 18 years after rupture of the anal sphincter during childbirth: I. Fecal incontinence. Am J Obstet Gynecol. 2006;194:1255-1259.
12. Plauche WC. Fetal cranial injuries related to delivery with the Malmsträm vacuum extractor. Obstet Gynecol. 1979;53:750-757.
13. Towner D, Castro MA, Eby-Wilkens E, et al. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
14. Sheiner E, Sarid L, Levy A, et al. Obstetric risk factors and outcome of pregnancies complicated with early postpartum hemorrhage: a population-based study. J Matern Fetal Neonatal Med. 2005;18:149-154.
15. Johanson R. Choice of instrument for vaginal delivery. Curr Opin Obstet Gynecol. 1997;9:361-365.
16. Chadwick LM, Pemberton PJ, Kurinczuk JJ. Neonatal subgaleal hematoma: associated risk factors, complications, and outcome. J Pediatr Child Health. 1996;32:228-232.
17. Ng PC, Siu YK, Lewindon PJ. Subaponeurotic hemorrhage in the 1990s: a 3-year surveillance. Acta Pediatr. 1995;84:1065-1069
18. Bofill JA, Rust OA, Devidas M, et al. Neonatal cephalohematoma from vacuum extraction. J Reprod Med. 1997;42:565-569.
19. Doumouchtsis SK, Arulkumaran S. Head injuries after instrumental vaginal deliveries. Curr Opin Obstet Gynecol. 2006;18:129-134.
20. Govaert P. Cranial Hemorrhage in the Term Newborn Infant. London: Mac Keith Press; 1993.
21. Hughes LA, May K, Talbot JF, Parsons MA. Incidence, distribution, and duration of birth-related retinal hemorrhages: a prospective study. J AAPOS. 2006;10:102-106.
22. Sheiner E, Levy A, Hershkovitz R, et al. Determining factors associated with shoulder dystocia: a population-based study. Eur J Obstet Gynecol Reprod Biol. 2006;126:11-15.
23. Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:15-18.
24. Mollberg M, Hagerg H, Bager B, et al. Risk factors for obstetric brachial plexus palsy among neonates delivered by vacuum extraction. Obstet Gynecol. 2005;106:913-918.
25. Caughey AB, Sandberg PL, Alantnik MG, et al. Forceps compared with vacuum: rates of neonatal and maternal morbidity. Obstet Gynecol. 2005;106:908-912.
26. Ngan HYS, Miu P, Ko L, et al. Long-term neurological sequelae following vacuum extractor delivery. Aust NZ J Obstet Gynecol. 1990;30:111-114.
27. Lyons AS, Petrucelli RJ. Medicine: An Illustrated History. New York: Harry N Abrams; 1978.
28. Speert H. Obstetric and Gynecologic Milestones Illustrated. Pearl River, NY: Parthenon Publishing; 1996.
29. Arnett N. Elements of Physics or Natural Philosophy, General and Medical, Explained Independently of Technical Mathematics and Containing New Disquisitions and Practical Suggestions. 2nd ed. Philadelphia: Carney and Lea; 1831.
30. Malmstrom T. The vacuum extractor, an obstetrical instrument. I. Acta Obstet Gynecol Scand. 1957;36(suppl 3):5-50.
31. O’Grady JP, Gimovsky ML, McIlhargie CJ. Vacuum Extraction in Modern Obstetric Practice. Pearl River, NY: Parthenon Publishing; 1995.
32. Plauche WC, Morrison JC, O’Sullivan MJ. Surgical Obstetrics. Philadelphia: WB Saunders; 1992.
33. Sjostedt JE. The vacuum extractor and forceps in obstetrics: a clinical study. Acta Obstet Gynecol Scand. 1967;48:638-639.
1. O’Grady JP, Gimovsky ML, McIlhargie CJ, eds. Operative Obstetrics. Pearl River, NY: Parthenon Publishing; 1995.
2. Johanson RB, Menon BKV. Vacuum extraction versus forceps for assisted vaginal delivery. Cochrane Database Syst Rev. 2000;(2):CD000224.-
3. Bloom SL, Casey BM, Schaffer JL, et al. Pushing in the second stage of labor. Am J Obstet Gynecol 2006;194:10-13.
4. Operative vaginal delivery. ACOG Practice Bulletin #17. Washington, DC: American College of Obstetricians and Gynecologists; June 2000.
5. Power D, Fitzpatrick M, O’Herlihy C. Obstetric anal sphincter injury: how to avoid, how to repair: a literature review. J Fam Pract 2006;55:193-200.
6. Chaliha C, Kalia V, Stanton S, et al. Antenatal prediction of postpartum urinary and fecal incontinence. Obstet Gynecol 1999;94:689-694.
7. Bofill JA, Rust OA, Schorr SJ, et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. Am J Obstet Gynecol 1996;175:1325-1330.
8. Salamalekis E, Loghis C, Pyrgiotis E, et al. Soft cup vacuum extractor versus forceps delivery. J Obstet Gynecol. 1995;15:245-246.
9. Zetterstrom JP, Lopez A, Anzen B, et al. Anal incontinence after vaginal delivery: a prospective study in primiparous women. Br J Obstet Gynaecol. 1999;106:324-330.
10. Johanson RB, Heycock E, Carter J, et al. Maternal and child health after assisted vaginal delivery: five-year follow up of a randomized controlled study comparing forceps and ventouse. Br J Obstet Gynaecol. 1999;106:544-549
11. Faltin DL, Otero M, Petignat P, et al. Women’s health 18 years after rupture of the anal sphincter during childbirth: I. Fecal incontinence. Am J Obstet Gynecol. 2006;194:1255-1259.
12. Plauche WC. Fetal cranial injuries related to delivery with the Malmsträm vacuum extractor. Obstet Gynecol. 1979;53:750-757.
13. Towner D, Castro MA, Eby-Wilkens E, et al. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709-1714.
14. Sheiner E, Sarid L, Levy A, et al. Obstetric risk factors and outcome of pregnancies complicated with early postpartum hemorrhage: a population-based study. J Matern Fetal Neonatal Med. 2005;18:149-154.
15. Johanson R. Choice of instrument for vaginal delivery. Curr Opin Obstet Gynecol. 1997;9:361-365.
16. Chadwick LM, Pemberton PJ, Kurinczuk JJ. Neonatal subgaleal hematoma: associated risk factors, complications, and outcome. J Pediatr Child Health. 1996;32:228-232.
17. Ng PC, Siu YK, Lewindon PJ. Subaponeurotic hemorrhage in the 1990s: a 3-year surveillance. Acta Pediatr. 1995;84:1065-1069
18. Bofill JA, Rust OA, Devidas M, et al. Neonatal cephalohematoma from vacuum extraction. J Reprod Med. 1997;42:565-569.
19. Doumouchtsis SK, Arulkumaran S. Head injuries after instrumental vaginal deliveries. Curr Opin Obstet Gynecol. 2006;18:129-134.
20. Govaert P. Cranial Hemorrhage in the Term Newborn Infant. London: Mac Keith Press; 1993.
21. Hughes LA, May K, Talbot JF, Parsons MA. Incidence, distribution, and duration of birth-related retinal hemorrhages: a prospective study. J AAPOS. 2006;10:102-106.
22. Sheiner E, Levy A, Hershkovitz R, et al. Determining factors associated with shoulder dystocia: a population-based study. Eur J Obstet Gynecol Reprod Biol. 2006;126:11-15.
23. Baskett TF, Allen AC. Perinatal implications of shoulder dystocia. Obstet Gynecol. 1995;86:15-18.
24. Mollberg M, Hagerg H, Bager B, et al. Risk factors for obstetric brachial plexus palsy among neonates delivered by vacuum extraction. Obstet Gynecol. 2005;106:913-918.
25. Caughey AB, Sandberg PL, Alantnik MG, et al. Forceps compared with vacuum: rates of neonatal and maternal morbidity. Obstet Gynecol. 2005;106:908-912.
26. Ngan HYS, Miu P, Ko L, et al. Long-term neurological sequelae following vacuum extractor delivery. Aust NZ J Obstet Gynecol. 1990;30:111-114.
27. Lyons AS, Petrucelli RJ. Medicine: An Illustrated History. New York: Harry N Abrams; 1978.
28. Speert H. Obstetric and Gynecologic Milestones Illustrated. Pearl River, NY: Parthenon Publishing; 1996.
29. Arnett N. Elements of Physics or Natural Philosophy, General and Medical, Explained Independently of Technical Mathematics and Containing New Disquisitions and Practical Suggestions. 2nd ed. Philadelphia: Carney and Lea; 1831.
30. Malmstrom T. The vacuum extractor, an obstetrical instrument. I. Acta Obstet Gynecol Scand. 1957;36(suppl 3):5-50.
31. O’Grady JP, Gimovsky ML, McIlhargie CJ. Vacuum Extraction in Modern Obstetric Practice. Pearl River, NY: Parthenon Publishing; 1995.
32. Plauche WC, Morrison JC, O’Sullivan MJ. Surgical Obstetrics. Philadelphia: WB Saunders; 1992.
33. Sjostedt JE. The vacuum extractor and forceps in obstetrics: a clinical study. Acta Obstet Gynecol Scand. 1967;48:638-639.
Radiologic Work By Gravidas Tied To Lymphoma
LOS ANGELES — Radiologic technicians who work during pregnancy have twice the risk of having a child who develops lymphoma than those who do not work during pregnancy, according to a poster presentation by Kimberly J. Johnson, at the annual meeting of the American Association for Cancer Research.
On the other hand, working as a radiologic technician during pregnancy did not significantly increase the risk of leukemia or solid tumors among the offspring, Ms. Johnson of the department of pediatrics, division of epidemiology/clinical research, at the University of Minnesota, Minneapolis, and her colleagues wrote.
The study used 63 years' worth of self-reported data involving 81,354 offspring of 38,239 female members of the U.S. Radiologic Technologists cohort. During that time, 230 of their offspring developed leukemia, lymphoma, or solid tumors before the age of 19.
A radiologic technician was considered to have worked during pregnancy if she reported having worked during the child's birth year as well as the prior year.
After adjusting for maternal age and birth year in a multivariate analysis, the investigators found no significant changes in the hazard ratio for leukemia or for solid tumors, but the hazard ratio for lymphoma was 1.99.
To account for occupational radiation exposures that differed by a work era, the investigators separated the data for children born between 1921 and 1959 from those born between 1960 and 1984. The investigators noted a significant increase in the risk of lymphoma only for those children born during the later era.
The investigators are trying to determine whether there is a significant relationship between the estimated dose of radiation received during pregnancy and cancers among the offspring.
The study was supported by the National Cancer Institute, the University of Minnesota, and the American Registry of Radiologic Technologists.
LOS ANGELES — Radiologic technicians who work during pregnancy have twice the risk of having a child who develops lymphoma than those who do not work during pregnancy, according to a poster presentation by Kimberly J. Johnson, at the annual meeting of the American Association for Cancer Research.
On the other hand, working as a radiologic technician during pregnancy did not significantly increase the risk of leukemia or solid tumors among the offspring, Ms. Johnson of the department of pediatrics, division of epidemiology/clinical research, at the University of Minnesota, Minneapolis, and her colleagues wrote.
The study used 63 years' worth of self-reported data involving 81,354 offspring of 38,239 female members of the U.S. Radiologic Technologists cohort. During that time, 230 of their offspring developed leukemia, lymphoma, or solid tumors before the age of 19.
A radiologic technician was considered to have worked during pregnancy if she reported having worked during the child's birth year as well as the prior year.
After adjusting for maternal age and birth year in a multivariate analysis, the investigators found no significant changes in the hazard ratio for leukemia or for solid tumors, but the hazard ratio for lymphoma was 1.99.
To account for occupational radiation exposures that differed by a work era, the investigators separated the data for children born between 1921 and 1959 from those born between 1960 and 1984. The investigators noted a significant increase in the risk of lymphoma only for those children born during the later era.
The investigators are trying to determine whether there is a significant relationship between the estimated dose of radiation received during pregnancy and cancers among the offspring.
The study was supported by the National Cancer Institute, the University of Minnesota, and the American Registry of Radiologic Technologists.
LOS ANGELES — Radiologic technicians who work during pregnancy have twice the risk of having a child who develops lymphoma than those who do not work during pregnancy, according to a poster presentation by Kimberly J. Johnson, at the annual meeting of the American Association for Cancer Research.
On the other hand, working as a radiologic technician during pregnancy did not significantly increase the risk of leukemia or solid tumors among the offspring, Ms. Johnson of the department of pediatrics, division of epidemiology/clinical research, at the University of Minnesota, Minneapolis, and her colleagues wrote.
The study used 63 years' worth of self-reported data involving 81,354 offspring of 38,239 female members of the U.S. Radiologic Technologists cohort. During that time, 230 of their offspring developed leukemia, lymphoma, or solid tumors before the age of 19.
A radiologic technician was considered to have worked during pregnancy if she reported having worked during the child's birth year as well as the prior year.
After adjusting for maternal age and birth year in a multivariate analysis, the investigators found no significant changes in the hazard ratio for leukemia or for solid tumors, but the hazard ratio for lymphoma was 1.99.
To account for occupational radiation exposures that differed by a work era, the investigators separated the data for children born between 1921 and 1959 from those born between 1960 and 1984. The investigators noted a significant increase in the risk of lymphoma only for those children born during the later era.
The investigators are trying to determine whether there is a significant relationship between the estimated dose of radiation received during pregnancy and cancers among the offspring.
The study was supported by the National Cancer Institute, the University of Minnesota, and the American Registry of Radiologic Technologists.
Race Drives Peripartum Cardiomyopathy Outcomes
NEW ORLEANS — Full recovery of left ventricular function is significantly less likely in black patients than in white patients with peripartum cardiomyopathy, Dr. Sorel Goland reported at the annual meeting of the American College of Cardiology.
There are other intriguing racial differences in the clinical profiles of patients with peripartum cardiomyopathy (PPCM).
Black patients are significantly younger at diagnosis, more likely to have gestational hypertension, and less likely to present with symptoms prior to delivery, and they tend to have worse outcomes, according to Dr. Goland of the University of Southern California, Los Angeles.
She presented a retrospective study involving 52 black and 104 white women with PPCM.
Baseline left ventricular ejection fraction averaged 28% in both groups, with half of all patients having an ejection fraction of 25% or less.
But only 18% of black patients experienced complete recovery of left ventricular function as defined by an ejection fraction of at least 50%, compared with 61% of white patients.
Mean age of the black women at diagnosis of peripartum cardiomyopathy was 26 years, compared with 30 years in white patients.
Two-thirds of black patients had gestational hypertension, as did 46% of white patients.
More than 80% of black women had PPCM symptoms after delivery, compared with half of white women.
Black patients also had a significantly greater mean left ventricular end diastolic diameter, both at the time of diagnosis and at last follow-up, an average of roughly 2 years later.
The combined end point of death or cardiac transplantation occurred in 31% of black patients, a significantly higher rate than was seen in white patients, Dr. Goland reported.
NEW ORLEANS — Full recovery of left ventricular function is significantly less likely in black patients than in white patients with peripartum cardiomyopathy, Dr. Sorel Goland reported at the annual meeting of the American College of Cardiology.
There are other intriguing racial differences in the clinical profiles of patients with peripartum cardiomyopathy (PPCM).
Black patients are significantly younger at diagnosis, more likely to have gestational hypertension, and less likely to present with symptoms prior to delivery, and they tend to have worse outcomes, according to Dr. Goland of the University of Southern California, Los Angeles.
She presented a retrospective study involving 52 black and 104 white women with PPCM.
Baseline left ventricular ejection fraction averaged 28% in both groups, with half of all patients having an ejection fraction of 25% or less.
But only 18% of black patients experienced complete recovery of left ventricular function as defined by an ejection fraction of at least 50%, compared with 61% of white patients.
Mean age of the black women at diagnosis of peripartum cardiomyopathy was 26 years, compared with 30 years in white patients.
Two-thirds of black patients had gestational hypertension, as did 46% of white patients.
More than 80% of black women had PPCM symptoms after delivery, compared with half of white women.
Black patients also had a significantly greater mean left ventricular end diastolic diameter, both at the time of diagnosis and at last follow-up, an average of roughly 2 years later.
The combined end point of death or cardiac transplantation occurred in 31% of black patients, a significantly higher rate than was seen in white patients, Dr. Goland reported.
NEW ORLEANS — Full recovery of left ventricular function is significantly less likely in black patients than in white patients with peripartum cardiomyopathy, Dr. Sorel Goland reported at the annual meeting of the American College of Cardiology.
There are other intriguing racial differences in the clinical profiles of patients with peripartum cardiomyopathy (PPCM).
Black patients are significantly younger at diagnosis, more likely to have gestational hypertension, and less likely to present with symptoms prior to delivery, and they tend to have worse outcomes, according to Dr. Goland of the University of Southern California, Los Angeles.
She presented a retrospective study involving 52 black and 104 white women with PPCM.
Baseline left ventricular ejection fraction averaged 28% in both groups, with half of all patients having an ejection fraction of 25% or less.
But only 18% of black patients experienced complete recovery of left ventricular function as defined by an ejection fraction of at least 50%, compared with 61% of white patients.
Mean age of the black women at diagnosis of peripartum cardiomyopathy was 26 years, compared with 30 years in white patients.
Two-thirds of black patients had gestational hypertension, as did 46% of white patients.
More than 80% of black women had PPCM symptoms after delivery, compared with half of white women.
Black patients also had a significantly greater mean left ventricular end diastolic diameter, both at the time of diagnosis and at last follow-up, an average of roughly 2 years later.
The combined end point of death or cardiac transplantation occurred in 31% of black patients, a significantly higher rate than was seen in white patients, Dr. Goland reported.
PPCM Mortality Unusually Low in Kaiser Study
NEW ORLEANS — Women with peripartum cardiomyopathy in a contemporary Southern California series had a markedly lower mortality than in previously reported studies, Dr. Somjot S. Brar said at the annual scientific session of the American College of Cardiology.
The overall incidence of peripartum cardiomyopathy (PPCM) was one case per 4,025 live births in this study of nearly 250,000 deliveries in the Southern California Kaiser Permanente health care system in 2000–2005. But there was a substantial racial variation.
The incidence of PPCM was highest in black women, at one case per 1,421 live births. This rate was 2.9-fold greater than in whites, 7-fold greater than in Hispanics, and twice that in Asians, according to Dr. Brar of Kaiser Permanente Medical Center, Los Angeles.
This observed marked racial variation in the incidence of PPMC may explain at least in part the wide range of rates reported in the literature, he added.
PPCM is a rare disorder of unclear etiology. The diagnosis is made in patients who experience left ventricular dysfunction with a documented ejection fraction of less than 50%, onset during the final month before or the first 5 months after delivery, and in whom no alternative cause for heart failure can be found.
The diagnostic requirement that systolic dysfunction be present prevents PPCM from being confused with preeclampsia, which is marked by diastolic dysfunction. Patients with PPCM commonly present with arrhythmias or thromboembolism rather than with classic heart failure symptoms.
In the Kaiser study, all of the nearly quarter-million women were initially screened using ICD-9 codes for hospitalization for heart failure from 6 months before to 12 months after delivery. Patients flagged in this way then underwent detailed chart review, which resulted in the diagnosis of PPCM in 60 cases.
No cardiac transplantations and only two deaths occurred in the PPCM group, for an all-cause mortality of 3.3% during an average follow-up of 4.7 years. One death occurred 5 months after delivery in a woman with complications of deep vein thrombosis, while the other was caused by respiratory failure more than 4 years after delivery in an asthmatic patient whose left ventricular function had recovered long before.
Other investigators have reported mortality rates as high as 50% in women with PPCM, with half of the deaths occurring within the first 3 months after delivery. Dr. Brar offered several potential explanations for the low rate in the Kaiser series. One is that many of the prior reports date from an era before routine use of modern evidence-based heart failure medications such as ACE inhibitors and β-blockers.
Another possibility is that earlier reports were biased by preferential detection of more advanced cases of PPCM. “Our process of screening by ICD-9 codes with individual chart review of all potential cases may have allowed for identification of less-sick patients,” he said.
Yet another potential explanation for the disparate results is that the improved outcomes in the Kaiser study stemmed from the selective nature of the prepaid health plan membership, in which the very poor and very rich are underrepresented.
Dr. Brar said this initial report is just the first phase of an ongoing Kaiser study. Now that he and his coinvestigators have reported on PPCM incidence and survival, they are focusing next on identification of risk factors and attempting to pin down the optimal duration of medical management.
Session Cochair Dr. Peter Liu of the University of Toronto noted that a number of risk factors for PPCM are already known. These include preeclampsia, black race, and advanced age at first pregnancy. There has also been speculation that PPCM might be a form of myocarditis.
'Our process of screening by ICD-9 codes … may have allowed for identification of less-sick patients.' DR. BRAR
NEW ORLEANS — Women with peripartum cardiomyopathy in a contemporary Southern California series had a markedly lower mortality than in previously reported studies, Dr. Somjot S. Brar said at the annual scientific session of the American College of Cardiology.
The overall incidence of peripartum cardiomyopathy (PPCM) was one case per 4,025 live births in this study of nearly 250,000 deliveries in the Southern California Kaiser Permanente health care system in 2000–2005. But there was a substantial racial variation.
The incidence of PPCM was highest in black women, at one case per 1,421 live births. This rate was 2.9-fold greater than in whites, 7-fold greater than in Hispanics, and twice that in Asians, according to Dr. Brar of Kaiser Permanente Medical Center, Los Angeles.
This observed marked racial variation in the incidence of PPMC may explain at least in part the wide range of rates reported in the literature, he added.
PPCM is a rare disorder of unclear etiology. The diagnosis is made in patients who experience left ventricular dysfunction with a documented ejection fraction of less than 50%, onset during the final month before or the first 5 months after delivery, and in whom no alternative cause for heart failure can be found.
The diagnostic requirement that systolic dysfunction be present prevents PPCM from being confused with preeclampsia, which is marked by diastolic dysfunction. Patients with PPCM commonly present with arrhythmias or thromboembolism rather than with classic heart failure symptoms.
In the Kaiser study, all of the nearly quarter-million women were initially screened using ICD-9 codes for hospitalization for heart failure from 6 months before to 12 months after delivery. Patients flagged in this way then underwent detailed chart review, which resulted in the diagnosis of PPCM in 60 cases.
No cardiac transplantations and only two deaths occurred in the PPCM group, for an all-cause mortality of 3.3% during an average follow-up of 4.7 years. One death occurred 5 months after delivery in a woman with complications of deep vein thrombosis, while the other was caused by respiratory failure more than 4 years after delivery in an asthmatic patient whose left ventricular function had recovered long before.
Other investigators have reported mortality rates as high as 50% in women with PPCM, with half of the deaths occurring within the first 3 months after delivery. Dr. Brar offered several potential explanations for the low rate in the Kaiser series. One is that many of the prior reports date from an era before routine use of modern evidence-based heart failure medications such as ACE inhibitors and β-blockers.
Another possibility is that earlier reports were biased by preferential detection of more advanced cases of PPCM. “Our process of screening by ICD-9 codes with individual chart review of all potential cases may have allowed for identification of less-sick patients,” he said.
Yet another potential explanation for the disparate results is that the improved outcomes in the Kaiser study stemmed from the selective nature of the prepaid health plan membership, in which the very poor and very rich are underrepresented.
Dr. Brar said this initial report is just the first phase of an ongoing Kaiser study. Now that he and his coinvestigators have reported on PPCM incidence and survival, they are focusing next on identification of risk factors and attempting to pin down the optimal duration of medical management.
Session Cochair Dr. Peter Liu of the University of Toronto noted that a number of risk factors for PPCM are already known. These include preeclampsia, black race, and advanced age at first pregnancy. There has also been speculation that PPCM might be a form of myocarditis.
'Our process of screening by ICD-9 codes … may have allowed for identification of less-sick patients.' DR. BRAR
NEW ORLEANS — Women with peripartum cardiomyopathy in a contemporary Southern California series had a markedly lower mortality than in previously reported studies, Dr. Somjot S. Brar said at the annual scientific session of the American College of Cardiology.
The overall incidence of peripartum cardiomyopathy (PPCM) was one case per 4,025 live births in this study of nearly 250,000 deliveries in the Southern California Kaiser Permanente health care system in 2000–2005. But there was a substantial racial variation.
The incidence of PPCM was highest in black women, at one case per 1,421 live births. This rate was 2.9-fold greater than in whites, 7-fold greater than in Hispanics, and twice that in Asians, according to Dr. Brar of Kaiser Permanente Medical Center, Los Angeles.
This observed marked racial variation in the incidence of PPMC may explain at least in part the wide range of rates reported in the literature, he added.
PPCM is a rare disorder of unclear etiology. The diagnosis is made in patients who experience left ventricular dysfunction with a documented ejection fraction of less than 50%, onset during the final month before or the first 5 months after delivery, and in whom no alternative cause for heart failure can be found.
The diagnostic requirement that systolic dysfunction be present prevents PPCM from being confused with preeclampsia, which is marked by diastolic dysfunction. Patients with PPCM commonly present with arrhythmias or thromboembolism rather than with classic heart failure symptoms.
In the Kaiser study, all of the nearly quarter-million women were initially screened using ICD-9 codes for hospitalization for heart failure from 6 months before to 12 months after delivery. Patients flagged in this way then underwent detailed chart review, which resulted in the diagnosis of PPCM in 60 cases.
No cardiac transplantations and only two deaths occurred in the PPCM group, for an all-cause mortality of 3.3% during an average follow-up of 4.7 years. One death occurred 5 months after delivery in a woman with complications of deep vein thrombosis, while the other was caused by respiratory failure more than 4 years after delivery in an asthmatic patient whose left ventricular function had recovered long before.
Other investigators have reported mortality rates as high as 50% in women with PPCM, with half of the deaths occurring within the first 3 months after delivery. Dr. Brar offered several potential explanations for the low rate in the Kaiser series. One is that many of the prior reports date from an era before routine use of modern evidence-based heart failure medications such as ACE inhibitors and β-blockers.
Another possibility is that earlier reports were biased by preferential detection of more advanced cases of PPCM. “Our process of screening by ICD-9 codes with individual chart review of all potential cases may have allowed for identification of less-sick patients,” he said.
Yet another potential explanation for the disparate results is that the improved outcomes in the Kaiser study stemmed from the selective nature of the prepaid health plan membership, in which the very poor and very rich are underrepresented.
Dr. Brar said this initial report is just the first phase of an ongoing Kaiser study. Now that he and his coinvestigators have reported on PPCM incidence and survival, they are focusing next on identification of risk factors and attempting to pin down the optimal duration of medical management.
Session Cochair Dr. Peter Liu of the University of Toronto noted that a number of risk factors for PPCM are already known. These include preeclampsia, black race, and advanced age at first pregnancy. There has also been speculation that PPCM might be a form of myocarditis.
'Our process of screening by ICD-9 codes … may have allowed for identification of less-sick patients.' DR. BRAR