User login
Data Suggest Rigorous Postpartum Testing in GDM
TORONTO — Postpartum testing in women who had gestational diabetes during pregnancy should include both an oral glucose-tolerance test and a lipid profile, Genevieve Dubé and her colleagues advised in a poster presented at the joint annual meeting of the Canadian Diabetes Association and the Canadian Society of Endocrinology and Metabolism.
Data from a retrospective analysis of 223 women who had gestational diabetes mellitus (GDM) during pregnancy revealed that postpartum glucose-tolerance abnormalities were common, affecting one-fourth of all women. Moreover, “Isolated fasting glucose testing would have failed to identify most cases of postpartum dysglycemia,” noted Ms. Dubé and her colleagues at the Centre Régional du Diabète de Laval (Que.).
The data also suggested that a lipid profile should be part of the assessment, because many of the women with previous GDM—including those with normal postpartum oral glucose-tolerance test (OGTT) results—have altered lipids suggestive of features of the cardiometabolic syndrome, they said.
The 223 women had received prenatal care between June 2004 and April 2005 at Laval's diabetic pregnancy clinic, which has had a program of routine postnatal GDM follow-up since 2002. The group had a mean age of 31 years and a mean body mass index (kg/m2) of 28.3. Two-thirds of the women were white. Insulin treatment was used by 34% during pregnancy.
All were told to return at 2 months—whether or not they were still breast-feeding—for postpartum lab testing, which included a 12-hour fasting glucose, a 75-g OGTT, a lipid profile, and a thyroid-stimulating hormone test. A total of 74% (165 patients) showed up, at a mean of 3 months following delivery.
Of the 164 who underwent the OGTT, some form of impaired glucose tolerance was detected in 25% (41 patients), including frank type 2 diabetes in 4% (7 patients), isolated impaired glucose tolerance in 16% (26 patients), isolated impaired fasting glucose in 2% (3 patients), and both impaired glucose tolerance and impaired fasting glucose in 3% (5 patients).
No matter what fasting blood glucose (FBG) cutoff was used, more than half of all dysglycemic women would have been missed if postpartum lab screening included only FBG instead of OGTT. Among the 41 women with abnormal 2-hour OGTT results, 49% had FBG values at or above 5.6 mmol/L, 41.5% had FBG levels at or above 5.8 mmol/L, and 32% had FBG levels at or above 6.1 mmol/L.
The need for insulin therapy and a first-trimester FBG above 6.1 mmol/L were the only risk factors analyzed that significantly predicted postpartum abnormal OGTT, with odds ratios of 1.89 and 3.41, respectively. Maternal age, BMI, parity, macrosomia, and nonwhite race were not predictive of postpartum glucose status, they said.
Among the 165 women who had postpartum lipid tests, 70% had at least one abnormality, defined as a triglyceride level of 1.7 mmol/L or higher, HDL cholesterol level at or lower than 1.3 mmol/L, or a total cholesterol/HDL cholesterol ratio of 5.0 or greater.
Cardiometabolic risk factors were not limited to women with abnormal OGTT results and diabetes. Indeed, two-thirds of the 123 women with normal postpartum glucose tolerance had at least one lipid abnormality; 23% had triglyceride levels of 1.7 mmol/L or higher, and 23% had HDL cholesterol of 1.3 mmol/L or lower. In fact, only when those two abnormalities were combined was there a significant correlation with OGTT results: The proportion of women with normal glucose tolerance who had both high triglycerides and low HDL cholesterol was 13%, compared with 24% of those with abnormal OGTT.
Among 129 of the women whose breast-feeding status was known, 63% were breast-feeding at the time of the postpartum visit. Breast-feeding was associated with significantly lower triglyceride level, higher HDL cholesterol, lower total cholesterol/HDL ratio, lower mean fasting glucose at the OGTT, and lower prevalence of any postpartum abnormality of glucose tolerance, including diabetes. Although these differences did not seem to be attributable to different maternal characteristics, there was a trend toward a lower prevalence of obesity (defined as a BMI of 27 or higher) among the breast-feeding women (49% vs. 62.5%).
TORONTO — Postpartum testing in women who had gestational diabetes during pregnancy should include both an oral glucose-tolerance test and a lipid profile, Genevieve Dubé and her colleagues advised in a poster presented at the joint annual meeting of the Canadian Diabetes Association and the Canadian Society of Endocrinology and Metabolism.
Data from a retrospective analysis of 223 women who had gestational diabetes mellitus (GDM) during pregnancy revealed that postpartum glucose-tolerance abnormalities were common, affecting one-fourth of all women. Moreover, “Isolated fasting glucose testing would have failed to identify most cases of postpartum dysglycemia,” noted Ms. Dubé and her colleagues at the Centre Régional du Diabète de Laval (Que.).
The data also suggested that a lipid profile should be part of the assessment, because many of the women with previous GDM—including those with normal postpartum oral glucose-tolerance test (OGTT) results—have altered lipids suggestive of features of the cardiometabolic syndrome, they said.
The 223 women had received prenatal care between June 2004 and April 2005 at Laval's diabetic pregnancy clinic, which has had a program of routine postnatal GDM follow-up since 2002. The group had a mean age of 31 years and a mean body mass index (kg/m2) of 28.3. Two-thirds of the women were white. Insulin treatment was used by 34% during pregnancy.
All were told to return at 2 months—whether or not they were still breast-feeding—for postpartum lab testing, which included a 12-hour fasting glucose, a 75-g OGTT, a lipid profile, and a thyroid-stimulating hormone test. A total of 74% (165 patients) showed up, at a mean of 3 months following delivery.
Of the 164 who underwent the OGTT, some form of impaired glucose tolerance was detected in 25% (41 patients), including frank type 2 diabetes in 4% (7 patients), isolated impaired glucose tolerance in 16% (26 patients), isolated impaired fasting glucose in 2% (3 patients), and both impaired glucose tolerance and impaired fasting glucose in 3% (5 patients).
No matter what fasting blood glucose (FBG) cutoff was used, more than half of all dysglycemic women would have been missed if postpartum lab screening included only FBG instead of OGTT. Among the 41 women with abnormal 2-hour OGTT results, 49% had FBG values at or above 5.6 mmol/L, 41.5% had FBG levels at or above 5.8 mmol/L, and 32% had FBG levels at or above 6.1 mmol/L.
The need for insulin therapy and a first-trimester FBG above 6.1 mmol/L were the only risk factors analyzed that significantly predicted postpartum abnormal OGTT, with odds ratios of 1.89 and 3.41, respectively. Maternal age, BMI, parity, macrosomia, and nonwhite race were not predictive of postpartum glucose status, they said.
Among the 165 women who had postpartum lipid tests, 70% had at least one abnormality, defined as a triglyceride level of 1.7 mmol/L or higher, HDL cholesterol level at or lower than 1.3 mmol/L, or a total cholesterol/HDL cholesterol ratio of 5.0 or greater.
Cardiometabolic risk factors were not limited to women with abnormal OGTT results and diabetes. Indeed, two-thirds of the 123 women with normal postpartum glucose tolerance had at least one lipid abnormality; 23% had triglyceride levels of 1.7 mmol/L or higher, and 23% had HDL cholesterol of 1.3 mmol/L or lower. In fact, only when those two abnormalities were combined was there a significant correlation with OGTT results: The proportion of women with normal glucose tolerance who had both high triglycerides and low HDL cholesterol was 13%, compared with 24% of those with abnormal OGTT.
Among 129 of the women whose breast-feeding status was known, 63% were breast-feeding at the time of the postpartum visit. Breast-feeding was associated with significantly lower triglyceride level, higher HDL cholesterol, lower total cholesterol/HDL ratio, lower mean fasting glucose at the OGTT, and lower prevalence of any postpartum abnormality of glucose tolerance, including diabetes. Although these differences did not seem to be attributable to different maternal characteristics, there was a trend toward a lower prevalence of obesity (defined as a BMI of 27 or higher) among the breast-feeding women (49% vs. 62.5%).
TORONTO — Postpartum testing in women who had gestational diabetes during pregnancy should include both an oral glucose-tolerance test and a lipid profile, Genevieve Dubé and her colleagues advised in a poster presented at the joint annual meeting of the Canadian Diabetes Association and the Canadian Society of Endocrinology and Metabolism.
Data from a retrospective analysis of 223 women who had gestational diabetes mellitus (GDM) during pregnancy revealed that postpartum glucose-tolerance abnormalities were common, affecting one-fourth of all women. Moreover, “Isolated fasting glucose testing would have failed to identify most cases of postpartum dysglycemia,” noted Ms. Dubé and her colleagues at the Centre Régional du Diabète de Laval (Que.).
The data also suggested that a lipid profile should be part of the assessment, because many of the women with previous GDM—including those with normal postpartum oral glucose-tolerance test (OGTT) results—have altered lipids suggestive of features of the cardiometabolic syndrome, they said.
The 223 women had received prenatal care between June 2004 and April 2005 at Laval's diabetic pregnancy clinic, which has had a program of routine postnatal GDM follow-up since 2002. The group had a mean age of 31 years and a mean body mass index (kg/m2) of 28.3. Two-thirds of the women were white. Insulin treatment was used by 34% during pregnancy.
All were told to return at 2 months—whether or not they were still breast-feeding—for postpartum lab testing, which included a 12-hour fasting glucose, a 75-g OGTT, a lipid profile, and a thyroid-stimulating hormone test. A total of 74% (165 patients) showed up, at a mean of 3 months following delivery.
Of the 164 who underwent the OGTT, some form of impaired glucose tolerance was detected in 25% (41 patients), including frank type 2 diabetes in 4% (7 patients), isolated impaired glucose tolerance in 16% (26 patients), isolated impaired fasting glucose in 2% (3 patients), and both impaired glucose tolerance and impaired fasting glucose in 3% (5 patients).
No matter what fasting blood glucose (FBG) cutoff was used, more than half of all dysglycemic women would have been missed if postpartum lab screening included only FBG instead of OGTT. Among the 41 women with abnormal 2-hour OGTT results, 49% had FBG values at or above 5.6 mmol/L, 41.5% had FBG levels at or above 5.8 mmol/L, and 32% had FBG levels at or above 6.1 mmol/L.
The need for insulin therapy and a first-trimester FBG above 6.1 mmol/L were the only risk factors analyzed that significantly predicted postpartum abnormal OGTT, with odds ratios of 1.89 and 3.41, respectively. Maternal age, BMI, parity, macrosomia, and nonwhite race were not predictive of postpartum glucose status, they said.
Among the 165 women who had postpartum lipid tests, 70% had at least one abnormality, defined as a triglyceride level of 1.7 mmol/L or higher, HDL cholesterol level at or lower than 1.3 mmol/L, or a total cholesterol/HDL cholesterol ratio of 5.0 or greater.
Cardiometabolic risk factors were not limited to women with abnormal OGTT results and diabetes. Indeed, two-thirds of the 123 women with normal postpartum glucose tolerance had at least one lipid abnormality; 23% had triglyceride levels of 1.7 mmol/L or higher, and 23% had HDL cholesterol of 1.3 mmol/L or lower. In fact, only when those two abnormalities were combined was there a significant correlation with OGTT results: The proportion of women with normal glucose tolerance who had both high triglycerides and low HDL cholesterol was 13%, compared with 24% of those with abnormal OGTT.
Among 129 of the women whose breast-feeding status was known, 63% were breast-feeding at the time of the postpartum visit. Breast-feeding was associated with significantly lower triglyceride level, higher HDL cholesterol, lower total cholesterol/HDL ratio, lower mean fasting glucose at the OGTT, and lower prevalence of any postpartum abnormality of glucose tolerance, including diabetes. Although these differences did not seem to be attributable to different maternal characteristics, there was a trend toward a lower prevalence of obesity (defined as a BMI of 27 or higher) among the breast-feeding women (49% vs. 62.5%).
Selenium Slows Chronic Thyroiditis in Pregnancy
VERONA, ITALY — For the first time, selenium supplementation has been shown to lessen the progression of autoimmune chronic thyroiditis in pregnant women.
Pregnant women who are positive for thyroid peroxidase antibodies are prone to develop postpartum thyroid dysfunction and permanent hypothyroidism.
Selenium supplementation during and after pregnancy reduced the incidence of both conditions in a large prospective, randomized controlled trial of euthyroid pregnant women, Dr. Roberto Negro and associates reported in an award-winning poster at a joint meeting of the Italian Association of Clinical Endocrinologists and the American Association of Clinical Endocrinologists.
“Giving adequate selenium supplementation may reduce the inflammatory action of the thyroid gland after delivery,” Dr. Negro said in an interview. “This is not sufficient to recommend this treatment for all pregnant women affected by chronic autoimmune thyroiditis, but it may be considered.”
Of the 2,143 women who participated in the study, 7.9% were positive for thyroid peroxidase antibodies (TPOAb). Of the TPOAb-positive women who both remained in the study and did not miscarry, Dr. Negro and associates randomized 77 to 200 mcg/day selenomethionine beginning at the 12th week of pregnancy until 12 months after delivery, and 74 to placebo. Of the TPOAb-negative women, 81 were age matched and served as the control. Thyroid function tests were performed at 20 and 30 weeks' gestation, at delivery, and after delivery at months 1, 2, 5, 9, and 12.
Blood selenium concentrations were measured at the first endocrinologic visit (at an average 9.4 weeks' gestation), at 20 and 30 weeks' gestation, at delivery, and at 6 and 12 months after delivery. Thyroid ultrasound scans were performed by an independent radiologist at the first endocrinologic visit during pregnancy, at delivery, and at 12 months after delivery.
At baseline, there were no significant differences between the three groups in average age (28 years, 28 years, and 27 years, respectively), in free thyroxine levels, and in the time supplementation began (average 12 weeks). Significant differences were noted between groups in baseline thyroid-stimulating hormone (1.6 mIU/;L, 1.7 mIU/;L, and 0.9 mIU/;L, respectively) and in those requiring levothyroxine during pregnancy (19.4%, 21.6%, and 2.5%, respectively).
At 12 months after delivery, rates of postpartum thyroid dysfunction (28.6% vs. 48.6%) and permanent hypothyroidism (11.7% vs. 20.3%) were significantly lower in women taking the selenium supplements than in placebo-treated patients, the authors reported.
In addition, TPOAb titers were significantly lower in the supplement group compared with placebo-treated patients, with a 62.4% versus 43.9% reduction during pregnancy and lower titers during the postpartum period (323.2 kIU/;L vs. 621.1 kIU/;L).
When the ultrasound echogenicity patterns of the two groups were compared, the selenium-supplemented group displayed a significantly lower percentage of moderate to advanced thyroiditis (grades 2–3) at the end of the postpartum period (27.3% vs. 44.6%), the authors reported.
No side effects were reported in the mothers and no families have been recalled for newborn thyroid dysfunction, said Dr. Negro, of the endocrinology department at Azienda Ospedaliera “Vito Fazzi,” Lecce, Italy.
“Relatively high doses are needed to obtain a significant response on postpartum thyroid dysfunction,” he said.
Further investigations are required to know whether these beneficial effects are reversed if selenium supplementation is interrupted or whether they can be maintained for a long time if selenium is continued, the authors concluded.
Postpartum thyroid problem rates were lower in women who took selenium supplements. DR. NEGRO
VERONA, ITALY — For the first time, selenium supplementation has been shown to lessen the progression of autoimmune chronic thyroiditis in pregnant women.
Pregnant women who are positive for thyroid peroxidase antibodies are prone to develop postpartum thyroid dysfunction and permanent hypothyroidism.
Selenium supplementation during and after pregnancy reduced the incidence of both conditions in a large prospective, randomized controlled trial of euthyroid pregnant women, Dr. Roberto Negro and associates reported in an award-winning poster at a joint meeting of the Italian Association of Clinical Endocrinologists and the American Association of Clinical Endocrinologists.
“Giving adequate selenium supplementation may reduce the inflammatory action of the thyroid gland after delivery,” Dr. Negro said in an interview. “This is not sufficient to recommend this treatment for all pregnant women affected by chronic autoimmune thyroiditis, but it may be considered.”
Of the 2,143 women who participated in the study, 7.9% were positive for thyroid peroxidase antibodies (TPOAb). Of the TPOAb-positive women who both remained in the study and did not miscarry, Dr. Negro and associates randomized 77 to 200 mcg/day selenomethionine beginning at the 12th week of pregnancy until 12 months after delivery, and 74 to placebo. Of the TPOAb-negative women, 81 were age matched and served as the control. Thyroid function tests were performed at 20 and 30 weeks' gestation, at delivery, and after delivery at months 1, 2, 5, 9, and 12.
Blood selenium concentrations were measured at the first endocrinologic visit (at an average 9.4 weeks' gestation), at 20 and 30 weeks' gestation, at delivery, and at 6 and 12 months after delivery. Thyroid ultrasound scans were performed by an independent radiologist at the first endocrinologic visit during pregnancy, at delivery, and at 12 months after delivery.
At baseline, there were no significant differences between the three groups in average age (28 years, 28 years, and 27 years, respectively), in free thyroxine levels, and in the time supplementation began (average 12 weeks). Significant differences were noted between groups in baseline thyroid-stimulating hormone (1.6 mIU/;L, 1.7 mIU/;L, and 0.9 mIU/;L, respectively) and in those requiring levothyroxine during pregnancy (19.4%, 21.6%, and 2.5%, respectively).
At 12 months after delivery, rates of postpartum thyroid dysfunction (28.6% vs. 48.6%) and permanent hypothyroidism (11.7% vs. 20.3%) were significantly lower in women taking the selenium supplements than in placebo-treated patients, the authors reported.
In addition, TPOAb titers were significantly lower in the supplement group compared with placebo-treated patients, with a 62.4% versus 43.9% reduction during pregnancy and lower titers during the postpartum period (323.2 kIU/;L vs. 621.1 kIU/;L).
When the ultrasound echogenicity patterns of the two groups were compared, the selenium-supplemented group displayed a significantly lower percentage of moderate to advanced thyroiditis (grades 2–3) at the end of the postpartum period (27.3% vs. 44.6%), the authors reported.
No side effects were reported in the mothers and no families have been recalled for newborn thyroid dysfunction, said Dr. Negro, of the endocrinology department at Azienda Ospedaliera “Vito Fazzi,” Lecce, Italy.
“Relatively high doses are needed to obtain a significant response on postpartum thyroid dysfunction,” he said.
Further investigations are required to know whether these beneficial effects are reversed if selenium supplementation is interrupted or whether they can be maintained for a long time if selenium is continued, the authors concluded.
Postpartum thyroid problem rates were lower in women who took selenium supplements. DR. NEGRO
VERONA, ITALY — For the first time, selenium supplementation has been shown to lessen the progression of autoimmune chronic thyroiditis in pregnant women.
Pregnant women who are positive for thyroid peroxidase antibodies are prone to develop postpartum thyroid dysfunction and permanent hypothyroidism.
Selenium supplementation during and after pregnancy reduced the incidence of both conditions in a large prospective, randomized controlled trial of euthyroid pregnant women, Dr. Roberto Negro and associates reported in an award-winning poster at a joint meeting of the Italian Association of Clinical Endocrinologists and the American Association of Clinical Endocrinologists.
“Giving adequate selenium supplementation may reduce the inflammatory action of the thyroid gland after delivery,” Dr. Negro said in an interview. “This is not sufficient to recommend this treatment for all pregnant women affected by chronic autoimmune thyroiditis, but it may be considered.”
Of the 2,143 women who participated in the study, 7.9% were positive for thyroid peroxidase antibodies (TPOAb). Of the TPOAb-positive women who both remained in the study and did not miscarry, Dr. Negro and associates randomized 77 to 200 mcg/day selenomethionine beginning at the 12th week of pregnancy until 12 months after delivery, and 74 to placebo. Of the TPOAb-negative women, 81 were age matched and served as the control. Thyroid function tests were performed at 20 and 30 weeks' gestation, at delivery, and after delivery at months 1, 2, 5, 9, and 12.
Blood selenium concentrations were measured at the first endocrinologic visit (at an average 9.4 weeks' gestation), at 20 and 30 weeks' gestation, at delivery, and at 6 and 12 months after delivery. Thyroid ultrasound scans were performed by an independent radiologist at the first endocrinologic visit during pregnancy, at delivery, and at 12 months after delivery.
At baseline, there were no significant differences between the three groups in average age (28 years, 28 years, and 27 years, respectively), in free thyroxine levels, and in the time supplementation began (average 12 weeks). Significant differences were noted between groups in baseline thyroid-stimulating hormone (1.6 mIU/;L, 1.7 mIU/;L, and 0.9 mIU/;L, respectively) and in those requiring levothyroxine during pregnancy (19.4%, 21.6%, and 2.5%, respectively).
At 12 months after delivery, rates of postpartum thyroid dysfunction (28.6% vs. 48.6%) and permanent hypothyroidism (11.7% vs. 20.3%) were significantly lower in women taking the selenium supplements than in placebo-treated patients, the authors reported.
In addition, TPOAb titers were significantly lower in the supplement group compared with placebo-treated patients, with a 62.4% versus 43.9% reduction during pregnancy and lower titers during the postpartum period (323.2 kIU/;L vs. 621.1 kIU/;L).
When the ultrasound echogenicity patterns of the two groups were compared, the selenium-supplemented group displayed a significantly lower percentage of moderate to advanced thyroiditis (grades 2–3) at the end of the postpartum period (27.3% vs. 44.6%), the authors reported.
No side effects were reported in the mothers and no families have been recalled for newborn thyroid dysfunction, said Dr. Negro, of the endocrinology department at Azienda Ospedaliera “Vito Fazzi,” Lecce, Italy.
“Relatively high doses are needed to obtain a significant response on postpartum thyroid dysfunction,” he said.
Further investigations are required to know whether these beneficial effects are reversed if selenium supplementation is interrupted or whether they can be maintained for a long time if selenium is continued, the authors concluded.
Postpartum thyroid problem rates were lower in women who took selenium supplements. DR. NEGRO
High Vitamin E in Pregnancy Lowers Asthma Risk in Kids
Children whose mothers consumed the highest levels of vitamin E during pregnancy had less asthma and wheezing than did their peers whose mothers consumed less vitamin E while pregnant, according to findings from a cohort study.
Dr. Graham Devereux and colleagues from the University of Aberdeen (Scotland) found that 5-year-old children of women with the highest intake of vitamin E during pregnancy had the lowest incidence of wheezing, physician visits because of wheezing, and both suspected and physician-diagnosed asthma (Am. J. Respir. Crit. Care Med. 2006;174:499–507).
A group of 2,000 expectant mothers was initially recruited in 1997 and 1999 from prenatal clinics, at a median of 12 weeks' gestation. A total of 1,856 women completed a questionnaire, underwent a skin-prick test to assess atopic status, and provided a blood sample. At 32 weeks' gestation, 1,704 women answered a food-frequency questionnaire (FFQ) to assess dietary intake during the preceding 3 months. Upon delivery, maternal and infant (cord blood) plasma was sampled in 1,134 mothers and 877 infants and analyzed for antioxidant content via liquid chromatography.
Six weeks after their singleton children turned 5 years old, a questionnaire was mailed to each child's family to assess history of wheezing and asthma; 1,253 were completed and received. A total of 1,120 parents who responded to this questionnaire filled out an additional FFQ based on the child's diet, and parents of 797 children accepted an invitation to take the child to the hospital for spirometry, skin-prick testing, and fraction of exhaled nitric oxide measurement.
Ultimately, “children born to mothers with the lowest quintile of vitamin E intake [were] 3.47 times more likely to be of the persistent wheezing phenotype than [were] children born to mothers with the highest quintile of vitamin E intake,” the investigators wrote.
They had reported an association between maternal vitamin E intake during pregnancy and asthma at age 2 years in the same cohort.
Children whose mothers consumed the highest levels of vitamin E during pregnancy had less asthma and wheezing than did their peers whose mothers consumed less vitamin E while pregnant, according to findings from a cohort study.
Dr. Graham Devereux and colleagues from the University of Aberdeen (Scotland) found that 5-year-old children of women with the highest intake of vitamin E during pregnancy had the lowest incidence of wheezing, physician visits because of wheezing, and both suspected and physician-diagnosed asthma (Am. J. Respir. Crit. Care Med. 2006;174:499–507).
A group of 2,000 expectant mothers was initially recruited in 1997 and 1999 from prenatal clinics, at a median of 12 weeks' gestation. A total of 1,856 women completed a questionnaire, underwent a skin-prick test to assess atopic status, and provided a blood sample. At 32 weeks' gestation, 1,704 women answered a food-frequency questionnaire (FFQ) to assess dietary intake during the preceding 3 months. Upon delivery, maternal and infant (cord blood) plasma was sampled in 1,134 mothers and 877 infants and analyzed for antioxidant content via liquid chromatography.
Six weeks after their singleton children turned 5 years old, a questionnaire was mailed to each child's family to assess history of wheezing and asthma; 1,253 were completed and received. A total of 1,120 parents who responded to this questionnaire filled out an additional FFQ based on the child's diet, and parents of 797 children accepted an invitation to take the child to the hospital for spirometry, skin-prick testing, and fraction of exhaled nitric oxide measurement.
Ultimately, “children born to mothers with the lowest quintile of vitamin E intake [were] 3.47 times more likely to be of the persistent wheezing phenotype than [were] children born to mothers with the highest quintile of vitamin E intake,” the investigators wrote.
They had reported an association between maternal vitamin E intake during pregnancy and asthma at age 2 years in the same cohort.
Children whose mothers consumed the highest levels of vitamin E during pregnancy had less asthma and wheezing than did their peers whose mothers consumed less vitamin E while pregnant, according to findings from a cohort study.
Dr. Graham Devereux and colleagues from the University of Aberdeen (Scotland) found that 5-year-old children of women with the highest intake of vitamin E during pregnancy had the lowest incidence of wheezing, physician visits because of wheezing, and both suspected and physician-diagnosed asthma (Am. J. Respir. Crit. Care Med. 2006;174:499–507).
A group of 2,000 expectant mothers was initially recruited in 1997 and 1999 from prenatal clinics, at a median of 12 weeks' gestation. A total of 1,856 women completed a questionnaire, underwent a skin-prick test to assess atopic status, and provided a blood sample. At 32 weeks' gestation, 1,704 women answered a food-frequency questionnaire (FFQ) to assess dietary intake during the preceding 3 months. Upon delivery, maternal and infant (cord blood) plasma was sampled in 1,134 mothers and 877 infants and analyzed for antioxidant content via liquid chromatography.
Six weeks after their singleton children turned 5 years old, a questionnaire was mailed to each child's family to assess history of wheezing and asthma; 1,253 were completed and received. A total of 1,120 parents who responded to this questionnaire filled out an additional FFQ based on the child's diet, and parents of 797 children accepted an invitation to take the child to the hospital for spirometry, skin-prick testing, and fraction of exhaled nitric oxide measurement.
Ultimately, “children born to mothers with the lowest quintile of vitamin E intake [were] 3.47 times more likely to be of the persistent wheezing phenotype than [were] children born to mothers with the highest quintile of vitamin E intake,” the investigators wrote.
They had reported an association between maternal vitamin E intake during pregnancy and asthma at age 2 years in the same cohort.
IV Diazoxide Effective In Hypertensive Crisis
LISBON — Intravenous diazoxide was as safe and effective as intravenous hydralazine for treating hypertensive crisis during pregnancy in a study with 124 patients.
Diazoxide has the advantage of working very quickly, and it may be a good option for physicians who are uncomfortable with hydralazine, Dr. Annemarie Hennessy said at the 15th World Congress of the International Society for the Study of Hypertension in Medicine. Intravenous β-blockers, another option for physicians in the United States, are not approved for use in Australia.
Women with severe hypertension at Royal Prince Alfred Hospital in Sydney were randomized so that 63 were assigned to treatment with diazoxide and 61 were scheduled to receive hydralazine. Treatment was actually administered to 59 women in the diazoxide group and 51 women in the hydralazine group, said Dr. Hennessy, a nephrologist at the University of Sydney and managing director of the preeclampsia research laboratory at the hospital.
The dosage used for diazoxide was a 15-mg bolus administered every 3 minutes to a maximum of 300 mg. In the hydralazine group, patients received 5 mg every 20 minutes to a maximum of 15 mg. The study's primary end point was need for cesarean section because of fetal deterioration as determined by cardiotocography.
The cesarean section rate was 70% in the hydralazine group and 76% in the diazoxide group, not a statistically significant difference.
The average time to reach target blood pressure was 34 minutes in the hydralazine group and 19 minutes in the diazoxide group, a statistically significant difference that is probably not very significant clinically, Dr. Hennessy said.
Episodes of persistent, severe hypertension occurred in 38% of women receving hydralazine and 16% of those receiving diazoxide, a statistically significant difference. There was one episode of severe hypotension in the hydralazine group and none in the diazoxide group. The incidence of other adverse events was 11% in both groups.
Dr. Hennessy attributed the absence of diazoxide-related hypotensive episodes in this study to the use of 15-mg boluses, which produced a controlled reduction in blood pressure.
LISBON — Intravenous diazoxide was as safe and effective as intravenous hydralazine for treating hypertensive crisis during pregnancy in a study with 124 patients.
Diazoxide has the advantage of working very quickly, and it may be a good option for physicians who are uncomfortable with hydralazine, Dr. Annemarie Hennessy said at the 15th World Congress of the International Society for the Study of Hypertension in Medicine. Intravenous β-blockers, another option for physicians in the United States, are not approved for use in Australia.
Women with severe hypertension at Royal Prince Alfred Hospital in Sydney were randomized so that 63 were assigned to treatment with diazoxide and 61 were scheduled to receive hydralazine. Treatment was actually administered to 59 women in the diazoxide group and 51 women in the hydralazine group, said Dr. Hennessy, a nephrologist at the University of Sydney and managing director of the preeclampsia research laboratory at the hospital.
The dosage used for diazoxide was a 15-mg bolus administered every 3 minutes to a maximum of 300 mg. In the hydralazine group, patients received 5 mg every 20 minutes to a maximum of 15 mg. The study's primary end point was need for cesarean section because of fetal deterioration as determined by cardiotocography.
The cesarean section rate was 70% in the hydralazine group and 76% in the diazoxide group, not a statistically significant difference.
The average time to reach target blood pressure was 34 minutes in the hydralazine group and 19 minutes in the diazoxide group, a statistically significant difference that is probably not very significant clinically, Dr. Hennessy said.
Episodes of persistent, severe hypertension occurred in 38% of women receving hydralazine and 16% of those receiving diazoxide, a statistically significant difference. There was one episode of severe hypotension in the hydralazine group and none in the diazoxide group. The incidence of other adverse events was 11% in both groups.
Dr. Hennessy attributed the absence of diazoxide-related hypotensive episodes in this study to the use of 15-mg boluses, which produced a controlled reduction in blood pressure.
LISBON — Intravenous diazoxide was as safe and effective as intravenous hydralazine for treating hypertensive crisis during pregnancy in a study with 124 patients.
Diazoxide has the advantage of working very quickly, and it may be a good option for physicians who are uncomfortable with hydralazine, Dr. Annemarie Hennessy said at the 15th World Congress of the International Society for the Study of Hypertension in Medicine. Intravenous β-blockers, another option for physicians in the United States, are not approved for use in Australia.
Women with severe hypertension at Royal Prince Alfred Hospital in Sydney were randomized so that 63 were assigned to treatment with diazoxide and 61 were scheduled to receive hydralazine. Treatment was actually administered to 59 women in the diazoxide group and 51 women in the hydralazine group, said Dr. Hennessy, a nephrologist at the University of Sydney and managing director of the preeclampsia research laboratory at the hospital.
The dosage used for diazoxide was a 15-mg bolus administered every 3 minutes to a maximum of 300 mg. In the hydralazine group, patients received 5 mg every 20 minutes to a maximum of 15 mg. The study's primary end point was need for cesarean section because of fetal deterioration as determined by cardiotocography.
The cesarean section rate was 70% in the hydralazine group and 76% in the diazoxide group, not a statistically significant difference.
The average time to reach target blood pressure was 34 minutes in the hydralazine group and 19 minutes in the diazoxide group, a statistically significant difference that is probably not very significant clinically, Dr. Hennessy said.
Episodes of persistent, severe hypertension occurred in 38% of women receving hydralazine and 16% of those receiving diazoxide, a statistically significant difference. There was one episode of severe hypotension in the hydralazine group and none in the diazoxide group. The incidence of other adverse events was 11% in both groups.
Dr. Hennessy attributed the absence of diazoxide-related hypotensive episodes in this study to the use of 15-mg boluses, which produced a controlled reduction in blood pressure.
Induction Ups Risk of Amniotic Fluid Embolism
Medical induction of labor appears to double the risk of amniotic fluid embolism, reported Dr. Michael S. Kramer of Montreal Children's Hospital and his associates.
Findings from their retrospective cohort study of approximately 3 million hospital births throughout Canada between 1991 and 2002 “support the long-standing, but heretofore unsubstantiated, suspicion that labour induction increases the risk of this rare but serious maternal complication.
“Although the small absolute risk of amniotic fluid embolism is unlikely to affect the decision to induce labour in the presence of compelling clinical indications, women and physicians should be aware of this risk if the decision is elective,” the researchers said in the Oct. 21 issue of the Lancet.
“We should emphasise that the absolute risk increase of amniotic fluid embolism for women undergoing medical induction of labour is very small: 4 or 5 total cases and 1 or 2 fatal cases per 100,000 women induced.
“However, with 4 million births per year and induction rates approaching 20% in the [United States], this practice could be causing amniotic fluid embolism in 30–40 women per year in the [United States] alone (including 10–15 deaths),” Dr. Kramer and his associates added (Lancet 2006;368:1444–8).
Despite being rare, amniotic fluid embolism is one of the leading causes of maternal mortality in developed countries, ahead of postpartum hemorrhage and other pulmonary embolisms. The cause of this catastrophic complication is not well understood. “It is thought to arise from a simultaneous tear in the fetal membranes and uterine vessels, through which amniotic fluid can pass into the uterine venous circulation and hence to the maternal pulmonary arterial circulation,” the researchers said.
Strong uterine contractions are believed to raise the risk of amniotic fluid embolism, and induction and augmentation of labor have been proposed as possible contributing factors.
In their epidemiologic study of the association between drug-induced labor and amniotic fluid embolism, there were 180 cases of this condition, including 24 fatal cases, yielding a total rate of 6/100,000 singleton deliveries and a fatal rate of 0.8/100,000 singleton deliveries.
The rate was almost twice as high in women who had undergone medical induction of labor as in those who had not. This association remained robust after the data were adjusted to account for many other potential risk factors, such as maternal age, presentation, delivery method, previous cesarean delivery, pregnancy complications, and labor complications.
This finding “should be a cause for concern in view of the increasing tendency for clinicians to induce labour, and especially for routine induction at term or after term,” the investigators said.
They also found that multiple pregnancy, older maternal age, cesarean delivery, forceps- or vacuum-assisted delivery, eclampsia, polyhydramnios, placenta previa, placental abruption, cervical laceration, uterine rupture, and fetal distress all raised the risk of amniotic fluid embolism, though not to the degree that drug-induced labor did. Many of these risk factors could be directly related to “the presumed causal roles of strong uterine contractions, excess amniotic fluid, and disruption of the uterine vasculature,” the researchers noted.
Moreover, the link between amniotic embolism on the one hand and cesarean or instrumental vaginal delivery on the other “might simply reflect the difficult labours that led to these procedures, rather than true effects of the procedures themselves.”
In particular, “the very strong association with caesarean delivery could also indicate reverse causality (i.e., the caesarean could have been a consequence of the signs and symptoms of amniotic fluid embolism,” Dr. Kramer and his associates pointed out.
The rates of total and fatal amniotic fluid embolism did not increase over time in this study, even though the rate of medically induced labor did increase. This probably reflects the rarity of the disorder, or it could be because of the concomitant decrease in other risk factors, such as forceps delivery, they added.
Medical induction of labor appears to double the risk of amniotic fluid embolism, reported Dr. Michael S. Kramer of Montreal Children's Hospital and his associates.
Findings from their retrospective cohort study of approximately 3 million hospital births throughout Canada between 1991 and 2002 “support the long-standing, but heretofore unsubstantiated, suspicion that labour induction increases the risk of this rare but serious maternal complication.
“Although the small absolute risk of amniotic fluid embolism is unlikely to affect the decision to induce labour in the presence of compelling clinical indications, women and physicians should be aware of this risk if the decision is elective,” the researchers said in the Oct. 21 issue of the Lancet.
“We should emphasise that the absolute risk increase of amniotic fluid embolism for women undergoing medical induction of labour is very small: 4 or 5 total cases and 1 or 2 fatal cases per 100,000 women induced.
“However, with 4 million births per year and induction rates approaching 20% in the [United States], this practice could be causing amniotic fluid embolism in 30–40 women per year in the [United States] alone (including 10–15 deaths),” Dr. Kramer and his associates added (Lancet 2006;368:1444–8).
Despite being rare, amniotic fluid embolism is one of the leading causes of maternal mortality in developed countries, ahead of postpartum hemorrhage and other pulmonary embolisms. The cause of this catastrophic complication is not well understood. “It is thought to arise from a simultaneous tear in the fetal membranes and uterine vessels, through which amniotic fluid can pass into the uterine venous circulation and hence to the maternal pulmonary arterial circulation,” the researchers said.
Strong uterine contractions are believed to raise the risk of amniotic fluid embolism, and induction and augmentation of labor have been proposed as possible contributing factors.
In their epidemiologic study of the association between drug-induced labor and amniotic fluid embolism, there were 180 cases of this condition, including 24 fatal cases, yielding a total rate of 6/100,000 singleton deliveries and a fatal rate of 0.8/100,000 singleton deliveries.
The rate was almost twice as high in women who had undergone medical induction of labor as in those who had not. This association remained robust after the data were adjusted to account for many other potential risk factors, such as maternal age, presentation, delivery method, previous cesarean delivery, pregnancy complications, and labor complications.
This finding “should be a cause for concern in view of the increasing tendency for clinicians to induce labour, and especially for routine induction at term or after term,” the investigators said.
They also found that multiple pregnancy, older maternal age, cesarean delivery, forceps- or vacuum-assisted delivery, eclampsia, polyhydramnios, placenta previa, placental abruption, cervical laceration, uterine rupture, and fetal distress all raised the risk of amniotic fluid embolism, though not to the degree that drug-induced labor did. Many of these risk factors could be directly related to “the presumed causal roles of strong uterine contractions, excess amniotic fluid, and disruption of the uterine vasculature,” the researchers noted.
Moreover, the link between amniotic embolism on the one hand and cesarean or instrumental vaginal delivery on the other “might simply reflect the difficult labours that led to these procedures, rather than true effects of the procedures themselves.”
In particular, “the very strong association with caesarean delivery could also indicate reverse causality (i.e., the caesarean could have been a consequence of the signs and symptoms of amniotic fluid embolism,” Dr. Kramer and his associates pointed out.
The rates of total and fatal amniotic fluid embolism did not increase over time in this study, even though the rate of medically induced labor did increase. This probably reflects the rarity of the disorder, or it could be because of the concomitant decrease in other risk factors, such as forceps delivery, they added.
Medical induction of labor appears to double the risk of amniotic fluid embolism, reported Dr. Michael S. Kramer of Montreal Children's Hospital and his associates.
Findings from their retrospective cohort study of approximately 3 million hospital births throughout Canada between 1991 and 2002 “support the long-standing, but heretofore unsubstantiated, suspicion that labour induction increases the risk of this rare but serious maternal complication.
“Although the small absolute risk of amniotic fluid embolism is unlikely to affect the decision to induce labour in the presence of compelling clinical indications, women and physicians should be aware of this risk if the decision is elective,” the researchers said in the Oct. 21 issue of the Lancet.
“We should emphasise that the absolute risk increase of amniotic fluid embolism for women undergoing medical induction of labour is very small: 4 or 5 total cases and 1 or 2 fatal cases per 100,000 women induced.
“However, with 4 million births per year and induction rates approaching 20% in the [United States], this practice could be causing amniotic fluid embolism in 30–40 women per year in the [United States] alone (including 10–15 deaths),” Dr. Kramer and his associates added (Lancet 2006;368:1444–8).
Despite being rare, amniotic fluid embolism is one of the leading causes of maternal mortality in developed countries, ahead of postpartum hemorrhage and other pulmonary embolisms. The cause of this catastrophic complication is not well understood. “It is thought to arise from a simultaneous tear in the fetal membranes and uterine vessels, through which amniotic fluid can pass into the uterine venous circulation and hence to the maternal pulmonary arterial circulation,” the researchers said.
Strong uterine contractions are believed to raise the risk of amniotic fluid embolism, and induction and augmentation of labor have been proposed as possible contributing factors.
In their epidemiologic study of the association between drug-induced labor and amniotic fluid embolism, there were 180 cases of this condition, including 24 fatal cases, yielding a total rate of 6/100,000 singleton deliveries and a fatal rate of 0.8/100,000 singleton deliveries.
The rate was almost twice as high in women who had undergone medical induction of labor as in those who had not. This association remained robust after the data were adjusted to account for many other potential risk factors, such as maternal age, presentation, delivery method, previous cesarean delivery, pregnancy complications, and labor complications.
This finding “should be a cause for concern in view of the increasing tendency for clinicians to induce labour, and especially for routine induction at term or after term,” the investigators said.
They also found that multiple pregnancy, older maternal age, cesarean delivery, forceps- or vacuum-assisted delivery, eclampsia, polyhydramnios, placenta previa, placental abruption, cervical laceration, uterine rupture, and fetal distress all raised the risk of amniotic fluid embolism, though not to the degree that drug-induced labor did. Many of these risk factors could be directly related to “the presumed causal roles of strong uterine contractions, excess amniotic fluid, and disruption of the uterine vasculature,” the researchers noted.
Moreover, the link between amniotic embolism on the one hand and cesarean or instrumental vaginal delivery on the other “might simply reflect the difficult labours that led to these procedures, rather than true effects of the procedures themselves.”
In particular, “the very strong association with caesarean delivery could also indicate reverse causality (i.e., the caesarean could have been a consequence of the signs and symptoms of amniotic fluid embolism,” Dr. Kramer and his associates pointed out.
The rates of total and fatal amniotic fluid embolism did not increase over time in this study, even though the rate of medically induced labor did increase. This probably reflects the rarity of the disorder, or it could be because of the concomitant decrease in other risk factors, such as forceps delivery, they added.
Management of prolonged decelerations
A prolonged deceleration may signal danger—or reflect a perfectly normal fetal response to maternal pelvic examination. Because of the wide range of possibilities, this fetal heart rate pattern justifies close attention. For example, repetitive prolonged decelerations may indicate cord compression from oligohydramnios. Even more troubling, a prolonged deceleration may occur for the first time during the evolution of a profound catastrophe, such as amniotic fluid embolism or uterine rupture during vaginal birth after cesarean delivery (VBAC). In some circumstances, a prolonged deceleration may be the terminus of a progression of nonreassuring fetal heart rate (FHR) changes, and becomes the immediate precursor to fetal death (TABLE 1).1
When FHR patterns exhibit these aberrations, we rightly worry about fetal well-being and the possible need for operative intervention. Unfortunately, the degree of fetal compromise is difficult to predict and depends on preexisting fetal condition, physiologic reserve, degree and duration of the insult, and other variables.
TABLE 1
Some causes of prolonged decelerations and bradycardias
| PROLONGED DECELERATION | BRADYCARDIA |
|---|---|
| Cord compression Oligohydramnios Cord prolapse Uteroplacental insufficiency Anesthesia (paracervical, spinal, epidural) Maternal valsalva Maternal supine hypotension Hypertonic or prolonged contractions Abruptio placentae Uterine rupture Cocaine ingestion Maternal hypoxia Maternal seizures, eclampsia Respiratory depression from medications Cardiopulmonary arrest Amniotic fluid embolism Fetal hemorrhage Vasa previa Traumatic amniocentesis Fetal vagal reaction Rapid descent, impending birth Cervical examination Fetal scalp electrode placement Fetal blood sampling Fetal central nervous system anomalies Idiopathic (cord compression?) | Congenital conduction abnormalities Complete heart block Long QT syndrome Congenital heart defects Tachyarrhythmia (Fetal tachyarrhythmia may produce an EFM tracing that appears to be a bradycardia and can only be distinguished by ultrasound) Medications Beta blockers Hypothermia Infection Chorioamnionitis Endotoxemia |
Ultimately, a judgment call
The 22nd edition of Williams Obstetrics2 summarizes the clinical challenges involved in the management of prolonged decelerations during labor: “Management of isolated prolonged decelerations is based on bedside clinical judgment, which inevitably will sometimes be imperfect given the unpredictability of these decelerations.”
“Fetal bradycardia” and “prolonged deceleration” are distinct entities
In general parlance, we often use the terms “fetal bradycardia” and “prolonged deceleration” loosely. In practice, we must differentiate these entities because underlying pathophysiologic mechanisms and clinical management may differ substantially.
The problem: Since the introduction of electronic fetal monitoring (EFM) in the 1960s, numerous descriptions of FHR patterns have been published, each slightly different from the others. The result: confusing nomenclature, miscommunication among clinicians, and stymied research efforts.
To standardize definitions of intrapartum FHR patterns so that the effectiveness of EFM could be better assessed in observational studies and clinical trials, the National Institute of Child Health and Human Development organized a workshop.3 Its recommendations were subsequently adopted by the American College of Obstetricians and Gynecologists (ACOG).4 Among the definitions:
Differentiation between the 2 entities is critical because, in many cases, bradycardias are chronic patterns that may not be associated with immediate fetal compromise and do not require immediate intervention. For example, a fetal bradycardia due to congenital heart block would not benefit from immediate delivery, especially prior to term.
“Moderate fetal bradycardia,” defined as a baseline of 100 to 119 bpm, was reported in 1.8% of 1,386 continuously monitored patients and is attributed to relative cephalopelvic disproportion, resolving after rotation of the fetal vertex and associated with normal neonatal outcome.5,6
Similar decelerations can reflect different events
The exact depth and duration of a prolonged deceleration leading to fetal compromise and requiring prompt delivery is difficult to define, although some observations warrant consideration. Experiments with fetal lambs show that the deceleration in response to umbilical vein occlusion is associated with a fall in fetal blood pressure, whereas deceleration in response to umbilical artery occlusion is associated with a rise in fetal blood pressure. This reflex can be abolished by vagotomy, but will eventually recur due to anoxia.7
Vital clue: What happened before the prolonged deceleration?
In clinical practice, it is important to appreciate characteristics of the FHR pattern preceding the prolonged deceleration.8 Williams and Galerneau9 correlated baseline FHR variability and duration of prolonged decelerations with neonatal acid–base status in 186 term gestations with an identified prolonged deceleration within 30 minutes of delivery. Patients were divided into 4 groups, based on FHR variability and recovery of the FHR baseline (TABLE 2).
The findings:
TABLE 2
Neonatal outcomes associated with variability and recovery of FHR patterns after prolonged deceleration
| UMBILICAL ARTERY | GROUP 1 V+ R+ (N=128) | GROUP 2 V+ R- (N=40) | GROUP 3 V- R+ (N=9) | GROUP 4 V- R- (N=9) | P VALUE |
|---|---|---|---|---|---|
| pH (mean±SD) | 7.17±0.09 | 7.13±0.15 | 7.11±0.11 | 6.83±0.16 | <.001 |
| Base deficit (mean±SD) | -6.5±3.9 | -7.2±5.1 | -10±4 | -20±6 | <.001 |
| pH <7.0 (%) | 2 | 18 | 44 | 78 | <.001 |
| pH <7.1 (%) | 22 | 33 | 56 | 89 | <.001 |
| Base deficit <16 (%) | 1 | 8 | 11 | 78 | <.001 |
| Base deficit <12 (%) | 5 | 13 | 22 | 89 | <.001 |
| V=variability | |||||
| R=recovery | |||||
| SOURCE: Williams and Galerneau9 | |||||
Acid–base changes likely begin within minutes of cord compression
Zilianti and colleagues10 evaluated 29 fetuses with normal FHR patterns during labor with FHR deceleration during the expulsion phase of delivery. When the FHR deceleration was prolonged (>120 seconds), umbilical artery pH significantly decreased (7.19 vs 7.27), umbilical vein pH remained unchanged (7.32), and the umbilical venous–arterial pH difference was significantly increased (0.13 vs 0.05). Thus, acid–base changes likely begin within minutes of cord compression.
The correlation between acidemia and loss of variability
In their review of 43 vacuum extractions, Gull and colleagues22 found that 27 infants were delivered for “end-stage bradycardia” (abrupt persistent decrease in FHR to less than 100 bpm for more than 2 minutes, or repeated deceleration more than 60 bpm below baseline with poor recovery), and 16 were delivered electively (controls). Umbilical-cord base deficit was greater in the newborns with bradycardia than in controls, and the length of time FHR variability was lost correlated with the degree of base deficit. Acidemic fetuses lost FHR variability during the bradycardia for more than 4 minutes, or started to lose FHR variability less than 3 minutes from the beginning of the bradycardia.
What is optimal interval between deceleration and delivery?
In a series of 106 cases of uterine rupture during VBAC, Leung et al11 found significant neonatal morbidity when 18 minutes or more lapsed between the onset of the prolonged deceleration and delivery.
First, remain calm when decelerations occur
Freeman and colleagues12 advocate staying calm and avoiding overreaction, because many cases will resolve spontaneously. Nonetheless, prolonged decelerations should prompt the physician to:
TABLE 3
6 pearls for managing prolonged decelerations
| GOAL | PEARL | |
|---|---|---|
| 1 | Reduce aorto-caval and/or cord compression | Change patient positioning |
| 2 | Restore intravascular volume | Administer intravenous fluid bolus |
| 3 | Reduce uterine activity | Discontinue oxytocin drip and give tocolytic therapy (terbutaline) |
| 4 | Enhance oxygen delivery to fetus | Give supplemental oxygen |
| 5 | Resolve hypotension | Administer vasopressor therapy (ephedrine) |
| 6 | Resolve oligohydramnios and cord compression | Perform transcervical amnioinfusion |
TABLE 4
Stepwise management of prolonged decelerations
| Examine the cervix Check for umbilical cord prolapse Check progress of dilation and descent Place internal monitors, if indicated |
| Determine probable cause |
| Start therapies |
| Prepare for intervention by operative delivery Intravenous access Blood type and screen Indwelling urinary catheter Obtain consents for operative vaginal delivery and cesarean delivery Notify appropriate personnel (eg, anesthesiology, pediatrics) |
| Deliver If fetal condition is nonreassuring despite therapies If prolonged decelerations recur and spontaneous delivery is remote (cases must be individualized) |
Consider amnioinfusion when cord compression is suspected
Many cases of prolonged decelerations are secondary to cord compression resulting from oligohydramnios. Miyazaki13 showed that saline amnioinfusion helped correct the FHR problem in most cases of repetitive variable decelerations (19 of 28) and prolonged decelerations (12 of 14 cases).
Several randomized clinical trials analyzed in a recent Cochrane Review14 suggest that amnioinfusion for cord compression reduces the occurrence of variable FHR decelerations and the need for cesarean section; this applies to settings in which nonreassuring FHR patterns were not further assessed by fetal blood sampling, which is reflective of practice in most US labor units.
The recent ACOG practice bulletin on intrapartum monitoring4 advocates amnioinfusion for recurrent variable FHR decelerations, but does not address prolonged decelerations specifically.
Although most data on amnioinfusion address treatment of recurrent variable FHR decelerations, it also seems reasonable to consider this option for prolonged decelerations when oligohydramnios is suspected.12
Other possible causes of prolonged decelerations
Vasa previa. A sudden prolonged deceleration following rupture of membranes with concomitant vaginal bleeding should prompt the physician to consider the possibility of a disrupted velamentous cord insertion (vasa previa), which can lead to rapid fetal exsanguination.15
Acute profound maternal hypoxemia may lead to a first prolonged FHR deceleration, often preceded by increased uterine tone, as described in both eclampsia16 and amniotic fluid embolism.17 With eclampsia, the prolonged deceleration is reversible; treatment and expectant management will allow for fetal recovery after the seizure abates.
When acute amniotic fluid embolism leads to profound cardiovascular collapse, prompt perimortem cesarean delivery may be required within minutes if CPR does not restore normal maternal cardiopulmonary function and recovery of FHR.
When is scalp stimulation helpful?
Stimulation of the fetal scalp is an effective technique for assessing fetal status during periods of nonreassuring FHR patterns.18 However, the technique is intended to be performed during periods of FHR baseline and is sometimes misapplied during prolonged decelerations. Scalp stimulation during a prolonged deceleration would not likely provide valid information or change clinical management and could in theory exacerbate fetal compromise if additional parasympathetic tone were elicited.
Avoid fetal pulse oximetry
Although fetal pulse oximetry is FDA-approved and commercially available in the United States, and may be well suited for monitoring fetal arrhythmias,19,20 a prolonged deceleration is an absolute contraindication to its use.21
Summary
Overall, in managing a delivery marked by prolonged decelerations, we should strive to minimize maternal–fetal complications by carefully assessing the clinical situation, correcting reversible problems, and preparing for expeditious delivery if the fetal condition is of sufficient concern that further expectant management is unlikely to allow for safe spontaneous delivery. Still, “…bedside judgment inevitably will sometimes be imperfect given the unpredictability of these decelerations.”2
The author reports no financial affiliations relevant to this article.
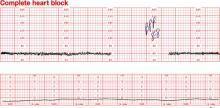
Dilemma: Fetal bradycardia due to congenital complete heart block secondary to anti-SSA/Ro and anti-SS-B/La antibodies. The fetal ventricular rate is fixed at 60 bpm
Management: At 30 weeks’ gestation, with no sonographic evidence of heart failure and a biophysical profile score of 8/8, expectant management is indicated
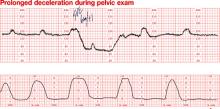
Dilemma: Prolonged deceleration during pelvic examination in an uncomplicated term pregnancy. Note that fetal heart rate (FHR) variability was maintained during recovery of the FHR baseline
Outcome: Uneventful spontaneous vaginal delivery
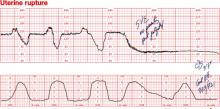
Dilemma: Prolonged deceleration due to uterine rupture during trial of labor after cesarean. Repetitive variable decelerations preceded the prolonged deceleration. FHR variability was lost after several minutes
Management: Emergency cesarean
1. Cetrulo CL, Schifrin BS. Fetal heart rate patterns preceding death in utero. Obstet Gynecol. 1976;48:521-527.
2. Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 22nd ed. New York: McGraw-Hill; 2005.
3. Electronic fetal heart rate monitoring: research guidelines for interpretation. National Institute of Child Health and Human Development Research Planning Workshop. Am J Obstet Gynecol. 1997;177:1385-1390.
4. Intrapartum fetal heart rate monitoring. ACOG Practice Bulletin #70. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2005;106:1453-1461.
5. Young BK, Weinstein HM. Moderate fetal bradycardia. Am J Obstet Gynecol. 1976;126:271-275.
6. Young BK, Katz M, et al. Fetal blood and tissue pH with moderate bradycardia. Am J Obstet Gynecol. 1979;135:45-47.
7. Reynolds SR. Bradycardia in the lamb fetus in response to circulatory distress. Am J Physiol. 1954;176:169-174.
8. Langer O, Sonnendecker EW. Characteristics and management of intrapartum prolonged fetal bradycardia. Br J Obstet Gynaecol. 1982;89:904-912.
9. Williams KP, Galerneau F. Fetal heart rate parameters predictive of neonatal outcome in the presence of a prolonged deceleration. Obstet Gynecol. 2002;100:951-954.
10. Zilianti M, Segura CL, et al. Studies on fetal bradycardia during birth process. II. Obstet Gynecol. 1973;42:840-843.
11. Leung AS, Leung EK, Paul RH. Uterine rupture after previous cesarean delivery: maternal and fetal consequences. Am J Obstet Gynecol. 1993;169:945-950.
12. Freeman RK, Garite TG, Nageotte MP. Fetal Heart Rate Monitoring. Philadelphia: Lippincott Williams & Wilkins; 2003.
13. Miyazaki FS, Taylor NA. Saline amnioinfusion for relief of variable or prolonged decelerations. A preliminary report. Am J Obstet Gynecol. 1983;146:670-678.
14. Hofmeyr GJ. Amnioinfusion for umbilical cord compression in labour. Cochrane Database Syst Rev. 2000;CD000013.-
15. Gabbe SG, Nelson LM, Paul RH. Fetal heart rate response to acute hemorrhage. Obstet Gynecol. 1977;49:247-251.
16. Paul RH, Koh KS, Bernstein SG. Changes in fetal heart rateuterine contraction patterns associated with eclampsia. Am J Obstet Gynecol. 1978;130:165-169.
17. Clark SL, Hankins GD, Dudley DA, Dildy GA, Porter TF. Amniotic fluid embolism: analysis of the national registry. Am J Obstet Gynecol. 1995;172:1158-1167;discussion 1167-1169.
18. Clark SL, Gimovsky ML, Miller FC. The scalp stimulation test: a clinical alternative to fetal scalp blood sampling. Am J Obstet Gynecol. 1984;148:274-277.
19. Dildy GA, Loucks CA, Clark SL. Intrapartum fetal pulse oximetry in the presence of fetal cardiac arrhythmia. Am J Obstet Gynecol. 1993;169:1609-1611.
20. van den Berg PP, Nijland R, van den Brand SF, Jongsma HW, Nijhuis JG. Intrapartum fetal surveillance of congenital heart block with pulse oximetry. Obstet Gynecol. 1994;84:683-686.
21. Garite TJ, Dildy GA, McNamara H, et al. A multicenter controlled trial of fetal pulse oximetry in the intrapartum management of nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2000;183:1049-1058.
22. Gull I, Jaffa AJ, Oren M, Grisaru D, Peyser MR, Lessing JB. Acid accumulation during end-stage bradycardia in term fetuses: how long is too long? Br J Obstet Gynaecol. 1996;103:1096-1101.
A prolonged deceleration may signal danger—or reflect a perfectly normal fetal response to maternal pelvic examination. Because of the wide range of possibilities, this fetal heart rate pattern justifies close attention. For example, repetitive prolonged decelerations may indicate cord compression from oligohydramnios. Even more troubling, a prolonged deceleration may occur for the first time during the evolution of a profound catastrophe, such as amniotic fluid embolism or uterine rupture during vaginal birth after cesarean delivery (VBAC). In some circumstances, a prolonged deceleration may be the terminus of a progression of nonreassuring fetal heart rate (FHR) changes, and becomes the immediate precursor to fetal death (TABLE 1).1
When FHR patterns exhibit these aberrations, we rightly worry about fetal well-being and the possible need for operative intervention. Unfortunately, the degree of fetal compromise is difficult to predict and depends on preexisting fetal condition, physiologic reserve, degree and duration of the insult, and other variables.
TABLE 1
Some causes of prolonged decelerations and bradycardias
| PROLONGED DECELERATION | BRADYCARDIA |
|---|---|
| Cord compression Oligohydramnios Cord prolapse Uteroplacental insufficiency Anesthesia (paracervical, spinal, epidural) Maternal valsalva Maternal supine hypotension Hypertonic or prolonged contractions Abruptio placentae Uterine rupture Cocaine ingestion Maternal hypoxia Maternal seizures, eclampsia Respiratory depression from medications Cardiopulmonary arrest Amniotic fluid embolism Fetal hemorrhage Vasa previa Traumatic amniocentesis Fetal vagal reaction Rapid descent, impending birth Cervical examination Fetal scalp electrode placement Fetal blood sampling Fetal central nervous system anomalies Idiopathic (cord compression?) | Congenital conduction abnormalities Complete heart block Long QT syndrome Congenital heart defects Tachyarrhythmia (Fetal tachyarrhythmia may produce an EFM tracing that appears to be a bradycardia and can only be distinguished by ultrasound) Medications Beta blockers Hypothermia Infection Chorioamnionitis Endotoxemia |
Ultimately, a judgment call
The 22nd edition of Williams Obstetrics2 summarizes the clinical challenges involved in the management of prolonged decelerations during labor: “Management of isolated prolonged decelerations is based on bedside clinical judgment, which inevitably will sometimes be imperfect given the unpredictability of these decelerations.”
“Fetal bradycardia” and “prolonged deceleration” are distinct entities
In general parlance, we often use the terms “fetal bradycardia” and “prolonged deceleration” loosely. In practice, we must differentiate these entities because underlying pathophysiologic mechanisms and clinical management may differ substantially.
The problem: Since the introduction of electronic fetal monitoring (EFM) in the 1960s, numerous descriptions of FHR patterns have been published, each slightly different from the others. The result: confusing nomenclature, miscommunication among clinicians, and stymied research efforts.
To standardize definitions of intrapartum FHR patterns so that the effectiveness of EFM could be better assessed in observational studies and clinical trials, the National Institute of Child Health and Human Development organized a workshop.3 Its recommendations were subsequently adopted by the American College of Obstetricians and Gynecologists (ACOG).4 Among the definitions:
Differentiation between the 2 entities is critical because, in many cases, bradycardias are chronic patterns that may not be associated with immediate fetal compromise and do not require immediate intervention. For example, a fetal bradycardia due to congenital heart block would not benefit from immediate delivery, especially prior to term.
“Moderate fetal bradycardia,” defined as a baseline of 100 to 119 bpm, was reported in 1.8% of 1,386 continuously monitored patients and is attributed to relative cephalopelvic disproportion, resolving after rotation of the fetal vertex and associated with normal neonatal outcome.5,6
Similar decelerations can reflect different events
The exact depth and duration of a prolonged deceleration leading to fetal compromise and requiring prompt delivery is difficult to define, although some observations warrant consideration. Experiments with fetal lambs show that the deceleration in response to umbilical vein occlusion is associated with a fall in fetal blood pressure, whereas deceleration in response to umbilical artery occlusion is associated with a rise in fetal blood pressure. This reflex can be abolished by vagotomy, but will eventually recur due to anoxia.7
Vital clue: What happened before the prolonged deceleration?
In clinical practice, it is important to appreciate characteristics of the FHR pattern preceding the prolonged deceleration.8 Williams and Galerneau9 correlated baseline FHR variability and duration of prolonged decelerations with neonatal acid–base status in 186 term gestations with an identified prolonged deceleration within 30 minutes of delivery. Patients were divided into 4 groups, based on FHR variability and recovery of the FHR baseline (TABLE 2).
The findings:
TABLE 2
Neonatal outcomes associated with variability and recovery of FHR patterns after prolonged deceleration
| UMBILICAL ARTERY | GROUP 1 V+ R+ (N=128) | GROUP 2 V+ R- (N=40) | GROUP 3 V- R+ (N=9) | GROUP 4 V- R- (N=9) | P VALUE |
|---|---|---|---|---|---|
| pH (mean±SD) | 7.17±0.09 | 7.13±0.15 | 7.11±0.11 | 6.83±0.16 | <.001 |
| Base deficit (mean±SD) | -6.5±3.9 | -7.2±5.1 | -10±4 | -20±6 | <.001 |
| pH <7.0 (%) | 2 | 18 | 44 | 78 | <.001 |
| pH <7.1 (%) | 22 | 33 | 56 | 89 | <.001 |
| Base deficit <16 (%) | 1 | 8 | 11 | 78 | <.001 |
| Base deficit <12 (%) | 5 | 13 | 22 | 89 | <.001 |
| V=variability | |||||
| R=recovery | |||||
| SOURCE: Williams and Galerneau9 | |||||
Acid–base changes likely begin within minutes of cord compression
Zilianti and colleagues10 evaluated 29 fetuses with normal FHR patterns during labor with FHR deceleration during the expulsion phase of delivery. When the FHR deceleration was prolonged (>120 seconds), umbilical artery pH significantly decreased (7.19 vs 7.27), umbilical vein pH remained unchanged (7.32), and the umbilical venous–arterial pH difference was significantly increased (0.13 vs 0.05). Thus, acid–base changes likely begin within minutes of cord compression.
The correlation between acidemia and loss of variability
In their review of 43 vacuum extractions, Gull and colleagues22 found that 27 infants were delivered for “end-stage bradycardia” (abrupt persistent decrease in FHR to less than 100 bpm for more than 2 minutes, or repeated deceleration more than 60 bpm below baseline with poor recovery), and 16 were delivered electively (controls). Umbilical-cord base deficit was greater in the newborns with bradycardia than in controls, and the length of time FHR variability was lost correlated with the degree of base deficit. Acidemic fetuses lost FHR variability during the bradycardia for more than 4 minutes, or started to lose FHR variability less than 3 minutes from the beginning of the bradycardia.
What is optimal interval between deceleration and delivery?
In a series of 106 cases of uterine rupture during VBAC, Leung et al11 found significant neonatal morbidity when 18 minutes or more lapsed between the onset of the prolonged deceleration and delivery.
First, remain calm when decelerations occur
Freeman and colleagues12 advocate staying calm and avoiding overreaction, because many cases will resolve spontaneously. Nonetheless, prolonged decelerations should prompt the physician to:
TABLE 3
6 pearls for managing prolonged decelerations
| GOAL | PEARL | |
|---|---|---|
| 1 | Reduce aorto-caval and/or cord compression | Change patient positioning |
| 2 | Restore intravascular volume | Administer intravenous fluid bolus |
| 3 | Reduce uterine activity | Discontinue oxytocin drip and give tocolytic therapy (terbutaline) |
| 4 | Enhance oxygen delivery to fetus | Give supplemental oxygen |
| 5 | Resolve hypotension | Administer vasopressor therapy (ephedrine) |
| 6 | Resolve oligohydramnios and cord compression | Perform transcervical amnioinfusion |
TABLE 4
Stepwise management of prolonged decelerations
| Examine the cervix Check for umbilical cord prolapse Check progress of dilation and descent Place internal monitors, if indicated |
| Determine probable cause |
| Start therapies |
| Prepare for intervention by operative delivery Intravenous access Blood type and screen Indwelling urinary catheter Obtain consents for operative vaginal delivery and cesarean delivery Notify appropriate personnel (eg, anesthesiology, pediatrics) |
| Deliver If fetal condition is nonreassuring despite therapies If prolonged decelerations recur and spontaneous delivery is remote (cases must be individualized) |
Consider amnioinfusion when cord compression is suspected
Many cases of prolonged decelerations are secondary to cord compression resulting from oligohydramnios. Miyazaki13 showed that saline amnioinfusion helped correct the FHR problem in most cases of repetitive variable decelerations (19 of 28) and prolonged decelerations (12 of 14 cases).
Several randomized clinical trials analyzed in a recent Cochrane Review14 suggest that amnioinfusion for cord compression reduces the occurrence of variable FHR decelerations and the need for cesarean section; this applies to settings in which nonreassuring FHR patterns were not further assessed by fetal blood sampling, which is reflective of practice in most US labor units.
The recent ACOG practice bulletin on intrapartum monitoring4 advocates amnioinfusion for recurrent variable FHR decelerations, but does not address prolonged decelerations specifically.
Although most data on amnioinfusion address treatment of recurrent variable FHR decelerations, it also seems reasonable to consider this option for prolonged decelerations when oligohydramnios is suspected.12
Other possible causes of prolonged decelerations
Vasa previa. A sudden prolonged deceleration following rupture of membranes with concomitant vaginal bleeding should prompt the physician to consider the possibility of a disrupted velamentous cord insertion (vasa previa), which can lead to rapid fetal exsanguination.15
Acute profound maternal hypoxemia may lead to a first prolonged FHR deceleration, often preceded by increased uterine tone, as described in both eclampsia16 and amniotic fluid embolism.17 With eclampsia, the prolonged deceleration is reversible; treatment and expectant management will allow for fetal recovery after the seizure abates.
When acute amniotic fluid embolism leads to profound cardiovascular collapse, prompt perimortem cesarean delivery may be required within minutes if CPR does not restore normal maternal cardiopulmonary function and recovery of FHR.
When is scalp stimulation helpful?
Stimulation of the fetal scalp is an effective technique for assessing fetal status during periods of nonreassuring FHR patterns.18 However, the technique is intended to be performed during periods of FHR baseline and is sometimes misapplied during prolonged decelerations. Scalp stimulation during a prolonged deceleration would not likely provide valid information or change clinical management and could in theory exacerbate fetal compromise if additional parasympathetic tone were elicited.
Avoid fetal pulse oximetry
Although fetal pulse oximetry is FDA-approved and commercially available in the United States, and may be well suited for monitoring fetal arrhythmias,19,20 a prolonged deceleration is an absolute contraindication to its use.21
Summary
Overall, in managing a delivery marked by prolonged decelerations, we should strive to minimize maternal–fetal complications by carefully assessing the clinical situation, correcting reversible problems, and preparing for expeditious delivery if the fetal condition is of sufficient concern that further expectant management is unlikely to allow for safe spontaneous delivery. Still, “…bedside judgment inevitably will sometimes be imperfect given the unpredictability of these decelerations.”2
The author reports no financial affiliations relevant to this article.
Dilemma: Fetal bradycardia due to congenital complete heart block secondary to anti-SSA/Ro and anti-SS-B/La antibodies. The fetal ventricular rate is fixed at 60 bpm
Management: At 30 weeks’ gestation, with no sonographic evidence of heart failure and a biophysical profile score of 8/8, expectant management is indicated
Dilemma: Prolonged deceleration during pelvic examination in an uncomplicated term pregnancy. Note that fetal heart rate (FHR) variability was maintained during recovery of the FHR baseline
Outcome: Uneventful spontaneous vaginal delivery
Dilemma: Prolonged deceleration due to uterine rupture during trial of labor after cesarean. Repetitive variable decelerations preceded the prolonged deceleration. FHR variability was lost after several minutes
Management: Emergency cesarean
A prolonged deceleration may signal danger—or reflect a perfectly normal fetal response to maternal pelvic examination. Because of the wide range of possibilities, this fetal heart rate pattern justifies close attention. For example, repetitive prolonged decelerations may indicate cord compression from oligohydramnios. Even more troubling, a prolonged deceleration may occur for the first time during the evolution of a profound catastrophe, such as amniotic fluid embolism or uterine rupture during vaginal birth after cesarean delivery (VBAC). In some circumstances, a prolonged deceleration may be the terminus of a progression of nonreassuring fetal heart rate (FHR) changes, and becomes the immediate precursor to fetal death (TABLE 1).1
When FHR patterns exhibit these aberrations, we rightly worry about fetal well-being and the possible need for operative intervention. Unfortunately, the degree of fetal compromise is difficult to predict and depends on preexisting fetal condition, physiologic reserve, degree and duration of the insult, and other variables.
TABLE 1
Some causes of prolonged decelerations and bradycardias
| PROLONGED DECELERATION | BRADYCARDIA |
|---|---|
| Cord compression Oligohydramnios Cord prolapse Uteroplacental insufficiency Anesthesia (paracervical, spinal, epidural) Maternal valsalva Maternal supine hypotension Hypertonic or prolonged contractions Abruptio placentae Uterine rupture Cocaine ingestion Maternal hypoxia Maternal seizures, eclampsia Respiratory depression from medications Cardiopulmonary arrest Amniotic fluid embolism Fetal hemorrhage Vasa previa Traumatic amniocentesis Fetal vagal reaction Rapid descent, impending birth Cervical examination Fetal scalp electrode placement Fetal blood sampling Fetal central nervous system anomalies Idiopathic (cord compression?) | Congenital conduction abnormalities Complete heart block Long QT syndrome Congenital heart defects Tachyarrhythmia (Fetal tachyarrhythmia may produce an EFM tracing that appears to be a bradycardia and can only be distinguished by ultrasound) Medications Beta blockers Hypothermia Infection Chorioamnionitis Endotoxemia |
Ultimately, a judgment call
The 22nd edition of Williams Obstetrics2 summarizes the clinical challenges involved in the management of prolonged decelerations during labor: “Management of isolated prolonged decelerations is based on bedside clinical judgment, which inevitably will sometimes be imperfect given the unpredictability of these decelerations.”
“Fetal bradycardia” and “prolonged deceleration” are distinct entities
In general parlance, we often use the terms “fetal bradycardia” and “prolonged deceleration” loosely. In practice, we must differentiate these entities because underlying pathophysiologic mechanisms and clinical management may differ substantially.
The problem: Since the introduction of electronic fetal monitoring (EFM) in the 1960s, numerous descriptions of FHR patterns have been published, each slightly different from the others. The result: confusing nomenclature, miscommunication among clinicians, and stymied research efforts.
To standardize definitions of intrapartum FHR patterns so that the effectiveness of EFM could be better assessed in observational studies and clinical trials, the National Institute of Child Health and Human Development organized a workshop.3 Its recommendations were subsequently adopted by the American College of Obstetricians and Gynecologists (ACOG).4 Among the definitions:
Differentiation between the 2 entities is critical because, in many cases, bradycardias are chronic patterns that may not be associated with immediate fetal compromise and do not require immediate intervention. For example, a fetal bradycardia due to congenital heart block would not benefit from immediate delivery, especially prior to term.
“Moderate fetal bradycardia,” defined as a baseline of 100 to 119 bpm, was reported in 1.8% of 1,386 continuously monitored patients and is attributed to relative cephalopelvic disproportion, resolving after rotation of the fetal vertex and associated with normal neonatal outcome.5,6
Similar decelerations can reflect different events
The exact depth and duration of a prolonged deceleration leading to fetal compromise and requiring prompt delivery is difficult to define, although some observations warrant consideration. Experiments with fetal lambs show that the deceleration in response to umbilical vein occlusion is associated with a fall in fetal blood pressure, whereas deceleration in response to umbilical artery occlusion is associated with a rise in fetal blood pressure. This reflex can be abolished by vagotomy, but will eventually recur due to anoxia.7
Vital clue: What happened before the prolonged deceleration?
In clinical practice, it is important to appreciate characteristics of the FHR pattern preceding the prolonged deceleration.8 Williams and Galerneau9 correlated baseline FHR variability and duration of prolonged decelerations with neonatal acid–base status in 186 term gestations with an identified prolonged deceleration within 30 minutes of delivery. Patients were divided into 4 groups, based on FHR variability and recovery of the FHR baseline (TABLE 2).
The findings:
TABLE 2
Neonatal outcomes associated with variability and recovery of FHR patterns after prolonged deceleration
| UMBILICAL ARTERY | GROUP 1 V+ R+ (N=128) | GROUP 2 V+ R- (N=40) | GROUP 3 V- R+ (N=9) | GROUP 4 V- R- (N=9) | P VALUE |
|---|---|---|---|---|---|
| pH (mean±SD) | 7.17±0.09 | 7.13±0.15 | 7.11±0.11 | 6.83±0.16 | <.001 |
| Base deficit (mean±SD) | -6.5±3.9 | -7.2±5.1 | -10±4 | -20±6 | <.001 |
| pH <7.0 (%) | 2 | 18 | 44 | 78 | <.001 |
| pH <7.1 (%) | 22 | 33 | 56 | 89 | <.001 |
| Base deficit <16 (%) | 1 | 8 | 11 | 78 | <.001 |
| Base deficit <12 (%) | 5 | 13 | 22 | 89 | <.001 |
| V=variability | |||||
| R=recovery | |||||
| SOURCE: Williams and Galerneau9 | |||||
Acid–base changes likely begin within minutes of cord compression
Zilianti and colleagues10 evaluated 29 fetuses with normal FHR patterns during labor with FHR deceleration during the expulsion phase of delivery. When the FHR deceleration was prolonged (>120 seconds), umbilical artery pH significantly decreased (7.19 vs 7.27), umbilical vein pH remained unchanged (7.32), and the umbilical venous–arterial pH difference was significantly increased (0.13 vs 0.05). Thus, acid–base changes likely begin within minutes of cord compression.
The correlation between acidemia and loss of variability
In their review of 43 vacuum extractions, Gull and colleagues22 found that 27 infants were delivered for “end-stage bradycardia” (abrupt persistent decrease in FHR to less than 100 bpm for more than 2 minutes, or repeated deceleration more than 60 bpm below baseline with poor recovery), and 16 were delivered electively (controls). Umbilical-cord base deficit was greater in the newborns with bradycardia than in controls, and the length of time FHR variability was lost correlated with the degree of base deficit. Acidemic fetuses lost FHR variability during the bradycardia for more than 4 minutes, or started to lose FHR variability less than 3 minutes from the beginning of the bradycardia.
What is optimal interval between deceleration and delivery?
In a series of 106 cases of uterine rupture during VBAC, Leung et al11 found significant neonatal morbidity when 18 minutes or more lapsed between the onset of the prolonged deceleration and delivery.
First, remain calm when decelerations occur
Freeman and colleagues12 advocate staying calm and avoiding overreaction, because many cases will resolve spontaneously. Nonetheless, prolonged decelerations should prompt the physician to:
TABLE 3
6 pearls for managing prolonged decelerations
| GOAL | PEARL | |
|---|---|---|
| 1 | Reduce aorto-caval and/or cord compression | Change patient positioning |
| 2 | Restore intravascular volume | Administer intravenous fluid bolus |
| 3 | Reduce uterine activity | Discontinue oxytocin drip and give tocolytic therapy (terbutaline) |
| 4 | Enhance oxygen delivery to fetus | Give supplemental oxygen |
| 5 | Resolve hypotension | Administer vasopressor therapy (ephedrine) |
| 6 | Resolve oligohydramnios and cord compression | Perform transcervical amnioinfusion |
TABLE 4
Stepwise management of prolonged decelerations
| Examine the cervix Check for umbilical cord prolapse Check progress of dilation and descent Place internal monitors, if indicated |
| Determine probable cause |
| Start therapies |
| Prepare for intervention by operative delivery Intravenous access Blood type and screen Indwelling urinary catheter Obtain consents for operative vaginal delivery and cesarean delivery Notify appropriate personnel (eg, anesthesiology, pediatrics) |
| Deliver If fetal condition is nonreassuring despite therapies If prolonged decelerations recur and spontaneous delivery is remote (cases must be individualized) |
Consider amnioinfusion when cord compression is suspected
Many cases of prolonged decelerations are secondary to cord compression resulting from oligohydramnios. Miyazaki13 showed that saline amnioinfusion helped correct the FHR problem in most cases of repetitive variable decelerations (19 of 28) and prolonged decelerations (12 of 14 cases).
Several randomized clinical trials analyzed in a recent Cochrane Review14 suggest that amnioinfusion for cord compression reduces the occurrence of variable FHR decelerations and the need for cesarean section; this applies to settings in which nonreassuring FHR patterns were not further assessed by fetal blood sampling, which is reflective of practice in most US labor units.
The recent ACOG practice bulletin on intrapartum monitoring4 advocates amnioinfusion for recurrent variable FHR decelerations, but does not address prolonged decelerations specifically.
Although most data on amnioinfusion address treatment of recurrent variable FHR decelerations, it also seems reasonable to consider this option for prolonged decelerations when oligohydramnios is suspected.12
Other possible causes of prolonged decelerations
Vasa previa. A sudden prolonged deceleration following rupture of membranes with concomitant vaginal bleeding should prompt the physician to consider the possibility of a disrupted velamentous cord insertion (vasa previa), which can lead to rapid fetal exsanguination.15
Acute profound maternal hypoxemia may lead to a first prolonged FHR deceleration, often preceded by increased uterine tone, as described in both eclampsia16 and amniotic fluid embolism.17 With eclampsia, the prolonged deceleration is reversible; treatment and expectant management will allow for fetal recovery after the seizure abates.
When acute amniotic fluid embolism leads to profound cardiovascular collapse, prompt perimortem cesarean delivery may be required within minutes if CPR does not restore normal maternal cardiopulmonary function and recovery of FHR.
When is scalp stimulation helpful?
Stimulation of the fetal scalp is an effective technique for assessing fetal status during periods of nonreassuring FHR patterns.18 However, the technique is intended to be performed during periods of FHR baseline and is sometimes misapplied during prolonged decelerations. Scalp stimulation during a prolonged deceleration would not likely provide valid information or change clinical management and could in theory exacerbate fetal compromise if additional parasympathetic tone were elicited.
Avoid fetal pulse oximetry
Although fetal pulse oximetry is FDA-approved and commercially available in the United States, and may be well suited for monitoring fetal arrhythmias,19,20 a prolonged deceleration is an absolute contraindication to its use.21
Summary
Overall, in managing a delivery marked by prolonged decelerations, we should strive to minimize maternal–fetal complications by carefully assessing the clinical situation, correcting reversible problems, and preparing for expeditious delivery if the fetal condition is of sufficient concern that further expectant management is unlikely to allow for safe spontaneous delivery. Still, “…bedside judgment inevitably will sometimes be imperfect given the unpredictability of these decelerations.”2
The author reports no financial affiliations relevant to this article.
Dilemma: Fetal bradycardia due to congenital complete heart block secondary to anti-SSA/Ro and anti-SS-B/La antibodies. The fetal ventricular rate is fixed at 60 bpm
Management: At 30 weeks’ gestation, with no sonographic evidence of heart failure and a biophysical profile score of 8/8, expectant management is indicated
Dilemma: Prolonged deceleration during pelvic examination in an uncomplicated term pregnancy. Note that fetal heart rate (FHR) variability was maintained during recovery of the FHR baseline
Outcome: Uneventful spontaneous vaginal delivery
Dilemma: Prolonged deceleration due to uterine rupture during trial of labor after cesarean. Repetitive variable decelerations preceded the prolonged deceleration. FHR variability was lost after several minutes
Management: Emergency cesarean
1. Cetrulo CL, Schifrin BS. Fetal heart rate patterns preceding death in utero. Obstet Gynecol. 1976;48:521-527.
2. Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 22nd ed. New York: McGraw-Hill; 2005.
3. Electronic fetal heart rate monitoring: research guidelines for interpretation. National Institute of Child Health and Human Development Research Planning Workshop. Am J Obstet Gynecol. 1997;177:1385-1390.
4. Intrapartum fetal heart rate monitoring. ACOG Practice Bulletin #70. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2005;106:1453-1461.
5. Young BK, Weinstein HM. Moderate fetal bradycardia. Am J Obstet Gynecol. 1976;126:271-275.
6. Young BK, Katz M, et al. Fetal blood and tissue pH with moderate bradycardia. Am J Obstet Gynecol. 1979;135:45-47.
7. Reynolds SR. Bradycardia in the lamb fetus in response to circulatory distress. Am J Physiol. 1954;176:169-174.
8. Langer O, Sonnendecker EW. Characteristics and management of intrapartum prolonged fetal bradycardia. Br J Obstet Gynaecol. 1982;89:904-912.
9. Williams KP, Galerneau F. Fetal heart rate parameters predictive of neonatal outcome in the presence of a prolonged deceleration. Obstet Gynecol. 2002;100:951-954.
10. Zilianti M, Segura CL, et al. Studies on fetal bradycardia during birth process. II. Obstet Gynecol. 1973;42:840-843.
11. Leung AS, Leung EK, Paul RH. Uterine rupture after previous cesarean delivery: maternal and fetal consequences. Am J Obstet Gynecol. 1993;169:945-950.
12. Freeman RK, Garite TG, Nageotte MP. Fetal Heart Rate Monitoring. Philadelphia: Lippincott Williams & Wilkins; 2003.
13. Miyazaki FS, Taylor NA. Saline amnioinfusion for relief of variable or prolonged decelerations. A preliminary report. Am J Obstet Gynecol. 1983;146:670-678.
14. Hofmeyr GJ. Amnioinfusion for umbilical cord compression in labour. Cochrane Database Syst Rev. 2000;CD000013.-
15. Gabbe SG, Nelson LM, Paul RH. Fetal heart rate response to acute hemorrhage. Obstet Gynecol. 1977;49:247-251.
16. Paul RH, Koh KS, Bernstein SG. Changes in fetal heart rateuterine contraction patterns associated with eclampsia. Am J Obstet Gynecol. 1978;130:165-169.
17. Clark SL, Hankins GD, Dudley DA, Dildy GA, Porter TF. Amniotic fluid embolism: analysis of the national registry. Am J Obstet Gynecol. 1995;172:1158-1167;discussion 1167-1169.
18. Clark SL, Gimovsky ML, Miller FC. The scalp stimulation test: a clinical alternative to fetal scalp blood sampling. Am J Obstet Gynecol. 1984;148:274-277.
19. Dildy GA, Loucks CA, Clark SL. Intrapartum fetal pulse oximetry in the presence of fetal cardiac arrhythmia. Am J Obstet Gynecol. 1993;169:1609-1611.
20. van den Berg PP, Nijland R, van den Brand SF, Jongsma HW, Nijhuis JG. Intrapartum fetal surveillance of congenital heart block with pulse oximetry. Obstet Gynecol. 1994;84:683-686.
21. Garite TJ, Dildy GA, McNamara H, et al. A multicenter controlled trial of fetal pulse oximetry in the intrapartum management of nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2000;183:1049-1058.
22. Gull I, Jaffa AJ, Oren M, Grisaru D, Peyser MR, Lessing JB. Acid accumulation during end-stage bradycardia in term fetuses: how long is too long? Br J Obstet Gynaecol. 1996;103:1096-1101.
1. Cetrulo CL, Schifrin BS. Fetal heart rate patterns preceding death in utero. Obstet Gynecol. 1976;48:521-527.
2. Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 22nd ed. New York: McGraw-Hill; 2005.
3. Electronic fetal heart rate monitoring: research guidelines for interpretation. National Institute of Child Health and Human Development Research Planning Workshop. Am J Obstet Gynecol. 1997;177:1385-1390.
4. Intrapartum fetal heart rate monitoring. ACOG Practice Bulletin #70. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2005;106:1453-1461.
5. Young BK, Weinstein HM. Moderate fetal bradycardia. Am J Obstet Gynecol. 1976;126:271-275.
6. Young BK, Katz M, et al. Fetal blood and tissue pH with moderate bradycardia. Am J Obstet Gynecol. 1979;135:45-47.
7. Reynolds SR. Bradycardia in the lamb fetus in response to circulatory distress. Am J Physiol. 1954;176:169-174.
8. Langer O, Sonnendecker EW. Characteristics and management of intrapartum prolonged fetal bradycardia. Br J Obstet Gynaecol. 1982;89:904-912.
9. Williams KP, Galerneau F. Fetal heart rate parameters predictive of neonatal outcome in the presence of a prolonged deceleration. Obstet Gynecol. 2002;100:951-954.
10. Zilianti M, Segura CL, et al. Studies on fetal bradycardia during birth process. II. Obstet Gynecol. 1973;42:840-843.
11. Leung AS, Leung EK, Paul RH. Uterine rupture after previous cesarean delivery: maternal and fetal consequences. Am J Obstet Gynecol. 1993;169:945-950.
12. Freeman RK, Garite TG, Nageotte MP. Fetal Heart Rate Monitoring. Philadelphia: Lippincott Williams & Wilkins; 2003.
13. Miyazaki FS, Taylor NA. Saline amnioinfusion for relief of variable or prolonged decelerations. A preliminary report. Am J Obstet Gynecol. 1983;146:670-678.
14. Hofmeyr GJ. Amnioinfusion for umbilical cord compression in labour. Cochrane Database Syst Rev. 2000;CD000013.-
15. Gabbe SG, Nelson LM, Paul RH. Fetal heart rate response to acute hemorrhage. Obstet Gynecol. 1977;49:247-251.
16. Paul RH, Koh KS, Bernstein SG. Changes in fetal heart rateuterine contraction patterns associated with eclampsia. Am J Obstet Gynecol. 1978;130:165-169.
17. Clark SL, Hankins GD, Dudley DA, Dildy GA, Porter TF. Amniotic fluid embolism: analysis of the national registry. Am J Obstet Gynecol. 1995;172:1158-1167;discussion 1167-1169.
18. Clark SL, Gimovsky ML, Miller FC. The scalp stimulation test: a clinical alternative to fetal scalp blood sampling. Am J Obstet Gynecol. 1984;148:274-277.
19. Dildy GA, Loucks CA, Clark SL. Intrapartum fetal pulse oximetry in the presence of fetal cardiac arrhythmia. Am J Obstet Gynecol. 1993;169:1609-1611.
20. van den Berg PP, Nijland R, van den Brand SF, Jongsma HW, Nijhuis JG. Intrapartum fetal surveillance of congenital heart block with pulse oximetry. Obstet Gynecol. 1994;84:683-686.
21. Garite TJ, Dildy GA, McNamara H, et al. A multicenter controlled trial of fetal pulse oximetry in the intrapartum management of nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2000;183:1049-1058.
22. Gull I, Jaffa AJ, Oren M, Grisaru D, Peyser MR, Lessing JB. Acid accumulation during end-stage bradycardia in term fetuses: how long is too long? Br J Obstet Gynaecol. 1996;103:1096-1101.
Major Adverse Events Hit 25% In Peripartum Cardiomyopathy
SEATTLE — One-fourth of 182 women with peripartum cardiomyopathy developed major adverse events, half of which were death or heart transplantation, Dr. Sorel Goland reported in a poster presentation at the annual meeting of the Heart Failure Society of America.
Women with peripartum cardiomyopathy who had very low ejection fractions of 25% or who were not white were most likely to develop major adverse events, 50% of which occurred before the diagnosis of peripartum cardiomyopathy was made, said Dr. Goland of the department of cardiology at Cedars-Sinai Medical Center, Los Angeles, and associates.
A delay in diagnosing peripartum cardiomyopathy of a week or longer increased the risk for death or heart transplant fivefold.
“Diagnosis of peripartum cardiomyopathy is often delayed and preceded by major adverse events. Early diagnosis and aggressive therapy, including treatment of heart failure, anticoagulation, and sudden death prevention, should improve the outcome of patients,” the investigators stated.
The clinical profile of peripartum cardiomyopathy and the risk factors for complications have not been well characterized previously because of the low incidence of this disorder. Peripartum cardiomyopathy occurs during pregnancy or the postpartum period because of unknown causes and can be associated with severe complications.
The retrospective review of data on 182 patients found that 25% died, underwent heart transplantation, developed cardiopulmonary arrest, required temporary circulatory support by an intra-aortic balloon pump or a left ventricular assist device, developed pulmonary edema or thromboembolic complications, or had a pacemaker or implantable cardioverter defibrillator implanted. Of the 46 major adverse events, 36 (78%) occurred within 6 months of the diagnosis of peripartum cardiomyopathy.
Of the 182 patients, 24 (13%) died or underwent heart transplantation; 16 of the 24 deaths or transplants happened within 6 months of diagnosis.
Patients with ejection fractions of 25% or less had quadruple the risk for major adverse events in general and for death or heart transplant, compared with patients who had higher ejection fractions. Nonwhite patients were three times more likely to develop major complications and four times more likely to die or need a heart transplant, compared with white patients.
ELSEVIER GLOBAL MEDICAL NEWS
SEATTLE — One-fourth of 182 women with peripartum cardiomyopathy developed major adverse events, half of which were death or heart transplantation, Dr. Sorel Goland reported in a poster presentation at the annual meeting of the Heart Failure Society of America.
Women with peripartum cardiomyopathy who had very low ejection fractions of 25% or who were not white were most likely to develop major adverse events, 50% of which occurred before the diagnosis of peripartum cardiomyopathy was made, said Dr. Goland of the department of cardiology at Cedars-Sinai Medical Center, Los Angeles, and associates.
A delay in diagnosing peripartum cardiomyopathy of a week or longer increased the risk for death or heart transplant fivefold.
“Diagnosis of peripartum cardiomyopathy is often delayed and preceded by major adverse events. Early diagnosis and aggressive therapy, including treatment of heart failure, anticoagulation, and sudden death prevention, should improve the outcome of patients,” the investigators stated.
The clinical profile of peripartum cardiomyopathy and the risk factors for complications have not been well characterized previously because of the low incidence of this disorder. Peripartum cardiomyopathy occurs during pregnancy or the postpartum period because of unknown causes and can be associated with severe complications.
The retrospective review of data on 182 patients found that 25% died, underwent heart transplantation, developed cardiopulmonary arrest, required temporary circulatory support by an intra-aortic balloon pump or a left ventricular assist device, developed pulmonary edema or thromboembolic complications, or had a pacemaker or implantable cardioverter defibrillator implanted. Of the 46 major adverse events, 36 (78%) occurred within 6 months of the diagnosis of peripartum cardiomyopathy.
Of the 182 patients, 24 (13%) died or underwent heart transplantation; 16 of the 24 deaths or transplants happened within 6 months of diagnosis.
Patients with ejection fractions of 25% or less had quadruple the risk for major adverse events in general and for death or heart transplant, compared with patients who had higher ejection fractions. Nonwhite patients were three times more likely to develop major complications and four times more likely to die or need a heart transplant, compared with white patients.
ELSEVIER GLOBAL MEDICAL NEWS
SEATTLE — One-fourth of 182 women with peripartum cardiomyopathy developed major adverse events, half of which were death or heart transplantation, Dr. Sorel Goland reported in a poster presentation at the annual meeting of the Heart Failure Society of America.
Women with peripartum cardiomyopathy who had very low ejection fractions of 25% or who were not white were most likely to develop major adverse events, 50% of which occurred before the diagnosis of peripartum cardiomyopathy was made, said Dr. Goland of the department of cardiology at Cedars-Sinai Medical Center, Los Angeles, and associates.
A delay in diagnosing peripartum cardiomyopathy of a week or longer increased the risk for death or heart transplant fivefold.
“Diagnosis of peripartum cardiomyopathy is often delayed and preceded by major adverse events. Early diagnosis and aggressive therapy, including treatment of heart failure, anticoagulation, and sudden death prevention, should improve the outcome of patients,” the investigators stated.
The clinical profile of peripartum cardiomyopathy and the risk factors for complications have not been well characterized previously because of the low incidence of this disorder. Peripartum cardiomyopathy occurs during pregnancy or the postpartum period because of unknown causes and can be associated with severe complications.
The retrospective review of data on 182 patients found that 25% died, underwent heart transplantation, developed cardiopulmonary arrest, required temporary circulatory support by an intra-aortic balloon pump or a left ventricular assist device, developed pulmonary edema or thromboembolic complications, or had a pacemaker or implantable cardioverter defibrillator implanted. Of the 46 major adverse events, 36 (78%) occurred within 6 months of the diagnosis of peripartum cardiomyopathy.
Of the 182 patients, 24 (13%) died or underwent heart transplantation; 16 of the 24 deaths or transplants happened within 6 months of diagnosis.
Patients with ejection fractions of 25% or less had quadruple the risk for major adverse events in general and for death or heart transplant, compared with patients who had higher ejection fractions. Nonwhite patients were three times more likely to develop major complications and four times more likely to die or need a heart transplant, compared with white patients.
ELSEVIER GLOBAL MEDICAL NEWS
Lupus Pregnancy Outcomes Depend on Multiple Factors
BOSTON — Successful pregnancies in women with systemic lupus erythematosus depend on a combination of factors, including disease activity at the time of conception, maternal renal function, the presence of lupus-related autoantibodies, and medication use, according to Dr. Lisa Sammaritano of Cornell University, Ithaca, N.Y.
In terms of disease activity, “it has been shown time and again that patients with inactive disease for 6 or more months prior to conception have a substantially reduced risk of experiencing a disease flare during pregnancy than women with active disease,” Dr. Sammaritano reported at a meeting on rheumatology sponsored by Harvard Medical School. “[Physicians] should have this in mind during prepregnancy consultations and advise patients to wait for periods of stable disease before trying to conceive.”
Maternal renal function should also be evaluated prior to conception, said Dr. Sammaritano. In women with renal insufficiency, pregnancy can accelerate a decline in renal function and worsen hypertension and proteinuria, thus increasing the risk of maternal and fetal complications, such as preeclampsia, intrauterine growth restriction, and intrauterine death.
Additionally, “it's essential to assess renal function before pregnancy in women with renal insufficiency in order to better differentiate worsening lupus-related renal disease from superimposed preeclampsia during pregnancy,” Dr. Sammaritano said. Kidney problems during pregnancy are more likely to be related to systemic lupus erythematosus (SLE) renal disease than to preeclampsia if the patient exhibits clinical symptoms of active SLE, has an elevated anti-double-stranded DNA antibody, or has detectable red blood cell casts in the urine, she said.
The presence and levels of certain lupus-related autoantibodies can also affect pregnancy outcome, Dr. Sammaritano noted. The antiphospholipid antibodies as well as lupus anticoagulant and medium to high anticardiolipin antibodies have been associated with recurrent pregnancy losses, poor fetal growth, preeclampsia, and stillbirths in women with lupus. Identifying the antibodies ahead of time “is critical, because studies have shown that treatment with medication, such as aspirin or heparin, during pregnancy can improve the viability of the fetus,” she added.
Two other lupus-related autoantibodies—anti-SS-A and anti-SS-B—can have an effect on the babies born to mothers with lupus. The presence of one or both of these IgG autoantibodies in the mother increases the risk of neonatal lupus erythematosus (NLE), which can cause rash or changes in blood counts or liver function and, in severe cases, can affect the conduction system of the heart, Dr. Sammaritano said.
For pregnant women who test positive for anti-SS-A or anti-SS-B, current management guidelines recommend weekly fetal echocardiographic monitoring between gestational weeks 16 and 26 and biweekly monitoring between weeks 27 and 34 to look for congenital heart block, she said.
In terms of medication during pregnancy for women with lupus, corticosteroids should be used for active disease only.
“If a patient is on a dose of steroid, in general we will continue at a low dose during the course of the pregnancy. However, we do not recommend prophylactic steroids in patients without active disease in an effort to prevent a flare,” Dr. Sammaritano said.
BOSTON — Successful pregnancies in women with systemic lupus erythematosus depend on a combination of factors, including disease activity at the time of conception, maternal renal function, the presence of lupus-related autoantibodies, and medication use, according to Dr. Lisa Sammaritano of Cornell University, Ithaca, N.Y.
In terms of disease activity, “it has been shown time and again that patients with inactive disease for 6 or more months prior to conception have a substantially reduced risk of experiencing a disease flare during pregnancy than women with active disease,” Dr. Sammaritano reported at a meeting on rheumatology sponsored by Harvard Medical School. “[Physicians] should have this in mind during prepregnancy consultations and advise patients to wait for periods of stable disease before trying to conceive.”
Maternal renal function should also be evaluated prior to conception, said Dr. Sammaritano. In women with renal insufficiency, pregnancy can accelerate a decline in renal function and worsen hypertension and proteinuria, thus increasing the risk of maternal and fetal complications, such as preeclampsia, intrauterine growth restriction, and intrauterine death.
Additionally, “it's essential to assess renal function before pregnancy in women with renal insufficiency in order to better differentiate worsening lupus-related renal disease from superimposed preeclampsia during pregnancy,” Dr. Sammaritano said. Kidney problems during pregnancy are more likely to be related to systemic lupus erythematosus (SLE) renal disease than to preeclampsia if the patient exhibits clinical symptoms of active SLE, has an elevated anti-double-stranded DNA antibody, or has detectable red blood cell casts in the urine, she said.
The presence and levels of certain lupus-related autoantibodies can also affect pregnancy outcome, Dr. Sammaritano noted. The antiphospholipid antibodies as well as lupus anticoagulant and medium to high anticardiolipin antibodies have been associated with recurrent pregnancy losses, poor fetal growth, preeclampsia, and stillbirths in women with lupus. Identifying the antibodies ahead of time “is critical, because studies have shown that treatment with medication, such as aspirin or heparin, during pregnancy can improve the viability of the fetus,” she added.
Two other lupus-related autoantibodies—anti-SS-A and anti-SS-B—can have an effect on the babies born to mothers with lupus. The presence of one or both of these IgG autoantibodies in the mother increases the risk of neonatal lupus erythematosus (NLE), which can cause rash or changes in blood counts or liver function and, in severe cases, can affect the conduction system of the heart, Dr. Sammaritano said.
For pregnant women who test positive for anti-SS-A or anti-SS-B, current management guidelines recommend weekly fetal echocardiographic monitoring between gestational weeks 16 and 26 and biweekly monitoring between weeks 27 and 34 to look for congenital heart block, she said.
In terms of medication during pregnancy for women with lupus, corticosteroids should be used for active disease only.
“If a patient is on a dose of steroid, in general we will continue at a low dose during the course of the pregnancy. However, we do not recommend prophylactic steroids in patients without active disease in an effort to prevent a flare,” Dr. Sammaritano said.
BOSTON — Successful pregnancies in women with systemic lupus erythematosus depend on a combination of factors, including disease activity at the time of conception, maternal renal function, the presence of lupus-related autoantibodies, and medication use, according to Dr. Lisa Sammaritano of Cornell University, Ithaca, N.Y.
In terms of disease activity, “it has been shown time and again that patients with inactive disease for 6 or more months prior to conception have a substantially reduced risk of experiencing a disease flare during pregnancy than women with active disease,” Dr. Sammaritano reported at a meeting on rheumatology sponsored by Harvard Medical School. “[Physicians] should have this in mind during prepregnancy consultations and advise patients to wait for periods of stable disease before trying to conceive.”
Maternal renal function should also be evaluated prior to conception, said Dr. Sammaritano. In women with renal insufficiency, pregnancy can accelerate a decline in renal function and worsen hypertension and proteinuria, thus increasing the risk of maternal and fetal complications, such as preeclampsia, intrauterine growth restriction, and intrauterine death.
Additionally, “it's essential to assess renal function before pregnancy in women with renal insufficiency in order to better differentiate worsening lupus-related renal disease from superimposed preeclampsia during pregnancy,” Dr. Sammaritano said. Kidney problems during pregnancy are more likely to be related to systemic lupus erythematosus (SLE) renal disease than to preeclampsia if the patient exhibits clinical symptoms of active SLE, has an elevated anti-double-stranded DNA antibody, or has detectable red blood cell casts in the urine, she said.
The presence and levels of certain lupus-related autoantibodies can also affect pregnancy outcome, Dr. Sammaritano noted. The antiphospholipid antibodies as well as lupus anticoagulant and medium to high anticardiolipin antibodies have been associated with recurrent pregnancy losses, poor fetal growth, preeclampsia, and stillbirths in women with lupus. Identifying the antibodies ahead of time “is critical, because studies have shown that treatment with medication, such as aspirin or heparin, during pregnancy can improve the viability of the fetus,” she added.
Two other lupus-related autoantibodies—anti-SS-A and anti-SS-B—can have an effect on the babies born to mothers with lupus. The presence of one or both of these IgG autoantibodies in the mother increases the risk of neonatal lupus erythematosus (NLE), which can cause rash or changes in blood counts or liver function and, in severe cases, can affect the conduction system of the heart, Dr. Sammaritano said.
For pregnant women who test positive for anti-SS-A or anti-SS-B, current management guidelines recommend weekly fetal echocardiographic monitoring between gestational weeks 16 and 26 and biweekly monitoring between weeks 27 and 34 to look for congenital heart block, she said.
In terms of medication during pregnancy for women with lupus, corticosteroids should be used for active disease only.
“If a patient is on a dose of steroid, in general we will continue at a low dose during the course of the pregnancy. However, we do not recommend prophylactic steroids in patients without active disease in an effort to prevent a flare,” Dr. Sammaritano said.
Gentamicin in Utero Does Not Cause Hearing Loss
MONTEREY, CALIF. — Neonates who were exposed to gentamicin in utero when their mothers were treated for pyelonephritis showed no increased risk of failing screening tests for hearing loss in a controlled study of 284 pregnancies, Dr. Tony Wen said.
The ongoing retrospective chart review analyzed cases of pregnant women who received gentamicin and ampicillin for pyelonephritis and delivered at 32 weeks' gestational age or later with the next gentamicin-free pregnancy matched by gestational age. All neonates underwent otoacoustic emissions testing after birth. Failure or incomplete results brought a second round of otoacoustic emissions testing before discharge. A second failure led to referral for more definitive hearing tests.
Among women with pyelonephritis, 92% received gentamicin, which crosses the placenta at term. Overall, 8% of newborns exposed to gentamicin in utero and 10% of controls failed otoacoustic emissions testing—opposite the trend that investigators expected to see because of concerns about gentamicin's potential effects on hearing, he said at the annual meeting of the Infectious Diseases Society for Obstetrics and Gynecology.
In the general population, 5%–8% of newborns fail otoacoustic emissions tests. The study was inspired by animal studies that found hearing loss in rats exposed in utero to gentamicin.
Gentamicin is an aminoglycoside with broad-spectrum coverage that is commonly used to treat women with pyelonephritis and chorioamnionitis, according to Dr. Wen of the University of Texas, Galveston, and his associates.
A subset analysis by trimester found that early-trimester exposure to gentamicin did not increase risk for otoacoustic emissions testing failure.
Neonates who were small for gestational age showed no increased risk for failing otoacoustic emissions testing from gentamicin exposure.
There was a statistically nonsignificant trend for longer courses of gentamicin to increase the risk for otoacoustic emissions test failure. After 4 days or less of gentamicin treatment, 2% of neonates failed the tests, compared with 12% of neonates whose mothers got gentamicin for 5 days or longer.
“If you have a patient who requires prolonged treatment, once you get the sensitivity testing results, you might consider changing antibiotics” if the mother is on gentamicin, Dr. Wen said.
Women treated with gentamicin and ampicillin received gentamicin in a loading dose of 120 mg followed by 80 mg gentamicin every 8 hours. Patients got 6–20 doses of gentamicin in the study.
The case and control groups did not differ in maternal or gestational age, gravidity, Apgar scores, birth weights, or mode of delivery, among other factors.
MONTEREY, CALIF. — Neonates who were exposed to gentamicin in utero when their mothers were treated for pyelonephritis showed no increased risk of failing screening tests for hearing loss in a controlled study of 284 pregnancies, Dr. Tony Wen said.
The ongoing retrospective chart review analyzed cases of pregnant women who received gentamicin and ampicillin for pyelonephritis and delivered at 32 weeks' gestational age or later with the next gentamicin-free pregnancy matched by gestational age. All neonates underwent otoacoustic emissions testing after birth. Failure or incomplete results brought a second round of otoacoustic emissions testing before discharge. A second failure led to referral for more definitive hearing tests.
Among women with pyelonephritis, 92% received gentamicin, which crosses the placenta at term. Overall, 8% of newborns exposed to gentamicin in utero and 10% of controls failed otoacoustic emissions testing—opposite the trend that investigators expected to see because of concerns about gentamicin's potential effects on hearing, he said at the annual meeting of the Infectious Diseases Society for Obstetrics and Gynecology.
In the general population, 5%–8% of newborns fail otoacoustic emissions tests. The study was inspired by animal studies that found hearing loss in rats exposed in utero to gentamicin.
Gentamicin is an aminoglycoside with broad-spectrum coverage that is commonly used to treat women with pyelonephritis and chorioamnionitis, according to Dr. Wen of the University of Texas, Galveston, and his associates.
A subset analysis by trimester found that early-trimester exposure to gentamicin did not increase risk for otoacoustic emissions testing failure.
Neonates who were small for gestational age showed no increased risk for failing otoacoustic emissions testing from gentamicin exposure.
There was a statistically nonsignificant trend for longer courses of gentamicin to increase the risk for otoacoustic emissions test failure. After 4 days or less of gentamicin treatment, 2% of neonates failed the tests, compared with 12% of neonates whose mothers got gentamicin for 5 days or longer.
“If you have a patient who requires prolonged treatment, once you get the sensitivity testing results, you might consider changing antibiotics” if the mother is on gentamicin, Dr. Wen said.
Women treated with gentamicin and ampicillin received gentamicin in a loading dose of 120 mg followed by 80 mg gentamicin every 8 hours. Patients got 6–20 doses of gentamicin in the study.
The case and control groups did not differ in maternal or gestational age, gravidity, Apgar scores, birth weights, or mode of delivery, among other factors.
MONTEREY, CALIF. — Neonates who were exposed to gentamicin in utero when their mothers were treated for pyelonephritis showed no increased risk of failing screening tests for hearing loss in a controlled study of 284 pregnancies, Dr. Tony Wen said.
The ongoing retrospective chart review analyzed cases of pregnant women who received gentamicin and ampicillin for pyelonephritis and delivered at 32 weeks' gestational age or later with the next gentamicin-free pregnancy matched by gestational age. All neonates underwent otoacoustic emissions testing after birth. Failure or incomplete results brought a second round of otoacoustic emissions testing before discharge. A second failure led to referral for more definitive hearing tests.
Among women with pyelonephritis, 92% received gentamicin, which crosses the placenta at term. Overall, 8% of newborns exposed to gentamicin in utero and 10% of controls failed otoacoustic emissions testing—opposite the trend that investigators expected to see because of concerns about gentamicin's potential effects on hearing, he said at the annual meeting of the Infectious Diseases Society for Obstetrics and Gynecology.
In the general population, 5%–8% of newborns fail otoacoustic emissions tests. The study was inspired by animal studies that found hearing loss in rats exposed in utero to gentamicin.
Gentamicin is an aminoglycoside with broad-spectrum coverage that is commonly used to treat women with pyelonephritis and chorioamnionitis, according to Dr. Wen of the University of Texas, Galveston, and his associates.
A subset analysis by trimester found that early-trimester exposure to gentamicin did not increase risk for otoacoustic emissions testing failure.
Neonates who were small for gestational age showed no increased risk for failing otoacoustic emissions testing from gentamicin exposure.
There was a statistically nonsignificant trend for longer courses of gentamicin to increase the risk for otoacoustic emissions test failure. After 4 days or less of gentamicin treatment, 2% of neonates failed the tests, compared with 12% of neonates whose mothers got gentamicin for 5 days or longer.
“If you have a patient who requires prolonged treatment, once you get the sensitivity testing results, you might consider changing antibiotics” if the mother is on gentamicin, Dr. Wen said.
Women treated with gentamicin and ampicillin received gentamicin in a loading dose of 120 mg followed by 80 mg gentamicin every 8 hours. Patients got 6–20 doses of gentamicin in the study.
The case and control groups did not differ in maternal or gestational age, gravidity, Apgar scores, birth weights, or mode of delivery, among other factors.
Study Challenges Breast-Feeding as IQ Boost for Kids
Breast-feeding has little or no effect on children's later intelligence, according to the largest study ever to address this question.
The influence of breast-feeding on cognitive ability has been debated for more than 70 years, with multiple potentially confounding factors having been identified. These include socioeconomic status, birth weight, maternal history of smoking, maternal and paternal education, and race or ethnicity. Maternal intelligence, however, has largely been overlooked as a potential confounder in studies investigating the effects of breast-feeding, according to Geoff Der, a statistician at the Medical Research Council's Social and Public Health Sciences Unit in Glasgow, Scotland.
Analyzing data from 5,475 children and their mothers in the population-based U.S. national longitudinal survey of youth that began in 1979, Mr. Der and his colleagues found that if maternal intelligence was not included as a potential confounder, breast-feeding did appear to exert beneficial effects on children's intelligence, adding approximately 4 points. However, when maternal intelligence was included in the analysis, breast-feeding was associated with an increase of less than half a point (BMJ 2006 Oct. 4 [doi:10.1136/bmj.38978.699583.55]). Previous studies' not having considered maternal intelligence was “surprising given the heritability of intelligence,” he observed.
The researchers also determined that an increase of 15 points in maternal intelligence more than doubled the likelihood that the child would be breast-fed. They furthermore observed that mothers who breast-fed tended to be older and more educated, and to provide a more stimulating home environment.
Despite the lack of association with children's intelligence, breast-feeding remains important for overall growth and development, Mr. Der noted.
Breast-feeding has little or no effect on children's later intelligence, according to the largest study ever to address this question.
The influence of breast-feeding on cognitive ability has been debated for more than 70 years, with multiple potentially confounding factors having been identified. These include socioeconomic status, birth weight, maternal history of smoking, maternal and paternal education, and race or ethnicity. Maternal intelligence, however, has largely been overlooked as a potential confounder in studies investigating the effects of breast-feeding, according to Geoff Der, a statistician at the Medical Research Council's Social and Public Health Sciences Unit in Glasgow, Scotland.
Analyzing data from 5,475 children and their mothers in the population-based U.S. national longitudinal survey of youth that began in 1979, Mr. Der and his colleagues found that if maternal intelligence was not included as a potential confounder, breast-feeding did appear to exert beneficial effects on children's intelligence, adding approximately 4 points. However, when maternal intelligence was included in the analysis, breast-feeding was associated with an increase of less than half a point (BMJ 2006 Oct. 4 [doi:10.1136/bmj.38978.699583.55]). Previous studies' not having considered maternal intelligence was “surprising given the heritability of intelligence,” he observed.
The researchers also determined that an increase of 15 points in maternal intelligence more than doubled the likelihood that the child would be breast-fed. They furthermore observed that mothers who breast-fed tended to be older and more educated, and to provide a more stimulating home environment.
Despite the lack of association with children's intelligence, breast-feeding remains important for overall growth and development, Mr. Der noted.
Breast-feeding has little or no effect on children's later intelligence, according to the largest study ever to address this question.
The influence of breast-feeding on cognitive ability has been debated for more than 70 years, with multiple potentially confounding factors having been identified. These include socioeconomic status, birth weight, maternal history of smoking, maternal and paternal education, and race or ethnicity. Maternal intelligence, however, has largely been overlooked as a potential confounder in studies investigating the effects of breast-feeding, according to Geoff Der, a statistician at the Medical Research Council's Social and Public Health Sciences Unit in Glasgow, Scotland.
Analyzing data from 5,475 children and their mothers in the population-based U.S. national longitudinal survey of youth that began in 1979, Mr. Der and his colleagues found that if maternal intelligence was not included as a potential confounder, breast-feeding did appear to exert beneficial effects on children's intelligence, adding approximately 4 points. However, when maternal intelligence was included in the analysis, breast-feeding was associated with an increase of less than half a point (BMJ 2006 Oct. 4 [doi:10.1136/bmj.38978.699583.55]). Previous studies' not having considered maternal intelligence was “surprising given the heritability of intelligence,” he observed.
The researchers also determined that an increase of 15 points in maternal intelligence more than doubled the likelihood that the child would be breast-fed. They furthermore observed that mothers who breast-fed tended to be older and more educated, and to provide a more stimulating home environment.
Despite the lack of association with children's intelligence, breast-feeding remains important for overall growth and development, Mr. Der noted.