User login
Apixaban Non-Inferior to Standard Therapy to Treat Acute VTE with Favorable Bleeding Risk
Clinical question: Is apixaban non-inferior to standard therapy for treating acute VTE?
Background: Apixaban, a direct Xa inhibitor, has not been tested for efficacy and safety in treating acute VTE. Rivaroxaban, another direct Xa inhibitor, is already FDA-approved for acute VTE treatment.
Study design: Randomized, double-blinded trial.
Setting: International multi-center.
Synopsis: Researchers randomized 5400 patients with acute VTE to receive either apixaban (10 mg po bid x 7 days, then 5 mg po bid x 6 months) + placebo, or lovenox with transition to coumadin, with a goal of international normalized ratio 2-3. Recurrent VTE occurred in 2.3% of the apixaban group, compared with 2.7% in the conventional therapy group (apixaban noninferior). Major bleeding occurred less in the apixaban group than in the conventional therapy group (0.6% compared to 1.8%), as did clinically relevant non-major bleeding (3.8% compared to 8%).
Bottom line: Apixaban is a safe alternative for treating acute VTE (pending FDA approval).
Citation: Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. New Engl J Med. 2013;369:799-808.
Clinical question: Is apixaban non-inferior to standard therapy for treating acute VTE?
Background: Apixaban, a direct Xa inhibitor, has not been tested for efficacy and safety in treating acute VTE. Rivaroxaban, another direct Xa inhibitor, is already FDA-approved for acute VTE treatment.
Study design: Randomized, double-blinded trial.
Setting: International multi-center.
Synopsis: Researchers randomized 5400 patients with acute VTE to receive either apixaban (10 mg po bid x 7 days, then 5 mg po bid x 6 months) + placebo, or lovenox with transition to coumadin, with a goal of international normalized ratio 2-3. Recurrent VTE occurred in 2.3% of the apixaban group, compared with 2.7% in the conventional therapy group (apixaban noninferior). Major bleeding occurred less in the apixaban group than in the conventional therapy group (0.6% compared to 1.8%), as did clinically relevant non-major bleeding (3.8% compared to 8%).
Bottom line: Apixaban is a safe alternative for treating acute VTE (pending FDA approval).
Citation: Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. New Engl J Med. 2013;369:799-808.
Clinical question: Is apixaban non-inferior to standard therapy for treating acute VTE?
Background: Apixaban, a direct Xa inhibitor, has not been tested for efficacy and safety in treating acute VTE. Rivaroxaban, another direct Xa inhibitor, is already FDA-approved for acute VTE treatment.
Study design: Randomized, double-blinded trial.
Setting: International multi-center.
Synopsis: Researchers randomized 5400 patients with acute VTE to receive either apixaban (10 mg po bid x 7 days, then 5 mg po bid x 6 months) + placebo, or lovenox with transition to coumadin, with a goal of international normalized ratio 2-3. Recurrent VTE occurred in 2.3% of the apixaban group, compared with 2.7% in the conventional therapy group (apixaban noninferior). Major bleeding occurred less in the apixaban group than in the conventional therapy group (0.6% compared to 1.8%), as did clinically relevant non-major bleeding (3.8% compared to 8%).
Bottom line: Apixaban is a safe alternative for treating acute VTE (pending FDA approval).
Citation: Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. New Engl J Med. 2013;369:799-808.
Adding Clopidogrel to Aspirin Prevents Recurrent CVA in a Defined Population
Clinical question: Does loading clopidogrel with aspirin reduce recurrent stroke after moderate to high-risk transient ischemic attack (TIA) or minor stroke if started within 24 hours of primary event?
Background: Recurrent stroke risk is highest during the first few weeks after TIA or minor stroke.
Study design: Randomized, double-blinded, placebo-controlled trial.
Setting: Multi-center health system in China.
Synopsis: More than 5100 patients were randomized within 24 hours after minor ischemic stroke (NIHSS<=3) or high-risk TIA (ABCD2>= 4) to loading dose clopidogrel 300 mg, then 75 mg po daily x 90 days in addition to aspirin 75 mg daily for the first 21 days or aspirin 75 mg po daily x 90 days + placebo. Within 90 days, recurrent stroke was higher in aspirin + placebo group compared to aspirin + clopidogrel (11.7% event rate compared with 8.2%). Moderate to severe bleeding risk was the same (0.3%) in both groups.
Strict eligibility criteria in this study might limit generalizability to the general public. This study occurred in China, where the recurrent stroke rate was higher (near 10%) than the rate seen in primary stroke centers in more developed countries (3% to 5%), perhaps because of less emphasis on secondary risk prevention (including hypertension and hyperlipidemia) in China.
Also, the distribution of stroke subtype in China (more intracranial atherosclerosis than in other populations) might have affected the study outcomes. Because of these limitations, more research needs to be done to confirm these findings for other populations.
Bottom line: Adding clopidogrel to aspirin reduced recurrent cerebrovascular event after high-risk TIA or minor ischemic stroke in China, but generalizability to other patient populations is not clear.
Citation: Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. New Engl J Med. 2013;369:11-19.
Clinical question: Does loading clopidogrel with aspirin reduce recurrent stroke after moderate to high-risk transient ischemic attack (TIA) or minor stroke if started within 24 hours of primary event?
Background: Recurrent stroke risk is highest during the first few weeks after TIA or minor stroke.
Study design: Randomized, double-blinded, placebo-controlled trial.
Setting: Multi-center health system in China.
Synopsis: More than 5100 patients were randomized within 24 hours after minor ischemic stroke (NIHSS<=3) or high-risk TIA (ABCD2>= 4) to loading dose clopidogrel 300 mg, then 75 mg po daily x 90 days in addition to aspirin 75 mg daily for the first 21 days or aspirin 75 mg po daily x 90 days + placebo. Within 90 days, recurrent stroke was higher in aspirin + placebo group compared to aspirin + clopidogrel (11.7% event rate compared with 8.2%). Moderate to severe bleeding risk was the same (0.3%) in both groups.
Strict eligibility criteria in this study might limit generalizability to the general public. This study occurred in China, where the recurrent stroke rate was higher (near 10%) than the rate seen in primary stroke centers in more developed countries (3% to 5%), perhaps because of less emphasis on secondary risk prevention (including hypertension and hyperlipidemia) in China.
Also, the distribution of stroke subtype in China (more intracranial atherosclerosis than in other populations) might have affected the study outcomes. Because of these limitations, more research needs to be done to confirm these findings for other populations.
Bottom line: Adding clopidogrel to aspirin reduced recurrent cerebrovascular event after high-risk TIA or minor ischemic stroke in China, but generalizability to other patient populations is not clear.
Citation: Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. New Engl J Med. 2013;369:11-19.
Clinical question: Does loading clopidogrel with aspirin reduce recurrent stroke after moderate to high-risk transient ischemic attack (TIA) or minor stroke if started within 24 hours of primary event?
Background: Recurrent stroke risk is highest during the first few weeks after TIA or minor stroke.
Study design: Randomized, double-blinded, placebo-controlled trial.
Setting: Multi-center health system in China.
Synopsis: More than 5100 patients were randomized within 24 hours after minor ischemic stroke (NIHSS<=3) or high-risk TIA (ABCD2>= 4) to loading dose clopidogrel 300 mg, then 75 mg po daily x 90 days in addition to aspirin 75 mg daily for the first 21 days or aspirin 75 mg po daily x 90 days + placebo. Within 90 days, recurrent stroke was higher in aspirin + placebo group compared to aspirin + clopidogrel (11.7% event rate compared with 8.2%). Moderate to severe bleeding risk was the same (0.3%) in both groups.
Strict eligibility criteria in this study might limit generalizability to the general public. This study occurred in China, where the recurrent stroke rate was higher (near 10%) than the rate seen in primary stroke centers in more developed countries (3% to 5%), perhaps because of less emphasis on secondary risk prevention (including hypertension and hyperlipidemia) in China.
Also, the distribution of stroke subtype in China (more intracranial atherosclerosis than in other populations) might have affected the study outcomes. Because of these limitations, more research needs to be done to confirm these findings for other populations.
Bottom line: Adding clopidogrel to aspirin reduced recurrent cerebrovascular event after high-risk TIA or minor ischemic stroke in China, but generalizability to other patient populations is not clear.
Citation: Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. New Engl J Med. 2013;369:11-19.
ITL: Physician Reviews of HM-Relevant Research
Clinical question: Is there a difference between aspirin and warfarin in preventing thromboembolic complications and risk of bleeding in patients with chronic kidney disease (CKD) and nonvalvular atrial fibrillation (NVAF)?
Background: Data are lacking on risks and benefits of aspirin and warfarin in CKD, as this group of patients largely has been excluded from anticoagulation therapy trials for NVAF. This study examined the risks and benefits of aspirin and warfarin in patients with CKD with NVAF.
Study design: Retrospective, observational cohort study.
Setting: Danish National Registries.
Synopsis: Of 132,372 patients with NVAF, 2.7% had CKD and 0.7% had end-stage renal disease (ESRD). Compared to patients with no CKD, there was increased risk of stroke or systemic thromboembolism in patients with ESRD (HR, 1.83; 95% CI, 1.57-2.14) and with non-end-stage CKD (HR 1.49; 95% CI 1.38-1.59).
In patients with CKD, warfarin significantly reduced stroke risk (HR, 0.76; 95% CI, 0.64-0.91) and significantly increased bleeding risk (HR, 1.33; 95% CI, 1.16-1.53); aspirin significantly increased bleeding risk (HR, 1.17; 95% CI, 1.02-1.34), with no reduction in stroke risk.
Bottom line: CKD was associated with an increased risk of stroke among NVAF patients. While both aspirin and warfarin were associated with increased risk of bleeding, there was a reduction in the risk of stroke with warfarin, but not with aspirin.
Citation: Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625-635.
Click here for more physician reviews of HM-relevant literature.
Clinical question: Is there a difference between aspirin and warfarin in preventing thromboembolic complications and risk of bleeding in patients with chronic kidney disease (CKD) and nonvalvular atrial fibrillation (NVAF)?
Background: Data are lacking on risks and benefits of aspirin and warfarin in CKD, as this group of patients largely has been excluded from anticoagulation therapy trials for NVAF. This study examined the risks and benefits of aspirin and warfarin in patients with CKD with NVAF.
Study design: Retrospective, observational cohort study.
Setting: Danish National Registries.
Synopsis: Of 132,372 patients with NVAF, 2.7% had CKD and 0.7% had end-stage renal disease (ESRD). Compared to patients with no CKD, there was increased risk of stroke or systemic thromboembolism in patients with ESRD (HR, 1.83; 95% CI, 1.57-2.14) and with non-end-stage CKD (HR 1.49; 95% CI 1.38-1.59).
In patients with CKD, warfarin significantly reduced stroke risk (HR, 0.76; 95% CI, 0.64-0.91) and significantly increased bleeding risk (HR, 1.33; 95% CI, 1.16-1.53); aspirin significantly increased bleeding risk (HR, 1.17; 95% CI, 1.02-1.34), with no reduction in stroke risk.
Bottom line: CKD was associated with an increased risk of stroke among NVAF patients. While both aspirin and warfarin were associated with increased risk of bleeding, there was a reduction in the risk of stroke with warfarin, but not with aspirin.
Citation: Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625-635.
Click here for more physician reviews of HM-relevant literature.
Clinical question: Is there a difference between aspirin and warfarin in preventing thromboembolic complications and risk of bleeding in patients with chronic kidney disease (CKD) and nonvalvular atrial fibrillation (NVAF)?
Background: Data are lacking on risks and benefits of aspirin and warfarin in CKD, as this group of patients largely has been excluded from anticoagulation therapy trials for NVAF. This study examined the risks and benefits of aspirin and warfarin in patients with CKD with NVAF.
Study design: Retrospective, observational cohort study.
Setting: Danish National Registries.
Synopsis: Of 132,372 patients with NVAF, 2.7% had CKD and 0.7% had end-stage renal disease (ESRD). Compared to patients with no CKD, there was increased risk of stroke or systemic thromboembolism in patients with ESRD (HR, 1.83; 95% CI, 1.57-2.14) and with non-end-stage CKD (HR 1.49; 95% CI 1.38-1.59).
In patients with CKD, warfarin significantly reduced stroke risk (HR, 0.76; 95% CI, 0.64-0.91) and significantly increased bleeding risk (HR, 1.33; 95% CI, 1.16-1.53); aspirin significantly increased bleeding risk (HR, 1.17; 95% CI, 1.02-1.34), with no reduction in stroke risk.
Bottom line: CKD was associated with an increased risk of stroke among NVAF patients. While both aspirin and warfarin were associated with increased risk of bleeding, there was a reduction in the risk of stroke with warfarin, but not with aspirin.
Citation: Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625-635.
Click here for more physician reviews of HM-relevant literature.
ITL: Physician Reviews of HM-Relevant Research
Clinical question: What are the relative predictive values of the HEMORR2HAGES, ATRIA, and HAS-BLED risk-prediction schemes?
Background: The tools predict bleeding risk in patients anticoagulated for atrial fibrillation (afib), but it is unknown which is the best for predicting clinically relevant bleeding.
Study design: Post-hoc analysis.
Setting: Data previously collected for the AMADEUS trial (2,293 patients taking warfarin; 251 had at least one clinically relevant bleeding event) were used to test each of the three bleeding-risk-prediction schemes on the same data set.
Synopsis: Using three analysis methods (net reclassification improvement, receiver-operating characteristic [ROC], and decision-curve analysis), the researchers compared the three schemes’ performance. HAS-BLED performed best in all three of the analysis methods.
The HAS-BLED score calculation requires the following patient information: history of hypertension, renal disease, liver disease, stroke, prior major bleeding event, and labile INR; age >65; and use of antiplatelet agents, aspirin, and alcohol.
Bottom line: HAS-BLED was the best of the three schemes, although all three had only modest ability to predict clinically relevant bleeding.
Citation: Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR2HAGES, ATRIA and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation. J Am Coll Cardiol. 2012;60(9):861-867.
Visit our website for more physician reviews of recent HM-relevant literature.
Clinical question: What are the relative predictive values of the HEMORR2HAGES, ATRIA, and HAS-BLED risk-prediction schemes?
Background: The tools predict bleeding risk in patients anticoagulated for atrial fibrillation (afib), but it is unknown which is the best for predicting clinically relevant bleeding.
Study design: Post-hoc analysis.
Setting: Data previously collected for the AMADEUS trial (2,293 patients taking warfarin; 251 had at least one clinically relevant bleeding event) were used to test each of the three bleeding-risk-prediction schemes on the same data set.
Synopsis: Using three analysis methods (net reclassification improvement, receiver-operating characteristic [ROC], and decision-curve analysis), the researchers compared the three schemes’ performance. HAS-BLED performed best in all three of the analysis methods.
The HAS-BLED score calculation requires the following patient information: history of hypertension, renal disease, liver disease, stroke, prior major bleeding event, and labile INR; age >65; and use of antiplatelet agents, aspirin, and alcohol.
Bottom line: HAS-BLED was the best of the three schemes, although all three had only modest ability to predict clinically relevant bleeding.
Citation: Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR2HAGES, ATRIA and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation. J Am Coll Cardiol. 2012;60(9):861-867.
Visit our website for more physician reviews of recent HM-relevant literature.
Clinical question: What are the relative predictive values of the HEMORR2HAGES, ATRIA, and HAS-BLED risk-prediction schemes?
Background: The tools predict bleeding risk in patients anticoagulated for atrial fibrillation (afib), but it is unknown which is the best for predicting clinically relevant bleeding.
Study design: Post-hoc analysis.
Setting: Data previously collected for the AMADEUS trial (2,293 patients taking warfarin; 251 had at least one clinically relevant bleeding event) were used to test each of the three bleeding-risk-prediction schemes on the same data set.
Synopsis: Using three analysis methods (net reclassification improvement, receiver-operating characteristic [ROC], and decision-curve analysis), the researchers compared the three schemes’ performance. HAS-BLED performed best in all three of the analysis methods.
The HAS-BLED score calculation requires the following patient information: history of hypertension, renal disease, liver disease, stroke, prior major bleeding event, and labile INR; age >65; and use of antiplatelet agents, aspirin, and alcohol.
Bottom line: HAS-BLED was the best of the three schemes, although all three had only modest ability to predict clinically relevant bleeding.
Citation: Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR2HAGES, ATRIA and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation. J Am Coll Cardiol. 2012;60(9):861-867.
Visit our website for more physician reviews of recent HM-relevant literature.
ITL: Physician Reviews of HM-Relevant Research
In This Edition
Literature At A Glance
A guide to this month’s studies
- Burnout among physicians and the general workforce
- Effects of clopidogrel added to aspirin in patients with recent lacunar stroke
- Performance of the HEMORR2AGES, ATRIA, and HAS-BLED bleeding risk prediction scores in patients with atrial fibrillation undergoing anticoagulation
- Probiotics for secondary prevention of hepatic encephalopathy
- Capsule endoscopy for acute obscure GI bleeding
- Perceptions of readmitted patients transitioning from hospital to home
- Thirty-day readmissions after acute myocardial infarction
- One-hour rule-out or rule-in for AMI patients in chest pain
- Aspirin increases bleed risk without reducing risk of stroke in CKD and NVAF patients
Burnout among Physicians and the General Workforce
Clinical question: What is the degree and distribution of burnout within the physician workforce, and how does that compare to the general U.S. workforce?
Background: Professional burnout, work satisfaction, and work-life balance are critical elements to understand in the physician workforce. It is well documented that physicians are at high risk for burnout; however, few extensive studies have looked at rates and the identification of high-risk subpopulations.
Study design: Cross-sectional survey.
Setting: U.S. workforce.
Synopsis: This study included 7,288 physicians (26.7% response rate) and 5,930 controls from the general U.S. population. Validated survey instruments were employed to assess the degree and presence of burnout, depression, and satisfaction with work-life balance.
In aggregate, using a validated, two-item burnout measure, 35.2% of physicians were characterized as having burnout, compared with 27.6% of the general population (P<0.001). Within the physician community, the specialties with the highest risk of burnout included emergency medicine, general internal medicine, family medicine, and neurology.
Important limitations of this study include that the physician and general population surveys were performed at different times (six months apart), that the groups were not ideally matched (age and sex, for example), and the overall response rate of the physician survey was low.
This study sheds light on an important topic for hospitalists. Future studies should continue to probe the problem of burnout and look for creative solutions to mitigate risks that might threaten professional longevity.
Bottom line: Burnout is prevalent among physicians, especially when compared to the general workforce. Physician specialties in front-line patient care are at highest risk.
Citation: Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;20 [Epub ahead of print].
Effects of Clopidogrel Added to Aspirin in Patients with Recent Lacunar Stroke
Clinical question: Does the addition of clopidogrel to aspirin reduce the risk of any type of recurrent stroke, or affect the risk of bleeding or death, in patients who recently suffered a lacunar stroke?
Background: There are no prior randomized, multicenter trials on secondary prevention of lacunar stroke; aspirin is the standard antiplatelet therapy in this setting.
Study design: Double-blind, randomized, multicenter trial.
Setting: Eighty-two clinical centers in North America, Latin America, and Spain.
Synopsis: Researchers enrolled 3,020 patients from 2003 to 2011; criteria included age >30 years old and symptomatic lacunar stroke (proven by MRI) in the preceding 180 days.
Results showed no significant difference between recurrent strokes (any type) in the aspirin-only group (2.7% per year) versus the aspirin-plus-clopidogrel group (2.5% per year). Major hemorrhage risk was much higher in the aspirin-plus-clopidogrel group (2.1% per year) versus aspirin-only group (1.1% per year). All-cause mortality also was much higher in the aspirin-plus-clopidogrel group (N=113) versus the aspirin-only group (N=77).
Bottom line: The addition of clopidogrel to aspirin for secondary prevention does not significantly reduce the risk of recurrent stroke, but it does significantly increase the risk of bleeding and death.
Citation: Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
Bleeding Risk Prediction Scores in Patients with Atrial Fibrillation Undergoing Anticoagulation
Clinical question: What are the relative predictive values of the HEMORR2AGES, ATRIA, and HAS-BLED risk-prediction schemes?
Background: The tools predict bleeding risk in patients anticoagulated for atrial fibrillation (afib), but it is unknown which is the best to predict clinically relevant bleeding.
Study design: Post-hoc analysis.
Setting: Data previously collected for the AMADEUS trial (2,293 patients taking warfarin; 251 had at least one clinically relevant bleeding event) were used to test each of the three bleeding risk-prediction schemes on the same data set.
Synopsis: Using three analysis methods (net reclassification improvement, receiver-operating characteristic [ROC], and decision-curve analysis), the researchers compared the three schemes’ performance. HAS-BLED performed best in all three of the analysis methods.
The HAS-BLED score calculation requires the following patient information: history of hypertension, renal disease, liver disease, stroke, prior major bleeding event, and labile INR; age >65; and use of antiplatelet agents, aspirin, and alcohol.
Bottom line: HAS-BLED was the best of the three schemes, although all three had only modest ability to predict clinically relevant bleeding.
Citation: Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORRAGES, ATRIA and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation. J Am Coll Cardiol. 2012;60(9):861-867
Probiotics for Secondary Prevention of Hepatic Encephalopathy
Clinical question: Are probiotics as effective as lactulose for secondary prevention of hepatic encephalopathy (HE)?
Background: Probiotics alter the gut flora, resulting in decreased ammonia production and absorption. Probiotics have been shown to reduce the incidence of low-grade HE. However, studies on probiotics usage for secondary prevention of HE are lacking.
Study design: Prospective, randomized, controlled, nonblinded, single-center study.
Setting: Tertiary-care center, New Delhi.
Synopsis: Three hundred sixty patients who had recovered from HE from October 2008 to December 2009 were screened; 235 met the inclusion criteria. They were randomized to receive either lactulose (Gp-L), probiotics (Gp-P), or no therapy (Gp-N). The Gp-L group received 30 to 60 ml of lactulose in two to three divided doses; the Gp-P group received three capsules per day containing lactobacillus, bifidobacterium, and Streptococcus salivarius strains.
The primary endpoints were the development of overt HE (assessed by the West Haven Criteria) or a follow-up of 12 months. Lactulose therapy was significantly more effective in secondary prophylaxis than no therapy (26.2% vs. 56.9%, P=0.001), as was probiotics therapy compared with no therapy (34.4% vs. 56.9%, P=0.02), but no significant difference was found between lactulose and probiotics therapy (26.2% vs. 34.4%, P=0.349).
The major limitation of the study was its open-label design. The study also used a high concentration of probiotics, and the results could be strain-specific and hence require validation with other probiotics combinations. The Gp-N group continued the previous therapy (excluding lactulose), with an unknown number on rifaximin.
Bottom line: Lactulose and probiotics are equally effective in secondary prophylaxis of hepatic encephalopathy.
Citation: Agrawal A, Sharma BC, Sharma P, Sarin SK. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics and no therapy. Am J Gastroenterol. 2012;107:1043-1050.
Capsule Endoscopy for Acute Obscure GI Bleeding
Clinical question: What testing modality is most appropriate for acute obscure GI bleeding: capsule endoscopy (CE) or angiography?
Background: Acute obscure GI bleeding (OGIB): remains a diagnostic challenge, accounting for 7% to 8% of patients presenting with GI bleeding. CE enables direct visualization of small bowel mucosa but lacks the ability for therapeutic intervention. Angiography is frequently chosen for massive bleeding; however, it is invasive and does not enable visualization of the bowel.
Study design: Prospective, randomized, controlled, blinded, single-center study.
Setting: Prince of Wales Hospital, Hong Kong.
Synopsis: Ninety-one patients with active OGIB from June 2005 to November 2007 were assessed for eligibility; 60 met the criteria and were randomized to either CE or angiography. Overt OGIB was defined as patients who had nondiagnostic upper endoscopy and colonoscopy.
The primary outcome was diagnostic yield of CE or mesenteric angiography in identifying the bleeding source. Secondary outcomes were long-term rebleeding rates, readmissions for bleeding or anemia, blood transfusions, and death.
CE was positive in 16 patients (53.3%) and angiography was positive in six patients (20%). The diagnostic yield of CE was significantly higher than angiography (difference=33.3%, 95% CI 8.9-52.8%, P=0.016). The mean follow-up period was 48.5 months. The cumulative risk of rebleeding was higher in the angiography group, but this was not statistically significant. There was no significant difference in rates of subsequent hospitalization, death, or transfusions between the two groups.
The study based the sample-size estimation on the diagnostic yield rather than clinical outcomes and, hence, was underpowered to detect any significant differences in clinical outcomes.
Bottom line: CE has a higher diagnostic yield than angiography in patients with active overt OGIB.
Citation: Leung WK, Ho S, Suen B, et al. Capsule endoscopy of angiography in patients with acute overt gastrointestinal bleeding: a prospective randomized study with long term follow up. Am J Gastroenterol. 2012;107:1370-1376.
Perceptions of Readmitted Patients Transitioning from Hospital to Home
Clinical question: What are patient-reported reasons for readmission to the hospital after discharge?
Background: Reducing readmissions is a critical component to improving the value of healthcare. While readmission reduction is a goal of all hospitals, there is much to be gleaned from evaluating patients’ view of the problem. This study used a survey to assess the patient’s viewpoint.
Study design: Cross-sectional survey.
Setting: The Hospital of the University of Pennsylvania and Penn Presbyterian Medical Center, Philadelphia.
Synopsis: A survey of 36 questions was posed to 1,084 patients who were readmitted within 30 days of discharge from November 2010 to July 2011 (32% of eligible patients). The data were subdivided based on socioeconomic status and medical versus surgical patients.
Some issues patients raised regarding discharge planning included difficulty with paying for medications, challenges with travel to pharmacies, and concern over medication side effects.
Patients with low socioeconomic status had more difficulty taking medications and following instructions, had more depression, and had less social support.
Bottom line: Readmission rates are affected by a patient’s social situation. A team approach to discharge planning might mitigate some of these factors.
Citation: Kangovi S, Grande D, Meehan P, Mitra N, Shannon R, Long JA. Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012 [Epub ahead of print].
30-Day Readmissions after Acute Myocardial Infarction
Clinical question: What are potential predictors of 30-day readmissions after acute myocardial infarction (MI)?
Background: Much attention has been given to evaluate the causes of readmissions of heart failure, acute MI, and pneumonia. This study looked at 30-day readmissions after an acute myocardial infarction (AMI).
Study design: Retrospective cohort study.
Setting: Olmstead County Hospital, Rochester, Minn.
Synopsis: A chart review of AMI based on ICD-9 codes from 1987 to 2010 identified 3,010 patients. Patients were verified using symptoms, cardiac enzymes, and EKG changes at the time of event. Interventions evaluated included fibrinolytic therapy, CABG, or primary PCI.
Survival increased to 96% from 89% during the period from 1987 to 2010. Researchers also noted more comorbid conditions, such as diabetes mellitus, COPD, and hypertension, noted over time. Of the patients evaluated, 643 readmissions occurred for 561 patients (18.6%). Of these, the most frequent causes were ischemic heart disease, respiratory symptoms, and heart failure. Comorbid conditions, such as diabetes, COPD, anemia, higher killip class on initial admission, duration of prior hospitalization, and procedural complications, independently increased the risk of readmission.
Bottom line: In addition to factors unrelated to an AMI, a patient’s comorbid conditions, post-procedure complications, and duration of hospitalization influence the risk of readmission.
Citation: Dunlay SM, Weston SA, Killian JM, et al. Thirty-day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157(1):11-18.
One-Hour Rule-Out or Rule-In for AMI in Chest Pain
Clinical question: How can we use the newly developed high-sensitivity cardiac troponin (hs-cTnT) to shorten the time to rule in and rule out AMI?
Background: The hs-cTnT assays available appear to improve the early diagnosis of AMI when compared to the regular cardiac troponins, but no clear guidelines are available as how to best use them in clinical practice.
Study design: Prospective, multicenter study.
Setting: Switzerland hospitals.
Synopsis: The study enrolled 872 unselected patients presenting to the ED with acute chest pain. Hs-cTnT level was measured in a blinded fashion at presentation and after one hour. Two independent cardiologists using all available medical records adjudicated the final AMI diagnosis. Optimal thresholds for rule-out were selected to allow for 100% sensitivity and negative predictive value. Rule-out criteria were defined as baseline hs-cTnT level <12 ng/L and an absolute change within the first hour of <3 ng/L. Rule-in criteria was defined as baseline hs-cTnT >52 ng/L or an absolute increase within the first hour of >5 ng/L.
AMI was the final diagnosis in 17% of patients; AMI was ruled out in 60%; and the remaining 23% were placed in observation.
Primary prognostic endpoint was 30-day mortality rate, which was 0.2% in the rule-out group, validating the suitability of these patients for early discharge.
Study limitations were that it was an observational study not used for clinical decision-making, no dialysis patients were included, and only one specific hs-cTnT assay was tested.
Bottom line: Using hs-cTnT levels at presentation and absolute changes within the first hour, a safe rule-out or rule-in of AMI can be performed in 77% of patients presenting with chest pain.
Citation: Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172(16):1-8.
Aspirin Increases Bleed Risk without Reducing Risk of Stroke in CKD and NVAF Patients
Clinical question: Is there a difference between aspirin and warfarin in preventing thromboembolic complications and risk of bleeding in patients with chronic kidney disease (CKD) and nonvalvular afib (NVAF)?
Background: Data are lacking on risks and benefits of aspirin and warfarin in CKD, as this group of patients largely has been excluded from anticoagulation therapy trials for NVAF. This study examined the risks and benefits of aspirin and warfarin in patients with CKD with NVAF.
Study design: Retrospective, observational cohort study.
Setting: Danish National Registries.
Synopsis: Of 132,372 patients with NVAF, 2.7% had CKD and 0.7% had end-stage renal disease (ESRD). Compared to patients with no CKD, there was increased risk of stroke or systemic thromboembolism in patients with ESRD (HR, 1.83; 95% CI, 1.57-2.14) and with non-end-stage CKD (HR 1.49; 95% CI 1.38-1.59).
In patients with CKD, warfarin significantly reduced stroke risk (HR, 0.76; 95% CI, 0.64-0.91) and significantly increased bleeding risk (HR, 1.33; 95% CI, 1.16-1.53); aspirin significantly increased bleeding risk (HR, 1.17; 95% CI, 1.02-1.34) with no reduction in stroke risk.
Bottom line: CKD was associated with an increased risk of stroke among NVAF patients. While both aspirin and warfarin were associated with increased risk of bleeding, there was a reduction in the risk of stroke with warfarin, but not with aspirin.
Citation: Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625-635.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Burnout among physicians and the general workforce
- Effects of clopidogrel added to aspirin in patients with recent lacunar stroke
- Performance of the HEMORR2AGES, ATRIA, and HAS-BLED bleeding risk prediction scores in patients with atrial fibrillation undergoing anticoagulation
- Probiotics for secondary prevention of hepatic encephalopathy
- Capsule endoscopy for acute obscure GI bleeding
- Perceptions of readmitted patients transitioning from hospital to home
- Thirty-day readmissions after acute myocardial infarction
- One-hour rule-out or rule-in for AMI patients in chest pain
- Aspirin increases bleed risk without reducing risk of stroke in CKD and NVAF patients
Burnout among Physicians and the General Workforce
Clinical question: What is the degree and distribution of burnout within the physician workforce, and how does that compare to the general U.S. workforce?
Background: Professional burnout, work satisfaction, and work-life balance are critical elements to understand in the physician workforce. It is well documented that physicians are at high risk for burnout; however, few extensive studies have looked at rates and the identification of high-risk subpopulations.
Study design: Cross-sectional survey.
Setting: U.S. workforce.
Synopsis: This study included 7,288 physicians (26.7% response rate) and 5,930 controls from the general U.S. population. Validated survey instruments were employed to assess the degree and presence of burnout, depression, and satisfaction with work-life balance.
In aggregate, using a validated, two-item burnout measure, 35.2% of physicians were characterized as having burnout, compared with 27.6% of the general population (P<0.001). Within the physician community, the specialties with the highest risk of burnout included emergency medicine, general internal medicine, family medicine, and neurology.
Important limitations of this study include that the physician and general population surveys were performed at different times (six months apart), that the groups were not ideally matched (age and sex, for example), and the overall response rate of the physician survey was low.
This study sheds light on an important topic for hospitalists. Future studies should continue to probe the problem of burnout and look for creative solutions to mitigate risks that might threaten professional longevity.
Bottom line: Burnout is prevalent among physicians, especially when compared to the general workforce. Physician specialties in front-line patient care are at highest risk.
Citation: Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;20 [Epub ahead of print].
Effects of Clopidogrel Added to Aspirin in Patients with Recent Lacunar Stroke
Clinical question: Does the addition of clopidogrel to aspirin reduce the risk of any type of recurrent stroke, or affect the risk of bleeding or death, in patients who recently suffered a lacunar stroke?
Background: There are no prior randomized, multicenter trials on secondary prevention of lacunar stroke; aspirin is the standard antiplatelet therapy in this setting.
Study design: Double-blind, randomized, multicenter trial.
Setting: Eighty-two clinical centers in North America, Latin America, and Spain.
Synopsis: Researchers enrolled 3,020 patients from 2003 to 2011; criteria included age >30 years old and symptomatic lacunar stroke (proven by MRI) in the preceding 180 days.
Results showed no significant difference between recurrent strokes (any type) in the aspirin-only group (2.7% per year) versus the aspirin-plus-clopidogrel group (2.5% per year). Major hemorrhage risk was much higher in the aspirin-plus-clopidogrel group (2.1% per year) versus aspirin-only group (1.1% per year). All-cause mortality also was much higher in the aspirin-plus-clopidogrel group (N=113) versus the aspirin-only group (N=77).
Bottom line: The addition of clopidogrel to aspirin for secondary prevention does not significantly reduce the risk of recurrent stroke, but it does significantly increase the risk of bleeding and death.
Citation: Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
Bleeding Risk Prediction Scores in Patients with Atrial Fibrillation Undergoing Anticoagulation
Clinical question: What are the relative predictive values of the HEMORR2AGES, ATRIA, and HAS-BLED risk-prediction schemes?
Background: The tools predict bleeding risk in patients anticoagulated for atrial fibrillation (afib), but it is unknown which is the best to predict clinically relevant bleeding.
Study design: Post-hoc analysis.
Setting: Data previously collected for the AMADEUS trial (2,293 patients taking warfarin; 251 had at least one clinically relevant bleeding event) were used to test each of the three bleeding risk-prediction schemes on the same data set.
Synopsis: Using three analysis methods (net reclassification improvement, receiver-operating characteristic [ROC], and decision-curve analysis), the researchers compared the three schemes’ performance. HAS-BLED performed best in all three of the analysis methods.
The HAS-BLED score calculation requires the following patient information: history of hypertension, renal disease, liver disease, stroke, prior major bleeding event, and labile INR; age >65; and use of antiplatelet agents, aspirin, and alcohol.
Bottom line: HAS-BLED was the best of the three schemes, although all three had only modest ability to predict clinically relevant bleeding.
Citation: Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORRAGES, ATRIA and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation. J Am Coll Cardiol. 2012;60(9):861-867
Probiotics for Secondary Prevention of Hepatic Encephalopathy
Clinical question: Are probiotics as effective as lactulose for secondary prevention of hepatic encephalopathy (HE)?
Background: Probiotics alter the gut flora, resulting in decreased ammonia production and absorption. Probiotics have been shown to reduce the incidence of low-grade HE. However, studies on probiotics usage for secondary prevention of HE are lacking.
Study design: Prospective, randomized, controlled, nonblinded, single-center study.
Setting: Tertiary-care center, New Delhi.
Synopsis: Three hundred sixty patients who had recovered from HE from October 2008 to December 2009 were screened; 235 met the inclusion criteria. They were randomized to receive either lactulose (Gp-L), probiotics (Gp-P), or no therapy (Gp-N). The Gp-L group received 30 to 60 ml of lactulose in two to three divided doses; the Gp-P group received three capsules per day containing lactobacillus, bifidobacterium, and Streptococcus salivarius strains.
The primary endpoints were the development of overt HE (assessed by the West Haven Criteria) or a follow-up of 12 months. Lactulose therapy was significantly more effective in secondary prophylaxis than no therapy (26.2% vs. 56.9%, P=0.001), as was probiotics therapy compared with no therapy (34.4% vs. 56.9%, P=0.02), but no significant difference was found between lactulose and probiotics therapy (26.2% vs. 34.4%, P=0.349).
The major limitation of the study was its open-label design. The study also used a high concentration of probiotics, and the results could be strain-specific and hence require validation with other probiotics combinations. The Gp-N group continued the previous therapy (excluding lactulose), with an unknown number on rifaximin.
Bottom line: Lactulose and probiotics are equally effective in secondary prophylaxis of hepatic encephalopathy.
Citation: Agrawal A, Sharma BC, Sharma P, Sarin SK. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics and no therapy. Am J Gastroenterol. 2012;107:1043-1050.
Capsule Endoscopy for Acute Obscure GI Bleeding
Clinical question: What testing modality is most appropriate for acute obscure GI bleeding: capsule endoscopy (CE) or angiography?
Background: Acute obscure GI bleeding (OGIB): remains a diagnostic challenge, accounting for 7% to 8% of patients presenting with GI bleeding. CE enables direct visualization of small bowel mucosa but lacks the ability for therapeutic intervention. Angiography is frequently chosen for massive bleeding; however, it is invasive and does not enable visualization of the bowel.
Study design: Prospective, randomized, controlled, blinded, single-center study.
Setting: Prince of Wales Hospital, Hong Kong.
Synopsis: Ninety-one patients with active OGIB from June 2005 to November 2007 were assessed for eligibility; 60 met the criteria and were randomized to either CE or angiography. Overt OGIB was defined as patients who had nondiagnostic upper endoscopy and colonoscopy.
The primary outcome was diagnostic yield of CE or mesenteric angiography in identifying the bleeding source. Secondary outcomes were long-term rebleeding rates, readmissions for bleeding or anemia, blood transfusions, and death.
CE was positive in 16 patients (53.3%) and angiography was positive in six patients (20%). The diagnostic yield of CE was significantly higher than angiography (difference=33.3%, 95% CI 8.9-52.8%, P=0.016). The mean follow-up period was 48.5 months. The cumulative risk of rebleeding was higher in the angiography group, but this was not statistically significant. There was no significant difference in rates of subsequent hospitalization, death, or transfusions between the two groups.
The study based the sample-size estimation on the diagnostic yield rather than clinical outcomes and, hence, was underpowered to detect any significant differences in clinical outcomes.
Bottom line: CE has a higher diagnostic yield than angiography in patients with active overt OGIB.
Citation: Leung WK, Ho S, Suen B, et al. Capsule endoscopy of angiography in patients with acute overt gastrointestinal bleeding: a prospective randomized study with long term follow up. Am J Gastroenterol. 2012;107:1370-1376.
Perceptions of Readmitted Patients Transitioning from Hospital to Home
Clinical question: What are patient-reported reasons for readmission to the hospital after discharge?
Background: Reducing readmissions is a critical component to improving the value of healthcare. While readmission reduction is a goal of all hospitals, there is much to be gleaned from evaluating patients’ view of the problem. This study used a survey to assess the patient’s viewpoint.
Study design: Cross-sectional survey.
Setting: The Hospital of the University of Pennsylvania and Penn Presbyterian Medical Center, Philadelphia.
Synopsis: A survey of 36 questions was posed to 1,084 patients who were readmitted within 30 days of discharge from November 2010 to July 2011 (32% of eligible patients). The data were subdivided based on socioeconomic status and medical versus surgical patients.
Some issues patients raised regarding discharge planning included difficulty with paying for medications, challenges with travel to pharmacies, and concern over medication side effects.
Patients with low socioeconomic status had more difficulty taking medications and following instructions, had more depression, and had less social support.
Bottom line: Readmission rates are affected by a patient’s social situation. A team approach to discharge planning might mitigate some of these factors.
Citation: Kangovi S, Grande D, Meehan P, Mitra N, Shannon R, Long JA. Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012 [Epub ahead of print].
30-Day Readmissions after Acute Myocardial Infarction
Clinical question: What are potential predictors of 30-day readmissions after acute myocardial infarction (MI)?
Background: Much attention has been given to evaluate the causes of readmissions of heart failure, acute MI, and pneumonia. This study looked at 30-day readmissions after an acute myocardial infarction (AMI).
Study design: Retrospective cohort study.
Setting: Olmstead County Hospital, Rochester, Minn.
Synopsis: A chart review of AMI based on ICD-9 codes from 1987 to 2010 identified 3,010 patients. Patients were verified using symptoms, cardiac enzymes, and EKG changes at the time of event. Interventions evaluated included fibrinolytic therapy, CABG, or primary PCI.
Survival increased to 96% from 89% during the period from 1987 to 2010. Researchers also noted more comorbid conditions, such as diabetes mellitus, COPD, and hypertension, noted over time. Of the patients evaluated, 643 readmissions occurred for 561 patients (18.6%). Of these, the most frequent causes were ischemic heart disease, respiratory symptoms, and heart failure. Comorbid conditions, such as diabetes, COPD, anemia, higher killip class on initial admission, duration of prior hospitalization, and procedural complications, independently increased the risk of readmission.
Bottom line: In addition to factors unrelated to an AMI, a patient’s comorbid conditions, post-procedure complications, and duration of hospitalization influence the risk of readmission.
Citation: Dunlay SM, Weston SA, Killian JM, et al. Thirty-day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157(1):11-18.
One-Hour Rule-Out or Rule-In for AMI in Chest Pain
Clinical question: How can we use the newly developed high-sensitivity cardiac troponin (hs-cTnT) to shorten the time to rule in and rule out AMI?
Background: The hs-cTnT assays available appear to improve the early diagnosis of AMI when compared to the regular cardiac troponins, but no clear guidelines are available as how to best use them in clinical practice.
Study design: Prospective, multicenter study.
Setting: Switzerland hospitals.
Synopsis: The study enrolled 872 unselected patients presenting to the ED with acute chest pain. Hs-cTnT level was measured in a blinded fashion at presentation and after one hour. Two independent cardiologists using all available medical records adjudicated the final AMI diagnosis. Optimal thresholds for rule-out were selected to allow for 100% sensitivity and negative predictive value. Rule-out criteria were defined as baseline hs-cTnT level <12 ng/L and an absolute change within the first hour of <3 ng/L. Rule-in criteria was defined as baseline hs-cTnT >52 ng/L or an absolute increase within the first hour of >5 ng/L.
AMI was the final diagnosis in 17% of patients; AMI was ruled out in 60%; and the remaining 23% were placed in observation.
Primary prognostic endpoint was 30-day mortality rate, which was 0.2% in the rule-out group, validating the suitability of these patients for early discharge.
Study limitations were that it was an observational study not used for clinical decision-making, no dialysis patients were included, and only one specific hs-cTnT assay was tested.
Bottom line: Using hs-cTnT levels at presentation and absolute changes within the first hour, a safe rule-out or rule-in of AMI can be performed in 77% of patients presenting with chest pain.
Citation: Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172(16):1-8.
Aspirin Increases Bleed Risk without Reducing Risk of Stroke in CKD and NVAF Patients
Clinical question: Is there a difference between aspirin and warfarin in preventing thromboembolic complications and risk of bleeding in patients with chronic kidney disease (CKD) and nonvalvular afib (NVAF)?
Background: Data are lacking on risks and benefits of aspirin and warfarin in CKD, as this group of patients largely has been excluded from anticoagulation therapy trials for NVAF. This study examined the risks and benefits of aspirin and warfarin in patients with CKD with NVAF.
Study design: Retrospective, observational cohort study.
Setting: Danish National Registries.
Synopsis: Of 132,372 patients with NVAF, 2.7% had CKD and 0.7% had end-stage renal disease (ESRD). Compared to patients with no CKD, there was increased risk of stroke or systemic thromboembolism in patients with ESRD (HR, 1.83; 95% CI, 1.57-2.14) and with non-end-stage CKD (HR 1.49; 95% CI 1.38-1.59).
In patients with CKD, warfarin significantly reduced stroke risk (HR, 0.76; 95% CI, 0.64-0.91) and significantly increased bleeding risk (HR, 1.33; 95% CI, 1.16-1.53); aspirin significantly increased bleeding risk (HR, 1.17; 95% CI, 1.02-1.34) with no reduction in stroke risk.
Bottom line: CKD was associated with an increased risk of stroke among NVAF patients. While both aspirin and warfarin were associated with increased risk of bleeding, there was a reduction in the risk of stroke with warfarin, but not with aspirin.
Citation: Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625-635.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Burnout among physicians and the general workforce
- Effects of clopidogrel added to aspirin in patients with recent lacunar stroke
- Performance of the HEMORR2AGES, ATRIA, and HAS-BLED bleeding risk prediction scores in patients with atrial fibrillation undergoing anticoagulation
- Probiotics for secondary prevention of hepatic encephalopathy
- Capsule endoscopy for acute obscure GI bleeding
- Perceptions of readmitted patients transitioning from hospital to home
- Thirty-day readmissions after acute myocardial infarction
- One-hour rule-out or rule-in for AMI patients in chest pain
- Aspirin increases bleed risk without reducing risk of stroke in CKD and NVAF patients
Burnout among Physicians and the General Workforce
Clinical question: What is the degree and distribution of burnout within the physician workforce, and how does that compare to the general U.S. workforce?
Background: Professional burnout, work satisfaction, and work-life balance are critical elements to understand in the physician workforce. It is well documented that physicians are at high risk for burnout; however, few extensive studies have looked at rates and the identification of high-risk subpopulations.
Study design: Cross-sectional survey.
Setting: U.S. workforce.
Synopsis: This study included 7,288 physicians (26.7% response rate) and 5,930 controls from the general U.S. population. Validated survey instruments were employed to assess the degree and presence of burnout, depression, and satisfaction with work-life balance.
In aggregate, using a validated, two-item burnout measure, 35.2% of physicians were characterized as having burnout, compared with 27.6% of the general population (P<0.001). Within the physician community, the specialties with the highest risk of burnout included emergency medicine, general internal medicine, family medicine, and neurology.
Important limitations of this study include that the physician and general population surveys were performed at different times (six months apart), that the groups were not ideally matched (age and sex, for example), and the overall response rate of the physician survey was low.
This study sheds light on an important topic for hospitalists. Future studies should continue to probe the problem of burnout and look for creative solutions to mitigate risks that might threaten professional longevity.
Bottom line: Burnout is prevalent among physicians, especially when compared to the general workforce. Physician specialties in front-line patient care are at highest risk.
Citation: Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012;20 [Epub ahead of print].
Effects of Clopidogrel Added to Aspirin in Patients with Recent Lacunar Stroke
Clinical question: Does the addition of clopidogrel to aspirin reduce the risk of any type of recurrent stroke, or affect the risk of bleeding or death, in patients who recently suffered a lacunar stroke?
Background: There are no prior randomized, multicenter trials on secondary prevention of lacunar stroke; aspirin is the standard antiplatelet therapy in this setting.
Study design: Double-blind, randomized, multicenter trial.
Setting: Eighty-two clinical centers in North America, Latin America, and Spain.
Synopsis: Researchers enrolled 3,020 patients from 2003 to 2011; criteria included age >30 years old and symptomatic lacunar stroke (proven by MRI) in the preceding 180 days.
Results showed no significant difference between recurrent strokes (any type) in the aspirin-only group (2.7% per year) versus the aspirin-plus-clopidogrel group (2.5% per year). Major hemorrhage risk was much higher in the aspirin-plus-clopidogrel group (2.1% per year) versus aspirin-only group (1.1% per year). All-cause mortality also was much higher in the aspirin-plus-clopidogrel group (N=113) versus the aspirin-only group (N=77).
Bottom line: The addition of clopidogrel to aspirin for secondary prevention does not significantly reduce the risk of recurrent stroke, but it does significantly increase the risk of bleeding and death.
Citation: Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
Bleeding Risk Prediction Scores in Patients with Atrial Fibrillation Undergoing Anticoagulation
Clinical question: What are the relative predictive values of the HEMORR2AGES, ATRIA, and HAS-BLED risk-prediction schemes?
Background: The tools predict bleeding risk in patients anticoagulated for atrial fibrillation (afib), but it is unknown which is the best to predict clinically relevant bleeding.
Study design: Post-hoc analysis.
Setting: Data previously collected for the AMADEUS trial (2,293 patients taking warfarin; 251 had at least one clinically relevant bleeding event) were used to test each of the three bleeding risk-prediction schemes on the same data set.
Synopsis: Using three analysis methods (net reclassification improvement, receiver-operating characteristic [ROC], and decision-curve analysis), the researchers compared the three schemes’ performance. HAS-BLED performed best in all three of the analysis methods.
The HAS-BLED score calculation requires the following patient information: history of hypertension, renal disease, liver disease, stroke, prior major bleeding event, and labile INR; age >65; and use of antiplatelet agents, aspirin, and alcohol.
Bottom line: HAS-BLED was the best of the three schemes, although all three had only modest ability to predict clinically relevant bleeding.
Citation: Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORRAGES, ATRIA and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation. J Am Coll Cardiol. 2012;60(9):861-867
Probiotics for Secondary Prevention of Hepatic Encephalopathy
Clinical question: Are probiotics as effective as lactulose for secondary prevention of hepatic encephalopathy (HE)?
Background: Probiotics alter the gut flora, resulting in decreased ammonia production and absorption. Probiotics have been shown to reduce the incidence of low-grade HE. However, studies on probiotics usage for secondary prevention of HE are lacking.
Study design: Prospective, randomized, controlled, nonblinded, single-center study.
Setting: Tertiary-care center, New Delhi.
Synopsis: Three hundred sixty patients who had recovered from HE from October 2008 to December 2009 were screened; 235 met the inclusion criteria. They were randomized to receive either lactulose (Gp-L), probiotics (Gp-P), or no therapy (Gp-N). The Gp-L group received 30 to 60 ml of lactulose in two to three divided doses; the Gp-P group received three capsules per day containing lactobacillus, bifidobacterium, and Streptococcus salivarius strains.
The primary endpoints were the development of overt HE (assessed by the West Haven Criteria) or a follow-up of 12 months. Lactulose therapy was significantly more effective in secondary prophylaxis than no therapy (26.2% vs. 56.9%, P=0.001), as was probiotics therapy compared with no therapy (34.4% vs. 56.9%, P=0.02), but no significant difference was found between lactulose and probiotics therapy (26.2% vs. 34.4%, P=0.349).
The major limitation of the study was its open-label design. The study also used a high concentration of probiotics, and the results could be strain-specific and hence require validation with other probiotics combinations. The Gp-N group continued the previous therapy (excluding lactulose), with an unknown number on rifaximin.
Bottom line: Lactulose and probiotics are equally effective in secondary prophylaxis of hepatic encephalopathy.
Citation: Agrawal A, Sharma BC, Sharma P, Sarin SK. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics and no therapy. Am J Gastroenterol. 2012;107:1043-1050.
Capsule Endoscopy for Acute Obscure GI Bleeding
Clinical question: What testing modality is most appropriate for acute obscure GI bleeding: capsule endoscopy (CE) or angiography?
Background: Acute obscure GI bleeding (OGIB): remains a diagnostic challenge, accounting for 7% to 8% of patients presenting with GI bleeding. CE enables direct visualization of small bowel mucosa but lacks the ability for therapeutic intervention. Angiography is frequently chosen for massive bleeding; however, it is invasive and does not enable visualization of the bowel.
Study design: Prospective, randomized, controlled, blinded, single-center study.
Setting: Prince of Wales Hospital, Hong Kong.
Synopsis: Ninety-one patients with active OGIB from June 2005 to November 2007 were assessed for eligibility; 60 met the criteria and were randomized to either CE or angiography. Overt OGIB was defined as patients who had nondiagnostic upper endoscopy and colonoscopy.
The primary outcome was diagnostic yield of CE or mesenteric angiography in identifying the bleeding source. Secondary outcomes were long-term rebleeding rates, readmissions for bleeding or anemia, blood transfusions, and death.
CE was positive in 16 patients (53.3%) and angiography was positive in six patients (20%). The diagnostic yield of CE was significantly higher than angiography (difference=33.3%, 95% CI 8.9-52.8%, P=0.016). The mean follow-up period was 48.5 months. The cumulative risk of rebleeding was higher in the angiography group, but this was not statistically significant. There was no significant difference in rates of subsequent hospitalization, death, or transfusions between the two groups.
The study based the sample-size estimation on the diagnostic yield rather than clinical outcomes and, hence, was underpowered to detect any significant differences in clinical outcomes.
Bottom line: CE has a higher diagnostic yield than angiography in patients with active overt OGIB.
Citation: Leung WK, Ho S, Suen B, et al. Capsule endoscopy of angiography in patients with acute overt gastrointestinal bleeding: a prospective randomized study with long term follow up. Am J Gastroenterol. 2012;107:1370-1376.
Perceptions of Readmitted Patients Transitioning from Hospital to Home
Clinical question: What are patient-reported reasons for readmission to the hospital after discharge?
Background: Reducing readmissions is a critical component to improving the value of healthcare. While readmission reduction is a goal of all hospitals, there is much to be gleaned from evaluating patients’ view of the problem. This study used a survey to assess the patient’s viewpoint.
Study design: Cross-sectional survey.
Setting: The Hospital of the University of Pennsylvania and Penn Presbyterian Medical Center, Philadelphia.
Synopsis: A survey of 36 questions was posed to 1,084 patients who were readmitted within 30 days of discharge from November 2010 to July 2011 (32% of eligible patients). The data were subdivided based on socioeconomic status and medical versus surgical patients.
Some issues patients raised regarding discharge planning included difficulty with paying for medications, challenges with travel to pharmacies, and concern over medication side effects.
Patients with low socioeconomic status had more difficulty taking medications and following instructions, had more depression, and had less social support.
Bottom line: Readmission rates are affected by a patient’s social situation. A team approach to discharge planning might mitigate some of these factors.
Citation: Kangovi S, Grande D, Meehan P, Mitra N, Shannon R, Long JA. Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012 [Epub ahead of print].
30-Day Readmissions after Acute Myocardial Infarction
Clinical question: What are potential predictors of 30-day readmissions after acute myocardial infarction (MI)?
Background: Much attention has been given to evaluate the causes of readmissions of heart failure, acute MI, and pneumonia. This study looked at 30-day readmissions after an acute myocardial infarction (AMI).
Study design: Retrospective cohort study.
Setting: Olmstead County Hospital, Rochester, Minn.
Synopsis: A chart review of AMI based on ICD-9 codes from 1987 to 2010 identified 3,010 patients. Patients were verified using symptoms, cardiac enzymes, and EKG changes at the time of event. Interventions evaluated included fibrinolytic therapy, CABG, or primary PCI.
Survival increased to 96% from 89% during the period from 1987 to 2010. Researchers also noted more comorbid conditions, such as diabetes mellitus, COPD, and hypertension, noted over time. Of the patients evaluated, 643 readmissions occurred for 561 patients (18.6%). Of these, the most frequent causes were ischemic heart disease, respiratory symptoms, and heart failure. Comorbid conditions, such as diabetes, COPD, anemia, higher killip class on initial admission, duration of prior hospitalization, and procedural complications, independently increased the risk of readmission.
Bottom line: In addition to factors unrelated to an AMI, a patient’s comorbid conditions, post-procedure complications, and duration of hospitalization influence the risk of readmission.
Citation: Dunlay SM, Weston SA, Killian JM, et al. Thirty-day rehospitalizations after acute myocardial infarction: a cohort study. Ann Intern Med. 2012;157(1):11-18.
One-Hour Rule-Out or Rule-In for AMI in Chest Pain
Clinical question: How can we use the newly developed high-sensitivity cardiac troponin (hs-cTnT) to shorten the time to rule in and rule out AMI?
Background: The hs-cTnT assays available appear to improve the early diagnosis of AMI when compared to the regular cardiac troponins, but no clear guidelines are available as how to best use them in clinical practice.
Study design: Prospective, multicenter study.
Setting: Switzerland hospitals.
Synopsis: The study enrolled 872 unselected patients presenting to the ED with acute chest pain. Hs-cTnT level was measured in a blinded fashion at presentation and after one hour. Two independent cardiologists using all available medical records adjudicated the final AMI diagnosis. Optimal thresholds for rule-out were selected to allow for 100% sensitivity and negative predictive value. Rule-out criteria were defined as baseline hs-cTnT level <12 ng/L and an absolute change within the first hour of <3 ng/L. Rule-in criteria was defined as baseline hs-cTnT >52 ng/L or an absolute increase within the first hour of >5 ng/L.
AMI was the final diagnosis in 17% of patients; AMI was ruled out in 60%; and the remaining 23% were placed in observation.
Primary prognostic endpoint was 30-day mortality rate, which was 0.2% in the rule-out group, validating the suitability of these patients for early discharge.
Study limitations were that it was an observational study not used for clinical decision-making, no dialysis patients were included, and only one specific hs-cTnT assay was tested.
Bottom line: Using hs-cTnT levels at presentation and absolute changes within the first hour, a safe rule-out or rule-in of AMI can be performed in 77% of patients presenting with chest pain.
Citation: Reichlin T, Schindler C, Drexler B, et al. One-hour rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med. 2012;172(16):1-8.
Aspirin Increases Bleed Risk without Reducing Risk of Stroke in CKD and NVAF Patients
Clinical question: Is there a difference between aspirin and warfarin in preventing thromboembolic complications and risk of bleeding in patients with chronic kidney disease (CKD) and nonvalvular afib (NVAF)?
Background: Data are lacking on risks and benefits of aspirin and warfarin in CKD, as this group of patients largely has been excluded from anticoagulation therapy trials for NVAF. This study examined the risks and benefits of aspirin and warfarin in patients with CKD with NVAF.
Study design: Retrospective, observational cohort study.
Setting: Danish National Registries.
Synopsis: Of 132,372 patients with NVAF, 2.7% had CKD and 0.7% had end-stage renal disease (ESRD). Compared to patients with no CKD, there was increased risk of stroke or systemic thromboembolism in patients with ESRD (HR, 1.83; 95% CI, 1.57-2.14) and with non-end-stage CKD (HR 1.49; 95% CI 1.38-1.59).
In patients with CKD, warfarin significantly reduced stroke risk (HR, 0.76; 95% CI, 0.64-0.91) and significantly increased bleeding risk (HR, 1.33; 95% CI, 1.16-1.53); aspirin significantly increased bleeding risk (HR, 1.17; 95% CI, 1.02-1.34) with no reduction in stroke risk.
Bottom line: CKD was associated with an increased risk of stroke among NVAF patients. While both aspirin and warfarin were associated with increased risk of bleeding, there was a reduction in the risk of stroke with warfarin, but not with aspirin.
Citation: Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625-635.
ITL: Physician Reviews of HM-Relevant Research
Clinical question: Does the addition of clopidogrel to aspirin reduce the risk of any type of recurrent stroke, or affect the risk of bleeding or death, in patients who recently suffered a lacunar stroke?
Background: There are no prior randomized, multicenter trials on secondary prevention of lacunar stroke; aspirin is the standard antiplatelet therapy in this setting.
Study design: Double-blind, randomized, multicenter trial.
Setting: Eighty-two clinical centers in North America, Latin America, and Spain.
Synopsis: Researchers enrolled 3,020 patients from 2003 to 2011; criteria included age >30 years old and symptomatic lacunar stroke (proven by MRI) in the preceding 180 days.
Results showed no significant difference between recurrent strokes (any type) in the aspirin-only group (2.7% per year) versus the aspirin-plus-clopidogrel group (2.5% per year). Major hemorrhage risk was much higher in the aspirin-plus-clopidogrel group (2.1% per year) versus aspirin-only group (1.1% per year). All-cause mortality also was much higher in the aspirin-plus-clopidogrel group (N=113) versus the aspirin-only group (N=77).
Bottom line: The addition of clopidogrel to aspirin for secondary prevention does not significantly reduce the risk of recurrent stroke, but it does significantly increase the risk of bleeding and death.
Citation: Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
For more physician reviews of recent HM-relevant literature, visit our website.
Clinical question: Does the addition of clopidogrel to aspirin reduce the risk of any type of recurrent stroke, or affect the risk of bleeding or death, in patients who recently suffered a lacunar stroke?
Background: There are no prior randomized, multicenter trials on secondary prevention of lacunar stroke; aspirin is the standard antiplatelet therapy in this setting.
Study design: Double-blind, randomized, multicenter trial.
Setting: Eighty-two clinical centers in North America, Latin America, and Spain.
Synopsis: Researchers enrolled 3,020 patients from 2003 to 2011; criteria included age >30 years old and symptomatic lacunar stroke (proven by MRI) in the preceding 180 days.
Results showed no significant difference between recurrent strokes (any type) in the aspirin-only group (2.7% per year) versus the aspirin-plus-clopidogrel group (2.5% per year). Major hemorrhage risk was much higher in the aspirin-plus-clopidogrel group (2.1% per year) versus aspirin-only group (1.1% per year). All-cause mortality also was much higher in the aspirin-plus-clopidogrel group (N=113) versus the aspirin-only group (N=77).
Bottom line: The addition of clopidogrel to aspirin for secondary prevention does not significantly reduce the risk of recurrent stroke, but it does significantly increase the risk of bleeding and death.
Citation: Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
For more physician reviews of recent HM-relevant literature, visit our website.
Clinical question: Does the addition of clopidogrel to aspirin reduce the risk of any type of recurrent stroke, or affect the risk of bleeding or death, in patients who recently suffered a lacunar stroke?
Background: There are no prior randomized, multicenter trials on secondary prevention of lacunar stroke; aspirin is the standard antiplatelet therapy in this setting.
Study design: Double-blind, randomized, multicenter trial.
Setting: Eighty-two clinical centers in North America, Latin America, and Spain.
Synopsis: Researchers enrolled 3,020 patients from 2003 to 2011; criteria included age >30 years old and symptomatic lacunar stroke (proven by MRI) in the preceding 180 days.
Results showed no significant difference between recurrent strokes (any type) in the aspirin-only group (2.7% per year) versus the aspirin-plus-clopidogrel group (2.5% per year). Major hemorrhage risk was much higher in the aspirin-plus-clopidogrel group (2.1% per year) versus aspirin-only group (1.1% per year). All-cause mortality also was much higher in the aspirin-plus-clopidogrel group (N=113) versus the aspirin-only group (N=77).
Bottom line: The addition of clopidogrel to aspirin for secondary prevention does not significantly reduce the risk of recurrent stroke, but it does significantly increase the risk of bleeding and death.
Citation: Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817-825.
For more physician reviews of recent HM-relevant literature, visit our website.
How is Graves' Disease Diagnosed and Evaluated?
Case
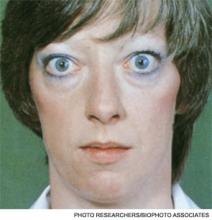
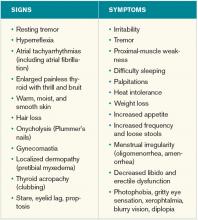
A 25-year-old, previously healthy woman presents with one month of anxiety, palpitations, intermittent loose non-dysenteriform stools, fine tremors, and hair loss. She has had a 20-pound weight loss in the previous four months, even though she reports an increased appetite. Her heart rate ranges from 115 to 130 beats per minute, and her temperature is 37.5oC. An exam is notable for mild bilateral proptosis, thin hair, and moist skin. A goiter is visible; it has increased consistency on palpation with an audible bruit over it. She has hyperreflexia and fine tremors. An EKG reveals sinus tachycardia. How should this patient be evaluated? What treatment should be initiated?
Overview
Graves’ disease, the most common cause of hyperthyroidism, is caused by autoimmune stimulation of the thyrotropin (TSH) receptor. It generally presents with a variety of signs and symptoms found with hyperthyroidism, but it can also carry unique clinical features unrelated to thyrotoxicosis, such as ophthalmopathy and dermopathy.
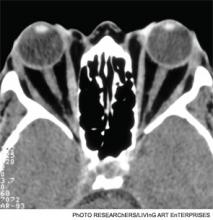
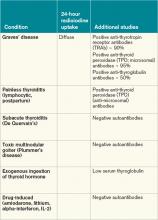
Graves’ disease diagnosis mainly is clinical, but also is supported by elevated free levels of thyroid hormones (mainly triiodothyronine [T3]) and suppressed TSH levels. Anti-thyrotropin receptor antibodies generally are present. Imaging in Graves’ disease is characterized by increased radioiodine uptake, as well as increased perfusion by Doppler ultrasonography.
Treatment can be pharmacologic, using anti-thyroid drugs, or ablative, with either radioiodine or thyroidectomy. Adjunctive therapy includes symptom control with beta-blocker agents, as well as steroid supplementation, especially in patients with orbitopathy undergoing radioablative treatment.
The Data
Epidemiology. Graves’ disease is the most common cause of hyperthyroidism, with a prevalence of ~0.5% of the population. Women are most commonly affected, with a prevalence five to 10 times higher than in male peers. The most common age of presentation is between the fifth and sixth decades of life.1-3
The fact that Graves’ disease occurs with higher incidence in patients with a family history of thyroid disease—and that concordance rates of up to 35% are seen with monozygotic twins—suggests that both genetic and environmental factors influence disease susceptibility.2,4
Pathophysiology. Graves’ disease occurs as a result of direct activation of the G-protein-coupled adenylate cyclase in the thyrotropin receptor by circulating IgG antibodies.2,3 Follicular hypertrophy and hyperplasia, and increased vascularity, cause goiter formation and an increased production of T3 and thyroxine (T4). The increase in T3 and T4 subsequently suppress TSH production.
Graves’ disease also is associated with unique clinical manifestations unrelated to the circulating levels of thyroid hormones, such as Graves’ ophthalmopathy and infiltrative dermopathy (localized or pretibial myxedema). Both of these occur as a result of local tissue infiltration by inflammatory cells and deposition of glycosaminglycanes.5
Clinical manifestations. Graves’ disease is characterized by a constellation of clinical findings and patient symptoms (see Table 1).1-3 The clinical presentation could differ in elderly patients, who present more commonly with weight loss or depression (also known as apathetic hyperthyroidism) and less commonly with tachycardia and tremor.2,3
Although clinically apparent, exophtalmos is detected in 30% to 50% of patients; when using orbital imaging, it is identified in ≥80% of patients.5 Ophthalmopathy has a clinical course typically independent of the thyroid activity; its manifestations include proptosis, periorbital edema and inflammation, exposure keratitis, photophobia, extraocular muscle infiltration, and eyelid lag.5-8
Thyroid dermopathy (localized dermal myxedema) can occur in 0.5% to 4.3% of patients with Graves’ disease; it occurs most commonly among patients with Graves’ ophthalmopathy, in whom it occurs in up to 13% of cases. About 20% of patients with dermal myxedema have associated thyroid acropachy.3,9
Hospitalists should be aware of thyroid storm. Although rare, occurring in only 1% to 2% of patients with hyperthyroidism, it can be a medical emergency. It is generally manifested by fever (due to severe thermogenesis), atrial tachyarrhythmias (due to hyperadrenergic response), mental status changes, and liver dysfunction.
In addition, patients with thyroid storm might present with hyperglycemia, hypercalcemia, hypocortisolism, and hypokalemia.10 Thyroid storm requires prompt treatment of both the clinical manifestations and the underlying condition.
Differential Diagnosis from Other Causes of Thyroiditis
Laboratory. The classic presentation of Graves’ disease is a suppressed TSH and elevated serum T3 and T4 levels.1-3 Generally, T3 is higher than T4, which also occurs in toxic multinodular goiter, solitary hyperfunctioning nodule, and iodine-induced hyperthyroidism.2,6 The free T3 and T4 levels should be obtained, as these are useful for monitoring response to therapy.1-3
Most patients with Graves’ disease also have anti-thyroid antibodies (see Table 2), although these are not required for the diagnosis.1-3,11
Following initiation of treatment, TSH levels remain suppressed for approximately two to three months, even after free T3 and T4 levels return to normal or below normal. After this period of suppression is over, TSH levels can be used to adjust therapy.1-3
Imaging. A thyroid radioiodine-uptake study provides a measure of iodine uptake, as well as an image of functioning thyroid tissue; the imaging is done 24 hours after the intake of iodine-123 or iodine-131. Generalized increased uptake is characteristic of Graves’ disease.1-3,12 In comparison, patients with thyroiditis have decreased radioiodine uptake as well as low blood flow in Doppler ultrasonography.13
In patients with large goiters, when there are signs or symptoms of upper airway or thoracic outlet obstruction, imaging with a neck and upper-chest CT scan is recommended.2 In patients with unilateral proptosis, asymmetric ophthalmopathy, or visual loss, orbital imaging is advised (CT scan or MRI).2,5 In patients with tachyarrhythmias, an electrocardiogram should evaluate for the presence of atrial fibrillation.2 Table 2 illustrates how Graves’ disease can be distinguished from other causes of thyroiditis.1-3
Initial Treatment
Treatment of Graves’ disease has two main tenets: treating the underlying thyroid disorder and quickly controlling symptoms. The underlying thyroid disorder can be treated with such anti-thyroid drugs as thionamides (methimazole or propylthiouracil), ablative radioiodine, or surgical excision of the thyroid. Adjunct symptom therapy can include beta-blockers, organic iodide, and glucocorticoids.11,14 Thionamides are preferred in young patients, pregnant women, and cases with orbital involvement.14
In pregnancy, treatment with propylthiouracil is preferred, especially during the first two trimesters due to the risk of teratogenicity with methimazole (there have been associated case reports of choanal atresia, aplasia cutis, and facial malformations).15
Steroid prophylaxis is used in patients with prominent ocular symptoms who undergo radioiodine ablation to minimize risk of worsening of ophthalmopathy.16
Back to the Case
The patient was admitted; free T3 and T4 levels were elevated, TSH was suppressed, and anti-thyroid antibodies (anti-TPO, anti-TG, and anti-TRAb) were positive. An I-123 radioiodine uptake scan showed diffuse thyroid gland uptake. Beta-blockers were initiated for heart-rate control (atenolol 25 mg) with adequate response.
Given the patient’s young age, it was decided to initiate thionamides. A pregnancy test was negative, so methimazole was initiated at a dose of 10 mg orally once daily.
Dr. Auron is a hospitalist in the Department of Hospital Medicine and the Center for Pediatric Hospital Medicine at Cleveland Clinic. Dr. Hamilton is a hospitalist in the Department of Hospital Medicine at Cleveland Clinic.
References
- Baskin HJ, Cobin RH, Duick DS, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. (2006 Amended version). Endocr Pract. 2002;8:457-469.
- Brent GA. Clinical practice. Graves’ disease. N Engl J Med. 2008;358:2594-2605.
- Nayak B, Hodak SP. Hyperthyroidism. Endocrinol Metab Clin North Am. 2007;36:617-656.
- Manji N, Carr-Smith JD, Boelaert K, et al. Influences of age, gender, smoking, and family history on autoimmune thyroid disease phenotype. J Clin Endocrinol Metab. 2006;91:4873-4880.
- Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726-738.
- Woeber KA. Triiodothyronine production in Graves’ hyperthyroidism. Thyroid. 2006;16:687-690.
- Osman F, Franklyn JA, Holder RL, Sheppard MC, Gammage MD. Cardiovascular manifestations of hyperthyroidism before and after antithyroid therapy: a matched case-control study. J Am Coll Cardiol. 2007;49:71-81.
- Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves’ ophthalmopathy. Thyroid. 2002;12:855-860.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- Chong HW, See KC, Phua J. Thyroid storm with multiorgan failure. Thyroid. 2010;20:333-336.
- De Groot L. Diagnosis and treatment of Graves’ disease. Thyroid Disease Manager website. Available at: http://www.thyroidmanager.org/chapter/diagnosis-and-treatment-of-graves-disease/. Accessed Jan. 20, 2012.
- Cappelli C, Pirola I, De Martino E, et al. The role of imaging in Graves’ disease: a cost-effectiveness analysis. Eur J Radiol. 2008;65:99-103.
- Ota H, Amino N, Morita S, et al. Quantitative measurement of thyroid blood flow for differentiation of painless thyroiditis from Graves’ disease. Clin Endocrinol (Oxf). 2007;67:41-45.
- Fumarola A, Di Fiore A, Dainelli M, Grani G, Calvanese A. Medical treatment of hyperthyroidism: state of the art. Exp Clin Endocrinol Diabetes. 2010;118:678-684.
- Fitzpatrick DL, Russell MA. Diagnosis and management of thyroid disease in pregnancy. Obstet Gynecol Clin North Am. 2010;37:173-193.
- Bartalena L. The dilemma of how to manage Graves’ hyperthyroidism in patients with associated orbitopathy. J Clin Endocrinol Metab. 2011;96:592-599.
Case
A 25-year-old, previously healthy woman presents with one month of anxiety, palpitations, intermittent loose non-dysenteriform stools, fine tremors, and hair loss. She has had a 20-pound weight loss in the previous four months, even though she reports an increased appetite. Her heart rate ranges from 115 to 130 beats per minute, and her temperature is 37.5oC. An exam is notable for mild bilateral proptosis, thin hair, and moist skin. A goiter is visible; it has increased consistency on palpation with an audible bruit over it. She has hyperreflexia and fine tremors. An EKG reveals sinus tachycardia. How should this patient be evaluated? What treatment should be initiated?
Overview
Graves’ disease, the most common cause of hyperthyroidism, is caused by autoimmune stimulation of the thyrotropin (TSH) receptor. It generally presents with a variety of signs and symptoms found with hyperthyroidism, but it can also carry unique clinical features unrelated to thyrotoxicosis, such as ophthalmopathy and dermopathy.
Graves’ disease diagnosis mainly is clinical, but also is supported by elevated free levels of thyroid hormones (mainly triiodothyronine [T3]) and suppressed TSH levels. Anti-thyrotropin receptor antibodies generally are present. Imaging in Graves’ disease is characterized by increased radioiodine uptake, as well as increased perfusion by Doppler ultrasonography.
Treatment can be pharmacologic, using anti-thyroid drugs, or ablative, with either radioiodine or thyroidectomy. Adjunctive therapy includes symptom control with beta-blocker agents, as well as steroid supplementation, especially in patients with orbitopathy undergoing radioablative treatment.
The Data
Epidemiology. Graves’ disease is the most common cause of hyperthyroidism, with a prevalence of ~0.5% of the population. Women are most commonly affected, with a prevalence five to 10 times higher than in male peers. The most common age of presentation is between the fifth and sixth decades of life.1-3
The fact that Graves’ disease occurs with higher incidence in patients with a family history of thyroid disease—and that concordance rates of up to 35% are seen with monozygotic twins—suggests that both genetic and environmental factors influence disease susceptibility.2,4
Pathophysiology. Graves’ disease occurs as a result of direct activation of the G-protein-coupled adenylate cyclase in the thyrotropin receptor by circulating IgG antibodies.2,3 Follicular hypertrophy and hyperplasia, and increased vascularity, cause goiter formation and an increased production of T3 and thyroxine (T4). The increase in T3 and T4 subsequently suppress TSH production.
Graves’ disease also is associated with unique clinical manifestations unrelated to the circulating levels of thyroid hormones, such as Graves’ ophthalmopathy and infiltrative dermopathy (localized or pretibial myxedema). Both of these occur as a result of local tissue infiltration by inflammatory cells and deposition of glycosaminglycanes.5
Clinical manifestations. Graves’ disease is characterized by a constellation of clinical findings and patient symptoms (see Table 1).1-3 The clinical presentation could differ in elderly patients, who present more commonly with weight loss or depression (also known as apathetic hyperthyroidism) and less commonly with tachycardia and tremor.2,3
Although clinically apparent, exophtalmos is detected in 30% to 50% of patients; when using orbital imaging, it is identified in ≥80% of patients.5 Ophthalmopathy has a clinical course typically independent of the thyroid activity; its manifestations include proptosis, periorbital edema and inflammation, exposure keratitis, photophobia, extraocular muscle infiltration, and eyelid lag.5-8
Thyroid dermopathy (localized dermal myxedema) can occur in 0.5% to 4.3% of patients with Graves’ disease; it occurs most commonly among patients with Graves’ ophthalmopathy, in whom it occurs in up to 13% of cases. About 20% of patients with dermal myxedema have associated thyroid acropachy.3,9
Hospitalists should be aware of thyroid storm. Although rare, occurring in only 1% to 2% of patients with hyperthyroidism, it can be a medical emergency. It is generally manifested by fever (due to severe thermogenesis), atrial tachyarrhythmias (due to hyperadrenergic response), mental status changes, and liver dysfunction.
In addition, patients with thyroid storm might present with hyperglycemia, hypercalcemia, hypocortisolism, and hypokalemia.10 Thyroid storm requires prompt treatment of both the clinical manifestations and the underlying condition.
Differential Diagnosis from Other Causes of Thyroiditis
Laboratory. The classic presentation of Graves’ disease is a suppressed TSH and elevated serum T3 and T4 levels.1-3 Generally, T3 is higher than T4, which also occurs in toxic multinodular goiter, solitary hyperfunctioning nodule, and iodine-induced hyperthyroidism.2,6 The free T3 and T4 levels should be obtained, as these are useful for monitoring response to therapy.1-3
Most patients with Graves’ disease also have anti-thyroid antibodies (see Table 2), although these are not required for the diagnosis.1-3,11
Following initiation of treatment, TSH levels remain suppressed for approximately two to three months, even after free T3 and T4 levels return to normal or below normal. After this period of suppression is over, TSH levels can be used to adjust therapy.1-3
Imaging. A thyroid radioiodine-uptake study provides a measure of iodine uptake, as well as an image of functioning thyroid tissue; the imaging is done 24 hours after the intake of iodine-123 or iodine-131. Generalized increased uptake is characteristic of Graves’ disease.1-3,12 In comparison, patients with thyroiditis have decreased radioiodine uptake as well as low blood flow in Doppler ultrasonography.13
In patients with large goiters, when there are signs or symptoms of upper airway or thoracic outlet obstruction, imaging with a neck and upper-chest CT scan is recommended.2 In patients with unilateral proptosis, asymmetric ophthalmopathy, or visual loss, orbital imaging is advised (CT scan or MRI).2,5 In patients with tachyarrhythmias, an electrocardiogram should evaluate for the presence of atrial fibrillation.2 Table 2 illustrates how Graves’ disease can be distinguished from other causes of thyroiditis.1-3
Initial Treatment
Treatment of Graves’ disease has two main tenets: treating the underlying thyroid disorder and quickly controlling symptoms. The underlying thyroid disorder can be treated with such anti-thyroid drugs as thionamides (methimazole or propylthiouracil), ablative radioiodine, or surgical excision of the thyroid. Adjunct symptom therapy can include beta-blockers, organic iodide, and glucocorticoids.11,14 Thionamides are preferred in young patients, pregnant women, and cases with orbital involvement.14
In pregnancy, treatment with propylthiouracil is preferred, especially during the first two trimesters due to the risk of teratogenicity with methimazole (there have been associated case reports of choanal atresia, aplasia cutis, and facial malformations).15
Steroid prophylaxis is used in patients with prominent ocular symptoms who undergo radioiodine ablation to minimize risk of worsening of ophthalmopathy.16
Back to the Case
The patient was admitted; free T3 and T4 levels were elevated, TSH was suppressed, and anti-thyroid antibodies (anti-TPO, anti-TG, and anti-TRAb) were positive. An I-123 radioiodine uptake scan showed diffuse thyroid gland uptake. Beta-blockers were initiated for heart-rate control (atenolol 25 mg) with adequate response.
Given the patient’s young age, it was decided to initiate thionamides. A pregnancy test was negative, so methimazole was initiated at a dose of 10 mg orally once daily.
Dr. Auron is a hospitalist in the Department of Hospital Medicine and the Center for Pediatric Hospital Medicine at Cleveland Clinic. Dr. Hamilton is a hospitalist in the Department of Hospital Medicine at Cleveland Clinic.
References
- Baskin HJ, Cobin RH, Duick DS, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. (2006 Amended version). Endocr Pract. 2002;8:457-469.
- Brent GA. Clinical practice. Graves’ disease. N Engl J Med. 2008;358:2594-2605.
- Nayak B, Hodak SP. Hyperthyroidism. Endocrinol Metab Clin North Am. 2007;36:617-656.
- Manji N, Carr-Smith JD, Boelaert K, et al. Influences of age, gender, smoking, and family history on autoimmune thyroid disease phenotype. J Clin Endocrinol Metab. 2006;91:4873-4880.
- Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726-738.
- Woeber KA. Triiodothyronine production in Graves’ hyperthyroidism. Thyroid. 2006;16:687-690.
- Osman F, Franklyn JA, Holder RL, Sheppard MC, Gammage MD. Cardiovascular manifestations of hyperthyroidism before and after antithyroid therapy: a matched case-control study. J Am Coll Cardiol. 2007;49:71-81.
- Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves’ ophthalmopathy. Thyroid. 2002;12:855-860.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- Chong HW, See KC, Phua J. Thyroid storm with multiorgan failure. Thyroid. 2010;20:333-336.
- De Groot L. Diagnosis and treatment of Graves’ disease. Thyroid Disease Manager website. Available at: http://www.thyroidmanager.org/chapter/diagnosis-and-treatment-of-graves-disease/. Accessed Jan. 20, 2012.
- Cappelli C, Pirola I, De Martino E, et al. The role of imaging in Graves’ disease: a cost-effectiveness analysis. Eur J Radiol. 2008;65:99-103.
- Ota H, Amino N, Morita S, et al. Quantitative measurement of thyroid blood flow for differentiation of painless thyroiditis from Graves’ disease. Clin Endocrinol (Oxf). 2007;67:41-45.
- Fumarola A, Di Fiore A, Dainelli M, Grani G, Calvanese A. Medical treatment of hyperthyroidism: state of the art. Exp Clin Endocrinol Diabetes. 2010;118:678-684.
- Fitzpatrick DL, Russell MA. Diagnosis and management of thyroid disease in pregnancy. Obstet Gynecol Clin North Am. 2010;37:173-193.
- Bartalena L. The dilemma of how to manage Graves’ hyperthyroidism in patients with associated orbitopathy. J Clin Endocrinol Metab. 2011;96:592-599.
Case
A 25-year-old, previously healthy woman presents with one month of anxiety, palpitations, intermittent loose non-dysenteriform stools, fine tremors, and hair loss. She has had a 20-pound weight loss in the previous four months, even though she reports an increased appetite. Her heart rate ranges from 115 to 130 beats per minute, and her temperature is 37.5oC. An exam is notable for mild bilateral proptosis, thin hair, and moist skin. A goiter is visible; it has increased consistency on palpation with an audible bruit over it. She has hyperreflexia and fine tremors. An EKG reveals sinus tachycardia. How should this patient be evaluated? What treatment should be initiated?
Overview
Graves’ disease, the most common cause of hyperthyroidism, is caused by autoimmune stimulation of the thyrotropin (TSH) receptor. It generally presents with a variety of signs and symptoms found with hyperthyroidism, but it can also carry unique clinical features unrelated to thyrotoxicosis, such as ophthalmopathy and dermopathy.
Graves’ disease diagnosis mainly is clinical, but also is supported by elevated free levels of thyroid hormones (mainly triiodothyronine [T3]) and suppressed TSH levels. Anti-thyrotropin receptor antibodies generally are present. Imaging in Graves’ disease is characterized by increased radioiodine uptake, as well as increased perfusion by Doppler ultrasonography.
Treatment can be pharmacologic, using anti-thyroid drugs, or ablative, with either radioiodine or thyroidectomy. Adjunctive therapy includes symptom control with beta-blocker agents, as well as steroid supplementation, especially in patients with orbitopathy undergoing radioablative treatment.
The Data
Epidemiology. Graves’ disease is the most common cause of hyperthyroidism, with a prevalence of ~0.5% of the population. Women are most commonly affected, with a prevalence five to 10 times higher than in male peers. The most common age of presentation is between the fifth and sixth decades of life.1-3
The fact that Graves’ disease occurs with higher incidence in patients with a family history of thyroid disease—and that concordance rates of up to 35% are seen with monozygotic twins—suggests that both genetic and environmental factors influence disease susceptibility.2,4
Pathophysiology. Graves’ disease occurs as a result of direct activation of the G-protein-coupled adenylate cyclase in the thyrotropin receptor by circulating IgG antibodies.2,3 Follicular hypertrophy and hyperplasia, and increased vascularity, cause goiter formation and an increased production of T3 and thyroxine (T4). The increase in T3 and T4 subsequently suppress TSH production.
Graves’ disease also is associated with unique clinical manifestations unrelated to the circulating levels of thyroid hormones, such as Graves’ ophthalmopathy and infiltrative dermopathy (localized or pretibial myxedema). Both of these occur as a result of local tissue infiltration by inflammatory cells and deposition of glycosaminglycanes.5
Clinical manifestations. Graves’ disease is characterized by a constellation of clinical findings and patient symptoms (see Table 1).1-3 The clinical presentation could differ in elderly patients, who present more commonly with weight loss or depression (also known as apathetic hyperthyroidism) and less commonly with tachycardia and tremor.2,3
Although clinically apparent, exophtalmos is detected in 30% to 50% of patients; when using orbital imaging, it is identified in ≥80% of patients.5 Ophthalmopathy has a clinical course typically independent of the thyroid activity; its manifestations include proptosis, periorbital edema and inflammation, exposure keratitis, photophobia, extraocular muscle infiltration, and eyelid lag.5-8
Thyroid dermopathy (localized dermal myxedema) can occur in 0.5% to 4.3% of patients with Graves’ disease; it occurs most commonly among patients with Graves’ ophthalmopathy, in whom it occurs in up to 13% of cases. About 20% of patients with dermal myxedema have associated thyroid acropachy.3,9
Hospitalists should be aware of thyroid storm. Although rare, occurring in only 1% to 2% of patients with hyperthyroidism, it can be a medical emergency. It is generally manifested by fever (due to severe thermogenesis), atrial tachyarrhythmias (due to hyperadrenergic response), mental status changes, and liver dysfunction.
In addition, patients with thyroid storm might present with hyperglycemia, hypercalcemia, hypocortisolism, and hypokalemia.10 Thyroid storm requires prompt treatment of both the clinical manifestations and the underlying condition.
Differential Diagnosis from Other Causes of Thyroiditis
Laboratory. The classic presentation of Graves’ disease is a suppressed TSH and elevated serum T3 and T4 levels.1-3 Generally, T3 is higher than T4, which also occurs in toxic multinodular goiter, solitary hyperfunctioning nodule, and iodine-induced hyperthyroidism.2,6 The free T3 and T4 levels should be obtained, as these are useful for monitoring response to therapy.1-3
Most patients with Graves’ disease also have anti-thyroid antibodies (see Table 2), although these are not required for the diagnosis.1-3,11
Following initiation of treatment, TSH levels remain suppressed for approximately two to three months, even after free T3 and T4 levels return to normal or below normal. After this period of suppression is over, TSH levels can be used to adjust therapy.1-3
Imaging. A thyroid radioiodine-uptake study provides a measure of iodine uptake, as well as an image of functioning thyroid tissue; the imaging is done 24 hours after the intake of iodine-123 or iodine-131. Generalized increased uptake is characteristic of Graves’ disease.1-3,12 In comparison, patients with thyroiditis have decreased radioiodine uptake as well as low blood flow in Doppler ultrasonography.13
In patients with large goiters, when there are signs or symptoms of upper airway or thoracic outlet obstruction, imaging with a neck and upper-chest CT scan is recommended.2 In patients with unilateral proptosis, asymmetric ophthalmopathy, or visual loss, orbital imaging is advised (CT scan or MRI).2,5 In patients with tachyarrhythmias, an electrocardiogram should evaluate for the presence of atrial fibrillation.2 Table 2 illustrates how Graves’ disease can be distinguished from other causes of thyroiditis.1-3
Initial Treatment
Treatment of Graves’ disease has two main tenets: treating the underlying thyroid disorder and quickly controlling symptoms. The underlying thyroid disorder can be treated with such anti-thyroid drugs as thionamides (methimazole or propylthiouracil), ablative radioiodine, or surgical excision of the thyroid. Adjunct symptom therapy can include beta-blockers, organic iodide, and glucocorticoids.11,14 Thionamides are preferred in young patients, pregnant women, and cases with orbital involvement.14
In pregnancy, treatment with propylthiouracil is preferred, especially during the first two trimesters due to the risk of teratogenicity with methimazole (there have been associated case reports of choanal atresia, aplasia cutis, and facial malformations).15
Steroid prophylaxis is used in patients with prominent ocular symptoms who undergo radioiodine ablation to minimize risk of worsening of ophthalmopathy.16
Back to the Case
The patient was admitted; free T3 and T4 levels were elevated, TSH was suppressed, and anti-thyroid antibodies (anti-TPO, anti-TG, and anti-TRAb) were positive. An I-123 radioiodine uptake scan showed diffuse thyroid gland uptake. Beta-blockers were initiated for heart-rate control (atenolol 25 mg) with adequate response.
Given the patient’s young age, it was decided to initiate thionamides. A pregnancy test was negative, so methimazole was initiated at a dose of 10 mg orally once daily.
Dr. Auron is a hospitalist in the Department of Hospital Medicine and the Center for Pediatric Hospital Medicine at Cleveland Clinic. Dr. Hamilton is a hospitalist in the Department of Hospital Medicine at Cleveland Clinic.
References
- Baskin HJ, Cobin RH, Duick DS, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. (2006 Amended version). Endocr Pract. 2002;8:457-469.
- Brent GA. Clinical practice. Graves’ disease. N Engl J Med. 2008;358:2594-2605.
- Nayak B, Hodak SP. Hyperthyroidism. Endocrinol Metab Clin North Am. 2007;36:617-656.
- Manji N, Carr-Smith JD, Boelaert K, et al. Influences of age, gender, smoking, and family history on autoimmune thyroid disease phenotype. J Clin Endocrinol Metab. 2006;91:4873-4880.
- Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726-738.
- Woeber KA. Triiodothyronine production in Graves’ hyperthyroidism. Thyroid. 2006;16:687-690.
- Osman F, Franklyn JA, Holder RL, Sheppard MC, Gammage MD. Cardiovascular manifestations of hyperthyroidism before and after antithyroid therapy: a matched case-control study. J Am Coll Cardiol. 2007;49:71-81.
- Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves’ ophthalmopathy. Thyroid. 2002;12:855-860.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- Chong HW, See KC, Phua J. Thyroid storm with multiorgan failure. Thyroid. 2010;20:333-336.
- De Groot L. Diagnosis and treatment of Graves’ disease. Thyroid Disease Manager website. Available at: http://www.thyroidmanager.org/chapter/diagnosis-and-treatment-of-graves-disease/. Accessed Jan. 20, 2012.
- Cappelli C, Pirola I, De Martino E, et al. The role of imaging in Graves’ disease: a cost-effectiveness analysis. Eur J Radiol. 2008;65:99-103.
- Ota H, Amino N, Morita S, et al. Quantitative measurement of thyroid blood flow for differentiation of painless thyroiditis from Graves’ disease. Clin Endocrinol (Oxf). 2007;67:41-45.
- Fumarola A, Di Fiore A, Dainelli M, Grani G, Calvanese A. Medical treatment of hyperthyroidism: state of the art. Exp Clin Endocrinol Diabetes. 2010;118:678-684.
- Fitzpatrick DL, Russell MA. Diagnosis and management of thyroid disease in pregnancy. Obstet Gynecol Clin North Am. 2010;37:173-193.
- Bartalena L. The dilemma of how to manage Graves’ hyperthyroidism in patients with associated orbitopathy. J Clin Endocrinol Metab. 2011;96:592-599.