User login
Inhibitor could be repurposed for MM
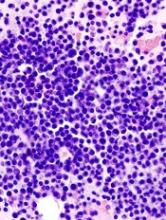
Tofacitinib, a pan-JAK inhibitor approved to treat rheumatoid arthritis, may advance as a potential treatment for multiple myeloma (MM) based on results from preclinical studies.
In these studies, tofacitinib was able to reverse proliferative effects in stromal-responsive human MM cell lines and reduce tumor growth in mouse models of MM.
Christine Lam, of University of California, San Francisco, and her colleagues conducted this research and reported the results in haematologica.
The researchers showed that, in co-cultures of MM cell lines and bone marrow stromal cells (BMSCs), tofacitinib inhibited the growth of MM cells in a dose-dependent manner.
RNA sequencing and phosphoproteonomics revealed an upregulation of 67 transcripts in MM cell lines co-cultured with BMSCs—most related to JAK-STAT and interleukin signaling.
Additional cell culture experiments showed that tofacitinib inhibited the downstream signaling molecule STAT3, which is responsible for proliferation through the JAK/STAT pathway.
The JAK1/2 inhibitor ruxolitinib did not replicate results seen with tofacitinib.
Further experiments showed that carfilzomib did not have synergistic effects with tofacitinib.
Venetoclax did demonstrate synergy with tofacitinib but only in MM cells cocultured with BMSCs, not in MM cells alone.
The researchers also tested tofacitinib in vivo. They injected mice with an MM cell line, and, after 2 weeks, mice were treated with tofacitinib for 4 weeks.
Mice treated with tofacitinib had lower tumor burden and a significant improvement in survival compared to untreated control mice.
Finally, the researchers tested tofacitinib in bone marrow mononuclear cells from patients. After stimulation with IL-6, the cells were exposed to tofacitinib.
The researchers observed “modest” viability against malignant plasma cells. They noted that because ex vivo MM plasma cells are minimally proliferative even with added cytokines or stromal stimulations, “these results may not fully reflect the potential therapeutic efficacy of tofacitinib in MM patients, where plasma cells are constantly proliferating within the [bone marrow].”
The researchers concluded that “tofacitinib is a promising agent to reverse the tumor-proliferative effects of the [bone marrow] microenvironment that can be rapidly repurposed to benefit MM patients.”
Tofacitinib, a pan-JAK inhibitor approved to treat rheumatoid arthritis, may advance as a potential treatment for multiple myeloma (MM) based on results from preclinical studies.
In these studies, tofacitinib was able to reverse proliferative effects in stromal-responsive human MM cell lines and reduce tumor growth in mouse models of MM.
Christine Lam, of University of California, San Francisco, and her colleagues conducted this research and reported the results in haematologica.
The researchers showed that, in co-cultures of MM cell lines and bone marrow stromal cells (BMSCs), tofacitinib inhibited the growth of MM cells in a dose-dependent manner.
RNA sequencing and phosphoproteonomics revealed an upregulation of 67 transcripts in MM cell lines co-cultured with BMSCs—most related to JAK-STAT and interleukin signaling.
Additional cell culture experiments showed that tofacitinib inhibited the downstream signaling molecule STAT3, which is responsible for proliferation through the JAK/STAT pathway.
The JAK1/2 inhibitor ruxolitinib did not replicate results seen with tofacitinib.
Further experiments showed that carfilzomib did not have synergistic effects with tofacitinib.
Venetoclax did demonstrate synergy with tofacitinib but only in MM cells cocultured with BMSCs, not in MM cells alone.
The researchers also tested tofacitinib in vivo. They injected mice with an MM cell line, and, after 2 weeks, mice were treated with tofacitinib for 4 weeks.
Mice treated with tofacitinib had lower tumor burden and a significant improvement in survival compared to untreated control mice.
Finally, the researchers tested tofacitinib in bone marrow mononuclear cells from patients. After stimulation with IL-6, the cells were exposed to tofacitinib.
The researchers observed “modest” viability against malignant plasma cells. They noted that because ex vivo MM plasma cells are minimally proliferative even with added cytokines or stromal stimulations, “these results may not fully reflect the potential therapeutic efficacy of tofacitinib in MM patients, where plasma cells are constantly proliferating within the [bone marrow].”
The researchers concluded that “tofacitinib is a promising agent to reverse the tumor-proliferative effects of the [bone marrow] microenvironment that can be rapidly repurposed to benefit MM patients.”
Tofacitinib, a pan-JAK inhibitor approved to treat rheumatoid arthritis, may advance as a potential treatment for multiple myeloma (MM) based on results from preclinical studies.
In these studies, tofacitinib was able to reverse proliferative effects in stromal-responsive human MM cell lines and reduce tumor growth in mouse models of MM.
Christine Lam, of University of California, San Francisco, and her colleagues conducted this research and reported the results in haematologica.
The researchers showed that, in co-cultures of MM cell lines and bone marrow stromal cells (BMSCs), tofacitinib inhibited the growth of MM cells in a dose-dependent manner.
RNA sequencing and phosphoproteonomics revealed an upregulation of 67 transcripts in MM cell lines co-cultured with BMSCs—most related to JAK-STAT and interleukin signaling.
Additional cell culture experiments showed that tofacitinib inhibited the downstream signaling molecule STAT3, which is responsible for proliferation through the JAK/STAT pathway.
The JAK1/2 inhibitor ruxolitinib did not replicate results seen with tofacitinib.
Further experiments showed that carfilzomib did not have synergistic effects with tofacitinib.
Venetoclax did demonstrate synergy with tofacitinib but only in MM cells cocultured with BMSCs, not in MM cells alone.
The researchers also tested tofacitinib in vivo. They injected mice with an MM cell line, and, after 2 weeks, mice were treated with tofacitinib for 4 weeks.
Mice treated with tofacitinib had lower tumor burden and a significant improvement in survival compared to untreated control mice.
Finally, the researchers tested tofacitinib in bone marrow mononuclear cells from patients. After stimulation with IL-6, the cells were exposed to tofacitinib.
The researchers observed “modest” viability against malignant plasma cells. They noted that because ex vivo MM plasma cells are minimally proliferative even with added cytokines or stromal stimulations, “these results may not fully reflect the potential therapeutic efficacy of tofacitinib in MM patients, where plasma cells are constantly proliferating within the [bone marrow].”
The researchers concluded that “tofacitinib is a promising agent to reverse the tumor-proliferative effects of the [bone marrow] microenvironment that can be rapidly repurposed to benefit MM patients.”
Transplant strategy not viable for aggressive B-NHL
Transplant with radioimmunotherapy (RIT)-based conditioning is a viable treatment option for patients with indolent—but not aggressive—B-cell non-Hodgkin lymphomas (NHLs), according to researchers.
Long-term follow-up data showed “excellent” outcomes in patients with indolent B-NHL who received conditioning with 90Y-ibritumomab tiuxetan plus fludarabine and low-dose total body irradiation (TBI) prior to HLA-matched hematopoietic stem cell transplant (HSCT).
However, long-term outcomes were inferior in patients with diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL).
Camille E. Puronen, MD, of the University of Washington in Seattle, and her colleagues reported these results in Biology of Blood and Marrow Transplantation.
The study enrolled 40 patients with high-risk B-NHL. This included DLBCL (n=14), chronic lymphocytic leukemia (CLL; n=10), MCL (n=8), follicular lymphoma (FL; n=6); hairy cell leukemia (HCL; n=1), and marginal zone lymphoma (MZL; n=1).
Patients were treated with 0.4 mCi/kg 90Y-ibritumomab tiuxetan, given 2 weeks prior to HSCT, to a maximum dose of 32 mCi.
Patients also received fludarabine at 30 mg/m2 on day 5, 6, and 7 prior to HSCT and 2 Gy TBI given on the day of transplant.
In an earlier report, the objective response rate (ORR) was 60%, and 35% of patients had a complete response (CR) or unconfirmed CR.
The researchers said early responses were not associated with disease bulk or chemoresistance, as the ORR was 59% in patients with bulky or chemoresistant disease.
However, responses were associated with histology, as the ORR was 38% in patients with DLBCL, 50% in those with MCL, 83% in those with FL, and 90% in those with CLL.
Long-term survival
In the current report, 11 of 40 patients were still alive at a median follow up of 9 years (range, 5.3 to 10.2). Fourteen patients died of disease progression, and 14 died from complications of HSCT.
The 5-year overall survival (OS) was 40%, and the 5-year progression-free survival (PFS) was 28%.
The best survival rates were in patients with indolent histology. The 5-year PFS was 44% in these patients, and the 5-year OS was 67%.
The researchers said early CR was not associated with long-term survival. However, patients who had at least stable disease (SD) at earlier time points did have the opportunity to achieve long-term survival. All patients who progressed before day 84 were dead by the 1-year mark.
Of the 11 patients who were still alive at a median follow up of 9 years, 4 had a CR or unconfirmed CR at day 84 (FL: 1; CLL: 2; MCL: 1); 6 were in partial response (CLL: 3; FL: 1; MCL: 1; MZL: 1); and 1 patient with FL had SD.
Among the 18 patients with indolent NHL, long-term PFS was observed in 5 of the 7 patients who achieved early CR and 8 of the 11 patients who did not achieve early CR.
Two of the 4 MCL patients who achieved an early CR had long-term PFS, but none of the MCL patients without an early CR had long-term PFS.
Among DLBCL patients, 1 of the 4 who achieved early CR had long-term PFS, but none of the patients without an early CR had long-term PFS. Only 1 DLBCL patient survived beyond 5 years. None survived beyond 8 years.
The researchers said the favorable outcomes in patients with indolent B-NHL are consistent with the known efficacy of RIT and the graft-versus-leukemia effect in these patients.
The team also noted that, since this trial began, several novel agents have been approved for the treatment of indolent B-NHL, which means allogeneic HSCT is often moved to later in the disease course.
The researchers concluded that 90Y-ibritumomab tiuxetan-based conditioning could “continue to play an important role in these settings,” but “improved strategies are needed” for patients with MCL and DLBCL.
Transplant with radioimmunotherapy (RIT)-based conditioning is a viable treatment option for patients with indolent—but not aggressive—B-cell non-Hodgkin lymphomas (NHLs), according to researchers.
Long-term follow-up data showed “excellent” outcomes in patients with indolent B-NHL who received conditioning with 90Y-ibritumomab tiuxetan plus fludarabine and low-dose total body irradiation (TBI) prior to HLA-matched hematopoietic stem cell transplant (HSCT).
However, long-term outcomes were inferior in patients with diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL).
Camille E. Puronen, MD, of the University of Washington in Seattle, and her colleagues reported these results in Biology of Blood and Marrow Transplantation.
The study enrolled 40 patients with high-risk B-NHL. This included DLBCL (n=14), chronic lymphocytic leukemia (CLL; n=10), MCL (n=8), follicular lymphoma (FL; n=6); hairy cell leukemia (HCL; n=1), and marginal zone lymphoma (MZL; n=1).
Patients were treated with 0.4 mCi/kg 90Y-ibritumomab tiuxetan, given 2 weeks prior to HSCT, to a maximum dose of 32 mCi.
Patients also received fludarabine at 30 mg/m2 on day 5, 6, and 7 prior to HSCT and 2 Gy TBI given on the day of transplant.
In an earlier report, the objective response rate (ORR) was 60%, and 35% of patients had a complete response (CR) or unconfirmed CR.
The researchers said early responses were not associated with disease bulk or chemoresistance, as the ORR was 59% in patients with bulky or chemoresistant disease.
However, responses were associated with histology, as the ORR was 38% in patients with DLBCL, 50% in those with MCL, 83% in those with FL, and 90% in those with CLL.
Long-term survival
In the current report, 11 of 40 patients were still alive at a median follow up of 9 years (range, 5.3 to 10.2). Fourteen patients died of disease progression, and 14 died from complications of HSCT.
The 5-year overall survival (OS) was 40%, and the 5-year progression-free survival (PFS) was 28%.
The best survival rates were in patients with indolent histology. The 5-year PFS was 44% in these patients, and the 5-year OS was 67%.
The researchers said early CR was not associated with long-term survival. However, patients who had at least stable disease (SD) at earlier time points did have the opportunity to achieve long-term survival. All patients who progressed before day 84 were dead by the 1-year mark.
Of the 11 patients who were still alive at a median follow up of 9 years, 4 had a CR or unconfirmed CR at day 84 (FL: 1; CLL: 2; MCL: 1); 6 were in partial response (CLL: 3; FL: 1; MCL: 1; MZL: 1); and 1 patient with FL had SD.
Among the 18 patients with indolent NHL, long-term PFS was observed in 5 of the 7 patients who achieved early CR and 8 of the 11 patients who did not achieve early CR.
Two of the 4 MCL patients who achieved an early CR had long-term PFS, but none of the MCL patients without an early CR had long-term PFS.
Among DLBCL patients, 1 of the 4 who achieved early CR had long-term PFS, but none of the patients without an early CR had long-term PFS. Only 1 DLBCL patient survived beyond 5 years. None survived beyond 8 years.
The researchers said the favorable outcomes in patients with indolent B-NHL are consistent with the known efficacy of RIT and the graft-versus-leukemia effect in these patients.
The team also noted that, since this trial began, several novel agents have been approved for the treatment of indolent B-NHL, which means allogeneic HSCT is often moved to later in the disease course.
The researchers concluded that 90Y-ibritumomab tiuxetan-based conditioning could “continue to play an important role in these settings,” but “improved strategies are needed” for patients with MCL and DLBCL.
Transplant with radioimmunotherapy (RIT)-based conditioning is a viable treatment option for patients with indolent—but not aggressive—B-cell non-Hodgkin lymphomas (NHLs), according to researchers.
Long-term follow-up data showed “excellent” outcomes in patients with indolent B-NHL who received conditioning with 90Y-ibritumomab tiuxetan plus fludarabine and low-dose total body irradiation (TBI) prior to HLA-matched hematopoietic stem cell transplant (HSCT).
However, long-term outcomes were inferior in patients with diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma (MCL).
Camille E. Puronen, MD, of the University of Washington in Seattle, and her colleagues reported these results in Biology of Blood and Marrow Transplantation.
The study enrolled 40 patients with high-risk B-NHL. This included DLBCL (n=14), chronic lymphocytic leukemia (CLL; n=10), MCL (n=8), follicular lymphoma (FL; n=6); hairy cell leukemia (HCL; n=1), and marginal zone lymphoma (MZL; n=1).
Patients were treated with 0.4 mCi/kg 90Y-ibritumomab tiuxetan, given 2 weeks prior to HSCT, to a maximum dose of 32 mCi.
Patients also received fludarabine at 30 mg/m2 on day 5, 6, and 7 prior to HSCT and 2 Gy TBI given on the day of transplant.
In an earlier report, the objective response rate (ORR) was 60%, and 35% of patients had a complete response (CR) or unconfirmed CR.
The researchers said early responses were not associated with disease bulk or chemoresistance, as the ORR was 59% in patients with bulky or chemoresistant disease.
However, responses were associated with histology, as the ORR was 38% in patients with DLBCL, 50% in those with MCL, 83% in those with FL, and 90% in those with CLL.
Long-term survival
In the current report, 11 of 40 patients were still alive at a median follow up of 9 years (range, 5.3 to 10.2). Fourteen patients died of disease progression, and 14 died from complications of HSCT.
The 5-year overall survival (OS) was 40%, and the 5-year progression-free survival (PFS) was 28%.
The best survival rates were in patients with indolent histology. The 5-year PFS was 44% in these patients, and the 5-year OS was 67%.
The researchers said early CR was not associated with long-term survival. However, patients who had at least stable disease (SD) at earlier time points did have the opportunity to achieve long-term survival. All patients who progressed before day 84 were dead by the 1-year mark.
Of the 11 patients who were still alive at a median follow up of 9 years, 4 had a CR or unconfirmed CR at day 84 (FL: 1; CLL: 2; MCL: 1); 6 were in partial response (CLL: 3; FL: 1; MCL: 1; MZL: 1); and 1 patient with FL had SD.
Among the 18 patients with indolent NHL, long-term PFS was observed in 5 of the 7 patients who achieved early CR and 8 of the 11 patients who did not achieve early CR.
Two of the 4 MCL patients who achieved an early CR had long-term PFS, but none of the MCL patients without an early CR had long-term PFS.
Among DLBCL patients, 1 of the 4 who achieved early CR had long-term PFS, but none of the patients without an early CR had long-term PFS. Only 1 DLBCL patient survived beyond 5 years. None survived beyond 8 years.
The researchers said the favorable outcomes in patients with indolent B-NHL are consistent with the known efficacy of RIT and the graft-versus-leukemia effect in these patients.
The team also noted that, since this trial began, several novel agents have been approved for the treatment of indolent B-NHL, which means allogeneic HSCT is often moved to later in the disease course.
The researchers concluded that 90Y-ibritumomab tiuxetan-based conditioning could “continue to play an important role in these settings,” but “improved strategies are needed” for patients with MCL and DLBCL.
Group updates guidelines on CLL
Recent advances in chronic lymphocytic leukemia (CLL) have prompted an update to the 2008 International Workshop in Chronic Lymphocytic Leukemia (iwCLL) consensus guidelines.
The updated iwCLL guidelines include new information on genomic alterations, the use of clinical staging and prognostic markers/scores, response assessment, minimal residual disease (MRD), and viral diseases in CLL patients.
The update was recently published in Blood.
Diagnosis, prognosis, and staging
To verify CLL diagnosis, the iwCLL guidelines recommend obtaining complete blood counts and differential counts as well as immunophenotyping of peripheral blood lymphocytes. A panel of CD19, CD5, CD20, CD23, κ, and λ typically suffices to establish a diagnosis.
Other tests that may help in prognosis or assessing tumor burden include molecular cytogenetics for del(13q), del(11q), del(17p), and add(12) in peripheral blood lymphocytes and determining TP53 and IGHV mutational status.
These tests can help identify poor-prognosis patients who are not likely to benefit from standard chemotherapy but are likely to benefit from small-molecule inhibitors of BTK, PI3K, or BCL2.
In addition, serum markers such as β2-microglogulin provide insight into overall survival and progression-free survival.
With regard to clinical staging, the guidelines highlight the Binet and Rai systems, which are routinely used in clinical practice and clinical trials.
However, the guidelines also note that “there are a large number of biomarkers that can provide additional prognostic information,” and, recently, “several prognostic scores and stratification systems have been proposed based on multivariate analyses.”
For example, the CLL international prognostic index (CLL-IPI) provides a weighted score that uses clinical stage, age, IGHV mutational status, β2-microglogulin, and the presence of del(17p) and/or TP53 mutations.
Indications for treatment
The guidelines note that active disease must be documented to initiate therapy. At least 1 of the following criteria must be met:
- Evidence of progressive marrow failure
- Massive, progressive, or symptomatic splenomegaly
- Progressive/symptomatic lymphadenopathy or massive nodes
- Progressive lymphocytosis
- Autoimmune complications
- Extranodal involvement
- Disease-related symptoms (unintentional weight loss, significant fatigue, fevers, night sweats for over a month without evidence of infection).
Following relapse, subsequent lines of treatment should follow the same principles as those used for initial treatment decisions.
Response, MRD, and more
The guidelines say 2 groups of parameters must be assessed to determine response to therapy:
- Group A: lymphoid tumor load and constitutional symptoms, including liver and/or spleen size, lymph node evaluation, and circulating lymphocyte count
- Group B: the hematopoietic system (platelet count, hemoglobin, and marrow).
For therapies with a defined treatment duration, response should be assessed at least 2 months after treatment is completed. For continued therapies or maintenance, response should be assessed at a predefined time point or at least 2 months after patients achieve their maximum response.
The guidelines also say MRD should be assessed in clinical trials aimed at maximizing the depth of remission. Furthermore, it “may be important” to confirm MRD negativity in the blood and marrow, as there are therapies that preferentially clear the blood but not the marrow (such as monoclonal antibodies).
In addition to the aforementioned recommendations, the updated iwCLL guidelines also include information on patient eligibility for clinical trials, guidance regarding treatment-related toxicities, and recommendations for supportive care and managing complications.
Recent advances in chronic lymphocytic leukemia (CLL) have prompted an update to the 2008 International Workshop in Chronic Lymphocytic Leukemia (iwCLL) consensus guidelines.
The updated iwCLL guidelines include new information on genomic alterations, the use of clinical staging and prognostic markers/scores, response assessment, minimal residual disease (MRD), and viral diseases in CLL patients.
The update was recently published in Blood.
Diagnosis, prognosis, and staging
To verify CLL diagnosis, the iwCLL guidelines recommend obtaining complete blood counts and differential counts as well as immunophenotyping of peripheral blood lymphocytes. A panel of CD19, CD5, CD20, CD23, κ, and λ typically suffices to establish a diagnosis.
Other tests that may help in prognosis or assessing tumor burden include molecular cytogenetics for del(13q), del(11q), del(17p), and add(12) in peripheral blood lymphocytes and determining TP53 and IGHV mutational status.
These tests can help identify poor-prognosis patients who are not likely to benefit from standard chemotherapy but are likely to benefit from small-molecule inhibitors of BTK, PI3K, or BCL2.
In addition, serum markers such as β2-microglogulin provide insight into overall survival and progression-free survival.
With regard to clinical staging, the guidelines highlight the Binet and Rai systems, which are routinely used in clinical practice and clinical trials.
However, the guidelines also note that “there are a large number of biomarkers that can provide additional prognostic information,” and, recently, “several prognostic scores and stratification systems have been proposed based on multivariate analyses.”
For example, the CLL international prognostic index (CLL-IPI) provides a weighted score that uses clinical stage, age, IGHV mutational status, β2-microglogulin, and the presence of del(17p) and/or TP53 mutations.
Indications for treatment
The guidelines note that active disease must be documented to initiate therapy. At least 1 of the following criteria must be met:
- Evidence of progressive marrow failure
- Massive, progressive, or symptomatic splenomegaly
- Progressive/symptomatic lymphadenopathy or massive nodes
- Progressive lymphocytosis
- Autoimmune complications
- Extranodal involvement
- Disease-related symptoms (unintentional weight loss, significant fatigue, fevers, night sweats for over a month without evidence of infection).
Following relapse, subsequent lines of treatment should follow the same principles as those used for initial treatment decisions.
Response, MRD, and more
The guidelines say 2 groups of parameters must be assessed to determine response to therapy:
- Group A: lymphoid tumor load and constitutional symptoms, including liver and/or spleen size, lymph node evaluation, and circulating lymphocyte count
- Group B: the hematopoietic system (platelet count, hemoglobin, and marrow).
For therapies with a defined treatment duration, response should be assessed at least 2 months after treatment is completed. For continued therapies or maintenance, response should be assessed at a predefined time point or at least 2 months after patients achieve their maximum response.
The guidelines also say MRD should be assessed in clinical trials aimed at maximizing the depth of remission. Furthermore, it “may be important” to confirm MRD negativity in the blood and marrow, as there are therapies that preferentially clear the blood but not the marrow (such as monoclonal antibodies).
In addition to the aforementioned recommendations, the updated iwCLL guidelines also include information on patient eligibility for clinical trials, guidance regarding treatment-related toxicities, and recommendations for supportive care and managing complications.
Recent advances in chronic lymphocytic leukemia (CLL) have prompted an update to the 2008 International Workshop in Chronic Lymphocytic Leukemia (iwCLL) consensus guidelines.
The updated iwCLL guidelines include new information on genomic alterations, the use of clinical staging and prognostic markers/scores, response assessment, minimal residual disease (MRD), and viral diseases in CLL patients.
The update was recently published in Blood.
Diagnosis, prognosis, and staging
To verify CLL diagnosis, the iwCLL guidelines recommend obtaining complete blood counts and differential counts as well as immunophenotyping of peripheral blood lymphocytes. A panel of CD19, CD5, CD20, CD23, κ, and λ typically suffices to establish a diagnosis.
Other tests that may help in prognosis or assessing tumor burden include molecular cytogenetics for del(13q), del(11q), del(17p), and add(12) in peripheral blood lymphocytes and determining TP53 and IGHV mutational status.
These tests can help identify poor-prognosis patients who are not likely to benefit from standard chemotherapy but are likely to benefit from small-molecule inhibitors of BTK, PI3K, or BCL2.
In addition, serum markers such as β2-microglogulin provide insight into overall survival and progression-free survival.
With regard to clinical staging, the guidelines highlight the Binet and Rai systems, which are routinely used in clinical practice and clinical trials.
However, the guidelines also note that “there are a large number of biomarkers that can provide additional prognostic information,” and, recently, “several prognostic scores and stratification systems have been proposed based on multivariate analyses.”
For example, the CLL international prognostic index (CLL-IPI) provides a weighted score that uses clinical stage, age, IGHV mutational status, β2-microglogulin, and the presence of del(17p) and/or TP53 mutations.
Indications for treatment
The guidelines note that active disease must be documented to initiate therapy. At least 1 of the following criteria must be met:
- Evidence of progressive marrow failure
- Massive, progressive, or symptomatic splenomegaly
- Progressive/symptomatic lymphadenopathy or massive nodes
- Progressive lymphocytosis
- Autoimmune complications
- Extranodal involvement
- Disease-related symptoms (unintentional weight loss, significant fatigue, fevers, night sweats for over a month without evidence of infection).
Following relapse, subsequent lines of treatment should follow the same principles as those used for initial treatment decisions.
Response, MRD, and more
The guidelines say 2 groups of parameters must be assessed to determine response to therapy:
- Group A: lymphoid tumor load and constitutional symptoms, including liver and/or spleen size, lymph node evaluation, and circulating lymphocyte count
- Group B: the hematopoietic system (platelet count, hemoglobin, and marrow).
For therapies with a defined treatment duration, response should be assessed at least 2 months after treatment is completed. For continued therapies or maintenance, response should be assessed at a predefined time point or at least 2 months after patients achieve their maximum response.
The guidelines also say MRD should be assessed in clinical trials aimed at maximizing the depth of remission. Furthermore, it “may be important” to confirm MRD negativity in the blood and marrow, as there are therapies that preferentially clear the blood but not the marrow (such as monoclonal antibodies).
In addition to the aforementioned recommendations, the updated iwCLL guidelines also include information on patient eligibility for clinical trials, guidance regarding treatment-related toxicities, and recommendations for supportive care and managing complications.
Ivosidenib active in R/R IDH1-mutated AML patients
CHICAGO—The investigational drug ivosidenib, an inhibitor of the mutant IDH1 enzyme, achieved complete remission (CR) rates of 32% and an overall response rate of 42% in relapsed/refractory (R/R) patients with acute myeloid leukemia (AML) and IDH1 mutation, according to investigators.
In addition, overall survival (OS) in patients who achieved CR more than doubled compared with those in the overall study population.
Fewer patients with CR had febrile neutropenia and infectious complications, and 25% of patients with CR were able to clear the IDH1 clone.
Duration of response was 6.5 months with the investigational drug.
Investigators reported the grade 3/4 toxicities could be managed with supportive care, were not fatal, and some patients still achieved responses.
IDH1 mutation, first identified almost 10 years ago with the sequencing of the first AML cancer genome, is a recurrent mutation in over 10% of patients with AML.
Mutated IDH1, reported in several malignancies, results in impaired cellular differentiation. Ivosidenib is a first-in-class oral therapy designed to inhibit the mutant IDH1 enzyme.
Phase 1 study (NCT02074839)
The phase 1 dose-escalation and dose expansion study specifically enrolled patients with R/RAML with mutated IDH1.
Daniel A. Pollyea, MD, of the Colorado University School of Medicine in Aurora, reported the data from 2 of the dose expansion cohorts as well as 35 patients from the dose escalation cohort at the 2018 ASCO Annual Meeting (abstract 7000).
All patients received ivosidenib 500 mg daily.
CR/CRh (CR with partial hematologic recovery; defined as morphologic remission with recovery of neutrophils to at least 500/mm3 and recovery of platelets to at least 50,000/µL) was the primary efficacy endpoint.
Of 179 patients in the primary efficacy cohort, 10% were still receiving treatment at the time of the presentation.
While most patients discontinued due to disease progression, 10% came off therapy for stem cell transplantation. Median duration of treatment was 4 months.
Patients were a median 67 years of age. Approximately 1/3 had secondary AML.
Patients had received a median of 2 prior therapies and approximately 1/4 had relapsed after transplantation.
Fifty-nine percent were refractory to induction or reinduction therapy.
Toxicity
Dr Pollyea considered adverse events to be as expected for a relapsed/refractory AML population.
However, he called out 3 for special mention—leukocytosis, ECG QT prolongation, and IDH differentiation syndrome—none of which was fatal.
Eight percent of patients had grade 3 or 4 leukocytosis, some of which were mechanistically induced from treatment.
About 10% of patients had grade 3 or 4 QT prolongation.
And grade 3 or 4 differentiation syndrome was reported for approximately 5% of patients.
In 19 patients with any grade differentiation syndrome, CR was reported for 5 patients. The message: patients experiencing this adverse event can be managed with supportive care, continue treatment, and still respond.
All adverse events were managed with supportive care measures, including concomitant medications, and ivosidenib dose modifications as required.
CR/CRh was 32% for the efficacy cohort; median time to response was 2 months and median time of response was 8.2 months. CR rate was 24%. Investigator-reported International Working Group categorized ORR was 42%.
The median OS was 9 months for the entire cohort and 18.8 months for patients who achieved CR/CRh.
Dr Pollyea reported that transfusion independence—defined as no need for transfusion for 56 days—was achieved in all CR patients, 75% of CRh patients, and even in a proportion of nonresponders.
Investigtors observed febrile neutropenia and grade 3 or 4 infectious complications in fewer patients who achieved CR/CRh.
Of note was the observation that 23% of patients who achieved CR/CRh were able to clear the mutant IDH1 clone. Patients who did not respond still harbored the IDH1 clone, Dr Pollyea reported.
These results reported at ASCO are an update from those simultaneously published in NEJM.
The study was supported by Agios Pharmaceuticals.
Ivosidenib is being evaluated alone and in combination in other clinical trials.
CHICAGO—The investigational drug ivosidenib, an inhibitor of the mutant IDH1 enzyme, achieved complete remission (CR) rates of 32% and an overall response rate of 42% in relapsed/refractory (R/R) patients with acute myeloid leukemia (AML) and IDH1 mutation, according to investigators.
In addition, overall survival (OS) in patients who achieved CR more than doubled compared with those in the overall study population.
Fewer patients with CR had febrile neutropenia and infectious complications, and 25% of patients with CR were able to clear the IDH1 clone.
Duration of response was 6.5 months with the investigational drug.
Investigators reported the grade 3/4 toxicities could be managed with supportive care, were not fatal, and some patients still achieved responses.
IDH1 mutation, first identified almost 10 years ago with the sequencing of the first AML cancer genome, is a recurrent mutation in over 10% of patients with AML.
Mutated IDH1, reported in several malignancies, results in impaired cellular differentiation. Ivosidenib is a first-in-class oral therapy designed to inhibit the mutant IDH1 enzyme.
Phase 1 study (NCT02074839)
The phase 1 dose-escalation and dose expansion study specifically enrolled patients with R/RAML with mutated IDH1.
Daniel A. Pollyea, MD, of the Colorado University School of Medicine in Aurora, reported the data from 2 of the dose expansion cohorts as well as 35 patients from the dose escalation cohort at the 2018 ASCO Annual Meeting (abstract 7000).
All patients received ivosidenib 500 mg daily.
CR/CRh (CR with partial hematologic recovery; defined as morphologic remission with recovery of neutrophils to at least 500/mm3 and recovery of platelets to at least 50,000/µL) was the primary efficacy endpoint.
Of 179 patients in the primary efficacy cohort, 10% were still receiving treatment at the time of the presentation.
While most patients discontinued due to disease progression, 10% came off therapy for stem cell transplantation. Median duration of treatment was 4 months.
Patients were a median 67 years of age. Approximately 1/3 had secondary AML.
Patients had received a median of 2 prior therapies and approximately 1/4 had relapsed after transplantation.
Fifty-nine percent were refractory to induction or reinduction therapy.
Toxicity
Dr Pollyea considered adverse events to be as expected for a relapsed/refractory AML population.
However, he called out 3 for special mention—leukocytosis, ECG QT prolongation, and IDH differentiation syndrome—none of which was fatal.
Eight percent of patients had grade 3 or 4 leukocytosis, some of which were mechanistically induced from treatment.
About 10% of patients had grade 3 or 4 QT prolongation.
And grade 3 or 4 differentiation syndrome was reported for approximately 5% of patients.
In 19 patients with any grade differentiation syndrome, CR was reported for 5 patients. The message: patients experiencing this adverse event can be managed with supportive care, continue treatment, and still respond.
All adverse events were managed with supportive care measures, including concomitant medications, and ivosidenib dose modifications as required.
CR/CRh was 32% for the efficacy cohort; median time to response was 2 months and median time of response was 8.2 months. CR rate was 24%. Investigator-reported International Working Group categorized ORR was 42%.
The median OS was 9 months for the entire cohort and 18.8 months for patients who achieved CR/CRh.
Dr Pollyea reported that transfusion independence—defined as no need for transfusion for 56 days—was achieved in all CR patients, 75% of CRh patients, and even in a proportion of nonresponders.
Investigtors observed febrile neutropenia and grade 3 or 4 infectious complications in fewer patients who achieved CR/CRh.
Of note was the observation that 23% of patients who achieved CR/CRh were able to clear the mutant IDH1 clone. Patients who did not respond still harbored the IDH1 clone, Dr Pollyea reported.
These results reported at ASCO are an update from those simultaneously published in NEJM.
The study was supported by Agios Pharmaceuticals.
Ivosidenib is being evaluated alone and in combination in other clinical trials.
CHICAGO—The investigational drug ivosidenib, an inhibitor of the mutant IDH1 enzyme, achieved complete remission (CR) rates of 32% and an overall response rate of 42% in relapsed/refractory (R/R) patients with acute myeloid leukemia (AML) and IDH1 mutation, according to investigators.
In addition, overall survival (OS) in patients who achieved CR more than doubled compared with those in the overall study population.
Fewer patients with CR had febrile neutropenia and infectious complications, and 25% of patients with CR were able to clear the IDH1 clone.
Duration of response was 6.5 months with the investigational drug.
Investigators reported the grade 3/4 toxicities could be managed with supportive care, were not fatal, and some patients still achieved responses.
IDH1 mutation, first identified almost 10 years ago with the sequencing of the first AML cancer genome, is a recurrent mutation in over 10% of patients with AML.
Mutated IDH1, reported in several malignancies, results in impaired cellular differentiation. Ivosidenib is a first-in-class oral therapy designed to inhibit the mutant IDH1 enzyme.
Phase 1 study (NCT02074839)
The phase 1 dose-escalation and dose expansion study specifically enrolled patients with R/RAML with mutated IDH1.
Daniel A. Pollyea, MD, of the Colorado University School of Medicine in Aurora, reported the data from 2 of the dose expansion cohorts as well as 35 patients from the dose escalation cohort at the 2018 ASCO Annual Meeting (abstract 7000).
All patients received ivosidenib 500 mg daily.
CR/CRh (CR with partial hematologic recovery; defined as morphologic remission with recovery of neutrophils to at least 500/mm3 and recovery of platelets to at least 50,000/µL) was the primary efficacy endpoint.
Of 179 patients in the primary efficacy cohort, 10% were still receiving treatment at the time of the presentation.
While most patients discontinued due to disease progression, 10% came off therapy for stem cell transplantation. Median duration of treatment was 4 months.
Patients were a median 67 years of age. Approximately 1/3 had secondary AML.
Patients had received a median of 2 prior therapies and approximately 1/4 had relapsed after transplantation.
Fifty-nine percent were refractory to induction or reinduction therapy.
Toxicity
Dr Pollyea considered adverse events to be as expected for a relapsed/refractory AML population.
However, he called out 3 for special mention—leukocytosis, ECG QT prolongation, and IDH differentiation syndrome—none of which was fatal.
Eight percent of patients had grade 3 or 4 leukocytosis, some of which were mechanistically induced from treatment.
About 10% of patients had grade 3 or 4 QT prolongation.
And grade 3 or 4 differentiation syndrome was reported for approximately 5% of patients.
In 19 patients with any grade differentiation syndrome, CR was reported for 5 patients. The message: patients experiencing this adverse event can be managed with supportive care, continue treatment, and still respond.
All adverse events were managed with supportive care measures, including concomitant medications, and ivosidenib dose modifications as required.
CR/CRh was 32% for the efficacy cohort; median time to response was 2 months and median time of response was 8.2 months. CR rate was 24%. Investigator-reported International Working Group categorized ORR was 42%.
The median OS was 9 months for the entire cohort and 18.8 months for patients who achieved CR/CRh.
Dr Pollyea reported that transfusion independence—defined as no need for transfusion for 56 days—was achieved in all CR patients, 75% of CRh patients, and even in a proportion of nonresponders.
Investigtors observed febrile neutropenia and grade 3 or 4 infectious complications in fewer patients who achieved CR/CRh.
Of note was the observation that 23% of patients who achieved CR/CRh were able to clear the mutant IDH1 clone. Patients who did not respond still harbored the IDH1 clone, Dr Pollyea reported.
These results reported at ASCO are an update from those simultaneously published in NEJM.
The study was supported by Agios Pharmaceuticals.
Ivosidenib is being evaluated alone and in combination in other clinical trials.
Ibrutinib and venetoclax combo promising in frontline CLL
CHICAGO—Ibrutinib combined with venetoclax is showing promising clinical activity in the frontline treatment of patients with chronic lymphocytic leukemia (CLL), according to investigators for the CAPTIVATE study.
In the first 30 patients, 77% of treatment-naïve patients had undetected minimal residual disease (MRD; <10-4 cells) in the blood and 86% showed a similar response in the bone marrow.
The overall response rate (ORR) was 100% in 11 evaluable patients. The investigators reported this initial data at the 2018 Annual Meeting of the American Society of Clinical Oncology (abstract 7502).
“These early results show a highly active and safe treatment with 12 cycles of combined treatment with ibrutinib and venetoclax,” said William G. Wierda, MD, PhD, of the MD Anderson Cancer Center in Houston, Texas, who presented the findings at ASCO.
Ibrutinib, a Bruton-kinase inhibitor, has already been approved for the treatment of CLL and venetoclax, a Bcl-2 inhibitor, is currently used to treat relapsed del 17p CLL.
Venetoclax in combination with rituximab was recently approved by the US Food and Drug Administration to treat patients with CLL or small lymphocytic lymphoma whether or not patients have del 17p.
With complementary mechanisms of action and preclinical studies suggesting synergy with the combination, CAPTIVATE was designed to test the efficacy of the oral combination given for 12 cycles.
Study design
CAPTIVATE (NCT02910583) is an ongoing phase 2 study that enrolled 164 patients with treatment-naïve CLL. Patients first received 3 cycles of ibrutinib monotherapy at the standard dose. This was intended to debulk the disease and reduce risk for venetoclax-associated tumor lysis syndrome (TLS).
Venetoclax 400 mg was initiated at cycle 4. After 12 cycles of the combination, patients with confirmed MRD negativity were randomized to receive ibrutinib with a placebo or to continue with the combination therapy.
In this initial report, Dr Wierda highlighted safety data for all 164 enrolled patients and efficacy data for the first 30 patients who had 6 cycles of combination therapy (MRD assessment cohort).
Dr Wierda also reported bone marrow data for the first 14 patients, who received a total of 12 cycles of the combination and represent the safety run-in cohort.
Ibrutinib and venetoclax show promising activity
Median age of patients was 58 years; about 2/3 of patients had unmutated IGHV and 1/3 had a creatine clearance of <80 mL/min.
Of 164 patients, 95% remain on therapy, with discontinuations reported for adverse events; one patient had disease progression to Richter’s transformation.
For the MRD evaluation, all 30 patients had 6 months of combination therapy and continue on treatment.
As expected, lead-in with ibrutinib monotherapy debulked the disease.
Investigators observed a reduction in the proportion of patients at high risk for TLS (24% to 3%) and an increase in the proportion of patients at low risk for TLS (12% to 29%).
A similar picture emerged for debulking of lymph node disease. No patient developed clinical TLS.
Other adverse events were consistent with the safety profile of single-agent ibrutinib and venetoclax. No new safety signals were seen.
After 6 cycles of the combination, blood MRD negativity was reported in 77% of the patients in the MRD assessment cohort.
In the safety-run in cohort of 14 patients, blood MRD negativity was reported in 86% of patients after 12 cycles and 93% of patients after 15 cycles of the combination. In these patients, bone marrow MRD negativity was achieved in 86%.
After 12 cycles of combination therapy, the objective response rate was 100% for 11 of the 14 evaluable patients from the safety run-in cohort: 6 patients showed complete remission (CR) or CR with incomplete blood count recovery (CRi) for a CR/CRi of 55%. All patients had confirmed undetectable MRD.
Investigators considered these responses promising and an assessment of the full treatment plan and durability of response are awaited.
The study was sponsored by Pharmacyclics.
CHICAGO—Ibrutinib combined with venetoclax is showing promising clinical activity in the frontline treatment of patients with chronic lymphocytic leukemia (CLL), according to investigators for the CAPTIVATE study.
In the first 30 patients, 77% of treatment-naïve patients had undetected minimal residual disease (MRD; <10-4 cells) in the blood and 86% showed a similar response in the bone marrow.
The overall response rate (ORR) was 100% in 11 evaluable patients. The investigators reported this initial data at the 2018 Annual Meeting of the American Society of Clinical Oncology (abstract 7502).
“These early results show a highly active and safe treatment with 12 cycles of combined treatment with ibrutinib and venetoclax,” said William G. Wierda, MD, PhD, of the MD Anderson Cancer Center in Houston, Texas, who presented the findings at ASCO.
Ibrutinib, a Bruton-kinase inhibitor, has already been approved for the treatment of CLL and venetoclax, a Bcl-2 inhibitor, is currently used to treat relapsed del 17p CLL.
Venetoclax in combination with rituximab was recently approved by the US Food and Drug Administration to treat patients with CLL or small lymphocytic lymphoma whether or not patients have del 17p.
With complementary mechanisms of action and preclinical studies suggesting synergy with the combination, CAPTIVATE was designed to test the efficacy of the oral combination given for 12 cycles.
Study design
CAPTIVATE (NCT02910583) is an ongoing phase 2 study that enrolled 164 patients with treatment-naïve CLL. Patients first received 3 cycles of ibrutinib monotherapy at the standard dose. This was intended to debulk the disease and reduce risk for venetoclax-associated tumor lysis syndrome (TLS).
Venetoclax 400 mg was initiated at cycle 4. After 12 cycles of the combination, patients with confirmed MRD negativity were randomized to receive ibrutinib with a placebo or to continue with the combination therapy.
In this initial report, Dr Wierda highlighted safety data for all 164 enrolled patients and efficacy data for the first 30 patients who had 6 cycles of combination therapy (MRD assessment cohort).
Dr Wierda also reported bone marrow data for the first 14 patients, who received a total of 12 cycles of the combination and represent the safety run-in cohort.
Ibrutinib and venetoclax show promising activity
Median age of patients was 58 years; about 2/3 of patients had unmutated IGHV and 1/3 had a creatine clearance of <80 mL/min.
Of 164 patients, 95% remain on therapy, with discontinuations reported for adverse events; one patient had disease progression to Richter’s transformation.
For the MRD evaluation, all 30 patients had 6 months of combination therapy and continue on treatment.
As expected, lead-in with ibrutinib monotherapy debulked the disease.
Investigators observed a reduction in the proportion of patients at high risk for TLS (24% to 3%) and an increase in the proportion of patients at low risk for TLS (12% to 29%).
A similar picture emerged for debulking of lymph node disease. No patient developed clinical TLS.
Other adverse events were consistent with the safety profile of single-agent ibrutinib and venetoclax. No new safety signals were seen.
After 6 cycles of the combination, blood MRD negativity was reported in 77% of the patients in the MRD assessment cohort.
In the safety-run in cohort of 14 patients, blood MRD negativity was reported in 86% of patients after 12 cycles and 93% of patients after 15 cycles of the combination. In these patients, bone marrow MRD negativity was achieved in 86%.
After 12 cycles of combination therapy, the objective response rate was 100% for 11 of the 14 evaluable patients from the safety run-in cohort: 6 patients showed complete remission (CR) or CR with incomplete blood count recovery (CRi) for a CR/CRi of 55%. All patients had confirmed undetectable MRD.
Investigators considered these responses promising and an assessment of the full treatment plan and durability of response are awaited.
The study was sponsored by Pharmacyclics.
CHICAGO—Ibrutinib combined with venetoclax is showing promising clinical activity in the frontline treatment of patients with chronic lymphocytic leukemia (CLL), according to investigators for the CAPTIVATE study.
In the first 30 patients, 77% of treatment-naïve patients had undetected minimal residual disease (MRD; <10-4 cells) in the blood and 86% showed a similar response in the bone marrow.
The overall response rate (ORR) was 100% in 11 evaluable patients. The investigators reported this initial data at the 2018 Annual Meeting of the American Society of Clinical Oncology (abstract 7502).
“These early results show a highly active and safe treatment with 12 cycles of combined treatment with ibrutinib and venetoclax,” said William G. Wierda, MD, PhD, of the MD Anderson Cancer Center in Houston, Texas, who presented the findings at ASCO.
Ibrutinib, a Bruton-kinase inhibitor, has already been approved for the treatment of CLL and venetoclax, a Bcl-2 inhibitor, is currently used to treat relapsed del 17p CLL.
Venetoclax in combination with rituximab was recently approved by the US Food and Drug Administration to treat patients with CLL or small lymphocytic lymphoma whether or not patients have del 17p.
With complementary mechanisms of action and preclinical studies suggesting synergy with the combination, CAPTIVATE was designed to test the efficacy of the oral combination given for 12 cycles.
Study design
CAPTIVATE (NCT02910583) is an ongoing phase 2 study that enrolled 164 patients with treatment-naïve CLL. Patients first received 3 cycles of ibrutinib monotherapy at the standard dose. This was intended to debulk the disease and reduce risk for venetoclax-associated tumor lysis syndrome (TLS).
Venetoclax 400 mg was initiated at cycle 4. After 12 cycles of the combination, patients with confirmed MRD negativity were randomized to receive ibrutinib with a placebo or to continue with the combination therapy.
In this initial report, Dr Wierda highlighted safety data for all 164 enrolled patients and efficacy data for the first 30 patients who had 6 cycles of combination therapy (MRD assessment cohort).
Dr Wierda also reported bone marrow data for the first 14 patients, who received a total of 12 cycles of the combination and represent the safety run-in cohort.
Ibrutinib and venetoclax show promising activity
Median age of patients was 58 years; about 2/3 of patients had unmutated IGHV and 1/3 had a creatine clearance of <80 mL/min.
Of 164 patients, 95% remain on therapy, with discontinuations reported for adverse events; one patient had disease progression to Richter’s transformation.
For the MRD evaluation, all 30 patients had 6 months of combination therapy and continue on treatment.
As expected, lead-in with ibrutinib monotherapy debulked the disease.
Investigators observed a reduction in the proportion of patients at high risk for TLS (24% to 3%) and an increase in the proportion of patients at low risk for TLS (12% to 29%).
A similar picture emerged for debulking of lymph node disease. No patient developed clinical TLS.
Other adverse events were consistent with the safety profile of single-agent ibrutinib and venetoclax. No new safety signals were seen.
After 6 cycles of the combination, blood MRD negativity was reported in 77% of the patients in the MRD assessment cohort.
In the safety-run in cohort of 14 patients, blood MRD negativity was reported in 86% of patients after 12 cycles and 93% of patients after 15 cycles of the combination. In these patients, bone marrow MRD negativity was achieved in 86%.
After 12 cycles of combination therapy, the objective response rate was 100% for 11 of the 14 evaluable patients from the safety run-in cohort: 6 patients showed complete remission (CR) or CR with incomplete blood count recovery (CRi) for a CR/CRi of 55%. All patients had confirmed undetectable MRD.
Investigators considered these responses promising and an assessment of the full treatment plan and durability of response are awaited.
The study was sponsored by Pharmacyclics.