User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Patient Navigators for Serious Illnesses Can Now Bill Under New Medicare Codes
In a move that acknowledges the gauntlet the US health system poses for people facing serious and fatal illnesses, Medicare will pay for a new class of workers to help patients manage treatments for conditions like cancer and heart failure.
The 2024 Medicare physician fee schedule includes new billing codes, including G0023, to pay for 60 minutes a month of care coordination by certified or trained auxiliary personnel working under the direction of a clinician.
A diagnosis of cancer or another serious illness takes a toll beyond the physical effects of the disease. Patients often scramble to make adjustments in family and work schedules to manage treatment, said Samyukta Mullangi, MD, MBA, medical director of oncology at Thyme Care, a Nashville, Tennessee–based firm that provides navigation and coordination services to oncology practices and insurers.
“It just really does create a bit of a pressure cooker for patients,” Dr. Mullangi told this news organization.
Medicare has for many years paid for medical professionals to help patients cope with the complexities of disease, such as chronic care management (CCM) provided by physicians, nurses, and physician assistants.
The new principal illness navigation (PIN) payments are intended to pay for work that to date typically has been done by people without medical degrees, including those involved in peer support networks and community health programs. The US Centers for Medicare and Medicaid Services(CMS) expects these navigators will undergo training and work under the supervision of clinicians.
The new navigators may coordinate care transitions between medical settings, follow up with patients after emergency department (ED) visits, or communicate with skilled nursing facilities regarding the psychosocial needs and functional deficits of a patient, among other functions.
CMS expects the new navigators may:
- Conduct assessments to understand a patient’s life story, strengths, needs, goals, preferences, and desired outcomes, including understanding cultural and linguistic factors.
- Provide support to accomplish the clinician’s treatment plan.
- Coordinate the receipt of needed services from healthcare facilities, home- and community-based service providers, and caregivers.
Peers as Navigators
The new navigators can be former patients who have undergone similar treatments for serious diseases, CMS said. This approach sets the new program apart from other care management services Medicare already covers, program officials wrote in the 2024 physician fee schedule.
“For some conditions, patients are best able to engage with the healthcare system and access care if they have assistance from a single, dedicated individual who has ‘lived experience,’ ” according to the rule.
The agency has taken a broad initial approach in defining what kinds of illnesses a patient may have to qualify for services. Patients must have a serious condition that is expected to last at least 3 months, such as cancer, heart failure, or substance use disorder.
But those without a definitive diagnosis may also qualify to receive navigator services.
In the rule, CMS cited a case in which a CT scan identified a suspicious mass in a patient’s colon. A clinician might decide this person would benefit from navigation services due to the potential risks for an undiagnosed illness.
“Regardless of the definitive diagnosis of the mass, presence of a colonic mass for that patient may be a serious high-risk condition that could, for example, cause obstruction and lead the patient to present to the emergency department, as well as be potentially indicative of an underlying life-threatening illness such as colon cancer,” CMS wrote in the rule.
Navigators often start their work when cancer patients are screened and guide them through initial diagnosis, potential surgery, radiation, or chemotherapy, said Sharon Gentry, MSN, RN, a former nurse navigator who is now the editor in chief of the Journal of the Academy of Oncology Nurse & Patient Navigators.
The navigators are meant to be a trusted and continual presence for patients, who otherwise might be left to start anew in finding help at each phase of care.
The navigators “see the whole picture. They see the whole journey the patient takes, from pre-diagnosis all the way through diagnosis care out through survival,” Ms. Gentry said.
Gaining a special Medicare payment for these kinds of services will elevate this work, she said.
Many newer drugs can target specific mechanisms and proteins of cancer. Often, oncology treatment involves testing to find out if mutations are allowing the cancer cells to evade a patient’s immune system.
Checking these biomarkers takes time, however. Patients sometimes become frustrated because they are anxious to begin treatment. Patients may receive inaccurate information from friends or family who went through treatment previously. Navigators can provide knowledge on the current state of care for a patient’s disease, helping them better manage anxieties.
“You have to explain to them that things have changed since the guy you drink coffee with was diagnosed with cancer, and there may be a drug that could target that,” Ms. Gentry said.
Potential Challenges
Initial uptake of the new PIN codes may be slow going, however, as clinicians and health systems may already use well-established codes. These include CCM and principal care management services, which may pay higher rates, Mullangi said.
“There might be sensitivity around not wanting to cannibalize existing programs with a new program,” Dr. Mullangi said.
In addition, many patients will have a copay for the services of principal illness navigators, Dr. Mullangi said.
While many patients have additional insurance that would cover the service, not all do. People with traditional Medicare coverage can sometimes pay 20% of the cost of some medical services.
“I think that may give patients pause, particularly if they’re already feeling the financial burden of a cancer treatment journey,” Dr. Mullangi said.
Pay rates for PIN services involve calculations of regional price differences, which are posted publicly by CMS, and potential added fees for services provided by hospital-affiliated organizations.
Consider payments for code G0023, covering 60 minutes of principal navigation services provided in a single month.
A set reimbursement for patients cared for in independent medical practices exists, with variation for local costs. Medicare’s non-facility price for G0023 would be $102.41 in some parts of Silicon Valley in California, including San Jose. In Arkansas, where costs are lower, reimbursement would be $73.14 for this same service.
Patients who get services covered by code G0023 in independent medical practices would have monthly copays of about $15-$20, depending on where they live.
The tab for patients tends to be higher for these same services if delivered through a medical practice owned by a hospital, as this would trigger the addition of facility fees to the payments made to cover the services. Facility fees are difficult for the public to ascertain before getting a treatment or service.
Dr. Mullangi and Ms. Gentry reported no relevant financial disclosures outside of their employers.
A version of this article first appeared on Medscape.com.
In a move that acknowledges the gauntlet the US health system poses for people facing serious and fatal illnesses, Medicare will pay for a new class of workers to help patients manage treatments for conditions like cancer and heart failure.
The 2024 Medicare physician fee schedule includes new billing codes, including G0023, to pay for 60 minutes a month of care coordination by certified or trained auxiliary personnel working under the direction of a clinician.
A diagnosis of cancer or another serious illness takes a toll beyond the physical effects of the disease. Patients often scramble to make adjustments in family and work schedules to manage treatment, said Samyukta Mullangi, MD, MBA, medical director of oncology at Thyme Care, a Nashville, Tennessee–based firm that provides navigation and coordination services to oncology practices and insurers.
“It just really does create a bit of a pressure cooker for patients,” Dr. Mullangi told this news organization.
Medicare has for many years paid for medical professionals to help patients cope with the complexities of disease, such as chronic care management (CCM) provided by physicians, nurses, and physician assistants.
The new principal illness navigation (PIN) payments are intended to pay for work that to date typically has been done by people without medical degrees, including those involved in peer support networks and community health programs. The US Centers for Medicare and Medicaid Services(CMS) expects these navigators will undergo training and work under the supervision of clinicians.
The new navigators may coordinate care transitions between medical settings, follow up with patients after emergency department (ED) visits, or communicate with skilled nursing facilities regarding the psychosocial needs and functional deficits of a patient, among other functions.
CMS expects the new navigators may:
- Conduct assessments to understand a patient’s life story, strengths, needs, goals, preferences, and desired outcomes, including understanding cultural and linguistic factors.
- Provide support to accomplish the clinician’s treatment plan.
- Coordinate the receipt of needed services from healthcare facilities, home- and community-based service providers, and caregivers.
Peers as Navigators
The new navigators can be former patients who have undergone similar treatments for serious diseases, CMS said. This approach sets the new program apart from other care management services Medicare already covers, program officials wrote in the 2024 physician fee schedule.
“For some conditions, patients are best able to engage with the healthcare system and access care if they have assistance from a single, dedicated individual who has ‘lived experience,’ ” according to the rule.
The agency has taken a broad initial approach in defining what kinds of illnesses a patient may have to qualify for services. Patients must have a serious condition that is expected to last at least 3 months, such as cancer, heart failure, or substance use disorder.
But those without a definitive diagnosis may also qualify to receive navigator services.
In the rule, CMS cited a case in which a CT scan identified a suspicious mass in a patient’s colon. A clinician might decide this person would benefit from navigation services due to the potential risks for an undiagnosed illness.
“Regardless of the definitive diagnosis of the mass, presence of a colonic mass for that patient may be a serious high-risk condition that could, for example, cause obstruction and lead the patient to present to the emergency department, as well as be potentially indicative of an underlying life-threatening illness such as colon cancer,” CMS wrote in the rule.
Navigators often start their work when cancer patients are screened and guide them through initial diagnosis, potential surgery, radiation, or chemotherapy, said Sharon Gentry, MSN, RN, a former nurse navigator who is now the editor in chief of the Journal of the Academy of Oncology Nurse & Patient Navigators.
The navigators are meant to be a trusted and continual presence for patients, who otherwise might be left to start anew in finding help at each phase of care.
The navigators “see the whole picture. They see the whole journey the patient takes, from pre-diagnosis all the way through diagnosis care out through survival,” Ms. Gentry said.
Gaining a special Medicare payment for these kinds of services will elevate this work, she said.
Many newer drugs can target specific mechanisms and proteins of cancer. Often, oncology treatment involves testing to find out if mutations are allowing the cancer cells to evade a patient’s immune system.
Checking these biomarkers takes time, however. Patients sometimes become frustrated because they are anxious to begin treatment. Patients may receive inaccurate information from friends or family who went through treatment previously. Navigators can provide knowledge on the current state of care for a patient’s disease, helping them better manage anxieties.
“You have to explain to them that things have changed since the guy you drink coffee with was diagnosed with cancer, and there may be a drug that could target that,” Ms. Gentry said.
Potential Challenges
Initial uptake of the new PIN codes may be slow going, however, as clinicians and health systems may already use well-established codes. These include CCM and principal care management services, which may pay higher rates, Mullangi said.
“There might be sensitivity around not wanting to cannibalize existing programs with a new program,” Dr. Mullangi said.
In addition, many patients will have a copay for the services of principal illness navigators, Dr. Mullangi said.
While many patients have additional insurance that would cover the service, not all do. People with traditional Medicare coverage can sometimes pay 20% of the cost of some medical services.
“I think that may give patients pause, particularly if they’re already feeling the financial burden of a cancer treatment journey,” Dr. Mullangi said.
Pay rates for PIN services involve calculations of regional price differences, which are posted publicly by CMS, and potential added fees for services provided by hospital-affiliated organizations.
Consider payments for code G0023, covering 60 minutes of principal navigation services provided in a single month.
A set reimbursement for patients cared for in independent medical practices exists, with variation for local costs. Medicare’s non-facility price for G0023 would be $102.41 in some parts of Silicon Valley in California, including San Jose. In Arkansas, where costs are lower, reimbursement would be $73.14 for this same service.
Patients who get services covered by code G0023 in independent medical practices would have monthly copays of about $15-$20, depending on where they live.
The tab for patients tends to be higher for these same services if delivered through a medical practice owned by a hospital, as this would trigger the addition of facility fees to the payments made to cover the services. Facility fees are difficult for the public to ascertain before getting a treatment or service.
Dr. Mullangi and Ms. Gentry reported no relevant financial disclosures outside of their employers.
A version of this article first appeared on Medscape.com.
In a move that acknowledges the gauntlet the US health system poses for people facing serious and fatal illnesses, Medicare will pay for a new class of workers to help patients manage treatments for conditions like cancer and heart failure.
The 2024 Medicare physician fee schedule includes new billing codes, including G0023, to pay for 60 minutes a month of care coordination by certified or trained auxiliary personnel working under the direction of a clinician.
A diagnosis of cancer or another serious illness takes a toll beyond the physical effects of the disease. Patients often scramble to make adjustments in family and work schedules to manage treatment, said Samyukta Mullangi, MD, MBA, medical director of oncology at Thyme Care, a Nashville, Tennessee–based firm that provides navigation and coordination services to oncology practices and insurers.
“It just really does create a bit of a pressure cooker for patients,” Dr. Mullangi told this news organization.
Medicare has for many years paid for medical professionals to help patients cope with the complexities of disease, such as chronic care management (CCM) provided by physicians, nurses, and physician assistants.
The new principal illness navigation (PIN) payments are intended to pay for work that to date typically has been done by people without medical degrees, including those involved in peer support networks and community health programs. The US Centers for Medicare and Medicaid Services(CMS) expects these navigators will undergo training and work under the supervision of clinicians.
The new navigators may coordinate care transitions between medical settings, follow up with patients after emergency department (ED) visits, or communicate with skilled nursing facilities regarding the psychosocial needs and functional deficits of a patient, among other functions.
CMS expects the new navigators may:
- Conduct assessments to understand a patient’s life story, strengths, needs, goals, preferences, and desired outcomes, including understanding cultural and linguistic factors.
- Provide support to accomplish the clinician’s treatment plan.
- Coordinate the receipt of needed services from healthcare facilities, home- and community-based service providers, and caregivers.
Peers as Navigators
The new navigators can be former patients who have undergone similar treatments for serious diseases, CMS said. This approach sets the new program apart from other care management services Medicare already covers, program officials wrote in the 2024 physician fee schedule.
“For some conditions, patients are best able to engage with the healthcare system and access care if they have assistance from a single, dedicated individual who has ‘lived experience,’ ” according to the rule.
The agency has taken a broad initial approach in defining what kinds of illnesses a patient may have to qualify for services. Patients must have a serious condition that is expected to last at least 3 months, such as cancer, heart failure, or substance use disorder.
But those without a definitive diagnosis may also qualify to receive navigator services.
In the rule, CMS cited a case in which a CT scan identified a suspicious mass in a patient’s colon. A clinician might decide this person would benefit from navigation services due to the potential risks for an undiagnosed illness.
“Regardless of the definitive diagnosis of the mass, presence of a colonic mass for that patient may be a serious high-risk condition that could, for example, cause obstruction and lead the patient to present to the emergency department, as well as be potentially indicative of an underlying life-threatening illness such as colon cancer,” CMS wrote in the rule.
Navigators often start their work when cancer patients are screened and guide them through initial diagnosis, potential surgery, radiation, or chemotherapy, said Sharon Gentry, MSN, RN, a former nurse navigator who is now the editor in chief of the Journal of the Academy of Oncology Nurse & Patient Navigators.
The navigators are meant to be a trusted and continual presence for patients, who otherwise might be left to start anew in finding help at each phase of care.
The navigators “see the whole picture. They see the whole journey the patient takes, from pre-diagnosis all the way through diagnosis care out through survival,” Ms. Gentry said.
Gaining a special Medicare payment for these kinds of services will elevate this work, she said.
Many newer drugs can target specific mechanisms and proteins of cancer. Often, oncology treatment involves testing to find out if mutations are allowing the cancer cells to evade a patient’s immune system.
Checking these biomarkers takes time, however. Patients sometimes become frustrated because they are anxious to begin treatment. Patients may receive inaccurate information from friends or family who went through treatment previously. Navigators can provide knowledge on the current state of care for a patient’s disease, helping them better manage anxieties.
“You have to explain to them that things have changed since the guy you drink coffee with was diagnosed with cancer, and there may be a drug that could target that,” Ms. Gentry said.
Potential Challenges
Initial uptake of the new PIN codes may be slow going, however, as clinicians and health systems may already use well-established codes. These include CCM and principal care management services, which may pay higher rates, Mullangi said.
“There might be sensitivity around not wanting to cannibalize existing programs with a new program,” Dr. Mullangi said.
In addition, many patients will have a copay for the services of principal illness navigators, Dr. Mullangi said.
While many patients have additional insurance that would cover the service, not all do. People with traditional Medicare coverage can sometimes pay 20% of the cost of some medical services.
“I think that may give patients pause, particularly if they’re already feeling the financial burden of a cancer treatment journey,” Dr. Mullangi said.
Pay rates for PIN services involve calculations of regional price differences, which are posted publicly by CMS, and potential added fees for services provided by hospital-affiliated organizations.
Consider payments for code G0023, covering 60 minutes of principal navigation services provided in a single month.
A set reimbursement for patients cared for in independent medical practices exists, with variation for local costs. Medicare’s non-facility price for G0023 would be $102.41 in some parts of Silicon Valley in California, including San Jose. In Arkansas, where costs are lower, reimbursement would be $73.14 for this same service.
Patients who get services covered by code G0023 in independent medical practices would have monthly copays of about $15-$20, depending on where they live.
The tab for patients tends to be higher for these same services if delivered through a medical practice owned by a hospital, as this would trigger the addition of facility fees to the payments made to cover the services. Facility fees are difficult for the public to ascertain before getting a treatment or service.
Dr. Mullangi and Ms. Gentry reported no relevant financial disclosures outside of their employers.
A version of this article first appeared on Medscape.com.

Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
Introduction
Large cell neuroendocrine carcinomas (LCNEC) of the lung are sufficiently rare that large trials to establish a standard of care are impractical. Treatment strategies effective for related malignancies, particularly small-cell lung cancer (SCLC), have been commonly applied to LCNEC of the lung, but it is important to recognize the unique features of LCNEC in order to make a diagnosis and to individualize treatment. As current long-term survival in patients with LCNEC of the lung remains poor, participation in clinical trials should be encouraged. Therapies under investigation include those targeted at the delta-like ligand 3 (DLL3), an antigen highly expressed in neuroendocrine (NE) tumors, and Seneca Valley oncolytic viral (SVV) therapy. Early introduction of palliative care should also be offered to optimize quality of life. High-quality data for LCNEC of the lung and novel breakthrough drugs are much needed.
Background
NE tumors can develop from NE cells in almost any organ.1 After the gastrointestinal tract, the lung is the most common site of NE malignancies. They account for only about 2% of all lung cancers but 25% of NE tumors.2 Criteria for differentiating NE tumors from other tumors in the lung were first proposed in 1991.3 In 2022, the World Health Organization described 5 major subtypes of NE lung malignancies.4 On a spectrum ranging from best to worst outcome among lung cancers, LCNEC has a significantly more aggressive course compared with typical carcinoids (TC) and atypical carcinoids (AC), approaching that of SCLC, which arguably has the worst outcome (Table).5
Table. Comparing NSCLC, SCLC, and LCNEC of the Lung
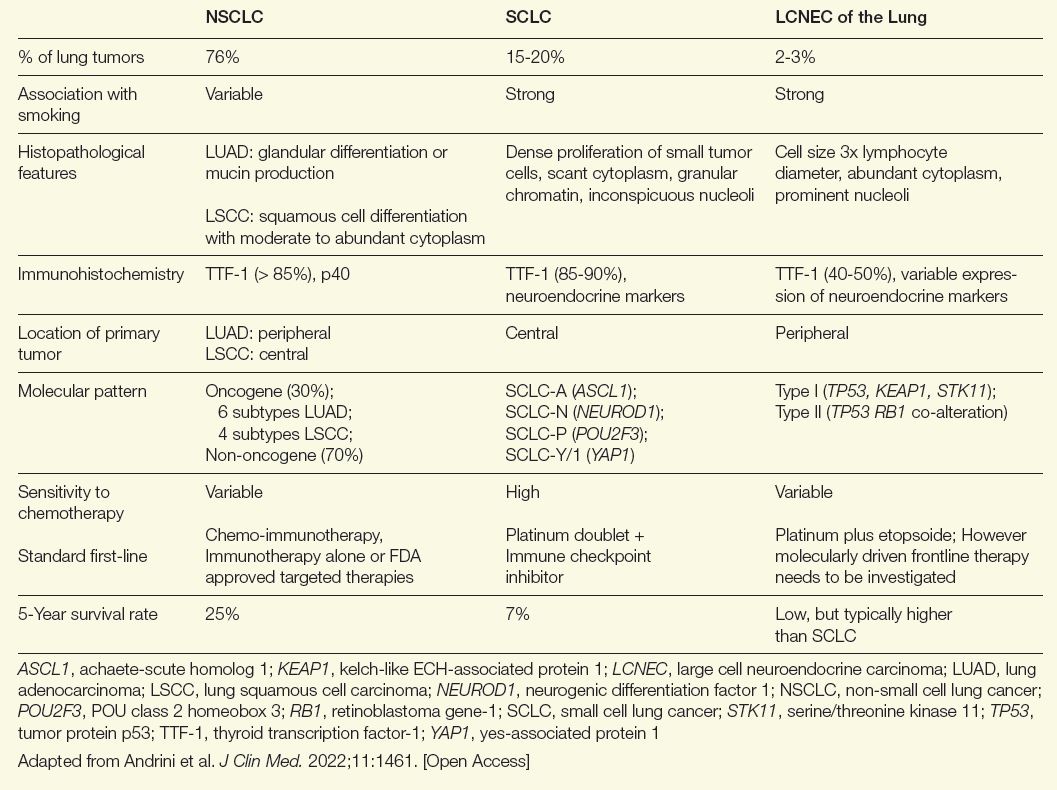
Similarities exist between LCNEC of the lung and other non-small cell lung cancer (NSCLC) types, but there are more parallels with SCLC. Both are more common in male patients and both are associated with a history of smoking.6 They also share a poor prognosis. If diagnosed at an advanced stage, 5-year survival rates for LCNEC of the lung and SCLC have been reported to be as low as 5% to 15%.6
The risk of a delay in establishing the correct diagnosis of LCNEC of the lung, even by experienced pathologists, is considerable. The key diagnostic criteria include expression of at least 1 NE marker, such as chromogranin-A or synaptophysin, a high proliferation rate (> 10 mitoses per high-power field), extensive necrosis, and NE morphology features, such as trabeculae and palisading and rosette formations.7 However, other lung cancers can also express NE markers and some features might be missed without relatively large tissue specimens.7
To improve diagnostic accuracy, additional criteria, such as absence of squamous or adenocarcinoma features or the demonstration of 2 or more NE markers are now being advocated in some reports,8 while others have advocated that terms such as “combined NSCLC/SCLC” should not be accepted as a substitute for differentiating and finalizing a diagnosis of LCNEC of the lung.7 Excisional or resection biopsies, as opposed to needle biopsies, might be required to obtain an adequate tissue sample to reach a definitive diagnosis.
Illustrating the potential for confusion with other lung cancers, LCNEC of the lung can be characterized by 2 subtypes.9 Type 1 is characterized by TP53 and STK11/KEAP1 alternations—similar to adenocarcinomas and squamous cell lung cancers—and it is associated with a higher expression of NE markers, such as ASCL1 and DLL3. Type 2 is typically characterized by inactivation of TP53 and RB1. Ultimately, type I LCNEC of the lung has a mutational pattern similar to NSCLC and type II has a pattern similar to SCLC. While LCNEC is typically located in the periphery of the lung, SCLC is typically centrally located and NSCLC can be found in either location. Complicated further by the fact that a proportion of these tumors have features shared with SCLC and rarer cancers, such as spindle-cell carcinoma and giant cell carcinoma, LCNEC should be considered in the differential diagnosis of any lung cancer with ambiguous features.7
For these reasons, a pathology review should be performed at a high-volume center whenever possible. As part of the diagnostic process, molecular testing should be gathered for all patients whether or not it is required to make or confirm the diagnosis. This information will be informative for guiding treatment, particularly second- and third-line interventions. Rather than being unique and definitive, the individual features of LCNEC of the lung—including the genetic, molecular, histologic, and morphologic characteristics—cumulatively support the diagnosis. After establishing a pathological diagnosis, staging of LCNEC of the lung is paramount. In addition, distinctions between the grades of LCNEC of the lung are relative. For example, tumors with a better relative prognosis typically have fewer gene mutations than tumors with a worse relative prognosis, but mutations are generally found in both.
Bronchoscopy with endobronchial ultrasound can be considered for both diagnosis and staging of locally advanced tumors, but a surgical specimen might still be required for a definitive diagnosis. Differentiating local LCNEC, which has been reported in about 25% of cases, from locally advanced and metastatic disease is critical for planning treatment. Fluorodeoxyglucose F18 (FDG) positron emission tomography (PET) plays an important role in staging LCNEC of the lung. Unlike TC and AC, for LCNEC of the lung there is a very limited role of somatostatin receptor agonist-based imaging or tetraazacyclododecanetetraacetic acid-DPhel-Tyr3-octreotate (DOTATATE) PET during diagnostic workup.
Therapeutic Strategies
In early stages, resection followed by adjuvant chemotherapy has long been used for LCNEC of the lung. Studies evaluating this approach, such as one that combined cisplatin and etoposide,10 suggest doublet chemotherapy after surgery offers a benefit in LCNEC of the lung comparable to that seen in SCLC. There is limited support for adjunctive radiotherapy in early-stage LCNEC of the lung,5 even if radiotherapy has shown benefit for patients ineligible for surgery.11
In locally advanced and advanced LCNEC (≥ stage III-B) ineligible for resection, chemoradiation has been associated with a survival advantage over chemotherapy alone,12 but due to the high rates of relapse and limited survival, efforts to move to novel therapies have been expanding for both LCNEC of the lung and SCLC. This includes immunotherapies used before or after chemoradiation. Again, much of the interest in immunotherapies has been derived from studies in SCLC, but several small studies have associated checkpoint inhibitors with substantial antitumor activity in patients with LCNEC.13,14 There are no large scale prospective trials to determine the optimal treatment in the first line setting for LCNEC of the lung and most data is extrapolated from treatment of ES-SCLC. In a retrospective study, however, comparing survival of palliative chemotherapy with a SCLC versus a NSCLC regimen, the SCLC regimen was favored.15
Following a pivotal trial of tarlatamab-dlle, that led to an accelerated approval for extensive-stage SCLC in May 2024,16 this drug has also been evaluated in a small group of patients with LCNEC of the lung. The parallels between LCNEC and SCLC have raised hope that this drug, which is a bispecific T-cell engager (BiTE) that binds to the DLL3 ligand and CD3, may provide benefit in LCNEC of the lung that is commensurate with the benefit seen in SCLC. A recently published LCNEC case study supports this potential.17 A high-grade NE-carcinoma-specific oncolytic virus called Seneca Valley virus holds promise. Preclinical data suggest encouraging anticancer activity when SVV is combined with immune checkpoint inhibitor therapy.18 SVV seems to attack cancer cells that express tumor endothelial marker 8 (TEM-8), making it an interesting target in future efforts for screening and tailoring treatment.19 Human studies are in development.
Due to the high frequency of relapse regardless of frontline therapies, there is also growing interest in maintenance strategies to extend disease control. Maintenance regimens that have been evaluated or are being considered include immunotherapies, even if the optimal sequence of treatment modalities remains unknown. The high rate of relapse also encourages early planning of sequential therapies based on molecular testing. Numerous studies of LCNEC of the lung have now identified activating mutations in targetable pathways, such as P13K/AKT/mTOR, KRAS, and FGFR1.18 Patients may also harbor a high tumor mutation burden, a characteristic that might favor treatment with immunotherapy. Each mutation is relevant to only a small proportion of patients with LCNEC. However, when all potentially targetable mutations are considered together, the proportion of patients with LCNEC who would benefit from an individualized therapy is substantial, thus supporting trials of individualized therapy, particularly in the second line.
The high rate of relapse with currently available therapies encourages enrollment in clinical trials, particularly among patients who have already failed a first-line strategy. In the United States, studies are enrolling patients with LCNEC of the lung for checkpoint inhibitors with or without combination chemotherapy, novel BiTE therapies, and novel therapies targeting specific activating pathways. Many of these trials offer enrollment to patients with either SCLC or LCNEC.
Due to poor survival, patients with advancing LCNEC of the lung should be considered for palliative care. Although no guideline protocol exists for palliative care, the American Society of Clinical Oncology recommends palliative care for all individuals with advanced cancer based on evidence of improved quality of life and, in some cases, survival.20
Summary
LCNEC is an uncommon lung malignancy with a poor prognosis in the advanced stages at which it is most often recognized. The risk of overlooking this cancer in the initial diagnosis emphasizes the need for an adequate index of suspicion and familiarity with its distinguishing characteristics. Treatments of LCNEC of the lung have been largely based on those used for SCLC, but there has been an evolution in the understanding of this disease, which includes a greater appreciation for heterogeneity among driving mutations, a growing interest in maintenance therapies to extend the time to relapse, and trials of a growing array of novel therapies, including immunotherapies and BiTEs. Early intervention with these novel therapies and an emphasis on palliative care is needed because LCNEC has such an aggressive course.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Sultana Q, Kar J, Verma A, et al. A comprehensive review on neuroendocrine neoplasms: presentation, pathophysiology and management. J Clin Med. 2023;12(15):5138. doi:10.3390/jcm12155138
- Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113(1):5-21. doi:10.1002/cncr.23542
- Travis WD, Linnoila RI, Tsokos MG, et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15(6):529-553. doi:10.1097/00000478-199106000-00003
- Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10(9):1240-1242. doi:10.1097/JTO.0000000000000663
- Andrini E, Marchese PV, De Biase D, et al. Large cell neuroendocrine carcinoma of the lung: current understanding and challenges. J Clin Med. 2022;11(5):1461. doi:10.3390/jcm11051461
- Lantuejoul S, Fernandez-Cuesta L, Damiola F, Girard N, McLeer A. New molecular classification of large cell neuroendocrine carcinoma and small cell lung carcinoma with potential therapeutic impacts. Transl Lung Cancer Res. 2020;9(5):2233-2244. doi:10.21037/tlcr-20-269
- Lindsay CR, Shaw EC, Moore DA, et al. Large cell neuroendocrine lung carcinoma: consensus statement from The British Thoracic Oncology Group and the Association of Pulmonary Pathologists. Br J Cancer. 2021;125(9):1210-1216. doi:10.1038/s41416-021-01407-9
- Derks JL, Dingemans AC, van Suylen RJ, et al. Is the sum of positive neuroendocrine immunohistochemical stains useful for diagnosis of large cell neuroendocrine carcinoma (LCNEC) on biopsy specimens? Histopathology. 2019;74(4):555-566. doi:10.1111/his.13800
- George J, Walter V, Peifer M, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun. 2018;9(1):1048. doi:10.1038/s41467-018-03099-x
- Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg. 2006;82(5):1802-1807. doi:10.1016/j.athoracsur.2006.05.109
- Cao L, Wu HF, Zhao L, et al. The role of radiotherapy in pulmonary large cell neuroendocrine carcinoma: propensity score matching analysis. J Radiat Res. 2020;61(4):594-601. doi:10.1093/jrr/rraa036
- Limonnik V, Abel S, Finley GG, Long GS, Wegner RE. Factors associated with treatment receipt and overall survival for patients with locally advanced large cell neuroendocrine carcinoma of the lung: a National Cancer Database analysis. Lung Cancer. 2020;150:107-113. doi:10.1016/j.lungcan.2020.10.001
- Shi Z, Wei J, Xu M, Song Z. Efficacy and safety of immune checkpoint inhibitors in lung large-cell neuroendocrine carcinoma. J Thorac Dis. 2023;15(8):4172-4181. doi:10.21037/jtd-23-348
- Chauhan A, Arnold SM, Kolesar J, Thomas HE, Evers M, Anthony L. Immune checkpoint inhibitors in large cell neuroendocrine carcinoma: current status. Oncotarget. 2018;9(18):14738-14740. doi:10.18632/oncotarget.24553
- Chen H, Ishihara M, Horita N, et al. Effect of adjuvant and palliative chemotherapy in large cell neuroendocrine carcinoma of the lung: a systematic review and metaanalysis. Cancers (Basel). 2021;13(23):5948. doi:10.3390/cancers13235948
- Ahn MJ, Cho BC, Felip E, et al. Tarlatamab for patients with previously treated small-cell lung cancer. N Engl J Med. 2023;389(22):2063-2075. doi:10.1056/NEJMoa2307980
- Patel SA, Whang Y, Medley C, et al. Tartalamab for large-cell neuroendocrine carcinoma in a young adult: a case report. JTO Clin Res Rep. 2024;5(10):100712. doi:10.1016/j.jtocrr.2024.100712
- Corbett V, Hallenbeck P, Rychahou P, Chauhan A. Evolving role of Seneca Valley virus and its biomarker TEM8/ANTXR1 in cancer therapeutics. Front Mol Biosci. 2022;9:930207. doi:10.3389/fmolb.2022.930207
- Kareff SA, Corbett V, Hallenbeck P, Chauhan A. TEM8 in oncogenesis: protein biology, pre-clinical agents, and clinical rationale. Cells. 2023;12(22):2623. doi:10.3390/cells12222623
- Sanders JJ, Temin S, Ghoshal A, et al. Palliative care for patients with cancer: ASCO guideline update. J Clin Oncol. 2024;42(19):2336-2357. doi:10.1200/JCO.24.00542
Introduction
Large cell neuroendocrine carcinomas (LCNEC) of the lung are sufficiently rare that large trials to establish a standard of care are impractical. Treatment strategies effective for related malignancies, particularly small-cell lung cancer (SCLC), have been commonly applied to LCNEC of the lung, but it is important to recognize the unique features of LCNEC in order to make a diagnosis and to individualize treatment. As current long-term survival in patients with LCNEC of the lung remains poor, participation in clinical trials should be encouraged. Therapies under investigation include those targeted at the delta-like ligand 3 (DLL3), an antigen highly expressed in neuroendocrine (NE) tumors, and Seneca Valley oncolytic viral (SVV) therapy. Early introduction of palliative care should also be offered to optimize quality of life. High-quality data for LCNEC of the lung and novel breakthrough drugs are much needed.
Background
NE tumors can develop from NE cells in almost any organ.1 After the gastrointestinal tract, the lung is the most common site of NE malignancies. They account for only about 2% of all lung cancers but 25% of NE tumors.2 Criteria for differentiating NE tumors from other tumors in the lung were first proposed in 1991.3 In 2022, the World Health Organization described 5 major subtypes of NE lung malignancies.4 On a spectrum ranging from best to worst outcome among lung cancers, LCNEC has a significantly more aggressive course compared with typical carcinoids (TC) and atypical carcinoids (AC), approaching that of SCLC, which arguably has the worst outcome (Table).5
Table. Comparing NSCLC, SCLC, and LCNEC of the Lung

Similarities exist between LCNEC of the lung and other non-small cell lung cancer (NSCLC) types, but there are more parallels with SCLC. Both are more common in male patients and both are associated with a history of smoking.6 They also share a poor prognosis. If diagnosed at an advanced stage, 5-year survival rates for LCNEC of the lung and SCLC have been reported to be as low as 5% to 15%.6
The risk of a delay in establishing the correct diagnosis of LCNEC of the lung, even by experienced pathologists, is considerable. The key diagnostic criteria include expression of at least 1 NE marker, such as chromogranin-A or synaptophysin, a high proliferation rate (> 10 mitoses per high-power field), extensive necrosis, and NE morphology features, such as trabeculae and palisading and rosette formations.7 However, other lung cancers can also express NE markers and some features might be missed without relatively large tissue specimens.7
To improve diagnostic accuracy, additional criteria, such as absence of squamous or adenocarcinoma features or the demonstration of 2 or more NE markers are now being advocated in some reports,8 while others have advocated that terms such as “combined NSCLC/SCLC” should not be accepted as a substitute for differentiating and finalizing a diagnosis of LCNEC of the lung.7 Excisional or resection biopsies, as opposed to needle biopsies, might be required to obtain an adequate tissue sample to reach a definitive diagnosis.
Illustrating the potential for confusion with other lung cancers, LCNEC of the lung can be characterized by 2 subtypes.9 Type 1 is characterized by TP53 and STK11/KEAP1 alternations—similar to adenocarcinomas and squamous cell lung cancers—and it is associated with a higher expression of NE markers, such as ASCL1 and DLL3. Type 2 is typically characterized by inactivation of TP53 and RB1. Ultimately, type I LCNEC of the lung has a mutational pattern similar to NSCLC and type II has a pattern similar to SCLC. While LCNEC is typically located in the periphery of the lung, SCLC is typically centrally located and NSCLC can be found in either location. Complicated further by the fact that a proportion of these tumors have features shared with SCLC and rarer cancers, such as spindle-cell carcinoma and giant cell carcinoma, LCNEC should be considered in the differential diagnosis of any lung cancer with ambiguous features.7
For these reasons, a pathology review should be performed at a high-volume center whenever possible. As part of the diagnostic process, molecular testing should be gathered for all patients whether or not it is required to make or confirm the diagnosis. This information will be informative for guiding treatment, particularly second- and third-line interventions. Rather than being unique and definitive, the individual features of LCNEC of the lung—including the genetic, molecular, histologic, and morphologic characteristics—cumulatively support the diagnosis. After establishing a pathological diagnosis, staging of LCNEC of the lung is paramount. In addition, distinctions between the grades of LCNEC of the lung are relative. For example, tumors with a better relative prognosis typically have fewer gene mutations than tumors with a worse relative prognosis, but mutations are generally found in both.
Bronchoscopy with endobronchial ultrasound can be considered for both diagnosis and staging of locally advanced tumors, but a surgical specimen might still be required for a definitive diagnosis. Differentiating local LCNEC, which has been reported in about 25% of cases, from locally advanced and metastatic disease is critical for planning treatment. Fluorodeoxyglucose F18 (FDG) positron emission tomography (PET) plays an important role in staging LCNEC of the lung. Unlike TC and AC, for LCNEC of the lung there is a very limited role of somatostatin receptor agonist-based imaging or tetraazacyclododecanetetraacetic acid-DPhel-Tyr3-octreotate (DOTATATE) PET during diagnostic workup.
Therapeutic Strategies
In early stages, resection followed by adjuvant chemotherapy has long been used for LCNEC of the lung. Studies evaluating this approach, such as one that combined cisplatin and etoposide,10 suggest doublet chemotherapy after surgery offers a benefit in LCNEC of the lung comparable to that seen in SCLC. There is limited support for adjunctive radiotherapy in early-stage LCNEC of the lung,5 even if radiotherapy has shown benefit for patients ineligible for surgery.11
In locally advanced and advanced LCNEC (≥ stage III-B) ineligible for resection, chemoradiation has been associated with a survival advantage over chemotherapy alone,12 but due to the high rates of relapse and limited survival, efforts to move to novel therapies have been expanding for both LCNEC of the lung and SCLC. This includes immunotherapies used before or after chemoradiation. Again, much of the interest in immunotherapies has been derived from studies in SCLC, but several small studies have associated checkpoint inhibitors with substantial antitumor activity in patients with LCNEC.13,14 There are no large scale prospective trials to determine the optimal treatment in the first line setting for LCNEC of the lung and most data is extrapolated from treatment of ES-SCLC. In a retrospective study, however, comparing survival of palliative chemotherapy with a SCLC versus a NSCLC regimen, the SCLC regimen was favored.15
Following a pivotal trial of tarlatamab-dlle, that led to an accelerated approval for extensive-stage SCLC in May 2024,16 this drug has also been evaluated in a small group of patients with LCNEC of the lung. The parallels between LCNEC and SCLC have raised hope that this drug, which is a bispecific T-cell engager (BiTE) that binds to the DLL3 ligand and CD3, may provide benefit in LCNEC of the lung that is commensurate with the benefit seen in SCLC. A recently published LCNEC case study supports this potential.17 A high-grade NE-carcinoma-specific oncolytic virus called Seneca Valley virus holds promise. Preclinical data suggest encouraging anticancer activity when SVV is combined with immune checkpoint inhibitor therapy.18 SVV seems to attack cancer cells that express tumor endothelial marker 8 (TEM-8), making it an interesting target in future efforts for screening and tailoring treatment.19 Human studies are in development.
Due to the high frequency of relapse regardless of frontline therapies, there is also growing interest in maintenance strategies to extend disease control. Maintenance regimens that have been evaluated or are being considered include immunotherapies, even if the optimal sequence of treatment modalities remains unknown. The high rate of relapse also encourages early planning of sequential therapies based on molecular testing. Numerous studies of LCNEC of the lung have now identified activating mutations in targetable pathways, such as P13K/AKT/mTOR, KRAS, and FGFR1.18 Patients may also harbor a high tumor mutation burden, a characteristic that might favor treatment with immunotherapy. Each mutation is relevant to only a small proportion of patients with LCNEC. However, when all potentially targetable mutations are considered together, the proportion of patients with LCNEC who would benefit from an individualized therapy is substantial, thus supporting trials of individualized therapy, particularly in the second line.
The high rate of relapse with currently available therapies encourages enrollment in clinical trials, particularly among patients who have already failed a first-line strategy. In the United States, studies are enrolling patients with LCNEC of the lung for checkpoint inhibitors with or without combination chemotherapy, novel BiTE therapies, and novel therapies targeting specific activating pathways. Many of these trials offer enrollment to patients with either SCLC or LCNEC.
Due to poor survival, patients with advancing LCNEC of the lung should be considered for palliative care. Although no guideline protocol exists for palliative care, the American Society of Clinical Oncology recommends palliative care for all individuals with advanced cancer based on evidence of improved quality of life and, in some cases, survival.20
Summary
LCNEC is an uncommon lung malignancy with a poor prognosis in the advanced stages at which it is most often recognized. The risk of overlooking this cancer in the initial diagnosis emphasizes the need for an adequate index of suspicion and familiarity with its distinguishing characteristics. Treatments of LCNEC of the lung have been largely based on those used for SCLC, but there has been an evolution in the understanding of this disease, which includes a greater appreciation for heterogeneity among driving mutations, a growing interest in maintenance therapies to extend the time to relapse, and trials of a growing array of novel therapies, including immunotherapies and BiTEs. Early intervention with these novel therapies and an emphasis on palliative care is needed because LCNEC has such an aggressive course.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
Introduction
Large cell neuroendocrine carcinomas (LCNEC) of the lung are sufficiently rare that large trials to establish a standard of care are impractical. Treatment strategies effective for related malignancies, particularly small-cell lung cancer (SCLC), have been commonly applied to LCNEC of the lung, but it is important to recognize the unique features of LCNEC in order to make a diagnosis and to individualize treatment. As current long-term survival in patients with LCNEC of the lung remains poor, participation in clinical trials should be encouraged. Therapies under investigation include those targeted at the delta-like ligand 3 (DLL3), an antigen highly expressed in neuroendocrine (NE) tumors, and Seneca Valley oncolytic viral (SVV) therapy. Early introduction of palliative care should also be offered to optimize quality of life. High-quality data for LCNEC of the lung and novel breakthrough drugs are much needed.
Background
NE tumors can develop from NE cells in almost any organ.1 After the gastrointestinal tract, the lung is the most common site of NE malignancies. They account for only about 2% of all lung cancers but 25% of NE tumors.2 Criteria for differentiating NE tumors from other tumors in the lung were first proposed in 1991.3 In 2022, the World Health Organization described 5 major subtypes of NE lung malignancies.4 On a spectrum ranging from best to worst outcome among lung cancers, LCNEC has a significantly more aggressive course compared with typical carcinoids (TC) and atypical carcinoids (AC), approaching that of SCLC, which arguably has the worst outcome (Table).5
Table. Comparing NSCLC, SCLC, and LCNEC of the Lung

Similarities exist between LCNEC of the lung and other non-small cell lung cancer (NSCLC) types, but there are more parallels with SCLC. Both are more common in male patients and both are associated with a history of smoking.6 They also share a poor prognosis. If diagnosed at an advanced stage, 5-year survival rates for LCNEC of the lung and SCLC have been reported to be as low as 5% to 15%.6
The risk of a delay in establishing the correct diagnosis of LCNEC of the lung, even by experienced pathologists, is considerable. The key diagnostic criteria include expression of at least 1 NE marker, such as chromogranin-A or synaptophysin, a high proliferation rate (> 10 mitoses per high-power field), extensive necrosis, and NE morphology features, such as trabeculae and palisading and rosette formations.7 However, other lung cancers can also express NE markers and some features might be missed without relatively large tissue specimens.7
To improve diagnostic accuracy, additional criteria, such as absence of squamous or adenocarcinoma features or the demonstration of 2 or more NE markers are now being advocated in some reports,8 while others have advocated that terms such as “combined NSCLC/SCLC” should not be accepted as a substitute for differentiating and finalizing a diagnosis of LCNEC of the lung.7 Excisional or resection biopsies, as opposed to needle biopsies, might be required to obtain an adequate tissue sample to reach a definitive diagnosis.
Illustrating the potential for confusion with other lung cancers, LCNEC of the lung can be characterized by 2 subtypes.9 Type 1 is characterized by TP53 and STK11/KEAP1 alternations—similar to adenocarcinomas and squamous cell lung cancers—and it is associated with a higher expression of NE markers, such as ASCL1 and DLL3. Type 2 is typically characterized by inactivation of TP53 and RB1. Ultimately, type I LCNEC of the lung has a mutational pattern similar to NSCLC and type II has a pattern similar to SCLC. While LCNEC is typically located in the periphery of the lung, SCLC is typically centrally located and NSCLC can be found in either location. Complicated further by the fact that a proportion of these tumors have features shared with SCLC and rarer cancers, such as spindle-cell carcinoma and giant cell carcinoma, LCNEC should be considered in the differential diagnosis of any lung cancer with ambiguous features.7
For these reasons, a pathology review should be performed at a high-volume center whenever possible. As part of the diagnostic process, molecular testing should be gathered for all patients whether or not it is required to make or confirm the diagnosis. This information will be informative for guiding treatment, particularly second- and third-line interventions. Rather than being unique and definitive, the individual features of LCNEC of the lung—including the genetic, molecular, histologic, and morphologic characteristics—cumulatively support the diagnosis. After establishing a pathological diagnosis, staging of LCNEC of the lung is paramount. In addition, distinctions between the grades of LCNEC of the lung are relative. For example, tumors with a better relative prognosis typically have fewer gene mutations than tumors with a worse relative prognosis, but mutations are generally found in both.
Bronchoscopy with endobronchial ultrasound can be considered for both diagnosis and staging of locally advanced tumors, but a surgical specimen might still be required for a definitive diagnosis. Differentiating local LCNEC, which has been reported in about 25% of cases, from locally advanced and metastatic disease is critical for planning treatment. Fluorodeoxyglucose F18 (FDG) positron emission tomography (PET) plays an important role in staging LCNEC of the lung. Unlike TC and AC, for LCNEC of the lung there is a very limited role of somatostatin receptor agonist-based imaging or tetraazacyclododecanetetraacetic acid-DPhel-Tyr3-octreotate (DOTATATE) PET during diagnostic workup.
Therapeutic Strategies
In early stages, resection followed by adjuvant chemotherapy has long been used for LCNEC of the lung. Studies evaluating this approach, such as one that combined cisplatin and etoposide,10 suggest doublet chemotherapy after surgery offers a benefit in LCNEC of the lung comparable to that seen in SCLC. There is limited support for adjunctive radiotherapy in early-stage LCNEC of the lung,5 even if radiotherapy has shown benefit for patients ineligible for surgery.11
In locally advanced and advanced LCNEC (≥ stage III-B) ineligible for resection, chemoradiation has been associated with a survival advantage over chemotherapy alone,12 but due to the high rates of relapse and limited survival, efforts to move to novel therapies have been expanding for both LCNEC of the lung and SCLC. This includes immunotherapies used before or after chemoradiation. Again, much of the interest in immunotherapies has been derived from studies in SCLC, but several small studies have associated checkpoint inhibitors with substantial antitumor activity in patients with LCNEC.13,14 There are no large scale prospective trials to determine the optimal treatment in the first line setting for LCNEC of the lung and most data is extrapolated from treatment of ES-SCLC. In a retrospective study, however, comparing survival of palliative chemotherapy with a SCLC versus a NSCLC regimen, the SCLC regimen was favored.15
Following a pivotal trial of tarlatamab-dlle, that led to an accelerated approval for extensive-stage SCLC in May 2024,16 this drug has also been evaluated in a small group of patients with LCNEC of the lung. The parallels between LCNEC and SCLC have raised hope that this drug, which is a bispecific T-cell engager (BiTE) that binds to the DLL3 ligand and CD3, may provide benefit in LCNEC of the lung that is commensurate with the benefit seen in SCLC. A recently published LCNEC case study supports this potential.17 A high-grade NE-carcinoma-specific oncolytic virus called Seneca Valley virus holds promise. Preclinical data suggest encouraging anticancer activity when SVV is combined with immune checkpoint inhibitor therapy.18 SVV seems to attack cancer cells that express tumor endothelial marker 8 (TEM-8), making it an interesting target in future efforts for screening and tailoring treatment.19 Human studies are in development.
Due to the high frequency of relapse regardless of frontline therapies, there is also growing interest in maintenance strategies to extend disease control. Maintenance regimens that have been evaluated or are being considered include immunotherapies, even if the optimal sequence of treatment modalities remains unknown. The high rate of relapse also encourages early planning of sequential therapies based on molecular testing. Numerous studies of LCNEC of the lung have now identified activating mutations in targetable pathways, such as P13K/AKT/mTOR, KRAS, and FGFR1.18 Patients may also harbor a high tumor mutation burden, a characteristic that might favor treatment with immunotherapy. Each mutation is relevant to only a small proportion of patients with LCNEC. However, when all potentially targetable mutations are considered together, the proportion of patients with LCNEC who would benefit from an individualized therapy is substantial, thus supporting trials of individualized therapy, particularly in the second line.
The high rate of relapse with currently available therapies encourages enrollment in clinical trials, particularly among patients who have already failed a first-line strategy. In the United States, studies are enrolling patients with LCNEC of the lung for checkpoint inhibitors with or without combination chemotherapy, novel BiTE therapies, and novel therapies targeting specific activating pathways. Many of these trials offer enrollment to patients with either SCLC or LCNEC.
Due to poor survival, patients with advancing LCNEC of the lung should be considered for palliative care. Although no guideline protocol exists for palliative care, the American Society of Clinical Oncology recommends palliative care for all individuals with advanced cancer based on evidence of improved quality of life and, in some cases, survival.20
Summary
LCNEC is an uncommon lung malignancy with a poor prognosis in the advanced stages at which it is most often recognized. The risk of overlooking this cancer in the initial diagnosis emphasizes the need for an adequate index of suspicion and familiarity with its distinguishing characteristics. Treatments of LCNEC of the lung have been largely based on those used for SCLC, but there has been an evolution in the understanding of this disease, which includes a greater appreciation for heterogeneity among driving mutations, a growing interest in maintenance therapies to extend the time to relapse, and trials of a growing array of novel therapies, including immunotherapies and BiTEs. Early intervention with these novel therapies and an emphasis on palliative care is needed because LCNEC has such an aggressive course.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Sultana Q, Kar J, Verma A, et al. A comprehensive review on neuroendocrine neoplasms: presentation, pathophysiology and management. J Clin Med. 2023;12(15):5138. doi:10.3390/jcm12155138
- Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113(1):5-21. doi:10.1002/cncr.23542
- Travis WD, Linnoila RI, Tsokos MG, et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15(6):529-553. doi:10.1097/00000478-199106000-00003
- Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10(9):1240-1242. doi:10.1097/JTO.0000000000000663
- Andrini E, Marchese PV, De Biase D, et al. Large cell neuroendocrine carcinoma of the lung: current understanding and challenges. J Clin Med. 2022;11(5):1461. doi:10.3390/jcm11051461
- Lantuejoul S, Fernandez-Cuesta L, Damiola F, Girard N, McLeer A. New molecular classification of large cell neuroendocrine carcinoma and small cell lung carcinoma with potential therapeutic impacts. Transl Lung Cancer Res. 2020;9(5):2233-2244. doi:10.21037/tlcr-20-269
- Lindsay CR, Shaw EC, Moore DA, et al. Large cell neuroendocrine lung carcinoma: consensus statement from The British Thoracic Oncology Group and the Association of Pulmonary Pathologists. Br J Cancer. 2021;125(9):1210-1216. doi:10.1038/s41416-021-01407-9
- Derks JL, Dingemans AC, van Suylen RJ, et al. Is the sum of positive neuroendocrine immunohistochemical stains useful for diagnosis of large cell neuroendocrine carcinoma (LCNEC) on biopsy specimens? Histopathology. 2019;74(4):555-566. doi:10.1111/his.13800
- George J, Walter V, Peifer M, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun. 2018;9(1):1048. doi:10.1038/s41467-018-03099-x
- Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg. 2006;82(5):1802-1807. doi:10.1016/j.athoracsur.2006.05.109
- Cao L, Wu HF, Zhao L, et al. The role of radiotherapy in pulmonary large cell neuroendocrine carcinoma: propensity score matching analysis. J Radiat Res. 2020;61(4):594-601. doi:10.1093/jrr/rraa036
- Limonnik V, Abel S, Finley GG, Long GS, Wegner RE. Factors associated with treatment receipt and overall survival for patients with locally advanced large cell neuroendocrine carcinoma of the lung: a National Cancer Database analysis. Lung Cancer. 2020;150:107-113. doi:10.1016/j.lungcan.2020.10.001
- Shi Z, Wei J, Xu M, Song Z. Efficacy and safety of immune checkpoint inhibitors in lung large-cell neuroendocrine carcinoma. J Thorac Dis. 2023;15(8):4172-4181. doi:10.21037/jtd-23-348
- Chauhan A, Arnold SM, Kolesar J, Thomas HE, Evers M, Anthony L. Immune checkpoint inhibitors in large cell neuroendocrine carcinoma: current status. Oncotarget. 2018;9(18):14738-14740. doi:10.18632/oncotarget.24553
- Chen H, Ishihara M, Horita N, et al. Effect of adjuvant and palliative chemotherapy in large cell neuroendocrine carcinoma of the lung: a systematic review and metaanalysis. Cancers (Basel). 2021;13(23):5948. doi:10.3390/cancers13235948
- Ahn MJ, Cho BC, Felip E, et al. Tarlatamab for patients with previously treated small-cell lung cancer. N Engl J Med. 2023;389(22):2063-2075. doi:10.1056/NEJMoa2307980
- Patel SA, Whang Y, Medley C, et al. Tartalamab for large-cell neuroendocrine carcinoma in a young adult: a case report. JTO Clin Res Rep. 2024;5(10):100712. doi:10.1016/j.jtocrr.2024.100712
- Corbett V, Hallenbeck P, Rychahou P, Chauhan A. Evolving role of Seneca Valley virus and its biomarker TEM8/ANTXR1 in cancer therapeutics. Front Mol Biosci. 2022;9:930207. doi:10.3389/fmolb.2022.930207
- Kareff SA, Corbett V, Hallenbeck P, Chauhan A. TEM8 in oncogenesis: protein biology, pre-clinical agents, and clinical rationale. Cells. 2023;12(22):2623. doi:10.3390/cells12222623
- Sanders JJ, Temin S, Ghoshal A, et al. Palliative care for patients with cancer: ASCO guideline update. J Clin Oncol. 2024;42(19):2336-2357. doi:10.1200/JCO.24.00542
- Sultana Q, Kar J, Verma A, et al. A comprehensive review on neuroendocrine neoplasms: presentation, pathophysiology and management. J Clin Med. 2023;12(15):5138. doi:10.3390/jcm12155138
- Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM. Bronchopulmonary neuroendocrine tumors. Cancer. 2008;113(1):5-21. doi:10.1002/cncr.23542
- Travis WD, Linnoila RI, Tsokos MG, et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15(6):529-553. doi:10.1097/00000478-199106000-00003
- Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10(9):1240-1242. doi:10.1097/JTO.0000000000000663
- Andrini E, Marchese PV, De Biase D, et al. Large cell neuroendocrine carcinoma of the lung: current understanding and challenges. J Clin Med. 2022;11(5):1461. doi:10.3390/jcm11051461
- Lantuejoul S, Fernandez-Cuesta L, Damiola F, Girard N, McLeer A. New molecular classification of large cell neuroendocrine carcinoma and small cell lung carcinoma with potential therapeutic impacts. Transl Lung Cancer Res. 2020;9(5):2233-2244. doi:10.21037/tlcr-20-269
- Lindsay CR, Shaw EC, Moore DA, et al. Large cell neuroendocrine lung carcinoma: consensus statement from The British Thoracic Oncology Group and the Association of Pulmonary Pathologists. Br J Cancer. 2021;125(9):1210-1216. doi:10.1038/s41416-021-01407-9
- Derks JL, Dingemans AC, van Suylen RJ, et al. Is the sum of positive neuroendocrine immunohistochemical stains useful for diagnosis of large cell neuroendocrine carcinoma (LCNEC) on biopsy specimens? Histopathology. 2019;74(4):555-566. doi:10.1111/his.13800
- George J, Walter V, Peifer M, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun. 2018;9(1):1048. doi:10.1038/s41467-018-03099-x
- Iyoda A, Hiroshima K, Moriya Y, et al. Prospective study of adjuvant chemotherapy for pulmonary large cell neuroendocrine carcinoma. Ann Thorac Surg. 2006;82(5):1802-1807. doi:10.1016/j.athoracsur.2006.05.109
- Cao L, Wu HF, Zhao L, et al. The role of radiotherapy in pulmonary large cell neuroendocrine carcinoma: propensity score matching analysis. J Radiat Res. 2020;61(4):594-601. doi:10.1093/jrr/rraa036
- Limonnik V, Abel S, Finley GG, Long GS, Wegner RE. Factors associated with treatment receipt and overall survival for patients with locally advanced large cell neuroendocrine carcinoma of the lung: a National Cancer Database analysis. Lung Cancer. 2020;150:107-113. doi:10.1016/j.lungcan.2020.10.001
- Shi Z, Wei J, Xu M, Song Z. Efficacy and safety of immune checkpoint inhibitors in lung large-cell neuroendocrine carcinoma. J Thorac Dis. 2023;15(8):4172-4181. doi:10.21037/jtd-23-348
- Chauhan A, Arnold SM, Kolesar J, Thomas HE, Evers M, Anthony L. Immune checkpoint inhibitors in large cell neuroendocrine carcinoma: current status. Oncotarget. 2018;9(18):14738-14740. doi:10.18632/oncotarget.24553
- Chen H, Ishihara M, Horita N, et al. Effect of adjuvant and palliative chemotherapy in large cell neuroendocrine carcinoma of the lung: a systematic review and metaanalysis. Cancers (Basel). 2021;13(23):5948. doi:10.3390/cancers13235948
- Ahn MJ, Cho BC, Felip E, et al. Tarlatamab for patients with previously treated small-cell lung cancer. N Engl J Med. 2023;389(22):2063-2075. doi:10.1056/NEJMoa2307980
- Patel SA, Whang Y, Medley C, et al. Tartalamab for large-cell neuroendocrine carcinoma in a young adult: a case report. JTO Clin Res Rep. 2024;5(10):100712. doi:10.1016/j.jtocrr.2024.100712
- Corbett V, Hallenbeck P, Rychahou P, Chauhan A. Evolving role of Seneca Valley virus and its biomarker TEM8/ANTXR1 in cancer therapeutics. Front Mol Biosci. 2022;9:930207. doi:10.3389/fmolb.2022.930207
- Kareff SA, Corbett V, Hallenbeck P, Chauhan A. TEM8 in oncogenesis: protein biology, pre-clinical agents, and clinical rationale. Cells. 2023;12(22):2623. doi:10.3390/cells12222623
- Sanders JJ, Temin S, Ghoshal A, et al. Palliative care for patients with cancer: ASCO guideline update. J Clin Oncol. 2024;42(19):2336-2357. doi:10.1200/JCO.24.00542
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
Treatment of Glioblastoma: A Potential Shift in Paradigm
Treatment of Glioblastoma: A Potential Shift in Paradigm
Introduction
The evolution toward targeted therapies for glioblastoma multiforme (GBM) accelerated in 2021 when the World Health Organization (WHO) reclassified malignancies of the central nervous system.1 By placing a greater emphasis on molecular rather than histological characteristics of brain cancers, the reclassification validated the progress in identifying potential targetable drivers of disease within GBM subtypes. At the time of this reclassification, the US Food and Drug Administration (FDA) was already granting more orphan drug designations to targeted small molecules and to immunotherapeutics than to cytotoxic drugs2; this evolution is ongoing. Several immunotherapeutic approaches look particularly promising in early clinical trials. For some GBM subtypes, a clinical trial might soon become a therapeutic choice, particularly in the second line.
Background
In the United States, the incidence of GBM is 3.23 cases per 100,000, representing nearly half (48.6%) of all primary malignant brain tumors.3 Relative to non-small cell lung cancer, which has an incidence of about 40 cases per 100,000,4 this incidence is a small burden, but GBM is highly lethal even relative to other aggressive tumors. Essentially all GBM patients relapse after first-line treatments, including patients with a complete response.5 The 5-year survival, which has changed little over decades, is estimated to be less than 5%.6
Following the 2021 WHO classification of tumors in the central nervous system (WHO CNS5),1 the histologically oriented categories of pro-neural, neural, classical, and mesenchymal disease were replaced by 3 major types of GBM that can each be further characterized. These are astrocytoma mutant for isocitrate dehydrogenase (IDH), oligodendroglioma, and glioblastoma IDH-wildtype. For the first time, a separate classification system was also developed for pediatric GBM. Although brain cancer is the second most common type of malignancy in children, it is rare. Most cases of GBM occur in adults. More than half of new GBM diagnoses are in people older than 65 years.7
No standard method for molecular testing was described in WHO CNS5, but further molecular differentiation through biologic and genetic testing is recommended.8 Testing can be performed with transcription profiles, gene alterations, or DNA methylation.9 In addition to the evaluation of IDH status, mutations in α-thalassemia X-linked intellectual disability (ATRX), cyclin dependent kinase inhibitor 2A (CDKN2A/B), tumor suppressor gene (TP53), mitogen-activated protein kinases (MAPK), epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), and histone H3 (H3) G34 have been identified as biomarkers with potential prognostic value.10 Some or all of these biomarkers might eventually prove targetable. Moreover, it is expected that more progress in describing the GBM molecular pathways will yield further modifications in prognostic assessment and, potentially, choice of treatment.
Despite the promise of some of these targets in laboratory and early clinical studies, none of the therapies in development have so far changed the standard of care, which is dominated by resection followed by radiation and temozolomide. However, several treatment categories support the premise that individualized therapies in GBM are plausible and might improve outcomes, including extended survival.
Selected Trials and Their Rationale
The distinction between IDH-wildtype GBM and IDH-mutant GBM, which has a better prognosis,11 was one of many factors that changed the perception of GBM as a relatively homogeneous tumor type to one characterized by an array of intricate signaling pathways. Overall and in the context of glioma stem cells—which are a cell population in the GBM tumor microenvironment now suspected to play an important role in resistance and subsequent relapse,10—several pathways hold considerable promise for interfering with GBM progression. Studies of immunotherapies have been among the most encouraging.
Following a substantial effort over the last decade to engage the immune response in the treatment of GBM through oncolytic virotherapy, the field, despite its promise, has yet to produce a viable treatment for GBM.12,13 This effort includes multiple studies with dendritic cell vaccination, including a phase 3 trial published in 2023,14 but no therapy has yet to be approved.15 Although some of these trials did generate signals of activity, there are no approved treatments, and, recently, greater attention has been drawn to other strategies to engage the patient’s immune response, including chimeric antigen receptor (CAR) T-cells and checkpoint inhibitors.
A phase 1 study published in April 2024 showed that a novel engineered CAR T-cell product called CARv3-TEAM-E elicited dramatic radiographic regression of tumors in all 3 patients treated within days of intravenous administration.16 Although only 1 of the responses was sustained over follow-up, this result showed that clinically significant responses can be achieved in patients with advanced intraparenchymal disease. The tested CAR T construct included T-cell engaging antibody molecules (TEAMS) against wildtype EGFR, which was credited with inducing a radiological response not seen with a prior CAR T-cell construct. Other CAR T-cell studies are ongoing. In another trial published this year, results were less promising. It also targeted EGFR as well as the interleukin-13 receptor alpha 1, but none of the reductions in tumor size met criteria for an objective response.17
The theoretical promise of checkpoint inhibitors in GBM has not yet been realized in studies so far, despite numerous case reports and small series supporting activity. For example, overall survival was not improved with the programmed cell death protein 1 (PD-1) inhibitor nivolumab relative to the vascular endothelial growth factor (VEGF) inhibitor bevacizumab in a phase 3 controlled trial conducted in patients with recurrent GBM.18 However, preclinical research suggests combination strategies, including checkpoint inhibitors added to other types of therapeutics, might yield greater activity.19 The unprecedented responses with checkpoint inhibitors in other solid tumors is one reason that this approach is still being pursued avidly in GBM.13
For all forms of pharmacologic therapy and immunotherapies, providing adequate levels of therapeutic agent to the location of the tumor has been challenging. Convection-enhanced delivery (CED) is an example of a novel approach supported by clinical studies. By bypassing the blood-brain barrier, CED involves the delivery of a drug through a catheter placed into
the tumor.20 While this method increases the concentration of the treatment at the malignancy, it also reduces the risk of systemic adverse effects. CED drug delivery for GBM has been evaluated across a diverse array of strategies, including oncolytic viruses, nucleotide-based therapies, and monoclonal antibodies, as well as immunotherapies. One potential advantage of pump-based CED is sustained drug delivery, which might prove to be an important variable in treatment success for a tumor that relapses almost uniformly after therapy.21
Despite the disappointments in the past, the enormous increase in the number of drugs and immunotherapies along with the array of available and potential GBM mechanisms is, by itself, a source of encouragement. This is because the growth in possible targets is representative of advances in GBM biology leading to new potential targets for disease control. For example, small molecule pathway inhibitors that have reached clinical trials include P13K pathway inhibitors, inhibitors of HGFR/MET and SGX532, and inhibitors of EGFR and PDGFR.12
Unfortunately, the failures of promising drugs in phase 3 trials have also continued. For example, the VEGF-targeted monoclonal antibody bevacizumab, did not provide an overall survival benefit despite an encouraging degree of activity in early clinical studies.22 Recently, the antibody-drug conjugate depatuxizumab mafodotin also failed to demonstrate a survival benefit in a recent phase 3 trial despite an improvement in progression-free survival.23 However, the failure of these drugs to extend survival as single agents does not preclude benefit in further studies when they are combined with other strategies or administered with novel methods of drug delivery. The poor response to conventional therapies has led to consideration of alternative strategies such as tumor-treating fields where low-intensity electrical fields delivered via an FDA-approved portable wearable device demonstrated a modest effect on survival when combined with temozolomide.24
Why Optimism for Advances in GBM Is Warranted
The standard for the first-line treatment of GBM has remained unchanged since the introduction of temozolomide about 25 years ago. The combination of surgical debulking, radiation, temozolomide, and adjuvant chemotherapy is recommended in joint guidelines from the Society of Neuro-Oncology and the European Society of Neuro-Oncology.25 This strategy also remains a recommendation in the most recent guidelines on central nervous system cancers from the National Comprehensive Cancer Network® (NCCN®).26
The absence of new treatment standards belies the substantial new detail in which the pathophysiology is understood and with which GBM is being characterized. In this short review, only a proportion of the work in this field could be included. The combination approaches being pursued in relapsed disease is an example of promising work that was not addressed.
Yet, a focus on first-line therapies might be particularly appropriate in GBM. In this malignancy, for which relapse after the standard therapy almost always occurs, the identification of effective early treatment might be the only practical opportunity to increase survival meaningfully. For most cancer types, patients are typically offered experimental therapies only after progression on the standard of care. With advances in understanding the biology and molecular pathways of GBM progression, a paradigm shift might be appropriate. For a tumor type that is rarely, if ever, controlled on the current standard, trials of promising therapies, individualized to the underlying biology of GBM, might be warranted in tumors newly diagnosed and at an early stage.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231-1251. doi:10.1093/neuonc/noab106
- Johann P, Lenz D, Ries M. The drug development pipeline for glioblastoma—a cross sectional assessment of the FDA Orphan Drug Product designation database. PLoS One. 2021;16(7):e0252924. doi:10.1371/journal.pone.0252924
- Stupp R, Tonn JC, Brada M, Pentheroudakis G, ESMO Guidelines Working Group. High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v190-v193. doi:10.1093/annonc/mdq187
- Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. 2021;7(12):1824-1832. doi:10.1001/jamaoncol.2021.4932
- Sherriff J, Tamangani J, Senthil L, et al. Patterns of relapse in glioblastoma multiforme following concomitant chemoradiotherapy with temozolomide. Br J Radiol. 2013;86(1022):20120414. doi:10.1259/bjr.20120414
- Holland EC. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci U S A. 2000;97(12):6242-6244. doi:10.1073/pnas.97.12.6242
- Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1-ii56. doi:10.1093/neuonc/not151
- Farsi Z, Allahyari Fard N. The identification of key genes and pathways in glioblastoma by bioinformatics analysis. Mol Cell Oncol. 2023;10(1):2246657. doi:10.1080/23723556.2023.2246657
- Zhang P, Xia Q, Liu L, Li S, Dong L. Current opinion on molecular characterization for GBM classification in guiding clinical diagnosis, prognosis, and therapy. Front Mol Biosci. 2020;7:562798. doi:10.3389/fmolb.2020.562798
- Agosti E, Antonietti S, Ius T, Fontanella MM, Zeppieri M, Panciani PP. Glioma stem cells as promoter of glioma progression: a systematic review of molecular pathways and targeted therapies. Int J Mol Sci. 2024;25(14):7979. doi:10.3390/ijms25147979
- Han S, Liu Y, Cai SJ, et al. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer. 2020;122(11):1580-1589. doi:10.1038/s41416-020-0814-x
- Taylor OG, Brzozowski JS, Skelding KA. Glioblastoma multiforme: an overview of emerging therapeutic targets. Front Oncol. 2019;9:963. doi:10.3389/fonc.2019.00963
- Rong L, Li N, Zhang Z. Emerging therapies for glioblastoma: current state and future directions. J Exp Clin Cancer Res. 2022;41(1):142. doi:10.1186/s13046-022-02349-7
- Liau LM, Ashkan K, Brem S, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: a phase 3 prospective externally controlled cohort trial. JAMA Oncol. 2023;9(1):112-121. doi:10.1001/jamaoncol.2022.5370
- Van Gool SW, Makalowski J, Kampers LFC, et al. Dendritic cell vaccination for glioblastoma multiforme patients: has a new milestone been reached? Transl Cancer Res. 2023;12(8):2224-2228. doi:10.21037/tcr-23-603
- Choi BD, Gerstner ER, Frigault MJ, et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N Engl J Med. 2024;390(14):1290-1298. doi:10.1056/NEJMoa2314390
- Bagley SJ, Logun M, Fraietta JA, et al. Intrathecal bivalent CAR T cells targeting EGFR and IL13R-2 in recurrent glioblastoma: phase 1 trial interim results. Nat Med. 2024;30(5):1320-1329. doi:10.1038/s41591-024-02893-z
- Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003-1010. doi:10.1001/jamaoncol.2020.1024
- Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290-5301. doi:10.1158/1078-0432. CCR-14-0514
- Sperring CP, Argenziano MG, Savage WM, et al. Convection-enhanced delivery of immunomodulatory therapy for high-grade glioma. Neurooncol Adv. 2023;5(1):vdad044. doi:10.1093/noajnl/vdad044
- Spinazzi EF, Argenziano MG, Upadhyayula PS, et al. Chronic convection-enhanced delivery of topotecan for patients with recurrent glioblastoma: a first-in-patient, singlecentre, single-arm, phase 1b trial. Lancet Oncol. 2022;23(11):1409-1418. doi:10.1016/S1470-2045(22)00599-X
- Fu M, Zhou Z, Huang X, et al. Use of bevacizumab in recurrent glioblastoma: a scoping review and evidence map. BMC Cancer. 2023;23(1):544. doi:10.1186/s12885-023-11043-6
- Lassman AB, Pugh SL, Wang TJC, et al. Depatuxizumab mafodotin in EGFR-amplified newly diagnosed glioblastoma: a phase III randomized clinical trial. Neuro Oncol. 2023;25(2):339-350. doi:10.1093/neuonc/noac173
- Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 2017; 318: 2306–16.
- Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073-1113. doi:10.1093/neuonc/noaa106
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: central nervous system cancers. Version 2.2024. July 25, 2024. Accessed September 3, 2024. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf
Introduction
The evolution toward targeted therapies for glioblastoma multiforme (GBM) accelerated in 2021 when the World Health Organization (WHO) reclassified malignancies of the central nervous system.1 By placing a greater emphasis on molecular rather than histological characteristics of brain cancers, the reclassification validated the progress in identifying potential targetable drivers of disease within GBM subtypes. At the time of this reclassification, the US Food and Drug Administration (FDA) was already granting more orphan drug designations to targeted small molecules and to immunotherapeutics than to cytotoxic drugs2; this evolution is ongoing. Several immunotherapeutic approaches look particularly promising in early clinical trials. For some GBM subtypes, a clinical trial might soon become a therapeutic choice, particularly in the second line.
Background
In the United States, the incidence of GBM is 3.23 cases per 100,000, representing nearly half (48.6%) of all primary malignant brain tumors.3 Relative to non-small cell lung cancer, which has an incidence of about 40 cases per 100,000,4 this incidence is a small burden, but GBM is highly lethal even relative to other aggressive tumors. Essentially all GBM patients relapse after first-line treatments, including patients with a complete response.5 The 5-year survival, which has changed little over decades, is estimated to be less than 5%.6
Following the 2021 WHO classification of tumors in the central nervous system (WHO CNS5),1 the histologically oriented categories of pro-neural, neural, classical, and mesenchymal disease were replaced by 3 major types of GBM that can each be further characterized. These are astrocytoma mutant for isocitrate dehydrogenase (IDH), oligodendroglioma, and glioblastoma IDH-wildtype. For the first time, a separate classification system was also developed for pediatric GBM. Although brain cancer is the second most common type of malignancy in children, it is rare. Most cases of GBM occur in adults. More than half of new GBM diagnoses are in people older than 65 years.7
No standard method for molecular testing was described in WHO CNS5, but further molecular differentiation through biologic and genetic testing is recommended.8 Testing can be performed with transcription profiles, gene alterations, or DNA methylation.9 In addition to the evaluation of IDH status, mutations in α-thalassemia X-linked intellectual disability (ATRX), cyclin dependent kinase inhibitor 2A (CDKN2A/B), tumor suppressor gene (TP53), mitogen-activated protein kinases (MAPK), epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), and histone H3 (H3) G34 have been identified as biomarkers with potential prognostic value.10 Some or all of these biomarkers might eventually prove targetable. Moreover, it is expected that more progress in describing the GBM molecular pathways will yield further modifications in prognostic assessment and, potentially, choice of treatment.
Despite the promise of some of these targets in laboratory and early clinical studies, none of the therapies in development have so far changed the standard of care, which is dominated by resection followed by radiation and temozolomide. However, several treatment categories support the premise that individualized therapies in GBM are plausible and might improve outcomes, including extended survival.
Selected Trials and Their Rationale
The distinction between IDH-wildtype GBM and IDH-mutant GBM, which has a better prognosis,11 was one of many factors that changed the perception of GBM as a relatively homogeneous tumor type to one characterized by an array of intricate signaling pathways. Overall and in the context of glioma stem cells—which are a cell population in the GBM tumor microenvironment now suspected to play an important role in resistance and subsequent relapse,10—several pathways hold considerable promise for interfering with GBM progression. Studies of immunotherapies have been among the most encouraging.
Following a substantial effort over the last decade to engage the immune response in the treatment of GBM through oncolytic virotherapy, the field, despite its promise, has yet to produce a viable treatment for GBM.12,13 This effort includes multiple studies with dendritic cell vaccination, including a phase 3 trial published in 2023,14 but no therapy has yet to be approved.15 Although some of these trials did generate signals of activity, there are no approved treatments, and, recently, greater attention has been drawn to other strategies to engage the patient’s immune response, including chimeric antigen receptor (CAR) T-cells and checkpoint inhibitors.
A phase 1 study published in April 2024 showed that a novel engineered CAR T-cell product called CARv3-TEAM-E elicited dramatic radiographic regression of tumors in all 3 patients treated within days of intravenous administration.16 Although only 1 of the responses was sustained over follow-up, this result showed that clinically significant responses can be achieved in patients with advanced intraparenchymal disease. The tested CAR T construct included T-cell engaging antibody molecules (TEAMS) against wildtype EGFR, which was credited with inducing a radiological response not seen with a prior CAR T-cell construct. Other CAR T-cell studies are ongoing. In another trial published this year, results were less promising. It also targeted EGFR as well as the interleukin-13 receptor alpha 1, but none of the reductions in tumor size met criteria for an objective response.17
The theoretical promise of checkpoint inhibitors in GBM has not yet been realized in studies so far, despite numerous case reports and small series supporting activity. For example, overall survival was not improved with the programmed cell death protein 1 (PD-1) inhibitor nivolumab relative to the vascular endothelial growth factor (VEGF) inhibitor bevacizumab in a phase 3 controlled trial conducted in patients with recurrent GBM.18 However, preclinical research suggests combination strategies, including checkpoint inhibitors added to other types of therapeutics, might yield greater activity.19 The unprecedented responses with checkpoint inhibitors in other solid tumors is one reason that this approach is still being pursued avidly in GBM.13
For all forms of pharmacologic therapy and immunotherapies, providing adequate levels of therapeutic agent to the location of the tumor has been challenging. Convection-enhanced delivery (CED) is an example of a novel approach supported by clinical studies. By bypassing the blood-brain barrier, CED involves the delivery of a drug through a catheter placed into
the tumor.20 While this method increases the concentration of the treatment at the malignancy, it also reduces the risk of systemic adverse effects. CED drug delivery for GBM has been evaluated across a diverse array of strategies, including oncolytic viruses, nucleotide-based therapies, and monoclonal antibodies, as well as immunotherapies. One potential advantage of pump-based CED is sustained drug delivery, which might prove to be an important variable in treatment success for a tumor that relapses almost uniformly after therapy.21
Despite the disappointments in the past, the enormous increase in the number of drugs and immunotherapies along with the array of available and potential GBM mechanisms is, by itself, a source of encouragement. This is because the growth in possible targets is representative of advances in GBM biology leading to new potential targets for disease control. For example, small molecule pathway inhibitors that have reached clinical trials include P13K pathway inhibitors, inhibitors of HGFR/MET and SGX532, and inhibitors of EGFR and PDGFR.12
Unfortunately, the failures of promising drugs in phase 3 trials have also continued. For example, the VEGF-targeted monoclonal antibody bevacizumab, did not provide an overall survival benefit despite an encouraging degree of activity in early clinical studies.22 Recently, the antibody-drug conjugate depatuxizumab mafodotin also failed to demonstrate a survival benefit in a recent phase 3 trial despite an improvement in progression-free survival.23 However, the failure of these drugs to extend survival as single agents does not preclude benefit in further studies when they are combined with other strategies or administered with novel methods of drug delivery. The poor response to conventional therapies has led to consideration of alternative strategies such as tumor-treating fields where low-intensity electrical fields delivered via an FDA-approved portable wearable device demonstrated a modest effect on survival when combined with temozolomide.24
Why Optimism for Advances in GBM Is Warranted
The standard for the first-line treatment of GBM has remained unchanged since the introduction of temozolomide about 25 years ago. The combination of surgical debulking, radiation, temozolomide, and adjuvant chemotherapy is recommended in joint guidelines from the Society of Neuro-Oncology and the European Society of Neuro-Oncology.25 This strategy also remains a recommendation in the most recent guidelines on central nervous system cancers from the National Comprehensive Cancer Network® (NCCN®).26
The absence of new treatment standards belies the substantial new detail in which the pathophysiology is understood and with which GBM is being characterized. In this short review, only a proportion of the work in this field could be included. The combination approaches being pursued in relapsed disease is an example of promising work that was not addressed.
Yet, a focus on first-line therapies might be particularly appropriate in GBM. In this malignancy, for which relapse after the standard therapy almost always occurs, the identification of effective early treatment might be the only practical opportunity to increase survival meaningfully. For most cancer types, patients are typically offered experimental therapies only after progression on the standard of care. With advances in understanding the biology and molecular pathways of GBM progression, a paradigm shift might be appropriate. For a tumor type that is rarely, if ever, controlled on the current standard, trials of promising therapies, individualized to the underlying biology of GBM, might be warranted in tumors newly diagnosed and at an early stage.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
Introduction
The evolution toward targeted therapies for glioblastoma multiforme (GBM) accelerated in 2021 when the World Health Organization (WHO) reclassified malignancies of the central nervous system.1 By placing a greater emphasis on molecular rather than histological characteristics of brain cancers, the reclassification validated the progress in identifying potential targetable drivers of disease within GBM subtypes. At the time of this reclassification, the US Food and Drug Administration (FDA) was already granting more orphan drug designations to targeted small molecules and to immunotherapeutics than to cytotoxic drugs2; this evolution is ongoing. Several immunotherapeutic approaches look particularly promising in early clinical trials. For some GBM subtypes, a clinical trial might soon become a therapeutic choice, particularly in the second line.
Background
In the United States, the incidence of GBM is 3.23 cases per 100,000, representing nearly half (48.6%) of all primary malignant brain tumors.3 Relative to non-small cell lung cancer, which has an incidence of about 40 cases per 100,000,4 this incidence is a small burden, but GBM is highly lethal even relative to other aggressive tumors. Essentially all GBM patients relapse after first-line treatments, including patients with a complete response.5 The 5-year survival, which has changed little over decades, is estimated to be less than 5%.6
Following the 2021 WHO classification of tumors in the central nervous system (WHO CNS5),1 the histologically oriented categories of pro-neural, neural, classical, and mesenchymal disease were replaced by 3 major types of GBM that can each be further characterized. These are astrocytoma mutant for isocitrate dehydrogenase (IDH), oligodendroglioma, and glioblastoma IDH-wildtype. For the first time, a separate classification system was also developed for pediatric GBM. Although brain cancer is the second most common type of malignancy in children, it is rare. Most cases of GBM occur in adults. More than half of new GBM diagnoses are in people older than 65 years.7
No standard method for molecular testing was described in WHO CNS5, but further molecular differentiation through biologic and genetic testing is recommended.8 Testing can be performed with transcription profiles, gene alterations, or DNA methylation.9 In addition to the evaluation of IDH status, mutations in α-thalassemia X-linked intellectual disability (ATRX), cyclin dependent kinase inhibitor 2A (CDKN2A/B), tumor suppressor gene (TP53), mitogen-activated protein kinases (MAPK), epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), and histone H3 (H3) G34 have been identified as biomarkers with potential prognostic value.10 Some or all of these biomarkers might eventually prove targetable. Moreover, it is expected that more progress in describing the GBM molecular pathways will yield further modifications in prognostic assessment and, potentially, choice of treatment.
Despite the promise of some of these targets in laboratory and early clinical studies, none of the therapies in development have so far changed the standard of care, which is dominated by resection followed by radiation and temozolomide. However, several treatment categories support the premise that individualized therapies in GBM are plausible and might improve outcomes, including extended survival.
Selected Trials and Their Rationale
The distinction between IDH-wildtype GBM and IDH-mutant GBM, which has a better prognosis,11 was one of many factors that changed the perception of GBM as a relatively homogeneous tumor type to one characterized by an array of intricate signaling pathways. Overall and in the context of glioma stem cells—which are a cell population in the GBM tumor microenvironment now suspected to play an important role in resistance and subsequent relapse,10—several pathways hold considerable promise for interfering with GBM progression. Studies of immunotherapies have been among the most encouraging.
Following a substantial effort over the last decade to engage the immune response in the treatment of GBM through oncolytic virotherapy, the field, despite its promise, has yet to produce a viable treatment for GBM.12,13 This effort includes multiple studies with dendritic cell vaccination, including a phase 3 trial published in 2023,14 but no therapy has yet to be approved.15 Although some of these trials did generate signals of activity, there are no approved treatments, and, recently, greater attention has been drawn to other strategies to engage the patient’s immune response, including chimeric antigen receptor (CAR) T-cells and checkpoint inhibitors.
A phase 1 study published in April 2024 showed that a novel engineered CAR T-cell product called CARv3-TEAM-E elicited dramatic radiographic regression of tumors in all 3 patients treated within days of intravenous administration.16 Although only 1 of the responses was sustained over follow-up, this result showed that clinically significant responses can be achieved in patients with advanced intraparenchymal disease. The tested CAR T construct included T-cell engaging antibody molecules (TEAMS) against wildtype EGFR, which was credited with inducing a radiological response not seen with a prior CAR T-cell construct. Other CAR T-cell studies are ongoing. In another trial published this year, results were less promising. It also targeted EGFR as well as the interleukin-13 receptor alpha 1, but none of the reductions in tumor size met criteria for an objective response.17
The theoretical promise of checkpoint inhibitors in GBM has not yet been realized in studies so far, despite numerous case reports and small series supporting activity. For example, overall survival was not improved with the programmed cell death protein 1 (PD-1) inhibitor nivolumab relative to the vascular endothelial growth factor (VEGF) inhibitor bevacizumab in a phase 3 controlled trial conducted in patients with recurrent GBM.18 However, preclinical research suggests combination strategies, including checkpoint inhibitors added to other types of therapeutics, might yield greater activity.19 The unprecedented responses with checkpoint inhibitors in other solid tumors is one reason that this approach is still being pursued avidly in GBM.13
For all forms of pharmacologic therapy and immunotherapies, providing adequate levels of therapeutic agent to the location of the tumor has been challenging. Convection-enhanced delivery (CED) is an example of a novel approach supported by clinical studies. By bypassing the blood-brain barrier, CED involves the delivery of a drug through a catheter placed into
the tumor.20 While this method increases the concentration of the treatment at the malignancy, it also reduces the risk of systemic adverse effects. CED drug delivery for GBM has been evaluated across a diverse array of strategies, including oncolytic viruses, nucleotide-based therapies, and monoclonal antibodies, as well as immunotherapies. One potential advantage of pump-based CED is sustained drug delivery, which might prove to be an important variable in treatment success for a tumor that relapses almost uniformly after therapy.21
Despite the disappointments in the past, the enormous increase in the number of drugs and immunotherapies along with the array of available and potential GBM mechanisms is, by itself, a source of encouragement. This is because the growth in possible targets is representative of advances in GBM biology leading to new potential targets for disease control. For example, small molecule pathway inhibitors that have reached clinical trials include P13K pathway inhibitors, inhibitors of HGFR/MET and SGX532, and inhibitors of EGFR and PDGFR.12
Unfortunately, the failures of promising drugs in phase 3 trials have also continued. For example, the VEGF-targeted monoclonal antibody bevacizumab, did not provide an overall survival benefit despite an encouraging degree of activity in early clinical studies.22 Recently, the antibody-drug conjugate depatuxizumab mafodotin also failed to demonstrate a survival benefit in a recent phase 3 trial despite an improvement in progression-free survival.23 However, the failure of these drugs to extend survival as single agents does not preclude benefit in further studies when they are combined with other strategies or administered with novel methods of drug delivery. The poor response to conventional therapies has led to consideration of alternative strategies such as tumor-treating fields where low-intensity electrical fields delivered via an FDA-approved portable wearable device demonstrated a modest effect on survival when combined with temozolomide.24
Why Optimism for Advances in GBM Is Warranted
The standard for the first-line treatment of GBM has remained unchanged since the introduction of temozolomide about 25 years ago. The combination of surgical debulking, radiation, temozolomide, and adjuvant chemotherapy is recommended in joint guidelines from the Society of Neuro-Oncology and the European Society of Neuro-Oncology.25 This strategy also remains a recommendation in the most recent guidelines on central nervous system cancers from the National Comprehensive Cancer Network® (NCCN®).26
The absence of new treatment standards belies the substantial new detail in which the pathophysiology is understood and with which GBM is being characterized. In this short review, only a proportion of the work in this field could be included. The combination approaches being pursued in relapsed disease is an example of promising work that was not addressed.
Yet, a focus on first-line therapies might be particularly appropriate in GBM. In this malignancy, for which relapse after the standard therapy almost always occurs, the identification of effective early treatment might be the only practical opportunity to increase survival meaningfully. For most cancer types, patients are typically offered experimental therapies only after progression on the standard of care. With advances in understanding the biology and molecular pathways of GBM progression, a paradigm shift might be appropriate. For a tumor type that is rarely, if ever, controlled on the current standard, trials of promising therapies, individualized to the underlying biology of GBM, might be warranted in tumors newly diagnosed and at an early stage.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231-1251. doi:10.1093/neuonc/noab106
- Johann P, Lenz D, Ries M. The drug development pipeline for glioblastoma—a cross sectional assessment of the FDA Orphan Drug Product designation database. PLoS One. 2021;16(7):e0252924. doi:10.1371/journal.pone.0252924
- Stupp R, Tonn JC, Brada M, Pentheroudakis G, ESMO Guidelines Working Group. High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v190-v193. doi:10.1093/annonc/mdq187
- Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. 2021;7(12):1824-1832. doi:10.1001/jamaoncol.2021.4932
- Sherriff J, Tamangani J, Senthil L, et al. Patterns of relapse in glioblastoma multiforme following concomitant chemoradiotherapy with temozolomide. Br J Radiol. 2013;86(1022):20120414. doi:10.1259/bjr.20120414
- Holland EC. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci U S A. 2000;97(12):6242-6244. doi:10.1073/pnas.97.12.6242
- Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1-ii56. doi:10.1093/neuonc/not151
- Farsi Z, Allahyari Fard N. The identification of key genes and pathways in glioblastoma by bioinformatics analysis. Mol Cell Oncol. 2023;10(1):2246657. doi:10.1080/23723556.2023.2246657
- Zhang P, Xia Q, Liu L, Li S, Dong L. Current opinion on molecular characterization for GBM classification in guiding clinical diagnosis, prognosis, and therapy. Front Mol Biosci. 2020;7:562798. doi:10.3389/fmolb.2020.562798
- Agosti E, Antonietti S, Ius T, Fontanella MM, Zeppieri M, Panciani PP. Glioma stem cells as promoter of glioma progression: a systematic review of molecular pathways and targeted therapies. Int J Mol Sci. 2024;25(14):7979. doi:10.3390/ijms25147979
- Han S, Liu Y, Cai SJ, et al. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer. 2020;122(11):1580-1589. doi:10.1038/s41416-020-0814-x
- Taylor OG, Brzozowski JS, Skelding KA. Glioblastoma multiforme: an overview of emerging therapeutic targets. Front Oncol. 2019;9:963. doi:10.3389/fonc.2019.00963
- Rong L, Li N, Zhang Z. Emerging therapies for glioblastoma: current state and future directions. J Exp Clin Cancer Res. 2022;41(1):142. doi:10.1186/s13046-022-02349-7
- Liau LM, Ashkan K, Brem S, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: a phase 3 prospective externally controlled cohort trial. JAMA Oncol. 2023;9(1):112-121. doi:10.1001/jamaoncol.2022.5370
- Van Gool SW, Makalowski J, Kampers LFC, et al. Dendritic cell vaccination for glioblastoma multiforme patients: has a new milestone been reached? Transl Cancer Res. 2023;12(8):2224-2228. doi:10.21037/tcr-23-603
- Choi BD, Gerstner ER, Frigault MJ, et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N Engl J Med. 2024;390(14):1290-1298. doi:10.1056/NEJMoa2314390
- Bagley SJ, Logun M, Fraietta JA, et al. Intrathecal bivalent CAR T cells targeting EGFR and IL13R-2 in recurrent glioblastoma: phase 1 trial interim results. Nat Med. 2024;30(5):1320-1329. doi:10.1038/s41591-024-02893-z
- Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003-1010. doi:10.1001/jamaoncol.2020.1024
- Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290-5301. doi:10.1158/1078-0432. CCR-14-0514
- Sperring CP, Argenziano MG, Savage WM, et al. Convection-enhanced delivery of immunomodulatory therapy for high-grade glioma. Neurooncol Adv. 2023;5(1):vdad044. doi:10.1093/noajnl/vdad044
- Spinazzi EF, Argenziano MG, Upadhyayula PS, et al. Chronic convection-enhanced delivery of topotecan for patients with recurrent glioblastoma: a first-in-patient, singlecentre, single-arm, phase 1b trial. Lancet Oncol. 2022;23(11):1409-1418. doi:10.1016/S1470-2045(22)00599-X
- Fu M, Zhou Z, Huang X, et al. Use of bevacizumab in recurrent glioblastoma: a scoping review and evidence map. BMC Cancer. 2023;23(1):544. doi:10.1186/s12885-023-11043-6
- Lassman AB, Pugh SL, Wang TJC, et al. Depatuxizumab mafodotin in EGFR-amplified newly diagnosed glioblastoma: a phase III randomized clinical trial. Neuro Oncol. 2023;25(2):339-350. doi:10.1093/neuonc/noac173
- Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 2017; 318: 2306–16.
- Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073-1113. doi:10.1093/neuonc/noaa106
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: central nervous system cancers. Version 2.2024. July 25, 2024. Accessed September 3, 2024. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231-1251. doi:10.1093/neuonc/noab106
- Johann P, Lenz D, Ries M. The drug development pipeline for glioblastoma—a cross sectional assessment of the FDA Orphan Drug Product designation database. PLoS One. 2021;16(7):e0252924. doi:10.1371/journal.pone.0252924
- Stupp R, Tonn JC, Brada M, Pentheroudakis G, ESMO Guidelines Working Group. High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v190-v193. doi:10.1093/annonc/mdq187
- Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. 2021;7(12):1824-1832. doi:10.1001/jamaoncol.2021.4932
- Sherriff J, Tamangani J, Senthil L, et al. Patterns of relapse in glioblastoma multiforme following concomitant chemoradiotherapy with temozolomide. Br J Radiol. 2013;86(1022):20120414. doi:10.1259/bjr.20120414
- Holland EC. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci U S A. 2000;97(12):6242-6244. doi:10.1073/pnas.97.12.6242
- Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1-ii56. doi:10.1093/neuonc/not151
- Farsi Z, Allahyari Fard N. The identification of key genes and pathways in glioblastoma by bioinformatics analysis. Mol Cell Oncol. 2023;10(1):2246657. doi:10.1080/23723556.2023.2246657
- Zhang P, Xia Q, Liu L, Li S, Dong L. Current opinion on molecular characterization for GBM classification in guiding clinical diagnosis, prognosis, and therapy. Front Mol Biosci. 2020;7:562798. doi:10.3389/fmolb.2020.562798
- Agosti E, Antonietti S, Ius T, Fontanella MM, Zeppieri M, Panciani PP. Glioma stem cells as promoter of glioma progression: a systematic review of molecular pathways and targeted therapies. Int J Mol Sci. 2024;25(14):7979. doi:10.3390/ijms25147979
- Han S, Liu Y, Cai SJ, et al. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer. 2020;122(11):1580-1589. doi:10.1038/s41416-020-0814-x
- Taylor OG, Brzozowski JS, Skelding KA. Glioblastoma multiforme: an overview of emerging therapeutic targets. Front Oncol. 2019;9:963. doi:10.3389/fonc.2019.00963
- Rong L, Li N, Zhang Z. Emerging therapies for glioblastoma: current state and future directions. J Exp Clin Cancer Res. 2022;41(1):142. doi:10.1186/s13046-022-02349-7
- Liau LM, Ashkan K, Brem S, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: a phase 3 prospective externally controlled cohort trial. JAMA Oncol. 2023;9(1):112-121. doi:10.1001/jamaoncol.2022.5370
- Van Gool SW, Makalowski J, Kampers LFC, et al. Dendritic cell vaccination for glioblastoma multiforme patients: has a new milestone been reached? Transl Cancer Res. 2023;12(8):2224-2228. doi:10.21037/tcr-23-603
- Choi BD, Gerstner ER, Frigault MJ, et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N Engl J Med. 2024;390(14):1290-1298. doi:10.1056/NEJMoa2314390
- Bagley SJ, Logun M, Fraietta JA, et al. Intrathecal bivalent CAR T cells targeting EGFR and IL13R-2 in recurrent glioblastoma: phase 1 trial interim results. Nat Med. 2024;30(5):1320-1329. doi:10.1038/s41591-024-02893-z
- Reardon DA, Brandes AA, Omuro A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003-1010. doi:10.1001/jamaoncol.2020.1024
- Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20(20):5290-5301. doi:10.1158/1078-0432. CCR-14-0514
- Sperring CP, Argenziano MG, Savage WM, et al. Convection-enhanced delivery of immunomodulatory therapy for high-grade glioma. Neurooncol Adv. 2023;5(1):vdad044. doi:10.1093/noajnl/vdad044
- Spinazzi EF, Argenziano MG, Upadhyayula PS, et al. Chronic convection-enhanced delivery of topotecan for patients with recurrent glioblastoma: a first-in-patient, singlecentre, single-arm, phase 1b trial. Lancet Oncol. 2022;23(11):1409-1418. doi:10.1016/S1470-2045(22)00599-X
- Fu M, Zhou Z, Huang X, et al. Use of bevacizumab in recurrent glioblastoma: a scoping review and evidence map. BMC Cancer. 2023;23(1):544. doi:10.1186/s12885-023-11043-6
- Lassman AB, Pugh SL, Wang TJC, et al. Depatuxizumab mafodotin in EGFR-amplified newly diagnosed glioblastoma: a phase III randomized clinical trial. Neuro Oncol. 2023;25(2):339-350. doi:10.1093/neuonc/noac173
- Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 2017; 318: 2306–16.
- Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073-1113. doi:10.1093/neuonc/noaa106
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: central nervous system cancers. Version 2.2024. July 25, 2024. Accessed September 3, 2024. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf
Treatment of Glioblastoma: A Potential Shift in Paradigm
Treatment of Glioblastoma: A Potential Shift in Paradigm
Advancements in the Treatment of Malignant PEComas with mTOR Inhibitors
Advancements in the Treatment of Malignant PEComas with mTOR Inhibitors
The PI3K/AKT/mTOR pathway is an attractive therapeutic target for soft tissue sarcomas, as dysregulation of mechanistic target of rapamycin (mTOR) can lead to the development of various cancer types. Recently, clinical trial data have demonstrated that mTOR inhibitors can significantly improve long-term outcomes in patients with malignant perivascular epithelioid cell tumors, or PEComas—a challenging disease to manage in the advanced stage.
Ultrarare Mesenchymal Tumors
PEComas are ultrarare soft tissue tumors that are mesenchymal in origin and are characterized histologically by distinctive epithelioid cells that express smooth muscle and melanocytic markers.1-3 Malignant PEComas affect fewer than 1/1,000,000 people per year,4,5 and have a predominance in women, as they are commonly found in the uterus.4 PEComas include several histological types, such as angiomyolipoma (the most prevalent type), lymphangioleiomyomatosis, clear cell (“sugar”) tumor, and other tumors with similar features.3
Detecting an Ultrarare Malignant PEComa
Most PEComas are diagnosed incidentally via imaging. Patients may also present with symptoms of abdominal pain, nausea, and unexplained weight loss.6,7 PEComas in the uterus are often detected through an ultrasound, in which they may have the appearance of fibroids.8 Diagnosis must be confirmed by biopsy, and histological analysis can determine the risk classification based on tumor characteristics.6 Many patients with PEComas harbor loss-of-function mutations in the TSC1 and TSC2 genes, resulting in overactivation of the PIK3/AKT/mTOR signaling pathway9; TP53 mutations and TFE3 rearrangements or fusions have also been identified.6,10
Therapeutic Strategies Are Limited
Because PEComas are often resistant to chemotherapy and radiotherapy, resection is considered standard-of-care treatment for localized disease.6 Patients with advanced disease should be considered for systemic therapy. However, there is a substantial unmet need for novel therapies due to the limited efficacy of existing treatment options. Agents that target mTOR have shown important potential in improving long-term outcomes in patients with metastatic PEComas.6 The PI3K/AKT/mTOR signaling pathway is a key signaling system that regulates cell proliferation and survival. TSC1 and TSC2 normally negatively regulate the mTOR complex 1 (mTORC1); however, alterations in TSC1 and TSC2 result in increased activity of this pathway, allowing tumors to proliferate (Figure).11,12 Clinical guidelines recommend using mTOR inhibitors for patients with locally advanced, unresectable, or metastatic malignant PEComas, and both on and off-label therapies are often used in the clinical setting.13 nab-Sirolimus, a nanoparticle albumin–bound sirolimus, is one such mTOR (previously known as mammalian target of rapamycin) inhibitor that binds to and blocks activation of the mechanistic target of rapamycin complex 1.11,14
Figure. mTOR Signaling Skin Diseases
The Promise of mTOR Inhibitors for Malignant PEComas
In 2021, the US Food and Drug Administration (FDA) approved nab-sirolimus to treat patients with locally advanced, unresectable, or metastatic malignant PEComas. This approval was based on results from the phase 2 Advanced Malignant Perivascular Epithelioid Cell Tumors (AMPECT) clinical trial (NCT02494570).14,15 AMPECT was a multicenter, open-label, single-arm trial that evaluated nab-sirolimus in 34 patients with metastatic or locally advanced (ineligible for surgery) malignant PEComa and measurable disease who had not been previously treated with an mTOR inhibitor. Most of the patients were women, and the most common site of disease was the uterus.14 Patients received nab-sirolimus (100 mg/m2 intravenously) on days 1 and 8 of a 21-day cycle. The primary outcome of the study was an overall response rate by 6 months, and secondary endpoints included duration of response, progression-free survival (PFS), PFS at 6 months (PFS6), overall survival (OS), and safety; tumor biomarkers were also evaluated as exploratory measures.14 At 6 months, nab-sirolimus demonstrated an overall response rate of 39%, with rapid and durable responses. The median PFS was 10.6 months, with a PFS6 of 70%; median OS was 40.8 months.
Of the 25 patients for whom tumor profiling was performed, 8 of 9 (89%) patients with a TSC2 mutation achieved a response compared with 2 of 16 (13%) without the mutation. The most common adverse events associated with treatment included mucositis, rash, fatigue, and anemia, which are consistent with the medication class.14 Long-term analysis from the AMPECT trial demonstrated a median OS of 53.1 months, with a median duration of response of 39.7 months. Taken together, these results indicate that nab-sirolimus may provide patients with positive long-term clinical benefits with an acceptable safety profile.15 nab-Sirolimus is currently being evaluated in clinical trials in patients harboring TSC1 and TSC2 mutations and is also being investigated as a therapeutic candidate for other cancer types, such as neuroendocrine tumors, endometrial cancer, and ovarian cancer (NCT05997056; NCT05997017; NCT06494150; NCT05103358).
Case Study Spotlight
A 70-year-old woman presented at a local emergency department with several episodes of tingling in her upper and lower extremities. A chest radiograph revealed multiple bilateral pulmonary nodules, and a computed tomography scan of the chest, abdomen, and pelvis revealed a 21-cm left abdominal mass, innumerable pulmonary nodules, and multiple hepatic lesions. The patient underwent palliative resection of the large left retroperitoneal mass. Pathology revealed malignant PEComa, and a liver biopsy confirmed metastatic disease.
Following referral, the patient was enrolled in the AMPECT clinical trial, during which she received nab-sirolimus treatment. An objective response was confirmed after the initial 6 weeks on therapy and serial imaging revealed continued shrinkage in lung and liver lesions over time; the nab-sirolimus dose was reduced by 25% due to grade 2 pneumonitis after ~18 months of treatment. The patient had a complete response after 4 years on treatment. Unfortunately, the patient died due to complications from an unrelated elective hernia repair. She was 74 at the time of her death, and there was no radiographic evidence of PEComa.
Future Directions
While mTOR inhibitors provide the most favorable outcomes in the advanced disease setting at this time, research is underway to evaluate the utility of additional novel targets to treat malignant PEComa. Anecdotal evidence from case reports indicates that anti-vascular endothelial growth factor (VEGF) tyrosine kinase inhibitors may be beneficial to patients with malignant PEComa, highlighting the VEGF/VEGF receptor signaling pathway as a potential therapeutic target.16 Some evidence has also suggested that programmed cell death (PD) protein 1/PD ligand 1 (PD-1/PD-L1) inhibitors may be effective for patients with metastatic disease with high PD-L1 levels.17 In addition to more treatment options, diagnostic markers could potentially improve prognosis by facilitating earlier detection, a key challenge in managing malignant PEComas, especially for uterine tumors that are often misdiagnosed.18 Future research may also help guide personalized treatment strategies based on tumor genetic composition.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Stacchiotti S, Frezza AM, Blay JY, et al. Ultra-rare sarcomas: a consensus paper from the Connective Tissue Oncology Society community of experts on the incidence threshold and the list of entities. Cancer. 2021;127(16):2934-2942. doi:10.1002/cncr.33618
- Bleeker JS, Quevedo JF, Folpe AL. “Malignant” perivascular epithelioid cell neoplasm: risk stratification and treatment strategies. Sarcoma. 2012;2012:541626. doi:10.1155/2012/541626
- Thway K, Fisher C. PEComa: morphology and genetics of a complex tumor family. Ann Diagn Pathol. 2015;19(5):359-368. doi:10.1016/j.anndiagpath.2015.06.003
- Battistella E, Pomba L, Mirabella M, et al. Metastatic adrenal PEComa: case report and short review of the literature. Medicina (Kaunas). 2023;59(1):149. doi:10.3390/medicina59010149
- Meredith L, Chao T, Nevler A, et al. A rare metastatic mesenteric malignant PEComa with TSC2 mutation treated with palliative surgical resection and nab-sirolimus: a case report. Diagn Pathol. 2023;18(1):45. doi:10.1186/s13000-023-01323-x
- Czarnecka AM, Skoczylas J, Bartnik E, Switaj T, Rutkowski P. Management strategies for adults with locally advanced, unresectable or metastatic malignant perivascular epithelioid cell tumor (PEComa): challenges and solutions. Cancer Manag Res. 2023;15:615-623. doi:10.2147/CMAR.S351284
- Kvietkauskas M, Samuolyte A, Rackauskas R, et al. Primary liver perivascular epithelioid cell tumor (PEComa): case report and literature review. Medicina (Kaunas). 2024;60(3):409. doi:10.3390/medicina60030409
- Giannella L, Delli Carpini G, Montik N, et al. Ultrasound features of a uterine perivascular epithelioid cell tumor (PEComa): case report and literature review. Diagnostic (Basel). 2020;10(8):553. doi:10.3390/diagnostics10080553
- Liu L, Dehner C, Grandhi N, et al. The impact of TSC-1 and -2 mutations on response to therapy in malignant PEComa: a multicenter retrospective analysis. Genes (Basel). 2022;13(11):1932. doi:10.3390/genes13111932
- Schoolmeester JK, Dao LN, Sukov WR, et al. TFE3 translocation-associated perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: morphology, immunophenotype, differential diagnosis. Am J Surg Pathol. 2015;39(3):394-404.doi:10.1097/PAS.0000000000000349
- Ali ES, Mitra K, Akter S, et al. Recent advances and limitations of mTOR inhibitors in the treatment of cancer. Cancer Cell Int. 2022;22(1):284. doi:10.1186/s12935-022-02706-8
- Sanfilippo R, Jones RL, Blay JY, et al. Role of chemotherapy, VEGFR inhibitors, and mTOR inhibitors in advanced perivascular epithelioid cell tumors (PEComas). Clin Cancer Res. 2019;25(17):5295-5300. doi:10.1158/1078-0432.CCR-19-0288
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: soft tissue sarcoma. Version 2.2024. July 31, 2024. Accessed September 10, 2024. https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf
- Wagner AJ, Ravi V, Riedel RF, et al. nab-Sirolimus for patients with malignant perivascular epithelioid cell tumors. J Clin Oncol. 2021;39(33):3660-3670. doi:10.1200/JCO.21.01728
- Wagner AJ, Ravi V, Riedel RF, et al. Phase II trial of nab-sirolimus in patients with advanced malignant perivascular epithelioid cell tumors (AMPECT): long-term efficacy and safety update. J Clin Oncol. 2024;42(13):1472-1476. doi:10.1200/JCO.23.02266
- Xu J, Gong XL, Wu H, Zhao L. Case report: gastrointestinal PEComa with TFE3 rearrangement treated with anti-VEGFR TKI apatinib. Front Oncol. 2020;10:582087. doi:10.3389/fonc.2020.582087
- McBride A, Garcia AJ, Sanders LJ, et al. Sustained response to pembrolizumab in recurrent perivascular epithelioid cell tumor with elevated expression of programmed death ligand: a case report. J Med Case Rep. 2021;15(1):400. doi:10.1186/s13256-021-02997-x
- Levin G, Capella MP, Meyer R, Brezinov Y, Gotlieb WH. Gynecologic perivascular epithelioid cell tumors (PEComas): a review of recent evidence. Arch Gynecol Obstet. 2024;309(6):2381-2386. doi:10.1007/s00404-024-07510-5
The PI3K/AKT/mTOR pathway is an attractive therapeutic target for soft tissue sarcomas, as dysregulation of mechanistic target of rapamycin (mTOR) can lead to the development of various cancer types. Recently, clinical trial data have demonstrated that mTOR inhibitors can significantly improve long-term outcomes in patients with malignant perivascular epithelioid cell tumors, or PEComas—a challenging disease to manage in the advanced stage.
Ultrarare Mesenchymal Tumors
PEComas are ultrarare soft tissue tumors that are mesenchymal in origin and are characterized histologically by distinctive epithelioid cells that express smooth muscle and melanocytic markers.1-3 Malignant PEComas affect fewer than 1/1,000,000 people per year,4,5 and have a predominance in women, as they are commonly found in the uterus.4 PEComas include several histological types, such as angiomyolipoma (the most prevalent type), lymphangioleiomyomatosis, clear cell (“sugar”) tumor, and other tumors with similar features.3
Detecting an Ultrarare Malignant PEComa
Most PEComas are diagnosed incidentally via imaging. Patients may also present with symptoms of abdominal pain, nausea, and unexplained weight loss.6,7 PEComas in the uterus are often detected through an ultrasound, in which they may have the appearance of fibroids.8 Diagnosis must be confirmed by biopsy, and histological analysis can determine the risk classification based on tumor characteristics.6 Many patients with PEComas harbor loss-of-function mutations in the TSC1 and TSC2 genes, resulting in overactivation of the PIK3/AKT/mTOR signaling pathway9; TP53 mutations and TFE3 rearrangements or fusions have also been identified.6,10
Therapeutic Strategies Are Limited
Because PEComas are often resistant to chemotherapy and radiotherapy, resection is considered standard-of-care treatment for localized disease.6 Patients with advanced disease should be considered for systemic therapy. However, there is a substantial unmet need for novel therapies due to the limited efficacy of existing treatment options. Agents that target mTOR have shown important potential in improving long-term outcomes in patients with metastatic PEComas.6 The PI3K/AKT/mTOR signaling pathway is a key signaling system that regulates cell proliferation and survival. TSC1 and TSC2 normally negatively regulate the mTOR complex 1 (mTORC1); however, alterations in TSC1 and TSC2 result in increased activity of this pathway, allowing tumors to proliferate (Figure).11,12 Clinical guidelines recommend using mTOR inhibitors for patients with locally advanced, unresectable, or metastatic malignant PEComas, and both on and off-label therapies are often used in the clinical setting.13 nab-Sirolimus, a nanoparticle albumin–bound sirolimus, is one such mTOR (previously known as mammalian target of rapamycin) inhibitor that binds to and blocks activation of the mechanistic target of rapamycin complex 1.11,14
Figure. mTOR Signaling Skin Diseases

The Promise of mTOR Inhibitors for Malignant PEComas
In 2021, the US Food and Drug Administration (FDA) approved nab-sirolimus to treat patients with locally advanced, unresectable, or metastatic malignant PEComas. This approval was based on results from the phase 2 Advanced Malignant Perivascular Epithelioid Cell Tumors (AMPECT) clinical trial (NCT02494570).14,15 AMPECT was a multicenter, open-label, single-arm trial that evaluated nab-sirolimus in 34 patients with metastatic or locally advanced (ineligible for surgery) malignant PEComa and measurable disease who had not been previously treated with an mTOR inhibitor. Most of the patients were women, and the most common site of disease was the uterus.14 Patients received nab-sirolimus (100 mg/m2 intravenously) on days 1 and 8 of a 21-day cycle. The primary outcome of the study was an overall response rate by 6 months, and secondary endpoints included duration of response, progression-free survival (PFS), PFS at 6 months (PFS6), overall survival (OS), and safety; tumor biomarkers were also evaluated as exploratory measures.14 At 6 months, nab-sirolimus demonstrated an overall response rate of 39%, with rapid and durable responses. The median PFS was 10.6 months, with a PFS6 of 70%; median OS was 40.8 months.
Of the 25 patients for whom tumor profiling was performed, 8 of 9 (89%) patients with a TSC2 mutation achieved a response compared with 2 of 16 (13%) without the mutation. The most common adverse events associated with treatment included mucositis, rash, fatigue, and anemia, which are consistent with the medication class.14 Long-term analysis from the AMPECT trial demonstrated a median OS of 53.1 months, with a median duration of response of 39.7 months. Taken together, these results indicate that nab-sirolimus may provide patients with positive long-term clinical benefits with an acceptable safety profile.15 nab-Sirolimus is currently being evaluated in clinical trials in patients harboring TSC1 and TSC2 mutations and is also being investigated as a therapeutic candidate for other cancer types, such as neuroendocrine tumors, endometrial cancer, and ovarian cancer (NCT05997056; NCT05997017; NCT06494150; NCT05103358).
Case Study Spotlight
A 70-year-old woman presented at a local emergency department with several episodes of tingling in her upper and lower extremities. A chest radiograph revealed multiple bilateral pulmonary nodules, and a computed tomography scan of the chest, abdomen, and pelvis revealed a 21-cm left abdominal mass, innumerable pulmonary nodules, and multiple hepatic lesions. The patient underwent palliative resection of the large left retroperitoneal mass. Pathology revealed malignant PEComa, and a liver biopsy confirmed metastatic disease.
Following referral, the patient was enrolled in the AMPECT clinical trial, during which she received nab-sirolimus treatment. An objective response was confirmed after the initial 6 weeks on therapy and serial imaging revealed continued shrinkage in lung and liver lesions over time; the nab-sirolimus dose was reduced by 25% due to grade 2 pneumonitis after ~18 months of treatment. The patient had a complete response after 4 years on treatment. Unfortunately, the patient died due to complications from an unrelated elective hernia repair. She was 74 at the time of her death, and there was no radiographic evidence of PEComa.
Future Directions
While mTOR inhibitors provide the most favorable outcomes in the advanced disease setting at this time, research is underway to evaluate the utility of additional novel targets to treat malignant PEComa. Anecdotal evidence from case reports indicates that anti-vascular endothelial growth factor (VEGF) tyrosine kinase inhibitors may be beneficial to patients with malignant PEComa, highlighting the VEGF/VEGF receptor signaling pathway as a potential therapeutic target.16 Some evidence has also suggested that programmed cell death (PD) protein 1/PD ligand 1 (PD-1/PD-L1) inhibitors may be effective for patients with metastatic disease with high PD-L1 levels.17 In addition to more treatment options, diagnostic markers could potentially improve prognosis by facilitating earlier detection, a key challenge in managing malignant PEComas, especially for uterine tumors that are often misdiagnosed.18 Future research may also help guide personalized treatment strategies based on tumor genetic composition.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
The PI3K/AKT/mTOR pathway is an attractive therapeutic target for soft tissue sarcomas, as dysregulation of mechanistic target of rapamycin (mTOR) can lead to the development of various cancer types. Recently, clinical trial data have demonstrated that mTOR inhibitors can significantly improve long-term outcomes in patients with malignant perivascular epithelioid cell tumors, or PEComas—a challenging disease to manage in the advanced stage.
Ultrarare Mesenchymal Tumors
PEComas are ultrarare soft tissue tumors that are mesenchymal in origin and are characterized histologically by distinctive epithelioid cells that express smooth muscle and melanocytic markers.1-3 Malignant PEComas affect fewer than 1/1,000,000 people per year,4,5 and have a predominance in women, as they are commonly found in the uterus.4 PEComas include several histological types, such as angiomyolipoma (the most prevalent type), lymphangioleiomyomatosis, clear cell (“sugar”) tumor, and other tumors with similar features.3
Detecting an Ultrarare Malignant PEComa
Most PEComas are diagnosed incidentally via imaging. Patients may also present with symptoms of abdominal pain, nausea, and unexplained weight loss.6,7 PEComas in the uterus are often detected through an ultrasound, in which they may have the appearance of fibroids.8 Diagnosis must be confirmed by biopsy, and histological analysis can determine the risk classification based on tumor characteristics.6 Many patients with PEComas harbor loss-of-function mutations in the TSC1 and TSC2 genes, resulting in overactivation of the PIK3/AKT/mTOR signaling pathway9; TP53 mutations and TFE3 rearrangements or fusions have also been identified.6,10
Therapeutic Strategies Are Limited
Because PEComas are often resistant to chemotherapy and radiotherapy, resection is considered standard-of-care treatment for localized disease.6 Patients with advanced disease should be considered for systemic therapy. However, there is a substantial unmet need for novel therapies due to the limited efficacy of existing treatment options. Agents that target mTOR have shown important potential in improving long-term outcomes in patients with metastatic PEComas.6 The PI3K/AKT/mTOR signaling pathway is a key signaling system that regulates cell proliferation and survival. TSC1 and TSC2 normally negatively regulate the mTOR complex 1 (mTORC1); however, alterations in TSC1 and TSC2 result in increased activity of this pathway, allowing tumors to proliferate (Figure).11,12 Clinical guidelines recommend using mTOR inhibitors for patients with locally advanced, unresectable, or metastatic malignant PEComas, and both on and off-label therapies are often used in the clinical setting.13 nab-Sirolimus, a nanoparticle albumin–bound sirolimus, is one such mTOR (previously known as mammalian target of rapamycin) inhibitor that binds to and blocks activation of the mechanistic target of rapamycin complex 1.11,14
Figure. mTOR Signaling Skin Diseases

The Promise of mTOR Inhibitors for Malignant PEComas
In 2021, the US Food and Drug Administration (FDA) approved nab-sirolimus to treat patients with locally advanced, unresectable, or metastatic malignant PEComas. This approval was based on results from the phase 2 Advanced Malignant Perivascular Epithelioid Cell Tumors (AMPECT) clinical trial (NCT02494570).14,15 AMPECT was a multicenter, open-label, single-arm trial that evaluated nab-sirolimus in 34 patients with metastatic or locally advanced (ineligible for surgery) malignant PEComa and measurable disease who had not been previously treated with an mTOR inhibitor. Most of the patients were women, and the most common site of disease was the uterus.14 Patients received nab-sirolimus (100 mg/m2 intravenously) on days 1 and 8 of a 21-day cycle. The primary outcome of the study was an overall response rate by 6 months, and secondary endpoints included duration of response, progression-free survival (PFS), PFS at 6 months (PFS6), overall survival (OS), and safety; tumor biomarkers were also evaluated as exploratory measures.14 At 6 months, nab-sirolimus demonstrated an overall response rate of 39%, with rapid and durable responses. The median PFS was 10.6 months, with a PFS6 of 70%; median OS was 40.8 months.
Of the 25 patients for whom tumor profiling was performed, 8 of 9 (89%) patients with a TSC2 mutation achieved a response compared with 2 of 16 (13%) without the mutation. The most common adverse events associated with treatment included mucositis, rash, fatigue, and anemia, which are consistent with the medication class.14 Long-term analysis from the AMPECT trial demonstrated a median OS of 53.1 months, with a median duration of response of 39.7 months. Taken together, these results indicate that nab-sirolimus may provide patients with positive long-term clinical benefits with an acceptable safety profile.15 nab-Sirolimus is currently being evaluated in clinical trials in patients harboring TSC1 and TSC2 mutations and is also being investigated as a therapeutic candidate for other cancer types, such as neuroendocrine tumors, endometrial cancer, and ovarian cancer (NCT05997056; NCT05997017; NCT06494150; NCT05103358).
Case Study Spotlight
A 70-year-old woman presented at a local emergency department with several episodes of tingling in her upper and lower extremities. A chest radiograph revealed multiple bilateral pulmonary nodules, and a computed tomography scan of the chest, abdomen, and pelvis revealed a 21-cm left abdominal mass, innumerable pulmonary nodules, and multiple hepatic lesions. The patient underwent palliative resection of the large left retroperitoneal mass. Pathology revealed malignant PEComa, and a liver biopsy confirmed metastatic disease.
Following referral, the patient was enrolled in the AMPECT clinical trial, during which she received nab-sirolimus treatment. An objective response was confirmed after the initial 6 weeks on therapy and serial imaging revealed continued shrinkage in lung and liver lesions over time; the nab-sirolimus dose was reduced by 25% due to grade 2 pneumonitis after ~18 months of treatment. The patient had a complete response after 4 years on treatment. Unfortunately, the patient died due to complications from an unrelated elective hernia repair. She was 74 at the time of her death, and there was no radiographic evidence of PEComa.
Future Directions
While mTOR inhibitors provide the most favorable outcomes in the advanced disease setting at this time, research is underway to evaluate the utility of additional novel targets to treat malignant PEComa. Anecdotal evidence from case reports indicates that anti-vascular endothelial growth factor (VEGF) tyrosine kinase inhibitors may be beneficial to patients with malignant PEComa, highlighting the VEGF/VEGF receptor signaling pathway as a potential therapeutic target.16 Some evidence has also suggested that programmed cell death (PD) protein 1/PD ligand 1 (PD-1/PD-L1) inhibitors may be effective for patients with metastatic disease with high PD-L1 levels.17 In addition to more treatment options, diagnostic markers could potentially improve prognosis by facilitating earlier detection, a key challenge in managing malignant PEComas, especially for uterine tumors that are often misdiagnosed.18 Future research may also help guide personalized treatment strategies based on tumor genetic composition.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Stacchiotti S, Frezza AM, Blay JY, et al. Ultra-rare sarcomas: a consensus paper from the Connective Tissue Oncology Society community of experts on the incidence threshold and the list of entities. Cancer. 2021;127(16):2934-2942. doi:10.1002/cncr.33618
- Bleeker JS, Quevedo JF, Folpe AL. “Malignant” perivascular epithelioid cell neoplasm: risk stratification and treatment strategies. Sarcoma. 2012;2012:541626. doi:10.1155/2012/541626
- Thway K, Fisher C. PEComa: morphology and genetics of a complex tumor family. Ann Diagn Pathol. 2015;19(5):359-368. doi:10.1016/j.anndiagpath.2015.06.003
- Battistella E, Pomba L, Mirabella M, et al. Metastatic adrenal PEComa: case report and short review of the literature. Medicina (Kaunas). 2023;59(1):149. doi:10.3390/medicina59010149
- Meredith L, Chao T, Nevler A, et al. A rare metastatic mesenteric malignant PEComa with TSC2 mutation treated with palliative surgical resection and nab-sirolimus: a case report. Diagn Pathol. 2023;18(1):45. doi:10.1186/s13000-023-01323-x
- Czarnecka AM, Skoczylas J, Bartnik E, Switaj T, Rutkowski P. Management strategies for adults with locally advanced, unresectable or metastatic malignant perivascular epithelioid cell tumor (PEComa): challenges and solutions. Cancer Manag Res. 2023;15:615-623. doi:10.2147/CMAR.S351284
- Kvietkauskas M, Samuolyte A, Rackauskas R, et al. Primary liver perivascular epithelioid cell tumor (PEComa): case report and literature review. Medicina (Kaunas). 2024;60(3):409. doi:10.3390/medicina60030409
- Giannella L, Delli Carpini G, Montik N, et al. Ultrasound features of a uterine perivascular epithelioid cell tumor (PEComa): case report and literature review. Diagnostic (Basel). 2020;10(8):553. doi:10.3390/diagnostics10080553
- Liu L, Dehner C, Grandhi N, et al. The impact of TSC-1 and -2 mutations on response to therapy in malignant PEComa: a multicenter retrospective analysis. Genes (Basel). 2022;13(11):1932. doi:10.3390/genes13111932
- Schoolmeester JK, Dao LN, Sukov WR, et al. TFE3 translocation-associated perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: morphology, immunophenotype, differential diagnosis. Am J Surg Pathol. 2015;39(3):394-404.doi:10.1097/PAS.0000000000000349
- Ali ES, Mitra K, Akter S, et al. Recent advances and limitations of mTOR inhibitors in the treatment of cancer. Cancer Cell Int. 2022;22(1):284. doi:10.1186/s12935-022-02706-8
- Sanfilippo R, Jones RL, Blay JY, et al. Role of chemotherapy, VEGFR inhibitors, and mTOR inhibitors in advanced perivascular epithelioid cell tumors (PEComas). Clin Cancer Res. 2019;25(17):5295-5300. doi:10.1158/1078-0432.CCR-19-0288
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: soft tissue sarcoma. Version 2.2024. July 31, 2024. Accessed September 10, 2024. https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf
- Wagner AJ, Ravi V, Riedel RF, et al. nab-Sirolimus for patients with malignant perivascular epithelioid cell tumors. J Clin Oncol. 2021;39(33):3660-3670. doi:10.1200/JCO.21.01728
- Wagner AJ, Ravi V, Riedel RF, et al. Phase II trial of nab-sirolimus in patients with advanced malignant perivascular epithelioid cell tumors (AMPECT): long-term efficacy and safety update. J Clin Oncol. 2024;42(13):1472-1476. doi:10.1200/JCO.23.02266
- Xu J, Gong XL, Wu H, Zhao L. Case report: gastrointestinal PEComa with TFE3 rearrangement treated with anti-VEGFR TKI apatinib. Front Oncol. 2020;10:582087. doi:10.3389/fonc.2020.582087
- McBride A, Garcia AJ, Sanders LJ, et al. Sustained response to pembrolizumab in recurrent perivascular epithelioid cell tumor with elevated expression of programmed death ligand: a case report. J Med Case Rep. 2021;15(1):400. doi:10.1186/s13256-021-02997-x
- Levin G, Capella MP, Meyer R, Brezinov Y, Gotlieb WH. Gynecologic perivascular epithelioid cell tumors (PEComas): a review of recent evidence. Arch Gynecol Obstet. 2024;309(6):2381-2386. doi:10.1007/s00404-024-07510-5
- Stacchiotti S, Frezza AM, Blay JY, et al. Ultra-rare sarcomas: a consensus paper from the Connective Tissue Oncology Society community of experts on the incidence threshold and the list of entities. Cancer. 2021;127(16):2934-2942. doi:10.1002/cncr.33618
- Bleeker JS, Quevedo JF, Folpe AL. “Malignant” perivascular epithelioid cell neoplasm: risk stratification and treatment strategies. Sarcoma. 2012;2012:541626. doi:10.1155/2012/541626
- Thway K, Fisher C. PEComa: morphology and genetics of a complex tumor family. Ann Diagn Pathol. 2015;19(5):359-368. doi:10.1016/j.anndiagpath.2015.06.003
- Battistella E, Pomba L, Mirabella M, et al. Metastatic adrenal PEComa: case report and short review of the literature. Medicina (Kaunas). 2023;59(1):149. doi:10.3390/medicina59010149
- Meredith L, Chao T, Nevler A, et al. A rare metastatic mesenteric malignant PEComa with TSC2 mutation treated with palliative surgical resection and nab-sirolimus: a case report. Diagn Pathol. 2023;18(1):45. doi:10.1186/s13000-023-01323-x
- Czarnecka AM, Skoczylas J, Bartnik E, Switaj T, Rutkowski P. Management strategies for adults with locally advanced, unresectable or metastatic malignant perivascular epithelioid cell tumor (PEComa): challenges and solutions. Cancer Manag Res. 2023;15:615-623. doi:10.2147/CMAR.S351284
- Kvietkauskas M, Samuolyte A, Rackauskas R, et al. Primary liver perivascular epithelioid cell tumor (PEComa): case report and literature review. Medicina (Kaunas). 2024;60(3):409. doi:10.3390/medicina60030409
- Giannella L, Delli Carpini G, Montik N, et al. Ultrasound features of a uterine perivascular epithelioid cell tumor (PEComa): case report and literature review. Diagnostic (Basel). 2020;10(8):553. doi:10.3390/diagnostics10080553
- Liu L, Dehner C, Grandhi N, et al. The impact of TSC-1 and -2 mutations on response to therapy in malignant PEComa: a multicenter retrospective analysis. Genes (Basel). 2022;13(11):1932. doi:10.3390/genes13111932
- Schoolmeester JK, Dao LN, Sukov WR, et al. TFE3 translocation-associated perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: morphology, immunophenotype, differential diagnosis. Am J Surg Pathol. 2015;39(3):394-404.doi:10.1097/PAS.0000000000000349
- Ali ES, Mitra K, Akter S, et al. Recent advances and limitations of mTOR inhibitors in the treatment of cancer. Cancer Cell Int. 2022;22(1):284. doi:10.1186/s12935-022-02706-8
- Sanfilippo R, Jones RL, Blay JY, et al. Role of chemotherapy, VEGFR inhibitors, and mTOR inhibitors in advanced perivascular epithelioid cell tumors (PEComas). Clin Cancer Res. 2019;25(17):5295-5300. doi:10.1158/1078-0432.CCR-19-0288
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: soft tissue sarcoma. Version 2.2024. July 31, 2024. Accessed September 10, 2024. https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf
- Wagner AJ, Ravi V, Riedel RF, et al. nab-Sirolimus for patients with malignant perivascular epithelioid cell tumors. J Clin Oncol. 2021;39(33):3660-3670. doi:10.1200/JCO.21.01728
- Wagner AJ, Ravi V, Riedel RF, et al. Phase II trial of nab-sirolimus in patients with advanced malignant perivascular epithelioid cell tumors (AMPECT): long-term efficacy and safety update. J Clin Oncol. 2024;42(13):1472-1476. doi:10.1200/JCO.23.02266
- Xu J, Gong XL, Wu H, Zhao L. Case report: gastrointestinal PEComa with TFE3 rearrangement treated with anti-VEGFR TKI apatinib. Front Oncol. 2020;10:582087. doi:10.3389/fonc.2020.582087
- McBride A, Garcia AJ, Sanders LJ, et al. Sustained response to pembrolizumab in recurrent perivascular epithelioid cell tumor with elevated expression of programmed death ligand: a case report. J Med Case Rep. 2021;15(1):400. doi:10.1186/s13256-021-02997-x
- Levin G, Capella MP, Meyer R, Brezinov Y, Gotlieb WH. Gynecologic perivascular epithelioid cell tumors (PEComas): a review of recent evidence. Arch Gynecol Obstet. 2024;309(6):2381-2386. doi:10.1007/s00404-024-07510-5
Advancements in the Treatment of Malignant PEComas with mTOR Inhibitors
Advancements in the Treatment of Malignant PEComas with mTOR Inhibitors
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
Introduction
Cutaneous T-cell lymphomas (CTCL) are a heterogenous group of rare extranodal non-Hodgkin lymphomas that are caused by the accumulation of neoplastic lymphocytes in the skin.1,2 According to the Surveillance, Epidemiology, and End Results database, a total of 14,942 CTCL cases were recorded between 2000 and 2018.3 The incidence rate for all CTCLs is 8.55 per million and appears to be rising. The causes of such an increase are multifactorial and may be related to better diagnostic tools and increased physician awareness.
The incidence of CTCLs also increases with age. The median age at diagnosis is mid-50s but the incidence of CTCLs is 4-fold greater in patients aged 70 years and older.2 Furthermore, men and Black individuals have the highest incidence rates for CTCLs.2,3 More than 10 types of CTCLs have been identified based on biology, histopathology, and clinical features. CTCL types can be either indolent or aggressive.1,4 Approximately 75% of all primary cutaneous lymphomas consist of CTCLs, including mycosis fungoides (MF), Sézary syndrome (SS), or CD30+ lymphoproliferative disorders (lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma).
The most common CTCL is MF, a clinically heterogeneous, often indolent disease that tends to progress over years or decades.1 This condition classically presents as cutaneous erythematous patches or plaques in sun-protected areas, ie, demonstrating a bathing suit distribution.5 Rarely, MF can present as or progress to more aggressive disease, with infiltrative plaques or tumors. For MF, 5- and 10-year survival ranges from 49% to 100% depending on the stage at diagnosis.1
The most common aggressive CTCL is SS, characterized by erythroderma, intractable pruritis, and the presence of neoplastic clonal T cells (eg, Sézary cells) in the skin, peripheral blood, and/or lymph nodes, with a Sézary cell absolute count of ≥ 1,000 cells/mm3.1,2 SS tends to progress more rapidly than MF and has a worse prognosis, with 5-year survival ranging from 10% to 50%.1,4
Definitive Diagnosis
Diagnosis of CTCL requires the neoplastic T cells be confined to the skin.2 Thus, diagnostic evaluation should involve a comprehensive physical examination, skin biopsy, and staging blood tests including a peripheral blood flow cytometry if indicated. Sometimes, radiologic imaging is needed, and if there are any abnormalities found on staging blood tests or imaging, lymph node and bone marrow biopsy may be necessary.1
MF
MF mimics a wide variety of dermatological diseases, with nearly 50 different clinical entities in the differential, making diagnosis challenging.5 Clinical findings are heterogenous, and symptoms may be attributed to benign diseases, eg, eczema, or psoriasis. Pathological features may be nonspecific and subtle in the early stages of the disease and overlap with reactive processes; therefore, multiple biopsies performed during the disease course may be required to reach a definitive diagnosis. Creating a further challenge is the potential for skin-directed therapies (such as topical steroids) to interfere with pathological assessment at the time of biopsy.2 Thus, obtaining a definitive diagnosis for MF, particularly in the patch or plaque stage, could take a median of 4 years but can take up to 4 decades.2,5
A definitive diagnosis for MF can be made using clinical and histopathological features. Possible ancillary studies (if indicated) include determination of T-cell clonality by polymerase chain reaction or next-generation sequencing methods, and assessment for aberrant loss of T-cell antigen expression by immunohistochemical staining.2
SS
Clinical features of SS may be similar to erythrodermic inflammatory dermatoses, and thus the gold standard for diagnosis is peripheral blood involvement and assessing for clonally related neoplastic T-cell populations.1 Histopathological findings on skin biopsy are often nonspecific.4 The currently proposed International Society for Cutaneous Lymphomas criteria for SS integrate clinical, histopathological, immunophenotyping, and molecular studies.2
Benefits of a Multidisciplinary Team Care Approach
Early-stage MF with limited disease can be managed by a dermatologist, but advanced cases often benefit from a multidisciplinary team care model, including hematology-oncology, dermatology, and radiation oncology.5,6 Several different CTCL care models exist that incorporate resource allocation, staffing availability, and institutional practices developed over time. Regardless of whether care is delivered in a specialized CTCL clinic or a community practice setting, a multidisciplinary team care approach is crucial for patients with advanced-stage CTCL. Dermatologists, hematologist-oncologists, and radiation oncologists may see a patient together or separately, depending on clinical context, and collaborate to formulate the assessment, treatment plan, and address the patient’s questions and concerns. In addition, supportive staff including patient assistance coordinators, pharmacists, behavior health specialists, and palliative care specialists may be included to address the patients’ mental health needs as considerable morbidity from pain, itching, and disfigurement occurs with MF and SS—putting patients at a greater risk for social isolation and depression.7
There are several benefits to using a multidisciplinary team care model for managing CTCLs. Different specialties can provide various services and treatment options for patients to consider. Dermatologists perform skin biopsies to monitor disease progression and can administer skin-directed treatments such as phototherapy; radiation oncologists can administer radiation treatment; and oncologists can administer systemic therapies that are outside the scope of dermatology.8 The coordination of specialty visits can improve patient satisfaction.
Treatment Goals and Disease Management
Goals for treatment include delaying progression, reducing disease burden, and improving or preserving quality of life.5 Decision-making for treating CTCLs should involve preserving potential active treatments for when they are needed during an extended disease course, and mitigating associated burdens of logistical, financial, and physical toxicity.1
A variety of therapeutic modalities are available for CTCL that target tumor cells and boost antitumor responses, including topical therapies, phototherapy, radiation, chemotherapy, retinoids, and immune-modulating drugs (Table). Because no specific driver mutations have been identified for CTCLs, recent targeted therapy development has focused on various immunomodulators, small molecule inhibitors, monoclonal antibodies, and antibody-drug conjugates.1 Lastly, for high-risk patients with persistent disease or disease that is refractory to multiple previous therapies, allogenic hematopoietic stem cell transplantation as a potential therapy to induce durable remission may be considered, with careful attention paid to the timing of its use as well as disease and patient characteristics.9
Table. Therapies for CTCL Care9,10,a
Alternatively for early-stage MF, a “watch-and-wait” approach depending on the site of lesions and disease evolution may be an option, as this approach is not associated with a worsening of the disease course or survival.1 Furthermore, aggressive treatments during early stages have not been found to modify the disease course or survival, emphasizing the need for tailoring treatments based on the extent of involvement of the skin and extracutaneous sites.1,10 New strategies in development to treat CTCL include immune-checkpoint inhibitors and chimeric antigen receptor T-cell therapies. Both strategies focus on engaging the immune system to better combat lymphoma.11,12
Outlook for Patients With CTCL
Using a multidisciplinary care approach is the optimal way to deliver the complex care required for CTCL.5 Such an approach can reduce the time to a definitive diagnosis and accurately stage and risk-stratify the disease. A stage-based treatment approach using sequential therapies in an escalated fashion can help reserve active treatments for advanced disease management and maintain quality of life for patients with CTCL.1,2
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Dummer R, Vermeer MH, Scarisbrick JJ, et al. Cutaneous T cell lymphoma. Nat Rev Dis Primers. 2021;7(1):61. doi:10.1038/s41572-021-00296-9
- Hristov AC, Tejasvi T, Wilcox RA. Cutaneous T-cell lymphomas: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(1):193-209. doi:10.1002/ajh.26760
- Cai ZR, Chen ML, Weinstock MA, Kim YH, Novoa RA, Linos E. Incidence trends of primary cutaneous T-cell lymphoma in the US from 2000 to 2018: a SEER population data analysis. JAMA Oncol. 2022;8(11):1690-1692. doi:10.1001/jamaoncol.2022.3236
- Saleh JS, Subtil A, Hristov AC. Primary cutaneous T-cell lymphoma: a review of the most common entities with focus on recent updates. Hum Pathol. 2023;140:75-100. doi:10.1016/j.humpath.2023.09.009
- Vitiello P, Sagnelli C, Ronchi A, et al. Multidisciplinary approach to the diagnosis and therapy of mycosis fungoides. Healthcare (Basel). 2023;11(4):614. doi:10.3390/healthcare11040614
- Morgenroth S, Roggo A, Pawlik L, Dummer R, Ramelyte E. What is new in cutaneous T cell lymphoma? Curr Oncol Rep. 2023;25(11):1397-1408. doi:10.1007/s11912-023-01464-8
- Molloy K, Jonak C, Woei-A-Jin FJSH, et al. Characteristics associated with significantly worse quality of life in mycosis fungoides/Sézary syndrome from the Prospective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2020;182(3):770-779. doi:10.1111/bjd.18089
- Tyler KH, Haverkos BM, Hastings J, et al. The role of an integrated multidisciplinary clinic in the management of patients with cutaneous lymphoma. Front Oncol. 2015;5:136. doi:10.3389/fonc.2015.00136
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: primary cutaneous lymphomas. Version 3.2024. August 22, 2024. Accessed October 6, 2024. https://www.nccn.org/professionals/physician_gls/pdf/primary_cutaneous.pdf
- Goel RR, Rook AH. Immunobiology and treatment of cutaneous T-cell lymphoma. Expert Rev Clin Immunol. 2024;20(8):985-996. doi:10.1080/1744666X.2024.2326035
- Iyer SP, Sica RA, Ho PJ, et al. S262: The COBALT-LYM study of CTX130: a phase 1 dose escalation study of CD70-targeted allogeneic CRISPR-Cas9–engineered CAR T cells in patients with relapsed/refractory (R/R) T-cell malignancies. HemaSphere. 2022;6(S3):163-164. doi:10.1097/01.HS9.0000843940.96598.e2
- Khodadoust MS, Rook AH, Porcu P, et al. Pembrolizumab in relapsed and refractory mycosis fungoides and Sézary syndrome: a multicenter phase II study. J Clin Oncol. 2020;38(1):20-28. doi:10.1200/JCO.19.01056
Introduction
Cutaneous T-cell lymphomas (CTCL) are a heterogenous group of rare extranodal non-Hodgkin lymphomas that are caused by the accumulation of neoplastic lymphocytes in the skin.1,2 According to the Surveillance, Epidemiology, and End Results database, a total of 14,942 CTCL cases were recorded between 2000 and 2018.3 The incidence rate for all CTCLs is 8.55 per million and appears to be rising. The causes of such an increase are multifactorial and may be related to better diagnostic tools and increased physician awareness.
The incidence of CTCLs also increases with age. The median age at diagnosis is mid-50s but the incidence of CTCLs is 4-fold greater in patients aged 70 years and older.2 Furthermore, men and Black individuals have the highest incidence rates for CTCLs.2,3 More than 10 types of CTCLs have been identified based on biology, histopathology, and clinical features. CTCL types can be either indolent or aggressive.1,4 Approximately 75% of all primary cutaneous lymphomas consist of CTCLs, including mycosis fungoides (MF), Sézary syndrome (SS), or CD30+ lymphoproliferative disorders (lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma).
The most common CTCL is MF, a clinically heterogeneous, often indolent disease that tends to progress over years or decades.1 This condition classically presents as cutaneous erythematous patches or plaques in sun-protected areas, ie, demonstrating a bathing suit distribution.5 Rarely, MF can present as or progress to more aggressive disease, with infiltrative plaques or tumors. For MF, 5- and 10-year survival ranges from 49% to 100% depending on the stage at diagnosis.1
The most common aggressive CTCL is SS, characterized by erythroderma, intractable pruritis, and the presence of neoplastic clonal T cells (eg, Sézary cells) in the skin, peripheral blood, and/or lymph nodes, with a Sézary cell absolute count of ≥ 1,000 cells/mm3.1,2 SS tends to progress more rapidly than MF and has a worse prognosis, with 5-year survival ranging from 10% to 50%.1,4
Definitive Diagnosis
Diagnosis of CTCL requires the neoplastic T cells be confined to the skin.2 Thus, diagnostic evaluation should involve a comprehensive physical examination, skin biopsy, and staging blood tests including a peripheral blood flow cytometry if indicated. Sometimes, radiologic imaging is needed, and if there are any abnormalities found on staging blood tests or imaging, lymph node and bone marrow biopsy may be necessary.1
MF
MF mimics a wide variety of dermatological diseases, with nearly 50 different clinical entities in the differential, making diagnosis challenging.5 Clinical findings are heterogenous, and symptoms may be attributed to benign diseases, eg, eczema, or psoriasis. Pathological features may be nonspecific and subtle in the early stages of the disease and overlap with reactive processes; therefore, multiple biopsies performed during the disease course may be required to reach a definitive diagnosis. Creating a further challenge is the potential for skin-directed therapies (such as topical steroids) to interfere with pathological assessment at the time of biopsy.2 Thus, obtaining a definitive diagnosis for MF, particularly in the patch or plaque stage, could take a median of 4 years but can take up to 4 decades.2,5
A definitive diagnosis for MF can be made using clinical and histopathological features. Possible ancillary studies (if indicated) include determination of T-cell clonality by polymerase chain reaction or next-generation sequencing methods, and assessment for aberrant loss of T-cell antigen expression by immunohistochemical staining.2
SS
Clinical features of SS may be similar to erythrodermic inflammatory dermatoses, and thus the gold standard for diagnosis is peripheral blood involvement and assessing for clonally related neoplastic T-cell populations.1 Histopathological findings on skin biopsy are often nonspecific.4 The currently proposed International Society for Cutaneous Lymphomas criteria for SS integrate clinical, histopathological, immunophenotyping, and molecular studies.2
Benefits of a Multidisciplinary Team Care Approach
Early-stage MF with limited disease can be managed by a dermatologist, but advanced cases often benefit from a multidisciplinary team care model, including hematology-oncology, dermatology, and radiation oncology.5,6 Several different CTCL care models exist that incorporate resource allocation, staffing availability, and institutional practices developed over time. Regardless of whether care is delivered in a specialized CTCL clinic or a community practice setting, a multidisciplinary team care approach is crucial for patients with advanced-stage CTCL. Dermatologists, hematologist-oncologists, and radiation oncologists may see a patient together or separately, depending on clinical context, and collaborate to formulate the assessment, treatment plan, and address the patient’s questions and concerns. In addition, supportive staff including patient assistance coordinators, pharmacists, behavior health specialists, and palliative care specialists may be included to address the patients’ mental health needs as considerable morbidity from pain, itching, and disfigurement occurs with MF and SS—putting patients at a greater risk for social isolation and depression.7
There are several benefits to using a multidisciplinary team care model for managing CTCLs. Different specialties can provide various services and treatment options for patients to consider. Dermatologists perform skin biopsies to monitor disease progression and can administer skin-directed treatments such as phototherapy; radiation oncologists can administer radiation treatment; and oncologists can administer systemic therapies that are outside the scope of dermatology.8 The coordination of specialty visits can improve patient satisfaction.
Treatment Goals and Disease Management
Goals for treatment include delaying progression, reducing disease burden, and improving or preserving quality of life.5 Decision-making for treating CTCLs should involve preserving potential active treatments for when they are needed during an extended disease course, and mitigating associated burdens of logistical, financial, and physical toxicity.1
A variety of therapeutic modalities are available for CTCL that target tumor cells and boost antitumor responses, including topical therapies, phototherapy, radiation, chemotherapy, retinoids, and immune-modulating drugs (Table). Because no specific driver mutations have been identified for CTCLs, recent targeted therapy development has focused on various immunomodulators, small molecule inhibitors, monoclonal antibodies, and antibody-drug conjugates.1 Lastly, for high-risk patients with persistent disease or disease that is refractory to multiple previous therapies, allogenic hematopoietic stem cell transplantation as a potential therapy to induce durable remission may be considered, with careful attention paid to the timing of its use as well as disease and patient characteristics.9
Table. Therapies for CTCL Care9,10,a

Alternatively for early-stage MF, a “watch-and-wait” approach depending on the site of lesions and disease evolution may be an option, as this approach is not associated with a worsening of the disease course or survival.1 Furthermore, aggressive treatments during early stages have not been found to modify the disease course or survival, emphasizing the need for tailoring treatments based on the extent of involvement of the skin and extracutaneous sites.1,10 New strategies in development to treat CTCL include immune-checkpoint inhibitors and chimeric antigen receptor T-cell therapies. Both strategies focus on engaging the immune system to better combat lymphoma.11,12
Outlook for Patients With CTCL
Using a multidisciplinary care approach is the optimal way to deliver the complex care required for CTCL.5 Such an approach can reduce the time to a definitive diagnosis and accurately stage and risk-stratify the disease. A stage-based treatment approach using sequential therapies in an escalated fashion can help reserve active treatments for advanced disease management and maintain quality of life for patients with CTCL.1,2
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
Introduction
Cutaneous T-cell lymphomas (CTCL) are a heterogenous group of rare extranodal non-Hodgkin lymphomas that are caused by the accumulation of neoplastic lymphocytes in the skin.1,2 According to the Surveillance, Epidemiology, and End Results database, a total of 14,942 CTCL cases were recorded between 2000 and 2018.3 The incidence rate for all CTCLs is 8.55 per million and appears to be rising. The causes of such an increase are multifactorial and may be related to better diagnostic tools and increased physician awareness.
The incidence of CTCLs also increases with age. The median age at diagnosis is mid-50s but the incidence of CTCLs is 4-fold greater in patients aged 70 years and older.2 Furthermore, men and Black individuals have the highest incidence rates for CTCLs.2,3 More than 10 types of CTCLs have been identified based on biology, histopathology, and clinical features. CTCL types can be either indolent or aggressive.1,4 Approximately 75% of all primary cutaneous lymphomas consist of CTCLs, including mycosis fungoides (MF), Sézary syndrome (SS), or CD30+ lymphoproliferative disorders (lymphomatoid papulosis and primary cutaneous anaplastic large cell lymphoma).
The most common CTCL is MF, a clinically heterogeneous, often indolent disease that tends to progress over years or decades.1 This condition classically presents as cutaneous erythematous patches or plaques in sun-protected areas, ie, demonstrating a bathing suit distribution.5 Rarely, MF can present as or progress to more aggressive disease, with infiltrative plaques or tumors. For MF, 5- and 10-year survival ranges from 49% to 100% depending on the stage at diagnosis.1
The most common aggressive CTCL is SS, characterized by erythroderma, intractable pruritis, and the presence of neoplastic clonal T cells (eg, Sézary cells) in the skin, peripheral blood, and/or lymph nodes, with a Sézary cell absolute count of ≥ 1,000 cells/mm3.1,2 SS tends to progress more rapidly than MF and has a worse prognosis, with 5-year survival ranging from 10% to 50%.1,4
Definitive Diagnosis
Diagnosis of CTCL requires the neoplastic T cells be confined to the skin.2 Thus, diagnostic evaluation should involve a comprehensive physical examination, skin biopsy, and staging blood tests including a peripheral blood flow cytometry if indicated. Sometimes, radiologic imaging is needed, and if there are any abnormalities found on staging blood tests or imaging, lymph node and bone marrow biopsy may be necessary.1
MF
MF mimics a wide variety of dermatological diseases, with nearly 50 different clinical entities in the differential, making diagnosis challenging.5 Clinical findings are heterogenous, and symptoms may be attributed to benign diseases, eg, eczema, or psoriasis. Pathological features may be nonspecific and subtle in the early stages of the disease and overlap with reactive processes; therefore, multiple biopsies performed during the disease course may be required to reach a definitive diagnosis. Creating a further challenge is the potential for skin-directed therapies (such as topical steroids) to interfere with pathological assessment at the time of biopsy.2 Thus, obtaining a definitive diagnosis for MF, particularly in the patch or plaque stage, could take a median of 4 years but can take up to 4 decades.2,5
A definitive diagnosis for MF can be made using clinical and histopathological features. Possible ancillary studies (if indicated) include determination of T-cell clonality by polymerase chain reaction or next-generation sequencing methods, and assessment for aberrant loss of T-cell antigen expression by immunohistochemical staining.2
SS
Clinical features of SS may be similar to erythrodermic inflammatory dermatoses, and thus the gold standard for diagnosis is peripheral blood involvement and assessing for clonally related neoplastic T-cell populations.1 Histopathological findings on skin biopsy are often nonspecific.4 The currently proposed International Society for Cutaneous Lymphomas criteria for SS integrate clinical, histopathological, immunophenotyping, and molecular studies.2
Benefits of a Multidisciplinary Team Care Approach
Early-stage MF with limited disease can be managed by a dermatologist, but advanced cases often benefit from a multidisciplinary team care model, including hematology-oncology, dermatology, and radiation oncology.5,6 Several different CTCL care models exist that incorporate resource allocation, staffing availability, and institutional practices developed over time. Regardless of whether care is delivered in a specialized CTCL clinic or a community practice setting, a multidisciplinary team care approach is crucial for patients with advanced-stage CTCL. Dermatologists, hematologist-oncologists, and radiation oncologists may see a patient together or separately, depending on clinical context, and collaborate to formulate the assessment, treatment plan, and address the patient’s questions and concerns. In addition, supportive staff including patient assistance coordinators, pharmacists, behavior health specialists, and palliative care specialists may be included to address the patients’ mental health needs as considerable morbidity from pain, itching, and disfigurement occurs with MF and SS—putting patients at a greater risk for social isolation and depression.7
There are several benefits to using a multidisciplinary team care model for managing CTCLs. Different specialties can provide various services and treatment options for patients to consider. Dermatologists perform skin biopsies to monitor disease progression and can administer skin-directed treatments such as phototherapy; radiation oncologists can administer radiation treatment; and oncologists can administer systemic therapies that are outside the scope of dermatology.8 The coordination of specialty visits can improve patient satisfaction.
Treatment Goals and Disease Management
Goals for treatment include delaying progression, reducing disease burden, and improving or preserving quality of life.5 Decision-making for treating CTCLs should involve preserving potential active treatments for when they are needed during an extended disease course, and mitigating associated burdens of logistical, financial, and physical toxicity.1
A variety of therapeutic modalities are available for CTCL that target tumor cells and boost antitumor responses, including topical therapies, phototherapy, radiation, chemotherapy, retinoids, and immune-modulating drugs (Table). Because no specific driver mutations have been identified for CTCLs, recent targeted therapy development has focused on various immunomodulators, small molecule inhibitors, monoclonal antibodies, and antibody-drug conjugates.1 Lastly, for high-risk patients with persistent disease or disease that is refractory to multiple previous therapies, allogenic hematopoietic stem cell transplantation as a potential therapy to induce durable remission may be considered, with careful attention paid to the timing of its use as well as disease and patient characteristics.9
Table. Therapies for CTCL Care9,10,a

Alternatively for early-stage MF, a “watch-and-wait” approach depending on the site of lesions and disease evolution may be an option, as this approach is not associated with a worsening of the disease course or survival.1 Furthermore, aggressive treatments during early stages have not been found to modify the disease course or survival, emphasizing the need for tailoring treatments based on the extent of involvement of the skin and extracutaneous sites.1,10 New strategies in development to treat CTCL include immune-checkpoint inhibitors and chimeric antigen receptor T-cell therapies. Both strategies focus on engaging the immune system to better combat lymphoma.11,12
Outlook for Patients With CTCL
Using a multidisciplinary care approach is the optimal way to deliver the complex care required for CTCL.5 Such an approach can reduce the time to a definitive diagnosis and accurately stage and risk-stratify the disease. A stage-based treatment approach using sequential therapies in an escalated fashion can help reserve active treatments for advanced disease management and maintain quality of life for patients with CTCL.1,2
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Dummer R, Vermeer MH, Scarisbrick JJ, et al. Cutaneous T cell lymphoma. Nat Rev Dis Primers. 2021;7(1):61. doi:10.1038/s41572-021-00296-9
- Hristov AC, Tejasvi T, Wilcox RA. Cutaneous T-cell lymphomas: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(1):193-209. doi:10.1002/ajh.26760
- Cai ZR, Chen ML, Weinstock MA, Kim YH, Novoa RA, Linos E. Incidence trends of primary cutaneous T-cell lymphoma in the US from 2000 to 2018: a SEER population data analysis. JAMA Oncol. 2022;8(11):1690-1692. doi:10.1001/jamaoncol.2022.3236
- Saleh JS, Subtil A, Hristov AC. Primary cutaneous T-cell lymphoma: a review of the most common entities with focus on recent updates. Hum Pathol. 2023;140:75-100. doi:10.1016/j.humpath.2023.09.009
- Vitiello P, Sagnelli C, Ronchi A, et al. Multidisciplinary approach to the diagnosis and therapy of mycosis fungoides. Healthcare (Basel). 2023;11(4):614. doi:10.3390/healthcare11040614
- Morgenroth S, Roggo A, Pawlik L, Dummer R, Ramelyte E. What is new in cutaneous T cell lymphoma? Curr Oncol Rep. 2023;25(11):1397-1408. doi:10.1007/s11912-023-01464-8
- Molloy K, Jonak C, Woei-A-Jin FJSH, et al. Characteristics associated with significantly worse quality of life in mycosis fungoides/Sézary syndrome from the Prospective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2020;182(3):770-779. doi:10.1111/bjd.18089
- Tyler KH, Haverkos BM, Hastings J, et al. The role of an integrated multidisciplinary clinic in the management of patients with cutaneous lymphoma. Front Oncol. 2015;5:136. doi:10.3389/fonc.2015.00136
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: primary cutaneous lymphomas. Version 3.2024. August 22, 2024. Accessed October 6, 2024. https://www.nccn.org/professionals/physician_gls/pdf/primary_cutaneous.pdf
- Goel RR, Rook AH. Immunobiology and treatment of cutaneous T-cell lymphoma. Expert Rev Clin Immunol. 2024;20(8):985-996. doi:10.1080/1744666X.2024.2326035
- Iyer SP, Sica RA, Ho PJ, et al. S262: The COBALT-LYM study of CTX130: a phase 1 dose escalation study of CD70-targeted allogeneic CRISPR-Cas9–engineered CAR T cells in patients with relapsed/refractory (R/R) T-cell malignancies. HemaSphere. 2022;6(S3):163-164. doi:10.1097/01.HS9.0000843940.96598.e2
- Khodadoust MS, Rook AH, Porcu P, et al. Pembrolizumab in relapsed and refractory mycosis fungoides and Sézary syndrome: a multicenter phase II study. J Clin Oncol. 2020;38(1):20-28. doi:10.1200/JCO.19.01056
- Dummer R, Vermeer MH, Scarisbrick JJ, et al. Cutaneous T cell lymphoma. Nat Rev Dis Primers. 2021;7(1):61. doi:10.1038/s41572-021-00296-9
- Hristov AC, Tejasvi T, Wilcox RA. Cutaneous T-cell lymphomas: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023;98(1):193-209. doi:10.1002/ajh.26760
- Cai ZR, Chen ML, Weinstock MA, Kim YH, Novoa RA, Linos E. Incidence trends of primary cutaneous T-cell lymphoma in the US from 2000 to 2018: a SEER population data analysis. JAMA Oncol. 2022;8(11):1690-1692. doi:10.1001/jamaoncol.2022.3236
- Saleh JS, Subtil A, Hristov AC. Primary cutaneous T-cell lymphoma: a review of the most common entities with focus on recent updates. Hum Pathol. 2023;140:75-100. doi:10.1016/j.humpath.2023.09.009
- Vitiello P, Sagnelli C, Ronchi A, et al. Multidisciplinary approach to the diagnosis and therapy of mycosis fungoides. Healthcare (Basel). 2023;11(4):614. doi:10.3390/healthcare11040614
- Morgenroth S, Roggo A, Pawlik L, Dummer R, Ramelyte E. What is new in cutaneous T cell lymphoma? Curr Oncol Rep. 2023;25(11):1397-1408. doi:10.1007/s11912-023-01464-8
- Molloy K, Jonak C, Woei-A-Jin FJSH, et al. Characteristics associated with significantly worse quality of life in mycosis fungoides/Sézary syndrome from the Prospective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2020;182(3):770-779. doi:10.1111/bjd.18089
- Tyler KH, Haverkos BM, Hastings J, et al. The role of an integrated multidisciplinary clinic in the management of patients with cutaneous lymphoma. Front Oncol. 2015;5:136. doi:10.3389/fonc.2015.00136
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: primary cutaneous lymphomas. Version 3.2024. August 22, 2024. Accessed October 6, 2024. https://www.nccn.org/professionals/physician_gls/pdf/primary_cutaneous.pdf
- Goel RR, Rook AH. Immunobiology and treatment of cutaneous T-cell lymphoma. Expert Rev Clin Immunol. 2024;20(8):985-996. doi:10.1080/1744666X.2024.2326035
- Iyer SP, Sica RA, Ho PJ, et al. S262: The COBALT-LYM study of CTX130: a phase 1 dose escalation study of CD70-targeted allogeneic CRISPR-Cas9–engineered CAR T cells in patients with relapsed/refractory (R/R) T-cell malignancies. HemaSphere. 2022;6(S3):163-164. doi:10.1097/01.HS9.0000843940.96598.e2
- Khodadoust MS, Rook AH, Porcu P, et al. Pembrolizumab in relapsed and refractory mycosis fungoides and Sézary syndrome: a multicenter phase II study. J Clin Oncol. 2020;38(1):20-28. doi:10.1200/JCO.19.01056
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
National Organization for Rare Disorders: Strengthening Rare Cancer Advocacy
National Organization for Rare Disorders: Strengthening Rare Cancer Advocacy
Rare cancers account for 25% to 30% of all cancer diagnoses and approximately 25% of all cancer deaths, thereby posing a significant public health burden.1 Recognizing the need for action to address this health crisis, the National Organization for Rare Disorders (NORD) established the Rare Cancer Coalition in 2017 to alleviate the challenges faced by people living with rare cancers. Since its inception, the Rare Cancer Coalition has reached millions of people through patient and caregiver education sessions, healthcare provider education, and public awareness campaigns. Since its inception, the Rare Cancer Coalition has reached millions of people through patient and caregiver education sessions, healthcare provider education, and public awareness campaigns. The Coalition Members have had an impact on other rare cancer advocacy groups, contributed to medical publications, and provided collaborative networking opportunities among patients, advocates and researchers.
Thanks to successful advocacy by the Rare Cancer Coalition, the United States Congress established “Rare Cancer Day.” This event takes place annually on September 30 and brings global awareness to rare cancers through mass media and public events. In recognition of Rare Cancer Day 2024, NORD focused its public education on the importance of patient participation in rare cancer research.
NORD and the Rare Cancer Coalition are deeply committed to addressing the unique challenges faced by the rare cancer community. Moving forward, we will focus on promoting the development of innovative and effective treatments, enhancing access to diagnostic testing, advancing new technologies, and fostering research that leads to improved medical approaches for patients with rare cancers.
To that end, we are pleased to present the 2024 Rare Disease Report: Hematology and Oncology in collaboration with our partners at MDedge. This issue will highlight some of the latest advances in rare cancer research, diagnosis, and treatments that are providing new hope for improved outcomes. In this issue, you will find articles that cover recent discoveries on specific rare cancers, including:
- The promise of mTOR inhibitors in improving malignant PEComas
- How novel immunotherapies are demonstrating the potential for improved outcomes for large cell neuroendocrine carcinoma of the lung
- Potential paradigm shifts in the treatment of glioblastoma leveraging CAR T-cell therapies and targeted inhibitors
- Future directions in the treatment of gallbladder cancer with molecular profiling, immunotherapies, and targeted treatments
- The benefits of a multidisciplinary approach in addressing cutaneous T-cell lymphomas
- The evolving role of JAK inhibitors in managing symptoms of myelofibrosis
- Advancements in staging and tailored treatments for hepatoblastoma
- And more!
We hope these articles will enhance your knowledge and enrich your day-to-day clinical practices. We invite you to explore NORD resources including digital CME sessions and disease-specific reports written in accessible language for patients and families. Additionally, you can sign up for our quarterly Caring for Rare newsletter, for timely updates on rare diseases.
Thank you for your commitment to advancing care for rare cancer patients. Your dedication to staying informed is vital for improving patient outcomes.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- International Agency for Research on Cancer (IARC). Rare Disease Day 2022: IARC Highlights the Burden of Rare Cancers. Published February 28, 2022. Accessed October 2, 2024. https://www.iarc.who.int/news-events/rare-disease-day-2022-iarc-highlights-the-burden-of-rare-cancers/
Rare cancers account for 25% to 30% of all cancer diagnoses and approximately 25% of all cancer deaths, thereby posing a significant public health burden.1 Recognizing the need for action to address this health crisis, the National Organization for Rare Disorders (NORD) established the Rare Cancer Coalition in 2017 to alleviate the challenges faced by people living with rare cancers. Since its inception, the Rare Cancer Coalition has reached millions of people through patient and caregiver education sessions, healthcare provider education, and public awareness campaigns. Since its inception, the Rare Cancer Coalition has reached millions of people through patient and caregiver education sessions, healthcare provider education, and public awareness campaigns. The Coalition Members have had an impact on other rare cancer advocacy groups, contributed to medical publications, and provided collaborative networking opportunities among patients, advocates and researchers.
Thanks to successful advocacy by the Rare Cancer Coalition, the United States Congress established “Rare Cancer Day.” This event takes place annually on September 30 and brings global awareness to rare cancers through mass media and public events. In recognition of Rare Cancer Day 2024, NORD focused its public education on the importance of patient participation in rare cancer research.
NORD and the Rare Cancer Coalition are deeply committed to addressing the unique challenges faced by the rare cancer community. Moving forward, we will focus on promoting the development of innovative and effective treatments, enhancing access to diagnostic testing, advancing new technologies, and fostering research that leads to improved medical approaches for patients with rare cancers.
To that end, we are pleased to present the 2024 Rare Disease Report: Hematology and Oncology in collaboration with our partners at MDedge. This issue will highlight some of the latest advances in rare cancer research, diagnosis, and treatments that are providing new hope for improved outcomes. In this issue, you will find articles that cover recent discoveries on specific rare cancers, including:
- The promise of mTOR inhibitors in improving malignant PEComas
- How novel immunotherapies are demonstrating the potential for improved outcomes for large cell neuroendocrine carcinoma of the lung
- Potential paradigm shifts in the treatment of glioblastoma leveraging CAR T-cell therapies and targeted inhibitors
- Future directions in the treatment of gallbladder cancer with molecular profiling, immunotherapies, and targeted treatments
- The benefits of a multidisciplinary approach in addressing cutaneous T-cell lymphomas
- The evolving role of JAK inhibitors in managing symptoms of myelofibrosis
- Advancements in staging and tailored treatments for hepatoblastoma
- And more!
We hope these articles will enhance your knowledge and enrich your day-to-day clinical practices. We invite you to explore NORD resources including digital CME sessions and disease-specific reports written in accessible language for patients and families. Additionally, you can sign up for our quarterly Caring for Rare newsletter, for timely updates on rare diseases.
Thank you for your commitment to advancing care for rare cancer patients. Your dedication to staying informed is vital for improving patient outcomes.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
Rare cancers account for 25% to 30% of all cancer diagnoses and approximately 25% of all cancer deaths, thereby posing a significant public health burden.1 Recognizing the need for action to address this health crisis, the National Organization for Rare Disorders (NORD) established the Rare Cancer Coalition in 2017 to alleviate the challenges faced by people living with rare cancers. Since its inception, the Rare Cancer Coalition has reached millions of people through patient and caregiver education sessions, healthcare provider education, and public awareness campaigns. Since its inception, the Rare Cancer Coalition has reached millions of people through patient and caregiver education sessions, healthcare provider education, and public awareness campaigns. The Coalition Members have had an impact on other rare cancer advocacy groups, contributed to medical publications, and provided collaborative networking opportunities among patients, advocates and researchers.
Thanks to successful advocacy by the Rare Cancer Coalition, the United States Congress established “Rare Cancer Day.” This event takes place annually on September 30 and brings global awareness to rare cancers through mass media and public events. In recognition of Rare Cancer Day 2024, NORD focused its public education on the importance of patient participation in rare cancer research.
NORD and the Rare Cancer Coalition are deeply committed to addressing the unique challenges faced by the rare cancer community. Moving forward, we will focus on promoting the development of innovative and effective treatments, enhancing access to diagnostic testing, advancing new technologies, and fostering research that leads to improved medical approaches for patients with rare cancers.
To that end, we are pleased to present the 2024 Rare Disease Report: Hematology and Oncology in collaboration with our partners at MDedge. This issue will highlight some of the latest advances in rare cancer research, diagnosis, and treatments that are providing new hope for improved outcomes. In this issue, you will find articles that cover recent discoveries on specific rare cancers, including:
- The promise of mTOR inhibitors in improving malignant PEComas
- How novel immunotherapies are demonstrating the potential for improved outcomes for large cell neuroendocrine carcinoma of the lung
- Potential paradigm shifts in the treatment of glioblastoma leveraging CAR T-cell therapies and targeted inhibitors
- Future directions in the treatment of gallbladder cancer with molecular profiling, immunotherapies, and targeted treatments
- The benefits of a multidisciplinary approach in addressing cutaneous T-cell lymphomas
- The evolving role of JAK inhibitors in managing symptoms of myelofibrosis
- Advancements in staging and tailored treatments for hepatoblastoma
- And more!
We hope these articles will enhance your knowledge and enrich your day-to-day clinical practices. We invite you to explore NORD resources including digital CME sessions and disease-specific reports written in accessible language for patients and families. Additionally, you can sign up for our quarterly Caring for Rare newsletter, for timely updates on rare diseases.
Thank you for your commitment to advancing care for rare cancer patients. Your dedication to staying informed is vital for improving patient outcomes.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- International Agency for Research on Cancer (IARC). Rare Disease Day 2022: IARC Highlights the Burden of Rare Cancers. Published February 28, 2022. Accessed October 2, 2024. https://www.iarc.who.int/news-events/rare-disease-day-2022-iarc-highlights-the-burden-of-rare-cancers/
- International Agency for Research on Cancer (IARC). Rare Disease Day 2022: IARC Highlights the Burden of Rare Cancers. Published February 28, 2022. Accessed October 2, 2024. https://www.iarc.who.int/news-events/rare-disease-day-2022-iarc-highlights-the-burden-of-rare-cancers/
National Organization for Rare Disorders: Strengthening Rare Cancer Advocacy
National Organization for Rare Disorders: Strengthening Rare Cancer Advocacy
2024 Rare Diseases Report: Hematology and Oncology
2024 Rare Diseases Report: Hematology and Oncology
National Organization for Rare Disorders: Strengthening Rare Cancer Advocacy
By Alli Ward
NORD's Rare Cancer Coalition has transformed advocacy and awareness efforts, offering education and fostering research to address the challenges of rare cancers.
Treatment of Glioblastoma: A Potential Shift in Paradigm
By Jeffrey N. Bruce, MD
Immunotherapies and molecular profiling are paving the way for more targeted approaches in treating glioblastoma.
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
By Robert A. Ramirez, DO, FACP, and Aman Chauhan, MD
New diagnostic tools and precision medicine approaches are addressing the unique challenges of this aggressive neuroendocrine cancer.
Advancements in the Treatment of Malignant PEComas with mTOR Inhibitors
By Richard F. Riedel, MD
The use of mTOR inhibitors marks significant progress in managing advanced malignant PEComas, offering new hope for patients.
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
By Jina Chung, MD, and Eric Mou, MD
A multidisciplinary care model ensures optimal outcomes for patients with cutaneous T-cell lymphomas, addressing both medical and emotional needs.
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
By Douglas Tremblay, MD
JAK inhibitors are central to myelofibrosis management, with personalized strategies helping to navigate resistance and improve quality of life.
Current Management and Future Directions in the Treatment of Gallbladder Cancer
By Ghassan K. Abou-Alfa, MD, MBA, JD, FASCO
Molecular profiling and immunotherapy are reshaping the treatment paradigm for gallbladder cancer, improving survival outcomes.
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
By Greg M. Tiao, MD
Risk stratification and individualized therapies are driving progress in treating hepatoblastoma, with promising advancements on the horizon.
National Organization for Rare Disorders: Strengthening Rare Cancer Advocacy
By Alli Ward
NORD's Rare Cancer Coalition has transformed advocacy and awareness efforts, offering education and fostering research to address the challenges of rare cancers.
Treatment of Glioblastoma: A Potential Shift in Paradigm
By Jeffrey N. Bruce, MD
Immunotherapies and molecular profiling are paving the way for more targeted approaches in treating glioblastoma.
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
By Robert A. Ramirez, DO, FACP, and Aman Chauhan, MD
New diagnostic tools and precision medicine approaches are addressing the unique challenges of this aggressive neuroendocrine cancer.
Advancements in the Treatment of Malignant PEComas with mTOR Inhibitors
By Richard F. Riedel, MD
The use of mTOR inhibitors marks significant progress in managing advanced malignant PEComas, offering new hope for patients.
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
By Jina Chung, MD, and Eric Mou, MD
A multidisciplinary care model ensures optimal outcomes for patients with cutaneous T-cell lymphomas, addressing both medical and emotional needs.
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
By Douglas Tremblay, MD
JAK inhibitors are central to myelofibrosis management, with personalized strategies helping to navigate resistance and improve quality of life.
Current Management and Future Directions in the Treatment of Gallbladder Cancer
By Ghassan K. Abou-Alfa, MD, MBA, JD, FASCO
Molecular profiling and immunotherapy are reshaping the treatment paradigm for gallbladder cancer, improving survival outcomes.
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
By Greg M. Tiao, MD
Risk stratification and individualized therapies are driving progress in treating hepatoblastoma, with promising advancements on the horizon.
National Organization for Rare Disorders: Strengthening Rare Cancer Advocacy
By Alli Ward
NORD's Rare Cancer Coalition has transformed advocacy and awareness efforts, offering education and fostering research to address the challenges of rare cancers.
Treatment of Glioblastoma: A Potential Shift in Paradigm
By Jeffrey N. Bruce, MD
Immunotherapies and molecular profiling are paving the way for more targeted approaches in treating glioblastoma.
Emerging Insights and Therapeutic Strategies for Large Cell Neuroendocrine Carcinoma of the Lung
By Robert A. Ramirez, DO, FACP, and Aman Chauhan, MD
New diagnostic tools and precision medicine approaches are addressing the unique challenges of this aggressive neuroendocrine cancer.
Advancements in the Treatment of Malignant PEComas with mTOR Inhibitors
By Richard F. Riedel, MD
The use of mTOR inhibitors marks significant progress in managing advanced malignant PEComas, offering new hope for patients.
Cutaneous T-Cell Lymphomas Update: Benefits of a Multidisciplinary Care Approach
By Jina Chung, MD, and Eric Mou, MD
A multidisciplinary care model ensures optimal outcomes for patients with cutaneous T-cell lymphomas, addressing both medical and emotional needs.
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
By Douglas Tremblay, MD
JAK inhibitors are central to myelofibrosis management, with personalized strategies helping to navigate resistance and improve quality of life.
Current Management and Future Directions in the Treatment of Gallbladder Cancer
By Ghassan K. Abou-Alfa, MD, MBA, JD, FASCO
Molecular profiling and immunotherapy are reshaping the treatment paradigm for gallbladder cancer, improving survival outcomes.
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
By Greg M. Tiao, MD
Risk stratification and individualized therapies are driving progress in treating hepatoblastoma, with promising advancements on the horizon.
2024 Rare Diseases Report: Hematology and Oncology
2024 Rare Diseases Report: Hematology and Oncology
Current Management and Future Directions in the Treatment of Gallbladder Cancer
Current Management and Future Directions in the Treatment of Gallbladder Cancer
Clinical outcomes of patients with gallbladder cancer have improved considerably with the advent of immunotherapy and targeted therapies. While specialists have gained tremendous insights into the disease over the last 10 years, significant knowledge gaps remain, as relapse rates remain high. Early referral to specialized treatment centers and timely molecular profiling can help guide therapeutic regimen choice and potentially improve patient outcomes.
Insights Into Disease Prevalence and Development
Gallbladder cancer is a rare malignancy with an aggressive course. Most gallbladder cancers are of epithelial origin, with adenocarcinoma being the most common type.1 Approximately 12,350 new cases of gallbladder cancer and nearby large bile duct cancers are anticipated in 2024 in the United States,2 predominantly affecting Southwestern Native Americans.3 The prevalence of gallbladder cancer varies greatly worldwide; rates are highest in South America (mainly in Chile) and Southeast Asia, including Eastern India.3,4
Multiple factors, including environment and genetics, contribute to the development of gallbladder cancer, which is driven primarily by chronic inflammation.5 While there are no defined risk factors, this malignancy is mostly associated with female sex, chronic gallbladder infections, and gallstones.4 Some evidence also suggests a dietary association with consuming mustard seed oil.6 Exposure to certain environmental toxins or heavy metals may also contribute to disease risk.1,4
Several genetic alterations have been identified in patients with gallbladder cancer that may be related to disease etiology; these include somatic mutations in the human epidermal growth factor receptor 2 (HER2), Kirsten rat sarcoma viral oncogene homolog (KRAS), and tumor protein p53 (TP53) genes, and many others.7,8 In addition to somatic mutations, gene overexpression, epigenetic changes, and microRNA-associated changes have also been linked to the disease.3
Challenges in Uncovering a “Hidden” Disease
While most gallbladder cancers are usually detected incidentally, patients may present with symptoms of abdominal pain, discomfort, and biliary obstruction–related symptoms like jaundice, itching, and dark urine.3,9,10 Cases may initially be misdiagnosed as inflammatory conditions such as cholecystitis or gallbladder stones; as a result, patients may be rushed into an inappropriate or incorrect surgical intervention.11 For these reasons, as well as the tight anatomical location of the gallbladder, cases are often not detected until advanced-stage disease.3,4
Patients diagnosed in stage 4 with distant metastases have an expected survival rate of less than 1 year,12 and low referral rates are associated with poor outcomes.13 Patients with suspected disease should therefore be referred to a specialized treatment center as soon as possible to confirm a diagnosis and initiate appropriate treatment. Core biopsies can provide histological confirmation, where feasible and safe, followed by imaging to determine extent of the disease.14
Evolving Management of Localized and Advanced Disease
Localized disease
Surgery with curative intent is the standard of care in patients with localized disease (Figure).14,15 Contraindications for resection include distant metastases and occlusion of blood vessels.4 Depending on tumor stage, eligible patients may undergo radical cholecystectomy and portal lymphadenectomy, as well as potential liver resection (segments 4b and 5).5
Figure. Biliary Tract Cancers (BTCs): Diagnosis and Management Algorithm14
From Lamarca A, Edeline J, Goyal L. How I treat biliary tract cancer. ESMO Open. 2022;7(1):100378. doi:10.1016/j.esmoop.2021.100378. [Open access].
As the nonencapsulated nature of the gallbladder renders local extension very likely, a peri-adjuvant approach including neoadjuvant and adjuvant arms should be initiated. Standard chemotherapy regimens may include capecitabine or gemcitabine + cisplatin/capecitabine.16,17
Advanced disease
Systemic therapy remains key in the setting of locally advanced or metastatic disease. In August 2024, the National Comprehensive Cancer Network (NCCN) updated its guidelines to strongly recommend durvalumab + gemcitabine + cisplatin or pembrolizumab + gemcitabine + cisplatin as the preferred regimens for primary treatment of these patients.17 Other regimens to consider based on both NCCN and European Society of Medical Oncology (ESMO) guidelines include gemcitabine + cisplatin or capecitabine + oxaliplatin.17,18 In addition, gemcitabine + S-1 (tegafur, gimeracil, and oteracil) may also be considered as part of first-line treatment based on data from clinical trials conducted in Japan.19 FOLFOX (folinic acid, fluorouracil, and oxaliplatin) is recommended for second-line treatment.16
While the American Society of Clinical Oncology (ASCO) guidelines are yet to be published, they have previously reviewed data on several potential novel agents and targeted therapies for first-line treatment.20
The addition of immunotherapies such as checkpoint inhibitors to the treatment algorithm has been monumental for the treatment of advanced gallbladder cancer. Durvalumab and pembrolizumab, programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PDL-1) receptor inhibitors, in combination with gemcitabine + cisplatin, significantly improve overall survival compared to gemcitabine + cisplatin alone.17,21,22 These regimens are strongly recommended as first-line therapy in eligible patients who have not previously been treated with a checkpoint inhibitor.
Biopsies should be performed as early as possible in all patients with unresectable or metastatic disease for genomic profiling. Next-generation sequencing can help inform response to targeted therapies in testing by identifying genetic mutations, potentially improving treatment response.1,23
In certain circumstances, patients with genetic mutations are eligible for molecularly targeted therapies17:
Unresectable or metastatic disease:
- Neurotrophic tyrosine receptor kinase (NTRK) gene fusion-positive tumors: entrectinib, larotrectinib, or repotrectinib
- High mutational burden (TMB-H) tumors: nivolumab + ipilimumab
Following disease progression:
- B-Raf Proto-Oncogene, Serine/Threonine Kinase (BRAF) V600E-mutated tumors: dabrafenib + trametinib
- Cholangiocarcinoma with fibroblast growth factor receptor 2 (FGFR2) fusions: futibatinib + pemigatinib
- Cholangiocarcinoma with rearrangements or isocitrate dehydrogenase 1 (IDH1) mutations: ivosidenib
It is important to note that further development of adjuvant strategies is greatly needed to better guide management across disease stages.16
Therapeutic candidates in testing
Despite the advancements achieved with immunotherapies and targeted treatments, therapeutic options have remained comparable to those of other biliary tumors such as intrahepatic cholangiocarcinoma. However, some novel candidates currently being evaluated in clinical trials have shown promise24-26:
HER2: Overexpression of the HER2 protein in gallbladder cancer causes abnormal cell survival and proliferation. Initial clinical trial data have suggested that agents targeting HER2 may improve outcomes in patients with advanced gallbladder cancer who harbor somatic HER2 mutations. In fact, the anti-HER2 agent zanidatamab provided clinical benefit and was well tolerated in patients with treatment-refractory, HER2-positive biliary tract cancer in a phase 2 single-arm trial.
Vascular endothelial growth factor (VEGF): The VEGF/ VEGF receptor pathway may also be a promising target due to its role in regulating epithelial cell differentiation and migration. Phase 2 studies of VEGF antibodies, such as bevacizumab, in combination with standard chemotherapy have demonstrated improved response rates; however, some of these studies have shown mixed results.
Phosphoinositide 3-kinases (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR): This key signaling pathway plays an important role in driving cancer growth and metastases. Early trials of an mTOR inhibitor in combination with standard chemotherapy have demonstrated an acceptable tolerability profile with potential signs of clinical benefit.
The immunotherapy landscape for gallbladder cancer may evolve beyond currently approved PD-1/PDL-1 receptor inhibitors with the development of agonist antibodies and chimeric antigen receptor T cell (CAR-T) candidates.27 Novel treatment approaches like vaccines and nanoparticle delivery systems are also under investigation.
Looking Toward the Future
Gallbladder cancer is challenging to detect, and earlier diagnosis is key to improving outcomes. It is critical to refer patients to specialized treatment centers as soon as the disease is suspected. Rapid development in advanced genetic testing and other analytical methods may lead to identification of diagnostic biomarkers to aid in detecting cases sooner.24
Despite the fast-evolving pipeline for therapeutic candidates, greater research is also needed to inform sequencing of chemotherapy regimens with immunotherapy and targeted therapy to achieve favorable long-term outcomes.27 As new candidates are approved, management may become remain less than ideal without this crucial guidance.
We hope the future will bring the opportunity to provide more tailored treatments to patients with novel candidates that can further engage the immune system beyond currently identified targets.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Okumura K, Gogna S, Gachabayov M, et al. Gallbladder cancer: historical treatment and new management options. World J Gastrointest Oncol. 2021;13(10):1317-1335. doi:10.4251/wjgo.v13.i10.1317
- Key statistics for gallbladder cancer. American Cancer Society. Updated May 22, 2024. Accessed August 26, 2024. https://www.cancer.org/cancer/types/gallbladder-cancer/about/key-statistics.html
- Nemunaitis JM, Brown-Glabeman U, Soares H, et al. Gallbladder cancer: review of a rare orphan gastrointestinal cancer with a focus on populations of New Mexico. BMC Cancer. 2018;18(1):665. doi:10.1186/s12885-018-4575-3
- Halaseh SA, Halaseh S, Shakman R. A review of the etiology and epidemiology of gallbladder cancer: what you need to know. Cureus. 2022;14(8):e28260. doi:10.7759/cureus.28260
- Menon G, Babiker HM. Gallbladder carcinoma. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Updated August 17, 2024. Accessed September 30, 2024. https://www.ncbi.nlm.nih.gov/books/NBK442002/
- Mhatre S, Rajaraman P, Chatterjee N, et al. Mustard oil consumption, cooking method, diet and gallbladder cancer risk in high- and low-risk regions of India. Int J Cancer. 2020;147(6):1621-1628. doi:10.1002/ijc.32952
- Sharma A, Sharma KL, Gupta A, Yadav A, Kumar A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: recent update. World J Gastroenterol. 2017;23(22):3978-3998. doi:10.3748/wjg.v23.i22.3978
- Kuipers H, de Bitter TJJ, de Boer MT, et al. Gallbladder cancer: current insights in genetic alterations and their possible therapeutic implications. Cancers (Basel). 2021;13(21):5257. doi:10.3390/cancers13215257
- Larson VA, Tang O, Ständer S, Kang S, Kwatra SG. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80(4):931-937. doi:10.1016/j.jaad.2018.08.044
- Neculoiu D, Neculoiu LC, Popa RM, Manea RM. The many hidden faces of gallbladder carcinoma on CT and MRI imaging—from A to Z. Diagnostics (Basel). 2024;14(5):475. doi:10.3390/diagnostics14050475
- Deo KB, Avudaiappan M, Shenvi S, et al. Misdiagnosis of carcinoma gallbladder in endemic regions. BMC Surg. 2022;22(1):343. doi:10.1186/s12893-022-01793-8
- Prieto M, Gastaca M, Ruiz P, et al. Long term recurrence free survival in a stage IV gallbladder cancer treated with chemotherapy plus trastuzumab and salvage liver resection. Ann Hepatobiliary Pancreat Surg. 2019;23(4):403-407. doi:10.14701/ahbps.2019.23.4.403
- van Dooren M, de Savornin Lohman EAJ, van der Post RS, et al. Referral rate of patients with incidental gallbladder cancer and survival: outcomes of a multicentre retrospective study. BJS Open. 2024;8(2):zrae013. doi:10.1093/bjsopen/zrae013
- Lamarca A, Edeline J, Goyal L. How I treat biliary tract cancer. ESMO Open. 2022;7(1):100378. doi:10.1016/j.esmoop.2021.100378
- Zhou Y, Yuan K, Yang Y, et al. Gallbladder cancer: current and future treatment options. Front Pharmacol. 2023;14:1183619. doi:10.3389/fphar.2023.1183619
- Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557-588. doi:10.1038/s41575-020-0310-z
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: biliary tract cancers. Version 4.2024. August 29, 2024. Accessed September 30, 2024. https://www.nccn.org/professionals/physician_gls/pdf/btc.pdf
- Vogel A, Bridgewater J, Edeline J, et al. Biliary tract cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(2):127-140. doi:10.1016/j.annonc.2022.10.506
- Nagino M, Hirano S, Yoshitomi H, et al. Clinical practice guidelines for the management of biliary tract cancers 2019: the 3rd English edition. J Hepatobiliary Pancreat Sci. 2021;28(1):26-54. doi:10.1002/jhbp.870
- Müller BG, De Aretxabala X, González Domingo M. A review of recent data in the treatment of gallbladder cancer: what we know, what we do, and what should be done. Am Soc Clin Oncol Educ Book. 2014;e165-e170. doi:10.14694/EdBook_AM.2014.34.e165
- Kelley RK, Ueno M, Yoo C, et al; for the KEYNOTE-966 investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10391):1853-1865. doi:10.1016/S0140-6736(23)00727-4
- Oh DY, Ruth He A, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8):EVIDoa2200015. doi:10.1056/EVIDoa2200015
- DiPeri TP, Javle MM, Meric-Bernstam F. Next generation sequencing for biliary tract cancers. Expert Rev Gastroenterol Hepatol. 2021;15(5):471-474. doi:10.1080/17474124.2021.1896967
- Song X, Hu Y, Li Y, Shao R, Liu F, Liu Y. Overview of current targeted therapy in gallbladder cancer. Signal Transduct Target Ther. 2020;5(1):230. doi:10.1038/s41392-020-00324-2
- LaPelusa M, Heumann T, Goff L, Agarwal R. Targeted therapies in advanced biliary tract cancers—a narrative review. Chin Clin Oncol. 2023;12(2):14. doi:10.21037/cco-22-93
- Harding JJ, Fan J, Oh DY, et al; for the HERIZON-BTC-01 study group. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol. 2023;24(7):772-782. doi:10.1016/S1470-2045(23)00242-5
- Lo JH, Agarwal R, Goff LW, Heumann TR. Immunotherapy in biliary tract cancers: current standard-of-care and emerging strategies. Cancers (Basel). 2023;15(13):3312. doi:10.3390/cancers15133312
Clinical outcomes of patients with gallbladder cancer have improved considerably with the advent of immunotherapy and targeted therapies. While specialists have gained tremendous insights into the disease over the last 10 years, significant knowledge gaps remain, as relapse rates remain high. Early referral to specialized treatment centers and timely molecular profiling can help guide therapeutic regimen choice and potentially improve patient outcomes.
Insights Into Disease Prevalence and Development
Gallbladder cancer is a rare malignancy with an aggressive course. Most gallbladder cancers are of epithelial origin, with adenocarcinoma being the most common type.1 Approximately 12,350 new cases of gallbladder cancer and nearby large bile duct cancers are anticipated in 2024 in the United States,2 predominantly affecting Southwestern Native Americans.3 The prevalence of gallbladder cancer varies greatly worldwide; rates are highest in South America (mainly in Chile) and Southeast Asia, including Eastern India.3,4
Multiple factors, including environment and genetics, contribute to the development of gallbladder cancer, which is driven primarily by chronic inflammation.5 While there are no defined risk factors, this malignancy is mostly associated with female sex, chronic gallbladder infections, and gallstones.4 Some evidence also suggests a dietary association with consuming mustard seed oil.6 Exposure to certain environmental toxins or heavy metals may also contribute to disease risk.1,4
Several genetic alterations have been identified in patients with gallbladder cancer that may be related to disease etiology; these include somatic mutations in the human epidermal growth factor receptor 2 (HER2), Kirsten rat sarcoma viral oncogene homolog (KRAS), and tumor protein p53 (TP53) genes, and many others.7,8 In addition to somatic mutations, gene overexpression, epigenetic changes, and microRNA-associated changes have also been linked to the disease.3
Challenges in Uncovering a “Hidden” Disease
While most gallbladder cancers are usually detected incidentally, patients may present with symptoms of abdominal pain, discomfort, and biliary obstruction–related symptoms like jaundice, itching, and dark urine.3,9,10 Cases may initially be misdiagnosed as inflammatory conditions such as cholecystitis or gallbladder stones; as a result, patients may be rushed into an inappropriate or incorrect surgical intervention.11 For these reasons, as well as the tight anatomical location of the gallbladder, cases are often not detected until advanced-stage disease.3,4
Patients diagnosed in stage 4 with distant metastases have an expected survival rate of less than 1 year,12 and low referral rates are associated with poor outcomes.13 Patients with suspected disease should therefore be referred to a specialized treatment center as soon as possible to confirm a diagnosis and initiate appropriate treatment. Core biopsies can provide histological confirmation, where feasible and safe, followed by imaging to determine extent of the disease.14
Evolving Management of Localized and Advanced Disease
Localized disease
Surgery with curative intent is the standard of care in patients with localized disease (Figure).14,15 Contraindications for resection include distant metastases and occlusion of blood vessels.4 Depending on tumor stage, eligible patients may undergo radical cholecystectomy and portal lymphadenectomy, as well as potential liver resection (segments 4b and 5).5
Figure. Biliary Tract Cancers (BTCs): Diagnosis and Management Algorithm14

From Lamarca A, Edeline J, Goyal L. How I treat biliary tract cancer. ESMO Open. 2022;7(1):100378. doi:10.1016/j.esmoop.2021.100378. [Open access].
As the nonencapsulated nature of the gallbladder renders local extension very likely, a peri-adjuvant approach including neoadjuvant and adjuvant arms should be initiated. Standard chemotherapy regimens may include capecitabine or gemcitabine + cisplatin/capecitabine.16,17
Advanced disease
Systemic therapy remains key in the setting of locally advanced or metastatic disease. In August 2024, the National Comprehensive Cancer Network (NCCN) updated its guidelines to strongly recommend durvalumab + gemcitabine + cisplatin or pembrolizumab + gemcitabine + cisplatin as the preferred regimens for primary treatment of these patients.17 Other regimens to consider based on both NCCN and European Society of Medical Oncology (ESMO) guidelines include gemcitabine + cisplatin or capecitabine + oxaliplatin.17,18 In addition, gemcitabine + S-1 (tegafur, gimeracil, and oteracil) may also be considered as part of first-line treatment based on data from clinical trials conducted in Japan.19 FOLFOX (folinic acid, fluorouracil, and oxaliplatin) is recommended for second-line treatment.16
While the American Society of Clinical Oncology (ASCO) guidelines are yet to be published, they have previously reviewed data on several potential novel agents and targeted therapies for first-line treatment.20
The addition of immunotherapies such as checkpoint inhibitors to the treatment algorithm has been monumental for the treatment of advanced gallbladder cancer. Durvalumab and pembrolizumab, programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PDL-1) receptor inhibitors, in combination with gemcitabine + cisplatin, significantly improve overall survival compared to gemcitabine + cisplatin alone.17,21,22 These regimens are strongly recommended as first-line therapy in eligible patients who have not previously been treated with a checkpoint inhibitor.
Biopsies should be performed as early as possible in all patients with unresectable or metastatic disease for genomic profiling. Next-generation sequencing can help inform response to targeted therapies in testing by identifying genetic mutations, potentially improving treatment response.1,23
In certain circumstances, patients with genetic mutations are eligible for molecularly targeted therapies17:
Unresectable or metastatic disease:
- Neurotrophic tyrosine receptor kinase (NTRK) gene fusion-positive tumors: entrectinib, larotrectinib, or repotrectinib
- High mutational burden (TMB-H) tumors: nivolumab + ipilimumab
Following disease progression:
- B-Raf Proto-Oncogene, Serine/Threonine Kinase (BRAF) V600E-mutated tumors: dabrafenib + trametinib
- Cholangiocarcinoma with fibroblast growth factor receptor 2 (FGFR2) fusions: futibatinib + pemigatinib
- Cholangiocarcinoma with rearrangements or isocitrate dehydrogenase 1 (IDH1) mutations: ivosidenib
It is important to note that further development of adjuvant strategies is greatly needed to better guide management across disease stages.16
Therapeutic candidates in testing
Despite the advancements achieved with immunotherapies and targeted treatments, therapeutic options have remained comparable to those of other biliary tumors such as intrahepatic cholangiocarcinoma. However, some novel candidates currently being evaluated in clinical trials have shown promise24-26:
HER2: Overexpression of the HER2 protein in gallbladder cancer causes abnormal cell survival and proliferation. Initial clinical trial data have suggested that agents targeting HER2 may improve outcomes in patients with advanced gallbladder cancer who harbor somatic HER2 mutations. In fact, the anti-HER2 agent zanidatamab provided clinical benefit and was well tolerated in patients with treatment-refractory, HER2-positive biliary tract cancer in a phase 2 single-arm trial.
Vascular endothelial growth factor (VEGF): The VEGF/ VEGF receptor pathway may also be a promising target due to its role in regulating epithelial cell differentiation and migration. Phase 2 studies of VEGF antibodies, such as bevacizumab, in combination with standard chemotherapy have demonstrated improved response rates; however, some of these studies have shown mixed results.
Phosphoinositide 3-kinases (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR): This key signaling pathway plays an important role in driving cancer growth and metastases. Early trials of an mTOR inhibitor in combination with standard chemotherapy have demonstrated an acceptable tolerability profile with potential signs of clinical benefit.
The immunotherapy landscape for gallbladder cancer may evolve beyond currently approved PD-1/PDL-1 receptor inhibitors with the development of agonist antibodies and chimeric antigen receptor T cell (CAR-T) candidates.27 Novel treatment approaches like vaccines and nanoparticle delivery systems are also under investigation.
Looking Toward the Future
Gallbladder cancer is challenging to detect, and earlier diagnosis is key to improving outcomes. It is critical to refer patients to specialized treatment centers as soon as the disease is suspected. Rapid development in advanced genetic testing and other analytical methods may lead to identification of diagnostic biomarkers to aid in detecting cases sooner.24
Despite the fast-evolving pipeline for therapeutic candidates, greater research is also needed to inform sequencing of chemotherapy regimens with immunotherapy and targeted therapy to achieve favorable long-term outcomes.27 As new candidates are approved, management may become remain less than ideal without this crucial guidance.
We hope the future will bring the opportunity to provide more tailored treatments to patients with novel candidates that can further engage the immune system beyond currently identified targets.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
Clinical outcomes of patients with gallbladder cancer have improved considerably with the advent of immunotherapy and targeted therapies. While specialists have gained tremendous insights into the disease over the last 10 years, significant knowledge gaps remain, as relapse rates remain high. Early referral to specialized treatment centers and timely molecular profiling can help guide therapeutic regimen choice and potentially improve patient outcomes.
Insights Into Disease Prevalence and Development
Gallbladder cancer is a rare malignancy with an aggressive course. Most gallbladder cancers are of epithelial origin, with adenocarcinoma being the most common type.1 Approximately 12,350 new cases of gallbladder cancer and nearby large bile duct cancers are anticipated in 2024 in the United States,2 predominantly affecting Southwestern Native Americans.3 The prevalence of gallbladder cancer varies greatly worldwide; rates are highest in South America (mainly in Chile) and Southeast Asia, including Eastern India.3,4
Multiple factors, including environment and genetics, contribute to the development of gallbladder cancer, which is driven primarily by chronic inflammation.5 While there are no defined risk factors, this malignancy is mostly associated with female sex, chronic gallbladder infections, and gallstones.4 Some evidence also suggests a dietary association with consuming mustard seed oil.6 Exposure to certain environmental toxins or heavy metals may also contribute to disease risk.1,4
Several genetic alterations have been identified in patients with gallbladder cancer that may be related to disease etiology; these include somatic mutations in the human epidermal growth factor receptor 2 (HER2), Kirsten rat sarcoma viral oncogene homolog (KRAS), and tumor protein p53 (TP53) genes, and many others.7,8 In addition to somatic mutations, gene overexpression, epigenetic changes, and microRNA-associated changes have also been linked to the disease.3
Challenges in Uncovering a “Hidden” Disease
While most gallbladder cancers are usually detected incidentally, patients may present with symptoms of abdominal pain, discomfort, and biliary obstruction–related symptoms like jaundice, itching, and dark urine.3,9,10 Cases may initially be misdiagnosed as inflammatory conditions such as cholecystitis or gallbladder stones; as a result, patients may be rushed into an inappropriate or incorrect surgical intervention.11 For these reasons, as well as the tight anatomical location of the gallbladder, cases are often not detected until advanced-stage disease.3,4
Patients diagnosed in stage 4 with distant metastases have an expected survival rate of less than 1 year,12 and low referral rates are associated with poor outcomes.13 Patients with suspected disease should therefore be referred to a specialized treatment center as soon as possible to confirm a diagnosis and initiate appropriate treatment. Core biopsies can provide histological confirmation, where feasible and safe, followed by imaging to determine extent of the disease.14
Evolving Management of Localized and Advanced Disease
Localized disease
Surgery with curative intent is the standard of care in patients with localized disease (Figure).14,15 Contraindications for resection include distant metastases and occlusion of blood vessels.4 Depending on tumor stage, eligible patients may undergo radical cholecystectomy and portal lymphadenectomy, as well as potential liver resection (segments 4b and 5).5
Figure. Biliary Tract Cancers (BTCs): Diagnosis and Management Algorithm14

From Lamarca A, Edeline J, Goyal L. How I treat biliary tract cancer. ESMO Open. 2022;7(1):100378. doi:10.1016/j.esmoop.2021.100378. [Open access].
As the nonencapsulated nature of the gallbladder renders local extension very likely, a peri-adjuvant approach including neoadjuvant and adjuvant arms should be initiated. Standard chemotherapy regimens may include capecitabine or gemcitabine + cisplatin/capecitabine.16,17
Advanced disease
Systemic therapy remains key in the setting of locally advanced or metastatic disease. In August 2024, the National Comprehensive Cancer Network (NCCN) updated its guidelines to strongly recommend durvalumab + gemcitabine + cisplatin or pembrolizumab + gemcitabine + cisplatin as the preferred regimens for primary treatment of these patients.17 Other regimens to consider based on both NCCN and European Society of Medical Oncology (ESMO) guidelines include gemcitabine + cisplatin or capecitabine + oxaliplatin.17,18 In addition, gemcitabine + S-1 (tegafur, gimeracil, and oteracil) may also be considered as part of first-line treatment based on data from clinical trials conducted in Japan.19 FOLFOX (folinic acid, fluorouracil, and oxaliplatin) is recommended for second-line treatment.16
While the American Society of Clinical Oncology (ASCO) guidelines are yet to be published, they have previously reviewed data on several potential novel agents and targeted therapies for first-line treatment.20
The addition of immunotherapies such as checkpoint inhibitors to the treatment algorithm has been monumental for the treatment of advanced gallbladder cancer. Durvalumab and pembrolizumab, programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PDL-1) receptor inhibitors, in combination with gemcitabine + cisplatin, significantly improve overall survival compared to gemcitabine + cisplatin alone.17,21,22 These regimens are strongly recommended as first-line therapy in eligible patients who have not previously been treated with a checkpoint inhibitor.
Biopsies should be performed as early as possible in all patients with unresectable or metastatic disease for genomic profiling. Next-generation sequencing can help inform response to targeted therapies in testing by identifying genetic mutations, potentially improving treatment response.1,23
In certain circumstances, patients with genetic mutations are eligible for molecularly targeted therapies17:
Unresectable or metastatic disease:
- Neurotrophic tyrosine receptor kinase (NTRK) gene fusion-positive tumors: entrectinib, larotrectinib, or repotrectinib
- High mutational burden (TMB-H) tumors: nivolumab + ipilimumab
Following disease progression:
- B-Raf Proto-Oncogene, Serine/Threonine Kinase (BRAF) V600E-mutated tumors: dabrafenib + trametinib
- Cholangiocarcinoma with fibroblast growth factor receptor 2 (FGFR2) fusions: futibatinib + pemigatinib
- Cholangiocarcinoma with rearrangements or isocitrate dehydrogenase 1 (IDH1) mutations: ivosidenib
It is important to note that further development of adjuvant strategies is greatly needed to better guide management across disease stages.16
Therapeutic candidates in testing
Despite the advancements achieved with immunotherapies and targeted treatments, therapeutic options have remained comparable to those of other biliary tumors such as intrahepatic cholangiocarcinoma. However, some novel candidates currently being evaluated in clinical trials have shown promise24-26:
HER2: Overexpression of the HER2 protein in gallbladder cancer causes abnormal cell survival and proliferation. Initial clinical trial data have suggested that agents targeting HER2 may improve outcomes in patients with advanced gallbladder cancer who harbor somatic HER2 mutations. In fact, the anti-HER2 agent zanidatamab provided clinical benefit and was well tolerated in patients with treatment-refractory, HER2-positive biliary tract cancer in a phase 2 single-arm trial.
Vascular endothelial growth factor (VEGF): The VEGF/ VEGF receptor pathway may also be a promising target due to its role in regulating epithelial cell differentiation and migration. Phase 2 studies of VEGF antibodies, such as bevacizumab, in combination with standard chemotherapy have demonstrated improved response rates; however, some of these studies have shown mixed results.
Phosphoinositide 3-kinases (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR): This key signaling pathway plays an important role in driving cancer growth and metastases. Early trials of an mTOR inhibitor in combination with standard chemotherapy have demonstrated an acceptable tolerability profile with potential signs of clinical benefit.
The immunotherapy landscape for gallbladder cancer may evolve beyond currently approved PD-1/PDL-1 receptor inhibitors with the development of agonist antibodies and chimeric antigen receptor T cell (CAR-T) candidates.27 Novel treatment approaches like vaccines and nanoparticle delivery systems are also under investigation.
Looking Toward the Future
Gallbladder cancer is challenging to detect, and earlier diagnosis is key to improving outcomes. It is critical to refer patients to specialized treatment centers as soon as the disease is suspected. Rapid development in advanced genetic testing and other analytical methods may lead to identification of diagnostic biomarkers to aid in detecting cases sooner.24
Despite the fast-evolving pipeline for therapeutic candidates, greater research is also needed to inform sequencing of chemotherapy regimens with immunotherapy and targeted therapy to achieve favorable long-term outcomes.27 As new candidates are approved, management may become remain less than ideal without this crucial guidance.
We hope the future will bring the opportunity to provide more tailored treatments to patients with novel candidates that can further engage the immune system beyond currently identified targets.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Okumura K, Gogna S, Gachabayov M, et al. Gallbladder cancer: historical treatment and new management options. World J Gastrointest Oncol. 2021;13(10):1317-1335. doi:10.4251/wjgo.v13.i10.1317
- Key statistics for gallbladder cancer. American Cancer Society. Updated May 22, 2024. Accessed August 26, 2024. https://www.cancer.org/cancer/types/gallbladder-cancer/about/key-statistics.html
- Nemunaitis JM, Brown-Glabeman U, Soares H, et al. Gallbladder cancer: review of a rare orphan gastrointestinal cancer with a focus on populations of New Mexico. BMC Cancer. 2018;18(1):665. doi:10.1186/s12885-018-4575-3
- Halaseh SA, Halaseh S, Shakman R. A review of the etiology and epidemiology of gallbladder cancer: what you need to know. Cureus. 2022;14(8):e28260. doi:10.7759/cureus.28260
- Menon G, Babiker HM. Gallbladder carcinoma. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Updated August 17, 2024. Accessed September 30, 2024. https://www.ncbi.nlm.nih.gov/books/NBK442002/
- Mhatre S, Rajaraman P, Chatterjee N, et al. Mustard oil consumption, cooking method, diet and gallbladder cancer risk in high- and low-risk regions of India. Int J Cancer. 2020;147(6):1621-1628. doi:10.1002/ijc.32952
- Sharma A, Sharma KL, Gupta A, Yadav A, Kumar A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: recent update. World J Gastroenterol. 2017;23(22):3978-3998. doi:10.3748/wjg.v23.i22.3978
- Kuipers H, de Bitter TJJ, de Boer MT, et al. Gallbladder cancer: current insights in genetic alterations and their possible therapeutic implications. Cancers (Basel). 2021;13(21):5257. doi:10.3390/cancers13215257
- Larson VA, Tang O, Ständer S, Kang S, Kwatra SG. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80(4):931-937. doi:10.1016/j.jaad.2018.08.044
- Neculoiu D, Neculoiu LC, Popa RM, Manea RM. The many hidden faces of gallbladder carcinoma on CT and MRI imaging—from A to Z. Diagnostics (Basel). 2024;14(5):475. doi:10.3390/diagnostics14050475
- Deo KB, Avudaiappan M, Shenvi S, et al. Misdiagnosis of carcinoma gallbladder in endemic regions. BMC Surg. 2022;22(1):343. doi:10.1186/s12893-022-01793-8
- Prieto M, Gastaca M, Ruiz P, et al. Long term recurrence free survival in a stage IV gallbladder cancer treated with chemotherapy plus trastuzumab and salvage liver resection. Ann Hepatobiliary Pancreat Surg. 2019;23(4):403-407. doi:10.14701/ahbps.2019.23.4.403
- van Dooren M, de Savornin Lohman EAJ, van der Post RS, et al. Referral rate of patients with incidental gallbladder cancer and survival: outcomes of a multicentre retrospective study. BJS Open. 2024;8(2):zrae013. doi:10.1093/bjsopen/zrae013
- Lamarca A, Edeline J, Goyal L. How I treat biliary tract cancer. ESMO Open. 2022;7(1):100378. doi:10.1016/j.esmoop.2021.100378
- Zhou Y, Yuan K, Yang Y, et al. Gallbladder cancer: current and future treatment options. Front Pharmacol. 2023;14:1183619. doi:10.3389/fphar.2023.1183619
- Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557-588. doi:10.1038/s41575-020-0310-z
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: biliary tract cancers. Version 4.2024. August 29, 2024. Accessed September 30, 2024. https://www.nccn.org/professionals/physician_gls/pdf/btc.pdf
- Vogel A, Bridgewater J, Edeline J, et al. Biliary tract cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(2):127-140. doi:10.1016/j.annonc.2022.10.506
- Nagino M, Hirano S, Yoshitomi H, et al. Clinical practice guidelines for the management of biliary tract cancers 2019: the 3rd English edition. J Hepatobiliary Pancreat Sci. 2021;28(1):26-54. doi:10.1002/jhbp.870
- Müller BG, De Aretxabala X, González Domingo M. A review of recent data in the treatment of gallbladder cancer: what we know, what we do, and what should be done. Am Soc Clin Oncol Educ Book. 2014;e165-e170. doi:10.14694/EdBook_AM.2014.34.e165
- Kelley RK, Ueno M, Yoo C, et al; for the KEYNOTE-966 investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10391):1853-1865. doi:10.1016/S0140-6736(23)00727-4
- Oh DY, Ruth He A, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8):EVIDoa2200015. doi:10.1056/EVIDoa2200015
- DiPeri TP, Javle MM, Meric-Bernstam F. Next generation sequencing for biliary tract cancers. Expert Rev Gastroenterol Hepatol. 2021;15(5):471-474. doi:10.1080/17474124.2021.1896967
- Song X, Hu Y, Li Y, Shao R, Liu F, Liu Y. Overview of current targeted therapy in gallbladder cancer. Signal Transduct Target Ther. 2020;5(1):230. doi:10.1038/s41392-020-00324-2
- LaPelusa M, Heumann T, Goff L, Agarwal R. Targeted therapies in advanced biliary tract cancers—a narrative review. Chin Clin Oncol. 2023;12(2):14. doi:10.21037/cco-22-93
- Harding JJ, Fan J, Oh DY, et al; for the HERIZON-BTC-01 study group. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol. 2023;24(7):772-782. doi:10.1016/S1470-2045(23)00242-5
- Lo JH, Agarwal R, Goff LW, Heumann TR. Immunotherapy in biliary tract cancers: current standard-of-care and emerging strategies. Cancers (Basel). 2023;15(13):3312. doi:10.3390/cancers15133312
- Okumura K, Gogna S, Gachabayov M, et al. Gallbladder cancer: historical treatment and new management options. World J Gastrointest Oncol. 2021;13(10):1317-1335. doi:10.4251/wjgo.v13.i10.1317
- Key statistics for gallbladder cancer. American Cancer Society. Updated May 22, 2024. Accessed August 26, 2024. https://www.cancer.org/cancer/types/gallbladder-cancer/about/key-statistics.html
- Nemunaitis JM, Brown-Glabeman U, Soares H, et al. Gallbladder cancer: review of a rare orphan gastrointestinal cancer with a focus on populations of New Mexico. BMC Cancer. 2018;18(1):665. doi:10.1186/s12885-018-4575-3
- Halaseh SA, Halaseh S, Shakman R. A review of the etiology and epidemiology of gallbladder cancer: what you need to know. Cureus. 2022;14(8):e28260. doi:10.7759/cureus.28260
- Menon G, Babiker HM. Gallbladder carcinoma. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Updated August 17, 2024. Accessed September 30, 2024. https://www.ncbi.nlm.nih.gov/books/NBK442002/
- Mhatre S, Rajaraman P, Chatterjee N, et al. Mustard oil consumption, cooking method, diet and gallbladder cancer risk in high- and low-risk regions of India. Int J Cancer. 2020;147(6):1621-1628. doi:10.1002/ijc.32952
- Sharma A, Sharma KL, Gupta A, Yadav A, Kumar A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: recent update. World J Gastroenterol. 2017;23(22):3978-3998. doi:10.3748/wjg.v23.i22.3978
- Kuipers H, de Bitter TJJ, de Boer MT, et al. Gallbladder cancer: current insights in genetic alterations and their possible therapeutic implications. Cancers (Basel). 2021;13(21):5257. doi:10.3390/cancers13215257
- Larson VA, Tang O, Ständer S, Kang S, Kwatra SG. Association between itch and cancer in 16,925 patients with pruritus: experience at a tertiary care center. J Am Acad Dermatol. 2019;80(4):931-937. doi:10.1016/j.jaad.2018.08.044
- Neculoiu D, Neculoiu LC, Popa RM, Manea RM. The many hidden faces of gallbladder carcinoma on CT and MRI imaging—from A to Z. Diagnostics (Basel). 2024;14(5):475. doi:10.3390/diagnostics14050475
- Deo KB, Avudaiappan M, Shenvi S, et al. Misdiagnosis of carcinoma gallbladder in endemic regions. BMC Surg. 2022;22(1):343. doi:10.1186/s12893-022-01793-8
- Prieto M, Gastaca M, Ruiz P, et al. Long term recurrence free survival in a stage IV gallbladder cancer treated with chemotherapy plus trastuzumab and salvage liver resection. Ann Hepatobiliary Pancreat Surg. 2019;23(4):403-407. doi:10.14701/ahbps.2019.23.4.403
- van Dooren M, de Savornin Lohman EAJ, van der Post RS, et al. Referral rate of patients with incidental gallbladder cancer and survival: outcomes of a multicentre retrospective study. BJS Open. 2024;8(2):zrae013. doi:10.1093/bjsopen/zrae013
- Lamarca A, Edeline J, Goyal L. How I treat biliary tract cancer. ESMO Open. 2022;7(1):100378. doi:10.1016/j.esmoop.2021.100378
- Zhou Y, Yuan K, Yang Y, et al. Gallbladder cancer: current and future treatment options. Front Pharmacol. 2023;14:1183619. doi:10.3389/fphar.2023.1183619
- Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557-588. doi:10.1038/s41575-020-0310-z
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: biliary tract cancers. Version 4.2024. August 29, 2024. Accessed September 30, 2024. https://www.nccn.org/professionals/physician_gls/pdf/btc.pdf
- Vogel A, Bridgewater J, Edeline J, et al. Biliary tract cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(2):127-140. doi:10.1016/j.annonc.2022.10.506
- Nagino M, Hirano S, Yoshitomi H, et al. Clinical practice guidelines for the management of biliary tract cancers 2019: the 3rd English edition. J Hepatobiliary Pancreat Sci. 2021;28(1):26-54. doi:10.1002/jhbp.870
- Müller BG, De Aretxabala X, González Domingo M. A review of recent data in the treatment of gallbladder cancer: what we know, what we do, and what should be done. Am Soc Clin Oncol Educ Book. 2014;e165-e170. doi:10.14694/EdBook_AM.2014.34.e165
- Kelley RK, Ueno M, Yoo C, et al; for the KEYNOTE-966 investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10391):1853-1865. doi:10.1016/S0140-6736(23)00727-4
- Oh DY, Ruth He A, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8):EVIDoa2200015. doi:10.1056/EVIDoa2200015
- DiPeri TP, Javle MM, Meric-Bernstam F. Next generation sequencing for biliary tract cancers. Expert Rev Gastroenterol Hepatol. 2021;15(5):471-474. doi:10.1080/17474124.2021.1896967
- Song X, Hu Y, Li Y, Shao R, Liu F, Liu Y. Overview of current targeted therapy in gallbladder cancer. Signal Transduct Target Ther. 2020;5(1):230. doi:10.1038/s41392-020-00324-2
- LaPelusa M, Heumann T, Goff L, Agarwal R. Targeted therapies in advanced biliary tract cancers—a narrative review. Chin Clin Oncol. 2023;12(2):14. doi:10.21037/cco-22-93
- Harding JJ, Fan J, Oh DY, et al; for the HERIZON-BTC-01 study group. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol. 2023;24(7):772-782. doi:10.1016/S1470-2045(23)00242-5
- Lo JH, Agarwal R, Goff LW, Heumann TR. Immunotherapy in biliary tract cancers: current standard-of-care and emerging strategies. Cancers (Basel). 2023;15(13):3312. doi:10.3390/cancers15133312
Current Management and Future Directions in the Treatment of Gallbladder Cancer
Current Management and Future Directions in the Treatment of Gallbladder Cancer
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
Introduction
Hepatoblastoma accounts for most pediatric liver cancers, but accounts for only 1% of all malignancies in children. Rates of hepatoblastoma have increased gradually over the past 20 years for unclear reasons, but it remains a rare malignancy. In the 1970s, only a small percentage of patients survived long-term. Today, 5-year survival rates range from 65% to over 90%, depending on risk factors, thanks to recent advancements in the understanding and treatment of hepatoblastoma.1-5 Improved risk stratification has led to better staging and more personalized treatment approaches. To further improve survival, current research is concentrated on improving outcomes in the most challenging patient subsets, such as those with metastatic disease and patients with disease relapse.
Background
Hepatoblastoma is typically diagnosed in the first 2 years of life.6 Accounting for more than 60% of pediatric hepatic malignancies worldwide, the incidence of hepatoblastoma is increasing. Results from a study evaluating the incidence between 2001 and 2017 showed a 2% annual increase documented in children aged from birth to 4 years in the United States, climbing to 5.8% annually among children aged 5 to 9 years.2 Risk factors for hepatoblastoma include maternal preeclampsia, premature birth, and parental smoking.7 The degree to which each of these factors plays a role is uncertain. A genetic etiology is suspected in a minority of hepatoblastoma cases, but it is associated with several genetic diseases, including Beckwith-Weidemann syndrome, familial adenomatous polyposis, and Prader-Willi syndrome.8 Genetic mutations in the Wnt signaling pathway that result in the accumulation of beta-catenin have also been found in sporadic, nonfamilial cases.9
Although this condition generally presents as a single abdominal mass in the right lobe of the liver, multifocal hepatoblastoma at diagnosis does occur.10 In most patients, alpha-fetoprotein (AFP) is significantly elevated.11 An estimated 20% of patients present with metastases, which are most commonly found in the lung.12 While ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) can be used to define the extent of the tumor in the liver, a chest CT is appropriate to look for metastases beyond the liver.13
Of the 2 broad histological categories commonly used to characterize hepatoblastoma, the more common epithelial form consists of fetal or embryonal liver cells. The mixed epithelial-mesenchymal form that accounts for 20% to 30% of hepatoblastomas features epithelial and primitive mesenchymal tissue, often with osteoid tissue or cartilage6; both have numerous histological subtypes. For example, the epithelial type can be further characterized by a well- or poorly-differentiated appearance, while the mixed type can be subdivided by the presence or absence of teratoid features.
Prior to 2017, there was considerable disparity in the way hepatoblastomas were characterized and staged among the major research consortiums. This issue was addressed when a consortium was established in which pediatric oncology groups pooled their data. The Children’s Hepatic tumors International Collaboration (CHIC) released the PRETEXT (PRETreatment EXTent of disease) approach.7,14 Based on comprehensive data from 1605 children participating in multicenter trials, the CHIC risk stratification defines and provides risk trees for very low-, low-, intermediate-, and high-risk groups. The most important predictors included AFP levels, patient age, extent of disease in the liver (particularly involving major hepatic veins), and the presence of metastases.
Further improvements to the diagnosis and staging of hepatoblastoma are credited to consensus-based recommendations for imaging that were created in the context of the PRETEXT staging system.13 While ultrasound is recommended for the initial approach to diagnosis, this consensus calls for MRI with hepatobiliary contrast to better characterize the lesion and detect satellite lesions. This form of imaging is also recommended for follow-up after treatment, but results should be interpreted in the context of biomarkers, such as AFP levels, pathologic grading, and tumor subtypes.
In patients with the most common familial disorders associated with a predisposition for hepatoblastoma, such as adenomatous polyposis, Beckwith-Weidemann spectrum, or trisomy 18, regular surveillance for hepatoblastoma is recommended during the early years of life.8 Characterization of the genetic and molecular features of patients who present with hepatoblastoma might be useful in determining prognosis. Of genetic features, mutations in the CTNNB1 gene are the most common, but several genes in the Wnt pathway are also linked to hepatoblastoma formation.9
Along with the progress in subtyping patients by genetics, epigenetics, and molecular features, there is a growing appreciation for the heterogeneity of hepatoblastoma and the likelihood that treatment strategies can be better individualized to improve outcomes in high-risk patients. This progress is expected to accelerate further when results from the results from the Pediatric Hepatic International Tumor Trial (PHITT) are published. These data are expected to be available in 2025, and may help with prognostication and understanding the biology of hepatoblastoma in relation to outcomes.
Treatment Strategies in Hepatoblastoma
For low-grade hepatoblastoma, the first-line therapy is surgery, which can be sufficient for cure without relapse in selected patients with PRETEXT group 1 disease. Although only 40% to 60% of patients have resectable disease at diagnosis,10 there are several strategies to shrink tumor bulk, particularly chemotherapy due to the relatively high sensitivity of hepatoblastoma to cytotoxic therapies. The intensity of chemotherapy is increased relative to risk.11 For example, cisplatin-based regimens are considered for low-risk patients, while additional therapies, such as doxorubicin, irinotecan, or both, are added in patients at higher risk. Cure is common if these regimens permit a margin-free resection, although relapse does occur in a subset of patients.
If adequate debulking of the tumor cannot be achieved with conventional surgery, liver transplantation is typically offered for patients without extrahepatic disease or after distant metastases have been successfully excised. With liver transplantation and combination therapies to inhibit relapse associated with seeding, long-term survival rates of 80% have been reported.3 Judicious use of transplantation in patients with high-risk disease that raises the potential for relapse has been credited with rates of long-term survival that exceed 80% in some series. However, there is concern of offering transplantation when it is not necessary. In patients who are high risk with multiple lesions in the liver, there is a general agreement that transplantation reduces the likelihood of subsequent relapse; however, as the precision of aggressive resection coupled with effective chemotherapy has improved, there are more patients in whom the optimal choice might not be debated by experts.
Review articles typically cite the likelihood of an overall 5-year survival in patients with hepatoblastoma as being on the order of 80%.1 This rate includes children with late-onset disease, which is generally associated with a worse prognosis, and patients who eventually experience disease relapse. Survival rates are now likely to be substantially higher, with progress developing better treatment protocols for both groups. In the absence of high-risk features, long-term survival rates of 90% or higher are now being reported in some centers with high relative volumes of hepatoblastoma, regardless of baseline risks.
PHITT
The rarity of hepatoblastoma poses a significant challenge to conducting prospective studies with sufficient sample sizes to evaluate the overall efficacy of treatments and their effectiveness in patient subgroups based on specific clinical characteristics and disease severity. PHITT is the first international collaborative liver tumors trial to use a consensus approach. Centers in Europe, Japan, and the United States are participating through regional cancer study consortia. The Cincinnati Children’s Hospital and Medical Center, a leader in hepatoblastoma management in the United States, is anchoring this effort for the Children’s Oncology Group.
In addition to assessing treatment strategies in larger patient cohorts, PHITT is expanding the data available to correlate outcomes across different stages and risk categories based on histological and biological classifications. Hepatoblastoma and hepatocellular carcinoma are being addressed in PHITT, but the design schema for these malignancies differs. For patients enrolled with hepatoblastoma, 4 risk groups have been defined, ranging from very low to high. Within these risk categories, flow charts provide guide selection of treatments based on clinical and disease features.
Cincinnati Children’s Hospital and Medical Center is one of the most active centers for the treatment of hepatoblastoma in the Unites States but manages only 15 to 20 cases of this rare disease per year. PHITT is expected to play a critical role in achieving a high level of valuable data, and the first sets of outcomes from this collaboration are anticipated to be available in early 2025. As the study progresses, meaningful data are expected for the most challenging and some of the rarest hepatoblastoma risk groups.
Summary
The rates of cure are now approaching 100% with surgery and chemotherapy in patients with localized or locally advanced hepatoblastoma. For more advanced, unresectable disease, liver transplantation is effective in most patients, providing high rates of long-term survival. For patients with relapsed disease, advanced treatment protocols at centers with high relative volumes
of hepatoblastoma are now regularly achieving a second remission—many of which are durable. Although prognosis is less favorable in patients who experience a second relapse, long-term survival is achieved even in a proportion of these children. Substantial rates of response and long-term survival have been common in hepatoblastoma diagnosed at early stages, but the recent progress in advanced hepatoblastoma is credited to more aggressive therapies based on a better understanding of the disease characteristics that allows for individualized therapy. There is hope that the larger pool of data becoming available in 2025 from PHITT will prove to be an additional source of information that guides further advances in managing this rare disease.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Koh KN, Namgoong JM, Yoon HM, et al. Recent improvement in survival outcomes and reappraisal of prognostic factors in hepatoblastoma. Cancer Med. 2021;10(10):3261-3273. doi:10.1002/cam4.3897
- Kahla JA, Siegel DA, Dai S, et al. Incidence and 5-year survival of children and adolescents with hepatoblastoma in the United States. Pediatr Blood Cancer. 2022;69(10):e29763. doi:10.1002/pbc.29763
- Ramos-Gonzalez G, LaQuaglia M, O’Neill AF, et al. Long-term outcomes of liver transplantation for hepatoblastoma: a single-center 14-year experience. Pediatr Transplant. 2018:e13250. doi:10.1111/petr.13250
- Zhou S, Malvar J, Chi YY, et al. Independent assessment of the Children’s Hepatic Tumors International Collaboration risk stratification for hepatoblastoma and the association of tumor histological characteristics with prognosis. JAMA Netw Open. 2022;5(2):e2148013. doi:10.1001/jamanetworkopen.2021.48013
- Feng J, Polychronidis G, Heger U, Frongia G, Mehrabi A, Hoffmann K. Incidence trends and survival prediction of hepatoblastoma in children: a population-based study. Cancer Commun (Lond). 2019;39(1):62. doi:10.1186/s40880-019-0411-7
- Sharma D, Subbarao G, Saxena R. Hepatoblastoma. Semin Diagn Pathol. 2017;34(2):192-200. doi:10.1053/j.semdp.2016.12.015
- Heck JE, Meyers TJ, Lombardi C, et al. Case-control study of birth characteristics and the risk of hepatoblastoma. Cancer Epidemiol. 2013;37(4):390-395. doi:10.1016/j.canep.2013.03.004
- Ranganathan S, Lopez-Terrada D, Alaggio R. Hepatoblastoma and pediatric hepatocellular carcinoma: an update. Pediatr Dev Pathol. 2020;23(2):79-95. doi:10.1177/1093526619875228
- Curia MC, Zuckermann M, De Lellis L, et al. Sporadic childhood hepatoblastomas show activation of beta-catenin, mismatch repair defects and p53 mutations. Mod Pathol. 2008;21(1):7-14. doi:10.1038/modpathol.3800977
- Fahy AS, Shaikh F, Gerstle JT. Multifocal hepatoblastoma: what is the risk of recurrent disease in the remnant liver? J Pediatr Surg. 2019;54(5):1035-1040. doi:10.1016/j.jpedsurg.2019.01.036
- Głowska-Ciemny J, Szymanski M, Kuszerska A, Rzepka R, von Kaisenberg CS, Kocyłowski R. Role of alpha-fetoprotein (AFP) in diagnosing childhood cancers and genetic-related chronic diseases. Cancers (Basel). 2023;15(17):4302. doi:10.3390/cancers15174302
- Angelico R, Grimaldi C, Gazia C, et al. How do synchronous lung metastases influence the surgical management of children with hepatoblastoma? An update and systematic review of the literature. Cancers (Basel). 2019;11(11):1693. doi:10.3390/cancers11111693
- Schooler GR, Infante JC, Acord M, et al. Imaging of pediatric liver tumors: A COG Diagnostic Imaging Committee/SPR Oncology Committee white paper. Pediatr Blood Cancer. 2023;70(suppl 4):e29965. doi:10.1002/pbc.29965
- Meyers RL, Maibach R, Hiyama E, et al. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol. 2017;18(1):122-131. doi:10.1016/S1470-2045(16)30598-8
Introduction
Hepatoblastoma accounts for most pediatric liver cancers, but accounts for only 1% of all malignancies in children. Rates of hepatoblastoma have increased gradually over the past 20 years for unclear reasons, but it remains a rare malignancy. In the 1970s, only a small percentage of patients survived long-term. Today, 5-year survival rates range from 65% to over 90%, depending on risk factors, thanks to recent advancements in the understanding and treatment of hepatoblastoma.1-5 Improved risk stratification has led to better staging and more personalized treatment approaches. To further improve survival, current research is concentrated on improving outcomes in the most challenging patient subsets, such as those with metastatic disease and patients with disease relapse.
Background
Hepatoblastoma is typically diagnosed in the first 2 years of life.6 Accounting for more than 60% of pediatric hepatic malignancies worldwide, the incidence of hepatoblastoma is increasing. Results from a study evaluating the incidence between 2001 and 2017 showed a 2% annual increase documented in children aged from birth to 4 years in the United States, climbing to 5.8% annually among children aged 5 to 9 years.2 Risk factors for hepatoblastoma include maternal preeclampsia, premature birth, and parental smoking.7 The degree to which each of these factors plays a role is uncertain. A genetic etiology is suspected in a minority of hepatoblastoma cases, but it is associated with several genetic diseases, including Beckwith-Weidemann syndrome, familial adenomatous polyposis, and Prader-Willi syndrome.8 Genetic mutations in the Wnt signaling pathway that result in the accumulation of beta-catenin have also been found in sporadic, nonfamilial cases.9
Although this condition generally presents as a single abdominal mass in the right lobe of the liver, multifocal hepatoblastoma at diagnosis does occur.10 In most patients, alpha-fetoprotein (AFP) is significantly elevated.11 An estimated 20% of patients present with metastases, which are most commonly found in the lung.12 While ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) can be used to define the extent of the tumor in the liver, a chest CT is appropriate to look for metastases beyond the liver.13
Of the 2 broad histological categories commonly used to characterize hepatoblastoma, the more common epithelial form consists of fetal or embryonal liver cells. The mixed epithelial-mesenchymal form that accounts for 20% to 30% of hepatoblastomas features epithelial and primitive mesenchymal tissue, often with osteoid tissue or cartilage6; both have numerous histological subtypes. For example, the epithelial type can be further characterized by a well- or poorly-differentiated appearance, while the mixed type can be subdivided by the presence or absence of teratoid features.
Prior to 2017, there was considerable disparity in the way hepatoblastomas were characterized and staged among the major research consortiums. This issue was addressed when a consortium was established in which pediatric oncology groups pooled their data. The Children’s Hepatic tumors International Collaboration (CHIC) released the PRETEXT (PRETreatment EXTent of disease) approach.7,14 Based on comprehensive data from 1605 children participating in multicenter trials, the CHIC risk stratification defines and provides risk trees for very low-, low-, intermediate-, and high-risk groups. The most important predictors included AFP levels, patient age, extent of disease in the liver (particularly involving major hepatic veins), and the presence of metastases.
Further improvements to the diagnosis and staging of hepatoblastoma are credited to consensus-based recommendations for imaging that were created in the context of the PRETEXT staging system.13 While ultrasound is recommended for the initial approach to diagnosis, this consensus calls for MRI with hepatobiliary contrast to better characterize the lesion and detect satellite lesions. This form of imaging is also recommended for follow-up after treatment, but results should be interpreted in the context of biomarkers, such as AFP levels, pathologic grading, and tumor subtypes.
In patients with the most common familial disorders associated with a predisposition for hepatoblastoma, such as adenomatous polyposis, Beckwith-Weidemann spectrum, or trisomy 18, regular surveillance for hepatoblastoma is recommended during the early years of life.8 Characterization of the genetic and molecular features of patients who present with hepatoblastoma might be useful in determining prognosis. Of genetic features, mutations in the CTNNB1 gene are the most common, but several genes in the Wnt pathway are also linked to hepatoblastoma formation.9
Along with the progress in subtyping patients by genetics, epigenetics, and molecular features, there is a growing appreciation for the heterogeneity of hepatoblastoma and the likelihood that treatment strategies can be better individualized to improve outcomes in high-risk patients. This progress is expected to accelerate further when results from the results from the Pediatric Hepatic International Tumor Trial (PHITT) are published. These data are expected to be available in 2025, and may help with prognostication and understanding the biology of hepatoblastoma in relation to outcomes.
Treatment Strategies in Hepatoblastoma
For low-grade hepatoblastoma, the first-line therapy is surgery, which can be sufficient for cure without relapse in selected patients with PRETEXT group 1 disease. Although only 40% to 60% of patients have resectable disease at diagnosis,10 there are several strategies to shrink tumor bulk, particularly chemotherapy due to the relatively high sensitivity of hepatoblastoma to cytotoxic therapies. The intensity of chemotherapy is increased relative to risk.11 For example, cisplatin-based regimens are considered for low-risk patients, while additional therapies, such as doxorubicin, irinotecan, or both, are added in patients at higher risk. Cure is common if these regimens permit a margin-free resection, although relapse does occur in a subset of patients.
If adequate debulking of the tumor cannot be achieved with conventional surgery, liver transplantation is typically offered for patients without extrahepatic disease or after distant metastases have been successfully excised. With liver transplantation and combination therapies to inhibit relapse associated with seeding, long-term survival rates of 80% have been reported.3 Judicious use of transplantation in patients with high-risk disease that raises the potential for relapse has been credited with rates of long-term survival that exceed 80% in some series. However, there is concern of offering transplantation when it is not necessary. In patients who are high risk with multiple lesions in the liver, there is a general agreement that transplantation reduces the likelihood of subsequent relapse; however, as the precision of aggressive resection coupled with effective chemotherapy has improved, there are more patients in whom the optimal choice might not be debated by experts.
Review articles typically cite the likelihood of an overall 5-year survival in patients with hepatoblastoma as being on the order of 80%.1 This rate includes children with late-onset disease, which is generally associated with a worse prognosis, and patients who eventually experience disease relapse. Survival rates are now likely to be substantially higher, with progress developing better treatment protocols for both groups. In the absence of high-risk features, long-term survival rates of 90% or higher are now being reported in some centers with high relative volumes of hepatoblastoma, regardless of baseline risks.
PHITT
The rarity of hepatoblastoma poses a significant challenge to conducting prospective studies with sufficient sample sizes to evaluate the overall efficacy of treatments and their effectiveness in patient subgroups based on specific clinical characteristics and disease severity. PHITT is the first international collaborative liver tumors trial to use a consensus approach. Centers in Europe, Japan, and the United States are participating through regional cancer study consortia. The Cincinnati Children’s Hospital and Medical Center, a leader in hepatoblastoma management in the United States, is anchoring this effort for the Children’s Oncology Group.
In addition to assessing treatment strategies in larger patient cohorts, PHITT is expanding the data available to correlate outcomes across different stages and risk categories based on histological and biological classifications. Hepatoblastoma and hepatocellular carcinoma are being addressed in PHITT, but the design schema for these malignancies differs. For patients enrolled with hepatoblastoma, 4 risk groups have been defined, ranging from very low to high. Within these risk categories, flow charts provide guide selection of treatments based on clinical and disease features.
Cincinnati Children’s Hospital and Medical Center is one of the most active centers for the treatment of hepatoblastoma in the Unites States but manages only 15 to 20 cases of this rare disease per year. PHITT is expected to play a critical role in achieving a high level of valuable data, and the first sets of outcomes from this collaboration are anticipated to be available in early 2025. As the study progresses, meaningful data are expected for the most challenging and some of the rarest hepatoblastoma risk groups.
Summary
The rates of cure are now approaching 100% with surgery and chemotherapy in patients with localized or locally advanced hepatoblastoma. For more advanced, unresectable disease, liver transplantation is effective in most patients, providing high rates of long-term survival. For patients with relapsed disease, advanced treatment protocols at centers with high relative volumes
of hepatoblastoma are now regularly achieving a second remission—many of which are durable. Although prognosis is less favorable in patients who experience a second relapse, long-term survival is achieved even in a proportion of these children. Substantial rates of response and long-term survival have been common in hepatoblastoma diagnosed at early stages, but the recent progress in advanced hepatoblastoma is credited to more aggressive therapies based on a better understanding of the disease characteristics that allows for individualized therapy. There is hope that the larger pool of data becoming available in 2025 from PHITT will prove to be an additional source of information that guides further advances in managing this rare disease.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
Introduction
Hepatoblastoma accounts for most pediatric liver cancers, but accounts for only 1% of all malignancies in children. Rates of hepatoblastoma have increased gradually over the past 20 years for unclear reasons, but it remains a rare malignancy. In the 1970s, only a small percentage of patients survived long-term. Today, 5-year survival rates range from 65% to over 90%, depending on risk factors, thanks to recent advancements in the understanding and treatment of hepatoblastoma.1-5 Improved risk stratification has led to better staging and more personalized treatment approaches. To further improve survival, current research is concentrated on improving outcomes in the most challenging patient subsets, such as those with metastatic disease and patients with disease relapse.
Background
Hepatoblastoma is typically diagnosed in the first 2 years of life.6 Accounting for more than 60% of pediatric hepatic malignancies worldwide, the incidence of hepatoblastoma is increasing. Results from a study evaluating the incidence between 2001 and 2017 showed a 2% annual increase documented in children aged from birth to 4 years in the United States, climbing to 5.8% annually among children aged 5 to 9 years.2 Risk factors for hepatoblastoma include maternal preeclampsia, premature birth, and parental smoking.7 The degree to which each of these factors plays a role is uncertain. A genetic etiology is suspected in a minority of hepatoblastoma cases, but it is associated with several genetic diseases, including Beckwith-Weidemann syndrome, familial adenomatous polyposis, and Prader-Willi syndrome.8 Genetic mutations in the Wnt signaling pathway that result in the accumulation of beta-catenin have also been found in sporadic, nonfamilial cases.9
Although this condition generally presents as a single abdominal mass in the right lobe of the liver, multifocal hepatoblastoma at diagnosis does occur.10 In most patients, alpha-fetoprotein (AFP) is significantly elevated.11 An estimated 20% of patients present with metastases, which are most commonly found in the lung.12 While ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) can be used to define the extent of the tumor in the liver, a chest CT is appropriate to look for metastases beyond the liver.13
Of the 2 broad histological categories commonly used to characterize hepatoblastoma, the more common epithelial form consists of fetal or embryonal liver cells. The mixed epithelial-mesenchymal form that accounts for 20% to 30% of hepatoblastomas features epithelial and primitive mesenchymal tissue, often with osteoid tissue or cartilage6; both have numerous histological subtypes. For example, the epithelial type can be further characterized by a well- or poorly-differentiated appearance, while the mixed type can be subdivided by the presence or absence of teratoid features.
Prior to 2017, there was considerable disparity in the way hepatoblastomas were characterized and staged among the major research consortiums. This issue was addressed when a consortium was established in which pediatric oncology groups pooled their data. The Children’s Hepatic tumors International Collaboration (CHIC) released the PRETEXT (PRETreatment EXTent of disease) approach.7,14 Based on comprehensive data from 1605 children participating in multicenter trials, the CHIC risk stratification defines and provides risk trees for very low-, low-, intermediate-, and high-risk groups. The most important predictors included AFP levels, patient age, extent of disease in the liver (particularly involving major hepatic veins), and the presence of metastases.
Further improvements to the diagnosis and staging of hepatoblastoma are credited to consensus-based recommendations for imaging that were created in the context of the PRETEXT staging system.13 While ultrasound is recommended for the initial approach to diagnosis, this consensus calls for MRI with hepatobiliary contrast to better characterize the lesion and detect satellite lesions. This form of imaging is also recommended for follow-up after treatment, but results should be interpreted in the context of biomarkers, such as AFP levels, pathologic grading, and tumor subtypes.
In patients with the most common familial disorders associated with a predisposition for hepatoblastoma, such as adenomatous polyposis, Beckwith-Weidemann spectrum, or trisomy 18, regular surveillance for hepatoblastoma is recommended during the early years of life.8 Characterization of the genetic and molecular features of patients who present with hepatoblastoma might be useful in determining prognosis. Of genetic features, mutations in the CTNNB1 gene are the most common, but several genes in the Wnt pathway are also linked to hepatoblastoma formation.9
Along with the progress in subtyping patients by genetics, epigenetics, and molecular features, there is a growing appreciation for the heterogeneity of hepatoblastoma and the likelihood that treatment strategies can be better individualized to improve outcomes in high-risk patients. This progress is expected to accelerate further when results from the results from the Pediatric Hepatic International Tumor Trial (PHITT) are published. These data are expected to be available in 2025, and may help with prognostication and understanding the biology of hepatoblastoma in relation to outcomes.
Treatment Strategies in Hepatoblastoma
For low-grade hepatoblastoma, the first-line therapy is surgery, which can be sufficient for cure without relapse in selected patients with PRETEXT group 1 disease. Although only 40% to 60% of patients have resectable disease at diagnosis,10 there are several strategies to shrink tumor bulk, particularly chemotherapy due to the relatively high sensitivity of hepatoblastoma to cytotoxic therapies. The intensity of chemotherapy is increased relative to risk.11 For example, cisplatin-based regimens are considered for low-risk patients, while additional therapies, such as doxorubicin, irinotecan, or both, are added in patients at higher risk. Cure is common if these regimens permit a margin-free resection, although relapse does occur in a subset of patients.
If adequate debulking of the tumor cannot be achieved with conventional surgery, liver transplantation is typically offered for patients without extrahepatic disease or after distant metastases have been successfully excised. With liver transplantation and combination therapies to inhibit relapse associated with seeding, long-term survival rates of 80% have been reported.3 Judicious use of transplantation in patients with high-risk disease that raises the potential for relapse has been credited with rates of long-term survival that exceed 80% in some series. However, there is concern of offering transplantation when it is not necessary. In patients who are high risk with multiple lesions in the liver, there is a general agreement that transplantation reduces the likelihood of subsequent relapse; however, as the precision of aggressive resection coupled with effective chemotherapy has improved, there are more patients in whom the optimal choice might not be debated by experts.
Review articles typically cite the likelihood of an overall 5-year survival in patients with hepatoblastoma as being on the order of 80%.1 This rate includes children with late-onset disease, which is generally associated with a worse prognosis, and patients who eventually experience disease relapse. Survival rates are now likely to be substantially higher, with progress developing better treatment protocols for both groups. In the absence of high-risk features, long-term survival rates of 90% or higher are now being reported in some centers with high relative volumes of hepatoblastoma, regardless of baseline risks.
PHITT
The rarity of hepatoblastoma poses a significant challenge to conducting prospective studies with sufficient sample sizes to evaluate the overall efficacy of treatments and their effectiveness in patient subgroups based on specific clinical characteristics and disease severity. PHITT is the first international collaborative liver tumors trial to use a consensus approach. Centers in Europe, Japan, and the United States are participating through regional cancer study consortia. The Cincinnati Children’s Hospital and Medical Center, a leader in hepatoblastoma management in the United States, is anchoring this effort for the Children’s Oncology Group.
In addition to assessing treatment strategies in larger patient cohorts, PHITT is expanding the data available to correlate outcomes across different stages and risk categories based on histological and biological classifications. Hepatoblastoma and hepatocellular carcinoma are being addressed in PHITT, but the design schema for these malignancies differs. For patients enrolled with hepatoblastoma, 4 risk groups have been defined, ranging from very low to high. Within these risk categories, flow charts provide guide selection of treatments based on clinical and disease features.
Cincinnati Children’s Hospital and Medical Center is one of the most active centers for the treatment of hepatoblastoma in the Unites States but manages only 15 to 20 cases of this rare disease per year. PHITT is expected to play a critical role in achieving a high level of valuable data, and the first sets of outcomes from this collaboration are anticipated to be available in early 2025. As the study progresses, meaningful data are expected for the most challenging and some of the rarest hepatoblastoma risk groups.
Summary
The rates of cure are now approaching 100% with surgery and chemotherapy in patients with localized or locally advanced hepatoblastoma. For more advanced, unresectable disease, liver transplantation is effective in most patients, providing high rates of long-term survival. For patients with relapsed disease, advanced treatment protocols at centers with high relative volumes
of hepatoblastoma are now regularly achieving a second remission—many of which are durable. Although prognosis is less favorable in patients who experience a second relapse, long-term survival is achieved even in a proportion of these children. Substantial rates of response and long-term survival have been common in hepatoblastoma diagnosed at early stages, but the recent progress in advanced hepatoblastoma is credited to more aggressive therapies based on a better understanding of the disease characteristics that allows for individualized therapy. There is hope that the larger pool of data becoming available in 2025 from PHITT will prove to be an additional source of information that guides further advances in managing this rare disease.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Koh KN, Namgoong JM, Yoon HM, et al. Recent improvement in survival outcomes and reappraisal of prognostic factors in hepatoblastoma. Cancer Med. 2021;10(10):3261-3273. doi:10.1002/cam4.3897
- Kahla JA, Siegel DA, Dai S, et al. Incidence and 5-year survival of children and adolescents with hepatoblastoma in the United States. Pediatr Blood Cancer. 2022;69(10):e29763. doi:10.1002/pbc.29763
- Ramos-Gonzalez G, LaQuaglia M, O’Neill AF, et al. Long-term outcomes of liver transplantation for hepatoblastoma: a single-center 14-year experience. Pediatr Transplant. 2018:e13250. doi:10.1111/petr.13250
- Zhou S, Malvar J, Chi YY, et al. Independent assessment of the Children’s Hepatic Tumors International Collaboration risk stratification for hepatoblastoma and the association of tumor histological characteristics with prognosis. JAMA Netw Open. 2022;5(2):e2148013. doi:10.1001/jamanetworkopen.2021.48013
- Feng J, Polychronidis G, Heger U, Frongia G, Mehrabi A, Hoffmann K. Incidence trends and survival prediction of hepatoblastoma in children: a population-based study. Cancer Commun (Lond). 2019;39(1):62. doi:10.1186/s40880-019-0411-7
- Sharma D, Subbarao G, Saxena R. Hepatoblastoma. Semin Diagn Pathol. 2017;34(2):192-200. doi:10.1053/j.semdp.2016.12.015
- Heck JE, Meyers TJ, Lombardi C, et al. Case-control study of birth characteristics and the risk of hepatoblastoma. Cancer Epidemiol. 2013;37(4):390-395. doi:10.1016/j.canep.2013.03.004
- Ranganathan S, Lopez-Terrada D, Alaggio R. Hepatoblastoma and pediatric hepatocellular carcinoma: an update. Pediatr Dev Pathol. 2020;23(2):79-95. doi:10.1177/1093526619875228
- Curia MC, Zuckermann M, De Lellis L, et al. Sporadic childhood hepatoblastomas show activation of beta-catenin, mismatch repair defects and p53 mutations. Mod Pathol. 2008;21(1):7-14. doi:10.1038/modpathol.3800977
- Fahy AS, Shaikh F, Gerstle JT. Multifocal hepatoblastoma: what is the risk of recurrent disease in the remnant liver? J Pediatr Surg. 2019;54(5):1035-1040. doi:10.1016/j.jpedsurg.2019.01.036
- Głowska-Ciemny J, Szymanski M, Kuszerska A, Rzepka R, von Kaisenberg CS, Kocyłowski R. Role of alpha-fetoprotein (AFP) in diagnosing childhood cancers and genetic-related chronic diseases. Cancers (Basel). 2023;15(17):4302. doi:10.3390/cancers15174302
- Angelico R, Grimaldi C, Gazia C, et al. How do synchronous lung metastases influence the surgical management of children with hepatoblastoma? An update and systematic review of the literature. Cancers (Basel). 2019;11(11):1693. doi:10.3390/cancers11111693
- Schooler GR, Infante JC, Acord M, et al. Imaging of pediatric liver tumors: A COG Diagnostic Imaging Committee/SPR Oncology Committee white paper. Pediatr Blood Cancer. 2023;70(suppl 4):e29965. doi:10.1002/pbc.29965
- Meyers RL, Maibach R, Hiyama E, et al. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol. 2017;18(1):122-131. doi:10.1016/S1470-2045(16)30598-8
- Koh KN, Namgoong JM, Yoon HM, et al. Recent improvement in survival outcomes and reappraisal of prognostic factors in hepatoblastoma. Cancer Med. 2021;10(10):3261-3273. doi:10.1002/cam4.3897
- Kahla JA, Siegel DA, Dai S, et al. Incidence and 5-year survival of children and adolescents with hepatoblastoma in the United States. Pediatr Blood Cancer. 2022;69(10):e29763. doi:10.1002/pbc.29763
- Ramos-Gonzalez G, LaQuaglia M, O’Neill AF, et al. Long-term outcomes of liver transplantation for hepatoblastoma: a single-center 14-year experience. Pediatr Transplant. 2018:e13250. doi:10.1111/petr.13250
- Zhou S, Malvar J, Chi YY, et al. Independent assessment of the Children’s Hepatic Tumors International Collaboration risk stratification for hepatoblastoma and the association of tumor histological characteristics with prognosis. JAMA Netw Open. 2022;5(2):e2148013. doi:10.1001/jamanetworkopen.2021.48013
- Feng J, Polychronidis G, Heger U, Frongia G, Mehrabi A, Hoffmann K. Incidence trends and survival prediction of hepatoblastoma in children: a population-based study. Cancer Commun (Lond). 2019;39(1):62. doi:10.1186/s40880-019-0411-7
- Sharma D, Subbarao G, Saxena R. Hepatoblastoma. Semin Diagn Pathol. 2017;34(2):192-200. doi:10.1053/j.semdp.2016.12.015
- Heck JE, Meyers TJ, Lombardi C, et al. Case-control study of birth characteristics and the risk of hepatoblastoma. Cancer Epidemiol. 2013;37(4):390-395. doi:10.1016/j.canep.2013.03.004
- Ranganathan S, Lopez-Terrada D, Alaggio R. Hepatoblastoma and pediatric hepatocellular carcinoma: an update. Pediatr Dev Pathol. 2020;23(2):79-95. doi:10.1177/1093526619875228
- Curia MC, Zuckermann M, De Lellis L, et al. Sporadic childhood hepatoblastomas show activation of beta-catenin, mismatch repair defects and p53 mutations. Mod Pathol. 2008;21(1):7-14. doi:10.1038/modpathol.3800977
- Fahy AS, Shaikh F, Gerstle JT. Multifocal hepatoblastoma: what is the risk of recurrent disease in the remnant liver? J Pediatr Surg. 2019;54(5):1035-1040. doi:10.1016/j.jpedsurg.2019.01.036
- Głowska-Ciemny J, Szymanski M, Kuszerska A, Rzepka R, von Kaisenberg CS, Kocyłowski R. Role of alpha-fetoprotein (AFP) in diagnosing childhood cancers and genetic-related chronic diseases. Cancers (Basel). 2023;15(17):4302. doi:10.3390/cancers15174302
- Angelico R, Grimaldi C, Gazia C, et al. How do synchronous lung metastases influence the surgical management of children with hepatoblastoma? An update and systematic review of the literature. Cancers (Basel). 2019;11(11):1693. doi:10.3390/cancers11111693
- Schooler GR, Infante JC, Acord M, et al. Imaging of pediatric liver tumors: A COG Diagnostic Imaging Committee/SPR Oncology Committee white paper. Pediatr Blood Cancer. 2023;70(suppl 4):e29965. doi:10.1002/pbc.29965
- Meyers RL, Maibach R, Hiyama E, et al. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol. 2017;18(1):122-131. doi:10.1016/S1470-2045(16)30598-8
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
Improving Prognosis in Hepatoblastoma: Evolving Risk Stratification and Treatment Strategies
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
How do you assess a patient’s prognosis at the time that they are diagnosed with myelofibrosis?
In the clinic, we use several scoring systems that have been developed based on the outcomes of hundreds of patients with myeloproliferative neoplasms (MPNs) to try to predict survival from time of diagnosis. Disease features associated with a poor prognosis include anemia, elevated white blood cell count, advanced age, constitutional symptoms, and increased peripheral blasts. Some of these scoring systems also incorporate chromosomal abnormalities as well as gene mutations to further refine prognostication.1
Determining prognosis can be important to creating a treatment plan, particularly to decide if curative allogeneic stem cell transplantation is necessary. However, I always caution patients that these prognostic scoring systems cannot tell the future and that each patient may respond differently to treatment.
How do you monitor for disease progression?
I will discuss with patients how they are feeling in order to determine if there are any new or developing symptoms that could be a sign that their disease is progressing. I will also review their laboratory work looking for changes in blood counts that could be a signal of disease evolution.
For instance, development of anemia or thrombocytopenia may signal worsening bone marrow function or progression to secondary acute leukemia. If there are concerning signs or symptoms, I will then perform a bone marrow biopsy with aspirate that will include assessment of mutations and chromosomal abnormalities to determine if their disease is progressing.
What are the first-line treatment options for a patient newly diagnosed with myelofibrosis, and how do you determine the best course of action?
For patients with myelofibrosis, the first-line treatment options include Janus kinase (JAK) inhibitors, which are effective at improving spleen size and reducing symptom burden. The US Food and Drug Administration (FDA) has approved 4 JAK inhibitors for the treatment of myelofibrosis: ruxolitinib, fedratinib, pacritinib, and momelotinib (Table).2-13 In general, ruxolitinib is the first-line treatment option unless there is thrombocytopenia, in which case pacritinib is more appropriate. In patients with baseline anemia, momelotinib may be the best choice.
Table. FDA-Approved JAK Inhibitors for Myelofibrosis2-13
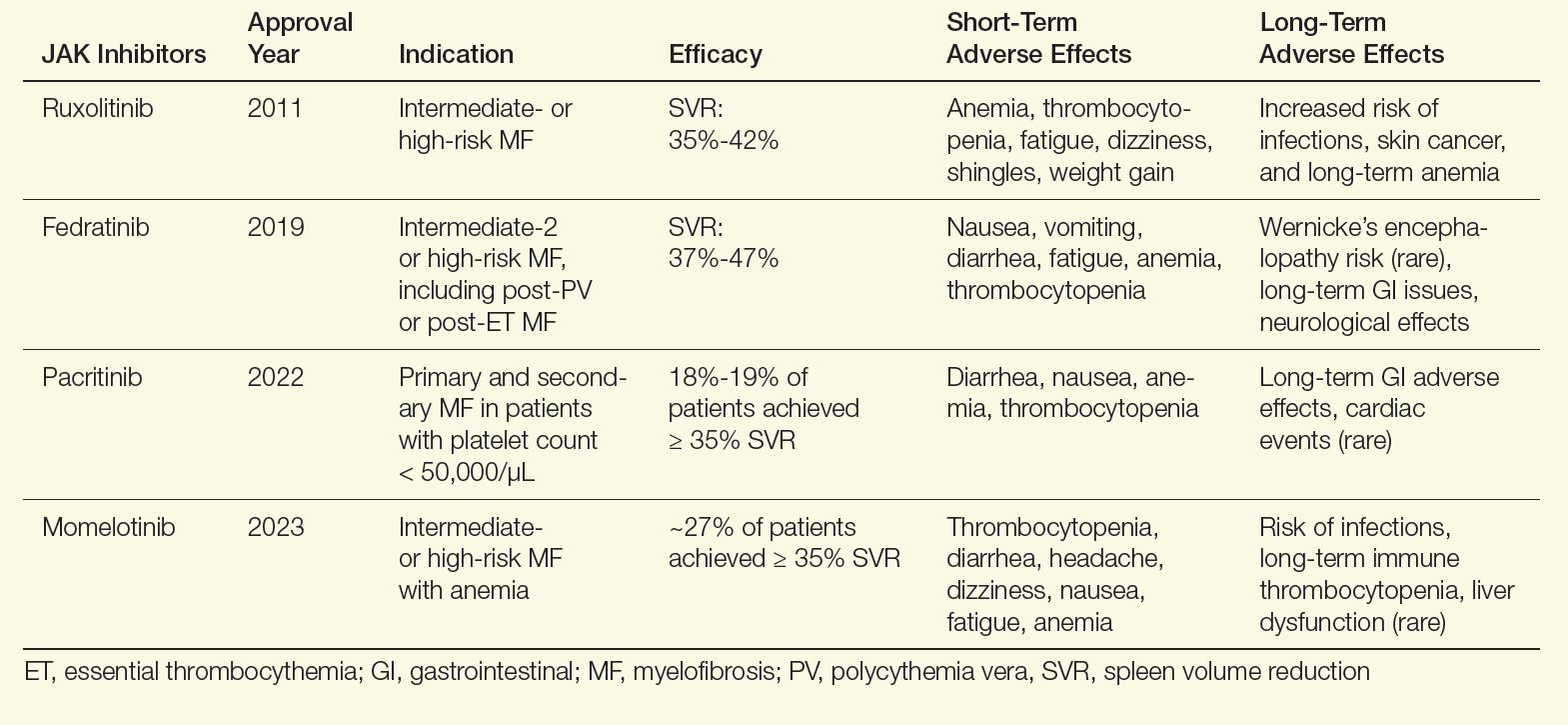
Although these agents are effective in reducing spleen size and improving symptoms, they do not affect disease progression. Therefore, I also evaluate all patients for allogeneic stem cell transplantation, which is the only curative modality. Appropriate patients are younger than age 75, with a low comorbidity burden and either intermediate-2 or high-risk disease. In addition, patients who do not respond to frontline JAK inhibitors should be considered for this approach. In patients who are transplant candidates, I will concurrently have them evaluated and start the process of finding a donor while initiating a JAK inhibitor.
What are the most common adverse effects of JAK inhibitors, and how do you help patients manage these issues?
There are short- and long-term effects of JAK inhibitors. Focusing on ruxolitinib, the most frequently used JAK inhibitor, patients can experience bruising, dizziness, and headaches, which generally resolves within a few weeks. Notable longer-term adverse events of ruxolitinib include increased rates of shingles infection, so I encourage my patients to get vaccinated for shingles before initiation.11 Weight gain has also been reported with ruxolitinib, but not with other JAK inhibitors.12,13 The other main adverse effect of ruxolitinib is worsening anemia and thrombocythemia, so I closely monitor blood counts during treatment.
What are some of the key reasons why patients may develop JAK inhibitor resistance or intolerance, and how do you address these problems in clinical practice?
There are a variety of reasons why patients discontinue a JAK inhibitor, but these can be lumped into 2 categories: (1) the medication has not achieved, or is no longer achieving, treatment goals, or (2) adverse effects from the JAK inhibitor require discontinuation. In a large series of patients with myelofibrosis who were treated with ruxolitinib, about 60% of discontinuations were because of JAK inhibitor refractoriness/resistance, and around 40% were from adverse events.14
Resistance can arise from several mechanisms, including activation of alternative pathways and clonal evolution that are ongoing regardless of JAK inhibition. In clinical practice, we are addressing JAK inhibitor resistance through clinical trials of novel therapies, particularly in combination with JAK inhibitors, which can potentially mitigate resistance. New JAK inhibitors are also being developed that may more effectively target the overactive JAK-signal transducer and activator of transcription (STAT) pathway and reduce resistance.
In terms of intolerance, there are many strategies to address nonhematological toxicities, and with the availability of pacritinib and momelotinib, patients in whom thrombocytopenia and anemia develop can be safely and effectively transitioned to an alternative JAK inhibitor if they experience adverse effects with ruxolitinib.
How do you incorporate patient-reported outcomes or quality-of-life measures into treatment planning?
Symptom assessment is a key component of the care for myelofibrosis. There are several well-validated patient-reported symptom assessment forms for myelofibrosis.15 These can be helpful both to quantify the burden of myelofibrosis-related symptoms, as well as to track progress of these symptoms over time. I generally incorporate these assessments into the initial evaluation and several times throughout therapy.
However, many symptoms are not captured on these assessments, and so I spend considerable time speaking to patients about how they are feeling and tracking their symptoms carefully over time. I also find it helpful to assess how a patient feels during treatment using the Patient’s Global Impression of Change (PGIC) questionnaire, which is a 7-point scale reflecting overall improvement compared with baseline.
Can you share any strategies for helping patients navigate the emotional and psychological challenges of living with a chronic disease like myelofibrosis?
In my experience, addressing emotional and psychological stress is one of the greatest challenges in caring for patients with myelofibrosis. Myelofibrosis significantly affects a patient’s quality of life and productivity.16 Some strategies that my patients have found helpful include engaging with the patient community and learning from others who have been living with this disease for years.
Anecdotally, I find that patients who exercise regularly and maintain an active lifestyle benefit psychologically. I have also observed that involving a support system is critical for dealing with the emotional stress of living with a chronic disease. It is particularly helpful if the patient brings a supportive friend or family member to appointments, as they can help the patient process the information discussed.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2018;36(4):310-318. doi:10.1200/JCO.2017.76.4886
- Jakafi [package insert]. Incyte Corporation; 2011. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202192lbl.pdf
- Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. doi:10.1056/NEJMoa1110557
- US Food and Drug Administration. FDA approves Inrebic for treatment of patients with myelofibrosis. FDA announcement. August 16, 2019. Accessed October 10, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approvesfedratinib-myelofibrosis
- Pardanani A, Tefferi A, Masszi T, et al. Updated results of the placebo-controlled, phase III JAKARTA trial of fedratinib in patients with intermediate-2 or high-risk myelofibrosis. Br J Haematol. 2021;195(2):244-248. doi:10.1111/bjh.17727
- Vonjo [package insert]. CTI BioPharma Corp; 2022. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Mesa RA, Vannucchi AM, Mead A, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol. 2017;4(5):e225-e236. doi:10.1016/S2352-3026(17)30027-3
- Mascarenhas J, Hoffman R, Talpaz M, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA Oncol. 2018;4(5):652-659. doi:10.1001/jamaoncol.2017.5818
- Ojjaara [package insert]. GSK; 2023. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216873s000lbl.pdf
- Mesa RA, Kiladjian JJ, Catalano JV, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor-naïve patients with myelofibrosis. J Clin Oncol. 2017;35(34):3844-3850. doi:10.1200/JCO.2017.73.4418
- Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib-associated infections: a systematic review and meta-analysis. Am J Hematol. 2018;93(3):339-347. doi:10.1002/ajh.24976
- Sapre M, Tremblay D, Wilck E, et al. Metabolic effects of JAK1/2 inhibition in patients with myeloproliferative neoplasms. Sci Rep. 2019;9(1):16609. doi:10.1038/s41598-019-53056-x
- Tremblay D, Cavalli L, Sy O, Rose S, Mascarenhas J. The effect of fedratinib, a selective inhibitor of Janus kinase 2, on weight and metabolic parameters in patients with intermediate- or high-risk myelofibrosis. Clin Lymphoma Myeloma Leuk. 2022;22(7):e463-e466. doi:10.1016/j.clml.2022.01.003
- Palandri F, Breccia M, Bonifacio M, et al. Life after ruxolitinib: reasons for discontinuation, impact of disease phase, and outcomes in 218 patients with myelofibrosis. Cancer. 2020;126(6):1243-1252. doi:10.1002/cncr.32664
- Tremblay D, Mesa R. Addressing symptom burden in myeloproliferative neoplasms. Best Pract Res Clin Haematol. 2022;35(2):101372. doi:10.1016/j.beha.2022.101372
- Harrison CN, Koschmieder S, Foltz L, et al. The impact of myeloproliferative neoplasms (MPNs) on patient quality of life and productivity: results from the international MPN Landmark survey. Ann Hematol. 2017;96(10):1653-1665. doi:10.1007/s00277-017-3082-y
How do you assess a patient’s prognosis at the time that they are diagnosed with myelofibrosis?
In the clinic, we use several scoring systems that have been developed based on the outcomes of hundreds of patients with myeloproliferative neoplasms (MPNs) to try to predict survival from time of diagnosis. Disease features associated with a poor prognosis include anemia, elevated white blood cell count, advanced age, constitutional symptoms, and increased peripheral blasts. Some of these scoring systems also incorporate chromosomal abnormalities as well as gene mutations to further refine prognostication.1
Determining prognosis can be important to creating a treatment plan, particularly to decide if curative allogeneic stem cell transplantation is necessary. However, I always caution patients that these prognostic scoring systems cannot tell the future and that each patient may respond differently to treatment.
How do you monitor for disease progression?
I will discuss with patients how they are feeling in order to determine if there are any new or developing symptoms that could be a sign that their disease is progressing. I will also review their laboratory work looking for changes in blood counts that could be a signal of disease evolution.
For instance, development of anemia or thrombocytopenia may signal worsening bone marrow function or progression to secondary acute leukemia. If there are concerning signs or symptoms, I will then perform a bone marrow biopsy with aspirate that will include assessment of mutations and chromosomal abnormalities to determine if their disease is progressing.
What are the first-line treatment options for a patient newly diagnosed with myelofibrosis, and how do you determine the best course of action?
For patients with myelofibrosis, the first-line treatment options include Janus kinase (JAK) inhibitors, which are effective at improving spleen size and reducing symptom burden. The US Food and Drug Administration (FDA) has approved 4 JAK inhibitors for the treatment of myelofibrosis: ruxolitinib, fedratinib, pacritinib, and momelotinib (Table).2-13 In general, ruxolitinib is the first-line treatment option unless there is thrombocytopenia, in which case pacritinib is more appropriate. In patients with baseline anemia, momelotinib may be the best choice.
Table. FDA-Approved JAK Inhibitors for Myelofibrosis2-13

Although these agents are effective in reducing spleen size and improving symptoms, they do not affect disease progression. Therefore, I also evaluate all patients for allogeneic stem cell transplantation, which is the only curative modality. Appropriate patients are younger than age 75, with a low comorbidity burden and either intermediate-2 or high-risk disease. In addition, patients who do not respond to frontline JAK inhibitors should be considered for this approach. In patients who are transplant candidates, I will concurrently have them evaluated and start the process of finding a donor while initiating a JAK inhibitor.
What are the most common adverse effects of JAK inhibitors, and how do you help patients manage these issues?
There are short- and long-term effects of JAK inhibitors. Focusing on ruxolitinib, the most frequently used JAK inhibitor, patients can experience bruising, dizziness, and headaches, which generally resolves within a few weeks. Notable longer-term adverse events of ruxolitinib include increased rates of shingles infection, so I encourage my patients to get vaccinated for shingles before initiation.11 Weight gain has also been reported with ruxolitinib, but not with other JAK inhibitors.12,13 The other main adverse effect of ruxolitinib is worsening anemia and thrombocythemia, so I closely monitor blood counts during treatment.
What are some of the key reasons why patients may develop JAK inhibitor resistance or intolerance, and how do you address these problems in clinical practice?
There are a variety of reasons why patients discontinue a JAK inhibitor, but these can be lumped into 2 categories: (1) the medication has not achieved, or is no longer achieving, treatment goals, or (2) adverse effects from the JAK inhibitor require discontinuation. In a large series of patients with myelofibrosis who were treated with ruxolitinib, about 60% of discontinuations were because of JAK inhibitor refractoriness/resistance, and around 40% were from adverse events.14
Resistance can arise from several mechanisms, including activation of alternative pathways and clonal evolution that are ongoing regardless of JAK inhibition. In clinical practice, we are addressing JAK inhibitor resistance through clinical trials of novel therapies, particularly in combination with JAK inhibitors, which can potentially mitigate resistance. New JAK inhibitors are also being developed that may more effectively target the overactive JAK-signal transducer and activator of transcription (STAT) pathway and reduce resistance.
In terms of intolerance, there are many strategies to address nonhematological toxicities, and with the availability of pacritinib and momelotinib, patients in whom thrombocytopenia and anemia develop can be safely and effectively transitioned to an alternative JAK inhibitor if they experience adverse effects with ruxolitinib.
How do you incorporate patient-reported outcomes or quality-of-life measures into treatment planning?
Symptom assessment is a key component of the care for myelofibrosis. There are several well-validated patient-reported symptom assessment forms for myelofibrosis.15 These can be helpful both to quantify the burden of myelofibrosis-related symptoms, as well as to track progress of these symptoms over time. I generally incorporate these assessments into the initial evaluation and several times throughout therapy.
However, many symptoms are not captured on these assessments, and so I spend considerable time speaking to patients about how they are feeling and tracking their symptoms carefully over time. I also find it helpful to assess how a patient feels during treatment using the Patient’s Global Impression of Change (PGIC) questionnaire, which is a 7-point scale reflecting overall improvement compared with baseline.
Can you share any strategies for helping patients navigate the emotional and psychological challenges of living with a chronic disease like myelofibrosis?
In my experience, addressing emotional and psychological stress is one of the greatest challenges in caring for patients with myelofibrosis. Myelofibrosis significantly affects a patient’s quality of life and productivity.16 Some strategies that my patients have found helpful include engaging with the patient community and learning from others who have been living with this disease for years.
Anecdotally, I find that patients who exercise regularly and maintain an active lifestyle benefit psychologically. I have also observed that involving a support system is critical for dealing with the emotional stress of living with a chronic disease. It is particularly helpful if the patient brings a supportive friend or family member to appointments, as they can help the patient process the information discussed.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
How do you assess a patient’s prognosis at the time that they are diagnosed with myelofibrosis?
In the clinic, we use several scoring systems that have been developed based on the outcomes of hundreds of patients with myeloproliferative neoplasms (MPNs) to try to predict survival from time of diagnosis. Disease features associated with a poor prognosis include anemia, elevated white blood cell count, advanced age, constitutional symptoms, and increased peripheral blasts. Some of these scoring systems also incorporate chromosomal abnormalities as well as gene mutations to further refine prognostication.1
Determining prognosis can be important to creating a treatment plan, particularly to decide if curative allogeneic stem cell transplantation is necessary. However, I always caution patients that these prognostic scoring systems cannot tell the future and that each patient may respond differently to treatment.
How do you monitor for disease progression?
I will discuss with patients how they are feeling in order to determine if there are any new or developing symptoms that could be a sign that their disease is progressing. I will also review their laboratory work looking for changes in blood counts that could be a signal of disease evolution.
For instance, development of anemia or thrombocytopenia may signal worsening bone marrow function or progression to secondary acute leukemia. If there are concerning signs or symptoms, I will then perform a bone marrow biopsy with aspirate that will include assessment of mutations and chromosomal abnormalities to determine if their disease is progressing.
What are the first-line treatment options for a patient newly diagnosed with myelofibrosis, and how do you determine the best course of action?
For patients with myelofibrosis, the first-line treatment options include Janus kinase (JAK) inhibitors, which are effective at improving spleen size and reducing symptom burden. The US Food and Drug Administration (FDA) has approved 4 JAK inhibitors for the treatment of myelofibrosis: ruxolitinib, fedratinib, pacritinib, and momelotinib (Table).2-13 In general, ruxolitinib is the first-line treatment option unless there is thrombocytopenia, in which case pacritinib is more appropriate. In patients with baseline anemia, momelotinib may be the best choice.
Table. FDA-Approved JAK Inhibitors for Myelofibrosis2-13

Although these agents are effective in reducing spleen size and improving symptoms, they do not affect disease progression. Therefore, I also evaluate all patients for allogeneic stem cell transplantation, which is the only curative modality. Appropriate patients are younger than age 75, with a low comorbidity burden and either intermediate-2 or high-risk disease. In addition, patients who do not respond to frontline JAK inhibitors should be considered for this approach. In patients who are transplant candidates, I will concurrently have them evaluated and start the process of finding a donor while initiating a JAK inhibitor.
What are the most common adverse effects of JAK inhibitors, and how do you help patients manage these issues?
There are short- and long-term effects of JAK inhibitors. Focusing on ruxolitinib, the most frequently used JAK inhibitor, patients can experience bruising, dizziness, and headaches, which generally resolves within a few weeks. Notable longer-term adverse events of ruxolitinib include increased rates of shingles infection, so I encourage my patients to get vaccinated for shingles before initiation.11 Weight gain has also been reported with ruxolitinib, but not with other JAK inhibitors.12,13 The other main adverse effect of ruxolitinib is worsening anemia and thrombocythemia, so I closely monitor blood counts during treatment.
What are some of the key reasons why patients may develop JAK inhibitor resistance or intolerance, and how do you address these problems in clinical practice?
There are a variety of reasons why patients discontinue a JAK inhibitor, but these can be lumped into 2 categories: (1) the medication has not achieved, or is no longer achieving, treatment goals, or (2) adverse effects from the JAK inhibitor require discontinuation. In a large series of patients with myelofibrosis who were treated with ruxolitinib, about 60% of discontinuations were because of JAK inhibitor refractoriness/resistance, and around 40% were from adverse events.14
Resistance can arise from several mechanisms, including activation of alternative pathways and clonal evolution that are ongoing regardless of JAK inhibition. In clinical practice, we are addressing JAK inhibitor resistance through clinical trials of novel therapies, particularly in combination with JAK inhibitors, which can potentially mitigate resistance. New JAK inhibitors are also being developed that may more effectively target the overactive JAK-signal transducer and activator of transcription (STAT) pathway and reduce resistance.
In terms of intolerance, there are many strategies to address nonhematological toxicities, and with the availability of pacritinib and momelotinib, patients in whom thrombocytopenia and anemia develop can be safely and effectively transitioned to an alternative JAK inhibitor if they experience adverse effects with ruxolitinib.
How do you incorporate patient-reported outcomes or quality-of-life measures into treatment planning?
Symptom assessment is a key component of the care for myelofibrosis. There are several well-validated patient-reported symptom assessment forms for myelofibrosis.15 These can be helpful both to quantify the burden of myelofibrosis-related symptoms, as well as to track progress of these symptoms over time. I generally incorporate these assessments into the initial evaluation and several times throughout therapy.
However, many symptoms are not captured on these assessments, and so I spend considerable time speaking to patients about how they are feeling and tracking their symptoms carefully over time. I also find it helpful to assess how a patient feels during treatment using the Patient’s Global Impression of Change (PGIC) questionnaire, which is a 7-point scale reflecting overall improvement compared with baseline.
Can you share any strategies for helping patients navigate the emotional and psychological challenges of living with a chronic disease like myelofibrosis?
In my experience, addressing emotional and psychological stress is one of the greatest challenges in caring for patients with myelofibrosis. Myelofibrosis significantly affects a patient’s quality of life and productivity.16 Some strategies that my patients have found helpful include engaging with the patient community and learning from others who have been living with this disease for years.
Anecdotally, I find that patients who exercise regularly and maintain an active lifestyle benefit psychologically. I have also observed that involving a support system is critical for dealing with the emotional stress of living with a chronic disease. It is particularly helpful if the patient brings a supportive friend or family member to appointments, as they can help the patient process the information discussed.
Read more from the 2024 Rare Diseases Report: Hematology and Oncology.
- Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2018;36(4):310-318. doi:10.1200/JCO.2017.76.4886
- Jakafi [package insert]. Incyte Corporation; 2011. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202192lbl.pdf
- Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. doi:10.1056/NEJMoa1110557
- US Food and Drug Administration. FDA approves Inrebic for treatment of patients with myelofibrosis. FDA announcement. August 16, 2019. Accessed October 10, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approvesfedratinib-myelofibrosis
- Pardanani A, Tefferi A, Masszi T, et al. Updated results of the placebo-controlled, phase III JAKARTA trial of fedratinib in patients with intermediate-2 or high-risk myelofibrosis. Br J Haematol. 2021;195(2):244-248. doi:10.1111/bjh.17727
- Vonjo [package insert]. CTI BioPharma Corp; 2022. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Mesa RA, Vannucchi AM, Mead A, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol. 2017;4(5):e225-e236. doi:10.1016/S2352-3026(17)30027-3
- Mascarenhas J, Hoffman R, Talpaz M, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA Oncol. 2018;4(5):652-659. doi:10.1001/jamaoncol.2017.5818
- Ojjaara [package insert]. GSK; 2023. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216873s000lbl.pdf
- Mesa RA, Kiladjian JJ, Catalano JV, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor-naïve patients with myelofibrosis. J Clin Oncol. 2017;35(34):3844-3850. doi:10.1200/JCO.2017.73.4418
- Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib-associated infections: a systematic review and meta-analysis. Am J Hematol. 2018;93(3):339-347. doi:10.1002/ajh.24976
- Sapre M, Tremblay D, Wilck E, et al. Metabolic effects of JAK1/2 inhibition in patients with myeloproliferative neoplasms. Sci Rep. 2019;9(1):16609. doi:10.1038/s41598-019-53056-x
- Tremblay D, Cavalli L, Sy O, Rose S, Mascarenhas J. The effect of fedratinib, a selective inhibitor of Janus kinase 2, on weight and metabolic parameters in patients with intermediate- or high-risk myelofibrosis. Clin Lymphoma Myeloma Leuk. 2022;22(7):e463-e466. doi:10.1016/j.clml.2022.01.003
- Palandri F, Breccia M, Bonifacio M, et al. Life after ruxolitinib: reasons for discontinuation, impact of disease phase, and outcomes in 218 patients with myelofibrosis. Cancer. 2020;126(6):1243-1252. doi:10.1002/cncr.32664
- Tremblay D, Mesa R. Addressing symptom burden in myeloproliferative neoplasms. Best Pract Res Clin Haematol. 2022;35(2):101372. doi:10.1016/j.beha.2022.101372
- Harrison CN, Koschmieder S, Foltz L, et al. The impact of myeloproliferative neoplasms (MPNs) on patient quality of life and productivity: results from the international MPN Landmark survey. Ann Hematol. 2017;96(10):1653-1665. doi:10.1007/s00277-017-3082-y
- Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2018;36(4):310-318. doi:10.1200/JCO.2017.76.4886
- Jakafi [package insert]. Incyte Corporation; 2011. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202192lbl.pdf
- Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. doi:10.1056/NEJMoa1110557
- US Food and Drug Administration. FDA approves Inrebic for treatment of patients with myelofibrosis. FDA announcement. August 16, 2019. Accessed October 10, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approvesfedratinib-myelofibrosis
- Pardanani A, Tefferi A, Masszi T, et al. Updated results of the placebo-controlled, phase III JAKARTA trial of fedratinib in patients with intermediate-2 or high-risk myelofibrosis. Br J Haematol. 2021;195(2):244-248. doi:10.1111/bjh.17727
- Vonjo [package insert]. CTI BioPharma Corp; 2022. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Mesa RA, Vannucchi AM, Mead A, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol. 2017;4(5):e225-e236. doi:10.1016/S2352-3026(17)30027-3
- Mascarenhas J, Hoffman R, Talpaz M, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA Oncol. 2018;4(5):652-659. doi:10.1001/jamaoncol.2017.5818
- Ojjaara [package insert]. GSK; 2023. Accessed October 10, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216873s000lbl.pdf
- Mesa RA, Kiladjian JJ, Catalano JV, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor-naïve patients with myelofibrosis. J Clin Oncol. 2017;35(34):3844-3850. doi:10.1200/JCO.2017.73.4418
- Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib-associated infections: a systematic review and meta-analysis. Am J Hematol. 2018;93(3):339-347. doi:10.1002/ajh.24976
- Sapre M, Tremblay D, Wilck E, et al. Metabolic effects of JAK1/2 inhibition in patients with myeloproliferative neoplasms. Sci Rep. 2019;9(1):16609. doi:10.1038/s41598-019-53056-x
- Tremblay D, Cavalli L, Sy O, Rose S, Mascarenhas J. The effect of fedratinib, a selective inhibitor of Janus kinase 2, on weight and metabolic parameters in patients with intermediate- or high-risk myelofibrosis. Clin Lymphoma Myeloma Leuk. 2022;22(7):e463-e466. doi:10.1016/j.clml.2022.01.003
- Palandri F, Breccia M, Bonifacio M, et al. Life after ruxolitinib: reasons for discontinuation, impact of disease phase, and outcomes in 218 patients with myelofibrosis. Cancer. 2020;126(6):1243-1252. doi:10.1002/cncr.32664
- Tremblay D, Mesa R. Addressing symptom burden in myeloproliferative neoplasms. Best Pract Res Clin Haematol. 2022;35(2):101372. doi:10.1016/j.beha.2022.101372
- Harrison CN, Koschmieder S, Foltz L, et al. The impact of myeloproliferative neoplasms (MPNs) on patient quality of life and productivity: results from the international MPN Landmark survey. Ann Hematol. 2017;96(10):1653-1665. doi:10.1007/s00277-017-3082-y
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors
Optimizing Myelofibrosis Care in the Age of JAK Inhibitors













