User login
A Case of Kidney Failure
An 84-year-old woman with history of coronary artery disease, hypertension, and hyperlipidemia presented with six months of anorexia, nausea, a five-pound weight loss, weakness, and nonbloody diarrhea. Over the past one to two weeks, she noticed decreased urine output despite her use of furosemide.
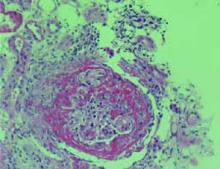
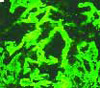
She was found to have a serum creatinine of 3.5 mg/dL on admission, increased from 1.5 mg/dL five days previously. She had no rash, dyspnea, cough, or abdominal pain. Urinalysis revealed >100 red blood cells (RBC), >100 white blood cells (WBC), occasional hyaline casts, and many gramnegative bacilli. Ciprofloxacin was started for her urinary tract infection. A renal biopsy was performed. The images shown are photomicrographs of light microscopy and immunofluorescence of the renal biopsy specimen. TH
Which of the following would be the most appropriate initial therapy for this condition?
- Increase dose of furosemide;
- Start fish oil;
- Initiate Low-dose dopamine;
- Discontinue ACE inhibitor; or
- Begin emergent plasmapheresis.
Discussion
The correct answer is e: plasmapheresis. The renal biopsy, as shown in the image at left, reveals crescents involving glomeruli on light microscopy and linear IgG staining on immunofluorescence. This patient has antiglomerular basement membrane (anti-GBM) glomerulonephritis (GN), which accounts for 10% to 20% of crescentic glomerulonephritides. It is characterized by circulating antibodies to the glomerular basement membrane with deposition of IgG or, rarely, IgA along the GBM.
The pulmonary-renal vasculitic syndrome is called Goodpasture’s syndrome, in which pulmonary hemorrhage occurs concurrently with GN. Anti-GBM disease has a bimodal distribution, with peaks in the second to third decades and the sixth to seventh decades of life.
Etiology is usually idiopathic, but hydrocarbon exposure has also been associated with the disease. Clinical presentation of renal anti-GBM disease is characterized by an acute onset of GN with severe oliguria or anuria. Urinalysis typically shows hematuria, dysmorphic red blood cells, and red blood cell casts. The diagnostic laboratory finding is circulating antibodies to GBM, specifically to the alpha-3 chain of type IV collagen; these are detected by radioimmunoassay or enzyme immunoassay in approximately 90% of patients.
The standard treatment for anti-GBM disease includes intensive plasmapheresis combined with corticosteroids and cyclophosphamide or azathioprine. Plasmapheresis consists of removal of two to four liters of plasma and replacement with fresh frozen plasma or a 5% albumin solution on a daily basis until circulating antibody levels become undetectable (usually two to three weeks). Steroids should be administered initially as pulse methylprednisolone (30 mg/kg or 1,000 mg intravenously over 20 minutes) for three doses (daily or every other day) followed by daily oral prednisone (1 mg/kg per day) for at least the first month, followed by a gradual taper. The initial cyclophosphamide dose is 2 mg/kg per day either orally or intravenously (0.5 g/m2 body surface area).
Selecting patients for treatment is based primarily on severity at presentation. Based on a large retrospective review of 71 patients treated with plasma exchange, prednisolone, and cyclophosphamide, those who presented with plasma creatinine (Cr) concentration of less than 5.7 mg/dL or those who had Cr greater than 5.7 mg/dL but did not require immediate dialysis had a favorable long-term patient and renal survival (approximately 70% to 80% at 90 months). Patients who required immediate dialysis had poor survival (approximately 35% at 90 months). Patients who had crescents in all glomeruli on renal biopsy required long-term maintenance dialysis. Therefore, plasma exchange, prednisone, and cyclophosphamide should be administered in the following settings:
- Pulmonary hemorrhage;
- Renal failure (Cr above 5-7 mg/dL) but not requiring immediate renal replacement therapy; and
- Less severe disease on renal biopsy (less than 30% to 50% crescents). Therapy is unlikely to be effective in patients who present with dialysisdependent renal failure without hemoptysis or if 100% of glomeruli have crescents on renal biopsy. In these settings, the risk of therapy may exceed the likelihood of benefit.
Fish oil is a potential therapy for IgA nephropathy, not anti-GBM disease. ACE inhibition may be useful in patients with nephrotic syndrome. IV hydration would be likely to cause volume overload and precipitate the need for acute dialysis. Low-dose dopamine has not proven effective in reversing acute renal failure. TH
REFERENCES
- Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney, 7th ed. Philadelphia, Pa: Elsevier Inc; 2005:198-199.
- Rose BD, Kaplan AA, Appel GB. Treatment of anti-GBM antibody disease (Goodpasture’s syndrome). UpToDate Online. Available at: www.uptodate.com/physicians/pulmonology_toclist.asp. Last accessed August 18, 2005.
- Levy JB, Turner AN, Rees AJ, et al. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033.
- Bolton WK. Goodpasture’s syndrome. Kidney Int. 1996;50:1753.
- Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63:1164.
An 84-year-old woman with history of coronary artery disease, hypertension, and hyperlipidemia presented with six months of anorexia, nausea, a five-pound weight loss, weakness, and nonbloody diarrhea. Over the past one to two weeks, she noticed decreased urine output despite her use of furosemide.
She was found to have a serum creatinine of 3.5 mg/dL on admission, increased from 1.5 mg/dL five days previously. She had no rash, dyspnea, cough, or abdominal pain. Urinalysis revealed >100 red blood cells (RBC), >100 white blood cells (WBC), occasional hyaline casts, and many gramnegative bacilli. Ciprofloxacin was started for her urinary tract infection. A renal biopsy was performed. The images shown are photomicrographs of light microscopy and immunofluorescence of the renal biopsy specimen. TH
Which of the following would be the most appropriate initial therapy for this condition?
- Increase dose of furosemide;
- Start fish oil;
- Initiate Low-dose dopamine;
- Discontinue ACE inhibitor; or
- Begin emergent plasmapheresis.
Discussion
The correct answer is e: plasmapheresis. The renal biopsy, as shown in the image at left, reveals crescents involving glomeruli on light microscopy and linear IgG staining on immunofluorescence. This patient has antiglomerular basement membrane (anti-GBM) glomerulonephritis (GN), which accounts for 10% to 20% of crescentic glomerulonephritides. It is characterized by circulating antibodies to the glomerular basement membrane with deposition of IgG or, rarely, IgA along the GBM.
The pulmonary-renal vasculitic syndrome is called Goodpasture’s syndrome, in which pulmonary hemorrhage occurs concurrently with GN. Anti-GBM disease has a bimodal distribution, with peaks in the second to third decades and the sixth to seventh decades of life.
Etiology is usually idiopathic, but hydrocarbon exposure has also been associated with the disease. Clinical presentation of renal anti-GBM disease is characterized by an acute onset of GN with severe oliguria or anuria. Urinalysis typically shows hematuria, dysmorphic red blood cells, and red blood cell casts. The diagnostic laboratory finding is circulating antibodies to GBM, specifically to the alpha-3 chain of type IV collagen; these are detected by radioimmunoassay or enzyme immunoassay in approximately 90% of patients.
The standard treatment for anti-GBM disease includes intensive plasmapheresis combined with corticosteroids and cyclophosphamide or azathioprine. Plasmapheresis consists of removal of two to four liters of plasma and replacement with fresh frozen plasma or a 5% albumin solution on a daily basis until circulating antibody levels become undetectable (usually two to three weeks). Steroids should be administered initially as pulse methylprednisolone (30 mg/kg or 1,000 mg intravenously over 20 minutes) for three doses (daily or every other day) followed by daily oral prednisone (1 mg/kg per day) for at least the first month, followed by a gradual taper. The initial cyclophosphamide dose is 2 mg/kg per day either orally or intravenously (0.5 g/m2 body surface area).
Selecting patients for treatment is based primarily on severity at presentation. Based on a large retrospective review of 71 patients treated with plasma exchange, prednisolone, and cyclophosphamide, those who presented with plasma creatinine (Cr) concentration of less than 5.7 mg/dL or those who had Cr greater than 5.7 mg/dL but did not require immediate dialysis had a favorable long-term patient and renal survival (approximately 70% to 80% at 90 months). Patients who required immediate dialysis had poor survival (approximately 35% at 90 months). Patients who had crescents in all glomeruli on renal biopsy required long-term maintenance dialysis. Therefore, plasma exchange, prednisone, and cyclophosphamide should be administered in the following settings:
- Pulmonary hemorrhage;
- Renal failure (Cr above 5-7 mg/dL) but not requiring immediate renal replacement therapy; and
- Less severe disease on renal biopsy (less than 30% to 50% crescents). Therapy is unlikely to be effective in patients who present with dialysisdependent renal failure without hemoptysis or if 100% of glomeruli have crescents on renal biopsy. In these settings, the risk of therapy may exceed the likelihood of benefit.
Fish oil is a potential therapy for IgA nephropathy, not anti-GBM disease. ACE inhibition may be useful in patients with nephrotic syndrome. IV hydration would be likely to cause volume overload and precipitate the need for acute dialysis. Low-dose dopamine has not proven effective in reversing acute renal failure. TH
REFERENCES
- Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney, 7th ed. Philadelphia, Pa: Elsevier Inc; 2005:198-199.
- Rose BD, Kaplan AA, Appel GB. Treatment of anti-GBM antibody disease (Goodpasture’s syndrome). UpToDate Online. Available at: www.uptodate.com/physicians/pulmonology_toclist.asp. Last accessed August 18, 2005.
- Levy JB, Turner AN, Rees AJ, et al. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033.
- Bolton WK. Goodpasture’s syndrome. Kidney Int. 1996;50:1753.
- Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63:1164.
An 84-year-old woman with history of coronary artery disease, hypertension, and hyperlipidemia presented with six months of anorexia, nausea, a five-pound weight loss, weakness, and nonbloody diarrhea. Over the past one to two weeks, she noticed decreased urine output despite her use of furosemide.
She was found to have a serum creatinine of 3.5 mg/dL on admission, increased from 1.5 mg/dL five days previously. She had no rash, dyspnea, cough, or abdominal pain. Urinalysis revealed >100 red blood cells (RBC), >100 white blood cells (WBC), occasional hyaline casts, and many gramnegative bacilli. Ciprofloxacin was started for her urinary tract infection. A renal biopsy was performed. The images shown are photomicrographs of light microscopy and immunofluorescence of the renal biopsy specimen. TH
Which of the following would be the most appropriate initial therapy for this condition?
- Increase dose of furosemide;
- Start fish oil;
- Initiate Low-dose dopamine;
- Discontinue ACE inhibitor; or
- Begin emergent plasmapheresis.
Discussion
The correct answer is e: plasmapheresis. The renal biopsy, as shown in the image at left, reveals crescents involving glomeruli on light microscopy and linear IgG staining on immunofluorescence. This patient has antiglomerular basement membrane (anti-GBM) glomerulonephritis (GN), which accounts for 10% to 20% of crescentic glomerulonephritides. It is characterized by circulating antibodies to the glomerular basement membrane with deposition of IgG or, rarely, IgA along the GBM.
The pulmonary-renal vasculitic syndrome is called Goodpasture’s syndrome, in which pulmonary hemorrhage occurs concurrently with GN. Anti-GBM disease has a bimodal distribution, with peaks in the second to third decades and the sixth to seventh decades of life.
Etiology is usually idiopathic, but hydrocarbon exposure has also been associated with the disease. Clinical presentation of renal anti-GBM disease is characterized by an acute onset of GN with severe oliguria or anuria. Urinalysis typically shows hematuria, dysmorphic red blood cells, and red blood cell casts. The diagnostic laboratory finding is circulating antibodies to GBM, specifically to the alpha-3 chain of type IV collagen; these are detected by radioimmunoassay or enzyme immunoassay in approximately 90% of patients.
The standard treatment for anti-GBM disease includes intensive plasmapheresis combined with corticosteroids and cyclophosphamide or azathioprine. Plasmapheresis consists of removal of two to four liters of plasma and replacement with fresh frozen plasma or a 5% albumin solution on a daily basis until circulating antibody levels become undetectable (usually two to three weeks). Steroids should be administered initially as pulse methylprednisolone (30 mg/kg or 1,000 mg intravenously over 20 minutes) for three doses (daily or every other day) followed by daily oral prednisone (1 mg/kg per day) for at least the first month, followed by a gradual taper. The initial cyclophosphamide dose is 2 mg/kg per day either orally or intravenously (0.5 g/m2 body surface area).
Selecting patients for treatment is based primarily on severity at presentation. Based on a large retrospective review of 71 patients treated with plasma exchange, prednisolone, and cyclophosphamide, those who presented with plasma creatinine (Cr) concentration of less than 5.7 mg/dL or those who had Cr greater than 5.7 mg/dL but did not require immediate dialysis had a favorable long-term patient and renal survival (approximately 70% to 80% at 90 months). Patients who required immediate dialysis had poor survival (approximately 35% at 90 months). Patients who had crescents in all glomeruli on renal biopsy required long-term maintenance dialysis. Therefore, plasma exchange, prednisone, and cyclophosphamide should be administered in the following settings:
- Pulmonary hemorrhage;
- Renal failure (Cr above 5-7 mg/dL) but not requiring immediate renal replacement therapy; and
- Less severe disease on renal biopsy (less than 30% to 50% crescents). Therapy is unlikely to be effective in patients who present with dialysisdependent renal failure without hemoptysis or if 100% of glomeruli have crescents on renal biopsy. In these settings, the risk of therapy may exceed the likelihood of benefit.
Fish oil is a potential therapy for IgA nephropathy, not anti-GBM disease. ACE inhibition may be useful in patients with nephrotic syndrome. IV hydration would be likely to cause volume overload and precipitate the need for acute dialysis. Low-dose dopamine has not proven effective in reversing acute renal failure. TH
REFERENCES
- Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney, 7th ed. Philadelphia, Pa: Elsevier Inc; 2005:198-199.
- Rose BD, Kaplan AA, Appel GB. Treatment of anti-GBM antibody disease (Goodpasture’s syndrome). UpToDate Online. Available at: www.uptodate.com/physicians/pulmonology_toclist.asp. Last accessed August 18, 2005.
- Levy JB, Turner AN, Rees AJ, et al. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med. 2001;134:1033.
- Bolton WK. Goodpasture’s syndrome. Kidney Int. 1996;50:1753.
- Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63:1164.