User login
Can safety and efficacy go hand in hand? Contraception for medically complex patients
The author reports no financial relationships relevant to this article.
CASE Multiple morbidities complicate choice of contraceptive
D.M. is a 27-year-old woman who has sickle cell disease, which led to a mild stroke during adolescence. She also has mild renal insufficiency and was given a diagnosis in adulthood of systemic lupus erythematosus, for which she takes prednisone on a maintenance basis.
D.M. is sexually active with her long-term boyfriend, and has undergone salpingectomy for ectopic pregnancy. Recently, she underwent exploratory laparotomy after a ruptured hemorrhagic ovarian cyst caused an intraperitoneal hemorrhage.
What method of birth control would be most appropriate for this patient?
The question is a daunting one, but it’s imperative for health-care providers to understand the nature and magnitude of contraceptive risks in medically complex women and provide the answers that these patients need.
In this article, I describe important considerations and sift the evidence regarding each of what I refer to here as highly effective contraceptive methods:
- safe hormonal contraceptives
- intrauterine contraceptives
- minimally invasive surgical sterilization.
These methods have given medically complex women greater control over their reproductive function and health, and a number of them offer benefits beyond contraception.
With some methods, such as progestin-only contraception, prospective data are lacking but retrospective studies show no elevated risk of cardiovascular events. And although combination hormonal contraceptives carry an elevated relative risk of cardiovascular events, absolute risk is very low.
First, who are these patients?
Women who have an extreme chronic medical condition, such as pulmonary hypertension, cardiomyopathy, or a dilated aortic root (>40 mm), face pregnancy-associated mortality as high as 10% to 50%—making unplanned pregnancy significantly more dangerous than any contraceptive. And even women who have a less severe medical condition stand to benefit from careful pregnancy timing: Those who have diabetes, lupus, or inflammatory bowel disease often need to optimize their medical condition before becoming pregnant. Still others may need to discontinue a teratogenic medication or treatment.
As for women who have multiple serious medical conditions, such as the patient described above, there is critical need to understand and prepare for the risks of pregnancy. These women deserve a contraceptive that has an efficacy rate approaching 100%.
All too often, however, these women settle for less effective barrier methods— or no method at all—out of concern that contraceptive and personal medical risks may interact adversely. Medical interests may drive these choices, but the unplanned pregnancies that result can pose more health risks than the rejected contraceptives.
A tool to weigh contraceptive risks
The World Health Organization (WHO) has categorized a large number of medical conditions according to their level of risk in regard to specific contraceptives.1 The four categories established by WHO range from no restrictions (category 1) to unacceptable health risks (category 4) (TABLE 1). With this system, you have a streamlined resource for weighing a contraceptive’s risks and benefits and finding an appropriate method for your patients.
TABLE 1
Four levels of risk in WHO categories
| CATEGORY | WHAT IT MEANS |
|---|---|
| 1 | A condition for which there is no restriction on the use of the contraceptive method |
| 2 | A condition in which the advantages of using the method generally outweigh the theoretical or proven risks |
| 3 | A condition in which the theoretical or proven risks usually outweigh the advantages of using the method |
| 4 | A condition that represents an unacceptable health risk if the contraceptive method is used |
Sifting risks and benefits of hormonal contraceptives
With typical use, hormonal contraceptive pills and injections prevent pregnancy in 92% to 97% of women who use one of these methods for 1 year.2 They also may decrease dysmenorrhea and menorrhagia, reduce the incidence of functional ovarian cysts, improve menstrual symptoms, and help prevent ovarian and endometrial cancers.2,3 In surveys in selected developed countries, the majority of women have used hormonal contraceptives at some time in their reproductive lives.2
Hormonal contraceptives also carry rare but potentially serious health risks that may deter their use—at times, inappropriately. Combined oral contraceptives (OCs) may double or triple the risk of myocardial infarction (MI)4 and stroke5,6 and triple or quadruple the risk of deep venous thrombosis (DVT) and venous thromboembolism (VTE).7
Recent data on the combined contraceptive patch suggest that it carries a risk of VTE twice as high as combined OCs.8 (Rates of MI and stroke were too small to compare accurately.8) We lack data on the vaginal ring contraceptive, but its medical risks are assumed to be similar to those of combined oral contraceptives.1
Putting the risks of OCs in context
It is very important to interpret these risks in light of the overall rarity of cardiovascular events and the opposing risks of pregnancy. TABLE 2 shows the low incidence of MI, stroke, and VTE among nonpregnant and pregnant women.
For every 100,000 woman-years, combined OCs are estimated to contribute three additional cases of MI, four additional cases of stroke, and 10 to 20 additional cases of VTE.3,5,9 For these severe conditions, the baseline incidence plus additional cases attributed to use of combination OCs still does not approach the risk of pregnancy itself. One study showed that women face a higher risk of cardiovascular death in pregnancy than when taking combined OCs, with the exception of smokers over the age of 35 years.9
For most women, combined OCs pose no greater cardiovascular risk than pregnancy does—but baseline cardiovascular risk factors augment that risk. Women who have hypertension, those who smoke, and those over age 35 face higher risks of MI and stroke while taking combined OCs.4,10 Diabetes and hypercholesterolemia further elevate the risk of MI,4 and migraine headache and thrombophilia raise the risk of stroke.6,11-13 Women with thrombophilia, a history of a clotting disorder, elevated body mass index (BMI), and, possibly, those who smoke face a higher risk of VTE when using a combined hormonal contraceptive.14-17
Because of these risks, the WHO classifies significant cardiovascular risk factors as category 4 (contraindicated) in regard to combined OCs (TABLE 3).
These risk factors include:
- known vascular disease
- ischemic heart disease
- history of stroke
- known thrombotic mutation
- complicated valvular disease.
When systolic blood pressure exceeds 160 mm Hg or diastolic blood pressure surpasses 100 mm Hg, combined OCs are again contraindicated. Use of combined OCs in women who have milder blood pressure elevations and adequately controlled hypertension is classified as category 3—theoretical or proven risks usually outweigh the advantages of using the method. Individual risk factors such as hyperlipidemia or uncomplicated diabetes are classified as category 3 in regard to combined OCs—unless multiple factors coexist, in which case they fall into category 4.
TABLE 2
Incidence of major cardiovascular events per 100,000 woman-years
| GROUP | MYOCARDIAL INFARCTION | STROKE | VENOUS THROMBOEMBOLISM3 |
|---|---|---|---|
| Nonpregnant | 0.2–530 | 4–1430 | 5 |
| Additional cases attributed to oral contraceptive use | 0.6–39 | 4.15 | 10–20 |
| Pregnant | 2.731 –6.232 | 2033 | 60 |
TABLE 3
Risk states in which combined hormonal contraceptives are contraindicated
| CARDIOVASCULAR RISK |
Multiple cardiovascular risk factors
|
| Systolic blood pressure >160 mm Hg |
| Diastolic blood pressure >100 mm Hg |
| Current vascular disease |
| History of ischemic heart disease |
Advanced diabetes
|
| CLOTTING RISK |
| History of deep venous thrombosis or pulmonary embolism |
| Major surgery with prolonged immobilization |
| Known thrombophilia |
| Complicated valvular heart disease |
| STROKE RISK |
| History of stroke |
| Migraine over age 35 |
| Migraine with aura |
| GASTROINTESTINAL ILLNESS |
| Active viral hepatitis |
| Decompensated cirrhosis |
| Liver tumor |
| CANCER RISK |
| Current breast cancer |
| SOURCE: World Health Organization |
Obese women may benefit from OCs—but efficacy may decline
Although obesity increases the risk of VTE17 and possibly MI4 during use of combined OCs, the WHO classifies it as category 2 in regard to this contraceptive method—advantages generally outweigh the theoretical or proven risks. This rating is based on the low number of major adverse events associated with use of low-dose combined OCs in obese women.1
However, combined OCs appear to be less effective in obese women than in their normal-weight peers. A recent case-control study showed diminished efficacy for women with a BMI over 27, and an even higher rate of contraceptive failure for those with a BMI over 32.18 Nevertheless, it is important for clinicians and patients to recognize the benefits likely to accrue from this method—probably at a higher rate than is seen with most barrier methods.
Obese women who suffer from oligoovulation may also benefit from the progestin in combined OCs, which can mitigate the effects of unopposed estrogen.
Nevertheless, it may be wise, when counseling these women, to consider a more effective method that carries less risk, such as a progestin-releasing intrauterine contraceptive.
Stroke risk in migraine sufferers may render OC option unwise
Patients who experience migraine have a higher risk of stroke than their migraine-free peers. The risk is even higher when the migraine is preceded by an aura (a 5- to 10-minute episode of moving lights in a visual field, speech disturbance, paresthesias, or weakness that precedes the headache).12,19 Risk is especially elevated when women who suffer migraines use a combined OC, with an odds ratio for stroke ranging from 6.6 to 8.7.
Because of these heightened risks, the WHO classifies migraine with aura as category 4 (contraindicated) for combined OCs. When no aura is present, the advisability of OC use depends on the woman’s age and whether her symptoms predate hormone use. Migraine without aura falls into category 4 for women over age 35 whose symptoms develop while on the contraceptive. It falls into category 2 if the woman is under age 35 and her symptoms predate contraceptive use. In other situations, migraine without aura falls into category 3.
Progestin-only options may be safer in women with cardiovascular risk
Women who face an unacceptable level of cardiovascular risk with combined OCs may still be candidates for progestin-only contraceptives. Although data are thin regarding the risks of progestins in the absence of estrogen, an international WHO study found no increased cardiovascular risk with the use of oral or injectable progestins.20
Current breast cancer is the only medical condition in which progestin-only contraception is contraindicated (category 4). Significant or multiple cardiac risk factors are classified as category 3 in regard to depot medroxyprogesterone acetate, and as category 1 or 2 for progestin-only pills.
Current DVT or VTE is classified as category 3 in regard to progestin-only contraception. A history of DVT or VTE is category 2 (TABLE 4).
TABLE 4
Risks of progestin-only contraceptives may outweigh benefits in these conditions
| CATEGORY 4 – CONTRAINDICATED |
| Current breast cancer |
| CATEGORY 3 – RISKS GENERALLY OUTWEIGH BENEFITS |
| Cardiovascular risk (for depot medroxyprogesterone acetate) |
Multiple CV risk factors
|
| Systolic BP >160 mm Hg |
| Diastolic BP >100 mm Hg |
| Current vascular disease |
Advanced diabetes
|
| Cardiovascular risk (for all progestin-only contraceptives) |
| History of ischemic heart disease while on the contraceptive |
| Clotting risk |
| Current deep venous thrombosis or pulmonary embolism |
| Stroke risk |
| History of stroke while on the contraceptive |
| Migraine with aura developing while on contraceptive |
| Gastrointestinal illness |
| Active viral hepatitis |
| Liver tumor |
| Decompensated cirrhosis |
| Cancer risk |
| History of breast cancer, remission up to 5 years |
| Unexplained vaginal bleeding |
| CV=cardiovascular |
| SOURCE: World Health Organization |
Liver disease, cancer may rule out use of hormones
Estrogens and progestins are metabolized by the liver, and women with significant liver dysfunction may accumulate medication. Hormones are also contraindicated in the setting of hormone-sensitive tumors, such as liver adenomas and breast cancer.
In addition, hormones may interact with—and should be avoided during use of—drugs that affect metabolic enzymes, such as certain anticonvulsants, rifampin, and some antiretrovirals.1
Intrauterine option is underused
Two types of intrauterine contraception (IUC) are available in the United States: the CuT-380A and the LNG-20. The former uses copper, whereas the latter delivers the progestin levonorgestrel directly to the endometrium. Both methods are extremely effective, with cumulative failure rates below 1% to 2% over 5 to 10 years.21 Unlike most hormonal contraceptives, IUCs do not require patient compliance, and the LNG-20 has the additional benefit of decreasing menstrual blood loss.21
Despite these advantages, fear of uterine infection has led to underuse of IUC in the United States.22 A worldwide review of prospective studies of IUC revealed that the risk of infection is limited to the first 20 days after insertion, when the risk of pelvic inflammatory disease (PID) is approximately 1%.23 Thereafter, the risk of infection is significantly lower and can be linked to other PID risk factors, such as young age and multiple partners.23,24 The risk may be even lower with the LNG-20 than with the copper system.25 The IUC’s safety and high level of effectiveness make it an excellent choice for many women with chronic medical conditions.
Picture is murky in immunocompromised women
Infection caused by IUC may be unlikely in a healthy woman, but use of IUC in immunocompromised patients carries uncertain risk. Data from HIV-infected women in Africa have been reassuring, demonstrating an acceptably low risk of infection.26 However, no studies have evaluated IUC among women on immunosuppressive drugs or those with otherwise impaired immune systems, and the WHO does not make formal recommendations for these patients. Two case reports of IUC failure in transplant patients led some to theorize that immune-mediated inflammation is necessary for IUC function, but this has not been proven.27
When immunocompromised women do not qualify for other highly effective contraceptives, the benefit of IUC may outweigh any theoretical risks. In this case, the LNG-20 may be preferable for its possibly lower risk of infection and decreased reliance on an inflammatory mechanism of action.
Contraindications to IUC include breast cancer, pelvic infection
Although the LNG-20 contains a hormone, the amount of levonorgestrel entering the circulation is very low, so the method is not restricted in women with cardiovascular risk factors. The only contraindications to IUC are:
- pelvic infection or sepsis
- pregnancy
- undiagnosed abnormal uterine bleeding or gynecologic cancer
- distorted uterine cavity
- breast cancer (for the LNG-20)
- Wilson’s disease (for the CuT-380A).
Sterilization is a safe option
For women ready to forego future childbearing, surgical sterilization is an excellent option. It requires no compliance or follow-up on the part of the patient, and efficacy rates approach that of IUC—at 98% to 99% or higher.
Beyond regret and sterilization failure, the risks of sterilization are limited to those of the surgical procedure itself. These may be negligible if tubal ligation is performed at the time of another indicated surgery, such as cesarean section.
Interval sterilization with laparoscopic tubal ligation is usually performed with general anesthesia. The rate of major morbidity is approximately 0.9%, including major bleeding, need for laparotomy, organ injury, and major infection. Complications may be higher in women with diabetes, a history of major surgery, and obesity.28
The WHO advises caution when using this method in the setting of severe diabetes, sickle cell disease, coagulopathy, severe renal disease, cardiovascular disease, or pulmonary disease.1
Insertion of intratubal coils is less invasive than tubal ligation
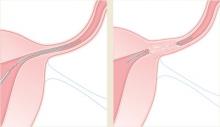
Hysteroscopic tubal sterilization with placement of titanium–Dacron intratubal coils (sold by the name Essure) is another option gaining use (FIGURE). Although large-scale studies have yet to be published, data from the largest phase III trial are consistent with smaller studies.29 In that multicenter trial, coil placement was successful in 92% of patients, and 99% of women completed the procedure without general anesthesia. Tubal perforation was identified in 1% of women, who went on to a laparoscopic procedure.
This less invasive method of permanent sterilization increases options for women who are poor laparoscopy candidates, although the 10% of women who experience technical failure will be forced to find an alternative method. Patient compliance is also an issue because the woman must use backup contraception for 3 months following the procedure, until tubal occlusion is confirmed by hysterosalpingography.
FIGURE Sterilization via insertion of intratubal coils
Delivery of the Essure device.
After 3 months, polyethylene (PET) fibers elicit ingrowth and proximal tubal occlusion.
Focusing on the patient’s partner may be the smartest approach
Male sterilization with vasectomy poses no medical risks to a woman with a complex medical history. However, long-term success requires that she keep the same sexual partner throughout her reproductive life or seek another form of contraception.
CASE RESOLVED Patient opts for progestin-only pills
Because of her sickle cell disease, D.M., the patient described at the beginning of this article, is not a good candidate for surgical sterilization, and neither is her boyfriend. According to WHO criteria, her sickle cell disease falls into category 2 in regard to combined OCs and category 1 for IUC—both effective methods. No guidance is available regarding concomitant use of steroids, which she is taking for lupus, with IUC, but her baseline risk for pelvic infection is thought to be relatively low. The noncontraceptive benefit of ovarian cyst suppression makes combined OCs even more attractive for this patient, but her history of stroke contraindicates this method (category 4). Depot medroxyprogesterone acetate may suppress ovarian function and is classified as category 3. She ultimately selects a combination of progestin-only pills and condoms and has successfully avoided pregnancy.
1. World Health Organization Medical Eligibility Criteria for Contraceptive Use. 3rd ed. Geneva: World Health Organization; 2004. Available at:www.who.int/reproductive-health/publications/mec/index.htm. Accessed Oct. 25, 2007.
2. Hatcher RA, Nelson A. Combined hormonal contraceptive methods. In: Hatcher RA et al, eds. Contraceptive Technology. 18th ed. New York: Ardent Media; 2004;391:460-
3. Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit First Prescription of Combined Oral Contraception. Royal College of Obstetricians and Gynaecologists: 2006.
4. Tanis BC, van den Bosch MA, Kemmeren JM, et al. Oral contraceptives and the risk of myocardial infarction. N Engl J Med. 2001;345:1787-1793.
5. Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: a meta-analysis. JAMA. 2000;284:72-78.
6. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet. 1996;348:498-505.
7. Vandenbroucke JP, Rosing J, Bloemenkamp KW, et al. Oral contraceptives and the risk of venous thrombosis. N Engl J Med. 2001;344:1527-1535.
8. Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109(2 Pt 1):339-346.
9. Schwingl PJ, Ory HW, Visness CM. Estimates of the risk of cardiovascular death attributable to low-dose oral contraceptives in the United States. Am J Obstet Gynecol. 1999;180(1 Pt 1):241-249.
10. Petitti DB, Sidney S, Quesenberry CP. Oral contraceptive use and myocardial infarction. Contraception. 1998;57:143-155.
11. Curtis KM, Mohllajee AP, Peterson HB. Use of combined oral contraceptives among women with migraine and nonmigrainous headaches: a systematic review. Contraception. 2006;73:189-194.
12. Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330:63.-
13. Slooter AJ, Rosendaal FR, Tanis BC, Kemmeren JM, van der Graaf Y, Algra A. Prothrombotic conditions, oral contraceptives, and the risk of ischemic stroke. J Thromb Haemost. 2005;3:1213-1217.
14. Mohllajee AP, Curtis KM, Martins SL, Peterson HB. Does use of hormonal contraceptives among women with thrombogenic mutations increase their risk of thromboembolism? A systematic review. Contraception. 2006;73:166-178.
15. Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293:2352-2361.
16. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception Effect of different progestagens in low oestrogen oral contraceptives on venous thromboembolic disease. Lancet. 1995;346:1582-1588.
17. Sidney S, Petitti DB, Soff GA, Cundiff DL, Tolan KK, Quesenberry CP, Jr. Venous thromboembolic disease in users of low-estrogen combined estrogen-progestin oral contraceptives. Contraception. 2004;70:3-10.
18. Holt VL, Scholes D, Wicklund KG, Cushing-Haugen KL, Daling JR. Body mass index, weight, and oral contraceptive failure risk. Obstet Gynecol. 2005;105:46-52.
19. Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318:13-18.
20. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception Cardiovascular disease and use of oral and injectable progestogen-only contraceptives and combined injectable contraceptives Results of an international, multicenter, case-control study. Contraception. 1998;57:315-324.
21. Grimes DA. Intrauterine devices. In: Hatcher RA et al, eds. Contraceptive Technology. 18th ed. New York: Ardent Media; 2004; 495-530.
22. Darney PD. Time to pardon the IUD? N Engl J Med. 2001;345:608-610.
23. Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339:785-788.
24. Lee NC, Rubin GL, Borucki R. The intrauterine device and pelvic inflammatory disease revisited: new results from the Women’s Health Study. Obstet Gynecol. 1988;72:1-6.
25. Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49:56-72.
26. Morrison CS, Sekadde-Kigondu C, Sinei SK, Weiner DH, Kwok C, Kokonya D. Is the intrauterine device appropriate contraception for HIV-1-infected women. BJOG. 2001;108:784-790.
27. Zerner J, Doil KL, Drewry J, Leeber DA. Intrauterine contraceptive device failures in renal transplant patients. J Reprod Med. 1981;26:99-102.
28. Jamieson DJ, Hillis SD, Duerr A, Marchbanks PA, Costello C, Peterson HB. Complications of interval laparoscopic tubal sterilization: findings from the United States Collaborative Review of Sterilization. Obstet Gynecol. 2000;96:997-1002.
29. Cooper JM, Carignan CS, Cher D, Kerin JF. Selective Tubal Occlusion Procedure Investigators Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59-67.
30. Petitti DB, Sidney S, Quesenberry CP, Jr, Bernstein A. Incidence of stroke and myocardial infarction in women of reproductive age. Stroke. 1997;28:280-283.
31. Ladner HE, Danielsen B, Gilbert WM. Acute myocardial infarction in pregnancy and the puerperium: a population-based study. Obstet Gynecol. 2005;105:480-484.
32. James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. 2006;113:1564-1571.
33. Kittner SJ, Stern BJ, Feeser BR, et al. Pregnancy and the risk of stroke. N Engl J Med. 1996;335:768-774.
The author reports no financial relationships relevant to this article.
CASE Multiple morbidities complicate choice of contraceptive
D.M. is a 27-year-old woman who has sickle cell disease, which led to a mild stroke during adolescence. She also has mild renal insufficiency and was given a diagnosis in adulthood of systemic lupus erythematosus, for which she takes prednisone on a maintenance basis.
D.M. is sexually active with her long-term boyfriend, and has undergone salpingectomy for ectopic pregnancy. Recently, she underwent exploratory laparotomy after a ruptured hemorrhagic ovarian cyst caused an intraperitoneal hemorrhage.
What method of birth control would be most appropriate for this patient?
The question is a daunting one, but it’s imperative for health-care providers to understand the nature and magnitude of contraceptive risks in medically complex women and provide the answers that these patients need.
In this article, I describe important considerations and sift the evidence regarding each of what I refer to here as highly effective contraceptive methods:
- safe hormonal contraceptives
- intrauterine contraceptives
- minimally invasive surgical sterilization.
These methods have given medically complex women greater control over their reproductive function and health, and a number of them offer benefits beyond contraception.
With some methods, such as progestin-only contraception, prospective data are lacking but retrospective studies show no elevated risk of cardiovascular events. And although combination hormonal contraceptives carry an elevated relative risk of cardiovascular events, absolute risk is very low.
First, who are these patients?
Women who have an extreme chronic medical condition, such as pulmonary hypertension, cardiomyopathy, or a dilated aortic root (>40 mm), face pregnancy-associated mortality as high as 10% to 50%—making unplanned pregnancy significantly more dangerous than any contraceptive. And even women who have a less severe medical condition stand to benefit from careful pregnancy timing: Those who have diabetes, lupus, or inflammatory bowel disease often need to optimize their medical condition before becoming pregnant. Still others may need to discontinue a teratogenic medication or treatment.
As for women who have multiple serious medical conditions, such as the patient described above, there is critical need to understand and prepare for the risks of pregnancy. These women deserve a contraceptive that has an efficacy rate approaching 100%.
All too often, however, these women settle for less effective barrier methods— or no method at all—out of concern that contraceptive and personal medical risks may interact adversely. Medical interests may drive these choices, but the unplanned pregnancies that result can pose more health risks than the rejected contraceptives.
A tool to weigh contraceptive risks
The World Health Organization (WHO) has categorized a large number of medical conditions according to their level of risk in regard to specific contraceptives.1 The four categories established by WHO range from no restrictions (category 1) to unacceptable health risks (category 4) (TABLE 1). With this system, you have a streamlined resource for weighing a contraceptive’s risks and benefits and finding an appropriate method for your patients.
TABLE 1
Four levels of risk in WHO categories
| CATEGORY | WHAT IT MEANS |
|---|---|
| 1 | A condition for which there is no restriction on the use of the contraceptive method |
| 2 | A condition in which the advantages of using the method generally outweigh the theoretical or proven risks |
| 3 | A condition in which the theoretical or proven risks usually outweigh the advantages of using the method |
| 4 | A condition that represents an unacceptable health risk if the contraceptive method is used |
Sifting risks and benefits of hormonal contraceptives
With typical use, hormonal contraceptive pills and injections prevent pregnancy in 92% to 97% of women who use one of these methods for 1 year.2 They also may decrease dysmenorrhea and menorrhagia, reduce the incidence of functional ovarian cysts, improve menstrual symptoms, and help prevent ovarian and endometrial cancers.2,3 In surveys in selected developed countries, the majority of women have used hormonal contraceptives at some time in their reproductive lives.2
Hormonal contraceptives also carry rare but potentially serious health risks that may deter their use—at times, inappropriately. Combined oral contraceptives (OCs) may double or triple the risk of myocardial infarction (MI)4 and stroke5,6 and triple or quadruple the risk of deep venous thrombosis (DVT) and venous thromboembolism (VTE).7
Recent data on the combined contraceptive patch suggest that it carries a risk of VTE twice as high as combined OCs.8 (Rates of MI and stroke were too small to compare accurately.8) We lack data on the vaginal ring contraceptive, but its medical risks are assumed to be similar to those of combined oral contraceptives.1
Putting the risks of OCs in context
It is very important to interpret these risks in light of the overall rarity of cardiovascular events and the opposing risks of pregnancy. TABLE 2 shows the low incidence of MI, stroke, and VTE among nonpregnant and pregnant women.
For every 100,000 woman-years, combined OCs are estimated to contribute three additional cases of MI, four additional cases of stroke, and 10 to 20 additional cases of VTE.3,5,9 For these severe conditions, the baseline incidence plus additional cases attributed to use of combination OCs still does not approach the risk of pregnancy itself. One study showed that women face a higher risk of cardiovascular death in pregnancy than when taking combined OCs, with the exception of smokers over the age of 35 years.9
For most women, combined OCs pose no greater cardiovascular risk than pregnancy does—but baseline cardiovascular risk factors augment that risk. Women who have hypertension, those who smoke, and those over age 35 face higher risks of MI and stroke while taking combined OCs.4,10 Diabetes and hypercholesterolemia further elevate the risk of MI,4 and migraine headache and thrombophilia raise the risk of stroke.6,11-13 Women with thrombophilia, a history of a clotting disorder, elevated body mass index (BMI), and, possibly, those who smoke face a higher risk of VTE when using a combined hormonal contraceptive.14-17
Because of these risks, the WHO classifies significant cardiovascular risk factors as category 4 (contraindicated) in regard to combined OCs (TABLE 3).
These risk factors include:
- known vascular disease
- ischemic heart disease
- history of stroke
- known thrombotic mutation
- complicated valvular disease.
When systolic blood pressure exceeds 160 mm Hg or diastolic blood pressure surpasses 100 mm Hg, combined OCs are again contraindicated. Use of combined OCs in women who have milder blood pressure elevations and adequately controlled hypertension is classified as category 3—theoretical or proven risks usually outweigh the advantages of using the method. Individual risk factors such as hyperlipidemia or uncomplicated diabetes are classified as category 3 in regard to combined OCs—unless multiple factors coexist, in which case they fall into category 4.
TABLE 2
Incidence of major cardiovascular events per 100,000 woman-years
| GROUP | MYOCARDIAL INFARCTION | STROKE | VENOUS THROMBOEMBOLISM3 |
|---|---|---|---|
| Nonpregnant | 0.2–530 | 4–1430 | 5 |
| Additional cases attributed to oral contraceptive use | 0.6–39 | 4.15 | 10–20 |
| Pregnant | 2.731 –6.232 | 2033 | 60 |
TABLE 3
Risk states in which combined hormonal contraceptives are contraindicated
| CARDIOVASCULAR RISK |
Multiple cardiovascular risk factors
|
| Systolic blood pressure >160 mm Hg |
| Diastolic blood pressure >100 mm Hg |
| Current vascular disease |
| History of ischemic heart disease |
Advanced diabetes
|
| CLOTTING RISK |
| History of deep venous thrombosis or pulmonary embolism |
| Major surgery with prolonged immobilization |
| Known thrombophilia |
| Complicated valvular heart disease |
| STROKE RISK |
| History of stroke |
| Migraine over age 35 |
| Migraine with aura |
| GASTROINTESTINAL ILLNESS |
| Active viral hepatitis |
| Decompensated cirrhosis |
| Liver tumor |
| CANCER RISK |
| Current breast cancer |
| SOURCE: World Health Organization |
Obese women may benefit from OCs—but efficacy may decline
Although obesity increases the risk of VTE17 and possibly MI4 during use of combined OCs, the WHO classifies it as category 2 in regard to this contraceptive method—advantages generally outweigh the theoretical or proven risks. This rating is based on the low number of major adverse events associated with use of low-dose combined OCs in obese women.1
However, combined OCs appear to be less effective in obese women than in their normal-weight peers. A recent case-control study showed diminished efficacy for women with a BMI over 27, and an even higher rate of contraceptive failure for those with a BMI over 32.18 Nevertheless, it is important for clinicians and patients to recognize the benefits likely to accrue from this method—probably at a higher rate than is seen with most barrier methods.
Obese women who suffer from oligoovulation may also benefit from the progestin in combined OCs, which can mitigate the effects of unopposed estrogen.
Nevertheless, it may be wise, when counseling these women, to consider a more effective method that carries less risk, such as a progestin-releasing intrauterine contraceptive.
Stroke risk in migraine sufferers may render OC option unwise
Patients who experience migraine have a higher risk of stroke than their migraine-free peers. The risk is even higher when the migraine is preceded by an aura (a 5- to 10-minute episode of moving lights in a visual field, speech disturbance, paresthesias, or weakness that precedes the headache).12,19 Risk is especially elevated when women who suffer migraines use a combined OC, with an odds ratio for stroke ranging from 6.6 to 8.7.
Because of these heightened risks, the WHO classifies migraine with aura as category 4 (contraindicated) for combined OCs. When no aura is present, the advisability of OC use depends on the woman’s age and whether her symptoms predate hormone use. Migraine without aura falls into category 4 for women over age 35 whose symptoms develop while on the contraceptive. It falls into category 2 if the woman is under age 35 and her symptoms predate contraceptive use. In other situations, migraine without aura falls into category 3.
Progestin-only options may be safer in women with cardiovascular risk
Women who face an unacceptable level of cardiovascular risk with combined OCs may still be candidates for progestin-only contraceptives. Although data are thin regarding the risks of progestins in the absence of estrogen, an international WHO study found no increased cardiovascular risk with the use of oral or injectable progestins.20
Current breast cancer is the only medical condition in which progestin-only contraception is contraindicated (category 4). Significant or multiple cardiac risk factors are classified as category 3 in regard to depot medroxyprogesterone acetate, and as category 1 or 2 for progestin-only pills.
Current DVT or VTE is classified as category 3 in regard to progestin-only contraception. A history of DVT or VTE is category 2 (TABLE 4).
TABLE 4
Risks of progestin-only contraceptives may outweigh benefits in these conditions
| CATEGORY 4 – CONTRAINDICATED |
| Current breast cancer |
| CATEGORY 3 – RISKS GENERALLY OUTWEIGH BENEFITS |
| Cardiovascular risk (for depot medroxyprogesterone acetate) |
Multiple CV risk factors
|
| Systolic BP >160 mm Hg |
| Diastolic BP >100 mm Hg |
| Current vascular disease |
Advanced diabetes
|
| Cardiovascular risk (for all progestin-only contraceptives) |
| History of ischemic heart disease while on the contraceptive |
| Clotting risk |
| Current deep venous thrombosis or pulmonary embolism |
| Stroke risk |
| History of stroke while on the contraceptive |
| Migraine with aura developing while on contraceptive |
| Gastrointestinal illness |
| Active viral hepatitis |
| Liver tumor |
| Decompensated cirrhosis |
| Cancer risk |
| History of breast cancer, remission up to 5 years |
| Unexplained vaginal bleeding |
| CV=cardiovascular |
| SOURCE: World Health Organization |
Liver disease, cancer may rule out use of hormones
Estrogens and progestins are metabolized by the liver, and women with significant liver dysfunction may accumulate medication. Hormones are also contraindicated in the setting of hormone-sensitive tumors, such as liver adenomas and breast cancer.
In addition, hormones may interact with—and should be avoided during use of—drugs that affect metabolic enzymes, such as certain anticonvulsants, rifampin, and some antiretrovirals.1
Intrauterine option is underused
Two types of intrauterine contraception (IUC) are available in the United States: the CuT-380A and the LNG-20. The former uses copper, whereas the latter delivers the progestin levonorgestrel directly to the endometrium. Both methods are extremely effective, with cumulative failure rates below 1% to 2% over 5 to 10 years.21 Unlike most hormonal contraceptives, IUCs do not require patient compliance, and the LNG-20 has the additional benefit of decreasing menstrual blood loss.21
Despite these advantages, fear of uterine infection has led to underuse of IUC in the United States.22 A worldwide review of prospective studies of IUC revealed that the risk of infection is limited to the first 20 days after insertion, when the risk of pelvic inflammatory disease (PID) is approximately 1%.23 Thereafter, the risk of infection is significantly lower and can be linked to other PID risk factors, such as young age and multiple partners.23,24 The risk may be even lower with the LNG-20 than with the copper system.25 The IUC’s safety and high level of effectiveness make it an excellent choice for many women with chronic medical conditions.
Picture is murky in immunocompromised women
Infection caused by IUC may be unlikely in a healthy woman, but use of IUC in immunocompromised patients carries uncertain risk. Data from HIV-infected women in Africa have been reassuring, demonstrating an acceptably low risk of infection.26 However, no studies have evaluated IUC among women on immunosuppressive drugs or those with otherwise impaired immune systems, and the WHO does not make formal recommendations for these patients. Two case reports of IUC failure in transplant patients led some to theorize that immune-mediated inflammation is necessary for IUC function, but this has not been proven.27
When immunocompromised women do not qualify for other highly effective contraceptives, the benefit of IUC may outweigh any theoretical risks. In this case, the LNG-20 may be preferable for its possibly lower risk of infection and decreased reliance on an inflammatory mechanism of action.
Contraindications to IUC include breast cancer, pelvic infection
Although the LNG-20 contains a hormone, the amount of levonorgestrel entering the circulation is very low, so the method is not restricted in women with cardiovascular risk factors. The only contraindications to IUC are:
- pelvic infection or sepsis
- pregnancy
- undiagnosed abnormal uterine bleeding or gynecologic cancer
- distorted uterine cavity
- breast cancer (for the LNG-20)
- Wilson’s disease (for the CuT-380A).
Sterilization is a safe option
For women ready to forego future childbearing, surgical sterilization is an excellent option. It requires no compliance or follow-up on the part of the patient, and efficacy rates approach that of IUC—at 98% to 99% or higher.
Beyond regret and sterilization failure, the risks of sterilization are limited to those of the surgical procedure itself. These may be negligible if tubal ligation is performed at the time of another indicated surgery, such as cesarean section.
Interval sterilization with laparoscopic tubal ligation is usually performed with general anesthesia. The rate of major morbidity is approximately 0.9%, including major bleeding, need for laparotomy, organ injury, and major infection. Complications may be higher in women with diabetes, a history of major surgery, and obesity.28
The WHO advises caution when using this method in the setting of severe diabetes, sickle cell disease, coagulopathy, severe renal disease, cardiovascular disease, or pulmonary disease.1
Insertion of intratubal coils is less invasive than tubal ligation
Hysteroscopic tubal sterilization with placement of titanium–Dacron intratubal coils (sold by the name Essure) is another option gaining use (FIGURE). Although large-scale studies have yet to be published, data from the largest phase III trial are consistent with smaller studies.29 In that multicenter trial, coil placement was successful in 92% of patients, and 99% of women completed the procedure without general anesthesia. Tubal perforation was identified in 1% of women, who went on to a laparoscopic procedure.
This less invasive method of permanent sterilization increases options for women who are poor laparoscopy candidates, although the 10% of women who experience technical failure will be forced to find an alternative method. Patient compliance is also an issue because the woman must use backup contraception for 3 months following the procedure, until tubal occlusion is confirmed by hysterosalpingography.
FIGURE Sterilization via insertion of intratubal coils
Delivery of the Essure device.
After 3 months, polyethylene (PET) fibers elicit ingrowth and proximal tubal occlusion.
Focusing on the patient’s partner may be the smartest approach
Male sterilization with vasectomy poses no medical risks to a woman with a complex medical history. However, long-term success requires that she keep the same sexual partner throughout her reproductive life or seek another form of contraception.
CASE RESOLVED Patient opts for progestin-only pills
Because of her sickle cell disease, D.M., the patient described at the beginning of this article, is not a good candidate for surgical sterilization, and neither is her boyfriend. According to WHO criteria, her sickle cell disease falls into category 2 in regard to combined OCs and category 1 for IUC—both effective methods. No guidance is available regarding concomitant use of steroids, which she is taking for lupus, with IUC, but her baseline risk for pelvic infection is thought to be relatively low. The noncontraceptive benefit of ovarian cyst suppression makes combined OCs even more attractive for this patient, but her history of stroke contraindicates this method (category 4). Depot medroxyprogesterone acetate may suppress ovarian function and is classified as category 3. She ultimately selects a combination of progestin-only pills and condoms and has successfully avoided pregnancy.
The author reports no financial relationships relevant to this article.
CASE Multiple morbidities complicate choice of contraceptive
D.M. is a 27-year-old woman who has sickle cell disease, which led to a mild stroke during adolescence. She also has mild renal insufficiency and was given a diagnosis in adulthood of systemic lupus erythematosus, for which she takes prednisone on a maintenance basis.
D.M. is sexually active with her long-term boyfriend, and has undergone salpingectomy for ectopic pregnancy. Recently, she underwent exploratory laparotomy after a ruptured hemorrhagic ovarian cyst caused an intraperitoneal hemorrhage.
What method of birth control would be most appropriate for this patient?
The question is a daunting one, but it’s imperative for health-care providers to understand the nature and magnitude of contraceptive risks in medically complex women and provide the answers that these patients need.
In this article, I describe important considerations and sift the evidence regarding each of what I refer to here as highly effective contraceptive methods:
- safe hormonal contraceptives
- intrauterine contraceptives
- minimally invasive surgical sterilization.
These methods have given medically complex women greater control over their reproductive function and health, and a number of them offer benefits beyond contraception.
With some methods, such as progestin-only contraception, prospective data are lacking but retrospective studies show no elevated risk of cardiovascular events. And although combination hormonal contraceptives carry an elevated relative risk of cardiovascular events, absolute risk is very low.
First, who are these patients?
Women who have an extreme chronic medical condition, such as pulmonary hypertension, cardiomyopathy, or a dilated aortic root (>40 mm), face pregnancy-associated mortality as high as 10% to 50%—making unplanned pregnancy significantly more dangerous than any contraceptive. And even women who have a less severe medical condition stand to benefit from careful pregnancy timing: Those who have diabetes, lupus, or inflammatory bowel disease often need to optimize their medical condition before becoming pregnant. Still others may need to discontinue a teratogenic medication or treatment.
As for women who have multiple serious medical conditions, such as the patient described above, there is critical need to understand and prepare for the risks of pregnancy. These women deserve a contraceptive that has an efficacy rate approaching 100%.
All too often, however, these women settle for less effective barrier methods— or no method at all—out of concern that contraceptive and personal medical risks may interact adversely. Medical interests may drive these choices, but the unplanned pregnancies that result can pose more health risks than the rejected contraceptives.
A tool to weigh contraceptive risks
The World Health Organization (WHO) has categorized a large number of medical conditions according to their level of risk in regard to specific contraceptives.1 The four categories established by WHO range from no restrictions (category 1) to unacceptable health risks (category 4) (TABLE 1). With this system, you have a streamlined resource for weighing a contraceptive’s risks and benefits and finding an appropriate method for your patients.
TABLE 1
Four levels of risk in WHO categories
| CATEGORY | WHAT IT MEANS |
|---|---|
| 1 | A condition for which there is no restriction on the use of the contraceptive method |
| 2 | A condition in which the advantages of using the method generally outweigh the theoretical or proven risks |
| 3 | A condition in which the theoretical or proven risks usually outweigh the advantages of using the method |
| 4 | A condition that represents an unacceptable health risk if the contraceptive method is used |
Sifting risks and benefits of hormonal contraceptives
With typical use, hormonal contraceptive pills and injections prevent pregnancy in 92% to 97% of women who use one of these methods for 1 year.2 They also may decrease dysmenorrhea and menorrhagia, reduce the incidence of functional ovarian cysts, improve menstrual symptoms, and help prevent ovarian and endometrial cancers.2,3 In surveys in selected developed countries, the majority of women have used hormonal contraceptives at some time in their reproductive lives.2
Hormonal contraceptives also carry rare but potentially serious health risks that may deter their use—at times, inappropriately. Combined oral contraceptives (OCs) may double or triple the risk of myocardial infarction (MI)4 and stroke5,6 and triple or quadruple the risk of deep venous thrombosis (DVT) and venous thromboembolism (VTE).7
Recent data on the combined contraceptive patch suggest that it carries a risk of VTE twice as high as combined OCs.8 (Rates of MI and stroke were too small to compare accurately.8) We lack data on the vaginal ring contraceptive, but its medical risks are assumed to be similar to those of combined oral contraceptives.1
Putting the risks of OCs in context
It is very important to interpret these risks in light of the overall rarity of cardiovascular events and the opposing risks of pregnancy. TABLE 2 shows the low incidence of MI, stroke, and VTE among nonpregnant and pregnant women.
For every 100,000 woman-years, combined OCs are estimated to contribute three additional cases of MI, four additional cases of stroke, and 10 to 20 additional cases of VTE.3,5,9 For these severe conditions, the baseline incidence plus additional cases attributed to use of combination OCs still does not approach the risk of pregnancy itself. One study showed that women face a higher risk of cardiovascular death in pregnancy than when taking combined OCs, with the exception of smokers over the age of 35 years.9
For most women, combined OCs pose no greater cardiovascular risk than pregnancy does—but baseline cardiovascular risk factors augment that risk. Women who have hypertension, those who smoke, and those over age 35 face higher risks of MI and stroke while taking combined OCs.4,10 Diabetes and hypercholesterolemia further elevate the risk of MI,4 and migraine headache and thrombophilia raise the risk of stroke.6,11-13 Women with thrombophilia, a history of a clotting disorder, elevated body mass index (BMI), and, possibly, those who smoke face a higher risk of VTE when using a combined hormonal contraceptive.14-17
Because of these risks, the WHO classifies significant cardiovascular risk factors as category 4 (contraindicated) in regard to combined OCs (TABLE 3).
These risk factors include:
- known vascular disease
- ischemic heart disease
- history of stroke
- known thrombotic mutation
- complicated valvular disease.
When systolic blood pressure exceeds 160 mm Hg or diastolic blood pressure surpasses 100 mm Hg, combined OCs are again contraindicated. Use of combined OCs in women who have milder blood pressure elevations and adequately controlled hypertension is classified as category 3—theoretical or proven risks usually outweigh the advantages of using the method. Individual risk factors such as hyperlipidemia or uncomplicated diabetes are classified as category 3 in regard to combined OCs—unless multiple factors coexist, in which case they fall into category 4.
TABLE 2
Incidence of major cardiovascular events per 100,000 woman-years
| GROUP | MYOCARDIAL INFARCTION | STROKE | VENOUS THROMBOEMBOLISM3 |
|---|---|---|---|
| Nonpregnant | 0.2–530 | 4–1430 | 5 |
| Additional cases attributed to oral contraceptive use | 0.6–39 | 4.15 | 10–20 |
| Pregnant | 2.731 –6.232 | 2033 | 60 |
TABLE 3
Risk states in which combined hormonal contraceptives are contraindicated
| CARDIOVASCULAR RISK |
Multiple cardiovascular risk factors
|
| Systolic blood pressure >160 mm Hg |
| Diastolic blood pressure >100 mm Hg |
| Current vascular disease |
| History of ischemic heart disease |
Advanced diabetes
|
| CLOTTING RISK |
| History of deep venous thrombosis or pulmonary embolism |
| Major surgery with prolonged immobilization |
| Known thrombophilia |
| Complicated valvular heart disease |
| STROKE RISK |
| History of stroke |
| Migraine over age 35 |
| Migraine with aura |
| GASTROINTESTINAL ILLNESS |
| Active viral hepatitis |
| Decompensated cirrhosis |
| Liver tumor |
| CANCER RISK |
| Current breast cancer |
| SOURCE: World Health Organization |
Obese women may benefit from OCs—but efficacy may decline
Although obesity increases the risk of VTE17 and possibly MI4 during use of combined OCs, the WHO classifies it as category 2 in regard to this contraceptive method—advantages generally outweigh the theoretical or proven risks. This rating is based on the low number of major adverse events associated with use of low-dose combined OCs in obese women.1
However, combined OCs appear to be less effective in obese women than in their normal-weight peers. A recent case-control study showed diminished efficacy for women with a BMI over 27, and an even higher rate of contraceptive failure for those with a BMI over 32.18 Nevertheless, it is important for clinicians and patients to recognize the benefits likely to accrue from this method—probably at a higher rate than is seen with most barrier methods.
Obese women who suffer from oligoovulation may also benefit from the progestin in combined OCs, which can mitigate the effects of unopposed estrogen.
Nevertheless, it may be wise, when counseling these women, to consider a more effective method that carries less risk, such as a progestin-releasing intrauterine contraceptive.
Stroke risk in migraine sufferers may render OC option unwise
Patients who experience migraine have a higher risk of stroke than their migraine-free peers. The risk is even higher when the migraine is preceded by an aura (a 5- to 10-minute episode of moving lights in a visual field, speech disturbance, paresthesias, or weakness that precedes the headache).12,19 Risk is especially elevated when women who suffer migraines use a combined OC, with an odds ratio for stroke ranging from 6.6 to 8.7.
Because of these heightened risks, the WHO classifies migraine with aura as category 4 (contraindicated) for combined OCs. When no aura is present, the advisability of OC use depends on the woman’s age and whether her symptoms predate hormone use. Migraine without aura falls into category 4 for women over age 35 whose symptoms develop while on the contraceptive. It falls into category 2 if the woman is under age 35 and her symptoms predate contraceptive use. In other situations, migraine without aura falls into category 3.
Progestin-only options may be safer in women with cardiovascular risk
Women who face an unacceptable level of cardiovascular risk with combined OCs may still be candidates for progestin-only contraceptives. Although data are thin regarding the risks of progestins in the absence of estrogen, an international WHO study found no increased cardiovascular risk with the use of oral or injectable progestins.20
Current breast cancer is the only medical condition in which progestin-only contraception is contraindicated (category 4). Significant or multiple cardiac risk factors are classified as category 3 in regard to depot medroxyprogesterone acetate, and as category 1 or 2 for progestin-only pills.
Current DVT or VTE is classified as category 3 in regard to progestin-only contraception. A history of DVT or VTE is category 2 (TABLE 4).
TABLE 4
Risks of progestin-only contraceptives may outweigh benefits in these conditions
| CATEGORY 4 – CONTRAINDICATED |
| Current breast cancer |
| CATEGORY 3 – RISKS GENERALLY OUTWEIGH BENEFITS |
| Cardiovascular risk (for depot medroxyprogesterone acetate) |
Multiple CV risk factors
|
| Systolic BP >160 mm Hg |
| Diastolic BP >100 mm Hg |
| Current vascular disease |
Advanced diabetes
|
| Cardiovascular risk (for all progestin-only contraceptives) |
| History of ischemic heart disease while on the contraceptive |
| Clotting risk |
| Current deep venous thrombosis or pulmonary embolism |
| Stroke risk |
| History of stroke while on the contraceptive |
| Migraine with aura developing while on contraceptive |
| Gastrointestinal illness |
| Active viral hepatitis |
| Liver tumor |
| Decompensated cirrhosis |
| Cancer risk |
| History of breast cancer, remission up to 5 years |
| Unexplained vaginal bleeding |
| CV=cardiovascular |
| SOURCE: World Health Organization |
Liver disease, cancer may rule out use of hormones
Estrogens and progestins are metabolized by the liver, and women with significant liver dysfunction may accumulate medication. Hormones are also contraindicated in the setting of hormone-sensitive tumors, such as liver adenomas and breast cancer.
In addition, hormones may interact with—and should be avoided during use of—drugs that affect metabolic enzymes, such as certain anticonvulsants, rifampin, and some antiretrovirals.1
Intrauterine option is underused
Two types of intrauterine contraception (IUC) are available in the United States: the CuT-380A and the LNG-20. The former uses copper, whereas the latter delivers the progestin levonorgestrel directly to the endometrium. Both methods are extremely effective, with cumulative failure rates below 1% to 2% over 5 to 10 years.21 Unlike most hormonal contraceptives, IUCs do not require patient compliance, and the LNG-20 has the additional benefit of decreasing menstrual blood loss.21
Despite these advantages, fear of uterine infection has led to underuse of IUC in the United States.22 A worldwide review of prospective studies of IUC revealed that the risk of infection is limited to the first 20 days after insertion, when the risk of pelvic inflammatory disease (PID) is approximately 1%.23 Thereafter, the risk of infection is significantly lower and can be linked to other PID risk factors, such as young age and multiple partners.23,24 The risk may be even lower with the LNG-20 than with the copper system.25 The IUC’s safety and high level of effectiveness make it an excellent choice for many women with chronic medical conditions.
Picture is murky in immunocompromised women
Infection caused by IUC may be unlikely in a healthy woman, but use of IUC in immunocompromised patients carries uncertain risk. Data from HIV-infected women in Africa have been reassuring, demonstrating an acceptably low risk of infection.26 However, no studies have evaluated IUC among women on immunosuppressive drugs or those with otherwise impaired immune systems, and the WHO does not make formal recommendations for these patients. Two case reports of IUC failure in transplant patients led some to theorize that immune-mediated inflammation is necessary for IUC function, but this has not been proven.27
When immunocompromised women do not qualify for other highly effective contraceptives, the benefit of IUC may outweigh any theoretical risks. In this case, the LNG-20 may be preferable for its possibly lower risk of infection and decreased reliance on an inflammatory mechanism of action.
Contraindications to IUC include breast cancer, pelvic infection
Although the LNG-20 contains a hormone, the amount of levonorgestrel entering the circulation is very low, so the method is not restricted in women with cardiovascular risk factors. The only contraindications to IUC are:
- pelvic infection or sepsis
- pregnancy
- undiagnosed abnormal uterine bleeding or gynecologic cancer
- distorted uterine cavity
- breast cancer (for the LNG-20)
- Wilson’s disease (for the CuT-380A).
Sterilization is a safe option
For women ready to forego future childbearing, surgical sterilization is an excellent option. It requires no compliance or follow-up on the part of the patient, and efficacy rates approach that of IUC—at 98% to 99% or higher.
Beyond regret and sterilization failure, the risks of sterilization are limited to those of the surgical procedure itself. These may be negligible if tubal ligation is performed at the time of another indicated surgery, such as cesarean section.
Interval sterilization with laparoscopic tubal ligation is usually performed with general anesthesia. The rate of major morbidity is approximately 0.9%, including major bleeding, need for laparotomy, organ injury, and major infection. Complications may be higher in women with diabetes, a history of major surgery, and obesity.28
The WHO advises caution when using this method in the setting of severe diabetes, sickle cell disease, coagulopathy, severe renal disease, cardiovascular disease, or pulmonary disease.1
Insertion of intratubal coils is less invasive than tubal ligation
Hysteroscopic tubal sterilization with placement of titanium–Dacron intratubal coils (sold by the name Essure) is another option gaining use (FIGURE). Although large-scale studies have yet to be published, data from the largest phase III trial are consistent with smaller studies.29 In that multicenter trial, coil placement was successful in 92% of patients, and 99% of women completed the procedure without general anesthesia. Tubal perforation was identified in 1% of women, who went on to a laparoscopic procedure.
This less invasive method of permanent sterilization increases options for women who are poor laparoscopy candidates, although the 10% of women who experience technical failure will be forced to find an alternative method. Patient compliance is also an issue because the woman must use backup contraception for 3 months following the procedure, until tubal occlusion is confirmed by hysterosalpingography.
FIGURE Sterilization via insertion of intratubal coils
Delivery of the Essure device.
After 3 months, polyethylene (PET) fibers elicit ingrowth and proximal tubal occlusion.
Focusing on the patient’s partner may be the smartest approach
Male sterilization with vasectomy poses no medical risks to a woman with a complex medical history. However, long-term success requires that she keep the same sexual partner throughout her reproductive life or seek another form of contraception.
CASE RESOLVED Patient opts for progestin-only pills
Because of her sickle cell disease, D.M., the patient described at the beginning of this article, is not a good candidate for surgical sterilization, and neither is her boyfriend. According to WHO criteria, her sickle cell disease falls into category 2 in regard to combined OCs and category 1 for IUC—both effective methods. No guidance is available regarding concomitant use of steroids, which she is taking for lupus, with IUC, but her baseline risk for pelvic infection is thought to be relatively low. The noncontraceptive benefit of ovarian cyst suppression makes combined OCs even more attractive for this patient, but her history of stroke contraindicates this method (category 4). Depot medroxyprogesterone acetate may suppress ovarian function and is classified as category 3. She ultimately selects a combination of progestin-only pills and condoms and has successfully avoided pregnancy.
1. World Health Organization Medical Eligibility Criteria for Contraceptive Use. 3rd ed. Geneva: World Health Organization; 2004. Available at:www.who.int/reproductive-health/publications/mec/index.htm. Accessed Oct. 25, 2007.
2. Hatcher RA, Nelson A. Combined hormonal contraceptive methods. In: Hatcher RA et al, eds. Contraceptive Technology. 18th ed. New York: Ardent Media; 2004;391:460-
3. Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit First Prescription of Combined Oral Contraception. Royal College of Obstetricians and Gynaecologists: 2006.
4. Tanis BC, van den Bosch MA, Kemmeren JM, et al. Oral contraceptives and the risk of myocardial infarction. N Engl J Med. 2001;345:1787-1793.
5. Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: a meta-analysis. JAMA. 2000;284:72-78.
6. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet. 1996;348:498-505.
7. Vandenbroucke JP, Rosing J, Bloemenkamp KW, et al. Oral contraceptives and the risk of venous thrombosis. N Engl J Med. 2001;344:1527-1535.
8. Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109(2 Pt 1):339-346.
9. Schwingl PJ, Ory HW, Visness CM. Estimates of the risk of cardiovascular death attributable to low-dose oral contraceptives in the United States. Am J Obstet Gynecol. 1999;180(1 Pt 1):241-249.
10. Petitti DB, Sidney S, Quesenberry CP. Oral contraceptive use and myocardial infarction. Contraception. 1998;57:143-155.
11. Curtis KM, Mohllajee AP, Peterson HB. Use of combined oral contraceptives among women with migraine and nonmigrainous headaches: a systematic review. Contraception. 2006;73:189-194.
12. Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330:63.-
13. Slooter AJ, Rosendaal FR, Tanis BC, Kemmeren JM, van der Graaf Y, Algra A. Prothrombotic conditions, oral contraceptives, and the risk of ischemic stroke. J Thromb Haemost. 2005;3:1213-1217.
14. Mohllajee AP, Curtis KM, Martins SL, Peterson HB. Does use of hormonal contraceptives among women with thrombogenic mutations increase their risk of thromboembolism? A systematic review. Contraception. 2006;73:166-178.
15. Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293:2352-2361.
16. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception Effect of different progestagens in low oestrogen oral contraceptives on venous thromboembolic disease. Lancet. 1995;346:1582-1588.
17. Sidney S, Petitti DB, Soff GA, Cundiff DL, Tolan KK, Quesenberry CP, Jr. Venous thromboembolic disease in users of low-estrogen combined estrogen-progestin oral contraceptives. Contraception. 2004;70:3-10.
18. Holt VL, Scholes D, Wicklund KG, Cushing-Haugen KL, Daling JR. Body mass index, weight, and oral contraceptive failure risk. Obstet Gynecol. 2005;105:46-52.
19. Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318:13-18.
20. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception Cardiovascular disease and use of oral and injectable progestogen-only contraceptives and combined injectable contraceptives Results of an international, multicenter, case-control study. Contraception. 1998;57:315-324.
21. Grimes DA. Intrauterine devices. In: Hatcher RA et al, eds. Contraceptive Technology. 18th ed. New York: Ardent Media; 2004; 495-530.
22. Darney PD. Time to pardon the IUD? N Engl J Med. 2001;345:608-610.
23. Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339:785-788.
24. Lee NC, Rubin GL, Borucki R. The intrauterine device and pelvic inflammatory disease revisited: new results from the Women’s Health Study. Obstet Gynecol. 1988;72:1-6.
25. Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49:56-72.
26. Morrison CS, Sekadde-Kigondu C, Sinei SK, Weiner DH, Kwok C, Kokonya D. Is the intrauterine device appropriate contraception for HIV-1-infected women. BJOG. 2001;108:784-790.
27. Zerner J, Doil KL, Drewry J, Leeber DA. Intrauterine contraceptive device failures in renal transplant patients. J Reprod Med. 1981;26:99-102.
28. Jamieson DJ, Hillis SD, Duerr A, Marchbanks PA, Costello C, Peterson HB. Complications of interval laparoscopic tubal sterilization: findings from the United States Collaborative Review of Sterilization. Obstet Gynecol. 2000;96:997-1002.
29. Cooper JM, Carignan CS, Cher D, Kerin JF. Selective Tubal Occlusion Procedure Investigators Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59-67.
30. Petitti DB, Sidney S, Quesenberry CP, Jr, Bernstein A. Incidence of stroke and myocardial infarction in women of reproductive age. Stroke. 1997;28:280-283.
31. Ladner HE, Danielsen B, Gilbert WM. Acute myocardial infarction in pregnancy and the puerperium: a population-based study. Obstet Gynecol. 2005;105:480-484.
32. James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. 2006;113:1564-1571.
33. Kittner SJ, Stern BJ, Feeser BR, et al. Pregnancy and the risk of stroke. N Engl J Med. 1996;335:768-774.
1. World Health Organization Medical Eligibility Criteria for Contraceptive Use. 3rd ed. Geneva: World Health Organization; 2004. Available at:www.who.int/reproductive-health/publications/mec/index.htm. Accessed Oct. 25, 2007.
2. Hatcher RA, Nelson A. Combined hormonal contraceptive methods. In: Hatcher RA et al, eds. Contraceptive Technology. 18th ed. New York: Ardent Media; 2004;391:460-
3. Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit First Prescription of Combined Oral Contraception. Royal College of Obstetricians and Gynaecologists: 2006.
4. Tanis BC, van den Bosch MA, Kemmeren JM, et al. Oral contraceptives and the risk of myocardial infarction. N Engl J Med. 2001;345:1787-1793.
5. Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: a meta-analysis. JAMA. 2000;284:72-78.
6. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet. 1996;348:498-505.
7. Vandenbroucke JP, Rosing J, Bloemenkamp KW, et al. Oral contraceptives and the risk of venous thrombosis. N Engl J Med. 2001;344:1527-1535.
8. Cole JA, Norman H, Doherty M, Walker AM. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109(2 Pt 1):339-346.
9. Schwingl PJ, Ory HW, Visness CM. Estimates of the risk of cardiovascular death attributable to low-dose oral contraceptives in the United States. Am J Obstet Gynecol. 1999;180(1 Pt 1):241-249.
10. Petitti DB, Sidney S, Quesenberry CP. Oral contraceptive use and myocardial infarction. Contraception. 1998;57:143-155.
11. Curtis KM, Mohllajee AP, Peterson HB. Use of combined oral contraceptives among women with migraine and nonmigrainous headaches: a systematic review. Contraception. 2006;73:189-194.
12. Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330:63.-
13. Slooter AJ, Rosendaal FR, Tanis BC, Kemmeren JM, van der Graaf Y, Algra A. Prothrombotic conditions, oral contraceptives, and the risk of ischemic stroke. J Thromb Haemost. 2005;3:1213-1217.
14. Mohllajee AP, Curtis KM, Martins SL, Peterson HB. Does use of hormonal contraceptives among women with thrombogenic mutations increase their risk of thromboembolism? A systematic review. Contraception. 2006;73:166-178.
15. Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293:2352-2361.
16. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception Effect of different progestagens in low oestrogen oral contraceptives on venous thromboembolic disease. Lancet. 1995;346:1582-1588.
17. Sidney S, Petitti DB, Soff GA, Cundiff DL, Tolan KK, Quesenberry CP, Jr. Venous thromboembolic disease in users of low-estrogen combined estrogen-progestin oral contraceptives. Contraception. 2004;70:3-10.
18. Holt VL, Scholes D, Wicklund KG, Cushing-Haugen KL, Daling JR. Body mass index, weight, and oral contraceptive failure risk. Obstet Gynecol. 2005;105:46-52.
19. Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318:13-18.
20. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception Cardiovascular disease and use of oral and injectable progestogen-only contraceptives and combined injectable contraceptives Results of an international, multicenter, case-control study. Contraception. 1998;57:315-324.
21. Grimes DA. Intrauterine devices. In: Hatcher RA et al, eds. Contraceptive Technology. 18th ed. New York: Ardent Media; 2004; 495-530.
22. Darney PD. Time to pardon the IUD? N Engl J Med. 2001;345:608-610.
23. Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339:785-788.
24. Lee NC, Rubin GL, Borucki R. The intrauterine device and pelvic inflammatory disease revisited: new results from the Women’s Health Study. Obstet Gynecol. 1988;72:1-6.
25. Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception. 1994;49:56-72.
26. Morrison CS, Sekadde-Kigondu C, Sinei SK, Weiner DH, Kwok C, Kokonya D. Is the intrauterine device appropriate contraception for HIV-1-infected women. BJOG. 2001;108:784-790.
27. Zerner J, Doil KL, Drewry J, Leeber DA. Intrauterine contraceptive device failures in renal transplant patients. J Reprod Med. 1981;26:99-102.
28. Jamieson DJ, Hillis SD, Duerr A, Marchbanks PA, Costello C, Peterson HB. Complications of interval laparoscopic tubal sterilization: findings from the United States Collaborative Review of Sterilization. Obstet Gynecol. 2000;96:997-1002.
29. Cooper JM, Carignan CS, Cher D, Kerin JF. Selective Tubal Occlusion Procedure Investigators Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59-67.
30. Petitti DB, Sidney S, Quesenberry CP, Jr, Bernstein A. Incidence of stroke and myocardial infarction in women of reproductive age. Stroke. 1997;28:280-283.
31. Ladner HE, Danielsen B, Gilbert WM. Acute myocardial infarction in pregnancy and the puerperium: a population-based study. Obstet Gynecol. 2005;105:480-484.
32. James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation. 2006;113:1564-1571.
33. Kittner SJ, Stern BJ, Feeser BR, et al. Pregnancy and the risk of stroke. N Engl J Med. 1996;335:768-774.