User login
How Can Tumor Lysis Syndrome Be Prevented and Managed in Cancer Patients?
Case
A 25-year-old male with HIV/AIDS and a CD4 count of 65 cells/μL presents to the ED with intractable nausea and vomiting for one week. Laboratory evaluation revealed a white blood cell of 67,000 cells/mm3. An extended chemistry panel reveals creatinine 3.5 mg/dL, potassium 3.0 mmol/L, LDH 250 IU/L, and uric acid 5mg/dL. Calcium and phosphorus were both normal. The patient was admitted for further evaluation and management, and was later diagnosed with Burkitt’s lymphoma.
Overview
Tumor lysis syndrome (TLS) is an acute cell lysis of tumor cells with the release of cell content into circulation either spontaneously or in response to therapy, leading to hyperurecemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.1-3
TLS is one of the most common oncology emergencies encountered by hospitalists caring for patients with hematologic malignancies. The incidence and severity of TLS depend on the cell burden, cell proliferation rate, potential for cell lysis or chemo sensitivity, baseline clinical characteristics, and preventive measures taken (see Table 1).2,4
TLS is classified as laboratory or clinical. Laboratory TLS is described as the presence of two or more of the following serum abnormalities at the same time, present within three days before or seven days after the start of therapy.5
- Uric acid >8 mg/dL (475.8 micromole/L) or 25% increase;
- Potassium >6 mEq/L (6 mmol/L) or 25% increase;
- Phosphorus >6.5 mg/dL (2.1 mmol/L) for children or >4.5 mg/dl (1.45 mmol/L) for adults or 25% increase; and
- Calcium >7 mg/dL (1.75 mmol/L) or 25% increase.
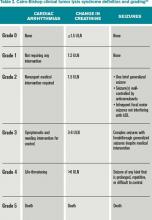
Clinical TLS is defined as laboratory TLS in association with increased creatinine levels, seizures, cardiac arrhythmias, or death (see Table 2).5
Pathogenesis
Tumor cell lysis releases DNA, cytokines, phosphate, and potassium. DNA is metabolized into adenosine and guanosine, which are then converted into xanthines. Xanthines are oxidized by xanthine oxidase into uric acid, which is then excreted through the kidneys.
TLS develops when the accumulation of xanthine, uric acid, potassium, and phosphorus exceeds the kidney’s capacity to excrete them. Cytokines cause hypotension, inflammation, and kidney injury, and worsen the kidney’s excretory capacity. Damage to the kidneys also occurs by renal precipitation of uric acid, xanthine, and calcium phosphate.4
Phosphorus concentrations in tumor cells are four times higher than in normal cells. When the calcium phosphorus product exceeds 60 mg2/dL2, there is an increased risk of calcium phosphate precipitation in the kidney tubules, which could lead to kidney failure. Accumulation of calcium phosphate product may also be cardiotoxic and can lead to cardiac arrhythmias. In addition, hyperphosphatemia can cause secondary hypocalcemia, which may lead to parasthesias, tetany, and cardiac arrhythmias.2,4
TLS is most common in tumors with high proliferative rates and high tumor burden, such as acute lymphoblastic leukemia and Burkitt’s lymphoma, but it can occur with other hematologic malignancies, such as T-cell precursor acute lymphocytic leukemia (ALL), B-cell precursor ALL, acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), anaplastic large cell lymphoma, and plasma cell disorders (e.g. multiple myeloma and plasmacytoma).6,7 TLS has also been reported with the treatment of solid organ nonhematologic tumors (see Table 3).
In hematologic tumors, TLS frequently is associated with cytotoxic chemotherapy, and less frequently with glucocorticoid treatment, monoclonal antibodies (eg, rituximab, bortezomab, imatinib), and radiation therapy.25-29
Patient factors, such as baseline kidney disease or lack of prophylactic/preventive measures for TLS, also increase the risk.4 TLS, however, can develop in patients classified as low risk (see Table 1.
TLS Prevention
Intravenous fluids. Every patient at intermediate or high risk of TLS should receive intravenous fluids (IVF) prior to cancer treatment; those at low risk may receive IVF based on the provider’s clinical judgment.30 The purpose of administering IVF is to generate high urine output to reduce the risk of precipitation of uric acid in the renal tubules.30 Both adults and children should receive approximately 2 to 3 L/m2 per day of IVF,30 and urine output should be maintained at 2 ml/kg/hr (or 4 to 6 ml/kg/hr for children <10kg).30 IVF should be cautiously administered in patients with renal insufficiency or heart failure, and diuretics may be used to maintain goal urine output. Recommended initial fluids are D51/4 normal saline, or normal saline for patients who are dehydrated or hyponatremic.30
Allopurinol. Allopurinol is usually also administered to patients at risk for developing TLS.30 Allopurinol inhibits the metabolism of hypoxanthine and xanthine to uric acid, which decreases the accumulation of uric acid in the renal tubules, thus preventing obstructive renal disease from precipitation of uric acid.4 The recommended dose of allopurinol is 100 mg/m2 every eight hours, and should not exceed 800 mg per day in adults. It should be started one to two days prior to induction chemotherapy and continued for three to seven days after the treatment and until uric acid levels and other electrolyte levels have returned to normal. The dose is adjusted to 50 mg/m2 every eight hours in patients with kidney failure.30
In some cases, allopurinol can lead to increased levels of xanthine crystals in the renal tubules, leading to acute kidney injury. Also, allopurinol does not have any effect on uric acid that has already been formed, so patients with elevated uric acid levels prior to the initiation of cancer therapy will not have any reduction in the levels of uric acid. Allopurinol reduces the degradation of other purines, so it can cause toxicity in patients on azathioprine and 6-mercaptopurine if the doses of these medications are not adjusted.
Rasburicase. Rasburicase is a recombinant urate oxidase, derived from aspergillus favus, which catalyzes the breakdown of uric acid to allantoin, which is a water-soluble product. Rasburicase is recommended as a first-line treatment for patients at high risk for clinical TLS.30 Rasburicase has an earlier onset than allopurinol and rapidly decreases serum levels of uric acid within four hours of administration.30,31 The recommended dose is 0.10 to 0.20 mg/kg once a day for five days in adults.30
A Phase III trial compared the efficiency and safety of rasburicase to rasburicase with allopurinol or allopurinol alone.32 A significantly higher normalization of uric acid was found in patients on rasburicase compared to allopurinol alone. The incidence of laboratory TLS was also significantly lower with rasburicase alone compared to allopurinol alone, and was even lower with allopurinol plus rasburicase. The incidence of acute kidney injury was the same with rasburicase alone or allopurinol alone but was higher with rasburicase plus allopurinol.
Serum uric acid, phosphorus, potassium, and calcium need to be monitored every four hours for 24 hours after the completion of chemotherapy in patients on rasburicase.4 The sample of blood drawn to check the uric acid levels has to be placed on ice and processed within four hours in order to avoid falsely lower levels of uric acid due to the conversion of uric acid to allantoin. Rasburicase is contraindicated in patients with G6PD deficiency and pregnant women, because one of the byproducts of uric acid breakdown is hydrogen peroxide, which can cause severe hemolysis and the formation of methemoglobin in these patients.30
Rasburicase has been approved for use in both children and adults, but there is more evidence for the use in children. Rasburicase has a black-box label for patients with anaphylaxis, methemoglobinemia, hemolysis, and hemoglobinuria, and there is a recommendation to check G6PD deficiency before use in high-risk patients.30
TLS Treatment
Alkalinization. Alkalinization of urine is controversial in the management of TLS. Urine alkalinization increases uric acid solubility but causes hyperphosphatemia and decreases calcium phosphate solubility, which can then deposit in the kidney once cancer treatment starts. Of note, hyperphosphatemia is much more difficult to correct than high levels of uric acid, and there are no clinical trials proving the superiority of urine alkalinization over normal saline.
Normalization of electrolytes. Electrolyte abnormalities should be corrected to avoid arrhythmias and seizures. Phosphorus levels >6.5 mg/dl (2.1 mmol/L) should be managed by restricting phosphorus intake, and by the use of phosphate binders (calcium acetate, calcium carbonate, sevelamer, lanthanum, or aluminum hydroxide). Aluminum hydroxide should be avoided in patients with renal insufficiency. In severe cases of hyperphosphatemia, dialysis should be considered.
Symptomatic hypocalcemia should be treated with calcium gluconate if changes are present on the electrocardiography (ECG). Hypocalcemia in the presence of hyperphosphatemia should be treated only in patients with tetany or cardiac arrhythmias; otherwise, hypocalcemia should not be treated until hyperphosphatemia has been corrected.
In cases of hyperkalemia, patients should be placed on a cardiac monitor and stabilized with calcium gluconate; kayexalate should be administered to reduce total body potassium. Other interventions, such as intravenous insulin given with dextrose, sodium bicarbonate, and albuterol, have a temporary effect on hyperkalemia and can be used as adjunct treatments in patients with severe hyperkalemia (>7). Hemodialysis should be strongly considered in severe cases of hyperkalemia, particularly in patients with persistently elevated potassium levels despite other treatments.
Back to the Case
Our patient was started on IVFs with close monitoring of his urine output. He was considered intermediate risk for developing TLS. Allopurinol, renally dosed, was administered for two days prior to initiating treatment with rituximab plus chemotherapy. His chemistry panel was monitored daily and he did not develop any form of TLS.
Bottom Line
TLS is a common oncology emergency in patients with hematologic malignancies. Preventative measures include starting IVF prior to cancer treatment, and administering allopurinol and/or rasburicase to patients at risk of developing TLS. Treatment should include normalizing electrolytes to avoid arrhythmias and seizures.
Dr. Akwe is assistant professor of medicine at the Emory University School of Medicine and a clinical instructor of medicine at the Morehouse School of Medicine, both in Atlanta. Dr. Smith is an assistant director for education in the division of hospital medicine at Emory. Both work as hospitalists at the Atlanta VA Medical Center.
References
- Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010;55:Suppl 3:S1-S13.
- Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
- Gertz MA. Managing tumor lysis syndrome in 2010. Leuk Lymphoma. 2010;51:179-180.
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844.
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3.
- Wössmann W, Schrappe M, Meyer U, et al. Incidence of tumor lysis syndrome in children with advanced stage Burkitt’s lymphoma/leukemia before and after introduction of prophylactic use of urate oxidase. Ann Hematol. 2003;82:160.
- Hussain K, Mazza JJ, Clouse LH. Tumor lysis syndrome (TLS) following fludarabine therapy Gemici C. Tumor lysis syndrome in solid tumors. J Clin Oncol. 2009;27:2738-2739
- Rostom AY, El-Hussainy G, Kandil A, Allam A. Tumor lysis syndrome following hemi-body irradiation for metastatic breast cancer. Ann Oncol. 2000;11:1349.
- Drakos P, Bar-Ziv J, Catane R. Tumor lysis syndrome in nonhematologic malignancies. Report of a case and review of the literature. Am J Clin Oncol. 1994;17:502.
- Baeksgaard L, Sørensen JB. Acute tumor lysis syndrome in solid tumors—a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187.
- Kalemkerian GP, Darwish B, Varterasian ML. Tumor lysis syndrome in small cell carcinoma and other solid tumors. Am J Med. 1997;103:363.
- Noh GY, Choe DH, Kim CH, Lee JC. Fatal tumor lysis syndrome during radiotherapy for non-small-cell lung cancer. J Clin Oncol. 2008;26:6005-6006.
- Pentheroudakis G, O’Neill VJ, Vasey P, Kaye SB. Spontaneous acute tumour lysis syndrome in patients with metastatic germ cell tumours. Report of two cases. Support Care Cancer. 2001;9:554.
- Joshita S, Yoshizawa K, Sano K, et al., A patient with advanced hepatocellular carcinoma treated with sorafenib tosylate showed massive tumor lysis with avoidance of tumor lysis syndrome. Intern Med. 2010;49:991-994.
- Huang WS, Yang CH. Sorafenib-induced tumor lysis syndrome in an advanced hepatocellular carcinoma patient. World J Gastroenterol. 2009;15:4464-4466.
- Bilgrami SF, Fallon BG. Tumor lysis syndrome after combination chemotherapy for ovarian cancer. Med Pediatr Oncol. 1993;21:521.
- Chan JK, Lin SS, McMeekin DS, Berman ML. Patients with malignancy requiring urgent therapy: CASE 3. Tumor lysis syndrome associated with chemotherapy in ovarian cancer. J Clin Oncol. 2005;23:6794.
- Godoy H, Kesterson JP, Lele S. Tumor lysis syndrome associated with carboplatin and paclitaxel in a woman with recurrent endometrial cancer. Int J Gynaecol Obstet. 2010;109:254.
- Shamseddine AI, Khalil AM, Wehbeh MH. Acute tumor lysis syndrome with squamous cell carcinoma of the vulva. Gynecol Oncol 1993;51:258
- Pinder EM, Atwal GS, Ayantunde AA, et al. Tumour lysis syndrome occurring in a patient with metastatic gastrointestinal stromal tumour treated with Glivec (imatinib mesylate, Gleevec, STI571). Sarcoma. 2007;2007:82012.
- Krishnan G, D’Silva K, Al-Janadi A. Cetuximab-related tumor lysis syndrome in metastatic colon carcinoma. J Clin Oncol. 2008;26:2406-2408.
- Oztop I, Demirkan B, Yaren A, et al. Rapid tumor lysis syndrome in a patient with metastatic colon cancer as a complication of treatment with 5-fluorouracil/leucoverin and irinotecan. Tumori. 2004;90:514.
- Lin CJ, Lim KH, Cheng YC, et al. Tumor lysis syndrome after treatment with gemcitabine for metastatic transitional cell carcinoma. Med Oncol. 2007;24:455.
- Malik IA, Abubakar S, Alam F, Khan A. Dexamethasone-induced tumor lysis syndrome in high-grade non-Hodgkin’s lymphoma. South Med J. 1994;87:409.
- Jabr FI. Acute tumor lysis syndrome induced by rituximab in diffuse large B-cell lymphoma. Int J Hematol. 2005;82:312.
- Sezer O, Vesole DH, Singhal S, et al. Bortezomib-induced tumor lysis syndrome in multiple myeloma. Clin Lymphoma Myeloma. 2006;7:233.
- Jensen M, Winkler U, Manzke O, et al. Rapid tumor lysis in a patient with B-cell chronic lymphocytic leukemia and lymphocytosis treated with an anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab). Ann Hematol. 1998;77:89.
- Linck D, Basara N, Tran V, et al. Peracute onset of severe tumor lysis syndrome immediately after 4 Gy fractionated TBI as part of reduced intensity preparative regimen in a patient with T-ALL with high tumor burden. Bone Marrow Transplant. 2003;31:935.
- Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767-2778. [Erratum, J Clin Oncol. 2010;28:708.]
- Cheuk DK, Chiang AK, Chan GC, Ha SY. Urate oxidase for the prevention and treatment of tumor lysis syndrome in children with cancer. Cochrane Database Syst Rev. 2010;(6):CD006945.
- Cortes J, Moore JO, Maziarz RT, et al. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone—results of a multicenter phase III study. J Clin Oncol. 2010;28:4207.
Case
A 25-year-old male with HIV/AIDS and a CD4 count of 65 cells/μL presents to the ED with intractable nausea and vomiting for one week. Laboratory evaluation revealed a white blood cell of 67,000 cells/mm3. An extended chemistry panel reveals creatinine 3.5 mg/dL, potassium 3.0 mmol/L, LDH 250 IU/L, and uric acid 5mg/dL. Calcium and phosphorus were both normal. The patient was admitted for further evaluation and management, and was later diagnosed with Burkitt’s lymphoma.
Overview
Tumor lysis syndrome (TLS) is an acute cell lysis of tumor cells with the release of cell content into circulation either spontaneously or in response to therapy, leading to hyperurecemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.1-3
TLS is one of the most common oncology emergencies encountered by hospitalists caring for patients with hematologic malignancies. The incidence and severity of TLS depend on the cell burden, cell proliferation rate, potential for cell lysis or chemo sensitivity, baseline clinical characteristics, and preventive measures taken (see Table 1).2,4
TLS is classified as laboratory or clinical. Laboratory TLS is described as the presence of two or more of the following serum abnormalities at the same time, present within three days before or seven days after the start of therapy.5
- Uric acid >8 mg/dL (475.8 micromole/L) or 25% increase;
- Potassium >6 mEq/L (6 mmol/L) or 25% increase;
- Phosphorus >6.5 mg/dL (2.1 mmol/L) for children or >4.5 mg/dl (1.45 mmol/L) for adults or 25% increase; and
- Calcium >7 mg/dL (1.75 mmol/L) or 25% increase.
Clinical TLS is defined as laboratory TLS in association with increased creatinine levels, seizures, cardiac arrhythmias, or death (see Table 2).5
Pathogenesis
Tumor cell lysis releases DNA, cytokines, phosphate, and potassium. DNA is metabolized into adenosine and guanosine, which are then converted into xanthines. Xanthines are oxidized by xanthine oxidase into uric acid, which is then excreted through the kidneys.
TLS develops when the accumulation of xanthine, uric acid, potassium, and phosphorus exceeds the kidney’s capacity to excrete them. Cytokines cause hypotension, inflammation, and kidney injury, and worsen the kidney’s excretory capacity. Damage to the kidneys also occurs by renal precipitation of uric acid, xanthine, and calcium phosphate.4
Phosphorus concentrations in tumor cells are four times higher than in normal cells. When the calcium phosphorus product exceeds 60 mg2/dL2, there is an increased risk of calcium phosphate precipitation in the kidney tubules, which could lead to kidney failure. Accumulation of calcium phosphate product may also be cardiotoxic and can lead to cardiac arrhythmias. In addition, hyperphosphatemia can cause secondary hypocalcemia, which may lead to parasthesias, tetany, and cardiac arrhythmias.2,4
TLS is most common in tumors with high proliferative rates and high tumor burden, such as acute lymphoblastic leukemia and Burkitt’s lymphoma, but it can occur with other hematologic malignancies, such as T-cell precursor acute lymphocytic leukemia (ALL), B-cell precursor ALL, acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), anaplastic large cell lymphoma, and plasma cell disorders (e.g. multiple myeloma and plasmacytoma).6,7 TLS has also been reported with the treatment of solid organ nonhematologic tumors (see Table 3).
In hematologic tumors, TLS frequently is associated with cytotoxic chemotherapy, and less frequently with glucocorticoid treatment, monoclonal antibodies (eg, rituximab, bortezomab, imatinib), and radiation therapy.25-29
Patient factors, such as baseline kidney disease or lack of prophylactic/preventive measures for TLS, also increase the risk.4 TLS, however, can develop in patients classified as low risk (see Table 1.
TLS Prevention
Intravenous fluids. Every patient at intermediate or high risk of TLS should receive intravenous fluids (IVF) prior to cancer treatment; those at low risk may receive IVF based on the provider’s clinical judgment.30 The purpose of administering IVF is to generate high urine output to reduce the risk of precipitation of uric acid in the renal tubules.30 Both adults and children should receive approximately 2 to 3 L/m2 per day of IVF,30 and urine output should be maintained at 2 ml/kg/hr (or 4 to 6 ml/kg/hr for children <10kg).30 IVF should be cautiously administered in patients with renal insufficiency or heart failure, and diuretics may be used to maintain goal urine output. Recommended initial fluids are D51/4 normal saline, or normal saline for patients who are dehydrated or hyponatremic.30
Allopurinol. Allopurinol is usually also administered to patients at risk for developing TLS.30 Allopurinol inhibits the metabolism of hypoxanthine and xanthine to uric acid, which decreases the accumulation of uric acid in the renal tubules, thus preventing obstructive renal disease from precipitation of uric acid.4 The recommended dose of allopurinol is 100 mg/m2 every eight hours, and should not exceed 800 mg per day in adults. It should be started one to two days prior to induction chemotherapy and continued for three to seven days after the treatment and until uric acid levels and other electrolyte levels have returned to normal. The dose is adjusted to 50 mg/m2 every eight hours in patients with kidney failure.30
In some cases, allopurinol can lead to increased levels of xanthine crystals in the renal tubules, leading to acute kidney injury. Also, allopurinol does not have any effect on uric acid that has already been formed, so patients with elevated uric acid levels prior to the initiation of cancer therapy will not have any reduction in the levels of uric acid. Allopurinol reduces the degradation of other purines, so it can cause toxicity in patients on azathioprine and 6-mercaptopurine if the doses of these medications are not adjusted.
Rasburicase. Rasburicase is a recombinant urate oxidase, derived from aspergillus favus, which catalyzes the breakdown of uric acid to allantoin, which is a water-soluble product. Rasburicase is recommended as a first-line treatment for patients at high risk for clinical TLS.30 Rasburicase has an earlier onset than allopurinol and rapidly decreases serum levels of uric acid within four hours of administration.30,31 The recommended dose is 0.10 to 0.20 mg/kg once a day for five days in adults.30
A Phase III trial compared the efficiency and safety of rasburicase to rasburicase with allopurinol or allopurinol alone.32 A significantly higher normalization of uric acid was found in patients on rasburicase compared to allopurinol alone. The incidence of laboratory TLS was also significantly lower with rasburicase alone compared to allopurinol alone, and was even lower with allopurinol plus rasburicase. The incidence of acute kidney injury was the same with rasburicase alone or allopurinol alone but was higher with rasburicase plus allopurinol.
Serum uric acid, phosphorus, potassium, and calcium need to be monitored every four hours for 24 hours after the completion of chemotherapy in patients on rasburicase.4 The sample of blood drawn to check the uric acid levels has to be placed on ice and processed within four hours in order to avoid falsely lower levels of uric acid due to the conversion of uric acid to allantoin. Rasburicase is contraindicated in patients with G6PD deficiency and pregnant women, because one of the byproducts of uric acid breakdown is hydrogen peroxide, which can cause severe hemolysis and the formation of methemoglobin in these patients.30
Rasburicase has been approved for use in both children and adults, but there is more evidence for the use in children. Rasburicase has a black-box label for patients with anaphylaxis, methemoglobinemia, hemolysis, and hemoglobinuria, and there is a recommendation to check G6PD deficiency before use in high-risk patients.30
TLS Treatment
Alkalinization. Alkalinization of urine is controversial in the management of TLS. Urine alkalinization increases uric acid solubility but causes hyperphosphatemia and decreases calcium phosphate solubility, which can then deposit in the kidney once cancer treatment starts. Of note, hyperphosphatemia is much more difficult to correct than high levels of uric acid, and there are no clinical trials proving the superiority of urine alkalinization over normal saline.
Normalization of electrolytes. Electrolyte abnormalities should be corrected to avoid arrhythmias and seizures. Phosphorus levels >6.5 mg/dl (2.1 mmol/L) should be managed by restricting phosphorus intake, and by the use of phosphate binders (calcium acetate, calcium carbonate, sevelamer, lanthanum, or aluminum hydroxide). Aluminum hydroxide should be avoided in patients with renal insufficiency. In severe cases of hyperphosphatemia, dialysis should be considered.
Symptomatic hypocalcemia should be treated with calcium gluconate if changes are present on the electrocardiography (ECG). Hypocalcemia in the presence of hyperphosphatemia should be treated only in patients with tetany or cardiac arrhythmias; otherwise, hypocalcemia should not be treated until hyperphosphatemia has been corrected.
In cases of hyperkalemia, patients should be placed on a cardiac monitor and stabilized with calcium gluconate; kayexalate should be administered to reduce total body potassium. Other interventions, such as intravenous insulin given with dextrose, sodium bicarbonate, and albuterol, have a temporary effect on hyperkalemia and can be used as adjunct treatments in patients with severe hyperkalemia (>7). Hemodialysis should be strongly considered in severe cases of hyperkalemia, particularly in patients with persistently elevated potassium levels despite other treatments.
Back to the Case
Our patient was started on IVFs with close monitoring of his urine output. He was considered intermediate risk for developing TLS. Allopurinol, renally dosed, was administered for two days prior to initiating treatment with rituximab plus chemotherapy. His chemistry panel was monitored daily and he did not develop any form of TLS.
Bottom Line
TLS is a common oncology emergency in patients with hematologic malignancies. Preventative measures include starting IVF prior to cancer treatment, and administering allopurinol and/or rasburicase to patients at risk of developing TLS. Treatment should include normalizing electrolytes to avoid arrhythmias and seizures.
Dr. Akwe is assistant professor of medicine at the Emory University School of Medicine and a clinical instructor of medicine at the Morehouse School of Medicine, both in Atlanta. Dr. Smith is an assistant director for education in the division of hospital medicine at Emory. Both work as hospitalists at the Atlanta VA Medical Center.
References
- Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010;55:Suppl 3:S1-S13.
- Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
- Gertz MA. Managing tumor lysis syndrome in 2010. Leuk Lymphoma. 2010;51:179-180.
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844.
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3.
- Wössmann W, Schrappe M, Meyer U, et al. Incidence of tumor lysis syndrome in children with advanced stage Burkitt’s lymphoma/leukemia before and after introduction of prophylactic use of urate oxidase. Ann Hematol. 2003;82:160.
- Hussain K, Mazza JJ, Clouse LH. Tumor lysis syndrome (TLS) following fludarabine therapy Gemici C. Tumor lysis syndrome in solid tumors. J Clin Oncol. 2009;27:2738-2739
- Rostom AY, El-Hussainy G, Kandil A, Allam A. Tumor lysis syndrome following hemi-body irradiation for metastatic breast cancer. Ann Oncol. 2000;11:1349.
- Drakos P, Bar-Ziv J, Catane R. Tumor lysis syndrome in nonhematologic malignancies. Report of a case and review of the literature. Am J Clin Oncol. 1994;17:502.
- Baeksgaard L, Sørensen JB. Acute tumor lysis syndrome in solid tumors—a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187.
- Kalemkerian GP, Darwish B, Varterasian ML. Tumor lysis syndrome in small cell carcinoma and other solid tumors. Am J Med. 1997;103:363.
- Noh GY, Choe DH, Kim CH, Lee JC. Fatal tumor lysis syndrome during radiotherapy for non-small-cell lung cancer. J Clin Oncol. 2008;26:6005-6006.
- Pentheroudakis G, O’Neill VJ, Vasey P, Kaye SB. Spontaneous acute tumour lysis syndrome in patients with metastatic germ cell tumours. Report of two cases. Support Care Cancer. 2001;9:554.
- Joshita S, Yoshizawa K, Sano K, et al., A patient with advanced hepatocellular carcinoma treated with sorafenib tosylate showed massive tumor lysis with avoidance of tumor lysis syndrome. Intern Med. 2010;49:991-994.
- Huang WS, Yang CH. Sorafenib-induced tumor lysis syndrome in an advanced hepatocellular carcinoma patient. World J Gastroenterol. 2009;15:4464-4466.
- Bilgrami SF, Fallon BG. Tumor lysis syndrome after combination chemotherapy for ovarian cancer. Med Pediatr Oncol. 1993;21:521.
- Chan JK, Lin SS, McMeekin DS, Berman ML. Patients with malignancy requiring urgent therapy: CASE 3. Tumor lysis syndrome associated with chemotherapy in ovarian cancer. J Clin Oncol. 2005;23:6794.
- Godoy H, Kesterson JP, Lele S. Tumor lysis syndrome associated with carboplatin and paclitaxel in a woman with recurrent endometrial cancer. Int J Gynaecol Obstet. 2010;109:254.
- Shamseddine AI, Khalil AM, Wehbeh MH. Acute tumor lysis syndrome with squamous cell carcinoma of the vulva. Gynecol Oncol 1993;51:258
- Pinder EM, Atwal GS, Ayantunde AA, et al. Tumour lysis syndrome occurring in a patient with metastatic gastrointestinal stromal tumour treated with Glivec (imatinib mesylate, Gleevec, STI571). Sarcoma. 2007;2007:82012.
- Krishnan G, D’Silva K, Al-Janadi A. Cetuximab-related tumor lysis syndrome in metastatic colon carcinoma. J Clin Oncol. 2008;26:2406-2408.
- Oztop I, Demirkan B, Yaren A, et al. Rapid tumor lysis syndrome in a patient with metastatic colon cancer as a complication of treatment with 5-fluorouracil/leucoverin and irinotecan. Tumori. 2004;90:514.
- Lin CJ, Lim KH, Cheng YC, et al. Tumor lysis syndrome after treatment with gemcitabine for metastatic transitional cell carcinoma. Med Oncol. 2007;24:455.
- Malik IA, Abubakar S, Alam F, Khan A. Dexamethasone-induced tumor lysis syndrome in high-grade non-Hodgkin’s lymphoma. South Med J. 1994;87:409.
- Jabr FI. Acute tumor lysis syndrome induced by rituximab in diffuse large B-cell lymphoma. Int J Hematol. 2005;82:312.
- Sezer O, Vesole DH, Singhal S, et al. Bortezomib-induced tumor lysis syndrome in multiple myeloma. Clin Lymphoma Myeloma. 2006;7:233.
- Jensen M, Winkler U, Manzke O, et al. Rapid tumor lysis in a patient with B-cell chronic lymphocytic leukemia and lymphocytosis treated with an anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab). Ann Hematol. 1998;77:89.
- Linck D, Basara N, Tran V, et al. Peracute onset of severe tumor lysis syndrome immediately after 4 Gy fractionated TBI as part of reduced intensity preparative regimen in a patient with T-ALL with high tumor burden. Bone Marrow Transplant. 2003;31:935.
- Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767-2778. [Erratum, J Clin Oncol. 2010;28:708.]
- Cheuk DK, Chiang AK, Chan GC, Ha SY. Urate oxidase for the prevention and treatment of tumor lysis syndrome in children with cancer. Cochrane Database Syst Rev. 2010;(6):CD006945.
- Cortes J, Moore JO, Maziarz RT, et al. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone—results of a multicenter phase III study. J Clin Oncol. 2010;28:4207.
Case
A 25-year-old male with HIV/AIDS and a CD4 count of 65 cells/μL presents to the ED with intractable nausea and vomiting for one week. Laboratory evaluation revealed a white blood cell of 67,000 cells/mm3. An extended chemistry panel reveals creatinine 3.5 mg/dL, potassium 3.0 mmol/L, LDH 250 IU/L, and uric acid 5mg/dL. Calcium and phosphorus were both normal. The patient was admitted for further evaluation and management, and was later diagnosed with Burkitt’s lymphoma.
Overview
Tumor lysis syndrome (TLS) is an acute cell lysis of tumor cells with the release of cell content into circulation either spontaneously or in response to therapy, leading to hyperurecemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.1-3
TLS is one of the most common oncology emergencies encountered by hospitalists caring for patients with hematologic malignancies. The incidence and severity of TLS depend on the cell burden, cell proliferation rate, potential for cell lysis or chemo sensitivity, baseline clinical characteristics, and preventive measures taken (see Table 1).2,4
TLS is classified as laboratory or clinical. Laboratory TLS is described as the presence of two or more of the following serum abnormalities at the same time, present within three days before or seven days after the start of therapy.5
- Uric acid >8 mg/dL (475.8 micromole/L) or 25% increase;
- Potassium >6 mEq/L (6 mmol/L) or 25% increase;
- Phosphorus >6.5 mg/dL (2.1 mmol/L) for children or >4.5 mg/dl (1.45 mmol/L) for adults or 25% increase; and
- Calcium >7 mg/dL (1.75 mmol/L) or 25% increase.
Clinical TLS is defined as laboratory TLS in association with increased creatinine levels, seizures, cardiac arrhythmias, or death (see Table 2).5
Pathogenesis
Tumor cell lysis releases DNA, cytokines, phosphate, and potassium. DNA is metabolized into adenosine and guanosine, which are then converted into xanthines. Xanthines are oxidized by xanthine oxidase into uric acid, which is then excreted through the kidneys.
TLS develops when the accumulation of xanthine, uric acid, potassium, and phosphorus exceeds the kidney’s capacity to excrete them. Cytokines cause hypotension, inflammation, and kidney injury, and worsen the kidney’s excretory capacity. Damage to the kidneys also occurs by renal precipitation of uric acid, xanthine, and calcium phosphate.4
Phosphorus concentrations in tumor cells are four times higher than in normal cells. When the calcium phosphorus product exceeds 60 mg2/dL2, there is an increased risk of calcium phosphate precipitation in the kidney tubules, which could lead to kidney failure. Accumulation of calcium phosphate product may also be cardiotoxic and can lead to cardiac arrhythmias. In addition, hyperphosphatemia can cause secondary hypocalcemia, which may lead to parasthesias, tetany, and cardiac arrhythmias.2,4
TLS is most common in tumors with high proliferative rates and high tumor burden, such as acute lymphoblastic leukemia and Burkitt’s lymphoma, but it can occur with other hematologic malignancies, such as T-cell precursor acute lymphocytic leukemia (ALL), B-cell precursor ALL, acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), anaplastic large cell lymphoma, and plasma cell disorders (e.g. multiple myeloma and plasmacytoma).6,7 TLS has also been reported with the treatment of solid organ nonhematologic tumors (see Table 3).
In hematologic tumors, TLS frequently is associated with cytotoxic chemotherapy, and less frequently with glucocorticoid treatment, monoclonal antibodies (eg, rituximab, bortezomab, imatinib), and radiation therapy.25-29
Patient factors, such as baseline kidney disease or lack of prophylactic/preventive measures for TLS, also increase the risk.4 TLS, however, can develop in patients classified as low risk (see Table 1.
TLS Prevention
Intravenous fluids. Every patient at intermediate or high risk of TLS should receive intravenous fluids (IVF) prior to cancer treatment; those at low risk may receive IVF based on the provider’s clinical judgment.30 The purpose of administering IVF is to generate high urine output to reduce the risk of precipitation of uric acid in the renal tubules.30 Both adults and children should receive approximately 2 to 3 L/m2 per day of IVF,30 and urine output should be maintained at 2 ml/kg/hr (or 4 to 6 ml/kg/hr for children <10kg).30 IVF should be cautiously administered in patients with renal insufficiency or heart failure, and diuretics may be used to maintain goal urine output. Recommended initial fluids are D51/4 normal saline, or normal saline for patients who are dehydrated or hyponatremic.30
Allopurinol. Allopurinol is usually also administered to patients at risk for developing TLS.30 Allopurinol inhibits the metabolism of hypoxanthine and xanthine to uric acid, which decreases the accumulation of uric acid in the renal tubules, thus preventing obstructive renal disease from precipitation of uric acid.4 The recommended dose of allopurinol is 100 mg/m2 every eight hours, and should not exceed 800 mg per day in adults. It should be started one to two days prior to induction chemotherapy and continued for three to seven days after the treatment and until uric acid levels and other electrolyte levels have returned to normal. The dose is adjusted to 50 mg/m2 every eight hours in patients with kidney failure.30
In some cases, allopurinol can lead to increased levels of xanthine crystals in the renal tubules, leading to acute kidney injury. Also, allopurinol does not have any effect on uric acid that has already been formed, so patients with elevated uric acid levels prior to the initiation of cancer therapy will not have any reduction in the levels of uric acid. Allopurinol reduces the degradation of other purines, so it can cause toxicity in patients on azathioprine and 6-mercaptopurine if the doses of these medications are not adjusted.
Rasburicase. Rasburicase is a recombinant urate oxidase, derived from aspergillus favus, which catalyzes the breakdown of uric acid to allantoin, which is a water-soluble product. Rasburicase is recommended as a first-line treatment for patients at high risk for clinical TLS.30 Rasburicase has an earlier onset than allopurinol and rapidly decreases serum levels of uric acid within four hours of administration.30,31 The recommended dose is 0.10 to 0.20 mg/kg once a day for five days in adults.30
A Phase III trial compared the efficiency and safety of rasburicase to rasburicase with allopurinol or allopurinol alone.32 A significantly higher normalization of uric acid was found in patients on rasburicase compared to allopurinol alone. The incidence of laboratory TLS was also significantly lower with rasburicase alone compared to allopurinol alone, and was even lower with allopurinol plus rasburicase. The incidence of acute kidney injury was the same with rasburicase alone or allopurinol alone but was higher with rasburicase plus allopurinol.
Serum uric acid, phosphorus, potassium, and calcium need to be monitored every four hours for 24 hours after the completion of chemotherapy in patients on rasburicase.4 The sample of blood drawn to check the uric acid levels has to be placed on ice and processed within four hours in order to avoid falsely lower levels of uric acid due to the conversion of uric acid to allantoin. Rasburicase is contraindicated in patients with G6PD deficiency and pregnant women, because one of the byproducts of uric acid breakdown is hydrogen peroxide, which can cause severe hemolysis and the formation of methemoglobin in these patients.30
Rasburicase has been approved for use in both children and adults, but there is more evidence for the use in children. Rasburicase has a black-box label for patients with anaphylaxis, methemoglobinemia, hemolysis, and hemoglobinuria, and there is a recommendation to check G6PD deficiency before use in high-risk patients.30
TLS Treatment
Alkalinization. Alkalinization of urine is controversial in the management of TLS. Urine alkalinization increases uric acid solubility but causes hyperphosphatemia and decreases calcium phosphate solubility, which can then deposit in the kidney once cancer treatment starts. Of note, hyperphosphatemia is much more difficult to correct than high levels of uric acid, and there are no clinical trials proving the superiority of urine alkalinization over normal saline.
Normalization of electrolytes. Electrolyte abnormalities should be corrected to avoid arrhythmias and seizures. Phosphorus levels >6.5 mg/dl (2.1 mmol/L) should be managed by restricting phosphorus intake, and by the use of phosphate binders (calcium acetate, calcium carbonate, sevelamer, lanthanum, or aluminum hydroxide). Aluminum hydroxide should be avoided in patients with renal insufficiency. In severe cases of hyperphosphatemia, dialysis should be considered.
Symptomatic hypocalcemia should be treated with calcium gluconate if changes are present on the electrocardiography (ECG). Hypocalcemia in the presence of hyperphosphatemia should be treated only in patients with tetany or cardiac arrhythmias; otherwise, hypocalcemia should not be treated until hyperphosphatemia has been corrected.
In cases of hyperkalemia, patients should be placed on a cardiac monitor and stabilized with calcium gluconate; kayexalate should be administered to reduce total body potassium. Other interventions, such as intravenous insulin given with dextrose, sodium bicarbonate, and albuterol, have a temporary effect on hyperkalemia and can be used as adjunct treatments in patients with severe hyperkalemia (>7). Hemodialysis should be strongly considered in severe cases of hyperkalemia, particularly in patients with persistently elevated potassium levels despite other treatments.
Back to the Case
Our patient was started on IVFs with close monitoring of his urine output. He was considered intermediate risk for developing TLS. Allopurinol, renally dosed, was administered for two days prior to initiating treatment with rituximab plus chemotherapy. His chemistry panel was monitored daily and he did not develop any form of TLS.
Bottom Line
TLS is a common oncology emergency in patients with hematologic malignancies. Preventative measures include starting IVF prior to cancer treatment, and administering allopurinol and/or rasburicase to patients at risk of developing TLS. Treatment should include normalizing electrolytes to avoid arrhythmias and seizures.
Dr. Akwe is assistant professor of medicine at the Emory University School of Medicine and a clinical instructor of medicine at the Morehouse School of Medicine, both in Atlanta. Dr. Smith is an assistant director for education in the division of hospital medicine at Emory. Both work as hospitalists at the Atlanta VA Medical Center.
References
- Abu-Alfa AK, Younes A. Tumor lysis syndrome and acute kidney injury: evaluation, prevention, and management. Am J Kidney Dis. 2010;55:Suppl 3:S1-S13.
- Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578-586.
- Gertz MA. Managing tumor lysis syndrome in 2010. Leuk Lymphoma. 2010;51:179-180.
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844.
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3.
- Wössmann W, Schrappe M, Meyer U, et al. Incidence of tumor lysis syndrome in children with advanced stage Burkitt’s lymphoma/leukemia before and after introduction of prophylactic use of urate oxidase. Ann Hematol. 2003;82:160.
- Hussain K, Mazza JJ, Clouse LH. Tumor lysis syndrome (TLS) following fludarabine therapy Gemici C. Tumor lysis syndrome in solid tumors. J Clin Oncol. 2009;27:2738-2739
- Rostom AY, El-Hussainy G, Kandil A, Allam A. Tumor lysis syndrome following hemi-body irradiation for metastatic breast cancer. Ann Oncol. 2000;11:1349.
- Drakos P, Bar-Ziv J, Catane R. Tumor lysis syndrome in nonhematologic malignancies. Report of a case and review of the literature. Am J Clin Oncol. 1994;17:502.
- Baeksgaard L, Sørensen JB. Acute tumor lysis syndrome in solid tumors—a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187.
- Kalemkerian GP, Darwish B, Varterasian ML. Tumor lysis syndrome in small cell carcinoma and other solid tumors. Am J Med. 1997;103:363.
- Noh GY, Choe DH, Kim CH, Lee JC. Fatal tumor lysis syndrome during radiotherapy for non-small-cell lung cancer. J Clin Oncol. 2008;26:6005-6006.
- Pentheroudakis G, O’Neill VJ, Vasey P, Kaye SB. Spontaneous acute tumour lysis syndrome in patients with metastatic germ cell tumours. Report of two cases. Support Care Cancer. 2001;9:554.
- Joshita S, Yoshizawa K, Sano K, et al., A patient with advanced hepatocellular carcinoma treated with sorafenib tosylate showed massive tumor lysis with avoidance of tumor lysis syndrome. Intern Med. 2010;49:991-994.
- Huang WS, Yang CH. Sorafenib-induced tumor lysis syndrome in an advanced hepatocellular carcinoma patient. World J Gastroenterol. 2009;15:4464-4466.
- Bilgrami SF, Fallon BG. Tumor lysis syndrome after combination chemotherapy for ovarian cancer. Med Pediatr Oncol. 1993;21:521.
- Chan JK, Lin SS, McMeekin DS, Berman ML. Patients with malignancy requiring urgent therapy: CASE 3. Tumor lysis syndrome associated with chemotherapy in ovarian cancer. J Clin Oncol. 2005;23:6794.
- Godoy H, Kesterson JP, Lele S. Tumor lysis syndrome associated with carboplatin and paclitaxel in a woman with recurrent endometrial cancer. Int J Gynaecol Obstet. 2010;109:254.
- Shamseddine AI, Khalil AM, Wehbeh MH. Acute tumor lysis syndrome with squamous cell carcinoma of the vulva. Gynecol Oncol 1993;51:258
- Pinder EM, Atwal GS, Ayantunde AA, et al. Tumour lysis syndrome occurring in a patient with metastatic gastrointestinal stromal tumour treated with Glivec (imatinib mesylate, Gleevec, STI571). Sarcoma. 2007;2007:82012.
- Krishnan G, D’Silva K, Al-Janadi A. Cetuximab-related tumor lysis syndrome in metastatic colon carcinoma. J Clin Oncol. 2008;26:2406-2408.
- Oztop I, Demirkan B, Yaren A, et al. Rapid tumor lysis syndrome in a patient with metastatic colon cancer as a complication of treatment with 5-fluorouracil/leucoverin and irinotecan. Tumori. 2004;90:514.
- Lin CJ, Lim KH, Cheng YC, et al. Tumor lysis syndrome after treatment with gemcitabine for metastatic transitional cell carcinoma. Med Oncol. 2007;24:455.
- Malik IA, Abubakar S, Alam F, Khan A. Dexamethasone-induced tumor lysis syndrome in high-grade non-Hodgkin’s lymphoma. South Med J. 1994;87:409.
- Jabr FI. Acute tumor lysis syndrome induced by rituximab in diffuse large B-cell lymphoma. Int J Hematol. 2005;82:312.
- Sezer O, Vesole DH, Singhal S, et al. Bortezomib-induced tumor lysis syndrome in multiple myeloma. Clin Lymphoma Myeloma. 2006;7:233.
- Jensen M, Winkler U, Manzke O, et al. Rapid tumor lysis in a patient with B-cell chronic lymphocytic leukemia and lymphocytosis treated with an anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab). Ann Hematol. 1998;77:89.
- Linck D, Basara N, Tran V, et al. Peracute onset of severe tumor lysis syndrome immediately after 4 Gy fractionated TBI as part of reduced intensity preparative regimen in a patient with T-ALL with high tumor burden. Bone Marrow Transplant. 2003;31:935.
- Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26(16):2767-2778. [Erratum, J Clin Oncol. 2010;28:708.]
- Cheuk DK, Chiang AK, Chan GC, Ha SY. Urate oxidase for the prevention and treatment of tumor lysis syndrome in children with cancer. Cochrane Database Syst Rev. 2010;(6):CD006945.
- Cortes J, Moore JO, Maziarz RT, et al. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone—results of a multicenter phase III study. J Clin Oncol. 2010;28:4207.
Southern Hospital Medicine Conference Drives Home the Value of Hospitalists
More than 300 hospitalists and other clinicians recently attended the 13th annual Southern Hospital Medicine Conference in Atlanta. The conference is a joint collaboration between the Emory University School of Medicine in Atlanta and Ochsner Health System New Orleans. The meeting site has alternated between the two cities each year since 2005.
The prevailing conference theme in 2012 was “Value and Values in Hospital Medicine,” alluding to the value that hospitalists bring to the medical community and hospitals and the values shared by hospitalists. The conference offered five pre-courses and more than 50 sessions focused on educating hospitalists on current best practices within core topic areas, including clinical care, quality improvement, healthcare information technology, innovative care models, systems of care, and transitions of care. A judged poster competition featured research and clinical vignettes abstracts, with interesting clinical cases as well as new research in hospital medicine.
One of the highlights of this year’s conference was the keynote address delivered by Dr. William A. Bornstein, chief quality and medical officer of Emory Healthcare. Dr. Bornstein discussed the various aspects of quality and cost in hospital care. He described the challenges in defining quality and measuring cost when trying to calculate the “value” equation in medicine (value=quality/cost). He outlined the Institute of Medicine’s previously described STEEEP (safe, timely, effective, efficient, equitable, patient-centered) aims of quality in 2001.
Dr. Bornstein’s own definition for quality is “partnering with patients and families to reliably, 100% of the time, deliver when, where, and how they want it—and with minimal waste—care based on the best available evidence and consistent with patient and family values and preferences.” To measure outcome, he said, we need to address system structure (what’s in place before the patient arrives), process (what we do for the patient), and culture (how we can get the buy-in from all stakeholders). The sum of these factors achieves outcome, which requires risk adjustment and, ideally, long-term follow-up data, he said.
Dr. Bornstein also discussed the need to develop standard processes whereby equivalent clinicians can follow similar processes to achieve the same results. When physicians “do it the same” (i.e. standardized protocols), error rates and cost decrease, he explained.
Dr. Bornstein also focused on transformative solutions to address problems in healthcare as a whole, rather than attempting to fix problems piecemeal.
Jason Stein, MD, SFHM, offered another conference highlight: a pre-conference program and plenary session on an innovative approach to improve hospital outcomes through implementation of the accountable-care unit (ACU). Dr. Stein, director of the clinical research program at Emory School of Medicine, described the current state of hospital care as asynchronous, with various providers caring for the patient without much coordination. For example, the physician sees the patient at 9 a.m., followed by the nurse at 10 a.m., and then finally the visiting family at 11 a.m. The ACU model of care would involve all the providers rounding with the patient and family at a scheduled time daily to provide synchronous care.
Dr. Stein described an ACU as a geographic inpatient area consistently responsible for the clinical, service, and cost outcomes it produces. Features of this unit include:
- Assignment of physicians by units to enhance predictability;
- Cohesiveness and communication;
- Structured interdisciplinary bedside rounds to consistently deliver evidence-based, patient-centered care;
- Evaluation of performance data by unit instead of facility or service line; and
- A dyad partnership involving a nurse unit director and a physician unit medical director.
ACU implementation at Emory has led to decreased mortality, reduced length of stay, and improved patient satisfaction compared to traditional units, according to Dr. Stein. While the ACU might not be suited for all, he said, all hospitals can learn from various components of this innovative approach to deliver better patient care.
The ever-changing state of HM in the U.S. remains a challenge, but it continues to generate innovation and excitement. The high number of engaged participants from 30 different states attending the 13th annual Southern Hospital Medicine Conference demonstrates that hospitalists are eager to learn and ready to improve their practice in order to provide high-value healthcare in U.S. hospitals today.
Dr. Lee is vice chairman in the department of hospital medicine at Ochsner Health System. Dr. Smith is an assistant director for education in the division of hospital medicine at Emory University. Dr. Deitelzweig is system chairman in the department of hospital medicine and medical director for regional business development at Ochsner Health System. Dr. Wang is the division director of hospital medicine at Emory University. Dr. Dressler is director for education in the division of hospital medicine and an associate program director for the J. Willis Hurst Internal Medicine Residency Program at Emory University.
More than 300 hospitalists and other clinicians recently attended the 13th annual Southern Hospital Medicine Conference in Atlanta. The conference is a joint collaboration between the Emory University School of Medicine in Atlanta and Ochsner Health System New Orleans. The meeting site has alternated between the two cities each year since 2005.
The prevailing conference theme in 2012 was “Value and Values in Hospital Medicine,” alluding to the value that hospitalists bring to the medical community and hospitals and the values shared by hospitalists. The conference offered five pre-courses and more than 50 sessions focused on educating hospitalists on current best practices within core topic areas, including clinical care, quality improvement, healthcare information technology, innovative care models, systems of care, and transitions of care. A judged poster competition featured research and clinical vignettes abstracts, with interesting clinical cases as well as new research in hospital medicine.
One of the highlights of this year’s conference was the keynote address delivered by Dr. William A. Bornstein, chief quality and medical officer of Emory Healthcare. Dr. Bornstein discussed the various aspects of quality and cost in hospital care. He described the challenges in defining quality and measuring cost when trying to calculate the “value” equation in medicine (value=quality/cost). He outlined the Institute of Medicine’s previously described STEEEP (safe, timely, effective, efficient, equitable, patient-centered) aims of quality in 2001.
Dr. Bornstein’s own definition for quality is “partnering with patients and families to reliably, 100% of the time, deliver when, where, and how they want it—and with minimal waste—care based on the best available evidence and consistent with patient and family values and preferences.” To measure outcome, he said, we need to address system structure (what’s in place before the patient arrives), process (what we do for the patient), and culture (how we can get the buy-in from all stakeholders). The sum of these factors achieves outcome, which requires risk adjustment and, ideally, long-term follow-up data, he said.
Dr. Bornstein also discussed the need to develop standard processes whereby equivalent clinicians can follow similar processes to achieve the same results. When physicians “do it the same” (i.e. standardized protocols), error rates and cost decrease, he explained.
Dr. Bornstein also focused on transformative solutions to address problems in healthcare as a whole, rather than attempting to fix problems piecemeal.
Jason Stein, MD, SFHM, offered another conference highlight: a pre-conference program and plenary session on an innovative approach to improve hospital outcomes through implementation of the accountable-care unit (ACU). Dr. Stein, director of the clinical research program at Emory School of Medicine, described the current state of hospital care as asynchronous, with various providers caring for the patient without much coordination. For example, the physician sees the patient at 9 a.m., followed by the nurse at 10 a.m., and then finally the visiting family at 11 a.m. The ACU model of care would involve all the providers rounding with the patient and family at a scheduled time daily to provide synchronous care.
Dr. Stein described an ACU as a geographic inpatient area consistently responsible for the clinical, service, and cost outcomes it produces. Features of this unit include:
- Assignment of physicians by units to enhance predictability;
- Cohesiveness and communication;
- Structured interdisciplinary bedside rounds to consistently deliver evidence-based, patient-centered care;
- Evaluation of performance data by unit instead of facility or service line; and
- A dyad partnership involving a nurse unit director and a physician unit medical director.
ACU implementation at Emory has led to decreased mortality, reduced length of stay, and improved patient satisfaction compared to traditional units, according to Dr. Stein. While the ACU might not be suited for all, he said, all hospitals can learn from various components of this innovative approach to deliver better patient care.
The ever-changing state of HM in the U.S. remains a challenge, but it continues to generate innovation and excitement. The high number of engaged participants from 30 different states attending the 13th annual Southern Hospital Medicine Conference demonstrates that hospitalists are eager to learn and ready to improve their practice in order to provide high-value healthcare in U.S. hospitals today.
Dr. Lee is vice chairman in the department of hospital medicine at Ochsner Health System. Dr. Smith is an assistant director for education in the division of hospital medicine at Emory University. Dr. Deitelzweig is system chairman in the department of hospital medicine and medical director for regional business development at Ochsner Health System. Dr. Wang is the division director of hospital medicine at Emory University. Dr. Dressler is director for education in the division of hospital medicine and an associate program director for the J. Willis Hurst Internal Medicine Residency Program at Emory University.
More than 300 hospitalists and other clinicians recently attended the 13th annual Southern Hospital Medicine Conference in Atlanta. The conference is a joint collaboration between the Emory University School of Medicine in Atlanta and Ochsner Health System New Orleans. The meeting site has alternated between the two cities each year since 2005.
The prevailing conference theme in 2012 was “Value and Values in Hospital Medicine,” alluding to the value that hospitalists bring to the medical community and hospitals and the values shared by hospitalists. The conference offered five pre-courses and more than 50 sessions focused on educating hospitalists on current best practices within core topic areas, including clinical care, quality improvement, healthcare information technology, innovative care models, systems of care, and transitions of care. A judged poster competition featured research and clinical vignettes abstracts, with interesting clinical cases as well as new research in hospital medicine.
One of the highlights of this year’s conference was the keynote address delivered by Dr. William A. Bornstein, chief quality and medical officer of Emory Healthcare. Dr. Bornstein discussed the various aspects of quality and cost in hospital care. He described the challenges in defining quality and measuring cost when trying to calculate the “value” equation in medicine (value=quality/cost). He outlined the Institute of Medicine’s previously described STEEEP (safe, timely, effective, efficient, equitable, patient-centered) aims of quality in 2001.
Dr. Bornstein’s own definition for quality is “partnering with patients and families to reliably, 100% of the time, deliver when, where, and how they want it—and with minimal waste—care based on the best available evidence and consistent with patient and family values and preferences.” To measure outcome, he said, we need to address system structure (what’s in place before the patient arrives), process (what we do for the patient), and culture (how we can get the buy-in from all stakeholders). The sum of these factors achieves outcome, which requires risk adjustment and, ideally, long-term follow-up data, he said.
Dr. Bornstein also discussed the need to develop standard processes whereby equivalent clinicians can follow similar processes to achieve the same results. When physicians “do it the same” (i.e. standardized protocols), error rates and cost decrease, he explained.
Dr. Bornstein also focused on transformative solutions to address problems in healthcare as a whole, rather than attempting to fix problems piecemeal.
Jason Stein, MD, SFHM, offered another conference highlight: a pre-conference program and plenary session on an innovative approach to improve hospital outcomes through implementation of the accountable-care unit (ACU). Dr. Stein, director of the clinical research program at Emory School of Medicine, described the current state of hospital care as asynchronous, with various providers caring for the patient without much coordination. For example, the physician sees the patient at 9 a.m., followed by the nurse at 10 a.m., and then finally the visiting family at 11 a.m. The ACU model of care would involve all the providers rounding with the patient and family at a scheduled time daily to provide synchronous care.
Dr. Stein described an ACU as a geographic inpatient area consistently responsible for the clinical, service, and cost outcomes it produces. Features of this unit include:
- Assignment of physicians by units to enhance predictability;
- Cohesiveness and communication;
- Structured interdisciplinary bedside rounds to consistently deliver evidence-based, patient-centered care;
- Evaluation of performance data by unit instead of facility or service line; and
- A dyad partnership involving a nurse unit director and a physician unit medical director.
ACU implementation at Emory has led to decreased mortality, reduced length of stay, and improved patient satisfaction compared to traditional units, according to Dr. Stein. While the ACU might not be suited for all, he said, all hospitals can learn from various components of this innovative approach to deliver better patient care.
The ever-changing state of HM in the U.S. remains a challenge, but it continues to generate innovation and excitement. The high number of engaged participants from 30 different states attending the 13th annual Southern Hospital Medicine Conference demonstrates that hospitalists are eager to learn and ready to improve their practice in order to provide high-value healthcare in U.S. hospitals today.
Dr. Lee is vice chairman in the department of hospital medicine at Ochsner Health System. Dr. Smith is an assistant director for education in the division of hospital medicine at Emory University. Dr. Deitelzweig is system chairman in the department of hospital medicine and medical director for regional business development at Ochsner Health System. Dr. Wang is the division director of hospital medicine at Emory University. Dr. Dressler is director for education in the division of hospital medicine and an associate program director for the J. Willis Hurst Internal Medicine Residency Program at Emory University.
ITL: Physician Reviews of HM-Relevant Research
Clinical question: Does treatment with drotrecogin alfa (activated) reduce mortality in patients with septic shock?
Background: Recombinant human activated protein C, or drotrecogin alfa (activated) (DrotAA), was approved for the treatment of patients with severe sepsis in 2001 on the basis of the Prospective Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study. Since its approval, conflicting reports about its efficacy have surfaced.
Study design: Double-blind, randomized-controlled trial.
Setting: Multicenter, multinational trial.
Synopsis: This trial enrolled 1,697 patients with septic shock to receive either DrotAA or placebo. At 28 days, 223 of 846 patients (26.4%) in the DrotAA group and 202 of 834 (24.2%) in the placebo group had died (relative risk in the DrotAA group, 1.09; 95% confidence interval, 0.92 to 1.28; P=0.31). At 90 days, there was still no significant difference in mortality. Mortality was also unchanged in patients with severe protein C deficiency at baseline. This lack of mortality benefit with either therapy persisted across all predefined subgroups in this study.
The incidence of nonserious bleeding was more common among patients who received DrotAA than among those in the placebo group (8.6% vs. 4.8%, P=0.002), but the incidence of serious bleeding events was similar in both groups. This study was appropriately powered after adjusting the sample size when aggregate mortality was found to be lower than anticipated.
Bottom line: DrotAA does not significantly reduce mortality at 28 or 90 days in patients with septic shock.
Citation: Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064.
Read more of our physician reviews of recent, HM-relevant literature.
Clinical question: Does treatment with drotrecogin alfa (activated) reduce mortality in patients with septic shock?
Background: Recombinant human activated protein C, or drotrecogin alfa (activated) (DrotAA), was approved for the treatment of patients with severe sepsis in 2001 on the basis of the Prospective Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study. Since its approval, conflicting reports about its efficacy have surfaced.
Study design: Double-blind, randomized-controlled trial.
Setting: Multicenter, multinational trial.
Synopsis: This trial enrolled 1,697 patients with septic shock to receive either DrotAA or placebo. At 28 days, 223 of 846 patients (26.4%) in the DrotAA group and 202 of 834 (24.2%) in the placebo group had died (relative risk in the DrotAA group, 1.09; 95% confidence interval, 0.92 to 1.28; P=0.31). At 90 days, there was still no significant difference in mortality. Mortality was also unchanged in patients with severe protein C deficiency at baseline. This lack of mortality benefit with either therapy persisted across all predefined subgroups in this study.
The incidence of nonserious bleeding was more common among patients who received DrotAA than among those in the placebo group (8.6% vs. 4.8%, P=0.002), but the incidence of serious bleeding events was similar in both groups. This study was appropriately powered after adjusting the sample size when aggregate mortality was found to be lower than anticipated.
Bottom line: DrotAA does not significantly reduce mortality at 28 or 90 days in patients with septic shock.
Citation: Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064.
Read more of our physician reviews of recent, HM-relevant literature.
Clinical question: Does treatment with drotrecogin alfa (activated) reduce mortality in patients with septic shock?
Background: Recombinant human activated protein C, or drotrecogin alfa (activated) (DrotAA), was approved for the treatment of patients with severe sepsis in 2001 on the basis of the Prospective Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study. Since its approval, conflicting reports about its efficacy have surfaced.
Study design: Double-blind, randomized-controlled trial.
Setting: Multicenter, multinational trial.
Synopsis: This trial enrolled 1,697 patients with septic shock to receive either DrotAA or placebo. At 28 days, 223 of 846 patients (26.4%) in the DrotAA group and 202 of 834 (24.2%) in the placebo group had died (relative risk in the DrotAA group, 1.09; 95% confidence interval, 0.92 to 1.28; P=0.31). At 90 days, there was still no significant difference in mortality. Mortality was also unchanged in patients with severe protein C deficiency at baseline. This lack of mortality benefit with either therapy persisted across all predefined subgroups in this study.
The incidence of nonserious bleeding was more common among patients who received DrotAA than among those in the placebo group (8.6% vs. 4.8%, P=0.002), but the incidence of serious bleeding events was similar in both groups. This study was appropriately powered after adjusting the sample size when aggregate mortality was found to be lower than anticipated.
Bottom line: DrotAA does not significantly reduce mortality at 28 or 90 days in patients with septic shock.
Citation: Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064.
Read more of our physician reviews of recent, HM-relevant literature.
ITL: Physician Reviews of HM-Relevant Research
Clinical question: Does treatment with drotrecogin alfa (activated) reduce mortality in patients with septic shock?
Background: Recombinant human activated protein C, or drotrecogin alfa (activated) (DrotAA), was approved for the treatment of patients with severe sepsis in 2001 on the basis of the Prospective Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study. Since approval, conflicting reports about its efficacy have surfaced.
Study design: Double-blind, randomized-controlled trial.
Setting: Multicenter, multinational trial.
Synopsis: This trial enrolled 1,697 patients with septic shock to receive either DrotAA or placebo. At 28 days, 223 of 846 patients (26.4%) in the DrotAA group and 202 of 834 (24.2%) in the placebo group had died (relative risk in the DrotAA group, 1.09; 95% confidence interval, 0.92 to 1.28; P=0.31). At 90 days, there was still no significant difference in mortality. Mortality was also unchanged in patients with severe protein C deficiency at baseline. This lack of mortality benefit with either therapy persisted across all predefined subgroups in this study.
The incidence of non-serious bleeding was more common among patients who received DrotAA than among those in the placebo group (8.6% vs. 4.8%, P=0.002), but the incidence of serious bleeding events was similar in both groups. This study was appropriately powered after adjusting the sample size when aggregate mortality was found to be lower than anticipated.
Bottom line: DrotAA does not significantly reduce mortality at 28 or 90 days in patients with septic shock.
Citation: Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064.
Read more of our physician reviews of recent, HM-relevant literature.
Clinical question: Does treatment with drotrecogin alfa (activated) reduce mortality in patients with septic shock?
Background: Recombinant human activated protein C, or drotrecogin alfa (activated) (DrotAA), was approved for the treatment of patients with severe sepsis in 2001 on the basis of the Prospective Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study. Since approval, conflicting reports about its efficacy have surfaced.
Study design: Double-blind, randomized-controlled trial.
Setting: Multicenter, multinational trial.
Synopsis: This trial enrolled 1,697 patients with septic shock to receive either DrotAA or placebo. At 28 days, 223 of 846 patients (26.4%) in the DrotAA group and 202 of 834 (24.2%) in the placebo group had died (relative risk in the DrotAA group, 1.09; 95% confidence interval, 0.92 to 1.28; P=0.31). At 90 days, there was still no significant difference in mortality. Mortality was also unchanged in patients with severe protein C deficiency at baseline. This lack of mortality benefit with either therapy persisted across all predefined subgroups in this study.
The incidence of non-serious bleeding was more common among patients who received DrotAA than among those in the placebo group (8.6% vs. 4.8%, P=0.002), but the incidence of serious bleeding events was similar in both groups. This study was appropriately powered after adjusting the sample size when aggregate mortality was found to be lower than anticipated.
Bottom line: DrotAA does not significantly reduce mortality at 28 or 90 days in patients with septic shock.
Citation: Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064.
Read more of our physician reviews of recent, HM-relevant literature.
Clinical question: Does treatment with drotrecogin alfa (activated) reduce mortality in patients with septic shock?
Background: Recombinant human activated protein C, or drotrecogin alfa (activated) (DrotAA), was approved for the treatment of patients with severe sepsis in 2001 on the basis of the Prospective Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study. Since approval, conflicting reports about its efficacy have surfaced.
Study design: Double-blind, randomized-controlled trial.
Setting: Multicenter, multinational trial.
Synopsis: This trial enrolled 1,697 patients with septic shock to receive either DrotAA or placebo. At 28 days, 223 of 846 patients (26.4%) in the DrotAA group and 202 of 834 (24.2%) in the placebo group had died (relative risk in the DrotAA group, 1.09; 95% confidence interval, 0.92 to 1.28; P=0.31). At 90 days, there was still no significant difference in mortality. Mortality was also unchanged in patients with severe protein C deficiency at baseline. This lack of mortality benefit with either therapy persisted across all predefined subgroups in this study.
The incidence of non-serious bleeding was more common among patients who received DrotAA than among those in the placebo group (8.6% vs. 4.8%, P=0.002), but the incidence of serious bleeding events was similar in both groups. This study was appropriately powered after adjusting the sample size when aggregate mortality was found to be lower than anticipated.
Bottom line: DrotAA does not significantly reduce mortality at 28 or 90 days in patients with septic shock.
Citation: Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055-2064.
Read more of our physician reviews of recent, HM-relevant literature.
ITL: Physician Reviews of HM-Relevant Research
In This Edition
Literature At A Glance
A guide to this month’s studies
- Warfarin vs. aspirin in heart-failure patients
- Aspirin after anticoagulation prevents VTE recurrence
- Novel oral anticoagulants vs. warfarin in afib patients
- Intravenous metronidazole for mild C. diff infection
- Frequency of Foley catheter complications vs. CAUTI
- Intensive hyperglycemia control in noncritical hospitalized patients
- Risk score predicts 30-day mortality after noncardiac surgery
- Temperature, white blood cell count are not sensitive predictors of bacteremia
Warfarin Reduces Stoke but Increases Hemorrhage Compared with Aspirin in Patients with Heart Failure and Sinus Rhythm
Clinical question: Is warfarin superior to aspirin with regard to clinical outcomes in patients with heart failure who are in sinus rhythm?
Background: Heart failure is associated with stroke and death potentially caused by atherothrombotic events. Anticoagulation is efficacious in some heart failure patients with atrial fibrillation or significant valvular disease, but the role of anticoagulation versus aspirin in patients with chronic heart failure and sinus rhythm is unclear.
Study design: Double-blind randomized controlled trial.
Setting: Multicenter, multinational trial involving outpatients.
Synopsis: This double-blind, double-dummy trial involving 2,305 patients with sinus rhythm and reduced left ventricular ejection fraction (<35%) showed no significant difference in the primary combined outcome (ischemic stroke, intracerebral hemorrhage, or death) in those treated with warfarin as compared with aspirin. Warfarin did significantly reduce the rate of ischemic stroke by 0.64 events per 100 patient-years (absolute risk reduction 2.2%, number needed to treat 45) when compared with aspirin, with no significant difference in the rate of intracerebral hemorrhage. This outcome was offset by an increased rate of major hemorrhage by 0.91 events per 100 patient-years (absolute risk increase 3.1%, number needed to harm 32).
This study included patients from all functional classes of heart failure, with a protocol to initiate treatment with other standard heart failure medications. Patients with an indication for either warfarin or aspirin were excluded. Due to recruitment difficulties, the power of this study was reduced. Other study limitations included a relatively low percentage of time that patients on warfarin were in therapeutic range and a substantial period of follow-up time in which patients did not receive the assigned study treatments.
Bottom line: The benefit of reduced stoke in patients with heart failure and sinus rhythm who take warfarin over aspirin is counteracted by an increased risk for serious bleeding outcomes.
Citation: Homma S, Thompson JLP, Pullicino PM, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366(20):1859-1869.
Aspirin Use after Recommended Anticoagulation Treatment Prevents Recurrence in VTE Patients
Clinical question: Does aspirin prevent recurrence in patients after treatment with anticoagulation following their first episode of unprovoked VTE?
Background: VTE recurrence is common following the discontinuation of anticoagulation, particularly in patients with a history of unprovoked pulmonary embolism (PE) or DVT. Extension of treatment with vitamin K antagonists decreases recurrence but also causes more bleeding. The role of aspirin in the secondary prevention of VTE is unknown.
Study design: Double-blind randomized controlled trial.
Setting: Multicenter international trial involving outpatients.
Synopsis: This trial compared treatment with aspirin versus placebo for approximately two years in 205 patients with a history of unprovoked VTE who had completed six to 18 months of anticoagulant therapy. The relative risk reduction of recurrent VTE in the aspirin versus the placebo group was approximately 40% per year (6.6% vs. 11.2% per year; absolute risk reduction 4.6% per year; number needed to treat 22). No difference in major or clinically relevant bleeding was observed between the two groups.
This study was appropriately powered to detect the treatment effect reported by the authors. Typically, anticoagulation is discontinued in this specific patient population once the risk of bleeding and/or the patient’s perceived inconvenience of therapy outweighs the expected benefit from continuing treatment to prevent VTE recurrence. Study results suggest aspirin is effective in preventing recurrence while not conferring an increased risk for hemorrhage. It is important to note that patients with cancer and symptomatic atherosclerosis were excluded from this study.
Bottom line: Following an appropriate treatment period with standard anticoagulation, aspirin appears to be a safe and effective therapy in the secondary prevention of recurrence in patients with a history of unprovoked VTE.
Citation: Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959-1967.
Meta-Analysis Supports New Oral Anticoagulants in Patients with Atrial Fibrillation
Clinical question: Are novel oral anticoagulants both efficacious and safe in comparison to warfarin in patients with atrial fibrillation (afib)?
Background: Three large clinical trials recently evaluated novel oral anticoagulants (dabigatran, rivaroxaban, and apixaban) as alternatives to warfarin in afib patients. Although the anticoagulants all appeared efficacious for primary outcomes, results regarding secondary and safety outcomes were either inconclusive or heterogeneous.
Study design: Systematic review and meta-analysis of randomized controlled trials.
Setting: Three diverse, clinical-trial settings.
Synopsis: A systematic review and meta-analysis of randomized controlled trials comparing novel oral anticoagulants to warfarin in afib patients found three large studies examining dabigatran, rivaroxaban, and apixaban that included 44,563 patients.
Using random-effects models to pool data from these studies, the authors found a 22% relative risk (RR) reduction of stroke or systemic embolism with the use of these new anticoagulants as compared to warfarin. The risks for ischemic and unidentified stroke (RR 0.87), hemorrhagic stroke (RR 0.45), intracranial bleeding (RR 0.49), and mortality (RR 0.88) were significantly reduced in patients taking these new anticoagulants. There was no significant reduction in major bleeding.
A trend in favor of warfarin was seen for gastrointestinal bleeding, but this trend was not statistically significant (RR 1.25; 95% confidence interval, 0.91-1.72).
This meta-analysis was limited to only three randomized controlled trials, each comparing a different oral anticoagulant to warfarin and using somewhat heterogeneous study designs and patient populations.
Bottom line: Three new oral anticoagulants appear safe and more efficacious than warfarin in the prevention of stroke and systemic embolism, as well as other important clinical outcomes in patients with atrial fibrillation.
Citation: Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110(3):453-60.
Observational Study Suggests Intravenous Metronidazole May Be Inferior for Mild Clostridium Difficile Infection
Clinical question: How do different treatment regimens (oral metronidazole vs. intravenous metronidazole vs. oral vancomycin) for mild Clostridium difficile infection (CDI) compare with regard to clinical outcomes?
Background: CDIs are increasing. Oral metronidazole or vancomycin can be used for the initial treatment of non-severe CDI. Additionally, a paucity of clinical data exists on intravenous metronidazole, which is used in some cases, typically in combination with vancomycin and in patients with severe CDI.
Study design: Prospective cohort study.
Setting: International hospital-based study.
Synopsis: This observational study assessed three different regimens (oral metronidazole, intravenous metronidazole, and oral vancomycin) for the treatment of mild CDI in 265 inpatients. Mortality at 30 days was higher in the intravenous metronidazole group (38.1%) than in the oral metronidazole (7.4%) and vancomycin (9.5%) groups. Patients receiving intravenous metronidazole also were less likely to recover from disease (52.4% versus >80% in the oral metronidazole and vancomycin groups). No statistically significant differences for other sequelae of CDI were found, except for higher risk of dehydration in the oral vancomycin group.
After controlling for age, sex, and comorbidity severity, patients in the intravenous metronidazole group were approximately four times more likely to die than patients in either the oral vancomycin group or the oral metronidazole group. There was no significant difference in mortality between the oral metronidazole and vancomycin groups.
Although this study was limited by its observational nature, no randomized controlled trial has yet to compare these three treatment regimens for mild CDI.
Bottom line: Treatment of mild C. diff infection with intravenous metronidazole appears to be associated with higher mortality and lower disease resolution than treatment with either oral metronidazole or vancomycin.
Citation: Wenisch JM, Schmid D, Kuo HW, et al. Prospective observational study comparing three different treatment regimes in patients with Clostridium difficile infection. Antimicrob Agents Chemother. 2012;56(4):1974-1978.
Traumatic Foley Catheter Complications Occur with Similar Frequency as Catheter-Associated Urinary Tract Infections
Clinical question: How does the frequency and clinical significance of urinary tract infection (UTI) compare to genitourinary trauma when associated with Foley catheterization?
Background: Foley catheter use in hospitalized patients is common and carries many associated risks. Although the risk of UTI when using catheters often is recognized, providers should consider other important complications of catheter use, such as genitourinary trauma.
Study design: Descriptive, prospective cohort study.
Setting: Single-center study in a Veterans Affairs hospital.
Synopsis: This surveillance study of 6,513 Foley catheter days compared the incidence of catheter-associated urinary tract infections (CAUTIs) to that of genitourinary trauma. Traumatic Foley events included gross hematuria, creation of false passage, ridging causing pain and/or difficulties with catheter removal, external genital trauma, catheter misplacement (ranging from prostatic to intraperitoneal), and catheter removal with the balloon inflated.
The incidence of possible UTI episodes was 1.8% of the Foley catheter days compared with 1.5% for catheter-associated trauma. Despite the fact that 72% of the UTI cases were asymptomatic, approximately 41% of these cases were treated with antibiotics, which accounted for 70% of all UTIs treated. Of the cases of Foley catheter trauma, 32% required further interventions (i.e. prolonged catheterization or cystoscopy). Trauma prompting intervention was as common as symptomatic UTIs.
The observational study design, use of a single center with a predominantly male population, and inclusion of patients undergoing urologic procedures who might have had valid indications for treatment of asymptomatic bacteriuria limit the study findings.
Bottom line: The complication of catheter-associated genitourinary trauma is just as common as CAUTIs in hospitalized patients, with each necessitating further intervention and treatment at similar rates.
Citation: Leuck AM, Wright D, Ellingson L, et al. Complications of Foley catheters—is infection the greatest risk? J Urol. 2012;187(5):1662-1666.
Intensive Control of Hyperglycemia in Noncritical, Hospitalized Patients Decreases Infection Risk, but No Significant Effect on Other Outcomes
Clinical question: What is the effect of tight glucose control in patients hospitalized in noncritical-care settings?
Background: Hyperglycemia is associated with increased in-hospital mortality and morbidity. Several trials have demonstrated the potential benefits of intensive glycemic control for patients in intensive-care settings, but this could lead to increased hypoglycemia. The effect of intensive therapy to achieve tight glycemic control in noncritically ill patients is unclear.
Study design: Systematic review and meta-analysis.
Setting: Various study sites of hospitalized patients.
Synopsis: This meta-analysis included 19 studies (nine randomized, 10 observational) published from 1995 to 2011. Intensive glycemic control (fasting blood glucose of 100-180 mg/dL) was not associated with significant effect on risk of death, myocardial infarction (MI), or stroke. There was a nonsignificant trend for increased risk of hypoglycemia (relative risk, 1.58; 95% CI, 0.97-2.57).
Intensive glycemic control was associated with a decreased risk of infection (relative risk, 0.41; 95% CI, 0.21–0.77), but this association mainly was derived from studies in surgical settings.
Subgroup analyses demonstrated an association between achieving intensive glycemic goals and an increased risk of hypoglycemia (P=0.01). Hypoglycemia also more commonly occurred in surgical patients.
There was substantial heterogeneity across studies included for all outcomes except infection. The quality of the current evidence supporting a reduction in infection is low and appears mostly derived from patients in surgical settings. The quality of evidence relating to all the other outcomes is also low and is limited by heterogeneity and imprecision.
Bottom line: Intensive control of hyperglycemia in noncritically ill hospitalized patients might reduce the risk of infection in some patients but does not appear to be significantly associated with improvement in other important clinical outcomes.
Citation: Murad MH, Coburn JA, Coto-Yglesias F, et al. Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97(1):49-58.
Simple Risk Score Predicts 30-Day Mortality after Noncardiac Surgery
Clinical question: Can 30-day mortality risk after noncardiac surgery be predicted by using a simple bedside-risk index?
Background: While indices exist to quantify the risk of cardiac complications in patients undergoing noncardiac surgery, there is significant perioperative mortality due to noncardiac causes. Therefore, a need exists for a simple risk index to predict all-cause mortality after noncardiac surgery.
Study design: Retrospective cohort study.
Setting: American College of Surgeons National Surgical Quality Improvement Program database of patients in more than 200 hospitals.
Synopsis: The nine-point Surgical Mortality Probability Model (S-MPM) 30-day mortality risk index was empirically created and applied to a randomly split portion of a database, which included 298,772 patients undergoing noncardiac surgery. Three risk factors were included: American Society of Anesthesiologists (ASA) physical status, surgery risk class, and emergency status. Patients with ASA physical status I, II, III, IV, or V were assigned zero, two, four, five, or six points, respectively. Patients undergoing intermediate- or high-risk procedures were assigned one or two points, respectively. Patients undergoing emergency procedures were assigned one point. The S-MPM then was applied to the validation portion of the data set.
The 30-day predicted risk of mortality was less than 0.50% for those patients with combined risk scores of less than five (S-MPM Class I), between 1.5% and 4.0% for risk scores of five to six (Class II), and more than 10% for risk scores greater than six (Class III).
The major limitation of this derived risk score is the reliance on ASA physical status, which has imprecise definitions and might lead to inconsistent ratings.
Bottom line: The S-MPM risk index is a simple bedside scoring system, which can accurately predict 30-day mortality in patients undergoing noncardiac surgery.
Citation: Glance LG, Lustik SJ, Hannan EL, et al. The Surgical Mortality Probability Model: Derivation and validation of a simple risk prediction rule for noncardiac surgery. Ann Surg. 2012;255(4):696-702.
Temperature, White Blood Cell Count Are Not Sensitive Predictors of Bacteremia
Clinical question: In ED patients with suspected infection, are temperature, white blood cell (WBC) count, and bandemia reliable predictors of bacteremia?
Background: Sepsis is a significant cause of morbidity and mortality. Early identification and treatment of sepsis improves patient outcomes. Although systemic inflammatory response syndrome criteria aids in the prompt recognition of sepsis, these markers have variable sensitivity and specificity for true infection or bacteremia, which places patients at high risk for sepsis.
Study design: Post-hoc data analysis of a prospective cohort study.
Setting: ED patients at a single tertiary-care center.
Synopsis: A retrospective analysis was performed on 3,563 patients who presented to the ED. Blood cultures obtained on patients with suspected infection revealed bacteremia in 289 patients (8.1%). Patients with bacteremia were reviewed for the presence of normal temperature (36.1° to 38° Celsius), normal WBC count (4,000 to 12,000 cells per µL), and presence of bandemia (>5% of WBC differential).
Among patients with bacteremia, 33% had a normal body temperature (67% sensitivity) and 52% had a normal WBC count (48% sensitivity). Of the 210 bacteremic patients who had a full differential performed, bandemia was present in 82% (sensitivity 82%). Bandemia was present in 80% of culture-positive patients with a normal temperature and 79% of culture-positive patients with a normal WBC count. Approximately 17% of patients with bacteremia had neither an abnormal temperature nor an abnormal WBC. This study was limited by the retrospective nature of analysis and the subjective designation of “suspected infection” by the original providers.
Bottom line: In patients presenting to the ED with suspected infection who are found to have culture-proven bacteremia, a significant percentage have normal temperature and WBC count, but the presence of bandemia could be more useful for identifying occult bacteremia.
Citation: Seigel TA, Cocchi MN, Salciccioli J, et al. Inadequacy of temperature and white blood cell count in predicting bacteremia in patients with suspected infection. J Emerg Med. 2012;42(3):254-259.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Warfarin vs. aspirin in heart-failure patients
- Aspirin after anticoagulation prevents VTE recurrence
- Novel oral anticoagulants vs. warfarin in afib patients
- Intravenous metronidazole for mild C. diff infection
- Frequency of Foley catheter complications vs. CAUTI
- Intensive hyperglycemia control in noncritical hospitalized patients
- Risk score predicts 30-day mortality after noncardiac surgery
- Temperature, white blood cell count are not sensitive predictors of bacteremia
Warfarin Reduces Stoke but Increases Hemorrhage Compared with Aspirin in Patients with Heart Failure and Sinus Rhythm
Clinical question: Is warfarin superior to aspirin with regard to clinical outcomes in patients with heart failure who are in sinus rhythm?
Background: Heart failure is associated with stroke and death potentially caused by atherothrombotic events. Anticoagulation is efficacious in some heart failure patients with atrial fibrillation or significant valvular disease, but the role of anticoagulation versus aspirin in patients with chronic heart failure and sinus rhythm is unclear.
Study design: Double-blind randomized controlled trial.
Setting: Multicenter, multinational trial involving outpatients.
Synopsis: This double-blind, double-dummy trial involving 2,305 patients with sinus rhythm and reduced left ventricular ejection fraction (<35%) showed no significant difference in the primary combined outcome (ischemic stroke, intracerebral hemorrhage, or death) in those treated with warfarin as compared with aspirin. Warfarin did significantly reduce the rate of ischemic stroke by 0.64 events per 100 patient-years (absolute risk reduction 2.2%, number needed to treat 45) when compared with aspirin, with no significant difference in the rate of intracerebral hemorrhage. This outcome was offset by an increased rate of major hemorrhage by 0.91 events per 100 patient-years (absolute risk increase 3.1%, number needed to harm 32).
This study included patients from all functional classes of heart failure, with a protocol to initiate treatment with other standard heart failure medications. Patients with an indication for either warfarin or aspirin were excluded. Due to recruitment difficulties, the power of this study was reduced. Other study limitations included a relatively low percentage of time that patients on warfarin were in therapeutic range and a substantial period of follow-up time in which patients did not receive the assigned study treatments.
Bottom line: The benefit of reduced stoke in patients with heart failure and sinus rhythm who take warfarin over aspirin is counteracted by an increased risk for serious bleeding outcomes.
Citation: Homma S, Thompson JLP, Pullicino PM, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366(20):1859-1869.
Aspirin Use after Recommended Anticoagulation Treatment Prevents Recurrence in VTE Patients
Clinical question: Does aspirin prevent recurrence in patients after treatment with anticoagulation following their first episode of unprovoked VTE?
Background: VTE recurrence is common following the discontinuation of anticoagulation, particularly in patients with a history of unprovoked pulmonary embolism (PE) or DVT. Extension of treatment with vitamin K antagonists decreases recurrence but also causes more bleeding. The role of aspirin in the secondary prevention of VTE is unknown.
Study design: Double-blind randomized controlled trial.
Setting: Multicenter international trial involving outpatients.
Synopsis: This trial compared treatment with aspirin versus placebo for approximately two years in 205 patients with a history of unprovoked VTE who had completed six to 18 months of anticoagulant therapy. The relative risk reduction of recurrent VTE in the aspirin versus the placebo group was approximately 40% per year (6.6% vs. 11.2% per year; absolute risk reduction 4.6% per year; number needed to treat 22). No difference in major or clinically relevant bleeding was observed between the two groups.
This study was appropriately powered to detect the treatment effect reported by the authors. Typically, anticoagulation is discontinued in this specific patient population once the risk of bleeding and/or the patient’s perceived inconvenience of therapy outweighs the expected benefit from continuing treatment to prevent VTE recurrence. Study results suggest aspirin is effective in preventing recurrence while not conferring an increased risk for hemorrhage. It is important to note that patients with cancer and symptomatic atherosclerosis were excluded from this study.
Bottom line: Following an appropriate treatment period with standard anticoagulation, aspirin appears to be a safe and effective therapy in the secondary prevention of recurrence in patients with a history of unprovoked VTE.
Citation: Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959-1967.
Meta-Analysis Supports New Oral Anticoagulants in Patients with Atrial Fibrillation
Clinical question: Are novel oral anticoagulants both efficacious and safe in comparison to warfarin in patients with atrial fibrillation (afib)?
Background: Three large clinical trials recently evaluated novel oral anticoagulants (dabigatran, rivaroxaban, and apixaban) as alternatives to warfarin in afib patients. Although the anticoagulants all appeared efficacious for primary outcomes, results regarding secondary and safety outcomes were either inconclusive or heterogeneous.
Study design: Systematic review and meta-analysis of randomized controlled trials.
Setting: Three diverse, clinical-trial settings.
Synopsis: A systematic review and meta-analysis of randomized controlled trials comparing novel oral anticoagulants to warfarin in afib patients found three large studies examining dabigatran, rivaroxaban, and apixaban that included 44,563 patients.
Using random-effects models to pool data from these studies, the authors found a 22% relative risk (RR) reduction of stroke or systemic embolism with the use of these new anticoagulants as compared to warfarin. The risks for ischemic and unidentified stroke (RR 0.87), hemorrhagic stroke (RR 0.45), intracranial bleeding (RR 0.49), and mortality (RR 0.88) were significantly reduced in patients taking these new anticoagulants. There was no significant reduction in major bleeding.
A trend in favor of warfarin was seen for gastrointestinal bleeding, but this trend was not statistically significant (RR 1.25; 95% confidence interval, 0.91-1.72).
This meta-analysis was limited to only three randomized controlled trials, each comparing a different oral anticoagulant to warfarin and using somewhat heterogeneous study designs and patient populations.
Bottom line: Three new oral anticoagulants appear safe and more efficacious than warfarin in the prevention of stroke and systemic embolism, as well as other important clinical outcomes in patients with atrial fibrillation.
Citation: Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110(3):453-60.
Observational Study Suggests Intravenous Metronidazole May Be Inferior for Mild Clostridium Difficile Infection
Clinical question: How do different treatment regimens (oral metronidazole vs. intravenous metronidazole vs. oral vancomycin) for mild Clostridium difficile infection (CDI) compare with regard to clinical outcomes?
Background: CDIs are increasing. Oral metronidazole or vancomycin can be used for the initial treatment of non-severe CDI. Additionally, a paucity of clinical data exists on intravenous metronidazole, which is used in some cases, typically in combination with vancomycin and in patients with severe CDI.
Study design: Prospective cohort study.
Setting: International hospital-based study.
Synopsis: This observational study assessed three different regimens (oral metronidazole, intravenous metronidazole, and oral vancomycin) for the treatment of mild CDI in 265 inpatients. Mortality at 30 days was higher in the intravenous metronidazole group (38.1%) than in the oral metronidazole (7.4%) and vancomycin (9.5%) groups. Patients receiving intravenous metronidazole also were less likely to recover from disease (52.4% versus >80% in the oral metronidazole and vancomycin groups). No statistically significant differences for other sequelae of CDI were found, except for higher risk of dehydration in the oral vancomycin group.
After controlling for age, sex, and comorbidity severity, patients in the intravenous metronidazole group were approximately four times more likely to die than patients in either the oral vancomycin group or the oral metronidazole group. There was no significant difference in mortality between the oral metronidazole and vancomycin groups.
Although this study was limited by its observational nature, no randomized controlled trial has yet to compare these three treatment regimens for mild CDI.
Bottom line: Treatment of mild C. diff infection with intravenous metronidazole appears to be associated with higher mortality and lower disease resolution than treatment with either oral metronidazole or vancomycin.
Citation: Wenisch JM, Schmid D, Kuo HW, et al. Prospective observational study comparing three different treatment regimes in patients with Clostridium difficile infection. Antimicrob Agents Chemother. 2012;56(4):1974-1978.
Traumatic Foley Catheter Complications Occur with Similar Frequency as Catheter-Associated Urinary Tract Infections
Clinical question: How does the frequency and clinical significance of urinary tract infection (UTI) compare to genitourinary trauma when associated with Foley catheterization?
Background: Foley catheter use in hospitalized patients is common and carries many associated risks. Although the risk of UTI when using catheters often is recognized, providers should consider other important complications of catheter use, such as genitourinary trauma.
Study design: Descriptive, prospective cohort study.
Setting: Single-center study in a Veterans Affairs hospital.
Synopsis: This surveillance study of 6,513 Foley catheter days compared the incidence of catheter-associated urinary tract infections (CAUTIs) to that of genitourinary trauma. Traumatic Foley events included gross hematuria, creation of false passage, ridging causing pain and/or difficulties with catheter removal, external genital trauma, catheter misplacement (ranging from prostatic to intraperitoneal), and catheter removal with the balloon inflated.
The incidence of possible UTI episodes was 1.8% of the Foley catheter days compared with 1.5% for catheter-associated trauma. Despite the fact that 72% of the UTI cases were asymptomatic, approximately 41% of these cases were treated with antibiotics, which accounted for 70% of all UTIs treated. Of the cases of Foley catheter trauma, 32% required further interventions (i.e. prolonged catheterization or cystoscopy). Trauma prompting intervention was as common as symptomatic UTIs.
The observational study design, use of a single center with a predominantly male population, and inclusion of patients undergoing urologic procedures who might have had valid indications for treatment of asymptomatic bacteriuria limit the study findings.
Bottom line: The complication of catheter-associated genitourinary trauma is just as common as CAUTIs in hospitalized patients, with each necessitating further intervention and treatment at similar rates.
Citation: Leuck AM, Wright D, Ellingson L, et al. Complications of Foley catheters—is infection the greatest risk? J Urol. 2012;187(5):1662-1666.
Intensive Control of Hyperglycemia in Noncritical, Hospitalized Patients Decreases Infection Risk, but No Significant Effect on Other Outcomes
Clinical question: What is the effect of tight glucose control in patients hospitalized in noncritical-care settings?
Background: Hyperglycemia is associated with increased in-hospital mortality and morbidity. Several trials have demonstrated the potential benefits of intensive glycemic control for patients in intensive-care settings, but this could lead to increased hypoglycemia. The effect of intensive therapy to achieve tight glycemic control in noncritically ill patients is unclear.
Study design: Systematic review and meta-analysis.
Setting: Various study sites of hospitalized patients.
Synopsis: This meta-analysis included 19 studies (nine randomized, 10 observational) published from 1995 to 2011. Intensive glycemic control (fasting blood glucose of 100-180 mg/dL) was not associated with significant effect on risk of death, myocardial infarction (MI), or stroke. There was a nonsignificant trend for increased risk of hypoglycemia (relative risk, 1.58; 95% CI, 0.97-2.57).
Intensive glycemic control was associated with a decreased risk of infection (relative risk, 0.41; 95% CI, 0.21–0.77), but this association mainly was derived from studies in surgical settings.
Subgroup analyses demonstrated an association between achieving intensive glycemic goals and an increased risk of hypoglycemia (P=0.01). Hypoglycemia also more commonly occurred in surgical patients.
There was substantial heterogeneity across studies included for all outcomes except infection. The quality of the current evidence supporting a reduction in infection is low and appears mostly derived from patients in surgical settings. The quality of evidence relating to all the other outcomes is also low and is limited by heterogeneity and imprecision.
Bottom line: Intensive control of hyperglycemia in noncritically ill hospitalized patients might reduce the risk of infection in some patients but does not appear to be significantly associated with improvement in other important clinical outcomes.
Citation: Murad MH, Coburn JA, Coto-Yglesias F, et al. Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97(1):49-58.
Simple Risk Score Predicts 30-Day Mortality after Noncardiac Surgery
Clinical question: Can 30-day mortality risk after noncardiac surgery be predicted by using a simple bedside-risk index?
Background: While indices exist to quantify the risk of cardiac complications in patients undergoing noncardiac surgery, there is significant perioperative mortality due to noncardiac causes. Therefore, a need exists for a simple risk index to predict all-cause mortality after noncardiac surgery.
Study design: Retrospective cohort study.
Setting: American College of Surgeons National Surgical Quality Improvement Program database of patients in more than 200 hospitals.
Synopsis: The nine-point Surgical Mortality Probability Model (S-MPM) 30-day mortality risk index was empirically created and applied to a randomly split portion of a database, which included 298,772 patients undergoing noncardiac surgery. Three risk factors were included: American Society of Anesthesiologists (ASA) physical status, surgery risk class, and emergency status. Patients with ASA physical status I, II, III, IV, or V were assigned zero, two, four, five, or six points, respectively. Patients undergoing intermediate- or high-risk procedures were assigned one or two points, respectively. Patients undergoing emergency procedures were assigned one point. The S-MPM then was applied to the validation portion of the data set.
The 30-day predicted risk of mortality was less than 0.50% for those patients with combined risk scores of less than five (S-MPM Class I), between 1.5% and 4.0% for risk scores of five to six (Class II), and more than 10% for risk scores greater than six (Class III).
The major limitation of this derived risk score is the reliance on ASA physical status, which has imprecise definitions and might lead to inconsistent ratings.
Bottom line: The S-MPM risk index is a simple bedside scoring system, which can accurately predict 30-day mortality in patients undergoing noncardiac surgery.
Citation: Glance LG, Lustik SJ, Hannan EL, et al. The Surgical Mortality Probability Model: Derivation and validation of a simple risk prediction rule for noncardiac surgery. Ann Surg. 2012;255(4):696-702.
Temperature, White Blood Cell Count Are Not Sensitive Predictors of Bacteremia
Clinical question: In ED patients with suspected infection, are temperature, white blood cell (WBC) count, and bandemia reliable predictors of bacteremia?
Background: Sepsis is a significant cause of morbidity and mortality. Early identification and treatment of sepsis improves patient outcomes. Although systemic inflammatory response syndrome criteria aids in the prompt recognition of sepsis, these markers have variable sensitivity and specificity for true infection or bacteremia, which places patients at high risk for sepsis.
Study design: Post-hoc data analysis of a prospective cohort study.
Setting: ED patients at a single tertiary-care center.
Synopsis: A retrospective analysis was performed on 3,563 patients who presented to the ED. Blood cultures obtained on patients with suspected infection revealed bacteremia in 289 patients (8.1%). Patients with bacteremia were reviewed for the presence of normal temperature (36.1° to 38° Celsius), normal WBC count (4,000 to 12,000 cells per µL), and presence of bandemia (>5% of WBC differential).
Among patients with bacteremia, 33% had a normal body temperature (67% sensitivity) and 52% had a normal WBC count (48% sensitivity). Of the 210 bacteremic patients who had a full differential performed, bandemia was present in 82% (sensitivity 82%). Bandemia was present in 80% of culture-positive patients with a normal temperature and 79% of culture-positive patients with a normal WBC count. Approximately 17% of patients with bacteremia had neither an abnormal temperature nor an abnormal WBC. This study was limited by the retrospective nature of analysis and the subjective designation of “suspected infection” by the original providers.
Bottom line: In patients presenting to the ED with suspected infection who are found to have culture-proven bacteremia, a significant percentage have normal temperature and WBC count, but the presence of bandemia could be more useful for identifying occult bacteremia.
Citation: Seigel TA, Cocchi MN, Salciccioli J, et al. Inadequacy of temperature and white blood cell count in predicting bacteremia in patients with suspected infection. J Emerg Med. 2012;42(3):254-259.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Warfarin vs. aspirin in heart-failure patients
- Aspirin after anticoagulation prevents VTE recurrence
- Novel oral anticoagulants vs. warfarin in afib patients
- Intravenous metronidazole for mild C. diff infection
- Frequency of Foley catheter complications vs. CAUTI
- Intensive hyperglycemia control in noncritical hospitalized patients
- Risk score predicts 30-day mortality after noncardiac surgery
- Temperature, white blood cell count are not sensitive predictors of bacteremia
Warfarin Reduces Stoke but Increases Hemorrhage Compared with Aspirin in Patients with Heart Failure and Sinus Rhythm
Clinical question: Is warfarin superior to aspirin with regard to clinical outcomes in patients with heart failure who are in sinus rhythm?
Background: Heart failure is associated with stroke and death potentially caused by atherothrombotic events. Anticoagulation is efficacious in some heart failure patients with atrial fibrillation or significant valvular disease, but the role of anticoagulation versus aspirin in patients with chronic heart failure and sinus rhythm is unclear.
Study design: Double-blind randomized controlled trial.
Setting: Multicenter, multinational trial involving outpatients.
Synopsis: This double-blind, double-dummy trial involving 2,305 patients with sinus rhythm and reduced left ventricular ejection fraction (<35%) showed no significant difference in the primary combined outcome (ischemic stroke, intracerebral hemorrhage, or death) in those treated with warfarin as compared with aspirin. Warfarin did significantly reduce the rate of ischemic stroke by 0.64 events per 100 patient-years (absolute risk reduction 2.2%, number needed to treat 45) when compared with aspirin, with no significant difference in the rate of intracerebral hemorrhage. This outcome was offset by an increased rate of major hemorrhage by 0.91 events per 100 patient-years (absolute risk increase 3.1%, number needed to harm 32).
This study included patients from all functional classes of heart failure, with a protocol to initiate treatment with other standard heart failure medications. Patients with an indication for either warfarin or aspirin were excluded. Due to recruitment difficulties, the power of this study was reduced. Other study limitations included a relatively low percentage of time that patients on warfarin were in therapeutic range and a substantial period of follow-up time in which patients did not receive the assigned study treatments.
Bottom line: The benefit of reduced stoke in patients with heart failure and sinus rhythm who take warfarin over aspirin is counteracted by an increased risk for serious bleeding outcomes.
Citation: Homma S, Thompson JLP, Pullicino PM, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366(20):1859-1869.
Aspirin Use after Recommended Anticoagulation Treatment Prevents Recurrence in VTE Patients
Clinical question: Does aspirin prevent recurrence in patients after treatment with anticoagulation following their first episode of unprovoked VTE?
Background: VTE recurrence is common following the discontinuation of anticoagulation, particularly in patients with a history of unprovoked pulmonary embolism (PE) or DVT. Extension of treatment with vitamin K antagonists decreases recurrence but also causes more bleeding. The role of aspirin in the secondary prevention of VTE is unknown.
Study design: Double-blind randomized controlled trial.
Setting: Multicenter international trial involving outpatients.
Synopsis: This trial compared treatment with aspirin versus placebo for approximately two years in 205 patients with a history of unprovoked VTE who had completed six to 18 months of anticoagulant therapy. The relative risk reduction of recurrent VTE in the aspirin versus the placebo group was approximately 40% per year (6.6% vs. 11.2% per year; absolute risk reduction 4.6% per year; number needed to treat 22). No difference in major or clinically relevant bleeding was observed between the two groups.
This study was appropriately powered to detect the treatment effect reported by the authors. Typically, anticoagulation is discontinued in this specific patient population once the risk of bleeding and/or the patient’s perceived inconvenience of therapy outweighs the expected benefit from continuing treatment to prevent VTE recurrence. Study results suggest aspirin is effective in preventing recurrence while not conferring an increased risk for hemorrhage. It is important to note that patients with cancer and symptomatic atherosclerosis were excluded from this study.
Bottom line: Following an appropriate treatment period with standard anticoagulation, aspirin appears to be a safe and effective therapy in the secondary prevention of recurrence in patients with a history of unprovoked VTE.
Citation: Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing recurrence of venous thromboembolism. N Engl J Med. 2012;366(21):1959-1967.
Meta-Analysis Supports New Oral Anticoagulants in Patients with Atrial Fibrillation
Clinical question: Are novel oral anticoagulants both efficacious and safe in comparison to warfarin in patients with atrial fibrillation (afib)?
Background: Three large clinical trials recently evaluated novel oral anticoagulants (dabigatran, rivaroxaban, and apixaban) as alternatives to warfarin in afib patients. Although the anticoagulants all appeared efficacious for primary outcomes, results regarding secondary and safety outcomes were either inconclusive or heterogeneous.
Study design: Systematic review and meta-analysis of randomized controlled trials.
Setting: Three diverse, clinical-trial settings.
Synopsis: A systematic review and meta-analysis of randomized controlled trials comparing novel oral anticoagulants to warfarin in afib patients found three large studies examining dabigatran, rivaroxaban, and apixaban that included 44,563 patients.
Using random-effects models to pool data from these studies, the authors found a 22% relative risk (RR) reduction of stroke or systemic embolism with the use of these new anticoagulants as compared to warfarin. The risks for ischemic and unidentified stroke (RR 0.87), hemorrhagic stroke (RR 0.45), intracranial bleeding (RR 0.49), and mortality (RR 0.88) were significantly reduced in patients taking these new anticoagulants. There was no significant reduction in major bleeding.
A trend in favor of warfarin was seen for gastrointestinal bleeding, but this trend was not statistically significant (RR 1.25; 95% confidence interval, 0.91-1.72).
This meta-analysis was limited to only three randomized controlled trials, each comparing a different oral anticoagulant to warfarin and using somewhat heterogeneous study designs and patient populations.
Bottom line: Three new oral anticoagulants appear safe and more efficacious than warfarin in the prevention of stroke and systemic embolism, as well as other important clinical outcomes in patients with atrial fibrillation.
Citation: Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110(3):453-60.
Observational Study Suggests Intravenous Metronidazole May Be Inferior for Mild Clostridium Difficile Infection
Clinical question: How do different treatment regimens (oral metronidazole vs. intravenous metronidazole vs. oral vancomycin) for mild Clostridium difficile infection (CDI) compare with regard to clinical outcomes?
Background: CDIs are increasing. Oral metronidazole or vancomycin can be used for the initial treatment of non-severe CDI. Additionally, a paucity of clinical data exists on intravenous metronidazole, which is used in some cases, typically in combination with vancomycin and in patients with severe CDI.
Study design: Prospective cohort study.
Setting: International hospital-based study.
Synopsis: This observational study assessed three different regimens (oral metronidazole, intravenous metronidazole, and oral vancomycin) for the treatment of mild CDI in 265 inpatients. Mortality at 30 days was higher in the intravenous metronidazole group (38.1%) than in the oral metronidazole (7.4%) and vancomycin (9.5%) groups. Patients receiving intravenous metronidazole also were less likely to recover from disease (52.4% versus >80% in the oral metronidazole and vancomycin groups). No statistically significant differences for other sequelae of CDI were found, except for higher risk of dehydration in the oral vancomycin group.
After controlling for age, sex, and comorbidity severity, patients in the intravenous metronidazole group were approximately four times more likely to die than patients in either the oral vancomycin group or the oral metronidazole group. There was no significant difference in mortality between the oral metronidazole and vancomycin groups.
Although this study was limited by its observational nature, no randomized controlled trial has yet to compare these three treatment regimens for mild CDI.
Bottom line: Treatment of mild C. diff infection with intravenous metronidazole appears to be associated with higher mortality and lower disease resolution than treatment with either oral metronidazole or vancomycin.
Citation: Wenisch JM, Schmid D, Kuo HW, et al. Prospective observational study comparing three different treatment regimes in patients with Clostridium difficile infection. Antimicrob Agents Chemother. 2012;56(4):1974-1978.
Traumatic Foley Catheter Complications Occur with Similar Frequency as Catheter-Associated Urinary Tract Infections
Clinical question: How does the frequency and clinical significance of urinary tract infection (UTI) compare to genitourinary trauma when associated with Foley catheterization?
Background: Foley catheter use in hospitalized patients is common and carries many associated risks. Although the risk of UTI when using catheters often is recognized, providers should consider other important complications of catheter use, such as genitourinary trauma.
Study design: Descriptive, prospective cohort study.
Setting: Single-center study in a Veterans Affairs hospital.
Synopsis: This surveillance study of 6,513 Foley catheter days compared the incidence of catheter-associated urinary tract infections (CAUTIs) to that of genitourinary trauma. Traumatic Foley events included gross hematuria, creation of false passage, ridging causing pain and/or difficulties with catheter removal, external genital trauma, catheter misplacement (ranging from prostatic to intraperitoneal), and catheter removal with the balloon inflated.
The incidence of possible UTI episodes was 1.8% of the Foley catheter days compared with 1.5% for catheter-associated trauma. Despite the fact that 72% of the UTI cases were asymptomatic, approximately 41% of these cases were treated with antibiotics, which accounted for 70% of all UTIs treated. Of the cases of Foley catheter trauma, 32% required further interventions (i.e. prolonged catheterization or cystoscopy). Trauma prompting intervention was as common as symptomatic UTIs.
The observational study design, use of a single center with a predominantly male population, and inclusion of patients undergoing urologic procedures who might have had valid indications for treatment of asymptomatic bacteriuria limit the study findings.
Bottom line: The complication of catheter-associated genitourinary trauma is just as common as CAUTIs in hospitalized patients, with each necessitating further intervention and treatment at similar rates.
Citation: Leuck AM, Wright D, Ellingson L, et al. Complications of Foley catheters—is infection the greatest risk? J Urol. 2012;187(5):1662-1666.
Intensive Control of Hyperglycemia in Noncritical, Hospitalized Patients Decreases Infection Risk, but No Significant Effect on Other Outcomes
Clinical question: What is the effect of tight glucose control in patients hospitalized in noncritical-care settings?
Background: Hyperglycemia is associated with increased in-hospital mortality and morbidity. Several trials have demonstrated the potential benefits of intensive glycemic control for patients in intensive-care settings, but this could lead to increased hypoglycemia. The effect of intensive therapy to achieve tight glycemic control in noncritically ill patients is unclear.
Study design: Systematic review and meta-analysis.
Setting: Various study sites of hospitalized patients.
Synopsis: This meta-analysis included 19 studies (nine randomized, 10 observational) published from 1995 to 2011. Intensive glycemic control (fasting blood glucose of 100-180 mg/dL) was not associated with significant effect on risk of death, myocardial infarction (MI), or stroke. There was a nonsignificant trend for increased risk of hypoglycemia (relative risk, 1.58; 95% CI, 0.97-2.57).
Intensive glycemic control was associated with a decreased risk of infection (relative risk, 0.41; 95% CI, 0.21–0.77), but this association mainly was derived from studies in surgical settings.
Subgroup analyses demonstrated an association between achieving intensive glycemic goals and an increased risk of hypoglycemia (P=0.01). Hypoglycemia also more commonly occurred in surgical patients.
There was substantial heterogeneity across studies included for all outcomes except infection. The quality of the current evidence supporting a reduction in infection is low and appears mostly derived from patients in surgical settings. The quality of evidence relating to all the other outcomes is also low and is limited by heterogeneity and imprecision.
Bottom line: Intensive control of hyperglycemia in noncritically ill hospitalized patients might reduce the risk of infection in some patients but does not appear to be significantly associated with improvement in other important clinical outcomes.
Citation: Murad MH, Coburn JA, Coto-Yglesias F, et al. Glycemic control in non-critically ill hospitalized patients: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012;97(1):49-58.
Simple Risk Score Predicts 30-Day Mortality after Noncardiac Surgery
Clinical question: Can 30-day mortality risk after noncardiac surgery be predicted by using a simple bedside-risk index?
Background: While indices exist to quantify the risk of cardiac complications in patients undergoing noncardiac surgery, there is significant perioperative mortality due to noncardiac causes. Therefore, a need exists for a simple risk index to predict all-cause mortality after noncardiac surgery.
Study design: Retrospective cohort study.
Setting: American College of Surgeons National Surgical Quality Improvement Program database of patients in more than 200 hospitals.
Synopsis: The nine-point Surgical Mortality Probability Model (S-MPM) 30-day mortality risk index was empirically created and applied to a randomly split portion of a database, which included 298,772 patients undergoing noncardiac surgery. Three risk factors were included: American Society of Anesthesiologists (ASA) physical status, surgery risk class, and emergency status. Patients with ASA physical status I, II, III, IV, or V were assigned zero, two, four, five, or six points, respectively. Patients undergoing intermediate- or high-risk procedures were assigned one or two points, respectively. Patients undergoing emergency procedures were assigned one point. The S-MPM then was applied to the validation portion of the data set.
The 30-day predicted risk of mortality was less than 0.50% for those patients with combined risk scores of less than five (S-MPM Class I), between 1.5% and 4.0% for risk scores of five to six (Class II), and more than 10% for risk scores greater than six (Class III).
The major limitation of this derived risk score is the reliance on ASA physical status, which has imprecise definitions and might lead to inconsistent ratings.
Bottom line: The S-MPM risk index is a simple bedside scoring system, which can accurately predict 30-day mortality in patients undergoing noncardiac surgery.
Citation: Glance LG, Lustik SJ, Hannan EL, et al. The Surgical Mortality Probability Model: Derivation and validation of a simple risk prediction rule for noncardiac surgery. Ann Surg. 2012;255(4):696-702.
Temperature, White Blood Cell Count Are Not Sensitive Predictors of Bacteremia
Clinical question: In ED patients with suspected infection, are temperature, white blood cell (WBC) count, and bandemia reliable predictors of bacteremia?
Background: Sepsis is a significant cause of morbidity and mortality. Early identification and treatment of sepsis improves patient outcomes. Although systemic inflammatory response syndrome criteria aids in the prompt recognition of sepsis, these markers have variable sensitivity and specificity for true infection or bacteremia, which places patients at high risk for sepsis.
Study design: Post-hoc data analysis of a prospective cohort study.
Setting: ED patients at a single tertiary-care center.
Synopsis: A retrospective analysis was performed on 3,563 patients who presented to the ED. Blood cultures obtained on patients with suspected infection revealed bacteremia in 289 patients (8.1%). Patients with bacteremia were reviewed for the presence of normal temperature (36.1° to 38° Celsius), normal WBC count (4,000 to 12,000 cells per µL), and presence of bandemia (>5% of WBC differential).
Among patients with bacteremia, 33% had a normal body temperature (67% sensitivity) and 52% had a normal WBC count (48% sensitivity). Of the 210 bacteremic patients who had a full differential performed, bandemia was present in 82% (sensitivity 82%). Bandemia was present in 80% of culture-positive patients with a normal temperature and 79% of culture-positive patients with a normal WBC count. Approximately 17% of patients with bacteremia had neither an abnormal temperature nor an abnormal WBC. This study was limited by the retrospective nature of analysis and the subjective designation of “suspected infection” by the original providers.
Bottom line: In patients presenting to the ED with suspected infection who are found to have culture-proven bacteremia, a significant percentage have normal temperature and WBC count, but the presence of bandemia could be more useful for identifying occult bacteremia.
Citation: Seigel TA, Cocchi MN, Salciccioli J, et al. Inadequacy of temperature and white blood cell count in predicting bacteremia in patients with suspected infection. J Emerg Med. 2012;42(3):254-259.
ITL: Physician Reviews of HM-Relevant Research
Clinical question: Is azithromycin use associated with an increased risk of cardiovascular death?
Background: Accumulating evidence suggests that azithromycin might have pro-arrhythmic effects on the heart. Other macrolides, including erythromycin and clarithromycin, can increase the risk for serious ventricular arrhythmias and are associated with an increased risk of sudden cardiac death. The risk of cardiac death associated with azithromycin use is unclear.
Study design: Retrospective cohort study.
Setting: Statewide database of patients enrolled in the Tennessee Medicaid program.
Synopsis: This study matched patients who took a five-day course of azithromycin (347,795 prescriptions) with those who took no antibiotics (1,391,180 control periods). Patients taking azithromycin had an increased risk of cardiovascular death (hazard ratio [HR], 2.88; P<0.001) and death from any cause (HR, 1.85; P=0.002).
Additional control groups of patients taking other antibiotics were included in this study for comparison. Patients who took amoxicillin did not have an increased risk of death. Relative to amoxicillin, azithromycin was associated with a significantly increased risk of cardiovascular death, with an estimated 47 additional cardiovascular deaths per 1 million courses. The risk of cardiovascular death was greater with azithromycin than with ciprofloxacin but did not differ significantly from levofloxacin.
Importantly, patients with factors conferring a high risk of death were excluded from analysis. The increased risk of death did not appear to persist after azithromycin therapy ended. A major limitation of this study was confounding associated with antibiotic use, which the authors attempted to mitigate with the use of multiple control groups.
Bottom line: A five-day treatment course of azithromycin is associated with a small absolute increase in cardiovascular deaths and deaths from any cause.
Citation: Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
Clinical question: Is azithromycin use associated with an increased risk of cardiovascular death?
Background: Accumulating evidence suggests that azithromycin might have pro-arrhythmic effects on the heart. Other macrolides, including erythromycin and clarithromycin, can increase the risk for serious ventricular arrhythmias and are associated with an increased risk of sudden cardiac death. The risk of cardiac death associated with azithromycin use is unclear.
Study design: Retrospective cohort study.
Setting: Statewide database of patients enrolled in the Tennessee Medicaid program.
Synopsis: This study matched patients who took a five-day course of azithromycin (347,795 prescriptions) with those who took no antibiotics (1,391,180 control periods). Patients taking azithromycin had an increased risk of cardiovascular death (hazard ratio [HR], 2.88; P<0.001) and death from any cause (HR, 1.85; P=0.002).
Additional control groups of patients taking other antibiotics were included in this study for comparison. Patients who took amoxicillin did not have an increased risk of death. Relative to amoxicillin, azithromycin was associated with a significantly increased risk of cardiovascular death, with an estimated 47 additional cardiovascular deaths per 1 million courses. The risk of cardiovascular death was greater with azithromycin than with ciprofloxacin but did not differ significantly from levofloxacin.
Importantly, patients with factors conferring a high risk of death were excluded from analysis. The increased risk of death did not appear to persist after azithromycin therapy ended. A major limitation of this study was confounding associated with antibiotic use, which the authors attempted to mitigate with the use of multiple control groups.
Bottom line: A five-day treatment course of azithromycin is associated with a small absolute increase in cardiovascular deaths and deaths from any cause.
Citation: Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
Clinical question: Is azithromycin use associated with an increased risk of cardiovascular death?
Background: Accumulating evidence suggests that azithromycin might have pro-arrhythmic effects on the heart. Other macrolides, including erythromycin and clarithromycin, can increase the risk for serious ventricular arrhythmias and are associated with an increased risk of sudden cardiac death. The risk of cardiac death associated with azithromycin use is unclear.
Study design: Retrospective cohort study.
Setting: Statewide database of patients enrolled in the Tennessee Medicaid program.
Synopsis: This study matched patients who took a five-day course of azithromycin (347,795 prescriptions) with those who took no antibiotics (1,391,180 control periods). Patients taking azithromycin had an increased risk of cardiovascular death (hazard ratio [HR], 2.88; P<0.001) and death from any cause (HR, 1.85; P=0.002).
Additional control groups of patients taking other antibiotics were included in this study for comparison. Patients who took amoxicillin did not have an increased risk of death. Relative to amoxicillin, azithromycin was associated with a significantly increased risk of cardiovascular death, with an estimated 47 additional cardiovascular deaths per 1 million courses. The risk of cardiovascular death was greater with azithromycin than with ciprofloxacin but did not differ significantly from levofloxacin.
Importantly, patients with factors conferring a high risk of death were excluded from analysis. The increased risk of death did not appear to persist after azithromycin therapy ended. A major limitation of this study was confounding associated with antibiotic use, which the authors attempted to mitigate with the use of multiple control groups.
Bottom line: A five-day treatment course of azithromycin is associated with a small absolute increase in cardiovascular deaths and deaths from any cause.
Citation: Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.