User login
How is Acute Pericarditis Diagnosed and Treated?
Case
A 32-year-old female with no significant past medical history is evaluated for sharp, left-sided chest pain for five days. Her pain is intermittent, worse with deep inspiration and in the supine position. She denies any shortness of breath. Her temperature is 100.8ºF, but otherwise her vital signs are normal. The physical exam and chest radiograph are unremarkable, but an electrocardiogram shows diffuse ST-segment elevations. The initial troponin is mildly elevated at 0.35 ng/ml.
Could this patient have acute pericarditis? If so, how should she be managed?
Background
Pericarditis is the most common pericardial disease encountered by hospitalists. As many as 5% of chest pain cases unattributable to myocardial infarction (MI) are diagnosed with pericarditis.1 In immunocompetent individuals, as many as 90% of acute pericarditis cases are viral or idiopathic in etiology.1,2 Human immunodeficiency virus (HIV) and tuberculosis are common culprits in developing countries and immunocompromised hosts.3 Other specific etiologies of acute pericarditis include autoimmune diseases, neoplasms, chest irradiation, trauma, and metabolic disturbances (e.g. uremia). An etiologic classification of acute pericarditis is shown in Table 2 (p. 16).
Pericarditis primarily is a clinical diagnosis. Most patients present with chest pain.4 A pericardial friction rub may or may not be heard (sensitivity 16% to 85%), but when present is nearly 100% specific for pericarditis.2,5 Diffuse ST-segment elevation on electrocardiogram (EKG) is present in 60% to 90% of cases, but it can be difficult to differentiate from ST-segment elevations in acute MI.4,6
Uncomplicated acute pericarditis often is treated successfully as an outpatient.4 However, patients with high-risk features (see Table 1, right) should be hospitalized for identification and treatment of specific underlying etiology and for monitoring of complications, such as tamponade.7
Our patient has features consistent with pericarditis. In the following sections, we will review the diagnosis and treatment of acute pericarditis.
Review of the Data
How is acute pericarditis diagnosed?
Acute pericarditis is a clinical diagnosis supported by EKG and echocardiogram. At least two of the following four criteria must be present for the diagnosis: pleuritic chest pain, pericardial rub, diffuse ST-segment elevation on EKG, and pericardial effusion.8
History. Patients may report fever (46% in one small study of 69 patients) or a recent history of respiratory or gastrointestinal infection (40%).5 Most patients will report pleuritic chest pain. Typically, the pain is improved when sitting up and leaning forward, and gets worse when lying supine.4 Pain might radiate to the trapezius muscle ridge due to the common phrenic nerve innervation of pericardium and trapezius.9 However, pain might be minimal or absent in patients with uremic, neoplastic, tuberculous, or post-irradiation pericarditis.
Physical exam. A pericardial friction rub is nearly 100% specific for a pericarditis diagnosis, but sensitivity can vary (16% to 85%) depending on the frequency of auscultation and underlying etiology.2,5 It is thought to be caused by friction between the parietal and visceral layers of inflamed pericardium. A pericardial rub classically is described as a superficial, high-pitched, scratchy, or squeaking sound best heard with the diaphragm of the stethoscope at the lower left sternal border with the patient leaning forward.
Laboratory data. A complete blood count, metabolic panel, and cardiac enzymes should be checked in all patients with suspected acute pericarditis. Troponin values are elevated in up to one-third of patients, indicating cardiac muscle injury or myopericarditis, but have not been shown to adversely impact hospital length of stay, readmission, or complication rates.5,10 Markers of inflammation (e.g. erythrocyte sedimentation rate or C-reactive protein) are frequently elevated but do not point to a specific underlying etiology. Routine viral cultures and antibody titers are not useful.11
Most cases of pericarditis are presumed idiopathic (viral); however, finding a specific etiology should be considered in patients who do not respond after one week of therapy. Anti-nuclear antibody, complement levels, and rheumatoid factor can serve as screening tests for autoimmune disease. Purified protein derivative or quantiferon testing and HIV testing might be indicated in patients with appropriate risk factors. In cases of suspected tuberculous or neoplastic pericarditis, pericardial fluid analysis and biopsy could be warranted.
Electrocardiography. The EKG is the most useful test in diagnosing acute pericarditis. EKG changes in acute pericarditis can progress over four stages:
- Stage 1: diffuse ST elevations with or without PR depressions, initially;
- Stage 2: normalization of ST and PR segments, typically after several days;
- Stage 3: diffuse T-wave inversions; and
- Stage 4: normalization of T-waves, typically after weeks or months.
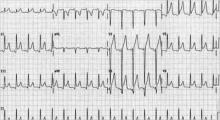
While all four stages are unlikely to be present in a given case, 80% of patients with pericarditis will demonstrate diffuse ST-segment elevations and PR-segment depression (see Figure 2, above).12
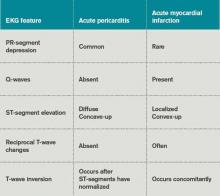
Table 3 lists EKG features helpful in differentiating acute pericarditis from acute myocardial infarction.
Chest radiography. Because a pericardial effusion often accompanies pericarditis, a chest radiograph (CXR) should be performed in all suspected cases. The CXR might show enlargement of the cardiac silhouette if more than 250 ml of pericardial fluid is present.3 A CXR also is helpful to diagnose concomitant pulmonary infection, pleural effusion, or mediastinal mass—all findings that could point to an underlying specific etiology of pericarditis and/or pericardial effusion.
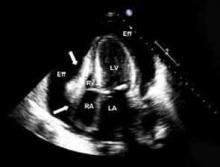
Echocardiography. An echocardiogram should be performed in all patients with suspected pericarditis to detect effusion, associated myocardial, or paracardial disease.13 The echocardiogram frequently is normal but could show an effusion in 60%, and tamponade (see Figure 1, p. 15) in 5%, of cases.4
Computed tomography (CT) and cardiac magnetic resonance imaging (CMR).CT or CMR are the imaging modalities of choice when an echocardiogram is inconclusive or in cases of pericarditis complicated by a hemorrhagic or localized effusion, pericardial thickening, or pericardial mass.14 They also help in precise imaging of neighboring structures, such as lungs or mediastinum.
Pericardial fluid analysis and pericardial biopsy. In cases of refractory pericarditis with effusion, pericardial fluid analysis might provide clues to the underlying etiology. Routine chemistry, cell count, gram and acid fast staining, culture, and cytology should be sent. In addition, acid-fast bacillus staining and culture, adenosine deaminase, and interferon-gamma testing should be ordered when tuberculous pericarditis is suspected. A pericardial biopsy may show granulomas or neoplastic cells. Overall, pericardial fluid analysis and biopsy reveal a diagnosis in roughly 20% of cases.11
How is acute pericarditis treated?
Most cases of uncomplicated acute pericarditis are viral and respond well to NSAID plus colchicine therapy.2,4 Failure to respond to NSAIDs plus colchicine—evidenced by persistent fever, pericardial chest pain, new pericardial effusion, or worsening of general illness—within a week of treatment should prompt a search for an underlying systemic illness. If found, treatment should be aimed at the causative illness.
Bacterial pericarditis usually requires surgical drainage in addition to treatment with appropriate antibiotics.11 Tuberculous pericarditis is treated with multidrug therapy; when underlying HIV is present, patients should receive highly active anti-retroviral therapy as well. Steroids and immunosuppressants should be considered in addition to NSAIDs and colchicine in autoimmune pericarditis.10 Neoplastic pericarditis may resolve with chemotherapy but it has a high recurrence rate.13 Uremic pericarditis requires intensified dialysis.
Treatment options for uncomplicated idiopathic or viral pericarditis include:
NSAIDs. It is important to adequately dose NSAIDs when treating acute pericarditis. Initial treatment options include ibuprofen (1,600 to 3,200 mg daily), indomethacin (75 to 150 mg daily) or aspirin (2 to 4 gm daily) for one week.11,15 Aspirin is preferred in patients with ischemic heart disease. For patients with symptoms that persist longer than a week, NSAIDS may be continued, but investigation for an underlying etiology is indicated. Concomitant proton-pump-inhibitor therapy should be considered in patients at high risk for peptic ulcer disease to minimize gastric side effects.
Colchicine. Colchicine has a favorable risk-benefit profile as an adjunct treatment for acute and recurrent pericarditis. Patients experience better symptom relief when treated with both colchicine and an NSAID, compared with NSAIDs alone (88% versus 63%). Recurrence rates are lower with combined therapy (11% versus 32%).16 Colchicine treatment (0.6 mg twice daily after a loading dose of up to 2 mg) is recommended for several months to greater than one year.13,16,17
Glucocorticoids. Routine glucocorticoid use should be avoided in the treatment of acute pericarditis, as it has been associated with an increased risk for recurrence (OR 4.3).16,18 Glucocorticoid use should be considered in cases of pericarditis refractory to NSAIDs and colchicine, cases in which NSAIDs and or colchicine are contraindicated, and in autoimmune or connective-tissue-disease-related pericarditis. Prednisone should be dosed up to 1 mg/kg/day for at least one month, depending on symptom resolution, then tapered after either NSAIDs or colchicine have been started.13 Smaller prednisone doses of up to 0.5 mg/kg/day could be as effective, with the added benefit of reduced side effects and recurrences.19
Invasive treatment. Pericardiocentesis and/or pericardiectomy should be considered when pericarditis is complicated by a large effusion or tamponade, constrictive physiology, or recurrent effusion.11 Pericardiocentesis is the least invasive option and helps provide immediate relief in cases of tamponade or large symptomatic effusions. It is the preferred modality for obtaining pericardial fluid for diagnostic analysis. However, effusions can recur and in those cases pericardial window is preferred, as it provides continued outflow of pericardial fluid. Pericardiectomy is recommended in cases of symptomatic constrictive pericarditis unresponsive to medical therapy.15
Back to the Case
The patient’s presentation—prodrome followed by fever and pleuritic chest pain—is characteristic of acute idiopathic pericarditis. No pericardial rub was heard, but EKG findings were typical. Troponin I elevation suggested underlying myopericarditis. An echocardiogram was unremarkable. Given the likely viral or idiopathic etiology, no further diagnostic tests were ordered to explore the possibility of an underlying systemic illness.
The patient was started on ibuprofen 600 mg every eight hours. She had significant relief of her symptoms within two days. A routine fever workup was negative. She was discharged the following day.
The patient was readmitted three months later with recurrent pleuritic chest pain, which did not improve with resumption of NSAID therapy. Initial troponin I was 0.22 ng/ml, electrocardiogram was unchanged, and an echocardiogram showed small effusion. She was started on ibuprofen 800 mg every eight hours, as well as colchicine 0.6 mg twice daily. Her symptoms resolved the next day and she was discharged with prescriptions for ibuprofen and colchicine. She was instructed to follow up with a primary-care doctor in one week.
At the clinic visit, ibuprofen was tapered but colchicine was continued for another six months. She remained asymptomatic at her six-month clinic follow-up.
Bottom Line
Acute pericarditis is a clinical diagnosis supported by EKG findings. Most cases are idiopathic or viral, and can be treated successfully with NSAIDs and colchicine. For cases that do not respond to initial therapy, or cases that present with high-risk features, a specific etiology should be sought.
Dr. Southern is chief of the division of hospital medicine at Montefiore Medical Center in Bronx, N.Y. Dr. Galhorta is an instructor and Drs. Martin, Korcak, and Stehlihova are assistant professors in the department of medicine at Albert Einstein.
References
- Lange RA, Hillis LD. Clinical practice. Acute pericarditis. N Engl J Med. 2004;351:2195-2202.
- Zayas R, Anguita M, Torres F, et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am J Cardiol. 1995;75:378-382.
- Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet. 2004;363:717-727.
- Imazio M, Demichelis B, Parrini I, et al. Day-hospital treatment of acute pericarditis: a management program for outpatient therapy. J Am Coll Cardiol. 2004;43:1042-1046.
- Bonnefoy E, Godon P, Kirkorian G, et al. Serum cardiac troponin I and ST-segment elevation in patients with acute pericarditis. Eur Heart J. 2000;21:832-836.
- Salisbury AC, Olalla-Gomez C, Rihal CS, et al. Frequency and predictors of urgent coronary angiography in patients with acute pericarditis. Mayo Clin Proc. 2009;84(1):11-15.
- Imazio M, Cecchi E, Demichelis B, et al. Indicators of poor prognosis of acute pericarditis. Circulation. 2007;115:2739-2744.
- Imazio M, Spodick DH, Brucato A, et al. Diagnostic issues in the clinical management of pericarditis. Int J Clin Pract. 2010;64(10):1384-1392.
- Spodick DH. Acute pericarditis: current concepts and practice. JAMA. 2003;289:1150-1153.
- Imazio M, Demichelis B, Cecchi E. Cardiac troponin I in acute pericarditis. J Am Coll Cardiol. 2003;42(12):2144-2148.
- Sagristà Sauleda J, Permanyer Miralda G, Soler Soler J. Diagnosis and management of pericardial syndromes. Rev Esp Cardiol. 2005;58(7):830-841.
- Bruce MA, Spodick DH. Atypical electrocardiogram in acute pericarditis: characteristics and prevalence. J Electrocardiol. 1980;13:61-66.
- Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004; 25(7):587-610.
- Verhaert D, Gabriel RS, Johnston D, et al. The role of multimodality imaging in the management of pericardial disease. Circ Cardiovasc Imaging. 2010;3:333-343.
- Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121:916-928.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the colchicine for acute pericarditis (COPE) trial. Circulation. 2005;112(13):2012-2016.
- Adler Y, Finkelstein Y, Guindo J, et al. Colchicine treatment for recurrent pericarditis: a decade of experience. Circulation. 1998;97:2183-185.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the colchicine for recurrent pericarditis (CORE) trial. Arch Intern Med. 2005;165:1987-1991.
- Imazio M, Brucato A, Cumetti D, et al. Corticosteroids for recurrent pericarditis: high versus low doses: a nonrandomized observation. Circulation. 2008;118:667-771.
Case
A 32-year-old female with no significant past medical history is evaluated for sharp, left-sided chest pain for five days. Her pain is intermittent, worse with deep inspiration and in the supine position. She denies any shortness of breath. Her temperature is 100.8ºF, but otherwise her vital signs are normal. The physical exam and chest radiograph are unremarkable, but an electrocardiogram shows diffuse ST-segment elevations. The initial troponin is mildly elevated at 0.35 ng/ml.
Could this patient have acute pericarditis? If so, how should she be managed?
Background
Pericarditis is the most common pericardial disease encountered by hospitalists. As many as 5% of chest pain cases unattributable to myocardial infarction (MI) are diagnosed with pericarditis.1 In immunocompetent individuals, as many as 90% of acute pericarditis cases are viral or idiopathic in etiology.1,2 Human immunodeficiency virus (HIV) and tuberculosis are common culprits in developing countries and immunocompromised hosts.3 Other specific etiologies of acute pericarditis include autoimmune diseases, neoplasms, chest irradiation, trauma, and metabolic disturbances (e.g. uremia). An etiologic classification of acute pericarditis is shown in Table 2 (p. 16).
Pericarditis primarily is a clinical diagnosis. Most patients present with chest pain.4 A pericardial friction rub may or may not be heard (sensitivity 16% to 85%), but when present is nearly 100% specific for pericarditis.2,5 Diffuse ST-segment elevation on electrocardiogram (EKG) is present in 60% to 90% of cases, but it can be difficult to differentiate from ST-segment elevations in acute MI.4,6
Uncomplicated acute pericarditis often is treated successfully as an outpatient.4 However, patients with high-risk features (see Table 1, right) should be hospitalized for identification and treatment of specific underlying etiology and for monitoring of complications, such as tamponade.7
Our patient has features consistent with pericarditis. In the following sections, we will review the diagnosis and treatment of acute pericarditis.
Review of the Data
How is acute pericarditis diagnosed?
Acute pericarditis is a clinical diagnosis supported by EKG and echocardiogram. At least two of the following four criteria must be present for the diagnosis: pleuritic chest pain, pericardial rub, diffuse ST-segment elevation on EKG, and pericardial effusion.8
History. Patients may report fever (46% in one small study of 69 patients) or a recent history of respiratory or gastrointestinal infection (40%).5 Most patients will report pleuritic chest pain. Typically, the pain is improved when sitting up and leaning forward, and gets worse when lying supine.4 Pain might radiate to the trapezius muscle ridge due to the common phrenic nerve innervation of pericardium and trapezius.9 However, pain might be minimal or absent in patients with uremic, neoplastic, tuberculous, or post-irradiation pericarditis.
Physical exam. A pericardial friction rub is nearly 100% specific for a pericarditis diagnosis, but sensitivity can vary (16% to 85%) depending on the frequency of auscultation and underlying etiology.2,5 It is thought to be caused by friction between the parietal and visceral layers of inflamed pericardium. A pericardial rub classically is described as a superficial, high-pitched, scratchy, or squeaking sound best heard with the diaphragm of the stethoscope at the lower left sternal border with the patient leaning forward.
Laboratory data. A complete blood count, metabolic panel, and cardiac enzymes should be checked in all patients with suspected acute pericarditis. Troponin values are elevated in up to one-third of patients, indicating cardiac muscle injury or myopericarditis, but have not been shown to adversely impact hospital length of stay, readmission, or complication rates.5,10 Markers of inflammation (e.g. erythrocyte sedimentation rate or C-reactive protein) are frequently elevated but do not point to a specific underlying etiology. Routine viral cultures and antibody titers are not useful.11
Most cases of pericarditis are presumed idiopathic (viral); however, finding a specific etiology should be considered in patients who do not respond after one week of therapy. Anti-nuclear antibody, complement levels, and rheumatoid factor can serve as screening tests for autoimmune disease. Purified protein derivative or quantiferon testing and HIV testing might be indicated in patients with appropriate risk factors. In cases of suspected tuberculous or neoplastic pericarditis, pericardial fluid analysis and biopsy could be warranted.
Electrocardiography. The EKG is the most useful test in diagnosing acute pericarditis. EKG changes in acute pericarditis can progress over four stages:
- Stage 1: diffuse ST elevations with or without PR depressions, initially;
- Stage 2: normalization of ST and PR segments, typically after several days;
- Stage 3: diffuse T-wave inversions; and
- Stage 4: normalization of T-waves, typically after weeks or months.
While all four stages are unlikely to be present in a given case, 80% of patients with pericarditis will demonstrate diffuse ST-segment elevations and PR-segment depression (see Figure 2, above).12
Table 3 lists EKG features helpful in differentiating acute pericarditis from acute myocardial infarction.
Chest radiography. Because a pericardial effusion often accompanies pericarditis, a chest radiograph (CXR) should be performed in all suspected cases. The CXR might show enlargement of the cardiac silhouette if more than 250 ml of pericardial fluid is present.3 A CXR also is helpful to diagnose concomitant pulmonary infection, pleural effusion, or mediastinal mass—all findings that could point to an underlying specific etiology of pericarditis and/or pericardial effusion.
Echocardiography. An echocardiogram should be performed in all patients with suspected pericarditis to detect effusion, associated myocardial, or paracardial disease.13 The echocardiogram frequently is normal but could show an effusion in 60%, and tamponade (see Figure 1, p. 15) in 5%, of cases.4
Computed tomography (CT) and cardiac magnetic resonance imaging (CMR).CT or CMR are the imaging modalities of choice when an echocardiogram is inconclusive or in cases of pericarditis complicated by a hemorrhagic or localized effusion, pericardial thickening, or pericardial mass.14 They also help in precise imaging of neighboring structures, such as lungs or mediastinum.
Pericardial fluid analysis and pericardial biopsy. In cases of refractory pericarditis with effusion, pericardial fluid analysis might provide clues to the underlying etiology. Routine chemistry, cell count, gram and acid fast staining, culture, and cytology should be sent. In addition, acid-fast bacillus staining and culture, adenosine deaminase, and interferon-gamma testing should be ordered when tuberculous pericarditis is suspected. A pericardial biopsy may show granulomas or neoplastic cells. Overall, pericardial fluid analysis and biopsy reveal a diagnosis in roughly 20% of cases.11
How is acute pericarditis treated?
Most cases of uncomplicated acute pericarditis are viral and respond well to NSAID plus colchicine therapy.2,4 Failure to respond to NSAIDs plus colchicine—evidenced by persistent fever, pericardial chest pain, new pericardial effusion, or worsening of general illness—within a week of treatment should prompt a search for an underlying systemic illness. If found, treatment should be aimed at the causative illness.
Bacterial pericarditis usually requires surgical drainage in addition to treatment with appropriate antibiotics.11 Tuberculous pericarditis is treated with multidrug therapy; when underlying HIV is present, patients should receive highly active anti-retroviral therapy as well. Steroids and immunosuppressants should be considered in addition to NSAIDs and colchicine in autoimmune pericarditis.10 Neoplastic pericarditis may resolve with chemotherapy but it has a high recurrence rate.13 Uremic pericarditis requires intensified dialysis.
Treatment options for uncomplicated idiopathic or viral pericarditis include:
NSAIDs. It is important to adequately dose NSAIDs when treating acute pericarditis. Initial treatment options include ibuprofen (1,600 to 3,200 mg daily), indomethacin (75 to 150 mg daily) or aspirin (2 to 4 gm daily) for one week.11,15 Aspirin is preferred in patients with ischemic heart disease. For patients with symptoms that persist longer than a week, NSAIDS may be continued, but investigation for an underlying etiology is indicated. Concomitant proton-pump-inhibitor therapy should be considered in patients at high risk for peptic ulcer disease to minimize gastric side effects.
Colchicine. Colchicine has a favorable risk-benefit profile as an adjunct treatment for acute and recurrent pericarditis. Patients experience better symptom relief when treated with both colchicine and an NSAID, compared with NSAIDs alone (88% versus 63%). Recurrence rates are lower with combined therapy (11% versus 32%).16 Colchicine treatment (0.6 mg twice daily after a loading dose of up to 2 mg) is recommended for several months to greater than one year.13,16,17
Glucocorticoids. Routine glucocorticoid use should be avoided in the treatment of acute pericarditis, as it has been associated with an increased risk for recurrence (OR 4.3).16,18 Glucocorticoid use should be considered in cases of pericarditis refractory to NSAIDs and colchicine, cases in which NSAIDs and or colchicine are contraindicated, and in autoimmune or connective-tissue-disease-related pericarditis. Prednisone should be dosed up to 1 mg/kg/day for at least one month, depending on symptom resolution, then tapered after either NSAIDs or colchicine have been started.13 Smaller prednisone doses of up to 0.5 mg/kg/day could be as effective, with the added benefit of reduced side effects and recurrences.19
Invasive treatment. Pericardiocentesis and/or pericardiectomy should be considered when pericarditis is complicated by a large effusion or tamponade, constrictive physiology, or recurrent effusion.11 Pericardiocentesis is the least invasive option and helps provide immediate relief in cases of tamponade or large symptomatic effusions. It is the preferred modality for obtaining pericardial fluid for diagnostic analysis. However, effusions can recur and in those cases pericardial window is preferred, as it provides continued outflow of pericardial fluid. Pericardiectomy is recommended in cases of symptomatic constrictive pericarditis unresponsive to medical therapy.15
Back to the Case
The patient’s presentation—prodrome followed by fever and pleuritic chest pain—is characteristic of acute idiopathic pericarditis. No pericardial rub was heard, but EKG findings were typical. Troponin I elevation suggested underlying myopericarditis. An echocardiogram was unremarkable. Given the likely viral or idiopathic etiology, no further diagnostic tests were ordered to explore the possibility of an underlying systemic illness.
The patient was started on ibuprofen 600 mg every eight hours. She had significant relief of her symptoms within two days. A routine fever workup was negative. She was discharged the following day.
The patient was readmitted three months later with recurrent pleuritic chest pain, which did not improve with resumption of NSAID therapy. Initial troponin I was 0.22 ng/ml, electrocardiogram was unchanged, and an echocardiogram showed small effusion. She was started on ibuprofen 800 mg every eight hours, as well as colchicine 0.6 mg twice daily. Her symptoms resolved the next day and she was discharged with prescriptions for ibuprofen and colchicine. She was instructed to follow up with a primary-care doctor in one week.
At the clinic visit, ibuprofen was tapered but colchicine was continued for another six months. She remained asymptomatic at her six-month clinic follow-up.
Bottom Line
Acute pericarditis is a clinical diagnosis supported by EKG findings. Most cases are idiopathic or viral, and can be treated successfully with NSAIDs and colchicine. For cases that do not respond to initial therapy, or cases that present with high-risk features, a specific etiology should be sought.
Dr. Southern is chief of the division of hospital medicine at Montefiore Medical Center in Bronx, N.Y. Dr. Galhorta is an instructor and Drs. Martin, Korcak, and Stehlihova are assistant professors in the department of medicine at Albert Einstein.
References
- Lange RA, Hillis LD. Clinical practice. Acute pericarditis. N Engl J Med. 2004;351:2195-2202.
- Zayas R, Anguita M, Torres F, et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am J Cardiol. 1995;75:378-382.
- Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet. 2004;363:717-727.
- Imazio M, Demichelis B, Parrini I, et al. Day-hospital treatment of acute pericarditis: a management program for outpatient therapy. J Am Coll Cardiol. 2004;43:1042-1046.
- Bonnefoy E, Godon P, Kirkorian G, et al. Serum cardiac troponin I and ST-segment elevation in patients with acute pericarditis. Eur Heart J. 2000;21:832-836.
- Salisbury AC, Olalla-Gomez C, Rihal CS, et al. Frequency and predictors of urgent coronary angiography in patients with acute pericarditis. Mayo Clin Proc. 2009;84(1):11-15.
- Imazio M, Cecchi E, Demichelis B, et al. Indicators of poor prognosis of acute pericarditis. Circulation. 2007;115:2739-2744.
- Imazio M, Spodick DH, Brucato A, et al. Diagnostic issues in the clinical management of pericarditis. Int J Clin Pract. 2010;64(10):1384-1392.
- Spodick DH. Acute pericarditis: current concepts and practice. JAMA. 2003;289:1150-1153.
- Imazio M, Demichelis B, Cecchi E. Cardiac troponin I in acute pericarditis. J Am Coll Cardiol. 2003;42(12):2144-2148.
- Sagristà Sauleda J, Permanyer Miralda G, Soler Soler J. Diagnosis and management of pericardial syndromes. Rev Esp Cardiol. 2005;58(7):830-841.
- Bruce MA, Spodick DH. Atypical electrocardiogram in acute pericarditis: characteristics and prevalence. J Electrocardiol. 1980;13:61-66.
- Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004; 25(7):587-610.
- Verhaert D, Gabriel RS, Johnston D, et al. The role of multimodality imaging in the management of pericardial disease. Circ Cardiovasc Imaging. 2010;3:333-343.
- Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121:916-928.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the colchicine for acute pericarditis (COPE) trial. Circulation. 2005;112(13):2012-2016.
- Adler Y, Finkelstein Y, Guindo J, et al. Colchicine treatment for recurrent pericarditis: a decade of experience. Circulation. 1998;97:2183-185.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the colchicine for recurrent pericarditis (CORE) trial. Arch Intern Med. 2005;165:1987-1991.
- Imazio M, Brucato A, Cumetti D, et al. Corticosteroids for recurrent pericarditis: high versus low doses: a nonrandomized observation. Circulation. 2008;118:667-771.
Case
A 32-year-old female with no significant past medical history is evaluated for sharp, left-sided chest pain for five days. Her pain is intermittent, worse with deep inspiration and in the supine position. She denies any shortness of breath. Her temperature is 100.8ºF, but otherwise her vital signs are normal. The physical exam and chest radiograph are unremarkable, but an electrocardiogram shows diffuse ST-segment elevations. The initial troponin is mildly elevated at 0.35 ng/ml.
Could this patient have acute pericarditis? If so, how should she be managed?
Background
Pericarditis is the most common pericardial disease encountered by hospitalists. As many as 5% of chest pain cases unattributable to myocardial infarction (MI) are diagnosed with pericarditis.1 In immunocompetent individuals, as many as 90% of acute pericarditis cases are viral or idiopathic in etiology.1,2 Human immunodeficiency virus (HIV) and tuberculosis are common culprits in developing countries and immunocompromised hosts.3 Other specific etiologies of acute pericarditis include autoimmune diseases, neoplasms, chest irradiation, trauma, and metabolic disturbances (e.g. uremia). An etiologic classification of acute pericarditis is shown in Table 2 (p. 16).
Pericarditis primarily is a clinical diagnosis. Most patients present with chest pain.4 A pericardial friction rub may or may not be heard (sensitivity 16% to 85%), but when present is nearly 100% specific for pericarditis.2,5 Diffuse ST-segment elevation on electrocardiogram (EKG) is present in 60% to 90% of cases, but it can be difficult to differentiate from ST-segment elevations in acute MI.4,6
Uncomplicated acute pericarditis often is treated successfully as an outpatient.4 However, patients with high-risk features (see Table 1, right) should be hospitalized for identification and treatment of specific underlying etiology and for monitoring of complications, such as tamponade.7
Our patient has features consistent with pericarditis. In the following sections, we will review the diagnosis and treatment of acute pericarditis.
Review of the Data
How is acute pericarditis diagnosed?
Acute pericarditis is a clinical diagnosis supported by EKG and echocardiogram. At least two of the following four criteria must be present for the diagnosis: pleuritic chest pain, pericardial rub, diffuse ST-segment elevation on EKG, and pericardial effusion.8
History. Patients may report fever (46% in one small study of 69 patients) or a recent history of respiratory or gastrointestinal infection (40%).5 Most patients will report pleuritic chest pain. Typically, the pain is improved when sitting up and leaning forward, and gets worse when lying supine.4 Pain might radiate to the trapezius muscle ridge due to the common phrenic nerve innervation of pericardium and trapezius.9 However, pain might be minimal or absent in patients with uremic, neoplastic, tuberculous, or post-irradiation pericarditis.
Physical exam. A pericardial friction rub is nearly 100% specific for a pericarditis diagnosis, but sensitivity can vary (16% to 85%) depending on the frequency of auscultation and underlying etiology.2,5 It is thought to be caused by friction between the parietal and visceral layers of inflamed pericardium. A pericardial rub classically is described as a superficial, high-pitched, scratchy, or squeaking sound best heard with the diaphragm of the stethoscope at the lower left sternal border with the patient leaning forward.
Laboratory data. A complete blood count, metabolic panel, and cardiac enzymes should be checked in all patients with suspected acute pericarditis. Troponin values are elevated in up to one-third of patients, indicating cardiac muscle injury or myopericarditis, but have not been shown to adversely impact hospital length of stay, readmission, or complication rates.5,10 Markers of inflammation (e.g. erythrocyte sedimentation rate or C-reactive protein) are frequently elevated but do not point to a specific underlying etiology. Routine viral cultures and antibody titers are not useful.11
Most cases of pericarditis are presumed idiopathic (viral); however, finding a specific etiology should be considered in patients who do not respond after one week of therapy. Anti-nuclear antibody, complement levels, and rheumatoid factor can serve as screening tests for autoimmune disease. Purified protein derivative or quantiferon testing and HIV testing might be indicated in patients with appropriate risk factors. In cases of suspected tuberculous or neoplastic pericarditis, pericardial fluid analysis and biopsy could be warranted.
Electrocardiography. The EKG is the most useful test in diagnosing acute pericarditis. EKG changes in acute pericarditis can progress over four stages:
- Stage 1: diffuse ST elevations with or without PR depressions, initially;
- Stage 2: normalization of ST and PR segments, typically after several days;
- Stage 3: diffuse T-wave inversions; and
- Stage 4: normalization of T-waves, typically after weeks or months.
While all four stages are unlikely to be present in a given case, 80% of patients with pericarditis will demonstrate diffuse ST-segment elevations and PR-segment depression (see Figure 2, above).12
Table 3 lists EKG features helpful in differentiating acute pericarditis from acute myocardial infarction.
Chest radiography. Because a pericardial effusion often accompanies pericarditis, a chest radiograph (CXR) should be performed in all suspected cases. The CXR might show enlargement of the cardiac silhouette if more than 250 ml of pericardial fluid is present.3 A CXR also is helpful to diagnose concomitant pulmonary infection, pleural effusion, or mediastinal mass—all findings that could point to an underlying specific etiology of pericarditis and/or pericardial effusion.
Echocardiography. An echocardiogram should be performed in all patients with suspected pericarditis to detect effusion, associated myocardial, or paracardial disease.13 The echocardiogram frequently is normal but could show an effusion in 60%, and tamponade (see Figure 1, p. 15) in 5%, of cases.4
Computed tomography (CT) and cardiac magnetic resonance imaging (CMR).CT or CMR are the imaging modalities of choice when an echocardiogram is inconclusive or in cases of pericarditis complicated by a hemorrhagic or localized effusion, pericardial thickening, or pericardial mass.14 They also help in precise imaging of neighboring structures, such as lungs or mediastinum.
Pericardial fluid analysis and pericardial biopsy. In cases of refractory pericarditis with effusion, pericardial fluid analysis might provide clues to the underlying etiology. Routine chemistry, cell count, gram and acid fast staining, culture, and cytology should be sent. In addition, acid-fast bacillus staining and culture, adenosine deaminase, and interferon-gamma testing should be ordered when tuberculous pericarditis is suspected. A pericardial biopsy may show granulomas or neoplastic cells. Overall, pericardial fluid analysis and biopsy reveal a diagnosis in roughly 20% of cases.11
How is acute pericarditis treated?
Most cases of uncomplicated acute pericarditis are viral and respond well to NSAID plus colchicine therapy.2,4 Failure to respond to NSAIDs plus colchicine—evidenced by persistent fever, pericardial chest pain, new pericardial effusion, or worsening of general illness—within a week of treatment should prompt a search for an underlying systemic illness. If found, treatment should be aimed at the causative illness.
Bacterial pericarditis usually requires surgical drainage in addition to treatment with appropriate antibiotics.11 Tuberculous pericarditis is treated with multidrug therapy; when underlying HIV is present, patients should receive highly active anti-retroviral therapy as well. Steroids and immunosuppressants should be considered in addition to NSAIDs and colchicine in autoimmune pericarditis.10 Neoplastic pericarditis may resolve with chemotherapy but it has a high recurrence rate.13 Uremic pericarditis requires intensified dialysis.
Treatment options for uncomplicated idiopathic or viral pericarditis include:
NSAIDs. It is important to adequately dose NSAIDs when treating acute pericarditis. Initial treatment options include ibuprofen (1,600 to 3,200 mg daily), indomethacin (75 to 150 mg daily) or aspirin (2 to 4 gm daily) for one week.11,15 Aspirin is preferred in patients with ischemic heart disease. For patients with symptoms that persist longer than a week, NSAIDS may be continued, but investigation for an underlying etiology is indicated. Concomitant proton-pump-inhibitor therapy should be considered in patients at high risk for peptic ulcer disease to minimize gastric side effects.
Colchicine. Colchicine has a favorable risk-benefit profile as an adjunct treatment for acute and recurrent pericarditis. Patients experience better symptom relief when treated with both colchicine and an NSAID, compared with NSAIDs alone (88% versus 63%). Recurrence rates are lower with combined therapy (11% versus 32%).16 Colchicine treatment (0.6 mg twice daily after a loading dose of up to 2 mg) is recommended for several months to greater than one year.13,16,17
Glucocorticoids. Routine glucocorticoid use should be avoided in the treatment of acute pericarditis, as it has been associated with an increased risk for recurrence (OR 4.3).16,18 Glucocorticoid use should be considered in cases of pericarditis refractory to NSAIDs and colchicine, cases in which NSAIDs and or colchicine are contraindicated, and in autoimmune or connective-tissue-disease-related pericarditis. Prednisone should be dosed up to 1 mg/kg/day for at least one month, depending on symptom resolution, then tapered after either NSAIDs or colchicine have been started.13 Smaller prednisone doses of up to 0.5 mg/kg/day could be as effective, with the added benefit of reduced side effects and recurrences.19
Invasive treatment. Pericardiocentesis and/or pericardiectomy should be considered when pericarditis is complicated by a large effusion or tamponade, constrictive physiology, or recurrent effusion.11 Pericardiocentesis is the least invasive option and helps provide immediate relief in cases of tamponade or large symptomatic effusions. It is the preferred modality for obtaining pericardial fluid for diagnostic analysis. However, effusions can recur and in those cases pericardial window is preferred, as it provides continued outflow of pericardial fluid. Pericardiectomy is recommended in cases of symptomatic constrictive pericarditis unresponsive to medical therapy.15
Back to the Case
The patient’s presentation—prodrome followed by fever and pleuritic chest pain—is characteristic of acute idiopathic pericarditis. No pericardial rub was heard, but EKG findings were typical. Troponin I elevation suggested underlying myopericarditis. An echocardiogram was unremarkable. Given the likely viral or idiopathic etiology, no further diagnostic tests were ordered to explore the possibility of an underlying systemic illness.
The patient was started on ibuprofen 600 mg every eight hours. She had significant relief of her symptoms within two days. A routine fever workup was negative. She was discharged the following day.
The patient was readmitted three months later with recurrent pleuritic chest pain, which did not improve with resumption of NSAID therapy. Initial troponin I was 0.22 ng/ml, electrocardiogram was unchanged, and an echocardiogram showed small effusion. She was started on ibuprofen 800 mg every eight hours, as well as colchicine 0.6 mg twice daily. Her symptoms resolved the next day and she was discharged with prescriptions for ibuprofen and colchicine. She was instructed to follow up with a primary-care doctor in one week.
At the clinic visit, ibuprofen was tapered but colchicine was continued for another six months. She remained asymptomatic at her six-month clinic follow-up.
Bottom Line
Acute pericarditis is a clinical diagnosis supported by EKG findings. Most cases are idiopathic or viral, and can be treated successfully with NSAIDs and colchicine. For cases that do not respond to initial therapy, or cases that present with high-risk features, a specific etiology should be sought.
Dr. Southern is chief of the division of hospital medicine at Montefiore Medical Center in Bronx, N.Y. Dr. Galhorta is an instructor and Drs. Martin, Korcak, and Stehlihova are assistant professors in the department of medicine at Albert Einstein.
References
- Lange RA, Hillis LD. Clinical practice. Acute pericarditis. N Engl J Med. 2004;351:2195-2202.
- Zayas R, Anguita M, Torres F, et al. Incidence of specific etiology and role of methods for specific etiologic diagnosis of primary acute pericarditis. Am J Cardiol. 1995;75:378-382.
- Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet. 2004;363:717-727.
- Imazio M, Demichelis B, Parrini I, et al. Day-hospital treatment of acute pericarditis: a management program for outpatient therapy. J Am Coll Cardiol. 2004;43:1042-1046.
- Bonnefoy E, Godon P, Kirkorian G, et al. Serum cardiac troponin I and ST-segment elevation in patients with acute pericarditis. Eur Heart J. 2000;21:832-836.
- Salisbury AC, Olalla-Gomez C, Rihal CS, et al. Frequency and predictors of urgent coronary angiography in patients with acute pericarditis. Mayo Clin Proc. 2009;84(1):11-15.
- Imazio M, Cecchi E, Demichelis B, et al. Indicators of poor prognosis of acute pericarditis. Circulation. 2007;115:2739-2744.
- Imazio M, Spodick DH, Brucato A, et al. Diagnostic issues in the clinical management of pericarditis. Int J Clin Pract. 2010;64(10):1384-1392.
- Spodick DH. Acute pericarditis: current concepts and practice. JAMA. 2003;289:1150-1153.
- Imazio M, Demichelis B, Cecchi E. Cardiac troponin I in acute pericarditis. J Am Coll Cardiol. 2003;42(12):2144-2148.
- Sagristà Sauleda J, Permanyer Miralda G, Soler Soler J. Diagnosis and management of pericardial syndromes. Rev Esp Cardiol. 2005;58(7):830-841.
- Bruce MA, Spodick DH. Atypical electrocardiogram in acute pericarditis: characteristics and prevalence. J Electrocardiol. 1980;13:61-66.
- Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004; 25(7):587-610.
- Verhaert D, Gabriel RS, Johnston D, et al. The role of multimodality imaging in the management of pericardial disease. Circ Cardiovasc Imaging. 2010;3:333-343.
- Imazio M, Spodick DH, Brucato A, et al. Controversial issues in the management of pericardial diseases. Circulation. 2010;121:916-928.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the colchicine for acute pericarditis (COPE) trial. Circulation. 2005;112(13):2012-2016.
- Adler Y, Finkelstein Y, Guindo J, et al. Colchicine treatment for recurrent pericarditis: a decade of experience. Circulation. 1998;97:2183-185.
- Imazio M, Bobbio M, Cecchi E, et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the colchicine for recurrent pericarditis (CORE) trial. Arch Intern Med. 2005;165:1987-1991.
- Imazio M, Brucato A, Cumetti D, et al. Corticosteroids for recurrent pericarditis: high versus low doses: a nonrandomized observation. Circulation. 2008;118:667-771.
When Is Testing for Thrombophilia Indicated?
The Case
A healthy 42-year-old woman presents to the hospital with acute-onset pleuritic chest pain and shortness of breath. She has not had any recent surgeries, takes no medications, and is very active. A lung ventilation-perfusion scan reveals a high probability of pulmonary embolism (PE). The patient’s history is notable for two second-trimester pregnancy losses. The patient is started on low-molecular heparin and warfarin (LMHW).
Should this patient be tested for thrombophilia?
Background
Thrombophilia can now be identified in more than half of all patients presenting with VTE, and testing for underlying causes of thrombophilia has become widespread.1 Physicians believe that thrombophilia testing frequently changes management of patients with VTE.2
Thrombophilias can be classified into three major categories: deficiency of natural inhibitors of coagulation, abnormal function or elevated level of coagulation factors, and acquired thrombophilias (see Table 1).
The prevalence of specific thrombophilias varies widely. For example, the prevalence of activated protein C resistance (the factor V Leiden mutation) is 3% to 7%. In comparison, the prevalence of antithrombin deficiency is estimated at 0.02%. Each thrombophilia is associated with an increased VTE risk, but the level of risk associated with a given thrombophilia varies greatly.1
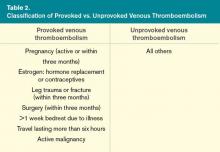
Before testing for thrombophilia in acute VTE, assess the risk of recurrent VTE by determining if the thrombosis was provoked or unprovoked. A VTE event is considered provoked if it occurs in the setting of pregnancy within the previous three months; estrogen therapy; immobility from acute illness for more than one week; travel lasting for more than six hours; leg trauma, fracture, or surgery within the previous three months; or active malignancy (see Table 2,).3 Unprovoked VTE has a recurrence rate of 7.4% per patient year, compared with 3.3% per patient year for a provoked VTE; the risk is even lower (0.7% per patient year) if the risk factor for the provoked VTE was surgical.4
Testing for thrombophilia is indicated if the results would add significant prognostic information beyond the clinical history, or if it would change patient management—in particular, the intensity or the duration of anticoagulation.
Review of the Data
Does presence of thrombophilia alter the intensity of anticoagulation for VTE?
If thrombophilia increases the risk of VTE recurrence while on anticoagulation, then a more intense level of anticoagulation might prevent future VTE. There are no studies investigating higher intensity of anticoagulation, but if standard anticoagulation were insufficient for patients with identifiable thrombophilia, one might expect to observe increased recurrence rates among patients with thrombophilia treated with standard warfarin therapy.
In a substudy of the Extended Low-Intensity Anticoagulation for Unprovoked Venous ThromboEmbolism (ELATE) trial, the risk of recurrence of VTE among treated subjects was very low overall, and the presence of thrombophilic abnormalities was not associated with significantly higher risk.5 Observational studies have found VTE recurrence rates are low in patients treated with warfarin, with or without thrombophilia.6-8
The impact of the initial level of anticoagulation on recurrence after the completion of the treatment period has been evaluated. Although one study suggested that patients with substandard levels of anticoagulation were at an increased risk of subsequent VTE, this was not confirmed in the Leiden Thrombophilia Study (LETS). 9,10
In sum, the majority of data do not suggest a significantly increased risk of recurrent VTE in patients with thrombophilia treated with standard anticoagulation. Therefore, treatment with warfarin to a goal INR of 2 to 3 is sufficient.
Does presence of thrombophilia alter duration of VTE treatment?
A major decision clinicians face when caring for VTE patients is the duration of anticoagulation treatment. The current ACCP recommendation for treatment of a provoked VTE is three months, with treatment for an unprovoked VTE three months or lifelong.11 If the presence of thrombophilia increases the risk of recurrence after cessation of anticoagulation treatment, longer duration of treatment might be indicated. One of the goals of thrombophilia testing should be to identify those patients.
Overall, the recurrence rate after first VTE is high, with a cumulative incidence of 25% at five years, 30% at eight years, and 56% at 20 years.12,13
Deficiency of natural inhibitors of coagulation.
Deficiency of a natural inhibitor of coagulation has been associated with a risk of recurrence of VTE of as much as 10% per year, according to some studies.6,14 However, the estimates are based on studies that include individuals from thrombosis-prone families, and selection bias might have contributed to the high recurrence rates.1 In the unselected population represented in the LETS study, only a modest elevation was seen in the estimated risk of recurrence for patients with inhibitor deficiencies.15
Testing for deficiency of inhibitors offers little prognostic information beyond that obtained when determining whether a VTE event is provoked or unprovoked. In studies that have separately examined subjects with provoked vs. unprovoked VTE, deficiency of an inhibitor is not associated with increased risk of recurrence.15,16
Abnormal function or level of anticoagulation factors.
Factor V Leiden (FVL) is the most common cause of inherited thrombophilia and is associated with as much as a sixfold increase in VTE risk, while the prothrombin gene mutation is associated with a twofold increase.17,18
In contrast, the evidence associating these mutations with recurrent VTE risk is not as consistent. Although a study conducted at a referral center in Italy found an increased risk of recurrence with either Factor V Leiden or prothrombin gene mutation, a large meta-analysis of 23 studies found increased risk only with Factor V Leiden.19,20 Another meta-analysis demonstrated only a modest increased risk of recurrence in subjects with Factor V Leiden or prothrombin gene mutation, and a prospective study from Austria found no increased risk of recurrence with Factor V Leiden two years after discontinuation of anticoagulation.18,21 Additionally, when using patients with unprovoked VTE as reference, there was no increased risk of recurrence among patients homozygous for Factor V Leiden or the prothrombin gene mutation.22
In summary, although Factor V Leiden and prothrombin gene defects are associated with increased risk of recurrent VTE, the magnitude of the risk increase is modest and, therefore, should not alter duration of therapy.
Acquired thrombophilia.
It appears that the only thrombophilic state that might have a significant impact on the risk of recurrence is the antiphospholipid syndrome. The cessation of warfarin therapy in patients with thrombosis associated with antiphospholipid antibodies carries a 69% risk of recurrent thrombosis within a year.23 Some studies have suggested that the presence of specific antibodies (i.e. anticardiolipin antibodies) is associated with increased risk in patients with antiphospholipid syndrome.24
However, at present, all patients with VTE and antiphospholipid syndrome should be candidates for lifelong anticoagulation. Antiphospholipid antibody testing should be performed in patients with a suggestive history, including those with recurrent fetal loss or a single fetal loss after 10 weeks, or known collagen vascular disease.25
The role of provoked vs. unprovoked VTE.
Identifying whether a VTE is provoked or unprovoked has been shown to be an important predictor of recurrence. For example, one prospective, cohort study found two-year recurrence rates of zero in patients with a surgery or pregnancy-related VTE, 9% with other provoked VTE, and 19% with unprovoked VTE.26 In the same study, thrombophilia testing failed to reliably predict recurrence risk. Patients with unprovoked VTE who were tested and found to not have a defect were at equally high risk of recurrent VTE as those found to have a thrombophilia.27
The most significant predictor for VTE recurrence is whether the first event was provoked, and thrombophilia testing offers little additional prognostic information.28
VTE as a multifactorial disorder.
It is becoming increasingly clear that VTE is multifactorial disorder, caused by the interactions of genotypic, phenotypic, and environmental factors. In the case of an unprovoked VTE, the patient already carries a significantly elevated risk for recurrence, and further testing for known causes of thrombophilia appears to add very little additional information. The optimal duration of anticoagulation for unprovoked VTE is unclear, but current guidelines suggest at least three months—and clinicians should consider lifelong treatment.
In the vast majority of cases, testing for thrombophilia has no impact on the management of VTE and is not warranted. In patients with antiphospholipid-antibody syndrome, given the high risk of recurrence, long-term anticoagulation after a first VTE might be indicated. In select patients with a clinical picture suggestive of antiphospholipid-antibody syndrome, or a strong family history, testing should be considered.
Back to the Case
Our patient appears to have an unprovoked VTE. She should receive regular anticoagulation with warfarin, with a goal INR of 2 to 3, for at least three months. Lifelong anticoagulation therapy should be considered. Testing for heritable thrombophilia will not change the current management or treatment duration and, hence, is not indicated. However, the patient’s history is suggestive of antiphospholipid-antibody syndrome, so she should be tested. If the diagnosis of antiphospholipid syndrome is made, lifelong anticoagulation should be considered.
Bottom Line
Unprovoked VTE provides the strongest predictor for recurrence. Thrombophilia testing adds little in predicting recurrence and rarely is indicated.
Dr. Stehlikova is a clinical hospitalist in the division of hospital medicine, department of medicine, at Albert Einstein College of Medicine and Montefiore Medical Center in Bronx, N.Y. Dr. Martin is director of the Einstein Hospitalist Service. Dr. Janakiram is a fellow in the department of hematology at Einstein, and Dr. Korcak is an instructor at Einstein in the department of medicine and director of the Weiler Medical Service. Dr. Galhotra is associate director for inpatient quality in the department of medicine at Einstein; Dr. Averbukh is an academic hospitalist; and Dr. Southern is chief of the division of hospital medicine at Einstein.
References
- Middeldorp S, van Hylckama Vlieg A. Does thrombophilia testing help in the clinical management of patients? Br J Haematol. 2008;143:321-335.
- Coppens M, van Mourik JA, Eckmann CM, Büller HR, Middeldorp S. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5:1979-1981.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92:199-205.
- Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010;170:1710-1716.
- Kearon C, Julian JA, Kovacs MJ, et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. 2008;112:4432-4436.
- Vossen CY, Walker ID, Svensson P, et al. Recurrence rate after a first venous thrombosis in patients with familial thrombophilia. Arterioscler Thromb Vasc Biol. 2005;25:1992-1997.
- Brown K, Luddington R, Williamson D, Baker P, Baglin T. Risk of venous thromboembolism associated with a G to A transition at position 20210 in the 3'-untranslated region of the prothrombin gene. Br J Haematol. 1997;98:907-909.
- Schulman S, Tengborn L. Treatment of venous thromboembolism in patients with congenital deficiency of antithrombin III. Thromb Haemost. 1992;68:634-636.
- Palareti G, Legnani C, Cosmi B, Guazzaloca G, Cini M, Mattarozzi S. Poor anticoagulation quality in the first 3 months after unprovoked venous thromboembolism is a risk factor for long-term recurrence. J Thromb Haemost. 2005;3:955-961.
- Gadisseur AP, Christiansen SC, van der Meer FJ, Rosendaal FR. The quality of oral anticoagulant therapy and recurrent venous thrombotic events in the Leiden Thrombophilia Study. J Thromb Haemost. 2007;5:931-936.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:454S-545S.
- Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1-7.
- Laczkovics C, Grafenhofer H, Kaider A, et al. Risk of recurrence after a first venous thromboembolic event in young women. Haematologica. 2007;92:1201-1207.
- Brouwer JL, Lijfering WM, Ten Kate MK, Kluin-Nelemans HC, Veeger NJ, van der Meer J. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost. 2009;101:93-99.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293:2352-2361.
- De Stefano V, Simioni P, Rossi E, et al. The risk of recurrent venous thromboembolism in patients with inherited deficiency of natural anticoagulants antithrombin, protein C and protein S. Haematologica. 2006;91:695-698.
- Price DT, Ridker PM. Factor V Leiden mutation and the risks for thromboembolic disease: a clinical perspective. Ann Intern Med. 1997;127:895-903.
- Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166:729-736.
- Simioni P, Prandoni P, Lensing AW, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood. 2000;96:3329-3333.
- Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301:2472-2485.
- Eichinger S, Pabinger I, Stumpflen A, et al. The risk of recurrent venous thromboembolism in patients with and without factor V Leiden. Thromb Haemost. 1997;77:624-628.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation. 2010;121(15):1706-1712.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993-997.
- Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med. 1998;104:332-338.
- Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2009;7:1737-1740.
- Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362:523-526.
- Rosendaal FR. Once and only once. Circulation. 2010;121:1688-1690.
- Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121:458-463.
The Case
A healthy 42-year-old woman presents to the hospital with acute-onset pleuritic chest pain and shortness of breath. She has not had any recent surgeries, takes no medications, and is very active. A lung ventilation-perfusion scan reveals a high probability of pulmonary embolism (PE). The patient’s history is notable for two second-trimester pregnancy losses. The patient is started on low-molecular heparin and warfarin (LMHW).
Should this patient be tested for thrombophilia?
Background
Thrombophilia can now be identified in more than half of all patients presenting with VTE, and testing for underlying causes of thrombophilia has become widespread.1 Physicians believe that thrombophilia testing frequently changes management of patients with VTE.2
Thrombophilias can be classified into three major categories: deficiency of natural inhibitors of coagulation, abnormal function or elevated level of coagulation factors, and acquired thrombophilias (see Table 1).
The prevalence of specific thrombophilias varies widely. For example, the prevalence of activated protein C resistance (the factor V Leiden mutation) is 3% to 7%. In comparison, the prevalence of antithrombin deficiency is estimated at 0.02%. Each thrombophilia is associated with an increased VTE risk, but the level of risk associated with a given thrombophilia varies greatly.1
Before testing for thrombophilia in acute VTE, assess the risk of recurrent VTE by determining if the thrombosis was provoked or unprovoked. A VTE event is considered provoked if it occurs in the setting of pregnancy within the previous three months; estrogen therapy; immobility from acute illness for more than one week; travel lasting for more than six hours; leg trauma, fracture, or surgery within the previous three months; or active malignancy (see Table 2,).3 Unprovoked VTE has a recurrence rate of 7.4% per patient year, compared with 3.3% per patient year for a provoked VTE; the risk is even lower (0.7% per patient year) if the risk factor for the provoked VTE was surgical.4
Testing for thrombophilia is indicated if the results would add significant prognostic information beyond the clinical history, or if it would change patient management—in particular, the intensity or the duration of anticoagulation.
Review of the Data
Does presence of thrombophilia alter the intensity of anticoagulation for VTE?
If thrombophilia increases the risk of VTE recurrence while on anticoagulation, then a more intense level of anticoagulation might prevent future VTE. There are no studies investigating higher intensity of anticoagulation, but if standard anticoagulation were insufficient for patients with identifiable thrombophilia, one might expect to observe increased recurrence rates among patients with thrombophilia treated with standard warfarin therapy.
In a substudy of the Extended Low-Intensity Anticoagulation for Unprovoked Venous ThromboEmbolism (ELATE) trial, the risk of recurrence of VTE among treated subjects was very low overall, and the presence of thrombophilic abnormalities was not associated with significantly higher risk.5 Observational studies have found VTE recurrence rates are low in patients treated with warfarin, with or without thrombophilia.6-8
The impact of the initial level of anticoagulation on recurrence after the completion of the treatment period has been evaluated. Although one study suggested that patients with substandard levels of anticoagulation were at an increased risk of subsequent VTE, this was not confirmed in the Leiden Thrombophilia Study (LETS). 9,10
In sum, the majority of data do not suggest a significantly increased risk of recurrent VTE in patients with thrombophilia treated with standard anticoagulation. Therefore, treatment with warfarin to a goal INR of 2 to 3 is sufficient.
Does presence of thrombophilia alter duration of VTE treatment?
A major decision clinicians face when caring for VTE patients is the duration of anticoagulation treatment. The current ACCP recommendation for treatment of a provoked VTE is three months, with treatment for an unprovoked VTE three months or lifelong.11 If the presence of thrombophilia increases the risk of recurrence after cessation of anticoagulation treatment, longer duration of treatment might be indicated. One of the goals of thrombophilia testing should be to identify those patients.
Overall, the recurrence rate after first VTE is high, with a cumulative incidence of 25% at five years, 30% at eight years, and 56% at 20 years.12,13
Deficiency of natural inhibitors of coagulation.
Deficiency of a natural inhibitor of coagulation has been associated with a risk of recurrence of VTE of as much as 10% per year, according to some studies.6,14 However, the estimates are based on studies that include individuals from thrombosis-prone families, and selection bias might have contributed to the high recurrence rates.1 In the unselected population represented in the LETS study, only a modest elevation was seen in the estimated risk of recurrence for patients with inhibitor deficiencies.15
Testing for deficiency of inhibitors offers little prognostic information beyond that obtained when determining whether a VTE event is provoked or unprovoked. In studies that have separately examined subjects with provoked vs. unprovoked VTE, deficiency of an inhibitor is not associated with increased risk of recurrence.15,16
Abnormal function or level of anticoagulation factors.
Factor V Leiden (FVL) is the most common cause of inherited thrombophilia and is associated with as much as a sixfold increase in VTE risk, while the prothrombin gene mutation is associated with a twofold increase.17,18
In contrast, the evidence associating these mutations with recurrent VTE risk is not as consistent. Although a study conducted at a referral center in Italy found an increased risk of recurrence with either Factor V Leiden or prothrombin gene mutation, a large meta-analysis of 23 studies found increased risk only with Factor V Leiden.19,20 Another meta-analysis demonstrated only a modest increased risk of recurrence in subjects with Factor V Leiden or prothrombin gene mutation, and a prospective study from Austria found no increased risk of recurrence with Factor V Leiden two years after discontinuation of anticoagulation.18,21 Additionally, when using patients with unprovoked VTE as reference, there was no increased risk of recurrence among patients homozygous for Factor V Leiden or the prothrombin gene mutation.22
In summary, although Factor V Leiden and prothrombin gene defects are associated with increased risk of recurrent VTE, the magnitude of the risk increase is modest and, therefore, should not alter duration of therapy.
Acquired thrombophilia.
It appears that the only thrombophilic state that might have a significant impact on the risk of recurrence is the antiphospholipid syndrome. The cessation of warfarin therapy in patients with thrombosis associated with antiphospholipid antibodies carries a 69% risk of recurrent thrombosis within a year.23 Some studies have suggested that the presence of specific antibodies (i.e. anticardiolipin antibodies) is associated with increased risk in patients with antiphospholipid syndrome.24
However, at present, all patients with VTE and antiphospholipid syndrome should be candidates for lifelong anticoagulation. Antiphospholipid antibody testing should be performed in patients with a suggestive history, including those with recurrent fetal loss or a single fetal loss after 10 weeks, or known collagen vascular disease.25
The role of provoked vs. unprovoked VTE.
Identifying whether a VTE is provoked or unprovoked has been shown to be an important predictor of recurrence. For example, one prospective, cohort study found two-year recurrence rates of zero in patients with a surgery or pregnancy-related VTE, 9% with other provoked VTE, and 19% with unprovoked VTE.26 In the same study, thrombophilia testing failed to reliably predict recurrence risk. Patients with unprovoked VTE who were tested and found to not have a defect were at equally high risk of recurrent VTE as those found to have a thrombophilia.27
The most significant predictor for VTE recurrence is whether the first event was provoked, and thrombophilia testing offers little additional prognostic information.28
VTE as a multifactorial disorder.
It is becoming increasingly clear that VTE is multifactorial disorder, caused by the interactions of genotypic, phenotypic, and environmental factors. In the case of an unprovoked VTE, the patient already carries a significantly elevated risk for recurrence, and further testing for known causes of thrombophilia appears to add very little additional information. The optimal duration of anticoagulation for unprovoked VTE is unclear, but current guidelines suggest at least three months—and clinicians should consider lifelong treatment.
In the vast majority of cases, testing for thrombophilia has no impact on the management of VTE and is not warranted. In patients with antiphospholipid-antibody syndrome, given the high risk of recurrence, long-term anticoagulation after a first VTE might be indicated. In select patients with a clinical picture suggestive of antiphospholipid-antibody syndrome, or a strong family history, testing should be considered.
Back to the Case
Our patient appears to have an unprovoked VTE. She should receive regular anticoagulation with warfarin, with a goal INR of 2 to 3, for at least three months. Lifelong anticoagulation therapy should be considered. Testing for heritable thrombophilia will not change the current management or treatment duration and, hence, is not indicated. However, the patient’s history is suggestive of antiphospholipid-antibody syndrome, so she should be tested. If the diagnosis of antiphospholipid syndrome is made, lifelong anticoagulation should be considered.
Bottom Line
Unprovoked VTE provides the strongest predictor for recurrence. Thrombophilia testing adds little in predicting recurrence and rarely is indicated.
Dr. Stehlikova is a clinical hospitalist in the division of hospital medicine, department of medicine, at Albert Einstein College of Medicine and Montefiore Medical Center in Bronx, N.Y. Dr. Martin is director of the Einstein Hospitalist Service. Dr. Janakiram is a fellow in the department of hematology at Einstein, and Dr. Korcak is an instructor at Einstein in the department of medicine and director of the Weiler Medical Service. Dr. Galhotra is associate director for inpatient quality in the department of medicine at Einstein; Dr. Averbukh is an academic hospitalist; and Dr. Southern is chief of the division of hospital medicine at Einstein.
References
- Middeldorp S, van Hylckama Vlieg A. Does thrombophilia testing help in the clinical management of patients? Br J Haematol. 2008;143:321-335.
- Coppens M, van Mourik JA, Eckmann CM, Büller HR, Middeldorp S. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5:1979-1981.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92:199-205.
- Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010;170:1710-1716.
- Kearon C, Julian JA, Kovacs MJ, et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. 2008;112:4432-4436.
- Vossen CY, Walker ID, Svensson P, et al. Recurrence rate after a first venous thrombosis in patients with familial thrombophilia. Arterioscler Thromb Vasc Biol. 2005;25:1992-1997.
- Brown K, Luddington R, Williamson D, Baker P, Baglin T. Risk of venous thromboembolism associated with a G to A transition at position 20210 in the 3'-untranslated region of the prothrombin gene. Br J Haematol. 1997;98:907-909.
- Schulman S, Tengborn L. Treatment of venous thromboembolism in patients with congenital deficiency of antithrombin III. Thromb Haemost. 1992;68:634-636.
- Palareti G, Legnani C, Cosmi B, Guazzaloca G, Cini M, Mattarozzi S. Poor anticoagulation quality in the first 3 months after unprovoked venous thromboembolism is a risk factor for long-term recurrence. J Thromb Haemost. 2005;3:955-961.
- Gadisseur AP, Christiansen SC, van der Meer FJ, Rosendaal FR. The quality of oral anticoagulant therapy and recurrent venous thrombotic events in the Leiden Thrombophilia Study. J Thromb Haemost. 2007;5:931-936.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:454S-545S.
- Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1-7.
- Laczkovics C, Grafenhofer H, Kaider A, et al. Risk of recurrence after a first venous thromboembolic event in young women. Haematologica. 2007;92:1201-1207.
- Brouwer JL, Lijfering WM, Ten Kate MK, Kluin-Nelemans HC, Veeger NJ, van der Meer J. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost. 2009;101:93-99.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293:2352-2361.
- De Stefano V, Simioni P, Rossi E, et al. The risk of recurrent venous thromboembolism in patients with inherited deficiency of natural anticoagulants antithrombin, protein C and protein S. Haematologica. 2006;91:695-698.
- Price DT, Ridker PM. Factor V Leiden mutation and the risks for thromboembolic disease: a clinical perspective. Ann Intern Med. 1997;127:895-903.
- Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166:729-736.
- Simioni P, Prandoni P, Lensing AW, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood. 2000;96:3329-3333.
- Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301:2472-2485.
- Eichinger S, Pabinger I, Stumpflen A, et al. The risk of recurrent venous thromboembolism in patients with and without factor V Leiden. Thromb Haemost. 1997;77:624-628.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation. 2010;121(15):1706-1712.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993-997.
- Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med. 1998;104:332-338.
- Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2009;7:1737-1740.
- Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362:523-526.
- Rosendaal FR. Once and only once. Circulation. 2010;121:1688-1690.
- Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121:458-463.
The Case
A healthy 42-year-old woman presents to the hospital with acute-onset pleuritic chest pain and shortness of breath. She has not had any recent surgeries, takes no medications, and is very active. A lung ventilation-perfusion scan reveals a high probability of pulmonary embolism (PE). The patient’s history is notable for two second-trimester pregnancy losses. The patient is started on low-molecular heparin and warfarin (LMHW).
Should this patient be tested for thrombophilia?
Background
Thrombophilia can now be identified in more than half of all patients presenting with VTE, and testing for underlying causes of thrombophilia has become widespread.1 Physicians believe that thrombophilia testing frequently changes management of patients with VTE.2
Thrombophilias can be classified into three major categories: deficiency of natural inhibitors of coagulation, abnormal function or elevated level of coagulation factors, and acquired thrombophilias (see Table 1).
The prevalence of specific thrombophilias varies widely. For example, the prevalence of activated protein C resistance (the factor V Leiden mutation) is 3% to 7%. In comparison, the prevalence of antithrombin deficiency is estimated at 0.02%. Each thrombophilia is associated with an increased VTE risk, but the level of risk associated with a given thrombophilia varies greatly.1
Before testing for thrombophilia in acute VTE, assess the risk of recurrent VTE by determining if the thrombosis was provoked or unprovoked. A VTE event is considered provoked if it occurs in the setting of pregnancy within the previous three months; estrogen therapy; immobility from acute illness for more than one week; travel lasting for more than six hours; leg trauma, fracture, or surgery within the previous three months; or active malignancy (see Table 2,).3 Unprovoked VTE has a recurrence rate of 7.4% per patient year, compared with 3.3% per patient year for a provoked VTE; the risk is even lower (0.7% per patient year) if the risk factor for the provoked VTE was surgical.4
Testing for thrombophilia is indicated if the results would add significant prognostic information beyond the clinical history, or if it would change patient management—in particular, the intensity or the duration of anticoagulation.
Review of the Data
Does presence of thrombophilia alter the intensity of anticoagulation for VTE?
If thrombophilia increases the risk of VTE recurrence while on anticoagulation, then a more intense level of anticoagulation might prevent future VTE. There are no studies investigating higher intensity of anticoagulation, but if standard anticoagulation were insufficient for patients with identifiable thrombophilia, one might expect to observe increased recurrence rates among patients with thrombophilia treated with standard warfarin therapy.
In a substudy of the Extended Low-Intensity Anticoagulation for Unprovoked Venous ThromboEmbolism (ELATE) trial, the risk of recurrence of VTE among treated subjects was very low overall, and the presence of thrombophilic abnormalities was not associated with significantly higher risk.5 Observational studies have found VTE recurrence rates are low in patients treated with warfarin, with or without thrombophilia.6-8
The impact of the initial level of anticoagulation on recurrence after the completion of the treatment period has been evaluated. Although one study suggested that patients with substandard levels of anticoagulation were at an increased risk of subsequent VTE, this was not confirmed in the Leiden Thrombophilia Study (LETS). 9,10
In sum, the majority of data do not suggest a significantly increased risk of recurrent VTE in patients with thrombophilia treated with standard anticoagulation. Therefore, treatment with warfarin to a goal INR of 2 to 3 is sufficient.
Does presence of thrombophilia alter duration of VTE treatment?
A major decision clinicians face when caring for VTE patients is the duration of anticoagulation treatment. The current ACCP recommendation for treatment of a provoked VTE is three months, with treatment for an unprovoked VTE three months or lifelong.11 If the presence of thrombophilia increases the risk of recurrence after cessation of anticoagulation treatment, longer duration of treatment might be indicated. One of the goals of thrombophilia testing should be to identify those patients.
Overall, the recurrence rate after first VTE is high, with a cumulative incidence of 25% at five years, 30% at eight years, and 56% at 20 years.12,13
Deficiency of natural inhibitors of coagulation.
Deficiency of a natural inhibitor of coagulation has been associated with a risk of recurrence of VTE of as much as 10% per year, according to some studies.6,14 However, the estimates are based on studies that include individuals from thrombosis-prone families, and selection bias might have contributed to the high recurrence rates.1 In the unselected population represented in the LETS study, only a modest elevation was seen in the estimated risk of recurrence for patients with inhibitor deficiencies.15
Testing for deficiency of inhibitors offers little prognostic information beyond that obtained when determining whether a VTE event is provoked or unprovoked. In studies that have separately examined subjects with provoked vs. unprovoked VTE, deficiency of an inhibitor is not associated with increased risk of recurrence.15,16
Abnormal function or level of anticoagulation factors.
Factor V Leiden (FVL) is the most common cause of inherited thrombophilia and is associated with as much as a sixfold increase in VTE risk, while the prothrombin gene mutation is associated with a twofold increase.17,18
In contrast, the evidence associating these mutations with recurrent VTE risk is not as consistent. Although a study conducted at a referral center in Italy found an increased risk of recurrence with either Factor V Leiden or prothrombin gene mutation, a large meta-analysis of 23 studies found increased risk only with Factor V Leiden.19,20 Another meta-analysis demonstrated only a modest increased risk of recurrence in subjects with Factor V Leiden or prothrombin gene mutation, and a prospective study from Austria found no increased risk of recurrence with Factor V Leiden two years after discontinuation of anticoagulation.18,21 Additionally, when using patients with unprovoked VTE as reference, there was no increased risk of recurrence among patients homozygous for Factor V Leiden or the prothrombin gene mutation.22
In summary, although Factor V Leiden and prothrombin gene defects are associated with increased risk of recurrent VTE, the magnitude of the risk increase is modest and, therefore, should not alter duration of therapy.
Acquired thrombophilia.
It appears that the only thrombophilic state that might have a significant impact on the risk of recurrence is the antiphospholipid syndrome. The cessation of warfarin therapy in patients with thrombosis associated with antiphospholipid antibodies carries a 69% risk of recurrent thrombosis within a year.23 Some studies have suggested that the presence of specific antibodies (i.e. anticardiolipin antibodies) is associated with increased risk in patients with antiphospholipid syndrome.24
However, at present, all patients with VTE and antiphospholipid syndrome should be candidates for lifelong anticoagulation. Antiphospholipid antibody testing should be performed in patients with a suggestive history, including those with recurrent fetal loss or a single fetal loss after 10 weeks, or known collagen vascular disease.25
The role of provoked vs. unprovoked VTE.
Identifying whether a VTE is provoked or unprovoked has been shown to be an important predictor of recurrence. For example, one prospective, cohort study found two-year recurrence rates of zero in patients with a surgery or pregnancy-related VTE, 9% with other provoked VTE, and 19% with unprovoked VTE.26 In the same study, thrombophilia testing failed to reliably predict recurrence risk. Patients with unprovoked VTE who were tested and found to not have a defect were at equally high risk of recurrent VTE as those found to have a thrombophilia.27
The most significant predictor for VTE recurrence is whether the first event was provoked, and thrombophilia testing offers little additional prognostic information.28
VTE as a multifactorial disorder.
It is becoming increasingly clear that VTE is multifactorial disorder, caused by the interactions of genotypic, phenotypic, and environmental factors. In the case of an unprovoked VTE, the patient already carries a significantly elevated risk for recurrence, and further testing for known causes of thrombophilia appears to add very little additional information. The optimal duration of anticoagulation for unprovoked VTE is unclear, but current guidelines suggest at least three months—and clinicians should consider lifelong treatment.
In the vast majority of cases, testing for thrombophilia has no impact on the management of VTE and is not warranted. In patients with antiphospholipid-antibody syndrome, given the high risk of recurrence, long-term anticoagulation after a first VTE might be indicated. In select patients with a clinical picture suggestive of antiphospholipid-antibody syndrome, or a strong family history, testing should be considered.
Back to the Case
Our patient appears to have an unprovoked VTE. She should receive regular anticoagulation with warfarin, with a goal INR of 2 to 3, for at least three months. Lifelong anticoagulation therapy should be considered. Testing for heritable thrombophilia will not change the current management or treatment duration and, hence, is not indicated. However, the patient’s history is suggestive of antiphospholipid-antibody syndrome, so she should be tested. If the diagnosis of antiphospholipid syndrome is made, lifelong anticoagulation should be considered.
Bottom Line
Unprovoked VTE provides the strongest predictor for recurrence. Thrombophilia testing adds little in predicting recurrence and rarely is indicated.
Dr. Stehlikova is a clinical hospitalist in the division of hospital medicine, department of medicine, at Albert Einstein College of Medicine and Montefiore Medical Center in Bronx, N.Y. Dr. Martin is director of the Einstein Hospitalist Service. Dr. Janakiram is a fellow in the department of hematology at Einstein, and Dr. Korcak is an instructor at Einstein in the department of medicine and director of the Weiler Medical Service. Dr. Galhotra is associate director for inpatient quality in the department of medicine at Einstein; Dr. Averbukh is an academic hospitalist; and Dr. Southern is chief of the division of hospital medicine at Einstein.
References
- Middeldorp S, van Hylckama Vlieg A. Does thrombophilia testing help in the clinical management of patients? Br J Haematol. 2008;143:321-335.
- Coppens M, van Mourik JA, Eckmann CM, Büller HR, Middeldorp S. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5:1979-1981.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92:199-205.
- Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010;170:1710-1716.
- Kearon C, Julian JA, Kovacs MJ, et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. 2008;112:4432-4436.
- Vossen CY, Walker ID, Svensson P, et al. Recurrence rate after a first venous thrombosis in patients with familial thrombophilia. Arterioscler Thromb Vasc Biol. 2005;25:1992-1997.
- Brown K, Luddington R, Williamson D, Baker P, Baglin T. Risk of venous thromboembolism associated with a G to A transition at position 20210 in the 3'-untranslated region of the prothrombin gene. Br J Haematol. 1997;98:907-909.
- Schulman S, Tengborn L. Treatment of venous thromboembolism in patients with congenital deficiency of antithrombin III. Thromb Haemost. 1992;68:634-636.
- Palareti G, Legnani C, Cosmi B, Guazzaloca G, Cini M, Mattarozzi S. Poor anticoagulation quality in the first 3 months after unprovoked venous thromboembolism is a risk factor for long-term recurrence. J Thromb Haemost. 2005;3:955-961.
- Gadisseur AP, Christiansen SC, van der Meer FJ, Rosendaal FR. The quality of oral anticoagulant therapy and recurrent venous thrombotic events in the Leiden Thrombophilia Study. J Thromb Haemost. 2007;5:931-936.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:454S-545S.
- Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1-7.
- Laczkovics C, Grafenhofer H, Kaider A, et al. Risk of recurrence after a first venous thromboembolic event in young women. Haematologica. 2007;92:1201-1207.
- Brouwer JL, Lijfering WM, Ten Kate MK, Kluin-Nelemans HC, Veeger NJ, van der Meer J. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost. 2009;101:93-99.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293:2352-2361.
- De Stefano V, Simioni P, Rossi E, et al. The risk of recurrent venous thromboembolism in patients with inherited deficiency of natural anticoagulants antithrombin, protein C and protein S. Haematologica. 2006;91:695-698.
- Price DT, Ridker PM. Factor V Leiden mutation and the risks for thromboembolic disease: a clinical perspective. Ann Intern Med. 1997;127:895-903.
- Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166:729-736.
- Simioni P, Prandoni P, Lensing AW, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood. 2000;96:3329-3333.
- Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301:2472-2485.
- Eichinger S, Pabinger I, Stumpflen A, et al. The risk of recurrent venous thromboembolism in patients with and without factor V Leiden. Thromb Haemost. 1997;77:624-628.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation. 2010;121(15):1706-1712.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993-997.
- Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med. 1998;104:332-338.
- Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2009;7:1737-1740.
- Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362:523-526.
- Rosendaal FR. Once and only once. Circulation. 2010;121:1688-1690.
- Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121:458-463.