User login
Ebola in the United States: Management considerations during pregnancy
“[Pregnant patients infected with the Ebola virus in West Africa] aren’t given preferential treatment...They aren’t even given beds.…They are assumed to die. Priority is given to the patients whom the health-care workers believe they can save. In effect, pregnant women are being triaged last.”
—Joshua Lang1
Ebola is a rare and potentially deadly disease caused by infection with a strain of the Ebola virus. First described in 1967,2 the Ebola virus has caused significant morbidity and mortality in many parts of sub-Saharan Africa. Awareness about this disease has increased dramatically in the United States as a result of the largest Ebola epidemic in history, which broke out in West Africa in 2014. The 3 countries most widely affected include Sierra Leone, Liberia, and Guinea. As of May 15, 2015, there were 26,798 cases of Ebola in this epidemic (with 14,971 laboratory confirmed), of whom 11,089 have died.3 There have been a total of 868 confirmed health care worker infections reported in Guinea, Liberia, and Sierra Leone since the start of the outbreak, with 507 reported deaths.4
It is highly unlikely that an Ebola epidemic of similar proportion will break out in any developed nation. However, isolated cases of Ebola have been identified among high-risk individuals in the United States (such as those who had recently served as medical volunteers in West Africa), which has raised concern about Ebola infection prevention and management in this country.
Little is known about Ebola infection in pregnancy. The few reports available suggest that pregnant women who become infected are highly contagious, with a maternal and perinatal mortality rate near 100%.5 Significant efforts were put in place in hospitals around the United States, including in labor and delivery units, to care for potentially or actively infected individuals, to protect health care workers, and to contain the spread of any new infections. It is important that clinicians be aware of the efforts and the recommended protocols—especially for the unique circumstance of infection during pregnancy. We review these protocols, as well as provide details on viral transmission and treatment.
What is Ebola virus and why are humans affected?
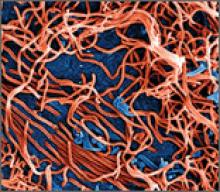
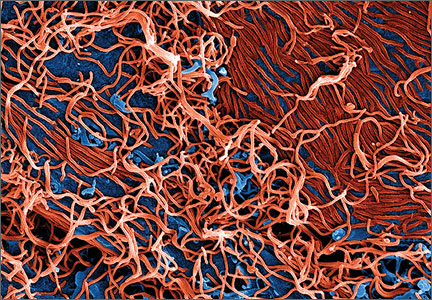
Ebola virus is a single-stranded RNA filovirus (FIGURE) with 5 independently identified species named after the countries or regions in which they were identified. Four of these are known to cause disease in humans, including Zaire Ebola virus, Sudan virus, Tai Forest virus (isolated in Ivory Coast), and Bundibugyo virus.1 Zaire Ebola virus is the most virulent species and has been responsible for most of the outbreaks in sub-Saharan Africa, with an overall mortality rate of around 70%.2 Sudan virus was responsible for outbreaks in the 1970s, 2000, and 2004; Bundibugyo virus caused a single outbreak in 2007, which had a lower mortality rate of around 30%. The fifth virus, Reston virus, does not appear to cause infection in humans, but does infect pigs and nonhuman primates.1
The natural reservoir of the Ebola virus is not known. It is unlikely to be primates, since the virus typically kills its primate host within a matter of days. Recent studies suggest that the natural host may be bats.3 Initial infections in humans may result from preparing and eating infected bush meat or from exposure to infected bat droppings during such activities as mining and spelunking.
References
- Bray M. Filoviridae. In: Clinical Virology, 2nd ed. Richman DD, Whitley RJ, Hayden FG, eds. Washington, DC: ASM Press; 2002:875.
- WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–1495.
- Centers for Disease Control and Prevention. Epidemiologic risk factors to consider when evaluating a person for exposure to Ebola virus. http://www.cdc.gov/vhf/ebola/exposure/risk-factors-when-evaluating-person-for-exposure.html. Updated May 1, 2015. Accessed May 14, 2015.
Transmission and clinical presentation
Ebola is not spread through air, water supply, food, or by mosquitoes.6 The Ebola virus is spread from person to person through direct contact with blood or bodily fluids from an infected individual who has developed disease symptoms. It is generally accepted that asymptomatic individuals are not infectious, likely due to their low circulating viral load. The incubation period is between 2 and 21 days.6,7
Sexual transmission. The Centers for Disease Control and Prevention (CDC) now recommends that contact with semen from male Ebola survivors be avoided “until more information regarding the duration and infectiousness of viral shedding in body fluids is known.” They recommend a condom be used (correctly and consistently) when male survivors have oral, vaginal, or anal sex.8
Following the initial inoculation, the virus spreads rapidly throughout the body, infecting many cell types, although it primarily targets macrophages (including the Kupffer cells of the liver), dendritic cells, and endothelial cells. Infected cells die and release more viral particles as well as proinflammatory mediators (tumor-necrosis-factor−a, interleukins, nitric oxide) leading to a massive systemic inflammatory response. Impaired dendritic cells are unable to mount an effective immune response to fight the infection.9
Symptoms develop rapidly, starting with fever and malaise, and progressing within a few days to vomiting, diarrhea, loss of appetite, abdominal pain, and rash. Signs include hypotension (due to vasodilation and increased vascular permeability), shock, multisystem organ failure, and coagulopathy (which occurs in 20% of cases due to activation of tissue factor). Leukopenia, thrombocytopenia, transaminitis, coagulation abnormalities, proteinuria, renal failure, and electrolyte abnormalities are commonly seen on laboratory analysis.10,11 Interestingly, the Ebola virus gains entry into human cells using the Niemann-Pick C1 cholesterol transporter, and cells from patients with Niemann-Pick type C disease are immune to infection with the Ebola virus.12
Ebola in pregnancy
Information about the true incidence and complications of Ebola disease is limited. Most infected patients have been cared for in community-based health care facilities in Africa with little access to diagnostic testing and unreliable medical records. The data we do have about risks factors, disease transmission, and mortality rates come mainly from epidemiologic studies conducted in the midst of an Ebola epidemic or from studies in nonhuman primates. Data on Ebola infection in pregnancy are even more limited.
Pregnant women are more vulnerable to and may have more complications as a result of certain infections, including malaria, varicella, and seasonal influenza. While data are limited, pregnant women and their fetuses infected with the Ebola virus also appear to have worse outcomes, with a maternal and perinatal mortality rate that approaches 100%.5 Under normal conditions, pregnant women are given priority within the medical system. However, given the overall poor prognosis, their increased infectivity, and concerns about the well-being of health care providers, many pregnant women infected with the Ebola virus during the recent epidemic were set aside and denied basic health care needs, including hospital admission. Whether improved infection control and more intensive medical care would improve the survival rate of infected pregnant women is not clear.
Most of what we know about Ebola infection in pregnancy comes from the 1995 epidemic in the Kikwit area of the Democratic Republic of the Congo (Zaire). Of the 202 people infected during that epidemic, 105 were women and 15 were pregnant (4 in the first trimester, 6 in the second trimester, and 5 in the third trimester). Pregnant women presented with vaginal bleeding and occasionally bleeding from other sites, including gum bleeding, hematemesis, hematuria, and melena. Of note, the diagnosis of Ebola during the Kikwit epidemic was based on clinical examination alone.5 Fourteen of these 15 women died, giving a mortality rate of 93.3%, with death occurring within 10 days in all instances. One woman delivered a live-born child, but both she and the baby died within 3 days. The woman who did survivehad a miscarriage in the first trimester.5
In the 1976 epidemic centered in Yambuku, Zaire, pregnant women fared slightly better, with a mortality rate of 89% (73/82), which was similar to the mortality rate for the population as a whole of 88%.5 Nineteen women (23%) had a spontaneous abortion. Ten women (12%) delivered live-born babies, but all died within 19 days. It is assumed that these infants contracted the Ebola virus, but whether this was indeed the case and when and how they contracted the infection is not known. The combined perinatal and infant mortality rate in these 2 epidemics was 100%.
During the recent epidemic in Guinea, there were 2 pregnant patients, both of whom presented with fetal demise in the third trimester. Their labors were induced and both mothers survived.13 During the height of the recent Liberian epidemic (between August and October 2014), 700 infected patients were admitted to the largest treatment center. Four women were pregnant, all in the latter half of gestation. Of these, 3 died (75% mortality rate). The remaining woman survived, but her fetus died.9 Taken together, the prevailing evidence suggests that maternal and perinatal outcomes of pregnant women infected with the Ebola virus are dismal, with mortality rates approaching 100%.
Protecting health care workers
Transmission of the Ebola virus to health care workers has emerged as a major concern during the most recent outbreak in West Africa. Frontline health care workers are usually the first to see such patients and are at high risk of exposure to infected bodily fluids. This is especially true of health care professionals working on labor and delivery units, where exposure to blood and amniotic fluid is commonplace at the time of delivery.
Contaminated needles and syringes also may play a role in transmission.14,15 And, in Africa, a large number of transmissions have been attributed to ritual washing of the body at funerals, since viral load is maximal at the time of death, but this is unlikely to play a significant role in transmission of the virus in developed countries. Ebola virus has been isolated from breast milk.16 While direct transmission of the virus through breastfeeding has not been documented, breast milk from infected individuals should be disposed of carefully.
Prophylaxis
Is a vaccine on the way?
Development of an Ebola vaccine is under way. The most promising vaccine to date is cAd3-ZEBOV (GlaxoSmithKline, Brentford, London, United Kingdom). This vaccine is derived from a chimpanzee adenovirus, called Chimp Adenovirus type 3 (ChAd3), which has been genetically engineered to express proteins from both the Zaire and Sudan species of Ebola virus to provoke an immune response against them. Phase 1 trials of this vaccine began in September 2014.17
Appropriate precautions
Until an effective vaccine is available, a number of recommendations have been put in place in an effort to prevent Ebola infection:
- Avoid all nonessential travel to West Africa, especially to Sierre Leone, Guinea, and Liberia.7
- Avoid exposure to bodily fluids of patients who have been exposed to or are at high risk of having Ebola. This includes individuals who are febrile or feeling unwell and who have traveled to West Africa within the previous 21 days, especially if they visited 1 of the 3 countries with the highest Ebola infection rates (Sierre Leone, Guinea, and Liberia).
- Introduce universal screening of all patients, family members, and employees entering labor and delivery units.
Classifying risk and risk-associated protocols
If an at-risk patient is identified, she should be placed in isolation and consultation with an infectious disease specialist should occur. Using appropriate personal protective equipment (PPE), a detailed history and physical examination should be performed, and the patient should be classified according to risk14,15:
- No risk—defined as those who traveled to an Ebola-affected country more than 21 days previously, those in contact with an asymptomatic person prior to them being diagnosed with Ebola, and those in contact with an asymptomatic person who in turn had contact with an infected individual.
- Low risk—including those who traveled to an Ebola-affected country within 21 days but are asymptomatic, those with brief contact with asymptomatic infected individuals, those exposed to infected individuals in countries without widespread disease while wearing PPE, and those in brief proximity to a symptomatic individual, such as being in the same room or on the same airplane.
- Some (moderate) risk—including those in close contact (within 1 m) with a symptomatic individual or those exposed to an infected individual in a country with widespread disease while wearing PPE.
- High risk—defined as those exposed to the bodily fluids of an infected individual without PPE.
When should a patient be tested for Ebola, and what does that testing entail?
Patients found to be at no risk should not be tested or monitored, regardless of whether or not they are symptomatic. Asymptomatic patients with risk factors should not be tested for the Ebola virus. However, they do need to be followed for signs and symptoms of infection. At this time, the CDC has decided that it will take on the responsibility of monitoring all such patients until they are out of the 21-day window.14,15
Symptomatic patients with risk factors should be tested for the Ebola virus, regardless of whether they are designated as being at low, moderate, or high risk of infection. Strict infection control precautions should be followed for such patients, and local/state health departments should be notified. Laboratory testing includes RT-PCR or Ebola immunoassay. A negative RT-PCR test result obtained more than 72 hours after the onset of symptoms effectively rules out Ebola infection. In general, patients can be discharged from the hospital if they are asymptomatic and have 2 negative RT-PCR test results within 48 hours.14,15
Other diagnoses that should be considered in these patients include influenza, malaria, Lassa fever, meningococcal infection, and typhoid. If a patient is asymptomatic but at risk, all nonemergent medical care should be deferred until they are out of the 21-day window. Repeat testing may be warranted in certain clinical scenarios.
Management of infected patients in a maternity ward
While no pregnant patient has yet been diagnosed with Ebola infection in the United States, it remains a possibility, and clinicians should be aware of appropriate management actions. Once the diagnosis is confirmed, patients and their families should be placed in strict isolation. In some states, specific regional centers have been designated to care for these patients. They should be cared for by a small, dedicated team of clinicians dressed in state-of-the-art PPE and fully trained in the technique of donning and doffing the gear. Some institutions have mandated that no medical students or residents be involved directly in the care of these patients. Infectious disease specialists should be actively involved. All medical equipment (such as stethoscopes, blood pressure cuffs, thermometers, and fetal heart rate monitors) should be dedicated to the care of this patient alone and should remain in the room, as the virus can remain viable on surfaces for “a few hours or days.”18
Treatment itself is largely supportive, with significant intravascular expansion and treatment of fever, nausea, vomiting, and diarrhea. Patients typically require 5 to 10 L of fluid replacement each day, along with regular electrolyte repletion. The development of coagulopathy is a real concern and should be carefully monitored for and corrected as needed. Since blood is highly infectious, every effort should be made to perform only critical blood tests and to do so at the bedside, if possible. Mobile devices are available that can be stationed in the room and provide basic hematologic and electrolyte measurements, thereby avoiding the need to transport the blood and the risk of potentially contaminating laboratory equipment. Dedicated staff should be trained on the use of such equipment. In all likelihood, radiologic imaging will not be available and management decisions will need to be made on the basis of clinical examination alone.
Treatment of the virus and the conditions it can cause
A number of experimental treatments are under investigation. These include some antiviral agents (such as the CMV antiviral drug brincidofovir and the influenza antiviral favipiravir), immune sera from Ebola survivors, and RNA interference agents (such as TKM-Ebola). Zmapp, a cocktail of 3 anti-Ebola monoclonal antibodies, has been shown to be protective in macaque monkeys in the late stages of the disease and has been given to 4 infected patients in the United States, with variable results.19 All of these options should be considered on an individual basis.
Some patients may experience renal or respiratory failure requiring advanced life support measures such as dialysis, mechanical ventilation, or cardiorespiratory resuscitation (CPR). The decision of whether or not to proceed with such interventions should be left to the discretion of the attending physician staff. Given the extremely poor prognosis for the patient and the attendant risks to the health care staff and potentially to subsequent patients using these same pieces of medical equipment, it would seem reasonable to withhold such interventions.
Unique considerations during pregnancy. In pregnant patients with Ebola, it may be reasonable to withhold the option of cesarean and offer only vaginal delivery in the event of labor. This is not just a theoretic concern. In 1 case in Zaire in 1995, an entire surgical team was infected after operating on an infected patient, with the infection spreading to outside hospital staff and family members.20
Survival rates are dismal
Reported survival rates are extremely low, especially for pregnant women. Patients who are younger, have lower viral loads, and do not have diarrhea or severe dehydration have a higher likelihood of surviving. Whether survival rates are higher in developed countries with more health care resources has yet to be confirmed. If patients do survive, the recovery period is long, with prolonged weakness, fatigue, and weight loss. While sexual transmission of the Ebola virus has not been documented, the CDC has recommended sexual abstinence for at least 3 months after recovery.14,15 Ebola survivors are thought to be immune to subsequent infections.
Education is the most important factor for most of us
In November 2014, the American College of Obstetricians and Gynecologists (ACOG) published a practice advisory on the care of obstetric patients during an Ebola virus outbreak.21 While the number of Ebola cases in the United States has been, and likely will continue to be low, especially among pregnant women, we should continue to focus on education and screening. Only providers who have undergone Ebola training and have proper PPE should be involved in the care of potentially infected or confirmed cases. The greatest potential for harm is suboptimal obstetric care leading to an adverse event in a patient suspected of having Ebola who subsequently tests negative. Once an Ebola infection has been confirmed, patients—regardless of whether or not they are pregnant—should be hospitalized in institutions with the requisite resources, protocols, and expertise to deal with such highly infectious patients.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Lang J. Ebola in the maternity ward. http://www.newyorker.com/tech/elements/ebola-maternity-ward. Published October 29, 2014. Accessed May 16, 2015.
2. Martini GA. Marburg agent disease in man. Trans R Soc Trop Med Hyg. 1969;63(3):295–302.
3. Centers for Disease Control and Prevention. 2014 Ebola Outbreak in West Africa - Case Counts. http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html. Updated May 15, 2015. Accessed May 17, 2015.
4. Centers for Disease Control and Prevention. 2014 Ebola outbreak in West Africa. http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/index.html. Updated May 15, 2015. Accessed May 17, 2015.
5. Mupapa K, Mukundu W, Bwaka MA, et al. Ebola hemorrhagic fever and pregnancy. J Infect Dis. 1999;179(suppl 1):S11–S12.
6. Centers for Disease Control and Prevention. Epidemiologic risk factors to consider when evaluating a person for exposure to Ebola virus. http://www.cdc.gov/vhf/ebola/exposure/risk-factors-when-evaluating-person-for-exposure.html. Updated May 1, 2015. Accessed May 16, 2015.
7. Centers for Disease Control and Prevention. Ebola (Ebola virus disease). http://www.cdc.gov/vhf/ebola/. Updated May 15, 2015. Accessed May 16, 2015.
8. Christie A, Davies-Wayne GJ, Cordier-Lasalle T, et al. Possible sexual transmission of Ebola virus — Liberia, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(17):479–481.
9. Chertow DS, Kleine C, Edwards JK, et al. Ebola virus disease in West Africa—clinical manifestations and management. N Engl J Med. 2014;371(22):2054–2057.
10. Mahanty S, Bray M. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis. 2004;4(8):487–498.
11. Bray M. Pathogenesis of viral hemorrhagic fever. Curr Opin Immunol. 2005;17(4):399–403.
12. Carette JE, Raaben M, Wong AC, et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477(7364):340–343.
13. Baggi FM, Taybi A, Kurth A, et al. Management of pregnant women infected with Ebola virus in a treatment centre in Guinea, June 2014. Euro Surveill. 2014;19(49). pii: 20983.
14. Centers for Disease Control and Prevention. Review of human-to-human transmission of Ebola virus. http://www.cdc.gov/vhf/ebola/transmission/human-transmission.html. Updated October 29, 2014. Accessed May 16, 2015.
15. Centers for Disease Control and Prevention. Ebola virus disease (EVD) information for clinicians in U.S. healthcare settings. http://www.cdc.gov/vhf/ebola/healthcare-us/preparing/clinicians.html. Updated April 1, 2015. Accessed May 16, 2015.
16. Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(suppl 2):S142–S147.
17. Ledgerwood JE, DeZure AD, Stanley DA, et al; VRC 207 Study Team. Chimpanzee adenovirus vector Ebola vaccine — preliminary report [published online ahead of print November 26, 2014]. N Engl J Med. http://www.nejm.org/doi/full/10.1056/NEJMoa1410863. Accessed May 17, 2015.
18. Centers for Disease Control and Prevention. Q&As on transmission. http://www.cdc.gov/vhf/ebola/transmission/qas.html. Updated April 24, 2015. Accessed May 17, 2015.
19. Lyon GM, Mehta AK, Varkey JB, et al; Emory Serious Communicable Diseases Unit. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med. 2014;371(25):2402–2409.
20. Khan AS, Tshioko FK, Heymann DL, et al. The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidémies à Kikwit. J Infect Dis. 1999;179(suppl 1):S76.
21. American College of Obstetricians and Gynecologists. Practice Advisory: Care of obstetric patients during an Ebola virus outbreak. http://www.acog.org/About-ACOG/News-Room/Practice-Advisories/ACOG-Practice-Advisory-Care-of-Obstetric-Patients-During-an-Ebola-Virus-Outbreak. Published November 3, 2014. Accessed May 17, 2015.
“[Pregnant patients infected with the Ebola virus in West Africa] aren’t given preferential treatment...They aren’t even given beds.…They are assumed to die. Priority is given to the patients whom the health-care workers believe they can save. In effect, pregnant women are being triaged last.”
—Joshua Lang1
Ebola is a rare and potentially deadly disease caused by infection with a strain of the Ebola virus. First described in 1967,2 the Ebola virus has caused significant morbidity and mortality in many parts of sub-Saharan Africa. Awareness about this disease has increased dramatically in the United States as a result of the largest Ebola epidemic in history, which broke out in West Africa in 2014. The 3 countries most widely affected include Sierra Leone, Liberia, and Guinea. As of May 15, 2015, there were 26,798 cases of Ebola in this epidemic (with 14,971 laboratory confirmed), of whom 11,089 have died.3 There have been a total of 868 confirmed health care worker infections reported in Guinea, Liberia, and Sierra Leone since the start of the outbreak, with 507 reported deaths.4
It is highly unlikely that an Ebola epidemic of similar proportion will break out in any developed nation. However, isolated cases of Ebola have been identified among high-risk individuals in the United States (such as those who had recently served as medical volunteers in West Africa), which has raised concern about Ebola infection prevention and management in this country.
Little is known about Ebola infection in pregnancy. The few reports available suggest that pregnant women who become infected are highly contagious, with a maternal and perinatal mortality rate near 100%.5 Significant efforts were put in place in hospitals around the United States, including in labor and delivery units, to care for potentially or actively infected individuals, to protect health care workers, and to contain the spread of any new infections. It is important that clinicians be aware of the efforts and the recommended protocols—especially for the unique circumstance of infection during pregnancy. We review these protocols, as well as provide details on viral transmission and treatment.
What is Ebola virus and why are humans affected?
Ebola virus is a single-stranded RNA filovirus (FIGURE) with 5 independently identified species named after the countries or regions in which they were identified. Four of these are known to cause disease in humans, including Zaire Ebola virus, Sudan virus, Tai Forest virus (isolated in Ivory Coast), and Bundibugyo virus.1 Zaire Ebola virus is the most virulent species and has been responsible for most of the outbreaks in sub-Saharan Africa, with an overall mortality rate of around 70%.2 Sudan virus was responsible for outbreaks in the 1970s, 2000, and 2004; Bundibugyo virus caused a single outbreak in 2007, which had a lower mortality rate of around 30%. The fifth virus, Reston virus, does not appear to cause infection in humans, but does infect pigs and nonhuman primates.1
The natural reservoir of the Ebola virus is not known. It is unlikely to be primates, since the virus typically kills its primate host within a matter of days. Recent studies suggest that the natural host may be bats.3 Initial infections in humans may result from preparing and eating infected bush meat or from exposure to infected bat droppings during such activities as mining and spelunking.
References
- Bray M. Filoviridae. In: Clinical Virology, 2nd ed. Richman DD, Whitley RJ, Hayden FG, eds. Washington, DC: ASM Press; 2002:875.
- WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–1495.
- Centers for Disease Control and Prevention. Epidemiologic risk factors to consider when evaluating a person for exposure to Ebola virus. http://www.cdc.gov/vhf/ebola/exposure/risk-factors-when-evaluating-person-for-exposure.html. Updated May 1, 2015. Accessed May 14, 2015.
Transmission and clinical presentation
Ebola is not spread through air, water supply, food, or by mosquitoes.6 The Ebola virus is spread from person to person through direct contact with blood or bodily fluids from an infected individual who has developed disease symptoms. It is generally accepted that asymptomatic individuals are not infectious, likely due to their low circulating viral load. The incubation period is between 2 and 21 days.6,7
Sexual transmission. The Centers for Disease Control and Prevention (CDC) now recommends that contact with semen from male Ebola survivors be avoided “until more information regarding the duration and infectiousness of viral shedding in body fluids is known.” They recommend a condom be used (correctly and consistently) when male survivors have oral, vaginal, or anal sex.8
Following the initial inoculation, the virus spreads rapidly throughout the body, infecting many cell types, although it primarily targets macrophages (including the Kupffer cells of the liver), dendritic cells, and endothelial cells. Infected cells die and release more viral particles as well as proinflammatory mediators (tumor-necrosis-factor−a, interleukins, nitric oxide) leading to a massive systemic inflammatory response. Impaired dendritic cells are unable to mount an effective immune response to fight the infection.9
Symptoms develop rapidly, starting with fever and malaise, and progressing within a few days to vomiting, diarrhea, loss of appetite, abdominal pain, and rash. Signs include hypotension (due to vasodilation and increased vascular permeability), shock, multisystem organ failure, and coagulopathy (which occurs in 20% of cases due to activation of tissue factor). Leukopenia, thrombocytopenia, transaminitis, coagulation abnormalities, proteinuria, renal failure, and electrolyte abnormalities are commonly seen on laboratory analysis.10,11 Interestingly, the Ebola virus gains entry into human cells using the Niemann-Pick C1 cholesterol transporter, and cells from patients with Niemann-Pick type C disease are immune to infection with the Ebola virus.12
Ebola in pregnancy
Information about the true incidence and complications of Ebola disease is limited. Most infected patients have been cared for in community-based health care facilities in Africa with little access to diagnostic testing and unreliable medical records. The data we do have about risks factors, disease transmission, and mortality rates come mainly from epidemiologic studies conducted in the midst of an Ebola epidemic or from studies in nonhuman primates. Data on Ebola infection in pregnancy are even more limited.
Pregnant women are more vulnerable to and may have more complications as a result of certain infections, including malaria, varicella, and seasonal influenza. While data are limited, pregnant women and their fetuses infected with the Ebola virus also appear to have worse outcomes, with a maternal and perinatal mortality rate that approaches 100%.5 Under normal conditions, pregnant women are given priority within the medical system. However, given the overall poor prognosis, their increased infectivity, and concerns about the well-being of health care providers, many pregnant women infected with the Ebola virus during the recent epidemic were set aside and denied basic health care needs, including hospital admission. Whether improved infection control and more intensive medical care would improve the survival rate of infected pregnant women is not clear.
Most of what we know about Ebola infection in pregnancy comes from the 1995 epidemic in the Kikwit area of the Democratic Republic of the Congo (Zaire). Of the 202 people infected during that epidemic, 105 were women and 15 were pregnant (4 in the first trimester, 6 in the second trimester, and 5 in the third trimester). Pregnant women presented with vaginal bleeding and occasionally bleeding from other sites, including gum bleeding, hematemesis, hematuria, and melena. Of note, the diagnosis of Ebola during the Kikwit epidemic was based on clinical examination alone.5 Fourteen of these 15 women died, giving a mortality rate of 93.3%, with death occurring within 10 days in all instances. One woman delivered a live-born child, but both she and the baby died within 3 days. The woman who did survivehad a miscarriage in the first trimester.5
In the 1976 epidemic centered in Yambuku, Zaire, pregnant women fared slightly better, with a mortality rate of 89% (73/82), which was similar to the mortality rate for the population as a whole of 88%.5 Nineteen women (23%) had a spontaneous abortion. Ten women (12%) delivered live-born babies, but all died within 19 days. It is assumed that these infants contracted the Ebola virus, but whether this was indeed the case and when and how they contracted the infection is not known. The combined perinatal and infant mortality rate in these 2 epidemics was 100%.
During the recent epidemic in Guinea, there were 2 pregnant patients, both of whom presented with fetal demise in the third trimester. Their labors were induced and both mothers survived.13 During the height of the recent Liberian epidemic (between August and October 2014), 700 infected patients were admitted to the largest treatment center. Four women were pregnant, all in the latter half of gestation. Of these, 3 died (75% mortality rate). The remaining woman survived, but her fetus died.9 Taken together, the prevailing evidence suggests that maternal and perinatal outcomes of pregnant women infected with the Ebola virus are dismal, with mortality rates approaching 100%.
Protecting health care workers
Transmission of the Ebola virus to health care workers has emerged as a major concern during the most recent outbreak in West Africa. Frontline health care workers are usually the first to see such patients and are at high risk of exposure to infected bodily fluids. This is especially true of health care professionals working on labor and delivery units, where exposure to blood and amniotic fluid is commonplace at the time of delivery.
Contaminated needles and syringes also may play a role in transmission.14,15 And, in Africa, a large number of transmissions have been attributed to ritual washing of the body at funerals, since viral load is maximal at the time of death, but this is unlikely to play a significant role in transmission of the virus in developed countries. Ebola virus has been isolated from breast milk.16 While direct transmission of the virus through breastfeeding has not been documented, breast milk from infected individuals should be disposed of carefully.
Prophylaxis
Is a vaccine on the way?
Development of an Ebola vaccine is under way. The most promising vaccine to date is cAd3-ZEBOV (GlaxoSmithKline, Brentford, London, United Kingdom). This vaccine is derived from a chimpanzee adenovirus, called Chimp Adenovirus type 3 (ChAd3), which has been genetically engineered to express proteins from both the Zaire and Sudan species of Ebola virus to provoke an immune response against them. Phase 1 trials of this vaccine began in September 2014.17
Appropriate precautions
Until an effective vaccine is available, a number of recommendations have been put in place in an effort to prevent Ebola infection:
- Avoid all nonessential travel to West Africa, especially to Sierre Leone, Guinea, and Liberia.7
- Avoid exposure to bodily fluids of patients who have been exposed to or are at high risk of having Ebola. This includes individuals who are febrile or feeling unwell and who have traveled to West Africa within the previous 21 days, especially if they visited 1 of the 3 countries with the highest Ebola infection rates (Sierre Leone, Guinea, and Liberia).
- Introduce universal screening of all patients, family members, and employees entering labor and delivery units.
Classifying risk and risk-associated protocols
If an at-risk patient is identified, she should be placed in isolation and consultation with an infectious disease specialist should occur. Using appropriate personal protective equipment (PPE), a detailed history and physical examination should be performed, and the patient should be classified according to risk14,15:
- No risk—defined as those who traveled to an Ebola-affected country more than 21 days previously, those in contact with an asymptomatic person prior to them being diagnosed with Ebola, and those in contact with an asymptomatic person who in turn had contact with an infected individual.
- Low risk—including those who traveled to an Ebola-affected country within 21 days but are asymptomatic, those with brief contact with asymptomatic infected individuals, those exposed to infected individuals in countries without widespread disease while wearing PPE, and those in brief proximity to a symptomatic individual, such as being in the same room or on the same airplane.
- Some (moderate) risk—including those in close contact (within 1 m) with a symptomatic individual or those exposed to an infected individual in a country with widespread disease while wearing PPE.
- High risk—defined as those exposed to the bodily fluids of an infected individual without PPE.
When should a patient be tested for Ebola, and what does that testing entail?
Patients found to be at no risk should not be tested or monitored, regardless of whether or not they are symptomatic. Asymptomatic patients with risk factors should not be tested for the Ebola virus. However, they do need to be followed for signs and symptoms of infection. At this time, the CDC has decided that it will take on the responsibility of monitoring all such patients until they are out of the 21-day window.14,15
Symptomatic patients with risk factors should be tested for the Ebola virus, regardless of whether they are designated as being at low, moderate, or high risk of infection. Strict infection control precautions should be followed for such patients, and local/state health departments should be notified. Laboratory testing includes RT-PCR or Ebola immunoassay. A negative RT-PCR test result obtained more than 72 hours after the onset of symptoms effectively rules out Ebola infection. In general, patients can be discharged from the hospital if they are asymptomatic and have 2 negative RT-PCR test results within 48 hours.14,15
Other diagnoses that should be considered in these patients include influenza, malaria, Lassa fever, meningococcal infection, and typhoid. If a patient is asymptomatic but at risk, all nonemergent medical care should be deferred until they are out of the 21-day window. Repeat testing may be warranted in certain clinical scenarios.
Management of infected patients in a maternity ward
While no pregnant patient has yet been diagnosed with Ebola infection in the United States, it remains a possibility, and clinicians should be aware of appropriate management actions. Once the diagnosis is confirmed, patients and their families should be placed in strict isolation. In some states, specific regional centers have been designated to care for these patients. They should be cared for by a small, dedicated team of clinicians dressed in state-of-the-art PPE and fully trained in the technique of donning and doffing the gear. Some institutions have mandated that no medical students or residents be involved directly in the care of these patients. Infectious disease specialists should be actively involved. All medical equipment (such as stethoscopes, blood pressure cuffs, thermometers, and fetal heart rate monitors) should be dedicated to the care of this patient alone and should remain in the room, as the virus can remain viable on surfaces for “a few hours or days.”18
Treatment itself is largely supportive, with significant intravascular expansion and treatment of fever, nausea, vomiting, and diarrhea. Patients typically require 5 to 10 L of fluid replacement each day, along with regular electrolyte repletion. The development of coagulopathy is a real concern and should be carefully monitored for and corrected as needed. Since blood is highly infectious, every effort should be made to perform only critical blood tests and to do so at the bedside, if possible. Mobile devices are available that can be stationed in the room and provide basic hematologic and electrolyte measurements, thereby avoiding the need to transport the blood and the risk of potentially contaminating laboratory equipment. Dedicated staff should be trained on the use of such equipment. In all likelihood, radiologic imaging will not be available and management decisions will need to be made on the basis of clinical examination alone.
Treatment of the virus and the conditions it can cause
A number of experimental treatments are under investigation. These include some antiviral agents (such as the CMV antiviral drug brincidofovir and the influenza antiviral favipiravir), immune sera from Ebola survivors, and RNA interference agents (such as TKM-Ebola). Zmapp, a cocktail of 3 anti-Ebola monoclonal antibodies, has been shown to be protective in macaque monkeys in the late stages of the disease and has been given to 4 infected patients in the United States, with variable results.19 All of these options should be considered on an individual basis.
Some patients may experience renal or respiratory failure requiring advanced life support measures such as dialysis, mechanical ventilation, or cardiorespiratory resuscitation (CPR). The decision of whether or not to proceed with such interventions should be left to the discretion of the attending physician staff. Given the extremely poor prognosis for the patient and the attendant risks to the health care staff and potentially to subsequent patients using these same pieces of medical equipment, it would seem reasonable to withhold such interventions.
Unique considerations during pregnancy. In pregnant patients with Ebola, it may be reasonable to withhold the option of cesarean and offer only vaginal delivery in the event of labor. This is not just a theoretic concern. In 1 case in Zaire in 1995, an entire surgical team was infected after operating on an infected patient, with the infection spreading to outside hospital staff and family members.20
Survival rates are dismal
Reported survival rates are extremely low, especially for pregnant women. Patients who are younger, have lower viral loads, and do not have diarrhea or severe dehydration have a higher likelihood of surviving. Whether survival rates are higher in developed countries with more health care resources has yet to be confirmed. If patients do survive, the recovery period is long, with prolonged weakness, fatigue, and weight loss. While sexual transmission of the Ebola virus has not been documented, the CDC has recommended sexual abstinence for at least 3 months after recovery.14,15 Ebola survivors are thought to be immune to subsequent infections.
Education is the most important factor for most of us
In November 2014, the American College of Obstetricians and Gynecologists (ACOG) published a practice advisory on the care of obstetric patients during an Ebola virus outbreak.21 While the number of Ebola cases in the United States has been, and likely will continue to be low, especially among pregnant women, we should continue to focus on education and screening. Only providers who have undergone Ebola training and have proper PPE should be involved in the care of potentially infected or confirmed cases. The greatest potential for harm is suboptimal obstetric care leading to an adverse event in a patient suspected of having Ebola who subsequently tests negative. Once an Ebola infection has been confirmed, patients—regardless of whether or not they are pregnant—should be hospitalized in institutions with the requisite resources, protocols, and expertise to deal with such highly infectious patients.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“[Pregnant patients infected with the Ebola virus in West Africa] aren’t given preferential treatment...They aren’t even given beds.…They are assumed to die. Priority is given to the patients whom the health-care workers believe they can save. In effect, pregnant women are being triaged last.”
—Joshua Lang1
Ebola is a rare and potentially deadly disease caused by infection with a strain of the Ebola virus. First described in 1967,2 the Ebola virus has caused significant morbidity and mortality in many parts of sub-Saharan Africa. Awareness about this disease has increased dramatically in the United States as a result of the largest Ebola epidemic in history, which broke out in West Africa in 2014. The 3 countries most widely affected include Sierra Leone, Liberia, and Guinea. As of May 15, 2015, there were 26,798 cases of Ebola in this epidemic (with 14,971 laboratory confirmed), of whom 11,089 have died.3 There have been a total of 868 confirmed health care worker infections reported in Guinea, Liberia, and Sierra Leone since the start of the outbreak, with 507 reported deaths.4
It is highly unlikely that an Ebola epidemic of similar proportion will break out in any developed nation. However, isolated cases of Ebola have been identified among high-risk individuals in the United States (such as those who had recently served as medical volunteers in West Africa), which has raised concern about Ebola infection prevention and management in this country.
Little is known about Ebola infection in pregnancy. The few reports available suggest that pregnant women who become infected are highly contagious, with a maternal and perinatal mortality rate near 100%.5 Significant efforts were put in place in hospitals around the United States, including in labor and delivery units, to care for potentially or actively infected individuals, to protect health care workers, and to contain the spread of any new infections. It is important that clinicians be aware of the efforts and the recommended protocols—especially for the unique circumstance of infection during pregnancy. We review these protocols, as well as provide details on viral transmission and treatment.
What is Ebola virus and why are humans affected?
Ebola virus is a single-stranded RNA filovirus (FIGURE) with 5 independently identified species named after the countries or regions in which they were identified. Four of these are known to cause disease in humans, including Zaire Ebola virus, Sudan virus, Tai Forest virus (isolated in Ivory Coast), and Bundibugyo virus.1 Zaire Ebola virus is the most virulent species and has been responsible for most of the outbreaks in sub-Saharan Africa, with an overall mortality rate of around 70%.2 Sudan virus was responsible for outbreaks in the 1970s, 2000, and 2004; Bundibugyo virus caused a single outbreak in 2007, which had a lower mortality rate of around 30%. The fifth virus, Reston virus, does not appear to cause infection in humans, but does infect pigs and nonhuman primates.1
The natural reservoir of the Ebola virus is not known. It is unlikely to be primates, since the virus typically kills its primate host within a matter of days. Recent studies suggest that the natural host may be bats.3 Initial infections in humans may result from preparing and eating infected bush meat or from exposure to infected bat droppings during such activities as mining and spelunking.
References
- Bray M. Filoviridae. In: Clinical Virology, 2nd ed. Richman DD, Whitley RJ, Hayden FG, eds. Washington, DC: ASM Press; 2002:875.
- WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371:1481–1495.
- Centers for Disease Control and Prevention. Epidemiologic risk factors to consider when evaluating a person for exposure to Ebola virus. http://www.cdc.gov/vhf/ebola/exposure/risk-factors-when-evaluating-person-for-exposure.html. Updated May 1, 2015. Accessed May 14, 2015.
Transmission and clinical presentation
Ebola is not spread through air, water supply, food, or by mosquitoes.6 The Ebola virus is spread from person to person through direct contact with blood or bodily fluids from an infected individual who has developed disease symptoms. It is generally accepted that asymptomatic individuals are not infectious, likely due to their low circulating viral load. The incubation period is between 2 and 21 days.6,7
Sexual transmission. The Centers for Disease Control and Prevention (CDC) now recommends that contact with semen from male Ebola survivors be avoided “until more information regarding the duration and infectiousness of viral shedding in body fluids is known.” They recommend a condom be used (correctly and consistently) when male survivors have oral, vaginal, or anal sex.8
Following the initial inoculation, the virus spreads rapidly throughout the body, infecting many cell types, although it primarily targets macrophages (including the Kupffer cells of the liver), dendritic cells, and endothelial cells. Infected cells die and release more viral particles as well as proinflammatory mediators (tumor-necrosis-factor−a, interleukins, nitric oxide) leading to a massive systemic inflammatory response. Impaired dendritic cells are unable to mount an effective immune response to fight the infection.9
Symptoms develop rapidly, starting with fever and malaise, and progressing within a few days to vomiting, diarrhea, loss of appetite, abdominal pain, and rash. Signs include hypotension (due to vasodilation and increased vascular permeability), shock, multisystem organ failure, and coagulopathy (which occurs in 20% of cases due to activation of tissue factor). Leukopenia, thrombocytopenia, transaminitis, coagulation abnormalities, proteinuria, renal failure, and electrolyte abnormalities are commonly seen on laboratory analysis.10,11 Interestingly, the Ebola virus gains entry into human cells using the Niemann-Pick C1 cholesterol transporter, and cells from patients with Niemann-Pick type C disease are immune to infection with the Ebola virus.12
Ebola in pregnancy
Information about the true incidence and complications of Ebola disease is limited. Most infected patients have been cared for in community-based health care facilities in Africa with little access to diagnostic testing and unreliable medical records. The data we do have about risks factors, disease transmission, and mortality rates come mainly from epidemiologic studies conducted in the midst of an Ebola epidemic or from studies in nonhuman primates. Data on Ebola infection in pregnancy are even more limited.
Pregnant women are more vulnerable to and may have more complications as a result of certain infections, including malaria, varicella, and seasonal influenza. While data are limited, pregnant women and their fetuses infected with the Ebola virus also appear to have worse outcomes, with a maternal and perinatal mortality rate that approaches 100%.5 Under normal conditions, pregnant women are given priority within the medical system. However, given the overall poor prognosis, their increased infectivity, and concerns about the well-being of health care providers, many pregnant women infected with the Ebola virus during the recent epidemic were set aside and denied basic health care needs, including hospital admission. Whether improved infection control and more intensive medical care would improve the survival rate of infected pregnant women is not clear.
Most of what we know about Ebola infection in pregnancy comes from the 1995 epidemic in the Kikwit area of the Democratic Republic of the Congo (Zaire). Of the 202 people infected during that epidemic, 105 were women and 15 were pregnant (4 in the first trimester, 6 in the second trimester, and 5 in the third trimester). Pregnant women presented with vaginal bleeding and occasionally bleeding from other sites, including gum bleeding, hematemesis, hematuria, and melena. Of note, the diagnosis of Ebola during the Kikwit epidemic was based on clinical examination alone.5 Fourteen of these 15 women died, giving a mortality rate of 93.3%, with death occurring within 10 days in all instances. One woman delivered a live-born child, but both she and the baby died within 3 days. The woman who did survivehad a miscarriage in the first trimester.5
In the 1976 epidemic centered in Yambuku, Zaire, pregnant women fared slightly better, with a mortality rate of 89% (73/82), which was similar to the mortality rate for the population as a whole of 88%.5 Nineteen women (23%) had a spontaneous abortion. Ten women (12%) delivered live-born babies, but all died within 19 days. It is assumed that these infants contracted the Ebola virus, but whether this was indeed the case and when and how they contracted the infection is not known. The combined perinatal and infant mortality rate in these 2 epidemics was 100%.
During the recent epidemic in Guinea, there were 2 pregnant patients, both of whom presented with fetal demise in the third trimester. Their labors were induced and both mothers survived.13 During the height of the recent Liberian epidemic (between August and October 2014), 700 infected patients were admitted to the largest treatment center. Four women were pregnant, all in the latter half of gestation. Of these, 3 died (75% mortality rate). The remaining woman survived, but her fetus died.9 Taken together, the prevailing evidence suggests that maternal and perinatal outcomes of pregnant women infected with the Ebola virus are dismal, with mortality rates approaching 100%.
Protecting health care workers
Transmission of the Ebola virus to health care workers has emerged as a major concern during the most recent outbreak in West Africa. Frontline health care workers are usually the first to see such patients and are at high risk of exposure to infected bodily fluids. This is especially true of health care professionals working on labor and delivery units, where exposure to blood and amniotic fluid is commonplace at the time of delivery.
Contaminated needles and syringes also may play a role in transmission.14,15 And, in Africa, a large number of transmissions have been attributed to ritual washing of the body at funerals, since viral load is maximal at the time of death, but this is unlikely to play a significant role in transmission of the virus in developed countries. Ebola virus has been isolated from breast milk.16 While direct transmission of the virus through breastfeeding has not been documented, breast milk from infected individuals should be disposed of carefully.
Prophylaxis
Is a vaccine on the way?
Development of an Ebola vaccine is under way. The most promising vaccine to date is cAd3-ZEBOV (GlaxoSmithKline, Brentford, London, United Kingdom). This vaccine is derived from a chimpanzee adenovirus, called Chimp Adenovirus type 3 (ChAd3), which has been genetically engineered to express proteins from both the Zaire and Sudan species of Ebola virus to provoke an immune response against them. Phase 1 trials of this vaccine began in September 2014.17
Appropriate precautions
Until an effective vaccine is available, a number of recommendations have been put in place in an effort to prevent Ebola infection:
- Avoid all nonessential travel to West Africa, especially to Sierre Leone, Guinea, and Liberia.7
- Avoid exposure to bodily fluids of patients who have been exposed to or are at high risk of having Ebola. This includes individuals who are febrile or feeling unwell and who have traveled to West Africa within the previous 21 days, especially if they visited 1 of the 3 countries with the highest Ebola infection rates (Sierre Leone, Guinea, and Liberia).
- Introduce universal screening of all patients, family members, and employees entering labor and delivery units.
Classifying risk and risk-associated protocols
If an at-risk patient is identified, she should be placed in isolation and consultation with an infectious disease specialist should occur. Using appropriate personal protective equipment (PPE), a detailed history and physical examination should be performed, and the patient should be classified according to risk14,15:
- No risk—defined as those who traveled to an Ebola-affected country more than 21 days previously, those in contact with an asymptomatic person prior to them being diagnosed with Ebola, and those in contact with an asymptomatic person who in turn had contact with an infected individual.
- Low risk—including those who traveled to an Ebola-affected country within 21 days but are asymptomatic, those with brief contact with asymptomatic infected individuals, those exposed to infected individuals in countries without widespread disease while wearing PPE, and those in brief proximity to a symptomatic individual, such as being in the same room or on the same airplane.
- Some (moderate) risk—including those in close contact (within 1 m) with a symptomatic individual or those exposed to an infected individual in a country with widespread disease while wearing PPE.
- High risk—defined as those exposed to the bodily fluids of an infected individual without PPE.
When should a patient be tested for Ebola, and what does that testing entail?
Patients found to be at no risk should not be tested or monitored, regardless of whether or not they are symptomatic. Asymptomatic patients with risk factors should not be tested for the Ebola virus. However, they do need to be followed for signs and symptoms of infection. At this time, the CDC has decided that it will take on the responsibility of monitoring all such patients until they are out of the 21-day window.14,15
Symptomatic patients with risk factors should be tested for the Ebola virus, regardless of whether they are designated as being at low, moderate, or high risk of infection. Strict infection control precautions should be followed for such patients, and local/state health departments should be notified. Laboratory testing includes RT-PCR or Ebola immunoassay. A negative RT-PCR test result obtained more than 72 hours after the onset of symptoms effectively rules out Ebola infection. In general, patients can be discharged from the hospital if they are asymptomatic and have 2 negative RT-PCR test results within 48 hours.14,15
Other diagnoses that should be considered in these patients include influenza, malaria, Lassa fever, meningococcal infection, and typhoid. If a patient is asymptomatic but at risk, all nonemergent medical care should be deferred until they are out of the 21-day window. Repeat testing may be warranted in certain clinical scenarios.
Management of infected patients in a maternity ward
While no pregnant patient has yet been diagnosed with Ebola infection in the United States, it remains a possibility, and clinicians should be aware of appropriate management actions. Once the diagnosis is confirmed, patients and their families should be placed in strict isolation. In some states, specific regional centers have been designated to care for these patients. They should be cared for by a small, dedicated team of clinicians dressed in state-of-the-art PPE and fully trained in the technique of donning and doffing the gear. Some institutions have mandated that no medical students or residents be involved directly in the care of these patients. Infectious disease specialists should be actively involved. All medical equipment (such as stethoscopes, blood pressure cuffs, thermometers, and fetal heart rate monitors) should be dedicated to the care of this patient alone and should remain in the room, as the virus can remain viable on surfaces for “a few hours or days.”18
Treatment itself is largely supportive, with significant intravascular expansion and treatment of fever, nausea, vomiting, and diarrhea. Patients typically require 5 to 10 L of fluid replacement each day, along with regular electrolyte repletion. The development of coagulopathy is a real concern and should be carefully monitored for and corrected as needed. Since blood is highly infectious, every effort should be made to perform only critical blood tests and to do so at the bedside, if possible. Mobile devices are available that can be stationed in the room and provide basic hematologic and electrolyte measurements, thereby avoiding the need to transport the blood and the risk of potentially contaminating laboratory equipment. Dedicated staff should be trained on the use of such equipment. In all likelihood, radiologic imaging will not be available and management decisions will need to be made on the basis of clinical examination alone.
Treatment of the virus and the conditions it can cause
A number of experimental treatments are under investigation. These include some antiviral agents (such as the CMV antiviral drug brincidofovir and the influenza antiviral favipiravir), immune sera from Ebola survivors, and RNA interference agents (such as TKM-Ebola). Zmapp, a cocktail of 3 anti-Ebola monoclonal antibodies, has been shown to be protective in macaque monkeys in the late stages of the disease and has been given to 4 infected patients in the United States, with variable results.19 All of these options should be considered on an individual basis.
Some patients may experience renal or respiratory failure requiring advanced life support measures such as dialysis, mechanical ventilation, or cardiorespiratory resuscitation (CPR). The decision of whether or not to proceed with such interventions should be left to the discretion of the attending physician staff. Given the extremely poor prognosis for the patient and the attendant risks to the health care staff and potentially to subsequent patients using these same pieces of medical equipment, it would seem reasonable to withhold such interventions.
Unique considerations during pregnancy. In pregnant patients with Ebola, it may be reasonable to withhold the option of cesarean and offer only vaginal delivery in the event of labor. This is not just a theoretic concern. In 1 case in Zaire in 1995, an entire surgical team was infected after operating on an infected patient, with the infection spreading to outside hospital staff and family members.20
Survival rates are dismal
Reported survival rates are extremely low, especially for pregnant women. Patients who are younger, have lower viral loads, and do not have diarrhea or severe dehydration have a higher likelihood of surviving. Whether survival rates are higher in developed countries with more health care resources has yet to be confirmed. If patients do survive, the recovery period is long, with prolonged weakness, fatigue, and weight loss. While sexual transmission of the Ebola virus has not been documented, the CDC has recommended sexual abstinence for at least 3 months after recovery.14,15 Ebola survivors are thought to be immune to subsequent infections.
Education is the most important factor for most of us
In November 2014, the American College of Obstetricians and Gynecologists (ACOG) published a practice advisory on the care of obstetric patients during an Ebola virus outbreak.21 While the number of Ebola cases in the United States has been, and likely will continue to be low, especially among pregnant women, we should continue to focus on education and screening. Only providers who have undergone Ebola training and have proper PPE should be involved in the care of potentially infected or confirmed cases. The greatest potential for harm is suboptimal obstetric care leading to an adverse event in a patient suspected of having Ebola who subsequently tests negative. Once an Ebola infection has been confirmed, patients—regardless of whether or not they are pregnant—should be hospitalized in institutions with the requisite resources, protocols, and expertise to deal with such highly infectious patients.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Lang J. Ebola in the maternity ward. http://www.newyorker.com/tech/elements/ebola-maternity-ward. Published October 29, 2014. Accessed May 16, 2015.
2. Martini GA. Marburg agent disease in man. Trans R Soc Trop Med Hyg. 1969;63(3):295–302.
3. Centers for Disease Control and Prevention. 2014 Ebola Outbreak in West Africa - Case Counts. http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html. Updated May 15, 2015. Accessed May 17, 2015.
4. Centers for Disease Control and Prevention. 2014 Ebola outbreak in West Africa. http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/index.html. Updated May 15, 2015. Accessed May 17, 2015.
5. Mupapa K, Mukundu W, Bwaka MA, et al. Ebola hemorrhagic fever and pregnancy. J Infect Dis. 1999;179(suppl 1):S11–S12.
6. Centers for Disease Control and Prevention. Epidemiologic risk factors to consider when evaluating a person for exposure to Ebola virus. http://www.cdc.gov/vhf/ebola/exposure/risk-factors-when-evaluating-person-for-exposure.html. Updated May 1, 2015. Accessed May 16, 2015.
7. Centers for Disease Control and Prevention. Ebola (Ebola virus disease). http://www.cdc.gov/vhf/ebola/. Updated May 15, 2015. Accessed May 16, 2015.
8. Christie A, Davies-Wayne GJ, Cordier-Lasalle T, et al. Possible sexual transmission of Ebola virus — Liberia, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(17):479–481.
9. Chertow DS, Kleine C, Edwards JK, et al. Ebola virus disease in West Africa—clinical manifestations and management. N Engl J Med. 2014;371(22):2054–2057.
10. Mahanty S, Bray M. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis. 2004;4(8):487–498.
11. Bray M. Pathogenesis of viral hemorrhagic fever. Curr Opin Immunol. 2005;17(4):399–403.
12. Carette JE, Raaben M, Wong AC, et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477(7364):340–343.
13. Baggi FM, Taybi A, Kurth A, et al. Management of pregnant women infected with Ebola virus in a treatment centre in Guinea, June 2014. Euro Surveill. 2014;19(49). pii: 20983.
14. Centers for Disease Control and Prevention. Review of human-to-human transmission of Ebola virus. http://www.cdc.gov/vhf/ebola/transmission/human-transmission.html. Updated October 29, 2014. Accessed May 16, 2015.
15. Centers for Disease Control and Prevention. Ebola virus disease (EVD) information for clinicians in U.S. healthcare settings. http://www.cdc.gov/vhf/ebola/healthcare-us/preparing/clinicians.html. Updated April 1, 2015. Accessed May 16, 2015.
16. Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(suppl 2):S142–S147.
17. Ledgerwood JE, DeZure AD, Stanley DA, et al; VRC 207 Study Team. Chimpanzee adenovirus vector Ebola vaccine — preliminary report [published online ahead of print November 26, 2014]. N Engl J Med. http://www.nejm.org/doi/full/10.1056/NEJMoa1410863. Accessed May 17, 2015.
18. Centers for Disease Control and Prevention. Q&As on transmission. http://www.cdc.gov/vhf/ebola/transmission/qas.html. Updated April 24, 2015. Accessed May 17, 2015.
19. Lyon GM, Mehta AK, Varkey JB, et al; Emory Serious Communicable Diseases Unit. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med. 2014;371(25):2402–2409.
20. Khan AS, Tshioko FK, Heymann DL, et al. The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidémies à Kikwit. J Infect Dis. 1999;179(suppl 1):S76.
21. American College of Obstetricians and Gynecologists. Practice Advisory: Care of obstetric patients during an Ebola virus outbreak. http://www.acog.org/About-ACOG/News-Room/Practice-Advisories/ACOG-Practice-Advisory-Care-of-Obstetric-Patients-During-an-Ebola-Virus-Outbreak. Published November 3, 2014. Accessed May 17, 2015.
1. Lang J. Ebola in the maternity ward. http://www.newyorker.com/tech/elements/ebola-maternity-ward. Published October 29, 2014. Accessed May 16, 2015.
2. Martini GA. Marburg agent disease in man. Trans R Soc Trop Med Hyg. 1969;63(3):295–302.
3. Centers for Disease Control and Prevention. 2014 Ebola Outbreak in West Africa - Case Counts. http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html. Updated May 15, 2015. Accessed May 17, 2015.
4. Centers for Disease Control and Prevention. 2014 Ebola outbreak in West Africa. http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/index.html. Updated May 15, 2015. Accessed May 17, 2015.
5. Mupapa K, Mukundu W, Bwaka MA, et al. Ebola hemorrhagic fever and pregnancy. J Infect Dis. 1999;179(suppl 1):S11–S12.
6. Centers for Disease Control and Prevention. Epidemiologic risk factors to consider when evaluating a person for exposure to Ebola virus. http://www.cdc.gov/vhf/ebola/exposure/risk-factors-when-evaluating-person-for-exposure.html. Updated May 1, 2015. Accessed May 16, 2015.
7. Centers for Disease Control and Prevention. Ebola (Ebola virus disease). http://www.cdc.gov/vhf/ebola/. Updated May 15, 2015. Accessed May 16, 2015.
8. Christie A, Davies-Wayne GJ, Cordier-Lasalle T, et al. Possible sexual transmission of Ebola virus — Liberia, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(17):479–481.
9. Chertow DS, Kleine C, Edwards JK, et al. Ebola virus disease in West Africa—clinical manifestations and management. N Engl J Med. 2014;371(22):2054–2057.
10. Mahanty S, Bray M. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect Dis. 2004;4(8):487–498.
11. Bray M. Pathogenesis of viral hemorrhagic fever. Curr Opin Immunol. 2005;17(4):399–403.
12. Carette JE, Raaben M, Wong AC, et al. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature. 2011;477(7364):340–343.
13. Baggi FM, Taybi A, Kurth A, et al. Management of pregnant women infected with Ebola virus in a treatment centre in Guinea, June 2014. Euro Surveill. 2014;19(49). pii: 20983.
14. Centers for Disease Control and Prevention. Review of human-to-human transmission of Ebola virus. http://www.cdc.gov/vhf/ebola/transmission/human-transmission.html. Updated October 29, 2014. Accessed May 16, 2015.
15. Centers for Disease Control and Prevention. Ebola virus disease (EVD) information for clinicians in U.S. healthcare settings. http://www.cdc.gov/vhf/ebola/healthcare-us/preparing/clinicians.html. Updated April 1, 2015. Accessed May 16, 2015.
16. Bausch DG, Towner JS, Dowell SF, et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis. 2007;196(suppl 2):S142–S147.
17. Ledgerwood JE, DeZure AD, Stanley DA, et al; VRC 207 Study Team. Chimpanzee adenovirus vector Ebola vaccine — preliminary report [published online ahead of print November 26, 2014]. N Engl J Med. http://www.nejm.org/doi/full/10.1056/NEJMoa1410863. Accessed May 17, 2015.
18. Centers for Disease Control and Prevention. Q&As on transmission. http://www.cdc.gov/vhf/ebola/transmission/qas.html. Updated April 24, 2015. Accessed May 17, 2015.
19. Lyon GM, Mehta AK, Varkey JB, et al; Emory Serious Communicable Diseases Unit. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med. 2014;371(25):2402–2409.
20. Khan AS, Tshioko FK, Heymann DL, et al. The reemergence of Ebola hemorrhagic fever, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidémies à Kikwit. J Infect Dis. 1999;179(suppl 1):S76.
21. American College of Obstetricians and Gynecologists. Practice Advisory: Care of obstetric patients during an Ebola virus outbreak. http://www.acog.org/About-ACOG/News-Room/Practice-Advisories/ACOG-Practice-Advisory-Care-of-Obstetric-Patients-During-an-Ebola-Virus-Outbreak. Published November 3, 2014. Accessed May 17, 2015.
In this article
- What we know about Ebola in pregnancy
- Is a vaccine on the way?
- Unique treatment considerations in pregnancy