User login
Does self-administered misoprostol prime the cervix for hysteroscopy?
In this randomized, placebo-controlled trial by Oppegaard and colleagues, when 1,000 μg of vaginal misoprostol was self-administered by premenopausal women at least 12 hours before operative hysteroscopy, significant cervical ripening occurred, with increased cervical dilatation and less difficulty with dilatation, compared with women given placebo.
The drug was ineffective in postmenopausal women.
EXPERT COMMENTARY
Operative hysteroscopy is a common, minimally invasive procedure used to treat a number of gynecologic pathologies.1 The procedure requires that the cervical canal be dilated enough to allow passage of the hysteroscope.
Misoprostol is a prostaglandin E1 analog. It also is an effective cervical-ripening and labor-induction agent used during pregnancy in the second and third trimesters.2,3 Earlier studies suggested that misoprostol may be promising as a cervical-ripening agent before hysteroscopy in premenopausal women, although further research is needed to determine the ideal dosage, route, and timing of administration.1,4-6 Most of the studies demonstrating benefit with misoprostol before hysteroscopy have involved intravaginal dosages of 200 to 400 μg given 8 to 12 hours before the procedure.1,4-6
Misoprostol enabled greater dilatation in more women
Oppegaard and colleagues found greater mean cervical dilatation with misoprostol in premenopausal women than with placebo (6.4±2.4 mm, compared with 4.8±2.2 mm), more women achieving at least 5-mm cervical dilatation (88% versus 65%), and fewer women being difficult to dilate for hysteroscopy (20% versus 32%). As in previous studies, they also found misoprostol to be an ineffective cervical-ripening agent in postmenopausal women.
Strengths of this study
Because this study was randomized and placebo-controlled, bias in the evaluation of outcomes was minimized. The sample size was based on a sequential trial design, which ensures adequate power to answer the question of interest using as few women as possible.
The medication was self-administered and therefore more convenient than physician administration. In addition, women were questioned afterward to determine the acceptability of the self-administered medication, and 83% of premenopausal subjects found self-administration fairly or completely acceptable.
Shortcomings
Oppegaard and colleagues recommend that 1,000 μg of vaginal misoprostol be offered to nulliparous premenopausal women before operative hysteroscopy, but they do not present data specific to nulliparous women.
Moreover, the use of 1,000 μg of misoprostol is higher than in most previous studies, and Oppegaard and colleagues do not compare different dosages. The use of a higher dosage (1,000 μg) may be expected to cause more side effects. Indeed, researchers found a higher incidence of vaginal bleeding with misoprostol, compared with placebo (21% versus 3%), and 42% of women receiving misoprostol experienced mild or moderate abdominal pain, with 7% reporting severe abdominal pain.
The use of self-administered vaginal misoprostol 12 hours before operative hysteroscopy in premenopausal women increases cervical dilatation and reduces the difficulty of dilatation. Oppegaard and colleagues used 1,000 μg of misoprostol, although earlier studies suggested benefit with 200 μg to 400 μg.
Although the route and timing of misoprostol for cervical ripening before hysteroscopy appears evident from the literature (vaginal administration 12 hours before the procedure), the ideal dosage is still unclear. Furthermore, misoprostol carries potential side effects, including vaginal bleeding and abdominal pain.—JOAN M.G. CRANE, MD, MSC
1. Crane JM, Healey S. Use of misoprostol before hysteroscopy: a systematic review. J Obstet Gynaecol Can. 2006;28:373-379.
2. Alfirevic Z, Weeks A. Oral misoprostol for induction of labour. Cochrane Database Syst Rev. 2006;(2):CD001338.-
3. Hofmeyr G, Gülmezoglu A. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2003;(1):CD000941.-
4. Barcaite E, Bartusevicius A, Railaite DR, Nadisauskiene R. Vaginal misoprostol for cervical priming before hysteroscopy in perimenopausal and postmenopausal women. Int J Gynaecol Obstet. 2005;91:141-145.
5. Darwish AM, Ahmad AM, Mohammad AM. Cervical priming prior to operative hysteroscopy: a randomized comparison of laminaria versus misoprostol. Hum Reprod. 2004;19:2391-2394.
6. Preutthipan S, Herabutya Y. A randomized comparison of vaginal misoprostol and dinoprostone for cervical priming in nulliparous women before operative hysteroscopy. Fertil Steril. 2006;86:990-994.
In this randomized, placebo-controlled trial by Oppegaard and colleagues, when 1,000 μg of vaginal misoprostol was self-administered by premenopausal women at least 12 hours before operative hysteroscopy, significant cervical ripening occurred, with increased cervical dilatation and less difficulty with dilatation, compared with women given placebo.
The drug was ineffective in postmenopausal women.
EXPERT COMMENTARY
Operative hysteroscopy is a common, minimally invasive procedure used to treat a number of gynecologic pathologies.1 The procedure requires that the cervical canal be dilated enough to allow passage of the hysteroscope.
Misoprostol is a prostaglandin E1 analog. It also is an effective cervical-ripening and labor-induction agent used during pregnancy in the second and third trimesters.2,3 Earlier studies suggested that misoprostol may be promising as a cervical-ripening agent before hysteroscopy in premenopausal women, although further research is needed to determine the ideal dosage, route, and timing of administration.1,4-6 Most of the studies demonstrating benefit with misoprostol before hysteroscopy have involved intravaginal dosages of 200 to 400 μg given 8 to 12 hours before the procedure.1,4-6
Misoprostol enabled greater dilatation in more women
Oppegaard and colleagues found greater mean cervical dilatation with misoprostol in premenopausal women than with placebo (6.4±2.4 mm, compared with 4.8±2.2 mm), more women achieving at least 5-mm cervical dilatation (88% versus 65%), and fewer women being difficult to dilate for hysteroscopy (20% versus 32%). As in previous studies, they also found misoprostol to be an ineffective cervical-ripening agent in postmenopausal women.
Strengths of this study
Because this study was randomized and placebo-controlled, bias in the evaluation of outcomes was minimized. The sample size was based on a sequential trial design, which ensures adequate power to answer the question of interest using as few women as possible.
The medication was self-administered and therefore more convenient than physician administration. In addition, women were questioned afterward to determine the acceptability of the self-administered medication, and 83% of premenopausal subjects found self-administration fairly or completely acceptable.
Shortcomings
Oppegaard and colleagues recommend that 1,000 μg of vaginal misoprostol be offered to nulliparous premenopausal women before operative hysteroscopy, but they do not present data specific to nulliparous women.
Moreover, the use of 1,000 μg of misoprostol is higher than in most previous studies, and Oppegaard and colleagues do not compare different dosages. The use of a higher dosage (1,000 μg) may be expected to cause more side effects. Indeed, researchers found a higher incidence of vaginal bleeding with misoprostol, compared with placebo (21% versus 3%), and 42% of women receiving misoprostol experienced mild or moderate abdominal pain, with 7% reporting severe abdominal pain.
The use of self-administered vaginal misoprostol 12 hours before operative hysteroscopy in premenopausal women increases cervical dilatation and reduces the difficulty of dilatation. Oppegaard and colleagues used 1,000 μg of misoprostol, although earlier studies suggested benefit with 200 μg to 400 μg.
Although the route and timing of misoprostol for cervical ripening before hysteroscopy appears evident from the literature (vaginal administration 12 hours before the procedure), the ideal dosage is still unclear. Furthermore, misoprostol carries potential side effects, including vaginal bleeding and abdominal pain.—JOAN M.G. CRANE, MD, MSC
In this randomized, placebo-controlled trial by Oppegaard and colleagues, when 1,000 μg of vaginal misoprostol was self-administered by premenopausal women at least 12 hours before operative hysteroscopy, significant cervical ripening occurred, with increased cervical dilatation and less difficulty with dilatation, compared with women given placebo.
The drug was ineffective in postmenopausal women.
EXPERT COMMENTARY
Operative hysteroscopy is a common, minimally invasive procedure used to treat a number of gynecologic pathologies.1 The procedure requires that the cervical canal be dilated enough to allow passage of the hysteroscope.
Misoprostol is a prostaglandin E1 analog. It also is an effective cervical-ripening and labor-induction agent used during pregnancy in the second and third trimesters.2,3 Earlier studies suggested that misoprostol may be promising as a cervical-ripening agent before hysteroscopy in premenopausal women, although further research is needed to determine the ideal dosage, route, and timing of administration.1,4-6 Most of the studies demonstrating benefit with misoprostol before hysteroscopy have involved intravaginal dosages of 200 to 400 μg given 8 to 12 hours before the procedure.1,4-6
Misoprostol enabled greater dilatation in more women
Oppegaard and colleagues found greater mean cervical dilatation with misoprostol in premenopausal women than with placebo (6.4±2.4 mm, compared with 4.8±2.2 mm), more women achieving at least 5-mm cervical dilatation (88% versus 65%), and fewer women being difficult to dilate for hysteroscopy (20% versus 32%). As in previous studies, they also found misoprostol to be an ineffective cervical-ripening agent in postmenopausal women.
Strengths of this study
Because this study was randomized and placebo-controlled, bias in the evaluation of outcomes was minimized. The sample size was based on a sequential trial design, which ensures adequate power to answer the question of interest using as few women as possible.
The medication was self-administered and therefore more convenient than physician administration. In addition, women were questioned afterward to determine the acceptability of the self-administered medication, and 83% of premenopausal subjects found self-administration fairly or completely acceptable.
Shortcomings
Oppegaard and colleagues recommend that 1,000 μg of vaginal misoprostol be offered to nulliparous premenopausal women before operative hysteroscopy, but they do not present data specific to nulliparous women.
Moreover, the use of 1,000 μg of misoprostol is higher than in most previous studies, and Oppegaard and colleagues do not compare different dosages. The use of a higher dosage (1,000 μg) may be expected to cause more side effects. Indeed, researchers found a higher incidence of vaginal bleeding with misoprostol, compared with placebo (21% versus 3%), and 42% of women receiving misoprostol experienced mild or moderate abdominal pain, with 7% reporting severe abdominal pain.
The use of self-administered vaginal misoprostol 12 hours before operative hysteroscopy in premenopausal women increases cervical dilatation and reduces the difficulty of dilatation. Oppegaard and colleagues used 1,000 μg of misoprostol, although earlier studies suggested benefit with 200 μg to 400 μg.
Although the route and timing of misoprostol for cervical ripening before hysteroscopy appears evident from the literature (vaginal administration 12 hours before the procedure), the ideal dosage is still unclear. Furthermore, misoprostol carries potential side effects, including vaginal bleeding and abdominal pain.—JOAN M.G. CRANE, MD, MSC
1. Crane JM, Healey S. Use of misoprostol before hysteroscopy: a systematic review. J Obstet Gynaecol Can. 2006;28:373-379.
2. Alfirevic Z, Weeks A. Oral misoprostol for induction of labour. Cochrane Database Syst Rev. 2006;(2):CD001338.-
3. Hofmeyr G, Gülmezoglu A. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2003;(1):CD000941.-
4. Barcaite E, Bartusevicius A, Railaite DR, Nadisauskiene R. Vaginal misoprostol for cervical priming before hysteroscopy in perimenopausal and postmenopausal women. Int J Gynaecol Obstet. 2005;91:141-145.
5. Darwish AM, Ahmad AM, Mohammad AM. Cervical priming prior to operative hysteroscopy: a randomized comparison of laminaria versus misoprostol. Hum Reprod. 2004;19:2391-2394.
6. Preutthipan S, Herabutya Y. A randomized comparison of vaginal misoprostol and dinoprostone for cervical priming in nulliparous women before operative hysteroscopy. Fertil Steril. 2006;86:990-994.
1. Crane JM, Healey S. Use of misoprostol before hysteroscopy: a systematic review. J Obstet Gynaecol Can. 2006;28:373-379.
2. Alfirevic Z, Weeks A. Oral misoprostol for induction of labour. Cochrane Database Syst Rev. 2006;(2):CD001338.-
3. Hofmeyr G, Gülmezoglu A. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2003;(1):CD000941.-
4. Barcaite E, Bartusevicius A, Railaite DR, Nadisauskiene R. Vaginal misoprostol for cervical priming before hysteroscopy in perimenopausal and postmenopausal women. Int J Gynaecol Obstet. 2005;91:141-145.
5. Darwish AM, Ahmad AM, Mohammad AM. Cervical priming prior to operative hysteroscopy: a randomized comparison of laminaria versus misoprostol. Hum Reprod. 2004;19:2391-2394.
6. Preutthipan S, Herabutya Y. A randomized comparison of vaginal misoprostol and dinoprostone for cervical priming in nulliparous women before operative hysteroscopy. Fertil Steril. 2006;86:990-994.
How to overcome a resistant cervix for hysteroscopy and endometrial biopsy
CASE: Difficulty inserting a catheter suggests an unyielding cervix
A.W. is a 38-year-old nulliparous woman who seeks treatment for persistent irregular vaginal bleeding. Her physician attempts an endometrial biopsy in the office but is unable to pass the catheter through the internal cervical os. She schedules office hysteroscopy as follow-up.
What steps can the ObGyn take to reduce the difficulty of the procedure, particularly insertion of the hysteroscope through the cervical canal?
Successful hysteroscopy requires a cervical canal sufficiently dilated to allow passage of the hysteroscope. And because of inevitable variation in anatomy—and even in models of hysteroscopes, which range in diameter from 2.7 to 10 mm—passage is not always easily accomplished. Many of the complications related to hysteroscopy, including cervical tears, creation of a false passage, uterine perforation, vasovagal reaction, pain, and inability to complete the procedure, are caused by inadequate cervical dilation and an inability to insert the hysteroscope.1-6 One study noted that almost half of complications were related to cervical entry.6
In this article, I describe ways to overcome the challenging cervix for hysteroscopic procedures and endometrial biopsy (TABLES 1 and 2).
TABLE 1
10 actions that can ease entry to the cervix for hysteroscopy
| ACTION | COMMENTS |
|---|---|
| Take a careful history and perform a rigorous physical exam | Identify risk factors for cervical stenosis and assess cervical/uterine position |
| Administer an oral nonsteroidal anti-inflammatory drug 60 minutes before the procedure | Helps to reduce discomfort, especially postprocedure pain |
| Provide an anxiolytic or conscious sedation, or both | Consider this option for women who are very anxious or unlikely to tolerate pain, especially for operative procedures |
| Use a tenaculum | Consider if the uterus is not in the axial position |
| Use Hagar dilators or a lacrimal duct probe | May be helpful if mechanical dilation is necessary |
| Proceed under ultrasonographic guidance | Consider transabdominal imaging to help guide cervical dilation in difficult cases, e.g., when the patient has a history of uterine perforation |
| Opt for a smaller hysteroscope | A smaller scope will require less cervical dilation |
| Administer a paracervical block | Consider this option if cervical dilation is expected to be difficult, especially in women at risk of significant pain. Be alert for complications such as bleeding, discomfort at the time of injection, and intravascular injection leading to bradycardia and hypotension |
| Administer a topical cervical anesthetic | May be appropriate when a tenaculum is used |
| Give misoprostol to prime the cervix | Consider giving 400 μg of intravaginal misoprostol 9 to 12 hours preoperatively in premenopausal women, particularly nulliparous women and those undergoing operative hysteroscopy |
TABLE 2
6 ways to prepare the cervix for endometrial biopsy
| ACTION | COMMENTS |
|---|---|
| Take a careful history and perform a thorough physical examination | Identify risk factors for cervical stenosis and assess uterine position |
| Administer an oral nonsteroidal anti-inflammatory drug 60 minutes prior to biopsy | Helps to reduce discomfort, especially postprocedure pain |
| Use a tenaculum | May be helpful if the uterus/cervix is not in the axial position |
| Apply a topical cervical anesthetic | May help alleviate discomfort associated with use of a tenaculum |
| Use Hagar dilators or lacrimal duct probes | Provide mechanical dilation |
| Use the smallest biopsy catheter possible | Reduces degree of cervical dilation necessary |
Hysteroscopy failure rate: 3.4% to 4.2%
Hysteroscopy is, of course, common in gynecologic practice, its indications extending across a range of investigations and treatments—for menstrual disorders, postmenopausal bleeding, infertility, and recurrent pregnancy loss.1,7 Flexible hysteroscopes range in diameter from 2.7 to 5 mm; rigid hysteroscopes, from 1 to 5 mm; and operative hysteroscopes can be as large as 8 to 10 mm.2,7
A systematic review of diagnostic hysteroscopy in more than 26,000 women reported a failure rate of 4.2% for ambulatory hysteroscopy and 3.4% for inpatient procedures.4 Failed ambulatory procedures were mainly attributed to technical problems, including:
- cervical stenosis
- anatomic and structural abnormalities
- pain and intolerance.4
Ideally, hysteroscopy is performed with minimal or no cervical dilation,7 but this may not always be possible.
Things to consider before embarking
Close attention to cervical and uterine anatomy is critical because insertion of the hysteroscope can be the most difficult aspect of the procedure. A bimanual examination is imperative to assess uterine size and position. It also is useful to sound the uterus to determine its depth.
An accurate medical, gynecologic, and obstetric history is essential, including information on pregnancies, dilation and curettage, cervical procedures such as cryotherapy, and any other procedures that may increase the risk of cervical stenosis, or difficulty dilating the cervix.
Is stenosis present? Stenosis is most common in nulliparous and postmenopausal women and in those who have undergone cervical procedures such as cryotherapy. Stenosis increases the risk of laceration and uterine perforation.
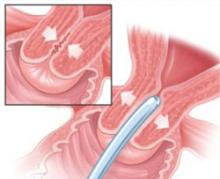
Consider a mechanical dilator. When cervical dilation is difficult, a series of small Hagar or lacrimal duct dilators may be helpful (FIGURE).
FIGURE Mechanical dilation is one antidote to cervical stenosis
In challenging cases, such as cervical stenosis, mechanical dilation with a series of Hagar or lacrimal duct dilators may facilitate entry into the cervix.
Pain can be mild—or it can thwart your work
Although many women tolerate placement of a small hysteroscope without analgesia or anesthesia, pain and vasovagal reaction sometimes occur. Indeed, the level of pain experienced by the patient is a major determinant of the overall success of the procedure.3,8-10 Pain can occur when a tenaculum is used to grasp the anterior cervix, as well as during cervical dilation, injection of local anesthetic, or insertion of the hysteroscope. In some cases, a smaller scope may be all that is needed to solve the problem.11
Analgesia may not always be necessary
Some researchers have studied office hysteroscopy without analgesia or anesthesia, finding a high level of acceptance.12,13 Others have found a significant percentage of women requesting anesthesia or analgesia (16.5%)10 or requiring local anesthesia (28.8%).8
Preoperative NSAIDs may suffice. Use of oral nonsteroidal anti-inflammatory drugs (NSAIDs) 1 hour before office hysteroscopy may reduce intraoperative and postoperative pain.7 Nagele and colleagues8 compared use of mefenamic acid 1 hour before the procedure with placebo in 95 women undergoing outpatient diagnostic hysteroscopy. Mefenamic acid reduced pain at 30 and 60 minutes after—but not during—the procedure. Other studies have found that pain is reduced when an oral NSAID is taken 1 to 2 hours before insertion of an intrauterine device and before suction curettage.14,15
Other perioperative medications may help reduce discomfort and patient anxiety, including anxiolytics, such as lorazepam, analgesics, and conscious sedation.3
Paracervical block may be appropriate when pain is very likely
A number of investigators have evaluated use of paracervical anesthesia during out-patient hysteroscopy.9,13,16,17 They injected lignocaine or mepivacaine using a 21- or 22-gauge needle at 3, 5, 7, and 9 o’clock or 4 and 8 o’clock paracervically.13 One study found paracervical block to be effective in reducing the pain of tenaculum placement and insertion of the hysteroscope.17 However, some studies suggested a reduction of pain in postmenopausal women only.9 These women may be more likely to have cervical stenosis.
Paracervical block does pose a risk of complications. Studies have reported bleeding in some women16 and pain with injection of the paracervical block, as well as bradycardia and hypotension possibly secondary to intravascular injection.17
Other methods are inconsistent
Intracervical injection. Some researchers have recommended injection of local anesthetic into the cervix.13 One study found no benefit—in fact, the injection appeared to be the most painful part of the procedure.18 A case series suggested that injection of local anesthetic may be effective, but the series lacked a placebo or control arm.13
Topical intrauterine anesthetic has been investigated after administration through the channel of the hysteroscope or by a catheter passed through the cervix into the uterine cavity.13 Findings have been mixed, with some researchers demonstrating reduced pain19,20 and others showing no relief.21
Topical cervical anesthesia. Some hysteroscopists have recommended application of anesthetic cream, gel, or spray directly to the cervix immediately before the procedure.13,22 The results have been mixed, with some studies noting decreased pain overall,13 one finding decreased pain only during tenaculum placement,22 and others finding no significant reduction in pain any time during the procedure.13,23,24 A review concluded that topical cervical lignocaine spray may reduce the discomfort of tenaculum placement.13
Topical anesthesia may minimize vasovagal reaction
In one study, 1.1% of women undergoing office hysteroscopy experienced a vasovagal reaction, caused by stimulation of the parasympathetic nervous system with cervical manipulation and passage of the scope through the internal os of the cervix.25 The reaction led to hypotension and bradycardia. Several studies have suggested that a local anesthetic can reduce this complication.19,20
Cicinelli and associates found that topical local anesthesia reduced the incidence of vasovagal reaction from 32.5% in the control arm to 5%.20 They suggest that a local anesthetic be considered in selected women, such as postmenopausal patients, who are at increased risk of vasovagal attack.
In contrast, Lau and associates17 found an increased rate of bradycardia and hypotension with paracervical lignocaine (31% versus 10%), but it may have been caused by inadvertent intravascular injection.17
Researchers have also suggested that the use of smaller hysteroscopes may reduce the incidence of vasovagal reactions.26
How to prime the cervix for hysteroscopy
The use of vaginal misoprostol, a prostaglandin E1 analogue, 9 to 12 hours before hysteroscopy may help increase preprocedural cervical dilation in premenopausal women, especially in nulliparas and women undergoing operative hysteroscopy. Misoprostol, used to prevent and treat NSAID-induced gastric ulcers, is gaining favor as a cervical ripening agent. We performed a meta-analysis to assess its effectiveness in dilating the cervix and reducing the need for mechanical dilation.5
We identified 10 studies that met inclusion criteria; five of them included premenopausal women, four included postmenopausal women or women receiving a gonadotropin-releasing hormone (GnRH) agonist, and one study included both groups.5 A variety of dosing protocols were used, with dosages ranging from 100 μg to 1,000 μg of intravaginal or oral misoprostol 4 to 24 hours preoperatively (most studies evaluated the vaginal route).
We found that misoprostol significantly reduced the need for further cervical dilation, and was associated with a lower rate of cervical laceration. However, this was true only for the premenopausal group: 42.6% of premenopausal women given misoprostol needed further dilation, compared with 71.7% in the control group, and 2% of premenopausal women given misoprostol suffered cervical laceration, compared with 11% in the control group. Among postmenopausal women and those receiving a GnRH agonist, misoprostol lacked clear benefit and was associated with side effects such as nausea, diarrhea, abdominal cramping, and fever.
For every premenopausal woman who received misoprostol before hysteroscopy, one woman avoided the need for further cervical dilation. For every 12 premenopausal women receiving misoprostol, one cervical laceration was avoided.
The ideal dosing regimen could not be determined because of variations in protocols. Nor was it clear whether misoprostol had any benefit among postmenopausal women or those receiving a GnRH agonist.
Most studies of misoprostol for cervical ripening have involved intravaginal administration, with dosages of 200 μg to 400 μg given 9 to 12 hours before hysteroscopy showing the greatest benefit.
Ultrasonography may help guide dilation
Transabdominal ultrasonography has been used to guide dilation in difficult dilation and curettage procedures, and is especially useful in women with a history of uterine perforation.27 It may be helpful in cases involving difficult cervical dilation during hysteroscopy or endometrial biopsy.
Steady the cervix. A tenaculum is not always required, but its use on the anterior lip of the cervix may help steady the cervix and provide countertraction during insertion of the hysteroscope through the cervical canal, especially if the cervix is not in an axial position.7
CASE Resolved!
Because she is nulliparous and may benefit from cervical priming, the patient is given 400 μg of intravaginal misoprostol 12 hours before hysteroscopy, as well as an oral NSAID 1 hour before the procedure. A bimanual examination reveals a sharply anteverted uterus, so a topical cervical anesthetic spray is applied to the anterior cervix, and a tenaculum is placed to help straighten the uterine position. The hysteroscope passes easily through the cervical canal, making further dilation unnecessary. The procedure is completed without difficulty and is well tolerated by the patient.
Difficult entry can also hamper endometrial biopsy
Every ObGyn has used endometrial biopsy to assess abnormal uterine bleeding, postmenopausal bleeding, infertility, or recurrent pregnancy loss, or to monitor women on hormone replacement therapy28,29 —so its advantages over dilation and curettage should come as no surprise. They include the ability to perform it in an office setting, usually with minimal cervical dilation, often without anesthesia, and at less expense.28 Complications include cramping and pain,29-32 vasovagal reaction,29 bleeding,29 and inability to pass the biopsy catheter through the cervix into the uterine cavity. Another rare complication is uterine perforation.29
As with hysteroscopy, many of these complications are related to difficulty entering the uterine cavity through the cervix.
Prerequisites include thorough assessment of the uterus
As with hysteroscopy, an accurate and detailed history is necessary to identify risk factors for a difficult procedure. Assess uterine size and position with a bimanual examination. Although a tenaculum is often unnecessary, its placement on the anterior lip of the cervix may help steady the cervix and allow the catheter to pass through the cervical canal into the uterine cavity, especially if the uterus is not in the axial position.28,29 Again, it is useful to sound the uterine cavity to ascertain its depth. This may be done with the biopsy catheter.
Cervical dilation may be necessary
Even when women with cervical stenosis were excluded in one study, it was difficult to pass the Pipelle endometrial biopsy through the cervix in 41.7% of women.30
If the sampling device does not pass easily through the cervix, use a tenaculum and a lacrimal duct probe or small Hagar dilators to dilate the cervix.28
Pain may again be an issue
Almost 50% of women experience moderate or severe pain during endometrial biopsy.32 Many clinicians recommend giving an oral NSAID 60 minutes before the procedure to decrease discomfort. One study found that the use of naproxen sodium before Vabra curettage reduced the severity of pain at 30 and 60 minutes after the procedure, but did not alleviate discomfort arising during the biopsy itself.14 Another study suggested the combination of naproxen sodium and intrauterine lidocaine (5 mL of 2% lidocaine) to reduce discomfort associated with the procedure.30
Use of anesthesia is controversial
A study by Lau and colleagues17 found paracervical lignocaine to be ineffective at reducing pain during hysteroscopy and endometrial biopsy, but the drug did increase the risk of bradycardia and hypotension. Another study demonstrated a decrease in procedure-related discomfort in postmenopausal women who were given 2 mL of 2% intrauterine mepivacaine.20 These findings are similar to those of Zupi and associates.19
Consider the tool
Discomfort may be related to the size of the biopsy catheter. Pain scores appear to be significantly lower with the Pipelle biopsy catheter than with the larger Novak biopsy curette.32
Vasovagal reaction usually resolves after the procedure
As with hysteroscopy, women may occasionally experience a vasovagal reaction during endometrial biopsy. This complication usually resolves quickly once the procedure is completed.29 Some clinicians suggest that the patient be allowed to eat and drink before the procedure and be given an analgesic before it begins.28
Cervical priming is not a proven strategy
Misoprostol has been considered as a preprocedure adjunct to endometrial biopsy. Only one small randomized, controlled trial involving 42 women has evaluated the drug for this indication. It found no benefit when 400 μg of misoprostol was given orally 3 hours before the procedure, as well as cramping and increased pain during the biopsy.33 This study had several shortcomings, including its small sample size and the inclusion of both pre- and postmenopausal women. Further research is needed—separately in premenopausal and postmenopausal women and with adequately large samples—to assess the use of misoprostol.
The author reports no financial relationships relevant to this article.
1. Bradley LD. Complications in hysteroscopy: prevention, treatment and legal risk. Curr Opin Obstet Gynecol. 2002;14:409-415.
2. American College of Obstetricians and Gynecologists. ACOG technology assessment in obstetrics and gynecology, number 4, August 2005: hysteroscopy. Obstet Gynecol. 2005;106:439-442.
3. Vilos GA, Abu-Rafea B. New developments in ambulatory hysteroscopic surgery. Best Pract Res Clin Obstet Gynaecol. 2005;19:727-742.
4. Clark TJ, Voit D, Gupta JK, Hyde C, Song F, Khan KS. Accuracy of hysteroscopy in the diagnosis of endometrial cancer and hyperplasia: a systematic quantitative review. JAMA. 2002;288:1610-1621.
5. Crane JM, Healey S. Use of misoprostol before hysteroscopy: a systematic review. J Obstet Gynaecol Can. 2006;28:373-379.
6. Jansen FW, Vredevoogd CB, van Ulzen K, Hermans J, Trimbos JB, Trimbos-Kemper TC. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol. 2000;96:266-270.
7. Guido R, Stovall D. Hysteroscopy Version 14.3. UpToDate [cited February 15, 2007]; Available from: www.uptodate.com.
8. Nagele F, Lockwood G, Magos AL. Randomised placebo controlled trial of mefenamic acid for premedication at outpatient hysteroscopy: a pilot study. Br J Obstet Gynaecol. 1997;104:842-844.
9. Cicinelli E, Didonna T, Schonauer LM, Stragapede S, Falco N, Pansini N. Paracervical anesthesia for hysteroscopy and endometrial biopsy in postmenopausal women. A randomized, double-blind, placebo-controlled study. J Reprod Med. 1998;43:1014-1018.
10. De Iaco P, Marabini A, Stefanetti M, Del Vecchio C, Bovicelli L. Acceptability and pain of outpatient hysteroscopy. J Am Assoc Gynecol Laparosc. 2000;7:71-75.
11. Marsh F, Jackson T, Duffy S. A case controlled study comparing 3.6 mm and 3.1 mm flexible hysteroscopes. Gynaecol Endosc. 2002;11:393-396.
12. Lau WC, Ho RY, Tsang MK, Yuen PM. Patient’s acceptance of outpatient hysteroscopy. Gynecol Obstet Invest. 1999;47:191-193.
13. Hassan L, Gannon MJ. Anaesthesia and analgesia for ambulatory hysteroscopic surgery. Best Pract Res Clin Obstet Gynaecol. 2005;19:681-691.
14. Siddle NC, Young O, Sledmere CM, Reading AE, Whitehead MI. A controlled trial of naproxen sodium for relief of pain associated with Vabra suction curettage. Br J Obstet Gynaecol. 1983;90:864-869.
15. Edgren RA, Morton CJ. Naproxen sodium for Ob/Gyn use, with special reference to pain states: a review. Int J Fertil. 1986;31:135-142.
16. Giorda G, Scarabelli C, Franceschi S, Campagnutta E. Feasibility and pain control in outpatient hysteroscopy in postmenopausal women: a randomized trial. Acta Obstet Gynecol Scand. 2000;79:593-597.
17. Lau WC, Lo WK, Tam WH, Yuen PM. Paracervical anaesthesia in outpatient hysteroscopy: a randomised double-blind placebo-controlled trial. Br J Obstet Gynaecol. 1999;106:356-359.
18. Broadbent JA, Hill NC, Molnar BG, Rolfe KJ, Magos AL. Randomized placebo controlled trial to assess the role of intracervical lignocaine in outpatient hysteroscopy. Br J Obstet Gynaecol. 1992;99:777-779.
19. Zupi E, Luciano AA, Valli E, Marconi D, Maneschi F, Romanini C. The use of topical anesthesia in diagnostic hysteroscopy and endometrial biopsy. Fertil Steril. 1995;63:414-416.
20. Cicinelli E, Didonna T, Ambrosi G, Schonauer LM, Fiore G, Matteo MG. Topical anaesthesia for diagnostic hysteroscopy and endometrial biopsy in postmenopausal women: a randomised placebo-controlled double-blind study. Br J Obstet Gynaecol. 1997;104:316-319.
21. Lau WC, Tam WH, Lo WK, Yuen PM. A randomised double-blind placebo-controlled trial of transcervical intrauterine local anaesthesia in outpatient hysteroscopy. BJOG. 2000;107:610-613.
22. Davies A, Richardson RE, O’Connor H, Baskett TF, Nagele F, Magos AL. Lignocaine aerosol spray in outpatient hysteroscopy: a randomized double-blind placebo-controlled trial. Fertil Steril. 1997;67:1019-1023.
23. Clark S, Vonau B, Macdonald R. Topical anaesthesia in out-patient hysteroscopy. Gynaecol Endosc. 1996;5:141-144.
24. Wong AY, Wong K, Tang LC. Stepwise pain score analysis of the effect of local lignocaine on outpatient hysteroscopy: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2000;73:1234-1237.
25. Bellingham FR. Outpatient hysteroscopy—problems. Aust N Z J Obstet Gynaecol. 1997;37:202-205.
26. Cicinelli E, Schonauer LM, Barba B, Tartagni M, Luisi D, Di Naro E. Tolerability and cardiovascular complications of outpatient diagnostic minihysteroscopy compared with conventional hysteroscopy. J Am Assoc Gynecol Laparosc. 2003;10:399-402.
27. Hunter RE, Reuter K, Kopin E. Use of ultrasonography in the difficult postmenopausal dilation and curettage. Obstet Gynecol. 1989;73:813-816.
28. Guido R, Stovall D. Endometrial sampling procedures Version 14.3. UpToDate [cited February 15, 2007]; Available from: www.uptodate.com.
29. Cooper JM, Erickson ML. Endometrial sampling techniques in the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:235-244.
30. Dogan E, Celiloglu M, Sarihan E, Demir A. Anesthetic effect of intrauterine lidocaine plus naproxen sodium in endometrial biopsy. Obstet Gynecol. 2004;103:347-351.
31. Trolice MP, Fishburne C, Jr, McGrady S. Anesthetic efficacy of intrauterine lidocaine for endometrial biopsy: a randomized double-masked trial. Obstet Gynecol. 2000;95:345-347.
32. Silver MM, Miles P, Rosa C. Comparison of Novak and Pipelle endometrial biopsy instruments. Obstet Gynecol. 1991;78:828-830.
33. Perrone JF, Caldito G, Mailhes JB, Tucker AN, Ford WR, London SN. Oral misoprostol before office endometrial biopsy. Obstet Gynecol. 2002;99:439-444.
CASE: Difficulty inserting a catheter suggests an unyielding cervix
A.W. is a 38-year-old nulliparous woman who seeks treatment for persistent irregular vaginal bleeding. Her physician attempts an endometrial biopsy in the office but is unable to pass the catheter through the internal cervical os. She schedules office hysteroscopy as follow-up.
What steps can the ObGyn take to reduce the difficulty of the procedure, particularly insertion of the hysteroscope through the cervical canal?
Successful hysteroscopy requires a cervical canal sufficiently dilated to allow passage of the hysteroscope. And because of inevitable variation in anatomy—and even in models of hysteroscopes, which range in diameter from 2.7 to 10 mm—passage is not always easily accomplished. Many of the complications related to hysteroscopy, including cervical tears, creation of a false passage, uterine perforation, vasovagal reaction, pain, and inability to complete the procedure, are caused by inadequate cervical dilation and an inability to insert the hysteroscope.1-6 One study noted that almost half of complications were related to cervical entry.6
In this article, I describe ways to overcome the challenging cervix for hysteroscopic procedures and endometrial biopsy (TABLES 1 and 2).
TABLE 1
10 actions that can ease entry to the cervix for hysteroscopy
| ACTION | COMMENTS |
|---|---|
| Take a careful history and perform a rigorous physical exam | Identify risk factors for cervical stenosis and assess cervical/uterine position |
| Administer an oral nonsteroidal anti-inflammatory drug 60 minutes before the procedure | Helps to reduce discomfort, especially postprocedure pain |
| Provide an anxiolytic or conscious sedation, or both | Consider this option for women who are very anxious or unlikely to tolerate pain, especially for operative procedures |
| Use a tenaculum | Consider if the uterus is not in the axial position |
| Use Hagar dilators or a lacrimal duct probe | May be helpful if mechanical dilation is necessary |
| Proceed under ultrasonographic guidance | Consider transabdominal imaging to help guide cervical dilation in difficult cases, e.g., when the patient has a history of uterine perforation |
| Opt for a smaller hysteroscope | A smaller scope will require less cervical dilation |
| Administer a paracervical block | Consider this option if cervical dilation is expected to be difficult, especially in women at risk of significant pain. Be alert for complications such as bleeding, discomfort at the time of injection, and intravascular injection leading to bradycardia and hypotension |
| Administer a topical cervical anesthetic | May be appropriate when a tenaculum is used |
| Give misoprostol to prime the cervix | Consider giving 400 μg of intravaginal misoprostol 9 to 12 hours preoperatively in premenopausal women, particularly nulliparous women and those undergoing operative hysteroscopy |
TABLE 2
6 ways to prepare the cervix for endometrial biopsy
| ACTION | COMMENTS |
|---|---|
| Take a careful history and perform a thorough physical examination | Identify risk factors for cervical stenosis and assess uterine position |
| Administer an oral nonsteroidal anti-inflammatory drug 60 minutes prior to biopsy | Helps to reduce discomfort, especially postprocedure pain |
| Use a tenaculum | May be helpful if the uterus/cervix is not in the axial position |
| Apply a topical cervical anesthetic | May help alleviate discomfort associated with use of a tenaculum |
| Use Hagar dilators or lacrimal duct probes | Provide mechanical dilation |
| Use the smallest biopsy catheter possible | Reduces degree of cervical dilation necessary |
Hysteroscopy failure rate: 3.4% to 4.2%
Hysteroscopy is, of course, common in gynecologic practice, its indications extending across a range of investigations and treatments—for menstrual disorders, postmenopausal bleeding, infertility, and recurrent pregnancy loss.1,7 Flexible hysteroscopes range in diameter from 2.7 to 5 mm; rigid hysteroscopes, from 1 to 5 mm; and operative hysteroscopes can be as large as 8 to 10 mm.2,7
A systematic review of diagnostic hysteroscopy in more than 26,000 women reported a failure rate of 4.2% for ambulatory hysteroscopy and 3.4% for inpatient procedures.4 Failed ambulatory procedures were mainly attributed to technical problems, including:
- cervical stenosis
- anatomic and structural abnormalities
- pain and intolerance.4
Ideally, hysteroscopy is performed with minimal or no cervical dilation,7 but this may not always be possible.
Things to consider before embarking
Close attention to cervical and uterine anatomy is critical because insertion of the hysteroscope can be the most difficult aspect of the procedure. A bimanual examination is imperative to assess uterine size and position. It also is useful to sound the uterus to determine its depth.
An accurate medical, gynecologic, and obstetric history is essential, including information on pregnancies, dilation and curettage, cervical procedures such as cryotherapy, and any other procedures that may increase the risk of cervical stenosis, or difficulty dilating the cervix.
Is stenosis present? Stenosis is most common in nulliparous and postmenopausal women and in those who have undergone cervical procedures such as cryotherapy. Stenosis increases the risk of laceration and uterine perforation.
Consider a mechanical dilator. When cervical dilation is difficult, a series of small Hagar or lacrimal duct dilators may be helpful (FIGURE).
FIGURE Mechanical dilation is one antidote to cervical stenosis
In challenging cases, such as cervical stenosis, mechanical dilation with a series of Hagar or lacrimal duct dilators may facilitate entry into the cervix.
Pain can be mild—or it can thwart your work
Although many women tolerate placement of a small hysteroscope without analgesia or anesthesia, pain and vasovagal reaction sometimes occur. Indeed, the level of pain experienced by the patient is a major determinant of the overall success of the procedure.3,8-10 Pain can occur when a tenaculum is used to grasp the anterior cervix, as well as during cervical dilation, injection of local anesthetic, or insertion of the hysteroscope. In some cases, a smaller scope may be all that is needed to solve the problem.11
Analgesia may not always be necessary
Some researchers have studied office hysteroscopy without analgesia or anesthesia, finding a high level of acceptance.12,13 Others have found a significant percentage of women requesting anesthesia or analgesia (16.5%)10 or requiring local anesthesia (28.8%).8
Preoperative NSAIDs may suffice. Use of oral nonsteroidal anti-inflammatory drugs (NSAIDs) 1 hour before office hysteroscopy may reduce intraoperative and postoperative pain.7 Nagele and colleagues8 compared use of mefenamic acid 1 hour before the procedure with placebo in 95 women undergoing outpatient diagnostic hysteroscopy. Mefenamic acid reduced pain at 30 and 60 minutes after—but not during—the procedure. Other studies have found that pain is reduced when an oral NSAID is taken 1 to 2 hours before insertion of an intrauterine device and before suction curettage.14,15
Other perioperative medications may help reduce discomfort and patient anxiety, including anxiolytics, such as lorazepam, analgesics, and conscious sedation.3
Paracervical block may be appropriate when pain is very likely
A number of investigators have evaluated use of paracervical anesthesia during out-patient hysteroscopy.9,13,16,17 They injected lignocaine or mepivacaine using a 21- or 22-gauge needle at 3, 5, 7, and 9 o’clock or 4 and 8 o’clock paracervically.13 One study found paracervical block to be effective in reducing the pain of tenaculum placement and insertion of the hysteroscope.17 However, some studies suggested a reduction of pain in postmenopausal women only.9 These women may be more likely to have cervical stenosis.
Paracervical block does pose a risk of complications. Studies have reported bleeding in some women16 and pain with injection of the paracervical block, as well as bradycardia and hypotension possibly secondary to intravascular injection.17
Other methods are inconsistent
Intracervical injection. Some researchers have recommended injection of local anesthetic into the cervix.13 One study found no benefit—in fact, the injection appeared to be the most painful part of the procedure.18 A case series suggested that injection of local anesthetic may be effective, but the series lacked a placebo or control arm.13
Topical intrauterine anesthetic has been investigated after administration through the channel of the hysteroscope or by a catheter passed through the cervix into the uterine cavity.13 Findings have been mixed, with some researchers demonstrating reduced pain19,20 and others showing no relief.21
Topical cervical anesthesia. Some hysteroscopists have recommended application of anesthetic cream, gel, or spray directly to the cervix immediately before the procedure.13,22 The results have been mixed, with some studies noting decreased pain overall,13 one finding decreased pain only during tenaculum placement,22 and others finding no significant reduction in pain any time during the procedure.13,23,24 A review concluded that topical cervical lignocaine spray may reduce the discomfort of tenaculum placement.13
Topical anesthesia may minimize vasovagal reaction
In one study, 1.1% of women undergoing office hysteroscopy experienced a vasovagal reaction, caused by stimulation of the parasympathetic nervous system with cervical manipulation and passage of the scope through the internal os of the cervix.25 The reaction led to hypotension and bradycardia. Several studies have suggested that a local anesthetic can reduce this complication.19,20
Cicinelli and associates found that topical local anesthesia reduced the incidence of vasovagal reaction from 32.5% in the control arm to 5%.20 They suggest that a local anesthetic be considered in selected women, such as postmenopausal patients, who are at increased risk of vasovagal attack.
In contrast, Lau and associates17 found an increased rate of bradycardia and hypotension with paracervical lignocaine (31% versus 10%), but it may have been caused by inadvertent intravascular injection.17
Researchers have also suggested that the use of smaller hysteroscopes may reduce the incidence of vasovagal reactions.26
How to prime the cervix for hysteroscopy
The use of vaginal misoprostol, a prostaglandin E1 analogue, 9 to 12 hours before hysteroscopy may help increase preprocedural cervical dilation in premenopausal women, especially in nulliparas and women undergoing operative hysteroscopy. Misoprostol, used to prevent and treat NSAID-induced gastric ulcers, is gaining favor as a cervical ripening agent. We performed a meta-analysis to assess its effectiveness in dilating the cervix and reducing the need for mechanical dilation.5
We identified 10 studies that met inclusion criteria; five of them included premenopausal women, four included postmenopausal women or women receiving a gonadotropin-releasing hormone (GnRH) agonist, and one study included both groups.5 A variety of dosing protocols were used, with dosages ranging from 100 μg to 1,000 μg of intravaginal or oral misoprostol 4 to 24 hours preoperatively (most studies evaluated the vaginal route).
We found that misoprostol significantly reduced the need for further cervical dilation, and was associated with a lower rate of cervical laceration. However, this was true only for the premenopausal group: 42.6% of premenopausal women given misoprostol needed further dilation, compared with 71.7% in the control group, and 2% of premenopausal women given misoprostol suffered cervical laceration, compared with 11% in the control group. Among postmenopausal women and those receiving a GnRH agonist, misoprostol lacked clear benefit and was associated with side effects such as nausea, diarrhea, abdominal cramping, and fever.
For every premenopausal woman who received misoprostol before hysteroscopy, one woman avoided the need for further cervical dilation. For every 12 premenopausal women receiving misoprostol, one cervical laceration was avoided.
The ideal dosing regimen could not be determined because of variations in protocols. Nor was it clear whether misoprostol had any benefit among postmenopausal women or those receiving a GnRH agonist.
Most studies of misoprostol for cervical ripening have involved intravaginal administration, with dosages of 200 μg to 400 μg given 9 to 12 hours before hysteroscopy showing the greatest benefit.
Ultrasonography may help guide dilation
Transabdominal ultrasonography has been used to guide dilation in difficult dilation and curettage procedures, and is especially useful in women with a history of uterine perforation.27 It may be helpful in cases involving difficult cervical dilation during hysteroscopy or endometrial biopsy.
Steady the cervix. A tenaculum is not always required, but its use on the anterior lip of the cervix may help steady the cervix and provide countertraction during insertion of the hysteroscope through the cervical canal, especially if the cervix is not in an axial position.7
CASE Resolved!
Because she is nulliparous and may benefit from cervical priming, the patient is given 400 μg of intravaginal misoprostol 12 hours before hysteroscopy, as well as an oral NSAID 1 hour before the procedure. A bimanual examination reveals a sharply anteverted uterus, so a topical cervical anesthetic spray is applied to the anterior cervix, and a tenaculum is placed to help straighten the uterine position. The hysteroscope passes easily through the cervical canal, making further dilation unnecessary. The procedure is completed without difficulty and is well tolerated by the patient.
Difficult entry can also hamper endometrial biopsy
Every ObGyn has used endometrial biopsy to assess abnormal uterine bleeding, postmenopausal bleeding, infertility, or recurrent pregnancy loss, or to monitor women on hormone replacement therapy28,29 —so its advantages over dilation and curettage should come as no surprise. They include the ability to perform it in an office setting, usually with minimal cervical dilation, often without anesthesia, and at less expense.28 Complications include cramping and pain,29-32 vasovagal reaction,29 bleeding,29 and inability to pass the biopsy catheter through the cervix into the uterine cavity. Another rare complication is uterine perforation.29
As with hysteroscopy, many of these complications are related to difficulty entering the uterine cavity through the cervix.
Prerequisites include thorough assessment of the uterus
As with hysteroscopy, an accurate and detailed history is necessary to identify risk factors for a difficult procedure. Assess uterine size and position with a bimanual examination. Although a tenaculum is often unnecessary, its placement on the anterior lip of the cervix may help steady the cervix and allow the catheter to pass through the cervical canal into the uterine cavity, especially if the uterus is not in the axial position.28,29 Again, it is useful to sound the uterine cavity to ascertain its depth. This may be done with the biopsy catheter.
Cervical dilation may be necessary
Even when women with cervical stenosis were excluded in one study, it was difficult to pass the Pipelle endometrial biopsy through the cervix in 41.7% of women.30
If the sampling device does not pass easily through the cervix, use a tenaculum and a lacrimal duct probe or small Hagar dilators to dilate the cervix.28
Pain may again be an issue
Almost 50% of women experience moderate or severe pain during endometrial biopsy.32 Many clinicians recommend giving an oral NSAID 60 minutes before the procedure to decrease discomfort. One study found that the use of naproxen sodium before Vabra curettage reduced the severity of pain at 30 and 60 minutes after the procedure, but did not alleviate discomfort arising during the biopsy itself.14 Another study suggested the combination of naproxen sodium and intrauterine lidocaine (5 mL of 2% lidocaine) to reduce discomfort associated with the procedure.30
Use of anesthesia is controversial
A study by Lau and colleagues17 found paracervical lignocaine to be ineffective at reducing pain during hysteroscopy and endometrial biopsy, but the drug did increase the risk of bradycardia and hypotension. Another study demonstrated a decrease in procedure-related discomfort in postmenopausal women who were given 2 mL of 2% intrauterine mepivacaine.20 These findings are similar to those of Zupi and associates.19
Consider the tool
Discomfort may be related to the size of the biopsy catheter. Pain scores appear to be significantly lower with the Pipelle biopsy catheter than with the larger Novak biopsy curette.32
Vasovagal reaction usually resolves after the procedure
As with hysteroscopy, women may occasionally experience a vasovagal reaction during endometrial biopsy. This complication usually resolves quickly once the procedure is completed.29 Some clinicians suggest that the patient be allowed to eat and drink before the procedure and be given an analgesic before it begins.28
Cervical priming is not a proven strategy
Misoprostol has been considered as a preprocedure adjunct to endometrial biopsy. Only one small randomized, controlled trial involving 42 women has evaluated the drug for this indication. It found no benefit when 400 μg of misoprostol was given orally 3 hours before the procedure, as well as cramping and increased pain during the biopsy.33 This study had several shortcomings, including its small sample size and the inclusion of both pre- and postmenopausal women. Further research is needed—separately in premenopausal and postmenopausal women and with adequately large samples—to assess the use of misoprostol.
The author reports no financial relationships relevant to this article.
CASE: Difficulty inserting a catheter suggests an unyielding cervix
A.W. is a 38-year-old nulliparous woman who seeks treatment for persistent irregular vaginal bleeding. Her physician attempts an endometrial biopsy in the office but is unable to pass the catheter through the internal cervical os. She schedules office hysteroscopy as follow-up.
What steps can the ObGyn take to reduce the difficulty of the procedure, particularly insertion of the hysteroscope through the cervical canal?
Successful hysteroscopy requires a cervical canal sufficiently dilated to allow passage of the hysteroscope. And because of inevitable variation in anatomy—and even in models of hysteroscopes, which range in diameter from 2.7 to 10 mm—passage is not always easily accomplished. Many of the complications related to hysteroscopy, including cervical tears, creation of a false passage, uterine perforation, vasovagal reaction, pain, and inability to complete the procedure, are caused by inadequate cervical dilation and an inability to insert the hysteroscope.1-6 One study noted that almost half of complications were related to cervical entry.6
In this article, I describe ways to overcome the challenging cervix for hysteroscopic procedures and endometrial biopsy (TABLES 1 and 2).
TABLE 1
10 actions that can ease entry to the cervix for hysteroscopy
| ACTION | COMMENTS |
|---|---|
| Take a careful history and perform a rigorous physical exam | Identify risk factors for cervical stenosis and assess cervical/uterine position |
| Administer an oral nonsteroidal anti-inflammatory drug 60 minutes before the procedure | Helps to reduce discomfort, especially postprocedure pain |
| Provide an anxiolytic or conscious sedation, or both | Consider this option for women who are very anxious or unlikely to tolerate pain, especially for operative procedures |
| Use a tenaculum | Consider if the uterus is not in the axial position |
| Use Hagar dilators or a lacrimal duct probe | May be helpful if mechanical dilation is necessary |
| Proceed under ultrasonographic guidance | Consider transabdominal imaging to help guide cervical dilation in difficult cases, e.g., when the patient has a history of uterine perforation |
| Opt for a smaller hysteroscope | A smaller scope will require less cervical dilation |
| Administer a paracervical block | Consider this option if cervical dilation is expected to be difficult, especially in women at risk of significant pain. Be alert for complications such as bleeding, discomfort at the time of injection, and intravascular injection leading to bradycardia and hypotension |
| Administer a topical cervical anesthetic | May be appropriate when a tenaculum is used |
| Give misoprostol to prime the cervix | Consider giving 400 μg of intravaginal misoprostol 9 to 12 hours preoperatively in premenopausal women, particularly nulliparous women and those undergoing operative hysteroscopy |
TABLE 2
6 ways to prepare the cervix for endometrial biopsy
| ACTION | COMMENTS |
|---|---|
| Take a careful history and perform a thorough physical examination | Identify risk factors for cervical stenosis and assess uterine position |
| Administer an oral nonsteroidal anti-inflammatory drug 60 minutes prior to biopsy | Helps to reduce discomfort, especially postprocedure pain |
| Use a tenaculum | May be helpful if the uterus/cervix is not in the axial position |
| Apply a topical cervical anesthetic | May help alleviate discomfort associated with use of a tenaculum |
| Use Hagar dilators or lacrimal duct probes | Provide mechanical dilation |
| Use the smallest biopsy catheter possible | Reduces degree of cervical dilation necessary |
Hysteroscopy failure rate: 3.4% to 4.2%
Hysteroscopy is, of course, common in gynecologic practice, its indications extending across a range of investigations and treatments—for menstrual disorders, postmenopausal bleeding, infertility, and recurrent pregnancy loss.1,7 Flexible hysteroscopes range in diameter from 2.7 to 5 mm; rigid hysteroscopes, from 1 to 5 mm; and operative hysteroscopes can be as large as 8 to 10 mm.2,7
A systematic review of diagnostic hysteroscopy in more than 26,000 women reported a failure rate of 4.2% for ambulatory hysteroscopy and 3.4% for inpatient procedures.4 Failed ambulatory procedures were mainly attributed to technical problems, including:
- cervical stenosis
- anatomic and structural abnormalities
- pain and intolerance.4
Ideally, hysteroscopy is performed with minimal or no cervical dilation,7 but this may not always be possible.
Things to consider before embarking
Close attention to cervical and uterine anatomy is critical because insertion of the hysteroscope can be the most difficult aspect of the procedure. A bimanual examination is imperative to assess uterine size and position. It also is useful to sound the uterus to determine its depth.
An accurate medical, gynecologic, and obstetric history is essential, including information on pregnancies, dilation and curettage, cervical procedures such as cryotherapy, and any other procedures that may increase the risk of cervical stenosis, or difficulty dilating the cervix.
Is stenosis present? Stenosis is most common in nulliparous and postmenopausal women and in those who have undergone cervical procedures such as cryotherapy. Stenosis increases the risk of laceration and uterine perforation.
Consider a mechanical dilator. When cervical dilation is difficult, a series of small Hagar or lacrimal duct dilators may be helpful (FIGURE).
FIGURE Mechanical dilation is one antidote to cervical stenosis
In challenging cases, such as cervical stenosis, mechanical dilation with a series of Hagar or lacrimal duct dilators may facilitate entry into the cervix.
Pain can be mild—or it can thwart your work
Although many women tolerate placement of a small hysteroscope without analgesia or anesthesia, pain and vasovagal reaction sometimes occur. Indeed, the level of pain experienced by the patient is a major determinant of the overall success of the procedure.3,8-10 Pain can occur when a tenaculum is used to grasp the anterior cervix, as well as during cervical dilation, injection of local anesthetic, or insertion of the hysteroscope. In some cases, a smaller scope may be all that is needed to solve the problem.11
Analgesia may not always be necessary
Some researchers have studied office hysteroscopy without analgesia or anesthesia, finding a high level of acceptance.12,13 Others have found a significant percentage of women requesting anesthesia or analgesia (16.5%)10 or requiring local anesthesia (28.8%).8
Preoperative NSAIDs may suffice. Use of oral nonsteroidal anti-inflammatory drugs (NSAIDs) 1 hour before office hysteroscopy may reduce intraoperative and postoperative pain.7 Nagele and colleagues8 compared use of mefenamic acid 1 hour before the procedure with placebo in 95 women undergoing outpatient diagnostic hysteroscopy. Mefenamic acid reduced pain at 30 and 60 minutes after—but not during—the procedure. Other studies have found that pain is reduced when an oral NSAID is taken 1 to 2 hours before insertion of an intrauterine device and before suction curettage.14,15
Other perioperative medications may help reduce discomfort and patient anxiety, including anxiolytics, such as lorazepam, analgesics, and conscious sedation.3
Paracervical block may be appropriate when pain is very likely
A number of investigators have evaluated use of paracervical anesthesia during out-patient hysteroscopy.9,13,16,17 They injected lignocaine or mepivacaine using a 21- or 22-gauge needle at 3, 5, 7, and 9 o’clock or 4 and 8 o’clock paracervically.13 One study found paracervical block to be effective in reducing the pain of tenaculum placement and insertion of the hysteroscope.17 However, some studies suggested a reduction of pain in postmenopausal women only.9 These women may be more likely to have cervical stenosis.
Paracervical block does pose a risk of complications. Studies have reported bleeding in some women16 and pain with injection of the paracervical block, as well as bradycardia and hypotension possibly secondary to intravascular injection.17
Other methods are inconsistent
Intracervical injection. Some researchers have recommended injection of local anesthetic into the cervix.13 One study found no benefit—in fact, the injection appeared to be the most painful part of the procedure.18 A case series suggested that injection of local anesthetic may be effective, but the series lacked a placebo or control arm.13
Topical intrauterine anesthetic has been investigated after administration through the channel of the hysteroscope or by a catheter passed through the cervix into the uterine cavity.13 Findings have been mixed, with some researchers demonstrating reduced pain19,20 and others showing no relief.21
Topical cervical anesthesia. Some hysteroscopists have recommended application of anesthetic cream, gel, or spray directly to the cervix immediately before the procedure.13,22 The results have been mixed, with some studies noting decreased pain overall,13 one finding decreased pain only during tenaculum placement,22 and others finding no significant reduction in pain any time during the procedure.13,23,24 A review concluded that topical cervical lignocaine spray may reduce the discomfort of tenaculum placement.13
Topical anesthesia may minimize vasovagal reaction
In one study, 1.1% of women undergoing office hysteroscopy experienced a vasovagal reaction, caused by stimulation of the parasympathetic nervous system with cervical manipulation and passage of the scope through the internal os of the cervix.25 The reaction led to hypotension and bradycardia. Several studies have suggested that a local anesthetic can reduce this complication.19,20
Cicinelli and associates found that topical local anesthesia reduced the incidence of vasovagal reaction from 32.5% in the control arm to 5%.20 They suggest that a local anesthetic be considered in selected women, such as postmenopausal patients, who are at increased risk of vasovagal attack.
In contrast, Lau and associates17 found an increased rate of bradycardia and hypotension with paracervical lignocaine (31% versus 10%), but it may have been caused by inadvertent intravascular injection.17
Researchers have also suggested that the use of smaller hysteroscopes may reduce the incidence of vasovagal reactions.26
How to prime the cervix for hysteroscopy
The use of vaginal misoprostol, a prostaglandin E1 analogue, 9 to 12 hours before hysteroscopy may help increase preprocedural cervical dilation in premenopausal women, especially in nulliparas and women undergoing operative hysteroscopy. Misoprostol, used to prevent and treat NSAID-induced gastric ulcers, is gaining favor as a cervical ripening agent. We performed a meta-analysis to assess its effectiveness in dilating the cervix and reducing the need for mechanical dilation.5
We identified 10 studies that met inclusion criteria; five of them included premenopausal women, four included postmenopausal women or women receiving a gonadotropin-releasing hormone (GnRH) agonist, and one study included both groups.5 A variety of dosing protocols were used, with dosages ranging from 100 μg to 1,000 μg of intravaginal or oral misoprostol 4 to 24 hours preoperatively (most studies evaluated the vaginal route).
We found that misoprostol significantly reduced the need for further cervical dilation, and was associated with a lower rate of cervical laceration. However, this was true only for the premenopausal group: 42.6% of premenopausal women given misoprostol needed further dilation, compared with 71.7% in the control group, and 2% of premenopausal women given misoprostol suffered cervical laceration, compared with 11% in the control group. Among postmenopausal women and those receiving a GnRH agonist, misoprostol lacked clear benefit and was associated with side effects such as nausea, diarrhea, abdominal cramping, and fever.
For every premenopausal woman who received misoprostol before hysteroscopy, one woman avoided the need for further cervical dilation. For every 12 premenopausal women receiving misoprostol, one cervical laceration was avoided.
The ideal dosing regimen could not be determined because of variations in protocols. Nor was it clear whether misoprostol had any benefit among postmenopausal women or those receiving a GnRH agonist.
Most studies of misoprostol for cervical ripening have involved intravaginal administration, with dosages of 200 μg to 400 μg given 9 to 12 hours before hysteroscopy showing the greatest benefit.
Ultrasonography may help guide dilation
Transabdominal ultrasonography has been used to guide dilation in difficult dilation and curettage procedures, and is especially useful in women with a history of uterine perforation.27 It may be helpful in cases involving difficult cervical dilation during hysteroscopy or endometrial biopsy.
Steady the cervix. A tenaculum is not always required, but its use on the anterior lip of the cervix may help steady the cervix and provide countertraction during insertion of the hysteroscope through the cervical canal, especially if the cervix is not in an axial position.7
CASE Resolved!
Because she is nulliparous and may benefit from cervical priming, the patient is given 400 μg of intravaginal misoprostol 12 hours before hysteroscopy, as well as an oral NSAID 1 hour before the procedure. A bimanual examination reveals a sharply anteverted uterus, so a topical cervical anesthetic spray is applied to the anterior cervix, and a tenaculum is placed to help straighten the uterine position. The hysteroscope passes easily through the cervical canal, making further dilation unnecessary. The procedure is completed without difficulty and is well tolerated by the patient.
Difficult entry can also hamper endometrial biopsy
Every ObGyn has used endometrial biopsy to assess abnormal uterine bleeding, postmenopausal bleeding, infertility, or recurrent pregnancy loss, or to monitor women on hormone replacement therapy28,29 —so its advantages over dilation and curettage should come as no surprise. They include the ability to perform it in an office setting, usually with minimal cervical dilation, often without anesthesia, and at less expense.28 Complications include cramping and pain,29-32 vasovagal reaction,29 bleeding,29 and inability to pass the biopsy catheter through the cervix into the uterine cavity. Another rare complication is uterine perforation.29
As with hysteroscopy, many of these complications are related to difficulty entering the uterine cavity through the cervix.
Prerequisites include thorough assessment of the uterus
As with hysteroscopy, an accurate and detailed history is necessary to identify risk factors for a difficult procedure. Assess uterine size and position with a bimanual examination. Although a tenaculum is often unnecessary, its placement on the anterior lip of the cervix may help steady the cervix and allow the catheter to pass through the cervical canal into the uterine cavity, especially if the uterus is not in the axial position.28,29 Again, it is useful to sound the uterine cavity to ascertain its depth. This may be done with the biopsy catheter.
Cervical dilation may be necessary
Even when women with cervical stenosis were excluded in one study, it was difficult to pass the Pipelle endometrial biopsy through the cervix in 41.7% of women.30
If the sampling device does not pass easily through the cervix, use a tenaculum and a lacrimal duct probe or small Hagar dilators to dilate the cervix.28
Pain may again be an issue
Almost 50% of women experience moderate or severe pain during endometrial biopsy.32 Many clinicians recommend giving an oral NSAID 60 minutes before the procedure to decrease discomfort. One study found that the use of naproxen sodium before Vabra curettage reduced the severity of pain at 30 and 60 minutes after the procedure, but did not alleviate discomfort arising during the biopsy itself.14 Another study suggested the combination of naproxen sodium and intrauterine lidocaine (5 mL of 2% lidocaine) to reduce discomfort associated with the procedure.30
Use of anesthesia is controversial
A study by Lau and colleagues17 found paracervical lignocaine to be ineffective at reducing pain during hysteroscopy and endometrial biopsy, but the drug did increase the risk of bradycardia and hypotension. Another study demonstrated a decrease in procedure-related discomfort in postmenopausal women who were given 2 mL of 2% intrauterine mepivacaine.20 These findings are similar to those of Zupi and associates.19
Consider the tool
Discomfort may be related to the size of the biopsy catheter. Pain scores appear to be significantly lower with the Pipelle biopsy catheter than with the larger Novak biopsy curette.32
Vasovagal reaction usually resolves after the procedure
As with hysteroscopy, women may occasionally experience a vasovagal reaction during endometrial biopsy. This complication usually resolves quickly once the procedure is completed.29 Some clinicians suggest that the patient be allowed to eat and drink before the procedure and be given an analgesic before it begins.28
Cervical priming is not a proven strategy
Misoprostol has been considered as a preprocedure adjunct to endometrial biopsy. Only one small randomized, controlled trial involving 42 women has evaluated the drug for this indication. It found no benefit when 400 μg of misoprostol was given orally 3 hours before the procedure, as well as cramping and increased pain during the biopsy.33 This study had several shortcomings, including its small sample size and the inclusion of both pre- and postmenopausal women. Further research is needed—separately in premenopausal and postmenopausal women and with adequately large samples—to assess the use of misoprostol.
The author reports no financial relationships relevant to this article.
1. Bradley LD. Complications in hysteroscopy: prevention, treatment and legal risk. Curr Opin Obstet Gynecol. 2002;14:409-415.
2. American College of Obstetricians and Gynecologists. ACOG technology assessment in obstetrics and gynecology, number 4, August 2005: hysteroscopy. Obstet Gynecol. 2005;106:439-442.
3. Vilos GA, Abu-Rafea B. New developments in ambulatory hysteroscopic surgery. Best Pract Res Clin Obstet Gynaecol. 2005;19:727-742.
4. Clark TJ, Voit D, Gupta JK, Hyde C, Song F, Khan KS. Accuracy of hysteroscopy in the diagnosis of endometrial cancer and hyperplasia: a systematic quantitative review. JAMA. 2002;288:1610-1621.
5. Crane JM, Healey S. Use of misoprostol before hysteroscopy: a systematic review. J Obstet Gynaecol Can. 2006;28:373-379.
6. Jansen FW, Vredevoogd CB, van Ulzen K, Hermans J, Trimbos JB, Trimbos-Kemper TC. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol. 2000;96:266-270.
7. Guido R, Stovall D. Hysteroscopy Version 14.3. UpToDate [cited February 15, 2007]; Available from: www.uptodate.com.
8. Nagele F, Lockwood G, Magos AL. Randomised placebo controlled trial of mefenamic acid for premedication at outpatient hysteroscopy: a pilot study. Br J Obstet Gynaecol. 1997;104:842-844.
9. Cicinelli E, Didonna T, Schonauer LM, Stragapede S, Falco N, Pansini N. Paracervical anesthesia for hysteroscopy and endometrial biopsy in postmenopausal women. A randomized, double-blind, placebo-controlled study. J Reprod Med. 1998;43:1014-1018.
10. De Iaco P, Marabini A, Stefanetti M, Del Vecchio C, Bovicelli L. Acceptability and pain of outpatient hysteroscopy. J Am Assoc Gynecol Laparosc. 2000;7:71-75.
11. Marsh F, Jackson T, Duffy S. A case controlled study comparing 3.6 mm and 3.1 mm flexible hysteroscopes. Gynaecol Endosc. 2002;11:393-396.
12. Lau WC, Ho RY, Tsang MK, Yuen PM. Patient’s acceptance of outpatient hysteroscopy. Gynecol Obstet Invest. 1999;47:191-193.
13. Hassan L, Gannon MJ. Anaesthesia and analgesia for ambulatory hysteroscopic surgery. Best Pract Res Clin Obstet Gynaecol. 2005;19:681-691.
14. Siddle NC, Young O, Sledmere CM, Reading AE, Whitehead MI. A controlled trial of naproxen sodium for relief of pain associated with Vabra suction curettage. Br J Obstet Gynaecol. 1983;90:864-869.
15. Edgren RA, Morton CJ. Naproxen sodium for Ob/Gyn use, with special reference to pain states: a review. Int J Fertil. 1986;31:135-142.
16. Giorda G, Scarabelli C, Franceschi S, Campagnutta E. Feasibility and pain control in outpatient hysteroscopy in postmenopausal women: a randomized trial. Acta Obstet Gynecol Scand. 2000;79:593-597.
17. Lau WC, Lo WK, Tam WH, Yuen PM. Paracervical anaesthesia in outpatient hysteroscopy: a randomised double-blind placebo-controlled trial. Br J Obstet Gynaecol. 1999;106:356-359.
18. Broadbent JA, Hill NC, Molnar BG, Rolfe KJ, Magos AL. Randomized placebo controlled trial to assess the role of intracervical lignocaine in outpatient hysteroscopy. Br J Obstet Gynaecol. 1992;99:777-779.
19. Zupi E, Luciano AA, Valli E, Marconi D, Maneschi F, Romanini C. The use of topical anesthesia in diagnostic hysteroscopy and endometrial biopsy. Fertil Steril. 1995;63:414-416.
20. Cicinelli E, Didonna T, Ambrosi G, Schonauer LM, Fiore G, Matteo MG. Topical anaesthesia for diagnostic hysteroscopy and endometrial biopsy in postmenopausal women: a randomised placebo-controlled double-blind study. Br J Obstet Gynaecol. 1997;104:316-319.
21. Lau WC, Tam WH, Lo WK, Yuen PM. A randomised double-blind placebo-controlled trial of transcervical intrauterine local anaesthesia in outpatient hysteroscopy. BJOG. 2000;107:610-613.
22. Davies A, Richardson RE, O’Connor H, Baskett TF, Nagele F, Magos AL. Lignocaine aerosol spray in outpatient hysteroscopy: a randomized double-blind placebo-controlled trial. Fertil Steril. 1997;67:1019-1023.
23. Clark S, Vonau B, Macdonald R. Topical anaesthesia in out-patient hysteroscopy. Gynaecol Endosc. 1996;5:141-144.
24. Wong AY, Wong K, Tang LC. Stepwise pain score analysis of the effect of local lignocaine on outpatient hysteroscopy: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2000;73:1234-1237.
25. Bellingham FR. Outpatient hysteroscopy—problems. Aust N Z J Obstet Gynaecol. 1997;37:202-205.
26. Cicinelli E, Schonauer LM, Barba B, Tartagni M, Luisi D, Di Naro E. Tolerability and cardiovascular complications of outpatient diagnostic minihysteroscopy compared with conventional hysteroscopy. J Am Assoc Gynecol Laparosc. 2003;10:399-402.
27. Hunter RE, Reuter K, Kopin E. Use of ultrasonography in the difficult postmenopausal dilation and curettage. Obstet Gynecol. 1989;73:813-816.
28. Guido R, Stovall D. Endometrial sampling procedures Version 14.3. UpToDate [cited February 15, 2007]; Available from: www.uptodate.com.
29. Cooper JM, Erickson ML. Endometrial sampling techniques in the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:235-244.
30. Dogan E, Celiloglu M, Sarihan E, Demir A. Anesthetic effect of intrauterine lidocaine plus naproxen sodium in endometrial biopsy. Obstet Gynecol. 2004;103:347-351.
31. Trolice MP, Fishburne C, Jr, McGrady S. Anesthetic efficacy of intrauterine lidocaine for endometrial biopsy: a randomized double-masked trial. Obstet Gynecol. 2000;95:345-347.
32. Silver MM, Miles P, Rosa C. Comparison of Novak and Pipelle endometrial biopsy instruments. Obstet Gynecol. 1991;78:828-830.
33. Perrone JF, Caldito G, Mailhes JB, Tucker AN, Ford WR, London SN. Oral misoprostol before office endometrial biopsy. Obstet Gynecol. 2002;99:439-444.
1. Bradley LD. Complications in hysteroscopy: prevention, treatment and legal risk. Curr Opin Obstet Gynecol. 2002;14:409-415.
2. American College of Obstetricians and Gynecologists. ACOG technology assessment in obstetrics and gynecology, number 4, August 2005: hysteroscopy. Obstet Gynecol. 2005;106:439-442.
3. Vilos GA, Abu-Rafea B. New developments in ambulatory hysteroscopic surgery. Best Pract Res Clin Obstet Gynaecol. 2005;19:727-742.
4. Clark TJ, Voit D, Gupta JK, Hyde C, Song F, Khan KS. Accuracy of hysteroscopy in the diagnosis of endometrial cancer and hyperplasia: a systematic quantitative review. JAMA. 2002;288:1610-1621.
5. Crane JM, Healey S. Use of misoprostol before hysteroscopy: a systematic review. J Obstet Gynaecol Can. 2006;28:373-379.
6. Jansen FW, Vredevoogd CB, van Ulzen K, Hermans J, Trimbos JB, Trimbos-Kemper TC. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol. 2000;96:266-270.
7. Guido R, Stovall D. Hysteroscopy Version 14.3. UpToDate [cited February 15, 2007]; Available from: www.uptodate.com.
8. Nagele F, Lockwood G, Magos AL. Randomised placebo controlled trial of mefenamic acid for premedication at outpatient hysteroscopy: a pilot study. Br J Obstet Gynaecol. 1997;104:842-844.
9. Cicinelli E, Didonna T, Schonauer LM, Stragapede S, Falco N, Pansini N. Paracervical anesthesia for hysteroscopy and endometrial biopsy in postmenopausal women. A randomized, double-blind, placebo-controlled study. J Reprod Med. 1998;43:1014-1018.
10. De Iaco P, Marabini A, Stefanetti M, Del Vecchio C, Bovicelli L. Acceptability and pain of outpatient hysteroscopy. J Am Assoc Gynecol Laparosc. 2000;7:71-75.
11. Marsh F, Jackson T, Duffy S. A case controlled study comparing 3.6 mm and 3.1 mm flexible hysteroscopes. Gynaecol Endosc. 2002;11:393-396.
12. Lau WC, Ho RY, Tsang MK, Yuen PM. Patient’s acceptance of outpatient hysteroscopy. Gynecol Obstet Invest. 1999;47:191-193.
13. Hassan L, Gannon MJ. Anaesthesia and analgesia for ambulatory hysteroscopic surgery. Best Pract Res Clin Obstet Gynaecol. 2005;19:681-691.
14. Siddle NC, Young O, Sledmere CM, Reading AE, Whitehead MI. A controlled trial of naproxen sodium for relief of pain associated with Vabra suction curettage. Br J Obstet Gynaecol. 1983;90:864-869.
15. Edgren RA, Morton CJ. Naproxen sodium for Ob/Gyn use, with special reference to pain states: a review. Int J Fertil. 1986;31:135-142.
16. Giorda G, Scarabelli C, Franceschi S, Campagnutta E. Feasibility and pain control in outpatient hysteroscopy in postmenopausal women: a randomized trial. Acta Obstet Gynecol Scand. 2000;79:593-597.
17. Lau WC, Lo WK, Tam WH, Yuen PM. Paracervical anaesthesia in outpatient hysteroscopy: a randomised double-blind placebo-controlled trial. Br J Obstet Gynaecol. 1999;106:356-359.
18. Broadbent JA, Hill NC, Molnar BG, Rolfe KJ, Magos AL. Randomized placebo controlled trial to assess the role of intracervical lignocaine in outpatient hysteroscopy. Br J Obstet Gynaecol. 1992;99:777-779.
19. Zupi E, Luciano AA, Valli E, Marconi D, Maneschi F, Romanini C. The use of topical anesthesia in diagnostic hysteroscopy and endometrial biopsy. Fertil Steril. 1995;63:414-416.
20. Cicinelli E, Didonna T, Ambrosi G, Schonauer LM, Fiore G, Matteo MG. Topical anaesthesia for diagnostic hysteroscopy and endometrial biopsy in postmenopausal women: a randomised placebo-controlled double-blind study. Br J Obstet Gynaecol. 1997;104:316-319.
21. Lau WC, Tam WH, Lo WK, Yuen PM. A randomised double-blind placebo-controlled trial of transcervical intrauterine local anaesthesia in outpatient hysteroscopy. BJOG. 2000;107:610-613.
22. Davies A, Richardson RE, O’Connor H, Baskett TF, Nagele F, Magos AL. Lignocaine aerosol spray in outpatient hysteroscopy: a randomized double-blind placebo-controlled trial. Fertil Steril. 1997;67:1019-1023.
23. Clark S, Vonau B, Macdonald R. Topical anaesthesia in out-patient hysteroscopy. Gynaecol Endosc. 1996;5:141-144.
24. Wong AY, Wong K, Tang LC. Stepwise pain score analysis of the effect of local lignocaine on outpatient hysteroscopy: a randomized, double-blind, placebo-controlled trial. Fertil Steril. 2000;73:1234-1237.
25. Bellingham FR. Outpatient hysteroscopy—problems. Aust N Z J Obstet Gynaecol. 1997;37:202-205.
26. Cicinelli E, Schonauer LM, Barba B, Tartagni M, Luisi D, Di Naro E. Tolerability and cardiovascular complications of outpatient diagnostic minihysteroscopy compared with conventional hysteroscopy. J Am Assoc Gynecol Laparosc. 2003;10:399-402.
27. Hunter RE, Reuter K, Kopin E. Use of ultrasonography in the difficult postmenopausal dilation and curettage. Obstet Gynecol. 1989;73:813-816.
28. Guido R, Stovall D. Endometrial sampling procedures Version 14.3. UpToDate [cited February 15, 2007]; Available from: www.uptodate.com.
29. Cooper JM, Erickson ML. Endometrial sampling techniques in the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000;27:235-244.
30. Dogan E, Celiloglu M, Sarihan E, Demir A. Anesthetic effect of intrauterine lidocaine plus naproxen sodium in endometrial biopsy. Obstet Gynecol. 2004;103:347-351.
31. Trolice MP, Fishburne C, Jr, McGrady S. Anesthetic efficacy of intrauterine lidocaine for endometrial biopsy: a randomized double-masked trial. Obstet Gynecol. 2000;95:345-347.
32. Silver MM, Miles P, Rosa C. Comparison of Novak and Pipelle endometrial biopsy instruments. Obstet Gynecol. 1991;78:828-830.
33. Perrone JF, Caldito G, Mailhes JB, Tucker AN, Ford WR, London SN. Oral misoprostol before office endometrial biopsy. Obstet Gynecol. 2002;99:439-444.