User login
Be an activist to prevent edentulism among the mentally ill
Poor dental hygiene is a serious and prevalent problem among people with mental illness or cognitive impairment: Dental caries and periodontal disease are 3.4 times more common among the mentally ill than among the general population.1 Little has been published on the causes and prevention of these diseases among the mentally ill, however. Interprofessional education provides the opportunity to reinforce the connection between oral health and systemic health.
Untreated dental disease can result in edentulism (partial or complete tooth loss). Often, this condition leads to embarrassment, poor self-image, and social isolation—all of which can exacerbate the psychotic state and its symptoms. Working with your patient to improve oral health can, in turn, lead to better mental and physical health.
CASE REPORT
Edentulism in a man with schizophrenia
A 34-year-old man, given a diagnosis of schizophrenia at age 17, is admitted to the inpatient psychiatry unit for bizarre behavior. The next day, 4 maxillary and incisor teeth fall out suddenly while he is brushing his teeth. The patient is brought to emergency dental services.
Factors contributing to his tooth loss include:
- schizophrenia
- neglected oral hygiene
- adverse effects of antipsychotic medication
- lack of advice on the importance of oral hygiene
- failure to recognize signs of a dental problem.
What else can lead to edentulism?
Breakdown of the periodontal attachment2 also can be caused by disinterest in oral hygiene practices; craving of, and preference for, carbohydrates because of reduced central serotonin activity3,4; and xerostomia.
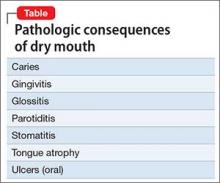
Xerostomia, or dry mouth, caused by psychotropic agents and an altered immune response, facilitates growth of pathogenic bacteria and can lead to several dental diseases (Table). These conditions are exacerbated by consumption of chewing gum, sweets, and sugary drinks in response to constantly feeling thirsty from xerostomia. Advise patients to take frequent sips of fluid or let ice cubes melt in their mouth.
Bruxism. Patients taking a selective serotonin reuptake inhibitor or an atypical antipsychotic can develop a movement disorder (eg, extrapyramidal symptoms or tardive dyskinesia) that includes clenching, grinding of the teeth (bruxism), or both, which can worsen their periodontal condition.
Lack of skills, physical dexterity, and motivation to maintain good oral hygiene are common among people with mental illness. Most patients visit a dentist only when they experience a serious oral problem or an emergency (ie, trauma). Many dentists treat psychiatric patients by extracting the tooth that is causing the pain, instead of pursuing complex tooth preservation or restoration techniques because of (1) the extent of the disease, (2) lack of knowledge related to psychiatric illnesses, and (3) frequent and timely follow-ups.5
Providing education about oral health to patients, implementing preventive steps, and educating other medical specialities about the link between oral health and systemic health can help to reduce the burden of dental problems among mentally ill patients.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products
1. Persson K, Axtelius B, Söderfeldt B, et al. Oral health-related quality of life and dental status in an outpatient psychiatric population: a multivariate approach. Int J Ment Health Nurs. 2010;19(1):62-70.
2. Lalloo R, Kisely S, Amarasinghe H, et al. Oral health of patients on psychotropic medications: a study of outpatients in Queensland. Australas Psychiatry. 2013;21(4):338-342.
3. O’Neil A, Berk M, Venugopal K, et al. The association between poor dental health and depression: findings from a large-scale, population-based study (the NHANES study). Gen Hosp Psychiatry. 2014;36(3):266-270.
4. Kisely S, Quek LH, Paris J, et al. Advanced dental disease in people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry. 2011;199(3):187-193.
5. Arnaiz A, Zumárraga M, Díez-Altuna I, et al. Oral health and the symptoms of schizophrenia. Psychiatry Res. 2011;188(1):24-28.
Poor dental hygiene is a serious and prevalent problem among people with mental illness or cognitive impairment: Dental caries and periodontal disease are 3.4 times more common among the mentally ill than among the general population.1 Little has been published on the causes and prevention of these diseases among the mentally ill, however. Interprofessional education provides the opportunity to reinforce the connection between oral health and systemic health.
Untreated dental disease can result in edentulism (partial or complete tooth loss). Often, this condition leads to embarrassment, poor self-image, and social isolation—all of which can exacerbate the psychotic state and its symptoms. Working with your patient to improve oral health can, in turn, lead to better mental and physical health.
CASE REPORT
Edentulism in a man with schizophrenia
A 34-year-old man, given a diagnosis of schizophrenia at age 17, is admitted to the inpatient psychiatry unit for bizarre behavior. The next day, 4 maxillary and incisor teeth fall out suddenly while he is brushing his teeth. The patient is brought to emergency dental services.
Factors contributing to his tooth loss include:
- schizophrenia
- neglected oral hygiene
- adverse effects of antipsychotic medication
- lack of advice on the importance of oral hygiene
- failure to recognize signs of a dental problem.
What else can lead to edentulism?
Breakdown of the periodontal attachment2 also can be caused by disinterest in oral hygiene practices; craving of, and preference for, carbohydrates because of reduced central serotonin activity3,4; and xerostomia.
Xerostomia, or dry mouth, caused by psychotropic agents and an altered immune response, facilitates growth of pathogenic bacteria and can lead to several dental diseases (Table). These conditions are exacerbated by consumption of chewing gum, sweets, and sugary drinks in response to constantly feeling thirsty from xerostomia. Advise patients to take frequent sips of fluid or let ice cubes melt in their mouth.
Bruxism. Patients taking a selective serotonin reuptake inhibitor or an atypical antipsychotic can develop a movement disorder (eg, extrapyramidal symptoms or tardive dyskinesia) that includes clenching, grinding of the teeth (bruxism), or both, which can worsen their periodontal condition.
Lack of skills, physical dexterity, and motivation to maintain good oral hygiene are common among people with mental illness. Most patients visit a dentist only when they experience a serious oral problem or an emergency (ie, trauma). Many dentists treat psychiatric patients by extracting the tooth that is causing the pain, instead of pursuing complex tooth preservation or restoration techniques because of (1) the extent of the disease, (2) lack of knowledge related to psychiatric illnesses, and (3) frequent and timely follow-ups.5
Providing education about oral health to patients, implementing preventive steps, and educating other medical specialities about the link between oral health and systemic health can help to reduce the burden of dental problems among mentally ill patients.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products
Poor dental hygiene is a serious and prevalent problem among people with mental illness or cognitive impairment: Dental caries and periodontal disease are 3.4 times more common among the mentally ill than among the general population.1 Little has been published on the causes and prevention of these diseases among the mentally ill, however. Interprofessional education provides the opportunity to reinforce the connection between oral health and systemic health.
Untreated dental disease can result in edentulism (partial or complete tooth loss). Often, this condition leads to embarrassment, poor self-image, and social isolation—all of which can exacerbate the psychotic state and its symptoms. Working with your patient to improve oral health can, in turn, lead to better mental and physical health.
CASE REPORT
Edentulism in a man with schizophrenia
A 34-year-old man, given a diagnosis of schizophrenia at age 17, is admitted to the inpatient psychiatry unit for bizarre behavior. The next day, 4 maxillary and incisor teeth fall out suddenly while he is brushing his teeth. The patient is brought to emergency dental services.
Factors contributing to his tooth loss include:
- schizophrenia
- neglected oral hygiene
- adverse effects of antipsychotic medication
- lack of advice on the importance of oral hygiene
- failure to recognize signs of a dental problem.
What else can lead to edentulism?
Breakdown of the periodontal attachment2 also can be caused by disinterest in oral hygiene practices; craving of, and preference for, carbohydrates because of reduced central serotonin activity3,4; and xerostomia.
Xerostomia, or dry mouth, caused by psychotropic agents and an altered immune response, facilitates growth of pathogenic bacteria and can lead to several dental diseases (Table). These conditions are exacerbated by consumption of chewing gum, sweets, and sugary drinks in response to constantly feeling thirsty from xerostomia. Advise patients to take frequent sips of fluid or let ice cubes melt in their mouth.
Bruxism. Patients taking a selective serotonin reuptake inhibitor or an atypical antipsychotic can develop a movement disorder (eg, extrapyramidal symptoms or tardive dyskinesia) that includes clenching, grinding of the teeth (bruxism), or both, which can worsen their periodontal condition.
Lack of skills, physical dexterity, and motivation to maintain good oral hygiene are common among people with mental illness. Most patients visit a dentist only when they experience a serious oral problem or an emergency (ie, trauma). Many dentists treat psychiatric patients by extracting the tooth that is causing the pain, instead of pursuing complex tooth preservation or restoration techniques because of (1) the extent of the disease, (2) lack of knowledge related to psychiatric illnesses, and (3) frequent and timely follow-ups.5
Providing education about oral health to patients, implementing preventive steps, and educating other medical specialities about the link between oral health and systemic health can help to reduce the burden of dental problems among mentally ill patients.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products
1. Persson K, Axtelius B, Söderfeldt B, et al. Oral health-related quality of life and dental status in an outpatient psychiatric population: a multivariate approach. Int J Ment Health Nurs. 2010;19(1):62-70.
2. Lalloo R, Kisely S, Amarasinghe H, et al. Oral health of patients on psychotropic medications: a study of outpatients in Queensland. Australas Psychiatry. 2013;21(4):338-342.
3. O’Neil A, Berk M, Venugopal K, et al. The association between poor dental health and depression: findings from a large-scale, population-based study (the NHANES study). Gen Hosp Psychiatry. 2014;36(3):266-270.
4. Kisely S, Quek LH, Paris J, et al. Advanced dental disease in people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry. 2011;199(3):187-193.
5. Arnaiz A, Zumárraga M, Díez-Altuna I, et al. Oral health and the symptoms of schizophrenia. Psychiatry Res. 2011;188(1):24-28.
1. Persson K, Axtelius B, Söderfeldt B, et al. Oral health-related quality of life and dental status in an outpatient psychiatric population: a multivariate approach. Int J Ment Health Nurs. 2010;19(1):62-70.
2. Lalloo R, Kisely S, Amarasinghe H, et al. Oral health of patients on psychotropic medications: a study of outpatients in Queensland. Australas Psychiatry. 2013;21(4):338-342.
3. O’Neil A, Berk M, Venugopal K, et al. The association between poor dental health and depression: findings from a large-scale, population-based study (the NHANES study). Gen Hosp Psychiatry. 2014;36(3):266-270.
4. Kisely S, Quek LH, Paris J, et al. Advanced dental disease in people with severe mental illness: systematic review and meta-analysis. Br J Psychiatry. 2011;199(3):187-193.
5. Arnaiz A, Zumárraga M, Díez-Altuna I, et al. Oral health and the symptoms of schizophrenia. Psychiatry Res. 2011;188(1):24-28.
Zolpidem may cause visual distortions and other psychotic symptoms
Zolpidem, an imidazopyridine hypnotic, has been used as an alternative to benzodiazepines for treating short-term insomnia because it has a relatively favorable side effect profile and less potential for abuse.1
However, several cases of zolpidemrelated psychotic symptoms have been reported,2 including a report of an association between zolpidem and hallucinations.1 The case illustrated here describes distortion of visual perception that can occur after ingestion of more than the recommended dosage of zolpidem.
CASE: Terrified and paranoid with distorted vision
Ms. K, age 33, an English-speaking woman from Portugal with a history of schizoaffective disorder, is brought to the emergency department in a terrified state. She describes visual distortion and paranoia because she fears losing her vision. She complains of suicidal ideation, depressed mood, insomnia, auditory
hallucinations, and distortion of visual perception. She reports seeing shadows, recurring movements of the ceiling bearing down on her, and molding or melting walls.
Ms. K reports that the visual distortions began when she started zolpidem, 10 mg/d, 2 months earlier, after her mother in Portugal gave it to her.
Ms. K describes feeling disoriented and disconnected from reality. She reports taking extra doses of zolpidem (40 mg)—recommended maximum dosage is 10 mg3—and clonazepam (1 mg) to address her insomnia.
On examination, Ms. K appears shaky and tremulous, and we note that her eyeballs roll upward. Vital signs are within normal limits, and she is awake, alert, and oriented to person, place, and time.
We diagnose exacerbation of schizoaffective disorder.
Ms. K is admitted to the inpatient psychiatric unit for observation and treatment. Quetiapine, 100 mg at bedtime, and sertraline, 100 mg/d, are started and zolpidem is discontinued.
The morning after admission, Ms. K reports that her vision has improved and that she no longer sees shadows or colored spots. All visual distortions resolve within 1 day after discontinuing zolpidem.
Discussion
Ms. K had no history of ophthalmologic or neurologic disease or alcohol or substance use. Physical and neurologic examination and laboratory testing failed to reveal any abnormality that accounts for her clinical presentation. Also, she reported visual distortions in the absence of drowsiness,4 thereby ruling out hypnagogic hallucination.5
Visual hallucinations are uncommon in schizoaffective disorder, but have been shown to occur with zolpidem use.1,4 The clinical manifestations in Ms. K’s case are
consistent with those of 3 reports, in which patients reported visual symptoms shortly after an initial dose of zolpidem, 10 mg.1,6 In those cases, symptoms resolved soon after zolpidem was discontinued.
Ms. K’s case is similar to other reports in regard to time of onset and manifestations of visual distortions. Iruela and colleagues documented that hallucinations could be reproduced with a lower challenge dose of zolpidem (5 or 2.5 mg), and that, with such a challenge, symptoms should be less severe.7 How zolpidem induces visual hallucinations remains unknown. Several studies have looked at variables that might predispose a patient taking zolpidem to visual
perceptual distortions.1
Sex might play an important role.1,7 After administering the same dosage of zolpidem, women age 20 to 40 had a blood concentration of the drug that was, on average, 45% higher than those measured in men.6 This difference in serum concentration is more remarkable in older women; the blood concentration of the drug in women age >60 was 63% higher than that of men.
Influence of hormones. Pharmacokinetics of zolpidem seem to be related to endocrine factors associated with cytochrome P450 (CYP) 3A4 metabolism. A low plasma concentration of free testosterone may contribute to lower CYP3A activity, with women achieving as much as a 50% higher plasma level of zolpidem, whereas exposure to testosterone activates biotransformation via CYP3A.8
Body weight is important when dosing zolpidem. Zolpidem-induced macropsia
has been reported in women with anorexia.7 The protein-binding capacity of zolpidem is approximately 92%, mainly to albumin (66%) and α1-acid glycoprotein (56.6%).4 In malnourished patients with anorexia nervosa, hypoalbuminemia is common and, therefore, unbound drug concentration is higher. This effect could account for, or contribute to, zolpidem toxicity.7
Ms. K did not have anorexia nervosa and was not underweight, and her liver function and kidney function were within normal limits. However, prescribing guidelines call for an initial dosage of 5 mg/d for women (10 mg/d for men)3; Ms. K ingested 8 times the recommended daily dosage.
The lesson for practitioners? When you encounter a patient who has a psychiatric disorder with psychotic features, explore causes unrelated to their primary disorder—such as taking a hypnotic or multiple psychoactive medications—as a possible source of the presenting symptoms (Box). In Ms. K’s case, her unusual symptoms resolved after a change in pharmacotherapy, leading us to conclude that her visual distortions likely were secondary to zolpidem and not to her schizoaffective disorder—a confounding factor.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Huang CL, Chang CJ, Hung CF, et al. Zolpidem-induced distortion in visual perception. Ann Pharmacother. 2003;37(5):683-686.
2. Ansseau M, Pitchot W, Hansenne M, et al. Psychotic reaction to zolpidem (letter). Lancet. 1992;339(8796):809.
3. Ambien [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2013.
4. van Puijenbroek EP, Egberts AC, Krom HJ. Visual hallucinations and amnesia associated with the use of zolpidem (letter). Int J Clin Pharmacol Ther. 1996;34(7):318.
5. Morselli PL. Zolpidem side-effects (letter). Lancet. 1993; 342(8875):868-869.
6. Salvà P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications. Clin Pharmacokinet. 1995;29(3):142-153.
7. Iruela LM, Ibañez-Rojo V, Baca E. Zolpidem-induced macropsia in anorexic woman (letter). Lancet. 1993; 342(8868):443-444.
8. Cubała WJ, Wiglusz M, Burkiewicz A, et al. Zolpidem pharmacokinetics and pharmacodynamics in metabolic interactions involving CYP3A: sex as a differentiating factor. Eur J Clin Pharmacol. 2010;66(9):955.
Zolpidem, an imidazopyridine hypnotic, has been used as an alternative to benzodiazepines for treating short-term insomnia because it has a relatively favorable side effect profile and less potential for abuse.1
However, several cases of zolpidemrelated psychotic symptoms have been reported,2 including a report of an association between zolpidem and hallucinations.1 The case illustrated here describes distortion of visual perception that can occur after ingestion of more than the recommended dosage of zolpidem.
CASE: Terrified and paranoid with distorted vision
Ms. K, age 33, an English-speaking woman from Portugal with a history of schizoaffective disorder, is brought to the emergency department in a terrified state. She describes visual distortion and paranoia because she fears losing her vision. She complains of suicidal ideation, depressed mood, insomnia, auditory
hallucinations, and distortion of visual perception. She reports seeing shadows, recurring movements of the ceiling bearing down on her, and molding or melting walls.
Ms. K reports that the visual distortions began when she started zolpidem, 10 mg/d, 2 months earlier, after her mother in Portugal gave it to her.
Ms. K describes feeling disoriented and disconnected from reality. She reports taking extra doses of zolpidem (40 mg)—recommended maximum dosage is 10 mg3—and clonazepam (1 mg) to address her insomnia.
On examination, Ms. K appears shaky and tremulous, and we note that her eyeballs roll upward. Vital signs are within normal limits, and she is awake, alert, and oriented to person, place, and time.
We diagnose exacerbation of schizoaffective disorder.
Ms. K is admitted to the inpatient psychiatric unit for observation and treatment. Quetiapine, 100 mg at bedtime, and sertraline, 100 mg/d, are started and zolpidem is discontinued.
The morning after admission, Ms. K reports that her vision has improved and that she no longer sees shadows or colored spots. All visual distortions resolve within 1 day after discontinuing zolpidem.
Discussion
Ms. K had no history of ophthalmologic or neurologic disease or alcohol or substance use. Physical and neurologic examination and laboratory testing failed to reveal any abnormality that accounts for her clinical presentation. Also, she reported visual distortions in the absence of drowsiness,4 thereby ruling out hypnagogic hallucination.5
Visual hallucinations are uncommon in schizoaffective disorder, but have been shown to occur with zolpidem use.1,4 The clinical manifestations in Ms. K’s case are
consistent with those of 3 reports, in which patients reported visual symptoms shortly after an initial dose of zolpidem, 10 mg.1,6 In those cases, symptoms resolved soon after zolpidem was discontinued.
Ms. K’s case is similar to other reports in regard to time of onset and manifestations of visual distortions. Iruela and colleagues documented that hallucinations could be reproduced with a lower challenge dose of zolpidem (5 or 2.5 mg), and that, with such a challenge, symptoms should be less severe.7 How zolpidem induces visual hallucinations remains unknown. Several studies have looked at variables that might predispose a patient taking zolpidem to visual
perceptual distortions.1
Sex might play an important role.1,7 After administering the same dosage of zolpidem, women age 20 to 40 had a blood concentration of the drug that was, on average, 45% higher than those measured in men.6 This difference in serum concentration is more remarkable in older women; the blood concentration of the drug in women age >60 was 63% higher than that of men.
Influence of hormones. Pharmacokinetics of zolpidem seem to be related to endocrine factors associated with cytochrome P450 (CYP) 3A4 metabolism. A low plasma concentration of free testosterone may contribute to lower CYP3A activity, with women achieving as much as a 50% higher plasma level of zolpidem, whereas exposure to testosterone activates biotransformation via CYP3A.8
Body weight is important when dosing zolpidem. Zolpidem-induced macropsia
has been reported in women with anorexia.7 The protein-binding capacity of zolpidem is approximately 92%, mainly to albumin (66%) and α1-acid glycoprotein (56.6%).4 In malnourished patients with anorexia nervosa, hypoalbuminemia is common and, therefore, unbound drug concentration is higher. This effect could account for, or contribute to, zolpidem toxicity.7
Ms. K did not have anorexia nervosa and was not underweight, and her liver function and kidney function were within normal limits. However, prescribing guidelines call for an initial dosage of 5 mg/d for women (10 mg/d for men)3; Ms. K ingested 8 times the recommended daily dosage.
The lesson for practitioners? When you encounter a patient who has a psychiatric disorder with psychotic features, explore causes unrelated to their primary disorder—such as taking a hypnotic or multiple psychoactive medications—as a possible source of the presenting symptoms (Box). In Ms. K’s case, her unusual symptoms resolved after a change in pharmacotherapy, leading us to conclude that her visual distortions likely were secondary to zolpidem and not to her schizoaffective disorder—a confounding factor.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Zolpidem, an imidazopyridine hypnotic, has been used as an alternative to benzodiazepines for treating short-term insomnia because it has a relatively favorable side effect profile and less potential for abuse.1
However, several cases of zolpidemrelated psychotic symptoms have been reported,2 including a report of an association between zolpidem and hallucinations.1 The case illustrated here describes distortion of visual perception that can occur after ingestion of more than the recommended dosage of zolpidem.
CASE: Terrified and paranoid with distorted vision
Ms. K, age 33, an English-speaking woman from Portugal with a history of schizoaffective disorder, is brought to the emergency department in a terrified state. She describes visual distortion and paranoia because she fears losing her vision. She complains of suicidal ideation, depressed mood, insomnia, auditory
hallucinations, and distortion of visual perception. She reports seeing shadows, recurring movements of the ceiling bearing down on her, and molding or melting walls.
Ms. K reports that the visual distortions began when she started zolpidem, 10 mg/d, 2 months earlier, after her mother in Portugal gave it to her.
Ms. K describes feeling disoriented and disconnected from reality. She reports taking extra doses of zolpidem (40 mg)—recommended maximum dosage is 10 mg3—and clonazepam (1 mg) to address her insomnia.
On examination, Ms. K appears shaky and tremulous, and we note that her eyeballs roll upward. Vital signs are within normal limits, and she is awake, alert, and oriented to person, place, and time.
We diagnose exacerbation of schizoaffective disorder.
Ms. K is admitted to the inpatient psychiatric unit for observation and treatment. Quetiapine, 100 mg at bedtime, and sertraline, 100 mg/d, are started and zolpidem is discontinued.
The morning after admission, Ms. K reports that her vision has improved and that she no longer sees shadows or colored spots. All visual distortions resolve within 1 day after discontinuing zolpidem.
Discussion
Ms. K had no history of ophthalmologic or neurologic disease or alcohol or substance use. Physical and neurologic examination and laboratory testing failed to reveal any abnormality that accounts for her clinical presentation. Also, she reported visual distortions in the absence of drowsiness,4 thereby ruling out hypnagogic hallucination.5
Visual hallucinations are uncommon in schizoaffective disorder, but have been shown to occur with zolpidem use.1,4 The clinical manifestations in Ms. K’s case are
consistent with those of 3 reports, in which patients reported visual symptoms shortly after an initial dose of zolpidem, 10 mg.1,6 In those cases, symptoms resolved soon after zolpidem was discontinued.
Ms. K’s case is similar to other reports in regard to time of onset and manifestations of visual distortions. Iruela and colleagues documented that hallucinations could be reproduced with a lower challenge dose of zolpidem (5 or 2.5 mg), and that, with such a challenge, symptoms should be less severe.7 How zolpidem induces visual hallucinations remains unknown. Several studies have looked at variables that might predispose a patient taking zolpidem to visual
perceptual distortions.1
Sex might play an important role.1,7 After administering the same dosage of zolpidem, women age 20 to 40 had a blood concentration of the drug that was, on average, 45% higher than those measured in men.6 This difference in serum concentration is more remarkable in older women; the blood concentration of the drug in women age >60 was 63% higher than that of men.
Influence of hormones. Pharmacokinetics of zolpidem seem to be related to endocrine factors associated with cytochrome P450 (CYP) 3A4 metabolism. A low plasma concentration of free testosterone may contribute to lower CYP3A activity, with women achieving as much as a 50% higher plasma level of zolpidem, whereas exposure to testosterone activates biotransformation via CYP3A.8
Body weight is important when dosing zolpidem. Zolpidem-induced macropsia
has been reported in women with anorexia.7 The protein-binding capacity of zolpidem is approximately 92%, mainly to albumin (66%) and α1-acid glycoprotein (56.6%).4 In malnourished patients with anorexia nervosa, hypoalbuminemia is common and, therefore, unbound drug concentration is higher. This effect could account for, or contribute to, zolpidem toxicity.7
Ms. K did not have anorexia nervosa and was not underweight, and her liver function and kidney function were within normal limits. However, prescribing guidelines call for an initial dosage of 5 mg/d for women (10 mg/d for men)3; Ms. K ingested 8 times the recommended daily dosage.
The lesson for practitioners? When you encounter a patient who has a psychiatric disorder with psychotic features, explore causes unrelated to their primary disorder—such as taking a hypnotic or multiple psychoactive medications—as a possible source of the presenting symptoms (Box). In Ms. K’s case, her unusual symptoms resolved after a change in pharmacotherapy, leading us to conclude that her visual distortions likely were secondary to zolpidem and not to her schizoaffective disorder—a confounding factor.
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Huang CL, Chang CJ, Hung CF, et al. Zolpidem-induced distortion in visual perception. Ann Pharmacother. 2003;37(5):683-686.
2. Ansseau M, Pitchot W, Hansenne M, et al. Psychotic reaction to zolpidem (letter). Lancet. 1992;339(8796):809.
3. Ambien [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2013.
4. van Puijenbroek EP, Egberts AC, Krom HJ. Visual hallucinations and amnesia associated with the use of zolpidem (letter). Int J Clin Pharmacol Ther. 1996;34(7):318.
5. Morselli PL. Zolpidem side-effects (letter). Lancet. 1993; 342(8875):868-869.
6. Salvà P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications. Clin Pharmacokinet. 1995;29(3):142-153.
7. Iruela LM, Ibañez-Rojo V, Baca E. Zolpidem-induced macropsia in anorexic woman (letter). Lancet. 1993; 342(8868):443-444.
8. Cubała WJ, Wiglusz M, Burkiewicz A, et al. Zolpidem pharmacokinetics and pharmacodynamics in metabolic interactions involving CYP3A: sex as a differentiating factor. Eur J Clin Pharmacol. 2010;66(9):955.
1. Huang CL, Chang CJ, Hung CF, et al. Zolpidem-induced distortion in visual perception. Ann Pharmacother. 2003;37(5):683-686.
2. Ansseau M, Pitchot W, Hansenne M, et al. Psychotic reaction to zolpidem (letter). Lancet. 1992;339(8796):809.
3. Ambien [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2013.
4. van Puijenbroek EP, Egberts AC, Krom HJ. Visual hallucinations and amnesia associated with the use of zolpidem (letter). Int J Clin Pharmacol Ther. 1996;34(7):318.
5. Morselli PL. Zolpidem side-effects (letter). Lancet. 1993; 342(8875):868-869.
6. Salvà P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem. Therapeutic implications. Clin Pharmacokinet. 1995;29(3):142-153.
7. Iruela LM, Ibañez-Rojo V, Baca E. Zolpidem-induced macropsia in anorexic woman (letter). Lancet. 1993; 342(8868):443-444.
8. Cubała WJ, Wiglusz M, Burkiewicz A, et al. Zolpidem pharmacokinetics and pharmacodynamics in metabolic interactions involving CYP3A: sex as a differentiating factor. Eur J Clin Pharmacol. 2010;66(9):955.