User login
Depression and inflammation: Examining the link
Sneezing, coughing, and a sore throat are hallmark symptoms of a common cold, but what keeps you in bed are the accompanying fatigue, inattentiveness, loss of appetite, change in sleep pattern, heightened perception of pain, and apathetic withdrawal. This “sickness behavior” is induced by inflammatory markers released in response to illness.1,2 These symptoms are similar to the constellation of symptoms that define depression. Within the inflammatory response to illness, we see the shadow of depression, but the precise relationship remains murky.
Is depression part of a normal somatic inflammatory response run amok? Some researchers have argued that “sickness behavior” is adaptive, forcing the body into a constricted pattern in order to funnel energy into healing.1,3 If depression and inflammation are related, depression pushes past these adaptive roots and is less a forced pause than a debilitating withdrawal. Perhaps depression, or a subtype, is a sign of inflammation along with heat, pain, redness, and swelling. In some instances, depression may be a sign of an underlying inflammatory process.4
In our progression toward understanding depression’s pathophysiology, we see factors that point to a relationship between depression and inflammation:
• depression frequently is comorbid with many inflammatory illnesses
• increased inflammatory biomarkers are associated with major depressive disorder (MDD)
• exposure to immunomodulating agents may increase the risk of developing depression
• stress can activate proinflammatory pathways
• antidepressants can decrease inflammatory response
• inhibition of inflammatory pathways can improve mood.
Exploring these factors and a possible pathway linking inflammation and neurobiologic changes found in depression allows us to look closer at the possible integration of the inflammatory process and depressive symptoms.
Illness and depression rates
Individuals with inflammatory illnesses—autoimmune diseases, cardiovascular disease, diabetes, and cancer—often struggle with depression. Nearly 1 in 5 persons with cardiovascular disease experiences MDD.5 A diabetes diagnosis doubles the odds of having depression.6 Up to 70% of patients with autoimmune diseases, such as rheumatoid arthritis or systemic lupus erythematosus, experience depression.7,8 In a large-scale longitudinal study, having a prior autoimmune disease increased the risk of depression by 45% and history of hospitalization with infection increased a patient’s risk by 62%; the risk more than doubled in individuals with both.9 Several studies show that 15% to 25% of cancer patients experience depression,10 compared with 9% in the general population.11
Role of inflammatory markers
During an inflammatory episode the body releases cytokines, which are small, cell-signaling protein molecules. These inflammatory markers launch signaling cascades that incite the immune system into action. Type 1 cytokines (interferon-ã, tumor necrosis factor-á [TNF-á], interleukin [IL]-1) enhance cellular immune responses, and type 2 cytokines (IL-6, IL-10, IL-13) engage antibody responses. These cytokines also induce acute phase proteins, such as C-reactive protein (CRP), which can activate the immune system. Significantly higher levels of inflammatory markers are associated with a range of depressive symptoms, which grants insight into disease severity and treatment response.3,12,13
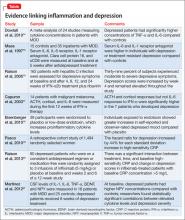
Multiple studies have explored the link between depression and inflammatory markers (Table).14-21 Peripheral inflammatory markers such as IL-6, IL-1â, CRP, and TNF-á are elevated in inflammatory diseases and in otherwise healthy individuals with MDD.12 In a meta-analysis of 24 studies measuring cytokines in depressed patients, Dowlati et al14 found individuals with MDD had significantly higher concentrations of TNF-á and IL-6 compared with controls. Increased peripheral inflammatory markers were found among antidepressant nonresponders more often than those who responded to treatment.15,22
Cytokines and depression risk
Administering immunomodulating agents has been shown to increase the risk of developing depression. Injecting animals with IL-1â or TNF-á causes sickness behavior in a dose- and time-related manner.1 As these inflammatory signaling proteins increase, sickness behaviors become more pronounced.
In humans, a natural model arises in the use of the cytokine interferon-á (INF-á) for treating hepatitis C, multiple sclerosis, malignant melanoma, and some blood cancers. Patients receiving INF-á have higher rates of depression than those not administered interferon.16 Patients receiving chronic immunotherapy treatment show long-term changes in monoamine neurotransmitters and along the HPA axis; these changes mimic those seen in depressed individuals.17,23 Acutely administered immunotherapeutic agents, such as the typhoid vaccine, have led to depressive symptoms with brain changes similar to those seen in MDD.18 Low levels of IL-6 and CRP independently predicted development of depression over several years.19
Immunotherapy-induced depression looks similar to any other major depressive episode through our current diagnostic framework and at the molecular and anatomical level.
Stress and inflammation
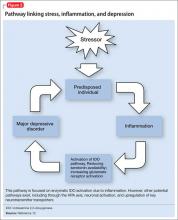
Depression can develop in the absence of inflammatory illness. Knowing that depressive symptoms may be associated with increased peripheral inflammatory markers, what induces the inflammatory process in some persons who are depressed but medically healthy? One theory is that psychological stress can activate inflammation.
Acute and chronic stress is associated with increased availability of proinflammatory cytokines and decreases in anti-inflammatory cytokines.3,24 One theory looks to glucocorticoid response to stress as an explanation. Miller et al25 found glucocorticoid sensitivity decreased among depressed women after exposure to a mock job interview stressor and increased among nondepressed controls. Because glucocorticoids normally stop the inflammatory cascade, this finding suggests depressed individuals may not be able to control inflammation during stress.26 At the level of genetic expression, there is increased transcription of proinflammatory genes in response to stress as a result of increased activation of nuclear factor kappa B.3,27
Shared pathways
If there is a relationship between inflammation and depression, what is the possible shared pathway?
There are 4 pathways by which cytokines effect changes in the CNS:12
• cytokines can activate primary afferent neurons (eg, vagal nerve)
• cytokines, released by macrophage-like cells in response to pathogens, diffuse through the brain’s circumventricular organs
• cytokine transporters saturate the blood-brain barrier
• cytokine IL-1 activates receptors on perivascular macrophages and endothelial cells of brain venules, causing local release of prostaglandin E2.
Through these pathways, cytokines initiate a cascade of reactions that lower serotonin levels and boost glutamatergic actions, possibly contributing to development of depressive symptoms. Depression correlates with a deficiency in serotonergic neurotransmission and increased glutamate receptor N-methyl-d-aspartate (NMDA) activation.28
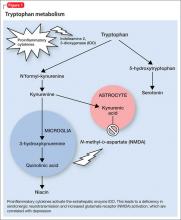
Proinflammatory cytokines activate theextrahepatic enzyme indoleamine 2,3-dioxygenase (IDO), which degrades tryptophan, a precursor to serotonin (Figure 1). Tryptophan is channeled increasingly toward production of kynurenine via IDO degradation, competing with the serotonin pathway. Within the microglia, which are preferentially activated over astrocytes during inflammatory states, kynurenine is metabolized into quinolinic acid, which is an agonist of glutamatergic NMDA receptors.28 Therefore, there is a serotonergic deficiency and glutamatergic overdrive in proinflammatory states that paves the way toward a likely depressive syndrome (Figure 2).
Antidepressants’ effects
The symptoms of cytokine-induced depression are no different from MDD with unknown etiology29 and both are effectively treated with antidepressants. Even sickness behavior can be improved with antidepressant treatment.30
Antidepressants not only decrease immunotherapy-induced depressive symptoms but have been shown to decrease inflammatory response and lower proinflammatory factors (IL-2, IL-6, TNF-á, and INF-ã).31-33 Electroconvulsive therapy has been shown to normalize elevated TNF-á levels.34
Enhancing depression treatment
Researchers are investigating whether treatment with anti-inflammatory agents can ease depressive symptoms. In animal studies, normal behavioral reactions to a stressor—similar to sickness behavior and overlapping with several features of depression—were reduced with administration of cytokine antagonists or anti-inflammatory cytokines directly into the brain.35 However, there have been few successful trials in humans. Both anti-inflammatory agents such as cyclooxygenase-2 (COX-2) inhibitors, acetylsalicylic acid (aspirin), and TNF receptor antagonists can enhance depression treatments. Persoons et al36 found that Crohn’s disease patients who had higher pretreatment CRP levels and MDD had greater remission of depressive symptoms after treatment with the TNF-á antagonist infliximab. In studies, depression within the context of other autoimmune disorders or any condition with increased inflammation has responded to treatment with TNF-á antagonists.37,38 COX-2 inhibitors added to a standard antidepressant regimen improved depressive symptoms in medically healthy individuals during an acute depressive episode.39 Aspirin has shown some benefits as an adjuvant agent in persons who have failed selective serotonin reuptake inhibitor monotherapy.40,41
These anti-inflammatory agents have shown benefits in treating depression in some persons, but not in all. The key difference between those subsets of patients is elusive, mired in the complex interactions of the many systems that contribute to the symptoms we label as depression.
Future clinical applications
The association between depression and inflammation raises the possibility of a tantalizing line of future theories and treatment options. However, when considered individually, these pieces are limited in defining the precise relationship - a task nearly impossible for such a diffuse symptom as inflammation and such a complex disease as depression.
It is evident that inflammation and depression form a strong relationship to each other in individuals, which suggests the possibility of an inflammatory subtype of depression. At least within that limited group, there is the possibility of successful intervention and treatment of depression by directly treating inflammation with anti-inflammatory agents.
Perhaps once the relationship between depression and inflammation is further defined and a high-risk population identified—maybe even by genotype—depressive symptoms might be used to flag a provider’s attention to a possible disease process and serve as a new tool for identifying dangerous inflammatory activity at an early stage. Managing stress and depression may become the next tool to prevent inflammatory diseases.
Given our current knowledge, clinicians treating patients with inflammatory conditions should be aware of the increased risk of depression and ensure that depression screening is routinely completed and treatment is initiated or referrals made as needed. Ensuring appropriate depression treatment may help improve patients’ quality of life and ease the inflammatory response itself.
For psychiatrists seeing patients with an inflammatory condition, brief explanations of the known links between depression and inflammation can provide patients—particularly those ambivalent about seeking mental health care—support for engaging in treatment and adhering to medication. Describing the links between inflammation and depression also can help encourage regular exercise and healthy diets rich in fruits, vegetables, and omega-3 fatty acids. In cases of treatment-resistant depression, particularly in those with known high inflammatory factors, it may be worthwhile to consider anti-inflammatory agents, such as infliximab, as an adjuvant treatment.
The relationship between inflammation and depression is rapidly unfolding, but the full intricacies have not yet described. However, this beginning awareness of the interplay among stress, inflammation, and depression can broaden our approach to care and treatment.
Bottom Line
Depression and inflammation are linked in many ways, although neither appears to be wholly necessary or sufficient for the other. Most likely there exists a particular subset of patients for whom inflammation will precipitate and perpetuate depression.
Related Resources
- The Emory University Mind-Body Program. www.
psychiatry.emory.edu/PROGRAMS/mindbody/index.html. - Gabriel B. The evolutionary advantage of depression. The Atlantic. October 2, 2012. www.theatlantic.com/health/archive/2012/10/the-evolutionary-advantage-of-depression/263124.
Drug Brand Names
Infliximab • Remicade Ribavirin • Rebetol, Virazole
Interferon-α • Intron
Disclosure
Dr. Almond reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
References
1. Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46-56.
2. Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12(2):123-137.
3. Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24-31.
4. Lamers F, Vogelzangs N, Merikangas KR, et al. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression [published online October 23, 2012]. Mol Psychiatry. doi: 10.1038/mp.2012.144.
5. Hoen P, Kupper N, de Jonge P. Depression and cardiovascular disease progression: epidemiology, mechanisms and treatment. In: Hjemdahl P, Rosengren A, Steptoe A, eds. Stress and cardiovascular disease. London, United Kingdom: Springer; 2012:211-233.
6. Anderson RJ, Freedland KE, Clouse RE, et al. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069-1078.
7. Bachen EA, Chesney MA, Criswell LA. Prevalence of mood and anxiety disorders in women with systemic lupus erythematosus. Arthritis Rheum. 2009;61(6):822-829.
8. Dickens C, McGowan L, Clark-Carter D, et al. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom Med. 2002;64(1):52-60.
9. Benros ME, Waltoft BL, Nordentoft M, et al. Autoimmunity and infections as risk factors for depression and other severe mental illnesses. Neurology, Psychiatry and Brain Research. 2012;18(2):40-41.
10. National Cancer Institute. Depression (PDQ). http://www.cancer.gov/cancertopics/pdq/supportivecare/depression/HealthProfessional/page1. Updated January 9, 2013. Accessed April 23, 2013.
11. Centers for Disease Control and Prevention. Current depression among adults—United States, 2006 and 2008. Morb Mortal Wkly Rep. 2010;59(38):1229-1235.
12. Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83(5):495-502.
13. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732-741.
14. Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446-457.
15. Maes M, Bosmans E, De Jongh R, et al. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9(11):853-858.
16. Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41-48.
17. Capuron L, Raison CL, Musselman DL, et al. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160(7):1342-1345.
18. Eisenberger NI, Berkman ET, Inagaki TK, et al. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68(8):748-754.
19. Pasco JA, Nicholson GC, Williams LJ, et al. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry. 2010;197(5):372-377.
20. Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):31-41.
21. Martinez JM, Garakani A, Yehuda R, et al. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress Anxiety. 2012;29(1):32-38.
22. Lanquillon S, Krieg JC, Bening-Abu-Shach U, et al. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22(4):370-379.
23. Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13(6):467-775.
24. Maes M, Song C, Lin A, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and Th1-like response in stress-induced anxiety. Cytokine. 1998;10(4):313-318.
25. Miller GE, Rohleder N, Stetler C, et al. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med. 2005;67(5):679-687.
26. Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160(9):1554-1565.
27. Tak PP, Firestein GS. NF-êB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7-12.
28. Müller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12(11):988-1000.
29. Capuron L, Fornwalt FB, Knight BT, et al. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord. 2009;119(1-3):181-185.
30. Yirmiya R, Pollak Y, Morag M, et al. Illness, cytokines, and depression. Ann N Y Acad Sci. 2000;917(1):478-487.
31. Maes M. The immunoregulatory effects of antidepressants. Hum Psychopharmacol. 2001;16(1):95-103.
32. Szuster-Ciesielska A, Tustanowska-Stachura A, Słotwin`ska M, et al. In vitro immunoregulatory effects of antidepressants in healthy volunteers. Pol J Pharmacol. 2003;55(3):353-362.
33. Maes M, Berk M, Goehler L, et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10(1):66.
34. Hestad KA, Tønseth S, Støen CD, et al. Raised plasma levels of tumor necrosis factor [alpha] in patients with depression: normalization during electroconvulsive therapy. J ECT. 2003;19(4):183-188.
35. Maier SF, Watkins LR. Intracerebroventricular interleukin-1 receptor antagonist blocks the enhancement of fear conditioning and interference with escape produced by inescapable shock. Brain Res. 1995;695(2):279-282.
36. Persoons P, Vermeire S, Demyttenaere K, et al. The impact of major depressive disorder on the short- and long-term outcome of Crohn’s disease treatment with infliximab. Aliment Pharmacol Ther. 2005;22(2):101-110.
37. Mathias SD, Colwell HH, Miller DP, et al. Health-related quality of life and functional status of patients with rheumatoid arthritis randomly assigned to receive etanercept or placebo. Clin Ther. 2000;22(1):128-139.
38. Raison C, Rutherford RE, Woolwine B, et al. The tumor necrosis factor-alpha antagonist infliximab reduces depressive symptoms in patients with treatment resistant depression and high inflammation. Brain, Behavior, and Immunity. 2012;26(suppl 1):S49.
39. Müller N, Schwarz MJ, Dehning S, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11(7):680-684.
40. Mendlewicz J, Kriwin P, Oswald P, et al. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int Clin Psychopharmacol. 2006;21(4):227-231.
41. Brunello N, Alboni S, Capone G, et al. Acetylsalicylic acid accelerates the antidepressant effect of fluoxetine in the chronic escape deficit model of depression. Int Clin Psychopharmacol. 2006;21(4):219-225.
Sneezing, coughing, and a sore throat are hallmark symptoms of a common cold, but what keeps you in bed are the accompanying fatigue, inattentiveness, loss of appetite, change in sleep pattern, heightened perception of pain, and apathetic withdrawal. This “sickness behavior” is induced by inflammatory markers released in response to illness.1,2 These symptoms are similar to the constellation of symptoms that define depression. Within the inflammatory response to illness, we see the shadow of depression, but the precise relationship remains murky.
Is depression part of a normal somatic inflammatory response run amok? Some researchers have argued that “sickness behavior” is adaptive, forcing the body into a constricted pattern in order to funnel energy into healing.1,3 If depression and inflammation are related, depression pushes past these adaptive roots and is less a forced pause than a debilitating withdrawal. Perhaps depression, or a subtype, is a sign of inflammation along with heat, pain, redness, and swelling. In some instances, depression may be a sign of an underlying inflammatory process.4
In our progression toward understanding depression’s pathophysiology, we see factors that point to a relationship between depression and inflammation:
• depression frequently is comorbid with many inflammatory illnesses
• increased inflammatory biomarkers are associated with major depressive disorder (MDD)
• exposure to immunomodulating agents may increase the risk of developing depression
• stress can activate proinflammatory pathways
• antidepressants can decrease inflammatory response
• inhibition of inflammatory pathways can improve mood.
Exploring these factors and a possible pathway linking inflammation and neurobiologic changes found in depression allows us to look closer at the possible integration of the inflammatory process and depressive symptoms.
Illness and depression rates
Individuals with inflammatory illnesses—autoimmune diseases, cardiovascular disease, diabetes, and cancer—often struggle with depression. Nearly 1 in 5 persons with cardiovascular disease experiences MDD.5 A diabetes diagnosis doubles the odds of having depression.6 Up to 70% of patients with autoimmune diseases, such as rheumatoid arthritis or systemic lupus erythematosus, experience depression.7,8 In a large-scale longitudinal study, having a prior autoimmune disease increased the risk of depression by 45% and history of hospitalization with infection increased a patient’s risk by 62%; the risk more than doubled in individuals with both.9 Several studies show that 15% to 25% of cancer patients experience depression,10 compared with 9% in the general population.11
Role of inflammatory markers
During an inflammatory episode the body releases cytokines, which are small, cell-signaling protein molecules. These inflammatory markers launch signaling cascades that incite the immune system into action. Type 1 cytokines (interferon-ã, tumor necrosis factor-á [TNF-á], interleukin [IL]-1) enhance cellular immune responses, and type 2 cytokines (IL-6, IL-10, IL-13) engage antibody responses. These cytokines also induce acute phase proteins, such as C-reactive protein (CRP), which can activate the immune system. Significantly higher levels of inflammatory markers are associated with a range of depressive symptoms, which grants insight into disease severity and treatment response.3,12,13
Multiple studies have explored the link between depression and inflammatory markers (Table).14-21 Peripheral inflammatory markers such as IL-6, IL-1â, CRP, and TNF-á are elevated in inflammatory diseases and in otherwise healthy individuals with MDD.12 In a meta-analysis of 24 studies measuring cytokines in depressed patients, Dowlati et al14 found individuals with MDD had significantly higher concentrations of TNF-á and IL-6 compared with controls. Increased peripheral inflammatory markers were found among antidepressant nonresponders more often than those who responded to treatment.15,22
Cytokines and depression risk
Administering immunomodulating agents has been shown to increase the risk of developing depression. Injecting animals with IL-1â or TNF-á causes sickness behavior in a dose- and time-related manner.1 As these inflammatory signaling proteins increase, sickness behaviors become more pronounced.
In humans, a natural model arises in the use of the cytokine interferon-á (INF-á) for treating hepatitis C, multiple sclerosis, malignant melanoma, and some blood cancers. Patients receiving INF-á have higher rates of depression than those not administered interferon.16 Patients receiving chronic immunotherapy treatment show long-term changes in monoamine neurotransmitters and along the HPA axis; these changes mimic those seen in depressed individuals.17,23 Acutely administered immunotherapeutic agents, such as the typhoid vaccine, have led to depressive symptoms with brain changes similar to those seen in MDD.18 Low levels of IL-6 and CRP independently predicted development of depression over several years.19
Immunotherapy-induced depression looks similar to any other major depressive episode through our current diagnostic framework and at the molecular and anatomical level.
Stress and inflammation
Depression can develop in the absence of inflammatory illness. Knowing that depressive symptoms may be associated with increased peripheral inflammatory markers, what induces the inflammatory process in some persons who are depressed but medically healthy? One theory is that psychological stress can activate inflammation.
Acute and chronic stress is associated with increased availability of proinflammatory cytokines and decreases in anti-inflammatory cytokines.3,24 One theory looks to glucocorticoid response to stress as an explanation. Miller et al25 found glucocorticoid sensitivity decreased among depressed women after exposure to a mock job interview stressor and increased among nondepressed controls. Because glucocorticoids normally stop the inflammatory cascade, this finding suggests depressed individuals may not be able to control inflammation during stress.26 At the level of genetic expression, there is increased transcription of proinflammatory genes in response to stress as a result of increased activation of nuclear factor kappa B.3,27
Shared pathways
If there is a relationship between inflammation and depression, what is the possible shared pathway?
There are 4 pathways by which cytokines effect changes in the CNS:12
• cytokines can activate primary afferent neurons (eg, vagal nerve)
• cytokines, released by macrophage-like cells in response to pathogens, diffuse through the brain’s circumventricular organs
• cytokine transporters saturate the blood-brain barrier
• cytokine IL-1 activates receptors on perivascular macrophages and endothelial cells of brain venules, causing local release of prostaglandin E2.
Through these pathways, cytokines initiate a cascade of reactions that lower serotonin levels and boost glutamatergic actions, possibly contributing to development of depressive symptoms. Depression correlates with a deficiency in serotonergic neurotransmission and increased glutamate receptor N-methyl-d-aspartate (NMDA) activation.28
Proinflammatory cytokines activate theextrahepatic enzyme indoleamine 2,3-dioxygenase (IDO), which degrades tryptophan, a precursor to serotonin (Figure 1). Tryptophan is channeled increasingly toward production of kynurenine via IDO degradation, competing with the serotonin pathway. Within the microglia, which are preferentially activated over astrocytes during inflammatory states, kynurenine is metabolized into quinolinic acid, which is an agonist of glutamatergic NMDA receptors.28 Therefore, there is a serotonergic deficiency and glutamatergic overdrive in proinflammatory states that paves the way toward a likely depressive syndrome (Figure 2).
Antidepressants’ effects
The symptoms of cytokine-induced depression are no different from MDD with unknown etiology29 and both are effectively treated with antidepressants. Even sickness behavior can be improved with antidepressant treatment.30
Antidepressants not only decrease immunotherapy-induced depressive symptoms but have been shown to decrease inflammatory response and lower proinflammatory factors (IL-2, IL-6, TNF-á, and INF-ã).31-33 Electroconvulsive therapy has been shown to normalize elevated TNF-á levels.34
Enhancing depression treatment
Researchers are investigating whether treatment with anti-inflammatory agents can ease depressive symptoms. In animal studies, normal behavioral reactions to a stressor—similar to sickness behavior and overlapping with several features of depression—were reduced with administration of cytokine antagonists or anti-inflammatory cytokines directly into the brain.35 However, there have been few successful trials in humans. Both anti-inflammatory agents such as cyclooxygenase-2 (COX-2) inhibitors, acetylsalicylic acid (aspirin), and TNF receptor antagonists can enhance depression treatments. Persoons et al36 found that Crohn’s disease patients who had higher pretreatment CRP levels and MDD had greater remission of depressive symptoms after treatment with the TNF-á antagonist infliximab. In studies, depression within the context of other autoimmune disorders or any condition with increased inflammation has responded to treatment with TNF-á antagonists.37,38 COX-2 inhibitors added to a standard antidepressant regimen improved depressive symptoms in medically healthy individuals during an acute depressive episode.39 Aspirin has shown some benefits as an adjuvant agent in persons who have failed selective serotonin reuptake inhibitor monotherapy.40,41
These anti-inflammatory agents have shown benefits in treating depression in some persons, but not in all. The key difference between those subsets of patients is elusive, mired in the complex interactions of the many systems that contribute to the symptoms we label as depression.
Future clinical applications
The association between depression and inflammation raises the possibility of a tantalizing line of future theories and treatment options. However, when considered individually, these pieces are limited in defining the precise relationship - a task nearly impossible for such a diffuse symptom as inflammation and such a complex disease as depression.
It is evident that inflammation and depression form a strong relationship to each other in individuals, which suggests the possibility of an inflammatory subtype of depression. At least within that limited group, there is the possibility of successful intervention and treatment of depression by directly treating inflammation with anti-inflammatory agents.
Perhaps once the relationship between depression and inflammation is further defined and a high-risk population identified—maybe even by genotype—depressive symptoms might be used to flag a provider’s attention to a possible disease process and serve as a new tool for identifying dangerous inflammatory activity at an early stage. Managing stress and depression may become the next tool to prevent inflammatory diseases.
Given our current knowledge, clinicians treating patients with inflammatory conditions should be aware of the increased risk of depression and ensure that depression screening is routinely completed and treatment is initiated or referrals made as needed. Ensuring appropriate depression treatment may help improve patients’ quality of life and ease the inflammatory response itself.
For psychiatrists seeing patients with an inflammatory condition, brief explanations of the known links between depression and inflammation can provide patients—particularly those ambivalent about seeking mental health care—support for engaging in treatment and adhering to medication. Describing the links between inflammation and depression also can help encourage regular exercise and healthy diets rich in fruits, vegetables, and omega-3 fatty acids. In cases of treatment-resistant depression, particularly in those with known high inflammatory factors, it may be worthwhile to consider anti-inflammatory agents, such as infliximab, as an adjuvant treatment.
The relationship between inflammation and depression is rapidly unfolding, but the full intricacies have not yet described. However, this beginning awareness of the interplay among stress, inflammation, and depression can broaden our approach to care and treatment.
Bottom Line
Depression and inflammation are linked in many ways, although neither appears to be wholly necessary or sufficient for the other. Most likely there exists a particular subset of patients for whom inflammation will precipitate and perpetuate depression.
Related Resources
- The Emory University Mind-Body Program. www.
psychiatry.emory.edu/PROGRAMS/mindbody/index.html. - Gabriel B. The evolutionary advantage of depression. The Atlantic. October 2, 2012. www.theatlantic.com/health/archive/2012/10/the-evolutionary-advantage-of-depression/263124.
Drug Brand Names
Infliximab • Remicade Ribavirin • Rebetol, Virazole
Interferon-α • Intron
Disclosure
Dr. Almond reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
References
1. Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46-56.
2. Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12(2):123-137.
3. Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24-31.
4. Lamers F, Vogelzangs N, Merikangas KR, et al. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression [published online October 23, 2012]. Mol Psychiatry. doi: 10.1038/mp.2012.144.
5. Hoen P, Kupper N, de Jonge P. Depression and cardiovascular disease progression: epidemiology, mechanisms and treatment. In: Hjemdahl P, Rosengren A, Steptoe A, eds. Stress and cardiovascular disease. London, United Kingdom: Springer; 2012:211-233.
6. Anderson RJ, Freedland KE, Clouse RE, et al. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069-1078.
7. Bachen EA, Chesney MA, Criswell LA. Prevalence of mood and anxiety disorders in women with systemic lupus erythematosus. Arthritis Rheum. 2009;61(6):822-829.
8. Dickens C, McGowan L, Clark-Carter D, et al. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom Med. 2002;64(1):52-60.
9. Benros ME, Waltoft BL, Nordentoft M, et al. Autoimmunity and infections as risk factors for depression and other severe mental illnesses. Neurology, Psychiatry and Brain Research. 2012;18(2):40-41.
10. National Cancer Institute. Depression (PDQ). http://www.cancer.gov/cancertopics/pdq/supportivecare/depression/HealthProfessional/page1. Updated January 9, 2013. Accessed April 23, 2013.
11. Centers for Disease Control and Prevention. Current depression among adults—United States, 2006 and 2008. Morb Mortal Wkly Rep. 2010;59(38):1229-1235.
12. Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83(5):495-502.
13. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732-741.
14. Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446-457.
15. Maes M, Bosmans E, De Jongh R, et al. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9(11):853-858.
16. Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41-48.
17. Capuron L, Raison CL, Musselman DL, et al. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160(7):1342-1345.
18. Eisenberger NI, Berkman ET, Inagaki TK, et al. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68(8):748-754.
19. Pasco JA, Nicholson GC, Williams LJ, et al. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry. 2010;197(5):372-377.
20. Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):31-41.
21. Martinez JM, Garakani A, Yehuda R, et al. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress Anxiety. 2012;29(1):32-38.
22. Lanquillon S, Krieg JC, Bening-Abu-Shach U, et al. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22(4):370-379.
23. Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13(6):467-775.
24. Maes M, Song C, Lin A, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and Th1-like response in stress-induced anxiety. Cytokine. 1998;10(4):313-318.
25. Miller GE, Rohleder N, Stetler C, et al. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med. 2005;67(5):679-687.
26. Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160(9):1554-1565.
27. Tak PP, Firestein GS. NF-êB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7-12.
28. Müller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12(11):988-1000.
29. Capuron L, Fornwalt FB, Knight BT, et al. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord. 2009;119(1-3):181-185.
30. Yirmiya R, Pollak Y, Morag M, et al. Illness, cytokines, and depression. Ann N Y Acad Sci. 2000;917(1):478-487.
31. Maes M. The immunoregulatory effects of antidepressants. Hum Psychopharmacol. 2001;16(1):95-103.
32. Szuster-Ciesielska A, Tustanowska-Stachura A, Słotwin`ska M, et al. In vitro immunoregulatory effects of antidepressants in healthy volunteers. Pol J Pharmacol. 2003;55(3):353-362.
33. Maes M, Berk M, Goehler L, et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10(1):66.
34. Hestad KA, Tønseth S, Støen CD, et al. Raised plasma levels of tumor necrosis factor [alpha] in patients with depression: normalization during electroconvulsive therapy. J ECT. 2003;19(4):183-188.
35. Maier SF, Watkins LR. Intracerebroventricular interleukin-1 receptor antagonist blocks the enhancement of fear conditioning and interference with escape produced by inescapable shock. Brain Res. 1995;695(2):279-282.
36. Persoons P, Vermeire S, Demyttenaere K, et al. The impact of major depressive disorder on the short- and long-term outcome of Crohn’s disease treatment with infliximab. Aliment Pharmacol Ther. 2005;22(2):101-110.
37. Mathias SD, Colwell HH, Miller DP, et al. Health-related quality of life and functional status of patients with rheumatoid arthritis randomly assigned to receive etanercept or placebo. Clin Ther. 2000;22(1):128-139.
38. Raison C, Rutherford RE, Woolwine B, et al. The tumor necrosis factor-alpha antagonist infliximab reduces depressive symptoms in patients with treatment resistant depression and high inflammation. Brain, Behavior, and Immunity. 2012;26(suppl 1):S49.
39. Müller N, Schwarz MJ, Dehning S, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11(7):680-684.
40. Mendlewicz J, Kriwin P, Oswald P, et al. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int Clin Psychopharmacol. 2006;21(4):227-231.
41. Brunello N, Alboni S, Capone G, et al. Acetylsalicylic acid accelerates the antidepressant effect of fluoxetine in the chronic escape deficit model of depression. Int Clin Psychopharmacol. 2006;21(4):219-225.
Sneezing, coughing, and a sore throat are hallmark symptoms of a common cold, but what keeps you in bed are the accompanying fatigue, inattentiveness, loss of appetite, change in sleep pattern, heightened perception of pain, and apathetic withdrawal. This “sickness behavior” is induced by inflammatory markers released in response to illness.1,2 These symptoms are similar to the constellation of symptoms that define depression. Within the inflammatory response to illness, we see the shadow of depression, but the precise relationship remains murky.
Is depression part of a normal somatic inflammatory response run amok? Some researchers have argued that “sickness behavior” is adaptive, forcing the body into a constricted pattern in order to funnel energy into healing.1,3 If depression and inflammation are related, depression pushes past these adaptive roots and is less a forced pause than a debilitating withdrawal. Perhaps depression, or a subtype, is a sign of inflammation along with heat, pain, redness, and swelling. In some instances, depression may be a sign of an underlying inflammatory process.4
In our progression toward understanding depression’s pathophysiology, we see factors that point to a relationship between depression and inflammation:
• depression frequently is comorbid with many inflammatory illnesses
• increased inflammatory biomarkers are associated with major depressive disorder (MDD)
• exposure to immunomodulating agents may increase the risk of developing depression
• stress can activate proinflammatory pathways
• antidepressants can decrease inflammatory response
• inhibition of inflammatory pathways can improve mood.
Exploring these factors and a possible pathway linking inflammation and neurobiologic changes found in depression allows us to look closer at the possible integration of the inflammatory process and depressive symptoms.
Illness and depression rates
Individuals with inflammatory illnesses—autoimmune diseases, cardiovascular disease, diabetes, and cancer—often struggle with depression. Nearly 1 in 5 persons with cardiovascular disease experiences MDD.5 A diabetes diagnosis doubles the odds of having depression.6 Up to 70% of patients with autoimmune diseases, such as rheumatoid arthritis or systemic lupus erythematosus, experience depression.7,8 In a large-scale longitudinal study, having a prior autoimmune disease increased the risk of depression by 45% and history of hospitalization with infection increased a patient’s risk by 62%; the risk more than doubled in individuals with both.9 Several studies show that 15% to 25% of cancer patients experience depression,10 compared with 9% in the general population.11
Role of inflammatory markers
During an inflammatory episode the body releases cytokines, which are small, cell-signaling protein molecules. These inflammatory markers launch signaling cascades that incite the immune system into action. Type 1 cytokines (interferon-ã, tumor necrosis factor-á [TNF-á], interleukin [IL]-1) enhance cellular immune responses, and type 2 cytokines (IL-6, IL-10, IL-13) engage antibody responses. These cytokines also induce acute phase proteins, such as C-reactive protein (CRP), which can activate the immune system. Significantly higher levels of inflammatory markers are associated with a range of depressive symptoms, which grants insight into disease severity and treatment response.3,12,13
Multiple studies have explored the link between depression and inflammatory markers (Table).14-21 Peripheral inflammatory markers such as IL-6, IL-1â, CRP, and TNF-á are elevated in inflammatory diseases and in otherwise healthy individuals with MDD.12 In a meta-analysis of 24 studies measuring cytokines in depressed patients, Dowlati et al14 found individuals with MDD had significantly higher concentrations of TNF-á and IL-6 compared with controls. Increased peripheral inflammatory markers were found among antidepressant nonresponders more often than those who responded to treatment.15,22
Cytokines and depression risk
Administering immunomodulating agents has been shown to increase the risk of developing depression. Injecting animals with IL-1â or TNF-á causes sickness behavior in a dose- and time-related manner.1 As these inflammatory signaling proteins increase, sickness behaviors become more pronounced.
In humans, a natural model arises in the use of the cytokine interferon-á (INF-á) for treating hepatitis C, multiple sclerosis, malignant melanoma, and some blood cancers. Patients receiving INF-á have higher rates of depression than those not administered interferon.16 Patients receiving chronic immunotherapy treatment show long-term changes in monoamine neurotransmitters and along the HPA axis; these changes mimic those seen in depressed individuals.17,23 Acutely administered immunotherapeutic agents, such as the typhoid vaccine, have led to depressive symptoms with brain changes similar to those seen in MDD.18 Low levels of IL-6 and CRP independently predicted development of depression over several years.19
Immunotherapy-induced depression looks similar to any other major depressive episode through our current diagnostic framework and at the molecular and anatomical level.
Stress and inflammation
Depression can develop in the absence of inflammatory illness. Knowing that depressive symptoms may be associated with increased peripheral inflammatory markers, what induces the inflammatory process in some persons who are depressed but medically healthy? One theory is that psychological stress can activate inflammation.
Acute and chronic stress is associated with increased availability of proinflammatory cytokines and decreases in anti-inflammatory cytokines.3,24 One theory looks to glucocorticoid response to stress as an explanation. Miller et al25 found glucocorticoid sensitivity decreased among depressed women after exposure to a mock job interview stressor and increased among nondepressed controls. Because glucocorticoids normally stop the inflammatory cascade, this finding suggests depressed individuals may not be able to control inflammation during stress.26 At the level of genetic expression, there is increased transcription of proinflammatory genes in response to stress as a result of increased activation of nuclear factor kappa B.3,27
Shared pathways
If there is a relationship between inflammation and depression, what is the possible shared pathway?
There are 4 pathways by which cytokines effect changes in the CNS:12
• cytokines can activate primary afferent neurons (eg, vagal nerve)
• cytokines, released by macrophage-like cells in response to pathogens, diffuse through the brain’s circumventricular organs
• cytokine transporters saturate the blood-brain barrier
• cytokine IL-1 activates receptors on perivascular macrophages and endothelial cells of brain venules, causing local release of prostaglandin E2.
Through these pathways, cytokines initiate a cascade of reactions that lower serotonin levels and boost glutamatergic actions, possibly contributing to development of depressive symptoms. Depression correlates with a deficiency in serotonergic neurotransmission and increased glutamate receptor N-methyl-d-aspartate (NMDA) activation.28
Proinflammatory cytokines activate theextrahepatic enzyme indoleamine 2,3-dioxygenase (IDO), which degrades tryptophan, a precursor to serotonin (Figure 1). Tryptophan is channeled increasingly toward production of kynurenine via IDO degradation, competing with the serotonin pathway. Within the microglia, which are preferentially activated over astrocytes during inflammatory states, kynurenine is metabolized into quinolinic acid, which is an agonist of glutamatergic NMDA receptors.28 Therefore, there is a serotonergic deficiency and glutamatergic overdrive in proinflammatory states that paves the way toward a likely depressive syndrome (Figure 2).
Antidepressants’ effects
The symptoms of cytokine-induced depression are no different from MDD with unknown etiology29 and both are effectively treated with antidepressants. Even sickness behavior can be improved with antidepressant treatment.30
Antidepressants not only decrease immunotherapy-induced depressive symptoms but have been shown to decrease inflammatory response and lower proinflammatory factors (IL-2, IL-6, TNF-á, and INF-ã).31-33 Electroconvulsive therapy has been shown to normalize elevated TNF-á levels.34
Enhancing depression treatment
Researchers are investigating whether treatment with anti-inflammatory agents can ease depressive symptoms. In animal studies, normal behavioral reactions to a stressor—similar to sickness behavior and overlapping with several features of depression—were reduced with administration of cytokine antagonists or anti-inflammatory cytokines directly into the brain.35 However, there have been few successful trials in humans. Both anti-inflammatory agents such as cyclooxygenase-2 (COX-2) inhibitors, acetylsalicylic acid (aspirin), and TNF receptor antagonists can enhance depression treatments. Persoons et al36 found that Crohn’s disease patients who had higher pretreatment CRP levels and MDD had greater remission of depressive symptoms after treatment with the TNF-á antagonist infliximab. In studies, depression within the context of other autoimmune disorders or any condition with increased inflammation has responded to treatment with TNF-á antagonists.37,38 COX-2 inhibitors added to a standard antidepressant regimen improved depressive symptoms in medically healthy individuals during an acute depressive episode.39 Aspirin has shown some benefits as an adjuvant agent in persons who have failed selective serotonin reuptake inhibitor monotherapy.40,41
These anti-inflammatory agents have shown benefits in treating depression in some persons, but not in all. The key difference between those subsets of patients is elusive, mired in the complex interactions of the many systems that contribute to the symptoms we label as depression.
Future clinical applications
The association between depression and inflammation raises the possibility of a tantalizing line of future theories and treatment options. However, when considered individually, these pieces are limited in defining the precise relationship - a task nearly impossible for such a diffuse symptom as inflammation and such a complex disease as depression.
It is evident that inflammation and depression form a strong relationship to each other in individuals, which suggests the possibility of an inflammatory subtype of depression. At least within that limited group, there is the possibility of successful intervention and treatment of depression by directly treating inflammation with anti-inflammatory agents.
Perhaps once the relationship between depression and inflammation is further defined and a high-risk population identified—maybe even by genotype—depressive symptoms might be used to flag a provider’s attention to a possible disease process and serve as a new tool for identifying dangerous inflammatory activity at an early stage. Managing stress and depression may become the next tool to prevent inflammatory diseases.
Given our current knowledge, clinicians treating patients with inflammatory conditions should be aware of the increased risk of depression and ensure that depression screening is routinely completed and treatment is initiated or referrals made as needed. Ensuring appropriate depression treatment may help improve patients’ quality of life and ease the inflammatory response itself.
For psychiatrists seeing patients with an inflammatory condition, brief explanations of the known links between depression and inflammation can provide patients—particularly those ambivalent about seeking mental health care—support for engaging in treatment and adhering to medication. Describing the links between inflammation and depression also can help encourage regular exercise and healthy diets rich in fruits, vegetables, and omega-3 fatty acids. In cases of treatment-resistant depression, particularly in those with known high inflammatory factors, it may be worthwhile to consider anti-inflammatory agents, such as infliximab, as an adjuvant treatment.
The relationship between inflammation and depression is rapidly unfolding, but the full intricacies have not yet described. However, this beginning awareness of the interplay among stress, inflammation, and depression can broaden our approach to care and treatment.
Bottom Line
Depression and inflammation are linked in many ways, although neither appears to be wholly necessary or sufficient for the other. Most likely there exists a particular subset of patients for whom inflammation will precipitate and perpetuate depression.
Related Resources
- The Emory University Mind-Body Program. www.
psychiatry.emory.edu/PROGRAMS/mindbody/index.html. - Gabriel B. The evolutionary advantage of depression. The Atlantic. October 2, 2012. www.theatlantic.com/health/archive/2012/10/the-evolutionary-advantage-of-depression/263124.
Drug Brand Names
Infliximab • Remicade Ribavirin • Rebetol, Virazole
Interferon-α • Intron
Disclosure
Dr. Almond reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
References
1. Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46-56.
2. Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12(2):123-137.
3. Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24-31.
4. Lamers F, Vogelzangs N, Merikangas KR, et al. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression [published online October 23, 2012]. Mol Psychiatry. doi: 10.1038/mp.2012.144.
5. Hoen P, Kupper N, de Jonge P. Depression and cardiovascular disease progression: epidemiology, mechanisms and treatment. In: Hjemdahl P, Rosengren A, Steptoe A, eds. Stress and cardiovascular disease. London, United Kingdom: Springer; 2012:211-233.
6. Anderson RJ, Freedland KE, Clouse RE, et al. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069-1078.
7. Bachen EA, Chesney MA, Criswell LA. Prevalence of mood and anxiety disorders in women with systemic lupus erythematosus. Arthritis Rheum. 2009;61(6):822-829.
8. Dickens C, McGowan L, Clark-Carter D, et al. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom Med. 2002;64(1):52-60.
9. Benros ME, Waltoft BL, Nordentoft M, et al. Autoimmunity and infections as risk factors for depression and other severe mental illnesses. Neurology, Psychiatry and Brain Research. 2012;18(2):40-41.
10. National Cancer Institute. Depression (PDQ). http://www.cancer.gov/cancertopics/pdq/supportivecare/depression/HealthProfessional/page1. Updated January 9, 2013. Accessed April 23, 2013.
11. Centers for Disease Control and Prevention. Current depression among adults—United States, 2006 and 2008. Morb Mortal Wkly Rep. 2010;59(38):1229-1235.
12. Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. 2012;83(5):495-502.
13. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732-741.
14. Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446-457.
15. Maes M, Bosmans E, De Jongh R, et al. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9(11):853-858.
16. Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66(1):41-48.
17. Capuron L, Raison CL, Musselman DL, et al. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160(7):1342-1345.
18. Eisenberger NI, Berkman ET, Inagaki TK, et al. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68(8):748-754.
19. Pasco JA, Nicholson GC, Williams LJ, et al. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry. 2010;197(5):372-377.
20. Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):31-41.
21. Martinez JM, Garakani A, Yehuda R, et al. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress Anxiety. 2012;29(1):32-38.
22. Lanquillon S, Krieg JC, Bening-Abu-Shach U, et al. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22(4):370-379.
23. Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13(6):467-775.
24. Maes M, Song C, Lin A, et al. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and Th1-like response in stress-induced anxiety. Cytokine. 1998;10(4):313-318.
25. Miller GE, Rohleder N, Stetler C, et al. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med. 2005;67(5):679-687.
26. Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160(9):1554-1565.
27. Tak PP, Firestein GS. NF-êB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7-12.
28. Müller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12(11):988-1000.
29. Capuron L, Fornwalt FB, Knight BT, et al. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord. 2009;119(1-3):181-185.
30. Yirmiya R, Pollak Y, Morag M, et al. Illness, cytokines, and depression. Ann N Y Acad Sci. 2000;917(1):478-487.
31. Maes M. The immunoregulatory effects of antidepressants. Hum Psychopharmacol. 2001;16(1):95-103.
32. Szuster-Ciesielska A, Tustanowska-Stachura A, Słotwin`ska M, et al. In vitro immunoregulatory effects of antidepressants in healthy volunteers. Pol J Pharmacol. 2003;55(3):353-362.
33. Maes M, Berk M, Goehler L, et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10(1):66.
34. Hestad KA, Tønseth S, Støen CD, et al. Raised plasma levels of tumor necrosis factor [alpha] in patients with depression: normalization during electroconvulsive therapy. J ECT. 2003;19(4):183-188.
35. Maier SF, Watkins LR. Intracerebroventricular interleukin-1 receptor antagonist blocks the enhancement of fear conditioning and interference with escape produced by inescapable shock. Brain Res. 1995;695(2):279-282.
36. Persoons P, Vermeire S, Demyttenaere K, et al. The impact of major depressive disorder on the short- and long-term outcome of Crohn’s disease treatment with infliximab. Aliment Pharmacol Ther. 2005;22(2):101-110.
37. Mathias SD, Colwell HH, Miller DP, et al. Health-related quality of life and functional status of patients with rheumatoid arthritis randomly assigned to receive etanercept or placebo. Clin Ther. 2000;22(1):128-139.
38. Raison C, Rutherford RE, Woolwine B, et al. The tumor necrosis factor-alpha antagonist infliximab reduces depressive symptoms in patients with treatment resistant depression and high inflammation. Brain, Behavior, and Immunity. 2012;26(suppl 1):S49.
39. Müller N, Schwarz MJ, Dehning S, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11(7):680-684.
40. Mendlewicz J, Kriwin P, Oswald P, et al. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int Clin Psychopharmacol. 2006;21(4):227-231.
41. Brunello N, Alboni S, Capone G, et al. Acetylsalicylic acid accelerates the antidepressant effect of fluoxetine in the chronic escape deficit model of depression. Int Clin Psychopharmacol. 2006;21(4):219-225.