User login
What you need to know about thyroid disorders in pregnancy
Until recently, thyroid dysfunction was thought to have little influence on pregnancy as long as it was treated, and management was straightforward. That was before case-control studies in prominent journals suggested an association between even subclinical hypothyroidism and impaired neonatal neurodevelopment.1-4
The risk associated with hyperthyroidism in pregnancy is less clear. Currently, it is believed to cause no adverse effects; the low thyroid-stimulating hormone (TSH) resolves in most women within 4 to 12 weeks.
As for the nonpregnant state, there is no agreement between the American College of Physicians and its British counterpart as to whether isolated, subclinical hyperthyroidism leads to morbidity or mortality, although some investigators have found an excess risk of atrial fibrillation and possibly increased bone loss in postmenopausal women. Treatment of hyperthyroidism in non-pregnant women is recommended only if low TSH persists after 4 to 12 weeks and the level is less than 0.1 mIU/L.5
This article discusses the detection and management of thyroid disease in pregnancy, concentrating on 2 representative cases. (See TABLE 1, for a list of the full spectrum of thyroid disorders.)
TABLE 1
The spectrum of thyroid disorders is wide
| Hypothyroidism |
| Hashimoto’s or subacute thyroiditis |
| Subclinical hypothyroidism |
| Subclinical hypothyroxemia |
| Postpartum thyroiditis |
| Secondary hypothyroidism |
|
| Hyperthyroidism |
| Grave’s disease |
| Subclinical hyperthyroidism |
| Thyroid storm |
| Secondary hyperthyroidism |
|
CASE 1: History of Graves’ disease
S.H., 32, is 6 weeks’ pregnant with her first child. She has a history of Graves’ disease, and underwent radioactive iodine treatment 10 years ago. She then became hypothyroid and has been on levothyroxine replacement for the past 9 years. She visits her endocrinologist annually and reports good control on 125 μg daily of oral levothyroxine sodium.
How should her pregnancy be managed?
When the mother has a history of Graves’ disease, regardless of her current thyroid state, 1% to 5% of newborns develop hyperthyroidism due to transplacental passage of thyroid-stimulating immunoglobulins (TSI). Fetal or neonatal hyperthyroidism is associated with fetal tachycardia (heart rate >160 bpm), poor growth, goiter, craniosynostosis, and advanced bone age. Therefore, fetal growth and heart rate should be monitored throughout pregnancy in these women. Investigators have published monograms on fetal thyroid measurement,6 and even argued that Doppler ultrasonography can differentiate between fetal hypo- and hyperthyroidism caused by drugs or disease processes.7 However, measurement of TSI levels (poor predictive value) and ultrasonography for fetal goiter (low yield) are controversial.
Another important consideration: The requirement for thyroxine hormone increases by approximately 30% in women on thyroid supplementation during pregnancy.8 This has been demonstrated in more than 9 studies, with athyrotic women experiencing greater increases than women with autoimmune hypothyroidism.9 The need for thyroxine increases as early as 5 weeks’ gestation and plateaus by 16 weeks.
Because hypothyroxemia or hypothyroidism (clinical or subclinical) may be associated with adverse neurodevelopment in the newborn, I recommend increasing the dosage of levothyroxine at the first encounter with this patient to 150 μg/day (<25% dosage increase). I also suggest measuring the baseline TSH level, if no reading is available from the past 3 months. If baseline TSH is less than 2.5 mIU/L, the dosage increase is probably adequate. If the TSH exceeds 2.5 mIU/L, however, I would ask the patient to take 1 extra pill (125 μg) on 2 days of the week (>30% dosage increase) and measure TSH again 4 to 6 weeks later (thyroxine takes 5 weeks to equilibrate after a change in dosage). Once the dosage has been adequately adjusted, I would monitor TSH every 6 to 8 weeks until delivery. At that time, the dosage should be reduced to the prepregnancy level, with TSH measured again in 4 to 6 weeks to confirm that the dosage is adequate.
Levothyroxine absorption is hampered by ferrous sulfate, aluminum hydroxide antacids, proton-pump inhibitors, and cholestyramine. Levothyroxine should be ingested at least 4 hours before or after the prenatal vitamin. The metabolism of levothyroxine is altered by phenytoin, carbamazepine, and rifampin.
Subclinical hypothyroidism can progress to overt disease
The majority of women with hypothyroidism are asymptomatic, with only 20% to 30% having any complaints, usually nonspecific (TABLE 2). Women with 1 or 2 symptoms are no more likely to have abnormal thyroid function tests than are asymptomatic women.
Overt hypothyroidism is primarily diagnosed with laboratory tests—specifically, low free thyroxine (FT4) or free triiodothyronine (FT3), or both, resulting in elevated TSH levels.
If untreated, overt hypothyroidism is associated with significant morbidity in both the nonpregnant and pregnant states (TABLE 3). Levothyroxine is easily administered and well tolerated, with no to few adverse effects with appropriate follow-up.10
In women with subclinical hypothyroidism, only 1 of the thyroid function tests is elevated—either elevated TSH with normal free thyroid hormone levels (mild thyroid failure) or normal TSH with low FT4 levels (hypothyroxemia). Most cases of mild thyroid failure are thought to be related to thyroid dysfunction, whereas hypothyroxemia is usually associated with a deficiency of iodine.
Subclinical hypothyroidism can occur in women with a history of thyroid disease, after surgery or radioactive iodine therapy for toxic goiter, or as the result of an inadequate dosage of thyroid medication. It can also occur in women with no history of thyroid dysfunction, detected in routine testing in women with no symptoms or with nonspecific complaints that could be related to thyroid disease. Experts agree that women with secondary subclinical disease should be treated to achieve a euthyroid state because approximately 5% per year will develop overt disease. Considerable controversy clouds management of women with primary subclinical hypothyroidism.
Subclinical hypothyroidism is more common among white women (~67%) than among black women.
TABLE 2
Know these signs and symptoms of thyroid dysfunction
| HYPOTHYROIDISM | HYPERTHYROIDISM |
|---|---|
| Fatigue | Resting tremors |
| Constipation | Hyperdefecation |
| Somnolence | Insomnia |
| Cold intolerance | Heat intolerance |
| Hair loss | Diaphoresis |
| Depression | Nervousness |
| Decreased libido | Palpitations |
| Menstrual irregularities | |
| Weight gain despite poor appetite | Weight loss |
| Dry skin | Warm, moist skin |
| Deafness | Ophthalmopathy |
| Hoarseness | Sinus tachycardia |
| Paresthesia | |
| Carpal tunnel syndrome | |
| Periorbital puffiness | |
| Slow cerebration or movement | |
| Slowing ankle jerk | Hyperreflexia |
| Goiter | Thyromegaly |
TABLE 3
Consequences of untreated thyroid dysfunction are significant
| HYPOTHYROIDISM | HYPERTHYROIDISM |
|---|---|
| Nonpregnant state | |
| Hyperlipidemia | Atrial fibrillation |
| Atherosclerosis | Congestive heart failure |
| Osteoporosis | |
| Neuropsychiatric disorders | Neuropsychiatric disorders with or without dementia/Alzheimer’s disease |
| Reduced functional status and quality of life | Reduced functional status and quality of life |
| Pregnancy | |
| Spontaneous abortion | Spontaneous abortion |
| Preterm delivery <32 weeks | Preterm labor |
| Low birth weight | Low birth weight |
| Perinatal morbidity and mortality | Stillbirth |
| Preeclampsia/gestational hypertension | Preeclampsia |
| Anovulation | |
| Cesarean delivery | |
| Postpartum hemorrhage | |
| Placental abruption | |
| Nonreassuring fetal heart rate tracing | |
| Impaired neurodevelopment | |
| Subclinical disease | |
| Risk factor for overt disease | Risk factor for overt disease |
Subclinical hyperthyroidism is more elusive
Overt hyperthyroidism can be detected through symptom-based screening (TABLE 2).
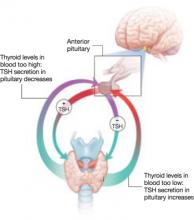
Subclinical hyperthyroidism is defined as low TSH with normal thyroid hormone levels. The pituitary appears to be more sensitive to the presence of thyroid hormones than to their absence. Subclinical hyperthyroidism is most common in black women and smokers. Approximately 50% of women with subclinical disease will have normal TSH levels several weeks to 1 year later.
Because subclinical hyperthyroidism can occur in up to 20% of women on thyroid replacement therapy, the dosage should be adjusted to achieve a euthyroid state.
CASE 2 Diabetes, with a family history of hypothyroidism
M.H., 30, is 12 weeks’ pregnant with her second child and reports a 12-year history of diabetes. Before she became pregnant, she was taking insulin, with HbA1C=8.5%. Her history includes a mother with hypothyroidism.
How should she be managed?
Besides the obvious need for good diabetes control, this case merits screening for thyroid dysfunction, as the patient has 2 risk factors (TABLE 4).
One prominent controversy of the 21st century is whether all pregnant women should undergo routine screening for hypothyroidism. The controversy extends to screening all women of childbearing age.
TABLE 4
Risk factors for hypothyroidism include other autoimmune disorders
| Family history of thyroid disease |
| More than 3 symptoms |
| History of postpartum thyroid disease |
| Type 1 diabetes mellitus |
| Recurrent spontaneous abortions |
| Unexplained intrauterine fetal demise |
| Other autoimmune disorders |
|
$64,000 question: Should all women be screened?
Screening would involve testing thyroid function in women with no history and few or no signs and symptoms of thyroid dysfunction. Such screening could be population-based (using special methods to recruit, contact, and follow patients) or case-finding (performed on patients who present for unrelated reasons). The decision to screen a woman who is pregnant or planning to conceive should be based on many factors, most notably whether treatment prevents impaired neonatal neurodevelopment and preterm delivery.
A mother’s elevated TSH level can have lasting effects in the child
Haddow and colleagues1 measured the IQ of 47 children, ages 7 to 9 years, whose mothers had had an elevated serum TSH concentration in the second trimester, 15 children whose mothers had high serum TSH values in combination with low thyroxine levels in the second trimester, and 124 children whose mothers had normal TSH values. None of these children had hypothyroidism at birth. The children of the women with an elevated TSH concentration had lower IQs. Interestingly, the group with hypothyroxemia was not evaluated at the time, and the mean FT4 level was low in the entire group, suggesting overt hypothyroidism rather than subclinical disease.
In a study from the Netherlands, Pop and associates2 found impaired psychomotor function in 22 infants (age 10 months) whose mothers had had FT4 below the 10th percentile at 12 weeks of gestation, compared with 194 infants whose mothers had normal readings. When these children were reevaluated at 2 years, no neurodevelopmental delay was found in the infants whose mothers had a spontaneously increased free thyroxine level after the first trimester.
There is much speculation about precisely when thyroid hormone is critical for fetal brain development. The study by Pop and associates2 would suggest it is important after the first trimester. That study also recommends exogenous thyroxine for FT4 values below 0.96 ng/mL (12 pmol/L).
In Italy, Vermiglio and coworkers11 conducted behavioral and neuropsychological testing in 27 children at ages 18 to 36 months and again at 8 to 10 years. Mothers of 16 of these children were from a moderately iodine-deficient area (group A), and the mothers of 11 children were from a marginally iodine-sufficient area and were monitored with thyroid function tests in the first trimester (group B). Attention-deficit and hyperactivity disorders were more prevalent in group A.
Two studies published in 2006 also suggest that maternal free thyroxine levels in the first trimester of pregnancy correlate with impaired neonatal behavior at 3 months, and impaired mental development at ages 6, 9, and 12 months.3,4
Thyroid disorders affect approximately 5% of the general population, two thirds of them women.17 Subclinical hypothyroidism occurs in an additional 4.3%, and subclinical hyperthyroidism in 0.7%.
In pregnancy, subclinical disease is present in 3.6% of women; overt hypothyroidism, in 2.5%; and overt hyperthyroidism, in 0.2%. In addition, thyroid disease affects 5% to 9% of postpartum women.14
No consensus on whom to test or what test is best
There is no clear agreement about which population should be targeted for screening or what test to use. Most medical societies do not recommend routine screening, including the American College of Obstetricians and Gynecologists, which recommends TSH testing only in women with a history of thyroid disease and in women with “symptoms” (but does not specify which symptoms or how many symptoms warrant testing). A majority of organizations agree that all high-risk women should be tested when pregnancy is planned or as soon as pregnancy is confirmed.
TABLE 5
Comparison of screening recommendations highlights lack of consensus (and, in pregnancy, the absence of guidance)
| YEAR | ORGANIZATION | NONPREGNANT STATE | PREGNANCY |
|---|---|---|---|
| 1994 | American Association of Clinical Endocrinologists (AACE), American Academy of Family Physicians | Periodic assessment via thyroid function tests in older women | No recommendation |
| 1998 | American College of Physicians | Office screening of women >50 years of age | No recommendation |
| 2000 | American Thyroid Association (ATA) | Measure TSH every 5 years in women age 35 and older (probably men also) | No recommendation |
| 2002 | American College of Obstetricians and Gynecologists | Measure TSH every 5 years in women age 65 and older | No screening recommended |
| 2003 | Institute of Medicine | Screening is not cost-effective in Medicare population | No recommendation |
| 2004 | United States Preventive Services Task Force | Routine screening of children and adults is not recommended | No recommendation |
| 2004 | AACE, ATA, the Endocrine Society Consensus Group | No population-based screening, but “aggressive case finding” in women at high risk and those over age 60 | Do not support routine testing; recommend “aggressive case finding” and screening pregnant women at high risk |
Proponents of routine screening argue that it may limit health risks to children and save money in the long run, and they point out that thyroid disease is easy to treat with pills. Opponents note that no cost-benefit analysis has been performed, the benefits of treating mild disease are unclear, and screening a large population could be a significant expense ($40–100 per person) and would necessitate a lifelong commitment to daily medication in asymptomatic patients.
As a diagnostic test, the TSH immunoassay has 98% sensitivity and 92% specificity, and the current third-generation test lacks biases between methods and does not require method-specific reference ranges. However, it has low predictive value as a screening test (7–25%), possibly because of multiple confounding variables. Despite being the “gold standard,” it can lead to falsely positive results.
TABLE 6
What makes a TSH measurement falsely high or low?
| ELEVATED TSH | LOW TSH |
|---|---|
| Recovery from nonthyroidal illness | Euthyroid sick syndrome |
| Late evening TSH surge | Recovery from normal pregnancy |
| Assay variability | |
| Adrenal insufficiency | |
| Drugs: metoclopramide, amiodarone, cholecystographic dye (sodium ipodate) | Drugs: glucocorticoids, dopamine |
As for the value of FT4 alone as a screening test, we lack sufficient data on its utility. Another problem is that equilibrium dialysis, the most accurate and reliable laboratory method to measure FT4, is too technically complex and expensive for routine use. The most widely used 2-step radioimmunoassay is automated, but different methods are used by different commercial laboratories, cutoffs vary for every laboratory, and the results are sensitive to abnormal binding-protein states such as pregnancy in a method-specific manner. Tandem mass spectrometry is as reliable as equilibrium dialysis, but is not yet readily available.12
Another consideration: The physiologic changes in pregnancy render the cutoffs for the nongravid state inapplicable. TSH is lower in pregnancy, whereas the FT4 level is probably slightly increased or unchanged (TT4 is 1.5 times the prepregnancy value).
The normal reference values in each trimester of pregnancy from iodine-sufficient, autoimmune thyroid antibody-negative women are becoming available for TSH,12 as are nomograms that adjust for fetal number and gestational age.12 The measurement of FT4 still needs to be standardized across laboratories (method-specific, trimester-specific, and, possibly, population-specific reference ranges) for pregnancy.
How to manage subclinical thyroid disorders
In the nonpregnant state, subclinical hyperthyroidism should be treated in the following groups if the abnormal thyroid levels persist beyond 4 to 12 weeks and the TSH level is less than 0.1 mIU/L:
- High-risk women: postmenopausal or over age 60
- Low-risk women with cardiac disease, low bone density, or nodular thyroid disease.
If the TSH level is between 0.1 and 0.5 mIU/L, treatment is recommended for high-risk women with cardiac disease, low bone density, or nodular thyroid disease.
Subclinical hypothyroidism with a TSH level of 4.5 to 10 mIU/L need not be treated even in an elderly woman or a patient with a high antibody titer. Treatment of any woman is beneficial when the TSH level exceeds 10 mIU/L because it can ease symptoms, reduce low-density lipoprotein cholesterol, and prevent progression to overt disease. However, treatment may not lower morbidity and mortality and carries a roughly 20% risk of causing subclinical hyperthyroidism. It also involves a lifelong commitment to daily medication.
How to treat subclinical hypothyroidism in pregnancy
It is clear that overt hypothyroidism warrants treatment in both the pregnant and nonpregnant states, but the management of subclinical disease remains controversial. No trials have assessed the benefits of thyroid hormone replacement on the neuropsychological development of the newborn. Expert opinion suggests that women be treated if they are planning a pregnancy, are already pregnant, or have high TSH or low FT4.
Until we have more data, pregnant women and those planning a pregnancy should be treated with levothyroxine (starting at 2 μg/kg/day) if they are found to have elevated TSH or low FT4.
Two studies will answer questions about effects in pregnancy
The Controlled Antenatal Thyroid Screening (CATS) study will be completed in 2009, and the National Institute of Child Health and Human Development Maternal–Fetal Medicine Units (NICHD-MFMU) study will conclude in 2014. The CATS study screened 22,000 pregnant women in the United Kingdom before 16 weeks’ gestation. Half these women were treated with levothyroxine in pregnancy if they had a TSH measurement above the 97.5th percentile or FT4 below the 2.5th percentile, and half had their blood samples stored and tested only after delivery.14 Cutoff values were derived from previously obtained antenatal sera from well-dated pregnancies, and were adjusted after every 2,000 to 3,000 samples.
The CATS study was conducted in an iodine-sufficient area with a median urinary iodine excretion of 100 μg/L (range: 11–240 μg/L). Each group contained 400 women with subclinical hypothyroidism (52% had low FT4, 45% had high TSH, and 3% had both). Antithyroid peroxidase antibodies were present in 50% of women with elevated TSH but in only 10% of women with low FT4. Neuropsychological development in their children is being tested at 3 years of age.
The NICHD-MFMU study plans to screen 110,000 women at 14 centers over 2 years, and will randomize roughly 1,000 women to thyroxine treatment or placebo. They plan to assess intellectual development of the infants yearly for 5 years, and test the mothers for postpartum thyroid dysfunction and follow them at 1 and 5 years to detect the rate of progression to overt hypothyroidism.
Postpartum dysfunction can be transient or permanent
Postpartum thyroid dysfunction (PPTD) is an autoimmune disorder that occurs at 13 to 19 weeks postpartum, affects 1 in 12 women worldwide, and is usually associated with psychiatric symptomatology.15
PPTD also is strongly associated with antithyroid peroxidase antibodies (TPOAbs).16 Premawardhana and colleagues found that 10% of women are TPOAbs-positive in the first trimester; of these, 50% develop PPTD. Of the women with PPTD, 20% to 30% develop permanent hypothyroidism, and an additional 30% to 40% develop it by 7 years. In contrast, only 5% of women without PPTD progress to overt disease by 7 years. These findings have generated considerable controversy about routine screening for PPTD. Proponents argue that PPTD is highly prevalent, linked to considerable morbidity, is easily diagnosed with relatively inexpensive tests, and is easy to treat effectively. Critics note the lack of consensus on the best screening test (thyroid function test versus TPOAbs), optimal timing of screening (early pregnancy or postpartum), and lack of high-quality, prospective cost-benefit analyses. The NICHD-MFMU hopes to resolve these controversies.
The author reports no financial relationships relevant to this article.
1. Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child [see comment]. N Engl J Med. 1999;341:549-555.
2. Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy [see comment]. Clin Endocrinol. 1999;50:149-155.
3. Kasatkina EP, Samsonova LN, Ivakhnenko VN, et al. Gestational hypothyroxinemia and cognitive function in offspring. Neurosci Behav Physiol. 2006;36:619-624.
4. Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics. 2006;117:161-167.
5. Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management [see comment]. JAMA. 2004;291:228-238.
6. Ranzini AC, Ananth CV, Smulian JC, Kung M, Limbachia A, Vintzileos AM. Ultrasonography of the fetal thyroid: nomograms based on biparietal diameter and gestational age. J Ultrasound Med. 2001;20:613-7.
7. Polak M, Le Gac I, Vuillard E, et al. Fetal and neonatal thyroid function in relation to maternal Graves’ disease. Best Practice & Research Clin Endocrinol Metab. 2004;18:289-302.
8. Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism [see comment]. N Engl J Med. 2004;351:241-249.
9. Mandel SJ, Spencer CA, Hollowell JG. Are detection and treatment of thyroid insufficiency in pregnancy feasible? Thyroid. 2005;15:44-53.
10. Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications [see comment]. J Clin Endocrinol Metab. 2006;91:2587-2591.
11. Vermiglio F, Lo Presti VP, Moleti M, et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89:6054-6060.
12. Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084-1090.
13. Dashe JS, Casey BM, Wells CE, et al. Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol. 2005;106:753-757.
14. Lazarus JH, Premawardhana LDKE. Screening for thyroid disease in pregnancy. J Clin Pathol. 2005;58:449-452.
15. Nicholson WK, Robinson KA, Smallridge RC, Ladenson PW, Powe NR. Prevalence of postpartum thyroid dysfunction: a quantitative review. Thyroid. 2006;16:573-582.
16. Premawardhana LDKE, Parkes AB, John R, Harris B, Lazarus JH. Thyroid peroxidase antibodies in early pregnancy: utility for prediction of postpartum thyroid dysfunction and implications for screening. Thyroid. 2004;14:610-615.
17. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) [see comment]. J Clin Endocrinol Metab. 2002;87:489-499.
Until recently, thyroid dysfunction was thought to have little influence on pregnancy as long as it was treated, and management was straightforward. That was before case-control studies in prominent journals suggested an association between even subclinical hypothyroidism and impaired neonatal neurodevelopment.1-4
The risk associated with hyperthyroidism in pregnancy is less clear. Currently, it is believed to cause no adverse effects; the low thyroid-stimulating hormone (TSH) resolves in most women within 4 to 12 weeks.
As for the nonpregnant state, there is no agreement between the American College of Physicians and its British counterpart as to whether isolated, subclinical hyperthyroidism leads to morbidity or mortality, although some investigators have found an excess risk of atrial fibrillation and possibly increased bone loss in postmenopausal women. Treatment of hyperthyroidism in non-pregnant women is recommended only if low TSH persists after 4 to 12 weeks and the level is less than 0.1 mIU/L.5
This article discusses the detection and management of thyroid disease in pregnancy, concentrating on 2 representative cases. (See TABLE 1, for a list of the full spectrum of thyroid disorders.)
TABLE 1
The spectrum of thyroid disorders is wide
| Hypothyroidism |
| Hashimoto’s or subacute thyroiditis |
| Subclinical hypothyroidism |
| Subclinical hypothyroxemia |
| Postpartum thyroiditis |
| Secondary hypothyroidism |
|
| Hyperthyroidism |
| Grave’s disease |
| Subclinical hyperthyroidism |
| Thyroid storm |
| Secondary hyperthyroidism |
|
CASE 1: History of Graves’ disease
S.H., 32, is 6 weeks’ pregnant with her first child. She has a history of Graves’ disease, and underwent radioactive iodine treatment 10 years ago. She then became hypothyroid and has been on levothyroxine replacement for the past 9 years. She visits her endocrinologist annually and reports good control on 125 μg daily of oral levothyroxine sodium.
How should her pregnancy be managed?
When the mother has a history of Graves’ disease, regardless of her current thyroid state, 1% to 5% of newborns develop hyperthyroidism due to transplacental passage of thyroid-stimulating immunoglobulins (TSI). Fetal or neonatal hyperthyroidism is associated with fetal tachycardia (heart rate >160 bpm), poor growth, goiter, craniosynostosis, and advanced bone age. Therefore, fetal growth and heart rate should be monitored throughout pregnancy in these women. Investigators have published monograms on fetal thyroid measurement,6 and even argued that Doppler ultrasonography can differentiate between fetal hypo- and hyperthyroidism caused by drugs or disease processes.7 However, measurement of TSI levels (poor predictive value) and ultrasonography for fetal goiter (low yield) are controversial.
Another important consideration: The requirement for thyroxine hormone increases by approximately 30% in women on thyroid supplementation during pregnancy.8 This has been demonstrated in more than 9 studies, with athyrotic women experiencing greater increases than women with autoimmune hypothyroidism.9 The need for thyroxine increases as early as 5 weeks’ gestation and plateaus by 16 weeks.
Because hypothyroxemia or hypothyroidism (clinical or subclinical) may be associated with adverse neurodevelopment in the newborn, I recommend increasing the dosage of levothyroxine at the first encounter with this patient to 150 μg/day (<25% dosage increase). I also suggest measuring the baseline TSH level, if no reading is available from the past 3 months. If baseline TSH is less than 2.5 mIU/L, the dosage increase is probably adequate. If the TSH exceeds 2.5 mIU/L, however, I would ask the patient to take 1 extra pill (125 μg) on 2 days of the week (>30% dosage increase) and measure TSH again 4 to 6 weeks later (thyroxine takes 5 weeks to equilibrate after a change in dosage). Once the dosage has been adequately adjusted, I would monitor TSH every 6 to 8 weeks until delivery. At that time, the dosage should be reduced to the prepregnancy level, with TSH measured again in 4 to 6 weeks to confirm that the dosage is adequate.
Levothyroxine absorption is hampered by ferrous sulfate, aluminum hydroxide antacids, proton-pump inhibitors, and cholestyramine. Levothyroxine should be ingested at least 4 hours before or after the prenatal vitamin. The metabolism of levothyroxine is altered by phenytoin, carbamazepine, and rifampin.
Subclinical hypothyroidism can progress to overt disease
The majority of women with hypothyroidism are asymptomatic, with only 20% to 30% having any complaints, usually nonspecific (TABLE 2). Women with 1 or 2 symptoms are no more likely to have abnormal thyroid function tests than are asymptomatic women.
Overt hypothyroidism is primarily diagnosed with laboratory tests—specifically, low free thyroxine (FT4) or free triiodothyronine (FT3), or both, resulting in elevated TSH levels.
If untreated, overt hypothyroidism is associated with significant morbidity in both the nonpregnant and pregnant states (TABLE 3). Levothyroxine is easily administered and well tolerated, with no to few adverse effects with appropriate follow-up.10
In women with subclinical hypothyroidism, only 1 of the thyroid function tests is elevated—either elevated TSH with normal free thyroid hormone levels (mild thyroid failure) or normal TSH with low FT4 levels (hypothyroxemia). Most cases of mild thyroid failure are thought to be related to thyroid dysfunction, whereas hypothyroxemia is usually associated with a deficiency of iodine.
Subclinical hypothyroidism can occur in women with a history of thyroid disease, after surgery or radioactive iodine therapy for toxic goiter, or as the result of an inadequate dosage of thyroid medication. It can also occur in women with no history of thyroid dysfunction, detected in routine testing in women with no symptoms or with nonspecific complaints that could be related to thyroid disease. Experts agree that women with secondary subclinical disease should be treated to achieve a euthyroid state because approximately 5% per year will develop overt disease. Considerable controversy clouds management of women with primary subclinical hypothyroidism.
Subclinical hypothyroidism is more common among white women (~67%) than among black women.
TABLE 2
Know these signs and symptoms of thyroid dysfunction
| HYPOTHYROIDISM | HYPERTHYROIDISM |
|---|---|
| Fatigue | Resting tremors |
| Constipation | Hyperdefecation |
| Somnolence | Insomnia |
| Cold intolerance | Heat intolerance |
| Hair loss | Diaphoresis |
| Depression | Nervousness |
| Decreased libido | Palpitations |
| Menstrual irregularities | |
| Weight gain despite poor appetite | Weight loss |
| Dry skin | Warm, moist skin |
| Deafness | Ophthalmopathy |
| Hoarseness | Sinus tachycardia |
| Paresthesia | |
| Carpal tunnel syndrome | |
| Periorbital puffiness | |
| Slow cerebration or movement | |
| Slowing ankle jerk | Hyperreflexia |
| Goiter | Thyromegaly |
TABLE 3
Consequences of untreated thyroid dysfunction are significant
| HYPOTHYROIDISM | HYPERTHYROIDISM |
|---|---|
| Nonpregnant state | |
| Hyperlipidemia | Atrial fibrillation |
| Atherosclerosis | Congestive heart failure |
| Osteoporosis | |
| Neuropsychiatric disorders | Neuropsychiatric disorders with or without dementia/Alzheimer’s disease |
| Reduced functional status and quality of life | Reduced functional status and quality of life |
| Pregnancy | |
| Spontaneous abortion | Spontaneous abortion |
| Preterm delivery <32 weeks | Preterm labor |
| Low birth weight | Low birth weight |
| Perinatal morbidity and mortality | Stillbirth |
| Preeclampsia/gestational hypertension | Preeclampsia |
| Anovulation | |
| Cesarean delivery | |
| Postpartum hemorrhage | |
| Placental abruption | |
| Nonreassuring fetal heart rate tracing | |
| Impaired neurodevelopment | |
| Subclinical disease | |
| Risk factor for overt disease | Risk factor for overt disease |
Subclinical hyperthyroidism is more elusive
Overt hyperthyroidism can be detected through symptom-based screening (TABLE 2).
Subclinical hyperthyroidism is defined as low TSH with normal thyroid hormone levels. The pituitary appears to be more sensitive to the presence of thyroid hormones than to their absence. Subclinical hyperthyroidism is most common in black women and smokers. Approximately 50% of women with subclinical disease will have normal TSH levels several weeks to 1 year later.
Because subclinical hyperthyroidism can occur in up to 20% of women on thyroid replacement therapy, the dosage should be adjusted to achieve a euthyroid state.
CASE 2 Diabetes, with a family history of hypothyroidism
M.H., 30, is 12 weeks’ pregnant with her second child and reports a 12-year history of diabetes. Before she became pregnant, she was taking insulin, with HbA1C=8.5%. Her history includes a mother with hypothyroidism.
How should she be managed?
Besides the obvious need for good diabetes control, this case merits screening for thyroid dysfunction, as the patient has 2 risk factors (TABLE 4).
One prominent controversy of the 21st century is whether all pregnant women should undergo routine screening for hypothyroidism. The controversy extends to screening all women of childbearing age.
TABLE 4
Risk factors for hypothyroidism include other autoimmune disorders
| Family history of thyroid disease |
| More than 3 symptoms |
| History of postpartum thyroid disease |
| Type 1 diabetes mellitus |
| Recurrent spontaneous abortions |
| Unexplained intrauterine fetal demise |
| Other autoimmune disorders |
|
$64,000 question: Should all women be screened?
Screening would involve testing thyroid function in women with no history and few or no signs and symptoms of thyroid dysfunction. Such screening could be population-based (using special methods to recruit, contact, and follow patients) or case-finding (performed on patients who present for unrelated reasons). The decision to screen a woman who is pregnant or planning to conceive should be based on many factors, most notably whether treatment prevents impaired neonatal neurodevelopment and preterm delivery.
A mother’s elevated TSH level can have lasting effects in the child
Haddow and colleagues1 measured the IQ of 47 children, ages 7 to 9 years, whose mothers had had an elevated serum TSH concentration in the second trimester, 15 children whose mothers had high serum TSH values in combination with low thyroxine levels in the second trimester, and 124 children whose mothers had normal TSH values. None of these children had hypothyroidism at birth. The children of the women with an elevated TSH concentration had lower IQs. Interestingly, the group with hypothyroxemia was not evaluated at the time, and the mean FT4 level was low in the entire group, suggesting overt hypothyroidism rather than subclinical disease.
In a study from the Netherlands, Pop and associates2 found impaired psychomotor function in 22 infants (age 10 months) whose mothers had had FT4 below the 10th percentile at 12 weeks of gestation, compared with 194 infants whose mothers had normal readings. When these children were reevaluated at 2 years, no neurodevelopmental delay was found in the infants whose mothers had a spontaneously increased free thyroxine level after the first trimester.
There is much speculation about precisely when thyroid hormone is critical for fetal brain development. The study by Pop and associates2 would suggest it is important after the first trimester. That study also recommends exogenous thyroxine for FT4 values below 0.96 ng/mL (12 pmol/L).
In Italy, Vermiglio and coworkers11 conducted behavioral and neuropsychological testing in 27 children at ages 18 to 36 months and again at 8 to 10 years. Mothers of 16 of these children were from a moderately iodine-deficient area (group A), and the mothers of 11 children were from a marginally iodine-sufficient area and were monitored with thyroid function tests in the first trimester (group B). Attention-deficit and hyperactivity disorders were more prevalent in group A.
Two studies published in 2006 also suggest that maternal free thyroxine levels in the first trimester of pregnancy correlate with impaired neonatal behavior at 3 months, and impaired mental development at ages 6, 9, and 12 months.3,4
Thyroid disorders affect approximately 5% of the general population, two thirds of them women.17 Subclinical hypothyroidism occurs in an additional 4.3%, and subclinical hyperthyroidism in 0.7%.
In pregnancy, subclinical disease is present in 3.6% of women; overt hypothyroidism, in 2.5%; and overt hyperthyroidism, in 0.2%. In addition, thyroid disease affects 5% to 9% of postpartum women.14
No consensus on whom to test or what test is best
There is no clear agreement about which population should be targeted for screening or what test to use. Most medical societies do not recommend routine screening, including the American College of Obstetricians and Gynecologists, which recommends TSH testing only in women with a history of thyroid disease and in women with “symptoms” (but does not specify which symptoms or how many symptoms warrant testing). A majority of organizations agree that all high-risk women should be tested when pregnancy is planned or as soon as pregnancy is confirmed.
TABLE 5
Comparison of screening recommendations highlights lack of consensus (and, in pregnancy, the absence of guidance)
| YEAR | ORGANIZATION | NONPREGNANT STATE | PREGNANCY |
|---|---|---|---|
| 1994 | American Association of Clinical Endocrinologists (AACE), American Academy of Family Physicians | Periodic assessment via thyroid function tests in older women | No recommendation |
| 1998 | American College of Physicians | Office screening of women >50 years of age | No recommendation |
| 2000 | American Thyroid Association (ATA) | Measure TSH every 5 years in women age 35 and older (probably men also) | No recommendation |
| 2002 | American College of Obstetricians and Gynecologists | Measure TSH every 5 years in women age 65 and older | No screening recommended |
| 2003 | Institute of Medicine | Screening is not cost-effective in Medicare population | No recommendation |
| 2004 | United States Preventive Services Task Force | Routine screening of children and adults is not recommended | No recommendation |
| 2004 | AACE, ATA, the Endocrine Society Consensus Group | No population-based screening, but “aggressive case finding” in women at high risk and those over age 60 | Do not support routine testing; recommend “aggressive case finding” and screening pregnant women at high risk |
Proponents of routine screening argue that it may limit health risks to children and save money in the long run, and they point out that thyroid disease is easy to treat with pills. Opponents note that no cost-benefit analysis has been performed, the benefits of treating mild disease are unclear, and screening a large population could be a significant expense ($40–100 per person) and would necessitate a lifelong commitment to daily medication in asymptomatic patients.
As a diagnostic test, the TSH immunoassay has 98% sensitivity and 92% specificity, and the current third-generation test lacks biases between methods and does not require method-specific reference ranges. However, it has low predictive value as a screening test (7–25%), possibly because of multiple confounding variables. Despite being the “gold standard,” it can lead to falsely positive results.
TABLE 6
What makes a TSH measurement falsely high or low?
| ELEVATED TSH | LOW TSH |
|---|---|
| Recovery from nonthyroidal illness | Euthyroid sick syndrome |
| Late evening TSH surge | Recovery from normal pregnancy |
| Assay variability | |
| Adrenal insufficiency | |
| Drugs: metoclopramide, amiodarone, cholecystographic dye (sodium ipodate) | Drugs: glucocorticoids, dopamine |
As for the value of FT4 alone as a screening test, we lack sufficient data on its utility. Another problem is that equilibrium dialysis, the most accurate and reliable laboratory method to measure FT4, is too technically complex and expensive for routine use. The most widely used 2-step radioimmunoassay is automated, but different methods are used by different commercial laboratories, cutoffs vary for every laboratory, and the results are sensitive to abnormal binding-protein states such as pregnancy in a method-specific manner. Tandem mass spectrometry is as reliable as equilibrium dialysis, but is not yet readily available.12
Another consideration: The physiologic changes in pregnancy render the cutoffs for the nongravid state inapplicable. TSH is lower in pregnancy, whereas the FT4 level is probably slightly increased or unchanged (TT4 is 1.5 times the prepregnancy value).
The normal reference values in each trimester of pregnancy from iodine-sufficient, autoimmune thyroid antibody-negative women are becoming available for TSH,12 as are nomograms that adjust for fetal number and gestational age.12 The measurement of FT4 still needs to be standardized across laboratories (method-specific, trimester-specific, and, possibly, population-specific reference ranges) for pregnancy.
How to manage subclinical thyroid disorders
In the nonpregnant state, subclinical hyperthyroidism should be treated in the following groups if the abnormal thyroid levels persist beyond 4 to 12 weeks and the TSH level is less than 0.1 mIU/L:
- High-risk women: postmenopausal or over age 60
- Low-risk women with cardiac disease, low bone density, or nodular thyroid disease.
If the TSH level is between 0.1 and 0.5 mIU/L, treatment is recommended for high-risk women with cardiac disease, low bone density, or nodular thyroid disease.
Subclinical hypothyroidism with a TSH level of 4.5 to 10 mIU/L need not be treated even in an elderly woman or a patient with a high antibody titer. Treatment of any woman is beneficial when the TSH level exceeds 10 mIU/L because it can ease symptoms, reduce low-density lipoprotein cholesterol, and prevent progression to overt disease. However, treatment may not lower morbidity and mortality and carries a roughly 20% risk of causing subclinical hyperthyroidism. It also involves a lifelong commitment to daily medication.
How to treat subclinical hypothyroidism in pregnancy
It is clear that overt hypothyroidism warrants treatment in both the pregnant and nonpregnant states, but the management of subclinical disease remains controversial. No trials have assessed the benefits of thyroid hormone replacement on the neuropsychological development of the newborn. Expert opinion suggests that women be treated if they are planning a pregnancy, are already pregnant, or have high TSH or low FT4.
Until we have more data, pregnant women and those planning a pregnancy should be treated with levothyroxine (starting at 2 μg/kg/day) if they are found to have elevated TSH or low FT4.
Two studies will answer questions about effects in pregnancy
The Controlled Antenatal Thyroid Screening (CATS) study will be completed in 2009, and the National Institute of Child Health and Human Development Maternal–Fetal Medicine Units (NICHD-MFMU) study will conclude in 2014. The CATS study screened 22,000 pregnant women in the United Kingdom before 16 weeks’ gestation. Half these women were treated with levothyroxine in pregnancy if they had a TSH measurement above the 97.5th percentile or FT4 below the 2.5th percentile, and half had their blood samples stored and tested only after delivery.14 Cutoff values were derived from previously obtained antenatal sera from well-dated pregnancies, and were adjusted after every 2,000 to 3,000 samples.
The CATS study was conducted in an iodine-sufficient area with a median urinary iodine excretion of 100 μg/L (range: 11–240 μg/L). Each group contained 400 women with subclinical hypothyroidism (52% had low FT4, 45% had high TSH, and 3% had both). Antithyroid peroxidase antibodies were present in 50% of women with elevated TSH but in only 10% of women with low FT4. Neuropsychological development in their children is being tested at 3 years of age.
The NICHD-MFMU study plans to screen 110,000 women at 14 centers over 2 years, and will randomize roughly 1,000 women to thyroxine treatment or placebo. They plan to assess intellectual development of the infants yearly for 5 years, and test the mothers for postpartum thyroid dysfunction and follow them at 1 and 5 years to detect the rate of progression to overt hypothyroidism.
Postpartum dysfunction can be transient or permanent
Postpartum thyroid dysfunction (PPTD) is an autoimmune disorder that occurs at 13 to 19 weeks postpartum, affects 1 in 12 women worldwide, and is usually associated with psychiatric symptomatology.15
PPTD also is strongly associated with antithyroid peroxidase antibodies (TPOAbs).16 Premawardhana and colleagues found that 10% of women are TPOAbs-positive in the first trimester; of these, 50% develop PPTD. Of the women with PPTD, 20% to 30% develop permanent hypothyroidism, and an additional 30% to 40% develop it by 7 years. In contrast, only 5% of women without PPTD progress to overt disease by 7 years. These findings have generated considerable controversy about routine screening for PPTD. Proponents argue that PPTD is highly prevalent, linked to considerable morbidity, is easily diagnosed with relatively inexpensive tests, and is easy to treat effectively. Critics note the lack of consensus on the best screening test (thyroid function test versus TPOAbs), optimal timing of screening (early pregnancy or postpartum), and lack of high-quality, prospective cost-benefit analyses. The NICHD-MFMU hopes to resolve these controversies.
The author reports no financial relationships relevant to this article.
Until recently, thyroid dysfunction was thought to have little influence on pregnancy as long as it was treated, and management was straightforward. That was before case-control studies in prominent journals suggested an association between even subclinical hypothyroidism and impaired neonatal neurodevelopment.1-4
The risk associated with hyperthyroidism in pregnancy is less clear. Currently, it is believed to cause no adverse effects; the low thyroid-stimulating hormone (TSH) resolves in most women within 4 to 12 weeks.
As for the nonpregnant state, there is no agreement between the American College of Physicians and its British counterpart as to whether isolated, subclinical hyperthyroidism leads to morbidity or mortality, although some investigators have found an excess risk of atrial fibrillation and possibly increased bone loss in postmenopausal women. Treatment of hyperthyroidism in non-pregnant women is recommended only if low TSH persists after 4 to 12 weeks and the level is less than 0.1 mIU/L.5
This article discusses the detection and management of thyroid disease in pregnancy, concentrating on 2 representative cases. (See TABLE 1, for a list of the full spectrum of thyroid disorders.)
TABLE 1
The spectrum of thyroid disorders is wide
| Hypothyroidism |
| Hashimoto’s or subacute thyroiditis |
| Subclinical hypothyroidism |
| Subclinical hypothyroxemia |
| Postpartum thyroiditis |
| Secondary hypothyroidism |
|
| Hyperthyroidism |
| Grave’s disease |
| Subclinical hyperthyroidism |
| Thyroid storm |
| Secondary hyperthyroidism |
|
CASE 1: History of Graves’ disease
S.H., 32, is 6 weeks’ pregnant with her first child. She has a history of Graves’ disease, and underwent radioactive iodine treatment 10 years ago. She then became hypothyroid and has been on levothyroxine replacement for the past 9 years. She visits her endocrinologist annually and reports good control on 125 μg daily of oral levothyroxine sodium.
How should her pregnancy be managed?
When the mother has a history of Graves’ disease, regardless of her current thyroid state, 1% to 5% of newborns develop hyperthyroidism due to transplacental passage of thyroid-stimulating immunoglobulins (TSI). Fetal or neonatal hyperthyroidism is associated with fetal tachycardia (heart rate >160 bpm), poor growth, goiter, craniosynostosis, and advanced bone age. Therefore, fetal growth and heart rate should be monitored throughout pregnancy in these women. Investigators have published monograms on fetal thyroid measurement,6 and even argued that Doppler ultrasonography can differentiate between fetal hypo- and hyperthyroidism caused by drugs or disease processes.7 However, measurement of TSI levels (poor predictive value) and ultrasonography for fetal goiter (low yield) are controversial.
Another important consideration: The requirement for thyroxine hormone increases by approximately 30% in women on thyroid supplementation during pregnancy.8 This has been demonstrated in more than 9 studies, with athyrotic women experiencing greater increases than women with autoimmune hypothyroidism.9 The need for thyroxine increases as early as 5 weeks’ gestation and plateaus by 16 weeks.
Because hypothyroxemia or hypothyroidism (clinical or subclinical) may be associated with adverse neurodevelopment in the newborn, I recommend increasing the dosage of levothyroxine at the first encounter with this patient to 150 μg/day (<25% dosage increase). I also suggest measuring the baseline TSH level, if no reading is available from the past 3 months. If baseline TSH is less than 2.5 mIU/L, the dosage increase is probably adequate. If the TSH exceeds 2.5 mIU/L, however, I would ask the patient to take 1 extra pill (125 μg) on 2 days of the week (>30% dosage increase) and measure TSH again 4 to 6 weeks later (thyroxine takes 5 weeks to equilibrate after a change in dosage). Once the dosage has been adequately adjusted, I would monitor TSH every 6 to 8 weeks until delivery. At that time, the dosage should be reduced to the prepregnancy level, with TSH measured again in 4 to 6 weeks to confirm that the dosage is adequate.
Levothyroxine absorption is hampered by ferrous sulfate, aluminum hydroxide antacids, proton-pump inhibitors, and cholestyramine. Levothyroxine should be ingested at least 4 hours before or after the prenatal vitamin. The metabolism of levothyroxine is altered by phenytoin, carbamazepine, and rifampin.
Subclinical hypothyroidism can progress to overt disease
The majority of women with hypothyroidism are asymptomatic, with only 20% to 30% having any complaints, usually nonspecific (TABLE 2). Women with 1 or 2 symptoms are no more likely to have abnormal thyroid function tests than are asymptomatic women.
Overt hypothyroidism is primarily diagnosed with laboratory tests—specifically, low free thyroxine (FT4) or free triiodothyronine (FT3), or both, resulting in elevated TSH levels.
If untreated, overt hypothyroidism is associated with significant morbidity in both the nonpregnant and pregnant states (TABLE 3). Levothyroxine is easily administered and well tolerated, with no to few adverse effects with appropriate follow-up.10
In women with subclinical hypothyroidism, only 1 of the thyroid function tests is elevated—either elevated TSH with normal free thyroid hormone levels (mild thyroid failure) or normal TSH with low FT4 levels (hypothyroxemia). Most cases of mild thyroid failure are thought to be related to thyroid dysfunction, whereas hypothyroxemia is usually associated with a deficiency of iodine.
Subclinical hypothyroidism can occur in women with a history of thyroid disease, after surgery or radioactive iodine therapy for toxic goiter, or as the result of an inadequate dosage of thyroid medication. It can also occur in women with no history of thyroid dysfunction, detected in routine testing in women with no symptoms or with nonspecific complaints that could be related to thyroid disease. Experts agree that women with secondary subclinical disease should be treated to achieve a euthyroid state because approximately 5% per year will develop overt disease. Considerable controversy clouds management of women with primary subclinical hypothyroidism.
Subclinical hypothyroidism is more common among white women (~67%) than among black women.
TABLE 2
Know these signs and symptoms of thyroid dysfunction
| HYPOTHYROIDISM | HYPERTHYROIDISM |
|---|---|
| Fatigue | Resting tremors |
| Constipation | Hyperdefecation |
| Somnolence | Insomnia |
| Cold intolerance | Heat intolerance |
| Hair loss | Diaphoresis |
| Depression | Nervousness |
| Decreased libido | Palpitations |
| Menstrual irregularities | |
| Weight gain despite poor appetite | Weight loss |
| Dry skin | Warm, moist skin |
| Deafness | Ophthalmopathy |
| Hoarseness | Sinus tachycardia |
| Paresthesia | |
| Carpal tunnel syndrome | |
| Periorbital puffiness | |
| Slow cerebration or movement | |
| Slowing ankle jerk | Hyperreflexia |
| Goiter | Thyromegaly |
TABLE 3
Consequences of untreated thyroid dysfunction are significant
| HYPOTHYROIDISM | HYPERTHYROIDISM |
|---|---|
| Nonpregnant state | |
| Hyperlipidemia | Atrial fibrillation |
| Atherosclerosis | Congestive heart failure |
| Osteoporosis | |
| Neuropsychiatric disorders | Neuropsychiatric disorders with or without dementia/Alzheimer’s disease |
| Reduced functional status and quality of life | Reduced functional status and quality of life |
| Pregnancy | |
| Spontaneous abortion | Spontaneous abortion |
| Preterm delivery <32 weeks | Preterm labor |
| Low birth weight | Low birth weight |
| Perinatal morbidity and mortality | Stillbirth |
| Preeclampsia/gestational hypertension | Preeclampsia |
| Anovulation | |
| Cesarean delivery | |
| Postpartum hemorrhage | |
| Placental abruption | |
| Nonreassuring fetal heart rate tracing | |
| Impaired neurodevelopment | |
| Subclinical disease | |
| Risk factor for overt disease | Risk factor for overt disease |
Subclinical hyperthyroidism is more elusive
Overt hyperthyroidism can be detected through symptom-based screening (TABLE 2).
Subclinical hyperthyroidism is defined as low TSH with normal thyroid hormone levels. The pituitary appears to be more sensitive to the presence of thyroid hormones than to their absence. Subclinical hyperthyroidism is most common in black women and smokers. Approximately 50% of women with subclinical disease will have normal TSH levels several weeks to 1 year later.
Because subclinical hyperthyroidism can occur in up to 20% of women on thyroid replacement therapy, the dosage should be adjusted to achieve a euthyroid state.
CASE 2 Diabetes, with a family history of hypothyroidism
M.H., 30, is 12 weeks’ pregnant with her second child and reports a 12-year history of diabetes. Before she became pregnant, she was taking insulin, with HbA1C=8.5%. Her history includes a mother with hypothyroidism.
How should she be managed?
Besides the obvious need for good diabetes control, this case merits screening for thyroid dysfunction, as the patient has 2 risk factors (TABLE 4).
One prominent controversy of the 21st century is whether all pregnant women should undergo routine screening for hypothyroidism. The controversy extends to screening all women of childbearing age.
TABLE 4
Risk factors for hypothyroidism include other autoimmune disorders
| Family history of thyroid disease |
| More than 3 symptoms |
| History of postpartum thyroid disease |
| Type 1 diabetes mellitus |
| Recurrent spontaneous abortions |
| Unexplained intrauterine fetal demise |
| Other autoimmune disorders |
|
$64,000 question: Should all women be screened?
Screening would involve testing thyroid function in women with no history and few or no signs and symptoms of thyroid dysfunction. Such screening could be population-based (using special methods to recruit, contact, and follow patients) or case-finding (performed on patients who present for unrelated reasons). The decision to screen a woman who is pregnant or planning to conceive should be based on many factors, most notably whether treatment prevents impaired neonatal neurodevelopment and preterm delivery.
A mother’s elevated TSH level can have lasting effects in the child
Haddow and colleagues1 measured the IQ of 47 children, ages 7 to 9 years, whose mothers had had an elevated serum TSH concentration in the second trimester, 15 children whose mothers had high serum TSH values in combination with low thyroxine levels in the second trimester, and 124 children whose mothers had normal TSH values. None of these children had hypothyroidism at birth. The children of the women with an elevated TSH concentration had lower IQs. Interestingly, the group with hypothyroxemia was not evaluated at the time, and the mean FT4 level was low in the entire group, suggesting overt hypothyroidism rather than subclinical disease.
In a study from the Netherlands, Pop and associates2 found impaired psychomotor function in 22 infants (age 10 months) whose mothers had had FT4 below the 10th percentile at 12 weeks of gestation, compared with 194 infants whose mothers had normal readings. When these children were reevaluated at 2 years, no neurodevelopmental delay was found in the infants whose mothers had a spontaneously increased free thyroxine level after the first trimester.
There is much speculation about precisely when thyroid hormone is critical for fetal brain development. The study by Pop and associates2 would suggest it is important after the first trimester. That study also recommends exogenous thyroxine for FT4 values below 0.96 ng/mL (12 pmol/L).
In Italy, Vermiglio and coworkers11 conducted behavioral and neuropsychological testing in 27 children at ages 18 to 36 months and again at 8 to 10 years. Mothers of 16 of these children were from a moderately iodine-deficient area (group A), and the mothers of 11 children were from a marginally iodine-sufficient area and were monitored with thyroid function tests in the first trimester (group B). Attention-deficit and hyperactivity disorders were more prevalent in group A.
Two studies published in 2006 also suggest that maternal free thyroxine levels in the first trimester of pregnancy correlate with impaired neonatal behavior at 3 months, and impaired mental development at ages 6, 9, and 12 months.3,4
Thyroid disorders affect approximately 5% of the general population, two thirds of them women.17 Subclinical hypothyroidism occurs in an additional 4.3%, and subclinical hyperthyroidism in 0.7%.
In pregnancy, subclinical disease is present in 3.6% of women; overt hypothyroidism, in 2.5%; and overt hyperthyroidism, in 0.2%. In addition, thyroid disease affects 5% to 9% of postpartum women.14
No consensus on whom to test or what test is best
There is no clear agreement about which population should be targeted for screening or what test to use. Most medical societies do not recommend routine screening, including the American College of Obstetricians and Gynecologists, which recommends TSH testing only in women with a history of thyroid disease and in women with “symptoms” (but does not specify which symptoms or how many symptoms warrant testing). A majority of organizations agree that all high-risk women should be tested when pregnancy is planned or as soon as pregnancy is confirmed.
TABLE 5
Comparison of screening recommendations highlights lack of consensus (and, in pregnancy, the absence of guidance)
| YEAR | ORGANIZATION | NONPREGNANT STATE | PREGNANCY |
|---|---|---|---|
| 1994 | American Association of Clinical Endocrinologists (AACE), American Academy of Family Physicians | Periodic assessment via thyroid function tests in older women | No recommendation |
| 1998 | American College of Physicians | Office screening of women >50 years of age | No recommendation |
| 2000 | American Thyroid Association (ATA) | Measure TSH every 5 years in women age 35 and older (probably men also) | No recommendation |
| 2002 | American College of Obstetricians and Gynecologists | Measure TSH every 5 years in women age 65 and older | No screening recommended |
| 2003 | Institute of Medicine | Screening is not cost-effective in Medicare population | No recommendation |
| 2004 | United States Preventive Services Task Force | Routine screening of children and adults is not recommended | No recommendation |
| 2004 | AACE, ATA, the Endocrine Society Consensus Group | No population-based screening, but “aggressive case finding” in women at high risk and those over age 60 | Do not support routine testing; recommend “aggressive case finding” and screening pregnant women at high risk |
Proponents of routine screening argue that it may limit health risks to children and save money in the long run, and they point out that thyroid disease is easy to treat with pills. Opponents note that no cost-benefit analysis has been performed, the benefits of treating mild disease are unclear, and screening a large population could be a significant expense ($40–100 per person) and would necessitate a lifelong commitment to daily medication in asymptomatic patients.
As a diagnostic test, the TSH immunoassay has 98% sensitivity and 92% specificity, and the current third-generation test lacks biases between methods and does not require method-specific reference ranges. However, it has low predictive value as a screening test (7–25%), possibly because of multiple confounding variables. Despite being the “gold standard,” it can lead to falsely positive results.
TABLE 6
What makes a TSH measurement falsely high or low?
| ELEVATED TSH | LOW TSH |
|---|---|
| Recovery from nonthyroidal illness | Euthyroid sick syndrome |
| Late evening TSH surge | Recovery from normal pregnancy |
| Assay variability | |
| Adrenal insufficiency | |
| Drugs: metoclopramide, amiodarone, cholecystographic dye (sodium ipodate) | Drugs: glucocorticoids, dopamine |
As for the value of FT4 alone as a screening test, we lack sufficient data on its utility. Another problem is that equilibrium dialysis, the most accurate and reliable laboratory method to measure FT4, is too technically complex and expensive for routine use. The most widely used 2-step radioimmunoassay is automated, but different methods are used by different commercial laboratories, cutoffs vary for every laboratory, and the results are sensitive to abnormal binding-protein states such as pregnancy in a method-specific manner. Tandem mass spectrometry is as reliable as equilibrium dialysis, but is not yet readily available.12
Another consideration: The physiologic changes in pregnancy render the cutoffs for the nongravid state inapplicable. TSH is lower in pregnancy, whereas the FT4 level is probably slightly increased or unchanged (TT4 is 1.5 times the prepregnancy value).
The normal reference values in each trimester of pregnancy from iodine-sufficient, autoimmune thyroid antibody-negative women are becoming available for TSH,12 as are nomograms that adjust for fetal number and gestational age.12 The measurement of FT4 still needs to be standardized across laboratories (method-specific, trimester-specific, and, possibly, population-specific reference ranges) for pregnancy.
How to manage subclinical thyroid disorders
In the nonpregnant state, subclinical hyperthyroidism should be treated in the following groups if the abnormal thyroid levels persist beyond 4 to 12 weeks and the TSH level is less than 0.1 mIU/L:
- High-risk women: postmenopausal or over age 60
- Low-risk women with cardiac disease, low bone density, or nodular thyroid disease.
If the TSH level is between 0.1 and 0.5 mIU/L, treatment is recommended for high-risk women with cardiac disease, low bone density, or nodular thyroid disease.
Subclinical hypothyroidism with a TSH level of 4.5 to 10 mIU/L need not be treated even in an elderly woman or a patient with a high antibody titer. Treatment of any woman is beneficial when the TSH level exceeds 10 mIU/L because it can ease symptoms, reduce low-density lipoprotein cholesterol, and prevent progression to overt disease. However, treatment may not lower morbidity and mortality and carries a roughly 20% risk of causing subclinical hyperthyroidism. It also involves a lifelong commitment to daily medication.
How to treat subclinical hypothyroidism in pregnancy
It is clear that overt hypothyroidism warrants treatment in both the pregnant and nonpregnant states, but the management of subclinical disease remains controversial. No trials have assessed the benefits of thyroid hormone replacement on the neuropsychological development of the newborn. Expert opinion suggests that women be treated if they are planning a pregnancy, are already pregnant, or have high TSH or low FT4.
Until we have more data, pregnant women and those planning a pregnancy should be treated with levothyroxine (starting at 2 μg/kg/day) if they are found to have elevated TSH or low FT4.
Two studies will answer questions about effects in pregnancy
The Controlled Antenatal Thyroid Screening (CATS) study will be completed in 2009, and the National Institute of Child Health and Human Development Maternal–Fetal Medicine Units (NICHD-MFMU) study will conclude in 2014. The CATS study screened 22,000 pregnant women in the United Kingdom before 16 weeks’ gestation. Half these women were treated with levothyroxine in pregnancy if they had a TSH measurement above the 97.5th percentile or FT4 below the 2.5th percentile, and half had their blood samples stored and tested only after delivery.14 Cutoff values were derived from previously obtained antenatal sera from well-dated pregnancies, and were adjusted after every 2,000 to 3,000 samples.
The CATS study was conducted in an iodine-sufficient area with a median urinary iodine excretion of 100 μg/L (range: 11–240 μg/L). Each group contained 400 women with subclinical hypothyroidism (52% had low FT4, 45% had high TSH, and 3% had both). Antithyroid peroxidase antibodies were present in 50% of women with elevated TSH but in only 10% of women with low FT4. Neuropsychological development in their children is being tested at 3 years of age.
The NICHD-MFMU study plans to screen 110,000 women at 14 centers over 2 years, and will randomize roughly 1,000 women to thyroxine treatment or placebo. They plan to assess intellectual development of the infants yearly for 5 years, and test the mothers for postpartum thyroid dysfunction and follow them at 1 and 5 years to detect the rate of progression to overt hypothyroidism.
Postpartum dysfunction can be transient or permanent
Postpartum thyroid dysfunction (PPTD) is an autoimmune disorder that occurs at 13 to 19 weeks postpartum, affects 1 in 12 women worldwide, and is usually associated with psychiatric symptomatology.15
PPTD also is strongly associated with antithyroid peroxidase antibodies (TPOAbs).16 Premawardhana and colleagues found that 10% of women are TPOAbs-positive in the first trimester; of these, 50% develop PPTD. Of the women with PPTD, 20% to 30% develop permanent hypothyroidism, and an additional 30% to 40% develop it by 7 years. In contrast, only 5% of women without PPTD progress to overt disease by 7 years. These findings have generated considerable controversy about routine screening for PPTD. Proponents argue that PPTD is highly prevalent, linked to considerable morbidity, is easily diagnosed with relatively inexpensive tests, and is easy to treat effectively. Critics note the lack of consensus on the best screening test (thyroid function test versus TPOAbs), optimal timing of screening (early pregnancy or postpartum), and lack of high-quality, prospective cost-benefit analyses. The NICHD-MFMU hopes to resolve these controversies.
The author reports no financial relationships relevant to this article.
1. Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child [see comment]. N Engl J Med. 1999;341:549-555.
2. Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy [see comment]. Clin Endocrinol. 1999;50:149-155.
3. Kasatkina EP, Samsonova LN, Ivakhnenko VN, et al. Gestational hypothyroxinemia and cognitive function in offspring. Neurosci Behav Physiol. 2006;36:619-624.
4. Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics. 2006;117:161-167.
5. Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management [see comment]. JAMA. 2004;291:228-238.
6. Ranzini AC, Ananth CV, Smulian JC, Kung M, Limbachia A, Vintzileos AM. Ultrasonography of the fetal thyroid: nomograms based on biparietal diameter and gestational age. J Ultrasound Med. 2001;20:613-7.
7. Polak M, Le Gac I, Vuillard E, et al. Fetal and neonatal thyroid function in relation to maternal Graves’ disease. Best Practice & Research Clin Endocrinol Metab. 2004;18:289-302.
8. Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism [see comment]. N Engl J Med. 2004;351:241-249.
9. Mandel SJ, Spencer CA, Hollowell JG. Are detection and treatment of thyroid insufficiency in pregnancy feasible? Thyroid. 2005;15:44-53.
10. Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications [see comment]. J Clin Endocrinol Metab. 2006;91:2587-2591.
11. Vermiglio F, Lo Presti VP, Moleti M, et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89:6054-6060.
12. Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084-1090.
13. Dashe JS, Casey BM, Wells CE, et al. Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol. 2005;106:753-757.
14. Lazarus JH, Premawardhana LDKE. Screening for thyroid disease in pregnancy. J Clin Pathol. 2005;58:449-452.
15. Nicholson WK, Robinson KA, Smallridge RC, Ladenson PW, Powe NR. Prevalence of postpartum thyroid dysfunction: a quantitative review. Thyroid. 2006;16:573-582.
16. Premawardhana LDKE, Parkes AB, John R, Harris B, Lazarus JH. Thyroid peroxidase antibodies in early pregnancy: utility for prediction of postpartum thyroid dysfunction and implications for screening. Thyroid. 2004;14:610-615.
17. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) [see comment]. J Clin Endocrinol Metab. 2002;87:489-499.
1. Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child [see comment]. N Engl J Med. 1999;341:549-555.
2. Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy [see comment]. Clin Endocrinol. 1999;50:149-155.
3. Kasatkina EP, Samsonova LN, Ivakhnenko VN, et al. Gestational hypothyroxinemia and cognitive function in offspring. Neurosci Behav Physiol. 2006;36:619-624.
4. Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics. 2006;117:161-167.
5. Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management [see comment]. JAMA. 2004;291:228-238.
6. Ranzini AC, Ananth CV, Smulian JC, Kung M, Limbachia A, Vintzileos AM. Ultrasonography of the fetal thyroid: nomograms based on biparietal diameter and gestational age. J Ultrasound Med. 2001;20:613-7.
7. Polak M, Le Gac I, Vuillard E, et al. Fetal and neonatal thyroid function in relation to maternal Graves’ disease. Best Practice & Research Clin Endocrinol Metab. 2004;18:289-302.
8. Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism [see comment]. N Engl J Med. 2004;351:241-249.
9. Mandel SJ, Spencer CA, Hollowell JG. Are detection and treatment of thyroid insufficiency in pregnancy feasible? Thyroid. 2005;15:44-53.
10. Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications [see comment]. J Clin Endocrinol Metab. 2006;91:2587-2591.
11. Vermiglio F, Lo Presti VP, Moleti M, et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89:6054-6060.
12. Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084-1090.
13. Dashe JS, Casey BM, Wells CE, et al. Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol. 2005;106:753-757.
14. Lazarus JH, Premawardhana LDKE. Screening for thyroid disease in pregnancy. J Clin Pathol. 2005;58:449-452.
15. Nicholson WK, Robinson KA, Smallridge RC, Ladenson PW, Powe NR. Prevalence of postpartum thyroid dysfunction: a quantitative review. Thyroid. 2006;16:573-582.
16. Premawardhana LDKE, Parkes AB, John R, Harris B, Lazarus JH. Thyroid peroxidase antibodies in early pregnancy: utility for prediction of postpartum thyroid dysfunction and implications for screening. Thyroid. 2004;14:610-615.
17. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) [see comment]. J Clin Endocrinol Metab. 2002;87:489-499.