User login
Chronic genital skin disorders: 6 challenging conditions
Six common dermatologic disorders of the vulva and vagina can present considerable challenges:
- Allergic contact dermatitis: 100% of the population may be at risk for this disorder, and the vulvar skin is especially vulnerable.
- Irritant contact dermatitis: Skin-barrier compromise due to chronic, low-level vulvar irritant dermatitis likely contributes to the acquisition of sexually transmitted disease.
- Lichen sclerosus: Women with this condition are at increased risk for persistent vulvar yeast infection and squamous vulvar cancer.
- Lichen planus: Antibiotic therapy is ineffective against this disorder; symptoms reappear as soon as antibiotics are stopped.
- Yeast infection: Yeast organisms release proteins that activate a local allergic response and perpetuate an environment that supports infection.
- Human papillomavirus (HPV): The small HPV particle easily gains entry to minimally traumatized vulvar skin.
These common conditions sometimes compromise the skin barrier and prevent an adequate immune response to invading microbes. Some degree of skin immune dysfunction is generally associated with each genital dermatologic disorder. Identifying and treating the underlying dermatologic disorder, then, often corrects the associated immune dysfunction and may restore the skin barrier and prevent further microbial invasion.
Allergic contact dermatitis
Allergic dermatitis, or atopic dermatitis (formerly called eczema), is a highly prevalent skin disorder (FIGURE 1). Depending on the environment and genetic factors, as many as 40% of adults have a history of atopic dermatitis, and essentially 100% of the population may be at risk. Women have a higher rate of atopic dermatitis than men do.
Recognized vulvar allergens or triggers include dry climate, elastic, latex, fragrances in soaps or body lotions, and residues of detergent and fabric softener in clothing. In most biopsy-proven cases of vulvar eczema, the patient is unable to identify specific allergens. Such a patient often has a history of asthma, allergic rhinitis, sinusitis, or atopic dermatitis on other parts of the body.
Patch testing is no help in identifying specific allergens in the pelvic area. Testing the tougher skin of the back may not disclose all vulvar sensitivities.
But biopsy is useful. Women with vulvar allergic contact dermatitis often complain of persistent itching. A history of allergy elsewhere on the body is diagnostically helpful, but a biopsy submitted to a dermatopathologist confirms the diagnosis.
Not all women with vulvar allergic dermatitis have atopy at other body sites. Hyperkeratosis and spongiosis in the pathology specimen are characteristic. A small (3-or 4-mm) biopsy at the most symptomatic site is appropriate in any woman with chronic vulvar pruritis.
Local immune dysfunction is involved. Allergic vulvar dermatitis is characterized by a locally dysfunctional cell-mediated immune response. Langerhans cells are involved in this allergic reaction, directed away from their normal protective role.1 (Read about the role of Langerhans cells in “Three barriers to microbial infection: The skin’s built-in defense system,”) Viruses, bacteria, and yeast that gain entry into the skin have greater freedom to proliferate and persist, and skin-cancer surveillance by Langerhans cells is also compromised, with an increased risk for squamous carcinoma. This may account for a large portion of the 50% of vulvar carcinomas that cannot be attributed to HPV infection. Langerhans-cell dysfunction also contributes to the progression of HPV-associated carcinoma.
Allergic dermatitis inhibits the production of human cathelicidin and the ß-defensins, natural skin microbicides. As a result, vulvar skin affected by allergic dermatitis has a higher yeast and bacterial colonization rate.
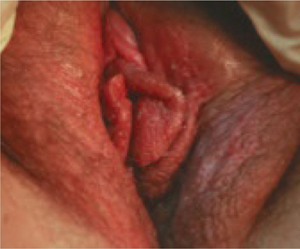
FIGURE 1 Allergic contact dermatitis
This case of severe allergic contact dermatitis has been aggravated by chronic scratching, especially of the left labia. Cases typically are much more subtle.
Look for telltale flaking skin
Allergic dermatitis involves flaking of the skin, probably due to allergic stimulation of epithelial cell proliferation. Flakes of skin are exfoliated before the desmosomes that hold individual skin cells together deteriorate. The flaking compromises the stratum corneum barrier, and likely facilitates skin invasion of yeast and bacteria that have colonized the surface.
Because the background rate of dermatitis in the general population is relatively high, skin flakes often appear in the saline wet prep and are referred to as “reactive, reparative” changes in the Papanicolaou smear.2
Other diagnostic clues. Chronic vulvar pruritus with a history of asthma, hay fever, sinusitis, atopic dermatitis, or dry skin is vulvar dermatitis until it is proved otherwise. Recurrent yeast infection is often reported as well.
In many cases, the dermatitis may exhibit no clinical signs beyond flakes of skin in the saline wet prep.
Start with a topical steroid
A trial of topical steroid ointment is appropriate, using a low-to medium-strength ointment such as 0.1% hydrocortisone butyrate, which may also lower the risk of yeast infection.
Several weeks of treatment may be necessary. It may take 4 to 6 weeks for a full layer of skin to be replaced. Subdermal atrophy, skin neovascularization, and other risks of topical steroids are of less concern during extended use of low-potency steroids, and may be more acceptable on an unexposed part of the body such as the vulva.
To test for therapeutic success, look for a reduction in pruritus and a lower incidence of yeast infection. Failure of steroid ointment and oral yeast suppression may justify vulvar biopsy, which should be submitted to a dermatopathologist.
Occasionally, a high-potency topical steroid such as clobetasol 0.05% ointment may be necessary (applied twice daily and rubbed in), but adrenal suppression may develop if therapy exceeds 3 to 4 weeks. The agent should be tapered rather than stopped abruptly.
Irritant contact dermatitis
This condition is characterized by a burning sensation. Common vulvar irritants include oxylate (in urine), propylene glycol (in medicated creams and lotions), and abrasive toilet paper. The list of potential irritants is long, and each irritant may have a different mechanism of action. A burning reaction after application of a topical cream suggests significant compromise of the skin barrier that would otherwise have prevented entry of the irritant. Skin-barrier compromise due to chronic, low-level vulvar irritant dermatitis likely contributes to acquisition of sexually transmitted disease.
Begin by identifying the culprits
The first step of treatment is recognizing and eliminating potential irritants such as bath soap, urine, topical creams that contain propylene glycol, and soap residue in clothing. Have the patient use a squirt bottle to rinse the genital area after urination to eliminate irritants such as oxylate. Also suggest that she rinse undergarments twice and use liquid rather than powder detergent. Cotton undergarments are more skin-friendly than synthetics.
Twice-daily or more frequent application of a skin moisturizer such as vegetable shortening, MimyX cream, or mineral oil/petrolatum cream (Eletone) helps to heal the skin, and continued use may prevent recurrence of symptoms.
When a patient complains of persistent vulvar pruritus and exhibits a figure-of-eight vulvar rash, suspect lichen sclerosus (FIGURE 2). The cause of this condition is unclear. Often, allergic contact dermatitis is superimposed on it. In older gynecologic terminology, this was referred to as mixed vulvar dystrophy.
Women with lichen sclerosus are at increased risk for persistent vulvar yeast infection and squamous vulvar cancer.
Vulvar biopsy occasionally discloses unexpected early lichen sclerosus.
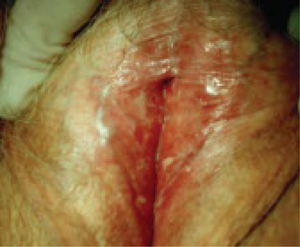
FIGURE 2 Lichen sclerosus
Note the labial agglutination (labia minora) and diffuse white epithelium, which are characteristic findings. If the areas of thickened epithelium do not resolve with topical steroid ointment, biopsy is appropriate.
Topical steroids are key to therapy
The degree of involvement that is grossly apparent determines the strength of the steroid ointment. If tissue is thickened, with areas of deep white change, a high-potency ointment such as clobetasol 0.05% may be necessary, applied twice daily for as long as several weeks. Milder cases may respond to a medium-strength topical steroid, such as fluticasone propionate 0.005% ointment.
If a higher-strength steroid is selected, it is appropriate to switch to a milder steroid as soon as symptoms resolve, with the goal of maintaining control with 1% hydrocortisone ointment or even continuous use of one of the skin moisturizers recommended for irritant dermatitis. Biopsy any thickened white patch, ulcerated area, or nonhealing skin fissure to check for squamous cancer.
Lichen planus
Erosive lichen planus (desquamative inflammatory vaginitis) of the vulva and vagina is an autoimmune skin disorder that causes superficial ulceration of the vaginal mucosa (FIGURE 3). An increase in vaginal discharge represents a shift in microflora away from lactobacillus dominance and an increase in the number of white blood cells and parabasal epithelial cells, with markedly heightened skin turnover. Local cellulitis does not develop, despite an overgrowth of various enteric organisms.
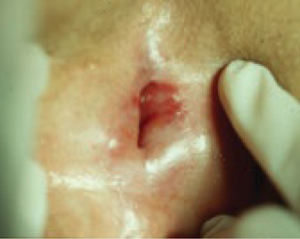
FIGURE 3 Lichen planus
This severe case resolved with a 3-month course of daily azathioprine (150 mg) but recurred after therapy ended.
A dermatologist may be required
Lichen planus is characterized by purulent discharge that contains bacteria, white blood cells, and parabasal cells. Unusual enteric microbes are often detected by routine culture, but antibiotic therapy is not helpful. Potent systemic anti-inflammatory therapy is often necessary rather than antimicrobial therapy. Daily azathioprine (Imuran) in doses ranging from 25 mg to 150 mg orally have been used, depending on the degree of vulvovaginal involvement. Tacrolimus ointment 0.1% applied twice daily may help in milder cases, but this agent typically causes an irritant reaction (burning) until the disorder partially resolves.
It may be helpful to seek the assistance of an experienced dermatologist if a biopsy demonstrates this disorder.
Vulvovaginal yeast infection is often found in conjunction with chronic vulvar eczema.3 Infection is promoted by:
- deficient skin microbicides
- skin-surface disruption with flaking
- ineffective Langerhans-cell response to invading yeast.
Yeast organisms release proteins that further activate a local allergic response to perpetuate an environment that supports infection (FIGURE 4). This represents a breakdown in the skin’s natural defenses.
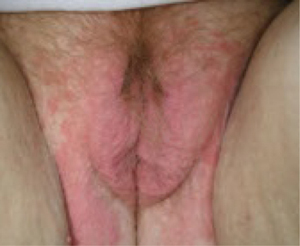
FIGURE 4 Yeast infection
Vulvar yeast rash in the normally occluded area of the vulva with large satellite lesions (erythematous patches around the margin of the vulvar rash). Satellite lesions are typically much smaller.
Oral therapy may be preferred
Topical anti-yeast creams often contain propylene glycol, an irritant to fragile skin, so oral therapy may be more appropriate. For oral therapy, 200 mg of fluconazole every 3 days for three doses is a useful starting point. For severe cases, this can be followed by a weekly 200-mg oral dose for 2 to 3 months to maintain yeast suppression while the underlying skin disorder begins to resolve. An extended course of oral fluconazole may not be appropriate during pregnancy or anticoagulation or while the patient is taking a statin drug to lower cholesterol. If oral therapy is not appropriate, 1% clotrimazole 7-day vaginal cream is the only topical agent in the United States that does not contain propylene glycol.
Beauty may be only skin deep, but that layer of epidermis is a pretty busy place. Among its activities is the production of hundreds of substances that regulate susceptibility to infection. More than 50 of these chemicals fall into the class of skin microbicides.8
We began to learn about these microbicides a decade ago, when researchers asked why eczema usually is secondarily infected with pathogenic staphylococcus, streptococcus, and yeast, and psoriatic skin isn’t. The answer: Both healthy and psoriatic skin produce natural microbicides, but allergic dermatitis (eczema) prevents their release on the skin surface.9
1. Proteins
Some proteins fight microbes better than pharmaceutical agents do. The most important antimicrobial proteins in the skin are defensins and cathelicidins, which are found in all epithelial structures, including the vulva and vagina.10 In the defensin category, human ß-defensins 2 and 3 are the most important proteins and are present in the surface epithelium. An inflammatory response triggers their release to inhibit microbes on the skin surface. The mean inhibitory concentration of human ß-defensin 3 against the relatively resistant yeast, Saccharomyces cerevisiae, is about 14 μg/mL.11 This inhibitory action is superior to many azole anti-yeast agents. Human cathelicidin is an equally effective skin microbicide, with antiviral, antifungal, and antibacterial activity. In normal function, these natural antimicrobial substances prevent colonization of pathogenic organisms in healthy skin.
2. Stratum corneum
The stratum corneum comprises the outer few microns of the epithelium. When it remains intact, the stratum corneum is an effective barrier to microbial invasion. Intact skin prevents substances with a molecular weight greater than 500 daltons from passing into the skin.
This barrier may be compromised by microtrauma or dermatologic disorders such as irritant or allergic dermatitis. Minimal microtrauma is all that is necessary to allow small microbes such as viruses to pass through the stratum corneum. Larger organisms (spirochetes, yeast) may require a greater degree of compromise, such as flaking skin. Environmental and dermatologic factors often compromise this natural barrier.
The vaginal epithelium is not keratinized and lacks an effective stratum corneum. Instead, vaginal tissue produces mucus, which floats on a thin transudate of intercellular fluid. Potential pathogens are captured in the mucus and drain out of the vagina. The vaginal epithelium produces several milliliters of mucus daily that is constantly draining out of the vaginal lumen.
3. Langerhans cells
The skin has a final layer of defense within its structure. Microbes that pass through the stratum corneum and enter the skin are attacked by defensive cells that reside there, known as antigen-presenting cells. Langerhans cells are the chief antigen-presenting cells in the skin. They originate in the bone marrow, but rest in the skin, awaiting microbial invasion.
Antigen-presenting cells kill intraepithelial microbes as they are detected, and then process the microbial antigens, enabling a cell-mediated immune response against the microbes. Langerhans cells also destroy individual cancer cells that appear randomly in the epithelium.
The genital skin and the skin around the mouth and eyes carry the highest concentration of Langerhans cells.12 Under normal conditions, Langerhans cells constitute as much as 8% of the cells in vulvar skin. In the genital area, the cervical transformation zone has the highest count of Langerhans cells13—possibly compensation for a highly vulnerable epithelial barrier, owing to the immature squamous epithelium at this site.
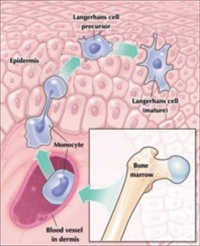
Maturation of a Langerhans cell
Langerhans cells, the main antigen-presenting cells in skin, defend it from microbes that breach the stratum corneum. Although Langerhans cells originate in bone marrow, mature cells reside in the epidermis.
With its high concentration of Langerhans cells, the cervical transformation zone may be the primary port of entry of HIV.14 Langerhans cells have a surface CD4 receptor to which HIV attaches. The Langerhans cells are unable to kill the HIV after phagocytosis. HIV-infected Langerhans cells then lead to systemic spread of the virus.
Overall, the antimicrobial function of Langerhans cells is imperfect. When a pathogen is located within a cell, some microbes, such as Chlamydia trachomatis, herpesvirus, and HPV, may evade detection. In addition, some dermatologic conditions are associated with significant dysfunction of Langerhans cells.
HPV infection
The small HPV particle easily gains entry to minimally traumatized vulvar skin—with a high transmission rate with even a single exposure—and the immature epithelium of the cervical transformation zone makes that site a naturally compromised barrier to infection.
Under normal conditions, a cell-mediated immune response eliminates the HPV virus within 12 months, with lasting protection from reinfection. If cell-mediated immunity is compromised, the virus cannot be eliminated. The result is genital warts, variable degrees of dysplasia, or cancer, depending on the degree of immune compromise (FIGURE 5).
Risk factors for HPV-associated warts and dysplasia include allergy, immunosuppressant drugs to prevent rejection of a transplanted organ, and smoking. Smoking cessation is particularly important because control of the virus is dependent on Langerhans-cell function.
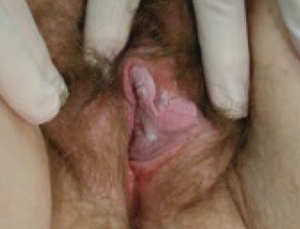
FIGURE 5 Genital warts
Debulking of warts with cryocautery or electrocautery may be appropriate, followed by imiquimod cream. It is prudent to biopsy persistent warts to exclude carcinoma, especially in an immunocompromised patient.
Begin by debulking warts
Electro- or cryocautery of large warts is an appropriate first step. Follow debulking with thrice-weekly application of one packet of imiquimod cream, to be washed off in the morning, for 4 to 6 weeks. This therapy may not eliminate genital warts in women who are taking immunosuppressant drugs to prevent organ-transplant rejection. Unfortunately, control of genital warts with monthly cautery of new warts may be the only useful option in these patients. Immunocompromised patients are at high risk for squamous carcinoma, and biopsy of persistent warts may be wise.
Vulnerabilities of vulvovaginal skin
The skin of the vulva and vagina is far from invincible. Some factors that affect it adversely are aging,4 tobacco use,5 estrogen deficiency,6 immunosuppressant drugs, and human immunodeficiency virus (HIV) infection.7 Look for these risk factors in women with persistent genital infection, so that the management plan can include treatment of the underlying dermatologic or immune disorder, as well as any microbes that are identified.
Immunosuppressed patients
Rejection of a transplanted organ is a function of cell-mediated immunity, so it is not surprising that drugs that suppress transplant rejection also inhibit vulvovaginal cell-mediated immunity. This increases the risk that HPV-associated disease will progress. Imiquimod cream promotes cell-mediated immunity by activating the release of interferon in the vulvar skin, and may compensate for depressed immune-cell function in the nontransplant population, but it is less effective in the transplant recipient. Sadly, there is no long-term solution to the effects of immunosuppressant therapy in this population; special surveillance for vulvar cancer and cervical dysplasia is necessary. Smoking cessation is also essential, especially for women with HPV-associated disease.
When HIV infection progresses to AIDS, Langerhans cells that carry HIV are depleted from the skin and substantially decrease in number, completely compromising cell-mediated immunity. This explains why AIDS patients often have severe genital herpes infections, severe chronic yeast vulvovaginitis, extensive molluscum disease, and unusual skin cancers. Antiretroviral therapy may restore some Langerhans-cell function.
Consider screening for HIV/AIDS when a woman has severe recurrent genital viral or yeast infection.
Aging and estrogen deficiency
Cell-mediated immune function declines with age. A higher risk of skin cancer, herpes (and its recurrence), and irritant and allergic vulvar dermatitis are the results. Increased surveillance for skin cancer, and varicella vaccination to lower the risk for herpes zoster, may be important.
Topical estrogen may be indicated if saline wet-prep evaluation reveals parabasal cells in vaginal secretions of a symptomatic postmenopausal woman. The estrogen may gradually alleviate burning and restrict potentially pathogenic bacterial flora. Be aware, however, that commercially available estrogen creams often contain propylene glycol, a recognized irritant of fragile skin. One solution: Have a compounding pharmacist formulate an equivalent cream (estradiol, 0.1 mg/g) for twice-daily topical application, using a base of petrolatum or solid vegetable oil.
1. Cruz PD. The epidermis: an outpost of the immune system. In: Freinkel RK, Woodley D, eds. The Biology of the Skin. New York: Parthenon Publishing Group; 2000:256-260.
2. Bonfiglio TA, Erozan YS. Gynecologic Cytopathology. Philadelphia: Lippincott-Raven; 1997:42-45.
3. Fidel PL, Sobel JD. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin Microbiol Rev. 1996;9:335-348.
4. Gilchrest B, Murphy G, Soter N. Effect of chronological aging and ultraviolet irradiation on Langerhans cells in human epidermis. J Invest Dermatol. 1982;79:85-88.
5. Ouyang Y, Virasch N, Hao P, et al. Suppression of human IL-1beta, IL-2, IFN-gamma, and TNF-alpha by cigarette smoke extracts. J Allergy Clin Immunol. 2000;106:280-287.
6. Mao A, Paharkova-Vatchkova V, Hardy J, et al. Estrogen selectively promotes the differentiation of dendritic cells with characteristics of Langerhans cells. J Immunol. 2005;175:5146-5151.
7. Memar OM, Geraminejad P, Arany I, Tyring SK. Cutaneous resistance to viral infections. In: Tyring SK, ed. Mucocutaneous Manifestations of Viral Diseases. New York: Marcel Dekker; 2002:25-28.
8. Braff MH, Bardan A, Nizet V, Gallo RL. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005;125:9-13.
9. Nomura I, Goleva E, Howell MD, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262-3269.
10. Harder J, Bartels J, Christophers E, Schroder JN. Isolation and characterization of human beta defensin-3, a novel inducible peptide antibiotic. J Biol Chem. 2001;276:5707-5713.
11. Garcia JR, Jaumann F, Schultz S, et al. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity. Cell Tissue Res. 2001;306:257-264.
12. Udey MC. Cadherins and Langerhans cell immunobiology. Clin Exp Immunol. 1997;107(suppl. 1):6-8.
13. Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253-1263.
14. Tschachler E, Groh V Popovic, et al. Epidermal Langerhans cells—a target for HTLV III/LAV infection. J Invest Dermatol. 1987;88:233-237.
Six common dermatologic disorders of the vulva and vagina can present considerable challenges:
- Allergic contact dermatitis: 100% of the population may be at risk for this disorder, and the vulvar skin is especially vulnerable.
- Irritant contact dermatitis: Skin-barrier compromise due to chronic, low-level vulvar irritant dermatitis likely contributes to the acquisition of sexually transmitted disease.
- Lichen sclerosus: Women with this condition are at increased risk for persistent vulvar yeast infection and squamous vulvar cancer.
- Lichen planus: Antibiotic therapy is ineffective against this disorder; symptoms reappear as soon as antibiotics are stopped.
- Yeast infection: Yeast organisms release proteins that activate a local allergic response and perpetuate an environment that supports infection.
- Human papillomavirus (HPV): The small HPV particle easily gains entry to minimally traumatized vulvar skin.
These common conditions sometimes compromise the skin barrier and prevent an adequate immune response to invading microbes. Some degree of skin immune dysfunction is generally associated with each genital dermatologic disorder. Identifying and treating the underlying dermatologic disorder, then, often corrects the associated immune dysfunction and may restore the skin barrier and prevent further microbial invasion.
Allergic contact dermatitis
Allergic dermatitis, or atopic dermatitis (formerly called eczema), is a highly prevalent skin disorder (FIGURE 1). Depending on the environment and genetic factors, as many as 40% of adults have a history of atopic dermatitis, and essentially 100% of the population may be at risk. Women have a higher rate of atopic dermatitis than men do.
Recognized vulvar allergens or triggers include dry climate, elastic, latex, fragrances in soaps or body lotions, and residues of detergent and fabric softener in clothing. In most biopsy-proven cases of vulvar eczema, the patient is unable to identify specific allergens. Such a patient often has a history of asthma, allergic rhinitis, sinusitis, or atopic dermatitis on other parts of the body.
Patch testing is no help in identifying specific allergens in the pelvic area. Testing the tougher skin of the back may not disclose all vulvar sensitivities.
But biopsy is useful. Women with vulvar allergic contact dermatitis often complain of persistent itching. A history of allergy elsewhere on the body is diagnostically helpful, but a biopsy submitted to a dermatopathologist confirms the diagnosis.
Not all women with vulvar allergic dermatitis have atopy at other body sites. Hyperkeratosis and spongiosis in the pathology specimen are characteristic. A small (3-or 4-mm) biopsy at the most symptomatic site is appropriate in any woman with chronic vulvar pruritis.
Local immune dysfunction is involved. Allergic vulvar dermatitis is characterized by a locally dysfunctional cell-mediated immune response. Langerhans cells are involved in this allergic reaction, directed away from their normal protective role.1 (Read about the role of Langerhans cells in “Three barriers to microbial infection: The skin’s built-in defense system,”) Viruses, bacteria, and yeast that gain entry into the skin have greater freedom to proliferate and persist, and skin-cancer surveillance by Langerhans cells is also compromised, with an increased risk for squamous carcinoma. This may account for a large portion of the 50% of vulvar carcinomas that cannot be attributed to HPV infection. Langerhans-cell dysfunction also contributes to the progression of HPV-associated carcinoma.
Allergic dermatitis inhibits the production of human cathelicidin and the ß-defensins, natural skin microbicides. As a result, vulvar skin affected by allergic dermatitis has a higher yeast and bacterial colonization rate.

FIGURE 1 Allergic contact dermatitis
This case of severe allergic contact dermatitis has been aggravated by chronic scratching, especially of the left labia. Cases typically are much more subtle.
Look for telltale flaking skin
Allergic dermatitis involves flaking of the skin, probably due to allergic stimulation of epithelial cell proliferation. Flakes of skin are exfoliated before the desmosomes that hold individual skin cells together deteriorate. The flaking compromises the stratum corneum barrier, and likely facilitates skin invasion of yeast and bacteria that have colonized the surface.
Because the background rate of dermatitis in the general population is relatively high, skin flakes often appear in the saline wet prep and are referred to as “reactive, reparative” changes in the Papanicolaou smear.2
Other diagnostic clues. Chronic vulvar pruritus with a history of asthma, hay fever, sinusitis, atopic dermatitis, or dry skin is vulvar dermatitis until it is proved otherwise. Recurrent yeast infection is often reported as well.
In many cases, the dermatitis may exhibit no clinical signs beyond flakes of skin in the saline wet prep.
Start with a topical steroid
A trial of topical steroid ointment is appropriate, using a low-to medium-strength ointment such as 0.1% hydrocortisone butyrate, which may also lower the risk of yeast infection.
Several weeks of treatment may be necessary. It may take 4 to 6 weeks for a full layer of skin to be replaced. Subdermal atrophy, skin neovascularization, and other risks of topical steroids are of less concern during extended use of low-potency steroids, and may be more acceptable on an unexposed part of the body such as the vulva.
To test for therapeutic success, look for a reduction in pruritus and a lower incidence of yeast infection. Failure of steroid ointment and oral yeast suppression may justify vulvar biopsy, which should be submitted to a dermatopathologist.
Occasionally, a high-potency topical steroid such as clobetasol 0.05% ointment may be necessary (applied twice daily and rubbed in), but adrenal suppression may develop if therapy exceeds 3 to 4 weeks. The agent should be tapered rather than stopped abruptly.
Irritant contact dermatitis
This condition is characterized by a burning sensation. Common vulvar irritants include oxylate (in urine), propylene glycol (in medicated creams and lotions), and abrasive toilet paper. The list of potential irritants is long, and each irritant may have a different mechanism of action. A burning reaction after application of a topical cream suggests significant compromise of the skin barrier that would otherwise have prevented entry of the irritant. Skin-barrier compromise due to chronic, low-level vulvar irritant dermatitis likely contributes to acquisition of sexually transmitted disease.
Begin by identifying the culprits
The first step of treatment is recognizing and eliminating potential irritants such as bath soap, urine, topical creams that contain propylene glycol, and soap residue in clothing. Have the patient use a squirt bottle to rinse the genital area after urination to eliminate irritants such as oxylate. Also suggest that she rinse undergarments twice and use liquid rather than powder detergent. Cotton undergarments are more skin-friendly than synthetics.
Twice-daily or more frequent application of a skin moisturizer such as vegetable shortening, MimyX cream, or mineral oil/petrolatum cream (Eletone) helps to heal the skin, and continued use may prevent recurrence of symptoms.
When a patient complains of persistent vulvar pruritus and exhibits a figure-of-eight vulvar rash, suspect lichen sclerosus (FIGURE 2). The cause of this condition is unclear. Often, allergic contact dermatitis is superimposed on it. In older gynecologic terminology, this was referred to as mixed vulvar dystrophy.
Women with lichen sclerosus are at increased risk for persistent vulvar yeast infection and squamous vulvar cancer.
Vulvar biopsy occasionally discloses unexpected early lichen sclerosus.

FIGURE 2 Lichen sclerosus
Note the labial agglutination (labia minora) and diffuse white epithelium, which are characteristic findings. If the areas of thickened epithelium do not resolve with topical steroid ointment, biopsy is appropriate.
Topical steroids are key to therapy
The degree of involvement that is grossly apparent determines the strength of the steroid ointment. If tissue is thickened, with areas of deep white change, a high-potency ointment such as clobetasol 0.05% may be necessary, applied twice daily for as long as several weeks. Milder cases may respond to a medium-strength topical steroid, such as fluticasone propionate 0.005% ointment.
If a higher-strength steroid is selected, it is appropriate to switch to a milder steroid as soon as symptoms resolve, with the goal of maintaining control with 1% hydrocortisone ointment or even continuous use of one of the skin moisturizers recommended for irritant dermatitis. Biopsy any thickened white patch, ulcerated area, or nonhealing skin fissure to check for squamous cancer.
Lichen planus
Erosive lichen planus (desquamative inflammatory vaginitis) of the vulva and vagina is an autoimmune skin disorder that causes superficial ulceration of the vaginal mucosa (FIGURE 3). An increase in vaginal discharge represents a shift in microflora away from lactobacillus dominance and an increase in the number of white blood cells and parabasal epithelial cells, with markedly heightened skin turnover. Local cellulitis does not develop, despite an overgrowth of various enteric organisms.

FIGURE 3 Lichen planus
This severe case resolved with a 3-month course of daily azathioprine (150 mg) but recurred after therapy ended.
A dermatologist may be required
Lichen planus is characterized by purulent discharge that contains bacteria, white blood cells, and parabasal cells. Unusual enteric microbes are often detected by routine culture, but antibiotic therapy is not helpful. Potent systemic anti-inflammatory therapy is often necessary rather than antimicrobial therapy. Daily azathioprine (Imuran) in doses ranging from 25 mg to 150 mg orally have been used, depending on the degree of vulvovaginal involvement. Tacrolimus ointment 0.1% applied twice daily may help in milder cases, but this agent typically causes an irritant reaction (burning) until the disorder partially resolves.
It may be helpful to seek the assistance of an experienced dermatologist if a biopsy demonstrates this disorder.
Vulvovaginal yeast infection is often found in conjunction with chronic vulvar eczema.3 Infection is promoted by:
- deficient skin microbicides
- skin-surface disruption with flaking
- ineffective Langerhans-cell response to invading yeast.
Yeast organisms release proteins that further activate a local allergic response to perpetuate an environment that supports infection (FIGURE 4). This represents a breakdown in the skin’s natural defenses.

FIGURE 4 Yeast infection
Vulvar yeast rash in the normally occluded area of the vulva with large satellite lesions (erythematous patches around the margin of the vulvar rash). Satellite lesions are typically much smaller.
Oral therapy may be preferred
Topical anti-yeast creams often contain propylene glycol, an irritant to fragile skin, so oral therapy may be more appropriate. For oral therapy, 200 mg of fluconazole every 3 days for three doses is a useful starting point. For severe cases, this can be followed by a weekly 200-mg oral dose for 2 to 3 months to maintain yeast suppression while the underlying skin disorder begins to resolve. An extended course of oral fluconazole may not be appropriate during pregnancy or anticoagulation or while the patient is taking a statin drug to lower cholesterol. If oral therapy is not appropriate, 1% clotrimazole 7-day vaginal cream is the only topical agent in the United States that does not contain propylene glycol.
Beauty may be only skin deep, but that layer of epidermis is a pretty busy place. Among its activities is the production of hundreds of substances that regulate susceptibility to infection. More than 50 of these chemicals fall into the class of skin microbicides.8
We began to learn about these microbicides a decade ago, when researchers asked why eczema usually is secondarily infected with pathogenic staphylococcus, streptococcus, and yeast, and psoriatic skin isn’t. The answer: Both healthy and psoriatic skin produce natural microbicides, but allergic dermatitis (eczema) prevents their release on the skin surface.9
1. Proteins
Some proteins fight microbes better than pharmaceutical agents do. The most important antimicrobial proteins in the skin are defensins and cathelicidins, which are found in all epithelial structures, including the vulva and vagina.10 In the defensin category, human ß-defensins 2 and 3 are the most important proteins and are present in the surface epithelium. An inflammatory response triggers their release to inhibit microbes on the skin surface. The mean inhibitory concentration of human ß-defensin 3 against the relatively resistant yeast, Saccharomyces cerevisiae, is about 14 μg/mL.11 This inhibitory action is superior to many azole anti-yeast agents. Human cathelicidin is an equally effective skin microbicide, with antiviral, antifungal, and antibacterial activity. In normal function, these natural antimicrobial substances prevent colonization of pathogenic organisms in healthy skin.
2. Stratum corneum
The stratum corneum comprises the outer few microns of the epithelium. When it remains intact, the stratum corneum is an effective barrier to microbial invasion. Intact skin prevents substances with a molecular weight greater than 500 daltons from passing into the skin.
This barrier may be compromised by microtrauma or dermatologic disorders such as irritant or allergic dermatitis. Minimal microtrauma is all that is necessary to allow small microbes such as viruses to pass through the stratum corneum. Larger organisms (spirochetes, yeast) may require a greater degree of compromise, such as flaking skin. Environmental and dermatologic factors often compromise this natural barrier.
The vaginal epithelium is not keratinized and lacks an effective stratum corneum. Instead, vaginal tissue produces mucus, which floats on a thin transudate of intercellular fluid. Potential pathogens are captured in the mucus and drain out of the vagina. The vaginal epithelium produces several milliliters of mucus daily that is constantly draining out of the vaginal lumen.
3. Langerhans cells
The skin has a final layer of defense within its structure. Microbes that pass through the stratum corneum and enter the skin are attacked by defensive cells that reside there, known as antigen-presenting cells. Langerhans cells are the chief antigen-presenting cells in the skin. They originate in the bone marrow, but rest in the skin, awaiting microbial invasion.
Antigen-presenting cells kill intraepithelial microbes as they are detected, and then process the microbial antigens, enabling a cell-mediated immune response against the microbes. Langerhans cells also destroy individual cancer cells that appear randomly in the epithelium.
The genital skin and the skin around the mouth and eyes carry the highest concentration of Langerhans cells.12 Under normal conditions, Langerhans cells constitute as much as 8% of the cells in vulvar skin. In the genital area, the cervical transformation zone has the highest count of Langerhans cells13—possibly compensation for a highly vulnerable epithelial barrier, owing to the immature squamous epithelium at this site.

Maturation of a Langerhans cell
Langerhans cells, the main antigen-presenting cells in skin, defend it from microbes that breach the stratum corneum. Although Langerhans cells originate in bone marrow, mature cells reside in the epidermis.
With its high concentration of Langerhans cells, the cervical transformation zone may be the primary port of entry of HIV.14 Langerhans cells have a surface CD4 receptor to which HIV attaches. The Langerhans cells are unable to kill the HIV after phagocytosis. HIV-infected Langerhans cells then lead to systemic spread of the virus.
Overall, the antimicrobial function of Langerhans cells is imperfect. When a pathogen is located within a cell, some microbes, such as Chlamydia trachomatis, herpesvirus, and HPV, may evade detection. In addition, some dermatologic conditions are associated with significant dysfunction of Langerhans cells.
HPV infection
The small HPV particle easily gains entry to minimally traumatized vulvar skin—with a high transmission rate with even a single exposure—and the immature epithelium of the cervical transformation zone makes that site a naturally compromised barrier to infection.
Under normal conditions, a cell-mediated immune response eliminates the HPV virus within 12 months, with lasting protection from reinfection. If cell-mediated immunity is compromised, the virus cannot be eliminated. The result is genital warts, variable degrees of dysplasia, or cancer, depending on the degree of immune compromise (FIGURE 5).
Risk factors for HPV-associated warts and dysplasia include allergy, immunosuppressant drugs to prevent rejection of a transplanted organ, and smoking. Smoking cessation is particularly important because control of the virus is dependent on Langerhans-cell function.

FIGURE 5 Genital warts
Debulking of warts with cryocautery or electrocautery may be appropriate, followed by imiquimod cream. It is prudent to biopsy persistent warts to exclude carcinoma, especially in an immunocompromised patient.
Begin by debulking warts
Electro- or cryocautery of large warts is an appropriate first step. Follow debulking with thrice-weekly application of one packet of imiquimod cream, to be washed off in the morning, for 4 to 6 weeks. This therapy may not eliminate genital warts in women who are taking immunosuppressant drugs to prevent organ-transplant rejection. Unfortunately, control of genital warts with monthly cautery of new warts may be the only useful option in these patients. Immunocompromised patients are at high risk for squamous carcinoma, and biopsy of persistent warts may be wise.
Vulnerabilities of vulvovaginal skin
The skin of the vulva and vagina is far from invincible. Some factors that affect it adversely are aging,4 tobacco use,5 estrogen deficiency,6 immunosuppressant drugs, and human immunodeficiency virus (HIV) infection.7 Look for these risk factors in women with persistent genital infection, so that the management plan can include treatment of the underlying dermatologic or immune disorder, as well as any microbes that are identified.
Immunosuppressed patients
Rejection of a transplanted organ is a function of cell-mediated immunity, so it is not surprising that drugs that suppress transplant rejection also inhibit vulvovaginal cell-mediated immunity. This increases the risk that HPV-associated disease will progress. Imiquimod cream promotes cell-mediated immunity by activating the release of interferon in the vulvar skin, and may compensate for depressed immune-cell function in the nontransplant population, but it is less effective in the transplant recipient. Sadly, there is no long-term solution to the effects of immunosuppressant therapy in this population; special surveillance for vulvar cancer and cervical dysplasia is necessary. Smoking cessation is also essential, especially for women with HPV-associated disease.
When HIV infection progresses to AIDS, Langerhans cells that carry HIV are depleted from the skin and substantially decrease in number, completely compromising cell-mediated immunity. This explains why AIDS patients often have severe genital herpes infections, severe chronic yeast vulvovaginitis, extensive molluscum disease, and unusual skin cancers. Antiretroviral therapy may restore some Langerhans-cell function.
Consider screening for HIV/AIDS when a woman has severe recurrent genital viral or yeast infection.
Aging and estrogen deficiency
Cell-mediated immune function declines with age. A higher risk of skin cancer, herpes (and its recurrence), and irritant and allergic vulvar dermatitis are the results. Increased surveillance for skin cancer, and varicella vaccination to lower the risk for herpes zoster, may be important.
Topical estrogen may be indicated if saline wet-prep evaluation reveals parabasal cells in vaginal secretions of a symptomatic postmenopausal woman. The estrogen may gradually alleviate burning and restrict potentially pathogenic bacterial flora. Be aware, however, that commercially available estrogen creams often contain propylene glycol, a recognized irritant of fragile skin. One solution: Have a compounding pharmacist formulate an equivalent cream (estradiol, 0.1 mg/g) for twice-daily topical application, using a base of petrolatum or solid vegetable oil.
Six common dermatologic disorders of the vulva and vagina can present considerable challenges:
- Allergic contact dermatitis: 100% of the population may be at risk for this disorder, and the vulvar skin is especially vulnerable.
- Irritant contact dermatitis: Skin-barrier compromise due to chronic, low-level vulvar irritant dermatitis likely contributes to the acquisition of sexually transmitted disease.
- Lichen sclerosus: Women with this condition are at increased risk for persistent vulvar yeast infection and squamous vulvar cancer.
- Lichen planus: Antibiotic therapy is ineffective against this disorder; symptoms reappear as soon as antibiotics are stopped.
- Yeast infection: Yeast organisms release proteins that activate a local allergic response and perpetuate an environment that supports infection.
- Human papillomavirus (HPV): The small HPV particle easily gains entry to minimally traumatized vulvar skin.
These common conditions sometimes compromise the skin barrier and prevent an adequate immune response to invading microbes. Some degree of skin immune dysfunction is generally associated with each genital dermatologic disorder. Identifying and treating the underlying dermatologic disorder, then, often corrects the associated immune dysfunction and may restore the skin barrier and prevent further microbial invasion.
Allergic contact dermatitis
Allergic dermatitis, or atopic dermatitis (formerly called eczema), is a highly prevalent skin disorder (FIGURE 1). Depending on the environment and genetic factors, as many as 40% of adults have a history of atopic dermatitis, and essentially 100% of the population may be at risk. Women have a higher rate of atopic dermatitis than men do.
Recognized vulvar allergens or triggers include dry climate, elastic, latex, fragrances in soaps or body lotions, and residues of detergent and fabric softener in clothing. In most biopsy-proven cases of vulvar eczema, the patient is unable to identify specific allergens. Such a patient often has a history of asthma, allergic rhinitis, sinusitis, or atopic dermatitis on other parts of the body.
Patch testing is no help in identifying specific allergens in the pelvic area. Testing the tougher skin of the back may not disclose all vulvar sensitivities.
But biopsy is useful. Women with vulvar allergic contact dermatitis often complain of persistent itching. A history of allergy elsewhere on the body is diagnostically helpful, but a biopsy submitted to a dermatopathologist confirms the diagnosis.
Not all women with vulvar allergic dermatitis have atopy at other body sites. Hyperkeratosis and spongiosis in the pathology specimen are characteristic. A small (3-or 4-mm) biopsy at the most symptomatic site is appropriate in any woman with chronic vulvar pruritis.
Local immune dysfunction is involved. Allergic vulvar dermatitis is characterized by a locally dysfunctional cell-mediated immune response. Langerhans cells are involved in this allergic reaction, directed away from their normal protective role.1 (Read about the role of Langerhans cells in “Three barriers to microbial infection: The skin’s built-in defense system,”) Viruses, bacteria, and yeast that gain entry into the skin have greater freedom to proliferate and persist, and skin-cancer surveillance by Langerhans cells is also compromised, with an increased risk for squamous carcinoma. This may account for a large portion of the 50% of vulvar carcinomas that cannot be attributed to HPV infection. Langerhans-cell dysfunction also contributes to the progression of HPV-associated carcinoma.
Allergic dermatitis inhibits the production of human cathelicidin and the ß-defensins, natural skin microbicides. As a result, vulvar skin affected by allergic dermatitis has a higher yeast and bacterial colonization rate.

FIGURE 1 Allergic contact dermatitis
This case of severe allergic contact dermatitis has been aggravated by chronic scratching, especially of the left labia. Cases typically are much more subtle.
Look for telltale flaking skin
Allergic dermatitis involves flaking of the skin, probably due to allergic stimulation of epithelial cell proliferation. Flakes of skin are exfoliated before the desmosomes that hold individual skin cells together deteriorate. The flaking compromises the stratum corneum barrier, and likely facilitates skin invasion of yeast and bacteria that have colonized the surface.
Because the background rate of dermatitis in the general population is relatively high, skin flakes often appear in the saline wet prep and are referred to as “reactive, reparative” changes in the Papanicolaou smear.2
Other diagnostic clues. Chronic vulvar pruritus with a history of asthma, hay fever, sinusitis, atopic dermatitis, or dry skin is vulvar dermatitis until it is proved otherwise. Recurrent yeast infection is often reported as well.
In many cases, the dermatitis may exhibit no clinical signs beyond flakes of skin in the saline wet prep.
Start with a topical steroid
A trial of topical steroid ointment is appropriate, using a low-to medium-strength ointment such as 0.1% hydrocortisone butyrate, which may also lower the risk of yeast infection.
Several weeks of treatment may be necessary. It may take 4 to 6 weeks for a full layer of skin to be replaced. Subdermal atrophy, skin neovascularization, and other risks of topical steroids are of less concern during extended use of low-potency steroids, and may be more acceptable on an unexposed part of the body such as the vulva.
To test for therapeutic success, look for a reduction in pruritus and a lower incidence of yeast infection. Failure of steroid ointment and oral yeast suppression may justify vulvar biopsy, which should be submitted to a dermatopathologist.
Occasionally, a high-potency topical steroid such as clobetasol 0.05% ointment may be necessary (applied twice daily and rubbed in), but adrenal suppression may develop if therapy exceeds 3 to 4 weeks. The agent should be tapered rather than stopped abruptly.
Irritant contact dermatitis
This condition is characterized by a burning sensation. Common vulvar irritants include oxylate (in urine), propylene glycol (in medicated creams and lotions), and abrasive toilet paper. The list of potential irritants is long, and each irritant may have a different mechanism of action. A burning reaction after application of a topical cream suggests significant compromise of the skin barrier that would otherwise have prevented entry of the irritant. Skin-barrier compromise due to chronic, low-level vulvar irritant dermatitis likely contributes to acquisition of sexually transmitted disease.
Begin by identifying the culprits
The first step of treatment is recognizing and eliminating potential irritants such as bath soap, urine, topical creams that contain propylene glycol, and soap residue in clothing. Have the patient use a squirt bottle to rinse the genital area after urination to eliminate irritants such as oxylate. Also suggest that she rinse undergarments twice and use liquid rather than powder detergent. Cotton undergarments are more skin-friendly than synthetics.
Twice-daily or more frequent application of a skin moisturizer such as vegetable shortening, MimyX cream, or mineral oil/petrolatum cream (Eletone) helps to heal the skin, and continued use may prevent recurrence of symptoms.
When a patient complains of persistent vulvar pruritus and exhibits a figure-of-eight vulvar rash, suspect lichen sclerosus (FIGURE 2). The cause of this condition is unclear. Often, allergic contact dermatitis is superimposed on it. In older gynecologic terminology, this was referred to as mixed vulvar dystrophy.
Women with lichen sclerosus are at increased risk for persistent vulvar yeast infection and squamous vulvar cancer.
Vulvar biopsy occasionally discloses unexpected early lichen sclerosus.

FIGURE 2 Lichen sclerosus
Note the labial agglutination (labia minora) and diffuse white epithelium, which are characteristic findings. If the areas of thickened epithelium do not resolve with topical steroid ointment, biopsy is appropriate.
Topical steroids are key to therapy
The degree of involvement that is grossly apparent determines the strength of the steroid ointment. If tissue is thickened, with areas of deep white change, a high-potency ointment such as clobetasol 0.05% may be necessary, applied twice daily for as long as several weeks. Milder cases may respond to a medium-strength topical steroid, such as fluticasone propionate 0.005% ointment.
If a higher-strength steroid is selected, it is appropriate to switch to a milder steroid as soon as symptoms resolve, with the goal of maintaining control with 1% hydrocortisone ointment or even continuous use of one of the skin moisturizers recommended for irritant dermatitis. Biopsy any thickened white patch, ulcerated area, or nonhealing skin fissure to check for squamous cancer.
Lichen planus
Erosive lichen planus (desquamative inflammatory vaginitis) of the vulva and vagina is an autoimmune skin disorder that causes superficial ulceration of the vaginal mucosa (FIGURE 3). An increase in vaginal discharge represents a shift in microflora away from lactobacillus dominance and an increase in the number of white blood cells and parabasal epithelial cells, with markedly heightened skin turnover. Local cellulitis does not develop, despite an overgrowth of various enteric organisms.

FIGURE 3 Lichen planus
This severe case resolved with a 3-month course of daily azathioprine (150 mg) but recurred after therapy ended.
A dermatologist may be required
Lichen planus is characterized by purulent discharge that contains bacteria, white blood cells, and parabasal cells. Unusual enteric microbes are often detected by routine culture, but antibiotic therapy is not helpful. Potent systemic anti-inflammatory therapy is often necessary rather than antimicrobial therapy. Daily azathioprine (Imuran) in doses ranging from 25 mg to 150 mg orally have been used, depending on the degree of vulvovaginal involvement. Tacrolimus ointment 0.1% applied twice daily may help in milder cases, but this agent typically causes an irritant reaction (burning) until the disorder partially resolves.
It may be helpful to seek the assistance of an experienced dermatologist if a biopsy demonstrates this disorder.
Vulvovaginal yeast infection is often found in conjunction with chronic vulvar eczema.3 Infection is promoted by:
- deficient skin microbicides
- skin-surface disruption with flaking
- ineffective Langerhans-cell response to invading yeast.
Yeast organisms release proteins that further activate a local allergic response to perpetuate an environment that supports infection (FIGURE 4). This represents a breakdown in the skin’s natural defenses.

FIGURE 4 Yeast infection
Vulvar yeast rash in the normally occluded area of the vulva with large satellite lesions (erythematous patches around the margin of the vulvar rash). Satellite lesions are typically much smaller.
Oral therapy may be preferred
Topical anti-yeast creams often contain propylene glycol, an irritant to fragile skin, so oral therapy may be more appropriate. For oral therapy, 200 mg of fluconazole every 3 days for three doses is a useful starting point. For severe cases, this can be followed by a weekly 200-mg oral dose for 2 to 3 months to maintain yeast suppression while the underlying skin disorder begins to resolve. An extended course of oral fluconazole may not be appropriate during pregnancy or anticoagulation or while the patient is taking a statin drug to lower cholesterol. If oral therapy is not appropriate, 1% clotrimazole 7-day vaginal cream is the only topical agent in the United States that does not contain propylene glycol.
Beauty may be only skin deep, but that layer of epidermis is a pretty busy place. Among its activities is the production of hundreds of substances that regulate susceptibility to infection. More than 50 of these chemicals fall into the class of skin microbicides.8
We began to learn about these microbicides a decade ago, when researchers asked why eczema usually is secondarily infected with pathogenic staphylococcus, streptococcus, and yeast, and psoriatic skin isn’t. The answer: Both healthy and psoriatic skin produce natural microbicides, but allergic dermatitis (eczema) prevents their release on the skin surface.9
1. Proteins
Some proteins fight microbes better than pharmaceutical agents do. The most important antimicrobial proteins in the skin are defensins and cathelicidins, which are found in all epithelial structures, including the vulva and vagina.10 In the defensin category, human ß-defensins 2 and 3 are the most important proteins and are present in the surface epithelium. An inflammatory response triggers their release to inhibit microbes on the skin surface. The mean inhibitory concentration of human ß-defensin 3 against the relatively resistant yeast, Saccharomyces cerevisiae, is about 14 μg/mL.11 This inhibitory action is superior to many azole anti-yeast agents. Human cathelicidin is an equally effective skin microbicide, with antiviral, antifungal, and antibacterial activity. In normal function, these natural antimicrobial substances prevent colonization of pathogenic organisms in healthy skin.
2. Stratum corneum
The stratum corneum comprises the outer few microns of the epithelium. When it remains intact, the stratum corneum is an effective barrier to microbial invasion. Intact skin prevents substances with a molecular weight greater than 500 daltons from passing into the skin.
This barrier may be compromised by microtrauma or dermatologic disorders such as irritant or allergic dermatitis. Minimal microtrauma is all that is necessary to allow small microbes such as viruses to pass through the stratum corneum. Larger organisms (spirochetes, yeast) may require a greater degree of compromise, such as flaking skin. Environmental and dermatologic factors often compromise this natural barrier.
The vaginal epithelium is not keratinized and lacks an effective stratum corneum. Instead, vaginal tissue produces mucus, which floats on a thin transudate of intercellular fluid. Potential pathogens are captured in the mucus and drain out of the vagina. The vaginal epithelium produces several milliliters of mucus daily that is constantly draining out of the vaginal lumen.
3. Langerhans cells
The skin has a final layer of defense within its structure. Microbes that pass through the stratum corneum and enter the skin are attacked by defensive cells that reside there, known as antigen-presenting cells. Langerhans cells are the chief antigen-presenting cells in the skin. They originate in the bone marrow, but rest in the skin, awaiting microbial invasion.
Antigen-presenting cells kill intraepithelial microbes as they are detected, and then process the microbial antigens, enabling a cell-mediated immune response against the microbes. Langerhans cells also destroy individual cancer cells that appear randomly in the epithelium.
The genital skin and the skin around the mouth and eyes carry the highest concentration of Langerhans cells.12 Under normal conditions, Langerhans cells constitute as much as 8% of the cells in vulvar skin. In the genital area, the cervical transformation zone has the highest count of Langerhans cells13—possibly compensation for a highly vulnerable epithelial barrier, owing to the immature squamous epithelium at this site.

Maturation of a Langerhans cell
Langerhans cells, the main antigen-presenting cells in skin, defend it from microbes that breach the stratum corneum. Although Langerhans cells originate in bone marrow, mature cells reside in the epidermis.
With its high concentration of Langerhans cells, the cervical transformation zone may be the primary port of entry of HIV.14 Langerhans cells have a surface CD4 receptor to which HIV attaches. The Langerhans cells are unable to kill the HIV after phagocytosis. HIV-infected Langerhans cells then lead to systemic spread of the virus.
Overall, the antimicrobial function of Langerhans cells is imperfect. When a pathogen is located within a cell, some microbes, such as Chlamydia trachomatis, herpesvirus, and HPV, may evade detection. In addition, some dermatologic conditions are associated with significant dysfunction of Langerhans cells.
HPV infection
The small HPV particle easily gains entry to minimally traumatized vulvar skin—with a high transmission rate with even a single exposure—and the immature epithelium of the cervical transformation zone makes that site a naturally compromised barrier to infection.
Under normal conditions, a cell-mediated immune response eliminates the HPV virus within 12 months, with lasting protection from reinfection. If cell-mediated immunity is compromised, the virus cannot be eliminated. The result is genital warts, variable degrees of dysplasia, or cancer, depending on the degree of immune compromise (FIGURE 5).
Risk factors for HPV-associated warts and dysplasia include allergy, immunosuppressant drugs to prevent rejection of a transplanted organ, and smoking. Smoking cessation is particularly important because control of the virus is dependent on Langerhans-cell function.

FIGURE 5 Genital warts
Debulking of warts with cryocautery or electrocautery may be appropriate, followed by imiquimod cream. It is prudent to biopsy persistent warts to exclude carcinoma, especially in an immunocompromised patient.
Begin by debulking warts
Electro- or cryocautery of large warts is an appropriate first step. Follow debulking with thrice-weekly application of one packet of imiquimod cream, to be washed off in the morning, for 4 to 6 weeks. This therapy may not eliminate genital warts in women who are taking immunosuppressant drugs to prevent organ-transplant rejection. Unfortunately, control of genital warts with monthly cautery of new warts may be the only useful option in these patients. Immunocompromised patients are at high risk for squamous carcinoma, and biopsy of persistent warts may be wise.
Vulnerabilities of vulvovaginal skin
The skin of the vulva and vagina is far from invincible. Some factors that affect it adversely are aging,4 tobacco use,5 estrogen deficiency,6 immunosuppressant drugs, and human immunodeficiency virus (HIV) infection.7 Look for these risk factors in women with persistent genital infection, so that the management plan can include treatment of the underlying dermatologic or immune disorder, as well as any microbes that are identified.
Immunosuppressed patients
Rejection of a transplanted organ is a function of cell-mediated immunity, so it is not surprising that drugs that suppress transplant rejection also inhibit vulvovaginal cell-mediated immunity. This increases the risk that HPV-associated disease will progress. Imiquimod cream promotes cell-mediated immunity by activating the release of interferon in the vulvar skin, and may compensate for depressed immune-cell function in the nontransplant population, but it is less effective in the transplant recipient. Sadly, there is no long-term solution to the effects of immunosuppressant therapy in this population; special surveillance for vulvar cancer and cervical dysplasia is necessary. Smoking cessation is also essential, especially for women with HPV-associated disease.
When HIV infection progresses to AIDS, Langerhans cells that carry HIV are depleted from the skin and substantially decrease in number, completely compromising cell-mediated immunity. This explains why AIDS patients often have severe genital herpes infections, severe chronic yeast vulvovaginitis, extensive molluscum disease, and unusual skin cancers. Antiretroviral therapy may restore some Langerhans-cell function.
Consider screening for HIV/AIDS when a woman has severe recurrent genital viral or yeast infection.
Aging and estrogen deficiency
Cell-mediated immune function declines with age. A higher risk of skin cancer, herpes (and its recurrence), and irritant and allergic vulvar dermatitis are the results. Increased surveillance for skin cancer, and varicella vaccination to lower the risk for herpes zoster, may be important.
Topical estrogen may be indicated if saline wet-prep evaluation reveals parabasal cells in vaginal secretions of a symptomatic postmenopausal woman. The estrogen may gradually alleviate burning and restrict potentially pathogenic bacterial flora. Be aware, however, that commercially available estrogen creams often contain propylene glycol, a recognized irritant of fragile skin. One solution: Have a compounding pharmacist formulate an equivalent cream (estradiol, 0.1 mg/g) for twice-daily topical application, using a base of petrolatum or solid vegetable oil.
1. Cruz PD. The epidermis: an outpost of the immune system. In: Freinkel RK, Woodley D, eds. The Biology of the Skin. New York: Parthenon Publishing Group; 2000:256-260.
2. Bonfiglio TA, Erozan YS. Gynecologic Cytopathology. Philadelphia: Lippincott-Raven; 1997:42-45.
3. Fidel PL, Sobel JD. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin Microbiol Rev. 1996;9:335-348.
4. Gilchrest B, Murphy G, Soter N. Effect of chronological aging and ultraviolet irradiation on Langerhans cells in human epidermis. J Invest Dermatol. 1982;79:85-88.
5. Ouyang Y, Virasch N, Hao P, et al. Suppression of human IL-1beta, IL-2, IFN-gamma, and TNF-alpha by cigarette smoke extracts. J Allergy Clin Immunol. 2000;106:280-287.
6. Mao A, Paharkova-Vatchkova V, Hardy J, et al. Estrogen selectively promotes the differentiation of dendritic cells with characteristics of Langerhans cells. J Immunol. 2005;175:5146-5151.
7. Memar OM, Geraminejad P, Arany I, Tyring SK. Cutaneous resistance to viral infections. In: Tyring SK, ed. Mucocutaneous Manifestations of Viral Diseases. New York: Marcel Dekker; 2002:25-28.
8. Braff MH, Bardan A, Nizet V, Gallo RL. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005;125:9-13.
9. Nomura I, Goleva E, Howell MD, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262-3269.
10. Harder J, Bartels J, Christophers E, Schroder JN. Isolation and characterization of human beta defensin-3, a novel inducible peptide antibiotic. J Biol Chem. 2001;276:5707-5713.
11. Garcia JR, Jaumann F, Schultz S, et al. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity. Cell Tissue Res. 2001;306:257-264.
12. Udey MC. Cadherins and Langerhans cell immunobiology. Clin Exp Immunol. 1997;107(suppl. 1):6-8.
13. Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253-1263.
14. Tschachler E, Groh V Popovic, et al. Epidermal Langerhans cells—a target for HTLV III/LAV infection. J Invest Dermatol. 1987;88:233-237.
1. Cruz PD. The epidermis: an outpost of the immune system. In: Freinkel RK, Woodley D, eds. The Biology of the Skin. New York: Parthenon Publishing Group; 2000:256-260.
2. Bonfiglio TA, Erozan YS. Gynecologic Cytopathology. Philadelphia: Lippincott-Raven; 1997:42-45.
3. Fidel PL, Sobel JD. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin Microbiol Rev. 1996;9:335-348.
4. Gilchrest B, Murphy G, Soter N. Effect of chronological aging and ultraviolet irradiation on Langerhans cells in human epidermis. J Invest Dermatol. 1982;79:85-88.
5. Ouyang Y, Virasch N, Hao P, et al. Suppression of human IL-1beta, IL-2, IFN-gamma, and TNF-alpha by cigarette smoke extracts. J Allergy Clin Immunol. 2000;106:280-287.
6. Mao A, Paharkova-Vatchkova V, Hardy J, et al. Estrogen selectively promotes the differentiation of dendritic cells with characteristics of Langerhans cells. J Immunol. 2005;175:5146-5151.
7. Memar OM, Geraminejad P, Arany I, Tyring SK. Cutaneous resistance to viral infections. In: Tyring SK, ed. Mucocutaneous Manifestations of Viral Diseases. New York: Marcel Dekker; 2002:25-28.
8. Braff MH, Bardan A, Nizet V, Gallo RL. Cutaneous defense mechanisms by antimicrobial peptides. J Invest Dermatol. 2005;125:9-13.
9. Nomura I, Goleva E, Howell MD, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262-3269.
10. Harder J, Bartels J, Christophers E, Schroder JN. Isolation and characterization of human beta defensin-3, a novel inducible peptide antibiotic. J Biol Chem. 2001;276:5707-5713.
11. Garcia JR, Jaumann F, Schultz S, et al. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity. Cell Tissue Res. 2001;306:257-264.
12. Udey MC. Cadherins and Langerhans cell immunobiology. Clin Exp Immunol. 1997;107(suppl. 1):6-8.
13. Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253-1263.
14. Tschachler E, Groh V Popovic, et al. Epidermal Langerhans cells—a target for HTLV III/LAV infection. J Invest Dermatol. 1987;88:233-237.