User login
CONTRACEPTION
Three new developments this year stand to make a difference in the high rate of unintended pregnancies in the United States. Increased use of highly effective, long-acting, user-independent methods is an effective way to lower the rate of unintended pregnancies in couples using contraception. Two such methods are the contraceptive implant and the intrauterine contraceptive. Emergency contraception is an effective way to reduce the risk of unintended pregnancy after failure of a contraceptive method or unprotected or forced sex. A randomized trial showed that direct access to EC does not increase high-risk behavior.
Half of pregnancies are unintended
- The United States has one of the highest rates of unintended births among industrialized countries.
- Of the 6 million pregnancies each year in the US, nearly 3 million are unintended, resulting in 1.4 million unintended births and 1.3 million abortions.
- Half of these unintended pregnancies are due to failure or incorrect or inconsistent use of a contraceptive method.1
REFERENCE
1. Contraception Counts: Ranking State Efforts. New York: Guttmacher Institute; February 2006.
Implanon essentials: How it works and what to tell patients
The single-rod implant (Implanon) is a new, highly effective, long-acting, rapidly reversible contraceptive, approved by the FDA, July 17, 2006.
A new single-rod implant that provides highly effective contraception for up to 3 years is expected to be widely available in the United States in 2007. Once inserted, Implanon is independent of user compliance and is rapidly effective and reversible. It is in use worldwide in more than 30 countries since 1998.
The new device is a nonbiodegradable 40 × 2.0 mm rod of 40% ethylene vinyl acetate (EVA) and 60% etonogestrel (ENG) covered with a rate-controlling EVA membrane.
The rod contains 68 mg of ENG, initially absorbed by the body at a rate of 60 μg/day, slowly declining to 30 μg/day after 3 years of use.2 Steady release of ENG into the circulation avoids first-pass effects on the liver.
Manufacturer-sponsored training: Call 1-877-IMPLANON
Before clinicians can order the implant, they must undergo training sponsored by the manufacturer, Organon. To take part in the training, which is set to begin in August, call 1-877-IMPLANON.
How the implant works
Ovarian and cervical mechanisms, which function prior to fertilization, provide high contraceptive efficacy.
Ovulation is suppressed. The ENG implant, unlike previous levonorgestrel-containing implants, works primarily by suppressing ovulation.3 ENG alters the hypothalamic–pituitary–ovarian axis and down-regulates the luteinizing hormone surge, which is required to support the production, growth, and maturation of ovarian follicles.
Ovulation returns rapidly after removal of the implant.3
Cervical mechanisms also prevent fertilization. Anti-estrogenic actions of ENG make the cervical mucus viscous, scanty, and impenetrable by sperm.
Cost-effectiveness depends on long-term use; early removal negates this benefit. At press time, the manufacturer had not released the price of Implanon.
Lack of protection against sexually transmitted infections is a disadvantage of the ENG implant, as well as all nonbarrier contraceptive methods.
Discontinuation rates have varied by region, but are usually due to bleeding pattern changes.
- In an international multicenter trial, 31% discontinued by 2 years and only 6% discontinued in the third year.4 Again, the most common reason was irregular bleeding.
- In a US series, 49% discontinued by 2 years. The most common reason was bleeding pattern changes (13%).5 The rate of discontinuation was highest during the first 8 months.
What to tell patients. To improve continuation, counseling should strongly stress the expected change in bleeding patterns.
Clinical trials
Outstanding efficacy. In an international multicenter trial, there were no intrauterine or ectopic pregnancies in a total of 1,200 woman-years (15,000 cycles of exposure, 2,000 of which were in the third year of use).4 The Pearl index was 0 (95% CI 0.0–0.2).4 In the US series, after a total exposure of 474 woman-years (6,186 cycles), no intrauterine or ectopic pregnancies were observed.5 It should be noted that phase III data from Indonesia were retracted by the manufacturer in 2004.6 The 2 trials noted above included a total of 965 women and were not included in this retraction.
Reasons for failures. Pregnancies were noted from postmarketing data in Australia; most of these pregnancies resulted from either incorrect timing at the initial insertion or failure to insert the implant. Based on the Australian phase IV data, with 204,486 devices inserted, the failure of the method itself was estimated to be 1 per 1,000 insertions.7 Implanon may be less effective in obese women or, as the Australian experience showed, with concomitant use of drugs that stimulate the liver’s cytochrome metabolism of steroids, such as some antibiotics (eg, rifampin) or anticonvulsants (eg, phenytoin).
Side effects
Infrequent bleeding. The main side effect is a change in bleeding patterns. In the US series, amenorrhea occurred in 14% to 20% of women. In the same series, women experienced infrequent bleeding (<3 episodes in 90 days) in 30% to 40% of the 90-day reference periods, making it the most common pattern experienced. Prolonged bleeding (>14 days of bleeding in one episode) varied from 14% to 36%, and frequent bleeding (>5 episodes in 90 days) varied from 7% to 14%.5 Anemia was not observed in the US series despite the irregular bleeding; in fact, hemoglobins rise.
Although the ENG implant is designed to facilitate rapid and simple insertion and removal, clinicians must first be trained. The manufacturer, Organon, announced last month that it would begin training doctors in August.
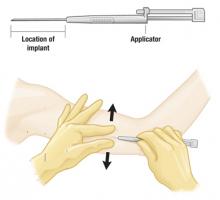
Insertion Average time: 1 minute4
The single-rod implant is preloaded in a disposable applicator. Insertion is done in the office using local anesthesia.
Place the 1.5-inch long implant on the inner aspect of the nondominant arm. Position the applicator needle subdermally and withdraw the cannula, leaving the implant rod in place.
After insertion, the implant may not be visible but should be palpable.
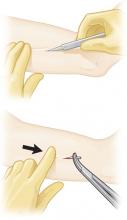
Removal Average time: 4 minutes4
Removal requires a 2- to 3-mm incision at the distal tip of the implant. Push the other end of the rod until it pops out.
Timing the insertion
- Between days 1 and 5 of menses, in women who either have not been using a contraceptive method or have been using a nonhormonal method
- During a hormone-free week, in women changing from a combination or progestin-only oral contraceptive, or from intrauterine contraception
- The day on which the next injection is scheduled, in women changing from injectable contraception
No backup contraceptive is necessary if timing of insertion occurs as detailed.
In all cases, exclude pregnancy before insertion.
Timing the removal
The ENG implant can be removed at any time, but must be removed after 3 years.
Return to ovulation is rapid following removal, so women still desiring contraception should begin another method immediately or have a new rod inserted through the removal incision.
Unpredictable bleeding pattern. Unlike with Norplant, there was no trend over time toward a particular bleeding pattern. Implanon patterns are irregular and unpredictable and vary from one 90-day reference period to the next. Similar results were noted in the multicenter international trial.4
Possible therapies include estrogen supplementation, nonsteroidal anti-inflammatory drugs, oral contraceptive pills, and observation.
Other side effects. In the US series, the most frequent nonmenstrual adverse effects possibly related to the ENG implant were acne (14.5%), headache (12.7%), weight gain (12.1%), and emotional lability (14.2%).
Contraindications
The ENG implant should not be placed in women with undiagnosed abnormal genital bleeding, known or suspected pregnancy, or hypersensitivity to any of the components in the ENG implant.
REFERENCES
Why the FDA removed ParaGard’s parity restriction
- An FDA labeling change for the ParaGard intrauterine device confirms what the evidence has long supported: The risk of pelvic infection is more related to sexual behavior than to age, contraceptive choice, or parity
- Evidence supports a link between cervical infection—but not IUD use—and pelvic inflammatory disease and infertility
The Food and Drug Administration has approved a less restrictive label for the ParaGard T380A copper intrauterine contraceptive. Evidence has long supported the conclusion that risk of pelvic infection is related more to a woman’s and her partner’s sexual behavior than to her age, contraceptive choice, or parity.
A woman with at least one child and in a mutually monogamous relationship is no longer listed as the recommended patient profile. Nor is ParaGard contraindicated for a woman with a history of sexually transmitted disease or pelvic inflammatory disease (PID), unless she has current acute PID or engages in sexual behavior suggesting a high risk for PID.
Why was the label restrictive to begin with?
Early studies8,9 that showed an increased risk of PID and infertility in intrauterine contraceptive users have been re-analyzed; most of the increased risk was associated with a single type of intrauterine contraceptive that is no longer on the market (Dalkon Shield), and with high-risk sexual behaviors.10-12 In most analyses of these studies, the increased risk of PID was present only in the first 20 days after insertion, indicating undiagnosed cervical infection at the time of insertion.
Furthermore, many studies had methodological flaws that introduced bias into the results, such as comparing intrauterine contraceptive users with users of combination oral contraceptives (who have a decreased risk of PID compared with nonusers).13
These early studies also equated nulliparity with high-risk sexual behavior. As young women are more likely to acquire sexually transmitted cervical infections, and because young age is associated with nulliparity, many studies erroneously concluded that the increased risk of PID and infertility was attributed to nulliparity.
A case-control study in nulliparous Mexican women who were seeking treatment for primary infertility found no association between tubal infertility and past copper IUD use. In this study, 358 women with primary infertility and documented tubal occlusion (cases) were compared with two sets of controls: 953 nulliparous women with primary infertility and no tubal occlusion, and 584 primigravid women. Past use of a copper IUD was not associated with tubal occlusion, compared with either infertile women without tubal occlusion or primigravid controls (P values 1.0 and 0.9, respectively).14 However, tubal infertility was associated with a past infection with Chlamydia (as evidenced by Chlamydia antibodies). This study further supports an association between PID and infertility and cervical infection—not IUD use.
Protective effect of progestin
The levonorgestrel-releasing intrauterine system (LNG-IUS) may even protect against PID. One of the primary physiologic effects of progestin contraception is thickening of the cervical mucus, which protects against ascending genital tract infection. This protective effect results in a decreased incidence of PID in women who use combination oral contraceptive pills, progestin implants, and progestin injectables.15 A randomized controlled trial found that the cumulative 36-month rate of PID was lower in users of a LNG-IUS contraceptive than in users of a copper IUD (Nova-T) (0.5 and 2.0, respectively; P< 0.013), in both parous and nulliparous women.16 This finding was more marked in women under the age of 25.
Prescribing IUDs in young women
When considering an intrauterine contraceptive for a young woman, it is therefore important to assess her risk of a STI, based on her and her partner’s sexual behavior, and not on parity or age. It is important to screen for STIs at the time of or prior to insertion of an intrauterine contraceptive, and to treat cervicitis prior to insertion.
Nulliparous women who are at low risk of STIs can be offered the intrauterine contraceptive as an effective, long-term, user-independent contraception.
The labeling for levonorgestrel intrauterine contraceptives should also reflect the evidence that the risk of pelvic infection is more related to a patient’s and/or her partner’s sexual behavior than to her age, contraceptive choice, or parity.
REFERENCES
Direct access to Plan B does not promote high-risk behavior
Raine TR, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54–62.
- Advance provision of emergency contraception results in increased usage of emergency contraception without an associated change in risky sexual behavior, sexually transmitted diseases, or use of long-term contraception
- Clinicians should provide emergency contraception in advance of need to ensure timely and appropriate emergency contraceptive use
Emergency contraception could significantly reduce the risk of unintended pregnancy after contraceptive method failure, or unprotected or forced sex. The newer progestin-only emergency contraceptive pills have now largely replaced the older combined (estrogen and progestin) pills because they are more effective and have fewer side effects.
Various emergency contraception regimens are effective
The only dedicated progestin-only emergency contraception pill product in the United States is Plan B, which contains 2 tablets of 0.75 mg of levonorgestrel. Although the recommended treatment schedule is an initial dose within 72 hours of unprotected intercourse and a second dose 12 hours later, a single dose of 1.5 mg of levonorgestrel is as effective as and causes no more side effects than 2 tablets of 0.75-mg doses 12 hours apart.17,18
The sooner the better?
Emergency contraception pills are more effective the sooner after sex that they are initiated. Both combination oral contraceptive pills and progestin-only regimens are moderately effective even if initiated more than 72 hours after unprotected intercourse. 18-20 No data are available on the efficacy of emergency contraception pills taken more than 120 hours (5 days) after unprotected intercourse.
Randomized trial
“It seems unreasonable to restrict access”
Raine et al21 added significantly to our knowledge of emergency contraception. This is the first randomized trial addressing the question of the effect of access on emergency contraceptive usage. A total of 2,117 young women (age 15 to 24 years) were randomly assigned to these 3 groups:
- Pharmacy access (without consulting a physician)
- Advance provision of 3 packs of Plan B
- Clinic access (ie, usual care, which required a clinic visit to obtain emergency contraception)
The study concluded: “While removing the requirement to go through pharmacists or clinics to obtain emergency contraception increases use, the public health impact may be negligible because of high rates of unprotected intercourse and relative underutilization of the method. Given that there is clear evidence that neither pharmacy access nor advance provision compromises contraceptive or sexual behavior, it seems unreasonable to restrict access to emergency contraception to clinics.” The conclusion reflected the following several outcomes, which were assessed after 6 months.
4 key outcomes
- Use of emergency contraception
- The advance provision group used emergency contraception at nearly twice the rate (37.4%) of the clinic access group (21.0%).
- Usage rates were similar in the pharmacy access (24.2%) and the clinic access group (21.0%).
- New sexually transmitted infection rates were similar in all groups
Levels of STIs, such as Chlamydia, were similar across all groups, and changes in HSV-2 serology were similar across all groups. - Many did not use the EC, even with advance provision
In this study, advance provision of emergency contraceptives did not lower pregnancy rates. This finding is disappointing; the likely explanation is that women at highest risk do not use emergency contraception often enough or at all. Thus, the overall pregnancy rate is unchanged. Nearly half (45%) of the women in the study who reported having unprotected sex did not use emergency contraception during the study period, even when they received it in advance. - High-risk sexual behavior did not increase in any group
Women who had increased access to emergency contraception did not have sex more frequently. Receiving emergency contraceptives in advance did not affect the number of sex partners, with most women having only one partner. Data on teens in the same study found that teens did not take more sexual risks than women aged 20 to 24.22
A concern with placing emergency contraception directly in the hands of women has been the theory that it would result in increased high-risk behavior and lower use of regular contraception.
This trial found:
- That women with pharmacy access and women given 3 packs of emergency contraceptives in advance were no more likely to change their regular contraceptive method than women who could obtain emergency contraception only via a clinic visit.
- That women with increased access to emergency contraceptives use their routine contraception with the same consistency as women without increased access.
No downside to easier, wider access to Plan B
This trial adds to the argument for wider and easier access to emergency contraception for women. There is no apparent downside from wide access to the current progestin-only emergency contraception regimen. The latest World Health Organization medical eligibility criteria describe no situation in which the risks of emergency contraception outweigh the benefits.23 The study by Raine et al21 provides evidence against concerns about the potential for increased high-risk sexual behavior.
Eight states (Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Washington) have passed legislation allowing pharmacists to prescribe emergency contraceptives without a prescription. Norway, Sweden, India, and the Netherlands allow emergency contraceptive availability over-the-counter.
17. Arowojolu AO, Okewole IA, Adekunle AO. Comparative evaluation of the effectiveness and safety of two regimens of levonorgestrel for emergency contraception in Nigerians. Contraception. 2002;66:269-273.
18. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803-1810.
19. Ellertson C, Evans M, Ferden S, et al. Extending the time limit for starting the Yuzpe regimen of emergency contraception to 120 hours. Obstet Gynecol. 2003;101:1168-1171.
20. Rodrigues I, Grou F, Joly J. Effectiveness of emergency contraceptive pills between 72 and 120 hours after unprotected sexual intercourse. Am J Obstet Gynecol. 2001;184:531-537.
21. Raine TR, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
22. Harper CC, Cheong M, Rocca CH, Darney PD, Raine TR. The effect of increased access to emergency contraception among young adolescents. Obstet Gynecol. 2005;106:483-491.
23. Medical Eligibilty Criteria for Contraceptive Use. Geneva: World Health Organisation; 2004
Dr. Parvataneni is a consultant to Organon. Dr. Darney is a consultant to Organon and is a speaker for Berlex and Organon.
Three new developments this year stand to make a difference in the high rate of unintended pregnancies in the United States. Increased use of highly effective, long-acting, user-independent methods is an effective way to lower the rate of unintended pregnancies in couples using contraception. Two such methods are the contraceptive implant and the intrauterine contraceptive. Emergency contraception is an effective way to reduce the risk of unintended pregnancy after failure of a contraceptive method or unprotected or forced sex. A randomized trial showed that direct access to EC does not increase high-risk behavior.
Half of pregnancies are unintended
- The United States has one of the highest rates of unintended births among industrialized countries.
- Of the 6 million pregnancies each year in the US, nearly 3 million are unintended, resulting in 1.4 million unintended births and 1.3 million abortions.
- Half of these unintended pregnancies are due to failure or incorrect or inconsistent use of a contraceptive method.1
REFERENCE
1. Contraception Counts: Ranking State Efforts. New York: Guttmacher Institute; February 2006.
Implanon essentials: How it works and what to tell patients
The single-rod implant (Implanon) is a new, highly effective, long-acting, rapidly reversible contraceptive, approved by the FDA, July 17, 2006.
A new single-rod implant that provides highly effective contraception for up to 3 years is expected to be widely available in the United States in 2007. Once inserted, Implanon is independent of user compliance and is rapidly effective and reversible. It is in use worldwide in more than 30 countries since 1998.
The new device is a nonbiodegradable 40 × 2.0 mm rod of 40% ethylene vinyl acetate (EVA) and 60% etonogestrel (ENG) covered with a rate-controlling EVA membrane.
The rod contains 68 mg of ENG, initially absorbed by the body at a rate of 60 μg/day, slowly declining to 30 μg/day after 3 years of use.2 Steady release of ENG into the circulation avoids first-pass effects on the liver.
Manufacturer-sponsored training: Call 1-877-IMPLANON
Before clinicians can order the implant, they must undergo training sponsored by the manufacturer, Organon. To take part in the training, which is set to begin in August, call 1-877-IMPLANON.
How the implant works
Ovarian and cervical mechanisms, which function prior to fertilization, provide high contraceptive efficacy.
Ovulation is suppressed. The ENG implant, unlike previous levonorgestrel-containing implants, works primarily by suppressing ovulation.3 ENG alters the hypothalamic–pituitary–ovarian axis and down-regulates the luteinizing hormone surge, which is required to support the production, growth, and maturation of ovarian follicles.
Ovulation returns rapidly after removal of the implant.3
Cervical mechanisms also prevent fertilization. Anti-estrogenic actions of ENG make the cervical mucus viscous, scanty, and impenetrable by sperm.
Cost-effectiveness depends on long-term use; early removal negates this benefit. At press time, the manufacturer had not released the price of Implanon.
Lack of protection against sexually transmitted infections is a disadvantage of the ENG implant, as well as all nonbarrier contraceptive methods.
Discontinuation rates have varied by region, but are usually due to bleeding pattern changes.
- In an international multicenter trial, 31% discontinued by 2 years and only 6% discontinued in the third year.4 Again, the most common reason was irregular bleeding.
- In a US series, 49% discontinued by 2 years. The most common reason was bleeding pattern changes (13%).5 The rate of discontinuation was highest during the first 8 months.
What to tell patients. To improve continuation, counseling should strongly stress the expected change in bleeding patterns.
Clinical trials
Outstanding efficacy. In an international multicenter trial, there were no intrauterine or ectopic pregnancies in a total of 1,200 woman-years (15,000 cycles of exposure, 2,000 of which were in the third year of use).4 The Pearl index was 0 (95% CI 0.0–0.2).4 In the US series, after a total exposure of 474 woman-years (6,186 cycles), no intrauterine or ectopic pregnancies were observed.5 It should be noted that phase III data from Indonesia were retracted by the manufacturer in 2004.6 The 2 trials noted above included a total of 965 women and were not included in this retraction.
Reasons for failures. Pregnancies were noted from postmarketing data in Australia; most of these pregnancies resulted from either incorrect timing at the initial insertion or failure to insert the implant. Based on the Australian phase IV data, with 204,486 devices inserted, the failure of the method itself was estimated to be 1 per 1,000 insertions.7 Implanon may be less effective in obese women or, as the Australian experience showed, with concomitant use of drugs that stimulate the liver’s cytochrome metabolism of steroids, such as some antibiotics (eg, rifampin) or anticonvulsants (eg, phenytoin).
Side effects
Infrequent bleeding. The main side effect is a change in bleeding patterns. In the US series, amenorrhea occurred in 14% to 20% of women. In the same series, women experienced infrequent bleeding (<3 episodes in 90 days) in 30% to 40% of the 90-day reference periods, making it the most common pattern experienced. Prolonged bleeding (>14 days of bleeding in one episode) varied from 14% to 36%, and frequent bleeding (>5 episodes in 90 days) varied from 7% to 14%.5 Anemia was not observed in the US series despite the irregular bleeding; in fact, hemoglobins rise.
Although the ENG implant is designed to facilitate rapid and simple insertion and removal, clinicians must first be trained. The manufacturer, Organon, announced last month that it would begin training doctors in August.
Insertion Average time: 1 minute4
The single-rod implant is preloaded in a disposable applicator. Insertion is done in the office using local anesthesia.
Place the 1.5-inch long implant on the inner aspect of the nondominant arm. Position the applicator needle subdermally and withdraw the cannula, leaving the implant rod in place.
After insertion, the implant may not be visible but should be palpable.
Removal Average time: 4 minutes4
Removal requires a 2- to 3-mm incision at the distal tip of the implant. Push the other end of the rod until it pops out.
Timing the insertion
- Between days 1 and 5 of menses, in women who either have not been using a contraceptive method or have been using a nonhormonal method
- During a hormone-free week, in women changing from a combination or progestin-only oral contraceptive, or from intrauterine contraception
- The day on which the next injection is scheduled, in women changing from injectable contraception
No backup contraceptive is necessary if timing of insertion occurs as detailed.
In all cases, exclude pregnancy before insertion.
Timing the removal
The ENG implant can be removed at any time, but must be removed after 3 years.
Return to ovulation is rapid following removal, so women still desiring contraception should begin another method immediately or have a new rod inserted through the removal incision.
Unpredictable bleeding pattern. Unlike with Norplant, there was no trend over time toward a particular bleeding pattern. Implanon patterns are irregular and unpredictable and vary from one 90-day reference period to the next. Similar results were noted in the multicenter international trial.4
Possible therapies include estrogen supplementation, nonsteroidal anti-inflammatory drugs, oral contraceptive pills, and observation.
Other side effects. In the US series, the most frequent nonmenstrual adverse effects possibly related to the ENG implant were acne (14.5%), headache (12.7%), weight gain (12.1%), and emotional lability (14.2%).
Contraindications
The ENG implant should not be placed in women with undiagnosed abnormal genital bleeding, known or suspected pregnancy, or hypersensitivity to any of the components in the ENG implant.
REFERENCES
Why the FDA removed ParaGard’s parity restriction
- An FDA labeling change for the ParaGard intrauterine device confirms what the evidence has long supported: The risk of pelvic infection is more related to sexual behavior than to age, contraceptive choice, or parity
- Evidence supports a link between cervical infection—but not IUD use—and pelvic inflammatory disease and infertility
The Food and Drug Administration has approved a less restrictive label for the ParaGard T380A copper intrauterine contraceptive. Evidence has long supported the conclusion that risk of pelvic infection is related more to a woman’s and her partner’s sexual behavior than to her age, contraceptive choice, or parity.
A woman with at least one child and in a mutually monogamous relationship is no longer listed as the recommended patient profile. Nor is ParaGard contraindicated for a woman with a history of sexually transmitted disease or pelvic inflammatory disease (PID), unless she has current acute PID or engages in sexual behavior suggesting a high risk for PID.
Why was the label restrictive to begin with?
Early studies8,9 that showed an increased risk of PID and infertility in intrauterine contraceptive users have been re-analyzed; most of the increased risk was associated with a single type of intrauterine contraceptive that is no longer on the market (Dalkon Shield), and with high-risk sexual behaviors.10-12 In most analyses of these studies, the increased risk of PID was present only in the first 20 days after insertion, indicating undiagnosed cervical infection at the time of insertion.
Furthermore, many studies had methodological flaws that introduced bias into the results, such as comparing intrauterine contraceptive users with users of combination oral contraceptives (who have a decreased risk of PID compared with nonusers).13
These early studies also equated nulliparity with high-risk sexual behavior. As young women are more likely to acquire sexually transmitted cervical infections, and because young age is associated with nulliparity, many studies erroneously concluded that the increased risk of PID and infertility was attributed to nulliparity.
A case-control study in nulliparous Mexican women who were seeking treatment for primary infertility found no association between tubal infertility and past copper IUD use. In this study, 358 women with primary infertility and documented tubal occlusion (cases) were compared with two sets of controls: 953 nulliparous women with primary infertility and no tubal occlusion, and 584 primigravid women. Past use of a copper IUD was not associated with tubal occlusion, compared with either infertile women without tubal occlusion or primigravid controls (P values 1.0 and 0.9, respectively).14 However, tubal infertility was associated with a past infection with Chlamydia (as evidenced by Chlamydia antibodies). This study further supports an association between PID and infertility and cervical infection—not IUD use.
Protective effect of progestin
The levonorgestrel-releasing intrauterine system (LNG-IUS) may even protect against PID. One of the primary physiologic effects of progestin contraception is thickening of the cervical mucus, which protects against ascending genital tract infection. This protective effect results in a decreased incidence of PID in women who use combination oral contraceptive pills, progestin implants, and progestin injectables.15 A randomized controlled trial found that the cumulative 36-month rate of PID was lower in users of a LNG-IUS contraceptive than in users of a copper IUD (Nova-T) (0.5 and 2.0, respectively; P< 0.013), in both parous and nulliparous women.16 This finding was more marked in women under the age of 25.
Prescribing IUDs in young women
When considering an intrauterine contraceptive for a young woman, it is therefore important to assess her risk of a STI, based on her and her partner’s sexual behavior, and not on parity or age. It is important to screen for STIs at the time of or prior to insertion of an intrauterine contraceptive, and to treat cervicitis prior to insertion.
Nulliparous women who are at low risk of STIs can be offered the intrauterine contraceptive as an effective, long-term, user-independent contraception.
The labeling for levonorgestrel intrauterine contraceptives should also reflect the evidence that the risk of pelvic infection is more related to a patient’s and/or her partner’s sexual behavior than to her age, contraceptive choice, or parity.
REFERENCES
Direct access to Plan B does not promote high-risk behavior
Raine TR, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54–62.
- Advance provision of emergency contraception results in increased usage of emergency contraception without an associated change in risky sexual behavior, sexually transmitted diseases, or use of long-term contraception
- Clinicians should provide emergency contraception in advance of need to ensure timely and appropriate emergency contraceptive use
Emergency contraception could significantly reduce the risk of unintended pregnancy after contraceptive method failure, or unprotected or forced sex. The newer progestin-only emergency contraceptive pills have now largely replaced the older combined (estrogen and progestin) pills because they are more effective and have fewer side effects.
Various emergency contraception regimens are effective
The only dedicated progestin-only emergency contraception pill product in the United States is Plan B, which contains 2 tablets of 0.75 mg of levonorgestrel. Although the recommended treatment schedule is an initial dose within 72 hours of unprotected intercourse and a second dose 12 hours later, a single dose of 1.5 mg of levonorgestrel is as effective as and causes no more side effects than 2 tablets of 0.75-mg doses 12 hours apart.17,18
The sooner the better?
Emergency contraception pills are more effective the sooner after sex that they are initiated. Both combination oral contraceptive pills and progestin-only regimens are moderately effective even if initiated more than 72 hours after unprotected intercourse. 18-20 No data are available on the efficacy of emergency contraception pills taken more than 120 hours (5 days) after unprotected intercourse.
Randomized trial
“It seems unreasonable to restrict access”
Raine et al21 added significantly to our knowledge of emergency contraception. This is the first randomized trial addressing the question of the effect of access on emergency contraceptive usage. A total of 2,117 young women (age 15 to 24 years) were randomly assigned to these 3 groups:
- Pharmacy access (without consulting a physician)
- Advance provision of 3 packs of Plan B
- Clinic access (ie, usual care, which required a clinic visit to obtain emergency contraception)
The study concluded: “While removing the requirement to go through pharmacists or clinics to obtain emergency contraception increases use, the public health impact may be negligible because of high rates of unprotected intercourse and relative underutilization of the method. Given that there is clear evidence that neither pharmacy access nor advance provision compromises contraceptive or sexual behavior, it seems unreasonable to restrict access to emergency contraception to clinics.” The conclusion reflected the following several outcomes, which were assessed after 6 months.
4 key outcomes
- Use of emergency contraception
- The advance provision group used emergency contraception at nearly twice the rate (37.4%) of the clinic access group (21.0%).
- Usage rates were similar in the pharmacy access (24.2%) and the clinic access group (21.0%).
- New sexually transmitted infection rates were similar in all groups
Levels of STIs, such as Chlamydia, were similar across all groups, and changes in HSV-2 serology were similar across all groups. - Many did not use the EC, even with advance provision
In this study, advance provision of emergency contraceptives did not lower pregnancy rates. This finding is disappointing; the likely explanation is that women at highest risk do not use emergency contraception often enough or at all. Thus, the overall pregnancy rate is unchanged. Nearly half (45%) of the women in the study who reported having unprotected sex did not use emergency contraception during the study period, even when they received it in advance. - High-risk sexual behavior did not increase in any group
Women who had increased access to emergency contraception did not have sex more frequently. Receiving emergency contraceptives in advance did not affect the number of sex partners, with most women having only one partner. Data on teens in the same study found that teens did not take more sexual risks than women aged 20 to 24.22
A concern with placing emergency contraception directly in the hands of women has been the theory that it would result in increased high-risk behavior and lower use of regular contraception.
This trial found:
- That women with pharmacy access and women given 3 packs of emergency contraceptives in advance were no more likely to change their regular contraceptive method than women who could obtain emergency contraception only via a clinic visit.
- That women with increased access to emergency contraceptives use their routine contraception with the same consistency as women without increased access.
No downside to easier, wider access to Plan B
This trial adds to the argument for wider and easier access to emergency contraception for women. There is no apparent downside from wide access to the current progestin-only emergency contraception regimen. The latest World Health Organization medical eligibility criteria describe no situation in which the risks of emergency contraception outweigh the benefits.23 The study by Raine et al21 provides evidence against concerns about the potential for increased high-risk sexual behavior.
Eight states (Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Washington) have passed legislation allowing pharmacists to prescribe emergency contraceptives without a prescription. Norway, Sweden, India, and the Netherlands allow emergency contraceptive availability over-the-counter.
Three new developments this year stand to make a difference in the high rate of unintended pregnancies in the United States. Increased use of highly effective, long-acting, user-independent methods is an effective way to lower the rate of unintended pregnancies in couples using contraception. Two such methods are the contraceptive implant and the intrauterine contraceptive. Emergency contraception is an effective way to reduce the risk of unintended pregnancy after failure of a contraceptive method or unprotected or forced sex. A randomized trial showed that direct access to EC does not increase high-risk behavior.
Half of pregnancies are unintended
- The United States has one of the highest rates of unintended births among industrialized countries.
- Of the 6 million pregnancies each year in the US, nearly 3 million are unintended, resulting in 1.4 million unintended births and 1.3 million abortions.
- Half of these unintended pregnancies are due to failure or incorrect or inconsistent use of a contraceptive method.1
REFERENCE
1. Contraception Counts: Ranking State Efforts. New York: Guttmacher Institute; February 2006.
Implanon essentials: How it works and what to tell patients
The single-rod implant (Implanon) is a new, highly effective, long-acting, rapidly reversible contraceptive, approved by the FDA, July 17, 2006.
A new single-rod implant that provides highly effective contraception for up to 3 years is expected to be widely available in the United States in 2007. Once inserted, Implanon is independent of user compliance and is rapidly effective and reversible. It is in use worldwide in more than 30 countries since 1998.
The new device is a nonbiodegradable 40 × 2.0 mm rod of 40% ethylene vinyl acetate (EVA) and 60% etonogestrel (ENG) covered with a rate-controlling EVA membrane.
The rod contains 68 mg of ENG, initially absorbed by the body at a rate of 60 μg/day, slowly declining to 30 μg/day after 3 years of use.2 Steady release of ENG into the circulation avoids first-pass effects on the liver.
Manufacturer-sponsored training: Call 1-877-IMPLANON
Before clinicians can order the implant, they must undergo training sponsored by the manufacturer, Organon. To take part in the training, which is set to begin in August, call 1-877-IMPLANON.
How the implant works
Ovarian and cervical mechanisms, which function prior to fertilization, provide high contraceptive efficacy.
Ovulation is suppressed. The ENG implant, unlike previous levonorgestrel-containing implants, works primarily by suppressing ovulation.3 ENG alters the hypothalamic–pituitary–ovarian axis and down-regulates the luteinizing hormone surge, which is required to support the production, growth, and maturation of ovarian follicles.
Ovulation returns rapidly after removal of the implant.3
Cervical mechanisms also prevent fertilization. Anti-estrogenic actions of ENG make the cervical mucus viscous, scanty, and impenetrable by sperm.
Cost-effectiveness depends on long-term use; early removal negates this benefit. At press time, the manufacturer had not released the price of Implanon.
Lack of protection against sexually transmitted infections is a disadvantage of the ENG implant, as well as all nonbarrier contraceptive methods.
Discontinuation rates have varied by region, but are usually due to bleeding pattern changes.
- In an international multicenter trial, 31% discontinued by 2 years and only 6% discontinued in the third year.4 Again, the most common reason was irregular bleeding.
- In a US series, 49% discontinued by 2 years. The most common reason was bleeding pattern changes (13%).5 The rate of discontinuation was highest during the first 8 months.
What to tell patients. To improve continuation, counseling should strongly stress the expected change in bleeding patterns.
Clinical trials
Outstanding efficacy. In an international multicenter trial, there were no intrauterine or ectopic pregnancies in a total of 1,200 woman-years (15,000 cycles of exposure, 2,000 of which were in the third year of use).4 The Pearl index was 0 (95% CI 0.0–0.2).4 In the US series, after a total exposure of 474 woman-years (6,186 cycles), no intrauterine or ectopic pregnancies were observed.5 It should be noted that phase III data from Indonesia were retracted by the manufacturer in 2004.6 The 2 trials noted above included a total of 965 women and were not included in this retraction.
Reasons for failures. Pregnancies were noted from postmarketing data in Australia; most of these pregnancies resulted from either incorrect timing at the initial insertion or failure to insert the implant. Based on the Australian phase IV data, with 204,486 devices inserted, the failure of the method itself was estimated to be 1 per 1,000 insertions.7 Implanon may be less effective in obese women or, as the Australian experience showed, with concomitant use of drugs that stimulate the liver’s cytochrome metabolism of steroids, such as some antibiotics (eg, rifampin) or anticonvulsants (eg, phenytoin).
Side effects
Infrequent bleeding. The main side effect is a change in bleeding patterns. In the US series, amenorrhea occurred in 14% to 20% of women. In the same series, women experienced infrequent bleeding (<3 episodes in 90 days) in 30% to 40% of the 90-day reference periods, making it the most common pattern experienced. Prolonged bleeding (>14 days of bleeding in one episode) varied from 14% to 36%, and frequent bleeding (>5 episodes in 90 days) varied from 7% to 14%.5 Anemia was not observed in the US series despite the irregular bleeding; in fact, hemoglobins rise.
Although the ENG implant is designed to facilitate rapid and simple insertion and removal, clinicians must first be trained. The manufacturer, Organon, announced last month that it would begin training doctors in August.
Insertion Average time: 1 minute4
The single-rod implant is preloaded in a disposable applicator. Insertion is done in the office using local anesthesia.
Place the 1.5-inch long implant on the inner aspect of the nondominant arm. Position the applicator needle subdermally and withdraw the cannula, leaving the implant rod in place.
After insertion, the implant may not be visible but should be palpable.
Removal Average time: 4 minutes4
Removal requires a 2- to 3-mm incision at the distal tip of the implant. Push the other end of the rod until it pops out.
Timing the insertion
- Between days 1 and 5 of menses, in women who either have not been using a contraceptive method or have been using a nonhormonal method
- During a hormone-free week, in women changing from a combination or progestin-only oral contraceptive, or from intrauterine contraception
- The day on which the next injection is scheduled, in women changing from injectable contraception
No backup contraceptive is necessary if timing of insertion occurs as detailed.
In all cases, exclude pregnancy before insertion.
Timing the removal
The ENG implant can be removed at any time, but must be removed after 3 years.
Return to ovulation is rapid following removal, so women still desiring contraception should begin another method immediately or have a new rod inserted through the removal incision.
Unpredictable bleeding pattern. Unlike with Norplant, there was no trend over time toward a particular bleeding pattern. Implanon patterns are irregular and unpredictable and vary from one 90-day reference period to the next. Similar results were noted in the multicenter international trial.4
Possible therapies include estrogen supplementation, nonsteroidal anti-inflammatory drugs, oral contraceptive pills, and observation.
Other side effects. In the US series, the most frequent nonmenstrual adverse effects possibly related to the ENG implant were acne (14.5%), headache (12.7%), weight gain (12.1%), and emotional lability (14.2%).
Contraindications
The ENG implant should not be placed in women with undiagnosed abnormal genital bleeding, known or suspected pregnancy, or hypersensitivity to any of the components in the ENG implant.
REFERENCES
Why the FDA removed ParaGard’s parity restriction
- An FDA labeling change for the ParaGard intrauterine device confirms what the evidence has long supported: The risk of pelvic infection is more related to sexual behavior than to age, contraceptive choice, or parity
- Evidence supports a link between cervical infection—but not IUD use—and pelvic inflammatory disease and infertility
The Food and Drug Administration has approved a less restrictive label for the ParaGard T380A copper intrauterine contraceptive. Evidence has long supported the conclusion that risk of pelvic infection is related more to a woman’s and her partner’s sexual behavior than to her age, contraceptive choice, or parity.
A woman with at least one child and in a mutually monogamous relationship is no longer listed as the recommended patient profile. Nor is ParaGard contraindicated for a woman with a history of sexually transmitted disease or pelvic inflammatory disease (PID), unless she has current acute PID or engages in sexual behavior suggesting a high risk for PID.
Why was the label restrictive to begin with?
Early studies8,9 that showed an increased risk of PID and infertility in intrauterine contraceptive users have been re-analyzed; most of the increased risk was associated with a single type of intrauterine contraceptive that is no longer on the market (Dalkon Shield), and with high-risk sexual behaviors.10-12 In most analyses of these studies, the increased risk of PID was present only in the first 20 days after insertion, indicating undiagnosed cervical infection at the time of insertion.
Furthermore, many studies had methodological flaws that introduced bias into the results, such as comparing intrauterine contraceptive users with users of combination oral contraceptives (who have a decreased risk of PID compared with nonusers).13
These early studies also equated nulliparity with high-risk sexual behavior. As young women are more likely to acquire sexually transmitted cervical infections, and because young age is associated with nulliparity, many studies erroneously concluded that the increased risk of PID and infertility was attributed to nulliparity.
A case-control study in nulliparous Mexican women who were seeking treatment for primary infertility found no association between tubal infertility and past copper IUD use. In this study, 358 women with primary infertility and documented tubal occlusion (cases) were compared with two sets of controls: 953 nulliparous women with primary infertility and no tubal occlusion, and 584 primigravid women. Past use of a copper IUD was not associated with tubal occlusion, compared with either infertile women without tubal occlusion or primigravid controls (P values 1.0 and 0.9, respectively).14 However, tubal infertility was associated with a past infection with Chlamydia (as evidenced by Chlamydia antibodies). This study further supports an association between PID and infertility and cervical infection—not IUD use.
Protective effect of progestin
The levonorgestrel-releasing intrauterine system (LNG-IUS) may even protect against PID. One of the primary physiologic effects of progestin contraception is thickening of the cervical mucus, which protects against ascending genital tract infection. This protective effect results in a decreased incidence of PID in women who use combination oral contraceptive pills, progestin implants, and progestin injectables.15 A randomized controlled trial found that the cumulative 36-month rate of PID was lower in users of a LNG-IUS contraceptive than in users of a copper IUD (Nova-T) (0.5 and 2.0, respectively; P< 0.013), in both parous and nulliparous women.16 This finding was more marked in women under the age of 25.
Prescribing IUDs in young women
When considering an intrauterine contraceptive for a young woman, it is therefore important to assess her risk of a STI, based on her and her partner’s sexual behavior, and not on parity or age. It is important to screen for STIs at the time of or prior to insertion of an intrauterine contraceptive, and to treat cervicitis prior to insertion.
Nulliparous women who are at low risk of STIs can be offered the intrauterine contraceptive as an effective, long-term, user-independent contraception.
The labeling for levonorgestrel intrauterine contraceptives should also reflect the evidence that the risk of pelvic infection is more related to a patient’s and/or her partner’s sexual behavior than to her age, contraceptive choice, or parity.
REFERENCES
Direct access to Plan B does not promote high-risk behavior
Raine TR, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54–62.
- Advance provision of emergency contraception results in increased usage of emergency contraception without an associated change in risky sexual behavior, sexually transmitted diseases, or use of long-term contraception
- Clinicians should provide emergency contraception in advance of need to ensure timely and appropriate emergency contraceptive use
Emergency contraception could significantly reduce the risk of unintended pregnancy after contraceptive method failure, or unprotected or forced sex. The newer progestin-only emergency contraceptive pills have now largely replaced the older combined (estrogen and progestin) pills because they are more effective and have fewer side effects.
Various emergency contraception regimens are effective
The only dedicated progestin-only emergency contraception pill product in the United States is Plan B, which contains 2 tablets of 0.75 mg of levonorgestrel. Although the recommended treatment schedule is an initial dose within 72 hours of unprotected intercourse and a second dose 12 hours later, a single dose of 1.5 mg of levonorgestrel is as effective as and causes no more side effects than 2 tablets of 0.75-mg doses 12 hours apart.17,18
The sooner the better?
Emergency contraception pills are more effective the sooner after sex that they are initiated. Both combination oral contraceptive pills and progestin-only regimens are moderately effective even if initiated more than 72 hours after unprotected intercourse. 18-20 No data are available on the efficacy of emergency contraception pills taken more than 120 hours (5 days) after unprotected intercourse.
Randomized trial
“It seems unreasonable to restrict access”
Raine et al21 added significantly to our knowledge of emergency contraception. This is the first randomized trial addressing the question of the effect of access on emergency contraceptive usage. A total of 2,117 young women (age 15 to 24 years) were randomly assigned to these 3 groups:
- Pharmacy access (without consulting a physician)
- Advance provision of 3 packs of Plan B
- Clinic access (ie, usual care, which required a clinic visit to obtain emergency contraception)
The study concluded: “While removing the requirement to go through pharmacists or clinics to obtain emergency contraception increases use, the public health impact may be negligible because of high rates of unprotected intercourse and relative underutilization of the method. Given that there is clear evidence that neither pharmacy access nor advance provision compromises contraceptive or sexual behavior, it seems unreasonable to restrict access to emergency contraception to clinics.” The conclusion reflected the following several outcomes, which were assessed after 6 months.
4 key outcomes
- Use of emergency contraception
- The advance provision group used emergency contraception at nearly twice the rate (37.4%) of the clinic access group (21.0%).
- Usage rates were similar in the pharmacy access (24.2%) and the clinic access group (21.0%).
- New sexually transmitted infection rates were similar in all groups
Levels of STIs, such as Chlamydia, were similar across all groups, and changes in HSV-2 serology were similar across all groups. - Many did not use the EC, even with advance provision
In this study, advance provision of emergency contraceptives did not lower pregnancy rates. This finding is disappointing; the likely explanation is that women at highest risk do not use emergency contraception often enough or at all. Thus, the overall pregnancy rate is unchanged. Nearly half (45%) of the women in the study who reported having unprotected sex did not use emergency contraception during the study period, even when they received it in advance. - High-risk sexual behavior did not increase in any group
Women who had increased access to emergency contraception did not have sex more frequently. Receiving emergency contraceptives in advance did not affect the number of sex partners, with most women having only one partner. Data on teens in the same study found that teens did not take more sexual risks than women aged 20 to 24.22
A concern with placing emergency contraception directly in the hands of women has been the theory that it would result in increased high-risk behavior and lower use of regular contraception.
This trial found:
- That women with pharmacy access and women given 3 packs of emergency contraceptives in advance were no more likely to change their regular contraceptive method than women who could obtain emergency contraception only via a clinic visit.
- That women with increased access to emergency contraceptives use their routine contraception with the same consistency as women without increased access.
No downside to easier, wider access to Plan B
This trial adds to the argument for wider and easier access to emergency contraception for women. There is no apparent downside from wide access to the current progestin-only emergency contraception regimen. The latest World Health Organization medical eligibility criteria describe no situation in which the risks of emergency contraception outweigh the benefits.23 The study by Raine et al21 provides evidence against concerns about the potential for increased high-risk sexual behavior.
Eight states (Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Washington) have passed legislation allowing pharmacists to prescribe emergency contraceptives without a prescription. Norway, Sweden, India, and the Netherlands allow emergency contraceptive availability over-the-counter.
17. Arowojolu AO, Okewole IA, Adekunle AO. Comparative evaluation of the effectiveness and safety of two regimens of levonorgestrel for emergency contraception in Nigerians. Contraception. 2002;66:269-273.
18. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803-1810.
19. Ellertson C, Evans M, Ferden S, et al. Extending the time limit for starting the Yuzpe regimen of emergency contraception to 120 hours. Obstet Gynecol. 2003;101:1168-1171.
20. Rodrigues I, Grou F, Joly J. Effectiveness of emergency contraceptive pills between 72 and 120 hours after unprotected sexual intercourse. Am J Obstet Gynecol. 2001;184:531-537.
21. Raine TR, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
22. Harper CC, Cheong M, Rocca CH, Darney PD, Raine TR. The effect of increased access to emergency contraception among young adolescents. Obstet Gynecol. 2005;106:483-491.
23. Medical Eligibilty Criteria for Contraceptive Use. Geneva: World Health Organisation; 2004
Dr. Parvataneni is a consultant to Organon. Dr. Darney is a consultant to Organon and is a speaker for Berlex and Organon.
17. Arowojolu AO, Okewole IA, Adekunle AO. Comparative evaluation of the effectiveness and safety of two regimens of levonorgestrel for emergency contraception in Nigerians. Contraception. 2002;66:269-273.
18. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803-1810.
19. Ellertson C, Evans M, Ferden S, et al. Extending the time limit for starting the Yuzpe regimen of emergency contraception to 120 hours. Obstet Gynecol. 2003;101:1168-1171.
20. Rodrigues I, Grou F, Joly J. Effectiveness of emergency contraceptive pills between 72 and 120 hours after unprotected sexual intercourse. Am J Obstet Gynecol. 2001;184:531-537.
21. Raine TR, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
22. Harper CC, Cheong M, Rocca CH, Darney PD, Raine TR. The effect of increased access to emergency contraception among young adolescents. Obstet Gynecol. 2005;106:483-491.
23. Medical Eligibilty Criteria for Contraceptive Use. Geneva: World Health Organisation; 2004
Dr. Parvataneni is a consultant to Organon. Dr. Darney is a consultant to Organon and is a speaker for Berlex and Organon.