User login
When Clozapine is not enough: Augment with lamotrigine?
Current antipsychotics are reasonably effective in treating positive symptoms, but they do less to improve the negative and cognitive symptoms1 that contribute to patients’ long-term poor functional capacity and quality of life.2 So what do psychiatrists do in clinical practice to mitigate antipsychotics’ limitations? We augment.
Schizophrenia patients routinely are treated with polypharmacy—often with antidepressants or anticonvulsants—in attempts to improve negative symptoms, aggression, and impulsivity.3 Most adjuncts, however—including divalproex, antidepressants, and lithium—have shown very small, inconsistent, or no effects.4,5 The only agent with a recent meta-analysis supporting its use as augmentation in treatment-resistant schizophrenia is lamotrigine,6 an anticonvulsant approved for use in epilepsy.7
This article examines the evidence supporting off-label use of lamotrigine as an augmenting agent in schizophrenia and explains the rationale, based on lamotrigine’s probable mechanism of action as a stabilizer of glutamate neurotransmission.
Is lamotrigine worth trying?
Some 20% of schizophrenia patients are considered treatment-resistant, with persistent positive symptoms despite having undergone ≥2 adequate antipsychotic trials.8 Evidence suggests clozapine then should be tried,4 but approximately one-half of treatment-resistant patients do not respond to clozapine. Treatment guidelines are limited for these 10% of schizophrenia patients with an inadequate response to available therapies, including clozapine.4
In a meta-analysis of 5 controlled trials in patients with treatment-resistant schizophrenia, adjunctive lamotrigine was shown to significantly reduce Positive and Negative Syndrome Scale (PANSS) total scores, positive symptom subscores, and negative symptom subscores.6 In these trials, lamotrigine was added to various antipsychotics, including clozapine. Based on the results—as outlined below—we suggest:
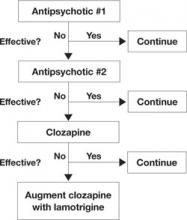
- In treatment-resistant patients with residual symptoms while taking clozapine, lamotrigine given in dosages ≥200 mg/d could be a first-line adjunct (Figure 1).
- Lamotrigine augmentation also might help patients whose positive symptoms are adequately controlled but who have persistent negative and/or cognitive symptoms.
- Evidence does not support routine use of lamotrigine in patients taking antipsychotics other than clozapine.
Managing side effects. Lamotrigine is generally well tolerated; in the meta-analysis, nausea was the only side effect more common with lamotrigine (9%) than with placebo (3.9%).6 Close follow-up is required, however, as a few case reports have noted worsening positive symptoms when lamotrigine was added to antipsychotics.9,10
Lamotrigine produces a skin rash in approximately 10% of patients; the rash usually is benign but may be severe, including the potentially fatal Stevens-Johnson syndrome.11 In the meta-analysis, rash was no more likely in patients receiving placebo (3%) than those receiving lamotrigine (2.2%), and no serious rashes were reported.6 Even so, lamotrigine needs to be titrated upwards very slowly over weeks, and patients must be able to monitor for rash.
Figure 1 An evidence-based approach to treatment-resistant schizophrenia
Treatment-resistant schizophrenia is defined as residual positive symptoms after ≥2 adequate antipsychotic trials. Evidence supports trying clozapine as the next step.4 When patients show an inadequate response to clozapine, a meta-analysis of 5 controlled trials6 indicates that lamotrigine may be a useful first-line adjunct.
Why consider lamotrigine?
During clinical trials of lamotrigine for epilepsy, patients showed improved mood12 as is seen with other anticonvulsants such as valproate and carbamazepine.13 A series of randomized trials then demonstrated lamotrigine’s effectiveness in treating patients with bipolar I disorder, especially during depressive episodes,14,15 and the FDA approved lamotrigine for maintenance treatment of bipolar I disorder.16 In those early studies, lamotrigine also improved bipolar patients’ quality of life and cognitive function in addition to showing mood-stabilizing properties.12
The glutamate hypothesis. Lamotrigine is an inhibitor of voltage-gated sodium channels and has been shown to inhibit the excessive synaptic release of glutamate.17 Glutamate is the primary excitatory neurotransmitter for at least 60% of neurons in the brain, including all cortical pyramidal neurons. A large body of evidence implicates dysfunctional glutamate signaling in the pathophysiology of schizophrenia.18
For example, phencyclidine (PCP) and ketamine—antagonists of one subtype of glutamate receptor, the N-methyl-D-aspartate (NMDA) receptor—are well known to produce positive psychotic symptoms, negative symptoms, and cognitive dysfunction.19 This led to a long-held hypothesis that schizophrenia is caused by too little glutamate. However, ketamine and PCP also increase the release of glutamate at synapses that then can act on glutamate receptors other than the NMDA receptor, which suggests that too much glutamate also may be involved in schizophrenia.
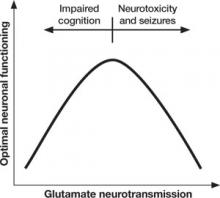
Too little or too much glutamate? These competing hypotheses could both be at least partially true, suggesting an “inverted-U” pattern of glutamate signaling (Figure 2). Because glutamate is involved in most cortical functions, too little glutamate can cause cognitive and processing deficits such as those seen in schizophrenia. On the other hand, too much glutamate can be toxic to neurons and may be a factor in neurodegeneration, such as in Alzheimer’s disease.20 Indeed, schizophrenia may be associated with gradual neurodegeneration.21
Figure 2 Inverted U-curve may explain dysfunctional glutamate signaling in schizophrenia
Both too little or too much glutamate may play a role in schizophrenia’s pathophysiology. Glutamate, the major excitatory neurotransmitter of the cerebral cortex, is involved in most cognitive functions. Too little (or glutamate inhibition) can impair cognition, whereas too much can lead to seizures, neurotoxicity, and cell death.
Glutamate stabilization?
Because lamotrigine prevents excessive glutamate release at synapses, it stabilizes neuronal membranes by preventing toxicity from too much glutamate without interfering with glutamate’s normal functions.22 Thus, lamotrigine may have potential to maintain optimal glutamate signaling in patients with schizophrenia.
In 16 healthy volunteers, a 300-mg dose of lamotrigine was significantly more effective than placebo in reducing ketamine-induced positive symptoms, as assessed by the Brief Psychiatric Rating Scale positive symptoms subscale (P < .001). Lamotrigine pretreatment also reduced negative symptoms and improved learning and memory.23
More recently, lamotrigine pretreatment was shown to prevent many ketamine-induced changes on functional MRI.24 Few antipsychotics have clinically significant effects on ketamine-induced symptoms—especially in a single dose—although repeated dosing with clozapine attenuates some ketamine-induced effects.25
Given the limitations of available antipsychotics, adding a drug such as lamotrigine—which may modulate and stabilize the glutamate system—could be effective in treatment-resistant schizophrenia.
What is the evidence?
Case reports and open-label case series first showed that lamotrigine augmentation could be effective in treatment-resistant schizophrenia patients receiving clozapine.26–28 One naturalistic case series also included patients receiving olanzapine or risperidone and suggested greater improvement with lamotrigine augmentation in patients on clozapine.26
Controlled trials. In a placebo-controlled trial, Tiihonen et al29 reported significantly lower ratings of positive symptoms—but not negative symptoms—after 38 treatment-resistant schizophrenia patients on clozapine received adjunctive lamotrigine, 200 mg/d, for 14 weeks (Table 1).
A subsequent controlled trial in which Kremer et al30 added lamotrigine, ≤400 mg/d, showed significant improvements in positive and negative symptoms among 31 treatment-resistant schizophrenia patients who completed the 10-week study. Patients were taking conventional and atypical antipsychotics, including clozapine. All groups improved, but the study was not powered to detect differences among the groups.
Table 1
Lamotrigine augmentation: 5 double-blind, placebo-controlled trials
| Trial duration | Patient diagnosis (number) | Antipsychotic(s) | Lamotrigine (mg/d) | Results |
|---|---|---|---|---|
| 14 weeks (Tiihonen et al, 200329) | Treatment-resistant schizophrenia (n=34) | Clozapine | 200 | Significantly reduced psychosis ratings, with no significant improvement in negative symptoms |
| 10 weeks (Kremer et al, 200430) | Treatment-resistant schizophrenia (n=38) | Conventional and atypical, including clozapine | ≤400 | Significant improvements with all antipsychotics, especially clozapine, in positive and negative symptoms* |
| 8 weeks (Akhondzadeh et al, 200531) | Schizophrenia (n=36) | Risperidone | 150 | Significant improvement in negative symptoms and cognition; less improvement in positive symptoms |
| 12 weeks, multicenter (Goff et al, 200732) | Schizophrenia, schizoaffective patients with residual symptoms (n=217+212) | Conventional and atypical, including clozapine | 100 to 400 | No significant improvement in any symptom domain; improved negative symptoms only in study 1 and cognitive symptoms only in study 2 |
| 24 weeks (Zoccali et al, 200733) | Treatment-resistant schizophrenia (n=51) | Clozapine | ≤200 | Significant improvement in positive and negative symptoms as well as some cognitive symptoms |
| * Significance achieved only in study completers, not in the last-observation-carried-forward analysis | ||||
A third trial by Akhondzadeh et al,31 augmenting risperidone with lamotrigine, 150 mg/d, resulted in modest improvements in negative and cognitive symptoms and slight improvement in positive symptoms.
Multicenter trials. Preliminary trials led to 2 randomized, double-blind, multicenter studies. In a total of 429 schizophrenia outpatients with residual psychotic symptoms on atypical antipsychotics, lamotrigine, 100 to 400 mg/d, or placebo was added for 12 weeks.32 The combined results failed to show significant improvement with adjunctive lamotrigine in any symptom domain compared with placebo. One study showed some improved negative symptoms, and the other showed improved cognitive symptoms.
Possible reasons for these negative results were unclear, although:
- a relatively large placebo response, compared with other studies, suggests a “failed” clinical trial
- the small number of patients receiving clozapine in this study suggests that they may have been less treatment-resistant than those enrolled in prior studies.
Meta-analysis. A meta-analysis of data from these 5 randomized, controlled trials found the “positive, negative, and general psychopathology subscale scores as measured with the PANSS … showed significant difference favoring adjuvant lamotrigine” (Table 2).6 As for study limitations, the authors noted that effectiveness data could be usefully analyzed in <70 of the 537 patients from the controlled trials, and “the small mean decrease in scores may not be really clinically relevant.”6 Thus, they said, caution is warranted in translating these results to clinical practice.
One more trial. Since the meta-analysis, an additional placebo-controlled trial has been reported.33 In this 24-week trial, lamotrigine augmentation, ≤200 mg/d, was statistically more effective than placebo in reducing positive and negative symptoms in 51 stable treatment-resistant patients on clozapine. Cognitive function also improved.
Table 2
How symptom scores changed with add-on lamotrigine in the meta-analysis of controlled trials
| PANSS subscales: Individual items scored 1 to 7, with 1=absent and 7=extreme | Change [95% CI]* |
|---|---|
| Positive symptom subscale (max 49) Delusions, conceptual disorganization, hallucinatory behavior, excitement, grandiosity, suspiciousness, hostility | -5.10 [-8.86, -1.34] |
| Negative symptom subscale (max 49) Blunted affect, emotional withdrawal, poor rapport, passive-apathetic social withdrawal, difficulty in abstract thinking, lack of spontaneity and flow of conversation, stereotyped thinking | -5.25 [-7.07, -3.43] |
| General psychopathology subscale (max 112) Somatic concern, anxiety, guilt feelings, tension, mannerisms and posturing, depression, motor retardation, uncooperativeness, unusual thought content, disorientation, poor attention, lack of judgment and insight, disturbance of volition, poor impulse control, preoccupation, active social avoidance | -10.74 [-16.53, -4.96] |
| * See text for limitations of the meta-analysis | |
| CI: confidence interval; PANSS: Positive and Negative Syndrome Scale | |
| Source: Reference 6 | |
Only treatment-resistant patients?
In controlled trials, lamotrigine augmentation has had the greatest effect on positive and negative symptoms in treatment-resistant schizophrenia patients, especially those on clozapine. Could lamotrigine augmentation be of benefit only in treatment-resistant schizophrenia?
Analysis of trial findings. As mentioned, outpatients who comprised the majority of subjects in the 2 large “negative” (or possibly failed) trials32 might have been less treatment-resistant than subjects in the other trials. Lower mean lamotrigine dosages (205 mg/d and 241 mg/d) also were used in the 2 negative trials and in the trial by Akhondzadeh et al (150 mg/d)31—compared with up to 400 mg/d in the trial by Kremer et al.30 This suggests that insufficient dosing might have caused the nonsignificant findings.
Given schizophrenia’s heterogeneity, treatment-resistant patients may represent a subgroup that has greater glutamatergic dysfunction, whereas patients who respond more completely to antipsychotics may have greater dopaminergic dysfunction. Thus, lamotrigine augmentation might be more beneficial in the subset of treatment-resistant patients. Lamotrigine or other glutamate stabilizers have been proposed to act as neuroprotective agents, slowing functional decline in chronic schizophrenia34 (although long-term studies needed to test this hypothesis are unlikely to occur because of cost and time constraints).
Another hypothetical, yet intriguing, explanation for the greater effects of lamotrigine augmentation in patients on clozapine is a pharmacodynamic interaction between these 2 drugs. Clozapine (and possibly olanzapine) have been shown to enhance cortical glutamatergic transmission.25 We propose that clozapine-induced boosting of glutamate in concert with stabilization of the glutamate system by lamotrigine improves neuronal functioning. Clinical trial data regarding lamotrigine augmentation of antipsychotics other than clozapine are needed to determine if the relationship between clozapine and lamotrigine is unique.
Related resources
- Lamotrigine prescribing information and patient handout. www.lamictal.com/bipolar/hcp/prescibing_information. html.
- Augmentation strategies for schizophrenia. IPAP Schizophrenia algorithm flowchart (online interactive version), node 11. www.ipap.org/algorithms.php.
Drug brand names
- Carbamazepine • Carbatrol, Equetro, Tegretol
- Clozapine • Clozaril
- Divalproex • Depakote
- Ketamine • Ketalar
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Valproate • Depacon, Depakene
Disclosures
Dr. Gray reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Risch receives research support from the National Institute of Mental Health and is a speaker for AstraZeneca and Pfizer Inc.
1. Gray JA, Roth BL. The pipeline and future of drug development in schizophrenia. Mol Psychiatry. 2007;12(10):904-922.
2. Agid Y, Buzsaki G, Diamond DM, et al. How can drug discovery for psychiatric disorders be improved? Nat Rev Drug Discov. 2007;6(3):189-201.
3. Stahl SM, Grady MM. A critical review of atypical antipsychotic utilization: comparing monotherapy with polypharmacy and augmentation. Curr Med Chem. 2004;11(3):313-327.
4. Miller AL, McEvoy SP, Jeste DV, et al. Treatment of chronic schizophrenia. In: Lieberman JA, Stroup TS, Perkins DO, eds. Textbook of schizophrenia. Arlington, VA: American Psychiatric Publishing; 2006:365-381.
5. Miller AL. Combination treatments for schizophrenia. CNS Spectr. 2004;9(9 suppl 9):19-23.
6. Premkumar TS, Pick J. Lamotrigine for schizophrenia. Cochrane Database Syst Rev. 2006;(4):CD005962.-
7. Brodie MJ, Richens A, Yuen AW. Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. UK lamotrigine/carbamazepine monotherapy trial group. Lancet. 1995;345(8948):476-479.
8. Buckley P, Miller A, Olsen J, et al. When symptoms persist: clozapine augmentation strategies. Schizophr Bull. 2001;27(4):615-628.
9. Chan YC, Miller KM, Shaheen N, et al. Worsening of psychotic symptoms in schizophrenia with addition of lamotrigine: a case report. Schizophr Res. 2005;78(2-3):343-345.
10. Konstantakopoulos G, Oulis P, Koulouris GC, et al. Lamotrigine-associated exacerbation of positive symptoms in paranoid schizophrenia. Schizophr Res. 2008;98(1-3):325-326.
11. Messenheimer J, Mullens EL, Giorgi L, et al. Safety review of adult clinical trial experience with lamotrigine. Drug Saf. 1998;18(4):281-296.
12. Smith D, Baker G, Davies G, et al. Outcomes of add-on treatment with lamotrigine in partial epilepsy. Epilepsia. 1993;34(2):312-322.
13. Post RM, Ketter TA, Denicoff K, et al. The place of anticonvulsant therapy in bipolar illness. Psychopharmacology (Berl). 1996;128(2):115-129.
14. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 study group. J Clin Psychiatry. 1999;60(2):79-88.
15. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. Lamictal 614 study group. J Clin Psychiatry. 2000;61(11):841-850.
16. Bowden CL, Calabrese JR, Sachs G, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60(4):392-400.
17. Large CH, Webster EL, Goff DC. The potential role of lamotrigine in schizophrenia. Psychopharmacology (Berl). 2005;181(3):415-436.
18. Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158(9):1367-1377.
19. Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry. 2004;9(11):984-997.
20. Chohan MO, Iqbal K. From tau to toxicity: emerging roles of NMDA receptor in Alzheimer’s disease. J Alzheimers Dis. 2006;10(1):81-87.
21. Konradi C, Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther. 2003;97(2):153-179.
22. Leach MJ, Baxter MG, Critchley MA. Neurochemical and behavioral aspects of lamotrigine. Epilepsia. 1991;32(suppl 2):S4-S8.
23. Anand A, Charney DS, Oren DA, et al. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of n-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57(3):270-276.
24. Deakin JF, Lees J, McKie S, et al. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65(2):154-164.
25. Large CH. Do NMDA receptor antagonist models of schizophrenia predict the clinical efficacy of antipsychotic drugs? J Psychopharmacol. 2007;21(3):283-301.
26. Dursun SM, Deakin JF. Augmenting antipsychotic treatment with lamotrigine or topiramate in patients with treatment-resistant schizophrenia: a naturalistic case-series outcome study. J Psychopharmacol. 2001;15(4):297-301.
27. Dursun SM, McIntosh D, Milliken H. Clozapine plus lamotrigine in treatment-resistant schizophrenia. Arch Gen Psychiatry. 1999;56(10):950.-
28. Saba G, Dumortier G, Kalalou K, et al. Lamotrigine-clozapine combination in refractory schizophrenia: three cases. J Neuropsychiatry Clin Neurosci. 2002;14(1):86.-
29. Tiihonen J, Hallikainen T, Ryynanen OP, et al. Lamotrigine in treatment-resistant schizophrenia: a randomized placebo-controlled crossover trial. Biol Psychiatry. 2003;54(11):1241-1248.
30. Kremer I, Vass A, Gorelik I, et al. Placebo-controlled trial of lamotrigine added to conventional and atypical antipsychotics in schizophrenia. Biol Psychiatry. 2004;56(6):441-446.
31. Akhondzadeh S, Mackinejad K, Ahmadi-Abhari SA, et al. Does the addition of lamotrigine to risperidone improve psychotic symptoms and cognitive impairments in chronic schizophrenia? Therapy. 2005;2(3):399-406.
32. Goff DC, Keefe R, Citrome L, et al. Lamotrigine as add-on therapy in schizophrenia: results of 2 placebo-controlled trials. J Clin Psychopharmacol. 2007;27(6):582-589.
33. Zoccali R, Muscatello MR, Bruno A, et al. The effect of lamotrigine augmentation of clozapine in a sample of treatment-resistant schizophrenic patients: a double-blind, placebo-controlled study. Schizophr Res. 2007;93(1-3):109-116.
34. Lieberman JA, Perkins DO, Jarskog LF. Neuroprotection: a therapeutic strategy to prevent deterioration associated with schizophrenia. CNS Spectr. 2007;12(3 suppl 4):1-13.
Current antipsychotics are reasonably effective in treating positive symptoms, but they do less to improve the negative and cognitive symptoms1 that contribute to patients’ long-term poor functional capacity and quality of life.2 So what do psychiatrists do in clinical practice to mitigate antipsychotics’ limitations? We augment.
Schizophrenia patients routinely are treated with polypharmacy—often with antidepressants or anticonvulsants—in attempts to improve negative symptoms, aggression, and impulsivity.3 Most adjuncts, however—including divalproex, antidepressants, and lithium—have shown very small, inconsistent, or no effects.4,5 The only agent with a recent meta-analysis supporting its use as augmentation in treatment-resistant schizophrenia is lamotrigine,6 an anticonvulsant approved for use in epilepsy.7
This article examines the evidence supporting off-label use of lamotrigine as an augmenting agent in schizophrenia and explains the rationale, based on lamotrigine’s probable mechanism of action as a stabilizer of glutamate neurotransmission.
Is lamotrigine worth trying?
Some 20% of schizophrenia patients are considered treatment-resistant, with persistent positive symptoms despite having undergone ≥2 adequate antipsychotic trials.8 Evidence suggests clozapine then should be tried,4 but approximately one-half of treatment-resistant patients do not respond to clozapine. Treatment guidelines are limited for these 10% of schizophrenia patients with an inadequate response to available therapies, including clozapine.4
In a meta-analysis of 5 controlled trials in patients with treatment-resistant schizophrenia, adjunctive lamotrigine was shown to significantly reduce Positive and Negative Syndrome Scale (PANSS) total scores, positive symptom subscores, and negative symptom subscores.6 In these trials, lamotrigine was added to various antipsychotics, including clozapine. Based on the results—as outlined below—we suggest:
- In treatment-resistant patients with residual symptoms while taking clozapine, lamotrigine given in dosages ≥200 mg/d could be a first-line adjunct (Figure 1).
- Lamotrigine augmentation also might help patients whose positive symptoms are adequately controlled but who have persistent negative and/or cognitive symptoms.
- Evidence does not support routine use of lamotrigine in patients taking antipsychotics other than clozapine.
Managing side effects. Lamotrigine is generally well tolerated; in the meta-analysis, nausea was the only side effect more common with lamotrigine (9%) than with placebo (3.9%).6 Close follow-up is required, however, as a few case reports have noted worsening positive symptoms when lamotrigine was added to antipsychotics.9,10
Lamotrigine produces a skin rash in approximately 10% of patients; the rash usually is benign but may be severe, including the potentially fatal Stevens-Johnson syndrome.11 In the meta-analysis, rash was no more likely in patients receiving placebo (3%) than those receiving lamotrigine (2.2%), and no serious rashes were reported.6 Even so, lamotrigine needs to be titrated upwards very slowly over weeks, and patients must be able to monitor for rash.
Figure 1 An evidence-based approach to treatment-resistant schizophrenia
Treatment-resistant schizophrenia is defined as residual positive symptoms after ≥2 adequate antipsychotic trials. Evidence supports trying clozapine as the next step.4 When patients show an inadequate response to clozapine, a meta-analysis of 5 controlled trials6 indicates that lamotrigine may be a useful first-line adjunct.
Why consider lamotrigine?
During clinical trials of lamotrigine for epilepsy, patients showed improved mood12 as is seen with other anticonvulsants such as valproate and carbamazepine.13 A series of randomized trials then demonstrated lamotrigine’s effectiveness in treating patients with bipolar I disorder, especially during depressive episodes,14,15 and the FDA approved lamotrigine for maintenance treatment of bipolar I disorder.16 In those early studies, lamotrigine also improved bipolar patients’ quality of life and cognitive function in addition to showing mood-stabilizing properties.12
The glutamate hypothesis. Lamotrigine is an inhibitor of voltage-gated sodium channels and has been shown to inhibit the excessive synaptic release of glutamate.17 Glutamate is the primary excitatory neurotransmitter for at least 60% of neurons in the brain, including all cortical pyramidal neurons. A large body of evidence implicates dysfunctional glutamate signaling in the pathophysiology of schizophrenia.18
For example, phencyclidine (PCP) and ketamine—antagonists of one subtype of glutamate receptor, the N-methyl-D-aspartate (NMDA) receptor—are well known to produce positive psychotic symptoms, negative symptoms, and cognitive dysfunction.19 This led to a long-held hypothesis that schizophrenia is caused by too little glutamate. However, ketamine and PCP also increase the release of glutamate at synapses that then can act on glutamate receptors other than the NMDA receptor, which suggests that too much glutamate also may be involved in schizophrenia.
Too little or too much glutamate? These competing hypotheses could both be at least partially true, suggesting an “inverted-U” pattern of glutamate signaling (Figure 2). Because glutamate is involved in most cortical functions, too little glutamate can cause cognitive and processing deficits such as those seen in schizophrenia. On the other hand, too much glutamate can be toxic to neurons and may be a factor in neurodegeneration, such as in Alzheimer’s disease.20 Indeed, schizophrenia may be associated with gradual neurodegeneration.21
Figure 2 Inverted U-curve may explain dysfunctional glutamate signaling in schizophrenia
Both too little or too much glutamate may play a role in schizophrenia’s pathophysiology. Glutamate, the major excitatory neurotransmitter of the cerebral cortex, is involved in most cognitive functions. Too little (or glutamate inhibition) can impair cognition, whereas too much can lead to seizures, neurotoxicity, and cell death.
Glutamate stabilization?
Because lamotrigine prevents excessive glutamate release at synapses, it stabilizes neuronal membranes by preventing toxicity from too much glutamate without interfering with glutamate’s normal functions.22 Thus, lamotrigine may have potential to maintain optimal glutamate signaling in patients with schizophrenia.
In 16 healthy volunteers, a 300-mg dose of lamotrigine was significantly more effective than placebo in reducing ketamine-induced positive symptoms, as assessed by the Brief Psychiatric Rating Scale positive symptoms subscale (P < .001). Lamotrigine pretreatment also reduced negative symptoms and improved learning and memory.23
More recently, lamotrigine pretreatment was shown to prevent many ketamine-induced changes on functional MRI.24 Few antipsychotics have clinically significant effects on ketamine-induced symptoms—especially in a single dose—although repeated dosing with clozapine attenuates some ketamine-induced effects.25
Given the limitations of available antipsychotics, adding a drug such as lamotrigine—which may modulate and stabilize the glutamate system—could be effective in treatment-resistant schizophrenia.
What is the evidence?
Case reports and open-label case series first showed that lamotrigine augmentation could be effective in treatment-resistant schizophrenia patients receiving clozapine.26–28 One naturalistic case series also included patients receiving olanzapine or risperidone and suggested greater improvement with lamotrigine augmentation in patients on clozapine.26
Controlled trials. In a placebo-controlled trial, Tiihonen et al29 reported significantly lower ratings of positive symptoms—but not negative symptoms—after 38 treatment-resistant schizophrenia patients on clozapine received adjunctive lamotrigine, 200 mg/d, for 14 weeks (Table 1).
A subsequent controlled trial in which Kremer et al30 added lamotrigine, ≤400 mg/d, showed significant improvements in positive and negative symptoms among 31 treatment-resistant schizophrenia patients who completed the 10-week study. Patients were taking conventional and atypical antipsychotics, including clozapine. All groups improved, but the study was not powered to detect differences among the groups.
Table 1
Lamotrigine augmentation: 5 double-blind, placebo-controlled trials
| Trial duration | Patient diagnosis (number) | Antipsychotic(s) | Lamotrigine (mg/d) | Results |
|---|---|---|---|---|
| 14 weeks (Tiihonen et al, 200329) | Treatment-resistant schizophrenia (n=34) | Clozapine | 200 | Significantly reduced psychosis ratings, with no significant improvement in negative symptoms |
| 10 weeks (Kremer et al, 200430) | Treatment-resistant schizophrenia (n=38) | Conventional and atypical, including clozapine | ≤400 | Significant improvements with all antipsychotics, especially clozapine, in positive and negative symptoms* |
| 8 weeks (Akhondzadeh et al, 200531) | Schizophrenia (n=36) | Risperidone | 150 | Significant improvement in negative symptoms and cognition; less improvement in positive symptoms |
| 12 weeks, multicenter (Goff et al, 200732) | Schizophrenia, schizoaffective patients with residual symptoms (n=217+212) | Conventional and atypical, including clozapine | 100 to 400 | No significant improvement in any symptom domain; improved negative symptoms only in study 1 and cognitive symptoms only in study 2 |
| 24 weeks (Zoccali et al, 200733) | Treatment-resistant schizophrenia (n=51) | Clozapine | ≤200 | Significant improvement in positive and negative symptoms as well as some cognitive symptoms |
| * Significance achieved only in study completers, not in the last-observation-carried-forward analysis | ||||
A third trial by Akhondzadeh et al,31 augmenting risperidone with lamotrigine, 150 mg/d, resulted in modest improvements in negative and cognitive symptoms and slight improvement in positive symptoms.
Multicenter trials. Preliminary trials led to 2 randomized, double-blind, multicenter studies. In a total of 429 schizophrenia outpatients with residual psychotic symptoms on atypical antipsychotics, lamotrigine, 100 to 400 mg/d, or placebo was added for 12 weeks.32 The combined results failed to show significant improvement with adjunctive lamotrigine in any symptom domain compared with placebo. One study showed some improved negative symptoms, and the other showed improved cognitive symptoms.
Possible reasons for these negative results were unclear, although:
- a relatively large placebo response, compared with other studies, suggests a “failed” clinical trial
- the small number of patients receiving clozapine in this study suggests that they may have been less treatment-resistant than those enrolled in prior studies.
Meta-analysis. A meta-analysis of data from these 5 randomized, controlled trials found the “positive, negative, and general psychopathology subscale scores as measured with the PANSS … showed significant difference favoring adjuvant lamotrigine” (Table 2).6 As for study limitations, the authors noted that effectiveness data could be usefully analyzed in <70 of the 537 patients from the controlled trials, and “the small mean decrease in scores may not be really clinically relevant.”6 Thus, they said, caution is warranted in translating these results to clinical practice.
One more trial. Since the meta-analysis, an additional placebo-controlled trial has been reported.33 In this 24-week trial, lamotrigine augmentation, ≤200 mg/d, was statistically more effective than placebo in reducing positive and negative symptoms in 51 stable treatment-resistant patients on clozapine. Cognitive function also improved.
Table 2
How symptom scores changed with add-on lamotrigine in the meta-analysis of controlled trials
| PANSS subscales: Individual items scored 1 to 7, with 1=absent and 7=extreme | Change [95% CI]* |
|---|---|
| Positive symptom subscale (max 49) Delusions, conceptual disorganization, hallucinatory behavior, excitement, grandiosity, suspiciousness, hostility | -5.10 [-8.86, -1.34] |
| Negative symptom subscale (max 49) Blunted affect, emotional withdrawal, poor rapport, passive-apathetic social withdrawal, difficulty in abstract thinking, lack of spontaneity and flow of conversation, stereotyped thinking | -5.25 [-7.07, -3.43] |
| General psychopathology subscale (max 112) Somatic concern, anxiety, guilt feelings, tension, mannerisms and posturing, depression, motor retardation, uncooperativeness, unusual thought content, disorientation, poor attention, lack of judgment and insight, disturbance of volition, poor impulse control, preoccupation, active social avoidance | -10.74 [-16.53, -4.96] |
| * See text for limitations of the meta-analysis | |
| CI: confidence interval; PANSS: Positive and Negative Syndrome Scale | |
| Source: Reference 6 | |
Only treatment-resistant patients?
In controlled trials, lamotrigine augmentation has had the greatest effect on positive and negative symptoms in treatment-resistant schizophrenia patients, especially those on clozapine. Could lamotrigine augmentation be of benefit only in treatment-resistant schizophrenia?
Analysis of trial findings. As mentioned, outpatients who comprised the majority of subjects in the 2 large “negative” (or possibly failed) trials32 might have been less treatment-resistant than subjects in the other trials. Lower mean lamotrigine dosages (205 mg/d and 241 mg/d) also were used in the 2 negative trials and in the trial by Akhondzadeh et al (150 mg/d)31—compared with up to 400 mg/d in the trial by Kremer et al.30 This suggests that insufficient dosing might have caused the nonsignificant findings.
Given schizophrenia’s heterogeneity, treatment-resistant patients may represent a subgroup that has greater glutamatergic dysfunction, whereas patients who respond more completely to antipsychotics may have greater dopaminergic dysfunction. Thus, lamotrigine augmentation might be more beneficial in the subset of treatment-resistant patients. Lamotrigine or other glutamate stabilizers have been proposed to act as neuroprotective agents, slowing functional decline in chronic schizophrenia34 (although long-term studies needed to test this hypothesis are unlikely to occur because of cost and time constraints).
Another hypothetical, yet intriguing, explanation for the greater effects of lamotrigine augmentation in patients on clozapine is a pharmacodynamic interaction between these 2 drugs. Clozapine (and possibly olanzapine) have been shown to enhance cortical glutamatergic transmission.25 We propose that clozapine-induced boosting of glutamate in concert with stabilization of the glutamate system by lamotrigine improves neuronal functioning. Clinical trial data regarding lamotrigine augmentation of antipsychotics other than clozapine are needed to determine if the relationship between clozapine and lamotrigine is unique.
Related resources
- Lamotrigine prescribing information and patient handout. www.lamictal.com/bipolar/hcp/prescibing_information. html.
- Augmentation strategies for schizophrenia. IPAP Schizophrenia algorithm flowchart (online interactive version), node 11. www.ipap.org/algorithms.php.
Drug brand names
- Carbamazepine • Carbatrol, Equetro, Tegretol
- Clozapine • Clozaril
- Divalproex • Depakote
- Ketamine • Ketalar
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Valproate • Depacon, Depakene
Disclosures
Dr. Gray reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Risch receives research support from the National Institute of Mental Health and is a speaker for AstraZeneca and Pfizer Inc.
Current antipsychotics are reasonably effective in treating positive symptoms, but they do less to improve the negative and cognitive symptoms1 that contribute to patients’ long-term poor functional capacity and quality of life.2 So what do psychiatrists do in clinical practice to mitigate antipsychotics’ limitations? We augment.
Schizophrenia patients routinely are treated with polypharmacy—often with antidepressants or anticonvulsants—in attempts to improve negative symptoms, aggression, and impulsivity.3 Most adjuncts, however—including divalproex, antidepressants, and lithium—have shown very small, inconsistent, or no effects.4,5 The only agent with a recent meta-analysis supporting its use as augmentation in treatment-resistant schizophrenia is lamotrigine,6 an anticonvulsant approved for use in epilepsy.7
This article examines the evidence supporting off-label use of lamotrigine as an augmenting agent in schizophrenia and explains the rationale, based on lamotrigine’s probable mechanism of action as a stabilizer of glutamate neurotransmission.
Is lamotrigine worth trying?
Some 20% of schizophrenia patients are considered treatment-resistant, with persistent positive symptoms despite having undergone ≥2 adequate antipsychotic trials.8 Evidence suggests clozapine then should be tried,4 but approximately one-half of treatment-resistant patients do not respond to clozapine. Treatment guidelines are limited for these 10% of schizophrenia patients with an inadequate response to available therapies, including clozapine.4
In a meta-analysis of 5 controlled trials in patients with treatment-resistant schizophrenia, adjunctive lamotrigine was shown to significantly reduce Positive and Negative Syndrome Scale (PANSS) total scores, positive symptom subscores, and negative symptom subscores.6 In these trials, lamotrigine was added to various antipsychotics, including clozapine. Based on the results—as outlined below—we suggest:
- In treatment-resistant patients with residual symptoms while taking clozapine, lamotrigine given in dosages ≥200 mg/d could be a first-line adjunct (Figure 1).
- Lamotrigine augmentation also might help patients whose positive symptoms are adequately controlled but who have persistent negative and/or cognitive symptoms.
- Evidence does not support routine use of lamotrigine in patients taking antipsychotics other than clozapine.
Managing side effects. Lamotrigine is generally well tolerated; in the meta-analysis, nausea was the only side effect more common with lamotrigine (9%) than with placebo (3.9%).6 Close follow-up is required, however, as a few case reports have noted worsening positive symptoms when lamotrigine was added to antipsychotics.9,10
Lamotrigine produces a skin rash in approximately 10% of patients; the rash usually is benign but may be severe, including the potentially fatal Stevens-Johnson syndrome.11 In the meta-analysis, rash was no more likely in patients receiving placebo (3%) than those receiving lamotrigine (2.2%), and no serious rashes were reported.6 Even so, lamotrigine needs to be titrated upwards very slowly over weeks, and patients must be able to monitor for rash.
Figure 1 An evidence-based approach to treatment-resistant schizophrenia
Treatment-resistant schizophrenia is defined as residual positive symptoms after ≥2 adequate antipsychotic trials. Evidence supports trying clozapine as the next step.4 When patients show an inadequate response to clozapine, a meta-analysis of 5 controlled trials6 indicates that lamotrigine may be a useful first-line adjunct.
Why consider lamotrigine?
During clinical trials of lamotrigine for epilepsy, patients showed improved mood12 as is seen with other anticonvulsants such as valproate and carbamazepine.13 A series of randomized trials then demonstrated lamotrigine’s effectiveness in treating patients with bipolar I disorder, especially during depressive episodes,14,15 and the FDA approved lamotrigine for maintenance treatment of bipolar I disorder.16 In those early studies, lamotrigine also improved bipolar patients’ quality of life and cognitive function in addition to showing mood-stabilizing properties.12
The glutamate hypothesis. Lamotrigine is an inhibitor of voltage-gated sodium channels and has been shown to inhibit the excessive synaptic release of glutamate.17 Glutamate is the primary excitatory neurotransmitter for at least 60% of neurons in the brain, including all cortical pyramidal neurons. A large body of evidence implicates dysfunctional glutamate signaling in the pathophysiology of schizophrenia.18
For example, phencyclidine (PCP) and ketamine—antagonists of one subtype of glutamate receptor, the N-methyl-D-aspartate (NMDA) receptor—are well known to produce positive psychotic symptoms, negative symptoms, and cognitive dysfunction.19 This led to a long-held hypothesis that schizophrenia is caused by too little glutamate. However, ketamine and PCP also increase the release of glutamate at synapses that then can act on glutamate receptors other than the NMDA receptor, which suggests that too much glutamate also may be involved in schizophrenia.
Too little or too much glutamate? These competing hypotheses could both be at least partially true, suggesting an “inverted-U” pattern of glutamate signaling (Figure 2). Because glutamate is involved in most cortical functions, too little glutamate can cause cognitive and processing deficits such as those seen in schizophrenia. On the other hand, too much glutamate can be toxic to neurons and may be a factor in neurodegeneration, such as in Alzheimer’s disease.20 Indeed, schizophrenia may be associated with gradual neurodegeneration.21
Figure 2 Inverted U-curve may explain dysfunctional glutamate signaling in schizophrenia
Both too little or too much glutamate may play a role in schizophrenia’s pathophysiology. Glutamate, the major excitatory neurotransmitter of the cerebral cortex, is involved in most cognitive functions. Too little (or glutamate inhibition) can impair cognition, whereas too much can lead to seizures, neurotoxicity, and cell death.
Glutamate stabilization?
Because lamotrigine prevents excessive glutamate release at synapses, it stabilizes neuronal membranes by preventing toxicity from too much glutamate without interfering with glutamate’s normal functions.22 Thus, lamotrigine may have potential to maintain optimal glutamate signaling in patients with schizophrenia.
In 16 healthy volunteers, a 300-mg dose of lamotrigine was significantly more effective than placebo in reducing ketamine-induced positive symptoms, as assessed by the Brief Psychiatric Rating Scale positive symptoms subscale (P < .001). Lamotrigine pretreatment also reduced negative symptoms and improved learning and memory.23
More recently, lamotrigine pretreatment was shown to prevent many ketamine-induced changes on functional MRI.24 Few antipsychotics have clinically significant effects on ketamine-induced symptoms—especially in a single dose—although repeated dosing with clozapine attenuates some ketamine-induced effects.25
Given the limitations of available antipsychotics, adding a drug such as lamotrigine—which may modulate and stabilize the glutamate system—could be effective in treatment-resistant schizophrenia.
What is the evidence?
Case reports and open-label case series first showed that lamotrigine augmentation could be effective in treatment-resistant schizophrenia patients receiving clozapine.26–28 One naturalistic case series also included patients receiving olanzapine or risperidone and suggested greater improvement with lamotrigine augmentation in patients on clozapine.26
Controlled trials. In a placebo-controlled trial, Tiihonen et al29 reported significantly lower ratings of positive symptoms—but not negative symptoms—after 38 treatment-resistant schizophrenia patients on clozapine received adjunctive lamotrigine, 200 mg/d, for 14 weeks (Table 1).
A subsequent controlled trial in which Kremer et al30 added lamotrigine, ≤400 mg/d, showed significant improvements in positive and negative symptoms among 31 treatment-resistant schizophrenia patients who completed the 10-week study. Patients were taking conventional and atypical antipsychotics, including clozapine. All groups improved, but the study was not powered to detect differences among the groups.
Table 1
Lamotrigine augmentation: 5 double-blind, placebo-controlled trials
| Trial duration | Patient diagnosis (number) | Antipsychotic(s) | Lamotrigine (mg/d) | Results |
|---|---|---|---|---|
| 14 weeks (Tiihonen et al, 200329) | Treatment-resistant schizophrenia (n=34) | Clozapine | 200 | Significantly reduced psychosis ratings, with no significant improvement in negative symptoms |
| 10 weeks (Kremer et al, 200430) | Treatment-resistant schizophrenia (n=38) | Conventional and atypical, including clozapine | ≤400 | Significant improvements with all antipsychotics, especially clozapine, in positive and negative symptoms* |
| 8 weeks (Akhondzadeh et al, 200531) | Schizophrenia (n=36) | Risperidone | 150 | Significant improvement in negative symptoms and cognition; less improvement in positive symptoms |
| 12 weeks, multicenter (Goff et al, 200732) | Schizophrenia, schizoaffective patients with residual symptoms (n=217+212) | Conventional and atypical, including clozapine | 100 to 400 | No significant improvement in any symptom domain; improved negative symptoms only in study 1 and cognitive symptoms only in study 2 |
| 24 weeks (Zoccali et al, 200733) | Treatment-resistant schizophrenia (n=51) | Clozapine | ≤200 | Significant improvement in positive and negative symptoms as well as some cognitive symptoms |
| * Significance achieved only in study completers, not in the last-observation-carried-forward analysis | ||||
A third trial by Akhondzadeh et al,31 augmenting risperidone with lamotrigine, 150 mg/d, resulted in modest improvements in negative and cognitive symptoms and slight improvement in positive symptoms.
Multicenter trials. Preliminary trials led to 2 randomized, double-blind, multicenter studies. In a total of 429 schizophrenia outpatients with residual psychotic symptoms on atypical antipsychotics, lamotrigine, 100 to 400 mg/d, or placebo was added for 12 weeks.32 The combined results failed to show significant improvement with adjunctive lamotrigine in any symptom domain compared with placebo. One study showed some improved negative symptoms, and the other showed improved cognitive symptoms.
Possible reasons for these negative results were unclear, although:
- a relatively large placebo response, compared with other studies, suggests a “failed” clinical trial
- the small number of patients receiving clozapine in this study suggests that they may have been less treatment-resistant than those enrolled in prior studies.
Meta-analysis. A meta-analysis of data from these 5 randomized, controlled trials found the “positive, negative, and general psychopathology subscale scores as measured with the PANSS … showed significant difference favoring adjuvant lamotrigine” (Table 2).6 As for study limitations, the authors noted that effectiveness data could be usefully analyzed in <70 of the 537 patients from the controlled trials, and “the small mean decrease in scores may not be really clinically relevant.”6 Thus, they said, caution is warranted in translating these results to clinical practice.
One more trial. Since the meta-analysis, an additional placebo-controlled trial has been reported.33 In this 24-week trial, lamotrigine augmentation, ≤200 mg/d, was statistically more effective than placebo in reducing positive and negative symptoms in 51 stable treatment-resistant patients on clozapine. Cognitive function also improved.
Table 2
How symptom scores changed with add-on lamotrigine in the meta-analysis of controlled trials
| PANSS subscales: Individual items scored 1 to 7, with 1=absent and 7=extreme | Change [95% CI]* |
|---|---|
| Positive symptom subscale (max 49) Delusions, conceptual disorganization, hallucinatory behavior, excitement, grandiosity, suspiciousness, hostility | -5.10 [-8.86, -1.34] |
| Negative symptom subscale (max 49) Blunted affect, emotional withdrawal, poor rapport, passive-apathetic social withdrawal, difficulty in abstract thinking, lack of spontaneity and flow of conversation, stereotyped thinking | -5.25 [-7.07, -3.43] |
| General psychopathology subscale (max 112) Somatic concern, anxiety, guilt feelings, tension, mannerisms and posturing, depression, motor retardation, uncooperativeness, unusual thought content, disorientation, poor attention, lack of judgment and insight, disturbance of volition, poor impulse control, preoccupation, active social avoidance | -10.74 [-16.53, -4.96] |
| * See text for limitations of the meta-analysis | |
| CI: confidence interval; PANSS: Positive and Negative Syndrome Scale | |
| Source: Reference 6 | |
Only treatment-resistant patients?
In controlled trials, lamotrigine augmentation has had the greatest effect on positive and negative symptoms in treatment-resistant schizophrenia patients, especially those on clozapine. Could lamotrigine augmentation be of benefit only in treatment-resistant schizophrenia?
Analysis of trial findings. As mentioned, outpatients who comprised the majority of subjects in the 2 large “negative” (or possibly failed) trials32 might have been less treatment-resistant than subjects in the other trials. Lower mean lamotrigine dosages (205 mg/d and 241 mg/d) also were used in the 2 negative trials and in the trial by Akhondzadeh et al (150 mg/d)31—compared with up to 400 mg/d in the trial by Kremer et al.30 This suggests that insufficient dosing might have caused the nonsignificant findings.
Given schizophrenia’s heterogeneity, treatment-resistant patients may represent a subgroup that has greater glutamatergic dysfunction, whereas patients who respond more completely to antipsychotics may have greater dopaminergic dysfunction. Thus, lamotrigine augmentation might be more beneficial in the subset of treatment-resistant patients. Lamotrigine or other glutamate stabilizers have been proposed to act as neuroprotective agents, slowing functional decline in chronic schizophrenia34 (although long-term studies needed to test this hypothesis are unlikely to occur because of cost and time constraints).
Another hypothetical, yet intriguing, explanation for the greater effects of lamotrigine augmentation in patients on clozapine is a pharmacodynamic interaction between these 2 drugs. Clozapine (and possibly olanzapine) have been shown to enhance cortical glutamatergic transmission.25 We propose that clozapine-induced boosting of glutamate in concert with stabilization of the glutamate system by lamotrigine improves neuronal functioning. Clinical trial data regarding lamotrigine augmentation of antipsychotics other than clozapine are needed to determine if the relationship between clozapine and lamotrigine is unique.
Related resources
- Lamotrigine prescribing information and patient handout. www.lamictal.com/bipolar/hcp/prescibing_information. html.
- Augmentation strategies for schizophrenia. IPAP Schizophrenia algorithm flowchart (online interactive version), node 11. www.ipap.org/algorithms.php.
Drug brand names
- Carbamazepine • Carbatrol, Equetro, Tegretol
- Clozapine • Clozaril
- Divalproex • Depakote
- Ketamine • Ketalar
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Risperidone • Risperdal
- Valproate • Depacon, Depakene
Disclosures
Dr. Gray reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Risch receives research support from the National Institute of Mental Health and is a speaker for AstraZeneca and Pfizer Inc.
1. Gray JA, Roth BL. The pipeline and future of drug development in schizophrenia. Mol Psychiatry. 2007;12(10):904-922.
2. Agid Y, Buzsaki G, Diamond DM, et al. How can drug discovery for psychiatric disorders be improved? Nat Rev Drug Discov. 2007;6(3):189-201.
3. Stahl SM, Grady MM. A critical review of atypical antipsychotic utilization: comparing monotherapy with polypharmacy and augmentation. Curr Med Chem. 2004;11(3):313-327.
4. Miller AL, McEvoy SP, Jeste DV, et al. Treatment of chronic schizophrenia. In: Lieberman JA, Stroup TS, Perkins DO, eds. Textbook of schizophrenia. Arlington, VA: American Psychiatric Publishing; 2006:365-381.
5. Miller AL. Combination treatments for schizophrenia. CNS Spectr. 2004;9(9 suppl 9):19-23.
6. Premkumar TS, Pick J. Lamotrigine for schizophrenia. Cochrane Database Syst Rev. 2006;(4):CD005962.-
7. Brodie MJ, Richens A, Yuen AW. Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. UK lamotrigine/carbamazepine monotherapy trial group. Lancet. 1995;345(8948):476-479.
8. Buckley P, Miller A, Olsen J, et al. When symptoms persist: clozapine augmentation strategies. Schizophr Bull. 2001;27(4):615-628.
9. Chan YC, Miller KM, Shaheen N, et al. Worsening of psychotic symptoms in schizophrenia with addition of lamotrigine: a case report. Schizophr Res. 2005;78(2-3):343-345.
10. Konstantakopoulos G, Oulis P, Koulouris GC, et al. Lamotrigine-associated exacerbation of positive symptoms in paranoid schizophrenia. Schizophr Res. 2008;98(1-3):325-326.
11. Messenheimer J, Mullens EL, Giorgi L, et al. Safety review of adult clinical trial experience with lamotrigine. Drug Saf. 1998;18(4):281-296.
12. Smith D, Baker G, Davies G, et al. Outcomes of add-on treatment with lamotrigine in partial epilepsy. Epilepsia. 1993;34(2):312-322.
13. Post RM, Ketter TA, Denicoff K, et al. The place of anticonvulsant therapy in bipolar illness. Psychopharmacology (Berl). 1996;128(2):115-129.
14. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 study group. J Clin Psychiatry. 1999;60(2):79-88.
15. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. Lamictal 614 study group. J Clin Psychiatry. 2000;61(11):841-850.
16. Bowden CL, Calabrese JR, Sachs G, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60(4):392-400.
17. Large CH, Webster EL, Goff DC. The potential role of lamotrigine in schizophrenia. Psychopharmacology (Berl). 2005;181(3):415-436.
18. Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158(9):1367-1377.
19. Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry. 2004;9(11):984-997.
20. Chohan MO, Iqbal K. From tau to toxicity: emerging roles of NMDA receptor in Alzheimer’s disease. J Alzheimers Dis. 2006;10(1):81-87.
21. Konradi C, Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther. 2003;97(2):153-179.
22. Leach MJ, Baxter MG, Critchley MA. Neurochemical and behavioral aspects of lamotrigine. Epilepsia. 1991;32(suppl 2):S4-S8.
23. Anand A, Charney DS, Oren DA, et al. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of n-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57(3):270-276.
24. Deakin JF, Lees J, McKie S, et al. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65(2):154-164.
25. Large CH. Do NMDA receptor antagonist models of schizophrenia predict the clinical efficacy of antipsychotic drugs? J Psychopharmacol. 2007;21(3):283-301.
26. Dursun SM, Deakin JF. Augmenting antipsychotic treatment with lamotrigine or topiramate in patients with treatment-resistant schizophrenia: a naturalistic case-series outcome study. J Psychopharmacol. 2001;15(4):297-301.
27. Dursun SM, McIntosh D, Milliken H. Clozapine plus lamotrigine in treatment-resistant schizophrenia. Arch Gen Psychiatry. 1999;56(10):950.-
28. Saba G, Dumortier G, Kalalou K, et al. Lamotrigine-clozapine combination in refractory schizophrenia: three cases. J Neuropsychiatry Clin Neurosci. 2002;14(1):86.-
29. Tiihonen J, Hallikainen T, Ryynanen OP, et al. Lamotrigine in treatment-resistant schizophrenia: a randomized placebo-controlled crossover trial. Biol Psychiatry. 2003;54(11):1241-1248.
30. Kremer I, Vass A, Gorelik I, et al. Placebo-controlled trial of lamotrigine added to conventional and atypical antipsychotics in schizophrenia. Biol Psychiatry. 2004;56(6):441-446.
31. Akhondzadeh S, Mackinejad K, Ahmadi-Abhari SA, et al. Does the addition of lamotrigine to risperidone improve psychotic symptoms and cognitive impairments in chronic schizophrenia? Therapy. 2005;2(3):399-406.
32. Goff DC, Keefe R, Citrome L, et al. Lamotrigine as add-on therapy in schizophrenia: results of 2 placebo-controlled trials. J Clin Psychopharmacol. 2007;27(6):582-589.
33. Zoccali R, Muscatello MR, Bruno A, et al. The effect of lamotrigine augmentation of clozapine in a sample of treatment-resistant schizophrenic patients: a double-blind, placebo-controlled study. Schizophr Res. 2007;93(1-3):109-116.
34. Lieberman JA, Perkins DO, Jarskog LF. Neuroprotection: a therapeutic strategy to prevent deterioration associated with schizophrenia. CNS Spectr. 2007;12(3 suppl 4):1-13.
1. Gray JA, Roth BL. The pipeline and future of drug development in schizophrenia. Mol Psychiatry. 2007;12(10):904-922.
2. Agid Y, Buzsaki G, Diamond DM, et al. How can drug discovery for psychiatric disorders be improved? Nat Rev Drug Discov. 2007;6(3):189-201.
3. Stahl SM, Grady MM. A critical review of atypical antipsychotic utilization: comparing monotherapy with polypharmacy and augmentation. Curr Med Chem. 2004;11(3):313-327.
4. Miller AL, McEvoy SP, Jeste DV, et al. Treatment of chronic schizophrenia. In: Lieberman JA, Stroup TS, Perkins DO, eds. Textbook of schizophrenia. Arlington, VA: American Psychiatric Publishing; 2006:365-381.
5. Miller AL. Combination treatments for schizophrenia. CNS Spectr. 2004;9(9 suppl 9):19-23.
6. Premkumar TS, Pick J. Lamotrigine for schizophrenia. Cochrane Database Syst Rev. 2006;(4):CD005962.-
7. Brodie MJ, Richens A, Yuen AW. Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. UK lamotrigine/carbamazepine monotherapy trial group. Lancet. 1995;345(8948):476-479.
8. Buckley P, Miller A, Olsen J, et al. When symptoms persist: clozapine augmentation strategies. Schizophr Bull. 2001;27(4):615-628.
9. Chan YC, Miller KM, Shaheen N, et al. Worsening of psychotic symptoms in schizophrenia with addition of lamotrigine: a case report. Schizophr Res. 2005;78(2-3):343-345.
10. Konstantakopoulos G, Oulis P, Koulouris GC, et al. Lamotrigine-associated exacerbation of positive symptoms in paranoid schizophrenia. Schizophr Res. 2008;98(1-3):325-326.
11. Messenheimer J, Mullens EL, Giorgi L, et al. Safety review of adult clinical trial experience with lamotrigine. Drug Saf. 1998;18(4):281-296.
12. Smith D, Baker G, Davies G, et al. Outcomes of add-on treatment with lamotrigine in partial epilepsy. Epilepsia. 1993;34(2):312-322.
13. Post RM, Ketter TA, Denicoff K, et al. The place of anticonvulsant therapy in bipolar illness. Psychopharmacology (Berl). 1996;128(2):115-129.
14. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 study group. J Clin Psychiatry. 1999;60(2):79-88.
15. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. Lamictal 614 study group. J Clin Psychiatry. 2000;61(11):841-850.
16. Bowden CL, Calabrese JR, Sachs G, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60(4):392-400.
17. Large CH, Webster EL, Goff DC. The potential role of lamotrigine in schizophrenia. Psychopharmacology (Berl). 2005;181(3):415-436.
18. Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158(9):1367-1377.
19. Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry. 2004;9(11):984-997.
20. Chohan MO, Iqbal K. From tau to toxicity: emerging roles of NMDA receptor in Alzheimer’s disease. J Alzheimers Dis. 2006;10(1):81-87.
21. Konradi C, Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther. 2003;97(2):153-179.
22. Leach MJ, Baxter MG, Critchley MA. Neurochemical and behavioral aspects of lamotrigine. Epilepsia. 1991;32(suppl 2):S4-S8.
23. Anand A, Charney DS, Oren DA, et al. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of n-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57(3):270-276.
24. Deakin JF, Lees J, McKie S, et al. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65(2):154-164.
25. Large CH. Do NMDA receptor antagonist models of schizophrenia predict the clinical efficacy of antipsychotic drugs? J Psychopharmacol. 2007;21(3):283-301.
26. Dursun SM, Deakin JF. Augmenting antipsychotic treatment with lamotrigine or topiramate in patients with treatment-resistant schizophrenia: a naturalistic case-series outcome study. J Psychopharmacol. 2001;15(4):297-301.
27. Dursun SM, McIntosh D, Milliken H. Clozapine plus lamotrigine in treatment-resistant schizophrenia. Arch Gen Psychiatry. 1999;56(10):950.-
28. Saba G, Dumortier G, Kalalou K, et al. Lamotrigine-clozapine combination in refractory schizophrenia: three cases. J Neuropsychiatry Clin Neurosci. 2002;14(1):86.-
29. Tiihonen J, Hallikainen T, Ryynanen OP, et al. Lamotrigine in treatment-resistant schizophrenia: a randomized placebo-controlled crossover trial. Biol Psychiatry. 2003;54(11):1241-1248.
30. Kremer I, Vass A, Gorelik I, et al. Placebo-controlled trial of lamotrigine added to conventional and atypical antipsychotics in schizophrenia. Biol Psychiatry. 2004;56(6):441-446.
31. Akhondzadeh S, Mackinejad K, Ahmadi-Abhari SA, et al. Does the addition of lamotrigine to risperidone improve psychotic symptoms and cognitive impairments in chronic schizophrenia? Therapy. 2005;2(3):399-406.
32. Goff DC, Keefe R, Citrome L, et al. Lamotrigine as add-on therapy in schizophrenia: results of 2 placebo-controlled trials. J Clin Psychopharmacol. 2007;27(6):582-589.
33. Zoccali R, Muscatello MR, Bruno A, et al. The effect of lamotrigine augmentation of clozapine in a sample of treatment-resistant schizophrenic patients: a double-blind, placebo-controlled study. Schizophr Res. 2007;93(1-3):109-116.
34. Lieberman JA, Perkins DO, Jarskog LF. Neuroprotection: a therapeutic strategy to prevent deterioration associated with schizophrenia. CNS Spectr. 2007;12(3 suppl 4):1-13.
Do cholinesterase inhibitors enhance cognition in schizophrenia?
Some schizophrenia patients have shown significant improvements in positive and negative symptoms when my colleagues and I added acetylcholinesterase inhibitors (AChEIs) to their anti-psychotic regimens. We cannot rule out these benefits as placebo effects, but nevertheless they have been sustained over time. When patients appear to have benefited from AChEIs but stopped them, the benefits rapidly disappeared. Then, when these patients restarted the medications, the benefits recurred.
Unfortunately, recent well-controlled clinical studies have not supported these anecdotal findings or the results of approximately 20 preliminary trials. Thus, this article explains:
- why we don’t recommend using off-label AChEIs as a “first choice” augmentation strategy in schizophrenia patients at this time
- under what circumstances the adjunctive use of these agents might be reasonable.
Why Alzheimer’s medications?
Schizophrenia and Alzheimer’s disease (AD) have dramatically different onset, symptoms, course, and pathophysiology. As reviewed below, schizophrenia patients are no more likely to develop AD than the general population, and AChEIs—even when effective—have a short-term, limited benefit in AD.
So why are psychiatrists trying AD medications in patients with schizophrenia? The answer has to do with the intriguing effects of cholinergic agents on cognition.
Toward cognitive enhancement
Schizophrenia’s cognitive impairments may occur at a very early age, often before other overt symptoms,1 then may worsen—sometimes to dementia levels—when obvious psychotic symptoms emerge.2
Positive symptoms (hallucinations, delusions, thought disorder, etc.) and—to a lesser extent—negative symptoms (anhedonia, asociality, blunted affect, etc.) often improve when patients are treated with antipsychotics. Antipsychotics do not significantly improve cognitive symptoms (attention, reaction time, working memory, verbal fluency, etc.), however, and cognitive symptoms are the strongest predictors of poor functional outcomes in our patients.
Heterogeneous disorder. In 2000, Cummings3 summarized evidence from case re-ports and small studies that AChEIs were useful in treating neuropsychiatric conditions other than AD (Table 1). Cholinergic agents, Cummings noted, “affect many aspects of cognition, which suggests that the primary effect may be on an attentional or executive system with a secondary, pan-intellectual modulating influence on memory, language, and visuospatial skills.”4
In schizophrenia, different patients have different types of cognitive impairment.5 Thus, broad-based cognitive enhancers such as AChEIs may be necessary for general use in this illness.
Acetyltransferase activity. Schizophrenia patients—even those meeting criteria for dementia—do not usually have typical AD neuropathology, and the incidence of AD is no different in elderly patients with or without comorbid schizophrenia.6 At autopsy, schizophrenia patients and normal controls have similar brain cortical choline acetyltransferase levels.
Nevertheless, persons with AD and those with schizophrenia show a similar, statistically significant negative correlation between premorbid Clinical Dementia Rating scale scores and brain cortical choline acetyltransferase activity (r=– 0.36, P 6 Furthermore, studies have found cholinergic neurotransmission alterations in schizophrenia patients, including:
- a deficit in regulation of the low-affinity alpha-7 nicotinic receptor in those with impaired sensory gating7
- altered high-affinity nicotinic receptor binding8
- decreased hippocampal muscarinic receptor binding compared with matched normal controls9
- reduced density of cholinergic inter-neurons in the ventral striatum.10
Table 1
Cholinesterase inhibitors have shown benefit in many neuropsychiatric conditions*
| Alcoholism with Wernicke’s encephalopathy |
| Attention-deficit/hyperactivity disorder |
| Autism |
| Bipolar disorder |
| Creutzfeldt-Jakob disease |
| Dementia pugilistica |
| Dementia with Lewy bodies |
| Olivopontocerebellar atrophy |
| Parkinson’s disease with dementia |
| Parkinsonism dementia complex of Guam |
| Pick’s disease |
| Progressive supranuclear palsy |
| Schizophrenia |
| Sleep disorders |
| Subacute sclerosing panencephalitis |
| Traumatic brain injury |
| Vascular dementia |
| * Data from case reports and small studies. Cholinesterase inhibitors are FDA-approved only for Alzheimer’s dementia. |
| Source: Reference 3 |
AChEI augmentation
Mixed results. A number of investigators—including myself—have published data indicating that adding AChEIs—most often donepezil, but also rivastigmine or galantamine—to antipsychotic regimens may improve some schizophrenia patients’ symptoms and general functioning. These benefits were modest, however, when they were seen in these relatively small case reports and studies (Box).
Approximately 20 published studies have reported clinically significant benefits (positive symptom, negative symptom, and/or cognitive improvement) when schizophrenia patients received cholinesterase inhibitors with their antipsychotic regimens. These include case reports, case series, and double-blind, placebo-controlled, crossover or parallel-design studies, most with relatively small numbers of subjects.a-o
Recent studies, however, have failed to show a clinically or statistically significant benefit from cholinesterase inhibitor augmentation in schizophrenia (Table 2). Some included larger sample sizes than earlier investigations and a placebo-active drug parallel design.
fMRI findings. A few crossover design studies of schizophrenia patients taking antipsychotics included functional magnetic resonance imaging (fMRI) at baseline and after cholinesterase inhibitor and placebo augmentation. Of interest, the basal “abnormal” pattern of the baseline fMR image became more “normal” when subjects were treated with donepezil.
Source: Click here to view references
Other studies of AChEI augmentation of typical or atypical antipsychotics have been:
- equivocal, reporting benefits in some but not all patients (with no clear statistical or clinical conclusions) or in schizophrenia patients with comorbid dementia11-14
- decisively negative, showing no benefits, particularly in comparatively larger, randomized, placebo-controlled trials (Table 2).15-19
The authors concluded that—based on preliminary data—adjunctive AChEIs seemed to have “some beneficial effects” on attention and memory for schizophrenia patients.
Both the treatment and placebo groups experienced statistically and clinically significant benefits from baseline in measures of cognition, positive symptoms, and negative symptoms. For all measures, placebo augmentation was equal to or superior to donepezil augmentation.
Table 2
Controlled trials: No benefit from AChEIs in schizophrenia
| Study design | Subjects | Drug (dosage) | Results |
|---|---|---|---|
| Friedman et al (2002),15 double-blind, placebo-controlled | 36 patients with schizophrenia | Donepezil, 5 or 10 mg/d for 12 weeks | Neither dose produced significant improvement in any cognitive measure |
| Tugal et al (2004),16 double-blind, placebo- controlled, crossover | 12 patients with stable schizophrenia | Donepezil, 5 mg/d for 6 weeks, with crossover to placebo for 6 weeks | Treatment effect was not significant in any cognitive measure |
| Freudenreich et al (2005),17 double-blind, placebo-controlled | 36 stable outpatients with schizophrenia | Donepezil, ≤10 mg/d for 8 weeks | No improvement in cognition or psychopathology measures |
| Sharma et al (2006),18 randomized, double-blind, placebo-controlled | 21 patients with stable schizophrenia | Rivastigmine, 12 mg/d for 24 weeks | No significant improvement in any cognitive measure |
| Fagerlund et al (2007),19 double-blind, placebo-controlled | 21 patients enrolled, 11 completed | Donepezil, 5 or 10 mg/d for 4 months added to ziprasidone | No differences in changes on PANSS scores or a global cognitive score |
| Keefe et al (2007),21 randomized, double-blind, placebo-controlled | 250 stable outpatients with schizophrenia or schizoaffective disorder | Donepezil, 5 mg for 6 weeks then 10 mg for 6 weeks | Donepezil was well-tolerated but did not improve cognition any more than placebo |
| PANSS: Positive and Negative Syndrome Scale | |||
Analyzing trial results
The large, well-designed clinical trial by Keefe et al21 suggests conclusively that donepezil augmentation is not more effective than placebo in most stable schizophrenia or schizoaffective disorder patients with mild to moderate cognitive impairment.
- Different dosages might have been more effective.
- Longer treatment (>3 months) might have been necessary for donepezil to “surpass” the large placebo effect.
- Other AChEIs—such as galantamine, which stimulates nicotinic receptors—might be more effective than donepezil, which is predominantly muscarinic.
If this hypothesis is true, clinicians would need to differentiate patients before giving them trials of AChEIs or other augmentation therapies. Genetic testing might identify different pathophysiologies among patients, but these technologies are not yet clinically available.
Recommendations
Clinical experience, case reports, and small case series indicate that occasional patients may benefit from AChEI augmentation. On the other hand, the only large, multi-center, placebo-controlled, parallel-design study found no difference between donepezil and placebo augmentation of atypical antipsychotics.21
Thus this review of available evidence does not support the routine use of AChEI augmentation of typical or atypical antipsychotics as a viable psychopharmacologic strategy. Until more supportive evidence has been reported, this reviewer cannot recommend AChEIs as a “first line” augmentation strategy. Furthermore, because these medications do not have an FDA-approved indication in schizophrenia and are expensive, a cost-benefit appraisal also would not support their routine use.
Nevertheless, AChEIs are relatively safe and occasionally have been dramatically effective in a small subgroup of schizophrenia patients when used as augmentation. They may represent a reasonable approach:
- when other adjuncts have failed
- as a supplement to other augmentation strategies, such as cognitive-behavioral therapy or family therapy.
- Mohamed S, Paulsen JS, O’Leary D, et al. Generalized cognitive deficits in schizophrenia. Arch Gen Psychiatry 1999;56:749-54.
- Risch SC, Horner MD, McGurk S, et al. Double-blind donepezil-placebo crossover augmentation study of atypical antipsychotics in chronic, stable schizophrenia: a pilot study. Schizophr Res 2007;93:131-5.
- Donepezil • Aricept
- Rivastigmine • Exelon
- Galantamine • Reminyl, Razadyne
- Ziprasidone • Geodon
Dr. Risch receives research support from the National Institute of Mental Health, Abbott Laboratories, GlaxoSmithKline, Bristol-Myers Squibb, and Forest Pharmaceuticals. He is a consultant to and speaker for AstraZeneca and Pfizer Inc.
1. Hans SL, Marcus J, Nuechterlein KH, et al. Neurobehavioral deficits at adolescence in children at risk for schizophrenia. The Jerusalem Infant Development Study. Arch Gen Psychiatry 1999;56:741-8.
2. Heaton R, Paulsen JS, McAdams LA, et al. Neuropsychological deficits in schizophrenia: relationship to age, chronicity and dementia. Arch Gen Psychiatry 1994;51(6):469-76.
3. Cummings JL. Cholinesterase inhibitors: a new class of psychotropic compounds. Am J Psychiatry 2000;157(1):4-15.
4. Lawrence AD, Sahakian BJ. Alzheimer disease, attention, and the cholinergic system. Alzheimer Dis Assoc Disord 1995;9(suppl 2):43-9.
5. Green MF, Kern RS, Broff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 2000;26:119-36.
6. Powchick P, Davidson M, Haroutunian V, et al. Postmortem studies in schizophrenia. Schizophr Bull 1998;24(3):325-41.
7. Breese CR, Lee MJ, Adams CE, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacol 2000;23(4):351-64.
8. Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Biol Psychiatry 2000;48(5):381-8.
9. Holt DJ, Bachus SE, Hyde TM, et al. Reduced density of cholinergic interneurons in the ventral striatum in schizophrenia: an in situ hybridization study. Biol Psychiatry 2005;58:408-16.
10. MacEwan GW, Ehmann TS, Khanbhai I, Wrixon C. Donepezil in schizophrenia—is it helpful? An experimental design case study. Acta Psychiatr Scand 2001;104(6):469-72.
11. Stryjer R, Strous RD, Bar F, et al. Beneficial effect of donepezil augmentation for the management of comorbid schizophrenia and dementia. Clin Neuropharmacol 2003;26:12-7.
12. Aasen I, Kumari V, Sharma T. Effects of rivastigmine on sustained attention in schizophrenia: an fMRI study. J Clin Psychopharmacol 2005;25:311-7.
13. Arnold DS, Rosse RB, Dickinson D, et al. Adjuvant therapeutic effects of galantamine on apathy in a schizophrenia patient. J Clin Psychiatry 2004;65:1723-4.
14. Friedman JI, Adler DN, Howanitz E, et al. A double blind placebo controlled trial of donepezil adjunctive treatment to risperidone for the cognitive impairment of schizophrenia. Biol Psychiatry 2002;51:349-57.
15. Tugal O, Yazici KM, Anil Y, Gögüs A. A double-blind placebo controlled, cross-over trial of adjunctive donepezil for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol 2004;7:117-23.
16. Freudenreich O, Herz L, Deckersbach T, et al. Added donepezil for stable schizophrenia: a double-blind, placebo-controlled trial. Psychopharmacology. (Berl) 2005;181:358-63.
17. Sharma T, Reed C, Aasen I, Kumari V. Cognitive effects of adjunctive 24-weeks rivastigmine treatment to antipsychotics in schizophrenia: a randomized, placebo-controlled, double-blind investigation. Schizophr Res 2006;85:73-83.
18. Fagerlund B, Soholm B, Fink-Jensen A, et al. Effects of donepezil adjunctive treatment ziprasidone on cognitive deficits in schizophrenia: a double-blind, placebo-controlled study. Clin Neuropharmacol 2007;30:3-12.
19. Chouinard S, Sepehry A, Amir A, et al. Oral cholinesterase inhibitor add-on therapy for cognitive enhancement in schizophrenia: a quantitative systematic review, part I. Clin Neuropharmacol 2007;30:169-82.
20. Keefe RS, Malhotra AK, Meltzer HY, et al. Efficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: significant placebo/practice effects in a 12-week, randomized, double-blind, placebo-controlled trial. Neuropharmacol 2007;July 11 [Epub ahead of print].
21. Maelicke A, Albuquerque EX. Allosteric modulation of nicotinic acetylcholine receptors as a treatment strategy for Alzheimer’s disease. Eur J Pharmacol 2000;393:165-70.
22. Miller AL. Combination treatments for schizophrenia. CNS. Spectr 2004;9(9):19-23.
Some schizophrenia patients have shown significant improvements in positive and negative symptoms when my colleagues and I added acetylcholinesterase inhibitors (AChEIs) to their anti-psychotic regimens. We cannot rule out these benefits as placebo effects, but nevertheless they have been sustained over time. When patients appear to have benefited from AChEIs but stopped them, the benefits rapidly disappeared. Then, when these patients restarted the medications, the benefits recurred.
Unfortunately, recent well-controlled clinical studies have not supported these anecdotal findings or the results of approximately 20 preliminary trials. Thus, this article explains:
- why we don’t recommend using off-label AChEIs as a “first choice” augmentation strategy in schizophrenia patients at this time
- under what circumstances the adjunctive use of these agents might be reasonable.
Why Alzheimer’s medications?
Schizophrenia and Alzheimer’s disease (AD) have dramatically different onset, symptoms, course, and pathophysiology. As reviewed below, schizophrenia patients are no more likely to develop AD than the general population, and AChEIs—even when effective—have a short-term, limited benefit in AD.
So why are psychiatrists trying AD medications in patients with schizophrenia? The answer has to do with the intriguing effects of cholinergic agents on cognition.
Toward cognitive enhancement
Schizophrenia’s cognitive impairments may occur at a very early age, often before other overt symptoms,1 then may worsen—sometimes to dementia levels—when obvious psychotic symptoms emerge.2
Positive symptoms (hallucinations, delusions, thought disorder, etc.) and—to a lesser extent—negative symptoms (anhedonia, asociality, blunted affect, etc.) often improve when patients are treated with antipsychotics. Antipsychotics do not significantly improve cognitive symptoms (attention, reaction time, working memory, verbal fluency, etc.), however, and cognitive symptoms are the strongest predictors of poor functional outcomes in our patients.
Heterogeneous disorder. In 2000, Cummings3 summarized evidence from case re-ports and small studies that AChEIs were useful in treating neuropsychiatric conditions other than AD (Table 1). Cholinergic agents, Cummings noted, “affect many aspects of cognition, which suggests that the primary effect may be on an attentional or executive system with a secondary, pan-intellectual modulating influence on memory, language, and visuospatial skills.”4
In schizophrenia, different patients have different types of cognitive impairment.5 Thus, broad-based cognitive enhancers such as AChEIs may be necessary for general use in this illness.
Acetyltransferase activity. Schizophrenia patients—even those meeting criteria for dementia—do not usually have typical AD neuropathology, and the incidence of AD is no different in elderly patients with or without comorbid schizophrenia.6 At autopsy, schizophrenia patients and normal controls have similar brain cortical choline acetyltransferase levels.
Nevertheless, persons with AD and those with schizophrenia show a similar, statistically significant negative correlation between premorbid Clinical Dementia Rating scale scores and brain cortical choline acetyltransferase activity (r=– 0.36, P 6 Furthermore, studies have found cholinergic neurotransmission alterations in schizophrenia patients, including:
- a deficit in regulation of the low-affinity alpha-7 nicotinic receptor in those with impaired sensory gating7
- altered high-affinity nicotinic receptor binding8
- decreased hippocampal muscarinic receptor binding compared with matched normal controls9
- reduced density of cholinergic inter-neurons in the ventral striatum.10
Table 1
Cholinesterase inhibitors have shown benefit in many neuropsychiatric conditions*
| Alcoholism with Wernicke’s encephalopathy |
| Attention-deficit/hyperactivity disorder |
| Autism |
| Bipolar disorder |
| Creutzfeldt-Jakob disease |
| Dementia pugilistica |
| Dementia with Lewy bodies |
| Olivopontocerebellar atrophy |
| Parkinson’s disease with dementia |
| Parkinsonism dementia complex of Guam |
| Pick’s disease |
| Progressive supranuclear palsy |
| Schizophrenia |
| Sleep disorders |
| Subacute sclerosing panencephalitis |
| Traumatic brain injury |
| Vascular dementia |
| * Data from case reports and small studies. Cholinesterase inhibitors are FDA-approved only for Alzheimer’s dementia. |
| Source: Reference 3 |
AChEI augmentation
Mixed results. A number of investigators—including myself—have published data indicating that adding AChEIs—most often donepezil, but also rivastigmine or galantamine—to antipsychotic regimens may improve some schizophrenia patients’ symptoms and general functioning. These benefits were modest, however, when they were seen in these relatively small case reports and studies (Box).
Approximately 20 published studies have reported clinically significant benefits (positive symptom, negative symptom, and/or cognitive improvement) when schizophrenia patients received cholinesterase inhibitors with their antipsychotic regimens. These include case reports, case series, and double-blind, placebo-controlled, crossover or parallel-design studies, most with relatively small numbers of subjects.a-o
Recent studies, however, have failed to show a clinically or statistically significant benefit from cholinesterase inhibitor augmentation in schizophrenia (Table 2). Some included larger sample sizes than earlier investigations and a placebo-active drug parallel design.
fMRI findings. A few crossover design studies of schizophrenia patients taking antipsychotics included functional magnetic resonance imaging (fMRI) at baseline and after cholinesterase inhibitor and placebo augmentation. Of interest, the basal “abnormal” pattern of the baseline fMR image became more “normal” when subjects were treated with donepezil.
Source: Click here to view references
Other studies of AChEI augmentation of typical or atypical antipsychotics have been:
- equivocal, reporting benefits in some but not all patients (with no clear statistical or clinical conclusions) or in schizophrenia patients with comorbid dementia11-14
- decisively negative, showing no benefits, particularly in comparatively larger, randomized, placebo-controlled trials (Table 2).15-19
The authors concluded that—based on preliminary data—adjunctive AChEIs seemed to have “some beneficial effects” on attention and memory for schizophrenia patients.
Both the treatment and placebo groups experienced statistically and clinically significant benefits from baseline in measures of cognition, positive symptoms, and negative symptoms. For all measures, placebo augmentation was equal to or superior to donepezil augmentation.
Table 2
Controlled trials: No benefit from AChEIs in schizophrenia
| Study design | Subjects | Drug (dosage) | Results |
|---|---|---|---|
| Friedman et al (2002),15 double-blind, placebo-controlled | 36 patients with schizophrenia | Donepezil, 5 or 10 mg/d for 12 weeks | Neither dose produced significant improvement in any cognitive measure |
| Tugal et al (2004),16 double-blind, placebo- controlled, crossover | 12 patients with stable schizophrenia | Donepezil, 5 mg/d for 6 weeks, with crossover to placebo for 6 weeks | Treatment effect was not significant in any cognitive measure |
| Freudenreich et al (2005),17 double-blind, placebo-controlled | 36 stable outpatients with schizophrenia | Donepezil, ≤10 mg/d for 8 weeks | No improvement in cognition or psychopathology measures |
| Sharma et al (2006),18 randomized, double-blind, placebo-controlled | 21 patients with stable schizophrenia | Rivastigmine, 12 mg/d for 24 weeks | No significant improvement in any cognitive measure |
| Fagerlund et al (2007),19 double-blind, placebo-controlled | 21 patients enrolled, 11 completed | Donepezil, 5 or 10 mg/d for 4 months added to ziprasidone | No differences in changes on PANSS scores or a global cognitive score |
| Keefe et al (2007),21 randomized, double-blind, placebo-controlled | 250 stable outpatients with schizophrenia or schizoaffective disorder | Donepezil, 5 mg for 6 weeks then 10 mg for 6 weeks | Donepezil was well-tolerated but did not improve cognition any more than placebo |
| PANSS: Positive and Negative Syndrome Scale | |||
Analyzing trial results
The large, well-designed clinical trial by Keefe et al21 suggests conclusively that donepezil augmentation is not more effective than placebo in most stable schizophrenia or schizoaffective disorder patients with mild to moderate cognitive impairment.
- Different dosages might have been more effective.
- Longer treatment (>3 months) might have been necessary for donepezil to “surpass” the large placebo effect.
- Other AChEIs—such as galantamine, which stimulates nicotinic receptors—might be more effective than donepezil, which is predominantly muscarinic.
If this hypothesis is true, clinicians would need to differentiate patients before giving them trials of AChEIs or other augmentation therapies. Genetic testing might identify different pathophysiologies among patients, but these technologies are not yet clinically available.
Recommendations
Clinical experience, case reports, and small case series indicate that occasional patients may benefit from AChEI augmentation. On the other hand, the only large, multi-center, placebo-controlled, parallel-design study found no difference between donepezil and placebo augmentation of atypical antipsychotics.21
Thus this review of available evidence does not support the routine use of AChEI augmentation of typical or atypical antipsychotics as a viable psychopharmacologic strategy. Until more supportive evidence has been reported, this reviewer cannot recommend AChEIs as a “first line” augmentation strategy. Furthermore, because these medications do not have an FDA-approved indication in schizophrenia and are expensive, a cost-benefit appraisal also would not support their routine use.
Nevertheless, AChEIs are relatively safe and occasionally have been dramatically effective in a small subgroup of schizophrenia patients when used as augmentation. They may represent a reasonable approach:
- when other adjuncts have failed
- as a supplement to other augmentation strategies, such as cognitive-behavioral therapy or family therapy.
- Mohamed S, Paulsen JS, O’Leary D, et al. Generalized cognitive deficits in schizophrenia. Arch Gen Psychiatry 1999;56:749-54.
- Risch SC, Horner MD, McGurk S, et al. Double-blind donepezil-placebo crossover augmentation study of atypical antipsychotics in chronic, stable schizophrenia: a pilot study. Schizophr Res 2007;93:131-5.
- Donepezil • Aricept
- Rivastigmine • Exelon
- Galantamine • Reminyl, Razadyne
- Ziprasidone • Geodon
Dr. Risch receives research support from the National Institute of Mental Health, Abbott Laboratories, GlaxoSmithKline, Bristol-Myers Squibb, and Forest Pharmaceuticals. He is a consultant to and speaker for AstraZeneca and Pfizer Inc.
Some schizophrenia patients have shown significant improvements in positive and negative symptoms when my colleagues and I added acetylcholinesterase inhibitors (AChEIs) to their anti-psychotic regimens. We cannot rule out these benefits as placebo effects, but nevertheless they have been sustained over time. When patients appear to have benefited from AChEIs but stopped them, the benefits rapidly disappeared. Then, when these patients restarted the medications, the benefits recurred.
Unfortunately, recent well-controlled clinical studies have not supported these anecdotal findings or the results of approximately 20 preliminary trials. Thus, this article explains:
- why we don’t recommend using off-label AChEIs as a “first choice” augmentation strategy in schizophrenia patients at this time
- under what circumstances the adjunctive use of these agents might be reasonable.
Why Alzheimer’s medications?
Schizophrenia and Alzheimer’s disease (AD) have dramatically different onset, symptoms, course, and pathophysiology. As reviewed below, schizophrenia patients are no more likely to develop AD than the general population, and AChEIs—even when effective—have a short-term, limited benefit in AD.
So why are psychiatrists trying AD medications in patients with schizophrenia? The answer has to do with the intriguing effects of cholinergic agents on cognition.
Toward cognitive enhancement
Schizophrenia’s cognitive impairments may occur at a very early age, often before other overt symptoms,1 then may worsen—sometimes to dementia levels—when obvious psychotic symptoms emerge.2
Positive symptoms (hallucinations, delusions, thought disorder, etc.) and—to a lesser extent—negative symptoms (anhedonia, asociality, blunted affect, etc.) often improve when patients are treated with antipsychotics. Antipsychotics do not significantly improve cognitive symptoms (attention, reaction time, working memory, verbal fluency, etc.), however, and cognitive symptoms are the strongest predictors of poor functional outcomes in our patients.
Heterogeneous disorder. In 2000, Cummings3 summarized evidence from case re-ports and small studies that AChEIs were useful in treating neuropsychiatric conditions other than AD (Table 1). Cholinergic agents, Cummings noted, “affect many aspects of cognition, which suggests that the primary effect may be on an attentional or executive system with a secondary, pan-intellectual modulating influence on memory, language, and visuospatial skills.”4
In schizophrenia, different patients have different types of cognitive impairment.5 Thus, broad-based cognitive enhancers such as AChEIs may be necessary for general use in this illness.
Acetyltransferase activity. Schizophrenia patients—even those meeting criteria for dementia—do not usually have typical AD neuropathology, and the incidence of AD is no different in elderly patients with or without comorbid schizophrenia.6 At autopsy, schizophrenia patients and normal controls have similar brain cortical choline acetyltransferase levels.
Nevertheless, persons with AD and those with schizophrenia show a similar, statistically significant negative correlation between premorbid Clinical Dementia Rating scale scores and brain cortical choline acetyltransferase activity (r=– 0.36, P 6 Furthermore, studies have found cholinergic neurotransmission alterations in schizophrenia patients, including:
- a deficit in regulation of the low-affinity alpha-7 nicotinic receptor in those with impaired sensory gating7
- altered high-affinity nicotinic receptor binding8
- decreased hippocampal muscarinic receptor binding compared with matched normal controls9
- reduced density of cholinergic inter-neurons in the ventral striatum.10
Table 1
Cholinesterase inhibitors have shown benefit in many neuropsychiatric conditions*
| Alcoholism with Wernicke’s encephalopathy |
| Attention-deficit/hyperactivity disorder |
| Autism |
| Bipolar disorder |
| Creutzfeldt-Jakob disease |
| Dementia pugilistica |
| Dementia with Lewy bodies |
| Olivopontocerebellar atrophy |
| Parkinson’s disease with dementia |
| Parkinsonism dementia complex of Guam |
| Pick’s disease |
| Progressive supranuclear palsy |
| Schizophrenia |
| Sleep disorders |
| Subacute sclerosing panencephalitis |
| Traumatic brain injury |
| Vascular dementia |
| * Data from case reports and small studies. Cholinesterase inhibitors are FDA-approved only for Alzheimer’s dementia. |
| Source: Reference 3 |
AChEI augmentation
Mixed results. A number of investigators—including myself—have published data indicating that adding AChEIs—most often donepezil, but also rivastigmine or galantamine—to antipsychotic regimens may improve some schizophrenia patients’ symptoms and general functioning. These benefits were modest, however, when they were seen in these relatively small case reports and studies (Box).
Approximately 20 published studies have reported clinically significant benefits (positive symptom, negative symptom, and/or cognitive improvement) when schizophrenia patients received cholinesterase inhibitors with their antipsychotic regimens. These include case reports, case series, and double-blind, placebo-controlled, crossover or parallel-design studies, most with relatively small numbers of subjects.a-o
Recent studies, however, have failed to show a clinically or statistically significant benefit from cholinesterase inhibitor augmentation in schizophrenia (Table 2). Some included larger sample sizes than earlier investigations and a placebo-active drug parallel design.
fMRI findings. A few crossover design studies of schizophrenia patients taking antipsychotics included functional magnetic resonance imaging (fMRI) at baseline and after cholinesterase inhibitor and placebo augmentation. Of interest, the basal “abnormal” pattern of the baseline fMR image became more “normal” when subjects were treated with donepezil.
Source: Click here to view references
Other studies of AChEI augmentation of typical or atypical antipsychotics have been:
- equivocal, reporting benefits in some but not all patients (with no clear statistical or clinical conclusions) or in schizophrenia patients with comorbid dementia11-14
- decisively negative, showing no benefits, particularly in comparatively larger, randomized, placebo-controlled trials (Table 2).15-19
The authors concluded that—based on preliminary data—adjunctive AChEIs seemed to have “some beneficial effects” on attention and memory for schizophrenia patients.
Both the treatment and placebo groups experienced statistically and clinically significant benefits from baseline in measures of cognition, positive symptoms, and negative symptoms. For all measures, placebo augmentation was equal to or superior to donepezil augmentation.
Table 2
Controlled trials: No benefit from AChEIs in schizophrenia
| Study design | Subjects | Drug (dosage) | Results |
|---|---|---|---|
| Friedman et al (2002),15 double-blind, placebo-controlled | 36 patients with schizophrenia | Donepezil, 5 or 10 mg/d for 12 weeks | Neither dose produced significant improvement in any cognitive measure |
| Tugal et al (2004),16 double-blind, placebo- controlled, crossover | 12 patients with stable schizophrenia | Donepezil, 5 mg/d for 6 weeks, with crossover to placebo for 6 weeks | Treatment effect was not significant in any cognitive measure |
| Freudenreich et al (2005),17 double-blind, placebo-controlled | 36 stable outpatients with schizophrenia | Donepezil, ≤10 mg/d for 8 weeks | No improvement in cognition or psychopathology measures |
| Sharma et al (2006),18 randomized, double-blind, placebo-controlled | 21 patients with stable schizophrenia | Rivastigmine, 12 mg/d for 24 weeks | No significant improvement in any cognitive measure |
| Fagerlund et al (2007),19 double-blind, placebo-controlled | 21 patients enrolled, 11 completed | Donepezil, 5 or 10 mg/d for 4 months added to ziprasidone | No differences in changes on PANSS scores or a global cognitive score |
| Keefe et al (2007),21 randomized, double-blind, placebo-controlled | 250 stable outpatients with schizophrenia or schizoaffective disorder | Donepezil, 5 mg for 6 weeks then 10 mg for 6 weeks | Donepezil was well-tolerated but did not improve cognition any more than placebo |
| PANSS: Positive and Negative Syndrome Scale | |||
Analyzing trial results
The large, well-designed clinical trial by Keefe et al21 suggests conclusively that donepezil augmentation is not more effective than placebo in most stable schizophrenia or schizoaffective disorder patients with mild to moderate cognitive impairment.
- Different dosages might have been more effective.
- Longer treatment (>3 months) might have been necessary for donepezil to “surpass” the large placebo effect.
- Other AChEIs—such as galantamine, which stimulates nicotinic receptors—might be more effective than donepezil, which is predominantly muscarinic.
If this hypothesis is true, clinicians would need to differentiate patients before giving them trials of AChEIs or other augmentation therapies. Genetic testing might identify different pathophysiologies among patients, but these technologies are not yet clinically available.
Recommendations
Clinical experience, case reports, and small case series indicate that occasional patients may benefit from AChEI augmentation. On the other hand, the only large, multi-center, placebo-controlled, parallel-design study found no difference between donepezil and placebo augmentation of atypical antipsychotics.21
Thus this review of available evidence does not support the routine use of AChEI augmentation of typical or atypical antipsychotics as a viable psychopharmacologic strategy. Until more supportive evidence has been reported, this reviewer cannot recommend AChEIs as a “first line” augmentation strategy. Furthermore, because these medications do not have an FDA-approved indication in schizophrenia and are expensive, a cost-benefit appraisal also would not support their routine use.
Nevertheless, AChEIs are relatively safe and occasionally have been dramatically effective in a small subgroup of schizophrenia patients when used as augmentation. They may represent a reasonable approach:
- when other adjuncts have failed
- as a supplement to other augmentation strategies, such as cognitive-behavioral therapy or family therapy.
- Mohamed S, Paulsen JS, O’Leary D, et al. Generalized cognitive deficits in schizophrenia. Arch Gen Psychiatry 1999;56:749-54.
- Risch SC, Horner MD, McGurk S, et al. Double-blind donepezil-placebo crossover augmentation study of atypical antipsychotics in chronic, stable schizophrenia: a pilot study. Schizophr Res 2007;93:131-5.
- Donepezil • Aricept
- Rivastigmine • Exelon
- Galantamine • Reminyl, Razadyne
- Ziprasidone • Geodon
Dr. Risch receives research support from the National Institute of Mental Health, Abbott Laboratories, GlaxoSmithKline, Bristol-Myers Squibb, and Forest Pharmaceuticals. He is a consultant to and speaker for AstraZeneca and Pfizer Inc.
1. Hans SL, Marcus J, Nuechterlein KH, et al. Neurobehavioral deficits at adolescence in children at risk for schizophrenia. The Jerusalem Infant Development Study. Arch Gen Psychiatry 1999;56:741-8.
2. Heaton R, Paulsen JS, McAdams LA, et al. Neuropsychological deficits in schizophrenia: relationship to age, chronicity and dementia. Arch Gen Psychiatry 1994;51(6):469-76.
3. Cummings JL. Cholinesterase inhibitors: a new class of psychotropic compounds. Am J Psychiatry 2000;157(1):4-15.
4. Lawrence AD, Sahakian BJ. Alzheimer disease, attention, and the cholinergic system. Alzheimer Dis Assoc Disord 1995;9(suppl 2):43-9.
5. Green MF, Kern RS, Broff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 2000;26:119-36.
6. Powchick P, Davidson M, Haroutunian V, et al. Postmortem studies in schizophrenia. Schizophr Bull 1998;24(3):325-41.
7. Breese CR, Lee MJ, Adams CE, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacol 2000;23(4):351-64.
8. Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Biol Psychiatry 2000;48(5):381-8.
9. Holt DJ, Bachus SE, Hyde TM, et al. Reduced density of cholinergic interneurons in the ventral striatum in schizophrenia: an in situ hybridization study. Biol Psychiatry 2005;58:408-16.
10. MacEwan GW, Ehmann TS, Khanbhai I, Wrixon C. Donepezil in schizophrenia—is it helpful? An experimental design case study. Acta Psychiatr Scand 2001;104(6):469-72.
11. Stryjer R, Strous RD, Bar F, et al. Beneficial effect of donepezil augmentation for the management of comorbid schizophrenia and dementia. Clin Neuropharmacol 2003;26:12-7.
12. Aasen I, Kumari V, Sharma T. Effects of rivastigmine on sustained attention in schizophrenia: an fMRI study. J Clin Psychopharmacol 2005;25:311-7.
13. Arnold DS, Rosse RB, Dickinson D, et al. Adjuvant therapeutic effects of galantamine on apathy in a schizophrenia patient. J Clin Psychiatry 2004;65:1723-4.
14. Friedman JI, Adler DN, Howanitz E, et al. A double blind placebo controlled trial of donepezil adjunctive treatment to risperidone for the cognitive impairment of schizophrenia. Biol Psychiatry 2002;51:349-57.
15. Tugal O, Yazici KM, Anil Y, Gögüs A. A double-blind placebo controlled, cross-over trial of adjunctive donepezil for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol 2004;7:117-23.
16. Freudenreich O, Herz L, Deckersbach T, et al. Added donepezil for stable schizophrenia: a double-blind, placebo-controlled trial. Psychopharmacology. (Berl) 2005;181:358-63.
17. Sharma T, Reed C, Aasen I, Kumari V. Cognitive effects of adjunctive 24-weeks rivastigmine treatment to antipsychotics in schizophrenia: a randomized, placebo-controlled, double-blind investigation. Schizophr Res 2006;85:73-83.
18. Fagerlund B, Soholm B, Fink-Jensen A, et al. Effects of donepezil adjunctive treatment ziprasidone on cognitive deficits in schizophrenia: a double-blind, placebo-controlled study. Clin Neuropharmacol 2007;30:3-12.
19. Chouinard S, Sepehry A, Amir A, et al. Oral cholinesterase inhibitor add-on therapy for cognitive enhancement in schizophrenia: a quantitative systematic review, part I. Clin Neuropharmacol 2007;30:169-82.
20. Keefe RS, Malhotra AK, Meltzer HY, et al. Efficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: significant placebo/practice effects in a 12-week, randomized, double-blind, placebo-controlled trial. Neuropharmacol 2007;July 11 [Epub ahead of print].
21. Maelicke A, Albuquerque EX. Allosteric modulation of nicotinic acetylcholine receptors as a treatment strategy for Alzheimer’s disease. Eur J Pharmacol 2000;393:165-70.
22. Miller AL. Combination treatments for schizophrenia. CNS. Spectr 2004;9(9):19-23.
1. Hans SL, Marcus J, Nuechterlein KH, et al. Neurobehavioral deficits at adolescence in children at risk for schizophrenia. The Jerusalem Infant Development Study. Arch Gen Psychiatry 1999;56:741-8.
2. Heaton R, Paulsen JS, McAdams LA, et al. Neuropsychological deficits in schizophrenia: relationship to age, chronicity and dementia. Arch Gen Psychiatry 1994;51(6):469-76.
3. Cummings JL. Cholinesterase inhibitors: a new class of psychotropic compounds. Am J Psychiatry 2000;157(1):4-15.
4. Lawrence AD, Sahakian BJ. Alzheimer disease, attention, and the cholinergic system. Alzheimer Dis Assoc Disord 1995;9(suppl 2):43-9.
5. Green MF, Kern RS, Broff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 2000;26:119-36.
6. Powchick P, Davidson M, Haroutunian V, et al. Postmortem studies in schizophrenia. Schizophr Bull 1998;24(3):325-41.
7. Breese CR, Lee MJ, Adams CE, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacol 2000;23(4):351-64.
8. Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Biol Psychiatry 2000;48(5):381-8.
9. Holt DJ, Bachus SE, Hyde TM, et al. Reduced density of cholinergic interneurons in the ventral striatum in schizophrenia: an in situ hybridization study. Biol Psychiatry 2005;58:408-16.
10. MacEwan GW, Ehmann TS, Khanbhai I, Wrixon C. Donepezil in schizophrenia—is it helpful? An experimental design case study. Acta Psychiatr Scand 2001;104(6):469-72.
11. Stryjer R, Strous RD, Bar F, et al. Beneficial effect of donepezil augmentation for the management of comorbid schizophrenia and dementia. Clin Neuropharmacol 2003;26:12-7.
12. Aasen I, Kumari V, Sharma T. Effects of rivastigmine on sustained attention in schizophrenia: an fMRI study. J Clin Psychopharmacol 2005;25:311-7.
13. Arnold DS, Rosse RB, Dickinson D, et al. Adjuvant therapeutic effects of galantamine on apathy in a schizophrenia patient. J Clin Psychiatry 2004;65:1723-4.
14. Friedman JI, Adler DN, Howanitz E, et al. A double blind placebo controlled trial of donepezil adjunctive treatment to risperidone for the cognitive impairment of schizophrenia. Biol Psychiatry 2002;51:349-57.
15. Tugal O, Yazici KM, Anil Y, Gögüs A. A double-blind placebo controlled, cross-over trial of adjunctive donepezil for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol 2004;7:117-23.
16. Freudenreich O, Herz L, Deckersbach T, et al. Added donepezil for stable schizophrenia: a double-blind, placebo-controlled trial. Psychopharmacology. (Berl) 2005;181:358-63.
17. Sharma T, Reed C, Aasen I, Kumari V. Cognitive effects of adjunctive 24-weeks rivastigmine treatment to antipsychotics in schizophrenia: a randomized, placebo-controlled, double-blind investigation. Schizophr Res 2006;85:73-83.
18. Fagerlund B, Soholm B, Fink-Jensen A, et al. Effects of donepezil adjunctive treatment ziprasidone on cognitive deficits in schizophrenia: a double-blind, placebo-controlled study. Clin Neuropharmacol 2007;30:3-12.
19. Chouinard S, Sepehry A, Amir A, et al. Oral cholinesterase inhibitor add-on therapy for cognitive enhancement in schizophrenia: a quantitative systematic review, part I. Clin Neuropharmacol 2007;30:169-82.
20. Keefe RS, Malhotra AK, Meltzer HY, et al. Efficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: significant placebo/practice effects in a 12-week, randomized, double-blind, placebo-controlled trial. Neuropharmacol 2007;July 11 [Epub ahead of print].
21. Maelicke A, Albuquerque EX. Allosteric modulation of nicotinic acetylcholine receptors as a treatment strategy for Alzheimer’s disease. Eur J Pharmacol 2000;393:165-70.
22. Miller AL. Combination treatments for schizophrenia. CNS. Spectr 2004;9(9):19-23.