User login
Strategies for treating depression in patients with hepatitis C
Mr. P, age 31, has been using heroin intravenously for 9 years. He smokes 1 pack of cigarettes daily, but denies using other substances, including alcohol. After an unintentional heroin overdose, Mr. P enrolls in a methadone maintenance treatment program (MMTP) that includes primary medical care and addiction medicine and psychiatric specialists, where he undergoes medical evaluation and screening for hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Laboratory data reveal that although Mr. P is HIV negative, he has been exposed to HCV and treatment is indicated.
Among the approximately 3 million people in the United States with chronic HCV—an enveloped, single-stranded RNA virus—there’s a high prevalence of premorbid psychopathology and substance abuse, as well as neuropsychiatric effects caused by HCV treatment.1-3 Because underdiagnosing and undertreating psychiatric disorders contributes to morbidity and mortality in HCV patients, early identification and prompt treatment is critical.
IV drug use is the most common route for HCV infection, accounting for 65% to 70% of infections.1 The prevalence of HCV among IV drug users is 28% to 90%.1 Once exposed to HCV, 75% to 85% of patients do not clear the initial infection and become chronically infected.
This article reviews the pathophysiology, identification, and management of psychiatric manifestations found among HCV patients and provides an understanding of how psychiatric symptoms manifest in HCV patients. This article also discusses HCV treatment and its neuropsychiatric side effects.
Testing for HCV
Chronically infected HCV patients may have few, if any, specific physical complaints, and often are diagnosed during screenings or other routine laboratory evaluations. The presence of risk factors, such as a history of injection drug use or receiving a blood transfusion before 1992,1 guides the decision to screen for HCV. Normal liver function test results should not preclude testing because many HCV-positive patients have transaminases within the normal range.4 Initial screening is via an antibody-mediated immunoassay that is highly specific and sensitive for past exposure to HCV (Table 1).4 However, a positive screen does not indicate the presence of active infection. Evidence of the virus via a viral assay will identify active HCV, but does not indicate need for treatment. Liver biopsy confirms the presence of liver injury and quantifies its extent. The severity of liver damage will determine whether treatment is needed. HCV genotyping determines the appropriate duration and dosage of pharmacotherapy.
Table 1
Tests to diagnose and evaluate HCV
| Test | Results |
|---|---|
| HCV antibody | Determines prior exposure to HCV |
| HCV viral assay | Evaluates for current HCV infection |
| Liver biopsy | Assesses level of liver damage |
| HCV genotyping | Provides data to determine duration and intensity of treatment and likelihood of treatment success |
| HCV: hepatitis C virus Source: Reference 4 | |
CASE CONTINUED: Mood improves, but fatigue persists
As part of pre-HCV treatment evaluation, Mr. P undergoes a psychiatric evaluation. He describes periods of low mood while actively engaged in drug use but has never received psychiatric treatment, experienced suicidal ideation, or attempted suicide. Since starting opioid agonist therapy, he reports improved mood but endorses continued mild fatigue and difficulty falling sleep. The psychiatrist determines Mr. P does not meet criteria for an axis I diagnosis other than a substance use disorder.
Although most HCV patients have few, if any, nonspecific physical symptoms, many have psychiatric symptoms or disorders before the HCV diagnosis is made or treatment is initiated; substance use disorders are most common. Batki et al1 found that 56% of HCV patients in an MMTP met criteria for a nonsubstance axis I disorder and 82% met criteria for such a disorder during their lifetime. Additionally, 66% of patients were taking psychiatric medications. Table 21,5,6 lists the rates of other psychiatric disorders found in patients with untreated HCV.
Table 2
Rates of psychiatric disorders in patients with untreated hepatitis C virus
| Disorder(s) | Current rate | Lifetime rate |
|---|---|---|
| Mood disorders | 34% to 35% | 67% |
| Major depressive disorder | 22% to 28% | 42% |
| Anxiety disorders | 26% to 44% | 63% |
| Antisocial personality disorder | No rates; lifetime diagnosis | 16% to 40% |
| Psychotic disorders | 9% to 17% | 11% |
| Substance use disorder | 56% | 56% to 86% |
| Source: References 1,5,6 | ||
Many patients with chronic HCV complain of chronic fatigue and deficiencies in attention, concentration, higher executive functions, learning ability, and memory that result in significant reduction in quality of life (Box 1).7-9 These findings have been found to be independent of the degree of liver disease and are seen in HCV patients with normal liver function.7,8
The pathophysiology of fatigue and neurocognitive dysfunction in hepatitis C virus (HCV) infection is unclear. However, the improvement of chronic fatigue in patients with HCV who receive ondansetron, a 5-hydroxytryptophan-3 receptor antagonist, has implicated abnormal monoaminergic function. Single-photon emission CT studies have found decreased midbrain serotonergic and striatal dopaminergic transmission in some HCV patients with cognitive deficits.7
Recently, data have been mounting on a direct neuropathic effect of HCV, with viral elements found in autopsy brain tissue and cerebrospinal fluid.8 Researchers have suggested that HCV may enter the CNS via a Trojan horse-like mechanism inside infected mononuclear cells.8 More recently, human brain microvascular endothelium, the major component of the blood-brain barrier, has been found to express all major viral receptors that would allow HCV infection of the CNS.9
CASE CONTINUED: Motivated and compliant
Since joining the MMTP 6 months ago, Mr. P has been motivated and compliant with all appointments and treatments. Routine urine toxicology screening supports his claim of abstinence. Mr. P begins HCV treatment while continuing follow-up with addiction medicine and psychiatric clinicians and maintains open communication with all treatment providers.
For many years the standard HCV treatment was pegylated interferon-α (IFN-α) and ribavirin. IFN-α is a proinflammatory cytokine with antiproliferative, antiviral, and immunoregulatory properties. The half-life of IFN-α significantly increases with pegylation, which allows for weekly injections.10,11 IFN-α usually is combined with ribavirin, which increases its efficacy as measured by the sustained virological response (SVR) compared with IFN-α alone. Depending on the virus genotype, treatment lasts 24 to 48 weeks; SVR rates range from 40% to 82%.11-13 In 2011, the FDA approved 2 agents—telaprevir and boceprevir—for adjunctive treatment of HCV genotype 1 infection. These 2 agents are protease inhibitors that when added to IFN-α and ribavirin increase the SVR rate in genotype 1 infection from 40% to 50% to approximately 75%.14,15
Although the neuropsychiatric side effects of telaprevir and boceprevir have not been determined, treating chronic HCV with IFN-α and ribavirin has been associated with multiple psychiatric symptoms, including depression, mania, suicidality, anxiety, and psychosis.11-14 Psychiatric symptoms are a common reason for discontinuing or reducing HCV treatment. Because of the high frequency of neuropsychiatric complications, some clinicians believe HCV patients with preexisting affective, psychotic, or substance use disorders should be excluded from HCV treatment. This has led to many HCV patients being untreated despite a lack of prospective, controlled data to support this opinion.12 To improve outcomes and decrease morbidity, providing appropriate psychiatric services appears to be more important than attempting to select lower-risk patients for antiviral therapy.1,12,16 The goals of psychiatric treatment should be to alleviate symptoms and allow patients to complete IFN-α therapy without interruption.16,17
Studies of high-risk patients who attend multidisciplinary treatment programs that can monitor adherence and efficacy and control side effects before and during HCV treatment have found psychiatric patients have similar adherence, compliance, and SVR rates and were not at increased risk of worsening depressive or psychotic symptoms compared with patients without a psychiatric history.12,18 Additionally, HCV patients with a psychiatric history are not at an increased risk of suicide.13,16 Similar findings have been observed in patients with active IV drug use or those receiving opioid agonist therapy. When HCV and substance use are treated simultaneously, patients can successfully complete HCV treatment with SVR rates comparable to those of patients not receiving opioid agonist therapy.19-21
CASE CONTINUED: Worsening symptoms
During a psychiatric follow-up 12 weeks after starting HCV treatment, Mr. P reports worsening depressive symptoms with low mood, decreased enjoyment of activities, poor sleep, low appetite, and fatigue. He shows no evidence of psychosis and denies suicidal ideation. We continue his HCV treatment, schedule more frequent psychiatric visits, and initiate citalopram, titrated to 40 mg/d.
Depressive symptoms, the most common neuropsychiatric manifestation of HCV, typically begin early in treatment, usually within the first 12 weeks. Two distinct symptom clusters are noted. A neurovegetative cluster characterized by reduced energy, anorexia, and psychomotor retardation typically begins within the first few months of treatment. Months later, a depression-specific syndrome appears that includes depressed mood, anxiety, and cognitive impairment.22
Depressive symptoms may occur in up to 60% of patients treated with IFN-α.11 When more rigorous depression measures are used, rates decrease to approximately 20% to 30%.11,13 Accurate diagnosis and treatment of emerging depressive symptoms is essential because untreated depression can lead to postponing or excluding patients from antiviral treatment.2 Screening instruments such as the Beck Depression Inventory-Second Edition (BDI-II) can be used to measure depressive symptoms in HCV patients with high sensitivity. However, because specificity has been low and somatic symptoms of chronic illness and depression often overlap, the BDI-II and other inventories may overestimate depression. Some researchers have suggested that focusing on questions targeting cognitive and affective symptoms rather than somatic ones may be a more valid measure of depression in patients undergoing immunotherapy for HCV.2
The immune system is implicated in IFN-α-induced depression because depressive symptoms share many features with a constellation of somatic and behavioral symptoms termed “sickness behavior.”11 These behaviors can occur when patients are exposed to cytokines that lead to a depressed level of functioning, which may allow the body to devote more energy to fighting illness. IFN-α, a cytokine, stimulates the immune system, which can lead to increases of interleukin (IL)-2, IL-6, and IL-10. Increased circulating levels of these ILs have been correlated with higher depression scores. Additionally, studies have found that patients who develop depression during IFN-α treatment have higher SVR rates, suggesting a more robust immune response.11,22 For a discussion of how serotonin metabolism and genetic polymorphisms also may help explain the prevalence of depression in patients with HCV, see Box 2.
Altered serotonin metabolism has been linked to depression in hepatitis C virus (HCV) patients treated with interferon-α (IFN-α). Tryptophan can be metabolized towards serotonin via tryptophan hydroxylase and niacin via indoleamine-2,3-dioxygenase (IDO) with kynurenine (KYN) and quinolinic acid (QUIN) as intermediaries. Introduction of IFN-α activates IDO, causing preferential conversion of tryptophan towards the niacin arm away from serotonin and leads to elevated levels of KYN and QUIN. KYN and QUIN are available centrally, are neurotoxic, and have been correlated with increased depressive symptoms in IFN-α-treated patients.a,b A tryptophan-deficient state is created, with less tryptophan being converted to serotonin and subsequently to its metabolite, 5-hydroxyindoleacetic acid (5-HIAA). Decreased levels of 5-HIAA in cerebrospinal fluid have been associated with higher depressive symptoms and higher rates of suicide.a,b
Several genetic polymorphisms may help identify patients at risk for developing IFN-α-induced depression. Genes for the 5’ promoter of the serotonin transporter (5-HTTLPR) have been investigated for roles in depression development in patients undergoing immunotherapy. Studies have found that persons with the short allele in the 5-HTTLPR gene are more likely to develop depression than those with the long allele. However, this has not been consistent across racial or ethnic groups.a,b Research also has associated the serotonin (5-HT) transporter, interferon receptor-A1, apolipoprotein ε4 allele, cyclooxygenase 2, and phospholipase A2 with development of a specific subgroup of symptoms.a
References
a. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
b. Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18(3):153-160.
Treating depressed HCV patients
Antidepressants are the treatment of choice for IFN-α-induced depression. Most currently used antidepressants are effective22 and selective serotonin reuptake inhibitors are considered first choice.16 Antidepressant choice should be guided by principles similar to those used for patients without HCV: using side effects profiles to target specific symptoms and being mindful of pharmacokinetic properties.
Two treatment approaches have been investigated: prophylactic and symptomatic. A 2012 study23 of 181 HCV patients with no history of mental illness determined escitalopram, 10 mg/d, effectively reduced the incidence and severity of interferon-associated depression. Other studies examining prophylactic treatment of all patients who were to undergo interferon treatment found this approach did not prevent depressive episodes.24,25 However, antidepressants have been beneficial for patients with subsyndromal depressive symptoms at baseline26 and after clinically significant depressive symptoms emerge.27 Electroconvulsive therapy also has been reported to effectively treat depression in HCV patients undergoing antiviral therapy.28
CASE CONTINUED: Lingering symptoms
Mr. P responds to citalopram with an improvement in mood, anhedonia, and appetite, but he continues to complain of low energy and poor concentration. In an effort to target these symptoms, methylphenidate, titrated to 30 mg/d in divided doses, is added to his regimen, which rapidly improves his symptoms. Insomnia is treated successfully with trazodone, 50 mg/d. Mr. P frequently visits his psychiatrist, who monitors his depressive symptoms using the BDI-II. Mr. P completes HCV treatment without recurrence of depressive symptoms or relapse to heroin use.
Although antidepressants are effective for treating affective and cognitive symptoms, they are not as effective for fatigue and other neurovegetative symptoms.16,29 The psychostimulants methylphenidate and dextroamphetamine and the nonstimulant modafinil have been studied for treating depressive symptoms in medically ill patients and can be used to treat IFN-α-induced fatigue.16,22,29
IFN-α’s effect on serotonin metabolism leads to a tryptophan-deficient state because of increased catabolism as a result of activation of indoleamine-2,3-dioxygenase (IDO). This has led to use of tryptophan supplementation, either as augmentation or monotherapy, for managing depressive symptoms in patients treated with IFN-α. Schaefer et al30 reported 3 cases where tryptophan supplementation significantly decreased depressive symptoms. Other researchers have argued that supplementing tryptophan in the context of IDO activation can lead to greater production of kynurenine and quinolinic acid, which have been linked to increased depressive symptoms in patients receiving IFN-α.31 They argue that supplementation of 5-HTP, which is available as a dietary supplement without a prescription, can lead to increased serotonin levels and improvement in depressive symptoms.31
IFN-α treatment also is associated with mania and psychosis. The incidence, pathophysiology, and management of these treatment-emergent symptoms are not as well studied as IFN-α-induced depression. Mania and hypomania have been reported with interferon treatment, discontinuation of interferon, and use of antidepressants for interferon-induced depression.29,32 Psychosis, in association with mood symptoms or alone, has been reported to occur in <1% of treated patients.33 Treatment for mania and psychosis consists of decreasing or discontinuing immunotherapy and adding mood stabilizers and antipsychotics. Once immunotherapy is discontinued, mania and psychosis usually resolve, but prolonged duration of symptoms has been reported.29,32,33
Related Resources
- Centers for Disease Control and Prevention. Hepatitis C information for health professionals. www.cdc.gov/hepatitis/hcv/index.htm.
- Hep C Connection. www.hepc-connection.org.
- United States Department of Veterans Affairs. Hepatitis C. www.hepatitis.va.gov.
Drug Brand Names
- Boceprevir • Victrelis
- Citalopram • Celexa
- Dextroamphetamine • Dexedrine
- Escitalopram • Lexapro
- Interferon-α • Intron
- Methadone • Dolophine, Methadose
- Methylphenidate • Ritalin, Methylin, others
- Modafinil • Provigil
- Ondansetron • Zofran
- Ribavirin • Copegus, Rebetol, others
- Telaprevir • Incivek
- Trazodone • Desyrel, Oleptro
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Batki SL, Canfield KM, Ploutz-Snyder R. Psychiatric and substance use disorders among methadone maintenance patients with chronic hepatitis C infection: effects on eligibility for hepatitis C treatment. Am J Addict. 2011;20(4):312-318.
2. Patterson AL, Morasco BJ, Fuller BE, et al. Screening for depression in patients with hepatitis C using the Beck Depression Inventory-II: do somatic symptoms compromise validity? Gen Hosp Psychiatry. 2011;33(4):354-362.
3. Maddur H, Kwo PY. Boceprevir. Hepatology. 2011;54(6):2254-2257.
4. Sylvestre D. Hepatitis C for addiction professionals. Addict Sci Clin Pract. 2007;4(1):34-41.
5. Dwight MM, Kowdley KV, Russo JE, et al. Depression, fatigue, and functional disability in patients with chronic hepatitis C. J Psychosom Res. 2000;49(5):311-317.
6. Yovtcheva SP, Rifai MA, Moles JK, et al. Psychiatric comorbidity among hepatitis C-positive patients. Psychosomatics. 2001;42(5):411-415.
7. Weissenborn K, Ennen JC, Bokemeyer M, et al. Monoaminergic neurotransmission is altered in hepatitis C virus infected patients with chronic fatigue and cognitive impairment. Gut. 2006;55(11):1624-1630.
8. Weissenborn K, Tryc AB, Heeren M, et al. Hepatitis C virus infection and the brain. Metab Brain Dis. 2009;24(1):197-210.
9. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142(3):634-643.e6.
10. Pawlotsky JM. Therapy of hepatitis C: from empiricism to eradication. Hepatology. 2006;43(2 suppl 1):S207-S220.
11. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
12. Schaefer M, Hinzpeter A, Mohmand A, et al. Hepatitis C treatment in “difficult-to-treat” psychiatric patients with pegylated interferon-alpha and ribavirin: response and psychiatric side effects. Hepatology. 2007;46(4):991-998.
13. Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18(3):153-160.
14. Telaprevir (Incivek) and boceprevir (Victrelis) for chronic hepatitis C. Med Lett Drugs Ther. 2011;53(1369):57-59.
15. Nelson DR. The role of triple therapy with protease inhibitors in hepatitis C virus genotype 1 naïve patients. Liver Int. 2011;31(suppl 1):53-57.
16. Spennati A, Pariante CM. Withdrawing interferon-α from psychiatric patients: clinical care or unjustifiable stigma? [published online September 14 2012] Psychol Med. doi: 10. 1017/S0033291712001808.
17. Baraldi S, Hepgul N, Mondelli V, et al. Symptomatic treatment of interferon-α-induced depression in hepatitis C: a systematic review. J Clin Psychopharmacol. 2012;32(4):531-543.
18. Schaefer M, Schmidt F, Folwaczny C, et al. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37(2):443-451.
19. Harris KA, Jr, Arnsten JH, Litwin AH. Successful integration of hepatitis C evaluation and treatment services with methadone maintenance. J Addict Med. 2010;4(1):20-26.
20. Litwin AH, Harris KA, Jr, Nahvi S, et al. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Subst Abuse Treat. 2009;37(1):32-40.
21. Sasadeusz JJ, Dore G, Kronborg I, et al. Clinical experience with the treatment of hepatitis C infection in patients on opioid pharmacotherapy. Addiction. 2011;106(5):977-984.
22. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
23. Schaefer M, Sarkar R, Knop V, et al. Escitalopram for the prevention of peginterferon-α2a-associated depression in hepatitis C virus-infected patients without previous psychiatric disease: a randomized trial. Ann Intern Med. 2012;157(2):94-103.
24. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
25. Morasco BJ, Loftis JM, Indest DW, et al. Prophylactic antidepressant treatment in patients with hepatitis C on antiviral therapy: a double-blind, placebo-controlled trial. Psychosomatics. 2010;51(5):401-408.
26. Raison CL, Woolwine BJ, Demetrashvili MF, et al. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther. 2007;25(10):1163-1174.
27. Kraus MR, Schäfer A, Schöttker K, et al. Therapy of interferon-induced depression in chronic hepatitis C with citalopram: a randomised, double-blind, placebo-controlled study. Gut. 2008;57(4):531-536.
28. Zincke MT, Kurani A, Istafanous R, et al. The successful use of electroconvulsive therapy in a patient with interferon-induced psychotic depression. J ECT. 2007;23(4):291-292.
29. Crone CC, Gabriel GM, Wise TN. Managing the neuropsychiatric side effects of interferon-based therapy for hepatitis C. Cleve Clin J Med. 2004;71(suppl 3):S27-S32.
30. Schaefer M, Winterer J, Sarkar R, et al. Three cases of successful tryptophan add-on or monotherapy of hepatitis C and IFNa-associated mood disorders. Psychosomatics. 2008;49(5):442-446.
31. Turner EH, Blackwell AD. 5-Hydroxytryptophan plus SSRIs for interferon-induced depression: synergistic mechanisms for normalizing synaptic serotonin. Med Hypotheses. 2005;65(1):138-144.
32. Onyike CU, Bonner JO, Lyketsos CG, et al. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry. 2004;161(3):429-435.
33. Cheng YC, Chen CC, Ho AS, et al. Prolonged psychosis associated with interferon therapy in a patient with hepatitis C: case study and literature review. Psychosomatics. 2009;50(5):538-542.
Mr. P, age 31, has been using heroin intravenously for 9 years. He smokes 1 pack of cigarettes daily, but denies using other substances, including alcohol. After an unintentional heroin overdose, Mr. P enrolls in a methadone maintenance treatment program (MMTP) that includes primary medical care and addiction medicine and psychiatric specialists, where he undergoes medical evaluation and screening for hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Laboratory data reveal that although Mr. P is HIV negative, he has been exposed to HCV and treatment is indicated.
Among the approximately 3 million people in the United States with chronic HCV—an enveloped, single-stranded RNA virus—there’s a high prevalence of premorbid psychopathology and substance abuse, as well as neuropsychiatric effects caused by HCV treatment.1-3 Because underdiagnosing and undertreating psychiatric disorders contributes to morbidity and mortality in HCV patients, early identification and prompt treatment is critical.
IV drug use is the most common route for HCV infection, accounting for 65% to 70% of infections.1 The prevalence of HCV among IV drug users is 28% to 90%.1 Once exposed to HCV, 75% to 85% of patients do not clear the initial infection and become chronically infected.
This article reviews the pathophysiology, identification, and management of psychiatric manifestations found among HCV patients and provides an understanding of how psychiatric symptoms manifest in HCV patients. This article also discusses HCV treatment and its neuropsychiatric side effects.
Testing for HCV
Chronically infected HCV patients may have few, if any, specific physical complaints, and often are diagnosed during screenings or other routine laboratory evaluations. The presence of risk factors, such as a history of injection drug use or receiving a blood transfusion before 1992,1 guides the decision to screen for HCV. Normal liver function test results should not preclude testing because many HCV-positive patients have transaminases within the normal range.4 Initial screening is via an antibody-mediated immunoassay that is highly specific and sensitive for past exposure to HCV (Table 1).4 However, a positive screen does not indicate the presence of active infection. Evidence of the virus via a viral assay will identify active HCV, but does not indicate need for treatment. Liver biopsy confirms the presence of liver injury and quantifies its extent. The severity of liver damage will determine whether treatment is needed. HCV genotyping determines the appropriate duration and dosage of pharmacotherapy.
Table 1
Tests to diagnose and evaluate HCV
| Test | Results |
|---|---|
| HCV antibody | Determines prior exposure to HCV |
| HCV viral assay | Evaluates for current HCV infection |
| Liver biopsy | Assesses level of liver damage |
| HCV genotyping | Provides data to determine duration and intensity of treatment and likelihood of treatment success |
| HCV: hepatitis C virus Source: Reference 4 | |
CASE CONTINUED: Mood improves, but fatigue persists
As part of pre-HCV treatment evaluation, Mr. P undergoes a psychiatric evaluation. He describes periods of low mood while actively engaged in drug use but has never received psychiatric treatment, experienced suicidal ideation, or attempted suicide. Since starting opioid agonist therapy, he reports improved mood but endorses continued mild fatigue and difficulty falling sleep. The psychiatrist determines Mr. P does not meet criteria for an axis I diagnosis other than a substance use disorder.
Although most HCV patients have few, if any, nonspecific physical symptoms, many have psychiatric symptoms or disorders before the HCV diagnosis is made or treatment is initiated; substance use disorders are most common. Batki et al1 found that 56% of HCV patients in an MMTP met criteria for a nonsubstance axis I disorder and 82% met criteria for such a disorder during their lifetime. Additionally, 66% of patients were taking psychiatric medications. Table 21,5,6 lists the rates of other psychiatric disorders found in patients with untreated HCV.
Table 2
Rates of psychiatric disorders in patients with untreated hepatitis C virus
| Disorder(s) | Current rate | Lifetime rate |
|---|---|---|
| Mood disorders | 34% to 35% | 67% |
| Major depressive disorder | 22% to 28% | 42% |
| Anxiety disorders | 26% to 44% | 63% |
| Antisocial personality disorder | No rates; lifetime diagnosis | 16% to 40% |
| Psychotic disorders | 9% to 17% | 11% |
| Substance use disorder | 56% | 56% to 86% |
| Source: References 1,5,6 | ||
Many patients with chronic HCV complain of chronic fatigue and deficiencies in attention, concentration, higher executive functions, learning ability, and memory that result in significant reduction in quality of life (Box 1).7-9 These findings have been found to be independent of the degree of liver disease and are seen in HCV patients with normal liver function.7,8
The pathophysiology of fatigue and neurocognitive dysfunction in hepatitis C virus (HCV) infection is unclear. However, the improvement of chronic fatigue in patients with HCV who receive ondansetron, a 5-hydroxytryptophan-3 receptor antagonist, has implicated abnormal monoaminergic function. Single-photon emission CT studies have found decreased midbrain serotonergic and striatal dopaminergic transmission in some HCV patients with cognitive deficits.7
Recently, data have been mounting on a direct neuropathic effect of HCV, with viral elements found in autopsy brain tissue and cerebrospinal fluid.8 Researchers have suggested that HCV may enter the CNS via a Trojan horse-like mechanism inside infected mononuclear cells.8 More recently, human brain microvascular endothelium, the major component of the blood-brain barrier, has been found to express all major viral receptors that would allow HCV infection of the CNS.9
CASE CONTINUED: Motivated and compliant
Since joining the MMTP 6 months ago, Mr. P has been motivated and compliant with all appointments and treatments. Routine urine toxicology screening supports his claim of abstinence. Mr. P begins HCV treatment while continuing follow-up with addiction medicine and psychiatric clinicians and maintains open communication with all treatment providers.
For many years the standard HCV treatment was pegylated interferon-α (IFN-α) and ribavirin. IFN-α is a proinflammatory cytokine with antiproliferative, antiviral, and immunoregulatory properties. The half-life of IFN-α significantly increases with pegylation, which allows for weekly injections.10,11 IFN-α usually is combined with ribavirin, which increases its efficacy as measured by the sustained virological response (SVR) compared with IFN-α alone. Depending on the virus genotype, treatment lasts 24 to 48 weeks; SVR rates range from 40% to 82%.11-13 In 2011, the FDA approved 2 agents—telaprevir and boceprevir—for adjunctive treatment of HCV genotype 1 infection. These 2 agents are protease inhibitors that when added to IFN-α and ribavirin increase the SVR rate in genotype 1 infection from 40% to 50% to approximately 75%.14,15
Although the neuropsychiatric side effects of telaprevir and boceprevir have not been determined, treating chronic HCV with IFN-α and ribavirin has been associated with multiple psychiatric symptoms, including depression, mania, suicidality, anxiety, and psychosis.11-14 Psychiatric symptoms are a common reason for discontinuing or reducing HCV treatment. Because of the high frequency of neuropsychiatric complications, some clinicians believe HCV patients with preexisting affective, psychotic, or substance use disorders should be excluded from HCV treatment. This has led to many HCV patients being untreated despite a lack of prospective, controlled data to support this opinion.12 To improve outcomes and decrease morbidity, providing appropriate psychiatric services appears to be more important than attempting to select lower-risk patients for antiviral therapy.1,12,16 The goals of psychiatric treatment should be to alleviate symptoms and allow patients to complete IFN-α therapy without interruption.16,17
Studies of high-risk patients who attend multidisciplinary treatment programs that can monitor adherence and efficacy and control side effects before and during HCV treatment have found psychiatric patients have similar adherence, compliance, and SVR rates and were not at increased risk of worsening depressive or psychotic symptoms compared with patients without a psychiatric history.12,18 Additionally, HCV patients with a psychiatric history are not at an increased risk of suicide.13,16 Similar findings have been observed in patients with active IV drug use or those receiving opioid agonist therapy. When HCV and substance use are treated simultaneously, patients can successfully complete HCV treatment with SVR rates comparable to those of patients not receiving opioid agonist therapy.19-21
CASE CONTINUED: Worsening symptoms
During a psychiatric follow-up 12 weeks after starting HCV treatment, Mr. P reports worsening depressive symptoms with low mood, decreased enjoyment of activities, poor sleep, low appetite, and fatigue. He shows no evidence of psychosis and denies suicidal ideation. We continue his HCV treatment, schedule more frequent psychiatric visits, and initiate citalopram, titrated to 40 mg/d.
Depressive symptoms, the most common neuropsychiatric manifestation of HCV, typically begin early in treatment, usually within the first 12 weeks. Two distinct symptom clusters are noted. A neurovegetative cluster characterized by reduced energy, anorexia, and psychomotor retardation typically begins within the first few months of treatment. Months later, a depression-specific syndrome appears that includes depressed mood, anxiety, and cognitive impairment.22
Depressive symptoms may occur in up to 60% of patients treated with IFN-α.11 When more rigorous depression measures are used, rates decrease to approximately 20% to 30%.11,13 Accurate diagnosis and treatment of emerging depressive symptoms is essential because untreated depression can lead to postponing or excluding patients from antiviral treatment.2 Screening instruments such as the Beck Depression Inventory-Second Edition (BDI-II) can be used to measure depressive symptoms in HCV patients with high sensitivity. However, because specificity has been low and somatic symptoms of chronic illness and depression often overlap, the BDI-II and other inventories may overestimate depression. Some researchers have suggested that focusing on questions targeting cognitive and affective symptoms rather than somatic ones may be a more valid measure of depression in patients undergoing immunotherapy for HCV.2
The immune system is implicated in IFN-α-induced depression because depressive symptoms share many features with a constellation of somatic and behavioral symptoms termed “sickness behavior.”11 These behaviors can occur when patients are exposed to cytokines that lead to a depressed level of functioning, which may allow the body to devote more energy to fighting illness. IFN-α, a cytokine, stimulates the immune system, which can lead to increases of interleukin (IL)-2, IL-6, and IL-10. Increased circulating levels of these ILs have been correlated with higher depression scores. Additionally, studies have found that patients who develop depression during IFN-α treatment have higher SVR rates, suggesting a more robust immune response.11,22 For a discussion of how serotonin metabolism and genetic polymorphisms also may help explain the prevalence of depression in patients with HCV, see Box 2.
Altered serotonin metabolism has been linked to depression in hepatitis C virus (HCV) patients treated with interferon-α (IFN-α). Tryptophan can be metabolized towards serotonin via tryptophan hydroxylase and niacin via indoleamine-2,3-dioxygenase (IDO) with kynurenine (KYN) and quinolinic acid (QUIN) as intermediaries. Introduction of IFN-α activates IDO, causing preferential conversion of tryptophan towards the niacin arm away from serotonin and leads to elevated levels of KYN and QUIN. KYN and QUIN are available centrally, are neurotoxic, and have been correlated with increased depressive symptoms in IFN-α-treated patients.a,b A tryptophan-deficient state is created, with less tryptophan being converted to serotonin and subsequently to its metabolite, 5-hydroxyindoleacetic acid (5-HIAA). Decreased levels of 5-HIAA in cerebrospinal fluid have been associated with higher depressive symptoms and higher rates of suicide.a,b
Several genetic polymorphisms may help identify patients at risk for developing IFN-α-induced depression. Genes for the 5’ promoter of the serotonin transporter (5-HTTLPR) have been investigated for roles in depression development in patients undergoing immunotherapy. Studies have found that persons with the short allele in the 5-HTTLPR gene are more likely to develop depression than those with the long allele. However, this has not been consistent across racial or ethnic groups.a,b Research also has associated the serotonin (5-HT) transporter, interferon receptor-A1, apolipoprotein ε4 allele, cyclooxygenase 2, and phospholipase A2 with development of a specific subgroup of symptoms.a
References
a. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
b. Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18(3):153-160.
Treating depressed HCV patients
Antidepressants are the treatment of choice for IFN-α-induced depression. Most currently used antidepressants are effective22 and selective serotonin reuptake inhibitors are considered first choice.16 Antidepressant choice should be guided by principles similar to those used for patients without HCV: using side effects profiles to target specific symptoms and being mindful of pharmacokinetic properties.
Two treatment approaches have been investigated: prophylactic and symptomatic. A 2012 study23 of 181 HCV patients with no history of mental illness determined escitalopram, 10 mg/d, effectively reduced the incidence and severity of interferon-associated depression. Other studies examining prophylactic treatment of all patients who were to undergo interferon treatment found this approach did not prevent depressive episodes.24,25 However, antidepressants have been beneficial for patients with subsyndromal depressive symptoms at baseline26 and after clinically significant depressive symptoms emerge.27 Electroconvulsive therapy also has been reported to effectively treat depression in HCV patients undergoing antiviral therapy.28
CASE CONTINUED: Lingering symptoms
Mr. P responds to citalopram with an improvement in mood, anhedonia, and appetite, but he continues to complain of low energy and poor concentration. In an effort to target these symptoms, methylphenidate, titrated to 30 mg/d in divided doses, is added to his regimen, which rapidly improves his symptoms. Insomnia is treated successfully with trazodone, 50 mg/d. Mr. P frequently visits his psychiatrist, who monitors his depressive symptoms using the BDI-II. Mr. P completes HCV treatment without recurrence of depressive symptoms or relapse to heroin use.
Although antidepressants are effective for treating affective and cognitive symptoms, they are not as effective for fatigue and other neurovegetative symptoms.16,29 The psychostimulants methylphenidate and dextroamphetamine and the nonstimulant modafinil have been studied for treating depressive symptoms in medically ill patients and can be used to treat IFN-α-induced fatigue.16,22,29
IFN-α’s effect on serotonin metabolism leads to a tryptophan-deficient state because of increased catabolism as a result of activation of indoleamine-2,3-dioxygenase (IDO). This has led to use of tryptophan supplementation, either as augmentation or monotherapy, for managing depressive symptoms in patients treated with IFN-α. Schaefer et al30 reported 3 cases where tryptophan supplementation significantly decreased depressive symptoms. Other researchers have argued that supplementing tryptophan in the context of IDO activation can lead to greater production of kynurenine and quinolinic acid, which have been linked to increased depressive symptoms in patients receiving IFN-α.31 They argue that supplementation of 5-HTP, which is available as a dietary supplement without a prescription, can lead to increased serotonin levels and improvement in depressive symptoms.31
IFN-α treatment also is associated with mania and psychosis. The incidence, pathophysiology, and management of these treatment-emergent symptoms are not as well studied as IFN-α-induced depression. Mania and hypomania have been reported with interferon treatment, discontinuation of interferon, and use of antidepressants for interferon-induced depression.29,32 Psychosis, in association with mood symptoms or alone, has been reported to occur in <1% of treated patients.33 Treatment for mania and psychosis consists of decreasing or discontinuing immunotherapy and adding mood stabilizers and antipsychotics. Once immunotherapy is discontinued, mania and psychosis usually resolve, but prolonged duration of symptoms has been reported.29,32,33
Related Resources
- Centers for Disease Control and Prevention. Hepatitis C information for health professionals. www.cdc.gov/hepatitis/hcv/index.htm.
- Hep C Connection. www.hepc-connection.org.
- United States Department of Veterans Affairs. Hepatitis C. www.hepatitis.va.gov.
Drug Brand Names
- Boceprevir • Victrelis
- Citalopram • Celexa
- Dextroamphetamine • Dexedrine
- Escitalopram • Lexapro
- Interferon-α • Intron
- Methadone • Dolophine, Methadose
- Methylphenidate • Ritalin, Methylin, others
- Modafinil • Provigil
- Ondansetron • Zofran
- Ribavirin • Copegus, Rebetol, others
- Telaprevir • Incivek
- Trazodone • Desyrel, Oleptro
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. P, age 31, has been using heroin intravenously for 9 years. He smokes 1 pack of cigarettes daily, but denies using other substances, including alcohol. After an unintentional heroin overdose, Mr. P enrolls in a methadone maintenance treatment program (MMTP) that includes primary medical care and addiction medicine and psychiatric specialists, where he undergoes medical evaluation and screening for hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Laboratory data reveal that although Mr. P is HIV negative, he has been exposed to HCV and treatment is indicated.
Among the approximately 3 million people in the United States with chronic HCV—an enveloped, single-stranded RNA virus—there’s a high prevalence of premorbid psychopathology and substance abuse, as well as neuropsychiatric effects caused by HCV treatment.1-3 Because underdiagnosing and undertreating psychiatric disorders contributes to morbidity and mortality in HCV patients, early identification and prompt treatment is critical.
IV drug use is the most common route for HCV infection, accounting for 65% to 70% of infections.1 The prevalence of HCV among IV drug users is 28% to 90%.1 Once exposed to HCV, 75% to 85% of patients do not clear the initial infection and become chronically infected.
This article reviews the pathophysiology, identification, and management of psychiatric manifestations found among HCV patients and provides an understanding of how psychiatric symptoms manifest in HCV patients. This article also discusses HCV treatment and its neuropsychiatric side effects.
Testing for HCV
Chronically infected HCV patients may have few, if any, specific physical complaints, and often are diagnosed during screenings or other routine laboratory evaluations. The presence of risk factors, such as a history of injection drug use or receiving a blood transfusion before 1992,1 guides the decision to screen for HCV. Normal liver function test results should not preclude testing because many HCV-positive patients have transaminases within the normal range.4 Initial screening is via an antibody-mediated immunoassay that is highly specific and sensitive for past exposure to HCV (Table 1).4 However, a positive screen does not indicate the presence of active infection. Evidence of the virus via a viral assay will identify active HCV, but does not indicate need for treatment. Liver biopsy confirms the presence of liver injury and quantifies its extent. The severity of liver damage will determine whether treatment is needed. HCV genotyping determines the appropriate duration and dosage of pharmacotherapy.
Table 1
Tests to diagnose and evaluate HCV
| Test | Results |
|---|---|
| HCV antibody | Determines prior exposure to HCV |
| HCV viral assay | Evaluates for current HCV infection |
| Liver biopsy | Assesses level of liver damage |
| HCV genotyping | Provides data to determine duration and intensity of treatment and likelihood of treatment success |
| HCV: hepatitis C virus Source: Reference 4 | |
CASE CONTINUED: Mood improves, but fatigue persists
As part of pre-HCV treatment evaluation, Mr. P undergoes a psychiatric evaluation. He describes periods of low mood while actively engaged in drug use but has never received psychiatric treatment, experienced suicidal ideation, or attempted suicide. Since starting opioid agonist therapy, he reports improved mood but endorses continued mild fatigue and difficulty falling sleep. The psychiatrist determines Mr. P does not meet criteria for an axis I diagnosis other than a substance use disorder.
Although most HCV patients have few, if any, nonspecific physical symptoms, many have psychiatric symptoms or disorders before the HCV diagnosis is made or treatment is initiated; substance use disorders are most common. Batki et al1 found that 56% of HCV patients in an MMTP met criteria for a nonsubstance axis I disorder and 82% met criteria for such a disorder during their lifetime. Additionally, 66% of patients were taking psychiatric medications. Table 21,5,6 lists the rates of other psychiatric disorders found in patients with untreated HCV.
Table 2
Rates of psychiatric disorders in patients with untreated hepatitis C virus
| Disorder(s) | Current rate | Lifetime rate |
|---|---|---|
| Mood disorders | 34% to 35% | 67% |
| Major depressive disorder | 22% to 28% | 42% |
| Anxiety disorders | 26% to 44% | 63% |
| Antisocial personality disorder | No rates; lifetime diagnosis | 16% to 40% |
| Psychotic disorders | 9% to 17% | 11% |
| Substance use disorder | 56% | 56% to 86% |
| Source: References 1,5,6 | ||
Many patients with chronic HCV complain of chronic fatigue and deficiencies in attention, concentration, higher executive functions, learning ability, and memory that result in significant reduction in quality of life (Box 1).7-9 These findings have been found to be independent of the degree of liver disease and are seen in HCV patients with normal liver function.7,8
The pathophysiology of fatigue and neurocognitive dysfunction in hepatitis C virus (HCV) infection is unclear. However, the improvement of chronic fatigue in patients with HCV who receive ondansetron, a 5-hydroxytryptophan-3 receptor antagonist, has implicated abnormal monoaminergic function. Single-photon emission CT studies have found decreased midbrain serotonergic and striatal dopaminergic transmission in some HCV patients with cognitive deficits.7
Recently, data have been mounting on a direct neuropathic effect of HCV, with viral elements found in autopsy brain tissue and cerebrospinal fluid.8 Researchers have suggested that HCV may enter the CNS via a Trojan horse-like mechanism inside infected mononuclear cells.8 More recently, human brain microvascular endothelium, the major component of the blood-brain barrier, has been found to express all major viral receptors that would allow HCV infection of the CNS.9
CASE CONTINUED: Motivated and compliant
Since joining the MMTP 6 months ago, Mr. P has been motivated and compliant with all appointments and treatments. Routine urine toxicology screening supports his claim of abstinence. Mr. P begins HCV treatment while continuing follow-up with addiction medicine and psychiatric clinicians and maintains open communication with all treatment providers.
For many years the standard HCV treatment was pegylated interferon-α (IFN-α) and ribavirin. IFN-α is a proinflammatory cytokine with antiproliferative, antiviral, and immunoregulatory properties. The half-life of IFN-α significantly increases with pegylation, which allows for weekly injections.10,11 IFN-α usually is combined with ribavirin, which increases its efficacy as measured by the sustained virological response (SVR) compared with IFN-α alone. Depending on the virus genotype, treatment lasts 24 to 48 weeks; SVR rates range from 40% to 82%.11-13 In 2011, the FDA approved 2 agents—telaprevir and boceprevir—for adjunctive treatment of HCV genotype 1 infection. These 2 agents are protease inhibitors that when added to IFN-α and ribavirin increase the SVR rate in genotype 1 infection from 40% to 50% to approximately 75%.14,15
Although the neuropsychiatric side effects of telaprevir and boceprevir have not been determined, treating chronic HCV with IFN-α and ribavirin has been associated with multiple psychiatric symptoms, including depression, mania, suicidality, anxiety, and psychosis.11-14 Psychiatric symptoms are a common reason for discontinuing or reducing HCV treatment. Because of the high frequency of neuropsychiatric complications, some clinicians believe HCV patients with preexisting affective, psychotic, or substance use disorders should be excluded from HCV treatment. This has led to many HCV patients being untreated despite a lack of prospective, controlled data to support this opinion.12 To improve outcomes and decrease morbidity, providing appropriate psychiatric services appears to be more important than attempting to select lower-risk patients for antiviral therapy.1,12,16 The goals of psychiatric treatment should be to alleviate symptoms and allow patients to complete IFN-α therapy without interruption.16,17
Studies of high-risk patients who attend multidisciplinary treatment programs that can monitor adherence and efficacy and control side effects before and during HCV treatment have found psychiatric patients have similar adherence, compliance, and SVR rates and were not at increased risk of worsening depressive or psychotic symptoms compared with patients without a psychiatric history.12,18 Additionally, HCV patients with a psychiatric history are not at an increased risk of suicide.13,16 Similar findings have been observed in patients with active IV drug use or those receiving opioid agonist therapy. When HCV and substance use are treated simultaneously, patients can successfully complete HCV treatment with SVR rates comparable to those of patients not receiving opioid agonist therapy.19-21
CASE CONTINUED: Worsening symptoms
During a psychiatric follow-up 12 weeks after starting HCV treatment, Mr. P reports worsening depressive symptoms with low mood, decreased enjoyment of activities, poor sleep, low appetite, and fatigue. He shows no evidence of psychosis and denies suicidal ideation. We continue his HCV treatment, schedule more frequent psychiatric visits, and initiate citalopram, titrated to 40 mg/d.
Depressive symptoms, the most common neuropsychiatric manifestation of HCV, typically begin early in treatment, usually within the first 12 weeks. Two distinct symptom clusters are noted. A neurovegetative cluster characterized by reduced energy, anorexia, and psychomotor retardation typically begins within the first few months of treatment. Months later, a depression-specific syndrome appears that includes depressed mood, anxiety, and cognitive impairment.22
Depressive symptoms may occur in up to 60% of patients treated with IFN-α.11 When more rigorous depression measures are used, rates decrease to approximately 20% to 30%.11,13 Accurate diagnosis and treatment of emerging depressive symptoms is essential because untreated depression can lead to postponing or excluding patients from antiviral treatment.2 Screening instruments such as the Beck Depression Inventory-Second Edition (BDI-II) can be used to measure depressive symptoms in HCV patients with high sensitivity. However, because specificity has been low and somatic symptoms of chronic illness and depression often overlap, the BDI-II and other inventories may overestimate depression. Some researchers have suggested that focusing on questions targeting cognitive and affective symptoms rather than somatic ones may be a more valid measure of depression in patients undergoing immunotherapy for HCV.2
The immune system is implicated in IFN-α-induced depression because depressive symptoms share many features with a constellation of somatic and behavioral symptoms termed “sickness behavior.”11 These behaviors can occur when patients are exposed to cytokines that lead to a depressed level of functioning, which may allow the body to devote more energy to fighting illness. IFN-α, a cytokine, stimulates the immune system, which can lead to increases of interleukin (IL)-2, IL-6, and IL-10. Increased circulating levels of these ILs have been correlated with higher depression scores. Additionally, studies have found that patients who develop depression during IFN-α treatment have higher SVR rates, suggesting a more robust immune response.11,22 For a discussion of how serotonin metabolism and genetic polymorphisms also may help explain the prevalence of depression in patients with HCV, see Box 2.
Altered serotonin metabolism has been linked to depression in hepatitis C virus (HCV) patients treated with interferon-α (IFN-α). Tryptophan can be metabolized towards serotonin via tryptophan hydroxylase and niacin via indoleamine-2,3-dioxygenase (IDO) with kynurenine (KYN) and quinolinic acid (QUIN) as intermediaries. Introduction of IFN-α activates IDO, causing preferential conversion of tryptophan towards the niacin arm away from serotonin and leads to elevated levels of KYN and QUIN. KYN and QUIN are available centrally, are neurotoxic, and have been correlated with increased depressive symptoms in IFN-α-treated patients.a,b A tryptophan-deficient state is created, with less tryptophan being converted to serotonin and subsequently to its metabolite, 5-hydroxyindoleacetic acid (5-HIAA). Decreased levels of 5-HIAA in cerebrospinal fluid have been associated with higher depressive symptoms and higher rates of suicide.a,b
Several genetic polymorphisms may help identify patients at risk for developing IFN-α-induced depression. Genes for the 5’ promoter of the serotonin transporter (5-HTTLPR) have been investigated for roles in depression development in patients undergoing immunotherapy. Studies have found that persons with the short allele in the 5-HTTLPR gene are more likely to develop depression than those with the long allele. However, this has not been consistent across racial or ethnic groups.a,b Research also has associated the serotonin (5-HT) transporter, interferon receptor-A1, apolipoprotein ε4 allele, cyclooxygenase 2, and phospholipase A2 with development of a specific subgroup of symptoms.a
References
a. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
b. Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18(3):153-160.
Treating depressed HCV patients
Antidepressants are the treatment of choice for IFN-α-induced depression. Most currently used antidepressants are effective22 and selective serotonin reuptake inhibitors are considered first choice.16 Antidepressant choice should be guided by principles similar to those used for patients without HCV: using side effects profiles to target specific symptoms and being mindful of pharmacokinetic properties.
Two treatment approaches have been investigated: prophylactic and symptomatic. A 2012 study23 of 181 HCV patients with no history of mental illness determined escitalopram, 10 mg/d, effectively reduced the incidence and severity of interferon-associated depression. Other studies examining prophylactic treatment of all patients who were to undergo interferon treatment found this approach did not prevent depressive episodes.24,25 However, antidepressants have been beneficial for patients with subsyndromal depressive symptoms at baseline26 and after clinically significant depressive symptoms emerge.27 Electroconvulsive therapy also has been reported to effectively treat depression in HCV patients undergoing antiviral therapy.28
CASE CONTINUED: Lingering symptoms
Mr. P responds to citalopram with an improvement in mood, anhedonia, and appetite, but he continues to complain of low energy and poor concentration. In an effort to target these symptoms, methylphenidate, titrated to 30 mg/d in divided doses, is added to his regimen, which rapidly improves his symptoms. Insomnia is treated successfully with trazodone, 50 mg/d. Mr. P frequently visits his psychiatrist, who monitors his depressive symptoms using the BDI-II. Mr. P completes HCV treatment without recurrence of depressive symptoms or relapse to heroin use.
Although antidepressants are effective for treating affective and cognitive symptoms, they are not as effective for fatigue and other neurovegetative symptoms.16,29 The psychostimulants methylphenidate and dextroamphetamine and the nonstimulant modafinil have been studied for treating depressive symptoms in medically ill patients and can be used to treat IFN-α-induced fatigue.16,22,29
IFN-α’s effect on serotonin metabolism leads to a tryptophan-deficient state because of increased catabolism as a result of activation of indoleamine-2,3-dioxygenase (IDO). This has led to use of tryptophan supplementation, either as augmentation or monotherapy, for managing depressive symptoms in patients treated with IFN-α. Schaefer et al30 reported 3 cases where tryptophan supplementation significantly decreased depressive symptoms. Other researchers have argued that supplementing tryptophan in the context of IDO activation can lead to greater production of kynurenine and quinolinic acid, which have been linked to increased depressive symptoms in patients receiving IFN-α.31 They argue that supplementation of 5-HTP, which is available as a dietary supplement without a prescription, can lead to increased serotonin levels and improvement in depressive symptoms.31
IFN-α treatment also is associated with mania and psychosis. The incidence, pathophysiology, and management of these treatment-emergent symptoms are not as well studied as IFN-α-induced depression. Mania and hypomania have been reported with interferon treatment, discontinuation of interferon, and use of antidepressants for interferon-induced depression.29,32 Psychosis, in association with mood symptoms or alone, has been reported to occur in <1% of treated patients.33 Treatment for mania and psychosis consists of decreasing or discontinuing immunotherapy and adding mood stabilizers and antipsychotics. Once immunotherapy is discontinued, mania and psychosis usually resolve, but prolonged duration of symptoms has been reported.29,32,33
Related Resources
- Centers for Disease Control and Prevention. Hepatitis C information for health professionals. www.cdc.gov/hepatitis/hcv/index.htm.
- Hep C Connection. www.hepc-connection.org.
- United States Department of Veterans Affairs. Hepatitis C. www.hepatitis.va.gov.
Drug Brand Names
- Boceprevir • Victrelis
- Citalopram • Celexa
- Dextroamphetamine • Dexedrine
- Escitalopram • Lexapro
- Interferon-α • Intron
- Methadone • Dolophine, Methadose
- Methylphenidate • Ritalin, Methylin, others
- Modafinil • Provigil
- Ondansetron • Zofran
- Ribavirin • Copegus, Rebetol, others
- Telaprevir • Incivek
- Trazodone • Desyrel, Oleptro
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Batki SL, Canfield KM, Ploutz-Snyder R. Psychiatric and substance use disorders among methadone maintenance patients with chronic hepatitis C infection: effects on eligibility for hepatitis C treatment. Am J Addict. 2011;20(4):312-318.
2. Patterson AL, Morasco BJ, Fuller BE, et al. Screening for depression in patients with hepatitis C using the Beck Depression Inventory-II: do somatic symptoms compromise validity? Gen Hosp Psychiatry. 2011;33(4):354-362.
3. Maddur H, Kwo PY. Boceprevir. Hepatology. 2011;54(6):2254-2257.
4. Sylvestre D. Hepatitis C for addiction professionals. Addict Sci Clin Pract. 2007;4(1):34-41.
5. Dwight MM, Kowdley KV, Russo JE, et al. Depression, fatigue, and functional disability in patients with chronic hepatitis C. J Psychosom Res. 2000;49(5):311-317.
6. Yovtcheva SP, Rifai MA, Moles JK, et al. Psychiatric comorbidity among hepatitis C-positive patients. Psychosomatics. 2001;42(5):411-415.
7. Weissenborn K, Ennen JC, Bokemeyer M, et al. Monoaminergic neurotransmission is altered in hepatitis C virus infected patients with chronic fatigue and cognitive impairment. Gut. 2006;55(11):1624-1630.
8. Weissenborn K, Tryc AB, Heeren M, et al. Hepatitis C virus infection and the brain. Metab Brain Dis. 2009;24(1):197-210.
9. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142(3):634-643.e6.
10. Pawlotsky JM. Therapy of hepatitis C: from empiricism to eradication. Hepatology. 2006;43(2 suppl 1):S207-S220.
11. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
12. Schaefer M, Hinzpeter A, Mohmand A, et al. Hepatitis C treatment in “difficult-to-treat” psychiatric patients with pegylated interferon-alpha and ribavirin: response and psychiatric side effects. Hepatology. 2007;46(4):991-998.
13. Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18(3):153-160.
14. Telaprevir (Incivek) and boceprevir (Victrelis) for chronic hepatitis C. Med Lett Drugs Ther. 2011;53(1369):57-59.
15. Nelson DR. The role of triple therapy with protease inhibitors in hepatitis C virus genotype 1 naïve patients. Liver Int. 2011;31(suppl 1):53-57.
16. Spennati A, Pariante CM. Withdrawing interferon-α from psychiatric patients: clinical care or unjustifiable stigma? [published online September 14 2012] Psychol Med. doi: 10. 1017/S0033291712001808.
17. Baraldi S, Hepgul N, Mondelli V, et al. Symptomatic treatment of interferon-α-induced depression in hepatitis C: a systematic review. J Clin Psychopharmacol. 2012;32(4):531-543.
18. Schaefer M, Schmidt F, Folwaczny C, et al. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37(2):443-451.
19. Harris KA, Jr, Arnsten JH, Litwin AH. Successful integration of hepatitis C evaluation and treatment services with methadone maintenance. J Addict Med. 2010;4(1):20-26.
20. Litwin AH, Harris KA, Jr, Nahvi S, et al. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Subst Abuse Treat. 2009;37(1):32-40.
21. Sasadeusz JJ, Dore G, Kronborg I, et al. Clinical experience with the treatment of hepatitis C infection in patients on opioid pharmacotherapy. Addiction. 2011;106(5):977-984.
22. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
23. Schaefer M, Sarkar R, Knop V, et al. Escitalopram for the prevention of peginterferon-α2a-associated depression in hepatitis C virus-infected patients without previous psychiatric disease: a randomized trial. Ann Intern Med. 2012;157(2):94-103.
24. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
25. Morasco BJ, Loftis JM, Indest DW, et al. Prophylactic antidepressant treatment in patients with hepatitis C on antiviral therapy: a double-blind, placebo-controlled trial. Psychosomatics. 2010;51(5):401-408.
26. Raison CL, Woolwine BJ, Demetrashvili MF, et al. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther. 2007;25(10):1163-1174.
27. Kraus MR, Schäfer A, Schöttker K, et al. Therapy of interferon-induced depression in chronic hepatitis C with citalopram: a randomised, double-blind, placebo-controlled study. Gut. 2008;57(4):531-536.
28. Zincke MT, Kurani A, Istafanous R, et al. The successful use of electroconvulsive therapy in a patient with interferon-induced psychotic depression. J ECT. 2007;23(4):291-292.
29. Crone CC, Gabriel GM, Wise TN. Managing the neuropsychiatric side effects of interferon-based therapy for hepatitis C. Cleve Clin J Med. 2004;71(suppl 3):S27-S32.
30. Schaefer M, Winterer J, Sarkar R, et al. Three cases of successful tryptophan add-on or monotherapy of hepatitis C and IFNa-associated mood disorders. Psychosomatics. 2008;49(5):442-446.
31. Turner EH, Blackwell AD. 5-Hydroxytryptophan plus SSRIs for interferon-induced depression: synergistic mechanisms for normalizing synaptic serotonin. Med Hypotheses. 2005;65(1):138-144.
32. Onyike CU, Bonner JO, Lyketsos CG, et al. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry. 2004;161(3):429-435.
33. Cheng YC, Chen CC, Ho AS, et al. Prolonged psychosis associated with interferon therapy in a patient with hepatitis C: case study and literature review. Psychosomatics. 2009;50(5):538-542.
1. Batki SL, Canfield KM, Ploutz-Snyder R. Psychiatric and substance use disorders among methadone maintenance patients with chronic hepatitis C infection: effects on eligibility for hepatitis C treatment. Am J Addict. 2011;20(4):312-318.
2. Patterson AL, Morasco BJ, Fuller BE, et al. Screening for depression in patients with hepatitis C using the Beck Depression Inventory-II: do somatic symptoms compromise validity? Gen Hosp Psychiatry. 2011;33(4):354-362.
3. Maddur H, Kwo PY. Boceprevir. Hepatology. 2011;54(6):2254-2257.
4. Sylvestre D. Hepatitis C for addiction professionals. Addict Sci Clin Pract. 2007;4(1):34-41.
5. Dwight MM, Kowdley KV, Russo JE, et al. Depression, fatigue, and functional disability in patients with chronic hepatitis C. J Psychosom Res. 2000;49(5):311-317.
6. Yovtcheva SP, Rifai MA, Moles JK, et al. Psychiatric comorbidity among hepatitis C-positive patients. Psychosomatics. 2001;42(5):411-415.
7. Weissenborn K, Ennen JC, Bokemeyer M, et al. Monoaminergic neurotransmission is altered in hepatitis C virus infected patients with chronic fatigue and cognitive impairment. Gut. 2006;55(11):1624-1630.
8. Weissenborn K, Tryc AB, Heeren M, et al. Hepatitis C virus infection and the brain. Metab Brain Dis. 2009;24(1):197-210.
9. Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142(3):634-643.e6.
10. Pawlotsky JM. Therapy of hepatitis C: from empiricism to eradication. Hepatology. 2006;43(2 suppl 1):S207-S220.
11. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
12. Schaefer M, Hinzpeter A, Mohmand A, et al. Hepatitis C treatment in “difficult-to-treat” psychiatric patients with pegylated interferon-alpha and ribavirin: response and psychiatric side effects. Hepatology. 2007;46(4):991-998.
13. Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18(3):153-160.
14. Telaprevir (Incivek) and boceprevir (Victrelis) for chronic hepatitis C. Med Lett Drugs Ther. 2011;53(1369):57-59.
15. Nelson DR. The role of triple therapy with protease inhibitors in hepatitis C virus genotype 1 naïve patients. Liver Int. 2011;31(suppl 1):53-57.
16. Spennati A, Pariante CM. Withdrawing interferon-α from psychiatric patients: clinical care or unjustifiable stigma? [published online September 14 2012] Psychol Med. doi: 10. 1017/S0033291712001808.
17. Baraldi S, Hepgul N, Mondelli V, et al. Symptomatic treatment of interferon-α-induced depression in hepatitis C: a systematic review. J Clin Psychopharmacol. 2012;32(4):531-543.
18. Schaefer M, Schmidt F, Folwaczny C, et al. Adherence and mental side effects during hepatitis C treatment with interferon alfa and ribavirin in psychiatric risk groups. Hepatology. 2003;37(2):443-451.
19. Harris KA, Jr, Arnsten JH, Litwin AH. Successful integration of hepatitis C evaluation and treatment services with methadone maintenance. J Addict Med. 2010;4(1):20-26.
20. Litwin AH, Harris KA, Jr, Nahvi S, et al. Successful treatment of chronic hepatitis C with pegylated interferon in combination with ribavirin in a methadone maintenance treatment program. J Subst Abuse Treat. 2009;37(1):32-40.
21. Sasadeusz JJ, Dore G, Kronborg I, et al. Clinical experience with the treatment of hepatitis C infection in patients on opioid pharmacotherapy. Addiction. 2011;106(5):977-984.
22. Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. Can J Psychiatry. 2009;54(9):614-625.
23. Schaefer M, Sarkar R, Knop V, et al. Escitalopram for the prevention of peginterferon-α2a-associated depression in hepatitis C virus-infected patients without previous psychiatric disease: a randomized trial. Ann Intern Med. 2012;157(2):94-103.
24. Galvão-de Almeida A, Guindalini C, Batista-Neves S, et al. Can antidepressants prevent interferon-alpha-induced depression? A review of the literature. Gen Hosp Psychiatry. 2010;32(4):401-405.
25. Morasco BJ, Loftis JM, Indest DW, et al. Prophylactic antidepressant treatment in patients with hepatitis C on antiviral therapy: a double-blind, placebo-controlled trial. Psychosomatics. 2010;51(5):401-408.
26. Raison CL, Woolwine BJ, Demetrashvili MF, et al. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther. 2007;25(10):1163-1174.
27. Kraus MR, Schäfer A, Schöttker K, et al. Therapy of interferon-induced depression in chronic hepatitis C with citalopram: a randomised, double-blind, placebo-controlled study. Gut. 2008;57(4):531-536.
28. Zincke MT, Kurani A, Istafanous R, et al. The successful use of electroconvulsive therapy in a patient with interferon-induced psychotic depression. J ECT. 2007;23(4):291-292.
29. Crone CC, Gabriel GM, Wise TN. Managing the neuropsychiatric side effects of interferon-based therapy for hepatitis C. Cleve Clin J Med. 2004;71(suppl 3):S27-S32.
30. Schaefer M, Winterer J, Sarkar R, et al. Three cases of successful tryptophan add-on or monotherapy of hepatitis C and IFNa-associated mood disorders. Psychosomatics. 2008;49(5):442-446.
31. Turner EH, Blackwell AD. 5-Hydroxytryptophan plus SSRIs for interferon-induced depression: synergistic mechanisms for normalizing synaptic serotonin. Med Hypotheses. 2005;65(1):138-144.
32. Onyike CU, Bonner JO, Lyketsos CG, et al. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry. 2004;161(3):429-435.
33. Cheng YC, Chen CC, Ho AS, et al. Prolonged psychosis associated with interferon therapy in a patient with hepatitis C: case study and literature review. Psychosomatics. 2009;50(5):538-542.
‘Morning sickness’ in pregnancy loses psychogenic stigma
Nausea and vomiting in pregnancy (NVP) is a misunderstood disorder associated with stress, anxiety, and depression. Prejudice toward women is thought to have guided the historical psychoanalytic concept of NVP as psychogenic, but this view is being replaced by newer biologic theories.
This article examines the evidence for psychological and organic causes of NVP to inform psychiatrists treating pregnant patients. We review guidelines for pharmacologic treatment of NVP and discuss potentially useful psychotherapies and alternative approaches.
Definitive cause unknown
“Morning sickness” affects 50% to 80% of pregnant women, occurring so commonly that NVP is often considered normal.1,2 Approximately 0.5% to 2% of women experience the most severe NVP—hyperemesis gravidarum (HG)3—characterized by intractable vomiting, weight loss, and electrolyte imbalance that can lead to hospitalization.
Without modern supportive care, HG can be lethal; although Charlotte Brontë’s death certificate states she died of “phthisis” (tuberculosis), the author of Jane Eyre is popularly believed to have succumbed to HG.4,5
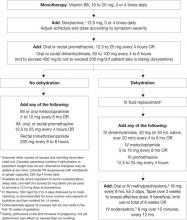
The search for effective NVP treatments has been disappointing, partly because no cause has been identified. After other conditions that may lead to nausea and vomiting are ruled out (Table 1), NVP medical management is supportive. Correcting dehydration and encouraging dietary and lifestyle changes (Table 2)6 are important adjuncts to step-wise pharmacologic treatment recommended by the American College of Obstetrics and Gynecology (Algorithm).7
Algorithm Pharmacologic treatment of nausea and vomiting in pregnancy*
Source: Adapted and reprinted with permission from Canadian Family Physician. Levichek Z, Atanackovic G, Oepkes D, et al. Nausea and vomiting of pregnancy. Evidence-based treatment algorithm. Can Fam Physician 2002;48:267-77Table 1
Medical causes of nausea and vomiting in pregnancy
| Possible cause | How to rule it out |
|---|---|
| Appendicitis | History; do physical, order imaging |
| Hepatitis | Jaundice; order liver function tests, antibody studies, imaging |
| Pancreatitis | History of alcohol use, abdominal pain; check amylase and lipase level |
| Gastrointestinal obstruction | History of surgeries; order imaging |
| Peptic ulcer disease | History; order upper GI series/endoscopy |
| Thyroid disease | Thyroid function tests |
| Urinary tract infection | Urinalysis, culture-sensitivity |
| Trophoblastic disease | Check hCG,* order ultrasound |
| * Elevated human chorionic gonadotropin (hCG) has shown evidence of an association with NVP20 | |
Table 2
Advice for patients: Strategies to manage NVP
| Correct dehydration |
| Drink small amount of fluids frequently |
| Dietary changes |
| Eat frequent, small meals |
| Avoid high-fat foods |
| Snack before getting out of bed and before going to sleep |
| Don’t force yourself to eat |
| Use candy and salty snacks to combat nausea |
| Avoid strong odors and scents; try cold foods, which may have less odor than hot foods |
| Take advantage of good days or good hours of the day for eating |
| Lifestyle changes |
| Get out of bed slowly |
| Lie down when nauseated |
| Avoid stressful situations |
| NVP: nausea and vomiting in pregnancy Source: Reference 6 |
Prejudice vs evidence
Psychological factors. Historically, psychological factors have been blamed for NVP, but support comes from a few poorly designed studies or case reports.3,4
Psychoanalytically, pregnancy and childbirth are significant events in a woman’s life and a rich environment for conflict that could lead to physical expression of symptoms. Freud believed pregnancy and childbirth involve the unconscious substitution of the penis with the child.8 Later writers viewed motherhood as woman’s most powerful wish and the primary organizer of her sexual drive and personality.8
NVP has been considered a conversion or somatization disorder in which symptoms are a “hysterical” expression of unconscious conflict. A psychoanalytic view contends that women who experience NVP are ambivalent about the pregnancy and seek to reject it.1,9 Vomiting, in this view, represents an oral abortion attempt.10 Others claim NVP is a rejection of femininity3 or that symptoms in women with overly attached maternal relationships mask unconscious aggressive feelings toward their mothers.11
Robertson11 proposed an association between NVP and a woman’s view of sexual experiences and her ability to achieve orgasm. He interviewed 100 women and found that 40 of 57 with NVP had “disturbed sexual functioning” or were “frigid” (defined as experiencing coitus as undesirable and unaccompanied by orgasm).
Higgins12 in 1887 proposed that the cause of NVP “is sexual intercourse, the husband too eager for it and the wife too adverse.” Additionally, NVP and HG have been associated with infantile, childish, immature, and hysterical personalities.12-14
Psychiatric comorbidities. Attempts have been made to associate NVP with other psychiatric disorders such as depression, bipolar disorder, schizophrenia, and anxiety disorders, including posttraumatic stress disorder (PTSD). No definitive association has been found between NVP and depressive illness, bipolar disorder, or schizophrenia15 or the use of antidepressants before or during early pregnancy.16 Studies reporting an association with depression have not established a cause-effect relationship.17
Seng18 reported increased NVP risk in women with PTSD. High levels of stress, anxiety, and depression found in women with NVP are thought to result from—rather than cause—NVP’s physical symptoms, however.3,19,20
Psychosocial stressors have been implicated, with higher NVP rates reported in unmarried women, those with unwanted or unplanned pregnancies, immigrants, and those living in crowded situations.21 NVP also is more frequent among women who experience emotionally disturbing events or interpersonal, economic, or occupational difficulties during pregnancy.22 Physical symptoms may provide secondary gain in attention and sympathy and a time-out from stressful home events.14
These psychosocial theories are poorly supported by data, but some clinicians may still believe NVP has a psychogenic cause. Lennane and Lennane23 proposed in 1973 that this perception may result from gender bias because:
- most conditions believed to have psychogenic causes affect women more than men
- the belief that NVP is psychogenic has been perpetuated primarily by male authors.
They argued that sexual prejudice may prevent women from receiving necessary symptomatic treatment and impede research into the cause of NVP.23
Gender bias continues to be found in the diagnosis of women with physical complaints. In 2006, Chiaramonte and Friend24 found strong, consistent gender bias among medical students and residents when evaluating women who reported coronary disease symptoms during stressful life events.
Organic theories
Organic theories view NVP as multifactorial, with contributions from evolution and multiple organ systems. Endocrine, vestibular, gastrointestinal, and CNS contributions have been described, but none have solved NVP’s etiologic mystery.
Evolutionary. NVP may provide an evolutionary advantage by protecting the embryo and mother. This theory states that potential toxins are present in many foods, especially if eaten in large quantities. NVP prevents the pregnant woman from eating very much and harming the embryo. Below-average miscarriage rates are seen in women with NVP.1,2,25
And because a woman’s immune system is depressed during pregnancy, NVP may be advantageous for the mother by limiting her ingestion of potential toxins.25
Endocrine. Human chorionic gonadotropin (hCG), estrogens, progesterone, and leptin, as well as adrenal cortex insufficiencies have been investigated for a role in NVP. Only hCG has shown clear evidence of an association, and some researchers believe it is the most likely cause of NVP.20
NVP rates are higher in pregnancies with elevated hCG. Molar and multiple-gestation pregnancies—each associated with elevated hCG—are complicated more frequently with the severest form of NVP.20,26 Conversely, NVP is less common in women who smoke, which is associated with lower hCG.26
During pregnancy, actions of hCG stimulate the thyroid. Hyperstimulation, leading to transient hyperthyroidism, has been implicated in NVP development.20,26 Symptom severity and the degree of thyroid stimulating hormone (TSH) suppression are closely correlated.26
Elevated hCG levels, hypersensitive TSH receptors, and the presence of a hyperactive hCG isoform have been proposed.20
Gastrointestinal disorders are believed to be involved in the pathogenesis of persistent NVP. Women with NVP usually lack structural or mucosal abnormalities and have normal endoscopic upper GI evaluations. They may, however, have disorders of the stomach’s neuromuscular function. Severe cases of gastric dysrhythmias and abnormalities of gastric tone may lead to gastroparesis.27
Stomach motility in pregnancy is influenced by neurohormonal changes, specifically in estrogen and thyroid hormones. Gastric motility abnormalities—evaluated by electrogastrography (EGG)—have been associated with NVP symptoms and normal EGGs with the absence of symptoms. Some women who had NVP and abnormal EGGs were retested after delivery when symptom-free and found to have normal myoelectric EGG patterns.27
Helicobacter pylori also may be involved in NVP, and at least 1 study found active H pylori infection and HG to be highly correlated. Pregnancy is not believed to predispose to H pylori infection, but active infection compounded by pregnancy’s hormonal changes may exacerbate NVP.28
NVP and motion sickness share many features, suggesting that NVP treatment could be targeted if a vestibular disorder could be discovered.29 Abnormal electroencephalography—particularly generalized slowing—that is not present in asymptomatic pregnant women has been reported in women with NVP.30
CNS contributions. Persistent NVP may be a learned behavior,24 a view based on findings of anticipatory nausea and vomiting in chemotherapy patients. Through conditioning, a pregnant woman may associate her physical symptoms with elements in her life that maintain the cycle of nausea and vomiting.31
Treating psychological symptoms
Brief psychotherapy to identify and correct sources of anxiety in pregnancy may alleviate a patient’s nausea and vomiting.32
- Progressive muscle relaxation training, often combined with guided imagery, can decrease nausea and vomiting associated with chemotherapy and may prevent anticipatory symptoms by decreasing anxiety.
- Systematic desensitization is successful in most chemotherapy patients who try it. In this technique, relaxation is counter-conditioned as a response to stimuli known to elicit symptoms.31
Hypnosis allows patients to achieve a physiologic state incompatible with nausea and vomiting31 and can terminate vomiting after 1 to 3 sessions.3
Medication. Similar to ondansetron, the antidepressant mirtazapine exhibits an antiemetic effect by blocking the 5-HT3 receptor. In treatment-resistant cases, mirtazapine, 30 mg/d, has been reported to ameliorate NVP symptoms, usually within 24 hours. Patients were able to return to normal diets and discontinue treatment after 6 to 10 days. Mirtazapine appears to be safe during pregnancy, based on animal studies using 17 and 20 times the maximum recommended human dose.33
For patients with anxiety symptoms, consider other medications—including selective serotonin reuptake inhibitors and benzodiazepines—only after counseling the patient about potential risks and benefits to her and the fetus.34
Alternative treatments. In traditional Indian medicine, a mixture of powdered ginger and honey is given to women with NVP. At least 2 studies demonstrate ginger’s efficacy.35
In traditional Chinese medicine, stimulating the Neiguan point (P6) on the wrist is believed to relieve nausea and vomiting. Although results are inconclusive, studies suggest that P6 stimulation can help control NVP.36 The FDA has approved wristbands that stimulate the P6 site, either electrically or by acupressure (Figure).36
Figure Wristband to manage nausea and vomiting in pregnancy
The FDA-cleared BioBand acupressure wristband may help control nausea and vomiting in pregnancy by stimulating the Neiguan point (P6) on the wrist.
Source: Reference 36Consider thiamine supplementation for women with severe symptoms, as Wernicke’s encephalopathy is a rare complication of prolonged NVP.19,37
Related resources
- Motherisk Program at The Hospital for Sick Children, Toronto, Ontario, Canada. Website with information on vomiting during pregnancy: www.motherisk.org/women/morningSickness.jsp. Nausea and vomiting of pregnancy (NVP) forum. www.motherisk.org/women/forum.jsp.
- BioBand acupuncture wristband. www.BioBands.com.
- Koren G, Bishai R, eds. Nausea and vomiting of pregnancy: state of the art 2000. Toronto, Ontario, Canada: The Motherisk Program; 2000.
Drug brand names
- Dimenhydrinate • Dramamine
- Doxylamine • Unisom
- Methylprednisolone • Medrol
- Metoclopramide • Reglan
- Mirtazapine • Remeron
- Ondansetron • Zofran
- Promethazine • Phenergan
- Trimethobenzamide • Tigan
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. el-Mallakh RS, Liebowitz NR, Hale MS. Hyperemesis gravidarum as conversion disorder. J Nerv Ment Dis 1990;178:655-9.
2. Davis M. Nausea and vomiting of pregnancy: an evidence-based review. J Perinat Neonatal Nurs 2004;18:312-28.
3. Buckwalter JG, Simpson SW. Psychological factors in the etiology and treatment of severe nausea and vomiting in pregnancy. Am J Obstet Gynecol 2002;186(5 suppl):S210-4.
4. Bogen JT. Neurosis: a Ms-diagnosis. Perspect Biol Med 1994;37(2):263-74.
5. Weiss G. The death of Charlotte Brontë. Obstet Gynecol 1991;78(4):705-8.
6. Lester EP, Notman MT. Pregnancy, developmental crisis and object relations: psychoanalytic considerations. Int J Psychoanal 1986;67(pt 3):357-66.
7. Sheehan P. Hyperemesis gravidarum—assessment and management. Aust Fam Physician 2007;36:698-701.
8. American College of Obstetrics and Gynecology. ACOG practice bulletin: nausea and vomiting of pregnancy. Obstet Gynecol 2004;103:803-14.
9. Iancu I, Kotler M, Spivak B, et al. Psychiatric aspects of hyperemesis gravidarum. Psychother Psychosom 1994;61:143-9.
10. Munch S. Chicken or the egg? The biological-psychological controversy surrounding hyperemesis gravidarum. Soc Sci Med 2002;55:1267-78.
11. Robertson GG. Nausea and vomiting of pregnancy: a study in psychosomatic and social medicine. Lancet 1946;336-45.
12. Fairweather DV. Nausea and vomiting in pregnancy. Am J Obstet Gynecol 1968;102:135-75.
13. Katon WJ, Ries RK, Bokan JA, Kleinman A. Hyperemesis gravidarum: a biopsychosocial perspective. Int J Psychiatry Med 1980;10:151-62.
14. Simpson SW, Goodwin TM, Robins SB, et al. Psychological factors and hyperemesis gravidarum. J Womens Health Gend Based Med 2001;10:471-7.
15. Majerus PW, Guze SB, Delong WB, Robins E. Psychologic factors and psychiatric disease in hyperemesis gravidarum: a follow-up study of 69 vomiters and 66 controls. Am J Psychiatry 1960;117:421-8.
16. Bozzo P, Koren G, Nava-Ocampo AA, Einarson A. The incidence of nausea and vomiting of pregnancy (NVP): a comparison between depressed women treated with antidepressants and non-depressed women. Clin Invest Med 2006;29(6):347-50.
17. Markl GE, Strunz-Lehner C, Egen-Lappe V, et al. The association of psychosocial factors with nausea and vomiting during pregnancy. J Psychosom Obstet Gynaecol 2007;1-6.
18. Seng JS, Oakley DJ, Sampselle CM, et al. Posttraumatic stress disorder and pregnancy complications. Obstet Gynecol 2001;97(1):17-22.
19. Ismail SK, Kenny L. Review on hyperemesis gravidarum. Best Pract Res Clin Gastroenterol 2007;21:755-69.
20. Verberg MF, Gillott DJ, Al-Fardan N, Grudzinskas JG. Hyperemesis gravidarum, a literature review. Hum Reprod Update 2005;11:527-39.
21. Deuchar N. Nausea and vomiting in pregnancy: a review of the problem with particular regard to psychological and social aspects. Br J Obstet Gynaecol 1995;102(1):6-8.
22. Iatrakis GM, Sakellaropoulos GG, Kourkoubas AH, Kabounia SE. Vomiting and nausea in the first 12 weeks of pregnancy. Psychother Psychosom 1988;49(1):22-4.
23. Lennane KJ, Lennane RJ. Alleged psychogenic disorders in women—a possible manifestation of sexual prejudice. N Engl J Med 1973;288(6):288-92.
24. Chiaramonte GR, Friend R. Medical students’ and residents’ gender bias in the diagnosis, treatment, and interpretation of coronary heart disease symptoms. Health Psychol 2006;25:255-66.
25. Sherman PW, Flaxman SM. Nausea and vomiting of pregnancy in an evolutionary perspective. Am J Obstet Gynecol 2002;186(5 suppl):S190-7.
26. Goodwin TM. Nausea and vomiting of pregnancy: an obstetric syndrome. Am J Obstet Gynecol 2002;186(5 suppl):S184-9.
27. Koch KL. Gastrointestinal factors in nausea and vomiting of pregnancy. Am J Obstet Gynecol 2002;186(5 suppl):S198-203.
28. Golberg D, Szilagyi A, Graves L. Hyperemesis gravidarum and Helicobacter pylori infection: a systematic review. Obstet Gynecol 2007;110:695-703.
29. Black FO. Maternal susceptibility to nausea and vomiting of pregnancy: is the vestibular system involved? Am J Obstet Gynecol 2002;186(5 suppl):S204-9.
30. Vaknin Z, Halperin R, Schneider D, et al. Hyperemesis gravidarum and nonspecific abnormal EEG findings: a preliminary report. J Reprod Med 2006;51:623-7.
31. Matteson S, Roscoe J, Hickok J, Morrow GR. The role of behavioral conditioning in the development of nausea. Am J Obstet Gynecol 2002;186(5 suppl):S239-43.
32. Zechnich R, Hammer T. Brief psychotherapy for hyperemesis gravidarum. Am Fam Physician 1982;26:179-81.
33. Guclu S, Gol M, Dogan E, Saygili U. Mirtazapine use in resistant hyperemesis gravidarum: report of three cases and review of the literature. Arch Gynecol Obstet 2005;272:298-300.
34. Raphael DB, Ross J, Brizendine L. Treating anxiety during pregnancy: Just how safe are SSRIs? Current Psychiatry 2008;7(2):39-52.
35. Niebyl JR, Goodwin TM. Overview of nausea and vomiting of pregnancy with an emphasis on vitamins and ginger. Am J Obstet Gynecol 2002;186(5 suppl):S253-5.
36. Roscoe JA, Matteson SE. Acupressure and acustimulation bands for control of nausea: a brief review. Am J Obstet Gynecol 2002;186(5 suppl):S244-7.
37. Chiossi G, Neri I, Cavazzuti M, et al. Hyperemesis gravidarum complicated by Wernicke encephalopathy: background, case report, and review of the literature. Obstet Gynecol Surv 2006;61:255-68.
Nausea and vomiting in pregnancy (NVP) is a misunderstood disorder associated with stress, anxiety, and depression. Prejudice toward women is thought to have guided the historical psychoanalytic concept of NVP as psychogenic, but this view is being replaced by newer biologic theories.
This article examines the evidence for psychological and organic causes of NVP to inform psychiatrists treating pregnant patients. We review guidelines for pharmacologic treatment of NVP and discuss potentially useful psychotherapies and alternative approaches.
Definitive cause unknown
“Morning sickness” affects 50% to 80% of pregnant women, occurring so commonly that NVP is often considered normal.1,2 Approximately 0.5% to 2% of women experience the most severe NVP—hyperemesis gravidarum (HG)3—characterized by intractable vomiting, weight loss, and electrolyte imbalance that can lead to hospitalization.
Without modern supportive care, HG can be lethal; although Charlotte Brontë’s death certificate states she died of “phthisis” (tuberculosis), the author of Jane Eyre is popularly believed to have succumbed to HG.4,5
The search for effective NVP treatments has been disappointing, partly because no cause has been identified. After other conditions that may lead to nausea and vomiting are ruled out (Table 1), NVP medical management is supportive. Correcting dehydration and encouraging dietary and lifestyle changes (Table 2)6 are important adjuncts to step-wise pharmacologic treatment recommended by the American College of Obstetrics and Gynecology (Algorithm).7
Algorithm Pharmacologic treatment of nausea and vomiting in pregnancy*
Source: Adapted and reprinted with permission from Canadian Family Physician. Levichek Z, Atanackovic G, Oepkes D, et al. Nausea and vomiting of pregnancy. Evidence-based treatment algorithm. Can Fam Physician 2002;48:267-77Table 1
Medical causes of nausea and vomiting in pregnancy
| Possible cause | How to rule it out |
|---|---|
| Appendicitis | History; do physical, order imaging |
| Hepatitis | Jaundice; order liver function tests, antibody studies, imaging |
| Pancreatitis | History of alcohol use, abdominal pain; check amylase and lipase level |
| Gastrointestinal obstruction | History of surgeries; order imaging |
| Peptic ulcer disease | History; order upper GI series/endoscopy |
| Thyroid disease | Thyroid function tests |
| Urinary tract infection | Urinalysis, culture-sensitivity |
| Trophoblastic disease | Check hCG,* order ultrasound |
| * Elevated human chorionic gonadotropin (hCG) has shown evidence of an association with NVP20 | |
Table 2
Advice for patients: Strategies to manage NVP
| Correct dehydration |
| Drink small amount of fluids frequently |
| Dietary changes |
| Eat frequent, small meals |
| Avoid high-fat foods |
| Snack before getting out of bed and before going to sleep |
| Don’t force yourself to eat |
| Use candy and salty snacks to combat nausea |
| Avoid strong odors and scents; try cold foods, which may have less odor than hot foods |
| Take advantage of good days or good hours of the day for eating |
| Lifestyle changes |
| Get out of bed slowly |
| Lie down when nauseated |
| Avoid stressful situations |
| NVP: nausea and vomiting in pregnancy Source: Reference 6 |
Prejudice vs evidence
Psychological factors. Historically, psychological factors have been blamed for NVP, but support comes from a few poorly designed studies or case reports.3,4
Psychoanalytically, pregnancy and childbirth are significant events in a woman’s life and a rich environment for conflict that could lead to physical expression of symptoms. Freud believed pregnancy and childbirth involve the unconscious substitution of the penis with the child.8 Later writers viewed motherhood as woman’s most powerful wish and the primary organizer of her sexual drive and personality.8
NVP has been considered a conversion or somatization disorder in which symptoms are a “hysterical” expression of unconscious conflict. A psychoanalytic view contends that women who experience NVP are ambivalent about the pregnancy and seek to reject it.1,9 Vomiting, in this view, represents an oral abortion attempt.10 Others claim NVP is a rejection of femininity3 or that symptoms in women with overly attached maternal relationships mask unconscious aggressive feelings toward their mothers.11
Robertson11 proposed an association between NVP and a woman’s view of sexual experiences and her ability to achieve orgasm. He interviewed 100 women and found that 40 of 57 with NVP had “disturbed sexual functioning” or were “frigid” (defined as experiencing coitus as undesirable and unaccompanied by orgasm).
Higgins12 in 1887 proposed that the cause of NVP “is sexual intercourse, the husband too eager for it and the wife too adverse.” Additionally, NVP and HG have been associated with infantile, childish, immature, and hysterical personalities.12-14
Psychiatric comorbidities. Attempts have been made to associate NVP with other psychiatric disorders such as depression, bipolar disorder, schizophrenia, and anxiety disorders, including posttraumatic stress disorder (PTSD). No definitive association has been found between NVP and depressive illness, bipolar disorder, or schizophrenia15 or the use of antidepressants before or during early pregnancy.16 Studies reporting an association with depression have not established a cause-effect relationship.17
Seng18 reported increased NVP risk in women with PTSD. High levels of stress, anxiety, and depression found in women with NVP are thought to result from—rather than cause—NVP’s physical symptoms, however.3,19,20
Psychosocial stressors have been implicated, with higher NVP rates reported in unmarried women, those with unwanted or unplanned pregnancies, immigrants, and those living in crowded situations.21 NVP also is more frequent among women who experience emotionally disturbing events or interpersonal, economic, or occupational difficulties during pregnancy.22 Physical symptoms may provide secondary gain in attention and sympathy and a time-out from stressful home events.14
These psychosocial theories are poorly supported by data, but some clinicians may still believe NVP has a psychogenic cause. Lennane and Lennane23 proposed in 1973 that this perception may result from gender bias because:
- most conditions believed to have psychogenic causes affect women more than men
- the belief that NVP is psychogenic has been perpetuated primarily by male authors.
They argued that sexual prejudice may prevent women from receiving necessary symptomatic treatment and impede research into the cause of NVP.23
Gender bias continues to be found in the diagnosis of women with physical complaints. In 2006, Chiaramonte and Friend24 found strong, consistent gender bias among medical students and residents when evaluating women who reported coronary disease symptoms during stressful life events.
Organic theories
Organic theories view NVP as multifactorial, with contributions from evolution and multiple organ systems. Endocrine, vestibular, gastrointestinal, and CNS contributions have been described, but none have solved NVP’s etiologic mystery.
Evolutionary. NVP may provide an evolutionary advantage by protecting the embryo and mother. This theory states that potential toxins are present in many foods, especially if eaten in large quantities. NVP prevents the pregnant woman from eating very much and harming the embryo. Below-average miscarriage rates are seen in women with NVP.1,2,25
And because a woman’s immune system is depressed during pregnancy, NVP may be advantageous for the mother by limiting her ingestion of potential toxins.25
Endocrine. Human chorionic gonadotropin (hCG), estrogens, progesterone, and leptin, as well as adrenal cortex insufficiencies have been investigated for a role in NVP. Only hCG has shown clear evidence of an association, and some researchers believe it is the most likely cause of NVP.20
NVP rates are higher in pregnancies with elevated hCG. Molar and multiple-gestation pregnancies—each associated with elevated hCG—are complicated more frequently with the severest form of NVP.20,26 Conversely, NVP is less common in women who smoke, which is associated with lower hCG.26
During pregnancy, actions of hCG stimulate the thyroid. Hyperstimulation, leading to transient hyperthyroidism, has been implicated in NVP development.20,26 Symptom severity and the degree of thyroid stimulating hormone (TSH) suppression are closely correlated.26
Elevated hCG levels, hypersensitive TSH receptors, and the presence of a hyperactive hCG isoform have been proposed.20
Gastrointestinal disorders are believed to be involved in the pathogenesis of persistent NVP. Women with NVP usually lack structural or mucosal abnormalities and have normal endoscopic upper GI evaluations. They may, however, have disorders of the stomach’s neuromuscular function. Severe cases of gastric dysrhythmias and abnormalities of gastric tone may lead to gastroparesis.27
Stomach motility in pregnancy is influenced by neurohormonal changes, specifically in estrogen and thyroid hormones. Gastric motility abnormalities—evaluated by electrogastrography (EGG)—have been associated with NVP symptoms and normal EGGs with the absence of symptoms. Some women who had NVP and abnormal EGGs were retested after delivery when symptom-free and found to have normal myoelectric EGG patterns.27
Helicobacter pylori also may be involved in NVP, and at least 1 study found active H pylori infection and HG to be highly correlated. Pregnancy is not believed to predispose to H pylori infection, but active infection compounded by pregnancy’s hormonal changes may exacerbate NVP.28
NVP and motion sickness share many features, suggesting that NVP treatment could be targeted if a vestibular disorder could be discovered.29 Abnormal electroencephalography—particularly generalized slowing—that is not present in asymptomatic pregnant women has been reported in women with NVP.30
CNS contributions. Persistent NVP may be a learned behavior,24 a view based on findings of anticipatory nausea and vomiting in chemotherapy patients. Through conditioning, a pregnant woman may associate her physical symptoms with elements in her life that maintain the cycle of nausea and vomiting.31
Treating psychological symptoms
Brief psychotherapy to identify and correct sources of anxiety in pregnancy may alleviate a patient’s nausea and vomiting.32
- Progressive muscle relaxation training, often combined with guided imagery, can decrease nausea and vomiting associated with chemotherapy and may prevent anticipatory symptoms by decreasing anxiety.
- Systematic desensitization is successful in most chemotherapy patients who try it. In this technique, relaxation is counter-conditioned as a response to stimuli known to elicit symptoms.31
Hypnosis allows patients to achieve a physiologic state incompatible with nausea and vomiting31 and can terminate vomiting after 1 to 3 sessions.3
Medication. Similar to ondansetron, the antidepressant mirtazapine exhibits an antiemetic effect by blocking the 5-HT3 receptor. In treatment-resistant cases, mirtazapine, 30 mg/d, has been reported to ameliorate NVP symptoms, usually within 24 hours. Patients were able to return to normal diets and discontinue treatment after 6 to 10 days. Mirtazapine appears to be safe during pregnancy, based on animal studies using 17 and 20 times the maximum recommended human dose.33
For patients with anxiety symptoms, consider other medications—including selective serotonin reuptake inhibitors and benzodiazepines—only after counseling the patient about potential risks and benefits to her and the fetus.34
Alternative treatments. In traditional Indian medicine, a mixture of powdered ginger and honey is given to women with NVP. At least 2 studies demonstrate ginger’s efficacy.35
In traditional Chinese medicine, stimulating the Neiguan point (P6) on the wrist is believed to relieve nausea and vomiting. Although results are inconclusive, studies suggest that P6 stimulation can help control NVP.36 The FDA has approved wristbands that stimulate the P6 site, either electrically or by acupressure (Figure).36
Figure Wristband to manage nausea and vomiting in pregnancy
The FDA-cleared BioBand acupressure wristband may help control nausea and vomiting in pregnancy by stimulating the Neiguan point (P6) on the wrist.
Source: Reference 36Consider thiamine supplementation for women with severe symptoms, as Wernicke’s encephalopathy is a rare complication of prolonged NVP.19,37
Related resources
- Motherisk Program at The Hospital for Sick Children, Toronto, Ontario, Canada. Website with information on vomiting during pregnancy: www.motherisk.org/women/morningSickness.jsp. Nausea and vomiting of pregnancy (NVP) forum. www.motherisk.org/women/forum.jsp.
- BioBand acupuncture wristband. www.BioBands.com.
- Koren G, Bishai R, eds. Nausea and vomiting of pregnancy: state of the art 2000. Toronto, Ontario, Canada: The Motherisk Program; 2000.
Drug brand names
- Dimenhydrinate • Dramamine
- Doxylamine • Unisom
- Methylprednisolone • Medrol
- Metoclopramide • Reglan
- Mirtazapine • Remeron
- Ondansetron • Zofran
- Promethazine • Phenergan
- Trimethobenzamide • Tigan
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Nausea and vomiting in pregnancy (NVP) is a misunderstood disorder associated with stress, anxiety, and depression. Prejudice toward women is thought to have guided the historical psychoanalytic concept of NVP as psychogenic, but this view is being replaced by newer biologic theories.
This article examines the evidence for psychological and organic causes of NVP to inform psychiatrists treating pregnant patients. We review guidelines for pharmacologic treatment of NVP and discuss potentially useful psychotherapies and alternative approaches.
Definitive cause unknown
“Morning sickness” affects 50% to 80% of pregnant women, occurring so commonly that NVP is often considered normal.1,2 Approximately 0.5% to 2% of women experience the most severe NVP—hyperemesis gravidarum (HG)3—characterized by intractable vomiting, weight loss, and electrolyte imbalance that can lead to hospitalization.
Without modern supportive care, HG can be lethal; although Charlotte Brontë’s death certificate states she died of “phthisis” (tuberculosis), the author of Jane Eyre is popularly believed to have succumbed to HG.4,5
The search for effective NVP treatments has been disappointing, partly because no cause has been identified. After other conditions that may lead to nausea and vomiting are ruled out (Table 1), NVP medical management is supportive. Correcting dehydration and encouraging dietary and lifestyle changes (Table 2)6 are important adjuncts to step-wise pharmacologic treatment recommended by the American College of Obstetrics and Gynecology (Algorithm).7
Algorithm Pharmacologic treatment of nausea and vomiting in pregnancy*
Source: Adapted and reprinted with permission from Canadian Family Physician. Levichek Z, Atanackovic G, Oepkes D, et al. Nausea and vomiting of pregnancy. Evidence-based treatment algorithm. Can Fam Physician 2002;48:267-77Table 1
Medical causes of nausea and vomiting in pregnancy
| Possible cause | How to rule it out |
|---|---|
| Appendicitis | History; do physical, order imaging |
| Hepatitis | Jaundice; order liver function tests, antibody studies, imaging |
| Pancreatitis | History of alcohol use, abdominal pain; check amylase and lipase level |
| Gastrointestinal obstruction | History of surgeries; order imaging |
| Peptic ulcer disease | History; order upper GI series/endoscopy |
| Thyroid disease | Thyroid function tests |
| Urinary tract infection | Urinalysis, culture-sensitivity |
| Trophoblastic disease | Check hCG,* order ultrasound |
| * Elevated human chorionic gonadotropin (hCG) has shown evidence of an association with NVP20 | |
Table 2
Advice for patients: Strategies to manage NVP
| Correct dehydration |
| Drink small amount of fluids frequently |
| Dietary changes |
| Eat frequent, small meals |
| Avoid high-fat foods |
| Snack before getting out of bed and before going to sleep |
| Don’t force yourself to eat |
| Use candy and salty snacks to combat nausea |
| Avoid strong odors and scents; try cold foods, which may have less odor than hot foods |
| Take advantage of good days or good hours of the day for eating |
| Lifestyle changes |
| Get out of bed slowly |
| Lie down when nauseated |
| Avoid stressful situations |
| NVP: nausea and vomiting in pregnancy Source: Reference 6 |
Prejudice vs evidence
Psychological factors. Historically, psychological factors have been blamed for NVP, but support comes from a few poorly designed studies or case reports.3,4
Psychoanalytically, pregnancy and childbirth are significant events in a woman’s life and a rich environment for conflict that could lead to physical expression of symptoms. Freud believed pregnancy and childbirth involve the unconscious substitution of the penis with the child.8 Later writers viewed motherhood as woman’s most powerful wish and the primary organizer of her sexual drive and personality.8
NVP has been considered a conversion or somatization disorder in which symptoms are a “hysterical” expression of unconscious conflict. A psychoanalytic view contends that women who experience NVP are ambivalent about the pregnancy and seek to reject it.1,9 Vomiting, in this view, represents an oral abortion attempt.10 Others claim NVP is a rejection of femininity3 or that symptoms in women with overly attached maternal relationships mask unconscious aggressive feelings toward their mothers.11
Robertson11 proposed an association between NVP and a woman’s view of sexual experiences and her ability to achieve orgasm. He interviewed 100 women and found that 40 of 57 with NVP had “disturbed sexual functioning” or were “frigid” (defined as experiencing coitus as undesirable and unaccompanied by orgasm).
Higgins12 in 1887 proposed that the cause of NVP “is sexual intercourse, the husband too eager for it and the wife too adverse.” Additionally, NVP and HG have been associated with infantile, childish, immature, and hysterical personalities.12-14
Psychiatric comorbidities. Attempts have been made to associate NVP with other psychiatric disorders such as depression, bipolar disorder, schizophrenia, and anxiety disorders, including posttraumatic stress disorder (PTSD). No definitive association has been found between NVP and depressive illness, bipolar disorder, or schizophrenia15 or the use of antidepressants before or during early pregnancy.16 Studies reporting an association with depression have not established a cause-effect relationship.17
Seng18 reported increased NVP risk in women with PTSD. High levels of stress, anxiety, and depression found in women with NVP are thought to result from—rather than cause—NVP’s physical symptoms, however.3,19,20
Psychosocial stressors have been implicated, with higher NVP rates reported in unmarried women, those with unwanted or unplanned pregnancies, immigrants, and those living in crowded situations.21 NVP also is more frequent among women who experience emotionally disturbing events or interpersonal, economic, or occupational difficulties during pregnancy.22 Physical symptoms may provide secondary gain in attention and sympathy and a time-out from stressful home events.14
These psychosocial theories are poorly supported by data, but some clinicians may still believe NVP has a psychogenic cause. Lennane and Lennane23 proposed in 1973 that this perception may result from gender bias because:
- most conditions believed to have psychogenic causes affect women more than men
- the belief that NVP is psychogenic has been perpetuated primarily by male authors.
They argued that sexual prejudice may prevent women from receiving necessary symptomatic treatment and impede research into the cause of NVP.23
Gender bias continues to be found in the diagnosis of women with physical complaints. In 2006, Chiaramonte and Friend24 found strong, consistent gender bias among medical students and residents when evaluating women who reported coronary disease symptoms during stressful life events.
Organic theories
Organic theories view NVP as multifactorial, with contributions from evolution and multiple organ systems. Endocrine, vestibular, gastrointestinal, and CNS contributions have been described, but none have solved NVP’s etiologic mystery.
Evolutionary. NVP may provide an evolutionary advantage by protecting the embryo and mother. This theory states that potential toxins are present in many foods, especially if eaten in large quantities. NVP prevents the pregnant woman from eating very much and harming the embryo. Below-average miscarriage rates are seen in women with NVP.1,2,25
And because a woman’s immune system is depressed during pregnancy, NVP may be advantageous for the mother by limiting her ingestion of potential toxins.25
Endocrine. Human chorionic gonadotropin (hCG), estrogens, progesterone, and leptin, as well as adrenal cortex insufficiencies have been investigated for a role in NVP. Only hCG has shown clear evidence of an association, and some researchers believe it is the most likely cause of NVP.20
NVP rates are higher in pregnancies with elevated hCG. Molar and multiple-gestation pregnancies—each associated with elevated hCG—are complicated more frequently with the severest form of NVP.20,26 Conversely, NVP is less common in women who smoke, which is associated with lower hCG.26
During pregnancy, actions of hCG stimulate the thyroid. Hyperstimulation, leading to transient hyperthyroidism, has been implicated in NVP development.20,26 Symptom severity and the degree of thyroid stimulating hormone (TSH) suppression are closely correlated.26
Elevated hCG levels, hypersensitive TSH receptors, and the presence of a hyperactive hCG isoform have been proposed.20
Gastrointestinal disorders are believed to be involved in the pathogenesis of persistent NVP. Women with NVP usually lack structural or mucosal abnormalities and have normal endoscopic upper GI evaluations. They may, however, have disorders of the stomach’s neuromuscular function. Severe cases of gastric dysrhythmias and abnormalities of gastric tone may lead to gastroparesis.27
Stomach motility in pregnancy is influenced by neurohormonal changes, specifically in estrogen and thyroid hormones. Gastric motility abnormalities—evaluated by electrogastrography (EGG)—have been associated with NVP symptoms and normal EGGs with the absence of symptoms. Some women who had NVP and abnormal EGGs were retested after delivery when symptom-free and found to have normal myoelectric EGG patterns.27
Helicobacter pylori also may be involved in NVP, and at least 1 study found active H pylori infection and HG to be highly correlated. Pregnancy is not believed to predispose to H pylori infection, but active infection compounded by pregnancy’s hormonal changes may exacerbate NVP.28
NVP and motion sickness share many features, suggesting that NVP treatment could be targeted if a vestibular disorder could be discovered.29 Abnormal electroencephalography—particularly generalized slowing—that is not present in asymptomatic pregnant women has been reported in women with NVP.30
CNS contributions. Persistent NVP may be a learned behavior,24 a view based on findings of anticipatory nausea and vomiting in chemotherapy patients. Through conditioning, a pregnant woman may associate her physical symptoms with elements in her life that maintain the cycle of nausea and vomiting.31
Treating psychological symptoms
Brief psychotherapy to identify and correct sources of anxiety in pregnancy may alleviate a patient’s nausea and vomiting.32
- Progressive muscle relaxation training, often combined with guided imagery, can decrease nausea and vomiting associated with chemotherapy and may prevent anticipatory symptoms by decreasing anxiety.
- Systematic desensitization is successful in most chemotherapy patients who try it. In this technique, relaxation is counter-conditioned as a response to stimuli known to elicit symptoms.31
Hypnosis allows patients to achieve a physiologic state incompatible with nausea and vomiting31 and can terminate vomiting after 1 to 3 sessions.3
Medication. Similar to ondansetron, the antidepressant mirtazapine exhibits an antiemetic effect by blocking the 5-HT3 receptor. In treatment-resistant cases, mirtazapine, 30 mg/d, has been reported to ameliorate NVP symptoms, usually within 24 hours. Patients were able to return to normal diets and discontinue treatment after 6 to 10 days. Mirtazapine appears to be safe during pregnancy, based on animal studies using 17 and 20 times the maximum recommended human dose.33
For patients with anxiety symptoms, consider other medications—including selective serotonin reuptake inhibitors and benzodiazepines—only after counseling the patient about potential risks and benefits to her and the fetus.34
Alternative treatments. In traditional Indian medicine, a mixture of powdered ginger and honey is given to women with NVP. At least 2 studies demonstrate ginger’s efficacy.35
In traditional Chinese medicine, stimulating the Neiguan point (P6) on the wrist is believed to relieve nausea and vomiting. Although results are inconclusive, studies suggest that P6 stimulation can help control NVP.36 The FDA has approved wristbands that stimulate the P6 site, either electrically or by acupressure (Figure).36
Figure Wristband to manage nausea and vomiting in pregnancy
The FDA-cleared BioBand acupressure wristband may help control nausea and vomiting in pregnancy by stimulating the Neiguan point (P6) on the wrist.
Source: Reference 36Consider thiamine supplementation for women with severe symptoms, as Wernicke’s encephalopathy is a rare complication of prolonged NVP.19,37
Related resources
- Motherisk Program at The Hospital for Sick Children, Toronto, Ontario, Canada. Website with information on vomiting during pregnancy: www.motherisk.org/women/morningSickness.jsp. Nausea and vomiting of pregnancy (NVP) forum. www.motherisk.org/women/forum.jsp.
- BioBand acupuncture wristband. www.BioBands.com.
- Koren G, Bishai R, eds. Nausea and vomiting of pregnancy: state of the art 2000. Toronto, Ontario, Canada: The Motherisk Program; 2000.
Drug brand names
- Dimenhydrinate • Dramamine
- Doxylamine • Unisom
- Methylprednisolone • Medrol
- Metoclopramide • Reglan
- Mirtazapine • Remeron
- Ondansetron • Zofran
- Promethazine • Phenergan
- Trimethobenzamide • Tigan
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. el-Mallakh RS, Liebowitz NR, Hale MS. Hyperemesis gravidarum as conversion disorder. J Nerv Ment Dis 1990;178:655-9.
2. Davis M. Nausea and vomiting of pregnancy: an evidence-based review. J Perinat Neonatal Nurs 2004;18:312-28.
3. Buckwalter JG, Simpson SW. Psychological factors in the etiology and treatment of severe nausea and vomiting in pregnancy. Am J Obstet Gynecol 2002;186(5 suppl):S210-4.
4. Bogen JT. Neurosis: a Ms-diagnosis. Perspect Biol Med 1994;37(2):263-74.
5. Weiss G. The death of Charlotte Brontë. Obstet Gynecol 1991;78(4):705-8.
6. Lester EP, Notman MT. Pregnancy, developmental crisis and object relations: psychoanalytic considerations. Int J Psychoanal 1986;67(pt 3):357-66.
7. Sheehan P. Hyperemesis gravidarum—assessment and management. Aust Fam Physician 2007;36:698-701.
8. American College of Obstetrics and Gynecology. ACOG practice bulletin: nausea and vomiting of pregnancy. Obstet Gynecol 2004;103:803-14.
9. Iancu I, Kotler M, Spivak B, et al. Psychiatric aspects of hyperemesis gravidarum. Psychother Psychosom 1994;61:143-9.
10. Munch S. Chicken or the egg? The biological-psychological controversy surrounding hyperemesis gravidarum. Soc Sci Med 2002;55:1267-78.
11. Robertson GG. Nausea and vomiting of pregnancy: a study in psychosomatic and social medicine. Lancet 1946;336-45.
12. Fairweather DV. Nausea and vomiting in pregnancy. Am J Obstet Gynecol 1968;102:135-75.
13. Katon WJ, Ries RK, Bokan JA, Kleinman A. Hyperemesis gravidarum: a biopsychosocial perspective. Int J Psychiatry Med 1980;10:151-62.
14. Simpson SW, Goodwin TM, Robins SB, et al. Psychological factors and hyperemesis gravidarum. J Womens Health Gend Based Med 2001;10:471-7.
15. Majerus PW, Guze SB, Delong WB, Robins E. Psychologic factors and psychiatric disease in hyperemesis gravidarum: a follow-up study of 69 vomiters and 66 controls. Am J Psychiatry 1960;117:421-8.
16. Bozzo P, Koren G, Nava-Ocampo AA, Einarson A. The incidence of nausea and vomiting of pregnancy (NVP): a comparison between depressed women treated with antidepressants and non-depressed women. Clin Invest Med 2006;29(6):347-50.
17. Markl GE, Strunz-Lehner C, Egen-Lappe V, et al. The association of psychosocial factors with nausea and vomiting during pregnancy. J Psychosom Obstet Gynaecol 2007;1-6.
18. Seng JS, Oakley DJ, Sampselle CM, et al. Posttraumatic stress disorder and pregnancy complications. Obstet Gynecol 2001;97(1):17-22.
19. Ismail SK, Kenny L. Review on hyperemesis gravidarum. Best Pract Res Clin Gastroenterol 2007;21:755-69.
20. Verberg MF, Gillott DJ, Al-Fardan N, Grudzinskas JG. Hyperemesis gravidarum, a literature review. Hum Reprod Update 2005;11:527-39.
21. Deuchar N. Nausea and vomiting in pregnancy: a review of the problem with particular regard to psychological and social aspects. Br J Obstet Gynaecol 1995;102(1):6-8.
22. Iatrakis GM, Sakellaropoulos GG, Kourkoubas AH, Kabounia SE. Vomiting and nausea in the first 12 weeks of pregnancy. Psychother Psychosom 1988;49(1):22-4.
23. Lennane KJ, Lennane RJ. Alleged psychogenic disorders in women—a possible manifestation of sexual prejudice. N Engl J Med 1973;288(6):288-92.
24. Chiaramonte GR, Friend R. Medical students’ and residents’ gender bias in the diagnosis, treatment, and interpretation of coronary heart disease symptoms. Health Psychol 2006;25:255-66.
25. Sherman PW, Flaxman SM. Nausea and vomiting of pregnancy in an evolutionary perspective. Am J Obstet Gynecol 2002;186(5 suppl):S190-7.
26. Goodwin TM. Nausea and vomiting of pregnancy: an obstetric syndrome. Am J Obstet Gynecol 2002;186(5 suppl):S184-9.
27. Koch KL. Gastrointestinal factors in nausea and vomiting of pregnancy. Am J Obstet Gynecol 2002;186(5 suppl):S198-203.
28. Golberg D, Szilagyi A, Graves L. Hyperemesis gravidarum and Helicobacter pylori infection: a systematic review. Obstet Gynecol 2007;110:695-703.
29. Black FO. Maternal susceptibility to nausea and vomiting of pregnancy: is the vestibular system involved? Am J Obstet Gynecol 2002;186(5 suppl):S204-9.
30. Vaknin Z, Halperin R, Schneider D, et al. Hyperemesis gravidarum and nonspecific abnormal EEG findings: a preliminary report. J Reprod Med 2006;51:623-7.
31. Matteson S, Roscoe J, Hickok J, Morrow GR. The role of behavioral conditioning in the development of nausea. Am J Obstet Gynecol 2002;186(5 suppl):S239-43.
32. Zechnich R, Hammer T. Brief psychotherapy for hyperemesis gravidarum. Am Fam Physician 1982;26:179-81.
33. Guclu S, Gol M, Dogan E, Saygili U. Mirtazapine use in resistant hyperemesis gravidarum: report of three cases and review of the literature. Arch Gynecol Obstet 2005;272:298-300.
34. Raphael DB, Ross J, Brizendine L. Treating anxiety during pregnancy: Just how safe are SSRIs? Current Psychiatry 2008;7(2):39-52.
35. Niebyl JR, Goodwin TM. Overview of nausea and vomiting of pregnancy with an emphasis on vitamins and ginger. Am J Obstet Gynecol 2002;186(5 suppl):S253-5.
36. Roscoe JA, Matteson SE. Acupressure and acustimulation bands for control of nausea: a brief review. Am J Obstet Gynecol 2002;186(5 suppl):S244-7.
37. Chiossi G, Neri I, Cavazzuti M, et al. Hyperemesis gravidarum complicated by Wernicke encephalopathy: background, case report, and review of the literature. Obstet Gynecol Surv 2006;61:255-68.
1. el-Mallakh RS, Liebowitz NR, Hale MS. Hyperemesis gravidarum as conversion disorder. J Nerv Ment Dis 1990;178:655-9.
2. Davis M. Nausea and vomiting of pregnancy: an evidence-based review. J Perinat Neonatal Nurs 2004;18:312-28.
3. Buckwalter JG, Simpson SW. Psychological factors in the etiology and treatment of severe nausea and vomiting in pregnancy. Am J Obstet Gynecol 2002;186(5 suppl):S210-4.
4. Bogen JT. Neurosis: a Ms-diagnosis. Perspect Biol Med 1994;37(2):263-74.
5. Weiss G. The death of Charlotte Brontë. Obstet Gynecol 1991;78(4):705-8.
6. Lester EP, Notman MT. Pregnancy, developmental crisis and object relations: psychoanalytic considerations. Int J Psychoanal 1986;67(pt 3):357-66.
7. Sheehan P. Hyperemesis gravidarum—assessment and management. Aust Fam Physician 2007;36:698-701.
8. American College of Obstetrics and Gynecology. ACOG practice bulletin: nausea and vomiting of pregnancy. Obstet Gynecol 2004;103:803-14.
9. Iancu I, Kotler M, Spivak B, et al. Psychiatric aspects of hyperemesis gravidarum. Psychother Psychosom 1994;61:143-9.
10. Munch S. Chicken or the egg? The biological-psychological controversy surrounding hyperemesis gravidarum. Soc Sci Med 2002;55:1267-78.
11. Robertson GG. Nausea and vomiting of pregnancy: a study in psychosomatic and social medicine. Lancet 1946;336-45.
12. Fairweather DV. Nausea and vomiting in pregnancy. Am J Obstet Gynecol 1968;102:135-75.
13. Katon WJ, Ries RK, Bokan JA, Kleinman A. Hyperemesis gravidarum: a biopsychosocial perspective. Int J Psychiatry Med 1980;10:151-62.
14. Simpson SW, Goodwin TM, Robins SB, et al. Psychological factors and hyperemesis gravidarum. J Womens Health Gend Based Med 2001;10:471-7.
15. Majerus PW, Guze SB, Delong WB, Robins E. Psychologic factors and psychiatric disease in hyperemesis gravidarum: a follow-up study of 69 vomiters and 66 controls. Am J Psychiatry 1960;117:421-8.
16. Bozzo P, Koren G, Nava-Ocampo AA, Einarson A. The incidence of nausea and vomiting of pregnancy (NVP): a comparison between depressed women treated with antidepressants and non-depressed women. Clin Invest Med 2006;29(6):347-50.
17. Markl GE, Strunz-Lehner C, Egen-Lappe V, et al. The association of psychosocial factors with nausea and vomiting during pregnancy. J Psychosom Obstet Gynaecol 2007;1-6.
18. Seng JS, Oakley DJ, Sampselle CM, et al. Posttraumatic stress disorder and pregnancy complications. Obstet Gynecol 2001;97(1):17-22.
19. Ismail SK, Kenny L. Review on hyperemesis gravidarum. Best Pract Res Clin Gastroenterol 2007;21:755-69.
20. Verberg MF, Gillott DJ, Al-Fardan N, Grudzinskas JG. Hyperemesis gravidarum, a literature review. Hum Reprod Update 2005;11:527-39.
21. Deuchar N. Nausea and vomiting in pregnancy: a review of the problem with particular regard to psychological and social aspects. Br J Obstet Gynaecol 1995;102(1):6-8.
22. Iatrakis GM, Sakellaropoulos GG, Kourkoubas AH, Kabounia SE. Vomiting and nausea in the first 12 weeks of pregnancy. Psychother Psychosom 1988;49(1):22-4.
23. Lennane KJ, Lennane RJ. Alleged psychogenic disorders in women—a possible manifestation of sexual prejudice. N Engl J Med 1973;288(6):288-92.
24. Chiaramonte GR, Friend R. Medical students’ and residents’ gender bias in the diagnosis, treatment, and interpretation of coronary heart disease symptoms. Health Psychol 2006;25:255-66.
25. Sherman PW, Flaxman SM. Nausea and vomiting of pregnancy in an evolutionary perspective. Am J Obstet Gynecol 2002;186(5 suppl):S190-7.
26. Goodwin TM. Nausea and vomiting of pregnancy: an obstetric syndrome. Am J Obstet Gynecol 2002;186(5 suppl):S184-9.
27. Koch KL. Gastrointestinal factors in nausea and vomiting of pregnancy. Am J Obstet Gynecol 2002;186(5 suppl):S198-203.
28. Golberg D, Szilagyi A, Graves L. Hyperemesis gravidarum and Helicobacter pylori infection: a systematic review. Obstet Gynecol 2007;110:695-703.
29. Black FO. Maternal susceptibility to nausea and vomiting of pregnancy: is the vestibular system involved? Am J Obstet Gynecol 2002;186(5 suppl):S204-9.
30. Vaknin Z, Halperin R, Schneider D, et al. Hyperemesis gravidarum and nonspecific abnormal EEG findings: a preliminary report. J Reprod Med 2006;51:623-7.
31. Matteson S, Roscoe J, Hickok J, Morrow GR. The role of behavioral conditioning in the development of nausea. Am J Obstet Gynecol 2002;186(5 suppl):S239-43.
32. Zechnich R, Hammer T. Brief psychotherapy for hyperemesis gravidarum. Am Fam Physician 1982;26:179-81.
33. Guclu S, Gol M, Dogan E, Saygili U. Mirtazapine use in resistant hyperemesis gravidarum: report of three cases and review of the literature. Arch Gynecol Obstet 2005;272:298-300.
34. Raphael DB, Ross J, Brizendine L. Treating anxiety during pregnancy: Just how safe are SSRIs? Current Psychiatry 2008;7(2):39-52.
35. Niebyl JR, Goodwin TM. Overview of nausea and vomiting of pregnancy with an emphasis on vitamins and ginger. Am J Obstet Gynecol 2002;186(5 suppl):S253-5.
36. Roscoe JA, Matteson SE. Acupressure and acustimulation bands for control of nausea: a brief review. Am J Obstet Gynecol 2002;186(5 suppl):S244-7.
37. Chiossi G, Neri I, Cavazzuti M, et al. Hyperemesis gravidarum complicated by Wernicke encephalopathy: background, case report, and review of the literature. Obstet Gynecol Surv 2006;61:255-68.