User login
4 Cases of Faulty Follow-Up: Cutting the legal risk of breast cancer screening
This article considers lessons to be learned from 4 malpractice cases involving allegations of inadequate follow-up and misdiagnosis of breast cancer. We focus on specific red flags, and a “systems approach” to adequate follow-up of breast screening findings and patient complaints. We focus on these questions:
- Which are the most important factors in risk management related to breast cancer?
- What can you do to reduce your risk?
We analyzed 132 breast cancer cases closed by ProMutual Group of Boston, the total number of breast cancer cases closed by the company between January 1999 and December 2004. These cases closed with an aggregate indemnity payment of over $47 million, including 12 cases with payments of $1 million or more.
Defendant lineup
The 132 cases involved 279 defendants, including:
- 129 radiologists (46%),
- 78 women’s health professionals (ObGyns, internists, and family physicians; 28%),
- 43 surgeons (15%),
- 2 pathologists (1%), and
- 1 physician each from several different specialties.
Red flag high-risk patients, screen early and in-depth
Diagnosing breast cancer in its earliest stages is the most effective way to reduce risk of litigation, as well as morbidity and mortality. The first step is to identify the patients at high risk and take care to perform earlier, and perhaps more in-depth, screening for these women.
Kern’s triad of errors portends litigation
Kern2 identified a “triad of errors” to beware:
- young age
- self-discovered breast mass
- negative mammogram.
Analyses of these breast cancer cases1,3 reveals that patients with an eventual breast cancer diagnosis of stage II or higher are more likely to file claims (though claims are not limited to this group).
LUCY’S CLAIM
35 office visits and no screening
Lucy, age 66, was seen 35 times by her physician and other health care providers over an 8-year period. Although she had a positive family history of breast cancer, no clinical breast examination was conducted at any of the visits. Ultimately, a mammogram was performed and found to be suspicious for cancer. A biopsy was positive. One year later, she had widespread metastases and filed a malpractice claim. Defense experts faulted the physician for “failing to undertake any preventive care.”
The case closed with an indemnity payment in the $500,000 range.
Completely omitting breast cancer screening invites a lawsuit, but avoiding litigation is not as simple as performing regular screening.
You must be prepared to question negative test results when the clinical examination is positive, to listen to the patient, and to follow through to diagnosis each positive clinical finding and every complaint that the patient brings up.
Inadequate follow-up: Many and varied
FIONA’S CLAIM
Negative aspirate and palpable mass
Fiona, 33, presented to her ObGyn with a painful breast mass of 3 months’ duration. The ObGyn referred her to a surgeon, who performed a fine-needle aspiration. Cytology revealed neither cells nor fluid, and the surgeon diagnosed “residual fibrocystic changes.” Eighteen months later Fiona was diagnosed with metastases to the liver. Defense experts faulted the ObGyn for not following up on the negative fine-needle aspiration: “When a needle aspirate is negative or does not reveal fluid and a mass is palpated, the mass is cancer until proven otherwise.”
This case closed with an indemnity payment of roughly $500,000.
Failure to perform adequate and timely follow-up lies at the root of many breast cancer cases, according to successive studies by the risk management department of ProMutual Group. In some instances, a missed screening or diagnostic test was not rescheduled; in others, a mammogram or slide was misread or the wrong breast mass was excised.
In some lawsuits, the issue was the physician’s assumption of benign disease before cancer was ruled out.
Reality: Patients do detect malignancies by self-exam
Breast cancer can be difficult to diagnose in its earliest stages. Correct diagnosis may be preceded by multiple complementary examinations, the first of which is likely to be the patient’s breast self-examination. Although self-examination has been derided in some professional circles of late, many physicians are alerted to the presence of a breast mass only after a patient reports finding a “lump.”
Each such mass requires clinical investigation, starting with a breast exam to evaluate symmetry, contour, texture, nodularity, the mass itself, tenderness, and any nipple discharge.
Mammography is imperfect
Although mammography is the most widely used screening tool, its reliability is limited, particularly in young women, whose dense, fibroglandular tissue can obscure the diagnosis. The number of false-negative reports may make mammography a questionable diagnostic tool for symptomatic women—unless the results are positive.
No “best” technology
Ultrasound yields information about variations in tissue sound transmission, while cytology reveals the microscopic appearance of the cells and other tissue components.
Each assessment tool has its place and limitations, and these vary from patient to patient.
Lawsuit is likely if screening ends too soon
OLIVIA’S CLAIM
Negative mammogram, palpable mass
Olivia was 31 when she reported finding a mass in her breast. Her ObGyn examined her and noted “dense fibroglandular breast tissue with specific nodularity.” A diagnostic mammogram followed and was negative. When the physician observed no changes at a visit 2 months later, Olivia was told she need not return until her next annual examination. When she did, she was diagnosed with infiltrating ductal carcinoma.
After reviewing the case, defense experts noted, “It is common to find negative mammograms and yet have palpable masses that require either ultrasound or core biopsy for diagnosis. This patient had specific complaints at numerous intervals in the course of her clinical history. The physician’s inaction may have [been responsible for the] change in her clinical course.”
Olivia’s case closed with an indemnity payment of just under $1 million.
In the absence of comprehensive follow-up, a malpractice suit alleging “failure to diagnose breast cancer” is likely, as in Olivia’s case.
Misdiagnosis is another common cause of litigation. According to surveys conducted by the Physician Insurers Association of America (PIAA) in 1990 and 1995,4,5 breast cancer is the number 1 most frequently misdiagnosed condition in malpractice claims. The most common reason given by expert reviewers in the PIAA study: “Physical findings failed to impress the physician.” Consider this example:
JACQUELINE’S CLAIM
Cancer or galactocele?
Jacqueline, 33, was told by her ObGyn that a breast mass discovered while she was in labor was a “clogged milk duct.” The medical record was not annotated. When the same mass was palpated postpartum, Jacqueline was told it was a sebaceous cyst. Again, the medical record failed to reflect the findings. One year later, Jacqueline changed physicians and underwent fine-needle aspiration, which confirmed malignancy.
Defense experts faulted the physician for his poor recordkeeping and failure to order a mammogram, ultrasound, or any follow-up despite the continued concerns of the patient.
This case closed with an indemnity payment in the $1 million range.
1. CLINICAL BREAST EXAM
Perform annually, at minimum
Develop and follow guidelines
Obtain medical history and identify high-risk patients
Ask the patient if she has any breast complaints
Pay special attention to any patient-detected abnormality
Ask patient if another clinician is currently providing breast care
Follow up previous complaints in both routine and episodic visits
Immediately evaluate gravidas with breast complaints
Follow to resolution any patient with a breast complaint
2. SCREENING MAMMOGRAM
Develop and follow screening guidelines
Consider screening at-risk women earlier than others
Compare current mammogram with previous films
Beware of false negatives
3. DIAGNOSTIC MAMMOGRAM (when abnormalities are present)
Have patient identify any lump or abnormality
Assume cancer until it is ruled out
Perform ultrasound if mammogram is inconclusive
If ultrasound is inconclusive, proceed to tissue diagnosis
4. TISSUE DIAGNOSIS
Perform tissue diagnosis or refer if abnormality does not resolve by follow-up breast exam or imaging studies
Correlate results of fine-needle aspiration or biopsy with clinical findings and mammography
5. OTHER IMPERATIVES
Communication
Identify which physician is coordinating care
Explain the benefits and limits of mammography to the patient
Develop and implement a system for tracking results and follow-up that includes all providers
Develop and implement a system for notifying patients of findings
Documentation
Record every step, including follow-up plan
Use a breast diagram to record physical findings
Source: ProMutual Group. Managing risk in breast care. Cambridge, Mass.
“Systems approach” to cutting risk of lawsuits
The cases of Lucy, Fiona, Olivia, and Jacqueline represent only some of the issues that give rise to malpractice claims. Since breast care is fragmented across medical specialties, a systematic approach is encouraged.
Systems form the basis of good risk management. The ProMutual Group advocates that every medical practice—whether office- or hospital-based—have a comprehensive risk management program that incorporates some or all of the following suggestions.
Annual exam not always enough
Even more frequent screening may be necessary if the patient has a specific breast complaint.
Every practice should have guidelines for these exams, including instructions on identifying women at risk because of personal or family history (ALGORITHM).
Ultrasound, MRI for young women
Younger, otherwise healthy women with unimpressive findings such as nodularities, nipple discharge, or tenderness deserve extra attention. The physician may want to consider additional diagnostic tests such as ultrasound or MRI for this group.
Never disregard a complaint
If a woman complains of discomfort or a self-detected mass, immediate evaluation is imperative. This includes pregnant patients; do not wait until after delivery to investigate an abnormality.
Use your own guidelines, but justify deviations from the norm
These may include earlier screening for women at risk, and need not match the recommendations of a nationally recognized group. However, in the event of a claim or suit, you will need to justify the reason for deviating from widely accepted guidelines.
Incorporate a systematic approach to diagnosis, treatment, referral (if required), and follow-up breast care.
Using an algorithm can help minimize error, confusion, and delays in care.
Assume cancer until it is ruled out
Consider diagnostic mammography and, when indicated, ultrasound imaging when the screening mammogram of a patient with a palpable mass is either negative or inconclusive.
A fine-needle aspiration or biopsy is a must to resolve indeterminate breast symptoms or inconclusive diagnostic breast imaging tests.
What not to say to patients
Do not assure the patient that a breast mass is benign until it is proven to be so.
Are you the “default PCP”? Screen for other cancers
When more than 1 physician is involved, someone needs to assume responsibility for the patient’s ongoing breast care. In a malpractice case, a doctor cannot simply claim that a routine mammogram or diagnostic test was deferred because it was assumed another physician would handle it.
In most cases, the patient’s primary care physician has the responsibility for her care.
ObGyn can be held to primary care physician standard. If the ObGyn is her only physician, the ObGyn may be held to a primary care standard—not only for breast and cervical cancer screening, but also for colorectal, skin, and other cancers.
Any physicians involved in a woman’s care should decide between them, as early in the process as possible, who will assume responsibility for ongoing care. According to the Agency for Healthcare Research and Quality,6-9 that physician oversees follow-up, monitoring, and tracking women with abnormal findings, including those for whom a biopsy is recommended.
The designation of responsibility and accountability should be documented in the medical record.
The radiology facility’s responsibility. The facility that performs the patient’s mammograms must share responsibility with the designated physician for follow-up, monitoring, and tracking abnormal test results. Each facility should have a system for tracking positive mammogram findings and, according to the Agency for Healthcare Research and Quality, “a process for correlating findings with biopsy results.” It also has the duty to communicate urgent or significant findings to physicians and, under certain circumstances, to the patient directly.
If more than 1 physician is involved, everyone should know who is responsible for coordinating the patient’s ongoing care. Good communication is especially critical when abnormal findings are involved and additional imaging or more invasive testing is needed.
Document, document, document
ProMutual Group’s studies repeatedly show that inadequate records—whether paper or electronic—substantially reduce the chance for a successful defense.
Dr. Zylstra reports no financial relationships relevant to this article. Ms. Greenwald and Ms. Mondor are employees of ProMutual Group, Boston.
1. Zylstra S, D’Orsi C, Ricci BA, et al. Defense of breast cancer malpractice claims. Breast J. 2001;7:76-90.
2. Kern KA. Breast cancer and malpractice: a surgeon’s perspective on risk prevention. Semin Breast Dis. 1998;1:22-31.
3. Zylstra S, Bors-Koefoed R, Mondor M, et al. A statistical model for predicting outcome in breast cancer malpractice lawsuits. Obstet Gynecol. 1994;84:392-398.
4. Physician Insurers Association of America. Breast Cancer Study. Lawrenceville, NJ: Physician Insurers Association of America; 1990.
5. Physician Insurers Association of America. Breast Cancer Study 1995. Washington, DC: Physician Insurers Association of America; 1995.
6. Mammography quality standards: proposed rules (CFR 900). Federal Regulations. 1996;65:4911-4912.
7. Berlin L. To telephone or not to telephone: how high is the standard? AJR Am J Roentgenol. 1992;159:1335-1339.
8. Berlin L. Communication of the urgent finding. AJR Am J Roentgenol. 1996;166:513-515.
9. Berlin L. Communication of the significant but not urgent finding. AJR Am J Roentgenol. 1997;168:329-331.
This article considers lessons to be learned from 4 malpractice cases involving allegations of inadequate follow-up and misdiagnosis of breast cancer. We focus on specific red flags, and a “systems approach” to adequate follow-up of breast screening findings and patient complaints. We focus on these questions:
- Which are the most important factors in risk management related to breast cancer?
- What can you do to reduce your risk?
We analyzed 132 breast cancer cases closed by ProMutual Group of Boston, the total number of breast cancer cases closed by the company between January 1999 and December 2004. These cases closed with an aggregate indemnity payment of over $47 million, including 12 cases with payments of $1 million or more.
Defendant lineup
The 132 cases involved 279 defendants, including:
- 129 radiologists (46%),
- 78 women’s health professionals (ObGyns, internists, and family physicians; 28%),
- 43 surgeons (15%),
- 2 pathologists (1%), and
- 1 physician each from several different specialties.
Red flag high-risk patients, screen early and in-depth
Diagnosing breast cancer in its earliest stages is the most effective way to reduce risk of litigation, as well as morbidity and mortality. The first step is to identify the patients at high risk and take care to perform earlier, and perhaps more in-depth, screening for these women.
Kern’s triad of errors portends litigation
Kern2 identified a “triad of errors” to beware:
- young age
- self-discovered breast mass
- negative mammogram.
Analyses of these breast cancer cases1,3 reveals that patients with an eventual breast cancer diagnosis of stage II or higher are more likely to file claims (though claims are not limited to this group).
LUCY’S CLAIM
35 office visits and no screening
Lucy, age 66, was seen 35 times by her physician and other health care providers over an 8-year period. Although she had a positive family history of breast cancer, no clinical breast examination was conducted at any of the visits. Ultimately, a mammogram was performed and found to be suspicious for cancer. A biopsy was positive. One year later, she had widespread metastases and filed a malpractice claim. Defense experts faulted the physician for “failing to undertake any preventive care.”
The case closed with an indemnity payment in the $500,000 range.
Completely omitting breast cancer screening invites a lawsuit, but avoiding litigation is not as simple as performing regular screening.
You must be prepared to question negative test results when the clinical examination is positive, to listen to the patient, and to follow through to diagnosis each positive clinical finding and every complaint that the patient brings up.
Inadequate follow-up: Many and varied
FIONA’S CLAIM
Negative aspirate and palpable mass
Fiona, 33, presented to her ObGyn with a painful breast mass of 3 months’ duration. The ObGyn referred her to a surgeon, who performed a fine-needle aspiration. Cytology revealed neither cells nor fluid, and the surgeon diagnosed “residual fibrocystic changes.” Eighteen months later Fiona was diagnosed with metastases to the liver. Defense experts faulted the ObGyn for not following up on the negative fine-needle aspiration: “When a needle aspirate is negative or does not reveal fluid and a mass is palpated, the mass is cancer until proven otherwise.”
This case closed with an indemnity payment of roughly $500,000.
Failure to perform adequate and timely follow-up lies at the root of many breast cancer cases, according to successive studies by the risk management department of ProMutual Group. In some instances, a missed screening or diagnostic test was not rescheduled; in others, a mammogram or slide was misread or the wrong breast mass was excised.
In some lawsuits, the issue was the physician’s assumption of benign disease before cancer was ruled out.
Reality: Patients do detect malignancies by self-exam
Breast cancer can be difficult to diagnose in its earliest stages. Correct diagnosis may be preceded by multiple complementary examinations, the first of which is likely to be the patient’s breast self-examination. Although self-examination has been derided in some professional circles of late, many physicians are alerted to the presence of a breast mass only after a patient reports finding a “lump.”
Each such mass requires clinical investigation, starting with a breast exam to evaluate symmetry, contour, texture, nodularity, the mass itself, tenderness, and any nipple discharge.
Mammography is imperfect
Although mammography is the most widely used screening tool, its reliability is limited, particularly in young women, whose dense, fibroglandular tissue can obscure the diagnosis. The number of false-negative reports may make mammography a questionable diagnostic tool for symptomatic women—unless the results are positive.
No “best” technology
Ultrasound yields information about variations in tissue sound transmission, while cytology reveals the microscopic appearance of the cells and other tissue components.
Each assessment tool has its place and limitations, and these vary from patient to patient.
Lawsuit is likely if screening ends too soon
OLIVIA’S CLAIM
Negative mammogram, palpable mass
Olivia was 31 when she reported finding a mass in her breast. Her ObGyn examined her and noted “dense fibroglandular breast tissue with specific nodularity.” A diagnostic mammogram followed and was negative. When the physician observed no changes at a visit 2 months later, Olivia was told she need not return until her next annual examination. When she did, she was diagnosed with infiltrating ductal carcinoma.
After reviewing the case, defense experts noted, “It is common to find negative mammograms and yet have palpable masses that require either ultrasound or core biopsy for diagnosis. This patient had specific complaints at numerous intervals in the course of her clinical history. The physician’s inaction may have [been responsible for the] change in her clinical course.”
Olivia’s case closed with an indemnity payment of just under $1 million.
In the absence of comprehensive follow-up, a malpractice suit alleging “failure to diagnose breast cancer” is likely, as in Olivia’s case.
Misdiagnosis is another common cause of litigation. According to surveys conducted by the Physician Insurers Association of America (PIAA) in 1990 and 1995,4,5 breast cancer is the number 1 most frequently misdiagnosed condition in malpractice claims. The most common reason given by expert reviewers in the PIAA study: “Physical findings failed to impress the physician.” Consider this example:
JACQUELINE’S CLAIM
Cancer or galactocele?
Jacqueline, 33, was told by her ObGyn that a breast mass discovered while she was in labor was a “clogged milk duct.” The medical record was not annotated. When the same mass was palpated postpartum, Jacqueline was told it was a sebaceous cyst. Again, the medical record failed to reflect the findings. One year later, Jacqueline changed physicians and underwent fine-needle aspiration, which confirmed malignancy.
Defense experts faulted the physician for his poor recordkeeping and failure to order a mammogram, ultrasound, or any follow-up despite the continued concerns of the patient.
This case closed with an indemnity payment in the $1 million range.
1. CLINICAL BREAST EXAM
Perform annually, at minimum
Develop and follow guidelines
Obtain medical history and identify high-risk patients
Ask the patient if she has any breast complaints
Pay special attention to any patient-detected abnormality
Ask patient if another clinician is currently providing breast care
Follow up previous complaints in both routine and episodic visits
Immediately evaluate gravidas with breast complaints
Follow to resolution any patient with a breast complaint
2. SCREENING MAMMOGRAM
Develop and follow screening guidelines
Consider screening at-risk women earlier than others
Compare current mammogram with previous films
Beware of false negatives
3. DIAGNOSTIC MAMMOGRAM (when abnormalities are present)
Have patient identify any lump or abnormality
Assume cancer until it is ruled out
Perform ultrasound if mammogram is inconclusive
If ultrasound is inconclusive, proceed to tissue diagnosis
4. TISSUE DIAGNOSIS
Perform tissue diagnosis or refer if abnormality does not resolve by follow-up breast exam or imaging studies
Correlate results of fine-needle aspiration or biopsy with clinical findings and mammography
5. OTHER IMPERATIVES
Communication
Identify which physician is coordinating care
Explain the benefits and limits of mammography to the patient
Develop and implement a system for tracking results and follow-up that includes all providers
Develop and implement a system for notifying patients of findings
Documentation
Record every step, including follow-up plan
Use a breast diagram to record physical findings
Source: ProMutual Group. Managing risk in breast care. Cambridge, Mass.
“Systems approach” to cutting risk of lawsuits
The cases of Lucy, Fiona, Olivia, and Jacqueline represent only some of the issues that give rise to malpractice claims. Since breast care is fragmented across medical specialties, a systematic approach is encouraged.
Systems form the basis of good risk management. The ProMutual Group advocates that every medical practice—whether office- or hospital-based—have a comprehensive risk management program that incorporates some or all of the following suggestions.
Annual exam not always enough
Even more frequent screening may be necessary if the patient has a specific breast complaint.
Every practice should have guidelines for these exams, including instructions on identifying women at risk because of personal or family history (ALGORITHM).
Ultrasound, MRI for young women
Younger, otherwise healthy women with unimpressive findings such as nodularities, nipple discharge, or tenderness deserve extra attention. The physician may want to consider additional diagnostic tests such as ultrasound or MRI for this group.
Never disregard a complaint
If a woman complains of discomfort or a self-detected mass, immediate evaluation is imperative. This includes pregnant patients; do not wait until after delivery to investigate an abnormality.
Use your own guidelines, but justify deviations from the norm
These may include earlier screening for women at risk, and need not match the recommendations of a nationally recognized group. However, in the event of a claim or suit, you will need to justify the reason for deviating from widely accepted guidelines.
Incorporate a systematic approach to diagnosis, treatment, referral (if required), and follow-up breast care.
Using an algorithm can help minimize error, confusion, and delays in care.
Assume cancer until it is ruled out
Consider diagnostic mammography and, when indicated, ultrasound imaging when the screening mammogram of a patient with a palpable mass is either negative or inconclusive.
A fine-needle aspiration or biopsy is a must to resolve indeterminate breast symptoms or inconclusive diagnostic breast imaging tests.
What not to say to patients
Do not assure the patient that a breast mass is benign until it is proven to be so.
Are you the “default PCP”? Screen for other cancers
When more than 1 physician is involved, someone needs to assume responsibility for the patient’s ongoing breast care. In a malpractice case, a doctor cannot simply claim that a routine mammogram or diagnostic test was deferred because it was assumed another physician would handle it.
In most cases, the patient’s primary care physician has the responsibility for her care.
ObGyn can be held to primary care physician standard. If the ObGyn is her only physician, the ObGyn may be held to a primary care standard—not only for breast and cervical cancer screening, but also for colorectal, skin, and other cancers.
Any physicians involved in a woman’s care should decide between them, as early in the process as possible, who will assume responsibility for ongoing care. According to the Agency for Healthcare Research and Quality,6-9 that physician oversees follow-up, monitoring, and tracking women with abnormal findings, including those for whom a biopsy is recommended.
The designation of responsibility and accountability should be documented in the medical record.
The radiology facility’s responsibility. The facility that performs the patient’s mammograms must share responsibility with the designated physician for follow-up, monitoring, and tracking abnormal test results. Each facility should have a system for tracking positive mammogram findings and, according to the Agency for Healthcare Research and Quality, “a process for correlating findings with biopsy results.” It also has the duty to communicate urgent or significant findings to physicians and, under certain circumstances, to the patient directly.
If more than 1 physician is involved, everyone should know who is responsible for coordinating the patient’s ongoing care. Good communication is especially critical when abnormal findings are involved and additional imaging or more invasive testing is needed.
Document, document, document
ProMutual Group’s studies repeatedly show that inadequate records—whether paper or electronic—substantially reduce the chance for a successful defense.
Dr. Zylstra reports no financial relationships relevant to this article. Ms. Greenwald and Ms. Mondor are employees of ProMutual Group, Boston.
This article considers lessons to be learned from 4 malpractice cases involving allegations of inadequate follow-up and misdiagnosis of breast cancer. We focus on specific red flags, and a “systems approach” to adequate follow-up of breast screening findings and patient complaints. We focus on these questions:
- Which are the most important factors in risk management related to breast cancer?
- What can you do to reduce your risk?
We analyzed 132 breast cancer cases closed by ProMutual Group of Boston, the total number of breast cancer cases closed by the company between January 1999 and December 2004. These cases closed with an aggregate indemnity payment of over $47 million, including 12 cases with payments of $1 million or more.
Defendant lineup
The 132 cases involved 279 defendants, including:
- 129 radiologists (46%),
- 78 women’s health professionals (ObGyns, internists, and family physicians; 28%),
- 43 surgeons (15%),
- 2 pathologists (1%), and
- 1 physician each from several different specialties.
Red flag high-risk patients, screen early and in-depth
Diagnosing breast cancer in its earliest stages is the most effective way to reduce risk of litigation, as well as morbidity and mortality. The first step is to identify the patients at high risk and take care to perform earlier, and perhaps more in-depth, screening for these women.
Kern’s triad of errors portends litigation
Kern2 identified a “triad of errors” to beware:
- young age
- self-discovered breast mass
- negative mammogram.
Analyses of these breast cancer cases1,3 reveals that patients with an eventual breast cancer diagnosis of stage II or higher are more likely to file claims (though claims are not limited to this group).
LUCY’S CLAIM
35 office visits and no screening
Lucy, age 66, was seen 35 times by her physician and other health care providers over an 8-year period. Although she had a positive family history of breast cancer, no clinical breast examination was conducted at any of the visits. Ultimately, a mammogram was performed and found to be suspicious for cancer. A biopsy was positive. One year later, she had widespread metastases and filed a malpractice claim. Defense experts faulted the physician for “failing to undertake any preventive care.”
The case closed with an indemnity payment in the $500,000 range.
Completely omitting breast cancer screening invites a lawsuit, but avoiding litigation is not as simple as performing regular screening.
You must be prepared to question negative test results when the clinical examination is positive, to listen to the patient, and to follow through to diagnosis each positive clinical finding and every complaint that the patient brings up.
Inadequate follow-up: Many and varied
FIONA’S CLAIM
Negative aspirate and palpable mass
Fiona, 33, presented to her ObGyn with a painful breast mass of 3 months’ duration. The ObGyn referred her to a surgeon, who performed a fine-needle aspiration. Cytology revealed neither cells nor fluid, and the surgeon diagnosed “residual fibrocystic changes.” Eighteen months later Fiona was diagnosed with metastases to the liver. Defense experts faulted the ObGyn for not following up on the negative fine-needle aspiration: “When a needle aspirate is negative or does not reveal fluid and a mass is palpated, the mass is cancer until proven otherwise.”
This case closed with an indemnity payment of roughly $500,000.
Failure to perform adequate and timely follow-up lies at the root of many breast cancer cases, according to successive studies by the risk management department of ProMutual Group. In some instances, a missed screening or diagnostic test was not rescheduled; in others, a mammogram or slide was misread or the wrong breast mass was excised.
In some lawsuits, the issue was the physician’s assumption of benign disease before cancer was ruled out.
Reality: Patients do detect malignancies by self-exam
Breast cancer can be difficult to diagnose in its earliest stages. Correct diagnosis may be preceded by multiple complementary examinations, the first of which is likely to be the patient’s breast self-examination. Although self-examination has been derided in some professional circles of late, many physicians are alerted to the presence of a breast mass only after a patient reports finding a “lump.”
Each such mass requires clinical investigation, starting with a breast exam to evaluate symmetry, contour, texture, nodularity, the mass itself, tenderness, and any nipple discharge.
Mammography is imperfect
Although mammography is the most widely used screening tool, its reliability is limited, particularly in young women, whose dense, fibroglandular tissue can obscure the diagnosis. The number of false-negative reports may make mammography a questionable diagnostic tool for symptomatic women—unless the results are positive.
No “best” technology
Ultrasound yields information about variations in tissue sound transmission, while cytology reveals the microscopic appearance of the cells and other tissue components.
Each assessment tool has its place and limitations, and these vary from patient to patient.
Lawsuit is likely if screening ends too soon
OLIVIA’S CLAIM
Negative mammogram, palpable mass
Olivia was 31 when she reported finding a mass in her breast. Her ObGyn examined her and noted “dense fibroglandular breast tissue with specific nodularity.” A diagnostic mammogram followed and was negative. When the physician observed no changes at a visit 2 months later, Olivia was told she need not return until her next annual examination. When she did, she was diagnosed with infiltrating ductal carcinoma.
After reviewing the case, defense experts noted, “It is common to find negative mammograms and yet have palpable masses that require either ultrasound or core biopsy for diagnosis. This patient had specific complaints at numerous intervals in the course of her clinical history. The physician’s inaction may have [been responsible for the] change in her clinical course.”
Olivia’s case closed with an indemnity payment of just under $1 million.
In the absence of comprehensive follow-up, a malpractice suit alleging “failure to diagnose breast cancer” is likely, as in Olivia’s case.
Misdiagnosis is another common cause of litigation. According to surveys conducted by the Physician Insurers Association of America (PIAA) in 1990 and 1995,4,5 breast cancer is the number 1 most frequently misdiagnosed condition in malpractice claims. The most common reason given by expert reviewers in the PIAA study: “Physical findings failed to impress the physician.” Consider this example:
JACQUELINE’S CLAIM
Cancer or galactocele?
Jacqueline, 33, was told by her ObGyn that a breast mass discovered while she was in labor was a “clogged milk duct.” The medical record was not annotated. When the same mass was palpated postpartum, Jacqueline was told it was a sebaceous cyst. Again, the medical record failed to reflect the findings. One year later, Jacqueline changed physicians and underwent fine-needle aspiration, which confirmed malignancy.
Defense experts faulted the physician for his poor recordkeeping and failure to order a mammogram, ultrasound, or any follow-up despite the continued concerns of the patient.
This case closed with an indemnity payment in the $1 million range.
1. CLINICAL BREAST EXAM
Perform annually, at minimum
Develop and follow guidelines
Obtain medical history and identify high-risk patients
Ask the patient if she has any breast complaints
Pay special attention to any patient-detected abnormality
Ask patient if another clinician is currently providing breast care
Follow up previous complaints in both routine and episodic visits
Immediately evaluate gravidas with breast complaints
Follow to resolution any patient with a breast complaint
2. SCREENING MAMMOGRAM
Develop and follow screening guidelines
Consider screening at-risk women earlier than others
Compare current mammogram with previous films
Beware of false negatives
3. DIAGNOSTIC MAMMOGRAM (when abnormalities are present)
Have patient identify any lump or abnormality
Assume cancer until it is ruled out
Perform ultrasound if mammogram is inconclusive
If ultrasound is inconclusive, proceed to tissue diagnosis
4. TISSUE DIAGNOSIS
Perform tissue diagnosis or refer if abnormality does not resolve by follow-up breast exam or imaging studies
Correlate results of fine-needle aspiration or biopsy with clinical findings and mammography
5. OTHER IMPERATIVES
Communication
Identify which physician is coordinating care
Explain the benefits and limits of mammography to the patient
Develop and implement a system for tracking results and follow-up that includes all providers
Develop and implement a system for notifying patients of findings
Documentation
Record every step, including follow-up plan
Use a breast diagram to record physical findings
Source: ProMutual Group. Managing risk in breast care. Cambridge, Mass.
“Systems approach” to cutting risk of lawsuits
The cases of Lucy, Fiona, Olivia, and Jacqueline represent only some of the issues that give rise to malpractice claims. Since breast care is fragmented across medical specialties, a systematic approach is encouraged.
Systems form the basis of good risk management. The ProMutual Group advocates that every medical practice—whether office- or hospital-based—have a comprehensive risk management program that incorporates some or all of the following suggestions.
Annual exam not always enough
Even more frequent screening may be necessary if the patient has a specific breast complaint.
Every practice should have guidelines for these exams, including instructions on identifying women at risk because of personal or family history (ALGORITHM).
Ultrasound, MRI for young women
Younger, otherwise healthy women with unimpressive findings such as nodularities, nipple discharge, or tenderness deserve extra attention. The physician may want to consider additional diagnostic tests such as ultrasound or MRI for this group.
Never disregard a complaint
If a woman complains of discomfort or a self-detected mass, immediate evaluation is imperative. This includes pregnant patients; do not wait until after delivery to investigate an abnormality.
Use your own guidelines, but justify deviations from the norm
These may include earlier screening for women at risk, and need not match the recommendations of a nationally recognized group. However, in the event of a claim or suit, you will need to justify the reason for deviating from widely accepted guidelines.
Incorporate a systematic approach to diagnosis, treatment, referral (if required), and follow-up breast care.
Using an algorithm can help minimize error, confusion, and delays in care.
Assume cancer until it is ruled out
Consider diagnostic mammography and, when indicated, ultrasound imaging when the screening mammogram of a patient with a palpable mass is either negative or inconclusive.
A fine-needle aspiration or biopsy is a must to resolve indeterminate breast symptoms or inconclusive diagnostic breast imaging tests.
What not to say to patients
Do not assure the patient that a breast mass is benign until it is proven to be so.
Are you the “default PCP”? Screen for other cancers
When more than 1 physician is involved, someone needs to assume responsibility for the patient’s ongoing breast care. In a malpractice case, a doctor cannot simply claim that a routine mammogram or diagnostic test was deferred because it was assumed another physician would handle it.
In most cases, the patient’s primary care physician has the responsibility for her care.
ObGyn can be held to primary care physician standard. If the ObGyn is her only physician, the ObGyn may be held to a primary care standard—not only for breast and cervical cancer screening, but also for colorectal, skin, and other cancers.
Any physicians involved in a woman’s care should decide between them, as early in the process as possible, who will assume responsibility for ongoing care. According to the Agency for Healthcare Research and Quality,6-9 that physician oversees follow-up, monitoring, and tracking women with abnormal findings, including those for whom a biopsy is recommended.
The designation of responsibility and accountability should be documented in the medical record.
The radiology facility’s responsibility. The facility that performs the patient’s mammograms must share responsibility with the designated physician for follow-up, monitoring, and tracking abnormal test results. Each facility should have a system for tracking positive mammogram findings and, according to the Agency for Healthcare Research and Quality, “a process for correlating findings with biopsy results.” It also has the duty to communicate urgent or significant findings to physicians and, under certain circumstances, to the patient directly.
If more than 1 physician is involved, everyone should know who is responsible for coordinating the patient’s ongoing care. Good communication is especially critical when abnormal findings are involved and additional imaging or more invasive testing is needed.
Document, document, document
ProMutual Group’s studies repeatedly show that inadequate records—whether paper or electronic—substantially reduce the chance for a successful defense.
Dr. Zylstra reports no financial relationships relevant to this article. Ms. Greenwald and Ms. Mondor are employees of ProMutual Group, Boston.
1. Zylstra S, D’Orsi C, Ricci BA, et al. Defense of breast cancer malpractice claims. Breast J. 2001;7:76-90.
2. Kern KA. Breast cancer and malpractice: a surgeon’s perspective on risk prevention. Semin Breast Dis. 1998;1:22-31.
3. Zylstra S, Bors-Koefoed R, Mondor M, et al. A statistical model for predicting outcome in breast cancer malpractice lawsuits. Obstet Gynecol. 1994;84:392-398.
4. Physician Insurers Association of America. Breast Cancer Study. Lawrenceville, NJ: Physician Insurers Association of America; 1990.
5. Physician Insurers Association of America. Breast Cancer Study 1995. Washington, DC: Physician Insurers Association of America; 1995.
6. Mammography quality standards: proposed rules (CFR 900). Federal Regulations. 1996;65:4911-4912.
7. Berlin L. To telephone or not to telephone: how high is the standard? AJR Am J Roentgenol. 1992;159:1335-1339.
8. Berlin L. Communication of the urgent finding. AJR Am J Roentgenol. 1996;166:513-515.
9. Berlin L. Communication of the significant but not urgent finding. AJR Am J Roentgenol. 1997;168:329-331.
1. Zylstra S, D’Orsi C, Ricci BA, et al. Defense of breast cancer malpractice claims. Breast J. 2001;7:76-90.
2. Kern KA. Breast cancer and malpractice: a surgeon’s perspective on risk prevention. Semin Breast Dis. 1998;1:22-31.
3. Zylstra S, Bors-Koefoed R, Mondor M, et al. A statistical model for predicting outcome in breast cancer malpractice lawsuits. Obstet Gynecol. 1994;84:392-398.
4. Physician Insurers Association of America. Breast Cancer Study. Lawrenceville, NJ: Physician Insurers Association of America; 1990.
5. Physician Insurers Association of America. Breast Cancer Study 1995. Washington, DC: Physician Insurers Association of America; 1995.
6. Mammography quality standards: proposed rules (CFR 900). Federal Regulations. 1996;65:4911-4912.
7. Berlin L. To telephone or not to telephone: how high is the standard? AJR Am J Roentgenol. 1992;159:1335-1339.
8. Berlin L. Communication of the urgent finding. AJR Am J Roentgenol. 1996;166:513-515.
9. Berlin L. Communication of the significant but not urgent finding. AJR Am J Roentgenol. 1997;168:329-331.
Cutting the medicolegal risk of shoulder dystocia
Clip-and-save shoulder dystocia documentation form
Practice recommendations
Among the intrapartum events that constitute bona fide emergencies, shoulder dystocia stands out. This obstetric emergency is the focus of an increasing number of medical liability cases. Most lawsuits involving shoulder dystocia allege negligence as the cause of the brachial plexus injury, fractured clavicle or humerus, or other injury. The defendant physicians named in these suits are often accused of inappropriately managing the prenatal or intrapartum course or the dystocia itself—or of inadequately documenting the steps taken to resolve the emergency.
To glean insights into the litigation process as it involves shoulder dystocia, we retrospectively reviewed all cases closed by the Boston-based ProMutual Group, a major liability insurance carrier, over a 7-year period. We wanted to learn more about the plaintiffs themselves, as well as the clinical and medicolegal factors that led to jury awards or indemnity payments. We also wanted data that could serve as the foundation for guidelines on how to proceed in the event of shoulder dystocia, as well as a documentation tool.
This shoulder dystocia case from an insurer’s closed claim file illustrates a problem often linked to litigation. Minor changes were made to conceal the identities of the involved parties.
Nurse and physician document different times
A 31-year-old woman in her 10th week of pregnancy had one prior uncomplicated vaginal delivery of a 9 lb 7 oz infant. Her prenatal course proceeds unremarkably, with a normal glucose tolerance test and total weight gain of 36 lb. At 41 weeks and 2 days, the estimated fetal weight is documented as 4,120 g. Labor is induced with oxytocin. Because of maternal fatigue, vacuum delivery is attempted.
Notes of the physician and the nurse differ regarding the time of the first of 3 vacuum applications.
After delivery of the head, shoulder dystocia is encountered. In a note handwritten immediately after delivery, the physician states that the head was “reconstituted as right occiput anterior with the left shoulder anterior.”
In a note dictated later, however, the same physician states the right shoulder was anterior.
Help is summoned and arrives 20 minutes after the dystocia is first encountered. The time that help was summoned is in question since there is an 18-minute discrepancy between the times the physician and the nurse note that assistance was called.
Despite the use of suprapubic pressure and maneuvers including McRoberts and Wood’s corkscrew, shoulder dystocia persists for 24 minutes. Apgars of the 11 lb 3 oz infant are 0, 1, and 3. The child is resuscitated but dies within 2 days of birth.
Outcome
Settled with a 7-figure indemnity payment.
What the defense experts said
The key issues involve documentation and summoning assistance. Discrepancies in documentation almost always cast doubt upon the credibility of a defendant. Ideally, there should be no discrepancies between nurse and physician notes and, certainly, no discrepancies between 2 notes on the same case by the same physician. If, in this case, the physician realized after writing the first note that the anterior shoulder had been incorrectly identified, a correction should have been written as a separate note.
Use of the shoulder dystocia documentation tool (see) helps create a chronology of events, which may prove vital to a successful defense.
The call for help might not have been delayed if the labor and delivery unit had had a shoulder dystocia protocol including “drills” for all team members. Help should be called as soon as a shoulder dystocia is encountered so that, when needed, it is available. Under no circumstances should it take 20 minutes for assistance to arrive.
Brachial plexus injury not always caused by shoulder dystocia
Between 21% and 42% of shoulder dystocias involve an injury1—usually brachial plexus injury. Plaintiff attorneys have manipulated this fact to attribute many cases of neonatal brachial plexus injury to mismanagement of shoulder dystocia by the obstetrician.
They fault the physician for failing to estimate fetal weight, perform a timely cesarean, use appropriate maneuvers correctly, or have a pediatrician present. They criticize nothing more resoundingly than use of “inappropriate” or “excessive” lateral traction to the fetal head.2
Nontraction injuries. The reality can be strikingly different, however. Some cases of brachial plexus injury involve no traction at all.
- Brachial plexus injuries have been reported in infants who had precipitate vaginal deliveries without any physical intervention by the obstetrician.2
- These injuries also have occurred in infants delivered via cesarean section.1,3,4
- In some cases, brachial plexus injuries have affected the posterior arm of neonates whose anterior arm was involved in shoulder dystocia.1,2,5-7
A retrospective study4 found that, of 39 cases of brachial plexus injury, only 17 were associated with shoulder dystocia. Similar findings have emerged from other studies.2,3,8
Other causes. It is unclear how brachial plexus injuries occur in the absence of shoulder dystocia. Some think they arise in response to infectious agents such as toxoplasmosis, coxsackievirus, mumps, pertussis, or mycoplasma pneumonia.2 Some assume a mechanical cause, such as fetal response to longstanding abnormal intrauterine pressure exerted by maternal conditions such as bicornate uterus and uterine fibroids, especially in the lower segment.1,2
When brachial plexus injuries occur in the absence of shoulder dystocia, they likely originated before labor and delivery.4 Some experts suggest serial electromyelograms within the first 7 days of life to establish a prenatal rather than intrapartum etiology. A positive electromyelogram within 1 week of birth would suggest antepartum causation.2,9
Recognizing risk factors for shoulder dystocia best way to reduce injury
Most brachial plexus injuries or impairments are associated with shoulder dystocia,9 and shoulder dystocia is the most common way brachial plexus injuries are introduced into litigation.
Decreasing the number of brachial plexusrelated liability cases, therefore, depends on decreasing the incidence of shoulder dystocia. Unfortunately, a failsafe method continues to elude both clinicians and researchers.10-12
Retrospective studies have identified certain factors that may—but do not necessarily—increase the risk of shoulder dystocia.
Prenatal risk factors include high maternal or paternal birth weight, maternal obesity, excessive weight gain during pregnancy, advanced age, short stature, multiparity, postdates, prior shoulder dystocia, small pelvis, prior delivery of a macrosomic infant, gestational diabetes in an earlier pregnancy, abnormal blood sugars in the current pregnancy, or fetal macrosomia.13,14-16
Intrapartum risk factors include a rapid or prolonged second stage, failure or arrest of descent, presence of considerable molding, and need for a midpelvic delivery.10,15
Predictability. Prospective studies have not established the predictability of shoulder dystocia. A 2000 study17 showed that 55% of cases with 1 or more risk factors experienced shoulder dystocia. Predictability increases somewhat when both maternal diabetes and fetal macrosomia complicate pregnancy, since the rate of shoulder dystocia in women with diabetes is consistently higher than in nondiabetic gravidas. This becomes a significant issue when the infant weighs more than 4,000 g.
Indications for prophylactic cesarean in women with diabetes
In 1999, Wagner et al9 found that 70% of shoulder dystocias in women with diabetes occurred when the fetal weight exceeded 4,000 g. They concluded that cesarean delivery for infants with an estimated weight over 4,250 g would reduce the rate of shoulder dystocia by 75% and increase the cesarean delivery rate by 1%.
Others are more conservative. Gross and colleagues11 suggested that, for every additional 26 cesarean deliveries, only 1 case of shoulder dystocia would be prevented.
Macrosomia. Most obstetricians and researchers still do not advocate prophylactic cesarean delivery for macrosomia alone because, by some estimates, 98% of macrosomic infants are delivered without difficulty.18 However, they do suggest that obstetricians at least consider the possibility of cesarean delivery for a macrosomic fetus when the woman has diabetes.
In a study completed in 2000, Skolbekken19 suggested a cutoff of 4,250 g for women with diabetes. Dildy20 suggested limits of more than 4,500 g for diabetic women and more than 5,000 g for nondiabetic gravidas. However, Conway and Langer21 assert that a cutoff of 4,500 g is too liberal for women with diabetes and maintain that, at this cutoff, 40% of shoulder dystocias would not be prevented.
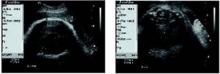
• Ultrasound measurements. Since estimates of fetal weight have a margin of error approaching 40%,9 others have chosen different parameters for determining fetal macrosomia in women with diabetes. In a retrospective study involving 31 women with gestational diabetes, Cohen et al22 found that subtracting the fetal biparietal diameter from the abdominal diameter—with both measurements obtained via ultrasound—yields a predictability score higher than estimated fetal weight. Specifically, if the difference between the 2 measurements is 2.6 cm or more, the rate of shoulder dystocia is high enough to warrant elective cesarean (FIGURE ).
FIGURE Using ultrasound measurements to predict macrosomia
A simple way to predict fetal macrosomia in women with diabetes is to subtract the fetal biparietal diameter (9.3 cm in the scan at left) from the abdominal diameter (average of 12.44 cm in the scan at right). If the difference exceeds 2.6 cm, elective cesarean is warranted. In this case it is 3.14 cm, indicating elective cesarean is warranted.
When dystocia occurs, have a plan and stick to it
Shoulder dystocia immediately places both mother and neonate at risk for temporary or permanent injury. Thus, it is imperative that all obstetricians and other health-care providers who deliver infants have a well developed plan of action for this emergency. They should immediately ask for obstetric assistance and instruct the mother to discontinue any pushing.
Attempts at vigorous downward traction should be avoided, and no fundal pressure should be applied, as these are known to increase the potential for brachial plexus injury. Gentle downward traction is considered the standard of care.17
The obstetrician’s goal is to free the impacted shoulder as quickly as possible, since a fetus can endure only 8 to 10 minutes of asphyxia before permanent neurologic damage occurs.17 The standard of care requires the obstetrician to know and use certain maneuvers to relieve shoulder dystocia. These maneuvers are designed to facilitate vaginal delivery and reduce the risk of permanent brachial plexus injury. The McRoberts maneuver, with flexion and slight rotation of the maternal hips onto the maternal abdomen, is the standard for initial relief of shoulder dystocia.17,23
This shoulder dystocia case from an insurer’s closed claim file illustrates a problem often linked to litigation. Minor changes were made to conceal the identities of the involved parties.
Prompt maneuvers, good outcome
A 28-year-old gravida weighing 214 lb has had 2 previous spontaneous deliveries of infants weighing 8 lb 5 oz and 9 lb 3 oz. Except for a weight gain of more than 60 lb, the pregnancy progressed without complications, and a 3-hour glucose tolerance test was normal. At 40 weeks, the obstetrician notes “concern” about an estimated fetal weight of 10 lb. Induction is planned, but spontaneous labor begins before oxytocin can be given. After 5 hours, the head is delivered without difficulty, but shoulder dystocia follows. The obstetrician extends the episiotomy and performs McRoberts and Wood’s corkscrew maneuvers, but the dystocia persists. Upon noting cyanosis, the obstetrician fractures the infant’s clavicle and quickly delivers a 10 lb 9 oz infant with Apgar scores of 8 and 10. Pediatricians examine the child immediately and diagnose Erb’s palsy, which subsequently resolves. X-rays confirm an undisplaced fracture of the right clavicle. Although the child recovers completely, the family sues, alleging a failure to perform cesarean delivery.
Outcome
Case closed with no payment.
What the defense experts said
Key issues are documentation and informed consent. The overriding opinion of 3 experts who reviewed the case for the defense was that cesarean delivery was not indicated and that in fracturing the clavicle, the physician acted responsibly, quickly, and within the standard of care. One defense expert said failure to document exact maneuvers used to relieve shoulder dystocia deviated from the standard of care. Another defense expert said the physician should have obtained informed consent from this at-risk patient, and explained the risks of shoulder dystocia, including neonatal injury, so that the she might have been better able to appreciate the fact that the obstetrician’s fast action may have saved her child from brain damage or death.
Factors that lead to litigation
In a review article, Hickson24 cited factors that prompted families to file medical liability claims following perinatal injury. Some families observed that, in their search for the cause of an injury, they found 1 or more aspects of care to be inappropriate.
The desire for information, perception of being misled, anger with the medical profession, desire to prevent injuries to others, recognition of long-term sequelae, and advice by knowledgeable acquaintances, as well as the need for money, all appeared to contribute to the decision to file medical liability claims.
Convey the risks, and listen carefully. Communication problems between physicians and patients are a contributing factor. Even when physicians provide technically adequate care, families expect answers to their questions and want to feel as though they have been consulted about important medical decisions.24 If these expectations are not met, even patients who have not experienced an adverse outcome may become angry and express dissatisfaction with care.24
The need for communication is critical when shoulder dystocia results in neonatal injury. Empathizing with the family, helping them understand that most brachial plexus injuries are not long-term, and offering to answer their questions both at the moment and later, may help prevent litigation.
This type of communication can be difficult. It helps to realize that an acknowledgment of distress and concern is not an admission of guilt, and an explanation is not an apology.15 However, an absence of communication or an attempt by the physician to place blame may be perceived as an admission of guilt that gives rise to a lawsuit.
Action plan is what counts in court. A review by Gross et al11 concluded that obstetricians should have a shoulder dystocia plan that enables an instant and orderly response. Also recommended is a protocol to help anticipate clinical problems and prevent medicolegal problems.
Gross and colleagues11 found that the Ob/Gyns with the most defensible cases paid close attention to the patient’s history and prenatal course and, upon encountering dystocia, implemented at least 2 maneuvers (if necessary) and thoroughly documented their actions immediately after delivery.
Fetterman15 agreed, asserting that what counts in court is not so much whether the obstetrician employed the McRoberts maneuver before or after the Wood’s corkscrew maneuver but whether he or she had an action plan in mind, implemented that plan properly, and thoroughly documented the actions taken and the reasons underlying them.
Dissecting legal cases for clues to reduced risk
In the review of medicolegal cases for this article, we limited our search to cases closed between Jan 1, 1995, and Dec 31, 2002, using a computer to search for codes specific to shoulder dystocia as well as the phrases “shoulder dystocia,” “Erb’s palsy,” and “brachial plexus injury.” We identified 61 cases involving 117 defendants and created a data sheet to gather information on patient and physician demographics, medical and obstetric history, description of the incident, analysis rendered by defense and plaintiff experts, and legal and financial outcomes.
Of 117 defendants, 76 were obstetricians. There also were 16 hospitals, 15 corporations, 5 certified nurse-midwives, 1 family physician, 1 emergency physician, and 3 persons categorized as “other.” Age, race, and parity of the 61 plaintiffs are given in TABLE 1.
Twenty-six of the 61 cases, involving 74 defendants, closed with no payment. That is, they were either dismissed or closed with a jury verdict for the defense. The remaining 35 cases involved 43 defendants and were closed with an aggregate indemnity payment of $19.2 million. The mean payment was $445,000 per defendant.
These guidelines are recommended to help prevent, predict, and manage shoulder dystocia and brachial plexus injury.
Obtain a prenatal history that includes the birth weights of both parents and any history of prior shoulder dystocia or cesarean delivery performed for “failure to progress.”
Estimate the fetal weight and take into account the risk for shoulder dystocia when the fetus is determined by ultrasound to be macrosomic.
Perform glucose testing on all patients, and follow up on even a single abnormal glucose reading. Discuss the possibility of shoulder dystocia and the accompanying risk of neonatal injury with “at risk” patients.
Consider obtaining informed consent for vaginal delivery of a patient with risk factors for shoulder dystocia.
Consider cesarean delivery for :
- nondiabetic women when the estimated fetal weight (EFW) exceeds 4,500 g,
- diabetic gravidas when the EFW exceeds 4,000 g,
- women with a prior delivery complicated by shoulder dystocia or brachial plexus palsy,
- gravidas with a prolonged second stage and nonprogression of labor, and
- patients who express fear and doubt about vaginal delivery.
Be sparing with the use of oxytocin when the fetus is known or suspected to be macrosomic, taking special care not to be aggressive with induction.
Use forceps or vacuum extraction with caution, and limit the number of attempts with each.
Minimize traction on the fetal head. Traction that is deemed to be “excessive” may be used against the physician in a liability suit. Gentle traction is acceptable.
Do not use or order fundal pressure. It will almost invariably be used against you in a lawsuit.
Be able to define and correctly describe maneuvers generally accepted as the standard for shoulder dystocia and, when necessary, use and document them appropriately. These include the McRoberts, Wood’s corkscrew, Rubin, and Zavanelli maneuvers; extended episiotomy; suprapubic pressure; and fracture of the anterior clavicle.
Be alert to the possibility of brachial plexus injury in the absence of shoulder dystocia. Obstetricians have been erroneously accused of causing brachial plexus injury by plaintiff attorneys who do not understand that this injury is not always the result of dystocia. Thorough contemporaneous documentation is key in these instances.
Request immediate pediatric assessment of a newborn involved in shoulder dystocia. Have the placenta sent for examination, and request cord blood gases.
Communicate openly and honestly with the parents of a child who has suffered a brachial plexus injury. This may be the single greatest tool for reducing the risk of liability litigation.
Consider serial electromyelograms during the first 7 days of life for a neonate with a brachial plexus palsy. These studies can help determine the etiology of the injury.
Use a shoulder dystocia documentation tool such as the one on page 91. Thorough documentation of all relevant prenatal and intrapartum events is critical to a successful defense. A 12-point detailed delivery note as recommended by Fetterman will also prevent or reduce legal risk.15
Schedule shoulder dystocia “drills” in the labor and delivery unit to familiarize obstetric team members with their roles.
Require comanagement of any midwifery patient at increased risk for shoulder dystocia.
Neonatal injury occurred in all 61 cases. Erb’s palsy was the overwhelming pediatric outcome (57 of 61 cases, or 93.4%), with an aggregate indemnity payment of $17.6 million. Fractured humerus was the outcome in 1 case, and 3 cases involved neonatal deaths.
Reasons for indemnity payments included:
- probable liability (18 defendants),
- plaintiff was sympathetic, likely to evoke an emotional response from the jury (8 defendants),
- clear liability (7 defendants),
- defendant would not have made a strong witness in his or her own defense (5 defendants),
- defendant had died or was too ill to stand trial (3 defendants),
- medical record had been altered (2 defendants),
- case was considered too inflammatory to risk a jury award (2 defendants), and
- policy limits were too low to risk a potentially high jury award (1 defendant).
Five defendants were involved in cases with multiple medicolegal issues that argued for settlement.
The effect of birth weight. Notably, 74% of infants involved in these cases were macrosomic (birth weight over 4,000 g). Except for neonatal injury (100%), no single maternal or fetal variable appears with greater frequency.
The mean indemnity in closed cases increased in direct proportion to fetal weight, ranging from $500,000 in cases involving infants whose birth weight was less than 4,000 g to $950,000 when the birth weight was 5,000 g or more.
Maternal factors seen with greatest frequency included obesity, excessive weight gain in pregnancy, and gestational diabetes. Analysis of prepregnant body mass index found that 19 women had a weight within the “obese” category, 18 of whom gave birth to macrosomic infants. Eight of these cases closed without indemnity payment and 10 closed with an aggregate indemnity payment of $6.5 million.
Forty-eight of the 61 plaintiffs (78.8%) exceeded the normal weight gain in pregnancy based on height and prepregnant weight. Twenty-nine of these cases closed with an aggregate indemnity payment of $16.6 million.
Influence of diabetes. Fifty-two of 61 plaintiffs underwent a glucose screening test. Of these, 23 went on to have a glucose tolerance test, with 12 testing positive for gestational diabetes. Further analysis revealed borderline screening glucose values in an additional 16 cases. These women were not considered diabetic by their obstetricians, were not retested for diabetes, and did not receive nutritional counseling. Macrosomic infants were born to 9 of the 12 patients with gestational diabetes and to 13 of the 16 borderline cases.
Of 28 cases with confirmed or suspected gestational diabetes, 14 women delivered infants weighing over 4,250 g; 11 of the 14 weighed more than 4,500 g.
In a comparison of cases involving diabetic and nondiabetic women who delivered infants weighing more than 4,250 g, 82% of the cases involving nondiabetic women closed without payment. Among cases involving diabetes (actual or borderline), the corresponding figure was 27.3%.
Labor and delivery interventions were cited in all cases. Oxytocin was used in 42 of the 61 cases (68.9%), forceps in 9 (14.8%), and vacuum extraction in 7 (11.5%). Suprapubic pressure was used in 37 cases (60.7%), fundal pressure in 9 (14.8%), and traction on the fetal head in 16 cases (26.2%).
In addition, defense experts determined that the McRoberts maneuver was used in 41 cases (67.2%) and the Wood’s corkscrew maneuver in 28 (45.9%).
Seven cases involved a second stage of labor exceeding 2.5 hours. The ratio of wins to losses decreased substantially with the use of oxytocin, forceps, fundal pressure, or a prolonged second stage.
Data were analyzed using selected variables thought to have an association with winning or losing cases and with indemnity (TABLE 2). No statistically significant models emerged. This is likely due to inadequate power (low number of cases) and the large number of interactions between variables relative to the outcomes evaluated.
TABLE 1
Plaintiff demographics
| CHARACTERISTIC | ALL CASES (n = 61) | CASES WITH INDEMNITY (n = 35) |
|---|---|---|
| Mean age, in years (range) | 28 (17–40) | 29 (18–38) |
| Race (%) | ||
| White | 35 (57) | 21 (60) |
| Black | 12 (20) | 7 (20) |
| Hispanic | 11 (18) | 6 (17) |
| Asian | 1 (2) | — |
| Unknown | 2 (3) | 1 (3) |
| Parity (%) | ||
| 0 | 19 (31) | 11 (31) |
| 1 | 26 (43) | 16 (46) |
| 2 | 11 (18) | 7 (20) |
| 3 | 2 (3) | 1 (3) |
| 4 | 2 (3) | — |
| 5 | 1 (2) | — |
TABLE 2
Litigation outcomes for selected prenatal and intrapartum variables
| CHARACTERISTIC | CLOSED WITHOUT INDEMNITY | CLOSED WITHOUT INDEMNITY PAYMENT | MEAN INDEMNITY ($) | TOTAL INDEMNITY ($) |
|---|---|---|---|---|
| Prenatal factors | ||||
| Gestational diabetes | 5 | 7 | 413,300 | 2,893,000 |
| Adjusted diabetes | 10 | 18 | 521,400 | 9,386,000 |
| Obesity | 8 | 13 | 651,300 | 8,466,000 |
| Intrapartum factors | ||||
| Prolonged second stage | 1 | 6 | 707,700 | 4,247,000 |
| Oxytocin induction | 2 | 10 | 406,500 | 4,065,300 |
| Oxytocin augmentation | 15 | 15 | 737,000 | 11,100,000 |
| Forceps delivery | 1 | 8 | 552,100 | 4,416,800 |
| Vacuum extraction | 3 | 4 | 531,300 | 2,125,000 |
| Episiotomy | 20 | 30 | 579,400 | 17,382,100 |
| McRoberts maneuver | 20 | 21 | 542,900 | 11,400,300 |
| Wood’s corkscrew maneuver | 12 | 16 | 652,300 | 10,436,200 |
| Suprapubic pressure | 17 | 20 | 629,000 | 12,579,400 |
| Traction to fetal head | 7 | 9 | 665,500 | 5,989,500 |
| Fundal pressure | 4 | 7 | 660,700 | 4,625,000 |
4 factors raise risk of litigation
After reviewing the literature and analyzing the ProMutual data, we concluded that shoulder dystocia remains largely unpredictable. However, certain clinical factors are clearly associated with an increased risk for litigation:
- prenatal factors,
- labor and delivery interventions,
- maneuvers performed at the time of the dystocia, and
- fetal outcomes.
Prenatal factors. The most significant prenatal factors were maternal obesity, excessive weight gain in pregnancy, and, especially, diabetes and fetal macrosomia.
• Fetal macrosomia. Although most macrosomic infants deliver without complication, fetal macrosomia was involved in more than 72% of the cases reviewed. Because macrosomia represents a risk not only for shoulder dystocia but also for the litigation that may arise from it, it is important to:
- know and document the estimated fetal weight (EFW);
- discuss the risk of dystocia and its sequelae with the mother and her partner; and
- consider cesarean delivery for estimated fetal weights that suggest macrosomia (see the recommendations on page 82 for specifics).
It also is advisable to mobilize a team for the possibility of shoulder dystocia if vaginal delivery is attempted.
Preparing for the increased risk of a macrosomic fetus can be successful only if macrosomia is both diagnosed and anticipated.
Because macrosomia often is accompanied by maternal diabetes, serum glucose testing is recommended for all gravidas, with follow-up of both abnormal and borderline values.
Labor and delivery interventions were cited in all cases, with data indicating a decreasing ratio of wins to losses with the use of oxytocin, forceps, or fundal pressure, and prolonged second stage.
- Use of oxytocin to augment an already established labor carried less risk than oxytocin for labor induction. Ten of the 12 cases (83.3%) in which oxytocin was used for induction closed with an indemnity payment. However, of the 30 cases in which oxytocin was used for labor augmentation, 50% closed with payment.
- • Forceps and a prolonged second stage. Eight of 9 cases (88.9%) involving forceps and 6 of 7 cases (85.7%) involving a prolonged second stage closed with indemnity payment.
Consider cesarean delivery when the second stage is prolonged or labor fails to progress. Be cautious using forceps and vacuum extraction in these circumstances, and limit the number of attempts with either.
Maneuvers performed at the time of dystocia. The most common maneuverswere McRoberts, suprapubic pressure, and Wood’s corkscrew. The ratio of wins to losses decreased with traction of the fetal head and use of fundal pressure.
Part of the risk-management protocol for obstetricians should be appropriate use of McRoberts maneuver, suprapubic pressure, and Wood’s corkscrew, and cutting a large episiotomy. In addition, be careful not to push, pull, rotate the head, or apply fundal pressure.
Fetal outcomes. All cases involved neonatal injury (Erb’s palsy, fractured humerus) or death.
For this reason, we recommend an action plan that includes immediate pediatric or neonatal assessment of neuromuscular function of the infant’s anterior shoulder. Assess the Moro reflex and the possibility of brachial plexus injury and fractures of the clavicle and humerus. Also examine the placenta, send it to pathology, and perform a cord blood gas analysis.
Last words
Shoulder dystocia is the unfortunate complication of a small number of deliveries, but the focus of an increasing number of lawsuits. Because the neonatal injuries that so often accompany shoulder dystocia often lead to litigation, obstetricians should prepare to identify risk and help patients make informed choices. We should be prepared to manage this emergency whenever it occurs and thoroughly document actions.
Dr. Zylstra reports no financial relationships relevant to this article.
1. Gherman RB, Goodwin TM, Ouzounian JG, Miller DA, Paul RH. Brachial plexus palsy associated with cesarean section: an in utero injury? Am J Obstet Gynecol. 1997;177:1162-1164.
2. Gherman RB, Ouzounian JG, Goodwin TM. Brachial plexus palsy: an in utero injury? Am J Obstet Gynecol. 1999;180:1303-1307.
3. Gilbert WM, Nesbitt TS, Danielsen B. Associated factors in 1611 cases of brachial plexus injury. Obstet Gynecol. 1999;93:536-540.
4. Jennett RJ, Tarby TJ, Kreinick CJ. Brachial plexus palsy: an old problem revisited. Am J Obstet Gynecol. 1992;166:1673-1677.
5. Hankins GDV, Clark SL. Brachial plexus palsy involving the posterior shoulder at spontaneous vaginal delivery. Am J Perinatol. 1995;12:44-45.
6. Gherman RB, Ouzounian JG, Miller DA, Kwok L, Goodwin TM. Spontaneous vaginal delivery: a risk factor for Erb’s palsy? Am J Obstet Gynecol. 1998;178:423-427.
7. Dodds SD, Wolfe SW. Perinatal brachial plexus palsy. Curr Opin Pediatrics. 2000;23:40-47.
8. Wade DM. A Comment on the Trial of Brachial Plexus/Erb’s Palsy Medical Malpractice Cases. Evidence Technologies, Inc. 1994.
9. Wagner RK, Nielsen PE, Gonik B. Controversies in labor management: shoulder dystocia. Obstet Gynecol Clin. 1999;26(2):371-382.
10. McFarland M, Hod M, Piper JM, Xenakis EM-J, Langer O. Are labor abnormalities more common in shoulder dystocia? Am J ObstetGynecol. 1995;173:1211-1214.
11. Gross TL, Sokol RJ, Williams T, Thompson K. Shoulder dystocia: A fetal-physician risk. Am J Obstet Gynecol. 1987;156:1408-1418.
12. Acker DB, Sachs BP, Friedman EA. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66:762-768.
13. Shoulder dystocia and Erb’s palsy. The Keenan Law Firm. 2002. Available at www.shoulderdystociaattorney.com. Accessed July 23, 2004.
14. Acker DB, Gregory KD, Sachs BP, Friedman EA. Risk factors for Erb-Duchenne palsy. Obstet Gynecol. 1988;71(Part l):390-392.
15. Fetterman HH. Cutting the legal risks of shoulder dystocia. OBG Management. March 1995;41-51.
16. Iffy L, Varadi V, Jakobovits A. Common intrapartum denominators of shoulder dystocia related birth injuries. Zentralbl Gynakol. 1994;116(1):33-37.
17. Calhoun BC, Hume RR, Walters JL. Shoulder Dystocia as a risk for obstetric liability. Legal Medicine. 2000;12-15.
18. Jennett RJ, Tarby TJ. Brachial plexus palsy: an old problem revisited again. Am J Obstet Gynecol. 1997;176:1354-1357.
19. Skolbekken JA. Shoulder dystocia—malpractice or acceptable risk? Acta Obstet Gynecol Scand. 2000;79:750-756.
20. Dildy GA. Fetal macrosomia [slide presentation]. Presented at the 18th Annual Conference on Obstetrics, Gynecology, Perinatal Medicine, and the Law, Boston University School of Medicine and the Center for Human Genetics, January 2002, Kauai, Hawaii.
21. Conway D, Langer O. Elective delivery of infants with macrosomia in diabetic women: reduced shoulder dystocia versus increased cesarean deliveries. Am J Obstet Gynecol. 1998;178(5):922-925.
22. Cohen B, Penning S, Major C, Ansley D, Porto M, Garite T. Sonographic prediction of shoulder dystocia in infants of diabetic mothers. Obstet Gynecol. 1998;88:10-13.
23. Gherman RB, Goodwin TM, Souter I, Neumann K, Ouzounian JG, Paul RH. The McRoberts’ maneuver for the alleviation of shoulder dystocia: how successful is it? Am J Obstet Gynecol. 1997;176:656-661.
24. Hickson GB, Clayton DW, Githens PB, Sloan FA. Factors that prompted families to file medical malpractice claims following perinatal injuries. JAMA. 1992;267:1359-1363.
Clip-and-save shoulder dystocia documentation form
Practice recommendations
Among the intrapartum events that constitute bona fide emergencies, shoulder dystocia stands out. This obstetric emergency is the focus of an increasing number of medical liability cases. Most lawsuits involving shoulder dystocia allege negligence as the cause of the brachial plexus injury, fractured clavicle or humerus, or other injury. The defendant physicians named in these suits are often accused of inappropriately managing the prenatal or intrapartum course or the dystocia itself—or of inadequately documenting the steps taken to resolve the emergency.
To glean insights into the litigation process as it involves shoulder dystocia, we retrospectively reviewed all cases closed by the Boston-based ProMutual Group, a major liability insurance carrier, over a 7-year period. We wanted to learn more about the plaintiffs themselves, as well as the clinical and medicolegal factors that led to jury awards or indemnity payments. We also wanted data that could serve as the foundation for guidelines on how to proceed in the event of shoulder dystocia, as well as a documentation tool.
This shoulder dystocia case from an insurer’s closed claim file illustrates a problem often linked to litigation. Minor changes were made to conceal the identities of the involved parties.
Nurse and physician document different times
A 31-year-old woman in her 10th week of pregnancy had one prior uncomplicated vaginal delivery of a 9 lb 7 oz infant. Her prenatal course proceeds unremarkably, with a normal glucose tolerance test and total weight gain of 36 lb. At 41 weeks and 2 days, the estimated fetal weight is documented as 4,120 g. Labor is induced with oxytocin. Because of maternal fatigue, vacuum delivery is attempted.
Notes of the physician and the nurse differ regarding the time of the first of 3 vacuum applications.
After delivery of the head, shoulder dystocia is encountered. In a note handwritten immediately after delivery, the physician states that the head was “reconstituted as right occiput anterior with the left shoulder anterior.”
In a note dictated later, however, the same physician states the right shoulder was anterior.
Help is summoned and arrives 20 minutes after the dystocia is first encountered. The time that help was summoned is in question since there is an 18-minute discrepancy between the times the physician and the nurse note that assistance was called.
Despite the use of suprapubic pressure and maneuvers including McRoberts and Wood’s corkscrew, shoulder dystocia persists for 24 minutes. Apgars of the 11 lb 3 oz infant are 0, 1, and 3. The child is resuscitated but dies within 2 days of birth.
Outcome
Settled with a 7-figure indemnity payment.
What the defense experts said
The key issues involve documentation and summoning assistance. Discrepancies in documentation almost always cast doubt upon the credibility of a defendant. Ideally, there should be no discrepancies between nurse and physician notes and, certainly, no discrepancies between 2 notes on the same case by the same physician. If, in this case, the physician realized after writing the first note that the anterior shoulder had been incorrectly identified, a correction should have been written as a separate note.
Use of the shoulder dystocia documentation tool (see) helps create a chronology of events, which may prove vital to a successful defense.
The call for help might not have been delayed if the labor and delivery unit had had a shoulder dystocia protocol including “drills” for all team members. Help should be called as soon as a shoulder dystocia is encountered so that, when needed, it is available. Under no circumstances should it take 20 minutes for assistance to arrive.
Brachial plexus injury not always caused by shoulder dystocia
Between 21% and 42% of shoulder dystocias involve an injury1—usually brachial plexus injury. Plaintiff attorneys have manipulated this fact to attribute many cases of neonatal brachial plexus injury to mismanagement of shoulder dystocia by the obstetrician.
They fault the physician for failing to estimate fetal weight, perform a timely cesarean, use appropriate maneuvers correctly, or have a pediatrician present. They criticize nothing more resoundingly than use of “inappropriate” or “excessive” lateral traction to the fetal head.2
Nontraction injuries. The reality can be strikingly different, however. Some cases of brachial plexus injury involve no traction at all.
- Brachial plexus injuries have been reported in infants who had precipitate vaginal deliveries without any physical intervention by the obstetrician.2
- These injuries also have occurred in infants delivered via cesarean section.1,3,4
- In some cases, brachial plexus injuries have affected the posterior arm of neonates whose anterior arm was involved in shoulder dystocia.1,2,5-7
A retrospective study4 found that, of 39 cases of brachial plexus injury, only 17 were associated with shoulder dystocia. Similar findings have emerged from other studies.2,3,8
Other causes. It is unclear how brachial plexus injuries occur in the absence of shoulder dystocia. Some think they arise in response to infectious agents such as toxoplasmosis, coxsackievirus, mumps, pertussis, or mycoplasma pneumonia.2 Some assume a mechanical cause, such as fetal response to longstanding abnormal intrauterine pressure exerted by maternal conditions such as bicornate uterus and uterine fibroids, especially in the lower segment.1,2
When brachial plexus injuries occur in the absence of shoulder dystocia, they likely originated before labor and delivery.4 Some experts suggest serial electromyelograms within the first 7 days of life to establish a prenatal rather than intrapartum etiology. A positive electromyelogram within 1 week of birth would suggest antepartum causation.2,9
Recognizing risk factors for shoulder dystocia best way to reduce injury
Most brachial plexus injuries or impairments are associated with shoulder dystocia,9 and shoulder dystocia is the most common way brachial plexus injuries are introduced into litigation.
Decreasing the number of brachial plexusrelated liability cases, therefore, depends on decreasing the incidence of shoulder dystocia. Unfortunately, a failsafe method continues to elude both clinicians and researchers.10-12
Retrospective studies have identified certain factors that may—but do not necessarily—increase the risk of shoulder dystocia.
Prenatal risk factors include high maternal or paternal birth weight, maternal obesity, excessive weight gain during pregnancy, advanced age, short stature, multiparity, postdates, prior shoulder dystocia, small pelvis, prior delivery of a macrosomic infant, gestational diabetes in an earlier pregnancy, abnormal blood sugars in the current pregnancy, or fetal macrosomia.13,14-16
Intrapartum risk factors include a rapid or prolonged second stage, failure or arrest of descent, presence of considerable molding, and need for a midpelvic delivery.10,15
Predictability. Prospective studies have not established the predictability of shoulder dystocia. A 2000 study17 showed that 55% of cases with 1 or more risk factors experienced shoulder dystocia. Predictability increases somewhat when both maternal diabetes and fetal macrosomia complicate pregnancy, since the rate of shoulder dystocia in women with diabetes is consistently higher than in nondiabetic gravidas. This becomes a significant issue when the infant weighs more than 4,000 g.
Indications for prophylactic cesarean in women with diabetes
In 1999, Wagner et al9 found that 70% of shoulder dystocias in women with diabetes occurred when the fetal weight exceeded 4,000 g. They concluded that cesarean delivery for infants with an estimated weight over 4,250 g would reduce the rate of shoulder dystocia by 75% and increase the cesarean delivery rate by 1%.
Others are more conservative. Gross and colleagues11 suggested that, for every additional 26 cesarean deliveries, only 1 case of shoulder dystocia would be prevented.
Macrosomia. Most obstetricians and researchers still do not advocate prophylactic cesarean delivery for macrosomia alone because, by some estimates, 98% of macrosomic infants are delivered without difficulty.18 However, they do suggest that obstetricians at least consider the possibility of cesarean delivery for a macrosomic fetus when the woman has diabetes.
In a study completed in 2000, Skolbekken19 suggested a cutoff of 4,250 g for women with diabetes. Dildy20 suggested limits of more than 4,500 g for diabetic women and more than 5,000 g for nondiabetic gravidas. However, Conway and Langer21 assert that a cutoff of 4,500 g is too liberal for women with diabetes and maintain that, at this cutoff, 40% of shoulder dystocias would not be prevented.
• Ultrasound measurements. Since estimates of fetal weight have a margin of error approaching 40%,9 others have chosen different parameters for determining fetal macrosomia in women with diabetes. In a retrospective study involving 31 women with gestational diabetes, Cohen et al22 found that subtracting the fetal biparietal diameter from the abdominal diameter—with both measurements obtained via ultrasound—yields a predictability score higher than estimated fetal weight. Specifically, if the difference between the 2 measurements is 2.6 cm or more, the rate of shoulder dystocia is high enough to warrant elective cesarean (FIGURE ).
FIGURE Using ultrasound measurements to predict macrosomia
A simple way to predict fetal macrosomia in women with diabetes is to subtract the fetal biparietal diameter (9.3 cm in the scan at left) from the abdominal diameter (average of 12.44 cm in the scan at right). If the difference exceeds 2.6 cm, elective cesarean is warranted. In this case it is 3.14 cm, indicating elective cesarean is warranted.
When dystocia occurs, have a plan and stick to it
Shoulder dystocia immediately places both mother and neonate at risk for temporary or permanent injury. Thus, it is imperative that all obstetricians and other health-care providers who deliver infants have a well developed plan of action for this emergency. They should immediately ask for obstetric assistance and instruct the mother to discontinue any pushing.
Attempts at vigorous downward traction should be avoided, and no fundal pressure should be applied, as these are known to increase the potential for brachial plexus injury. Gentle downward traction is considered the standard of care.17
The obstetrician’s goal is to free the impacted shoulder as quickly as possible, since a fetus can endure only 8 to 10 minutes of asphyxia before permanent neurologic damage occurs.17 The standard of care requires the obstetrician to know and use certain maneuvers to relieve shoulder dystocia. These maneuvers are designed to facilitate vaginal delivery and reduce the risk of permanent brachial plexus injury. The McRoberts maneuver, with flexion and slight rotation of the maternal hips onto the maternal abdomen, is the standard for initial relief of shoulder dystocia.17,23
This shoulder dystocia case from an insurer’s closed claim file illustrates a problem often linked to litigation. Minor changes were made to conceal the identities of the involved parties.
Prompt maneuvers, good outcome
A 28-year-old gravida weighing 214 lb has had 2 previous spontaneous deliveries of infants weighing 8 lb 5 oz and 9 lb 3 oz. Except for a weight gain of more than 60 lb, the pregnancy progressed without complications, and a 3-hour glucose tolerance test was normal. At 40 weeks, the obstetrician notes “concern” about an estimated fetal weight of 10 lb. Induction is planned, but spontaneous labor begins before oxytocin can be given. After 5 hours, the head is delivered without difficulty, but shoulder dystocia follows. The obstetrician extends the episiotomy and performs McRoberts and Wood’s corkscrew maneuvers, but the dystocia persists. Upon noting cyanosis, the obstetrician fractures the infant’s clavicle and quickly delivers a 10 lb 9 oz infant with Apgar scores of 8 and 10. Pediatricians examine the child immediately and diagnose Erb’s palsy, which subsequently resolves. X-rays confirm an undisplaced fracture of the right clavicle. Although the child recovers completely, the family sues, alleging a failure to perform cesarean delivery.
Outcome
Case closed with no payment.
What the defense experts said
Key issues are documentation and informed consent. The overriding opinion of 3 experts who reviewed the case for the defense was that cesarean delivery was not indicated and that in fracturing the clavicle, the physician acted responsibly, quickly, and within the standard of care. One defense expert said failure to document exact maneuvers used to relieve shoulder dystocia deviated from the standard of care. Another defense expert said the physician should have obtained informed consent from this at-risk patient, and explained the risks of shoulder dystocia, including neonatal injury, so that the she might have been better able to appreciate the fact that the obstetrician’s fast action may have saved her child from brain damage or death.
Factors that lead to litigation
In a review article, Hickson24 cited factors that prompted families to file medical liability claims following perinatal injury. Some families observed that, in their search for the cause of an injury, they found 1 or more aspects of care to be inappropriate.
The desire for information, perception of being misled, anger with the medical profession, desire to prevent injuries to others, recognition of long-term sequelae, and advice by knowledgeable acquaintances, as well as the need for money, all appeared to contribute to the decision to file medical liability claims.
Convey the risks, and listen carefully. Communication problems between physicians and patients are a contributing factor. Even when physicians provide technically adequate care, families expect answers to their questions and want to feel as though they have been consulted about important medical decisions.24 If these expectations are not met, even patients who have not experienced an adverse outcome may become angry and express dissatisfaction with care.24
The need for communication is critical when shoulder dystocia results in neonatal injury. Empathizing with the family, helping them understand that most brachial plexus injuries are not long-term, and offering to answer their questions both at the moment and later, may help prevent litigation.
This type of communication can be difficult. It helps to realize that an acknowledgment of distress and concern is not an admission of guilt, and an explanation is not an apology.15 However, an absence of communication or an attempt by the physician to place blame may be perceived as an admission of guilt that gives rise to a lawsuit.
Action plan is what counts in court. A review by Gross et al11 concluded that obstetricians should have a shoulder dystocia plan that enables an instant and orderly response. Also recommended is a protocol to help anticipate clinical problems and prevent medicolegal problems.
Gross and colleagues11 found that the Ob/Gyns with the most defensible cases paid close attention to the patient’s history and prenatal course and, upon encountering dystocia, implemented at least 2 maneuvers (if necessary) and thoroughly documented their actions immediately after delivery.
Fetterman15 agreed, asserting that what counts in court is not so much whether the obstetrician employed the McRoberts maneuver before or after the Wood’s corkscrew maneuver but whether he or she had an action plan in mind, implemented that plan properly, and thoroughly documented the actions taken and the reasons underlying them.
Dissecting legal cases for clues to reduced risk
In the review of medicolegal cases for this article, we limited our search to cases closed between Jan 1, 1995, and Dec 31, 2002, using a computer to search for codes specific to shoulder dystocia as well as the phrases “shoulder dystocia,” “Erb’s palsy,” and “brachial plexus injury.” We identified 61 cases involving 117 defendants and created a data sheet to gather information on patient and physician demographics, medical and obstetric history, description of the incident, analysis rendered by defense and plaintiff experts, and legal and financial outcomes.
Of 117 defendants, 76 were obstetricians. There also were 16 hospitals, 15 corporations, 5 certified nurse-midwives, 1 family physician, 1 emergency physician, and 3 persons categorized as “other.” Age, race, and parity of the 61 plaintiffs are given in TABLE 1.
Twenty-six of the 61 cases, involving 74 defendants, closed with no payment. That is, they were either dismissed or closed with a jury verdict for the defense. The remaining 35 cases involved 43 defendants and were closed with an aggregate indemnity payment of $19.2 million. The mean payment was $445,000 per defendant.
These guidelines are recommended to help prevent, predict, and manage shoulder dystocia and brachial plexus injury.
Obtain a prenatal history that includes the birth weights of both parents and any history of prior shoulder dystocia or cesarean delivery performed for “failure to progress.”
Estimate the fetal weight and take into account the risk for shoulder dystocia when the fetus is determined by ultrasound to be macrosomic.
Perform glucose testing on all patients, and follow up on even a single abnormal glucose reading. Discuss the possibility of shoulder dystocia and the accompanying risk of neonatal injury with “at risk” patients.
Consider obtaining informed consent for vaginal delivery of a patient with risk factors for shoulder dystocia.
Consider cesarean delivery for :
- nondiabetic women when the estimated fetal weight (EFW) exceeds 4,500 g,
- diabetic gravidas when the EFW exceeds 4,000 g,
- women with a prior delivery complicated by shoulder dystocia or brachial plexus palsy,
- gravidas with a prolonged second stage and nonprogression of labor, and
- patients who express fear and doubt about vaginal delivery.
Be sparing with the use of oxytocin when the fetus is known or suspected to be macrosomic, taking special care not to be aggressive with induction.
Use forceps or vacuum extraction with caution, and limit the number of attempts with each.
Minimize traction on the fetal head. Traction that is deemed to be “excessive” may be used against the physician in a liability suit. Gentle traction is acceptable.
Do not use or order fundal pressure. It will almost invariably be used against you in a lawsuit.
Be able to define and correctly describe maneuvers generally accepted as the standard for shoulder dystocia and, when necessary, use and document them appropriately. These include the McRoberts, Wood’s corkscrew, Rubin, and Zavanelli maneuvers; extended episiotomy; suprapubic pressure; and fracture of the anterior clavicle.
Be alert to the possibility of brachial plexus injury in the absence of shoulder dystocia. Obstetricians have been erroneously accused of causing brachial plexus injury by plaintiff attorneys who do not understand that this injury is not always the result of dystocia. Thorough contemporaneous documentation is key in these instances.
Request immediate pediatric assessment of a newborn involved in shoulder dystocia. Have the placenta sent for examination, and request cord blood gases.
Communicate openly and honestly with the parents of a child who has suffered a brachial plexus injury. This may be the single greatest tool for reducing the risk of liability litigation.
Consider serial electromyelograms during the first 7 days of life for a neonate with a brachial plexus palsy. These studies can help determine the etiology of the injury.
Use a shoulder dystocia documentation tool such as the one on page 91. Thorough documentation of all relevant prenatal and intrapartum events is critical to a successful defense. A 12-point detailed delivery note as recommended by Fetterman will also prevent or reduce legal risk.15
Schedule shoulder dystocia “drills” in the labor and delivery unit to familiarize obstetric team members with their roles.
Require comanagement of any midwifery patient at increased risk for shoulder dystocia.
Neonatal injury occurred in all 61 cases. Erb’s palsy was the overwhelming pediatric outcome (57 of 61 cases, or 93.4%), with an aggregate indemnity payment of $17.6 million. Fractured humerus was the outcome in 1 case, and 3 cases involved neonatal deaths.
Reasons for indemnity payments included:
- probable liability (18 defendants),
- plaintiff was sympathetic, likely to evoke an emotional response from the jury (8 defendants),
- clear liability (7 defendants),
- defendant would not have made a strong witness in his or her own defense (5 defendants),
- defendant had died or was too ill to stand trial (3 defendants),
- medical record had been altered (2 defendants),
- case was considered too inflammatory to risk a jury award (2 defendants), and
- policy limits were too low to risk a potentially high jury award (1 defendant).
Five defendants were involved in cases with multiple medicolegal issues that argued for settlement.
The effect of birth weight. Notably, 74% of infants involved in these cases were macrosomic (birth weight over 4,000 g). Except for neonatal injury (100%), no single maternal or fetal variable appears with greater frequency.
The mean indemnity in closed cases increased in direct proportion to fetal weight, ranging from $500,000 in cases involving infants whose birth weight was less than 4,000 g to $950,000 when the birth weight was 5,000 g or more.
Maternal factors seen with greatest frequency included obesity, excessive weight gain in pregnancy, and gestational diabetes. Analysis of prepregnant body mass index found that 19 women had a weight within the “obese” category, 18 of whom gave birth to macrosomic infants. Eight of these cases closed without indemnity payment and 10 closed with an aggregate indemnity payment of $6.5 million.
Forty-eight of the 61 plaintiffs (78.8%) exceeded the normal weight gain in pregnancy based on height and prepregnant weight. Twenty-nine of these cases closed with an aggregate indemnity payment of $16.6 million.
Influence of diabetes. Fifty-two of 61 plaintiffs underwent a glucose screening test. Of these, 23 went on to have a glucose tolerance test, with 12 testing positive for gestational diabetes. Further analysis revealed borderline screening glucose values in an additional 16 cases. These women were not considered diabetic by their obstetricians, were not retested for diabetes, and did not receive nutritional counseling. Macrosomic infants were born to 9 of the 12 patients with gestational diabetes and to 13 of the 16 borderline cases.
Of 28 cases with confirmed or suspected gestational diabetes, 14 women delivered infants weighing over 4,250 g; 11 of the 14 weighed more than 4,500 g.
In a comparison of cases involving diabetic and nondiabetic women who delivered infants weighing more than 4,250 g, 82% of the cases involving nondiabetic women closed without payment. Among cases involving diabetes (actual or borderline), the corresponding figure was 27.3%.
Labor and delivery interventions were cited in all cases. Oxytocin was used in 42 of the 61 cases (68.9%), forceps in 9 (14.8%), and vacuum extraction in 7 (11.5%). Suprapubic pressure was used in 37 cases (60.7%), fundal pressure in 9 (14.8%), and traction on the fetal head in 16 cases (26.2%).
In addition, defense experts determined that the McRoberts maneuver was used in 41 cases (67.2%) and the Wood’s corkscrew maneuver in 28 (45.9%).
Seven cases involved a second stage of labor exceeding 2.5 hours. The ratio of wins to losses decreased substantially with the use of oxytocin, forceps, fundal pressure, or a prolonged second stage.
Data were analyzed using selected variables thought to have an association with winning or losing cases and with indemnity (TABLE 2). No statistically significant models emerged. This is likely due to inadequate power (low number of cases) and the large number of interactions between variables relative to the outcomes evaluated.
TABLE 1
Plaintiff demographics
| CHARACTERISTIC | ALL CASES (n = 61) | CASES WITH INDEMNITY (n = 35) |
|---|---|---|
| Mean age, in years (range) | 28 (17–40) | 29 (18–38) |
| Race (%) | ||
| White | 35 (57) | 21 (60) |
| Black | 12 (20) | 7 (20) |
| Hispanic | 11 (18) | 6 (17) |
| Asian | 1 (2) | — |
| Unknown | 2 (3) | 1 (3) |
| Parity (%) | ||
| 0 | 19 (31) | 11 (31) |
| 1 | 26 (43) | 16 (46) |
| 2 | 11 (18) | 7 (20) |
| 3 | 2 (3) | 1 (3) |
| 4 | 2 (3) | — |
| 5 | 1 (2) | — |
TABLE 2
Litigation outcomes for selected prenatal and intrapartum variables
| CHARACTERISTIC | CLOSED WITHOUT INDEMNITY | CLOSED WITHOUT INDEMNITY PAYMENT | MEAN INDEMNITY ($) | TOTAL INDEMNITY ($) |
|---|---|---|---|---|
| Prenatal factors | ||||
| Gestational diabetes | 5 | 7 | 413,300 | 2,893,000 |
| Adjusted diabetes | 10 | 18 | 521,400 | 9,386,000 |
| Obesity | 8 | 13 | 651,300 | 8,466,000 |
| Intrapartum factors | ||||
| Prolonged second stage | 1 | 6 | 707,700 | 4,247,000 |
| Oxytocin induction | 2 | 10 | 406,500 | 4,065,300 |
| Oxytocin augmentation | 15 | 15 | 737,000 | 11,100,000 |
| Forceps delivery | 1 | 8 | 552,100 | 4,416,800 |
| Vacuum extraction | 3 | 4 | 531,300 | 2,125,000 |
| Episiotomy | 20 | 30 | 579,400 | 17,382,100 |
| McRoberts maneuver | 20 | 21 | 542,900 | 11,400,300 |
| Wood’s corkscrew maneuver | 12 | 16 | 652,300 | 10,436,200 |
| Suprapubic pressure | 17 | 20 | 629,000 | 12,579,400 |
| Traction to fetal head | 7 | 9 | 665,500 | 5,989,500 |
| Fundal pressure | 4 | 7 | 660,700 | 4,625,000 |
4 factors raise risk of litigation
After reviewing the literature and analyzing the ProMutual data, we concluded that shoulder dystocia remains largely unpredictable. However, certain clinical factors are clearly associated with an increased risk for litigation:
- prenatal factors,
- labor and delivery interventions,
- maneuvers performed at the time of the dystocia, and
- fetal outcomes.
Prenatal factors. The most significant prenatal factors were maternal obesity, excessive weight gain in pregnancy, and, especially, diabetes and fetal macrosomia.
• Fetal macrosomia. Although most macrosomic infants deliver without complication, fetal macrosomia was involved in more than 72% of the cases reviewed. Because macrosomia represents a risk not only for shoulder dystocia but also for the litigation that may arise from it, it is important to:
- know and document the estimated fetal weight (EFW);
- discuss the risk of dystocia and its sequelae with the mother and her partner; and
- consider cesarean delivery for estimated fetal weights that suggest macrosomia (see the recommendations on page 82 for specifics).
It also is advisable to mobilize a team for the possibility of shoulder dystocia if vaginal delivery is attempted.
Preparing for the increased risk of a macrosomic fetus can be successful only if macrosomia is both diagnosed and anticipated.
Because macrosomia often is accompanied by maternal diabetes, serum glucose testing is recommended for all gravidas, with follow-up of both abnormal and borderline values.
Labor and delivery interventions were cited in all cases, with data indicating a decreasing ratio of wins to losses with the use of oxytocin, forceps, or fundal pressure, and prolonged second stage.
- Use of oxytocin to augment an already established labor carried less risk than oxytocin for labor induction. Ten of the 12 cases (83.3%) in which oxytocin was used for induction closed with an indemnity payment. However, of the 30 cases in which oxytocin was used for labor augmentation, 50% closed with payment.
- • Forceps and a prolonged second stage. Eight of 9 cases (88.9%) involving forceps and 6 of 7 cases (85.7%) involving a prolonged second stage closed with indemnity payment.
Consider cesarean delivery when the second stage is prolonged or labor fails to progress. Be cautious using forceps and vacuum extraction in these circumstances, and limit the number of attempts with either.
Maneuvers performed at the time of dystocia. The most common maneuverswere McRoberts, suprapubic pressure, and Wood’s corkscrew. The ratio of wins to losses decreased with traction of the fetal head and use of fundal pressure.
Part of the risk-management protocol for obstetricians should be appropriate use of McRoberts maneuver, suprapubic pressure, and Wood’s corkscrew, and cutting a large episiotomy. In addition, be careful not to push, pull, rotate the head, or apply fundal pressure.
Fetal outcomes. All cases involved neonatal injury (Erb’s palsy, fractured humerus) or death.
For this reason, we recommend an action plan that includes immediate pediatric or neonatal assessment of neuromuscular function of the infant’s anterior shoulder. Assess the Moro reflex and the possibility of brachial plexus injury and fractures of the clavicle and humerus. Also examine the placenta, send it to pathology, and perform a cord blood gas analysis.
Last words
Shoulder dystocia is the unfortunate complication of a small number of deliveries, but the focus of an increasing number of lawsuits. Because the neonatal injuries that so often accompany shoulder dystocia often lead to litigation, obstetricians should prepare to identify risk and help patients make informed choices. We should be prepared to manage this emergency whenever it occurs and thoroughly document actions.
Dr. Zylstra reports no financial relationships relevant to this article.
Clip-and-save shoulder dystocia documentation form
Practice recommendations
Among the intrapartum events that constitute bona fide emergencies, shoulder dystocia stands out. This obstetric emergency is the focus of an increasing number of medical liability cases. Most lawsuits involving shoulder dystocia allege negligence as the cause of the brachial plexus injury, fractured clavicle or humerus, or other injury. The defendant physicians named in these suits are often accused of inappropriately managing the prenatal or intrapartum course or the dystocia itself—or of inadequately documenting the steps taken to resolve the emergency.
To glean insights into the litigation process as it involves shoulder dystocia, we retrospectively reviewed all cases closed by the Boston-based ProMutual Group, a major liability insurance carrier, over a 7-year period. We wanted to learn more about the plaintiffs themselves, as well as the clinical and medicolegal factors that led to jury awards or indemnity payments. We also wanted data that could serve as the foundation for guidelines on how to proceed in the event of shoulder dystocia, as well as a documentation tool.
This shoulder dystocia case from an insurer’s closed claim file illustrates a problem often linked to litigation. Minor changes were made to conceal the identities of the involved parties.
Nurse and physician document different times
A 31-year-old woman in her 10th week of pregnancy had one prior uncomplicated vaginal delivery of a 9 lb 7 oz infant. Her prenatal course proceeds unremarkably, with a normal glucose tolerance test and total weight gain of 36 lb. At 41 weeks and 2 days, the estimated fetal weight is documented as 4,120 g. Labor is induced with oxytocin. Because of maternal fatigue, vacuum delivery is attempted.
Notes of the physician and the nurse differ regarding the time of the first of 3 vacuum applications.
After delivery of the head, shoulder dystocia is encountered. In a note handwritten immediately after delivery, the physician states that the head was “reconstituted as right occiput anterior with the left shoulder anterior.”
In a note dictated later, however, the same physician states the right shoulder was anterior.
Help is summoned and arrives 20 minutes after the dystocia is first encountered. The time that help was summoned is in question since there is an 18-minute discrepancy between the times the physician and the nurse note that assistance was called.
Despite the use of suprapubic pressure and maneuvers including McRoberts and Wood’s corkscrew, shoulder dystocia persists for 24 minutes. Apgars of the 11 lb 3 oz infant are 0, 1, and 3. The child is resuscitated but dies within 2 days of birth.
Outcome
Settled with a 7-figure indemnity payment.
What the defense experts said
The key issues involve documentation and summoning assistance. Discrepancies in documentation almost always cast doubt upon the credibility of a defendant. Ideally, there should be no discrepancies between nurse and physician notes and, certainly, no discrepancies between 2 notes on the same case by the same physician. If, in this case, the physician realized after writing the first note that the anterior shoulder had been incorrectly identified, a correction should have been written as a separate note.
Use of the shoulder dystocia documentation tool (see) helps create a chronology of events, which may prove vital to a successful defense.
The call for help might not have been delayed if the labor and delivery unit had had a shoulder dystocia protocol including “drills” for all team members. Help should be called as soon as a shoulder dystocia is encountered so that, when needed, it is available. Under no circumstances should it take 20 minutes for assistance to arrive.
Brachial plexus injury not always caused by shoulder dystocia
Between 21% and 42% of shoulder dystocias involve an injury1—usually brachial plexus injury. Plaintiff attorneys have manipulated this fact to attribute many cases of neonatal brachial plexus injury to mismanagement of shoulder dystocia by the obstetrician.
They fault the physician for failing to estimate fetal weight, perform a timely cesarean, use appropriate maneuvers correctly, or have a pediatrician present. They criticize nothing more resoundingly than use of “inappropriate” or “excessive” lateral traction to the fetal head.2
Nontraction injuries. The reality can be strikingly different, however. Some cases of brachial plexus injury involve no traction at all.
- Brachial plexus injuries have been reported in infants who had precipitate vaginal deliveries without any physical intervention by the obstetrician.2
- These injuries also have occurred in infants delivered via cesarean section.1,3,4
- In some cases, brachial plexus injuries have affected the posterior arm of neonates whose anterior arm was involved in shoulder dystocia.1,2,5-7
A retrospective study4 found that, of 39 cases of brachial plexus injury, only 17 were associated with shoulder dystocia. Similar findings have emerged from other studies.2,3,8
Other causes. It is unclear how brachial plexus injuries occur in the absence of shoulder dystocia. Some think they arise in response to infectious agents such as toxoplasmosis, coxsackievirus, mumps, pertussis, or mycoplasma pneumonia.2 Some assume a mechanical cause, such as fetal response to longstanding abnormal intrauterine pressure exerted by maternal conditions such as bicornate uterus and uterine fibroids, especially in the lower segment.1,2
When brachial plexus injuries occur in the absence of shoulder dystocia, they likely originated before labor and delivery.4 Some experts suggest serial electromyelograms within the first 7 days of life to establish a prenatal rather than intrapartum etiology. A positive electromyelogram within 1 week of birth would suggest antepartum causation.2,9
Recognizing risk factors for shoulder dystocia best way to reduce injury
Most brachial plexus injuries or impairments are associated with shoulder dystocia,9 and shoulder dystocia is the most common way brachial plexus injuries are introduced into litigation.
Decreasing the number of brachial plexusrelated liability cases, therefore, depends on decreasing the incidence of shoulder dystocia. Unfortunately, a failsafe method continues to elude both clinicians and researchers.10-12
Retrospective studies have identified certain factors that may—but do not necessarily—increase the risk of shoulder dystocia.
Prenatal risk factors include high maternal or paternal birth weight, maternal obesity, excessive weight gain during pregnancy, advanced age, short stature, multiparity, postdates, prior shoulder dystocia, small pelvis, prior delivery of a macrosomic infant, gestational diabetes in an earlier pregnancy, abnormal blood sugars in the current pregnancy, or fetal macrosomia.13,14-16
Intrapartum risk factors include a rapid or prolonged second stage, failure or arrest of descent, presence of considerable molding, and need for a midpelvic delivery.10,15
Predictability. Prospective studies have not established the predictability of shoulder dystocia. A 2000 study17 showed that 55% of cases with 1 or more risk factors experienced shoulder dystocia. Predictability increases somewhat when both maternal diabetes and fetal macrosomia complicate pregnancy, since the rate of shoulder dystocia in women with diabetes is consistently higher than in nondiabetic gravidas. This becomes a significant issue when the infant weighs more than 4,000 g.
Indications for prophylactic cesarean in women with diabetes
In 1999, Wagner et al9 found that 70% of shoulder dystocias in women with diabetes occurred when the fetal weight exceeded 4,000 g. They concluded that cesarean delivery for infants with an estimated weight over 4,250 g would reduce the rate of shoulder dystocia by 75% and increase the cesarean delivery rate by 1%.
Others are more conservative. Gross and colleagues11 suggested that, for every additional 26 cesarean deliveries, only 1 case of shoulder dystocia would be prevented.
Macrosomia. Most obstetricians and researchers still do not advocate prophylactic cesarean delivery for macrosomia alone because, by some estimates, 98% of macrosomic infants are delivered without difficulty.18 However, they do suggest that obstetricians at least consider the possibility of cesarean delivery for a macrosomic fetus when the woman has diabetes.
In a study completed in 2000, Skolbekken19 suggested a cutoff of 4,250 g for women with diabetes. Dildy20 suggested limits of more than 4,500 g for diabetic women and more than 5,000 g for nondiabetic gravidas. However, Conway and Langer21 assert that a cutoff of 4,500 g is too liberal for women with diabetes and maintain that, at this cutoff, 40% of shoulder dystocias would not be prevented.
• Ultrasound measurements. Since estimates of fetal weight have a margin of error approaching 40%,9 others have chosen different parameters for determining fetal macrosomia in women with diabetes. In a retrospective study involving 31 women with gestational diabetes, Cohen et al22 found that subtracting the fetal biparietal diameter from the abdominal diameter—with both measurements obtained via ultrasound—yields a predictability score higher than estimated fetal weight. Specifically, if the difference between the 2 measurements is 2.6 cm or more, the rate of shoulder dystocia is high enough to warrant elective cesarean (FIGURE ).
FIGURE Using ultrasound measurements to predict macrosomia
A simple way to predict fetal macrosomia in women with diabetes is to subtract the fetal biparietal diameter (9.3 cm in the scan at left) from the abdominal diameter (average of 12.44 cm in the scan at right). If the difference exceeds 2.6 cm, elective cesarean is warranted. In this case it is 3.14 cm, indicating elective cesarean is warranted.
When dystocia occurs, have a plan and stick to it
Shoulder dystocia immediately places both mother and neonate at risk for temporary or permanent injury. Thus, it is imperative that all obstetricians and other health-care providers who deliver infants have a well developed plan of action for this emergency. They should immediately ask for obstetric assistance and instruct the mother to discontinue any pushing.
Attempts at vigorous downward traction should be avoided, and no fundal pressure should be applied, as these are known to increase the potential for brachial plexus injury. Gentle downward traction is considered the standard of care.17
The obstetrician’s goal is to free the impacted shoulder as quickly as possible, since a fetus can endure only 8 to 10 minutes of asphyxia before permanent neurologic damage occurs.17 The standard of care requires the obstetrician to know and use certain maneuvers to relieve shoulder dystocia. These maneuvers are designed to facilitate vaginal delivery and reduce the risk of permanent brachial plexus injury. The McRoberts maneuver, with flexion and slight rotation of the maternal hips onto the maternal abdomen, is the standard for initial relief of shoulder dystocia.17,23
This shoulder dystocia case from an insurer’s closed claim file illustrates a problem often linked to litigation. Minor changes were made to conceal the identities of the involved parties.
Prompt maneuvers, good outcome
A 28-year-old gravida weighing 214 lb has had 2 previous spontaneous deliveries of infants weighing 8 lb 5 oz and 9 lb 3 oz. Except for a weight gain of more than 60 lb, the pregnancy progressed without complications, and a 3-hour glucose tolerance test was normal. At 40 weeks, the obstetrician notes “concern” about an estimated fetal weight of 10 lb. Induction is planned, but spontaneous labor begins before oxytocin can be given. After 5 hours, the head is delivered without difficulty, but shoulder dystocia follows. The obstetrician extends the episiotomy and performs McRoberts and Wood’s corkscrew maneuvers, but the dystocia persists. Upon noting cyanosis, the obstetrician fractures the infant’s clavicle and quickly delivers a 10 lb 9 oz infant with Apgar scores of 8 and 10. Pediatricians examine the child immediately and diagnose Erb’s palsy, which subsequently resolves. X-rays confirm an undisplaced fracture of the right clavicle. Although the child recovers completely, the family sues, alleging a failure to perform cesarean delivery.
Outcome
Case closed with no payment.
What the defense experts said
Key issues are documentation and informed consent. The overriding opinion of 3 experts who reviewed the case for the defense was that cesarean delivery was not indicated and that in fracturing the clavicle, the physician acted responsibly, quickly, and within the standard of care. One defense expert said failure to document exact maneuvers used to relieve shoulder dystocia deviated from the standard of care. Another defense expert said the physician should have obtained informed consent from this at-risk patient, and explained the risks of shoulder dystocia, including neonatal injury, so that the she might have been better able to appreciate the fact that the obstetrician’s fast action may have saved her child from brain damage or death.
Factors that lead to litigation
In a review article, Hickson24 cited factors that prompted families to file medical liability claims following perinatal injury. Some families observed that, in their search for the cause of an injury, they found 1 or more aspects of care to be inappropriate.
The desire for information, perception of being misled, anger with the medical profession, desire to prevent injuries to others, recognition of long-term sequelae, and advice by knowledgeable acquaintances, as well as the need for money, all appeared to contribute to the decision to file medical liability claims.
Convey the risks, and listen carefully. Communication problems between physicians and patients are a contributing factor. Even when physicians provide technically adequate care, families expect answers to their questions and want to feel as though they have been consulted about important medical decisions.24 If these expectations are not met, even patients who have not experienced an adverse outcome may become angry and express dissatisfaction with care.24
The need for communication is critical when shoulder dystocia results in neonatal injury. Empathizing with the family, helping them understand that most brachial plexus injuries are not long-term, and offering to answer their questions both at the moment and later, may help prevent litigation.
This type of communication can be difficult. It helps to realize that an acknowledgment of distress and concern is not an admission of guilt, and an explanation is not an apology.15 However, an absence of communication or an attempt by the physician to place blame may be perceived as an admission of guilt that gives rise to a lawsuit.
Action plan is what counts in court. A review by Gross et al11 concluded that obstetricians should have a shoulder dystocia plan that enables an instant and orderly response. Also recommended is a protocol to help anticipate clinical problems and prevent medicolegal problems.
Gross and colleagues11 found that the Ob/Gyns with the most defensible cases paid close attention to the patient’s history and prenatal course and, upon encountering dystocia, implemented at least 2 maneuvers (if necessary) and thoroughly documented their actions immediately after delivery.
Fetterman15 agreed, asserting that what counts in court is not so much whether the obstetrician employed the McRoberts maneuver before or after the Wood’s corkscrew maneuver but whether he or she had an action plan in mind, implemented that plan properly, and thoroughly documented the actions taken and the reasons underlying them.
Dissecting legal cases for clues to reduced risk
In the review of medicolegal cases for this article, we limited our search to cases closed between Jan 1, 1995, and Dec 31, 2002, using a computer to search for codes specific to shoulder dystocia as well as the phrases “shoulder dystocia,” “Erb’s palsy,” and “brachial plexus injury.” We identified 61 cases involving 117 defendants and created a data sheet to gather information on patient and physician demographics, medical and obstetric history, description of the incident, analysis rendered by defense and plaintiff experts, and legal and financial outcomes.
Of 117 defendants, 76 were obstetricians. There also were 16 hospitals, 15 corporations, 5 certified nurse-midwives, 1 family physician, 1 emergency physician, and 3 persons categorized as “other.” Age, race, and parity of the 61 plaintiffs are given in TABLE 1.
Twenty-six of the 61 cases, involving 74 defendants, closed with no payment. That is, they were either dismissed or closed with a jury verdict for the defense. The remaining 35 cases involved 43 defendants and were closed with an aggregate indemnity payment of $19.2 million. The mean payment was $445,000 per defendant.
These guidelines are recommended to help prevent, predict, and manage shoulder dystocia and brachial plexus injury.
Obtain a prenatal history that includes the birth weights of both parents and any history of prior shoulder dystocia or cesarean delivery performed for “failure to progress.”
Estimate the fetal weight and take into account the risk for shoulder dystocia when the fetus is determined by ultrasound to be macrosomic.
Perform glucose testing on all patients, and follow up on even a single abnormal glucose reading. Discuss the possibility of shoulder dystocia and the accompanying risk of neonatal injury with “at risk” patients.
Consider obtaining informed consent for vaginal delivery of a patient with risk factors for shoulder dystocia.
Consider cesarean delivery for :
- nondiabetic women when the estimated fetal weight (EFW) exceeds 4,500 g,
- diabetic gravidas when the EFW exceeds 4,000 g,
- women with a prior delivery complicated by shoulder dystocia or brachial plexus palsy,
- gravidas with a prolonged second stage and nonprogression of labor, and
- patients who express fear and doubt about vaginal delivery.
Be sparing with the use of oxytocin when the fetus is known or suspected to be macrosomic, taking special care not to be aggressive with induction.
Use forceps or vacuum extraction with caution, and limit the number of attempts with each.
Minimize traction on the fetal head. Traction that is deemed to be “excessive” may be used against the physician in a liability suit. Gentle traction is acceptable.
Do not use or order fundal pressure. It will almost invariably be used against you in a lawsuit.
Be able to define and correctly describe maneuvers generally accepted as the standard for shoulder dystocia and, when necessary, use and document them appropriately. These include the McRoberts, Wood’s corkscrew, Rubin, and Zavanelli maneuvers; extended episiotomy; suprapubic pressure; and fracture of the anterior clavicle.
Be alert to the possibility of brachial plexus injury in the absence of shoulder dystocia. Obstetricians have been erroneously accused of causing brachial plexus injury by plaintiff attorneys who do not understand that this injury is not always the result of dystocia. Thorough contemporaneous documentation is key in these instances.
Request immediate pediatric assessment of a newborn involved in shoulder dystocia. Have the placenta sent for examination, and request cord blood gases.
Communicate openly and honestly with the parents of a child who has suffered a brachial plexus injury. This may be the single greatest tool for reducing the risk of liability litigation.
Consider serial electromyelograms during the first 7 days of life for a neonate with a brachial plexus palsy. These studies can help determine the etiology of the injury.
Use a shoulder dystocia documentation tool such as the one on page 91. Thorough documentation of all relevant prenatal and intrapartum events is critical to a successful defense. A 12-point detailed delivery note as recommended by Fetterman will also prevent or reduce legal risk.15
Schedule shoulder dystocia “drills” in the labor and delivery unit to familiarize obstetric team members with their roles.
Require comanagement of any midwifery patient at increased risk for shoulder dystocia.
Neonatal injury occurred in all 61 cases. Erb’s palsy was the overwhelming pediatric outcome (57 of 61 cases, or 93.4%), with an aggregate indemnity payment of $17.6 million. Fractured humerus was the outcome in 1 case, and 3 cases involved neonatal deaths.
Reasons for indemnity payments included:
- probable liability (18 defendants),
- plaintiff was sympathetic, likely to evoke an emotional response from the jury (8 defendants),
- clear liability (7 defendants),
- defendant would not have made a strong witness in his or her own defense (5 defendants),
- defendant had died or was too ill to stand trial (3 defendants),
- medical record had been altered (2 defendants),
- case was considered too inflammatory to risk a jury award (2 defendants), and
- policy limits were too low to risk a potentially high jury award (1 defendant).
Five defendants were involved in cases with multiple medicolegal issues that argued for settlement.
The effect of birth weight. Notably, 74% of infants involved in these cases were macrosomic (birth weight over 4,000 g). Except for neonatal injury (100%), no single maternal or fetal variable appears with greater frequency.
The mean indemnity in closed cases increased in direct proportion to fetal weight, ranging from $500,000 in cases involving infants whose birth weight was less than 4,000 g to $950,000 when the birth weight was 5,000 g or more.
Maternal factors seen with greatest frequency included obesity, excessive weight gain in pregnancy, and gestational diabetes. Analysis of prepregnant body mass index found that 19 women had a weight within the “obese” category, 18 of whom gave birth to macrosomic infants. Eight of these cases closed without indemnity payment and 10 closed with an aggregate indemnity payment of $6.5 million.
Forty-eight of the 61 plaintiffs (78.8%) exceeded the normal weight gain in pregnancy based on height and prepregnant weight. Twenty-nine of these cases closed with an aggregate indemnity payment of $16.6 million.
Influence of diabetes. Fifty-two of 61 plaintiffs underwent a glucose screening test. Of these, 23 went on to have a glucose tolerance test, with 12 testing positive for gestational diabetes. Further analysis revealed borderline screening glucose values in an additional 16 cases. These women were not considered diabetic by their obstetricians, were not retested for diabetes, and did not receive nutritional counseling. Macrosomic infants were born to 9 of the 12 patients with gestational diabetes and to 13 of the 16 borderline cases.
Of 28 cases with confirmed or suspected gestational diabetes, 14 women delivered infants weighing over 4,250 g; 11 of the 14 weighed more than 4,500 g.
In a comparison of cases involving diabetic and nondiabetic women who delivered infants weighing more than 4,250 g, 82% of the cases involving nondiabetic women closed without payment. Among cases involving diabetes (actual or borderline), the corresponding figure was 27.3%.
Labor and delivery interventions were cited in all cases. Oxytocin was used in 42 of the 61 cases (68.9%), forceps in 9 (14.8%), and vacuum extraction in 7 (11.5%). Suprapubic pressure was used in 37 cases (60.7%), fundal pressure in 9 (14.8%), and traction on the fetal head in 16 cases (26.2%).
In addition, defense experts determined that the McRoberts maneuver was used in 41 cases (67.2%) and the Wood’s corkscrew maneuver in 28 (45.9%).
Seven cases involved a second stage of labor exceeding 2.5 hours. The ratio of wins to losses decreased substantially with the use of oxytocin, forceps, fundal pressure, or a prolonged second stage.
Data were analyzed using selected variables thought to have an association with winning or losing cases and with indemnity (TABLE 2). No statistically significant models emerged. This is likely due to inadequate power (low number of cases) and the large number of interactions between variables relative to the outcomes evaluated.
TABLE 1
Plaintiff demographics
| CHARACTERISTIC | ALL CASES (n = 61) | CASES WITH INDEMNITY (n = 35) |
|---|---|---|
| Mean age, in years (range) | 28 (17–40) | 29 (18–38) |
| Race (%) | ||
| White | 35 (57) | 21 (60) |
| Black | 12 (20) | 7 (20) |
| Hispanic | 11 (18) | 6 (17) |
| Asian | 1 (2) | — |
| Unknown | 2 (3) | 1 (3) |
| Parity (%) | ||
| 0 | 19 (31) | 11 (31) |
| 1 | 26 (43) | 16 (46) |
| 2 | 11 (18) | 7 (20) |
| 3 | 2 (3) | 1 (3) |
| 4 | 2 (3) | — |
| 5 | 1 (2) | — |
TABLE 2
Litigation outcomes for selected prenatal and intrapartum variables
| CHARACTERISTIC | CLOSED WITHOUT INDEMNITY | CLOSED WITHOUT INDEMNITY PAYMENT | MEAN INDEMNITY ($) | TOTAL INDEMNITY ($) |
|---|---|---|---|---|
| Prenatal factors | ||||
| Gestational diabetes | 5 | 7 | 413,300 | 2,893,000 |
| Adjusted diabetes | 10 | 18 | 521,400 | 9,386,000 |
| Obesity | 8 | 13 | 651,300 | 8,466,000 |
| Intrapartum factors | ||||
| Prolonged second stage | 1 | 6 | 707,700 | 4,247,000 |
| Oxytocin induction | 2 | 10 | 406,500 | 4,065,300 |
| Oxytocin augmentation | 15 | 15 | 737,000 | 11,100,000 |
| Forceps delivery | 1 | 8 | 552,100 | 4,416,800 |
| Vacuum extraction | 3 | 4 | 531,300 | 2,125,000 |
| Episiotomy | 20 | 30 | 579,400 | 17,382,100 |
| McRoberts maneuver | 20 | 21 | 542,900 | 11,400,300 |
| Wood’s corkscrew maneuver | 12 | 16 | 652,300 | 10,436,200 |
| Suprapubic pressure | 17 | 20 | 629,000 | 12,579,400 |
| Traction to fetal head | 7 | 9 | 665,500 | 5,989,500 |
| Fundal pressure | 4 | 7 | 660,700 | 4,625,000 |
4 factors raise risk of litigation
After reviewing the literature and analyzing the ProMutual data, we concluded that shoulder dystocia remains largely unpredictable. However, certain clinical factors are clearly associated with an increased risk for litigation:
- prenatal factors,
- labor and delivery interventions,
- maneuvers performed at the time of the dystocia, and
- fetal outcomes.
Prenatal factors. The most significant prenatal factors were maternal obesity, excessive weight gain in pregnancy, and, especially, diabetes and fetal macrosomia.
• Fetal macrosomia. Although most macrosomic infants deliver without complication, fetal macrosomia was involved in more than 72% of the cases reviewed. Because macrosomia represents a risk not only for shoulder dystocia but also for the litigation that may arise from it, it is important to:
- know and document the estimated fetal weight (EFW);
- discuss the risk of dystocia and its sequelae with the mother and her partner; and
- consider cesarean delivery for estimated fetal weights that suggest macrosomia (see the recommendations on page 82 for specifics).
It also is advisable to mobilize a team for the possibility of shoulder dystocia if vaginal delivery is attempted.
Preparing for the increased risk of a macrosomic fetus can be successful only if macrosomia is both diagnosed and anticipated.
Because macrosomia often is accompanied by maternal diabetes, serum glucose testing is recommended for all gravidas, with follow-up of both abnormal and borderline values.
Labor and delivery interventions were cited in all cases, with data indicating a decreasing ratio of wins to losses with the use of oxytocin, forceps, or fundal pressure, and prolonged second stage.
- Use of oxytocin to augment an already established labor carried less risk than oxytocin for labor induction. Ten of the 12 cases (83.3%) in which oxytocin was used for induction closed with an indemnity payment. However, of the 30 cases in which oxytocin was used for labor augmentation, 50% closed with payment.
- • Forceps and a prolonged second stage. Eight of 9 cases (88.9%) involving forceps and 6 of 7 cases (85.7%) involving a prolonged second stage closed with indemnity payment.
Consider cesarean delivery when the second stage is prolonged or labor fails to progress. Be cautious using forceps and vacuum extraction in these circumstances, and limit the number of attempts with either.
Maneuvers performed at the time of dystocia. The most common maneuverswere McRoberts, suprapubic pressure, and Wood’s corkscrew. The ratio of wins to losses decreased with traction of the fetal head and use of fundal pressure.
Part of the risk-management protocol for obstetricians should be appropriate use of McRoberts maneuver, suprapubic pressure, and Wood’s corkscrew, and cutting a large episiotomy. In addition, be careful not to push, pull, rotate the head, or apply fundal pressure.
Fetal outcomes. All cases involved neonatal injury (Erb’s palsy, fractured humerus) or death.
For this reason, we recommend an action plan that includes immediate pediatric or neonatal assessment of neuromuscular function of the infant’s anterior shoulder. Assess the Moro reflex and the possibility of brachial plexus injury and fractures of the clavicle and humerus. Also examine the placenta, send it to pathology, and perform a cord blood gas analysis.
Last words
Shoulder dystocia is the unfortunate complication of a small number of deliveries, but the focus of an increasing number of lawsuits. Because the neonatal injuries that so often accompany shoulder dystocia often lead to litigation, obstetricians should prepare to identify risk and help patients make informed choices. We should be prepared to manage this emergency whenever it occurs and thoroughly document actions.
Dr. Zylstra reports no financial relationships relevant to this article.
1. Gherman RB, Goodwin TM, Ouzounian JG, Miller DA, Paul RH. Brachial plexus palsy associated with cesarean section: an in utero injury? Am J Obstet Gynecol. 1997;177:1162-1164.
2. Gherman RB, Ouzounian JG, Goodwin TM. Brachial plexus palsy: an in utero injury? Am J Obstet Gynecol. 1999;180:1303-1307.
3. Gilbert WM, Nesbitt TS, Danielsen B. Associated factors in 1611 cases of brachial plexus injury. Obstet Gynecol. 1999;93:536-540.
4. Jennett RJ, Tarby TJ, Kreinick CJ. Brachial plexus palsy: an old problem revisited. Am J Obstet Gynecol. 1992;166:1673-1677.
5. Hankins GDV, Clark SL. Brachial plexus palsy involving the posterior shoulder at spontaneous vaginal delivery. Am J Perinatol. 1995;12:44-45.
6. Gherman RB, Ouzounian JG, Miller DA, Kwok L, Goodwin TM. Spontaneous vaginal delivery: a risk factor for Erb’s palsy? Am J Obstet Gynecol. 1998;178:423-427.
7. Dodds SD, Wolfe SW. Perinatal brachial plexus palsy. Curr Opin Pediatrics. 2000;23:40-47.
8. Wade DM. A Comment on the Trial of Brachial Plexus/Erb’s Palsy Medical Malpractice Cases. Evidence Technologies, Inc. 1994.
9. Wagner RK, Nielsen PE, Gonik B. Controversies in labor management: shoulder dystocia. Obstet Gynecol Clin. 1999;26(2):371-382.
10. McFarland M, Hod M, Piper JM, Xenakis EM-J, Langer O. Are labor abnormalities more common in shoulder dystocia? Am J ObstetGynecol. 1995;173:1211-1214.
11. Gross TL, Sokol RJ, Williams T, Thompson K. Shoulder dystocia: A fetal-physician risk. Am J Obstet Gynecol. 1987;156:1408-1418.
12. Acker DB, Sachs BP, Friedman EA. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66:762-768.
13. Shoulder dystocia and Erb’s palsy. The Keenan Law Firm. 2002. Available at www.shoulderdystociaattorney.com. Accessed July 23, 2004.
14. Acker DB, Gregory KD, Sachs BP, Friedman EA. Risk factors for Erb-Duchenne palsy. Obstet Gynecol. 1988;71(Part l):390-392.
15. Fetterman HH. Cutting the legal risks of shoulder dystocia. OBG Management. March 1995;41-51.
16. Iffy L, Varadi V, Jakobovits A. Common intrapartum denominators of shoulder dystocia related birth injuries. Zentralbl Gynakol. 1994;116(1):33-37.
17. Calhoun BC, Hume RR, Walters JL. Shoulder Dystocia as a risk for obstetric liability. Legal Medicine. 2000;12-15.
18. Jennett RJ, Tarby TJ. Brachial plexus palsy: an old problem revisited again. Am J Obstet Gynecol. 1997;176:1354-1357.
19. Skolbekken JA. Shoulder dystocia—malpractice or acceptable risk? Acta Obstet Gynecol Scand. 2000;79:750-756.
20. Dildy GA. Fetal macrosomia [slide presentation]. Presented at the 18th Annual Conference on Obstetrics, Gynecology, Perinatal Medicine, and the Law, Boston University School of Medicine and the Center for Human Genetics, January 2002, Kauai, Hawaii.
21. Conway D, Langer O. Elective delivery of infants with macrosomia in diabetic women: reduced shoulder dystocia versus increased cesarean deliveries. Am J Obstet Gynecol. 1998;178(5):922-925.
22. Cohen B, Penning S, Major C, Ansley D, Porto M, Garite T. Sonographic prediction of shoulder dystocia in infants of diabetic mothers. Obstet Gynecol. 1998;88:10-13.
23. Gherman RB, Goodwin TM, Souter I, Neumann K, Ouzounian JG, Paul RH. The McRoberts’ maneuver for the alleviation of shoulder dystocia: how successful is it? Am J Obstet Gynecol. 1997;176:656-661.
24. Hickson GB, Clayton DW, Githens PB, Sloan FA. Factors that prompted families to file medical malpractice claims following perinatal injuries. JAMA. 1992;267:1359-1363.
1. Gherman RB, Goodwin TM, Ouzounian JG, Miller DA, Paul RH. Brachial plexus palsy associated with cesarean section: an in utero injury? Am J Obstet Gynecol. 1997;177:1162-1164.
2. Gherman RB, Ouzounian JG, Goodwin TM. Brachial plexus palsy: an in utero injury? Am J Obstet Gynecol. 1999;180:1303-1307.
3. Gilbert WM, Nesbitt TS, Danielsen B. Associated factors in 1611 cases of brachial plexus injury. Obstet Gynecol. 1999;93:536-540.
4. Jennett RJ, Tarby TJ, Kreinick CJ. Brachial plexus palsy: an old problem revisited. Am J Obstet Gynecol. 1992;166:1673-1677.
5. Hankins GDV, Clark SL. Brachial plexus palsy involving the posterior shoulder at spontaneous vaginal delivery. Am J Perinatol. 1995;12:44-45.
6. Gherman RB, Ouzounian JG, Miller DA, Kwok L, Goodwin TM. Spontaneous vaginal delivery: a risk factor for Erb’s palsy? Am J Obstet Gynecol. 1998;178:423-427.
7. Dodds SD, Wolfe SW. Perinatal brachial plexus palsy. Curr Opin Pediatrics. 2000;23:40-47.
8. Wade DM. A Comment on the Trial of Brachial Plexus/Erb’s Palsy Medical Malpractice Cases. Evidence Technologies, Inc. 1994.
9. Wagner RK, Nielsen PE, Gonik B. Controversies in labor management: shoulder dystocia. Obstet Gynecol Clin. 1999;26(2):371-382.
10. McFarland M, Hod M, Piper JM, Xenakis EM-J, Langer O. Are labor abnormalities more common in shoulder dystocia? Am J ObstetGynecol. 1995;173:1211-1214.
11. Gross TL, Sokol RJ, Williams T, Thompson K. Shoulder dystocia: A fetal-physician risk. Am J Obstet Gynecol. 1987;156:1408-1418.
12. Acker DB, Sachs BP, Friedman EA. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66:762-768.
13. Shoulder dystocia and Erb’s palsy. The Keenan Law Firm. 2002. Available at www.shoulderdystociaattorney.com. Accessed July 23, 2004.
14. Acker DB, Gregory KD, Sachs BP, Friedman EA. Risk factors for Erb-Duchenne palsy. Obstet Gynecol. 1988;71(Part l):390-392.
15. Fetterman HH. Cutting the legal risks of shoulder dystocia. OBG Management. March 1995;41-51.
16. Iffy L, Varadi V, Jakobovits A. Common intrapartum denominators of shoulder dystocia related birth injuries. Zentralbl Gynakol. 1994;116(1):33-37.
17. Calhoun BC, Hume RR, Walters JL. Shoulder Dystocia as a risk for obstetric liability. Legal Medicine. 2000;12-15.
18. Jennett RJ, Tarby TJ. Brachial plexus palsy: an old problem revisited again. Am J Obstet Gynecol. 1997;176:1354-1357.
19. Skolbekken JA. Shoulder dystocia—malpractice or acceptable risk? Acta Obstet Gynecol Scand. 2000;79:750-756.
20. Dildy GA. Fetal macrosomia [slide presentation]. Presented at the 18th Annual Conference on Obstetrics, Gynecology, Perinatal Medicine, and the Law, Boston University School of Medicine and the Center for Human Genetics, January 2002, Kauai, Hawaii.
21. Conway D, Langer O. Elective delivery of infants with macrosomia in diabetic women: reduced shoulder dystocia versus increased cesarean deliveries. Am J Obstet Gynecol. 1998;178(5):922-925.
22. Cohen B, Penning S, Major C, Ansley D, Porto M, Garite T. Sonographic prediction of shoulder dystocia in infants of diabetic mothers. Obstet Gynecol. 1998;88:10-13.
23. Gherman RB, Goodwin TM, Souter I, Neumann K, Ouzounian JG, Paul RH. The McRoberts’ maneuver for the alleviation of shoulder dystocia: how successful is it? Am J Obstet Gynecol. 1997;176:656-661.
24. Hickson GB, Clayton DW, Githens PB, Sloan FA. Factors that prompted families to file medical malpractice claims following perinatal injuries. JAMA. 1992;267:1359-1363.