User login
Anticoagulation in pregnancy: Q&A on low molecular weight heparin
- Low molecular weight heparin appears to be as safe as unfractionated heparin in pregnancy, with longer-lasting effects and reduced need for monitoring. Both the American College pregnancy with appropriate counseling.
- Although warfarin is the anticoagulant of choice in the nonpregnant state, it crosses the placenta and has been linked to structural birth defects known as “warfarin embryopathy.”
- A single subcutaneous, prophylactic 40-mg dose of the low molecular weight heparin enoxaparin costs about $30, compared with about $1 for an equivalent dose of unfractionated heparin.
What are the attributes of the ideal anticoagulant in pregnancy? Low molecular weight heparin fills the bill in many ways: It is safe for both mother and fetus, as effective in pregnancy as in the nongravid population, and side effects are minimal. It also has a favorable dosing route and interval, with less need for monitoring than with unfractionated heparin (UH).
In other ways, low molecular weight heparin (LMWH) is distinctly inferior. This article describes its strengths and weaknesses, addressing 10 common clinical questions.
Assessing the heightened risks of pregnancy
Pregnant women have 5 times the risk of venous thromboembolism (VTE) of nongravid patients.1 The increased risk is due to physiologic, mechanical and, sometimes, iatrogenic factors (TABLE 1):
- Gravidas have greater concentrations of factors I, VII, VIII, IX, and X; decreased fibrinolytic activity; and increased platelet activation. These changes in the coagulation system predispose the gravida to clot formation. Although they may protect against hemorrhage, they also heighten the risk for VTE during pregnancy and the postpartum period.
- The enlarging uterus can compress venous drainage from the lower extremities, resulting in stasis. Further, prolonged immobilization in the form of bed rest is often prescribed for obstetric complications such as hypertension, preterm labor, hemorrhage, and preterm premature rupture of membranes.
- Both abdominal and vaginal operative delivery can predispose to vascular endothelial injury.
LMWH deactivates more slowly than UH, exposing patients to fewer periods of subtherapeutic anticoagulation.
These factors—singularly or in combination–can lead to a thrombotic or embolic event.2
TABLE 1
Pregnancy-associated risk factors for venous thromboembolism
| RISK FACTOR | CAUSES |
|---|---|
| Changes in the coagulation system | Increased factors I, VII, VIII, IX, X |
| Decreased fibrinolytic activity | |
| Increased platelet activation | |
| Venous stasis | Enlarging uterus compresses venous return from lower extremities |
| Endothelial injury | Vacuum delivery |
| Forceps delivery | |
| Cesarean delivery | |
| Prolonged immobilization | Preterm labor |
| Preterm premature rupture of membranes | |
| Obstetric hemorrhage | |
| Hypertensive disorders of pregnancy |
Question 1When is anticoagulation warranted in pregnancy?
It is indicated in women who:
- experience a thromboembolic event,
- become pregnant while being treated for VTE,
- have a previous history of unprovoked VTE (unrelated to trauma, immobilization, etc),
- have a known hereditary thrombophilia such as antithrombin III deficiency, factor V Leiden mutation, or the prothrombin G20210A mutation, with or without a personal history of thrombosis, or
- have a connective tissue disorder such as antiphospholipid syndrome.
Anticoagulation in pregnancy is common, and usually is given for the duration of pregnancy, into the postpartum period.
Question 2What are the options for anticoagulation?
Heparin is the sole choice for long-term anticoagulation, since warfarin is contraindicated in pregnancy.3 (See “Dangers of warfarin”.)
Unfortunately, heparin has disadvantages that render it a second-line agent in the nonpregnant population. For example, because of enzymatic degradation, heparins cannot be given orally. In addition, because of its large size and strongly positive charge, the parent heparin molecule—known as “unfractionated” heparin—is rapidly deactivated by tissue proteins, making for an unpredictable anticoagulation response. Underdosing and overdosing are typical, and frequent monitoring is necessary.
For these and other reasons, investigators have sought a more predictable, reliable agent for long-term anticoagulation in patients who cannot take warfarin. Interest has focused on a derivative of the parent heparin molecule: LMWH.
Snapshot of LMWH. This agent is produced by the controlled enzymatic degradation of unfractionated heparin (molecular weight of approximately 10,000 to 15,000 daltons) into approximately 5,000-dalton molecules. Although they are much smaller than the parent molecule, these polymers still carry a strong positive charge.
This polarity is probably why LMWH does not cross the placenta—a major advantage over warfarin for anticoagulation during pregnancy.5
In addition, accumulating evidence6,7 suggests that LMWH is at least as safe and effective as UH in pregnancy, although more research is needed. As with UH, there appears to be no transplacental passage.8
Pregnancy category. According to the manufacturer, the LMWH enoxaparin falls into pregnancy category B.9 Another LMWH, dalteparin, also falls into pregnancy category B. Both the American College of Obstetricians and Gynecologists2 and the Society for Maternal-Fetal Medicine10 endorse the use of LMWH in pregnancy with appropriate counseling.8
Although it is the drug of choice in the nonpregnant population, warfarin is contraindicated in pregnancy because it can cross the placenta and has been linked to adverse pregnancy outcomes.
Several studies have demonstrated an association between first-trimester warfarin exposure and a constellation of structural birth defects, termed “warfarin embryopathy,” which includes craniofacial and skeletal defects. Exposure in any trimester is associated with fetal and neonatal intracranial hemorrhage.3
For these reasons, warfarin is contraindicated in pregnancy with the rare exception of women with mechanical prosthetic heart valves.4
Question 3How does LMWH differ from unfractionated heparin?
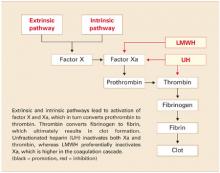
LMWH is more efficient. Both UH and LMWH contain an essential pentasaccharide within their polymer structure that binds to and enhances antithrombin III, which in turn inhibits thrombin and activated factor X (Xa). Because of its smaller size, LMWH preferentially inhibits Xa, which is higher in the coagulation cascade. Inhibition of a single molecule of Xa prevents the formation of many molecules of thrombin. Molecule for molecule, LMWH is a more efficient anticoagulant than UH (FIGURE).11
The second way that LMWH differs from UH also relates to the molecule’s size. Smaller heparin molecules are less likely to be deactivated by tissue proteins. This results in improved bioavailability of the administered dose. Greater bioavailability translates to a more predictable dose-response relationship, a long half-life, and better anticoagulation.12
FIGURE Simplified schematic of the coagulation cascade
Question 4What are the clinical advantages of LMWH?
LMWH has longer-lasting effects and subcutaneous dosing. It also has fewer side effects than UH.
Because of its large size and positive charge, UH has an unfavorable pharmacokinetic profile. Tissue proteins interfere with and deactivate it, and many of these proteins increase in pregnancy and with advancing gestation. Heparin tissue levels are therefore erratic and unpredictable and often lead to periods of subtherapeutic coverage. This is true even with intravenous (IV) dosing.
Rapid absorption, no intravenous dosing. In contrast, because of its smaller size, LMWH is rapidly and predictably absorbed from a subcutaneous injection. Intravenous dosing is not necessary to obtain adequate tissue levels. Once in tissue, it is deactivated more slowly and therefore maintains its anticoagulation effect longer. Consequently, patients are exposed to fewer periods of subtherapeutic anticoagulation. A longer half-life also translates to more favorable dosing routes (subcutaneous rather than IV) and regimens (daily versus twice daily). Similarly, since the dose-response is predictable and tissue levels are more constant, frequent monitoring of treatment response is not routinely necessary.
Fewer side effects. Another advantage of LMWH over UH is the improved side-effect profile. Patients on LMWH have decreased risk of hemorrhage, osteoporosis, and antibody-mediated thrombocytopenia.11 Although most data regarding these advantages come from the nonpregnant population, it is plausible to speculate that these traits also are present in pregnant women (TABLE 2).
TABLE 2
Advantages and disadvantages of LMWH in pregnancy
| ADVANTAGES |
| More effective anticoagulation |
| Better dose-response |
| Longer half-life |
| Better dosing route |
| Decreased need for monitoring |
| Fewer side effects |
| DISADVANTAGES |
| Longer half-life |
| Risk of hematoma with epidural anesthesia |
| Not fully reversible with protamine sulfate |
| Anticoagulation effect difficult to monitor |
| Higher cost |
Question 5What are the disadvantages?
They include the long half-life, risk of hematoma with epidural anesthesia, lower efficacy of the antidote, monitoring difficulty, and higher cost.
The long half-life of LMWH is both an advantage and disadvantage. For example, when UH is administered intravenously, it has a half-life of 30 to 60 minutes. When it is given subcutaneously, the half-life is 1 to 2 hours. This means that a patient can undergo vaginal delivery or even surgery within hours of her last subcutaneous UH injection.
In contrast, LMWH has a half-life of approximately 4 hours. A recent dose may increase the risk of operative morbidity in the form of overt or delayed hemorrhage, hematoma, or wound dehiscence.
Not for use with epidural anesthesia. Case reports of epidural hematomas after regional anesthesia during orthopedic procedures have caused considerable concern about the use of LMWH and the placement of a neuraxial block such as a spinal or epidural.13 Many anesthesiologists will not place an epidural or spinal within 24 hours of a LMWH dose.14 However, as experience with these agents in the nonpregnant population expands, a more evidence-based approach is likely to develop.
Antidote less effective. Protamine sulfate is a strong base that binds with the positively charged UH molecule, thereby serving as an antidote through competitive inhibition. Because of its smaller size, LMWH is reversed by protamine to a lesser degree (approximately 60% effective).14 Therefore, hemorrhage associated with LMWH may require replacement of blood components, which carries the risks of infection and transfusion reactions.
Difficult to monitor. The anticoagulation effect of UH can be reliably monitored by the activated partial thromboplastin time (aPTT), which is a widely available test with a rapid turnaround. However, the anticoagulation effect of LMWH is not reflected by the aPTT. Assessment of LMWH activity requires assessment of the antifactor Xa level, a test that is not universally available and has a longer turnaround.
Higher cost. Another limitation of LMWH is its cost. A single, subcutaneous, prophylactic 40-mg dose of enoxaparin costs about $30, compared with about $1 for an equivalent dose of unfractionated heparin (5,000 U subcutaneously twice a day). Because of its increased cost, many insurance companies do not authorize the use of LMWH for prolonged periods such as pregnancy and the postpartum period. However, studies in the nonpregnant population have demonstrated overall decreased cost over UH due to the reduced need for monitoring, shorter length of hospital stay, and diminished treatment failure.
Question 6What is the dose for prophylaxis and treatment?
The standard dose of enoxaparin for prophylaxis in pregnancy and the postpartum period is 40 mg administered subcutaneously every 24 hours (TABLE 3). Therapeutic anticoagulation (sometimes referred to as a “weightadjusted” dose) is usually achieved with 1 mg/kg every 12 hours.
Dalteparin can be given in a prophylactic dose of 5,000 U subcutaneously every 24 hours and a therapeutic dose of 200 U/kg every 24 hours.15 Dosing may need to be adjusted with advancing gestation as plasma volume, renal clearance, and tissue proteins increase.
The various LMWH preparations are not equivalent in their pharmacokinetics. Generally, clinicians familiarize themselves with a single agent. It also is important to note that dosing regimens in pregnancy are not evidence-based but largely “borrowed” from nonpregnant regimens. They also vary widely in the available literature.
TABLE 3
Common low molecular weight heparins
| BRAND NAME | GENERIC NAME | PROPHYLACTIC DOSE | THERAPEUTIC DOSE |
|---|---|---|---|
| Lovenox | Enoxaparin | 40 mg every 24 hours | 1 mg/kg every 12 hours |
| Fragmin | Dalteparin | 5,000 U every 24 hours | 200 U/kg every 24 hours |
| Note: All doses are subcutaneous | |||
Question 7Under what conditions can LMWH be given?
Generally, LMWH can replace UH in any condition that warrants prophylactic or therapeutic anticoagulation in pregnancy, except the acute management of pulmonary embolism and in women with mechanical heart valves.
Prophylactic dosing can be offered to women with a previous thromboembolic event such as deep vein thrombosis (DVT) or pulmonary embolism that was not associated with a reversible and temporary predisposing risk factor such as immobilization or trauma. (In general, pregnancy is not seen as a reversible or temporary risk factor.)
Prophylactic or therapeutic dosing is sometimes offered to women with a hereditary thrombophilia, antiphospholipid syndrome, or other vasculopathies and connective-tissue diseases. In addition, LMWH is an accepted first-line therapeutic anticoagulant for acute DVT in pregnancy.
Not for use in treating acute pulmonary embolism. Evidence is insufficient to support the use of LMWH as a first-line anticoagulant for acute pulmonary embolism. To date, IV loading with UH is the standard of care, with conversion to LMWH after 4 to 5 days of therapeutic UH anticoagulation. This may change as experience with LMWH increases.
Not for use with mechanical prosthetic heart valves. Because of case reports of recurrent thromboembolism resulting in maternal and fetal death in pregnant women with mechanical prosthetic heart valves on therapeutic LMWH, the manufacturer of enoxaparin warns against its use in pregnancy in these women.9 However, similar outcomes have been reported with UH and warfarin. This forces clinicians to consider potentially less effective and more problematic agents for anticoagulation in this fortunately rare circumstance.
Contemporary management involves converting from warfarin to subcutaneous heparin or the heparin pump once pregnancy is established and before organogenesis (at approximately 6 weeks’ gestation), followed by frequent monitoring of aPTT. Alternatively, warfarin can be resumed after organogenesis (at about 12 weeks) and continued into the third trimester, followed by conversion to heparin pump, subcutaneous injections, or an IV drip near term. Both options carry significant maternal and fetal risk and should be performed in a multidisciplinary fashion along with cardiology and vascular medicine.16
Question 8How do I start and stop LMWH?
Start LMWH as a subcutaneous injection without IV loading. A baseline complete blood count for platelet count is reasonable. Patient education is straightforward.
Prophylactic dosing can be initiated on an outpatient basis immediately after patient education and procurement of the medicine.
Therapeutic dosing for DVT usually is begun during hospitalization, with 1 mg per kilogram given subcutaneously every 12 hours. Barring other comorbidities, discharge can be achieved after patient education. The exception is treatment of acute pulmonary embolism.Conversion to LMWH is achieved once the patient is fully anticoagulated.
Discontinuation. Pregnancy is a period of shifting maternal and fetal status, when indications for delivery can develop suddenly and with little warning (eg, abruptio placenta, nonreassuring antenatal testing).
LMWH dosing regimens in pregnancy are not evidence-based but largely “borrowed” from nonpregnant regimens.
A major consideration is the long half-life of LMWH. If a patient has had a recent subcutaneous injection of LMWH followed by an urgent indication for delivery, she may be anticoagulated during her delivery and be at increased risk for hemorrhage. Similarly, she may not be a candidate for epidural or spinal anesthesia because of the risk of epidural hematoma. If cesarean section is indicated, she may need general anesthesia, which is associated with increased maternal morbidity. If hemorrhage occurs, she may require transfusion of blood products, which carries the risks of infection and transfusion reactions.
Three strategies for peripartum management with LMWH are:
- Leave the patient on LMWH until the onset of labor. Depending on the timing and dose of her last injection, she may or may not be a candidate for regional anesthesia in labor. With prophylactic dosing, the risk of significant hemorrhage is low.
- Time delivery so that the patient withholds her PMinjection and presents for delivery the following morning, 24 hours after her last dose. This option is attractive when cesarean delivery is planned. The advantage of this approach is that it keeps the window of thrombosis as narrow as possible. The disadvantage is that the patient may enter labor or require delivery before the chosen delivery date, in which case she may be at risk for hemorrhage.
- Convert the patient to UH at approximately 36 to 37 weeks’ gestation. The advantage? The shorter half-life of UH increases the possibility for regional anesthesia and decreases the risk for hemorrhage. The disadvantage: The risk for thrombosis as well as complications (thrombocytopenia, osteoporosis, thrombosis) may be greater with UH for the reasons described earlier.
Restarting after delivery. Strategies for continued thromboprophylaxis in the postpartum period include continuing LMWH for an additional 6 to 8 weeks or converting to warfarin. Warfarin can be taken orally but requires frequent monitoring and adjustment of dosing, which is unnecessary with LMWH. Many patients choose to continue LMWH because they are familiar with the routine of daily injections. There is little data to support or refute either approach.
There also is little data to guide the decision of when to reinitiate prophylactic or therapeutic doses of LMWH after vaginal or cesarean delivery. Translating from other surgical subspecialties, most obstetricians are comfortable resuming both prophylactic and therapeutic doses at 6 hours after vaginal delivery and 8 hours after cesarean section.
Question 9How do I monitor the effectiveness of LMWH?
The frequent monitoring necessary with UH is not required with LMWH, since the increased bioavailability of LMWH leads to reliable tissue levels. The aPTT level does not correlate well with the anticoagulation effect of LMWH.
Antifactor Xa levels—sometimes referred to as the LMWH assay—of 0.5 to 1.2 IU/mL are considered adequate for therapeutic anticoagulation in the nonpregnant population. Peak anti-Xa activity is achieved approximately 4 hours after subcutaneous injection. AntiXa levels should therefore be drawn 4 hours after the last dose.17 Prophylactic subcutaneous dosing of enoxaparin 40 mg daily generally does not require monitoring of anti-Xa levels.
In nonpregnant women, routine monitoring is seldom necessary. Gravidas, however, are constantly changing in terms of weight, plasma volume, renal clearance, and amount of heparin-binding proteins. Because of this, periodic monitoring (every 4 weeks) is reasonable until an evidence-based recommendation can be made.
Question 10When can I provide neuraxial anesthesia?
Most anesthesiologists are reluctant to perform neuraxial anesthesia within 12 hours of the last prophylactic dose or 24 hours of the last therapeutic dose of LMWH because of the risk of epidural and spinal hematoma.
The American Society of Regional Anesthesia recommends that neuraxial anesthesia be withheld for 24 hours after the last therapeutic dose and 12 hours after a prophylactic dose. That organization did not recommend checking anti-Xa levels, since they do not adequately predict the risk of bleeding.9 However, as experience with LMWH broadens in nonobstetric surgical cases, such as orthopedic and cardiac procedures, it is likely that greater familiarity with the medication will lead to better evidence and broader acceptance.
Dr. Emery reports no financial relationships relevant to this article.
1. Gherman RB, Goodwin TM, Leung JD, Byrne JD, Hethumumi R, Montoro M. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol. 1999;94:730-734.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #19: Thromboembolism in Pregnancy. Washington, DC: ACOG; 2000.
3. Hall JG, Pauli RM, Wilson KM. Maternal and fetal sequelae of anticoagulation during pregnancy. Am J Med. 1980;68:122-140.
4. Born D, Martinez EE, Almeida PAM, et al. Pregnancy in patients with mechanical heart valves: the effects of anticoagulation on mother, fetus and neonate. Am Heart J. 1992;124:413-417.
5. Omri A, Delayoye JF, Anderson H, Bachmann F. Low molecular weight heparin Novo (LHN-1) does not cross the placenta during the second trimester of pregnancy. Thromb Haemost. 1989;61:55-56.
6. Sanson BJ, Lensing AWA, Prins MH, et al. Safety of low molecular weight heparin in pregnancy: a systematic review. Thromb Haemost. 1999;81:668-672.
7. Nelson-Piercy C, Letsky EA, de Sweit M. Low molecular weight heparin for obstetric thromboprophylaxis: experience of sixty-nine pregnancies in sixty-one women at risk. Am J Obstet Gynecol. 1997;176:1062-1068.
8. Forestier F, Daffos F, Rainaut M, et al. Low molecular weight heparin (CY 216) does not cross the placenta during the third trimester of pregnancy. Thromb Haemost. 1987;57:234.-
9. Lovenox injection [package insert]. Bridgewater, NJ: Aventis; 2003.
10. Enoxaprin sodium (Lovenox) and pregnancy. SMFM Practice Committee Announcement posted at http://www.smfm.org
11. Hirsh J, Warkentin TE, Raschke R, Granger C, Ohman EM, Dalen JE. Heparin and low molecular weight heparin: mechanisms of action, pharmacokinetics, dosing considerations, monitoring, efficacy and safety. Chest. 1998;114:489S-510S.
12. Weitz JI. Low-molecular-weight heparins. N Engl J Med. 1997;337:688-698.
13. US Department of Health and Human Services. FDA Public Health Advisory. Reports of Epidural and Spinal Hematomas with Concurrent Use of Low Molecular Weight Heparin and Spinal/Epidural Anesthesia or Spinal Puncture. Rockville, MD: Food and Drug Administration; December 1997.
14. American Society of Regional Anesthesia. Recommendations for Neuraxial Anesthesia and Anticoagulation. Richmond, Va: ASRA; 1998.
15. Hirsh J, Raschke R, Warkentin TE, et al. Heparin: mechanism of action, pharmacokinetics, dosing considerations, efficacy, and safety. Chest. 1995;108:258S-275S.
16. Anticoagulation and Enoxaparin Use in Patients with Prosthetic Heart Valves and/or Pregnancy. Clinical Cardiology Consensus Reports. Atlanta, Ga: American Health Consultants; 2002.
17. Ginsberg JS, Greer I, Hirsh J. Use of antithrombotic agents during pregnancy. Chest. 2001;119:122S-131S.
- Low molecular weight heparin appears to be as safe as unfractionated heparin in pregnancy, with longer-lasting effects and reduced need for monitoring. Both the American College pregnancy with appropriate counseling.
- Although warfarin is the anticoagulant of choice in the nonpregnant state, it crosses the placenta and has been linked to structural birth defects known as “warfarin embryopathy.”
- A single subcutaneous, prophylactic 40-mg dose of the low molecular weight heparin enoxaparin costs about $30, compared with about $1 for an equivalent dose of unfractionated heparin.
What are the attributes of the ideal anticoagulant in pregnancy? Low molecular weight heparin fills the bill in many ways: It is safe for both mother and fetus, as effective in pregnancy as in the nongravid population, and side effects are minimal. It also has a favorable dosing route and interval, with less need for monitoring than with unfractionated heparin (UH).
In other ways, low molecular weight heparin (LMWH) is distinctly inferior. This article describes its strengths and weaknesses, addressing 10 common clinical questions.
Assessing the heightened risks of pregnancy
Pregnant women have 5 times the risk of venous thromboembolism (VTE) of nongravid patients.1 The increased risk is due to physiologic, mechanical and, sometimes, iatrogenic factors (TABLE 1):
- Gravidas have greater concentrations of factors I, VII, VIII, IX, and X; decreased fibrinolytic activity; and increased platelet activation. These changes in the coagulation system predispose the gravida to clot formation. Although they may protect against hemorrhage, they also heighten the risk for VTE during pregnancy and the postpartum period.
- The enlarging uterus can compress venous drainage from the lower extremities, resulting in stasis. Further, prolonged immobilization in the form of bed rest is often prescribed for obstetric complications such as hypertension, preterm labor, hemorrhage, and preterm premature rupture of membranes.
- Both abdominal and vaginal operative delivery can predispose to vascular endothelial injury.
LMWH deactivates more slowly than UH, exposing patients to fewer periods of subtherapeutic anticoagulation.
These factors—singularly or in combination–can lead to a thrombotic or embolic event.2
TABLE 1
Pregnancy-associated risk factors for venous thromboembolism
| RISK FACTOR | CAUSES |
|---|---|
| Changes in the coagulation system | Increased factors I, VII, VIII, IX, X |
| Decreased fibrinolytic activity | |
| Increased platelet activation | |
| Venous stasis | Enlarging uterus compresses venous return from lower extremities |
| Endothelial injury | Vacuum delivery |
| Forceps delivery | |
| Cesarean delivery | |
| Prolonged immobilization | Preterm labor |
| Preterm premature rupture of membranes | |
| Obstetric hemorrhage | |
| Hypertensive disorders of pregnancy |
Question 1When is anticoagulation warranted in pregnancy?
It is indicated in women who:
- experience a thromboembolic event,
- become pregnant while being treated for VTE,
- have a previous history of unprovoked VTE (unrelated to trauma, immobilization, etc),
- have a known hereditary thrombophilia such as antithrombin III deficiency, factor V Leiden mutation, or the prothrombin G20210A mutation, with or without a personal history of thrombosis, or
- have a connective tissue disorder such as antiphospholipid syndrome.
Anticoagulation in pregnancy is common, and usually is given for the duration of pregnancy, into the postpartum period.
Question 2What are the options for anticoagulation?
Heparin is the sole choice for long-term anticoagulation, since warfarin is contraindicated in pregnancy.3 (See “Dangers of warfarin”.)
Unfortunately, heparin has disadvantages that render it a second-line agent in the nonpregnant population. For example, because of enzymatic degradation, heparins cannot be given orally. In addition, because of its large size and strongly positive charge, the parent heparin molecule—known as “unfractionated” heparin—is rapidly deactivated by tissue proteins, making for an unpredictable anticoagulation response. Underdosing and overdosing are typical, and frequent monitoring is necessary.
For these and other reasons, investigators have sought a more predictable, reliable agent for long-term anticoagulation in patients who cannot take warfarin. Interest has focused on a derivative of the parent heparin molecule: LMWH.
Snapshot of LMWH. This agent is produced by the controlled enzymatic degradation of unfractionated heparin (molecular weight of approximately 10,000 to 15,000 daltons) into approximately 5,000-dalton molecules. Although they are much smaller than the parent molecule, these polymers still carry a strong positive charge.
This polarity is probably why LMWH does not cross the placenta—a major advantage over warfarin for anticoagulation during pregnancy.5
In addition, accumulating evidence6,7 suggests that LMWH is at least as safe and effective as UH in pregnancy, although more research is needed. As with UH, there appears to be no transplacental passage.8
Pregnancy category. According to the manufacturer, the LMWH enoxaparin falls into pregnancy category B.9 Another LMWH, dalteparin, also falls into pregnancy category B. Both the American College of Obstetricians and Gynecologists2 and the Society for Maternal-Fetal Medicine10 endorse the use of LMWH in pregnancy with appropriate counseling.8
Although it is the drug of choice in the nonpregnant population, warfarin is contraindicated in pregnancy because it can cross the placenta and has been linked to adverse pregnancy outcomes.
Several studies have demonstrated an association between first-trimester warfarin exposure and a constellation of structural birth defects, termed “warfarin embryopathy,” which includes craniofacial and skeletal defects. Exposure in any trimester is associated with fetal and neonatal intracranial hemorrhage.3
For these reasons, warfarin is contraindicated in pregnancy with the rare exception of women with mechanical prosthetic heart valves.4
Question 3How does LMWH differ from unfractionated heparin?
LMWH is more efficient. Both UH and LMWH contain an essential pentasaccharide within their polymer structure that binds to and enhances antithrombin III, which in turn inhibits thrombin and activated factor X (Xa). Because of its smaller size, LMWH preferentially inhibits Xa, which is higher in the coagulation cascade. Inhibition of a single molecule of Xa prevents the formation of many molecules of thrombin. Molecule for molecule, LMWH is a more efficient anticoagulant than UH (FIGURE).11
The second way that LMWH differs from UH also relates to the molecule’s size. Smaller heparin molecules are less likely to be deactivated by tissue proteins. This results in improved bioavailability of the administered dose. Greater bioavailability translates to a more predictable dose-response relationship, a long half-life, and better anticoagulation.12
FIGURE Simplified schematic of the coagulation cascade
Question 4What are the clinical advantages of LMWH?
LMWH has longer-lasting effects and subcutaneous dosing. It also has fewer side effects than UH.
Because of its large size and positive charge, UH has an unfavorable pharmacokinetic profile. Tissue proteins interfere with and deactivate it, and many of these proteins increase in pregnancy and with advancing gestation. Heparin tissue levels are therefore erratic and unpredictable and often lead to periods of subtherapeutic coverage. This is true even with intravenous (IV) dosing.
Rapid absorption, no intravenous dosing. In contrast, because of its smaller size, LMWH is rapidly and predictably absorbed from a subcutaneous injection. Intravenous dosing is not necessary to obtain adequate tissue levels. Once in tissue, it is deactivated more slowly and therefore maintains its anticoagulation effect longer. Consequently, patients are exposed to fewer periods of subtherapeutic anticoagulation. A longer half-life also translates to more favorable dosing routes (subcutaneous rather than IV) and regimens (daily versus twice daily). Similarly, since the dose-response is predictable and tissue levels are more constant, frequent monitoring of treatment response is not routinely necessary.
Fewer side effects. Another advantage of LMWH over UH is the improved side-effect profile. Patients on LMWH have decreased risk of hemorrhage, osteoporosis, and antibody-mediated thrombocytopenia.11 Although most data regarding these advantages come from the nonpregnant population, it is plausible to speculate that these traits also are present in pregnant women (TABLE 2).
TABLE 2
Advantages and disadvantages of LMWH in pregnancy
| ADVANTAGES |
| More effective anticoagulation |
| Better dose-response |
| Longer half-life |
| Better dosing route |
| Decreased need for monitoring |
| Fewer side effects |
| DISADVANTAGES |
| Longer half-life |
| Risk of hematoma with epidural anesthesia |
| Not fully reversible with protamine sulfate |
| Anticoagulation effect difficult to monitor |
| Higher cost |
Question 5What are the disadvantages?
They include the long half-life, risk of hematoma with epidural anesthesia, lower efficacy of the antidote, monitoring difficulty, and higher cost.
The long half-life of LMWH is both an advantage and disadvantage. For example, when UH is administered intravenously, it has a half-life of 30 to 60 minutes. When it is given subcutaneously, the half-life is 1 to 2 hours. This means that a patient can undergo vaginal delivery or even surgery within hours of her last subcutaneous UH injection.
In contrast, LMWH has a half-life of approximately 4 hours. A recent dose may increase the risk of operative morbidity in the form of overt or delayed hemorrhage, hematoma, or wound dehiscence.
Not for use with epidural anesthesia. Case reports of epidural hematomas after regional anesthesia during orthopedic procedures have caused considerable concern about the use of LMWH and the placement of a neuraxial block such as a spinal or epidural.13 Many anesthesiologists will not place an epidural or spinal within 24 hours of a LMWH dose.14 However, as experience with these agents in the nonpregnant population expands, a more evidence-based approach is likely to develop.
Antidote less effective. Protamine sulfate is a strong base that binds with the positively charged UH molecule, thereby serving as an antidote through competitive inhibition. Because of its smaller size, LMWH is reversed by protamine to a lesser degree (approximately 60% effective).14 Therefore, hemorrhage associated with LMWH may require replacement of blood components, which carries the risks of infection and transfusion reactions.
Difficult to monitor. The anticoagulation effect of UH can be reliably monitored by the activated partial thromboplastin time (aPTT), which is a widely available test with a rapid turnaround. However, the anticoagulation effect of LMWH is not reflected by the aPTT. Assessment of LMWH activity requires assessment of the antifactor Xa level, a test that is not universally available and has a longer turnaround.
Higher cost. Another limitation of LMWH is its cost. A single, subcutaneous, prophylactic 40-mg dose of enoxaparin costs about $30, compared with about $1 for an equivalent dose of unfractionated heparin (5,000 U subcutaneously twice a day). Because of its increased cost, many insurance companies do not authorize the use of LMWH for prolonged periods such as pregnancy and the postpartum period. However, studies in the nonpregnant population have demonstrated overall decreased cost over UH due to the reduced need for monitoring, shorter length of hospital stay, and diminished treatment failure.
Question 6What is the dose for prophylaxis and treatment?
The standard dose of enoxaparin for prophylaxis in pregnancy and the postpartum period is 40 mg administered subcutaneously every 24 hours (TABLE 3). Therapeutic anticoagulation (sometimes referred to as a “weightadjusted” dose) is usually achieved with 1 mg/kg every 12 hours.
Dalteparin can be given in a prophylactic dose of 5,000 U subcutaneously every 24 hours and a therapeutic dose of 200 U/kg every 24 hours.15 Dosing may need to be adjusted with advancing gestation as plasma volume, renal clearance, and tissue proteins increase.
The various LMWH preparations are not equivalent in their pharmacokinetics. Generally, clinicians familiarize themselves with a single agent. It also is important to note that dosing regimens in pregnancy are not evidence-based but largely “borrowed” from nonpregnant regimens. They also vary widely in the available literature.
TABLE 3
Common low molecular weight heparins
| BRAND NAME | GENERIC NAME | PROPHYLACTIC DOSE | THERAPEUTIC DOSE |
|---|---|---|---|
| Lovenox | Enoxaparin | 40 mg every 24 hours | 1 mg/kg every 12 hours |
| Fragmin | Dalteparin | 5,000 U every 24 hours | 200 U/kg every 24 hours |
| Note: All doses are subcutaneous | |||
Question 7Under what conditions can LMWH be given?
Generally, LMWH can replace UH in any condition that warrants prophylactic or therapeutic anticoagulation in pregnancy, except the acute management of pulmonary embolism and in women with mechanical heart valves.
Prophylactic dosing can be offered to women with a previous thromboembolic event such as deep vein thrombosis (DVT) or pulmonary embolism that was not associated with a reversible and temporary predisposing risk factor such as immobilization or trauma. (In general, pregnancy is not seen as a reversible or temporary risk factor.)
Prophylactic or therapeutic dosing is sometimes offered to women with a hereditary thrombophilia, antiphospholipid syndrome, or other vasculopathies and connective-tissue diseases. In addition, LMWH is an accepted first-line therapeutic anticoagulant for acute DVT in pregnancy.
Not for use in treating acute pulmonary embolism. Evidence is insufficient to support the use of LMWH as a first-line anticoagulant for acute pulmonary embolism. To date, IV loading with UH is the standard of care, with conversion to LMWH after 4 to 5 days of therapeutic UH anticoagulation. This may change as experience with LMWH increases.
Not for use with mechanical prosthetic heart valves. Because of case reports of recurrent thromboembolism resulting in maternal and fetal death in pregnant women with mechanical prosthetic heart valves on therapeutic LMWH, the manufacturer of enoxaparin warns against its use in pregnancy in these women.9 However, similar outcomes have been reported with UH and warfarin. This forces clinicians to consider potentially less effective and more problematic agents for anticoagulation in this fortunately rare circumstance.
Contemporary management involves converting from warfarin to subcutaneous heparin or the heparin pump once pregnancy is established and before organogenesis (at approximately 6 weeks’ gestation), followed by frequent monitoring of aPTT. Alternatively, warfarin can be resumed after organogenesis (at about 12 weeks) and continued into the third trimester, followed by conversion to heparin pump, subcutaneous injections, or an IV drip near term. Both options carry significant maternal and fetal risk and should be performed in a multidisciplinary fashion along with cardiology and vascular medicine.16
Question 8How do I start and stop LMWH?
Start LMWH as a subcutaneous injection without IV loading. A baseline complete blood count for platelet count is reasonable. Patient education is straightforward.
Prophylactic dosing can be initiated on an outpatient basis immediately after patient education and procurement of the medicine.
Therapeutic dosing for DVT usually is begun during hospitalization, with 1 mg per kilogram given subcutaneously every 12 hours. Barring other comorbidities, discharge can be achieved after patient education. The exception is treatment of acute pulmonary embolism.Conversion to LMWH is achieved once the patient is fully anticoagulated.
Discontinuation. Pregnancy is a period of shifting maternal and fetal status, when indications for delivery can develop suddenly and with little warning (eg, abruptio placenta, nonreassuring antenatal testing).
LMWH dosing regimens in pregnancy are not evidence-based but largely “borrowed” from nonpregnant regimens.
A major consideration is the long half-life of LMWH. If a patient has had a recent subcutaneous injection of LMWH followed by an urgent indication for delivery, she may be anticoagulated during her delivery and be at increased risk for hemorrhage. Similarly, she may not be a candidate for epidural or spinal anesthesia because of the risk of epidural hematoma. If cesarean section is indicated, she may need general anesthesia, which is associated with increased maternal morbidity. If hemorrhage occurs, she may require transfusion of blood products, which carries the risks of infection and transfusion reactions.
Three strategies for peripartum management with LMWH are:
- Leave the patient on LMWH until the onset of labor. Depending on the timing and dose of her last injection, she may or may not be a candidate for regional anesthesia in labor. With prophylactic dosing, the risk of significant hemorrhage is low.
- Time delivery so that the patient withholds her PMinjection and presents for delivery the following morning, 24 hours after her last dose. This option is attractive when cesarean delivery is planned. The advantage of this approach is that it keeps the window of thrombosis as narrow as possible. The disadvantage is that the patient may enter labor or require delivery before the chosen delivery date, in which case she may be at risk for hemorrhage.
- Convert the patient to UH at approximately 36 to 37 weeks’ gestation. The advantage? The shorter half-life of UH increases the possibility for regional anesthesia and decreases the risk for hemorrhage. The disadvantage: The risk for thrombosis as well as complications (thrombocytopenia, osteoporosis, thrombosis) may be greater with UH for the reasons described earlier.
Restarting after delivery. Strategies for continued thromboprophylaxis in the postpartum period include continuing LMWH for an additional 6 to 8 weeks or converting to warfarin. Warfarin can be taken orally but requires frequent monitoring and adjustment of dosing, which is unnecessary with LMWH. Many patients choose to continue LMWH because they are familiar with the routine of daily injections. There is little data to support or refute either approach.
There also is little data to guide the decision of when to reinitiate prophylactic or therapeutic doses of LMWH after vaginal or cesarean delivery. Translating from other surgical subspecialties, most obstetricians are comfortable resuming both prophylactic and therapeutic doses at 6 hours after vaginal delivery and 8 hours after cesarean section.
Question 9How do I monitor the effectiveness of LMWH?
The frequent monitoring necessary with UH is not required with LMWH, since the increased bioavailability of LMWH leads to reliable tissue levels. The aPTT level does not correlate well with the anticoagulation effect of LMWH.
Antifactor Xa levels—sometimes referred to as the LMWH assay—of 0.5 to 1.2 IU/mL are considered adequate for therapeutic anticoagulation in the nonpregnant population. Peak anti-Xa activity is achieved approximately 4 hours after subcutaneous injection. AntiXa levels should therefore be drawn 4 hours after the last dose.17 Prophylactic subcutaneous dosing of enoxaparin 40 mg daily generally does not require monitoring of anti-Xa levels.
In nonpregnant women, routine monitoring is seldom necessary. Gravidas, however, are constantly changing in terms of weight, plasma volume, renal clearance, and amount of heparin-binding proteins. Because of this, periodic monitoring (every 4 weeks) is reasonable until an evidence-based recommendation can be made.
Question 10When can I provide neuraxial anesthesia?
Most anesthesiologists are reluctant to perform neuraxial anesthesia within 12 hours of the last prophylactic dose or 24 hours of the last therapeutic dose of LMWH because of the risk of epidural and spinal hematoma.
The American Society of Regional Anesthesia recommends that neuraxial anesthesia be withheld for 24 hours after the last therapeutic dose and 12 hours after a prophylactic dose. That organization did not recommend checking anti-Xa levels, since they do not adequately predict the risk of bleeding.9 However, as experience with LMWH broadens in nonobstetric surgical cases, such as orthopedic and cardiac procedures, it is likely that greater familiarity with the medication will lead to better evidence and broader acceptance.
Dr. Emery reports no financial relationships relevant to this article.
- Low molecular weight heparin appears to be as safe as unfractionated heparin in pregnancy, with longer-lasting effects and reduced need for monitoring. Both the American College pregnancy with appropriate counseling.
- Although warfarin is the anticoagulant of choice in the nonpregnant state, it crosses the placenta and has been linked to structural birth defects known as “warfarin embryopathy.”
- A single subcutaneous, prophylactic 40-mg dose of the low molecular weight heparin enoxaparin costs about $30, compared with about $1 for an equivalent dose of unfractionated heparin.
What are the attributes of the ideal anticoagulant in pregnancy? Low molecular weight heparin fills the bill in many ways: It is safe for both mother and fetus, as effective in pregnancy as in the nongravid population, and side effects are minimal. It also has a favorable dosing route and interval, with less need for monitoring than with unfractionated heparin (UH).
In other ways, low molecular weight heparin (LMWH) is distinctly inferior. This article describes its strengths and weaknesses, addressing 10 common clinical questions.
Assessing the heightened risks of pregnancy
Pregnant women have 5 times the risk of venous thromboembolism (VTE) of nongravid patients.1 The increased risk is due to physiologic, mechanical and, sometimes, iatrogenic factors (TABLE 1):
- Gravidas have greater concentrations of factors I, VII, VIII, IX, and X; decreased fibrinolytic activity; and increased platelet activation. These changes in the coagulation system predispose the gravida to clot formation. Although they may protect against hemorrhage, they also heighten the risk for VTE during pregnancy and the postpartum period.
- The enlarging uterus can compress venous drainage from the lower extremities, resulting in stasis. Further, prolonged immobilization in the form of bed rest is often prescribed for obstetric complications such as hypertension, preterm labor, hemorrhage, and preterm premature rupture of membranes.
- Both abdominal and vaginal operative delivery can predispose to vascular endothelial injury.
LMWH deactivates more slowly than UH, exposing patients to fewer periods of subtherapeutic anticoagulation.
These factors—singularly or in combination–can lead to a thrombotic or embolic event.2
TABLE 1
Pregnancy-associated risk factors for venous thromboembolism
| RISK FACTOR | CAUSES |
|---|---|
| Changes in the coagulation system | Increased factors I, VII, VIII, IX, X |
| Decreased fibrinolytic activity | |
| Increased platelet activation | |
| Venous stasis | Enlarging uterus compresses venous return from lower extremities |
| Endothelial injury | Vacuum delivery |
| Forceps delivery | |
| Cesarean delivery | |
| Prolonged immobilization | Preterm labor |
| Preterm premature rupture of membranes | |
| Obstetric hemorrhage | |
| Hypertensive disorders of pregnancy |
Question 1When is anticoagulation warranted in pregnancy?
It is indicated in women who:
- experience a thromboembolic event,
- become pregnant while being treated for VTE,
- have a previous history of unprovoked VTE (unrelated to trauma, immobilization, etc),
- have a known hereditary thrombophilia such as antithrombin III deficiency, factor V Leiden mutation, or the prothrombin G20210A mutation, with or without a personal history of thrombosis, or
- have a connective tissue disorder such as antiphospholipid syndrome.
Anticoagulation in pregnancy is common, and usually is given for the duration of pregnancy, into the postpartum period.
Question 2What are the options for anticoagulation?
Heparin is the sole choice for long-term anticoagulation, since warfarin is contraindicated in pregnancy.3 (See “Dangers of warfarin”.)
Unfortunately, heparin has disadvantages that render it a second-line agent in the nonpregnant population. For example, because of enzymatic degradation, heparins cannot be given orally. In addition, because of its large size and strongly positive charge, the parent heparin molecule—known as “unfractionated” heparin—is rapidly deactivated by tissue proteins, making for an unpredictable anticoagulation response. Underdosing and overdosing are typical, and frequent monitoring is necessary.
For these and other reasons, investigators have sought a more predictable, reliable agent for long-term anticoagulation in patients who cannot take warfarin. Interest has focused on a derivative of the parent heparin molecule: LMWH.
Snapshot of LMWH. This agent is produced by the controlled enzymatic degradation of unfractionated heparin (molecular weight of approximately 10,000 to 15,000 daltons) into approximately 5,000-dalton molecules. Although they are much smaller than the parent molecule, these polymers still carry a strong positive charge.
This polarity is probably why LMWH does not cross the placenta—a major advantage over warfarin for anticoagulation during pregnancy.5
In addition, accumulating evidence6,7 suggests that LMWH is at least as safe and effective as UH in pregnancy, although more research is needed. As with UH, there appears to be no transplacental passage.8
Pregnancy category. According to the manufacturer, the LMWH enoxaparin falls into pregnancy category B.9 Another LMWH, dalteparin, also falls into pregnancy category B. Both the American College of Obstetricians and Gynecologists2 and the Society for Maternal-Fetal Medicine10 endorse the use of LMWH in pregnancy with appropriate counseling.8
Although it is the drug of choice in the nonpregnant population, warfarin is contraindicated in pregnancy because it can cross the placenta and has been linked to adverse pregnancy outcomes.
Several studies have demonstrated an association between first-trimester warfarin exposure and a constellation of structural birth defects, termed “warfarin embryopathy,” which includes craniofacial and skeletal defects. Exposure in any trimester is associated with fetal and neonatal intracranial hemorrhage.3
For these reasons, warfarin is contraindicated in pregnancy with the rare exception of women with mechanical prosthetic heart valves.4
Question 3How does LMWH differ from unfractionated heparin?
LMWH is more efficient. Both UH and LMWH contain an essential pentasaccharide within their polymer structure that binds to and enhances antithrombin III, which in turn inhibits thrombin and activated factor X (Xa). Because of its smaller size, LMWH preferentially inhibits Xa, which is higher in the coagulation cascade. Inhibition of a single molecule of Xa prevents the formation of many molecules of thrombin. Molecule for molecule, LMWH is a more efficient anticoagulant than UH (FIGURE).11
The second way that LMWH differs from UH also relates to the molecule’s size. Smaller heparin molecules are less likely to be deactivated by tissue proteins. This results in improved bioavailability of the administered dose. Greater bioavailability translates to a more predictable dose-response relationship, a long half-life, and better anticoagulation.12
FIGURE Simplified schematic of the coagulation cascade
Question 4What are the clinical advantages of LMWH?
LMWH has longer-lasting effects and subcutaneous dosing. It also has fewer side effects than UH.
Because of its large size and positive charge, UH has an unfavorable pharmacokinetic profile. Tissue proteins interfere with and deactivate it, and many of these proteins increase in pregnancy and with advancing gestation. Heparin tissue levels are therefore erratic and unpredictable and often lead to periods of subtherapeutic coverage. This is true even with intravenous (IV) dosing.
Rapid absorption, no intravenous dosing. In contrast, because of its smaller size, LMWH is rapidly and predictably absorbed from a subcutaneous injection. Intravenous dosing is not necessary to obtain adequate tissue levels. Once in tissue, it is deactivated more slowly and therefore maintains its anticoagulation effect longer. Consequently, patients are exposed to fewer periods of subtherapeutic anticoagulation. A longer half-life also translates to more favorable dosing routes (subcutaneous rather than IV) and regimens (daily versus twice daily). Similarly, since the dose-response is predictable and tissue levels are more constant, frequent monitoring of treatment response is not routinely necessary.
Fewer side effects. Another advantage of LMWH over UH is the improved side-effect profile. Patients on LMWH have decreased risk of hemorrhage, osteoporosis, and antibody-mediated thrombocytopenia.11 Although most data regarding these advantages come from the nonpregnant population, it is plausible to speculate that these traits also are present in pregnant women (TABLE 2).
TABLE 2
Advantages and disadvantages of LMWH in pregnancy
| ADVANTAGES |
| More effective anticoagulation |
| Better dose-response |
| Longer half-life |
| Better dosing route |
| Decreased need for monitoring |
| Fewer side effects |
| DISADVANTAGES |
| Longer half-life |
| Risk of hematoma with epidural anesthesia |
| Not fully reversible with protamine sulfate |
| Anticoagulation effect difficult to monitor |
| Higher cost |
Question 5What are the disadvantages?
They include the long half-life, risk of hematoma with epidural anesthesia, lower efficacy of the antidote, monitoring difficulty, and higher cost.
The long half-life of LMWH is both an advantage and disadvantage. For example, when UH is administered intravenously, it has a half-life of 30 to 60 minutes. When it is given subcutaneously, the half-life is 1 to 2 hours. This means that a patient can undergo vaginal delivery or even surgery within hours of her last subcutaneous UH injection.
In contrast, LMWH has a half-life of approximately 4 hours. A recent dose may increase the risk of operative morbidity in the form of overt or delayed hemorrhage, hematoma, or wound dehiscence.
Not for use with epidural anesthesia. Case reports of epidural hematomas after regional anesthesia during orthopedic procedures have caused considerable concern about the use of LMWH and the placement of a neuraxial block such as a spinal or epidural.13 Many anesthesiologists will not place an epidural or spinal within 24 hours of a LMWH dose.14 However, as experience with these agents in the nonpregnant population expands, a more evidence-based approach is likely to develop.
Antidote less effective. Protamine sulfate is a strong base that binds with the positively charged UH molecule, thereby serving as an antidote through competitive inhibition. Because of its smaller size, LMWH is reversed by protamine to a lesser degree (approximately 60% effective).14 Therefore, hemorrhage associated with LMWH may require replacement of blood components, which carries the risks of infection and transfusion reactions.
Difficult to monitor. The anticoagulation effect of UH can be reliably monitored by the activated partial thromboplastin time (aPTT), which is a widely available test with a rapid turnaround. However, the anticoagulation effect of LMWH is not reflected by the aPTT. Assessment of LMWH activity requires assessment of the antifactor Xa level, a test that is not universally available and has a longer turnaround.
Higher cost. Another limitation of LMWH is its cost. A single, subcutaneous, prophylactic 40-mg dose of enoxaparin costs about $30, compared with about $1 for an equivalent dose of unfractionated heparin (5,000 U subcutaneously twice a day). Because of its increased cost, many insurance companies do not authorize the use of LMWH for prolonged periods such as pregnancy and the postpartum period. However, studies in the nonpregnant population have demonstrated overall decreased cost over UH due to the reduced need for monitoring, shorter length of hospital stay, and diminished treatment failure.
Question 6What is the dose for prophylaxis and treatment?
The standard dose of enoxaparin for prophylaxis in pregnancy and the postpartum period is 40 mg administered subcutaneously every 24 hours (TABLE 3). Therapeutic anticoagulation (sometimes referred to as a “weightadjusted” dose) is usually achieved with 1 mg/kg every 12 hours.
Dalteparin can be given in a prophylactic dose of 5,000 U subcutaneously every 24 hours and a therapeutic dose of 200 U/kg every 24 hours.15 Dosing may need to be adjusted with advancing gestation as plasma volume, renal clearance, and tissue proteins increase.
The various LMWH preparations are not equivalent in their pharmacokinetics. Generally, clinicians familiarize themselves with a single agent. It also is important to note that dosing regimens in pregnancy are not evidence-based but largely “borrowed” from nonpregnant regimens. They also vary widely in the available literature.
TABLE 3
Common low molecular weight heparins
| BRAND NAME | GENERIC NAME | PROPHYLACTIC DOSE | THERAPEUTIC DOSE |
|---|---|---|---|
| Lovenox | Enoxaparin | 40 mg every 24 hours | 1 mg/kg every 12 hours |
| Fragmin | Dalteparin | 5,000 U every 24 hours | 200 U/kg every 24 hours |
| Note: All doses are subcutaneous | |||
Question 7Under what conditions can LMWH be given?
Generally, LMWH can replace UH in any condition that warrants prophylactic or therapeutic anticoagulation in pregnancy, except the acute management of pulmonary embolism and in women with mechanical heart valves.
Prophylactic dosing can be offered to women with a previous thromboembolic event such as deep vein thrombosis (DVT) or pulmonary embolism that was not associated with a reversible and temporary predisposing risk factor such as immobilization or trauma. (In general, pregnancy is not seen as a reversible or temporary risk factor.)
Prophylactic or therapeutic dosing is sometimes offered to women with a hereditary thrombophilia, antiphospholipid syndrome, or other vasculopathies and connective-tissue diseases. In addition, LMWH is an accepted first-line therapeutic anticoagulant for acute DVT in pregnancy.
Not for use in treating acute pulmonary embolism. Evidence is insufficient to support the use of LMWH as a first-line anticoagulant for acute pulmonary embolism. To date, IV loading with UH is the standard of care, with conversion to LMWH after 4 to 5 days of therapeutic UH anticoagulation. This may change as experience with LMWH increases.
Not for use with mechanical prosthetic heart valves. Because of case reports of recurrent thromboembolism resulting in maternal and fetal death in pregnant women with mechanical prosthetic heart valves on therapeutic LMWH, the manufacturer of enoxaparin warns against its use in pregnancy in these women.9 However, similar outcomes have been reported with UH and warfarin. This forces clinicians to consider potentially less effective and more problematic agents for anticoagulation in this fortunately rare circumstance.
Contemporary management involves converting from warfarin to subcutaneous heparin or the heparin pump once pregnancy is established and before organogenesis (at approximately 6 weeks’ gestation), followed by frequent monitoring of aPTT. Alternatively, warfarin can be resumed after organogenesis (at about 12 weeks) and continued into the third trimester, followed by conversion to heparin pump, subcutaneous injections, or an IV drip near term. Both options carry significant maternal and fetal risk and should be performed in a multidisciplinary fashion along with cardiology and vascular medicine.16
Question 8How do I start and stop LMWH?
Start LMWH as a subcutaneous injection without IV loading. A baseline complete blood count for platelet count is reasonable. Patient education is straightforward.
Prophylactic dosing can be initiated on an outpatient basis immediately after patient education and procurement of the medicine.
Therapeutic dosing for DVT usually is begun during hospitalization, with 1 mg per kilogram given subcutaneously every 12 hours. Barring other comorbidities, discharge can be achieved after patient education. The exception is treatment of acute pulmonary embolism.Conversion to LMWH is achieved once the patient is fully anticoagulated.
Discontinuation. Pregnancy is a period of shifting maternal and fetal status, when indications for delivery can develop suddenly and with little warning (eg, abruptio placenta, nonreassuring antenatal testing).
LMWH dosing regimens in pregnancy are not evidence-based but largely “borrowed” from nonpregnant regimens.
A major consideration is the long half-life of LMWH. If a patient has had a recent subcutaneous injection of LMWH followed by an urgent indication for delivery, she may be anticoagulated during her delivery and be at increased risk for hemorrhage. Similarly, she may not be a candidate for epidural or spinal anesthesia because of the risk of epidural hematoma. If cesarean section is indicated, she may need general anesthesia, which is associated with increased maternal morbidity. If hemorrhage occurs, she may require transfusion of blood products, which carries the risks of infection and transfusion reactions.
Three strategies for peripartum management with LMWH are:
- Leave the patient on LMWH until the onset of labor. Depending on the timing and dose of her last injection, she may or may not be a candidate for regional anesthesia in labor. With prophylactic dosing, the risk of significant hemorrhage is low.
- Time delivery so that the patient withholds her PMinjection and presents for delivery the following morning, 24 hours after her last dose. This option is attractive when cesarean delivery is planned. The advantage of this approach is that it keeps the window of thrombosis as narrow as possible. The disadvantage is that the patient may enter labor or require delivery before the chosen delivery date, in which case she may be at risk for hemorrhage.
- Convert the patient to UH at approximately 36 to 37 weeks’ gestation. The advantage? The shorter half-life of UH increases the possibility for regional anesthesia and decreases the risk for hemorrhage. The disadvantage: The risk for thrombosis as well as complications (thrombocytopenia, osteoporosis, thrombosis) may be greater with UH for the reasons described earlier.
Restarting after delivery. Strategies for continued thromboprophylaxis in the postpartum period include continuing LMWH for an additional 6 to 8 weeks or converting to warfarin. Warfarin can be taken orally but requires frequent monitoring and adjustment of dosing, which is unnecessary with LMWH. Many patients choose to continue LMWH because they are familiar with the routine of daily injections. There is little data to support or refute either approach.
There also is little data to guide the decision of when to reinitiate prophylactic or therapeutic doses of LMWH after vaginal or cesarean delivery. Translating from other surgical subspecialties, most obstetricians are comfortable resuming both prophylactic and therapeutic doses at 6 hours after vaginal delivery and 8 hours after cesarean section.
Question 9How do I monitor the effectiveness of LMWH?
The frequent monitoring necessary with UH is not required with LMWH, since the increased bioavailability of LMWH leads to reliable tissue levels. The aPTT level does not correlate well with the anticoagulation effect of LMWH.
Antifactor Xa levels—sometimes referred to as the LMWH assay—of 0.5 to 1.2 IU/mL are considered adequate for therapeutic anticoagulation in the nonpregnant population. Peak anti-Xa activity is achieved approximately 4 hours after subcutaneous injection. AntiXa levels should therefore be drawn 4 hours after the last dose.17 Prophylactic subcutaneous dosing of enoxaparin 40 mg daily generally does not require monitoring of anti-Xa levels.
In nonpregnant women, routine monitoring is seldom necessary. Gravidas, however, are constantly changing in terms of weight, plasma volume, renal clearance, and amount of heparin-binding proteins. Because of this, periodic monitoring (every 4 weeks) is reasonable until an evidence-based recommendation can be made.
Question 10When can I provide neuraxial anesthesia?
Most anesthesiologists are reluctant to perform neuraxial anesthesia within 12 hours of the last prophylactic dose or 24 hours of the last therapeutic dose of LMWH because of the risk of epidural and spinal hematoma.
The American Society of Regional Anesthesia recommends that neuraxial anesthesia be withheld for 24 hours after the last therapeutic dose and 12 hours after a prophylactic dose. That organization did not recommend checking anti-Xa levels, since they do not adequately predict the risk of bleeding.9 However, as experience with LMWH broadens in nonobstetric surgical cases, such as orthopedic and cardiac procedures, it is likely that greater familiarity with the medication will lead to better evidence and broader acceptance.
Dr. Emery reports no financial relationships relevant to this article.
1. Gherman RB, Goodwin TM, Leung JD, Byrne JD, Hethumumi R, Montoro M. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol. 1999;94:730-734.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #19: Thromboembolism in Pregnancy. Washington, DC: ACOG; 2000.
3. Hall JG, Pauli RM, Wilson KM. Maternal and fetal sequelae of anticoagulation during pregnancy. Am J Med. 1980;68:122-140.
4. Born D, Martinez EE, Almeida PAM, et al. Pregnancy in patients with mechanical heart valves: the effects of anticoagulation on mother, fetus and neonate. Am Heart J. 1992;124:413-417.
5. Omri A, Delayoye JF, Anderson H, Bachmann F. Low molecular weight heparin Novo (LHN-1) does not cross the placenta during the second trimester of pregnancy. Thromb Haemost. 1989;61:55-56.
6. Sanson BJ, Lensing AWA, Prins MH, et al. Safety of low molecular weight heparin in pregnancy: a systematic review. Thromb Haemost. 1999;81:668-672.
7. Nelson-Piercy C, Letsky EA, de Sweit M. Low molecular weight heparin for obstetric thromboprophylaxis: experience of sixty-nine pregnancies in sixty-one women at risk. Am J Obstet Gynecol. 1997;176:1062-1068.
8. Forestier F, Daffos F, Rainaut M, et al. Low molecular weight heparin (CY 216) does not cross the placenta during the third trimester of pregnancy. Thromb Haemost. 1987;57:234.-
9. Lovenox injection [package insert]. Bridgewater, NJ: Aventis; 2003.
10. Enoxaprin sodium (Lovenox) and pregnancy. SMFM Practice Committee Announcement posted at http://www.smfm.org
11. Hirsh J, Warkentin TE, Raschke R, Granger C, Ohman EM, Dalen JE. Heparin and low molecular weight heparin: mechanisms of action, pharmacokinetics, dosing considerations, monitoring, efficacy and safety. Chest. 1998;114:489S-510S.
12. Weitz JI. Low-molecular-weight heparins. N Engl J Med. 1997;337:688-698.
13. US Department of Health and Human Services. FDA Public Health Advisory. Reports of Epidural and Spinal Hematomas with Concurrent Use of Low Molecular Weight Heparin and Spinal/Epidural Anesthesia or Spinal Puncture. Rockville, MD: Food and Drug Administration; December 1997.
14. American Society of Regional Anesthesia. Recommendations for Neuraxial Anesthesia and Anticoagulation. Richmond, Va: ASRA; 1998.
15. Hirsh J, Raschke R, Warkentin TE, et al. Heparin: mechanism of action, pharmacokinetics, dosing considerations, efficacy, and safety. Chest. 1995;108:258S-275S.
16. Anticoagulation and Enoxaparin Use in Patients with Prosthetic Heart Valves and/or Pregnancy. Clinical Cardiology Consensus Reports. Atlanta, Ga: American Health Consultants; 2002.
17. Ginsberg JS, Greer I, Hirsh J. Use of antithrombotic agents during pregnancy. Chest. 2001;119:122S-131S.
1. Gherman RB, Goodwin TM, Leung JD, Byrne JD, Hethumumi R, Montoro M. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol. 1999;94:730-734.
2. American College of Obstetricians and Gynecologists. Practice Bulletin #19: Thromboembolism in Pregnancy. Washington, DC: ACOG; 2000.
3. Hall JG, Pauli RM, Wilson KM. Maternal and fetal sequelae of anticoagulation during pregnancy. Am J Med. 1980;68:122-140.
4. Born D, Martinez EE, Almeida PAM, et al. Pregnancy in patients with mechanical heart valves: the effects of anticoagulation on mother, fetus and neonate. Am Heart J. 1992;124:413-417.
5. Omri A, Delayoye JF, Anderson H, Bachmann F. Low molecular weight heparin Novo (LHN-1) does not cross the placenta during the second trimester of pregnancy. Thromb Haemost. 1989;61:55-56.
6. Sanson BJ, Lensing AWA, Prins MH, et al. Safety of low molecular weight heparin in pregnancy: a systematic review. Thromb Haemost. 1999;81:668-672.
7. Nelson-Piercy C, Letsky EA, de Sweit M. Low molecular weight heparin for obstetric thromboprophylaxis: experience of sixty-nine pregnancies in sixty-one women at risk. Am J Obstet Gynecol. 1997;176:1062-1068.
8. Forestier F, Daffos F, Rainaut M, et al. Low molecular weight heparin (CY 216) does not cross the placenta during the third trimester of pregnancy. Thromb Haemost. 1987;57:234.-
9. Lovenox injection [package insert]. Bridgewater, NJ: Aventis; 2003.
10. Enoxaprin sodium (Lovenox) and pregnancy. SMFM Practice Committee Announcement posted at http://www.smfm.org
11. Hirsh J, Warkentin TE, Raschke R, Granger C, Ohman EM, Dalen JE. Heparin and low molecular weight heparin: mechanisms of action, pharmacokinetics, dosing considerations, monitoring, efficacy and safety. Chest. 1998;114:489S-510S.
12. Weitz JI. Low-molecular-weight heparins. N Engl J Med. 1997;337:688-698.
13. US Department of Health and Human Services. FDA Public Health Advisory. Reports of Epidural and Spinal Hematomas with Concurrent Use of Low Molecular Weight Heparin and Spinal/Epidural Anesthesia or Spinal Puncture. Rockville, MD: Food and Drug Administration; December 1997.
14. American Society of Regional Anesthesia. Recommendations for Neuraxial Anesthesia and Anticoagulation. Richmond, Va: ASRA; 1998.
15. Hirsh J, Raschke R, Warkentin TE, et al. Heparin: mechanism of action, pharmacokinetics, dosing considerations, efficacy, and safety. Chest. 1995;108:258S-275S.
16. Anticoagulation and Enoxaparin Use in Patients with Prosthetic Heart Valves and/or Pregnancy. Clinical Cardiology Consensus Reports. Atlanta, Ga: American Health Consultants; 2002.
17. Ginsberg JS, Greer I, Hirsh J. Use of antithrombotic agents during pregnancy. Chest. 2001;119:122S-131S.