User login
Treating methamphetamine abuse disorder: Experience from research and practice
Methamphetamine and other amphetamine-type stimulants are the world’s second most widely used group of illicit substances (after Cannabis), with prevalence of abuse varying by region and by locales within nations. As prescription use of stimulants has grown dramatically in recent years, so has abuse of these substances.
Given the widespread and growing misuse of amphetamine-type stimulants (Box,1-3), clinicians are faced with the need to learn how to recognize and manage methamphetamine abuse. Both prescribed and non-prescribed uses of stimulants present complex challenges; in this article, we examine effects, manifestations, and current evidence-based behavioral and medical treatments of methamphetamine misuse and abuse, and look ahead to investigational therapies that hold promise for improving the limited existing approaches to management.
Effects and manifestations of methamphetamine use
Different routes of administration produce different consequences, in terms of medical comorbidity and propensity to induce addiction. Smoked or injected, methamphetamine enters the brain in seconds; snorted or taken by mouth, the drug produces its effects in several minutes and a half hour, respectively.
Rapid uptake and effects of methamphetamine result from its ability to cross the blood−brain barrier. Its primary effects are caused by inhibition of dopamine storage and release of intracellular dopamine.
Methamphetamine stimulates the CNS and the cardiovascular system through release of dopamine and norepinephrine, which increases blood pressure, body temperature, and heart rate, and, occasionally, induces arrhythmia that can contribute to heart attack and stroke. Users experience euphoria, hypervigilance, suppressed appetite, and increased libido.
Binge use is common to sustain euphoria and other reinforcing effects, which subside with rapidly developing tolerance. After days of repeated dosing, elevated methamphetamine blood levels can lead to mood disturbances, repetitive motor activities, and psychotic symptoms such as hallucinations, delusions, and paranoia. Acute psychosis can bring on violence and other injurious behaviors that involve law enforcement and emergency medical services.
When methamphetamine is used over months or years, health consequences include anorexia, tremor, so-called meth mouth (broken teeth, infections, cavities, burns), insomnia, panic attacks, confusion, depression, irritability, and impaired memory and other cognitive processes.
Treating methamphetamine intoxication and withdrawal
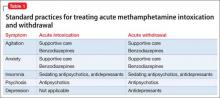
At initial clinical contact with a person who abuses methamphetamine, practitioners may face several acute consequences requiring attention. Prominent among presenting conditions, especially during acute intoxication, are agitation, anxiety, and psychotic symptoms, which may improve by providing the patient with calming reassurance in a quiet space. In more severe cases, a benzodiazepine, antipsychotic, or both might be indicated4,5 (Table 1).
Methamphetamine withdrawal is characterized by anxiety, depression, and insomnia. These symptoms generally resolve in a matter of days after the start of withdrawal without pharmacotherapy. In some cases, depression or psychosis becomes chronic, as a result of methamphetamine use itself6 or as an emergent concomitant psychiatric condition.
A sedative-hypnotic medication or an anxiolytic can be used as necessary to ameliorate insomnia or anxiety, respectively. Prolonged depression can be treated with an antidepressant. An antipsychotic might be indicated for long-term management of patients who have persistent psychosis.
Therapy for methamphetamine abuse
Treatment of methamphetamine abuse— with the goal of stopping drug use—is a complicated matter on 2 counts:
• No medications are FDA-approved for treating methamphetamine addiction.
• There are no accepted substitution medications (ie, stimulants that can be used in place of methamphetamine, as is available for opioid addiction).
Pharmacotherapeutic possibilities. The rationale for considering replacement pharmacotherapy is that psychostimulants can counter the cravings, dysphoria, and fatigue produced by methamphetamine withdrawal and can alleviate methamphetamine-related cognitive impairment. Although dextroamphetamine and other psychostimulants have been evaluated in small trials as replacement medication, most countries are reluctant to consider their use, because of the potential for abuse and accompanying liability.
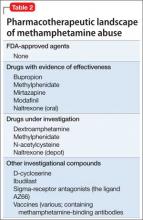
After decades of medication research, several drugs have shown promise for reducing methamphetamine abuse, although results have not been robust (Table 2):
• Bupropion has shown benefit in reducing methamphetamine use among users with less severe addiction.7,8
• Methylphenidate, a psychostimulant FDA-approved for attention-deficit/hyperactivity disorder, was found to reduce methamphetamine use compared with placebo in a European sample of amphetamine injectors who had attained abstinence in a residential program.9 Those results were not replicated in a recent study by Miles et al, however.10 A study with a more clinically realistic approach (ie, not requiring daily clinic attendance, as in the Miles trial) vs placebo for methamphetamine abuse was recently published, with promising results that require confirmation in further study.11
• Mirtazapine, an antidepressant, has demonstrated efficacy in reducing methamphetamine use compared with placebo.12
• Modafinil, another medication with stimulant properties, reduced methamphetamine use in a subgroup analysis of heavy users, compared with placebo.13
• Dextroamphetamine, 60 mg/d, showed no difference in reducing methamphetamine compared with placebo, but did diminish cravings and withdrawal symptoms.14
A trial of the phosphodiesterase inhibitor ibudilast (not available in the United States) for methamphetamine abuse is underway. Ibudilast has anti-inflammatory activity in the peripheral immune system and the central nervous system, including modulating the activity of glial cells.15
Many medications have yielded negligible results in studies: selegiline, baclofen, sertraline, topiramate, gabapentin, rivastigmine, risperidone, and ondansetron.16 Recent evaluation of disulfiram, vigabatrin, and lobeline also has yielded inconsistent findings.17
No drug has proved effective for preventing relapse; research continues, focusing on several types of compounds that target various mechanisms: the dopamine system, the opioid system (by way of the γ-aminobutyric acid inhibitory system), and cortico-limbic reward circuitry.
Once-monthly injectable naltrexone has potential for ameliorating craving and relapse by modulating the opioid receptor system. However, the drug has not been adequately explored in generalizable settings of methamphetamine users.
Trials of oral naltrexone in Sweden have shown encouraging results, including reduced subjective effects and amphetamine use in open-label trials18,19; results were replicated in a subsequent placebo-controlled trial.20 In an unpublished study, however, no differences in amphetamine use were found among users randomized to depot naltrexone or placebo.21
Depot naltrexone with assured dosing might have a role in treating methamphetamine abuse, however; a combination of depot naltrexone and oral bupropion is being examined in a National Institute on Drug Abuse Clinical Trials Networks study that commenced in 2013. Pairing medications that have different mechanistic targets might work toward promoting cessation of methamphetamine abuse and reducing relapse once patients are abstinent.
In an early phase of research, but showing promise based on their ability to target different systems, are:
• N-acetylcysteine, modulator of the glutamate system
• D3 antagonists and partial agonists22
• varenicline.23
Potential “vaccines” against methamphetamine are in preclinical development, including use of a protein carrier or other immune-stimulating molecule to create antibodies that bind methamphetamine in the bloodstream and block its psychoactive effects.24,25
Sigma receptor effects are being studied in rodents as potential targets to mitigate effects of methamphetamine. The ligand AZ66, a sigma receptor antagonist, has demonstrated efficacy in reducing methamphetamine-induced cognitive impairment—suggesting that the sigma receptor has a potential role in ameliorating methamphetamine-related neurotoxicity.26
Psychosocial and behavioral interventions. Among the non-drug treatments that have demonstrated efficacy for treating methamphetamine abuse, cognitive-behavioral therapy (CBT) and contingency management (CM) have been most widely studied and applied in treatment settings.
CBT involves individual or group counseling that focuses on relapse prevention skills, including identification of relapse triggers, strategies to diminish cravings, and engagement in alternative non-drug activities27,28 (Table 3).
CM, which is based on positive reinforcement, offers tangible reinforcers, or rewards, for behaviors (eg, clinic attendance, providing a drug-free urine sample) according to guidelines set by the practitioner. CM-based interventions are the most reliably documented approaches for treating methamphetamine abuse,29,30 but their utility might prove to be most efficient in combination with medication— once suitable pharmacotherapeutic options emerge.
Although CBT and CM remain accepted standard treatments for methamphetamine abuse, outcomes are suboptimal.27 Both interventions have a high rate of dropout during the first month of treatment and a >50% relapse rate 6 to 19 months after treatment ends.31-33
As with treatment of other substance use disorders, patients who abuse methamphetamine can benefit from residential treatment in a drug-free setting for ≥30 days.34 In the residential approach, removing access to drugs, drug cues, and drug-using acquaintances combined with group and individual counseling reaches an inevitable end: discharge into the community. Then the patient’s battle to avoid relapse begins.
Because cognitive impairment is common among patients who abuse methamphetamine, even after they stop using,35 researchers have examined the potential for increasing participation in psychosocial interventions such as CBT by using medications that might have potential to increase cognitive function, such as modafinil.36 Increased attention and concentration afforded by medication could enhance efficacy of CBT. Results of trials and new drug development have been mixed37; no clear candidate for preventing relapse through any of the putative mechanisms of action has emerged.
Relapse is a problematic target for treatment
Ending methamphetamine abuse and sustaining abstinence from stimulants require a change in the cognitive associations that have been laid down in a drug user’s memory. Relapse occurs because of recalled memories that can be cued, or triggered, by internal or external stimuli. Eliminating drug memories, perhaps assisted by medications such as d-cycloserine (an antagonist of the N-methyl-d-aspartate receptor), could be useful for suppressing the inclination to relapse.
Last, alternative, non-drug forms of cognitive amendment have shown efficacy in preventing relapse: for example, incorporating mindfulness meditation, which has shown promise in managing craving for methamphetamine and decreasing reactivity to environmental cues for drug use.38
Bottom Line
Practitioners who work in emergency, inpatient, and outpatient settings will be called on more and more to treat acute stimulant intoxication and withdrawal, stimulant-induced psychosis, and methamphetamine abuse. Few evidence-based treatments and no FDA-approved medications are available to treat this addiction; many drugs and a few psychotherapeutic techniques have shown promise. Ongoing research promises to deliver medical and behavioral interventions to help patients quit using methamphetamine.
Related Resources
• Karch SB, Drummer O. Karch’s pathology of drug abuse, fifth ed. Boca Raton, FL: CRC Press/Taylor & David; 2013.
• Roll J, Rawson RA, Ling W, eds. Methamphetamine addiction: from basic science to treatment. New York, NY: Guilford Press; 2009.
• Sheff D. Beautiful boy: a father’s journey through his son’s addiction. New York, NY: Houghton Mifflin Harcourt Publishing Company; 2008.
• Sheff N. Tweak: growing up on methamphetamines. New York, NY: Antheneum Books for Young Readers; 2007.
• National Institute on Drug Abuse. Drugs of abuse. www. drugabuse.gov/drugs-abuse/methamphetamine.
Drug Brand Names
Baclofen • Lioresal Naltrexone (depot) • Vivitrol
Bupropion • Wellbutrin Naltrexone (oral) • ReVia
D-cycloserine • Seromycin Ondansetron • Zofran
Dexreoamphetaime • Adderall Risperidone • Risperadal
Disulfiram • Antabuse Rivastigimine • Exelon
Gabapentin • Neurontin Selegiline• EMSAM
Methylphenidate • Ritalin Sertraline • Zoloft
Mirtazapine • Remeron Topiramate • Topamax
Modafinil • Provigil Varenicline • Chantix
N-acetylcysteine • Mucomyst Vigabatrin • Sabril
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. UNODC. World Drug Report 2012 (United Nations publication, Sales No. E.12.XI.1). http://www.unodc. org/documents/data-and-analysis/WDR2012/ WDR_2012_web_small.pdf. Published 2012. Accessed August 4, 2014.
2. UNODC. World Drug Report 2010 (United Nations publication, Sales No. E.10.XI.13). http://www.unodc. org/documents/wdr/WDR_2010/World_Drug_ Report_2010_lo-res.pdf. Published 2010. Accessed August 4, 2014.
3. Rawson RA, Gonzales R, Brecht M, et al. Evaluation of the California Outcomes Measurement System (CalOMS): Final Report 2008. http://www.uclaisap.org/assets/documents/ California-ADP-DHCS-Evals/2007-2008_CalOMS%20 Report.pdf. Published 2008. Accessed August 4, 2014.
4. Shoptaw SJ, Kao U, Ling W. Treatment for amphetamine psychosis. Cochrane Database Syst Rev. 2009;(1):CD003026.
5. Leelahanaj T, Kongsakon R, Netrakom P. A 4-week, double-blind comparison of olanzapine with haloperidol in the treatment of amphetamine psychosis. J Med Assoc Thai. 2005;88(suppl 3):S43-S52.
6. McKetin R, McLaren J, Lubman D, et al. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101(10):1473-1478.
7. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
8. McCann DJ, Li SH. A novel, nonbinary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: reanalysis of a multisite trial. CNS Neurosci Ther. 2012;18(5):414-418.
9. Tiihonen J, Kuoppasalmi K, Föhr J, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164(1): 160-162.
10. Miles SW, Sheridan J, Russell B, et al. Extended-release methylphenidate for treatment of amphetamine/ methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108(7): 1279-1286.
11. Ling W, Chang L, Hillhouse M, et al. Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder. Addiction. 2014;109(9): 1489-1500.
12. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175.
13. Heinzerling KG, Swanson AN, Kim S, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109(1-3):20-29.
14. Galloway GP, Buscemi R, Coyle JR, et al. A randomized, placebo-controlled trial of sustained-release dextro-amphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther. 2011;89(2):276-282.
15. Snider SE, Hendrick ES, Beardsley PM. Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur J Pharmacol. 2013;701(1-3):124-130.
16. Ling W, Rawson R, Shoptaw S. Management of methamphetamine abuse and dependence. Curr Psychiatry Rep. 2006;8(5):345-354.
17. Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24(6):541-550.
18. Jayaram-Lindström N, Wennberg P, Beck O, et al. An open clinical trial of naltrexone for amphetamine dependence: compliance and tolerability. Nord J Psychiatry. 2005;59(3):167-171.
19. Jayaram-Lindström N, Konstenius M, Eksborg S, et al. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2007;33(8):1856-1863.
20. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448.
21. Woody GE, Tyrfingsoon P. Symposium XI: Emerging data on efficacy and clinical applications of extended-release naltrexone formulations. 75th Annual Meeting, College on Problems of Drug Dependence. June 19, 2013; San Diego, CA.
22. Newman AH, Blaylock BL, Nader MA, et al. Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochem Pharmacol. 2012;84(7):882-890.
23. Verrico CD, Mahoney JJ 3rd, Thompson-Lake DG, et al. Safety and efficacy of varenicline to reduce positive subjective effects produced by methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol. 2014;17(2):223-233.
24. Miller ML, Moreno AY, Aarde S, et al. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol Psychiatry. 2013;73(8):721-728.
25. Shen XY, Kosten TA, Lopez AY, et al. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013;129(1-2):41-48.
26. Seminerio MJ, Robson MJ, Abdelazeem AH, et al. Synthesis and pharmacological characterization of a novel sigma receptor ligand with improved metabolic stability and antagonistic effects against methamphetamine. AAPS J. 2012;14(1):43-51.
27. Rawson RA, Marinelli-Casey P, Anglin M, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99(6):708-717.
28. Vocci FJ, Montoya ID. Psychological treatments for stimulant misuse, comparing and contrasting those for amphetamine dependence and those for cocaine dependence. Curr Opin Psychiatry. 2009;22(3):263-268.
29. Rawson RA, McCann MJ, Flammino F, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267-274.
30. Roll JM, Petry NM, Stitzer ML, et al. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry. 2006;163(11):1993-1999.
31. Brecht ML, von Mayrhauser C, Anglin MD. Predictors of relapse after treatment for methamphetamine use. J Psychoactive Drugs. 2000;32(2):211-220.
32. Smout MF, Longo M, Harrison S, et al. Psychosocial treatment for methamphetamine use disorders: a preliminary randomized controlled trial of cognitive behavior therapy and Acceptance and Commitment Therapy. Subst Abus. 2010;31(2):98-107.
33. Wang G, Shi J, Chen N, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;24;8(7):e68791. doi: 10.1371/ journal.pone.0068791.
34. McKetin R, Lubman DI, Baker AL, et al. Dose-related psychotic symptoms in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry. 2013;70(3):319-324.
35. Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35(6):593-598.
36. Dean AC, Sevak RJ, Monterosso JR, et al. Acute modafinil effects on attention and inhibitory control in methamphetamine-dependent humans. J Stud Alcohol Drugs. 2011;72(6):943-953.
37. Zullino DF, Benguettat D, Khazaal Y. Improvement of cognitive performance by topiramate: blockage of automatic processes may be the underlying mechanism [Comment on: Effects of topiramate on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition in recently abstinent methamphetamine-dependent individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2007.] Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):787.
38. Witkiewitz K, Lustyk M, Bowen S. Retraining the addicted brain: a review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychol Addict Behav. 2013;27(2):351-365.
Methamphetamine and other amphetamine-type stimulants are the world’s second most widely used group of illicit substances (after Cannabis), with prevalence of abuse varying by region and by locales within nations. As prescription use of stimulants has grown dramatically in recent years, so has abuse of these substances.
Given the widespread and growing misuse of amphetamine-type stimulants (Box,1-3), clinicians are faced with the need to learn how to recognize and manage methamphetamine abuse. Both prescribed and non-prescribed uses of stimulants present complex challenges; in this article, we examine effects, manifestations, and current evidence-based behavioral and medical treatments of methamphetamine misuse and abuse, and look ahead to investigational therapies that hold promise for improving the limited existing approaches to management.
Effects and manifestations of methamphetamine use
Different routes of administration produce different consequences, in terms of medical comorbidity and propensity to induce addiction. Smoked or injected, methamphetamine enters the brain in seconds; snorted or taken by mouth, the drug produces its effects in several minutes and a half hour, respectively.
Rapid uptake and effects of methamphetamine result from its ability to cross the blood−brain barrier. Its primary effects are caused by inhibition of dopamine storage and release of intracellular dopamine.
Methamphetamine stimulates the CNS and the cardiovascular system through release of dopamine and norepinephrine, which increases blood pressure, body temperature, and heart rate, and, occasionally, induces arrhythmia that can contribute to heart attack and stroke. Users experience euphoria, hypervigilance, suppressed appetite, and increased libido.
Binge use is common to sustain euphoria and other reinforcing effects, which subside with rapidly developing tolerance. After days of repeated dosing, elevated methamphetamine blood levels can lead to mood disturbances, repetitive motor activities, and psychotic symptoms such as hallucinations, delusions, and paranoia. Acute psychosis can bring on violence and other injurious behaviors that involve law enforcement and emergency medical services.
When methamphetamine is used over months or years, health consequences include anorexia, tremor, so-called meth mouth (broken teeth, infections, cavities, burns), insomnia, panic attacks, confusion, depression, irritability, and impaired memory and other cognitive processes.
Treating methamphetamine intoxication and withdrawal
At initial clinical contact with a person who abuses methamphetamine, practitioners may face several acute consequences requiring attention. Prominent among presenting conditions, especially during acute intoxication, are agitation, anxiety, and psychotic symptoms, which may improve by providing the patient with calming reassurance in a quiet space. In more severe cases, a benzodiazepine, antipsychotic, or both might be indicated4,5 (Table 1).
Methamphetamine withdrawal is characterized by anxiety, depression, and insomnia. These symptoms generally resolve in a matter of days after the start of withdrawal without pharmacotherapy. In some cases, depression or psychosis becomes chronic, as a result of methamphetamine use itself6 or as an emergent concomitant psychiatric condition.
A sedative-hypnotic medication or an anxiolytic can be used as necessary to ameliorate insomnia or anxiety, respectively. Prolonged depression can be treated with an antidepressant. An antipsychotic might be indicated for long-term management of patients who have persistent psychosis.
Therapy for methamphetamine abuse
Treatment of methamphetamine abuse— with the goal of stopping drug use—is a complicated matter on 2 counts:
• No medications are FDA-approved for treating methamphetamine addiction.
• There are no accepted substitution medications (ie, stimulants that can be used in place of methamphetamine, as is available for opioid addiction).
Pharmacotherapeutic possibilities. The rationale for considering replacement pharmacotherapy is that psychostimulants can counter the cravings, dysphoria, and fatigue produced by methamphetamine withdrawal and can alleviate methamphetamine-related cognitive impairment. Although dextroamphetamine and other psychostimulants have been evaluated in small trials as replacement medication, most countries are reluctant to consider their use, because of the potential for abuse and accompanying liability.
After decades of medication research, several drugs have shown promise for reducing methamphetamine abuse, although results have not been robust (Table 2):
• Bupropion has shown benefit in reducing methamphetamine use among users with less severe addiction.7,8
• Methylphenidate, a psychostimulant FDA-approved for attention-deficit/hyperactivity disorder, was found to reduce methamphetamine use compared with placebo in a European sample of amphetamine injectors who had attained abstinence in a residential program.9 Those results were not replicated in a recent study by Miles et al, however.10 A study with a more clinically realistic approach (ie, not requiring daily clinic attendance, as in the Miles trial) vs placebo for methamphetamine abuse was recently published, with promising results that require confirmation in further study.11
• Mirtazapine, an antidepressant, has demonstrated efficacy in reducing methamphetamine use compared with placebo.12
• Modafinil, another medication with stimulant properties, reduced methamphetamine use in a subgroup analysis of heavy users, compared with placebo.13
• Dextroamphetamine, 60 mg/d, showed no difference in reducing methamphetamine compared with placebo, but did diminish cravings and withdrawal symptoms.14
A trial of the phosphodiesterase inhibitor ibudilast (not available in the United States) for methamphetamine abuse is underway. Ibudilast has anti-inflammatory activity in the peripheral immune system and the central nervous system, including modulating the activity of glial cells.15
Many medications have yielded negligible results in studies: selegiline, baclofen, sertraline, topiramate, gabapentin, rivastigmine, risperidone, and ondansetron.16 Recent evaluation of disulfiram, vigabatrin, and lobeline also has yielded inconsistent findings.17
No drug has proved effective for preventing relapse; research continues, focusing on several types of compounds that target various mechanisms: the dopamine system, the opioid system (by way of the γ-aminobutyric acid inhibitory system), and cortico-limbic reward circuitry.
Once-monthly injectable naltrexone has potential for ameliorating craving and relapse by modulating the opioid receptor system. However, the drug has not been adequately explored in generalizable settings of methamphetamine users.
Trials of oral naltrexone in Sweden have shown encouraging results, including reduced subjective effects and amphetamine use in open-label trials18,19; results were replicated in a subsequent placebo-controlled trial.20 In an unpublished study, however, no differences in amphetamine use were found among users randomized to depot naltrexone or placebo.21
Depot naltrexone with assured dosing might have a role in treating methamphetamine abuse, however; a combination of depot naltrexone and oral bupropion is being examined in a National Institute on Drug Abuse Clinical Trials Networks study that commenced in 2013. Pairing medications that have different mechanistic targets might work toward promoting cessation of methamphetamine abuse and reducing relapse once patients are abstinent.
In an early phase of research, but showing promise based on their ability to target different systems, are:
• N-acetylcysteine, modulator of the glutamate system
• D3 antagonists and partial agonists22
• varenicline.23
Potential “vaccines” against methamphetamine are in preclinical development, including use of a protein carrier or other immune-stimulating molecule to create antibodies that bind methamphetamine in the bloodstream and block its psychoactive effects.24,25
Sigma receptor effects are being studied in rodents as potential targets to mitigate effects of methamphetamine. The ligand AZ66, a sigma receptor antagonist, has demonstrated efficacy in reducing methamphetamine-induced cognitive impairment—suggesting that the sigma receptor has a potential role in ameliorating methamphetamine-related neurotoxicity.26
Psychosocial and behavioral interventions. Among the non-drug treatments that have demonstrated efficacy for treating methamphetamine abuse, cognitive-behavioral therapy (CBT) and contingency management (CM) have been most widely studied and applied in treatment settings.
CBT involves individual or group counseling that focuses on relapse prevention skills, including identification of relapse triggers, strategies to diminish cravings, and engagement in alternative non-drug activities27,28 (Table 3).
CM, which is based on positive reinforcement, offers tangible reinforcers, or rewards, for behaviors (eg, clinic attendance, providing a drug-free urine sample) according to guidelines set by the practitioner. CM-based interventions are the most reliably documented approaches for treating methamphetamine abuse,29,30 but their utility might prove to be most efficient in combination with medication— once suitable pharmacotherapeutic options emerge.
Although CBT and CM remain accepted standard treatments for methamphetamine abuse, outcomes are suboptimal.27 Both interventions have a high rate of dropout during the first month of treatment and a >50% relapse rate 6 to 19 months after treatment ends.31-33
As with treatment of other substance use disorders, patients who abuse methamphetamine can benefit from residential treatment in a drug-free setting for ≥30 days.34 In the residential approach, removing access to drugs, drug cues, and drug-using acquaintances combined with group and individual counseling reaches an inevitable end: discharge into the community. Then the patient’s battle to avoid relapse begins.
Because cognitive impairment is common among patients who abuse methamphetamine, even after they stop using,35 researchers have examined the potential for increasing participation in psychosocial interventions such as CBT by using medications that might have potential to increase cognitive function, such as modafinil.36 Increased attention and concentration afforded by medication could enhance efficacy of CBT. Results of trials and new drug development have been mixed37; no clear candidate for preventing relapse through any of the putative mechanisms of action has emerged.
Relapse is a problematic target for treatment
Ending methamphetamine abuse and sustaining abstinence from stimulants require a change in the cognitive associations that have been laid down in a drug user’s memory. Relapse occurs because of recalled memories that can be cued, or triggered, by internal or external stimuli. Eliminating drug memories, perhaps assisted by medications such as d-cycloserine (an antagonist of the N-methyl-d-aspartate receptor), could be useful for suppressing the inclination to relapse.
Last, alternative, non-drug forms of cognitive amendment have shown efficacy in preventing relapse: for example, incorporating mindfulness meditation, which has shown promise in managing craving for methamphetamine and decreasing reactivity to environmental cues for drug use.38
Bottom Line
Practitioners who work in emergency, inpatient, and outpatient settings will be called on more and more to treat acute stimulant intoxication and withdrawal, stimulant-induced psychosis, and methamphetamine abuse. Few evidence-based treatments and no FDA-approved medications are available to treat this addiction; many drugs and a few psychotherapeutic techniques have shown promise. Ongoing research promises to deliver medical and behavioral interventions to help patients quit using methamphetamine.
Related Resources
• Karch SB, Drummer O. Karch’s pathology of drug abuse, fifth ed. Boca Raton, FL: CRC Press/Taylor & David; 2013.
• Roll J, Rawson RA, Ling W, eds. Methamphetamine addiction: from basic science to treatment. New York, NY: Guilford Press; 2009.
• Sheff D. Beautiful boy: a father’s journey through his son’s addiction. New York, NY: Houghton Mifflin Harcourt Publishing Company; 2008.
• Sheff N. Tweak: growing up on methamphetamines. New York, NY: Antheneum Books for Young Readers; 2007.
• National Institute on Drug Abuse. Drugs of abuse. www. drugabuse.gov/drugs-abuse/methamphetamine.
Drug Brand Names
Baclofen • Lioresal Naltrexone (depot) • Vivitrol
Bupropion • Wellbutrin Naltrexone (oral) • ReVia
D-cycloserine • Seromycin Ondansetron • Zofran
Dexreoamphetaime • Adderall Risperidone • Risperadal
Disulfiram • Antabuse Rivastigimine • Exelon
Gabapentin • Neurontin Selegiline• EMSAM
Methylphenidate • Ritalin Sertraline • Zoloft
Mirtazapine • Remeron Topiramate • Topamax
Modafinil • Provigil Varenicline • Chantix
N-acetylcysteine • Mucomyst Vigabatrin • Sabril
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Methamphetamine and other amphetamine-type stimulants are the world’s second most widely used group of illicit substances (after Cannabis), with prevalence of abuse varying by region and by locales within nations. As prescription use of stimulants has grown dramatically in recent years, so has abuse of these substances.
Given the widespread and growing misuse of amphetamine-type stimulants (Box,1-3), clinicians are faced with the need to learn how to recognize and manage methamphetamine abuse. Both prescribed and non-prescribed uses of stimulants present complex challenges; in this article, we examine effects, manifestations, and current evidence-based behavioral and medical treatments of methamphetamine misuse and abuse, and look ahead to investigational therapies that hold promise for improving the limited existing approaches to management.
Effects and manifestations of methamphetamine use
Different routes of administration produce different consequences, in terms of medical comorbidity and propensity to induce addiction. Smoked or injected, methamphetamine enters the brain in seconds; snorted or taken by mouth, the drug produces its effects in several minutes and a half hour, respectively.
Rapid uptake and effects of methamphetamine result from its ability to cross the blood−brain barrier. Its primary effects are caused by inhibition of dopamine storage and release of intracellular dopamine.
Methamphetamine stimulates the CNS and the cardiovascular system through release of dopamine and norepinephrine, which increases blood pressure, body temperature, and heart rate, and, occasionally, induces arrhythmia that can contribute to heart attack and stroke. Users experience euphoria, hypervigilance, suppressed appetite, and increased libido.
Binge use is common to sustain euphoria and other reinforcing effects, which subside with rapidly developing tolerance. After days of repeated dosing, elevated methamphetamine blood levels can lead to mood disturbances, repetitive motor activities, and psychotic symptoms such as hallucinations, delusions, and paranoia. Acute psychosis can bring on violence and other injurious behaviors that involve law enforcement and emergency medical services.
When methamphetamine is used over months or years, health consequences include anorexia, tremor, so-called meth mouth (broken teeth, infections, cavities, burns), insomnia, panic attacks, confusion, depression, irritability, and impaired memory and other cognitive processes.
Treating methamphetamine intoxication and withdrawal
At initial clinical contact with a person who abuses methamphetamine, practitioners may face several acute consequences requiring attention. Prominent among presenting conditions, especially during acute intoxication, are agitation, anxiety, and psychotic symptoms, which may improve by providing the patient with calming reassurance in a quiet space. In more severe cases, a benzodiazepine, antipsychotic, or both might be indicated4,5 (Table 1).
Methamphetamine withdrawal is characterized by anxiety, depression, and insomnia. These symptoms generally resolve in a matter of days after the start of withdrawal without pharmacotherapy. In some cases, depression or psychosis becomes chronic, as a result of methamphetamine use itself6 or as an emergent concomitant psychiatric condition.
A sedative-hypnotic medication or an anxiolytic can be used as necessary to ameliorate insomnia or anxiety, respectively. Prolonged depression can be treated with an antidepressant. An antipsychotic might be indicated for long-term management of patients who have persistent psychosis.
Therapy for methamphetamine abuse
Treatment of methamphetamine abuse— with the goal of stopping drug use—is a complicated matter on 2 counts:
• No medications are FDA-approved for treating methamphetamine addiction.
• There are no accepted substitution medications (ie, stimulants that can be used in place of methamphetamine, as is available for opioid addiction).
Pharmacotherapeutic possibilities. The rationale for considering replacement pharmacotherapy is that psychostimulants can counter the cravings, dysphoria, and fatigue produced by methamphetamine withdrawal and can alleviate methamphetamine-related cognitive impairment. Although dextroamphetamine and other psychostimulants have been evaluated in small trials as replacement medication, most countries are reluctant to consider their use, because of the potential for abuse and accompanying liability.
After decades of medication research, several drugs have shown promise for reducing methamphetamine abuse, although results have not been robust (Table 2):
• Bupropion has shown benefit in reducing methamphetamine use among users with less severe addiction.7,8
• Methylphenidate, a psychostimulant FDA-approved for attention-deficit/hyperactivity disorder, was found to reduce methamphetamine use compared with placebo in a European sample of amphetamine injectors who had attained abstinence in a residential program.9 Those results were not replicated in a recent study by Miles et al, however.10 A study with a more clinically realistic approach (ie, not requiring daily clinic attendance, as in the Miles trial) vs placebo for methamphetamine abuse was recently published, with promising results that require confirmation in further study.11
• Mirtazapine, an antidepressant, has demonstrated efficacy in reducing methamphetamine use compared with placebo.12
• Modafinil, another medication with stimulant properties, reduced methamphetamine use in a subgroup analysis of heavy users, compared with placebo.13
• Dextroamphetamine, 60 mg/d, showed no difference in reducing methamphetamine compared with placebo, but did diminish cravings and withdrawal symptoms.14
A trial of the phosphodiesterase inhibitor ibudilast (not available in the United States) for methamphetamine abuse is underway. Ibudilast has anti-inflammatory activity in the peripheral immune system and the central nervous system, including modulating the activity of glial cells.15
Many medications have yielded negligible results in studies: selegiline, baclofen, sertraline, topiramate, gabapentin, rivastigmine, risperidone, and ondansetron.16 Recent evaluation of disulfiram, vigabatrin, and lobeline also has yielded inconsistent findings.17
No drug has proved effective for preventing relapse; research continues, focusing on several types of compounds that target various mechanisms: the dopamine system, the opioid system (by way of the γ-aminobutyric acid inhibitory system), and cortico-limbic reward circuitry.
Once-monthly injectable naltrexone has potential for ameliorating craving and relapse by modulating the opioid receptor system. However, the drug has not been adequately explored in generalizable settings of methamphetamine users.
Trials of oral naltrexone in Sweden have shown encouraging results, including reduced subjective effects and amphetamine use in open-label trials18,19; results were replicated in a subsequent placebo-controlled trial.20 In an unpublished study, however, no differences in amphetamine use were found among users randomized to depot naltrexone or placebo.21
Depot naltrexone with assured dosing might have a role in treating methamphetamine abuse, however; a combination of depot naltrexone and oral bupropion is being examined in a National Institute on Drug Abuse Clinical Trials Networks study that commenced in 2013. Pairing medications that have different mechanistic targets might work toward promoting cessation of methamphetamine abuse and reducing relapse once patients are abstinent.
In an early phase of research, but showing promise based on their ability to target different systems, are:
• N-acetylcysteine, modulator of the glutamate system
• D3 antagonists and partial agonists22
• varenicline.23
Potential “vaccines” against methamphetamine are in preclinical development, including use of a protein carrier or other immune-stimulating molecule to create antibodies that bind methamphetamine in the bloodstream and block its psychoactive effects.24,25
Sigma receptor effects are being studied in rodents as potential targets to mitigate effects of methamphetamine. The ligand AZ66, a sigma receptor antagonist, has demonstrated efficacy in reducing methamphetamine-induced cognitive impairment—suggesting that the sigma receptor has a potential role in ameliorating methamphetamine-related neurotoxicity.26
Psychosocial and behavioral interventions. Among the non-drug treatments that have demonstrated efficacy for treating methamphetamine abuse, cognitive-behavioral therapy (CBT) and contingency management (CM) have been most widely studied and applied in treatment settings.
CBT involves individual or group counseling that focuses on relapse prevention skills, including identification of relapse triggers, strategies to diminish cravings, and engagement in alternative non-drug activities27,28 (Table 3).
CM, which is based on positive reinforcement, offers tangible reinforcers, or rewards, for behaviors (eg, clinic attendance, providing a drug-free urine sample) according to guidelines set by the practitioner. CM-based interventions are the most reliably documented approaches for treating methamphetamine abuse,29,30 but their utility might prove to be most efficient in combination with medication— once suitable pharmacotherapeutic options emerge.
Although CBT and CM remain accepted standard treatments for methamphetamine abuse, outcomes are suboptimal.27 Both interventions have a high rate of dropout during the first month of treatment and a >50% relapse rate 6 to 19 months after treatment ends.31-33
As with treatment of other substance use disorders, patients who abuse methamphetamine can benefit from residential treatment in a drug-free setting for ≥30 days.34 In the residential approach, removing access to drugs, drug cues, and drug-using acquaintances combined with group and individual counseling reaches an inevitable end: discharge into the community. Then the patient’s battle to avoid relapse begins.
Because cognitive impairment is common among patients who abuse methamphetamine, even after they stop using,35 researchers have examined the potential for increasing participation in psychosocial interventions such as CBT by using medications that might have potential to increase cognitive function, such as modafinil.36 Increased attention and concentration afforded by medication could enhance efficacy of CBT. Results of trials and new drug development have been mixed37; no clear candidate for preventing relapse through any of the putative mechanisms of action has emerged.
Relapse is a problematic target for treatment
Ending methamphetamine abuse and sustaining abstinence from stimulants require a change in the cognitive associations that have been laid down in a drug user’s memory. Relapse occurs because of recalled memories that can be cued, or triggered, by internal or external stimuli. Eliminating drug memories, perhaps assisted by medications such as d-cycloserine (an antagonist of the N-methyl-d-aspartate receptor), could be useful for suppressing the inclination to relapse.
Last, alternative, non-drug forms of cognitive amendment have shown efficacy in preventing relapse: for example, incorporating mindfulness meditation, which has shown promise in managing craving for methamphetamine and decreasing reactivity to environmental cues for drug use.38
Bottom Line
Practitioners who work in emergency, inpatient, and outpatient settings will be called on more and more to treat acute stimulant intoxication and withdrawal, stimulant-induced psychosis, and methamphetamine abuse. Few evidence-based treatments and no FDA-approved medications are available to treat this addiction; many drugs and a few psychotherapeutic techniques have shown promise. Ongoing research promises to deliver medical and behavioral interventions to help patients quit using methamphetamine.
Related Resources
• Karch SB, Drummer O. Karch’s pathology of drug abuse, fifth ed. Boca Raton, FL: CRC Press/Taylor & David; 2013.
• Roll J, Rawson RA, Ling W, eds. Methamphetamine addiction: from basic science to treatment. New York, NY: Guilford Press; 2009.
• Sheff D. Beautiful boy: a father’s journey through his son’s addiction. New York, NY: Houghton Mifflin Harcourt Publishing Company; 2008.
• Sheff N. Tweak: growing up on methamphetamines. New York, NY: Antheneum Books for Young Readers; 2007.
• National Institute on Drug Abuse. Drugs of abuse. www. drugabuse.gov/drugs-abuse/methamphetamine.
Drug Brand Names
Baclofen • Lioresal Naltrexone (depot) • Vivitrol
Bupropion • Wellbutrin Naltrexone (oral) • ReVia
D-cycloserine • Seromycin Ondansetron • Zofran
Dexreoamphetaime • Adderall Risperidone • Risperadal
Disulfiram • Antabuse Rivastigimine • Exelon
Gabapentin • Neurontin Selegiline• EMSAM
Methylphenidate • Ritalin Sertraline • Zoloft
Mirtazapine • Remeron Topiramate • Topamax
Modafinil • Provigil Varenicline • Chantix
N-acetylcysteine • Mucomyst Vigabatrin • Sabril
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. UNODC. World Drug Report 2012 (United Nations publication, Sales No. E.12.XI.1). http://www.unodc. org/documents/data-and-analysis/WDR2012/ WDR_2012_web_small.pdf. Published 2012. Accessed August 4, 2014.
2. UNODC. World Drug Report 2010 (United Nations publication, Sales No. E.10.XI.13). http://www.unodc. org/documents/wdr/WDR_2010/World_Drug_ Report_2010_lo-res.pdf. Published 2010. Accessed August 4, 2014.
3. Rawson RA, Gonzales R, Brecht M, et al. Evaluation of the California Outcomes Measurement System (CalOMS): Final Report 2008. http://www.uclaisap.org/assets/documents/ California-ADP-DHCS-Evals/2007-2008_CalOMS%20 Report.pdf. Published 2008. Accessed August 4, 2014.
4. Shoptaw SJ, Kao U, Ling W. Treatment for amphetamine psychosis. Cochrane Database Syst Rev. 2009;(1):CD003026.
5. Leelahanaj T, Kongsakon R, Netrakom P. A 4-week, double-blind comparison of olanzapine with haloperidol in the treatment of amphetamine psychosis. J Med Assoc Thai. 2005;88(suppl 3):S43-S52.
6. McKetin R, McLaren J, Lubman D, et al. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101(10):1473-1478.
7. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
8. McCann DJ, Li SH. A novel, nonbinary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: reanalysis of a multisite trial. CNS Neurosci Ther. 2012;18(5):414-418.
9. Tiihonen J, Kuoppasalmi K, Föhr J, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164(1): 160-162.
10. Miles SW, Sheridan J, Russell B, et al. Extended-release methylphenidate for treatment of amphetamine/ methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108(7): 1279-1286.
11. Ling W, Chang L, Hillhouse M, et al. Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder. Addiction. 2014;109(9): 1489-1500.
12. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175.
13. Heinzerling KG, Swanson AN, Kim S, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109(1-3):20-29.
14. Galloway GP, Buscemi R, Coyle JR, et al. A randomized, placebo-controlled trial of sustained-release dextro-amphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther. 2011;89(2):276-282.
15. Snider SE, Hendrick ES, Beardsley PM. Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur J Pharmacol. 2013;701(1-3):124-130.
16. Ling W, Rawson R, Shoptaw S. Management of methamphetamine abuse and dependence. Curr Psychiatry Rep. 2006;8(5):345-354.
17. Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24(6):541-550.
18. Jayaram-Lindström N, Wennberg P, Beck O, et al. An open clinical trial of naltrexone for amphetamine dependence: compliance and tolerability. Nord J Psychiatry. 2005;59(3):167-171.
19. Jayaram-Lindström N, Konstenius M, Eksborg S, et al. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2007;33(8):1856-1863.
20. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448.
21. Woody GE, Tyrfingsoon P. Symposium XI: Emerging data on efficacy and clinical applications of extended-release naltrexone formulations. 75th Annual Meeting, College on Problems of Drug Dependence. June 19, 2013; San Diego, CA.
22. Newman AH, Blaylock BL, Nader MA, et al. Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochem Pharmacol. 2012;84(7):882-890.
23. Verrico CD, Mahoney JJ 3rd, Thompson-Lake DG, et al. Safety and efficacy of varenicline to reduce positive subjective effects produced by methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol. 2014;17(2):223-233.
24. Miller ML, Moreno AY, Aarde S, et al. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol Psychiatry. 2013;73(8):721-728.
25. Shen XY, Kosten TA, Lopez AY, et al. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013;129(1-2):41-48.
26. Seminerio MJ, Robson MJ, Abdelazeem AH, et al. Synthesis and pharmacological characterization of a novel sigma receptor ligand with improved metabolic stability and antagonistic effects against methamphetamine. AAPS J. 2012;14(1):43-51.
27. Rawson RA, Marinelli-Casey P, Anglin M, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99(6):708-717.
28. Vocci FJ, Montoya ID. Psychological treatments for stimulant misuse, comparing and contrasting those for amphetamine dependence and those for cocaine dependence. Curr Opin Psychiatry. 2009;22(3):263-268.
29. Rawson RA, McCann MJ, Flammino F, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267-274.
30. Roll JM, Petry NM, Stitzer ML, et al. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry. 2006;163(11):1993-1999.
31. Brecht ML, von Mayrhauser C, Anglin MD. Predictors of relapse after treatment for methamphetamine use. J Psychoactive Drugs. 2000;32(2):211-220.
32. Smout MF, Longo M, Harrison S, et al. Psychosocial treatment for methamphetamine use disorders: a preliminary randomized controlled trial of cognitive behavior therapy and Acceptance and Commitment Therapy. Subst Abus. 2010;31(2):98-107.
33. Wang G, Shi J, Chen N, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;24;8(7):e68791. doi: 10.1371/ journal.pone.0068791.
34. McKetin R, Lubman DI, Baker AL, et al. Dose-related psychotic symptoms in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry. 2013;70(3):319-324.
35. Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35(6):593-598.
36. Dean AC, Sevak RJ, Monterosso JR, et al. Acute modafinil effects on attention and inhibitory control in methamphetamine-dependent humans. J Stud Alcohol Drugs. 2011;72(6):943-953.
37. Zullino DF, Benguettat D, Khazaal Y. Improvement of cognitive performance by topiramate: blockage of automatic processes may be the underlying mechanism [Comment on: Effects of topiramate on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition in recently abstinent methamphetamine-dependent individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2007.] Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):787.
38. Witkiewitz K, Lustyk M, Bowen S. Retraining the addicted brain: a review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychol Addict Behav. 2013;27(2):351-365.
1. UNODC. World Drug Report 2012 (United Nations publication, Sales No. E.12.XI.1). http://www.unodc. org/documents/data-and-analysis/WDR2012/ WDR_2012_web_small.pdf. Published 2012. Accessed August 4, 2014.
2. UNODC. World Drug Report 2010 (United Nations publication, Sales No. E.10.XI.13). http://www.unodc. org/documents/wdr/WDR_2010/World_Drug_ Report_2010_lo-res.pdf. Published 2010. Accessed August 4, 2014.
3. Rawson RA, Gonzales R, Brecht M, et al. Evaluation of the California Outcomes Measurement System (CalOMS): Final Report 2008. http://www.uclaisap.org/assets/documents/ California-ADP-DHCS-Evals/2007-2008_CalOMS%20 Report.pdf. Published 2008. Accessed August 4, 2014.
4. Shoptaw SJ, Kao U, Ling W. Treatment for amphetamine psychosis. Cochrane Database Syst Rev. 2009;(1):CD003026.
5. Leelahanaj T, Kongsakon R, Netrakom P. A 4-week, double-blind comparison of olanzapine with haloperidol in the treatment of amphetamine psychosis. J Med Assoc Thai. 2005;88(suppl 3):S43-S52.
6. McKetin R, McLaren J, Lubman D, et al. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101(10):1473-1478.
7. Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162-1170.
8. McCann DJ, Li SH. A novel, nonbinary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: reanalysis of a multisite trial. CNS Neurosci Ther. 2012;18(5):414-418.
9. Tiihonen J, Kuoppasalmi K, Föhr J, et al. A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry. 2007;164(1): 160-162.
10. Miles SW, Sheridan J, Russell B, et al. Extended-release methylphenidate for treatment of amphetamine/ methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108(7): 1279-1286.
11. Ling W, Chang L, Hillhouse M, et al. Sustained-release methylphenidate in a randomized trial of treatment of methamphetamine use disorder. Addiction. 2014;109(9): 1489-1500.
12. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175.
13. Heinzerling KG, Swanson AN, Kim S, et al. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109(1-3):20-29.
14. Galloway GP, Buscemi R, Coyle JR, et al. A randomized, placebo-controlled trial of sustained-release dextro-amphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther. 2011;89(2):276-282.
15. Snider SE, Hendrick ES, Beardsley PM. Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur J Pharmacol. 2013;701(1-3):124-130.
16. Ling W, Rawson R, Shoptaw S. Management of methamphetamine abuse and dependence. Curr Psychiatry Rep. 2006;8(5):345-354.
17. Brackins T, Brahm NC, Kissack JC. Treatments for methamphetamine abuse: a literature review for the clinician. J Pharm Pract. 2011;24(6):541-550.
18. Jayaram-Lindström N, Wennberg P, Beck O, et al. An open clinical trial of naltrexone for amphetamine dependence: compliance and tolerability. Nord J Psychiatry. 2005;59(3):167-171.
19. Jayaram-Lindström N, Konstenius M, Eksborg S, et al. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2007;33(8):1856-1863.
20. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448.
21. Woody GE, Tyrfingsoon P. Symposium XI: Emerging data on efficacy and clinical applications of extended-release naltrexone formulations. 75th Annual Meeting, College on Problems of Drug Dependence. June 19, 2013; San Diego, CA.
22. Newman AH, Blaylock BL, Nader MA, et al. Medication discovery for addiction: translating the dopamine D3 receptor hypothesis. Biochem Pharmacol. 2012;84(7):882-890.
23. Verrico CD, Mahoney JJ 3rd, Thompson-Lake DG, et al. Safety and efficacy of varenicline to reduce positive subjective effects produced by methamphetamine in methamphetamine-dependent volunteers. Int J Neuropsychopharmacol. 2014;17(2):223-233.
24. Miller ML, Moreno AY, Aarde S, et al. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol Psychiatry. 2013;73(8):721-728.
25. Shen XY, Kosten TA, Lopez AY, et al. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013;129(1-2):41-48.
26. Seminerio MJ, Robson MJ, Abdelazeem AH, et al. Synthesis and pharmacological characterization of a novel sigma receptor ligand with improved metabolic stability and antagonistic effects against methamphetamine. AAPS J. 2012;14(1):43-51.
27. Rawson RA, Marinelli-Casey P, Anglin M, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99(6):708-717.
28. Vocci FJ, Montoya ID. Psychological treatments for stimulant misuse, comparing and contrasting those for amphetamine dependence and those for cocaine dependence. Curr Opin Psychiatry. 2009;22(3):263-268.
29. Rawson RA, McCann MJ, Flammino F, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267-274.
30. Roll JM, Petry NM, Stitzer ML, et al. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry. 2006;163(11):1993-1999.
31. Brecht ML, von Mayrhauser C, Anglin MD. Predictors of relapse after treatment for methamphetamine use. J Psychoactive Drugs. 2000;32(2):211-220.
32. Smout MF, Longo M, Harrison S, et al. Psychosocial treatment for methamphetamine use disorders: a preliminary randomized controlled trial of cognitive behavior therapy and Acceptance and Commitment Therapy. Subst Abus. 2010;31(2):98-107.
33. Wang G, Shi J, Chen N, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;24;8(7):e68791. doi: 10.1371/ journal.pone.0068791.
34. McKetin R, Lubman DI, Baker AL, et al. Dose-related psychotic symptoms in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry. 2013;70(3):319-324.
35. Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addict Behav. 2010;35(6):593-598.
36. Dean AC, Sevak RJ, Monterosso JR, et al. Acute modafinil effects on attention and inhibitory control in methamphetamine-dependent humans. J Stud Alcohol Drugs. 2011;72(6):943-953.
37. Zullino DF, Benguettat D, Khazaal Y. Improvement of cognitive performance by topiramate: blockage of automatic processes may be the underlying mechanism [Comment on: Effects of topiramate on methamphetamine-induced changes in attentional and perceptual-motor skills of cognition in recently abstinent methamphetamine-dependent individuals. Prog Neuropsychopharmacol Biol Psychiatry. 2007.] Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):787.
38. Witkiewitz K, Lustyk M, Bowen S. Retraining the addicted brain: a review of hypothesized neurobiological mechanisms of mindfulness-based relapse prevention. Psychol Addict Behav. 2013;27(2):351-365.