User login
What you need to know about medication safety in pregnancy
The author reports no financial relationships relevant to this article.
Fifty years ago, the thalidomide experience—a high incidence of major birth defects following prenatal use of the drug—made clear the devastating potential of drug exposure during pregnancy. Since that disaster, healthcare providers and patients have adopted a conservative approach to medication use during pregnancy, especially during the first trimester and lactation. That is a wise strategy, although very few medications are associated with abnormal fetal development.
In this article, I’ll guide you through some of the issues that must be considered when assessing a drug’s teratogenicity, help you find information on a host of medications, and familiarize you with some of the challenges involved in counseling the patient. I also present a table listing the adverse effects known to be associated with selected drugs during the first, second, and third trimesters and lactation (TABLE). We are fortunate that a large body of information about medication use during pregnancy and lactation is readily available on the Web and in books and medical journals. This information is far from definitive, however, because much of the evidence concerning prescribed drugs is anecdotal or presented with insufficient warning about their use during pregnancy and lactation.
A discussion of these issues with the patient will help set the risks and benefits of a particular drug into proper perspective, alleviate fears, and improve compliance. Nonprescription medications should also be discussed, and the patient should be advised that we have very little data concerning their use during pregnancy.
Assignment of risk is an uncertain science
Major structural defects are apparent at birth in about 3% of all pregnancies and in about 4.5% of all children by the age of 5 years.1 A cause or proposed mechanism for the defects can be determined in fewer than 50% of these cases. Nor can we count on expert consensus about the safety of many medications during pregnancy because it rarely occurs and, in some cases, may be impossible to achieve.
Animal studies are the means of assessing the teratogenicity of most drugs. Animals commonly used to study fetal effects include rodents (fertility, birth defects, birth weight, behavior), rabbits (birth defects), baboons (uterine blood flow), and sheep (uterine blood flow, cardiovascular effects, fetal hypoxia, and acidosis). Dosages are often much higher (in relation to body weight or surface area) to “test the systems” for any possible reproductive harm. Although these studies may be helpful, they do not reliably predict the human response.
Even when humans are the subject of study, conclusions must be viewed with caution. To determine the risk of teratogenesis, it is necessary to know the stage of development during which the exposure occurred, as well as the identity and dose of the medication and the genetic susceptibility of the mother and fetus.
Three critical stages. In utero exposure to a drug occurs in one of three periods of fetal development:
- ovum – from fertilization to implantation
- embryo – from the second through the eighth week of gestation
- fetus – from the eighth completed week until delivery.
An “all-or-none” effect (i.e., spontaneous abortion or not) is believed to arise from exposure during the first period, but the embryo stage is the most critical time because it involves organogenesis. Detrimental effects may occur even beyond this period as cells continue to divide in the hematologic, reproductive, and central nervous systems.
Many fine points of exposure are difficult to clarify
Retrospective and uncontrolled studies, as well as individual case reports or small series, may overestimate the risk to the fetus of exposure to a specific drug or combination of medications. Case reports do not establish causation.
It can also be difficult to differentiate between the risks of a specific drug and the hazards of maternal illness to explain an unfavorable outcome. For example, is a particular case of stillbirth the result of fetal exposure to enoxaparin or maternal thrombophilia, or both? Can fetal growth restriction be attributed to use of azathioprine during pregnancy or to the mother’s underlying illness? And so on.
In addition, it is necessary to distinguish between a defect’s natural prevalence—i.e., the rate at which it occurs in a population—and the additional risk posed by exposure to a particular drug. Studies in large populations are needed—but usually unattainable—to determine the relative risk from specific potential teratogens.
Finally, it is very difficult to assess neurobehavioral effects of in utero exposure to centrally acting drugs beyond the immediate neonatal period. The dose, offspring’s age and gender, and behavioral test system must all be considered.
Few drugs are implicated in restricting fetal growth or reducing organ size. We also lack consistent information about long-term effects such as learning or behavioral problems (i.e., functional teratogenesis) that may result from chronic prenatal exposure to a certain medication.
In 1979, the Food and Drug Administration created five pregnancy risk categories to be used by manufacturers to rate their products in the drug formulary for use during pregnancy: categories A, B, C, D, and X, which range from no evidence of damage to the fetus (category A) to clear teratogenicity (D and X).
The D rating is generally reserved for drugs with no safer alternatives, such as secobarbital, doxycycline, and lorazepam. The X rating means there is absolutely no reason to risk using the drug in pregnancy, as in the case of oral contraceptives, benzodiazepines, and misoprostol.
Approximately 2% of drugs fall into category A, 50% in category B, 38% in category C, 3–5% in category D, and 1–5% in category X.3 These categories do not often accurately reflect the available information on risk to the fetus. A major initiative is under way to declare these categories obsolete and provide more informative drug labeling. Pregnancy labels of the future will likely address three important areas:
- clinical considerations–issues relevant to prescription of a particular drug in pregnancy, including the risk of disease versus no treatment. Also included will be information of use when counseling a patient whose fetus was inadvertently exposed to a medication in early gestation
- summary risk assessment–a narrative text that describes, as comprehensively as possible, the risk of exposure based on animal and human data
- data to support the assessment.
All drugs cross the placenta
Most medications are easily absorbed during pregnancy, and serum concentrations of albumin for drug binding are lower than in the nonpregnant state. Pharmacokinetic changes during pregnancy include:
- higher volume of distribution
- lower maximum plasma concentration
- lower steady-state serum concentration
- shorter plasma half-life
- higher clearance rate.1
The small spatial configuration and high lipid solubility of most medications permit easy transfer of an unbound drug or its metabolite across the placenta or into breast milk. Virtually all drugs and their end products cross the placenta, with unbound concentrations of the drug in the fetal serum similar to the level in maternal serum—sometimes even higher (FIGURE).
A few drugs with high molecular weight do not cross the placenta in significant amounts (e.g., glyburide, interferon, thyroid supplements, insulin).
FIGURE An elaborate nutrient (and drug) delivery system
The placenta and umbilical cord deliver the nutrients and oxygen the fetus needs for normal growth—as well as most medications used by the mother.
Medication use tends to increase as pregnancy progresses
The drugs most commonly taken during pregnancy include vitamins, iron preparations, calcium, analgesics, antibiotics, and antacids. Excluding vitamins and mineral supplements, an average of one to two medications are taken during gestation. Over-the-counter formulations account for about half of these drugs, with acetaminophen being the single most commonly used medication during pregnancy. Antibiotics are the most widely prescribed drugs.
Although caffeine, tobacco, alcohol, and illicit substance use tends to diminish as pregnancy progresses, medications are usually taken at the same frequency or more often during gestation.
My colleagues and I found a significantly higher mean number of medications (3.3 and 4.1, respectively) used during the second and third trimesters of gestation than were taken before pregnancy (2.6).2
How to counsel the patient
Counseling a woman before or during pregnancy about the continuation or initiation of a medication should take place in an open, supportive, and informative manner. Most inquiries relate to exposures involving very low levels of relative and absolute risk.
A detailed fetal ultrasonographic examination is often used to accurately date the pregnancy and, if possible, screen for any structural defects. The patient should be advised that first-trimester screening, chorionic villus sampling, maternal serum quadruple screening, amniocentesis, and fetal blood sampling are not very predictive of a drug’s fetal effects. Exceptions may be the observation of open neural tube defects (approximate 1% risk associated with valproic acid and carbamazepine) by maternal serum quadruple screening and facial clefting by targeted ultrasonography.
When a patient inquires about a particular drug, it is important to gather the following information:
- When did she take the medication?
- Why did she take it?
- For how long did she take the medication?
- Did she take other medications, or any substances of abuse, at the same time?
A number of sources of information about potential teratogens are available.3-5 These include national computerized databases that are accessible on the Web:
- National Library of Medicine (http://sis.nlm.nih.gov/enviro.html)
- pregnancy exposure registries (www.fda.gov/womens/registries/default.htm)
- Reproductive Toxicology Center (http://reprotox.org) (access requires a paid subscription)
- LactMed, National Library of Medicine guide to drug safety in lactation (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT)
- Organization of Teratology Information Specialists (OTIS) (www.otispregnancy.org).
The last source (OTIS) consolidates teratology information nationwide and reports it by state or region. It also publishes a host of fact sheets on various drugs that may be useful to dispense to the patient during counseling. In addition, many teratogen information services or poison control centers (often at children’s hospitals) are available throughout the United States to serve specific geographic areas. And teratogen registries at pharmaceutical companies may provide limited information about newer medications.
The Physician’s Desk Reference (PDR) is a common source of information about the use of prescription drugs in pregnancy.3 But be aware that, to avoid liability, pharmaceutical manufacturers do not encourage use of their drugs during pregnancy unless the benefit clearly outweighs the risk. It would be unrealistic for them to market a medication for specific use during pregnancy because it would require considerable time and cost, and raise ethical objections, to conduct research in a vulnerable population that is limited in number.
Effects of agents used more than 40 years ago were reported by the Collaborative Perinatal Project or the Boston Collaborative Drug Surveillance Program.6 Those findings are often inconclusive, reflect bias in study designs, and do not help a clinician evaluate current medications or those less commonly prescribed during pregnancy.
The risks and experience associated with new drugs are usually not well explored in regard to pregnancy. As a result, older medications are more likely to be prescribed as maintenance therapy during gestation for the simple reason that they have a larger body of information regarding their effects. These older drugs may no longer be preferred once the patient delivers.
Most drugs are not teratogens
The TABLE lists adverse effects in the human fetus known to arise from exposure to specific drugs. The information comes largely from the Reprotox database, which was reviewed as recently as 2006, describes human data only, and is reported by first trimester (anomalies, abortion) and the second and third trimesters (fetal growth restriction, stillbirth, low birth weight, preterm delivery, immediate neonatal problems).7 Typical dosages of most drugs are not anticipated to increase the risk of congenital anomalies.
Most human data come from small series or case reports. Although these types of studies are helpful, they tend to be biased or reflect the pregnancy’s background risk of birth defects rather than the risk posed by a specific drug. In addition, case reports of malformation after prenatal exposure to a certain drug may involve exposures to other agents and a lack of uniformity of abnormalities, making the association between adverse effects and a single agent unlikely. Dissimilarities in the dosage and route of delivery also limit interpretation. for example, short-term intravenous or sublingual administration of a drug may pose a different risk than taking that medication orally or vaginally, in a lower dose, for a longer period, or at a different period of gestation.
Randomized controlled trials of drugs are rare during pregnancy, as are prospective cohort investigations. Because a control population is often impossible to identify, it becomes difficult to separate any heightened risk identified during use of the medication from the underlying disease. When the gravida has significant medical problems, it is important to assess the potential risk of a drug—or its omission—in her as well as her fetus. The lowest effective dose is preferred, but keep in mind that inadequate treatment may lead to minimal benefit and potentially greater risk to the pregnancy.
When reviewing or planning maintenance drug therapy, follow the same principles as you would in a nonpregnant patient. Be familiar with more than one medication for each disorder. Also be aware that some drugs may need to be prescribed at a higher dose or greater frequency to attain a therapeutic concentration in the expanded intravascular volume of pregnancy. In addition, side effects such as nausea, fatigue, and gastrointestinal disturbance may mimic symptoms arising from physiologic changes of pregnancy.
Assessing the risks associated with over-the-counter medications and natural food products is even harder. The PDR for Nonprescription Drugs, Dietary Supplements, and Herbs8 contains little or no information about the reproductive hazards of most of these products. Many agents contain multiple ingredients, both active and inactive, thereby complicating counseling about their risks during pregnancy. Although the recommended dosage is usually low, many product labels do not specify what it should be.
Most drugs enter breast milk
The amount of drug that an infant consumes from breast milk depends on the medication’s chemical properties as well as the dosage, frequency, and duration of exposure.2 Contraindications and cautions are usually either theoretical or based on findings from case reports that often conflict or confuse. In theory, it is safer for the mother to take the medication just after infant feeding or just before the infant’s longest sleep period.
The TABLE also lists the effects of drugs in the breastfed human infant. Again, the information comes from the Reprotox database, access to which requires a subscription. For additional information, try the free LactMed site at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT.
Nearly all drugs are excreted in breast milk, usually in small amounts (often less than 5% of the weight-adjusted maternal daily dose). The amount of drug or metabolite in an infant’s serum also is determined by the volume of breast milk, age of the infant, and other exposures.
Avoid prescribing multiple medications, if possible, and choose “safe” drugs from among the options in categories that include a number of teratogenic medications, such as anticonvulsants.
Determine the best method to monitor therapy. For example, use a peak flow meter for asthma, a portable blood pressure monitor for hypertension, and so on.
Focus on keeping the patient healthy. The healthiest mother is most likely to deliver the healthiest infant.
Keep the underlying disorder in mind, as well as the drug, when choosing a drug.
Know which drugs are clearly linked to birth defects. These include phenytoin, warfarin, alcohol, methotrexate, diethylstilbestrol, cis-retinoic acid, valproic acid, and carbamazepine.
Pay special attention to the first trimester. Too little is known about the first-trimester effects of the vast majority of drugs for them to be considered safe.
Suspect a drug-related effect
A medication may be the cause in any newborn manifesting signs of anemia, hepatitis, hepatotoxicity, hepatorenal dysfunction, and hyperbilirubinemia. This includes breastfed infants. An adverse drug-related effect should also be suspected when an infant exhibits signs of jaundice, floppiness, jitteriness, poor suck, diarrhea, or growth restriction.
Reference
1. American College of Obstetricians and Gynecologists. Teratology. ACOG Educational Bulletin #236. Washington, DC: ACOG; 1997.
2. Splinter M, Nightingale B, Sawgraves R, Rayburn W. Medication use during pregnancy by women delivering at a tertiary university hospital. South Med J. 1997;90:498-502.
3. Physician’s Desk Reference. 61st ed. Montvale, NJ: Medical Economics; 2007.
4. Briggs GG, Freeman RK, Yaffee FJ. Drugs in Pregnancy and Lactation: Reference Guide to Fetal and Neonatal Risk. 7th ed. Baltimore: Williams & Wilkins; 2005.
5. Briggs GG, Freeman RK, Yaffee FJ. ReproTox Database. Vol. 13. No. 1. Bethesda, Md: Reproductive Toxicology Center; 2000.
6. Heinonen OP, Sloan ED, Shapiro S. Birth Defects and Drugs in Pregnancy. Boston: John Wright PSG; 1973.
7. Reproductive Toxicology Center. Bethesda, Md. Available at http://reprotox.org. Accessed Oct. 5, 2007.
8. PDR for Nonprescription Drugs, Dietary Supplements, and Herbs. 28th ed. Montvale, NJ: Medical Economics; 2007.
<b>How selected drugs affect the human fetus and breastfed infant</b><cs cn="1" a="l"> <cs cn="2" a="l"> <cs cn="3" a="l"> <cs cn="4" a="l"> </cs></cs></cs></cs><row><entry v="t">DRUG</entry> <entry v="t">FIRST-TRIMESTER EFFECTS</entry> <entry v="t">EFFECTS DURING SECOND AND THIRD TRIMESTER</entry> <entry v="t">SAFETY DURING BREASTFEEDING</entry></row><row><entry cs="4">ANALGESICS</entry></row><row><entry v="t">Acetaminophen</entry> <entry v="t">None known</entry> <entry v="t">Hepatotoxicity/nephrotoxicity</entry> <entry v="t">Safe</entry></row><row><entry v="t">Ibuprofen</entry> <entry v="t">Gastroschisis (?)</entry> <entry v="t">Closure of ductus</entry> <entry v="t">Small amount passed; no other information</entry></row><row><entry v="t">Narcotics</entry> <entry v="t">None known</entry> <entry v="t">Depression, withdrawal</entry> <entry v="t">Not recommended if dosing is repetitive</entry></row><row><entry v="t">Salicylates</entry> <entry v="t">None known</entry> <entry v="t">Prolonged pregnancy and labor, hemorrhage, altered hemostasis, intracranial hemorrhage</entry> <entry v="t">Use with caution; may have adverse effects in newborn</entry></row><row><entry cs="4">ANESTHETICS</entry></row><row><entry v="t">General</entry> <entry v="t">Anomalies (?), abortion (?)</entry> <entry v="t">Depression</entry> <entry v="t"> </entry></row><row><entry v="t">Local</entry> <entry v="t">None known</entry> <entry v="t">Bradycardia, seizures</entry> <entry v="t"> </entry></row><row><entry cs="4">ANTI-ASTHMATICS</entry></row><row><entry v="t">Metaproterenol, salmeterol, albuterol</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">Terbutaline</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, and hyperglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Theophylline</entry> <entry v="t">None known</entry> <entry v="t">Jitteriness, tachycardia</entry> <entry v="t">May produce jitteriness, poor feeding, vomiting, cardiac arrhythmias</entry></row><row><entry cs="4">ANTICOAGULANTS</entry></row><row><entry v="t">Warfarin</entry> <entry v="t">Nasal hypoplasia, ophthalmic abnormalities, epiphyseal stippling</entry> <entry v="t">Hemorrhage, stillbirth</entry> <entry v="t">Safe</entry></row><row><entry v="t">Heparin, low molecular weight</entry> <entry v="t">None known</entry> <entry v="t">Hemorrhage (?), stillbirth (?)</entry> <entry v="t">Safe</entry></row><row><entry cs="4">ANTICONVULSANTS</entry></row><row><entry v="t">Barbiturates</entry> <entry v="t">Malformations (?)</entry> <entry v="t">Bleeding, withdrawal</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Carbamazepine, oxcarbazepine</entry> <entry v="t">Craniofacial, neural tube (?)</entry> <entry v="t">Bleeding, withdrawal, growth restriction</entry> <entry v="t">Probably safe</entry></row><row><entry v="t">Clonazepam</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, depression</entry> <entry v="t">Not recommended (potential for apnea, cyanosis, or hypotonia); serum levels should be monitored</entry></row><row><entry v="t">Ethosuximide</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Gabapentin</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Phenytoin*</entry> <entry v="t">Craniofacial abnormalities, mental retardation, hypoplasia of phalanges</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors</entry> <entry v="t">Probably safe</entry></row><row><entry v="t">Primidone</entry> <entry v="t">Orofacial clefts</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors, intrauterine growth restriction</entry> <entry v="t">May produce significant adverse effects in infants; use with caution</entry></row><row><entry v="t">Trimethadione*</entry> <entry v="t">Mental retardation, facial dysmorphogenesis, cardiovascular effects</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors, intrauterine growth restriction</entry> <entry v="t">No information available</entry></row><row><entry v="t">Valproic acid*</entry> <entry v="t">Spina bifida, facial dysmorphogenesis</entry> <entry v="t">Perinatal distress, behavioral abnormalities</entry> <entry v="t">Safe</entry></row><row><entry cs="4">ANTI-EMETICS</entry></row><row><entry v="t">Diphenhydramine</entry> <entry v="t">None known, clefting unlikely</entry> <entry v="t">None known</entry> <entry v="t">Safe, but may cause drowsiness</entry></row><row><entry v="t">Doxylamine (with pyridoxine)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown; probably sedating</entry></row><row><entry v="t">Meclizine</entry> <entry v="t">None known</entry> <entry v="t">Retrolental fibrosis in premature infant</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Metoclopramide</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Potential central nervous system effects</entry></row><row><entry v="t">Ondansetron</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Promethazine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Scopolamine</entry> <entry v="t">None known</entry> <entry v="t">Fetal heart rate changes</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIBACTERIALS</entry></row><row><entry v="t">Aminoglycosides</entry> <entry v="t">None known</entry> <entry v="t">Nephrotoxic (?), ototoxic (?)</entry> <entry v="t">Depends on level of exposure and renal function of infant</entry></row><row><entry v="t">Azithromycin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Cephalosporins</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Probably Compatible</entry></row><row><entry v="t">Chloramphenicol</entry> <entry v="t">None known</entry> <entry v="t">Vascular collapse</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Ciprofloxacin</entry> <entry v="t">Toxic to developing cartilage (?)</entry> <entry v="t">Toxic to developing cartilage (?)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Clindamycin</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Compatible, but potential modification of bowel flora, interference with culture interpretation after fever work-up in infants</entry></row><row><entry v="t">Erythromycin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Isoniazid</entry> <entry v="t">Malformations (?)</entry> <entry v="t">Behavioral abnormality</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Metronidazole</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Use with caution because of mutagenic and carcinogenic effects in some species; abstain from breastfeeding for 12–24 hours after single dose</entry></row><row><entry v="t">Nitrofurantoin</entry> <entry v="t">None known</entry> <entry v="t">Hemolysis (?)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Penicillins</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Rifampin</entry> <entry v="t">Risk of malformation not greater than in general population</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Sulfonamides</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Generally Compatible, but avoid in infants with hyperbilirubinemia, premature infants, and infants with G6PD deficiency</entry></row><row><entry v="t">Tetracyclines</entry> <entry v="t">None known</entry> <entry v="t">Stained deciduous teeth (enamel hypoplasia)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Trimethoprim</entry> <entry v="t">Cleft palate, micrognathia, limb shortening</entry> <entry v="t">Unknown</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIFUNGALS</entry></row><row><entry v="t">Amphotericin-B</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Fluconazole</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIRETROVIRALS</entry></row><row><entry v="t">Class of drugs in general</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Contraindicated (HIV)</entry></row><row><entry cs="4">ANTIVIRALS</entry></row><row><entry v="t">Acyclovir</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Interferon</entry> <entry v="t">None known</entry> <entry v="t">Intrauterine growth restriction (?)</entry> <entry v="t">Likely safe</entry></row><row><entry cs="4">CANCER CHEMOTHERAPY</entry></row><row><entry v="t">Alkylating agents</entry> <entry v="t">Abortion, anomalies</entry> <entry v="t">Hypoplastic gonads, growth restriction and delay</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Antimetabolites <list type="bullet"> <item><para>Folic acid analogues (methotrexate)</para></item> <item><para>Purine analogues</para></item> <item><para>Pyrimidine analogues (cytosine arabinoside, 5-fluorouracil)</para></item> </list></entry> <entry v="t"><list type="bullet"> <item><para>Abortion, intrauterine growth restriction, cranial anomalies</para></item> <item><para>Same as above</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Hypoplastic gonads, growth restriction and delay</para></item> <item><para>Same as above, plus transient anemia</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item></list></entry></row><row><entry v="t">Antibiotics <list type="bullet"> <item><para>Actinomycin</para></item> <item><para>Vinca alkaloids (vincristine)</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Abortion, intrauterine growth restriction, cranial anomalies</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Hypoplastic gonads, growth restriction and delay</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item></list></entry></row><row><entry cs="4">CARDIOVASCULAR DRUGS</entry></row><row><entry v="t">ACE inhibitors</entry> <entry v="t">None known</entry> <entry v="t">Oliguria, skull defects, death</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Adenosine</entry> <entry v="t">None known</entry> <entry v="t">No effects on fetal heart rate</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Beta-sympathomimetics</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, and hyperglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Calcium channel blockers</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Digitalis</entry> <entry v="t">None known</entry> <entry v="t">Lower heart rate</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Hydralazine</entry> <entry v="t">Skeletal defects (?)</entry> <entry v="t">Tachycardia, thrombocytopenia, fetal distress</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Methyldopa</entry> <entry v="t">None known</entry> <entry v="t">Hemolytic anemia, tremor, hypotension</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Propranolol, labetalol</entry> <entry v="t">Unknown</entry> <entry v="t">Lower heart rate, intrauterine growth restriction (?), hypoglycemia, respiratory distress</entry> <entry v="t">Compatible; hypoglycemia (?)</entry></row><row><entry v="t">Reserpine</entry> <entry v="t">None known</entry> <entry v="t">Lethargy, respiratory distress</entry> <entry v="t">Unknown</entry></row><row><entry cs="4">COLD AND COUGH PREPARATIONS</entry></row><row><entry v="t">Antihistamines</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Reduced milk (?); drowsiness</entry></row><row><entry v="t">Cough suppressants</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Decongestants</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Dextromethorphan</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Expectorants</entry> <entry v="t">Fetal goiter (?)</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Loratadine</entry> <entry v="t">Likely none</entry> <entry v="t">Likely none</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">DIURETICS</entry></row><row><entry v="t">Furosemide</entry> <entry v="t">Unknown</entry> <entry v="t">Death from sudden hypoperfusion, electrolyte imbalance</entry> <entry v="t">Found to suppress lactation</entry></row><row><entry v="t">Thiazides</entry> <entry v="t">None known</entry> <entry v="t">Thrombocytopenia, hypokalemia, hyperbilirubinemia, hyponatremia</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">FERTILITY DRUGS</entry></row><row><entry v="t">Clomiphene</entry> <entry v="t">Meiotic nondisjunction (?), neural tube defects (?)</entry> <entry v="t">Unknown</entry> <entry v="t">No data available</entry></row><row><entry cs="4">GASTROINTESTINAL AGENTS</entry></row><row><entry v="t">Bisacodyl</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">No reports of adverse effects</entry></row><row><entry v="t">Cholestyramine</entry> <entry v="t">None known</entry> <entry v="t">None known, but fat-soluble vitamins are depleted</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Colestipol</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown, but minimal absorption</entry> <entry v="t">No data available</entry></row><row><entry v="t">Docusate</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">H2-histamine receptor blockers</entry> <entry v="t">None known</entry> <entry v="t">Anti-androgen effect (cimetidine)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Magnesium hydroxide (Milk of Magnesia)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Mineral oil</entry> <entry v="t">Decreased maternal vitamin absorption</entry> <entry v="t">Decreased maternal vitamin absorption</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Proton pump inhibitors</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sulfasalazine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Caution with ill infants</entry></row><row><entry cs="4">HORMONES</entry></row><row><entry v="t">Androgens*</entry> <entry v="t">Virilization of female fetus</entry> <entry v="t">Adrenal suppression</entry> <entry v="t">No adverse effects reported</entry></row><row><entry v="t">Corticosteroids</entry> <entry v="t">Orofacial cleft in animals, not in humans</entry> <entry v="t">No adverse effects in humans</entry> <entry v="t">No data available</entry></row><row><entry v="t">Danazol</entry> <entry v="t">Virilization of female fetus (?)</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">Estrogens</entry> <entry v="t">Cardiovascular anomalies (?)</entry> <entry v="t">None known</entry> <entry v="t">No reported adverse effects</entry></row><row><entry v="t">Progestins</entry> <entry v="t">Limb and cardiovascular anomalies (?), VACTERL syndrome (?), masculinization of female fetus (?)</entry> <entry v="t">None known</entry> <entry v="t">No reported adverse effects</entry></row><row><entry cs="4">DIABETES CARE</entry></row><row><entry v="t">Glucagon</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Glyburide</entry> <entry v="t">None known</entry> <entry v="t">Not thought to cross the placenta in significant amounts; no neonatal hypoglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Insulin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Safe</entry></row><row><entry v="t">Metformin</entry> <entry v="t">None known</entry> <entry v="t">Neonatal hypoglycemia</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sulfonylureas</entry> <entry v="t">Anomalies (?)</entry> <entry v="t">Suppressed insulin secretion</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">MIGRAINE REMEDIES</entry></row><row><entry v="t">Ergotamine</entry> <entry v="t">None known</entry> <entry v="t">May stimulate contractions</entry> <entry v="t">Use with caution</entry></row><row><entry v="t">Sumatriptan</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">PSYCHOACTIVE DRUGS, ANTIDEPRESSANTS</entry></row><row><entry v="t">Amphetamine</entry> <entry v="t">Inconsistent; likely none</entry> <entry v="t">Reduced weight</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Benzodiazepines</entry> <entry v="t">Facial dysmorphism (?)</entry> <entry v="t">Depression, floppy infant, hypothermia, withdrawal</entry> <entry v="t">Some concern about central nervous system toxicity with long-term use</entry></row><row><entry v="t">Fluoxetine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Symptoms of colic</entry></row><row><entry v="t">Hydroxyzine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Lithium</entry> <entry v="t">Facial clefts; cardiovascular anomaly</entry> <entry v="t">Lithium toxicity (neurologic and hepatic dysfunction)</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Meprobamate</entry> <entry v="t">Cardiac anomalies (?), major malformations (?)</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Phenothiazines</entry> <entry v="t">None known</entry> <entry v="t">Muscle rigidity, hypothermia, tremor</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sedatives</entry> <entry v="t">None known</entry> <entry v="t">Depression, slow learning</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Thalidomide*</entry> <entry v="t">Phocomelia in 20% of cases</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Tricyclics</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown/caution</entry></row><row><entry v="t">Zolpiden</entry> <entry v="t">Unknown</entry> <entry v="t">Withdrawal or floppy infant (?)</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">RADIOLABELED DIAGNOSTICS</entry></row><row><entry v="t">Albumin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">I131 (diagnostic)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Not recommended during exposure; may continue 24 hours after exposure</entry></row><row><entry v="t">Technetium</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Not recommended during exposure; may continue 24 hours after exposure</entry></row><row><entry cs="4">SMOKING CESSATION</entry></row><row><entry v="t">Bupropion</entry> <entry v="t">Likely none</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Nicotine</entry> <entry v="t">Spontaneous abortion (?)</entry> <entry v="t">Impaired growth (?)</entry> <entry v="t">Consistent with passive smoking</entry></row><row><entry cs="4">THYROID MEDICATION</entry></row><row><entry v="t">I131 (therapeutic)</entry> <entry v="t">Goiter, abortion, anomalies</entry> <entry v="t">Goiter, airway obstruction, hyperthyroid, mental retardation</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methimazole</entry> <entry v="t">Aplasia cutis (?), goiter</entry> <entry v="t">Goiter, airway obstruction, hyperthyroid, mental retardation, aplasia cutis (?)</entry> <entry v="t">Compatible, but monitor fetal thyroid function</entry></row><row><entry v="t">Propylthiouracil</entry> <entry v="t">Goiter</entry> <entry v="t">Same as above</entry> <entry v="t">Safe, but monitor baby’s thyroid status</entry></row><row><entry v="t">Thyroid USP</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Thyroxine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">TOCOLYTICS</entry></row><row><entry v="t">Beta-sympathomimetics</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, hyperglycemia</entry> <entry v="t">—</entry></row><row><entry v="t">Indomethacin</entry> <entry v="t">None known</entry> <entry v="t">Oligohydramnios (>48 hours of use)</entry> <entry v="t">—</entry></row><row><entry v="t">Magnesium sulfate</entry> <entry v="t">None known</entry> <entry v="t">Hypermagnesemia, respiratory depression</entry> <entry v="t">—</entry></row><row><entry v="t">Nifedipine</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">—</entry></row><row><entry cs="4">VACCINATIONS</entry></row><row><entry v="t">Influenza</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Pneumovaccine</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Tetanus toxoid</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">VAGINAL PREPARATIONS</entry></row><row><entry v="t">Antifungal agents</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Podophyllin</entry> <entry v="t">Mutagenesis (?)</entry> <entry v="t">Central nervous system effects (?)</entry> <entry v="t">Contraindicated</entry></row><row><entry cs="4">VITAMINS (high dose)</entry></row><row><entry v="t">A</entry> <entry v="t">Urogenital and craniofacial anomalies (?)</entry> <entry v="t">None known</entry> <entry v="t">No data available</entry></row><row><entry v="t">C</entry> <entry v="t">None known</entry> <entry v="t">Scurvy after delivery</entry> <entry v="t">Compatible</entry></row><row><entry v="t">D</entry> <entry v="t">Supravalvular aortic stenosis (?)</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">E</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">K</entry> <entry v="t">Unknown</entry> <entry v="t">Hemorrhage, if deficiency</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">“STREET” DRUGS</entry></row><row><entry v="t">Cocaine</entry> <entry v="t">Placental abruption, vascular disruption, urinary tract anomalies</entry> <entry v="t">Withdrawal, placental abruption, vascular disruption, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Heroin</entry> <entry v="t">None known</entry> <entry v="t">Depression, withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">LSD</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, behavioral effects</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Marijuana</entry> <entry v="t">None known</entry> <entry v="t">Behavioral effects, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methadone</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methamphetamine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Pentazocine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Phencyclidine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, neurobehavioral effects, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry cs="4">OTHER DRUGS</entry></row><row><entry v="t">Azathioprine</entry> <entry v="t">Abortion</entry> <entry v="t">Anemia, thrombocytopenia, lymphopenia, growth retardation</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Bromocriptine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Caffeine</entry> <entry v="t">Anomalies (?) in high doses, abortion (?)</entry> <entry v="t">Jitteriness</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Immune gamma globulin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Isotretinoin*</entry> <entry v="t">Central nervous system, cardiac, facial anomalies</entry> <entry v="t">Stillbirth, mental retardation (?)</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Misoprostol</entry> <entry v="t">Abortion; variety of anomalies (cranium, limb, oral cleft); Mobius sequence</entry> <entry v="t">None with low dose for cervical ripening; placental abruption</entry> <entry v="t">Contraindicated, especially if diarrhea occurs</entry></row><row><entry v="t">Spermicides</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry cs="4">* Proven teratogen.</entry></row><row><entry cs="4">Unknown=no studies to investigate fetal effects; none known=no malformations reported in human studies or no consistent malformations in animal studies; (?)=conflicting information</entry></row><row><entry cs="4">Source: Reprotox data from humans, last reviewed in 2006.</entry></row>
The author reports no financial relationships relevant to this article.
Fifty years ago, the thalidomide experience—a high incidence of major birth defects following prenatal use of the drug—made clear the devastating potential of drug exposure during pregnancy. Since that disaster, healthcare providers and patients have adopted a conservative approach to medication use during pregnancy, especially during the first trimester and lactation. That is a wise strategy, although very few medications are associated with abnormal fetal development.
In this article, I’ll guide you through some of the issues that must be considered when assessing a drug’s teratogenicity, help you find information on a host of medications, and familiarize you with some of the challenges involved in counseling the patient. I also present a table listing the adverse effects known to be associated with selected drugs during the first, second, and third trimesters and lactation (TABLE). We are fortunate that a large body of information about medication use during pregnancy and lactation is readily available on the Web and in books and medical journals. This information is far from definitive, however, because much of the evidence concerning prescribed drugs is anecdotal or presented with insufficient warning about their use during pregnancy and lactation.
A discussion of these issues with the patient will help set the risks and benefits of a particular drug into proper perspective, alleviate fears, and improve compliance. Nonprescription medications should also be discussed, and the patient should be advised that we have very little data concerning their use during pregnancy.
Assignment of risk is an uncertain science
Major structural defects are apparent at birth in about 3% of all pregnancies and in about 4.5% of all children by the age of 5 years.1 A cause or proposed mechanism for the defects can be determined in fewer than 50% of these cases. Nor can we count on expert consensus about the safety of many medications during pregnancy because it rarely occurs and, in some cases, may be impossible to achieve.
Animal studies are the means of assessing the teratogenicity of most drugs. Animals commonly used to study fetal effects include rodents (fertility, birth defects, birth weight, behavior), rabbits (birth defects), baboons (uterine blood flow), and sheep (uterine blood flow, cardiovascular effects, fetal hypoxia, and acidosis). Dosages are often much higher (in relation to body weight or surface area) to “test the systems” for any possible reproductive harm. Although these studies may be helpful, they do not reliably predict the human response.
Even when humans are the subject of study, conclusions must be viewed with caution. To determine the risk of teratogenesis, it is necessary to know the stage of development during which the exposure occurred, as well as the identity and dose of the medication and the genetic susceptibility of the mother and fetus.
Three critical stages. In utero exposure to a drug occurs in one of three periods of fetal development:
- ovum – from fertilization to implantation
- embryo – from the second through the eighth week of gestation
- fetus – from the eighth completed week until delivery.
An “all-or-none” effect (i.e., spontaneous abortion or not) is believed to arise from exposure during the first period, but the embryo stage is the most critical time because it involves organogenesis. Detrimental effects may occur even beyond this period as cells continue to divide in the hematologic, reproductive, and central nervous systems.
Many fine points of exposure are difficult to clarify
Retrospective and uncontrolled studies, as well as individual case reports or small series, may overestimate the risk to the fetus of exposure to a specific drug or combination of medications. Case reports do not establish causation.
It can also be difficult to differentiate between the risks of a specific drug and the hazards of maternal illness to explain an unfavorable outcome. For example, is a particular case of stillbirth the result of fetal exposure to enoxaparin or maternal thrombophilia, or both? Can fetal growth restriction be attributed to use of azathioprine during pregnancy or to the mother’s underlying illness? And so on.
In addition, it is necessary to distinguish between a defect’s natural prevalence—i.e., the rate at which it occurs in a population—and the additional risk posed by exposure to a particular drug. Studies in large populations are needed—but usually unattainable—to determine the relative risk from specific potential teratogens.
Finally, it is very difficult to assess neurobehavioral effects of in utero exposure to centrally acting drugs beyond the immediate neonatal period. The dose, offspring’s age and gender, and behavioral test system must all be considered.
Few drugs are implicated in restricting fetal growth or reducing organ size. We also lack consistent information about long-term effects such as learning or behavioral problems (i.e., functional teratogenesis) that may result from chronic prenatal exposure to a certain medication.
In 1979, the Food and Drug Administration created five pregnancy risk categories to be used by manufacturers to rate their products in the drug formulary for use during pregnancy: categories A, B, C, D, and X, which range from no evidence of damage to the fetus (category A) to clear teratogenicity (D and X).
The D rating is generally reserved for drugs with no safer alternatives, such as secobarbital, doxycycline, and lorazepam. The X rating means there is absolutely no reason to risk using the drug in pregnancy, as in the case of oral contraceptives, benzodiazepines, and misoprostol.
Approximately 2% of drugs fall into category A, 50% in category B, 38% in category C, 3–5% in category D, and 1–5% in category X.3 These categories do not often accurately reflect the available information on risk to the fetus. A major initiative is under way to declare these categories obsolete and provide more informative drug labeling. Pregnancy labels of the future will likely address three important areas:
- clinical considerations–issues relevant to prescription of a particular drug in pregnancy, including the risk of disease versus no treatment. Also included will be information of use when counseling a patient whose fetus was inadvertently exposed to a medication in early gestation
- summary risk assessment–a narrative text that describes, as comprehensively as possible, the risk of exposure based on animal and human data
- data to support the assessment.
All drugs cross the placenta
Most medications are easily absorbed during pregnancy, and serum concentrations of albumin for drug binding are lower than in the nonpregnant state. Pharmacokinetic changes during pregnancy include:
- higher volume of distribution
- lower maximum plasma concentration
- lower steady-state serum concentration
- shorter plasma half-life
- higher clearance rate.1
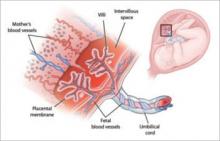
The small spatial configuration and high lipid solubility of most medications permit easy transfer of an unbound drug or its metabolite across the placenta or into breast milk. Virtually all drugs and their end products cross the placenta, with unbound concentrations of the drug in the fetal serum similar to the level in maternal serum—sometimes even higher (FIGURE).
A few drugs with high molecular weight do not cross the placenta in significant amounts (e.g., glyburide, interferon, thyroid supplements, insulin).
FIGURE An elaborate nutrient (and drug) delivery system
The placenta and umbilical cord deliver the nutrients and oxygen the fetus needs for normal growth—as well as most medications used by the mother.
Medication use tends to increase as pregnancy progresses
The drugs most commonly taken during pregnancy include vitamins, iron preparations, calcium, analgesics, antibiotics, and antacids. Excluding vitamins and mineral supplements, an average of one to two medications are taken during gestation. Over-the-counter formulations account for about half of these drugs, with acetaminophen being the single most commonly used medication during pregnancy. Antibiotics are the most widely prescribed drugs.
Although caffeine, tobacco, alcohol, and illicit substance use tends to diminish as pregnancy progresses, medications are usually taken at the same frequency or more often during gestation.
My colleagues and I found a significantly higher mean number of medications (3.3 and 4.1, respectively) used during the second and third trimesters of gestation than were taken before pregnancy (2.6).2
How to counsel the patient
Counseling a woman before or during pregnancy about the continuation or initiation of a medication should take place in an open, supportive, and informative manner. Most inquiries relate to exposures involving very low levels of relative and absolute risk.
A detailed fetal ultrasonographic examination is often used to accurately date the pregnancy and, if possible, screen for any structural defects. The patient should be advised that first-trimester screening, chorionic villus sampling, maternal serum quadruple screening, amniocentesis, and fetal blood sampling are not very predictive of a drug’s fetal effects. Exceptions may be the observation of open neural tube defects (approximate 1% risk associated with valproic acid and carbamazepine) by maternal serum quadruple screening and facial clefting by targeted ultrasonography.
When a patient inquires about a particular drug, it is important to gather the following information:
- When did she take the medication?
- Why did she take it?
- For how long did she take the medication?
- Did she take other medications, or any substances of abuse, at the same time?
A number of sources of information about potential teratogens are available.3-5 These include national computerized databases that are accessible on the Web:
- National Library of Medicine (http://sis.nlm.nih.gov/enviro.html)
- pregnancy exposure registries (www.fda.gov/womens/registries/default.htm)
- Reproductive Toxicology Center (http://reprotox.org) (access requires a paid subscription)
- LactMed, National Library of Medicine guide to drug safety in lactation (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT)
- Organization of Teratology Information Specialists (OTIS) (www.otispregnancy.org).
The last source (OTIS) consolidates teratology information nationwide and reports it by state or region. It also publishes a host of fact sheets on various drugs that may be useful to dispense to the patient during counseling. In addition, many teratogen information services or poison control centers (often at children’s hospitals) are available throughout the United States to serve specific geographic areas. And teratogen registries at pharmaceutical companies may provide limited information about newer medications.
The Physician’s Desk Reference (PDR) is a common source of information about the use of prescription drugs in pregnancy.3 But be aware that, to avoid liability, pharmaceutical manufacturers do not encourage use of their drugs during pregnancy unless the benefit clearly outweighs the risk. It would be unrealistic for them to market a medication for specific use during pregnancy because it would require considerable time and cost, and raise ethical objections, to conduct research in a vulnerable population that is limited in number.
Effects of agents used more than 40 years ago were reported by the Collaborative Perinatal Project or the Boston Collaborative Drug Surveillance Program.6 Those findings are often inconclusive, reflect bias in study designs, and do not help a clinician evaluate current medications or those less commonly prescribed during pregnancy.
The risks and experience associated with new drugs are usually not well explored in regard to pregnancy. As a result, older medications are more likely to be prescribed as maintenance therapy during gestation for the simple reason that they have a larger body of information regarding their effects. These older drugs may no longer be preferred once the patient delivers.
Most drugs are not teratogens
The TABLE lists adverse effects in the human fetus known to arise from exposure to specific drugs. The information comes largely from the Reprotox database, which was reviewed as recently as 2006, describes human data only, and is reported by first trimester (anomalies, abortion) and the second and third trimesters (fetal growth restriction, stillbirth, low birth weight, preterm delivery, immediate neonatal problems).7 Typical dosages of most drugs are not anticipated to increase the risk of congenital anomalies.
Most human data come from small series or case reports. Although these types of studies are helpful, they tend to be biased or reflect the pregnancy’s background risk of birth defects rather than the risk posed by a specific drug. In addition, case reports of malformation after prenatal exposure to a certain drug may involve exposures to other agents and a lack of uniformity of abnormalities, making the association between adverse effects and a single agent unlikely. Dissimilarities in the dosage and route of delivery also limit interpretation. for example, short-term intravenous or sublingual administration of a drug may pose a different risk than taking that medication orally or vaginally, in a lower dose, for a longer period, or at a different period of gestation.
Randomized controlled trials of drugs are rare during pregnancy, as are prospective cohort investigations. Because a control population is often impossible to identify, it becomes difficult to separate any heightened risk identified during use of the medication from the underlying disease. When the gravida has significant medical problems, it is important to assess the potential risk of a drug—or its omission—in her as well as her fetus. The lowest effective dose is preferred, but keep in mind that inadequate treatment may lead to minimal benefit and potentially greater risk to the pregnancy.
When reviewing or planning maintenance drug therapy, follow the same principles as you would in a nonpregnant patient. Be familiar with more than one medication for each disorder. Also be aware that some drugs may need to be prescribed at a higher dose or greater frequency to attain a therapeutic concentration in the expanded intravascular volume of pregnancy. In addition, side effects such as nausea, fatigue, and gastrointestinal disturbance may mimic symptoms arising from physiologic changes of pregnancy.
Assessing the risks associated with over-the-counter medications and natural food products is even harder. The PDR for Nonprescription Drugs, Dietary Supplements, and Herbs8 contains little or no information about the reproductive hazards of most of these products. Many agents contain multiple ingredients, both active and inactive, thereby complicating counseling about their risks during pregnancy. Although the recommended dosage is usually low, many product labels do not specify what it should be.
Most drugs enter breast milk
The amount of drug that an infant consumes from breast milk depends on the medication’s chemical properties as well as the dosage, frequency, and duration of exposure.2 Contraindications and cautions are usually either theoretical or based on findings from case reports that often conflict or confuse. In theory, it is safer for the mother to take the medication just after infant feeding or just before the infant’s longest sleep period.
The TABLE also lists the effects of drugs in the breastfed human infant. Again, the information comes from the Reprotox database, access to which requires a subscription. For additional information, try the free LactMed site at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT.
Nearly all drugs are excreted in breast milk, usually in small amounts (often less than 5% of the weight-adjusted maternal daily dose). The amount of drug or metabolite in an infant’s serum also is determined by the volume of breast milk, age of the infant, and other exposures.
Avoid prescribing multiple medications, if possible, and choose “safe” drugs from among the options in categories that include a number of teratogenic medications, such as anticonvulsants.
Determine the best method to monitor therapy. For example, use a peak flow meter for asthma, a portable blood pressure monitor for hypertension, and so on.
Focus on keeping the patient healthy. The healthiest mother is most likely to deliver the healthiest infant.
Keep the underlying disorder in mind, as well as the drug, when choosing a drug.
Know which drugs are clearly linked to birth defects. These include phenytoin, warfarin, alcohol, methotrexate, diethylstilbestrol, cis-retinoic acid, valproic acid, and carbamazepine.
Pay special attention to the first trimester. Too little is known about the first-trimester effects of the vast majority of drugs for them to be considered safe.
Suspect a drug-related effect
A medication may be the cause in any newborn manifesting signs of anemia, hepatitis, hepatotoxicity, hepatorenal dysfunction, and hyperbilirubinemia. This includes breastfed infants. An adverse drug-related effect should also be suspected when an infant exhibits signs of jaundice, floppiness, jitteriness, poor suck, diarrhea, or growth restriction.
The author reports no financial relationships relevant to this article.
Fifty years ago, the thalidomide experience—a high incidence of major birth defects following prenatal use of the drug—made clear the devastating potential of drug exposure during pregnancy. Since that disaster, healthcare providers and patients have adopted a conservative approach to medication use during pregnancy, especially during the first trimester and lactation. That is a wise strategy, although very few medications are associated with abnormal fetal development.
In this article, I’ll guide you through some of the issues that must be considered when assessing a drug’s teratogenicity, help you find information on a host of medications, and familiarize you with some of the challenges involved in counseling the patient. I also present a table listing the adverse effects known to be associated with selected drugs during the first, second, and third trimesters and lactation (TABLE). We are fortunate that a large body of information about medication use during pregnancy and lactation is readily available on the Web and in books and medical journals. This information is far from definitive, however, because much of the evidence concerning prescribed drugs is anecdotal or presented with insufficient warning about their use during pregnancy and lactation.
A discussion of these issues with the patient will help set the risks and benefits of a particular drug into proper perspective, alleviate fears, and improve compliance. Nonprescription medications should also be discussed, and the patient should be advised that we have very little data concerning their use during pregnancy.
Assignment of risk is an uncertain science
Major structural defects are apparent at birth in about 3% of all pregnancies and in about 4.5% of all children by the age of 5 years.1 A cause or proposed mechanism for the defects can be determined in fewer than 50% of these cases. Nor can we count on expert consensus about the safety of many medications during pregnancy because it rarely occurs and, in some cases, may be impossible to achieve.
Animal studies are the means of assessing the teratogenicity of most drugs. Animals commonly used to study fetal effects include rodents (fertility, birth defects, birth weight, behavior), rabbits (birth defects), baboons (uterine blood flow), and sheep (uterine blood flow, cardiovascular effects, fetal hypoxia, and acidosis). Dosages are often much higher (in relation to body weight or surface area) to “test the systems” for any possible reproductive harm. Although these studies may be helpful, they do not reliably predict the human response.
Even when humans are the subject of study, conclusions must be viewed with caution. To determine the risk of teratogenesis, it is necessary to know the stage of development during which the exposure occurred, as well as the identity and dose of the medication and the genetic susceptibility of the mother and fetus.
Three critical stages. In utero exposure to a drug occurs in one of three periods of fetal development:
- ovum – from fertilization to implantation
- embryo – from the second through the eighth week of gestation
- fetus – from the eighth completed week until delivery.
An “all-or-none” effect (i.e., spontaneous abortion or not) is believed to arise from exposure during the first period, but the embryo stage is the most critical time because it involves organogenesis. Detrimental effects may occur even beyond this period as cells continue to divide in the hematologic, reproductive, and central nervous systems.
Many fine points of exposure are difficult to clarify
Retrospective and uncontrolled studies, as well as individual case reports or small series, may overestimate the risk to the fetus of exposure to a specific drug or combination of medications. Case reports do not establish causation.
It can also be difficult to differentiate between the risks of a specific drug and the hazards of maternal illness to explain an unfavorable outcome. For example, is a particular case of stillbirth the result of fetal exposure to enoxaparin or maternal thrombophilia, or both? Can fetal growth restriction be attributed to use of azathioprine during pregnancy or to the mother’s underlying illness? And so on.
In addition, it is necessary to distinguish between a defect’s natural prevalence—i.e., the rate at which it occurs in a population—and the additional risk posed by exposure to a particular drug. Studies in large populations are needed—but usually unattainable—to determine the relative risk from specific potential teratogens.
Finally, it is very difficult to assess neurobehavioral effects of in utero exposure to centrally acting drugs beyond the immediate neonatal period. The dose, offspring’s age and gender, and behavioral test system must all be considered.
Few drugs are implicated in restricting fetal growth or reducing organ size. We also lack consistent information about long-term effects such as learning or behavioral problems (i.e., functional teratogenesis) that may result from chronic prenatal exposure to a certain medication.
In 1979, the Food and Drug Administration created five pregnancy risk categories to be used by manufacturers to rate their products in the drug formulary for use during pregnancy: categories A, B, C, D, and X, which range from no evidence of damage to the fetus (category A) to clear teratogenicity (D and X).
The D rating is generally reserved for drugs with no safer alternatives, such as secobarbital, doxycycline, and lorazepam. The X rating means there is absolutely no reason to risk using the drug in pregnancy, as in the case of oral contraceptives, benzodiazepines, and misoprostol.
Approximately 2% of drugs fall into category A, 50% in category B, 38% in category C, 3–5% in category D, and 1–5% in category X.3 These categories do not often accurately reflect the available information on risk to the fetus. A major initiative is under way to declare these categories obsolete and provide more informative drug labeling. Pregnancy labels of the future will likely address three important areas:
- clinical considerations–issues relevant to prescription of a particular drug in pregnancy, including the risk of disease versus no treatment. Also included will be information of use when counseling a patient whose fetus was inadvertently exposed to a medication in early gestation
- summary risk assessment–a narrative text that describes, as comprehensively as possible, the risk of exposure based on animal and human data
- data to support the assessment.
All drugs cross the placenta
Most medications are easily absorbed during pregnancy, and serum concentrations of albumin for drug binding are lower than in the nonpregnant state. Pharmacokinetic changes during pregnancy include:
- higher volume of distribution
- lower maximum plasma concentration
- lower steady-state serum concentration
- shorter plasma half-life
- higher clearance rate.1
The small spatial configuration and high lipid solubility of most medications permit easy transfer of an unbound drug or its metabolite across the placenta or into breast milk. Virtually all drugs and their end products cross the placenta, with unbound concentrations of the drug in the fetal serum similar to the level in maternal serum—sometimes even higher (FIGURE).
A few drugs with high molecular weight do not cross the placenta in significant amounts (e.g., glyburide, interferon, thyroid supplements, insulin).
FIGURE An elaborate nutrient (and drug) delivery system
The placenta and umbilical cord deliver the nutrients and oxygen the fetus needs for normal growth—as well as most medications used by the mother.
Medication use tends to increase as pregnancy progresses
The drugs most commonly taken during pregnancy include vitamins, iron preparations, calcium, analgesics, antibiotics, and antacids. Excluding vitamins and mineral supplements, an average of one to two medications are taken during gestation. Over-the-counter formulations account for about half of these drugs, with acetaminophen being the single most commonly used medication during pregnancy. Antibiotics are the most widely prescribed drugs.
Although caffeine, tobacco, alcohol, and illicit substance use tends to diminish as pregnancy progresses, medications are usually taken at the same frequency or more often during gestation.
My colleagues and I found a significantly higher mean number of medications (3.3 and 4.1, respectively) used during the second and third trimesters of gestation than were taken before pregnancy (2.6).2
How to counsel the patient
Counseling a woman before or during pregnancy about the continuation or initiation of a medication should take place in an open, supportive, and informative manner. Most inquiries relate to exposures involving very low levels of relative and absolute risk.
A detailed fetal ultrasonographic examination is often used to accurately date the pregnancy and, if possible, screen for any structural defects. The patient should be advised that first-trimester screening, chorionic villus sampling, maternal serum quadruple screening, amniocentesis, and fetal blood sampling are not very predictive of a drug’s fetal effects. Exceptions may be the observation of open neural tube defects (approximate 1% risk associated with valproic acid and carbamazepine) by maternal serum quadruple screening and facial clefting by targeted ultrasonography.
When a patient inquires about a particular drug, it is important to gather the following information:
- When did she take the medication?
- Why did she take it?
- For how long did she take the medication?
- Did she take other medications, or any substances of abuse, at the same time?
A number of sources of information about potential teratogens are available.3-5 These include national computerized databases that are accessible on the Web:
- National Library of Medicine (http://sis.nlm.nih.gov/enviro.html)
- pregnancy exposure registries (www.fda.gov/womens/registries/default.htm)
- Reproductive Toxicology Center (http://reprotox.org) (access requires a paid subscription)
- LactMed, National Library of Medicine guide to drug safety in lactation (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT)
- Organization of Teratology Information Specialists (OTIS) (www.otispregnancy.org).
The last source (OTIS) consolidates teratology information nationwide and reports it by state or region. It also publishes a host of fact sheets on various drugs that may be useful to dispense to the patient during counseling. In addition, many teratogen information services or poison control centers (often at children’s hospitals) are available throughout the United States to serve specific geographic areas. And teratogen registries at pharmaceutical companies may provide limited information about newer medications.
The Physician’s Desk Reference (PDR) is a common source of information about the use of prescription drugs in pregnancy.3 But be aware that, to avoid liability, pharmaceutical manufacturers do not encourage use of their drugs during pregnancy unless the benefit clearly outweighs the risk. It would be unrealistic for them to market a medication for specific use during pregnancy because it would require considerable time and cost, and raise ethical objections, to conduct research in a vulnerable population that is limited in number.
Effects of agents used more than 40 years ago were reported by the Collaborative Perinatal Project or the Boston Collaborative Drug Surveillance Program.6 Those findings are often inconclusive, reflect bias in study designs, and do not help a clinician evaluate current medications or those less commonly prescribed during pregnancy.
The risks and experience associated with new drugs are usually not well explored in regard to pregnancy. As a result, older medications are more likely to be prescribed as maintenance therapy during gestation for the simple reason that they have a larger body of information regarding their effects. These older drugs may no longer be preferred once the patient delivers.
Most drugs are not teratogens
The TABLE lists adverse effects in the human fetus known to arise from exposure to specific drugs. The information comes largely from the Reprotox database, which was reviewed as recently as 2006, describes human data only, and is reported by first trimester (anomalies, abortion) and the second and third trimesters (fetal growth restriction, stillbirth, low birth weight, preterm delivery, immediate neonatal problems).7 Typical dosages of most drugs are not anticipated to increase the risk of congenital anomalies.
Most human data come from small series or case reports. Although these types of studies are helpful, they tend to be biased or reflect the pregnancy’s background risk of birth defects rather than the risk posed by a specific drug. In addition, case reports of malformation after prenatal exposure to a certain drug may involve exposures to other agents and a lack of uniformity of abnormalities, making the association between adverse effects and a single agent unlikely. Dissimilarities in the dosage and route of delivery also limit interpretation. for example, short-term intravenous or sublingual administration of a drug may pose a different risk than taking that medication orally or vaginally, in a lower dose, for a longer period, or at a different period of gestation.
Randomized controlled trials of drugs are rare during pregnancy, as are prospective cohort investigations. Because a control population is often impossible to identify, it becomes difficult to separate any heightened risk identified during use of the medication from the underlying disease. When the gravida has significant medical problems, it is important to assess the potential risk of a drug—or its omission—in her as well as her fetus. The lowest effective dose is preferred, but keep in mind that inadequate treatment may lead to minimal benefit and potentially greater risk to the pregnancy.
When reviewing or planning maintenance drug therapy, follow the same principles as you would in a nonpregnant patient. Be familiar with more than one medication for each disorder. Also be aware that some drugs may need to be prescribed at a higher dose or greater frequency to attain a therapeutic concentration in the expanded intravascular volume of pregnancy. In addition, side effects such as nausea, fatigue, and gastrointestinal disturbance may mimic symptoms arising from physiologic changes of pregnancy.
Assessing the risks associated with over-the-counter medications and natural food products is even harder. The PDR for Nonprescription Drugs, Dietary Supplements, and Herbs8 contains little or no information about the reproductive hazards of most of these products. Many agents contain multiple ingredients, both active and inactive, thereby complicating counseling about their risks during pregnancy. Although the recommended dosage is usually low, many product labels do not specify what it should be.
Most drugs enter breast milk
The amount of drug that an infant consumes from breast milk depends on the medication’s chemical properties as well as the dosage, frequency, and duration of exposure.2 Contraindications and cautions are usually either theoretical or based on findings from case reports that often conflict or confuse. In theory, it is safer for the mother to take the medication just after infant feeding or just before the infant’s longest sleep period.
The TABLE also lists the effects of drugs in the breastfed human infant. Again, the information comes from the Reprotox database, access to which requires a subscription. For additional information, try the free LactMed site at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT.
Nearly all drugs are excreted in breast milk, usually in small amounts (often less than 5% of the weight-adjusted maternal daily dose). The amount of drug or metabolite in an infant’s serum also is determined by the volume of breast milk, age of the infant, and other exposures.
Avoid prescribing multiple medications, if possible, and choose “safe” drugs from among the options in categories that include a number of teratogenic medications, such as anticonvulsants.
Determine the best method to monitor therapy. For example, use a peak flow meter for asthma, a portable blood pressure monitor for hypertension, and so on.
Focus on keeping the patient healthy. The healthiest mother is most likely to deliver the healthiest infant.
Keep the underlying disorder in mind, as well as the drug, when choosing a drug.
Know which drugs are clearly linked to birth defects. These include phenytoin, warfarin, alcohol, methotrexate, diethylstilbestrol, cis-retinoic acid, valproic acid, and carbamazepine.
Pay special attention to the first trimester. Too little is known about the first-trimester effects of the vast majority of drugs for them to be considered safe.
Suspect a drug-related effect
A medication may be the cause in any newborn manifesting signs of anemia, hepatitis, hepatotoxicity, hepatorenal dysfunction, and hyperbilirubinemia. This includes breastfed infants. An adverse drug-related effect should also be suspected when an infant exhibits signs of jaundice, floppiness, jitteriness, poor suck, diarrhea, or growth restriction.
Reference
1. American College of Obstetricians and Gynecologists. Teratology. ACOG Educational Bulletin #236. Washington, DC: ACOG; 1997.
2. Splinter M, Nightingale B, Sawgraves R, Rayburn W. Medication use during pregnancy by women delivering at a tertiary university hospital. South Med J. 1997;90:498-502.
3. Physician’s Desk Reference. 61st ed. Montvale, NJ: Medical Economics; 2007.
4. Briggs GG, Freeman RK, Yaffee FJ. Drugs in Pregnancy and Lactation: Reference Guide to Fetal and Neonatal Risk. 7th ed. Baltimore: Williams & Wilkins; 2005.
5. Briggs GG, Freeman RK, Yaffee FJ. ReproTox Database. Vol. 13. No. 1. Bethesda, Md: Reproductive Toxicology Center; 2000.
6. Heinonen OP, Sloan ED, Shapiro S. Birth Defects and Drugs in Pregnancy. Boston: John Wright PSG; 1973.
7. Reproductive Toxicology Center. Bethesda, Md. Available at http://reprotox.org. Accessed Oct. 5, 2007.
8. PDR for Nonprescription Drugs, Dietary Supplements, and Herbs. 28th ed. Montvale, NJ: Medical Economics; 2007.
<b>How selected drugs affect the human fetus and breastfed infant</b><cs cn="1" a="l"> <cs cn="2" a="l"> <cs cn="3" a="l"> <cs cn="4" a="l"> </cs></cs></cs></cs><row><entry v="t">DRUG</entry> <entry v="t">FIRST-TRIMESTER EFFECTS</entry> <entry v="t">EFFECTS DURING SECOND AND THIRD TRIMESTER</entry> <entry v="t">SAFETY DURING BREASTFEEDING</entry></row><row><entry cs="4">ANALGESICS</entry></row><row><entry v="t">Acetaminophen</entry> <entry v="t">None known</entry> <entry v="t">Hepatotoxicity/nephrotoxicity</entry> <entry v="t">Safe</entry></row><row><entry v="t">Ibuprofen</entry> <entry v="t">Gastroschisis (?)</entry> <entry v="t">Closure of ductus</entry> <entry v="t">Small amount passed; no other information</entry></row><row><entry v="t">Narcotics</entry> <entry v="t">None known</entry> <entry v="t">Depression, withdrawal</entry> <entry v="t">Not recommended if dosing is repetitive</entry></row><row><entry v="t">Salicylates</entry> <entry v="t">None known</entry> <entry v="t">Prolonged pregnancy and labor, hemorrhage, altered hemostasis, intracranial hemorrhage</entry> <entry v="t">Use with caution; may have adverse effects in newborn</entry></row><row><entry cs="4">ANESTHETICS</entry></row><row><entry v="t">General</entry> <entry v="t">Anomalies (?), abortion (?)</entry> <entry v="t">Depression</entry> <entry v="t"> </entry></row><row><entry v="t">Local</entry> <entry v="t">None known</entry> <entry v="t">Bradycardia, seizures</entry> <entry v="t"> </entry></row><row><entry cs="4">ANTI-ASTHMATICS</entry></row><row><entry v="t">Metaproterenol, salmeterol, albuterol</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">Terbutaline</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, and hyperglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Theophylline</entry> <entry v="t">None known</entry> <entry v="t">Jitteriness, tachycardia</entry> <entry v="t">May produce jitteriness, poor feeding, vomiting, cardiac arrhythmias</entry></row><row><entry cs="4">ANTICOAGULANTS</entry></row><row><entry v="t">Warfarin</entry> <entry v="t">Nasal hypoplasia, ophthalmic abnormalities, epiphyseal stippling</entry> <entry v="t">Hemorrhage, stillbirth</entry> <entry v="t">Safe</entry></row><row><entry v="t">Heparin, low molecular weight</entry> <entry v="t">None known</entry> <entry v="t">Hemorrhage (?), stillbirth (?)</entry> <entry v="t">Safe</entry></row><row><entry cs="4">ANTICONVULSANTS</entry></row><row><entry v="t">Barbiturates</entry> <entry v="t">Malformations (?)</entry> <entry v="t">Bleeding, withdrawal</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Carbamazepine, oxcarbazepine</entry> <entry v="t">Craniofacial, neural tube (?)</entry> <entry v="t">Bleeding, withdrawal, growth restriction</entry> <entry v="t">Probably safe</entry></row><row><entry v="t">Clonazepam</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, depression</entry> <entry v="t">Not recommended (potential for apnea, cyanosis, or hypotonia); serum levels should be monitored</entry></row><row><entry v="t">Ethosuximide</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Gabapentin</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Phenytoin*</entry> <entry v="t">Craniofacial abnormalities, mental retardation, hypoplasia of phalanges</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors</entry> <entry v="t">Probably safe</entry></row><row><entry v="t">Primidone</entry> <entry v="t">Orofacial clefts</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors, intrauterine growth restriction</entry> <entry v="t">May produce significant adverse effects in infants; use with caution</entry></row><row><entry v="t">Trimethadione*</entry> <entry v="t">Mental retardation, facial dysmorphogenesis, cardiovascular effects</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors, intrauterine growth restriction</entry> <entry v="t">No information available</entry></row><row><entry v="t">Valproic acid*</entry> <entry v="t">Spina bifida, facial dysmorphogenesis</entry> <entry v="t">Perinatal distress, behavioral abnormalities</entry> <entry v="t">Safe</entry></row><row><entry cs="4">ANTI-EMETICS</entry></row><row><entry v="t">Diphenhydramine</entry> <entry v="t">None known, clefting unlikely</entry> <entry v="t">None known</entry> <entry v="t">Safe, but may cause drowsiness</entry></row><row><entry v="t">Doxylamine (with pyridoxine)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown; probably sedating</entry></row><row><entry v="t">Meclizine</entry> <entry v="t">None known</entry> <entry v="t">Retrolental fibrosis in premature infant</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Metoclopramide</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Potential central nervous system effects</entry></row><row><entry v="t">Ondansetron</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Promethazine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Scopolamine</entry> <entry v="t">None known</entry> <entry v="t">Fetal heart rate changes</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIBACTERIALS</entry></row><row><entry v="t">Aminoglycosides</entry> <entry v="t">None known</entry> <entry v="t">Nephrotoxic (?), ototoxic (?)</entry> <entry v="t">Depends on level of exposure and renal function of infant</entry></row><row><entry v="t">Azithromycin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Cephalosporins</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Probably Compatible</entry></row><row><entry v="t">Chloramphenicol</entry> <entry v="t">None known</entry> <entry v="t">Vascular collapse</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Ciprofloxacin</entry> <entry v="t">Toxic to developing cartilage (?)</entry> <entry v="t">Toxic to developing cartilage (?)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Clindamycin</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Compatible, but potential modification of bowel flora, interference with culture interpretation after fever work-up in infants</entry></row><row><entry v="t">Erythromycin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Isoniazid</entry> <entry v="t">Malformations (?)</entry> <entry v="t">Behavioral abnormality</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Metronidazole</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Use with caution because of mutagenic and carcinogenic effects in some species; abstain from breastfeeding for 12–24 hours after single dose</entry></row><row><entry v="t">Nitrofurantoin</entry> <entry v="t">None known</entry> <entry v="t">Hemolysis (?)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Penicillins</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Rifampin</entry> <entry v="t">Risk of malformation not greater than in general population</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Sulfonamides</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Generally Compatible, but avoid in infants with hyperbilirubinemia, premature infants, and infants with G6PD deficiency</entry></row><row><entry v="t">Tetracyclines</entry> <entry v="t">None known</entry> <entry v="t">Stained deciduous teeth (enamel hypoplasia)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Trimethoprim</entry> <entry v="t">Cleft palate, micrognathia, limb shortening</entry> <entry v="t">Unknown</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIFUNGALS</entry></row><row><entry v="t">Amphotericin-B</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Fluconazole</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIRETROVIRALS</entry></row><row><entry v="t">Class of drugs in general</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Contraindicated (HIV)</entry></row><row><entry cs="4">ANTIVIRALS</entry></row><row><entry v="t">Acyclovir</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Interferon</entry> <entry v="t">None known</entry> <entry v="t">Intrauterine growth restriction (?)</entry> <entry v="t">Likely safe</entry></row><row><entry cs="4">CANCER CHEMOTHERAPY</entry></row><row><entry v="t">Alkylating agents</entry> <entry v="t">Abortion, anomalies</entry> <entry v="t">Hypoplastic gonads, growth restriction and delay</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Antimetabolites <list type="bullet"> <item><para>Folic acid analogues (methotrexate)</para></item> <item><para>Purine analogues</para></item> <item><para>Pyrimidine analogues (cytosine arabinoside, 5-fluorouracil)</para></item> </list></entry> <entry v="t"><list type="bullet"> <item><para>Abortion, intrauterine growth restriction, cranial anomalies</para></item> <item><para>Same as above</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Hypoplastic gonads, growth restriction and delay</para></item> <item><para>Same as above, plus transient anemia</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item></list></entry></row><row><entry v="t">Antibiotics <list type="bullet"> <item><para>Actinomycin</para></item> <item><para>Vinca alkaloids (vincristine)</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Abortion, intrauterine growth restriction, cranial anomalies</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Hypoplastic gonads, growth restriction and delay</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item></list></entry></row><row><entry cs="4">CARDIOVASCULAR DRUGS</entry></row><row><entry v="t">ACE inhibitors</entry> <entry v="t">None known</entry> <entry v="t">Oliguria, skull defects, death</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Adenosine</entry> <entry v="t">None known</entry> <entry v="t">No effects on fetal heart rate</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Beta-sympathomimetics</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, and hyperglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Calcium channel blockers</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Digitalis</entry> <entry v="t">None known</entry> <entry v="t">Lower heart rate</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Hydralazine</entry> <entry v="t">Skeletal defects (?)</entry> <entry v="t">Tachycardia, thrombocytopenia, fetal distress</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Methyldopa</entry> <entry v="t">None known</entry> <entry v="t">Hemolytic anemia, tremor, hypotension</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Propranolol, labetalol</entry> <entry v="t">Unknown</entry> <entry v="t">Lower heart rate, intrauterine growth restriction (?), hypoglycemia, respiratory distress</entry> <entry v="t">Compatible; hypoglycemia (?)</entry></row><row><entry v="t">Reserpine</entry> <entry v="t">None known</entry> <entry v="t">Lethargy, respiratory distress</entry> <entry v="t">Unknown</entry></row><row><entry cs="4">COLD AND COUGH PREPARATIONS</entry></row><row><entry v="t">Antihistamines</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Reduced milk (?); drowsiness</entry></row><row><entry v="t">Cough suppressants</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Decongestants</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Dextromethorphan</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Expectorants</entry> <entry v="t">Fetal goiter (?)</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Loratadine</entry> <entry v="t">Likely none</entry> <entry v="t">Likely none</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">DIURETICS</entry></row><row><entry v="t">Furosemide</entry> <entry v="t">Unknown</entry> <entry v="t">Death from sudden hypoperfusion, electrolyte imbalance</entry> <entry v="t">Found to suppress lactation</entry></row><row><entry v="t">Thiazides</entry> <entry v="t">None known</entry> <entry v="t">Thrombocytopenia, hypokalemia, hyperbilirubinemia, hyponatremia</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">FERTILITY DRUGS</entry></row><row><entry v="t">Clomiphene</entry> <entry v="t">Meiotic nondisjunction (?), neural tube defects (?)</entry> <entry v="t">Unknown</entry> <entry v="t">No data available</entry></row><row><entry cs="4">GASTROINTESTINAL AGENTS</entry></row><row><entry v="t">Bisacodyl</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">No reports of adverse effects</entry></row><row><entry v="t">Cholestyramine</entry> <entry v="t">None known</entry> <entry v="t">None known, but fat-soluble vitamins are depleted</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Colestipol</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown, but minimal absorption</entry> <entry v="t">No data available</entry></row><row><entry v="t">Docusate</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">H2-histamine receptor blockers</entry> <entry v="t">None known</entry> <entry v="t">Anti-androgen effect (cimetidine)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Magnesium hydroxide (Milk of Magnesia)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Mineral oil</entry> <entry v="t">Decreased maternal vitamin absorption</entry> <entry v="t">Decreased maternal vitamin absorption</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Proton pump inhibitors</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sulfasalazine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Caution with ill infants</entry></row><row><entry cs="4">HORMONES</entry></row><row><entry v="t">Androgens*</entry> <entry v="t">Virilization of female fetus</entry> <entry v="t">Adrenal suppression</entry> <entry v="t">No adverse effects reported</entry></row><row><entry v="t">Corticosteroids</entry> <entry v="t">Orofacial cleft in animals, not in humans</entry> <entry v="t">No adverse effects in humans</entry> <entry v="t">No data available</entry></row><row><entry v="t">Danazol</entry> <entry v="t">Virilization of female fetus (?)</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">Estrogens</entry> <entry v="t">Cardiovascular anomalies (?)</entry> <entry v="t">None known</entry> <entry v="t">No reported adverse effects</entry></row><row><entry v="t">Progestins</entry> <entry v="t">Limb and cardiovascular anomalies (?), VACTERL syndrome (?), masculinization of female fetus (?)</entry> <entry v="t">None known</entry> <entry v="t">No reported adverse effects</entry></row><row><entry cs="4">DIABETES CARE</entry></row><row><entry v="t">Glucagon</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Glyburide</entry> <entry v="t">None known</entry> <entry v="t">Not thought to cross the placenta in significant amounts; no neonatal hypoglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Insulin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Safe</entry></row><row><entry v="t">Metformin</entry> <entry v="t">None known</entry> <entry v="t">Neonatal hypoglycemia</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sulfonylureas</entry> <entry v="t">Anomalies (?)</entry> <entry v="t">Suppressed insulin secretion</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">MIGRAINE REMEDIES</entry></row><row><entry v="t">Ergotamine</entry> <entry v="t">None known</entry> <entry v="t">May stimulate contractions</entry> <entry v="t">Use with caution</entry></row><row><entry v="t">Sumatriptan</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">PSYCHOACTIVE DRUGS, ANTIDEPRESSANTS</entry></row><row><entry v="t">Amphetamine</entry> <entry v="t">Inconsistent; likely none</entry> <entry v="t">Reduced weight</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Benzodiazepines</entry> <entry v="t">Facial dysmorphism (?)</entry> <entry v="t">Depression, floppy infant, hypothermia, withdrawal</entry> <entry v="t">Some concern about central nervous system toxicity with long-term use</entry></row><row><entry v="t">Fluoxetine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Symptoms of colic</entry></row><row><entry v="t">Hydroxyzine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Lithium</entry> <entry v="t">Facial clefts; cardiovascular anomaly</entry> <entry v="t">Lithium toxicity (neurologic and hepatic dysfunction)</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Meprobamate</entry> <entry v="t">Cardiac anomalies (?), major malformations (?)</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Phenothiazines</entry> <entry v="t">None known</entry> <entry v="t">Muscle rigidity, hypothermia, tremor</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sedatives</entry> <entry v="t">None known</entry> <entry v="t">Depression, slow learning</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Thalidomide*</entry> <entry v="t">Phocomelia in 20% of cases</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Tricyclics</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown/caution</entry></row><row><entry v="t">Zolpiden</entry> <entry v="t">Unknown</entry> <entry v="t">Withdrawal or floppy infant (?)</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">RADIOLABELED DIAGNOSTICS</entry></row><row><entry v="t">Albumin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">I131 (diagnostic)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Not recommended during exposure; may continue 24 hours after exposure</entry></row><row><entry v="t">Technetium</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Not recommended during exposure; may continue 24 hours after exposure</entry></row><row><entry cs="4">SMOKING CESSATION</entry></row><row><entry v="t">Bupropion</entry> <entry v="t">Likely none</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Nicotine</entry> <entry v="t">Spontaneous abortion (?)</entry> <entry v="t">Impaired growth (?)</entry> <entry v="t">Consistent with passive smoking</entry></row><row><entry cs="4">THYROID MEDICATION</entry></row><row><entry v="t">I131 (therapeutic)</entry> <entry v="t">Goiter, abortion, anomalies</entry> <entry v="t">Goiter, airway obstruction, hyperthyroid, mental retardation</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methimazole</entry> <entry v="t">Aplasia cutis (?), goiter</entry> <entry v="t">Goiter, airway obstruction, hyperthyroid, mental retardation, aplasia cutis (?)</entry> <entry v="t">Compatible, but monitor fetal thyroid function</entry></row><row><entry v="t">Propylthiouracil</entry> <entry v="t">Goiter</entry> <entry v="t">Same as above</entry> <entry v="t">Safe, but monitor baby’s thyroid status</entry></row><row><entry v="t">Thyroid USP</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Thyroxine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">TOCOLYTICS</entry></row><row><entry v="t">Beta-sympathomimetics</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, hyperglycemia</entry> <entry v="t">—</entry></row><row><entry v="t">Indomethacin</entry> <entry v="t">None known</entry> <entry v="t">Oligohydramnios (>48 hours of use)</entry> <entry v="t">—</entry></row><row><entry v="t">Magnesium sulfate</entry> <entry v="t">None known</entry> <entry v="t">Hypermagnesemia, respiratory depression</entry> <entry v="t">—</entry></row><row><entry v="t">Nifedipine</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">—</entry></row><row><entry cs="4">VACCINATIONS</entry></row><row><entry v="t">Influenza</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Pneumovaccine</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Tetanus toxoid</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">VAGINAL PREPARATIONS</entry></row><row><entry v="t">Antifungal agents</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Podophyllin</entry> <entry v="t">Mutagenesis (?)</entry> <entry v="t">Central nervous system effects (?)</entry> <entry v="t">Contraindicated</entry></row><row><entry cs="4">VITAMINS (high dose)</entry></row><row><entry v="t">A</entry> <entry v="t">Urogenital and craniofacial anomalies (?)</entry> <entry v="t">None known</entry> <entry v="t">No data available</entry></row><row><entry v="t">C</entry> <entry v="t">None known</entry> <entry v="t">Scurvy after delivery</entry> <entry v="t">Compatible</entry></row><row><entry v="t">D</entry> <entry v="t">Supravalvular aortic stenosis (?)</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">E</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">K</entry> <entry v="t">Unknown</entry> <entry v="t">Hemorrhage, if deficiency</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">“STREET” DRUGS</entry></row><row><entry v="t">Cocaine</entry> <entry v="t">Placental abruption, vascular disruption, urinary tract anomalies</entry> <entry v="t">Withdrawal, placental abruption, vascular disruption, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Heroin</entry> <entry v="t">None known</entry> <entry v="t">Depression, withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">LSD</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, behavioral effects</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Marijuana</entry> <entry v="t">None known</entry> <entry v="t">Behavioral effects, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methadone</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methamphetamine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Pentazocine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Phencyclidine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, neurobehavioral effects, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry cs="4">OTHER DRUGS</entry></row><row><entry v="t">Azathioprine</entry> <entry v="t">Abortion</entry> <entry v="t">Anemia, thrombocytopenia, lymphopenia, growth retardation</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Bromocriptine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Caffeine</entry> <entry v="t">Anomalies (?) in high doses, abortion (?)</entry> <entry v="t">Jitteriness</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Immune gamma globulin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Isotretinoin*</entry> <entry v="t">Central nervous system, cardiac, facial anomalies</entry> <entry v="t">Stillbirth, mental retardation (?)</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Misoprostol</entry> <entry v="t">Abortion; variety of anomalies (cranium, limb, oral cleft); Mobius sequence</entry> <entry v="t">None with low dose for cervical ripening; placental abruption</entry> <entry v="t">Contraindicated, especially if diarrhea occurs</entry></row><row><entry v="t">Spermicides</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry cs="4">* Proven teratogen.</entry></row><row><entry cs="4">Unknown=no studies to investigate fetal effects; none known=no malformations reported in human studies or no consistent malformations in animal studies; (?)=conflicting information</entry></row><row><entry cs="4">Source: Reprotox data from humans, last reviewed in 2006.</entry></row>
Reference
1. American College of Obstetricians and Gynecologists. Teratology. ACOG Educational Bulletin #236. Washington, DC: ACOG; 1997.
2. Splinter M, Nightingale B, Sawgraves R, Rayburn W. Medication use during pregnancy by women delivering at a tertiary university hospital. South Med J. 1997;90:498-502.
3. Physician’s Desk Reference. 61st ed. Montvale, NJ: Medical Economics; 2007.
4. Briggs GG, Freeman RK, Yaffee FJ. Drugs in Pregnancy and Lactation: Reference Guide to Fetal and Neonatal Risk. 7th ed. Baltimore: Williams & Wilkins; 2005.
5. Briggs GG, Freeman RK, Yaffee FJ. ReproTox Database. Vol. 13. No. 1. Bethesda, Md: Reproductive Toxicology Center; 2000.
6. Heinonen OP, Sloan ED, Shapiro S. Birth Defects and Drugs in Pregnancy. Boston: John Wright PSG; 1973.
7. Reproductive Toxicology Center. Bethesda, Md. Available at http://reprotox.org. Accessed Oct. 5, 2007.
8. PDR for Nonprescription Drugs, Dietary Supplements, and Herbs. 28th ed. Montvale, NJ: Medical Economics; 2007.
<b>How selected drugs affect the human fetus and breastfed infant</b><cs cn="1" a="l"> <cs cn="2" a="l"> <cs cn="3" a="l"> <cs cn="4" a="l"> </cs></cs></cs></cs><row><entry v="t">DRUG</entry> <entry v="t">FIRST-TRIMESTER EFFECTS</entry> <entry v="t">EFFECTS DURING SECOND AND THIRD TRIMESTER</entry> <entry v="t">SAFETY DURING BREASTFEEDING</entry></row><row><entry cs="4">ANALGESICS</entry></row><row><entry v="t">Acetaminophen</entry> <entry v="t">None known</entry> <entry v="t">Hepatotoxicity/nephrotoxicity</entry> <entry v="t">Safe</entry></row><row><entry v="t">Ibuprofen</entry> <entry v="t">Gastroschisis (?)</entry> <entry v="t">Closure of ductus</entry> <entry v="t">Small amount passed; no other information</entry></row><row><entry v="t">Narcotics</entry> <entry v="t">None known</entry> <entry v="t">Depression, withdrawal</entry> <entry v="t">Not recommended if dosing is repetitive</entry></row><row><entry v="t">Salicylates</entry> <entry v="t">None known</entry> <entry v="t">Prolonged pregnancy and labor, hemorrhage, altered hemostasis, intracranial hemorrhage</entry> <entry v="t">Use with caution; may have adverse effects in newborn</entry></row><row><entry cs="4">ANESTHETICS</entry></row><row><entry v="t">General</entry> <entry v="t">Anomalies (?), abortion (?)</entry> <entry v="t">Depression</entry> <entry v="t"> </entry></row><row><entry v="t">Local</entry> <entry v="t">None known</entry> <entry v="t">Bradycardia, seizures</entry> <entry v="t"> </entry></row><row><entry cs="4">ANTI-ASTHMATICS</entry></row><row><entry v="t">Metaproterenol, salmeterol, albuterol</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">Terbutaline</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, and hyperglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Theophylline</entry> <entry v="t">None known</entry> <entry v="t">Jitteriness, tachycardia</entry> <entry v="t">May produce jitteriness, poor feeding, vomiting, cardiac arrhythmias</entry></row><row><entry cs="4">ANTICOAGULANTS</entry></row><row><entry v="t">Warfarin</entry> <entry v="t">Nasal hypoplasia, ophthalmic abnormalities, epiphyseal stippling</entry> <entry v="t">Hemorrhage, stillbirth</entry> <entry v="t">Safe</entry></row><row><entry v="t">Heparin, low molecular weight</entry> <entry v="t">None known</entry> <entry v="t">Hemorrhage (?), stillbirth (?)</entry> <entry v="t">Safe</entry></row><row><entry cs="4">ANTICONVULSANTS</entry></row><row><entry v="t">Barbiturates</entry> <entry v="t">Malformations (?)</entry> <entry v="t">Bleeding, withdrawal</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Carbamazepine, oxcarbazepine</entry> <entry v="t">Craniofacial, neural tube (?)</entry> <entry v="t">Bleeding, withdrawal, growth restriction</entry> <entry v="t">Probably safe</entry></row><row><entry v="t">Clonazepam</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, depression</entry> <entry v="t">Not recommended (potential for apnea, cyanosis, or hypotonia); serum levels should be monitored</entry></row><row><entry v="t">Ethosuximide</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Gabapentin</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Phenytoin*</entry> <entry v="t">Craniofacial abnormalities, mental retardation, hypoplasia of phalanges</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors</entry> <entry v="t">Probably safe</entry></row><row><entry v="t">Primidone</entry> <entry v="t">Orofacial clefts</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors, intrauterine growth restriction</entry> <entry v="t">May produce significant adverse effects in infants; use with caution</entry></row><row><entry v="t">Trimethadione*</entry> <entry v="t">Mental retardation, facial dysmorphogenesis, cardiovascular effects</entry> <entry v="t">Hemorrhage, depletion of vitamin K-dependent clotting factors, intrauterine growth restriction</entry> <entry v="t">No information available</entry></row><row><entry v="t">Valproic acid*</entry> <entry v="t">Spina bifida, facial dysmorphogenesis</entry> <entry v="t">Perinatal distress, behavioral abnormalities</entry> <entry v="t">Safe</entry></row><row><entry cs="4">ANTI-EMETICS</entry></row><row><entry v="t">Diphenhydramine</entry> <entry v="t">None known, clefting unlikely</entry> <entry v="t">None known</entry> <entry v="t">Safe, but may cause drowsiness</entry></row><row><entry v="t">Doxylamine (with pyridoxine)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown; probably sedating</entry></row><row><entry v="t">Meclizine</entry> <entry v="t">None known</entry> <entry v="t">Retrolental fibrosis in premature infant</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Metoclopramide</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Potential central nervous system effects</entry></row><row><entry v="t">Ondansetron</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Promethazine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Scopolamine</entry> <entry v="t">None known</entry> <entry v="t">Fetal heart rate changes</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIBACTERIALS</entry></row><row><entry v="t">Aminoglycosides</entry> <entry v="t">None known</entry> <entry v="t">Nephrotoxic (?), ototoxic (?)</entry> <entry v="t">Depends on level of exposure and renal function of infant</entry></row><row><entry v="t">Azithromycin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Cephalosporins</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Probably Compatible</entry></row><row><entry v="t">Chloramphenicol</entry> <entry v="t">None known</entry> <entry v="t">Vascular collapse</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Ciprofloxacin</entry> <entry v="t">Toxic to developing cartilage (?)</entry> <entry v="t">Toxic to developing cartilage (?)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Clindamycin</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Compatible, but potential modification of bowel flora, interference with culture interpretation after fever work-up in infants</entry></row><row><entry v="t">Erythromycin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Isoniazid</entry> <entry v="t">Malformations (?)</entry> <entry v="t">Behavioral abnormality</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Metronidazole</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Use with caution because of mutagenic and carcinogenic effects in some species; abstain from breastfeeding for 12–24 hours after single dose</entry></row><row><entry v="t">Nitrofurantoin</entry> <entry v="t">None known</entry> <entry v="t">Hemolysis (?)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Penicillins</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Rifampin</entry> <entry v="t">Risk of malformation not greater than in general population</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Sulfonamides</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Generally Compatible, but avoid in infants with hyperbilirubinemia, premature infants, and infants with G6PD deficiency</entry></row><row><entry v="t">Tetracyclines</entry> <entry v="t">None known</entry> <entry v="t">Stained deciduous teeth (enamel hypoplasia)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Trimethoprim</entry> <entry v="t">Cleft palate, micrognathia, limb shortening</entry> <entry v="t">Unknown</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIFUNGALS</entry></row><row><entry v="t">Amphotericin-B</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Fluconazole</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">ANTIRETROVIRALS</entry></row><row><entry v="t">Class of drugs in general</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Contraindicated (HIV)</entry></row><row><entry cs="4">ANTIVIRALS</entry></row><row><entry v="t">Acyclovir</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Interferon</entry> <entry v="t">None known</entry> <entry v="t">Intrauterine growth restriction (?)</entry> <entry v="t">Likely safe</entry></row><row><entry cs="4">CANCER CHEMOTHERAPY</entry></row><row><entry v="t">Alkylating agents</entry> <entry v="t">Abortion, anomalies</entry> <entry v="t">Hypoplastic gonads, growth restriction and delay</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Antimetabolites <list type="bullet"> <item><para>Folic acid analogues (methotrexate)</para></item> <item><para>Purine analogues</para></item> <item><para>Pyrimidine analogues (cytosine arabinoside, 5-fluorouracil)</para></item> </list></entry> <entry v="t"><list type="bullet"> <item><para>Abortion, intrauterine growth restriction, cranial anomalies</para></item> <item><para>Same as above</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Hypoplastic gonads, growth restriction and delay</para></item> <item><para>Same as above, plus transient anemia</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item></list></entry></row><row><entry v="t">Antibiotics <list type="bullet"> <item><para>Actinomycin</para></item> <item><para>Vinca alkaloids (vincristine)</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Abortion, intrauterine growth restriction, cranial anomalies</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Hypoplastic gonads, growth restriction and delay</para></item> <item><para>Same as above</para></item></list></entry> <entry v="t"><list type="bullet"> <item><para>Contraindicated</para></item> <item><para>Contraindicated</para></item></list></entry></row><row><entry cs="4">CARDIOVASCULAR DRUGS</entry></row><row><entry v="t">ACE inhibitors</entry> <entry v="t">None known</entry> <entry v="t">Oliguria, skull defects, death</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Adenosine</entry> <entry v="t">None known</entry> <entry v="t">No effects on fetal heart rate</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Beta-sympathomimetics</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, and hyperglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Calcium channel blockers</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Digitalis</entry> <entry v="t">None known</entry> <entry v="t">Lower heart rate</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Hydralazine</entry> <entry v="t">Skeletal defects (?)</entry> <entry v="t">Tachycardia, thrombocytopenia, fetal distress</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Methyldopa</entry> <entry v="t">None known</entry> <entry v="t">Hemolytic anemia, tremor, hypotension</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Propranolol, labetalol</entry> <entry v="t">Unknown</entry> <entry v="t">Lower heart rate, intrauterine growth restriction (?), hypoglycemia, respiratory distress</entry> <entry v="t">Compatible; hypoglycemia (?)</entry></row><row><entry v="t">Reserpine</entry> <entry v="t">None known</entry> <entry v="t">Lethargy, respiratory distress</entry> <entry v="t">Unknown</entry></row><row><entry cs="4">COLD AND COUGH PREPARATIONS</entry></row><row><entry v="t">Antihistamines</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Reduced milk (?); drowsiness</entry></row><row><entry v="t">Cough suppressants</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Decongestants</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Dextromethorphan</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Expectorants</entry> <entry v="t">Fetal goiter (?)</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Loratadine</entry> <entry v="t">Likely none</entry> <entry v="t">Likely none</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">DIURETICS</entry></row><row><entry v="t">Furosemide</entry> <entry v="t">Unknown</entry> <entry v="t">Death from sudden hypoperfusion, electrolyte imbalance</entry> <entry v="t">Found to suppress lactation</entry></row><row><entry v="t">Thiazides</entry> <entry v="t">None known</entry> <entry v="t">Thrombocytopenia, hypokalemia, hyperbilirubinemia, hyponatremia</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">FERTILITY DRUGS</entry></row><row><entry v="t">Clomiphene</entry> <entry v="t">Meiotic nondisjunction (?), neural tube defects (?)</entry> <entry v="t">Unknown</entry> <entry v="t">No data available</entry></row><row><entry cs="4">GASTROINTESTINAL AGENTS</entry></row><row><entry v="t">Bisacodyl</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown</entry> <entry v="t">No reports of adverse effects</entry></row><row><entry v="t">Cholestyramine</entry> <entry v="t">None known</entry> <entry v="t">None known, but fat-soluble vitamins are depleted</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Colestipol</entry> <entry v="t">Unknown</entry> <entry v="t">Unknown, but minimal absorption</entry> <entry v="t">No data available</entry></row><row><entry v="t">Docusate</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">H2-histamine receptor blockers</entry> <entry v="t">None known</entry> <entry v="t">Anti-androgen effect (cimetidine)</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Magnesium hydroxide (Milk of Magnesia)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Mineral oil</entry> <entry v="t">Decreased maternal vitamin absorption</entry> <entry v="t">Decreased maternal vitamin absorption</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Proton pump inhibitors</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sulfasalazine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Caution with ill infants</entry></row><row><entry cs="4">HORMONES</entry></row><row><entry v="t">Androgens*</entry> <entry v="t">Virilization of female fetus</entry> <entry v="t">Adrenal suppression</entry> <entry v="t">No adverse effects reported</entry></row><row><entry v="t">Corticosteroids</entry> <entry v="t">Orofacial cleft in animals, not in humans</entry> <entry v="t">No adverse effects in humans</entry> <entry v="t">No data available</entry></row><row><entry v="t">Danazol</entry> <entry v="t">Virilization of female fetus (?)</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">Estrogens</entry> <entry v="t">Cardiovascular anomalies (?)</entry> <entry v="t">None known</entry> <entry v="t">No reported adverse effects</entry></row><row><entry v="t">Progestins</entry> <entry v="t">Limb and cardiovascular anomalies (?), VACTERL syndrome (?), masculinization of female fetus (?)</entry> <entry v="t">None known</entry> <entry v="t">No reported adverse effects</entry></row><row><entry cs="4">DIABETES CARE</entry></row><row><entry v="t">Glucagon</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Glyburide</entry> <entry v="t">None known</entry> <entry v="t">Not thought to cross the placenta in significant amounts; no neonatal hypoglycemia</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Insulin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Safe</entry></row><row><entry v="t">Metformin</entry> <entry v="t">None known</entry> <entry v="t">Neonatal hypoglycemia</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sulfonylureas</entry> <entry v="t">Anomalies (?)</entry> <entry v="t">Suppressed insulin secretion</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">MIGRAINE REMEDIES</entry></row><row><entry v="t">Ergotamine</entry> <entry v="t">None known</entry> <entry v="t">May stimulate contractions</entry> <entry v="t">Use with caution</entry></row><row><entry v="t">Sumatriptan</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">PSYCHOACTIVE DRUGS, ANTIDEPRESSANTS</entry></row><row><entry v="t">Amphetamine</entry> <entry v="t">Inconsistent; likely none</entry> <entry v="t">Reduced weight</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Benzodiazepines</entry> <entry v="t">Facial dysmorphism (?)</entry> <entry v="t">Depression, floppy infant, hypothermia, withdrawal</entry> <entry v="t">Some concern about central nervous system toxicity with long-term use</entry></row><row><entry v="t">Fluoxetine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Symptoms of colic</entry></row><row><entry v="t">Hydroxyzine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Lithium</entry> <entry v="t">Facial clefts; cardiovascular anomaly</entry> <entry v="t">Lithium toxicity (neurologic and hepatic dysfunction)</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Meprobamate</entry> <entry v="t">Cardiac anomalies (?), major malformations (?)</entry> <entry v="t">None known</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Phenothiazines</entry> <entry v="t">None known</entry> <entry v="t">Muscle rigidity, hypothermia, tremor</entry> <entry v="t">Unknown</entry></row><row><entry v="t">Sedatives</entry> <entry v="t">None known</entry> <entry v="t">Depression, slow learning</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Thalidomide*</entry> <entry v="t">Phocomelia in 20% of cases</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry v="t">Tricyclics</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Unknown/caution</entry></row><row><entry v="t">Zolpiden</entry> <entry v="t">Unknown</entry> <entry v="t">Withdrawal or floppy infant (?)</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">RADIOLABELED DIAGNOSTICS</entry></row><row><entry v="t">Albumin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information available</entry></row><row><entry v="t">I131 (diagnostic)</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Not recommended during exposure; may continue 24 hours after exposure</entry></row><row><entry v="t">Technetium</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Not recommended during exposure; may continue 24 hours after exposure</entry></row><row><entry cs="4">SMOKING CESSATION</entry></row><row><entry v="t">Bupropion</entry> <entry v="t">Likely none</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Nicotine</entry> <entry v="t">Spontaneous abortion (?)</entry> <entry v="t">Impaired growth (?)</entry> <entry v="t">Consistent with passive smoking</entry></row><row><entry cs="4">THYROID MEDICATION</entry></row><row><entry v="t">I131 (therapeutic)</entry> <entry v="t">Goiter, abortion, anomalies</entry> <entry v="t">Goiter, airway obstruction, hyperthyroid, mental retardation</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methimazole</entry> <entry v="t">Aplasia cutis (?), goiter</entry> <entry v="t">Goiter, airway obstruction, hyperthyroid, mental retardation, aplasia cutis (?)</entry> <entry v="t">Compatible, but monitor fetal thyroid function</entry></row><row><entry v="t">Propylthiouracil</entry> <entry v="t">Goiter</entry> <entry v="t">Same as above</entry> <entry v="t">Safe, but monitor baby’s thyroid status</entry></row><row><entry v="t">Thyroid USP</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Thyroxine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">TOCOLYTICS</entry></row><row><entry v="t">Beta-sympathomimetics</entry> <entry v="t">None known</entry> <entry v="t">Tachycardia, hypothermia, hypocalcemia, hypoglycemia, hyperglycemia</entry> <entry v="t">—</entry></row><row><entry v="t">Indomethacin</entry> <entry v="t">None known</entry> <entry v="t">Oligohydramnios (>48 hours of use)</entry> <entry v="t">—</entry></row><row><entry v="t">Magnesium sulfate</entry> <entry v="t">None known</entry> <entry v="t">Hypermagnesemia, respiratory depression</entry> <entry v="t">—</entry></row><row><entry v="t">Nifedipine</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">—</entry></row><row><entry cs="4">VACCINATIONS</entry></row><row><entry v="t">Influenza</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Pneumovaccine</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Tetanus toxoid</entry> <entry v="t">None known</entry> <entry v="t">Passive immunization</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">VAGINAL PREPARATIONS</entry></row><row><entry v="t">Antifungal agents</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Podophyllin</entry> <entry v="t">Mutagenesis (?)</entry> <entry v="t">Central nervous system effects (?)</entry> <entry v="t">Contraindicated</entry></row><row><entry cs="4">VITAMINS (high dose)</entry></row><row><entry v="t">A</entry> <entry v="t">Urogenital and craniofacial anomalies (?)</entry> <entry v="t">None known</entry> <entry v="t">No data available</entry></row><row><entry v="t">C</entry> <entry v="t">None known</entry> <entry v="t">Scurvy after delivery</entry> <entry v="t">Compatible</entry></row><row><entry v="t">D</entry> <entry v="t">Supravalvular aortic stenosis (?)</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">E</entry> <entry v="t">Unknown</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">K</entry> <entry v="t">Unknown</entry> <entry v="t">Hemorrhage, if deficiency</entry> <entry v="t">Compatible</entry></row><row><entry cs="4">“STREET” DRUGS</entry></row><row><entry v="t">Cocaine</entry> <entry v="t">Placental abruption, vascular disruption, urinary tract anomalies</entry> <entry v="t">Withdrawal, placental abruption, vascular disruption, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Heroin</entry> <entry v="t">None known</entry> <entry v="t">Depression, withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">LSD</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, behavioral effects</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Marijuana</entry> <entry v="t">None known</entry> <entry v="t">Behavioral effects, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methadone</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Methamphetamine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Pentazocine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Phencyclidine</entry> <entry v="t">None known</entry> <entry v="t">Withdrawal, neurobehavioral effects, growth restriction</entry> <entry v="t">Contraindicated</entry></row><row><entry cs="4">OTHER DRUGS</entry></row><row><entry v="t">Azathioprine</entry> <entry v="t">Abortion</entry> <entry v="t">Anemia, thrombocytopenia, lymphopenia, growth retardation</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Bromocriptine</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Caffeine</entry> <entry v="t">Anomalies (?) in high doses, abortion (?)</entry> <entry v="t">Jitteriness</entry> <entry v="t">Not recommended</entry></row><row><entry v="t">Immune gamma globulin</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">Compatible</entry></row><row><entry v="t">Isotretinoin*</entry> <entry v="t">Central nervous system, cardiac, facial anomalies</entry> <entry v="t">Stillbirth, mental retardation (?)</entry> <entry v="t">Contraindicated</entry></row><row><entry v="t">Misoprostol</entry> <entry v="t">Abortion; variety of anomalies (cranium, limb, oral cleft); Mobius sequence</entry> <entry v="t">None with low dose for cervical ripening; placental abruption</entry> <entry v="t">Contraindicated, especially if diarrhea occurs</entry></row><row><entry v="t">Spermicides</entry> <entry v="t">None known</entry> <entry v="t">None known</entry> <entry v="t">No information</entry></row><row><entry cs="4">* Proven teratogen.</entry></row><row><entry cs="4">Unknown=no studies to investigate fetal effects; none known=no malformations reported in human studies or no consistent malformations in animal studies; (?)=conflicting information</entry></row><row><entry cs="4">Source: Reprotox data from humans, last reviewed in 2006.</entry></row>