User login
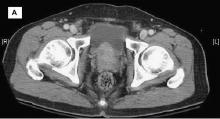
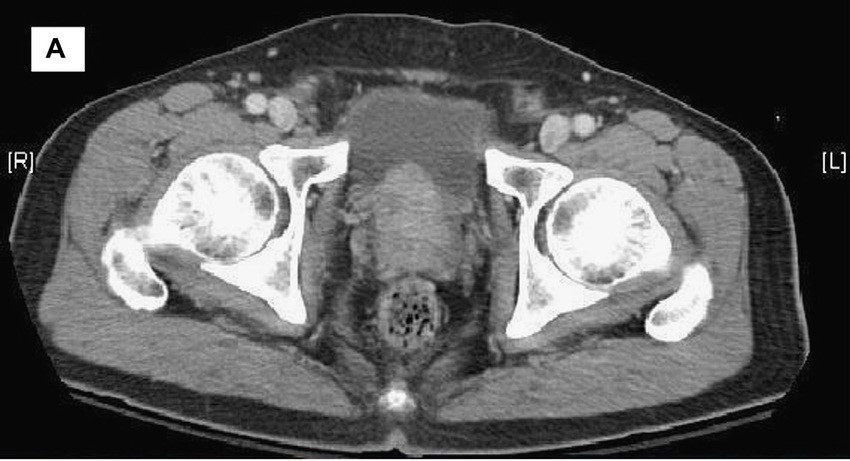
Transrectal ultrasonography (TRUS)–guided needle biopsy of the prostate confirms a diagnosis of high-grade prostate cancer, and the digital rectal exam and CT scan are concerning for extracapsular invasion. Genetic and molecular biomarker testing is recommended.
According to GLOBOCAN 2020 data, prostate cancer is the second most common type of cancer in men (second only to lung cancer) and the fifth leading cause of death globally. Compared with other races, the incidence of prostate cancer in the United States is highest in Black men, and mortality rates are more than double than those reported in White men. In its early stages, prostate cancer is often asymptomatic and has an indolent course. Locally advanced prostate cancer is a clinical scenario in which the cancer has extended beyond the prostatic capsule. It involves invasion of the pericapsular tissue, bladder neck, or seminal vesicles, without lymph node involvement or distant metastases. Biological recurrence, metastatic progression, and poor survival are associated with locally advanced prostate cancer.
In the presence of advanced disease, troublesome lower urinary tract symptoms — particularly abnormal growth of prostate cancer–induced bladder outlet obstruction — are often reported. Such symptoms have a significant impact on patients' quality of life. Other symptoms of locally advanced disease may include hematuria, pain, urinary retention, urinary incontinence, hematospermia, painful ejaculation, anejaculation, constipation, and hematochezia.
Guideline-based approaches to the management of prostate cancer begin with appropriate risk stratification based on biopsy, physical examination, and imaging evaluation. In patients with advanced prostate cancer, treatment decisions should incorporate a multidisciplinary approach and include consideration of life expectancy, comorbidities, patient preferences, and tumor characteristics. Establishing whether the patient has widely advanced disease vs locally advanced disease (clinical stage T3) is helpful for ascertaining which treatment options are available. Pain control and other supportive therapies should be optimized in cases involving advanced prostate cancer.
Androgen deprivation therapy (ADT), combined with luteinizing hormone–releasing hormone (LHRH) agonists or surgical castration, is considered first-line treatment for advanced metastatic prostate cancer. Abundant data show that ADT in advanced symptomatic metastatic prostate cancer, either in the form of surgical castration or LHRH analogues, is beneficial chiefly for palliation of symptoms. However, the combination of ADT with radical prostatectomy or radiation therapy has been shown to improve overall and cancer-specific survival in selected patients with nonmetastatic but locally advanced prostate cancer. Recently, a prospective study showed a significant improvement in urodynamic variables and International Prostate Symptom Score (IPSS) questionnaire results, including IPSS-related quality of life, in patients with advanced cancer who received ADT, although lower urinary tract symptoms persist in some patients.
For patients with metastatic hormone-sensitive prostate cancer (mHSPC), continued treatment with ADT in combination with either androgen pathway–directed therapy (abiraterone acetate plus prednisone, apalutamide, enzalutamide) or chemotherapy (docetaxel) is generally recommended. A recent meta-analysis found that the next-generation androgen receptor inhibitors abiraterone, apalutamide, and enzalutamide appear to be significantly more effective than ADT and more effective than docetaxel for mHSPC; apalutamide was the best tolerated. For selected patients with mHSPC with low-volume metastatic disease, primary radiation therapy to the prostate in combination with ADT may be offered. First-generation antiandrogens (bicalutamide, flutamide, nilutamide) in combination with LHRH agonists are not recommended for patients with mHSPC, unless needed to block testosterone flare. In addition, oral androgen pathway–directed therapy (eg, abiraterone acetate plus prednisone, apalutamide, bicalutamide, darolutamide, enzalutamide, flutamide, nilutamide) without ADT is not recommended for patients with mHSPC.
In patients with nonmetastatic castration-resistant prostate cancer (nmCRPC), darolutamide, apalutamide, and enzalutamide with continued ADT have been shown to postpone the onset of metastases and death. Unless within the context of a clinical trial, systemic chemotherapy or immunotherapy should not be offered to patients with nmCRPC.
For patients with newly diagnosed metastatic castration-resistant prostate cancer (mCRPC), continued ADT with abiraterone acetate plus prednisone, docetaxel, or enzalutamide is recommended. For patients with mCRPC who are asymptomatic or minimally symptomatic, sipuleucel-T may be offered. At present, radium-223 is the only available therapy for mCRPC that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. On the basis of results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for patients with castration-resistant prostate cancer with bone lesions. The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Complete recommendations on sequencing agents and selecting therapies for patients with advanced prostate cancer can be found in guidelines from the American Urological Association, National Comprehensive Cancer Network, and the European Association of Urology.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin
Kyle A. Richards, MD, has disclosed no relevant financial relationships
Transrectal ultrasonography (TRUS)–guided needle biopsy of the prostate confirms a diagnosis of high-grade prostate cancer, and the digital rectal exam and CT scan are concerning for extracapsular invasion. Genetic and molecular biomarker testing is recommended.
According to GLOBOCAN 2020 data, prostate cancer is the second most common type of cancer in men (second only to lung cancer) and the fifth leading cause of death globally. Compared with other races, the incidence of prostate cancer in the United States is highest in Black men, and mortality rates are more than double than those reported in White men. In its early stages, prostate cancer is often asymptomatic and has an indolent course. Locally advanced prostate cancer is a clinical scenario in which the cancer has extended beyond the prostatic capsule. It involves invasion of the pericapsular tissue, bladder neck, or seminal vesicles, without lymph node involvement or distant metastases. Biological recurrence, metastatic progression, and poor survival are associated with locally advanced prostate cancer.
In the presence of advanced disease, troublesome lower urinary tract symptoms — particularly abnormal growth of prostate cancer–induced bladder outlet obstruction — are often reported. Such symptoms have a significant impact on patients' quality of life. Other symptoms of locally advanced disease may include hematuria, pain, urinary retention, urinary incontinence, hematospermia, painful ejaculation, anejaculation, constipation, and hematochezia.
Guideline-based approaches to the management of prostate cancer begin with appropriate risk stratification based on biopsy, physical examination, and imaging evaluation. In patients with advanced prostate cancer, treatment decisions should incorporate a multidisciplinary approach and include consideration of life expectancy, comorbidities, patient preferences, and tumor characteristics. Establishing whether the patient has widely advanced disease vs locally advanced disease (clinical stage T3) is helpful for ascertaining which treatment options are available. Pain control and other supportive therapies should be optimized in cases involving advanced prostate cancer.
Androgen deprivation therapy (ADT), combined with luteinizing hormone–releasing hormone (LHRH) agonists or surgical castration, is considered first-line treatment for advanced metastatic prostate cancer. Abundant data show that ADT in advanced symptomatic metastatic prostate cancer, either in the form of surgical castration or LHRH analogues, is beneficial chiefly for palliation of symptoms. However, the combination of ADT with radical prostatectomy or radiation therapy has been shown to improve overall and cancer-specific survival in selected patients with nonmetastatic but locally advanced prostate cancer. Recently, a prospective study showed a significant improvement in urodynamic variables and International Prostate Symptom Score (IPSS) questionnaire results, including IPSS-related quality of life, in patients with advanced cancer who received ADT, although lower urinary tract symptoms persist in some patients.
For patients with metastatic hormone-sensitive prostate cancer (mHSPC), continued treatment with ADT in combination with either androgen pathway–directed therapy (abiraterone acetate plus prednisone, apalutamide, enzalutamide) or chemotherapy (docetaxel) is generally recommended. A recent meta-analysis found that the next-generation androgen receptor inhibitors abiraterone, apalutamide, and enzalutamide appear to be significantly more effective than ADT and more effective than docetaxel for mHSPC; apalutamide was the best tolerated. For selected patients with mHSPC with low-volume metastatic disease, primary radiation therapy to the prostate in combination with ADT may be offered. First-generation antiandrogens (bicalutamide, flutamide, nilutamide) in combination with LHRH agonists are not recommended for patients with mHSPC, unless needed to block testosterone flare. In addition, oral androgen pathway–directed therapy (eg, abiraterone acetate plus prednisone, apalutamide, bicalutamide, darolutamide, enzalutamide, flutamide, nilutamide) without ADT is not recommended for patients with mHSPC.
In patients with nonmetastatic castration-resistant prostate cancer (nmCRPC), darolutamide, apalutamide, and enzalutamide with continued ADT have been shown to postpone the onset of metastases and death. Unless within the context of a clinical trial, systemic chemotherapy or immunotherapy should not be offered to patients with nmCRPC.
For patients with newly diagnosed metastatic castration-resistant prostate cancer (mCRPC), continued ADT with abiraterone acetate plus prednisone, docetaxel, or enzalutamide is recommended. For patients with mCRPC who are asymptomatic or minimally symptomatic, sipuleucel-T may be offered. At present, radium-223 is the only available therapy for mCRPC that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. On the basis of results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for patients with castration-resistant prostate cancer with bone lesions. The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Complete recommendations on sequencing agents and selecting therapies for patients with advanced prostate cancer can be found in guidelines from the American Urological Association, National Comprehensive Cancer Network, and the European Association of Urology.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin
Kyle A. Richards, MD, has disclosed no relevant financial relationships
Transrectal ultrasonography (TRUS)–guided needle biopsy of the prostate confirms a diagnosis of high-grade prostate cancer, and the digital rectal exam and CT scan are concerning for extracapsular invasion. Genetic and molecular biomarker testing is recommended.
According to GLOBOCAN 2020 data, prostate cancer is the second most common type of cancer in men (second only to lung cancer) and the fifth leading cause of death globally. Compared with other races, the incidence of prostate cancer in the United States is highest in Black men, and mortality rates are more than double than those reported in White men. In its early stages, prostate cancer is often asymptomatic and has an indolent course. Locally advanced prostate cancer is a clinical scenario in which the cancer has extended beyond the prostatic capsule. It involves invasion of the pericapsular tissue, bladder neck, or seminal vesicles, without lymph node involvement or distant metastases. Biological recurrence, metastatic progression, and poor survival are associated with locally advanced prostate cancer.
In the presence of advanced disease, troublesome lower urinary tract symptoms — particularly abnormal growth of prostate cancer–induced bladder outlet obstruction — are often reported. Such symptoms have a significant impact on patients' quality of life. Other symptoms of locally advanced disease may include hematuria, pain, urinary retention, urinary incontinence, hematospermia, painful ejaculation, anejaculation, constipation, and hematochezia.
Guideline-based approaches to the management of prostate cancer begin with appropriate risk stratification based on biopsy, physical examination, and imaging evaluation. In patients with advanced prostate cancer, treatment decisions should incorporate a multidisciplinary approach and include consideration of life expectancy, comorbidities, patient preferences, and tumor characteristics. Establishing whether the patient has widely advanced disease vs locally advanced disease (clinical stage T3) is helpful for ascertaining which treatment options are available. Pain control and other supportive therapies should be optimized in cases involving advanced prostate cancer.
Androgen deprivation therapy (ADT), combined with luteinizing hormone–releasing hormone (LHRH) agonists or surgical castration, is considered first-line treatment for advanced metastatic prostate cancer. Abundant data show that ADT in advanced symptomatic metastatic prostate cancer, either in the form of surgical castration or LHRH analogues, is beneficial chiefly for palliation of symptoms. However, the combination of ADT with radical prostatectomy or radiation therapy has been shown to improve overall and cancer-specific survival in selected patients with nonmetastatic but locally advanced prostate cancer. Recently, a prospective study showed a significant improvement in urodynamic variables and International Prostate Symptom Score (IPSS) questionnaire results, including IPSS-related quality of life, in patients with advanced cancer who received ADT, although lower urinary tract symptoms persist in some patients.
For patients with metastatic hormone-sensitive prostate cancer (mHSPC), continued treatment with ADT in combination with either androgen pathway–directed therapy (abiraterone acetate plus prednisone, apalutamide, enzalutamide) or chemotherapy (docetaxel) is generally recommended. A recent meta-analysis found that the next-generation androgen receptor inhibitors abiraterone, apalutamide, and enzalutamide appear to be significantly more effective than ADT and more effective than docetaxel for mHSPC; apalutamide was the best tolerated. For selected patients with mHSPC with low-volume metastatic disease, primary radiation therapy to the prostate in combination with ADT may be offered. First-generation antiandrogens (bicalutamide, flutamide, nilutamide) in combination with LHRH agonists are not recommended for patients with mHSPC, unless needed to block testosterone flare. In addition, oral androgen pathway–directed therapy (eg, abiraterone acetate plus prednisone, apalutamide, bicalutamide, darolutamide, enzalutamide, flutamide, nilutamide) without ADT is not recommended for patients with mHSPC.
In patients with nonmetastatic castration-resistant prostate cancer (nmCRPC), darolutamide, apalutamide, and enzalutamide with continued ADT have been shown to postpone the onset of metastases and death. Unless within the context of a clinical trial, systemic chemotherapy or immunotherapy should not be offered to patients with nmCRPC.
For patients with newly diagnosed metastatic castration-resistant prostate cancer (mCRPC), continued ADT with abiraterone acetate plus prednisone, docetaxel, or enzalutamide is recommended. For patients with mCRPC who are asymptomatic or minimally symptomatic, sipuleucel-T may be offered. At present, radium-223 is the only available therapy for mCRPC that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. On the basis of results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for patients with castration-resistant prostate cancer with bone lesions. The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Complete recommendations on sequencing agents and selecting therapies for patients with advanced prostate cancer can be found in guidelines from the American Urological Association, National Comprehensive Cancer Network, and the European Association of Urology.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin
Kyle A. Richards, MD, has disclosed no relevant financial relationships
A 58-year-old Black man presents with abdominal pain, urinary frequency and urgency, dysuria, incomplete voiding, and postmicturition dribble. The patient's medical history is unremarkable apart from stage 1 hypertension, for which he receives losartan plus amlodipine. Physical examination findings reveal an overdistended bladder with associated tenderness and a mildly enlarged prostate with a large, firm nodule on digital rectal exam. Urinalysis shows hematuria. Complete blood count and chemistry panel are normal. The total prostate-specific antigen level is 22 ng/mL.