User login
- Approximately 15% to 20% of office gynecologic visits are for the evaluation of abnormal uterine bleeding (AUB), and 25% to 50% of gynecologic surgeries are performed to address menstrual dysfunction.
- Office hysteroscopy and saline infusion sonography are essential skills for the practicing gynecologist. Learn them and use them liberally.
- Inherited and acquired disorders of coagulation, as well as liver and renal diseases, frequently present with symptoms of abnormal uterine bleeding.
- Liberal use of endometrial biopsy is encouraged in women over 35 years of age at risk for endometrial hyperplasia and cancer.
- About 20% to 30% of teens with irregular heavy menses have a major bleeding diathesis.
- Medical therapy is the standard unless uterine pathology is present.
Half of all hysterectomies in the United States are performed to treat abnormal uterine bleeding. Of these, approximately 20% are performed in women with a normal uterine size.1 However, when the uterus appears normal, without adenomyosis or uterine pathology, it is imperative that the clinician perform a thorough evaluation before resorting to hysterectomy.
Abnormal uterine bleeding is defined as excessive, erratic, or irregular bleeding in the presence or absence of intracavitary or uterine pathology. It may be associated with structural or systemic abnormalities. In contrast, dysfunctional uterine bleeding (DUB) is associated with anovulatory menstrual cycles. It is not caused by pelvic pathology, medications, systemic disease, or pregnancy.
Abnormal bleeding is associated with an array of symptoms. Frequent complaints include heavy or prolonged menstrual flow, social embarrassment, diminished quality of life, sexual compromise, and the need to alter lifestyle. Pain is not a common presenting symptom unless it is associated with the passage of large blood clots.
The following menstrual patterns are associated with DUB:
- Oligomenorrhea. A cycle length of more than 35 days
- Polymenorrhea. A cycle length of less than 21 days
- Amenorrhea. The absence of menses for 6 months or 3 consecutive cycles
- Menorrhagia. Heavy or increased flow occurring at regular intervals, or a loss of more than 80 mL of blood
- Metrorrhagia. Irregular episodes of bleeding
- Menometrorrhagia. A longer duration of flow occurring at unpredictable intervals
- Postmenopausal bleeding. Bleeding that occurs more than 12 months after the last menstrual cycle
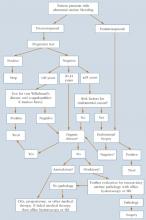
Basic evaluation and management of abnormal uterine bleeding
*If still bleeds, then SIS or hysteroscopy
SIS=saline infusion sonography
Prevalence and pathophysiology
Although we lack precise figures regarding the prevalence of abnormal uterine bleeding, it is estimated that 9% to 30% of reproductive-age women have menstrual irregularities requiring medical evaluation.2 Approximately 15% to 20% of office gynecologic visits are scheduled for the evaluation of abnormal uterine bleeding, which is exceeded only by vaginitis as a chief complaint. In addition, 25% to 50% of gynecologic surgical procedures are performed to address menstrual dysfunction.
Normal menstruation is triggered by fluctuations in the hypothalamic-pituitary-ovarian axis that lead to denudation and sloughing of the endometrium. This hemorrhage is followed by prompt hemostasis and repair. Low physiologic levels of estrogen prime the endometrium, while the normal secretion of progesterone from the corpus luteum stabilizes it, decreasing vascular fragility and supporting the endometrial stroma. Platelets and fibrin are necessary for endometrial hemostasis. Deficiencies in either factor may result in heavier menstruation.
DUB occurs when there is inadequate progesterone secretion to stabilize the endometrium. Anovulatory bleeding can be episodic or continuous. Patients with anovulatory cycles typically do not experience premenstrual tension, breast discomfort, increased mucoid vaginal discharge, or cramping and bloating, all characteristic of ovulatory cycles. Although ovulatory cycles are predictable, erratic bleeding may occur when they coexist with intracavitary lesions, including polyps and fibroids.
Anovulatory cycles typically are associated with puberty and the perimenopausal years. In puberty, the immature hypothalamic-pituitary-ovarian axis has not yet developed the necessary hormonal feedback to sustain the endometrium. In perimenopause, the decline of inhibin and rise in follicle-stimulating hormone (FSH) levels reflect the loss of follicular activity and competence.
In some cases, severe anemia can cause incessant menstrual blood loss. Typical complaints of anemia include fatigue, unusual food cravings (pica), and headaches. Severe anemia also can cause fainting, exercise-induced fatigue, shortness of breath, congestive heart failure, and/or the inability to perform routine activities. Unless it is a chronic condition, DUB is rarely associated with the need for a blood transfusion. Hemorrhagic shock and death are rare sequelae.
Diagnosis
Diagnosis involves 3 main components:
- A detailed medical history and review of systems. This should alert the physician to the possible etiology of a patient’s menstrual dysfunction (Table 1). Inherited and acquired disorders of coagulation, as well as liver and renal diseases, frequently present with symptoms of abnormal uterine bleeding.
- A physical examination. The exam must be comprehensive, even in the presence of heavy bleeding, focusing on the vagina, cervix, uterus, and adnexa to exclude pathology.
- Appropriate laboratory studies based on the focused clinical history and any physical findings. Pregnancy testing is necessary in all sexually active premenopausal women. In addition, women with profuse menorrhagia and a normal uterine size should be screened for von Willebrand’s disease, since 13% to 20% of women offered surgical intervention have the subtle form of Type I disease. (In women, von Willebrand’s disease most commonly presents as DUB.) Obviously, medical therapy is paramount for women with von Willebrand’s disease; hysterectomy or other surgical treatment should not be the first option.
TABLE 1
Causes of menstrual dysfunction
| ANATOMIC |
| Polyps |
| Fibroids |
| Adenomyosis |
| Vaginitis |
| Endometritis |
| Retained products of conception |
| Endometriosis |
| Hyperplasia |
| Malignancy |
| ENDOCRINE |
| Thyroid dysfunction |
| Elevated prolactin levels |
| Adrenal dysfunction |
| Hypothalamic/pituitary dysfunction |
| Estrogen-producing tumors |
| HEMATOLOGIC |
| Anemia |
Coagulopathy
|
| Leukemia |
| SYSTEMIC |
| Renal impairment |
| Liver disorders |
| Obesity |
| Anorexia |
| Chronic illness |
| Rapid fluctuations in weight |
| MEDICATIONS |
| Anticoagulants |
| Steroids |
| Progesterone withdrawal |
| Herbal and soy products |
| MISCELLANEOUS |
| Smoking |
| Depression |
| Excessive alcohol intake |
| Sexually transmitted diseases |
Special populations
Adolescents. Teens with irregular heavy menses should be evaluated for coagulopathies, since 20% to 30% have a major bleeding diathesis.3 This is especially true if the patient presents with a hemoglobin level of less than 10 g/dL or if hospitalization is required. Specifically, adolescents should be evaluated for von Willebrand’s disease with the ristocetin cofactor assay, the single best screening test for the disease. This prevents false-negative results. Other laboratory tests should include:
- Serum human chorionic gonadotropin (hCG)
- Bleeding time
- Partial time (PT) and partial thromboplastin time (PTT)
- Complete blood count (CBC) with platelets
Successful medical therapies for von Willebrand’s disease include oral contraceptives (OCs), which have an 88% success rate; desmopressin acetate; antifibrinolytic agents; and plasma-derived concentrates rich in the high-molecular-weight multimers of von Willebrand factor (vWf).4
Perimenopausal women. Women entering perimenopause may have recurrent bouts of DUB and associated physical complaints due to changes in the hypothalamic-pituitaryovarian axis. The hormonal milieu is associated with decreased inhibin, variable estradiol, normal FSH, and menstrual cycles that can be episodically ovulatory.5 Many menstrual complaints occur in perimenopausal women, including menometrorrhagia, amenorrhea, and oligomenorrheic cycles. Decreased mental clarity and concentration, vaginal dryness, hot flushes, and night sweats are classic symptoms of perimenopause.
Oral contraceptive (OC) therapy is quite useful in these women and should be the first line of intervention, rather than conventional hormone replacement therapy (HRT).6 The usual postmenopausal doses of HRT do not suppress ovulation or prevent pregnancy, while OCs do. In healthy, nonsmoking women over 35 years of age, OCs regulate menstrual cycles, decrease vasomotor symptoms, improve bone mineral density (BMD), and reduce the need for surgical intervention for DUB. They also reduce endometrial and ovarian cancer rates.
Postmenopausal patients. Bleeding that occurs with HRT or tamoxifen use more than 1 year after the cessation of menses requires thorough evaluation. While the most common cause of postmenopausal bleeding is atrophy, it is important to rule out intracavitary pathology, endometrial hyperplasia, and cancer. Approximately 10% of women with postmenopausal bleeding have endometrial cancer. Because the risk of this cancer increases with each decade of life, its exclusion is critical.
Focal intracavitary lesions, including polyps, submucosal fibroids, and endometrial hyperplasia, account for 20% to 40% of cases of abnormal uterine bleeding in this population.7
Organic diseases. Women with renal or liver disease also may have abnormal uterine bleeding. Patients with liver disease may have higher circulating levels of estrogen due to hepatic dysfunction and an inability to metabolize estrogen. Coagulopathies also may occur with liver disease, while renal failure is associated with hypothalamic-pituitaryovarian axis irregularities due to gonadal resistance to hormones, platelet dysfunction, and abnormal factor VIII activity.
Medical therapy
Once the likely cause of abnormal bleeding is identified, appropriate treatment should be instituted. For anovulatory cycles, medical therapy with OCs or progesterone is the standard. Patients with ovulatory abnormal bleeding should be evaluated for intracavitary uterine pathology, since hormonal dysfunction is probably not the cause. Patients whose abnormal bleeding is anatomic in origin usually are managed surgically.
Medical therapy should be tailored to the individual after reviewing her risks, benefits, contraindications, and individual concerns. It is important to determine which facet of the menstrual cycle the patient wants improved, e.g., length, duration, clotting, pain, quantity, in order to target treatment appropriately.
Objective measurements (alkaline hematin assay) of menstrual blood loss are impractical in an office setting.
Oral contraceptives. OCs have many roles in the treatment of menorrhagia and other forms of DUB. Short-term, high-dose therapy is valuable when excessive bleeding occurs in an emergency situation or when heavy menstrual bleeding occurs in adolescent and perimenopausal women. Any low-dose (30 to 35 mcg) ethinyl estradiol product can be given at 6-hour intervals for 5 days to stabilize bleeding. This should be followed by a tapering regimen of 1 low-dose OC pill at 8-hour intervals for 5 days, 12-hour intervals for 5 more days, and then daily for 5 days. This regimen quickly halts heavy menses and controls bleeding. It also prepares the patient for a withdrawal menses.
After the withdrawal bleed, the patient should continue on a maintenance dose (1 pill daily) to ensure regular menstrual cycles and contraception. Low-dose OCs are safe and effective for women over 35 who do not smoke and lack a history of thromboembolic disease.
Progesterone therapy. Women with anovulatory menstrual cycles also may benefit from progesterone therapy, which stabilizes the proliferative endometrium and establishes regular sloughing. Cyclical progesterone is useful in women with contraindications to estrogen therapy, e.g., women over 35 who smoke or have a history of deep venous thrombosis (DVT) or cardiovascular risk factors. Generally, 10 mg of medroxyprogesterone acetate for 10 to 14 days each month will induce a regular withdrawal bleed, although it does not provide contraception.
Long-acting progesterone therapy in the form of medroxyprogesterone (Depo-Provera; Pharmacia Corp, Peapack, NJ) will stop menses in the majority of patients. Standard dosing is 150 mg administered intramuscularly (IM) every 3 months. Approximately 80% to 90% of patients who complete 12 months of Depo-Provera therapy will be amenorrheic. Potential side effects include weight gain, irregular bleeding, and depression.
Danazol. This pituitary suppressant creates a hypoestrogenic state and decreases menstrual blood loss by 70% to 80%. A daily dose of 50 to 100 mg may be adequate in some cases; otherwise, the conventional 400 to 800 mg is recommended. Potential side effects include weight gain, acne, and alteration of lipids.8
GnRH therapy. Gonadotropin-releasing hormone (GnRH) therapy with leuprolide or nafarelin creates a hypoestrogenic menopause-like condition, with menstruation usually ceasing within 3 months. Menopausal symptoms may include hot flushes, night sweats, vaginal dryness, bone loss, decreased concentration, and diminished libido. Nevertheless, compliance generally is good. Because prolonged therapy can lead to osteoporosis, treatment usually is limited to 6 months unless estrogen “addback” therapy is instituted.
GnRH therapy is a valuable option for the late perimenopausal woman who has significant contraindications to other medical regimens. For most of these women, the cessation of menses is a relief. After therapy, many patients spontaneously transition into the menopause. An intermittent 6-month course of leuprolide is an option for women with uterine fibroids. Data indicate that it provides an additional 9 months of symptom control (range: 2 to longer than 25 months).9
Progesterone intrauterine system. The recently introduced levonorgestrel-releasing intrauterine system (IUS) (Mirena, Berlex Laboratories, Montville, NJ) also is effective therapy for DUB. This IUS causes pseudodecidual changes and amenorrhea, decreasing menstrual blood loss by 65% to 98% within 12 months, with little systemic absorption of progesterone. It is likely to prove quite valuable for women with menorrhagia who need contraception, have a normal uterine size, and wish to avoid surgery.10
NSAIDs. Nonsteroidal anti-inflammatory drugs (NSAIDs) decrease the rate of dysmenorrhea, improve clotting, and reduce menstrual blood loss. Some studies have demonstrated a 50% to 80% reduction in blood loss with proper use.11 Patients are advised to begin therapy 1 to 2 days before their period is expected and continue throughout the menses. NSAIDs may be combined with OCs, if necessary.
Most menstrual cycles occur every 21 to 35 days. Normal menstrual flow lasts 3 to 7 days, with most blood lost within the first 3 days. The typical menstrual flow averages 35 mL and consists of effluent debris and blood. Women with normal menstrual cycles use an average of 5 to 6 pads or tampons each day. Social obligations, sexual activity, hobbies, work, and travel are not interrupted with normal menstrual function.
When menorrhagia is present, a woman may lose more than 80 mL of blood with each menstrual cycle. Since approximately 16 mg of iron is lost in normal cycles, women with menorrhagia often develop anemia. They also typically have an imbalance of prostaglandin levels and increased fibrinolytic activity.
It is important to note that more than 50% of women who complain of menorrhagia do not have heavy menses. Some patients change their sanitary products more often not because of heavy flow, but for reasons concerning hygiene, personal preference, or fear of toxic shock syndrome.—Linda D. Bradley, MD
FIGURE 5
When medical therapy fails
When the patient fails to improve after 3 months of medical therapy, additional evaluation such as endometrial biopsy is warranted. For hemodynamically stable patients with normal laboratory evaluation, imaging may be a valuable adjunct. In fact, imaging is increasingly used during the initial workup.12
Biopsy. The endometrium generally is sampled in an office setting using a Pipelle instrument. The biopsy can be performed quickly and generally is well-tolerated by the patient, with few complications. While it has a high sensitivity for detecting endometrial cancer and hyperplasia, it is not as effective in detecting intracavitary lesions, including polyps and submucosal fibroids. Lesions that encompass a small surface area are likely to be missed, as the instrument samples only 10% to 25% of the endometrial cavity. Patients with persistent symptoms despite a normal biopsy require further evaluation.
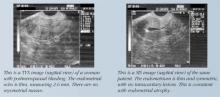
Transvaginal sonography (TVS). This imaging modality is extremely helpful in evaluating women with postmenopausal bleeding. TVS enhances the detection of uterine fibroids and aids in determining their size and position. Adnexal pathology also can be assessed. If the uterine size is greater than 12 to 14 gestational weeks, transabdominal scanning is preferred.
Measurement of the endometrial echo is helpful in determining whether endometrial biopsy or further imaging studies are necessary. Normally, the postmenopausal endometrial echo measures less than 5 mm. Greater thicknesses are associated with endometrial hyperplasia, polyps, fibroids, and cancer. When the endometrial echo exceeds 5 mm or is indistinct or indeterminate, an enhanced view using saline infusion sonography (SIS) or hysteroscopy is advised. When the endometrial echo is less than 5 mm, malignancy is present in fewer than 0.5% of cases.
Weigel et al prospectively evaluated 200 postmenopausal women with an endometrial echo of 3 to 10 mm and found that homogeneity of the echo, a low echo, and a “sonographically depictable central echo between symmetrical endometrial leaves” were associated with an absence of pathology, while heterogeneity and high echogenicity were associated with pathology.13
During the reproductive years, TVS also is useful in assessing myometrial echotexture, adnexal pathology, and, less consistently, endometrial echo. The endometrial thickness varies daily during a normal menstrual cycle. It is thinnest during menses, increases during the follicular phase, and achieves the greatest endometrial height (10 to 14 mm) during the secretory phase. By correlating these measurements with ovarian activity (corpus luteum vs follicular activity), the physician is better able to assess endometrial morphology observed in the midfollicular and secretory phases. However, ancillary testing with SIS is more sensitive for the detection of intracavitary pathology in these women.
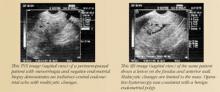
Saline infusion sonography (SIS). With SIS, saline is infused into the endometrial cavity during TVS to enhance the image. Many terms have been used to describe this technique, but I prefer SIS because it defines the technique more precisely.14
SIS allows for more accurate evaluation of the uterus for intracavitary lesions than TVS and makes it easier to differentiate the causes of increased endometrial thickness. Indications for SIS include:
- Abnormal bleeding in premenopausal or postmenopausal patients
- Evaluation of an endometrium that is thickened, irregular, immeasurable, or poorly defined on conventional TVS
- Evaluation of an endometrium that appears irregular on TVS in women on tamoxifen
- Differentiating between sessile and pedunculated masses of the endometrium
- Preoperative evaluation of intracavitary fibroids
Increasingly, gynecologists are embracing the concept of “one-stop” evaluation for menstrual disorders by combining the physical exam and basic laboratory studies (CBC and thyroid-stimulating hormone [TSH]) with TVS unless the clinical history dictates otherwise. However, when TVS is indeterminate, SIS should be performed. Also, all women over 40 who have a suspicious TVS exam should undergo SIS and endometrial biopsy. When such evaluation suggests endometrial polyps or submucosal fibroids, the patient needs operative intervention instead of medical therapy, the patient can be referred directly to a surgeon.
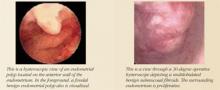
Hysteroscopy. Office hysteroscopy has revolutionized the practice of gynecology. Thin operative hysteroscopes with outer diameters ranging from 3 to 5 mm can be utilized comfortably in an office setting. The procedure permits full visualization of the endometrial cavity and endocervix and facilitates the accurate diagnosis of atrophy, endometrial hyperplasia, polyps, fibroids, and endometrial cancer. Directed endometrial biopsies are possible with some hysteroscopes.
Office hysteroscopy accurately diagnoses many endometrial lesions associated with abnormal bleeding. When the endometrial cavity appears normal at hysteroscopy, aggressive medical therapy should be considered.
Surgical options
Among the surgical treatments for abnormal uterine bleeding are myomectomy, polypectomy, endometrial ablation, and hysterectomy. Dilatation and curettage (D&C), commonly used in the past to treat menstrual aberrations, is no longer preferred. D&C is highly inaccurate, resulting in missed diagnoses, incomplete removal of intracavitary pathology, and a high false-negative rate.15 Operative hysteroscopy with directed endometrial sampling is now the gold standard for surgical evaluation of the uterine cavity. With it, full evaluation is possible even in the presence of heavy bleeding; coexisting intrauterine pathology also can be removed. Because the range of surgical options has broadened in recent years, hysterectomy should be the last resort.
To a large extent, the best surgical modality for a given patient depends on her specific pathology, fertility, and contraceptive needs.
Normal uterine cavity. Endometrial ablation typically is offered after failed medical therapy in women with a normal uterine cavity and negative laboratory workup, provided they have completed childbearing. Hysteroscopic and global endometrial ablation procedures destroy the endometrium, preventing regeneration. This creates Asherman’s syndrome, which leads to hypomenorrhea, eumenorrhea, or amenorrhea.
Endometrial ablation is an outpatient procedure associated with a rapid return to work, minimal complications, and high patient satisfaction. Approximately 20% to 30% of patients undergoing endometrial ablation will become amenorrheic, 65% to 70% will become hypomenorrheic, and 5% to 10% of the procedures will fail. Approximately 30% of patients treated by endometrial ablation will require a subsequent operation.16
If the woman wants to preserve her fertility, I generally order magnetic resonance imaging (MRI) to rule out adenomyosis, since TVS, SIS, and hysteroscopy usually cannot detect it. Adenomyosis can be focal or diffuse and is associated with irregular menses and dysmenorrhea. I also suggest an aggressive trial of medical therapy for at least 3 or 4 cycles, with extensive laboratory evaluation. That is, if the history is suggestive of systemic disease, I order liver function tests. If renal disease is suspected, blood urea nitrogen (BUN) and creatinine levels are helpful, as are luteinizing hormone (LH), FSH, and androgen levels to diagnose PCOS. Adrenal function tests (cortisol, 17-alpha hydroxyprogesterone [17-OHP]) are useful in the diagnosis of hyperandrogensim with suspected adrenal tumors, and congenital adrenal hyperplasia (CAH) is diagnosed by an abnormal 17-OHP level.
Women who smoke often have difficulties with abnormal bleeding. Nicotine is detrimental to the ovaries and is associated with irregular menses and premature ovarian failure. I pressure smokers to stop, as they seem to fail medical therapy more frequently than any other group. I also encourage overweight patients to lose weight. Stress, depression, eating disorders, and excessive exercise also should be addressed.
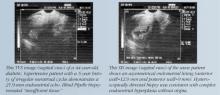
Submucosal fibroids and endometrial polyps. These lesions vary in number, location, and size. When they are present, the altered endometrial surface area, increased fragility and vascularity, other endometrial irregularities, and atypical prostaglandin levels contribute to abnormal bleeding. Intracavitary lesions also may coexist with anovulatory and ovulatory cycles.
As I mentioned earlier, office hysteroscopy and SIS are the most accurate methods of detecting these lesions. Treatment generally consists of outpatient hysteroscopic myomectomy or polypectomy, which is quick, safe, eases symptoms, and is associated with high levels of patient satisfaction.17
Intramural fibroids. Intramural fibroids can cause disturbances in menstrual flow. Although the mechanisms of action are unclear, these disturbances probably result from topographic endometrial abnormalities, glandular atrophy overlying the fibroid, venous congestion, increased endometrial surface area, or an alteration in prostaglandin levels.
The type of therapy offered depends on the patient’s desire for pregnancy or preservation of the uterus. Basically, there are 3 options: removing or destroying the fibroids or removing the uterus. When future fertility is desired, the patient typically undergoes abdominal or laparoscopic myomectomy, with the surgical route contingent upon the number, size, and location of fibroids, as well as the surgeon’s level of skill and experience.
Attempts to resect large intramural fibroids hysteroscopically should be avoided in patients who have not completed childbearing, since scarring and/or the obliteration of a significant portion of the endometrium overlying the fibroid can lead to infertility.
When the patient experiences heavy menstrual bleeding and does not wish to preserve her fertility, she may be offered a minimally invasive outpatient procedure called uterine artery embolization (UAE). In this procedure, a catheter is inserted transcutaneously and threaded through the femoral artery into the aortic bifurcation and then to the contralateral uterine artery, which is then occluded. Several products are used for UAE, including Embosphere Microspheres (BioSphere Medical, Rockland, Mass), polyvinyl alcohol (PVA) particles, coils, or gelfoam, cutting off blood flow to the fibroid. The fibroid then necroses and shrinks in size and volume. Of the many symptoms associated with fibroids, menorrhagia is most effectively controlled with UAE. In fact, patients have an 85% to 95% chance of resolving symptoms of menorrhagia after undergoing this procedure.18
Hysterectomy offers definitive therapy for patients who have completed childbearing and have no desire for uterine preservation. Either vaginal, laparoscopic-assisted, or abdominal hysterectomy is an option. Factors influencing selection of the surgical route include the number, size, and location of the fibroids, as well as any concomitant pelvic pathology and the surgeon’s skill. (See “Complex hysterectomy: opting for the vaginal approach”.)
Conclusion
After the initial history, physical examination, and laboratory evaluation, the factors involved in abnormal uterine bleeding usually are well defined. Medical management is the standard unless uterine pathology is present. Most patients respond favorably to hormonal manipulation with OCs or progesterone. Another effective option, particularly for women who cannot tolerate traditional medical therapy, is the levonorgestrel-releasing IUS.
Endometrial ablation offers 90% success for the treatment of menorrhagia and dysfunctional bleeding in women with a normal uterine cavity and negative laboratory workup, provided they do not desire children.
Patients with intrauterine polyps and submucosal fibroids have excellent relief of symptoms following operative hysteroscopy. Also, uterine artery embolization is an excellent nonsurgical intervention for patients with fibroids and menorrhagia who wish to avoid major surgery. Fortunately, in this era of many alternative medical and surgical treatments, hysterectomy is the last resort for abnormal uterine bleeding.
Dr. Bradley reports that she serves as a speaker and consultant/preceptor for Olympus.
1. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Engl J Med. 1993;328(12):856-860.
2. Coulter A, Bradlow J, Agass M, et al. Outcomes of referrals to gynecology outpatient clinics for menstrual problems: an audit of general practice records. Br J Obstet Gynaecol. 1991;98:789-796.
3. Claessens E, Cowell CA. Acute adolescent menorrhagia. Am J Obstet Gynecol. 1981;139:277-280.
4. American College of Obstetricians and Gynecologists. ACOG Committee Opinion #263. von Willebrand’s disease in gynecologic practice. Obstet Gynecol. 2001;98:1185-1186.
5. Santoro N, Adel T, Skurnick JH. Decreased inhibin tone and increased activin A secretion characterize reproductive aging in women. Fertil Steril. 1999;71:658-662.
6. Kaunitz A. Oral contraceptive use in perimenopause. Am J Obstet Gynecol. 2001;185(2):S32-S37.
7. Jones H. Clinical pathway for evaluating women with abnormal uterine bleeding. Obstet Gynecol Surv. 2002;57(1):22-24.
8. Higham JM, Shaw RW. A comparative study of danazol, a regimen of decreasing doses of danazol and norethindrone in the treatment of objectively proven unexplained menorrhagia. Am J Obstet Gynecol. 1993;169:1134-1139.
9. Scialli A, Levi A. Intermittent leuprolide acetate for the nonsurgical management of women with leiomyomata uteri. Fertil Steril. 2000;74(3):540-546.
10. Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomized trial. Lancet. 2001;357:273-277.
11. Vargyas JM, Campeau JD, Mishell DR. Treatment of menorrhagia with meclofenamate sodium. Am J Obstet Gynecol. 1987;157:944-950.
12. Jones K, Bourne T. The feasibility of a “one-stop” ultrasound-based clinic for the diagnosis and management of abnormal uterine bleeding. Ultrasound Obstet Gynecol. 2001;176:517-521.
13. Weigel M, Friese K, Strittmatter HJ, et al. Measuring the thickness—is that all we have to do for sonographic assessment of endometrium in postmenopausal women? Ultrasound Obstet Gynecol. 1995;6:97-102.
14. Widrich T, Bradley L, Mitchinson AR, Collins R. Comparison of saline infusion sonography with office hysteroscopy for the evaluation of the endometrium. Am J Obstet Gynecol. 1996;174:1327-1334.
15. Bettocchi S, Ceci O, Vicino M, et al. Diagnostic inadequacy of dilation and curettage. Fertil Steril. 2001;75(4):803-805.
16. Stabinsky S, Einstein M, Breen J. Modern treatments of menorrhagia attributable to dysfunctional uterine bleeding. Obstet Gynecol Surv. 1999;54(11):251-262.
17. Pasqualotto EB, Margossian H, Priul L, Bradley LD, et al. Accuracy of preoperative diagnostic tools and outcome of hysteroscopic management of menstrual dysfunction. J Am Assoc Gynecol Laparosc. 2000;7(2):201-209.
18. Hurst B, Stackhouse D, Matthews M, Marshburn P. Uterine artery embolization for symptomatic uterine myomas. Fertil Steril. 2000;74(5):855-869.
- Approximately 15% to 20% of office gynecologic visits are for the evaluation of abnormal uterine bleeding (AUB), and 25% to 50% of gynecologic surgeries are performed to address menstrual dysfunction.
- Office hysteroscopy and saline infusion sonography are essential skills for the practicing gynecologist. Learn them and use them liberally.
- Inherited and acquired disorders of coagulation, as well as liver and renal diseases, frequently present with symptoms of abnormal uterine bleeding.
- Liberal use of endometrial biopsy is encouraged in women over 35 years of age at risk for endometrial hyperplasia and cancer.
- About 20% to 30% of teens with irregular heavy menses have a major bleeding diathesis.
- Medical therapy is the standard unless uterine pathology is present.
Half of all hysterectomies in the United States are performed to treat abnormal uterine bleeding. Of these, approximately 20% are performed in women with a normal uterine size.1 However, when the uterus appears normal, without adenomyosis or uterine pathology, it is imperative that the clinician perform a thorough evaluation before resorting to hysterectomy.
Abnormal uterine bleeding is defined as excessive, erratic, or irregular bleeding in the presence or absence of intracavitary or uterine pathology. It may be associated with structural or systemic abnormalities. In contrast, dysfunctional uterine bleeding (DUB) is associated with anovulatory menstrual cycles. It is not caused by pelvic pathology, medications, systemic disease, or pregnancy.
Abnormal bleeding is associated with an array of symptoms. Frequent complaints include heavy or prolonged menstrual flow, social embarrassment, diminished quality of life, sexual compromise, and the need to alter lifestyle. Pain is not a common presenting symptom unless it is associated with the passage of large blood clots.
The following menstrual patterns are associated with DUB:
- Oligomenorrhea. A cycle length of more than 35 days
- Polymenorrhea. A cycle length of less than 21 days
- Amenorrhea. The absence of menses for 6 months or 3 consecutive cycles
- Menorrhagia. Heavy or increased flow occurring at regular intervals, or a loss of more than 80 mL of blood
- Metrorrhagia. Irregular episodes of bleeding
- Menometrorrhagia. A longer duration of flow occurring at unpredictable intervals
- Postmenopausal bleeding. Bleeding that occurs more than 12 months after the last menstrual cycle
Basic evaluation and management of abnormal uterine bleeding
*If still bleeds, then SIS or hysteroscopy
SIS=saline infusion sonography
Prevalence and pathophysiology
Although we lack precise figures regarding the prevalence of abnormal uterine bleeding, it is estimated that 9% to 30% of reproductive-age women have menstrual irregularities requiring medical evaluation.2 Approximately 15% to 20% of office gynecologic visits are scheduled for the evaluation of abnormal uterine bleeding, which is exceeded only by vaginitis as a chief complaint. In addition, 25% to 50% of gynecologic surgical procedures are performed to address menstrual dysfunction.
Normal menstruation is triggered by fluctuations in the hypothalamic-pituitary-ovarian axis that lead to denudation and sloughing of the endometrium. This hemorrhage is followed by prompt hemostasis and repair. Low physiologic levels of estrogen prime the endometrium, while the normal secretion of progesterone from the corpus luteum stabilizes it, decreasing vascular fragility and supporting the endometrial stroma. Platelets and fibrin are necessary for endometrial hemostasis. Deficiencies in either factor may result in heavier menstruation.
DUB occurs when there is inadequate progesterone secretion to stabilize the endometrium. Anovulatory bleeding can be episodic or continuous. Patients with anovulatory cycles typically do not experience premenstrual tension, breast discomfort, increased mucoid vaginal discharge, or cramping and bloating, all characteristic of ovulatory cycles. Although ovulatory cycles are predictable, erratic bleeding may occur when they coexist with intracavitary lesions, including polyps and fibroids.
Anovulatory cycles typically are associated with puberty and the perimenopausal years. In puberty, the immature hypothalamic-pituitary-ovarian axis has not yet developed the necessary hormonal feedback to sustain the endometrium. In perimenopause, the decline of inhibin and rise in follicle-stimulating hormone (FSH) levels reflect the loss of follicular activity and competence.
In some cases, severe anemia can cause incessant menstrual blood loss. Typical complaints of anemia include fatigue, unusual food cravings (pica), and headaches. Severe anemia also can cause fainting, exercise-induced fatigue, shortness of breath, congestive heart failure, and/or the inability to perform routine activities. Unless it is a chronic condition, DUB is rarely associated with the need for a blood transfusion. Hemorrhagic shock and death are rare sequelae.
Diagnosis
Diagnosis involves 3 main components:
- A detailed medical history and review of systems. This should alert the physician to the possible etiology of a patient’s menstrual dysfunction (Table 1). Inherited and acquired disorders of coagulation, as well as liver and renal diseases, frequently present with symptoms of abnormal uterine bleeding.
- A physical examination. The exam must be comprehensive, even in the presence of heavy bleeding, focusing on the vagina, cervix, uterus, and adnexa to exclude pathology.
- Appropriate laboratory studies based on the focused clinical history and any physical findings. Pregnancy testing is necessary in all sexually active premenopausal women. In addition, women with profuse menorrhagia and a normal uterine size should be screened for von Willebrand’s disease, since 13% to 20% of women offered surgical intervention have the subtle form of Type I disease. (In women, von Willebrand’s disease most commonly presents as DUB.) Obviously, medical therapy is paramount for women with von Willebrand’s disease; hysterectomy or other surgical treatment should not be the first option.
TABLE 1
Causes of menstrual dysfunction
| ANATOMIC |
| Polyps |
| Fibroids |
| Adenomyosis |
| Vaginitis |
| Endometritis |
| Retained products of conception |
| Endometriosis |
| Hyperplasia |
| Malignancy |
| ENDOCRINE |
| Thyroid dysfunction |
| Elevated prolactin levels |
| Adrenal dysfunction |
| Hypothalamic/pituitary dysfunction |
| Estrogen-producing tumors |
| HEMATOLOGIC |
| Anemia |
Coagulopathy
|
| Leukemia |
| SYSTEMIC |
| Renal impairment |
| Liver disorders |
| Obesity |
| Anorexia |
| Chronic illness |
| Rapid fluctuations in weight |
| MEDICATIONS |
| Anticoagulants |
| Steroids |
| Progesterone withdrawal |
| Herbal and soy products |
| MISCELLANEOUS |
| Smoking |
| Depression |
| Excessive alcohol intake |
| Sexually transmitted diseases |
Special populations
Adolescents. Teens with irregular heavy menses should be evaluated for coagulopathies, since 20% to 30% have a major bleeding diathesis.3 This is especially true if the patient presents with a hemoglobin level of less than 10 g/dL or if hospitalization is required. Specifically, adolescents should be evaluated for von Willebrand’s disease with the ristocetin cofactor assay, the single best screening test for the disease. This prevents false-negative results. Other laboratory tests should include:
- Serum human chorionic gonadotropin (hCG)
- Bleeding time
- Partial time (PT) and partial thromboplastin time (PTT)
- Complete blood count (CBC) with platelets
Successful medical therapies for von Willebrand’s disease include oral contraceptives (OCs), which have an 88% success rate; desmopressin acetate; antifibrinolytic agents; and plasma-derived concentrates rich in the high-molecular-weight multimers of von Willebrand factor (vWf).4
Perimenopausal women. Women entering perimenopause may have recurrent bouts of DUB and associated physical complaints due to changes in the hypothalamic-pituitaryovarian axis. The hormonal milieu is associated with decreased inhibin, variable estradiol, normal FSH, and menstrual cycles that can be episodically ovulatory.5 Many menstrual complaints occur in perimenopausal women, including menometrorrhagia, amenorrhea, and oligomenorrheic cycles. Decreased mental clarity and concentration, vaginal dryness, hot flushes, and night sweats are classic symptoms of perimenopause.
Oral contraceptive (OC) therapy is quite useful in these women and should be the first line of intervention, rather than conventional hormone replacement therapy (HRT).6 The usual postmenopausal doses of HRT do not suppress ovulation or prevent pregnancy, while OCs do. In healthy, nonsmoking women over 35 years of age, OCs regulate menstrual cycles, decrease vasomotor symptoms, improve bone mineral density (BMD), and reduce the need for surgical intervention for DUB. They also reduce endometrial and ovarian cancer rates.
Postmenopausal patients. Bleeding that occurs with HRT or tamoxifen use more than 1 year after the cessation of menses requires thorough evaluation. While the most common cause of postmenopausal bleeding is atrophy, it is important to rule out intracavitary pathology, endometrial hyperplasia, and cancer. Approximately 10% of women with postmenopausal bleeding have endometrial cancer. Because the risk of this cancer increases with each decade of life, its exclusion is critical.
Focal intracavitary lesions, including polyps, submucosal fibroids, and endometrial hyperplasia, account for 20% to 40% of cases of abnormal uterine bleeding in this population.7
Organic diseases. Women with renal or liver disease also may have abnormal uterine bleeding. Patients with liver disease may have higher circulating levels of estrogen due to hepatic dysfunction and an inability to metabolize estrogen. Coagulopathies also may occur with liver disease, while renal failure is associated with hypothalamic-pituitaryovarian axis irregularities due to gonadal resistance to hormones, platelet dysfunction, and abnormal factor VIII activity.
Medical therapy
Once the likely cause of abnormal bleeding is identified, appropriate treatment should be instituted. For anovulatory cycles, medical therapy with OCs or progesterone is the standard. Patients with ovulatory abnormal bleeding should be evaluated for intracavitary uterine pathology, since hormonal dysfunction is probably not the cause. Patients whose abnormal bleeding is anatomic in origin usually are managed surgically.
Medical therapy should be tailored to the individual after reviewing her risks, benefits, contraindications, and individual concerns. It is important to determine which facet of the menstrual cycle the patient wants improved, e.g., length, duration, clotting, pain, quantity, in order to target treatment appropriately.
Objective measurements (alkaline hematin assay) of menstrual blood loss are impractical in an office setting.
Oral contraceptives. OCs have many roles in the treatment of menorrhagia and other forms of DUB. Short-term, high-dose therapy is valuable when excessive bleeding occurs in an emergency situation or when heavy menstrual bleeding occurs in adolescent and perimenopausal women. Any low-dose (30 to 35 mcg) ethinyl estradiol product can be given at 6-hour intervals for 5 days to stabilize bleeding. This should be followed by a tapering regimen of 1 low-dose OC pill at 8-hour intervals for 5 days, 12-hour intervals for 5 more days, and then daily for 5 days. This regimen quickly halts heavy menses and controls bleeding. It also prepares the patient for a withdrawal menses.
After the withdrawal bleed, the patient should continue on a maintenance dose (1 pill daily) to ensure regular menstrual cycles and contraception. Low-dose OCs are safe and effective for women over 35 who do not smoke and lack a history of thromboembolic disease.
Progesterone therapy. Women with anovulatory menstrual cycles also may benefit from progesterone therapy, which stabilizes the proliferative endometrium and establishes regular sloughing. Cyclical progesterone is useful in women with contraindications to estrogen therapy, e.g., women over 35 who smoke or have a history of deep venous thrombosis (DVT) or cardiovascular risk factors. Generally, 10 mg of medroxyprogesterone acetate for 10 to 14 days each month will induce a regular withdrawal bleed, although it does not provide contraception.
Long-acting progesterone therapy in the form of medroxyprogesterone (Depo-Provera; Pharmacia Corp, Peapack, NJ) will stop menses in the majority of patients. Standard dosing is 150 mg administered intramuscularly (IM) every 3 months. Approximately 80% to 90% of patients who complete 12 months of Depo-Provera therapy will be amenorrheic. Potential side effects include weight gain, irregular bleeding, and depression.
Danazol. This pituitary suppressant creates a hypoestrogenic state and decreases menstrual blood loss by 70% to 80%. A daily dose of 50 to 100 mg may be adequate in some cases; otherwise, the conventional 400 to 800 mg is recommended. Potential side effects include weight gain, acne, and alteration of lipids.8
GnRH therapy. Gonadotropin-releasing hormone (GnRH) therapy with leuprolide or nafarelin creates a hypoestrogenic menopause-like condition, with menstruation usually ceasing within 3 months. Menopausal symptoms may include hot flushes, night sweats, vaginal dryness, bone loss, decreased concentration, and diminished libido. Nevertheless, compliance generally is good. Because prolonged therapy can lead to osteoporosis, treatment usually is limited to 6 months unless estrogen “addback” therapy is instituted.
GnRH therapy is a valuable option for the late perimenopausal woman who has significant contraindications to other medical regimens. For most of these women, the cessation of menses is a relief. After therapy, many patients spontaneously transition into the menopause. An intermittent 6-month course of leuprolide is an option for women with uterine fibroids. Data indicate that it provides an additional 9 months of symptom control (range: 2 to longer than 25 months).9
Progesterone intrauterine system. The recently introduced levonorgestrel-releasing intrauterine system (IUS) (Mirena, Berlex Laboratories, Montville, NJ) also is effective therapy for DUB. This IUS causes pseudodecidual changes and amenorrhea, decreasing menstrual blood loss by 65% to 98% within 12 months, with little systemic absorption of progesterone. It is likely to prove quite valuable for women with menorrhagia who need contraception, have a normal uterine size, and wish to avoid surgery.10
NSAIDs. Nonsteroidal anti-inflammatory drugs (NSAIDs) decrease the rate of dysmenorrhea, improve clotting, and reduce menstrual blood loss. Some studies have demonstrated a 50% to 80% reduction in blood loss with proper use.11 Patients are advised to begin therapy 1 to 2 days before their period is expected and continue throughout the menses. NSAIDs may be combined with OCs, if necessary.
Most menstrual cycles occur every 21 to 35 days. Normal menstrual flow lasts 3 to 7 days, with most blood lost within the first 3 days. The typical menstrual flow averages 35 mL and consists of effluent debris and blood. Women with normal menstrual cycles use an average of 5 to 6 pads or tampons each day. Social obligations, sexual activity, hobbies, work, and travel are not interrupted with normal menstrual function.
When menorrhagia is present, a woman may lose more than 80 mL of blood with each menstrual cycle. Since approximately 16 mg of iron is lost in normal cycles, women with menorrhagia often develop anemia. They also typically have an imbalance of prostaglandin levels and increased fibrinolytic activity.
It is important to note that more than 50% of women who complain of menorrhagia do not have heavy menses. Some patients change their sanitary products more often not because of heavy flow, but for reasons concerning hygiene, personal preference, or fear of toxic shock syndrome.—Linda D. Bradley, MD
FIGURE 5
When medical therapy fails
When the patient fails to improve after 3 months of medical therapy, additional evaluation such as endometrial biopsy is warranted. For hemodynamically stable patients with normal laboratory evaluation, imaging may be a valuable adjunct. In fact, imaging is increasingly used during the initial workup.12
Biopsy. The endometrium generally is sampled in an office setting using a Pipelle instrument. The biopsy can be performed quickly and generally is well-tolerated by the patient, with few complications. While it has a high sensitivity for detecting endometrial cancer and hyperplasia, it is not as effective in detecting intracavitary lesions, including polyps and submucosal fibroids. Lesions that encompass a small surface area are likely to be missed, as the instrument samples only 10% to 25% of the endometrial cavity. Patients with persistent symptoms despite a normal biopsy require further evaluation.
Transvaginal sonography (TVS). This imaging modality is extremely helpful in evaluating women with postmenopausal bleeding. TVS enhances the detection of uterine fibroids and aids in determining their size and position. Adnexal pathology also can be assessed. If the uterine size is greater than 12 to 14 gestational weeks, transabdominal scanning is preferred.
Measurement of the endometrial echo is helpful in determining whether endometrial biopsy or further imaging studies are necessary. Normally, the postmenopausal endometrial echo measures less than 5 mm. Greater thicknesses are associated with endometrial hyperplasia, polyps, fibroids, and cancer. When the endometrial echo exceeds 5 mm or is indistinct or indeterminate, an enhanced view using saline infusion sonography (SIS) or hysteroscopy is advised. When the endometrial echo is less than 5 mm, malignancy is present in fewer than 0.5% of cases.
Weigel et al prospectively evaluated 200 postmenopausal women with an endometrial echo of 3 to 10 mm and found that homogeneity of the echo, a low echo, and a “sonographically depictable central echo between symmetrical endometrial leaves” were associated with an absence of pathology, while heterogeneity and high echogenicity were associated with pathology.13
During the reproductive years, TVS also is useful in assessing myometrial echotexture, adnexal pathology, and, less consistently, endometrial echo. The endometrial thickness varies daily during a normal menstrual cycle. It is thinnest during menses, increases during the follicular phase, and achieves the greatest endometrial height (10 to 14 mm) during the secretory phase. By correlating these measurements with ovarian activity (corpus luteum vs follicular activity), the physician is better able to assess endometrial morphology observed in the midfollicular and secretory phases. However, ancillary testing with SIS is more sensitive for the detection of intracavitary pathology in these women.
Saline infusion sonography (SIS). With SIS, saline is infused into the endometrial cavity during TVS to enhance the image. Many terms have been used to describe this technique, but I prefer SIS because it defines the technique more precisely.14
SIS allows for more accurate evaluation of the uterus for intracavitary lesions than TVS and makes it easier to differentiate the causes of increased endometrial thickness. Indications for SIS include:
- Abnormal bleeding in premenopausal or postmenopausal patients
- Evaluation of an endometrium that is thickened, irregular, immeasurable, or poorly defined on conventional TVS
- Evaluation of an endometrium that appears irregular on TVS in women on tamoxifen
- Differentiating between sessile and pedunculated masses of the endometrium
- Preoperative evaluation of intracavitary fibroids
Increasingly, gynecologists are embracing the concept of “one-stop” evaluation for menstrual disorders by combining the physical exam and basic laboratory studies (CBC and thyroid-stimulating hormone [TSH]) with TVS unless the clinical history dictates otherwise. However, when TVS is indeterminate, SIS should be performed. Also, all women over 40 who have a suspicious TVS exam should undergo SIS and endometrial biopsy. When such evaluation suggests endometrial polyps or submucosal fibroids, the patient needs operative intervention instead of medical therapy, the patient can be referred directly to a surgeon.
Hysteroscopy. Office hysteroscopy has revolutionized the practice of gynecology. Thin operative hysteroscopes with outer diameters ranging from 3 to 5 mm can be utilized comfortably in an office setting. The procedure permits full visualization of the endometrial cavity and endocervix and facilitates the accurate diagnosis of atrophy, endometrial hyperplasia, polyps, fibroids, and endometrial cancer. Directed endometrial biopsies are possible with some hysteroscopes.
Office hysteroscopy accurately diagnoses many endometrial lesions associated with abnormal bleeding. When the endometrial cavity appears normal at hysteroscopy, aggressive medical therapy should be considered.
Surgical options
Among the surgical treatments for abnormal uterine bleeding are myomectomy, polypectomy, endometrial ablation, and hysterectomy. Dilatation and curettage (D&C), commonly used in the past to treat menstrual aberrations, is no longer preferred. D&C is highly inaccurate, resulting in missed diagnoses, incomplete removal of intracavitary pathology, and a high false-negative rate.15 Operative hysteroscopy with directed endometrial sampling is now the gold standard for surgical evaluation of the uterine cavity. With it, full evaluation is possible even in the presence of heavy bleeding; coexisting intrauterine pathology also can be removed. Because the range of surgical options has broadened in recent years, hysterectomy should be the last resort.
To a large extent, the best surgical modality for a given patient depends on her specific pathology, fertility, and contraceptive needs.
Normal uterine cavity. Endometrial ablation typically is offered after failed medical therapy in women with a normal uterine cavity and negative laboratory workup, provided they have completed childbearing. Hysteroscopic and global endometrial ablation procedures destroy the endometrium, preventing regeneration. This creates Asherman’s syndrome, which leads to hypomenorrhea, eumenorrhea, or amenorrhea.
Endometrial ablation is an outpatient procedure associated with a rapid return to work, minimal complications, and high patient satisfaction. Approximately 20% to 30% of patients undergoing endometrial ablation will become amenorrheic, 65% to 70% will become hypomenorrheic, and 5% to 10% of the procedures will fail. Approximately 30% of patients treated by endometrial ablation will require a subsequent operation.16
If the woman wants to preserve her fertility, I generally order magnetic resonance imaging (MRI) to rule out adenomyosis, since TVS, SIS, and hysteroscopy usually cannot detect it. Adenomyosis can be focal or diffuse and is associated with irregular menses and dysmenorrhea. I also suggest an aggressive trial of medical therapy for at least 3 or 4 cycles, with extensive laboratory evaluation. That is, if the history is suggestive of systemic disease, I order liver function tests. If renal disease is suspected, blood urea nitrogen (BUN) and creatinine levels are helpful, as are luteinizing hormone (LH), FSH, and androgen levels to diagnose PCOS. Adrenal function tests (cortisol, 17-alpha hydroxyprogesterone [17-OHP]) are useful in the diagnosis of hyperandrogensim with suspected adrenal tumors, and congenital adrenal hyperplasia (CAH) is diagnosed by an abnormal 17-OHP level.
Women who smoke often have difficulties with abnormal bleeding. Nicotine is detrimental to the ovaries and is associated with irregular menses and premature ovarian failure. I pressure smokers to stop, as they seem to fail medical therapy more frequently than any other group. I also encourage overweight patients to lose weight. Stress, depression, eating disorders, and excessive exercise also should be addressed.
Submucosal fibroids and endometrial polyps. These lesions vary in number, location, and size. When they are present, the altered endometrial surface area, increased fragility and vascularity, other endometrial irregularities, and atypical prostaglandin levels contribute to abnormal bleeding. Intracavitary lesions also may coexist with anovulatory and ovulatory cycles.
As I mentioned earlier, office hysteroscopy and SIS are the most accurate methods of detecting these lesions. Treatment generally consists of outpatient hysteroscopic myomectomy or polypectomy, which is quick, safe, eases symptoms, and is associated with high levels of patient satisfaction.17
Intramural fibroids. Intramural fibroids can cause disturbances in menstrual flow. Although the mechanisms of action are unclear, these disturbances probably result from topographic endometrial abnormalities, glandular atrophy overlying the fibroid, venous congestion, increased endometrial surface area, or an alteration in prostaglandin levels.
The type of therapy offered depends on the patient’s desire for pregnancy or preservation of the uterus. Basically, there are 3 options: removing or destroying the fibroids or removing the uterus. When future fertility is desired, the patient typically undergoes abdominal or laparoscopic myomectomy, with the surgical route contingent upon the number, size, and location of fibroids, as well as the surgeon’s level of skill and experience.
Attempts to resect large intramural fibroids hysteroscopically should be avoided in patients who have not completed childbearing, since scarring and/or the obliteration of a significant portion of the endometrium overlying the fibroid can lead to infertility.
When the patient experiences heavy menstrual bleeding and does not wish to preserve her fertility, she may be offered a minimally invasive outpatient procedure called uterine artery embolization (UAE). In this procedure, a catheter is inserted transcutaneously and threaded through the femoral artery into the aortic bifurcation and then to the contralateral uterine artery, which is then occluded. Several products are used for UAE, including Embosphere Microspheres (BioSphere Medical, Rockland, Mass), polyvinyl alcohol (PVA) particles, coils, or gelfoam, cutting off blood flow to the fibroid. The fibroid then necroses and shrinks in size and volume. Of the many symptoms associated with fibroids, menorrhagia is most effectively controlled with UAE. In fact, patients have an 85% to 95% chance of resolving symptoms of menorrhagia after undergoing this procedure.18
Hysterectomy offers definitive therapy for patients who have completed childbearing and have no desire for uterine preservation. Either vaginal, laparoscopic-assisted, or abdominal hysterectomy is an option. Factors influencing selection of the surgical route include the number, size, and location of the fibroids, as well as any concomitant pelvic pathology and the surgeon’s skill. (See “Complex hysterectomy: opting for the vaginal approach”.)
Conclusion
After the initial history, physical examination, and laboratory evaluation, the factors involved in abnormal uterine bleeding usually are well defined. Medical management is the standard unless uterine pathology is present. Most patients respond favorably to hormonal manipulation with OCs or progesterone. Another effective option, particularly for women who cannot tolerate traditional medical therapy, is the levonorgestrel-releasing IUS.
Endometrial ablation offers 90% success for the treatment of menorrhagia and dysfunctional bleeding in women with a normal uterine cavity and negative laboratory workup, provided they do not desire children.
Patients with intrauterine polyps and submucosal fibroids have excellent relief of symptoms following operative hysteroscopy. Also, uterine artery embolization is an excellent nonsurgical intervention for patients with fibroids and menorrhagia who wish to avoid major surgery. Fortunately, in this era of many alternative medical and surgical treatments, hysterectomy is the last resort for abnormal uterine bleeding.
Dr. Bradley reports that she serves as a speaker and consultant/preceptor for Olympus.
- Approximately 15% to 20% of office gynecologic visits are for the evaluation of abnormal uterine bleeding (AUB), and 25% to 50% of gynecologic surgeries are performed to address menstrual dysfunction.
- Office hysteroscopy and saline infusion sonography are essential skills for the practicing gynecologist. Learn them and use them liberally.
- Inherited and acquired disorders of coagulation, as well as liver and renal diseases, frequently present with symptoms of abnormal uterine bleeding.
- Liberal use of endometrial biopsy is encouraged in women over 35 years of age at risk for endometrial hyperplasia and cancer.
- About 20% to 30% of teens with irregular heavy menses have a major bleeding diathesis.
- Medical therapy is the standard unless uterine pathology is present.
Half of all hysterectomies in the United States are performed to treat abnormal uterine bleeding. Of these, approximately 20% are performed in women with a normal uterine size.1 However, when the uterus appears normal, without adenomyosis or uterine pathology, it is imperative that the clinician perform a thorough evaluation before resorting to hysterectomy.
Abnormal uterine bleeding is defined as excessive, erratic, or irregular bleeding in the presence or absence of intracavitary or uterine pathology. It may be associated with structural or systemic abnormalities. In contrast, dysfunctional uterine bleeding (DUB) is associated with anovulatory menstrual cycles. It is not caused by pelvic pathology, medications, systemic disease, or pregnancy.
Abnormal bleeding is associated with an array of symptoms. Frequent complaints include heavy or prolonged menstrual flow, social embarrassment, diminished quality of life, sexual compromise, and the need to alter lifestyle. Pain is not a common presenting symptom unless it is associated with the passage of large blood clots.
The following menstrual patterns are associated with DUB:
- Oligomenorrhea. A cycle length of more than 35 days
- Polymenorrhea. A cycle length of less than 21 days
- Amenorrhea. The absence of menses for 6 months or 3 consecutive cycles
- Menorrhagia. Heavy or increased flow occurring at regular intervals, or a loss of more than 80 mL of blood
- Metrorrhagia. Irregular episodes of bleeding
- Menometrorrhagia. A longer duration of flow occurring at unpredictable intervals
- Postmenopausal bleeding. Bleeding that occurs more than 12 months after the last menstrual cycle
Basic evaluation and management of abnormal uterine bleeding
*If still bleeds, then SIS or hysteroscopy
SIS=saline infusion sonography
Prevalence and pathophysiology
Although we lack precise figures regarding the prevalence of abnormal uterine bleeding, it is estimated that 9% to 30% of reproductive-age women have menstrual irregularities requiring medical evaluation.2 Approximately 15% to 20% of office gynecologic visits are scheduled for the evaluation of abnormal uterine bleeding, which is exceeded only by vaginitis as a chief complaint. In addition, 25% to 50% of gynecologic surgical procedures are performed to address menstrual dysfunction.
Normal menstruation is triggered by fluctuations in the hypothalamic-pituitary-ovarian axis that lead to denudation and sloughing of the endometrium. This hemorrhage is followed by prompt hemostasis and repair. Low physiologic levels of estrogen prime the endometrium, while the normal secretion of progesterone from the corpus luteum stabilizes it, decreasing vascular fragility and supporting the endometrial stroma. Platelets and fibrin are necessary for endometrial hemostasis. Deficiencies in either factor may result in heavier menstruation.
DUB occurs when there is inadequate progesterone secretion to stabilize the endometrium. Anovulatory bleeding can be episodic or continuous. Patients with anovulatory cycles typically do not experience premenstrual tension, breast discomfort, increased mucoid vaginal discharge, or cramping and bloating, all characteristic of ovulatory cycles. Although ovulatory cycles are predictable, erratic bleeding may occur when they coexist with intracavitary lesions, including polyps and fibroids.
Anovulatory cycles typically are associated with puberty and the perimenopausal years. In puberty, the immature hypothalamic-pituitary-ovarian axis has not yet developed the necessary hormonal feedback to sustain the endometrium. In perimenopause, the decline of inhibin and rise in follicle-stimulating hormone (FSH) levels reflect the loss of follicular activity and competence.
In some cases, severe anemia can cause incessant menstrual blood loss. Typical complaints of anemia include fatigue, unusual food cravings (pica), and headaches. Severe anemia also can cause fainting, exercise-induced fatigue, shortness of breath, congestive heart failure, and/or the inability to perform routine activities. Unless it is a chronic condition, DUB is rarely associated with the need for a blood transfusion. Hemorrhagic shock and death are rare sequelae.
Diagnosis
Diagnosis involves 3 main components:
- A detailed medical history and review of systems. This should alert the physician to the possible etiology of a patient’s menstrual dysfunction (Table 1). Inherited and acquired disorders of coagulation, as well as liver and renal diseases, frequently present with symptoms of abnormal uterine bleeding.
- A physical examination. The exam must be comprehensive, even in the presence of heavy bleeding, focusing on the vagina, cervix, uterus, and adnexa to exclude pathology.
- Appropriate laboratory studies based on the focused clinical history and any physical findings. Pregnancy testing is necessary in all sexually active premenopausal women. In addition, women with profuse menorrhagia and a normal uterine size should be screened for von Willebrand’s disease, since 13% to 20% of women offered surgical intervention have the subtle form of Type I disease. (In women, von Willebrand’s disease most commonly presents as DUB.) Obviously, medical therapy is paramount for women with von Willebrand’s disease; hysterectomy or other surgical treatment should not be the first option.
TABLE 1
Causes of menstrual dysfunction
| ANATOMIC |
| Polyps |
| Fibroids |
| Adenomyosis |
| Vaginitis |
| Endometritis |
| Retained products of conception |
| Endometriosis |
| Hyperplasia |
| Malignancy |
| ENDOCRINE |
| Thyroid dysfunction |
| Elevated prolactin levels |
| Adrenal dysfunction |
| Hypothalamic/pituitary dysfunction |
| Estrogen-producing tumors |
| HEMATOLOGIC |
| Anemia |
Coagulopathy
|
| Leukemia |
| SYSTEMIC |
| Renal impairment |
| Liver disorders |
| Obesity |
| Anorexia |
| Chronic illness |
| Rapid fluctuations in weight |
| MEDICATIONS |
| Anticoagulants |
| Steroids |
| Progesterone withdrawal |
| Herbal and soy products |
| MISCELLANEOUS |
| Smoking |
| Depression |
| Excessive alcohol intake |
| Sexually transmitted diseases |
Special populations
Adolescents. Teens with irregular heavy menses should be evaluated for coagulopathies, since 20% to 30% have a major bleeding diathesis.3 This is especially true if the patient presents with a hemoglobin level of less than 10 g/dL or if hospitalization is required. Specifically, adolescents should be evaluated for von Willebrand’s disease with the ristocetin cofactor assay, the single best screening test for the disease. This prevents false-negative results. Other laboratory tests should include:
- Serum human chorionic gonadotropin (hCG)
- Bleeding time
- Partial time (PT) and partial thromboplastin time (PTT)
- Complete blood count (CBC) with platelets
Successful medical therapies for von Willebrand’s disease include oral contraceptives (OCs), which have an 88% success rate; desmopressin acetate; antifibrinolytic agents; and plasma-derived concentrates rich in the high-molecular-weight multimers of von Willebrand factor (vWf).4
Perimenopausal women. Women entering perimenopause may have recurrent bouts of DUB and associated physical complaints due to changes in the hypothalamic-pituitaryovarian axis. The hormonal milieu is associated with decreased inhibin, variable estradiol, normal FSH, and menstrual cycles that can be episodically ovulatory.5 Many menstrual complaints occur in perimenopausal women, including menometrorrhagia, amenorrhea, and oligomenorrheic cycles. Decreased mental clarity and concentration, vaginal dryness, hot flushes, and night sweats are classic symptoms of perimenopause.
Oral contraceptive (OC) therapy is quite useful in these women and should be the first line of intervention, rather than conventional hormone replacement therapy (HRT).6 The usual postmenopausal doses of HRT do not suppress ovulation or prevent pregnancy, while OCs do. In healthy, nonsmoking women over 35 years of age, OCs regulate menstrual cycles, decrease vasomotor symptoms, improve bone mineral density (BMD), and reduce the need for surgical intervention for DUB. They also reduce endometrial and ovarian cancer rates.
Postmenopausal patients. Bleeding that occurs with HRT or tamoxifen use more than 1 year after the cessation of menses requires thorough evaluation. While the most common cause of postmenopausal bleeding is atrophy, it is important to rule out intracavitary pathology, endometrial hyperplasia, and cancer. Approximately 10% of women with postmenopausal bleeding have endometrial cancer. Because the risk of this cancer increases with each decade of life, its exclusion is critical.
Focal intracavitary lesions, including polyps, submucosal fibroids, and endometrial hyperplasia, account for 20% to 40% of cases of abnormal uterine bleeding in this population.7
Organic diseases. Women with renal or liver disease also may have abnormal uterine bleeding. Patients with liver disease may have higher circulating levels of estrogen due to hepatic dysfunction and an inability to metabolize estrogen. Coagulopathies also may occur with liver disease, while renal failure is associated with hypothalamic-pituitaryovarian axis irregularities due to gonadal resistance to hormones, platelet dysfunction, and abnormal factor VIII activity.
Medical therapy
Once the likely cause of abnormal bleeding is identified, appropriate treatment should be instituted. For anovulatory cycles, medical therapy with OCs or progesterone is the standard. Patients with ovulatory abnormal bleeding should be evaluated for intracavitary uterine pathology, since hormonal dysfunction is probably not the cause. Patients whose abnormal bleeding is anatomic in origin usually are managed surgically.
Medical therapy should be tailored to the individual after reviewing her risks, benefits, contraindications, and individual concerns. It is important to determine which facet of the menstrual cycle the patient wants improved, e.g., length, duration, clotting, pain, quantity, in order to target treatment appropriately.
Objective measurements (alkaline hematin assay) of menstrual blood loss are impractical in an office setting.
Oral contraceptives. OCs have many roles in the treatment of menorrhagia and other forms of DUB. Short-term, high-dose therapy is valuable when excessive bleeding occurs in an emergency situation or when heavy menstrual bleeding occurs in adolescent and perimenopausal women. Any low-dose (30 to 35 mcg) ethinyl estradiol product can be given at 6-hour intervals for 5 days to stabilize bleeding. This should be followed by a tapering regimen of 1 low-dose OC pill at 8-hour intervals for 5 days, 12-hour intervals for 5 more days, and then daily for 5 days. This regimen quickly halts heavy menses and controls bleeding. It also prepares the patient for a withdrawal menses.
After the withdrawal bleed, the patient should continue on a maintenance dose (1 pill daily) to ensure regular menstrual cycles and contraception. Low-dose OCs are safe and effective for women over 35 who do not smoke and lack a history of thromboembolic disease.
Progesterone therapy. Women with anovulatory menstrual cycles also may benefit from progesterone therapy, which stabilizes the proliferative endometrium and establishes regular sloughing. Cyclical progesterone is useful in women with contraindications to estrogen therapy, e.g., women over 35 who smoke or have a history of deep venous thrombosis (DVT) or cardiovascular risk factors. Generally, 10 mg of medroxyprogesterone acetate for 10 to 14 days each month will induce a regular withdrawal bleed, although it does not provide contraception.
Long-acting progesterone therapy in the form of medroxyprogesterone (Depo-Provera; Pharmacia Corp, Peapack, NJ) will stop menses in the majority of patients. Standard dosing is 150 mg administered intramuscularly (IM) every 3 months. Approximately 80% to 90% of patients who complete 12 months of Depo-Provera therapy will be amenorrheic. Potential side effects include weight gain, irregular bleeding, and depression.
Danazol. This pituitary suppressant creates a hypoestrogenic state and decreases menstrual blood loss by 70% to 80%. A daily dose of 50 to 100 mg may be adequate in some cases; otherwise, the conventional 400 to 800 mg is recommended. Potential side effects include weight gain, acne, and alteration of lipids.8
GnRH therapy. Gonadotropin-releasing hormone (GnRH) therapy with leuprolide or nafarelin creates a hypoestrogenic menopause-like condition, with menstruation usually ceasing within 3 months. Menopausal symptoms may include hot flushes, night sweats, vaginal dryness, bone loss, decreased concentration, and diminished libido. Nevertheless, compliance generally is good. Because prolonged therapy can lead to osteoporosis, treatment usually is limited to 6 months unless estrogen “addback” therapy is instituted.
GnRH therapy is a valuable option for the late perimenopausal woman who has significant contraindications to other medical regimens. For most of these women, the cessation of menses is a relief. After therapy, many patients spontaneously transition into the menopause. An intermittent 6-month course of leuprolide is an option for women with uterine fibroids. Data indicate that it provides an additional 9 months of symptom control (range: 2 to longer than 25 months).9
Progesterone intrauterine system. The recently introduced levonorgestrel-releasing intrauterine system (IUS) (Mirena, Berlex Laboratories, Montville, NJ) also is effective therapy for DUB. This IUS causes pseudodecidual changes and amenorrhea, decreasing menstrual blood loss by 65% to 98% within 12 months, with little systemic absorption of progesterone. It is likely to prove quite valuable for women with menorrhagia who need contraception, have a normal uterine size, and wish to avoid surgery.10
NSAIDs. Nonsteroidal anti-inflammatory drugs (NSAIDs) decrease the rate of dysmenorrhea, improve clotting, and reduce menstrual blood loss. Some studies have demonstrated a 50% to 80% reduction in blood loss with proper use.11 Patients are advised to begin therapy 1 to 2 days before their period is expected and continue throughout the menses. NSAIDs may be combined with OCs, if necessary.
Most menstrual cycles occur every 21 to 35 days. Normal menstrual flow lasts 3 to 7 days, with most blood lost within the first 3 days. The typical menstrual flow averages 35 mL and consists of effluent debris and blood. Women with normal menstrual cycles use an average of 5 to 6 pads or tampons each day. Social obligations, sexual activity, hobbies, work, and travel are not interrupted with normal menstrual function.
When menorrhagia is present, a woman may lose more than 80 mL of blood with each menstrual cycle. Since approximately 16 mg of iron is lost in normal cycles, women with menorrhagia often develop anemia. They also typically have an imbalance of prostaglandin levels and increased fibrinolytic activity.
It is important to note that more than 50% of women who complain of menorrhagia do not have heavy menses. Some patients change their sanitary products more often not because of heavy flow, but for reasons concerning hygiene, personal preference, or fear of toxic shock syndrome.—Linda D. Bradley, MD
FIGURE 5
When medical therapy fails
When the patient fails to improve after 3 months of medical therapy, additional evaluation such as endometrial biopsy is warranted. For hemodynamically stable patients with normal laboratory evaluation, imaging may be a valuable adjunct. In fact, imaging is increasingly used during the initial workup.12
Biopsy. The endometrium generally is sampled in an office setting using a Pipelle instrument. The biopsy can be performed quickly and generally is well-tolerated by the patient, with few complications. While it has a high sensitivity for detecting endometrial cancer and hyperplasia, it is not as effective in detecting intracavitary lesions, including polyps and submucosal fibroids. Lesions that encompass a small surface area are likely to be missed, as the instrument samples only 10% to 25% of the endometrial cavity. Patients with persistent symptoms despite a normal biopsy require further evaluation.
Transvaginal sonography (TVS). This imaging modality is extremely helpful in evaluating women with postmenopausal bleeding. TVS enhances the detection of uterine fibroids and aids in determining their size and position. Adnexal pathology also can be assessed. If the uterine size is greater than 12 to 14 gestational weeks, transabdominal scanning is preferred.
Measurement of the endometrial echo is helpful in determining whether endometrial biopsy or further imaging studies are necessary. Normally, the postmenopausal endometrial echo measures less than 5 mm. Greater thicknesses are associated with endometrial hyperplasia, polyps, fibroids, and cancer. When the endometrial echo exceeds 5 mm or is indistinct or indeterminate, an enhanced view using saline infusion sonography (SIS) or hysteroscopy is advised. When the endometrial echo is less than 5 mm, malignancy is present in fewer than 0.5% of cases.
Weigel et al prospectively evaluated 200 postmenopausal women with an endometrial echo of 3 to 10 mm and found that homogeneity of the echo, a low echo, and a “sonographically depictable central echo between symmetrical endometrial leaves” were associated with an absence of pathology, while heterogeneity and high echogenicity were associated with pathology.13
During the reproductive years, TVS also is useful in assessing myometrial echotexture, adnexal pathology, and, less consistently, endometrial echo. The endometrial thickness varies daily during a normal menstrual cycle. It is thinnest during menses, increases during the follicular phase, and achieves the greatest endometrial height (10 to 14 mm) during the secretory phase. By correlating these measurements with ovarian activity (corpus luteum vs follicular activity), the physician is better able to assess endometrial morphology observed in the midfollicular and secretory phases. However, ancillary testing with SIS is more sensitive for the detection of intracavitary pathology in these women.
Saline infusion sonography (SIS). With SIS, saline is infused into the endometrial cavity during TVS to enhance the image. Many terms have been used to describe this technique, but I prefer SIS because it defines the technique more precisely.14
SIS allows for more accurate evaluation of the uterus for intracavitary lesions than TVS and makes it easier to differentiate the causes of increased endometrial thickness. Indications for SIS include:
- Abnormal bleeding in premenopausal or postmenopausal patients
- Evaluation of an endometrium that is thickened, irregular, immeasurable, or poorly defined on conventional TVS
- Evaluation of an endometrium that appears irregular on TVS in women on tamoxifen
- Differentiating between sessile and pedunculated masses of the endometrium
- Preoperative evaluation of intracavitary fibroids
Increasingly, gynecologists are embracing the concept of “one-stop” evaluation for menstrual disorders by combining the physical exam and basic laboratory studies (CBC and thyroid-stimulating hormone [TSH]) with TVS unless the clinical history dictates otherwise. However, when TVS is indeterminate, SIS should be performed. Also, all women over 40 who have a suspicious TVS exam should undergo SIS and endometrial biopsy. When such evaluation suggests endometrial polyps or submucosal fibroids, the patient needs operative intervention instead of medical therapy, the patient can be referred directly to a surgeon.
Hysteroscopy. Office hysteroscopy has revolutionized the practice of gynecology. Thin operative hysteroscopes with outer diameters ranging from 3 to 5 mm can be utilized comfortably in an office setting. The procedure permits full visualization of the endometrial cavity and endocervix and facilitates the accurate diagnosis of atrophy, endometrial hyperplasia, polyps, fibroids, and endometrial cancer. Directed endometrial biopsies are possible with some hysteroscopes.
Office hysteroscopy accurately diagnoses many endometrial lesions associated with abnormal bleeding. When the endometrial cavity appears normal at hysteroscopy, aggressive medical therapy should be considered.
Surgical options
Among the surgical treatments for abnormal uterine bleeding are myomectomy, polypectomy, endometrial ablation, and hysterectomy. Dilatation and curettage (D&C), commonly used in the past to treat menstrual aberrations, is no longer preferred. D&C is highly inaccurate, resulting in missed diagnoses, incomplete removal of intracavitary pathology, and a high false-negative rate.15 Operative hysteroscopy with directed endometrial sampling is now the gold standard for surgical evaluation of the uterine cavity. With it, full evaluation is possible even in the presence of heavy bleeding; coexisting intrauterine pathology also can be removed. Because the range of surgical options has broadened in recent years, hysterectomy should be the last resort.
To a large extent, the best surgical modality for a given patient depends on her specific pathology, fertility, and contraceptive needs.
Normal uterine cavity. Endometrial ablation typically is offered after failed medical therapy in women with a normal uterine cavity and negative laboratory workup, provided they have completed childbearing. Hysteroscopic and global endometrial ablation procedures destroy the endometrium, preventing regeneration. This creates Asherman’s syndrome, which leads to hypomenorrhea, eumenorrhea, or amenorrhea.
Endometrial ablation is an outpatient procedure associated with a rapid return to work, minimal complications, and high patient satisfaction. Approximately 20% to 30% of patients undergoing endometrial ablation will become amenorrheic, 65% to 70% will become hypomenorrheic, and 5% to 10% of the procedures will fail. Approximately 30% of patients treated by endometrial ablation will require a subsequent operation.16
If the woman wants to preserve her fertility, I generally order magnetic resonance imaging (MRI) to rule out adenomyosis, since TVS, SIS, and hysteroscopy usually cannot detect it. Adenomyosis can be focal or diffuse and is associated with irregular menses and dysmenorrhea. I also suggest an aggressive trial of medical therapy for at least 3 or 4 cycles, with extensive laboratory evaluation. That is, if the history is suggestive of systemic disease, I order liver function tests. If renal disease is suspected, blood urea nitrogen (BUN) and creatinine levels are helpful, as are luteinizing hormone (LH), FSH, and androgen levels to diagnose PCOS. Adrenal function tests (cortisol, 17-alpha hydroxyprogesterone [17-OHP]) are useful in the diagnosis of hyperandrogensim with suspected adrenal tumors, and congenital adrenal hyperplasia (CAH) is diagnosed by an abnormal 17-OHP level.
Women who smoke often have difficulties with abnormal bleeding. Nicotine is detrimental to the ovaries and is associated with irregular menses and premature ovarian failure. I pressure smokers to stop, as they seem to fail medical therapy more frequently than any other group. I also encourage overweight patients to lose weight. Stress, depression, eating disorders, and excessive exercise also should be addressed.
Submucosal fibroids and endometrial polyps. These lesions vary in number, location, and size. When they are present, the altered endometrial surface area, increased fragility and vascularity, other endometrial irregularities, and atypical prostaglandin levels contribute to abnormal bleeding. Intracavitary lesions also may coexist with anovulatory and ovulatory cycles.
As I mentioned earlier, office hysteroscopy and SIS are the most accurate methods of detecting these lesions. Treatment generally consists of outpatient hysteroscopic myomectomy or polypectomy, which is quick, safe, eases symptoms, and is associated with high levels of patient satisfaction.17
Intramural fibroids. Intramural fibroids can cause disturbances in menstrual flow. Although the mechanisms of action are unclear, these disturbances probably result from topographic endometrial abnormalities, glandular atrophy overlying the fibroid, venous congestion, increased endometrial surface area, or an alteration in prostaglandin levels.
The type of therapy offered depends on the patient’s desire for pregnancy or preservation of the uterus. Basically, there are 3 options: removing or destroying the fibroids or removing the uterus. When future fertility is desired, the patient typically undergoes abdominal or laparoscopic myomectomy, with the surgical route contingent upon the number, size, and location of fibroids, as well as the surgeon’s level of skill and experience.
Attempts to resect large intramural fibroids hysteroscopically should be avoided in patients who have not completed childbearing, since scarring and/or the obliteration of a significant portion of the endometrium overlying the fibroid can lead to infertility.
When the patient experiences heavy menstrual bleeding and does not wish to preserve her fertility, she may be offered a minimally invasive outpatient procedure called uterine artery embolization (UAE). In this procedure, a catheter is inserted transcutaneously and threaded through the femoral artery into the aortic bifurcation and then to the contralateral uterine artery, which is then occluded. Several products are used for UAE, including Embosphere Microspheres (BioSphere Medical, Rockland, Mass), polyvinyl alcohol (PVA) particles, coils, or gelfoam, cutting off blood flow to the fibroid. The fibroid then necroses and shrinks in size and volume. Of the many symptoms associated with fibroids, menorrhagia is most effectively controlled with UAE. In fact, patients have an 85% to 95% chance of resolving symptoms of menorrhagia after undergoing this procedure.18
Hysterectomy offers definitive therapy for patients who have completed childbearing and have no desire for uterine preservation. Either vaginal, laparoscopic-assisted, or abdominal hysterectomy is an option. Factors influencing selection of the surgical route include the number, size, and location of the fibroids, as well as any concomitant pelvic pathology and the surgeon’s skill. (See “Complex hysterectomy: opting for the vaginal approach”.)
Conclusion
After the initial history, physical examination, and laboratory evaluation, the factors involved in abnormal uterine bleeding usually are well defined. Medical management is the standard unless uterine pathology is present. Most patients respond favorably to hormonal manipulation with OCs or progesterone. Another effective option, particularly for women who cannot tolerate traditional medical therapy, is the levonorgestrel-releasing IUS.
Endometrial ablation offers 90% success for the treatment of menorrhagia and dysfunctional bleeding in women with a normal uterine cavity and negative laboratory workup, provided they do not desire children.
Patients with intrauterine polyps and submucosal fibroids have excellent relief of symptoms following operative hysteroscopy. Also, uterine artery embolization is an excellent nonsurgical intervention for patients with fibroids and menorrhagia who wish to avoid major surgery. Fortunately, in this era of many alternative medical and surgical treatments, hysterectomy is the last resort for abnormal uterine bleeding.
Dr. Bradley reports that she serves as a speaker and consultant/preceptor for Olympus.
1. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Engl J Med. 1993;328(12):856-860.
2. Coulter A, Bradlow J, Agass M, et al. Outcomes of referrals to gynecology outpatient clinics for menstrual problems: an audit of general practice records. Br J Obstet Gynaecol. 1991;98:789-796.
3. Claessens E, Cowell CA. Acute adolescent menorrhagia. Am J Obstet Gynecol. 1981;139:277-280.
4. American College of Obstetricians and Gynecologists. ACOG Committee Opinion #263. von Willebrand’s disease in gynecologic practice. Obstet Gynecol. 2001;98:1185-1186.
5. Santoro N, Adel T, Skurnick JH. Decreased inhibin tone and increased activin A secretion characterize reproductive aging in women. Fertil Steril. 1999;71:658-662.
6. Kaunitz A. Oral contraceptive use in perimenopause. Am J Obstet Gynecol. 2001;185(2):S32-S37.
7. Jones H. Clinical pathway for evaluating women with abnormal uterine bleeding. Obstet Gynecol Surv. 2002;57(1):22-24.
8. Higham JM, Shaw RW. A comparative study of danazol, a regimen of decreasing doses of danazol and norethindrone in the treatment of objectively proven unexplained menorrhagia. Am J Obstet Gynecol. 1993;169:1134-1139.
9. Scialli A, Levi A. Intermittent leuprolide acetate for the nonsurgical management of women with leiomyomata uteri. Fertil Steril. 2000;74(3):540-546.
10. Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomized trial. Lancet. 2001;357:273-277.
11. Vargyas JM, Campeau JD, Mishell DR. Treatment of menorrhagia with meclofenamate sodium. Am J Obstet Gynecol. 1987;157:944-950.
12. Jones K, Bourne T. The feasibility of a “one-stop” ultrasound-based clinic for the diagnosis and management of abnormal uterine bleeding. Ultrasound Obstet Gynecol. 2001;176:517-521.
13. Weigel M, Friese K, Strittmatter HJ, et al. Measuring the thickness—is that all we have to do for sonographic assessment of endometrium in postmenopausal women? Ultrasound Obstet Gynecol. 1995;6:97-102.
14. Widrich T, Bradley L, Mitchinson AR, Collins R. Comparison of saline infusion sonography with office hysteroscopy for the evaluation of the endometrium. Am J Obstet Gynecol. 1996;174:1327-1334.
15. Bettocchi S, Ceci O, Vicino M, et al. Diagnostic inadequacy of dilation and curettage. Fertil Steril. 2001;75(4):803-805.
16. Stabinsky S, Einstein M, Breen J. Modern treatments of menorrhagia attributable to dysfunctional uterine bleeding. Obstet Gynecol Surv. 1999;54(11):251-262.
17. Pasqualotto EB, Margossian H, Priul L, Bradley LD, et al. Accuracy of preoperative diagnostic tools and outcome of hysteroscopic management of menstrual dysfunction. J Am Assoc Gynecol Laparosc. 2000;7(2):201-209.
18. Hurst B, Stackhouse D, Matthews M, Marshburn P. Uterine artery embolization for symptomatic uterine myomas. Fertil Steril. 2000;74(5):855-869.
1. Carlson KJ, Nichols DH, Schiff I. Indications for hysterectomy. N Engl J Med. 1993;328(12):856-860.
2. Coulter A, Bradlow J, Agass M, et al. Outcomes of referrals to gynecology outpatient clinics for menstrual problems: an audit of general practice records. Br J Obstet Gynaecol. 1991;98:789-796.
3. Claessens E, Cowell CA. Acute adolescent menorrhagia. Am J Obstet Gynecol. 1981;139:277-280.
4. American College of Obstetricians and Gynecologists. ACOG Committee Opinion #263. von Willebrand’s disease in gynecologic practice. Obstet Gynecol. 2001;98:1185-1186.
5. Santoro N, Adel T, Skurnick JH. Decreased inhibin tone and increased activin A secretion characterize reproductive aging in women. Fertil Steril. 1999;71:658-662.
6. Kaunitz A. Oral contraceptive use in perimenopause. Am J Obstet Gynecol. 2001;185(2):S32-S37.
7. Jones H. Clinical pathway for evaluating women with abnormal uterine bleeding. Obstet Gynecol Surv. 2002;57(1):22-24.
8. Higham JM, Shaw RW. A comparative study of danazol, a regimen of decreasing doses of danazol and norethindrone in the treatment of objectively proven unexplained menorrhagia. Am J Obstet Gynecol. 1993;169:1134-1139.
9. Scialli A, Levi A. Intermittent leuprolide acetate for the nonsurgical management of women with leiomyomata uteri. Fertil Steril. 2000;74(3):540-546.
10. Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomized trial. Lancet. 2001;357:273-277.
11. Vargyas JM, Campeau JD, Mishell DR. Treatment of menorrhagia with meclofenamate sodium. Am J Obstet Gynecol. 1987;157:944-950.
12. Jones K, Bourne T. The feasibility of a “one-stop” ultrasound-based clinic for the diagnosis and management of abnormal uterine bleeding. Ultrasound Obstet Gynecol. 2001;176:517-521.
13. Weigel M, Friese K, Strittmatter HJ, et al. Measuring the thickness—is that all we have to do for sonographic assessment of endometrium in postmenopausal women? Ultrasound Obstet Gynecol. 1995;6:97-102.
14. Widrich T, Bradley L, Mitchinson AR, Collins R. Comparison of saline infusion sonography with office hysteroscopy for the evaluation of the endometrium. Am J Obstet Gynecol. 1996;174:1327-1334.
15. Bettocchi S, Ceci O, Vicino M, et al. Diagnostic inadequacy of dilation and curettage. Fertil Steril. 2001;75(4):803-805.
16. Stabinsky S, Einstein M, Breen J. Modern treatments of menorrhagia attributable to dysfunctional uterine bleeding. Obstet Gynecol Surv. 1999;54(11):251-262.
17. Pasqualotto EB, Margossian H, Priul L, Bradley LD, et al. Accuracy of preoperative diagnostic tools and outcome of hysteroscopic management of menstrual dysfunction. J Am Assoc Gynecol Laparosc. 2000;7(2):201-209.
18. Hurst B, Stackhouse D, Matthews M, Marshburn P. Uterine artery embolization for symptomatic uterine myomas. Fertil Steril. 2000;74(5):855-869.