User login
Fibroids: Patient considerations in medical and surgical management
Uterine fibroids (myomas or leiomyomas) are common and can cause considerable morbidity, including infertility, in reproductive-aged women. In this roundtable discussion, moderated by OBG
Perspectives on a pervasive problem
Joseph S. Sanfilippo, MD, MBA: First let’s discuss the scope of the problem. How prevalent are uterine fibroids, and what are their effects on quality of life?
Linda D. Bradley, MD: Fibroids are extremely prevalent. Depending on age and race, between 60% and 80% of women have them.1 About 50% of women with fibroids have no symptoms2; in symptomatic women, the symptoms may vary based on age. Fibroids are more common in women from the African diaspora, who have earlier onset of symptoms, very large or more numerous fibroids, and more symptomatic fibroids, according to some clinical studies.3 While it is a very common disease state, about half of women with fibroids may not have significant symptoms that warrant anything more than watchful waiting or some minimally invasive options.
Ted L. Anderson, MD, PhD: We probably underestimate the scope because we see people coming in with fibroids only when they have a specific problem. There probably are a lot of asymptomatic women out there that we do not know about.
Case 1: Abnormal uterine bleeding in a young woman desiring pregnancy in the near future
Dr. Sanfilippo: Abnormal uterine bleeding is a common dilemma in my practice. Consider the following case example.
A 24-year-old woman (G1P1) presents with heavy, irregular menses over 6 months’ duration. She is interested in pregnancy, not immediately but in several months. She passes clots, soaks a pad in an hour, and has dysmenorrhea and fatigue. She uses no birth control. She is very distraught, as this bleeding truly has changed her lifestyle.
What is your approach to counseling this patient?
Dr. Bradley: You described a woman whose quality of life is very poor—frequent pad changes, clotting, pain. And she wants to have a child. A patient coming to me with those symptoms does not need to wait 4 to 6 months. I would immediately do some early evaluation.
Dr. Anderson: Sometimes a patient comes to us and already has had an ultrasonography exam. That is helpful, but I am driven by the fact that this patient is interested in pregnancy. I want to look at the uterine cavity and will probably do an office hysteroscopy to see if she has fibroids that distort the uterine cavity. Are there fibroids inside the cavity? To what degree does that possibly play a role? The presence of fibroids does not necessarily mean there is distortion of the cavity, and some evidence suggests that you do not need to do anything about those fibroids.4 Fibroids actually may not be the source of bleeding. We need to keep an open mind when we do the evaluation.
Continue to: Imaging technologies and classification aids...
Imaging technologies and classification aids
Dr. Sanfilippo: Apropos to your comment, is there a role for a sonohysterography in this population?
Dr. Anderson: That is a great technique. Some clinicians prefer to use sonohysterography while others prefer hysteroscopy. I tend to use hysteroscopy, and I have the equipment in the office. Both are great techniques and they answer the same question with respect to cavity evaluation.
Dr. Bradley: We once studied about 150 patients who, on the same day, with 2 separate examiners (one being me), would first undergo saline infusion sonohysterography (SIS) and then hysteroscopy, or vice versa. The sensitivity of identifying an intracavitary lesion is quite good with both. The additional benefit with SIS is that you can look at the adnexa.
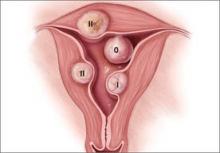
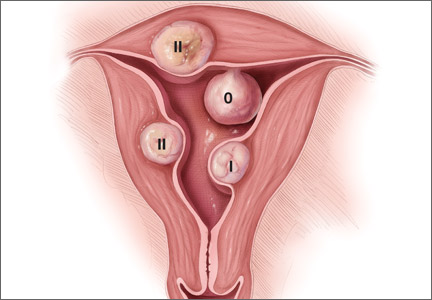
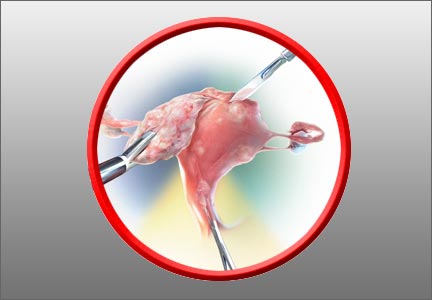
In terms of the classification by the International Federation of Gynaecology and Obstetrics (FIGO), sometimes when we do a hysteroscopy, we are not sure how deep a fibroid is—whether it is a type 1 or type 2 or how close it is to the serosa (see illustration, page 26). Are we seeing just the tip of the iceberg? There is a role for imaging, and it is not always an “either/or” situation. There are times, for example, that hysteroscopy will show a type 0. Other times it may not show that, and you look for other things in terms of whether a fibroid abuts the endometrium. The take-home message is that physicians should abandon endometrial biopsy alone and, in this case, not offer a D&C.
In evaluating the endometrium, as gynecologists we should be facile in both technologies. In our workplaces we need to advocate to get trained, to be certified, and to be able to offer both technologies, because sometimes you need both to obtain the right answer.
Dr. Sanfilippo: Let’s talk about the FIGO classification, because it is important to have a communication method not only between physicians but with the patient. If we determine that a fibroid is a type 0, and therefore totally intracavitary, management is different than if the fibroid is a type 1 (less than 50% into the myometrium) or type 2 (more than 50%). What is the role for a classification system such as the FIGO?
Dr. Anderson: I like the FIGO classification system. We can show the patient fibroid classification diagrammatically and she will be able to understand exactly what we are talking about. It’s helpful for patient education and for surgical planning. The approach to a type 0 fibroid is a no-brainer, but with type 1 and more specifically with type 2, where the bulk of the fibroid is intramural and only a portion of that is intracavitary, fibroid size begins to matter a lot in terms of treatment approach.
Sometimes although a fibroid is intracavitary, a laparoscopic rather than hysteroscopic approach is preferred, as long as you can dissect the fibroid away from the endometrium. FIGO classification is very helpful, but I agree with Dr. Bradley that first you need to do a thorough evaluation to make your operative plan.
Continue to: Dr. Sanfilippo...
Dr. Sanfilippo: I encourage residents to go through an orderly sequence of assessment for evaluating abnormal uterine bleeding, including anatomic and endocrinologic factors. The PALM-COEIN classification system is a great mnemonic for use in evaluating abnormal uterine bleeding (TABLE).5 Is there a role for an aid such as PALM-COEIN in your practice?
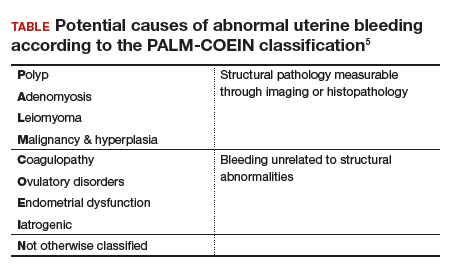
Dr. Bradley: I totally agree. In 2011, Malcolm Munro and colleagues in the FIGO Working Group on Menstrual Disorders helped us to have a reporting on outcomes by knowing the size, number, and location of fibroids.5 This helps us to look for structural causes and then, to get to the answer, we often use imaging such as ultrasonography or saline infusion, sometimes magnetic resonance imaging (MRI), because other conditions can coexist—endometrial polyps, adenomyosis, and so on.
The PALM-COEIN system helps us to look at 2 things. One is that in addition to structural causes, there can be hematologic causes. While it is rare in a 24-year-old, we all have had the anecdotal patient who came in 6 months ago, had a fibroid, but had a platelet count of 6,000. Second, we have to look at the patient as a whole. My residents, myself, and our fellows look at any bleeding. Does she have a bleeding diathesis, bruising, nose bleeds; has she been anemic, does she have pica? Has she had a blood transfusion, is she on certain medications? We do not want to create a “silo” and think that the patient can have only a fibroid, because then we may miss an opportunity to treat other disease states. She can have a fibroid coexisting with polycystic ovary syndrome (PCOS), for instance. I like to look at everything so we can offer appropriate treatment modalities.
Dr. Sanfilippo: You bring up a very important point. Coagulopathies are more common statistically at the earlier part of a woman’s reproductive age group, soon after menarche, but they also occur toward menopause. We have to be cognizant that a woman can develop a coagulopathy throughout the reproductive years.
Dr. Anderson: You have to look at other medical causes. That is where the PALM-COEIN system can help. It helps you take the blinders off. If you focus on the fibroid and treat the fibroid and the patient still has bleeding, you missed something. You have to consider the whole patient and think of all the nonclassical or nonanatomical things, for example, thyroid disease. The PALM-COEIN helps us to evaluate the patient in a methodical way—every patient every time—so you do not miss something.
The value of MRI
Dr. Sanfilippo: What is the role for MRI, and when do you use it? Is it for only when you do a procedure—laparoscopically, robotically, open—so you have a detailed map of the fibroids?
Dr. Anderson: I love MRI, especially for hysteroscopy. I will print out the MRI image and trace the fibroid because there are things I want to know: exactly how much of the fibroid is inside or outside, where this fibroid is in the uterus, and how much of a normal buffer there is between the edge of that fibroid and the serosa. How aggressive can I be, or how cautious do I need to be, during the resection? Maybe this will be a planned 2-stage resection. MRIs are wonderful for fibroid disease, not only for diagnosis but also for surgical planning and patient counseling.
Dr. Bradley: SIS is also very useful. If the patient has an intracavitary fibroid that is larger than 4.5 to 5 cm and we insert the catheter, however, sometimes you cannot distend the cavity very well. Sometimes large intramural fibroids can compress the cavity, making the procedure difficult in an office setting. You cannot see the limits to help you as a surgical option. Although SIS generally is associated with little pain, some patients may have pain, and some patients cannot tolerate the test.
Continue to: I would order an MRI for surgical planning when...
I would order an MRI for surgical planning when a hysteroscopy is equivocal and if I cannot do an SIS. Also, if a patient who had a hysteroscopic resection with incomplete removal comes to me and is still symptomatic, I want to know the depth of penetration.
Obtaining an MRI may sometimes be difficult at a particular institution, and some clinicians have to go through the hurdles of getting an ultrasound to get certified and approved. We have to be our patient’s advocate and do the peer phone calls; any other specialty would require presurgical planning, and we are no different from other surgeons in that regard.
Dr. Sanfilippo: Yes, that can be a stumbling block. In the operating room, I like to have the images right in front of me, ideally an MRI or an ultrasound scan, as I know how to proceed. Having that visual helps me understand how close the fibroid is to the lining of the uterus.
Tapping into radiologists’ expertise
Dr. Bradley: Every quarter we meet with our radiologists, who are very interested in our MRI and SIS reports. They will describe the count and say how many fibroids—that is very helpful instead of just saying she has a bunch of fibroids—but they also will tell us when there is a type 0, a type 2, a type 7 fibroid. The team looks for adenomyosis and for endometriosis that can coexist.
Dr. Anderson: One caution about reading radiology reports is that often someone will come in with a report from an outside hospital or from a small community hospital that may say, “There is a 2-cm submucosal fibroid.” Some people might be tempted to take this person right to the OR, but you need to look at the images yourself, because in a radiologist’s mind “submucosal” truly means under the mucosa, which in our liturgy would be “intramural.” So we need to make sure that we are talking the same language. You should look at the images yourself.
Dr. Sanfilippo: I totally agree. It is also not unreasonable to speak with the radiologists and educate them about the FIGO classification.
Dr. Bradley: I prefer the word “intracavitary” for fibroids. When I see a typed report without the picture, “submucosal” can mean in the cavity or abutting the endometrium.
Case 2: Woman with heavy bleeding and fibroids seeks nonsurgical treatment
Dr. Sanfilippo: A 39-year-old (G3P3) woman is referred for evaluation for heavy vaginal bleeding, soaking a pad in an hour, which has been going on for months. Her primary ObGyn obtained a pelvic sonogram and noted multiple intramural and subserosal fibroids. A sonohysterogram reveals a submucosal myoma.
The patient is not interested in a hysterectomy. She was treated with birth control pills, with no improvement. She is interested in nonsurgical options. Dr. Bradley, what medical treatments might you offer this patient?
Medical treatment options
Dr. Bradley: If oral contraceptives have not worked, a good option would be tranexamic acid. Years ago our hospital was involved with enrolling patients in the multicenter clinical trial of this drug. The classic patient enrolled had regular, predictable, heavy menstrual cycles with alkaline hematin assay of greater than 80. If the case patient described has regular and predictable heavy bleeding every month at the same time, for the same duration, I would consider the use of tranexamic acid. There are several contraindications for the drug, so those exclusion issues would need to be reviewed. Contraindications include subarachnoid hemorrhage. Cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients. Other contraindications include active intravascular clotting and hypersensitivity.
Continue to: Another option is to see if a progestin-releasing intrauterine system...
Another option is to see if a progestin-releasing intrauterine system (IUS) like the levonorgestrel (LNG) IUS would fit into this patient’s uterine cavity. Like Ted, I want to look into that cavity. I am not sure what “submucosal fibroid” means. If it has not distorted the cavity, or is totally within the uterine cavity, or abuts the endometrial cavity. The LNG-IUS cannot be placed into a uterine cavity that has intracavitary fibroids or sounds to greater than 12 cm. We are not going to put an LNG-IUS in somebody, at least in general, with a globally enlarged uterine cavity. I could ask, do you do that? You do a bimanual exam, and it is 18-weeks in size. I am not sure that I would put it in, but does it meet those criteria? The package insert for the LNG-IUS specifies upper and lower limits of uterine size for placement. I would start with those 2 options (tranexamic acid and LNG-IUS), and also get some more imaging.
Dr. Anderson: I agree with Linda. The submucosal fibroid could be contributing to this patient’s bleeding, but it is not the total contribution. The other fibroids may be completely irrelevant as far as her bleeding is concerned. We may need to deal with that one surgically, which we can do without a hysterectomy, most of the time.
I am a big fan of the LNG-IUS, it has been great in my experience. There are some other treatments available as well, such as gonadotropin–releasing hormone (GnRH) agonists. I tell patients that, while GnRH does work, it is not designed to be long-term therapy. If I have, for example, a 49-year-old patient, I just need to get her to menopause. Longer-term GnRH agonists might be a good option in this case. Otherwise, we could use short-term a GnRH agonist to stop the bleeding for a while so that we can reset the clock and get her started on something like levonorgestrel, tranexamic acid, or one of the other medical therapies. That may be a 2-step combination therapy.
Dr. Sanfilippo: There is a whole category of agents available—selective progesterone receptor modulators (SPRMs), pure progesterone receptor antagonists, ulipristal comes to mind. Clinicians need to know that options are available beyond birth control pills.
Dr. Anderson: As I tell patients, there are also “bridge” options. These are interventional procedures that are not hysterectomy, such as uterine fibroid embolization or endometrial ablation if bleeding is really the problem. We might consider a variety of different approaches. Obviously, we do not typically use fibroid embolization for submucosal fibroids, but it depends on how much of the fibroid is intracavitary and how big it is. Other options are a little more aggressive than medical therapy but they do not involve a hysterectomy.
Pros and cons of uterine artery embolization
Dr. Sanfilippo: If a woman desires future childbearing, is there a role for uterine artery embolization? How would you counsel her about the pros and cons?
Dr. Bradley: At the Cleveland Clinic, we generally do not offer uterine artery embolization if the patient wants a child. While it is an excellent method for treating heavy bleeding and bulk symptoms, the endometrium can be impacted. Patients can develop fistula, adhesions, or concentric narrowing, and changes in anti-Müllerian hormone levels, and there is potential for an Asherman-like syndrome and poor perfusion. I have many hysteroscopic images where the anterior wall of the uterus is nice and pink and the posterior wall is totally pale. The embolic microsphere particles can reach the endometrium—I have seen particles in the endometrium when doing a fibroid resection.
Continue to: A good early study looked at 555 women for almost a year...
A good early study looked at 555 women for almost a year.6 If women became pregnant, they had a higher rate of postpartum hemorrhage; placenta accreta, increta, and percreta; and emergent hysterectomy. It was recommended that these women deliver at a tertiary care center due to higher rates of preterm labor and malposition.
If a patient wants a baby, she should find a gynecologic surgeon who does minimally invasive laparoscopic, robotic, or open surgery, because she is more likely to have a take-home baby with a surgical approach than with embolization. In my experience, there is always going to be a patient who wants to keep her uterus at age 49 and who has every comorbidity. I might offer her the embolization just knowing what the odds of pregnancy are.
Dr. Anderson: I agree with Linda but I take a more liberal approach. Sometimes we do a myomectomy because we are trying to enhance fertility, while other times we do a myomectomy to address fibroid-related symptoms. These patients are having specific symptoms, and we want to leave the embolization option open.
If I have a patient who is 39 and becoming pregnant is not necessarily her goal, but she does not want to have a hysterectomy and if she got pregnant it would be okay, I am going to treat her a little different with respect to fibroid embolization than I would treat someone who is actively trying to have a baby. This goes back to what you were saying, let’s treat the patient, not just the fibroid.
Dr. Bradley: That is so important and sentinel. If she really does not want a hysterectomy but does not want a baby, I will ask, “Would you go through in vitro fertilization? Would you take clomiphene?” If she answers no, then I feel more comfortable, like you, with referring the patient for uterine fibroid embolization. The point is to get the patient with the right team to get the best outcomes.
Surgical approaches, intraoperative agents, and suture technique
Dr. Sanfilippo: Dr. Anderson, tell us about your surgical approaches to fibroids.
Dr. Anderson: At my institution we do have a fellowship in minimally invasive surgery, but I still do a lot of open myomectomies. I have a few guidelines to determine whether I am going to proceed laparoscopically, do a little minilaparotomy incision, or if a gigantic uterus is going to require a big incision. My mantra to my fellows has always been, “minimally invasive is the impact on the patient, not the size of the incision.”
Sometimes, prolonged anesthesia and Trendelenburg create more morbidity than a minilaparotomy. If a patient has 4 or 5 fibroids and most of them are intramural and I cannot see them but I want to be able to feel them, and to get a really good closure of the myometrium, I might choose to do a minilaparotomy. But if it is a case of a solitary fibroid, I would be more inclined to operate laparoscopically.
Continue to: Dr. Bradley...
Dr. Bradley: Our protocol is similar. We use MRI liberally. If patients have 4 or more fibroids and they are larger than 8 cm, most will have open surgery. I do not do robotic or laparoscopic procedures, so my referral source is for the larger myomas. We do not put retractors in; we can make incisions. Even if we do a huge Maylard incision, it is cosmetically wonderful. We use a loading dose of IV tranexamic acid with tranexamic acid throughout the surgery, and misoprostol intravaginally prior to surgery, to control uterine bleeding.
Dr. Sanfilippo: Dr. Anderson, is there a role for agents such as vasopressin, and what about routes of administration?
Dr. Anderson: When I do a laparoscopic or open procedure, I inject vasopressin (dilute 20 U in 100 mL of saline) into the pseudocapsule around the fibroid. I also administer rectal misoprostol (400 µg) just before the patient prep is done, which is amazing in reducing blood loss. There is also a role for a GnRH agonist, not necessarily to reduce the size of the uterus but to reduce blood flow in the pelvis and blood loss. Many different techniques are available. I do not use tourniquets, however. If bleeding does occur, I want to see it so I can fix it—not after I have sewn up the uterus and taken off a tourniquet.
Dr. Bradley: Do you use Floseal hemostatic matrix or any other agent to control bleeding?
Dr. Anderson: I do, for local hemostasis.
Dr. Bradley: Some surgeons will use barbed suture.
Dr. Anderson: I do like barbed sutures. In teaching residents to do myomectomy, it is very beneficial. But I am still a big fan of the good old figure-of-8 stitch because it is compressive and you get a good apposition of the tissue, good hemostasis, and strong closure.
Dr. Sanfilippo: We hope that this conversation will change your management of uterine fibroids. I thank Dr. Bradley and Dr. Anderson for a lively and very informative discussion.
Watch the video: Video roundtable–Fibroids: Patient considerations in medical and surgical management
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95-114.
- Divakars H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:643-654.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. 2013;22:807-816.
- Hartmann KE, Velez Edwards DR, Savitz DA, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017;186:1140-1148.
- Munro MG, Critchley HOD, Fraser IS, for the FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204-2208.
- Pron G, Mocarski E, Bennett J, et al; Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
Uterine fibroids (myomas or leiomyomas) are common and can cause considerable morbidity, including infertility, in reproductive-aged women. In this roundtable discussion, moderated by OBG
Perspectives on a pervasive problem
Joseph S. Sanfilippo, MD, MBA: First let’s discuss the scope of the problem. How prevalent are uterine fibroids, and what are their effects on quality of life?
Linda D. Bradley, MD: Fibroids are extremely prevalent. Depending on age and race, between 60% and 80% of women have them.1 About 50% of women with fibroids have no symptoms2; in symptomatic women, the symptoms may vary based on age. Fibroids are more common in women from the African diaspora, who have earlier onset of symptoms, very large or more numerous fibroids, and more symptomatic fibroids, according to some clinical studies.3 While it is a very common disease state, about half of women with fibroids may not have significant symptoms that warrant anything more than watchful waiting or some minimally invasive options.
Ted L. Anderson, MD, PhD: We probably underestimate the scope because we see people coming in with fibroids only when they have a specific problem. There probably are a lot of asymptomatic women out there that we do not know about.
Case 1: Abnormal uterine bleeding in a young woman desiring pregnancy in the near future
Dr. Sanfilippo: Abnormal uterine bleeding is a common dilemma in my practice. Consider the following case example.
A 24-year-old woman (G1P1) presents with heavy, irregular menses over 6 months’ duration. She is interested in pregnancy, not immediately but in several months. She passes clots, soaks a pad in an hour, and has dysmenorrhea and fatigue. She uses no birth control. She is very distraught, as this bleeding truly has changed her lifestyle.
What is your approach to counseling this patient?
Dr. Bradley: You described a woman whose quality of life is very poor—frequent pad changes, clotting, pain. And she wants to have a child. A patient coming to me with those symptoms does not need to wait 4 to 6 months. I would immediately do some early evaluation.
Dr. Anderson: Sometimes a patient comes to us and already has had an ultrasonography exam. That is helpful, but I am driven by the fact that this patient is interested in pregnancy. I want to look at the uterine cavity and will probably do an office hysteroscopy to see if she has fibroids that distort the uterine cavity. Are there fibroids inside the cavity? To what degree does that possibly play a role? The presence of fibroids does not necessarily mean there is distortion of the cavity, and some evidence suggests that you do not need to do anything about those fibroids.4 Fibroids actually may not be the source of bleeding. We need to keep an open mind when we do the evaluation.
Continue to: Imaging technologies and classification aids...
Imaging technologies and classification aids
Dr. Sanfilippo: Apropos to your comment, is there a role for a sonohysterography in this population?
Dr. Anderson: That is a great technique. Some clinicians prefer to use sonohysterography while others prefer hysteroscopy. I tend to use hysteroscopy, and I have the equipment in the office. Both are great techniques and they answer the same question with respect to cavity evaluation.
Dr. Bradley: We once studied about 150 patients who, on the same day, with 2 separate examiners (one being me), would first undergo saline infusion sonohysterography (SIS) and then hysteroscopy, or vice versa. The sensitivity of identifying an intracavitary lesion is quite good with both. The additional benefit with SIS is that you can look at the adnexa.
In terms of the classification by the International Federation of Gynaecology and Obstetrics (FIGO), sometimes when we do a hysteroscopy, we are not sure how deep a fibroid is—whether it is a type 1 or type 2 or how close it is to the serosa (see illustration, page 26). Are we seeing just the tip of the iceberg? There is a role for imaging, and it is not always an “either/or” situation. There are times, for example, that hysteroscopy will show a type 0. Other times it may not show that, and you look for other things in terms of whether a fibroid abuts the endometrium. The take-home message is that physicians should abandon endometrial biopsy alone and, in this case, not offer a D&C.
In evaluating the endometrium, as gynecologists we should be facile in both technologies. In our workplaces we need to advocate to get trained, to be certified, and to be able to offer both technologies, because sometimes you need both to obtain the right answer.
Dr. Sanfilippo: Let’s talk about the FIGO classification, because it is important to have a communication method not only between physicians but with the patient. If we determine that a fibroid is a type 0, and therefore totally intracavitary, management is different than if the fibroid is a type 1 (less than 50% into the myometrium) or type 2 (more than 50%). What is the role for a classification system such as the FIGO?
Dr. Anderson: I like the FIGO classification system. We can show the patient fibroid classification diagrammatically and she will be able to understand exactly what we are talking about. It’s helpful for patient education and for surgical planning. The approach to a type 0 fibroid is a no-brainer, but with type 1 and more specifically with type 2, where the bulk of the fibroid is intramural and only a portion of that is intracavitary, fibroid size begins to matter a lot in terms of treatment approach.
Sometimes although a fibroid is intracavitary, a laparoscopic rather than hysteroscopic approach is preferred, as long as you can dissect the fibroid away from the endometrium. FIGO classification is very helpful, but I agree with Dr. Bradley that first you need to do a thorough evaluation to make your operative plan.
Continue to: Dr. Sanfilippo...
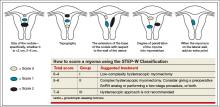
Dr. Sanfilippo: I encourage residents to go through an orderly sequence of assessment for evaluating abnormal uterine bleeding, including anatomic and endocrinologic factors. The PALM-COEIN classification system is a great mnemonic for use in evaluating abnormal uterine bleeding (TABLE).5 Is there a role for an aid such as PALM-COEIN in your practice?

Dr. Bradley: I totally agree. In 2011, Malcolm Munro and colleagues in the FIGO Working Group on Menstrual Disorders helped us to have a reporting on outcomes by knowing the size, number, and location of fibroids.5 This helps us to look for structural causes and then, to get to the answer, we often use imaging such as ultrasonography or saline infusion, sometimes magnetic resonance imaging (MRI), because other conditions can coexist—endometrial polyps, adenomyosis, and so on.
The PALM-COEIN system helps us to look at 2 things. One is that in addition to structural causes, there can be hematologic causes. While it is rare in a 24-year-old, we all have had the anecdotal patient who came in 6 months ago, had a fibroid, but had a platelet count of 6,000. Second, we have to look at the patient as a whole. My residents, myself, and our fellows look at any bleeding. Does she have a bleeding diathesis, bruising, nose bleeds; has she been anemic, does she have pica? Has she had a blood transfusion, is she on certain medications? We do not want to create a “silo” and think that the patient can have only a fibroid, because then we may miss an opportunity to treat other disease states. She can have a fibroid coexisting with polycystic ovary syndrome (PCOS), for instance. I like to look at everything so we can offer appropriate treatment modalities.
Dr. Sanfilippo: You bring up a very important point. Coagulopathies are more common statistically at the earlier part of a woman’s reproductive age group, soon after menarche, but they also occur toward menopause. We have to be cognizant that a woman can develop a coagulopathy throughout the reproductive years.
Dr. Anderson: You have to look at other medical causes. That is where the PALM-COEIN system can help. It helps you take the blinders off. If you focus on the fibroid and treat the fibroid and the patient still has bleeding, you missed something. You have to consider the whole patient and think of all the nonclassical or nonanatomical things, for example, thyroid disease. The PALM-COEIN helps us to evaluate the patient in a methodical way—every patient every time—so you do not miss something.
The value of MRI
Dr. Sanfilippo: What is the role for MRI, and when do you use it? Is it for only when you do a procedure—laparoscopically, robotically, open—so you have a detailed map of the fibroids?
Dr. Anderson: I love MRI, especially for hysteroscopy. I will print out the MRI image and trace the fibroid because there are things I want to know: exactly how much of the fibroid is inside or outside, where this fibroid is in the uterus, and how much of a normal buffer there is between the edge of that fibroid and the serosa. How aggressive can I be, or how cautious do I need to be, during the resection? Maybe this will be a planned 2-stage resection. MRIs are wonderful for fibroid disease, not only for diagnosis but also for surgical planning and patient counseling.
Dr. Bradley: SIS is also very useful. If the patient has an intracavitary fibroid that is larger than 4.5 to 5 cm and we insert the catheter, however, sometimes you cannot distend the cavity very well. Sometimes large intramural fibroids can compress the cavity, making the procedure difficult in an office setting. You cannot see the limits to help you as a surgical option. Although SIS generally is associated with little pain, some patients may have pain, and some patients cannot tolerate the test.
Continue to: I would order an MRI for surgical planning when...
I would order an MRI for surgical planning when a hysteroscopy is equivocal and if I cannot do an SIS. Also, if a patient who had a hysteroscopic resection with incomplete removal comes to me and is still symptomatic, I want to know the depth of penetration.
Obtaining an MRI may sometimes be difficult at a particular institution, and some clinicians have to go through the hurdles of getting an ultrasound to get certified and approved. We have to be our patient’s advocate and do the peer phone calls; any other specialty would require presurgical planning, and we are no different from other surgeons in that regard.
Dr. Sanfilippo: Yes, that can be a stumbling block. In the operating room, I like to have the images right in front of me, ideally an MRI or an ultrasound scan, as I know how to proceed. Having that visual helps me understand how close the fibroid is to the lining of the uterus.
Tapping into radiologists’ expertise
Dr. Bradley: Every quarter we meet with our radiologists, who are very interested in our MRI and SIS reports. They will describe the count and say how many fibroids—that is very helpful instead of just saying she has a bunch of fibroids—but they also will tell us when there is a type 0, a type 2, a type 7 fibroid. The team looks for adenomyosis and for endometriosis that can coexist.
Dr. Anderson: One caution about reading radiology reports is that often someone will come in with a report from an outside hospital or from a small community hospital that may say, “There is a 2-cm submucosal fibroid.” Some people might be tempted to take this person right to the OR, but you need to look at the images yourself, because in a radiologist’s mind “submucosal” truly means under the mucosa, which in our liturgy would be “intramural.” So we need to make sure that we are talking the same language. You should look at the images yourself.
Dr. Sanfilippo: I totally agree. It is also not unreasonable to speak with the radiologists and educate them about the FIGO classification.
Dr. Bradley: I prefer the word “intracavitary” for fibroids. When I see a typed report without the picture, “submucosal” can mean in the cavity or abutting the endometrium.
Case 2: Woman with heavy bleeding and fibroids seeks nonsurgical treatment
Dr. Sanfilippo: A 39-year-old (G3P3) woman is referred for evaluation for heavy vaginal bleeding, soaking a pad in an hour, which has been going on for months. Her primary ObGyn obtained a pelvic sonogram and noted multiple intramural and subserosal fibroids. A sonohysterogram reveals a submucosal myoma.
The patient is not interested in a hysterectomy. She was treated with birth control pills, with no improvement. She is interested in nonsurgical options. Dr. Bradley, what medical treatments might you offer this patient?
Medical treatment options
Dr. Bradley: If oral contraceptives have not worked, a good option would be tranexamic acid. Years ago our hospital was involved with enrolling patients in the multicenter clinical trial of this drug. The classic patient enrolled had regular, predictable, heavy menstrual cycles with alkaline hematin assay of greater than 80. If the case patient described has regular and predictable heavy bleeding every month at the same time, for the same duration, I would consider the use of tranexamic acid. There are several contraindications for the drug, so those exclusion issues would need to be reviewed. Contraindications include subarachnoid hemorrhage. Cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients. Other contraindications include active intravascular clotting and hypersensitivity.
Continue to: Another option is to see if a progestin-releasing intrauterine system...
Another option is to see if a progestin-releasing intrauterine system (IUS) like the levonorgestrel (LNG) IUS would fit into this patient’s uterine cavity. Like Ted, I want to look into that cavity. I am not sure what “submucosal fibroid” means. If it has not distorted the cavity, or is totally within the uterine cavity, or abuts the endometrial cavity. The LNG-IUS cannot be placed into a uterine cavity that has intracavitary fibroids or sounds to greater than 12 cm. We are not going to put an LNG-IUS in somebody, at least in general, with a globally enlarged uterine cavity. I could ask, do you do that? You do a bimanual exam, and it is 18-weeks in size. I am not sure that I would put it in, but does it meet those criteria? The package insert for the LNG-IUS specifies upper and lower limits of uterine size for placement. I would start with those 2 options (tranexamic acid and LNG-IUS), and also get some more imaging.
Dr. Anderson: I agree with Linda. The submucosal fibroid could be contributing to this patient’s bleeding, but it is not the total contribution. The other fibroids may be completely irrelevant as far as her bleeding is concerned. We may need to deal with that one surgically, which we can do without a hysterectomy, most of the time.
I am a big fan of the LNG-IUS, it has been great in my experience. There are some other treatments available as well, such as gonadotropin–releasing hormone (GnRH) agonists. I tell patients that, while GnRH does work, it is not designed to be long-term therapy. If I have, for example, a 49-year-old patient, I just need to get her to menopause. Longer-term GnRH agonists might be a good option in this case. Otherwise, we could use short-term a GnRH agonist to stop the bleeding for a while so that we can reset the clock and get her started on something like levonorgestrel, tranexamic acid, or one of the other medical therapies. That may be a 2-step combination therapy.
Dr. Sanfilippo: There is a whole category of agents available—selective progesterone receptor modulators (SPRMs), pure progesterone receptor antagonists, ulipristal comes to mind. Clinicians need to know that options are available beyond birth control pills.
Dr. Anderson: As I tell patients, there are also “bridge” options. These are interventional procedures that are not hysterectomy, such as uterine fibroid embolization or endometrial ablation if bleeding is really the problem. We might consider a variety of different approaches. Obviously, we do not typically use fibroid embolization for submucosal fibroids, but it depends on how much of the fibroid is intracavitary and how big it is. Other options are a little more aggressive than medical therapy but they do not involve a hysterectomy.
Pros and cons of uterine artery embolization
Dr. Sanfilippo: If a woman desires future childbearing, is there a role for uterine artery embolization? How would you counsel her about the pros and cons?
Dr. Bradley: At the Cleveland Clinic, we generally do not offer uterine artery embolization if the patient wants a child. While it is an excellent method for treating heavy bleeding and bulk symptoms, the endometrium can be impacted. Patients can develop fistula, adhesions, or concentric narrowing, and changes in anti-Müllerian hormone levels, and there is potential for an Asherman-like syndrome and poor perfusion. I have many hysteroscopic images where the anterior wall of the uterus is nice and pink and the posterior wall is totally pale. The embolic microsphere particles can reach the endometrium—I have seen particles in the endometrium when doing a fibroid resection.
Continue to: A good early study looked at 555 women for almost a year...
A good early study looked at 555 women for almost a year.6 If women became pregnant, they had a higher rate of postpartum hemorrhage; placenta accreta, increta, and percreta; and emergent hysterectomy. It was recommended that these women deliver at a tertiary care center due to higher rates of preterm labor and malposition.
If a patient wants a baby, she should find a gynecologic surgeon who does minimally invasive laparoscopic, robotic, or open surgery, because she is more likely to have a take-home baby with a surgical approach than with embolization. In my experience, there is always going to be a patient who wants to keep her uterus at age 49 and who has every comorbidity. I might offer her the embolization just knowing what the odds of pregnancy are.
Dr. Anderson: I agree with Linda but I take a more liberal approach. Sometimes we do a myomectomy because we are trying to enhance fertility, while other times we do a myomectomy to address fibroid-related symptoms. These patients are having specific symptoms, and we want to leave the embolization option open.
If I have a patient who is 39 and becoming pregnant is not necessarily her goal, but she does not want to have a hysterectomy and if she got pregnant it would be okay, I am going to treat her a little different with respect to fibroid embolization than I would treat someone who is actively trying to have a baby. This goes back to what you were saying, let’s treat the patient, not just the fibroid.
Dr. Bradley: That is so important and sentinel. If she really does not want a hysterectomy but does not want a baby, I will ask, “Would you go through in vitro fertilization? Would you take clomiphene?” If she answers no, then I feel more comfortable, like you, with referring the patient for uterine fibroid embolization. The point is to get the patient with the right team to get the best outcomes.
Surgical approaches, intraoperative agents, and suture technique
Dr. Sanfilippo: Dr. Anderson, tell us about your surgical approaches to fibroids.
Dr. Anderson: At my institution we do have a fellowship in minimally invasive surgery, but I still do a lot of open myomectomies. I have a few guidelines to determine whether I am going to proceed laparoscopically, do a little minilaparotomy incision, or if a gigantic uterus is going to require a big incision. My mantra to my fellows has always been, “minimally invasive is the impact on the patient, not the size of the incision.”
Sometimes, prolonged anesthesia and Trendelenburg create more morbidity than a minilaparotomy. If a patient has 4 or 5 fibroids and most of them are intramural and I cannot see them but I want to be able to feel them, and to get a really good closure of the myometrium, I might choose to do a minilaparotomy. But if it is a case of a solitary fibroid, I would be more inclined to operate laparoscopically.
Continue to: Dr. Bradley...
Dr. Bradley: Our protocol is similar. We use MRI liberally. If patients have 4 or more fibroids and they are larger than 8 cm, most will have open surgery. I do not do robotic or laparoscopic procedures, so my referral source is for the larger myomas. We do not put retractors in; we can make incisions. Even if we do a huge Maylard incision, it is cosmetically wonderful. We use a loading dose of IV tranexamic acid with tranexamic acid throughout the surgery, and misoprostol intravaginally prior to surgery, to control uterine bleeding.
Dr. Sanfilippo: Dr. Anderson, is there a role for agents such as vasopressin, and what about routes of administration?
Dr. Anderson: When I do a laparoscopic or open procedure, I inject vasopressin (dilute 20 U in 100 mL of saline) into the pseudocapsule around the fibroid. I also administer rectal misoprostol (400 µg) just before the patient prep is done, which is amazing in reducing blood loss. There is also a role for a GnRH agonist, not necessarily to reduce the size of the uterus but to reduce blood flow in the pelvis and blood loss. Many different techniques are available. I do not use tourniquets, however. If bleeding does occur, I want to see it so I can fix it—not after I have sewn up the uterus and taken off a tourniquet.
Dr. Bradley: Do you use Floseal hemostatic matrix or any other agent to control bleeding?
Dr. Anderson: I do, for local hemostasis.
Dr. Bradley: Some surgeons will use barbed suture.
Dr. Anderson: I do like barbed sutures. In teaching residents to do myomectomy, it is very beneficial. But I am still a big fan of the good old figure-of-8 stitch because it is compressive and you get a good apposition of the tissue, good hemostasis, and strong closure.
Dr. Sanfilippo: We hope that this conversation will change your management of uterine fibroids. I thank Dr. Bradley and Dr. Anderson for a lively and very informative discussion.
Watch the video: Video roundtable–Fibroids: Patient considerations in medical and surgical management
Uterine fibroids (myomas or leiomyomas) are common and can cause considerable morbidity, including infertility, in reproductive-aged women. In this roundtable discussion, moderated by OBG
Perspectives on a pervasive problem
Joseph S. Sanfilippo, MD, MBA: First let’s discuss the scope of the problem. How prevalent are uterine fibroids, and what are their effects on quality of life?
Linda D. Bradley, MD: Fibroids are extremely prevalent. Depending on age and race, between 60% and 80% of women have them.1 About 50% of women with fibroids have no symptoms2; in symptomatic women, the symptoms may vary based on age. Fibroids are more common in women from the African diaspora, who have earlier onset of symptoms, very large or more numerous fibroids, and more symptomatic fibroids, according to some clinical studies.3 While it is a very common disease state, about half of women with fibroids may not have significant symptoms that warrant anything more than watchful waiting or some minimally invasive options.
Ted L. Anderson, MD, PhD: We probably underestimate the scope because we see people coming in with fibroids only when they have a specific problem. There probably are a lot of asymptomatic women out there that we do not know about.
Case 1: Abnormal uterine bleeding in a young woman desiring pregnancy in the near future
Dr. Sanfilippo: Abnormal uterine bleeding is a common dilemma in my practice. Consider the following case example.
A 24-year-old woman (G1P1) presents with heavy, irregular menses over 6 months’ duration. She is interested in pregnancy, not immediately but in several months. She passes clots, soaks a pad in an hour, and has dysmenorrhea and fatigue. She uses no birth control. She is very distraught, as this bleeding truly has changed her lifestyle.
What is your approach to counseling this patient?
Dr. Bradley: You described a woman whose quality of life is very poor—frequent pad changes, clotting, pain. And she wants to have a child. A patient coming to me with those symptoms does not need to wait 4 to 6 months. I would immediately do some early evaluation.
Dr. Anderson: Sometimes a patient comes to us and already has had an ultrasonography exam. That is helpful, but I am driven by the fact that this patient is interested in pregnancy. I want to look at the uterine cavity and will probably do an office hysteroscopy to see if she has fibroids that distort the uterine cavity. Are there fibroids inside the cavity? To what degree does that possibly play a role? The presence of fibroids does not necessarily mean there is distortion of the cavity, and some evidence suggests that you do not need to do anything about those fibroids.4 Fibroids actually may not be the source of bleeding. We need to keep an open mind when we do the evaluation.
Continue to: Imaging technologies and classification aids...
Imaging technologies and classification aids
Dr. Sanfilippo: Apropos to your comment, is there a role for a sonohysterography in this population?
Dr. Anderson: That is a great technique. Some clinicians prefer to use sonohysterography while others prefer hysteroscopy. I tend to use hysteroscopy, and I have the equipment in the office. Both are great techniques and they answer the same question with respect to cavity evaluation.
Dr. Bradley: We once studied about 150 patients who, on the same day, with 2 separate examiners (one being me), would first undergo saline infusion sonohysterography (SIS) and then hysteroscopy, or vice versa. The sensitivity of identifying an intracavitary lesion is quite good with both. The additional benefit with SIS is that you can look at the adnexa.
In terms of the classification by the International Federation of Gynaecology and Obstetrics (FIGO), sometimes when we do a hysteroscopy, we are not sure how deep a fibroid is—whether it is a type 1 or type 2 or how close it is to the serosa (see illustration, page 26). Are we seeing just the tip of the iceberg? There is a role for imaging, and it is not always an “either/or” situation. There are times, for example, that hysteroscopy will show a type 0. Other times it may not show that, and you look for other things in terms of whether a fibroid abuts the endometrium. The take-home message is that physicians should abandon endometrial biopsy alone and, in this case, not offer a D&C.
In evaluating the endometrium, as gynecologists we should be facile in both technologies. In our workplaces we need to advocate to get trained, to be certified, and to be able to offer both technologies, because sometimes you need both to obtain the right answer.
Dr. Sanfilippo: Let’s talk about the FIGO classification, because it is important to have a communication method not only between physicians but with the patient. If we determine that a fibroid is a type 0, and therefore totally intracavitary, management is different than if the fibroid is a type 1 (less than 50% into the myometrium) or type 2 (more than 50%). What is the role for a classification system such as the FIGO?
Dr. Anderson: I like the FIGO classification system. We can show the patient fibroid classification diagrammatically and she will be able to understand exactly what we are talking about. It’s helpful for patient education and for surgical planning. The approach to a type 0 fibroid is a no-brainer, but with type 1 and more specifically with type 2, where the bulk of the fibroid is intramural and only a portion of that is intracavitary, fibroid size begins to matter a lot in terms of treatment approach.
Sometimes although a fibroid is intracavitary, a laparoscopic rather than hysteroscopic approach is preferred, as long as you can dissect the fibroid away from the endometrium. FIGO classification is very helpful, but I agree with Dr. Bradley that first you need to do a thorough evaluation to make your operative plan.
Continue to: Dr. Sanfilippo...
Dr. Sanfilippo: I encourage residents to go through an orderly sequence of assessment for evaluating abnormal uterine bleeding, including anatomic and endocrinologic factors. The PALM-COEIN classification system is a great mnemonic for use in evaluating abnormal uterine bleeding (TABLE).5 Is there a role for an aid such as PALM-COEIN in your practice?

Dr. Bradley: I totally agree. In 2011, Malcolm Munro and colleagues in the FIGO Working Group on Menstrual Disorders helped us to have a reporting on outcomes by knowing the size, number, and location of fibroids.5 This helps us to look for structural causes and then, to get to the answer, we often use imaging such as ultrasonography or saline infusion, sometimes magnetic resonance imaging (MRI), because other conditions can coexist—endometrial polyps, adenomyosis, and so on.
The PALM-COEIN system helps us to look at 2 things. One is that in addition to structural causes, there can be hematologic causes. While it is rare in a 24-year-old, we all have had the anecdotal patient who came in 6 months ago, had a fibroid, but had a platelet count of 6,000. Second, we have to look at the patient as a whole. My residents, myself, and our fellows look at any bleeding. Does she have a bleeding diathesis, bruising, nose bleeds; has she been anemic, does she have pica? Has she had a blood transfusion, is she on certain medications? We do not want to create a “silo” and think that the patient can have only a fibroid, because then we may miss an opportunity to treat other disease states. She can have a fibroid coexisting with polycystic ovary syndrome (PCOS), for instance. I like to look at everything so we can offer appropriate treatment modalities.
Dr. Sanfilippo: You bring up a very important point. Coagulopathies are more common statistically at the earlier part of a woman’s reproductive age group, soon after menarche, but they also occur toward menopause. We have to be cognizant that a woman can develop a coagulopathy throughout the reproductive years.
Dr. Anderson: You have to look at other medical causes. That is where the PALM-COEIN system can help. It helps you take the blinders off. If you focus on the fibroid and treat the fibroid and the patient still has bleeding, you missed something. You have to consider the whole patient and think of all the nonclassical or nonanatomical things, for example, thyroid disease. The PALM-COEIN helps us to evaluate the patient in a methodical way—every patient every time—so you do not miss something.
The value of MRI
Dr. Sanfilippo: What is the role for MRI, and when do you use it? Is it for only when you do a procedure—laparoscopically, robotically, open—so you have a detailed map of the fibroids?
Dr. Anderson: I love MRI, especially for hysteroscopy. I will print out the MRI image and trace the fibroid because there are things I want to know: exactly how much of the fibroid is inside or outside, where this fibroid is in the uterus, and how much of a normal buffer there is between the edge of that fibroid and the serosa. How aggressive can I be, or how cautious do I need to be, during the resection? Maybe this will be a planned 2-stage resection. MRIs are wonderful for fibroid disease, not only for diagnosis but also for surgical planning and patient counseling.
Dr. Bradley: SIS is also very useful. If the patient has an intracavitary fibroid that is larger than 4.5 to 5 cm and we insert the catheter, however, sometimes you cannot distend the cavity very well. Sometimes large intramural fibroids can compress the cavity, making the procedure difficult in an office setting. You cannot see the limits to help you as a surgical option. Although SIS generally is associated with little pain, some patients may have pain, and some patients cannot tolerate the test.
Continue to: I would order an MRI for surgical planning when...
I would order an MRI for surgical planning when a hysteroscopy is equivocal and if I cannot do an SIS. Also, if a patient who had a hysteroscopic resection with incomplete removal comes to me and is still symptomatic, I want to know the depth of penetration.
Obtaining an MRI may sometimes be difficult at a particular institution, and some clinicians have to go through the hurdles of getting an ultrasound to get certified and approved. We have to be our patient’s advocate and do the peer phone calls; any other specialty would require presurgical planning, and we are no different from other surgeons in that regard.
Dr. Sanfilippo: Yes, that can be a stumbling block. In the operating room, I like to have the images right in front of me, ideally an MRI or an ultrasound scan, as I know how to proceed. Having that visual helps me understand how close the fibroid is to the lining of the uterus.
Tapping into radiologists’ expertise
Dr. Bradley: Every quarter we meet with our radiologists, who are very interested in our MRI and SIS reports. They will describe the count and say how many fibroids—that is very helpful instead of just saying she has a bunch of fibroids—but they also will tell us when there is a type 0, a type 2, a type 7 fibroid. The team looks for adenomyosis and for endometriosis that can coexist.
Dr. Anderson: One caution about reading radiology reports is that often someone will come in with a report from an outside hospital or from a small community hospital that may say, “There is a 2-cm submucosal fibroid.” Some people might be tempted to take this person right to the OR, but you need to look at the images yourself, because in a radiologist’s mind “submucosal” truly means under the mucosa, which in our liturgy would be “intramural.” So we need to make sure that we are talking the same language. You should look at the images yourself.
Dr. Sanfilippo: I totally agree. It is also not unreasonable to speak with the radiologists and educate them about the FIGO classification.
Dr. Bradley: I prefer the word “intracavitary” for fibroids. When I see a typed report without the picture, “submucosal” can mean in the cavity or abutting the endometrium.
Case 2: Woman with heavy bleeding and fibroids seeks nonsurgical treatment
Dr. Sanfilippo: A 39-year-old (G3P3) woman is referred for evaluation for heavy vaginal bleeding, soaking a pad in an hour, which has been going on for months. Her primary ObGyn obtained a pelvic sonogram and noted multiple intramural and subserosal fibroids. A sonohysterogram reveals a submucosal myoma.
The patient is not interested in a hysterectomy. She was treated with birth control pills, with no improvement. She is interested in nonsurgical options. Dr. Bradley, what medical treatments might you offer this patient?
Medical treatment options
Dr. Bradley: If oral contraceptives have not worked, a good option would be tranexamic acid. Years ago our hospital was involved with enrolling patients in the multicenter clinical trial of this drug. The classic patient enrolled had regular, predictable, heavy menstrual cycles with alkaline hematin assay of greater than 80. If the case patient described has regular and predictable heavy bleeding every month at the same time, for the same duration, I would consider the use of tranexamic acid. There are several contraindications for the drug, so those exclusion issues would need to be reviewed. Contraindications include subarachnoid hemorrhage. Cerebral edema and cerebral infarction may be caused by tranexamic acid in such patients. Other contraindications include active intravascular clotting and hypersensitivity.
Continue to: Another option is to see if a progestin-releasing intrauterine system...
Another option is to see if a progestin-releasing intrauterine system (IUS) like the levonorgestrel (LNG) IUS would fit into this patient’s uterine cavity. Like Ted, I want to look into that cavity. I am not sure what “submucosal fibroid” means. If it has not distorted the cavity, or is totally within the uterine cavity, or abuts the endometrial cavity. The LNG-IUS cannot be placed into a uterine cavity that has intracavitary fibroids or sounds to greater than 12 cm. We are not going to put an LNG-IUS in somebody, at least in general, with a globally enlarged uterine cavity. I could ask, do you do that? You do a bimanual exam, and it is 18-weeks in size. I am not sure that I would put it in, but does it meet those criteria? The package insert for the LNG-IUS specifies upper and lower limits of uterine size for placement. I would start with those 2 options (tranexamic acid and LNG-IUS), and also get some more imaging.
Dr. Anderson: I agree with Linda. The submucosal fibroid could be contributing to this patient’s bleeding, but it is not the total contribution. The other fibroids may be completely irrelevant as far as her bleeding is concerned. We may need to deal with that one surgically, which we can do without a hysterectomy, most of the time.
I am a big fan of the LNG-IUS, it has been great in my experience. There are some other treatments available as well, such as gonadotropin–releasing hormone (GnRH) agonists. I tell patients that, while GnRH does work, it is not designed to be long-term therapy. If I have, for example, a 49-year-old patient, I just need to get her to menopause. Longer-term GnRH agonists might be a good option in this case. Otherwise, we could use short-term a GnRH agonist to stop the bleeding for a while so that we can reset the clock and get her started on something like levonorgestrel, tranexamic acid, or one of the other medical therapies. That may be a 2-step combination therapy.
Dr. Sanfilippo: There is a whole category of agents available—selective progesterone receptor modulators (SPRMs), pure progesterone receptor antagonists, ulipristal comes to mind. Clinicians need to know that options are available beyond birth control pills.
Dr. Anderson: As I tell patients, there are also “bridge” options. These are interventional procedures that are not hysterectomy, such as uterine fibroid embolization or endometrial ablation if bleeding is really the problem. We might consider a variety of different approaches. Obviously, we do not typically use fibroid embolization for submucosal fibroids, but it depends on how much of the fibroid is intracavitary and how big it is. Other options are a little more aggressive than medical therapy but they do not involve a hysterectomy.
Pros and cons of uterine artery embolization
Dr. Sanfilippo: If a woman desires future childbearing, is there a role for uterine artery embolization? How would you counsel her about the pros and cons?
Dr. Bradley: At the Cleveland Clinic, we generally do not offer uterine artery embolization if the patient wants a child. While it is an excellent method for treating heavy bleeding and bulk symptoms, the endometrium can be impacted. Patients can develop fistula, adhesions, or concentric narrowing, and changes in anti-Müllerian hormone levels, and there is potential for an Asherman-like syndrome and poor perfusion. I have many hysteroscopic images where the anterior wall of the uterus is nice and pink and the posterior wall is totally pale. The embolic microsphere particles can reach the endometrium—I have seen particles in the endometrium when doing a fibroid resection.
Continue to: A good early study looked at 555 women for almost a year...
A good early study looked at 555 women for almost a year.6 If women became pregnant, they had a higher rate of postpartum hemorrhage; placenta accreta, increta, and percreta; and emergent hysterectomy. It was recommended that these women deliver at a tertiary care center due to higher rates of preterm labor and malposition.
If a patient wants a baby, she should find a gynecologic surgeon who does minimally invasive laparoscopic, robotic, or open surgery, because she is more likely to have a take-home baby with a surgical approach than with embolization. In my experience, there is always going to be a patient who wants to keep her uterus at age 49 and who has every comorbidity. I might offer her the embolization just knowing what the odds of pregnancy are.
Dr. Anderson: I agree with Linda but I take a more liberal approach. Sometimes we do a myomectomy because we are trying to enhance fertility, while other times we do a myomectomy to address fibroid-related symptoms. These patients are having specific symptoms, and we want to leave the embolization option open.
If I have a patient who is 39 and becoming pregnant is not necessarily her goal, but she does not want to have a hysterectomy and if she got pregnant it would be okay, I am going to treat her a little different with respect to fibroid embolization than I would treat someone who is actively trying to have a baby. This goes back to what you were saying, let’s treat the patient, not just the fibroid.
Dr. Bradley: That is so important and sentinel. If she really does not want a hysterectomy but does not want a baby, I will ask, “Would you go through in vitro fertilization? Would you take clomiphene?” If she answers no, then I feel more comfortable, like you, with referring the patient for uterine fibroid embolization. The point is to get the patient with the right team to get the best outcomes.
Surgical approaches, intraoperative agents, and suture technique
Dr. Sanfilippo: Dr. Anderson, tell us about your surgical approaches to fibroids.
Dr. Anderson: At my institution we do have a fellowship in minimally invasive surgery, but I still do a lot of open myomectomies. I have a few guidelines to determine whether I am going to proceed laparoscopically, do a little minilaparotomy incision, or if a gigantic uterus is going to require a big incision. My mantra to my fellows has always been, “minimally invasive is the impact on the patient, not the size of the incision.”
Sometimes, prolonged anesthesia and Trendelenburg create more morbidity than a minilaparotomy. If a patient has 4 or 5 fibroids and most of them are intramural and I cannot see them but I want to be able to feel them, and to get a really good closure of the myometrium, I might choose to do a minilaparotomy. But if it is a case of a solitary fibroid, I would be more inclined to operate laparoscopically.
Continue to: Dr. Bradley...
Dr. Bradley: Our protocol is similar. We use MRI liberally. If patients have 4 or more fibroids and they are larger than 8 cm, most will have open surgery. I do not do robotic or laparoscopic procedures, so my referral source is for the larger myomas. We do not put retractors in; we can make incisions. Even if we do a huge Maylard incision, it is cosmetically wonderful. We use a loading dose of IV tranexamic acid with tranexamic acid throughout the surgery, and misoprostol intravaginally prior to surgery, to control uterine bleeding.
Dr. Sanfilippo: Dr. Anderson, is there a role for agents such as vasopressin, and what about routes of administration?
Dr. Anderson: When I do a laparoscopic or open procedure, I inject vasopressin (dilute 20 U in 100 mL of saline) into the pseudocapsule around the fibroid. I also administer rectal misoprostol (400 µg) just before the patient prep is done, which is amazing in reducing blood loss. There is also a role for a GnRH agonist, not necessarily to reduce the size of the uterus but to reduce blood flow in the pelvis and blood loss. Many different techniques are available. I do not use tourniquets, however. If bleeding does occur, I want to see it so I can fix it—not after I have sewn up the uterus and taken off a tourniquet.
Dr. Bradley: Do you use Floseal hemostatic matrix or any other agent to control bleeding?
Dr. Anderson: I do, for local hemostasis.
Dr. Bradley: Some surgeons will use barbed suture.
Dr. Anderson: I do like barbed sutures. In teaching residents to do myomectomy, it is very beneficial. But I am still a big fan of the good old figure-of-8 stitch because it is compressive and you get a good apposition of the tissue, good hemostasis, and strong closure.
Dr. Sanfilippo: We hope that this conversation will change your management of uterine fibroids. I thank Dr. Bradley and Dr. Anderson for a lively and very informative discussion.
Watch the video: Video roundtable–Fibroids: Patient considerations in medical and surgical management
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95-114.
- Divakars H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:643-654.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. 2013;22:807-816.
- Hartmann KE, Velez Edwards DR, Savitz DA, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017;186:1140-1148.
- Munro MG, Critchley HOD, Fraser IS, for the FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204-2208.
- Pron G, Mocarski E, Bennett J, et al; Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
- Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014;6:95-114.
- Divakars H. Asymptomatic uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2008;22:643-654.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health. 2013;22:807-816.
- Hartmann KE, Velez Edwards DR, Savitz DA, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol. 2017;186:1140-1148.
- Munro MG, Critchley HOD, Fraser IS, for the FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011;95:2204-2208.
- Pron G, Mocarski E, Bennett J, et al; Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
Video roundtable–Fibroids: Patient considerations in medical and surgical management
Read the article: Fibroids: Patient considerations in medical and surgical management
Read the article: Fibroids: Patient considerations in medical and surgical management
Read the article: Fibroids: Patient considerations in medical and surgical management
The importance of weight management and exercise: Practical advice for your patients
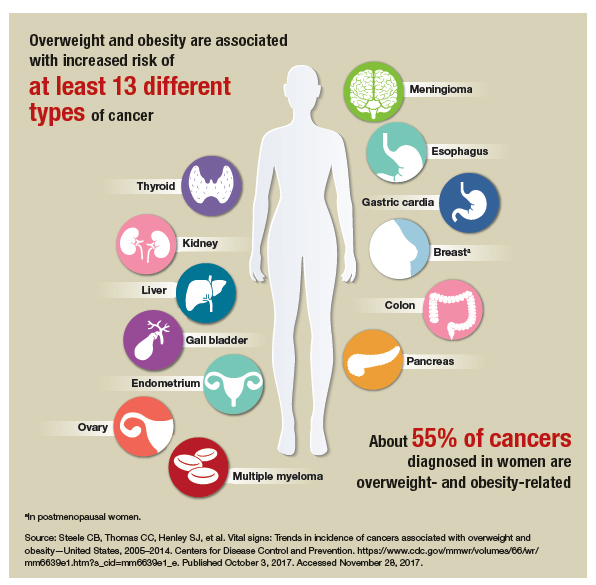
Over the past 3 decades, the prevalence of overweight and obesity has increased dramatically in the United States. A study published in 2016 showed the age-adjusted prevalence of obesity in 2013–2014 was 35% among men and 40.4% among women.1 It comes as no surprise that increased reliance on inexpensive fast foods coupled with progressively more sedentary lifestyles have been implicated as causative factors.2
With the rise in obesity also has come an attendant rise in related chronic diseases, such as type 2 diabetes mellitus and cardiovascular disease. Women who are obese are also at risk for certain women’s health conditions, such as polycystic ovary syndrome, breast cancer, and endometrial cancer.
It is clear that curbing this public health crisis will require concerted efforts from individuals, clinicians, and policy makers, as well as changes in societal norms.
Linda D. Bradley, MD: I think it is important for us not to lecture our patients. I could list all of the things that patients should or could do to prevent or even reverse disease states, in terms of eating right and exercising, but I think motivational interviewing is a more productive approach to elicit and evoke change (see “Principles and practice of motivational interviewing”). I used to preach to my patients. I would say, “You know, if you stay at this weight, you’re going to get diabetes, you’re going to increase your breast cancer risk, you’re going to have abnormal bleeding, you’re not going to be able to get pregnant,” and so on. It is easy to slip into that in the 7 minutes that you have with your patient, but to me, that is not the right way.
With motivational interviewing, our interactions with patients are shaped by:
- asking
- advising
- assisting
- arranging.
We begin by asking permission: “Do you mind if we talk about your weight?” or “Can we talk about your level of exercise?” Once the patient has granted permission, we ask open-ended questions and use reflective listening: “What I hear you saying is that you are concerned you will not be able to lose the weight,” or “It sounds like you don’t like to exercise, but you are worried about the health consequences of that.”
Utilizing motivational interviewing to help patients identify thoughts and feelings that contribute to unhealthy behaviors--and replacing those thoughts and feelings with new thought patterns that aid in behavior change--has been shown to be an effective and efficient facilitator for change. By incorporating the following principles of motivational interviewing into practice, clinicians can have an important impact on the prevention or management of serious diseases in women1:
- Express empathy and avoid arguments. "I know it has been difficult for you to take the first step to losing weight. That is something that is difficult for a lot of my patients. How can I help you take that first step?"
- Develop discrepancies to help the patient understand the difference between her behavior and her goals. "You have said that you would like to lose some weight. I think you know that exercise would help with that. Why do you think it has been hard for you to start exercising more?"
- Roll with resistance and provide personalized feedback to help the patient find ways to succeed. "What I hear you saying is your work schedule does not allow you time to work out at the gym. What about walking during lunch breaks or taking the stairs instead of the elevator--is that something you think you can commit to doing?"
- Support self-efficacy and elicit self-motivation. "What would you like to see differently about your health? What makes you think you need to change? What happens if you don't change?"
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 423: Motivational interviewing: a tool for behavioral change. Obstet Gynecol. 2009;113(1):243-246.
I find these skills useful for addressing anything from smoking to drinking to weight management to excessive shopping—any extreme behavior that is affecting a patient negatively. When a patient is not ready to talk about her clinical problems or make changes, I let her know my door is always open to her and that I have many resources available to help her when she is ready (TABLE).4 In those cases, I might say something like, “I have many patients who really don’t want to talk about this when I first ask them, but I just want you to know, Mrs. Jones, that I want you to succeed and I want you to be healthy. We have a team approach to taking care of all of you, and when you are ready, we are here to help.”
Related article:
2017 Update on fertility: Effects of obesity on reproduction
It is important to provide practical advice to patients—including how much to exercise, the importance of keeping a food journal, and determining a goal for slow, safe weight loss—and provide resources as necessary (such as for Weight Watchers, nutrition, and dieticians). Each day we have more than 30 opportunities to select foods to eat, drink, or purchase. Have a plan and advise your patients do the same. Recommend patients cook their own meals. Suggest weight loss apps. Counsel them to celebrate successes, find a buddy (for social support), practice positive self-talk (positive language), and plan for challenges (travel, parties, working late) and setbacks, which do not need to become a fall. Find an activity or exercise that the patient enjoys and tell them to seek professional help if needed.
Read about how to educate your patients on wellness.
Dr. Bradley: About 86% of the health care dollars spent in the United States are due to chronic diseases, and chronic diseases are the leading cause of death and disability in the country.5 The most common chronic diseases—cardiovascular disease, hypertension, type 2 diabetes, colon cancer, depression, dementia, cognitive problems, higher rates of fractures—all have been associated, at least in part, with unhealthy food choices and lack of exercise. That applies to breast cancer, too.
The good news is, we can prevent and even reverse disease. As Hippocrates said, let food be thy medicine and medicine be thy food. We have all seen success stories where consistent exercise and dietary changes definitely change the paradigm for what the disease state represents. A multiplicity of factors affect poor health—noncompliance, obesity, smoking—but when we begin to make consistent, healthy changes with diet and exercise, this creates a sort of domino effect.
In the book Us! Our Life. Our Health. Our Legacy,1 co-authored by Dr. Bradley and her colleague, Margaret L. McKenzie, MD, the authors highlight the 10 healthiest behaviors to bring about youthfulness and robust health:
- Walk at least 30-45 minutes per day most days of the week.
- Engage in resistance training 2-3 days per week.
- Eat a primarily plant-based diet made up of a variety of whole foods.
- Do not smoke.
- Maintain a waist line that measures less than half your height.
- Drink alcohol only in moderation.
- Get 7-8 hours of sleep most nights.
- Forgive.
- Have gratitude.
- Believe in something greater than yourself.
- Bradley LD, McKenzie ML. Us! Our life. Our health. Our legacy. Las Vegas, Nevada: The Literary Front Publishing Co., LLC; 2016.
Dr. Bradley: I think we need to get to the root cause of these clinical problems and provide the resources and support that patients need to reverse or even prevent these diseases. Clinicians need to become more aware—be an example and a role model. Our patients are watching us as much as we are watching them. Together, we can form good partnerships in order to promote better health.
Dr. Bradley: I think when you are about to be a change agent for your body and become what I call the best version of yourself, you can have these great ideas, but you need to turn those ideas into actions and make them consistent. And we know that is difficult to do, so I do try to have patients write down specific goals, their plan for achieving them, and list the reasons why it is important for them to reach their goals. That gives them something tangible to look at when the going gets tough. It is also important to work into the contract ways to reward positive behaviors when goals are met, and to plan for challenges and setbacks and how to get back on track.
I also encourage patients to document their progress and learn how to make quick adjustments when necessary to get back on track. Another important element involves setting milestones—by what date are you going to reach this goal? Like any other contract, I have my patients date and sign their wellness contracts. I also encourage them to visualize what their new self is going to look like, how they will feel when they reach their goal, what they will wear, and what activities they will engage in.
Related article:
Obesity medicine: How to incorporate it into your practice
Dr. Bradley: I do, but the amount of nutrition education that most of us get in medical school is minimal to nonexistent and not practical. As physicians, we know that food is health, exercise is fitness, and that our patients need both of them. We also know that we did not get this information in school and that our education was more about treating disease than preventing disease. Many of us were not trained in robot surgery either, because it did not exist. So what did we do? We took classes, attended lectures, read books, and learned. We can do the same with wellness. There are many courses around the country. We have to begin to relearn and reteach ourselves about health, nutrition, and exercise and then pass that information on to our patients—be a resource and a guide. We should be able to write a prescription for health as quickly as we can write a prescription for insulin or a statin.
I also bring up portion distortion with my patients. The National Institutes of Health has resources on their website (https://www.nhlbi.nih.gov/health/educational /wecan/eat-right/portion-distortion.html) that include great visuals that show portion sizes 20 years ago and what they are now. For instance, 20 years ago a bagel was 3 inches and 140 calories; today’s bagel is 6 inches and 350 calories (plus whatever toppings are added). I tell that to my patients and then explain how much more exercise is needed to burn off just that 1 bagel.
Related article:
How to help your patients control gestational weight gain
Dr. Bradley: They may not know that term directly, but I think people understand that you have the potential to pass on poor lifestyle and/or health issues related to how things are when you are in utero and later in life. It gets back to letting people know to be healthy in pregnancy and even pre-pregnancy, and that includes one’s emotional state, physical state, and spiritual state. We are what we are in our mother’s womb. Getting the best start in life starts with a healthy mom, healthy dad, and a healthy environment.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA.2016;315(21):2284Arial–2291.
- Sturm R, An R. Obesity and economic environments. CA Cancer J Clin. 2014;64(5):337Arial–350.
- Bradley LD, McKenzie ML. Us! Our life. Our health. Our legacy. Las Vegas, Nevada: The Literary Front Publishing Co., LLC; 2016.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 423: Motivational interviewing: a tool for behavioral change. Obstet Gynecol. 2009;113(1):243–246.
- Centers for Disease Control and Prevention. Chronic disease overview. https://www.cdc.gov/chronicdisease/overview/index.htm. Updated June 28, 2017. Accessed November 3, 2017.
Over the past 3 decades, the prevalence of overweight and obesity has increased dramatically in the United States. A study published in 2016 showed the age-adjusted prevalence of obesity in 2013–2014 was 35% among men and 40.4% among women.1 It comes as no surprise that increased reliance on inexpensive fast foods coupled with progressively more sedentary lifestyles have been implicated as causative factors.2
With the rise in obesity also has come an attendant rise in related chronic diseases, such as type 2 diabetes mellitus and cardiovascular disease. Women who are obese are also at risk for certain women’s health conditions, such as polycystic ovary syndrome, breast cancer, and endometrial cancer.
It is clear that curbing this public health crisis will require concerted efforts from individuals, clinicians, and policy makers, as well as changes in societal norms.
Linda D. Bradley, MD: I think it is important for us not to lecture our patients. I could list all of the things that patients should or could do to prevent or even reverse disease states, in terms of eating right and exercising, but I think motivational interviewing is a more productive approach to elicit and evoke change (see “Principles and practice of motivational interviewing”). I used to preach to my patients. I would say, “You know, if you stay at this weight, you’re going to get diabetes, you’re going to increase your breast cancer risk, you’re going to have abnormal bleeding, you’re not going to be able to get pregnant,” and so on. It is easy to slip into that in the 7 minutes that you have with your patient, but to me, that is not the right way.
With motivational interviewing, our interactions with patients are shaped by:
- asking
- advising
- assisting
- arranging.
We begin by asking permission: “Do you mind if we talk about your weight?” or “Can we talk about your level of exercise?” Once the patient has granted permission, we ask open-ended questions and use reflective listening: “What I hear you saying is that you are concerned you will not be able to lose the weight,” or “It sounds like you don’t like to exercise, but you are worried about the health consequences of that.”
Utilizing motivational interviewing to help patients identify thoughts and feelings that contribute to unhealthy behaviors--and replacing those thoughts and feelings with new thought patterns that aid in behavior change--has been shown to be an effective and efficient facilitator for change. By incorporating the following principles of motivational interviewing into practice, clinicians can have an important impact on the prevention or management of serious diseases in women1:
- Express empathy and avoid arguments. "I know it has been difficult for you to take the first step to losing weight. That is something that is difficult for a lot of my patients. How can I help you take that first step?"
- Develop discrepancies to help the patient understand the difference between her behavior and her goals. "You have said that you would like to lose some weight. I think you know that exercise would help with that. Why do you think it has been hard for you to start exercising more?"
- Roll with resistance and provide personalized feedback to help the patient find ways to succeed. "What I hear you saying is your work schedule does not allow you time to work out at the gym. What about walking during lunch breaks or taking the stairs instead of the elevator--is that something you think you can commit to doing?"
- Support self-efficacy and elicit self-motivation. "What would you like to see differently about your health? What makes you think you need to change? What happens if you don't change?"
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 423: Motivational interviewing: a tool for behavioral change. Obstet Gynecol. 2009;113(1):243-246.
I find these skills useful for addressing anything from smoking to drinking to weight management to excessive shopping—any extreme behavior that is affecting a patient negatively. When a patient is not ready to talk about her clinical problems or make changes, I let her know my door is always open to her and that I have many resources available to help her when she is ready (TABLE).4 In those cases, I might say something like, “I have many patients who really don’t want to talk about this when I first ask them, but I just want you to know, Mrs. Jones, that I want you to succeed and I want you to be healthy. We have a team approach to taking care of all of you, and when you are ready, we are here to help.”

Related article:
2017 Update on fertility: Effects of obesity on reproduction
It is important to provide practical advice to patients—including how much to exercise, the importance of keeping a food journal, and determining a goal for slow, safe weight loss—and provide resources as necessary (such as for Weight Watchers, nutrition, and dieticians). Each day we have more than 30 opportunities to select foods to eat, drink, or purchase. Have a plan and advise your patients do the same. Recommend patients cook their own meals. Suggest weight loss apps. Counsel them to celebrate successes, find a buddy (for social support), practice positive self-talk (positive language), and plan for challenges (travel, parties, working late) and setbacks, which do not need to become a fall. Find an activity or exercise that the patient enjoys and tell them to seek professional help if needed.
Read about how to educate your patients on wellness.
Dr. Bradley: About 86% of the health care dollars spent in the United States are due to chronic diseases, and chronic diseases are the leading cause of death and disability in the country.5 The most common chronic diseases—cardiovascular disease, hypertension, type 2 diabetes, colon cancer, depression, dementia, cognitive problems, higher rates of fractures—all have been associated, at least in part, with unhealthy food choices and lack of exercise. That applies to breast cancer, too.
The good news is, we can prevent and even reverse disease. As Hippocrates said, let food be thy medicine and medicine be thy food. We have all seen success stories where consistent exercise and dietary changes definitely change the paradigm for what the disease state represents. A multiplicity of factors affect poor health—noncompliance, obesity, smoking—but when we begin to make consistent, healthy changes with diet and exercise, this creates a sort of domino effect.
In the book Us! Our Life. Our Health. Our Legacy,1 co-authored by Dr. Bradley and her colleague, Margaret L. McKenzie, MD, the authors highlight the 10 healthiest behaviors to bring about youthfulness and robust health:
- Walk at least 30-45 minutes per day most days of the week.
- Engage in resistance training 2-3 days per week.
- Eat a primarily plant-based diet made up of a variety of whole foods.
- Do not smoke.
- Maintain a waist line that measures less than half your height.
- Drink alcohol only in moderation.
- Get 7-8 hours of sleep most nights.
- Forgive.
- Have gratitude.
- Believe in something greater than yourself.
- Bradley LD, McKenzie ML. Us! Our life. Our health. Our legacy. Las Vegas, Nevada: The Literary Front Publishing Co., LLC; 2016.
Dr. Bradley: I think we need to get to the root cause of these clinical problems and provide the resources and support that patients need to reverse or even prevent these diseases. Clinicians need to become more aware—be an example and a role model. Our patients are watching us as much as we are watching them. Together, we can form good partnerships in order to promote better health.

Dr. Bradley: I think when you are about to be a change agent for your body and become what I call the best version of yourself, you can have these great ideas, but you need to turn those ideas into actions and make them consistent. And we know that is difficult to do, so I do try to have patients write down specific goals, their plan for achieving them, and list the reasons why it is important for them to reach their goals. That gives them something tangible to look at when the going gets tough. It is also important to work into the contract ways to reward positive behaviors when goals are met, and to plan for challenges and setbacks and how to get back on track.
I also encourage patients to document their progress and learn how to make quick adjustments when necessary to get back on track. Another important element involves setting milestones—by what date are you going to reach this goal? Like any other contract, I have my patients date and sign their wellness contracts. I also encourage them to visualize what their new self is going to look like, how they will feel when they reach their goal, what they will wear, and what activities they will engage in.
Related article:
Obesity medicine: How to incorporate it into your practice
Dr. Bradley: I do, but the amount of nutrition education that most of us get in medical school is minimal to nonexistent and not practical. As physicians, we know that food is health, exercise is fitness, and that our patients need both of them. We also know that we did not get this information in school and that our education was more about treating disease than preventing disease. Many of us were not trained in robot surgery either, because it did not exist. So what did we do? We took classes, attended lectures, read books, and learned. We can do the same with wellness. There are many courses around the country. We have to begin to relearn and reteach ourselves about health, nutrition, and exercise and then pass that information on to our patients—be a resource and a guide. We should be able to write a prescription for health as quickly as we can write a prescription for insulin or a statin.
I also bring up portion distortion with my patients. The National Institutes of Health has resources on their website (https://www.nhlbi.nih.gov/health/educational /wecan/eat-right/portion-distortion.html) that include great visuals that show portion sizes 20 years ago and what they are now. For instance, 20 years ago a bagel was 3 inches and 140 calories; today’s bagel is 6 inches and 350 calories (plus whatever toppings are added). I tell that to my patients and then explain how much more exercise is needed to burn off just that 1 bagel.
Related article:
How to help your patients control gestational weight gain
Dr. Bradley: They may not know that term directly, but I think people understand that you have the potential to pass on poor lifestyle and/or health issues related to how things are when you are in utero and later in life. It gets back to letting people know to be healthy in pregnancy and even pre-pregnancy, and that includes one’s emotional state, physical state, and spiritual state. We are what we are in our mother’s womb. Getting the best start in life starts with a healthy mom, healthy dad, and a healthy environment.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Over the past 3 decades, the prevalence of overweight and obesity has increased dramatically in the United States. A study published in 2016 showed the age-adjusted prevalence of obesity in 2013–2014 was 35% among men and 40.4% among women.1 It comes as no surprise that increased reliance on inexpensive fast foods coupled with progressively more sedentary lifestyles have been implicated as causative factors.2
With the rise in obesity also has come an attendant rise in related chronic diseases, such as type 2 diabetes mellitus and cardiovascular disease. Women who are obese are also at risk for certain women’s health conditions, such as polycystic ovary syndrome, breast cancer, and endometrial cancer.
It is clear that curbing this public health crisis will require concerted efforts from individuals, clinicians, and policy makers, as well as changes in societal norms.
Linda D. Bradley, MD: I think it is important for us not to lecture our patients. I could list all of the things that patients should or could do to prevent or even reverse disease states, in terms of eating right and exercising, but I think motivational interviewing is a more productive approach to elicit and evoke change (see “Principles and practice of motivational interviewing”). I used to preach to my patients. I would say, “You know, if you stay at this weight, you’re going to get diabetes, you’re going to increase your breast cancer risk, you’re going to have abnormal bleeding, you’re not going to be able to get pregnant,” and so on. It is easy to slip into that in the 7 minutes that you have with your patient, but to me, that is not the right way.
With motivational interviewing, our interactions with patients are shaped by:
- asking
- advising
- assisting
- arranging.
We begin by asking permission: “Do you mind if we talk about your weight?” or “Can we talk about your level of exercise?” Once the patient has granted permission, we ask open-ended questions and use reflective listening: “What I hear you saying is that you are concerned you will not be able to lose the weight,” or “It sounds like you don’t like to exercise, but you are worried about the health consequences of that.”
Utilizing motivational interviewing to help patients identify thoughts and feelings that contribute to unhealthy behaviors--and replacing those thoughts and feelings with new thought patterns that aid in behavior change--has been shown to be an effective and efficient facilitator for change. By incorporating the following principles of motivational interviewing into practice, clinicians can have an important impact on the prevention or management of serious diseases in women1:
- Express empathy and avoid arguments. "I know it has been difficult for you to take the first step to losing weight. That is something that is difficult for a lot of my patients. How can I help you take that first step?"
- Develop discrepancies to help the patient understand the difference between her behavior and her goals. "You have said that you would like to lose some weight. I think you know that exercise would help with that. Why do you think it has been hard for you to start exercising more?"
- Roll with resistance and provide personalized feedback to help the patient find ways to succeed. "What I hear you saying is your work schedule does not allow you time to work out at the gym. What about walking during lunch breaks or taking the stairs instead of the elevator--is that something you think you can commit to doing?"
- Support self-efficacy and elicit self-motivation. "What would you like to see differently about your health? What makes you think you need to change? What happens if you don't change?"
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 423: Motivational interviewing: a tool for behavioral change. Obstet Gynecol. 2009;113(1):243-246.
I find these skills useful for addressing anything from smoking to drinking to weight management to excessive shopping—any extreme behavior that is affecting a patient negatively. When a patient is not ready to talk about her clinical problems or make changes, I let her know my door is always open to her and that I have many resources available to help her when she is ready (TABLE).4 In those cases, I might say something like, “I have many patients who really don’t want to talk about this when I first ask them, but I just want you to know, Mrs. Jones, that I want you to succeed and I want you to be healthy. We have a team approach to taking care of all of you, and when you are ready, we are here to help.”

Related article:
2017 Update on fertility: Effects of obesity on reproduction
It is important to provide practical advice to patients—including how much to exercise, the importance of keeping a food journal, and determining a goal for slow, safe weight loss—and provide resources as necessary (such as for Weight Watchers, nutrition, and dieticians). Each day we have more than 30 opportunities to select foods to eat, drink, or purchase. Have a plan and advise your patients do the same. Recommend patients cook their own meals. Suggest weight loss apps. Counsel them to celebrate successes, find a buddy (for social support), practice positive self-talk (positive language), and plan for challenges (travel, parties, working late) and setbacks, which do not need to become a fall. Find an activity or exercise that the patient enjoys and tell them to seek professional help if needed.
Read about how to educate your patients on wellness.
Dr. Bradley: About 86% of the health care dollars spent in the United States are due to chronic diseases, and chronic diseases are the leading cause of death and disability in the country.5 The most common chronic diseases—cardiovascular disease, hypertension, type 2 diabetes, colon cancer, depression, dementia, cognitive problems, higher rates of fractures—all have been associated, at least in part, with unhealthy food choices and lack of exercise. That applies to breast cancer, too.
The good news is, we can prevent and even reverse disease. As Hippocrates said, let food be thy medicine and medicine be thy food. We have all seen success stories where consistent exercise and dietary changes definitely change the paradigm for what the disease state represents. A multiplicity of factors affect poor health—noncompliance, obesity, smoking—but when we begin to make consistent, healthy changes with diet and exercise, this creates a sort of domino effect.
In the book Us! Our Life. Our Health. Our Legacy,1 co-authored by Dr. Bradley and her colleague, Margaret L. McKenzie, MD, the authors highlight the 10 healthiest behaviors to bring about youthfulness and robust health:
- Walk at least 30-45 minutes per day most days of the week.
- Engage in resistance training 2-3 days per week.
- Eat a primarily plant-based diet made up of a variety of whole foods.
- Do not smoke.
- Maintain a waist line that measures less than half your height.
- Drink alcohol only in moderation.
- Get 7-8 hours of sleep most nights.
- Forgive.
- Have gratitude.
- Believe in something greater than yourself.
- Bradley LD, McKenzie ML. Us! Our life. Our health. Our legacy. Las Vegas, Nevada: The Literary Front Publishing Co., LLC; 2016.
Dr. Bradley: I think we need to get to the root cause of these clinical problems and provide the resources and support that patients need to reverse or even prevent these diseases. Clinicians need to become more aware—be an example and a role model. Our patients are watching us as much as we are watching them. Together, we can form good partnerships in order to promote better health.

Dr. Bradley: I think when you are about to be a change agent for your body and become what I call the best version of yourself, you can have these great ideas, but you need to turn those ideas into actions and make them consistent. And we know that is difficult to do, so I do try to have patients write down specific goals, their plan for achieving them, and list the reasons why it is important for them to reach their goals. That gives them something tangible to look at when the going gets tough. It is also important to work into the contract ways to reward positive behaviors when goals are met, and to plan for challenges and setbacks and how to get back on track.
I also encourage patients to document their progress and learn how to make quick adjustments when necessary to get back on track. Another important element involves setting milestones—by what date are you going to reach this goal? Like any other contract, I have my patients date and sign their wellness contracts. I also encourage them to visualize what their new self is going to look like, how they will feel when they reach their goal, what they will wear, and what activities they will engage in.
Related article:
Obesity medicine: How to incorporate it into your practice
Dr. Bradley: I do, but the amount of nutrition education that most of us get in medical school is minimal to nonexistent and not practical. As physicians, we know that food is health, exercise is fitness, and that our patients need both of them. We also know that we did not get this information in school and that our education was more about treating disease than preventing disease. Many of us were not trained in robot surgery either, because it did not exist. So what did we do? We took classes, attended lectures, read books, and learned. We can do the same with wellness. There are many courses around the country. We have to begin to relearn and reteach ourselves about health, nutrition, and exercise and then pass that information on to our patients—be a resource and a guide. We should be able to write a prescription for health as quickly as we can write a prescription for insulin or a statin.
I also bring up portion distortion with my patients. The National Institutes of Health has resources on their website (https://www.nhlbi.nih.gov/health/educational /wecan/eat-right/portion-distortion.html) that include great visuals that show portion sizes 20 years ago and what they are now. For instance, 20 years ago a bagel was 3 inches and 140 calories; today’s bagel is 6 inches and 350 calories (plus whatever toppings are added). I tell that to my patients and then explain how much more exercise is needed to burn off just that 1 bagel.
Related article:
How to help your patients control gestational weight gain
Dr. Bradley: They may not know that term directly, but I think people understand that you have the potential to pass on poor lifestyle and/or health issues related to how things are when you are in utero and later in life. It gets back to letting people know to be healthy in pregnancy and even pre-pregnancy, and that includes one’s emotional state, physical state, and spiritual state. We are what we are in our mother’s womb. Getting the best start in life starts with a healthy mom, healthy dad, and a healthy environment.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA.2016;315(21):2284Arial–2291.
- Sturm R, An R. Obesity and economic environments. CA Cancer J Clin. 2014;64(5):337Arial–350.
- Bradley LD, McKenzie ML. Us! Our life. Our health. Our legacy. Las Vegas, Nevada: The Literary Front Publishing Co., LLC; 2016.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 423: Motivational interviewing: a tool for behavioral change. Obstet Gynecol. 2009;113(1):243–246.
- Centers for Disease Control and Prevention. Chronic disease overview. https://www.cdc.gov/chronicdisease/overview/index.htm. Updated June 28, 2017. Accessed November 3, 2017.
- Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA.2016;315(21):2284Arial–2291.
- Sturm R, An R. Obesity and economic environments. CA Cancer J Clin. 2014;64(5):337Arial–350.
- Bradley LD, McKenzie ML. Us! Our life. Our health. Our legacy. Las Vegas, Nevada: The Literary Front Publishing Co., LLC; 2016.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 423: Motivational interviewing: a tool for behavioral change. Obstet Gynecol. 2009;113(1):243–246.
- Centers for Disease Control and Prevention. Chronic disease overview. https://www.cdc.gov/chronicdisease/overview/index.htm. Updated June 28, 2017. Accessed November 3, 2017.
Audiocast: Hysteroscopic tubal occlusion: How new product labeling can be a resource for patient counseling
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Resources
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Resources
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Resources
Hysteroscopic tubal occlusion: How new product labeling can be a resource for patient counseling
In November 2016, Bayer, the manufacturer of the permanent birth control tubal implant system (Essure), revised the Essure product labeling in accordance with a US Food and Drug Administration (FDA) guidance document.1 The FDA developed its labeling guidance based on its examination of an increasing number of reported adverse events associated with the system’s use (such as persistent pain, perforation of the uterus and/or fallopian tubes, intra-abdominal or pelvic device migration, abnormal or irregular bleeding, and allergy or hypersensitivity reactions) and its evaluation of a trade complaint regarding allegations initially made in a Citizen Petition.
Changes to the new FDA-approved labeling for Essure include:
- the addition of a boxed warning listing adverse events that have been reported either in clinical studies or through postmarket surveillance (see Box)
- updated Instructions for Use document for clinicians and Patient Information Booklet, which contain additional information on safety (contraindications, warnings, and precautions), clinical data, and instructions2,3
- a Patient-Doctor Discussion Checklist (included within the Patient Information Booklet), designed to support appropriate patient counseling, facilitate the patient’s understanding of birth control options, and explain the benefits and risks associated with the device and what to expect during and after the implantation procedure.3
How will these labeling changes impact clinicians and patients? OBG
Reference
1. Essure permanent birth control (Bayer) Instructions for use. http://www.hcp.essure-us.com/assets/pdf/Link%20Essure%20IFU.pdf. Accessed January 5, 2017.
OBG Management: What does the new product labeling mean for clinicians who offer tubal implants as an option for permanent sterilization?
Linda D. Bradley, MD: The FDA-approved revised labeling for the Essure system means that physicians should have a very detailed, in-depth conversation with their patients who are contemplating hysteroscopic tubal insert placement for permanent sterilization. This counseling really should not differ from what doctors were doing before the label was revised. However, physicians can now use the new Patient-Doctor Discussion Checklist as a guide in reviewing the benefits of the device, its known risks and potential risks, outcomes of the insertion procedure, and the possible need for future surgical intervention if device placement–related issues arise.
For clinicians, this counseling adds just a few more minutes to the visit. The Patient-Doctor Discussion Checklist will become an inherent part of the informed consent process, aiding in the review of the device’s benefits, potential risks, and more importantly its permanence.
In the past, there was some concern that perhaps patients did not receive enough guidance for informed consent, so one of the first things listed on the checklist is confirmation—in the form of a printed line where the patient can sign her initials—that she understands that Essure is a permanent form of birth control. The checklist covers additional important issues, including that the doctor has indeed shared with the patient other options for birth control or sterilization, such as laparoscopic sterilization, vasectomy for her male partner, an intrauterine device (IUD), and birth control pills. This is an opportunity to reinforce the fact that tubal implants are a permanent form of birth control, and if the patient is uncertain about ending her fertility, the clinician can inform her about reversible options. The checklist also includes for discussion the pregnancy risk with use of the device, what the patient can expect during the implant insertion procedure and for the days afterwards (such as cramping, mild to moderate pain, nausea and vomiting), and the need for a confirmation test 3 months after device placement.
Other discussion points covered include long-term risks and benefits of the device, the potential for complications, and the possibility (due to pelvic pain) that the hysteroscopically placed devices may need to be removed with a surgical procedure requiring general anesthesia.
Incorporating the checklist into our clinical practice shows that we have listened to patients and complied with recommendations made by the FDA review panel, and we can use this document to have a more complete discussion with our patients.
OBG Management: Do you agree with some clinicians who say that physicians who place the device also should have the skills required to remove it if necessary?
Dr. Bradley: Essure placement—which is a hysteroscopic procedure—is done very differently than a laparoscopic procedure. In the past, among women who needed to have the Essure system removed, most procedures would be done laparoscopically. Since we work collaboratively in teams, someone within the team or division would have the clinical expertise to remove the devices. An ObGyn who does laparoscopy with salpingectomy and/or cornual resection would best be able to remove the devices.
The clinician who does hysteroscopy is not always the same one who does laparoscopy. Someone within the division who is interested in removing the device will develop an expertise and algorithm that suits the practice, so that person in the practice becomes the expert. This is no different from many other things that physicians do. In our clinical practice, for example, we have a pelvic pain specialist, a sexual counselor, someone interested in menopause and management, and someone interested in alternatives to hysterectomies. Those who practice their craft and their art become proficient at it. So if you do not perform a particular procedure such as a tubal implant removal, know the expert to whom you can make a referral.
OBG Management: How do you now advise your colleagues to counsel patients on permanent sterilization?
Dr. Bradley: Hysteroscopic tubal implant sterilization, a minimally invasive procedure, is an excellent and viable option for women who meet the inclusion criteria and who do not have the exclusion criteria for placement. It is overall safe and extremely effective. If a patient has issues after undergoing implant placement—just like with any other surgery or procedure—for example, if she is not feeling better or is not doing as well as anticipated, we must not forget the patient. It is important for our patients to be listened to and to be heard. Postprocedure issues are generally transient and related to pain and discomfort or abnormal bleeding. If they are persistent, then further evaluation is needed.
Tell the patient to contact you if she has questions or issues, and have a tiered approach for working up any problems that she may present with. In addition, reiterate that the patient must use another form of birth control for 3 months until she undergoes the confirmation test and until the results verify that the implants can be relied on for contraception. I am still placing the device. Before I perform the procedure, I speak with my patients—as I did before the checklist was developed—about all of the informed consent issues, the risk−benefit profile, and ruling out contraindications to use. I think this is good medical and surgical practice. The new labeling means we need to have a critical conversation with our patients, and we should be doing that for all procedures.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization: guidance for industry and Food and Drug Administration staff. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM488020.pdf. Published October 31, 2016. Accessed January 5, 2017.
- Essure permanent birth control (Bayer) Instructions for use. http://www.hcp.essure-us.com/assets/pdf/Link_Essure_IFU.pdf. Accessed January 5, 2017.
- Essure patient information booklet. http://labeling.bayerhealthcare.com/html/products/pi/essure_pib_en.pdf. Accessed January 5, 2017.
In November 2016, Bayer, the manufacturer of the permanent birth control tubal implant system (Essure), revised the Essure product labeling in accordance with a US Food and Drug Administration (FDA) guidance document.1 The FDA developed its labeling guidance based on its examination of an increasing number of reported adverse events associated with the system’s use (such as persistent pain, perforation of the uterus and/or fallopian tubes, intra-abdominal or pelvic device migration, abnormal or irregular bleeding, and allergy or hypersensitivity reactions) and its evaluation of a trade complaint regarding allegations initially made in a Citizen Petition.
Changes to the new FDA-approved labeling for Essure include:
- the addition of a boxed warning listing adverse events that have been reported either in clinical studies or through postmarket surveillance (see Box)
- updated Instructions for Use document for clinicians and Patient Information Booklet, which contain additional information on safety (contraindications, warnings, and precautions), clinical data, and instructions2,3
- a Patient-Doctor Discussion Checklist (included within the Patient Information Booklet), designed to support appropriate patient counseling, facilitate the patient’s understanding of birth control options, and explain the benefits and risks associated with the device and what to expect during and after the implantation procedure.3
How will these labeling changes impact clinicians and patients? OBG
Reference
1. Essure permanent birth control (Bayer) Instructions for use. http://www.hcp.essure-us.com/assets/pdf/Link%20Essure%20IFU.pdf. Accessed January 5, 2017.
OBG Management: What does the new product labeling mean for clinicians who offer tubal implants as an option for permanent sterilization?
Linda D. Bradley, MD: The FDA-approved revised labeling for the Essure system means that physicians should have a very detailed, in-depth conversation with their patients who are contemplating hysteroscopic tubal insert placement for permanent sterilization. This counseling really should not differ from what doctors were doing before the label was revised. However, physicians can now use the new Patient-Doctor Discussion Checklist as a guide in reviewing the benefits of the device, its known risks and potential risks, outcomes of the insertion procedure, and the possible need for future surgical intervention if device placement–related issues arise.
For clinicians, this counseling adds just a few more minutes to the visit. The Patient-Doctor Discussion Checklist will become an inherent part of the informed consent process, aiding in the review of the device’s benefits, potential risks, and more importantly its permanence.
In the past, there was some concern that perhaps patients did not receive enough guidance for informed consent, so one of the first things listed on the checklist is confirmation—in the form of a printed line where the patient can sign her initials—that she understands that Essure is a permanent form of birth control. The checklist covers additional important issues, including that the doctor has indeed shared with the patient other options for birth control or sterilization, such as laparoscopic sterilization, vasectomy for her male partner, an intrauterine device (IUD), and birth control pills. This is an opportunity to reinforce the fact that tubal implants are a permanent form of birth control, and if the patient is uncertain about ending her fertility, the clinician can inform her about reversible options. The checklist also includes for discussion the pregnancy risk with use of the device, what the patient can expect during the implant insertion procedure and for the days afterwards (such as cramping, mild to moderate pain, nausea and vomiting), and the need for a confirmation test 3 months after device placement.
Other discussion points covered include long-term risks and benefits of the device, the potential for complications, and the possibility (due to pelvic pain) that the hysteroscopically placed devices may need to be removed with a surgical procedure requiring general anesthesia.
Incorporating the checklist into our clinical practice shows that we have listened to patients and complied with recommendations made by the FDA review panel, and we can use this document to have a more complete discussion with our patients.
OBG Management: Do you agree with some clinicians who say that physicians who place the device also should have the skills required to remove it if necessary?
Dr. Bradley: Essure placement—which is a hysteroscopic procedure—is done very differently than a laparoscopic procedure. In the past, among women who needed to have the Essure system removed, most procedures would be done laparoscopically. Since we work collaboratively in teams, someone within the team or division would have the clinical expertise to remove the devices. An ObGyn who does laparoscopy with salpingectomy and/or cornual resection would best be able to remove the devices.
The clinician who does hysteroscopy is not always the same one who does laparoscopy. Someone within the division who is interested in removing the device will develop an expertise and algorithm that suits the practice, so that person in the practice becomes the expert. This is no different from many other things that physicians do. In our clinical practice, for example, we have a pelvic pain specialist, a sexual counselor, someone interested in menopause and management, and someone interested in alternatives to hysterectomies. Those who practice their craft and their art become proficient at it. So if you do not perform a particular procedure such as a tubal implant removal, know the expert to whom you can make a referral.
OBG Management: How do you now advise your colleagues to counsel patients on permanent sterilization?
Dr. Bradley: Hysteroscopic tubal implant sterilization, a minimally invasive procedure, is an excellent and viable option for women who meet the inclusion criteria and who do not have the exclusion criteria for placement. It is overall safe and extremely effective. If a patient has issues after undergoing implant placement—just like with any other surgery or procedure—for example, if she is not feeling better or is not doing as well as anticipated, we must not forget the patient. It is important for our patients to be listened to and to be heard. Postprocedure issues are generally transient and related to pain and discomfort or abnormal bleeding. If they are persistent, then further evaluation is needed.
Tell the patient to contact you if she has questions or issues, and have a tiered approach for working up any problems that she may present with. In addition, reiterate that the patient must use another form of birth control for 3 months until she undergoes the confirmation test and until the results verify that the implants can be relied on for contraception. I am still placing the device. Before I perform the procedure, I speak with my patients—as I did before the checklist was developed—about all of the informed consent issues, the risk−benefit profile, and ruling out contraindications to use. I think this is good medical and surgical practice. The new labeling means we need to have a critical conversation with our patients, and we should be doing that for all procedures.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In November 2016, Bayer, the manufacturer of the permanent birth control tubal implant system (Essure), revised the Essure product labeling in accordance with a US Food and Drug Administration (FDA) guidance document.1 The FDA developed its labeling guidance based on its examination of an increasing number of reported adverse events associated with the system’s use (such as persistent pain, perforation of the uterus and/or fallopian tubes, intra-abdominal or pelvic device migration, abnormal or irregular bleeding, and allergy or hypersensitivity reactions) and its evaluation of a trade complaint regarding allegations initially made in a Citizen Petition.
Changes to the new FDA-approved labeling for Essure include:
- the addition of a boxed warning listing adverse events that have been reported either in clinical studies or through postmarket surveillance (see Box)
- updated Instructions for Use document for clinicians and Patient Information Booklet, which contain additional information on safety (contraindications, warnings, and precautions), clinical data, and instructions2,3
- a Patient-Doctor Discussion Checklist (included within the Patient Information Booklet), designed to support appropriate patient counseling, facilitate the patient’s understanding of birth control options, and explain the benefits and risks associated with the device and what to expect during and after the implantation procedure.3
How will these labeling changes impact clinicians and patients? OBG
Reference
1. Essure permanent birth control (Bayer) Instructions for use. http://www.hcp.essure-us.com/assets/pdf/Link%20Essure%20IFU.pdf. Accessed January 5, 2017.
OBG Management: What does the new product labeling mean for clinicians who offer tubal implants as an option for permanent sterilization?
Linda D. Bradley, MD: The FDA-approved revised labeling for the Essure system means that physicians should have a very detailed, in-depth conversation with their patients who are contemplating hysteroscopic tubal insert placement for permanent sterilization. This counseling really should not differ from what doctors were doing before the label was revised. However, physicians can now use the new Patient-Doctor Discussion Checklist as a guide in reviewing the benefits of the device, its known risks and potential risks, outcomes of the insertion procedure, and the possible need for future surgical intervention if device placement–related issues arise.
For clinicians, this counseling adds just a few more minutes to the visit. The Patient-Doctor Discussion Checklist will become an inherent part of the informed consent process, aiding in the review of the device’s benefits, potential risks, and more importantly its permanence.
In the past, there was some concern that perhaps patients did not receive enough guidance for informed consent, so one of the first things listed on the checklist is confirmation—in the form of a printed line where the patient can sign her initials—that she understands that Essure is a permanent form of birth control. The checklist covers additional important issues, including that the doctor has indeed shared with the patient other options for birth control or sterilization, such as laparoscopic sterilization, vasectomy for her male partner, an intrauterine device (IUD), and birth control pills. This is an opportunity to reinforce the fact that tubal implants are a permanent form of birth control, and if the patient is uncertain about ending her fertility, the clinician can inform her about reversible options. The checklist also includes for discussion the pregnancy risk with use of the device, what the patient can expect during the implant insertion procedure and for the days afterwards (such as cramping, mild to moderate pain, nausea and vomiting), and the need for a confirmation test 3 months after device placement.
Other discussion points covered include long-term risks and benefits of the device, the potential for complications, and the possibility (due to pelvic pain) that the hysteroscopically placed devices may need to be removed with a surgical procedure requiring general anesthesia.
Incorporating the checklist into our clinical practice shows that we have listened to patients and complied with recommendations made by the FDA review panel, and we can use this document to have a more complete discussion with our patients.
OBG Management: Do you agree with some clinicians who say that physicians who place the device also should have the skills required to remove it if necessary?
Dr. Bradley: Essure placement—which is a hysteroscopic procedure—is done very differently than a laparoscopic procedure. In the past, among women who needed to have the Essure system removed, most procedures would be done laparoscopically. Since we work collaboratively in teams, someone within the team or division would have the clinical expertise to remove the devices. An ObGyn who does laparoscopy with salpingectomy and/or cornual resection would best be able to remove the devices.
The clinician who does hysteroscopy is not always the same one who does laparoscopy. Someone within the division who is interested in removing the device will develop an expertise and algorithm that suits the practice, so that person in the practice becomes the expert. This is no different from many other things that physicians do. In our clinical practice, for example, we have a pelvic pain specialist, a sexual counselor, someone interested in menopause and management, and someone interested in alternatives to hysterectomies. Those who practice their craft and their art become proficient at it. So if you do not perform a particular procedure such as a tubal implant removal, know the expert to whom you can make a referral.
OBG Management: How do you now advise your colleagues to counsel patients on permanent sterilization?
Dr. Bradley: Hysteroscopic tubal implant sterilization, a minimally invasive procedure, is an excellent and viable option for women who meet the inclusion criteria and who do not have the exclusion criteria for placement. It is overall safe and extremely effective. If a patient has issues after undergoing implant placement—just like with any other surgery or procedure—for example, if she is not feeling better or is not doing as well as anticipated, we must not forget the patient. It is important for our patients to be listened to and to be heard. Postprocedure issues are generally transient and related to pain and discomfort or abnormal bleeding. If they are persistent, then further evaluation is needed.
Tell the patient to contact you if she has questions or issues, and have a tiered approach for working up any problems that she may present with. In addition, reiterate that the patient must use another form of birth control for 3 months until she undergoes the confirmation test and until the results verify that the implants can be relied on for contraception. I am still placing the device. Before I perform the procedure, I speak with my patients—as I did before the checklist was developed—about all of the informed consent issues, the risk−benefit profile, and ruling out contraindications to use. I think this is good medical and surgical practice. The new labeling means we need to have a critical conversation with our patients, and we should be doing that for all procedures.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization: guidance for industry and Food and Drug Administration staff. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM488020.pdf. Published October 31, 2016. Accessed January 5, 2017.
- Essure permanent birth control (Bayer) Instructions for use. http://www.hcp.essure-us.com/assets/pdf/Link_Essure_IFU.pdf. Accessed January 5, 2017.
- Essure patient information booklet. http://labeling.bayerhealthcare.com/html/products/pi/essure_pib_en.pdf. Accessed January 5, 2017.
- US Food and Drug Administration. Labeling for permanent hysteroscopically-placed tubal implants intended for sterilization: guidance for industry and Food and Drug Administration staff. http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM488020.pdf. Published October 31, 2016. Accessed January 5, 2017.
- Essure permanent birth control (Bayer) Instructions for use. http://www.hcp.essure-us.com/assets/pdf/Link_Essure_IFU.pdf. Accessed January 5, 2017.
- Essure patient information booklet. http://labeling.bayerhealthcare.com/html/products/pi/essure_pib_en.pdf. Accessed January 5, 2017.
Hysteroscopic myomectomy using a mechanical approach
Uterine fibroids are a common complaint in gynecology, with an incidence of approximately 30% in women aged 25 to 45 years and a cumulative incidence of 70% to 80% by age 50.1,2 They are more prevalent in women of African descent and are a leading indication for hysterectomy.
Although they can be asymptomatic, submucosal fibroids are frequently associated with:
- abnormal uterine bleeding (AUB)
- dysmenorrhea
- expulsion of an intrauterine device (IUD)
- leukorrhea
- pelvic pain
- urinary frequency
- infertility
- premature labor
- reproductive wastage
- bleeding during hormone replacement therapy.
In postmenopausal women, the risk of malignancy in a leiomyoma ranges from 0.2% to 0.5%.1 The risk is lower in premenopausal women.
In this article, I describe the technique for hysteroscopic myomectomy using a mechanical approach (Truclear Tissue Removal System, Smith & Nephew, Andover, MA), which offers hysteroscopic morcellation as well as quick resection and efficient fluid management. (Note: Unlike open intraperitoneal morcellation, hysteroscopic morcellation carries a low risk of tissue spread.)
Classification of fibroids
Preoperative classification of leiomyomas makes it possible to determine the best route for surgery. The most commonly used classification system was developed by the European Society of Gynaecological Endoscopy (ESGE) (FIGURE 1), which considers the extent of intramural extension. Each fibroid under that system is classified as:
- Type 0 – no intramural extension
- Type I – less than 50% extension
- Type II – more than 50% extension.
A second classification system recently was devised to take into account additional features of the fibroid. The STEP-W classification considers size, topography, extension, penetration, and the lateral wall (FIGURE 2). In general, the lower the score, the less complex the procedure will be, with a lower risk of fluid intravasation, shorter operative time, and a greater likelihood of complete removal of the fibroid.
A multicenter, prospective study of 449 women who underwent hysteroscopic resection of their fibroids correlated the ESGE and STEP-W systems. All 320 fibroids (100%) with a score of 4 or below on the STEP-W classification system were completely removed, compared with 112 of 145 fibroids (77.2%) with a score greater than 4. All 33 cases of incomplete hysteroscopic resection (100%) had a STEP-W score above 4.3
In the same study, 85 of 86 cases (98.9%) with Type 0 fibroids under the ESGE system had complete resection, along with 278 of 298 Type I fibroids (93.3%), and 69 of 81 Type II fibroids (85.2%).3 Complete removal is a goal because it relieves symptoms and averts the need for additional procedures.
Patient selection
Proper patient selection for hysteroscopic myomectomy is extremely important. The most common indications are AUB, pelvic pain or discomfort, recurrent pregnancy loss, and infertility. In addition, the patient should have a strong wish for uterine preservation and desire a minimally invasive transcervical approach.
AAGL guidelines on the diagnosis and management of submucous fibroids note that, in general, submucous leiomyomas as large as 4 or 5 cm in diameter can be removed hysteroscopically by experienced surgeons.4
A hysteroscopic approach is not advised for women in whom hysteroscopic surgery is contraindicated, such as women with intrauterine pregnancy, active pelvic infection, active herpes infection, or cervical or uterine cancer. Women who have medical comorbidities such as coronary heart disease, significant renal disease, or bleeding diathesis may need perioperative clearance from anesthesia or hematology prior to hysteroscopic surgery and close fluid monitoring during the procedure.
Consider the leiomyoma
Penetration into the myometrium. Women who have a fibroid that penetrates more than 50% into the myometrium may benefit from hysteroscopic myomectomy, provided the surgeon is highly experienced. A skilled hysteroscopist can ensure complete enucleation of a penetrating fibroid in these cases.
If you are still in the learning process for hysteroscopy, however, start with easier cases—ie, polyps and Type 0 and Type I fibroids. Type II fibroids require longer operative time, are associated with increased fluid absorption and intravasation, carry an increased risk of perioperative complications, and may not always be completely resected.
Size of the fibroid also is relevant. As size increases, so does the volume of tissue needing to be removed, adding to overall operative time.
Presence of other fibroids. When a woman has an intracavitary fibroid as well as myomas in other locations, the surgeon should consider whether hysteroscopic removal of the intracavitary lesion alone can provide significant relief of all fibroid-related symptoms. In such cases, laparoscopic, robotic, or abdominal myomectomy may be preferable, especially if the volume of the additional myomas is considerable.
To determine the optimal surgical route, the physician must consider the symptoms present—is AUB the only symptom, or are other fibroid-related conditions present as well, such as bulk, pelvic pain, and other quality-of-life issues? If multiple symptoms exist, then other approaches may be better.
How fibroids affect fertility
Fibroids are present in 5% to 10% of women with infertility. In this population, fibroids are the only abnormal finding in 1.0% to 2.4% of cases.4
In a meta-analysis of 23 studies evaluating women with fibroids and infertility, Pritts and colleagues found nine studies involving submucosal fibroids.5 These studies included one randomized controlled trial, two prospective studies, and six retrospective analyses. They found that women who had fibroids with a submucosal component had lower pregnancy and implantation rates, compared with their infertile, myoma-free counterparts. Pritts and colleagues concluded that myomectomy is likely to improve fertility in these cases (TABLE).5
Instrumentation
Among the options are monopolar and bipolar resectoscopy and the mechanical approach using the Truclear System, which includes a morcellator. With conventional resectoscopy all chips must be removed, necessitating multiple insertions of the hysteroscope. Monopolar instrumentation, in particular, carries a risk of energy discharge to healthy tissue. The monopolar resectoscope also has a longer learning curve, compared with the mechanical approach.6
In contrast, the Truclear System requires fewer insertions, has a short learning curve, and omits the need for capture of individual chips, as the mechanical morcellator suctions and captures them throughout the procedure.7 In addition, because resection is performed mechanically, there is no risk of energy discharge to healthy tissue.
The Truclear system also is associated with a significantly shorter operative time, compared with resectoscopy, which may be advantageous for residents, fellows, and other physicians learning the procedure (FIGURE 3).7 Shorter operative time also may result in lower fluid deficits. In addition, saline distension may reduce the risk of fluid absorption and hyponatremia. The tissue-capture feature allows evaluation of the entire pathologic specimen.
Besides hysteroscopic myomectomy, the Truclear System is appropriate for visual dilatation and curettage (D&C), adhesiolysis, polypectomy, and evacuation of retained products of conception.
Preoperative evaluation
A complete history is vital to document which fibroid-related symptoms are present and how they affect quality of life.
Preoperative imaging also is imperative—using either 2D or 3D saline infusion sonography or a combination of diagnostic hysteroscopy and transvaginal ultrasound—to select patients for hysteroscopy, anticipate blood loss, and ensure that the proper instrumentation is available at the time of surgery. Magnetic resonance imaging, computed tomography, and hysterosalpingography are either prohibitively expensive or of limited value in the initial preoperative assessment of uterine fibroids.
Any woman who has AUB and a risk for endometrial hyperplasia or cancer should undergo endometrial assessment as well.
Use of preoperative medications
In most cases, prophylactic administration of antibiotics is not warranted to prevent infection or endocarditis.
Although some clinicians give gonadotropin-releasing hormone (GnRH) agonists to reduce the size of large fibroids, the drug complicates dissection of the fibroid from the surrounding capsule. For this reason, and because we lack data demonstrating that GnRH agonists decrease blood loss and limit absorption of distension media, I do not administer them to patients.8–12 Moreover, this drug can cause vasomotor symptoms, cervical stenosis, and vaginal hemorrhage (related to estrogen flare).
GnRH agonists may be of value to stimulate transient amenorrhea for several months preoperatively in order to correct iron-deficiency anemia. Intravenous iron also can be administered during this interval.
The risk of bleeding in hysteroscopic myomectomy is 2% to 3%.1 When the mechanical approach is used, rather than resectoscopy, continuous flow coupled with suctioning of the chips during the procedure keeps the image clear. Post-procedure contraction of the uterus stops most bleeding. Intrauterine pressure of the pump can be increased to help tamponade any oozing.
Misoprostol. Cervical stenosis is not uncommon in menopausal women. It can also pose a challenge in nulliparous women. Attempting hysteroscopy in the setting of cervical stenosis increases the risk of cervical laceration, creation of a false passage, and uterine perforation. For this reason, I prescribe oral or vaginal misoprostol 200 to 400 µg nightly for 1 to 2 days before the procedure.
Vasopressin can reduce blood loss during hysteroscopic myomectomy when it is injected into the cervical stroma preoperatively. It also reduces absorption of distension fluid and facilitates cervical dilation.
However, vasopressin must be injected with extreme care, with aspiration to confirm the absence of blood prior to each injection, as intravascular injection can lead to bradycardia, profound hypertension, and even death.13 Always notify the anesthesiologist prior to injection when vasopressin will be administered.
I routinely use vasopressin before hysteroscopic myomectomy (0.5 mg in 20 cc of saline or 20 U in 100 cc), injecting 5 cc of the solution at 3, 6, 9, and 12 o’clock positions.
Anesthesia during hysteroscopic myomectomy typically is “monitored anesthesia care,” or MAC, which consists of local anesthesia with sedation and analgesia. The need for regional or general anesthesia is rare. Consider adding a pericervical block or intravenous ketorolac (Toradol) to provide postoperative analgesia.
Surgical technique
Strict attention to fluid management is required throughout the procedure, preferably in accordance with AAGL guidelines on the management of hysteroscopic distending media.14 With the mechanical approach, because the distension fluid is isotonic (normal saline), it does not increase the risk of hyponatremia but can cause pulmonary edema or congestive heart failure. Intravasation usually is the result of excessive operative time, treatment of deeper myometrial fibroids (Type I or II), or high intrauterine pressure. I operate using intrauterine pressure in the range of 75 to 125 mm Hg.
The steps involved in the mechanical hysteroscopy approach are:
- Insert the hysteroscope into the uterus under direct visualization. In general, the greater the number of insertions, the greater the risk of uterine perforation. Preoperative cervical ripening helps facilitate insertion (see “Misoprostol” above).
- Distend the uterus with saline and inspect the uterine cavity, noting again the size and location of the fibroids and whether they are sessile or pedunculated.
- Locate the fibroid or other pathology to be removed, and place the morcellator window against it to begin cutting. Use the tip of the morcellator to elevate the fibroid for easier cutting. Enucleation is accomplished largely by varying the intrauterine pressure, which permits uterine decompression and myometrial contraction and renders the fibroid capsule more visible. If necessary, the hysteroscope can be withdrawn to stimulate myometrial contraction, which also helps to delineate the fibroid capsule.
- Reinspect the uterus to rule out perforation and remove any additional intrauterine pathology with a targeted view.
- Once all designated fibroids have been removed, withdraw the morcellator and hysteroscope from the uterus.
- Inspect the endocervical landscape to rule out injury and other pathology.
- Careful preoperative evaluation is important, preferably using diagnostic hysteroscopy or saline infusion sonography, to choose the optimal route of myomectomy and plan the surgical approach.
- During the myomectomy, pay close attention to fluid management and adhere strictly to predetermined limits.
- Complete removal of the fibroid is essential to relieve symptoms and avert the need for additional procedures.
Postoperative care
A nonsteroidal anti-inflammatory drug or limited use of narcotics usually is sufficient to relieve any postoperative cramping or vaginal discomfort.
Advise the patient to notify you in the event of increasing pain, foul-smelling vaginal discharge, or fever.
Also counsel her that she can return to most normal activities within 24 to 48 hours. Sexual activity is permissible 1 week after surgery. Early and frequent ambulation is important.
Schedule a follow-up visit 4 to 6 weeks after the procedure.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Perez-Medina T, Font EC, eds. Diagnostic and Operative Hysteroscopy. Tunbridge Wells, Kent, UK: Anshan Publishing; 2007:13.
2. Management of uterine fibroids: an update of the evidence. Agency for Healthcare Research and Quality. http://archive.ahrq.gov/clinic/tp/uteruptp.htm. Published July 2007. Accessed January 14, 2015.
3. Lasmar RB, Zinmei Z, Indman PD, Celeste RK, Di Spiezo Sardo A. Feasibility of a new system of classification of submucous myomas: a multicenter study. Fertil Steril. 2011;95(6):2073–2077.
4. AAGL Practice Report: practice guidelines for the diagnosis and management of submucous leiomyomas. J Minim Invasive Gynecol. 2012;19(2):152–171.
5. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215–1223.
6. Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15(4):466–471.
7. Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12(1):62–66.
8. Emanuel MH, Hart A, Wamsteker K, Lammes F. An analysis of fluid loss during transcervical resection of submucous myomas. Fertil Steril. 1997;68(5):881–886.
9. Taskin O, Sadik S, Onoglu A, et al. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J Am Assoc Gynecol Laparosc. 2000;7(3):351.
10. Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96(4):517–520.
11. Perino A, Chianchiano N, Petronio M, Cittadini E. Role of leuprolide acetate depot in hysteroscopic surgery: a controlled study. Fertil Steril. 1993;59(3):507–510.
12. Mencaglia L, Tantini C. GnRH agonist analogs and hysteroscopic resection of myomas. Int J Gynaecol Obstet. 1993;43(3):285–288.
13. Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 Pt 2):484–486.
14. Munro MD, Storz K, Abbott JA, et al; AAGL. AAGL Practice Report: practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013;20(2):137–148.
Uterine fibroids are a common complaint in gynecology, with an incidence of approximately 30% in women aged 25 to 45 years and a cumulative incidence of 70% to 80% by age 50.1,2 They are more prevalent in women of African descent and are a leading indication for hysterectomy.
Although they can be asymptomatic, submucosal fibroids are frequently associated with:
- abnormal uterine bleeding (AUB)
- dysmenorrhea
- expulsion of an intrauterine device (IUD)
- leukorrhea
- pelvic pain
- urinary frequency
- infertility
- premature labor
- reproductive wastage
- bleeding during hormone replacement therapy.
In postmenopausal women, the risk of malignancy in a leiomyoma ranges from 0.2% to 0.5%.1 The risk is lower in premenopausal women.
In this article, I describe the technique for hysteroscopic myomectomy using a mechanical approach (Truclear Tissue Removal System, Smith & Nephew, Andover, MA), which offers hysteroscopic morcellation as well as quick resection and efficient fluid management. (Note: Unlike open intraperitoneal morcellation, hysteroscopic morcellation carries a low risk of tissue spread.)
Classification of fibroids
Preoperative classification of leiomyomas makes it possible to determine the best route for surgery. The most commonly used classification system was developed by the European Society of Gynaecological Endoscopy (ESGE) (FIGURE 1), which considers the extent of intramural extension. Each fibroid under that system is classified as:
- Type 0 – no intramural extension
- Type I – less than 50% extension
- Type II – more than 50% extension.
A second classification system recently was devised to take into account additional features of the fibroid. The STEP-W classification considers size, topography, extension, penetration, and the lateral wall (FIGURE 2). In general, the lower the score, the less complex the procedure will be, with a lower risk of fluid intravasation, shorter operative time, and a greater likelihood of complete removal of the fibroid.
A multicenter, prospective study of 449 women who underwent hysteroscopic resection of their fibroids correlated the ESGE and STEP-W systems. All 320 fibroids (100%) with a score of 4 or below on the STEP-W classification system were completely removed, compared with 112 of 145 fibroids (77.2%) with a score greater than 4. All 33 cases of incomplete hysteroscopic resection (100%) had a STEP-W score above 4.3
In the same study, 85 of 86 cases (98.9%) with Type 0 fibroids under the ESGE system had complete resection, along with 278 of 298 Type I fibroids (93.3%), and 69 of 81 Type II fibroids (85.2%).3 Complete removal is a goal because it relieves symptoms and averts the need for additional procedures.
Patient selection
Proper patient selection for hysteroscopic myomectomy is extremely important. The most common indications are AUB, pelvic pain or discomfort, recurrent pregnancy loss, and infertility. In addition, the patient should have a strong wish for uterine preservation and desire a minimally invasive transcervical approach.
AAGL guidelines on the diagnosis and management of submucous fibroids note that, in general, submucous leiomyomas as large as 4 or 5 cm in diameter can be removed hysteroscopically by experienced surgeons.4
A hysteroscopic approach is not advised for women in whom hysteroscopic surgery is contraindicated, such as women with intrauterine pregnancy, active pelvic infection, active herpes infection, or cervical or uterine cancer. Women who have medical comorbidities such as coronary heart disease, significant renal disease, or bleeding diathesis may need perioperative clearance from anesthesia or hematology prior to hysteroscopic surgery and close fluid monitoring during the procedure.
Consider the leiomyoma
Penetration into the myometrium. Women who have a fibroid that penetrates more than 50% into the myometrium may benefit from hysteroscopic myomectomy, provided the surgeon is highly experienced. A skilled hysteroscopist can ensure complete enucleation of a penetrating fibroid in these cases.
If you are still in the learning process for hysteroscopy, however, start with easier cases—ie, polyps and Type 0 and Type I fibroids. Type II fibroids require longer operative time, are associated with increased fluid absorption and intravasation, carry an increased risk of perioperative complications, and may not always be completely resected.
Size of the fibroid also is relevant. As size increases, so does the volume of tissue needing to be removed, adding to overall operative time.
Presence of other fibroids. When a woman has an intracavitary fibroid as well as myomas in other locations, the surgeon should consider whether hysteroscopic removal of the intracavitary lesion alone can provide significant relief of all fibroid-related symptoms. In such cases, laparoscopic, robotic, or abdominal myomectomy may be preferable, especially if the volume of the additional myomas is considerable.
To determine the optimal surgical route, the physician must consider the symptoms present—is AUB the only symptom, or are other fibroid-related conditions present as well, such as bulk, pelvic pain, and other quality-of-life issues? If multiple symptoms exist, then other approaches may be better.
How fibroids affect fertility
Fibroids are present in 5% to 10% of women with infertility. In this population, fibroids are the only abnormal finding in 1.0% to 2.4% of cases.4
In a meta-analysis of 23 studies evaluating women with fibroids and infertility, Pritts and colleagues found nine studies involving submucosal fibroids.5 These studies included one randomized controlled trial, two prospective studies, and six retrospective analyses. They found that women who had fibroids with a submucosal component had lower pregnancy and implantation rates, compared with their infertile, myoma-free counterparts. Pritts and colleagues concluded that myomectomy is likely to improve fertility in these cases (TABLE).5
Instrumentation
Among the options are monopolar and bipolar resectoscopy and the mechanical approach using the Truclear System, which includes a morcellator. With conventional resectoscopy all chips must be removed, necessitating multiple insertions of the hysteroscope. Monopolar instrumentation, in particular, carries a risk of energy discharge to healthy tissue. The monopolar resectoscope also has a longer learning curve, compared with the mechanical approach.6
In contrast, the Truclear System requires fewer insertions, has a short learning curve, and omits the need for capture of individual chips, as the mechanical morcellator suctions and captures them throughout the procedure.7 In addition, because resection is performed mechanically, there is no risk of energy discharge to healthy tissue.
The Truclear system also is associated with a significantly shorter operative time, compared with resectoscopy, which may be advantageous for residents, fellows, and other physicians learning the procedure (FIGURE 3).7 Shorter operative time also may result in lower fluid deficits. In addition, saline distension may reduce the risk of fluid absorption and hyponatremia. The tissue-capture feature allows evaluation of the entire pathologic specimen.
Besides hysteroscopic myomectomy, the Truclear System is appropriate for visual dilatation and curettage (D&C), adhesiolysis, polypectomy, and evacuation of retained products of conception.
Preoperative evaluation
A complete history is vital to document which fibroid-related symptoms are present and how they affect quality of life.
Preoperative imaging also is imperative—using either 2D or 3D saline infusion sonography or a combination of diagnostic hysteroscopy and transvaginal ultrasound—to select patients for hysteroscopy, anticipate blood loss, and ensure that the proper instrumentation is available at the time of surgery. Magnetic resonance imaging, computed tomography, and hysterosalpingography are either prohibitively expensive or of limited value in the initial preoperative assessment of uterine fibroids.
Any woman who has AUB and a risk for endometrial hyperplasia or cancer should undergo endometrial assessment as well.
Use of preoperative medications
In most cases, prophylactic administration of antibiotics is not warranted to prevent infection or endocarditis.
Although some clinicians give gonadotropin-releasing hormone (GnRH) agonists to reduce the size of large fibroids, the drug complicates dissection of the fibroid from the surrounding capsule. For this reason, and because we lack data demonstrating that GnRH agonists decrease blood loss and limit absorption of distension media, I do not administer them to patients.8–12 Moreover, this drug can cause vasomotor symptoms, cervical stenosis, and vaginal hemorrhage (related to estrogen flare).
GnRH agonists may be of value to stimulate transient amenorrhea for several months preoperatively in order to correct iron-deficiency anemia. Intravenous iron also can be administered during this interval.
The risk of bleeding in hysteroscopic myomectomy is 2% to 3%.1 When the mechanical approach is used, rather than resectoscopy, continuous flow coupled with suctioning of the chips during the procedure keeps the image clear. Post-procedure contraction of the uterus stops most bleeding. Intrauterine pressure of the pump can be increased to help tamponade any oozing.
Misoprostol. Cervical stenosis is not uncommon in menopausal women. It can also pose a challenge in nulliparous women. Attempting hysteroscopy in the setting of cervical stenosis increases the risk of cervical laceration, creation of a false passage, and uterine perforation. For this reason, I prescribe oral or vaginal misoprostol 200 to 400 µg nightly for 1 to 2 days before the procedure.
Vasopressin can reduce blood loss during hysteroscopic myomectomy when it is injected into the cervical stroma preoperatively. It also reduces absorption of distension fluid and facilitates cervical dilation.
However, vasopressin must be injected with extreme care, with aspiration to confirm the absence of blood prior to each injection, as intravascular injection can lead to bradycardia, profound hypertension, and even death.13 Always notify the anesthesiologist prior to injection when vasopressin will be administered.
I routinely use vasopressin before hysteroscopic myomectomy (0.5 mg in 20 cc of saline or 20 U in 100 cc), injecting 5 cc of the solution at 3, 6, 9, and 12 o’clock positions.
Anesthesia during hysteroscopic myomectomy typically is “monitored anesthesia care,” or MAC, which consists of local anesthesia with sedation and analgesia. The need for regional or general anesthesia is rare. Consider adding a pericervical block or intravenous ketorolac (Toradol) to provide postoperative analgesia.
Surgical technique
Strict attention to fluid management is required throughout the procedure, preferably in accordance with AAGL guidelines on the management of hysteroscopic distending media.14 With the mechanical approach, because the distension fluid is isotonic (normal saline), it does not increase the risk of hyponatremia but can cause pulmonary edema or congestive heart failure. Intravasation usually is the result of excessive operative time, treatment of deeper myometrial fibroids (Type I or II), or high intrauterine pressure. I operate using intrauterine pressure in the range of 75 to 125 mm Hg.
The steps involved in the mechanical hysteroscopy approach are:
- Insert the hysteroscope into the uterus under direct visualization. In general, the greater the number of insertions, the greater the risk of uterine perforation. Preoperative cervical ripening helps facilitate insertion (see “Misoprostol” above).
- Distend the uterus with saline and inspect the uterine cavity, noting again the size and location of the fibroids and whether they are sessile or pedunculated.
- Locate the fibroid or other pathology to be removed, and place the morcellator window against it to begin cutting. Use the tip of the morcellator to elevate the fibroid for easier cutting. Enucleation is accomplished largely by varying the intrauterine pressure, which permits uterine decompression and myometrial contraction and renders the fibroid capsule more visible. If necessary, the hysteroscope can be withdrawn to stimulate myometrial contraction, which also helps to delineate the fibroid capsule.
- Reinspect the uterus to rule out perforation and remove any additional intrauterine pathology with a targeted view.
- Once all designated fibroids have been removed, withdraw the morcellator and hysteroscope from the uterus.
- Inspect the endocervical landscape to rule out injury and other pathology.
- Careful preoperative evaluation is important, preferably using diagnostic hysteroscopy or saline infusion sonography, to choose the optimal route of myomectomy and plan the surgical approach.
- During the myomectomy, pay close attention to fluid management and adhere strictly to predetermined limits.
- Complete removal of the fibroid is essential to relieve symptoms and avert the need for additional procedures.
Postoperative care
A nonsteroidal anti-inflammatory drug or limited use of narcotics usually is sufficient to relieve any postoperative cramping or vaginal discomfort.
Advise the patient to notify you in the event of increasing pain, foul-smelling vaginal discharge, or fever.
Also counsel her that she can return to most normal activities within 24 to 48 hours. Sexual activity is permissible 1 week after surgery. Early and frequent ambulation is important.
Schedule a follow-up visit 4 to 6 weeks after the procedure.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Uterine fibroids are a common complaint in gynecology, with an incidence of approximately 30% in women aged 25 to 45 years and a cumulative incidence of 70% to 80% by age 50.1,2 They are more prevalent in women of African descent and are a leading indication for hysterectomy.
Although they can be asymptomatic, submucosal fibroids are frequently associated with:
- abnormal uterine bleeding (AUB)
- dysmenorrhea
- expulsion of an intrauterine device (IUD)
- leukorrhea
- pelvic pain
- urinary frequency
- infertility
- premature labor
- reproductive wastage
- bleeding during hormone replacement therapy.
In postmenopausal women, the risk of malignancy in a leiomyoma ranges from 0.2% to 0.5%.1 The risk is lower in premenopausal women.
In this article, I describe the technique for hysteroscopic myomectomy using a mechanical approach (Truclear Tissue Removal System, Smith & Nephew, Andover, MA), which offers hysteroscopic morcellation as well as quick resection and efficient fluid management. (Note: Unlike open intraperitoneal morcellation, hysteroscopic morcellation carries a low risk of tissue spread.)
Classification of fibroids
Preoperative classification of leiomyomas makes it possible to determine the best route for surgery. The most commonly used classification system was developed by the European Society of Gynaecological Endoscopy (ESGE) (FIGURE 1), which considers the extent of intramural extension. Each fibroid under that system is classified as:
- Type 0 – no intramural extension
- Type I – less than 50% extension
- Type II – more than 50% extension.
A second classification system recently was devised to take into account additional features of the fibroid. The STEP-W classification considers size, topography, extension, penetration, and the lateral wall (FIGURE 2). In general, the lower the score, the less complex the procedure will be, with a lower risk of fluid intravasation, shorter operative time, and a greater likelihood of complete removal of the fibroid.
A multicenter, prospective study of 449 women who underwent hysteroscopic resection of their fibroids correlated the ESGE and STEP-W systems. All 320 fibroids (100%) with a score of 4 or below on the STEP-W classification system were completely removed, compared with 112 of 145 fibroids (77.2%) with a score greater than 4. All 33 cases of incomplete hysteroscopic resection (100%) had a STEP-W score above 4.3
In the same study, 85 of 86 cases (98.9%) with Type 0 fibroids under the ESGE system had complete resection, along with 278 of 298 Type I fibroids (93.3%), and 69 of 81 Type II fibroids (85.2%).3 Complete removal is a goal because it relieves symptoms and averts the need for additional procedures.
Patient selection
Proper patient selection for hysteroscopic myomectomy is extremely important. The most common indications are AUB, pelvic pain or discomfort, recurrent pregnancy loss, and infertility. In addition, the patient should have a strong wish for uterine preservation and desire a minimally invasive transcervical approach.
AAGL guidelines on the diagnosis and management of submucous fibroids note that, in general, submucous leiomyomas as large as 4 or 5 cm in diameter can be removed hysteroscopically by experienced surgeons.4
A hysteroscopic approach is not advised for women in whom hysteroscopic surgery is contraindicated, such as women with intrauterine pregnancy, active pelvic infection, active herpes infection, or cervical or uterine cancer. Women who have medical comorbidities such as coronary heart disease, significant renal disease, or bleeding diathesis may need perioperative clearance from anesthesia or hematology prior to hysteroscopic surgery and close fluid monitoring during the procedure.
Consider the leiomyoma
Penetration into the myometrium. Women who have a fibroid that penetrates more than 50% into the myometrium may benefit from hysteroscopic myomectomy, provided the surgeon is highly experienced. A skilled hysteroscopist can ensure complete enucleation of a penetrating fibroid in these cases.
If you are still in the learning process for hysteroscopy, however, start with easier cases—ie, polyps and Type 0 and Type I fibroids. Type II fibroids require longer operative time, are associated with increased fluid absorption and intravasation, carry an increased risk of perioperative complications, and may not always be completely resected.
Size of the fibroid also is relevant. As size increases, so does the volume of tissue needing to be removed, adding to overall operative time.
Presence of other fibroids. When a woman has an intracavitary fibroid as well as myomas in other locations, the surgeon should consider whether hysteroscopic removal of the intracavitary lesion alone can provide significant relief of all fibroid-related symptoms. In such cases, laparoscopic, robotic, or abdominal myomectomy may be preferable, especially if the volume of the additional myomas is considerable.
To determine the optimal surgical route, the physician must consider the symptoms present—is AUB the only symptom, or are other fibroid-related conditions present as well, such as bulk, pelvic pain, and other quality-of-life issues? If multiple symptoms exist, then other approaches may be better.
How fibroids affect fertility
Fibroids are present in 5% to 10% of women with infertility. In this population, fibroids are the only abnormal finding in 1.0% to 2.4% of cases.4
In a meta-analysis of 23 studies evaluating women with fibroids and infertility, Pritts and colleagues found nine studies involving submucosal fibroids.5 These studies included one randomized controlled trial, two prospective studies, and six retrospective analyses. They found that women who had fibroids with a submucosal component had lower pregnancy and implantation rates, compared with their infertile, myoma-free counterparts. Pritts and colleagues concluded that myomectomy is likely to improve fertility in these cases (TABLE).5
Instrumentation
Among the options are monopolar and bipolar resectoscopy and the mechanical approach using the Truclear System, which includes a morcellator. With conventional resectoscopy all chips must be removed, necessitating multiple insertions of the hysteroscope. Monopolar instrumentation, in particular, carries a risk of energy discharge to healthy tissue. The monopolar resectoscope also has a longer learning curve, compared with the mechanical approach.6
In contrast, the Truclear System requires fewer insertions, has a short learning curve, and omits the need for capture of individual chips, as the mechanical morcellator suctions and captures them throughout the procedure.7 In addition, because resection is performed mechanically, there is no risk of energy discharge to healthy tissue.
The Truclear system also is associated with a significantly shorter operative time, compared with resectoscopy, which may be advantageous for residents, fellows, and other physicians learning the procedure (FIGURE 3).7 Shorter operative time also may result in lower fluid deficits. In addition, saline distension may reduce the risk of fluid absorption and hyponatremia. The tissue-capture feature allows evaluation of the entire pathologic specimen.
Besides hysteroscopic myomectomy, the Truclear System is appropriate for visual dilatation and curettage (D&C), adhesiolysis, polypectomy, and evacuation of retained products of conception.
Preoperative evaluation
A complete history is vital to document which fibroid-related symptoms are present and how they affect quality of life.
Preoperative imaging also is imperative—using either 2D or 3D saline infusion sonography or a combination of diagnostic hysteroscopy and transvaginal ultrasound—to select patients for hysteroscopy, anticipate blood loss, and ensure that the proper instrumentation is available at the time of surgery. Magnetic resonance imaging, computed tomography, and hysterosalpingography are either prohibitively expensive or of limited value in the initial preoperative assessment of uterine fibroids.
Any woman who has AUB and a risk for endometrial hyperplasia or cancer should undergo endometrial assessment as well.
Use of preoperative medications
In most cases, prophylactic administration of antibiotics is not warranted to prevent infection or endocarditis.
Although some clinicians give gonadotropin-releasing hormone (GnRH) agonists to reduce the size of large fibroids, the drug complicates dissection of the fibroid from the surrounding capsule. For this reason, and because we lack data demonstrating that GnRH agonists decrease blood loss and limit absorption of distension media, I do not administer them to patients.8–12 Moreover, this drug can cause vasomotor symptoms, cervical stenosis, and vaginal hemorrhage (related to estrogen flare).
GnRH agonists may be of value to stimulate transient amenorrhea for several months preoperatively in order to correct iron-deficiency anemia. Intravenous iron also can be administered during this interval.
The risk of bleeding in hysteroscopic myomectomy is 2% to 3%.1 When the mechanical approach is used, rather than resectoscopy, continuous flow coupled with suctioning of the chips during the procedure keeps the image clear. Post-procedure contraction of the uterus stops most bleeding. Intrauterine pressure of the pump can be increased to help tamponade any oozing.
Misoprostol. Cervical stenosis is not uncommon in menopausal women. It can also pose a challenge in nulliparous women. Attempting hysteroscopy in the setting of cervical stenosis increases the risk of cervical laceration, creation of a false passage, and uterine perforation. For this reason, I prescribe oral or vaginal misoprostol 200 to 400 µg nightly for 1 to 2 days before the procedure.
Vasopressin can reduce blood loss during hysteroscopic myomectomy when it is injected into the cervical stroma preoperatively. It also reduces absorption of distension fluid and facilitates cervical dilation.
However, vasopressin must be injected with extreme care, with aspiration to confirm the absence of blood prior to each injection, as intravascular injection can lead to bradycardia, profound hypertension, and even death.13 Always notify the anesthesiologist prior to injection when vasopressin will be administered.
I routinely use vasopressin before hysteroscopic myomectomy (0.5 mg in 20 cc of saline or 20 U in 100 cc), injecting 5 cc of the solution at 3, 6, 9, and 12 o’clock positions.
Anesthesia during hysteroscopic myomectomy typically is “monitored anesthesia care,” or MAC, which consists of local anesthesia with sedation and analgesia. The need for regional or general anesthesia is rare. Consider adding a pericervical block or intravenous ketorolac (Toradol) to provide postoperative analgesia.
Surgical technique
Strict attention to fluid management is required throughout the procedure, preferably in accordance with AAGL guidelines on the management of hysteroscopic distending media.14 With the mechanical approach, because the distension fluid is isotonic (normal saline), it does not increase the risk of hyponatremia but can cause pulmonary edema or congestive heart failure. Intravasation usually is the result of excessive operative time, treatment of deeper myometrial fibroids (Type I or II), or high intrauterine pressure. I operate using intrauterine pressure in the range of 75 to 125 mm Hg.
The steps involved in the mechanical hysteroscopy approach are:
- Insert the hysteroscope into the uterus under direct visualization. In general, the greater the number of insertions, the greater the risk of uterine perforation. Preoperative cervical ripening helps facilitate insertion (see “Misoprostol” above).
- Distend the uterus with saline and inspect the uterine cavity, noting again the size and location of the fibroids and whether they are sessile or pedunculated.
- Locate the fibroid or other pathology to be removed, and place the morcellator window against it to begin cutting. Use the tip of the morcellator to elevate the fibroid for easier cutting. Enucleation is accomplished largely by varying the intrauterine pressure, which permits uterine decompression and myometrial contraction and renders the fibroid capsule more visible. If necessary, the hysteroscope can be withdrawn to stimulate myometrial contraction, which also helps to delineate the fibroid capsule.
- Reinspect the uterus to rule out perforation and remove any additional intrauterine pathology with a targeted view.
- Once all designated fibroids have been removed, withdraw the morcellator and hysteroscope from the uterus.
- Inspect the endocervical landscape to rule out injury and other pathology.
- Careful preoperative evaluation is important, preferably using diagnostic hysteroscopy or saline infusion sonography, to choose the optimal route of myomectomy and plan the surgical approach.
- During the myomectomy, pay close attention to fluid management and adhere strictly to predetermined limits.
- Complete removal of the fibroid is essential to relieve symptoms and avert the need for additional procedures.
Postoperative care
A nonsteroidal anti-inflammatory drug or limited use of narcotics usually is sufficient to relieve any postoperative cramping or vaginal discomfort.
Advise the patient to notify you in the event of increasing pain, foul-smelling vaginal discharge, or fever.
Also counsel her that she can return to most normal activities within 24 to 48 hours. Sexual activity is permissible 1 week after surgery. Early and frequent ambulation is important.
Schedule a follow-up visit 4 to 6 weeks after the procedure.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Perez-Medina T, Font EC, eds. Diagnostic and Operative Hysteroscopy. Tunbridge Wells, Kent, UK: Anshan Publishing; 2007:13.
2. Management of uterine fibroids: an update of the evidence. Agency for Healthcare Research and Quality. http://archive.ahrq.gov/clinic/tp/uteruptp.htm. Published July 2007. Accessed January 14, 2015.
3. Lasmar RB, Zinmei Z, Indman PD, Celeste RK, Di Spiezo Sardo A. Feasibility of a new system of classification of submucous myomas: a multicenter study. Fertil Steril. 2011;95(6):2073–2077.
4. AAGL Practice Report: practice guidelines for the diagnosis and management of submucous leiomyomas. J Minim Invasive Gynecol. 2012;19(2):152–171.
5. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215–1223.
6. Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15(4):466–471.
7. Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12(1):62–66.
8. Emanuel MH, Hart A, Wamsteker K, Lammes F. An analysis of fluid loss during transcervical resection of submucous myomas. Fertil Steril. 1997;68(5):881–886.
9. Taskin O, Sadik S, Onoglu A, et al. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J Am Assoc Gynecol Laparosc. 2000;7(3):351.
10. Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96(4):517–520.
11. Perino A, Chianchiano N, Petronio M, Cittadini E. Role of leuprolide acetate depot in hysteroscopic surgery: a controlled study. Fertil Steril. 1993;59(3):507–510.
12. Mencaglia L, Tantini C. GnRH agonist analogs and hysteroscopic resection of myomas. Int J Gynaecol Obstet. 1993;43(3):285–288.
13. Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 Pt 2):484–486.
14. Munro MD, Storz K, Abbott JA, et al; AAGL. AAGL Practice Report: practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013;20(2):137–148.
1. Perez-Medina T, Font EC, eds. Diagnostic and Operative Hysteroscopy. Tunbridge Wells, Kent, UK: Anshan Publishing; 2007:13.
2. Management of uterine fibroids: an update of the evidence. Agency for Healthcare Research and Quality. http://archive.ahrq.gov/clinic/tp/uteruptp.htm. Published July 2007. Accessed January 14, 2015.
3. Lasmar RB, Zinmei Z, Indman PD, Celeste RK, Di Spiezo Sardo A. Feasibility of a new system of classification of submucous myomas: a multicenter study. Fertil Steril. 2011;95(6):2073–2077.
4. AAGL Practice Report: practice guidelines for the diagnosis and management of submucous leiomyomas. J Minim Invasive Gynecol. 2012;19(2):152–171.
5. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009;91(4):1215–1223.
6. Van Dongen H, Emanuel MH, Wolterbeek R, Trimbos JB, Jansen FW. Hysteroscopic morcellator for removal of intrauterine polyps and myomas: a randomized controlled pilot study among residents in training. J Minim Invasive Gynecol. 2008;15(4):466–471.
7. Emanuel MH, Wamsteker K. The intra uterine morcellator: a new hysteroscopic operating technique to remove intrauterine polyps and myomas. J Minim Invasive Gynecol. 2005;12(1):62–66.
8. Emanuel MH, Hart A, Wamsteker K, Lammes F. An analysis of fluid loss during transcervical resection of submucous myomas. Fertil Steril. 1997;68(5):881–886.
9. Taskin O, Sadik S, Onoglu A, et al. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J Am Assoc Gynecol Laparosc. 2000;7(3):351.
10. Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96(4):517–520.
11. Perino A, Chianchiano N, Petronio M, Cittadini E. Role of leuprolide acetate depot in hysteroscopic surgery: a controlled study. Fertil Steril. 1993;59(3):507–510.
12. Mencaglia L, Tantini C. GnRH agonist analogs and hysteroscopic resection of myomas. Int J Gynaecol Obstet. 1993;43(3):285–288.
13. Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 Pt 2):484–486.
14. Munro MD, Storz K, Abbott JA, et al; AAGL. AAGL Practice Report: practice guidelines for the management of hysteroscopic distending media. J Minim Invasive Gynecol. 2013;20(2):137–148.
Tissue extraction during minimally invasive Gyn surgery. Second of 2 Parts: Counseling the patient
In the absence of a definitive FDA decision on the future of power morcellation in minimally invasive gynecologic surgery, many surgeons have stopped offering the option, often in response to constraints placed by their institutions, or have greatly expanded the informed consent discussion.
In Part 1 of this two-part roundtable discussion, which appeared in the September 2014 issue of OBG Management, our expert panelists discussed their current approach to tissue extraction during hysterectomy and myomectomy, as well as their preferred approach to both procedures amid this changing surgical environment. Here, in Part 2, they discuss patient counseling and the likely effects of FDA action.
How has your counseling changed?
OBG Management: Given recent concerns about the use of power morcellation, how has your counseling of the patient changed?
Kimberly Kho, MD, MPH: Though I look forward to the development of instruments and techniques that will make contained power morcellation safer, I am not using it currently and have been able to find minimally invasive alternatives such as minilaparotomy and vaginal removal of masses for the cases I would have considered for power morcellation.
Certainly, with power morcellation or any type of morcellation, it’s important to discuss the risks and benefits, as well as alternatives. Discussion should include the potential for:
- iatrogenic injury and tissue seeding of both benign and malignant tissue
- exacerbation of any occult malignancy and possible worsening of prognosis
- missing or mischaracterizing an occult malignancy.
Although there is no surefire way to avoid cellular dissemination with any type of surgery, I think it’s equally important to explain that, often, the only way to completely avoid fragmenting a large mass is to remove it en bloc, which would mean a large laparotomy for many patients. Women should understand the risks of laparotomy as well, including more frequent wound complications, longer hospitalization, and slower recovery.
Arnold P. Advincula, MD: If a clinician anticipates or plans the use of power morcellation, he or she certainly needs to go through an informed consent process with the patient. This process may include a separate form specific to power morcellation as well as detailed documentation during the preoperative visit.
OBG Management: What elements of the preoperative visit do you believe are important to document?
Dr. Advincula: It is important to clearly document the indications and alternatives for the surgery, as well as the decision-making process that led to the selection of a particular procedure and route of access. If any type of morcellation (power-driven or not) is anticipated, then the risks associated with it must be thoroughly discussed and documented in addition to the standard risks associated with any type of abdominal-pelvic surgery. No surgical procedure is without risks. Therefore, the process of informed consent cannot be taken lightly and is a critical part of the process that allows a patient to decide upon a particular intervention.
Jason D. Wright, MD: I believe the current role of power morcellation is limited. Patients considering the procedure should be counseled about the risks of cancer as well as other adverse pathologic abnormalities, including smooth muscle tumors of uncertain malignant potential, disseminated leiomyomatosis, and endometrial hyperplasia that may be associated with an occult cancer.
OBG Management: Do you recommend a separate consent form for power morcellation, as Dr. Advincula suggested?
Dr. Wright: Given the risk of adverse pathology, I think the role of electric power morcellation is limited. Patients should be carefully counseled about alternative surgical approaches that avoid tissue disruption and understand that the sensitivity of preoperative testing and intraoperative evaluation of smooth muscle neoplasms is limited. Further, patients considering contained morcellation also should be informed that the data examining the efficacy of these techniques are sparse.
Linda D. Bradley, MD: As I mentioned in Part 1 of our discussion, I’m giving patients new information about our concerns regarding occult malignancy, quoting the risk estimates given by the FDA this year.1 And the fact that we no longer use power morcellation at the Cleveland Clinic means that I no longer discuss it as an option, although one or two patients have asked for it in recent months.
I think many patients have read about it in the news or, once hysterectomy or myomectomy was planned, found discussion of the controversy surrounding it during their research. I’ve even had patients who underwent hysteroscopic myomectomy 2 or more years ago contacting me to find out whether power morcellation was used, and I have had to explain that hysteroscopic morcellation is different from the laparoscopic variant.
Patients are critical readers and are much more knowledgeable as a result of social media, so I do find myself spending more time discussing their procedure with them.
For myomectomy in particular, we send for a frozen section intraoperatively. Although that approach still is not 100% sensitive, it does guide what we do during surgery. If a sarcoma is found, for example, we call in the oncologists. I discuss that possibility with the patient as well. So I am spending more time with patients, but I don’t go into power morcellation because that is no longer an option for me.
OBG Management: Dr. Iglesia, has your counseling of patients changed in any way?
Cheryl Iglesia, MD: I do not routinely use power morcellation. However, the findings from the FDA and Dr. Wright about the higher risk of occult malignancy in fibroids is information I share with patients preoperatively.1,2
For women with fibroids who want uterine conservation procedures or who desire medical management, such as focused ultrasound or uterine fibroid embolization, MRI is routine. However, we make patients aware that this imaging modality is not 100% sensitive in detecting occult cancer—and neither are random biopsies of fibroids. Patients also need to be made aware that treatment with fibroid embolization or other medical options also could delay the detection of cancer and sarcoma. Any morcellation technique (power, hand, vaginal) does have the risk of potential cancer spread and upstaging, so morcellation should not be used in any women with suspected or known malignancy.
Effects of likely FDA actions
OBG Management: If the FDA decides to ban power morcellation outright, in some ways the approach to patient counseling will be simpler, as one option will have been permanently eliminated. But if the FDA allows power morcellation to continue, with stricter labeling, would that affect how you counsel patients? And would you reconsider power morcellation in that light?
Dr. Kho: I think the current discussion has highlighted again how important the informed consent process is as an opportunity for information sharing. It’s an ongoing discussion of risks, benefits, and alternatives. It also offers us an opportunity to understand the patient’s values and perspectives throughout the process of surgical planning. So, no, I don’t think the FDA’s actions will change how I counsel patients. Regardless of the FDA’s decisions, I think open power morcellation as we currently know it may be obviated as new instruments for contained morcellation—as well as other techniques we’ve discussed—become more popular. But it’s critical that we meaningfully monitor these techniques for long-term safety. In order to make evidence-based decisions, we will need good data.
Dr. Iglesia: I cannot comment on a final FDA decision. However, my feeling is that any information that patients can use to become educated about treatment alternatives—including the risks and benefits of each option—will help inform and improve the shared decision-making process.
Dr. Advincula: Regardless of the verdict rendered by the FDA, the way we approach tissue extraction in minimally invasive surgery has been changed forever. It is always important to take a critical look at the way things are done, but not at the expense of throwing the proverbial baby out with the bath water. If power morcellation were to remain a viable option, my counseling would remain as is, as it already has been modified and quite detailed in the wake of this whole controversy. I still believe there is a role for power morcellation, albeit modified from its current iteration, when applied by the right physician in a properly evaluated patient with the right indication.
Summing up
OBG Management: Do you have any additional comments about this issue?
Dr. Advincula: The ability to accurately and reliably detect an occult uterine malignancy—specifically, leiomyosarcoma—is lacking at present. Whether or not power morcellation remains a viable option in the future, the bottom line is that patients will still present with occult uterine malignancy. Minimizing the mishandling of this unfortunate diagnosis will depend on sound clinical judgment as well as improvements in diagnosis. It always will be important to avoid blaming the lack of sound clinical practice on surgical devices that, when used appropriately, have the potential to benefit the majority of women.
Dr. Kho: The current attention on power morcellators presents an opportunity to improve upon our current practices and find solutions to the issues we are encountering. I think this is an exciting time for examining preoperative risk stratification, the innovation of new techniques, repopularization and improvement of older ones such as vaginal tissue extraction, and, overall, to improve our system of safety monitoring and surgical device surveillance.
Dr. Iglesia: Intraperitoneal power morcellation should not be used in cases of malignancy or suspected malignancy or in postmenopausal patients with bleeding or growing fibroids. The availability of power morcellators may be limited as manufacturers
cease distribution, hospitals ban use, or insurers refuse payment for use.
Alternative minimally invasive approaches—especially the transvaginal approach—should be considered, since there are fewer complications associated with vaginal surgery, especially compared with open and laparoscopic surgery.
Dr. Wright: Although electric power morcellation may allow some women to undergo a minimally invasive procedure, the data currently available clearly suggest that adverse pathology is more common in women who undergo morcellation than was previously thought.
Although the debate around morcellation has focused on leiomyosarcoma, epithelial endometrial tumors and other preinvasive abnormalities are also common. These unexpected pathologic findings in women who underwent electric power morcellation highlight the importance of performing more rigorous evaluation of new methods of tissue extraction.
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page, and then see how your peers have voted. Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication.http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed September 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi:10.1001/jama.2014.9005.
In the absence of a definitive FDA decision on the future of power morcellation in minimally invasive gynecologic surgery, many surgeons have stopped offering the option, often in response to constraints placed by their institutions, or have greatly expanded the informed consent discussion.
In Part 1 of this two-part roundtable discussion, which appeared in the September 2014 issue of OBG Management, our expert panelists discussed their current approach to tissue extraction during hysterectomy and myomectomy, as well as their preferred approach to both procedures amid this changing surgical environment. Here, in Part 2, they discuss patient counseling and the likely effects of FDA action.
How has your counseling changed?
OBG Management: Given recent concerns about the use of power morcellation, how has your counseling of the patient changed?
Kimberly Kho, MD, MPH: Though I look forward to the development of instruments and techniques that will make contained power morcellation safer, I am not using it currently and have been able to find minimally invasive alternatives such as minilaparotomy and vaginal removal of masses for the cases I would have considered for power morcellation.
Certainly, with power morcellation or any type of morcellation, it’s important to discuss the risks and benefits, as well as alternatives. Discussion should include the potential for:
- iatrogenic injury and tissue seeding of both benign and malignant tissue
- exacerbation of any occult malignancy and possible worsening of prognosis
- missing or mischaracterizing an occult malignancy.
Although there is no surefire way to avoid cellular dissemination with any type of surgery, I think it’s equally important to explain that, often, the only way to completely avoid fragmenting a large mass is to remove it en bloc, which would mean a large laparotomy for many patients. Women should understand the risks of laparotomy as well, including more frequent wound complications, longer hospitalization, and slower recovery.
Arnold P. Advincula, MD: If a clinician anticipates or plans the use of power morcellation, he or she certainly needs to go through an informed consent process with the patient. This process may include a separate form specific to power morcellation as well as detailed documentation during the preoperative visit.
OBG Management: What elements of the preoperative visit do you believe are important to document?
Dr. Advincula: It is important to clearly document the indications and alternatives for the surgery, as well as the decision-making process that led to the selection of a particular procedure and route of access. If any type of morcellation (power-driven or not) is anticipated, then the risks associated with it must be thoroughly discussed and documented in addition to the standard risks associated with any type of abdominal-pelvic surgery. No surgical procedure is without risks. Therefore, the process of informed consent cannot be taken lightly and is a critical part of the process that allows a patient to decide upon a particular intervention.
Jason D. Wright, MD: I believe the current role of power morcellation is limited. Patients considering the procedure should be counseled about the risks of cancer as well as other adverse pathologic abnormalities, including smooth muscle tumors of uncertain malignant potential, disseminated leiomyomatosis, and endometrial hyperplasia that may be associated with an occult cancer.
OBG Management: Do you recommend a separate consent form for power morcellation, as Dr. Advincula suggested?
Dr. Wright: Given the risk of adverse pathology, I think the role of electric power morcellation is limited. Patients should be carefully counseled about alternative surgical approaches that avoid tissue disruption and understand that the sensitivity of preoperative testing and intraoperative evaluation of smooth muscle neoplasms is limited. Further, patients considering contained morcellation also should be informed that the data examining the efficacy of these techniques are sparse.
Linda D. Bradley, MD: As I mentioned in Part 1 of our discussion, I’m giving patients new information about our concerns regarding occult malignancy, quoting the risk estimates given by the FDA this year.1 And the fact that we no longer use power morcellation at the Cleveland Clinic means that I no longer discuss it as an option, although one or two patients have asked for it in recent months.
I think many patients have read about it in the news or, once hysterectomy or myomectomy was planned, found discussion of the controversy surrounding it during their research. I’ve even had patients who underwent hysteroscopic myomectomy 2 or more years ago contacting me to find out whether power morcellation was used, and I have had to explain that hysteroscopic morcellation is different from the laparoscopic variant.
Patients are critical readers and are much more knowledgeable as a result of social media, so I do find myself spending more time discussing their procedure with them.
For myomectomy in particular, we send for a frozen section intraoperatively. Although that approach still is not 100% sensitive, it does guide what we do during surgery. If a sarcoma is found, for example, we call in the oncologists. I discuss that possibility with the patient as well. So I am spending more time with patients, but I don’t go into power morcellation because that is no longer an option for me.
OBG Management: Dr. Iglesia, has your counseling of patients changed in any way?
Cheryl Iglesia, MD: I do not routinely use power morcellation. However, the findings from the FDA and Dr. Wright about the higher risk of occult malignancy in fibroids is information I share with patients preoperatively.1,2
For women with fibroids who want uterine conservation procedures or who desire medical management, such as focused ultrasound or uterine fibroid embolization, MRI is routine. However, we make patients aware that this imaging modality is not 100% sensitive in detecting occult cancer—and neither are random biopsies of fibroids. Patients also need to be made aware that treatment with fibroid embolization or other medical options also could delay the detection of cancer and sarcoma. Any morcellation technique (power, hand, vaginal) does have the risk of potential cancer spread and upstaging, so morcellation should not be used in any women with suspected or known malignancy.
Effects of likely FDA actions
OBG Management: If the FDA decides to ban power morcellation outright, in some ways the approach to patient counseling will be simpler, as one option will have been permanently eliminated. But if the FDA allows power morcellation to continue, with stricter labeling, would that affect how you counsel patients? And would you reconsider power morcellation in that light?
Dr. Kho: I think the current discussion has highlighted again how important the informed consent process is as an opportunity for information sharing. It’s an ongoing discussion of risks, benefits, and alternatives. It also offers us an opportunity to understand the patient’s values and perspectives throughout the process of surgical planning. So, no, I don’t think the FDA’s actions will change how I counsel patients. Regardless of the FDA’s decisions, I think open power morcellation as we currently know it may be obviated as new instruments for contained morcellation—as well as other techniques we’ve discussed—become more popular. But it’s critical that we meaningfully monitor these techniques for long-term safety. In order to make evidence-based decisions, we will need good data.
Dr. Iglesia: I cannot comment on a final FDA decision. However, my feeling is that any information that patients can use to become educated about treatment alternatives—including the risks and benefits of each option—will help inform and improve the shared decision-making process.
Dr. Advincula: Regardless of the verdict rendered by the FDA, the way we approach tissue extraction in minimally invasive surgery has been changed forever. It is always important to take a critical look at the way things are done, but not at the expense of throwing the proverbial baby out with the bath water. If power morcellation were to remain a viable option, my counseling would remain as is, as it already has been modified and quite detailed in the wake of this whole controversy. I still believe there is a role for power morcellation, albeit modified from its current iteration, when applied by the right physician in a properly evaluated patient with the right indication.
Summing up
OBG Management: Do you have any additional comments about this issue?
Dr. Advincula: The ability to accurately and reliably detect an occult uterine malignancy—specifically, leiomyosarcoma—is lacking at present. Whether or not power morcellation remains a viable option in the future, the bottom line is that patients will still present with occult uterine malignancy. Minimizing the mishandling of this unfortunate diagnosis will depend on sound clinical judgment as well as improvements in diagnosis. It always will be important to avoid blaming the lack of sound clinical practice on surgical devices that, when used appropriately, have the potential to benefit the majority of women.
Dr. Kho: The current attention on power morcellators presents an opportunity to improve upon our current practices and find solutions to the issues we are encountering. I think this is an exciting time for examining preoperative risk stratification, the innovation of new techniques, repopularization and improvement of older ones such as vaginal tissue extraction, and, overall, to improve our system of safety monitoring and surgical device surveillance.
Dr. Iglesia: Intraperitoneal power morcellation should not be used in cases of malignancy or suspected malignancy or in postmenopausal patients with bleeding or growing fibroids. The availability of power morcellators may be limited as manufacturers
cease distribution, hospitals ban use, or insurers refuse payment for use.
Alternative minimally invasive approaches—especially the transvaginal approach—should be considered, since there are fewer complications associated with vaginal surgery, especially compared with open and laparoscopic surgery.
Dr. Wright: Although electric power morcellation may allow some women to undergo a minimally invasive procedure, the data currently available clearly suggest that adverse pathology is more common in women who undergo morcellation than was previously thought.
Although the debate around morcellation has focused on leiomyosarcoma, epithelial endometrial tumors and other preinvasive abnormalities are also common. These unexpected pathologic findings in women who underwent electric power morcellation highlight the importance of performing more rigorous evaluation of new methods of tissue extraction.
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page, and then see how your peers have voted. Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In the absence of a definitive FDA decision on the future of power morcellation in minimally invasive gynecologic surgery, many surgeons have stopped offering the option, often in response to constraints placed by their institutions, or have greatly expanded the informed consent discussion.
In Part 1 of this two-part roundtable discussion, which appeared in the September 2014 issue of OBG Management, our expert panelists discussed their current approach to tissue extraction during hysterectomy and myomectomy, as well as their preferred approach to both procedures amid this changing surgical environment. Here, in Part 2, they discuss patient counseling and the likely effects of FDA action.
How has your counseling changed?
OBG Management: Given recent concerns about the use of power morcellation, how has your counseling of the patient changed?
Kimberly Kho, MD, MPH: Though I look forward to the development of instruments and techniques that will make contained power morcellation safer, I am not using it currently and have been able to find minimally invasive alternatives such as minilaparotomy and vaginal removal of masses for the cases I would have considered for power morcellation.
Certainly, with power morcellation or any type of morcellation, it’s important to discuss the risks and benefits, as well as alternatives. Discussion should include the potential for:
- iatrogenic injury and tissue seeding of both benign and malignant tissue
- exacerbation of any occult malignancy and possible worsening of prognosis
- missing or mischaracterizing an occult malignancy.
Although there is no surefire way to avoid cellular dissemination with any type of surgery, I think it’s equally important to explain that, often, the only way to completely avoid fragmenting a large mass is to remove it en bloc, which would mean a large laparotomy for many patients. Women should understand the risks of laparotomy as well, including more frequent wound complications, longer hospitalization, and slower recovery.
Arnold P. Advincula, MD: If a clinician anticipates or plans the use of power morcellation, he or she certainly needs to go through an informed consent process with the patient. This process may include a separate form specific to power morcellation as well as detailed documentation during the preoperative visit.
OBG Management: What elements of the preoperative visit do you believe are important to document?
Dr. Advincula: It is important to clearly document the indications and alternatives for the surgery, as well as the decision-making process that led to the selection of a particular procedure and route of access. If any type of morcellation (power-driven or not) is anticipated, then the risks associated with it must be thoroughly discussed and documented in addition to the standard risks associated with any type of abdominal-pelvic surgery. No surgical procedure is without risks. Therefore, the process of informed consent cannot be taken lightly and is a critical part of the process that allows a patient to decide upon a particular intervention.
Jason D. Wright, MD: I believe the current role of power morcellation is limited. Patients considering the procedure should be counseled about the risks of cancer as well as other adverse pathologic abnormalities, including smooth muscle tumors of uncertain malignant potential, disseminated leiomyomatosis, and endometrial hyperplasia that may be associated with an occult cancer.
OBG Management: Do you recommend a separate consent form for power morcellation, as Dr. Advincula suggested?
Dr. Wright: Given the risk of adverse pathology, I think the role of electric power morcellation is limited. Patients should be carefully counseled about alternative surgical approaches that avoid tissue disruption and understand that the sensitivity of preoperative testing and intraoperative evaluation of smooth muscle neoplasms is limited. Further, patients considering contained morcellation also should be informed that the data examining the efficacy of these techniques are sparse.
Linda D. Bradley, MD: As I mentioned in Part 1 of our discussion, I’m giving patients new information about our concerns regarding occult malignancy, quoting the risk estimates given by the FDA this year.1 And the fact that we no longer use power morcellation at the Cleveland Clinic means that I no longer discuss it as an option, although one or two patients have asked for it in recent months.
I think many patients have read about it in the news or, once hysterectomy or myomectomy was planned, found discussion of the controversy surrounding it during their research. I’ve even had patients who underwent hysteroscopic myomectomy 2 or more years ago contacting me to find out whether power morcellation was used, and I have had to explain that hysteroscopic morcellation is different from the laparoscopic variant.
Patients are critical readers and are much more knowledgeable as a result of social media, so I do find myself spending more time discussing their procedure with them.
For myomectomy in particular, we send for a frozen section intraoperatively. Although that approach still is not 100% sensitive, it does guide what we do during surgery. If a sarcoma is found, for example, we call in the oncologists. I discuss that possibility with the patient as well. So I am spending more time with patients, but I don’t go into power morcellation because that is no longer an option for me.
OBG Management: Dr. Iglesia, has your counseling of patients changed in any way?
Cheryl Iglesia, MD: I do not routinely use power morcellation. However, the findings from the FDA and Dr. Wright about the higher risk of occult malignancy in fibroids is information I share with patients preoperatively.1,2
For women with fibroids who want uterine conservation procedures or who desire medical management, such as focused ultrasound or uterine fibroid embolization, MRI is routine. However, we make patients aware that this imaging modality is not 100% sensitive in detecting occult cancer—and neither are random biopsies of fibroids. Patients also need to be made aware that treatment with fibroid embolization or other medical options also could delay the detection of cancer and sarcoma. Any morcellation technique (power, hand, vaginal) does have the risk of potential cancer spread and upstaging, so morcellation should not be used in any women with suspected or known malignancy.
Effects of likely FDA actions
OBG Management: If the FDA decides to ban power morcellation outright, in some ways the approach to patient counseling will be simpler, as one option will have been permanently eliminated. But if the FDA allows power morcellation to continue, with stricter labeling, would that affect how you counsel patients? And would you reconsider power morcellation in that light?
Dr. Kho: I think the current discussion has highlighted again how important the informed consent process is as an opportunity for information sharing. It’s an ongoing discussion of risks, benefits, and alternatives. It also offers us an opportunity to understand the patient’s values and perspectives throughout the process of surgical planning. So, no, I don’t think the FDA’s actions will change how I counsel patients. Regardless of the FDA’s decisions, I think open power morcellation as we currently know it may be obviated as new instruments for contained morcellation—as well as other techniques we’ve discussed—become more popular. But it’s critical that we meaningfully monitor these techniques for long-term safety. In order to make evidence-based decisions, we will need good data.
Dr. Iglesia: I cannot comment on a final FDA decision. However, my feeling is that any information that patients can use to become educated about treatment alternatives—including the risks and benefits of each option—will help inform and improve the shared decision-making process.
Dr. Advincula: Regardless of the verdict rendered by the FDA, the way we approach tissue extraction in minimally invasive surgery has been changed forever. It is always important to take a critical look at the way things are done, but not at the expense of throwing the proverbial baby out with the bath water. If power morcellation were to remain a viable option, my counseling would remain as is, as it already has been modified and quite detailed in the wake of this whole controversy. I still believe there is a role for power morcellation, albeit modified from its current iteration, when applied by the right physician in a properly evaluated patient with the right indication.
Summing up
OBG Management: Do you have any additional comments about this issue?
Dr. Advincula: The ability to accurately and reliably detect an occult uterine malignancy—specifically, leiomyosarcoma—is lacking at present. Whether or not power morcellation remains a viable option in the future, the bottom line is that patients will still present with occult uterine malignancy. Minimizing the mishandling of this unfortunate diagnosis will depend on sound clinical judgment as well as improvements in diagnosis. It always will be important to avoid blaming the lack of sound clinical practice on surgical devices that, when used appropriately, have the potential to benefit the majority of women.
Dr. Kho: The current attention on power morcellators presents an opportunity to improve upon our current practices and find solutions to the issues we are encountering. I think this is an exciting time for examining preoperative risk stratification, the innovation of new techniques, repopularization and improvement of older ones such as vaginal tissue extraction, and, overall, to improve our system of safety monitoring and surgical device surveillance.
Dr. Iglesia: Intraperitoneal power morcellation should not be used in cases of malignancy or suspected malignancy or in postmenopausal patients with bleeding or growing fibroids. The availability of power morcellators may be limited as manufacturers
cease distribution, hospitals ban use, or insurers refuse payment for use.
Alternative minimally invasive approaches—especially the transvaginal approach—should be considered, since there are fewer complications associated with vaginal surgery, especially compared with open and laparoscopic surgery.
Dr. Wright: Although electric power morcellation may allow some women to undergo a minimally invasive procedure, the data currently available clearly suggest that adverse pathology is more common in women who undergo morcellation than was previously thought.
Although the debate around morcellation has focused on leiomyosarcoma, epithelial endometrial tumors and other preinvasive abnormalities are also common. These unexpected pathologic findings in women who underwent electric power morcellation highlight the importance of performing more rigorous evaluation of new methods of tissue extraction.
Quick Poll:
If you are using, plan to use, or anticipate the possibility of using power morcellation during minimally invasive gynecologic surgery, does your consent process include a separate form specific to power morcellation?
Please provide your answer to this question in the Quick Poll on the OBG Management home page, and then see how your peers have voted. Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication.http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed September 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi:10.1001/jama.2014.9005.
1. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication.http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed September 18, 2014.
2. Wright JD, Tergas AI, Burke WM, et al. Uterine pathology in women undergoing minimally invasive hysterectomy with morcellation [published online ahead of print July 22, 2014]. JAMA. doi:10.1001/jama.2014.9005.
Does misoprostol have value in reducing pain during outpatient hysteroscopy?
Dilation of the cervix is the most challenging aspect of both diagnostic and operative hysteroscopy. The most common complications of operative hysteroscopy relate to this challenge; they are perforation of the uterus, laceration of the cervix, creation of false tracts, and inability to perform the procedure. In comparison, complications such as fluid overload, uterine hemorrhage, and postoperative infection are rare.
Historically, laminaria (osmotic dilators) have been used to dilate the cervix in women undergoing hysteroscopic procedures. Although this practice is useful, the placement of laminaria requires an additional office visit and may not always be practical (e.g., when scheduling is difficult or the cervix is extremely stenotic).
Numerous investigators in the past decade have attempted to determine the benefits and risks of vaginal or oral misoprostol to improve cervical dilation and minimize complications of operative and diagnostic hysteroscopy.1-5 In this systematic review, Cooper and colleagues selected six of 585 relevant studies that they identified in the literature to assess pain associated with outpatient hysteroscopy and determine whether misoprostol provides relief. They found some evidence that prostaglandins reduce the force required for hysteroscopy, as well as the need to dilate the cervix beyond 5 mm. They also concluded that vaginal misoprostol may be helpful in postmenopausal patients when the hysteroscope is larger than 5 mm.
In addition, the investigators found that most patients in the selected studies underwent hysteroscopy with a rigid hysteroscope that ranged in diameter from 2.9 mm to 5.5 mm.
Study overlooks many issues
This study has several practical limitations that prevent me from recommending that you base clinical decisions on it. The study:
- does not mention the type of distention media utilized (saline or carbon dioxide), which can affect the pain score. (Saline is associated with lower pain scores.6)
- does not mention whether hormone replacement was utilized among menopausal women
- does not mention whether a nonsteroidal agent was given before the procedure or whether a paracervical block was used during the procedure
- does not include any cases involving flexible hysteroscopy with a small-caliber hysteroscope (3–5 mm), which may be associated with less pain.
These omissions are concerning because a number of variables besides misoprostol contribute to the patient’s experience of hysteroscopy and influence her satisfaction and degree of pain. It is difficult to tabulate the many nuances that may affect pain, in particular, but they include:
- The type of patient education provided before the procedure
- Whether a vaginoscopic approach was considered or performed. Performing hysteroscopy without a speculum has been shown to produce less discomfort.7
- Whether a tenaculum was used routinely before the procedure
- Whether the physician was a seasoned hysteroscopist or a trainee
- Whether a gentle technique was utilized
- The ambiance of the procedure room. (Did it feature music or aromatherapy? Was a supportive relative or friend present?)
- Whether a video monitor was present to allow the patient to visually participate in the procedure. (Many patients find it enjoyable to watch the monitor during the procedure. It offers distraction and, combined with a calm environment and excellent nursing support [a “vocal local”], may reduce pain during the procedure.)
There are an increasing number of clinically useful algorithms available to minimize procedural pain during outpatient and office-based gynecologic procedures.8
We need additional studies
Because of conflicting results in the studies included in this systematic review, we need more investigations of the use of misoprostol for diagnostic and operative hysteroscopy. We also need to evaluate the timing, route of administration, cost, and side effects of misoprostol in this context. Studies that utilize a tonometer to measure the force of dilation would be ideal.
Vaginal bleeding, cramping, and diarrhea have been observed during use of misoprostol, but it would be interesting to determine whether intracavitary pathology plays a role in these effects. Subgroup analyses would also help clarify the ideal candidate for misoprostol and should focus on women who are nulliparous, menopausal, have a history of one or more cesarean deliveries, or have undergone loop electrosurgical excision procedure (LEEP) or cone biopsy, as these are the women at greatest risk of cervical stenosis.
Myometrial contractions often occur during use of misoprostol. It would be clinically useful to determine whether Type-1 leiomyomas are more often removed completely during hysteroscopic myomectomy when misoprostol is given. Anecdotally, I have found it easier to completely excise myomas when the patient has been pretreated with misoprostol.
Until we have additional data, I plan to continue using misoprostol to prepare the cervix for hysteroscopy. I also endorse patient education, a nurturing outpatient environment, and a trained ancillary staff to reduce pain and improve the outpatient hysteroscopy experience.
Who knows whether the magic is in the misoprostol or the magician?
Linda D. Bradley, MD
We want to hear from you! Tell us what you think.
1. Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: a randomized controlled trial. Obstet Gynecol. 2000;96(6):890-894.
2. Waddell G, Desindes S, Takser L, Beauchemin MC, Bessette P. Cervical ripening using vaginal misoprostol before hysteroscopy: a double-blind randomized trial. J Min Invasive Gynecol. 2008;15(6):739-744.
3. Mazny EA, Abou-Salem N. A double-blind randomized controlled trial of vaginal misoprostol for cervical priming before outpatient hysteroscopy. Fertil Steril. 2011;96(4):962-965.
4. Lee YY, Kim TJ, Kang H, et al. The use of misoprostol before hysteroscopic surgery in non-pregnant premenopausal women: a randomized comparison of sublingual, oral and vaginal administrations. Human Reprod. 2010;25(8):1942.-
5. da Costa AR, Pinto-Neto AM, Amorim M, et al. Use of misoprostol prior to hysteroscopy in postmenopausal women: a randomized, placebo-controlled clinical trial. J Min Invasive Gynecol. 2008;15(1):67-73.
6. Shankar M, Davidson A, Taub N, Habiba M. Randomised comparison of distension media for outpatient hysteroscopy. BJOG. 2004;111:57-62.
7. Bettocchi S, Selvaggi L. A vaginoscopic approach to reduce the pain of office hysteroscopy. J Am Assoc Gynecol Laparosc. 1997;4(2):255-258.
8. Chudnoff S, Einstein M, Levie M. Paracervical block efficacy in office hysteroscopic sterilization. Obstet Gynecol. 2010;115:26-34.
Dilation of the cervix is the most challenging aspect of both diagnostic and operative hysteroscopy. The most common complications of operative hysteroscopy relate to this challenge; they are perforation of the uterus, laceration of the cervix, creation of false tracts, and inability to perform the procedure. In comparison, complications such as fluid overload, uterine hemorrhage, and postoperative infection are rare.
Historically, laminaria (osmotic dilators) have been used to dilate the cervix in women undergoing hysteroscopic procedures. Although this practice is useful, the placement of laminaria requires an additional office visit and may not always be practical (e.g., when scheduling is difficult or the cervix is extremely stenotic).
Numerous investigators in the past decade have attempted to determine the benefits and risks of vaginal or oral misoprostol to improve cervical dilation and minimize complications of operative and diagnostic hysteroscopy.1-5 In this systematic review, Cooper and colleagues selected six of 585 relevant studies that they identified in the literature to assess pain associated with outpatient hysteroscopy and determine whether misoprostol provides relief. They found some evidence that prostaglandins reduce the force required for hysteroscopy, as well as the need to dilate the cervix beyond 5 mm. They also concluded that vaginal misoprostol may be helpful in postmenopausal patients when the hysteroscope is larger than 5 mm.
In addition, the investigators found that most patients in the selected studies underwent hysteroscopy with a rigid hysteroscope that ranged in diameter from 2.9 mm to 5.5 mm.
Study overlooks many issues
This study has several practical limitations that prevent me from recommending that you base clinical decisions on it. The study:
- does not mention the type of distention media utilized (saline or carbon dioxide), which can affect the pain score. (Saline is associated with lower pain scores.6)
- does not mention whether hormone replacement was utilized among menopausal women
- does not mention whether a nonsteroidal agent was given before the procedure or whether a paracervical block was used during the procedure
- does not include any cases involving flexible hysteroscopy with a small-caliber hysteroscope (3–5 mm), which may be associated with less pain.
These omissions are concerning because a number of variables besides misoprostol contribute to the patient’s experience of hysteroscopy and influence her satisfaction and degree of pain. It is difficult to tabulate the many nuances that may affect pain, in particular, but they include:
- The type of patient education provided before the procedure
- Whether a vaginoscopic approach was considered or performed. Performing hysteroscopy without a speculum has been shown to produce less discomfort.7
- Whether a tenaculum was used routinely before the procedure
- Whether the physician was a seasoned hysteroscopist or a trainee
- Whether a gentle technique was utilized
- The ambiance of the procedure room. (Did it feature music or aromatherapy? Was a supportive relative or friend present?)
- Whether a video monitor was present to allow the patient to visually participate in the procedure. (Many patients find it enjoyable to watch the monitor during the procedure. It offers distraction and, combined with a calm environment and excellent nursing support [a “vocal local”], may reduce pain during the procedure.)
There are an increasing number of clinically useful algorithms available to minimize procedural pain during outpatient and office-based gynecologic procedures.8
We need additional studies
Because of conflicting results in the studies included in this systematic review, we need more investigations of the use of misoprostol for diagnostic and operative hysteroscopy. We also need to evaluate the timing, route of administration, cost, and side effects of misoprostol in this context. Studies that utilize a tonometer to measure the force of dilation would be ideal.
Vaginal bleeding, cramping, and diarrhea have been observed during use of misoprostol, but it would be interesting to determine whether intracavitary pathology plays a role in these effects. Subgroup analyses would also help clarify the ideal candidate for misoprostol and should focus on women who are nulliparous, menopausal, have a history of one or more cesarean deliveries, or have undergone loop electrosurgical excision procedure (LEEP) or cone biopsy, as these are the women at greatest risk of cervical stenosis.
Myometrial contractions often occur during use of misoprostol. It would be clinically useful to determine whether Type-1 leiomyomas are more often removed completely during hysteroscopic myomectomy when misoprostol is given. Anecdotally, I have found it easier to completely excise myomas when the patient has been pretreated with misoprostol.
Until we have additional data, I plan to continue using misoprostol to prepare the cervix for hysteroscopy. I also endorse patient education, a nurturing outpatient environment, and a trained ancillary staff to reduce pain and improve the outpatient hysteroscopy experience.
Who knows whether the magic is in the misoprostol or the magician?
Linda D. Bradley, MD
We want to hear from you! Tell us what you think.
Dilation of the cervix is the most challenging aspect of both diagnostic and operative hysteroscopy. The most common complications of operative hysteroscopy relate to this challenge; they are perforation of the uterus, laceration of the cervix, creation of false tracts, and inability to perform the procedure. In comparison, complications such as fluid overload, uterine hemorrhage, and postoperative infection are rare.
Historically, laminaria (osmotic dilators) have been used to dilate the cervix in women undergoing hysteroscopic procedures. Although this practice is useful, the placement of laminaria requires an additional office visit and may not always be practical (e.g., when scheduling is difficult or the cervix is extremely stenotic).
Numerous investigators in the past decade have attempted to determine the benefits and risks of vaginal or oral misoprostol to improve cervical dilation and minimize complications of operative and diagnostic hysteroscopy.1-5 In this systematic review, Cooper and colleagues selected six of 585 relevant studies that they identified in the literature to assess pain associated with outpatient hysteroscopy and determine whether misoprostol provides relief. They found some evidence that prostaglandins reduce the force required for hysteroscopy, as well as the need to dilate the cervix beyond 5 mm. They also concluded that vaginal misoprostol may be helpful in postmenopausal patients when the hysteroscope is larger than 5 mm.
In addition, the investigators found that most patients in the selected studies underwent hysteroscopy with a rigid hysteroscope that ranged in diameter from 2.9 mm to 5.5 mm.
Study overlooks many issues
This study has several practical limitations that prevent me from recommending that you base clinical decisions on it. The study:
- does not mention the type of distention media utilized (saline or carbon dioxide), which can affect the pain score. (Saline is associated with lower pain scores.6)
- does not mention whether hormone replacement was utilized among menopausal women
- does not mention whether a nonsteroidal agent was given before the procedure or whether a paracervical block was used during the procedure
- does not include any cases involving flexible hysteroscopy with a small-caliber hysteroscope (3–5 mm), which may be associated with less pain.
These omissions are concerning because a number of variables besides misoprostol contribute to the patient’s experience of hysteroscopy and influence her satisfaction and degree of pain. It is difficult to tabulate the many nuances that may affect pain, in particular, but they include:
- The type of patient education provided before the procedure
- Whether a vaginoscopic approach was considered or performed. Performing hysteroscopy without a speculum has been shown to produce less discomfort.7
- Whether a tenaculum was used routinely before the procedure
- Whether the physician was a seasoned hysteroscopist or a trainee
- Whether a gentle technique was utilized
- The ambiance of the procedure room. (Did it feature music or aromatherapy? Was a supportive relative or friend present?)
- Whether a video monitor was present to allow the patient to visually participate in the procedure. (Many patients find it enjoyable to watch the monitor during the procedure. It offers distraction and, combined with a calm environment and excellent nursing support [a “vocal local”], may reduce pain during the procedure.)
There are an increasing number of clinically useful algorithms available to minimize procedural pain during outpatient and office-based gynecologic procedures.8
We need additional studies
Because of conflicting results in the studies included in this systematic review, we need more investigations of the use of misoprostol for diagnostic and operative hysteroscopy. We also need to evaluate the timing, route of administration, cost, and side effects of misoprostol in this context. Studies that utilize a tonometer to measure the force of dilation would be ideal.
Vaginal bleeding, cramping, and diarrhea have been observed during use of misoprostol, but it would be interesting to determine whether intracavitary pathology plays a role in these effects. Subgroup analyses would also help clarify the ideal candidate for misoprostol and should focus on women who are nulliparous, menopausal, have a history of one or more cesarean deliveries, or have undergone loop electrosurgical excision procedure (LEEP) or cone biopsy, as these are the women at greatest risk of cervical stenosis.
Myometrial contractions often occur during use of misoprostol. It would be clinically useful to determine whether Type-1 leiomyomas are more often removed completely during hysteroscopic myomectomy when misoprostol is given. Anecdotally, I have found it easier to completely excise myomas when the patient has been pretreated with misoprostol.
Until we have additional data, I plan to continue using misoprostol to prepare the cervix for hysteroscopy. I also endorse patient education, a nurturing outpatient environment, and a trained ancillary staff to reduce pain and improve the outpatient hysteroscopy experience.
Who knows whether the magic is in the misoprostol or the magician?
Linda D. Bradley, MD
We want to hear from you! Tell us what you think.
1. Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: a randomized controlled trial. Obstet Gynecol. 2000;96(6):890-894.
2. Waddell G, Desindes S, Takser L, Beauchemin MC, Bessette P. Cervical ripening using vaginal misoprostol before hysteroscopy: a double-blind randomized trial. J Min Invasive Gynecol. 2008;15(6):739-744.
3. Mazny EA, Abou-Salem N. A double-blind randomized controlled trial of vaginal misoprostol for cervical priming before outpatient hysteroscopy. Fertil Steril. 2011;96(4):962-965.
4. Lee YY, Kim TJ, Kang H, et al. The use of misoprostol before hysteroscopic surgery in non-pregnant premenopausal women: a randomized comparison of sublingual, oral and vaginal administrations. Human Reprod. 2010;25(8):1942.-
5. da Costa AR, Pinto-Neto AM, Amorim M, et al. Use of misoprostol prior to hysteroscopy in postmenopausal women: a randomized, placebo-controlled clinical trial. J Min Invasive Gynecol. 2008;15(1):67-73.
6. Shankar M, Davidson A, Taub N, Habiba M. Randomised comparison of distension media for outpatient hysteroscopy. BJOG. 2004;111:57-62.
7. Bettocchi S, Selvaggi L. A vaginoscopic approach to reduce the pain of office hysteroscopy. J Am Assoc Gynecol Laparosc. 1997;4(2):255-258.
8. Chudnoff S, Einstein M, Levie M. Paracervical block efficacy in office hysteroscopic sterilization. Obstet Gynecol. 2010;115:26-34.
1. Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: a randomized controlled trial. Obstet Gynecol. 2000;96(6):890-894.
2. Waddell G, Desindes S, Takser L, Beauchemin MC, Bessette P. Cervical ripening using vaginal misoprostol before hysteroscopy: a double-blind randomized trial. J Min Invasive Gynecol. 2008;15(6):739-744.
3. Mazny EA, Abou-Salem N. A double-blind randomized controlled trial of vaginal misoprostol for cervical priming before outpatient hysteroscopy. Fertil Steril. 2011;96(4):962-965.
4. Lee YY, Kim TJ, Kang H, et al. The use of misoprostol before hysteroscopic surgery in non-pregnant premenopausal women: a randomized comparison of sublingual, oral and vaginal administrations. Human Reprod. 2010;25(8):1942.-
5. da Costa AR, Pinto-Neto AM, Amorim M, et al. Use of misoprostol prior to hysteroscopy in postmenopausal women: a randomized, placebo-controlled clinical trial. J Min Invasive Gynecol. 2008;15(1):67-73.
6. Shankar M, Davidson A, Taub N, Habiba M. Randomised comparison of distension media for outpatient hysteroscopy. BJOG. 2004;111:57-62.
7. Bettocchi S, Selvaggi L. A vaginoscopic approach to reduce the pain of office hysteroscopy. J Am Assoc Gynecol Laparosc. 1997;4(2):255-258.
8. Chudnoff S, Einstein M, Levie M. Paracervical block efficacy in office hysteroscopic sterilization. Obstet Gynecol. 2010;115:26-34.
What is the 5-year cumulative failure rate of global endometrial ablation?
Abnormal uterine bleeding (AUB) among women of reproductive age has an enormous impact on quality of life and sexual function and consumes many health-care dollars in its evaluation and management.
If a woman has completed childbearing and has a uterus of normal size without intracavitary pathology, options include:
- the levonorgestrel-releasing intrauterine system (Mirena)
- hormonal contraception (both combination and progestin-only)
- nonsteroidal anti-inflammatory drugs
- cyclic progesterone therapy.
If the patient fails, refuses, or has contra-indications to nonsurgical therapy and seeks surgical intervention, endometrial ablation is a viable option. However, she should be informed that she may need additional treatment, resume menstruation, or develop a complication. She also should be apprised of the potential for pregnancy.
If she demands amenorrhea, total hysterectomy is the only option.
Age and other variables were predictors of outcome
The ability to predict outcomes of global endometrial ablation is clinically useful and may help the patient decide between ablation and hysterectomy. In the study by ElNashar and colleagues, women were more likely to achieve amenorrhea if they:
- were 45 years of age or older
- had a uterus shorter than 9 cm
- had endometrium thinner than 4 mm
- underwent radiofrequency ablation.
Women who were more likely to fail:
- were younger than 45 years
- had parity of 5 or higher
- had a history of tubal ligation
- had a history of dysmenorrhea.
The study included 816 women who underwent global endometrial ablation—455 in the model-development arm, and 361 in the validation arm. Three pregnancies occurred (all ended in spontaneous first-trimester abortion), 23 women (5%) complained of pelvic pain, and no patients died or developed endometrial cancer. Overall, 45 women in the model-development arm underwent hysterectomy—28 for persistent bleeding, 12 for persistent pain, and five for other indications.
Study size was a strength
Also valuable was long-term follow-up using an established registry. Among the weaknesses of the study was the fact that only two types of ablation were used.
This study confirms what many people have intuitively believed about endometrial ablation: It rarely causes permanent amenorrhea regardless of the system selected. In the original FDA clinical trials that included three other devices, the amenorrhea rate ranged from 22% to 55%, but patient satisfaction was greater than 90% in all devices studied.
Because most of the women in this study were white, further validation of this model among other races is needed.
When a patient seeks surgical intervention for AUB, assess her expectations. Ask her, “If we can make your periods return to normal or reduce monthly blood flow below normal, would you be happy with the outcome?” If she answers, “Yes,” endometrial ablation is an option. The younger the patient, the greater is the likelihood that additional surgery will eventually be necessary. If, on the other hand, she demands amenorrhea, the only option is hysterectomy with removal of the cervix.
When endometrial ablation is planned, perform preoperative imaging with saline infusion sonography or hysteroscopy to exclude intracavitary pathology, and perform preoperative endometrial biopsy to exclude pre-malignant or malignant disease. In addition, assess preoperative dysmenorrhea closely to avoid ablation in a woman who may have adenomyosis.
Also evaluate women for bleeding diathesis, such as von Willebrand’s disease, prior to ablation.- LINDA D. BRADLEY, MD
Abnormal uterine bleeding (AUB) among women of reproductive age has an enormous impact on quality of life and sexual function and consumes many health-care dollars in its evaluation and management.
If a woman has completed childbearing and has a uterus of normal size without intracavitary pathology, options include:
- the levonorgestrel-releasing intrauterine system (Mirena)
- hormonal contraception (both combination and progestin-only)
- nonsteroidal anti-inflammatory drugs
- cyclic progesterone therapy.
If the patient fails, refuses, or has contra-indications to nonsurgical therapy and seeks surgical intervention, endometrial ablation is a viable option. However, she should be informed that she may need additional treatment, resume menstruation, or develop a complication. She also should be apprised of the potential for pregnancy.
If she demands amenorrhea, total hysterectomy is the only option.
Age and other variables were predictors of outcome
The ability to predict outcomes of global endometrial ablation is clinically useful and may help the patient decide between ablation and hysterectomy. In the study by ElNashar and colleagues, women were more likely to achieve amenorrhea if they:
- were 45 years of age or older
- had a uterus shorter than 9 cm
- had endometrium thinner than 4 mm
- underwent radiofrequency ablation.
Women who were more likely to fail:
- were younger than 45 years
- had parity of 5 or higher
- had a history of tubal ligation
- had a history of dysmenorrhea.
The study included 816 women who underwent global endometrial ablation—455 in the model-development arm, and 361 in the validation arm. Three pregnancies occurred (all ended in spontaneous first-trimester abortion), 23 women (5%) complained of pelvic pain, and no patients died or developed endometrial cancer. Overall, 45 women in the model-development arm underwent hysterectomy—28 for persistent bleeding, 12 for persistent pain, and five for other indications.
Study size was a strength
Also valuable was long-term follow-up using an established registry. Among the weaknesses of the study was the fact that only two types of ablation were used.
This study confirms what many people have intuitively believed about endometrial ablation: It rarely causes permanent amenorrhea regardless of the system selected. In the original FDA clinical trials that included three other devices, the amenorrhea rate ranged from 22% to 55%, but patient satisfaction was greater than 90% in all devices studied.
Because most of the women in this study were white, further validation of this model among other races is needed.
When a patient seeks surgical intervention for AUB, assess her expectations. Ask her, “If we can make your periods return to normal or reduce monthly blood flow below normal, would you be happy with the outcome?” If she answers, “Yes,” endometrial ablation is an option. The younger the patient, the greater is the likelihood that additional surgery will eventually be necessary. If, on the other hand, she demands amenorrhea, the only option is hysterectomy with removal of the cervix.
When endometrial ablation is planned, perform preoperative imaging with saline infusion sonography or hysteroscopy to exclude intracavitary pathology, and perform preoperative endometrial biopsy to exclude pre-malignant or malignant disease. In addition, assess preoperative dysmenorrhea closely to avoid ablation in a woman who may have adenomyosis.
Also evaluate women for bleeding diathesis, such as von Willebrand’s disease, prior to ablation.- LINDA D. BRADLEY, MD
Abnormal uterine bleeding (AUB) among women of reproductive age has an enormous impact on quality of life and sexual function and consumes many health-care dollars in its evaluation and management.
If a woman has completed childbearing and has a uterus of normal size without intracavitary pathology, options include:
- the levonorgestrel-releasing intrauterine system (Mirena)
- hormonal contraception (both combination and progestin-only)
- nonsteroidal anti-inflammatory drugs
- cyclic progesterone therapy.
If the patient fails, refuses, or has contra-indications to nonsurgical therapy and seeks surgical intervention, endometrial ablation is a viable option. However, she should be informed that she may need additional treatment, resume menstruation, or develop a complication. She also should be apprised of the potential for pregnancy.
If she demands amenorrhea, total hysterectomy is the only option.
Age and other variables were predictors of outcome
The ability to predict outcomes of global endometrial ablation is clinically useful and may help the patient decide between ablation and hysterectomy. In the study by ElNashar and colleagues, women were more likely to achieve amenorrhea if they:
- were 45 years of age or older
- had a uterus shorter than 9 cm
- had endometrium thinner than 4 mm
- underwent radiofrequency ablation.
Women who were more likely to fail:
- were younger than 45 years
- had parity of 5 or higher
- had a history of tubal ligation
- had a history of dysmenorrhea.
The study included 816 women who underwent global endometrial ablation—455 in the model-development arm, and 361 in the validation arm. Three pregnancies occurred (all ended in spontaneous first-trimester abortion), 23 women (5%) complained of pelvic pain, and no patients died or developed endometrial cancer. Overall, 45 women in the model-development arm underwent hysterectomy—28 for persistent bleeding, 12 for persistent pain, and five for other indications.
Study size was a strength
Also valuable was long-term follow-up using an established registry. Among the weaknesses of the study was the fact that only two types of ablation were used.
This study confirms what many people have intuitively believed about endometrial ablation: It rarely causes permanent amenorrhea regardless of the system selected. In the original FDA clinical trials that included three other devices, the amenorrhea rate ranged from 22% to 55%, but patient satisfaction was greater than 90% in all devices studied.
Because most of the women in this study were white, further validation of this model among other races is needed.
When a patient seeks surgical intervention for AUB, assess her expectations. Ask her, “If we can make your periods return to normal or reduce monthly blood flow below normal, would you be happy with the outcome?” If she answers, “Yes,” endometrial ablation is an option. The younger the patient, the greater is the likelihood that additional surgery will eventually be necessary. If, on the other hand, she demands amenorrhea, the only option is hysterectomy with removal of the cervix.
When endometrial ablation is planned, perform preoperative imaging with saline infusion sonography or hysteroscopy to exclude intracavitary pathology, and perform preoperative endometrial biopsy to exclude pre-malignant or malignant disease. In addition, assess preoperative dysmenorrhea closely to avoid ablation in a woman who may have adenomyosis.
Also evaluate women for bleeding diathesis, such as von Willebrand’s disease, prior to ablation.- LINDA D. BRADLEY, MD
Cutting the risk of hysteroscopic complications
- Preoperative treatment with a gonadotropinreleasing hormone agonist increases the odds of operative complications by a factor of 4 to 7.
- Preoperative cervical ripening reduced the need for cervical dilation, minimized cervical complications, and reduced operative time.
- CO2 should never be used for operative hysteroscopic procedures because of the high risk of CO2 embolism.
- Ultrasound guidance may improve outcomes in selected hysteroscopic procedures.
It is a valuable tool in the evaluation and treatment of infertility, recurrent pregnancy loss, and abnormal and postmenopausal uterine bleeding, and is useful when saline infusion sonography findings are equivocal.
Further, if a global ablation device fails, the surgeon can convert to hysteroscopic ablation rather than abandon the procedure altogether. This is not as unusual as it might appear: In US Food and Drug Administration trials, there was a staggering 10% to 22% malfunction of global ablation technology.1
Safe, easily learned
Although gynecologists are beginning to embrace this modality, many physicians avoid it because of inadequate training or exaggerated fears of complications. In reality, hysteroscopy is one of the safest and most easily acquired surgical skills in gynecology. For example, in a prospective evaluation of 13,600 diagnostic and operative hysteroscopic procedures performed at 63 hospitals in the Netherlands—which involved both established surgeons and residents—Jansen et al2 found an astonishingly low complication rate of 0.28%, with no deaths.
Proper selection and treatment of patients and prompt intervention minimize complications as well as legal risks. Surgical misadventures and lawsuits occur with delayed intervention, failure to recognize pathology or risky conditions, and inadequate preventive maneuvers.
Overall, emphasis on safety is vital to success, and thorough awareness of potential complications is just as important.
Three types of complications
Complications fall into 3 categories (TABLE):
- Procedure-related
- Media-related
- Postoperative
PROCEDURE-RELATED COMPLICATIONS
Complication rates
In a retrospective investigation, Propst et al3 determined the rate of complications associated with specific hysteroscopic procedures. Demographic data and medical histories were collected for 925 women who had operative hysteroscopy in 1995 and 1996. The overall complication rate was 2.7%. Myomectomy and resection of uterine septa carried the greatest odds of complications; polypectomy and endometrial ablation had the lowest. Preoperative treatment with a gonadotropinreleasing hormone (GnRH) agonist increased the odds of complications by a factor of 4 to 7. Women under age 50 were more likely to experience complications than those over 50.
In the study by Jansen et al,2 38 complica-tions occurred in the 13,600 procedures. The greatest risk of complications occurred with adhesiolysis (4.48%), followed by endometrial resection (0.81%), myomectomy (0.75%), and polypectomy (0.38%).
Cervical entry requires special attention
Almost half of the complications in the Jansen study were related to cervical entry, so caution and, perhaps, preoperative cervical ripening are advised. Many premenopausal subjects were given GnRH analogues, which may render the cervix more resistant to dilation. Complications associated with a stenotic cervix include a cervical tear, creation of a false cervical passage, and uterine perforation.
Cervical ripening may help prevent uterine perforation. The most common complication, occurring in 14.2 cases per thousand, is uterine perforation.2 The risk of this is highest in postpartum procedures, followed by procedures in postmenopausal, then perimenopausal, women. Patients with endometrial cancer also have a higher rate of perforation.
Risk factors for uterine perforation include:
- nulliparity
- menopause
- use of GnRH agonists
- prior cone biopsy
- markedly retroverted uterus
- undue force
Vaginal or oral misoprostol for cervical ripening prior to operative hysteroscopy was evaluated in a randomized trial.4 Researchers found a reduced need for cervical dilation, a minimum of cervical complications, and reduced operative time in study patients compared with controls.
When 400 μg oral misoprostol is given 12 and 24 hours before surgery, it also softens the cervix and eases dilation.5 Although misoprostol has several bothersome side effects (such as lower abdominal pain and slight vaginal bleeding), few if any prevent its use.
Signs of perforation. Patients who sustain uterine perforation with subsequent intraperitoneal bleeding often complain of pain in the abdomen and shoulder, and experience hemodynamic instability. A quick sonographic survey of the abdomen will demonstrate free intraperitoneal fluid. (It is rare for much intraperitoneal fluid to accumulate by transtubal regurgitation during operative hysteroscopy, despite the quantity of fluid used.)
If perforation is suspected, laparoscopy or laparotomy is necessary to clarify the cause of pain, unstable vital signs, or free fluid visualized by ultrasound.6
Exercise extra care and precautions in women who have had a prior cesarean section, myomectomy, or uterine perforation. Complete visualization of uterine landmarks is necessary during operative hysteroscopy to exclude uterine dehiscence, sacculation, and perforation. Prior uterine surgery may cause myometrial weakness and lead to possible perforation. Do not proceed if abnormal uterine morphology is detected. If uterine perforation occurs, injury to bladder and bowel is possible when electrical energy is applied to a uterine wall compromised by prior surgery. Strict visualization of uterine anatomy is critical in this population so that bowel or bladder burns can be avoided.
MEDIA-RELATED COMPLICATIONS
Notorious complications and several recent lawsuits have stemmed from fluid overload. A common element has been the physician’s lack of awareness of how rapidly complications can arise, and what signs and symptoms are specific to the fluid used.
Monitor fluids vigilantly
Operative hysteroscopy must be performed in a fluid medium. The type of fluid depends on the surgeon’s preference and the instrument utilized, but any fluid can be associated with complications. Fluid choices with monopolar instruments include glycine 1.5%, a mixture of sorbitol 3% and mannitol 0.54%, and mannitol 5%. These are frequently used with the continuous-flow resectoscope. Bipolar operative hysteroscopy can be performed using saline.
The solution to media-related complications is basic: vigilant monitoring of fluids. A cavalier attitude, poor fluid documentation, and failure to respond to complications can lead to trouble. If fluid overload occurs, comanagement and consultation with an intensive care specialist is advised.
Distention media
Among the options for distention media in operative and diagnostic hysteroscopy are high-viscosity dextran 70 and low-viscosity fluids such as hypotonic, electrolyte-free and isotonic, electrolyte-containing solutions. The popularity of dextran 70 is waning, however. While it is immiscible with blood, significant complications have been reported.
Signs of anaphylactic reactions to dextran 70 include acute hypotension, hypoxia, pulmonary edema, fluid overload, fulminant coagulopathies, and anemia. The surgeon must operate quickly, minimize endometrial trauma, use continuous pulse oximetry, and obtain a preoperative coagulation panel.
Dextran 70 also can ruin operative hysteroscopes if they are not cleaned promptly and thoroughly after use.
Hypotonic, electrolyte-free solutions. With hypotonic, electrolyte-free solutions such as glycine 1.5%, early recognition of possible complications, including hyponatremic hypervolemia, is vital. For example, when glycine and sorbitol are metabolized, free water accumulates and the body attempts to achieve homeostasis through compensatory mechanisms such as osmosis, which moves free water into extracellular and intracellular spaces. This can lead to increased free water in the brain, resulting in cerebral edema, rising intracranial pressure, and cellular necrosis.
The cerebral cation pump normally pumps osmotically active cations into the extracellular space, thereby minimizing cerebral edema. However, this pump is inhibited by estrogen, so the compensatory mechanism is diminished.
Classic clinical features of hyponatremic hypervolemia include apprehension, confusion, fatigue, headache, mental agitation, nausea, visual disturbances (including blindness), vomiting, and weakness. These complications are more readily apparent when regional anesthesia is used rather than general anesthesia.
If hyponatremic hypervolemia goes unrecognized, bradycardia and hypertension can ensue, followed rapidly by cerebral and pulmonary edema and cardiovascular collapse. In addition, glycine 1.5% is metabolized to glycolic acid and ammonia. Free ammonia is associated with central nervous system disorders. Recognition and prompt treatment by an intensivist may prevent permanent neurologic sequelae, death, and lawsuits.7
Isotonic, electrolyte-containing solutions. Mannitol 5% is electrolyte poor but isotonic, creating less risk for hypo-osmolality. However, dilutional hyponatremia (ie, low sodium levels) can still occur.
Advantages of bipolar instruments. To minimize complications from hypotonic, electrolyte-free solutions, manufacturers developed operative hysteroscopes that can function in a bipolar environment. Bipolar instruments can operate in isotonic, physiologic, electrolyte-containing media. Hyponatremia and hypo-osmolality cannot occur with normal saline or Ringer’s lactate, but fluid overload can. (Fluid overload with saline can cause pulmonary edema and congestive heart failure.)
How much fluid will be absorbed? The answer depends on factors including surface area of the surgical field, duration of surgery, opened venous channels, type of irrigation fluid used, and pressure of the delivery system. Modern gynecologic suites employ fluid irrigation systems that continuously measure input and output, with alarms that signal a predetermined fluid deficit. The alarm indicates the need to halt the procedure and quickly evaluate the patient. Careful attention to the recommendations of Loffer et al8 would lead to fewer complications from fluid mismanagement.
Appropriate use of CO2
High risk of embolism with CO2 in operative procedures. Although diagnostic hysteroscopic procedures often are performed with carbon dioxide (CO2), operative procedures never should be. The reason: the high risk of CO2 embolism that occurs with open venous channels and vascular endometrium. The choice between CO2 and fluid medium for diagnostic hysteroscopy often is determined by physician preference and the presence of uterine bleeding. Many gynecologists prefer CO2 for its optical clarity and patient comfort during insufflation.9
Purge tubing of room air before each procedure. Embolic complications with CO2 have been recorded with use of the neodymium: yttrium aluminum garnet (Nd:YAG) laser and during operative procedures. Less well known are the adverse sequelae that can occur with room air prior to beginning the procedure. It is critical to purge the entire tubal system with CO2 prior to instrumentation, since up to 40 cm3 of room air may be insufflated into a patient when 200 cm of connective tubing with a 0.5-cm lumen is used.10 Wait for several minutes before starting the procedure so that the whole system is purged.
One of the greatest concerns about endometrial ablation is that diagnosis of endometrial cancer will be delayed because the endometrial cavity has been obliterated. Vilos19 recently reviewed the salient characteristics and findings in women treated by endometrial ablation who subsequently developed endometrial cancer. A review of the individual cases revealed that most of these patients had numerous risk factors for endometrial cancer.
Review risk factors, chronic conditions
Many patients with abnormal bleeding also have risk factors for endometrial cancer, as well as medical conditions that increase the likelihood of morbidity with surgery, such as obesity, hypertension, diabetes, and advanced age. In these cases, hysterectomy may be a better option than endometrial ablation. It would be far better to have such high-risk patients cleared for hysterectomy than to chance their becoming an endometrial-ablation “statistic.” If endometrial ablation is performed in these cases, we prevent the egress of blood, foster development of synechiae, render endometrial biopsy difficult or impossible and, potentially, “bury” endometrial cells deeper within the myometrium—all of which contribute to a delayed and “upstaged” diagnosis of endometrial cancer.
Patients at risk of endometrial cancer should undergo a scrupulous and unambiguous work-up and evaluation. Indeterminate endometrial echo and office evaluation that generates biopsy samples designated as “insufficient for diagnosis,” “no endometrial tissue seen,” or “atrophy” should raise suspicion. These patients require full visualization of the endometrium.
Heightened risk during perimenopause
Newer ablation techniques that utilize global therapy make it paramount that perimenopausal women undergo scrupulous evaluation. Until much more information is available, endometrial ablation should be avoided in patients with endometrial hyperplasia, particularly with atypia. While some gynecologists may be persuaded to consider endometrial ablation as a minimally invasive procedure compared to hysterectomy, the risk of delayed diagnosis of endometrial cancer is of paramount concern. The treatment of choice for these patients remains medical therapy with oral progesterone and, possibly, longterm use of a levonorgestrel-releasing intrauterine system. If this fails, hysterectomy is advisable. As Cooper20 aptly states, “Conservative, nonextirpative procedures offer no life raft” compared with hysterectomy, which covers many missed diagnoses.
No risk of spreading cancer cells
Some gynecologists have worried about the risk of disseminating endometrial cancer cells during hysteroscopy. However, Kudela and Pilka21 studied the true risk in women undergoing blind dilation and curettage and hysteroscopy performed with a fluid medium. Cul-de-sac aspiration prior to instrumentation and at the conclusion of the procedure demonstrated no increased risk of positive cytology. They are continuing a Phase II trial comparing outcomes of both groups over 5 years.
6 most common symptoms of venous or air emboli. Anesthesiologists and gynecologists must be vigilant to prevent venous or air emboli. Munro et al12 succinctly outline the 6 most common symptoms:
- pulmonary hypertension
- hypercarbia
- hypoxia
- arrhythmias
- tachypnea
- systemic hypotension
Beware of a drop in end-tidal CO2. The most common sign of impending cardiovascular collapse is a sudden decrease in end-tidal CO2, when the right cardiac outflow tract is obstructed by CO2, which leads to arterial oxygen (O2) desaturation. If such a decrease is suspected, stop the procedure immediately and administer 100% O2. (Also stop nitrous oxide, if used.) Turn the patient to the left lateral decubitis position and use a central venous catheter to aspirate gas, if necessary. Cardiac massage and a precordial thump may dislodge CO2; unfortunately, high false-positive rates of pre-cordial Doppler make its use impractical.
How to minimize risks
- Avoid coaxial gas cooling tips associated with Nd:YAG crystal lasers
- Avoid a steep Trendelenburg position
- Keep cervix covered with sponge or dilator when operative hysteroscope is removed to minimize air embolism
- Deaerate the equipment prior to surgery Use a low-pressure hysteroscopic CO2 insufflator
- Carefully monitor the patient
- Be highly suspicious when vital signs are unstable
POSTOPERATIVE COMPLICATIONS
Some complications of hysteroscopy may not become clinically evident for months or even years. The most common complications of hysteroscopic endometrial ablation include pregnancy, postablation tubal sterilization syndrome, new or worsening dysmenorrhea, hematometra, endometrial cancer, and failure to completely treat symptoms.
Patients scheduled for hysteroscopy must be informed of potential delayed risks of the procedure. In addition, all reproductive-aged women should be advised that pregnancy is possible after endometrial ablation or operative removal of an intracavitary mass; thus, contraception is crucial. The endometrial tissue is resilient and may regenerate after ablation.
Hematometra: Avoid cervical canal
Hematometra is an infrequent late complication of operative hysteroscopy. If menstruating women or those taking hormone replacement therapy experience cyclic or chronic lower pelvic pain after surgery, scarring or narrowing of the endometrial cavity may be the cause. Approximately 1% to 2% of women who undergo operative hysteroscopy experience this phenomenon. Most cases can be treated with cervical dilation alone.
Since the cervical canal contains no endometrial glands, there is no need to treat this area in women undergoing endometrial ablation. In fact, avoiding this area during treatment is a critical component of successful surgery.
Tubal sterilization syndrome possible after endometrial ablation
Consider this syndrome when a patient undergoing endometrial ablation complains of crampy, cyclic, unilateral or bilateral pelvic pain, possibly accompanied by vaginal spotting. Sometimes a unilateral mass can be palpated, but more commonly tenderness is elicited on pelvic examination.
Ultrasound may demonstrate fluid near the cornual region. Laparoscopy confirms the diagnosis by visualizing a swollen, edematous proximal fallopian tube. Salpingectomy may confirm hematosalpinx, chronic or acute inflammation, or hemosiderin deposits.
Treatment includes bilateral cornual resection and reablation of proximal endometrium, or hysterectomy.14
Pregnancy complications
Endometrial ablation is not to be regarded as a method of contraception. Patients needing birth control should consider concurrent tubal ligation or other reliable methods after this procedure.
The frequency of pregnancy after endometrial ablation ranges from 0.2% to 1.6%, though this data may represent underreporting. Pregnancy outcomes have been dismal in women conceiving after endometrial ablation. Complications include preterm labor, premature delivery, intrauterine growth retardation, prenatal death, postpartum hemorrhage, and placentation problems such as placenta accreta, increta, or percreta, as well as placental abruption.15
Uterine dehiscence and sacculation and extremely thin myometrium have been reported after uterine adhesiolysis, uterine perforation during operative hysteroscopy, and with myoma resections. A high index of suspicion is vital when a gravida presents with pelvic pain, decreased fetal movement, vaginal bleeding, or abnormal uterine masses detected ultrasonographically.
Signs of uterine rupture. Pregnancyrelated complications of operative hysteroscopy can be dramatic and fatal if not recognized quickly, as in the case of uterine rupture. Kerimis et al16 describe uterine rupture in a term pregnancy after hysteroscopic resection of a uterine septum. Severe fetal distress, maternal shoulder pain, and abdominal pain led to an emergency cesarean section. Intraoperative findings included a 7-cm tear from left cornua to right cornua. The original metroplasty, performed with cutting diathermy and laparoscopy, was not accompanied by complications or perforation.
Patients who experience intraoperative complications during metroplasty or deep resection of intramural fibroids should be informed of the risk of uterine rupture so they may consider elective cesarean. Regardless of the mode of delivery, prompt attention is vital if fetal distress is suspected.
Postablation warning signs
Patients undergoing endometrial ablation generally have a quick postoperative return to activity, minimal need for postoperative pain medication, and limited complaints. Beware of patients who make frequent postoperative phone calls and have escalating requirements for pain medication. While bowel and bladder injuries are infrequent—as is postoperative endometritis—these must be vigilantly considered and evaluated when patients complain of persistent pain, fever, and general malaise. Office evaluation is necessary, including thorough abdominal and pelvic examinations. Laboratory testing should include electrolytes, complete blood count, sedimentation rate, ultrasound, and a flat plate of the abdomen (kidneys, ureter, and bladder; upright) may be required. Sometimes a computed tomography scan of the pelvis/abdomen may be needed if perforation with bowel or bladder injury is suspected.
Hysteroscopic fibroid removal may be necessary after UAE
Uterine artery embolization (UAE) is gaining popularity for the treatment of symptomatic uterine fibroids. Transcatheter embolization of the uterine artery leads to occlusion of the fibroid, ischemic shrinkage of the fibroid, and shrinkage of residual myometrial tissue. Fibroids may migrate weeks to months after the procedure as the myometrium contracts and the treated fibroid degenerates, leading to delayed discharge, passage of necrotic fibroids, cramps, and heavy bleeding if the fibroid migrates to a submucosal location. Hysteroscopic removal is an obvious option.
Recently, De Iaco et al17 reported the development of a uterine fistula and discontinuity of the myometrium after hysteroscopic resection of an embolized migrated fibroid. They speculated this was due to the development of an avascular myometrium after UAE. The patient was asymptomatic, but routine diagnostic hysteroscopy revealed a 2-cm discontinuity of the uterine wall at the site of the previous resectoscopic myomectomy. The myometrium was white and less than the full thickness.
Ultrasound guidance improves outcomes
Coccia et al18 described the benefits of intraoperative ultrasound guidance during operative hysteroscopy in fibroid treatment and uterine septum removal. Prospective evaluation of 81 patients involved an experienced ultrasonographer who mapped the limits of treatment. Patients were compared to 45 historical controls who had been similarly treated with laparoscopic monitoring. Satisfactory outcomes included relief of menorrhagia, complete resection of fibroids (including full resection of intramural fibroids), and thorough metroplasty of uterine septum.
Ultrasound guidance made it possible to extend the resection beyond the limit conventionally defined by hysteroscopy; none of the patients in the ultrasound group required reintervention. Among controls, a second operation was necessary in 4 cases. Investigators concluded that a wider resection (10 to 15 mm distance from the external surface of the uterus) of fibroids was achieved using ultrasound guidance.
Dr. Bradley reports that she serves as a consultant to Karl Storz, ACMI, Olympus, and Gynecare, and as a lecturer for Novacept.
1. Gurtcheff SE, Sharp HT. Complications associated with global endometrial ablation: the utility of the MAUDE database. Obstet Gynecol. 2003;102:1278-1282.
2. Jansen FW, Vredevoogd CB, Ulzen K, et al. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol. 2000;96:266-270.
3. Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96:517-520.
4. Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: a randomized controlled trial. Obstet Gynecol. 2000;96:890-894.
5. Thomas JA, Leyland N, Durand N, Windrim RD. The use of oral misoprostol as a cervical ripening agent in operative hysteroscopy: a double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2002;186:876-879.
6. Stotz M, Lampart A, Kochli OR, Schneider M. Intraabdominal bleeding masked by hemodilution after hysteroscopy. Anesthesiology. 2000;93:569-570.
7. Cooper JM, Brady RM. Intraoperative and early postoperative complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:347-366.
8. Loffer FD, Bradley LD, Brill AL, Brooks PG, Cooper JM. Hysteroscopic fluid monitoring guidelines. J Am Assoc Gynecol Laparosc. 2000;7:167-168.
9. Bradley LD, Widrich T. Flexible hysteroscopy: a state-of-the-art procedure for gynecologic evaluation. J Am Assoc Gynecol Laparosc. 1995;2:263-267.
10. Neis KJ, Brandner P, Lindemann HJ. Room air as a cause of gas embolism in diagnostic CO2hysteroscopy. Zentralbl Gynakol. 2000;122:222-225.
11. Bradner P, Neis KJ, Ehmer C. The etiology, frequency, and prevention of gas embolism during CO2hysteroscopy. J Am Assoc Gynecol Laparosc. 1999;6:421-428.
12. Munro MG, Weisberg M, Rubinstein E. Gas and air embolization during hysteroscopic electrosurgical vaporization: comparison of gas generation using bipolar and monopolar electrodes in an experimental model. J Am Assoc Gynecol Laparosc. 2001;8:488-494.
13. Murdoch JAC, Gan TJ. Anesthesia for hysteroscopy. Anesthesiol Clin North Am. 2001;1:125-140.
14. Cooper JM, Brady RM. Late complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:367-374.
15. Rogerson L, Gannon B, O’Donovan P. Outcome of pregnancy following endometrial ablation. J Gynecol Surg. 1997;13:155-160.
16. Kerimis P, Zolti M, Sinwany G, Mashiach S, Carp H. Uterine rupture after hysteroscopic resection of uterine septum. Fertil Steril. 2002;77:618-620.
17. De Iaco P, Golfieri R, Ghi T, Muzzupapa G, Ceccarini M, Bovicelli L. Uterine fistula induced by hysteroscopic resection of an embolized migrated fibroid: a rare complication after embolization of uterine fibroids. Fertil Steril. 2001;75:818-820.
18. Coccia ME, Becattini C, Bracco GL, et al. Intraoperative ultrasound guidance for operative hysteroscopy. J Reprod Med. 2000;45:413-418.
19. Brooks-Carter GN, Killackey MA, Neuwirth RS. Adenocarcinoma of the endometrium after endometrial ablation. Obstet Gynecol. 2000;96:836-837.
20. Cooper JM. Swimming lessons: check the water before jumping in. J Am Assoc Gynecol Laparosc. 1998;5:87-90.
21. Kudela M, Pilka R. Is there a real risk in patients with endometrial carcinoma undergoing diagnostic hysteroscopy (HSC)? Eur J Gynecol Oncol. 2001;22:342-344.
- Preoperative treatment with a gonadotropinreleasing hormone agonist increases the odds of operative complications by a factor of 4 to 7.
- Preoperative cervical ripening reduced the need for cervical dilation, minimized cervical complications, and reduced operative time.
- CO2 should never be used for operative hysteroscopic procedures because of the high risk of CO2 embolism.
- Ultrasound guidance may improve outcomes in selected hysteroscopic procedures.
It is a valuable tool in the evaluation and treatment of infertility, recurrent pregnancy loss, and abnormal and postmenopausal uterine bleeding, and is useful when saline infusion sonography findings are equivocal.
Further, if a global ablation device fails, the surgeon can convert to hysteroscopic ablation rather than abandon the procedure altogether. This is not as unusual as it might appear: In US Food and Drug Administration trials, there was a staggering 10% to 22% malfunction of global ablation technology.1
Safe, easily learned
Although gynecologists are beginning to embrace this modality, many physicians avoid it because of inadequate training or exaggerated fears of complications. In reality, hysteroscopy is one of the safest and most easily acquired surgical skills in gynecology. For example, in a prospective evaluation of 13,600 diagnostic and operative hysteroscopic procedures performed at 63 hospitals in the Netherlands—which involved both established surgeons and residents—Jansen et al2 found an astonishingly low complication rate of 0.28%, with no deaths.
Proper selection and treatment of patients and prompt intervention minimize complications as well as legal risks. Surgical misadventures and lawsuits occur with delayed intervention, failure to recognize pathology or risky conditions, and inadequate preventive maneuvers.
Overall, emphasis on safety is vital to success, and thorough awareness of potential complications is just as important.
Three types of complications
Complications fall into 3 categories (TABLE):
- Procedure-related
- Media-related
- Postoperative
PROCEDURE-RELATED COMPLICATIONS
Complication rates
In a retrospective investigation, Propst et al3 determined the rate of complications associated with specific hysteroscopic procedures. Demographic data and medical histories were collected for 925 women who had operative hysteroscopy in 1995 and 1996. The overall complication rate was 2.7%. Myomectomy and resection of uterine septa carried the greatest odds of complications; polypectomy and endometrial ablation had the lowest. Preoperative treatment with a gonadotropinreleasing hormone (GnRH) agonist increased the odds of complications by a factor of 4 to 7. Women under age 50 were more likely to experience complications than those over 50.
In the study by Jansen et al,2 38 complica-tions occurred in the 13,600 procedures. The greatest risk of complications occurred with adhesiolysis (4.48%), followed by endometrial resection (0.81%), myomectomy (0.75%), and polypectomy (0.38%).
Cervical entry requires special attention
Almost half of the complications in the Jansen study were related to cervical entry, so caution and, perhaps, preoperative cervical ripening are advised. Many premenopausal subjects were given GnRH analogues, which may render the cervix more resistant to dilation. Complications associated with a stenotic cervix include a cervical tear, creation of a false cervical passage, and uterine perforation.
Cervical ripening may help prevent uterine perforation. The most common complication, occurring in 14.2 cases per thousand, is uterine perforation.2 The risk of this is highest in postpartum procedures, followed by procedures in postmenopausal, then perimenopausal, women. Patients with endometrial cancer also have a higher rate of perforation.
Risk factors for uterine perforation include:
- nulliparity
- menopause
- use of GnRH agonists
- prior cone biopsy
- markedly retroverted uterus
- undue force
Vaginal or oral misoprostol for cervical ripening prior to operative hysteroscopy was evaluated in a randomized trial.4 Researchers found a reduced need for cervical dilation, a minimum of cervical complications, and reduced operative time in study patients compared with controls.
When 400 μg oral misoprostol is given 12 and 24 hours before surgery, it also softens the cervix and eases dilation.5 Although misoprostol has several bothersome side effects (such as lower abdominal pain and slight vaginal bleeding), few if any prevent its use.
Signs of perforation. Patients who sustain uterine perforation with subsequent intraperitoneal bleeding often complain of pain in the abdomen and shoulder, and experience hemodynamic instability. A quick sonographic survey of the abdomen will demonstrate free intraperitoneal fluid. (It is rare for much intraperitoneal fluid to accumulate by transtubal regurgitation during operative hysteroscopy, despite the quantity of fluid used.)
If perforation is suspected, laparoscopy or laparotomy is necessary to clarify the cause of pain, unstable vital signs, or free fluid visualized by ultrasound.6
Exercise extra care and precautions in women who have had a prior cesarean section, myomectomy, or uterine perforation. Complete visualization of uterine landmarks is necessary during operative hysteroscopy to exclude uterine dehiscence, sacculation, and perforation. Prior uterine surgery may cause myometrial weakness and lead to possible perforation. Do not proceed if abnormal uterine morphology is detected. If uterine perforation occurs, injury to bladder and bowel is possible when electrical energy is applied to a uterine wall compromised by prior surgery. Strict visualization of uterine anatomy is critical in this population so that bowel or bladder burns can be avoided.
MEDIA-RELATED COMPLICATIONS
Notorious complications and several recent lawsuits have stemmed from fluid overload. A common element has been the physician’s lack of awareness of how rapidly complications can arise, and what signs and symptoms are specific to the fluid used.
Monitor fluids vigilantly
Operative hysteroscopy must be performed in a fluid medium. The type of fluid depends on the surgeon’s preference and the instrument utilized, but any fluid can be associated with complications. Fluid choices with monopolar instruments include glycine 1.5%, a mixture of sorbitol 3% and mannitol 0.54%, and mannitol 5%. These are frequently used with the continuous-flow resectoscope. Bipolar operative hysteroscopy can be performed using saline.
The solution to media-related complications is basic: vigilant monitoring of fluids. A cavalier attitude, poor fluid documentation, and failure to respond to complications can lead to trouble. If fluid overload occurs, comanagement and consultation with an intensive care specialist is advised.
Distention media
Among the options for distention media in operative and diagnostic hysteroscopy are high-viscosity dextran 70 and low-viscosity fluids such as hypotonic, electrolyte-free and isotonic, electrolyte-containing solutions. The popularity of dextran 70 is waning, however. While it is immiscible with blood, significant complications have been reported.
Signs of anaphylactic reactions to dextran 70 include acute hypotension, hypoxia, pulmonary edema, fluid overload, fulminant coagulopathies, and anemia. The surgeon must operate quickly, minimize endometrial trauma, use continuous pulse oximetry, and obtain a preoperative coagulation panel.
Dextran 70 also can ruin operative hysteroscopes if they are not cleaned promptly and thoroughly after use.
Hypotonic, electrolyte-free solutions. With hypotonic, electrolyte-free solutions such as glycine 1.5%, early recognition of possible complications, including hyponatremic hypervolemia, is vital. For example, when glycine and sorbitol are metabolized, free water accumulates and the body attempts to achieve homeostasis through compensatory mechanisms such as osmosis, which moves free water into extracellular and intracellular spaces. This can lead to increased free water in the brain, resulting in cerebral edema, rising intracranial pressure, and cellular necrosis.
The cerebral cation pump normally pumps osmotically active cations into the extracellular space, thereby minimizing cerebral edema. However, this pump is inhibited by estrogen, so the compensatory mechanism is diminished.
Classic clinical features of hyponatremic hypervolemia include apprehension, confusion, fatigue, headache, mental agitation, nausea, visual disturbances (including blindness), vomiting, and weakness. These complications are more readily apparent when regional anesthesia is used rather than general anesthesia.
If hyponatremic hypervolemia goes unrecognized, bradycardia and hypertension can ensue, followed rapidly by cerebral and pulmonary edema and cardiovascular collapse. In addition, glycine 1.5% is metabolized to glycolic acid and ammonia. Free ammonia is associated with central nervous system disorders. Recognition and prompt treatment by an intensivist may prevent permanent neurologic sequelae, death, and lawsuits.7
Isotonic, electrolyte-containing solutions. Mannitol 5% is electrolyte poor but isotonic, creating less risk for hypo-osmolality. However, dilutional hyponatremia (ie, low sodium levels) can still occur.
Advantages of bipolar instruments. To minimize complications from hypotonic, electrolyte-free solutions, manufacturers developed operative hysteroscopes that can function in a bipolar environment. Bipolar instruments can operate in isotonic, physiologic, electrolyte-containing media. Hyponatremia and hypo-osmolality cannot occur with normal saline or Ringer’s lactate, but fluid overload can. (Fluid overload with saline can cause pulmonary edema and congestive heart failure.)
How much fluid will be absorbed? The answer depends on factors including surface area of the surgical field, duration of surgery, opened venous channels, type of irrigation fluid used, and pressure of the delivery system. Modern gynecologic suites employ fluid irrigation systems that continuously measure input and output, with alarms that signal a predetermined fluid deficit. The alarm indicates the need to halt the procedure and quickly evaluate the patient. Careful attention to the recommendations of Loffer et al8 would lead to fewer complications from fluid mismanagement.
Appropriate use of CO2
High risk of embolism with CO2 in operative procedures. Although diagnostic hysteroscopic procedures often are performed with carbon dioxide (CO2), operative procedures never should be. The reason: the high risk of CO2 embolism that occurs with open venous channels and vascular endometrium. The choice between CO2 and fluid medium for diagnostic hysteroscopy often is determined by physician preference and the presence of uterine bleeding. Many gynecologists prefer CO2 for its optical clarity and patient comfort during insufflation.9
Purge tubing of room air before each procedure. Embolic complications with CO2 have been recorded with use of the neodymium: yttrium aluminum garnet (Nd:YAG) laser and during operative procedures. Less well known are the adverse sequelae that can occur with room air prior to beginning the procedure. It is critical to purge the entire tubal system with CO2 prior to instrumentation, since up to 40 cm3 of room air may be insufflated into a patient when 200 cm of connective tubing with a 0.5-cm lumen is used.10 Wait for several minutes before starting the procedure so that the whole system is purged.
One of the greatest concerns about endometrial ablation is that diagnosis of endometrial cancer will be delayed because the endometrial cavity has been obliterated. Vilos19 recently reviewed the salient characteristics and findings in women treated by endometrial ablation who subsequently developed endometrial cancer. A review of the individual cases revealed that most of these patients had numerous risk factors for endometrial cancer.
Review risk factors, chronic conditions
Many patients with abnormal bleeding also have risk factors for endometrial cancer, as well as medical conditions that increase the likelihood of morbidity with surgery, such as obesity, hypertension, diabetes, and advanced age. In these cases, hysterectomy may be a better option than endometrial ablation. It would be far better to have such high-risk patients cleared for hysterectomy than to chance their becoming an endometrial-ablation “statistic.” If endometrial ablation is performed in these cases, we prevent the egress of blood, foster development of synechiae, render endometrial biopsy difficult or impossible and, potentially, “bury” endometrial cells deeper within the myometrium—all of which contribute to a delayed and “upstaged” diagnosis of endometrial cancer.
Patients at risk of endometrial cancer should undergo a scrupulous and unambiguous work-up and evaluation. Indeterminate endometrial echo and office evaluation that generates biopsy samples designated as “insufficient for diagnosis,” “no endometrial tissue seen,” or “atrophy” should raise suspicion. These patients require full visualization of the endometrium.
Heightened risk during perimenopause
Newer ablation techniques that utilize global therapy make it paramount that perimenopausal women undergo scrupulous evaluation. Until much more information is available, endometrial ablation should be avoided in patients with endometrial hyperplasia, particularly with atypia. While some gynecologists may be persuaded to consider endometrial ablation as a minimally invasive procedure compared to hysterectomy, the risk of delayed diagnosis of endometrial cancer is of paramount concern. The treatment of choice for these patients remains medical therapy with oral progesterone and, possibly, longterm use of a levonorgestrel-releasing intrauterine system. If this fails, hysterectomy is advisable. As Cooper20 aptly states, “Conservative, nonextirpative procedures offer no life raft” compared with hysterectomy, which covers many missed diagnoses.
No risk of spreading cancer cells
Some gynecologists have worried about the risk of disseminating endometrial cancer cells during hysteroscopy. However, Kudela and Pilka21 studied the true risk in women undergoing blind dilation and curettage and hysteroscopy performed with a fluid medium. Cul-de-sac aspiration prior to instrumentation and at the conclusion of the procedure demonstrated no increased risk of positive cytology. They are continuing a Phase II trial comparing outcomes of both groups over 5 years.
6 most common symptoms of venous or air emboli. Anesthesiologists and gynecologists must be vigilant to prevent venous or air emboli. Munro et al12 succinctly outline the 6 most common symptoms:
- pulmonary hypertension
- hypercarbia
- hypoxia
- arrhythmias
- tachypnea
- systemic hypotension
Beware of a drop in end-tidal CO2. The most common sign of impending cardiovascular collapse is a sudden decrease in end-tidal CO2, when the right cardiac outflow tract is obstructed by CO2, which leads to arterial oxygen (O2) desaturation. If such a decrease is suspected, stop the procedure immediately and administer 100% O2. (Also stop nitrous oxide, if used.) Turn the patient to the left lateral decubitis position and use a central venous catheter to aspirate gas, if necessary. Cardiac massage and a precordial thump may dislodge CO2; unfortunately, high false-positive rates of pre-cordial Doppler make its use impractical.
How to minimize risks
- Avoid coaxial gas cooling tips associated with Nd:YAG crystal lasers
- Avoid a steep Trendelenburg position
- Keep cervix covered with sponge or dilator when operative hysteroscope is removed to minimize air embolism
- Deaerate the equipment prior to surgery Use a low-pressure hysteroscopic CO2 insufflator
- Carefully monitor the patient
- Be highly suspicious when vital signs are unstable
POSTOPERATIVE COMPLICATIONS
Some complications of hysteroscopy may not become clinically evident for months or even years. The most common complications of hysteroscopic endometrial ablation include pregnancy, postablation tubal sterilization syndrome, new or worsening dysmenorrhea, hematometra, endometrial cancer, and failure to completely treat symptoms.
Patients scheduled for hysteroscopy must be informed of potential delayed risks of the procedure. In addition, all reproductive-aged women should be advised that pregnancy is possible after endometrial ablation or operative removal of an intracavitary mass; thus, contraception is crucial. The endometrial tissue is resilient and may regenerate after ablation.
Hematometra: Avoid cervical canal
Hematometra is an infrequent late complication of operative hysteroscopy. If menstruating women or those taking hormone replacement therapy experience cyclic or chronic lower pelvic pain after surgery, scarring or narrowing of the endometrial cavity may be the cause. Approximately 1% to 2% of women who undergo operative hysteroscopy experience this phenomenon. Most cases can be treated with cervical dilation alone.
Since the cervical canal contains no endometrial glands, there is no need to treat this area in women undergoing endometrial ablation. In fact, avoiding this area during treatment is a critical component of successful surgery.
Tubal sterilization syndrome possible after endometrial ablation
Consider this syndrome when a patient undergoing endometrial ablation complains of crampy, cyclic, unilateral or bilateral pelvic pain, possibly accompanied by vaginal spotting. Sometimes a unilateral mass can be palpated, but more commonly tenderness is elicited on pelvic examination.
Ultrasound may demonstrate fluid near the cornual region. Laparoscopy confirms the diagnosis by visualizing a swollen, edematous proximal fallopian tube. Salpingectomy may confirm hematosalpinx, chronic or acute inflammation, or hemosiderin deposits.
Treatment includes bilateral cornual resection and reablation of proximal endometrium, or hysterectomy.14
Pregnancy complications
Endometrial ablation is not to be regarded as a method of contraception. Patients needing birth control should consider concurrent tubal ligation or other reliable methods after this procedure.
The frequency of pregnancy after endometrial ablation ranges from 0.2% to 1.6%, though this data may represent underreporting. Pregnancy outcomes have been dismal in women conceiving after endometrial ablation. Complications include preterm labor, premature delivery, intrauterine growth retardation, prenatal death, postpartum hemorrhage, and placentation problems such as placenta accreta, increta, or percreta, as well as placental abruption.15
Uterine dehiscence and sacculation and extremely thin myometrium have been reported after uterine adhesiolysis, uterine perforation during operative hysteroscopy, and with myoma resections. A high index of suspicion is vital when a gravida presents with pelvic pain, decreased fetal movement, vaginal bleeding, or abnormal uterine masses detected ultrasonographically.
Signs of uterine rupture. Pregnancyrelated complications of operative hysteroscopy can be dramatic and fatal if not recognized quickly, as in the case of uterine rupture. Kerimis et al16 describe uterine rupture in a term pregnancy after hysteroscopic resection of a uterine septum. Severe fetal distress, maternal shoulder pain, and abdominal pain led to an emergency cesarean section. Intraoperative findings included a 7-cm tear from left cornua to right cornua. The original metroplasty, performed with cutting diathermy and laparoscopy, was not accompanied by complications or perforation.
Patients who experience intraoperative complications during metroplasty or deep resection of intramural fibroids should be informed of the risk of uterine rupture so they may consider elective cesarean. Regardless of the mode of delivery, prompt attention is vital if fetal distress is suspected.
Postablation warning signs
Patients undergoing endometrial ablation generally have a quick postoperative return to activity, minimal need for postoperative pain medication, and limited complaints. Beware of patients who make frequent postoperative phone calls and have escalating requirements for pain medication. While bowel and bladder injuries are infrequent—as is postoperative endometritis—these must be vigilantly considered and evaluated when patients complain of persistent pain, fever, and general malaise. Office evaluation is necessary, including thorough abdominal and pelvic examinations. Laboratory testing should include electrolytes, complete blood count, sedimentation rate, ultrasound, and a flat plate of the abdomen (kidneys, ureter, and bladder; upright) may be required. Sometimes a computed tomography scan of the pelvis/abdomen may be needed if perforation with bowel or bladder injury is suspected.
Hysteroscopic fibroid removal may be necessary after UAE
Uterine artery embolization (UAE) is gaining popularity for the treatment of symptomatic uterine fibroids. Transcatheter embolization of the uterine artery leads to occlusion of the fibroid, ischemic shrinkage of the fibroid, and shrinkage of residual myometrial tissue. Fibroids may migrate weeks to months after the procedure as the myometrium contracts and the treated fibroid degenerates, leading to delayed discharge, passage of necrotic fibroids, cramps, and heavy bleeding if the fibroid migrates to a submucosal location. Hysteroscopic removal is an obvious option.
Recently, De Iaco et al17 reported the development of a uterine fistula and discontinuity of the myometrium after hysteroscopic resection of an embolized migrated fibroid. They speculated this was due to the development of an avascular myometrium after UAE. The patient was asymptomatic, but routine diagnostic hysteroscopy revealed a 2-cm discontinuity of the uterine wall at the site of the previous resectoscopic myomectomy. The myometrium was white and less than the full thickness.
Ultrasound guidance improves outcomes
Coccia et al18 described the benefits of intraoperative ultrasound guidance during operative hysteroscopy in fibroid treatment and uterine septum removal. Prospective evaluation of 81 patients involved an experienced ultrasonographer who mapped the limits of treatment. Patients were compared to 45 historical controls who had been similarly treated with laparoscopic monitoring. Satisfactory outcomes included relief of menorrhagia, complete resection of fibroids (including full resection of intramural fibroids), and thorough metroplasty of uterine septum.
Ultrasound guidance made it possible to extend the resection beyond the limit conventionally defined by hysteroscopy; none of the patients in the ultrasound group required reintervention. Among controls, a second operation was necessary in 4 cases. Investigators concluded that a wider resection (10 to 15 mm distance from the external surface of the uterus) of fibroids was achieved using ultrasound guidance.
Dr. Bradley reports that she serves as a consultant to Karl Storz, ACMI, Olympus, and Gynecare, and as a lecturer for Novacept.
- Preoperative treatment with a gonadotropinreleasing hormone agonist increases the odds of operative complications by a factor of 4 to 7.
- Preoperative cervical ripening reduced the need for cervical dilation, minimized cervical complications, and reduced operative time.
- CO2 should never be used for operative hysteroscopic procedures because of the high risk of CO2 embolism.
- Ultrasound guidance may improve outcomes in selected hysteroscopic procedures.
It is a valuable tool in the evaluation and treatment of infertility, recurrent pregnancy loss, and abnormal and postmenopausal uterine bleeding, and is useful when saline infusion sonography findings are equivocal.
Further, if a global ablation device fails, the surgeon can convert to hysteroscopic ablation rather than abandon the procedure altogether. This is not as unusual as it might appear: In US Food and Drug Administration trials, there was a staggering 10% to 22% malfunction of global ablation technology.1
Safe, easily learned
Although gynecologists are beginning to embrace this modality, many physicians avoid it because of inadequate training or exaggerated fears of complications. In reality, hysteroscopy is one of the safest and most easily acquired surgical skills in gynecology. For example, in a prospective evaluation of 13,600 diagnostic and operative hysteroscopic procedures performed at 63 hospitals in the Netherlands—which involved both established surgeons and residents—Jansen et al2 found an astonishingly low complication rate of 0.28%, with no deaths.
Proper selection and treatment of patients and prompt intervention minimize complications as well as legal risks. Surgical misadventures and lawsuits occur with delayed intervention, failure to recognize pathology or risky conditions, and inadequate preventive maneuvers.
Overall, emphasis on safety is vital to success, and thorough awareness of potential complications is just as important.
Three types of complications
Complications fall into 3 categories (TABLE):
- Procedure-related
- Media-related
- Postoperative
PROCEDURE-RELATED COMPLICATIONS
Complication rates
In a retrospective investigation, Propst et al3 determined the rate of complications associated with specific hysteroscopic procedures. Demographic data and medical histories were collected for 925 women who had operative hysteroscopy in 1995 and 1996. The overall complication rate was 2.7%. Myomectomy and resection of uterine septa carried the greatest odds of complications; polypectomy and endometrial ablation had the lowest. Preoperative treatment with a gonadotropinreleasing hormone (GnRH) agonist increased the odds of complications by a factor of 4 to 7. Women under age 50 were more likely to experience complications than those over 50.
In the study by Jansen et al,2 38 complica-tions occurred in the 13,600 procedures. The greatest risk of complications occurred with adhesiolysis (4.48%), followed by endometrial resection (0.81%), myomectomy (0.75%), and polypectomy (0.38%).
Cervical entry requires special attention
Almost half of the complications in the Jansen study were related to cervical entry, so caution and, perhaps, preoperative cervical ripening are advised. Many premenopausal subjects were given GnRH analogues, which may render the cervix more resistant to dilation. Complications associated with a stenotic cervix include a cervical tear, creation of a false cervical passage, and uterine perforation.
Cervical ripening may help prevent uterine perforation. The most common complication, occurring in 14.2 cases per thousand, is uterine perforation.2 The risk of this is highest in postpartum procedures, followed by procedures in postmenopausal, then perimenopausal, women. Patients with endometrial cancer also have a higher rate of perforation.
Risk factors for uterine perforation include:
- nulliparity
- menopause
- use of GnRH agonists
- prior cone biopsy
- markedly retroverted uterus
- undue force
Vaginal or oral misoprostol for cervical ripening prior to operative hysteroscopy was evaluated in a randomized trial.4 Researchers found a reduced need for cervical dilation, a minimum of cervical complications, and reduced operative time in study patients compared with controls.
When 400 μg oral misoprostol is given 12 and 24 hours before surgery, it also softens the cervix and eases dilation.5 Although misoprostol has several bothersome side effects (such as lower abdominal pain and slight vaginal bleeding), few if any prevent its use.
Signs of perforation. Patients who sustain uterine perforation with subsequent intraperitoneal bleeding often complain of pain in the abdomen and shoulder, and experience hemodynamic instability. A quick sonographic survey of the abdomen will demonstrate free intraperitoneal fluid. (It is rare for much intraperitoneal fluid to accumulate by transtubal regurgitation during operative hysteroscopy, despite the quantity of fluid used.)
If perforation is suspected, laparoscopy or laparotomy is necessary to clarify the cause of pain, unstable vital signs, or free fluid visualized by ultrasound.6
Exercise extra care and precautions in women who have had a prior cesarean section, myomectomy, or uterine perforation. Complete visualization of uterine landmarks is necessary during operative hysteroscopy to exclude uterine dehiscence, sacculation, and perforation. Prior uterine surgery may cause myometrial weakness and lead to possible perforation. Do not proceed if abnormal uterine morphology is detected. If uterine perforation occurs, injury to bladder and bowel is possible when electrical energy is applied to a uterine wall compromised by prior surgery. Strict visualization of uterine anatomy is critical in this population so that bowel or bladder burns can be avoided.
MEDIA-RELATED COMPLICATIONS
Notorious complications and several recent lawsuits have stemmed from fluid overload. A common element has been the physician’s lack of awareness of how rapidly complications can arise, and what signs and symptoms are specific to the fluid used.
Monitor fluids vigilantly
Operative hysteroscopy must be performed in a fluid medium. The type of fluid depends on the surgeon’s preference and the instrument utilized, but any fluid can be associated with complications. Fluid choices with monopolar instruments include glycine 1.5%, a mixture of sorbitol 3% and mannitol 0.54%, and mannitol 5%. These are frequently used with the continuous-flow resectoscope. Bipolar operative hysteroscopy can be performed using saline.
The solution to media-related complications is basic: vigilant monitoring of fluids. A cavalier attitude, poor fluid documentation, and failure to respond to complications can lead to trouble. If fluid overload occurs, comanagement and consultation with an intensive care specialist is advised.
Distention media
Among the options for distention media in operative and diagnostic hysteroscopy are high-viscosity dextran 70 and low-viscosity fluids such as hypotonic, electrolyte-free and isotonic, electrolyte-containing solutions. The popularity of dextran 70 is waning, however. While it is immiscible with blood, significant complications have been reported.
Signs of anaphylactic reactions to dextran 70 include acute hypotension, hypoxia, pulmonary edema, fluid overload, fulminant coagulopathies, and anemia. The surgeon must operate quickly, minimize endometrial trauma, use continuous pulse oximetry, and obtain a preoperative coagulation panel.
Dextran 70 also can ruin operative hysteroscopes if they are not cleaned promptly and thoroughly after use.
Hypotonic, electrolyte-free solutions. With hypotonic, electrolyte-free solutions such as glycine 1.5%, early recognition of possible complications, including hyponatremic hypervolemia, is vital. For example, when glycine and sorbitol are metabolized, free water accumulates and the body attempts to achieve homeostasis through compensatory mechanisms such as osmosis, which moves free water into extracellular and intracellular spaces. This can lead to increased free water in the brain, resulting in cerebral edema, rising intracranial pressure, and cellular necrosis.
The cerebral cation pump normally pumps osmotically active cations into the extracellular space, thereby minimizing cerebral edema. However, this pump is inhibited by estrogen, so the compensatory mechanism is diminished.
Classic clinical features of hyponatremic hypervolemia include apprehension, confusion, fatigue, headache, mental agitation, nausea, visual disturbances (including blindness), vomiting, and weakness. These complications are more readily apparent when regional anesthesia is used rather than general anesthesia.
If hyponatremic hypervolemia goes unrecognized, bradycardia and hypertension can ensue, followed rapidly by cerebral and pulmonary edema and cardiovascular collapse. In addition, glycine 1.5% is metabolized to glycolic acid and ammonia. Free ammonia is associated with central nervous system disorders. Recognition and prompt treatment by an intensivist may prevent permanent neurologic sequelae, death, and lawsuits.7
Isotonic, electrolyte-containing solutions. Mannitol 5% is electrolyte poor but isotonic, creating less risk for hypo-osmolality. However, dilutional hyponatremia (ie, low sodium levels) can still occur.
Advantages of bipolar instruments. To minimize complications from hypotonic, electrolyte-free solutions, manufacturers developed operative hysteroscopes that can function in a bipolar environment. Bipolar instruments can operate in isotonic, physiologic, electrolyte-containing media. Hyponatremia and hypo-osmolality cannot occur with normal saline or Ringer’s lactate, but fluid overload can. (Fluid overload with saline can cause pulmonary edema and congestive heart failure.)
How much fluid will be absorbed? The answer depends on factors including surface area of the surgical field, duration of surgery, opened venous channels, type of irrigation fluid used, and pressure of the delivery system. Modern gynecologic suites employ fluid irrigation systems that continuously measure input and output, with alarms that signal a predetermined fluid deficit. The alarm indicates the need to halt the procedure and quickly evaluate the patient. Careful attention to the recommendations of Loffer et al8 would lead to fewer complications from fluid mismanagement.
Appropriate use of CO2
High risk of embolism with CO2 in operative procedures. Although diagnostic hysteroscopic procedures often are performed with carbon dioxide (CO2), operative procedures never should be. The reason: the high risk of CO2 embolism that occurs with open venous channels and vascular endometrium. The choice between CO2 and fluid medium for diagnostic hysteroscopy often is determined by physician preference and the presence of uterine bleeding. Many gynecologists prefer CO2 for its optical clarity and patient comfort during insufflation.9
Purge tubing of room air before each procedure. Embolic complications with CO2 have been recorded with use of the neodymium: yttrium aluminum garnet (Nd:YAG) laser and during operative procedures. Less well known are the adverse sequelae that can occur with room air prior to beginning the procedure. It is critical to purge the entire tubal system with CO2 prior to instrumentation, since up to 40 cm3 of room air may be insufflated into a patient when 200 cm of connective tubing with a 0.5-cm lumen is used.10 Wait for several minutes before starting the procedure so that the whole system is purged.
One of the greatest concerns about endometrial ablation is that diagnosis of endometrial cancer will be delayed because the endometrial cavity has been obliterated. Vilos19 recently reviewed the salient characteristics and findings in women treated by endometrial ablation who subsequently developed endometrial cancer. A review of the individual cases revealed that most of these patients had numerous risk factors for endometrial cancer.
Review risk factors, chronic conditions
Many patients with abnormal bleeding also have risk factors for endometrial cancer, as well as medical conditions that increase the likelihood of morbidity with surgery, such as obesity, hypertension, diabetes, and advanced age. In these cases, hysterectomy may be a better option than endometrial ablation. It would be far better to have such high-risk patients cleared for hysterectomy than to chance their becoming an endometrial-ablation “statistic.” If endometrial ablation is performed in these cases, we prevent the egress of blood, foster development of synechiae, render endometrial biopsy difficult or impossible and, potentially, “bury” endometrial cells deeper within the myometrium—all of which contribute to a delayed and “upstaged” diagnosis of endometrial cancer.
Patients at risk of endometrial cancer should undergo a scrupulous and unambiguous work-up and evaluation. Indeterminate endometrial echo and office evaluation that generates biopsy samples designated as “insufficient for diagnosis,” “no endometrial tissue seen,” or “atrophy” should raise suspicion. These patients require full visualization of the endometrium.
Heightened risk during perimenopause
Newer ablation techniques that utilize global therapy make it paramount that perimenopausal women undergo scrupulous evaluation. Until much more information is available, endometrial ablation should be avoided in patients with endometrial hyperplasia, particularly with atypia. While some gynecologists may be persuaded to consider endometrial ablation as a minimally invasive procedure compared to hysterectomy, the risk of delayed diagnosis of endometrial cancer is of paramount concern. The treatment of choice for these patients remains medical therapy with oral progesterone and, possibly, longterm use of a levonorgestrel-releasing intrauterine system. If this fails, hysterectomy is advisable. As Cooper20 aptly states, “Conservative, nonextirpative procedures offer no life raft” compared with hysterectomy, which covers many missed diagnoses.
No risk of spreading cancer cells
Some gynecologists have worried about the risk of disseminating endometrial cancer cells during hysteroscopy. However, Kudela and Pilka21 studied the true risk in women undergoing blind dilation and curettage and hysteroscopy performed with a fluid medium. Cul-de-sac aspiration prior to instrumentation and at the conclusion of the procedure demonstrated no increased risk of positive cytology. They are continuing a Phase II trial comparing outcomes of both groups over 5 years.
6 most common symptoms of venous or air emboli. Anesthesiologists and gynecologists must be vigilant to prevent venous or air emboli. Munro et al12 succinctly outline the 6 most common symptoms:
- pulmonary hypertension
- hypercarbia
- hypoxia
- arrhythmias
- tachypnea
- systemic hypotension
Beware of a drop in end-tidal CO2. The most common sign of impending cardiovascular collapse is a sudden decrease in end-tidal CO2, when the right cardiac outflow tract is obstructed by CO2, which leads to arterial oxygen (O2) desaturation. If such a decrease is suspected, stop the procedure immediately and administer 100% O2. (Also stop nitrous oxide, if used.) Turn the patient to the left lateral decubitis position and use a central venous catheter to aspirate gas, if necessary. Cardiac massage and a precordial thump may dislodge CO2; unfortunately, high false-positive rates of pre-cordial Doppler make its use impractical.
How to minimize risks
- Avoid coaxial gas cooling tips associated with Nd:YAG crystal lasers
- Avoid a steep Trendelenburg position
- Keep cervix covered with sponge or dilator when operative hysteroscope is removed to minimize air embolism
- Deaerate the equipment prior to surgery Use a low-pressure hysteroscopic CO2 insufflator
- Carefully monitor the patient
- Be highly suspicious when vital signs are unstable
POSTOPERATIVE COMPLICATIONS
Some complications of hysteroscopy may not become clinically evident for months or even years. The most common complications of hysteroscopic endometrial ablation include pregnancy, postablation tubal sterilization syndrome, new or worsening dysmenorrhea, hematometra, endometrial cancer, and failure to completely treat symptoms.
Patients scheduled for hysteroscopy must be informed of potential delayed risks of the procedure. In addition, all reproductive-aged women should be advised that pregnancy is possible after endometrial ablation or operative removal of an intracavitary mass; thus, contraception is crucial. The endometrial tissue is resilient and may regenerate after ablation.
Hematometra: Avoid cervical canal
Hematometra is an infrequent late complication of operative hysteroscopy. If menstruating women or those taking hormone replacement therapy experience cyclic or chronic lower pelvic pain after surgery, scarring or narrowing of the endometrial cavity may be the cause. Approximately 1% to 2% of women who undergo operative hysteroscopy experience this phenomenon. Most cases can be treated with cervical dilation alone.
Since the cervical canal contains no endometrial glands, there is no need to treat this area in women undergoing endometrial ablation. In fact, avoiding this area during treatment is a critical component of successful surgery.
Tubal sterilization syndrome possible after endometrial ablation
Consider this syndrome when a patient undergoing endometrial ablation complains of crampy, cyclic, unilateral or bilateral pelvic pain, possibly accompanied by vaginal spotting. Sometimes a unilateral mass can be palpated, but more commonly tenderness is elicited on pelvic examination.
Ultrasound may demonstrate fluid near the cornual region. Laparoscopy confirms the diagnosis by visualizing a swollen, edematous proximal fallopian tube. Salpingectomy may confirm hematosalpinx, chronic or acute inflammation, or hemosiderin deposits.
Treatment includes bilateral cornual resection and reablation of proximal endometrium, or hysterectomy.14
Pregnancy complications
Endometrial ablation is not to be regarded as a method of contraception. Patients needing birth control should consider concurrent tubal ligation or other reliable methods after this procedure.
The frequency of pregnancy after endometrial ablation ranges from 0.2% to 1.6%, though this data may represent underreporting. Pregnancy outcomes have been dismal in women conceiving after endometrial ablation. Complications include preterm labor, premature delivery, intrauterine growth retardation, prenatal death, postpartum hemorrhage, and placentation problems such as placenta accreta, increta, or percreta, as well as placental abruption.15
Uterine dehiscence and sacculation and extremely thin myometrium have been reported after uterine adhesiolysis, uterine perforation during operative hysteroscopy, and with myoma resections. A high index of suspicion is vital when a gravida presents with pelvic pain, decreased fetal movement, vaginal bleeding, or abnormal uterine masses detected ultrasonographically.
Signs of uterine rupture. Pregnancyrelated complications of operative hysteroscopy can be dramatic and fatal if not recognized quickly, as in the case of uterine rupture. Kerimis et al16 describe uterine rupture in a term pregnancy after hysteroscopic resection of a uterine septum. Severe fetal distress, maternal shoulder pain, and abdominal pain led to an emergency cesarean section. Intraoperative findings included a 7-cm tear from left cornua to right cornua. The original metroplasty, performed with cutting diathermy and laparoscopy, was not accompanied by complications or perforation.
Patients who experience intraoperative complications during metroplasty or deep resection of intramural fibroids should be informed of the risk of uterine rupture so they may consider elective cesarean. Regardless of the mode of delivery, prompt attention is vital if fetal distress is suspected.
Postablation warning signs
Patients undergoing endometrial ablation generally have a quick postoperative return to activity, minimal need for postoperative pain medication, and limited complaints. Beware of patients who make frequent postoperative phone calls and have escalating requirements for pain medication. While bowel and bladder injuries are infrequent—as is postoperative endometritis—these must be vigilantly considered and evaluated when patients complain of persistent pain, fever, and general malaise. Office evaluation is necessary, including thorough abdominal and pelvic examinations. Laboratory testing should include electrolytes, complete blood count, sedimentation rate, ultrasound, and a flat plate of the abdomen (kidneys, ureter, and bladder; upright) may be required. Sometimes a computed tomography scan of the pelvis/abdomen may be needed if perforation with bowel or bladder injury is suspected.
Hysteroscopic fibroid removal may be necessary after UAE
Uterine artery embolization (UAE) is gaining popularity for the treatment of symptomatic uterine fibroids. Transcatheter embolization of the uterine artery leads to occlusion of the fibroid, ischemic shrinkage of the fibroid, and shrinkage of residual myometrial tissue. Fibroids may migrate weeks to months after the procedure as the myometrium contracts and the treated fibroid degenerates, leading to delayed discharge, passage of necrotic fibroids, cramps, and heavy bleeding if the fibroid migrates to a submucosal location. Hysteroscopic removal is an obvious option.
Recently, De Iaco et al17 reported the development of a uterine fistula and discontinuity of the myometrium after hysteroscopic resection of an embolized migrated fibroid. They speculated this was due to the development of an avascular myometrium after UAE. The patient was asymptomatic, but routine diagnostic hysteroscopy revealed a 2-cm discontinuity of the uterine wall at the site of the previous resectoscopic myomectomy. The myometrium was white and less than the full thickness.
Ultrasound guidance improves outcomes
Coccia et al18 described the benefits of intraoperative ultrasound guidance during operative hysteroscopy in fibroid treatment and uterine septum removal. Prospective evaluation of 81 patients involved an experienced ultrasonographer who mapped the limits of treatment. Patients were compared to 45 historical controls who had been similarly treated with laparoscopic monitoring. Satisfactory outcomes included relief of menorrhagia, complete resection of fibroids (including full resection of intramural fibroids), and thorough metroplasty of uterine septum.
Ultrasound guidance made it possible to extend the resection beyond the limit conventionally defined by hysteroscopy; none of the patients in the ultrasound group required reintervention. Among controls, a second operation was necessary in 4 cases. Investigators concluded that a wider resection (10 to 15 mm distance from the external surface of the uterus) of fibroids was achieved using ultrasound guidance.
Dr. Bradley reports that she serves as a consultant to Karl Storz, ACMI, Olympus, and Gynecare, and as a lecturer for Novacept.
1. Gurtcheff SE, Sharp HT. Complications associated with global endometrial ablation: the utility of the MAUDE database. Obstet Gynecol. 2003;102:1278-1282.
2. Jansen FW, Vredevoogd CB, Ulzen K, et al. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol. 2000;96:266-270.
3. Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96:517-520.
4. Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: a randomized controlled trial. Obstet Gynecol. 2000;96:890-894.
5. Thomas JA, Leyland N, Durand N, Windrim RD. The use of oral misoprostol as a cervical ripening agent in operative hysteroscopy: a double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2002;186:876-879.
6. Stotz M, Lampart A, Kochli OR, Schneider M. Intraabdominal bleeding masked by hemodilution after hysteroscopy. Anesthesiology. 2000;93:569-570.
7. Cooper JM, Brady RM. Intraoperative and early postoperative complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:347-366.
8. Loffer FD, Bradley LD, Brill AL, Brooks PG, Cooper JM. Hysteroscopic fluid monitoring guidelines. J Am Assoc Gynecol Laparosc. 2000;7:167-168.
9. Bradley LD, Widrich T. Flexible hysteroscopy: a state-of-the-art procedure for gynecologic evaluation. J Am Assoc Gynecol Laparosc. 1995;2:263-267.
10. Neis KJ, Brandner P, Lindemann HJ. Room air as a cause of gas embolism in diagnostic CO2hysteroscopy. Zentralbl Gynakol. 2000;122:222-225.
11. Bradner P, Neis KJ, Ehmer C. The etiology, frequency, and prevention of gas embolism during CO2hysteroscopy. J Am Assoc Gynecol Laparosc. 1999;6:421-428.
12. Munro MG, Weisberg M, Rubinstein E. Gas and air embolization during hysteroscopic electrosurgical vaporization: comparison of gas generation using bipolar and monopolar electrodes in an experimental model. J Am Assoc Gynecol Laparosc. 2001;8:488-494.
13. Murdoch JAC, Gan TJ. Anesthesia for hysteroscopy. Anesthesiol Clin North Am. 2001;1:125-140.
14. Cooper JM, Brady RM. Late complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:367-374.
15. Rogerson L, Gannon B, O’Donovan P. Outcome of pregnancy following endometrial ablation. J Gynecol Surg. 1997;13:155-160.
16. Kerimis P, Zolti M, Sinwany G, Mashiach S, Carp H. Uterine rupture after hysteroscopic resection of uterine septum. Fertil Steril. 2002;77:618-620.
17. De Iaco P, Golfieri R, Ghi T, Muzzupapa G, Ceccarini M, Bovicelli L. Uterine fistula induced by hysteroscopic resection of an embolized migrated fibroid: a rare complication after embolization of uterine fibroids. Fertil Steril. 2001;75:818-820.
18. Coccia ME, Becattini C, Bracco GL, et al. Intraoperative ultrasound guidance for operative hysteroscopy. J Reprod Med. 2000;45:413-418.
19. Brooks-Carter GN, Killackey MA, Neuwirth RS. Adenocarcinoma of the endometrium after endometrial ablation. Obstet Gynecol. 2000;96:836-837.
20. Cooper JM. Swimming lessons: check the water before jumping in. J Am Assoc Gynecol Laparosc. 1998;5:87-90.
21. Kudela M, Pilka R. Is there a real risk in patients with endometrial carcinoma undergoing diagnostic hysteroscopy (HSC)? Eur J Gynecol Oncol. 2001;22:342-344.
1. Gurtcheff SE, Sharp HT. Complications associated with global endometrial ablation: the utility of the MAUDE database. Obstet Gynecol. 2003;102:1278-1282.
2. Jansen FW, Vredevoogd CB, Ulzen K, et al. Complications of hysteroscopy: a prospective, multicenter study. Obstet Gynecol. 2000;96:266-270.
3. Propst AM, Liberman RF, Harlow BL, Ginsburg ES. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96:517-520.
4. Preutthipan S, Herabutya Y. Vaginal misoprostol for cervical priming before operative hysteroscopy: a randomized controlled trial. Obstet Gynecol. 2000;96:890-894.
5. Thomas JA, Leyland N, Durand N, Windrim RD. The use of oral misoprostol as a cervical ripening agent in operative hysteroscopy: a double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2002;186:876-879.
6. Stotz M, Lampart A, Kochli OR, Schneider M. Intraabdominal bleeding masked by hemodilution after hysteroscopy. Anesthesiology. 2000;93:569-570.
7. Cooper JM, Brady RM. Intraoperative and early postoperative complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:347-366.
8. Loffer FD, Bradley LD, Brill AL, Brooks PG, Cooper JM. Hysteroscopic fluid monitoring guidelines. J Am Assoc Gynecol Laparosc. 2000;7:167-168.
9. Bradley LD, Widrich T. Flexible hysteroscopy: a state-of-the-art procedure for gynecologic evaluation. J Am Assoc Gynecol Laparosc. 1995;2:263-267.
10. Neis KJ, Brandner P, Lindemann HJ. Room air as a cause of gas embolism in diagnostic CO2hysteroscopy. Zentralbl Gynakol. 2000;122:222-225.
11. Bradner P, Neis KJ, Ehmer C. The etiology, frequency, and prevention of gas embolism during CO2hysteroscopy. J Am Assoc Gynecol Laparosc. 1999;6:421-428.
12. Munro MG, Weisberg M, Rubinstein E. Gas and air embolization during hysteroscopic electrosurgical vaporization: comparison of gas generation using bipolar and monopolar electrodes in an experimental model. J Am Assoc Gynecol Laparosc. 2001;8:488-494.
13. Murdoch JAC, Gan TJ. Anesthesia for hysteroscopy. Anesthesiol Clin North Am. 2001;1:125-140.
14. Cooper JM, Brady RM. Late complications of operative hysteroscopy. Obstet Gynecol Clin North Am. 2000;27:367-374.
15. Rogerson L, Gannon B, O’Donovan P. Outcome of pregnancy following endometrial ablation. J Gynecol Surg. 1997;13:155-160.
16. Kerimis P, Zolti M, Sinwany G, Mashiach S, Carp H. Uterine rupture after hysteroscopic resection of uterine septum. Fertil Steril. 2002;77:618-620.
17. De Iaco P, Golfieri R, Ghi T, Muzzupapa G, Ceccarini M, Bovicelli L. Uterine fistula induced by hysteroscopic resection of an embolized migrated fibroid: a rare complication after embolization of uterine fibroids. Fertil Steril. 2001;75:818-820.
18. Coccia ME, Becattini C, Bracco GL, et al. Intraoperative ultrasound guidance for operative hysteroscopy. J Reprod Med. 2000;45:413-418.
19. Brooks-Carter GN, Killackey MA, Neuwirth RS. Adenocarcinoma of the endometrium after endometrial ablation. Obstet Gynecol. 2000;96:836-837.
20. Cooper JM. Swimming lessons: check the water before jumping in. J Am Assoc Gynecol Laparosc. 1998;5:87-90.
21. Kudela M, Pilka R. Is there a real risk in patients with endometrial carcinoma undergoing diagnostic hysteroscopy (HSC)? Eur J Gynecol Oncol. 2001;22:342-344.