User login
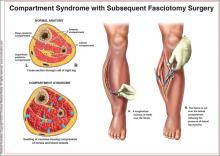
Acute compartment syndrome (ACS) is a condition in which elevated pressures in the confined space of a closed fascial compartment lead to vascular compromise. Typically, ACS develops in the distal extremities after a traumatic event, such as a fracture, crush, or burn injury. A dangerous cycle ensues, involving increased compartmental pressure, decreased tissue perfusion, and continuing ischemia; fluids leak from the vasculature, perpetuating the process.1
Diagnosis of ACS requires a high level of clinical suspicion combined with a keen understanding of the risk factors for ACS and its pathophysiology, as well as astute awareness of the subtle clinical exam findings that usually accompany objective pressure measurements. Though most common in the extremities, ACS may also develop in the buttock, pelvis, or abdominal or spinal musculature.2-5
Prompt recognition and management of ACS are critical: This condition is considered a surgical emergency requiring immediate attention. Urgent decompression by fasciotomy is the definitive treatment6—an essential intervention to prevent critical tissue ischemia and necrosis. Failure to release the fascia in a timely manner can result in poor outcomes for patients, including but not limited to chronic pain, paralysis, sensory or motor deficits, loss of limb, deformity, and renal failure secondary to rhabdomyolysis.7,8
Clinical assessment and severity of injury are the two main factors that lead to prompt diagnosis and treatment of ACS. However, distracting injuries or an unconsciousness patient may interfere with the assessment, decreasing its accuracy.7 Heightened awareness of ACS is paramount so that clinicians can better recognize the condition before complications arise.
EPIDEMIOLOGY
ACS is typically associated with long bone injuries after significant trauma or crush injuries (eg, in a motor vehicle collision). Less often, severe burns, gun shot wounds, snake bites, poor anticoagulation, prolonged surgery (including procedures involving prolonged elevation of a limb),9 nephrotic syndrome, IV infiltrations, and other volume-expanding pathologies can present a similar risk.10-15 Essentially, ACS is a self-perpetuating process resulting from either increased compartmental content (eg, bleeding, edema) or reduced compartment size (eg, tight casting, burns).7,16
Ninety percent of cases of ACS in the extremities occur in men,15 and males younger than 35 have the greatest incidence of posttraumatic ACS.15,17,18 Although fractures account for nearly 70% of confirmed cases of ACS, soft-tissue injuries (particularly vascular injuries15) are also associated with ACS.18 Clinicians should be aware of a key misconception: that open fractures relieve intracompartmental pressure, reducing the risk for ACS. Rather, the damage and inflammation that occur in open fractures pose the same risk for ACS as do closed fractures.15
PATIENT PRESENTATION
A key consideration, in addition to the history and mechanism of injury or illness, is that patient’s description of the pain: The conscious patient will complain of pain that worsens progressively over time and that usually seems out of proportion to the physical exam findings or apparent injury.19 For this reason, serial assessments are recommended; worsening pain is indicative of rapid evolution of ACS, with the possibility of irreversible necrosis.20
Pain with passive stretch is an excellent indicator that the pathology is progressing.2 The “classic P” signs and symptoms of ACS are progressively later findings that represent markedly significant ischemia and injury already in progress: paresthesia, paresis/paralysis, poikilothermia (the inability of the patient to maintain a constant core temperature), pulselessness, and pallor.17
Diminishment of pain suggests a poor prognosis, as this indicates that the tissue is likely nonviable and necrotic by that point.1
Clinicians must be mindful that, in patients with central or peripheral nerve deficits or in those who have undergone nerve blocks or regional anesthesia, pain may be absent; in these instances, the risk for delayed diagnosis is increased.14,21 Furthermore, pain tolerance varies among patients, and distracting injuries may also cloud the clinical picture.
The diagnosis of ACS is not easily made. Thus, clinical suspicion should be elevated in any high-energy scenario: an extensive burn, overly restrictive casting, or volume-expanding disorder—especially in patients who present with pain that is out of proportion to the injury or is worsening progressively.1,7
PHYSICAL EXAMINATION
Physical examination and assessment should be repeated every two to four hours. The examining clinician should focus on pain characteristics, the skin, and the “classic P” signs mentioned above. Marked tenderness out of proportion to the injury, unmanageable pain, and pain on passive movement of the limb are the strongest indicators of developing ACS. These findings are nearly universal in the conscious patient, but they can be confused with primary pain of the injury itself. Additional findings of sensory or motor impairments may help raise the clinician’s suspicion for ACS but are nonspecific.1,7,23 Palpable tenseness is a common observation in ACS, but a clinician’s subjective measurement of skin firmness has little value as an objective strategy.22 Absence of distal pulses, decreased skin temperature, and pallor are very late signs that pressures are sufficiently high to cause complete arterial occlusion.20 Thus, clinicians should not wait for absent pulses or pallor to begin treatment. Suspicion for ACS should arise as soon as pain associated with trauma or surgery intensifies or becomes unmanageable.
DIAGNOSTIC TOOLS
ACS is diagnosed based on the previously mentioned clinical signs, along with objective evidence of decreased tissue perfusion in the affected limbs.24 Direct intracompartmental pressure measurements, obtained using a needle- or catheter-based technique, are the most common means of identifying ACS.7 However, near infrared spectroscopy and infrared imaging have also been found helpful.7, 25
Direct Intracompartmental Pressure Monitoring
Supported by clinical findings, direct measurement of the intracompartmental pressure (ICP) using a needle transducer is the most accurate method for detecting ACS and guiding treatment choices. Several measurement options are available, including a handheld needle manometer, which records single-pressure readings, or a wick or slit catheter, which can record continuous pressures.13 The choice between devices is based on facility/provider preference, as any commercial pressure device can be used with similar accuracies. When no such device is available, an 18-gauge needle with a set-up resembling an arterial line is another option.1
When using these instruments, it is essential for the provider to implement proper sterile technique and to record pressures from compartments within a 5-cm radius of the injury site.17 Furthermore, clinicians should be aware that the ICP can vary greatly and that tolerance to increased pressure varies with the patient’s diastolic blood pressure.
Normal capillary perfusion pressure (diastolic blood pressure minus compartment pressure) is approximately 30 mm Hg. Therefore, absolute ICP readings above 30 mm Hg can be diagnostic of ACS and indicative of fasciotomy. Likewise, a perfusion pressure below 30 mm Hg is also diagnostic. Based on consensus, either calculation can be used.16,17
Infrared Spectroscopy and Imaging
Barker and colleagues7 describe use of near infrared spectroscopy (NIRS) as a method to diagnose lower-extremity ACS. Noninvasive skin probes can detect the absorption spectra of mixed venous hemoglobin levels beneath the skin to determine oxygen saturation (StO2). StO2 measured by NIRS is location dependent, enabling the clinician to monitor oxygenation levels in any illuminated tissue. NIRS has great potential for use in confirming a diagnosis of ACS since decreased perfusion pressure from elevated ICP correlates well with decreased StO2. However, this technique has limitations that currently prevent its widespread use as a diagnostic tool.7
Use of noninvasive infrared imaging has also been investigated. Katz and colleagues25 describe the use of a long-wave infrared camera and thermographic imaging analysis software to detect differences in surface temperatures in patients’ limbs after blunt trauma. The researchers were able to correlate declines in temperature with decreased blood flow. They proposed that this modality be used to make the diagnosis of ACS before the development of muscle ischemia and necrosis. This software is still being investigated.25
Imaging Studies
CT or MRI can be helpful in identifying swelling, hematomas, or areas of necrosis, but their specificity is not sufficient to confirm elevated compartmental pressures24; additionally, MRI cannot distinguish between swelling resulting from soft-tissue injury and swelling in muscles affected by ACS.26, 27
Ultrasound also has potential as a diagnostic tool, as it helps clinicians visualize soft-tissue structures and assess the patency of large arteries and veins; the absence of venous outflow may suggest ACS. However, the efficacy of ultrasound in diagnosing ACS has proven inconsistent, and it is not recommended over direct ICP measurements. Instead, ultrasound can be relied on as an adjunctive modality.13
Blood Tests
Laboratory tests cannot contribute toward the diagnosis of ACS, but assessment of renal function and skeletal muscle breakdown is important to identify potential complications of ACS. Obtaining baseline levels in blood urea nitrogen and creatinine will help identify changes in kidney function, while potassium, urates, creatine phosphokinase (CPK), and myoglobin can be measured to assess for muscle breakdown. Findings of myoglobinuria with elevated CPK are strongly indicative of rhabdomyolysis, which can easily precipitate acute renal failure.8
Results from a complete blood count or prothrombin time/partial thromboplastin time may facilitate monitoring for blood loss or identification of contributing bleeding disorders. Because surgery remains the definitive treatment for ACS, a type and screen is essential in the workup, as blood products and transfusion are likely to be required during treatment.
MANAGEMENT
Fasciotomy remains the standard of care for patients presenting with the clinical signs and symptoms of increased compartment pressure consistent with ACS.16 Researchers engaged in animal studies and human case reports have shown that fasciotomy must be performed within six hours of injury to prevent adverse outcomes.8,16,28
As definitive treatment for ACS, a complete fasciotomy of all compartments in the vicinity of the injury should be performed. The most effective approach for fasciotomy consists of two long skin incisions (ie, double-incision radical dissection, to prevent concomitant increased pressure within the boundary of the skin), on opposite aspects of the affected limb, to ensure that all compartmental fascia can be decompressed.17,26,28
Delayed primary intention on postop day 5 is the preferred method for fasciotomy wound closure. Wounds should remain open to allow limb swelling to subside. In severe cases, when delayed primary intention cannot be performed in the window of time described, split-thickness skin grafts can be used. Postoperative wounds should be packed open with bulky dressings and changed daily. Negative-pressure wound dressings can be used for improved and accelerated wound closure.29
In the setting of ACS or limb ischemia, it is important to consider the possibility that tissue destruction will lead to significant myoglobinuria and potentially rhabdomyolysis.8 In patients presenting with crush injuries, or trauma patients who experience a significant rise in creatine kinase, the kidneys should be protected via extracellular resuscitation with isotonic fluids. The goal of resuscitation is to maintain urinary output of at least 200 mL/h to prevent renal failure.29
PATIENT EDUCATION
Patients who undergo fasciotomy within 12 hours of onset of signs and symptoms of ACS retain normal limb function in 68% of cases. However, this outcome falls to 8% if fasciotomy is delayed longer than 12 hours.30 Patients should understand that return to normal function usually takes two to three months and requires active participation by the patient. Furthermore, 20% of patients have some motor and sensory deficits at one year postfasciotomy.17
FOLLOW-UP
After fasciotomy, patients will require adequate pain control and an extensive rehabilitation program. Early physical therapy should progress slowly, with focus on range-of-motion and stretching exercises. Once patients have regained the ability to ambulate, resistance exercises and moderate exercise activities should be implemented to return them to their regular activities.31
CONCLUSION
ACS figures significantly in the long-term morbidity and mortality associated with trauma. Clinical research and laboratory science have indicated that ACS must be treated within six hours to prevent life-long deformity and disability. New diagnostic and therapeutic approaches must be investigated to improve outcomes. The most widely accepted surgical approach is the double-incision radical dissection of all fascia within the affected limb.
Appropriate management must include protection of the patient’s kidneys, given the risk for rhabdomyolysis, as well as extensive postoperative physical therapy. Given the invasive treatment required for ACS, progression toward full recovery is a long and difficult process. However, with prompt recognition and early intervention, full return to normal function is possible, with little to no deformity or dysfunction.
REFERECNES
1. Murdock M, Murdoch MM. Compartment syndrome: a review of the literature. Clin Podiatr Med Surg. 2012;29:301-310, viii.
2. Osteen KD, Haque SH. Bilateral gluteal compartment syndrome following right total knee revision: a case report. Ochsner J. 2012;12:141-144.
3. Paryavi E, Jobin CM, Ludwig SC, et al. Acute exertional lumbar paraspinal compartment syndrome. Spine. 2010;35:E1529-E1533.
4. Bosch U, Tscherne H. The pelvic compartment syndrome. Arch Ortho Trauma Surg. 1992;111:314-317.
5. Maeda A, Wakabayashi K, Suzuki H. Acute limb ischemia due to abdominal compartment syndrome. Catheter Cardiovasc Interv. 2013 Jun 19. [Epub ahead of print]
6. Masquelet AC. Acute compartment syndrome of the leg: pressure measurement and fasciotomy. Orthop Trumatol Surg Res. 2010;96:913-917.
7. Barker T, Midwinter M, Porter K. The diagnosis of acute lower limb compartment syndrome: applications of near infrared spectroscopy. Trauma. 2011;13:125-136.
8. Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62-72.
9. Karmaniolou I, Staikou C. Compartment syndrome as a complication of the lithotomy position. West Indian Med J. 2010;59:698-701.
10. Pietrangiolillo Z, Frassoldati R, Leonelli V, et al. Compartment syndrome after viper-bite in toddler: case report and review of literature. Acta Biomed. 2012;83:44-50.
11. Kakkar R, Ellis M, Fearon PV. Compartment syndrome of the thigh as a complication of anticoagulant therapy in a patient with a left ventricular assist device (Berlin Heart). Gen Thorac Cardiovasc Surg. 2010;58: 477-479.
12. Balogh ZJ, Leppäniemi A. Patient populations at risk for intra-abdominal hypertension and abdominal compartment syndrome. Am Surg. 2011;77 suppl 1:S12-S16.
13. Zimmerman DC, Kapoor T, Elfond M, Scott P. Spontaneous compartment syndrome of the upper arm in a patient receiving anticoagulation therapy. J Emerg Med. 2013;44:e53-e56.
14. Olson SA. Acute compartment syndrome in lower extremity musculoskeletal trauma. J Am Acad Orthop Surg. 2005;13(7):436-444.
15. Branco BC, Inaba K, Barmparas G, et al. Incidence and predictors for the need for fasciotomy after extremity trauma: a 10-year review in a mature level I trauma centre. Injury. 2011;42:1157-1163.
16. Wall CJ, Richardson, Lowe AJ, et al. Survey of management of acute, traumatic compartment syndrome of the leg in Australia. ANZ J Surg. 2007;77:733-737.
17. Wall CJ, Lynch J, Harris IA, et al; Liverpool (Sydney) and Royal Melbourne Hospitals. Clinical practice guidelines for the management of acute limb compartment syndrome following trauma. ANZ J Surg. 2010;80:151-156.
18. McQueen MM, Gaston P, Court-Brown CM. Acute compartment syndrome: who is at risk? J Bone Joint Surg. 2000;82:200-203.
19. Khan M, Hodkinson SL. Acute compartment syndrome—presenting as severe pain in an extremity out of proportion with the injury. J R Army Med Corps. 1997;143:165-166.
20. Percival TJ, White JM, Ricci MA. Compartment syndrome in the setting of vascular injury. Perspect Vasc Surg Endovasc Ther. 2011;23(2):119-124.
21. Badhe S, Baiju D, Elliot R, et al. The ‘silent’ compartment syndrome. Injury. 2009;40:220-222.
22. Shuler FD, Dietz MJ. Physicians’ ability to manually detect isolated elevations in leg intracompartmental pressure. J Bone Joint Surg Am. 2010;92:361-367.
23. Taylor RM, Sullivan MP, Mehta S. Acute compartment syndrome: obtaining diagnosis, providing treatment, and minimizing medicolegal risk. Curr Rev Musculoskelet Med. 2012;5(3):206-213.
24. McDonald S, Bearcroft P. Compartment syndromes. Semin Musculoskelet Radiol. 2010;14:236-244.
25. Katz LM, Nauriyal V, Nagaraj S, et al. Infrared imaging of trauma patients for detection of acute compartment syndrome of the leg. Crit Care Med. 2008;36:1756-1761.
26. Shadgan B, Menon M, Sanders D, et al. Current thinking about acute compartment syndrome of the lower extremity. Can J Surg. 2010;53:329-334.
27. Rominger M, Lukosch C, Bachmann G, et al. Compartment syndrome: value of MR imaging. Radiology. 1995;197:296.
28. Kashuk JL, Moore EE, Pinski S, et al. Lower extremity compartment syndrome in the acute care surgery paradigm: safety lessons learned. Patient Saf Surg. 2009;3(1):11.
29. Rasul AT Jr. Acute compartment syndrome (2011). emedicine.medscape.com/article/307668-overview#showall. Accessed June 25, 2013.
30. Sheridan GW, Matsen FA 3rd. Fasciotomy in the treatment of the acute compartment syndrome. J Bone Joint Surg Am. 1976;58(1):112-115.
31. Schubert AG. Exertional compartment syndrome: review of the literature and proposed rehabilitation guidelines following surgical release. Intern J Sports Phys Ther. 2011;6:126-141.
Acute compartment syndrome (ACS) is a condition in which elevated pressures in the confined space of a closed fascial compartment lead to vascular compromise. Typically, ACS develops in the distal extremities after a traumatic event, such as a fracture, crush, or burn injury. A dangerous cycle ensues, involving increased compartmental pressure, decreased tissue perfusion, and continuing ischemia; fluids leak from the vasculature, perpetuating the process.1
Diagnosis of ACS requires a high level of clinical suspicion combined with a keen understanding of the risk factors for ACS and its pathophysiology, as well as astute awareness of the subtle clinical exam findings that usually accompany objective pressure measurements. Though most common in the extremities, ACS may also develop in the buttock, pelvis, or abdominal or spinal musculature.2-5
Prompt recognition and management of ACS are critical: This condition is considered a surgical emergency requiring immediate attention. Urgent decompression by fasciotomy is the definitive treatment6—an essential intervention to prevent critical tissue ischemia and necrosis. Failure to release the fascia in a timely manner can result in poor outcomes for patients, including but not limited to chronic pain, paralysis, sensory or motor deficits, loss of limb, deformity, and renal failure secondary to rhabdomyolysis.7,8
Clinical assessment and severity of injury are the two main factors that lead to prompt diagnosis and treatment of ACS. However, distracting injuries or an unconsciousness patient may interfere with the assessment, decreasing its accuracy.7 Heightened awareness of ACS is paramount so that clinicians can better recognize the condition before complications arise.
EPIDEMIOLOGY
ACS is typically associated with long bone injuries after significant trauma or crush injuries (eg, in a motor vehicle collision). Less often, severe burns, gun shot wounds, snake bites, poor anticoagulation, prolonged surgery (including procedures involving prolonged elevation of a limb),9 nephrotic syndrome, IV infiltrations, and other volume-expanding pathologies can present a similar risk.10-15 Essentially, ACS is a self-perpetuating process resulting from either increased compartmental content (eg, bleeding, edema) or reduced compartment size (eg, tight casting, burns).7,16
Ninety percent of cases of ACS in the extremities occur in men,15 and males younger than 35 have the greatest incidence of posttraumatic ACS.15,17,18 Although fractures account for nearly 70% of confirmed cases of ACS, soft-tissue injuries (particularly vascular injuries15) are also associated with ACS.18 Clinicians should be aware of a key misconception: that open fractures relieve intracompartmental pressure, reducing the risk for ACS. Rather, the damage and inflammation that occur in open fractures pose the same risk for ACS as do closed fractures.15
PATIENT PRESENTATION
A key consideration, in addition to the history and mechanism of injury or illness, is that patient’s description of the pain: The conscious patient will complain of pain that worsens progressively over time and that usually seems out of proportion to the physical exam findings or apparent injury.19 For this reason, serial assessments are recommended; worsening pain is indicative of rapid evolution of ACS, with the possibility of irreversible necrosis.20
Pain with passive stretch is an excellent indicator that the pathology is progressing.2 The “classic P” signs and symptoms of ACS are progressively later findings that represent markedly significant ischemia and injury already in progress: paresthesia, paresis/paralysis, poikilothermia (the inability of the patient to maintain a constant core temperature), pulselessness, and pallor.17
Diminishment of pain suggests a poor prognosis, as this indicates that the tissue is likely nonviable and necrotic by that point.1
Clinicians must be mindful that, in patients with central or peripheral nerve deficits or in those who have undergone nerve blocks or regional anesthesia, pain may be absent; in these instances, the risk for delayed diagnosis is increased.14,21 Furthermore, pain tolerance varies among patients, and distracting injuries may also cloud the clinical picture.
The diagnosis of ACS is not easily made. Thus, clinical suspicion should be elevated in any high-energy scenario: an extensive burn, overly restrictive casting, or volume-expanding disorder—especially in patients who present with pain that is out of proportion to the injury or is worsening progressively.1,7
PHYSICAL EXAMINATION
Physical examination and assessment should be repeated every two to four hours. The examining clinician should focus on pain characteristics, the skin, and the “classic P” signs mentioned above. Marked tenderness out of proportion to the injury, unmanageable pain, and pain on passive movement of the limb are the strongest indicators of developing ACS. These findings are nearly universal in the conscious patient, but they can be confused with primary pain of the injury itself. Additional findings of sensory or motor impairments may help raise the clinician’s suspicion for ACS but are nonspecific.1,7,23 Palpable tenseness is a common observation in ACS, but a clinician’s subjective measurement of skin firmness has little value as an objective strategy.22 Absence of distal pulses, decreased skin temperature, and pallor are very late signs that pressures are sufficiently high to cause complete arterial occlusion.20 Thus, clinicians should not wait for absent pulses or pallor to begin treatment. Suspicion for ACS should arise as soon as pain associated with trauma or surgery intensifies or becomes unmanageable.
DIAGNOSTIC TOOLS
ACS is diagnosed based on the previously mentioned clinical signs, along with objective evidence of decreased tissue perfusion in the affected limbs.24 Direct intracompartmental pressure measurements, obtained using a needle- or catheter-based technique, are the most common means of identifying ACS.7 However, near infrared spectroscopy and infrared imaging have also been found helpful.7, 25
Direct Intracompartmental Pressure Monitoring
Supported by clinical findings, direct measurement of the intracompartmental pressure (ICP) using a needle transducer is the most accurate method for detecting ACS and guiding treatment choices. Several measurement options are available, including a handheld needle manometer, which records single-pressure readings, or a wick or slit catheter, which can record continuous pressures.13 The choice between devices is based on facility/provider preference, as any commercial pressure device can be used with similar accuracies. When no such device is available, an 18-gauge needle with a set-up resembling an arterial line is another option.1
When using these instruments, it is essential for the provider to implement proper sterile technique and to record pressures from compartments within a 5-cm radius of the injury site.17 Furthermore, clinicians should be aware that the ICP can vary greatly and that tolerance to increased pressure varies with the patient’s diastolic blood pressure.
Normal capillary perfusion pressure (diastolic blood pressure minus compartment pressure) is approximately 30 mm Hg. Therefore, absolute ICP readings above 30 mm Hg can be diagnostic of ACS and indicative of fasciotomy. Likewise, a perfusion pressure below 30 mm Hg is also diagnostic. Based on consensus, either calculation can be used.16,17
Infrared Spectroscopy and Imaging
Barker and colleagues7 describe use of near infrared spectroscopy (NIRS) as a method to diagnose lower-extremity ACS. Noninvasive skin probes can detect the absorption spectra of mixed venous hemoglobin levels beneath the skin to determine oxygen saturation (StO2). StO2 measured by NIRS is location dependent, enabling the clinician to monitor oxygenation levels in any illuminated tissue. NIRS has great potential for use in confirming a diagnosis of ACS since decreased perfusion pressure from elevated ICP correlates well with decreased StO2. However, this technique has limitations that currently prevent its widespread use as a diagnostic tool.7
Use of noninvasive infrared imaging has also been investigated. Katz and colleagues25 describe the use of a long-wave infrared camera and thermographic imaging analysis software to detect differences in surface temperatures in patients’ limbs after blunt trauma. The researchers were able to correlate declines in temperature with decreased blood flow. They proposed that this modality be used to make the diagnosis of ACS before the development of muscle ischemia and necrosis. This software is still being investigated.25
Imaging Studies
CT or MRI can be helpful in identifying swelling, hematomas, or areas of necrosis, but their specificity is not sufficient to confirm elevated compartmental pressures24; additionally, MRI cannot distinguish between swelling resulting from soft-tissue injury and swelling in muscles affected by ACS.26, 27
Ultrasound also has potential as a diagnostic tool, as it helps clinicians visualize soft-tissue structures and assess the patency of large arteries and veins; the absence of venous outflow may suggest ACS. However, the efficacy of ultrasound in diagnosing ACS has proven inconsistent, and it is not recommended over direct ICP measurements. Instead, ultrasound can be relied on as an adjunctive modality.13
Blood Tests
Laboratory tests cannot contribute toward the diagnosis of ACS, but assessment of renal function and skeletal muscle breakdown is important to identify potential complications of ACS. Obtaining baseline levels in blood urea nitrogen and creatinine will help identify changes in kidney function, while potassium, urates, creatine phosphokinase (CPK), and myoglobin can be measured to assess for muscle breakdown. Findings of myoglobinuria with elevated CPK are strongly indicative of rhabdomyolysis, which can easily precipitate acute renal failure.8
Results from a complete blood count or prothrombin time/partial thromboplastin time may facilitate monitoring for blood loss or identification of contributing bleeding disorders. Because surgery remains the definitive treatment for ACS, a type and screen is essential in the workup, as blood products and transfusion are likely to be required during treatment.
MANAGEMENT
Fasciotomy remains the standard of care for patients presenting with the clinical signs and symptoms of increased compartment pressure consistent with ACS.16 Researchers engaged in animal studies and human case reports have shown that fasciotomy must be performed within six hours of injury to prevent adverse outcomes.8,16,28
As definitive treatment for ACS, a complete fasciotomy of all compartments in the vicinity of the injury should be performed. The most effective approach for fasciotomy consists of two long skin incisions (ie, double-incision radical dissection, to prevent concomitant increased pressure within the boundary of the skin), on opposite aspects of the affected limb, to ensure that all compartmental fascia can be decompressed.17,26,28
Delayed primary intention on postop day 5 is the preferred method for fasciotomy wound closure. Wounds should remain open to allow limb swelling to subside. In severe cases, when delayed primary intention cannot be performed in the window of time described, split-thickness skin grafts can be used. Postoperative wounds should be packed open with bulky dressings and changed daily. Negative-pressure wound dressings can be used for improved and accelerated wound closure.29
In the setting of ACS or limb ischemia, it is important to consider the possibility that tissue destruction will lead to significant myoglobinuria and potentially rhabdomyolysis.8 In patients presenting with crush injuries, or trauma patients who experience a significant rise in creatine kinase, the kidneys should be protected via extracellular resuscitation with isotonic fluids. The goal of resuscitation is to maintain urinary output of at least 200 mL/h to prevent renal failure.29
PATIENT EDUCATION
Patients who undergo fasciotomy within 12 hours of onset of signs and symptoms of ACS retain normal limb function in 68% of cases. However, this outcome falls to 8% if fasciotomy is delayed longer than 12 hours.30 Patients should understand that return to normal function usually takes two to three months and requires active participation by the patient. Furthermore, 20% of patients have some motor and sensory deficits at one year postfasciotomy.17
FOLLOW-UP
After fasciotomy, patients will require adequate pain control and an extensive rehabilitation program. Early physical therapy should progress slowly, with focus on range-of-motion and stretching exercises. Once patients have regained the ability to ambulate, resistance exercises and moderate exercise activities should be implemented to return them to their regular activities.31
CONCLUSION
ACS figures significantly in the long-term morbidity and mortality associated with trauma. Clinical research and laboratory science have indicated that ACS must be treated within six hours to prevent life-long deformity and disability. New diagnostic and therapeutic approaches must be investigated to improve outcomes. The most widely accepted surgical approach is the double-incision radical dissection of all fascia within the affected limb.
Appropriate management must include protection of the patient’s kidneys, given the risk for rhabdomyolysis, as well as extensive postoperative physical therapy. Given the invasive treatment required for ACS, progression toward full recovery is a long and difficult process. However, with prompt recognition and early intervention, full return to normal function is possible, with little to no deformity or dysfunction.
REFERECNES
1. Murdock M, Murdoch MM. Compartment syndrome: a review of the literature. Clin Podiatr Med Surg. 2012;29:301-310, viii.
2. Osteen KD, Haque SH. Bilateral gluteal compartment syndrome following right total knee revision: a case report. Ochsner J. 2012;12:141-144.
3. Paryavi E, Jobin CM, Ludwig SC, et al. Acute exertional lumbar paraspinal compartment syndrome. Spine. 2010;35:E1529-E1533.
4. Bosch U, Tscherne H. The pelvic compartment syndrome. Arch Ortho Trauma Surg. 1992;111:314-317.
5. Maeda A, Wakabayashi K, Suzuki H. Acute limb ischemia due to abdominal compartment syndrome. Catheter Cardiovasc Interv. 2013 Jun 19. [Epub ahead of print]
6. Masquelet AC. Acute compartment syndrome of the leg: pressure measurement and fasciotomy. Orthop Trumatol Surg Res. 2010;96:913-917.
7. Barker T, Midwinter M, Porter K. The diagnosis of acute lower limb compartment syndrome: applications of near infrared spectroscopy. Trauma. 2011;13:125-136.
8. Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62-72.
9. Karmaniolou I, Staikou C. Compartment syndrome as a complication of the lithotomy position. West Indian Med J. 2010;59:698-701.
10. Pietrangiolillo Z, Frassoldati R, Leonelli V, et al. Compartment syndrome after viper-bite in toddler: case report and review of literature. Acta Biomed. 2012;83:44-50.
11. Kakkar R, Ellis M, Fearon PV. Compartment syndrome of the thigh as a complication of anticoagulant therapy in a patient with a left ventricular assist device (Berlin Heart). Gen Thorac Cardiovasc Surg. 2010;58: 477-479.
12. Balogh ZJ, Leppäniemi A. Patient populations at risk for intra-abdominal hypertension and abdominal compartment syndrome. Am Surg. 2011;77 suppl 1:S12-S16.
13. Zimmerman DC, Kapoor T, Elfond M, Scott P. Spontaneous compartment syndrome of the upper arm in a patient receiving anticoagulation therapy. J Emerg Med. 2013;44:e53-e56.
14. Olson SA. Acute compartment syndrome in lower extremity musculoskeletal trauma. J Am Acad Orthop Surg. 2005;13(7):436-444.
15. Branco BC, Inaba K, Barmparas G, et al. Incidence and predictors for the need for fasciotomy after extremity trauma: a 10-year review in a mature level I trauma centre. Injury. 2011;42:1157-1163.
16. Wall CJ, Richardson, Lowe AJ, et al. Survey of management of acute, traumatic compartment syndrome of the leg in Australia. ANZ J Surg. 2007;77:733-737.
17. Wall CJ, Lynch J, Harris IA, et al; Liverpool (Sydney) and Royal Melbourne Hospitals. Clinical practice guidelines for the management of acute limb compartment syndrome following trauma. ANZ J Surg. 2010;80:151-156.
18. McQueen MM, Gaston P, Court-Brown CM. Acute compartment syndrome: who is at risk? J Bone Joint Surg. 2000;82:200-203.
19. Khan M, Hodkinson SL. Acute compartment syndrome—presenting as severe pain in an extremity out of proportion with the injury. J R Army Med Corps. 1997;143:165-166.
20. Percival TJ, White JM, Ricci MA. Compartment syndrome in the setting of vascular injury. Perspect Vasc Surg Endovasc Ther. 2011;23(2):119-124.
21. Badhe S, Baiju D, Elliot R, et al. The ‘silent’ compartment syndrome. Injury. 2009;40:220-222.
22. Shuler FD, Dietz MJ. Physicians’ ability to manually detect isolated elevations in leg intracompartmental pressure. J Bone Joint Surg Am. 2010;92:361-367.
23. Taylor RM, Sullivan MP, Mehta S. Acute compartment syndrome: obtaining diagnosis, providing treatment, and minimizing medicolegal risk. Curr Rev Musculoskelet Med. 2012;5(3):206-213.
24. McDonald S, Bearcroft P. Compartment syndromes. Semin Musculoskelet Radiol. 2010;14:236-244.
25. Katz LM, Nauriyal V, Nagaraj S, et al. Infrared imaging of trauma patients for detection of acute compartment syndrome of the leg. Crit Care Med. 2008;36:1756-1761.
26. Shadgan B, Menon M, Sanders D, et al. Current thinking about acute compartment syndrome of the lower extremity. Can J Surg. 2010;53:329-334.
27. Rominger M, Lukosch C, Bachmann G, et al. Compartment syndrome: value of MR imaging. Radiology. 1995;197:296.
28. Kashuk JL, Moore EE, Pinski S, et al. Lower extremity compartment syndrome in the acute care surgery paradigm: safety lessons learned. Patient Saf Surg. 2009;3(1):11.
29. Rasul AT Jr. Acute compartment syndrome (2011). emedicine.medscape.com/article/307668-overview#showall. Accessed June 25, 2013.
30. Sheridan GW, Matsen FA 3rd. Fasciotomy in the treatment of the acute compartment syndrome. J Bone Joint Surg Am. 1976;58(1):112-115.
31. Schubert AG. Exertional compartment syndrome: review of the literature and proposed rehabilitation guidelines following surgical release. Intern J Sports Phys Ther. 2011;6:126-141.
Acute compartment syndrome (ACS) is a condition in which elevated pressures in the confined space of a closed fascial compartment lead to vascular compromise. Typically, ACS develops in the distal extremities after a traumatic event, such as a fracture, crush, or burn injury. A dangerous cycle ensues, involving increased compartmental pressure, decreased tissue perfusion, and continuing ischemia; fluids leak from the vasculature, perpetuating the process.1
Diagnosis of ACS requires a high level of clinical suspicion combined with a keen understanding of the risk factors for ACS and its pathophysiology, as well as astute awareness of the subtle clinical exam findings that usually accompany objective pressure measurements. Though most common in the extremities, ACS may also develop in the buttock, pelvis, or abdominal or spinal musculature.2-5
Prompt recognition and management of ACS are critical: This condition is considered a surgical emergency requiring immediate attention. Urgent decompression by fasciotomy is the definitive treatment6—an essential intervention to prevent critical tissue ischemia and necrosis. Failure to release the fascia in a timely manner can result in poor outcomes for patients, including but not limited to chronic pain, paralysis, sensory or motor deficits, loss of limb, deformity, and renal failure secondary to rhabdomyolysis.7,8
Clinical assessment and severity of injury are the two main factors that lead to prompt diagnosis and treatment of ACS. However, distracting injuries or an unconsciousness patient may interfere with the assessment, decreasing its accuracy.7 Heightened awareness of ACS is paramount so that clinicians can better recognize the condition before complications arise.
EPIDEMIOLOGY
ACS is typically associated with long bone injuries after significant trauma or crush injuries (eg, in a motor vehicle collision). Less often, severe burns, gun shot wounds, snake bites, poor anticoagulation, prolonged surgery (including procedures involving prolonged elevation of a limb),9 nephrotic syndrome, IV infiltrations, and other volume-expanding pathologies can present a similar risk.10-15 Essentially, ACS is a self-perpetuating process resulting from either increased compartmental content (eg, bleeding, edema) or reduced compartment size (eg, tight casting, burns).7,16
Ninety percent of cases of ACS in the extremities occur in men,15 and males younger than 35 have the greatest incidence of posttraumatic ACS.15,17,18 Although fractures account for nearly 70% of confirmed cases of ACS, soft-tissue injuries (particularly vascular injuries15) are also associated with ACS.18 Clinicians should be aware of a key misconception: that open fractures relieve intracompartmental pressure, reducing the risk for ACS. Rather, the damage and inflammation that occur in open fractures pose the same risk for ACS as do closed fractures.15
PATIENT PRESENTATION
A key consideration, in addition to the history and mechanism of injury or illness, is that patient’s description of the pain: The conscious patient will complain of pain that worsens progressively over time and that usually seems out of proportion to the physical exam findings or apparent injury.19 For this reason, serial assessments are recommended; worsening pain is indicative of rapid evolution of ACS, with the possibility of irreversible necrosis.20
Pain with passive stretch is an excellent indicator that the pathology is progressing.2 The “classic P” signs and symptoms of ACS are progressively later findings that represent markedly significant ischemia and injury already in progress: paresthesia, paresis/paralysis, poikilothermia (the inability of the patient to maintain a constant core temperature), pulselessness, and pallor.17
Diminishment of pain suggests a poor prognosis, as this indicates that the tissue is likely nonviable and necrotic by that point.1
Clinicians must be mindful that, in patients with central or peripheral nerve deficits or in those who have undergone nerve blocks or regional anesthesia, pain may be absent; in these instances, the risk for delayed diagnosis is increased.14,21 Furthermore, pain tolerance varies among patients, and distracting injuries may also cloud the clinical picture.
The diagnosis of ACS is not easily made. Thus, clinical suspicion should be elevated in any high-energy scenario: an extensive burn, overly restrictive casting, or volume-expanding disorder—especially in patients who present with pain that is out of proportion to the injury or is worsening progressively.1,7
PHYSICAL EXAMINATION
Physical examination and assessment should be repeated every two to four hours. The examining clinician should focus on pain characteristics, the skin, and the “classic P” signs mentioned above. Marked tenderness out of proportion to the injury, unmanageable pain, and pain on passive movement of the limb are the strongest indicators of developing ACS. These findings are nearly universal in the conscious patient, but they can be confused with primary pain of the injury itself. Additional findings of sensory or motor impairments may help raise the clinician’s suspicion for ACS but are nonspecific.1,7,23 Palpable tenseness is a common observation in ACS, but a clinician’s subjective measurement of skin firmness has little value as an objective strategy.22 Absence of distal pulses, decreased skin temperature, and pallor are very late signs that pressures are sufficiently high to cause complete arterial occlusion.20 Thus, clinicians should not wait for absent pulses or pallor to begin treatment. Suspicion for ACS should arise as soon as pain associated with trauma or surgery intensifies or becomes unmanageable.
DIAGNOSTIC TOOLS
ACS is diagnosed based on the previously mentioned clinical signs, along with objective evidence of decreased tissue perfusion in the affected limbs.24 Direct intracompartmental pressure measurements, obtained using a needle- or catheter-based technique, are the most common means of identifying ACS.7 However, near infrared spectroscopy and infrared imaging have also been found helpful.7, 25
Direct Intracompartmental Pressure Monitoring
Supported by clinical findings, direct measurement of the intracompartmental pressure (ICP) using a needle transducer is the most accurate method for detecting ACS and guiding treatment choices. Several measurement options are available, including a handheld needle manometer, which records single-pressure readings, or a wick or slit catheter, which can record continuous pressures.13 The choice between devices is based on facility/provider preference, as any commercial pressure device can be used with similar accuracies. When no such device is available, an 18-gauge needle with a set-up resembling an arterial line is another option.1
When using these instruments, it is essential for the provider to implement proper sterile technique and to record pressures from compartments within a 5-cm radius of the injury site.17 Furthermore, clinicians should be aware that the ICP can vary greatly and that tolerance to increased pressure varies with the patient’s diastolic blood pressure.
Normal capillary perfusion pressure (diastolic blood pressure minus compartment pressure) is approximately 30 mm Hg. Therefore, absolute ICP readings above 30 mm Hg can be diagnostic of ACS and indicative of fasciotomy. Likewise, a perfusion pressure below 30 mm Hg is also diagnostic. Based on consensus, either calculation can be used.16,17
Infrared Spectroscopy and Imaging
Barker and colleagues7 describe use of near infrared spectroscopy (NIRS) as a method to diagnose lower-extremity ACS. Noninvasive skin probes can detect the absorption spectra of mixed venous hemoglobin levels beneath the skin to determine oxygen saturation (StO2). StO2 measured by NIRS is location dependent, enabling the clinician to monitor oxygenation levels in any illuminated tissue. NIRS has great potential for use in confirming a diagnosis of ACS since decreased perfusion pressure from elevated ICP correlates well with decreased StO2. However, this technique has limitations that currently prevent its widespread use as a diagnostic tool.7
Use of noninvasive infrared imaging has also been investigated. Katz and colleagues25 describe the use of a long-wave infrared camera and thermographic imaging analysis software to detect differences in surface temperatures in patients’ limbs after blunt trauma. The researchers were able to correlate declines in temperature with decreased blood flow. They proposed that this modality be used to make the diagnosis of ACS before the development of muscle ischemia and necrosis. This software is still being investigated.25
Imaging Studies
CT or MRI can be helpful in identifying swelling, hematomas, or areas of necrosis, but their specificity is not sufficient to confirm elevated compartmental pressures24; additionally, MRI cannot distinguish between swelling resulting from soft-tissue injury and swelling in muscles affected by ACS.26, 27
Ultrasound also has potential as a diagnostic tool, as it helps clinicians visualize soft-tissue structures and assess the patency of large arteries and veins; the absence of venous outflow may suggest ACS. However, the efficacy of ultrasound in diagnosing ACS has proven inconsistent, and it is not recommended over direct ICP measurements. Instead, ultrasound can be relied on as an adjunctive modality.13
Blood Tests
Laboratory tests cannot contribute toward the diagnosis of ACS, but assessment of renal function and skeletal muscle breakdown is important to identify potential complications of ACS. Obtaining baseline levels in blood urea nitrogen and creatinine will help identify changes in kidney function, while potassium, urates, creatine phosphokinase (CPK), and myoglobin can be measured to assess for muscle breakdown. Findings of myoglobinuria with elevated CPK are strongly indicative of rhabdomyolysis, which can easily precipitate acute renal failure.8
Results from a complete blood count or prothrombin time/partial thromboplastin time may facilitate monitoring for blood loss or identification of contributing bleeding disorders. Because surgery remains the definitive treatment for ACS, a type and screen is essential in the workup, as blood products and transfusion are likely to be required during treatment.
MANAGEMENT
Fasciotomy remains the standard of care for patients presenting with the clinical signs and symptoms of increased compartment pressure consistent with ACS.16 Researchers engaged in animal studies and human case reports have shown that fasciotomy must be performed within six hours of injury to prevent adverse outcomes.8,16,28
As definitive treatment for ACS, a complete fasciotomy of all compartments in the vicinity of the injury should be performed. The most effective approach for fasciotomy consists of two long skin incisions (ie, double-incision radical dissection, to prevent concomitant increased pressure within the boundary of the skin), on opposite aspects of the affected limb, to ensure that all compartmental fascia can be decompressed.17,26,28
Delayed primary intention on postop day 5 is the preferred method for fasciotomy wound closure. Wounds should remain open to allow limb swelling to subside. In severe cases, when delayed primary intention cannot be performed in the window of time described, split-thickness skin grafts can be used. Postoperative wounds should be packed open with bulky dressings and changed daily. Negative-pressure wound dressings can be used for improved and accelerated wound closure.29
In the setting of ACS or limb ischemia, it is important to consider the possibility that tissue destruction will lead to significant myoglobinuria and potentially rhabdomyolysis.8 In patients presenting with crush injuries, or trauma patients who experience a significant rise in creatine kinase, the kidneys should be protected via extracellular resuscitation with isotonic fluids. The goal of resuscitation is to maintain urinary output of at least 200 mL/h to prevent renal failure.29
PATIENT EDUCATION
Patients who undergo fasciotomy within 12 hours of onset of signs and symptoms of ACS retain normal limb function in 68% of cases. However, this outcome falls to 8% if fasciotomy is delayed longer than 12 hours.30 Patients should understand that return to normal function usually takes two to three months and requires active participation by the patient. Furthermore, 20% of patients have some motor and sensory deficits at one year postfasciotomy.17
FOLLOW-UP
After fasciotomy, patients will require adequate pain control and an extensive rehabilitation program. Early physical therapy should progress slowly, with focus on range-of-motion and stretching exercises. Once patients have regained the ability to ambulate, resistance exercises and moderate exercise activities should be implemented to return them to their regular activities.31
CONCLUSION
ACS figures significantly in the long-term morbidity and mortality associated with trauma. Clinical research and laboratory science have indicated that ACS must be treated within six hours to prevent life-long deformity and disability. New diagnostic and therapeutic approaches must be investigated to improve outcomes. The most widely accepted surgical approach is the double-incision radical dissection of all fascia within the affected limb.
Appropriate management must include protection of the patient’s kidneys, given the risk for rhabdomyolysis, as well as extensive postoperative physical therapy. Given the invasive treatment required for ACS, progression toward full recovery is a long and difficult process. However, with prompt recognition and early intervention, full return to normal function is possible, with little to no deformity or dysfunction.
REFERECNES
1. Murdock M, Murdoch MM. Compartment syndrome: a review of the literature. Clin Podiatr Med Surg. 2012;29:301-310, viii.
2. Osteen KD, Haque SH. Bilateral gluteal compartment syndrome following right total knee revision: a case report. Ochsner J. 2012;12:141-144.
3. Paryavi E, Jobin CM, Ludwig SC, et al. Acute exertional lumbar paraspinal compartment syndrome. Spine. 2010;35:E1529-E1533.
4. Bosch U, Tscherne H. The pelvic compartment syndrome. Arch Ortho Trauma Surg. 1992;111:314-317.
5. Maeda A, Wakabayashi K, Suzuki H. Acute limb ischemia due to abdominal compartment syndrome. Catheter Cardiovasc Interv. 2013 Jun 19. [Epub ahead of print]
6. Masquelet AC. Acute compartment syndrome of the leg: pressure measurement and fasciotomy. Orthop Trumatol Surg Res. 2010;96:913-917.
7. Barker T, Midwinter M, Porter K. The diagnosis of acute lower limb compartment syndrome: applications of near infrared spectroscopy. Trauma. 2011;13:125-136.
8. Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62-72.
9. Karmaniolou I, Staikou C. Compartment syndrome as a complication of the lithotomy position. West Indian Med J. 2010;59:698-701.
10. Pietrangiolillo Z, Frassoldati R, Leonelli V, et al. Compartment syndrome after viper-bite in toddler: case report and review of literature. Acta Biomed. 2012;83:44-50.
11. Kakkar R, Ellis M, Fearon PV. Compartment syndrome of the thigh as a complication of anticoagulant therapy in a patient with a left ventricular assist device (Berlin Heart). Gen Thorac Cardiovasc Surg. 2010;58: 477-479.
12. Balogh ZJ, Leppäniemi A. Patient populations at risk for intra-abdominal hypertension and abdominal compartment syndrome. Am Surg. 2011;77 suppl 1:S12-S16.
13. Zimmerman DC, Kapoor T, Elfond M, Scott P. Spontaneous compartment syndrome of the upper arm in a patient receiving anticoagulation therapy. J Emerg Med. 2013;44:e53-e56.
14. Olson SA. Acute compartment syndrome in lower extremity musculoskeletal trauma. J Am Acad Orthop Surg. 2005;13(7):436-444.
15. Branco BC, Inaba K, Barmparas G, et al. Incidence and predictors for the need for fasciotomy after extremity trauma: a 10-year review in a mature level I trauma centre. Injury. 2011;42:1157-1163.
16. Wall CJ, Richardson, Lowe AJ, et al. Survey of management of acute, traumatic compartment syndrome of the leg in Australia. ANZ J Surg. 2007;77:733-737.
17. Wall CJ, Lynch J, Harris IA, et al; Liverpool (Sydney) and Royal Melbourne Hospitals. Clinical practice guidelines for the management of acute limb compartment syndrome following trauma. ANZ J Surg. 2010;80:151-156.
18. McQueen MM, Gaston P, Court-Brown CM. Acute compartment syndrome: who is at risk? J Bone Joint Surg. 2000;82:200-203.
19. Khan M, Hodkinson SL. Acute compartment syndrome—presenting as severe pain in an extremity out of proportion with the injury. J R Army Med Corps. 1997;143:165-166.
20. Percival TJ, White JM, Ricci MA. Compartment syndrome in the setting of vascular injury. Perspect Vasc Surg Endovasc Ther. 2011;23(2):119-124.
21. Badhe S, Baiju D, Elliot R, et al. The ‘silent’ compartment syndrome. Injury. 2009;40:220-222.
22. Shuler FD, Dietz MJ. Physicians’ ability to manually detect isolated elevations in leg intracompartmental pressure. J Bone Joint Surg Am. 2010;92:361-367.
23. Taylor RM, Sullivan MP, Mehta S. Acute compartment syndrome: obtaining diagnosis, providing treatment, and minimizing medicolegal risk. Curr Rev Musculoskelet Med. 2012;5(3):206-213.
24. McDonald S, Bearcroft P. Compartment syndromes. Semin Musculoskelet Radiol. 2010;14:236-244.
25. Katz LM, Nauriyal V, Nagaraj S, et al. Infrared imaging of trauma patients for detection of acute compartment syndrome of the leg. Crit Care Med. 2008;36:1756-1761.
26. Shadgan B, Menon M, Sanders D, et al. Current thinking about acute compartment syndrome of the lower extremity. Can J Surg. 2010;53:329-334.
27. Rominger M, Lukosch C, Bachmann G, et al. Compartment syndrome: value of MR imaging. Radiology. 1995;197:296.
28. Kashuk JL, Moore EE, Pinski S, et al. Lower extremity compartment syndrome in the acute care surgery paradigm: safety lessons learned. Patient Saf Surg. 2009;3(1):11.
29. Rasul AT Jr. Acute compartment syndrome (2011). emedicine.medscape.com/article/307668-overview#showall. Accessed June 25, 2013.
30. Sheridan GW, Matsen FA 3rd. Fasciotomy in the treatment of the acute compartment syndrome. J Bone Joint Surg Am. 1976;58(1):112-115.
31. Schubert AG. Exertional compartment syndrome: review of the literature and proposed rehabilitation guidelines following surgical release. Intern J Sports Phys Ther. 2011;6:126-141.