User login
• Use nasal steroids to treat allergic rhinitis (AR) in adults. A
• Recommend nasal saline irrigation to reduce symptoms in children and adults with seasonal rhinitis. A
• Consider immunotherapy for adults and children with severe AR that does not respond to conventional pharmacotherapy or allergen avoidance measures. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A man in his 30s with allergic rhinitis (AR) at predictable times of the year with high pollen counts reports only modest symptom relief with a nasal steroid preparation after 3 weeks of use. He comes to see you because he’s “tired of feeling lousy all of the time.”
What management options would you consider?
There is a plethora of treatment options for patients like this one, and considerable variation in clinical practice when it comes to AR.1 The good news is that there are several recent guidelines for treating AR patients, whose symptoms (and underlying cause) can vary widely.
The following review—and accompanying algorithm—provides evidence-based recommendations that can help you refine your approach to AR.
Two guidelines, and several Cochrane reviews
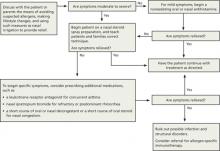
Allergic Rhinitis and its Impact on Asthma (ARIA), a sentinel rhinitis treatment guideline, was published in 2001 and updated in 2008 and 2010.2-4 The British Society for Allergy and Clinical Immunology Standards of Care Committee (BSACI) published guidelines for rhinitis management in 2008 and guidelines for immunotherapy in 2011.5,6 In addition, several Cochrane reviews have been performed.7-12 The ALGORITHM1-6 combines these recommendations. The TABLE2-12 itemizes the recommendations made by each guideline.
ALGORITHM
An evidence-based approach to treating allergic rhinitis1-6
Based on recommendations from ARIA and BSACI guidelines and Cochrane reviews
ARIA, Allergic Rhinitis and its Impact on Asthma; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee.
TABLE
Treatment recommendations/suggestions for allergic rhinitis2-12
| TREATMENT RECOMMENDATIONS/SUGGESTIONS | ARIA 2001 | ARIA 2008 | ARIA 2010 | BSACI 2008 | BSACI 2011 | COCHRANE REVIEWS |
|---|---|---|---|---|---|---|
| General principles of treatment | ||||||
| Maintenance therapy is required for persistent AR as medications have little effect after cessation. | X | |||||
| Patient education | ||||||
| Standardized patient education improves disease-specific quality of life. | X | |||||
| Nasal steroids | ||||||
| NS are the most effective monotherapy for all symptoms of AR, seasonal and perennial,* including nasal congestion. | X | |||||
| NS are recommended for AR treatment in adults and suggested for children. | X | |||||
| NS are the treatment of choice for moderate to severe persistent* AR and for treatment failures with antihistamines alone. | X | |||||
| NS are suggested over oral antihistamines in adults and children for seasonal AR. | X | |||||
| NS are suggested over oral antihistamines for adults and children with persistent AR. | X | |||||
| NS are recommended rather than nasal antihistamines. | X | |||||
| NS are recommended over oral leukotriene receptor antagonists for seasonal AR. | X | |||||
| NS are the most effective treatment of AR for children. | X | |||||
| There is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR. | X (2010) | |||||
| Intermittent* NS use may be beneficial in children. | X | |||||
| Avoid NS with high bioavailability (betamethasone) in children, as regular use for >1 year may decrease growth rate. | X | |||||
| Antihistamines | ||||||
| New-generation oral nonsedating antihistamines that do not affect cytochrome P450 are recommended for the treatment of patients with AR. | X | |||||
| Oral or topical antihistamines are first-line treatment for mild to moderate intermittent and moderate persistent AR. | X | |||||
| When NS alone do not control moderate to severe persistent AR, may add oral or topical antihistamines. | X | |||||
| New-generation oral antihistamines are suggested over nasal antihistamines for children and adults, and for children with seasonal or persistent AR. | X | |||||
| Oral antihistamines are suggested over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR. | X | |||||
| Nasal antihistamines are suggested over nasal chromones (the need to use chromones 4 times daily may limit adherence). | X | |||||
| Nasal antihistamine use is suggested for children and adults with seasonal AR. | X | |||||
| Patients with persistent AR should avoid using nasal antihistamines until more data on efficacy and safety are available. | X | |||||
| In children, weigh adverse effects of antihistamines against the general malaise caused by AR. | X | |||||
| Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children. | X | |||||
| Continuous administration of antihistamines is optimal in children, rather than as needed. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for patients with ocular symptoms. | X | |||||
| Oral leukotriene receptor antagonists | ||||||
| Oral leukotriene receptor antagonists are suggested for children and adults with seasonal AR and for preschool children with persistent AR. | X | |||||
| Avoid oral leukotriene receptor antagonists in adults with persistent AR. | X | |||||
| Decongestants | ||||||
| For adults with severe nasal obstruction, a short course (<5 days) of a nasal decongestant, along with other drugs, is suggested. | X | |||||
| Nasal decongestants may be useful for eustachian tube dysfunction when flying, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency prior to NS use. | X | |||||
| Regular oral decongestant use is not suggested. | X | X | ||||
| Avoid decongestants in pregnant patients. | X | |||||
| Avoid using nasal decongestants in preschool children. | X | |||||
| Chromones | ||||||
| Limited use of chromones is recommended for children and adults with mild symptoms. | X | |||||
| Chromones are less effective than NS or antihistamines. | X | |||||
| Nasal antihistamines are suggested over nasal chromones. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for ocular symptoms. Due to the excellent safety of these agents, chromones may be tried before antihistamines. | X | |||||
| Nasal saline | ||||||
| Nasal saline irrigation reduces symptoms in children and adults with seasonal rhinitis. | X | |||||
| Oral, intramuscular steroids | ||||||
| A short course of oral glucocorticosteroids is suggested for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments. | X | |||||
| Oral steroids are rarely indicated, but a short course (5-10 days) may be used for severe nasal congestion, uncontrolled symptoms on conventional pharmacotherapy, or important social/work events. | X | |||||
| Avoid intramuscular steroids. | X | X | ||||
| Ipratropium | ||||||
| Nasal ipratropium is suggested for treatment of rhinorrhea for patients with persistent AR. | X | |||||
| Allergen-specific immunotherapy | ||||||
| Immunotherapy is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy or allergen avoidance measures. | X | |||||
| SCIT is suggested for adults with seasonal AR and those with persistent AR due to house dust mites. | X | |||||
| SCIT is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few severe adverse reactions. | X (2007) | |||||
| SLIT is suggested for adults with AR due to pollen, although other alternatives may be equally reasonable. | X | |||||
| SLIT is safe and efficacious for AR treatment, decreasing symptoms and medication requirements. | X (2003) | |||||
| Nasal immunotherapy is suggested for adults with AR due to pollens. | X | |||||
| For pregnant patients, maintenance ASI may be continued, but starting ASI or increasing the dose is contraindicated. | X | |||||
| SCIT is suggested for children with AR. | X | |||||
| SCIT should not be started before 5 years of age. | X | |||||
| Based on preliminary studies, SLIT is safe, but more studies are needed in children. | X | |||||
| SLIT and NIT are suggested for children with AR due to pollens, acknowledging that other alternatives may be equally reasonable. SLIT should not be given to children with AR due to HDM unless being done for research. | X | |||||
| Lifestyle changes | ||||||
| Avoid single chemical or physical preventive and combination preventive methods to reduce HDM exposure. | X | |||||
| Allergen avoidance may decrease AR symptoms, but more research is needed. | X (2010) | |||||
| Achieving substantial reductions in HDM load may decrease AR symptoms. | X (2012) | |||||
| Avoidance of mold or animal dander is recommended for patients who are allergic to them. | X | |||||
| Nasal filters can reduce symptoms of AR during ragweed and grass pollen seasons. | X | |||||
| Complementary and alternative medicine | ||||||
| Avoid homeopathy, acupuncture, butterbur, herbal medicines, and phototherapy. | X | |||||
| AR, allergic rhinitis; ARIA, Allergic Rhinitis and its Impact on Asthma; ASI, allergen-specific immunotherapy; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee; HDM, house dust mites; NIT, nasal immunotherapy; NS, nasal steroids; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy. *ARIA 2008 recommended changing the classification of AR from seasonal and perennial (frequent nonseasonal nasal or ocular symptoms) to intermittent (symptoms lasting <4 days per week or <4 weeks per year) or persistent (symptoms >4 days per week and >4 weeks per year).3 AR severity is classified as mild or moderate to severe.2,3 | ||||||
The summary that follows provides a more detailed look at the recommendations, with a review of the pathophysiology of AR (“Phases of allergic rhinitis”2,3,5,8,13-15).
The early phase of allergic rhinitis (AR) occurs within minutes of allergen exposure. Mast cell degranulation releases histamine and other inflammatory mediators that cause sneezing, pruritus, rhinorrhea, and nasal congestion.3,8,13 The late phase, beginning at 4 hours and peaking 6 to 12 hours after exposure, is believed to be due to recruitment of circulating leukocytes—particularly eosinophils. Leukocyte activation causes additional inflammatory mediators to be released, which primarily causes nasal congestion—often the most bothersome symptom of AR.2,5,8,13,14 Other presenting symptoms may include feeling “fuzzy” or tired, chronic viral infections, sniffing, eye rubbing, blinking, congested voice, snoring, or dark skin beneath the eyes (allergic shiners).15
Of note: This summary preserves the terminology used in ARIA 2010. Specifically, the ARIA guideline uses the term suggest for conditional recommendations and recommend for strong recommendations.4 That same language is used here.
Nasal steroids: First-line Tx for moderate to severe symptoms
BSACI indicates that nasal steroids (NS) are the treatment of choice for moderate to severe persistent AR (symptoms lasting >4 days per week or >4 weeks per year).5 ARIA 2010 suggests NS as first-line treatment rather than oral antihistamines for adults and children with seasonal (related to outdoor allergens such as pollens or molds) and persistent AR.4 ARIA 2008 finds NS are the most effective treatment for children.3 Steroids reduce inflammation by decreasing inflammatory cell migration and inhibiting cytokine release.16 They are the most effective monotherapy for all symptoms of AR, including nasal congestion, which antihistamines do not treat effectively.13,16 NS also treat ocular symptoms of allergy effectively.15,17
The ARIA 2010 guideline also recommends using NS rather than nasal antihistamines and leukotriene receptor antagonists.4 Combination therapy (eg, NS with the addition of nasal antihistamines) is an option for severe or persistent AR, but it appears to be no more effective than monotherapy with NS.16 A 2010 Cochrane review determined there is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR.7 Intermittent steroid use may be beneficial in children.5
Steroids begin working 6 to 8 hours after the first dose, although symptom reduction may take days and maximal effect up to 2 weeks.5 Treatment failure may be due to poor technique that can cause local adverse effects (ie, dryness, irritation, epistaxis). Technique-related failure occurs in up to 10% of users.5,15 Educating patients and families about correct technique with steroid spray may decrease nonadherence due to irritation and epistaxis.18 Tell them to shake the bottle well, look down, aim the nozzle toward the outside wall of the nostril using the opposite hand, and spray while sniffing lightly.5
Any steroid is appropriate for adults. For children ≥2 years of age, consider fluticasone propionate, mometasone furoate, or triamcinolone acetonide.3 These medications have lower systemic bioavailability and a decreased risk of such adverse effects as hypothalamic-pituitary-adrenal axis suppression and growth retardation.15 Budesonide is appropriate for those ≥6 years.19-21 Avoid regular use of betamethasone, which has high bioavailability, for >1 year in children, as it may decrease their growth rate.3 Beclomethasone, fluticasone, and budesonide have been used widely and safely for pregnant women with asthma.5
Antihistamines are first-line Tx for mild symptoms
ARIA 2010 recommends new-generation oral nonsedating antihistamines that do not affect cytochrome P450 for mild AR,4 such as cetirizine, levocetirizine, loratadine, desloratadine, and fexofenadine. First-generation antihistamines can reduce symptoms, but are not first-line treatment as they cause sedation, fatigue, decreased cognitive function, and reduced academic and work performance.3-5 ARIA 2010 further suggests choosing oral antihistamines over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR.4
BSACI recommends oral or topical antihistamines as first-line treatment for mild to moderate symptoms lasting <4 days per week or <4 weeks per year and moderate persistent AR.5 When steroids alone do not control moderate to severe persistent AR, BSACI recommends adding oral or topical antihistamines.5 Oral and topical antihistamines decrease histamine-related symptoms of itching, rhinorrhea, and sneezing, but do not significantly decrease nasal congestion.15
Nasal antihistamines (levocabastine, azelastine) have a rapid onset of action and few adverse effects.3 ARIA 2010 suggests nasal antihistamines over nasal chromones (inhibitors of mast cell degranulation) and notes that the need to use chromones 4 times daily may limit adherence.4 The same guidelines suggest nasal antihistamine use for children and adults with seasonal AR and suggest not using nasal antihistamines for patients with persistent AR until more data on efficacy and safety are available.4
Alezastine is approved for individuals ≥5 years, and olopatadine is approved for individuals ≥6 years for the treatment of AR.16,22,23 A pediatric review article noted nasal antihistamine (azelastine) plus nasal fluticasone was more efficacious than NS alone.15
In children, weigh adverse effects of antihistamines against the general malaise caused by AR.3 Do not use first-generation antihistamines due to the sedation that may interfere with learning.15 Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children.5 Continuous administration, rather than as needed, is optimal treatment in children.5 Cetirizine, loratadine, and levocetirizine have been studied and are effective and safe in children.3 Levocetirizine has proven safe and efficacious for children ≥2 years.24 Fexofenadine was found to be effective and safe for those ≥6 years.25
For children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones.4 Due to the safety of these agents, chromones may be used first, then antihistamines.4 Just as with nasal chromones, the need to use intraocular chromones 4 times daily may limit their use in children.4
Pregnant patients. Antihistamines do cross the placenta.5 Agents that appear to be safe for pregnant patients are chlorphenamine (first-generation), loratadine, and cetirizine.5
Leukotriene receptor antagonists: Always pair with antihistamines
As adjunctive therapy for additional symptom control, ARIA 2010 suggests oral leukotriene receptor antagonists for children and adults with seasonal AR, and for preschool children with persistent AR. These agents may also be helpful in children with concurrent asthma.15 Always pair leukotriene receptor antagonists with antihistamines. Montelukast is approved for seasonal AR in children ≥2 years and for frequent nonseasonal nasal or ocular AR symptoms in children ≥6 months.26
ARIA 2010 recommends against the use of oral leukotriene receptor antagonists in adults with persistent AR.4
Decongestants are for limited use only
For adults with severe nasal obstruction, ARIA 2010 suggests a short course (<5 days) of nasal decongestant along with other drugs.4 Limiting use of nasal decongestants to <10 days helps prevent rhinitis medicamentosa.5,27 BSACI notes nasal decongestants may be useful for eustachian tube dysfunction experienced aboard airplanes, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency before NS use.5 Both guidelines suggest against regular oral decongestant use.4,5
Avoid decongestants in pregnant patients.5 ARIA 2010 suggests against nasal decongestant use in preschool children.4
Chromones may help, but require multiple daily dosing
Chromones inhibit mast cell degranulation, are weakly effective for reducing nasal obstruction in AR, and have a high safety profile.3-5,28 As noted earlier, they must be used 4 times daily, which may reduce adherence—particularly in children.4
ARIA 2008 notes that disodium cromoglycate is less effective than NS or antihistamines.3 The 2010 update suggests nasal antihistamines over nasal chromones.4 For adults as well as children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones. BSACI recommends limited use of chromones for children and adults with mild symptoms.5
Nasal saline helpful as adjunct to medication
Nasal saline irrigation improves symptoms of AR, clears nasal passages, and is helpful for pregnant patients, for whom medications should be used with caution.2,3,5 Nasal irrigation using a neti pot or squeeze bottle is efficacious for chronic rhinorrhea, as solo or complementary treatment, and for children.5,16,27
Oral steroids: Use only rarely
ARIA 2010 suggests a short course of oral glucocorticosteroids for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments.4 BSACI notes oral steroids are rarely indicated, but that their use over 5 to 10 days may help with severe nasal congestion, symptoms uncontrolled by conventional pharmacotherapy, or before important social or work events.5 Both guidelines recommend against intramuscular steroids.4 ARIA 2008 notes oral and depot preparations of steroids affect growth in young children.3
Ipratropium when rhinorrhea is severe
Nasal ipratropium bromide, a topical anticholinergic, is helpful for excessive or refractory rhinorrhea. Consider using ipratropium with NS for patients for whom rhinorrhea is the dominant symptom.5,16,28 ARIA 2010 suggests using nasal ipratropium to treat rhinorrhea in patients with persistent AR.4
Allergen-specific immunotherapy: When other treatments fail
Allergen-specific immunotherapy (ASI) consists of repeated exposure to an allergen to induce immunomodulation, which prevents or reduces allergy symptoms and actually changes the natural course of AR. (For more on identifying the offending agent, see “Time for allergen testing?”2,5,15,18,29.) This treatment process decreases medication needs, prevents new allergen sensitization, and results in long-lasting improvement.2,5,6,30 BSACI 2011 notes that ASI is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy and allergen avoidance measures.6
When a patient’s symptoms are poorly controlled or persist after treatment, consider allergen testing.29 Skin prick testing (SPT) is the best means of eliciting specific allergen sensitization. However, limit testing to allergens most likely causing the patient’s symptoms rather than ordering a random panel; 15% of those with a positive SPT to specific allergens do not have symptoms when exposed to those allergens in their environment.5 And always interpret results of allergy testing in light of the patient’s history.2,15,18
SPT has a high negative predictive value, which can prevent unnecessary lifestyle changes.29 However, keep in mind that SPT results may be suppressed if the patient is using antihistamines, tricyclic antidepressants, or topical steroids.29 If SPT is not feasible or the patient is taking medications that may suppress results, consider arranging for serum-specific IgE testing, also known as radioallergosorbent testing, or RAST.5 RAST and SPT have similar sensitivities for house dust mites, but RAST is not as sensitive as SPT for other inhalants (eg, cat epithelium, mold, grass pollen).5
ASI methods developed to date use subcutaneous, sublingual, or nasal routes of administration. However, the US Food and Drug Administration has yet to approve commercial sublingual or nasal products for use in the United States.16
Subcutaneous immunotherapy may cause local adverse reactions (pruritus and swelling) and systemic reactions that can be severe or life threatening (anaphylaxis) and thus must be given in a doctor’s office prepared to treat anaphylaxis.6,16,30 Adrenaline administration has been necessary in 0.13% of those being treated.9 Subcutaneous immunotherapy must be done for 3 to 5 years for sustained effective treatment.15
ARIA 2010 suggests subcutaneous immunotherapy for adults with seasonal AR and with persistent AR due to house dust mites.4 A 2007 Cochrane review found subcutaneous immunotherapy is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few significant severe adverse reactions.9 A meta-analysis showed subcutaneous immunotherapy is as potent as pharmacotherapy in controlling seasonal AR symptoms as early as the first season of treatment.31
What if the patient is pregnant—or a child? BSACI notes that maintenance ASI may be continued in a patient who becomes pregnant, but starting ASI or increasing the dose is contraindicated.5
Based on ARIA 2008 and 2010, consider subcutaneous immunotherapy for children—but not for those <5 years.3,4 Care must be used in selecting patients, as 3 to 5 years of treatment are necessary for sustained benefit.15
Lifestyle changes: Limited benefit may be achievable
ARIA 2010 recommends mold avoidance and animal dander avoidance for patients so affected.4 Allergens from pets can persist in homes for months after pet removal.15 BSACI found that commercially available nasal filters (filters or screens placed over or within both nares) reduced symptoms of AR during ragweed and grass pollen seasons.5 Allergen avoidance for children with persistent AR has not shown consistent benefit.15 A 2010 Cochrane review concluded that allergen avoidance may decrease AR symptoms, but more research is needed.11
House dust mites. The 2010 Cochrane review also reported on 2 trials that assessed high-efficiency particulate air (HEPA) filters specifically for patients allergic to house dust mites.11 The studies, which had methodological limitations (inconsistent randomization, small sample size, and short duration), concluded that HEPA filters alone will not likely reduce symptoms of house dust mite allergy. But HEPA filters may be beneficial as one component of an extensive bedroom-based environmental control program.11
Impermeable bedding has been shown to reduce dust mite load by 50% to 70%, leaving residual allergen that may still trigger symptoms.11 A 2012 Cochrane review concluded that achieving substantial reductions in house dust mite load using a combination approach of multiple interventions, including acaricides and extensive bedroom-based environmental control programs, may decrease AR symptoms.12 However, ARIA 2010 recommends against single chemical or physical preventive methods and against combination preventive methods to reduce house dust mite exposure.4
Total elimination of house dust mites may be impossible, and recommending use of impermeable covers and HEPA filters, removal of rugs and curtains, and frequent cleaning must take into account a patient’s symptoms and a family’s motivation and finances.11,18
Complementary and alternative medicine: Too little evidence
ARIA 2010 suggests against patients using homeopathy, acupuncture, butterbur, herbal medicines, or phototherapy for AR.4 While one systematic review of acupuncture for AR demonstrated mixed results with no specific effects for seasonal AR and some improvement of frequent nonseasonal symptoms,32 another review concluded evidence was insufficient to make any recommendation.32,33 The benefit of ear acupressure is unknown, as supporting studies are of low methodological quality, although it appeared to provide some benefit for AR.34
Due to lack of data, probiotics should not be recommended.27 A pediatric review article noted that probiotics may alter cytokine production in patients with seasonal AR and may be more helpful in AR than in asthma, although more research was needed.15 Another review showed that probiotics may reduce AR symptoms and medication use.35
CASE Since the nasal steroid you prescribed for your patient did not provide adequate relief, you opt to add cetirizine 10 mg to his NS regimen. This step relieved his symptoms within 2 to 3 days. Had his symptoms persisted, the patient would have been a candidate for a one-week course of oral decongestant, such as pseudoephedrine 120 mg orally every 12 hours, as needed; and then for allergen testing, specifically for pollens corresponding to the seasonality of his AR. Appropriate follow-up would be to monitor the patient until his symptoms resolved or became manageable.
1. Bousquet J, Schünemann HJ, Zuberbier T, et al. Development and implementation of guidelines in allergic rhinitis – an ARIA-GA2LEN paper. Allergy. 2010;65:1212-1221.
2. Bousquet J, Van-Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(suppl):S147-S334.
3. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008. Allergy. 2008;63(suppl 86):S8-S160.
4. Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466-476.
5. Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008;38:19-42.
6. Walker SM, Durham SR, Till SJ, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41:1177-1200.
7. Al Sayyad JJ, Fedorowicz Z, Alhashimi D, et al. Topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2007;(1):CD003163.-
8. Nasser M, Fedorowicz, Alijufairi H, et al. Antihistamines used in addition to topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2010;(7):CD006989.-
9. Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;(1):CD001936.-
10. Wilson D, Torres-Lima M, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003;(2):CD002893.-
11. Sheikh A, Hurwitz B, Nurmatov U, et al. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database Syst Rev. 2010;(7):CD001563.-
12. Nurmatov U, van Schayck CP, Hurwitz B, et al. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158-165.
13. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108(suppl):S2-S8.
14. Nathan RA. The pathophysiology, clinical impact, and management of nasal congestion in allergic rhinitis. Clin Ther. 2008;30:573-586.
15. Kemp AS. Allergic rhinitis. Paediatric Respir Rev. 2009;10:63-68.
16. Sur DK, Scandale S. Treatment of allergic rhinitis. Am Fam Physician. 2010;81:1440-1446.
17. Hong J, Bielory B, Rosenberg JL, et al. Efficacy of intranasal corticosteroids for the ocular symptoms of allergic rhinitis: a systematic review. Allergy Asthma Proc. 2011;32:22-35.
18. Hu W, Katelaris CH, Kemp AS. Allergic rhinitis – practical management strategies. Aust Fam Physician. 2008;37:214-220.
19. Veramyst (fluticasone furoate) nasal spray [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; August 2012. Available at: http://us.gsk.com/products/assets/us_veramyst.pdf. Accessed January 16, 2013.
20. Nasacort AQ (triamcinolone acetonide) nasal spray [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/nasacort_aq/nasacort_aq.html. Accessed January 16, 2013.
21. Rhinocort AQUA 32 mcg (budesonide) nasal spray [prescribing information]. Wilmington, Del: AstraZeneca; revised December 2010. Available at: http://www1.astrazeneca-us.com/pi/Rhinocort_Aqua.pdf. Accessed January 16, 2013.
22. Astelin (azelastine hydrochloride) spray, metered [prescribing information]. Somerset, NJ: Meda Pharmaceuticals; revised July 2011. Available at: http://www.astelin.com/pdf/astelin_pi.pdf. Accessed January 16, 2013.
23. Patanase (olopatadine hydrochloride) nasal spray [prescribing information]. Fort Worth, Tex: Alcon Laboratories; revised February 2012. Available at: http://ecatalog.alcon.com/PI/Patanase_us_en.pdf. Accessed January 16, 2013.
24. Xyzal (levocetirizine dihydrochloride) tablets and oral solution [prescribing information]. Smyrna, Ga: UCB and Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/xyzal/xyzal.pdf. Accessed January 16, 2013.
25. Allegra (fexofenadine hydrochloride) tablets, ODT, and oral suspension [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2007. Available at: http://products.sanofi.us/allegra/allegra.html. Accessed January 16, 2013.
26. Singulair (montelukast sodium) tablets, chewable tablets, and oral granules [prescribing information]. Whitehouse Station, NJ: Merck; revised November 2012. Available at: http://www.merck.com/product/usa/pi_circulars/s/singulair/singulair_pi.pdf. Accessed January 16, 2013.
27. Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106(suppl):S12-S16.
28. Lim MY, Leong JM. Allergic rhinitis: evidence-based practice. Singapore Med J. 2010;51:542-550.
29. Angier E, Willington J, Scadding G, et al. Management of allergic and non-allergic rhinitis: a primary care summary of the BSACI guideline. Prim Care Respir J. 2010;19:217-222.
30. Radulovic S, Wilson D, Calderon M, et al. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011;66:740-752.
31. Matricardi PM, Kuna P, Panetta V, et al. Subcutaneous immunotherapy and pharmacology in seasonal allergic rhinitis: a comparison based on meta-analyses. J Allergy Clin Immunol. 2011;128:791-799.
32. Lee MS, Pittler MH, Shin B, et al. Acupuncture for allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2009;102:269-279.
33. Roberts J, Huissoon A, Dretzke J, et al. A systematic review of the clinical effectiveness of acupuncture for allergic rhinitis. BMC Complement Altern Med. 2008;8:13.-
34. Zhang CS, Yang AW, Zhang AL, et al. Ear-acupressure for allergic rhinitis: a systematic review. Clin Otolaryngol. 2010;35:6-12.
35. Vliagoftis H, Kouranos VD, Betsi GI, et al. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101:570-579.
CORRESPONDENCE Suzanne Minor, MD, Florida International University Herbert Wertheim College of Medicine; 11200 SW 8th Street, AHC II 361A, Miami, FL 33199; [email protected]
• Use nasal steroids to treat allergic rhinitis (AR) in adults. A
• Recommend nasal saline irrigation to reduce symptoms in children and adults with seasonal rhinitis. A
• Consider immunotherapy for adults and children with severe AR that does not respond to conventional pharmacotherapy or allergen avoidance measures. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A man in his 30s with allergic rhinitis (AR) at predictable times of the year with high pollen counts reports only modest symptom relief with a nasal steroid preparation after 3 weeks of use. He comes to see you because he’s “tired of feeling lousy all of the time.”
What management options would you consider?
There is a plethora of treatment options for patients like this one, and considerable variation in clinical practice when it comes to AR.1 The good news is that there are several recent guidelines for treating AR patients, whose symptoms (and underlying cause) can vary widely.
The following review—and accompanying algorithm—provides evidence-based recommendations that can help you refine your approach to AR.
Two guidelines, and several Cochrane reviews
Allergic Rhinitis and its Impact on Asthma (ARIA), a sentinel rhinitis treatment guideline, was published in 2001 and updated in 2008 and 2010.2-4 The British Society for Allergy and Clinical Immunology Standards of Care Committee (BSACI) published guidelines for rhinitis management in 2008 and guidelines for immunotherapy in 2011.5,6 In addition, several Cochrane reviews have been performed.7-12 The ALGORITHM1-6 combines these recommendations. The TABLE2-12 itemizes the recommendations made by each guideline.
ALGORITHM
An evidence-based approach to treating allergic rhinitis1-6
Based on recommendations from ARIA and BSACI guidelines and Cochrane reviews
ARIA, Allergic Rhinitis and its Impact on Asthma; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee.
TABLE
Treatment recommendations/suggestions for allergic rhinitis2-12
| TREATMENT RECOMMENDATIONS/SUGGESTIONS | ARIA 2001 | ARIA 2008 | ARIA 2010 | BSACI 2008 | BSACI 2011 | COCHRANE REVIEWS |
|---|---|---|---|---|---|---|
| General principles of treatment | ||||||
| Maintenance therapy is required for persistent AR as medications have little effect after cessation. | X | |||||
| Patient education | ||||||
| Standardized patient education improves disease-specific quality of life. | X | |||||
| Nasal steroids | ||||||
| NS are the most effective monotherapy for all symptoms of AR, seasonal and perennial,* including nasal congestion. | X | |||||
| NS are recommended for AR treatment in adults and suggested for children. | X | |||||
| NS are the treatment of choice for moderate to severe persistent* AR and for treatment failures with antihistamines alone. | X | |||||
| NS are suggested over oral antihistamines in adults and children for seasonal AR. | X | |||||
| NS are suggested over oral antihistamines for adults and children with persistent AR. | X | |||||
| NS are recommended rather than nasal antihistamines. | X | |||||
| NS are recommended over oral leukotriene receptor antagonists for seasonal AR. | X | |||||
| NS are the most effective treatment of AR for children. | X | |||||
| There is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR. | X (2010) | |||||
| Intermittent* NS use may be beneficial in children. | X | |||||
| Avoid NS with high bioavailability (betamethasone) in children, as regular use for >1 year may decrease growth rate. | X | |||||
| Antihistamines | ||||||
| New-generation oral nonsedating antihistamines that do not affect cytochrome P450 are recommended for the treatment of patients with AR. | X | |||||
| Oral or topical antihistamines are first-line treatment for mild to moderate intermittent and moderate persistent AR. | X | |||||
| When NS alone do not control moderate to severe persistent AR, may add oral or topical antihistamines. | X | |||||
| New-generation oral antihistamines are suggested over nasal antihistamines for children and adults, and for children with seasonal or persistent AR. | X | |||||
| Oral antihistamines are suggested over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR. | X | |||||
| Nasal antihistamines are suggested over nasal chromones (the need to use chromones 4 times daily may limit adherence). | X | |||||
| Nasal antihistamine use is suggested for children and adults with seasonal AR. | X | |||||
| Patients with persistent AR should avoid using nasal antihistamines until more data on efficacy and safety are available. | X | |||||
| In children, weigh adverse effects of antihistamines against the general malaise caused by AR. | X | |||||
| Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children. | X | |||||
| Continuous administration of antihistamines is optimal in children, rather than as needed. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for patients with ocular symptoms. | X | |||||
| Oral leukotriene receptor antagonists | ||||||
| Oral leukotriene receptor antagonists are suggested for children and adults with seasonal AR and for preschool children with persistent AR. | X | |||||
| Avoid oral leukotriene receptor antagonists in adults with persistent AR. | X | |||||
| Decongestants | ||||||
| For adults with severe nasal obstruction, a short course (<5 days) of a nasal decongestant, along with other drugs, is suggested. | X | |||||
| Nasal decongestants may be useful for eustachian tube dysfunction when flying, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency prior to NS use. | X | |||||
| Regular oral decongestant use is not suggested. | X | X | ||||
| Avoid decongestants in pregnant patients. | X | |||||
| Avoid using nasal decongestants in preschool children. | X | |||||
| Chromones | ||||||
| Limited use of chromones is recommended for children and adults with mild symptoms. | X | |||||
| Chromones are less effective than NS or antihistamines. | X | |||||
| Nasal antihistamines are suggested over nasal chromones. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for ocular symptoms. Due to the excellent safety of these agents, chromones may be tried before antihistamines. | X | |||||
| Nasal saline | ||||||
| Nasal saline irrigation reduces symptoms in children and adults with seasonal rhinitis. | X | |||||
| Oral, intramuscular steroids | ||||||
| A short course of oral glucocorticosteroids is suggested for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments. | X | |||||
| Oral steroids are rarely indicated, but a short course (5-10 days) may be used for severe nasal congestion, uncontrolled symptoms on conventional pharmacotherapy, or important social/work events. | X | |||||
| Avoid intramuscular steroids. | X | X | ||||
| Ipratropium | ||||||
| Nasal ipratropium is suggested for treatment of rhinorrhea for patients with persistent AR. | X | |||||
| Allergen-specific immunotherapy | ||||||
| Immunotherapy is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy or allergen avoidance measures. | X | |||||
| SCIT is suggested for adults with seasonal AR and those with persistent AR due to house dust mites. | X | |||||
| SCIT is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few severe adverse reactions. | X (2007) | |||||
| SLIT is suggested for adults with AR due to pollen, although other alternatives may be equally reasonable. | X | |||||
| SLIT is safe and efficacious for AR treatment, decreasing symptoms and medication requirements. | X (2003) | |||||
| Nasal immunotherapy is suggested for adults with AR due to pollens. | X | |||||
| For pregnant patients, maintenance ASI may be continued, but starting ASI or increasing the dose is contraindicated. | X | |||||
| SCIT is suggested for children with AR. | X | |||||
| SCIT should not be started before 5 years of age. | X | |||||
| Based on preliminary studies, SLIT is safe, but more studies are needed in children. | X | |||||
| SLIT and NIT are suggested for children with AR due to pollens, acknowledging that other alternatives may be equally reasonable. SLIT should not be given to children with AR due to HDM unless being done for research. | X | |||||
| Lifestyle changes | ||||||
| Avoid single chemical or physical preventive and combination preventive methods to reduce HDM exposure. | X | |||||
| Allergen avoidance may decrease AR symptoms, but more research is needed. | X (2010) | |||||
| Achieving substantial reductions in HDM load may decrease AR symptoms. | X (2012) | |||||
| Avoidance of mold or animal dander is recommended for patients who are allergic to them. | X | |||||
| Nasal filters can reduce symptoms of AR during ragweed and grass pollen seasons. | X | |||||
| Complementary and alternative medicine | ||||||
| Avoid homeopathy, acupuncture, butterbur, herbal medicines, and phototherapy. | X | |||||
| AR, allergic rhinitis; ARIA, Allergic Rhinitis and its Impact on Asthma; ASI, allergen-specific immunotherapy; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee; HDM, house dust mites; NIT, nasal immunotherapy; NS, nasal steroids; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy. *ARIA 2008 recommended changing the classification of AR from seasonal and perennial (frequent nonseasonal nasal or ocular symptoms) to intermittent (symptoms lasting <4 days per week or <4 weeks per year) or persistent (symptoms >4 days per week and >4 weeks per year).3 AR severity is classified as mild or moderate to severe.2,3 | ||||||
The summary that follows provides a more detailed look at the recommendations, with a review of the pathophysiology of AR (“Phases of allergic rhinitis”2,3,5,8,13-15).
The early phase of allergic rhinitis (AR) occurs within minutes of allergen exposure. Mast cell degranulation releases histamine and other inflammatory mediators that cause sneezing, pruritus, rhinorrhea, and nasal congestion.3,8,13 The late phase, beginning at 4 hours and peaking 6 to 12 hours after exposure, is believed to be due to recruitment of circulating leukocytes—particularly eosinophils. Leukocyte activation causes additional inflammatory mediators to be released, which primarily causes nasal congestion—often the most bothersome symptom of AR.2,5,8,13,14 Other presenting symptoms may include feeling “fuzzy” or tired, chronic viral infections, sniffing, eye rubbing, blinking, congested voice, snoring, or dark skin beneath the eyes (allergic shiners).15
Of note: This summary preserves the terminology used in ARIA 2010. Specifically, the ARIA guideline uses the term suggest for conditional recommendations and recommend for strong recommendations.4 That same language is used here.
Nasal steroids: First-line Tx for moderate to severe symptoms
BSACI indicates that nasal steroids (NS) are the treatment of choice for moderate to severe persistent AR (symptoms lasting >4 days per week or >4 weeks per year).5 ARIA 2010 suggests NS as first-line treatment rather than oral antihistamines for adults and children with seasonal (related to outdoor allergens such as pollens or molds) and persistent AR.4 ARIA 2008 finds NS are the most effective treatment for children.3 Steroids reduce inflammation by decreasing inflammatory cell migration and inhibiting cytokine release.16 They are the most effective monotherapy for all symptoms of AR, including nasal congestion, which antihistamines do not treat effectively.13,16 NS also treat ocular symptoms of allergy effectively.15,17
The ARIA 2010 guideline also recommends using NS rather than nasal antihistamines and leukotriene receptor antagonists.4 Combination therapy (eg, NS with the addition of nasal antihistamines) is an option for severe or persistent AR, but it appears to be no more effective than monotherapy with NS.16 A 2010 Cochrane review determined there is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR.7 Intermittent steroid use may be beneficial in children.5
Steroids begin working 6 to 8 hours after the first dose, although symptom reduction may take days and maximal effect up to 2 weeks.5 Treatment failure may be due to poor technique that can cause local adverse effects (ie, dryness, irritation, epistaxis). Technique-related failure occurs in up to 10% of users.5,15 Educating patients and families about correct technique with steroid spray may decrease nonadherence due to irritation and epistaxis.18 Tell them to shake the bottle well, look down, aim the nozzle toward the outside wall of the nostril using the opposite hand, and spray while sniffing lightly.5
Any steroid is appropriate for adults. For children ≥2 years of age, consider fluticasone propionate, mometasone furoate, or triamcinolone acetonide.3 These medications have lower systemic bioavailability and a decreased risk of such adverse effects as hypothalamic-pituitary-adrenal axis suppression and growth retardation.15 Budesonide is appropriate for those ≥6 years.19-21 Avoid regular use of betamethasone, which has high bioavailability, for >1 year in children, as it may decrease their growth rate.3 Beclomethasone, fluticasone, and budesonide have been used widely and safely for pregnant women with asthma.5
Antihistamines are first-line Tx for mild symptoms
ARIA 2010 recommends new-generation oral nonsedating antihistamines that do not affect cytochrome P450 for mild AR,4 such as cetirizine, levocetirizine, loratadine, desloratadine, and fexofenadine. First-generation antihistamines can reduce symptoms, but are not first-line treatment as they cause sedation, fatigue, decreased cognitive function, and reduced academic and work performance.3-5 ARIA 2010 further suggests choosing oral antihistamines over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR.4
BSACI recommends oral or topical antihistamines as first-line treatment for mild to moderate symptoms lasting <4 days per week or <4 weeks per year and moderate persistent AR.5 When steroids alone do not control moderate to severe persistent AR, BSACI recommends adding oral or topical antihistamines.5 Oral and topical antihistamines decrease histamine-related symptoms of itching, rhinorrhea, and sneezing, but do not significantly decrease nasal congestion.15
Nasal antihistamines (levocabastine, azelastine) have a rapid onset of action and few adverse effects.3 ARIA 2010 suggests nasal antihistamines over nasal chromones (inhibitors of mast cell degranulation) and notes that the need to use chromones 4 times daily may limit adherence.4 The same guidelines suggest nasal antihistamine use for children and adults with seasonal AR and suggest not using nasal antihistamines for patients with persistent AR until more data on efficacy and safety are available.4
Alezastine is approved for individuals ≥5 years, and olopatadine is approved for individuals ≥6 years for the treatment of AR.16,22,23 A pediatric review article noted nasal antihistamine (azelastine) plus nasal fluticasone was more efficacious than NS alone.15
In children, weigh adverse effects of antihistamines against the general malaise caused by AR.3 Do not use first-generation antihistamines due to the sedation that may interfere with learning.15 Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children.5 Continuous administration, rather than as needed, is optimal treatment in children.5 Cetirizine, loratadine, and levocetirizine have been studied and are effective and safe in children.3 Levocetirizine has proven safe and efficacious for children ≥2 years.24 Fexofenadine was found to be effective and safe for those ≥6 years.25
For children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones.4 Due to the safety of these agents, chromones may be used first, then antihistamines.4 Just as with nasal chromones, the need to use intraocular chromones 4 times daily may limit their use in children.4
Pregnant patients. Antihistamines do cross the placenta.5 Agents that appear to be safe for pregnant patients are chlorphenamine (first-generation), loratadine, and cetirizine.5
Leukotriene receptor antagonists: Always pair with antihistamines
As adjunctive therapy for additional symptom control, ARIA 2010 suggests oral leukotriene receptor antagonists for children and adults with seasonal AR, and for preschool children with persistent AR. These agents may also be helpful in children with concurrent asthma.15 Always pair leukotriene receptor antagonists with antihistamines. Montelukast is approved for seasonal AR in children ≥2 years and for frequent nonseasonal nasal or ocular AR symptoms in children ≥6 months.26
ARIA 2010 recommends against the use of oral leukotriene receptor antagonists in adults with persistent AR.4
Decongestants are for limited use only
For adults with severe nasal obstruction, ARIA 2010 suggests a short course (<5 days) of nasal decongestant along with other drugs.4 Limiting use of nasal decongestants to <10 days helps prevent rhinitis medicamentosa.5,27 BSACI notes nasal decongestants may be useful for eustachian tube dysfunction experienced aboard airplanes, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency before NS use.5 Both guidelines suggest against regular oral decongestant use.4,5
Avoid decongestants in pregnant patients.5 ARIA 2010 suggests against nasal decongestant use in preschool children.4
Chromones may help, but require multiple daily dosing
Chromones inhibit mast cell degranulation, are weakly effective for reducing nasal obstruction in AR, and have a high safety profile.3-5,28 As noted earlier, they must be used 4 times daily, which may reduce adherence—particularly in children.4
ARIA 2008 notes that disodium cromoglycate is less effective than NS or antihistamines.3 The 2010 update suggests nasal antihistamines over nasal chromones.4 For adults as well as children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones. BSACI recommends limited use of chromones for children and adults with mild symptoms.5
Nasal saline helpful as adjunct to medication
Nasal saline irrigation improves symptoms of AR, clears nasal passages, and is helpful for pregnant patients, for whom medications should be used with caution.2,3,5 Nasal irrigation using a neti pot or squeeze bottle is efficacious for chronic rhinorrhea, as solo or complementary treatment, and for children.5,16,27
Oral steroids: Use only rarely
ARIA 2010 suggests a short course of oral glucocorticosteroids for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments.4 BSACI notes oral steroids are rarely indicated, but that their use over 5 to 10 days may help with severe nasal congestion, symptoms uncontrolled by conventional pharmacotherapy, or before important social or work events.5 Both guidelines recommend against intramuscular steroids.4 ARIA 2008 notes oral and depot preparations of steroids affect growth in young children.3
Ipratropium when rhinorrhea is severe
Nasal ipratropium bromide, a topical anticholinergic, is helpful for excessive or refractory rhinorrhea. Consider using ipratropium with NS for patients for whom rhinorrhea is the dominant symptom.5,16,28 ARIA 2010 suggests using nasal ipratropium to treat rhinorrhea in patients with persistent AR.4
Allergen-specific immunotherapy: When other treatments fail
Allergen-specific immunotherapy (ASI) consists of repeated exposure to an allergen to induce immunomodulation, which prevents or reduces allergy symptoms and actually changes the natural course of AR. (For more on identifying the offending agent, see “Time for allergen testing?”2,5,15,18,29.) This treatment process decreases medication needs, prevents new allergen sensitization, and results in long-lasting improvement.2,5,6,30 BSACI 2011 notes that ASI is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy and allergen avoidance measures.6
When a patient’s symptoms are poorly controlled or persist after treatment, consider allergen testing.29 Skin prick testing (SPT) is the best means of eliciting specific allergen sensitization. However, limit testing to allergens most likely causing the patient’s symptoms rather than ordering a random panel; 15% of those with a positive SPT to specific allergens do not have symptoms when exposed to those allergens in their environment.5 And always interpret results of allergy testing in light of the patient’s history.2,15,18
SPT has a high negative predictive value, which can prevent unnecessary lifestyle changes.29 However, keep in mind that SPT results may be suppressed if the patient is using antihistamines, tricyclic antidepressants, or topical steroids.29 If SPT is not feasible or the patient is taking medications that may suppress results, consider arranging for serum-specific IgE testing, also known as radioallergosorbent testing, or RAST.5 RAST and SPT have similar sensitivities for house dust mites, but RAST is not as sensitive as SPT for other inhalants (eg, cat epithelium, mold, grass pollen).5
ASI methods developed to date use subcutaneous, sublingual, or nasal routes of administration. However, the US Food and Drug Administration has yet to approve commercial sublingual or nasal products for use in the United States.16
Subcutaneous immunotherapy may cause local adverse reactions (pruritus and swelling) and systemic reactions that can be severe or life threatening (anaphylaxis) and thus must be given in a doctor’s office prepared to treat anaphylaxis.6,16,30 Adrenaline administration has been necessary in 0.13% of those being treated.9 Subcutaneous immunotherapy must be done for 3 to 5 years for sustained effective treatment.15
ARIA 2010 suggests subcutaneous immunotherapy for adults with seasonal AR and with persistent AR due to house dust mites.4 A 2007 Cochrane review found subcutaneous immunotherapy is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few significant severe adverse reactions.9 A meta-analysis showed subcutaneous immunotherapy is as potent as pharmacotherapy in controlling seasonal AR symptoms as early as the first season of treatment.31
What if the patient is pregnant—or a child? BSACI notes that maintenance ASI may be continued in a patient who becomes pregnant, but starting ASI or increasing the dose is contraindicated.5
Based on ARIA 2008 and 2010, consider subcutaneous immunotherapy for children—but not for those <5 years.3,4 Care must be used in selecting patients, as 3 to 5 years of treatment are necessary for sustained benefit.15
Lifestyle changes: Limited benefit may be achievable
ARIA 2010 recommends mold avoidance and animal dander avoidance for patients so affected.4 Allergens from pets can persist in homes for months after pet removal.15 BSACI found that commercially available nasal filters (filters or screens placed over or within both nares) reduced symptoms of AR during ragweed and grass pollen seasons.5 Allergen avoidance for children with persistent AR has not shown consistent benefit.15 A 2010 Cochrane review concluded that allergen avoidance may decrease AR symptoms, but more research is needed.11
House dust mites. The 2010 Cochrane review also reported on 2 trials that assessed high-efficiency particulate air (HEPA) filters specifically for patients allergic to house dust mites.11 The studies, which had methodological limitations (inconsistent randomization, small sample size, and short duration), concluded that HEPA filters alone will not likely reduce symptoms of house dust mite allergy. But HEPA filters may be beneficial as one component of an extensive bedroom-based environmental control program.11
Impermeable bedding has been shown to reduce dust mite load by 50% to 70%, leaving residual allergen that may still trigger symptoms.11 A 2012 Cochrane review concluded that achieving substantial reductions in house dust mite load using a combination approach of multiple interventions, including acaricides and extensive bedroom-based environmental control programs, may decrease AR symptoms.12 However, ARIA 2010 recommends against single chemical or physical preventive methods and against combination preventive methods to reduce house dust mite exposure.4
Total elimination of house dust mites may be impossible, and recommending use of impermeable covers and HEPA filters, removal of rugs and curtains, and frequent cleaning must take into account a patient’s symptoms and a family’s motivation and finances.11,18
Complementary and alternative medicine: Too little evidence
ARIA 2010 suggests against patients using homeopathy, acupuncture, butterbur, herbal medicines, or phototherapy for AR.4 While one systematic review of acupuncture for AR demonstrated mixed results with no specific effects for seasonal AR and some improvement of frequent nonseasonal symptoms,32 another review concluded evidence was insufficient to make any recommendation.32,33 The benefit of ear acupressure is unknown, as supporting studies are of low methodological quality, although it appeared to provide some benefit for AR.34
Due to lack of data, probiotics should not be recommended.27 A pediatric review article noted that probiotics may alter cytokine production in patients with seasonal AR and may be more helpful in AR than in asthma, although more research was needed.15 Another review showed that probiotics may reduce AR symptoms and medication use.35
CASE Since the nasal steroid you prescribed for your patient did not provide adequate relief, you opt to add cetirizine 10 mg to his NS regimen. This step relieved his symptoms within 2 to 3 days. Had his symptoms persisted, the patient would have been a candidate for a one-week course of oral decongestant, such as pseudoephedrine 120 mg orally every 12 hours, as needed; and then for allergen testing, specifically for pollens corresponding to the seasonality of his AR. Appropriate follow-up would be to monitor the patient until his symptoms resolved or became manageable.
• Use nasal steroids to treat allergic rhinitis (AR) in adults. A
• Recommend nasal saline irrigation to reduce symptoms in children and adults with seasonal rhinitis. A
• Consider immunotherapy for adults and children with severe AR that does not respond to conventional pharmacotherapy or allergen avoidance measures. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE A man in his 30s with allergic rhinitis (AR) at predictable times of the year with high pollen counts reports only modest symptom relief with a nasal steroid preparation after 3 weeks of use. He comes to see you because he’s “tired of feeling lousy all of the time.”
What management options would you consider?
There is a plethora of treatment options for patients like this one, and considerable variation in clinical practice when it comes to AR.1 The good news is that there are several recent guidelines for treating AR patients, whose symptoms (and underlying cause) can vary widely.
The following review—and accompanying algorithm—provides evidence-based recommendations that can help you refine your approach to AR.
Two guidelines, and several Cochrane reviews
Allergic Rhinitis and its Impact on Asthma (ARIA), a sentinel rhinitis treatment guideline, was published in 2001 and updated in 2008 and 2010.2-4 The British Society for Allergy and Clinical Immunology Standards of Care Committee (BSACI) published guidelines for rhinitis management in 2008 and guidelines for immunotherapy in 2011.5,6 In addition, several Cochrane reviews have been performed.7-12 The ALGORITHM1-6 combines these recommendations. The TABLE2-12 itemizes the recommendations made by each guideline.
ALGORITHM
An evidence-based approach to treating allergic rhinitis1-6
Based on recommendations from ARIA and BSACI guidelines and Cochrane reviews
ARIA, Allergic Rhinitis and its Impact on Asthma; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee.
TABLE
Treatment recommendations/suggestions for allergic rhinitis2-12
| TREATMENT RECOMMENDATIONS/SUGGESTIONS | ARIA 2001 | ARIA 2008 | ARIA 2010 | BSACI 2008 | BSACI 2011 | COCHRANE REVIEWS |
|---|---|---|---|---|---|---|
| General principles of treatment | ||||||
| Maintenance therapy is required for persistent AR as medications have little effect after cessation. | X | |||||
| Patient education | ||||||
| Standardized patient education improves disease-specific quality of life. | X | |||||
| Nasal steroids | ||||||
| NS are the most effective monotherapy for all symptoms of AR, seasonal and perennial,* including nasal congestion. | X | |||||
| NS are recommended for AR treatment in adults and suggested for children. | X | |||||
| NS are the treatment of choice for moderate to severe persistent* AR and for treatment failures with antihistamines alone. | X | |||||
| NS are suggested over oral antihistamines in adults and children for seasonal AR. | X | |||||
| NS are suggested over oral antihistamines for adults and children with persistent AR. | X | |||||
| NS are recommended rather than nasal antihistamines. | X | |||||
| NS are recommended over oral leukotriene receptor antagonists for seasonal AR. | X | |||||
| NS are the most effective treatment of AR for children. | X | |||||
| There is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR. | X (2010) | |||||
| Intermittent* NS use may be beneficial in children. | X | |||||
| Avoid NS with high bioavailability (betamethasone) in children, as regular use for >1 year may decrease growth rate. | X | |||||
| Antihistamines | ||||||
| New-generation oral nonsedating antihistamines that do not affect cytochrome P450 are recommended for the treatment of patients with AR. | X | |||||
| Oral or topical antihistamines are first-line treatment for mild to moderate intermittent and moderate persistent AR. | X | |||||
| When NS alone do not control moderate to severe persistent AR, may add oral or topical antihistamines. | X | |||||
| New-generation oral antihistamines are suggested over nasal antihistamines for children and adults, and for children with seasonal or persistent AR. | X | |||||
| Oral antihistamines are suggested over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR. | X | |||||
| Nasal antihistamines are suggested over nasal chromones (the need to use chromones 4 times daily may limit adherence). | X | |||||
| Nasal antihistamine use is suggested for children and adults with seasonal AR. | X | |||||
| Patients with persistent AR should avoid using nasal antihistamines until more data on efficacy and safety are available. | X | |||||
| In children, weigh adverse effects of antihistamines against the general malaise caused by AR. | X | |||||
| Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children. | X | |||||
| Continuous administration of antihistamines is optimal in children, rather than as needed. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for patients with ocular symptoms. | X | |||||
| Oral leukotriene receptor antagonists | ||||||
| Oral leukotriene receptor antagonists are suggested for children and adults with seasonal AR and for preschool children with persistent AR. | X | |||||
| Avoid oral leukotriene receptor antagonists in adults with persistent AR. | X | |||||
| Decongestants | ||||||
| For adults with severe nasal obstruction, a short course (<5 days) of a nasal decongestant, along with other drugs, is suggested. | X | |||||
| Nasal decongestants may be useful for eustachian tube dysfunction when flying, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency prior to NS use. | X | |||||
| Regular oral decongestant use is not suggested. | X | X | ||||
| Avoid decongestants in pregnant patients. | X | |||||
| Avoid using nasal decongestants in preschool children. | X | |||||
| Chromones | ||||||
| Limited use of chromones is recommended for children and adults with mild symptoms. | X | |||||
| Chromones are less effective than NS or antihistamines. | X | |||||
| Nasal antihistamines are suggested over nasal chromones. | X | |||||
| Intraocular antihistamines or intraocular chromones are suggested for ocular symptoms. Due to the excellent safety of these agents, chromones may be tried before antihistamines. | X | |||||
| Nasal saline | ||||||
| Nasal saline irrigation reduces symptoms in children and adults with seasonal rhinitis. | X | |||||
| Oral, intramuscular steroids | ||||||
| A short course of oral glucocorticosteroids is suggested for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments. | X | |||||
| Oral steroids are rarely indicated, but a short course (5-10 days) may be used for severe nasal congestion, uncontrolled symptoms on conventional pharmacotherapy, or important social/work events. | X | |||||
| Avoid intramuscular steroids. | X | X | ||||
| Ipratropium | ||||||
| Nasal ipratropium is suggested for treatment of rhinorrhea for patients with persistent AR. | X | |||||
| Allergen-specific immunotherapy | ||||||
| Immunotherapy is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy or allergen avoidance measures. | X | |||||
| SCIT is suggested for adults with seasonal AR and those with persistent AR due to house dust mites. | X | |||||
| SCIT is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few severe adverse reactions. | X (2007) | |||||
| SLIT is suggested for adults with AR due to pollen, although other alternatives may be equally reasonable. | X | |||||
| SLIT is safe and efficacious for AR treatment, decreasing symptoms and medication requirements. | X (2003) | |||||
| Nasal immunotherapy is suggested for adults with AR due to pollens. | X | |||||
| For pregnant patients, maintenance ASI may be continued, but starting ASI or increasing the dose is contraindicated. | X | |||||
| SCIT is suggested for children with AR. | X | |||||
| SCIT should not be started before 5 years of age. | X | |||||
| Based on preliminary studies, SLIT is safe, but more studies are needed in children. | X | |||||
| SLIT and NIT are suggested for children with AR due to pollens, acknowledging that other alternatives may be equally reasonable. SLIT should not be given to children with AR due to HDM unless being done for research. | X | |||||
| Lifestyle changes | ||||||
| Avoid single chemical or physical preventive and combination preventive methods to reduce HDM exposure. | X | |||||
| Allergen avoidance may decrease AR symptoms, but more research is needed. | X (2010) | |||||
| Achieving substantial reductions in HDM load may decrease AR symptoms. | X (2012) | |||||
| Avoidance of mold or animal dander is recommended for patients who are allergic to them. | X | |||||
| Nasal filters can reduce symptoms of AR during ragweed and grass pollen seasons. | X | |||||
| Complementary and alternative medicine | ||||||
| Avoid homeopathy, acupuncture, butterbur, herbal medicines, and phototherapy. | X | |||||
| AR, allergic rhinitis; ARIA, Allergic Rhinitis and its Impact on Asthma; ASI, allergen-specific immunotherapy; BSACI, British Society for Allergy and Clinical Immunology Standards of Care Committee; HDM, house dust mites; NIT, nasal immunotherapy; NS, nasal steroids; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy. *ARIA 2008 recommended changing the classification of AR from seasonal and perennial (frequent nonseasonal nasal or ocular symptoms) to intermittent (symptoms lasting <4 days per week or <4 weeks per year) or persistent (symptoms >4 days per week and >4 weeks per year).3 AR severity is classified as mild or moderate to severe.2,3 | ||||||
The summary that follows provides a more detailed look at the recommendations, with a review of the pathophysiology of AR (“Phases of allergic rhinitis”2,3,5,8,13-15).
The early phase of allergic rhinitis (AR) occurs within minutes of allergen exposure. Mast cell degranulation releases histamine and other inflammatory mediators that cause sneezing, pruritus, rhinorrhea, and nasal congestion.3,8,13 The late phase, beginning at 4 hours and peaking 6 to 12 hours after exposure, is believed to be due to recruitment of circulating leukocytes—particularly eosinophils. Leukocyte activation causes additional inflammatory mediators to be released, which primarily causes nasal congestion—often the most bothersome symptom of AR.2,5,8,13,14 Other presenting symptoms may include feeling “fuzzy” or tired, chronic viral infections, sniffing, eye rubbing, blinking, congested voice, snoring, or dark skin beneath the eyes (allergic shiners).15
Of note: This summary preserves the terminology used in ARIA 2010. Specifically, the ARIA guideline uses the term suggest for conditional recommendations and recommend for strong recommendations.4 That same language is used here.
Nasal steroids: First-line Tx for moderate to severe symptoms
BSACI indicates that nasal steroids (NS) are the treatment of choice for moderate to severe persistent AR (symptoms lasting >4 days per week or >4 weeks per year).5 ARIA 2010 suggests NS as first-line treatment rather than oral antihistamines for adults and children with seasonal (related to outdoor allergens such as pollens or molds) and persistent AR.4 ARIA 2008 finds NS are the most effective treatment for children.3 Steroids reduce inflammation by decreasing inflammatory cell migration and inhibiting cytokine release.16 They are the most effective monotherapy for all symptoms of AR, including nasal congestion, which antihistamines do not treat effectively.13,16 NS also treat ocular symptoms of allergy effectively.15,17
The ARIA 2010 guideline also recommends using NS rather than nasal antihistamines and leukotriene receptor antagonists.4 Combination therapy (eg, NS with the addition of nasal antihistamines) is an option for severe or persistent AR, but it appears to be no more effective than monotherapy with NS.16 A 2010 Cochrane review determined there is insufficient evidence for or against the use of oral antihistamines plus NS vs NS alone in children with AR.7 Intermittent steroid use may be beneficial in children.5
Steroids begin working 6 to 8 hours after the first dose, although symptom reduction may take days and maximal effect up to 2 weeks.5 Treatment failure may be due to poor technique that can cause local adverse effects (ie, dryness, irritation, epistaxis). Technique-related failure occurs in up to 10% of users.5,15 Educating patients and families about correct technique with steroid spray may decrease nonadherence due to irritation and epistaxis.18 Tell them to shake the bottle well, look down, aim the nozzle toward the outside wall of the nostril using the opposite hand, and spray while sniffing lightly.5
Any steroid is appropriate for adults. For children ≥2 years of age, consider fluticasone propionate, mometasone furoate, or triamcinolone acetonide.3 These medications have lower systemic bioavailability and a decreased risk of such adverse effects as hypothalamic-pituitary-adrenal axis suppression and growth retardation.15 Budesonide is appropriate for those ≥6 years.19-21 Avoid regular use of betamethasone, which has high bioavailability, for >1 year in children, as it may decrease their growth rate.3 Beclomethasone, fluticasone, and budesonide have been used widely and safely for pregnant women with asthma.5
Antihistamines are first-line Tx for mild symptoms
ARIA 2010 recommends new-generation oral nonsedating antihistamines that do not affect cytochrome P450 for mild AR,4 such as cetirizine, levocetirizine, loratadine, desloratadine, and fexofenadine. First-generation antihistamines can reduce symptoms, but are not first-line treatment as they cause sedation, fatigue, decreased cognitive function, and reduced academic and work performance.3-5 ARIA 2010 further suggests choosing oral antihistamines over oral leukotriene receptor antagonists in patients with seasonal AR and in preschool children with persistent AR.4
BSACI recommends oral or topical antihistamines as first-line treatment for mild to moderate symptoms lasting <4 days per week or <4 weeks per year and moderate persistent AR.5 When steroids alone do not control moderate to severe persistent AR, BSACI recommends adding oral or topical antihistamines.5 Oral and topical antihistamines decrease histamine-related symptoms of itching, rhinorrhea, and sneezing, but do not significantly decrease nasal congestion.15
Nasal antihistamines (levocabastine, azelastine) have a rapid onset of action and few adverse effects.3 ARIA 2010 suggests nasal antihistamines over nasal chromones (inhibitors of mast cell degranulation) and notes that the need to use chromones 4 times daily may limit adherence.4 The same guidelines suggest nasal antihistamine use for children and adults with seasonal AR and suggest not using nasal antihistamines for patients with persistent AR until more data on efficacy and safety are available.4
Alezastine is approved for individuals ≥5 years, and olopatadine is approved for individuals ≥6 years for the treatment of AR.16,22,23 A pediatric review article noted nasal antihistamine (azelastine) plus nasal fluticasone was more efficacious than NS alone.15
In children, weigh adverse effects of antihistamines against the general malaise caused by AR.3 Do not use first-generation antihistamines due to the sedation that may interfere with learning.15 Treatment with once-daily, long-acting antihistamines rather than multiple daily dosing may improve adherence in children.5 Continuous administration, rather than as needed, is optimal treatment in children.5 Cetirizine, loratadine, and levocetirizine have been studied and are effective and safe in children.3 Levocetirizine has proven safe and efficacious for children ≥2 years.24 Fexofenadine was found to be effective and safe for those ≥6 years.25
For children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones.4 Due to the safety of these agents, chromones may be used first, then antihistamines.4 Just as with nasal chromones, the need to use intraocular chromones 4 times daily may limit their use in children.4
Pregnant patients. Antihistamines do cross the placenta.5 Agents that appear to be safe for pregnant patients are chlorphenamine (first-generation), loratadine, and cetirizine.5
Leukotriene receptor antagonists: Always pair with antihistamines
As adjunctive therapy for additional symptom control, ARIA 2010 suggests oral leukotriene receptor antagonists for children and adults with seasonal AR, and for preschool children with persistent AR. These agents may also be helpful in children with concurrent asthma.15 Always pair leukotriene receptor antagonists with antihistamines. Montelukast is approved for seasonal AR in children ≥2 years and for frequent nonseasonal nasal or ocular AR symptoms in children ≥6 months.26
ARIA 2010 recommends against the use of oral leukotriene receptor antagonists in adults with persistent AR.4
Decongestants are for limited use only
For adults with severe nasal obstruction, ARIA 2010 suggests a short course (<5 days) of nasal decongestant along with other drugs.4 Limiting use of nasal decongestants to <10 days helps prevent rhinitis medicamentosa.5,27 BSACI notes nasal decongestants may be useful for eustachian tube dysfunction experienced aboard airplanes, for children with acute otitis media with middle ear pain, to relieve congestion after an upper respiratory infection, and to improve nasal patency before NS use.5 Both guidelines suggest against regular oral decongestant use.4,5
Avoid decongestants in pregnant patients.5 ARIA 2010 suggests against nasal decongestant use in preschool children.4
Chromones may help, but require multiple daily dosing
Chromones inhibit mast cell degranulation, are weakly effective for reducing nasal obstruction in AR, and have a high safety profile.3-5,28 As noted earlier, they must be used 4 times daily, which may reduce adherence—particularly in children.4
ARIA 2008 notes that disodium cromoglycate is less effective than NS or antihistamines.3 The 2010 update suggests nasal antihistamines over nasal chromones.4 For adults as well as children with ocular symptoms, ARIA 2010 suggests intraocular antihistamines or intraocular chromones. BSACI recommends limited use of chromones for children and adults with mild symptoms.5
Nasal saline helpful as adjunct to medication
Nasal saline irrigation improves symptoms of AR, clears nasal passages, and is helpful for pregnant patients, for whom medications should be used with caution.2,3,5 Nasal irrigation using a neti pot or squeeze bottle is efficacious for chronic rhinorrhea, as solo or complementary treatment, and for children.5,16,27
Oral steroids: Use only rarely
ARIA 2010 suggests a short course of oral glucocorticosteroids for patients with AR and moderate to severe nasal or ocular symptoms not controlled with other treatments.4 BSACI notes oral steroids are rarely indicated, but that their use over 5 to 10 days may help with severe nasal congestion, symptoms uncontrolled by conventional pharmacotherapy, or before important social or work events.5 Both guidelines recommend against intramuscular steroids.4 ARIA 2008 notes oral and depot preparations of steroids affect growth in young children.3
Ipratropium when rhinorrhea is severe
Nasal ipratropium bromide, a topical anticholinergic, is helpful for excessive or refractory rhinorrhea. Consider using ipratropium with NS for patients for whom rhinorrhea is the dominant symptom.5,16,28 ARIA 2010 suggests using nasal ipratropium to treat rhinorrhea in patients with persistent AR.4
Allergen-specific immunotherapy: When other treatments fail
Allergen-specific immunotherapy (ASI) consists of repeated exposure to an allergen to induce immunomodulation, which prevents or reduces allergy symptoms and actually changes the natural course of AR. (For more on identifying the offending agent, see “Time for allergen testing?”2,5,15,18,29.) This treatment process decreases medication needs, prevents new allergen sensitization, and results in long-lasting improvement.2,5,6,30 BSACI 2011 notes that ASI is effective for adults and children with severe AR who do not respond to conventional pharmacotherapy and allergen avoidance measures.6
When a patient’s symptoms are poorly controlled or persist after treatment, consider allergen testing.29 Skin prick testing (SPT) is the best means of eliciting specific allergen sensitization. However, limit testing to allergens most likely causing the patient’s symptoms rather than ordering a random panel; 15% of those with a positive SPT to specific allergens do not have symptoms when exposed to those allergens in their environment.5 And always interpret results of allergy testing in light of the patient’s history.2,15,18
SPT has a high negative predictive value, which can prevent unnecessary lifestyle changes.29 However, keep in mind that SPT results may be suppressed if the patient is using antihistamines, tricyclic antidepressants, or topical steroids.29 If SPT is not feasible or the patient is taking medications that may suppress results, consider arranging for serum-specific IgE testing, also known as radioallergosorbent testing, or RAST.5 RAST and SPT have similar sensitivities for house dust mites, but RAST is not as sensitive as SPT for other inhalants (eg, cat epithelium, mold, grass pollen).5
ASI methods developed to date use subcutaneous, sublingual, or nasal routes of administration. However, the US Food and Drug Administration has yet to approve commercial sublingual or nasal products for use in the United States.16
Subcutaneous immunotherapy may cause local adverse reactions (pruritus and swelling) and systemic reactions that can be severe or life threatening (anaphylaxis) and thus must be given in a doctor’s office prepared to treat anaphylaxis.6,16,30 Adrenaline administration has been necessary in 0.13% of those being treated.9 Subcutaneous immunotherapy must be done for 3 to 5 years for sustained effective treatment.15
ARIA 2010 suggests subcutaneous immunotherapy for adults with seasonal AR and with persistent AR due to house dust mites.4 A 2007 Cochrane review found subcutaneous immunotherapy is efficacious for patients with seasonal AR due to pollens, resulting in decreased symptoms and medication use with few significant severe adverse reactions.9 A meta-analysis showed subcutaneous immunotherapy is as potent as pharmacotherapy in controlling seasonal AR symptoms as early as the first season of treatment.31
What if the patient is pregnant—or a child? BSACI notes that maintenance ASI may be continued in a patient who becomes pregnant, but starting ASI or increasing the dose is contraindicated.5
Based on ARIA 2008 and 2010, consider subcutaneous immunotherapy for children—but not for those <5 years.3,4 Care must be used in selecting patients, as 3 to 5 years of treatment are necessary for sustained benefit.15
Lifestyle changes: Limited benefit may be achievable
ARIA 2010 recommends mold avoidance and animal dander avoidance for patients so affected.4 Allergens from pets can persist in homes for months after pet removal.15 BSACI found that commercially available nasal filters (filters or screens placed over or within both nares) reduced symptoms of AR during ragweed and grass pollen seasons.5 Allergen avoidance for children with persistent AR has not shown consistent benefit.15 A 2010 Cochrane review concluded that allergen avoidance may decrease AR symptoms, but more research is needed.11
House dust mites. The 2010 Cochrane review also reported on 2 trials that assessed high-efficiency particulate air (HEPA) filters specifically for patients allergic to house dust mites.11 The studies, which had methodological limitations (inconsistent randomization, small sample size, and short duration), concluded that HEPA filters alone will not likely reduce symptoms of house dust mite allergy. But HEPA filters may be beneficial as one component of an extensive bedroom-based environmental control program.11
Impermeable bedding has been shown to reduce dust mite load by 50% to 70%, leaving residual allergen that may still trigger symptoms.11 A 2012 Cochrane review concluded that achieving substantial reductions in house dust mite load using a combination approach of multiple interventions, including acaricides and extensive bedroom-based environmental control programs, may decrease AR symptoms.12 However, ARIA 2010 recommends against single chemical or physical preventive methods and against combination preventive methods to reduce house dust mite exposure.4
Total elimination of house dust mites may be impossible, and recommending use of impermeable covers and HEPA filters, removal of rugs and curtains, and frequent cleaning must take into account a patient’s symptoms and a family’s motivation and finances.11,18
Complementary and alternative medicine: Too little evidence
ARIA 2010 suggests against patients using homeopathy, acupuncture, butterbur, herbal medicines, or phototherapy for AR.4 While one systematic review of acupuncture for AR demonstrated mixed results with no specific effects for seasonal AR and some improvement of frequent nonseasonal symptoms,32 another review concluded evidence was insufficient to make any recommendation.32,33 The benefit of ear acupressure is unknown, as supporting studies are of low methodological quality, although it appeared to provide some benefit for AR.34
Due to lack of data, probiotics should not be recommended.27 A pediatric review article noted that probiotics may alter cytokine production in patients with seasonal AR and may be more helpful in AR than in asthma, although more research was needed.15 Another review showed that probiotics may reduce AR symptoms and medication use.35
CASE Since the nasal steroid you prescribed for your patient did not provide adequate relief, you opt to add cetirizine 10 mg to his NS regimen. This step relieved his symptoms within 2 to 3 days. Had his symptoms persisted, the patient would have been a candidate for a one-week course of oral decongestant, such as pseudoephedrine 120 mg orally every 12 hours, as needed; and then for allergen testing, specifically for pollens corresponding to the seasonality of his AR. Appropriate follow-up would be to monitor the patient until his symptoms resolved or became manageable.
1. Bousquet J, Schünemann HJ, Zuberbier T, et al. Development and implementation of guidelines in allergic rhinitis – an ARIA-GA2LEN paper. Allergy. 2010;65:1212-1221.
2. Bousquet J, Van-Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(suppl):S147-S334.
3. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008. Allergy. 2008;63(suppl 86):S8-S160.
4. Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466-476.
5. Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008;38:19-42.
6. Walker SM, Durham SR, Till SJ, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41:1177-1200.
7. Al Sayyad JJ, Fedorowicz Z, Alhashimi D, et al. Topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2007;(1):CD003163.-
8. Nasser M, Fedorowicz, Alijufairi H, et al. Antihistamines used in addition to topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2010;(7):CD006989.-
9. Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;(1):CD001936.-
10. Wilson D, Torres-Lima M, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003;(2):CD002893.-
11. Sheikh A, Hurwitz B, Nurmatov U, et al. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database Syst Rev. 2010;(7):CD001563.-
12. Nurmatov U, van Schayck CP, Hurwitz B, et al. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158-165.
13. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108(suppl):S2-S8.
14. Nathan RA. The pathophysiology, clinical impact, and management of nasal congestion in allergic rhinitis. Clin Ther. 2008;30:573-586.
15. Kemp AS. Allergic rhinitis. Paediatric Respir Rev. 2009;10:63-68.
16. Sur DK, Scandale S. Treatment of allergic rhinitis. Am Fam Physician. 2010;81:1440-1446.
17. Hong J, Bielory B, Rosenberg JL, et al. Efficacy of intranasal corticosteroids for the ocular symptoms of allergic rhinitis: a systematic review. Allergy Asthma Proc. 2011;32:22-35.
18. Hu W, Katelaris CH, Kemp AS. Allergic rhinitis – practical management strategies. Aust Fam Physician. 2008;37:214-220.
19. Veramyst (fluticasone furoate) nasal spray [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; August 2012. Available at: http://us.gsk.com/products/assets/us_veramyst.pdf. Accessed January 16, 2013.
20. Nasacort AQ (triamcinolone acetonide) nasal spray [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/nasacort_aq/nasacort_aq.html. Accessed January 16, 2013.
21. Rhinocort AQUA 32 mcg (budesonide) nasal spray [prescribing information]. Wilmington, Del: AstraZeneca; revised December 2010. Available at: http://www1.astrazeneca-us.com/pi/Rhinocort_Aqua.pdf. Accessed January 16, 2013.
22. Astelin (azelastine hydrochloride) spray, metered [prescribing information]. Somerset, NJ: Meda Pharmaceuticals; revised July 2011. Available at: http://www.astelin.com/pdf/astelin_pi.pdf. Accessed January 16, 2013.
23. Patanase (olopatadine hydrochloride) nasal spray [prescribing information]. Fort Worth, Tex: Alcon Laboratories; revised February 2012. Available at: http://ecatalog.alcon.com/PI/Patanase_us_en.pdf. Accessed January 16, 2013.
24. Xyzal (levocetirizine dihydrochloride) tablets and oral solution [prescribing information]. Smyrna, Ga: UCB and Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/xyzal/xyzal.pdf. Accessed January 16, 2013.
25. Allegra (fexofenadine hydrochloride) tablets, ODT, and oral suspension [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2007. Available at: http://products.sanofi.us/allegra/allegra.html. Accessed January 16, 2013.
26. Singulair (montelukast sodium) tablets, chewable tablets, and oral granules [prescribing information]. Whitehouse Station, NJ: Merck; revised November 2012. Available at: http://www.merck.com/product/usa/pi_circulars/s/singulair/singulair_pi.pdf. Accessed January 16, 2013.
27. Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106(suppl):S12-S16.
28. Lim MY, Leong JM. Allergic rhinitis: evidence-based practice. Singapore Med J. 2010;51:542-550.
29. Angier E, Willington J, Scadding G, et al. Management of allergic and non-allergic rhinitis: a primary care summary of the BSACI guideline. Prim Care Respir J. 2010;19:217-222.
30. Radulovic S, Wilson D, Calderon M, et al. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011;66:740-752.
31. Matricardi PM, Kuna P, Panetta V, et al. Subcutaneous immunotherapy and pharmacology in seasonal allergic rhinitis: a comparison based on meta-analyses. J Allergy Clin Immunol. 2011;128:791-799.
32. Lee MS, Pittler MH, Shin B, et al. Acupuncture for allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2009;102:269-279.
33. Roberts J, Huissoon A, Dretzke J, et al. A systematic review of the clinical effectiveness of acupuncture for allergic rhinitis. BMC Complement Altern Med. 2008;8:13.-
34. Zhang CS, Yang AW, Zhang AL, et al. Ear-acupressure for allergic rhinitis: a systematic review. Clin Otolaryngol. 2010;35:6-12.
35. Vliagoftis H, Kouranos VD, Betsi GI, et al. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101:570-579.
CORRESPONDENCE Suzanne Minor, MD, Florida International University Herbert Wertheim College of Medicine; 11200 SW 8th Street, AHC II 361A, Miami, FL 33199; [email protected]
1. Bousquet J, Schünemann HJ, Zuberbier T, et al. Development and implementation of guidelines in allergic rhinitis – an ARIA-GA2LEN paper. Allergy. 2010;65:1212-1221.
2. Bousquet J, Van-Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(suppl):S147-S334.
3. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008. Allergy. 2008;63(suppl 86):S8-S160.
4. Brozek JL, Bousquet J, Baena-Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466-476.
5. Scadding GK, Durham SR, Mirakian R, et al. BSACI guidelines for the management of allergic and non-allergic rhinitis. Clin Exp Allergy. 2008;38:19-42.
6. Walker SM, Durham SR, Till SJ, et al. Immunotherapy for allergic rhinitis. Clin Exp Allergy. 2011;41:1177-1200.
7. Al Sayyad JJ, Fedorowicz Z, Alhashimi D, et al. Topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2007;(1):CD003163.-
8. Nasser M, Fedorowicz, Alijufairi H, et al. Antihistamines used in addition to topical nasal steroids for intermittent and persistent allergic rhinitis in children. Cochrane Database Syst Rev. 2010;(7):CD006989.-
9. Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;(1):CD001936.-
10. Wilson D, Torres-Lima M, Durham S. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev. 2003;(2):CD002893.-
11. Sheikh A, Hurwitz B, Nurmatov U, et al. House dust mite avoidance measures for perennial allergic rhinitis. Cochrane Database Syst Rev. 2010;(7):CD001563.-
12. Nurmatov U, van Schayck CP, Hurwitz B, et al. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67:158-165.
13. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. 2001;108(suppl):S2-S8.
14. Nathan RA. The pathophysiology, clinical impact, and management of nasal congestion in allergic rhinitis. Clin Ther. 2008;30:573-586.
15. Kemp AS. Allergic rhinitis. Paediatric Respir Rev. 2009;10:63-68.
16. Sur DK, Scandale S. Treatment of allergic rhinitis. Am Fam Physician. 2010;81:1440-1446.
17. Hong J, Bielory B, Rosenberg JL, et al. Efficacy of intranasal corticosteroids for the ocular symptoms of allergic rhinitis: a systematic review. Allergy Asthma Proc. 2011;32:22-35.
18. Hu W, Katelaris CH, Kemp AS. Allergic rhinitis – practical management strategies. Aust Fam Physician. 2008;37:214-220.
19. Veramyst (fluticasone furoate) nasal spray [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; August 2012. Available at: http://us.gsk.com/products/assets/us_veramyst.pdf. Accessed January 16, 2013.
20. Nasacort AQ (triamcinolone acetonide) nasal spray [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/nasacort_aq/nasacort_aq.html. Accessed January 16, 2013.
21. Rhinocort AQUA 32 mcg (budesonide) nasal spray [prescribing information]. Wilmington, Del: AstraZeneca; revised December 2010. Available at: http://www1.astrazeneca-us.com/pi/Rhinocort_Aqua.pdf. Accessed January 16, 2013.
22. Astelin (azelastine hydrochloride) spray, metered [prescribing information]. Somerset, NJ: Meda Pharmaceuticals; revised July 2011. Available at: http://www.astelin.com/pdf/astelin_pi.pdf. Accessed January 16, 2013.
23. Patanase (olopatadine hydrochloride) nasal spray [prescribing information]. Fort Worth, Tex: Alcon Laboratories; revised February 2012. Available at: http://ecatalog.alcon.com/PI/Patanase_us_en.pdf. Accessed January 16, 2013.
24. Xyzal (levocetirizine dihydrochloride) tablets and oral solution [prescribing information]. Smyrna, Ga: UCB and Bridgewater, NJ: sanofi-aventis; 2010. Available at: http://products.sanofi.us/xyzal/xyzal.pdf. Accessed January 16, 2013.
25. Allegra (fexofenadine hydrochloride) tablets, ODT, and oral suspension [prescribing information]. Bridgewater, NJ: sanofi-aventis; 2007. Available at: http://products.sanofi.us/allegra/allegra.html. Accessed January 16, 2013.
26. Singulair (montelukast sodium) tablets, chewable tablets, and oral granules [prescribing information]. Whitehouse Station, NJ: Merck; revised November 2012. Available at: http://www.merck.com/product/usa/pi_circulars/s/singulair/singulair_pi.pdf. Accessed January 16, 2013.
27. Meltzer EO, Bukstein DA. The economic impact of allergic rhinitis and current guidelines for treatment. Ann Allergy Asthma Immunol. 2011;106(suppl):S12-S16.
28. Lim MY, Leong JM. Allergic rhinitis: evidence-based practice. Singapore Med J. 2010;51:542-550.
29. Angier E, Willington J, Scadding G, et al. Management of allergic and non-allergic rhinitis: a primary care summary of the BSACI guideline. Prim Care Respir J. 2010;19:217-222.
30. Radulovic S, Wilson D, Calderon M, et al. Systematic reviews of sublingual immunotherapy (SLIT). Allergy. 2011;66:740-752.
31. Matricardi PM, Kuna P, Panetta V, et al. Subcutaneous immunotherapy and pharmacology in seasonal allergic rhinitis: a comparison based on meta-analyses. J Allergy Clin Immunol. 2011;128:791-799.
32. Lee MS, Pittler MH, Shin B, et al. Acupuncture for allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2009;102:269-279.
33. Roberts J, Huissoon A, Dretzke J, et al. A systematic review of the clinical effectiveness of acupuncture for allergic rhinitis. BMC Complement Altern Med. 2008;8:13.-
34. Zhang CS, Yang AW, Zhang AL, et al. Ear-acupressure for allergic rhinitis: a systematic review. Clin Otolaryngol. 2010;35:6-12.
35. Vliagoftis H, Kouranos VD, Betsi GI, et al. Probiotics for the treatment of allergic rhinitis and asthma: systematic review of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101:570-579.
CORRESPONDENCE Suzanne Minor, MD, Florida International University Herbert Wertheim College of Medicine; 11200 SW 8th Street, AHC II 361A, Miami, FL 33199; [email protected]