User login
In 2008, the American Heart Association (AHA) published a science advisory on depression and coronary heart disease (CHD).1 Since its publication, the advisory has evoked substantial commentary. The purpose of this article is threefold: (1) to explain the aims of the AHA science advisory, (2) to briefly discuss its content, and (3) to examine some of the comments it has provoked.
WHAT THE ADVISORY SET OUT TO DO
The purpose of an AHA science advisory is to provide rapid, clear, and consistent AHA positioning on a scientific issue. Advisories are statements on an evolving, prominent scientific issue of great interest to the public and health professionals. All AHA science advisories undergo peer review and are also reviewed and approved by the AHA Science Advisory and Coordinating Committee, AHA’s highest science body. Because this particular advisory addressed the interaction of cardiovascular and mental health, the AHA asked the American Psychiatric Association (APA) to review the document; the APA endorsed the AHA advisory.
Two points are worth emphasizing:
- An AHA science advisory is not a treatment guideline.
- Advisories usually are brief and therefore do not exhaustively discuss their topic.
After discussing epidemiologic studies that elucidated the relationships between depression and CHD, the AHA advisory on depression and CHD focuses on screening, referral, and treatment of depression from a cardiology perspective.
The 1-year prevalence of major depressive disorder in the US general population is 7%, and the lifetime prevalence is about 16%.2 Depression in otherwise healthy persons almost doubles the risk of developing CHD.3 About 20% of patients hospitalized for acute coronary syndromes (ACS) have major depressive disorder on admission or within a few weeks thereafter, and these patients have about 2.5 times the mortality rate as patients who are not depressed, after adjusting for infarct severity and cardiovascular risk factors.4–6
ASSESSMENT OF DEPRESSION AND DEPRESSIVE SYMPTOMS
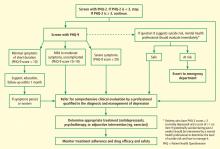
The AHA advisory discusses use of the 2-question Patient Health Questionnaire (PHQ-2) as the first step in screening for depression.7,8 The PHQ-2 inquires about the frequency of depressed mood and anhedonia by asking the following:
Over the past 2 weeks, how often have you been bothered by either of the following problems?
(1) Little interest or pleasure in doing things
(2) Feeling down, depressed, or hopeless.
For each item the response options are “not at all” (scored as 0), “several days” (scored as 1), “more than half the days” (scored as 2), and “nearly every day” (scored as 3). Thus, the total PHQ-2 score can range from 0 to 6.
Using a structured psychiatric interview as the standard, a total PHQ-2 score of 3 or greater has been shown to have a sensitivity of 83% and a specificity of 92% for major depression.7 A PHQ-2 score of 3 is the optimal cut point for screening purposes. A PHQ-2 score of 0 virtually excludes depression.
If a patient’s PHQ-2 score is 3 or greater, it is recommended that answers be obtained for a full 9-item PHQ. The PHQ-9 provides the sensitivity and specificity suitable for assigning a provisional diagnosis of major depressive disorder and a symptom severity score that can be used to identify patients for further evaluation and to make decisions about therapy.9–11
The US Preventive Services Task Force (USPSTF) currently recommends that clinicians screen adults for depression in clinical practices that have systems in place to assure accurate diagnosis, effective treatment, and follow-up.12 The USPSTF concluded that there is good evidence that screening improves the accurate identification of depressed patients in primary care settings and that treating depressed adults identified in such settings reduces clinical morbidity. Results of trials that have directly evaluated the effect of screening on clinical outcomes depend on follow-up. Limited benefits have been demonstrated in studies that simply feed screening results back to clinicians. Larger benefits have been seen in studies in which the communication of screening results is coordinated with effective follow-up and treatment. The USPSTF concluded that the benefits of screening and treating are likely to outweigh any potential harms.12
DEPRESSION TREATMENT
Drug therapy
The safety of fluoxetine, sertraline (SADHART, ENRICHD), citalopram (CREATE), and mirtazapine (MIND-IT) has been evaluated in clinical trials.4,17–21 By far the strongest evidence of safety is for selective serotonin reuptake inhibitors (SSRIs), especially sertraline.
The Sertraline Antidepressant Heart Attack Randomized Trial (SADHART; N = 369), a randomized study of depression after ACS, found no difference in cardiovascular adverse events between the sertraline and placebo groups after 16 weeks of therapy, and there were no significant differences in left ventricular ejection fraction, heart rate, blood pressure, ventricular premature complexes, or electrocardiogram changes.17 In the group assigned to sertraline, life-threatening cardiovascular events occurred less frequently (15% vs 22%, P = NS).
The Enhancing Recovery in Coronary Heart Disease study (ENRICHD) was a large trial (N = 2,481) to evaluate the effect of cognitive behavioral therapy (CBT) on depression or low perceived social support in patients enrolled within 28 days of myocardial infarction (MI).4 The ENRICHD protocol required patients randomized to CBT with Hamilton Depression Rating Scale (HAM-D) scores greater than 24, or who showed less than 50% reduction in Beck Depression Inventory (BDI) scores after 5 weeks of CBT, to be referred to a study psychiatrist for consideration of pharmacotherapy, usually sertraline. Of the overall ENRICHD population, 1,834 participants (74%) had a diagnosis of depression, and 446 of these participants (24%) were treated with antidepressant drugs, 301 with an SSRI and 145 with other antidepressants.18 During mean follow-up of 29 months, the SSRI-treated group had a statistically significant 43% reduction in death or MI.4
The Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) recruited 284 patients with chronic CHD, major depressive disorder, and a 24-item HAM-D score of 20 or greater and randomly assigned half to citalopram (another SSRI) and half to placebo.19 Citalopram showed antidepressant efficacy and no evidence of harm.
The Myocardial Infarction and Depression Intervention Trial (MIND-IT) recruited 91 patients within 30 days of hospital admission for MI with depression and randomized them in a 1:1 ratio to mirtazapine or placebo. Mirtazapine showed some evidence of antidepressant efficacy and no evidence of harm.20
Strik et al in the Netherlands recruited 54 patients who had major depression after a first MI and randomized them 1:1 to the SSRI fluoxetine or placebo 3 months after the MI.21 The fluoxetine group showed a trend toward antidepressant efficacy, and no cardiovascular safety problems were detected clinically or by either electrocardiogram or echocardiogram.
Psychotherapy
APA practice guidelines for major depressive disorder indicate that among psycho therapeutic approaches, CBT and interpersonal psychotherapy have the best-documented efficacy for treatment of major depressive disorder.22 CBT aims to solve problems related to dysfunctional emotions, behaviors, and cognition and is an umbrella term for various techniques that share a theoretical basis in behavioristic learning theory and cognitive psychology. Aaron T. Beck proposed that depressed people are quick to make negative evaluations of themselves and the world, and he designed treatment to reduce these negative cognitions.23 Interpersonal psychotherapy stems from the work of Harry Stack Sullivan, who believed that emotional reactions were triggered by interpersonal behaviors.24 Gerald Klerman and Myrna Weissman used this method to treat adults diagnosed with moderate or severe nondelusional clinical depression.25
CBT was used in ENRICHD,4 interpersonal psychotherapy in CREATE,19 and problem-solving therapy in the Coronary Psychosocial Evaluation Studies (COPES).26,27 Unintended therapy can also be a confounder in antidepressant drug trials; education and supportive care (eg, frequent visits or telephone calls with monitoring of depressive symptoms and counseling) are often provided for both the intervention and control (placebo) groups. If a study is blinded, education, supportive care, and attention will be identical for both groups and thus may reduce the likelihood of finding a difference between the drug and placebo, if one exists.
Psychotherapy can be helpful for depression, is preferred over antidepressant drugs by some patients, and can be combined with drugs to increase antidepressant efficacy. We still have much to learn about timing and choice of therapy, as well as about sequencing and combination of antidepressant drugs and psychotherapy.
Physical activity and exercise
Aerobic exercise28 and cardiac rehabilitation29 can reduce depressive symptoms in addition to improving cardiovascular fitness. Depression can serve as a barrier to participation in cardiac rehabilitation and exercise programs, but cardiologists can help depressed patients overcome this barrier by offering encouragement and follow-up contacts. Cardiologists also should enlist the help of spouses or other family members and friends to promote adherence. The prescription of exercise needs to be based on the cardiac status and exercise capacity of each individual.30
SUMMARY OF ADVISORY RECOMMENDATIONS
The AHA advisory1 summarized its recommendations as follows:
1. Routine screening for depression in patients with CHD should be considered in a variety of settings, including the hospital, the physician’s office, clinics, and cardiac rehabilitation centers. The opportunity to screen for and treat depression in cardiac patients should not be missed, as effective depression treatment may improve health outcomes.
2. Patients with positive screening results should be evaluated by a professional qualified in diagnosis and management of depression. Such a clinician can determine whether depression is present and needs treatment, as well as how to connect a patient to an effective care program in the local area.
3. Patients with heart disease who are being treated for depression should undergo careful monitoring for adherence to their medical care and for the efficacy and safety of drug therapy for their medical and mental health conditions.
4. Coordination of care among health care providers is essential for patients with coexisting medical and mental health issues.
COMMENTS ON THE ADVISORY
Since AHA advisories usually address evolving scientific issues, the knowledge base on these issues is constantly growing and a range of opinions and hypotheses are tenable, pending new information. Soon after the AHA science advisory on depression and CHD was issued, a systematic review on depression screening and patient outcomes in cardiac care was published.31 This review posed three key questions that are explored below.
Three key questions
Key question 1: What’s the accuracy of screening instruments for depression in cardiovascular care populations? To answer this question, a case-finding method such as a questionnaire (eg, BDI or PHQ) must be compared with a structured interview by mental health personnel as the “gold standard” for diagnosis (ie, the truth).
To estimate the accuracy of clinical diagnosis by general practitioners, Mitchell et al pooled data from 50,371 primary care patients across 41 studies in Europe or the United States to evaluate general practitioners’ ability to make an unassisted diagnosis of depression (ie, without specific help from severity scales, diagnostic instruments, education programs, etc).32 They reported a sensitivity of about 50% and a specificity of 81% when the prevalence of depression was about 20%. In other words, general practitioners missed about half the cases of depression when no case-finding tool was used. These researchers pointed out that a low prevalence of depression favors identification of nondepressed cases (false-positive diagnoses), whereas a high prevalence favors diagnosis of depression (true-positive diagnoses).32
Cardiologists focused on treatment of ACS are probably less likely to make a clinical diagnosis of depression and may attribute emotional symptoms to rapidly evolving cardiovascular events. The simple 2-question PHQ-2 case-finding instrument takes only a few minutes to administer and is recommended for use by primary care physicians or cardiologists to evaluate patients at high risk for depression or who manifest symptoms suggestive of depression.7
In the United States, most patients with depression are cared for in primary care venues. The AHA advisory recommends referring ACS patients who screen positive on a PHQ to a professional qualified in the diagnosis and management of depression.1 Enhanced care—using outreach, monitoring, adjustment of therapy, and psychiatric backup—produces significant improvement in depression.33
Key question 2: Is treatment of depression in cardiovascular care patients effective in improving depression? Cardiac outcomes? The evidence for benefit of SSRI therapy for depression detected at the time of ACS is consistent and was discussed above.4,17–21 However, the antidepressant effect of SSRIs is modest in placebo-controlled trials. Most such trials excluded patients who were taking antidepressant drugs when screened, and many patients recruited for antidepressant clinical trials had no previous episodes of depression, had relatively mild symptoms of short duration, and were recruited by screening (ie, they were not seeking treatment for depression).34 Patients with brief and short episodes are likely to remit spontaneously and to respond to psychotherapy or supportive care.34 Moreover, in most antidepressant trials, both the intervention and “control” groups received elements of enhanced depression care, which will reduce the apparent benefit of antidepressant drugs. For instance, because the primary goal of SADHART was to evaluate the safety of sertraline in patients with ACS, monitoring for adverse effects included six or seven visits during 16 weeks of follow-up plus six or seven phone calls, providing several elements of enhanced depression care, including face-to-face education, frequent follow-up, support by a case manager (research coordinator) with a mental health background, and support from a psychiatrist or psychologist.17 Randomization to sertraline or placebo in SADHART was blinded, so the frequent contact and support was the same in both groups.
Whether SSRI treatment for depression will improve survival and cardiovascular outcomes is not established by adequately powered randomized trials. Most randomized (SADHART, CREATE) or nonrandomized (ENRICHD) studies suggest that SSRIs reduce cardiovascular events, but only ENRICHD produced a statistically significant result. SSRIs—certainly sertraline and citalopram—are not associated with significant cardiovascular adverse effects, even during ACS, when the cardiovascular system is unstable and multiple drugs are being started and titrated. Patients who do not improve significantly during antidepressant drug therapy or psychotherapy have a two-to threefold increase in cardiovascular events compared with patients who do improve substantially.35
Key question 3: Is systematic screening for depression more effective than usual care for identifying patients with depression? Facilitating treatment of depression? Reducing depressive symptoms? Improving cardiac outcomes? Pignone et al conducted a literature review and meta-analysis on behalf of the USPSTF to clarify whether screening adults for depression in primary care venues improves recognition, treatment, and clinical outcomes.36 They reviewed randomized trials conducted in primary care settings that evaluated the effect of screening for depression on identification, treatment, or health outcomes, including trials that examined integrated, systematic support for treatment after identification of depression. The meta-analysis suggested that screening and feedback of screening results reduced the risk of persistent depression. Stronger effects were observed with programs that integrated interventions to improve recognition and treatment of depressed patients and that incorporated quality improvements into clinic systems as compared with programs that provided only screening and feedback.
Screening patients with CHD, especially those with ACS, is more effective than usual care (no screening). Indeed, many depressed patients go undetected unless identified by screening. Not surprisingly, those identified by screening tend to have milder depression. For example, half the participants in SADHART had a HAM-D score less than 18 (≥ 25 is considered severe). However, a considerable number identified by screening had above-average HAM-D scores but either viewed their symptoms as appropriate for their medical condition or were simply denying their psychiatric illness. If patients had not been screened, their depression would not have been detected or treated. Even among SADHART participants with baseline HAM-D scores less than 18, those whose depression failed to respond to sertraline/placebo had twice the 7-year mortality rate as did those whose depression remitted.16
The Prevention of Suicide in Primary Care Elderly: Collaborative Trial (PROSPECT) examined the impact of a care management intervention on suicidal ideation and depression in older primary care patients and reported outcomes over a 2-year follow-up.37 PROSPECT screened 9,072 patients in 20 primary care practices to find 599 study participants aged 60 years or older with major or minor depression (not all had cardiovascular disease). Participants were randomly assigned to either usual care or the PROSPECT intervention, which consisted of services rendered by trained care managers who offered algorithm-based recommendations to physicians and helped patients adhere to treatment during the 24-month trial. Compared with patients receiving usual care, those receiving the intervention had a greater likelihood of receiving antidepressant drugs and/or psychotherapy (85%–89% vs 49%–62%) and had a 2.2-fold greater decline in suicidal ideation over 24 months. Treatment response started sooner in the intervention group and continued to improve at 18 and 24 months; no appreciable increase in treatment response was observed in the usual-care group during the same period. Among patients with major depression, a significantly greater percentage of the intervention group achieved remission compared with the usual-care group at 4, 8, and 24 months. Patients with minor depression had favorable mental health outcomes regardless of treatment assignment. Sustained collaborative care maintained high utilization of depression treatment, reduced suicidal ideation, and improved the outcomes of major depression during 2 years of follow-up.37
No randomized trial of SSRIs has been designed to show an effect on mortality or cardiovascular events. ENRICHD has produced the strongest evidence that an SSRI can reduce mortality or cardiovascular outcomes. The study was designed with approximately 85% power to detect a 25% to 30% reduction in the primary end point (death or MI) as a result of CBT; as noted above, 2,481 patients (1,834 with depression) were randomized to usual care or CBT within 28 days of MI.4 Depression significantly improved with CBT, but the rate of death or MI did not: during mean follow-up of 29 months, death or MI occurred in 299 patients in the CBT group versus 300 in the usual-care group.4 A post hoc analysis specific to the 1,834 ENRICHD participants who had depression aimed to determine the effects of antidepressant drugs on morbidity and mortality.18 The protocol required patients in the intervention group with scores of 25 or higher on the 17-item HAM-D, or those who had less than a 50% reduction in BDI scores after 5 weeks of treatment, to be referred to study psychiatrists for consideration of pharmacotherapy. Study psychiatrists met with patients who were being treated with antidepressant drugs to monitor medication use. Unless contraindicated or previously ineffective or poorly tolerated, sertraline was the first antidepressant used. Using a time-dependent multivariable Cox proportional hazards regression model to adjust for baseline depression score and cardiac risk factors, the researchers found SSRI use to be associated with a statistically significant 43% lower risk of death or nonfatal MI. Like other SSRI studies, ENRICHD found SSRIs safe for post-MI patients and to possibly, but not certainly, reduce death and MI.18
Medication adherence
One obvious but important way antidepressant drug therapy could prevent death or MI is by improving adherence to post-MI cardiovascular drugs. Four or five classes of cardiac drugs have each been proven to improve survival following ACS (aspirin, beta-blockers, statins, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers), and when all are taken regularly, mortality is reduced by about half.38,39 In addition, antihypertensive and antidiabetic drugs are often needed to control blood pressure or blood glucose. However, patients have to actually take these drugs to receive their benefit. Depression in the setting of CHD, especially ACS, is a risk indicator for lack of adherence to medical therapy, mental health therapy, or both.
DiMatteo et al conducted a meta-analysis to test the hypothesis that anxiety and depression might explain poor adherence to treatment recommendations and result in poor medical outcomes.15 Of the 25 trials that met the inclusion criteria, 13 studied anxiety and 12 studied depression. The associations between anxiety and nonadherence were small and not statistically significant, but depression was strongly associated with nonadherence to medications (odds ratio = 3.03; 95% confidence interval, 1.96–4.89). In other words, depressed patients were three times as likely as nondepressed patients to be nonadherent to treatment recommendations. The authors speculated that depression might increase nonadherence in the following ways: (1) the hopelessness of depression might reduce patients’ hope in the therapy, (2) depression may cause withdrawal from family and social networks that otherwise would provide support and assistance, or (3) the impaired cognitive dysfunction associated with depression may impair memory and follow through on treatment recommendations.15
The findings from this meta-analysis could reflect depression causing medication nonadherence or vice versa. To establish the sequence, Rieckmann et al measured adherence to aspirin therapy during a 3-month period in a consecutive cohort of 172 patients (25 to 85 years old) recruited within 1 week of hospitalization for ACS.40 Severity of depressive symptoms was quantified using the BDI during hospitalization and at 1 and 3 months after discharge. Adherence was defined as taking aspirin as prescribed on at least 80% of days. Using an electronic monitoring system that recorded the date and time when the aspirin bottle cap was opened, the study found that more than 30% of patients with post-ACS depression were nonadherent to their aspirin therapy compared with only 15% of nondepressed patients.40 The more severe patients’ depressive symptoms were, the greater the nonadherence to aspirin therapy. Moreover, a lagged correlation statistical model determined that improvement in depression preceded improvement in medication adherence.
SADHART was conducted under the new drug application for sertraline,16 which required that the use of trial drugs be under strict compliance to protocol, sponsor monitoring, and auditing by the US Food and Drug Administration. Drug use data were complete in 98.1% of participants. Adherence was measured using tablet counts. Depressed patients who had a large improvement in depression during blinded drug therapy (sertraline or placebo) showed improved adherence to the blinded therapy. To determine whether depression improved before medication adherence improved, researchers compared responders’ medication adherence before and after their improvement in depression. Medication adherence increased following remission of depression in 128 of 187 participants (68.4%) who remitted on trial medication (remission was defined as a Clinical Global Impression–Improvement score of 1). This sequence of change (improved depression before improved medication adherence) occurred significantly more often than would be expected by chance (P < .001). This finding suggests that improvement in depression is driving improved medication adherence.
Because persistent depression is associated with increased mortality rates and reduced medication adherence, physicians need to not only aggressively treat depression but also diligently promote adherence to guideline-defined cardiovascular drug therapy. If depression doesn’t improve, additional measures should be initiated not only to improve depression but also to achieve adherence to cardiovascular drug therapy (eg, assistance from spouse, child, or visiting nurse; calls by case manager; electronic health record monitoring of drug prescription refills). When depression is found during clinical encounters or by screening, nonadherence to drug treatment is much more likely and should be sought vigilantly.
Adherence to lifestyle recommendations
Ziegelstein et al have shown that depressed patients have poorer adherence to lifestyle recommendations (diet, exercise, smoking cessation).41 The Heart and Soul Study, a prospective cohort study of 1,017 outpatients with stable CHD, attempted to determine why depressive symptoms (as determined by PHQ-9 self-report) are associated with an increased risk of cardiovascular events.42 Participants were predominantly older men, about half of whom were recruited from Veterans Administration hospitals. A total of 341 cardiovascular events occurred during a mean follow-up of 4.8 years. Participants with baseline depressive symptoms had a 50% greater rate of cardiovascular events during the study period compared with participants without depressive symptoms. However, no significant association between depressive symptoms and cardiovascular events remained after adjustment for health behaviors—most strikingly, physical activity.42 This finding was consistent with an earlier study that found that exercise therapy plus antidepressant medication could reduce the risk of cardiovascular events in patients with depression.43 The ongoing Understanding Prognostic Benefits of Exercise and Antidepressant Therapy (UPBEAT) study is comparing the effects of exercise and antidepressant medication on depression and biomarkers of cardiovascular risk in patients with depressive symptoms and CHD.44 The study’s longer-term goal is to identify an intervention that improves both depression and cardiovascular disease outcomes.
CONCLUSIONS
The USPSTF recommends screening for depression in adults. The PHQ-2 is an efficient first-step screening tool that can identify many depressed patients who would otherwise go undetected. It is clear that SSRIs are safe in cardiac patients, can reduce depression, and can improve medication adherence, but it is not enough to screen and report depression. Optimal benefit depends on having (1) a primary care provider who is familiar with managing depression, (2) a case manager with a mental health background to follow and support patients, and (3) regular supervision of the case manager by a psychiatrist or psychologist. Cardiologists see large numbers of patients with chronic CHD, ACS, or recent coronary artery bypass graft surgery who are at high risk for depression. The AHA advisory recommends a care model that is practical for CHD patients with depression. Such a care model must be based on detection of depression and referral to a practice that has resources and knowledge to manage it well.
- Lichtman JH, Bigger JT, Blumenthal JA, et al Depression and coronary heart disease: recommendations for screening, referral, and treatment. A science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research; endorsed by the American Psychiatric Association. Circulation 2008; 118:1768–1775.
- Kessler RC, Merikangas KR, Wang PS. Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annu Rev Clin Psychol 2007; 3:137–158.
- Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med 2003; 65:201–210.
- Berkman LF, Blumenthal J, Burg M, et al Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA 2003; 289:3106–3116.
- Thombs BD, Bass EB, Ford DE, et al Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med 2006; 21:30–38.
- van Melle JP, de Jonge P, Spijkerman TA, et al Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004; 66:814–822.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003; 41:1284–1292.
- Whooley M, Avins AL, Miranda J, Brown WS. Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med 1997; 12:289–290.
- Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med 2007; 22:1596–1602.
- Stafford L, Berk M, Jackson HJ. Validity of the Hospital Anxiety and Depression Scale and Patient Health Questionnaire-9 to screen for depression in patients with coronary artery disease. Gen Hosp Psychiatry 2007; 29:417–424.
- McManus D, Pipkin SS, Whooley MA. Screening for depression in patients with coronary heart disease (data from the Heart and Soul Study). Am J Cardiol 2005; 96:1076–1081.
- U.S. Preventive Services Task Force. Screening for depression: recommendations and rationale. Rockville, MD: Agency for Healthcare Research and Quality; May 2002. http://www.ahrq.gov/clinic/3rduspstf/depression/depressrr.htm. Accessed January 8, 2010.
- Depression Guideline Panel. Clinical Practice Guideline Number 5: Depression in Primary Care, 1: Detection and Diagnosis. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; April 1993. AHCPR publication 93-0550.
- Depression Guideline Panel. Clinical Practice Guideline Number 5: Depression in Primary Care, 2: Treatment of Major Depression. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; April 1993. AHCPR publication 93-0551.
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000; 160:2101–2107.
- Glassman AH, Bigger JT, Gaffney M. Psychiatric characteristics associated with long-term mortality among 361 patients having an acute coronary syndrome and major depression: seven-year follow-up of SADHART participants. Arch Gen Psychiatry 2009; 66:1022–1029.
- Glassman AH, O’Connor CM, Califf RM, et al Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709.
- Taylor CB, Youngblood ME, Catellier D, et al Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry 2005; 62:792–798.
- Lesperance F, Frasure-Smith N, Koszycki D, et al Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007; 297:367–379.
- Honig A, Kuyper A, Schene AH, et al Treatment of post-myocardial infarction depressive disorder: a randomized, placebo-controlled trial with mirtazapine. Psychosom Med 2007; 69:606–613.
- Strik J, Honig A, Lousberg R, et al Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: findings from a double-blind, placebo-controlled trial. Psychosom Med 2000; 62:783–789.
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000; 157( suppl 4):1–45.
- Beck AT. Cognitive Therapy and the Emotional Disorders. Madison, CT: International Universities Press Inc; 1975.
- Weissman MM. A brief history of interpersonal psychotherapy. Psychiatr Ann 2006; 36:553–557.
- Klerman GL, Weissmann MM. Interpersonal psychotherapy (IPT) and drugs in the treatment of depression. Pharmacopsychiatry 1987; 20:3–7.
- Burg MM, Lespérance F, Rieckmann N, Clemow L, Skotzko C, Davidson KW. Treating persistent depressive symptoms in post-ACS patients: the project COPES phase-I randomized controlled trial. Contemp Clin Trials 2008; 29:231–240.
- Davidson KW, Rieckmann N, Clemow L, et al Collaborative depression care for acute coronary syndrome patients with persistent depression: Coronary Psychosocial Evaluation Studies (COPES) randomized controlled trial. Arch Intern Med. In press.
- Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med 2002; 32:741–760.
- Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. Am J Med 2007; 120:799–806.
- Smith SC, Allen J, Blair SN, et al AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update; endorsed by the National Heart, Lung, and Blood Institute. Circulation 2006; 113:2363–2372.
- Thombs BD, de Jonge P, Coyne JC, et al Ziegelstein RC. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA 2008; 300:2161–2171.
- Mitchell AJ, Vaze A, Rao S. Clinical diagnosis of depression in primary care: a meta-analysis. Lancet 2009; 374:609–619.
- Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006; 166:2314–2321.
- Glassman AH, Bigger JT, Gaffney M, Shapiro PA, Swenson JR. Onset of major depression associated with acute coronary syndromes: relationship of onset, major depressive disorder history, and episode severity to sertraline benefit. Arch Gen Psychiatry 2006; 63:283–288.
- Carney RM, Freedland KE. Treatment-resistant depression and mortality after acute coronary syndrome. Am J Psychiatry 2009; 166:410–417.
- Pignone MP, Gaynes BN, Rushton JL, et al Screening for depression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 136:765–776.
- Alexopoulos GS, Reynolds CF, Bruce ML, et al Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry 2009; 166:882–890.
- Mukherjee D, Fang J, Chetcuti S, Moscucci M, Kline-Rogers E, Eagle KA. Impact of combination evidence-based medical therapy on mortality in patients with acute coronary syndromes. Circulation 2004; 109:745–749.
- Eagle KA, Kline-Rogers E, Goodman SG, et al Adherence to evidence-based therapies after discharge for acute coronary syndromes: an ongoing prospective, observational study. Am J Med 2004; 117:73–81.
- Rieckmann N, Gerin W, Kronish IA, et al Course of depressive symptoms and medication adherence after acute coronary syndromes: an electronic medication monitoring study. J Am Coll Cardiol 2006; 48:2218–2222.
- Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med 2000; 160:1818–1823.
- Whooley MA, de Jonge P, Vittinghoff E, et al Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA 2008; 300:2379–2388.
- Blumenthal JA, Babyak MA, Doraiswamy PM, et al Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 2007; 69:587–596.
- Blumenthal JA, Sherwood A, Rogers SD, et al Understanding prognostic benefits of exercise and antidepressant therapy for persons with depression and heart disease: the UPBEAT study—rationale, design, and methodological issues. Clin Trials 2007; 4:548–559.
In 2008, the American Heart Association (AHA) published a science advisory on depression and coronary heart disease (CHD).1 Since its publication, the advisory has evoked substantial commentary. The purpose of this article is threefold: (1) to explain the aims of the AHA science advisory, (2) to briefly discuss its content, and (3) to examine some of the comments it has provoked.
WHAT THE ADVISORY SET OUT TO DO
The purpose of an AHA science advisory is to provide rapid, clear, and consistent AHA positioning on a scientific issue. Advisories are statements on an evolving, prominent scientific issue of great interest to the public and health professionals. All AHA science advisories undergo peer review and are also reviewed and approved by the AHA Science Advisory and Coordinating Committee, AHA’s highest science body. Because this particular advisory addressed the interaction of cardiovascular and mental health, the AHA asked the American Psychiatric Association (APA) to review the document; the APA endorsed the AHA advisory.
Two points are worth emphasizing:
- An AHA science advisory is not a treatment guideline.
- Advisories usually are brief and therefore do not exhaustively discuss their topic.
After discussing epidemiologic studies that elucidated the relationships between depression and CHD, the AHA advisory on depression and CHD focuses on screening, referral, and treatment of depression from a cardiology perspective.
The 1-year prevalence of major depressive disorder in the US general population is 7%, and the lifetime prevalence is about 16%.2 Depression in otherwise healthy persons almost doubles the risk of developing CHD.3 About 20% of patients hospitalized for acute coronary syndromes (ACS) have major depressive disorder on admission or within a few weeks thereafter, and these patients have about 2.5 times the mortality rate as patients who are not depressed, after adjusting for infarct severity and cardiovascular risk factors.4–6
ASSESSMENT OF DEPRESSION AND DEPRESSIVE SYMPTOMS
The AHA advisory discusses use of the 2-question Patient Health Questionnaire (PHQ-2) as the first step in screening for depression.7,8 The PHQ-2 inquires about the frequency of depressed mood and anhedonia by asking the following:
Over the past 2 weeks, how often have you been bothered by either of the following problems?
(1) Little interest or pleasure in doing things
(2) Feeling down, depressed, or hopeless.
For each item the response options are “not at all” (scored as 0), “several days” (scored as 1), “more than half the days” (scored as 2), and “nearly every day” (scored as 3). Thus, the total PHQ-2 score can range from 0 to 6.
Using a structured psychiatric interview as the standard, a total PHQ-2 score of 3 or greater has been shown to have a sensitivity of 83% and a specificity of 92% for major depression.7 A PHQ-2 score of 3 is the optimal cut point for screening purposes. A PHQ-2 score of 0 virtually excludes depression.
If a patient’s PHQ-2 score is 3 or greater, it is recommended that answers be obtained for a full 9-item PHQ. The PHQ-9 provides the sensitivity and specificity suitable for assigning a provisional diagnosis of major depressive disorder and a symptom severity score that can be used to identify patients for further evaluation and to make decisions about therapy.9–11
The US Preventive Services Task Force (USPSTF) currently recommends that clinicians screen adults for depression in clinical practices that have systems in place to assure accurate diagnosis, effective treatment, and follow-up.12 The USPSTF concluded that there is good evidence that screening improves the accurate identification of depressed patients in primary care settings and that treating depressed adults identified in such settings reduces clinical morbidity. Results of trials that have directly evaluated the effect of screening on clinical outcomes depend on follow-up. Limited benefits have been demonstrated in studies that simply feed screening results back to clinicians. Larger benefits have been seen in studies in which the communication of screening results is coordinated with effective follow-up and treatment. The USPSTF concluded that the benefits of screening and treating are likely to outweigh any potential harms.12
DEPRESSION TREATMENT
Drug therapy
The safety of fluoxetine, sertraline (SADHART, ENRICHD), citalopram (CREATE), and mirtazapine (MIND-IT) has been evaluated in clinical trials.4,17–21 By far the strongest evidence of safety is for selective serotonin reuptake inhibitors (SSRIs), especially sertraline.
The Sertraline Antidepressant Heart Attack Randomized Trial (SADHART; N = 369), a randomized study of depression after ACS, found no difference in cardiovascular adverse events between the sertraline and placebo groups after 16 weeks of therapy, and there were no significant differences in left ventricular ejection fraction, heart rate, blood pressure, ventricular premature complexes, or electrocardiogram changes.17 In the group assigned to sertraline, life-threatening cardiovascular events occurred less frequently (15% vs 22%, P = NS).
The Enhancing Recovery in Coronary Heart Disease study (ENRICHD) was a large trial (N = 2,481) to evaluate the effect of cognitive behavioral therapy (CBT) on depression or low perceived social support in patients enrolled within 28 days of myocardial infarction (MI).4 The ENRICHD protocol required patients randomized to CBT with Hamilton Depression Rating Scale (HAM-D) scores greater than 24, or who showed less than 50% reduction in Beck Depression Inventory (BDI) scores after 5 weeks of CBT, to be referred to a study psychiatrist for consideration of pharmacotherapy, usually sertraline. Of the overall ENRICHD population, 1,834 participants (74%) had a diagnosis of depression, and 446 of these participants (24%) were treated with antidepressant drugs, 301 with an SSRI and 145 with other antidepressants.18 During mean follow-up of 29 months, the SSRI-treated group had a statistically significant 43% reduction in death or MI.4
The Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) recruited 284 patients with chronic CHD, major depressive disorder, and a 24-item HAM-D score of 20 or greater and randomly assigned half to citalopram (another SSRI) and half to placebo.19 Citalopram showed antidepressant efficacy and no evidence of harm.
The Myocardial Infarction and Depression Intervention Trial (MIND-IT) recruited 91 patients within 30 days of hospital admission for MI with depression and randomized them in a 1:1 ratio to mirtazapine or placebo. Mirtazapine showed some evidence of antidepressant efficacy and no evidence of harm.20
Strik et al in the Netherlands recruited 54 patients who had major depression after a first MI and randomized them 1:1 to the SSRI fluoxetine or placebo 3 months after the MI.21 The fluoxetine group showed a trend toward antidepressant efficacy, and no cardiovascular safety problems were detected clinically or by either electrocardiogram or echocardiogram.
Psychotherapy
APA practice guidelines for major depressive disorder indicate that among psycho therapeutic approaches, CBT and interpersonal psychotherapy have the best-documented efficacy for treatment of major depressive disorder.22 CBT aims to solve problems related to dysfunctional emotions, behaviors, and cognition and is an umbrella term for various techniques that share a theoretical basis in behavioristic learning theory and cognitive psychology. Aaron T. Beck proposed that depressed people are quick to make negative evaluations of themselves and the world, and he designed treatment to reduce these negative cognitions.23 Interpersonal psychotherapy stems from the work of Harry Stack Sullivan, who believed that emotional reactions were triggered by interpersonal behaviors.24 Gerald Klerman and Myrna Weissman used this method to treat adults diagnosed with moderate or severe nondelusional clinical depression.25
CBT was used in ENRICHD,4 interpersonal psychotherapy in CREATE,19 and problem-solving therapy in the Coronary Psychosocial Evaluation Studies (COPES).26,27 Unintended therapy can also be a confounder in antidepressant drug trials; education and supportive care (eg, frequent visits or telephone calls with monitoring of depressive symptoms and counseling) are often provided for both the intervention and control (placebo) groups. If a study is blinded, education, supportive care, and attention will be identical for both groups and thus may reduce the likelihood of finding a difference between the drug and placebo, if one exists.
Psychotherapy can be helpful for depression, is preferred over antidepressant drugs by some patients, and can be combined with drugs to increase antidepressant efficacy. We still have much to learn about timing and choice of therapy, as well as about sequencing and combination of antidepressant drugs and psychotherapy.
Physical activity and exercise
Aerobic exercise28 and cardiac rehabilitation29 can reduce depressive symptoms in addition to improving cardiovascular fitness. Depression can serve as a barrier to participation in cardiac rehabilitation and exercise programs, but cardiologists can help depressed patients overcome this barrier by offering encouragement and follow-up contacts. Cardiologists also should enlist the help of spouses or other family members and friends to promote adherence. The prescription of exercise needs to be based on the cardiac status and exercise capacity of each individual.30
SUMMARY OF ADVISORY RECOMMENDATIONS
The AHA advisory1 summarized its recommendations as follows:
1. Routine screening for depression in patients with CHD should be considered in a variety of settings, including the hospital, the physician’s office, clinics, and cardiac rehabilitation centers. The opportunity to screen for and treat depression in cardiac patients should not be missed, as effective depression treatment may improve health outcomes.
2. Patients with positive screening results should be evaluated by a professional qualified in diagnosis and management of depression. Such a clinician can determine whether depression is present and needs treatment, as well as how to connect a patient to an effective care program in the local area.
3. Patients with heart disease who are being treated for depression should undergo careful monitoring for adherence to their medical care and for the efficacy and safety of drug therapy for their medical and mental health conditions.
4. Coordination of care among health care providers is essential for patients with coexisting medical and mental health issues.
COMMENTS ON THE ADVISORY
Since AHA advisories usually address evolving scientific issues, the knowledge base on these issues is constantly growing and a range of opinions and hypotheses are tenable, pending new information. Soon after the AHA science advisory on depression and CHD was issued, a systematic review on depression screening and patient outcomes in cardiac care was published.31 This review posed three key questions that are explored below.
Three key questions
Key question 1: What’s the accuracy of screening instruments for depression in cardiovascular care populations? To answer this question, a case-finding method such as a questionnaire (eg, BDI or PHQ) must be compared with a structured interview by mental health personnel as the “gold standard” for diagnosis (ie, the truth).
To estimate the accuracy of clinical diagnosis by general practitioners, Mitchell et al pooled data from 50,371 primary care patients across 41 studies in Europe or the United States to evaluate general practitioners’ ability to make an unassisted diagnosis of depression (ie, without specific help from severity scales, diagnostic instruments, education programs, etc).32 They reported a sensitivity of about 50% and a specificity of 81% when the prevalence of depression was about 20%. In other words, general practitioners missed about half the cases of depression when no case-finding tool was used. These researchers pointed out that a low prevalence of depression favors identification of nondepressed cases (false-positive diagnoses), whereas a high prevalence favors diagnosis of depression (true-positive diagnoses).32
Cardiologists focused on treatment of ACS are probably less likely to make a clinical diagnosis of depression and may attribute emotional symptoms to rapidly evolving cardiovascular events. The simple 2-question PHQ-2 case-finding instrument takes only a few minutes to administer and is recommended for use by primary care physicians or cardiologists to evaluate patients at high risk for depression or who manifest symptoms suggestive of depression.7
In the United States, most patients with depression are cared for in primary care venues. The AHA advisory recommends referring ACS patients who screen positive on a PHQ to a professional qualified in the diagnosis and management of depression.1 Enhanced care—using outreach, monitoring, adjustment of therapy, and psychiatric backup—produces significant improvement in depression.33
Key question 2: Is treatment of depression in cardiovascular care patients effective in improving depression? Cardiac outcomes? The evidence for benefit of SSRI therapy for depression detected at the time of ACS is consistent and was discussed above.4,17–21 However, the antidepressant effect of SSRIs is modest in placebo-controlled trials. Most such trials excluded patients who were taking antidepressant drugs when screened, and many patients recruited for antidepressant clinical trials had no previous episodes of depression, had relatively mild symptoms of short duration, and were recruited by screening (ie, they were not seeking treatment for depression).34 Patients with brief and short episodes are likely to remit spontaneously and to respond to psychotherapy or supportive care.34 Moreover, in most antidepressant trials, both the intervention and “control” groups received elements of enhanced depression care, which will reduce the apparent benefit of antidepressant drugs. For instance, because the primary goal of SADHART was to evaluate the safety of sertraline in patients with ACS, monitoring for adverse effects included six or seven visits during 16 weeks of follow-up plus six or seven phone calls, providing several elements of enhanced depression care, including face-to-face education, frequent follow-up, support by a case manager (research coordinator) with a mental health background, and support from a psychiatrist or psychologist.17 Randomization to sertraline or placebo in SADHART was blinded, so the frequent contact and support was the same in both groups.
Whether SSRI treatment for depression will improve survival and cardiovascular outcomes is not established by adequately powered randomized trials. Most randomized (SADHART, CREATE) or nonrandomized (ENRICHD) studies suggest that SSRIs reduce cardiovascular events, but only ENRICHD produced a statistically significant result. SSRIs—certainly sertraline and citalopram—are not associated with significant cardiovascular adverse effects, even during ACS, when the cardiovascular system is unstable and multiple drugs are being started and titrated. Patients who do not improve significantly during antidepressant drug therapy or psychotherapy have a two-to threefold increase in cardiovascular events compared with patients who do improve substantially.35
Key question 3: Is systematic screening for depression more effective than usual care for identifying patients with depression? Facilitating treatment of depression? Reducing depressive symptoms? Improving cardiac outcomes? Pignone et al conducted a literature review and meta-analysis on behalf of the USPSTF to clarify whether screening adults for depression in primary care venues improves recognition, treatment, and clinical outcomes.36 They reviewed randomized trials conducted in primary care settings that evaluated the effect of screening for depression on identification, treatment, or health outcomes, including trials that examined integrated, systematic support for treatment after identification of depression. The meta-analysis suggested that screening and feedback of screening results reduced the risk of persistent depression. Stronger effects were observed with programs that integrated interventions to improve recognition and treatment of depressed patients and that incorporated quality improvements into clinic systems as compared with programs that provided only screening and feedback.
Screening patients with CHD, especially those with ACS, is more effective than usual care (no screening). Indeed, many depressed patients go undetected unless identified by screening. Not surprisingly, those identified by screening tend to have milder depression. For example, half the participants in SADHART had a HAM-D score less than 18 (≥ 25 is considered severe). However, a considerable number identified by screening had above-average HAM-D scores but either viewed their symptoms as appropriate for their medical condition or were simply denying their psychiatric illness. If patients had not been screened, their depression would not have been detected or treated. Even among SADHART participants with baseline HAM-D scores less than 18, those whose depression failed to respond to sertraline/placebo had twice the 7-year mortality rate as did those whose depression remitted.16
The Prevention of Suicide in Primary Care Elderly: Collaborative Trial (PROSPECT) examined the impact of a care management intervention on suicidal ideation and depression in older primary care patients and reported outcomes over a 2-year follow-up.37 PROSPECT screened 9,072 patients in 20 primary care practices to find 599 study participants aged 60 years or older with major or minor depression (not all had cardiovascular disease). Participants were randomly assigned to either usual care or the PROSPECT intervention, which consisted of services rendered by trained care managers who offered algorithm-based recommendations to physicians and helped patients adhere to treatment during the 24-month trial. Compared with patients receiving usual care, those receiving the intervention had a greater likelihood of receiving antidepressant drugs and/or psychotherapy (85%–89% vs 49%–62%) and had a 2.2-fold greater decline in suicidal ideation over 24 months. Treatment response started sooner in the intervention group and continued to improve at 18 and 24 months; no appreciable increase in treatment response was observed in the usual-care group during the same period. Among patients with major depression, a significantly greater percentage of the intervention group achieved remission compared with the usual-care group at 4, 8, and 24 months. Patients with minor depression had favorable mental health outcomes regardless of treatment assignment. Sustained collaborative care maintained high utilization of depression treatment, reduced suicidal ideation, and improved the outcomes of major depression during 2 years of follow-up.37
No randomized trial of SSRIs has been designed to show an effect on mortality or cardiovascular events. ENRICHD has produced the strongest evidence that an SSRI can reduce mortality or cardiovascular outcomes. The study was designed with approximately 85% power to detect a 25% to 30% reduction in the primary end point (death or MI) as a result of CBT; as noted above, 2,481 patients (1,834 with depression) were randomized to usual care or CBT within 28 days of MI.4 Depression significantly improved with CBT, but the rate of death or MI did not: during mean follow-up of 29 months, death or MI occurred in 299 patients in the CBT group versus 300 in the usual-care group.4 A post hoc analysis specific to the 1,834 ENRICHD participants who had depression aimed to determine the effects of antidepressant drugs on morbidity and mortality.18 The protocol required patients in the intervention group with scores of 25 or higher on the 17-item HAM-D, or those who had less than a 50% reduction in BDI scores after 5 weeks of treatment, to be referred to study psychiatrists for consideration of pharmacotherapy. Study psychiatrists met with patients who were being treated with antidepressant drugs to monitor medication use. Unless contraindicated or previously ineffective or poorly tolerated, sertraline was the first antidepressant used. Using a time-dependent multivariable Cox proportional hazards regression model to adjust for baseline depression score and cardiac risk factors, the researchers found SSRI use to be associated with a statistically significant 43% lower risk of death or nonfatal MI. Like other SSRI studies, ENRICHD found SSRIs safe for post-MI patients and to possibly, but not certainly, reduce death and MI.18
Medication adherence
One obvious but important way antidepressant drug therapy could prevent death or MI is by improving adherence to post-MI cardiovascular drugs. Four or five classes of cardiac drugs have each been proven to improve survival following ACS (aspirin, beta-blockers, statins, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers), and when all are taken regularly, mortality is reduced by about half.38,39 In addition, antihypertensive and antidiabetic drugs are often needed to control blood pressure or blood glucose. However, patients have to actually take these drugs to receive their benefit. Depression in the setting of CHD, especially ACS, is a risk indicator for lack of adherence to medical therapy, mental health therapy, or both.
DiMatteo et al conducted a meta-analysis to test the hypothesis that anxiety and depression might explain poor adherence to treatment recommendations and result in poor medical outcomes.15 Of the 25 trials that met the inclusion criteria, 13 studied anxiety and 12 studied depression. The associations between anxiety and nonadherence were small and not statistically significant, but depression was strongly associated with nonadherence to medications (odds ratio = 3.03; 95% confidence interval, 1.96–4.89). In other words, depressed patients were three times as likely as nondepressed patients to be nonadherent to treatment recommendations. The authors speculated that depression might increase nonadherence in the following ways: (1) the hopelessness of depression might reduce patients’ hope in the therapy, (2) depression may cause withdrawal from family and social networks that otherwise would provide support and assistance, or (3) the impaired cognitive dysfunction associated with depression may impair memory and follow through on treatment recommendations.15
The findings from this meta-analysis could reflect depression causing medication nonadherence or vice versa. To establish the sequence, Rieckmann et al measured adherence to aspirin therapy during a 3-month period in a consecutive cohort of 172 patients (25 to 85 years old) recruited within 1 week of hospitalization for ACS.40 Severity of depressive symptoms was quantified using the BDI during hospitalization and at 1 and 3 months after discharge. Adherence was defined as taking aspirin as prescribed on at least 80% of days. Using an electronic monitoring system that recorded the date and time when the aspirin bottle cap was opened, the study found that more than 30% of patients with post-ACS depression were nonadherent to their aspirin therapy compared with only 15% of nondepressed patients.40 The more severe patients’ depressive symptoms were, the greater the nonadherence to aspirin therapy. Moreover, a lagged correlation statistical model determined that improvement in depression preceded improvement in medication adherence.
SADHART was conducted under the new drug application for sertraline,16 which required that the use of trial drugs be under strict compliance to protocol, sponsor monitoring, and auditing by the US Food and Drug Administration. Drug use data were complete in 98.1% of participants. Adherence was measured using tablet counts. Depressed patients who had a large improvement in depression during blinded drug therapy (sertraline or placebo) showed improved adherence to the blinded therapy. To determine whether depression improved before medication adherence improved, researchers compared responders’ medication adherence before and after their improvement in depression. Medication adherence increased following remission of depression in 128 of 187 participants (68.4%) who remitted on trial medication (remission was defined as a Clinical Global Impression–Improvement score of 1). This sequence of change (improved depression before improved medication adherence) occurred significantly more often than would be expected by chance (P < .001). This finding suggests that improvement in depression is driving improved medication adherence.
Because persistent depression is associated with increased mortality rates and reduced medication adherence, physicians need to not only aggressively treat depression but also diligently promote adherence to guideline-defined cardiovascular drug therapy. If depression doesn’t improve, additional measures should be initiated not only to improve depression but also to achieve adherence to cardiovascular drug therapy (eg, assistance from spouse, child, or visiting nurse; calls by case manager; electronic health record monitoring of drug prescription refills). When depression is found during clinical encounters or by screening, nonadherence to drug treatment is much more likely and should be sought vigilantly.
Adherence to lifestyle recommendations
Ziegelstein et al have shown that depressed patients have poorer adherence to lifestyle recommendations (diet, exercise, smoking cessation).41 The Heart and Soul Study, a prospective cohort study of 1,017 outpatients with stable CHD, attempted to determine why depressive symptoms (as determined by PHQ-9 self-report) are associated with an increased risk of cardiovascular events.42 Participants were predominantly older men, about half of whom were recruited from Veterans Administration hospitals. A total of 341 cardiovascular events occurred during a mean follow-up of 4.8 years. Participants with baseline depressive symptoms had a 50% greater rate of cardiovascular events during the study period compared with participants without depressive symptoms. However, no significant association between depressive symptoms and cardiovascular events remained after adjustment for health behaviors—most strikingly, physical activity.42 This finding was consistent with an earlier study that found that exercise therapy plus antidepressant medication could reduce the risk of cardiovascular events in patients with depression.43 The ongoing Understanding Prognostic Benefits of Exercise and Antidepressant Therapy (UPBEAT) study is comparing the effects of exercise and antidepressant medication on depression and biomarkers of cardiovascular risk in patients with depressive symptoms and CHD.44 The study’s longer-term goal is to identify an intervention that improves both depression and cardiovascular disease outcomes.
CONCLUSIONS
The USPSTF recommends screening for depression in adults. The PHQ-2 is an efficient first-step screening tool that can identify many depressed patients who would otherwise go undetected. It is clear that SSRIs are safe in cardiac patients, can reduce depression, and can improve medication adherence, but it is not enough to screen and report depression. Optimal benefit depends on having (1) a primary care provider who is familiar with managing depression, (2) a case manager with a mental health background to follow and support patients, and (3) regular supervision of the case manager by a psychiatrist or psychologist. Cardiologists see large numbers of patients with chronic CHD, ACS, or recent coronary artery bypass graft surgery who are at high risk for depression. The AHA advisory recommends a care model that is practical for CHD patients with depression. Such a care model must be based on detection of depression and referral to a practice that has resources and knowledge to manage it well.
In 2008, the American Heart Association (AHA) published a science advisory on depression and coronary heart disease (CHD).1 Since its publication, the advisory has evoked substantial commentary. The purpose of this article is threefold: (1) to explain the aims of the AHA science advisory, (2) to briefly discuss its content, and (3) to examine some of the comments it has provoked.
WHAT THE ADVISORY SET OUT TO DO
The purpose of an AHA science advisory is to provide rapid, clear, and consistent AHA positioning on a scientific issue. Advisories are statements on an evolving, prominent scientific issue of great interest to the public and health professionals. All AHA science advisories undergo peer review and are also reviewed and approved by the AHA Science Advisory and Coordinating Committee, AHA’s highest science body. Because this particular advisory addressed the interaction of cardiovascular and mental health, the AHA asked the American Psychiatric Association (APA) to review the document; the APA endorsed the AHA advisory.
Two points are worth emphasizing:
- An AHA science advisory is not a treatment guideline.
- Advisories usually are brief and therefore do not exhaustively discuss their topic.
After discussing epidemiologic studies that elucidated the relationships between depression and CHD, the AHA advisory on depression and CHD focuses on screening, referral, and treatment of depression from a cardiology perspective.
The 1-year prevalence of major depressive disorder in the US general population is 7%, and the lifetime prevalence is about 16%.2 Depression in otherwise healthy persons almost doubles the risk of developing CHD.3 About 20% of patients hospitalized for acute coronary syndromes (ACS) have major depressive disorder on admission or within a few weeks thereafter, and these patients have about 2.5 times the mortality rate as patients who are not depressed, after adjusting for infarct severity and cardiovascular risk factors.4–6
ASSESSMENT OF DEPRESSION AND DEPRESSIVE SYMPTOMS
The AHA advisory discusses use of the 2-question Patient Health Questionnaire (PHQ-2) as the first step in screening for depression.7,8 The PHQ-2 inquires about the frequency of depressed mood and anhedonia by asking the following:
Over the past 2 weeks, how often have you been bothered by either of the following problems?
(1) Little interest or pleasure in doing things
(2) Feeling down, depressed, or hopeless.
For each item the response options are “not at all” (scored as 0), “several days” (scored as 1), “more than half the days” (scored as 2), and “nearly every day” (scored as 3). Thus, the total PHQ-2 score can range from 0 to 6.
Using a structured psychiatric interview as the standard, a total PHQ-2 score of 3 or greater has been shown to have a sensitivity of 83% and a specificity of 92% for major depression.7 A PHQ-2 score of 3 is the optimal cut point for screening purposes. A PHQ-2 score of 0 virtually excludes depression.
If a patient’s PHQ-2 score is 3 or greater, it is recommended that answers be obtained for a full 9-item PHQ. The PHQ-9 provides the sensitivity and specificity suitable for assigning a provisional diagnosis of major depressive disorder and a symptom severity score that can be used to identify patients for further evaluation and to make decisions about therapy.9–11
The US Preventive Services Task Force (USPSTF) currently recommends that clinicians screen adults for depression in clinical practices that have systems in place to assure accurate diagnosis, effective treatment, and follow-up.12 The USPSTF concluded that there is good evidence that screening improves the accurate identification of depressed patients in primary care settings and that treating depressed adults identified in such settings reduces clinical morbidity. Results of trials that have directly evaluated the effect of screening on clinical outcomes depend on follow-up. Limited benefits have been demonstrated in studies that simply feed screening results back to clinicians. Larger benefits have been seen in studies in which the communication of screening results is coordinated with effective follow-up and treatment. The USPSTF concluded that the benefits of screening and treating are likely to outweigh any potential harms.12
DEPRESSION TREATMENT
Drug therapy
The safety of fluoxetine, sertraline (SADHART, ENRICHD), citalopram (CREATE), and mirtazapine (MIND-IT) has been evaluated in clinical trials.4,17–21 By far the strongest evidence of safety is for selective serotonin reuptake inhibitors (SSRIs), especially sertraline.
The Sertraline Antidepressant Heart Attack Randomized Trial (SADHART; N = 369), a randomized study of depression after ACS, found no difference in cardiovascular adverse events between the sertraline and placebo groups after 16 weeks of therapy, and there were no significant differences in left ventricular ejection fraction, heart rate, blood pressure, ventricular premature complexes, or electrocardiogram changes.17 In the group assigned to sertraline, life-threatening cardiovascular events occurred less frequently (15% vs 22%, P = NS).
The Enhancing Recovery in Coronary Heart Disease study (ENRICHD) was a large trial (N = 2,481) to evaluate the effect of cognitive behavioral therapy (CBT) on depression or low perceived social support in patients enrolled within 28 days of myocardial infarction (MI).4 The ENRICHD protocol required patients randomized to CBT with Hamilton Depression Rating Scale (HAM-D) scores greater than 24, or who showed less than 50% reduction in Beck Depression Inventory (BDI) scores after 5 weeks of CBT, to be referred to a study psychiatrist for consideration of pharmacotherapy, usually sertraline. Of the overall ENRICHD population, 1,834 participants (74%) had a diagnosis of depression, and 446 of these participants (24%) were treated with antidepressant drugs, 301 with an SSRI and 145 with other antidepressants.18 During mean follow-up of 29 months, the SSRI-treated group had a statistically significant 43% reduction in death or MI.4
The Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) recruited 284 patients with chronic CHD, major depressive disorder, and a 24-item HAM-D score of 20 or greater and randomly assigned half to citalopram (another SSRI) and half to placebo.19 Citalopram showed antidepressant efficacy and no evidence of harm.
The Myocardial Infarction and Depression Intervention Trial (MIND-IT) recruited 91 patients within 30 days of hospital admission for MI with depression and randomized them in a 1:1 ratio to mirtazapine or placebo. Mirtazapine showed some evidence of antidepressant efficacy and no evidence of harm.20
Strik et al in the Netherlands recruited 54 patients who had major depression after a first MI and randomized them 1:1 to the SSRI fluoxetine or placebo 3 months after the MI.21 The fluoxetine group showed a trend toward antidepressant efficacy, and no cardiovascular safety problems were detected clinically or by either electrocardiogram or echocardiogram.
Psychotherapy
APA practice guidelines for major depressive disorder indicate that among psycho therapeutic approaches, CBT and interpersonal psychotherapy have the best-documented efficacy for treatment of major depressive disorder.22 CBT aims to solve problems related to dysfunctional emotions, behaviors, and cognition and is an umbrella term for various techniques that share a theoretical basis in behavioristic learning theory and cognitive psychology. Aaron T. Beck proposed that depressed people are quick to make negative evaluations of themselves and the world, and he designed treatment to reduce these negative cognitions.23 Interpersonal psychotherapy stems from the work of Harry Stack Sullivan, who believed that emotional reactions were triggered by interpersonal behaviors.24 Gerald Klerman and Myrna Weissman used this method to treat adults diagnosed with moderate or severe nondelusional clinical depression.25
CBT was used in ENRICHD,4 interpersonal psychotherapy in CREATE,19 and problem-solving therapy in the Coronary Psychosocial Evaluation Studies (COPES).26,27 Unintended therapy can also be a confounder in antidepressant drug trials; education and supportive care (eg, frequent visits or telephone calls with monitoring of depressive symptoms and counseling) are often provided for both the intervention and control (placebo) groups. If a study is blinded, education, supportive care, and attention will be identical for both groups and thus may reduce the likelihood of finding a difference between the drug and placebo, if one exists.
Psychotherapy can be helpful for depression, is preferred over antidepressant drugs by some patients, and can be combined with drugs to increase antidepressant efficacy. We still have much to learn about timing and choice of therapy, as well as about sequencing and combination of antidepressant drugs and psychotherapy.
Physical activity and exercise
Aerobic exercise28 and cardiac rehabilitation29 can reduce depressive symptoms in addition to improving cardiovascular fitness. Depression can serve as a barrier to participation in cardiac rehabilitation and exercise programs, but cardiologists can help depressed patients overcome this barrier by offering encouragement and follow-up contacts. Cardiologists also should enlist the help of spouses or other family members and friends to promote adherence. The prescription of exercise needs to be based on the cardiac status and exercise capacity of each individual.30
SUMMARY OF ADVISORY RECOMMENDATIONS
The AHA advisory1 summarized its recommendations as follows:
1. Routine screening for depression in patients with CHD should be considered in a variety of settings, including the hospital, the physician’s office, clinics, and cardiac rehabilitation centers. The opportunity to screen for and treat depression in cardiac patients should not be missed, as effective depression treatment may improve health outcomes.
2. Patients with positive screening results should be evaluated by a professional qualified in diagnosis and management of depression. Such a clinician can determine whether depression is present and needs treatment, as well as how to connect a patient to an effective care program in the local area.
3. Patients with heart disease who are being treated for depression should undergo careful monitoring for adherence to their medical care and for the efficacy and safety of drug therapy for their medical and mental health conditions.
4. Coordination of care among health care providers is essential for patients with coexisting medical and mental health issues.
COMMENTS ON THE ADVISORY
Since AHA advisories usually address evolving scientific issues, the knowledge base on these issues is constantly growing and a range of opinions and hypotheses are tenable, pending new information. Soon after the AHA science advisory on depression and CHD was issued, a systematic review on depression screening and patient outcomes in cardiac care was published.31 This review posed three key questions that are explored below.
Three key questions
Key question 1: What’s the accuracy of screening instruments for depression in cardiovascular care populations? To answer this question, a case-finding method such as a questionnaire (eg, BDI or PHQ) must be compared with a structured interview by mental health personnel as the “gold standard” for diagnosis (ie, the truth).
To estimate the accuracy of clinical diagnosis by general practitioners, Mitchell et al pooled data from 50,371 primary care patients across 41 studies in Europe or the United States to evaluate general practitioners’ ability to make an unassisted diagnosis of depression (ie, without specific help from severity scales, diagnostic instruments, education programs, etc).32 They reported a sensitivity of about 50% and a specificity of 81% when the prevalence of depression was about 20%. In other words, general practitioners missed about half the cases of depression when no case-finding tool was used. These researchers pointed out that a low prevalence of depression favors identification of nondepressed cases (false-positive diagnoses), whereas a high prevalence favors diagnosis of depression (true-positive diagnoses).32
Cardiologists focused on treatment of ACS are probably less likely to make a clinical diagnosis of depression and may attribute emotional symptoms to rapidly evolving cardiovascular events. The simple 2-question PHQ-2 case-finding instrument takes only a few minutes to administer and is recommended for use by primary care physicians or cardiologists to evaluate patients at high risk for depression or who manifest symptoms suggestive of depression.7
In the United States, most patients with depression are cared for in primary care venues. The AHA advisory recommends referring ACS patients who screen positive on a PHQ to a professional qualified in the diagnosis and management of depression.1 Enhanced care—using outreach, monitoring, adjustment of therapy, and psychiatric backup—produces significant improvement in depression.33
Key question 2: Is treatment of depression in cardiovascular care patients effective in improving depression? Cardiac outcomes? The evidence for benefit of SSRI therapy for depression detected at the time of ACS is consistent and was discussed above.4,17–21 However, the antidepressant effect of SSRIs is modest in placebo-controlled trials. Most such trials excluded patients who were taking antidepressant drugs when screened, and many patients recruited for antidepressant clinical trials had no previous episodes of depression, had relatively mild symptoms of short duration, and were recruited by screening (ie, they were not seeking treatment for depression).34 Patients with brief and short episodes are likely to remit spontaneously and to respond to psychotherapy or supportive care.34 Moreover, in most antidepressant trials, both the intervention and “control” groups received elements of enhanced depression care, which will reduce the apparent benefit of antidepressant drugs. For instance, because the primary goal of SADHART was to evaluate the safety of sertraline in patients with ACS, monitoring for adverse effects included six or seven visits during 16 weeks of follow-up plus six or seven phone calls, providing several elements of enhanced depression care, including face-to-face education, frequent follow-up, support by a case manager (research coordinator) with a mental health background, and support from a psychiatrist or psychologist.17 Randomization to sertraline or placebo in SADHART was blinded, so the frequent contact and support was the same in both groups.
Whether SSRI treatment for depression will improve survival and cardiovascular outcomes is not established by adequately powered randomized trials. Most randomized (SADHART, CREATE) or nonrandomized (ENRICHD) studies suggest that SSRIs reduce cardiovascular events, but only ENRICHD produced a statistically significant result. SSRIs—certainly sertraline and citalopram—are not associated with significant cardiovascular adverse effects, even during ACS, when the cardiovascular system is unstable and multiple drugs are being started and titrated. Patients who do not improve significantly during antidepressant drug therapy or psychotherapy have a two-to threefold increase in cardiovascular events compared with patients who do improve substantially.35
Key question 3: Is systematic screening for depression more effective than usual care for identifying patients with depression? Facilitating treatment of depression? Reducing depressive symptoms? Improving cardiac outcomes? Pignone et al conducted a literature review and meta-analysis on behalf of the USPSTF to clarify whether screening adults for depression in primary care venues improves recognition, treatment, and clinical outcomes.36 They reviewed randomized trials conducted in primary care settings that evaluated the effect of screening for depression on identification, treatment, or health outcomes, including trials that examined integrated, systematic support for treatment after identification of depression. The meta-analysis suggested that screening and feedback of screening results reduced the risk of persistent depression. Stronger effects were observed with programs that integrated interventions to improve recognition and treatment of depressed patients and that incorporated quality improvements into clinic systems as compared with programs that provided only screening and feedback.
Screening patients with CHD, especially those with ACS, is more effective than usual care (no screening). Indeed, many depressed patients go undetected unless identified by screening. Not surprisingly, those identified by screening tend to have milder depression. For example, half the participants in SADHART had a HAM-D score less than 18 (≥ 25 is considered severe). However, a considerable number identified by screening had above-average HAM-D scores but either viewed their symptoms as appropriate for their medical condition or were simply denying their psychiatric illness. If patients had not been screened, their depression would not have been detected or treated. Even among SADHART participants with baseline HAM-D scores less than 18, those whose depression failed to respond to sertraline/placebo had twice the 7-year mortality rate as did those whose depression remitted.16
The Prevention of Suicide in Primary Care Elderly: Collaborative Trial (PROSPECT) examined the impact of a care management intervention on suicidal ideation and depression in older primary care patients and reported outcomes over a 2-year follow-up.37 PROSPECT screened 9,072 patients in 20 primary care practices to find 599 study participants aged 60 years or older with major or minor depression (not all had cardiovascular disease). Participants were randomly assigned to either usual care or the PROSPECT intervention, which consisted of services rendered by trained care managers who offered algorithm-based recommendations to physicians and helped patients adhere to treatment during the 24-month trial. Compared with patients receiving usual care, those receiving the intervention had a greater likelihood of receiving antidepressant drugs and/or psychotherapy (85%–89% vs 49%–62%) and had a 2.2-fold greater decline in suicidal ideation over 24 months. Treatment response started sooner in the intervention group and continued to improve at 18 and 24 months; no appreciable increase in treatment response was observed in the usual-care group during the same period. Among patients with major depression, a significantly greater percentage of the intervention group achieved remission compared with the usual-care group at 4, 8, and 24 months. Patients with minor depression had favorable mental health outcomes regardless of treatment assignment. Sustained collaborative care maintained high utilization of depression treatment, reduced suicidal ideation, and improved the outcomes of major depression during 2 years of follow-up.37
No randomized trial of SSRIs has been designed to show an effect on mortality or cardiovascular events. ENRICHD has produced the strongest evidence that an SSRI can reduce mortality or cardiovascular outcomes. The study was designed with approximately 85% power to detect a 25% to 30% reduction in the primary end point (death or MI) as a result of CBT; as noted above, 2,481 patients (1,834 with depression) were randomized to usual care or CBT within 28 days of MI.4 Depression significantly improved with CBT, but the rate of death or MI did not: during mean follow-up of 29 months, death or MI occurred in 299 patients in the CBT group versus 300 in the usual-care group.4 A post hoc analysis specific to the 1,834 ENRICHD participants who had depression aimed to determine the effects of antidepressant drugs on morbidity and mortality.18 The protocol required patients in the intervention group with scores of 25 or higher on the 17-item HAM-D, or those who had less than a 50% reduction in BDI scores after 5 weeks of treatment, to be referred to study psychiatrists for consideration of pharmacotherapy. Study psychiatrists met with patients who were being treated with antidepressant drugs to monitor medication use. Unless contraindicated or previously ineffective or poorly tolerated, sertraline was the first antidepressant used. Using a time-dependent multivariable Cox proportional hazards regression model to adjust for baseline depression score and cardiac risk factors, the researchers found SSRI use to be associated with a statistically significant 43% lower risk of death or nonfatal MI. Like other SSRI studies, ENRICHD found SSRIs safe for post-MI patients and to possibly, but not certainly, reduce death and MI.18
Medication adherence
One obvious but important way antidepressant drug therapy could prevent death or MI is by improving adherence to post-MI cardiovascular drugs. Four or five classes of cardiac drugs have each been proven to improve survival following ACS (aspirin, beta-blockers, statins, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers), and when all are taken regularly, mortality is reduced by about half.38,39 In addition, antihypertensive and antidiabetic drugs are often needed to control blood pressure or blood glucose. However, patients have to actually take these drugs to receive their benefit. Depression in the setting of CHD, especially ACS, is a risk indicator for lack of adherence to medical therapy, mental health therapy, or both.
DiMatteo et al conducted a meta-analysis to test the hypothesis that anxiety and depression might explain poor adherence to treatment recommendations and result in poor medical outcomes.15 Of the 25 trials that met the inclusion criteria, 13 studied anxiety and 12 studied depression. The associations between anxiety and nonadherence were small and not statistically significant, but depression was strongly associated with nonadherence to medications (odds ratio = 3.03; 95% confidence interval, 1.96–4.89). In other words, depressed patients were three times as likely as nondepressed patients to be nonadherent to treatment recommendations. The authors speculated that depression might increase nonadherence in the following ways: (1) the hopelessness of depression might reduce patients’ hope in the therapy, (2) depression may cause withdrawal from family and social networks that otherwise would provide support and assistance, or (3) the impaired cognitive dysfunction associated with depression may impair memory and follow through on treatment recommendations.15
The findings from this meta-analysis could reflect depression causing medication nonadherence or vice versa. To establish the sequence, Rieckmann et al measured adherence to aspirin therapy during a 3-month period in a consecutive cohort of 172 patients (25 to 85 years old) recruited within 1 week of hospitalization for ACS.40 Severity of depressive symptoms was quantified using the BDI during hospitalization and at 1 and 3 months after discharge. Adherence was defined as taking aspirin as prescribed on at least 80% of days. Using an electronic monitoring system that recorded the date and time when the aspirin bottle cap was opened, the study found that more than 30% of patients with post-ACS depression were nonadherent to their aspirin therapy compared with only 15% of nondepressed patients.40 The more severe patients’ depressive symptoms were, the greater the nonadherence to aspirin therapy. Moreover, a lagged correlation statistical model determined that improvement in depression preceded improvement in medication adherence.
SADHART was conducted under the new drug application for sertraline,16 which required that the use of trial drugs be under strict compliance to protocol, sponsor monitoring, and auditing by the US Food and Drug Administration. Drug use data were complete in 98.1% of participants. Adherence was measured using tablet counts. Depressed patients who had a large improvement in depression during blinded drug therapy (sertraline or placebo) showed improved adherence to the blinded therapy. To determine whether depression improved before medication adherence improved, researchers compared responders’ medication adherence before and after their improvement in depression. Medication adherence increased following remission of depression in 128 of 187 participants (68.4%) who remitted on trial medication (remission was defined as a Clinical Global Impression–Improvement score of 1). This sequence of change (improved depression before improved medication adherence) occurred significantly more often than would be expected by chance (P < .001). This finding suggests that improvement in depression is driving improved medication adherence.
Because persistent depression is associated with increased mortality rates and reduced medication adherence, physicians need to not only aggressively treat depression but also diligently promote adherence to guideline-defined cardiovascular drug therapy. If depression doesn’t improve, additional measures should be initiated not only to improve depression but also to achieve adherence to cardiovascular drug therapy (eg, assistance from spouse, child, or visiting nurse; calls by case manager; electronic health record monitoring of drug prescription refills). When depression is found during clinical encounters or by screening, nonadherence to drug treatment is much more likely and should be sought vigilantly.
Adherence to lifestyle recommendations
Ziegelstein et al have shown that depressed patients have poorer adherence to lifestyle recommendations (diet, exercise, smoking cessation).41 The Heart and Soul Study, a prospective cohort study of 1,017 outpatients with stable CHD, attempted to determine why depressive symptoms (as determined by PHQ-9 self-report) are associated with an increased risk of cardiovascular events.42 Participants were predominantly older men, about half of whom were recruited from Veterans Administration hospitals. A total of 341 cardiovascular events occurred during a mean follow-up of 4.8 years. Participants with baseline depressive symptoms had a 50% greater rate of cardiovascular events during the study period compared with participants without depressive symptoms. However, no significant association between depressive symptoms and cardiovascular events remained after adjustment for health behaviors—most strikingly, physical activity.42 This finding was consistent with an earlier study that found that exercise therapy plus antidepressant medication could reduce the risk of cardiovascular events in patients with depression.43 The ongoing Understanding Prognostic Benefits of Exercise and Antidepressant Therapy (UPBEAT) study is comparing the effects of exercise and antidepressant medication on depression and biomarkers of cardiovascular risk in patients with depressive symptoms and CHD.44 The study’s longer-term goal is to identify an intervention that improves both depression and cardiovascular disease outcomes.
CONCLUSIONS
The USPSTF recommends screening for depression in adults. The PHQ-2 is an efficient first-step screening tool that can identify many depressed patients who would otherwise go undetected. It is clear that SSRIs are safe in cardiac patients, can reduce depression, and can improve medication adherence, but it is not enough to screen and report depression. Optimal benefit depends on having (1) a primary care provider who is familiar with managing depression, (2) a case manager with a mental health background to follow and support patients, and (3) regular supervision of the case manager by a psychiatrist or psychologist. Cardiologists see large numbers of patients with chronic CHD, ACS, or recent coronary artery bypass graft surgery who are at high risk for depression. The AHA advisory recommends a care model that is practical for CHD patients with depression. Such a care model must be based on detection of depression and referral to a practice that has resources and knowledge to manage it well.
- Lichtman JH, Bigger JT, Blumenthal JA, et al Depression and coronary heart disease: recommendations for screening, referral, and treatment. A science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research; endorsed by the American Psychiatric Association. Circulation 2008; 118:1768–1775.
- Kessler RC, Merikangas KR, Wang PS. Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annu Rev Clin Psychol 2007; 3:137–158.
- Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med 2003; 65:201–210.
- Berkman LF, Blumenthal J, Burg M, et al Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA 2003; 289:3106–3116.
- Thombs BD, Bass EB, Ford DE, et al Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med 2006; 21:30–38.
- van Melle JP, de Jonge P, Spijkerman TA, et al Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004; 66:814–822.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003; 41:1284–1292.
- Whooley M, Avins AL, Miranda J, Brown WS. Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med 1997; 12:289–290.
- Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med 2007; 22:1596–1602.
- Stafford L, Berk M, Jackson HJ. Validity of the Hospital Anxiety and Depression Scale and Patient Health Questionnaire-9 to screen for depression in patients with coronary artery disease. Gen Hosp Psychiatry 2007; 29:417–424.
- McManus D, Pipkin SS, Whooley MA. Screening for depression in patients with coronary heart disease (data from the Heart and Soul Study). Am J Cardiol 2005; 96:1076–1081.
- U.S. Preventive Services Task Force. Screening for depression: recommendations and rationale. Rockville, MD: Agency for Healthcare Research and Quality; May 2002. http://www.ahrq.gov/clinic/3rduspstf/depression/depressrr.htm. Accessed January 8, 2010.
- Depression Guideline Panel. Clinical Practice Guideline Number 5: Depression in Primary Care, 1: Detection and Diagnosis. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; April 1993. AHCPR publication 93-0550.
- Depression Guideline Panel. Clinical Practice Guideline Number 5: Depression in Primary Care, 2: Treatment of Major Depression. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; April 1993. AHCPR publication 93-0551.
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000; 160:2101–2107.
- Glassman AH, Bigger JT, Gaffney M. Psychiatric characteristics associated with long-term mortality among 361 patients having an acute coronary syndrome and major depression: seven-year follow-up of SADHART participants. Arch Gen Psychiatry 2009; 66:1022–1029.
- Glassman AH, O’Connor CM, Califf RM, et al Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709.
- Taylor CB, Youngblood ME, Catellier D, et al Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry 2005; 62:792–798.
- Lesperance F, Frasure-Smith N, Koszycki D, et al Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007; 297:367–379.
- Honig A, Kuyper A, Schene AH, et al Treatment of post-myocardial infarction depressive disorder: a randomized, placebo-controlled trial with mirtazapine. Psychosom Med 2007; 69:606–613.
- Strik J, Honig A, Lousberg R, et al Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: findings from a double-blind, placebo-controlled trial. Psychosom Med 2000; 62:783–789.
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000; 157( suppl 4):1–45.
- Beck AT. Cognitive Therapy and the Emotional Disorders. Madison, CT: International Universities Press Inc; 1975.
- Weissman MM. A brief history of interpersonal psychotherapy. Psychiatr Ann 2006; 36:553–557.
- Klerman GL, Weissmann MM. Interpersonal psychotherapy (IPT) and drugs in the treatment of depression. Pharmacopsychiatry 1987; 20:3–7.
- Burg MM, Lespérance F, Rieckmann N, Clemow L, Skotzko C, Davidson KW. Treating persistent depressive symptoms in post-ACS patients: the project COPES phase-I randomized controlled trial. Contemp Clin Trials 2008; 29:231–240.
- Davidson KW, Rieckmann N, Clemow L, et al Collaborative depression care for acute coronary syndrome patients with persistent depression: Coronary Psychosocial Evaluation Studies (COPES) randomized controlled trial. Arch Intern Med. In press.
- Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med 2002; 32:741–760.
- Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. Am J Med 2007; 120:799–806.
- Smith SC, Allen J, Blair SN, et al AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update; endorsed by the National Heart, Lung, and Blood Institute. Circulation 2006; 113:2363–2372.
- Thombs BD, de Jonge P, Coyne JC, et al Ziegelstein RC. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA 2008; 300:2161–2171.
- Mitchell AJ, Vaze A, Rao S. Clinical diagnosis of depression in primary care: a meta-analysis. Lancet 2009; 374:609–619.
- Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006; 166:2314–2321.
- Glassman AH, Bigger JT, Gaffney M, Shapiro PA, Swenson JR. Onset of major depression associated with acute coronary syndromes: relationship of onset, major depressive disorder history, and episode severity to sertraline benefit. Arch Gen Psychiatry 2006; 63:283–288.
- Carney RM, Freedland KE. Treatment-resistant depression and mortality after acute coronary syndrome. Am J Psychiatry 2009; 166:410–417.
- Pignone MP, Gaynes BN, Rushton JL, et al Screening for depression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 136:765–776.
- Alexopoulos GS, Reynolds CF, Bruce ML, et al Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry 2009; 166:882–890.
- Mukherjee D, Fang J, Chetcuti S, Moscucci M, Kline-Rogers E, Eagle KA. Impact of combination evidence-based medical therapy on mortality in patients with acute coronary syndromes. Circulation 2004; 109:745–749.
- Eagle KA, Kline-Rogers E, Goodman SG, et al Adherence to evidence-based therapies after discharge for acute coronary syndromes: an ongoing prospective, observational study. Am J Med 2004; 117:73–81.
- Rieckmann N, Gerin W, Kronish IA, et al Course of depressive symptoms and medication adherence after acute coronary syndromes: an electronic medication monitoring study. J Am Coll Cardiol 2006; 48:2218–2222.
- Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med 2000; 160:1818–1823.
- Whooley MA, de Jonge P, Vittinghoff E, et al Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA 2008; 300:2379–2388.
- Blumenthal JA, Babyak MA, Doraiswamy PM, et al Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 2007; 69:587–596.
- Blumenthal JA, Sherwood A, Rogers SD, et al Understanding prognostic benefits of exercise and antidepressant therapy for persons with depression and heart disease: the UPBEAT study—rationale, design, and methodological issues. Clin Trials 2007; 4:548–559.
- Lichtman JH, Bigger JT, Blumenthal JA, et al Depression and coronary heart disease: recommendations for screening, referral, and treatment. A science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research; endorsed by the American Psychiatric Association. Circulation 2008; 118:1768–1775.
- Kessler RC, Merikangas KR, Wang PS. Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annu Rev Clin Psychol 2007; 3:137–158.
- Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med 2003; 65:201–210.
- Berkman LF, Blumenthal J, Burg M, et al Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) randomized trial. JAMA 2003; 289:3106–3116.
- Thombs BD, Bass EB, Ford DE, et al Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med 2006; 21:30–38.
- van Melle JP, de Jonge P, Spijkerman TA, et al Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004; 66:814–822.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003; 41:1284–1292.
- Whooley M, Avins AL, Miranda J, Brown WS. Case-finding instruments for depression: two questions are as good as many. J Gen Intern Med 1997; 12:289–290.
- Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med 2007; 22:1596–1602.
- Stafford L, Berk M, Jackson HJ. Validity of the Hospital Anxiety and Depression Scale and Patient Health Questionnaire-9 to screen for depression in patients with coronary artery disease. Gen Hosp Psychiatry 2007; 29:417–424.
- McManus D, Pipkin SS, Whooley MA. Screening for depression in patients with coronary heart disease (data from the Heart and Soul Study). Am J Cardiol 2005; 96:1076–1081.
- U.S. Preventive Services Task Force. Screening for depression: recommendations and rationale. Rockville, MD: Agency for Healthcare Research and Quality; May 2002. http://www.ahrq.gov/clinic/3rduspstf/depression/depressrr.htm. Accessed January 8, 2010.
- Depression Guideline Panel. Clinical Practice Guideline Number 5: Depression in Primary Care, 1: Detection and Diagnosis. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; April 1993. AHCPR publication 93-0550.
- Depression Guideline Panel. Clinical Practice Guideline Number 5: Depression in Primary Care, 2: Treatment of Major Depression. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research; April 1993. AHCPR publication 93-0551.
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000; 160:2101–2107.
- Glassman AH, Bigger JT, Gaffney M. Psychiatric characteristics associated with long-term mortality among 361 patients having an acute coronary syndrome and major depression: seven-year follow-up of SADHART participants. Arch Gen Psychiatry 2009; 66:1022–1029.
- Glassman AH, O’Connor CM, Califf RM, et al Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709.
- Taylor CB, Youngblood ME, Catellier D, et al Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry 2005; 62:792–798.
- Lesperance F, Frasure-Smith N, Koszycki D, et al Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007; 297:367–379.
- Honig A, Kuyper A, Schene AH, et al Treatment of post-myocardial infarction depressive disorder: a randomized, placebo-controlled trial with mirtazapine. Psychosom Med 2007; 69:606–613.
- Strik J, Honig A, Lousberg R, et al Efficacy and safety of fluoxetine in the treatment of patients with major depression after first myocardial infarction: findings from a double-blind, placebo-controlled trial. Psychosom Med 2000; 62:783–789.
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000; 157( suppl 4):1–45.
- Beck AT. Cognitive Therapy and the Emotional Disorders. Madison, CT: International Universities Press Inc; 1975.
- Weissman MM. A brief history of interpersonal psychotherapy. Psychiatr Ann 2006; 36:553–557.
- Klerman GL, Weissmann MM. Interpersonal psychotherapy (IPT) and drugs in the treatment of depression. Pharmacopsychiatry 1987; 20:3–7.
- Burg MM, Lespérance F, Rieckmann N, Clemow L, Skotzko C, Davidson KW. Treating persistent depressive symptoms in post-ACS patients: the project COPES phase-I randomized controlled trial. Contemp Clin Trials 2008; 29:231–240.
- Davidson KW, Rieckmann N, Clemow L, et al Collaborative depression care for acute coronary syndrome patients with persistent depression: Coronary Psychosocial Evaluation Studies (COPES) randomized controlled trial. Arch Intern Med. In press.
- Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med 2002; 32:741–760.
- Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. Am J Med 2007; 120:799–806.
- Smith SC, Allen J, Blair SN, et al AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update; endorsed by the National Heart, Lung, and Blood Institute. Circulation 2006; 113:2363–2372.
- Thombs BD, de Jonge P, Coyne JC, et al Ziegelstein RC. Depression screening and patient outcomes in cardiovascular care: a systematic review. JAMA 2008; 300:2161–2171.
- Mitchell AJ, Vaze A, Rao S. Clinical diagnosis of depression in primary care: a meta-analysis. Lancet 2009; 374:609–619.
- Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006; 166:2314–2321.
- Glassman AH, Bigger JT, Gaffney M, Shapiro PA, Swenson JR. Onset of major depression associated with acute coronary syndromes: relationship of onset, major depressive disorder history, and episode severity to sertraline benefit. Arch Gen Psychiatry 2006; 63:283–288.
- Carney RM, Freedland KE. Treatment-resistant depression and mortality after acute coronary syndrome. Am J Psychiatry 2009; 166:410–417.
- Pignone MP, Gaynes BN, Rushton JL, et al Screening for depression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 136:765–776.
- Alexopoulos GS, Reynolds CF, Bruce ML, et al Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry 2009; 166:882–890.
- Mukherjee D, Fang J, Chetcuti S, Moscucci M, Kline-Rogers E, Eagle KA. Impact of combination evidence-based medical therapy on mortality in patients with acute coronary syndromes. Circulation 2004; 109:745–749.
- Eagle KA, Kline-Rogers E, Goodman SG, et al Adherence to evidence-based therapies after discharge for acute coronary syndromes: an ongoing prospective, observational study. Am J Med 2004; 117:73–81.
- Rieckmann N, Gerin W, Kronish IA, et al Course of depressive symptoms and medication adherence after acute coronary syndromes: an electronic medication monitoring study. J Am Coll Cardiol 2006; 48:2218–2222.
- Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med 2000; 160:1818–1823.
- Whooley MA, de Jonge P, Vittinghoff E, et al Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA 2008; 300:2379–2388.
- Blumenthal JA, Babyak MA, Doraiswamy PM, et al Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med 2007; 69:587–596.
- Blumenthal JA, Sherwood A, Rogers SD, et al Understanding prognostic benefits of exercise and antidepressant therapy for persons with depression and heart disease: the UPBEAT study—rationale, design, and methodological issues. Clin Trials 2007; 4:548–559.