User login
› Reserve antinuclear antibody testing for instances of clinically suggestive connective tissue diseases (CTD) and for assessing CTD prognosis. It can also be useful in monitoring disease progression. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Antinuclear antibodies (ANA) are a spectrum of autoantibodies that react with various nuclear and cytoplasmic components of normal human cells. Their detection is important in the diagnosis of some connective tissue diseases (CTD)—eg, systemic lupus erythematosus (SLE), Sjögren’s syndrome (SS), scleroderma, polymyositis, or mixed connective tissue disease (MCTD). Unfortunately, ANA tests are often used indiscriminately in daily clinical practice.1
When is ANA testing warranted?
Indiscriminate use of ANA testing can yield positive results that falsely point to CTD in a high proportion of patients and thereby lead to further inappropriate testing and errant management decisions. To wit: The presence of ANA in the serum can be associated with any number of factors, such as genetic predisposition (eg, through histocompatibility locus DR3), environmental agents (viruses, drugs), chronic infections, neoplasms, and advancing age.1 Therefore, the test should not be ordered in a patient with low pre-test probability of CTD. Moreover, higher titers of ANA are more clinically significant than lower titers. In one multicenter study, 31.7% of healthy individuals were ANA-positive at a serum dilution of 1:40, but only 5% were ANA-positive at a dilution of 1:160.2
What is the clinical significance of different immunofluorescent patterns?
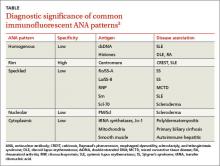
Immunofluorescent ANA testing not only determines if such antibodies are present in a patient’s serum but also reveals informative antibody patterns. Five distinct patterns of fluorescence are possible and can help differentiate between various CTDs (TABLE3):
1. Homogenous, in which the entire nucleus fluoresces, is seen in SLE and discoid lupus erythematosus (DLE).
2. Rim, in which the nuclear perimeter fluoresces, is seen most often in CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) syndrome and SLE.
3. Speckled, in which the nucleus fluoresces in a speckled pattern, can be seen in a variety of CTDs, including Sjögren’s syndrome, MCTD, SLE, and scleroderma.
4. Nucleolar, in which the nucleolus fluoresces, is associated with scleroderma.
5. Cytoplasmic, in which fluorescence occurs outside the nucleus, typically occurs with poly/dermatomyositis, primary biliary cirrhosis, or autoimmune hepatitis.
What is the next step if ANA is positive?
A positive ANA result warrants additional studies to identify specific autoantibodies suggested by the fluorescence pattern and by a patient’s signs and symptoms.
Following up diagnostic clues
Most systemic autoimmune diseases have a highly characteristic profile of autoantibodies to cellular antigens. A patient’s clinical features and ANA fluorescence pattern should direct additional testing.
Photosensitive butterfly rash, arthralgias/arthritis, pleuritic chest pain, fever of unknown cause, and urine sediment consistent with nephritis point to a diagnosis of SLE. Order an assay for anti-double-stranded DNA (dsDNA) antibodies, which, if present, confirm the diagnosis.4 Also order an assay for anti-Sm antibodies, which are highly specific for SLE but found only in 30% to 40% of SLE patients.4
Raynaud’s phenomenon, skin hardening or thickening, stiffness and tightening of the skin on the fingers, hands and forearms, tight and mask-like skin on the face, dry cough, shortness of breath, and difficulty in swallowing are features of scleroderma. If you suspect this disorder, order an assay for anti-Scl-70 antibodies. These antibodies are highly specific for scleroderma, but sensitivity of the assay is only 15% to 20%.5
Calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia indicate CREST syndrome. Anti-centromere antibodies are highly specific for CREST syndrome; sensitivity on assay is 50% to 90%.6
MCTD combines features of rheumatoid arthritis, SLE, myositis, and scleroderma. Order an assay of anti-RNP (ribonucleoprotein) antibodies. Although anti-RNP antibodies are also found in 25% to 30% of patients with SLE, they typically appear in the company of anti-Sm antibodies.7 Isolated high titers of anti-RNP antibodies point to MCTD, and sensitivity on assay is 100%.8 Their absence on testing, therefore, excludes the diagnosis of MCTD.
RNP, anti-Ro/SS-A, La/SS-B, and Sm are also referred to as extractable nuclear antigens (ENA). Assays of antibodies to ENA and anti-dsDNA are warranted only if the ANA assay result is positive. It is rare to have a positive anti-ENA antibody test (with the exception of antibodies to cytoplasmic antigens) in the absence of a positive ANA test.9
Dry eyes, dry mouth, joint pain and swelling, and swelling of parotid glands point to Sjögren’s syndrome. Anti-Ro/SS-A and La/SS-B antibodies are associated with Sjögren’s syndrome, but are also found in seronegative SLE.10 Therefore, if patients with features suggestive of SLE have a negative result on a dsDNA antibody assay, test for anti-Ro/SS-A and La/SS-B antibodies.
Muscle weakness and soreness, purplish discoloration of the upper eyelids, and purplish-red discoloration of the knuckles suggest dermatomyositis. Muscle biopsy and electromyography will clinch the diagnosis. Also test for anti–Jo-1 antibodies, which are associated with pulmonary involvement in polymyositis.11
ANA’s continuing role—prognosis and disease activity
Besides confirming a diagnosis of CTD in patients with suggestive clinical features, ANA testing serves 2 additional purposes: to help determine a patient’s prognosis and to monitor CTD activity. Consider the following:
- Patients with Sjögren’s syndrome who test positive for anti-Ro/SS-A antibodies have aggressive, extra-glandular disease that can cause vasculitis, purpura, lymphadenopathy, leukopenia, and thrombocytopenia.12
- The presence of anti-Ro/SS-A in the circulation of pregnant women with SLE confers a higher risk of neonatal lupus erythematosus and of congenital heart block in their newborns.13
- Severe interstitial lung disease is frequently found in scleroderma patients who test positive for anti-Scl-70.14 Antibodies to aminoacyl-tRNA synthetases—including anti–Jo-1, as mentioned earlier—are associated with pulmonary involvement in polymyositis patients.11
- A positive ANA test result in Raynaud’s phenomenon increases the likelihood that the patient will develop a systemic rheumatic disease; a negative result reduces this likelihood.15
- While the ANA test is not useful for diagnosing juvenile chronic arthritis (JCA), it is useful to test for ANA in patients with known JCA. A positive test result should prompt screening for uveitis.16
- An ANA test is not necessary for diagnosing antiphospholipid antibody syndrome (APS). However, the presence of ANA in a patient with APS increases the likelihood that APS is secondary to SLE.17
Monitoring disease activity
Documenting titers of anti-dsDNA antibodies may help in monitoring the disease activity of SLE in some patients. However, changes in titers of anti-dsDNA should be interpreted in the clinical context of the SLE Disease Activity Index.18
CORRESPONDENCE
Habib U. Rehman, MB, Department of Medicine, Regina Qu’Appelle Health Region, Regina General Hospital, 1440–14th Avenue, Regina, SK, S4P 0W5, Canada; [email protected]
1. Volkmann ER, Taylor M, Ben-Artzi A. Using the antinuclear antibody test to diagnose rheumatic disease: when does a positive test warrant further investigation? South Med J. 2012;105:100-104.
2. Giannouli E, Chatzidimitriou D, Gerou S, et al. Frequency and specificity of antibodies against nuclear and cytoplasmic antigens in healthy individuals by classic and new methods. Clin Rheumatol. 2013;32:1541-1546.
3. O’Sullivan M, McLean-Tooke A, Loh RK. Antinuclear antibody test. Aust Fam Physician. 2013;42:718-721.
4. Kurien BT, Scofield RH. Autoantibody determination in the diagnosis of systemic lupus erythematosus. Scand J Immunol. 2006;64:227-235.
5. Basu D, Reveille JD. Ant-scl-70. Autoimmunity. 2005;38:65-72.
6. Caramaschi P, Biasi D, Manzo T, et al. Anticentromere antibody—clinical associations. A study of 44 patients. Rheumatol Int. 1995;14:253-255.
7. Migliorini P, Baldini C, Rocchi V, et al. Anti-Sm and anti-RNP antibodies. Autoimmunity. 2005;38:47-54.
8. Alarcón-Segovia D, Cardiel MH. Comparison between 3 diagnostic criteria for mixed connective tissue disease. Study of 593 patients. J Rheumatol. 1989;16:328-334.
9. Phan TG, Wong RC, Adelstein S. Autoantibodies to extractable nuclear antigens: making detection and interpretation more meaningful. Clin Diagn Lab Immunol. 2002;9:1-7.
10. Cross LS, Aslam A, Misbah SA. Antinuclear antibody-negative lupus as a distinct diagnostic entity—does it no longer exist? QJM. 2004;97:303-308.
11. Miller FW, Waite KA, Biswat T, et al. The role of an autoantigen, histidyl-tRNA synthetase, in the induction and maintenance of autoimmunity. Proc Natl Acad Sci USA. 1990;87:9933-9937.
12. Brito-Zerón P, Ramos-Casals M, Bove A, et al. Predicting adverse outcomes in primary Sjogren’s syndrome: identification of prognostic factors. Rheumatology. 2007;46:1359-1362.
13. Lindop R, Arentz G, Thurgood LA, et al. Pathogenicity and proteomic signatures of autoantibodies to Ro and La. Immunol Cell Biol. 2012;90:304-309.
14. Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35:35-42.
15. Spencer-Green G. Outcomes in primary Raynaud phenomenon: a meta-analysis of the frequency, rates, and predictors of transition to secondary diseases. Arch Intern Med. 1998;158:595-600.
16. Grassi A, Corona F, Casellato A, et al. Prevalence and outcome of juvenile idiopathic arthritis-associated uveitis and relation to articular disease. J Rheumatol. 2007;34:1139-1145.
17. Petri M. Diagnosis of antiphospholipid antibody syndrome. Rheum Dis Clin North Am. 1994;20:443.
18. Kavanaugh A, Tomar R, Reveille J, et al. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med. 2000;124:71-81.
› Reserve antinuclear antibody testing for instances of clinically suggestive connective tissue diseases (CTD) and for assessing CTD prognosis. It can also be useful in monitoring disease progression. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Antinuclear antibodies (ANA) are a spectrum of autoantibodies that react with various nuclear and cytoplasmic components of normal human cells. Their detection is important in the diagnosis of some connective tissue diseases (CTD)—eg, systemic lupus erythematosus (SLE), Sjögren’s syndrome (SS), scleroderma, polymyositis, or mixed connective tissue disease (MCTD). Unfortunately, ANA tests are often used indiscriminately in daily clinical practice.1
When is ANA testing warranted?
Indiscriminate use of ANA testing can yield positive results that falsely point to CTD in a high proportion of patients and thereby lead to further inappropriate testing and errant management decisions. To wit: The presence of ANA in the serum can be associated with any number of factors, such as genetic predisposition (eg, through histocompatibility locus DR3), environmental agents (viruses, drugs), chronic infections, neoplasms, and advancing age.1 Therefore, the test should not be ordered in a patient with low pre-test probability of CTD. Moreover, higher titers of ANA are more clinically significant than lower titers. In one multicenter study, 31.7% of healthy individuals were ANA-positive at a serum dilution of 1:40, but only 5% were ANA-positive at a dilution of 1:160.2
What is the clinical significance of different immunofluorescent patterns?
Immunofluorescent ANA testing not only determines if such antibodies are present in a patient’s serum but also reveals informative antibody patterns. Five distinct patterns of fluorescence are possible and can help differentiate between various CTDs (TABLE3):
1. Homogenous, in which the entire nucleus fluoresces, is seen in SLE and discoid lupus erythematosus (DLE).
2. Rim, in which the nuclear perimeter fluoresces, is seen most often in CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) syndrome and SLE.
3. Speckled, in which the nucleus fluoresces in a speckled pattern, can be seen in a variety of CTDs, including Sjögren’s syndrome, MCTD, SLE, and scleroderma.
4. Nucleolar, in which the nucleolus fluoresces, is associated with scleroderma.
5. Cytoplasmic, in which fluorescence occurs outside the nucleus, typically occurs with poly/dermatomyositis, primary biliary cirrhosis, or autoimmune hepatitis.
What is the next step if ANA is positive?
A positive ANA result warrants additional studies to identify specific autoantibodies suggested by the fluorescence pattern and by a patient’s signs and symptoms.
Following up diagnostic clues
Most systemic autoimmune diseases have a highly characteristic profile of autoantibodies to cellular antigens. A patient’s clinical features and ANA fluorescence pattern should direct additional testing.
Photosensitive butterfly rash, arthralgias/arthritis, pleuritic chest pain, fever of unknown cause, and urine sediment consistent with nephritis point to a diagnosis of SLE. Order an assay for anti-double-stranded DNA (dsDNA) antibodies, which, if present, confirm the diagnosis.4 Also order an assay for anti-Sm antibodies, which are highly specific for SLE but found only in 30% to 40% of SLE patients.4
Raynaud’s phenomenon, skin hardening or thickening, stiffness and tightening of the skin on the fingers, hands and forearms, tight and mask-like skin on the face, dry cough, shortness of breath, and difficulty in swallowing are features of scleroderma. If you suspect this disorder, order an assay for anti-Scl-70 antibodies. These antibodies are highly specific for scleroderma, but sensitivity of the assay is only 15% to 20%.5
Calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia indicate CREST syndrome. Anti-centromere antibodies are highly specific for CREST syndrome; sensitivity on assay is 50% to 90%.6
MCTD combines features of rheumatoid arthritis, SLE, myositis, and scleroderma. Order an assay of anti-RNP (ribonucleoprotein) antibodies. Although anti-RNP antibodies are also found in 25% to 30% of patients with SLE, they typically appear in the company of anti-Sm antibodies.7 Isolated high titers of anti-RNP antibodies point to MCTD, and sensitivity on assay is 100%.8 Their absence on testing, therefore, excludes the diagnosis of MCTD.
RNP, anti-Ro/SS-A, La/SS-B, and Sm are also referred to as extractable nuclear antigens (ENA). Assays of antibodies to ENA and anti-dsDNA are warranted only if the ANA assay result is positive. It is rare to have a positive anti-ENA antibody test (with the exception of antibodies to cytoplasmic antigens) in the absence of a positive ANA test.9
Dry eyes, dry mouth, joint pain and swelling, and swelling of parotid glands point to Sjögren’s syndrome. Anti-Ro/SS-A and La/SS-B antibodies are associated with Sjögren’s syndrome, but are also found in seronegative SLE.10 Therefore, if patients with features suggestive of SLE have a negative result on a dsDNA antibody assay, test for anti-Ro/SS-A and La/SS-B antibodies.
Muscle weakness and soreness, purplish discoloration of the upper eyelids, and purplish-red discoloration of the knuckles suggest dermatomyositis. Muscle biopsy and electromyography will clinch the diagnosis. Also test for anti–Jo-1 antibodies, which are associated with pulmonary involvement in polymyositis.11
ANA’s continuing role—prognosis and disease activity
Besides confirming a diagnosis of CTD in patients with suggestive clinical features, ANA testing serves 2 additional purposes: to help determine a patient’s prognosis and to monitor CTD activity. Consider the following:
- Patients with Sjögren’s syndrome who test positive for anti-Ro/SS-A antibodies have aggressive, extra-glandular disease that can cause vasculitis, purpura, lymphadenopathy, leukopenia, and thrombocytopenia.12
- The presence of anti-Ro/SS-A in the circulation of pregnant women with SLE confers a higher risk of neonatal lupus erythematosus and of congenital heart block in their newborns.13
- Severe interstitial lung disease is frequently found in scleroderma patients who test positive for anti-Scl-70.14 Antibodies to aminoacyl-tRNA synthetases—including anti–Jo-1, as mentioned earlier—are associated with pulmonary involvement in polymyositis patients.11
- A positive ANA test result in Raynaud’s phenomenon increases the likelihood that the patient will develop a systemic rheumatic disease; a negative result reduces this likelihood.15
- While the ANA test is not useful for diagnosing juvenile chronic arthritis (JCA), it is useful to test for ANA in patients with known JCA. A positive test result should prompt screening for uveitis.16
- An ANA test is not necessary for diagnosing antiphospholipid antibody syndrome (APS). However, the presence of ANA in a patient with APS increases the likelihood that APS is secondary to SLE.17
Monitoring disease activity
Documenting titers of anti-dsDNA antibodies may help in monitoring the disease activity of SLE in some patients. However, changes in titers of anti-dsDNA should be interpreted in the clinical context of the SLE Disease Activity Index.18
CORRESPONDENCE
Habib U. Rehman, MB, Department of Medicine, Regina Qu’Appelle Health Region, Regina General Hospital, 1440–14th Avenue, Regina, SK, S4P 0W5, Canada; [email protected]
› Reserve antinuclear antibody testing for instances of clinically suggestive connective tissue diseases (CTD) and for assessing CTD prognosis. It can also be useful in monitoring disease progression. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Antinuclear antibodies (ANA) are a spectrum of autoantibodies that react with various nuclear and cytoplasmic components of normal human cells. Their detection is important in the diagnosis of some connective tissue diseases (CTD)—eg, systemic lupus erythematosus (SLE), Sjögren’s syndrome (SS), scleroderma, polymyositis, or mixed connective tissue disease (MCTD). Unfortunately, ANA tests are often used indiscriminately in daily clinical practice.1
When is ANA testing warranted?
Indiscriminate use of ANA testing can yield positive results that falsely point to CTD in a high proportion of patients and thereby lead to further inappropriate testing and errant management decisions. To wit: The presence of ANA in the serum can be associated with any number of factors, such as genetic predisposition (eg, through histocompatibility locus DR3), environmental agents (viruses, drugs), chronic infections, neoplasms, and advancing age.1 Therefore, the test should not be ordered in a patient with low pre-test probability of CTD. Moreover, higher titers of ANA are more clinically significant than lower titers. In one multicenter study, 31.7% of healthy individuals were ANA-positive at a serum dilution of 1:40, but only 5% were ANA-positive at a dilution of 1:160.2
What is the clinical significance of different immunofluorescent patterns?
Immunofluorescent ANA testing not only determines if such antibodies are present in a patient’s serum but also reveals informative antibody patterns. Five distinct patterns of fluorescence are possible and can help differentiate between various CTDs (TABLE3):
1. Homogenous, in which the entire nucleus fluoresces, is seen in SLE and discoid lupus erythematosus (DLE).
2. Rim, in which the nuclear perimeter fluoresces, is seen most often in CREST (calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) syndrome and SLE.
3. Speckled, in which the nucleus fluoresces in a speckled pattern, can be seen in a variety of CTDs, including Sjögren’s syndrome, MCTD, SLE, and scleroderma.
4. Nucleolar, in which the nucleolus fluoresces, is associated with scleroderma.
5. Cytoplasmic, in which fluorescence occurs outside the nucleus, typically occurs with poly/dermatomyositis, primary biliary cirrhosis, or autoimmune hepatitis.
What is the next step if ANA is positive?
A positive ANA result warrants additional studies to identify specific autoantibodies suggested by the fluorescence pattern and by a patient’s signs and symptoms.
Following up diagnostic clues
Most systemic autoimmune diseases have a highly characteristic profile of autoantibodies to cellular antigens. A patient’s clinical features and ANA fluorescence pattern should direct additional testing.
Photosensitive butterfly rash, arthralgias/arthritis, pleuritic chest pain, fever of unknown cause, and urine sediment consistent with nephritis point to a diagnosis of SLE. Order an assay for anti-double-stranded DNA (dsDNA) antibodies, which, if present, confirm the diagnosis.4 Also order an assay for anti-Sm antibodies, which are highly specific for SLE but found only in 30% to 40% of SLE patients.4
Raynaud’s phenomenon, skin hardening or thickening, stiffness and tightening of the skin on the fingers, hands and forearms, tight and mask-like skin on the face, dry cough, shortness of breath, and difficulty in swallowing are features of scleroderma. If you suspect this disorder, order an assay for anti-Scl-70 antibodies. These antibodies are highly specific for scleroderma, but sensitivity of the assay is only 15% to 20%.5
Calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia indicate CREST syndrome. Anti-centromere antibodies are highly specific for CREST syndrome; sensitivity on assay is 50% to 90%.6
MCTD combines features of rheumatoid arthritis, SLE, myositis, and scleroderma. Order an assay of anti-RNP (ribonucleoprotein) antibodies. Although anti-RNP antibodies are also found in 25% to 30% of patients with SLE, they typically appear in the company of anti-Sm antibodies.7 Isolated high titers of anti-RNP antibodies point to MCTD, and sensitivity on assay is 100%.8 Their absence on testing, therefore, excludes the diagnosis of MCTD.
RNP, anti-Ro/SS-A, La/SS-B, and Sm are also referred to as extractable nuclear antigens (ENA). Assays of antibodies to ENA and anti-dsDNA are warranted only if the ANA assay result is positive. It is rare to have a positive anti-ENA antibody test (with the exception of antibodies to cytoplasmic antigens) in the absence of a positive ANA test.9
Dry eyes, dry mouth, joint pain and swelling, and swelling of parotid glands point to Sjögren’s syndrome. Anti-Ro/SS-A and La/SS-B antibodies are associated with Sjögren’s syndrome, but are also found in seronegative SLE.10 Therefore, if patients with features suggestive of SLE have a negative result on a dsDNA antibody assay, test for anti-Ro/SS-A and La/SS-B antibodies.
Muscle weakness and soreness, purplish discoloration of the upper eyelids, and purplish-red discoloration of the knuckles suggest dermatomyositis. Muscle biopsy and electromyography will clinch the diagnosis. Also test for anti–Jo-1 antibodies, which are associated with pulmonary involvement in polymyositis.11
ANA’s continuing role—prognosis and disease activity
Besides confirming a diagnosis of CTD in patients with suggestive clinical features, ANA testing serves 2 additional purposes: to help determine a patient’s prognosis and to monitor CTD activity. Consider the following:
- Patients with Sjögren’s syndrome who test positive for anti-Ro/SS-A antibodies have aggressive, extra-glandular disease that can cause vasculitis, purpura, lymphadenopathy, leukopenia, and thrombocytopenia.12
- The presence of anti-Ro/SS-A in the circulation of pregnant women with SLE confers a higher risk of neonatal lupus erythematosus and of congenital heart block in their newborns.13
- Severe interstitial lung disease is frequently found in scleroderma patients who test positive for anti-Scl-70.14 Antibodies to aminoacyl-tRNA synthetases—including anti–Jo-1, as mentioned earlier—are associated with pulmonary involvement in polymyositis patients.11
- A positive ANA test result in Raynaud’s phenomenon increases the likelihood that the patient will develop a systemic rheumatic disease; a negative result reduces this likelihood.15
- While the ANA test is not useful for diagnosing juvenile chronic arthritis (JCA), it is useful to test for ANA in patients with known JCA. A positive test result should prompt screening for uveitis.16
- An ANA test is not necessary for diagnosing antiphospholipid antibody syndrome (APS). However, the presence of ANA in a patient with APS increases the likelihood that APS is secondary to SLE.17
Monitoring disease activity
Documenting titers of anti-dsDNA antibodies may help in monitoring the disease activity of SLE in some patients. However, changes in titers of anti-dsDNA should be interpreted in the clinical context of the SLE Disease Activity Index.18
CORRESPONDENCE
Habib U. Rehman, MB, Department of Medicine, Regina Qu’Appelle Health Region, Regina General Hospital, 1440–14th Avenue, Regina, SK, S4P 0W5, Canada; [email protected]
1. Volkmann ER, Taylor M, Ben-Artzi A. Using the antinuclear antibody test to diagnose rheumatic disease: when does a positive test warrant further investigation? South Med J. 2012;105:100-104.
2. Giannouli E, Chatzidimitriou D, Gerou S, et al. Frequency and specificity of antibodies against nuclear and cytoplasmic antigens in healthy individuals by classic and new methods. Clin Rheumatol. 2013;32:1541-1546.
3. O’Sullivan M, McLean-Tooke A, Loh RK. Antinuclear antibody test. Aust Fam Physician. 2013;42:718-721.
4. Kurien BT, Scofield RH. Autoantibody determination in the diagnosis of systemic lupus erythematosus. Scand J Immunol. 2006;64:227-235.
5. Basu D, Reveille JD. Ant-scl-70. Autoimmunity. 2005;38:65-72.
6. Caramaschi P, Biasi D, Manzo T, et al. Anticentromere antibody—clinical associations. A study of 44 patients. Rheumatol Int. 1995;14:253-255.
7. Migliorini P, Baldini C, Rocchi V, et al. Anti-Sm and anti-RNP antibodies. Autoimmunity. 2005;38:47-54.
8. Alarcón-Segovia D, Cardiel MH. Comparison between 3 diagnostic criteria for mixed connective tissue disease. Study of 593 patients. J Rheumatol. 1989;16:328-334.
9. Phan TG, Wong RC, Adelstein S. Autoantibodies to extractable nuclear antigens: making detection and interpretation more meaningful. Clin Diagn Lab Immunol. 2002;9:1-7.
10. Cross LS, Aslam A, Misbah SA. Antinuclear antibody-negative lupus as a distinct diagnostic entity—does it no longer exist? QJM. 2004;97:303-308.
11. Miller FW, Waite KA, Biswat T, et al. The role of an autoantigen, histidyl-tRNA synthetase, in the induction and maintenance of autoimmunity. Proc Natl Acad Sci USA. 1990;87:9933-9937.
12. Brito-Zerón P, Ramos-Casals M, Bove A, et al. Predicting adverse outcomes in primary Sjogren’s syndrome: identification of prognostic factors. Rheumatology. 2007;46:1359-1362.
13. Lindop R, Arentz G, Thurgood LA, et al. Pathogenicity and proteomic signatures of autoantibodies to Ro and La. Immunol Cell Biol. 2012;90:304-309.
14. Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35:35-42.
15. Spencer-Green G. Outcomes in primary Raynaud phenomenon: a meta-analysis of the frequency, rates, and predictors of transition to secondary diseases. Arch Intern Med. 1998;158:595-600.
16. Grassi A, Corona F, Casellato A, et al. Prevalence and outcome of juvenile idiopathic arthritis-associated uveitis and relation to articular disease. J Rheumatol. 2007;34:1139-1145.
17. Petri M. Diagnosis of antiphospholipid antibody syndrome. Rheum Dis Clin North Am. 1994;20:443.
18. Kavanaugh A, Tomar R, Reveille J, et al. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med. 2000;124:71-81.
1. Volkmann ER, Taylor M, Ben-Artzi A. Using the antinuclear antibody test to diagnose rheumatic disease: when does a positive test warrant further investigation? South Med J. 2012;105:100-104.
2. Giannouli E, Chatzidimitriou D, Gerou S, et al. Frequency and specificity of antibodies against nuclear and cytoplasmic antigens in healthy individuals by classic and new methods. Clin Rheumatol. 2013;32:1541-1546.
3. O’Sullivan M, McLean-Tooke A, Loh RK. Antinuclear antibody test. Aust Fam Physician. 2013;42:718-721.
4. Kurien BT, Scofield RH. Autoantibody determination in the diagnosis of systemic lupus erythematosus. Scand J Immunol. 2006;64:227-235.
5. Basu D, Reveille JD. Ant-scl-70. Autoimmunity. 2005;38:65-72.
6. Caramaschi P, Biasi D, Manzo T, et al. Anticentromere antibody—clinical associations. A study of 44 patients. Rheumatol Int. 1995;14:253-255.
7. Migliorini P, Baldini C, Rocchi V, et al. Anti-Sm and anti-RNP antibodies. Autoimmunity. 2005;38:47-54.
8. Alarcón-Segovia D, Cardiel MH. Comparison between 3 diagnostic criteria for mixed connective tissue disease. Study of 593 patients. J Rheumatol. 1989;16:328-334.
9. Phan TG, Wong RC, Adelstein S. Autoantibodies to extractable nuclear antigens: making detection and interpretation more meaningful. Clin Diagn Lab Immunol. 2002;9:1-7.
10. Cross LS, Aslam A, Misbah SA. Antinuclear antibody-negative lupus as a distinct diagnostic entity—does it no longer exist? QJM. 2004;97:303-308.
11. Miller FW, Waite KA, Biswat T, et al. The role of an autoantigen, histidyl-tRNA synthetase, in the induction and maintenance of autoimmunity. Proc Natl Acad Sci USA. 1990;87:9933-9937.
12. Brito-Zerón P, Ramos-Casals M, Bove A, et al. Predicting adverse outcomes in primary Sjogren’s syndrome: identification of prognostic factors. Rheumatology. 2007;46:1359-1362.
13. Lindop R, Arentz G, Thurgood LA, et al. Pathogenicity and proteomic signatures of autoantibodies to Ro and La. Immunol Cell Biol. 2012;90:304-309.
14. Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35:35-42.
15. Spencer-Green G. Outcomes in primary Raynaud phenomenon: a meta-analysis of the frequency, rates, and predictors of transition to secondary diseases. Arch Intern Med. 1998;158:595-600.
16. Grassi A, Corona F, Casellato A, et al. Prevalence and outcome of juvenile idiopathic arthritis-associated uveitis and relation to articular disease. J Rheumatol. 2007;34:1139-1145.
17. Petri M. Diagnosis of antiphospholipid antibody syndrome. Rheum Dis Clin North Am. 1994;20:443.
18. Kavanaugh A, Tomar R, Reveille J, et al. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med. 2000;124:71-81.