User login
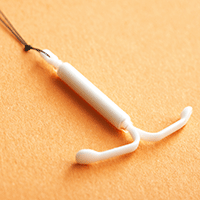
Fear of potential pain caused by insertion of an intrauterine device (IUD) prevents some women from using this highly effective and safe contraceptive method. Recently, investigators conducted a randomized, placebo-controlled trial to assess whether vaginal lidocaine gel administered shortly before IUD placement was associated with a decrease from baseline in patient-reported pain scores.1
In this blinded trial, Rapkinand colleagues randomly assigned nulliparous women presenting for IUD placement (either the copper T380A IUD or the 52-mg levonorgestrel-releasing IUD) at faculty and resident clinics at a US urban academic center to place 4 mL of 2% lidocaine gel or placebo gel vaginally (using an applicator) 5 to 15 minutes prior to IUD placement.1 A 100-mm visual analog scale (VAS) was used to assess pain at each step of the procedure, including at baseline (before speculum insertion), after speculum placement, tenaculum placement, uterine sound, IUD insertion, and 5 minutes after speculum removal.
Among the 58 evaluable participants, the mean age was 23 years in the lidocaine group and 24 years in the placebo group; more than 80% of the women were white.
The study’s primary outcome was change in pain experience from baseline to IUD insertion. Pain was measured on a VAS from 0 mm (no pain) to 100 mm (worst pain in my life). Secondary outcomes included patient acceptability of gel self-insertion, physician-reported ease of IUD insertion, and need for pain medication for up to 7 days after IUD insertion.
Related article:
Liletta gets a new inserter: Steps for successful placement
What the investigators found
The mean change in pain scores with IUD placement was 61 mm for the lidocaine group and 69 mm for the placebo group (P = .06). Thus, no difference in the primary outcome was found between the 2 groups. However, women who received the lidocaine gel treatment experiencedsignificantly less pain with tenaculum placement than those who received placebo gel (32 mm vs 56 mm; P = .02), and they were substantially less likely to require cervical dilation (3.3% vs 34.5%; P = .002), an often painful procedure.
Related article:
Does the injection of ketorolac prior to IUD placement reduce pain?
Patient acceptability and satisfaction. Five minutes after the IUD placement procedure, approximately two-thirds of women in both groups indicated that they experienced an acceptable level of discomfort, and more than three-quarters indicated that they were satisfied with the placement procedure. Fully 67% of the lidocaine group and 68% of the placebo group indicated definitely or probably yes when asked if the level of discomfort they experienced was acceptable, with 27% and 21%, respectively, responding as neutral. When asked if getting the IUD was worth the level of discomfort experienced, 73% of the lidocaine group and 82% of the placebo group responded “yes,” while 23% and 18%, respectively, were unsure.
Pearls for practice
As this study showed, self-administered lidocaine vaginal gel did not alter the primary outcome (pain with IUD placement), but the reduced need for cervical dilation is a promising finding and warrants additional study.
Tip. Interestingly, the placebo-treated women experienced pain intensity with cervical tenaculum placement similar to that associated with IUD placement. This finding illuminates the fact that IUD placement is not the only action that can produce pain. For this reason, I use a finer, single-tooth tenaculum designed for use with sonohysterograms (Goldstein Grasp Cervical Stabilizer).2 This instrument appears to cause less pain and bleeding than conventional tenacula.
Related article:
How to identify and localize IUDs on ultrasound
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Rapkin RB, Achilles SL, Schwarz B, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128(3):621–628.
- Goldstein Grasp Cervical Stabilzer. CooperSurgical, Inc. website. http://www.coopersurgical.com/Products/Detail/Goldstein-Grasp-Cervical-Stabilizer. Accessed November 16, 2016.
Fear of potential pain caused by insertion of an intrauterine device (IUD) prevents some women from using this highly effective and safe contraceptive method. Recently, investigators conducted a randomized, placebo-controlled trial to assess whether vaginal lidocaine gel administered shortly before IUD placement was associated with a decrease from baseline in patient-reported pain scores.1
In this blinded trial, Rapkinand colleagues randomly assigned nulliparous women presenting for IUD placement (either the copper T380A IUD or the 52-mg levonorgestrel-releasing IUD) at faculty and resident clinics at a US urban academic center to place 4 mL of 2% lidocaine gel or placebo gel vaginally (using an applicator) 5 to 15 minutes prior to IUD placement.1 A 100-mm visual analog scale (VAS) was used to assess pain at each step of the procedure, including at baseline (before speculum insertion), after speculum placement, tenaculum placement, uterine sound, IUD insertion, and 5 minutes after speculum removal.
Among the 58 evaluable participants, the mean age was 23 years in the lidocaine group and 24 years in the placebo group; more than 80% of the women were white.
The study’s primary outcome was change in pain experience from baseline to IUD insertion. Pain was measured on a VAS from 0 mm (no pain) to 100 mm (worst pain in my life). Secondary outcomes included patient acceptability of gel self-insertion, physician-reported ease of IUD insertion, and need for pain medication for up to 7 days after IUD insertion.
Related article:
Liletta gets a new inserter: Steps for successful placement
What the investigators found
The mean change in pain scores with IUD placement was 61 mm for the lidocaine group and 69 mm for the placebo group (P = .06). Thus, no difference in the primary outcome was found between the 2 groups. However, women who received the lidocaine gel treatment experiencedsignificantly less pain with tenaculum placement than those who received placebo gel (32 mm vs 56 mm; P = .02), and they were substantially less likely to require cervical dilation (3.3% vs 34.5%; P = .002), an often painful procedure.
Related article:
Does the injection of ketorolac prior to IUD placement reduce pain?
Patient acceptability and satisfaction. Five minutes after the IUD placement procedure, approximately two-thirds of women in both groups indicated that they experienced an acceptable level of discomfort, and more than three-quarters indicated that they were satisfied with the placement procedure. Fully 67% of the lidocaine group and 68% of the placebo group indicated definitely or probably yes when asked if the level of discomfort they experienced was acceptable, with 27% and 21%, respectively, responding as neutral. When asked if getting the IUD was worth the level of discomfort experienced, 73% of the lidocaine group and 82% of the placebo group responded “yes,” while 23% and 18%, respectively, were unsure.
Pearls for practice
As this study showed, self-administered lidocaine vaginal gel did not alter the primary outcome (pain with IUD placement), but the reduced need for cervical dilation is a promising finding and warrants additional study.
Tip. Interestingly, the placebo-treated women experienced pain intensity with cervical tenaculum placement similar to that associated with IUD placement. This finding illuminates the fact that IUD placement is not the only action that can produce pain. For this reason, I use a finer, single-tooth tenaculum designed for use with sonohysterograms (Goldstein Grasp Cervical Stabilizer).2 This instrument appears to cause less pain and bleeding than conventional tenacula.
Related article:
How to identify and localize IUDs on ultrasound
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Fear of potential pain caused by insertion of an intrauterine device (IUD) prevents some women from using this highly effective and safe contraceptive method. Recently, investigators conducted a randomized, placebo-controlled trial to assess whether vaginal lidocaine gel administered shortly before IUD placement was associated with a decrease from baseline in patient-reported pain scores.1
In this blinded trial, Rapkinand colleagues randomly assigned nulliparous women presenting for IUD placement (either the copper T380A IUD or the 52-mg levonorgestrel-releasing IUD) at faculty and resident clinics at a US urban academic center to place 4 mL of 2% lidocaine gel or placebo gel vaginally (using an applicator) 5 to 15 minutes prior to IUD placement.1 A 100-mm visual analog scale (VAS) was used to assess pain at each step of the procedure, including at baseline (before speculum insertion), after speculum placement, tenaculum placement, uterine sound, IUD insertion, and 5 minutes after speculum removal.
Among the 58 evaluable participants, the mean age was 23 years in the lidocaine group and 24 years in the placebo group; more than 80% of the women were white.
The study’s primary outcome was change in pain experience from baseline to IUD insertion. Pain was measured on a VAS from 0 mm (no pain) to 100 mm (worst pain in my life). Secondary outcomes included patient acceptability of gel self-insertion, physician-reported ease of IUD insertion, and need for pain medication for up to 7 days after IUD insertion.
Related article:
Liletta gets a new inserter: Steps for successful placement
What the investigators found
The mean change in pain scores with IUD placement was 61 mm for the lidocaine group and 69 mm for the placebo group (P = .06). Thus, no difference in the primary outcome was found between the 2 groups. However, women who received the lidocaine gel treatment experiencedsignificantly less pain with tenaculum placement than those who received placebo gel (32 mm vs 56 mm; P = .02), and they were substantially less likely to require cervical dilation (3.3% vs 34.5%; P = .002), an often painful procedure.
Related article:
Does the injection of ketorolac prior to IUD placement reduce pain?
Patient acceptability and satisfaction. Five minutes after the IUD placement procedure, approximately two-thirds of women in both groups indicated that they experienced an acceptable level of discomfort, and more than three-quarters indicated that they were satisfied with the placement procedure. Fully 67% of the lidocaine group and 68% of the placebo group indicated definitely or probably yes when asked if the level of discomfort they experienced was acceptable, with 27% and 21%, respectively, responding as neutral. When asked if getting the IUD was worth the level of discomfort experienced, 73% of the lidocaine group and 82% of the placebo group responded “yes,” while 23% and 18%, respectively, were unsure.
Pearls for practice
As this study showed, self-administered lidocaine vaginal gel did not alter the primary outcome (pain with IUD placement), but the reduced need for cervical dilation is a promising finding and warrants additional study.
Tip. Interestingly, the placebo-treated women experienced pain intensity with cervical tenaculum placement similar to that associated with IUD placement. This finding illuminates the fact that IUD placement is not the only action that can produce pain. For this reason, I use a finer, single-tooth tenaculum designed for use with sonohysterograms (Goldstein Grasp Cervical Stabilizer).2 This instrument appears to cause less pain and bleeding than conventional tenacula.
Related article:
How to identify and localize IUDs on ultrasound
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Rapkin RB, Achilles SL, Schwarz B, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128(3):621–628.
- Goldstein Grasp Cervical Stabilzer. CooperSurgical, Inc. website. http://www.coopersurgical.com/Products/Detail/Goldstein-Grasp-Cervical-Stabilizer. Accessed November 16, 2016.
- Rapkin RB, Achilles SL, Schwarz B, et al. Self-administered lidocaine gel for intrauterine device insertion in nulliparous women: a randomized controlled trial. Obstet Gynecol. 2016;128(3):621–628.
- Goldstein Grasp Cervical Stabilzer. CooperSurgical, Inc. website. http://www.coopersurgical.com/Products/Detail/Goldstein-Grasp-Cervical-Stabilizer. Accessed November 16, 2016.
In this article