User login
The attempt to alter electroencephalographic (EEG) frequency/amplitude patterns and their underlying brain mechanisms using contingent operant conditioning methods is today referred to variously as EEG biofeedback, neurofeedback, or neurotherapy. This article traces the history of the clinical application of EEG operant conditioning from empirical animal investigations to its emergence as a treatment option for major seizure types. In light of the diversity of the clinical applications of this method in general, and the complexity of seizure disorders in particular, I also present an overview of specific methods used in our EEG biofeedback program.
INITIAL APPLICATION IN HUMANS
BACKDROP TO THE CLINICAL APPLICATION: KEY ANIMAL STUDIES
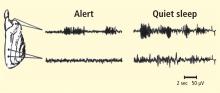
To accomplish this, we attempted to facilitate the SMR during wakefulness using an operant conditioning paradigm with a liquid food reward, and then study any resulting changes in sleep spindle activity and sleep structure. Necessary quality controls included alternate training to suppress this rhythm and a counterbalanced design employing two separate groups of cats. Six weeks of three training sessions per week to satiation led to profound and differential changes in sleep EEG and sleep architecture. SMR training, whether it preceded or followed suppression training, led to a significant increase in EEG sleep spindle density, as well as a significant reduction in sleep period fragmentation due to arousals. No changes occurred in the control condition.3
A more profound finding in the cat
Platform for a dual research approach
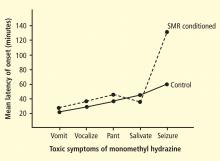
These two studies provided several interesting conclusions that directed our subsequent scientific efforts. First, in the cat study we observed a common prodrome in both SMR-trained and control animals even though the SMR-trained animals had acquired protection against seizures. This suggested a direct effect on the seizure process and not on MMH toxicity in general. Second, in our human epileptic patient, the seizures that were suppressed arose out of the unconscious state of sleep, a fact that eliminated the possibility of any voluntary countermeasure and again indicated a direct effect on the seizure mechanism. Accordingly, we undertook a dual approach to understanding the basis of this effect, involving both additional animal electrophysiologic and human clinical studies.
Animal studies evaluated motor behavior, motor reflexes, motor and thalamic unit firing, and somatosensory pathway correlates of the SMR response. Clinical studies, as reviewed in the following section, sought to further document the anticonvulsant effects of SMR operant conditioning and examine this effect on various seizure types. Possible alternative explanations, such as altered medication compliance and placebo effects, were also addressed in several comprehensive studies. Additionally, by this time other laboratories were beginning to add to the research literature in this new field.
CLINICAL STUDIES
A series of human studies followed our initial clinical report, including group studies involving crossover and placebo-controlled designs. These studies consistently reported significant seizure reductions in epileptic patients in response to reward for increasing sensorimotor EEG rhythmic activity.
Two independent meta-analyses of the peer-reviewed papers in this literature have appeared in the last decade.8,10 In a review of 24 studies involving 243 patients with predominantly partial complex seizures provided with central cortical SMR feedback training, Sterman determined that 82% of these subjects registered seizure reductions greater than 50%.8 More recently, Tan and colleagues evaluated data from 63 studies and selected for comprehensive analysis 10 studies that met stringent criteria for controls and population and seizure details.10 They reported that 79% of the patients treated with SMR feedback training experienced a statistically significant reduction in seizure frequency despite a collective history of failed medication therapy.
- One group simply tabulated their seizure experiences for 6 weeks using a comprehensive logging method.
- The second group received EEG feedback training for 1 hour three times a week for 6 weeks; however, the EEG signal responsible for reward was previously recorded from a different individual. This noncontingent feedback constituted a “yoked control” group.
- The third group received 6 weeks of contingent training for increasing SMR activity in somatosensory cortex while simultaneously suppressing slower 4- to 8-Hz activity.
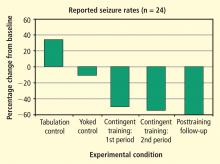
After the initial 6 weeks, all 24 subjects were combined into one group that received 6 more weeks of contingent training only. This was followed by a 4-week period of gradual withdrawal from training and then by a final tabulation of seizure incidence during a 6-week period after training was terminated. As can be seen in Figure 5, the seizure tabulation control was associated with an increased seizure count and the “yoked control” noncontingent SMR training was associated with no significant change in seizure incidence, whereas contingent SMR training was associated with a statistically significant reduction in seizures. The statistical significance of this reduction increased progressively as subjects from the other two groups were added to a second 6-week period of contingent training, and after an additional 6 weeks following withdrawal from training. In addition to this exclusive seizure reduction after SMR contingent training, pre-/post-training neuropsychological testing showed that responding SMR-trained subjects also improved significantly in performance of tasks specific to the hemisphere contralateral to their frontotemporal lesion, indicating a reduced corrosive disturbance from the seizure focus.11
EEG BIOFEEDBACK IN PRACTICE: PROFILE OF THE AUTHOR’S PROGRAM
EEG operant conditioning methods for biofeedback training have diversified as various hardware and software products have emerged and as individuals with differing backgrounds and credentials have entered the field. A lack of methodologic standards and professional regulations has contributed to an undesirable inconsistency in the competence and effectiveness of therapeutic applications. Nevertheless, abundant peer-reviewed research by qualified investigators has proven the worth of this method as a viable alternative treatment for seizure disorders, so I will attempt to provide some idea of a systematic and evidence-guided approach to treatment as used in our program.
With each box monitoring the same electrode site and each frequency tuned to the same band, thresholds can be set to promote facilitation or suppression through “successive approximation,” or sequencing from left to right with sequentially more difficult thresholds. Numerous other configurations are possible. In the case shown in Figure 7, the band-pass at the far left is set at 12 to 15 Hz (SMR) for the C3 electrode site, and the remaining three bands to the right are set to 3 to 5 Hz at the left medial frontal location Fz, with successively lower thresholds to promote suppression of this band at this site.
A RATIONAL MODEL FROM RECENT NEUROIMAGING STUDIES
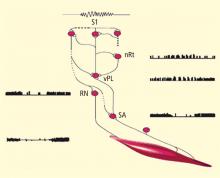
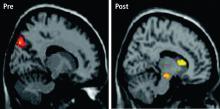
While it is difficult to evaluate neurophysiologic changes in human subjects to a degree similar to that in animals, certain parallels can be drawn. Further, new imaging methods allow for assessment of localized metabolic changes in the human brain during and after EEG feedback training. Behaviorally, during successful SMR training, human subjects become behaviorally quiet and direct their attention to the task. It is safe to presume that the SMR response develops as a result of reduced motor excitation and resulting intrathalamic ventrobasal oscillations, since this mechanism is well established as a basis for mammalian sensorimotor EEG rhythm generation.12 These changes, as well as others documented in our animal studies, set the stage for the development of SMR activity and are likely collectively initiated by altered input from some other executive system.
These facts provide a rational model for a threshold-altering process that could affect seizure discharge propagation to motor networks. Although there are many different neurotransmitters used within the basal ganglia (principally acetylcholine, gamma-aminobutyric acid, and dopamine), the overall effect on thalamus and premotor networks in the mesencephalic tegmentum and superior colliculus is inhibitory.14–16 If activation of these inhibitory basal ganglia networks can become labeled by the SMR through contingent feedback training, and if responsible circuits can be potentiated by this association, motor inhibitory regulation would be generally facilitated.
CONCLUSIONS
Despite the encouraging findings and concepts reviewed here, there are significant issues at virtually every step of the thinking and practice behind this new therapy. This method depends on a comprehensive understanding of the EEG signal and the technical requirements of valid quantitative analysis and feedback applications. This includes a basic knowledge of the principles essential for effective operant conditioning. Further, in light of the complexity of seizure disorders, accurate history and seizure classification must be evaluated and understood.
Alternative explanations for therapeutic results include such considerations as short-lasting expectation effects and changes in patient behavior. However, it must again be noted that the prolonged anticonvulsant effect documented in our animal studies, as well as in relation to nocturnal seizures arising out of sleep in a human subject, would seem to rule out placebo or nonspecific effects. This conclusion is supported further by the finding of improved neuropsychological performance after SMR training in tasks mediated by the hemisphere contralateral to disrupting localized epileptogenic lesions. Additionally, an alternative explanation for improved seizure control based on increased medication compliance has been rejected through studies that carefully monitored blood levels of prescribed anticonvulsant drugs before, during, and after training.
Finally, the epileptic patients who have demonstrated clinical improvement in EEG biofeedback research studies, along with many who seek this treatment today, represent unquestionable failures of anticonvulsant drug therapy. Notably, positive outcomes have frequently been achieved in patients with complex-partial seizures, an extremely difficult-to-treat seizure type. It is therefore unfortunate that some professionals still criticize neurofeedback therapy for a lack of more consistent or successful outcomes. On the contrary, as noted here, evidence has shown that most of these difficult-to-treat patients benefit beyond any chance or placebo outcome, in some cases dramatically so. In light of the frequent adverse effects and costs associated with lifelong pharmacotherapy, we view EEG biofeedback therapy not as a “last resort” option to be restricted solely to pharmacotherapy-resistant cases but rather as a generally viable consideration for any patient suffering from seizures. Moreover, in contrast to drug-dependent management approaches, the altered modulation of striatal and thalamocortical inhibition that is possible through neurofeedback training may sufficiently raise seizure thresholds to greatly increase the prospects for the long-term nondependent management of epilepsy.
- Sterman MB, Friar L. Suppression of seizures in an epileptic following sensorimotor EEG feedback training. Electroencephalogr Clin Neurophysiol 1972; 33:89–95.
- Sterman MB. Effects of sensorimotor EEG feedback training on sleep and clinical manifestations of epilepsy. In:Beatty J, Legewie H, eds. Biofeedback and Behavior. New York, NY: Plenum Press; 1977:167–200.
- Sterman MB, Howe RD, Macdonald LR. Facilitation of spindle-burst sleep by conditioning of electroencephalographic activity while awake. Science 1970; 167:1146–1148.
- Sterman MB, LoPresti RW, Fairchild MD. Electroencephalographic and behavioral studies of monomethyl hydrazine toxicity in the cat. Aerospace Medical Research Laboratory 1969; AMRL-TR-69-3:1–8.
- Sterman MB. Studies of EEG biofeedback training in man and cats. In: Veterans Administration Studies in Mental Health and Behavioral Sciences: Highlights of the 17th Annual Conference. Perry Point, MD: US Veterans Administration; 1972:50–60.
- Sterman MB, Bowersox SS. Sensorimotor EEG rhythmic activity: a functional gate mechanism. Sleep 1981; 4:408–422.
- Sterman MB. Physiological origins and functional correlates of EEG rhythmic activities: implications for self-regulation. Biofeedack Self Reg 1996; 21:3–33.
- Sterman MB. Basic concepts and clinical findings in the treatment of seizure disorders with EEG operant conditioning. Clin Electroencephalogr 2000; 31:45–55.
- Egner T, Sterman MB. Neurofeedback treatment of epilepsy: from basic rationale to practical application. Expert Rev Neurother 2006; 6:247–257.
- Tan G, Thornby J, Hammond D, et al Meta-analysis of EEG biofeedback in treating epilepsy. Clin EEG Neurosci 2009; 40:173–179.
- Lantz D, Sterman MB. Neuropsychological assessment of subjects with uncontrolled epilepsy: effects of EEG biofeedback training. Epilepsia 1988; 29:163–171.
- Steriade M, McCormick DA, Seinowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 1993; 262:679–685.
- Lévesque J, Beauregard M, Mensour B. Effect of neurofeedback training on the neural substrates of selective attention in children with attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study. Neurosci Lett 2006; 394:216–221.
- Brodal P. The basal ganglia. In: The Central Nervous System: Structure and Function. New York, NY: Oxford University Press; 1992:246–261.
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci 1990; 13:277–280.
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 1990; 13:281–285.
The attempt to alter electroencephalographic (EEG) frequency/amplitude patterns and their underlying brain mechanisms using contingent operant conditioning methods is today referred to variously as EEG biofeedback, neurofeedback, or neurotherapy. This article traces the history of the clinical application of EEG operant conditioning from empirical animal investigations to its emergence as a treatment option for major seizure types. In light of the diversity of the clinical applications of this method in general, and the complexity of seizure disorders in particular, I also present an overview of specific methods used in our EEG biofeedback program.
INITIAL APPLICATION IN HUMANS
BACKDROP TO THE CLINICAL APPLICATION: KEY ANIMAL STUDIES
To accomplish this, we attempted to facilitate the SMR during wakefulness using an operant conditioning paradigm with a liquid food reward, and then study any resulting changes in sleep spindle activity and sleep structure. Necessary quality controls included alternate training to suppress this rhythm and a counterbalanced design employing two separate groups of cats. Six weeks of three training sessions per week to satiation led to profound and differential changes in sleep EEG and sleep architecture. SMR training, whether it preceded or followed suppression training, led to a significant increase in EEG sleep spindle density, as well as a significant reduction in sleep period fragmentation due to arousals. No changes occurred in the control condition.3
A more profound finding in the cat
Platform for a dual research approach
These two studies provided several interesting conclusions that directed our subsequent scientific efforts. First, in the cat study we observed a common prodrome in both SMR-trained and control animals even though the SMR-trained animals had acquired protection against seizures. This suggested a direct effect on the seizure process and not on MMH toxicity in general. Second, in our human epileptic patient, the seizures that were suppressed arose out of the unconscious state of sleep, a fact that eliminated the possibility of any voluntary countermeasure and again indicated a direct effect on the seizure mechanism. Accordingly, we undertook a dual approach to understanding the basis of this effect, involving both additional animal electrophysiologic and human clinical studies.
Animal studies evaluated motor behavior, motor reflexes, motor and thalamic unit firing, and somatosensory pathway correlates of the SMR response. Clinical studies, as reviewed in the following section, sought to further document the anticonvulsant effects of SMR operant conditioning and examine this effect on various seizure types. Possible alternative explanations, such as altered medication compliance and placebo effects, were also addressed in several comprehensive studies. Additionally, by this time other laboratories were beginning to add to the research literature in this new field.
CLINICAL STUDIES
A series of human studies followed our initial clinical report, including group studies involving crossover and placebo-controlled designs. These studies consistently reported significant seizure reductions in epileptic patients in response to reward for increasing sensorimotor EEG rhythmic activity.
Two independent meta-analyses of the peer-reviewed papers in this literature have appeared in the last decade.8,10 In a review of 24 studies involving 243 patients with predominantly partial complex seizures provided with central cortical SMR feedback training, Sterman determined that 82% of these subjects registered seizure reductions greater than 50%.8 More recently, Tan and colleagues evaluated data from 63 studies and selected for comprehensive analysis 10 studies that met stringent criteria for controls and population and seizure details.10 They reported that 79% of the patients treated with SMR feedback training experienced a statistically significant reduction in seizure frequency despite a collective history of failed medication therapy.
- One group simply tabulated their seizure experiences for 6 weeks using a comprehensive logging method.
- The second group received EEG feedback training for 1 hour three times a week for 6 weeks; however, the EEG signal responsible for reward was previously recorded from a different individual. This noncontingent feedback constituted a “yoked control” group.
- The third group received 6 weeks of contingent training for increasing SMR activity in somatosensory cortex while simultaneously suppressing slower 4- to 8-Hz activity.
After the initial 6 weeks, all 24 subjects were combined into one group that received 6 more weeks of contingent training only. This was followed by a 4-week period of gradual withdrawal from training and then by a final tabulation of seizure incidence during a 6-week period after training was terminated. As can be seen in Figure 5, the seizure tabulation control was associated with an increased seizure count and the “yoked control” noncontingent SMR training was associated with no significant change in seizure incidence, whereas contingent SMR training was associated with a statistically significant reduction in seizures. The statistical significance of this reduction increased progressively as subjects from the other two groups were added to a second 6-week period of contingent training, and after an additional 6 weeks following withdrawal from training. In addition to this exclusive seizure reduction after SMR contingent training, pre-/post-training neuropsychological testing showed that responding SMR-trained subjects also improved significantly in performance of tasks specific to the hemisphere contralateral to their frontotemporal lesion, indicating a reduced corrosive disturbance from the seizure focus.11
EEG BIOFEEDBACK IN PRACTICE: PROFILE OF THE AUTHOR’S PROGRAM
EEG operant conditioning methods for biofeedback training have diversified as various hardware and software products have emerged and as individuals with differing backgrounds and credentials have entered the field. A lack of methodologic standards and professional regulations has contributed to an undesirable inconsistency in the competence and effectiveness of therapeutic applications. Nevertheless, abundant peer-reviewed research by qualified investigators has proven the worth of this method as a viable alternative treatment for seizure disorders, so I will attempt to provide some idea of a systematic and evidence-guided approach to treatment as used in our program.
With each box monitoring the same electrode site and each frequency tuned to the same band, thresholds can be set to promote facilitation or suppression through “successive approximation,” or sequencing from left to right with sequentially more difficult thresholds. Numerous other configurations are possible. In the case shown in Figure 7, the band-pass at the far left is set at 12 to 15 Hz (SMR) for the C3 electrode site, and the remaining three bands to the right are set to 3 to 5 Hz at the left medial frontal location Fz, with successively lower thresholds to promote suppression of this band at this site.
A RATIONAL MODEL FROM RECENT NEUROIMAGING STUDIES
While it is difficult to evaluate neurophysiologic changes in human subjects to a degree similar to that in animals, certain parallels can be drawn. Further, new imaging methods allow for assessment of localized metabolic changes in the human brain during and after EEG feedback training. Behaviorally, during successful SMR training, human subjects become behaviorally quiet and direct their attention to the task. It is safe to presume that the SMR response develops as a result of reduced motor excitation and resulting intrathalamic ventrobasal oscillations, since this mechanism is well established as a basis for mammalian sensorimotor EEG rhythm generation.12 These changes, as well as others documented in our animal studies, set the stage for the development of SMR activity and are likely collectively initiated by altered input from some other executive system.
These facts provide a rational model for a threshold-altering process that could affect seizure discharge propagation to motor networks. Although there are many different neurotransmitters used within the basal ganglia (principally acetylcholine, gamma-aminobutyric acid, and dopamine), the overall effect on thalamus and premotor networks in the mesencephalic tegmentum and superior colliculus is inhibitory.14–16 If activation of these inhibitory basal ganglia networks can become labeled by the SMR through contingent feedback training, and if responsible circuits can be potentiated by this association, motor inhibitory regulation would be generally facilitated.
CONCLUSIONS
Despite the encouraging findings and concepts reviewed here, there are significant issues at virtually every step of the thinking and practice behind this new therapy. This method depends on a comprehensive understanding of the EEG signal and the technical requirements of valid quantitative analysis and feedback applications. This includes a basic knowledge of the principles essential for effective operant conditioning. Further, in light of the complexity of seizure disorders, accurate history and seizure classification must be evaluated and understood.
Alternative explanations for therapeutic results include such considerations as short-lasting expectation effects and changes in patient behavior. However, it must again be noted that the prolonged anticonvulsant effect documented in our animal studies, as well as in relation to nocturnal seizures arising out of sleep in a human subject, would seem to rule out placebo or nonspecific effects. This conclusion is supported further by the finding of improved neuropsychological performance after SMR training in tasks mediated by the hemisphere contralateral to disrupting localized epileptogenic lesions. Additionally, an alternative explanation for improved seizure control based on increased medication compliance has been rejected through studies that carefully monitored blood levels of prescribed anticonvulsant drugs before, during, and after training.
Finally, the epileptic patients who have demonstrated clinical improvement in EEG biofeedback research studies, along with many who seek this treatment today, represent unquestionable failures of anticonvulsant drug therapy. Notably, positive outcomes have frequently been achieved in patients with complex-partial seizures, an extremely difficult-to-treat seizure type. It is therefore unfortunate that some professionals still criticize neurofeedback therapy for a lack of more consistent or successful outcomes. On the contrary, as noted here, evidence has shown that most of these difficult-to-treat patients benefit beyond any chance or placebo outcome, in some cases dramatically so. In light of the frequent adverse effects and costs associated with lifelong pharmacotherapy, we view EEG biofeedback therapy not as a “last resort” option to be restricted solely to pharmacotherapy-resistant cases but rather as a generally viable consideration for any patient suffering from seizures. Moreover, in contrast to drug-dependent management approaches, the altered modulation of striatal and thalamocortical inhibition that is possible through neurofeedback training may sufficiently raise seizure thresholds to greatly increase the prospects for the long-term nondependent management of epilepsy.
The attempt to alter electroencephalographic (EEG) frequency/amplitude patterns and their underlying brain mechanisms using contingent operant conditioning methods is today referred to variously as EEG biofeedback, neurofeedback, or neurotherapy. This article traces the history of the clinical application of EEG operant conditioning from empirical animal investigations to its emergence as a treatment option for major seizure types. In light of the diversity of the clinical applications of this method in general, and the complexity of seizure disorders in particular, I also present an overview of specific methods used in our EEG biofeedback program.
INITIAL APPLICATION IN HUMANS
BACKDROP TO THE CLINICAL APPLICATION: KEY ANIMAL STUDIES
To accomplish this, we attempted to facilitate the SMR during wakefulness using an operant conditioning paradigm with a liquid food reward, and then study any resulting changes in sleep spindle activity and sleep structure. Necessary quality controls included alternate training to suppress this rhythm and a counterbalanced design employing two separate groups of cats. Six weeks of three training sessions per week to satiation led to profound and differential changes in sleep EEG and sleep architecture. SMR training, whether it preceded or followed suppression training, led to a significant increase in EEG sleep spindle density, as well as a significant reduction in sleep period fragmentation due to arousals. No changes occurred in the control condition.3
A more profound finding in the cat
Platform for a dual research approach
These two studies provided several interesting conclusions that directed our subsequent scientific efforts. First, in the cat study we observed a common prodrome in both SMR-trained and control animals even though the SMR-trained animals had acquired protection against seizures. This suggested a direct effect on the seizure process and not on MMH toxicity in general. Second, in our human epileptic patient, the seizures that were suppressed arose out of the unconscious state of sleep, a fact that eliminated the possibility of any voluntary countermeasure and again indicated a direct effect on the seizure mechanism. Accordingly, we undertook a dual approach to understanding the basis of this effect, involving both additional animal electrophysiologic and human clinical studies.
Animal studies evaluated motor behavior, motor reflexes, motor and thalamic unit firing, and somatosensory pathway correlates of the SMR response. Clinical studies, as reviewed in the following section, sought to further document the anticonvulsant effects of SMR operant conditioning and examine this effect on various seizure types. Possible alternative explanations, such as altered medication compliance and placebo effects, were also addressed in several comprehensive studies. Additionally, by this time other laboratories were beginning to add to the research literature in this new field.
CLINICAL STUDIES
A series of human studies followed our initial clinical report, including group studies involving crossover and placebo-controlled designs. These studies consistently reported significant seizure reductions in epileptic patients in response to reward for increasing sensorimotor EEG rhythmic activity.
Two independent meta-analyses of the peer-reviewed papers in this literature have appeared in the last decade.8,10 In a review of 24 studies involving 243 patients with predominantly partial complex seizures provided with central cortical SMR feedback training, Sterman determined that 82% of these subjects registered seizure reductions greater than 50%.8 More recently, Tan and colleagues evaluated data from 63 studies and selected for comprehensive analysis 10 studies that met stringent criteria for controls and population and seizure details.10 They reported that 79% of the patients treated with SMR feedback training experienced a statistically significant reduction in seizure frequency despite a collective history of failed medication therapy.
- One group simply tabulated their seizure experiences for 6 weeks using a comprehensive logging method.
- The second group received EEG feedback training for 1 hour three times a week for 6 weeks; however, the EEG signal responsible for reward was previously recorded from a different individual. This noncontingent feedback constituted a “yoked control” group.
- The third group received 6 weeks of contingent training for increasing SMR activity in somatosensory cortex while simultaneously suppressing slower 4- to 8-Hz activity.
After the initial 6 weeks, all 24 subjects were combined into one group that received 6 more weeks of contingent training only. This was followed by a 4-week period of gradual withdrawal from training and then by a final tabulation of seizure incidence during a 6-week period after training was terminated. As can be seen in Figure 5, the seizure tabulation control was associated with an increased seizure count and the “yoked control” noncontingent SMR training was associated with no significant change in seizure incidence, whereas contingent SMR training was associated with a statistically significant reduction in seizures. The statistical significance of this reduction increased progressively as subjects from the other two groups were added to a second 6-week period of contingent training, and after an additional 6 weeks following withdrawal from training. In addition to this exclusive seizure reduction after SMR contingent training, pre-/post-training neuropsychological testing showed that responding SMR-trained subjects also improved significantly in performance of tasks specific to the hemisphere contralateral to their frontotemporal lesion, indicating a reduced corrosive disturbance from the seizure focus.11
EEG BIOFEEDBACK IN PRACTICE: PROFILE OF THE AUTHOR’S PROGRAM
EEG operant conditioning methods for biofeedback training have diversified as various hardware and software products have emerged and as individuals with differing backgrounds and credentials have entered the field. A lack of methodologic standards and professional regulations has contributed to an undesirable inconsistency in the competence and effectiveness of therapeutic applications. Nevertheless, abundant peer-reviewed research by qualified investigators has proven the worth of this method as a viable alternative treatment for seizure disorders, so I will attempt to provide some idea of a systematic and evidence-guided approach to treatment as used in our program.
With each box monitoring the same electrode site and each frequency tuned to the same band, thresholds can be set to promote facilitation or suppression through “successive approximation,” or sequencing from left to right with sequentially more difficult thresholds. Numerous other configurations are possible. In the case shown in Figure 7, the band-pass at the far left is set at 12 to 15 Hz (SMR) for the C3 electrode site, and the remaining three bands to the right are set to 3 to 5 Hz at the left medial frontal location Fz, with successively lower thresholds to promote suppression of this band at this site.
A RATIONAL MODEL FROM RECENT NEUROIMAGING STUDIES
While it is difficult to evaluate neurophysiologic changes in human subjects to a degree similar to that in animals, certain parallels can be drawn. Further, new imaging methods allow for assessment of localized metabolic changes in the human brain during and after EEG feedback training. Behaviorally, during successful SMR training, human subjects become behaviorally quiet and direct their attention to the task. It is safe to presume that the SMR response develops as a result of reduced motor excitation and resulting intrathalamic ventrobasal oscillations, since this mechanism is well established as a basis for mammalian sensorimotor EEG rhythm generation.12 These changes, as well as others documented in our animal studies, set the stage for the development of SMR activity and are likely collectively initiated by altered input from some other executive system.
These facts provide a rational model for a threshold-altering process that could affect seizure discharge propagation to motor networks. Although there are many different neurotransmitters used within the basal ganglia (principally acetylcholine, gamma-aminobutyric acid, and dopamine), the overall effect on thalamus and premotor networks in the mesencephalic tegmentum and superior colliculus is inhibitory.14–16 If activation of these inhibitory basal ganglia networks can become labeled by the SMR through contingent feedback training, and if responsible circuits can be potentiated by this association, motor inhibitory regulation would be generally facilitated.
CONCLUSIONS
Despite the encouraging findings and concepts reviewed here, there are significant issues at virtually every step of the thinking and practice behind this new therapy. This method depends on a comprehensive understanding of the EEG signal and the technical requirements of valid quantitative analysis and feedback applications. This includes a basic knowledge of the principles essential for effective operant conditioning. Further, in light of the complexity of seizure disorders, accurate history and seizure classification must be evaluated and understood.
Alternative explanations for therapeutic results include such considerations as short-lasting expectation effects and changes in patient behavior. However, it must again be noted that the prolonged anticonvulsant effect documented in our animal studies, as well as in relation to nocturnal seizures arising out of sleep in a human subject, would seem to rule out placebo or nonspecific effects. This conclusion is supported further by the finding of improved neuropsychological performance after SMR training in tasks mediated by the hemisphere contralateral to disrupting localized epileptogenic lesions. Additionally, an alternative explanation for improved seizure control based on increased medication compliance has been rejected through studies that carefully monitored blood levels of prescribed anticonvulsant drugs before, during, and after training.
Finally, the epileptic patients who have demonstrated clinical improvement in EEG biofeedback research studies, along with many who seek this treatment today, represent unquestionable failures of anticonvulsant drug therapy. Notably, positive outcomes have frequently been achieved in patients with complex-partial seizures, an extremely difficult-to-treat seizure type. It is therefore unfortunate that some professionals still criticize neurofeedback therapy for a lack of more consistent or successful outcomes. On the contrary, as noted here, evidence has shown that most of these difficult-to-treat patients benefit beyond any chance or placebo outcome, in some cases dramatically so. In light of the frequent adverse effects and costs associated with lifelong pharmacotherapy, we view EEG biofeedback therapy not as a “last resort” option to be restricted solely to pharmacotherapy-resistant cases but rather as a generally viable consideration for any patient suffering from seizures. Moreover, in contrast to drug-dependent management approaches, the altered modulation of striatal and thalamocortical inhibition that is possible through neurofeedback training may sufficiently raise seizure thresholds to greatly increase the prospects for the long-term nondependent management of epilepsy.
- Sterman MB, Friar L. Suppression of seizures in an epileptic following sensorimotor EEG feedback training. Electroencephalogr Clin Neurophysiol 1972; 33:89–95.
- Sterman MB. Effects of sensorimotor EEG feedback training on sleep and clinical manifestations of epilepsy. In:Beatty J, Legewie H, eds. Biofeedback and Behavior. New York, NY: Plenum Press; 1977:167–200.
- Sterman MB, Howe RD, Macdonald LR. Facilitation of spindle-burst sleep by conditioning of electroencephalographic activity while awake. Science 1970; 167:1146–1148.
- Sterman MB, LoPresti RW, Fairchild MD. Electroencephalographic and behavioral studies of monomethyl hydrazine toxicity in the cat. Aerospace Medical Research Laboratory 1969; AMRL-TR-69-3:1–8.
- Sterman MB. Studies of EEG biofeedback training in man and cats. In: Veterans Administration Studies in Mental Health and Behavioral Sciences: Highlights of the 17th Annual Conference. Perry Point, MD: US Veterans Administration; 1972:50–60.
- Sterman MB, Bowersox SS. Sensorimotor EEG rhythmic activity: a functional gate mechanism. Sleep 1981; 4:408–422.
- Sterman MB. Physiological origins and functional correlates of EEG rhythmic activities: implications for self-regulation. Biofeedack Self Reg 1996; 21:3–33.
- Sterman MB. Basic concepts and clinical findings in the treatment of seizure disorders with EEG operant conditioning. Clin Electroencephalogr 2000; 31:45–55.
- Egner T, Sterman MB. Neurofeedback treatment of epilepsy: from basic rationale to practical application. Expert Rev Neurother 2006; 6:247–257.
- Tan G, Thornby J, Hammond D, et al Meta-analysis of EEG biofeedback in treating epilepsy. Clin EEG Neurosci 2009; 40:173–179.
- Lantz D, Sterman MB. Neuropsychological assessment of subjects with uncontrolled epilepsy: effects of EEG biofeedback training. Epilepsia 1988; 29:163–171.
- Steriade M, McCormick DA, Seinowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 1993; 262:679–685.
- Lévesque J, Beauregard M, Mensour B. Effect of neurofeedback training on the neural substrates of selective attention in children with attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study. Neurosci Lett 2006; 394:216–221.
- Brodal P. The basal ganglia. In: The Central Nervous System: Structure and Function. New York, NY: Oxford University Press; 1992:246–261.
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci 1990; 13:277–280.
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 1990; 13:281–285.
- Sterman MB, Friar L. Suppression of seizures in an epileptic following sensorimotor EEG feedback training. Electroencephalogr Clin Neurophysiol 1972; 33:89–95.
- Sterman MB. Effects of sensorimotor EEG feedback training on sleep and clinical manifestations of epilepsy. In:Beatty J, Legewie H, eds. Biofeedback and Behavior. New York, NY: Plenum Press; 1977:167–200.
- Sterman MB, Howe RD, Macdonald LR. Facilitation of spindle-burst sleep by conditioning of electroencephalographic activity while awake. Science 1970; 167:1146–1148.
- Sterman MB, LoPresti RW, Fairchild MD. Electroencephalographic and behavioral studies of monomethyl hydrazine toxicity in the cat. Aerospace Medical Research Laboratory 1969; AMRL-TR-69-3:1–8.
- Sterman MB. Studies of EEG biofeedback training in man and cats. In: Veterans Administration Studies in Mental Health and Behavioral Sciences: Highlights of the 17th Annual Conference. Perry Point, MD: US Veterans Administration; 1972:50–60.
- Sterman MB, Bowersox SS. Sensorimotor EEG rhythmic activity: a functional gate mechanism. Sleep 1981; 4:408–422.
- Sterman MB. Physiological origins and functional correlates of EEG rhythmic activities: implications for self-regulation. Biofeedack Self Reg 1996; 21:3–33.
- Sterman MB. Basic concepts and clinical findings in the treatment of seizure disorders with EEG operant conditioning. Clin Electroencephalogr 2000; 31:45–55.
- Egner T, Sterman MB. Neurofeedback treatment of epilepsy: from basic rationale to practical application. Expert Rev Neurother 2006; 6:247–257.
- Tan G, Thornby J, Hammond D, et al Meta-analysis of EEG biofeedback in treating epilepsy. Clin EEG Neurosci 2009; 40:173–179.
- Lantz D, Sterman MB. Neuropsychological assessment of subjects with uncontrolled epilepsy: effects of EEG biofeedback training. Epilepsia 1988; 29:163–171.
- Steriade M, McCormick DA, Seinowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 1993; 262:679–685.
- Lévesque J, Beauregard M, Mensour B. Effect of neurofeedback training on the neural substrates of selective attention in children with attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study. Neurosci Lett 2006; 394:216–221.
- Brodal P. The basal ganglia. In: The Central Nervous System: Structure and Function. New York, NY: Oxford University Press; 1992:246–261.
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci 1990; 13:277–280.
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci 1990; 13:281–285.