User login
Many well-controlled trials in the past 4 years have evaluated new medications for treating bipolar disorder. It’s time to build a consensus on how this data may apply to clinical practice.
This year, our group will re-examine the Texas Medication Algorithm Project (TMAP) treatment algorithms for bipolar I disorder.
What makes TMAP unique? It is the first project to evaluate treatment algorithm use in community mental health settings for patients with a history of mania (see Box).1-5 Severely, persistently ill outpatients such as these are seldom included in research but are frequently seen in clinical practice.
To preview for psychiatrists the changes expected in 2004, this article describes the goals of TMAP and the controlled study on which the medication algorithms are based. We review the medication algorithms of 2000 as a starting point and present the evidence that is changing clinical practice.
Guiding principles of TMAP
A treatment algorithm is no substitute for clinical judgment; rather, medication guidelines and algorithms are guideposts to help the clinician and patient collaboratively develop the most effective medication strategy with the fewest side effects.
The Texas Medication Algorithm Project (TMAP)1-3 is a public and academic collaboration started in 1996 to develop evidence- and consensus-based medication treatment algorithms for schizophrenia, major depressive disorder, and bipolar disorder.
TMAP’s goal is to establish “best practices” to encourage uniformity of care, achieve the best possible patient outcomes, and use mental health care dollars most efficiently. The project includes four phases, in which the treatment algorithms were developed, compared with treatment-as-usual, put into practice, and will undergo periodic updates.4 The next update begins this year.
The comparison of algorithms for treating bipolar mania/hypomania and depression included 409 patients (mean age 38 to 40) with bipolar I disorder or schizoaffective disorder, bipolar type. These patients were severely and persistently mentally ill, from a diverse ethnic population, and significantly impaired in functioning.
During 12 months of treatment, psychiatric symptoms diminished more rapidly in patients in the algorithm group—as measured by the Brief Psychiatric Rating Scale (BPRS-24)—compared with those receiving usual treatment. After the first 3 months, the usual-treatment patients also showed diminished symptoms. At study’s end, symptom severity between the groups was not significantly different; both groups showed improvement.
Manic and psychotic symptoms—measured by Clinician-Administered Rating Scale subscales (CARS-M)5—improved significantly more in the algorithm group in the first 3 months, and this gap between the two groups was sustained for 12 months. Depressive symptoms declined, but no overall differences were noted between the two groups. Side effect rates and functioning were also similar.
TMAP’s treatment manual (see Related resources) describes clinicians’ preferred tactics and decision points, which we summarize here. The guidelines are an ongoing effort to apply evidence-based medicine to everyday practice and are meant to be adapted to patient needs.
Treatment goals that guided TMAP algorithm development are:
- symptomatic remission
- full return of psychosocial functioning
- prevention of relapse and recurrence.
Suggestions came from controlled clinical trials, open trials, retrospective data analyses, expert clinical consensus, and input from consumers.
Treatment selection. Initial algorithm stages recommend simple treatments (in terms of safety, tolerability, and side effects), whereas later stages recommend more-complicated regimens. A patient’s symptoms, comorbid conditions, and treatment history guide treatment selection. Patients may enter an algorithm at any stage, depending on their clinical presentation and medication history.
The clinician may consider patient preference when deciding among equivalent medications. The algorithm strongly encourages patients and families to participate, such as by keeping daily mood charts and completing symptom and side-effect checklists. When clinicians face a choice among medication brands, generics, or forms (such as immediate- versus slow-release), agents with greater tolerability are preferred.
Patient management. When patients enter the algorithm, clinic visits are frequent (such as every 2 weeks). Follow-up appointments address medication adherence, dosage adjustments, and side effects or adverse reactions.
If a patient’s symptoms show no change after two treatment stages, re-evaluate the diagnosis and consider mitigating factors such as substance abuse. Patients who complete acute treatment should receive continuation treatment.
Documentation. Clinicians are advised to document decision points and the rationale for treatment choices made outside the algorithm package.
Treating mania or hypomania
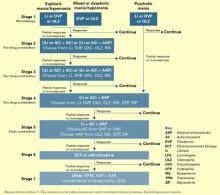
After clinical evaluation confirms the diagnosis of bipolar illness,4 the TMAP mania/hypomania algorithm (Algorithm 1) splits into three treatment pathways:
- euphoric mania/hypomania
- mixed or dysphoric mania/hypomania
- psychotic mania.
These pathways recognize the need for differing approaches to initial monotherapy and later two-drug combinations. If a patient develops persistent or severe depressive symptoms, the bipolar algorithm for a major depressive episode (Algorithm 2) is used during depressive periods with the primary mania algorithm.
Treatment recommendations. The key to using mood stabilizers is to achieve the optimum response—assuming good tolerability—before switching to another agent. Adjust medication dosages one at a time to allow adequate response and assessment.
When switching medications, use an overlap-and-taper strategy, assuming there is no medical necessity to stop a drug abruptly. Add the new medication, then gradually taper the one that is being discontinued. Monitor serum levels.
Discontinue antidepressants when appropriate in patients with hypomania/mania or rapid cycling, and continually evaluate suicide and homicide potential of patients in mixed or depressive states.
Stage 1: Monotherapy. First medication choices are lithium, divalproex, or olanzapine. For mixed or dysphoric mania, the algorithm recommends divalproex (preferred over valproic acid because of tolerability and side effects) or olanzapine.6 Data suggest dysphoric manic patients are less likely to respond to lithium.7 A Consensus Panel minority expressed concern about using olanzapine as first-line monotherapy for acute mania because of limited data on the drug’s long-term safety. Patients with partial response or residual symptoms may move to stage 2 or switch to other medication options within stage 1.
Patients with psychotic mania move directly to stage 4 for a broader range of combination therapy.
Stage 2: Combination therapy. Combination therapy has become the standard of care in treating most patients with bipolar disorder. The algorithm recommends using two agents:
- lithium or an anticonvulsant plus another anticonvulsant ([Li or AC]+AC)
- or lithium or an anticonvulsant plus an atypical antipsychotic ([Li or AC]+AAP).8
Recommended agents include lithium, divalproex, oxcarbazepine, olanzapine, or risperidone. The experts recommended oxcarbazepine as first choice because it is better tolerated and interacts with fewer drugs than carbamazepine and does not require serum level monitoring.9
A Consensus Panel minority expressed concern that few studies had examined using oxcarbazepine in bipolar disorder. Carbamazepine was also considered an option.
Stages 3 and 4: Other two-drug combinations. Other two-drug combinations are tried at these stages, drawing from the same pool of medication classes described in stage 2.
Stage 4 broadens the choice of atypical antipsychotic by adding quetiapine10 and ziprasidone11 to the recommended stage-2 agents olanzapine and risperidone. When the 2000 algorithm was developed, limited data were available on using some newer atypicals in patients with bipolar mania. Based on recent, high-quality studies of mono- and combination therapy—including quetiapine,10 ziprasidone,11 risperidone,12,13 and aripiprazole14 —the 2004 algorithm update panel will likely recommend using atypicals earlier, including at stage 1.
Algorithm 1 Treating mania/hypomania in patients with bipolar I disorder
Stage 5: Triple-drug combination. Lithium, an anticonvulsant (divalproex or oxcarbazepine), and an atypical antipsychotic (olanzapine, risperidone, quetiapine, or ziprasidone) is a recommended triple-drug combination. In the 2004 update, the choices will likely expand to include all the newer atypicals and will list carbamazepine as an option.
Stage 6: ECT or clozapine. For patients with inadequate response to triple-drug combinations, the algorithm recommends adding electroconvulsive therapy (ECT) or clozapine.
ECT15 is recommended three times a week until the patient achieves remission of manic symptoms or fails to achieve a sustained response over three to six treatment cycles. Treatment resistance is declared if no response is seen after 6 to 10 treatment cycles.
Clozapine’s16 recommendation at this stage is consistent with its use in patients who fail to respond to other atypical antipsychotics. Blood monitoring for agranulocytosis is required; other adverse effects include an increased risk of seizures, myocarditis, and orthostatic hypotension.
Stage 7: Other. Treatment options such as topiramate17,18 and lamotrigine19 are recommended at this stage. These recommendations also will be reviewed and likely revised.
Treating bipolar depression
The TMAP algorithm for treating depression in bipolar disorder (Algorithm 2) assumes that anti-depressants will be used only with optimum mood-stabilizer levels because of the risk of inducing manic symptoms. The bipolar depression algorithm is always used with the primary algorithm for mania/hypomania.
The patient’s clinical presentation guides medication selection. For the “pure” bipolar I patient with a major depressive episode but little mood lability or hypomania, starting an antide-pressant is a clear decision. On the other hand, patients with predominant depressive symptoms plus dysphoric hypomania, mood lability, and irritability need a balance of mood-stabilizing drugs and antidepressants.
Stage 1: Mood stabilizer. Initiate a mood stabilizer and optimize the dosage. Choices are the same mood stabilizers listed in the hypomania/mania treatment algorithm.
Stage 2: Antidepressant. Adding an antidepressant implies that depressive symptoms are severe enough to change treatment. Antidepressant options include a selective serotonin reuptake inhibitor (SSRI), sustained-release bupropion, or lamotrigine.20
Using SSRIs is supported by widespread clinical experience and offers the convenience of once-daily dosing. Recommended SSRIs include fluoxetine, paroxetine, fluvoxamine, sertraline, and citalopram. The SSRI escitalopram was introduced after the 2000 algorithms were published; evidence for using it and other newer medications will be reviewed for the 2004 update.
The recommendation for sustained-release bupropion is consistent with the algorithm principle to use medications in the most well-tolerated form when accessible and available.
With lamotrigine, review with patients the risk of serious rash. To minimize rash risk, start lamotrigine slowly and follow the recommended titration schedule.
Stage 3: Multiple choices. At this stage, no definitive studies, safety data, or tolerability issues are available to rank the medication choices. The algorithm suggests:
- adding lithium21 or a second antidepressant
- or switching to an alternate antidepressant such as venlafaxine or nefazodone.
If a patient moves to stage 3 because of side effects with one antidepressant class, a different class—preferably with a contrasting side-effect profile—is recommended.
Algorithm 2 Treating depression in bipolar I disorder*
Stage 4: Two antidepressants. To enhance clinical response, the algorithm recommends combining two antidepressants, preferably from different classes. Monitor patients closely for side effects.
Stage 5. Antipsychotic or MAOI. At this stage, the algorithm recommends adding an atypical antipsychotic22 or switching to a monoamine oxidase inhibitor (MAOI).
Early evidence supported the efficacy of MAOIs in bipolar depression. However, the panel ranked MAOIs lower in the algorithm because they are associated with more bothersome side effects than SSRIs and other antidepressants. When using MAOIs, provide patients with dietary restriction guidelines.
Stage 6. Other therapies. Therapies such as ECT or “other” interventions are recommended at this stage. ECT has proven efficacy in bipolar depression and is appropriate for patients with limited medication response. The panel gave ECT a low ranking because of limited availability, lack of patient acceptance, and newer options.
Medication options include experimental treatments with limited evidence, such as inositol, dopamine agonists, stimulants, thyroid supplementation, conventional antipsychotics, and tri-cyclic antidepressants.
Acute to maintenance treatment
Adjunctive treatments for agitation, insomnia, GI upset, sedation, headache, and tremor are recommended in the physician manual supporting the TMAP guidelines (see Related resources). The manual also suggests ways to manage medication side effects and modify the algorithms for inpatients.
Patient and family psychoeducation plays an important role in helping the patient:
- identify prodromal bipolar symptoms
- understand the need to take medications as prescribed.
Continuation treatment. After mania or hypomania remits, continue medication(s) at the effective acute-phase dosages for at least 3 months. Use follow-up visits to enhance patient adherence, detect early symptoms of relapse, and monitor for side effects.
During the late continuation phase, after a period of sustained stability, clinicians can try to simplify the medications. When discontinuing a medication, taper the dosage by no more than 25% per week. If symptoms recur, promptly return to acute-phase treatment. Consider restarting medications and titrating up to the dosage(s) that resulted in remission.
In a depressive episode, continue the antidepressant(s) for 1 to 3 months at the effective acutephase dosage(s). Follow up frequently, and educate patients to watch for symptom recurrence and to communicate with you to assess when medication changes are needed.
Maintenance treatment. Relatively few well-controlled studies on long-term management of bipolar patients were available for the 2000 algorithm update.23 In general, all patients need mood stabilizer(s) to prevent relapse, using the lowest dosage that maintains therapeutic efficacy. Based on new evidence for lamotrigine and atypical antipsychotics—including FDAapproval of olanzapine for bipolar maintenace therapy—we anticipate recommendations will be expanded and more delineated in the 2004 update.
Discontinuing antidepressants after 3 to 6 months of initial treatment is now recommended. However, a recent retrospective case series suggests that continuing antidepressants at least 1 year after initial successful therapy may protect against depressive relapse. During this study, continuing antidepressants more than 3 to 6 months did not appear to increase the risk of switching to mania.24
Should antidepressants be continued or discontinued after successful acute treatment of a bipolar I depressive episode? This is an active area of research and debate as to the most appropriate strategy. The 2004 algorithm update panel will consider recent evidence that supports continuing antidepressants after symptom remission.24
- Texas Medication Algorithm Project algorithms and physician manual. Texas Department of Mental Health and Mental Retardation. http://www.mhmr.state.tx.us/centraloffice/medicaldirector/TIMA.html
- American Psychiatric Association. Practice guidelines for the treatment of patients with bipolar disorder. Am J Psychiatry 2002; 159(4):suppl 2.
- Depression and Bipolar Support Alliance. www.dbsalliance.org
Drug brand names
- Bupropion • Wellbutrin SR
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Inositol • Various
- Lamotrigine • Lamictal
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Topiramate • Topamax
- Tranylcypromine • Parnate
- Valproic acid • Depakene
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
Dr. Shivakumar reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Suppes receives research support from or is a consultant to Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Co., GlaxoSmithKline, Janssen Pharmaceutica, Johnson & Johnson, National Institutes of Mental Health, Novartis Pharmaceuticals Corp., Pfizer Inc., Pharmaceutical Research Institute, Ortho-McNeil Pharmaceutical, Robert Wood Johnson Pharmaceutical Research Institute, The Stanley Medical Research Institute, and UCB Pharma.
1. Gilbert DA, Altshuler KZ, Rago WV, et al. Texas Medication Algorithm Project: definitions, rationale, and methods to develop medication algorithms. J Clin Psychiatry 1998;59:345-51.
2. Altshuler KZ, Rush AJ. Computerized Texas Medication Algorithm Project undergoes testing. Outcomes Accountability Alert 1999;4(1):10-11.
3. Rush AJ, Crismon ML, Kashner TM, et al. Texas Medication Algorithm Project, Phase 3 (TMAP-3): rationale and study design. J Clin Psychiatry 2003;64(4):357-69.
4. Altman E, Hedeker D, Janicak P, et al. The Clinician-Administered Rating Scale For Mania (CARS-M): development, reliability, and validity. Biol Psychiatry 1994;36:124-34.
5. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas consensus conference panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry 2002;63:288-99.
6. Dennehy EB, Doyle K, Suppes T. The efficacy of olanzapine monotherapy for acute hypomania or mania in an outpatient setting. Int Clin Psychopharmacol 2003;18(3):143-5.
7. Dilsaver SC, Swann AC, Shoaib AM, et al. The manic syndrome: factors which may predict a patient’s response to lithium, carbamazepine and valproate. J Psych Neurosci 1993;18:61-6.
8. Tohen M, Chengappa KNR, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59(1):62-9.
9. Emrich HM. Studies with oxcarbazepine (Trileptal) in acute mania. Int Clin Psychopharmacol 1990;190(5,suppl):83-8.
10. Vieta E, Parramon G, Padrell E, et al. Quetiapine in the treatment of rapid cycling bipolar disorder. Bipolar Disord 2002;4(5):335-40.
11. Keck PE, Versiani M, Potkin S, et al. Ziprasidone in the treatment of acute bipolar mania: a three week placebo-controlled, double-blinded randomized trial. Am J Psychiatry 2003;160(4):741-8.
12. Yatham LN, Grossman F, Augustyns I, et al. Mood stabilisers plus risperidone or placebo in the treatment of acute mania. International, double-blinded, randomised controlled trial. Br J Psychiatry 2003;182:141-7.
13. Sachs GS, Grossman F, Ghaemi SN, et al. Combination of a mood stabilizer with risperidone or haloperidol for treatment of acute mania: a double-blind, placebo-controlled comparison of efficacy and safety. Am J Psychiatry 2002;159(7):1146-54.
14. Keck PE, Jr, Marcus R, Tourkodimitris S, et al. Aripiprazole study group. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160(9):1651-8.
15. Mukherjee S, Sackeim HA, Schnur DB. Electroconvulsive therapy of acute manic episode: a review of 50 years’ experience. Am J Psychiatry 1988;45:727-32.
16. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trail of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry 1999;156(8):1164-9.
17. Guille C, Sachs G. Clinical outcome of adjunctive topiramate treatment in a sample of refractory bipolar patients with comorbid conditions. Prog Neuropsychopharmacol Biol Psychiatry 2002;26(6):1035-9.
18. Vieta E, Torrent C, Garcia-Ribas G, et al. Use of topiramate in treatment-resistant bipolar spectrum disorders. J Clin Psychopharmacol 2002;22(4):431-5.
19. Calabrese JR, Suppes T, Bowden CL, et al. A double blinded, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. J Clin Psychiatry 2000;61:841-50.
20. Calabrese JR, Bowden CL, Sachs GS, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry 2003;64(9):1013-24.
21. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacology 1999;19:427-34.
22. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
23. Baldessarini RJ, Tohen M, Tondo L. Maintenance treatment in bipolar disorder (comment). Arch Gen Psychiatry 2000;57:490-2.
24. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 2003;160:1252-62.
Many well-controlled trials in the past 4 years have evaluated new medications for treating bipolar disorder. It’s time to build a consensus on how this data may apply to clinical practice.
This year, our group will re-examine the Texas Medication Algorithm Project (TMAP) treatment algorithms for bipolar I disorder.
What makes TMAP unique? It is the first project to evaluate treatment algorithm use in community mental health settings for patients with a history of mania (see Box).1-5 Severely, persistently ill outpatients such as these are seldom included in research but are frequently seen in clinical practice.
To preview for psychiatrists the changes expected in 2004, this article describes the goals of TMAP and the controlled study on which the medication algorithms are based. We review the medication algorithms of 2000 as a starting point and present the evidence that is changing clinical practice.
Guiding principles of TMAP
A treatment algorithm is no substitute for clinical judgment; rather, medication guidelines and algorithms are guideposts to help the clinician and patient collaboratively develop the most effective medication strategy with the fewest side effects.
The Texas Medication Algorithm Project (TMAP)1-3 is a public and academic collaboration started in 1996 to develop evidence- and consensus-based medication treatment algorithms for schizophrenia, major depressive disorder, and bipolar disorder.
TMAP’s goal is to establish “best practices” to encourage uniformity of care, achieve the best possible patient outcomes, and use mental health care dollars most efficiently. The project includes four phases, in which the treatment algorithms were developed, compared with treatment-as-usual, put into practice, and will undergo periodic updates.4 The next update begins this year.
The comparison of algorithms for treating bipolar mania/hypomania and depression included 409 patients (mean age 38 to 40) with bipolar I disorder or schizoaffective disorder, bipolar type. These patients were severely and persistently mentally ill, from a diverse ethnic population, and significantly impaired in functioning.
During 12 months of treatment, psychiatric symptoms diminished more rapidly in patients in the algorithm group—as measured by the Brief Psychiatric Rating Scale (BPRS-24)—compared with those receiving usual treatment. After the first 3 months, the usual-treatment patients also showed diminished symptoms. At study’s end, symptom severity between the groups was not significantly different; both groups showed improvement.
Manic and psychotic symptoms—measured by Clinician-Administered Rating Scale subscales (CARS-M)5—improved significantly more in the algorithm group in the first 3 months, and this gap between the two groups was sustained for 12 months. Depressive symptoms declined, but no overall differences were noted between the two groups. Side effect rates and functioning were also similar.
TMAP’s treatment manual (see Related resources) describes clinicians’ preferred tactics and decision points, which we summarize here. The guidelines are an ongoing effort to apply evidence-based medicine to everyday practice and are meant to be adapted to patient needs.
Treatment goals that guided TMAP algorithm development are:
- symptomatic remission
- full return of psychosocial functioning
- prevention of relapse and recurrence.
Suggestions came from controlled clinical trials, open trials, retrospective data analyses, expert clinical consensus, and input from consumers.
Treatment selection. Initial algorithm stages recommend simple treatments (in terms of safety, tolerability, and side effects), whereas later stages recommend more-complicated regimens. A patient’s symptoms, comorbid conditions, and treatment history guide treatment selection. Patients may enter an algorithm at any stage, depending on their clinical presentation and medication history.
The clinician may consider patient preference when deciding among equivalent medications. The algorithm strongly encourages patients and families to participate, such as by keeping daily mood charts and completing symptom and side-effect checklists. When clinicians face a choice among medication brands, generics, or forms (such as immediate- versus slow-release), agents with greater tolerability are preferred.
Patient management. When patients enter the algorithm, clinic visits are frequent (such as every 2 weeks). Follow-up appointments address medication adherence, dosage adjustments, and side effects or adverse reactions.
If a patient’s symptoms show no change after two treatment stages, re-evaluate the diagnosis and consider mitigating factors such as substance abuse. Patients who complete acute treatment should receive continuation treatment.
Documentation. Clinicians are advised to document decision points and the rationale for treatment choices made outside the algorithm package.
Treating mania or hypomania
After clinical evaluation confirms the diagnosis of bipolar illness,4 the TMAP mania/hypomania algorithm (Algorithm 1) splits into three treatment pathways:
- euphoric mania/hypomania
- mixed or dysphoric mania/hypomania
- psychotic mania.
These pathways recognize the need for differing approaches to initial monotherapy and later two-drug combinations. If a patient develops persistent or severe depressive symptoms, the bipolar algorithm for a major depressive episode (Algorithm 2) is used during depressive periods with the primary mania algorithm.
Treatment recommendations. The key to using mood stabilizers is to achieve the optimum response—assuming good tolerability—before switching to another agent. Adjust medication dosages one at a time to allow adequate response and assessment.
When switching medications, use an overlap-and-taper strategy, assuming there is no medical necessity to stop a drug abruptly. Add the new medication, then gradually taper the one that is being discontinued. Monitor serum levels.
Discontinue antidepressants when appropriate in patients with hypomania/mania or rapid cycling, and continually evaluate suicide and homicide potential of patients in mixed or depressive states.
Stage 1: Monotherapy. First medication choices are lithium, divalproex, or olanzapine. For mixed or dysphoric mania, the algorithm recommends divalproex (preferred over valproic acid because of tolerability and side effects) or olanzapine.6 Data suggest dysphoric manic patients are less likely to respond to lithium.7 A Consensus Panel minority expressed concern about using olanzapine as first-line monotherapy for acute mania because of limited data on the drug’s long-term safety. Patients with partial response or residual symptoms may move to stage 2 or switch to other medication options within stage 1.
Patients with psychotic mania move directly to stage 4 for a broader range of combination therapy.
Stage 2: Combination therapy. Combination therapy has become the standard of care in treating most patients with bipolar disorder. The algorithm recommends using two agents:
- lithium or an anticonvulsant plus another anticonvulsant ([Li or AC]+AC)
- or lithium or an anticonvulsant plus an atypical antipsychotic ([Li or AC]+AAP).8
Recommended agents include lithium, divalproex, oxcarbazepine, olanzapine, or risperidone. The experts recommended oxcarbazepine as first choice because it is better tolerated and interacts with fewer drugs than carbamazepine and does not require serum level monitoring.9
A Consensus Panel minority expressed concern that few studies had examined using oxcarbazepine in bipolar disorder. Carbamazepine was also considered an option.
Stages 3 and 4: Other two-drug combinations. Other two-drug combinations are tried at these stages, drawing from the same pool of medication classes described in stage 2.
Stage 4 broadens the choice of atypical antipsychotic by adding quetiapine10 and ziprasidone11 to the recommended stage-2 agents olanzapine and risperidone. When the 2000 algorithm was developed, limited data were available on using some newer atypicals in patients with bipolar mania. Based on recent, high-quality studies of mono- and combination therapy—including quetiapine,10 ziprasidone,11 risperidone,12,13 and aripiprazole14 —the 2004 algorithm update panel will likely recommend using atypicals earlier, including at stage 1.
Algorithm 1 Treating mania/hypomania in patients with bipolar I disorder
Stage 5: Triple-drug combination. Lithium, an anticonvulsant (divalproex or oxcarbazepine), and an atypical antipsychotic (olanzapine, risperidone, quetiapine, or ziprasidone) is a recommended triple-drug combination. In the 2004 update, the choices will likely expand to include all the newer atypicals and will list carbamazepine as an option.
Stage 6: ECT or clozapine. For patients with inadequate response to triple-drug combinations, the algorithm recommends adding electroconvulsive therapy (ECT) or clozapine.
ECT15 is recommended three times a week until the patient achieves remission of manic symptoms or fails to achieve a sustained response over three to six treatment cycles. Treatment resistance is declared if no response is seen after 6 to 10 treatment cycles.
Clozapine’s16 recommendation at this stage is consistent with its use in patients who fail to respond to other atypical antipsychotics. Blood monitoring for agranulocytosis is required; other adverse effects include an increased risk of seizures, myocarditis, and orthostatic hypotension.
Stage 7: Other. Treatment options such as topiramate17,18 and lamotrigine19 are recommended at this stage. These recommendations also will be reviewed and likely revised.
Treating bipolar depression
The TMAP algorithm for treating depression in bipolar disorder (Algorithm 2) assumes that anti-depressants will be used only with optimum mood-stabilizer levels because of the risk of inducing manic symptoms. The bipolar depression algorithm is always used with the primary algorithm for mania/hypomania.
The patient’s clinical presentation guides medication selection. For the “pure” bipolar I patient with a major depressive episode but little mood lability or hypomania, starting an antide-pressant is a clear decision. On the other hand, patients with predominant depressive symptoms plus dysphoric hypomania, mood lability, and irritability need a balance of mood-stabilizing drugs and antidepressants.
Stage 1: Mood stabilizer. Initiate a mood stabilizer and optimize the dosage. Choices are the same mood stabilizers listed in the hypomania/mania treatment algorithm.
Stage 2: Antidepressant. Adding an antidepressant implies that depressive symptoms are severe enough to change treatment. Antidepressant options include a selective serotonin reuptake inhibitor (SSRI), sustained-release bupropion, or lamotrigine.20
Using SSRIs is supported by widespread clinical experience and offers the convenience of once-daily dosing. Recommended SSRIs include fluoxetine, paroxetine, fluvoxamine, sertraline, and citalopram. The SSRI escitalopram was introduced after the 2000 algorithms were published; evidence for using it and other newer medications will be reviewed for the 2004 update.
The recommendation for sustained-release bupropion is consistent with the algorithm principle to use medications in the most well-tolerated form when accessible and available.
With lamotrigine, review with patients the risk of serious rash. To minimize rash risk, start lamotrigine slowly and follow the recommended titration schedule.
Stage 3: Multiple choices. At this stage, no definitive studies, safety data, or tolerability issues are available to rank the medication choices. The algorithm suggests:
- adding lithium21 or a second antidepressant
- or switching to an alternate antidepressant such as venlafaxine or nefazodone.
If a patient moves to stage 3 because of side effects with one antidepressant class, a different class—preferably with a contrasting side-effect profile—is recommended.
Algorithm 2 Treating depression in bipolar I disorder*
Stage 4: Two antidepressants. To enhance clinical response, the algorithm recommends combining two antidepressants, preferably from different classes. Monitor patients closely for side effects.
Stage 5. Antipsychotic or MAOI. At this stage, the algorithm recommends adding an atypical antipsychotic22 or switching to a monoamine oxidase inhibitor (MAOI).
Early evidence supported the efficacy of MAOIs in bipolar depression. However, the panel ranked MAOIs lower in the algorithm because they are associated with more bothersome side effects than SSRIs and other antidepressants. When using MAOIs, provide patients with dietary restriction guidelines.
Stage 6. Other therapies. Therapies such as ECT or “other” interventions are recommended at this stage. ECT has proven efficacy in bipolar depression and is appropriate for patients with limited medication response. The panel gave ECT a low ranking because of limited availability, lack of patient acceptance, and newer options.
Medication options include experimental treatments with limited evidence, such as inositol, dopamine agonists, stimulants, thyroid supplementation, conventional antipsychotics, and tri-cyclic antidepressants.
Acute to maintenance treatment
Adjunctive treatments for agitation, insomnia, GI upset, sedation, headache, and tremor are recommended in the physician manual supporting the TMAP guidelines (see Related resources). The manual also suggests ways to manage medication side effects and modify the algorithms for inpatients.
Patient and family psychoeducation plays an important role in helping the patient:
- identify prodromal bipolar symptoms
- understand the need to take medications as prescribed.
Continuation treatment. After mania or hypomania remits, continue medication(s) at the effective acute-phase dosages for at least 3 months. Use follow-up visits to enhance patient adherence, detect early symptoms of relapse, and monitor for side effects.
During the late continuation phase, after a period of sustained stability, clinicians can try to simplify the medications. When discontinuing a medication, taper the dosage by no more than 25% per week. If symptoms recur, promptly return to acute-phase treatment. Consider restarting medications and titrating up to the dosage(s) that resulted in remission.
In a depressive episode, continue the antidepressant(s) for 1 to 3 months at the effective acutephase dosage(s). Follow up frequently, and educate patients to watch for symptom recurrence and to communicate with you to assess when medication changes are needed.
Maintenance treatment. Relatively few well-controlled studies on long-term management of bipolar patients were available for the 2000 algorithm update.23 In general, all patients need mood stabilizer(s) to prevent relapse, using the lowest dosage that maintains therapeutic efficacy. Based on new evidence for lamotrigine and atypical antipsychotics—including FDAapproval of olanzapine for bipolar maintenace therapy—we anticipate recommendations will be expanded and more delineated in the 2004 update.
Discontinuing antidepressants after 3 to 6 months of initial treatment is now recommended. However, a recent retrospective case series suggests that continuing antidepressants at least 1 year after initial successful therapy may protect against depressive relapse. During this study, continuing antidepressants more than 3 to 6 months did not appear to increase the risk of switching to mania.24
Should antidepressants be continued or discontinued after successful acute treatment of a bipolar I depressive episode? This is an active area of research and debate as to the most appropriate strategy. The 2004 algorithm update panel will consider recent evidence that supports continuing antidepressants after symptom remission.24
- Texas Medication Algorithm Project algorithms and physician manual. Texas Department of Mental Health and Mental Retardation. http://www.mhmr.state.tx.us/centraloffice/medicaldirector/TIMA.html
- American Psychiatric Association. Practice guidelines for the treatment of patients with bipolar disorder. Am J Psychiatry 2002; 159(4):suppl 2.
- Depression and Bipolar Support Alliance. www.dbsalliance.org
Drug brand names
- Bupropion • Wellbutrin SR
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Inositol • Various
- Lamotrigine • Lamictal
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Topiramate • Topamax
- Tranylcypromine • Parnate
- Valproic acid • Depakene
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
Dr. Shivakumar reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Suppes receives research support from or is a consultant to Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Co., GlaxoSmithKline, Janssen Pharmaceutica, Johnson & Johnson, National Institutes of Mental Health, Novartis Pharmaceuticals Corp., Pfizer Inc., Pharmaceutical Research Institute, Ortho-McNeil Pharmaceutical, Robert Wood Johnson Pharmaceutical Research Institute, The Stanley Medical Research Institute, and UCB Pharma.
Many well-controlled trials in the past 4 years have evaluated new medications for treating bipolar disorder. It’s time to build a consensus on how this data may apply to clinical practice.
This year, our group will re-examine the Texas Medication Algorithm Project (TMAP) treatment algorithms for bipolar I disorder.
What makes TMAP unique? It is the first project to evaluate treatment algorithm use in community mental health settings for patients with a history of mania (see Box).1-5 Severely, persistently ill outpatients such as these are seldom included in research but are frequently seen in clinical practice.
To preview for psychiatrists the changes expected in 2004, this article describes the goals of TMAP and the controlled study on which the medication algorithms are based. We review the medication algorithms of 2000 as a starting point and present the evidence that is changing clinical practice.
Guiding principles of TMAP
A treatment algorithm is no substitute for clinical judgment; rather, medication guidelines and algorithms are guideposts to help the clinician and patient collaboratively develop the most effective medication strategy with the fewest side effects.
The Texas Medication Algorithm Project (TMAP)1-3 is a public and academic collaboration started in 1996 to develop evidence- and consensus-based medication treatment algorithms for schizophrenia, major depressive disorder, and bipolar disorder.
TMAP’s goal is to establish “best practices” to encourage uniformity of care, achieve the best possible patient outcomes, and use mental health care dollars most efficiently. The project includes four phases, in which the treatment algorithms were developed, compared with treatment-as-usual, put into practice, and will undergo periodic updates.4 The next update begins this year.
The comparison of algorithms for treating bipolar mania/hypomania and depression included 409 patients (mean age 38 to 40) with bipolar I disorder or schizoaffective disorder, bipolar type. These patients were severely and persistently mentally ill, from a diverse ethnic population, and significantly impaired in functioning.
During 12 months of treatment, psychiatric symptoms diminished more rapidly in patients in the algorithm group—as measured by the Brief Psychiatric Rating Scale (BPRS-24)—compared with those receiving usual treatment. After the first 3 months, the usual-treatment patients also showed diminished symptoms. At study’s end, symptom severity between the groups was not significantly different; both groups showed improvement.
Manic and psychotic symptoms—measured by Clinician-Administered Rating Scale subscales (CARS-M)5—improved significantly more in the algorithm group in the first 3 months, and this gap between the two groups was sustained for 12 months. Depressive symptoms declined, but no overall differences were noted between the two groups. Side effect rates and functioning were also similar.
TMAP’s treatment manual (see Related resources) describes clinicians’ preferred tactics and decision points, which we summarize here. The guidelines are an ongoing effort to apply evidence-based medicine to everyday practice and are meant to be adapted to patient needs.
Treatment goals that guided TMAP algorithm development are:
- symptomatic remission
- full return of psychosocial functioning
- prevention of relapse and recurrence.
Suggestions came from controlled clinical trials, open trials, retrospective data analyses, expert clinical consensus, and input from consumers.
Treatment selection. Initial algorithm stages recommend simple treatments (in terms of safety, tolerability, and side effects), whereas later stages recommend more-complicated regimens. A patient’s symptoms, comorbid conditions, and treatment history guide treatment selection. Patients may enter an algorithm at any stage, depending on their clinical presentation and medication history.
The clinician may consider patient preference when deciding among equivalent medications. The algorithm strongly encourages patients and families to participate, such as by keeping daily mood charts and completing symptom and side-effect checklists. When clinicians face a choice among medication brands, generics, or forms (such as immediate- versus slow-release), agents with greater tolerability are preferred.
Patient management. When patients enter the algorithm, clinic visits are frequent (such as every 2 weeks). Follow-up appointments address medication adherence, dosage adjustments, and side effects or adverse reactions.
If a patient’s symptoms show no change after two treatment stages, re-evaluate the diagnosis and consider mitigating factors such as substance abuse. Patients who complete acute treatment should receive continuation treatment.
Documentation. Clinicians are advised to document decision points and the rationale for treatment choices made outside the algorithm package.
Treating mania or hypomania
After clinical evaluation confirms the diagnosis of bipolar illness,4 the TMAP mania/hypomania algorithm (Algorithm 1) splits into three treatment pathways:
- euphoric mania/hypomania
- mixed or dysphoric mania/hypomania
- psychotic mania.
These pathways recognize the need for differing approaches to initial monotherapy and later two-drug combinations. If a patient develops persistent or severe depressive symptoms, the bipolar algorithm for a major depressive episode (Algorithm 2) is used during depressive periods with the primary mania algorithm.
Treatment recommendations. The key to using mood stabilizers is to achieve the optimum response—assuming good tolerability—before switching to another agent. Adjust medication dosages one at a time to allow adequate response and assessment.
When switching medications, use an overlap-and-taper strategy, assuming there is no medical necessity to stop a drug abruptly. Add the new medication, then gradually taper the one that is being discontinued. Monitor serum levels.
Discontinue antidepressants when appropriate in patients with hypomania/mania or rapid cycling, and continually evaluate suicide and homicide potential of patients in mixed or depressive states.
Stage 1: Monotherapy. First medication choices are lithium, divalproex, or olanzapine. For mixed or dysphoric mania, the algorithm recommends divalproex (preferred over valproic acid because of tolerability and side effects) or olanzapine.6 Data suggest dysphoric manic patients are less likely to respond to lithium.7 A Consensus Panel minority expressed concern about using olanzapine as first-line monotherapy for acute mania because of limited data on the drug’s long-term safety. Patients with partial response or residual symptoms may move to stage 2 or switch to other medication options within stage 1.
Patients with psychotic mania move directly to stage 4 for a broader range of combination therapy.
Stage 2: Combination therapy. Combination therapy has become the standard of care in treating most patients with bipolar disorder. The algorithm recommends using two agents:
- lithium or an anticonvulsant plus another anticonvulsant ([Li or AC]+AC)
- or lithium or an anticonvulsant plus an atypical antipsychotic ([Li or AC]+AAP).8
Recommended agents include lithium, divalproex, oxcarbazepine, olanzapine, or risperidone. The experts recommended oxcarbazepine as first choice because it is better tolerated and interacts with fewer drugs than carbamazepine and does not require serum level monitoring.9
A Consensus Panel minority expressed concern that few studies had examined using oxcarbazepine in bipolar disorder. Carbamazepine was also considered an option.
Stages 3 and 4: Other two-drug combinations. Other two-drug combinations are tried at these stages, drawing from the same pool of medication classes described in stage 2.
Stage 4 broadens the choice of atypical antipsychotic by adding quetiapine10 and ziprasidone11 to the recommended stage-2 agents olanzapine and risperidone. When the 2000 algorithm was developed, limited data were available on using some newer atypicals in patients with bipolar mania. Based on recent, high-quality studies of mono- and combination therapy—including quetiapine,10 ziprasidone,11 risperidone,12,13 and aripiprazole14 —the 2004 algorithm update panel will likely recommend using atypicals earlier, including at stage 1.
Algorithm 1 Treating mania/hypomania in patients with bipolar I disorder
Stage 5: Triple-drug combination. Lithium, an anticonvulsant (divalproex or oxcarbazepine), and an atypical antipsychotic (olanzapine, risperidone, quetiapine, or ziprasidone) is a recommended triple-drug combination. In the 2004 update, the choices will likely expand to include all the newer atypicals and will list carbamazepine as an option.
Stage 6: ECT or clozapine. For patients with inadequate response to triple-drug combinations, the algorithm recommends adding electroconvulsive therapy (ECT) or clozapine.
ECT15 is recommended three times a week until the patient achieves remission of manic symptoms or fails to achieve a sustained response over three to six treatment cycles. Treatment resistance is declared if no response is seen after 6 to 10 treatment cycles.
Clozapine’s16 recommendation at this stage is consistent with its use in patients who fail to respond to other atypical antipsychotics. Blood monitoring for agranulocytosis is required; other adverse effects include an increased risk of seizures, myocarditis, and orthostatic hypotension.
Stage 7: Other. Treatment options such as topiramate17,18 and lamotrigine19 are recommended at this stage. These recommendations also will be reviewed and likely revised.
Treating bipolar depression
The TMAP algorithm for treating depression in bipolar disorder (Algorithm 2) assumes that anti-depressants will be used only with optimum mood-stabilizer levels because of the risk of inducing manic symptoms. The bipolar depression algorithm is always used with the primary algorithm for mania/hypomania.
The patient’s clinical presentation guides medication selection. For the “pure” bipolar I patient with a major depressive episode but little mood lability or hypomania, starting an antide-pressant is a clear decision. On the other hand, patients with predominant depressive symptoms plus dysphoric hypomania, mood lability, and irritability need a balance of mood-stabilizing drugs and antidepressants.
Stage 1: Mood stabilizer. Initiate a mood stabilizer and optimize the dosage. Choices are the same mood stabilizers listed in the hypomania/mania treatment algorithm.
Stage 2: Antidepressant. Adding an antidepressant implies that depressive symptoms are severe enough to change treatment. Antidepressant options include a selective serotonin reuptake inhibitor (SSRI), sustained-release bupropion, or lamotrigine.20
Using SSRIs is supported by widespread clinical experience and offers the convenience of once-daily dosing. Recommended SSRIs include fluoxetine, paroxetine, fluvoxamine, sertraline, and citalopram. The SSRI escitalopram was introduced after the 2000 algorithms were published; evidence for using it and other newer medications will be reviewed for the 2004 update.
The recommendation for sustained-release bupropion is consistent with the algorithm principle to use medications in the most well-tolerated form when accessible and available.
With lamotrigine, review with patients the risk of serious rash. To minimize rash risk, start lamotrigine slowly and follow the recommended titration schedule.
Stage 3: Multiple choices. At this stage, no definitive studies, safety data, or tolerability issues are available to rank the medication choices. The algorithm suggests:
- adding lithium21 or a second antidepressant
- or switching to an alternate antidepressant such as venlafaxine or nefazodone.
If a patient moves to stage 3 because of side effects with one antidepressant class, a different class—preferably with a contrasting side-effect profile—is recommended.
Algorithm 2 Treating depression in bipolar I disorder*
Stage 4: Two antidepressants. To enhance clinical response, the algorithm recommends combining two antidepressants, preferably from different classes. Monitor patients closely for side effects.
Stage 5. Antipsychotic or MAOI. At this stage, the algorithm recommends adding an atypical antipsychotic22 or switching to a monoamine oxidase inhibitor (MAOI).
Early evidence supported the efficacy of MAOIs in bipolar depression. However, the panel ranked MAOIs lower in the algorithm because they are associated with more bothersome side effects than SSRIs and other antidepressants. When using MAOIs, provide patients with dietary restriction guidelines.
Stage 6. Other therapies. Therapies such as ECT or “other” interventions are recommended at this stage. ECT has proven efficacy in bipolar depression and is appropriate for patients with limited medication response. The panel gave ECT a low ranking because of limited availability, lack of patient acceptance, and newer options.
Medication options include experimental treatments with limited evidence, such as inositol, dopamine agonists, stimulants, thyroid supplementation, conventional antipsychotics, and tri-cyclic antidepressants.
Acute to maintenance treatment
Adjunctive treatments for agitation, insomnia, GI upset, sedation, headache, and tremor are recommended in the physician manual supporting the TMAP guidelines (see Related resources). The manual also suggests ways to manage medication side effects and modify the algorithms for inpatients.
Patient and family psychoeducation plays an important role in helping the patient:
- identify prodromal bipolar symptoms
- understand the need to take medications as prescribed.
Continuation treatment. After mania or hypomania remits, continue medication(s) at the effective acute-phase dosages for at least 3 months. Use follow-up visits to enhance patient adherence, detect early symptoms of relapse, and monitor for side effects.
During the late continuation phase, after a period of sustained stability, clinicians can try to simplify the medications. When discontinuing a medication, taper the dosage by no more than 25% per week. If symptoms recur, promptly return to acute-phase treatment. Consider restarting medications and titrating up to the dosage(s) that resulted in remission.
In a depressive episode, continue the antidepressant(s) for 1 to 3 months at the effective acutephase dosage(s). Follow up frequently, and educate patients to watch for symptom recurrence and to communicate with you to assess when medication changes are needed.
Maintenance treatment. Relatively few well-controlled studies on long-term management of bipolar patients were available for the 2000 algorithm update.23 In general, all patients need mood stabilizer(s) to prevent relapse, using the lowest dosage that maintains therapeutic efficacy. Based on new evidence for lamotrigine and atypical antipsychotics—including FDAapproval of olanzapine for bipolar maintenace therapy—we anticipate recommendations will be expanded and more delineated in the 2004 update.
Discontinuing antidepressants after 3 to 6 months of initial treatment is now recommended. However, a recent retrospective case series suggests that continuing antidepressants at least 1 year after initial successful therapy may protect against depressive relapse. During this study, continuing antidepressants more than 3 to 6 months did not appear to increase the risk of switching to mania.24
Should antidepressants be continued or discontinued after successful acute treatment of a bipolar I depressive episode? This is an active area of research and debate as to the most appropriate strategy. The 2004 algorithm update panel will consider recent evidence that supports continuing antidepressants after symptom remission.24
- Texas Medication Algorithm Project algorithms and physician manual. Texas Department of Mental Health and Mental Retardation. http://www.mhmr.state.tx.us/centraloffice/medicaldirector/TIMA.html
- American Psychiatric Association. Practice guidelines for the treatment of patients with bipolar disorder. Am J Psychiatry 2002; 159(4):suppl 2.
- Depression and Bipolar Support Alliance. www.dbsalliance.org
Drug brand names
- Bupropion • Wellbutrin SR
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Inositol • Various
- Lamotrigine • Lamictal
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Topiramate • Topamax
- Tranylcypromine • Parnate
- Valproic acid • Depakene
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
Dr. Shivakumar reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Suppes receives research support from or is a consultant to Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Co., GlaxoSmithKline, Janssen Pharmaceutica, Johnson & Johnson, National Institutes of Mental Health, Novartis Pharmaceuticals Corp., Pfizer Inc., Pharmaceutical Research Institute, Ortho-McNeil Pharmaceutical, Robert Wood Johnson Pharmaceutical Research Institute, The Stanley Medical Research Institute, and UCB Pharma.
1. Gilbert DA, Altshuler KZ, Rago WV, et al. Texas Medication Algorithm Project: definitions, rationale, and methods to develop medication algorithms. J Clin Psychiatry 1998;59:345-51.
2. Altshuler KZ, Rush AJ. Computerized Texas Medication Algorithm Project undergoes testing. Outcomes Accountability Alert 1999;4(1):10-11.
3. Rush AJ, Crismon ML, Kashner TM, et al. Texas Medication Algorithm Project, Phase 3 (TMAP-3): rationale and study design. J Clin Psychiatry 2003;64(4):357-69.
4. Altman E, Hedeker D, Janicak P, et al. The Clinician-Administered Rating Scale For Mania (CARS-M): development, reliability, and validity. Biol Psychiatry 1994;36:124-34.
5. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas consensus conference panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry 2002;63:288-99.
6. Dennehy EB, Doyle K, Suppes T. The efficacy of olanzapine monotherapy for acute hypomania or mania in an outpatient setting. Int Clin Psychopharmacol 2003;18(3):143-5.
7. Dilsaver SC, Swann AC, Shoaib AM, et al. The manic syndrome: factors which may predict a patient’s response to lithium, carbamazepine and valproate. J Psych Neurosci 1993;18:61-6.
8. Tohen M, Chengappa KNR, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59(1):62-9.
9. Emrich HM. Studies with oxcarbazepine (Trileptal) in acute mania. Int Clin Psychopharmacol 1990;190(5,suppl):83-8.
10. Vieta E, Parramon G, Padrell E, et al. Quetiapine in the treatment of rapid cycling bipolar disorder. Bipolar Disord 2002;4(5):335-40.
11. Keck PE, Versiani M, Potkin S, et al. Ziprasidone in the treatment of acute bipolar mania: a three week placebo-controlled, double-blinded randomized trial. Am J Psychiatry 2003;160(4):741-8.
12. Yatham LN, Grossman F, Augustyns I, et al. Mood stabilisers plus risperidone or placebo in the treatment of acute mania. International, double-blinded, randomised controlled trial. Br J Psychiatry 2003;182:141-7.
13. Sachs GS, Grossman F, Ghaemi SN, et al. Combination of a mood stabilizer with risperidone or haloperidol for treatment of acute mania: a double-blind, placebo-controlled comparison of efficacy and safety. Am J Psychiatry 2002;159(7):1146-54.
14. Keck PE, Jr, Marcus R, Tourkodimitris S, et al. Aripiprazole study group. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160(9):1651-8.
15. Mukherjee S, Sackeim HA, Schnur DB. Electroconvulsive therapy of acute manic episode: a review of 50 years’ experience. Am J Psychiatry 1988;45:727-32.
16. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trail of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry 1999;156(8):1164-9.
17. Guille C, Sachs G. Clinical outcome of adjunctive topiramate treatment in a sample of refractory bipolar patients with comorbid conditions. Prog Neuropsychopharmacol Biol Psychiatry 2002;26(6):1035-9.
18. Vieta E, Torrent C, Garcia-Ribas G, et al. Use of topiramate in treatment-resistant bipolar spectrum disorders. J Clin Psychopharmacol 2002;22(4):431-5.
19. Calabrese JR, Suppes T, Bowden CL, et al. A double blinded, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. J Clin Psychiatry 2000;61:841-50.
20. Calabrese JR, Bowden CL, Sachs GS, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry 2003;64(9):1013-24.
21. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacology 1999;19:427-34.
22. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
23. Baldessarini RJ, Tohen M, Tondo L. Maintenance treatment in bipolar disorder (comment). Arch Gen Psychiatry 2000;57:490-2.
24. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 2003;160:1252-62.
1. Gilbert DA, Altshuler KZ, Rago WV, et al. Texas Medication Algorithm Project: definitions, rationale, and methods to develop medication algorithms. J Clin Psychiatry 1998;59:345-51.
2. Altshuler KZ, Rush AJ. Computerized Texas Medication Algorithm Project undergoes testing. Outcomes Accountability Alert 1999;4(1):10-11.
3. Rush AJ, Crismon ML, Kashner TM, et al. Texas Medication Algorithm Project, Phase 3 (TMAP-3): rationale and study design. J Clin Psychiatry 2003;64(4):357-69.
4. Altman E, Hedeker D, Janicak P, et al. The Clinician-Administered Rating Scale For Mania (CARS-M): development, reliability, and validity. Biol Psychiatry 1994;36:124-34.
5. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas consensus conference panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry 2002;63:288-99.
6. Dennehy EB, Doyle K, Suppes T. The efficacy of olanzapine monotherapy for acute hypomania or mania in an outpatient setting. Int Clin Psychopharmacol 2003;18(3):143-5.
7. Dilsaver SC, Swann AC, Shoaib AM, et al. The manic syndrome: factors which may predict a patient’s response to lithium, carbamazepine and valproate. J Psych Neurosci 1993;18:61-6.
8. Tohen M, Chengappa KNR, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59(1):62-9.
9. Emrich HM. Studies with oxcarbazepine (Trileptal) in acute mania. Int Clin Psychopharmacol 1990;190(5,suppl):83-8.
10. Vieta E, Parramon G, Padrell E, et al. Quetiapine in the treatment of rapid cycling bipolar disorder. Bipolar Disord 2002;4(5):335-40.
11. Keck PE, Versiani M, Potkin S, et al. Ziprasidone in the treatment of acute bipolar mania: a three week placebo-controlled, double-blinded randomized trial. Am J Psychiatry 2003;160(4):741-8.
12. Yatham LN, Grossman F, Augustyns I, et al. Mood stabilisers plus risperidone or placebo in the treatment of acute mania. International, double-blinded, randomised controlled trial. Br J Psychiatry 2003;182:141-7.
13. Sachs GS, Grossman F, Ghaemi SN, et al. Combination of a mood stabilizer with risperidone or haloperidol for treatment of acute mania: a double-blind, placebo-controlled comparison of efficacy and safety. Am J Psychiatry 2002;159(7):1146-54.
14. Keck PE, Jr, Marcus R, Tourkodimitris S, et al. Aripiprazole study group. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160(9):1651-8.
15. Mukherjee S, Sackeim HA, Schnur DB. Electroconvulsive therapy of acute manic episode: a review of 50 years’ experience. Am J Psychiatry 1988;45:727-32.
16. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trail of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry 1999;156(8):1164-9.
17. Guille C, Sachs G. Clinical outcome of adjunctive topiramate treatment in a sample of refractory bipolar patients with comorbid conditions. Prog Neuropsychopharmacol Biol Psychiatry 2002;26(6):1035-9.
18. Vieta E, Torrent C, Garcia-Ribas G, et al. Use of topiramate in treatment-resistant bipolar spectrum disorders. J Clin Psychopharmacol 2002;22(4):431-5.
19. Calabrese JR, Suppes T, Bowden CL, et al. A double blinded, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. J Clin Psychiatry 2000;61:841-50.
20. Calabrese JR, Bowden CL, Sachs GS, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry 2003;64(9):1013-24.
21. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacology 1999;19:427-34.
22. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
23. Baldessarini RJ, Tohen M, Tondo L. Maintenance treatment in bipolar disorder (comment). Arch Gen Psychiatry 2000;57:490-2.
24. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 2003;160:1252-62.