User login
Stimulants for adult bipolar disorder?
Patients with bipolar disorder show an unpredictable range of responses to stimulants, from virtually no ill effects to emerging manic-like symptoms.1 Thus, although stimulants may be beneficial to some bipolar patients, there is a great deal of concern about using stimulants in this population. Even so, stimulants may be a rational adjunct for treating certain aspects of bipolar illness, particularly resistant depression, iatrogenic sedation, and comorbid attention-deficit/hyperactivity disorder (ADHD).
To help you decide if and when your patient might be a candidate for stimulant therapy, this article:
- reviews the evidence on stimulants’ safety and tolerability for patients with bipolar disorder
- weighs potential benefits and risks of using stimulants in this population
- addresses stimulants’ possible adverse effects on illness course and from interactions with other psychotropics
- discusses treatment options based on the limited evidence and our clinical experience.
Limited support
We are aware that using stimulants to treat patients with bipolar disorder is not an uncommon clinical practice, but supportive evidence is limited (Table 1). In searching the literature, we found only 2 randomized controlled studies—Frye et al2 and Scheffer et al3—that addressed this practice. (One author of this review [TS] participated as a coinvestigator with Frye et al.2) Other evidence that suggests a role for stimulants in bipolar disorder comes from case reports, retrospective case series, and open-label studies.4-11
- “traditional” stimulants (including amphetamine-based compounds such as dextroamphetamine, methylphenidate, dexmethylphenidate, and lisdexamfetamine) thought to affect the dopamine transporter, resulting in increased dopamine in nerve terminals
- the “novel” psychostimulant modafinil, thought to affect multiple neurotransmitter systems (dopamine, GABA, serotonin, histamine, and glutamate), although its mechanism of action is unclear.
Table 1
Clinical studies of stimulant use in patients with bipolar disorder
| Stimulant(s) studied | Study design | Patients studied | Clinical outcomes |
|---|---|---|---|
| Traditional stimulants | |||
| Adjunctive methylphenidate | Chart review, naturalistic12 | 16 adults (5 with comorbid ADHD, 11 with bipolar depression) | Improvements in depression, overall functioning, and ability to concentrate; sleep disturbance, irritability/agitation reported |
| Adjunctive methylphenidate or racemic mixture of AMPH salts | Chart review of sedation and depressive symptoms13 | 8 adults (BD II) | Improved clinical impression of bipolar illness; no manic switches, changes in cycling patterns, or substance abuse noted |
| Adjunctive methylphenidate | 12-week open study, bipolar depression14 | 12 adults (10 BD I, 2 BD II) | Significant clinical improvements in depressive symptoms; no change in manic symptoms; anxiety, agitation, and hypomania reported |
| Multiple stimulants | Chart review, history of stimulants and bipolar illness course25 | 34 hospitalized adolescents | Prior stimulant treatment associated with earlier age of illness onset |
| Adjunctive mixed amphetamine salts | Randomized, placebo-controlled; comorbid BD and ADHD3 | 30 children with ADHD symptoms stabilized on divalproex sodium | Decrease in ADHD symptoms with adjunctive amphetamine treatment but not with divalproex sodium alone; 1 case of mania |
| Novel stimulant | |||
| Adjunctive modafinil | Case series15 | Mixed sample of depressed adults (4 unipolar, 3 bipolar) | Significant improvement in depressive symptoms |
| Adjunctive modafinil | Randomized, double-blind, placebo-controlled2 | 85 adults with bipolar depression | Treatment group showed greater response and remission of depressive symptoms compared with placebo group; no difference in development of manic symptoms |
| ADHD: attention-deficit/hyperactivity disorder; AMPH: amphetamine; BD: bipolar disorder; NOS: not otherwise specified | |||
Depression and iatrogenic sedation
Small, uncontrolled trials have reported some benefit and tolerability in bipolar disorder patients when stimulants are used to treat residual depressive symptoms or iatrogenic sedation associated with mood stabilizers.
Traditional stimulants. A retrospective chart review of 16 patients treated with adjunctive methylphenidate noted improved functioning, as measured by the Global Assessment of Functioning scale. Some patients’ depressive symptoms and concentration also appeared to improve, but how these parameters were assessed is not clear. Some patients tolerated stimulants well, whereas others experienced irritability, agitation, and sleep disturbances.12
Another retrospective chart review described 8 patients with iatrogenic sedation or depression who received adjunctive methylphenidate, mean 20 to 40 mg/d, or a racemic mixture of amphetamine salts, mean 20 to 40 mg/d. Overall bipolar symptoms decreased in severity, as measured by Clinical Global Impression (CGI) scores, but the authors did not directly measure sedation or depression. The stimulants were well-tolerated, with no evidence of stimulant-induced mania.13
In a 12-week open-label trial of methylphenidate in 14 patients with bipolar disorder, depressive symptoms improved as measured by the Hamilton Depression Rating Scale (HAM-D). Mean doses were 10 mg/d for the 3 patients who discontinued because of anxiety, agitation, or hypomania and 16.6 mg/d for those who completed the trial.14
Modafinil may have some efficacy in treating bipolar depression. In a case series of 7 depressed patients (4 unipolar and 3 bipolar), 5 patients showed a 50% decrease in HAM-D scores with adjunctive modafinil. Dosages ranged from 100 to 200 mg/d, although most patients took 200 mg/d. In this series, modafinil was added to a variety of treatments, including bupropion, nefazodone, paroxetine, venlafaxine, an unspecified tricyclic antidepressant (TCA), divalproex sodium, lamotrigine, lithium, electroconvulsive therapy, olanzapine, and gabapentin.15
The only randomized, double-blind, placebo-controlled trial of adjunctive modafinil for bipolar depression enrolled 85 patients with moderate or more severe depression. In this 6-week trial by Frye et al,2 41 patients received modafinil, 100 to 200 mg/d (mean dose 174.2 mg/d), and 44 received placebo.
Bipolar disorder plus ADHD
An estimated 10% to 21% of bipolar patients meet criteria for ADHD,16-19 although at times the line differentiating these 2 disorders is unclear. Co-occurring ADHD worsens the course of bipolar illness,20-22 and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial suggest that only 2% of dual-diagnosis patients are receiving treatment specifically for ADHD symptoms.23
Theoretically, overlapping symptoms such as talkativeness, distractibility, and physical activity remain relatively constant in ADHD but wax and wane with bipolar disorder’s manic and depressive phases. Recent evidence suggests, however, that many bipolar patients experience prodromal symptoms that may resemble ADHD, including cognitive impairment, distractibility, and increased psychomotor activity.24 In addition, medications used to treat bipolar disorder may impair cognitive function, making ADHD diagnosis difficult in this population.
We are not aware of any clinical trials that examined stimulants’ safety and efficacy in adult bipolar patients with co-occurring ADHD. One of the only studies to examine stimulant treatment of ADHD symptoms in a bipolar population was a retrospective chart review of 34 adolescents hospitalized with bipolar mania. An earlier age of bipolar illness onset was reported in adolescents who had been exposed to stimulants, whether or not they also had ADHD.25
Possible adverse events
Some bipolar disorder patients tolerate stimulants well, whereas others experience serious side effects, toxicities, and illness destabilization (Table 2). Because mood-stabilizer treatment may attenuate stimulants’ undesirable effects in bipolar disorder patients,26,27 be sure to use adequate dosing of a mood stabilizer if you determine a stimulant trial is warranted in your patient.
Destabilization. Stimulants can have a direct negative effect on mood; they can cause restlessness, irritability, anxiety, and mood lability. Some bipolar patients may be more sensitive to these adverse effects than others. Particularly concerning is the possibility of switching to mania or worsening of manic symptoms.28,29 Other potential destabilizing effects include:
- changing cycling patterns, such as inducing rapid cycling
- sleep disturbance because stimulants promote wakefulness.
If you are considering stimulant treatment for a bipolar disorder patient in whom substance abuse is a concern, modafinil or lisdexamfetamine may have a lower abuse potential compared with immediate-release psychostimulants. Lisdexamfetamine is metabolized in the GI tract and does not produce high d-amphetamine blood levels or cause reinforcing effects if injected or snorted.34
Table 2
Possible stimulant side effects, signs of toxicity, and contraindications
| Stimulant class | Possible side effects | Signs of toxicity/overdose | Contraindications/cautions |
|---|---|---|---|
| Traditional (amphetamine mixtures, dexmethylphenidate, dextroamphetamine, lisdexamfetamine methylphenidate)* | Restlessness, insomnia, mood lability, anxiety | Agitation, confusion, tremor, tachycardia, hyperreflexia, hypertension, sweating, psychomotor agitation, seizure, arrhythmia, coma, psychosis | Cardiovascular disease, hypertension, hyperthyroidism, glaucoma, Tourette’s syndrome/motor tics, history of seizure disorder, hypersensitivity to medication class |
| Novel (modafinil) | Restlessness, insomnia, mood lability, anxiety | Agitation, tremor, nausea, diarrhea, confusion | Cardiovascular disease, hepatic impairment, psychosis |
| * Amphetamines and dextroamphetamine (Adderall, Adderall XR); dexmethylphenidate (Focalin, Focalin XR), dextroamphetamine (Dexedrine, DextroStat); lisdexamfetamine (Vyvanse); methylphenidate (Concerta, Daytrana, Metadate CD, Methylin, Methylin ER, Ritalin, Ritalin LA, Ritalin SR) | |||
Drug-drug interactions
Polypharmacy is the rule in treating bipolar disorder, and stimulants can interact with many other psychotropics (Table 3).
Antidepressants. Never use traditional stimulants with monoamine oxidase inhibitors, as this combination may precipitate a hypertensive crisis. Coadministered stimulants also may decrease the metabolism of serotonergic agents—such as selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs)—and cause side effects associated with increased serotonin neurotransmission, including serotonin syndrome.
Combining traditional stimulants with TCAs may increase TCA concentrations. When coadministered with bupropion, stimulants can increase the risk of seizures.
Modafinil is both an inducer and inhibitor of cytochrome P450 isoenzymes. Because it induces CYP3A4 and inhibits CYP2C19 and CYP2C9, modafinil interacts with many other psychopharmacologic agents:
- Its induction of CYP3A4 may increase the metabolism of commonly used medications such as carbamazepine, aripiprazole, and triazolam.
- Its inhibition of CYP2C19 may decrease the metabolism of many SSRIs, TCAs, diazepam, and clozapine, increasing these drugs’ effects and adverse events.
Possible stimulant interactions with other psychotropics
| Stimulant class | Psychotropic medication | Possible adverse effects |
|---|---|---|
| Traditional (amphetamine mixtures, dexmethylphenidate, dextroamphetamine, lisdexamfetamine methylphenidate)* | MAOIs | Hypertensive crisis |
| CBZ | Reduced methylphenidate levels; abruptly stopping CBZ increases methylphenidate’s effect | |
| TCAs | Increased TCA concentration | |
| SSRIs, SNRIs | Possible decreased metabolism of antidepressants; potential for serotonin syndrome or NMS-like syndrome | |
| Typical and atypical antipsychotics | Each may interfere with the other’s therapeutic action | |
| Novel (modafinil) | CBZ | Decreased modafinil efficacy; decreased CBZ levels |
| Triazolam | Decreased triazolam efficacy; increased effects of triazolam with modafinil discontinuation | |
| Fluoxetine, fluvoxamine | Decreased modafinil clearance | |
| Citalopram, escitalopram, sertraline | Prolonged elimination and increased levels of antidepressant | |
| MAOIs | Hypertensive crisis(?); not recommended | |
| Diazepam | Prolonged elimination and increased levels of diazepam | |
| TCAs | Prolonged elimination and increased levels of TCAs | |
| Clozapine | Increased clozapine concentration (case report) | |
| Aripiprazole | Decreased levels of aripiprazole | |
| * Amphetamines and dextroamphetamine (Adderall, Adderall XR); dexmethylphenidate (Focalin, Focalin XR), dextroamphetamine (Dexedrine, DextroStat); lisdexamfetamine (Vyvanse); methylphenidate (Concerta, Daytrana, Metadate CD, Methylin, Methylin ER, Ritalin, Ritalin LA, Ritalin SR) | ||
| CBZ: carbamazepine; MAOIs: monoamine oxidase inhibitors; NMS: neuroleptic malignant syndrome; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants | ||
Treatment considerations
Without evidence to support stimulants’ safety and efficacy in patients with bipolar disorder, we cannot make specific recommendations. We would, however, like to offer some general recommendations if you decide to use stimulants when treating patients with bipolar disorder (Table 4).
Carefully assess and—in many cases—reassess the patient’s symptoms to clarify the diagnosis. As mentioned, ADHD and bipolar disorder share many symptoms, particularly in the manic phase of bipolar illness. Overlapping symptoms include decreased ability to concentrate and focus, distractibility, hyperactivity and psychomotor agitation, racing thoughts, and impulsivity.
Substance abuse can negatively impact bipolar illness and present as clinical scenarios in which stimulants are used (such as treatment-resistant depression, impulsivity, somnolence, or fatigue).
Treat medical conditions such as thyroid disease, diabetes, and sleep apnea, which may worsen depression, cause somnolence and sedation, and present with symptoms similar to those of ADHD.
When possible, use lifestyle techniques to help patients manage the course of bipolar illness. Encourage good sleep hygiene, exercise, stable social rhythms, and limited use of alcohol and caffeine (both of which can impair sleep quality, which affects illness stability).
The next step. When you have explored all medication options and ruled out all other causes for the patient’s symptoms, stimulant treatment may be an appropriate next step. In these cases:
Encourage patients to participate in treatment, particularly in monitoring mood changes (as with life charts), symptoms associated with mood episodes, and emergence of side effects. When possible, involve family members in monitoring for adverse events.
Administration. Start stimulants only when bipolar illness is well-stabilized, especially regarding manic symptoms. We highly caution against using stimulants in patients with manic or hypomanic symptoms, including mixed states. We recommend not using stimulants in patients with:
- clinically significant insomnia or sleep fragmentation
- active suicidal ideation or psychotic symptoms, particularly if associated with manic symptoms.
Schedule frequent office visits when prescribing stimulants. At least initially, see patients every other week to assess for the emergence of adverse events.
Table 4
6 recommendations when using stimulants in bipolar disorder
| Carefully assess patient’s symptoms | Manic symptoms vs ADHD; medical conditions such as thyroid disorders, diabetes, or sleep apnea |
| Review possible iatrogenic causes of symptoms | Somnolence, decreased energy/fatigue, sedation, difficulty with concentration/focus |
| Engage patient in the therapeutic process | Discuss risks and benefits; monitor mood with life charts; enlist help of family, significant others when appropriate |
| Use caution in clinical scenarios that may herald adverse response to stimulants | Manic/hypomanic symptoms; sleep disturbances; psychosis; history of substance abuse |
| Administer stimulants with caution | Start low and go slow; always use stimulants in conjunction with a mood-stabilizing agent; be aware of possible interactions with patient’s other medications; schedule more frequent visits when starting stimulants |
| Monitor for adverse events associated with stimulant administration | Manic symptoms, changes in cycling patterns, sleep disturbances, substance abuse |
| ADHD: attention-deficit/hyperactivity disorder | |
- The Texas Medication Algorithm Project. Texas Department of State Health Services. www.dshs.state.tx.us/mhprograms/tmapover.shtm.
- The Cochrane Collaboration. www.cochrane.org.
- Amphetamine and dextroamphetamine • Adderall
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clozapine • Clozaril
- Dexmethylphenidate • Focalin
- Dextroamphetamine • Dexedrine, DextroStat
- Diazepam • Valium
- Divalproex sodium • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Lisdexamfetamine • Vyvanse
- Lithium • various
- Methylphenidate • Ritalin, Concerta, others
- Modafinil • Provigil
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Triazolam • Halcion
- Valproic acid • Depakene
- Venlafaxine • Effexor
Dr. Gonzalez reports no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products. He is a recipient of a T32 Ruth L. Kirschstein National Research Service Awards training fellowship sponsored by the National Institutes of Health.
Dr. Suppes receives grants/research support from Abbott Laboratories, AstraZeneca, GlaxoSmithKline, JDS Pharmaceuticals, Janssen Pharmaceutica, National Institute of Mental Health, Novartis, Pfizer Inc., and the Stanley Medical Research Institute.
1. Silberman EK, Reus VI, Jimerson DC, et al. Heterogeneity of amphetamine response in depressed patients. Am J Psychiatry 1981;138(10):1302-7.
2. Frye MA, Grunze H, Suppes T, et al. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry 2007;164(8):1242-9.
3. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
4. Meyers B. Treatment of imipramine-resistant depression and lithium-refractory mania through drug interactions. Am J Psychiatry 1978;135(11):1420-1.
5. Bannet J, Ebstein RP, Belmaker RH. Clinical aspects of the interaction of lithium and stimulants. Br J Psychiatry 1980;136:204.-
6. Drimmer EJ, Gitlin MJ, Gwirtsman HE. Desipramine and methylphenidate combination treatment for depression: case report. Am J Psychiatry 1983;140(2):241-2.
7. Fernandes PP, Petty F. Modafinil for remitted bipolar depression with hypersomnia. Ann Pharmacother 2003;37(12):1807-9.
8. Berigan TR. Augmentation with modafinil to achieve remission in depression: a case report. Prim Care Companion J Clin Psychiatry 2001;3(1):32.-
9. Berigan TR. Modafinil treatment of excessive daytime sedation and fatigue associated with topiramate. Prim Care Companion J Clin Psychiatry 2002;4(6):249-50.
10. Berigan T. Modafinil treatment of excessive sedation associated with divalproex sodium. Can J Psychiatry 2004;49(1):72-3.
11. Even C, Thuile J, Santos J, Bourgin P. Modafinil as an adjunctive treatment to sleep deprivation in depression. J Psychiatry Neurosci 2005;30(6):432-3.
12. Lydon E, El-Mallakh RS. Naturalistic long-term use of methylphenidate in bipolar disorder. J Clin Psychopharmacol 2006;26(5):516-8.
13. Carlson PJ, Merlock MC, Suppes T. Adjunctive stimulant use in patients with bipolar disorder: treatment of residual depression and sedation. Bipolar Disord 2004;6(5):416-20.
14. El-Mallakh RS. An open study of methylphenidate in bipolar depression. Bipolar Disord 2000;2(1):56-9.
15. Menza MA, Kaufman KR, Castellanos A. Modafinil augmentation of antidepressant treatment in depression. J Clin Psychiatry 2000;61(5):378-81.
16. Wingo AP, Ghaemi SN. A systematic review of rates and diagnostic validity of comorbid adult attention-deficit/hyperactivity disorder and bipolar disorder. J Clin Psychiatry 2007;68(11):1776-84.
17. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163(4):716-23.
18. Nierenberg AA, Miyahara S, Spencer T, et al. Clinical and diagnostic implications of lifetime attention-deficit/hyperactivity disorder comorbidity in adults with bipolar disorder: data from the first 1000 STEP-BD participants. Biol Psychiatry 2005;57(11):1467-73.
19. Tamam L, Tuglu C, Karatas G, Ozcan S. Adult attention-deficit hyperactivity disorder in patients with bipolar I disorder in remission: preliminary study. Psychiatry Clin Neurosci 2006;60(4):480-5.
20. Faraone SV, Biederman J, Mennin D, et al. Attention-deficit hyperactivity disorder with bipolar disorder: a familial subtype? J Am Acad Child Adolesc Psychiatry 1997;36(10):1378-87; discussion 1387-90.
21. Faraone SV, Biederman J, Monuteaux MC. Attention deficit hyperactivity disorder with bipolar disorder in girls: further evidence for a familial subtype? J Affect Disord 2001;64(1):19-26.
22. Faraone SV, Glatt SJ, Tsuang MT. The genetics of pediatriconset bipolar disorder. Biol Psychiatry 2003;53(11):970-7.
23. Simon NM, Otto MW, Weiss RD, et al. Pharmacotherapy for bipolar disorder and comorbid conditions: baseline data from STEP-BD. J Clin Psychopharmacol 2004;24(5):512-20.
24. Calabrese JR. Overview of patient care issues and treatment in bipolar spectrum and bipolar II disorder. J Clin Psychiatry 2008;69(6):e18.-
25. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001;3(2):53-7.
26. Van Kammen DP, Murphy DL. Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia 1975;44(3):215-24.
27. Huey LY, Janowsky DS, Judd LL, et al. Effects of lithium carbonate on methylphenidate-induced mood, behavior, and cognitive processes. Psychopharmacology (Berl) 1981;73(2):161-4.
28. Gerner RH, Post RM, Bunney WE, Jr. A dopaminergic mechanism in mania. Am J Psychiatry 1976;133(10):1177-80.
29. Koehler-Troy C, Strober M, Malenbaum R. Methylphenidateinduced mania in a prepubertal child. J Clin Psychiatry 1986;47(11):566-7.
30. Brady KT, Sonne SC. The relationship between substance abuse and bipolar disorder. J Clin Psychiatry 1995;56(suppl 3):19-24.
31. Sonne SC, Brady KT. Substance abuse and bipolar comorbidity. Psychiatr Clin North Am 1999;22(3):609-27,ix.
32. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990;264(19):2511-8.
33. Estroff TW, Dackis CA, Gold MS, Pottash AL. Drug abuse and bipolar disorders. Int J Psychiatry Med 1985-1986;15(1):37-40.
34. Faraone SV. Lisdexamfetamine dimesylate: the first longacting prodrug stimulant treatment for attention deficit/hyperactivity disorder. Expert Opin Pharmacother 2008;9(9):1565-74.
Patients with bipolar disorder show an unpredictable range of responses to stimulants, from virtually no ill effects to emerging manic-like symptoms.1 Thus, although stimulants may be beneficial to some bipolar patients, there is a great deal of concern about using stimulants in this population. Even so, stimulants may be a rational adjunct for treating certain aspects of bipolar illness, particularly resistant depression, iatrogenic sedation, and comorbid attention-deficit/hyperactivity disorder (ADHD).
To help you decide if and when your patient might be a candidate for stimulant therapy, this article:
- reviews the evidence on stimulants’ safety and tolerability for patients with bipolar disorder
- weighs potential benefits and risks of using stimulants in this population
- addresses stimulants’ possible adverse effects on illness course and from interactions with other psychotropics
- discusses treatment options based on the limited evidence and our clinical experience.
Limited support
We are aware that using stimulants to treat patients with bipolar disorder is not an uncommon clinical practice, but supportive evidence is limited (Table 1). In searching the literature, we found only 2 randomized controlled studies—Frye et al2 and Scheffer et al3—that addressed this practice. (One author of this review [TS] participated as a coinvestigator with Frye et al.2) Other evidence that suggests a role for stimulants in bipolar disorder comes from case reports, retrospective case series, and open-label studies.4-11
- “traditional” stimulants (including amphetamine-based compounds such as dextroamphetamine, methylphenidate, dexmethylphenidate, and lisdexamfetamine) thought to affect the dopamine transporter, resulting in increased dopamine in nerve terminals
- the “novel” psychostimulant modafinil, thought to affect multiple neurotransmitter systems (dopamine, GABA, serotonin, histamine, and glutamate), although its mechanism of action is unclear.
Table 1
Clinical studies of stimulant use in patients with bipolar disorder
| Stimulant(s) studied | Study design | Patients studied | Clinical outcomes |
|---|---|---|---|
| Traditional stimulants | |||
| Adjunctive methylphenidate | Chart review, naturalistic12 | 16 adults (5 with comorbid ADHD, 11 with bipolar depression) | Improvements in depression, overall functioning, and ability to concentrate; sleep disturbance, irritability/agitation reported |
| Adjunctive methylphenidate or racemic mixture of AMPH salts | Chart review of sedation and depressive symptoms13 | 8 adults (BD II) | Improved clinical impression of bipolar illness; no manic switches, changes in cycling patterns, or substance abuse noted |
| Adjunctive methylphenidate | 12-week open study, bipolar depression14 | 12 adults (10 BD I, 2 BD II) | Significant clinical improvements in depressive symptoms; no change in manic symptoms; anxiety, agitation, and hypomania reported |
| Multiple stimulants | Chart review, history of stimulants and bipolar illness course25 | 34 hospitalized adolescents | Prior stimulant treatment associated with earlier age of illness onset |
| Adjunctive mixed amphetamine salts | Randomized, placebo-controlled; comorbid BD and ADHD3 | 30 children with ADHD symptoms stabilized on divalproex sodium | Decrease in ADHD symptoms with adjunctive amphetamine treatment but not with divalproex sodium alone; 1 case of mania |
| Novel stimulant | |||
| Adjunctive modafinil | Case series15 | Mixed sample of depressed adults (4 unipolar, 3 bipolar) | Significant improvement in depressive symptoms |
| Adjunctive modafinil | Randomized, double-blind, placebo-controlled2 | 85 adults with bipolar depression | Treatment group showed greater response and remission of depressive symptoms compared with placebo group; no difference in development of manic symptoms |
| ADHD: attention-deficit/hyperactivity disorder; AMPH: amphetamine; BD: bipolar disorder; NOS: not otherwise specified | |||
Depression and iatrogenic sedation
Small, uncontrolled trials have reported some benefit and tolerability in bipolar disorder patients when stimulants are used to treat residual depressive symptoms or iatrogenic sedation associated with mood stabilizers.
Traditional stimulants. A retrospective chart review of 16 patients treated with adjunctive methylphenidate noted improved functioning, as measured by the Global Assessment of Functioning scale. Some patients’ depressive symptoms and concentration also appeared to improve, but how these parameters were assessed is not clear. Some patients tolerated stimulants well, whereas others experienced irritability, agitation, and sleep disturbances.12
Another retrospective chart review described 8 patients with iatrogenic sedation or depression who received adjunctive methylphenidate, mean 20 to 40 mg/d, or a racemic mixture of amphetamine salts, mean 20 to 40 mg/d. Overall bipolar symptoms decreased in severity, as measured by Clinical Global Impression (CGI) scores, but the authors did not directly measure sedation or depression. The stimulants were well-tolerated, with no evidence of stimulant-induced mania.13
In a 12-week open-label trial of methylphenidate in 14 patients with bipolar disorder, depressive symptoms improved as measured by the Hamilton Depression Rating Scale (HAM-D). Mean doses were 10 mg/d for the 3 patients who discontinued because of anxiety, agitation, or hypomania and 16.6 mg/d for those who completed the trial.14
Modafinil may have some efficacy in treating bipolar depression. In a case series of 7 depressed patients (4 unipolar and 3 bipolar), 5 patients showed a 50% decrease in HAM-D scores with adjunctive modafinil. Dosages ranged from 100 to 200 mg/d, although most patients took 200 mg/d. In this series, modafinil was added to a variety of treatments, including bupropion, nefazodone, paroxetine, venlafaxine, an unspecified tricyclic antidepressant (TCA), divalproex sodium, lamotrigine, lithium, electroconvulsive therapy, olanzapine, and gabapentin.15
The only randomized, double-blind, placebo-controlled trial of adjunctive modafinil for bipolar depression enrolled 85 patients with moderate or more severe depression. In this 6-week trial by Frye et al,2 41 patients received modafinil, 100 to 200 mg/d (mean dose 174.2 mg/d), and 44 received placebo.
Bipolar disorder plus ADHD
An estimated 10% to 21% of bipolar patients meet criteria for ADHD,16-19 although at times the line differentiating these 2 disorders is unclear. Co-occurring ADHD worsens the course of bipolar illness,20-22 and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial suggest that only 2% of dual-diagnosis patients are receiving treatment specifically for ADHD symptoms.23
Theoretically, overlapping symptoms such as talkativeness, distractibility, and physical activity remain relatively constant in ADHD but wax and wane with bipolar disorder’s manic and depressive phases. Recent evidence suggests, however, that many bipolar patients experience prodromal symptoms that may resemble ADHD, including cognitive impairment, distractibility, and increased psychomotor activity.24 In addition, medications used to treat bipolar disorder may impair cognitive function, making ADHD diagnosis difficult in this population.
We are not aware of any clinical trials that examined stimulants’ safety and efficacy in adult bipolar patients with co-occurring ADHD. One of the only studies to examine stimulant treatment of ADHD symptoms in a bipolar population was a retrospective chart review of 34 adolescents hospitalized with bipolar mania. An earlier age of bipolar illness onset was reported in adolescents who had been exposed to stimulants, whether or not they also had ADHD.25
Possible adverse events
Some bipolar disorder patients tolerate stimulants well, whereas others experience serious side effects, toxicities, and illness destabilization (Table 2). Because mood-stabilizer treatment may attenuate stimulants’ undesirable effects in bipolar disorder patients,26,27 be sure to use adequate dosing of a mood stabilizer if you determine a stimulant trial is warranted in your patient.
Destabilization. Stimulants can have a direct negative effect on mood; they can cause restlessness, irritability, anxiety, and mood lability. Some bipolar patients may be more sensitive to these adverse effects than others. Particularly concerning is the possibility of switching to mania or worsening of manic symptoms.28,29 Other potential destabilizing effects include:
- changing cycling patterns, such as inducing rapid cycling
- sleep disturbance because stimulants promote wakefulness.
If you are considering stimulant treatment for a bipolar disorder patient in whom substance abuse is a concern, modafinil or lisdexamfetamine may have a lower abuse potential compared with immediate-release psychostimulants. Lisdexamfetamine is metabolized in the GI tract and does not produce high d-amphetamine blood levels or cause reinforcing effects if injected or snorted.34
Table 2
Possible stimulant side effects, signs of toxicity, and contraindications
| Stimulant class | Possible side effects | Signs of toxicity/overdose | Contraindications/cautions |
|---|---|---|---|
| Traditional (amphetamine mixtures, dexmethylphenidate, dextroamphetamine, lisdexamfetamine methylphenidate)* | Restlessness, insomnia, mood lability, anxiety | Agitation, confusion, tremor, tachycardia, hyperreflexia, hypertension, sweating, psychomotor agitation, seizure, arrhythmia, coma, psychosis | Cardiovascular disease, hypertension, hyperthyroidism, glaucoma, Tourette’s syndrome/motor tics, history of seizure disorder, hypersensitivity to medication class |
| Novel (modafinil) | Restlessness, insomnia, mood lability, anxiety | Agitation, tremor, nausea, diarrhea, confusion | Cardiovascular disease, hepatic impairment, psychosis |
| * Amphetamines and dextroamphetamine (Adderall, Adderall XR); dexmethylphenidate (Focalin, Focalin XR), dextroamphetamine (Dexedrine, DextroStat); lisdexamfetamine (Vyvanse); methylphenidate (Concerta, Daytrana, Metadate CD, Methylin, Methylin ER, Ritalin, Ritalin LA, Ritalin SR) | |||
Drug-drug interactions
Polypharmacy is the rule in treating bipolar disorder, and stimulants can interact with many other psychotropics (Table 3).
Antidepressants. Never use traditional stimulants with monoamine oxidase inhibitors, as this combination may precipitate a hypertensive crisis. Coadministered stimulants also may decrease the metabolism of serotonergic agents—such as selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs)—and cause side effects associated with increased serotonin neurotransmission, including serotonin syndrome.
Combining traditional stimulants with TCAs may increase TCA concentrations. When coadministered with bupropion, stimulants can increase the risk of seizures.
Modafinil is both an inducer and inhibitor of cytochrome P450 isoenzymes. Because it induces CYP3A4 and inhibits CYP2C19 and CYP2C9, modafinil interacts with many other psychopharmacologic agents:
- Its induction of CYP3A4 may increase the metabolism of commonly used medications such as carbamazepine, aripiprazole, and triazolam.
- Its inhibition of CYP2C19 may decrease the metabolism of many SSRIs, TCAs, diazepam, and clozapine, increasing these drugs’ effects and adverse events.
Possible stimulant interactions with other psychotropics
| Stimulant class | Psychotropic medication | Possible adverse effects |
|---|---|---|
| Traditional (amphetamine mixtures, dexmethylphenidate, dextroamphetamine, lisdexamfetamine methylphenidate)* | MAOIs | Hypertensive crisis |
| CBZ | Reduced methylphenidate levels; abruptly stopping CBZ increases methylphenidate’s effect | |
| TCAs | Increased TCA concentration | |
| SSRIs, SNRIs | Possible decreased metabolism of antidepressants; potential for serotonin syndrome or NMS-like syndrome | |
| Typical and atypical antipsychotics | Each may interfere with the other’s therapeutic action | |
| Novel (modafinil) | CBZ | Decreased modafinil efficacy; decreased CBZ levels |
| Triazolam | Decreased triazolam efficacy; increased effects of triazolam with modafinil discontinuation | |
| Fluoxetine, fluvoxamine | Decreased modafinil clearance | |
| Citalopram, escitalopram, sertraline | Prolonged elimination and increased levels of antidepressant | |
| MAOIs | Hypertensive crisis(?); not recommended | |
| Diazepam | Prolonged elimination and increased levels of diazepam | |
| TCAs | Prolonged elimination and increased levels of TCAs | |
| Clozapine | Increased clozapine concentration (case report) | |
| Aripiprazole | Decreased levels of aripiprazole | |
| * Amphetamines and dextroamphetamine (Adderall, Adderall XR); dexmethylphenidate (Focalin, Focalin XR), dextroamphetamine (Dexedrine, DextroStat); lisdexamfetamine (Vyvanse); methylphenidate (Concerta, Daytrana, Metadate CD, Methylin, Methylin ER, Ritalin, Ritalin LA, Ritalin SR) | ||
| CBZ: carbamazepine; MAOIs: monoamine oxidase inhibitors; NMS: neuroleptic malignant syndrome; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants | ||
Treatment considerations
Without evidence to support stimulants’ safety and efficacy in patients with bipolar disorder, we cannot make specific recommendations. We would, however, like to offer some general recommendations if you decide to use stimulants when treating patients with bipolar disorder (Table 4).
Carefully assess and—in many cases—reassess the patient’s symptoms to clarify the diagnosis. As mentioned, ADHD and bipolar disorder share many symptoms, particularly in the manic phase of bipolar illness. Overlapping symptoms include decreased ability to concentrate and focus, distractibility, hyperactivity and psychomotor agitation, racing thoughts, and impulsivity.
Substance abuse can negatively impact bipolar illness and present as clinical scenarios in which stimulants are used (such as treatment-resistant depression, impulsivity, somnolence, or fatigue).
Treat medical conditions such as thyroid disease, diabetes, and sleep apnea, which may worsen depression, cause somnolence and sedation, and present with symptoms similar to those of ADHD.
When possible, use lifestyle techniques to help patients manage the course of bipolar illness. Encourage good sleep hygiene, exercise, stable social rhythms, and limited use of alcohol and caffeine (both of which can impair sleep quality, which affects illness stability).
The next step. When you have explored all medication options and ruled out all other causes for the patient’s symptoms, stimulant treatment may be an appropriate next step. In these cases:
Encourage patients to participate in treatment, particularly in monitoring mood changes (as with life charts), symptoms associated with mood episodes, and emergence of side effects. When possible, involve family members in monitoring for adverse events.
Administration. Start stimulants only when bipolar illness is well-stabilized, especially regarding manic symptoms. We highly caution against using stimulants in patients with manic or hypomanic symptoms, including mixed states. We recommend not using stimulants in patients with:
- clinically significant insomnia or sleep fragmentation
- active suicidal ideation or psychotic symptoms, particularly if associated with manic symptoms.
Schedule frequent office visits when prescribing stimulants. At least initially, see patients every other week to assess for the emergence of adverse events.
Table 4
6 recommendations when using stimulants in bipolar disorder
| Carefully assess patient’s symptoms | Manic symptoms vs ADHD; medical conditions such as thyroid disorders, diabetes, or sleep apnea |
| Review possible iatrogenic causes of symptoms | Somnolence, decreased energy/fatigue, sedation, difficulty with concentration/focus |
| Engage patient in the therapeutic process | Discuss risks and benefits; monitor mood with life charts; enlist help of family, significant others when appropriate |
| Use caution in clinical scenarios that may herald adverse response to stimulants | Manic/hypomanic symptoms; sleep disturbances; psychosis; history of substance abuse |
| Administer stimulants with caution | Start low and go slow; always use stimulants in conjunction with a mood-stabilizing agent; be aware of possible interactions with patient’s other medications; schedule more frequent visits when starting stimulants |
| Monitor for adverse events associated with stimulant administration | Manic symptoms, changes in cycling patterns, sleep disturbances, substance abuse |
| ADHD: attention-deficit/hyperactivity disorder | |
- The Texas Medication Algorithm Project. Texas Department of State Health Services. www.dshs.state.tx.us/mhprograms/tmapover.shtm.
- The Cochrane Collaboration. www.cochrane.org.
- Amphetamine and dextroamphetamine • Adderall
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clozapine • Clozaril
- Dexmethylphenidate • Focalin
- Dextroamphetamine • Dexedrine, DextroStat
- Diazepam • Valium
- Divalproex sodium • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Lisdexamfetamine • Vyvanse
- Lithium • various
- Methylphenidate • Ritalin, Concerta, others
- Modafinil • Provigil
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Triazolam • Halcion
- Valproic acid • Depakene
- Venlafaxine • Effexor
Dr. Gonzalez reports no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products. He is a recipient of a T32 Ruth L. Kirschstein National Research Service Awards training fellowship sponsored by the National Institutes of Health.
Dr. Suppes receives grants/research support from Abbott Laboratories, AstraZeneca, GlaxoSmithKline, JDS Pharmaceuticals, Janssen Pharmaceutica, National Institute of Mental Health, Novartis, Pfizer Inc., and the Stanley Medical Research Institute.
Patients with bipolar disorder show an unpredictable range of responses to stimulants, from virtually no ill effects to emerging manic-like symptoms.1 Thus, although stimulants may be beneficial to some bipolar patients, there is a great deal of concern about using stimulants in this population. Even so, stimulants may be a rational adjunct for treating certain aspects of bipolar illness, particularly resistant depression, iatrogenic sedation, and comorbid attention-deficit/hyperactivity disorder (ADHD).
To help you decide if and when your patient might be a candidate for stimulant therapy, this article:
- reviews the evidence on stimulants’ safety and tolerability for patients with bipolar disorder
- weighs potential benefits and risks of using stimulants in this population
- addresses stimulants’ possible adverse effects on illness course and from interactions with other psychotropics
- discusses treatment options based on the limited evidence and our clinical experience.
Limited support
We are aware that using stimulants to treat patients with bipolar disorder is not an uncommon clinical practice, but supportive evidence is limited (Table 1). In searching the literature, we found only 2 randomized controlled studies—Frye et al2 and Scheffer et al3—that addressed this practice. (One author of this review [TS] participated as a coinvestigator with Frye et al.2) Other evidence that suggests a role for stimulants in bipolar disorder comes from case reports, retrospective case series, and open-label studies.4-11
- “traditional” stimulants (including amphetamine-based compounds such as dextroamphetamine, methylphenidate, dexmethylphenidate, and lisdexamfetamine) thought to affect the dopamine transporter, resulting in increased dopamine in nerve terminals
- the “novel” psychostimulant modafinil, thought to affect multiple neurotransmitter systems (dopamine, GABA, serotonin, histamine, and glutamate), although its mechanism of action is unclear.
Table 1
Clinical studies of stimulant use in patients with bipolar disorder
| Stimulant(s) studied | Study design | Patients studied | Clinical outcomes |
|---|---|---|---|
| Traditional stimulants | |||
| Adjunctive methylphenidate | Chart review, naturalistic12 | 16 adults (5 with comorbid ADHD, 11 with bipolar depression) | Improvements in depression, overall functioning, and ability to concentrate; sleep disturbance, irritability/agitation reported |
| Adjunctive methylphenidate or racemic mixture of AMPH salts | Chart review of sedation and depressive symptoms13 | 8 adults (BD II) | Improved clinical impression of bipolar illness; no manic switches, changes in cycling patterns, or substance abuse noted |
| Adjunctive methylphenidate | 12-week open study, bipolar depression14 | 12 adults (10 BD I, 2 BD II) | Significant clinical improvements in depressive symptoms; no change in manic symptoms; anxiety, agitation, and hypomania reported |
| Multiple stimulants | Chart review, history of stimulants and bipolar illness course25 | 34 hospitalized adolescents | Prior stimulant treatment associated with earlier age of illness onset |
| Adjunctive mixed amphetamine salts | Randomized, placebo-controlled; comorbid BD and ADHD3 | 30 children with ADHD symptoms stabilized on divalproex sodium | Decrease in ADHD symptoms with adjunctive amphetamine treatment but not with divalproex sodium alone; 1 case of mania |
| Novel stimulant | |||
| Adjunctive modafinil | Case series15 | Mixed sample of depressed adults (4 unipolar, 3 bipolar) | Significant improvement in depressive symptoms |
| Adjunctive modafinil | Randomized, double-blind, placebo-controlled2 | 85 adults with bipolar depression | Treatment group showed greater response and remission of depressive symptoms compared with placebo group; no difference in development of manic symptoms |
| ADHD: attention-deficit/hyperactivity disorder; AMPH: amphetamine; BD: bipolar disorder; NOS: not otherwise specified | |||
Depression and iatrogenic sedation
Small, uncontrolled trials have reported some benefit and tolerability in bipolar disorder patients when stimulants are used to treat residual depressive symptoms or iatrogenic sedation associated with mood stabilizers.
Traditional stimulants. A retrospective chart review of 16 patients treated with adjunctive methylphenidate noted improved functioning, as measured by the Global Assessment of Functioning scale. Some patients’ depressive symptoms and concentration also appeared to improve, but how these parameters were assessed is not clear. Some patients tolerated stimulants well, whereas others experienced irritability, agitation, and sleep disturbances.12
Another retrospective chart review described 8 patients with iatrogenic sedation or depression who received adjunctive methylphenidate, mean 20 to 40 mg/d, or a racemic mixture of amphetamine salts, mean 20 to 40 mg/d. Overall bipolar symptoms decreased in severity, as measured by Clinical Global Impression (CGI) scores, but the authors did not directly measure sedation or depression. The stimulants were well-tolerated, with no evidence of stimulant-induced mania.13
In a 12-week open-label trial of methylphenidate in 14 patients with bipolar disorder, depressive symptoms improved as measured by the Hamilton Depression Rating Scale (HAM-D). Mean doses were 10 mg/d for the 3 patients who discontinued because of anxiety, agitation, or hypomania and 16.6 mg/d for those who completed the trial.14
Modafinil may have some efficacy in treating bipolar depression. In a case series of 7 depressed patients (4 unipolar and 3 bipolar), 5 patients showed a 50% decrease in HAM-D scores with adjunctive modafinil. Dosages ranged from 100 to 200 mg/d, although most patients took 200 mg/d. In this series, modafinil was added to a variety of treatments, including bupropion, nefazodone, paroxetine, venlafaxine, an unspecified tricyclic antidepressant (TCA), divalproex sodium, lamotrigine, lithium, electroconvulsive therapy, olanzapine, and gabapentin.15
The only randomized, double-blind, placebo-controlled trial of adjunctive modafinil for bipolar depression enrolled 85 patients with moderate or more severe depression. In this 6-week trial by Frye et al,2 41 patients received modafinil, 100 to 200 mg/d (mean dose 174.2 mg/d), and 44 received placebo.
Bipolar disorder plus ADHD
An estimated 10% to 21% of bipolar patients meet criteria for ADHD,16-19 although at times the line differentiating these 2 disorders is unclear. Co-occurring ADHD worsens the course of bipolar illness,20-22 and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial suggest that only 2% of dual-diagnosis patients are receiving treatment specifically for ADHD symptoms.23
Theoretically, overlapping symptoms such as talkativeness, distractibility, and physical activity remain relatively constant in ADHD but wax and wane with bipolar disorder’s manic and depressive phases. Recent evidence suggests, however, that many bipolar patients experience prodromal symptoms that may resemble ADHD, including cognitive impairment, distractibility, and increased psychomotor activity.24 In addition, medications used to treat bipolar disorder may impair cognitive function, making ADHD diagnosis difficult in this population.
We are not aware of any clinical trials that examined stimulants’ safety and efficacy in adult bipolar patients with co-occurring ADHD. One of the only studies to examine stimulant treatment of ADHD symptoms in a bipolar population was a retrospective chart review of 34 adolescents hospitalized with bipolar mania. An earlier age of bipolar illness onset was reported in adolescents who had been exposed to stimulants, whether or not they also had ADHD.25
Possible adverse events
Some bipolar disorder patients tolerate stimulants well, whereas others experience serious side effects, toxicities, and illness destabilization (Table 2). Because mood-stabilizer treatment may attenuate stimulants’ undesirable effects in bipolar disorder patients,26,27 be sure to use adequate dosing of a mood stabilizer if you determine a stimulant trial is warranted in your patient.
Destabilization. Stimulants can have a direct negative effect on mood; they can cause restlessness, irritability, anxiety, and mood lability. Some bipolar patients may be more sensitive to these adverse effects than others. Particularly concerning is the possibility of switching to mania or worsening of manic symptoms.28,29 Other potential destabilizing effects include:
- changing cycling patterns, such as inducing rapid cycling
- sleep disturbance because stimulants promote wakefulness.
If you are considering stimulant treatment for a bipolar disorder patient in whom substance abuse is a concern, modafinil or lisdexamfetamine may have a lower abuse potential compared with immediate-release psychostimulants. Lisdexamfetamine is metabolized in the GI tract and does not produce high d-amphetamine blood levels or cause reinforcing effects if injected or snorted.34
Table 2
Possible stimulant side effects, signs of toxicity, and contraindications
| Stimulant class | Possible side effects | Signs of toxicity/overdose | Contraindications/cautions |
|---|---|---|---|
| Traditional (amphetamine mixtures, dexmethylphenidate, dextroamphetamine, lisdexamfetamine methylphenidate)* | Restlessness, insomnia, mood lability, anxiety | Agitation, confusion, tremor, tachycardia, hyperreflexia, hypertension, sweating, psychomotor agitation, seizure, arrhythmia, coma, psychosis | Cardiovascular disease, hypertension, hyperthyroidism, glaucoma, Tourette’s syndrome/motor tics, history of seizure disorder, hypersensitivity to medication class |
| Novel (modafinil) | Restlessness, insomnia, mood lability, anxiety | Agitation, tremor, nausea, diarrhea, confusion | Cardiovascular disease, hepatic impairment, psychosis |
| * Amphetamines and dextroamphetamine (Adderall, Adderall XR); dexmethylphenidate (Focalin, Focalin XR), dextroamphetamine (Dexedrine, DextroStat); lisdexamfetamine (Vyvanse); methylphenidate (Concerta, Daytrana, Metadate CD, Methylin, Methylin ER, Ritalin, Ritalin LA, Ritalin SR) | |||
Drug-drug interactions
Polypharmacy is the rule in treating bipolar disorder, and stimulants can interact with many other psychotropics (Table 3).
Antidepressants. Never use traditional stimulants with monoamine oxidase inhibitors, as this combination may precipitate a hypertensive crisis. Coadministered stimulants also may decrease the metabolism of serotonergic agents—such as selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs)—and cause side effects associated with increased serotonin neurotransmission, including serotonin syndrome.
Combining traditional stimulants with TCAs may increase TCA concentrations. When coadministered with bupropion, stimulants can increase the risk of seizures.
Modafinil is both an inducer and inhibitor of cytochrome P450 isoenzymes. Because it induces CYP3A4 and inhibits CYP2C19 and CYP2C9, modafinil interacts with many other psychopharmacologic agents:
- Its induction of CYP3A4 may increase the metabolism of commonly used medications such as carbamazepine, aripiprazole, and triazolam.
- Its inhibition of CYP2C19 may decrease the metabolism of many SSRIs, TCAs, diazepam, and clozapine, increasing these drugs’ effects and adverse events.
Possible stimulant interactions with other psychotropics
| Stimulant class | Psychotropic medication | Possible adverse effects |
|---|---|---|
| Traditional (amphetamine mixtures, dexmethylphenidate, dextroamphetamine, lisdexamfetamine methylphenidate)* | MAOIs | Hypertensive crisis |
| CBZ | Reduced methylphenidate levels; abruptly stopping CBZ increases methylphenidate’s effect | |
| TCAs | Increased TCA concentration | |
| SSRIs, SNRIs | Possible decreased metabolism of antidepressants; potential for serotonin syndrome or NMS-like syndrome | |
| Typical and atypical antipsychotics | Each may interfere with the other’s therapeutic action | |
| Novel (modafinil) | CBZ | Decreased modafinil efficacy; decreased CBZ levels |
| Triazolam | Decreased triazolam efficacy; increased effects of triazolam with modafinil discontinuation | |
| Fluoxetine, fluvoxamine | Decreased modafinil clearance | |
| Citalopram, escitalopram, sertraline | Prolonged elimination and increased levels of antidepressant | |
| MAOIs | Hypertensive crisis(?); not recommended | |
| Diazepam | Prolonged elimination and increased levels of diazepam | |
| TCAs | Prolonged elimination and increased levels of TCAs | |
| Clozapine | Increased clozapine concentration (case report) | |
| Aripiprazole | Decreased levels of aripiprazole | |
| * Amphetamines and dextroamphetamine (Adderall, Adderall XR); dexmethylphenidate (Focalin, Focalin XR), dextroamphetamine (Dexedrine, DextroStat); lisdexamfetamine (Vyvanse); methylphenidate (Concerta, Daytrana, Metadate CD, Methylin, Methylin ER, Ritalin, Ritalin LA, Ritalin SR) | ||
| CBZ: carbamazepine; MAOIs: monoamine oxidase inhibitors; NMS: neuroleptic malignant syndrome; SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors; TCAs: tricyclic antidepressants | ||
Treatment considerations
Without evidence to support stimulants’ safety and efficacy in patients with bipolar disorder, we cannot make specific recommendations. We would, however, like to offer some general recommendations if you decide to use stimulants when treating patients with bipolar disorder (Table 4).
Carefully assess and—in many cases—reassess the patient’s symptoms to clarify the diagnosis. As mentioned, ADHD and bipolar disorder share many symptoms, particularly in the manic phase of bipolar illness. Overlapping symptoms include decreased ability to concentrate and focus, distractibility, hyperactivity and psychomotor agitation, racing thoughts, and impulsivity.
Substance abuse can negatively impact bipolar illness and present as clinical scenarios in which stimulants are used (such as treatment-resistant depression, impulsivity, somnolence, or fatigue).
Treat medical conditions such as thyroid disease, diabetes, and sleep apnea, which may worsen depression, cause somnolence and sedation, and present with symptoms similar to those of ADHD.
When possible, use lifestyle techniques to help patients manage the course of bipolar illness. Encourage good sleep hygiene, exercise, stable social rhythms, and limited use of alcohol and caffeine (both of which can impair sleep quality, which affects illness stability).
The next step. When you have explored all medication options and ruled out all other causes for the patient’s symptoms, stimulant treatment may be an appropriate next step. In these cases:
Encourage patients to participate in treatment, particularly in monitoring mood changes (as with life charts), symptoms associated with mood episodes, and emergence of side effects. When possible, involve family members in monitoring for adverse events.
Administration. Start stimulants only when bipolar illness is well-stabilized, especially regarding manic symptoms. We highly caution against using stimulants in patients with manic or hypomanic symptoms, including mixed states. We recommend not using stimulants in patients with:
- clinically significant insomnia or sleep fragmentation
- active suicidal ideation or psychotic symptoms, particularly if associated with manic symptoms.
Schedule frequent office visits when prescribing stimulants. At least initially, see patients every other week to assess for the emergence of adverse events.
Table 4
6 recommendations when using stimulants in bipolar disorder
| Carefully assess patient’s symptoms | Manic symptoms vs ADHD; medical conditions such as thyroid disorders, diabetes, or sleep apnea |
| Review possible iatrogenic causes of symptoms | Somnolence, decreased energy/fatigue, sedation, difficulty with concentration/focus |
| Engage patient in the therapeutic process | Discuss risks and benefits; monitor mood with life charts; enlist help of family, significant others when appropriate |
| Use caution in clinical scenarios that may herald adverse response to stimulants | Manic/hypomanic symptoms; sleep disturbances; psychosis; history of substance abuse |
| Administer stimulants with caution | Start low and go slow; always use stimulants in conjunction with a mood-stabilizing agent; be aware of possible interactions with patient’s other medications; schedule more frequent visits when starting stimulants |
| Monitor for adverse events associated with stimulant administration | Manic symptoms, changes in cycling patterns, sleep disturbances, substance abuse |
| ADHD: attention-deficit/hyperactivity disorder | |
- The Texas Medication Algorithm Project. Texas Department of State Health Services. www.dshs.state.tx.us/mhprograms/tmapover.shtm.
- The Cochrane Collaboration. www.cochrane.org.
- Amphetamine and dextroamphetamine • Adderall
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clozapine • Clozaril
- Dexmethylphenidate • Focalin
- Dextroamphetamine • Dexedrine, DextroStat
- Diazepam • Valium
- Divalproex sodium • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Lisdexamfetamine • Vyvanse
- Lithium • various
- Methylphenidate • Ritalin, Concerta, others
- Modafinil • Provigil
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Triazolam • Halcion
- Valproic acid • Depakene
- Venlafaxine • Effexor
Dr. Gonzalez reports no financial relationship with any company whose products are mentioned in the article or with manufacturers of competing products. He is a recipient of a T32 Ruth L. Kirschstein National Research Service Awards training fellowship sponsored by the National Institutes of Health.
Dr. Suppes receives grants/research support from Abbott Laboratories, AstraZeneca, GlaxoSmithKline, JDS Pharmaceuticals, Janssen Pharmaceutica, National Institute of Mental Health, Novartis, Pfizer Inc., and the Stanley Medical Research Institute.
1. Silberman EK, Reus VI, Jimerson DC, et al. Heterogeneity of amphetamine response in depressed patients. Am J Psychiatry 1981;138(10):1302-7.
2. Frye MA, Grunze H, Suppes T, et al. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry 2007;164(8):1242-9.
3. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
4. Meyers B. Treatment of imipramine-resistant depression and lithium-refractory mania through drug interactions. Am J Psychiatry 1978;135(11):1420-1.
5. Bannet J, Ebstein RP, Belmaker RH. Clinical aspects of the interaction of lithium and stimulants. Br J Psychiatry 1980;136:204.-
6. Drimmer EJ, Gitlin MJ, Gwirtsman HE. Desipramine and methylphenidate combination treatment for depression: case report. Am J Psychiatry 1983;140(2):241-2.
7. Fernandes PP, Petty F. Modafinil for remitted bipolar depression with hypersomnia. Ann Pharmacother 2003;37(12):1807-9.
8. Berigan TR. Augmentation with modafinil to achieve remission in depression: a case report. Prim Care Companion J Clin Psychiatry 2001;3(1):32.-
9. Berigan TR. Modafinil treatment of excessive daytime sedation and fatigue associated with topiramate. Prim Care Companion J Clin Psychiatry 2002;4(6):249-50.
10. Berigan T. Modafinil treatment of excessive sedation associated with divalproex sodium. Can J Psychiatry 2004;49(1):72-3.
11. Even C, Thuile J, Santos J, Bourgin P. Modafinil as an adjunctive treatment to sleep deprivation in depression. J Psychiatry Neurosci 2005;30(6):432-3.
12. Lydon E, El-Mallakh RS. Naturalistic long-term use of methylphenidate in bipolar disorder. J Clin Psychopharmacol 2006;26(5):516-8.
13. Carlson PJ, Merlock MC, Suppes T. Adjunctive stimulant use in patients with bipolar disorder: treatment of residual depression and sedation. Bipolar Disord 2004;6(5):416-20.
14. El-Mallakh RS. An open study of methylphenidate in bipolar depression. Bipolar Disord 2000;2(1):56-9.
15. Menza MA, Kaufman KR, Castellanos A. Modafinil augmentation of antidepressant treatment in depression. J Clin Psychiatry 2000;61(5):378-81.
16. Wingo AP, Ghaemi SN. A systematic review of rates and diagnostic validity of comorbid adult attention-deficit/hyperactivity disorder and bipolar disorder. J Clin Psychiatry 2007;68(11):1776-84.
17. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163(4):716-23.
18. Nierenberg AA, Miyahara S, Spencer T, et al. Clinical and diagnostic implications of lifetime attention-deficit/hyperactivity disorder comorbidity in adults with bipolar disorder: data from the first 1000 STEP-BD participants. Biol Psychiatry 2005;57(11):1467-73.
19. Tamam L, Tuglu C, Karatas G, Ozcan S. Adult attention-deficit hyperactivity disorder in patients with bipolar I disorder in remission: preliminary study. Psychiatry Clin Neurosci 2006;60(4):480-5.
20. Faraone SV, Biederman J, Mennin D, et al. Attention-deficit hyperactivity disorder with bipolar disorder: a familial subtype? J Am Acad Child Adolesc Psychiatry 1997;36(10):1378-87; discussion 1387-90.
21. Faraone SV, Biederman J, Monuteaux MC. Attention deficit hyperactivity disorder with bipolar disorder in girls: further evidence for a familial subtype? J Affect Disord 2001;64(1):19-26.
22. Faraone SV, Glatt SJ, Tsuang MT. The genetics of pediatriconset bipolar disorder. Biol Psychiatry 2003;53(11):970-7.
23. Simon NM, Otto MW, Weiss RD, et al. Pharmacotherapy for bipolar disorder and comorbid conditions: baseline data from STEP-BD. J Clin Psychopharmacol 2004;24(5):512-20.
24. Calabrese JR. Overview of patient care issues and treatment in bipolar spectrum and bipolar II disorder. J Clin Psychiatry 2008;69(6):e18.-
25. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001;3(2):53-7.
26. Van Kammen DP, Murphy DL. Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia 1975;44(3):215-24.
27. Huey LY, Janowsky DS, Judd LL, et al. Effects of lithium carbonate on methylphenidate-induced mood, behavior, and cognitive processes. Psychopharmacology (Berl) 1981;73(2):161-4.
28. Gerner RH, Post RM, Bunney WE, Jr. A dopaminergic mechanism in mania. Am J Psychiatry 1976;133(10):1177-80.
29. Koehler-Troy C, Strober M, Malenbaum R. Methylphenidateinduced mania in a prepubertal child. J Clin Psychiatry 1986;47(11):566-7.
30. Brady KT, Sonne SC. The relationship between substance abuse and bipolar disorder. J Clin Psychiatry 1995;56(suppl 3):19-24.
31. Sonne SC, Brady KT. Substance abuse and bipolar comorbidity. Psychiatr Clin North Am 1999;22(3):609-27,ix.
32. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990;264(19):2511-8.
33. Estroff TW, Dackis CA, Gold MS, Pottash AL. Drug abuse and bipolar disorders. Int J Psychiatry Med 1985-1986;15(1):37-40.
34. Faraone SV. Lisdexamfetamine dimesylate: the first longacting prodrug stimulant treatment for attention deficit/hyperactivity disorder. Expert Opin Pharmacother 2008;9(9):1565-74.
1. Silberman EK, Reus VI, Jimerson DC, et al. Heterogeneity of amphetamine response in depressed patients. Am J Psychiatry 1981;138(10):1302-7.
2. Frye MA, Grunze H, Suppes T, et al. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry 2007;164(8):1242-9.
3. Scheffer RE, Kowatch RA, Carmody T, Rush AJ. Randomized, placebo-controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry 2005;162(1):58-64.
4. Meyers B. Treatment of imipramine-resistant depression and lithium-refractory mania through drug interactions. Am J Psychiatry 1978;135(11):1420-1.
5. Bannet J, Ebstein RP, Belmaker RH. Clinical aspects of the interaction of lithium and stimulants. Br J Psychiatry 1980;136:204.-
6. Drimmer EJ, Gitlin MJ, Gwirtsman HE. Desipramine and methylphenidate combination treatment for depression: case report. Am J Psychiatry 1983;140(2):241-2.
7. Fernandes PP, Petty F. Modafinil for remitted bipolar depression with hypersomnia. Ann Pharmacother 2003;37(12):1807-9.
8. Berigan TR. Augmentation with modafinil to achieve remission in depression: a case report. Prim Care Companion J Clin Psychiatry 2001;3(1):32.-
9. Berigan TR. Modafinil treatment of excessive daytime sedation and fatigue associated with topiramate. Prim Care Companion J Clin Psychiatry 2002;4(6):249-50.
10. Berigan T. Modafinil treatment of excessive sedation associated with divalproex sodium. Can J Psychiatry 2004;49(1):72-3.
11. Even C, Thuile J, Santos J, Bourgin P. Modafinil as an adjunctive treatment to sleep deprivation in depression. J Psychiatry Neurosci 2005;30(6):432-3.
12. Lydon E, El-Mallakh RS. Naturalistic long-term use of methylphenidate in bipolar disorder. J Clin Psychopharmacol 2006;26(5):516-8.
13. Carlson PJ, Merlock MC, Suppes T. Adjunctive stimulant use in patients with bipolar disorder: treatment of residual depression and sedation. Bipolar Disord 2004;6(5):416-20.
14. El-Mallakh RS. An open study of methylphenidate in bipolar depression. Bipolar Disord 2000;2(1):56-9.
15. Menza MA, Kaufman KR, Castellanos A. Modafinil augmentation of antidepressant treatment in depression. J Clin Psychiatry 2000;61(5):378-81.
16. Wingo AP, Ghaemi SN. A systematic review of rates and diagnostic validity of comorbid adult attention-deficit/hyperactivity disorder and bipolar disorder. J Clin Psychiatry 2007;68(11):1776-84.
17. Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163(4):716-23.
18. Nierenberg AA, Miyahara S, Spencer T, et al. Clinical and diagnostic implications of lifetime attention-deficit/hyperactivity disorder comorbidity in adults with bipolar disorder: data from the first 1000 STEP-BD participants. Biol Psychiatry 2005;57(11):1467-73.
19. Tamam L, Tuglu C, Karatas G, Ozcan S. Adult attention-deficit hyperactivity disorder in patients with bipolar I disorder in remission: preliminary study. Psychiatry Clin Neurosci 2006;60(4):480-5.
20. Faraone SV, Biederman J, Mennin D, et al. Attention-deficit hyperactivity disorder with bipolar disorder: a familial subtype? J Am Acad Child Adolesc Psychiatry 1997;36(10):1378-87; discussion 1387-90.
21. Faraone SV, Biederman J, Monuteaux MC. Attention deficit hyperactivity disorder with bipolar disorder in girls: further evidence for a familial subtype? J Affect Disord 2001;64(1):19-26.
22. Faraone SV, Glatt SJ, Tsuang MT. The genetics of pediatriconset bipolar disorder. Biol Psychiatry 2003;53(11):970-7.
23. Simon NM, Otto MW, Weiss RD, et al. Pharmacotherapy for bipolar disorder and comorbid conditions: baseline data from STEP-BD. J Clin Psychopharmacol 2004;24(5):512-20.
24. Calabrese JR. Overview of patient care issues and treatment in bipolar spectrum and bipolar II disorder. J Clin Psychiatry 2008;69(6):e18.-
25. DelBello MP, Soutullo CA, Hendricks W, et al. Prior stimulant treatment in adolescents with bipolar disorder: association with age at onset. Bipolar Disord 2001;3(2):53-7.
26. Van Kammen DP, Murphy DL. Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia 1975;44(3):215-24.
27. Huey LY, Janowsky DS, Judd LL, et al. Effects of lithium carbonate on methylphenidate-induced mood, behavior, and cognitive processes. Psychopharmacology (Berl) 1981;73(2):161-4.
28. Gerner RH, Post RM, Bunney WE, Jr. A dopaminergic mechanism in mania. Am J Psychiatry 1976;133(10):1177-80.
29. Koehler-Troy C, Strober M, Malenbaum R. Methylphenidateinduced mania in a prepubertal child. J Clin Psychiatry 1986;47(11):566-7.
30. Brady KT, Sonne SC. The relationship between substance abuse and bipolar disorder. J Clin Psychiatry 1995;56(suppl 3):19-24.
31. Sonne SC, Brady KT. Substance abuse and bipolar comorbidity. Psychiatr Clin North Am 1999;22(3):609-27,ix.
32. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990;264(19):2511-8.
33. Estroff TW, Dackis CA, Gold MS, Pottash AL. Drug abuse and bipolar disorders. Int J Psychiatry Med 1985-1986;15(1):37-40.
34. Faraone SV. Lisdexamfetamine dimesylate: the first longacting prodrug stimulant treatment for attention deficit/hyperactivity disorder. Expert Opin Pharmacother 2008;9(9):1565-74.
Bipolar treatment update: Evidence is driving change in mania, depression algorithms
Many well-controlled trials in the past 4 years have evaluated new medications for treating bipolar disorder. It’s time to build a consensus on how this data may apply to clinical practice.
This year, our group will re-examine the Texas Medication Algorithm Project (TMAP) treatment algorithms for bipolar I disorder.
What makes TMAP unique? It is the first project to evaluate treatment algorithm use in community mental health settings for patients with a history of mania (see Box).1-5 Severely, persistently ill outpatients such as these are seldom included in research but are frequently seen in clinical practice.
To preview for psychiatrists the changes expected in 2004, this article describes the goals of TMAP and the controlled study on which the medication algorithms are based. We review the medication algorithms of 2000 as a starting point and present the evidence that is changing clinical practice.
Guiding principles of TMAP
A treatment algorithm is no substitute for clinical judgment; rather, medication guidelines and algorithms are guideposts to help the clinician and patient collaboratively develop the most effective medication strategy with the fewest side effects.
The Texas Medication Algorithm Project (TMAP)1-3 is a public and academic collaboration started in 1996 to develop evidence- and consensus-based medication treatment algorithms for schizophrenia, major depressive disorder, and bipolar disorder.
TMAP’s goal is to establish “best practices” to encourage uniformity of care, achieve the best possible patient outcomes, and use mental health care dollars most efficiently. The project includes four phases, in which the treatment algorithms were developed, compared with treatment-as-usual, put into practice, and will undergo periodic updates.4 The next update begins this year.
The comparison of algorithms for treating bipolar mania/hypomania and depression included 409 patients (mean age 38 to 40) with bipolar I disorder or schizoaffective disorder, bipolar type. These patients were severely and persistently mentally ill, from a diverse ethnic population, and significantly impaired in functioning.
During 12 months of treatment, psychiatric symptoms diminished more rapidly in patients in the algorithm group—as measured by the Brief Psychiatric Rating Scale (BPRS-24)—compared with those receiving usual treatment. After the first 3 months, the usual-treatment patients also showed diminished symptoms. At study’s end, symptom severity between the groups was not significantly different; both groups showed improvement.
Manic and psychotic symptoms—measured by Clinician-Administered Rating Scale subscales (CARS-M)5—improved significantly more in the algorithm group in the first 3 months, and this gap between the two groups was sustained for 12 months. Depressive symptoms declined, but no overall differences were noted between the two groups. Side effect rates and functioning were also similar.
TMAP’s treatment manual (see Related resources) describes clinicians’ preferred tactics and decision points, which we summarize here. The guidelines are an ongoing effort to apply evidence-based medicine to everyday practice and are meant to be adapted to patient needs.
Treatment goals that guided TMAP algorithm development are:
- symptomatic remission
- full return of psychosocial functioning
- prevention of relapse and recurrence.
Suggestions came from controlled clinical trials, open trials, retrospective data analyses, expert clinical consensus, and input from consumers.
Treatment selection. Initial algorithm stages recommend simple treatments (in terms of safety, tolerability, and side effects), whereas later stages recommend more-complicated regimens. A patient’s symptoms, comorbid conditions, and treatment history guide treatment selection. Patients may enter an algorithm at any stage, depending on their clinical presentation and medication history.
The clinician may consider patient preference when deciding among equivalent medications. The algorithm strongly encourages patients and families to participate, such as by keeping daily mood charts and completing symptom and side-effect checklists. When clinicians face a choice among medication brands, generics, or forms (such as immediate- versus slow-release), agents with greater tolerability are preferred.
Patient management. When patients enter the algorithm, clinic visits are frequent (such as every 2 weeks). Follow-up appointments address medication adherence, dosage adjustments, and side effects or adverse reactions.
If a patient’s symptoms show no change after two treatment stages, re-evaluate the diagnosis and consider mitigating factors such as substance abuse. Patients who complete acute treatment should receive continuation treatment.
Documentation. Clinicians are advised to document decision points and the rationale for treatment choices made outside the algorithm package.
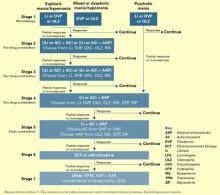
Treating mania or hypomania
After clinical evaluation confirms the diagnosis of bipolar illness,4 the TMAP mania/hypomania algorithm (Algorithm 1) splits into three treatment pathways:
- euphoric mania/hypomania
- mixed or dysphoric mania/hypomania
- psychotic mania.
These pathways recognize the need for differing approaches to initial monotherapy and later two-drug combinations. If a patient develops persistent or severe depressive symptoms, the bipolar algorithm for a major depressive episode (Algorithm 2) is used during depressive periods with the primary mania algorithm.
Treatment recommendations. The key to using mood stabilizers is to achieve the optimum response—assuming good tolerability—before switching to another agent. Adjust medication dosages one at a time to allow adequate response and assessment.
When switching medications, use an overlap-and-taper strategy, assuming there is no medical necessity to stop a drug abruptly. Add the new medication, then gradually taper the one that is being discontinued. Monitor serum levels.
Discontinue antidepressants when appropriate in patients with hypomania/mania or rapid cycling, and continually evaluate suicide and homicide potential of patients in mixed or depressive states.
Stage 1: Monotherapy. First medication choices are lithium, divalproex, or olanzapine. For mixed or dysphoric mania, the algorithm recommends divalproex (preferred over valproic acid because of tolerability and side effects) or olanzapine.6 Data suggest dysphoric manic patients are less likely to respond to lithium.7 A Consensus Panel minority expressed concern about using olanzapine as first-line monotherapy for acute mania because of limited data on the drug’s long-term safety. Patients with partial response or residual symptoms may move to stage 2 or switch to other medication options within stage 1.
Patients with psychotic mania move directly to stage 4 for a broader range of combination therapy.
Stage 2: Combination therapy. Combination therapy has become the standard of care in treating most patients with bipolar disorder. The algorithm recommends using two agents:
- lithium or an anticonvulsant plus another anticonvulsant ([Li or AC]+AC)
- or lithium or an anticonvulsant plus an atypical antipsychotic ([Li or AC]+AAP).8
Recommended agents include lithium, divalproex, oxcarbazepine, olanzapine, or risperidone. The experts recommended oxcarbazepine as first choice because it is better tolerated and interacts with fewer drugs than carbamazepine and does not require serum level monitoring.9
A Consensus Panel minority expressed concern that few studies had examined using oxcarbazepine in bipolar disorder. Carbamazepine was also considered an option.
Stages 3 and 4: Other two-drug combinations. Other two-drug combinations are tried at these stages, drawing from the same pool of medication classes described in stage 2.
Stage 4 broadens the choice of atypical antipsychotic by adding quetiapine10 and ziprasidone11 to the recommended stage-2 agents olanzapine and risperidone. When the 2000 algorithm was developed, limited data were available on using some newer atypicals in patients with bipolar mania. Based on recent, high-quality studies of mono- and combination therapy—including quetiapine,10 ziprasidone,11 risperidone,12,13 and aripiprazole14 —the 2004 algorithm update panel will likely recommend using atypicals earlier, including at stage 1.
Algorithm 1 Treating mania/hypomania in patients with bipolar I disorder
Stage 5: Triple-drug combination. Lithium, an anticonvulsant (divalproex or oxcarbazepine), and an atypical antipsychotic (olanzapine, risperidone, quetiapine, or ziprasidone) is a recommended triple-drug combination. In the 2004 update, the choices will likely expand to include all the newer atypicals and will list carbamazepine as an option.
Stage 6: ECT or clozapine. For patients with inadequate response to triple-drug combinations, the algorithm recommends adding electroconvulsive therapy (ECT) or clozapine.
ECT15 is recommended three times a week until the patient achieves remission of manic symptoms or fails to achieve a sustained response over three to six treatment cycles. Treatment resistance is declared if no response is seen after 6 to 10 treatment cycles.
Clozapine’s16 recommendation at this stage is consistent with its use in patients who fail to respond to other atypical antipsychotics. Blood monitoring for agranulocytosis is required; other adverse effects include an increased risk of seizures, myocarditis, and orthostatic hypotension.
Stage 7: Other. Treatment options such as topiramate17,18 and lamotrigine19 are recommended at this stage. These recommendations also will be reviewed and likely revised.
Treating bipolar depression
The TMAP algorithm for treating depression in bipolar disorder (Algorithm 2) assumes that anti-depressants will be used only with optimum mood-stabilizer levels because of the risk of inducing manic symptoms. The bipolar depression algorithm is always used with the primary algorithm for mania/hypomania.
The patient’s clinical presentation guides medication selection. For the “pure” bipolar I patient with a major depressive episode but little mood lability or hypomania, starting an antide-pressant is a clear decision. On the other hand, patients with predominant depressive symptoms plus dysphoric hypomania, mood lability, and irritability need a balance of mood-stabilizing drugs and antidepressants.
Stage 1: Mood stabilizer. Initiate a mood stabilizer and optimize the dosage. Choices are the same mood stabilizers listed in the hypomania/mania treatment algorithm.
Stage 2: Antidepressant. Adding an antidepressant implies that depressive symptoms are severe enough to change treatment. Antidepressant options include a selective serotonin reuptake inhibitor (SSRI), sustained-release bupropion, or lamotrigine.20
Using SSRIs is supported by widespread clinical experience and offers the convenience of once-daily dosing. Recommended SSRIs include fluoxetine, paroxetine, fluvoxamine, sertraline, and citalopram. The SSRI escitalopram was introduced after the 2000 algorithms were published; evidence for using it and other newer medications will be reviewed for the 2004 update.
The recommendation for sustained-release bupropion is consistent with the algorithm principle to use medications in the most well-tolerated form when accessible and available.
With lamotrigine, review with patients the risk of serious rash. To minimize rash risk, start lamotrigine slowly and follow the recommended titration schedule.
Stage 3: Multiple choices. At this stage, no definitive studies, safety data, or tolerability issues are available to rank the medication choices. The algorithm suggests:
- adding lithium21 or a second antidepressant
- or switching to an alternate antidepressant such as venlafaxine or nefazodone.
If a patient moves to stage 3 because of side effects with one antidepressant class, a different class—preferably with a contrasting side-effect profile—is recommended.
Algorithm 2 Treating depression in bipolar I disorder*
Stage 4: Two antidepressants. To enhance clinical response, the algorithm recommends combining two antidepressants, preferably from different classes. Monitor patients closely for side effects.
Stage 5. Antipsychotic or MAOI. At this stage, the algorithm recommends adding an atypical antipsychotic22 or switching to a monoamine oxidase inhibitor (MAOI).
Early evidence supported the efficacy of MAOIs in bipolar depression. However, the panel ranked MAOIs lower in the algorithm because they are associated with more bothersome side effects than SSRIs and other antidepressants. When using MAOIs, provide patients with dietary restriction guidelines.
Stage 6. Other therapies. Therapies such as ECT or “other” interventions are recommended at this stage. ECT has proven efficacy in bipolar depression and is appropriate for patients with limited medication response. The panel gave ECT a low ranking because of limited availability, lack of patient acceptance, and newer options.
Medication options include experimental treatments with limited evidence, such as inositol, dopamine agonists, stimulants, thyroid supplementation, conventional antipsychotics, and tri-cyclic antidepressants.
Acute to maintenance treatment
Adjunctive treatments for agitation, insomnia, GI upset, sedation, headache, and tremor are recommended in the physician manual supporting the TMAP guidelines (see Related resources). The manual also suggests ways to manage medication side effects and modify the algorithms for inpatients.
Patient and family psychoeducation plays an important role in helping the patient:
- identify prodromal bipolar symptoms
- understand the need to take medications as prescribed.
Continuation treatment. After mania or hypomania remits, continue medication(s) at the effective acute-phase dosages for at least 3 months. Use follow-up visits to enhance patient adherence, detect early symptoms of relapse, and monitor for side effects.
During the late continuation phase, after a period of sustained stability, clinicians can try to simplify the medications. When discontinuing a medication, taper the dosage by no more than 25% per week. If symptoms recur, promptly return to acute-phase treatment. Consider restarting medications and titrating up to the dosage(s) that resulted in remission.
In a depressive episode, continue the antidepressant(s) for 1 to 3 months at the effective acutephase dosage(s). Follow up frequently, and educate patients to watch for symptom recurrence and to communicate with you to assess when medication changes are needed.
Maintenance treatment. Relatively few well-controlled studies on long-term management of bipolar patients were available for the 2000 algorithm update.23 In general, all patients need mood stabilizer(s) to prevent relapse, using the lowest dosage that maintains therapeutic efficacy. Based on new evidence for lamotrigine and atypical antipsychotics—including FDAapproval of olanzapine for bipolar maintenace therapy—we anticipate recommendations will be expanded and more delineated in the 2004 update.
Discontinuing antidepressants after 3 to 6 months of initial treatment is now recommended. However, a recent retrospective case series suggests that continuing antidepressants at least 1 year after initial successful therapy may protect against depressive relapse. During this study, continuing antidepressants more than 3 to 6 months did not appear to increase the risk of switching to mania.24
Should antidepressants be continued or discontinued after successful acute treatment of a bipolar I depressive episode? This is an active area of research and debate as to the most appropriate strategy. The 2004 algorithm update panel will consider recent evidence that supports continuing antidepressants after symptom remission.24
- Texas Medication Algorithm Project algorithms and physician manual. Texas Department of Mental Health and Mental Retardation. http://www.mhmr.state.tx.us/centraloffice/medicaldirector/TIMA.html
- American Psychiatric Association. Practice guidelines for the treatment of patients with bipolar disorder. Am J Psychiatry 2002; 159(4):suppl 2.
- Depression and Bipolar Support Alliance. www.dbsalliance.org
Drug brand names
- Bupropion • Wellbutrin SR
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Inositol • Various
- Lamotrigine • Lamictal
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Topiramate • Topamax
- Tranylcypromine • Parnate
- Valproic acid • Depakene
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
Dr. Shivakumar reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Suppes receives research support from or is a consultant to Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Co., GlaxoSmithKline, Janssen Pharmaceutica, Johnson & Johnson, National Institutes of Mental Health, Novartis Pharmaceuticals Corp., Pfizer Inc., Pharmaceutical Research Institute, Ortho-McNeil Pharmaceutical, Robert Wood Johnson Pharmaceutical Research Institute, The Stanley Medical Research Institute, and UCB Pharma.
1. Gilbert DA, Altshuler KZ, Rago WV, et al. Texas Medication Algorithm Project: definitions, rationale, and methods to develop medication algorithms. J Clin Psychiatry 1998;59:345-51.
2. Altshuler KZ, Rush AJ. Computerized Texas Medication Algorithm Project undergoes testing. Outcomes Accountability Alert 1999;4(1):10-11.
3. Rush AJ, Crismon ML, Kashner TM, et al. Texas Medication Algorithm Project, Phase 3 (TMAP-3): rationale and study design. J Clin Psychiatry 2003;64(4):357-69.
4. Altman E, Hedeker D, Janicak P, et al. The Clinician-Administered Rating Scale For Mania (CARS-M): development, reliability, and validity. Biol Psychiatry 1994;36:124-34.
5. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas consensus conference panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry 2002;63:288-99.
6. Dennehy EB, Doyle K, Suppes T. The efficacy of olanzapine monotherapy for acute hypomania or mania in an outpatient setting. Int Clin Psychopharmacol 2003;18(3):143-5.
7. Dilsaver SC, Swann AC, Shoaib AM, et al. The manic syndrome: factors which may predict a patient’s response to lithium, carbamazepine and valproate. J Psych Neurosci 1993;18:61-6.
8. Tohen M, Chengappa KNR, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59(1):62-9.
9. Emrich HM. Studies with oxcarbazepine (Trileptal) in acute mania. Int Clin Psychopharmacol 1990;190(5,suppl):83-8.
10. Vieta E, Parramon G, Padrell E, et al. Quetiapine in the treatment of rapid cycling bipolar disorder. Bipolar Disord 2002;4(5):335-40.
11. Keck PE, Versiani M, Potkin S, et al. Ziprasidone in the treatment of acute bipolar mania: a three week placebo-controlled, double-blinded randomized trial. Am J Psychiatry 2003;160(4):741-8.
12. Yatham LN, Grossman F, Augustyns I, et al. Mood stabilisers plus risperidone or placebo in the treatment of acute mania. International, double-blinded, randomised controlled trial. Br J Psychiatry 2003;182:141-7.
13. Sachs GS, Grossman F, Ghaemi SN, et al. Combination of a mood stabilizer with risperidone or haloperidol for treatment of acute mania: a double-blind, placebo-controlled comparison of efficacy and safety. Am J Psychiatry 2002;159(7):1146-54.
14. Keck PE, Jr, Marcus R, Tourkodimitris S, et al. Aripiprazole study group. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160(9):1651-8.
15. Mukherjee S, Sackeim HA, Schnur DB. Electroconvulsive therapy of acute manic episode: a review of 50 years’ experience. Am J Psychiatry 1988;45:727-32.
16. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trail of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry 1999;156(8):1164-9.
17. Guille C, Sachs G. Clinical outcome of adjunctive topiramate treatment in a sample of refractory bipolar patients with comorbid conditions. Prog Neuropsychopharmacol Biol Psychiatry 2002;26(6):1035-9.
18. Vieta E, Torrent C, Garcia-Ribas G, et al. Use of topiramate in treatment-resistant bipolar spectrum disorders. J Clin Psychopharmacol 2002;22(4):431-5.
19. Calabrese JR, Suppes T, Bowden CL, et al. A double blinded, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. J Clin Psychiatry 2000;61:841-50.
20. Calabrese JR, Bowden CL, Sachs GS, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry 2003;64(9):1013-24.
21. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacology 1999;19:427-34.
22. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
23. Baldessarini RJ, Tohen M, Tondo L. Maintenance treatment in bipolar disorder (comment). Arch Gen Psychiatry 2000;57:490-2.
24. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 2003;160:1252-62.
Many well-controlled trials in the past 4 years have evaluated new medications for treating bipolar disorder. It’s time to build a consensus on how this data may apply to clinical practice.
This year, our group will re-examine the Texas Medication Algorithm Project (TMAP) treatment algorithms for bipolar I disorder.
What makes TMAP unique? It is the first project to evaluate treatment algorithm use in community mental health settings for patients with a history of mania (see Box).1-5 Severely, persistently ill outpatients such as these are seldom included in research but are frequently seen in clinical practice.
To preview for psychiatrists the changes expected in 2004, this article describes the goals of TMAP and the controlled study on which the medication algorithms are based. We review the medication algorithms of 2000 as a starting point and present the evidence that is changing clinical practice.
Guiding principles of TMAP
A treatment algorithm is no substitute for clinical judgment; rather, medication guidelines and algorithms are guideposts to help the clinician and patient collaboratively develop the most effective medication strategy with the fewest side effects.
The Texas Medication Algorithm Project (TMAP)1-3 is a public and academic collaboration started in 1996 to develop evidence- and consensus-based medication treatment algorithms for schizophrenia, major depressive disorder, and bipolar disorder.
TMAP’s goal is to establish “best practices” to encourage uniformity of care, achieve the best possible patient outcomes, and use mental health care dollars most efficiently. The project includes four phases, in which the treatment algorithms were developed, compared with treatment-as-usual, put into practice, and will undergo periodic updates.4 The next update begins this year.
The comparison of algorithms for treating bipolar mania/hypomania and depression included 409 patients (mean age 38 to 40) with bipolar I disorder or schizoaffective disorder, bipolar type. These patients were severely and persistently mentally ill, from a diverse ethnic population, and significantly impaired in functioning.
During 12 months of treatment, psychiatric symptoms diminished more rapidly in patients in the algorithm group—as measured by the Brief Psychiatric Rating Scale (BPRS-24)—compared with those receiving usual treatment. After the first 3 months, the usual-treatment patients also showed diminished symptoms. At study’s end, symptom severity between the groups was not significantly different; both groups showed improvement.
Manic and psychotic symptoms—measured by Clinician-Administered Rating Scale subscales (CARS-M)5—improved significantly more in the algorithm group in the first 3 months, and this gap between the two groups was sustained for 12 months. Depressive symptoms declined, but no overall differences were noted between the two groups. Side effect rates and functioning were also similar.
TMAP’s treatment manual (see Related resources) describes clinicians’ preferred tactics and decision points, which we summarize here. The guidelines are an ongoing effort to apply evidence-based medicine to everyday practice and are meant to be adapted to patient needs.
Treatment goals that guided TMAP algorithm development are:
- symptomatic remission
- full return of psychosocial functioning
- prevention of relapse and recurrence.
Suggestions came from controlled clinical trials, open trials, retrospective data analyses, expert clinical consensus, and input from consumers.
Treatment selection. Initial algorithm stages recommend simple treatments (in terms of safety, tolerability, and side effects), whereas later stages recommend more-complicated regimens. A patient’s symptoms, comorbid conditions, and treatment history guide treatment selection. Patients may enter an algorithm at any stage, depending on their clinical presentation and medication history.
The clinician may consider patient preference when deciding among equivalent medications. The algorithm strongly encourages patients and families to participate, such as by keeping daily mood charts and completing symptom and side-effect checklists. When clinicians face a choice among medication brands, generics, or forms (such as immediate- versus slow-release), agents with greater tolerability are preferred.
Patient management. When patients enter the algorithm, clinic visits are frequent (such as every 2 weeks). Follow-up appointments address medication adherence, dosage adjustments, and side effects or adverse reactions.
If a patient’s symptoms show no change after two treatment stages, re-evaluate the diagnosis and consider mitigating factors such as substance abuse. Patients who complete acute treatment should receive continuation treatment.
Documentation. Clinicians are advised to document decision points and the rationale for treatment choices made outside the algorithm package.
Treating mania or hypomania
After clinical evaluation confirms the diagnosis of bipolar illness,4 the TMAP mania/hypomania algorithm (Algorithm 1) splits into three treatment pathways:
- euphoric mania/hypomania
- mixed or dysphoric mania/hypomania
- psychotic mania.
These pathways recognize the need for differing approaches to initial monotherapy and later two-drug combinations. If a patient develops persistent or severe depressive symptoms, the bipolar algorithm for a major depressive episode (Algorithm 2) is used during depressive periods with the primary mania algorithm.
Treatment recommendations. The key to using mood stabilizers is to achieve the optimum response—assuming good tolerability—before switching to another agent. Adjust medication dosages one at a time to allow adequate response and assessment.
When switching medications, use an overlap-and-taper strategy, assuming there is no medical necessity to stop a drug abruptly. Add the new medication, then gradually taper the one that is being discontinued. Monitor serum levels.
Discontinue antidepressants when appropriate in patients with hypomania/mania or rapid cycling, and continually evaluate suicide and homicide potential of patients in mixed or depressive states.
Stage 1: Monotherapy. First medication choices are lithium, divalproex, or olanzapine. For mixed or dysphoric mania, the algorithm recommends divalproex (preferred over valproic acid because of tolerability and side effects) or olanzapine.6 Data suggest dysphoric manic patients are less likely to respond to lithium.7 A Consensus Panel minority expressed concern about using olanzapine as first-line monotherapy for acute mania because of limited data on the drug’s long-term safety. Patients with partial response or residual symptoms may move to stage 2 or switch to other medication options within stage 1.
Patients with psychotic mania move directly to stage 4 for a broader range of combination therapy.
Stage 2: Combination therapy. Combination therapy has become the standard of care in treating most patients with bipolar disorder. The algorithm recommends using two agents:
- lithium or an anticonvulsant plus another anticonvulsant ([Li or AC]+AC)
- or lithium or an anticonvulsant plus an atypical antipsychotic ([Li or AC]+AAP).8
Recommended agents include lithium, divalproex, oxcarbazepine, olanzapine, or risperidone. The experts recommended oxcarbazepine as first choice because it is better tolerated and interacts with fewer drugs than carbamazepine and does not require serum level monitoring.9
A Consensus Panel minority expressed concern that few studies had examined using oxcarbazepine in bipolar disorder. Carbamazepine was also considered an option.
Stages 3 and 4: Other two-drug combinations. Other two-drug combinations are tried at these stages, drawing from the same pool of medication classes described in stage 2.
Stage 4 broadens the choice of atypical antipsychotic by adding quetiapine10 and ziprasidone11 to the recommended stage-2 agents olanzapine and risperidone. When the 2000 algorithm was developed, limited data were available on using some newer atypicals in patients with bipolar mania. Based on recent, high-quality studies of mono- and combination therapy—including quetiapine,10 ziprasidone,11 risperidone,12,13 and aripiprazole14 —the 2004 algorithm update panel will likely recommend using atypicals earlier, including at stage 1.
Algorithm 1 Treating mania/hypomania in patients with bipolar I disorder
Stage 5: Triple-drug combination. Lithium, an anticonvulsant (divalproex or oxcarbazepine), and an atypical antipsychotic (olanzapine, risperidone, quetiapine, or ziprasidone) is a recommended triple-drug combination. In the 2004 update, the choices will likely expand to include all the newer atypicals and will list carbamazepine as an option.
Stage 6: ECT or clozapine. For patients with inadequate response to triple-drug combinations, the algorithm recommends adding electroconvulsive therapy (ECT) or clozapine.
ECT15 is recommended three times a week until the patient achieves remission of manic symptoms or fails to achieve a sustained response over three to six treatment cycles. Treatment resistance is declared if no response is seen after 6 to 10 treatment cycles.
Clozapine’s16 recommendation at this stage is consistent with its use in patients who fail to respond to other atypical antipsychotics. Blood monitoring for agranulocytosis is required; other adverse effects include an increased risk of seizures, myocarditis, and orthostatic hypotension.
Stage 7: Other. Treatment options such as topiramate17,18 and lamotrigine19 are recommended at this stage. These recommendations also will be reviewed and likely revised.
Treating bipolar depression
The TMAP algorithm for treating depression in bipolar disorder (Algorithm 2) assumes that anti-depressants will be used only with optimum mood-stabilizer levels because of the risk of inducing manic symptoms. The bipolar depression algorithm is always used with the primary algorithm for mania/hypomania.
The patient’s clinical presentation guides medication selection. For the “pure” bipolar I patient with a major depressive episode but little mood lability or hypomania, starting an antide-pressant is a clear decision. On the other hand, patients with predominant depressive symptoms plus dysphoric hypomania, mood lability, and irritability need a balance of mood-stabilizing drugs and antidepressants.
Stage 1: Mood stabilizer. Initiate a mood stabilizer and optimize the dosage. Choices are the same mood stabilizers listed in the hypomania/mania treatment algorithm.
Stage 2: Antidepressant. Adding an antidepressant implies that depressive symptoms are severe enough to change treatment. Antidepressant options include a selective serotonin reuptake inhibitor (SSRI), sustained-release bupropion, or lamotrigine.20
Using SSRIs is supported by widespread clinical experience and offers the convenience of once-daily dosing. Recommended SSRIs include fluoxetine, paroxetine, fluvoxamine, sertraline, and citalopram. The SSRI escitalopram was introduced after the 2000 algorithms were published; evidence for using it and other newer medications will be reviewed for the 2004 update.
The recommendation for sustained-release bupropion is consistent with the algorithm principle to use medications in the most well-tolerated form when accessible and available.
With lamotrigine, review with patients the risk of serious rash. To minimize rash risk, start lamotrigine slowly and follow the recommended titration schedule.
Stage 3: Multiple choices. At this stage, no definitive studies, safety data, or tolerability issues are available to rank the medication choices. The algorithm suggests:
- adding lithium21 or a second antidepressant
- or switching to an alternate antidepressant such as venlafaxine or nefazodone.
If a patient moves to stage 3 because of side effects with one antidepressant class, a different class—preferably with a contrasting side-effect profile—is recommended.
Algorithm 2 Treating depression in bipolar I disorder*
Stage 4: Two antidepressants. To enhance clinical response, the algorithm recommends combining two antidepressants, preferably from different classes. Monitor patients closely for side effects.
Stage 5. Antipsychotic or MAOI. At this stage, the algorithm recommends adding an atypical antipsychotic22 or switching to a monoamine oxidase inhibitor (MAOI).
Early evidence supported the efficacy of MAOIs in bipolar depression. However, the panel ranked MAOIs lower in the algorithm because they are associated with more bothersome side effects than SSRIs and other antidepressants. When using MAOIs, provide patients with dietary restriction guidelines.
Stage 6. Other therapies. Therapies such as ECT or “other” interventions are recommended at this stage. ECT has proven efficacy in bipolar depression and is appropriate for patients with limited medication response. The panel gave ECT a low ranking because of limited availability, lack of patient acceptance, and newer options.
Medication options include experimental treatments with limited evidence, such as inositol, dopamine agonists, stimulants, thyroid supplementation, conventional antipsychotics, and tri-cyclic antidepressants.
Acute to maintenance treatment
Adjunctive treatments for agitation, insomnia, GI upset, sedation, headache, and tremor are recommended in the physician manual supporting the TMAP guidelines (see Related resources). The manual also suggests ways to manage medication side effects and modify the algorithms for inpatients.
Patient and family psychoeducation plays an important role in helping the patient:
- identify prodromal bipolar symptoms
- understand the need to take medications as prescribed.
Continuation treatment. After mania or hypomania remits, continue medication(s) at the effective acute-phase dosages for at least 3 months. Use follow-up visits to enhance patient adherence, detect early symptoms of relapse, and monitor for side effects.
During the late continuation phase, after a period of sustained stability, clinicians can try to simplify the medications. When discontinuing a medication, taper the dosage by no more than 25% per week. If symptoms recur, promptly return to acute-phase treatment. Consider restarting medications and titrating up to the dosage(s) that resulted in remission.
In a depressive episode, continue the antidepressant(s) for 1 to 3 months at the effective acutephase dosage(s). Follow up frequently, and educate patients to watch for symptom recurrence and to communicate with you to assess when medication changes are needed.
Maintenance treatment. Relatively few well-controlled studies on long-term management of bipolar patients were available for the 2000 algorithm update.23 In general, all patients need mood stabilizer(s) to prevent relapse, using the lowest dosage that maintains therapeutic efficacy. Based on new evidence for lamotrigine and atypical antipsychotics—including FDAapproval of olanzapine for bipolar maintenace therapy—we anticipate recommendations will be expanded and more delineated in the 2004 update.
Discontinuing antidepressants after 3 to 6 months of initial treatment is now recommended. However, a recent retrospective case series suggests that continuing antidepressants at least 1 year after initial successful therapy may protect against depressive relapse. During this study, continuing antidepressants more than 3 to 6 months did not appear to increase the risk of switching to mania.24
Should antidepressants be continued or discontinued after successful acute treatment of a bipolar I depressive episode? This is an active area of research and debate as to the most appropriate strategy. The 2004 algorithm update panel will consider recent evidence that supports continuing antidepressants after symptom remission.24
- Texas Medication Algorithm Project algorithms and physician manual. Texas Department of Mental Health and Mental Retardation. http://www.mhmr.state.tx.us/centraloffice/medicaldirector/TIMA.html
- American Psychiatric Association. Practice guidelines for the treatment of patients with bipolar disorder. Am J Psychiatry 2002; 159(4):suppl 2.
- Depression and Bipolar Support Alliance. www.dbsalliance.org
Drug brand names
- Bupropion • Wellbutrin SR
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Inositol • Various
- Lamotrigine • Lamictal
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Topiramate • Topamax
- Tranylcypromine • Parnate
- Valproic acid • Depakene
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
Dr. Shivakumar reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Suppes receives research support from or is a consultant to Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Co., GlaxoSmithKline, Janssen Pharmaceutica, Johnson & Johnson, National Institutes of Mental Health, Novartis Pharmaceuticals Corp., Pfizer Inc., Pharmaceutical Research Institute, Ortho-McNeil Pharmaceutical, Robert Wood Johnson Pharmaceutical Research Institute, The Stanley Medical Research Institute, and UCB Pharma.
Many well-controlled trials in the past 4 years have evaluated new medications for treating bipolar disorder. It’s time to build a consensus on how this data may apply to clinical practice.
This year, our group will re-examine the Texas Medication Algorithm Project (TMAP) treatment algorithms for bipolar I disorder.
What makes TMAP unique? It is the first project to evaluate treatment algorithm use in community mental health settings for patients with a history of mania (see Box).1-5 Severely, persistently ill outpatients such as these are seldom included in research but are frequently seen in clinical practice.
To preview for psychiatrists the changes expected in 2004, this article describes the goals of TMAP and the controlled study on which the medication algorithms are based. We review the medication algorithms of 2000 as a starting point and present the evidence that is changing clinical practice.
Guiding principles of TMAP
A treatment algorithm is no substitute for clinical judgment; rather, medication guidelines and algorithms are guideposts to help the clinician and patient collaboratively develop the most effective medication strategy with the fewest side effects.
The Texas Medication Algorithm Project (TMAP)1-3 is a public and academic collaboration started in 1996 to develop evidence- and consensus-based medication treatment algorithms for schizophrenia, major depressive disorder, and bipolar disorder.
TMAP’s goal is to establish “best practices” to encourage uniformity of care, achieve the best possible patient outcomes, and use mental health care dollars most efficiently. The project includes four phases, in which the treatment algorithms were developed, compared with treatment-as-usual, put into practice, and will undergo periodic updates.4 The next update begins this year.
The comparison of algorithms for treating bipolar mania/hypomania and depression included 409 patients (mean age 38 to 40) with bipolar I disorder or schizoaffective disorder, bipolar type. These patients were severely and persistently mentally ill, from a diverse ethnic population, and significantly impaired in functioning.
During 12 months of treatment, psychiatric symptoms diminished more rapidly in patients in the algorithm group—as measured by the Brief Psychiatric Rating Scale (BPRS-24)—compared with those receiving usual treatment. After the first 3 months, the usual-treatment patients also showed diminished symptoms. At study’s end, symptom severity between the groups was not significantly different; both groups showed improvement.
Manic and psychotic symptoms—measured by Clinician-Administered Rating Scale subscales (CARS-M)5—improved significantly more in the algorithm group in the first 3 months, and this gap between the two groups was sustained for 12 months. Depressive symptoms declined, but no overall differences were noted between the two groups. Side effect rates and functioning were also similar.
TMAP’s treatment manual (see Related resources) describes clinicians’ preferred tactics and decision points, which we summarize here. The guidelines are an ongoing effort to apply evidence-based medicine to everyday practice and are meant to be adapted to patient needs.
Treatment goals that guided TMAP algorithm development are:
- symptomatic remission
- full return of psychosocial functioning
- prevention of relapse and recurrence.
Suggestions came from controlled clinical trials, open trials, retrospective data analyses, expert clinical consensus, and input from consumers.
Treatment selection. Initial algorithm stages recommend simple treatments (in terms of safety, tolerability, and side effects), whereas later stages recommend more-complicated regimens. A patient’s symptoms, comorbid conditions, and treatment history guide treatment selection. Patients may enter an algorithm at any stage, depending on their clinical presentation and medication history.
The clinician may consider patient preference when deciding among equivalent medications. The algorithm strongly encourages patients and families to participate, such as by keeping daily mood charts and completing symptom and side-effect checklists. When clinicians face a choice among medication brands, generics, or forms (such as immediate- versus slow-release), agents with greater tolerability are preferred.
Patient management. When patients enter the algorithm, clinic visits are frequent (such as every 2 weeks). Follow-up appointments address medication adherence, dosage adjustments, and side effects or adverse reactions.
If a patient’s symptoms show no change after two treatment stages, re-evaluate the diagnosis and consider mitigating factors such as substance abuse. Patients who complete acute treatment should receive continuation treatment.
Documentation. Clinicians are advised to document decision points and the rationale for treatment choices made outside the algorithm package.
Treating mania or hypomania
After clinical evaluation confirms the diagnosis of bipolar illness,4 the TMAP mania/hypomania algorithm (Algorithm 1) splits into three treatment pathways:
- euphoric mania/hypomania
- mixed or dysphoric mania/hypomania
- psychotic mania.
These pathways recognize the need for differing approaches to initial monotherapy and later two-drug combinations. If a patient develops persistent or severe depressive symptoms, the bipolar algorithm for a major depressive episode (Algorithm 2) is used during depressive periods with the primary mania algorithm.
Treatment recommendations. The key to using mood stabilizers is to achieve the optimum response—assuming good tolerability—before switching to another agent. Adjust medication dosages one at a time to allow adequate response and assessment.
When switching medications, use an overlap-and-taper strategy, assuming there is no medical necessity to stop a drug abruptly. Add the new medication, then gradually taper the one that is being discontinued. Monitor serum levels.
Discontinue antidepressants when appropriate in patients with hypomania/mania or rapid cycling, and continually evaluate suicide and homicide potential of patients in mixed or depressive states.
Stage 1: Monotherapy. First medication choices are lithium, divalproex, or olanzapine. For mixed or dysphoric mania, the algorithm recommends divalproex (preferred over valproic acid because of tolerability and side effects) or olanzapine.6 Data suggest dysphoric manic patients are less likely to respond to lithium.7 A Consensus Panel minority expressed concern about using olanzapine as first-line monotherapy for acute mania because of limited data on the drug’s long-term safety. Patients with partial response or residual symptoms may move to stage 2 or switch to other medication options within stage 1.
Patients with psychotic mania move directly to stage 4 for a broader range of combination therapy.
Stage 2: Combination therapy. Combination therapy has become the standard of care in treating most patients with bipolar disorder. The algorithm recommends using two agents:
- lithium or an anticonvulsant plus another anticonvulsant ([Li or AC]+AC)
- or lithium or an anticonvulsant plus an atypical antipsychotic ([Li or AC]+AAP).8
Recommended agents include lithium, divalproex, oxcarbazepine, olanzapine, or risperidone. The experts recommended oxcarbazepine as first choice because it is better tolerated and interacts with fewer drugs than carbamazepine and does not require serum level monitoring.9
A Consensus Panel minority expressed concern that few studies had examined using oxcarbazepine in bipolar disorder. Carbamazepine was also considered an option.
Stages 3 and 4: Other two-drug combinations. Other two-drug combinations are tried at these stages, drawing from the same pool of medication classes described in stage 2.
Stage 4 broadens the choice of atypical antipsychotic by adding quetiapine10 and ziprasidone11 to the recommended stage-2 agents olanzapine and risperidone. When the 2000 algorithm was developed, limited data were available on using some newer atypicals in patients with bipolar mania. Based on recent, high-quality studies of mono- and combination therapy—including quetiapine,10 ziprasidone,11 risperidone,12,13 and aripiprazole14 —the 2004 algorithm update panel will likely recommend using atypicals earlier, including at stage 1.
Algorithm 1 Treating mania/hypomania in patients with bipolar I disorder
Stage 5: Triple-drug combination. Lithium, an anticonvulsant (divalproex or oxcarbazepine), and an atypical antipsychotic (olanzapine, risperidone, quetiapine, or ziprasidone) is a recommended triple-drug combination. In the 2004 update, the choices will likely expand to include all the newer atypicals and will list carbamazepine as an option.
Stage 6: ECT or clozapine. For patients with inadequate response to triple-drug combinations, the algorithm recommends adding electroconvulsive therapy (ECT) or clozapine.
ECT15 is recommended three times a week until the patient achieves remission of manic symptoms or fails to achieve a sustained response over three to six treatment cycles. Treatment resistance is declared if no response is seen after 6 to 10 treatment cycles.
Clozapine’s16 recommendation at this stage is consistent with its use in patients who fail to respond to other atypical antipsychotics. Blood monitoring for agranulocytosis is required; other adverse effects include an increased risk of seizures, myocarditis, and orthostatic hypotension.
Stage 7: Other. Treatment options such as topiramate17,18 and lamotrigine19 are recommended at this stage. These recommendations also will be reviewed and likely revised.
Treating bipolar depression
The TMAP algorithm for treating depression in bipolar disorder (Algorithm 2) assumes that anti-depressants will be used only with optimum mood-stabilizer levels because of the risk of inducing manic symptoms. The bipolar depression algorithm is always used with the primary algorithm for mania/hypomania.
The patient’s clinical presentation guides medication selection. For the “pure” bipolar I patient with a major depressive episode but little mood lability or hypomania, starting an antide-pressant is a clear decision. On the other hand, patients with predominant depressive symptoms plus dysphoric hypomania, mood lability, and irritability need a balance of mood-stabilizing drugs and antidepressants.
Stage 1: Mood stabilizer. Initiate a mood stabilizer and optimize the dosage. Choices are the same mood stabilizers listed in the hypomania/mania treatment algorithm.
Stage 2: Antidepressant. Adding an antidepressant implies that depressive symptoms are severe enough to change treatment. Antidepressant options include a selective serotonin reuptake inhibitor (SSRI), sustained-release bupropion, or lamotrigine.20
Using SSRIs is supported by widespread clinical experience and offers the convenience of once-daily dosing. Recommended SSRIs include fluoxetine, paroxetine, fluvoxamine, sertraline, and citalopram. The SSRI escitalopram was introduced after the 2000 algorithms were published; evidence for using it and other newer medications will be reviewed for the 2004 update.
The recommendation for sustained-release bupropion is consistent with the algorithm principle to use medications in the most well-tolerated form when accessible and available.
With lamotrigine, review with patients the risk of serious rash. To minimize rash risk, start lamotrigine slowly and follow the recommended titration schedule.
Stage 3: Multiple choices. At this stage, no definitive studies, safety data, or tolerability issues are available to rank the medication choices. The algorithm suggests:
- adding lithium21 or a second antidepressant
- or switching to an alternate antidepressant such as venlafaxine or nefazodone.
If a patient moves to stage 3 because of side effects with one antidepressant class, a different class—preferably with a contrasting side-effect profile—is recommended.
Algorithm 2 Treating depression in bipolar I disorder*
Stage 4: Two antidepressants. To enhance clinical response, the algorithm recommends combining two antidepressants, preferably from different classes. Monitor patients closely for side effects.
Stage 5. Antipsychotic or MAOI. At this stage, the algorithm recommends adding an atypical antipsychotic22 or switching to a monoamine oxidase inhibitor (MAOI).
Early evidence supported the efficacy of MAOIs in bipolar depression. However, the panel ranked MAOIs lower in the algorithm because they are associated with more bothersome side effects than SSRIs and other antidepressants. When using MAOIs, provide patients with dietary restriction guidelines.
Stage 6. Other therapies. Therapies such as ECT or “other” interventions are recommended at this stage. ECT has proven efficacy in bipolar depression and is appropriate for patients with limited medication response. The panel gave ECT a low ranking because of limited availability, lack of patient acceptance, and newer options.
Medication options include experimental treatments with limited evidence, such as inositol, dopamine agonists, stimulants, thyroid supplementation, conventional antipsychotics, and tri-cyclic antidepressants.
Acute to maintenance treatment
Adjunctive treatments for agitation, insomnia, GI upset, sedation, headache, and tremor are recommended in the physician manual supporting the TMAP guidelines (see Related resources). The manual also suggests ways to manage medication side effects and modify the algorithms for inpatients.
Patient and family psychoeducation plays an important role in helping the patient:
- identify prodromal bipolar symptoms
- understand the need to take medications as prescribed.
Continuation treatment. After mania or hypomania remits, continue medication(s) at the effective acute-phase dosages for at least 3 months. Use follow-up visits to enhance patient adherence, detect early symptoms of relapse, and monitor for side effects.
During the late continuation phase, after a period of sustained stability, clinicians can try to simplify the medications. When discontinuing a medication, taper the dosage by no more than 25% per week. If symptoms recur, promptly return to acute-phase treatment. Consider restarting medications and titrating up to the dosage(s) that resulted in remission.
In a depressive episode, continue the antidepressant(s) for 1 to 3 months at the effective acutephase dosage(s). Follow up frequently, and educate patients to watch for symptom recurrence and to communicate with you to assess when medication changes are needed.
Maintenance treatment. Relatively few well-controlled studies on long-term management of bipolar patients were available for the 2000 algorithm update.23 In general, all patients need mood stabilizer(s) to prevent relapse, using the lowest dosage that maintains therapeutic efficacy. Based on new evidence for lamotrigine and atypical antipsychotics—including FDAapproval of olanzapine for bipolar maintenace therapy—we anticipate recommendations will be expanded and more delineated in the 2004 update.
Discontinuing antidepressants after 3 to 6 months of initial treatment is now recommended. However, a recent retrospective case series suggests that continuing antidepressants at least 1 year after initial successful therapy may protect against depressive relapse. During this study, continuing antidepressants more than 3 to 6 months did not appear to increase the risk of switching to mania.24
Should antidepressants be continued or discontinued after successful acute treatment of a bipolar I depressive episode? This is an active area of research and debate as to the most appropriate strategy. The 2004 algorithm update panel will consider recent evidence that supports continuing antidepressants after symptom remission.24
- Texas Medication Algorithm Project algorithms and physician manual. Texas Department of Mental Health and Mental Retardation. http://www.mhmr.state.tx.us/centraloffice/medicaldirector/TIMA.html
- American Psychiatric Association. Practice guidelines for the treatment of patients with bipolar disorder. Am J Psychiatry 2002; 159(4):suppl 2.
- Depression and Bipolar Support Alliance. www.dbsalliance.org
Drug brand names
- Bupropion • Wellbutrin SR
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Inositol • Various
- Lamotrigine • Lamictal
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Topiramate • Topamax
- Tranylcypromine • Parnate
- Valproic acid • Depakene
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
Dr. Shivakumar reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Suppes receives research support from or is a consultant to Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Co., GlaxoSmithKline, Janssen Pharmaceutica, Johnson & Johnson, National Institutes of Mental Health, Novartis Pharmaceuticals Corp., Pfizer Inc., Pharmaceutical Research Institute, Ortho-McNeil Pharmaceutical, Robert Wood Johnson Pharmaceutical Research Institute, The Stanley Medical Research Institute, and UCB Pharma.
1. Gilbert DA, Altshuler KZ, Rago WV, et al. Texas Medication Algorithm Project: definitions, rationale, and methods to develop medication algorithms. J Clin Psychiatry 1998;59:345-51.
2. Altshuler KZ, Rush AJ. Computerized Texas Medication Algorithm Project undergoes testing. Outcomes Accountability Alert 1999;4(1):10-11.
3. Rush AJ, Crismon ML, Kashner TM, et al. Texas Medication Algorithm Project, Phase 3 (TMAP-3): rationale and study design. J Clin Psychiatry 2003;64(4):357-69.
4. Altman E, Hedeker D, Janicak P, et al. The Clinician-Administered Rating Scale For Mania (CARS-M): development, reliability, and validity. Biol Psychiatry 1994;36:124-34.
5. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas consensus conference panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry 2002;63:288-99.
6. Dennehy EB, Doyle K, Suppes T. The efficacy of olanzapine monotherapy for acute hypomania or mania in an outpatient setting. Int Clin Psychopharmacol 2003;18(3):143-5.
7. Dilsaver SC, Swann AC, Shoaib AM, et al. The manic syndrome: factors which may predict a patient’s response to lithium, carbamazepine and valproate. J Psych Neurosci 1993;18:61-6.
8. Tohen M, Chengappa KNR, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59(1):62-9.
9. Emrich HM. Studies with oxcarbazepine (Trileptal) in acute mania. Int Clin Psychopharmacol 1990;190(5,suppl):83-8.
10. Vieta E, Parramon G, Padrell E, et al. Quetiapine in the treatment of rapid cycling bipolar disorder. Bipolar Disord 2002;4(5):335-40.
11. Keck PE, Versiani M, Potkin S, et al. Ziprasidone in the treatment of acute bipolar mania: a three week placebo-controlled, double-blinded randomized trial. Am J Psychiatry 2003;160(4):741-8.
12. Yatham LN, Grossman F, Augustyns I, et al. Mood stabilisers plus risperidone or placebo in the treatment of acute mania. International, double-blinded, randomised controlled trial. Br J Psychiatry 2003;182:141-7.
13. Sachs GS, Grossman F, Ghaemi SN, et al. Combination of a mood stabilizer with risperidone or haloperidol for treatment of acute mania: a double-blind, placebo-controlled comparison of efficacy and safety. Am J Psychiatry 2002;159(7):1146-54.
14. Keck PE, Jr, Marcus R, Tourkodimitris S, et al. Aripiprazole study group. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160(9):1651-8.
15. Mukherjee S, Sackeim HA, Schnur DB. Electroconvulsive therapy of acute manic episode: a review of 50 years’ experience. Am J Psychiatry 1988;45:727-32.
16. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trail of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry 1999;156(8):1164-9.
17. Guille C, Sachs G. Clinical outcome of adjunctive topiramate treatment in a sample of refractory bipolar patients with comorbid conditions. Prog Neuropsychopharmacol Biol Psychiatry 2002;26(6):1035-9.
18. Vieta E, Torrent C, Garcia-Ribas G, et al. Use of topiramate in treatment-resistant bipolar spectrum disorders. J Clin Psychopharmacol 2002;22(4):431-5.
19. Calabrese JR, Suppes T, Bowden CL, et al. A double blinded, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. J Clin Psychiatry 2000;61:841-50.
20. Calabrese JR, Bowden CL, Sachs GS, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry 2003;64(9):1013-24.
21. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacology 1999;19:427-34.
22. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
23. Baldessarini RJ, Tohen M, Tondo L. Maintenance treatment in bipolar disorder (comment). Arch Gen Psychiatry 2000;57:490-2.
24. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 2003;160:1252-62.
1. Gilbert DA, Altshuler KZ, Rago WV, et al. Texas Medication Algorithm Project: definitions, rationale, and methods to develop medication algorithms. J Clin Psychiatry 1998;59:345-51.
2. Altshuler KZ, Rush AJ. Computerized Texas Medication Algorithm Project undergoes testing. Outcomes Accountability Alert 1999;4(1):10-11.
3. Rush AJ, Crismon ML, Kashner TM, et al. Texas Medication Algorithm Project, Phase 3 (TMAP-3): rationale and study design. J Clin Psychiatry 2003;64(4):357-69.
4. Altman E, Hedeker D, Janicak P, et al. The Clinician-Administered Rating Scale For Mania (CARS-M): development, reliability, and validity. Biol Psychiatry 1994;36:124-34.
5. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas consensus conference panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry 2002;63:288-99.
6. Dennehy EB, Doyle K, Suppes T. The efficacy of olanzapine monotherapy for acute hypomania or mania in an outpatient setting. Int Clin Psychopharmacol 2003;18(3):143-5.
7. Dilsaver SC, Swann AC, Shoaib AM, et al. The manic syndrome: factors which may predict a patient’s response to lithium, carbamazepine and valproate. J Psych Neurosci 1993;18:61-6.
8. Tohen M, Chengappa KNR, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59(1):62-9.
9. Emrich HM. Studies with oxcarbazepine (Trileptal) in acute mania. Int Clin Psychopharmacol 1990;190(5,suppl):83-8.
10. Vieta E, Parramon G, Padrell E, et al. Quetiapine in the treatment of rapid cycling bipolar disorder. Bipolar Disord 2002;4(5):335-40.
11. Keck PE, Versiani M, Potkin S, et al. Ziprasidone in the treatment of acute bipolar mania: a three week placebo-controlled, double-blinded randomized trial. Am J Psychiatry 2003;160(4):741-8.
12. Yatham LN, Grossman F, Augustyns I, et al. Mood stabilisers plus risperidone or placebo in the treatment of acute mania. International, double-blinded, randomised controlled trial. Br J Psychiatry 2003;182:141-7.
13. Sachs GS, Grossman F, Ghaemi SN, et al. Combination of a mood stabilizer with risperidone or haloperidol for treatment of acute mania: a double-blind, placebo-controlled comparison of efficacy and safety. Am J Psychiatry 2002;159(7):1146-54.
14. Keck PE, Jr, Marcus R, Tourkodimitris S, et al. Aripiprazole study group. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160(9):1651-8.
15. Mukherjee S, Sackeim HA, Schnur DB. Electroconvulsive therapy of acute manic episode: a review of 50 years’ experience. Am J Psychiatry 1988;45:727-32.
16. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trail of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry 1999;156(8):1164-9.
17. Guille C, Sachs G. Clinical outcome of adjunctive topiramate treatment in a sample of refractory bipolar patients with comorbid conditions. Prog Neuropsychopharmacol Biol Psychiatry 2002;26(6):1035-9.
18. Vieta E, Torrent C, Garcia-Ribas G, et al. Use of topiramate in treatment-resistant bipolar spectrum disorders. J Clin Psychopharmacol 2002;22(4):431-5.
19. Calabrese JR, Suppes T, Bowden CL, et al. A double blinded, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. J Clin Psychiatry 2000;61:841-50.
20. Calabrese JR, Bowden CL, Sachs GS, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry 2003;64(9):1013-24.
21. Bauer M, Dopfmer S. Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacology 1999;19:427-34.
22. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
23. Baldessarini RJ, Tohen M, Tondo L. Maintenance treatment in bipolar disorder (comment). Arch Gen Psychiatry 2000;57:490-2.
24. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 2003;160:1252-62.