User login
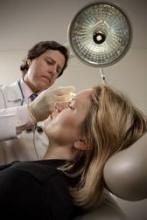
Botulinum toxins, including Botox and Dysport, are being associated with increasing reports of paralysis away from injection sites, difficulties in swallowing, incontinence, and breathing problems, according to the Institute for Safe Medication Practices.
The institute reviews and analyzes reports to the Food and Drug Administration's MedWatch Safety Information and Adverse Event Reporting Program.
The group cited 6 deaths, 18 cases of disability, and 100 other serious injuries in the first quarter of 2010. Previous quarters have averaged 30-50 adverse events and one patient death associated with the toxin. Seventy-nine of the cases (64%) in the latest quarter were associated with Botox, 26 (21%) with Botox Cosmetic, 17 (14%) with Dysport, and 2 cases with undetermined brands.
The watchdog group charged that the Botox label downplays the potential for the drug to spread during dermatologic use.
Botulinum toxins, including Botox and Dysport, are being associated with increasing reports of paralysis away from injection sites, difficulties in swallowing, incontinence, and breathing problems, according to the Institute for Safe Medication Practices.
The institute reviews and analyzes reports to the Food and Drug Administration's MedWatch Safety Information and Adverse Event Reporting Program.
The group cited 6 deaths, 18 cases of disability, and 100 other serious injuries in the first quarter of 2010. Previous quarters have averaged 30-50 adverse events and one patient death associated with the toxin. Seventy-nine of the cases (64%) in the latest quarter were associated with Botox, 26 (21%) with Botox Cosmetic, 17 (14%) with Dysport, and 2 cases with undetermined brands.
The watchdog group charged that the Botox label downplays the potential for the drug to spread during dermatologic use.
Botulinum toxins, including Botox and Dysport, are being associated with increasing reports of paralysis away from injection sites, difficulties in swallowing, incontinence, and breathing problems, according to the Institute for Safe Medication Practices.
The institute reviews and analyzes reports to the Food and Drug Administration's MedWatch Safety Information and Adverse Event Reporting Program.
The group cited 6 deaths, 18 cases of disability, and 100 other serious injuries in the first quarter of 2010. Previous quarters have averaged 30-50 adverse events and one patient death associated with the toxin. Seventy-nine of the cases (64%) in the latest quarter were associated with Botox, 26 (21%) with Botox Cosmetic, 17 (14%) with Dysport, and 2 cases with undetermined brands.
The watchdog group charged that the Botox label downplays the potential for the drug to spread during dermatologic use.
FROM THE INSTITUTE FOR SAFE MEDICATION PRACTICES