User login
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Topical application or injection of cosmeceuticals in conjunction with procedures such as facial microneedling (MN) has been associated with local and systemic complications.1
Although at-home options may be more accessible and affordable for patients, they also increase the risk for improper use and subsequent infection. Additionally, the use of cosmeceuticals such as vitamin E oil in conjunction with MN to enhance the effects of the procedure can lead to further complications. We report the case of a 44-year-old woman who developed a necrotic ulcer on the chin following self-treatment with vitamin E oil and an at-home MN device. While MN has been reported to be relatively safe when performed by board-certified dermatologists, clinicians should be vigilant in correlating clinical history and recent cosmetic procedures with the histologic findings for timely diagnosis and treatment of unusual lesions on the face.
Case Report
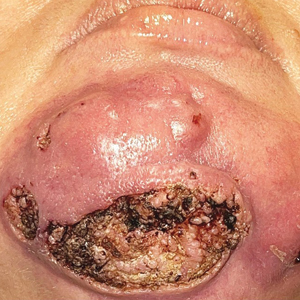
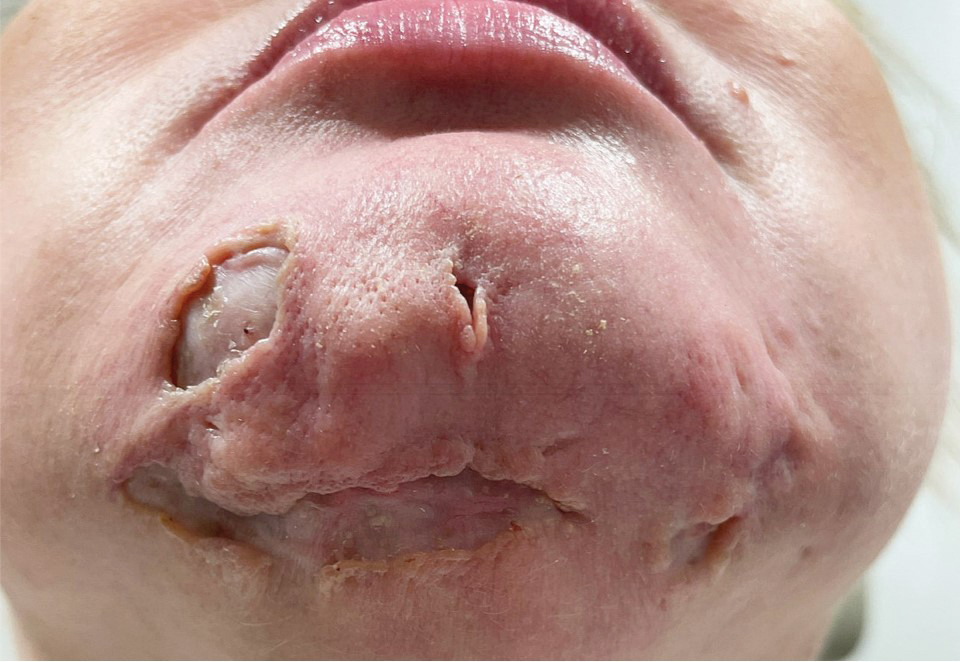
A 44-year-old woman presented to the emergency department with a progressively enlarging, necrotic, ulcerative lesion on the midline chin of 4 months’ duration. The patient reported that the lesion started as redness that developed into a painful oozing ulcer following application of vitamin E oil in conjunction with an at-home MN device (Figure 1). She purchased the vitamin E oil and MN device online and performed the procedure herself, applying the vitamin E oil to her whole face before, during, and after using the MN device, which contained 0.25-mm titanium needles. She denied undergoing any other recent cosmetic procedures.
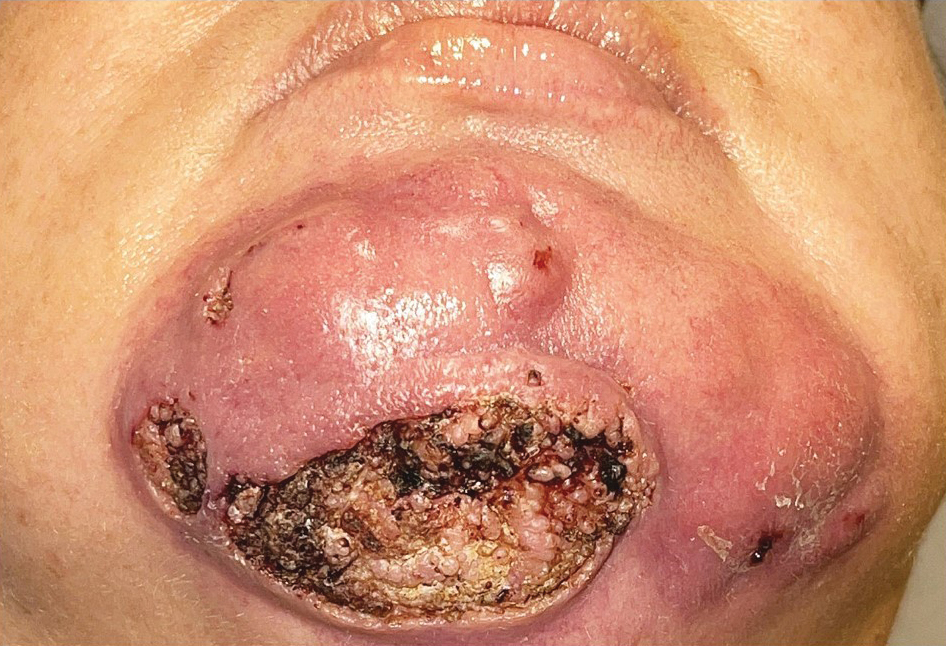
The lesion initially was treated by the patient’s primary care physician with oral doxycycline for 6 weeks, followed by oral cephalexin and clindamycin for 2 weeks. Although the redness stabilized, the lesion continued to enlarge, which prompted her initial visit to our hospital 1 month after seeing her primary care physician. During this visit, the patient was given penicillin, and the ulcer was debrided and biopsied; however, no clinical improvement was seen.
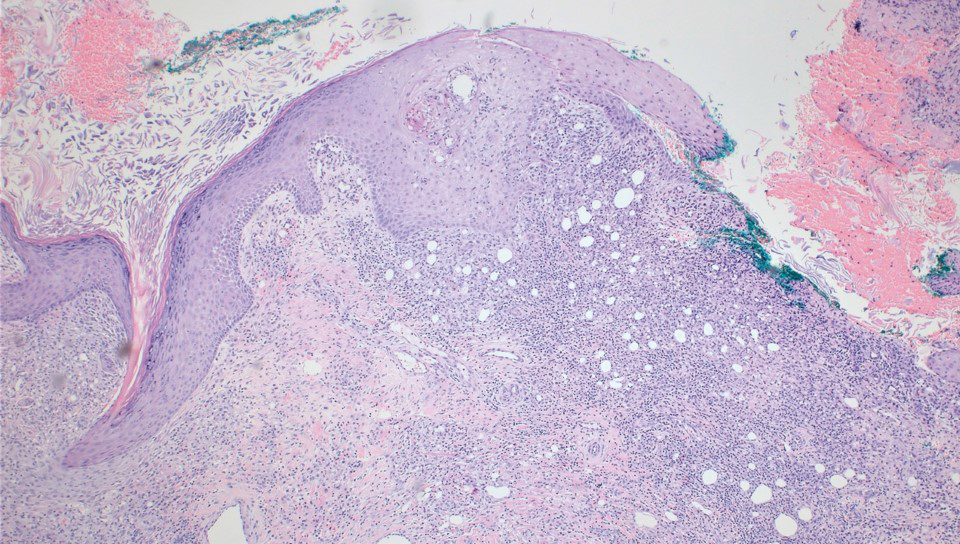
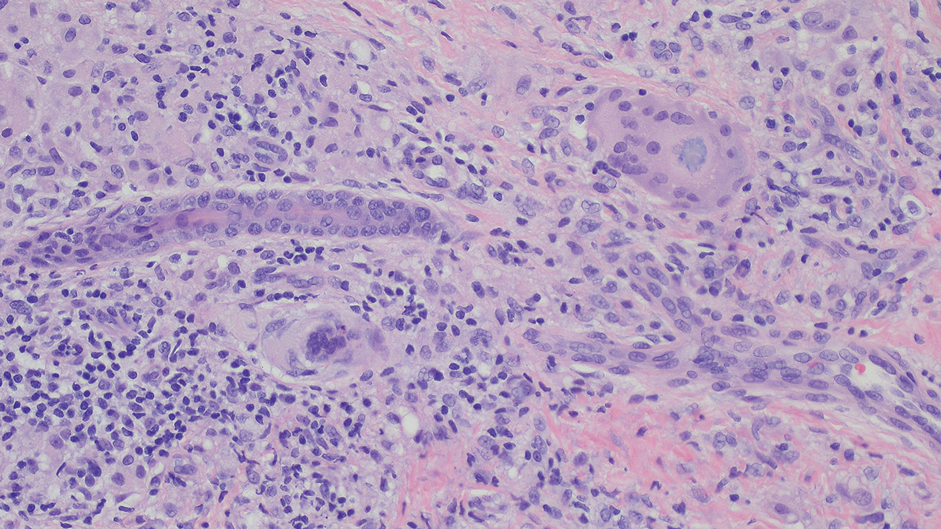
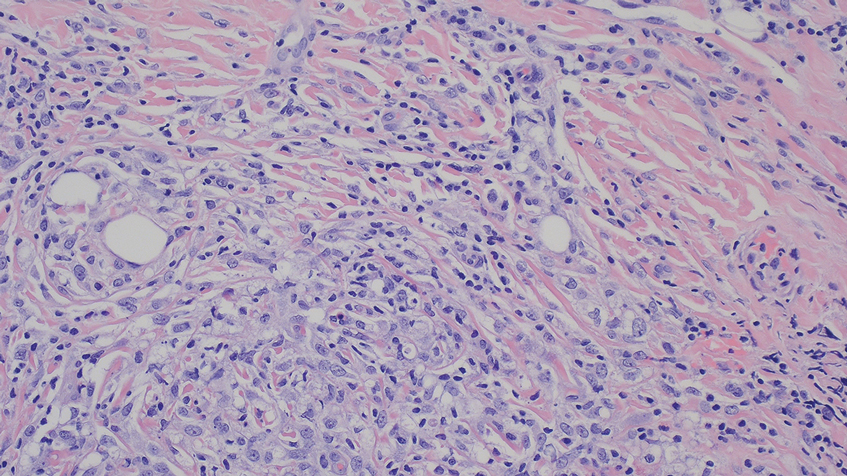
A biopsy during her initial emergency department visit and a repeat biopsy after 1 month showed similar findings of diffuse lymphohistiocytic and eosinophilic inflammation in the dermis (Figure 2) with poorly defined granulomas and multinucleated giant cells containing nonpolarizable exogenous material (Figure 3). Similar detached exogenous materials also were identified adjacent to the tissue. Diffuse re-epithelialization was seen, featuring pseudoepitheliomatous hyperplasia in association with the inflammatory process and granulation tissue (Figures 3 and 4). A higher-power view of the dermis showed foci of sclerosing lipogranuloma (Figure 4). Periodic acid–Schiff, Grocott methenamine silver, acid-fast bacilli, Fite, and Wright-Giemsa stains all were negative for microorganisms, and pancytokeratin staining was negative for carcinoma. These findings supported the diagnosis of a foreign body granulomatous reaction to an exogenous material—in this case, the vitamin E oil. Subsequent treatment with intralesional triamcinolone 10 mg/mL injection over 18 months resulted in progressive and drastic improvement of the lesion (Figure 5). A scar excision was performed, which further improved the lesion’s cosmetic appearance.
Comment
Application of various topical cosmeceuticals before, during, or after MN to enhance the effects of the procedure can introduce particles into the dermis, resulting in local or systemic hypersensitivity reactions. The associated adverse events can be divided into 2 main categories: adverse reactions related to the topical product or to the materials of the MN device itself.
A study showed that topical application of vitamin E oil to wounds on the skin does not improve the cosmetic appearance of scars.3 Instead, it is associated with a high incidence of contact dermatitis. A similar case of vitamin E injection, although without the concurrent use of an MN device, complicated by a facial lipogranuloma has been described.4 Sclerodermoid reaction, subcutaneous nodules, persistent edema, and ulceration at the site of vitamin E injection also have been described following the injection.5,6 Because vitamin E is a lipid-soluble vitamin, its absorption in the human body is dependent on the presence of lipid or oil-like substances. The reactions mentioned above are associated with the vitamin E oil, which acts as a helper vehicle for lipid-soluble vitamins to be absorbed.7 Other ingredients in topical vitamin E oil include a combination of D-alpha-tocopherol, D-alpha-tocopheryl acetate, D-alpha-tocopheryl succinate, or mixed tocopherols.8 These ester conjugate forms of vitamin E also may play a role in its immunogenic properties and
Hyaluronic acid is a relatively safe and commonly used topical treatment that acts as a lubricant during MN procedures to help the needles glide across the skin and prevent dragging. It also can be applied after the procedure for hydration purposes. Other common alternatives include peptides, ceramides, and epidermal growth factors. Topical products to avoid before, during, and 48 hours after undergoing MN include retinoids, vitamin C, vitamin E, exfoliants, serums that contain acids (eg, alpha hydroxy acids, beta hydroxy acids, glycolic acid, and lactic acid), serums that contain fragrance, and oil-based serums because they are associated with similar adverse effects.8-10 A granulomatous reaction after an MN procedure also has been reported with the use of vitamin C serum.11
The
Most MN devices are made of nickel and various other metals. Cases of contact dermatitis and delayed-type hypersensitivity granulomatous reaction with systemic symptoms have been reported after MN procedures due to the material of the MN device.1,13,14
Conclusion
Microneedling is a minimally invasive procedure that causes nominal damage to the epidermis and superficial papillary dermis, stimulating a wound-healing cascade for collagen production.15,16 Although not approved by the US Food and Drug Administration, MN performed at dermatology offices sometimes can be used in conjunction with topical products to enhance their absorption; however, while vitamin E is known for its antioxidant properties and potential skin benefits, the lipid substance acting as the vehicle is not absorbable by the skin and may cause a granulomatous reaction as the body attempts to encapsulate and digest the foreign substance.10,17 Although rarely reported, the use of topical vitamins with MN—through intradermal injection or combined with MN—can be associated with severe complications, including local, sometimes systemic, and life-threatening complications. Clinicians should be vigilant in order to correlate clinical background and history of recent cosmetic procedures with the histologic findings for prompt diagnosis and timely treatment.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150:68-72. doi:10.1001/jamadermatol.2013.6955
- Microneedling market. The Brainy Insights. Published January, 2023. Accessed September 9, 2023. https://www.thebrainyinsights.com/report/microneedling-market-13269
- Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg. 1999;25:311-315. doi:10.1046/j.1524-4725.1999.08223.x
- Abtahi-Naeini B, Rastegarnasab F, Saffaei A. Liquid vitamin E injection for cosmetic facial rejuvenation: a disaster report of lipogranuloma. J Cosmet Dermatol. 2022;21:5549-5554. doi:10.1111/jocd.15294
- Kamouna B, Litov I, Bardarov E, et al. Granuloma formation after oil-soluble vitamin D injection for lip augmentation - case report. J Eur Acad Dermatol Venereol. 2016;30:1435-1436. doi:10.1111/jdv.13277
- Kamouna B, Darlenski R, Kazandjieva J, et al. Complications of injected vitamin E as a filler for lip augmentation: case series and therapeutic approach. Dermatol Ther. 2015;28:94-97. doi:10.1111/dth.12203
- Kosari P, Alikhan A, Sockolov M, et al. Vitamin E and allergic contact dermatitis. Dermatitis. 2010;21:148-153
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667. doi:10.1016/j.mam.2007.06.001
- Spataro EA, Dierks K, Carniol PJ. Microneedling-associated procedures to enhance facial rejuvenation. Facial Plast Surg Clin North Am. 2022;30:389-397. doi:10.1016/j.fsc.2022.03.012
- Setterfield L. The Concise Guide to Dermal Needling. Acacia Dermacare; 2017.
- Handal M, Kyriakides K, Cohen J, et al. Sarcoidal granulomatous reaction to microneedling with vitamin C serum. JAAD Case Rep. 2023;36:67-69. doi:10.1016/j.jdcr.2023.04.015
- Microneedling devices. U.S. Food and Drug Administration. Published 2020. Accessed September 9, 2025. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/microneedling-devices#risks
- Gowda A, Healey B, Ezaldein H, et al. A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol. 2021;14:45-54.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339. doi:10.1097/DSS.0000000000000924
- Hogan S, Velez MW, Ibrahim O. Microneedling: a new approach for treating textural abnormalities and scars. Semin Cutan Med Surg. 2017;36:155-163. doi:10.12788/j.sder.2017.042
- Schmitt L, Marquardt Y, Amann P, et al. Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model. PLoS One. 2018;13:e0204318. doi:10.1371/journal.pone.0204318
- Friedmann DP, Mehta E, Verma KK, et al. Granulomatous reactions from microneedling: a systematic review of the literature. Dermatol Surg. 2025;51:263-266. doi:10.1097/DSS.0000000000004450
Topical application or injection of cosmeceuticals in conjunction with procedures such as facial microneedling (MN) has been associated with local and systemic complications.1
Although at-home options may be more accessible and affordable for patients, they also increase the risk for improper use and subsequent infection. Additionally, the use of cosmeceuticals such as vitamin E oil in conjunction with MN to enhance the effects of the procedure can lead to further complications. We report the case of a 44-year-old woman who developed a necrotic ulcer on the chin following self-treatment with vitamin E oil and an at-home MN device. While MN has been reported to be relatively safe when performed by board-certified dermatologists, clinicians should be vigilant in correlating clinical history and recent cosmetic procedures with the histologic findings for timely diagnosis and treatment of unusual lesions on the face.
Case Report
A 44-year-old woman presented to the emergency department with a progressively enlarging, necrotic, ulcerative lesion on the midline chin of 4 months’ duration. The patient reported that the lesion started as redness that developed into a painful oozing ulcer following application of vitamin E oil in conjunction with an at-home MN device (Figure 1). She purchased the vitamin E oil and MN device online and performed the procedure herself, applying the vitamin E oil to her whole face before, during, and after using the MN device, which contained 0.25-mm titanium needles. She denied undergoing any other recent cosmetic procedures.

The lesion initially was treated by the patient’s primary care physician with oral doxycycline for 6 weeks, followed by oral cephalexin and clindamycin for 2 weeks. Although the redness stabilized, the lesion continued to enlarge, which prompted her initial visit to our hospital 1 month after seeing her primary care physician. During this visit, the patient was given penicillin, and the ulcer was debrided and biopsied; however, no clinical improvement was seen.
A biopsy during her initial emergency department visit and a repeat biopsy after 1 month showed similar findings of diffuse lymphohistiocytic and eosinophilic inflammation in the dermis (Figure 2) with poorly defined granulomas and multinucleated giant cells containing nonpolarizable exogenous material (Figure 3). Similar detached exogenous materials also were identified adjacent to the tissue. Diffuse re-epithelialization was seen, featuring pseudoepitheliomatous hyperplasia in association with the inflammatory process and granulation tissue (Figures 3 and 4). A higher-power view of the dermis showed foci of sclerosing lipogranuloma (Figure 4). Periodic acid–Schiff, Grocott methenamine silver, acid-fast bacilli, Fite, and Wright-Giemsa stains all were negative for microorganisms, and pancytokeratin staining was negative for carcinoma. These findings supported the diagnosis of a foreign body granulomatous reaction to an exogenous material—in this case, the vitamin E oil. Subsequent treatment with intralesional triamcinolone 10 mg/mL injection over 18 months resulted in progressive and drastic improvement of the lesion (Figure 5). A scar excision was performed, which further improved the lesion’s cosmetic appearance.




Comment
Application of various topical cosmeceuticals before, during, or after MN to enhance the effects of the procedure can introduce particles into the dermis, resulting in local or systemic hypersensitivity reactions. The associated adverse events can be divided into 2 main categories: adverse reactions related to the topical product or to the materials of the MN device itself.
A study showed that topical application of vitamin E oil to wounds on the skin does not improve the cosmetic appearance of scars.3 Instead, it is associated with a high incidence of contact dermatitis. A similar case of vitamin E injection, although without the concurrent use of an MN device, complicated by a facial lipogranuloma has been described.4 Sclerodermoid reaction, subcutaneous nodules, persistent edema, and ulceration at the site of vitamin E injection also have been described following the injection.5,6 Because vitamin E is a lipid-soluble vitamin, its absorption in the human body is dependent on the presence of lipid or oil-like substances. The reactions mentioned above are associated with the vitamin E oil, which acts as a helper vehicle for lipid-soluble vitamins to be absorbed.7 Other ingredients in topical vitamin E oil include a combination of D-alpha-tocopherol, D-alpha-tocopheryl acetate, D-alpha-tocopheryl succinate, or mixed tocopherols.8 These ester conjugate forms of vitamin E also may play a role in its immunogenic properties and
Hyaluronic acid is a relatively safe and commonly used topical treatment that acts as a lubricant during MN procedures to help the needles glide across the skin and prevent dragging. It also can be applied after the procedure for hydration purposes. Other common alternatives include peptides, ceramides, and epidermal growth factors. Topical products to avoid before, during, and 48 hours after undergoing MN include retinoids, vitamin C, vitamin E, exfoliants, serums that contain acids (eg, alpha hydroxy acids, beta hydroxy acids, glycolic acid, and lactic acid), serums that contain fragrance, and oil-based serums because they are associated with similar adverse effects.8-10 A granulomatous reaction after an MN procedure also has been reported with the use of vitamin C serum.11
The
Most MN devices are made of nickel and various other metals. Cases of contact dermatitis and delayed-type hypersensitivity granulomatous reaction with systemic symptoms have been reported after MN procedures due to the material of the MN device.1,13,14
Conclusion
Microneedling is a minimally invasive procedure that causes nominal damage to the epidermis and superficial papillary dermis, stimulating a wound-healing cascade for collagen production.15,16 Although not approved by the US Food and Drug Administration, MN performed at dermatology offices sometimes can be used in conjunction with topical products to enhance their absorption; however, while vitamin E is known for its antioxidant properties and potential skin benefits, the lipid substance acting as the vehicle is not absorbable by the skin and may cause a granulomatous reaction as the body attempts to encapsulate and digest the foreign substance.10,17 Although rarely reported, the use of topical vitamins with MN—through intradermal injection or combined with MN—can be associated with severe complications, including local, sometimes systemic, and life-threatening complications. Clinicians should be vigilant in order to correlate clinical background and history of recent cosmetic procedures with the histologic findings for prompt diagnosis and timely treatment.
Topical application or injection of cosmeceuticals in conjunction with procedures such as facial microneedling (MN) has been associated with local and systemic complications.1
Although at-home options may be more accessible and affordable for patients, they also increase the risk for improper use and subsequent infection. Additionally, the use of cosmeceuticals such as vitamin E oil in conjunction with MN to enhance the effects of the procedure can lead to further complications. We report the case of a 44-year-old woman who developed a necrotic ulcer on the chin following self-treatment with vitamin E oil and an at-home MN device. While MN has been reported to be relatively safe when performed by board-certified dermatologists, clinicians should be vigilant in correlating clinical history and recent cosmetic procedures with the histologic findings for timely diagnosis and treatment of unusual lesions on the face.
Case Report
A 44-year-old woman presented to the emergency department with a progressively enlarging, necrotic, ulcerative lesion on the midline chin of 4 months’ duration. The patient reported that the lesion started as redness that developed into a painful oozing ulcer following application of vitamin E oil in conjunction with an at-home MN device (Figure 1). She purchased the vitamin E oil and MN device online and performed the procedure herself, applying the vitamin E oil to her whole face before, during, and after using the MN device, which contained 0.25-mm titanium needles. She denied undergoing any other recent cosmetic procedures.

The lesion initially was treated by the patient’s primary care physician with oral doxycycline for 6 weeks, followed by oral cephalexin and clindamycin for 2 weeks. Although the redness stabilized, the lesion continued to enlarge, which prompted her initial visit to our hospital 1 month after seeing her primary care physician. During this visit, the patient was given penicillin, and the ulcer was debrided and biopsied; however, no clinical improvement was seen.
A biopsy during her initial emergency department visit and a repeat biopsy after 1 month showed similar findings of diffuse lymphohistiocytic and eosinophilic inflammation in the dermis (Figure 2) with poorly defined granulomas and multinucleated giant cells containing nonpolarizable exogenous material (Figure 3). Similar detached exogenous materials also were identified adjacent to the tissue. Diffuse re-epithelialization was seen, featuring pseudoepitheliomatous hyperplasia in association with the inflammatory process and granulation tissue (Figures 3 and 4). A higher-power view of the dermis showed foci of sclerosing lipogranuloma (Figure 4). Periodic acid–Schiff, Grocott methenamine silver, acid-fast bacilli, Fite, and Wright-Giemsa stains all were negative for microorganisms, and pancytokeratin staining was negative for carcinoma. These findings supported the diagnosis of a foreign body granulomatous reaction to an exogenous material—in this case, the vitamin E oil. Subsequent treatment with intralesional triamcinolone 10 mg/mL injection over 18 months resulted in progressive and drastic improvement of the lesion (Figure 5). A scar excision was performed, which further improved the lesion’s cosmetic appearance.




Comment
Application of various topical cosmeceuticals before, during, or after MN to enhance the effects of the procedure can introduce particles into the dermis, resulting in local or systemic hypersensitivity reactions. The associated adverse events can be divided into 2 main categories: adverse reactions related to the topical product or to the materials of the MN device itself.
A study showed that topical application of vitamin E oil to wounds on the skin does not improve the cosmetic appearance of scars.3 Instead, it is associated with a high incidence of contact dermatitis. A similar case of vitamin E injection, although without the concurrent use of an MN device, complicated by a facial lipogranuloma has been described.4 Sclerodermoid reaction, subcutaneous nodules, persistent edema, and ulceration at the site of vitamin E injection also have been described following the injection.5,6 Because vitamin E is a lipid-soluble vitamin, its absorption in the human body is dependent on the presence of lipid or oil-like substances. The reactions mentioned above are associated with the vitamin E oil, which acts as a helper vehicle for lipid-soluble vitamins to be absorbed.7 Other ingredients in topical vitamin E oil include a combination of D-alpha-tocopherol, D-alpha-tocopheryl acetate, D-alpha-tocopheryl succinate, or mixed tocopherols.8 These ester conjugate forms of vitamin E also may play a role in its immunogenic properties and
Hyaluronic acid is a relatively safe and commonly used topical treatment that acts as a lubricant during MN procedures to help the needles glide across the skin and prevent dragging. It also can be applied after the procedure for hydration purposes. Other common alternatives include peptides, ceramides, and epidermal growth factors. Topical products to avoid before, during, and 48 hours after undergoing MN include retinoids, vitamin C, vitamin E, exfoliants, serums that contain acids (eg, alpha hydroxy acids, beta hydroxy acids, glycolic acid, and lactic acid), serums that contain fragrance, and oil-based serums because they are associated with similar adverse effects.8-10 A granulomatous reaction after an MN procedure also has been reported with the use of vitamin C serum.11
The
Most MN devices are made of nickel and various other metals. Cases of contact dermatitis and delayed-type hypersensitivity granulomatous reaction with systemic symptoms have been reported after MN procedures due to the material of the MN device.1,13,14
Conclusion
Microneedling is a minimally invasive procedure that causes nominal damage to the epidermis and superficial papillary dermis, stimulating a wound-healing cascade for collagen production.15,16 Although not approved by the US Food and Drug Administration, MN performed at dermatology offices sometimes can be used in conjunction with topical products to enhance their absorption; however, while vitamin E is known for its antioxidant properties and potential skin benefits, the lipid substance acting as the vehicle is not absorbable by the skin and may cause a granulomatous reaction as the body attempts to encapsulate and digest the foreign substance.10,17 Although rarely reported, the use of topical vitamins with MN—through intradermal injection or combined with MN—can be associated with severe complications, including local, sometimes systemic, and life-threatening complications. Clinicians should be vigilant in order to correlate clinical background and history of recent cosmetic procedures with the histologic findings for prompt diagnosis and timely treatment.
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150:68-72. doi:10.1001/jamadermatol.2013.6955
- Microneedling market. The Brainy Insights. Published January, 2023. Accessed September 9, 2023. https://www.thebrainyinsights.com/report/microneedling-market-13269
- Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg. 1999;25:311-315. doi:10.1046/j.1524-4725.1999.08223.x
- Abtahi-Naeini B, Rastegarnasab F, Saffaei A. Liquid vitamin E injection for cosmetic facial rejuvenation: a disaster report of lipogranuloma. J Cosmet Dermatol. 2022;21:5549-5554. doi:10.1111/jocd.15294
- Kamouna B, Litov I, Bardarov E, et al. Granuloma formation after oil-soluble vitamin D injection for lip augmentation - case report. J Eur Acad Dermatol Venereol. 2016;30:1435-1436. doi:10.1111/jdv.13277
- Kamouna B, Darlenski R, Kazandjieva J, et al. Complications of injected vitamin E as a filler for lip augmentation: case series and therapeutic approach. Dermatol Ther. 2015;28:94-97. doi:10.1111/dth.12203
- Kosari P, Alikhan A, Sockolov M, et al. Vitamin E and allergic contact dermatitis. Dermatitis. 2010;21:148-153
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667. doi:10.1016/j.mam.2007.06.001
- Spataro EA, Dierks K, Carniol PJ. Microneedling-associated procedures to enhance facial rejuvenation. Facial Plast Surg Clin North Am. 2022;30:389-397. doi:10.1016/j.fsc.2022.03.012
- Setterfield L. The Concise Guide to Dermal Needling. Acacia Dermacare; 2017.
- Handal M, Kyriakides K, Cohen J, et al. Sarcoidal granulomatous reaction to microneedling with vitamin C serum. JAAD Case Rep. 2023;36:67-69. doi:10.1016/j.jdcr.2023.04.015
- Microneedling devices. U.S. Food and Drug Administration. Published 2020. Accessed September 9, 2025. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/microneedling-devices#risks
- Gowda A, Healey B, Ezaldein H, et al. A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol. 2021;14:45-54.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339. doi:10.1097/DSS.0000000000000924
- Hogan S, Velez MW, Ibrahim O. Microneedling: a new approach for treating textural abnormalities and scars. Semin Cutan Med Surg. 2017;36:155-163. doi:10.12788/j.sder.2017.042
- Schmitt L, Marquardt Y, Amann P, et al. Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model. PLoS One. 2018;13:e0204318. doi:10.1371/journal.pone.0204318
- Friedmann DP, Mehta E, Verma KK, et al. Granulomatous reactions from microneedling: a systematic review of the literature. Dermatol Surg. 2025;51:263-266. doi:10.1097/DSS.0000000000004450
- Soltani-Arabshahi R, Wong JW, Duffy KL, et al. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014;150:68-72. doi:10.1001/jamadermatol.2013.6955
- Microneedling market. The Brainy Insights. Published January, 2023. Accessed September 9, 2023. https://www.thebrainyinsights.com/report/microneedling-market-13269
- Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg. 1999;25:311-315. doi:10.1046/j.1524-4725.1999.08223.x
- Abtahi-Naeini B, Rastegarnasab F, Saffaei A. Liquid vitamin E injection for cosmetic facial rejuvenation: a disaster report of lipogranuloma. J Cosmet Dermatol. 2022;21:5549-5554. doi:10.1111/jocd.15294
- Kamouna B, Litov I, Bardarov E, et al. Granuloma formation after oil-soluble vitamin D injection for lip augmentation - case report. J Eur Acad Dermatol Venereol. 2016;30:1435-1436. doi:10.1111/jdv.13277
- Kamouna B, Darlenski R, Kazandjieva J, et al. Complications of injected vitamin E as a filler for lip augmentation: case series and therapeutic approach. Dermatol Ther. 2015;28:94-97. doi:10.1111/dth.12203
- Kosari P, Alikhan A, Sockolov M, et al. Vitamin E and allergic contact dermatitis. Dermatitis. 2010;21:148-153
- Thiele JJ, Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med. 2007;28:646-667. doi:10.1016/j.mam.2007.06.001
- Spataro EA, Dierks K, Carniol PJ. Microneedling-associated procedures to enhance facial rejuvenation. Facial Plast Surg Clin North Am. 2022;30:389-397. doi:10.1016/j.fsc.2022.03.012
- Setterfield L. The Concise Guide to Dermal Needling. Acacia Dermacare; 2017.
- Handal M, Kyriakides K, Cohen J, et al. Sarcoidal granulomatous reaction to microneedling with vitamin C serum. JAAD Case Rep. 2023;36:67-69. doi:10.1016/j.jdcr.2023.04.015
- Microneedling devices. U.S. Food and Drug Administration. Published 2020. Accessed September 9, 2025. https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/microneedling-devices#risks
- Gowda A, Healey B, Ezaldein H, et al. A systematic review examining the potential adverse effects of microneedling. J Clin Aesthet Dermatol. 2021;14:45-54.
- Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321-339. doi:10.1097/DSS.0000000000000924
- Hogan S, Velez MW, Ibrahim O. Microneedling: a new approach for treating textural abnormalities and scars. Semin Cutan Med Surg. 2017;36:155-163. doi:10.12788/j.sder.2017.042
- Schmitt L, Marquardt Y, Amann P, et al. Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model. PLoS One. 2018;13:e0204318. doi:10.1371/journal.pone.0204318
- Friedmann DP, Mehta E, Verma KK, et al. Granulomatous reactions from microneedling: a systematic review of the literature. Dermatol Surg. 2025;51:263-266. doi:10.1097/DSS.0000000000004450
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Destructive Facial Granuloma Following Self-Treatment With Vitamin E Oil and an At-Home Microneedling Device
Practice Points
- Severe complications can potentially arise from at-home microneedling procedures when combined with cosmeceuticals such as vitamin E oil.
- Clinicopathologic correlation with cosmetic procedures is imperative to prompt diagnosis and treatment of these skin reactions.
- Microneedling procedures should be performed under the supervision of a board-certified dermatologist to avoid complications, and clinicians should inquire specifically about skin care routines and cosmetic procedures when patients present with unusual lesions on the face.
Axolotl Salamander Holds Potential for Cosmeceuticals, Wound Healing
For over 200 years, researchers have been captivated by axolotl salamanders (Ambystoma mexicanum) and their remarkable regenerative abilities, seeking to uncover secrets that could revolutionize regenerative medicine, including the scarless healing of wounds.
“The axolotl salamander is the most studied animal ever in science for its neotenic ability to regenerate,” Jill S. Waibel, MD, dermatologist and researcher in Miami, Florida, said in an interview. Neotenic tissue retains a juvenile or immature state throughout an organism’s life. In the case of the axolotl, “it can regenerate limbs, part of its heart, even its brain.”
A 2019 review of several studies on the regenerative abilities of axolotls highlights the importance of gene activity in controlling its skin regeneration. Specifically, growth factors such as fibroblast growth factors, transforming growth factor beta, and Wnt play a key role in guiding how the creature’s skin cells behave during healing and regrowth. The immune response , particularly the actions of macrophages and neutrophils, is also crucial in the early stages of regeneration, as these cells clear away dead tissue and kickstart the healing process.
without harming the animal. In axolotls, Waibel explained, damaged neotenic tissue “still thinks it’s in fetal mode, so if it injures its muscle, bone, nerves, collagen, or skin, everything will redevelop. After a few months in utero, that process stops in humans, but it never stops in the axolotl. The axolotl has scarless healing and immunity because of antimicrobial properties found in the neotenic tissue.”
RegenX scientists have developed a proprietary decellularization process that renders the urodele collagen extract safe and effective for use in humans. “We then harnessed a reservoir of bioactive peptides, which are small proteins that come from the axolotl, but they don’t contain any RNA or DNA that could confer the risk of any diseases or cancer,” she added.
According to Waibel, who is also subsection chief of dermatology at Baptist Hospital and past medical director of the Miami Cancer Institute’s Multidisciplinary Skin Cancer Clinic, genetic analysis of the axolotl revealed genes that have not been seen in humans. The urodele collagen extract also has anti-inflammatory and analgesic properties. “It decreases TNF [tumor necrosis factor] and IL [interleukin]–23 and stimulates regenerative pathways like FETUB (Fetuin-B), which is a gene involved in tissue regeneration,” she said. “We’re exploring these for some products.”
Institutional Review Board–approved human clinical trials at three US sites are nearly complete for evaluating an antiaging hydrating daily serum, an antiaging serum for damaged skin, and a restorative serum to be applied following cosmetic procedures, all containing the extract. The product furthest along is a “super gel” that contains properties of the urodele collagen extract.
In a proof-of-concept study using a third-degree burn model in two pigs, Waibel and colleagues at the University of Miami, found that 3 days after the injury was induced, application of the gel led to 92% reepithelialization of the pig’s skin, compared with only 54% in untreated skin.
Shortly after this study was conducted, a burn patient was referred to Waibel — 4 years after he was struck by lightning while fishing on a boat in Mississippi, an accident that resulted in the loss of his right arm and both legs. During a telemedicine consultation, Waibel noticed open ulcers on his chest. “What are those from?” she asked. “They’re from my accident 4 years ago,” he replied.
After the man flew to Miami for an in-person evaluation, Waibel treated his ulcers with a fractional laser to debride the wound, then applied the gel as part of a proof-of-concept approach, testing its potential in a real-world patient setting. Within 3 weeks, the long-standing ulcerated area had healed completely, marking the first time a human was treated with the super gel.
Looking ahead, the million-dollar question, Waibel noted, is how much healing can be achieved in humans with formulations of axolotl-derived technology. “For example, can we help a spinal cord injury patient? That sounds like a science fiction movie, but there are proteins in genes in this animal that we have turned off that potentially can be turned on in a human,” she said. “It’s very exciting.”
Arisa E. Ortiz, MD, director of Laser and Cosmetic Dermatology at the University of California, San Diego, and current president of the American Society for Laser Medicine and Surgery, who was asked to comment on this work, said that the use of urodele collagen extract derived from axolotl tissue “is an exciting innovation, especially given its unique properties like scarless healing and antimicrobial activity.”
While the results from preclinical and proof-of-concept studies are promising, “a key limitation lies in understanding the extent to which these findings will translate to human applications,” Ortiz said. “Overall, this research contributes significantly to the fields of regenerative medicine and dermatology, offering hope for more effective treatments in the future.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to provide her insights on the topic, said that, if researchers could replicate the axolotl salamander’s ability to regenerate its own limbs and organs, “medicine would be transformed. Rather than transplant another person’s organ with lifelong immunosuppression, a regenerative treatment could program a patient’s own body to create a needed organ.
“On a simpler level,” she continued, “regenerating skin and its underlying structures could hasten wound healing and potentially even treat hair loss. This is not a pipe dream, as Waibel has successfully treated severe ulcers using a super gel containing urodele collagen extract. Urodele collagen is type XII collagen, important in the salamander’s capacity to heal and regenerate.”
Waibel disclosed that she is a scientific adviser to RegenX and is a member of the company’s board of directors. Ortiz and Ko reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
For over 200 years, researchers have been captivated by axolotl salamanders (Ambystoma mexicanum) and their remarkable regenerative abilities, seeking to uncover secrets that could revolutionize regenerative medicine, including the scarless healing of wounds.
“The axolotl salamander is the most studied animal ever in science for its neotenic ability to regenerate,” Jill S. Waibel, MD, dermatologist and researcher in Miami, Florida, said in an interview. Neotenic tissue retains a juvenile or immature state throughout an organism’s life. In the case of the axolotl, “it can regenerate limbs, part of its heart, even its brain.”
A 2019 review of several studies on the regenerative abilities of axolotls highlights the importance of gene activity in controlling its skin regeneration. Specifically, growth factors such as fibroblast growth factors, transforming growth factor beta, and Wnt play a key role in guiding how the creature’s skin cells behave during healing and regrowth. The immune response , particularly the actions of macrophages and neutrophils, is also crucial in the early stages of regeneration, as these cells clear away dead tissue and kickstart the healing process.
without harming the animal. In axolotls, Waibel explained, damaged neotenic tissue “still thinks it’s in fetal mode, so if it injures its muscle, bone, nerves, collagen, or skin, everything will redevelop. After a few months in utero, that process stops in humans, but it never stops in the axolotl. The axolotl has scarless healing and immunity because of antimicrobial properties found in the neotenic tissue.”
RegenX scientists have developed a proprietary decellularization process that renders the urodele collagen extract safe and effective for use in humans. “We then harnessed a reservoir of bioactive peptides, which are small proteins that come from the axolotl, but they don’t contain any RNA or DNA that could confer the risk of any diseases or cancer,” she added.
According to Waibel, who is also subsection chief of dermatology at Baptist Hospital and past medical director of the Miami Cancer Institute’s Multidisciplinary Skin Cancer Clinic, genetic analysis of the axolotl revealed genes that have not been seen in humans. The urodele collagen extract also has anti-inflammatory and analgesic properties. “It decreases TNF [tumor necrosis factor] and IL [interleukin]–23 and stimulates regenerative pathways like FETUB (Fetuin-B), which is a gene involved in tissue regeneration,” she said. “We’re exploring these for some products.”
Institutional Review Board–approved human clinical trials at three US sites are nearly complete for evaluating an antiaging hydrating daily serum, an antiaging serum for damaged skin, and a restorative serum to be applied following cosmetic procedures, all containing the extract. The product furthest along is a “super gel” that contains properties of the urodele collagen extract.
In a proof-of-concept study using a third-degree burn model in two pigs, Waibel and colleagues at the University of Miami, found that 3 days after the injury was induced, application of the gel led to 92% reepithelialization of the pig’s skin, compared with only 54% in untreated skin.
Shortly after this study was conducted, a burn patient was referred to Waibel — 4 years after he was struck by lightning while fishing on a boat in Mississippi, an accident that resulted in the loss of his right arm and both legs. During a telemedicine consultation, Waibel noticed open ulcers on his chest. “What are those from?” she asked. “They’re from my accident 4 years ago,” he replied.
After the man flew to Miami for an in-person evaluation, Waibel treated his ulcers with a fractional laser to debride the wound, then applied the gel as part of a proof-of-concept approach, testing its potential in a real-world patient setting. Within 3 weeks, the long-standing ulcerated area had healed completely, marking the first time a human was treated with the super gel.
Looking ahead, the million-dollar question, Waibel noted, is how much healing can be achieved in humans with formulations of axolotl-derived technology. “For example, can we help a spinal cord injury patient? That sounds like a science fiction movie, but there are proteins in genes in this animal that we have turned off that potentially can be turned on in a human,” she said. “It’s very exciting.”
Arisa E. Ortiz, MD, director of Laser and Cosmetic Dermatology at the University of California, San Diego, and current president of the American Society for Laser Medicine and Surgery, who was asked to comment on this work, said that the use of urodele collagen extract derived from axolotl tissue “is an exciting innovation, especially given its unique properties like scarless healing and antimicrobial activity.”
While the results from preclinical and proof-of-concept studies are promising, “a key limitation lies in understanding the extent to which these findings will translate to human applications,” Ortiz said. “Overall, this research contributes significantly to the fields of regenerative medicine and dermatology, offering hope for more effective treatments in the future.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to provide her insights on the topic, said that, if researchers could replicate the axolotl salamander’s ability to regenerate its own limbs and organs, “medicine would be transformed. Rather than transplant another person’s organ with lifelong immunosuppression, a regenerative treatment could program a patient’s own body to create a needed organ.
“On a simpler level,” she continued, “regenerating skin and its underlying structures could hasten wound healing and potentially even treat hair loss. This is not a pipe dream, as Waibel has successfully treated severe ulcers using a super gel containing urodele collagen extract. Urodele collagen is type XII collagen, important in the salamander’s capacity to heal and regenerate.”
Waibel disclosed that she is a scientific adviser to RegenX and is a member of the company’s board of directors. Ortiz and Ko reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
For over 200 years, researchers have been captivated by axolotl salamanders (Ambystoma mexicanum) and their remarkable regenerative abilities, seeking to uncover secrets that could revolutionize regenerative medicine, including the scarless healing of wounds.
“The axolotl salamander is the most studied animal ever in science for its neotenic ability to regenerate,” Jill S. Waibel, MD, dermatologist and researcher in Miami, Florida, said in an interview. Neotenic tissue retains a juvenile or immature state throughout an organism’s life. In the case of the axolotl, “it can regenerate limbs, part of its heart, even its brain.”
A 2019 review of several studies on the regenerative abilities of axolotls highlights the importance of gene activity in controlling its skin regeneration. Specifically, growth factors such as fibroblast growth factors, transforming growth factor beta, and Wnt play a key role in guiding how the creature’s skin cells behave during healing and regrowth. The immune response , particularly the actions of macrophages and neutrophils, is also crucial in the early stages of regeneration, as these cells clear away dead tissue and kickstart the healing process.
without harming the animal. In axolotls, Waibel explained, damaged neotenic tissue “still thinks it’s in fetal mode, so if it injures its muscle, bone, nerves, collagen, or skin, everything will redevelop. After a few months in utero, that process stops in humans, but it never stops in the axolotl. The axolotl has scarless healing and immunity because of antimicrobial properties found in the neotenic tissue.”
RegenX scientists have developed a proprietary decellularization process that renders the urodele collagen extract safe and effective for use in humans. “We then harnessed a reservoir of bioactive peptides, which are small proteins that come from the axolotl, but they don’t contain any RNA or DNA that could confer the risk of any diseases or cancer,” she added.
According to Waibel, who is also subsection chief of dermatology at Baptist Hospital and past medical director of the Miami Cancer Institute’s Multidisciplinary Skin Cancer Clinic, genetic analysis of the axolotl revealed genes that have not been seen in humans. The urodele collagen extract also has anti-inflammatory and analgesic properties. “It decreases TNF [tumor necrosis factor] and IL [interleukin]–23 and stimulates regenerative pathways like FETUB (Fetuin-B), which is a gene involved in tissue regeneration,” she said. “We’re exploring these for some products.”
Institutional Review Board–approved human clinical trials at three US sites are nearly complete for evaluating an antiaging hydrating daily serum, an antiaging serum for damaged skin, and a restorative serum to be applied following cosmetic procedures, all containing the extract. The product furthest along is a “super gel” that contains properties of the urodele collagen extract.
In a proof-of-concept study using a third-degree burn model in two pigs, Waibel and colleagues at the University of Miami, found that 3 days after the injury was induced, application of the gel led to 92% reepithelialization of the pig’s skin, compared with only 54% in untreated skin.
Shortly after this study was conducted, a burn patient was referred to Waibel — 4 years after he was struck by lightning while fishing on a boat in Mississippi, an accident that resulted in the loss of his right arm and both legs. During a telemedicine consultation, Waibel noticed open ulcers on his chest. “What are those from?” she asked. “They’re from my accident 4 years ago,” he replied.
After the man flew to Miami for an in-person evaluation, Waibel treated his ulcers with a fractional laser to debride the wound, then applied the gel as part of a proof-of-concept approach, testing its potential in a real-world patient setting. Within 3 weeks, the long-standing ulcerated area had healed completely, marking the first time a human was treated with the super gel.
Looking ahead, the million-dollar question, Waibel noted, is how much healing can be achieved in humans with formulations of axolotl-derived technology. “For example, can we help a spinal cord injury patient? That sounds like a science fiction movie, but there are proteins in genes in this animal that we have turned off that potentially can be turned on in a human,” she said. “It’s very exciting.”
Arisa E. Ortiz, MD, director of Laser and Cosmetic Dermatology at the University of California, San Diego, and current president of the American Society for Laser Medicine and Surgery, who was asked to comment on this work, said that the use of urodele collagen extract derived from axolotl tissue “is an exciting innovation, especially given its unique properties like scarless healing and antimicrobial activity.”
While the results from preclinical and proof-of-concept studies are promising, “a key limitation lies in understanding the extent to which these findings will translate to human applications,” Ortiz said. “Overall, this research contributes significantly to the fields of regenerative medicine and dermatology, offering hope for more effective treatments in the future.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to provide her insights on the topic, said that, if researchers could replicate the axolotl salamander’s ability to regenerate its own limbs and organs, “medicine would be transformed. Rather than transplant another person’s organ with lifelong immunosuppression, a regenerative treatment could program a patient’s own body to create a needed organ.
“On a simpler level,” she continued, “regenerating skin and its underlying structures could hasten wound healing and potentially even treat hair loss. This is not a pipe dream, as Waibel has successfully treated severe ulcers using a super gel containing urodele collagen extract. Urodele collagen is type XII collagen, important in the salamander’s capacity to heal and regenerate.”
Waibel disclosed that she is a scientific adviser to RegenX and is a member of the company’s board of directors. Ortiz and Ko reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
Probiotics, Prebiotics, and Provocative Claims About Bacillus Lysate
Outrageous assertions with little evidence are not new. Even the famous statement “There’s a sucker born every minute,” long attributed to 1800s showman P.T. Barnum, lacks evidence that the circus founder uttered the remark. The message itself and the snippet of a story about the message may be pertinent, though, when we consider the touted benefits of Bacillus lysate for the skin. The focus of this column will be the foundation for the use of probiotics and prebiotics in skin care and then claims made about this skin care ingredient derived from a particular strain of Bacillus bacteria.
. Typically, this topic is broached in the context of the gut-skin axis and the skin and gut microbiomes.1-3 In 2014, Miyazaki et al. found that phenols produced by gut bacteria spurred skin disorders and that decreasing phenols with probiotics and/or prebiotics can restore or maintain cutaneous health.4
Probiotics have been associated with antioxidant activity, primarily because of the presence of antioxidant enzymes (eg, superoxide dismutase), the delivery of antioxidant substances (eg, glutathione), and extracellular polysaccharide synthesis.5-8 Further, probiotics are known to synthesize a cascade of substances with anti-inflammatory, antibacterial, immunomodulatory, and angiogenetic functions that can contribute to wound healing.9 The use of probiotics in skin health largely relies on applying inactivated beneficial bacteria.10 Prebiotics, which are non-digestible plant-based carbohydrates that aid digestion, inhibit pathogens, and support beneficial bacteria, are known to rebalance the skin microflora.10 In addition, prebiotics are considered a robust option to replace live bacteria in skin formulations.11 Bacterial cell lysates, which include bacterial metabolites, cell walls, and dead bacteria, are incorporated into skin care products as well.12
Probiotics and Wound Healing
In 2020, Ashoori et al. reported on their study of three formulations composed of probiotic supernatant (Lactobacillus reuteri, L. fermentum, and Bacillus subtilis sp. natto)-loaded chitosan nanogels prepared from cultures. They evaluated the effectiveness and dressing activity of the formulations by gauging wound closure and histological results in Sprague-Dawley rats. The researchers found that all probiotic lysate preparations conferred healing properties, with the Bacillus subtilis natto yielding the best wound healing quality. They concluded that probiotic lysate nanogels impart a range of benefits, such as favorable wound closure rates, improved appearance, and suitable histological results upon in vivo examination, supporting the potential use of such formulations to treat wounds.9
Probiotics and Treating Skin Disorders
A 2015 review by Roudsari et al. suggests that probiotics display the potential for preventing and treating various skin disorders, including acne, atopic dermatitis, allergic inflammation or hypersensitivity, eczema, photodamage, and wounds.8 They reported that in a US patent, Gueniche revealed ways to employ at least one probiotic microorganism (from Lactobacillus and/or Bifidobacterium) as an active agent to prevent or treat skin irritation.8,13 In addition, they noted that L. brevis was used successfully by DeSimone in 2003 to promote apoptosis and/or diminish inflammation, particularly in creams and ointments to alleviate inflammation.8
At around the same time, Miyazaki et al. reported that Bifidobacterium-fermented soy milk extract stimulated the production of hyaluronic acid (HA) in organotypic cultures of human keratinocytes, cultures of human skin fibroblasts, and hairless mouse skin after 2 weeks of topical application and has the potential to promote HA synthesis in the epidermis and dermis and thus act as an anti-aging agent.14 In another study, Miyazaki et al. investigated the impact of Bifidobacterium-fermented soy milk extract containing genistein and daidzein on the HA content of hairless mouse as well as human skin. After 6 weeks of topical application in mice, skin elasticity, viscoelasticity, hydration, and thickness improved, and HA content increased. In addition, after 3 months of topical application of a 10% Bifidobacterium-fermented soy milk extract gel to the human forearm, decreases in skin elasticity were significantly mitigated.15More recently, in 2023, Xie et al. reviewed clinical and experimental data on the use of various species of Lactobacillus for the treatment and prevention of atopic dermatitis (AD). They found evidence that multiple species (L. rhamnosus in animal and clinical experiments) appeared to be effective in preventing and treating AD, with L. acidophilus lessening symptoms and reported to be safe, L. plantarum improving symptoms through immunomodulatory activity, and L. sakei demonstrating anti-inflammatory and skin barrier protective activity. The authors also noted that L. paracasei exhibited anti-inflammatory effects on AD-like skin lesions, and L. reuteri supplementation prevented AD development. Overall, they called for more in vivo studies and randomized controlled clinical trials to fully elucidate the wide-ranging potential of Lactobacillus species in treating and preventing AD.16
The Darker Side of Using Prebiotic Species in Skin Care?
According to manufacturer Delavie Sciences, its Aeonia product line was based on research conducted on the International Space Station, which allowed for its patented microorganism to be exposed to the conditions of outer space. This cornerstone ingredient, Bacillus lysate, once returned to Earth, reportedly exhibited anti-aging and UV-protective characteristics. The product line has been described as a prebiotic that contributes to a healthy skin barrier.17
In a September 2023 interview in CosmeticsDesign, the president of Delavie Sciences clarified that its Bacillus lysate contains no live bacteria and that it is not a probiotic, but rather, the certified prebiotic lysate is a Bacillus extract that has been used to strengthen the SPF potency of skin care formulations.18 Because of the research performed on the International Space Station, the manufacturers are claiming these ingredients could be “out-of-this-world” as a way to promote results that have, as yet, not been verified by peer review.
Conclusion
Probiotics and prebiotics continue to be the focus of multiple lines of research for their applications and further potential in skin care. In the case of the Bacillus lysate prebiotic compound, there is a kernel of an interesting idea here, at the very least. But proprietary research limits our ability to render a comprehensive evaluation at this time. Such bold and outrageous claims spur more skepticism than optimism. However, lysates are the latest thing in skin care — so we need to keep watch on the developments to stay current. But that’s what you have me for, I’ll help keep you current on new ingredient findings. If you are on LinkedIn, come connect with me. I post breaking ingredient news and skin care trends there to help you answer patient questions. When you are asked if these lysates work, the answer is: All the data we have on bacillus extract are from computer analysis of the ingredient properties and not on the actual formulations or products. Stay tuned.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. Mahmud MR et al. Gut Microbes. 2022 Jan-Dec;14(1):2096995. doi: 10.1080/19490976.2022.2096995.
2. Sinha S et al. Clin Dermatol. 2021 Sep-Oct;39(5):829-839. doi: 10.1016/j.clindermatol.2021.08.021.
3. Gao T et al. Nutrients. 2023 Jul 13;15(14):3123. doi: 10.3390/nu15143123.
4. Miyazaki K et al. Benef Microbes. 2014 Jun 1;5(2):121-128. doi: 10.3920/BM2012.0066.
5. Shen Q et al. Anaerobe. 2010 Aug;16(4):380-386. doi: 10.1016/j.anaerobe.2010.06.006.
6. Peran L et al. Int J Colorectal Dis. 2006 Dec;21(8):737-746. doi: 10.1007/s00384-005-0773-y.
7. Kodali VP, Sen R. Biotechnol J. 2008 Feb;3(2):245-251. doi: 10.1002/biot.200700208.
8. Roudsari MR et al. Health effects of probiotics on the skin. Crit Rev Food Sci Nutr. 2015;55(9):1219-40. doi: 10.1080/10408398.2012.680078.
9. Ashoori Y et al. Biomed Res Int. 2020 Dec 28;2020:8868618. doi: 10.1155/2020/8868618.
10. Simmering R, Breves R. Hautarzt. 2009 Oct;60(10):809-814. doi: 10.1007/s00105-009-1759-4.
11. Bockmuhl D. IFSSC Mag. 2006 Sep 30;9[3]:1-5.
12. Lew LC, Liong MT. J Appl Microbiol. 2013 May;114(5):1241-1253. doi: 10.1111/jam.12137.
13. Gueniche A. US Patent, US 20100226892. 2010.
14. Miyazaki K et al. Skin Pharmacol Appl Skin Physiol. 2003 Mar-Apr;16(2):108-116. doi: 10.1159/000069031.
15. Miyazaki et al. J Cosmet Sci. 2004 Sep-Oct;55(5):473-479.16. Xie A et al. Front Cell Infect Microbiol. 2023 Feb 16;13:1137275. doi: 10.3389/fcimb.2023.1137275.
17. Delavie Sciences. Skincare Science: Aeonia. Skincare from the Stars.
. Accessed December 12, 2024.
18. Stern C. CosmeticsDesign USA. September 7, 2023.
Outrageous assertions with little evidence are not new. Even the famous statement “There’s a sucker born every minute,” long attributed to 1800s showman P.T. Barnum, lacks evidence that the circus founder uttered the remark. The message itself and the snippet of a story about the message may be pertinent, though, when we consider the touted benefits of Bacillus lysate for the skin. The focus of this column will be the foundation for the use of probiotics and prebiotics in skin care and then claims made about this skin care ingredient derived from a particular strain of Bacillus bacteria.
. Typically, this topic is broached in the context of the gut-skin axis and the skin and gut microbiomes.1-3 In 2014, Miyazaki et al. found that phenols produced by gut bacteria spurred skin disorders and that decreasing phenols with probiotics and/or prebiotics can restore or maintain cutaneous health.4
Probiotics have been associated with antioxidant activity, primarily because of the presence of antioxidant enzymes (eg, superoxide dismutase), the delivery of antioxidant substances (eg, glutathione), and extracellular polysaccharide synthesis.5-8 Further, probiotics are known to synthesize a cascade of substances with anti-inflammatory, antibacterial, immunomodulatory, and angiogenetic functions that can contribute to wound healing.9 The use of probiotics in skin health largely relies on applying inactivated beneficial bacteria.10 Prebiotics, which are non-digestible plant-based carbohydrates that aid digestion, inhibit pathogens, and support beneficial bacteria, are known to rebalance the skin microflora.10 In addition, prebiotics are considered a robust option to replace live bacteria in skin formulations.11 Bacterial cell lysates, which include bacterial metabolites, cell walls, and dead bacteria, are incorporated into skin care products as well.12
Probiotics and Wound Healing
In 2020, Ashoori et al. reported on their study of three formulations composed of probiotic supernatant (Lactobacillus reuteri, L. fermentum, and Bacillus subtilis sp. natto)-loaded chitosan nanogels prepared from cultures. They evaluated the effectiveness and dressing activity of the formulations by gauging wound closure and histological results in Sprague-Dawley rats. The researchers found that all probiotic lysate preparations conferred healing properties, with the Bacillus subtilis natto yielding the best wound healing quality. They concluded that probiotic lysate nanogels impart a range of benefits, such as favorable wound closure rates, improved appearance, and suitable histological results upon in vivo examination, supporting the potential use of such formulations to treat wounds.9
Probiotics and Treating Skin Disorders
A 2015 review by Roudsari et al. suggests that probiotics display the potential for preventing and treating various skin disorders, including acne, atopic dermatitis, allergic inflammation or hypersensitivity, eczema, photodamage, and wounds.8 They reported that in a US patent, Gueniche revealed ways to employ at least one probiotic microorganism (from Lactobacillus and/or Bifidobacterium) as an active agent to prevent or treat skin irritation.8,13 In addition, they noted that L. brevis was used successfully by DeSimone in 2003 to promote apoptosis and/or diminish inflammation, particularly in creams and ointments to alleviate inflammation.8
At around the same time, Miyazaki et al. reported that Bifidobacterium-fermented soy milk extract stimulated the production of hyaluronic acid (HA) in organotypic cultures of human keratinocytes, cultures of human skin fibroblasts, and hairless mouse skin after 2 weeks of topical application and has the potential to promote HA synthesis in the epidermis and dermis and thus act as an anti-aging agent.14 In another study, Miyazaki et al. investigated the impact of Bifidobacterium-fermented soy milk extract containing genistein and daidzein on the HA content of hairless mouse as well as human skin. After 6 weeks of topical application in mice, skin elasticity, viscoelasticity, hydration, and thickness improved, and HA content increased. In addition, after 3 months of topical application of a 10% Bifidobacterium-fermented soy milk extract gel to the human forearm, decreases in skin elasticity were significantly mitigated.15More recently, in 2023, Xie et al. reviewed clinical and experimental data on the use of various species of Lactobacillus for the treatment and prevention of atopic dermatitis (AD). They found evidence that multiple species (L. rhamnosus in animal and clinical experiments) appeared to be effective in preventing and treating AD, with L. acidophilus lessening symptoms and reported to be safe, L. plantarum improving symptoms through immunomodulatory activity, and L. sakei demonstrating anti-inflammatory and skin barrier protective activity. The authors also noted that L. paracasei exhibited anti-inflammatory effects on AD-like skin lesions, and L. reuteri supplementation prevented AD development. Overall, they called for more in vivo studies and randomized controlled clinical trials to fully elucidate the wide-ranging potential of Lactobacillus species in treating and preventing AD.16
The Darker Side of Using Prebiotic Species in Skin Care?
According to manufacturer Delavie Sciences, its Aeonia product line was based on research conducted on the International Space Station, which allowed for its patented microorganism to be exposed to the conditions of outer space. This cornerstone ingredient, Bacillus lysate, once returned to Earth, reportedly exhibited anti-aging and UV-protective characteristics. The product line has been described as a prebiotic that contributes to a healthy skin barrier.17
In a September 2023 interview in CosmeticsDesign, the president of Delavie Sciences clarified that its Bacillus lysate contains no live bacteria and that it is not a probiotic, but rather, the certified prebiotic lysate is a Bacillus extract that has been used to strengthen the SPF potency of skin care formulations.18 Because of the research performed on the International Space Station, the manufacturers are claiming these ingredients could be “out-of-this-world” as a way to promote results that have, as yet, not been verified by peer review.
Conclusion
Probiotics and prebiotics continue to be the focus of multiple lines of research for their applications and further potential in skin care. In the case of the Bacillus lysate prebiotic compound, there is a kernel of an interesting idea here, at the very least. But proprietary research limits our ability to render a comprehensive evaluation at this time. Such bold and outrageous claims spur more skepticism than optimism. However, lysates are the latest thing in skin care — so we need to keep watch on the developments to stay current. But that’s what you have me for, I’ll help keep you current on new ingredient findings. If you are on LinkedIn, come connect with me. I post breaking ingredient news and skin care trends there to help you answer patient questions. When you are asked if these lysates work, the answer is: All the data we have on bacillus extract are from computer analysis of the ingredient properties and not on the actual formulations or products. Stay tuned.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. Mahmud MR et al. Gut Microbes. 2022 Jan-Dec;14(1):2096995. doi: 10.1080/19490976.2022.2096995.
2. Sinha S et al. Clin Dermatol. 2021 Sep-Oct;39(5):829-839. doi: 10.1016/j.clindermatol.2021.08.021.
3. Gao T et al. Nutrients. 2023 Jul 13;15(14):3123. doi: 10.3390/nu15143123.
4. Miyazaki K et al. Benef Microbes. 2014 Jun 1;5(2):121-128. doi: 10.3920/BM2012.0066.
5. Shen Q et al. Anaerobe. 2010 Aug;16(4):380-386. doi: 10.1016/j.anaerobe.2010.06.006.
6. Peran L et al. Int J Colorectal Dis. 2006 Dec;21(8):737-746. doi: 10.1007/s00384-005-0773-y.
7. Kodali VP, Sen R. Biotechnol J. 2008 Feb;3(2):245-251. doi: 10.1002/biot.200700208.
8. Roudsari MR et al. Health effects of probiotics on the skin. Crit Rev Food Sci Nutr. 2015;55(9):1219-40. doi: 10.1080/10408398.2012.680078.
9. Ashoori Y et al. Biomed Res Int. 2020 Dec 28;2020:8868618. doi: 10.1155/2020/8868618.
10. Simmering R, Breves R. Hautarzt. 2009 Oct;60(10):809-814. doi: 10.1007/s00105-009-1759-4.
11. Bockmuhl D. IFSSC Mag. 2006 Sep 30;9[3]:1-5.
12. Lew LC, Liong MT. J Appl Microbiol. 2013 May;114(5):1241-1253. doi: 10.1111/jam.12137.
13. Gueniche A. US Patent, US 20100226892. 2010.
14. Miyazaki K et al. Skin Pharmacol Appl Skin Physiol. 2003 Mar-Apr;16(2):108-116. doi: 10.1159/000069031.
15. Miyazaki et al. J Cosmet Sci. 2004 Sep-Oct;55(5):473-479.16. Xie A et al. Front Cell Infect Microbiol. 2023 Feb 16;13:1137275. doi: 10.3389/fcimb.2023.1137275.
17. Delavie Sciences. Skincare Science: Aeonia. Skincare from the Stars.
. Accessed December 12, 2024.
18. Stern C. CosmeticsDesign USA. September 7, 2023.
Outrageous assertions with little evidence are not new. Even the famous statement “There’s a sucker born every minute,” long attributed to 1800s showman P.T. Barnum, lacks evidence that the circus founder uttered the remark. The message itself and the snippet of a story about the message may be pertinent, though, when we consider the touted benefits of Bacillus lysate for the skin. The focus of this column will be the foundation for the use of probiotics and prebiotics in skin care and then claims made about this skin care ingredient derived from a particular strain of Bacillus bacteria.
. Typically, this topic is broached in the context of the gut-skin axis and the skin and gut microbiomes.1-3 In 2014, Miyazaki et al. found that phenols produced by gut bacteria spurred skin disorders and that decreasing phenols with probiotics and/or prebiotics can restore or maintain cutaneous health.4
Probiotics have been associated with antioxidant activity, primarily because of the presence of antioxidant enzymes (eg, superoxide dismutase), the delivery of antioxidant substances (eg, glutathione), and extracellular polysaccharide synthesis.5-8 Further, probiotics are known to synthesize a cascade of substances with anti-inflammatory, antibacterial, immunomodulatory, and angiogenetic functions that can contribute to wound healing.9 The use of probiotics in skin health largely relies on applying inactivated beneficial bacteria.10 Prebiotics, which are non-digestible plant-based carbohydrates that aid digestion, inhibit pathogens, and support beneficial bacteria, are known to rebalance the skin microflora.10 In addition, prebiotics are considered a robust option to replace live bacteria in skin formulations.11 Bacterial cell lysates, which include bacterial metabolites, cell walls, and dead bacteria, are incorporated into skin care products as well.12
Probiotics and Wound Healing
In 2020, Ashoori et al. reported on their study of three formulations composed of probiotic supernatant (Lactobacillus reuteri, L. fermentum, and Bacillus subtilis sp. natto)-loaded chitosan nanogels prepared from cultures. They evaluated the effectiveness and dressing activity of the formulations by gauging wound closure and histological results in Sprague-Dawley rats. The researchers found that all probiotic lysate preparations conferred healing properties, with the Bacillus subtilis natto yielding the best wound healing quality. They concluded that probiotic lysate nanogels impart a range of benefits, such as favorable wound closure rates, improved appearance, and suitable histological results upon in vivo examination, supporting the potential use of such formulations to treat wounds.9
Probiotics and Treating Skin Disorders
A 2015 review by Roudsari et al. suggests that probiotics display the potential for preventing and treating various skin disorders, including acne, atopic dermatitis, allergic inflammation or hypersensitivity, eczema, photodamage, and wounds.8 They reported that in a US patent, Gueniche revealed ways to employ at least one probiotic microorganism (from Lactobacillus and/or Bifidobacterium) as an active agent to prevent or treat skin irritation.8,13 In addition, they noted that L. brevis was used successfully by DeSimone in 2003 to promote apoptosis and/or diminish inflammation, particularly in creams and ointments to alleviate inflammation.8
At around the same time, Miyazaki et al. reported that Bifidobacterium-fermented soy milk extract stimulated the production of hyaluronic acid (HA) in organotypic cultures of human keratinocytes, cultures of human skin fibroblasts, and hairless mouse skin after 2 weeks of topical application and has the potential to promote HA synthesis in the epidermis and dermis and thus act as an anti-aging agent.14 In another study, Miyazaki et al. investigated the impact of Bifidobacterium-fermented soy milk extract containing genistein and daidzein on the HA content of hairless mouse as well as human skin. After 6 weeks of topical application in mice, skin elasticity, viscoelasticity, hydration, and thickness improved, and HA content increased. In addition, after 3 months of topical application of a 10% Bifidobacterium-fermented soy milk extract gel to the human forearm, decreases in skin elasticity were significantly mitigated.15More recently, in 2023, Xie et al. reviewed clinical and experimental data on the use of various species of Lactobacillus for the treatment and prevention of atopic dermatitis (AD). They found evidence that multiple species (L. rhamnosus in animal and clinical experiments) appeared to be effective in preventing and treating AD, with L. acidophilus lessening symptoms and reported to be safe, L. plantarum improving symptoms through immunomodulatory activity, and L. sakei demonstrating anti-inflammatory and skin barrier protective activity. The authors also noted that L. paracasei exhibited anti-inflammatory effects on AD-like skin lesions, and L. reuteri supplementation prevented AD development. Overall, they called for more in vivo studies and randomized controlled clinical trials to fully elucidate the wide-ranging potential of Lactobacillus species in treating and preventing AD.16
The Darker Side of Using Prebiotic Species in Skin Care?
According to manufacturer Delavie Sciences, its Aeonia product line was based on research conducted on the International Space Station, which allowed for its patented microorganism to be exposed to the conditions of outer space. This cornerstone ingredient, Bacillus lysate, once returned to Earth, reportedly exhibited anti-aging and UV-protective characteristics. The product line has been described as a prebiotic that contributes to a healthy skin barrier.17
In a September 2023 interview in CosmeticsDesign, the president of Delavie Sciences clarified that its Bacillus lysate contains no live bacteria and that it is not a probiotic, but rather, the certified prebiotic lysate is a Bacillus extract that has been used to strengthen the SPF potency of skin care formulations.18 Because of the research performed on the International Space Station, the manufacturers are claiming these ingredients could be “out-of-this-world” as a way to promote results that have, as yet, not been verified by peer review.
Conclusion
Probiotics and prebiotics continue to be the focus of multiple lines of research for their applications and further potential in skin care. In the case of the Bacillus lysate prebiotic compound, there is a kernel of an interesting idea here, at the very least. But proprietary research limits our ability to render a comprehensive evaluation at this time. Such bold and outrageous claims spur more skepticism than optimism. However, lysates are the latest thing in skin care — so we need to keep watch on the developments to stay current. But that’s what you have me for, I’ll help keep you current on new ingredient findings. If you are on LinkedIn, come connect with me. I post breaking ingredient news and skin care trends there to help you answer patient questions. When you are asked if these lysates work, the answer is: All the data we have on bacillus extract are from computer analysis of the ingredient properties and not on the actual formulations or products. Stay tuned.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. Mahmud MR et al. Gut Microbes. 2022 Jan-Dec;14(1):2096995. doi: 10.1080/19490976.2022.2096995.
2. Sinha S et al. Clin Dermatol. 2021 Sep-Oct;39(5):829-839. doi: 10.1016/j.clindermatol.2021.08.021.
3. Gao T et al. Nutrients. 2023 Jul 13;15(14):3123. doi: 10.3390/nu15143123.
4. Miyazaki K et al. Benef Microbes. 2014 Jun 1;5(2):121-128. doi: 10.3920/BM2012.0066.
5. Shen Q et al. Anaerobe. 2010 Aug;16(4):380-386. doi: 10.1016/j.anaerobe.2010.06.006.
6. Peran L et al. Int J Colorectal Dis. 2006 Dec;21(8):737-746. doi: 10.1007/s00384-005-0773-y.
7. Kodali VP, Sen R. Biotechnol J. 2008 Feb;3(2):245-251. doi: 10.1002/biot.200700208.
8. Roudsari MR et al. Health effects of probiotics on the skin. Crit Rev Food Sci Nutr. 2015;55(9):1219-40. doi: 10.1080/10408398.2012.680078.
9. Ashoori Y et al. Biomed Res Int. 2020 Dec 28;2020:8868618. doi: 10.1155/2020/8868618.
10. Simmering R, Breves R. Hautarzt. 2009 Oct;60(10):809-814. doi: 10.1007/s00105-009-1759-4.
11. Bockmuhl D. IFSSC Mag. 2006 Sep 30;9[3]:1-5.
12. Lew LC, Liong MT. J Appl Microbiol. 2013 May;114(5):1241-1253. doi: 10.1111/jam.12137.
13. Gueniche A. US Patent, US 20100226892. 2010.
14. Miyazaki K et al. Skin Pharmacol Appl Skin Physiol. 2003 Mar-Apr;16(2):108-116. doi: 10.1159/000069031.
15. Miyazaki et al. J Cosmet Sci. 2004 Sep-Oct;55(5):473-479.16. Xie A et al. Front Cell Infect Microbiol. 2023 Feb 16;13:1137275. doi: 10.3389/fcimb.2023.1137275.
17. Delavie Sciences. Skincare Science: Aeonia. Skincare from the Stars.
. Accessed December 12, 2024.
18. Stern C. CosmeticsDesign USA. September 7, 2023.
Sea Buckthorn
A member of the Elaeagnaceae family, Hippophae rhamnoides, better known as sea buckthorn, is a high-altitude wild shrub endemic to Europe and Asia with edible fruits and a lengthy record of use in traditional Chinese medicine.1-6 Used as a health supplement and consumed in the diet throughout the world,5 sea buckthorn berries, seeds, and leaves have been used in traditional medicine to treat burns/injuries, edema, hypertension, inflammation, skin grafts, ulcers, and wounds.4,7
This hardy plant is associated with a wide range of biologic activities, including anti-atherogenic, anti-atopic dermatitis, antibacterial, anticancer, antifungal, anti-inflammatory, antimicrobial, antioxidant, anti-psoriasis, anti-sebum, anti-stress, anti-tumor, cytoprotective, hepatoprotective, immunomodulatory, neuroprotective, radioprotective, and tissue regenerative functions.4,5,8-11
Key Constituents
Functional constituents identified in sea buckthorn include alkaloids, carotenoids, flavonoids, lignans, organic acids, phenolic acids, proanthocyanidins, polyunsaturated acids (including omega-3, -6, -7, and -9), steroids, tannins, terpenoids, and volatile oils, as well as nutritional compounds such as minerals, proteins, and vitamins.4,5,11 Sea buckthorn pericarp oil contains copious amounts of saturated palmitic acid (29%-36%) and omega-7 unsaturated palmitoleic acid (36%-48%), which fosters cutaneous and mucosal epithelialization, as well as linoleic (10%-12%) and oleic (4%-6%) acids.12,6 Significant amounts of carotenoids as well as alpha‐linolenic fatty acid (38%), linoleic (36%), oleic (13%), and palmitic (7%) acids are present in sea buckthorn seed oil.6
Polysaccharides
In an expansive review on the pharmacological activities of sea buckthorn polysaccharides, Teng and colleagues reported in April 2024 that 20 diverse polysaccharides have been culled from sea buckthorn and exhibited various healthy activities, including antioxidant, anti-fatigue, anti-inflammatory, anti-obesity, anti-tumor, hepatoprotective, hypoglycemic, and immunoregulation, and regulation of intestinal flora activities.1
Proanthocyanidins and Anti-Aging
In 2023, Liu and colleagues investigated the anti–skin aging impact of sea buckthorn proanthocyanidins in D-galactose-induced aging in mice given the known free radical scavenging activity of these compounds. They found the proanthocyanidins mitigated D-galactose-induced aging and can augment the total antioxidant capacity of the body. Sea buckthorn proanthocyanidins can further attenuate the effects of skin aging by regulating the TGF-beta1/Smads pathway and MMPs/TIMP system, thus amplifying collagen I and tropoelastin content.13
A year earlier, many of the same investigators assessed the possible protective activity of sea buckthorn proanthocyanidins against cutaneous aging engendered by oxidative stress from hydrogen peroxide. The compounds amplified superoxide dismutase and glutathione antioxidant functions. The extracts also fostered collagen I production in aging human skin fibroblasts via the TGF-beta1/Smads pathway and hindered collagen I degradation by regulating the MMPs/TIMPs system, which maintained extracellular matrix integrity. Senescent cell migration was also promoted with 100 mcg/mL of sea buckthorn proanthocyanidins. The researchers concluded that this sets the stage for investigating how sea buckthorn proanthocyanidins can be incorporated in cosmetic formulations.14 In a separate study, Liu and colleagues demonstrated that sea buckthorn proanthocyanidins can attenuate oxidative damage and protect mitochondrial function.9
Acne and Barrier Functions
The extracts of H rhamnoides and Cassia fistula in a combined formulation were found to be effective in lowering skin sebum content in humans with grade I and grade II acne vulgaris in a 2014 single-blind, randomized, placebo-controlled, split-face study with two groups of 25 patients each (aged 18-37 years).15 Khan and colleagues have also reported that a sea buckthorn oil-in-water emulsion improved barrier function in human skin as tested by a tewameter and corneometer (noninvasive probes) in 13 healthy males with a mean age of 27 ± 4.8 years.16
Anti-Aging, Antioxidant, Antibacterial, Skin-Whitening Activity
Zaman and colleagues reported in 2011 that results from an in vivo study of the effects of a sea buckthorn fruit extract topical cream on stratum corneum water content and transepidermal water loss indicated that the formulation enhanced cell surface integrin expression thus facilitating collagen contraction.17
In 2012, Khan and colleagues reported amelioration in skin elasticity, thus achieving an anti-aging result, from the use of a water-in-oil–based hydroalcoholic cream loaded with fruit extract of H rhamnoides, as measured with a Cutometer.18 The previous year, some of the same researchers reported that the antioxidants and flavonoids found in a topical sea buckthorn formulation could decrease cutaneous melanin and erythema levels.
More recently, Gęgotek and colleagues found that sea buckthorn seed oil prevented redox balance and lipid metabolism disturbances in skin fibroblasts and keratinocytes caused by UVA or UVB. They suggested that such findings point to the potential of this natural agent to confer anti-inflammatory properties and photoprotection to the skin.19
In 2020, Ivanišová and colleagues investigated the antioxidant and antimicrobial activities of H rhamnoides 100% oil, 100% juice, dry berries, and tea (dry berries, leaves, and twigs). They found that all of the studied sea buckthorn products displayed high antioxidant activity (identified through DPPH radical scavenging and molybdenum reducing antioxidant power tests). Sea buckthorn juice contained the highest total content of polyphenols, flavonoids, and carotenoids. All of the tested products also exhibited substantial antibacterial activity against the tested microbes.20
Burns and Wound Healing
In a preclinical study of the effects of sea buckthorn leaf extracts on wound healing in albino rats using an excision-punch wound model in 2005, Gupta and colleagues found that twice daily topical application of the aqueous leaf extract fostered wound healing. This was indicated by higher hydroxyproline and protein levels, a diminished wound area, and lower lipid peroxide levels. The investigators suggested that sea buckthorn may facilitate wound healing at least in part because of elevated antioxidant activity in the granulation tissue.3
A year later, Wang and colleagues reported on observations of using H rhamnoides oil, a traditional Chinese herbal medicine derived from sea buckthorn fruit, as a burn treatment. In the study, 151 burn patients received an H rhamnoides oil dressing (changed every other day until wound healing) that was covered with a disinfecting dressing. The dressing reduced swelling and effusion, and alleviated pain, with patients receiving the sea buckthorn dressing experiencing greater apparent exudation reduction, pain reduction, and more rapid epithelial cell growth and wound healing than controls (treated only with Vaseline gauze). The difference between the two groups was statistically significant.21
Conclusion
Sea buckthorn has been used for hundreds if not thousands of years in traditional medical applications, including for dermatologic purposes. Emerging data appear to support the use of this dynamic plant for consideration in dermatologic applications. As is often the case, much more work is necessary in the form of randomized controlled trials to determine the effectiveness of sea buckthorn formulations as well as the most appropriate avenues of research or uses for dermatologic application of this traditionally used botanical agent.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at [email protected].
References
1. Teng H et al. J Ethnopharmacol. 2024 Apr 24;324:117809. doi: 10.1016/j.jep.2024.117809.
2. Wang Z et al. Int J Biol Macromol. 2024 Apr;263(Pt 1):130206. doi: 10.1016/j.ijbiomac.2024.130206.
3. Gupta A et al. Int J Low Extrem Wounds. 2005 Jun;4(2):88-92. doi: 10.1177/1534734605277401.
4. Pundir S et al. J Ethnopharmacol. 2021 Feb 10;266:113434. doi: 10.1016/j.jep.2020.113434.
5. Ma QG et al. J Agric Food Chem. 2023 Mar 29;71(12):4769-4788. doi: 10.1021/acs.jafc.2c06916.
6. Poljšak N et al. Phytother Res. 2020 Feb;34(2):254-269. doi: 10.1002/ptr.6524. doi: 10.1002/ptr.6524.
7. Upadhyay NK et al. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189.
8. Suryakumar G, Gupta A. J Ethnopharmacol. 2011 Nov 18;138(2):268-78. doi: 10.1016/j.jep.2011.09.024.
9. Liu K et al. Front Pharmacol. 2022 Jul 8;13:914146. doi: 10.3389/fphar.2022.914146.
10. Akhtar N et al. J Pharm Bioallied Sci. 2010 Jan;2(1):13-7. doi: 10.4103/0975-7406.62698.
11. Ren R et al. RSC Adv. 2020 Dec 17;10(73):44654-44671. doi: 10.1039/d0ra06488b.
12. Ito H et al. Burns. 2014 May;40(3):511-9. doi: 10.1016/j.burns.2013.08.011.
13. Liu X et al. Food Sci Nutr. 2023 Dec 7;12(2):1082-1094. doi: 10.1002/fsn3.3823.
14. Liu X at al. Antioxidants (Basel). 2022 Sep 25;11(10):1900. doi: 10.3390/antiox11101900.
15. Khan BA, Akhtar N. Postepy Dermatol Alergol. 2014 Aug;31(4):229-234. doi: 10.5114/pdia.2014.40934.
16. Khan BA, Akhtar N. Pak J Pharm Sci. 2014 Nov;27(6):1919-22.
17. Khan AB et al. African J Pharm Pharmacol. 2011 Aug;5(8):1092-5.
18. Khan BA, Akhtar N, Braga VA. Trop J Pharm Res. 2012;11(6):955-62.
19. Gęgotek A et al. Antioxidants (Basel). 2018 Aug 23;7(9):110. doi: 10.3390/antiox7090110.
20. Ivanišová E et al. Acta Sci Pol Technol Aliment. 2020 Apr-Jun;19(2):195-205. doi: 10.17306/J.AFS.0809.
21. Wang ZY, Luo XL, He CP. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):124-5.
A member of the Elaeagnaceae family, Hippophae rhamnoides, better known as sea buckthorn, is a high-altitude wild shrub endemic to Europe and Asia with edible fruits and a lengthy record of use in traditional Chinese medicine.1-6 Used as a health supplement and consumed in the diet throughout the world,5 sea buckthorn berries, seeds, and leaves have been used in traditional medicine to treat burns/injuries, edema, hypertension, inflammation, skin grafts, ulcers, and wounds.4,7
This hardy plant is associated with a wide range of biologic activities, including anti-atherogenic, anti-atopic dermatitis, antibacterial, anticancer, antifungal, anti-inflammatory, antimicrobial, antioxidant, anti-psoriasis, anti-sebum, anti-stress, anti-tumor, cytoprotective, hepatoprotective, immunomodulatory, neuroprotective, radioprotective, and tissue regenerative functions.4,5,8-11
Key Constituents
Functional constituents identified in sea buckthorn include alkaloids, carotenoids, flavonoids, lignans, organic acids, phenolic acids, proanthocyanidins, polyunsaturated acids (including omega-3, -6, -7, and -9), steroids, tannins, terpenoids, and volatile oils, as well as nutritional compounds such as minerals, proteins, and vitamins.4,5,11 Sea buckthorn pericarp oil contains copious amounts of saturated palmitic acid (29%-36%) and omega-7 unsaturated palmitoleic acid (36%-48%), which fosters cutaneous and mucosal epithelialization, as well as linoleic (10%-12%) and oleic (4%-6%) acids.12,6 Significant amounts of carotenoids as well as alpha‐linolenic fatty acid (38%), linoleic (36%), oleic (13%), and palmitic (7%) acids are present in sea buckthorn seed oil.6
Polysaccharides
In an expansive review on the pharmacological activities of sea buckthorn polysaccharides, Teng and colleagues reported in April 2024 that 20 diverse polysaccharides have been culled from sea buckthorn and exhibited various healthy activities, including antioxidant, anti-fatigue, anti-inflammatory, anti-obesity, anti-tumor, hepatoprotective, hypoglycemic, and immunoregulation, and regulation of intestinal flora activities.1
Proanthocyanidins and Anti-Aging
In 2023, Liu and colleagues investigated the anti–skin aging impact of sea buckthorn proanthocyanidins in D-galactose-induced aging in mice given the known free radical scavenging activity of these compounds. They found the proanthocyanidins mitigated D-galactose-induced aging and can augment the total antioxidant capacity of the body. Sea buckthorn proanthocyanidins can further attenuate the effects of skin aging by regulating the TGF-beta1/Smads pathway and MMPs/TIMP system, thus amplifying collagen I and tropoelastin content.13
A year earlier, many of the same investigators assessed the possible protective activity of sea buckthorn proanthocyanidins against cutaneous aging engendered by oxidative stress from hydrogen peroxide. The compounds amplified superoxide dismutase and glutathione antioxidant functions. The extracts also fostered collagen I production in aging human skin fibroblasts via the TGF-beta1/Smads pathway and hindered collagen I degradation by regulating the MMPs/TIMPs system, which maintained extracellular matrix integrity. Senescent cell migration was also promoted with 100 mcg/mL of sea buckthorn proanthocyanidins. The researchers concluded that this sets the stage for investigating how sea buckthorn proanthocyanidins can be incorporated in cosmetic formulations.14 In a separate study, Liu and colleagues demonstrated that sea buckthorn proanthocyanidins can attenuate oxidative damage and protect mitochondrial function.9
Acne and Barrier Functions
The extracts of H rhamnoides and Cassia fistula in a combined formulation were found to be effective in lowering skin sebum content in humans with grade I and grade II acne vulgaris in a 2014 single-blind, randomized, placebo-controlled, split-face study with two groups of 25 patients each (aged 18-37 years).15 Khan and colleagues have also reported that a sea buckthorn oil-in-water emulsion improved barrier function in human skin as tested by a tewameter and corneometer (noninvasive probes) in 13 healthy males with a mean age of 27 ± 4.8 years.16
Anti-Aging, Antioxidant, Antibacterial, Skin-Whitening Activity
Zaman and colleagues reported in 2011 that results from an in vivo study of the effects of a sea buckthorn fruit extract topical cream on stratum corneum water content and transepidermal water loss indicated that the formulation enhanced cell surface integrin expression thus facilitating collagen contraction.17
In 2012, Khan and colleagues reported amelioration in skin elasticity, thus achieving an anti-aging result, from the use of a water-in-oil–based hydroalcoholic cream loaded with fruit extract of H rhamnoides, as measured with a Cutometer.18 The previous year, some of the same researchers reported that the antioxidants and flavonoids found in a topical sea buckthorn formulation could decrease cutaneous melanin and erythema levels.
More recently, Gęgotek and colleagues found that sea buckthorn seed oil prevented redox balance and lipid metabolism disturbances in skin fibroblasts and keratinocytes caused by UVA or UVB. They suggested that such findings point to the potential of this natural agent to confer anti-inflammatory properties and photoprotection to the skin.19
In 2020, Ivanišová and colleagues investigated the antioxidant and antimicrobial activities of H rhamnoides 100% oil, 100% juice, dry berries, and tea (dry berries, leaves, and twigs). They found that all of the studied sea buckthorn products displayed high antioxidant activity (identified through DPPH radical scavenging and molybdenum reducing antioxidant power tests). Sea buckthorn juice contained the highest total content of polyphenols, flavonoids, and carotenoids. All of the tested products also exhibited substantial antibacterial activity against the tested microbes.20
Burns and Wound Healing
In a preclinical study of the effects of sea buckthorn leaf extracts on wound healing in albino rats using an excision-punch wound model in 2005, Gupta and colleagues found that twice daily topical application of the aqueous leaf extract fostered wound healing. This was indicated by higher hydroxyproline and protein levels, a diminished wound area, and lower lipid peroxide levels. The investigators suggested that sea buckthorn may facilitate wound healing at least in part because of elevated antioxidant activity in the granulation tissue.3
A year later, Wang and colleagues reported on observations of using H rhamnoides oil, a traditional Chinese herbal medicine derived from sea buckthorn fruit, as a burn treatment. In the study, 151 burn patients received an H rhamnoides oil dressing (changed every other day until wound healing) that was covered with a disinfecting dressing. The dressing reduced swelling and effusion, and alleviated pain, with patients receiving the sea buckthorn dressing experiencing greater apparent exudation reduction, pain reduction, and more rapid epithelial cell growth and wound healing than controls (treated only with Vaseline gauze). The difference between the two groups was statistically significant.21
Conclusion
Sea buckthorn has been used for hundreds if not thousands of years in traditional medical applications, including for dermatologic purposes. Emerging data appear to support the use of this dynamic plant for consideration in dermatologic applications. As is often the case, much more work is necessary in the form of randomized controlled trials to determine the effectiveness of sea buckthorn formulations as well as the most appropriate avenues of research or uses for dermatologic application of this traditionally used botanical agent.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at [email protected].
References
1. Teng H et al. J Ethnopharmacol. 2024 Apr 24;324:117809. doi: 10.1016/j.jep.2024.117809.
2. Wang Z et al. Int J Biol Macromol. 2024 Apr;263(Pt 1):130206. doi: 10.1016/j.ijbiomac.2024.130206.
3. Gupta A et al. Int J Low Extrem Wounds. 2005 Jun;4(2):88-92. doi: 10.1177/1534734605277401.
4. Pundir S et al. J Ethnopharmacol. 2021 Feb 10;266:113434. doi: 10.1016/j.jep.2020.113434.
5. Ma QG et al. J Agric Food Chem. 2023 Mar 29;71(12):4769-4788. doi: 10.1021/acs.jafc.2c06916.
6. Poljšak N et al. Phytother Res. 2020 Feb;34(2):254-269. doi: 10.1002/ptr.6524. doi: 10.1002/ptr.6524.
7. Upadhyay NK et al. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189.
8. Suryakumar G, Gupta A. J Ethnopharmacol. 2011 Nov 18;138(2):268-78. doi: 10.1016/j.jep.2011.09.024.
9. Liu K et al. Front Pharmacol. 2022 Jul 8;13:914146. doi: 10.3389/fphar.2022.914146.
10. Akhtar N et al. J Pharm Bioallied Sci. 2010 Jan;2(1):13-7. doi: 10.4103/0975-7406.62698.
11. Ren R et al. RSC Adv. 2020 Dec 17;10(73):44654-44671. doi: 10.1039/d0ra06488b.
12. Ito H et al. Burns. 2014 May;40(3):511-9. doi: 10.1016/j.burns.2013.08.011.
13. Liu X et al. Food Sci Nutr. 2023 Dec 7;12(2):1082-1094. doi: 10.1002/fsn3.3823.
14. Liu X at al. Antioxidants (Basel). 2022 Sep 25;11(10):1900. doi: 10.3390/antiox11101900.
15. Khan BA, Akhtar N. Postepy Dermatol Alergol. 2014 Aug;31(4):229-234. doi: 10.5114/pdia.2014.40934.
16. Khan BA, Akhtar N. Pak J Pharm Sci. 2014 Nov;27(6):1919-22.
17. Khan AB et al. African J Pharm Pharmacol. 2011 Aug;5(8):1092-5.
18. Khan BA, Akhtar N, Braga VA. Trop J Pharm Res. 2012;11(6):955-62.
19. Gęgotek A et al. Antioxidants (Basel). 2018 Aug 23;7(9):110. doi: 10.3390/antiox7090110.
20. Ivanišová E et al. Acta Sci Pol Technol Aliment. 2020 Apr-Jun;19(2):195-205. doi: 10.17306/J.AFS.0809.
21. Wang ZY, Luo XL, He CP. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):124-5.
A member of the Elaeagnaceae family, Hippophae rhamnoides, better known as sea buckthorn, is a high-altitude wild shrub endemic to Europe and Asia with edible fruits and a lengthy record of use in traditional Chinese medicine.1-6 Used as a health supplement and consumed in the diet throughout the world,5 sea buckthorn berries, seeds, and leaves have been used in traditional medicine to treat burns/injuries, edema, hypertension, inflammation, skin grafts, ulcers, and wounds.4,7
This hardy plant is associated with a wide range of biologic activities, including anti-atherogenic, anti-atopic dermatitis, antibacterial, anticancer, antifungal, anti-inflammatory, antimicrobial, antioxidant, anti-psoriasis, anti-sebum, anti-stress, anti-tumor, cytoprotective, hepatoprotective, immunomodulatory, neuroprotective, radioprotective, and tissue regenerative functions.4,5,8-11
Key Constituents
Functional constituents identified in sea buckthorn include alkaloids, carotenoids, flavonoids, lignans, organic acids, phenolic acids, proanthocyanidins, polyunsaturated acids (including omega-3, -6, -7, and -9), steroids, tannins, terpenoids, and volatile oils, as well as nutritional compounds such as minerals, proteins, and vitamins.4,5,11 Sea buckthorn pericarp oil contains copious amounts of saturated palmitic acid (29%-36%) and omega-7 unsaturated palmitoleic acid (36%-48%), which fosters cutaneous and mucosal epithelialization, as well as linoleic (10%-12%) and oleic (4%-6%) acids.12,6 Significant amounts of carotenoids as well as alpha‐linolenic fatty acid (38%), linoleic (36%), oleic (13%), and palmitic (7%) acids are present in sea buckthorn seed oil.6
Polysaccharides
In an expansive review on the pharmacological activities of sea buckthorn polysaccharides, Teng and colleagues reported in April 2024 that 20 diverse polysaccharides have been culled from sea buckthorn and exhibited various healthy activities, including antioxidant, anti-fatigue, anti-inflammatory, anti-obesity, anti-tumor, hepatoprotective, hypoglycemic, and immunoregulation, and regulation of intestinal flora activities.1
Proanthocyanidins and Anti-Aging
In 2023, Liu and colleagues investigated the anti–skin aging impact of sea buckthorn proanthocyanidins in D-galactose-induced aging in mice given the known free radical scavenging activity of these compounds. They found the proanthocyanidins mitigated D-galactose-induced aging and can augment the total antioxidant capacity of the body. Sea buckthorn proanthocyanidins can further attenuate the effects of skin aging by regulating the TGF-beta1/Smads pathway and MMPs/TIMP system, thus amplifying collagen I and tropoelastin content.13
A year earlier, many of the same investigators assessed the possible protective activity of sea buckthorn proanthocyanidins against cutaneous aging engendered by oxidative stress from hydrogen peroxide. The compounds amplified superoxide dismutase and glutathione antioxidant functions. The extracts also fostered collagen I production in aging human skin fibroblasts via the TGF-beta1/Smads pathway and hindered collagen I degradation by regulating the MMPs/TIMPs system, which maintained extracellular matrix integrity. Senescent cell migration was also promoted with 100 mcg/mL of sea buckthorn proanthocyanidins. The researchers concluded that this sets the stage for investigating how sea buckthorn proanthocyanidins can be incorporated in cosmetic formulations.14 In a separate study, Liu and colleagues demonstrated that sea buckthorn proanthocyanidins can attenuate oxidative damage and protect mitochondrial function.9
Acne and Barrier Functions
The extracts of H rhamnoides and Cassia fistula in a combined formulation were found to be effective in lowering skin sebum content in humans with grade I and grade II acne vulgaris in a 2014 single-blind, randomized, placebo-controlled, split-face study with two groups of 25 patients each (aged 18-37 years).15 Khan and colleagues have also reported that a sea buckthorn oil-in-water emulsion improved barrier function in human skin as tested by a tewameter and corneometer (noninvasive probes) in 13 healthy males with a mean age of 27 ± 4.8 years.16
Anti-Aging, Antioxidant, Antibacterial, Skin-Whitening Activity
Zaman and colleagues reported in 2011 that results from an in vivo study of the effects of a sea buckthorn fruit extract topical cream on stratum corneum water content and transepidermal water loss indicated that the formulation enhanced cell surface integrin expression thus facilitating collagen contraction.17
In 2012, Khan and colleagues reported amelioration in skin elasticity, thus achieving an anti-aging result, from the use of a water-in-oil–based hydroalcoholic cream loaded with fruit extract of H rhamnoides, as measured with a Cutometer.18 The previous year, some of the same researchers reported that the antioxidants and flavonoids found in a topical sea buckthorn formulation could decrease cutaneous melanin and erythema levels.
More recently, Gęgotek and colleagues found that sea buckthorn seed oil prevented redox balance and lipid metabolism disturbances in skin fibroblasts and keratinocytes caused by UVA or UVB. They suggested that such findings point to the potential of this natural agent to confer anti-inflammatory properties and photoprotection to the skin.19
In 2020, Ivanišová and colleagues investigated the antioxidant and antimicrobial activities of H rhamnoides 100% oil, 100% juice, dry berries, and tea (dry berries, leaves, and twigs). They found that all of the studied sea buckthorn products displayed high antioxidant activity (identified through DPPH radical scavenging and molybdenum reducing antioxidant power tests). Sea buckthorn juice contained the highest total content of polyphenols, flavonoids, and carotenoids. All of the tested products also exhibited substantial antibacterial activity against the tested microbes.20
Burns and Wound Healing
In a preclinical study of the effects of sea buckthorn leaf extracts on wound healing in albino rats using an excision-punch wound model in 2005, Gupta and colleagues found that twice daily topical application of the aqueous leaf extract fostered wound healing. This was indicated by higher hydroxyproline and protein levels, a diminished wound area, and lower lipid peroxide levels. The investigators suggested that sea buckthorn may facilitate wound healing at least in part because of elevated antioxidant activity in the granulation tissue.3
A year later, Wang and colleagues reported on observations of using H rhamnoides oil, a traditional Chinese herbal medicine derived from sea buckthorn fruit, as a burn treatment. In the study, 151 burn patients received an H rhamnoides oil dressing (changed every other day until wound healing) that was covered with a disinfecting dressing. The dressing reduced swelling and effusion, and alleviated pain, with patients receiving the sea buckthorn dressing experiencing greater apparent exudation reduction, pain reduction, and more rapid epithelial cell growth and wound healing than controls (treated only with Vaseline gauze). The difference between the two groups was statistically significant.21
Conclusion
Sea buckthorn has been used for hundreds if not thousands of years in traditional medical applications, including for dermatologic purposes. Emerging data appear to support the use of this dynamic plant for consideration in dermatologic applications. As is often the case, much more work is necessary in the form of randomized controlled trials to determine the effectiveness of sea buckthorn formulations as well as the most appropriate avenues of research or uses for dermatologic application of this traditionally used botanical agent.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at [email protected].
References
1. Teng H et al. J Ethnopharmacol. 2024 Apr 24;324:117809. doi: 10.1016/j.jep.2024.117809.
2. Wang Z et al. Int J Biol Macromol. 2024 Apr;263(Pt 1):130206. doi: 10.1016/j.ijbiomac.2024.130206.
3. Gupta A et al. Int J Low Extrem Wounds. 2005 Jun;4(2):88-92. doi: 10.1177/1534734605277401.
4. Pundir S et al. J Ethnopharmacol. 2021 Feb 10;266:113434. doi: 10.1016/j.jep.2020.113434.
5. Ma QG et al. J Agric Food Chem. 2023 Mar 29;71(12):4769-4788. doi: 10.1021/acs.jafc.2c06916.
6. Poljšak N et al. Phytother Res. 2020 Feb;34(2):254-269. doi: 10.1002/ptr.6524. doi: 10.1002/ptr.6524.
7. Upadhyay NK et al. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189.
8. Suryakumar G, Gupta A. J Ethnopharmacol. 2011 Nov 18;138(2):268-78. doi: 10.1016/j.jep.2011.09.024.
9. Liu K et al. Front Pharmacol. 2022 Jul 8;13:914146. doi: 10.3389/fphar.2022.914146.
10. Akhtar N et al. J Pharm Bioallied Sci. 2010 Jan;2(1):13-7. doi: 10.4103/0975-7406.62698.
11. Ren R et al. RSC Adv. 2020 Dec 17;10(73):44654-44671. doi: 10.1039/d0ra06488b.
12. Ito H et al. Burns. 2014 May;40(3):511-9. doi: 10.1016/j.burns.2013.08.011.
13. Liu X et al. Food Sci Nutr. 2023 Dec 7;12(2):1082-1094. doi: 10.1002/fsn3.3823.
14. Liu X at al. Antioxidants (Basel). 2022 Sep 25;11(10):1900. doi: 10.3390/antiox11101900.
15. Khan BA, Akhtar N. Postepy Dermatol Alergol. 2014 Aug;31(4):229-234. doi: 10.5114/pdia.2014.40934.
16. Khan BA, Akhtar N. Pak J Pharm Sci. 2014 Nov;27(6):1919-22.
17. Khan AB et al. African J Pharm Pharmacol. 2011 Aug;5(8):1092-5.
18. Khan BA, Akhtar N, Braga VA. Trop J Pharm Res. 2012;11(6):955-62.
19. Gęgotek A et al. Antioxidants (Basel). 2018 Aug 23;7(9):110. doi: 10.3390/antiox7090110.
20. Ivanišová E et al. Acta Sci Pol Technol Aliment. 2020 Apr-Jun;19(2):195-205. doi: 10.17306/J.AFS.0809.
21. Wang ZY, Luo XL, He CP. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):124-5.
Cosmetic Dermatology Product Recalls Still Common, Analysis Finds
TOPLINE:
Between 2011 and 2023, the US Food and Drug Administration (FDA) reported recalls of 334 cosmetic dermatology products in the United States, affecting over 77 million units, predominantly due to bacterial contamination.
METHODOLOGY:
- Researchers conducted a cross-sectional analysis of the FDA Enforcement Report database for cosmetic dermatology products from 2011 to 2023.
- Cosmetic products are any article “intended for body cleaning or beauty enhancement,” as defined by the FDA.
- Recalls were categorized by product type, reason for the recall, microbial contaminant, inorganic contaminant, distribution, and risk classification.
TAKEAWAY:
- During the study period, 334 voluntary and manufacturer-initiated recalls of cosmetic products were reported, affecting 77,135,700 units.
- A total of 297 recalls (88.9%) were categorized as Class II, indicating that they caused “medically reversible health consequences.” The median recall duration was 307 days.
- Hygiene and cleaning products accounted for most of the recalls (51.5%). Makeup gels, soaps, shampoos, tattoo ink, wipes, and lotions were the most recalled product categories. Nearly 51% of the products were distributed internationally.
- Microbial and inorganic contamination accounted for 76.8% and 10.2% of the recalls (the two most common reasons for the recall), respectively, with bacteria (80%) the most common contaminating pathogen (primarily Pseudomonas and Burkholderia species).
IN PRACTICE:
With 77 million units recalled by the FDA over 12 years, cosmetic recalls have remained common, the authors concluded, adding that “dermatologists should be key voices in pharmacovigilance given scientific expertise and frontline experience managing products and associated concerns.” Dermatologists, they added, “should also be aware of FDA enforcement reports for recall updates given that average recall termination took approximately 1 year.”
SOURCE:
The study was led by Kaushik P. Venkatesh, MBA, MPH, Harvard Medical School, Boston, and was published online on October 29 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations include the potential underreporting of Class III recalls (products that are unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws) and lack of complete information on contaminants.
DISCLOSURES:
No information on funding was provided in the study. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Between 2011 and 2023, the US Food and Drug Administration (FDA) reported recalls of 334 cosmetic dermatology products in the United States, affecting over 77 million units, predominantly due to bacterial contamination.
METHODOLOGY:
- Researchers conducted a cross-sectional analysis of the FDA Enforcement Report database for cosmetic dermatology products from 2011 to 2023.
- Cosmetic products are any article “intended for body cleaning or beauty enhancement,” as defined by the FDA.
- Recalls were categorized by product type, reason for the recall, microbial contaminant, inorganic contaminant, distribution, and risk classification.
TAKEAWAY:
- During the study period, 334 voluntary and manufacturer-initiated recalls of cosmetic products were reported, affecting 77,135,700 units.
- A total of 297 recalls (88.9%) were categorized as Class II, indicating that they caused “medically reversible health consequences.” The median recall duration was 307 days.
- Hygiene and cleaning products accounted for most of the recalls (51.5%). Makeup gels, soaps, shampoos, tattoo ink, wipes, and lotions were the most recalled product categories. Nearly 51% of the products were distributed internationally.
- Microbial and inorganic contamination accounted for 76.8% and 10.2% of the recalls (the two most common reasons for the recall), respectively, with bacteria (80%) the most common contaminating pathogen (primarily Pseudomonas and Burkholderia species).
IN PRACTICE:
With 77 million units recalled by the FDA over 12 years, cosmetic recalls have remained common, the authors concluded, adding that “dermatologists should be key voices in pharmacovigilance given scientific expertise and frontline experience managing products and associated concerns.” Dermatologists, they added, “should also be aware of FDA enforcement reports for recall updates given that average recall termination took approximately 1 year.”
SOURCE:
The study was led by Kaushik P. Venkatesh, MBA, MPH, Harvard Medical School, Boston, and was published online on October 29 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations include the potential underreporting of Class III recalls (products that are unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws) and lack of complete information on contaminants.
DISCLOSURES:
No information on funding was provided in the study. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Between 2011 and 2023, the US Food and Drug Administration (FDA) reported recalls of 334 cosmetic dermatology products in the United States, affecting over 77 million units, predominantly due to bacterial contamination.
METHODOLOGY:
- Researchers conducted a cross-sectional analysis of the FDA Enforcement Report database for cosmetic dermatology products from 2011 to 2023.
- Cosmetic products are any article “intended for body cleaning or beauty enhancement,” as defined by the FDA.
- Recalls were categorized by product type, reason for the recall, microbial contaminant, inorganic contaminant, distribution, and risk classification.
TAKEAWAY:
- During the study period, 334 voluntary and manufacturer-initiated recalls of cosmetic products were reported, affecting 77,135,700 units.
- A total of 297 recalls (88.9%) were categorized as Class II, indicating that they caused “medically reversible health consequences.” The median recall duration was 307 days.
- Hygiene and cleaning products accounted for most of the recalls (51.5%). Makeup gels, soaps, shampoos, tattoo ink, wipes, and lotions were the most recalled product categories. Nearly 51% of the products were distributed internationally.
- Microbial and inorganic contamination accounted for 76.8% and 10.2% of the recalls (the two most common reasons for the recall), respectively, with bacteria (80%) the most common contaminating pathogen (primarily Pseudomonas and Burkholderia species).
IN PRACTICE:
With 77 million units recalled by the FDA over 12 years, cosmetic recalls have remained common, the authors concluded, adding that “dermatologists should be key voices in pharmacovigilance given scientific expertise and frontline experience managing products and associated concerns.” Dermatologists, they added, “should also be aware of FDA enforcement reports for recall updates given that average recall termination took approximately 1 year.”
SOURCE:
The study was led by Kaushik P. Venkatesh, MBA, MPH, Harvard Medical School, Boston, and was published online on October 29 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations include the potential underreporting of Class III recalls (products that are unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws) and lack of complete information on contaminants.
DISCLOSURES:
No information on funding was provided in the study. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
FDA Approves OnabotulinumtoxinA for Improving Platysma Bands
The in adults.
According to a press release from Allergan Aesthetics, which developed onabotulinumtoxinA, by injecting along the jawline and the vertical bands connecting the jaw and neck with one of the FDA-approved doses of the product based on severity, onabotulinumtoxinA temporarily reduces underlying muscle activity.
The company cited results from phase 3 clinical studies, which demonstrated statistical significance for the improvement in appearance of platysma bands from baseline with onabotulinumtoxinA compared with placebo on both investigator and patient assessment (P < .0001).
All secondary endpoints were also met, as measured by multiple validated, proprietary patient-reported outcome instruments. In two of the clinical studies, for example, 65% and 62% of patients reported being “very satisfied” or “satisfied,” respectively, with their neck and jawline definition 14 days after treatment with a dose of 26, 31, or 36 units of onabotulinumtoxinA, compared with 12% with placebo in both studies.
The development marks the fourth indication for onabotulinumtoxinA. The others are for moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity, moderate to severe lateral canthal lines associated with orbicularis oculi activity, and moderate to severe forehead lines associated with frontalis activity.
A version of this article appeared on Medscape.com.
The in adults.
According to a press release from Allergan Aesthetics, which developed onabotulinumtoxinA, by injecting along the jawline and the vertical bands connecting the jaw and neck with one of the FDA-approved doses of the product based on severity, onabotulinumtoxinA temporarily reduces underlying muscle activity.
The company cited results from phase 3 clinical studies, which demonstrated statistical significance for the improvement in appearance of platysma bands from baseline with onabotulinumtoxinA compared with placebo on both investigator and patient assessment (P < .0001).
All secondary endpoints were also met, as measured by multiple validated, proprietary patient-reported outcome instruments. In two of the clinical studies, for example, 65% and 62% of patients reported being “very satisfied” or “satisfied,” respectively, with their neck and jawline definition 14 days after treatment with a dose of 26, 31, or 36 units of onabotulinumtoxinA, compared with 12% with placebo in both studies.
The development marks the fourth indication for onabotulinumtoxinA. The others are for moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity, moderate to severe lateral canthal lines associated with orbicularis oculi activity, and moderate to severe forehead lines associated with frontalis activity.
A version of this article appeared on Medscape.com.
The in adults.
According to a press release from Allergan Aesthetics, which developed onabotulinumtoxinA, by injecting along the jawline and the vertical bands connecting the jaw and neck with one of the FDA-approved doses of the product based on severity, onabotulinumtoxinA temporarily reduces underlying muscle activity.
The company cited results from phase 3 clinical studies, which demonstrated statistical significance for the improvement in appearance of platysma bands from baseline with onabotulinumtoxinA compared with placebo on both investigator and patient assessment (P < .0001).
All secondary endpoints were also met, as measured by multiple validated, proprietary patient-reported outcome instruments. In two of the clinical studies, for example, 65% and 62% of patients reported being “very satisfied” or “satisfied,” respectively, with their neck and jawline definition 14 days after treatment with a dose of 26, 31, or 36 units of onabotulinumtoxinA, compared with 12% with placebo in both studies.
The development marks the fourth indication for onabotulinumtoxinA. The others are for moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity, moderate to severe lateral canthal lines associated with orbicularis oculi activity, and moderate to severe forehead lines associated with frontalis activity.
A version of this article appeared on Medscape.com.
Wrinkles, Dyspigmentation Improve with PDT, in Small Study
.
“Our study helps capture and quantify a phenomenon that clinicians who use PDT in their practice have already noticed: Patients experience a visible improvement across several cosmetically important metrics including but not limited to fine lines, wrinkles, and skin tightness following PDT,” one of the study authors, Luke Horton, MD, a fourth-year dermatology resident at the University of California, Irvine, said in an interview following the annual meeting of the American Society for Dermatologic Surgery, where he presented the results during an oral abstract session.
For the study, 11 patients underwent a 120-minute incubation period with 17% 5-aminolevulinic acid over the face, followed by visible blue light PDT exposure for 16 minutes, to reduce rhytides. The researchers used a Vectra imaging system to capture three-dimensional images of the patients before the procedure and during the follow-up. Three dermatologists analyzed the pre-procedure and post-procedure images and used a validated five-point Merz wrinkle severity scale to grade various regions of the face including the forehead, glabella, lateral canthal rhytides, melolabial folds, nasolabial folds, and perioral rhytides.
They also used a five-point solar lentigines scale to evaluate the change in degree of pigmentation and quantity of age spots as well as the change in rhytid severity before and after PDT and the change in the seven-point Global Aesthetic Improvement Scale (GAIS) to gauge overall improvement of fine lines and wrinkles.
After a mean follow-up of 4.25 months, rhytid severity among the 11 patients was reduced by an average of 0.65 points on the Merz scale, with an SD of 0.20. Broken down by region, rhytid severity scores decreased by 0.2 points (SD, 0.42) for the forehead, 0.7 points (SD, 0.48) for the glabella and lateral canthal rhytides, 0.88 points (SD, 0.35) for the melolabial folds and perioral rhytides, and 0.8 points (SD, 0.42) for the nasolabial folds. (The researchers excluded ratings for the melolabial folds and perioral rhytides in two patients with beards.)
In other findings, solar lentigines grading showed an average reduction of 1 point (SD, 0.45), while the GAIS score improved by 1 or more for every patient, with an average of score of 1.45 (SD, 0.52), showing that some degree of improvement in facial rhytides was noted for all patients following PDT.
“The degree of improvement as measured by our independent physician graders was impressive and not far off from those reported with CO2 ablative laser,” Horton said. “Further, the effect was not isolated to actinic keratoses but extended to improved appearance of fine lines, some deep lines, and lentigines. Although we are not implying that PDT is superior to and should replace lasers or other energy-based devices, it does provide a real, measurable cosmetic benefit.”
Clinicians, he added, can use these findings “to counsel their patients when discussing field cancerization treatment options, especially for patients who may be hesitant to undergo PDT as it can be a painful therapy with a considerable downtime for some.”
Lawrence J. Green, MD, clinical professor of dermatology, The George Washington University, Washington, DC, who was asked to comment on the study results, said that the findings “shine more light on the long-standing off-label use of PDT for lessening signs of photoaging. Like studies done before it, I think this adds an additional benefit to discuss for those who are considering PDT treatment for their actinic keratoses.”
Horton acknowledged certain limitations of the study including its small sample size and the fact that physician graders were not blinded to which images were pre- and post-treatment, “which could introduce an element of bias in the data,” he said. “But this being an unfunded project born out of clinical observation, we hope to later expand its size. Furthermore, we invite other physicians to join us to better study these effects and to design protocols that minimize adverse effects and maximize clinical outcomes.”
His co-authors were Milan Hirpara; Sarah Choe; Joel Cohen, MD; and Natasha A. Mesinkovska, MD, PhD.
No relevant disclosures were reported. Green had no relevant disclosures.
A version of this article appeared on Medscape.com.
.
“Our study helps capture and quantify a phenomenon that clinicians who use PDT in their practice have already noticed: Patients experience a visible improvement across several cosmetically important metrics including but not limited to fine lines, wrinkles, and skin tightness following PDT,” one of the study authors, Luke Horton, MD, a fourth-year dermatology resident at the University of California, Irvine, said in an interview following the annual meeting of the American Society for Dermatologic Surgery, where he presented the results during an oral abstract session.
For the study, 11 patients underwent a 120-minute incubation period with 17% 5-aminolevulinic acid over the face, followed by visible blue light PDT exposure for 16 minutes, to reduce rhytides. The researchers used a Vectra imaging system to capture three-dimensional images of the patients before the procedure and during the follow-up. Three dermatologists analyzed the pre-procedure and post-procedure images and used a validated five-point Merz wrinkle severity scale to grade various regions of the face including the forehead, glabella, lateral canthal rhytides, melolabial folds, nasolabial folds, and perioral rhytides.
They also used a five-point solar lentigines scale to evaluate the change in degree of pigmentation and quantity of age spots as well as the change in rhytid severity before and after PDT and the change in the seven-point Global Aesthetic Improvement Scale (GAIS) to gauge overall improvement of fine lines and wrinkles.
After a mean follow-up of 4.25 months, rhytid severity among the 11 patients was reduced by an average of 0.65 points on the Merz scale, with an SD of 0.20. Broken down by region, rhytid severity scores decreased by 0.2 points (SD, 0.42) for the forehead, 0.7 points (SD, 0.48) for the glabella and lateral canthal rhytides, 0.88 points (SD, 0.35) for the melolabial folds and perioral rhytides, and 0.8 points (SD, 0.42) for the nasolabial folds. (The researchers excluded ratings for the melolabial folds and perioral rhytides in two patients with beards.)
In other findings, solar lentigines grading showed an average reduction of 1 point (SD, 0.45), while the GAIS score improved by 1 or more for every patient, with an average of score of 1.45 (SD, 0.52), showing that some degree of improvement in facial rhytides was noted for all patients following PDT.
“The degree of improvement as measured by our independent physician graders was impressive and not far off from those reported with CO2 ablative laser,” Horton said. “Further, the effect was not isolated to actinic keratoses but extended to improved appearance of fine lines, some deep lines, and lentigines. Although we are not implying that PDT is superior to and should replace lasers or other energy-based devices, it does provide a real, measurable cosmetic benefit.”
Clinicians, he added, can use these findings “to counsel their patients when discussing field cancerization treatment options, especially for patients who may be hesitant to undergo PDT as it can be a painful therapy with a considerable downtime for some.”
Lawrence J. Green, MD, clinical professor of dermatology, The George Washington University, Washington, DC, who was asked to comment on the study results, said that the findings “shine more light on the long-standing off-label use of PDT for lessening signs of photoaging. Like studies done before it, I think this adds an additional benefit to discuss for those who are considering PDT treatment for their actinic keratoses.”
Horton acknowledged certain limitations of the study including its small sample size and the fact that physician graders were not blinded to which images were pre- and post-treatment, “which could introduce an element of bias in the data,” he said. “But this being an unfunded project born out of clinical observation, we hope to later expand its size. Furthermore, we invite other physicians to join us to better study these effects and to design protocols that minimize adverse effects and maximize clinical outcomes.”
His co-authors were Milan Hirpara; Sarah Choe; Joel Cohen, MD; and Natasha A. Mesinkovska, MD, PhD.
No relevant disclosures were reported. Green had no relevant disclosures.
A version of this article appeared on Medscape.com.
.
“Our study helps capture and quantify a phenomenon that clinicians who use PDT in their practice have already noticed: Patients experience a visible improvement across several cosmetically important metrics including but not limited to fine lines, wrinkles, and skin tightness following PDT,” one of the study authors, Luke Horton, MD, a fourth-year dermatology resident at the University of California, Irvine, said in an interview following the annual meeting of the American Society for Dermatologic Surgery, where he presented the results during an oral abstract session.
For the study, 11 patients underwent a 120-minute incubation period with 17% 5-aminolevulinic acid over the face, followed by visible blue light PDT exposure for 16 minutes, to reduce rhytides. The researchers used a Vectra imaging system to capture three-dimensional images of the patients before the procedure and during the follow-up. Three dermatologists analyzed the pre-procedure and post-procedure images and used a validated five-point Merz wrinkle severity scale to grade various regions of the face including the forehead, glabella, lateral canthal rhytides, melolabial folds, nasolabial folds, and perioral rhytides.
They also used a five-point solar lentigines scale to evaluate the change in degree of pigmentation and quantity of age spots as well as the change in rhytid severity before and after PDT and the change in the seven-point Global Aesthetic Improvement Scale (GAIS) to gauge overall improvement of fine lines and wrinkles.
After a mean follow-up of 4.25 months, rhytid severity among the 11 patients was reduced by an average of 0.65 points on the Merz scale, with an SD of 0.20. Broken down by region, rhytid severity scores decreased by 0.2 points (SD, 0.42) for the forehead, 0.7 points (SD, 0.48) for the glabella and lateral canthal rhytides, 0.88 points (SD, 0.35) for the melolabial folds and perioral rhytides, and 0.8 points (SD, 0.42) for the nasolabial folds. (The researchers excluded ratings for the melolabial folds and perioral rhytides in two patients with beards.)
In other findings, solar lentigines grading showed an average reduction of 1 point (SD, 0.45), while the GAIS score improved by 1 or more for every patient, with an average of score of 1.45 (SD, 0.52), showing that some degree of improvement in facial rhytides was noted for all patients following PDT.
“The degree of improvement as measured by our independent physician graders was impressive and not far off from those reported with CO2 ablative laser,” Horton said. “Further, the effect was not isolated to actinic keratoses but extended to improved appearance of fine lines, some deep lines, and lentigines. Although we are not implying that PDT is superior to and should replace lasers or other energy-based devices, it does provide a real, measurable cosmetic benefit.”
Clinicians, he added, can use these findings “to counsel their patients when discussing field cancerization treatment options, especially for patients who may be hesitant to undergo PDT as it can be a painful therapy with a considerable downtime for some.”
Lawrence J. Green, MD, clinical professor of dermatology, The George Washington University, Washington, DC, who was asked to comment on the study results, said that the findings “shine more light on the long-standing off-label use of PDT for lessening signs of photoaging. Like studies done before it, I think this adds an additional benefit to discuss for those who are considering PDT treatment for their actinic keratoses.”
Horton acknowledged certain limitations of the study including its small sample size and the fact that physician graders were not blinded to which images were pre- and post-treatment, “which could introduce an element of bias in the data,” he said. “But this being an unfunded project born out of clinical observation, we hope to later expand its size. Furthermore, we invite other physicians to join us to better study these effects and to design protocols that minimize adverse effects and maximize clinical outcomes.”
His co-authors were Milan Hirpara; Sarah Choe; Joel Cohen, MD; and Natasha A. Mesinkovska, MD, PhD.
No relevant disclosures were reported. Green had no relevant disclosures.
A version of this article appeared on Medscape.com.
New Cosmeceutical as Effective as Cysteamine for Facial Melasma
A presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
“Melasyl is a new potent melanogenesis inhibitor that exhibits a unique mode of action while preserving melanocyte integrity,” Mukta Sachdev, MD, head of the Department of Dermatology at Manipal Hospital in Bangalore, India, said at a late-breaking news session.
Both the serum and the cysteamine cream lightened participants’ skin to a similar extent, according to the modified Melasma Area and Severity Index (mMASI), with respective reductions of 4.19 and 3.81 points over a period of 4 months from baseline values of 11.15 and 10.93.
The mMASI score ranges from 0 to 24, with the lowest score representing the least and the highest score the most severe hyperpigmentation of the skin.
But the serum performed better than the cream by another measure. Judged by investigators blinded to which preparation study participants had been using, there was a significantly higher reduction in the Investigator Global Assessment (IGA) score from baseline among those treated with the serum than among those treated with the cream (−51.85% vs −39.06%; P = .0163).
Moreover, after 4 months of treatment, there were significantly more participants with clear or almost clear skin with the serum than with the cream (17.46% vs 7.81%; P = .0163), Sachdev reported.
Other skin parameters relative to melasma, such as the brightness of skin tone and evenness of the improvement, improved more in the participants using the serum vs cream, she said.
With “no side effects, no local skin reactions,” Sachdev said, “quality of life improved significantly and similarly, and almost all subjects in both groups were very satisfied with their treatment options.”
Active Ingredients
Margarida Gonçalo, MD, PhD, professor of dermatology at the University of Coimbra, in Portugal, who co-chaired the late-breaking news session, commented: “It’s really nice to have new products to treat such a devastating disease.”
Session co-chair, Lidia Rudnicka, MD, head of the Department of Dermatology, Medical University of Warsaw, in Poland, and president of the Polish Dermatological Society, wanted to know more about the active ingredients of the serum and the study’s design.
Sachdev replied that the serum also contains other ingredients that provide “antioxidant protection” and moisturization. These include retinyl palmitate, which works on the dermal-epidermal junction, and hyaluronic acid, as well as “soothing agents,” such as the medicinal herb Centella asiatica, she said.
Study Design
Conducted at a single center in India, the study involved 127 adults aged 20-50 years with melasma. For inclusion, the participants had to have facial epidermal or mixed melasma (phototypes III-V) for more than 1 year; those with dermal melasma were excluded.
Participants were randomly allocated to receive either the serum, which was applied topically to the areas of interest twice a day in the morning and then at bedtime (n = 63), or cysteamine cream (n = 64), which was applied once a day in addition to a neutral moisturizer. Treatment was for 4 months, with an on-site visit every month.
All participants were supplied with the same sunscreen/ultraviolet protector applied twice a day (once in the morning and again at midday) and a neutral hydrating cleanser that was used in the morning and evening.
Practical Implications
Over 4 months, both products showed significant improvement in melasma without reaching a plateau, Sachdev reported, with the serum demonstrating superior efficacy and tolerability, as judged by the investigators.
The study suggests that the serum is a promising non-hydroquinone treatment for melasma, she said. Hydroquinone-containing topical preparations are used to depigment the skin, but their long-term use can be limited for safety reasons.
“When products like this demonstrate improvement, it is something for the dermatologist to think about because we now have newer ingredients, which are safer and well tolerated,” she continued, noting that there appeared to be no risk for exogenous ochronosis, which can occur with long-term application of hydroquinone.
“So, I think the armamentarium of non-hydroquinone products for the treatment of melasma is rapidly expanding, and there are studies now with clinically proven efficacy,” Sachdev concluded.
The study was supported by L’Oréal France La Roche-Posay, which launched Melasyl in March 2024. Sachdev reported receipt of research support and honoraria from the company. Gonçalo and Rudnicka were not involved in the study and had no relevant conflicts of interest to report.
A version of this article appeared on Medscape.com.
A presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
“Melasyl is a new potent melanogenesis inhibitor that exhibits a unique mode of action while preserving melanocyte integrity,” Mukta Sachdev, MD, head of the Department of Dermatology at Manipal Hospital in Bangalore, India, said at a late-breaking news session.
Both the serum and the cysteamine cream lightened participants’ skin to a similar extent, according to the modified Melasma Area and Severity Index (mMASI), with respective reductions of 4.19 and 3.81 points over a period of 4 months from baseline values of 11.15 and 10.93.
The mMASI score ranges from 0 to 24, with the lowest score representing the least and the highest score the most severe hyperpigmentation of the skin.
But the serum performed better than the cream by another measure. Judged by investigators blinded to which preparation study participants had been using, there was a significantly higher reduction in the Investigator Global Assessment (IGA) score from baseline among those treated with the serum than among those treated with the cream (−51.85% vs −39.06%; P = .0163).
Moreover, after 4 months of treatment, there were significantly more participants with clear or almost clear skin with the serum than with the cream (17.46% vs 7.81%; P = .0163), Sachdev reported.
Other skin parameters relative to melasma, such as the brightness of skin tone and evenness of the improvement, improved more in the participants using the serum vs cream, she said.
With “no side effects, no local skin reactions,” Sachdev said, “quality of life improved significantly and similarly, and almost all subjects in both groups were very satisfied with their treatment options.”
Active Ingredients
Margarida Gonçalo, MD, PhD, professor of dermatology at the University of Coimbra, in Portugal, who co-chaired the late-breaking news session, commented: “It’s really nice to have new products to treat such a devastating disease.”
Session co-chair, Lidia Rudnicka, MD, head of the Department of Dermatology, Medical University of Warsaw, in Poland, and president of the Polish Dermatological Society, wanted to know more about the active ingredients of the serum and the study’s design.
Sachdev replied that the serum also contains other ingredients that provide “antioxidant protection” and moisturization. These include retinyl palmitate, which works on the dermal-epidermal junction, and hyaluronic acid, as well as “soothing agents,” such as the medicinal herb Centella asiatica, she said.
Study Design
Conducted at a single center in India, the study involved 127 adults aged 20-50 years with melasma. For inclusion, the participants had to have facial epidermal or mixed melasma (phototypes III-V) for more than 1 year; those with dermal melasma were excluded.
Participants were randomly allocated to receive either the serum, which was applied topically to the areas of interest twice a day in the morning and then at bedtime (n = 63), or cysteamine cream (n = 64), which was applied once a day in addition to a neutral moisturizer. Treatment was for 4 months, with an on-site visit every month.
All participants were supplied with the same sunscreen/ultraviolet protector applied twice a day (once in the morning and again at midday) and a neutral hydrating cleanser that was used in the morning and evening.
Practical Implications
Over 4 months, both products showed significant improvement in melasma without reaching a plateau, Sachdev reported, with the serum demonstrating superior efficacy and tolerability, as judged by the investigators.
The study suggests that the serum is a promising non-hydroquinone treatment for melasma, she said. Hydroquinone-containing topical preparations are used to depigment the skin, but their long-term use can be limited for safety reasons.
“When products like this demonstrate improvement, it is something for the dermatologist to think about because we now have newer ingredients, which are safer and well tolerated,” she continued, noting that there appeared to be no risk for exogenous ochronosis, which can occur with long-term application of hydroquinone.
“So, I think the armamentarium of non-hydroquinone products for the treatment of melasma is rapidly expanding, and there are studies now with clinically proven efficacy,” Sachdev concluded.
The study was supported by L’Oréal France La Roche-Posay, which launched Melasyl in March 2024. Sachdev reported receipt of research support and honoraria from the company. Gonçalo and Rudnicka were not involved in the study and had no relevant conflicts of interest to report.
A version of this article appeared on Medscape.com.
A presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress.
“Melasyl is a new potent melanogenesis inhibitor that exhibits a unique mode of action while preserving melanocyte integrity,” Mukta Sachdev, MD, head of the Department of Dermatology at Manipal Hospital in Bangalore, India, said at a late-breaking news session.
Both the serum and the cysteamine cream lightened participants’ skin to a similar extent, according to the modified Melasma Area and Severity Index (mMASI), with respective reductions of 4.19 and 3.81 points over a period of 4 months from baseline values of 11.15 and 10.93.
The mMASI score ranges from 0 to 24, with the lowest score representing the least and the highest score the most severe hyperpigmentation of the skin.
But the serum performed better than the cream by another measure. Judged by investigators blinded to which preparation study participants had been using, there was a significantly higher reduction in the Investigator Global Assessment (IGA) score from baseline among those treated with the serum than among those treated with the cream (−51.85% vs −39.06%; P = .0163).
Moreover, after 4 months of treatment, there were significantly more participants with clear or almost clear skin with the serum than with the cream (17.46% vs 7.81%; P = .0163), Sachdev reported.
Other skin parameters relative to melasma, such as the brightness of skin tone and evenness of the improvement, improved more in the participants using the serum vs cream, she said.
With “no side effects, no local skin reactions,” Sachdev said, “quality of life improved significantly and similarly, and almost all subjects in both groups were very satisfied with their treatment options.”
Active Ingredients
Margarida Gonçalo, MD, PhD, professor of dermatology at the University of Coimbra, in Portugal, who co-chaired the late-breaking news session, commented: “It’s really nice to have new products to treat such a devastating disease.”
Session co-chair, Lidia Rudnicka, MD, head of the Department of Dermatology, Medical University of Warsaw, in Poland, and president of the Polish Dermatological Society, wanted to know more about the active ingredients of the serum and the study’s design.
Sachdev replied that the serum also contains other ingredients that provide “antioxidant protection” and moisturization. These include retinyl palmitate, which works on the dermal-epidermal junction, and hyaluronic acid, as well as “soothing agents,” such as the medicinal herb Centella asiatica, she said.
Study Design
Conducted at a single center in India, the study involved 127 adults aged 20-50 years with melasma. For inclusion, the participants had to have facial epidermal or mixed melasma (phototypes III-V) for more than 1 year; those with dermal melasma were excluded.
Participants were randomly allocated to receive either the serum, which was applied topically to the areas of interest twice a day in the morning and then at bedtime (n = 63), or cysteamine cream (n = 64), which was applied once a day in addition to a neutral moisturizer. Treatment was for 4 months, with an on-site visit every month.
All participants were supplied with the same sunscreen/ultraviolet protector applied twice a day (once in the morning and again at midday) and a neutral hydrating cleanser that was used in the morning and evening.
Practical Implications
Over 4 months, both products showed significant improvement in melasma without reaching a plateau, Sachdev reported, with the serum demonstrating superior efficacy and tolerability, as judged by the investigators.
The study suggests that the serum is a promising non-hydroquinone treatment for melasma, she said. Hydroquinone-containing topical preparations are used to depigment the skin, but their long-term use can be limited for safety reasons.
“When products like this demonstrate improvement, it is something for the dermatologist to think about because we now have newer ingredients, which are safer and well tolerated,” she continued, noting that there appeared to be no risk for exogenous ochronosis, which can occur with long-term application of hydroquinone.
“So, I think the armamentarium of non-hydroquinone products for the treatment of melasma is rapidly expanding, and there are studies now with clinically proven efficacy,” Sachdev concluded.
The study was supported by L’Oréal France La Roche-Posay, which launched Melasyl in March 2024. Sachdev reported receipt of research support and honoraria from the company. Gonçalo and Rudnicka were not involved in the study and had no relevant conflicts of interest to report.
A version of this article appeared on Medscape.com.
FROM EADV 2024
What We Know About Salmon Sperm in Dermatology
It may not have an aesthetic-sounding appeal to most people, but salmon sperm is indeed one of the novel ingredients featured in products for human skin. These products also reportedly enhance and promote skin regeneration.1 This column will focus on the innovative approach to skin care involving purified polynucleotides derived from salmon sperm.
The Properties and Activities of PDRNs, PNs
PDRNs contain DNA fragments primarily derived from Pacific or chum salmon (Oncorhynchus keta), and salmon trout (Oncorhynchus mykiss) sperm cells.2 Through preclinical and clinical trials, PDRN has demonstrated a wide range of salutary functions, including antiallodynic, antiapoptotic, anti-inflammatory, antimelanogenetic, antiosteonecrotic, antiosteoporotic, antiulcerative, bone-regenerative, tissue damage–preventive, and wound-healing activities through adenosine A2A receptor and salvage pathways activation. Indeed, PDRNs have been shown in vitro to spur the proliferation of preadipocytes and, in vivo, to be effective in treating wounds and ulcers.3,4 In particular, atrophic, hypertrophic, surgical, and various acne scars have been treated with such injections.2,5,6 PDRN is thought to affect cutaneous health more directly by facilitating angiogenesis, cellular functions, especially fibroblast stimulation, collagen production, soft-tissue regeneration, and skin revitalization. Further, it has been used successfully to treat hyperpigmentation.7
PNs, derived from the same fish species as PDRNs, have been used effectively to ameliorate skin elasticity, hydration, pore size, thickness, wrinkles, as well as pigmentation and, specifically, in treating periorbital rhytides and postsurgical scars.5,6,8 Beyond skin rejuvenation, PNs have been recognized for effectiveness in treating stretch marks and achieving vulvovaginal revitalization; guidelines for its use have been established and implemented in recent years.6,9,10 In South Korea, PNs have become a popular treatment for facial erythema even though preclinical and clinical data are sparse.11 Nevertheless, the use of these novel substances is thought to foster tissue regeneration and a more natural rejuvenation than achieved through more traditional fillers.6
Skin Rejuvenation
Park and colleagues conducted a small study with five patients in 2016 in which long-chain polynucleotide filler was used for skin rejuvenation. Over a 2-week period, five Korean women received four injections of the filler (0.05 mL) on one side of the face. No adverse side effects were reported. In the patients in their 30s, pore and skin thickness significantly improved with treatment. For patients in their 40s, observable improvements were noted in melanin, sagging, skin tone, and wrinkles. Despite the small study size, the investigators concluded that this intradermal injection material is a safe and effective product for skin rejuvenation therapy.1 The product is also available in Europe and reportedly spurs the regeneration of damaged tissues and yields a more natural appearance.1
A Hybrid HA-PN Filler
Given that the most common filling agent, hyaluronic acid (HA), is associated with multiple side effects, JH Kim and colleagues set out in 2020 to compare HA with a new HA-PN dermal filler that has displayed notable biocompatibility and promoted tissue regeneration. The investigators observed that the combination filler provoked greater cell migration in a wound healing assay and was more effective in promoting collagen production in human and mouse fibroblasts. To their knowledge, this was the first study showing the efficacy, safety, and durability of a hybrid HA-PN filler. They concluded that fillers containing both HA and PN were more effective than HA alone in suppressing cutaneous aging and may represent the next step in the evolution of dermal filling agents.12
Most Recent Findings
In August 2023, MJ Kim and colleagues became the first to report on the successful use of PNs derived from fish sperm as a volumizing treatment for fat atrophy in vivo (in the temple in one case, and the cheek in the other). Injections were made into the subcutaneous layer to treat iatrogenic volume loss resulting from lipolysis injections. In one case, a depression in the left temple of a 53-year-old female lipolysis patient was treated with a series of 1 cc PN injections in a 20 mg/mL concentration. At 1 month after the final series of injections (four treatments), significant clinical improvement was observed, with the result (barely visible depression) maintained at 11 months and 21 months after the last treatment. The second patient, a 34-year-old female, presented with two depressed areas on the left cheek 2 months after steroid injections for two acne lesions. A series of PN filler injections also with a concentration of 20 mg/mL was administered (four treatments) at 1-month intervals. Significant improvement was seen 2 months after the last treatment, with maintenance of complete healing noted at 5 months and 12 months after the final treatment. No adverse effects were reported in either case. The investigators concluded that long-chain PN fillers appear to be effective in treating depressions in the skin, but more data, particularly from controlled studies, is necessary to determine the safety and efficacy as a lone therapeutic approach for soft-tissue depression.6
A month later, Lee and colleagues reported on the results of their survey of clinicians in South Korea who use PNs in clinical practice. The goal was to understand current practices and perceptions of effectiveness in treating facial erythema. Of the 557 physicians who participated, 84.4% used PNs for facial erythema provoked by inflammatory facial dermatosis, 66.4% for facial erythema induced by repeated laser/microneedle radiofrequency, and 47.4% for facial erythema caused by steroid overuse. In these same classifications, 88.1%, 90%, and 83.7%, respectively, found PNs to be “highly effective” or “effective.” Survey respondents also characterized PNs as imparting wound healing/regeneration (95.8%), skin barrier protection (92.2%), hydration (90.5%), vascular stabilization (81.0%), and anti-inflammatory activity (79.5%).11
Conclusion
The use of salmon sperm cells is an example of the recent trend toward a cellular approach in which cutaneous components are activated with the intention of stimulating tissue regeneration. It is commonly used in Brazil and my Brazilian patients seem to know all about it. This innovative outlook is intriguing as are a spate of recently reported results. Nevertheless, much more evidence is required to ascertain safety and effectiveness in large sample sizes and, ideally, to establish maintenance of corrections over longer periods whether these ingredients are used in filling agents or topical formulations.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at [email protected].
References
1. Park KY et al. Dermatol Ther. 2016 Jan;29(1):37-40. .
2. Kim TH et al. Mar Drugs. 2021 May 22;19(6):296.
3. Raposio E et al. Cell Prolif. 2008 Oct;41(5):739-54.
4. Veronesi F et al. J Cell Physiol. 2017 Sep;232(9):2299-2307.
5. Kim JH et al. Lasers Surg Med. 2018 Mar 25.
6. Kim MJ et al. Skin Res Technol. 2023 Aug;29(8):e13439.
7. Khan A et al. Chinese Journal of Plastic and Reconstructive Surgery. 2022 Dec;4(4):187-193.
8. Lee YJ et al. J Dermatolog Treat. 2022 Feb;33(1):254-260.
9. De Caridi G et al. Int Wound J. 2016 Oct;13(5):754-8.
10. Cavallini M et al. J Cosmet Dermatol. 2021 Mar;20(3):922-928.
11. Lee D. Skin Res Technol. 2023 Sep;29(9):e13466. doi: 10.1111/srt.13466.
12. Kim JH et al. Sci Rep. 2020 Mar 20;10(1):5127. .
It may not have an aesthetic-sounding appeal to most people, but salmon sperm is indeed one of the novel ingredients featured in products for human skin. These products also reportedly enhance and promote skin regeneration.1 This column will focus on the innovative approach to skin care involving purified polynucleotides derived from salmon sperm.
The Properties and Activities of PDRNs, PNs
PDRNs contain DNA fragments primarily derived from Pacific or chum salmon (Oncorhynchus keta), and salmon trout (Oncorhynchus mykiss) sperm cells.2 Through preclinical and clinical trials, PDRN has demonstrated a wide range of salutary functions, including antiallodynic, antiapoptotic, anti-inflammatory, antimelanogenetic, antiosteonecrotic, antiosteoporotic, antiulcerative, bone-regenerative, tissue damage–preventive, and wound-healing activities through adenosine A2A receptor and salvage pathways activation. Indeed, PDRNs have been shown in vitro to spur the proliferation of preadipocytes and, in vivo, to be effective in treating wounds and ulcers.3,4 In particular, atrophic, hypertrophic, surgical, and various acne scars have been treated with such injections.2,5,6 PDRN is thought to affect cutaneous health more directly by facilitating angiogenesis, cellular functions, especially fibroblast stimulation, collagen production, soft-tissue regeneration, and skin revitalization. Further, it has been used successfully to treat hyperpigmentation.7
PNs, derived from the same fish species as PDRNs, have been used effectively to ameliorate skin elasticity, hydration, pore size, thickness, wrinkles, as well as pigmentation and, specifically, in treating periorbital rhytides and postsurgical scars.5,6,8 Beyond skin rejuvenation, PNs have been recognized for effectiveness in treating stretch marks and achieving vulvovaginal revitalization; guidelines for its use have been established and implemented in recent years.6,9,10 In South Korea, PNs have become a popular treatment for facial erythema even though preclinical and clinical data are sparse.11 Nevertheless, the use of these novel substances is thought to foster tissue regeneration and a more natural rejuvenation than achieved through more traditional fillers.6
Skin Rejuvenation
Park and colleagues conducted a small study with five patients in 2016 in which long-chain polynucleotide filler was used for skin rejuvenation. Over a 2-week period, five Korean women received four injections of the filler (0.05 mL) on one side of the face. No adverse side effects were reported. In the patients in their 30s, pore and skin thickness significantly improved with treatment. For patients in their 40s, observable improvements were noted in melanin, sagging, skin tone, and wrinkles. Despite the small study size, the investigators concluded that this intradermal injection material is a safe and effective product for skin rejuvenation therapy.1 The product is also available in Europe and reportedly spurs the regeneration of damaged tissues and yields a more natural appearance.1
A Hybrid HA-PN Filler
Given that the most common filling agent, hyaluronic acid (HA), is associated with multiple side effects, JH Kim and colleagues set out in 2020 to compare HA with a new HA-PN dermal filler that has displayed notable biocompatibility and promoted tissue regeneration. The investigators observed that the combination filler provoked greater cell migration in a wound healing assay and was more effective in promoting collagen production in human and mouse fibroblasts. To their knowledge, this was the first study showing the efficacy, safety, and durability of a hybrid HA-PN filler. They concluded that fillers containing both HA and PN were more effective than HA alone in suppressing cutaneous aging and may represent the next step in the evolution of dermal filling agents.12
Most Recent Findings
In August 2023, MJ Kim and colleagues became the first to report on the successful use of PNs derived from fish sperm as a volumizing treatment for fat atrophy in vivo (in the temple in one case, and the cheek in the other). Injections were made into the subcutaneous layer to treat iatrogenic volume loss resulting from lipolysis injections. In one case, a depression in the left temple of a 53-year-old female lipolysis patient was treated with a series of 1 cc PN injections in a 20 mg/mL concentration. At 1 month after the final series of injections (four treatments), significant clinical improvement was observed, with the result (barely visible depression) maintained at 11 months and 21 months after the last treatment. The second patient, a 34-year-old female, presented with two depressed areas on the left cheek 2 months after steroid injections for two acne lesions. A series of PN filler injections also with a concentration of 20 mg/mL was administered (four treatments) at 1-month intervals. Significant improvement was seen 2 months after the last treatment, with maintenance of complete healing noted at 5 months and 12 months after the final treatment. No adverse effects were reported in either case. The investigators concluded that long-chain PN fillers appear to be effective in treating depressions in the skin, but more data, particularly from controlled studies, is necessary to determine the safety and efficacy as a lone therapeutic approach for soft-tissue depression.6
A month later, Lee and colleagues reported on the results of their survey of clinicians in South Korea who use PNs in clinical practice. The goal was to understand current practices and perceptions of effectiveness in treating facial erythema. Of the 557 physicians who participated, 84.4% used PNs for facial erythema provoked by inflammatory facial dermatosis, 66.4% for facial erythema induced by repeated laser/microneedle radiofrequency, and 47.4% for facial erythema caused by steroid overuse. In these same classifications, 88.1%, 90%, and 83.7%, respectively, found PNs to be “highly effective” or “effective.” Survey respondents also characterized PNs as imparting wound healing/regeneration (95.8%), skin barrier protection (92.2%), hydration (90.5%), vascular stabilization (81.0%), and anti-inflammatory activity (79.5%).11
Conclusion
The use of salmon sperm cells is an example of the recent trend toward a cellular approach in which cutaneous components are activated with the intention of stimulating tissue regeneration. It is commonly used in Brazil and my Brazilian patients seem to know all about it. This innovative outlook is intriguing as are a spate of recently reported results. Nevertheless, much more evidence is required to ascertain safety and effectiveness in large sample sizes and, ideally, to establish maintenance of corrections over longer periods whether these ingredients are used in filling agents or topical formulations.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at [email protected].
References
1. Park KY et al. Dermatol Ther. 2016 Jan;29(1):37-40. .
2. Kim TH et al. Mar Drugs. 2021 May 22;19(6):296.
3. Raposio E et al. Cell Prolif. 2008 Oct;41(5):739-54.
4. Veronesi F et al. J Cell Physiol. 2017 Sep;232(9):2299-2307.
5. Kim JH et al. Lasers Surg Med. 2018 Mar 25.
6. Kim MJ et al. Skin Res Technol. 2023 Aug;29(8):e13439.
7. Khan A et al. Chinese Journal of Plastic and Reconstructive Surgery. 2022 Dec;4(4):187-193.
8. Lee YJ et al. J Dermatolog Treat. 2022 Feb;33(1):254-260.
9. De Caridi G et al. Int Wound J. 2016 Oct;13(5):754-8.
10. Cavallini M et al. J Cosmet Dermatol. 2021 Mar;20(3):922-928.
11. Lee D. Skin Res Technol. 2023 Sep;29(9):e13466. doi: 10.1111/srt.13466.
12. Kim JH et al. Sci Rep. 2020 Mar 20;10(1):5127. .
It may not have an aesthetic-sounding appeal to most people, but salmon sperm is indeed one of the novel ingredients featured in products for human skin. These products also reportedly enhance and promote skin regeneration.1 This column will focus on the innovative approach to skin care involving purified polynucleotides derived from salmon sperm.
The Properties and Activities of PDRNs, PNs
PDRNs contain DNA fragments primarily derived from Pacific or chum salmon (Oncorhynchus keta), and salmon trout (Oncorhynchus mykiss) sperm cells.2 Through preclinical and clinical trials, PDRN has demonstrated a wide range of salutary functions, including antiallodynic, antiapoptotic, anti-inflammatory, antimelanogenetic, antiosteonecrotic, antiosteoporotic, antiulcerative, bone-regenerative, tissue damage–preventive, and wound-healing activities through adenosine A2A receptor and salvage pathways activation. Indeed, PDRNs have been shown in vitro to spur the proliferation of preadipocytes and, in vivo, to be effective in treating wounds and ulcers.3,4 In particular, atrophic, hypertrophic, surgical, and various acne scars have been treated with such injections.2,5,6 PDRN is thought to affect cutaneous health more directly by facilitating angiogenesis, cellular functions, especially fibroblast stimulation, collagen production, soft-tissue regeneration, and skin revitalization. Further, it has been used successfully to treat hyperpigmentation.7
PNs, derived from the same fish species as PDRNs, have been used effectively to ameliorate skin elasticity, hydration, pore size, thickness, wrinkles, as well as pigmentation and, specifically, in treating periorbital rhytides and postsurgical scars.5,6,8 Beyond skin rejuvenation, PNs have been recognized for effectiveness in treating stretch marks and achieving vulvovaginal revitalization; guidelines for its use have been established and implemented in recent years.6,9,10 In South Korea, PNs have become a popular treatment for facial erythema even though preclinical and clinical data are sparse.11 Nevertheless, the use of these novel substances is thought to foster tissue regeneration and a more natural rejuvenation than achieved through more traditional fillers.6
Skin Rejuvenation
Park and colleagues conducted a small study with five patients in 2016 in which long-chain polynucleotide filler was used for skin rejuvenation. Over a 2-week period, five Korean women received four injections of the filler (0.05 mL) on one side of the face. No adverse side effects were reported. In the patients in their 30s, pore and skin thickness significantly improved with treatment. For patients in their 40s, observable improvements were noted in melanin, sagging, skin tone, and wrinkles. Despite the small study size, the investigators concluded that this intradermal injection material is a safe and effective product for skin rejuvenation therapy.1 The product is also available in Europe and reportedly spurs the regeneration of damaged tissues and yields a more natural appearance.1
A Hybrid HA-PN Filler
Given that the most common filling agent, hyaluronic acid (HA), is associated with multiple side effects, JH Kim and colleagues set out in 2020 to compare HA with a new HA-PN dermal filler that has displayed notable biocompatibility and promoted tissue regeneration. The investigators observed that the combination filler provoked greater cell migration in a wound healing assay and was more effective in promoting collagen production in human and mouse fibroblasts. To their knowledge, this was the first study showing the efficacy, safety, and durability of a hybrid HA-PN filler. They concluded that fillers containing both HA and PN were more effective than HA alone in suppressing cutaneous aging and may represent the next step in the evolution of dermal filling agents.12
Most Recent Findings
In August 2023, MJ Kim and colleagues became the first to report on the successful use of PNs derived from fish sperm as a volumizing treatment for fat atrophy in vivo (in the temple in one case, and the cheek in the other). Injections were made into the subcutaneous layer to treat iatrogenic volume loss resulting from lipolysis injections. In one case, a depression in the left temple of a 53-year-old female lipolysis patient was treated with a series of 1 cc PN injections in a 20 mg/mL concentration. At 1 month after the final series of injections (four treatments), significant clinical improvement was observed, with the result (barely visible depression) maintained at 11 months and 21 months after the last treatment. The second patient, a 34-year-old female, presented with two depressed areas on the left cheek 2 months after steroid injections for two acne lesions. A series of PN filler injections also with a concentration of 20 mg/mL was administered (four treatments) at 1-month intervals. Significant improvement was seen 2 months after the last treatment, with maintenance of complete healing noted at 5 months and 12 months after the final treatment. No adverse effects were reported in either case. The investigators concluded that long-chain PN fillers appear to be effective in treating depressions in the skin, but more data, particularly from controlled studies, is necessary to determine the safety and efficacy as a lone therapeutic approach for soft-tissue depression.6
A month later, Lee and colleagues reported on the results of their survey of clinicians in South Korea who use PNs in clinical practice. The goal was to understand current practices and perceptions of effectiveness in treating facial erythema. Of the 557 physicians who participated, 84.4% used PNs for facial erythema provoked by inflammatory facial dermatosis, 66.4% for facial erythema induced by repeated laser/microneedle radiofrequency, and 47.4% for facial erythema caused by steroid overuse. In these same classifications, 88.1%, 90%, and 83.7%, respectively, found PNs to be “highly effective” or “effective.” Survey respondents also characterized PNs as imparting wound healing/regeneration (95.8%), skin barrier protection (92.2%), hydration (90.5%), vascular stabilization (81.0%), and anti-inflammatory activity (79.5%).11
Conclusion
The use of salmon sperm cells is an example of the recent trend toward a cellular approach in which cutaneous components are activated with the intention of stimulating tissue regeneration. It is commonly used in Brazil and my Brazilian patients seem to know all about it. This innovative outlook is intriguing as are a spate of recently reported results. Nevertheless, much more evidence is required to ascertain safety and effectiveness in large sample sizes and, ideally, to establish maintenance of corrections over longer periods whether these ingredients are used in filling agents or topical formulations.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at [email protected].
References
1. Park KY et al. Dermatol Ther. 2016 Jan;29(1):37-40. .
2. Kim TH et al. Mar Drugs. 2021 May 22;19(6):296.
3. Raposio E et al. Cell Prolif. 2008 Oct;41(5):739-54.
4. Veronesi F et al. J Cell Physiol. 2017 Sep;232(9):2299-2307.
5. Kim JH et al. Lasers Surg Med. 2018 Mar 25.
6. Kim MJ et al. Skin Res Technol. 2023 Aug;29(8):e13439.
7. Khan A et al. Chinese Journal of Plastic and Reconstructive Surgery. 2022 Dec;4(4):187-193.
8. Lee YJ et al. J Dermatolog Treat. 2022 Feb;33(1):254-260.
9. De Caridi G et al. Int Wound J. 2016 Oct;13(5):754-8.
10. Cavallini M et al. J Cosmet Dermatol. 2021 Mar;20(3):922-928.
11. Lee D. Skin Res Technol. 2023 Sep;29(9):e13466. doi: 10.1111/srt.13466.
12. Kim JH et al. Sci Rep. 2020 Mar 20;10(1):5127. .
FDA Approves IL-13 inhibitor for Atopic Dermatitis
The that is not well controlled, despite treatment with topical prescription therapies.
The recommended initial starting dose of lebrikizumab consists of 500 mg (two 250 mg injections) at baseline and week 2, followed by 250 mg every 2 weeks until week 16 or later when adequate clinical response is achieved. Then, maintenance dosing is recommended with one monthly injection (250 mg every 4 weeks). Children aged 12-17 years must weigh at least 88 pounds (40 kg) to be eligible for lebrikizumab treatment.
According to a press release from Lilly, which has been developing lebrikizumab, approval was based on results from the ADvocate 1, ADvocate 2, and ADhere studies, which included over 1000 adults and children aged 12 and older with moderate to severe AD. The primary endpoint for these studies was evaluated at 16 weeks and measured clear or almost clear skin (IGA score of 0 or 1).
According to Lilly, 38% of people in ADvocate 1 and 2 who took lebrikizumab achieved clear or almost-clear skin at 16 weeks, compared with 12% of those in the placebo arm, and 10% experienced these results as early as 4 weeks. Of those treated with lebrikizumab who experienced clear or almost-clear skin at week 16, 77% maintained those results at 1 year on the once-monthly dose. In addition, on average, 43% of those on lebrikizumab experienced relief of itch at 16 weeks, compared with 12% of those on placebo, according to the press release.
The most common side effects of lebrikizumab observed in the clinical trials include eye and eyelid inflammation, such as redness, swelling, and itching; injection-site reactions; and herpes zoster (shingles).
Lebrikizumab was approved in Japan in January 2024, and by the European Commission in 2023.
A version of this article first appeared on Medscape.com.
The that is not well controlled, despite treatment with topical prescription therapies.
The recommended initial starting dose of lebrikizumab consists of 500 mg (two 250 mg injections) at baseline and week 2, followed by 250 mg every 2 weeks until week 16 or later when adequate clinical response is achieved. Then, maintenance dosing is recommended with one monthly injection (250 mg every 4 weeks). Children aged 12-17 years must weigh at least 88 pounds (40 kg) to be eligible for lebrikizumab treatment.
According to a press release from Lilly, which has been developing lebrikizumab, approval was based on results from the ADvocate 1, ADvocate 2, and ADhere studies, which included over 1000 adults and children aged 12 and older with moderate to severe AD. The primary endpoint for these studies was evaluated at 16 weeks and measured clear or almost clear skin (IGA score of 0 or 1).
According to Lilly, 38% of people in ADvocate 1 and 2 who took lebrikizumab achieved clear or almost-clear skin at 16 weeks, compared with 12% of those in the placebo arm, and 10% experienced these results as early as 4 weeks. Of those treated with lebrikizumab who experienced clear or almost-clear skin at week 16, 77% maintained those results at 1 year on the once-monthly dose. In addition, on average, 43% of those on lebrikizumab experienced relief of itch at 16 weeks, compared with 12% of those on placebo, according to the press release.
The most common side effects of lebrikizumab observed in the clinical trials include eye and eyelid inflammation, such as redness, swelling, and itching; injection-site reactions; and herpes zoster (shingles).
Lebrikizumab was approved in Japan in January 2024, and by the European Commission in 2023.
A version of this article first appeared on Medscape.com.
The that is not well controlled, despite treatment with topical prescription therapies.
The recommended initial starting dose of lebrikizumab consists of 500 mg (two 250 mg injections) at baseline and week 2, followed by 250 mg every 2 weeks until week 16 or later when adequate clinical response is achieved. Then, maintenance dosing is recommended with one monthly injection (250 mg every 4 weeks). Children aged 12-17 years must weigh at least 88 pounds (40 kg) to be eligible for lebrikizumab treatment.
According to a press release from Lilly, which has been developing lebrikizumab, approval was based on results from the ADvocate 1, ADvocate 2, and ADhere studies, which included over 1000 adults and children aged 12 and older with moderate to severe AD. The primary endpoint for these studies was evaluated at 16 weeks and measured clear or almost clear skin (IGA score of 0 or 1).
According to Lilly, 38% of people in ADvocate 1 and 2 who took lebrikizumab achieved clear or almost-clear skin at 16 weeks, compared with 12% of those in the placebo arm, and 10% experienced these results as early as 4 weeks. Of those treated with lebrikizumab who experienced clear or almost-clear skin at week 16, 77% maintained those results at 1 year on the once-monthly dose. In addition, on average, 43% of those on lebrikizumab experienced relief of itch at 16 weeks, compared with 12% of those on placebo, according to the press release.
The most common side effects of lebrikizumab observed in the clinical trials include eye and eyelid inflammation, such as redness, swelling, and itching; injection-site reactions; and herpes zoster (shingles).
Lebrikizumab was approved in Japan in January 2024, and by the European Commission in 2023.
A version of this article first appeared on Medscape.com.