User login
Deposition of amyloid-β peptide (Aβ) is believed to contribute to Alzheimer’s disease (AD) pathogenesis. Derived from a larger precursor protein, Aβ aggregates into plaques, and may promote neuronal death and, ultimately, dementia.
Current treatments alleviate symptoms without slowing underlying neurodegeneration. The prospect of harnessing the immune system to target the Aβ peptide offers an intriguing option for preventing this devastating, increasingly common disease.
Anti-a BETA antibodies
Transgenic mice bred to overexpress AD genes have responded remarkably in studies using the immune system to target the amyloid-β peptide.3 Several mouse groups have shown plaque reduction (Figure 1) and improved cognitive performance. These findings substantiate the amyloid hypothesis in AD pathogenesis.
A host could acquire anti-Aβ antibodies though two basic approaches (Figure 2):2
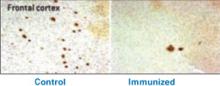
Figure 1 Differences in amyloid deposition between control and immunized mice
Frontal cortex of an unvaccinated mouse (left) shows more amyloid deposits (dark spots) than that of a mouse producing antibodies against the amyloid-β peptide (right).
Source: Image by Cynthia A. Lemere, PhD. Used with permission.Active immunization exposes the subject to the antigen (in this case the Aβ peptide) and allows T cells and B cells to produce anti-Aβ antibodies. This approach has been studied in humans, but adverse effects have stymied its development.
Passive immunization, which involves developing anti-Aβ antibodies in a separate source, aims to clear Aβ peptide without requiring an immunologic response from the host. Large doses of antibodies administered weekly or monthly would be needed to build adequate plasma levels in the CNS, and large quantities of circulating antibodies could cause hemorrhagic stroke.
A troublesome trial
After successful preclinical and phase 1 testing of a vaccine against the Aβ peptide (called AN-1792), a phase 2a placebo-controlled trial in 2001 followed patients with mild to moderate AD. Drug administration was halted after 18 patients (6%) developed meningoencephalitis after several months.4 However, 300 patients with AD and 72 control patients had received at least one injection, and double-blind assessments were maintained for 12 months.
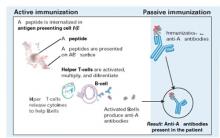
Figure 2 Methods for immunizing against Aβ peptide
Active immunization produces anti-Aβ antibodies via immunologic response to vaccination. With passive immunization, anti-Aβ antibodies are administered directly.
Illustration by Rich LaRocco.All patients with meningoencephalitis had received the vaccine but not all developed an immune response, suggesting that something other than the antibodies—such as T cells—caused the encephalitis. Twelve patients recovered, but six had persistent cognitive and neurologic deficits.
More-optimistic news
Of the 300 patients who received an active vaccine, 20% developed an adequate antibody response.5
The responders showed no significant difference from the placebo group in most outcome measures but showed less worsening in the nine-component Neuropsychological Test Battery (NTB) (P=0.02). Of particular interest, antibody responders showed significant improvement in the NTB—s memory domain (P=0.03). Further, subjects with higher IgG antibody titers showed greater improvement than did other responders.
More work ahead
Although the outcome of this initial AN-1792 trial is disappointing because of its discontinuation and mixed results, T cell infiltration and amyloid depletion were found during postmortem examinations of two vaccine recipients.6
Pharmaceutical companies are testing two compounds for AD immunotherapy:7
- AAB-001, a human monoclonal antibody, targets all 42 Aβ amino acids via passive immunization and has entered phase 2 trials.
- ACC-001, an Aβ immuno-conjugate designed to elicit an active antibody response, began phase 1 testing last fall.
These efforts suggest that an “Alzheimer’s vaccine” could be produced, provided it could attack the Aβ peptide without inducing a significant cellular reaction.
1. Neugroschl JA, Kolevzor A, Samuels SC, Marir DB. Dementia. In: Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive text-book of psychiatry (8th ed). Philadelphia: Lippincott Williams & Wilkins; 2005:1068-93.
2. Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci 2002;3:824-8.
3. Schenk D, Hagen M, Seubert P. Current progress in beta-amyloid immunotherapy. Current Opin Immunol 2004;16:599-606.
4. Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoen-cephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003;61:46-54.
5. Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005;64:1553-62.
6. Ferrer I, Boada Rovira M, Sanchez Guerra ML, et al. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathology 2004;14(1):11-20.
7. Sullivan MG. Immunotherapy studies for AD back on track. Psychiatry News 2005;33(11):69.-
Deposition of amyloid-β peptide (Aβ) is believed to contribute to Alzheimer’s disease (AD) pathogenesis. Derived from a larger precursor protein, Aβ aggregates into plaques, and may promote neuronal death and, ultimately, dementia.
Current treatments alleviate symptoms without slowing underlying neurodegeneration. The prospect of harnessing the immune system to target the Aβ peptide offers an intriguing option for preventing this devastating, increasingly common disease.
Anti-a BETA antibodies
Transgenic mice bred to overexpress AD genes have responded remarkably in studies using the immune system to target the amyloid-β peptide.3 Several mouse groups have shown plaque reduction (Figure 1) and improved cognitive performance. These findings substantiate the amyloid hypothesis in AD pathogenesis.
A host could acquire anti-Aβ antibodies though two basic approaches (Figure 2):2
Figure 1 Differences in amyloid deposition between control and immunized mice
Frontal cortex of an unvaccinated mouse (left) shows more amyloid deposits (dark spots) than that of a mouse producing antibodies against the amyloid-β peptide (right).
Source: Image by Cynthia A. Lemere, PhD. Used with permission.Active immunization exposes the subject to the antigen (in this case the Aβ peptide) and allows T cells and B cells to produce anti-Aβ antibodies. This approach has been studied in humans, but adverse effects have stymied its development.
Passive immunization, which involves developing anti-Aβ antibodies in a separate source, aims to clear Aβ peptide without requiring an immunologic response from the host. Large doses of antibodies administered weekly or monthly would be needed to build adequate plasma levels in the CNS, and large quantities of circulating antibodies could cause hemorrhagic stroke.
A troublesome trial
After successful preclinical and phase 1 testing of a vaccine against the Aβ peptide (called AN-1792), a phase 2a placebo-controlled trial in 2001 followed patients with mild to moderate AD. Drug administration was halted after 18 patients (6%) developed meningoencephalitis after several months.4 However, 300 patients with AD and 72 control patients had received at least one injection, and double-blind assessments were maintained for 12 months.
Figure 2 Methods for immunizing against Aβ peptide
Active immunization produces anti-Aβ antibodies via immunologic response to vaccination. With passive immunization, anti-Aβ antibodies are administered directly.
Illustration by Rich LaRocco.All patients with meningoencephalitis had received the vaccine but not all developed an immune response, suggesting that something other than the antibodies—such as T cells—caused the encephalitis. Twelve patients recovered, but six had persistent cognitive and neurologic deficits.
More-optimistic news
Of the 300 patients who received an active vaccine, 20% developed an adequate antibody response.5
The responders showed no significant difference from the placebo group in most outcome measures but showed less worsening in the nine-component Neuropsychological Test Battery (NTB) (P=0.02). Of particular interest, antibody responders showed significant improvement in the NTB—s memory domain (P=0.03). Further, subjects with higher IgG antibody titers showed greater improvement than did other responders.
More work ahead
Although the outcome of this initial AN-1792 trial is disappointing because of its discontinuation and mixed results, T cell infiltration and amyloid depletion were found during postmortem examinations of two vaccine recipients.6
Pharmaceutical companies are testing two compounds for AD immunotherapy:7
- AAB-001, a human monoclonal antibody, targets all 42 Aβ amino acids via passive immunization and has entered phase 2 trials.
- ACC-001, an Aβ immuno-conjugate designed to elicit an active antibody response, began phase 1 testing last fall.
These efforts suggest that an “Alzheimer’s vaccine” could be produced, provided it could attack the Aβ peptide without inducing a significant cellular reaction.
Deposition of amyloid-β peptide (Aβ) is believed to contribute to Alzheimer’s disease (AD) pathogenesis. Derived from a larger precursor protein, Aβ aggregates into plaques, and may promote neuronal death and, ultimately, dementia.
Current treatments alleviate symptoms without slowing underlying neurodegeneration. The prospect of harnessing the immune system to target the Aβ peptide offers an intriguing option for preventing this devastating, increasingly common disease.
Anti-a BETA antibodies
Transgenic mice bred to overexpress AD genes have responded remarkably in studies using the immune system to target the amyloid-β peptide.3 Several mouse groups have shown plaque reduction (Figure 1) and improved cognitive performance. These findings substantiate the amyloid hypothesis in AD pathogenesis.
A host could acquire anti-Aβ antibodies though two basic approaches (Figure 2):2
Figure 1 Differences in amyloid deposition between control and immunized mice
Frontal cortex of an unvaccinated mouse (left) shows more amyloid deposits (dark spots) than that of a mouse producing antibodies against the amyloid-β peptide (right).
Source: Image by Cynthia A. Lemere, PhD. Used with permission.Active immunization exposes the subject to the antigen (in this case the Aβ peptide) and allows T cells and B cells to produce anti-Aβ antibodies. This approach has been studied in humans, but adverse effects have stymied its development.
Passive immunization, which involves developing anti-Aβ antibodies in a separate source, aims to clear Aβ peptide without requiring an immunologic response from the host. Large doses of antibodies administered weekly or monthly would be needed to build adequate plasma levels in the CNS, and large quantities of circulating antibodies could cause hemorrhagic stroke.
A troublesome trial
After successful preclinical and phase 1 testing of a vaccine against the Aβ peptide (called AN-1792), a phase 2a placebo-controlled trial in 2001 followed patients with mild to moderate AD. Drug administration was halted after 18 patients (6%) developed meningoencephalitis after several months.4 However, 300 patients with AD and 72 control patients had received at least one injection, and double-blind assessments were maintained for 12 months.
Figure 2 Methods for immunizing against Aβ peptide
Active immunization produces anti-Aβ antibodies via immunologic response to vaccination. With passive immunization, anti-Aβ antibodies are administered directly.
Illustration by Rich LaRocco.All patients with meningoencephalitis had received the vaccine but not all developed an immune response, suggesting that something other than the antibodies—such as T cells—caused the encephalitis. Twelve patients recovered, but six had persistent cognitive and neurologic deficits.
More-optimistic news
Of the 300 patients who received an active vaccine, 20% developed an adequate antibody response.5
The responders showed no significant difference from the placebo group in most outcome measures but showed less worsening in the nine-component Neuropsychological Test Battery (NTB) (P=0.02). Of particular interest, antibody responders showed significant improvement in the NTB—s memory domain (P=0.03). Further, subjects with higher IgG antibody titers showed greater improvement than did other responders.
More work ahead
Although the outcome of this initial AN-1792 trial is disappointing because of its discontinuation and mixed results, T cell infiltration and amyloid depletion were found during postmortem examinations of two vaccine recipients.6
Pharmaceutical companies are testing two compounds for AD immunotherapy:7
- AAB-001, a human monoclonal antibody, targets all 42 Aβ amino acids via passive immunization and has entered phase 2 trials.
- ACC-001, an Aβ immuno-conjugate designed to elicit an active antibody response, began phase 1 testing last fall.
These efforts suggest that an “Alzheimer’s vaccine” could be produced, provided it could attack the Aβ peptide without inducing a significant cellular reaction.
1. Neugroschl JA, Kolevzor A, Samuels SC, Marir DB. Dementia. In: Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive text-book of psychiatry (8th ed). Philadelphia: Lippincott Williams & Wilkins; 2005:1068-93.
2. Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci 2002;3:824-8.
3. Schenk D, Hagen M, Seubert P. Current progress in beta-amyloid immunotherapy. Current Opin Immunol 2004;16:599-606.
4. Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoen-cephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003;61:46-54.
5. Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005;64:1553-62.
6. Ferrer I, Boada Rovira M, Sanchez Guerra ML, et al. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathology 2004;14(1):11-20.
7. Sullivan MG. Immunotherapy studies for AD back on track. Psychiatry News 2005;33(11):69.-
1. Neugroschl JA, Kolevzor A, Samuels SC, Marir DB. Dementia. In: Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive text-book of psychiatry (8th ed). Philadelphia: Lippincott Williams & Wilkins; 2005:1068-93.
2. Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci 2002;3:824-8.
3. Schenk D, Hagen M, Seubert P. Current progress in beta-amyloid immunotherapy. Current Opin Immunol 2004;16:599-606.
4. Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoen-cephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003;61:46-54.
5. Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005;64:1553-62.
6. Ferrer I, Boada Rovira M, Sanchez Guerra ML, et al. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathology 2004;14(1):11-20.
7. Sullivan MG. Immunotherapy studies for AD back on track. Psychiatry News 2005;33(11):69.-