User login
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
The Veterans Health Administration (VHA) is the largest US integrated health care system, providing health care to > 9 million veterans annually. Dementia affects > 7.2 million Americans, and an estimated 450,000 veterans live with Alzheimer disease (AD).1,2 Compared with the general population, veterans have a higher burden of chronic medical conditions and are disproportionately affected by AD due to exposure to military-related risk factors (eg, traumatic brain injury and posttraumatic stress disorder) and the high prevalence of nonmilitary risk factors, such as cardiovascular disease. The VHA is a pioneer in dementia care, having established a Dementia System of Care to provide primary and specialty care to veterans with dementia. The VHA also is leading the way in implementing the Institute for Healthcare Improvement Age-Friendly Health Systems (AFHS) framework for providing goal-concordant care in > 100 VHA medical centers. The VHA aims to be the largest AFHS in the country.
AD profoundly affects individuals and their families. The progressive nature of the most common form of dementia diminishes the quality of life for patients as well as their care partners in an ongoing fashion, often leading to emotional, physical, and financial strain. Costs for health and long-term care for people living with AD and other dementias were projected at $360 billion in 2024, largely due to the need for nursing home care.1 Although several oral medications are available, their capacity to effectively mitigate the negative effects of AD is limited. Cholinesterase inhibitors and memantine may offer temporary symptomatic relief, but they do not alter disease progression.3 The use of these agents is relatively low, with about one-third of patients diagnosed with AD receiving these medications.4
Amyloid-Targeting Therapies
Recent advancements in biologics, particularly amyloid-targeting therapies, such as lecanemab and donanemab, offer new hope for managing AD. Older adults treated with these medications show less decline on measures of cognition and function than those receiving a placebo at 18 months.5,6 However, accessing and using these medications is challenging.
Use of amyloid-targeting therapies poses challenges. The medications are expensive, potentially placing a financial burden on patients, families, and health care systems.7 Determining initial eligibility for treatment requires a battery of cognitive assessments, laboratory tests, advanced radiologic studies (eg, magnetic resonance imaging [MRI] of the brain and amyloid positron emission tomography [PET] scans), and possible cerebrospinal fluid (CSF) testing. Frequent ongoing assessments are necessary to monitor safety and efficacy. These treatments carry substantial risks, particularly amyloid-related imaging abnormalities (ARIA) such as cerebral edema, microhemorrhages, and superficial siderosis. Therefore, follow-up assessments typically occur around months 2, 3, 4, and 7, depending on which medication is selected. Finally, at present, both agents must be intravenous (IV)-administered in a monitored clinical setting, which requires additional coordination, transportation, and cost.
Ongoing evaluations and in-person administration particularly affect patients and care partners with limitations regarding transportation, time off work, and navigating complex health care systems.8 VHA clinicians at sites that have implemented or are interested in implementing amyloid-targeting therapy programs endorse similar challenges when implementing these therapies in their US Department of Veterans Affairs (VA) medical centers (VAMCs).9
The VHA was one of the first health care systems to use amyloid-targeting therapies, covering the cost of lecanemab and donanemab, in addition to costs associated with concomitant evaluation and testing. However, given the safety concerns with this novel class of medications, the VHA National Formulary Committee developed criteria for use and recommended the VA Center for Medication Safety (VAMedSAFE) conduct a mandatory real-time medication use evaluation (MUE). VAMedSAFE developed the MUE to monitor the safe and appropriate use of amyloid-targeting therapy for AD. Two authors (AJO, SMH) partnered with VAMedSAFE through the VA Pittsburgh Healthcare System Technology Enhancing Cognition and Health–Geriatric Research, Education, and Clinical Center (TECH-GRECC) to provide clinical expertise, substantive feedback for the development of the MUE, and guidance for VHA sites starting amyloid targeting-therapy programs. We started a VHA Amyloid-Targeting Therapy for AD SharePoint collaborative platform and VHA AD Therapeutics Community of Practice (CoP) for shared learning (Figure). The private SharePoint platform houses an array of implementation materials for VAMCs starting programs: key documents and links; educational materials; sample guidelines; note templates; and electronic health record screenshots. The CoP allows VHAs to share best practices and discuss challenges.
Even with these advantages, we found that ensuring the safe and appropriate use of amyloid-targeting therapies did not overcome the barriers associated with their complexity. This was especially true for veterans living in rural areas. Only 4 VAMCs had administered amyloid-targeting therapies in the first year they were available. Preliminary data demonstrated that 27 (84%) of 32 veterans who initiated lecanemab in the VHA between October 2023 and September 2024 resided in urban areas.10 To address the underutilization of amyloid-targeting therapy, we propose leveraging the strengths of VHA telehealth to facilitate expansion of access to these medications for veterans with early AD. Telehealth may substantially increase access to evaluation for veterans with early dementia and, when medically appropriate, to receive amyloid-targeting therapies by reducing transportation needs and mitigating costs while ensuring appropriate monitoring through ongoing clinical assessments.
Using Telehealth
The VHA is a pioneer in telehealth, with programs dating back to 2003.11 Between October 1, 2018, and September 30, 2019, the VHA served > 900,000 veterans through the provision of > 2.6 million episodes of care via telehealth.12 The COVID-19 pandemic further cemented the role of telemedicine as an essential component of health care. Telehealth has demonstrated success in the assessment and management of individuals living with dementia. At the VHA, the GRECC-Connect Project is a partnership between 9 urban GRECC sites that seek to provide consultative geriatric and dementia care to rural veterans through telehealth.13 Additional evidence supports the potential to leverage telehealth to effectively communicate results of amyloid PET scans.14
This approach is not without limitations such as the digital divide, or the gap that separates technology-enabled individuals and those unprepared to adopt technology due to limited digital literacy levels or access to needed hardware, software, and connectivity. The VHA has taken steps to address these digital divide barriers by broadly providing tools—such as tablets and broadband connectivity—to veterans. Specifically, the VHA has instituted digital divide consults to determine whether telehealth could be a potential solution for appropriate veterans and to provide an iPad (if eligible) to connect with VA clinicians. Complementary to the digital divide consult, a VHA-specific telehealth preparedness assessment tool is under development and being tested by 2 authors (JF, SMH). This telehealth preparedness assessment tool is designed to aid in the seamless integration of telehealth services with the support of tailored education materials specific to gaps in digital literacy that a veteran might experience.
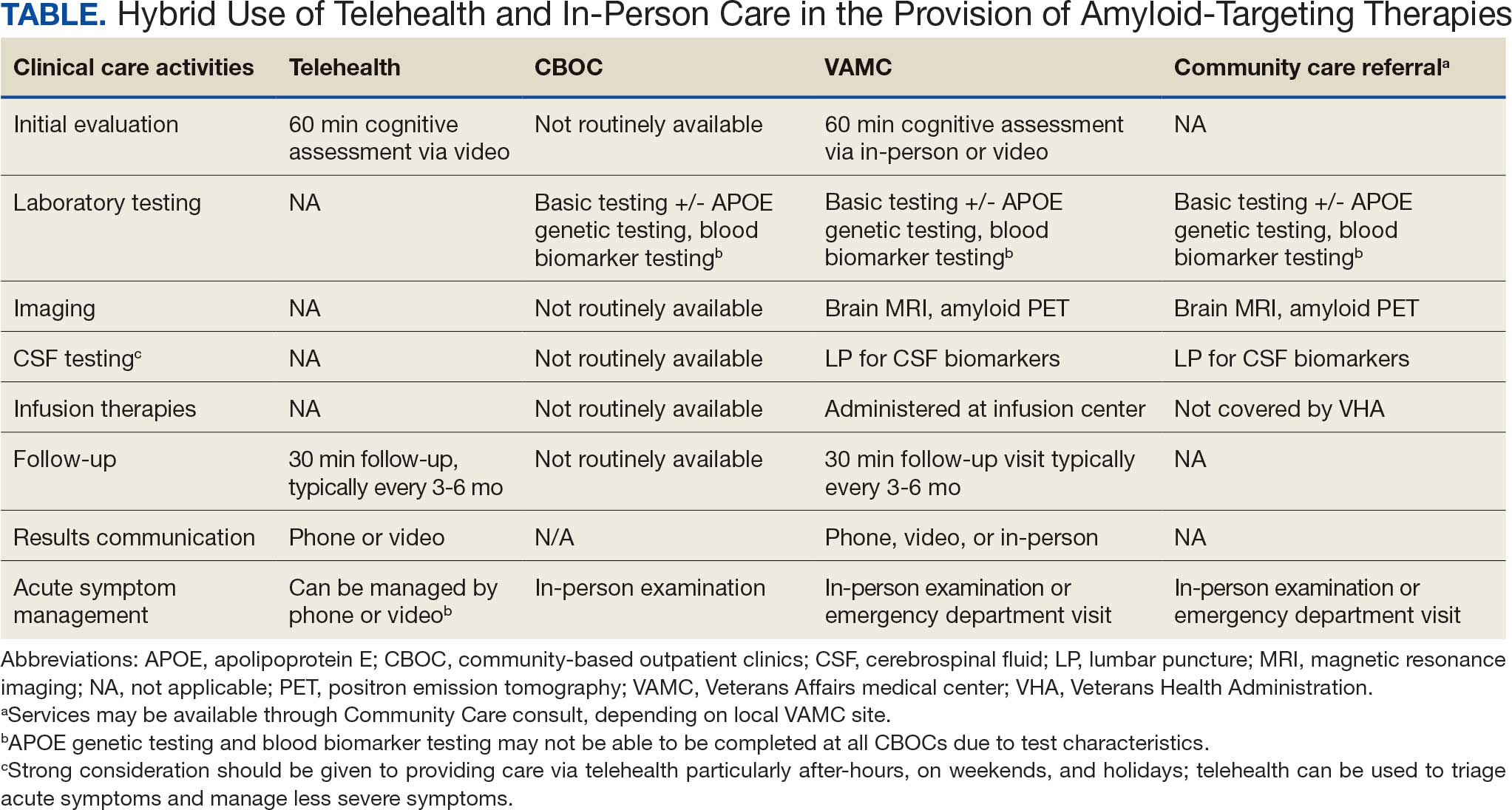
Building on these initiatives, there is an opportunity to expand access to amyloid-targeting therapies, regardless of distance to large VAMCs, by leveraging telehealth as an alternative method of connecting patients with specialty care. Specifically, a hybrid approach could be used to accomplish the myriad initial and follow-up tasks involved in the provision of amyloid-targeting therapies (Table). Not all VHA facilities possess the specialty expertise to prescribe these medications, and local clinicians may not have sufficient knowledge and clinical support to prescribe and monitor these therapies.
The first step is identifying local and regional subject matter experts, followed by the development and expansion of these networks. The National TeleNeurology Program is a good example of a national telehealth program that leverages technology to bring specialty services to rural areas with limited access to care. Although amyloid-targeting therapies often require more complex logistics, such as laboratory tests and imaging, these initial hurdles can be overcome through localized services and collaboration between VAMCs.
While treatment and imaging will most likely need to occur at a VAMC, most basic laboratory studies can be performed at community-based outpatient clinics (CBOCs). Some CBOCs may not be able to process more specialized laboratory tests such as apolipoprotein E genetic testing. Samples for these tests can be collected and processed at VAMCs, which usually have contracts with outside laboratories capable of performing these studies. Most, although not all, VAMCs offer advanced imaging, including MRI of the brain and amyloid PETs. VAMCs without those modalities may need to coordinate with other regional VAMCs. Additionally, a pilot program is already underway whereby VAMCs without the ability to quantify the amount of amyloid on PETs are able to leverage technology and collaborations with other VAMCs to obtain these data.
Once the initial phases of evaluation and care are completed, telemedicine can be leveraged for follow-up and ongoing management. Interdisciplinary teams can help facilitate care related to amyloid-targeting therapies, including the close monitoring of veterans for development of ARIA.15 To achieve this monitoring, specialty clinic teams prescribing amyloid-targeting therapies, which may be geographically distant, need to coordinate with local primary care clinical teams and emergency clinicians. All of these health care team members, along with neurologists and neurosurgeons, should be involved in the development and implementation of protocols in the event that patients present to their local primary or specialty care clinics or emergency department with ARIA symptoms.
If amyloid-targeting therapies are to be provided along with other emerging treatments for rural veterans, telehealth must be part of the solution. There is a pressing need to explore innovative evaluation and delivery models for these therapies, particularly as we expect additional diagnostics and therapeutics to be available in the future. With the advent of commercially available blood tests (ie, blood biomarkers) for AD, there is hope for a transition away from PETs and CSF testing given their cost, limited access, and invasiveness for diagnosis and monitoring of AD. These advances will increase the utility of telehealth to help rural veterans access amyloid-targeting therapies.
Additionally, administering the drug at home or at local clinics, supported by a dedicated health care team or home health agency, could further improve accessibility. Telehealth can be leveraged in this scenario, allowing specialty clinics and specialists to connect with patients and clinicians based out of local clinics or even home health agencies. In this scenario, specialists can provide hands-on care guidance and oversight even though they may be geographically distant from care recipients. Transitioning from IV administration to subcutaneous formulations would further enhance convenience and reduce barriers; these formulations may be available soon.16 Addressing logistical challenges to care and access through technology-based solutions will require coordinated efforts and continued VHA investment.
Conclusions
The VHA has a large population of veterans with dementia, and the costs to care for these veterans will only increase. While the current benefits of amyloid-targeting therapies are modest, now is the time to establish care processes that will support future innovations in amyloid-targeting therapies and other treatments and diagnostics. We are developing better ways to detect AD using clinical decision support tools, improving care pathways and the management of AD, and leveraging telehealth to improve access. The VA is conducting research to investigate whether a cognitive screening and laboratory evaluation that includes a telehealth preparedness assessment will be feasible and effective for improving the detection of AD and access to treatment, and we plan to publish the results.
The lessons learned can be extended to non-VHA care settings to help achieve potential benefits for other patients with early AD. Emerging therapies have the potential to improve the quality of life for both patients and care partners, adding life to years and not just years to life. Policymakers and payors must prioritize research funding to evaluate the safety and efficacy of these approaches to the delivery of health services, ensuring that emerging therapies are accessible for all individuals affected by AD.
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025;21(4):e70235. doi:10.1002/alz.70235
- US Department of Veterans Affairs. Statistical Projections of Alzheimer’s Dementia for VA Patients, VA Enrollees, and US Veterans. December 18, 2020. Accessed November 2, 2025. https://www.va.gov/GERIATRICS/docs/VHA_ALZHEIMERS_DEMENTIA_Statistical_Projections_FY21_and_FY33_sgc121820.pdf
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? P T. 2010;35(4):208-211.
- Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579-589. doi:10.3233/JAD-200133
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi:10.1001/jama.2023.13239
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
- Tanne JH. Lecanemab: US Veterans Health Administration will cover cost of new Alzheimer’s drug. BMJ. 2023;380:p628. doi:10.1136/bmj.p628
- Nadeau SE. Lecanemab questions. Neurology. 2024;102(7):e209320. doi:10.1212/WNL.0000000000209320 9. O’Donnell AJ, Fortunato AT, Spitznogle BL, et al. Implementation of lecanemab for Alzheimer’s disease: facilitators and barriers. Presented at: American Geriatrics Society 2025 Annual Scientific Meeting, Chicago. May 2025.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- Hopp F, Whitten P, Subramanian U, et al. Perspectives from the Veterans Health Administration about opportunities and barriers in telemedicine. J Telemed Telecare. 2006;12(8):404-409. doi:10.1258/135763306779378717
- VA reports significant increase in veteran use of telehealth services. News release. US Department of Veterans Affairs. November 22, 2019. Accessed November 19, 2025. https://news.va.gov/press-room/va-reports-significant-increase-in-veteran-use-of-telehealth-services/
- Powers BB, Homer MC, Morone N, et al. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
- Erickson CM, Chin NA, Rosario HL, et al. Feasibility of virtual Alzheimer’s biomarker disclosure: findings from an observational cohort. Alzheimers Dement (N Y). 2023;9(3):e12413. doi:10.1002/trc2.12413
- Turk KW, Knobel MD, Nothern A, et al. An interprofessional team for disease-modifying therapy in Alzheimer disease implementation. Neurol Clin Pract. 2024;14(6):e200346. doi:10.1212/CPJ.0000000000200346
- FDA accepts LEQEMBI® (lecanemab-irmb) biologics license application for subcutaneous maintenance dosing for the treatment of early Alzheimer’s disease. News release. Elsai US. January 13, 2025. Accessed November 2, 2025. https://media-us.eisai.com/2025-01-13-FDA-Accepts-LEQEMBI-R-lecanemab-irmb-Biologics-License-Application-for-Subcutaneous-Maintenance-Dosing-for-the-Treatment-of-Early-Alzheimers-Disease
The Veterans Health Administration (VHA) is the largest US integrated health care system, providing health care to > 9 million veterans annually. Dementia affects > 7.2 million Americans, and an estimated 450,000 veterans live with Alzheimer disease (AD).1,2 Compared with the general population, veterans have a higher burden of chronic medical conditions and are disproportionately affected by AD due to exposure to military-related risk factors (eg, traumatic brain injury and posttraumatic stress disorder) and the high prevalence of nonmilitary risk factors, such as cardiovascular disease. The VHA is a pioneer in dementia care, having established a Dementia System of Care to provide primary and specialty care to veterans with dementia. The VHA also is leading the way in implementing the Institute for Healthcare Improvement Age-Friendly Health Systems (AFHS) framework for providing goal-concordant care in > 100 VHA medical centers. The VHA aims to be the largest AFHS in the country.
AD profoundly affects individuals and their families. The progressive nature of the most common form of dementia diminishes the quality of life for patients as well as their care partners in an ongoing fashion, often leading to emotional, physical, and financial strain. Costs for health and long-term care for people living with AD and other dementias were projected at $360 billion in 2024, largely due to the need for nursing home care.1 Although several oral medications are available, their capacity to effectively mitigate the negative effects of AD is limited. Cholinesterase inhibitors and memantine may offer temporary symptomatic relief, but they do not alter disease progression.3 The use of these agents is relatively low, with about one-third of patients diagnosed with AD receiving these medications.4
Amyloid-Targeting Therapies
Recent advancements in biologics, particularly amyloid-targeting therapies, such as lecanemab and donanemab, offer new hope for managing AD. Older adults treated with these medications show less decline on measures of cognition and function than those receiving a placebo at 18 months.5,6 However, accessing and using these medications is challenging.
Use of amyloid-targeting therapies poses challenges. The medications are expensive, potentially placing a financial burden on patients, families, and health care systems.7 Determining initial eligibility for treatment requires a battery of cognitive assessments, laboratory tests, advanced radiologic studies (eg, magnetic resonance imaging [MRI] of the brain and amyloid positron emission tomography [PET] scans), and possible cerebrospinal fluid (CSF) testing. Frequent ongoing assessments are necessary to monitor safety and efficacy. These treatments carry substantial risks, particularly amyloid-related imaging abnormalities (ARIA) such as cerebral edema, microhemorrhages, and superficial siderosis. Therefore, follow-up assessments typically occur around months 2, 3, 4, and 7, depending on which medication is selected. Finally, at present, both agents must be intravenous (IV)-administered in a monitored clinical setting, which requires additional coordination, transportation, and cost.
Ongoing evaluations and in-person administration particularly affect patients and care partners with limitations regarding transportation, time off work, and navigating complex health care systems.8 VHA clinicians at sites that have implemented or are interested in implementing amyloid-targeting therapy programs endorse similar challenges when implementing these therapies in their US Department of Veterans Affairs (VA) medical centers (VAMCs).9
The VHA was one of the first health care systems to use amyloid-targeting therapies, covering the cost of lecanemab and donanemab, in addition to costs associated with concomitant evaluation and testing. However, given the safety concerns with this novel class of medications, the VHA National Formulary Committee developed criteria for use and recommended the VA Center for Medication Safety (VAMedSAFE) conduct a mandatory real-time medication use evaluation (MUE). VAMedSAFE developed the MUE to monitor the safe and appropriate use of amyloid-targeting therapy for AD. Two authors (AJO, SMH) partnered with VAMedSAFE through the VA Pittsburgh Healthcare System Technology Enhancing Cognition and Health–Geriatric Research, Education, and Clinical Center (TECH-GRECC) to provide clinical expertise, substantive feedback for the development of the MUE, and guidance for VHA sites starting amyloid targeting-therapy programs. We started a VHA Amyloid-Targeting Therapy for AD SharePoint collaborative platform and VHA AD Therapeutics Community of Practice (CoP) for shared learning (Figure). The private SharePoint platform houses an array of implementation materials for VAMCs starting programs: key documents and links; educational materials; sample guidelines; note templates; and electronic health record screenshots. The CoP allows VHAs to share best practices and discuss challenges.

Even with these advantages, we found that ensuring the safe and appropriate use of amyloid-targeting therapies did not overcome the barriers associated with their complexity. This was especially true for veterans living in rural areas. Only 4 VAMCs had administered amyloid-targeting therapies in the first year they were available. Preliminary data demonstrated that 27 (84%) of 32 veterans who initiated lecanemab in the VHA between October 2023 and September 2024 resided in urban areas.10 To address the underutilization of amyloid-targeting therapy, we propose leveraging the strengths of VHA telehealth to facilitate expansion of access to these medications for veterans with early AD. Telehealth may substantially increase access to evaluation for veterans with early dementia and, when medically appropriate, to receive amyloid-targeting therapies by reducing transportation needs and mitigating costs while ensuring appropriate monitoring through ongoing clinical assessments.
Using Telehealth
The VHA is a pioneer in telehealth, with programs dating back to 2003.11 Between October 1, 2018, and September 30, 2019, the VHA served > 900,000 veterans through the provision of > 2.6 million episodes of care via telehealth.12 The COVID-19 pandemic further cemented the role of telemedicine as an essential component of health care. Telehealth has demonstrated success in the assessment and management of individuals living with dementia. At the VHA, the GRECC-Connect Project is a partnership between 9 urban GRECC sites that seek to provide consultative geriatric and dementia care to rural veterans through telehealth.13 Additional evidence supports the potential to leverage telehealth to effectively communicate results of amyloid PET scans.14
This approach is not without limitations such as the digital divide, or the gap that separates technology-enabled individuals and those unprepared to adopt technology due to limited digital literacy levels or access to needed hardware, software, and connectivity. The VHA has taken steps to address these digital divide barriers by broadly providing tools—such as tablets and broadband connectivity—to veterans. Specifically, the VHA has instituted digital divide consults to determine whether telehealth could be a potential solution for appropriate veterans and to provide an iPad (if eligible) to connect with VA clinicians. Complementary to the digital divide consult, a VHA-specific telehealth preparedness assessment tool is under development and being tested by 2 authors (JF, SMH). This telehealth preparedness assessment tool is designed to aid in the seamless integration of telehealth services with the support of tailored education materials specific to gaps in digital literacy that a veteran might experience.
Building on these initiatives, there is an opportunity to expand access to amyloid-targeting therapies, regardless of distance to large VAMCs, by leveraging telehealth as an alternative method of connecting patients with specialty care. Specifically, a hybrid approach could be used to accomplish the myriad initial and follow-up tasks involved in the provision of amyloid-targeting therapies (Table). Not all VHA facilities possess the specialty expertise to prescribe these medications, and local clinicians may not have sufficient knowledge and clinical support to prescribe and monitor these therapies.

The first step is identifying local and regional subject matter experts, followed by the development and expansion of these networks. The National TeleNeurology Program is a good example of a national telehealth program that leverages technology to bring specialty services to rural areas with limited access to care. Although amyloid-targeting therapies often require more complex logistics, such as laboratory tests and imaging, these initial hurdles can be overcome through localized services and collaboration between VAMCs.
While treatment and imaging will most likely need to occur at a VAMC, most basic laboratory studies can be performed at community-based outpatient clinics (CBOCs). Some CBOCs may not be able to process more specialized laboratory tests such as apolipoprotein E genetic testing. Samples for these tests can be collected and processed at VAMCs, which usually have contracts with outside laboratories capable of performing these studies. Most, although not all, VAMCs offer advanced imaging, including MRI of the brain and amyloid PETs. VAMCs without those modalities may need to coordinate with other regional VAMCs. Additionally, a pilot program is already underway whereby VAMCs without the ability to quantify the amount of amyloid on PETs are able to leverage technology and collaborations with other VAMCs to obtain these data.
Once the initial phases of evaluation and care are completed, telemedicine can be leveraged for follow-up and ongoing management. Interdisciplinary teams can help facilitate care related to amyloid-targeting therapies, including the close monitoring of veterans for development of ARIA.15 To achieve this monitoring, specialty clinic teams prescribing amyloid-targeting therapies, which may be geographically distant, need to coordinate with local primary care clinical teams and emergency clinicians. All of these health care team members, along with neurologists and neurosurgeons, should be involved in the development and implementation of protocols in the event that patients present to their local primary or specialty care clinics or emergency department with ARIA symptoms.
If amyloid-targeting therapies are to be provided along with other emerging treatments for rural veterans, telehealth must be part of the solution. There is a pressing need to explore innovative evaluation and delivery models for these therapies, particularly as we expect additional diagnostics and therapeutics to be available in the future. With the advent of commercially available blood tests (ie, blood biomarkers) for AD, there is hope for a transition away from PETs and CSF testing given their cost, limited access, and invasiveness for diagnosis and monitoring of AD. These advances will increase the utility of telehealth to help rural veterans access amyloid-targeting therapies.
Additionally, administering the drug at home or at local clinics, supported by a dedicated health care team or home health agency, could further improve accessibility. Telehealth can be leveraged in this scenario, allowing specialty clinics and specialists to connect with patients and clinicians based out of local clinics or even home health agencies. In this scenario, specialists can provide hands-on care guidance and oversight even though they may be geographically distant from care recipients. Transitioning from IV administration to subcutaneous formulations would further enhance convenience and reduce barriers; these formulations may be available soon.16 Addressing logistical challenges to care and access through technology-based solutions will require coordinated efforts and continued VHA investment.
Conclusions
The VHA has a large population of veterans with dementia, and the costs to care for these veterans will only increase. While the current benefits of amyloid-targeting therapies are modest, now is the time to establish care processes that will support future innovations in amyloid-targeting therapies and other treatments and diagnostics. We are developing better ways to detect AD using clinical decision support tools, improving care pathways and the management of AD, and leveraging telehealth to improve access. The VA is conducting research to investigate whether a cognitive screening and laboratory evaluation that includes a telehealth preparedness assessment will be feasible and effective for improving the detection of AD and access to treatment, and we plan to publish the results.
The lessons learned can be extended to non-VHA care settings to help achieve potential benefits for other patients with early AD. Emerging therapies have the potential to improve the quality of life for both patients and care partners, adding life to years and not just years to life. Policymakers and payors must prioritize research funding to evaluate the safety and efficacy of these approaches to the delivery of health services, ensuring that emerging therapies are accessible for all individuals affected by AD.
The Veterans Health Administration (VHA) is the largest US integrated health care system, providing health care to > 9 million veterans annually. Dementia affects > 7.2 million Americans, and an estimated 450,000 veterans live with Alzheimer disease (AD).1,2 Compared with the general population, veterans have a higher burden of chronic medical conditions and are disproportionately affected by AD due to exposure to military-related risk factors (eg, traumatic brain injury and posttraumatic stress disorder) and the high prevalence of nonmilitary risk factors, such as cardiovascular disease. The VHA is a pioneer in dementia care, having established a Dementia System of Care to provide primary and specialty care to veterans with dementia. The VHA also is leading the way in implementing the Institute for Healthcare Improvement Age-Friendly Health Systems (AFHS) framework for providing goal-concordant care in > 100 VHA medical centers. The VHA aims to be the largest AFHS in the country.
AD profoundly affects individuals and their families. The progressive nature of the most common form of dementia diminishes the quality of life for patients as well as their care partners in an ongoing fashion, often leading to emotional, physical, and financial strain. Costs for health and long-term care for people living with AD and other dementias were projected at $360 billion in 2024, largely due to the need for nursing home care.1 Although several oral medications are available, their capacity to effectively mitigate the negative effects of AD is limited. Cholinesterase inhibitors and memantine may offer temporary symptomatic relief, but they do not alter disease progression.3 The use of these agents is relatively low, with about one-third of patients diagnosed with AD receiving these medications.4
Amyloid-Targeting Therapies
Recent advancements in biologics, particularly amyloid-targeting therapies, such as lecanemab and donanemab, offer new hope for managing AD. Older adults treated with these medications show less decline on measures of cognition and function than those receiving a placebo at 18 months.5,6 However, accessing and using these medications is challenging.
Use of amyloid-targeting therapies poses challenges. The medications are expensive, potentially placing a financial burden on patients, families, and health care systems.7 Determining initial eligibility for treatment requires a battery of cognitive assessments, laboratory tests, advanced radiologic studies (eg, magnetic resonance imaging [MRI] of the brain and amyloid positron emission tomography [PET] scans), and possible cerebrospinal fluid (CSF) testing. Frequent ongoing assessments are necessary to monitor safety and efficacy. These treatments carry substantial risks, particularly amyloid-related imaging abnormalities (ARIA) such as cerebral edema, microhemorrhages, and superficial siderosis. Therefore, follow-up assessments typically occur around months 2, 3, 4, and 7, depending on which medication is selected. Finally, at present, both agents must be intravenous (IV)-administered in a monitored clinical setting, which requires additional coordination, transportation, and cost.
Ongoing evaluations and in-person administration particularly affect patients and care partners with limitations regarding transportation, time off work, and navigating complex health care systems.8 VHA clinicians at sites that have implemented or are interested in implementing amyloid-targeting therapy programs endorse similar challenges when implementing these therapies in their US Department of Veterans Affairs (VA) medical centers (VAMCs).9
The VHA was one of the first health care systems to use amyloid-targeting therapies, covering the cost of lecanemab and donanemab, in addition to costs associated with concomitant evaluation and testing. However, given the safety concerns with this novel class of medications, the VHA National Formulary Committee developed criteria for use and recommended the VA Center for Medication Safety (VAMedSAFE) conduct a mandatory real-time medication use evaluation (MUE). VAMedSAFE developed the MUE to monitor the safe and appropriate use of amyloid-targeting therapy for AD. Two authors (AJO, SMH) partnered with VAMedSAFE through the VA Pittsburgh Healthcare System Technology Enhancing Cognition and Health–Geriatric Research, Education, and Clinical Center (TECH-GRECC) to provide clinical expertise, substantive feedback for the development of the MUE, and guidance for VHA sites starting amyloid targeting-therapy programs. We started a VHA Amyloid-Targeting Therapy for AD SharePoint collaborative platform and VHA AD Therapeutics Community of Practice (CoP) for shared learning (Figure). The private SharePoint platform houses an array of implementation materials for VAMCs starting programs: key documents and links; educational materials; sample guidelines; note templates; and electronic health record screenshots. The CoP allows VHAs to share best practices and discuss challenges.

Even with these advantages, we found that ensuring the safe and appropriate use of amyloid-targeting therapies did not overcome the barriers associated with their complexity. This was especially true for veterans living in rural areas. Only 4 VAMCs had administered amyloid-targeting therapies in the first year they were available. Preliminary data demonstrated that 27 (84%) of 32 veterans who initiated lecanemab in the VHA between October 2023 and September 2024 resided in urban areas.10 To address the underutilization of amyloid-targeting therapy, we propose leveraging the strengths of VHA telehealth to facilitate expansion of access to these medications for veterans with early AD. Telehealth may substantially increase access to evaluation for veterans with early dementia and, when medically appropriate, to receive amyloid-targeting therapies by reducing transportation needs and mitigating costs while ensuring appropriate monitoring through ongoing clinical assessments.
Using Telehealth
The VHA is a pioneer in telehealth, with programs dating back to 2003.11 Between October 1, 2018, and September 30, 2019, the VHA served > 900,000 veterans through the provision of > 2.6 million episodes of care via telehealth.12 The COVID-19 pandemic further cemented the role of telemedicine as an essential component of health care. Telehealth has demonstrated success in the assessment and management of individuals living with dementia. At the VHA, the GRECC-Connect Project is a partnership between 9 urban GRECC sites that seek to provide consultative geriatric and dementia care to rural veterans through telehealth.13 Additional evidence supports the potential to leverage telehealth to effectively communicate results of amyloid PET scans.14
This approach is not without limitations such as the digital divide, or the gap that separates technology-enabled individuals and those unprepared to adopt technology due to limited digital literacy levels or access to needed hardware, software, and connectivity. The VHA has taken steps to address these digital divide barriers by broadly providing tools—such as tablets and broadband connectivity—to veterans. Specifically, the VHA has instituted digital divide consults to determine whether telehealth could be a potential solution for appropriate veterans and to provide an iPad (if eligible) to connect with VA clinicians. Complementary to the digital divide consult, a VHA-specific telehealth preparedness assessment tool is under development and being tested by 2 authors (JF, SMH). This telehealth preparedness assessment tool is designed to aid in the seamless integration of telehealth services with the support of tailored education materials specific to gaps in digital literacy that a veteran might experience.
Building on these initiatives, there is an opportunity to expand access to amyloid-targeting therapies, regardless of distance to large VAMCs, by leveraging telehealth as an alternative method of connecting patients with specialty care. Specifically, a hybrid approach could be used to accomplish the myriad initial and follow-up tasks involved in the provision of amyloid-targeting therapies (Table). Not all VHA facilities possess the specialty expertise to prescribe these medications, and local clinicians may not have sufficient knowledge and clinical support to prescribe and monitor these therapies.

The first step is identifying local and regional subject matter experts, followed by the development and expansion of these networks. The National TeleNeurology Program is a good example of a national telehealth program that leverages technology to bring specialty services to rural areas with limited access to care. Although amyloid-targeting therapies often require more complex logistics, such as laboratory tests and imaging, these initial hurdles can be overcome through localized services and collaboration between VAMCs.
While treatment and imaging will most likely need to occur at a VAMC, most basic laboratory studies can be performed at community-based outpatient clinics (CBOCs). Some CBOCs may not be able to process more specialized laboratory tests such as apolipoprotein E genetic testing. Samples for these tests can be collected and processed at VAMCs, which usually have contracts with outside laboratories capable of performing these studies. Most, although not all, VAMCs offer advanced imaging, including MRI of the brain and amyloid PETs. VAMCs without those modalities may need to coordinate with other regional VAMCs. Additionally, a pilot program is already underway whereby VAMCs without the ability to quantify the amount of amyloid on PETs are able to leverage technology and collaborations with other VAMCs to obtain these data.
Once the initial phases of evaluation and care are completed, telemedicine can be leveraged for follow-up and ongoing management. Interdisciplinary teams can help facilitate care related to amyloid-targeting therapies, including the close monitoring of veterans for development of ARIA.15 To achieve this monitoring, specialty clinic teams prescribing amyloid-targeting therapies, which may be geographically distant, need to coordinate with local primary care clinical teams and emergency clinicians. All of these health care team members, along with neurologists and neurosurgeons, should be involved in the development and implementation of protocols in the event that patients present to their local primary or specialty care clinics or emergency department with ARIA symptoms.
If amyloid-targeting therapies are to be provided along with other emerging treatments for rural veterans, telehealth must be part of the solution. There is a pressing need to explore innovative evaluation and delivery models for these therapies, particularly as we expect additional diagnostics and therapeutics to be available in the future. With the advent of commercially available blood tests (ie, blood biomarkers) for AD, there is hope for a transition away from PETs and CSF testing given their cost, limited access, and invasiveness for diagnosis and monitoring of AD. These advances will increase the utility of telehealth to help rural veterans access amyloid-targeting therapies.
Additionally, administering the drug at home or at local clinics, supported by a dedicated health care team or home health agency, could further improve accessibility. Telehealth can be leveraged in this scenario, allowing specialty clinics and specialists to connect with patients and clinicians based out of local clinics or even home health agencies. In this scenario, specialists can provide hands-on care guidance and oversight even though they may be geographically distant from care recipients. Transitioning from IV administration to subcutaneous formulations would further enhance convenience and reduce barriers; these formulations may be available soon.16 Addressing logistical challenges to care and access through technology-based solutions will require coordinated efforts and continued VHA investment.
Conclusions
The VHA has a large population of veterans with dementia, and the costs to care for these veterans will only increase. While the current benefits of amyloid-targeting therapies are modest, now is the time to establish care processes that will support future innovations in amyloid-targeting therapies and other treatments and diagnostics. We are developing better ways to detect AD using clinical decision support tools, improving care pathways and the management of AD, and leveraging telehealth to improve access. The VA is conducting research to investigate whether a cognitive screening and laboratory evaluation that includes a telehealth preparedness assessment will be feasible and effective for improving the detection of AD and access to treatment, and we plan to publish the results.
The lessons learned can be extended to non-VHA care settings to help achieve potential benefits for other patients with early AD. Emerging therapies have the potential to improve the quality of life for both patients and care partners, adding life to years and not just years to life. Policymakers and payors must prioritize research funding to evaluate the safety and efficacy of these approaches to the delivery of health services, ensuring that emerging therapies are accessible for all individuals affected by AD.
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025;21(4):e70235. doi:10.1002/alz.70235
- US Department of Veterans Affairs. Statistical Projections of Alzheimer’s Dementia for VA Patients, VA Enrollees, and US Veterans. December 18, 2020. Accessed November 2, 2025. https://www.va.gov/GERIATRICS/docs/VHA_ALZHEIMERS_DEMENTIA_Statistical_Projections_FY21_and_FY33_sgc121820.pdf
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? P T. 2010;35(4):208-211.
- Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579-589. doi:10.3233/JAD-200133
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi:10.1001/jama.2023.13239
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
- Tanne JH. Lecanemab: US Veterans Health Administration will cover cost of new Alzheimer’s drug. BMJ. 2023;380:p628. doi:10.1136/bmj.p628
- Nadeau SE. Lecanemab questions. Neurology. 2024;102(7):e209320. doi:10.1212/WNL.0000000000209320 9. O’Donnell AJ, Fortunato AT, Spitznogle BL, et al. Implementation of lecanemab for Alzheimer’s disease: facilitators and barriers. Presented at: American Geriatrics Society 2025 Annual Scientific Meeting, Chicago. May 2025.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- Hopp F, Whitten P, Subramanian U, et al. Perspectives from the Veterans Health Administration about opportunities and barriers in telemedicine. J Telemed Telecare. 2006;12(8):404-409. doi:10.1258/135763306779378717
- VA reports significant increase in veteran use of telehealth services. News release. US Department of Veterans Affairs. November 22, 2019. Accessed November 19, 2025. https://news.va.gov/press-room/va-reports-significant-increase-in-veteran-use-of-telehealth-services/
- Powers BB, Homer MC, Morone N, et al. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
- Erickson CM, Chin NA, Rosario HL, et al. Feasibility of virtual Alzheimer’s biomarker disclosure: findings from an observational cohort. Alzheimers Dement (N Y). 2023;9(3):e12413. doi:10.1002/trc2.12413
- Turk KW, Knobel MD, Nothern A, et al. An interprofessional team for disease-modifying therapy in Alzheimer disease implementation. Neurol Clin Pract. 2024;14(6):e200346. doi:10.1212/CPJ.0000000000200346
- FDA accepts LEQEMBI® (lecanemab-irmb) biologics license application for subcutaneous maintenance dosing for the treatment of early Alzheimer’s disease. News release. Elsai US. January 13, 2025. Accessed November 2, 2025. https://media-us.eisai.com/2025-01-13-FDA-Accepts-LEQEMBI-R-lecanemab-irmb-Biologics-License-Application-for-Subcutaneous-Maintenance-Dosing-for-the-Treatment-of-Early-Alzheimers-Disease
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025;21(4):e70235. doi:10.1002/alz.70235
- US Department of Veterans Affairs. Statistical Projections of Alzheimer’s Dementia for VA Patients, VA Enrollees, and US Veterans. December 18, 2020. Accessed November 2, 2025. https://www.va.gov/GERIATRICS/docs/VHA_ALZHEIMERS_DEMENTIA_Statistical_Projections_FY21_and_FY33_sgc121820.pdf
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? P T. 2010;35(4):208-211.
- Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579-589. doi:10.3233/JAD-200133
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi:10.1001/jama.2023.13239
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
- Tanne JH. Lecanemab: US Veterans Health Administration will cover cost of new Alzheimer’s drug. BMJ. 2023;380:p628. doi:10.1136/bmj.p628
- Nadeau SE. Lecanemab questions. Neurology. 2024;102(7):e209320. doi:10.1212/WNL.0000000000209320 9. O’Donnell AJ, Fortunato AT, Spitznogle BL, et al. Implementation of lecanemab for Alzheimer’s disease: facilitators and barriers. Presented at: American Geriatrics Society 2025 Annual Scientific Meeting, Chicago. May 2025.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- Hopp F, Whitten P, Subramanian U, et al. Perspectives from the Veterans Health Administration about opportunities and barriers in telemedicine. J Telemed Telecare. 2006;12(8):404-409. doi:10.1258/135763306779378717
- VA reports significant increase in veteran use of telehealth services. News release. US Department of Veterans Affairs. November 22, 2019. Accessed November 19, 2025. https://news.va.gov/press-room/va-reports-significant-increase-in-veteran-use-of-telehealth-services/
- Powers BB, Homer MC, Morone N, et al. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
- Erickson CM, Chin NA, Rosario HL, et al. Feasibility of virtual Alzheimer’s biomarker disclosure: findings from an observational cohort. Alzheimers Dement (N Y). 2023;9(3):e12413. doi:10.1002/trc2.12413
- Turk KW, Knobel MD, Nothern A, et al. An interprofessional team for disease-modifying therapy in Alzheimer disease implementation. Neurol Clin Pract. 2024;14(6):e200346. doi:10.1212/CPJ.0000000000200346
- FDA accepts LEQEMBI® (lecanemab-irmb) biologics license application for subcutaneous maintenance dosing for the treatment of early Alzheimer’s disease. News release. Elsai US. January 13, 2025. Accessed November 2, 2025. https://media-us.eisai.com/2025-01-13-FDA-Accepts-LEQEMBI-R-lecanemab-irmb-Biologics-License-Application-for-Subcutaneous-Maintenance-Dosing-for-the-Treatment-of-Early-Alzheimers-Disease
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
Thoracic Intramedullary Mass Causing Neurologic Weakness
Thoracic Intramedullary Mass Causing Neurologic Weakness
Discussion
A diagnosis of dural arteriovenous fistula (dAVF) was made. Lesions involving the spinal cord are traditionally classified by location as extradural, intradural/extramedullary, or intramedullary. Intramedullary spinal cord abnormalities pose considerable diagnostic and management challenges because of the risks of biopsy in this location and the added potential for morbidity and mortality from improperly treated lesions. Although MRI is the preferred imaging modality, PET/CT and magnetic resonance angiography (MRA) may also help narrow the differential diagnosis and potentially avoid complications from an invasive biopsy.1 This patient’s intramedullary lesion, which represented a dAVF, posed a diagnostic challenge; after diagnosis, it was successfully managed conservatively with dexamethasone and physical therapy.
Intradural tumors account for 2% to 4% of all primary central nervous system (CNS) tumors.2 Ependymomas account for 50% to 60% of intramedullary tumors in adults, while astrocytomas account for about 60% of all lesions in children and adolescents.3,4 The differential diagnosis for intramedullary tumors also includes hemangioblastoma, metastases, primary CNS lymphoma, germ cell tumors, and gangliogliomas.5,6
Intramedullary metastases remain rare, although the incidence is rising with improvements in oncologic and supportive treatments. Autopsy studies conducted decades ago demonstrated that about 0.9% to 2.1% of patients with systemic cancer have intramedullary metastases at death.7,8 In patients with an established history of malignancy, a metastatic intramedullary tumor should be placed higher on the differential diagnosis. Intramedullary metastases most often occur in the setting of widespread metastatic disease. A systematic review of the literature on patients with lung cancer (small cell and non-small cell lung carcinomas) and ≥ 1 intramedullary spinal cord metastasis demonstrated that 55.8% of patients had concurrent brain metastases, 20.0% had leptomeningeal carcinomatosis, and 19.5% had vertebral metastases.9 While about half of all intramedullary metastases are associated with lung cancer, other common malignancies that metastasize to this area include colorectal, breast, and renal cell carcinoma, as well as lymphoma and melanoma primaries.10,11
On imaging, intramedullary metastases often appear as several short, studded segments with surrounding edema, typically out of proportion to the size of the lesion.1 By contrast, astrocytomas and ependymomas often span multiple segments, and enhancement patterns can vary depending on the subtype and grade. Glioblastoma multiforme, or grade 4 IDH wild-type astrocytomas, demonstrate an irregular, heterogeneous pattern of enhancement. Hemangioblastomas vary in size and are classically hypointense to isointense on T1-weighted sequences, isointense to hyperintense on T2-weighted sequences, and demonstrate avid enhancement on T1- postcontrast images. In large hemangioblastomas, flow voids due to prominent vasculature may be visualized.
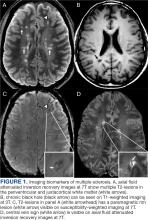
Numerous nonneoplastic tumor mimics can obscure the differential diagnosis. Vascular malformations, including cavernomas and dAVFs, can also present with enhancement and edema. dAVFs are the most common type of spinal vascular malformation, accounting for about 70% of cases.12 They are supplied by the radiculomeningeal arteries, whereas pial arteriovenous malformations (AVMs) are supplied by the radiculomedullary and radiculopial arteries. On MRI, dAVFs usually have venous congestion with intramedullary edema, which appears as an ill-defined centromedullary hyperintensity on T2-weighted imaging over multiple segments. The spinal cord may appear swollen with atrophic changes in chronic cases. Spinal cord AVMs are rarer and have an intramedullary nidus. They usually demonstrate mixed heterogeneous signal on T1- and T2-weighted imaging due to blood products, while the nidus demonstrates a variable degree of enhancement. Serpiginous flow voids are seen both within the nidus and at the cord surface.
Demyelinating lesions of the spine may be seen in neuroinflammatory conditions such as multiple sclerosis, neuromyelitis optica spectrum disorder, acute transverse myelitis, and acute disseminated encephalomyelitis. In multiple sclerosis, lesions typically extend ≤ 2 vertebral segments in length, cover less than half of the vertebral cross-sectional area, and have a dorsolateral predilection.13 Active lesions may demonstrate enhancement along the rim or in a patchy pattern. In the presence of demyelinating lesions, there may occasionally appear to be an expansile mass with a syrinx.14
Infections such as tuberculosis and neurosarcoidosis should also remain on the differential diagnosis. On MRI, tuberculosis usually involves the thoracic cord and is typically rim-enhancing.15 If there are caseating granulomas, T2-weighted images may also demonstrate rim enhancement.16 Spinal sarcoidosis is unusual without intracranial involvement, and its appearance may include leptomeningeal enhancement, cord expansion, and hyperintense signal on T2- weighted imaging.17
Finally, iatrogenic causes are also possible, including radiation myelopathy and mechanical spinal cord injury. For radiation myelopathy, it is important to ascertain whether a patient has undergone prior radiotherapy in the region and to obtain the pertinent dosimetry. Spinal cord injury may cause a focal signal abnormality within the cord, with T2 hyperintensity; these foci may or may not present with enhancement, edema, or hematoma and therefore may resemble tumors.13
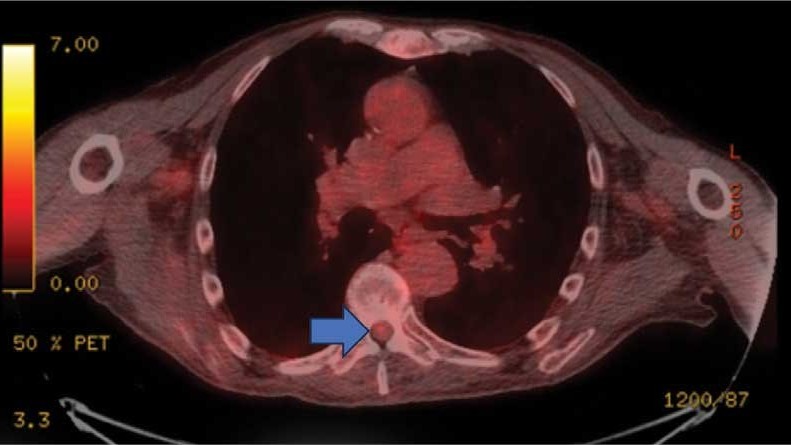
This patient presented with progressive right-sided lower extremity weakness and hypoesthesia and a history of a low-grade right renal/pelvic ureteral tumor. The immediate impression was that the thoracic intramedullary lesion represented a metastatic lesion. However, in the absence of any systemic or intracranial metastases, this progression was much less likely. An extensive interdisciplinary workup was conducted that included medical oncology, neurology, neuroradiology, neuro-oncology, neurosurgery, nuclear medicine, and radiation oncology. Neuroradiology and nuclear medicine identified a slightly hypermetabolic focus on the PET/CT from 1.5 years prior that correlated exactly with the same location as the lesion on the recent spinal MRI. This finding, along with the MRA, confirmed the diagnosis of a dAVF, which was successfully managed conservatively with dexamethasone and physical therapy, rather than through oncologic treatments such as radiotherapy
There remains debate regarding the utility of steroids in treating patients with dAVF. Although there are some case reports documenting that the edema associated with the dAVF responds to steroids, other case series have found that steroids may worsen outcomes in patients with dAVF, possibly due to increased venous hydrostatic pressure.
This case demonstrates the importance of an interdisciplinary workup when evaluating an intramedullary lesion, as well as maintaining a wide differential diagnosis, particularly in the absence of a history of polymetastatic cancer. All the clues (such as the slightly hypermetabolic focus on a PET/CT from 1.5 years prior) need to be obtained to comfortably reach a diagnosis in the absence of pathologic confirmation. These cases can be especially challenging due to the lack of pathologic confirmation, but by understanding the main differentiating features among the various etiologies and obtaining all available information, a correct diagnosis can be made without unnecessary interventions.
- Moghaddam SM, Bhatt AA. Location, length, and enhancement: systematic approach to differentiating intramedullary spinal cord lesions. Insights Imaging. 2018;9:511-526. doi:10.1007/s13244-018-0608-3
- Grimm S, Chamberlain MC. Adult primary spinal cord tumors. Expert Rev Neurother. 2009;9:1487-1495. doi:10.1586/ern.09.101
- Miller DJ, McCutcheon IE. Hemangioblastomas and other uncommon intramedullary tumors. J Neurooncol. 2000;47:253- 270. doi:10.1023/a:1006403500801
- Mottl H, Koutecky J. Treatment of spinal cord tumors in children. Med Pediatr Oncol. 1997;29:293-295.
- Kandemirli SG, Reddy A, Hitchon P, et al. Intramedullary tumours and tumour mimics. Clin Radiol. 2020;75:876.e17-876. e32. doi:10.1016/j.crad.2020.05.010
- Tobin MK, Geraghty JR, Engelhard HH, et al. Intramedullary spinal cord tumors: a review of current and future treatment strategies. Neurosurg Focus. 2015;39:E14. doi:10.3171/2015.5.FOCUS15158
- Chason JL, Walker FB, Landers JW. Metastatic carcinoma in the central nervous system and dorsal root ganglia. A prospective autopsy study. Cancer. 1963;16:781-787.
- Costigan DA, Winkelman MD. Intramedullary spinal cord metastasis. A clinicopathological study of 13 cases. J Neurosurg. 1985;62:227-233.
- Wu L, Wang L, Yang J, et al. Clinical features, treatments, and prognosis of intramedullary spinal cord metastases from lung cancer: a case series and systematic review. Neurospine. 2022;19:65-76. doi:10.14245/ns.2142910.455
- Lv J, Liu B, Quan X, et al. Intramedullary spinal cord metastasis in malignancies: an institutional analysis and review. Onco Targets Ther. 2019;12:4741-4753. doi:10.2147/OTT.S193235
- Goyal A, Yolcu Y, Kerezoudis P, et al. Intramedullary spinal cord metastases: an institutional review of survival and outcomes. J Neurooncol. 2019;142:347-354. doi:10.1007/s11060-019-03105-2
- Krings T. Vascular malformations of the spine and spinal cord: anatomy, classification, treatment. Clin Neuroradiol. 2010;20:5-24. doi:10.1007/s00062-010-9036-6
- Maj E, Wojtowicz K, Aleksandra PP, et al. Intramedullary spinal tumor-like lesions. Acta Radiol. 2019;60:994-1010. doi:10.1177/0284185118809540
- Waziri A, Vonsattel JP, Kaiser MG, et al. Expansile, enhancing cervical cord lesion with an associated syrinx secondary to demyelination. Case report and review of the literature. J Neurosurg Spine. 2007;6:52-56. doi:10.3171/spi.2007.6.1.52
- Nussbaum ES, Rockswold GL, Bergman TA, et al. Spinal tuberculosis: a diagnostic and management challenge. J Neurosurg. 1995;83:243-247. doi:10.3171/jns.1995.83.2.0243
- Lu M. Imaging diagnosis of spinal intramedullary tuberculoma: case reports and literature review. J Spinal Cord Med. 2010;33:159-162. doi:10.1080/10790268.2010.11689691
- Do-Dai DD, Brooks MK, Goldkamp A, et al. Magnetic resonance imaging of intramedullary spinal cord lesions: a pictorial review. Curr Probl Diagn Radiol. 2010;39:160-185. doi:10.1067/j.cpradiol.2009.05.004
Discussion
A diagnosis of dural arteriovenous fistula (dAVF) was made. Lesions involving the spinal cord are traditionally classified by location as extradural, intradural/extramedullary, or intramedullary. Intramedullary spinal cord abnormalities pose considerable diagnostic and management challenges because of the risks of biopsy in this location and the added potential for morbidity and mortality from improperly treated lesions. Although MRI is the preferred imaging modality, PET/CT and magnetic resonance angiography (MRA) may also help narrow the differential diagnosis and potentially avoid complications from an invasive biopsy.1 This patient’s intramedullary lesion, which represented a dAVF, posed a diagnostic challenge; after diagnosis, it was successfully managed conservatively with dexamethasone and physical therapy.
Intradural tumors account for 2% to 4% of all primary central nervous system (CNS) tumors.2 Ependymomas account for 50% to 60% of intramedullary tumors in adults, while astrocytomas account for about 60% of all lesions in children and adolescents.3,4 The differential diagnosis for intramedullary tumors also includes hemangioblastoma, metastases, primary CNS lymphoma, germ cell tumors, and gangliogliomas.5,6
Intramedullary metastases remain rare, although the incidence is rising with improvements in oncologic and supportive treatments. Autopsy studies conducted decades ago demonstrated that about 0.9% to 2.1% of patients with systemic cancer have intramedullary metastases at death.7,8 In patients with an established history of malignancy, a metastatic intramedullary tumor should be placed higher on the differential diagnosis. Intramedullary metastases most often occur in the setting of widespread metastatic disease. A systematic review of the literature on patients with lung cancer (small cell and non-small cell lung carcinomas) and ≥ 1 intramedullary spinal cord metastasis demonstrated that 55.8% of patients had concurrent brain metastases, 20.0% had leptomeningeal carcinomatosis, and 19.5% had vertebral metastases.9 While about half of all intramedullary metastases are associated with lung cancer, other common malignancies that metastasize to this area include colorectal, breast, and renal cell carcinoma, as well as lymphoma and melanoma primaries.10,11
On imaging, intramedullary metastases often appear as several short, studded segments with surrounding edema, typically out of proportion to the size of the lesion.1 By contrast, astrocytomas and ependymomas often span multiple segments, and enhancement patterns can vary depending on the subtype and grade. Glioblastoma multiforme, or grade 4 IDH wild-type astrocytomas, demonstrate an irregular, heterogeneous pattern of enhancement. Hemangioblastomas vary in size and are classically hypointense to isointense on T1-weighted sequences, isointense to hyperintense on T2-weighted sequences, and demonstrate avid enhancement on T1- postcontrast images. In large hemangioblastomas, flow voids due to prominent vasculature may be visualized.
Numerous nonneoplastic tumor mimics can obscure the differential diagnosis. Vascular malformations, including cavernomas and dAVFs, can also present with enhancement and edema. dAVFs are the most common type of spinal vascular malformation, accounting for about 70% of cases.12 They are supplied by the radiculomeningeal arteries, whereas pial arteriovenous malformations (AVMs) are supplied by the radiculomedullary and radiculopial arteries. On MRI, dAVFs usually have venous congestion with intramedullary edema, which appears as an ill-defined centromedullary hyperintensity on T2-weighted imaging over multiple segments. The spinal cord may appear swollen with atrophic changes in chronic cases. Spinal cord AVMs are rarer and have an intramedullary nidus. They usually demonstrate mixed heterogeneous signal on T1- and T2-weighted imaging due to blood products, while the nidus demonstrates a variable degree of enhancement. Serpiginous flow voids are seen both within the nidus and at the cord surface.
Demyelinating lesions of the spine may be seen in neuroinflammatory conditions such as multiple sclerosis, neuromyelitis optica spectrum disorder, acute transverse myelitis, and acute disseminated encephalomyelitis. In multiple sclerosis, lesions typically extend ≤ 2 vertebral segments in length, cover less than half of the vertebral cross-sectional area, and have a dorsolateral predilection.13 Active lesions may demonstrate enhancement along the rim or in a patchy pattern. In the presence of demyelinating lesions, there may occasionally appear to be an expansile mass with a syrinx.14
Infections such as tuberculosis and neurosarcoidosis should also remain on the differential diagnosis. On MRI, tuberculosis usually involves the thoracic cord and is typically rim-enhancing.15 If there are caseating granulomas, T2-weighted images may also demonstrate rim enhancement.16 Spinal sarcoidosis is unusual without intracranial involvement, and its appearance may include leptomeningeal enhancement, cord expansion, and hyperintense signal on T2- weighted imaging.17
Finally, iatrogenic causes are also possible, including radiation myelopathy and mechanical spinal cord injury. For radiation myelopathy, it is important to ascertain whether a patient has undergone prior radiotherapy in the region and to obtain the pertinent dosimetry. Spinal cord injury may cause a focal signal abnormality within the cord, with T2 hyperintensity; these foci may or may not present with enhancement, edema, or hematoma and therefore may resemble tumors.13
This patient presented with progressive right-sided lower extremity weakness and hypoesthesia and a history of a low-grade right renal/pelvic ureteral tumor. The immediate impression was that the thoracic intramedullary lesion represented a metastatic lesion. However, in the absence of any systemic or intracranial metastases, this progression was much less likely. An extensive interdisciplinary workup was conducted that included medical oncology, neurology, neuroradiology, neuro-oncology, neurosurgery, nuclear medicine, and radiation oncology. Neuroradiology and nuclear medicine identified a slightly hypermetabolic focus on the PET/CT from 1.5 years prior that correlated exactly with the same location as the lesion on the recent spinal MRI. This finding, along with the MRA, confirmed the diagnosis of a dAVF, which was successfully managed conservatively with dexamethasone and physical therapy, rather than through oncologic treatments such as radiotherapy
There remains debate regarding the utility of steroids in treating patients with dAVF. Although there are some case reports documenting that the edema associated with the dAVF responds to steroids, other case series have found that steroids may worsen outcomes in patients with dAVF, possibly due to increased venous hydrostatic pressure.
This case demonstrates the importance of an interdisciplinary workup when evaluating an intramedullary lesion, as well as maintaining a wide differential diagnosis, particularly in the absence of a history of polymetastatic cancer. All the clues (such as the slightly hypermetabolic focus on a PET/CT from 1.5 years prior) need to be obtained to comfortably reach a diagnosis in the absence of pathologic confirmation. These cases can be especially challenging due to the lack of pathologic confirmation, but by understanding the main differentiating features among the various etiologies and obtaining all available information, a correct diagnosis can be made without unnecessary interventions.
Discussion
A diagnosis of dural arteriovenous fistula (dAVF) was made. Lesions involving the spinal cord are traditionally classified by location as extradural, intradural/extramedullary, or intramedullary. Intramedullary spinal cord abnormalities pose considerable diagnostic and management challenges because of the risks of biopsy in this location and the added potential for morbidity and mortality from improperly treated lesions. Although MRI is the preferred imaging modality, PET/CT and magnetic resonance angiography (MRA) may also help narrow the differential diagnosis and potentially avoid complications from an invasive biopsy.1 This patient’s intramedullary lesion, which represented a dAVF, posed a diagnostic challenge; after diagnosis, it was successfully managed conservatively with dexamethasone and physical therapy.
Intradural tumors account for 2% to 4% of all primary central nervous system (CNS) tumors.2 Ependymomas account for 50% to 60% of intramedullary tumors in adults, while astrocytomas account for about 60% of all lesions in children and adolescents.3,4 The differential diagnosis for intramedullary tumors also includes hemangioblastoma, metastases, primary CNS lymphoma, germ cell tumors, and gangliogliomas.5,6
Intramedullary metastases remain rare, although the incidence is rising with improvements in oncologic and supportive treatments. Autopsy studies conducted decades ago demonstrated that about 0.9% to 2.1% of patients with systemic cancer have intramedullary metastases at death.7,8 In patients with an established history of malignancy, a metastatic intramedullary tumor should be placed higher on the differential diagnosis. Intramedullary metastases most often occur in the setting of widespread metastatic disease. A systematic review of the literature on patients with lung cancer (small cell and non-small cell lung carcinomas) and ≥ 1 intramedullary spinal cord metastasis demonstrated that 55.8% of patients had concurrent brain metastases, 20.0% had leptomeningeal carcinomatosis, and 19.5% had vertebral metastases.9 While about half of all intramedullary metastases are associated with lung cancer, other common malignancies that metastasize to this area include colorectal, breast, and renal cell carcinoma, as well as lymphoma and melanoma primaries.10,11
On imaging, intramedullary metastases often appear as several short, studded segments with surrounding edema, typically out of proportion to the size of the lesion.1 By contrast, astrocytomas and ependymomas often span multiple segments, and enhancement patterns can vary depending on the subtype and grade. Glioblastoma multiforme, or grade 4 IDH wild-type astrocytomas, demonstrate an irregular, heterogeneous pattern of enhancement. Hemangioblastomas vary in size and are classically hypointense to isointense on T1-weighted sequences, isointense to hyperintense on T2-weighted sequences, and demonstrate avid enhancement on T1- postcontrast images. In large hemangioblastomas, flow voids due to prominent vasculature may be visualized.
Numerous nonneoplastic tumor mimics can obscure the differential diagnosis. Vascular malformations, including cavernomas and dAVFs, can also present with enhancement and edema. dAVFs are the most common type of spinal vascular malformation, accounting for about 70% of cases.12 They are supplied by the radiculomeningeal arteries, whereas pial arteriovenous malformations (AVMs) are supplied by the radiculomedullary and radiculopial arteries. On MRI, dAVFs usually have venous congestion with intramedullary edema, which appears as an ill-defined centromedullary hyperintensity on T2-weighted imaging over multiple segments. The spinal cord may appear swollen with atrophic changes in chronic cases. Spinal cord AVMs are rarer and have an intramedullary nidus. They usually demonstrate mixed heterogeneous signal on T1- and T2-weighted imaging due to blood products, while the nidus demonstrates a variable degree of enhancement. Serpiginous flow voids are seen both within the nidus and at the cord surface.
Demyelinating lesions of the spine may be seen in neuroinflammatory conditions such as multiple sclerosis, neuromyelitis optica spectrum disorder, acute transverse myelitis, and acute disseminated encephalomyelitis. In multiple sclerosis, lesions typically extend ≤ 2 vertebral segments in length, cover less than half of the vertebral cross-sectional area, and have a dorsolateral predilection.13 Active lesions may demonstrate enhancement along the rim or in a patchy pattern. In the presence of demyelinating lesions, there may occasionally appear to be an expansile mass with a syrinx.14
Infections such as tuberculosis and neurosarcoidosis should also remain on the differential diagnosis. On MRI, tuberculosis usually involves the thoracic cord and is typically rim-enhancing.15 If there are caseating granulomas, T2-weighted images may also demonstrate rim enhancement.16 Spinal sarcoidosis is unusual without intracranial involvement, and its appearance may include leptomeningeal enhancement, cord expansion, and hyperintense signal on T2- weighted imaging.17
Finally, iatrogenic causes are also possible, including radiation myelopathy and mechanical spinal cord injury. For radiation myelopathy, it is important to ascertain whether a patient has undergone prior radiotherapy in the region and to obtain the pertinent dosimetry. Spinal cord injury may cause a focal signal abnormality within the cord, with T2 hyperintensity; these foci may or may not present with enhancement, edema, or hematoma and therefore may resemble tumors.13
This patient presented with progressive right-sided lower extremity weakness and hypoesthesia and a history of a low-grade right renal/pelvic ureteral tumor. The immediate impression was that the thoracic intramedullary lesion represented a metastatic lesion. However, in the absence of any systemic or intracranial metastases, this progression was much less likely. An extensive interdisciplinary workup was conducted that included medical oncology, neurology, neuroradiology, neuro-oncology, neurosurgery, nuclear medicine, and radiation oncology. Neuroradiology and nuclear medicine identified a slightly hypermetabolic focus on the PET/CT from 1.5 years prior that correlated exactly with the same location as the lesion on the recent spinal MRI. This finding, along with the MRA, confirmed the diagnosis of a dAVF, which was successfully managed conservatively with dexamethasone and physical therapy, rather than through oncologic treatments such as radiotherapy
There remains debate regarding the utility of steroids in treating patients with dAVF. Although there are some case reports documenting that the edema associated with the dAVF responds to steroids, other case series have found that steroids may worsen outcomes in patients with dAVF, possibly due to increased venous hydrostatic pressure.
This case demonstrates the importance of an interdisciplinary workup when evaluating an intramedullary lesion, as well as maintaining a wide differential diagnosis, particularly in the absence of a history of polymetastatic cancer. All the clues (such as the slightly hypermetabolic focus on a PET/CT from 1.5 years prior) need to be obtained to comfortably reach a diagnosis in the absence of pathologic confirmation. These cases can be especially challenging due to the lack of pathologic confirmation, but by understanding the main differentiating features among the various etiologies and obtaining all available information, a correct diagnosis can be made without unnecessary interventions.
- Moghaddam SM, Bhatt AA. Location, length, and enhancement: systematic approach to differentiating intramedullary spinal cord lesions. Insights Imaging. 2018;9:511-526. doi:10.1007/s13244-018-0608-3
- Grimm S, Chamberlain MC. Adult primary spinal cord tumors. Expert Rev Neurother. 2009;9:1487-1495. doi:10.1586/ern.09.101
- Miller DJ, McCutcheon IE. Hemangioblastomas and other uncommon intramedullary tumors. J Neurooncol. 2000;47:253- 270. doi:10.1023/a:1006403500801
- Mottl H, Koutecky J. Treatment of spinal cord tumors in children. Med Pediatr Oncol. 1997;29:293-295.
- Kandemirli SG, Reddy A, Hitchon P, et al. Intramedullary tumours and tumour mimics. Clin Radiol. 2020;75:876.e17-876. e32. doi:10.1016/j.crad.2020.05.010
- Tobin MK, Geraghty JR, Engelhard HH, et al. Intramedullary spinal cord tumors: a review of current and future treatment strategies. Neurosurg Focus. 2015;39:E14. doi:10.3171/2015.5.FOCUS15158
- Chason JL, Walker FB, Landers JW. Metastatic carcinoma in the central nervous system and dorsal root ganglia. A prospective autopsy study. Cancer. 1963;16:781-787.
- Costigan DA, Winkelman MD. Intramedullary spinal cord metastasis. A clinicopathological study of 13 cases. J Neurosurg. 1985;62:227-233.
- Wu L, Wang L, Yang J, et al. Clinical features, treatments, and prognosis of intramedullary spinal cord metastases from lung cancer: a case series and systematic review. Neurospine. 2022;19:65-76. doi:10.14245/ns.2142910.455
- Lv J, Liu B, Quan X, et al. Intramedullary spinal cord metastasis in malignancies: an institutional analysis and review. Onco Targets Ther. 2019;12:4741-4753. doi:10.2147/OTT.S193235
- Goyal A, Yolcu Y, Kerezoudis P, et al. Intramedullary spinal cord metastases: an institutional review of survival and outcomes. J Neurooncol. 2019;142:347-354. doi:10.1007/s11060-019-03105-2
- Krings T. Vascular malformations of the spine and spinal cord: anatomy, classification, treatment. Clin Neuroradiol. 2010;20:5-24. doi:10.1007/s00062-010-9036-6
- Maj E, Wojtowicz K, Aleksandra PP, et al. Intramedullary spinal tumor-like lesions. Acta Radiol. 2019;60:994-1010. doi:10.1177/0284185118809540
- Waziri A, Vonsattel JP, Kaiser MG, et al. Expansile, enhancing cervical cord lesion with an associated syrinx secondary to demyelination. Case report and review of the literature. J Neurosurg Spine. 2007;6:52-56. doi:10.3171/spi.2007.6.1.52
- Nussbaum ES, Rockswold GL, Bergman TA, et al. Spinal tuberculosis: a diagnostic and management challenge. J Neurosurg. 1995;83:243-247. doi:10.3171/jns.1995.83.2.0243
- Lu M. Imaging diagnosis of spinal intramedullary tuberculoma: case reports and literature review. J Spinal Cord Med. 2010;33:159-162. doi:10.1080/10790268.2010.11689691
- Do-Dai DD, Brooks MK, Goldkamp A, et al. Magnetic resonance imaging of intramedullary spinal cord lesions: a pictorial review. Curr Probl Diagn Radiol. 2010;39:160-185. doi:10.1067/j.cpradiol.2009.05.004
- Moghaddam SM, Bhatt AA. Location, length, and enhancement: systematic approach to differentiating intramedullary spinal cord lesions. Insights Imaging. 2018;9:511-526. doi:10.1007/s13244-018-0608-3
- Grimm S, Chamberlain MC. Adult primary spinal cord tumors. Expert Rev Neurother. 2009;9:1487-1495. doi:10.1586/ern.09.101
- Miller DJ, McCutcheon IE. Hemangioblastomas and other uncommon intramedullary tumors. J Neurooncol. 2000;47:253- 270. doi:10.1023/a:1006403500801
- Mottl H, Koutecky J. Treatment of spinal cord tumors in children. Med Pediatr Oncol. 1997;29:293-295.
- Kandemirli SG, Reddy A, Hitchon P, et al. Intramedullary tumours and tumour mimics. Clin Radiol. 2020;75:876.e17-876. e32. doi:10.1016/j.crad.2020.05.010
- Tobin MK, Geraghty JR, Engelhard HH, et al. Intramedullary spinal cord tumors: a review of current and future treatment strategies. Neurosurg Focus. 2015;39:E14. doi:10.3171/2015.5.FOCUS15158
- Chason JL, Walker FB, Landers JW. Metastatic carcinoma in the central nervous system and dorsal root ganglia. A prospective autopsy study. Cancer. 1963;16:781-787.
- Costigan DA, Winkelman MD. Intramedullary spinal cord metastasis. A clinicopathological study of 13 cases. J Neurosurg. 1985;62:227-233.
- Wu L, Wang L, Yang J, et al. Clinical features, treatments, and prognosis of intramedullary spinal cord metastases from lung cancer: a case series and systematic review. Neurospine. 2022;19:65-76. doi:10.14245/ns.2142910.455
- Lv J, Liu B, Quan X, et al. Intramedullary spinal cord metastasis in malignancies: an institutional analysis and review. Onco Targets Ther. 2019;12:4741-4753. doi:10.2147/OTT.S193235
- Goyal A, Yolcu Y, Kerezoudis P, et al. Intramedullary spinal cord metastases: an institutional review of survival and outcomes. J Neurooncol. 2019;142:347-354. doi:10.1007/s11060-019-03105-2
- Krings T. Vascular malformations of the spine and spinal cord: anatomy, classification, treatment. Clin Neuroradiol. 2010;20:5-24. doi:10.1007/s00062-010-9036-6
- Maj E, Wojtowicz K, Aleksandra PP, et al. Intramedullary spinal tumor-like lesions. Acta Radiol. 2019;60:994-1010. doi:10.1177/0284185118809540
- Waziri A, Vonsattel JP, Kaiser MG, et al. Expansile, enhancing cervical cord lesion with an associated syrinx secondary to demyelination. Case report and review of the literature. J Neurosurg Spine. 2007;6:52-56. doi:10.3171/spi.2007.6.1.52
- Nussbaum ES, Rockswold GL, Bergman TA, et al. Spinal tuberculosis: a diagnostic and management challenge. J Neurosurg. 1995;83:243-247. doi:10.3171/jns.1995.83.2.0243
- Lu M. Imaging diagnosis of spinal intramedullary tuberculoma: case reports and literature review. J Spinal Cord Med. 2010;33:159-162. doi:10.1080/10790268.2010.11689691
- Do-Dai DD, Brooks MK, Goldkamp A, et al. Magnetic resonance imaging of intramedullary spinal cord lesions: a pictorial review. Curr Probl Diagn Radiol. 2010;39:160-185. doi:10.1067/j.cpradiol.2009.05.004
Thoracic Intramedullary Mass Causing Neurologic Weakness
Thoracic Intramedullary Mass Causing Neurologic Weakness
An 87-year-old man presented to the emergency department reporting a 1-month history of right lower extremity weakness, progressing to an inability to ambulate. The patient had a history of hyperlipidemia, hypertension, benign prostatic hyperplasia, chronic obstructive pulmonary disease, low-grade right urothelial carcinoma status postbiopsy 2 years earlier, and atrial fibrillation following cardioversion 6 years earlier without anticoagulation therapy. He also reported severe right groin pain and increasing urinary obstruction.
On admission, neurology evaluated the patient’s lower extremity strength as 5/5 on his left, 1/5 on his right hip, and 2/5 on his right knee, with hypoesthesia of his right lower extremity. Computed tomography (CT) with contrast of the chest, abdomen, and pelvis demonstrated moderate to severe right-sided hydronephrosis, possibly due to a proximal right ureteric mass; no evidence of systemic metastases was found. He underwent a gadolinium-enhanced magnetic resonance imaging (MRI) of the cervical, thoracic, and lumbar spine, which showed a mass at T7-T8, a mass effect in the central cord, and abnormal spinal cord enhancement from T7 through the conus medullaris. A review of fluorodeoxyglucose- 18 (FDG-18) positron emission tomography (PET)-CT imaging from 1.5 years prior showed a low-grade focus (Figures 1-3). A gadolinium-enhanced brain MRI did not demonstrate any intracranial metastatic disease, acute infarct, hemorrhage, mass effect, or extra-axial fluid collections.
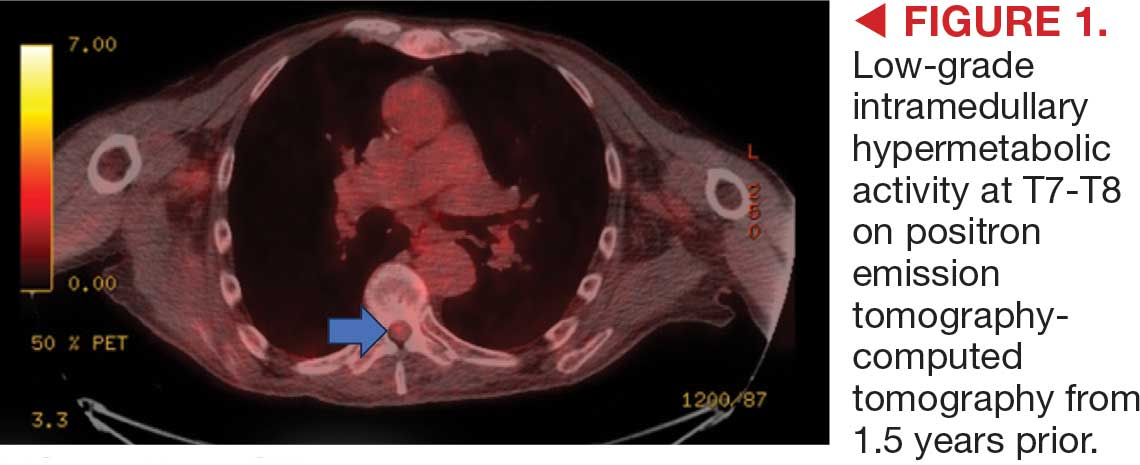


Higher Epilepsy Mortality in Posttraumatic Cases, VA Study Finds
The risk of death in patients with posttraumatic epilepsy (PTE) varies dramatically by type of brain injury, with some facing twice the mortality rate as those with other forms of epilepsy, according to a new study of Veterans Health Administration data.
Of 210,182 veterans with epilepsy followed for a median of 6 years, those who developed PTE after diffuse cerebral injury, focal cerebral injury, or skull/facial fractures had 16% to 18% higher mortality rates than veterans with nontraumatic epilepsy (NTE) the study found. Published in Neurology, the analysis was completed by Zulfi Haneef, MBBS, MD, of Baylor College of Medicine Medical Center, and colleagues.
Young patients who developed PTE after extracerebral hemorrhage faced the highest risk — double the mortality rate of those with NTE.
“These numbers are striking considering that the group against which these rates are compared — other causes of epilepsy — itself suffers from a high mortality rate,” Haneef said in an interview with Federal Practitioner. “Our findings argue for risk-stratified follow-up in PTE based on the underlying TBI [traumatic brain injury] mechanism and age at epilepsy onset.”
How Common is PTE?
PTE is defined as “long-term predisposition to developing recurrent and unprovoked seizures caused by a traumatic brain injury,” according to neurologist Edilberto Amorim, MD, of University of California at San Francisco Weill Institute for Neurosciences, who was not involved with the study but is familiar with its findings. “We do not fully understand why some people with a traumatic brain injury develop epilepsy and others do not, but the risk is higher with more severe types of TBI.”
PTE accounts for about 5% of all epilepsy cases, Amorim said. The study cites research linking PTE to mortality risk that’s 1.75 to 2.30 higher than in people without epilepsy.
Haneef said the study aimed to shed light on mortality in PTE. “Although epilepsy and TBI are each linked to higher mortality, it had never been conclusively shown that PTE specifically carries higher mortality than nontraumatic epilepsy,” he said. “We set out to answer that question in a large national veterans cohort and to see whether mortality differs by the type of antecedent TBI.”
Methodology and Findings
Researchers tracked 210,182 veterans diagnosed with epilepsy from 2005 to 2022 through the end of 2024: 28,832 with PTE (mean onset age 52.6 years, 7.4% female, 74.2% White, 16.2% Black) and 181,350 with NTE (mean onset age 60.9 years, 8.5% female, 71.0% White, 21.4% Black).
Patients with PTE were defined as having had documentation of TBI within 5 years previous to receiving an epilepsy diagnosis.
Among those with NTE (median follow-up, 6.0 years), 51.1% died. In the PTE group (median follow-up, 6.4 years), 37.3% died.
After adjustment for differences in age, sex, and comorbidities, the risk of mortality in PTE was slightly higher than in NTE (adjusted hazard ratio [aHR], 1.02); the risk was lower for the concussive TBI subtype (aHR, 0.91, both P < .05). “The underlying injury in concussion
is likely to be less severe compared with structural TBI, which may have led to the lower relative mortality observed,” the authors wrote.
However, risk of mortality in PTE was higher than in NTE for cases with underlying TBI subtypes of skull/facial fracture (aHR, 1.18), diffuse cerebral injury (aHR, 1.17), and focal cerebral injury (aHR, 1.16).
“These injuries are associated with greater structural brain damage and sustained neuroinflammation, which are factors linked to harder-to-treat (drug-resistant) epilepsy, which carries higher mortality,” Haneef said. “They may also coexist with extracranial trauma and medical comorbidity that compound long-term risk.”
Among various age groups, there was a notably higher risk of mortality linked to patients aged 18 to 39 years at onset with extracerebral PTE (aHR, 2.02, vs NTE): “In younger patients, extracerebral bleeds (eg, subdural, epidural, and subarachnoid) may reflect higher-energy trauma and more aggressive secondary cascades, amplifying epilepsy severity and longer lifetime exposure to risk. Mechanistic differences in hemorrhage types across ages may also contribute,” Haneef said.
Perspective on Findings
Amorim said the new research is “very useful,” although it has limitations that are common in large database studies. “A key point that this study highlights is the variability in the impact of TBI type on mortality and the differential risk across different age groups,” he said.
As for the higher risk in younger people, Amorim said this may be related to severity of injury: “Older patients often have TBI after falls, while younger patients are more frequently involved in traffic accidents or victims of violence,” he said
In the big picture, Amorim said, “studies like this highlight the importance of moving beyond a one-size-fits-all approach in epilepsy care. Understanding the nuances of posttraumatic epilepsy—how the type of injury, age, and other factors affect outcomes—can help us personalize treatment and counseling and maybe even guide future research into preventing or mitigating epilepsy after brain injury. New methods to automate review of medical records with higher resolution, such as large language models and natural language processing, may make this type of study with large databases even more comprehensive and impactful.”
Haneef said the findings highlight the importance of recognizing PTE as a higher-risk epilepsy and prioritizing early specialty care, especially after focal/diffuse brain injury or fracture. “Screen proactively for drug resistance and fast-track definitive therapies—surgery and device-based therapies—when indicated,” Haneef said. “Management should also include optimized antiseizure therapy, comorbidity control, and safety counseling, since many deaths may be preventable with coordinated multidisciplinary care.”
Haneef added that clinicians should “pay particular attention to younger PTE patients with extracerebral hemorrhage, who showed the greatest relative mortality.”
He also noted that the US Department of Veterans Affairs has comprehensive Epilepsy Centers of Excellence across the country.
The US Department of Defense (DoD) funded the study. Haneef discloses DoD funding, and another author discloses DoD and VA funding. Other authors have no disclosures.
Amorim discloses funding from DoD, NIH, American Heart Association, Regents of the University of California, Cures Within Reach, Zoll Foundation, and Hellman Foundation.
The risk of death in patients with posttraumatic epilepsy (PTE) varies dramatically by type of brain injury, with some facing twice the mortality rate as those with other forms of epilepsy, according to a new study of Veterans Health Administration data.
Of 210,182 veterans with epilepsy followed for a median of 6 years, those who developed PTE after diffuse cerebral injury, focal cerebral injury, or skull/facial fractures had 16% to 18% higher mortality rates than veterans with nontraumatic epilepsy (NTE) the study found. Published in Neurology, the analysis was completed by Zulfi Haneef, MBBS, MD, of Baylor College of Medicine Medical Center, and colleagues.
Young patients who developed PTE after extracerebral hemorrhage faced the highest risk — double the mortality rate of those with NTE.
“These numbers are striking considering that the group against which these rates are compared — other causes of epilepsy — itself suffers from a high mortality rate,” Haneef said in an interview with Federal Practitioner. “Our findings argue for risk-stratified follow-up in PTE based on the underlying TBI [traumatic brain injury] mechanism and age at epilepsy onset.”
How Common is PTE?
PTE is defined as “long-term predisposition to developing recurrent and unprovoked seizures caused by a traumatic brain injury,” according to neurologist Edilberto Amorim, MD, of University of California at San Francisco Weill Institute for Neurosciences, who was not involved with the study but is familiar with its findings. “We do not fully understand why some people with a traumatic brain injury develop epilepsy and others do not, but the risk is higher with more severe types of TBI.”
PTE accounts for about 5% of all epilepsy cases, Amorim said. The study cites research linking PTE to mortality risk that’s 1.75 to 2.30 higher than in people without epilepsy.
Haneef said the study aimed to shed light on mortality in PTE. “Although epilepsy and TBI are each linked to higher mortality, it had never been conclusively shown that PTE specifically carries higher mortality than nontraumatic epilepsy,” he said. “We set out to answer that question in a large national veterans cohort and to see whether mortality differs by the type of antecedent TBI.”
Methodology and Findings
Researchers tracked 210,182 veterans diagnosed with epilepsy from 2005 to 2022 through the end of 2024: 28,832 with PTE (mean onset age 52.6 years, 7.4% female, 74.2% White, 16.2% Black) and 181,350 with NTE (mean onset age 60.9 years, 8.5% female, 71.0% White, 21.4% Black).
Patients with PTE were defined as having had documentation of TBI within 5 years previous to receiving an epilepsy diagnosis.
Among those with NTE (median follow-up, 6.0 years), 51.1% died. In the PTE group (median follow-up, 6.4 years), 37.3% died.
After adjustment for differences in age, sex, and comorbidities, the risk of mortality in PTE was slightly higher than in NTE (adjusted hazard ratio [aHR], 1.02); the risk was lower for the concussive TBI subtype (aHR, 0.91, both P < .05). “The underlying injury in concussion
is likely to be less severe compared with structural TBI, which may have led to the lower relative mortality observed,” the authors wrote.
However, risk of mortality in PTE was higher than in NTE for cases with underlying TBI subtypes of skull/facial fracture (aHR, 1.18), diffuse cerebral injury (aHR, 1.17), and focal cerebral injury (aHR, 1.16).
“These injuries are associated with greater structural brain damage and sustained neuroinflammation, which are factors linked to harder-to-treat (drug-resistant) epilepsy, which carries higher mortality,” Haneef said. “They may also coexist with extracranial trauma and medical comorbidity that compound long-term risk.”
Among various age groups, there was a notably higher risk of mortality linked to patients aged 18 to 39 years at onset with extracerebral PTE (aHR, 2.02, vs NTE): “In younger patients, extracerebral bleeds (eg, subdural, epidural, and subarachnoid) may reflect higher-energy trauma and more aggressive secondary cascades, amplifying epilepsy severity and longer lifetime exposure to risk. Mechanistic differences in hemorrhage types across ages may also contribute,” Haneef said.
Perspective on Findings
Amorim said the new research is “very useful,” although it has limitations that are common in large database studies. “A key point that this study highlights is the variability in the impact of TBI type on mortality and the differential risk across different age groups,” he said.
As for the higher risk in younger people, Amorim said this may be related to severity of injury: “Older patients often have TBI after falls, while younger patients are more frequently involved in traffic accidents or victims of violence,” he said
In the big picture, Amorim said, “studies like this highlight the importance of moving beyond a one-size-fits-all approach in epilepsy care. Understanding the nuances of posttraumatic epilepsy—how the type of injury, age, and other factors affect outcomes—can help us personalize treatment and counseling and maybe even guide future research into preventing or mitigating epilepsy after brain injury. New methods to automate review of medical records with higher resolution, such as large language models and natural language processing, may make this type of study with large databases even more comprehensive and impactful.”
Haneef said the findings highlight the importance of recognizing PTE as a higher-risk epilepsy and prioritizing early specialty care, especially after focal/diffuse brain injury or fracture. “Screen proactively for drug resistance and fast-track definitive therapies—surgery and device-based therapies—when indicated,” Haneef said. “Management should also include optimized antiseizure therapy, comorbidity control, and safety counseling, since many deaths may be preventable with coordinated multidisciplinary care.”
Haneef added that clinicians should “pay particular attention to younger PTE patients with extracerebral hemorrhage, who showed the greatest relative mortality.”
He also noted that the US Department of Veterans Affairs has comprehensive Epilepsy Centers of Excellence across the country.
The US Department of Defense (DoD) funded the study. Haneef discloses DoD funding, and another author discloses DoD and VA funding. Other authors have no disclosures.
Amorim discloses funding from DoD, NIH, American Heart Association, Regents of the University of California, Cures Within Reach, Zoll Foundation, and Hellman Foundation.
The risk of death in patients with posttraumatic epilepsy (PTE) varies dramatically by type of brain injury, with some facing twice the mortality rate as those with other forms of epilepsy, according to a new study of Veterans Health Administration data.
Of 210,182 veterans with epilepsy followed for a median of 6 years, those who developed PTE after diffuse cerebral injury, focal cerebral injury, or skull/facial fractures had 16% to 18% higher mortality rates than veterans with nontraumatic epilepsy (NTE) the study found. Published in Neurology, the analysis was completed by Zulfi Haneef, MBBS, MD, of Baylor College of Medicine Medical Center, and colleagues.
Young patients who developed PTE after extracerebral hemorrhage faced the highest risk — double the mortality rate of those with NTE.
“These numbers are striking considering that the group against which these rates are compared — other causes of epilepsy — itself suffers from a high mortality rate,” Haneef said in an interview with Federal Practitioner. “Our findings argue for risk-stratified follow-up in PTE based on the underlying TBI [traumatic brain injury] mechanism and age at epilepsy onset.”
How Common is PTE?
PTE is defined as “long-term predisposition to developing recurrent and unprovoked seizures caused by a traumatic brain injury,” according to neurologist Edilberto Amorim, MD, of University of California at San Francisco Weill Institute for Neurosciences, who was not involved with the study but is familiar with its findings. “We do not fully understand why some people with a traumatic brain injury develop epilepsy and others do not, but the risk is higher with more severe types of TBI.”
PTE accounts for about 5% of all epilepsy cases, Amorim said. The study cites research linking PTE to mortality risk that’s 1.75 to 2.30 higher than in people without epilepsy.
Haneef said the study aimed to shed light on mortality in PTE. “Although epilepsy and TBI are each linked to higher mortality, it had never been conclusively shown that PTE specifically carries higher mortality than nontraumatic epilepsy,” he said. “We set out to answer that question in a large national veterans cohort and to see whether mortality differs by the type of antecedent TBI.”
Methodology and Findings
Researchers tracked 210,182 veterans diagnosed with epilepsy from 2005 to 2022 through the end of 2024: 28,832 with PTE (mean onset age 52.6 years, 7.4% female, 74.2% White, 16.2% Black) and 181,350 with NTE (mean onset age 60.9 years, 8.5% female, 71.0% White, 21.4% Black).
Patients with PTE were defined as having had documentation of TBI within 5 years previous to receiving an epilepsy diagnosis.
Among those with NTE (median follow-up, 6.0 years), 51.1% died. In the PTE group (median follow-up, 6.4 years), 37.3% died.
After adjustment for differences in age, sex, and comorbidities, the risk of mortality in PTE was slightly higher than in NTE (adjusted hazard ratio [aHR], 1.02); the risk was lower for the concussive TBI subtype (aHR, 0.91, both P < .05). “The underlying injury in concussion
is likely to be less severe compared with structural TBI, which may have led to the lower relative mortality observed,” the authors wrote.
However, risk of mortality in PTE was higher than in NTE for cases with underlying TBI subtypes of skull/facial fracture (aHR, 1.18), diffuse cerebral injury (aHR, 1.17), and focal cerebral injury (aHR, 1.16).
“These injuries are associated with greater structural brain damage and sustained neuroinflammation, which are factors linked to harder-to-treat (drug-resistant) epilepsy, which carries higher mortality,” Haneef said. “They may also coexist with extracranial trauma and medical comorbidity that compound long-term risk.”
Among various age groups, there was a notably higher risk of mortality linked to patients aged 18 to 39 years at onset with extracerebral PTE (aHR, 2.02, vs NTE): “In younger patients, extracerebral bleeds (eg, subdural, epidural, and subarachnoid) may reflect higher-energy trauma and more aggressive secondary cascades, amplifying epilepsy severity and longer lifetime exposure to risk. Mechanistic differences in hemorrhage types across ages may also contribute,” Haneef said.
Perspective on Findings
Amorim said the new research is “very useful,” although it has limitations that are common in large database studies. “A key point that this study highlights is the variability in the impact of TBI type on mortality and the differential risk across different age groups,” he said.
As for the higher risk in younger people, Amorim said this may be related to severity of injury: “Older patients often have TBI after falls, while younger patients are more frequently involved in traffic accidents or victims of violence,” he said
In the big picture, Amorim said, “studies like this highlight the importance of moving beyond a one-size-fits-all approach in epilepsy care. Understanding the nuances of posttraumatic epilepsy—how the type of injury, age, and other factors affect outcomes—can help us personalize treatment and counseling and maybe even guide future research into preventing or mitigating epilepsy after brain injury. New methods to automate review of medical records with higher resolution, such as large language models and natural language processing, may make this type of study with large databases even more comprehensive and impactful.”
Haneef said the findings highlight the importance of recognizing PTE as a higher-risk epilepsy and prioritizing early specialty care, especially after focal/diffuse brain injury or fracture. “Screen proactively for drug resistance and fast-track definitive therapies—surgery and device-based therapies—when indicated,” Haneef said. “Management should also include optimized antiseizure therapy, comorbidity control, and safety counseling, since many deaths may be preventable with coordinated multidisciplinary care.”
Haneef added that clinicians should “pay particular attention to younger PTE patients with extracerebral hemorrhage, who showed the greatest relative mortality.”
He also noted that the US Department of Veterans Affairs has comprehensive Epilepsy Centers of Excellence across the country.
The US Department of Defense (DoD) funded the study. Haneef discloses DoD funding, and another author discloses DoD and VA funding. Other authors have no disclosures.
Amorim discloses funding from DoD, NIH, American Heart Association, Regents of the University of California, Cures Within Reach, Zoll Foundation, and Hellman Foundation.
Staff Perspectives on the VISN 20 Tele-Neuropsychology Program
Staff Perspectives on the VISN 20 Tele-Neuropsychology Program
There are 2.7 million (48%) rural veterans enrolled in the Veterans Health Administration (VHA).1 Many VHA-enrolled rural veterans are aged ≥ 65 years (54%), a medically complex population that requires more extensive health care.1 These veterans may live far from US Department of Veterans Affairs (VA) medical centers (VAMCs) and often receive most of their care at rural community-based outpatient clinics (CBOCs). In addition to face-to-face (F2F) services provided at these clinics, many patient care needs may be met using telehealth technology, which can connect veterans at CBOCs with remote health care practitioners (HCPs).
This technology is used across medical specialties throughout the VA and has expanded into neuropsychology services to improve access amid the shortage of rural neuropsychologists. Prior research suggests that access to neuropsychology services improves the functional outcomes of people with diverse medical conditions, including dementia, brain injury, and epilepsy, and reduces emergency department visits, hospitalization duration, and health care costs.2-6 Given that veterans unable to access neuropsychology services may be at risk for poorer outcomes, identifying ways to improve access is a priority. Tele-neuropsychology (teleNP) has been used to expand access for rural veterans in need of these services.7,8
TeleNP is the application of audiovisual technologies to enable remote clinical encounters for neuropsychological assessments.9 TeleNP has been shown to be generally equivalent to F2F care, without significant differences compared with in-person visits.10-13 TeleNP was increasingly implemented following the COVID-19 pandemic and remains an enduring and expanding feature of neuropsychology care delivery.8,14-18 TeleNP services can increase access to care, especially for rural veterans and those with limited transportation.
Research in non-VA samples suggests a high level of clinician satisfaction with teleNP.16 In VA samples, research has found high levels of patient satisfaction with teleNP both within Veterans Integrated Services Network (VISN) 20 and in a VA health care system outside VISN 20.7,19 Investigating staff perceptions of these services and their utility compared with non-VA F2F visits is pertinent to the overall feasibility and effectiveness of teleNP.
TELE-NEUROPSYCHOLOGY PROGRAM
A clinical resource hub (CRH) is a VISN-governed program that provides veteran health care when local VHA facilities have service gaps.20,21 CRH 20 serves several Pacific Northwest VISN 20 health care systems and began providing teleNP in 2015. The CRH 20 teleNP service serves older adults in rural settings with > 570 teleNP evaluations completed over a recent 12-month period (May 2023 to May 2024). In the CRH 20 teleNP program, veterans are offered services by CRH 20 neuropsychologists via telehealth to a patient’s local VAMC, larger health care clinic, CBOC, or via Veterans Video Connect to the home.
Referral pathways to the CRH 20 teleNP program differ across sites. For VISN 20 sites that do not have any in-house neuropsychology services, referrals are initiated by HCPs from any discipline. At 2 sites with in-house neuropsychology programs, CRH 20 teleNP referrals typically are forwarded from the inhouse service whenever the veteran prefers to be seen at an outlying clinic. All sites, including the CBOCs, are equipped fully for testing, and the HCP encounters veterans in a private office via video-based telehealth technology after a telehealth technician orients them to the space. The private office minimizes environmental disruptions and uses standardized technology to ensure valid results. A limited number of evaluations are offered at home (< 5% of the evaluations) if the veteran is unable to come to a VHA facility, has access to reliable internet, and a minimally distracting home setting.
In VISN 20, teleNP is a routine practice for delivering services to rural sites, most of which lack neuropsychologists. However, there is limited information about the extent to which the referral sources find the service useful. This quality improvement (QI) project aimed to better understand how well-established teleNP services were received by referral sources/stakeholders and how services could be improved. Prior to the advent of the CRH 20 teleNP program, staff had the option of referring for F2F evaluations in the local community (outside the VA) at some sites, an option that remains. This QI project examined staff perspectives on the usefulness of CRH 20 teleNP services compared with non-VA F2F services. We administered an anonymous, confidential survey examining these factors to VISN 20 staff within 4 VA health care systems.
METHODS
This QI project used a mixed quantitative and qualitative descriptive survey design to elicit feedback. The authors (3 neuropsychologists, 1 geropsychologist, and 1 research coordinator) developed the survey questions. The 13-question survey was voluntary, anonymous, and confidential, and respondents were given an opportunity to ask questions, with the first author serving as the point of contact.
The survey ascertained information about respondents and their work setting (ie, facility type, specific work setting and location, profession, and rurality of patients). First respondents were asked whether they have referred patients to neuropsychology services in the past year. Those who had not referred patients during the past year were asked about reasons for nonreferral with an option to provide an open-ended response. Respondents who did refer were asked how they refer for neuropsychology services and about the usefulness and timeliness of both teleNP and non-VA F2F services. Respondents were asked to respond with their preference for teleNP vs non-VA F2F with an open-ended prompt. Finally, respondents were invited to share any feedback for improvement regarding teleNP services.
A link to the survey, hosted on the VA Research Electronic Data Capture system, was emailed to facility and service line leaders at the 4 VISN 20 health care systems for distribution to the staff. All staff were included because in many of the facilities, particularly those that are highly rural with low staffing, it is not uncommon for technicians, nurses, and other support staff to assist with placing consults. In particular, VISN 20 nurses often have an optimal understanding of referral pathways to care for patients and are positioned to give and receive feedback about the utility of neuropsychological evaluations. The Research and Development Committee at the Boise VA Medical Center determined this project to be QI and exempt from institutional review board oversight. The VISN 20 employee labor relations HR supervisor approved this survey, with union awareness. Responses were anonymous.
Data were imported into Microsoft Excel and IBM SPSS Statistics for further analysis. Data were summarized using descriptive statistics, frequencies, and percentages. Nonparametric χ2 and Wilcoxon signed-rank tests were used to test for differences. An inductive approach to develop codes was used for the 3 open-ended questions. Two authors (CC, CEG) independently coded the responses and reviewed discrepancies. Final code applications were based on consensus.
RESULTS
The survey was deployed for 1 month between February 7, 2024, and June 15, 2024, at each of the 4 health care systems. Thirty-three staff members responded; of these, 1 person did not respond to an item on whether they referred for neuropsychology services. Eighteen of 33 respondents reported referring patients to teleNP or F2F neuropsychology services in the past year. Fourteen of the 33 respondents stated they did not refer; of these, 2 were unfamiliar with the teleNP service and 12 provided other reasons (eg, new to VA, not in their professional scope to order consults, did not have patients needing services).
The analysis focused on the 18 respondents who referred for neuropsychology services. Thirteen were within health care system A, and 5 were within health care system B (which had no nearby non-VA contracted neuropsychology services) and none were in the other 2 health care systems. Ten of 18 respondents (56%) stated they practiced primarily in a rural setting. Five respondents worked in a CBOC, 12 in a main VA facility, 9 in a primary care setting, 8 in a mental health setting, and 3 in other settings (eg, domiciliary). Participants could select > 1 setting. The 18 respondents who referred to neuropsychology services included 7 psychologists, 1 nurse, 2 social workers, 1 social services assistant, 4 nurse practitioners, 2 physicians, and 1 unknown HCP.
When asked to categorize the usefulness of services, more respondents characterized teleNP as very much so (1 on a 5-point scale) than F2F referrals (Figure). The mean (SD) of 1.5 (0.8) for teleNP usefulness fell between very much so and mostly and 1 respondent indicated not applicable. Similarly, the mean (SD) for non-VA F2F usefulness was 1.7 (0.9); 9 respondents rated this item as not applicable. A Wilcoxon signed-rank test of related samples indicated no significant differences between the pairs of ratings (Z = 1.50; P = .41).
Respondents with rural patients were more likely to refer them to teleNP services compared with respondents with nonrural patients (χ2 = 5.7; P = .02). However, ratings of teleNP usefulness did not significantly differ for those serving rural vs with nonrural patients (χ2 = 1.4; P = .49). Mean (SD) rating of teleNP usefulness was 1.3 (0.7) for the 9 rural subgroup respondents (between very much so and mostly) vs 1.8 (0.9) for the 8 nonrural subgroup respondents (between very much so and mostly). The mean (SD) rating for non-VA F2F usefulness was 1.8 (1.0) for the 4 rural subgroup respondents and 1.6 (0.8) for the 5 nonrural subgroup, between very much so and mostly for both groups.
Most respondents had no preference between teleNP or F2F. Notably, the responses underlying this group were multifaceted and corresponded to multiple codes (ie, access, preference for in-person services, technology, space and logistics, and service boundaries and requirements). According to 1 respondent, “the logistics of scheduling/room availability, technological challenges, and client behavioral issues that are likely to occur could possibly be more easily addressed via in-person sessions for some clients and providers.”
Six of 18 respondents preferred teleNP, citing timeliness, ease of access, and evaluation quality. One respondent noted that the “majority of my veterans live in extremely remote areas” and may need to take a plane for their visit. The 3 respondents who preferred in-person neuropsychology services cited veterans’ preference for in-person services.
Open-Ended Feedback
Thirteen respondents offered feedback on what is working well with teleNP services. Reasons mentioned were related to the service (ie, timeliness, access, quality) and the neuropsychologist (ie, communication and HCP skills). One respondent described the service and neuropsychologists positively, stating that they were “responsive, notes are readily available, clear assessments and recommendations, being available by [Microsoft] Teams/email.”
Ten respondents provided suggestions for improvement. Suggestions focused on expanding services, such as to “all veterans with cognitive/memory concerns that desire testing,” individuals with attention-deficit/hyperactivity disorder and co-occurring mental health concerns, and those in residential programs. Suggestions included hiring psychology technicians or more staff and providing education at local clinics.
DISCUSSION
This QI project examines VA staff perspectives on the usefulness of CRH 20 teleNP services and non-VA F2F services. While the small sample size limits generalizability, this preliminary study suggests that VA teleNP evaluations were similar to those conducted F2F in non-VA settings. While ratings of teleNP usefulness did not differ significantly for those serving rural vs nonrural veterans, respondents serving rural patients were more likely to refer patients to teleNP, suggesting that teleNP may increase access in rural settings, consistent with other studies.7,8,13 This article also presents qualitative suggestions for improving teleNP delivery within the VHA. This is the first known initiative to report on VHA staff satisfaction with a teleNP service and expands the limited literature to date on satisfaction with teleNP services. The findings provide initial support for continued use and, potentially, expansion of teleNP services within this CRH remote hub-and-spoke model.
Limitations
A significant limitation of the current work is the small sample size of survey respondents. In particular, while teleNP turnaround time was perceived as faster than non-VA F2F care, only 8 respondents reported on timeliness of F2F evaluation results, which renders it difficult to draw conclusions. Interestingly, not all respondents reported referring to neuropsychology services within the previous year; the most common reasons reflect the perception that referral to neuropsychology was outside of that staff member’s role or not clinically indicated.
One additional possible explanation for the absence of reporting on utility of teleNP specifically is that respondents did not track whether their patient was seen by teleNP or F2F services, based on how the referral process varies at each health care system. For example, in health care system C, a large number of referrals are forwarded to the service by local VA F2F neuropsychologists. This may speak to the seamlessness of the teleNP process, such that local staff and/or referring HCPs are unaware of the modality over which neuropsychology is being conducted. It is plausible that the reason behind this smaller response rate in health care systems B and C relates to how neuropsychology consults are processed at these local VAMCs. We suspect that in these settings, the HCPs referring for neuropsychological evaluations (eg, primary care, mental health) may be unaware that their referrals are being triaged to neuropsychologists in a different program (CRH 20 teleNP). Therefore, they would not necessarily know that they used teleNP and didn’t complete the survey.
The referral process for these 2 sites contrasts with the process for other VISN 20 sites where there is no local neuropsychology program triaging. In these settings, referrals from local HCPs come directly to teleNP; thus, it is more likely that these HCPs are aware of teleNP services. There were only 2 physicians who completed the survey, which may relate to their workload and a workflow where other staff have been increasingly requested to order the consults for the physician. This type of workflow results in an increase in the number of VHA staff involved in patient care. Ratings of usefulness were highest in health care system B, which does not have neuropsychology services at the facility or in the community; this may relate to elevated teleNP satisfaction ratings.
Further work may help identify which aspects of a teleNP service make it more useful than F2F care for this population or determine whether there were HCPor setting-specific factors that influenced the ratings (ie, preference for VA care or comparison of favorability ratings for the HCPs who conduct teleNP and F2F within the same system). The latter comparisons could not be drawn in the current systems due to the absence of HCPs who provide both teleNP and F2F modalities within VISN 20. Another consideration for future work would be to use a previously published/validated survey measure and piloting of questions with a naive sample before implementation.
CONCLUSIONS
This analysis provides initial support for feasibility and acceptability of teleNP as an alternative to traditional in-person neuropsychological evaluations. The small number of survey respondents may reflect the multiple pathways through which consults are forwarded to CRH 20, which includes both direct HCP referrals and forwarded consults from local neuropsychology services. CRH 20 has completed > 570 teleNP evaluations within 1 year, suggesting that lack of awareness may not be hindering veteran access to the service. Replication with a larger sample that is more broadly representative of key stakeholders in veteran care, identification of populations that would benefit most from teleNP services, and dissemination studies of the expansion of teleNP services are all important directions for future work. The robustness and longevity of the VISN 20 teleNP program, coupled with the preliminary positive findings from this project, demonstrate support for further assessment of the potential impact of telehealth on neuropsychological care within the VHA and show that barriers associated with access to health care services in remote settings may be mitigated through teleNP service delivery.
- US Department of Veterans Affairs, Office of Rural Health. Rural veterans. Updated March 10, 2025. Accessed July 7, 2025. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- Braun M, Tupper D, Kaufmann P, et al. Neuropsychological assessment: a valuable tool in the diagnosis and management of neurological, neurodevelopmental, medical, and psychiatric disorders. Cogn Behav Neurol. 2011;24(3):107-114. doi:10.1097/wnn.0b013e3182351289
- Donders J. The incremental value of neuropsychological assessment: a critical review. Clin Neuropsychol. 2020;34(1):56-87. doi:10.1080/13854046.2019.1575471
- Williams MW, Rapport LJ, Hanks RA, et al. Incremental value of neuropsychological evaluations to computed tomography in predicting long-term outcomes after traumatic brain injury. Clin Neuropsychol. 2013;27(3):356-375. doi:10.1080/13854046.2013.765507
- Sieg E, Mai Q, Mosti C, Brook M. The utility of neuropsychological consultation in identifying medical inpatients with suspected cognitive impairment at risk for greater hospital utilization. Clin Neuropsychol. 2019;33(1):75-89. doi:10.1080/13854046.2018.1465124
- Vankirk KM, Horner MD, Turner TH, et al. CE hospital service utilization is reduced following neuropsychological evaluation in a sample of U.S. veterans. Clin Neuropsychol. 2013;27(5):750-761. doi:10.1080/13854046.2013.783122
- Appleman ER, O’Connor MK, Boucher SJ, et al. Teleneuropsychology clinic development and patient satisfaction. Clin Neuropsychol. 2021;35(4):819-837. doi:10.1080/13854046.2020.1871515
- Stelmokas J, Ratcliffe LN, Lengu K, et al. Evaluation of teleneuropsychology services in veterans during COVID-19. Psychol Serv. 2024;21(1):65-72. doi:10.1037/ser0000810
- Bilder R Postal KS, Barisa M, et al. Inter Organizational Practice Committee recommendations/guidance for teleneuropsychology in response to the COVID-19 pandemic. Arch Clin Neuropsychol. 2020;35(6):647-659. doi:10.1093/arclin/acaa046
- Brearly TW, Shura RD, Martindale SL, et al. Neuropsychological test administration by videoconference: a systematic review and meta-analysis. Neuropsychol Rev. 2017;27(2):174-186. doi:10.1007/s11065-017-9349-1
- Brown AD, Kelso W, Eratne D, et al. Investigating equivalence of in-person and telehealth-based neuropsychological assessment performance for individuals being investigated for younger onset dementia. Arch Clin Neuropsychol. 2024;39(5):594-607. doi:10.1093/arclin/acad108
- Chapman JE, Ponsford J, Bagot KL, et al. The use of videoconferencing in clinical neuropsychology practice: a mixed methods evaluation of neuropsychologists’ experiences and views. Aust Psychol. 2020;55(6):618-633. doi:10.1111/ap.12471
- Marra DE, Hamlet KM, Bauer RM, et al. Validity of teleneuropsychology for older adults in response to COVID-19: a systematic and critical review. Clin Neuropsychol. 2020;34:1411-1452. doi:10.1080/13854046.2020.1769192
- Hammers DB, Stolwyk R, Harder L, et al. A survey of international clinical teleneuropsychology service provision prior to COVID-19. Clin Neuropsychol. 2020;34(7-8):1267- 1283. doi:10.1080/13854046.2020.1810323
- Marra DE, Hoelzle JB, Davis JJ, et al. Initial changes in neuropsychologists’ clinical practice during the COVID-19 pandemic: a survey study. Clin Neuropsychol. 2020;34(7- 8):1251-1266. doi:10.1080/13854046.2020.1800098
- Parsons MW, Gardner MM, Sherman, JC et al. Feasibility and acceptance of direct-to-home teleneuropsychology services during the COVID-19 pandemic. J Int Neuropsychol Soc. 2022;28(2):210-215. doi:10.1017/s1355617721000436
- Rochette AD, Rahman-Filipiak A, Spencer RJ, et al. Teleneuropsychology practice survey during COVID-19 within the United States. Appl Neuropsychol Adult. 2022;29(6):1312- 1322. doi:10.1080/23279095.2021.1872576
- Messler AC, Hargrave DD, Trittschuh EH, et al. National survey of telehealth neuropsychology practices: current attitudes, practices, and relevance of tele-neuropsychology three years after the onset of COVID-19. Clin Neuropsychol. 2023;39:1017-1036. doi:10.1080/13854046.2023.2192422
- Rautman L, Sordahl JA. Veteran satisfaction with tele-neuropsychology services. Clin Neuropsychol. 2018;32:1453949. doi:10.1080/13854046.2018.1453949
- US Department of Veterans Affairs. Patient care services: clinical resource hubs. Updated March 20, 2024. Accessed August 4, 2025. https://www.patientcare .va.gov/primarycare/CRH.asp
- Burnett K, Stockdale SE, Yoon J, et al. The Clinical Resource Hub initiative: first-year implementation of the Veterans Health Administration regional telehealth contingency staffing program. Ambul Care Manage. 2023;46(3):228-239. doi:10.1097/JAC.0000000000000468
There are 2.7 million (48%) rural veterans enrolled in the Veterans Health Administration (VHA).1 Many VHA-enrolled rural veterans are aged ≥ 65 years (54%), a medically complex population that requires more extensive health care.1 These veterans may live far from US Department of Veterans Affairs (VA) medical centers (VAMCs) and often receive most of their care at rural community-based outpatient clinics (CBOCs). In addition to face-to-face (F2F) services provided at these clinics, many patient care needs may be met using telehealth technology, which can connect veterans at CBOCs with remote health care practitioners (HCPs).
This technology is used across medical specialties throughout the VA and has expanded into neuropsychology services to improve access amid the shortage of rural neuropsychologists. Prior research suggests that access to neuropsychology services improves the functional outcomes of people with diverse medical conditions, including dementia, brain injury, and epilepsy, and reduces emergency department visits, hospitalization duration, and health care costs.2-6 Given that veterans unable to access neuropsychology services may be at risk for poorer outcomes, identifying ways to improve access is a priority. Tele-neuropsychology (teleNP) has been used to expand access for rural veterans in need of these services.7,8
TeleNP is the application of audiovisual technologies to enable remote clinical encounters for neuropsychological assessments.9 TeleNP has been shown to be generally equivalent to F2F care, without significant differences compared with in-person visits.10-13 TeleNP was increasingly implemented following the COVID-19 pandemic and remains an enduring and expanding feature of neuropsychology care delivery.8,14-18 TeleNP services can increase access to care, especially for rural veterans and those with limited transportation.
Research in non-VA samples suggests a high level of clinician satisfaction with teleNP.16 In VA samples, research has found high levels of patient satisfaction with teleNP both within Veterans Integrated Services Network (VISN) 20 and in a VA health care system outside VISN 20.7,19 Investigating staff perceptions of these services and their utility compared with non-VA F2F visits is pertinent to the overall feasibility and effectiveness of teleNP.
TELE-NEUROPSYCHOLOGY PROGRAM
A clinical resource hub (CRH) is a VISN-governed program that provides veteran health care when local VHA facilities have service gaps.20,21 CRH 20 serves several Pacific Northwest VISN 20 health care systems and began providing teleNP in 2015. The CRH 20 teleNP service serves older adults in rural settings with > 570 teleNP evaluations completed over a recent 12-month period (May 2023 to May 2024). In the CRH 20 teleNP program, veterans are offered services by CRH 20 neuropsychologists via telehealth to a patient’s local VAMC, larger health care clinic, CBOC, or via Veterans Video Connect to the home.

Referral pathways to the CRH 20 teleNP program differ across sites. For VISN 20 sites that do not have any in-house neuropsychology services, referrals are initiated by HCPs from any discipline. At 2 sites with in-house neuropsychology programs, CRH 20 teleNP referrals typically are forwarded from the inhouse service whenever the veteran prefers to be seen at an outlying clinic. All sites, including the CBOCs, are equipped fully for testing, and the HCP encounters veterans in a private office via video-based telehealth technology after a telehealth technician orients them to the space. The private office minimizes environmental disruptions and uses standardized technology to ensure valid results. A limited number of evaluations are offered at home (< 5% of the evaluations) if the veteran is unable to come to a VHA facility, has access to reliable internet, and a minimally distracting home setting.
In VISN 20, teleNP is a routine practice for delivering services to rural sites, most of which lack neuropsychologists. However, there is limited information about the extent to which the referral sources find the service useful. This quality improvement (QI) project aimed to better understand how well-established teleNP services were received by referral sources/stakeholders and how services could be improved. Prior to the advent of the CRH 20 teleNP program, staff had the option of referring for F2F evaluations in the local community (outside the VA) at some sites, an option that remains. This QI project examined staff perspectives on the usefulness of CRH 20 teleNP services compared with non-VA F2F services. We administered an anonymous, confidential survey examining these factors to VISN 20 staff within 4 VA health care systems.
METHODS
This QI project used a mixed quantitative and qualitative descriptive survey design to elicit feedback. The authors (3 neuropsychologists, 1 geropsychologist, and 1 research coordinator) developed the survey questions. The 13-question survey was voluntary, anonymous, and confidential, and respondents were given an opportunity to ask questions, with the first author serving as the point of contact.
The survey ascertained information about respondents and their work setting (ie, facility type, specific work setting and location, profession, and rurality of patients). First respondents were asked whether they have referred patients to neuropsychology services in the past year. Those who had not referred patients during the past year were asked about reasons for nonreferral with an option to provide an open-ended response. Respondents who did refer were asked how they refer for neuropsychology services and about the usefulness and timeliness of both teleNP and non-VA F2F services. Respondents were asked to respond with their preference for teleNP vs non-VA F2F with an open-ended prompt. Finally, respondents were invited to share any feedback for improvement regarding teleNP services.
A link to the survey, hosted on the VA Research Electronic Data Capture system, was emailed to facility and service line leaders at the 4 VISN 20 health care systems for distribution to the staff. All staff were included because in many of the facilities, particularly those that are highly rural with low staffing, it is not uncommon for technicians, nurses, and other support staff to assist with placing consults. In particular, VISN 20 nurses often have an optimal understanding of referral pathways to care for patients and are positioned to give and receive feedback about the utility of neuropsychological evaluations. The Research and Development Committee at the Boise VA Medical Center determined this project to be QI and exempt from institutional review board oversight. The VISN 20 employee labor relations HR supervisor approved this survey, with union awareness. Responses were anonymous.
Data were imported into Microsoft Excel and IBM SPSS Statistics for further analysis. Data were summarized using descriptive statistics, frequencies, and percentages. Nonparametric χ2 and Wilcoxon signed-rank tests were used to test for differences. An inductive approach to develop codes was used for the 3 open-ended questions. Two authors (CC, CEG) independently coded the responses and reviewed discrepancies. Final code applications were based on consensus.
RESULTS
The survey was deployed for 1 month between February 7, 2024, and June 15, 2024, at each of the 4 health care systems. Thirty-three staff members responded; of these, 1 person did not respond to an item on whether they referred for neuropsychology services. Eighteen of 33 respondents reported referring patients to teleNP or F2F neuropsychology services in the past year. Fourteen of the 33 respondents stated they did not refer; of these, 2 were unfamiliar with the teleNP service and 12 provided other reasons (eg, new to VA, not in their professional scope to order consults, did not have patients needing services).
The analysis focused on the 18 respondents who referred for neuropsychology services. Thirteen were within health care system A, and 5 were within health care system B (which had no nearby non-VA contracted neuropsychology services) and none were in the other 2 health care systems. Ten of 18 respondents (56%) stated they practiced primarily in a rural setting. Five respondents worked in a CBOC, 12 in a main VA facility, 9 in a primary care setting, 8 in a mental health setting, and 3 in other settings (eg, domiciliary). Participants could select > 1 setting. The 18 respondents who referred to neuropsychology services included 7 psychologists, 1 nurse, 2 social workers, 1 social services assistant, 4 nurse practitioners, 2 physicians, and 1 unknown HCP.
When asked to categorize the usefulness of services, more respondents characterized teleNP as very much so (1 on a 5-point scale) than F2F referrals (Figure). The mean (SD) of 1.5 (0.8) for teleNP usefulness fell between very much so and mostly and 1 respondent indicated not applicable. Similarly, the mean (SD) for non-VA F2F usefulness was 1.7 (0.9); 9 respondents rated this item as not applicable. A Wilcoxon signed-rank test of related samples indicated no significant differences between the pairs of ratings (Z = 1.50; P = .41).
Respondents with rural patients were more likely to refer them to teleNP services compared with respondents with nonrural patients (χ2 = 5.7; P = .02). However, ratings of teleNP usefulness did not significantly differ for those serving rural vs with nonrural patients (χ2 = 1.4; P = .49). Mean (SD) rating of teleNP usefulness was 1.3 (0.7) for the 9 rural subgroup respondents (between very much so and mostly) vs 1.8 (0.9) for the 8 nonrural subgroup respondents (between very much so and mostly). The mean (SD) rating for non-VA F2F usefulness was 1.8 (1.0) for the 4 rural subgroup respondents and 1.6 (0.8) for the 5 nonrural subgroup, between very much so and mostly for both groups.
Most respondents had no preference between teleNP or F2F. Notably, the responses underlying this group were multifaceted and corresponded to multiple codes (ie, access, preference for in-person services, technology, space and logistics, and service boundaries and requirements). According to 1 respondent, “the logistics of scheduling/room availability, technological challenges, and client behavioral issues that are likely to occur could possibly be more easily addressed via in-person sessions for some clients and providers.”
Six of 18 respondents preferred teleNP, citing timeliness, ease of access, and evaluation quality. One respondent noted that the “majority of my veterans live in extremely remote areas” and may need to take a plane for their visit. The 3 respondents who preferred in-person neuropsychology services cited veterans’ preference for in-person services.
Open-Ended Feedback
Thirteen respondents offered feedback on what is working well with teleNP services. Reasons mentioned were related to the service (ie, timeliness, access, quality) and the neuropsychologist (ie, communication and HCP skills). One respondent described the service and neuropsychologists positively, stating that they were “responsive, notes are readily available, clear assessments and recommendations, being available by [Microsoft] Teams/email.”
Ten respondents provided suggestions for improvement. Suggestions focused on expanding services, such as to “all veterans with cognitive/memory concerns that desire testing,” individuals with attention-deficit/hyperactivity disorder and co-occurring mental health concerns, and those in residential programs. Suggestions included hiring psychology technicians or more staff and providing education at local clinics.
DISCUSSION
This QI project examines VA staff perspectives on the usefulness of CRH 20 teleNP services and non-VA F2F services. While the small sample size limits generalizability, this preliminary study suggests that VA teleNP evaluations were similar to those conducted F2F in non-VA settings. While ratings of teleNP usefulness did not differ significantly for those serving rural vs nonrural veterans, respondents serving rural patients were more likely to refer patients to teleNP, suggesting that teleNP may increase access in rural settings, consistent with other studies.7,8,13 This article also presents qualitative suggestions for improving teleNP delivery within the VHA. This is the first known initiative to report on VHA staff satisfaction with a teleNP service and expands the limited literature to date on satisfaction with teleNP services. The findings provide initial support for continued use and, potentially, expansion of teleNP services within this CRH remote hub-and-spoke model.
Limitations
A significant limitation of the current work is the small sample size of survey respondents. In particular, while teleNP turnaround time was perceived as faster than non-VA F2F care, only 8 respondents reported on timeliness of F2F evaluation results, which renders it difficult to draw conclusions. Interestingly, not all respondents reported referring to neuropsychology services within the previous year; the most common reasons reflect the perception that referral to neuropsychology was outside of that staff member’s role or not clinically indicated.
One additional possible explanation for the absence of reporting on utility of teleNP specifically is that respondents did not track whether their patient was seen by teleNP or F2F services, based on how the referral process varies at each health care system. For example, in health care system C, a large number of referrals are forwarded to the service by local VA F2F neuropsychologists. This may speak to the seamlessness of the teleNP process, such that local staff and/or referring HCPs are unaware of the modality over which neuropsychology is being conducted. It is plausible that the reason behind this smaller response rate in health care systems B and C relates to how neuropsychology consults are processed at these local VAMCs. We suspect that in these settings, the HCPs referring for neuropsychological evaluations (eg, primary care, mental health) may be unaware that their referrals are being triaged to neuropsychologists in a different program (CRH 20 teleNP). Therefore, they would not necessarily know that they used teleNP and didn’t complete the survey.
The referral process for these 2 sites contrasts with the process for other VISN 20 sites where there is no local neuropsychology program triaging. In these settings, referrals from local HCPs come directly to teleNP; thus, it is more likely that these HCPs are aware of teleNP services. There were only 2 physicians who completed the survey, which may relate to their workload and a workflow where other staff have been increasingly requested to order the consults for the physician. This type of workflow results in an increase in the number of VHA staff involved in patient care. Ratings of usefulness were highest in health care system B, which does not have neuropsychology services at the facility or in the community; this may relate to elevated teleNP satisfaction ratings.
Further work may help identify which aspects of a teleNP service make it more useful than F2F care for this population or determine whether there were HCPor setting-specific factors that influenced the ratings (ie, preference for VA care or comparison of favorability ratings for the HCPs who conduct teleNP and F2F within the same system). The latter comparisons could not be drawn in the current systems due to the absence of HCPs who provide both teleNP and F2F modalities within VISN 20. Another consideration for future work would be to use a previously published/validated survey measure and piloting of questions with a naive sample before implementation.
CONCLUSIONS
This analysis provides initial support for feasibility and acceptability of teleNP as an alternative to traditional in-person neuropsychological evaluations. The small number of survey respondents may reflect the multiple pathways through which consults are forwarded to CRH 20, which includes both direct HCP referrals and forwarded consults from local neuropsychology services. CRH 20 has completed > 570 teleNP evaluations within 1 year, suggesting that lack of awareness may not be hindering veteran access to the service. Replication with a larger sample that is more broadly representative of key stakeholders in veteran care, identification of populations that would benefit most from teleNP services, and dissemination studies of the expansion of teleNP services are all important directions for future work. The robustness and longevity of the VISN 20 teleNP program, coupled with the preliminary positive findings from this project, demonstrate support for further assessment of the potential impact of telehealth on neuropsychological care within the VHA and show that barriers associated with access to health care services in remote settings may be mitigated through teleNP service delivery.
There are 2.7 million (48%) rural veterans enrolled in the Veterans Health Administration (VHA).1 Many VHA-enrolled rural veterans are aged ≥ 65 years (54%), a medically complex population that requires more extensive health care.1 These veterans may live far from US Department of Veterans Affairs (VA) medical centers (VAMCs) and often receive most of their care at rural community-based outpatient clinics (CBOCs). In addition to face-to-face (F2F) services provided at these clinics, many patient care needs may be met using telehealth technology, which can connect veterans at CBOCs with remote health care practitioners (HCPs).
This technology is used across medical specialties throughout the VA and has expanded into neuropsychology services to improve access amid the shortage of rural neuropsychologists. Prior research suggests that access to neuropsychology services improves the functional outcomes of people with diverse medical conditions, including dementia, brain injury, and epilepsy, and reduces emergency department visits, hospitalization duration, and health care costs.2-6 Given that veterans unable to access neuropsychology services may be at risk for poorer outcomes, identifying ways to improve access is a priority. Tele-neuropsychology (teleNP) has been used to expand access for rural veterans in need of these services.7,8
TeleNP is the application of audiovisual technologies to enable remote clinical encounters for neuropsychological assessments.9 TeleNP has been shown to be generally equivalent to F2F care, without significant differences compared with in-person visits.10-13 TeleNP was increasingly implemented following the COVID-19 pandemic and remains an enduring and expanding feature of neuropsychology care delivery.8,14-18 TeleNP services can increase access to care, especially for rural veterans and those with limited transportation.
Research in non-VA samples suggests a high level of clinician satisfaction with teleNP.16 In VA samples, research has found high levels of patient satisfaction with teleNP both within Veterans Integrated Services Network (VISN) 20 and in a VA health care system outside VISN 20.7,19 Investigating staff perceptions of these services and their utility compared with non-VA F2F visits is pertinent to the overall feasibility and effectiveness of teleNP.
TELE-NEUROPSYCHOLOGY PROGRAM
A clinical resource hub (CRH) is a VISN-governed program that provides veteran health care when local VHA facilities have service gaps.20,21 CRH 20 serves several Pacific Northwest VISN 20 health care systems and began providing teleNP in 2015. The CRH 20 teleNP service serves older adults in rural settings with > 570 teleNP evaluations completed over a recent 12-month period (May 2023 to May 2024). In the CRH 20 teleNP program, veterans are offered services by CRH 20 neuropsychologists via telehealth to a patient’s local VAMC, larger health care clinic, CBOC, or via Veterans Video Connect to the home.

Referral pathways to the CRH 20 teleNP program differ across sites. For VISN 20 sites that do not have any in-house neuropsychology services, referrals are initiated by HCPs from any discipline. At 2 sites with in-house neuropsychology programs, CRH 20 teleNP referrals typically are forwarded from the inhouse service whenever the veteran prefers to be seen at an outlying clinic. All sites, including the CBOCs, are equipped fully for testing, and the HCP encounters veterans in a private office via video-based telehealth technology after a telehealth technician orients them to the space. The private office minimizes environmental disruptions and uses standardized technology to ensure valid results. A limited number of evaluations are offered at home (< 5% of the evaluations) if the veteran is unable to come to a VHA facility, has access to reliable internet, and a minimally distracting home setting.
In VISN 20, teleNP is a routine practice for delivering services to rural sites, most of which lack neuropsychologists. However, there is limited information about the extent to which the referral sources find the service useful. This quality improvement (QI) project aimed to better understand how well-established teleNP services were received by referral sources/stakeholders and how services could be improved. Prior to the advent of the CRH 20 teleNP program, staff had the option of referring for F2F evaluations in the local community (outside the VA) at some sites, an option that remains. This QI project examined staff perspectives on the usefulness of CRH 20 teleNP services compared with non-VA F2F services. We administered an anonymous, confidential survey examining these factors to VISN 20 staff within 4 VA health care systems.
METHODS
This QI project used a mixed quantitative and qualitative descriptive survey design to elicit feedback. The authors (3 neuropsychologists, 1 geropsychologist, and 1 research coordinator) developed the survey questions. The 13-question survey was voluntary, anonymous, and confidential, and respondents were given an opportunity to ask questions, with the first author serving as the point of contact.
The survey ascertained information about respondents and their work setting (ie, facility type, specific work setting and location, profession, and rurality of patients). First respondents were asked whether they have referred patients to neuropsychology services in the past year. Those who had not referred patients during the past year were asked about reasons for nonreferral with an option to provide an open-ended response. Respondents who did refer were asked how they refer for neuropsychology services and about the usefulness and timeliness of both teleNP and non-VA F2F services. Respondents were asked to respond with their preference for teleNP vs non-VA F2F with an open-ended prompt. Finally, respondents were invited to share any feedback for improvement regarding teleNP services.
A link to the survey, hosted on the VA Research Electronic Data Capture system, was emailed to facility and service line leaders at the 4 VISN 20 health care systems for distribution to the staff. All staff were included because in many of the facilities, particularly those that are highly rural with low staffing, it is not uncommon for technicians, nurses, and other support staff to assist with placing consults. In particular, VISN 20 nurses often have an optimal understanding of referral pathways to care for patients and are positioned to give and receive feedback about the utility of neuropsychological evaluations. The Research and Development Committee at the Boise VA Medical Center determined this project to be QI and exempt from institutional review board oversight. The VISN 20 employee labor relations HR supervisor approved this survey, with union awareness. Responses were anonymous.
Data were imported into Microsoft Excel and IBM SPSS Statistics for further analysis. Data were summarized using descriptive statistics, frequencies, and percentages. Nonparametric χ2 and Wilcoxon signed-rank tests were used to test for differences. An inductive approach to develop codes was used for the 3 open-ended questions. Two authors (CC, CEG) independently coded the responses and reviewed discrepancies. Final code applications were based on consensus.
RESULTS
The survey was deployed for 1 month between February 7, 2024, and June 15, 2024, at each of the 4 health care systems. Thirty-three staff members responded; of these, 1 person did not respond to an item on whether they referred for neuropsychology services. Eighteen of 33 respondents reported referring patients to teleNP or F2F neuropsychology services in the past year. Fourteen of the 33 respondents stated they did not refer; of these, 2 were unfamiliar with the teleNP service and 12 provided other reasons (eg, new to VA, not in their professional scope to order consults, did not have patients needing services).
The analysis focused on the 18 respondents who referred for neuropsychology services. Thirteen were within health care system A, and 5 were within health care system B (which had no nearby non-VA contracted neuropsychology services) and none were in the other 2 health care systems. Ten of 18 respondents (56%) stated they practiced primarily in a rural setting. Five respondents worked in a CBOC, 12 in a main VA facility, 9 in a primary care setting, 8 in a mental health setting, and 3 in other settings (eg, domiciliary). Participants could select > 1 setting. The 18 respondents who referred to neuropsychology services included 7 psychologists, 1 nurse, 2 social workers, 1 social services assistant, 4 nurse practitioners, 2 physicians, and 1 unknown HCP.
When asked to categorize the usefulness of services, more respondents characterized teleNP as very much so (1 on a 5-point scale) than F2F referrals (Figure). The mean (SD) of 1.5 (0.8) for teleNP usefulness fell between very much so and mostly and 1 respondent indicated not applicable. Similarly, the mean (SD) for non-VA F2F usefulness was 1.7 (0.9); 9 respondents rated this item as not applicable. A Wilcoxon signed-rank test of related samples indicated no significant differences between the pairs of ratings (Z = 1.50; P = .41).
Respondents with rural patients were more likely to refer them to teleNP services compared with respondents with nonrural patients (χ2 = 5.7; P = .02). However, ratings of teleNP usefulness did not significantly differ for those serving rural vs with nonrural patients (χ2 = 1.4; P = .49). Mean (SD) rating of teleNP usefulness was 1.3 (0.7) for the 9 rural subgroup respondents (between very much so and mostly) vs 1.8 (0.9) for the 8 nonrural subgroup respondents (between very much so and mostly). The mean (SD) rating for non-VA F2F usefulness was 1.8 (1.0) for the 4 rural subgroup respondents and 1.6 (0.8) for the 5 nonrural subgroup, between very much so and mostly for both groups.
Most respondents had no preference between teleNP or F2F. Notably, the responses underlying this group were multifaceted and corresponded to multiple codes (ie, access, preference for in-person services, technology, space and logistics, and service boundaries and requirements). According to 1 respondent, “the logistics of scheduling/room availability, technological challenges, and client behavioral issues that are likely to occur could possibly be more easily addressed via in-person sessions for some clients and providers.”
Six of 18 respondents preferred teleNP, citing timeliness, ease of access, and evaluation quality. One respondent noted that the “majority of my veterans live in extremely remote areas” and may need to take a plane for their visit. The 3 respondents who preferred in-person neuropsychology services cited veterans’ preference for in-person services.
Open-Ended Feedback
Thirteen respondents offered feedback on what is working well with teleNP services. Reasons mentioned were related to the service (ie, timeliness, access, quality) and the neuropsychologist (ie, communication and HCP skills). One respondent described the service and neuropsychologists positively, stating that they were “responsive, notes are readily available, clear assessments and recommendations, being available by [Microsoft] Teams/email.”
Ten respondents provided suggestions for improvement. Suggestions focused on expanding services, such as to “all veterans with cognitive/memory concerns that desire testing,” individuals with attention-deficit/hyperactivity disorder and co-occurring mental health concerns, and those in residential programs. Suggestions included hiring psychology technicians or more staff and providing education at local clinics.
DISCUSSION
This QI project examines VA staff perspectives on the usefulness of CRH 20 teleNP services and non-VA F2F services. While the small sample size limits generalizability, this preliminary study suggests that VA teleNP evaluations were similar to those conducted F2F in non-VA settings. While ratings of teleNP usefulness did not differ significantly for those serving rural vs nonrural veterans, respondents serving rural patients were more likely to refer patients to teleNP, suggesting that teleNP may increase access in rural settings, consistent with other studies.7,8,13 This article also presents qualitative suggestions for improving teleNP delivery within the VHA. This is the first known initiative to report on VHA staff satisfaction with a teleNP service and expands the limited literature to date on satisfaction with teleNP services. The findings provide initial support for continued use and, potentially, expansion of teleNP services within this CRH remote hub-and-spoke model.
Limitations
A significant limitation of the current work is the small sample size of survey respondents. In particular, while teleNP turnaround time was perceived as faster than non-VA F2F care, only 8 respondents reported on timeliness of F2F evaluation results, which renders it difficult to draw conclusions. Interestingly, not all respondents reported referring to neuropsychology services within the previous year; the most common reasons reflect the perception that referral to neuropsychology was outside of that staff member’s role or not clinically indicated.
One additional possible explanation for the absence of reporting on utility of teleNP specifically is that respondents did not track whether their patient was seen by teleNP or F2F services, based on how the referral process varies at each health care system. For example, in health care system C, a large number of referrals are forwarded to the service by local VA F2F neuropsychologists. This may speak to the seamlessness of the teleNP process, such that local staff and/or referring HCPs are unaware of the modality over which neuropsychology is being conducted. It is plausible that the reason behind this smaller response rate in health care systems B and C relates to how neuropsychology consults are processed at these local VAMCs. We suspect that in these settings, the HCPs referring for neuropsychological evaluations (eg, primary care, mental health) may be unaware that their referrals are being triaged to neuropsychologists in a different program (CRH 20 teleNP). Therefore, they would not necessarily know that they used teleNP and didn’t complete the survey.
The referral process for these 2 sites contrasts with the process for other VISN 20 sites where there is no local neuropsychology program triaging. In these settings, referrals from local HCPs come directly to teleNP; thus, it is more likely that these HCPs are aware of teleNP services. There were only 2 physicians who completed the survey, which may relate to their workload and a workflow where other staff have been increasingly requested to order the consults for the physician. This type of workflow results in an increase in the number of VHA staff involved in patient care. Ratings of usefulness were highest in health care system B, which does not have neuropsychology services at the facility or in the community; this may relate to elevated teleNP satisfaction ratings.
Further work may help identify which aspects of a teleNP service make it more useful than F2F care for this population or determine whether there were HCPor setting-specific factors that influenced the ratings (ie, preference for VA care or comparison of favorability ratings for the HCPs who conduct teleNP and F2F within the same system). The latter comparisons could not be drawn in the current systems due to the absence of HCPs who provide both teleNP and F2F modalities within VISN 20. Another consideration for future work would be to use a previously published/validated survey measure and piloting of questions with a naive sample before implementation.
CONCLUSIONS
This analysis provides initial support for feasibility and acceptability of teleNP as an alternative to traditional in-person neuropsychological evaluations. The small number of survey respondents may reflect the multiple pathways through which consults are forwarded to CRH 20, which includes both direct HCP referrals and forwarded consults from local neuropsychology services. CRH 20 has completed > 570 teleNP evaluations within 1 year, suggesting that lack of awareness may not be hindering veteran access to the service. Replication with a larger sample that is more broadly representative of key stakeholders in veteran care, identification of populations that would benefit most from teleNP services, and dissemination studies of the expansion of teleNP services are all important directions for future work. The robustness and longevity of the VISN 20 teleNP program, coupled with the preliminary positive findings from this project, demonstrate support for further assessment of the potential impact of telehealth on neuropsychological care within the VHA and show that barriers associated with access to health care services in remote settings may be mitigated through teleNP service delivery.
- US Department of Veterans Affairs, Office of Rural Health. Rural veterans. Updated March 10, 2025. Accessed July 7, 2025. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- Braun M, Tupper D, Kaufmann P, et al. Neuropsychological assessment: a valuable tool in the diagnosis and management of neurological, neurodevelopmental, medical, and psychiatric disorders. Cogn Behav Neurol. 2011;24(3):107-114. doi:10.1097/wnn.0b013e3182351289
- Donders J. The incremental value of neuropsychological assessment: a critical review. Clin Neuropsychol. 2020;34(1):56-87. doi:10.1080/13854046.2019.1575471
- Williams MW, Rapport LJ, Hanks RA, et al. Incremental value of neuropsychological evaluations to computed tomography in predicting long-term outcomes after traumatic brain injury. Clin Neuropsychol. 2013;27(3):356-375. doi:10.1080/13854046.2013.765507
- Sieg E, Mai Q, Mosti C, Brook M. The utility of neuropsychological consultation in identifying medical inpatients with suspected cognitive impairment at risk for greater hospital utilization. Clin Neuropsychol. 2019;33(1):75-89. doi:10.1080/13854046.2018.1465124
- Vankirk KM, Horner MD, Turner TH, et al. CE hospital service utilization is reduced following neuropsychological evaluation in a sample of U.S. veterans. Clin Neuropsychol. 2013;27(5):750-761. doi:10.1080/13854046.2013.783122
- Appleman ER, O’Connor MK, Boucher SJ, et al. Teleneuropsychology clinic development and patient satisfaction. Clin Neuropsychol. 2021;35(4):819-837. doi:10.1080/13854046.2020.1871515
- Stelmokas J, Ratcliffe LN, Lengu K, et al. Evaluation of teleneuropsychology services in veterans during COVID-19. Psychol Serv. 2024;21(1):65-72. doi:10.1037/ser0000810
- Bilder R Postal KS, Barisa M, et al. Inter Organizational Practice Committee recommendations/guidance for teleneuropsychology in response to the COVID-19 pandemic. Arch Clin Neuropsychol. 2020;35(6):647-659. doi:10.1093/arclin/acaa046
- Brearly TW, Shura RD, Martindale SL, et al. Neuropsychological test administration by videoconference: a systematic review and meta-analysis. Neuropsychol Rev. 2017;27(2):174-186. doi:10.1007/s11065-017-9349-1
- Brown AD, Kelso W, Eratne D, et al. Investigating equivalence of in-person and telehealth-based neuropsychological assessment performance for individuals being investigated for younger onset dementia. Arch Clin Neuropsychol. 2024;39(5):594-607. doi:10.1093/arclin/acad108
- Chapman JE, Ponsford J, Bagot KL, et al. The use of videoconferencing in clinical neuropsychology practice: a mixed methods evaluation of neuropsychologists’ experiences and views. Aust Psychol. 2020;55(6):618-633. doi:10.1111/ap.12471
- Marra DE, Hamlet KM, Bauer RM, et al. Validity of teleneuropsychology for older adults in response to COVID-19: a systematic and critical review. Clin Neuropsychol. 2020;34:1411-1452. doi:10.1080/13854046.2020.1769192
- Hammers DB, Stolwyk R, Harder L, et al. A survey of international clinical teleneuropsychology service provision prior to COVID-19. Clin Neuropsychol. 2020;34(7-8):1267- 1283. doi:10.1080/13854046.2020.1810323
- Marra DE, Hoelzle JB, Davis JJ, et al. Initial changes in neuropsychologists’ clinical practice during the COVID-19 pandemic: a survey study. Clin Neuropsychol. 2020;34(7- 8):1251-1266. doi:10.1080/13854046.2020.1800098
- Parsons MW, Gardner MM, Sherman, JC et al. Feasibility and acceptance of direct-to-home teleneuropsychology services during the COVID-19 pandemic. J Int Neuropsychol Soc. 2022;28(2):210-215. doi:10.1017/s1355617721000436
- Rochette AD, Rahman-Filipiak A, Spencer RJ, et al. Teleneuropsychology practice survey during COVID-19 within the United States. Appl Neuropsychol Adult. 2022;29(6):1312- 1322. doi:10.1080/23279095.2021.1872576
- Messler AC, Hargrave DD, Trittschuh EH, et al. National survey of telehealth neuropsychology practices: current attitudes, practices, and relevance of tele-neuropsychology three years after the onset of COVID-19. Clin Neuropsychol. 2023;39:1017-1036. doi:10.1080/13854046.2023.2192422
- Rautman L, Sordahl JA. Veteran satisfaction with tele-neuropsychology services. Clin Neuropsychol. 2018;32:1453949. doi:10.1080/13854046.2018.1453949
- US Department of Veterans Affairs. Patient care services: clinical resource hubs. Updated March 20, 2024. Accessed August 4, 2025. https://www.patientcare .va.gov/primarycare/CRH.asp
- Burnett K, Stockdale SE, Yoon J, et al. The Clinical Resource Hub initiative: first-year implementation of the Veterans Health Administration regional telehealth contingency staffing program. Ambul Care Manage. 2023;46(3):228-239. doi:10.1097/JAC.0000000000000468
- US Department of Veterans Affairs, Office of Rural Health. Rural veterans. Updated March 10, 2025. Accessed July 7, 2025. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- Braun M, Tupper D, Kaufmann P, et al. Neuropsychological assessment: a valuable tool in the diagnosis and management of neurological, neurodevelopmental, medical, and psychiatric disorders. Cogn Behav Neurol. 2011;24(3):107-114. doi:10.1097/wnn.0b013e3182351289
- Donders J. The incremental value of neuropsychological assessment: a critical review. Clin Neuropsychol. 2020;34(1):56-87. doi:10.1080/13854046.2019.1575471
- Williams MW, Rapport LJ, Hanks RA, et al. Incremental value of neuropsychological evaluations to computed tomography in predicting long-term outcomes after traumatic brain injury. Clin Neuropsychol. 2013;27(3):356-375. doi:10.1080/13854046.2013.765507
- Sieg E, Mai Q, Mosti C, Brook M. The utility of neuropsychological consultation in identifying medical inpatients with suspected cognitive impairment at risk for greater hospital utilization. Clin Neuropsychol. 2019;33(1):75-89. doi:10.1080/13854046.2018.1465124
- Vankirk KM, Horner MD, Turner TH, et al. CE hospital service utilization is reduced following neuropsychological evaluation in a sample of U.S. veterans. Clin Neuropsychol. 2013;27(5):750-761. doi:10.1080/13854046.2013.783122
- Appleman ER, O’Connor MK, Boucher SJ, et al. Teleneuropsychology clinic development and patient satisfaction. Clin Neuropsychol. 2021;35(4):819-837. doi:10.1080/13854046.2020.1871515
- Stelmokas J, Ratcliffe LN, Lengu K, et al. Evaluation of teleneuropsychology services in veterans during COVID-19. Psychol Serv. 2024;21(1):65-72. doi:10.1037/ser0000810
- Bilder R Postal KS, Barisa M, et al. Inter Organizational Practice Committee recommendations/guidance for teleneuropsychology in response to the COVID-19 pandemic. Arch Clin Neuropsychol. 2020;35(6):647-659. doi:10.1093/arclin/acaa046
- Brearly TW, Shura RD, Martindale SL, et al. Neuropsychological test administration by videoconference: a systematic review and meta-analysis. Neuropsychol Rev. 2017;27(2):174-186. doi:10.1007/s11065-017-9349-1
- Brown AD, Kelso W, Eratne D, et al. Investigating equivalence of in-person and telehealth-based neuropsychological assessment performance for individuals being investigated for younger onset dementia. Arch Clin Neuropsychol. 2024;39(5):594-607. doi:10.1093/arclin/acad108
- Chapman JE, Ponsford J, Bagot KL, et al. The use of videoconferencing in clinical neuropsychology practice: a mixed methods evaluation of neuropsychologists’ experiences and views. Aust Psychol. 2020;55(6):618-633. doi:10.1111/ap.12471
- Marra DE, Hamlet KM, Bauer RM, et al. Validity of teleneuropsychology for older adults in response to COVID-19: a systematic and critical review. Clin Neuropsychol. 2020;34:1411-1452. doi:10.1080/13854046.2020.1769192
- Hammers DB, Stolwyk R, Harder L, et al. A survey of international clinical teleneuropsychology service provision prior to COVID-19. Clin Neuropsychol. 2020;34(7-8):1267- 1283. doi:10.1080/13854046.2020.1810323
- Marra DE, Hoelzle JB, Davis JJ, et al. Initial changes in neuropsychologists’ clinical practice during the COVID-19 pandemic: a survey study. Clin Neuropsychol. 2020;34(7- 8):1251-1266. doi:10.1080/13854046.2020.1800098
- Parsons MW, Gardner MM, Sherman, JC et al. Feasibility and acceptance of direct-to-home teleneuropsychology services during the COVID-19 pandemic. J Int Neuropsychol Soc. 2022;28(2):210-215. doi:10.1017/s1355617721000436
- Rochette AD, Rahman-Filipiak A, Spencer RJ, et al. Teleneuropsychology practice survey during COVID-19 within the United States. Appl Neuropsychol Adult. 2022;29(6):1312- 1322. doi:10.1080/23279095.2021.1872576
- Messler AC, Hargrave DD, Trittschuh EH, et al. National survey of telehealth neuropsychology practices: current attitudes, practices, and relevance of tele-neuropsychology three years after the onset of COVID-19. Clin Neuropsychol. 2023;39:1017-1036. doi:10.1080/13854046.2023.2192422
- Rautman L, Sordahl JA. Veteran satisfaction with tele-neuropsychology services. Clin Neuropsychol. 2018;32:1453949. doi:10.1080/13854046.2018.1453949
- US Department of Veterans Affairs. Patient care services: clinical resource hubs. Updated March 20, 2024. Accessed August 4, 2025. https://www.patientcare .va.gov/primarycare/CRH.asp
- Burnett K, Stockdale SE, Yoon J, et al. The Clinical Resource Hub initiative: first-year implementation of the Veterans Health Administration regional telehealth contingency staffing program. Ambul Care Manage. 2023;46(3):228-239. doi:10.1097/JAC.0000000000000468
Staff Perspectives on the VISN 20 Tele-Neuropsychology Program
Staff Perspectives on the VISN 20 Tele-Neuropsychology Program
Sim and Learn: Simulation and its Value in Neurology Education
Sim and Learn: Simulation and its Value in Neurology Education
Clinical simulation is a technique, not a technology, used to replace or amplify real experiences with guided experiences that evoke or replicate substantial aspects of the real world in a fully interactive fashion.1 Simulation is widely used in medical education and spans a spectrum of sophistication, from simple reproduction of isolated body parts to high-fidelity human patient simulators that replicate whole body appearance and variable physiological parameters.2,3
Simulation-based medical education can be a valuable tool for safe health care delivery.4Simulation-based education is typically provided via 5 modalities: mannequins, computer-based mannequins, standardized patients, computer-based simulators, and software-based simulations. Simulation technology increases procedural skill by allowing for deliberate practice in a safe environment.5 Mastery learning is a stringent form of competency-based education that requires trainees to acquire clinical skill measured against a fixed achievement standard.6 In mastery learning, educational practice time varies but results are uniform. This approach improves patient outcomes and is more effective than clinical training alone.7-9
Advanced simulation models are helpful tools for neurologic education and training, especially for emergency department encounters.10 In recent years, advanced simulation models have been applied in various fields of medicine, especially emergency medicine and anesthesia.11-14
Acute neurology
In acute neurologic conditions (eg, stroke, intracerebral hemorrhage, status epilepticus, and neuromuscular respiratory failure) clinical outcomes are highly time dependent; consequently, a reduction in treatment delays can improve patient care. The application of simulation methodology allows trainees to address acute and potentially life-threatening emergencies in a safe, controlled, and reproducible environment. In addition to improving trainees’ knowledge base, simulation also helps to enhance team skills, communication, multidisciplinary collaboration, and leadership. Research has shown that deliberate practice leads to a decrease in clinical errors and improved procedural performance in the operating room.8,15 These results can be extrapolated to acute neurology settings to improve adherence to set protocols, thus streamlining management in acute settings.
Scenarios can be built to teach skills such as eliciting an appropriate history, establishing inclusion or exclusion criteria for the use of certain medications, evaluating neuroimaging and laboratory studies (while avoiding related common pitfalls), and managing treatment complications. Simulation also provides an opportunity for interprofessional education by training nurses and collaborative staff. It can be used to enhance nontechnical skills (eg, communication, situation awareness, decision making, and leadership) that further contribute to patient safety.
Simulation can be performed with the help of mannequins such as the SimMan 3G(Laerdal), which can display neurologic symptoms and physiological findings, or live actors who portray a patient by mimicking focal neurologic deficits.16,17 A briefing familiarizes the trainees with the equipment and explains the simulation process. The documentation and equipment are the same as that which is used in emergency departments or intensive care units.
Once the simulation is completed, a trainee’s performance is checked against a critical action checklist before a debriefing process during which the scenario is reviewed and learning goals are assessed. Immediate feedback is given to trainees to identify weaknesses and the simulation is repeated if multiple critical action items are missed. (Figure).17
RESIDENCY TRAINING
Simulation training in stroke is mandatory in some residency programs for neurology postgraduate year (PGY) 2 residents.18 These simulations are a part of a boot camp for incoming neurology residents after completing an internal medicine internship. The simulation program is not standardized across various training programs. The European Stroke Organization Simulation Committee has published an opinion paper with a consensus of experts about the implementation of simulation techniques in the stroke field.19,20 Residents participating in these mandatory programs are required to complete certification in the National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Scale, including a pretest that assesses their knowledge of acute stroke protocols prior to live simulation.17 A stepwise algorithm that incorporates faculty specialized in the field is used to evaluate and debrief the simulation.
Stroke vignettes are typically selected by the vascular neurology attending physician to cover thrombolytic therapy (indications and contraindications), mechanical thrombectomy, early arterial blood pressure management, anticoagulant reversal protocols, and management of thrombolytic complications (eg, neurologic worsening). Nursing staff is educated on the acute stroke protocol. Computed tomography (CT) and CT angiography scans are retrieved from teaching files. These are provided as live responses along with pertinent laboratory work, vital signs, and electrocardiogram tracings. Trainee performance is based on adherence to a critical action checklist, which includes (but is not limited to) identification of relative and absolute contraindications of thrombolytic treatments, estimation of NIHSS within 5 minutes of arrival, and consideration of candidacy for endovascular intervention.17
EVIDENCE FOR SIMULATION TRAINING
Simulations for acute ischemic stroke also improve cohesive teamwork to improve the door-to-needle and door-to-puncture time. A retrospective analysis involving first-year neurology residents at a comprehensive stroke center that compared patient cohort data before and after implementation of simulation training found that there was an improvement in door-to-needle time after implementation of stroke simulation training program by nearly 10 minutes.17 This was likely due to improvement in the comfort of the flow of management across multidisciplinary teams.
Discussing goals of care, communicating poor prognosis or complex decisions with distraught family members or patients requires practice. Simulation programs with video playback help focus on trainee’s body language, avoiding medical jargon and handling ethical dilemmas while adjusting the communication style to the patient’s personality.20 Enhanced communication skills improve patient satisfaction, trust, and adherence to treatments, all of which lead to better outcomes.21
Simulation has been effectively used as a training tool for recognizing and managing acute neuromuscular respiratory failure. These scenarios emphasize the importance of obtaining a focused clinical history, performing key neurological assessments (such as neck flexion strength and breath counting), evaluating pulmonary function tests, and identifying when to initiate ventilatory support.22 In a study designed as a simulation-based learning curriculum for status epilepticus, there was an improvement in the performance of PGY-2 residents after completing the curriculum from a median of 44.2% at pretest to 94.2% at posttest.23 In this curriculum, an emphasis was placed on the following: recognizing the delay in identification and treatment of status epilepticus; evaluating contraindications of certain antiseizure medication (ASM) based on history or laboratory work; giving first-line ASM within 5 minutes of seizure onset; airway and blood pressure assessment; suctioning the patient; use of second-line ASMs after first-line has failed; ordering a head CT and re-evaluating the case with postload ASM level; ordering a stat electroencephalography (EEG); and communicating the decision regarding patient disposition/level of care.24
There is a growing need for well designed simulation education programs targeted at the management of disorders requiring acute neurologic care, including not only stroke and status epilepticus, but also traumatic brain injury, subarachnoid hemorrhage, neuromuscular respiratory failure, flare of multiple sclerosis, acutely elevated intracranial pressure, malignant cerebral infarction, deterioration of Parkinson disease, and brain death evaluation with family counseling.25 This novel approach to teaching provides an opportunity to learn in addition to remediation with repetition of scenario and might be used for maintenance of recertification programs.
PROCEDURAL SKILLs
Perhaps one of the most studied uses for simulation in neurology is in procedural skills. This extends beyond neurology trainees and can include pulmonary critical care fellows, pediatric residents, and internal medicine residents receiving training in neurology-based procedures such as lumbar punctures (LPs). Other examples of neurology procedures and protocols in which simulation has been studied include fundoscopy, brain death evaluation, EEG interpretation in context of status epilepticus, and simulated stroke code responses. Additional procedures that lack research but may benefit from simulation-based training include the use of Doppler ultrasound and botulinum toxin injections practiced on mannequins.
Proficiency in LP procedural skills has been extensively studied by multiple institutions, with trainee levels ranging from medical students to fellows. One study in France enrolled 115 medical students without prior LP experience and randomized them to either a simulation or a control group.26 Those in the simulation group received instruction using a mannequin, and those in the control group received clinical training through hospital rotations. Both groups received an email containing literature-based information on the procedure as well as a self-assessment questionnaire before participating in either educational program.
The study showed that those students who received simulation training had a success rate of 67% on their first LP on a live patient compared with a success rate of 14% in those with traditional training. Students receiving simulation training required less assistance during the procedure from a supervisor and had higher satisfaction rates and confidence in their procedural skills.26
Another study of 128 medical students at the University of Pittsburgh found that a hybrid LP simulation significantly improved students’ confidence and perceived skill in performing LPs, obtaining informed consent, and electronic order entry. For example, confidence with LP increased from 5.95% presimulation to 90% postsimulation, with 58.24% of students reporting an improvement from minimal or no confidence to average or better (P < .001). Similarly, the proportion of students who felt able to perform LP with minimal or no assistance rose from 0% to 38.57% (P < .001). Confidence and perceived skill in obtaining informed consent and electronic order entry also saw significant gains. Although real-world skill assessments were limited by low survey response rates, preceptor evaluations and follow-up surveys suggested that students who participated in the simulation were more likely to perform these tasks independently or with minimal supervision during clinical rotations.27
Research on simulation training involving nonneurology residents is also encouraging. One study compared the LP skills of traditionally trained neurology residents (PGY-2 to PGY-4) to internal medicine residents (PGY-1) who underwent simulation on a mannequin.28 The internal medicine residents first underwent a pretest on LP performance, watched an educational video, underwent an LP demonstration, and practiced on a mannequin with feedback. The neurology residents completed the checklist-style pretest and performed an LP on a mannequin. Internal medicine residents were found to increase their pretest scores from a mean of 46.3% to 95.7% following training, whereas neurology residents scored a mean of 65.4%. More than half of neurology residents were unable to identify the correct anatomic location or standard cerebrospinal fluid (CSF) tests to be ordered on a routine LP.28
A pediatric resident study in Canada found that following simulation-based training, LP procedural skill improved in 15 of 16 residents tested, and PGY-1 residents showed a reduction in anxiety related to performing the procedure.29
Virtual Reality
An additional tool for simulation is the use of virtual reality (VR) in combination with mannequins. A French study used videos of LPs on actual patients, from equipment set up to final CSF collection and termination of the procedure.30 These videos included a 360-degree view of the procedure. The short video was administered through a VR device (the Oculus Go headset by Microsoft) or by a YouTube video (if VR was not desired).
Participants in the study watched the video then performed an LP on a mannequin. Those who used the VR option had minimal adverse effects (eg, low rates of cybersickness, blurred vision, nausea) and high satisfaction regarding their training environment.30Another VR-based program is the vascular intervention system trainer, which allows clinicians to use endovascular devices and simulate procedures such as thrombectomies. VR simulation is used for trainees and to retrain experienced physicians in performance of high-risk procedures.31
Fundoscopic and Ultrasound Simulations
The AR403 eye stimulator device for fundoscopic examinations is a mannequin-based simulation.32 In a single-center, prospective, single-blind study of neurology and pediatric neurology residents, trainees were split into control and intervention groups, with the intervention group receiving simulator training. Both groups received video lectures on fundoscopy techniques. Pre- and postintervention measurements included knowledge, skill, and total scores on the skills assessment. Of the 48 trainees who participated, the intervention group demonstrated significantly higher increases in skills (P = .01) and total (P = .02) scores, although knowledge scores did not improve. The intervention group also reported higher comfort levels, higher confidence, and higher success rates.
Areas that would benefit from simulation training and development include ultrasound training, such as transcranial Doppler evaluation. In a national survey of residents in anesthesia and critical care, trainees reported that simulation was not frequently used in ultrasound training and that bedside teaching was more common. Interestingly, there was a discrepancy between the opinions of residents and program directors. The program directors felt simulation was in fact used (18.2% of program directors reported this vs 5.3% of trainees).33
A new program, the NewroSim (Gaumard), is a computer-based model of cerebral perfusion that may be a useful tool in this setting. It can simulate blood flow velocities, including pathologic ones, both with a mannequin or without.34
Another potential area for development is the use of mannequins to teach botulinum toxin injections for migraine, dystonia and spasticity in a training environment This is typically led by pharmaceutical representatives who are not necessarily clinicians. Residents and fellows may benefit instead from clinician-led education during their training programs.
Simulation in Patient Communication
Simulation provides a realistic environment for teaching rapid decision-making, leadership, and appropriate management of acutely ill neurologic patients; this includes the communication skills needed in response to neurologic injury.35 Simulation can be particularly useful in situations involving brain death determination, where the communication techniques differ significantly from those used in shared decision-making. Simulation provides a low-stakes setting for clinicians to practice the process of brain death determination and communication, leading to improved confidence and knowledge.36
In the context of acute neurologic emergencies, simulation exercises have been used to investigate the consistency of prognostication across a spectrum of neurology physicians. These exercises revealed that acute neuroprognostication is highly variable and often inaccurate among neurology clinicians, suggesting a potential area for improvement through further simulation training.37
FUTURE DIRECTIONS
Simulation education in neurology can be directed towards learners at all levels, including medical students, residents, fellows, nurses, and medical technologists. In addition, simulation has great value to different disciplines, including emergency medicine, intensive care, and psychiatry. In our view simulation is not being used to full potential in neurology.
Simulation can be used to expose clinicians to rare pathology, play an integral role in competency-based evaluations, and serve as the foundation for simulation-based neurology curriculums, teleneurology simulation training programs, and team training for neurologic emergencies.38Another under-recognized aspect of neurology education is teaching interpersonal communication and professionalism. A survey conducted at a neurology department (20 residents and 73 faculty respondents) asked about residents’ comfort level in performing a number of interpersonal communication and professionalism tasks.38 While none of the residents said they were “very uncomfortable” with these tasks, only 1 resident reported being “very comfortable.” In addition, fewer than 50% noted that they had been directly observed by a faculty member while performing these tasks. The results prompted the facility to develop a simulation curriculum that including observation and feedback from 8 objective structured clinical examinations at a simulation center. A standardized professional simulated the role of a patient, caregiver, medical student, or a faculty member. Residents indicated in postsimulation surveys that it was very useful, and a majority voted for the activity to be repeated for future classes.38
Simulation models may also provide a more objective method to evaluate neurology residents. Accreditation Council for Graduate Medical Education has provided Milestones that are used for assessment of neurology residents. Most of the programs rely on end-of-rotation faculty evaluations. These are subjective evaluations, rely on chance evaluations and may not reflect the exact caliber of a trainee in different clinical situations. Simulation models can serve as alternatives to provide an objective and accurate assessment of resident’s competency in different neurologic scenarios.
In a study of PGY-4 neurology residents from 3 tertiary care academic medical centers were evaluated using simulation-based assessment. Their skills in identifying and managing status epilepticus were assessed via a simulation-based model and compared with clinical experience. No graduating neurology residents were able to meet or exceed the minimum passing score during the testing. It was suggested that end-of-rotation evaluations are inadequate for assigning level of Milestones.24 To move forward with use of simulation-based assessments, these models need to be trialed more extensively and validated.
Morris et al developed simulations for assessment in neurocritical care.39 Ten evaluative simulation cases were developed. Researchers reported on 64 trainee participants in 274 evaluative simulation scenarios. The participants were very satisfied with the cases, found them to be very realistic and appropriately difficult. Interrater reliability was acceptable for both checklist action items and global rating scales. The researchers concluded that they were able to demonstrate validity evidence via the 10 simulation cases for assessment in neurologic emergencies.39 It is the authors’ belief that the future of residents’ competency assessment should include more widespread use of similar simulation models.
Finally, VR and augmented reality (AR) have shown promise in various fields, including neurology. In neurology, these technologies are being explored for applications in rehabilitation, therapy, and medical training. Ongoing research aims to leverage these technologies for improved patient outcomes and medical education. Virtual simulations can recreate neurologic scenarios, allowing learners to interact with 3-dimensional (3D) models of the brain or experience virtual patient cases. AR can enhance traditional learning materials by overlaying digital information onto real-world objects, aiding in the understanding of complex neuroanatomy and medical concepts. These technologies contribute to more engaging and effective neurology education.39In a study of 84 graduate medical students divided into 3 groups, the first group attended a traditional lecture on neuroanatomy, the second group was shown VR-based 3D images, and the third group had a VR-based, interactive and stereoscopic session.40 Groups 2 and 3 showed the highest mean scores in evaluations and differed significantly from Group 1 (P < .05). Groups 2 and 3 did not differ significantly from each other. The researchers concluded that VR-based resources for teaching neuroanatomy fostered significantly higher learning when compared to the traditional methods.40
- Corvetto M, Bravo MP, Montaña R, et al. Simulación en educación médica: una sinopsis. Rev Med Chil. 2013;141:70-79. doi:10.4067/S0034-98872013000100010
- Lane JL, Slavin S, Ziv A. Simulation in medical education: a review. Simul Gaming. 2001;32:297-314. doi:10.1177/104687810103200302
- Bradley P. The history of simulation in medical education and possible future directions. Med Educ. 2006;40:254-262. doi:10.1111/j.1365-2929.2006.02394.x
- Jones F, Passos-Neto C, Melro Braghiroli O. Simulation in medical education: brief history and methodology. Princ Pract Clin Res J. 2015;1:46-54. doi:10.21801/ppcrj.2015.12.8
- Issenberg SB. Simulation technology for health care professional skills training and assessment. JAMA. 1999;28:861-866. doi:10.1001/jama.282.9.861
- McGaghie WC, Miller GE, Sajid AW, et al. Competency-based curriculum development on medical education: an introduction. Public Health Pap. 1978;68:11-91.
- Barsuk JH, Cohen ER, Feinglass J, et al. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169:1420-1423. doi:10.1001/archinternmed.2009.215
- Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133:56-61. doi:10.1378/chest.07-0131
- McGaghie WC, Issenberg SB, Cohen ER, et al. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86:706-711. doi:10.1097/ACM.0b013e318217e119
- Micieli G, Cavallini A, Santalucia P, et al. Simulation in neurology. Neurol Sci. 2015;36:1967-1971. doi:10.1007/s10072-015-2228-8
- Bond WF, Lammers RL, Spillane LL, et al. The use of simulation in emergency medicine: a research agenda. Acad Emerg Med. 2007;14:353-363. doi:10.1197/j.aem.2006.11.02112.
- McLaughlin SA, Doezema D, Sklar DP. Human simulation in emergency medicine training: a model curriculum. Acad Emerg Med. 2002;9:1310-1318. doi:10.1111/j.1553-2712.2002.tb01593.x
- Howard SK, Gaba DM, Fish KJ, et al. Anesthesia crisis resource management training: teaching anesthesiologists to handle critical incidents. Aviat Space Environ Med. 1992;63:763-770.
- Gaba DM. Anaesthesiology as a model for patient safety in health care. BMJ. 2000;320:785-788. doi:10.1136/bmj.320.7237.785
- Sedlack RE, Kolars JC. Computer simulator training enhances the competency of gastroenterology fellows at colonoscopy: results of a pilot study. Am J Gastroenterol. 2004;99:33-37. doi:10.1111/j.1572-0241.2004.04007.x
- Tchopev ZN, Nelson AE, Hunninghake JC, et al. Curriculum innovations: high-fidelity simulation of acute neurology enhances rising resident confidence: results from a multicohort study. Neurol Educ. 2022;1:e200022. doi:10.1212/ne9.0000000000200022
- Mehta T, Strauss S, Beland D, et al. Stroke simulation improves acute stroke management: a systems-based practice experience. J Grad Med Educ. 2018;10:57-62. doi:10.4300/JGME-D-17-00167.1
- Pergakis MB, Chang WTW, Tabatabai A, et al. Simulation-based assessment of graduate neurology trainees’ performance managing acute ischemic stroke. Neurology. 2021;97:e2414-e2422. doi:10.1212/WNL.0000000000012972
- Casolla B. Simulation for neurology training: acute setting and beyond. Rev Neurol (Paris). 2021;177:1207-1213. doi:10.1016/j.neurol.2021.03.008
- Casolla B, de Leciñana MA, Neves R, et al. Simulation training programs for acute stroke care: Objectives and standards of methodology. Eur Stroke J. 2020;5:328-335. doi:10.1177/2396987320971105
- Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826-834.doi:10.1097/MLR.0b013e31819a5acc
- Patel RA, Mohl L, Paetow G, Maiser S. Acute neuromuscular respiratory weakness due to acute inflammatory demyelinating polyneuropathy (AIDP): a simulation scenario for neurology providers. MedEdPORTAL. 2019;15:10811. doi:10.15766/mep_2374-8265.10811
- Mikhaeil-Demo Y, Barsuk JH, Culler GW, et al. Use of a simulation-based mastery learning curriculum for neurology residents to improve the identification and management of status epilepticus. Epilepsy Behav. 2020;111:107247. doi:10.1016/j.yebeh.2020.107247
- Mikhaeil-Demo Y, Holmboe E, Gerard EE, et al. Simulation-based assessments and graduating neurology residents’ milestones: status epilepticus milestones. J Grad Med Educ. 2021;13:223-230. doi:10.4300/JGME-D-20-00832.1
- Hocker S, Wijdicks EFM, Feske SK, et al. Use of simulation in acute neurology training: point and counterpoint. Ann Neurol. 2015;78:337-342. doi:10.1002/ana.24473
- Gaubert S, Blet A, Dib F, et al. Positive effects of lumbar puncture simulation training for medical students in clinical practice. BMC Med Educ. 2021;21:1-6. doi:10.1186/S12909-020-02452-327.
- Yanta C, Knepper L, Van Deusen R, et al. The use of hybrid lumbar puncture simulation to teach entrustable professional activities during a medical student neurology clerkship. MedEdPublish (2016). 2021;9:266. doi:10.15694/mep.2020.000266.2
- Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79:132-137. doi:10.1212/WNL.0B013E31825DD39D
- McMillan HJ, Writer H, Moreau KA, et al. Lumbar puncture simulation in pediatric residency training: improving procedural competence and decreasing anxiety. BMC Med Educ. 2016;16:198. doi:10.1186/S12909-016-0722-1
- Vrillon A, Gonzales-Marabal L, Ceccaldi PF, et al. Using virtual reality in lumbar puncture training improves students learning experience. BMC Med Educ. 2022;22:244. doi:10.1186/S12909-022-03317-7
- Liebig T, Holtmannspötter M, Crossley R, et al. Metric-based virtual reality simulation: a paradigm shift in training for mechanical thrombectomy in acute stroke. Stroke. 2018;49:e239-e242.doi:10.1161/STROKEAHA.118.021089
- Gupta DK, Khandker N, Stacy K, et al. Utility of combining a simulation-based method with a lecture-based method for fundoscopy training in neurology residency. JAMA Neurol. 2017;74:1223-1227. doi:10.1001/JAMANEUROL.2017.2073
- Mongodi S, Bonomi F, Vaschetto R, et al. Point-of-care ultrasound training for residents in anaesthesia and critical care: results of a national survey comparing residents and training program directors’ perspectives. BMC Med Educ. 2022;22:647. doi:10.1186/S12909-022-03708-W
- Morris NA, Czeisler BM, Sarwal A. Simulation in neurocritical care: past, present, and future. Neurocrit Care. 2019;30:522-533. doi:10.1007/S12028-018-0629-2
- Wijdicks EFM, Hocker SE. A future for simulation in acute neurology. Semin Neurol. 2018;38:465-470. doi:10.1055/s-0038-1666986
- Kramer NM, O’Mahony S, Deamant C. Brain death determination and communication: an innovative approach using simulation and standardized patients. J Pain Symptom Manage. 2022;63:e765-e772. doi:10.1016/j.jpainsymman.2022.01.020
- Sloane KL, Miller JJ, Piquet A, et al. Prognostication in acute neurological emergencies. J Stroke Cerebrovasc Dis. 2022;31:106277. doi:10.1016/J.JSTROKECEREBROVASDIS.2021.106277
- Kurzweil AM, Lewis A, Pleninger P, et al. Education research: teaching and assessing communication and professionalism in neurology residency with simulation. Neurology. 2020;94:229-232. doi:10.1212/WNL.0000000000008895
- Morris NA, Chang WT, Tabatabai A, et al. Development of neurological emergency simulations for assessment: content evidence and response process. Neurocrit Care. 2021;35:389-396. doi:10.1007/S12028-020-01176-Y
- De Faria JWV, Teixeira MJ, De Moura Sousa Júnior L, et al. Virtual and stereoscopic anatomy: when virtual reality meets medical education. J Neurosurg. 2016;125:1105-1111. doi:10.3171/2015.8.JNS141563
Clinical simulation is a technique, not a technology, used to replace or amplify real experiences with guided experiences that evoke or replicate substantial aspects of the real world in a fully interactive fashion.1 Simulation is widely used in medical education and spans a spectrum of sophistication, from simple reproduction of isolated body parts to high-fidelity human patient simulators that replicate whole body appearance and variable physiological parameters.2,3
Simulation-based medical education can be a valuable tool for safe health care delivery.4Simulation-based education is typically provided via 5 modalities: mannequins, computer-based mannequins, standardized patients, computer-based simulators, and software-based simulations. Simulation technology increases procedural skill by allowing for deliberate practice in a safe environment.5 Mastery learning is a stringent form of competency-based education that requires trainees to acquire clinical skill measured against a fixed achievement standard.6 In mastery learning, educational practice time varies but results are uniform. This approach improves patient outcomes and is more effective than clinical training alone.7-9
Advanced simulation models are helpful tools for neurologic education and training, especially for emergency department encounters.10 In recent years, advanced simulation models have been applied in various fields of medicine, especially emergency medicine and anesthesia.11-14
Acute neurology
In acute neurologic conditions (eg, stroke, intracerebral hemorrhage, status epilepticus, and neuromuscular respiratory failure) clinical outcomes are highly time dependent; consequently, a reduction in treatment delays can improve patient care. The application of simulation methodology allows trainees to address acute and potentially life-threatening emergencies in a safe, controlled, and reproducible environment. In addition to improving trainees’ knowledge base, simulation also helps to enhance team skills, communication, multidisciplinary collaboration, and leadership. Research has shown that deliberate practice leads to a decrease in clinical errors and improved procedural performance in the operating room.8,15 These results can be extrapolated to acute neurology settings to improve adherence to set protocols, thus streamlining management in acute settings.
Scenarios can be built to teach skills such as eliciting an appropriate history, establishing inclusion or exclusion criteria for the use of certain medications, evaluating neuroimaging and laboratory studies (while avoiding related common pitfalls), and managing treatment complications. Simulation also provides an opportunity for interprofessional education by training nurses and collaborative staff. It can be used to enhance nontechnical skills (eg, communication, situation awareness, decision making, and leadership) that further contribute to patient safety.
Simulation can be performed with the help of mannequins such as the SimMan 3G(Laerdal), which can display neurologic symptoms and physiological findings, or live actors who portray a patient by mimicking focal neurologic deficits.16,17 A briefing familiarizes the trainees with the equipment and explains the simulation process. The documentation and equipment are the same as that which is used in emergency departments or intensive care units.
Once the simulation is completed, a trainee’s performance is checked against a critical action checklist before a debriefing process during which the scenario is reviewed and learning goals are assessed. Immediate feedback is given to trainees to identify weaknesses and the simulation is repeated if multiple critical action items are missed. (Figure).17

RESIDENCY TRAINING
Simulation training in stroke is mandatory in some residency programs for neurology postgraduate year (PGY) 2 residents.18 These simulations are a part of a boot camp for incoming neurology residents after completing an internal medicine internship. The simulation program is not standardized across various training programs. The European Stroke Organization Simulation Committee has published an opinion paper with a consensus of experts about the implementation of simulation techniques in the stroke field.19,20 Residents participating in these mandatory programs are required to complete certification in the National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Scale, including a pretest that assesses their knowledge of acute stroke protocols prior to live simulation.17 A stepwise algorithm that incorporates faculty specialized in the field is used to evaluate and debrief the simulation.
Stroke vignettes are typically selected by the vascular neurology attending physician to cover thrombolytic therapy (indications and contraindications), mechanical thrombectomy, early arterial blood pressure management, anticoagulant reversal protocols, and management of thrombolytic complications (eg, neurologic worsening). Nursing staff is educated on the acute stroke protocol. Computed tomography (CT) and CT angiography scans are retrieved from teaching files. These are provided as live responses along with pertinent laboratory work, vital signs, and electrocardiogram tracings. Trainee performance is based on adherence to a critical action checklist, which includes (but is not limited to) identification of relative and absolute contraindications of thrombolytic treatments, estimation of NIHSS within 5 minutes of arrival, and consideration of candidacy for endovascular intervention.17
EVIDENCE FOR SIMULATION TRAINING
Simulations for acute ischemic stroke also improve cohesive teamwork to improve the door-to-needle and door-to-puncture time. A retrospective analysis involving first-year neurology residents at a comprehensive stroke center that compared patient cohort data before and after implementation of simulation training found that there was an improvement in door-to-needle time after implementation of stroke simulation training program by nearly 10 minutes.17 This was likely due to improvement in the comfort of the flow of management across multidisciplinary teams.
Discussing goals of care, communicating poor prognosis or complex decisions with distraught family members or patients requires practice. Simulation programs with video playback help focus on trainee’s body language, avoiding medical jargon and handling ethical dilemmas while adjusting the communication style to the patient’s personality.20 Enhanced communication skills improve patient satisfaction, trust, and adherence to treatments, all of which lead to better outcomes.21
Simulation has been effectively used as a training tool for recognizing and managing acute neuromuscular respiratory failure. These scenarios emphasize the importance of obtaining a focused clinical history, performing key neurological assessments (such as neck flexion strength and breath counting), evaluating pulmonary function tests, and identifying when to initiate ventilatory support.22 In a study designed as a simulation-based learning curriculum for status epilepticus, there was an improvement in the performance of PGY-2 residents after completing the curriculum from a median of 44.2% at pretest to 94.2% at posttest.23 In this curriculum, an emphasis was placed on the following: recognizing the delay in identification and treatment of status epilepticus; evaluating contraindications of certain antiseizure medication (ASM) based on history or laboratory work; giving first-line ASM within 5 minutes of seizure onset; airway and blood pressure assessment; suctioning the patient; use of second-line ASMs after first-line has failed; ordering a head CT and re-evaluating the case with postload ASM level; ordering a stat electroencephalography (EEG); and communicating the decision regarding patient disposition/level of care.24
There is a growing need for well designed simulation education programs targeted at the management of disorders requiring acute neurologic care, including not only stroke and status epilepticus, but also traumatic brain injury, subarachnoid hemorrhage, neuromuscular respiratory failure, flare of multiple sclerosis, acutely elevated intracranial pressure, malignant cerebral infarction, deterioration of Parkinson disease, and brain death evaluation with family counseling.25 This novel approach to teaching provides an opportunity to learn in addition to remediation with repetition of scenario and might be used for maintenance of recertification programs.
PROCEDURAL SKILLs
Perhaps one of the most studied uses for simulation in neurology is in procedural skills. This extends beyond neurology trainees and can include pulmonary critical care fellows, pediatric residents, and internal medicine residents receiving training in neurology-based procedures such as lumbar punctures (LPs). Other examples of neurology procedures and protocols in which simulation has been studied include fundoscopy, brain death evaluation, EEG interpretation in context of status epilepticus, and simulated stroke code responses. Additional procedures that lack research but may benefit from simulation-based training include the use of Doppler ultrasound and botulinum toxin injections practiced on mannequins.
Proficiency in LP procedural skills has been extensively studied by multiple institutions, with trainee levels ranging from medical students to fellows. One study in France enrolled 115 medical students without prior LP experience and randomized them to either a simulation or a control group.26 Those in the simulation group received instruction using a mannequin, and those in the control group received clinical training through hospital rotations. Both groups received an email containing literature-based information on the procedure as well as a self-assessment questionnaire before participating in either educational program.
The study showed that those students who received simulation training had a success rate of 67% on their first LP on a live patient compared with a success rate of 14% in those with traditional training. Students receiving simulation training required less assistance during the procedure from a supervisor and had higher satisfaction rates and confidence in their procedural skills.26
Another study of 128 medical students at the University of Pittsburgh found that a hybrid LP simulation significantly improved students’ confidence and perceived skill in performing LPs, obtaining informed consent, and electronic order entry. For example, confidence with LP increased from 5.95% presimulation to 90% postsimulation, with 58.24% of students reporting an improvement from minimal or no confidence to average or better (P < .001). Similarly, the proportion of students who felt able to perform LP with minimal or no assistance rose from 0% to 38.57% (P < .001). Confidence and perceived skill in obtaining informed consent and electronic order entry also saw significant gains. Although real-world skill assessments were limited by low survey response rates, preceptor evaluations and follow-up surveys suggested that students who participated in the simulation were more likely to perform these tasks independently or with minimal supervision during clinical rotations.27
Research on simulation training involving nonneurology residents is also encouraging. One study compared the LP skills of traditionally trained neurology residents (PGY-2 to PGY-4) to internal medicine residents (PGY-1) who underwent simulation on a mannequin.28 The internal medicine residents first underwent a pretest on LP performance, watched an educational video, underwent an LP demonstration, and practiced on a mannequin with feedback. The neurology residents completed the checklist-style pretest and performed an LP on a mannequin. Internal medicine residents were found to increase their pretest scores from a mean of 46.3% to 95.7% following training, whereas neurology residents scored a mean of 65.4%. More than half of neurology residents were unable to identify the correct anatomic location or standard cerebrospinal fluid (CSF) tests to be ordered on a routine LP.28
A pediatric resident study in Canada found that following simulation-based training, LP procedural skill improved in 15 of 16 residents tested, and PGY-1 residents showed a reduction in anxiety related to performing the procedure.29
Virtual Reality
An additional tool for simulation is the use of virtual reality (VR) in combination with mannequins. A French study used videos of LPs on actual patients, from equipment set up to final CSF collection and termination of the procedure.30 These videos included a 360-degree view of the procedure. The short video was administered through a VR device (the Oculus Go headset by Microsoft) or by a YouTube video (if VR was not desired).
Participants in the study watched the video then performed an LP on a mannequin. Those who used the VR option had minimal adverse effects (eg, low rates of cybersickness, blurred vision, nausea) and high satisfaction regarding their training environment.30Another VR-based program is the vascular intervention system trainer, which allows clinicians to use endovascular devices and simulate procedures such as thrombectomies. VR simulation is used for trainees and to retrain experienced physicians in performance of high-risk procedures.31
Fundoscopic and Ultrasound Simulations
The AR403 eye stimulator device for fundoscopic examinations is a mannequin-based simulation.32 In a single-center, prospective, single-blind study of neurology and pediatric neurology residents, trainees were split into control and intervention groups, with the intervention group receiving simulator training. Both groups received video lectures on fundoscopy techniques. Pre- and postintervention measurements included knowledge, skill, and total scores on the skills assessment. Of the 48 trainees who participated, the intervention group demonstrated significantly higher increases in skills (P = .01) and total (P = .02) scores, although knowledge scores did not improve. The intervention group also reported higher comfort levels, higher confidence, and higher success rates.
Areas that would benefit from simulation training and development include ultrasound training, such as transcranial Doppler evaluation. In a national survey of residents in anesthesia and critical care, trainees reported that simulation was not frequently used in ultrasound training and that bedside teaching was more common. Interestingly, there was a discrepancy between the opinions of residents and program directors. The program directors felt simulation was in fact used (18.2% of program directors reported this vs 5.3% of trainees).33
A new program, the NewroSim (Gaumard), is a computer-based model of cerebral perfusion that may be a useful tool in this setting. It can simulate blood flow velocities, including pathologic ones, both with a mannequin or without.34
Another potential area for development is the use of mannequins to teach botulinum toxin injections for migraine, dystonia and spasticity in a training environment This is typically led by pharmaceutical representatives who are not necessarily clinicians. Residents and fellows may benefit instead from clinician-led education during their training programs.
Simulation in Patient Communication
Simulation provides a realistic environment for teaching rapid decision-making, leadership, and appropriate management of acutely ill neurologic patients; this includes the communication skills needed in response to neurologic injury.35 Simulation can be particularly useful in situations involving brain death determination, where the communication techniques differ significantly from those used in shared decision-making. Simulation provides a low-stakes setting for clinicians to practice the process of brain death determination and communication, leading to improved confidence and knowledge.36
In the context of acute neurologic emergencies, simulation exercises have been used to investigate the consistency of prognostication across a spectrum of neurology physicians. These exercises revealed that acute neuroprognostication is highly variable and often inaccurate among neurology clinicians, suggesting a potential area for improvement through further simulation training.37
FUTURE DIRECTIONS
Simulation education in neurology can be directed towards learners at all levels, including medical students, residents, fellows, nurses, and medical technologists. In addition, simulation has great value to different disciplines, including emergency medicine, intensive care, and psychiatry. In our view simulation is not being used to full potential in neurology.
Simulation can be used to expose clinicians to rare pathology, play an integral role in competency-based evaluations, and serve as the foundation for simulation-based neurology curriculums, teleneurology simulation training programs, and team training for neurologic emergencies.38Another under-recognized aspect of neurology education is teaching interpersonal communication and professionalism. A survey conducted at a neurology department (20 residents and 73 faculty respondents) asked about residents’ comfort level in performing a number of interpersonal communication and professionalism tasks.38 While none of the residents said they were “very uncomfortable” with these tasks, only 1 resident reported being “very comfortable.” In addition, fewer than 50% noted that they had been directly observed by a faculty member while performing these tasks. The results prompted the facility to develop a simulation curriculum that including observation and feedback from 8 objective structured clinical examinations at a simulation center. A standardized professional simulated the role of a patient, caregiver, medical student, or a faculty member. Residents indicated in postsimulation surveys that it was very useful, and a majority voted for the activity to be repeated for future classes.38
Simulation models may also provide a more objective method to evaluate neurology residents. Accreditation Council for Graduate Medical Education has provided Milestones that are used for assessment of neurology residents. Most of the programs rely on end-of-rotation faculty evaluations. These are subjective evaluations, rely on chance evaluations and may not reflect the exact caliber of a trainee in different clinical situations. Simulation models can serve as alternatives to provide an objective and accurate assessment of resident’s competency in different neurologic scenarios.
In a study of PGY-4 neurology residents from 3 tertiary care academic medical centers were evaluated using simulation-based assessment. Their skills in identifying and managing status epilepticus were assessed via a simulation-based model and compared with clinical experience. No graduating neurology residents were able to meet or exceed the minimum passing score during the testing. It was suggested that end-of-rotation evaluations are inadequate for assigning level of Milestones.24 To move forward with use of simulation-based assessments, these models need to be trialed more extensively and validated.
Morris et al developed simulations for assessment in neurocritical care.39 Ten evaluative simulation cases were developed. Researchers reported on 64 trainee participants in 274 evaluative simulation scenarios. The participants were very satisfied with the cases, found them to be very realistic and appropriately difficult. Interrater reliability was acceptable for both checklist action items and global rating scales. The researchers concluded that they were able to demonstrate validity evidence via the 10 simulation cases for assessment in neurologic emergencies.39 It is the authors’ belief that the future of residents’ competency assessment should include more widespread use of similar simulation models.
Finally, VR and augmented reality (AR) have shown promise in various fields, including neurology. In neurology, these technologies are being explored for applications in rehabilitation, therapy, and medical training. Ongoing research aims to leverage these technologies for improved patient outcomes and medical education. Virtual simulations can recreate neurologic scenarios, allowing learners to interact with 3-dimensional (3D) models of the brain or experience virtual patient cases. AR can enhance traditional learning materials by overlaying digital information onto real-world objects, aiding in the understanding of complex neuroanatomy and medical concepts. These technologies contribute to more engaging and effective neurology education.39In a study of 84 graduate medical students divided into 3 groups, the first group attended a traditional lecture on neuroanatomy, the second group was shown VR-based 3D images, and the third group had a VR-based, interactive and stereoscopic session.40 Groups 2 and 3 showed the highest mean scores in evaluations and differed significantly from Group 1 (P < .05). Groups 2 and 3 did not differ significantly from each other. The researchers concluded that VR-based resources for teaching neuroanatomy fostered significantly higher learning when compared to the traditional methods.40
Clinical simulation is a technique, not a technology, used to replace or amplify real experiences with guided experiences that evoke or replicate substantial aspects of the real world in a fully interactive fashion.1 Simulation is widely used in medical education and spans a spectrum of sophistication, from simple reproduction of isolated body parts to high-fidelity human patient simulators that replicate whole body appearance and variable physiological parameters.2,3
Simulation-based medical education can be a valuable tool for safe health care delivery.4Simulation-based education is typically provided via 5 modalities: mannequins, computer-based mannequins, standardized patients, computer-based simulators, and software-based simulations. Simulation technology increases procedural skill by allowing for deliberate practice in a safe environment.5 Mastery learning is a stringent form of competency-based education that requires trainees to acquire clinical skill measured against a fixed achievement standard.6 In mastery learning, educational practice time varies but results are uniform. This approach improves patient outcomes and is more effective than clinical training alone.7-9
Advanced simulation models are helpful tools for neurologic education and training, especially for emergency department encounters.10 In recent years, advanced simulation models have been applied in various fields of medicine, especially emergency medicine and anesthesia.11-14
Acute neurology
In acute neurologic conditions (eg, stroke, intracerebral hemorrhage, status epilepticus, and neuromuscular respiratory failure) clinical outcomes are highly time dependent; consequently, a reduction in treatment delays can improve patient care. The application of simulation methodology allows trainees to address acute and potentially life-threatening emergencies in a safe, controlled, and reproducible environment. In addition to improving trainees’ knowledge base, simulation also helps to enhance team skills, communication, multidisciplinary collaboration, and leadership. Research has shown that deliberate practice leads to a decrease in clinical errors and improved procedural performance in the operating room.8,15 These results can be extrapolated to acute neurology settings to improve adherence to set protocols, thus streamlining management in acute settings.
Scenarios can be built to teach skills such as eliciting an appropriate history, establishing inclusion or exclusion criteria for the use of certain medications, evaluating neuroimaging and laboratory studies (while avoiding related common pitfalls), and managing treatment complications. Simulation also provides an opportunity for interprofessional education by training nurses and collaborative staff. It can be used to enhance nontechnical skills (eg, communication, situation awareness, decision making, and leadership) that further contribute to patient safety.
Simulation can be performed with the help of mannequins such as the SimMan 3G(Laerdal), which can display neurologic symptoms and physiological findings, or live actors who portray a patient by mimicking focal neurologic deficits.16,17 A briefing familiarizes the trainees with the equipment and explains the simulation process. The documentation and equipment are the same as that which is used in emergency departments or intensive care units.
Once the simulation is completed, a trainee’s performance is checked against a critical action checklist before a debriefing process during which the scenario is reviewed and learning goals are assessed. Immediate feedback is given to trainees to identify weaknesses and the simulation is repeated if multiple critical action items are missed. (Figure).17

RESIDENCY TRAINING
Simulation training in stroke is mandatory in some residency programs for neurology postgraduate year (PGY) 2 residents.18 These simulations are a part of a boot camp for incoming neurology residents after completing an internal medicine internship. The simulation program is not standardized across various training programs. The European Stroke Organization Simulation Committee has published an opinion paper with a consensus of experts about the implementation of simulation techniques in the stroke field.19,20 Residents participating in these mandatory programs are required to complete certification in the National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Scale, including a pretest that assesses their knowledge of acute stroke protocols prior to live simulation.17 A stepwise algorithm that incorporates faculty specialized in the field is used to evaluate and debrief the simulation.
Stroke vignettes are typically selected by the vascular neurology attending physician to cover thrombolytic therapy (indications and contraindications), mechanical thrombectomy, early arterial blood pressure management, anticoagulant reversal protocols, and management of thrombolytic complications (eg, neurologic worsening). Nursing staff is educated on the acute stroke protocol. Computed tomography (CT) and CT angiography scans are retrieved from teaching files. These are provided as live responses along with pertinent laboratory work, vital signs, and electrocardiogram tracings. Trainee performance is based on adherence to a critical action checklist, which includes (but is not limited to) identification of relative and absolute contraindications of thrombolytic treatments, estimation of NIHSS within 5 minutes of arrival, and consideration of candidacy for endovascular intervention.17
EVIDENCE FOR SIMULATION TRAINING
Simulations for acute ischemic stroke also improve cohesive teamwork to improve the door-to-needle and door-to-puncture time. A retrospective analysis involving first-year neurology residents at a comprehensive stroke center that compared patient cohort data before and after implementation of simulation training found that there was an improvement in door-to-needle time after implementation of stroke simulation training program by nearly 10 minutes.17 This was likely due to improvement in the comfort of the flow of management across multidisciplinary teams.
Discussing goals of care, communicating poor prognosis or complex decisions with distraught family members or patients requires practice. Simulation programs with video playback help focus on trainee’s body language, avoiding medical jargon and handling ethical dilemmas while adjusting the communication style to the patient’s personality.20 Enhanced communication skills improve patient satisfaction, trust, and adherence to treatments, all of which lead to better outcomes.21
Simulation has been effectively used as a training tool for recognizing and managing acute neuromuscular respiratory failure. These scenarios emphasize the importance of obtaining a focused clinical history, performing key neurological assessments (such as neck flexion strength and breath counting), evaluating pulmonary function tests, and identifying when to initiate ventilatory support.22 In a study designed as a simulation-based learning curriculum for status epilepticus, there was an improvement in the performance of PGY-2 residents after completing the curriculum from a median of 44.2% at pretest to 94.2% at posttest.23 In this curriculum, an emphasis was placed on the following: recognizing the delay in identification and treatment of status epilepticus; evaluating contraindications of certain antiseizure medication (ASM) based on history or laboratory work; giving first-line ASM within 5 minutes of seizure onset; airway and blood pressure assessment; suctioning the patient; use of second-line ASMs after first-line has failed; ordering a head CT and re-evaluating the case with postload ASM level; ordering a stat electroencephalography (EEG); and communicating the decision regarding patient disposition/level of care.24
There is a growing need for well designed simulation education programs targeted at the management of disorders requiring acute neurologic care, including not only stroke and status epilepticus, but also traumatic brain injury, subarachnoid hemorrhage, neuromuscular respiratory failure, flare of multiple sclerosis, acutely elevated intracranial pressure, malignant cerebral infarction, deterioration of Parkinson disease, and brain death evaluation with family counseling.25 This novel approach to teaching provides an opportunity to learn in addition to remediation with repetition of scenario and might be used for maintenance of recertification programs.
PROCEDURAL SKILLs
Perhaps one of the most studied uses for simulation in neurology is in procedural skills. This extends beyond neurology trainees and can include pulmonary critical care fellows, pediatric residents, and internal medicine residents receiving training in neurology-based procedures such as lumbar punctures (LPs). Other examples of neurology procedures and protocols in which simulation has been studied include fundoscopy, brain death evaluation, EEG interpretation in context of status epilepticus, and simulated stroke code responses. Additional procedures that lack research but may benefit from simulation-based training include the use of Doppler ultrasound and botulinum toxin injections practiced on mannequins.
Proficiency in LP procedural skills has been extensively studied by multiple institutions, with trainee levels ranging from medical students to fellows. One study in France enrolled 115 medical students without prior LP experience and randomized them to either a simulation or a control group.26 Those in the simulation group received instruction using a mannequin, and those in the control group received clinical training through hospital rotations. Both groups received an email containing literature-based information on the procedure as well as a self-assessment questionnaire before participating in either educational program.
The study showed that those students who received simulation training had a success rate of 67% on their first LP on a live patient compared with a success rate of 14% in those with traditional training. Students receiving simulation training required less assistance during the procedure from a supervisor and had higher satisfaction rates and confidence in their procedural skills.26
Another study of 128 medical students at the University of Pittsburgh found that a hybrid LP simulation significantly improved students’ confidence and perceived skill in performing LPs, obtaining informed consent, and electronic order entry. For example, confidence with LP increased from 5.95% presimulation to 90% postsimulation, with 58.24% of students reporting an improvement from minimal or no confidence to average or better (P < .001). Similarly, the proportion of students who felt able to perform LP with minimal or no assistance rose from 0% to 38.57% (P < .001). Confidence and perceived skill in obtaining informed consent and electronic order entry also saw significant gains. Although real-world skill assessments were limited by low survey response rates, preceptor evaluations and follow-up surveys suggested that students who participated in the simulation were more likely to perform these tasks independently or with minimal supervision during clinical rotations.27
Research on simulation training involving nonneurology residents is also encouraging. One study compared the LP skills of traditionally trained neurology residents (PGY-2 to PGY-4) to internal medicine residents (PGY-1) who underwent simulation on a mannequin.28 The internal medicine residents first underwent a pretest on LP performance, watched an educational video, underwent an LP demonstration, and practiced on a mannequin with feedback. The neurology residents completed the checklist-style pretest and performed an LP on a mannequin. Internal medicine residents were found to increase their pretest scores from a mean of 46.3% to 95.7% following training, whereas neurology residents scored a mean of 65.4%. More than half of neurology residents were unable to identify the correct anatomic location or standard cerebrospinal fluid (CSF) tests to be ordered on a routine LP.28
A pediatric resident study in Canada found that following simulation-based training, LP procedural skill improved in 15 of 16 residents tested, and PGY-1 residents showed a reduction in anxiety related to performing the procedure.29
Virtual Reality
An additional tool for simulation is the use of virtual reality (VR) in combination with mannequins. A French study used videos of LPs on actual patients, from equipment set up to final CSF collection and termination of the procedure.30 These videos included a 360-degree view of the procedure. The short video was administered through a VR device (the Oculus Go headset by Microsoft) or by a YouTube video (if VR was not desired).
Participants in the study watched the video then performed an LP on a mannequin. Those who used the VR option had minimal adverse effects (eg, low rates of cybersickness, blurred vision, nausea) and high satisfaction regarding their training environment.30Another VR-based program is the vascular intervention system trainer, which allows clinicians to use endovascular devices and simulate procedures such as thrombectomies. VR simulation is used for trainees and to retrain experienced physicians in performance of high-risk procedures.31
Fundoscopic and Ultrasound Simulations
The AR403 eye stimulator device for fundoscopic examinations is a mannequin-based simulation.32 In a single-center, prospective, single-blind study of neurology and pediatric neurology residents, trainees were split into control and intervention groups, with the intervention group receiving simulator training. Both groups received video lectures on fundoscopy techniques. Pre- and postintervention measurements included knowledge, skill, and total scores on the skills assessment. Of the 48 trainees who participated, the intervention group demonstrated significantly higher increases in skills (P = .01) and total (P = .02) scores, although knowledge scores did not improve. The intervention group also reported higher comfort levels, higher confidence, and higher success rates.
Areas that would benefit from simulation training and development include ultrasound training, such as transcranial Doppler evaluation. In a national survey of residents in anesthesia and critical care, trainees reported that simulation was not frequently used in ultrasound training and that bedside teaching was more common. Interestingly, there was a discrepancy between the opinions of residents and program directors. The program directors felt simulation was in fact used (18.2% of program directors reported this vs 5.3% of trainees).33
A new program, the NewroSim (Gaumard), is a computer-based model of cerebral perfusion that may be a useful tool in this setting. It can simulate blood flow velocities, including pathologic ones, both with a mannequin or without.34
Another potential area for development is the use of mannequins to teach botulinum toxin injections for migraine, dystonia and spasticity in a training environment This is typically led by pharmaceutical representatives who are not necessarily clinicians. Residents and fellows may benefit instead from clinician-led education during their training programs.
Simulation in Patient Communication
Simulation provides a realistic environment for teaching rapid decision-making, leadership, and appropriate management of acutely ill neurologic patients; this includes the communication skills needed in response to neurologic injury.35 Simulation can be particularly useful in situations involving brain death determination, where the communication techniques differ significantly from those used in shared decision-making. Simulation provides a low-stakes setting for clinicians to practice the process of brain death determination and communication, leading to improved confidence and knowledge.36
In the context of acute neurologic emergencies, simulation exercises have been used to investigate the consistency of prognostication across a spectrum of neurology physicians. These exercises revealed that acute neuroprognostication is highly variable and often inaccurate among neurology clinicians, suggesting a potential area for improvement through further simulation training.37
FUTURE DIRECTIONS
Simulation education in neurology can be directed towards learners at all levels, including medical students, residents, fellows, nurses, and medical technologists. In addition, simulation has great value to different disciplines, including emergency medicine, intensive care, and psychiatry. In our view simulation is not being used to full potential in neurology.
Simulation can be used to expose clinicians to rare pathology, play an integral role in competency-based evaluations, and serve as the foundation for simulation-based neurology curriculums, teleneurology simulation training programs, and team training for neurologic emergencies.38Another under-recognized aspect of neurology education is teaching interpersonal communication and professionalism. A survey conducted at a neurology department (20 residents and 73 faculty respondents) asked about residents’ comfort level in performing a number of interpersonal communication and professionalism tasks.38 While none of the residents said they were “very uncomfortable” with these tasks, only 1 resident reported being “very comfortable.” In addition, fewer than 50% noted that they had been directly observed by a faculty member while performing these tasks. The results prompted the facility to develop a simulation curriculum that including observation and feedback from 8 objective structured clinical examinations at a simulation center. A standardized professional simulated the role of a patient, caregiver, medical student, or a faculty member. Residents indicated in postsimulation surveys that it was very useful, and a majority voted for the activity to be repeated for future classes.38
Simulation models may also provide a more objective method to evaluate neurology residents. Accreditation Council for Graduate Medical Education has provided Milestones that are used for assessment of neurology residents. Most of the programs rely on end-of-rotation faculty evaluations. These are subjective evaluations, rely on chance evaluations and may not reflect the exact caliber of a trainee in different clinical situations. Simulation models can serve as alternatives to provide an objective and accurate assessment of resident’s competency in different neurologic scenarios.
In a study of PGY-4 neurology residents from 3 tertiary care academic medical centers were evaluated using simulation-based assessment. Their skills in identifying and managing status epilepticus were assessed via a simulation-based model and compared with clinical experience. No graduating neurology residents were able to meet or exceed the minimum passing score during the testing. It was suggested that end-of-rotation evaluations are inadequate for assigning level of Milestones.24 To move forward with use of simulation-based assessments, these models need to be trialed more extensively and validated.
Morris et al developed simulations for assessment in neurocritical care.39 Ten evaluative simulation cases were developed. Researchers reported on 64 trainee participants in 274 evaluative simulation scenarios. The participants were very satisfied with the cases, found them to be very realistic and appropriately difficult. Interrater reliability was acceptable for both checklist action items and global rating scales. The researchers concluded that they were able to demonstrate validity evidence via the 10 simulation cases for assessment in neurologic emergencies.39 It is the authors’ belief that the future of residents’ competency assessment should include more widespread use of similar simulation models.
Finally, VR and augmented reality (AR) have shown promise in various fields, including neurology. In neurology, these technologies are being explored for applications in rehabilitation, therapy, and medical training. Ongoing research aims to leverage these technologies for improved patient outcomes and medical education. Virtual simulations can recreate neurologic scenarios, allowing learners to interact with 3-dimensional (3D) models of the brain or experience virtual patient cases. AR can enhance traditional learning materials by overlaying digital information onto real-world objects, aiding in the understanding of complex neuroanatomy and medical concepts. These technologies contribute to more engaging and effective neurology education.39In a study of 84 graduate medical students divided into 3 groups, the first group attended a traditional lecture on neuroanatomy, the second group was shown VR-based 3D images, and the third group had a VR-based, interactive and stereoscopic session.40 Groups 2 and 3 showed the highest mean scores in evaluations and differed significantly from Group 1 (P < .05). Groups 2 and 3 did not differ significantly from each other. The researchers concluded that VR-based resources for teaching neuroanatomy fostered significantly higher learning when compared to the traditional methods.40
- Corvetto M, Bravo MP, Montaña R, et al. Simulación en educación médica: una sinopsis. Rev Med Chil. 2013;141:70-79. doi:10.4067/S0034-98872013000100010
- Lane JL, Slavin S, Ziv A. Simulation in medical education: a review. Simul Gaming. 2001;32:297-314. doi:10.1177/104687810103200302
- Bradley P. The history of simulation in medical education and possible future directions. Med Educ. 2006;40:254-262. doi:10.1111/j.1365-2929.2006.02394.x
- Jones F, Passos-Neto C, Melro Braghiroli O. Simulation in medical education: brief history and methodology. Princ Pract Clin Res J. 2015;1:46-54. doi:10.21801/ppcrj.2015.12.8
- Issenberg SB. Simulation technology for health care professional skills training and assessment. JAMA. 1999;28:861-866. doi:10.1001/jama.282.9.861
- McGaghie WC, Miller GE, Sajid AW, et al. Competency-based curriculum development on medical education: an introduction. Public Health Pap. 1978;68:11-91.
- Barsuk JH, Cohen ER, Feinglass J, et al. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169:1420-1423. doi:10.1001/archinternmed.2009.215
- Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133:56-61. doi:10.1378/chest.07-0131
- McGaghie WC, Issenberg SB, Cohen ER, et al. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86:706-711. doi:10.1097/ACM.0b013e318217e119
- Micieli G, Cavallini A, Santalucia P, et al. Simulation in neurology. Neurol Sci. 2015;36:1967-1971. doi:10.1007/s10072-015-2228-8
- Bond WF, Lammers RL, Spillane LL, et al. The use of simulation in emergency medicine: a research agenda. Acad Emerg Med. 2007;14:353-363. doi:10.1197/j.aem.2006.11.02112.
- McLaughlin SA, Doezema D, Sklar DP. Human simulation in emergency medicine training: a model curriculum. Acad Emerg Med. 2002;9:1310-1318. doi:10.1111/j.1553-2712.2002.tb01593.x
- Howard SK, Gaba DM, Fish KJ, et al. Anesthesia crisis resource management training: teaching anesthesiologists to handle critical incidents. Aviat Space Environ Med. 1992;63:763-770.
- Gaba DM. Anaesthesiology as a model for patient safety in health care. BMJ. 2000;320:785-788. doi:10.1136/bmj.320.7237.785
- Sedlack RE, Kolars JC. Computer simulator training enhances the competency of gastroenterology fellows at colonoscopy: results of a pilot study. Am J Gastroenterol. 2004;99:33-37. doi:10.1111/j.1572-0241.2004.04007.x
- Tchopev ZN, Nelson AE, Hunninghake JC, et al. Curriculum innovations: high-fidelity simulation of acute neurology enhances rising resident confidence: results from a multicohort study. Neurol Educ. 2022;1:e200022. doi:10.1212/ne9.0000000000200022
- Mehta T, Strauss S, Beland D, et al. Stroke simulation improves acute stroke management: a systems-based practice experience. J Grad Med Educ. 2018;10:57-62. doi:10.4300/JGME-D-17-00167.1
- Pergakis MB, Chang WTW, Tabatabai A, et al. Simulation-based assessment of graduate neurology trainees’ performance managing acute ischemic stroke. Neurology. 2021;97:e2414-e2422. doi:10.1212/WNL.0000000000012972
- Casolla B. Simulation for neurology training: acute setting and beyond. Rev Neurol (Paris). 2021;177:1207-1213. doi:10.1016/j.neurol.2021.03.008
- Casolla B, de Leciñana MA, Neves R, et al. Simulation training programs for acute stroke care: Objectives and standards of methodology. Eur Stroke J. 2020;5:328-335. doi:10.1177/2396987320971105
- Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826-834.doi:10.1097/MLR.0b013e31819a5acc
- Patel RA, Mohl L, Paetow G, Maiser S. Acute neuromuscular respiratory weakness due to acute inflammatory demyelinating polyneuropathy (AIDP): a simulation scenario for neurology providers. MedEdPORTAL. 2019;15:10811. doi:10.15766/mep_2374-8265.10811
- Mikhaeil-Demo Y, Barsuk JH, Culler GW, et al. Use of a simulation-based mastery learning curriculum for neurology residents to improve the identification and management of status epilepticus. Epilepsy Behav. 2020;111:107247. doi:10.1016/j.yebeh.2020.107247
- Mikhaeil-Demo Y, Holmboe E, Gerard EE, et al. Simulation-based assessments and graduating neurology residents’ milestones: status epilepticus milestones. J Grad Med Educ. 2021;13:223-230. doi:10.4300/JGME-D-20-00832.1
- Hocker S, Wijdicks EFM, Feske SK, et al. Use of simulation in acute neurology training: point and counterpoint. Ann Neurol. 2015;78:337-342. doi:10.1002/ana.24473
- Gaubert S, Blet A, Dib F, et al. Positive effects of lumbar puncture simulation training for medical students in clinical practice. BMC Med Educ. 2021;21:1-6. doi:10.1186/S12909-020-02452-327.
- Yanta C, Knepper L, Van Deusen R, et al. The use of hybrid lumbar puncture simulation to teach entrustable professional activities during a medical student neurology clerkship. MedEdPublish (2016). 2021;9:266. doi:10.15694/mep.2020.000266.2
- Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79:132-137. doi:10.1212/WNL.0B013E31825DD39D
- McMillan HJ, Writer H, Moreau KA, et al. Lumbar puncture simulation in pediatric residency training: improving procedural competence and decreasing anxiety. BMC Med Educ. 2016;16:198. doi:10.1186/S12909-016-0722-1
- Vrillon A, Gonzales-Marabal L, Ceccaldi PF, et al. Using virtual reality in lumbar puncture training improves students learning experience. BMC Med Educ. 2022;22:244. doi:10.1186/S12909-022-03317-7
- Liebig T, Holtmannspötter M, Crossley R, et al. Metric-based virtual reality simulation: a paradigm shift in training for mechanical thrombectomy in acute stroke. Stroke. 2018;49:e239-e242.doi:10.1161/STROKEAHA.118.021089
- Gupta DK, Khandker N, Stacy K, et al. Utility of combining a simulation-based method with a lecture-based method for fundoscopy training in neurology residency. JAMA Neurol. 2017;74:1223-1227. doi:10.1001/JAMANEUROL.2017.2073
- Mongodi S, Bonomi F, Vaschetto R, et al. Point-of-care ultrasound training for residents in anaesthesia and critical care: results of a national survey comparing residents and training program directors’ perspectives. BMC Med Educ. 2022;22:647. doi:10.1186/S12909-022-03708-W
- Morris NA, Czeisler BM, Sarwal A. Simulation in neurocritical care: past, present, and future. Neurocrit Care. 2019;30:522-533. doi:10.1007/S12028-018-0629-2
- Wijdicks EFM, Hocker SE. A future for simulation in acute neurology. Semin Neurol. 2018;38:465-470. doi:10.1055/s-0038-1666986
- Kramer NM, O’Mahony S, Deamant C. Brain death determination and communication: an innovative approach using simulation and standardized patients. J Pain Symptom Manage. 2022;63:e765-e772. doi:10.1016/j.jpainsymman.2022.01.020
- Sloane KL, Miller JJ, Piquet A, et al. Prognostication in acute neurological emergencies. J Stroke Cerebrovasc Dis. 2022;31:106277. doi:10.1016/J.JSTROKECEREBROVASDIS.2021.106277
- Kurzweil AM, Lewis A, Pleninger P, et al. Education research: teaching and assessing communication and professionalism in neurology residency with simulation. Neurology. 2020;94:229-232. doi:10.1212/WNL.0000000000008895
- Morris NA, Chang WT, Tabatabai A, et al. Development of neurological emergency simulations for assessment: content evidence and response process. Neurocrit Care. 2021;35:389-396. doi:10.1007/S12028-020-01176-Y
- De Faria JWV, Teixeira MJ, De Moura Sousa Júnior L, et al. Virtual and stereoscopic anatomy: when virtual reality meets medical education. J Neurosurg. 2016;125:1105-1111. doi:10.3171/2015.8.JNS141563
- Corvetto M, Bravo MP, Montaña R, et al. Simulación en educación médica: una sinopsis. Rev Med Chil. 2013;141:70-79. doi:10.4067/S0034-98872013000100010
- Lane JL, Slavin S, Ziv A. Simulation in medical education: a review. Simul Gaming. 2001;32:297-314. doi:10.1177/104687810103200302
- Bradley P. The history of simulation in medical education and possible future directions. Med Educ. 2006;40:254-262. doi:10.1111/j.1365-2929.2006.02394.x
- Jones F, Passos-Neto C, Melro Braghiroli O. Simulation in medical education: brief history and methodology. Princ Pract Clin Res J. 2015;1:46-54. doi:10.21801/ppcrj.2015.12.8
- Issenberg SB. Simulation technology for health care professional skills training and assessment. JAMA. 1999;28:861-866. doi:10.1001/jama.282.9.861
- McGaghie WC, Miller GE, Sajid AW, et al. Competency-based curriculum development on medical education: an introduction. Public Health Pap. 1978;68:11-91.
- Barsuk JH, Cohen ER, Feinglass J, et al. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169:1420-1423. doi:10.1001/archinternmed.2009.215
- Wayne DB, Didwania A, Feinglass J, et al. Simulation-based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case-control study. Chest. 2008;133:56-61. doi:10.1378/chest.07-0131
- McGaghie WC, Issenberg SB, Cohen ER, et al. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86:706-711. doi:10.1097/ACM.0b013e318217e119
- Micieli G, Cavallini A, Santalucia P, et al. Simulation in neurology. Neurol Sci. 2015;36:1967-1971. doi:10.1007/s10072-015-2228-8
- Bond WF, Lammers RL, Spillane LL, et al. The use of simulation in emergency medicine: a research agenda. Acad Emerg Med. 2007;14:353-363. doi:10.1197/j.aem.2006.11.02112.
- McLaughlin SA, Doezema D, Sklar DP. Human simulation in emergency medicine training: a model curriculum. Acad Emerg Med. 2002;9:1310-1318. doi:10.1111/j.1553-2712.2002.tb01593.x
- Howard SK, Gaba DM, Fish KJ, et al. Anesthesia crisis resource management training: teaching anesthesiologists to handle critical incidents. Aviat Space Environ Med. 1992;63:763-770.
- Gaba DM. Anaesthesiology as a model for patient safety in health care. BMJ. 2000;320:785-788. doi:10.1136/bmj.320.7237.785
- Sedlack RE, Kolars JC. Computer simulator training enhances the competency of gastroenterology fellows at colonoscopy: results of a pilot study. Am J Gastroenterol. 2004;99:33-37. doi:10.1111/j.1572-0241.2004.04007.x
- Tchopev ZN, Nelson AE, Hunninghake JC, et al. Curriculum innovations: high-fidelity simulation of acute neurology enhances rising resident confidence: results from a multicohort study. Neurol Educ. 2022;1:e200022. doi:10.1212/ne9.0000000000200022
- Mehta T, Strauss S, Beland D, et al. Stroke simulation improves acute stroke management: a systems-based practice experience. J Grad Med Educ. 2018;10:57-62. doi:10.4300/JGME-D-17-00167.1
- Pergakis MB, Chang WTW, Tabatabai A, et al. Simulation-based assessment of graduate neurology trainees’ performance managing acute ischemic stroke. Neurology. 2021;97:e2414-e2422. doi:10.1212/WNL.0000000000012972
- Casolla B. Simulation for neurology training: acute setting and beyond. Rev Neurol (Paris). 2021;177:1207-1213. doi:10.1016/j.neurol.2021.03.008
- Casolla B, de Leciñana MA, Neves R, et al. Simulation training programs for acute stroke care: Objectives and standards of methodology. Eur Stroke J. 2020;5:328-335. doi:10.1177/2396987320971105
- Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826-834.doi:10.1097/MLR.0b013e31819a5acc
- Patel RA, Mohl L, Paetow G, Maiser S. Acute neuromuscular respiratory weakness due to acute inflammatory demyelinating polyneuropathy (AIDP): a simulation scenario for neurology providers. MedEdPORTAL. 2019;15:10811. doi:10.15766/mep_2374-8265.10811
- Mikhaeil-Demo Y, Barsuk JH, Culler GW, et al. Use of a simulation-based mastery learning curriculum for neurology residents to improve the identification and management of status epilepticus. Epilepsy Behav. 2020;111:107247. doi:10.1016/j.yebeh.2020.107247
- Mikhaeil-Demo Y, Holmboe E, Gerard EE, et al. Simulation-based assessments and graduating neurology residents’ milestones: status epilepticus milestones. J Grad Med Educ. 2021;13:223-230. doi:10.4300/JGME-D-20-00832.1
- Hocker S, Wijdicks EFM, Feske SK, et al. Use of simulation in acute neurology training: point and counterpoint. Ann Neurol. 2015;78:337-342. doi:10.1002/ana.24473
- Gaubert S, Blet A, Dib F, et al. Positive effects of lumbar puncture simulation training for medical students in clinical practice. BMC Med Educ. 2021;21:1-6. doi:10.1186/S12909-020-02452-327.
- Yanta C, Knepper L, Van Deusen R, et al. The use of hybrid lumbar puncture simulation to teach entrustable professional activities during a medical student neurology clerkship. MedEdPublish (2016). 2021;9:266. doi:10.15694/mep.2020.000266.2
- Barsuk JH, Cohen ER, Caprio T, et al. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79:132-137. doi:10.1212/WNL.0B013E31825DD39D
- McMillan HJ, Writer H, Moreau KA, et al. Lumbar puncture simulation in pediatric residency training: improving procedural competence and decreasing anxiety. BMC Med Educ. 2016;16:198. doi:10.1186/S12909-016-0722-1
- Vrillon A, Gonzales-Marabal L, Ceccaldi PF, et al. Using virtual reality in lumbar puncture training improves students learning experience. BMC Med Educ. 2022;22:244. doi:10.1186/S12909-022-03317-7
- Liebig T, Holtmannspötter M, Crossley R, et al. Metric-based virtual reality simulation: a paradigm shift in training for mechanical thrombectomy in acute stroke. Stroke. 2018;49:e239-e242.doi:10.1161/STROKEAHA.118.021089
- Gupta DK, Khandker N, Stacy K, et al. Utility of combining a simulation-based method with a lecture-based method for fundoscopy training in neurology residency. JAMA Neurol. 2017;74:1223-1227. doi:10.1001/JAMANEUROL.2017.2073
- Mongodi S, Bonomi F, Vaschetto R, et al. Point-of-care ultrasound training for residents in anaesthesia and critical care: results of a national survey comparing residents and training program directors’ perspectives. BMC Med Educ. 2022;22:647. doi:10.1186/S12909-022-03708-W
- Morris NA, Czeisler BM, Sarwal A. Simulation in neurocritical care: past, present, and future. Neurocrit Care. 2019;30:522-533. doi:10.1007/S12028-018-0629-2
- Wijdicks EFM, Hocker SE. A future for simulation in acute neurology. Semin Neurol. 2018;38:465-470. doi:10.1055/s-0038-1666986
- Kramer NM, O’Mahony S, Deamant C. Brain death determination and communication: an innovative approach using simulation and standardized patients. J Pain Symptom Manage. 2022;63:e765-e772. doi:10.1016/j.jpainsymman.2022.01.020
- Sloane KL, Miller JJ, Piquet A, et al. Prognostication in acute neurological emergencies. J Stroke Cerebrovasc Dis. 2022;31:106277. doi:10.1016/J.JSTROKECEREBROVASDIS.2021.106277
- Kurzweil AM, Lewis A, Pleninger P, et al. Education research: teaching and assessing communication and professionalism in neurology residency with simulation. Neurology. 2020;94:229-232. doi:10.1212/WNL.0000000000008895
- Morris NA, Chang WT, Tabatabai A, et al. Development of neurological emergency simulations for assessment: content evidence and response process. Neurocrit Care. 2021;35:389-396. doi:10.1007/S12028-020-01176-Y
- De Faria JWV, Teixeira MJ, De Moura Sousa Júnior L, et al. Virtual and stereoscopic anatomy: when virtual reality meets medical education. J Neurosurg. 2016;125:1105-1111. doi:10.3171/2015.8.JNS141563
Sim and Learn: Simulation and its Value in Neurology Education
Sim and Learn: Simulation and its Value in Neurology Education
Updates in Multiple Sclerosis Imaging
Updates in Multiple Sclerosis Imaging
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
Updates in Multiple Sclerosis Imaging
Updates in Multiple Sclerosis Imaging
Checkpoint Inhibitor-Associated Optic Neuritis: A Rare irAE With Reversible Vision Loss
Background
Immune-related adverse events (irAEs) associated with checkpoint inhibitors can involve virtually any organ system. Optic neuritis is a rare but potentially reversible toxicity, with limited reports in the literature.
Case Presentation
A 57-year-old male with Stage IV poorly-differentiated neuroendocrine carcinoma presented with progressive bilateral vision loss following a near-complete response to four cycles of atezolizumab, carboplatin, and etoposide chemotherapy, and one cycle of maintenance atezolizumab. Symptoms began in the right eye and progressed to the left over 12 days. Neurological and ophthalmological evaluations included brain and orbital MRI, autoimmune panels, and infectious workup, all of which were unrevealing. The clinical picture remained consistent with isolated, immunemediated optic neuritis.
Discussion
High-dose intravenous methylprednisolone was initiated, resulting in gradual improvement and partial visual recovery by day four. An oral prednisone taper was prescribed for continued treatment. This is the second reported case of isolated optic neuritis associated with PD-L1 inhibitor therapy and the second with negative imaging findings. The rarity of this irAE and the absence of radiographic abnormalities may delay diagnosis and treatment.
Conclusions
Checkpoint-inhibitor-induced optic neuritis should be considered in patients with visual symptoms on immunotherapy, even in the setting of negative imaging. Early recognition and corticosteroid therapy are critical in preserving visual function.
Background
Immune-related adverse events (irAEs) associated with checkpoint inhibitors can involve virtually any organ system. Optic neuritis is a rare but potentially reversible toxicity, with limited reports in the literature.
Case Presentation
A 57-year-old male with Stage IV poorly-differentiated neuroendocrine carcinoma presented with progressive bilateral vision loss following a near-complete response to four cycles of atezolizumab, carboplatin, and etoposide chemotherapy, and one cycle of maintenance atezolizumab. Symptoms began in the right eye and progressed to the left over 12 days. Neurological and ophthalmological evaluations included brain and orbital MRI, autoimmune panels, and infectious workup, all of which were unrevealing. The clinical picture remained consistent with isolated, immunemediated optic neuritis.
Discussion
High-dose intravenous methylprednisolone was initiated, resulting in gradual improvement and partial visual recovery by day four. An oral prednisone taper was prescribed for continued treatment. This is the second reported case of isolated optic neuritis associated with PD-L1 inhibitor therapy and the second with negative imaging findings. The rarity of this irAE and the absence of radiographic abnormalities may delay diagnosis and treatment.
Conclusions
Checkpoint-inhibitor-induced optic neuritis should be considered in patients with visual symptoms on immunotherapy, even in the setting of negative imaging. Early recognition and corticosteroid therapy are critical in preserving visual function.
Background
Immune-related adverse events (irAEs) associated with checkpoint inhibitors can involve virtually any organ system. Optic neuritis is a rare but potentially reversible toxicity, with limited reports in the literature.
Case Presentation
A 57-year-old male with Stage IV poorly-differentiated neuroendocrine carcinoma presented with progressive bilateral vision loss following a near-complete response to four cycles of atezolizumab, carboplatin, and etoposide chemotherapy, and one cycle of maintenance atezolizumab. Symptoms began in the right eye and progressed to the left over 12 days. Neurological and ophthalmological evaluations included brain and orbital MRI, autoimmune panels, and infectious workup, all of which were unrevealing. The clinical picture remained consistent with isolated, immunemediated optic neuritis.
Discussion
High-dose intravenous methylprednisolone was initiated, resulting in gradual improvement and partial visual recovery by day four. An oral prednisone taper was prescribed for continued treatment. This is the second reported case of isolated optic neuritis associated with PD-L1 inhibitor therapy and the second with negative imaging findings. The rarity of this irAE and the absence of radiographic abnormalities may delay diagnosis and treatment.
Conclusions
Checkpoint-inhibitor-induced optic neuritis should be considered in patients with visual symptoms on immunotherapy, even in the setting of negative imaging. Early recognition and corticosteroid therapy are critical in preserving visual function.
Data Trends 2025: Neurology
Data Trends 2025: Neurology
Click to view more from Federal Health Care Data Trends 2025.
- Lin C, et al. Front Neurol. 2024;15:1392721. Published 2024 Mar 12. doi:10.3389/fneur.2024.1392721
- Defense Medical Surveillance System, Theater Medical Data Store provided by the Armed Forces Health Surveillance Division. Prepared by the Traumatic Brain Injury Center of Excellence. Accessed April 2, 2025. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Traumatic-Brain-Injury-Center-of-Excellence/DODTBI-Worldwide-Numbers
- Karr JE, et al. Arch Phys Med Rehabil. 2025;106(4):537-547. doi:10.1016/j.apmr.2024.11.010
- Howard JT, et al. J Racial Ethn Health Disparities. 2025;12(3):1745-1756. doi:10.1007/s40615-024-02004-1
- Gasperi M, et al. JAMA Netw Open. 2024;7(3):e242299. doi:10.1001/jamanetworkopen.2024.2299
- Roghani A, et al. Epilepsia. 2024;65(8):2255-2269. doi:10.1111/epi.18026
- Herbert MS, et al. Headache. 2025;65(3):430-438. doi:10.1111/head.14815
- Fleming NH, et al. Mult Scler Relat Disord. 2024;82:105372. doi:10.1016/j.msard.2023.105372
- Silveira SL, et al. CNS Spectr. 2024;29(6):1-8. doi:10.1017/S1092852924002165
- Whiteneck G, et al. J Head Trauma Rehabil. 2024;39(5):E462-E469. doi:10.1097/HTR.0000000000000952
Seng EK, et al. Headache. 2024;64(10):1273-1284. doi:10.1111/head.14842
Click to view more from Federal Health Care Data Trends 2025.
Click to view more from Federal Health Care Data Trends 2025.
- Lin C, et al. Front Neurol. 2024;15:1392721. Published 2024 Mar 12. doi:10.3389/fneur.2024.1392721
- Defense Medical Surveillance System, Theater Medical Data Store provided by the Armed Forces Health Surveillance Division. Prepared by the Traumatic Brain Injury Center of Excellence. Accessed April 2, 2025. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Traumatic-Brain-Injury-Center-of-Excellence/DODTBI-Worldwide-Numbers
- Karr JE, et al. Arch Phys Med Rehabil. 2025;106(4):537-547. doi:10.1016/j.apmr.2024.11.010
- Howard JT, et al. J Racial Ethn Health Disparities. 2025;12(3):1745-1756. doi:10.1007/s40615-024-02004-1
- Gasperi M, et al. JAMA Netw Open. 2024;7(3):e242299. doi:10.1001/jamanetworkopen.2024.2299
- Roghani A, et al. Epilepsia. 2024;65(8):2255-2269. doi:10.1111/epi.18026
- Herbert MS, et al. Headache. 2025;65(3):430-438. doi:10.1111/head.14815
- Fleming NH, et al. Mult Scler Relat Disord. 2024;82:105372. doi:10.1016/j.msard.2023.105372
- Silveira SL, et al. CNS Spectr. 2024;29(6):1-8. doi:10.1017/S1092852924002165
- Whiteneck G, et al. J Head Trauma Rehabil. 2024;39(5):E462-E469. doi:10.1097/HTR.0000000000000952
Seng EK, et al. Headache. 2024;64(10):1273-1284. doi:10.1111/head.14842
- Lin C, et al. Front Neurol. 2024;15:1392721. Published 2024 Mar 12. doi:10.3389/fneur.2024.1392721
- Defense Medical Surveillance System, Theater Medical Data Store provided by the Armed Forces Health Surveillance Division. Prepared by the Traumatic Brain Injury Center of Excellence. Accessed April 2, 2025. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Traumatic-Brain-Injury-Center-of-Excellence/DODTBI-Worldwide-Numbers
- Karr JE, et al. Arch Phys Med Rehabil. 2025;106(4):537-547. doi:10.1016/j.apmr.2024.11.010
- Howard JT, et al. J Racial Ethn Health Disparities. 2025;12(3):1745-1756. doi:10.1007/s40615-024-02004-1
- Gasperi M, et al. JAMA Netw Open. 2024;7(3):e242299. doi:10.1001/jamanetworkopen.2024.2299
- Roghani A, et al. Epilepsia. 2024;65(8):2255-2269. doi:10.1111/epi.18026
- Herbert MS, et al. Headache. 2025;65(3):430-438. doi:10.1111/head.14815
- Fleming NH, et al. Mult Scler Relat Disord. 2024;82:105372. doi:10.1016/j.msard.2023.105372
- Silveira SL, et al. CNS Spectr. 2024;29(6):1-8. doi:10.1017/S1092852924002165
- Whiteneck G, et al. J Head Trauma Rehabil. 2024;39(5):E462-E469. doi:10.1097/HTR.0000000000000952
Seng EK, et al. Headache. 2024;64(10):1273-1284. doi:10.1111/head.14842
Data Trends 2025: Neurology
Data Trends 2025: Neurology
Post-COVID Cough Linked to Neurological Dysfunction
Chronic cough remains a common reason for consultation in pulmonology post–COVID-19. But what do we really know about this condition, now 5 years after the pandemic’s onset? This topic was discussed at the recent French-Speaking Pneumology Congress held in Marseille, France, from January 24-26, 2025.
Before discussing post-COVID cough, it is crucial to differentiate between an acute cough, often viral in origin (including those associated with SARS-CoV-2), a subacute cough (lasting 3-8 weeks), and a chronic cough (persisting over 8 weeks).
“This distinction allows us to tailor treatment and prescribe the appropriate investigations, according to the duration and the probability of symptom resolution,” explained Laurent Guilleminault, MD, PhD, pulmonologist at Toulouse University Hospital Centre, Toulouse, France.
In the case of an acute cough, for instance, after a viral infection, the probability of spontaneous resolution is very high. It is often unnecessary to carry out additional examinations or initiate specific treatments because none has proven its effectiveness in shortening this type of cough. On the other hand, when a cough persists beyond 8 weeks, the chance of spontaneous resolution decreases considerably. “This is when an assessment is necessary to identify a possible underlying cause,” Guilleminault noted.
“The absence of coughing during the consultation should not lead to ruling out a diagnosis,” he added.
Neurological Link
A large-scale French study of 70,000 patients examined the demographic profiles of patients with COVID-19. It revealed a lower frequency of coughing among children and older individuals, with a notable prevalence among adults aged 30-60 years.
Furthermore, during the acute phase of COVID, coughing did not appear to indicate severity. A comparison between survivors and nonsurvivors revealed no significant differences in the frequency and severity of coughing. Another study concluded that, contrary to expectations, COVID-related pneumonia, although potentially severe, does not necessarily involve severe cough.
These findings highlight the absence of a direct link between coughing and pulmonary involvement in patients with COVID-19.
“Coughing appears to be more closely linked to neurological dysfunction than to classic respiratory involvement. A distinction that is essential for better understanding the pathophysiology of the disease and guiding therapeutic strategies,” Guilleminault noted.
Cough Mechanism
“The analysis of cough in the context of phylogenetic evolution is fascinating,” explained Guilleminault. “It illustrates how this reflex has provided an advantage to the virus for its propagation.” Studies on the transmission of SARS-CoV-2 have confirmed that coughing plays a key role in the spread of viral particles. However, this mechanism does not involve severe pulmonary damage. The primary goal of the virus is to induce neurological dysfunction in the host by triggering a cough reflex. This neurological activation enables the virus to trigger a cough reflex for dissemination even without significant pulmonary damage. This mechanism provides an evolutionary advantage by enhancing the ability of the virus to spread and colonize new hosts.
The cough mechanism remains partially understood and involves cough hypersensitivity, characterized by increased neural responsivity to a range of stimuli that affect the airways, lungs, and other tissues innervated by common nerve supplies. The cough reflex begins with the activation of sensitive peripheral receptors located mainly in the respiratory tract that detect irritants or abnormalities.
These receptors, such as P2X2, P2X3, and others, transmit information to the brainstem, which coordinates the reflex response. This process is modulated by cortical controls that normally inhibit spontaneous coughing, explaining why we do not cough constantly even in the presence of moderate stimuli.
However, when there is an imbalance in this inhibition mechanism, coughing can be triggered either excessively or uncontrollably. SARS-CoV-2 appears to interact directly with these peripheral receptors, stimulating the cough reflex. The widespread presence and density of these receptors make this mechanism highly effective for the virus’s transmission.
Additionally, the vagus nerve likely plays a central role in triggering cough, particularly in viral infections. Studies of influenza have shown the involvement of sensory cells associated with the vagus nerve.
The virus stimulates the vagus nerve, which activates the cough reflex. Research suggests that neurotropism, neuroinflammation, and neuroimmunomodulation via the vagal sensory nerves, which are involved in SARS-CoV-2 infection, lead to cough hypersensitivity.
One question remains: Could vagus nerve involvement prolong coughing beyond the active phase of viral infection? The data indicate that viral infection significantly increases the sensitivity of the cough reflex, regardless of the level of irritation. The brain areas involved in inhibiting this reflex appear less effective during viral infection, resulting in reduced inhibitory control and easier triggering of cough. This phenomenon reflects temporary dysfunction of the neurological modulation system, which gradually recovers after recovery.
Long-Term Effects
The epidemiology of post-COVID cough and its integration into the framework of the long COVID framework remain subjects of ongoing debate. Early studies have revealed that cough could be either an isolated symptom or associated with other manifestations of long COVID. These studies were often conducted over relatively short periods (14-110 days) and estimated that approximately 19% of patients with long COVID experienced persistent cough. Another study found that 14% of patients reported cough between 3 weeks and 3 months after hospital discharge for COVID-19.
Longer follow-up periods showed a significant decrease in the prevalence of cough over time. For instance, a 1-year study reported that only 2.5% of patients had episodes of chronic cough.
However, a 2023 study published in JAMA found that the prevalence of post-COVID chronic cough exceeded 30% in some groups of patients.
“It is not relevant to wait so long before acting,” Guilleminault said. A reasonable threshold for evaluation and treatment is 8-12 weeks postinfection to begin investigations and consider appropriate treatment. What should be done when a patient presents with “Doctor, I had COVID, I have a cough, and it hasn’t stopped?” These situations are common in clinical practice. In terms of severity, quality of life, and overall impact, patients with chronic post-COVID cough are not significantly different from those with other chronic coughs. Moreover, both conditions involve a real neurological dysfunction.
Same Diagnostic Steps
Management should follow existing guidelines, including the recent French recommendations for chronic cough.
A visual analog scale can be used, and possible complications should be assessed. A chest x-ray is recommended to identify any warning signs, such as cough, although linked to COVID — may coincide with other conditions, such as bronchial cancer. In smokers, chest CT should be considered to rule out neoplastic pathology. The presence of interstitial lesions, particularly fibrosing lesions, suggests that fibrosing interstitial pneumonia requires specialized management.
Smoking, which is an aggravating factor, should be discontinued. Discontinuing angiotensin-converting enzyme inhibitors for 4 weeks can help determine if they contribute to cough.
The three most common causes of chronic cough — rhinosinusitis, asthma, and gastroesophageal reflux disease — should be ruled out. Diagnosis is based on history, physical examination, and specific tests: Nasofibroscopy for rhinosinusitis, spirometry, fractional exhaled nitric oxide for asthma and clinical history of gastroesophageal reflux disease. Studies have indicated that asthma may develop after a COVID infection.
Laryngeal abnormalities are also common in chronic post-COVID cough. One study found that a quarter of patients had increased laryngeal sensitivity or voice changes. “The larynx, a highly cough-producing organ, causes more coughing than the lungs,” Guilleminault explained.
Laryngeal abnormalities are frequently observed. A study found that 63% of patients experienced dysphonia, 56% had a sensation of a foreign body in the larynx, and 10% experienced laryngospasms.
These issues are common in patients with post-COVID cough and are often associated with neurological dysfunction. Innervation of the larynx is complex and can be affected by viruses, leading to hypersensitivity, paresthesia, and other sensory disturbances, which may explain the laryngeal symptoms observed in these patients.
Next Steps
If common causes such as asthma, abnormal imaging findings, or laryngeal pathology are ruled out, the condition may be classified as a chronic refractory or unexplained cough. In these cases, the neurological origin is likely due to nervous system dysfunction. Neuromodulatory treatments including amitriptyline, pregabalin, and gabapentin may be considered in some cases. Corticosteroids are generally ineffective against chronic coughs.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Chronic cough remains a common reason for consultation in pulmonology post–COVID-19. But what do we really know about this condition, now 5 years after the pandemic’s onset? This topic was discussed at the recent French-Speaking Pneumology Congress held in Marseille, France, from January 24-26, 2025.
Before discussing post-COVID cough, it is crucial to differentiate between an acute cough, often viral in origin (including those associated with SARS-CoV-2), a subacute cough (lasting 3-8 weeks), and a chronic cough (persisting over 8 weeks).
“This distinction allows us to tailor treatment and prescribe the appropriate investigations, according to the duration and the probability of symptom resolution,” explained Laurent Guilleminault, MD, PhD, pulmonologist at Toulouse University Hospital Centre, Toulouse, France.
In the case of an acute cough, for instance, after a viral infection, the probability of spontaneous resolution is very high. It is often unnecessary to carry out additional examinations or initiate specific treatments because none has proven its effectiveness in shortening this type of cough. On the other hand, when a cough persists beyond 8 weeks, the chance of spontaneous resolution decreases considerably. “This is when an assessment is necessary to identify a possible underlying cause,” Guilleminault noted.
“The absence of coughing during the consultation should not lead to ruling out a diagnosis,” he added.
Neurological Link
A large-scale French study of 70,000 patients examined the demographic profiles of patients with COVID-19. It revealed a lower frequency of coughing among children and older individuals, with a notable prevalence among adults aged 30-60 years.
Furthermore, during the acute phase of COVID, coughing did not appear to indicate severity. A comparison between survivors and nonsurvivors revealed no significant differences in the frequency and severity of coughing. Another study concluded that, contrary to expectations, COVID-related pneumonia, although potentially severe, does not necessarily involve severe cough.
These findings highlight the absence of a direct link between coughing and pulmonary involvement in patients with COVID-19.
“Coughing appears to be more closely linked to neurological dysfunction than to classic respiratory involvement. A distinction that is essential for better understanding the pathophysiology of the disease and guiding therapeutic strategies,” Guilleminault noted.
Cough Mechanism
“The analysis of cough in the context of phylogenetic evolution is fascinating,” explained Guilleminault. “It illustrates how this reflex has provided an advantage to the virus for its propagation.” Studies on the transmission of SARS-CoV-2 have confirmed that coughing plays a key role in the spread of viral particles. However, this mechanism does not involve severe pulmonary damage. The primary goal of the virus is to induce neurological dysfunction in the host by triggering a cough reflex. This neurological activation enables the virus to trigger a cough reflex for dissemination even without significant pulmonary damage. This mechanism provides an evolutionary advantage by enhancing the ability of the virus to spread and colonize new hosts.
The cough mechanism remains partially understood and involves cough hypersensitivity, characterized by increased neural responsivity to a range of stimuli that affect the airways, lungs, and other tissues innervated by common nerve supplies. The cough reflex begins with the activation of sensitive peripheral receptors located mainly in the respiratory tract that detect irritants or abnormalities.
These receptors, such as P2X2, P2X3, and others, transmit information to the brainstem, which coordinates the reflex response. This process is modulated by cortical controls that normally inhibit spontaneous coughing, explaining why we do not cough constantly even in the presence of moderate stimuli.
However, when there is an imbalance in this inhibition mechanism, coughing can be triggered either excessively or uncontrollably. SARS-CoV-2 appears to interact directly with these peripheral receptors, stimulating the cough reflex. The widespread presence and density of these receptors make this mechanism highly effective for the virus’s transmission.
Additionally, the vagus nerve likely plays a central role in triggering cough, particularly in viral infections. Studies of influenza have shown the involvement of sensory cells associated with the vagus nerve.
The virus stimulates the vagus nerve, which activates the cough reflex. Research suggests that neurotropism, neuroinflammation, and neuroimmunomodulation via the vagal sensory nerves, which are involved in SARS-CoV-2 infection, lead to cough hypersensitivity.
One question remains: Could vagus nerve involvement prolong coughing beyond the active phase of viral infection? The data indicate that viral infection significantly increases the sensitivity of the cough reflex, regardless of the level of irritation. The brain areas involved in inhibiting this reflex appear less effective during viral infection, resulting in reduced inhibitory control and easier triggering of cough. This phenomenon reflects temporary dysfunction of the neurological modulation system, which gradually recovers after recovery.
Long-Term Effects
The epidemiology of post-COVID cough and its integration into the framework of the long COVID framework remain subjects of ongoing debate. Early studies have revealed that cough could be either an isolated symptom or associated with other manifestations of long COVID. These studies were often conducted over relatively short periods (14-110 days) and estimated that approximately 19% of patients with long COVID experienced persistent cough. Another study found that 14% of patients reported cough between 3 weeks and 3 months after hospital discharge for COVID-19.
Longer follow-up periods showed a significant decrease in the prevalence of cough over time. For instance, a 1-year study reported that only 2.5% of patients had episodes of chronic cough.
However, a 2023 study published in JAMA found that the prevalence of post-COVID chronic cough exceeded 30% in some groups of patients.
“It is not relevant to wait so long before acting,” Guilleminault said. A reasonable threshold for evaluation and treatment is 8-12 weeks postinfection to begin investigations and consider appropriate treatment. What should be done when a patient presents with “Doctor, I had COVID, I have a cough, and it hasn’t stopped?” These situations are common in clinical practice. In terms of severity, quality of life, and overall impact, patients with chronic post-COVID cough are not significantly different from those with other chronic coughs. Moreover, both conditions involve a real neurological dysfunction.
Same Diagnostic Steps
Management should follow existing guidelines, including the recent French recommendations for chronic cough.
A visual analog scale can be used, and possible complications should be assessed. A chest x-ray is recommended to identify any warning signs, such as cough, although linked to COVID — may coincide with other conditions, such as bronchial cancer. In smokers, chest CT should be considered to rule out neoplastic pathology. The presence of interstitial lesions, particularly fibrosing lesions, suggests that fibrosing interstitial pneumonia requires specialized management.
Smoking, which is an aggravating factor, should be discontinued. Discontinuing angiotensin-converting enzyme inhibitors for 4 weeks can help determine if they contribute to cough.
The three most common causes of chronic cough — rhinosinusitis, asthma, and gastroesophageal reflux disease — should be ruled out. Diagnosis is based on history, physical examination, and specific tests: Nasofibroscopy for rhinosinusitis, spirometry, fractional exhaled nitric oxide for asthma and clinical history of gastroesophageal reflux disease. Studies have indicated that asthma may develop after a COVID infection.
Laryngeal abnormalities are also common in chronic post-COVID cough. One study found that a quarter of patients had increased laryngeal sensitivity or voice changes. “The larynx, a highly cough-producing organ, causes more coughing than the lungs,” Guilleminault explained.
Laryngeal abnormalities are frequently observed. A study found that 63% of patients experienced dysphonia, 56% had a sensation of a foreign body in the larynx, and 10% experienced laryngospasms.
These issues are common in patients with post-COVID cough and are often associated with neurological dysfunction. Innervation of the larynx is complex and can be affected by viruses, leading to hypersensitivity, paresthesia, and other sensory disturbances, which may explain the laryngeal symptoms observed in these patients.
Next Steps
If common causes such as asthma, abnormal imaging findings, or laryngeal pathology are ruled out, the condition may be classified as a chronic refractory or unexplained cough. In these cases, the neurological origin is likely due to nervous system dysfunction. Neuromodulatory treatments including amitriptyline, pregabalin, and gabapentin may be considered in some cases. Corticosteroids are generally ineffective against chronic coughs.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Chronic cough remains a common reason for consultation in pulmonology post–COVID-19. But what do we really know about this condition, now 5 years after the pandemic’s onset? This topic was discussed at the recent French-Speaking Pneumology Congress held in Marseille, France, from January 24-26, 2025.
Before discussing post-COVID cough, it is crucial to differentiate between an acute cough, often viral in origin (including those associated with SARS-CoV-2), a subacute cough (lasting 3-8 weeks), and a chronic cough (persisting over 8 weeks).
“This distinction allows us to tailor treatment and prescribe the appropriate investigations, according to the duration and the probability of symptom resolution,” explained Laurent Guilleminault, MD, PhD, pulmonologist at Toulouse University Hospital Centre, Toulouse, France.
In the case of an acute cough, for instance, after a viral infection, the probability of spontaneous resolution is very high. It is often unnecessary to carry out additional examinations or initiate specific treatments because none has proven its effectiveness in shortening this type of cough. On the other hand, when a cough persists beyond 8 weeks, the chance of spontaneous resolution decreases considerably. “This is when an assessment is necessary to identify a possible underlying cause,” Guilleminault noted.
“The absence of coughing during the consultation should not lead to ruling out a diagnosis,” he added.
Neurological Link
A large-scale French study of 70,000 patients examined the demographic profiles of patients with COVID-19. It revealed a lower frequency of coughing among children and older individuals, with a notable prevalence among adults aged 30-60 years.
Furthermore, during the acute phase of COVID, coughing did not appear to indicate severity. A comparison between survivors and nonsurvivors revealed no significant differences in the frequency and severity of coughing. Another study concluded that, contrary to expectations, COVID-related pneumonia, although potentially severe, does not necessarily involve severe cough.
These findings highlight the absence of a direct link between coughing and pulmonary involvement in patients with COVID-19.
“Coughing appears to be more closely linked to neurological dysfunction than to classic respiratory involvement. A distinction that is essential for better understanding the pathophysiology of the disease and guiding therapeutic strategies,” Guilleminault noted.
Cough Mechanism
“The analysis of cough in the context of phylogenetic evolution is fascinating,” explained Guilleminault. “It illustrates how this reflex has provided an advantage to the virus for its propagation.” Studies on the transmission of SARS-CoV-2 have confirmed that coughing plays a key role in the spread of viral particles. However, this mechanism does not involve severe pulmonary damage. The primary goal of the virus is to induce neurological dysfunction in the host by triggering a cough reflex. This neurological activation enables the virus to trigger a cough reflex for dissemination even without significant pulmonary damage. This mechanism provides an evolutionary advantage by enhancing the ability of the virus to spread and colonize new hosts.
The cough mechanism remains partially understood and involves cough hypersensitivity, characterized by increased neural responsivity to a range of stimuli that affect the airways, lungs, and other tissues innervated by common nerve supplies. The cough reflex begins with the activation of sensitive peripheral receptors located mainly in the respiratory tract that detect irritants or abnormalities.
These receptors, such as P2X2, P2X3, and others, transmit information to the brainstem, which coordinates the reflex response. This process is modulated by cortical controls that normally inhibit spontaneous coughing, explaining why we do not cough constantly even in the presence of moderate stimuli.
However, when there is an imbalance in this inhibition mechanism, coughing can be triggered either excessively or uncontrollably. SARS-CoV-2 appears to interact directly with these peripheral receptors, stimulating the cough reflex. The widespread presence and density of these receptors make this mechanism highly effective for the virus’s transmission.
Additionally, the vagus nerve likely plays a central role in triggering cough, particularly in viral infections. Studies of influenza have shown the involvement of sensory cells associated with the vagus nerve.
The virus stimulates the vagus nerve, which activates the cough reflex. Research suggests that neurotropism, neuroinflammation, and neuroimmunomodulation via the vagal sensory nerves, which are involved in SARS-CoV-2 infection, lead to cough hypersensitivity.
One question remains: Could vagus nerve involvement prolong coughing beyond the active phase of viral infection? The data indicate that viral infection significantly increases the sensitivity of the cough reflex, regardless of the level of irritation. The brain areas involved in inhibiting this reflex appear less effective during viral infection, resulting in reduced inhibitory control and easier triggering of cough. This phenomenon reflects temporary dysfunction of the neurological modulation system, which gradually recovers after recovery.
Long-Term Effects
The epidemiology of post-COVID cough and its integration into the framework of the long COVID framework remain subjects of ongoing debate. Early studies have revealed that cough could be either an isolated symptom or associated with other manifestations of long COVID. These studies were often conducted over relatively short periods (14-110 days) and estimated that approximately 19% of patients with long COVID experienced persistent cough. Another study found that 14% of patients reported cough between 3 weeks and 3 months after hospital discharge for COVID-19.
Longer follow-up periods showed a significant decrease in the prevalence of cough over time. For instance, a 1-year study reported that only 2.5% of patients had episodes of chronic cough.
However, a 2023 study published in JAMA found that the prevalence of post-COVID chronic cough exceeded 30% in some groups of patients.
“It is not relevant to wait so long before acting,” Guilleminault said. A reasonable threshold for evaluation and treatment is 8-12 weeks postinfection to begin investigations and consider appropriate treatment. What should be done when a patient presents with “Doctor, I had COVID, I have a cough, and it hasn’t stopped?” These situations are common in clinical practice. In terms of severity, quality of life, and overall impact, patients with chronic post-COVID cough are not significantly different from those with other chronic coughs. Moreover, both conditions involve a real neurological dysfunction.
Same Diagnostic Steps
Management should follow existing guidelines, including the recent French recommendations for chronic cough.
A visual analog scale can be used, and possible complications should be assessed. A chest x-ray is recommended to identify any warning signs, such as cough, although linked to COVID — may coincide with other conditions, such as bronchial cancer. In smokers, chest CT should be considered to rule out neoplastic pathology. The presence of interstitial lesions, particularly fibrosing lesions, suggests that fibrosing interstitial pneumonia requires specialized management.
Smoking, which is an aggravating factor, should be discontinued. Discontinuing angiotensin-converting enzyme inhibitors for 4 weeks can help determine if they contribute to cough.
The three most common causes of chronic cough — rhinosinusitis, asthma, and gastroesophageal reflux disease — should be ruled out. Diagnosis is based on history, physical examination, and specific tests: Nasofibroscopy for rhinosinusitis, spirometry, fractional exhaled nitric oxide for asthma and clinical history of gastroesophageal reflux disease. Studies have indicated that asthma may develop after a COVID infection.
Laryngeal abnormalities are also common in chronic post-COVID cough. One study found that a quarter of patients had increased laryngeal sensitivity or voice changes. “The larynx, a highly cough-producing organ, causes more coughing than the lungs,” Guilleminault explained.
Laryngeal abnormalities are frequently observed. A study found that 63% of patients experienced dysphonia, 56% had a sensation of a foreign body in the larynx, and 10% experienced laryngospasms.
These issues are common in patients with post-COVID cough and are often associated with neurological dysfunction. Innervation of the larynx is complex and can be affected by viruses, leading to hypersensitivity, paresthesia, and other sensory disturbances, which may explain the laryngeal symptoms observed in these patients.
Next Steps
If common causes such as asthma, abnormal imaging findings, or laryngeal pathology are ruled out, the condition may be classified as a chronic refractory or unexplained cough. In these cases, the neurological origin is likely due to nervous system dysfunction. Neuromodulatory treatments including amitriptyline, pregabalin, and gabapentin may be considered in some cases. Corticosteroids are generally ineffective against chronic coughs.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Updated Alzheimer’s Guidelines Chart the Full Diagnostic Journey
This is the first update since 2001 for specialists and the first guideline for primary care physicians. Executive summaries of the guidelines were published in three articles online on December 23 in a special issue of Alzheimer’s & Dementia.
What’s New?
“With this guideline, we expand the scope of prior guidelines by providing recommendations for practicing clinicians on the process from start to finish,” coauthor Brad Dickerson, MD, director of the Massachusetts General Hospital Frontotemporal Disorders Unit and professor of neurology at Harvard Medical School, Boston, said in a statement.
“If clinicians adopt these recommendations and healthcare systems provide adequate resources, outcomes should improve in most patients in most practice settings,” Dickerson added in an interview.
Through a modified-Delphi approach and guideline-development process, an expert workgroup representing primary and specialty care reviewed 7374 publications, of which 133 met inclusion criteria.
Based on the information, the workgroup outlined a three-step patient-centered evaluation process, which includes assessing cognitive functional status, identifying the cognitive-behavioral syndrome based on specific symptoms, and determining the likely brain diseases or conditions causing the symptoms.
What Are the Key Recommendations?
The guidelines include 19 “practical” recommendations that are applicable to any practice setting. They capture the core elements of a high-quality evaluation and disclosure process, the author said. Here is a brief summary of the recommendations:
Initial evaluation: Perform a multitiered evaluation for patients who self-report or whose care partner or clinician reports cognitive, behavioral, or functional changes.
Patient-centered communication: Partner with the patient and/or care partner to establish shared goals for the evaluation process; assess the patient’s capacity to engage in goal setting.
Diagnostic formulation: Use a tiered approach to assessments and tests based on individual presentation, risk factors, and profile, aiming to determine the level of impairment, cognitive-behavioral syndrome, and likely causes and contributing factors.
History taking: Gather reliable information from informants about changes in cognition, activities of daily living, mood, neuropsychiatric symptoms, and sensory/motor functions. Document individualized risk factors for cognitive decline.
Examination: Conduct a comprehensive examination of cognition, mood, behavior, and a dementia-focused neurologic evaluation using validated tools.
Laboratory tests: Perform tiered, individualized laboratory evaluations, starting with routine tests for all patients.
Structural imaging: Obtain structural brain imaging (MRI preferred, CT as an alternative) to help establish a cause.
Ongoing communication: Engage in ongoing dialogue with patient/care partner to guide them throughout the diagnostic process.
Diagnostic disclosure: Share findings honestly and compassionately, explaining the syndrome, its severity, probable cause, prognosis, treatment options and support resources.
Specialist referral: Refer patients with atypical, uncertain, early-onset, or rapidly progressing symptoms to a dementia subspecialist.
Neuropsychological testing: Use in instances of diagnostic uncertainty or patients with complex clinical profiles. At a minimum, the neuropsychological evaluation should include normed neuropsychological testing of the domains of learning and memory (in particular delayed free and cued recall/recognition), attention, executive function, visuospatial function, and language.
Advanced diagnostic testing: When diagnostic uncertainty remains, obtain additional laboratory tests tailored to individual patient profiles.
Molecular imaging: In a patient with an established cognitive-behavioral syndrome in whom there is continued diagnostic uncertainty regarding cause(s) after structural imaging, a dementia specialist can obtain molecular imaging with fluorodeoxyglucose PET to improve diagnostic accuracy.
Cerebrospinal fluid (CSF) analysis: Utilize CSF biomarkers to evaluate amyloid beta and tau profiles in cases with unresolved diagnostic uncertainty.
Amyloid PET imaging: Perform amyloid PET scans for patients with persistent diagnostic uncertainty after other assessments.
Genetic counseling and testing: Consider genetic testing for patients with strong autosomal dominant family histories and involve a genetic counselor.
Future Directions?
Maria C. Carrillo, PhD, chief science officer and medical affairs lead for the Alzheimer’s Association, encourages clinicians to incorporate these guidelines into their practice.
“These guidelines are important because they guide clinicians in the evaluation of memory complaints, which could have many underlying causes. That is the necessary start for an early and accurate Alzheimer’s diagnosis,” Carrillo said in a statement.
Dickerson said the new guidelines do not address blood-based biomarkers “because nobody really feels that they are ready for prime time yet, even though they’re getting rolled out as clinical products.”
However, the recommendations will be revised as needed. “That’s one of the values of setting this up as a process; whenever any new development occurs, it will be easy to update the guidelines to show where that new test or new biomarker fits in the overall process,” he said.
New Appropriate Use Guidance
A separate workgroup, jointly convened by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging, has revised appropriate use criteria (AUC) for amyloid PET imaging and developed AUC for tau PET imaging.
They were simultaneously published online in Alzheimer’s & Dementia and The Journal of Nuclear Medicine. They are the first revision since the initial AUC for amyloid PET was introduced in 2013.
“The updated amyloid/tau appropriate use criteria will help ensure these tracers are used in a cost-effective manner and the scan results will be used appropriately to add value to the diagnosis and management of dementia,” said workgroup members Kevin Donohoe, MD, with Beth Israel Deaconess Medical Center, Boston, and Phillip Kuo, MD, with City of Hope National Medical Center, Duarte, California.
The AUC include 17 real-world scenarios in which amyloid or tau PET may be considered, with the two tests considered separately and given their own rating for each scenario.
Overall, the strongest evidence for their use includes assessment and prognosis for people with mild cognitive impairment; assessment of people with dementia when the cause is not clearly known; and determining eligibility for treatment with new disease-modifying therapies, and monitoring response to these treatments, the workgroup said.
“Whereas the prior AUC was written at a time when only the deposition of amyloid could be documented, the new therapeutic agents allow us to demonstrate the actual clearance of amyloid during therapy,” Donohoe and Kuo explained.
“These new therapeutic agents are expensive and, as with most medications, may cause unwanted side effects. The most recent version of the AUC includes information about the appropriate use of amyloid imaging for both documenting the presence of amyloid deposits in the brain, making anti-amyloid therapy an option, as well as documenting the effectiveness of the therapeutic agents as amyloid is (or is not) cleared from the brain,” Donahoe and Kuo noted.
The revised AUC also state that, in most cases, amyloid and tau PET tests should not be used for people who do not have cognitive impairment, even if they carry the APOE4 risk-related gene for Alzheimer’s disease; nonmedical use such as for legal concerns, insurance coverage, or employment screening; and in place of genetic testing in patients suspected of carrying a disease-causing genetic mutation.
In a statement, lead author Gil D. Rabinovici, MD, with University of California, San Francisco, emphasized that the AUC “should be considered guidelines for clinicians, not a substitute for careful clinical judgment that considers the full clinical context for each patient with cognitive complaints.”
This research was funded by the Alzheimer’s Association. Disclosures for guideline authors are available with the original articles.
A version of this article first appeared on Medscape.com.
This is the first update since 2001 for specialists and the first guideline for primary care physicians. Executive summaries of the guidelines were published in three articles online on December 23 in a special issue of Alzheimer’s & Dementia.
What’s New?
“With this guideline, we expand the scope of prior guidelines by providing recommendations for practicing clinicians on the process from start to finish,” coauthor Brad Dickerson, MD, director of the Massachusetts General Hospital Frontotemporal Disorders Unit and professor of neurology at Harvard Medical School, Boston, said in a statement.
“If clinicians adopt these recommendations and healthcare systems provide adequate resources, outcomes should improve in most patients in most practice settings,” Dickerson added in an interview.
Through a modified-Delphi approach and guideline-development process, an expert workgroup representing primary and specialty care reviewed 7374 publications, of which 133 met inclusion criteria.
Based on the information, the workgroup outlined a three-step patient-centered evaluation process, which includes assessing cognitive functional status, identifying the cognitive-behavioral syndrome based on specific symptoms, and determining the likely brain diseases or conditions causing the symptoms.
What Are the Key Recommendations?
The guidelines include 19 “practical” recommendations that are applicable to any practice setting. They capture the core elements of a high-quality evaluation and disclosure process, the author said. Here is a brief summary of the recommendations:
Initial evaluation: Perform a multitiered evaluation for patients who self-report or whose care partner or clinician reports cognitive, behavioral, or functional changes.
Patient-centered communication: Partner with the patient and/or care partner to establish shared goals for the evaluation process; assess the patient’s capacity to engage in goal setting.
Diagnostic formulation: Use a tiered approach to assessments and tests based on individual presentation, risk factors, and profile, aiming to determine the level of impairment, cognitive-behavioral syndrome, and likely causes and contributing factors.
History taking: Gather reliable information from informants about changes in cognition, activities of daily living, mood, neuropsychiatric symptoms, and sensory/motor functions. Document individualized risk factors for cognitive decline.
Examination: Conduct a comprehensive examination of cognition, mood, behavior, and a dementia-focused neurologic evaluation using validated tools.
Laboratory tests: Perform tiered, individualized laboratory evaluations, starting with routine tests for all patients.
Structural imaging: Obtain structural brain imaging (MRI preferred, CT as an alternative) to help establish a cause.
Ongoing communication: Engage in ongoing dialogue with patient/care partner to guide them throughout the diagnostic process.
Diagnostic disclosure: Share findings honestly and compassionately, explaining the syndrome, its severity, probable cause, prognosis, treatment options and support resources.
Specialist referral: Refer patients with atypical, uncertain, early-onset, or rapidly progressing symptoms to a dementia subspecialist.
Neuropsychological testing: Use in instances of diagnostic uncertainty or patients with complex clinical profiles. At a minimum, the neuropsychological evaluation should include normed neuropsychological testing of the domains of learning and memory (in particular delayed free and cued recall/recognition), attention, executive function, visuospatial function, and language.
Advanced diagnostic testing: When diagnostic uncertainty remains, obtain additional laboratory tests tailored to individual patient profiles.
Molecular imaging: In a patient with an established cognitive-behavioral syndrome in whom there is continued diagnostic uncertainty regarding cause(s) after structural imaging, a dementia specialist can obtain molecular imaging with fluorodeoxyglucose PET to improve diagnostic accuracy.
Cerebrospinal fluid (CSF) analysis: Utilize CSF biomarkers to evaluate amyloid beta and tau profiles in cases with unresolved diagnostic uncertainty.
Amyloid PET imaging: Perform amyloid PET scans for patients with persistent diagnostic uncertainty after other assessments.
Genetic counseling and testing: Consider genetic testing for patients with strong autosomal dominant family histories and involve a genetic counselor.
Future Directions?
Maria C. Carrillo, PhD, chief science officer and medical affairs lead for the Alzheimer’s Association, encourages clinicians to incorporate these guidelines into their practice.
“These guidelines are important because they guide clinicians in the evaluation of memory complaints, which could have many underlying causes. That is the necessary start for an early and accurate Alzheimer’s diagnosis,” Carrillo said in a statement.
Dickerson said the new guidelines do not address blood-based biomarkers “because nobody really feels that they are ready for prime time yet, even though they’re getting rolled out as clinical products.”
However, the recommendations will be revised as needed. “That’s one of the values of setting this up as a process; whenever any new development occurs, it will be easy to update the guidelines to show where that new test or new biomarker fits in the overall process,” he said.
New Appropriate Use Guidance
A separate workgroup, jointly convened by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging, has revised appropriate use criteria (AUC) for amyloid PET imaging and developed AUC for tau PET imaging.
They were simultaneously published online in Alzheimer’s & Dementia and The Journal of Nuclear Medicine. They are the first revision since the initial AUC for amyloid PET was introduced in 2013.
“The updated amyloid/tau appropriate use criteria will help ensure these tracers are used in a cost-effective manner and the scan results will be used appropriately to add value to the diagnosis and management of dementia,” said workgroup members Kevin Donohoe, MD, with Beth Israel Deaconess Medical Center, Boston, and Phillip Kuo, MD, with City of Hope National Medical Center, Duarte, California.
The AUC include 17 real-world scenarios in which amyloid or tau PET may be considered, with the two tests considered separately and given their own rating for each scenario.
Overall, the strongest evidence for their use includes assessment and prognosis for people with mild cognitive impairment; assessment of people with dementia when the cause is not clearly known; and determining eligibility for treatment with new disease-modifying therapies, and monitoring response to these treatments, the workgroup said.
“Whereas the prior AUC was written at a time when only the deposition of amyloid could be documented, the new therapeutic agents allow us to demonstrate the actual clearance of amyloid during therapy,” Donohoe and Kuo explained.
“These new therapeutic agents are expensive and, as with most medications, may cause unwanted side effects. The most recent version of the AUC includes information about the appropriate use of amyloid imaging for both documenting the presence of amyloid deposits in the brain, making anti-amyloid therapy an option, as well as documenting the effectiveness of the therapeutic agents as amyloid is (or is not) cleared from the brain,” Donahoe and Kuo noted.
The revised AUC also state that, in most cases, amyloid and tau PET tests should not be used for people who do not have cognitive impairment, even if they carry the APOE4 risk-related gene for Alzheimer’s disease; nonmedical use such as for legal concerns, insurance coverage, or employment screening; and in place of genetic testing in patients suspected of carrying a disease-causing genetic mutation.
In a statement, lead author Gil D. Rabinovici, MD, with University of California, San Francisco, emphasized that the AUC “should be considered guidelines for clinicians, not a substitute for careful clinical judgment that considers the full clinical context for each patient with cognitive complaints.”
This research was funded by the Alzheimer’s Association. Disclosures for guideline authors are available with the original articles.
A version of this article first appeared on Medscape.com.
This is the first update since 2001 for specialists and the first guideline for primary care physicians. Executive summaries of the guidelines were published in three articles online on December 23 in a special issue of Alzheimer’s & Dementia.
What’s New?
“With this guideline, we expand the scope of prior guidelines by providing recommendations for practicing clinicians on the process from start to finish,” coauthor Brad Dickerson, MD, director of the Massachusetts General Hospital Frontotemporal Disorders Unit and professor of neurology at Harvard Medical School, Boston, said in a statement.
“If clinicians adopt these recommendations and healthcare systems provide adequate resources, outcomes should improve in most patients in most practice settings,” Dickerson added in an interview.
Through a modified-Delphi approach and guideline-development process, an expert workgroup representing primary and specialty care reviewed 7374 publications, of which 133 met inclusion criteria.
Based on the information, the workgroup outlined a three-step patient-centered evaluation process, which includes assessing cognitive functional status, identifying the cognitive-behavioral syndrome based on specific symptoms, and determining the likely brain diseases or conditions causing the symptoms.
What Are the Key Recommendations?
The guidelines include 19 “practical” recommendations that are applicable to any practice setting. They capture the core elements of a high-quality evaluation and disclosure process, the author said. Here is a brief summary of the recommendations:
Initial evaluation: Perform a multitiered evaluation for patients who self-report or whose care partner or clinician reports cognitive, behavioral, or functional changes.
Patient-centered communication: Partner with the patient and/or care partner to establish shared goals for the evaluation process; assess the patient’s capacity to engage in goal setting.
Diagnostic formulation: Use a tiered approach to assessments and tests based on individual presentation, risk factors, and profile, aiming to determine the level of impairment, cognitive-behavioral syndrome, and likely causes and contributing factors.
History taking: Gather reliable information from informants about changes in cognition, activities of daily living, mood, neuropsychiatric symptoms, and sensory/motor functions. Document individualized risk factors for cognitive decline.
Examination: Conduct a comprehensive examination of cognition, mood, behavior, and a dementia-focused neurologic evaluation using validated tools.
Laboratory tests: Perform tiered, individualized laboratory evaluations, starting with routine tests for all patients.
Structural imaging: Obtain structural brain imaging (MRI preferred, CT as an alternative) to help establish a cause.
Ongoing communication: Engage in ongoing dialogue with patient/care partner to guide them throughout the diagnostic process.
Diagnostic disclosure: Share findings honestly and compassionately, explaining the syndrome, its severity, probable cause, prognosis, treatment options and support resources.
Specialist referral: Refer patients with atypical, uncertain, early-onset, or rapidly progressing symptoms to a dementia subspecialist.
Neuropsychological testing: Use in instances of diagnostic uncertainty or patients with complex clinical profiles. At a minimum, the neuropsychological evaluation should include normed neuropsychological testing of the domains of learning and memory (in particular delayed free and cued recall/recognition), attention, executive function, visuospatial function, and language.
Advanced diagnostic testing: When diagnostic uncertainty remains, obtain additional laboratory tests tailored to individual patient profiles.
Molecular imaging: In a patient with an established cognitive-behavioral syndrome in whom there is continued diagnostic uncertainty regarding cause(s) after structural imaging, a dementia specialist can obtain molecular imaging with fluorodeoxyglucose PET to improve diagnostic accuracy.
Cerebrospinal fluid (CSF) analysis: Utilize CSF biomarkers to evaluate amyloid beta and tau profiles in cases with unresolved diagnostic uncertainty.
Amyloid PET imaging: Perform amyloid PET scans for patients with persistent diagnostic uncertainty after other assessments.
Genetic counseling and testing: Consider genetic testing for patients with strong autosomal dominant family histories and involve a genetic counselor.
Future Directions?
Maria C. Carrillo, PhD, chief science officer and medical affairs lead for the Alzheimer’s Association, encourages clinicians to incorporate these guidelines into their practice.
“These guidelines are important because they guide clinicians in the evaluation of memory complaints, which could have many underlying causes. That is the necessary start for an early and accurate Alzheimer’s diagnosis,” Carrillo said in a statement.
Dickerson said the new guidelines do not address blood-based biomarkers “because nobody really feels that they are ready for prime time yet, even though they’re getting rolled out as clinical products.”
However, the recommendations will be revised as needed. “That’s one of the values of setting this up as a process; whenever any new development occurs, it will be easy to update the guidelines to show where that new test or new biomarker fits in the overall process,” he said.
New Appropriate Use Guidance
A separate workgroup, jointly convened by the Alzheimer’s Association and the Society of Nuclear Medicine and Molecular Imaging, has revised appropriate use criteria (AUC) for amyloid PET imaging and developed AUC for tau PET imaging.
They were simultaneously published online in Alzheimer’s & Dementia and The Journal of Nuclear Medicine. They are the first revision since the initial AUC for amyloid PET was introduced in 2013.
“The updated amyloid/tau appropriate use criteria will help ensure these tracers are used in a cost-effective manner and the scan results will be used appropriately to add value to the diagnosis and management of dementia,” said workgroup members Kevin Donohoe, MD, with Beth Israel Deaconess Medical Center, Boston, and Phillip Kuo, MD, with City of Hope National Medical Center, Duarte, California.
The AUC include 17 real-world scenarios in which amyloid or tau PET may be considered, with the two tests considered separately and given their own rating for each scenario.
Overall, the strongest evidence for their use includes assessment and prognosis for people with mild cognitive impairment; assessment of people with dementia when the cause is not clearly known; and determining eligibility for treatment with new disease-modifying therapies, and monitoring response to these treatments, the workgroup said.
“Whereas the prior AUC was written at a time when only the deposition of amyloid could be documented, the new therapeutic agents allow us to demonstrate the actual clearance of amyloid during therapy,” Donohoe and Kuo explained.
“These new therapeutic agents are expensive and, as with most medications, may cause unwanted side effects. The most recent version of the AUC includes information about the appropriate use of amyloid imaging for both documenting the presence of amyloid deposits in the brain, making anti-amyloid therapy an option, as well as documenting the effectiveness of the therapeutic agents as amyloid is (or is not) cleared from the brain,” Donahoe and Kuo noted.
The revised AUC also state that, in most cases, amyloid and tau PET tests should not be used for people who do not have cognitive impairment, even if they carry the APOE4 risk-related gene for Alzheimer’s disease; nonmedical use such as for legal concerns, insurance coverage, or employment screening; and in place of genetic testing in patients suspected of carrying a disease-causing genetic mutation.
In a statement, lead author Gil D. Rabinovici, MD, with University of California, San Francisco, emphasized that the AUC “should be considered guidelines for clinicians, not a substitute for careful clinical judgment that considers the full clinical context for each patient with cognitive complaints.”
This research was funded by the Alzheimer’s Association. Disclosures for guideline authors are available with the original articles.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S & DEMENTIA