User login
Chimeric antigen receptor (CAR) T-cell technology has been successfully used in the treatment of hematologic malignancies such as leukemias and lymphomas. Now, this technology has come to the forefront in multiple myeloma (MM).
Researchers at the National Cancer Institute, led by James N. Kochenderfer, MD, generated CAR T cells expressing the B-cell maturation antigen (BCMA), which is uniquely found on MM cells.
In the first in humans study, 24 patients received CAR-BCMA T cells at doses ranging between 0.3 and 3 x 106 CAR+ T cells/kg. Sixteen patients were treated at the highest dose. These patients had a median of 9.5 lines of prior therapy and 63% were refractory to their last treatment.
Thirteen patients had responses that were either partial or better and the overall response rate was 81%. Median event-free survival was 31 weeks. At the time of the report, 6 patients still have ongoing responses.
Patient cases
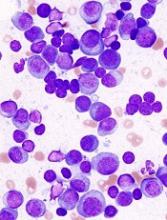
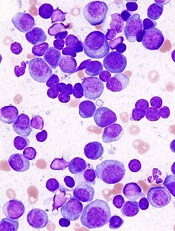
The report highlights a case study of a patient who had a large abdominal mass that resolved on computed tomography imaging, with λ light chains decreasing dramatically and becoming undetectable after CAR T-cell infusion. Recovery of normal plasma cells was noticeable with λ chain increases, but the ratio of κ to λ ratio remained normal 6 months after CAR T-cell infusion.
Using immunohistochemistry staining of CS138 before and 2 months after CAR-BMCA infusion, the researchers found effective depletion in bone marrow plasma cells in patients who were evaluated.
In patients who responded to treatment, decline in serum BCMA was also observed. However, in a patient who later progressed, BCMA increases were seen, leading the researchers to suggest that BCMA may be a tumor marker for MM.
The researchers noted that peak levels of CAR T cells occurred between 7 and 14 days after infusion and highest levels were seen in patients who showed antimyeloma responses.
Toxicity
CAR T-cell technology is also associated with accompanying toxicities.
At the highest dose, cytokine release syndrome (CRS) was a substantial toxicity especially in 2 patients who had a significant MM burden: in one case 80% of bone marrow cells were MM plasma cells and in the other the MM burden was 90%.
Six of the 16 patients required vasopressor support for hypotension and 1 patient required mechanical ventilation. The researchers noted that CRS of grade 3/4 was seen in patients who had a higher MM plasma cell burden.
The researchers indicated there is room for improvement. “The importance of persistence of CAR T cells in treating MM requires additional study,” they stated.
The sometimes low or absence of BCMA expression on MM cells may prompt the search for other antigens. “Treatment outcomes varied substantially between patients, and much room for improvement remains in improving the durability of antimyeloma responses and in reducing toxicity,” they concluded.
The researchers reported their findings in the Journal of Clinical Oncology.
Chimeric antigen receptor (CAR) T-cell technology has been successfully used in the treatment of hematologic malignancies such as leukemias and lymphomas. Now, this technology has come to the forefront in multiple myeloma (MM).
Researchers at the National Cancer Institute, led by James N. Kochenderfer, MD, generated CAR T cells expressing the B-cell maturation antigen (BCMA), which is uniquely found on MM cells.
In the first in humans study, 24 patients received CAR-BCMA T cells at doses ranging between 0.3 and 3 x 106 CAR+ T cells/kg. Sixteen patients were treated at the highest dose. These patients had a median of 9.5 lines of prior therapy and 63% were refractory to their last treatment.
Thirteen patients had responses that were either partial or better and the overall response rate was 81%. Median event-free survival was 31 weeks. At the time of the report, 6 patients still have ongoing responses.
Patient cases
The report highlights a case study of a patient who had a large abdominal mass that resolved on computed tomography imaging, with λ light chains decreasing dramatically and becoming undetectable after CAR T-cell infusion. Recovery of normal plasma cells was noticeable with λ chain increases, but the ratio of κ to λ ratio remained normal 6 months after CAR T-cell infusion.
Using immunohistochemistry staining of CS138 before and 2 months after CAR-BMCA infusion, the researchers found effective depletion in bone marrow plasma cells in patients who were evaluated.
In patients who responded to treatment, decline in serum BCMA was also observed. However, in a patient who later progressed, BCMA increases were seen, leading the researchers to suggest that BCMA may be a tumor marker for MM.
The researchers noted that peak levels of CAR T cells occurred between 7 and 14 days after infusion and highest levels were seen in patients who showed antimyeloma responses.
Toxicity
CAR T-cell technology is also associated with accompanying toxicities.
At the highest dose, cytokine release syndrome (CRS) was a substantial toxicity especially in 2 patients who had a significant MM burden: in one case 80% of bone marrow cells were MM plasma cells and in the other the MM burden was 90%.
Six of the 16 patients required vasopressor support for hypotension and 1 patient required mechanical ventilation. The researchers noted that CRS of grade 3/4 was seen in patients who had a higher MM plasma cell burden.
The researchers indicated there is room for improvement. “The importance of persistence of CAR T cells in treating MM requires additional study,” they stated.
The sometimes low or absence of BCMA expression on MM cells may prompt the search for other antigens. “Treatment outcomes varied substantially between patients, and much room for improvement remains in improving the durability of antimyeloma responses and in reducing toxicity,” they concluded.
The researchers reported their findings in the Journal of Clinical Oncology.
Chimeric antigen receptor (CAR) T-cell technology has been successfully used in the treatment of hematologic malignancies such as leukemias and lymphomas. Now, this technology has come to the forefront in multiple myeloma (MM).
Researchers at the National Cancer Institute, led by James N. Kochenderfer, MD, generated CAR T cells expressing the B-cell maturation antigen (BCMA), which is uniquely found on MM cells.
In the first in humans study, 24 patients received CAR-BCMA T cells at doses ranging between 0.3 and 3 x 106 CAR+ T cells/kg. Sixteen patients were treated at the highest dose. These patients had a median of 9.5 lines of prior therapy and 63% were refractory to their last treatment.
Thirteen patients had responses that were either partial or better and the overall response rate was 81%. Median event-free survival was 31 weeks. At the time of the report, 6 patients still have ongoing responses.
Patient cases
The report highlights a case study of a patient who had a large abdominal mass that resolved on computed tomography imaging, with λ light chains decreasing dramatically and becoming undetectable after CAR T-cell infusion. Recovery of normal plasma cells was noticeable with λ chain increases, but the ratio of κ to λ ratio remained normal 6 months after CAR T-cell infusion.
Using immunohistochemistry staining of CS138 before and 2 months after CAR-BMCA infusion, the researchers found effective depletion in bone marrow plasma cells in patients who were evaluated.
In patients who responded to treatment, decline in serum BCMA was also observed. However, in a patient who later progressed, BCMA increases were seen, leading the researchers to suggest that BCMA may be a tumor marker for MM.
The researchers noted that peak levels of CAR T cells occurred between 7 and 14 days after infusion and highest levels were seen in patients who showed antimyeloma responses.
Toxicity
CAR T-cell technology is also associated with accompanying toxicities.
At the highest dose, cytokine release syndrome (CRS) was a substantial toxicity especially in 2 patients who had a significant MM burden: in one case 80% of bone marrow cells were MM plasma cells and in the other the MM burden was 90%.
Six of the 16 patients required vasopressor support for hypotension and 1 patient required mechanical ventilation. The researchers noted that CRS of grade 3/4 was seen in patients who had a higher MM plasma cell burden.
The researchers indicated there is room for improvement. “The importance of persistence of CAR T cells in treating MM requires additional study,” they stated.
The sometimes low or absence of BCMA expression on MM cells may prompt the search for other antigens. “Treatment outcomes varied substantially between patients, and much room for improvement remains in improving the durability of antimyeloma responses and in reducing toxicity,” they concluded.
The researchers reported their findings in the Journal of Clinical Oncology.