User login
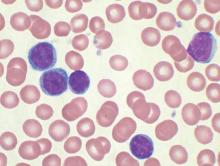
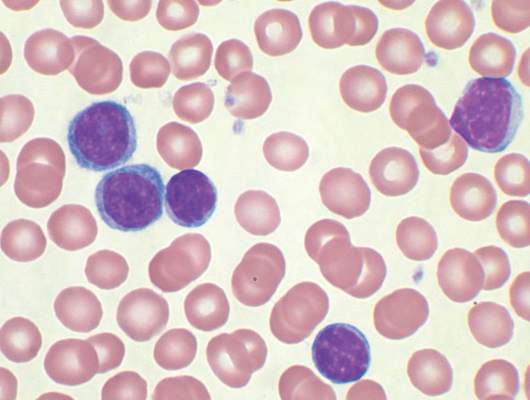
Four of eight patients with residual chronic lymphocytic leukemia (CLL) following initial chemotherapy had complete or partial responses to an outpatient therapy that used autologous T cells genetically targeted to the B cell–specific antigen CD19, Mark Blaine Geyer, MD, of Memorial Sloan Kettering Cancer Center, New York, reported at the annual meeting of the American Society of Clinical Oncology.
The therapy employing T cells genetically modified to express CD19-targeted 19-28z chimeric antigen receptors (CARs) was well tolerated but had limited observed efficacy, especially in patients with enlarged lymph nodes. The study goal was to find a safe dose of modified T cells for patients who have disease remaining after initial chemotherapy.
For the phase I dose escalation study (NCT01416974), Dr. Geyer and his associates enrolled eight CLL patients who had residual disease after upfront therapy consisting of six cycles of pentostatin, cyclophosphamide, and rituximab.
Five patients had clearly enlarged lymph nodes prior to T cell infusion.
Patients received cyclophosphamide 600 mg/m2 followed 2 days later by escalating doses of 19-28z T cells. Four of the five patients who received at least a 1 × 107 dose of 19-28z T cells/kg were admitted with fevers and mild cytokine release syndrome.
Maximal levels of CAR T cell persistence were detected at 8 weeks. With a median patient follow-up of 32 months, clinical complete response has been seen in two patients, partial response in two patients, and stable disease in one patient. Disease has progressed in three patients: one had a rising absolute lymphocyte count by the time of infusion and two had marrow response with progressive disease in lymph nodes. The median time to disease progression was 13.6 months, Dr. Geyer said.
Five of seven evaluable patients have received further CLL-directed therapy.
The researchers speculated that low-dose cyclophosphamide monotherapy, used before the CAR T-cell therapy, may be insufficient for lymphodepletion. Additionally, CAR T cell expansion and antitumor efficacy may be limited by a hostile CLL microenvironment. Strategies to enhance CAR T cell expansion and efficacy in patients with CLL are in preparation, Dr. Geyer reported.
Dr. Geyer had no financial disclosures. His colleagues reported various financial relationships with Juno Therapeutics, a developer of CAR technology.
On Twitter @maryjodales
Four of eight patients with residual chronic lymphocytic leukemia (CLL) following initial chemotherapy had complete or partial responses to an outpatient therapy that used autologous T cells genetically targeted to the B cell–specific antigen CD19, Mark Blaine Geyer, MD, of Memorial Sloan Kettering Cancer Center, New York, reported at the annual meeting of the American Society of Clinical Oncology.
The therapy employing T cells genetically modified to express CD19-targeted 19-28z chimeric antigen receptors (CARs) was well tolerated but had limited observed efficacy, especially in patients with enlarged lymph nodes. The study goal was to find a safe dose of modified T cells for patients who have disease remaining after initial chemotherapy.
For the phase I dose escalation study (NCT01416974), Dr. Geyer and his associates enrolled eight CLL patients who had residual disease after upfront therapy consisting of six cycles of pentostatin, cyclophosphamide, and rituximab.
Five patients had clearly enlarged lymph nodes prior to T cell infusion.
Patients received cyclophosphamide 600 mg/m2 followed 2 days later by escalating doses of 19-28z T cells. Four of the five patients who received at least a 1 × 107 dose of 19-28z T cells/kg were admitted with fevers and mild cytokine release syndrome.
Maximal levels of CAR T cell persistence were detected at 8 weeks. With a median patient follow-up of 32 months, clinical complete response has been seen in two patients, partial response in two patients, and stable disease in one patient. Disease has progressed in three patients: one had a rising absolute lymphocyte count by the time of infusion and two had marrow response with progressive disease in lymph nodes. The median time to disease progression was 13.6 months, Dr. Geyer said.
Five of seven evaluable patients have received further CLL-directed therapy.
The researchers speculated that low-dose cyclophosphamide monotherapy, used before the CAR T-cell therapy, may be insufficient for lymphodepletion. Additionally, CAR T cell expansion and antitumor efficacy may be limited by a hostile CLL microenvironment. Strategies to enhance CAR T cell expansion and efficacy in patients with CLL are in preparation, Dr. Geyer reported.
Dr. Geyer had no financial disclosures. His colleagues reported various financial relationships with Juno Therapeutics, a developer of CAR technology.
On Twitter @maryjodales
Four of eight patients with residual chronic lymphocytic leukemia (CLL) following initial chemotherapy had complete or partial responses to an outpatient therapy that used autologous T cells genetically targeted to the B cell–specific antigen CD19, Mark Blaine Geyer, MD, of Memorial Sloan Kettering Cancer Center, New York, reported at the annual meeting of the American Society of Clinical Oncology.
The therapy employing T cells genetically modified to express CD19-targeted 19-28z chimeric antigen receptors (CARs) was well tolerated but had limited observed efficacy, especially in patients with enlarged lymph nodes. The study goal was to find a safe dose of modified T cells for patients who have disease remaining after initial chemotherapy.
For the phase I dose escalation study (NCT01416974), Dr. Geyer and his associates enrolled eight CLL patients who had residual disease after upfront therapy consisting of six cycles of pentostatin, cyclophosphamide, and rituximab.
Five patients had clearly enlarged lymph nodes prior to T cell infusion.
Patients received cyclophosphamide 600 mg/m2 followed 2 days later by escalating doses of 19-28z T cells. Four of the five patients who received at least a 1 × 107 dose of 19-28z T cells/kg were admitted with fevers and mild cytokine release syndrome.
Maximal levels of CAR T cell persistence were detected at 8 weeks. With a median patient follow-up of 32 months, clinical complete response has been seen in two patients, partial response in two patients, and stable disease in one patient. Disease has progressed in three patients: one had a rising absolute lymphocyte count by the time of infusion and two had marrow response with progressive disease in lymph nodes. The median time to disease progression was 13.6 months, Dr. Geyer said.
Five of seven evaluable patients have received further CLL-directed therapy.
The researchers speculated that low-dose cyclophosphamide monotherapy, used before the CAR T-cell therapy, may be insufficient for lymphodepletion. Additionally, CAR T cell expansion and antitumor efficacy may be limited by a hostile CLL microenvironment. Strategies to enhance CAR T cell expansion and efficacy in patients with CLL are in preparation, Dr. Geyer reported.
Dr. Geyer had no financial disclosures. His colleagues reported various financial relationships with Juno Therapeutics, a developer of CAR technology.
On Twitter @maryjodales
FROM THE 2016 ASCO ANNUAL MEETING
Key clinical point: CAR T-cell therapy may be an option for chronic lymphocytic leukemia patients with residual disease after upfront therapy.
Major finding: Four of eight patients with residual CLL following initial chemotherapy had complete or partial responses to an outpatient therapy that used autologous T cells genetically targeted to the B cell–specific antigen CD19.
Data source: A phase I dose-finding and efficacy study in 8 patients with CLL.
Disclosures: Dr. Geyer had no financial disclosures. His colleagues reported various financial relationships with Juno Therapeutics, a developer of CAR technology.