User login
The Lewy body is the pathologic hallmark of both Parkinson disease and dementia with Lewy bodies. Lewy bodies are seen microscopically as neuronal inclusions containing alpha-synuclein and associated proteins. In contrast, glial inclusions involving alpha-synuclein are seen in multiple system atrophy. Because Lewy bodies are observed in autonomic regulatory regions of the brain, they are of interest in the study of the autonomic dysfunction that figures prominently in several parkinsonian syndromes. Cardiovascular autonomic dysfunction in parkinsonian syndromes includes orthostatic hypotension, postprandial hypotension, and supine hypertension.
This article will describe the major clinical and pathologic features of movement disorders with Lewy body pathology, the likelihood of autonomic dysregulation in these disorders, and issues involved in the treatment of autonomic dysfunction in patients with these movement disorders.
OVERVIEW OF PARKINSONIAN SYNDROMES
Tauopathies versus synucleinopathies
Pathologically, parkinsonian syndromes can be divided into two groups: the tauopathies and the synucleinopathies.
The tauopathies, so named because of the presence of hyperphosphylated tau protein, include progressive supranuclear palsy and corticobasal ganglionic degeneration as well as a number of other neurodegenerative conditions (ie, Pick disease, FTDP-17, primary progressive aphasia, argyrophilic grain disease) that do not cause parkinsonian features.
Synucleinopathies, the focus of this article, are disorders in which the protein alpha-synuclein accumulates in the cytoplasm. They include idiopathic Parkinson disease, multiple system atrophy, dementia with Lewy bodies, and pure autonomic failure. In multiple system atrophy, deposits of alpha-synuclein are prominent in glial cytoplasmic inclusions. In both Parkinson disease and dementia with Lewy bodies, alpha-synuclein is present in Lewy bodies. Although primary autonomic failure is not a movement disorder, its pathology is similar to that of the other synucleinopathies, with alpha-synuclein accumulation in both the central and peripheral nervous systems, as well as the presence of Lewy bodies.
IDIOPATHIC PARKINSON DISEASE
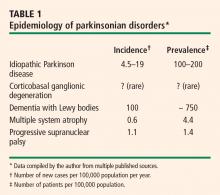
Autonomic dysfunction usually occurs late in idiopathic Parkinson disease, and its severity is less than that observed with other parkinsonian syndromes. However, the lifetime risk of significant autonomic dysfunction in patients with Parkinson disease is approximately 1 in 3.1 Almost 60% of patients with idiopathic Parkinson disease meet the criterion for a diagnosis of orthostatic hypotension—ie, a fall in systolic blood pressure of at least 20 mm Hg—and orthostatic hypotension is symptomatic in about 20% of patients.1
MULTIPLE SYSTEM ATROPHY
The nomenclature of multiple system atrophy has been evolving slowly. In 1900, Dejerine and Thomas described olivopontocerebellar atrophy, a progressive cerebellar degeneration with parkinsonism. In 1960, Shy and Drager described the Shy Drager syndrome, which has prominent autonomic features common to Parkinson disease, such as orthostatic hypotension, urinary and fecal incontinence, loss of sweating, iris atrophy, external ocular palsies, rigidity, tremor, loss of associated movement, and impotence.2
Also in 1960, Van der Eecken described striatonigral degeneration, an akinetic, rigid, parkinsonian syndrome that did not respond well to medications and was associated with autonomic dysfunction.3
In 1969, Graham and Oppenheimer realized an overlap to these syndromes and coined the term multiple system atrophy. They used it to refer to a gradually progressive idiopathic neurodegenerative process of adult onset characterized by varying proportions of cerebellar dysfunction, autonomic failure, and parkinsonism, and which is poorly responsive to levodopa therapy.4
Newer terminology is more specific for the predominant symptoms in the syndrome. A predominance of parkinsonism with this syndrome is referred to as “parkinsonian type of multiple system atrophy” (MSA-P), whereas a predominance of cerebellar signs is termed “multiple system atrophy with cerebellar-predominant symptoms” (MSA-C). The parkinsonian type is about four times as common as the cerebellar type.5 Autonomic dysfunction is common to both types, and its severity varies.
Parkinsonism is the most common symptom in multiple system atrophy, followed by autonomic failure and cerebellar signs. Approximately one fourth of patients with multiple system atrophy have all three categories of symptoms. Pyramidal signs are present in approximately 60% of patients, and help distinguish this syndrome primarily from idiopathic Parkinson disease.6
Diagnostic criteria
Autonomic dysfunction in the form of orthostatic hypotension and/or urinary incontinence is a key diagnostic criterion for multiple system atrophy.
Parkinsonism. Parkinsonian features of the syndrome are bradykinesia, rigidity, postural instability, and tremor.
Cerebellar dysfunction. Features of cerebellar dysfunction include gait ataxia, ataxic dysarthria, limb ataxia, and sustained gaze-evoked nystagmus.
Corticospinal tract dysfunction (extensor plantar response with hyperreflexia) also helps establish the diagnosis because this feature separates multiple system atrophy from idiopathic Parkinson disease as well as some of the other parkinsonian syndromes.
Diagnostic categories
The above diagnostic criteria can be combined to make a diagnosis of possible, probable, or definite multiple system atrophy.
Possible. For a diagnosis of possible multiple system atrophy, one of the above diagnostic criteria must be present along with two features from separate domains. If the case meets the criteria for parkinsonism (bradykinesia plus at least one of the other aforementioned features of parkinsonism), a poor levodopa response qualifies as a feature.
Probable. A diagnosis of probable multiple system atrophy must meet the criterion for autonomic dysfunction plus either the criterion for parkinsonism (with poor levodopa response) or the criterion for cerebellar dysfunction.
Definite. Definite multiple system atrophy requires pathological confirmation.
Extrapyramidal features in multiple system atrophy
In addition to prominent autonomic and/or cerebellar dysfunction, differences in extrapyramidal features help distinguish multiple system atrophy from idiopathic Parkinson disease. Tremor is less common in multiple system atrophy than in idiopathic Parkinson disease, and the akinetic/rigid symptoms tend to be symmetric in multiple system atrophy, rather than asymmetric as in Parkinson disease. Postural instability occurs early in multiple system atrophy but does not occur until late in idiopathic Parkinson disease. Moreover, multiple system atrophy responds poorly to levodopa and is characterized by more rapid disease progression. The presence of early autonomic and cerebellar symptoms is diagnostic for multiple system atrophy.
Pathology
The pathologic hallmark of multiple system atrophy is alpha-synuclein deposits in the glial or glial cytoplasmic inclusions (Papp-Lantos inclusions), which are diffuse through the central nervous system but are present particularly in the brainstem and spinal cord.
DEMENTIA WITH LEWY BODIES
Dementia with Lewy bodies is also known as diffuse Lewy body disease, senile dementia of the Lewy body type, Lewy body variant of Alzheimer disease, and Parkinson disease with dementia.
Dementia with Lewy bodies is descriptive for the entire series of these diseases. Pathologically, it is identical to Parkinson disease with dementia, with the only difference being an objective criterion based on the duration of dementia. Dementia less than 1 year after onset of parkinsonism is considered dementia with Lewy bodies, whereas dementia more than 1 year after the onset of parkinsonism is considered Parkinson disease with dementia. Whether these are separate disorders or two ends of a spectrum of disease is unclear.
Clinical criteria
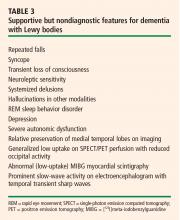
Central feature: progressive cognitive decline. Dementia with Lewy bodies is characterized by prominent progressive cognitive decline that is uncharacteristic of Parkinson disease. In particular, patients with dementia with Lewy bodies have fluctuating cognition, pronounced variations in attention, and early hallucinations when either off medications or on low doses of dopamimetic medications.
Pattern of dementia is more subcortical than cortical. The cognitive changes are different from those present in Alzheimer disease. In contrast to patients with Alzheimer disease, those with dementia with Lewy bodies have more subcortical than cortical dementia, resulting in executive dysfunction and inattention, whereas patients with Alzheimer disease have dysfunction of naming and memory.8
Pathology: diffuse distribution of Lewy bodies. As in Parkinson disease, the pathology is characterized by the appearance of Lewy bodies (positive stain for alpha-synuclein), but their distribution is more diffuse than in Parkinson disease and includes the brainstem, subcortical nuclei, limbic cortex, and neocortex, which may lead to hallucinations in affected patients.
Autonomic features are also diffuse. Autonomic features are also more common in dementia with Lewy bodies than in idiopathic Parkinson disease, which may relate to the different distribution of pathology in these diseases. Significant autonomic failure is present in 62% of patients with dementia with Lewy bodies,9 and the autonomic failure is believed to result from dysfunction of peripheral postganglionic neurons in addition to numerous cortical and brainstem Lewy bodies. Patients with dementia with Lewy bodies also have significant deposits in intermediolateral columns of the spinal cord and autonomic ganglia and sympathetic neurons.
PURE AUTONOMIC FAILURE
The pathology of pure autonomic failure is similar to that of dementia with Lewy bodies and idiopathic Parkinson disease. In contrast to these disorders, however, pure autonomic failure is characterized by a less significant presence of Lewy bodies in the cortex and brainstem, although the pathology in the spinal cord and peripheral nervous system is quite prominent.
Pure autonomic failure is a sporadic disease with onset after age 60 years. It is characterized by slowly progressive isolated impairment of the autonomic nervous system, which manifests particularly as orthostatic hypotension and also as significant bladder and sexual dysfunction. The condition is ultimately disabling as a result of the orthostatic hypotension.
CARDIOVASCULAR AUTONOMIC DYSFUNCTION
Orthostatic hypotension is the most limiting of the cardiovascular autonomic dysfunctions in the neurodegenerative disorders discussed here. Postprandial hypotension is also prevalent in these disorders, as is supine hypertension, which makes successful treatment of cardiovascular autonomic dysfunction difficult.
Orthostatic hypotension is defined as a decrease in systolic blood pressure of at least 20 mm Hg, or a decrease in diastolic blood pressure of at least 10 mm Hg, upon tilting or standing.
In contrast, in normal subjects the initial response upon standing is a pooling of 500 to 1,000 mL of blood and a reduction in venous return and cardiac output. A resultant decrease in blood pressure would occur if not for the baroreceptor reflex, which increases sympathetic tone and decreases vagal parasympathetic tone. Vasopressin is then released from the posterior pituitary, which increases peripheral vascular resistance, venous return, and cardiac output. As a result, the normal response to standing is a modest decrease in systolic blood pressure—ie, by 5 to 10 mm Hg— and an increase in diastolic blood pressure by a similar amount, as well as a compensatory increase in pulse rate of 10 to 25 beats per minute.
Approaches to therapy for orthostatic hypotension
Nonpharmacologic approaches to orthostatic hypotension include raising the head of the patient’s bed by 30 degrees, use of compression stockings, and liberalizing the use of fluids and salt.
Often, however, patients require pharmacologic therapy. Fluorohydrocortisone and midodrine are the primary drugs used for this purpose, but pyrodostigmine also has shown some efficacy in doses of 60 mg or greater in small clinical trials. Less-effective options include nonsteroidal anti-inflammatory drugs, vasopressin analogs, erythropoietin, and caffeine.
Management of postprandial hypotension
Reducing meal size while increasing the frequency of meals and adding caffeine are dietary approaches to treat postprandial hypotension. Somatostatin analogs may be helpful, although data to support their use for this indication are limited.
Treatment of supine hypertension is more difficult
The management of supine hypertension is difficult in patients with neurodegenerative disorders. Supine hypertension is defined as a blood pressure greater than 140/90 mm Hg, but the threshold for concern is uncertain. Most patients with neurodegenerative disorders are plagued more by hypotension than by hypertension, but the hypertension can be deleterious to their health, particularly when they are being treated for their hypotension during the day. Some clinicians choose to treat the hypertension if it is significant in the evening. Most important is to remove the midodrine at night and minimize the use of fluorohydrocortisone. Other options are nitrate derivatives, hydralazine, and calcium channel blockers. The proposed benefits of minoxidil and clonidine are controversial.
Autonomic complications of dopaminergic therapy
Complicating the management of autonomic dysfunction in patients with parkinsonian features is that drug therapies for Parkinson disease exacerbate orthostatic hypotension to varying degrees. Selegiline, amantadine, and dopamine agonists exacerbate orthostasis to a greater degree than levodopa does. Therefore, we are apt to start treatment with levodopa as patients develop more features of autonomic dysfunction, as well as in patients with advanced Parkinson disease or in patients who are older than 70 years of age.
Multiple system atrophy may respond only to high doses of levodopa (> 1 g), and when autonomic symptoms are prominent, patients with multiple system atrophy may not tolerate dopaminergic therapy at all. In patients with dementia with Lewy bodies, the use of dopaminergic therapies is limited not so much by autonomic dysfunction but because of hallucinations and cognitive decline.
SUMMARY
Central autonomic dysfunction predominates in patients with multiple system atrophy. Peripheral autonomic dysfunction predominates in the other parkinsonian disorders with Lewy body pathology, and this includes idiopathic Parkinson disease, dementia with Lewy bodies, and the related disorder, pure autonomic failure, in which there are no parkinsonian features.
- Magalhaes M, Wenning GK, Daniel SE, Quinn NP. Autonomic dysfunction in pathologically confirmed multiple system atrophy and idiopathic Parkinson’s disease—a retrospective comparison. Acta Neurol Scand 1995; 91:98–102.
- Shy GM, Drager GA. A neurological syndrome associated with orthostatic hypotension: a clinical-pathologic study. Arch Neurol 1960; 2:511–527.
- Van der Eecken H, Adams RD, van Bogaert L. Striopallidal-nigral degeneration. An hitherto undescribed lesion in paralysis agitans. J Neuropathol Exp Neurol 1960; 19:159–160.
- Graham JG, Oppenheimer DR. Orthostatic hypotension and nicotine sensitivity in a case of multiple system atrophy. J Neurol Neurosurg Psychiatry 1969; 32:28–34.
- Wenning GK, Geser F, Stampfer-Kountchev M, Tison F. Multiple system atrophy: an update. Mov Disord 2003; 18(Suppl 6):S34–S42.
- Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 2002; 125:861–870.
- McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65:1863–1872.
- Kraybill ML, Larson EB, Tsuang DW, et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology 2005; 64:2069–2073.
- Horimoto Y, Matsumoto M, Akatsu H, et al. Autonomic dysfunctions in dementia with Lewy bodies. J Neurol 2003; 250:530–533.
The Lewy body is the pathologic hallmark of both Parkinson disease and dementia with Lewy bodies. Lewy bodies are seen microscopically as neuronal inclusions containing alpha-synuclein and associated proteins. In contrast, glial inclusions involving alpha-synuclein are seen in multiple system atrophy. Because Lewy bodies are observed in autonomic regulatory regions of the brain, they are of interest in the study of the autonomic dysfunction that figures prominently in several parkinsonian syndromes. Cardiovascular autonomic dysfunction in parkinsonian syndromes includes orthostatic hypotension, postprandial hypotension, and supine hypertension.
This article will describe the major clinical and pathologic features of movement disorders with Lewy body pathology, the likelihood of autonomic dysregulation in these disorders, and issues involved in the treatment of autonomic dysfunction in patients with these movement disorders.
OVERVIEW OF PARKINSONIAN SYNDROMES
Tauopathies versus synucleinopathies
Pathologically, parkinsonian syndromes can be divided into two groups: the tauopathies and the synucleinopathies.
The tauopathies, so named because of the presence of hyperphosphylated tau protein, include progressive supranuclear palsy and corticobasal ganglionic degeneration as well as a number of other neurodegenerative conditions (ie, Pick disease, FTDP-17, primary progressive aphasia, argyrophilic grain disease) that do not cause parkinsonian features.
Synucleinopathies, the focus of this article, are disorders in which the protein alpha-synuclein accumulates in the cytoplasm. They include idiopathic Parkinson disease, multiple system atrophy, dementia with Lewy bodies, and pure autonomic failure. In multiple system atrophy, deposits of alpha-synuclein are prominent in glial cytoplasmic inclusions. In both Parkinson disease and dementia with Lewy bodies, alpha-synuclein is present in Lewy bodies. Although primary autonomic failure is not a movement disorder, its pathology is similar to that of the other synucleinopathies, with alpha-synuclein accumulation in both the central and peripheral nervous systems, as well as the presence of Lewy bodies.
IDIOPATHIC PARKINSON DISEASE
Autonomic dysfunction usually occurs late in idiopathic Parkinson disease, and its severity is less than that observed with other parkinsonian syndromes. However, the lifetime risk of significant autonomic dysfunction in patients with Parkinson disease is approximately 1 in 3.1 Almost 60% of patients with idiopathic Parkinson disease meet the criterion for a diagnosis of orthostatic hypotension—ie, a fall in systolic blood pressure of at least 20 mm Hg—and orthostatic hypotension is symptomatic in about 20% of patients.1
MULTIPLE SYSTEM ATROPHY
The nomenclature of multiple system atrophy has been evolving slowly. In 1900, Dejerine and Thomas described olivopontocerebellar atrophy, a progressive cerebellar degeneration with parkinsonism. In 1960, Shy and Drager described the Shy Drager syndrome, which has prominent autonomic features common to Parkinson disease, such as orthostatic hypotension, urinary and fecal incontinence, loss of sweating, iris atrophy, external ocular palsies, rigidity, tremor, loss of associated movement, and impotence.2
Also in 1960, Van der Eecken described striatonigral degeneration, an akinetic, rigid, parkinsonian syndrome that did not respond well to medications and was associated with autonomic dysfunction.3
In 1969, Graham and Oppenheimer realized an overlap to these syndromes and coined the term multiple system atrophy. They used it to refer to a gradually progressive idiopathic neurodegenerative process of adult onset characterized by varying proportions of cerebellar dysfunction, autonomic failure, and parkinsonism, and which is poorly responsive to levodopa therapy.4
Newer terminology is more specific for the predominant symptoms in the syndrome. A predominance of parkinsonism with this syndrome is referred to as “parkinsonian type of multiple system atrophy” (MSA-P), whereas a predominance of cerebellar signs is termed “multiple system atrophy with cerebellar-predominant symptoms” (MSA-C). The parkinsonian type is about four times as common as the cerebellar type.5 Autonomic dysfunction is common to both types, and its severity varies.
Parkinsonism is the most common symptom in multiple system atrophy, followed by autonomic failure and cerebellar signs. Approximately one fourth of patients with multiple system atrophy have all three categories of symptoms. Pyramidal signs are present in approximately 60% of patients, and help distinguish this syndrome primarily from idiopathic Parkinson disease.6
Diagnostic criteria
Autonomic dysfunction in the form of orthostatic hypotension and/or urinary incontinence is a key diagnostic criterion for multiple system atrophy.
Parkinsonism. Parkinsonian features of the syndrome are bradykinesia, rigidity, postural instability, and tremor.
Cerebellar dysfunction. Features of cerebellar dysfunction include gait ataxia, ataxic dysarthria, limb ataxia, and sustained gaze-evoked nystagmus.
Corticospinal tract dysfunction (extensor plantar response with hyperreflexia) also helps establish the diagnosis because this feature separates multiple system atrophy from idiopathic Parkinson disease as well as some of the other parkinsonian syndromes.
Diagnostic categories
The above diagnostic criteria can be combined to make a diagnosis of possible, probable, or definite multiple system atrophy.
Possible. For a diagnosis of possible multiple system atrophy, one of the above diagnostic criteria must be present along with two features from separate domains. If the case meets the criteria for parkinsonism (bradykinesia plus at least one of the other aforementioned features of parkinsonism), a poor levodopa response qualifies as a feature.
Probable. A diagnosis of probable multiple system atrophy must meet the criterion for autonomic dysfunction plus either the criterion for parkinsonism (with poor levodopa response) or the criterion for cerebellar dysfunction.
Definite. Definite multiple system atrophy requires pathological confirmation.
Extrapyramidal features in multiple system atrophy
In addition to prominent autonomic and/or cerebellar dysfunction, differences in extrapyramidal features help distinguish multiple system atrophy from idiopathic Parkinson disease. Tremor is less common in multiple system atrophy than in idiopathic Parkinson disease, and the akinetic/rigid symptoms tend to be symmetric in multiple system atrophy, rather than asymmetric as in Parkinson disease. Postural instability occurs early in multiple system atrophy but does not occur until late in idiopathic Parkinson disease. Moreover, multiple system atrophy responds poorly to levodopa and is characterized by more rapid disease progression. The presence of early autonomic and cerebellar symptoms is diagnostic for multiple system atrophy.
Pathology
The pathologic hallmark of multiple system atrophy is alpha-synuclein deposits in the glial or glial cytoplasmic inclusions (Papp-Lantos inclusions), which are diffuse through the central nervous system but are present particularly in the brainstem and spinal cord.
DEMENTIA WITH LEWY BODIES
Dementia with Lewy bodies is also known as diffuse Lewy body disease, senile dementia of the Lewy body type, Lewy body variant of Alzheimer disease, and Parkinson disease with dementia.
Dementia with Lewy bodies is descriptive for the entire series of these diseases. Pathologically, it is identical to Parkinson disease with dementia, with the only difference being an objective criterion based on the duration of dementia. Dementia less than 1 year after onset of parkinsonism is considered dementia with Lewy bodies, whereas dementia more than 1 year after the onset of parkinsonism is considered Parkinson disease with dementia. Whether these are separate disorders or two ends of a spectrum of disease is unclear.
Clinical criteria
Central feature: progressive cognitive decline. Dementia with Lewy bodies is characterized by prominent progressive cognitive decline that is uncharacteristic of Parkinson disease. In particular, patients with dementia with Lewy bodies have fluctuating cognition, pronounced variations in attention, and early hallucinations when either off medications or on low doses of dopamimetic medications.
Pattern of dementia is more subcortical than cortical. The cognitive changes are different from those present in Alzheimer disease. In contrast to patients with Alzheimer disease, those with dementia with Lewy bodies have more subcortical than cortical dementia, resulting in executive dysfunction and inattention, whereas patients with Alzheimer disease have dysfunction of naming and memory.8
Pathology: diffuse distribution of Lewy bodies. As in Parkinson disease, the pathology is characterized by the appearance of Lewy bodies (positive stain for alpha-synuclein), but their distribution is more diffuse than in Parkinson disease and includes the brainstem, subcortical nuclei, limbic cortex, and neocortex, which may lead to hallucinations in affected patients.
Autonomic features are also diffuse. Autonomic features are also more common in dementia with Lewy bodies than in idiopathic Parkinson disease, which may relate to the different distribution of pathology in these diseases. Significant autonomic failure is present in 62% of patients with dementia with Lewy bodies,9 and the autonomic failure is believed to result from dysfunction of peripheral postganglionic neurons in addition to numerous cortical and brainstem Lewy bodies. Patients with dementia with Lewy bodies also have significant deposits in intermediolateral columns of the spinal cord and autonomic ganglia and sympathetic neurons.
PURE AUTONOMIC FAILURE
The pathology of pure autonomic failure is similar to that of dementia with Lewy bodies and idiopathic Parkinson disease. In contrast to these disorders, however, pure autonomic failure is characterized by a less significant presence of Lewy bodies in the cortex and brainstem, although the pathology in the spinal cord and peripheral nervous system is quite prominent.
Pure autonomic failure is a sporadic disease with onset after age 60 years. It is characterized by slowly progressive isolated impairment of the autonomic nervous system, which manifests particularly as orthostatic hypotension and also as significant bladder and sexual dysfunction. The condition is ultimately disabling as a result of the orthostatic hypotension.
CARDIOVASCULAR AUTONOMIC DYSFUNCTION
Orthostatic hypotension is the most limiting of the cardiovascular autonomic dysfunctions in the neurodegenerative disorders discussed here. Postprandial hypotension is also prevalent in these disorders, as is supine hypertension, which makes successful treatment of cardiovascular autonomic dysfunction difficult.
Orthostatic hypotension is defined as a decrease in systolic blood pressure of at least 20 mm Hg, or a decrease in diastolic blood pressure of at least 10 mm Hg, upon tilting or standing.
In contrast, in normal subjects the initial response upon standing is a pooling of 500 to 1,000 mL of blood and a reduction in venous return and cardiac output. A resultant decrease in blood pressure would occur if not for the baroreceptor reflex, which increases sympathetic tone and decreases vagal parasympathetic tone. Vasopressin is then released from the posterior pituitary, which increases peripheral vascular resistance, venous return, and cardiac output. As a result, the normal response to standing is a modest decrease in systolic blood pressure—ie, by 5 to 10 mm Hg— and an increase in diastolic blood pressure by a similar amount, as well as a compensatory increase in pulse rate of 10 to 25 beats per minute.
Approaches to therapy for orthostatic hypotension
Nonpharmacologic approaches to orthostatic hypotension include raising the head of the patient’s bed by 30 degrees, use of compression stockings, and liberalizing the use of fluids and salt.
Often, however, patients require pharmacologic therapy. Fluorohydrocortisone and midodrine are the primary drugs used for this purpose, but pyrodostigmine also has shown some efficacy in doses of 60 mg or greater in small clinical trials. Less-effective options include nonsteroidal anti-inflammatory drugs, vasopressin analogs, erythropoietin, and caffeine.
Management of postprandial hypotension
Reducing meal size while increasing the frequency of meals and adding caffeine are dietary approaches to treat postprandial hypotension. Somatostatin analogs may be helpful, although data to support their use for this indication are limited.
Treatment of supine hypertension is more difficult
The management of supine hypertension is difficult in patients with neurodegenerative disorders. Supine hypertension is defined as a blood pressure greater than 140/90 mm Hg, but the threshold for concern is uncertain. Most patients with neurodegenerative disorders are plagued more by hypotension than by hypertension, but the hypertension can be deleterious to their health, particularly when they are being treated for their hypotension during the day. Some clinicians choose to treat the hypertension if it is significant in the evening. Most important is to remove the midodrine at night and minimize the use of fluorohydrocortisone. Other options are nitrate derivatives, hydralazine, and calcium channel blockers. The proposed benefits of minoxidil and clonidine are controversial.
Autonomic complications of dopaminergic therapy
Complicating the management of autonomic dysfunction in patients with parkinsonian features is that drug therapies for Parkinson disease exacerbate orthostatic hypotension to varying degrees. Selegiline, amantadine, and dopamine agonists exacerbate orthostasis to a greater degree than levodopa does. Therefore, we are apt to start treatment with levodopa as patients develop more features of autonomic dysfunction, as well as in patients with advanced Parkinson disease or in patients who are older than 70 years of age.
Multiple system atrophy may respond only to high doses of levodopa (> 1 g), and when autonomic symptoms are prominent, patients with multiple system atrophy may not tolerate dopaminergic therapy at all. In patients with dementia with Lewy bodies, the use of dopaminergic therapies is limited not so much by autonomic dysfunction but because of hallucinations and cognitive decline.
SUMMARY
Central autonomic dysfunction predominates in patients with multiple system atrophy. Peripheral autonomic dysfunction predominates in the other parkinsonian disorders with Lewy body pathology, and this includes idiopathic Parkinson disease, dementia with Lewy bodies, and the related disorder, pure autonomic failure, in which there are no parkinsonian features.
The Lewy body is the pathologic hallmark of both Parkinson disease and dementia with Lewy bodies. Lewy bodies are seen microscopically as neuronal inclusions containing alpha-synuclein and associated proteins. In contrast, glial inclusions involving alpha-synuclein are seen in multiple system atrophy. Because Lewy bodies are observed in autonomic regulatory regions of the brain, they are of interest in the study of the autonomic dysfunction that figures prominently in several parkinsonian syndromes. Cardiovascular autonomic dysfunction in parkinsonian syndromes includes orthostatic hypotension, postprandial hypotension, and supine hypertension.
This article will describe the major clinical and pathologic features of movement disorders with Lewy body pathology, the likelihood of autonomic dysregulation in these disorders, and issues involved in the treatment of autonomic dysfunction in patients with these movement disorders.
OVERVIEW OF PARKINSONIAN SYNDROMES
Tauopathies versus synucleinopathies
Pathologically, parkinsonian syndromes can be divided into two groups: the tauopathies and the synucleinopathies.
The tauopathies, so named because of the presence of hyperphosphylated tau protein, include progressive supranuclear palsy and corticobasal ganglionic degeneration as well as a number of other neurodegenerative conditions (ie, Pick disease, FTDP-17, primary progressive aphasia, argyrophilic grain disease) that do not cause parkinsonian features.
Synucleinopathies, the focus of this article, are disorders in which the protein alpha-synuclein accumulates in the cytoplasm. They include idiopathic Parkinson disease, multiple system atrophy, dementia with Lewy bodies, and pure autonomic failure. In multiple system atrophy, deposits of alpha-synuclein are prominent in glial cytoplasmic inclusions. In both Parkinson disease and dementia with Lewy bodies, alpha-synuclein is present in Lewy bodies. Although primary autonomic failure is not a movement disorder, its pathology is similar to that of the other synucleinopathies, with alpha-synuclein accumulation in both the central and peripheral nervous systems, as well as the presence of Lewy bodies.
IDIOPATHIC PARKINSON DISEASE
Autonomic dysfunction usually occurs late in idiopathic Parkinson disease, and its severity is less than that observed with other parkinsonian syndromes. However, the lifetime risk of significant autonomic dysfunction in patients with Parkinson disease is approximately 1 in 3.1 Almost 60% of patients with idiopathic Parkinson disease meet the criterion for a diagnosis of orthostatic hypotension—ie, a fall in systolic blood pressure of at least 20 mm Hg—and orthostatic hypotension is symptomatic in about 20% of patients.1
MULTIPLE SYSTEM ATROPHY
The nomenclature of multiple system atrophy has been evolving slowly. In 1900, Dejerine and Thomas described olivopontocerebellar atrophy, a progressive cerebellar degeneration with parkinsonism. In 1960, Shy and Drager described the Shy Drager syndrome, which has prominent autonomic features common to Parkinson disease, such as orthostatic hypotension, urinary and fecal incontinence, loss of sweating, iris atrophy, external ocular palsies, rigidity, tremor, loss of associated movement, and impotence.2
Also in 1960, Van der Eecken described striatonigral degeneration, an akinetic, rigid, parkinsonian syndrome that did not respond well to medications and was associated with autonomic dysfunction.3
In 1969, Graham and Oppenheimer realized an overlap to these syndromes and coined the term multiple system atrophy. They used it to refer to a gradually progressive idiopathic neurodegenerative process of adult onset characterized by varying proportions of cerebellar dysfunction, autonomic failure, and parkinsonism, and which is poorly responsive to levodopa therapy.4
Newer terminology is more specific for the predominant symptoms in the syndrome. A predominance of parkinsonism with this syndrome is referred to as “parkinsonian type of multiple system atrophy” (MSA-P), whereas a predominance of cerebellar signs is termed “multiple system atrophy with cerebellar-predominant symptoms” (MSA-C). The parkinsonian type is about four times as common as the cerebellar type.5 Autonomic dysfunction is common to both types, and its severity varies.
Parkinsonism is the most common symptom in multiple system atrophy, followed by autonomic failure and cerebellar signs. Approximately one fourth of patients with multiple system atrophy have all three categories of symptoms. Pyramidal signs are present in approximately 60% of patients, and help distinguish this syndrome primarily from idiopathic Parkinson disease.6
Diagnostic criteria
Autonomic dysfunction in the form of orthostatic hypotension and/or urinary incontinence is a key diagnostic criterion for multiple system atrophy.
Parkinsonism. Parkinsonian features of the syndrome are bradykinesia, rigidity, postural instability, and tremor.
Cerebellar dysfunction. Features of cerebellar dysfunction include gait ataxia, ataxic dysarthria, limb ataxia, and sustained gaze-evoked nystagmus.
Corticospinal tract dysfunction (extensor plantar response with hyperreflexia) also helps establish the diagnosis because this feature separates multiple system atrophy from idiopathic Parkinson disease as well as some of the other parkinsonian syndromes.
Diagnostic categories
The above diagnostic criteria can be combined to make a diagnosis of possible, probable, or definite multiple system atrophy.
Possible. For a diagnosis of possible multiple system atrophy, one of the above diagnostic criteria must be present along with two features from separate domains. If the case meets the criteria for parkinsonism (bradykinesia plus at least one of the other aforementioned features of parkinsonism), a poor levodopa response qualifies as a feature.
Probable. A diagnosis of probable multiple system atrophy must meet the criterion for autonomic dysfunction plus either the criterion for parkinsonism (with poor levodopa response) or the criterion for cerebellar dysfunction.
Definite. Definite multiple system atrophy requires pathological confirmation.
Extrapyramidal features in multiple system atrophy
In addition to prominent autonomic and/or cerebellar dysfunction, differences in extrapyramidal features help distinguish multiple system atrophy from idiopathic Parkinson disease. Tremor is less common in multiple system atrophy than in idiopathic Parkinson disease, and the akinetic/rigid symptoms tend to be symmetric in multiple system atrophy, rather than asymmetric as in Parkinson disease. Postural instability occurs early in multiple system atrophy but does not occur until late in idiopathic Parkinson disease. Moreover, multiple system atrophy responds poorly to levodopa and is characterized by more rapid disease progression. The presence of early autonomic and cerebellar symptoms is diagnostic for multiple system atrophy.
Pathology
The pathologic hallmark of multiple system atrophy is alpha-synuclein deposits in the glial or glial cytoplasmic inclusions (Papp-Lantos inclusions), which are diffuse through the central nervous system but are present particularly in the brainstem and spinal cord.
DEMENTIA WITH LEWY BODIES
Dementia with Lewy bodies is also known as diffuse Lewy body disease, senile dementia of the Lewy body type, Lewy body variant of Alzheimer disease, and Parkinson disease with dementia.
Dementia with Lewy bodies is descriptive for the entire series of these diseases. Pathologically, it is identical to Parkinson disease with dementia, with the only difference being an objective criterion based on the duration of dementia. Dementia less than 1 year after onset of parkinsonism is considered dementia with Lewy bodies, whereas dementia more than 1 year after the onset of parkinsonism is considered Parkinson disease with dementia. Whether these are separate disorders or two ends of a spectrum of disease is unclear.
Clinical criteria
Central feature: progressive cognitive decline. Dementia with Lewy bodies is characterized by prominent progressive cognitive decline that is uncharacteristic of Parkinson disease. In particular, patients with dementia with Lewy bodies have fluctuating cognition, pronounced variations in attention, and early hallucinations when either off medications or on low doses of dopamimetic medications.
Pattern of dementia is more subcortical than cortical. The cognitive changes are different from those present in Alzheimer disease. In contrast to patients with Alzheimer disease, those with dementia with Lewy bodies have more subcortical than cortical dementia, resulting in executive dysfunction and inattention, whereas patients with Alzheimer disease have dysfunction of naming and memory.8
Pathology: diffuse distribution of Lewy bodies. As in Parkinson disease, the pathology is characterized by the appearance of Lewy bodies (positive stain for alpha-synuclein), but their distribution is more diffuse than in Parkinson disease and includes the brainstem, subcortical nuclei, limbic cortex, and neocortex, which may lead to hallucinations in affected patients.
Autonomic features are also diffuse. Autonomic features are also more common in dementia with Lewy bodies than in idiopathic Parkinson disease, which may relate to the different distribution of pathology in these diseases. Significant autonomic failure is present in 62% of patients with dementia with Lewy bodies,9 and the autonomic failure is believed to result from dysfunction of peripheral postganglionic neurons in addition to numerous cortical and brainstem Lewy bodies. Patients with dementia with Lewy bodies also have significant deposits in intermediolateral columns of the spinal cord and autonomic ganglia and sympathetic neurons.
PURE AUTONOMIC FAILURE
The pathology of pure autonomic failure is similar to that of dementia with Lewy bodies and idiopathic Parkinson disease. In contrast to these disorders, however, pure autonomic failure is characterized by a less significant presence of Lewy bodies in the cortex and brainstem, although the pathology in the spinal cord and peripheral nervous system is quite prominent.
Pure autonomic failure is a sporadic disease with onset after age 60 years. It is characterized by slowly progressive isolated impairment of the autonomic nervous system, which manifests particularly as orthostatic hypotension and also as significant bladder and sexual dysfunction. The condition is ultimately disabling as a result of the orthostatic hypotension.
CARDIOVASCULAR AUTONOMIC DYSFUNCTION
Orthostatic hypotension is the most limiting of the cardiovascular autonomic dysfunctions in the neurodegenerative disorders discussed here. Postprandial hypotension is also prevalent in these disorders, as is supine hypertension, which makes successful treatment of cardiovascular autonomic dysfunction difficult.
Orthostatic hypotension is defined as a decrease in systolic blood pressure of at least 20 mm Hg, or a decrease in diastolic blood pressure of at least 10 mm Hg, upon tilting or standing.
In contrast, in normal subjects the initial response upon standing is a pooling of 500 to 1,000 mL of blood and a reduction in venous return and cardiac output. A resultant decrease in blood pressure would occur if not for the baroreceptor reflex, which increases sympathetic tone and decreases vagal parasympathetic tone. Vasopressin is then released from the posterior pituitary, which increases peripheral vascular resistance, venous return, and cardiac output. As a result, the normal response to standing is a modest decrease in systolic blood pressure—ie, by 5 to 10 mm Hg— and an increase in diastolic blood pressure by a similar amount, as well as a compensatory increase in pulse rate of 10 to 25 beats per minute.
Approaches to therapy for orthostatic hypotension
Nonpharmacologic approaches to orthostatic hypotension include raising the head of the patient’s bed by 30 degrees, use of compression stockings, and liberalizing the use of fluids and salt.
Often, however, patients require pharmacologic therapy. Fluorohydrocortisone and midodrine are the primary drugs used for this purpose, but pyrodostigmine also has shown some efficacy in doses of 60 mg or greater in small clinical trials. Less-effective options include nonsteroidal anti-inflammatory drugs, vasopressin analogs, erythropoietin, and caffeine.
Management of postprandial hypotension
Reducing meal size while increasing the frequency of meals and adding caffeine are dietary approaches to treat postprandial hypotension. Somatostatin analogs may be helpful, although data to support their use for this indication are limited.
Treatment of supine hypertension is more difficult
The management of supine hypertension is difficult in patients with neurodegenerative disorders. Supine hypertension is defined as a blood pressure greater than 140/90 mm Hg, but the threshold for concern is uncertain. Most patients with neurodegenerative disorders are plagued more by hypotension than by hypertension, but the hypertension can be deleterious to their health, particularly when they are being treated for their hypotension during the day. Some clinicians choose to treat the hypertension if it is significant in the evening. Most important is to remove the midodrine at night and minimize the use of fluorohydrocortisone. Other options are nitrate derivatives, hydralazine, and calcium channel blockers. The proposed benefits of minoxidil and clonidine are controversial.
Autonomic complications of dopaminergic therapy
Complicating the management of autonomic dysfunction in patients with parkinsonian features is that drug therapies for Parkinson disease exacerbate orthostatic hypotension to varying degrees. Selegiline, amantadine, and dopamine agonists exacerbate orthostasis to a greater degree than levodopa does. Therefore, we are apt to start treatment with levodopa as patients develop more features of autonomic dysfunction, as well as in patients with advanced Parkinson disease or in patients who are older than 70 years of age.
Multiple system atrophy may respond only to high doses of levodopa (> 1 g), and when autonomic symptoms are prominent, patients with multiple system atrophy may not tolerate dopaminergic therapy at all. In patients with dementia with Lewy bodies, the use of dopaminergic therapies is limited not so much by autonomic dysfunction but because of hallucinations and cognitive decline.
SUMMARY
Central autonomic dysfunction predominates in patients with multiple system atrophy. Peripheral autonomic dysfunction predominates in the other parkinsonian disorders with Lewy body pathology, and this includes idiopathic Parkinson disease, dementia with Lewy bodies, and the related disorder, pure autonomic failure, in which there are no parkinsonian features.
- Magalhaes M, Wenning GK, Daniel SE, Quinn NP. Autonomic dysfunction in pathologically confirmed multiple system atrophy and idiopathic Parkinson’s disease—a retrospective comparison. Acta Neurol Scand 1995; 91:98–102.
- Shy GM, Drager GA. A neurological syndrome associated with orthostatic hypotension: a clinical-pathologic study. Arch Neurol 1960; 2:511–527.
- Van der Eecken H, Adams RD, van Bogaert L. Striopallidal-nigral degeneration. An hitherto undescribed lesion in paralysis agitans. J Neuropathol Exp Neurol 1960; 19:159–160.
- Graham JG, Oppenheimer DR. Orthostatic hypotension and nicotine sensitivity in a case of multiple system atrophy. J Neurol Neurosurg Psychiatry 1969; 32:28–34.
- Wenning GK, Geser F, Stampfer-Kountchev M, Tison F. Multiple system atrophy: an update. Mov Disord 2003; 18(Suppl 6):S34–S42.
- Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 2002; 125:861–870.
- McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65:1863–1872.
- Kraybill ML, Larson EB, Tsuang DW, et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology 2005; 64:2069–2073.
- Horimoto Y, Matsumoto M, Akatsu H, et al. Autonomic dysfunctions in dementia with Lewy bodies. J Neurol 2003; 250:530–533.
- Magalhaes M, Wenning GK, Daniel SE, Quinn NP. Autonomic dysfunction in pathologically confirmed multiple system atrophy and idiopathic Parkinson’s disease—a retrospective comparison. Acta Neurol Scand 1995; 91:98–102.
- Shy GM, Drager GA. A neurological syndrome associated with orthostatic hypotension: a clinical-pathologic study. Arch Neurol 1960; 2:511–527.
- Van der Eecken H, Adams RD, van Bogaert L. Striopallidal-nigral degeneration. An hitherto undescribed lesion in paralysis agitans. J Neuropathol Exp Neurol 1960; 19:159–160.
- Graham JG, Oppenheimer DR. Orthostatic hypotension and nicotine sensitivity in a case of multiple system atrophy. J Neurol Neurosurg Psychiatry 1969; 32:28–34.
- Wenning GK, Geser F, Stampfer-Kountchev M, Tison F. Multiple system atrophy: an update. Mov Disord 2003; 18(Suppl 6):S34–S42.
- Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 2002; 125:861–870.
- McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65:1863–1872.
- Kraybill ML, Larson EB, Tsuang DW, et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology 2005; 64:2069–2073.
- Horimoto Y, Matsumoto M, Akatsu H, et al. Autonomic dysfunctions in dementia with Lewy bodies. J Neurol 2003; 250:530–533.