User login
New technologies that solve some old problems could make cardiovascular CT the go-to imaging tool to assess both anatomy and function.
Cardiovascular CT imaging has been a welcome tool in patient assessment due to its noninvasive nature, ease, and quickness. Likewise, cardiovascular CT angiography (CCTA) has provided noninvasive information about vessel stenoses, with lower costs and radiation exposure compared with invasive angiography.
Perhaps the biggest criticism about cardiovascular CT is that, while it provides very good anatomic detail, functional information – a crucial piece to the overall picture – is not discernable.
"Historically, coronary CT angiography has been an extremely anatomic method for documenting the severity of coronary artery stenoses or luminal compromise," said Dr. James K. Min, immediate past president of SCCT. This has left CTA open to the criticism that the more useful evaluation of coronary artery disease is not only to document high-grade stenoses – as with coronary CT angiography – but also to determine the physiologic significance of those stenoses.
However, at this year’s meeting of the Society of Cardiovascular Computed Tomography in Baltimore, "we saw the emergence of two robust methods for physiologic assessment of coronary disease by cardiac CT," he said. These are fractional flow reserve CT and CT-based myocardial perfusion imaging.
Evidence regarding these methods was then bolstered at the annual congress of the European Society of Cardiology in Munich, with the presentation of two imaging trials. The DeFACTO study, comparing CT-based FFR with FFR obtained by conventional angiography, did not show diagnostic superiority of CT-based FFR over angiography, but it did enhance diagnoses compared with CT without FFR. The CORE320 trial, assessing the diagnostic accuracy CT-based myocardial perfusion imaging in patients with suspected coronary artery disease, showed that CT-based myocardial perfusion imaging significantly increased the diagnostic accuracy of CTA alone to delineate flow-limiting disease.
Cardiovascular CT also has been hamstrung by a number of technical limitations. Calcium blooming artifacts make it difficult to get an accurate measurement of calcium lesion components and lumen size and motion artifacts also limit the clarity of CT images, leaving some vessels simply unreadable.
Two new technological advances – dual-energy CT and intelligent motion correction using snapshot freeze – are already changing practice, according to leaders at this year’s annual meeting of the SCCT.
CT Fractional Flow Reserve
Fractional flow reserve (FFR) is defined as the maximal blood flow through a diseased artery to the blood flow in the hypothetical case that this artery is normal. CT-based FFR relies on the use of some complicated math and modeling to calculate FFR from CT data and actually traces its roots back to the air flow modeling that goes into designing airplane wings.
All of the data needed can be gleaned from a single-phase static CT image. Geometry can be extracted from CCTA anatomic data. Boundary conditions can also be determined. Resting coronary blood flow can be calculated from myocardial mass. Mean blood pressure can be estimated from brachial artery pressure. Coronary microcirculatory resistance can be derived from morphometry data. Lastly, fluid properties include the viscosity and density of blood. "Putting it all together, we can come out with a calculated assessment of things like coronary flow and FFR ... now, we can do anatomy and function," said Dr. Matthew J. Budoff, director of cardiology at the University of California at Los Angeles School of Medicine in Torrance.
Until now, FFR was determined through invasive coronary angiography, which was the only method for specific determination of coronary artery lesions (lesion-specific ischemia). Values of 0.80 and lower or 0.75 and lower are considered diagnostic of lesion-specific ischemia. "It tells us whether there is a physiologic significance of the lesion," Dr. Budoff said.
At the same time, stenosis seen on CCTA has been an unreliable predictor of lesion-specific ischemia in trials. "Just seeing that anatomical stenosis doesn’t mean it’s functionally significant," said Dr. Budoff. CCTA results in a lot of false positives when it comes to physiologic significance.
FFR estimates throughout the entire coronary tree. "Because it relies on the entire coronary anatomy, it’s actually less sensitive to artifacts and less sensitive to things like calcification than individual segment assessment."
The first trial of FFR-CT was in the Diagnosis of Ischemia-Causing Stenoses Obtained via Noninvasive Fractional Flow Reserve (DISCOVER-FLOW) trial. In the study, the researchers compared the accuracy of CCTA with FFR-CT (invasive FFR served as the reference).
The results showed that the coronary stenoses that cause ischemia can be identified noninvasively with computer analysis of CCTA to construct FFR for specific lesions (Eur. Heart J. Cardiovasc. Imaging 2012 Jul 15 [doi: 10.1093/ehjci/jes130]).
The upshot of this study is that "when we think it’s a high-grade stenosis [with FFR-CT], it is a physiologically significant stenosis," Dr. Budoff said. "So we’ve dramatically improved the ability of CT to correlate with the most invasive and probably the most definitive way, currently, of assessing physiologic significance, and that’s FFR."
CT-based fractional flow reserve (FFR) is now a step closer to clinical use with the results of the Determination of FFR by Anatomic CT Angiography (DeFACTO) trial presented at the ESC meeting and simultaneously published (JAMA 2012 Aug. 26 [doi:10.1001/2012.jama.11274]). The addition of CT-based FFR information to CT alone improved diagnostic accuracy of stenoses, compared with invasive angiography and FFR.
"We’ve dramatically improved the ability of CT to correlate with ... FFR," said Dr. Matthew J. Budoff.
Although FFR-CT plus CT narrowly failed to meet the trial’s primary end point – diagnostic accuracy greater than 70% for the lower bound of the 95% confidence interval, the per-patient performance diagnostic accuracy of FFR-CT plus CT was 73% with a 95% CI of 67%-78%.
Nevertheless, the addition of FFR-CT "demonstrated superior diagnostic performance characteristics, as compared with CT stenosis alone in all patients, in all vessels and also in vessels of intermediate stenosis severity," Dr. Min said during a press conference.
Myocardial Perfusion
"The second method of physiologic assessment of coronary disease, which is emerging, is myocardial perfusion imaging – performing a typical stress test with a CT angiogram," said Dr. Min.
This allows the visualization of perfusion/ischemia at specific lesions identified by CTA, providing functional and anatomic information. The data for perfusion images are extracted from a standard CTA scan.
With CT perfusion, a rest CT angiogram is performed to document the coronary artery stenoses that are within the coronary artery bed and also to look at the rest perfusion of the myocardium to determine whether it’s normal or abnormal, according to Dr. Min.
Before or after the rest CTA, a stress CT would be performed via pharmacologic means using a traditional 64-row cardiac CT.
It’s known that patients who have a normal SPECT myocardial perfusion examination have a very low rate of cardiovascular events over the next year, according to Dr. Richard T. George. "However, those patients with an abnormal nuclear scan actually have a quite high event rate over the next year."
In addition, it’s also known that CCTA has great prognostic value "and probably for a longer period than SPECT does, but SPECT myocardial perfusion imaging probably tells you more about the intermediate time period in the future about the event rate in the patient," he said.
Several studies have looked at the additional value of CT perfusion testing.
One of the first studies that compared CCTA and CT perfusion (CTP) with quantitative coronary angiography and SPECT perfusion showed a sensitivity with CTA/CTP of 88% and a specificity of 91% (Circ. Cardiovasc. Imaging 2009;2:174-82). "This study demonstrates some of the additional value of stress CT myocardial perfusion imaging," said Dr. George, assistant professor of medicine at the Heart and Vascular Institute at Johns Hopkins Hospital in Baltimore.
In another study, researchers assessed the additional value of dipyridamole stress myocardial perfusion by 64-row CT in patients with coronary stents (J. Cardiovasc. Comput. Tomogr. 2011;5:449-58). It is often difficult in many of these patients to assess whether in-stent restenosis is present, said Dr. George. The researchers in this study found that the addition of CT myocardial perfusion imaging to CCTA improved accuracy.
In a study targeting reversible ischemia, researchers assessed CT myocardial perfusion imaging with 320-row detector CT in 50 patients with an intermediate- to high-risk for CAD (Circ. Cardiovasc. Imaging 2012;5:333-40).
"The important part of this study is that they actually looked at reversible ischemia. A lot of our studies with CT perfusion imaging just lump all perfusion deficits together." In it, 40% of patients had an abnormal SPECT scan; 90% of those abnormal scans were reversible ischemia," said Dr. George.
In a per-patient analysis of CT perfusion imaging vs. CTA stenosis greater than 50% in the setting of a territorial SPECT myocardial perfusion deficit, sensitivity was 100% and specificity was 81%. The study shows the effectiveness of CT perfusion for assessing lesion-specific ischemia, Dr. George noted.
"What we’re finding is that CT perfusion or stress testing with CT can be as accurate, if not more accurate, than the conventional stress testing methods that we’ve used until this day," Dr. Min said.
"CT perfusion or stress testing with CT can be as accurate, if not more accurate, than the conventional stress testing method," said Dr. James K. Min.
In the Combined Non-invasive Coronary Angiography and Myocardial Perfusion Imaging Using 320 Detector Computed Tomography (Core320) trial, researchers compared the diagnostic accuracy of CTA plus CT-based myocardial perfusion against ICA plus SPECT myocardial perfusion imaging in 381 patients with suspected or diagnosed coronary artery disease with a clinical indication for coronary angiography.
"When we add perfusion [to CTA alone] we gain power to diagnose flow-limiting stenosis," lead author Dr. Joao A.C. Lima said at the ESC meeting. Together, "they have the same power as invasive angiography and SPECT MPI in defining who are the patients who end up going through revascularization," he said.
The area under the receiver operating characteristic curve (ROC) – an effective method of evaluating the performance of diagnostic tests – was 0.79 for CTA-CTP and 0.81 for ICA-SPECT.
"At this point, the [CT perfusion] technology could be used precisely to find the patients who have flow-limiting disease and, therefore, are going to need revascularization," said Dr. Lima, director of cardiovascular imaging at the Johns Hopkins University in Baltimore.
Dual-Energy CT
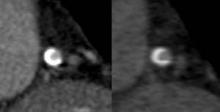
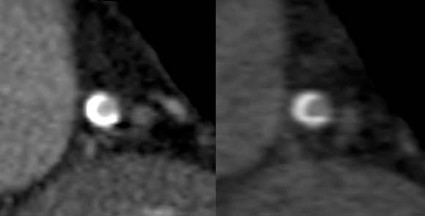
Dual-energy CT (also known as dual-source CT) "interleaves sets of high- and low-keV images. The energy alternates between two energies (80 keV to 140 keV) every 0.5 msec," said Dr. James P. Earls. The result is two competing sets that can be used simultaneously. The importance of this dual-energy approach is that with higher energies and a software algorithm, calcium blooming is decreased. This means a more accurate size of calcium in lesions and lumen size.
Back-end software produces a range of monochromatic images (images at a single energy). "In terms of calcium reduction, the monochromatic images are probably more important," said Dr. Earls, codirector of the cardiac CT program at Inova Heart and Vascular Institute in Falls Church, Va.
Monochromatic images range from 40 keV to 140 keV. "At 40 keV you’re very close to the k-edge of iodine, so you have significant attenuation of anything that is enhancing ... at about 75 keV you have something that looks akin to a 120-keV image that we would normally get." As the energy increases, "you get away from what we would do on a routine clinical basis."
The ATLANTA I study (Assessment of Tissue Characteristics, Lesion Morphology, and Hemodynamics by Angiography With Fractional Flow Reserve, Intravascular Ultrasound and Virtual Histology, and Noninvasive Computed Tomography in Atherosclerotic Plaques) showed that with conventional single-energy CT calcium, plaque is overestimated and lumen size is therefore underestimated (J. Am. Coll. Cardiol. Intv. 2011;4:198-208 [doi:10.1016/j.jcin.2010.10.008]).
The ATLANTA I study tells us that "when we do routine single-energy CT, calcium plaques are significantly overportrayed." In this study, researchers found that calcified plaque volume is 104% greater than its true volume as determined by intravascular ultrasound. As a result, the minimal luminal diameter is underestimated by 21% and the percentage diameter stenosis is overrepresented by 39%. "Lots of false positives [are] caused by calcified plaque in the area," Dr. Earls observed.
The reason for this is that as energy increases, the calcium blooming decreases. This accounts for the enlargement of the lumen.
Noncalcified plaques don’t show as dramatic an effect, though. Soft plaque size does not change much when seen at different energies, Dr. Earls noted. "Ultimately it may be that we use a combination of monochromatic images and material density images, as we approach this going forward. These are both available every time that you do a scan," said Dr. Earls.
Dual-source CT does have limitations, though. There is no retrospective gating; there are limited milliAmpere (electrical charge) presets, and there is no high-resolution mode.
Intelligent Motion Correction
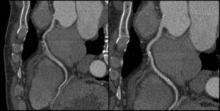
Motion artifacts make it difficult or impossible to evaluate vessels, leaving clinicians to treat the vessels as narrowed by default. A new technology is designed to overcome the problem of motion artifacts in cardiovascular CT.
"The images are acquired in a standard fashion, but instead of reconstructing them in a standard fashion, there is integration of this intelligent motion correction that tracks the motion of the vessel over two or three phases of the cardiac cycle," Dr. Jonathon A. Leipsic said in an interview. "When we acquire the study, we get a couple of phases beyond just the minimal acquisition – which is standard. It sees where the vessel is over a couple of phases and how it looks. Based on the known velocity of the artery, how it appears, and the patient’s known heart rate, [the algorithm] then corrects for the expected motion."
The technology is already changing practice. It is used on Europe and Japan; it is also being used in many centers in the United States. The motion correction algorithm has wide range of applications. "Anywhere there is motion, anyone with a sudden ectopic pace or premature ventricular contraction, anyone with an arrhythmia – all of those patients will benefit from this motion correction," said Dr. Leipsic, chairman of the department of radiology for Providence Health Care and vice chairman of research for the department of radiology at the University of British Columbia, Vancouver.
Importantly, the scan time and radiation dose don’t increase appreciably with the acquisition of vessel velocity data. "It doesn’t change the acquisition. You can just wait and see. If you have a study that has no motion, then you don’t need to use [motion correction]. But if you have a study with motion, then you can use it. You can make that choice after you see the initial data," Dr. Leipsic explained.
Dr. Leipsic was the lead author on one of the first studies to assess the accuracy this of this method (J. Cardiovasc. Comput. Tomography 2012;6:164-71. Epub 2012 Apr 6). In the study, "we looked at a population of convenience patients that happened to be going to the cath lab. We chose very difficult patients – patients undergoing transcatheter valve replacement – who have very high heart rates. We saw a significant improvement in interpretability, overall image quality, and diagnostic accuracy" with this technology. The researchers also noted significant improvements in right coronary evaluation, as well as other coronary territories.
Some have argued that all that is needed to avoid motion artifacts is to rate-control patients. However, "we aggressively rate-controlled patients ... there’s just too much motion," Dr. Leipsic countered. "It’s hard to anticipate some problems – an irregular beat or some irregular rhythm – and having this in your back pocket ... has exciting potential."
Additional trials to assess accuracy are expected, perhaps most prominently the VICTORY trial (Validation of an Intracycle CT Motion Correction Algorithm for Diagnostic Accuracy: A Prospective Multicenter Study). In the study, CCTA will be compared with invasive coronary angiography (ICA) for diagnostic sensitivity and specificity, positive predictive value, negative predictive value, diagnostic accuracy, and positive and negative likelihood ratios.
Coronary segments will be assessed for "significance" of coronary artery luminal diameter obstruction. Individual segments will be graded based on image quality, with the third reader used to achieve consensus. Dr. Leipsic and Dr. James Min are the principal investigators for the study.
For now, the intelligent motion correction algorithm, called SnapShot Freez4e, is available only from GE Healthcare, as part of its Discovery CT750 HD FREEdom Edition, which was granted 510(k) clearance in June. "I think that other companies are going to come up with something similar but I think that each type will be adequately different enough that [these technologies] will require their own validation studies," said Dr. Leipsic.
Siemens is reportedly developing a similar technology.
Dr. Min is a speaker for GE Healthcare. Dr. Budoff receives grant support from HeartFlow. Dr. George has received research support from Toshiba Medical Systems and GE Healthcare, is on the advisory board of GE Healthcare, and is a consultant to ICON Medical Imaging. Dr. Earls is on the speakers bureau for and has research funded by GE Healthcare. Dr. Leipsic is a consultant/speaker for Edwards Lifesciences and GE Healthcare.
New technologies that solve some old problems could make cardiovascular CT the go-to imaging tool to assess both anatomy and function.
Cardiovascular CT imaging has been a welcome tool in patient assessment due to its noninvasive nature, ease, and quickness. Likewise, cardiovascular CT angiography (CCTA) has provided noninvasive information about vessel stenoses, with lower costs and radiation exposure compared with invasive angiography.
Perhaps the biggest criticism about cardiovascular CT is that, while it provides very good anatomic detail, functional information – a crucial piece to the overall picture – is not discernable.
"Historically, coronary CT angiography has been an extremely anatomic method for documenting the severity of coronary artery stenoses or luminal compromise," said Dr. James K. Min, immediate past president of SCCT. This has left CTA open to the criticism that the more useful evaluation of coronary artery disease is not only to document high-grade stenoses – as with coronary CT angiography – but also to determine the physiologic significance of those stenoses.
However, at this year’s meeting of the Society of Cardiovascular Computed Tomography in Baltimore, "we saw the emergence of two robust methods for physiologic assessment of coronary disease by cardiac CT," he said. These are fractional flow reserve CT and CT-based myocardial perfusion imaging.
Evidence regarding these methods was then bolstered at the annual congress of the European Society of Cardiology in Munich, with the presentation of two imaging trials. The DeFACTO study, comparing CT-based FFR with FFR obtained by conventional angiography, did not show diagnostic superiority of CT-based FFR over angiography, but it did enhance diagnoses compared with CT without FFR. The CORE320 trial, assessing the diagnostic accuracy CT-based myocardial perfusion imaging in patients with suspected coronary artery disease, showed that CT-based myocardial perfusion imaging significantly increased the diagnostic accuracy of CTA alone to delineate flow-limiting disease.
Cardiovascular CT also has been hamstrung by a number of technical limitations. Calcium blooming artifacts make it difficult to get an accurate measurement of calcium lesion components and lumen size and motion artifacts also limit the clarity of CT images, leaving some vessels simply unreadable.
Two new technological advances – dual-energy CT and intelligent motion correction using snapshot freeze – are already changing practice, according to leaders at this year’s annual meeting of the SCCT.
CT Fractional Flow Reserve
Fractional flow reserve (FFR) is defined as the maximal blood flow through a diseased artery to the blood flow in the hypothetical case that this artery is normal. CT-based FFR relies on the use of some complicated math and modeling to calculate FFR from CT data and actually traces its roots back to the air flow modeling that goes into designing airplane wings.
All of the data needed can be gleaned from a single-phase static CT image. Geometry can be extracted from CCTA anatomic data. Boundary conditions can also be determined. Resting coronary blood flow can be calculated from myocardial mass. Mean blood pressure can be estimated from brachial artery pressure. Coronary microcirculatory resistance can be derived from morphometry data. Lastly, fluid properties include the viscosity and density of blood. "Putting it all together, we can come out with a calculated assessment of things like coronary flow and FFR ... now, we can do anatomy and function," said Dr. Matthew J. Budoff, director of cardiology at the University of California at Los Angeles School of Medicine in Torrance.
Until now, FFR was determined through invasive coronary angiography, which was the only method for specific determination of coronary artery lesions (lesion-specific ischemia). Values of 0.80 and lower or 0.75 and lower are considered diagnostic of lesion-specific ischemia. "It tells us whether there is a physiologic significance of the lesion," Dr. Budoff said.
At the same time, stenosis seen on CCTA has been an unreliable predictor of lesion-specific ischemia in trials. "Just seeing that anatomical stenosis doesn’t mean it’s functionally significant," said Dr. Budoff. CCTA results in a lot of false positives when it comes to physiologic significance.
FFR estimates throughout the entire coronary tree. "Because it relies on the entire coronary anatomy, it’s actually less sensitive to artifacts and less sensitive to things like calcification than individual segment assessment."
The first trial of FFR-CT was in the Diagnosis of Ischemia-Causing Stenoses Obtained via Noninvasive Fractional Flow Reserve (DISCOVER-FLOW) trial. In the study, the researchers compared the accuracy of CCTA with FFR-CT (invasive FFR served as the reference).
The results showed that the coronary stenoses that cause ischemia can be identified noninvasively with computer analysis of CCTA to construct FFR for specific lesions (Eur. Heart J. Cardiovasc. Imaging 2012 Jul 15 [doi: 10.1093/ehjci/jes130]).
The upshot of this study is that "when we think it’s a high-grade stenosis [with FFR-CT], it is a physiologically significant stenosis," Dr. Budoff said. "So we’ve dramatically improved the ability of CT to correlate with the most invasive and probably the most definitive way, currently, of assessing physiologic significance, and that’s FFR."
CT-based fractional flow reserve (FFR) is now a step closer to clinical use with the results of the Determination of FFR by Anatomic CT Angiography (DeFACTO) trial presented at the ESC meeting and simultaneously published (JAMA 2012 Aug. 26 [doi:10.1001/2012.jama.11274]). The addition of CT-based FFR information to CT alone improved diagnostic accuracy of stenoses, compared with invasive angiography and FFR.
"We’ve dramatically improved the ability of CT to correlate with ... FFR," said Dr. Matthew J. Budoff.
Although FFR-CT plus CT narrowly failed to meet the trial’s primary end point – diagnostic accuracy greater than 70% for the lower bound of the 95% confidence interval, the per-patient performance diagnostic accuracy of FFR-CT plus CT was 73% with a 95% CI of 67%-78%.
Nevertheless, the addition of FFR-CT "demonstrated superior diagnostic performance characteristics, as compared with CT stenosis alone in all patients, in all vessels and also in vessels of intermediate stenosis severity," Dr. Min said during a press conference.
Myocardial Perfusion
"The second method of physiologic assessment of coronary disease, which is emerging, is myocardial perfusion imaging – performing a typical stress test with a CT angiogram," said Dr. Min.
This allows the visualization of perfusion/ischemia at specific lesions identified by CTA, providing functional and anatomic information. The data for perfusion images are extracted from a standard CTA scan.
With CT perfusion, a rest CT angiogram is performed to document the coronary artery stenoses that are within the coronary artery bed and also to look at the rest perfusion of the myocardium to determine whether it’s normal or abnormal, according to Dr. Min.
Before or after the rest CTA, a stress CT would be performed via pharmacologic means using a traditional 64-row cardiac CT.
It’s known that patients who have a normal SPECT myocardial perfusion examination have a very low rate of cardiovascular events over the next year, according to Dr. Richard T. George. "However, those patients with an abnormal nuclear scan actually have a quite high event rate over the next year."
In addition, it’s also known that CCTA has great prognostic value "and probably for a longer period than SPECT does, but SPECT myocardial perfusion imaging probably tells you more about the intermediate time period in the future about the event rate in the patient," he said.
Several studies have looked at the additional value of CT perfusion testing.
One of the first studies that compared CCTA and CT perfusion (CTP) with quantitative coronary angiography and SPECT perfusion showed a sensitivity with CTA/CTP of 88% and a specificity of 91% (Circ. Cardiovasc. Imaging 2009;2:174-82). "This study demonstrates some of the additional value of stress CT myocardial perfusion imaging," said Dr. George, assistant professor of medicine at the Heart and Vascular Institute at Johns Hopkins Hospital in Baltimore.
In another study, researchers assessed the additional value of dipyridamole stress myocardial perfusion by 64-row CT in patients with coronary stents (J. Cardiovasc. Comput. Tomogr. 2011;5:449-58). It is often difficult in many of these patients to assess whether in-stent restenosis is present, said Dr. George. The researchers in this study found that the addition of CT myocardial perfusion imaging to CCTA improved accuracy.
In a study targeting reversible ischemia, researchers assessed CT myocardial perfusion imaging with 320-row detector CT in 50 patients with an intermediate- to high-risk for CAD (Circ. Cardiovasc. Imaging 2012;5:333-40).
"The important part of this study is that they actually looked at reversible ischemia. A lot of our studies with CT perfusion imaging just lump all perfusion deficits together." In it, 40% of patients had an abnormal SPECT scan; 90% of those abnormal scans were reversible ischemia," said Dr. George.
In a per-patient analysis of CT perfusion imaging vs. CTA stenosis greater than 50% in the setting of a territorial SPECT myocardial perfusion deficit, sensitivity was 100% and specificity was 81%. The study shows the effectiveness of CT perfusion for assessing lesion-specific ischemia, Dr. George noted.
"What we’re finding is that CT perfusion or stress testing with CT can be as accurate, if not more accurate, than the conventional stress testing methods that we’ve used until this day," Dr. Min said.
"CT perfusion or stress testing with CT can be as accurate, if not more accurate, than the conventional stress testing method," said Dr. James K. Min.
In the Combined Non-invasive Coronary Angiography and Myocardial Perfusion Imaging Using 320 Detector Computed Tomography (Core320) trial, researchers compared the diagnostic accuracy of CTA plus CT-based myocardial perfusion against ICA plus SPECT myocardial perfusion imaging in 381 patients with suspected or diagnosed coronary artery disease with a clinical indication for coronary angiography.
"When we add perfusion [to CTA alone] we gain power to diagnose flow-limiting stenosis," lead author Dr. Joao A.C. Lima said at the ESC meeting. Together, "they have the same power as invasive angiography and SPECT MPI in defining who are the patients who end up going through revascularization," he said.
The area under the receiver operating characteristic curve (ROC) – an effective method of evaluating the performance of diagnostic tests – was 0.79 for CTA-CTP and 0.81 for ICA-SPECT.
"At this point, the [CT perfusion] technology could be used precisely to find the patients who have flow-limiting disease and, therefore, are going to need revascularization," said Dr. Lima, director of cardiovascular imaging at the Johns Hopkins University in Baltimore.
Dual-Energy CT
Dual-energy CT (also known as dual-source CT) "interleaves sets of high- and low-keV images. The energy alternates between two energies (80 keV to 140 keV) every 0.5 msec," said Dr. James P. Earls. The result is two competing sets that can be used simultaneously. The importance of this dual-energy approach is that with higher energies and a software algorithm, calcium blooming is decreased. This means a more accurate size of calcium in lesions and lumen size.
Back-end software produces a range of monochromatic images (images at a single energy). "In terms of calcium reduction, the monochromatic images are probably more important," said Dr. Earls, codirector of the cardiac CT program at Inova Heart and Vascular Institute in Falls Church, Va.
Monochromatic images range from 40 keV to 140 keV. "At 40 keV you’re very close to the k-edge of iodine, so you have significant attenuation of anything that is enhancing ... at about 75 keV you have something that looks akin to a 120-keV image that we would normally get." As the energy increases, "you get away from what we would do on a routine clinical basis."
The ATLANTA I study (Assessment of Tissue Characteristics, Lesion Morphology, and Hemodynamics by Angiography With Fractional Flow Reserve, Intravascular Ultrasound and Virtual Histology, and Noninvasive Computed Tomography in Atherosclerotic Plaques) showed that with conventional single-energy CT calcium, plaque is overestimated and lumen size is therefore underestimated (J. Am. Coll. Cardiol. Intv. 2011;4:198-208 [doi:10.1016/j.jcin.2010.10.008]).
The ATLANTA I study tells us that "when we do routine single-energy CT, calcium plaques are significantly overportrayed." In this study, researchers found that calcified plaque volume is 104% greater than its true volume as determined by intravascular ultrasound. As a result, the minimal luminal diameter is underestimated by 21% and the percentage diameter stenosis is overrepresented by 39%. "Lots of false positives [are] caused by calcified plaque in the area," Dr. Earls observed.
The reason for this is that as energy increases, the calcium blooming decreases. This accounts for the enlargement of the lumen.
Noncalcified plaques don’t show as dramatic an effect, though. Soft plaque size does not change much when seen at different energies, Dr. Earls noted. "Ultimately it may be that we use a combination of monochromatic images and material density images, as we approach this going forward. These are both available every time that you do a scan," said Dr. Earls.
Dual-source CT does have limitations, though. There is no retrospective gating; there are limited milliAmpere (electrical charge) presets, and there is no high-resolution mode.
Intelligent Motion Correction
Motion artifacts make it difficult or impossible to evaluate vessels, leaving clinicians to treat the vessels as narrowed by default. A new technology is designed to overcome the problem of motion artifacts in cardiovascular CT.
"The images are acquired in a standard fashion, but instead of reconstructing them in a standard fashion, there is integration of this intelligent motion correction that tracks the motion of the vessel over two or three phases of the cardiac cycle," Dr. Jonathon A. Leipsic said in an interview. "When we acquire the study, we get a couple of phases beyond just the minimal acquisition – which is standard. It sees where the vessel is over a couple of phases and how it looks. Based on the known velocity of the artery, how it appears, and the patient’s known heart rate, [the algorithm] then corrects for the expected motion."
The technology is already changing practice. It is used on Europe and Japan; it is also being used in many centers in the United States. The motion correction algorithm has wide range of applications. "Anywhere there is motion, anyone with a sudden ectopic pace or premature ventricular contraction, anyone with an arrhythmia – all of those patients will benefit from this motion correction," said Dr. Leipsic, chairman of the department of radiology for Providence Health Care and vice chairman of research for the department of radiology at the University of British Columbia, Vancouver.
Importantly, the scan time and radiation dose don’t increase appreciably with the acquisition of vessel velocity data. "It doesn’t change the acquisition. You can just wait and see. If you have a study that has no motion, then you don’t need to use [motion correction]. But if you have a study with motion, then you can use it. You can make that choice after you see the initial data," Dr. Leipsic explained.
Dr. Leipsic was the lead author on one of the first studies to assess the accuracy this of this method (J. Cardiovasc. Comput. Tomography 2012;6:164-71. Epub 2012 Apr 6). In the study, "we looked at a population of convenience patients that happened to be going to the cath lab. We chose very difficult patients – patients undergoing transcatheter valve replacement – who have very high heart rates. We saw a significant improvement in interpretability, overall image quality, and diagnostic accuracy" with this technology. The researchers also noted significant improvements in right coronary evaluation, as well as other coronary territories.
Some have argued that all that is needed to avoid motion artifacts is to rate-control patients. However, "we aggressively rate-controlled patients ... there’s just too much motion," Dr. Leipsic countered. "It’s hard to anticipate some problems – an irregular beat or some irregular rhythm – and having this in your back pocket ... has exciting potential."
Additional trials to assess accuracy are expected, perhaps most prominently the VICTORY trial (Validation of an Intracycle CT Motion Correction Algorithm for Diagnostic Accuracy: A Prospective Multicenter Study). In the study, CCTA will be compared with invasive coronary angiography (ICA) for diagnostic sensitivity and specificity, positive predictive value, negative predictive value, diagnostic accuracy, and positive and negative likelihood ratios.
Coronary segments will be assessed for "significance" of coronary artery luminal diameter obstruction. Individual segments will be graded based on image quality, with the third reader used to achieve consensus. Dr. Leipsic and Dr. James Min are the principal investigators for the study.
For now, the intelligent motion correction algorithm, called SnapShot Freez4e, is available only from GE Healthcare, as part of its Discovery CT750 HD FREEdom Edition, which was granted 510(k) clearance in June. "I think that other companies are going to come up with something similar but I think that each type will be adequately different enough that [these technologies] will require their own validation studies," said Dr. Leipsic.
Siemens is reportedly developing a similar technology.
Dr. Min is a speaker for GE Healthcare. Dr. Budoff receives grant support from HeartFlow. Dr. George has received research support from Toshiba Medical Systems and GE Healthcare, is on the advisory board of GE Healthcare, and is a consultant to ICON Medical Imaging. Dr. Earls is on the speakers bureau for and has research funded by GE Healthcare. Dr. Leipsic is a consultant/speaker for Edwards Lifesciences and GE Healthcare.
New technologies that solve some old problems could make cardiovascular CT the go-to imaging tool to assess both anatomy and function.
Cardiovascular CT imaging has been a welcome tool in patient assessment due to its noninvasive nature, ease, and quickness. Likewise, cardiovascular CT angiography (CCTA) has provided noninvasive information about vessel stenoses, with lower costs and radiation exposure compared with invasive angiography.
Perhaps the biggest criticism about cardiovascular CT is that, while it provides very good anatomic detail, functional information – a crucial piece to the overall picture – is not discernable.
"Historically, coronary CT angiography has been an extremely anatomic method for documenting the severity of coronary artery stenoses or luminal compromise," said Dr. James K. Min, immediate past president of SCCT. This has left CTA open to the criticism that the more useful evaluation of coronary artery disease is not only to document high-grade stenoses – as with coronary CT angiography – but also to determine the physiologic significance of those stenoses.
However, at this year’s meeting of the Society of Cardiovascular Computed Tomography in Baltimore, "we saw the emergence of two robust methods for physiologic assessment of coronary disease by cardiac CT," he said. These are fractional flow reserve CT and CT-based myocardial perfusion imaging.
Evidence regarding these methods was then bolstered at the annual congress of the European Society of Cardiology in Munich, with the presentation of two imaging trials. The DeFACTO study, comparing CT-based FFR with FFR obtained by conventional angiography, did not show diagnostic superiority of CT-based FFR over angiography, but it did enhance diagnoses compared with CT without FFR. The CORE320 trial, assessing the diagnostic accuracy CT-based myocardial perfusion imaging in patients with suspected coronary artery disease, showed that CT-based myocardial perfusion imaging significantly increased the diagnostic accuracy of CTA alone to delineate flow-limiting disease.
Cardiovascular CT also has been hamstrung by a number of technical limitations. Calcium blooming artifacts make it difficult to get an accurate measurement of calcium lesion components and lumen size and motion artifacts also limit the clarity of CT images, leaving some vessels simply unreadable.
Two new technological advances – dual-energy CT and intelligent motion correction using snapshot freeze – are already changing practice, according to leaders at this year’s annual meeting of the SCCT.
CT Fractional Flow Reserve
Fractional flow reserve (FFR) is defined as the maximal blood flow through a diseased artery to the blood flow in the hypothetical case that this artery is normal. CT-based FFR relies on the use of some complicated math and modeling to calculate FFR from CT data and actually traces its roots back to the air flow modeling that goes into designing airplane wings.
All of the data needed can be gleaned from a single-phase static CT image. Geometry can be extracted from CCTA anatomic data. Boundary conditions can also be determined. Resting coronary blood flow can be calculated from myocardial mass. Mean blood pressure can be estimated from brachial artery pressure. Coronary microcirculatory resistance can be derived from morphometry data. Lastly, fluid properties include the viscosity and density of blood. "Putting it all together, we can come out with a calculated assessment of things like coronary flow and FFR ... now, we can do anatomy and function," said Dr. Matthew J. Budoff, director of cardiology at the University of California at Los Angeles School of Medicine in Torrance.
Until now, FFR was determined through invasive coronary angiography, which was the only method for specific determination of coronary artery lesions (lesion-specific ischemia). Values of 0.80 and lower or 0.75 and lower are considered diagnostic of lesion-specific ischemia. "It tells us whether there is a physiologic significance of the lesion," Dr. Budoff said.
At the same time, stenosis seen on CCTA has been an unreliable predictor of lesion-specific ischemia in trials. "Just seeing that anatomical stenosis doesn’t mean it’s functionally significant," said Dr. Budoff. CCTA results in a lot of false positives when it comes to physiologic significance.
FFR estimates throughout the entire coronary tree. "Because it relies on the entire coronary anatomy, it’s actually less sensitive to artifacts and less sensitive to things like calcification than individual segment assessment."
The first trial of FFR-CT was in the Diagnosis of Ischemia-Causing Stenoses Obtained via Noninvasive Fractional Flow Reserve (DISCOVER-FLOW) trial. In the study, the researchers compared the accuracy of CCTA with FFR-CT (invasive FFR served as the reference).
The results showed that the coronary stenoses that cause ischemia can be identified noninvasively with computer analysis of CCTA to construct FFR for specific lesions (Eur. Heart J. Cardiovasc. Imaging 2012 Jul 15 [doi: 10.1093/ehjci/jes130]).
The upshot of this study is that "when we think it’s a high-grade stenosis [with FFR-CT], it is a physiologically significant stenosis," Dr. Budoff said. "So we’ve dramatically improved the ability of CT to correlate with the most invasive and probably the most definitive way, currently, of assessing physiologic significance, and that’s FFR."
CT-based fractional flow reserve (FFR) is now a step closer to clinical use with the results of the Determination of FFR by Anatomic CT Angiography (DeFACTO) trial presented at the ESC meeting and simultaneously published (JAMA 2012 Aug. 26 [doi:10.1001/2012.jama.11274]). The addition of CT-based FFR information to CT alone improved diagnostic accuracy of stenoses, compared with invasive angiography and FFR.
"We’ve dramatically improved the ability of CT to correlate with ... FFR," said Dr. Matthew J. Budoff.
Although FFR-CT plus CT narrowly failed to meet the trial’s primary end point – diagnostic accuracy greater than 70% for the lower bound of the 95% confidence interval, the per-patient performance diagnostic accuracy of FFR-CT plus CT was 73% with a 95% CI of 67%-78%.
Nevertheless, the addition of FFR-CT "demonstrated superior diagnostic performance characteristics, as compared with CT stenosis alone in all patients, in all vessels and also in vessels of intermediate stenosis severity," Dr. Min said during a press conference.
Myocardial Perfusion
"The second method of physiologic assessment of coronary disease, which is emerging, is myocardial perfusion imaging – performing a typical stress test with a CT angiogram," said Dr. Min.
This allows the visualization of perfusion/ischemia at specific lesions identified by CTA, providing functional and anatomic information. The data for perfusion images are extracted from a standard CTA scan.
With CT perfusion, a rest CT angiogram is performed to document the coronary artery stenoses that are within the coronary artery bed and also to look at the rest perfusion of the myocardium to determine whether it’s normal or abnormal, according to Dr. Min.
Before or after the rest CTA, a stress CT would be performed via pharmacologic means using a traditional 64-row cardiac CT.
It’s known that patients who have a normal SPECT myocardial perfusion examination have a very low rate of cardiovascular events over the next year, according to Dr. Richard T. George. "However, those patients with an abnormal nuclear scan actually have a quite high event rate over the next year."
In addition, it’s also known that CCTA has great prognostic value "and probably for a longer period than SPECT does, but SPECT myocardial perfusion imaging probably tells you more about the intermediate time period in the future about the event rate in the patient," he said.
Several studies have looked at the additional value of CT perfusion testing.
One of the first studies that compared CCTA and CT perfusion (CTP) with quantitative coronary angiography and SPECT perfusion showed a sensitivity with CTA/CTP of 88% and a specificity of 91% (Circ. Cardiovasc. Imaging 2009;2:174-82). "This study demonstrates some of the additional value of stress CT myocardial perfusion imaging," said Dr. George, assistant professor of medicine at the Heart and Vascular Institute at Johns Hopkins Hospital in Baltimore.
In another study, researchers assessed the additional value of dipyridamole stress myocardial perfusion by 64-row CT in patients with coronary stents (J. Cardiovasc. Comput. Tomogr. 2011;5:449-58). It is often difficult in many of these patients to assess whether in-stent restenosis is present, said Dr. George. The researchers in this study found that the addition of CT myocardial perfusion imaging to CCTA improved accuracy.
In a study targeting reversible ischemia, researchers assessed CT myocardial perfusion imaging with 320-row detector CT in 50 patients with an intermediate- to high-risk for CAD (Circ. Cardiovasc. Imaging 2012;5:333-40).
"The important part of this study is that they actually looked at reversible ischemia. A lot of our studies with CT perfusion imaging just lump all perfusion deficits together." In it, 40% of patients had an abnormal SPECT scan; 90% of those abnormal scans were reversible ischemia," said Dr. George.
In a per-patient analysis of CT perfusion imaging vs. CTA stenosis greater than 50% in the setting of a territorial SPECT myocardial perfusion deficit, sensitivity was 100% and specificity was 81%. The study shows the effectiveness of CT perfusion for assessing lesion-specific ischemia, Dr. George noted.
"What we’re finding is that CT perfusion or stress testing with CT can be as accurate, if not more accurate, than the conventional stress testing methods that we’ve used until this day," Dr. Min said.
"CT perfusion or stress testing with CT can be as accurate, if not more accurate, than the conventional stress testing method," said Dr. James K. Min.
In the Combined Non-invasive Coronary Angiography and Myocardial Perfusion Imaging Using 320 Detector Computed Tomography (Core320) trial, researchers compared the diagnostic accuracy of CTA plus CT-based myocardial perfusion against ICA plus SPECT myocardial perfusion imaging in 381 patients with suspected or diagnosed coronary artery disease with a clinical indication for coronary angiography.
"When we add perfusion [to CTA alone] we gain power to diagnose flow-limiting stenosis," lead author Dr. Joao A.C. Lima said at the ESC meeting. Together, "they have the same power as invasive angiography and SPECT MPI in defining who are the patients who end up going through revascularization," he said.
The area under the receiver operating characteristic curve (ROC) – an effective method of evaluating the performance of diagnostic tests – was 0.79 for CTA-CTP and 0.81 for ICA-SPECT.
"At this point, the [CT perfusion] technology could be used precisely to find the patients who have flow-limiting disease and, therefore, are going to need revascularization," said Dr. Lima, director of cardiovascular imaging at the Johns Hopkins University in Baltimore.
Dual-Energy CT
Dual-energy CT (also known as dual-source CT) "interleaves sets of high- and low-keV images. The energy alternates between two energies (80 keV to 140 keV) every 0.5 msec," said Dr. James P. Earls. The result is two competing sets that can be used simultaneously. The importance of this dual-energy approach is that with higher energies and a software algorithm, calcium blooming is decreased. This means a more accurate size of calcium in lesions and lumen size.
Back-end software produces a range of monochromatic images (images at a single energy). "In terms of calcium reduction, the monochromatic images are probably more important," said Dr. Earls, codirector of the cardiac CT program at Inova Heart and Vascular Institute in Falls Church, Va.
Monochromatic images range from 40 keV to 140 keV. "At 40 keV you’re very close to the k-edge of iodine, so you have significant attenuation of anything that is enhancing ... at about 75 keV you have something that looks akin to a 120-keV image that we would normally get." As the energy increases, "you get away from what we would do on a routine clinical basis."
The ATLANTA I study (Assessment of Tissue Characteristics, Lesion Morphology, and Hemodynamics by Angiography With Fractional Flow Reserve, Intravascular Ultrasound and Virtual Histology, and Noninvasive Computed Tomography in Atherosclerotic Plaques) showed that with conventional single-energy CT calcium, plaque is overestimated and lumen size is therefore underestimated (J. Am. Coll. Cardiol. Intv. 2011;4:198-208 [doi:10.1016/j.jcin.2010.10.008]).
The ATLANTA I study tells us that "when we do routine single-energy CT, calcium plaques are significantly overportrayed." In this study, researchers found that calcified plaque volume is 104% greater than its true volume as determined by intravascular ultrasound. As a result, the minimal luminal diameter is underestimated by 21% and the percentage diameter stenosis is overrepresented by 39%. "Lots of false positives [are] caused by calcified plaque in the area," Dr. Earls observed.
The reason for this is that as energy increases, the calcium blooming decreases. This accounts for the enlargement of the lumen.
Noncalcified plaques don’t show as dramatic an effect, though. Soft plaque size does not change much when seen at different energies, Dr. Earls noted. "Ultimately it may be that we use a combination of monochromatic images and material density images, as we approach this going forward. These are both available every time that you do a scan," said Dr. Earls.
Dual-source CT does have limitations, though. There is no retrospective gating; there are limited milliAmpere (electrical charge) presets, and there is no high-resolution mode.
Intelligent Motion Correction
Motion artifacts make it difficult or impossible to evaluate vessels, leaving clinicians to treat the vessels as narrowed by default. A new technology is designed to overcome the problem of motion artifacts in cardiovascular CT.
"The images are acquired in a standard fashion, but instead of reconstructing them in a standard fashion, there is integration of this intelligent motion correction that tracks the motion of the vessel over two or three phases of the cardiac cycle," Dr. Jonathon A. Leipsic said in an interview. "When we acquire the study, we get a couple of phases beyond just the minimal acquisition – which is standard. It sees where the vessel is over a couple of phases and how it looks. Based on the known velocity of the artery, how it appears, and the patient’s known heart rate, [the algorithm] then corrects for the expected motion."
The technology is already changing practice. It is used on Europe and Japan; it is also being used in many centers in the United States. The motion correction algorithm has wide range of applications. "Anywhere there is motion, anyone with a sudden ectopic pace or premature ventricular contraction, anyone with an arrhythmia – all of those patients will benefit from this motion correction," said Dr. Leipsic, chairman of the department of radiology for Providence Health Care and vice chairman of research for the department of radiology at the University of British Columbia, Vancouver.
Importantly, the scan time and radiation dose don’t increase appreciably with the acquisition of vessel velocity data. "It doesn’t change the acquisition. You can just wait and see. If you have a study that has no motion, then you don’t need to use [motion correction]. But if you have a study with motion, then you can use it. You can make that choice after you see the initial data," Dr. Leipsic explained.
Dr. Leipsic was the lead author on one of the first studies to assess the accuracy this of this method (J. Cardiovasc. Comput. Tomography 2012;6:164-71. Epub 2012 Apr 6). In the study, "we looked at a population of convenience patients that happened to be going to the cath lab. We chose very difficult patients – patients undergoing transcatheter valve replacement – who have very high heart rates. We saw a significant improvement in interpretability, overall image quality, and diagnostic accuracy" with this technology. The researchers also noted significant improvements in right coronary evaluation, as well as other coronary territories.
Some have argued that all that is needed to avoid motion artifacts is to rate-control patients. However, "we aggressively rate-controlled patients ... there’s just too much motion," Dr. Leipsic countered. "It’s hard to anticipate some problems – an irregular beat or some irregular rhythm – and having this in your back pocket ... has exciting potential."
Additional trials to assess accuracy are expected, perhaps most prominently the VICTORY trial (Validation of an Intracycle CT Motion Correction Algorithm for Diagnostic Accuracy: A Prospective Multicenter Study). In the study, CCTA will be compared with invasive coronary angiography (ICA) for diagnostic sensitivity and specificity, positive predictive value, negative predictive value, diagnostic accuracy, and positive and negative likelihood ratios.
Coronary segments will be assessed for "significance" of coronary artery luminal diameter obstruction. Individual segments will be graded based on image quality, with the third reader used to achieve consensus. Dr. Leipsic and Dr. James Min are the principal investigators for the study.
For now, the intelligent motion correction algorithm, called SnapShot Freez4e, is available only from GE Healthcare, as part of its Discovery CT750 HD FREEdom Edition, which was granted 510(k) clearance in June. "I think that other companies are going to come up with something similar but I think that each type will be adequately different enough that [these technologies] will require their own validation studies," said Dr. Leipsic.
Siemens is reportedly developing a similar technology.
Dr. Min is a speaker for GE Healthcare. Dr. Budoff receives grant support from HeartFlow. Dr. George has received research support from Toshiba Medical Systems and GE Healthcare, is on the advisory board of GE Healthcare, and is a consultant to ICON Medical Imaging. Dr. Earls is on the speakers bureau for and has research funded by GE Healthcare. Dr. Leipsic is a consultant/speaker for Edwards Lifesciences and GE Healthcare.