User login
Gadolinium Intermediate Elimination and Persistent Symptoms After Magnetic Resonance Imaging Contrast Agent Exposure
Gadolinium Intermediate Elimination and Persistent Symptoms After Magnetic Resonance Imaging Contrast Agent Exposure
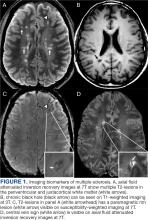
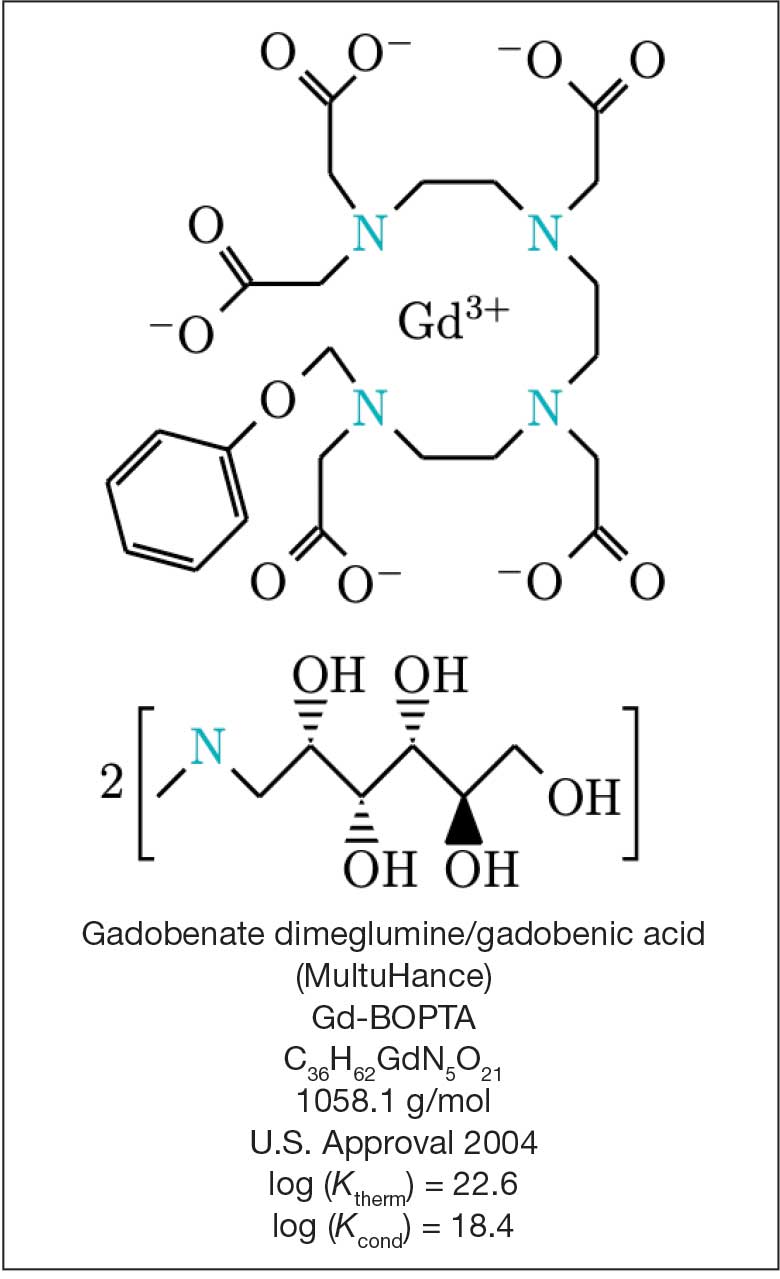
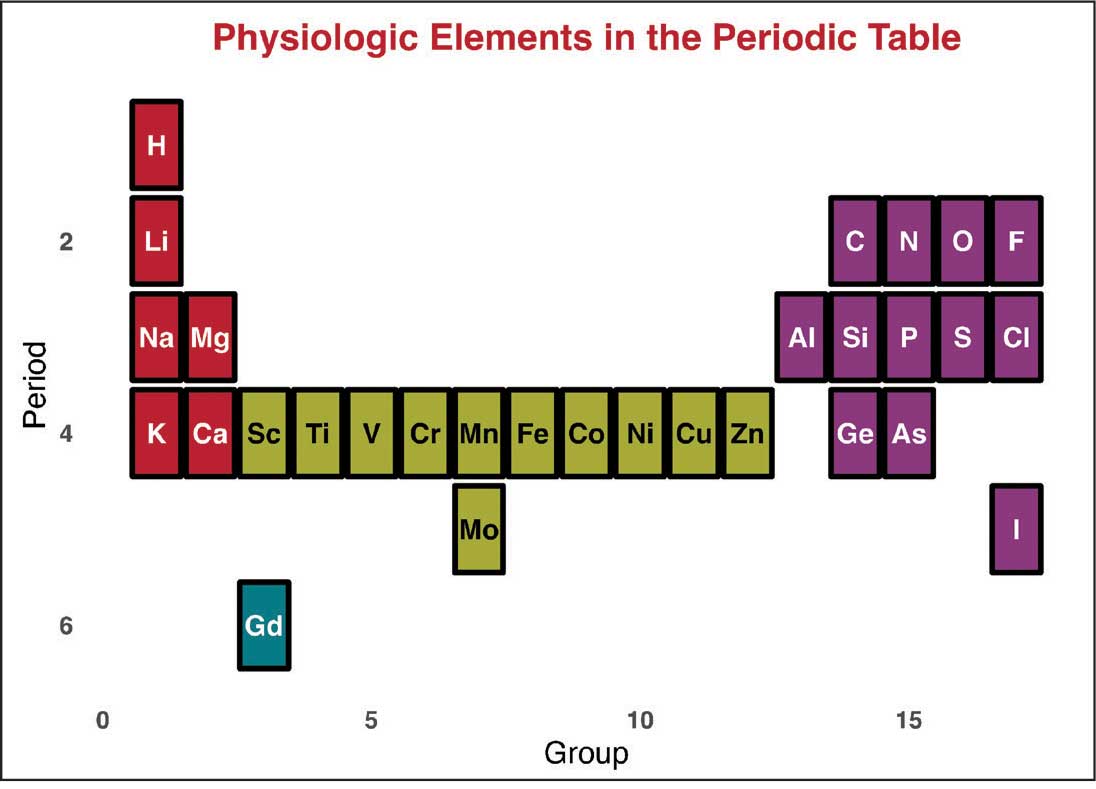
Magnetic resonance image (MRI) contrast agents can induce profound complications, including gadolinium encephalopathy, kidney injury, gadolinium-associated plaques, and progressive systemic fibrosis, which can be fatal.1-10 About 50% of MRIs use gadolinium-based contrast (Gd3+), a toxic rare earth metal ion that enhances imaging but requires binding with pharmaceutical ligands to reduce toxicity and promote renal elimination (Figure 1). Despite these measures, Gd3+ can persist in the body, including the brain.11,12 Wastewater treatment fails to remove these agents, making Gd3+ a growing pollutant in water and the food chain.13-15 Because Gd3+ is a rare earth metal ion in the milieu intérieur, there is an urgent need to study its biological and long-term effects (Appendix 1).
Case Presentation
A 65-year-old Vietnam-era veteran presented to nephrology at the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, for evaluation of gadolinium-induced symptoms. His medical history included metabolic syndrome, hypertension, hyperlipidemia, hypogonadism, cervical spondylosis, and an elevated prostate-specific antigen, previously assessed with a contrast-enhanced MRI in 2019 (Gadobenic acid, 19 mL). Surgical history included cervical fusion and ankle hardware.
The patient had a scheduled MRI 25 days earlier, following an elevated prostate specific antigen test result, prompting urologic surveillance and concern for malignancy. In preparation for the contrast-enhanced MRI, his right arm was cannulated with a line primed with gadobenic acid contrast. Though the technician stated the infusion had not started, the patient’s symptoms began shortly after entry into the scanner, before any programmed pulse sequences. The patient experienced claustrophobia, diaphoresis, palpitations, xerostomia, dysgeusia, shortness of breath, and a sensation of heat in his groin, chest, “kidneys,” and lower back. The MRI was terminated prematurely in response to the patient’s acute symptomatology. The patient continued experiencing new symptoms intermittently during the following week, including lightheadedness, headaches, right clavicular pain, raspy voice, edema, and a sense of doom.
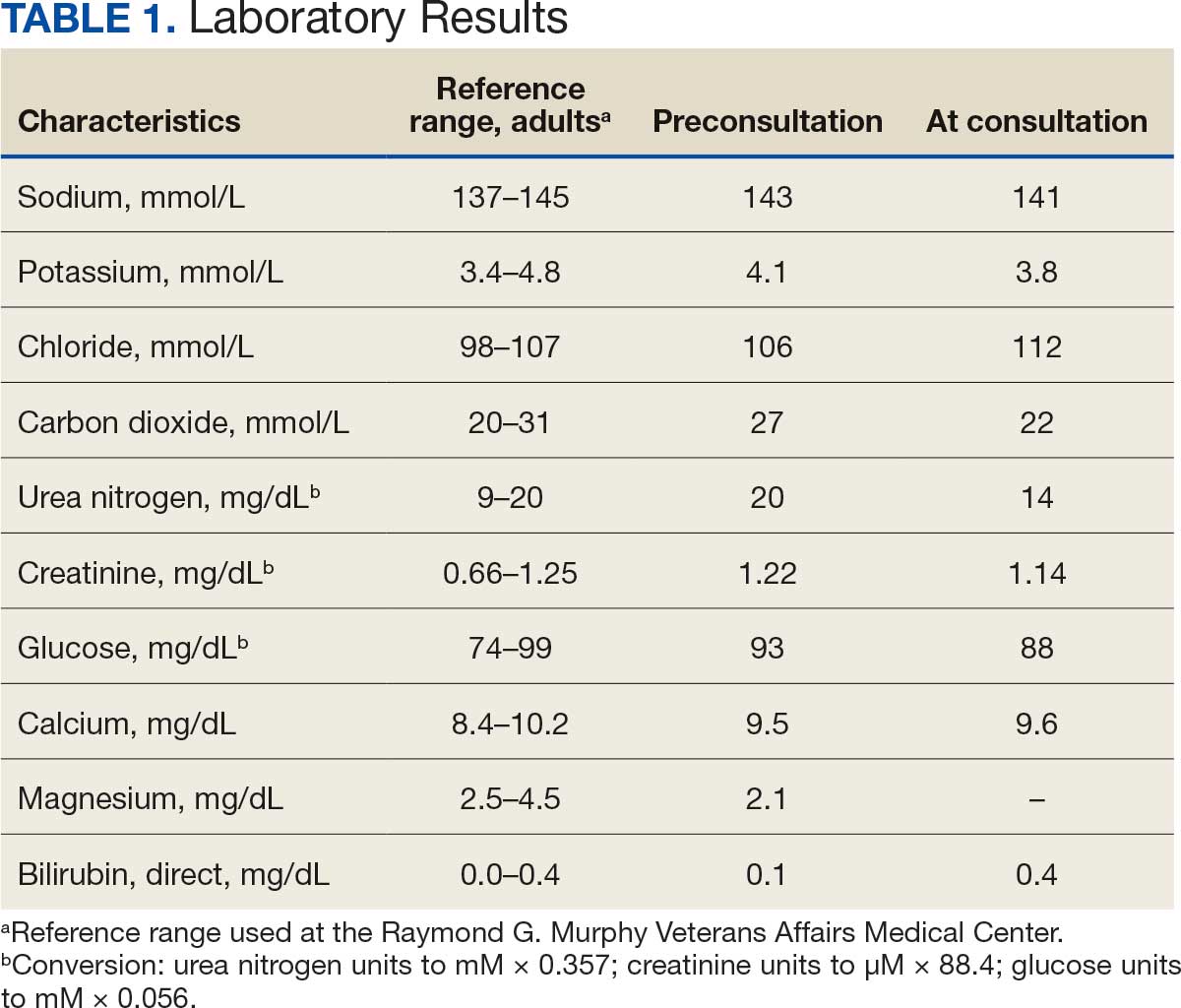
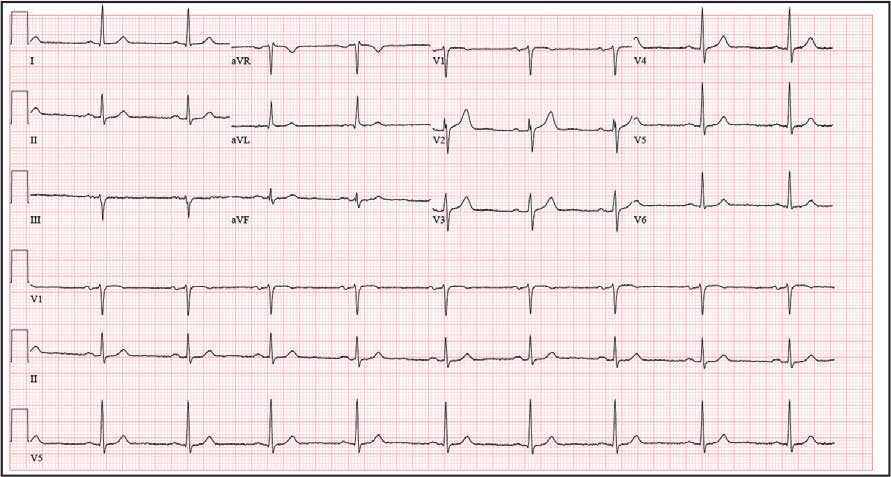
The patient presented to the RGMVAMC emergency department (ED) 8 days after the MRI with worsening symptoms and was hospitalized for 10 days. During this time, he was referred to nephrology for outpatient evaluation. While awaiting his nephrology appointment, the patient presented to the RGMVAMC ED 20 days after the initial episode with ongoing symptoms. “I thought I was dying,” he said. Laboratory results and a 12-lead electrocardiogram showed a finely static background, wide P waves (> 80 ms) with notching in lead II, sinusoidal P waves in V1, R transition in V2, RR’ in V2, ST flat in lead III, and sinus bradycardia (Table 1 and Appendix 2).
The patient’s medical and surgical histories were reviewed at the nephrology evaluation 25 days following the MRI. He reported that household water was sourced from a well and that he filtered his drinking water with a reverse osmosis system. He served in the US Army for 10 years as an engineer specializing in mechanical systems, power generation, and vehicles. Following Army retirement, the patient served in the US Air Force Reserves for 15 years, working as a crew chief in pneudraulics. The patient reported stopping tobacco use 1 year before and also reported regular use of a broad array of prescription medications and dietary supplements, including dexamethasone (4 mg twice daily), fluticasone nasal spray (50 mcg per nostril, twice daily), ibuprofen (400 mg twice daily, as needed), loratadine (10 mg daily), aspirin (81 mg daily), and metoprolol succinate (50 mg nightly). In addition, he reported consistent use of cholecalciferol (3000 IU daily), another supplemental vitamin D preparation, chelated magnesium glycinate (3 tablets daily for bone issues), turmeric (1 tablet daily), a multivitamin (Living Green Liquid Gel, daily), and a mega-B complex.
Physical examination revealed a well-nourished, tall man with hypertension (145/87 mmHg) and bilateral lower extremity edema. Oral examination showed poor dentition, including missing molars (#1-3, #14-16, #17-19, #30-31), with the anterior teeth replaced by bridges supported by dental implants. The review of systems was otherwise unremarkable, with nocturia noted before the consultation.
Serum and urine gadolinium testing, (Mayo Clinic Laboratories) revealed gadolinium levels of 0.3 mcg/24 h in the urine and 0.1 ng/mL in the serum. Nonzero values indicated detectable gadolinium, suggesting retention. The patient had a prior gadolinium exposure during a 2019 MRI (about 1340 days before) and suspected a repeat exposure on day 0, although the MRI technician stated that no contrast was administered. Given his elevated vitamin D levels, the patient was advised to minimize dietary supplements, particularly vitamin D, to avoid confounding symptoms. The plan included monitoring symptoms and a follow-up evaluation with repeat laboratory tests on day 116.
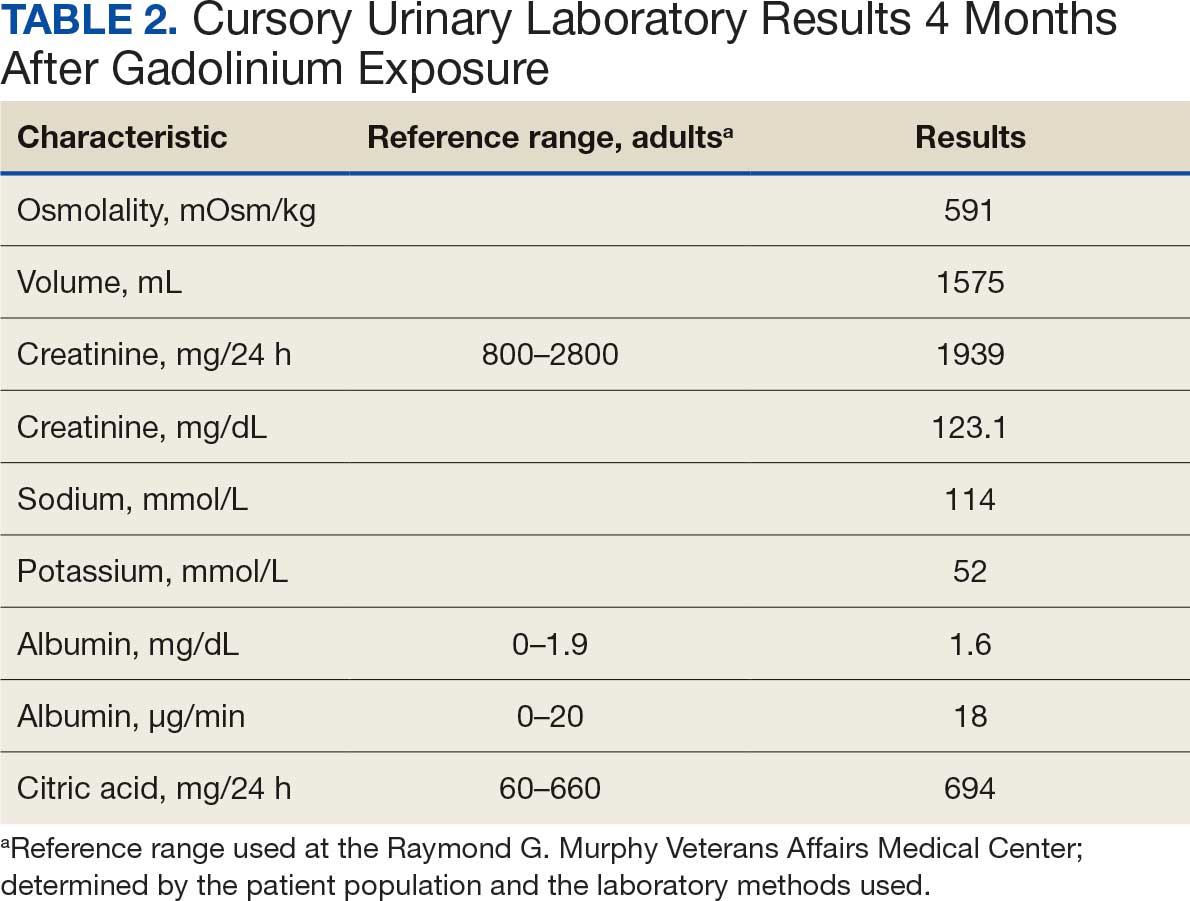
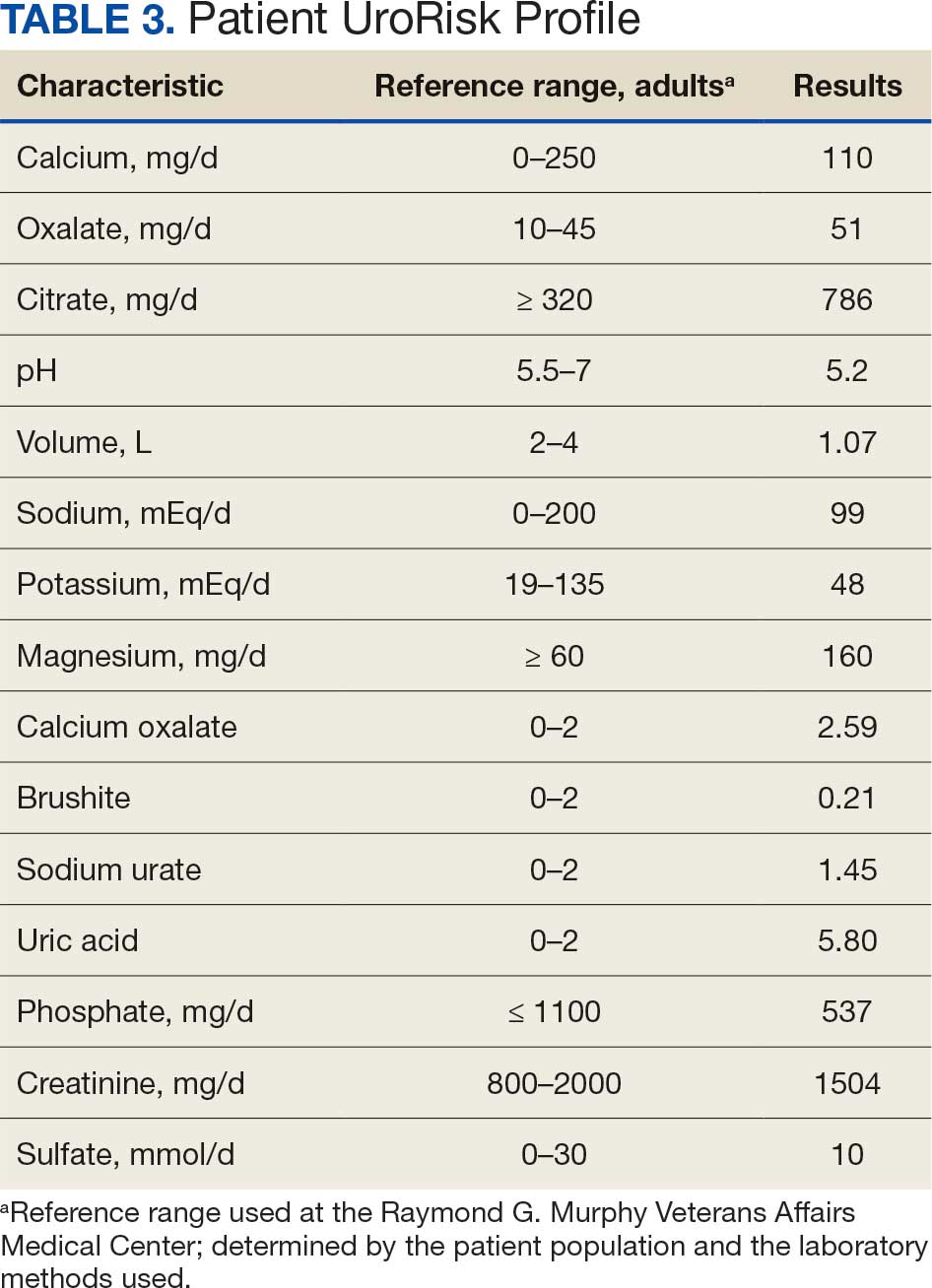
At the nephrology follow-up 4 months postexposure, the patient's symptoms had primarily abated, with a marked reduction in the previously noted metallic dysgeusia. Physical examination remained consistent with prior findings. He was afebrile (97.7 °F) with a blood pressure of 111/72 mmHg, a pulse of 63 beats per minute, and an oxygen saturation of 98% on ambient air. Laboratory analysis revealed serum and urine gadolinium levels below detectable thresholds (< 0.1 ng/mL and < 0.1 mcg/24 h). A 24-hour creatinine clearance, calculated from a urine volume of 1300 mL, measured at an optimal 106 mL/min, indicating preserved renal function (Tables 2 and 3). Of note, his 24-hour oxalate was above the reference range, with a urine pH below the reference range and a high supersaturation index for calcium oxalate.
Discussion
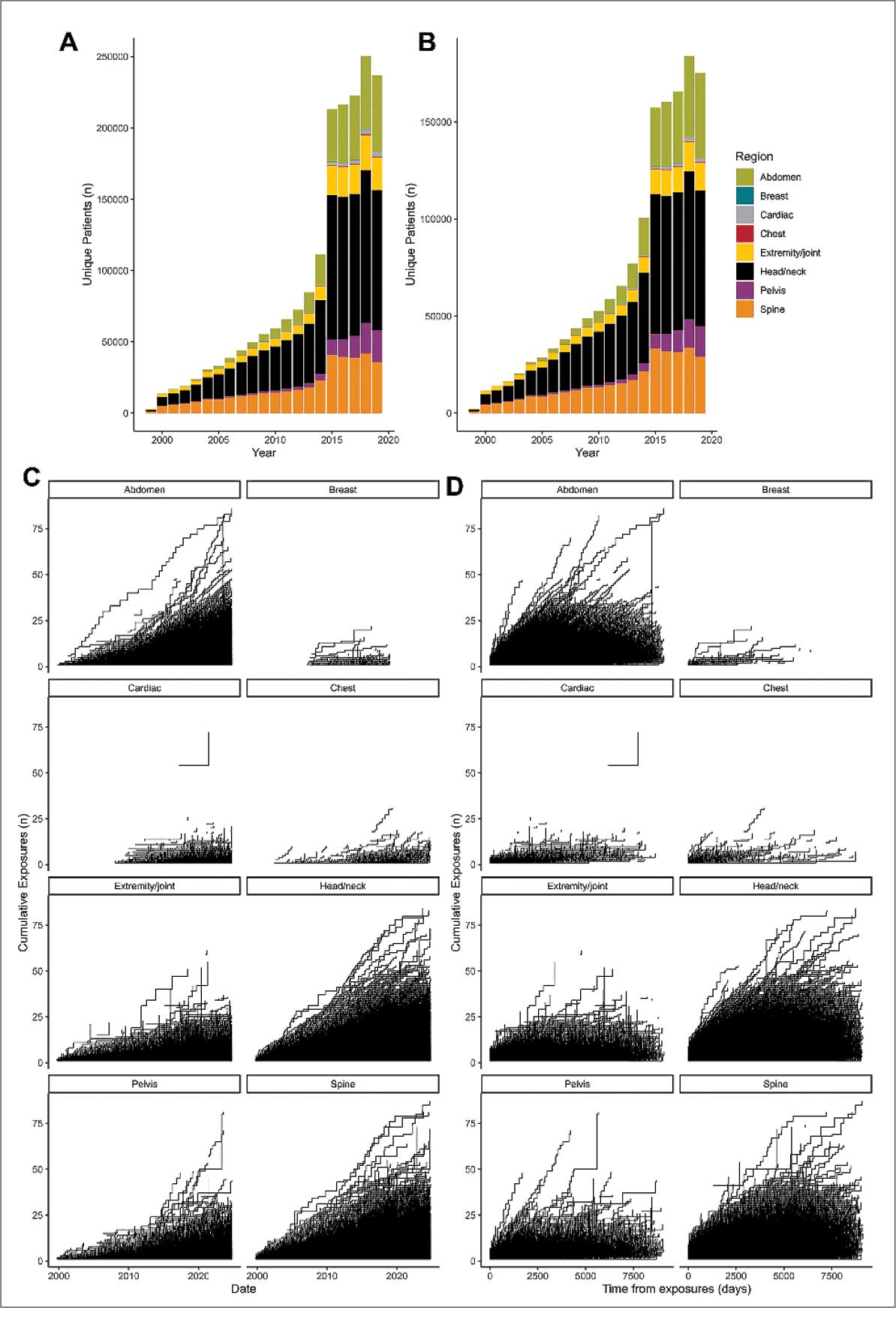
Use of enhanced MRI has increased in the Veterans Health Administration (Figure 2). A growing range of indications for enhanced procedures (eg, cardiac MRI) has contributed to this rise. The market has grown with new gadolinium-based contrast agents, such as gadopiclenol. However, reliance on untested assumptions about the safety of newer agents and need for robust clinical trials pose potential risks to patient safety.
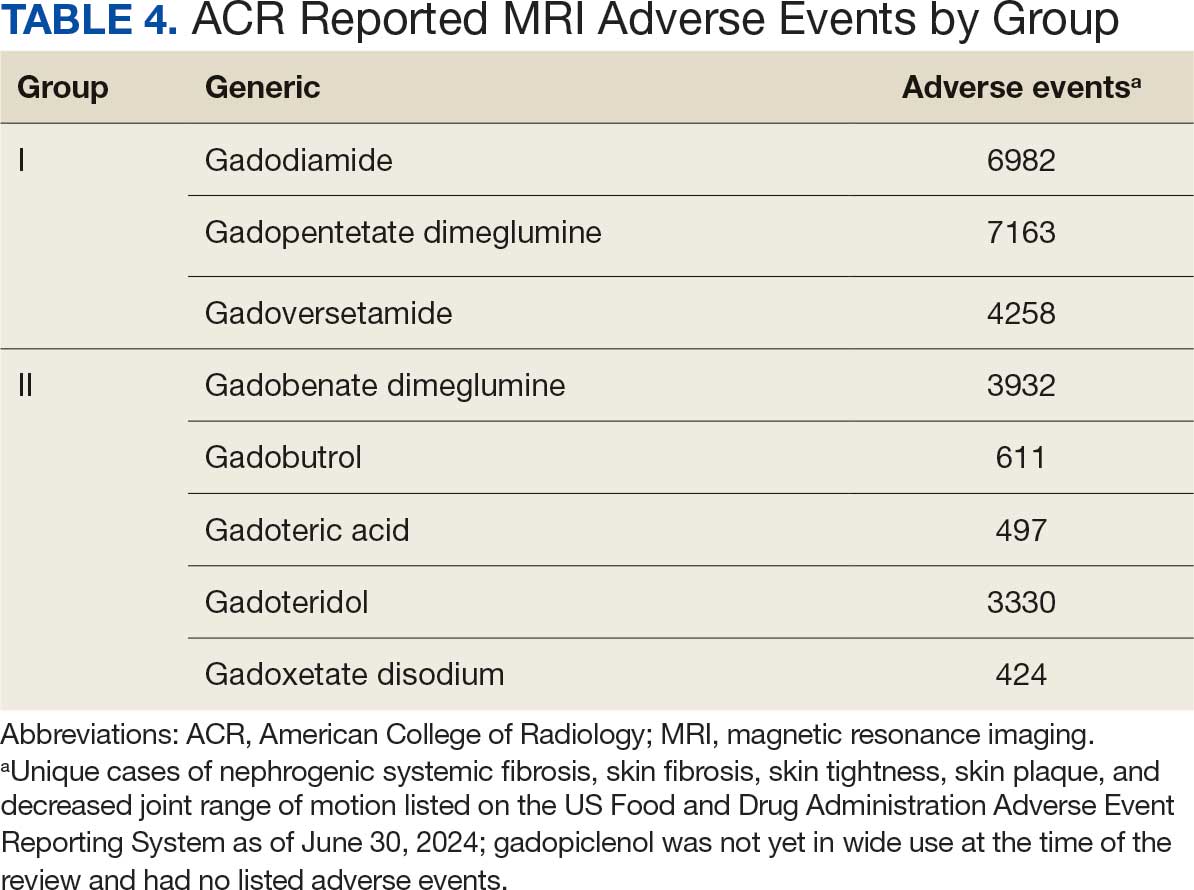
Without prospective evidence, the American College of Radiology (ACR) classifies gadolinium-based contrast agents into 3 groups: Group 1, associated with the highest number of nephrogenic systemic fibrosis cases; Group 2, linked to few, if any, unconfounded cases; and Group 3, where data on nephrogenic systemic fibrosis risk have been limited. As of April 2024, the ACR reclassified Group 3 agents (Ablavar/Vasovist/Angiomark and Primovist/Eovist) into Group 2. Curiously, Vueway and Elucirem were approved in late 2022 and should clearly be categorized as Group 3 (Table 4).There were 19 cases of nephrogenic systemic fibrosis or similar manifestations, 8 of which were unconfounded by other factors. These patients had been exposed to gadobutrol, often combined with other agents. Gadobutrol—like other Group 2 agents—has been associated with nephrogenic systemic fibrosis.16,17 Despite US Food and Drug Administration (FDA) documentation of rising reports, many clinicians remain unaware that nephrogenic systemic fibrosis is increasingly linked to Group 2 agents classified by the ACR.18 While declines in reported cases of nephrogenic systemic fibrosis may suggest reduced incidence, this trend may reflect diminished clinical vigilance and underreporting, particularly given emerging evidence implicating even Group 2 gadolinium-based contrast agents in delayed and underrecognized presentations. This information has yet to permeate the medical community, particularly among nephrologists. Considering these cases, revisiting the ACR guidelines may be prudent.
To address this growing concern, clinicians must adopt stricter vigilance and actively pursue updated information to mitigate patient risks tied to these contrast agents.
There exists an illusion of knowledge in disregarding the confounded exposures of MRI contrast agents. Ten distinct brands of contrast agents have been approved for clinical use. With repeated imaging, patients are often exposed to varying formulations of gadolinium-based agents. Yet investigators commonly discard these data points when assessing risk. By doing so, they assume—without evidence—that some formulations are inherently less likely to provoke adverse effects (AEs) than others. This untested presumption becomes perilous, especially given the limited understanding of the mechanisms underlying gadolinium-induced pathologies. As Aldous Huxley warned, “Facts do not cease to exist because they are ignored.”19
Gadolinium Persistence
Contrary to expectations, gadolinium persists in the body far longer than initially presumed. Symptoms associated with gadolinium exposure (SAGE) encapsulate the chronic, often enigmatic maladies tied to MRI contrast agents.20 The prolonged retention of this rare earth metal offers a compelling hypothesis for the etiology of SAGE. It has been hypothesized that Lewis base-rich metabolites increase susceptibility to gadolinium-based contrast agent complications.21
The blood and urine concentration elimination curves of gadolinium are exponential and categorized as fast, intermediate, and long-term.1 For urinary elimination, the function of the curves is exponential. The quantity of gadolinium in the urine at a time (t) after exposure (D[Gd](t)) is equal to the product of the amount of gadolinium in the sample (urine or blood) at the end of the fast elimination period (D[Gd](t0)) and the exponential decay with k being a rate constant.
To the authors’ knowledge, we are the only research team currently investigating the rate constant for the intermediate- and long-term phase gadolinium elimination. The Retention and Toxicity of Gadolinium-based Contrast Agents study was approved by the University of New Mexico Health Sciences Center Institutional Review Board on May 27, 2020 (IRB ID 19-660). The data for the patient in this case were compared with preliminary results for patients with exposure-to-measurement intervals < 100 days.
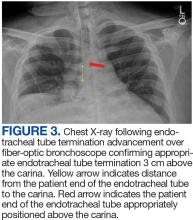
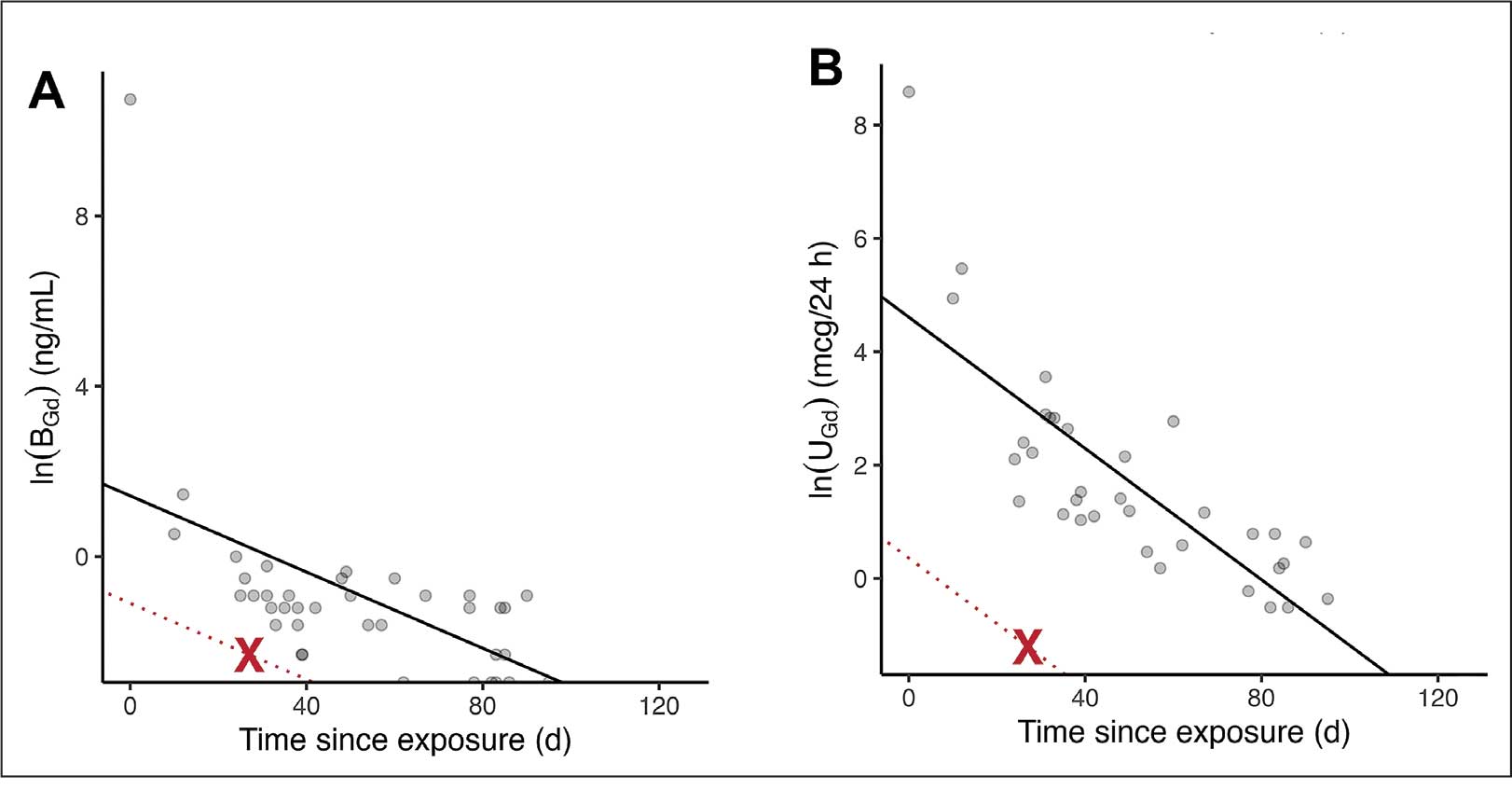
The patient in this case presented with detectable gadolinium levels in urine and serum shortly after an attempted contrast-enhanced MRI procedure (Figure 3). The presence of detectable gadolinium levels in the patient’s urine and serum suggests a likely exposure to a contrast agent about 27 days before his consultation. While the technician reported that no contrast was administered during the attempted MRI, it remains possible that a small amount was introduced during cannulation, potentially triggering the patient’s symptoms. Linear modeling of semilogarithmic plots for participants exposed to contrast agents within 100 days (urine: P = 1.8 × 10ˉ8, adjusted r² = 0.62; blood: P = .005, adjusted r² = 0.21) provided clearance rates (k values) for urine and blood. Extrapolating from these models to the presumed exposure date, the intercepts estimate that the patient received between 0.5% and 8% of a standard contrast dose.
MRI contrast agents can cause skin disease. Systemic fibrosis is considered one of the most severe AEs. Skin pathophysiology involving myeloid cells is driven by elevated levels of monocyte chemoattractant protein-1, which recruits circulating fibroblasts via the C-C chemokine receptor 2.22,23 This occurs alongside activation of NADPH oxidase Nox4.4,24,25 Intracellular gadolinium-rich nanoparticles likely serve as catalysts for this reactive cascade.2,18,22,26,27 These particles assemble around intracellular lipid droplets and ferrule them in spiculated rare earth-rich shells that compromise cellular architecture.2,18,21,22,26,27 Frequently sequestered within endosomal compartments, they disrupt vesicular integrity and threaten cellular homeostasis. Interference with degradative systems such as the endolysosomal axis perturbs energy-recycling pathways—an insidious disturbance, particularly in cells with high metabolic demand. Skin-related symptoms are among the most frequently reported AEs, according to the FDA AE reporting system.18
Studies indicate repeated exposure to MRI contrast agents can lead to permanent gadolinium retention in the brain and other vital organs. Intravenous (IV) contrast agents cross the blood-brain barrier rapidly, while intrathecal administration has been linked to significant and lasting neurologic effects.18
Gadolinium is chemically bound to pharmaceutical ligands to enhance renal clearance and reduce toxicity. However, available data from human samples suggest potential ligand exchanges with undefined physiologic substances. This exchange may facilitate gadolinium precipitation and accumulation within cells into spiculated nanoparticles. Transmission electron microscopy reveals the formation of unilamellar bodies associated with mitochondriopathy and cellular damage, particularly in renal proximal tubules.2,18,22,26,27 It is proposed that intracellular nanoparticle formation represents a key mechanism driving the systemic symptoms observed in patients.1,2,18, 22,26,27
Any hypothesis based on free soluble gadolinium—or concept derived from it—should be discarded. The high affinity of pharmaceutical ligands for gadolinium suggests that the cationic rare earth metal remains predominantly in a ligand-bound, soluble form. It is hypothesized that gadolinium undergoes ligand exchange with physiologic substances, directly leading to nanoparticle formation. Current data demonstrate gadolinium precipitation according to the Le Chatelier’s principle. Since precipitated gadolinium does not readily re-equilibrate with pharmaceutical ligands, repeated administration of different contrast agent brands may contribute to nanoparticle growth.26
Meanwhile, a growing number of patients are turning to chelation therapy, a largely untested treatment. The premise of chelation therapy is rooted in several unproven assumptions.18,21 First, it assumes that clinically significant amounts of gadolinium persist in compartments such as the extracellular space, where they can be effectively chelated and cleared. Second, it presumes that free gadolinium is the primary driver of chronic symptoms, an assertion that remains scientifically unsubstantiated. Finally, chelation proponents overlook the potential harm caused by depleting essential physiological metals during the process, assuming without evidence that the scant removal of gadolinium outweighs the risk of physiological mineral depletion.
These assumptions underpin an unproven remedy that demands critical scrutiny. Recent findings reveal that gadolinium deposits in the skin and kidney often take the form of intracellular nanoparticles, directly challenging the foundation of chelation therapy. Chelation advocates must demonstrate that these intracellular gadolinium deposits neither trigger cellular toxicity nor initiate a cytokine cascade. Chelation supporters must prove that the systemic response to these foreign particles is unrelated to the symptoms reported by patients. Until then, the validity of chelation therapy remains highly questionable.
The causality of the symptoms, mainly whether IV gadolinium was administered, was examined. The null hypothesis stated that the patient was not exposed to gadolinium. However, this hypothesis was contradicted by the detection of gadolinium in the serum and urine 27 days after the potential exposure.
Two plausible explanations exist for the nonzero gadolinium levels detected in the serum and urine. The first possibility is that minute quantities of gadolinium were introduced during cannulation, with the amount being sufficient to persist in measurable concentrations 27 days postexposure. The second possibility is that the gadolinium originated from an MRI contrast agent administered 4 years earlier. In this scenario, gadolinium stored in organ reservoirs such as bone, liver, or kidneys may have been mobilized into the extracellular fluid compartment due to the administration of high-dose steroids 20 days after the recent contrast-enhanced MRI procedure attempt. Coyte et al reported elevated gadolinium levels in the serum, cord blood, breast milk, and placenta of pregnant women with prior exposure to MRI contrast agents.28 These findings suggest that gadolinium, stored in organs such as bone may be remobilized by variables affecting bone remodeling (eg, high-dose steroids).
Significantly, the patient exhibited elevated urinary oxalate levels. Previous research has found that oxalic acid reacts rapidly with MRI contrast agents, forming digadolinium trioxalate. While the gadolinium-rich nanoparticles identified in tissues such as the skin and kidney (including the human kidney) are amorphous, these in vitro findings establish a proof-of-concept: the intracellular environment facilitates gadolinium dissociation from pharmaceutical chelates.
Furthermore, in vitro experiments show that proteins and lysosomal pH promote this dissociation, underscoring how human metabolic conditions—particularly oxalic acid concentration—may drive intracellular gadolinium deposition.
Patient Perspective
“They put something into my body that they cannot get out.” This stark realization underpins the patient’s profound concern about gadolinium-based contrast agents and their potential long-term effects. Reflecting on his experience, the patient expressed deep fears about the unknown future impacts: “I’m concerned about my kidneys, I’m concerned about my heart, and I’m concerned about my brain. I don’t know how this stuff is going to affect me in the future.”
He drew an unsettling parallel between gadolinium and heavy metals: “Heavy metal is poison. The body does not produce this kind of stuff on its own.” His reaction to the procedure left a lasting impression, prompting him to question the logic of using a substance that cannot be purged: “Why would you put something into someone’s body that you cannot extract? Nobody—nobody—should experience what I went through.”
The patient emphasized the lack of clear research on long-term outcomes, which compounds his anxiety: “If there was research that said, ‘Well, this is only going to affect these organs for this long,’ OK, I might be able to accept that. But there is no research like that. Nobody can tell me what’s going to happen in 5 years.”
Strengths and Limitations
A significant strength of this approach is the ability to track gadolinium elimination and symptom resolution over time, supported by unique access to intermediate and long-term clearance data from our ongoing research protocol. The investigators were equipped to back-extrapolate the exposure, which provided a rare opportunity to correlate gadolinium levels with clinical outcomes. The primary limitation is the lack of a defined clinical case definition for gadolinium toxicity and limited mechanistic understanding of SAGE, which hinders diagnosis and management.
Metabolites, proteins, and lipids rich in Lewis bases could initiate this process as substrates for intracellular gadolinium sedimentation. Future studies should investigate whether metabolic conditions such as oxalate burden or altered parathyroid hormone levels modulate gadolinium compartmentalization and tissue retention. If gadolinium-rich nanoparticle formation and accumulation disrupt cellular equilibrium, it underscores an urgent need to understand the implications of long-term gadolinium retention. The research team continues to gather evidence that the gadolinium cation remains chelated from the moment MRI contrast agents are administered through to the formation of intracellular nanoparticles. Retained gadolinium nanoparticles may act as a nidus, triggering cellular signaling cascades that lead to multisymptomatic illnesses. Intracellular and insoluble retained gadolinium challenges proponents of untested chelation therapies.
Conclusions
This case highlights emerging clinical and ethical concerns surrounding gadolinium-based contrast agent use. Clinicians may benefit from considering gadolinium retention as a contributor to persistent, unexplained symptoms—particularly in patients with recent imaging exposure. As contrast use continues to rise within federal health systems, regulatory and administrative stakeholders would do well to re-examine current safety frameworks. Informed consent should reflect what is known: gadolinium can remain in the body long after administration, potentially indefinitely. The long-term consequences of cumulative exposure remain poorly defined, but the presence of a lanthanide element in human tissue warrants greater attention from researchers and regulators alike. Interest in alternative imaging modalities and long-term safety monitoring would mark progress toward more transparent, accountable care.
Jackson DB, MacIntyre T, Duarte-Miramontes V, et al. Gadolinium deposition disease: a case report and the prevalence of enhanced MRI procedures within the Veterans Health Administration. Fed Pract. 2022;39:218-225. doi:10.12788/fp.0258
Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1:561-568.doi:10.34067/kid.0000272019
Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28:154-162. doi:10.1097/MNH.0000000000000475
Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311:F1-F11. doi:10.1152/ajprenal.00166.2016
Maramattom BV, Manno EM, Wijdicks EF, et al. Gadolinium encephalopathy in a patient with renal failure. Neurology. 2005;64:1276-1278.doi:10.1212/01.WNL.0000156805.45547.6E
Sam AD II, Morasch MD, Collins J, et al. Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J Vasc Surg. 2003;38:313-318. doi:10.1016/s0741-5214(03)00315-x
Schenker MP, Solomon JA, Roberts DA. Gadolinium arteriography complicated by acute pancreatitis and acute renal failure. J Vasc Interv Radiol. 2001;12:393. doi:10.1016/s1051-0443(07)61925-3
Gemery J, Idelson B, Reid S, et al. Acute renal failure after arteriography with a gadolinium-based contrast agent. AJR Am J Roentgenol. 1998;171:1277-1278. doi:10.2214/ajr.171.5.9798860
Akgun H, Gonlusen G, Cartwright J Jr, et al. Are gadolinium-based contrast media nephrotoxic? A renal biopsy study. Arch Pathol Lab Med. 2006;130:1354-1357. doi:10.5858/2006-130-1354-AGCMNA
Gathings RM, Reddy R, Santa Cruz D, et al. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151:316-319. doi:10.1001/jamadermatol.2014.2660
McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834-841. doi:10.1148/radiol.13131669
Schmidt K, Bau M, Merschel G, et al. Anthropogenic gadolinium in tap water and in tap water-based beverages from fast-food franchises in six major cities in Germany. Sci Total Environ. 2019;687:1401-1408. doi:10.1016/j.scitotenv.2019.07.075
Kulaksız S, Bau M. Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Appl Geochem. 2011;26:1877-1885.
Brunjes R, Hofmann T. Anthropogenic gadolinium in freshwater and drinking water systems. Water Res. 2020;182:115966. doi:10.1016/j.watres.2020.115966
Endrikat J, Gutberlet M, Hoffmann KT, et al. Clinical safety of gadobutrol: review of over 25 years of use exceeding 100 million administrations. Invest Radiol. 2024;59(9):605-613. doi:10.1097/RLI.0000000000001072
Elmholdt TR, Jørgensen B, Ramsing M, et al. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287. doi:10.1093/ndtplus/sfq028
Cunningham A, Kirk M, Hong E, et al. The safety of magnetic resonance imaging contrast agents. Front Toxicol. 2024;6:1376587. doi:10.3389/ftox.2024.1376587
Huxley A. Complete Essays. Volume II, 1926-1929. Chicago; 2000:227.
McDonald RJ, Weinreb JC, Davenport MS. Symptoms associated with gadolinium exposure (SAGE): a suggested term. Radiology. 2022;302(2):270-273. doi:10.1148/radiol.2021211349
Henderson IM, Benevidez AD, Mowry CD, et al. Precipitation of gadolinium from magnetic resonance imaging contrast agents may be the Brass tacks of toxicity. Magn Reson Imaging. 2025;119:110383. doi:10.1016/j.mri.2025.110383
Do C, Drel V, Tan C, et al. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143. doi:10.1016/j.jid.2019.03.1145
Drel VR, Tan C, Barnes JL, et al. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH oxidase 4 for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
DeAguero J, Howard T, Kusewitt D, et al. The onset of rare earth metallosis begins with renal gadolinium-rich nanoparticles from magnetic resonance imaging contrast agent exposure. Sci Rep. 2023;13(1):2025. doi:10.1038/s41598-023-28666-1
Do C, Ford B, Lee DY, et al. Gadolinium-based contrast agents: Stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
Coyte RM, Darrah T, Olesik J, et al. Gadolinium during human pregnancy following administration of gadolinium chelate before pregnancy. Birth Defects Res. 2023;115(14):1264-1273. doi:10.1002/bdr2.2209
Magnetic resonance image (MRI) contrast agents can induce profound complications, including gadolinium encephalopathy, kidney injury, gadolinium-associated plaques, and progressive systemic fibrosis, which can be fatal.1-10 About 50% of MRIs use gadolinium-based contrast (Gd3+), a toxic rare earth metal ion that enhances imaging but requires binding with pharmaceutical ligands to reduce toxicity and promote renal elimination (Figure 1). Despite these measures, Gd3+ can persist in the body, including the brain.11,12 Wastewater treatment fails to remove these agents, making Gd3+ a growing pollutant in water and the food chain.13-15 Because Gd3+ is a rare earth metal ion in the milieu intérieur, there is an urgent need to study its biological and long-term effects (Appendix 1).
Case Presentation
A 65-year-old Vietnam-era veteran presented to nephrology at the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, for evaluation of gadolinium-induced symptoms. His medical history included metabolic syndrome, hypertension, hyperlipidemia, hypogonadism, cervical spondylosis, and an elevated prostate-specific antigen, previously assessed with a contrast-enhanced MRI in 2019 (Gadobenic acid, 19 mL). Surgical history included cervical fusion and ankle hardware.
The patient had a scheduled MRI 25 days earlier, following an elevated prostate specific antigen test result, prompting urologic surveillance and concern for malignancy. In preparation for the contrast-enhanced MRI, his right arm was cannulated with a line primed with gadobenic acid contrast. Though the technician stated the infusion had not started, the patient’s symptoms began shortly after entry into the scanner, before any programmed pulse sequences. The patient experienced claustrophobia, diaphoresis, palpitations, xerostomia, dysgeusia, shortness of breath, and a sensation of heat in his groin, chest, “kidneys,” and lower back. The MRI was terminated prematurely in response to the patient’s acute symptomatology. The patient continued experiencing new symptoms intermittently during the following week, including lightheadedness, headaches, right clavicular pain, raspy voice, edema, and a sense of doom.


The patient presented to the RGMVAMC emergency department (ED) 8 days after the MRI with worsening symptoms and was hospitalized for 10 days. During this time, he was referred to nephrology for outpatient evaluation. While awaiting his nephrology appointment, the patient presented to the RGMVAMC ED 20 days after the initial episode with ongoing symptoms. “I thought I was dying,” he said. Laboratory results and a 12-lead electrocardiogram showed a finely static background, wide P waves (> 80 ms) with notching in lead II, sinusoidal P waves in V1, R transition in V2, RR’ in V2, ST flat in lead III, and sinus bradycardia (Table 1 and Appendix 2).
The patient’s medical and surgical histories were reviewed at the nephrology evaluation 25 days following the MRI. He reported that household water was sourced from a well and that he filtered his drinking water with a reverse osmosis system. He served in the US Army for 10 years as an engineer specializing in mechanical systems, power generation, and vehicles. Following Army retirement, the patient served in the US Air Force Reserves for 15 years, working as a crew chief in pneudraulics. The patient reported stopping tobacco use 1 year before and also reported regular use of a broad array of prescription medications and dietary supplements, including dexamethasone (4 mg twice daily), fluticasone nasal spray (50 mcg per nostril, twice daily), ibuprofen (400 mg twice daily, as needed), loratadine (10 mg daily), aspirin (81 mg daily), and metoprolol succinate (50 mg nightly). In addition, he reported consistent use of cholecalciferol (3000 IU daily), another supplemental vitamin D preparation, chelated magnesium glycinate (3 tablets daily for bone issues), turmeric (1 tablet daily), a multivitamin (Living Green Liquid Gel, daily), and a mega-B complex.
Physical examination revealed a well-nourished, tall man with hypertension (145/87 mmHg) and bilateral lower extremity edema. Oral examination showed poor dentition, including missing molars (#1-3, #14-16, #17-19, #30-31), with the anterior teeth replaced by bridges supported by dental implants. The review of systems was otherwise unremarkable, with nocturia noted before the consultation.

Serum and urine gadolinium testing, (Mayo Clinic Laboratories) revealed gadolinium levels of 0.3 mcg/24 h in the urine and 0.1 ng/mL in the serum. Nonzero values indicated detectable gadolinium, suggesting retention. The patient had a prior gadolinium exposure during a 2019 MRI (about 1340 days before) and suspected a repeat exposure on day 0, although the MRI technician stated that no contrast was administered. Given his elevated vitamin D levels, the patient was advised to minimize dietary supplements, particularly vitamin D, to avoid confounding symptoms. The plan included monitoring symptoms and a follow-up evaluation with repeat laboratory tests on day 116.
At the nephrology follow-up 4 months postexposure, the patient's symptoms had primarily abated, with a marked reduction in the previously noted metallic dysgeusia. Physical examination remained consistent with prior findings. He was afebrile (97.7 °F) with a blood pressure of 111/72 mmHg, a pulse of 63 beats per minute, and an oxygen saturation of 98% on ambient air. Laboratory analysis revealed serum and urine gadolinium levels below detectable thresholds (< 0.1 ng/mL and < 0.1 mcg/24 h). A 24-hour creatinine clearance, calculated from a urine volume of 1300 mL, measured at an optimal 106 mL/min, indicating preserved renal function (Tables 2 and 3). Of note, his 24-hour oxalate was above the reference range, with a urine pH below the reference range and a high supersaturation index for calcium oxalate.
Discussion
Use of enhanced MRI has increased in the Veterans Health Administration (Figure 2). A growing range of indications for enhanced procedures (eg, cardiac MRI) has contributed to this rise. The market has grown with new gadolinium-based contrast agents, such as gadopiclenol. However, reliance on untested assumptions about the safety of newer agents and need for robust clinical trials pose potential risks to patient safety.
Without prospective evidence, the American College of Radiology (ACR) classifies gadolinium-based contrast agents into 3 groups: Group 1, associated with the highest number of nephrogenic systemic fibrosis cases; Group 2, linked to few, if any, unconfounded cases; and Group 3, where data on nephrogenic systemic fibrosis risk have been limited. As of April 2024, the ACR reclassified Group 3 agents (Ablavar/Vasovist/Angiomark and Primovist/Eovist) into Group 2. Curiously, Vueway and Elucirem were approved in late 2022 and should clearly be categorized as Group 3 (Table 4).There were 19 cases of nephrogenic systemic fibrosis or similar manifestations, 8 of which were unconfounded by other factors. These patients had been exposed to gadobutrol, often combined with other agents. Gadobutrol—like other Group 2 agents—has been associated with nephrogenic systemic fibrosis.16,17 Despite US Food and Drug Administration (FDA) documentation of rising reports, many clinicians remain unaware that nephrogenic systemic fibrosis is increasingly linked to Group 2 agents classified by the ACR.18 While declines in reported cases of nephrogenic systemic fibrosis may suggest reduced incidence, this trend may reflect diminished clinical vigilance and underreporting, particularly given emerging evidence implicating even Group 2 gadolinium-based contrast agents in delayed and underrecognized presentations. This information has yet to permeate the medical community, particularly among nephrologists. Considering these cases, revisiting the ACR guidelines may be prudent.

To address this growing concern, clinicians must adopt stricter vigilance and actively pursue updated information to mitigate patient risks tied to these contrast agents.
There exists an illusion of knowledge in disregarding the confounded exposures of MRI contrast agents. Ten distinct brands of contrast agents have been approved for clinical use. With repeated imaging, patients are often exposed to varying formulations of gadolinium-based agents. Yet investigators commonly discard these data points when assessing risk. By doing so, they assume—without evidence—that some formulations are inherently less likely to provoke adverse effects (AEs) than others. This untested presumption becomes perilous, especially given the limited understanding of the mechanisms underlying gadolinium-induced pathologies. As Aldous Huxley warned, “Facts do not cease to exist because they are ignored.”19
Gadolinium Persistence
Contrary to expectations, gadolinium persists in the body far longer than initially presumed. Symptoms associated with gadolinium exposure (SAGE) encapsulate the chronic, often enigmatic maladies tied to MRI contrast agents.20 The prolonged retention of this rare earth metal offers a compelling hypothesis for the etiology of SAGE. It has been hypothesized that Lewis base-rich metabolites increase susceptibility to gadolinium-based contrast agent complications.21
The blood and urine concentration elimination curves of gadolinium are exponential and categorized as fast, intermediate, and long-term.1 For urinary elimination, the function of the curves is exponential. The quantity of gadolinium in the urine at a time (t) after exposure (D[Gd](t)) is equal to the product of the amount of gadolinium in the sample (urine or blood) at the end of the fast elimination period (D[Gd](t0)) and the exponential decay with k being a rate constant.
To the authors’ knowledge, we are the only research team currently investigating the rate constant for the intermediate- and long-term phase gadolinium elimination. The Retention and Toxicity of Gadolinium-based Contrast Agents study was approved by the University of New Mexico Health Sciences Center Institutional Review Board on May 27, 2020 (IRB ID 19-660). The data for the patient in this case were compared with preliminary results for patients with exposure-to-measurement intervals < 100 days.
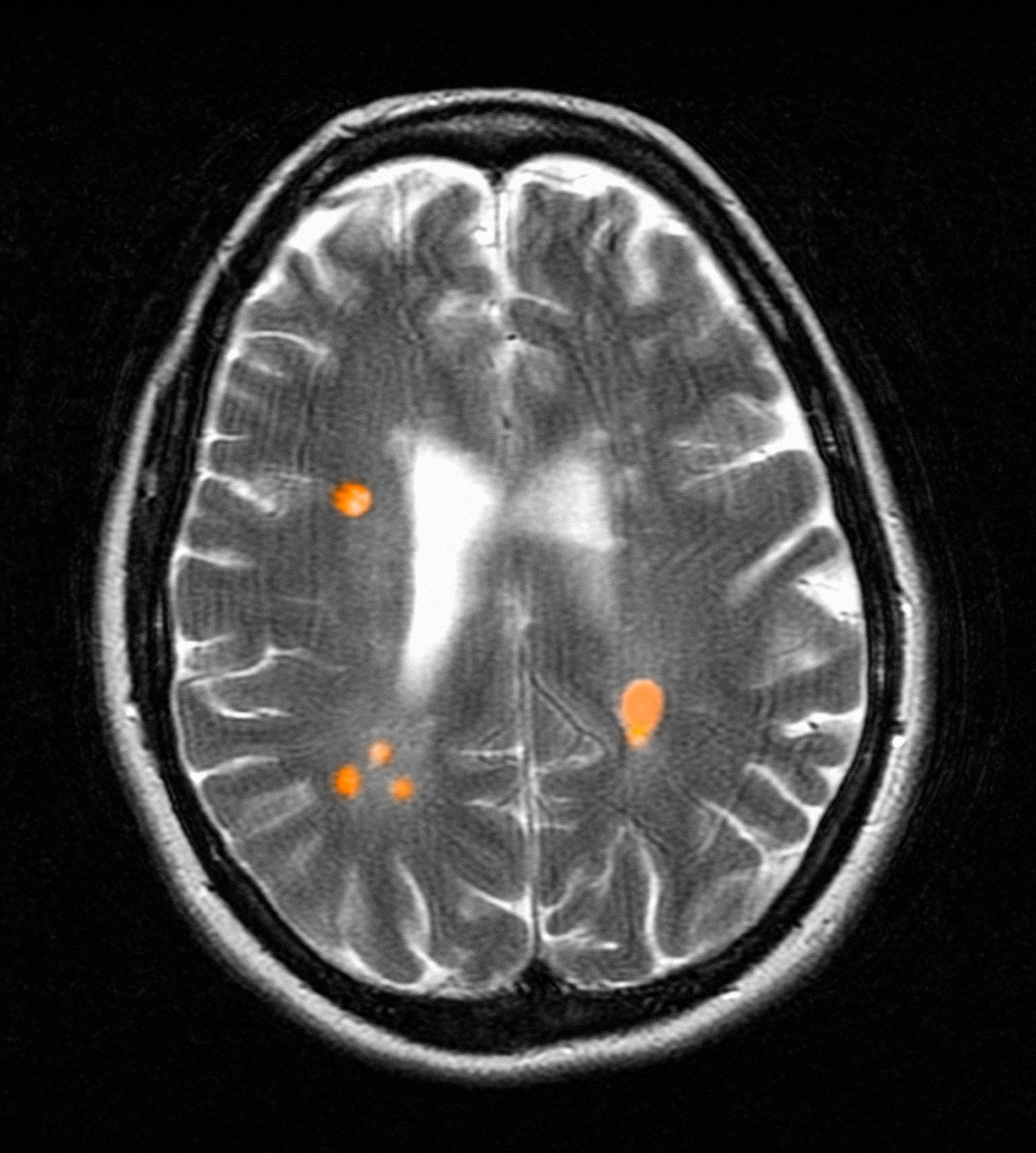
The patient in this case presented with detectable gadolinium levels in urine and serum shortly after an attempted contrast-enhanced MRI procedure (Figure 3). The presence of detectable gadolinium levels in the patient’s urine and serum suggests a likely exposure to a contrast agent about 27 days before his consultation. While the technician reported that no contrast was administered during the attempted MRI, it remains possible that a small amount was introduced during cannulation, potentially triggering the patient’s symptoms. Linear modeling of semilogarithmic plots for participants exposed to contrast agents within 100 days (urine: P = 1.8 × 10ˉ8, adjusted r² = 0.62; blood: P = .005, adjusted r² = 0.21) provided clearance rates (k values) for urine and blood. Extrapolating from these models to the presumed exposure date, the intercepts estimate that the patient received between 0.5% and 8% of a standard contrast dose.

MRI contrast agents can cause skin disease. Systemic fibrosis is considered one of the most severe AEs. Skin pathophysiology involving myeloid cells is driven by elevated levels of monocyte chemoattractant protein-1, which recruits circulating fibroblasts via the C-C chemokine receptor 2.22,23 This occurs alongside activation of NADPH oxidase Nox4.4,24,25 Intracellular gadolinium-rich nanoparticles likely serve as catalysts for this reactive cascade.2,18,22,26,27 These particles assemble around intracellular lipid droplets and ferrule them in spiculated rare earth-rich shells that compromise cellular architecture.2,18,21,22,26,27 Frequently sequestered within endosomal compartments, they disrupt vesicular integrity and threaten cellular homeostasis. Interference with degradative systems such as the endolysosomal axis perturbs energy-recycling pathways—an insidious disturbance, particularly in cells with high metabolic demand. Skin-related symptoms are among the most frequently reported AEs, according to the FDA AE reporting system.18
Studies indicate repeated exposure to MRI contrast agents can lead to permanent gadolinium retention in the brain and other vital organs. Intravenous (IV) contrast agents cross the blood-brain barrier rapidly, while intrathecal administration has been linked to significant and lasting neurologic effects.18
Gadolinium is chemically bound to pharmaceutical ligands to enhance renal clearance and reduce toxicity. However, available data from human samples suggest potential ligand exchanges with undefined physiologic substances. This exchange may facilitate gadolinium precipitation and accumulation within cells into spiculated nanoparticles. Transmission electron microscopy reveals the formation of unilamellar bodies associated with mitochondriopathy and cellular damage, particularly in renal proximal tubules.2,18,22,26,27 It is proposed that intracellular nanoparticle formation represents a key mechanism driving the systemic symptoms observed in patients.1,2,18, 22,26,27
Any hypothesis based on free soluble gadolinium—or concept derived from it—should be discarded. The high affinity of pharmaceutical ligands for gadolinium suggests that the cationic rare earth metal remains predominantly in a ligand-bound, soluble form. It is hypothesized that gadolinium undergoes ligand exchange with physiologic substances, directly leading to nanoparticle formation. Current data demonstrate gadolinium precipitation according to the Le Chatelier’s principle. Since precipitated gadolinium does not readily re-equilibrate with pharmaceutical ligands, repeated administration of different contrast agent brands may contribute to nanoparticle growth.26
Meanwhile, a growing number of patients are turning to chelation therapy, a largely untested treatment. The premise of chelation therapy is rooted in several unproven assumptions.18,21 First, it assumes that clinically significant amounts of gadolinium persist in compartments such as the extracellular space, where they can be effectively chelated and cleared. Second, it presumes that free gadolinium is the primary driver of chronic symptoms, an assertion that remains scientifically unsubstantiated. Finally, chelation proponents overlook the potential harm caused by depleting essential physiological metals during the process, assuming without evidence that the scant removal of gadolinium outweighs the risk of physiological mineral depletion.

These assumptions underpin an unproven remedy that demands critical scrutiny. Recent findings reveal that gadolinium deposits in the skin and kidney often take the form of intracellular nanoparticles, directly challenging the foundation of chelation therapy. Chelation advocates must demonstrate that these intracellular gadolinium deposits neither trigger cellular toxicity nor initiate a cytokine cascade. Chelation supporters must prove that the systemic response to these foreign particles is unrelated to the symptoms reported by patients. Until then, the validity of chelation therapy remains highly questionable.
The causality of the symptoms, mainly whether IV gadolinium was administered, was examined. The null hypothesis stated that the patient was not exposed to gadolinium. However, this hypothesis was contradicted by the detection of gadolinium in the serum and urine 27 days after the potential exposure.
Two plausible explanations exist for the nonzero gadolinium levels detected in the serum and urine. The first possibility is that minute quantities of gadolinium were introduced during cannulation, with the amount being sufficient to persist in measurable concentrations 27 days postexposure. The second possibility is that the gadolinium originated from an MRI contrast agent administered 4 years earlier. In this scenario, gadolinium stored in organ reservoirs such as bone, liver, or kidneys may have been mobilized into the extracellular fluid compartment due to the administration of high-dose steroids 20 days after the recent contrast-enhanced MRI procedure attempt. Coyte et al reported elevated gadolinium levels in the serum, cord blood, breast milk, and placenta of pregnant women with prior exposure to MRI contrast agents.28 These findings suggest that gadolinium, stored in organs such as bone may be remobilized by variables affecting bone remodeling (eg, high-dose steroids).
Significantly, the patient exhibited elevated urinary oxalate levels. Previous research has found that oxalic acid reacts rapidly with MRI contrast agents, forming digadolinium trioxalate. While the gadolinium-rich nanoparticles identified in tissues such as the skin and kidney (including the human kidney) are amorphous, these in vitro findings establish a proof-of-concept: the intracellular environment facilitates gadolinium dissociation from pharmaceutical chelates.

Furthermore, in vitro experiments show that proteins and lysosomal pH promote this dissociation, underscoring how human metabolic conditions—particularly oxalic acid concentration—may drive intracellular gadolinium deposition.
Patient Perspective
“They put something into my body that they cannot get out.” This stark realization underpins the patient’s profound concern about gadolinium-based contrast agents and their potential long-term effects. Reflecting on his experience, the patient expressed deep fears about the unknown future impacts: “I’m concerned about my kidneys, I’m concerned about my heart, and I’m concerned about my brain. I don’t know how this stuff is going to affect me in the future.”
He drew an unsettling parallel between gadolinium and heavy metals: “Heavy metal is poison. The body does not produce this kind of stuff on its own.” His reaction to the procedure left a lasting impression, prompting him to question the logic of using a substance that cannot be purged: “Why would you put something into someone’s body that you cannot extract? Nobody—nobody—should experience what I went through.”
The patient emphasized the lack of clear research on long-term outcomes, which compounds his anxiety: “If there was research that said, ‘Well, this is only going to affect these organs for this long,’ OK, I might be able to accept that. But there is no research like that. Nobody can tell me what’s going to happen in 5 years.”
Strengths and Limitations
A significant strength of this approach is the ability to track gadolinium elimination and symptom resolution over time, supported by unique access to intermediate and long-term clearance data from our ongoing research protocol. The investigators were equipped to back-extrapolate the exposure, which provided a rare opportunity to correlate gadolinium levels with clinical outcomes. The primary limitation is the lack of a defined clinical case definition for gadolinium toxicity and limited mechanistic understanding of SAGE, which hinders diagnosis and management.
Metabolites, proteins, and lipids rich in Lewis bases could initiate this process as substrates for intracellular gadolinium sedimentation. Future studies should investigate whether metabolic conditions such as oxalate burden or altered parathyroid hormone levels modulate gadolinium compartmentalization and tissue retention. If gadolinium-rich nanoparticle formation and accumulation disrupt cellular equilibrium, it underscores an urgent need to understand the implications of long-term gadolinium retention. The research team continues to gather evidence that the gadolinium cation remains chelated from the moment MRI contrast agents are administered through to the formation of intracellular nanoparticles. Retained gadolinium nanoparticles may act as a nidus, triggering cellular signaling cascades that lead to multisymptomatic illnesses. Intracellular and insoluble retained gadolinium challenges proponents of untested chelation therapies.
Conclusions
This case highlights emerging clinical and ethical concerns surrounding gadolinium-based contrast agent use. Clinicians may benefit from considering gadolinium retention as a contributor to persistent, unexplained symptoms—particularly in patients with recent imaging exposure. As contrast use continues to rise within federal health systems, regulatory and administrative stakeholders would do well to re-examine current safety frameworks. Informed consent should reflect what is known: gadolinium can remain in the body long after administration, potentially indefinitely. The long-term consequences of cumulative exposure remain poorly defined, but the presence of a lanthanide element in human tissue warrants greater attention from researchers and regulators alike. Interest in alternative imaging modalities and long-term safety monitoring would mark progress toward more transparent, accountable care.


Magnetic resonance image (MRI) contrast agents can induce profound complications, including gadolinium encephalopathy, kidney injury, gadolinium-associated plaques, and progressive systemic fibrosis, which can be fatal.1-10 About 50% of MRIs use gadolinium-based contrast (Gd3+), a toxic rare earth metal ion that enhances imaging but requires binding with pharmaceutical ligands to reduce toxicity and promote renal elimination (Figure 1). Despite these measures, Gd3+ can persist in the body, including the brain.11,12 Wastewater treatment fails to remove these agents, making Gd3+ a growing pollutant in water and the food chain.13-15 Because Gd3+ is a rare earth metal ion in the milieu intérieur, there is an urgent need to study its biological and long-term effects (Appendix 1).
Case Presentation
A 65-year-old Vietnam-era veteran presented to nephrology at the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, for evaluation of gadolinium-induced symptoms. His medical history included metabolic syndrome, hypertension, hyperlipidemia, hypogonadism, cervical spondylosis, and an elevated prostate-specific antigen, previously assessed with a contrast-enhanced MRI in 2019 (Gadobenic acid, 19 mL). Surgical history included cervical fusion and ankle hardware.
The patient had a scheduled MRI 25 days earlier, following an elevated prostate specific antigen test result, prompting urologic surveillance and concern for malignancy. In preparation for the contrast-enhanced MRI, his right arm was cannulated with a line primed with gadobenic acid contrast. Though the technician stated the infusion had not started, the patient’s symptoms began shortly after entry into the scanner, before any programmed pulse sequences. The patient experienced claustrophobia, diaphoresis, palpitations, xerostomia, dysgeusia, shortness of breath, and a sensation of heat in his groin, chest, “kidneys,” and lower back. The MRI was terminated prematurely in response to the patient’s acute symptomatology. The patient continued experiencing new symptoms intermittently during the following week, including lightheadedness, headaches, right clavicular pain, raspy voice, edema, and a sense of doom.


The patient presented to the RGMVAMC emergency department (ED) 8 days after the MRI with worsening symptoms and was hospitalized for 10 days. During this time, he was referred to nephrology for outpatient evaluation. While awaiting his nephrology appointment, the patient presented to the RGMVAMC ED 20 days after the initial episode with ongoing symptoms. “I thought I was dying,” he said. Laboratory results and a 12-lead electrocardiogram showed a finely static background, wide P waves (> 80 ms) with notching in lead II, sinusoidal P waves in V1, R transition in V2, RR’ in V2, ST flat in lead III, and sinus bradycardia (Table 1 and Appendix 2).
The patient’s medical and surgical histories were reviewed at the nephrology evaluation 25 days following the MRI. He reported that household water was sourced from a well and that he filtered his drinking water with a reverse osmosis system. He served in the US Army for 10 years as an engineer specializing in mechanical systems, power generation, and vehicles. Following Army retirement, the patient served in the US Air Force Reserves for 15 years, working as a crew chief in pneudraulics. The patient reported stopping tobacco use 1 year before and also reported regular use of a broad array of prescription medications and dietary supplements, including dexamethasone (4 mg twice daily), fluticasone nasal spray (50 mcg per nostril, twice daily), ibuprofen (400 mg twice daily, as needed), loratadine (10 mg daily), aspirin (81 mg daily), and metoprolol succinate (50 mg nightly). In addition, he reported consistent use of cholecalciferol (3000 IU daily), another supplemental vitamin D preparation, chelated magnesium glycinate (3 tablets daily for bone issues), turmeric (1 tablet daily), a multivitamin (Living Green Liquid Gel, daily), and a mega-B complex.
Physical examination revealed a well-nourished, tall man with hypertension (145/87 mmHg) and bilateral lower extremity edema. Oral examination showed poor dentition, including missing molars (#1-3, #14-16, #17-19, #30-31), with the anterior teeth replaced by bridges supported by dental implants. The review of systems was otherwise unremarkable, with nocturia noted before the consultation.

Serum and urine gadolinium testing, (Mayo Clinic Laboratories) revealed gadolinium levels of 0.3 mcg/24 h in the urine and 0.1 ng/mL in the serum. Nonzero values indicated detectable gadolinium, suggesting retention. The patient had a prior gadolinium exposure during a 2019 MRI (about 1340 days before) and suspected a repeat exposure on day 0, although the MRI technician stated that no contrast was administered. Given his elevated vitamin D levels, the patient was advised to minimize dietary supplements, particularly vitamin D, to avoid confounding symptoms. The plan included monitoring symptoms and a follow-up evaluation with repeat laboratory tests on day 116.
At the nephrology follow-up 4 months postexposure, the patient's symptoms had primarily abated, with a marked reduction in the previously noted metallic dysgeusia. Physical examination remained consistent with prior findings. He was afebrile (97.7 °F) with a blood pressure of 111/72 mmHg, a pulse of 63 beats per minute, and an oxygen saturation of 98% on ambient air. Laboratory analysis revealed serum and urine gadolinium levels below detectable thresholds (< 0.1 ng/mL and < 0.1 mcg/24 h). A 24-hour creatinine clearance, calculated from a urine volume of 1300 mL, measured at an optimal 106 mL/min, indicating preserved renal function (Tables 2 and 3). Of note, his 24-hour oxalate was above the reference range, with a urine pH below the reference range and a high supersaturation index for calcium oxalate.
Discussion
Use of enhanced MRI has increased in the Veterans Health Administration (Figure 2). A growing range of indications for enhanced procedures (eg, cardiac MRI) has contributed to this rise. The market has grown with new gadolinium-based contrast agents, such as gadopiclenol. However, reliance on untested assumptions about the safety of newer agents and need for robust clinical trials pose potential risks to patient safety.
Without prospective evidence, the American College of Radiology (ACR) classifies gadolinium-based contrast agents into 3 groups: Group 1, associated with the highest number of nephrogenic systemic fibrosis cases; Group 2, linked to few, if any, unconfounded cases; and Group 3, where data on nephrogenic systemic fibrosis risk have been limited. As of April 2024, the ACR reclassified Group 3 agents (Ablavar/Vasovist/Angiomark and Primovist/Eovist) into Group 2. Curiously, Vueway and Elucirem were approved in late 2022 and should clearly be categorized as Group 3 (Table 4).There were 19 cases of nephrogenic systemic fibrosis or similar manifestations, 8 of which were unconfounded by other factors. These patients had been exposed to gadobutrol, often combined with other agents. Gadobutrol—like other Group 2 agents—has been associated with nephrogenic systemic fibrosis.16,17 Despite US Food and Drug Administration (FDA) documentation of rising reports, many clinicians remain unaware that nephrogenic systemic fibrosis is increasingly linked to Group 2 agents classified by the ACR.18 While declines in reported cases of nephrogenic systemic fibrosis may suggest reduced incidence, this trend may reflect diminished clinical vigilance and underreporting, particularly given emerging evidence implicating even Group 2 gadolinium-based contrast agents in delayed and underrecognized presentations. This information has yet to permeate the medical community, particularly among nephrologists. Considering these cases, revisiting the ACR guidelines may be prudent.

To address this growing concern, clinicians must adopt stricter vigilance and actively pursue updated information to mitigate patient risks tied to these contrast agents.
There exists an illusion of knowledge in disregarding the confounded exposures of MRI contrast agents. Ten distinct brands of contrast agents have been approved for clinical use. With repeated imaging, patients are often exposed to varying formulations of gadolinium-based agents. Yet investigators commonly discard these data points when assessing risk. By doing so, they assume—without evidence—that some formulations are inherently less likely to provoke adverse effects (AEs) than others. This untested presumption becomes perilous, especially given the limited understanding of the mechanisms underlying gadolinium-induced pathologies. As Aldous Huxley warned, “Facts do not cease to exist because they are ignored.”19
Gadolinium Persistence
Contrary to expectations, gadolinium persists in the body far longer than initially presumed. Symptoms associated with gadolinium exposure (SAGE) encapsulate the chronic, often enigmatic maladies tied to MRI contrast agents.20 The prolonged retention of this rare earth metal offers a compelling hypothesis for the etiology of SAGE. It has been hypothesized that Lewis base-rich metabolites increase susceptibility to gadolinium-based contrast agent complications.21
The blood and urine concentration elimination curves of gadolinium are exponential and categorized as fast, intermediate, and long-term.1 For urinary elimination, the function of the curves is exponential. The quantity of gadolinium in the urine at a time (t) after exposure (D[Gd](t)) is equal to the product of the amount of gadolinium in the sample (urine or blood) at the end of the fast elimination period (D[Gd](t0)) and the exponential decay with k being a rate constant.
To the authors’ knowledge, we are the only research team currently investigating the rate constant for the intermediate- and long-term phase gadolinium elimination. The Retention and Toxicity of Gadolinium-based Contrast Agents study was approved by the University of New Mexico Health Sciences Center Institutional Review Board on May 27, 2020 (IRB ID 19-660). The data for the patient in this case were compared with preliminary results for patients with exposure-to-measurement intervals < 100 days.
The patient in this case presented with detectable gadolinium levels in urine and serum shortly after an attempted contrast-enhanced MRI procedure (Figure 3). The presence of detectable gadolinium levels in the patient’s urine and serum suggests a likely exposure to a contrast agent about 27 days before his consultation. While the technician reported that no contrast was administered during the attempted MRI, it remains possible that a small amount was introduced during cannulation, potentially triggering the patient’s symptoms. Linear modeling of semilogarithmic plots for participants exposed to contrast agents within 100 days (urine: P = 1.8 × 10ˉ8, adjusted r² = 0.62; blood: P = .005, adjusted r² = 0.21) provided clearance rates (k values) for urine and blood. Extrapolating from these models to the presumed exposure date, the intercepts estimate that the patient received between 0.5% and 8% of a standard contrast dose.

MRI contrast agents can cause skin disease. Systemic fibrosis is considered one of the most severe AEs. Skin pathophysiology involving myeloid cells is driven by elevated levels of monocyte chemoattractant protein-1, which recruits circulating fibroblasts via the C-C chemokine receptor 2.22,23 This occurs alongside activation of NADPH oxidase Nox4.4,24,25 Intracellular gadolinium-rich nanoparticles likely serve as catalysts for this reactive cascade.2,18,22,26,27 These particles assemble around intracellular lipid droplets and ferrule them in spiculated rare earth-rich shells that compromise cellular architecture.2,18,21,22,26,27 Frequently sequestered within endosomal compartments, they disrupt vesicular integrity and threaten cellular homeostasis. Interference with degradative systems such as the endolysosomal axis perturbs energy-recycling pathways—an insidious disturbance, particularly in cells with high metabolic demand. Skin-related symptoms are among the most frequently reported AEs, according to the FDA AE reporting system.18
Studies indicate repeated exposure to MRI contrast agents can lead to permanent gadolinium retention in the brain and other vital organs. Intravenous (IV) contrast agents cross the blood-brain barrier rapidly, while intrathecal administration has been linked to significant and lasting neurologic effects.18
Gadolinium is chemically bound to pharmaceutical ligands to enhance renal clearance and reduce toxicity. However, available data from human samples suggest potential ligand exchanges with undefined physiologic substances. This exchange may facilitate gadolinium precipitation and accumulation within cells into spiculated nanoparticles. Transmission electron microscopy reveals the formation of unilamellar bodies associated with mitochondriopathy and cellular damage, particularly in renal proximal tubules.2,18,22,26,27 It is proposed that intracellular nanoparticle formation represents a key mechanism driving the systemic symptoms observed in patients.1,2,18, 22,26,27
Any hypothesis based on free soluble gadolinium—or concept derived from it—should be discarded. The high affinity of pharmaceutical ligands for gadolinium suggests that the cationic rare earth metal remains predominantly in a ligand-bound, soluble form. It is hypothesized that gadolinium undergoes ligand exchange with physiologic substances, directly leading to nanoparticle formation. Current data demonstrate gadolinium precipitation according to the Le Chatelier’s principle. Since precipitated gadolinium does not readily re-equilibrate with pharmaceutical ligands, repeated administration of different contrast agent brands may contribute to nanoparticle growth.26
Meanwhile, a growing number of patients are turning to chelation therapy, a largely untested treatment. The premise of chelation therapy is rooted in several unproven assumptions.18,21 First, it assumes that clinically significant amounts of gadolinium persist in compartments such as the extracellular space, where they can be effectively chelated and cleared. Second, it presumes that free gadolinium is the primary driver of chronic symptoms, an assertion that remains scientifically unsubstantiated. Finally, chelation proponents overlook the potential harm caused by depleting essential physiological metals during the process, assuming without evidence that the scant removal of gadolinium outweighs the risk of physiological mineral depletion.

These assumptions underpin an unproven remedy that demands critical scrutiny. Recent findings reveal that gadolinium deposits in the skin and kidney often take the form of intracellular nanoparticles, directly challenging the foundation of chelation therapy. Chelation advocates must demonstrate that these intracellular gadolinium deposits neither trigger cellular toxicity nor initiate a cytokine cascade. Chelation supporters must prove that the systemic response to these foreign particles is unrelated to the symptoms reported by patients. Until then, the validity of chelation therapy remains highly questionable.
The causality of the symptoms, mainly whether IV gadolinium was administered, was examined. The null hypothesis stated that the patient was not exposed to gadolinium. However, this hypothesis was contradicted by the detection of gadolinium in the serum and urine 27 days after the potential exposure.
Two plausible explanations exist for the nonzero gadolinium levels detected in the serum and urine. The first possibility is that minute quantities of gadolinium were introduced during cannulation, with the amount being sufficient to persist in measurable concentrations 27 days postexposure. The second possibility is that the gadolinium originated from an MRI contrast agent administered 4 years earlier. In this scenario, gadolinium stored in organ reservoirs such as bone, liver, or kidneys may have been mobilized into the extracellular fluid compartment due to the administration of high-dose steroids 20 days after the recent contrast-enhanced MRI procedure attempt. Coyte et al reported elevated gadolinium levels in the serum, cord blood, breast milk, and placenta of pregnant women with prior exposure to MRI contrast agents.28 These findings suggest that gadolinium, stored in organs such as bone may be remobilized by variables affecting bone remodeling (eg, high-dose steroids).
Significantly, the patient exhibited elevated urinary oxalate levels. Previous research has found that oxalic acid reacts rapidly with MRI contrast agents, forming digadolinium trioxalate. While the gadolinium-rich nanoparticles identified in tissues such as the skin and kidney (including the human kidney) are amorphous, these in vitro findings establish a proof-of-concept: the intracellular environment facilitates gadolinium dissociation from pharmaceutical chelates.

Furthermore, in vitro experiments show that proteins and lysosomal pH promote this dissociation, underscoring how human metabolic conditions—particularly oxalic acid concentration—may drive intracellular gadolinium deposition.
Patient Perspective
“They put something into my body that they cannot get out.” This stark realization underpins the patient’s profound concern about gadolinium-based contrast agents and their potential long-term effects. Reflecting on his experience, the patient expressed deep fears about the unknown future impacts: “I’m concerned about my kidneys, I’m concerned about my heart, and I’m concerned about my brain. I don’t know how this stuff is going to affect me in the future.”
He drew an unsettling parallel between gadolinium and heavy metals: “Heavy metal is poison. The body does not produce this kind of stuff on its own.” His reaction to the procedure left a lasting impression, prompting him to question the logic of using a substance that cannot be purged: “Why would you put something into someone’s body that you cannot extract? Nobody—nobody—should experience what I went through.”
The patient emphasized the lack of clear research on long-term outcomes, which compounds his anxiety: “If there was research that said, ‘Well, this is only going to affect these organs for this long,’ OK, I might be able to accept that. But there is no research like that. Nobody can tell me what’s going to happen in 5 years.”
Strengths and Limitations
A significant strength of this approach is the ability to track gadolinium elimination and symptom resolution over time, supported by unique access to intermediate and long-term clearance data from our ongoing research protocol. The investigators were equipped to back-extrapolate the exposure, which provided a rare opportunity to correlate gadolinium levels with clinical outcomes. The primary limitation is the lack of a defined clinical case definition for gadolinium toxicity and limited mechanistic understanding of SAGE, which hinders diagnosis and management.
Metabolites, proteins, and lipids rich in Lewis bases could initiate this process as substrates for intracellular gadolinium sedimentation. Future studies should investigate whether metabolic conditions such as oxalate burden or altered parathyroid hormone levels modulate gadolinium compartmentalization and tissue retention. If gadolinium-rich nanoparticle formation and accumulation disrupt cellular equilibrium, it underscores an urgent need to understand the implications of long-term gadolinium retention. The research team continues to gather evidence that the gadolinium cation remains chelated from the moment MRI contrast agents are administered through to the formation of intracellular nanoparticles. Retained gadolinium nanoparticles may act as a nidus, triggering cellular signaling cascades that lead to multisymptomatic illnesses. Intracellular and insoluble retained gadolinium challenges proponents of untested chelation therapies.
Conclusions
This case highlights emerging clinical and ethical concerns surrounding gadolinium-based contrast agent use. Clinicians may benefit from considering gadolinium retention as a contributor to persistent, unexplained symptoms—particularly in patients with recent imaging exposure. As contrast use continues to rise within federal health systems, regulatory and administrative stakeholders would do well to re-examine current safety frameworks. Informed consent should reflect what is known: gadolinium can remain in the body long after administration, potentially indefinitely. The long-term consequences of cumulative exposure remain poorly defined, but the presence of a lanthanide element in human tissue warrants greater attention from researchers and regulators alike. Interest in alternative imaging modalities and long-term safety monitoring would mark progress toward more transparent, accountable care.


Jackson DB, MacIntyre T, Duarte-Miramontes V, et al. Gadolinium deposition disease: a case report and the prevalence of enhanced MRI procedures within the Veterans Health Administration. Fed Pract. 2022;39:218-225. doi:10.12788/fp.0258
Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1:561-568.doi:10.34067/kid.0000272019
Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28:154-162. doi:10.1097/MNH.0000000000000475
Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311:F1-F11. doi:10.1152/ajprenal.00166.2016
Maramattom BV, Manno EM, Wijdicks EF, et al. Gadolinium encephalopathy in a patient with renal failure. Neurology. 2005;64:1276-1278.doi:10.1212/01.WNL.0000156805.45547.6E
Sam AD II, Morasch MD, Collins J, et al. Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J Vasc Surg. 2003;38:313-318. doi:10.1016/s0741-5214(03)00315-x
Schenker MP, Solomon JA, Roberts DA. Gadolinium arteriography complicated by acute pancreatitis and acute renal failure. J Vasc Interv Radiol. 2001;12:393. doi:10.1016/s1051-0443(07)61925-3
Gemery J, Idelson B, Reid S, et al. Acute renal failure after arteriography with a gadolinium-based contrast agent. AJR Am J Roentgenol. 1998;171:1277-1278. doi:10.2214/ajr.171.5.9798860
Akgun H, Gonlusen G, Cartwright J Jr, et al. Are gadolinium-based contrast media nephrotoxic? A renal biopsy study. Arch Pathol Lab Med. 2006;130:1354-1357. doi:10.5858/2006-130-1354-AGCMNA
Gathings RM, Reddy R, Santa Cruz D, et al. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151:316-319. doi:10.1001/jamadermatol.2014.2660
McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834-841. doi:10.1148/radiol.13131669
Schmidt K, Bau M, Merschel G, et al. Anthropogenic gadolinium in tap water and in tap water-based beverages from fast-food franchises in six major cities in Germany. Sci Total Environ. 2019;687:1401-1408. doi:10.1016/j.scitotenv.2019.07.075
Kulaksız S, Bau M. Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Appl Geochem. 2011;26:1877-1885.
Brunjes R, Hofmann T. Anthropogenic gadolinium in freshwater and drinking water systems. Water Res. 2020;182:115966. doi:10.1016/j.watres.2020.115966
Endrikat J, Gutberlet M, Hoffmann KT, et al. Clinical safety of gadobutrol: review of over 25 years of use exceeding 100 million administrations. Invest Radiol. 2024;59(9):605-613. doi:10.1097/RLI.0000000000001072
Elmholdt TR, Jørgensen B, Ramsing M, et al. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287. doi:10.1093/ndtplus/sfq028
Cunningham A, Kirk M, Hong E, et al. The safety of magnetic resonance imaging contrast agents. Front Toxicol. 2024;6:1376587. doi:10.3389/ftox.2024.1376587
Huxley A. Complete Essays. Volume II, 1926-1929. Chicago; 2000:227.
McDonald RJ, Weinreb JC, Davenport MS. Symptoms associated with gadolinium exposure (SAGE): a suggested term. Radiology. 2022;302(2):270-273. doi:10.1148/radiol.2021211349
Henderson IM, Benevidez AD, Mowry CD, et al. Precipitation of gadolinium from magnetic resonance imaging contrast agents may be the Brass tacks of toxicity. Magn Reson Imaging. 2025;119:110383. doi:10.1016/j.mri.2025.110383
Do C, Drel V, Tan C, et al. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143. doi:10.1016/j.jid.2019.03.1145
Drel VR, Tan C, Barnes JL, et al. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH oxidase 4 for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
DeAguero J, Howard T, Kusewitt D, et al. The onset of rare earth metallosis begins with renal gadolinium-rich nanoparticles from magnetic resonance imaging contrast agent exposure. Sci Rep. 2023;13(1):2025. doi:10.1038/s41598-023-28666-1
Do C, Ford B, Lee DY, et al. Gadolinium-based contrast agents: Stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
Coyte RM, Darrah T, Olesik J, et al. Gadolinium during human pregnancy following administration of gadolinium chelate before pregnancy. Birth Defects Res. 2023;115(14):1264-1273. doi:10.1002/bdr2.2209
Jackson DB, MacIntyre T, Duarte-Miramontes V, et al. Gadolinium deposition disease: a case report and the prevalence of enhanced MRI procedures within the Veterans Health Administration. Fed Pract. 2022;39:218-225. doi:10.12788/fp.0258
Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1:561-568.doi:10.34067/kid.0000272019
Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28:154-162. doi:10.1097/MNH.0000000000000475
Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311:F1-F11. doi:10.1152/ajprenal.00166.2016
Maramattom BV, Manno EM, Wijdicks EF, et al. Gadolinium encephalopathy in a patient with renal failure. Neurology. 2005;64:1276-1278.doi:10.1212/01.WNL.0000156805.45547.6E
Sam AD II, Morasch MD, Collins J, et al. Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J Vasc Surg. 2003;38:313-318. doi:10.1016/s0741-5214(03)00315-x
Schenker MP, Solomon JA, Roberts DA. Gadolinium arteriography complicated by acute pancreatitis and acute renal failure. J Vasc Interv Radiol. 2001;12:393. doi:10.1016/s1051-0443(07)61925-3
Gemery J, Idelson B, Reid S, et al. Acute renal failure after arteriography with a gadolinium-based contrast agent. AJR Am J Roentgenol. 1998;171:1277-1278. doi:10.2214/ajr.171.5.9798860
Akgun H, Gonlusen G, Cartwright J Jr, et al. Are gadolinium-based contrast media nephrotoxic? A renal biopsy study. Arch Pathol Lab Med. 2006;130:1354-1357. doi:10.5858/2006-130-1354-AGCMNA
Gathings RM, Reddy R, Santa Cruz D, et al. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151:316-319. doi:10.1001/jamadermatol.2014.2660
McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834-841. doi:10.1148/radiol.13131669
Schmidt K, Bau M, Merschel G, et al. Anthropogenic gadolinium in tap water and in tap water-based beverages from fast-food franchises in six major cities in Germany. Sci Total Environ. 2019;687:1401-1408. doi:10.1016/j.scitotenv.2019.07.075
Kulaksız S, Bau M. Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Appl Geochem. 2011;26:1877-1885.
Brunjes R, Hofmann T. Anthropogenic gadolinium in freshwater and drinking water systems. Water Res. 2020;182:115966. doi:10.1016/j.watres.2020.115966
Endrikat J, Gutberlet M, Hoffmann KT, et al. Clinical safety of gadobutrol: review of over 25 years of use exceeding 100 million administrations. Invest Radiol. 2024;59(9):605-613. doi:10.1097/RLI.0000000000001072
Elmholdt TR, Jørgensen B, Ramsing M, et al. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287. doi:10.1093/ndtplus/sfq028
Cunningham A, Kirk M, Hong E, et al. The safety of magnetic resonance imaging contrast agents. Front Toxicol. 2024;6:1376587. doi:10.3389/ftox.2024.1376587
Huxley A. Complete Essays. Volume II, 1926-1929. Chicago; 2000:227.
McDonald RJ, Weinreb JC, Davenport MS. Symptoms associated with gadolinium exposure (SAGE): a suggested term. Radiology. 2022;302(2):270-273. doi:10.1148/radiol.2021211349
Henderson IM, Benevidez AD, Mowry CD, et al. Precipitation of gadolinium from magnetic resonance imaging contrast agents may be the Brass tacks of toxicity. Magn Reson Imaging. 2025;119:110383. doi:10.1016/j.mri.2025.110383
Do C, Drel V, Tan C, et al. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143. doi:10.1016/j.jid.2019.03.1145
Drel VR, Tan C, Barnes JL, et al. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH oxidase 4 for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
DeAguero J, Howard T, Kusewitt D, et al. The onset of rare earth metallosis begins with renal gadolinium-rich nanoparticles from magnetic resonance imaging contrast agent exposure. Sci Rep. 2023;13(1):2025. doi:10.1038/s41598-023-28666-1
Do C, Ford B, Lee DY, et al. Gadolinium-based contrast agents: Stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
Coyte RM, Darrah T, Olesik J, et al. Gadolinium during human pregnancy following administration of gadolinium chelate before pregnancy. Birth Defects Res. 2023;115(14):1264-1273. doi:10.1002/bdr2.2209
Gadolinium Intermediate Elimination and Persistent Symptoms After Magnetic Resonance Imaging Contrast Agent Exposure
Gadolinium Intermediate Elimination and Persistent Symptoms After Magnetic Resonance Imaging Contrast Agent Exposure
Updates in Multiple Sclerosis Imaging
Updates in Multiple Sclerosis Imaging
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
Multiple sclerosis (MS) is a complex, chronic immune-mediated disease of the central nervous system characterized by focal inflammation, demyelination, and neurodegeneration. Magnetic resonance imaging (MRI), first incorporated into the McDonald Criteria for the diagnosis of MS in 2001, is an integral tool in the diagnosis, prognosis, and therapeutic monitoring of people with MS (PwMS).1
MRI research in MS is rapidly expanding and offers insights into the pathophysiology of MS with important implications for the routine clinical care of PwMS. At the Consortium of Multiple Sclerosis Centers 2024 Annual Meeting, the US Department of Veterans Affairs (VA) MS Centers of Excellence hosted an educational symposium highlighting MRI biomarkers in MS, including T2-lesions, chronic black holes (cBHs), brain atrophy, paramagnetic rim lesions (PRLs), and the central vein sign (CVS). The symposium also provided a brief overview of quantitative MRI techniques used to characterize MS lesion severity and research applications of these techniques. This clinical review summarizes the main points of that symposium with the goal of introducing key concepts to federal health care practitioners caring for PwMS.
MRI Biomarkers in MS
T2-lesions, Chronic Black Holes, and Brain Atrophy
Focal immune-mediated inflammation and demyelination in MS may be detected by MRI as hyperintense foci on T2-weighted (T2-w) imaging (eg, T2-w turbo spin echo or T2-w fluid attenuated inversion recovery sequences). These T2-lesions, critical for diagnosing MS, are typically ovoid and occur in the periventricular, juxtacortical, infratentorial spinal cord white matter (Figure 1A). T2-lesion number and volume show some association with disability and optic nerve.
Wattjes et al highlight 2 cases to demonstrate this point: a man aged 52 years with MS for 23 years and a woman aged 50 years with MS for 11 years. Despite having MS for a much shorter duration, the woman had worse disability due to a higher lesion number and volume.2 T2-lesion volume also impacts disability progression in PwMS. Gauthier et al compared the probability of progression in 3 women, all of whom were aged 39 years and had MS for 6 years. The profile with highest probability of disability progression had the highest quartile of T2-lesion volume.3 T2-lesion volume over 2 years correlates with worse scores on disability metrics such as the MS functional composite, paced auditory serial addition task, and brain volume.4 A 2024 systematic review and meta-analysis demonstrated that T2-lesion volume is significantly correlated with clinical disability in PwMS.5
Select T2-lesions are also hypointense on T1-w spin echo images and are known as cBHs (Figure 1B). Histologically, T2-lesions with cBHs have more severe architectural disruption than those without cBHs.6 cBH number and volume are significantly correlated with disability, regardless of the degree of hypointensity on T1-w imaging.5,7 A 10-year longitudinal study demonstrated that cBHs were associated with disease progression after 5 years while T2-lesion volume was not, indicating that cBHs may be a more accurate predictor of disability.8
Brain atrophy, another imaging biomarker of MS, affects both the cerebral white and gray matter. White matter fraction (the volume of white matter relative to the intracranial compartment volume) and gray matter fraction (the volume of gray matter relative to the intracranial compartment) are significantly lower among PwMS compared with healthy controls. In addition, gray matter fraction is lower among patients with primary and secondary progressive MS compared with those with relapsing-remitting MS, clinically isolated syndrome (CIS), and radiologically isolated syndrome (RIS). Gray matter fraction is also correlated with several motor and cognitive disability indices.9
Paramagnetic Rim Lesions
Neurologic worsening in PwMS occurs by 2 distinct mechanisms: relapse-associated worsening, a stepwise worsening of symptoms due to incomplete recovery following a relapse; and progression independent of relapse activity (PIRA), which is an irreversible neurologic deterioration in the absence of clinical or radiological relapses.10 PIRA is associated with neurodegeneration and predominates in both primary and secondary progressive MS. However, recent data demonstrated that PIRA may contribute to as much as 50% of disability worsening in relapsing MS and occurs early in the RMS disease course.10,11 Current high-efficacy disease modifying therapy, such as ocrelizumab, are extraordinarily successful at preventing focal inflammation and relapses but are less effective for preventing the slow march of disability progression characterizing PIRA.12,13 The prevention of PIRA is therefore an unmet treatment need.
Chronic active lesions (CALs) are an important driver of PIRA. When an acute gadolinium-enhancing lesion develops in PwMS, there are 3 possible fates of this lesion. The lesion may become chronically inactive, remyelinate, or transition to CALs.14 The histopathologic signature of CALs is compartmentalized, low-grade inflammation behind an intact blood-brain barrier with evidence of both active and chronic components.15 CALs may be found not only in cerebral white matter but also in the cerebral cortex and spinal cord.16,17 Combined MRI and histopathological studies have shown that iron-laden microglia/macrophages can be detected by susceptibility-based MRI as a rim of paramagnetic signal surrounding select T2-lesions.19 These PRLs represent an in vivo imaging biomarker of CAL (Figure 1C). According to the North American Imaging in MS Cooperative (NAIMS) consensus criteria, a PRL must surround at least two-thirds of the outer edge of a T2-lesion, be visible in ≥ 2 consecutive MRI slices, and cannot be contrast enhancing.20
PRLs can be visualized on multiple susceptibility-based imaging methods, including multiecho derived R2*/T2*, phase maps, susceptibility-weighted imaging, and quantitative susceptibility mapping.21-23 Retrospective analyses have shown no significant differences in sensitivity across these imaging modalities.24 Although first visualized with 7T MRI, PRLs may also be detected by 1.5T and 3T MRI with comparable sensitivities.25-27 However, there remains a significant knowledge gap regarding the accuracy of each imaging modality. Systematic, prospectively designed studies are needed to ascertain the comparative value of each method.
The presence of PRL is a poor prognostic indicator. PwMS without PRLs have higher levels of disability, are more likely to progress, and demonstrate greater gray matter atrophy and cognitive dysfunction when compared with PwMS with PRLs.27-29 Lesions with PRL tend to slowly expand, exhibit greater demyelination, and have diminished white matter integrity.21,22,30
PRLs may also be used as a diagnostic tool. PRLs are highly specific for MS/CIS with a 99.7% specificity and 98.4% positive predictive value, although the sensitivity is limited to 24%.31 Taken together, these data indicate that the presence of a PRL substantially increases the likelihood of an MS/CIS diagnosis, whereas the absence of a PRL does not exclude these diagnoses.
Several unanswered questions remain: Why do select acute MS lesions transition to CALs? How may investigators utilize PRLs as outcome measures in future clinical trials? How should PRLs be incorporated into the routine care of PwMS? As the role of this imaging biomarker is clarified both in the research and clinical settings, clinicians caring for PwMS can expect to increasingly encounter the topic of PRLs in the near future.
Central Vein Sign
A CVS is defined by the presence of a central vessel within a demyelinating plaque (Figure 1D). As early as the 1820s, MS plaques on gross pathology were noted to follow the course of a vessel. Early histological studies reported that up to 91% of MS plaques had a central vessel present.32 Lesion formation is dependent on the movement of lymphocytes and other inflammatory cells from the systemic circulation across the blood brain barrier into the perivascular space, a privileged site where immune cells interact with antigen presenting cells to launch an inflammatory cascade and eventual demyelinating lesion.33
CVS can be visualized on 1.5T, 3T and 7T MRI. However, 7T MRI is superior to 3T in the detection of CVS, with 85% of MS lesions having CVS visible compared with 45% on 3T.34 With advances in 7T MRI, fluid attenuated inversion recovery and T2* susceptibility, weighted sequences can be overlaid, allowing simultaneous visualization of the vessel and the demyelinating lesion. With higher density of parenchymal veins in the periventricular regions, the CVS is most seen in lesions of this territory but can also be present in juxtacortical, thalamic and infratentorial lesions with decreasing prevalence as these approach the cortex.35
MS lesions are more likely to have CVS than T2 hyperintense white matter lesions of other causes, with a large study reporting 78% of MS lesions were CVS positive. Further, CVS positive lesions can be found across all MS phenotypes including relapsing remitting, primary progressive, and secondary progressive.35 The CVS is also specific to MS lesions and is an effective tool for differentiating MS lesions from other common causes of T2 hyperintense lesions including chronic ischemic white matter disease,36 migraines,37 neuromyelitis optica spectrum disorders,38,39 Susac syndrome,40 and systemic autoimmune diseases (Behcet disease, systemic lupus erythematosus, and antiphospholipid syndrome).41
With CVS emerging as a promising radiographic biomarker for MS, NAIMS issued a consensus statement on necessary properties of a CVS. These criteria included appearance of a thin hypointense line or small dot, visualized in ≥ 2 perpendicular planes, with diameter < 2 mm, and running partially or entirely through the center of the lesion. They also clarified that lesions < 3 mm, confluent lesions, lesions with multiple vessels present or poorly visualized lesions were excluded.42
A shared CVS definition was a necessary step toward routine use of CVS as a radiographic biomarker and its incorporation in the 2024 revised McDonald criteria.43 Remaining limitations including 7T MRI is primarily available in research settings and the lack of consensus on a diagnostic threshold. There have been many proposed methods, including a 40% cut off,44 60% cut off,45 and Select 3* or Select 6* methods.46 The goal of each method is to optimize sensitivity and specificity while not compromising efficiency of MRI review for both neurologists and radiologists.
The CVS has significant potential as a radiographic biomarker for MS and may allow the early stages of MS to be differentiated from other common causes of white matter lesions on MRI. However, it remains unclear whether CVS holds prognostic value for patients, if CVS is suggestive of differing underlying pathology, or if the presence of a CVS is dynamic over time. Progress in these areas is anticipated as CVS is incorporated into routine clinical practice.
Quantitative MRI Techniques
In the research setting, several imaging modalities can be used to quantify the degree of microstructural injury in PwMS. The goal of these methods is to identify and quantify myelin and axonal damage, the major drivers of neurodegeneration. Among these methods, diffusion-based imaging is a measure of the amount of diffusion or fluid mobility across the tissues of the brain.47 Diffusion-weighted imaging (DWI) yields several parametric maps including axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (Figure 2 A, B, and C). These parametric maps provide information on different directions of water molecules’ movements. Myelin surrounds the axons preventing water molecules diffusion perpendicular to axons (RD) while axonal content prevents water diffusion horizontal to the axons (AD).Thus, AD is considered more specific to axonal injury, whereas RD is specific to myelin content.48 A higher value of any of these metrics is associated with a higher degree of tissue injury.
Although sensitive to axonal and myelin injury, AD and RD computed from single b-shell DWI experience several limitations including being affected by nonpathologic factors such as fiber orientation, distribution, and crossing, and by various nonmyelin specific pathologies including fluid accumulation during inflammation, myelin sheath thickness, and axonal intactness.48 Several multi b-shell methods have been developed to overcome diffusion imaging limitations. For example, work at the Nashville VA MS Center of Excellence has focused on the use of the multicompartment diffusion MRI with spherical mean technique (SMT). This method removes the orientation dependency of the diffusion MRI signal, increasing the signal-to-noise ratio and reducing biases from fiber undulation, crossing, and dispersion.49 SMT generates the apparent axonal volume fraction (Vax), which is a direct measure of axonal integrity with lower values indicating lower axonal content and higher tissue destruction (Figure 2D). Vax was previously validated in MS as a measure of axonal integrity.49
In terms of myelin, several other specific measures have been developed. Magnetization transfer ratio (MTR) is another measure of tissue integrity that has been validated as a measure of tissue injury in MS (Figure 2E).50,51 Zheng et al found that the percentage of lesions with low MTR was significantly higher among patients whose disease disability progressed compared with patients who did not.52Selective inversion recovery with quantitative magnetization transfer (SIR-qMT) was developed to account for the limitations of MTR, including its sensitivity to edema and axonal density.52 Germane to myelin measurements, SIR-qMT generates the macromolecular to free size ratio (PSR). PSR represents the ratio of protons bound to macromolecules (myelin) to free protons (Figure 2F). PSR is considered a marker of myelin integrity, with lower values correlating with disability severity and indicating higher tissue damage and lower myelin content. Previous studies from the Nashville VA MS Center of Excellence validated the use of SIR-qMT among patients with MS, CIS, RIS, and healthy controls.53
Quantitative MRI has several research applications in the field of MS. We demonstrated that PRL harbor a higher degree of myelin injury indicated by PSR compared with rimless lesions.54 These MRI techniques are also helpful to investigate tissues surrounding the lesions, called normal appearing white matter (NAWM). Using quantitative MRI techniques such as MTR,52 PSR,53 and Vax,49 investigators have demonstrated that NAWM is injured in PwMS, and proximal NAWM may have higher degree of tissue damage compared with distant NAWM.55
Anticipated Innovations and Challenges
In the field of quantitative MRI, several new techniques are being adopted. Researchers are developing techniques such as myelin water fraction which evaluates the interaction between water and protons to measure myelin content. This is considered an advancement as it takes into account edema resulting from MS injury.56 Another example is multicompartment diffusion imaging, such as standard model imaging,57 and neurite orientation dispersion and density imaging,58 which considers water as an additional compartment compared with the SMT derived Vax. For PRL identification, more advanced methodologic techniques are developing such quantitative susceptibility mapping (QSM), which can detect iron deposits that surround the lesions with relatively high sensitivity and specificity of identifying PRL.59
Despite these innovations, several challenges remain before possible incorporation into the clinical setting. These limitations include longer scan time, familiarity of clinicians in using these maps, higher financial cost, and the necessity of advanced imaging processing skills. Artificial intelligence is a promising tool that may overcome these challenges through creating automated processing pipelines and developing synthetic maps without the need for additional acquisition.60
Conclusions
MRI is the most important tool for diagnosing and treating PwMS. Imaging biomarkers such as T2-lesions, cBHs, brain atrophy, PRLs, and CVS provide insight into the disease’s pathogenesis and are invaluable for the accurate diagnosis and prognostication of MS. Quantitative MRI techniques, while not available in the clinical setting, are important tools for translational research that may help direct the development of future therapeutics. In the near future, clinicians caring for PwMS should expect to encounter these imaging biomarkers more frequently in the clinical setting, especially with the inclusion of PRLs and CVS in the next iteration of the McDonald diagnostic criteria.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121-127. doi:10.1002/ana.1032
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25:157-165. doi:10.1007/s00062-015-0430-y
Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology. 2007;68:2059-2065.doi:10.1212/01.wnl.0000264890.97479.b1
Rudick RA, Lee JC, Simon J, Fisher E. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol. 2006;60:236-242. doi:10.1002/ana.20883
Nabizadeh F, Zafari R, Mohamadi M, et al. MRI features and disability in multiple sclerosis: a systematic review and meta-analysis. J Neuroradiol. 2024;51:24-37. doi:10.1016/j.neurad.2023.11.007
Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain. 2003;126:1782-1789. doi:10.1093/brain/awg182
Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. 2014;85:1109-1115. doi:10.1136/jnnp-2013-306906
Rovaris M, Gass A, Bammer R, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526-1532. doi:10.1212/01.wnl.0000184471.83948.e0
Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247-254. doi:10.1002/ana.21423
Lublin FD, Häring DA, Ganjgahi H, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147-3161. doi:10.1093/brain/awac016
Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132-1140. doi:10.1001/jamaneurol.2020.1568
Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221-234. doi:10.1056/NEJMoa1601277
Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376:209-220. doi:10.1056/NEJMoa1606468
Prineas JW, Kwon EE, Cho ES, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646-657. doi:10.1002/ana.1255
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13-24. doi:10.1007/s00401-016-1653-y
Pitt D, Boster A, Pei W, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812-818. doi:10.1001/archneurol.2010.148
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15:180-188. doi:10.1177/1352458508096876
Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210-218. doi:10.1111/j.1750-3639.2007.00064.x
Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602-3615. doi:10.1093/brain/awr278
Bagnato F, Sati P, Hemond CC, et al. Imaging chronic active lesions in multiple sclerosis: a consensus statement. Brain. 2024;147:2913-2933. doi:10.1093/brain/awae013
Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133:25-42. doi:10.1007/s00401-016-1636-z
Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126:2597-2609. doi:10.1172/JCI86198
Gillen KM, Mubarak M, Park C, et al. QSM is an imaging biomarker for chronic glial activation in multiple sclerosis lesions. Ann Clin Transl Neurol. 2021;8:877-886. doi:10.1002/acn3.51338
Ng Kee Kwong KC, Mollison D, Meijboom R, et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One. 2021;16:e0256845. doi:10.1371/journal.pone.0256845
Absinta M, Sati P, Fechner A, et al. Identification of chronic active multiple sclerosis lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39:1233-1238. doi:10.3174/ajnr.A5660
Hemond CC, Reich DS, Dundamadappa SK. Paramagnetic rim lesions in multiple sclerosis: comparison of visualization at 1.5-T and 3-T MRI. AJR Am J Roentgenol. 2022;219:120-131. doi:10.2214/AJR.21.26777
Altokhis AI, Hibbert AM, Allen CM, et al. Longitudinal clinical study of patients with iron rim lesions in multiple sclerosis. Mult Scler. 2022;28:2202-2211. doi:10.1177/13524585221114750
Choi S, Lake S, Harrison DM. Evaluation of the blood-brain barrier, demyelination, and neurodegeneration in paramagnetic rim lesions in multiple sclerosis on 7 tesla MRI. J Magn Reson Imaging. 2024;59:941-951. doi:10.1002/jmri.28847
Kazimuddin HF, Wang J, Hernandez B, et al. Paramagnetic rim lesions and their relationship with neurodegeneration and clinical disability at the time of multiple sclerosis diagnosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Rohm Z, Koch C, Kazimuddin H, et al. Longitudinal characterization of paramagnetic rim lesions in early multiple sclerosis. Poster presented at: 2024 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum; February 26-March 2; West Palm Beach, FL.
Meaton I, Altokhis A, Allen CM, et al. Paramagnetic rims are a promising diagnostic imaging biomarker in multiple sclerosis. Mult Scler. 2022;28:2212-2220. doi:10.1177/13524585221118677
Fog T. On the vessel-plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl. 1964;40:9-15.
Ineichen BV, Okar SV, Proulx ST, et al. Perivascular spaces and their role in neuroinflammation. Neuron. 2022;110:3566-3581. doi:10.1016/j.neuron.2022.10.024
Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol. 2009;44:491-494. doi:10.1097/RLI.0b013e3181b4c144
Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol. 2014;261:1356-1364. doi:10.1007/s00415-014-7351-6
Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534-539. doi:10.1212/WNL.0b013e31820b7630
Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82-87. doi:10.1002/acn3.273
Sinnecker T, Dörr J, Pfueller CF, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708-714. doi:10.1212/WNL.0b013e3182648bc8
Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013;2013:398259. doi:10.1155/2013/398259
Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592-1599. doi:10.1177/1352458512441270
Massacesi L. Perivenular distribution of white matter lesions evaluated by MRI can differentiate MS lesions from inflammatory small vessel diseases. Eur J Neurol. 2016;23:86. doi:10.1212/WNL.86.16_supplement.P6.121
Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol. 2016;12:714-722. doi:10.1038/nrneurol.2016.166
Montalban X, Lebrun-Frénay C, Oh J, et al. Diagnosis of multiple sclerosis: 2024 revisions of the McDonald criteria. Lancet Neurol. 2025;24:850-865. doi:10.1016/S1474-4422(25)00270-4
Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high-field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol. 2013;70:623-628. doi:10.1001/jamaneurol.2013.1405
Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol. 2017;27:4257-4263. doi:10.1007/s00330-017-4822-z
Solomon AJ, Watts R, Ontaneda D, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler. 2018;24:750-757. doi:10.1177/1352458517726383
Cercignani M, Bozzali M, Iannucci G, Comi G, Filippi M. Intra-voxel and inter-voxel coherence in patients with multiple sclerosis assessed using diffusion tensor MRI. J Neurol. 2002;249:875-883. doi:10.1007/s00415-002-0752-y
Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132-140. doi:10.1016/j.neuroimage.2005.01.028
Bagnato F, Franco G, Li H, et al. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595-1605. doi:10.1002/acn3.50836
Filippi M, Cercignani M, Inglese M, et al. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304-311. doi:10.1212/wnl.56.3.304
Bagnato F. Clinical application of magnetization transfer imaging. In: Advanced Neuro MR Techniques and Applications. Elsevier; 2022:403-417. doi:10.1016/B978-0-12-822479-3.00041-5
Zheng Y, Lee JC, Rudick R, Fisher E. Long-term magnetization transfer ratio evolution in multiple sclerosis white matter lesions. J Neuroimaging. 2018;28:191-198. doi:10.1111/jon.12480
Bagnato F, Hametner S, Franco G, et al. Selective inversion recovery quantitative magnetization transfer brain MRI at 7T: clinical and postmortem validation in multiple sclerosis. J Neuroimaging. 2018;28:380-388. doi:10.1111/jon.12511
Clarke MA, Cheek R, Hernandez B, et al. Paramagnetic rim lesions and the central vein sign: characterizing multiple sclerosis imaging markers. J Neuroimaging. 2024;34:86-94. doi:10.1111/jon.13173
Clarke MA, Lakhani DA, Wen S, et al. Perilesional neurodegenerative injury in multiple sclerosis: relation to focal lesions and impact on disability. Mult Scler Relat Disord. 2021;49:102738. doi:10.1016/j.msard.2021.102738
Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28:750-764. doi:10.1111/bpa.12645
Coelho S, Baete SH, Lemberskiy G, et al. Reproducibility of the standard model of diffusion in white matter on clinical MRI systems. Neuroimage. 2022;257:119290. doi:10.1016/j.neuroimage.2022.119290
Novikov DS, Veraart J, Jelescu IO, et al. Rotationally-invariant mapping of scalar and orientational metrics of neuronal microstructure with diffusion MRI. Neuroimage. 2018;174:518-538. doi:10.1016/j.neuroimage.2018.03.006
Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology. 2013;267:551-559. doi:10.1148/radiol.12120707
Collorone S, Coll L, Lorenzi M, et al. Artificial intelligence applied to MRI data to tackle key challenges in multiple sclerosis. Mult Scler. 2024;30:767-784. doi:10.1177/13524585241249422
Updates in Multiple Sclerosis Imaging
Updates in Multiple Sclerosis Imaging
A Case Report of Unanticipated Difficult Intubation Due to Posterior Tracheal Angulation
A Case Report of Unanticipated Difficult Intubation Due to Posterior Tracheal Angulation
Tracheal deviation mostly occurs from mechanical compression of the trachea, and can be caused by a variety of clinical conditions, including trauma,¹ pharyngeal abscess,² neck hematoma,³ thyroid enlargement,4 and kyphoscoliosis.5 These conditions often result in lateral tracheal deviation, which can be associated with tracheal compression and reduction in tracheal caliber.
Anterior-posterior (A-P) tracheal deviation has rarely been reported. Kyphoscoliosis, scarring after a tracheostomy, or innominate vein compression are probable causes of A-P tracheal deviation and can be associated with tracheal narrowing and vascular fistula formation. This report describes a case of difficult endotracheal tube (ETT) advancement secondary to unexpected acute posterior tracheal deviation encountered during cardiopulmonary resuscitation (CPR). A waiver of patient consent was obtained from the Human Research Protection Program at the US Department of Veterans Affairs (VA) Puget Sound Health Care System.
Case Presentation
A 50-year-old male with a history of chronic cerebral venous sinus thrombosis and taking enoxaparin, presented to the emergency department for recurrent headaches. He experienced sudden cardiac arrest, and CPR in the form of chest compression and bag mask ventilation was immediately initiated. With the patient's head in an extended position and using a video laryngoscope, a Cormack–Lehane grade 1 view of the glottic opening was obtained and the trachea was intubated with an 8 mm (internal diameter) polyvinyl chloride ETT. Tracheal intubation was confirmed by utilizing continuous EtCO2 monitoring. The ETT was secured at 22 cm measured at the teeth.
After about 40 minutes of CPR, spontaneous circulation restarted and a portable A-P chest X-ray with the head in a neutral position indicated the ETT tip was at the level of the first rib (Figure 1). This finding, along with a persistent air leak, prompted blind advancement of the ETT to 26 cm at the teeth, but resistance to advancement was noted. A subsequent chest computed tomography (CT) with the head in a neutral position revealed the ETT remained inappropriately positioned with the tip measured 8.2 cm above the carina (Figure 2A). Concurrently, a sagittal CT view demonstrated significant posterior deviation of the mid and lower trachea. This deviation was determined to be the most likely cause of the difficulty encountered in advancing the ETT. No masses or lesions contributing to the acute tracheal angulation could be identified. Comparing CT imaging from 2 months prior, the trachea was of normal caliber and ordinarily aligned with the vertebral column (Figure 2B).
With the patient in Fowler position with the head midline, a flexible fiber-optic bronchoscopy was performed. Acute, almost 90-degree tracheal angulation was encountered and navigated by retroflexion of the flexible bronchoscope. Once the posterior tracheal wall was encountered, retroflexion was relaxed and the carina was visualized. The bronchoscope tip was placed near the carina, and the ETT was advanced over the fiber-optic bronchoscope to terminate 3 cm above the carina. A subsequent chest X-ray confirmed appropriate ETT position (Figure 3).
Discussion
Tracheal deviation in the A-P dimension resulting in difficult tracheal intubation has rarely been reported. Previous reports have described anatomical lesions contributing to similar tracheal deviation, such as retro-tracheal thyroid tissue, pronounced cervical lordosis, and severe kyphoscoliosis with destructive cervical fusion.5-8 In a study of the anatomical correlation of double lumen tube placement while using positron emission tomography CT, Cameron et al evaluated the size and angulation of the glottis and proximal trachea using calibrated CT measurements and an online digital protractor and note nearly perfect alignment of the pharynx and glottis.9 However, the trachea turned posteriorly relative to the glottis, resulting in an overall posterior angle of the proximal trachea compared to the glottis of 30.4 to 50.1 degrees, with no sex differences. The need to maneuver similar proximal tracheal angulation during endotracheal intubation has been reported as a cause of difficult intubation.10
In this case, the posterior angulation was not encountered in the proximal trachea but rather in the more distal trachea. The extreme A-P tracheal deviation was not associated with any identifiable masses or lesions. A CT performed 2 months prior demonstrated normal tracheal anatomy, and there was no interval history of neck trauma or tracheal obstruction suggestive of a likely cause for this deviation. This change in the patient’s tracheal anatomy was only discovered after CPR had been performed and as part of the workup for cardiac arrest. Iatrogenic injuries are known to occur during CPR. Common CPR-related airway injuries include tracheal mucosal injury from traumatic intubation and bony injuries to the chest wall from compressions.11 Laryngeal cartilage damage from intubation may also occur, but tracheal displacement following CPR has not been previously reported.11
This case of tracheal deviation is unlikely to be related to patient positioning, as the A-P deviation persisted in 3 separate head and neck alignments. First, during indirect laryngoscopy, performed in a standard sniffing position. Second, during the CT, performed in the supine position, with no head support. The acute A-P deviation seen in Figure 2 was clearly noted in this position. Lastly, flexible fiber-optic bronchoscopy was performed in a semiupright position with the head supported on a pillow. A-P deviation was encountered and navigated in this position during flexible fiber-optic guided ETT repositioning.
Using magnetic resonance imaging, alterations in the alignment of pharyngeal and tracheal axes have been described with changes in neck positioning; however, tracheal deviation has not been described with changes in head and neck alignment.12 Although the clinical presentation in this case was consistent with prior reports, we were unable to identify any previously reported anatomic cause for the tracheal deviation.5,6,8 Initial glottic visualization with a video laryngoscope was unremarkable, but resistance to sufficient ETT advancement past the vocal cords and a persistent air leak due to cuff herniation through the glottic opening was noticeable. The ETT was maneuvered to an appropriate position in the trachea using a flexible fiber-optic bronchoscope. The acute angulation of the trachea that was appreciated on bronchoscopy did not result in kinking of the ETT both initially and after in-situ thermosoftening of the polyvinyl chloride tube.13 Previously reported instances of A-P tracheal deviation have outlined the necessity of using alternative techniques to establish a patent airway, including the use of a laryngeal mask airway and a cuffless ETT with saline-soaked gauze packing.5,8 In 1 reported case, awake fiber-optic intubation was performed when difficult tracheal intubation was anticipated due to known A-P tracheal deviation.6
Failure of ETT advancement can be due to obstruction from the arytenoids and at the level of the vocal cords.14 When the ETT has been visualized to have traversed the vocal cords, tracheal A-P deviation should be considered as a cause of difficult ETT advancement. If an adequate endotracheal airway cannot be established, prompt consideration should be given to placement of a supraglottic airway. Early fiber-optic bronchoscopy should be used to establish the diagnosis and assist with proper ETT positioning.
Conclusions
This case illustrates the rare occurrence of A-P tracheal deviation leading to difficult intubation during CPR. The findings underscore the importance of considering A-P deviation as a potential cause of airway complications in emergency settings, especially in patients with previously normal tracheal anatomy. The successful use of flexible fiber-optic bronchoscopy in this case provides a valuable technique for addressing acute tracheal angulation. This report contributes to the limited literature on A-P tracheal deviation and serves as a reminder for clinicians to maintain a high index of suspicion for unusual airway challenges during critical interventions.
Creasy JD, Chiles C, Routh WD, et al. Overview of traumatic injury of the thoracic aorta. Radiogr Rev Publ Radiol Soc N Am Inc. 1997;17:27-45. doi:10.1148/radiographics.17.1.9017797
Yee AM, Christensen DN, Waterbrook AL, et al. Parapharyngeal abscess with tracheal deviation. Intern Emerg Med. 2017;12:1077-1078.doi:10.1007/s11739-017-1634-8
Querney J, Singh SI, Sebbag I. Tracheal deviation with phrenic nerve palsy after brachial plexus block. Anaesth Rep. 2021;9:41-43. doi:10.1002/anr3.12100
Geissler B, Wagner T, Dorn R, et al. Extensive sterile abscess in an invasive fibrous thyroiditis (Riedel’s thyroiditis) caused by an occlusive vasculitis. J Endocrinol Invest. 2001;24:111-115. doi:10.1007/BF03343824
Kim HJ, Choi YS, Park SH, et al. Difficult endotracheal intubation secondary to tracheal deviation and stenosis in a patient with severe kyphoscoliosis: a case report. Korean J Anesthesiol. 2016;69:386-389. doi:10.4097/kjae.2016.69.4.386
Crabb IJ. Anterior deviation of the trachea. Anaesthesia. 2001;56:284-286.doi:10.1046/j.1365-2044.2001.01918-17.x
De Cassai A, Boscolo A, Rose K, et al. Predictive parameters of difficult intubation in thyroid surgery: a meta-analysis. Minerva Anestesiol. 2020;86:317-326. doi:10.23736/S0375-9393.19.14127-2
Davies R. Difficult tracheal intubation secondary to a tracheal diverticulum and a 90 degree deviation in the trachea. Anaesthesia. 2000;55:923-925. doi:10.1046/j.1365-2044.2000.01664-18.x
Cameron RB, Peacock WJ, Chang XG, et al. Double lumen endobronchial tube intubation: lessons learned from anatomy. BMC Anesthesiol. 2024;24:150. doi:10.1186/s12871-024-02517-6
Walls RM, Samuels-Kalow M, Perkins A. A new maneuver for endotracheal tube insertion during difficult GlideScope intubation. J Emerg Med. 2010;39:86-88. doi:10.1016/j.jemermed.2009.11.005
Buschmann CT, Tsokos M. Frequent and rare complications of resuscitation attempts. Intensive Care Med. 2009;35:397-404. doi:10.1007/s00134-008-1255-9
Greenland KB, Edwards MJ, Hutton NJ, et al. Changes in airway configuration with different head and neck positions using magnetic resonance imaging of normal airways: a new concept with possible clinical applications. Br J Anaesth. 2010;105:683-690. doi:10.1093/bja/aeq239
Takasugi Y, Futagawa K, Umeda T, et al. Thermophysical Properties of Thermosoftening Nasotracheal Tubes. Anesth Prog. 2018;65:100-105. doi:10.2344/anpr-65-02-06
Phelan MP. Use of the endotracheal bougie introducer for difficult intubations. Am J Emerg Med. 2004;22:479-482. doi:10.1016/j.ajem.2004.07.017
Tracheal deviation mostly occurs from mechanical compression of the trachea, and can be caused by a variety of clinical conditions, including trauma,¹ pharyngeal abscess,² neck hematoma,³ thyroid enlargement,4 and kyphoscoliosis.5 These conditions often result in lateral tracheal deviation, which can be associated with tracheal compression and reduction in tracheal caliber.
Anterior-posterior (A-P) tracheal deviation has rarely been reported. Kyphoscoliosis, scarring after a tracheostomy, or innominate vein compression are probable causes of A-P tracheal deviation and can be associated with tracheal narrowing and vascular fistula formation. This report describes a case of difficult endotracheal tube (ETT) advancement secondary to unexpected acute posterior tracheal deviation encountered during cardiopulmonary resuscitation (CPR). A waiver of patient consent was obtained from the Human Research Protection Program at the US Department of Veterans Affairs (VA) Puget Sound Health Care System.
Case Presentation
A 50-year-old male with a history of chronic cerebral venous sinus thrombosis and taking enoxaparin, presented to the emergency department for recurrent headaches. He experienced sudden cardiac arrest, and CPR in the form of chest compression and bag mask ventilation was immediately initiated. With the patient's head in an extended position and using a video laryngoscope, a Cormack–Lehane grade 1 view of the glottic opening was obtained and the trachea was intubated with an 8 mm (internal diameter) polyvinyl chloride ETT. Tracheal intubation was confirmed by utilizing continuous EtCO2 monitoring. The ETT was secured at 22 cm measured at the teeth.
After about 40 minutes of CPR, spontaneous circulation restarted and a portable A-P chest X-ray with the head in a neutral position indicated the ETT tip was at the level of the first rib (Figure 1). This finding, along with a persistent air leak, prompted blind advancement of the ETT to 26 cm at the teeth, but resistance to advancement was noted. A subsequent chest computed tomography (CT) with the head in a neutral position revealed the ETT remained inappropriately positioned with the tip measured 8.2 cm above the carina (Figure 2A). Concurrently, a sagittal CT view demonstrated significant posterior deviation of the mid and lower trachea. This deviation was determined to be the most likely cause of the difficulty encountered in advancing the ETT. No masses or lesions contributing to the acute tracheal angulation could be identified. Comparing CT imaging from 2 months prior, the trachea was of normal caliber and ordinarily aligned with the vertebral column (Figure 2B).
With the patient in Fowler position with the head midline, a flexible fiber-optic bronchoscopy was performed. Acute, almost 90-degree tracheal angulation was encountered and navigated by retroflexion of the flexible bronchoscope. Once the posterior tracheal wall was encountered, retroflexion was relaxed and the carina was visualized. The bronchoscope tip was placed near the carina, and the ETT was advanced over the fiber-optic bronchoscope to terminate 3 cm above the carina. A subsequent chest X-ray confirmed appropriate ETT position (Figure 3).
Discussion
Tracheal deviation in the A-P dimension resulting in difficult tracheal intubation has rarely been reported. Previous reports have described anatomical lesions contributing to similar tracheal deviation, such as retro-tracheal thyroid tissue, pronounced cervical lordosis, and severe kyphoscoliosis with destructive cervical fusion.5-8 In a study of the anatomical correlation of double lumen tube placement while using positron emission tomography CT, Cameron et al evaluated the size and angulation of the glottis and proximal trachea using calibrated CT measurements and an online digital protractor and note nearly perfect alignment of the pharynx and glottis.9 However, the trachea turned posteriorly relative to the glottis, resulting in an overall posterior angle of the proximal trachea compared to the glottis of 30.4 to 50.1 degrees, with no sex differences. The need to maneuver similar proximal tracheal angulation during endotracheal intubation has been reported as a cause of difficult intubation.10
In this case, the posterior angulation was not encountered in the proximal trachea but rather in the more distal trachea. The extreme A-P tracheal deviation was not associated with any identifiable masses or lesions. A CT performed 2 months prior demonstrated normal tracheal anatomy, and there was no interval history of neck trauma or tracheal obstruction suggestive of a likely cause for this deviation. This change in the patient’s tracheal anatomy was only discovered after CPR had been performed and as part of the workup for cardiac arrest. Iatrogenic injuries are known to occur during CPR. Common CPR-related airway injuries include tracheal mucosal injury from traumatic intubation and bony injuries to the chest wall from compressions.11 Laryngeal cartilage damage from intubation may also occur, but tracheal displacement following CPR has not been previously reported.11
This case of tracheal deviation is unlikely to be related to patient positioning, as the A-P deviation persisted in 3 separate head and neck alignments. First, during indirect laryngoscopy, performed in a standard sniffing position. Second, during the CT, performed in the supine position, with no head support. The acute A-P deviation seen in Figure 2 was clearly noted in this position. Lastly, flexible fiber-optic bronchoscopy was performed in a semiupright position with the head supported on a pillow. A-P deviation was encountered and navigated in this position during flexible fiber-optic guided ETT repositioning.
Using magnetic resonance imaging, alterations in the alignment of pharyngeal and tracheal axes have been described with changes in neck positioning; however, tracheal deviation has not been described with changes in head and neck alignment.12 Although the clinical presentation in this case was consistent with prior reports, we were unable to identify any previously reported anatomic cause for the tracheal deviation.5,6,8 Initial glottic visualization with a video laryngoscope was unremarkable, but resistance to sufficient ETT advancement past the vocal cords and a persistent air leak due to cuff herniation through the glottic opening was noticeable. The ETT was maneuvered to an appropriate position in the trachea using a flexible fiber-optic bronchoscope. The acute angulation of the trachea that was appreciated on bronchoscopy did not result in kinking of the ETT both initially and after in-situ thermosoftening of the polyvinyl chloride tube.13 Previously reported instances of A-P tracheal deviation have outlined the necessity of using alternative techniques to establish a patent airway, including the use of a laryngeal mask airway and a cuffless ETT with saline-soaked gauze packing.5,8 In 1 reported case, awake fiber-optic intubation was performed when difficult tracheal intubation was anticipated due to known A-P tracheal deviation.6
Failure of ETT advancement can be due to obstruction from the arytenoids and at the level of the vocal cords.14 When the ETT has been visualized to have traversed the vocal cords, tracheal A-P deviation should be considered as a cause of difficult ETT advancement. If an adequate endotracheal airway cannot be established, prompt consideration should be given to placement of a supraglottic airway. Early fiber-optic bronchoscopy should be used to establish the diagnosis and assist with proper ETT positioning.
Conclusions
This case illustrates the rare occurrence of A-P tracheal deviation leading to difficult intubation during CPR. The findings underscore the importance of considering A-P deviation as a potential cause of airway complications in emergency settings, especially in patients with previously normal tracheal anatomy. The successful use of flexible fiber-optic bronchoscopy in this case provides a valuable technique for addressing acute tracheal angulation. This report contributes to the limited literature on A-P tracheal deviation and serves as a reminder for clinicians to maintain a high index of suspicion for unusual airway challenges during critical interventions.
Tracheal deviation mostly occurs from mechanical compression of the trachea, and can be caused by a variety of clinical conditions, including trauma,¹ pharyngeal abscess,² neck hematoma,³ thyroid enlargement,4 and kyphoscoliosis.5 These conditions often result in lateral tracheal deviation, which can be associated with tracheal compression and reduction in tracheal caliber.
Anterior-posterior (A-P) tracheal deviation has rarely been reported. Kyphoscoliosis, scarring after a tracheostomy, or innominate vein compression are probable causes of A-P tracheal deviation and can be associated with tracheal narrowing and vascular fistula formation. This report describes a case of difficult endotracheal tube (ETT) advancement secondary to unexpected acute posterior tracheal deviation encountered during cardiopulmonary resuscitation (CPR). A waiver of patient consent was obtained from the Human Research Protection Program at the US Department of Veterans Affairs (VA) Puget Sound Health Care System.
Case Presentation
A 50-year-old male with a history of chronic cerebral venous sinus thrombosis and taking enoxaparin, presented to the emergency department for recurrent headaches. He experienced sudden cardiac arrest, and CPR in the form of chest compression and bag mask ventilation was immediately initiated. With the patient's head in an extended position and using a video laryngoscope, a Cormack–Lehane grade 1 view of the glottic opening was obtained and the trachea was intubated with an 8 mm (internal diameter) polyvinyl chloride ETT. Tracheal intubation was confirmed by utilizing continuous EtCO2 monitoring. The ETT was secured at 22 cm measured at the teeth.
After about 40 minutes of CPR, spontaneous circulation restarted and a portable A-P chest X-ray with the head in a neutral position indicated the ETT tip was at the level of the first rib (Figure 1). This finding, along with a persistent air leak, prompted blind advancement of the ETT to 26 cm at the teeth, but resistance to advancement was noted. A subsequent chest computed tomography (CT) with the head in a neutral position revealed the ETT remained inappropriately positioned with the tip measured 8.2 cm above the carina (Figure 2A). Concurrently, a sagittal CT view demonstrated significant posterior deviation of the mid and lower trachea. This deviation was determined to be the most likely cause of the difficulty encountered in advancing the ETT. No masses or lesions contributing to the acute tracheal angulation could be identified. Comparing CT imaging from 2 months prior, the trachea was of normal caliber and ordinarily aligned with the vertebral column (Figure 2B).
With the patient in Fowler position with the head midline, a flexible fiber-optic bronchoscopy was performed. Acute, almost 90-degree tracheal angulation was encountered and navigated by retroflexion of the flexible bronchoscope. Once the posterior tracheal wall was encountered, retroflexion was relaxed and the carina was visualized. The bronchoscope tip was placed near the carina, and the ETT was advanced over the fiber-optic bronchoscope to terminate 3 cm above the carina. A subsequent chest X-ray confirmed appropriate ETT position (Figure 3).
Discussion
Tracheal deviation in the A-P dimension resulting in difficult tracheal intubation has rarely been reported. Previous reports have described anatomical lesions contributing to similar tracheal deviation, such as retro-tracheal thyroid tissue, pronounced cervical lordosis, and severe kyphoscoliosis with destructive cervical fusion.5-8 In a study of the anatomical correlation of double lumen tube placement while using positron emission tomography CT, Cameron et al evaluated the size and angulation of the glottis and proximal trachea using calibrated CT measurements and an online digital protractor and note nearly perfect alignment of the pharynx and glottis.9 However, the trachea turned posteriorly relative to the glottis, resulting in an overall posterior angle of the proximal trachea compared to the glottis of 30.4 to 50.1 degrees, with no sex differences. The need to maneuver similar proximal tracheal angulation during endotracheal intubation has been reported as a cause of difficult intubation.10
In this case, the posterior angulation was not encountered in the proximal trachea but rather in the more distal trachea. The extreme A-P tracheal deviation was not associated with any identifiable masses or lesions. A CT performed 2 months prior demonstrated normal tracheal anatomy, and there was no interval history of neck trauma or tracheal obstruction suggestive of a likely cause for this deviation. This change in the patient’s tracheal anatomy was only discovered after CPR had been performed and as part of the workup for cardiac arrest. Iatrogenic injuries are known to occur during CPR. Common CPR-related airway injuries include tracheal mucosal injury from traumatic intubation and bony injuries to the chest wall from compressions.11 Laryngeal cartilage damage from intubation may also occur, but tracheal displacement following CPR has not been previously reported.11
This case of tracheal deviation is unlikely to be related to patient positioning, as the A-P deviation persisted in 3 separate head and neck alignments. First, during indirect laryngoscopy, performed in a standard sniffing position. Second, during the CT, performed in the supine position, with no head support. The acute A-P deviation seen in Figure 2 was clearly noted in this position. Lastly, flexible fiber-optic bronchoscopy was performed in a semiupright position with the head supported on a pillow. A-P deviation was encountered and navigated in this position during flexible fiber-optic guided ETT repositioning.
Using magnetic resonance imaging, alterations in the alignment of pharyngeal and tracheal axes have been described with changes in neck positioning; however, tracheal deviation has not been described with changes in head and neck alignment.12 Although the clinical presentation in this case was consistent with prior reports, we were unable to identify any previously reported anatomic cause for the tracheal deviation.5,6,8 Initial glottic visualization with a video laryngoscope was unremarkable, but resistance to sufficient ETT advancement past the vocal cords and a persistent air leak due to cuff herniation through the glottic opening was noticeable. The ETT was maneuvered to an appropriate position in the trachea using a flexible fiber-optic bronchoscope. The acute angulation of the trachea that was appreciated on bronchoscopy did not result in kinking of the ETT both initially and after in-situ thermosoftening of the polyvinyl chloride tube.13 Previously reported instances of A-P tracheal deviation have outlined the necessity of using alternative techniques to establish a patent airway, including the use of a laryngeal mask airway and a cuffless ETT with saline-soaked gauze packing.5,8 In 1 reported case, awake fiber-optic intubation was performed when difficult tracheal intubation was anticipated due to known A-P tracheal deviation.6
Failure of ETT advancement can be due to obstruction from the arytenoids and at the level of the vocal cords.14 When the ETT has been visualized to have traversed the vocal cords, tracheal A-P deviation should be considered as a cause of difficult ETT advancement. If an adequate endotracheal airway cannot be established, prompt consideration should be given to placement of a supraglottic airway. Early fiber-optic bronchoscopy should be used to establish the diagnosis and assist with proper ETT positioning.
Conclusions
This case illustrates the rare occurrence of A-P tracheal deviation leading to difficult intubation during CPR. The findings underscore the importance of considering A-P deviation as a potential cause of airway complications in emergency settings, especially in patients with previously normal tracheal anatomy. The successful use of flexible fiber-optic bronchoscopy in this case provides a valuable technique for addressing acute tracheal angulation. This report contributes to the limited literature on A-P tracheal deviation and serves as a reminder for clinicians to maintain a high index of suspicion for unusual airway challenges during critical interventions.
Creasy JD, Chiles C, Routh WD, et al. Overview of traumatic injury of the thoracic aorta. Radiogr Rev Publ Radiol Soc N Am Inc. 1997;17:27-45. doi:10.1148/radiographics.17.1.9017797
Yee AM, Christensen DN, Waterbrook AL, et al. Parapharyngeal abscess with tracheal deviation. Intern Emerg Med. 2017;12:1077-1078.doi:10.1007/s11739-017-1634-8
Querney J, Singh SI, Sebbag I. Tracheal deviation with phrenic nerve palsy after brachial plexus block. Anaesth Rep. 2021;9:41-43. doi:10.1002/anr3.12100
Geissler B, Wagner T, Dorn R, et al. Extensive sterile abscess in an invasive fibrous thyroiditis (Riedel’s thyroiditis) caused by an occlusive vasculitis. J Endocrinol Invest. 2001;24:111-115. doi:10.1007/BF03343824
Kim HJ, Choi YS, Park SH, et al. Difficult endotracheal intubation secondary to tracheal deviation and stenosis in a patient with severe kyphoscoliosis: a case report. Korean J Anesthesiol. 2016;69:386-389. doi:10.4097/kjae.2016.69.4.386
Crabb IJ. Anterior deviation of the trachea. Anaesthesia. 2001;56:284-286.doi:10.1046/j.1365-2044.2001.01918-17.x
De Cassai A, Boscolo A, Rose K, et al. Predictive parameters of difficult intubation in thyroid surgery: a meta-analysis. Minerva Anestesiol. 2020;86:317-326. doi:10.23736/S0375-9393.19.14127-2
Davies R. Difficult tracheal intubation secondary to a tracheal diverticulum and a 90 degree deviation in the trachea. Anaesthesia. 2000;55:923-925. doi:10.1046/j.1365-2044.2000.01664-18.x
Cameron RB, Peacock WJ, Chang XG, et al. Double lumen endobronchial tube intubation: lessons learned from anatomy. BMC Anesthesiol. 2024;24:150. doi:10.1186/s12871-024-02517-6
Walls RM, Samuels-Kalow M, Perkins A. A new maneuver for endotracheal tube insertion during difficult GlideScope intubation. J Emerg Med. 2010;39:86-88. doi:10.1016/j.jemermed.2009.11.005
Buschmann CT, Tsokos M. Frequent and rare complications of resuscitation attempts. Intensive Care Med. 2009;35:397-404. doi:10.1007/s00134-008-1255-9
Greenland KB, Edwards MJ, Hutton NJ, et al. Changes in airway configuration with different head and neck positions using magnetic resonance imaging of normal airways: a new concept with possible clinical applications. Br J Anaesth. 2010;105:683-690. doi:10.1093/bja/aeq239
Takasugi Y, Futagawa K, Umeda T, et al. Thermophysical Properties of Thermosoftening Nasotracheal Tubes. Anesth Prog. 2018;65:100-105. doi:10.2344/anpr-65-02-06
Phelan MP. Use of the endotracheal bougie introducer for difficult intubations. Am J Emerg Med. 2004;22:479-482. doi:10.1016/j.ajem.2004.07.017
Creasy JD, Chiles C, Routh WD, et al. Overview of traumatic injury of the thoracic aorta. Radiogr Rev Publ Radiol Soc N Am Inc. 1997;17:27-45. doi:10.1148/radiographics.17.1.9017797
Yee AM, Christensen DN, Waterbrook AL, et al. Parapharyngeal abscess with tracheal deviation. Intern Emerg Med. 2017;12:1077-1078.doi:10.1007/s11739-017-1634-8
Querney J, Singh SI, Sebbag I. Tracheal deviation with phrenic nerve palsy after brachial plexus block. Anaesth Rep. 2021;9:41-43. doi:10.1002/anr3.12100
Geissler B, Wagner T, Dorn R, et al. Extensive sterile abscess in an invasive fibrous thyroiditis (Riedel’s thyroiditis) caused by an occlusive vasculitis. J Endocrinol Invest. 2001;24:111-115. doi:10.1007/BF03343824
Kim HJ, Choi YS, Park SH, et al. Difficult endotracheal intubation secondary to tracheal deviation and stenosis in a patient with severe kyphoscoliosis: a case report. Korean J Anesthesiol. 2016;69:386-389. doi:10.4097/kjae.2016.69.4.386
Crabb IJ. Anterior deviation of the trachea. Anaesthesia. 2001;56:284-286.doi:10.1046/j.1365-2044.2001.01918-17.x
De Cassai A, Boscolo A, Rose K, et al. Predictive parameters of difficult intubation in thyroid surgery: a meta-analysis. Minerva Anestesiol. 2020;86:317-326. doi:10.23736/S0375-9393.19.14127-2
Davies R. Difficult tracheal intubation secondary to a tracheal diverticulum and a 90 degree deviation in the trachea. Anaesthesia. 2000;55:923-925. doi:10.1046/j.1365-2044.2000.01664-18.x
Cameron RB, Peacock WJ, Chang XG, et al. Double lumen endobronchial tube intubation: lessons learned from anatomy. BMC Anesthesiol. 2024;24:150. doi:10.1186/s12871-024-02517-6
Walls RM, Samuels-Kalow M, Perkins A. A new maneuver for endotracheal tube insertion during difficult GlideScope intubation. J Emerg Med. 2010;39:86-88. doi:10.1016/j.jemermed.2009.11.005
Buschmann CT, Tsokos M. Frequent and rare complications of resuscitation attempts. Intensive Care Med. 2009;35:397-404. doi:10.1007/s00134-008-1255-9
Greenland KB, Edwards MJ, Hutton NJ, et al. Changes in airway configuration with different head and neck positions using magnetic resonance imaging of normal airways: a new concept with possible clinical applications. Br J Anaesth. 2010;105:683-690. doi:10.1093/bja/aeq239
Takasugi Y, Futagawa K, Umeda T, et al. Thermophysical Properties of Thermosoftening Nasotracheal Tubes. Anesth Prog. 2018;65:100-105. doi:10.2344/anpr-65-02-06
Phelan MP. Use of the endotracheal bougie introducer for difficult intubations. Am J Emerg Med. 2004;22:479-482. doi:10.1016/j.ajem.2004.07.017
A Case Report of Unanticipated Difficult Intubation Due to Posterior Tracheal Angulation
A Case Report of Unanticipated Difficult Intubation Due to Posterior Tracheal Angulation
PET/CT Imaging Study Reveals Differing Views on How to Manage Incidental Findings
Disparate views on managing incidental imaging findings made during clinical research — particularly for unclear results — signal a need for standardized guidance, according to recent survey results.
Respondents were split on whether it was the site primary investigator’s responsibility to decide which incidental findings should be reported back to the patient, and the most commonly cited challenges included adequately explaining these findings and the follow-up required. These issues were most present when dealing with nonspecific incidental findings or findings of unclear importance, said lead author Jane S. Kang, MD, a bioethicist and associate professor of medicine in the Division of Rheumatology at Columbia University Irving Medical Center, New York City.
“It can be difficult to have a clear approach” when it comes to these situations that are not black and white, and it is hard to get a clear answer, she said in an interview.
The survey included responses from investigators from the Treatments Against Rheumatoid Arthritis and Effect on 18F-fluorodeoxyglucose (FDG) PET/CT (TARGET) trial, conducted between 2015 and 2021. The 24-week trial included patients from 28 centers in the United States to investigate how different disease-modifying antirheumatic drugs can reduce cardiovascular and joint inflammation, assessed via whole body FDG PET/CT. The survey was a planned substudy of the TARGET trial and is “the first study that examines researchers’ attitudes and beliefs regarding incidental research findings from whole body FDG PET/CT,” Kang and her coauthors wrote.
This news organization reported the main results of the TARGET trial in 2022.
Eighteen of the 28 site primary investigators (PIs) of the TARGET trial participated in the survey, which was published in Arthritis Care & Research in September 2024.
TARGET Trial Incidental Findings
The TARGET trial enrolled 159 patients, of whom 82% had at least one incidental finding and 62% had one or more FDG-avid incidental findings. There were 46 “clinically actionable findings” for 40 participants overall; the reading radiologists recommended additional imaging for 28 findings and specialist consultation or procedural evaluation for 15 findings.
Details on these incidental findings were presented in a poster at the annual meeting of the American College of Rheumatology (ACR), held in Washington, DC.
The most common non–FDG-avid findings were pulmonary nodules, diverticulosis, cholelithiasis, sinus disease, and vascular calcifications. The most common FDG-avid findings were hypermetabolic lymphadenopathy, increased gastric/esophageal uptake, increased bowel uptake, and increased pharyngeal uptake.
In the related survey, 11 respondents (61%) said they returned any incidental findings to participants and 5 (28%) did not; the remaining 2 respondents did not know.
Across all study PIs, 22% felt that incidental findings were beneficial, 39% said they were potentially beneficial, and 11% said they were potentially detrimental. PIs that ranked incidental findings as potentially detrimental pointed to how these findings led to invasive additional testing.
“One of my subjects was found to have diverticulosis, which needed an invasive procedure to rule out malignancy,” one respondent wrote. “However, the subject had already had a colonoscopy months prior to the PET findings, which was still not deemed sufficient by the nuclear radiologist and GI consultant, so he had to have another colonoscopy, which was benign, but uncomfortable.”
Obligation to Return Findings
All investigators agreed that incidental findings should be shared with patients if they revealed a high-risk medical condition that can be treated; had important health implications such as premature death or substantial morbidity; and their health could be improved with proven preventive or therapeutic interventions.
There was more disagreement on whether to share that the FDG PET/CT revealed no findings or if the test revealed a finding without clear medical importance of which the research participant may not be aware.
An example of a less-specific finding could be something like increased FDG uptake in a particular area, like the bowel, Kang explained.
“The question is: What does that mean?” she said. “How do you interpret that?”
While some PIs might feel obligated to share all results with patients, sharing ambiguous incidental findings will likely not be helpful to the patient, said Arthur Caplan, PhD, of the Division of Medical Ethics at New York University (NYU) Grossman School of Medicine, New York City.
“Dealing in unknowns and uncertainties when you’re diagnosing doesn’t really do people very much good,” he said in an interview.
While most survey respondents said they were at least moderately obligated to disclose incidental research findings if a patient requests them, Caplan noted that it was ultimately the researchers’ decision.
“Patient preferences are something to take into account, but they’re not final. If the research team says, ‘we don’t know, it’s too uncertain, it’s too new,’ then I don’t think they have any obligation to return that [information],” he said. “You can’t tell somebody what you don’t understand.”
Conversely, the clearer the incidental finding, the stronger the obligation to share that information with research participants, he continued.
Need for a Standardized Approach
The TARGET study, like many research studies, left the management of incidental imaging findings to individual research sites and investigators.
It’s possible that different sites responded to these ambiguous clinical findings in different ways, Kang noted.
“If there’s a situation that’s difficult to interpret as it is, you can imagine that the resulting actions that may result from that can vary, too,” she said, which highlights the need for more specific and standardized guidance.
One way to approach this, Caplan noted, is establishing an agreed-upon approach for dealing with any incidental findings across all research sites before a study begins.
“If there is going to be a common study at many sites, then they should have a common response on what they are going to do,” he noted, and how they will share that information effectively with the research participants to ensure it’s understandable. However, in a lot of research studies, each site has its own approach.
“Right now, it’s all over the place and that shouldn’t be,” he said.
Institutional review boards (IRBs) could be one resource to help build detailed guidance on managing unclear incidental findings in future research, wrote Kang and coauthors.
“For incidental findings from whole body FDG PET/CT that are not clearly actionable or less straightforward, IRBs may consider requiring a certain level of follow-up for different categories or types of incidental findings or require that all incidental findings are reviewed by an independent group that would provide timely recommendations on the most appropriate return and management of those findings,” Kang and colleagues wrote. “With IRB guidance, very specific and detailed policies and procedures for returning and managing incidental findings should be established for every study, with consistency among the research sites of multicenter trials.”
The TARGET trial and survey were funded by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Kang reported receiving research funding from the National Institutes of Health and the Rheumatology Research Foundation. Caplan serves as a contributing author for this news organization and served on an independent bioethics panel for compassionate drug use that was funded by Johnson & Johnson through the NYU Grossman School of Medicine.
A version of this article first appeared on Medscape.com.
Disparate views on managing incidental imaging findings made during clinical research — particularly for unclear results — signal a need for standardized guidance, according to recent survey results.
Respondents were split on whether it was the site primary investigator’s responsibility to decide which incidental findings should be reported back to the patient, and the most commonly cited challenges included adequately explaining these findings and the follow-up required. These issues were most present when dealing with nonspecific incidental findings or findings of unclear importance, said lead author Jane S. Kang, MD, a bioethicist and associate professor of medicine in the Division of Rheumatology at Columbia University Irving Medical Center, New York City.
“It can be difficult to have a clear approach” when it comes to these situations that are not black and white, and it is hard to get a clear answer, she said in an interview.
The survey included responses from investigators from the Treatments Against Rheumatoid Arthritis and Effect on 18F-fluorodeoxyglucose (FDG) PET/CT (TARGET) trial, conducted between 2015 and 2021. The 24-week trial included patients from 28 centers in the United States to investigate how different disease-modifying antirheumatic drugs can reduce cardiovascular and joint inflammation, assessed via whole body FDG PET/CT. The survey was a planned substudy of the TARGET trial and is “the first study that examines researchers’ attitudes and beliefs regarding incidental research findings from whole body FDG PET/CT,” Kang and her coauthors wrote.
This news organization reported the main results of the TARGET trial in 2022.
Eighteen of the 28 site primary investigators (PIs) of the TARGET trial participated in the survey, which was published in Arthritis Care & Research in September 2024.
TARGET Trial Incidental Findings
The TARGET trial enrolled 159 patients, of whom 82% had at least one incidental finding and 62% had one or more FDG-avid incidental findings. There were 46 “clinically actionable findings” for 40 participants overall; the reading radiologists recommended additional imaging for 28 findings and specialist consultation or procedural evaluation for 15 findings.
Details on these incidental findings were presented in a poster at the annual meeting of the American College of Rheumatology (ACR), held in Washington, DC.
The most common non–FDG-avid findings were pulmonary nodules, diverticulosis, cholelithiasis, sinus disease, and vascular calcifications. The most common FDG-avid findings were hypermetabolic lymphadenopathy, increased gastric/esophageal uptake, increased bowel uptake, and increased pharyngeal uptake.
In the related survey, 11 respondents (61%) said they returned any incidental findings to participants and 5 (28%) did not; the remaining 2 respondents did not know.
Across all study PIs, 22% felt that incidental findings were beneficial, 39% said they were potentially beneficial, and 11% said they were potentially detrimental. PIs that ranked incidental findings as potentially detrimental pointed to how these findings led to invasive additional testing.
“One of my subjects was found to have diverticulosis, which needed an invasive procedure to rule out malignancy,” one respondent wrote. “However, the subject had already had a colonoscopy months prior to the PET findings, which was still not deemed sufficient by the nuclear radiologist and GI consultant, so he had to have another colonoscopy, which was benign, but uncomfortable.”
Obligation to Return Findings
All investigators agreed that incidental findings should be shared with patients if they revealed a high-risk medical condition that can be treated; had important health implications such as premature death or substantial morbidity; and their health could be improved with proven preventive or therapeutic interventions.
There was more disagreement on whether to share that the FDG PET/CT revealed no findings or if the test revealed a finding without clear medical importance of which the research participant may not be aware.
An example of a less-specific finding could be something like increased FDG uptake in a particular area, like the bowel, Kang explained.
“The question is: What does that mean?” she said. “How do you interpret that?”
While some PIs might feel obligated to share all results with patients, sharing ambiguous incidental findings will likely not be helpful to the patient, said Arthur Caplan, PhD, of the Division of Medical Ethics at New York University (NYU) Grossman School of Medicine, New York City.
“Dealing in unknowns and uncertainties when you’re diagnosing doesn’t really do people very much good,” he said in an interview.
While most survey respondents said they were at least moderately obligated to disclose incidental research findings if a patient requests them, Caplan noted that it was ultimately the researchers’ decision.
“Patient preferences are something to take into account, but they’re not final. If the research team says, ‘we don’t know, it’s too uncertain, it’s too new,’ then I don’t think they have any obligation to return that [information],” he said. “You can’t tell somebody what you don’t understand.”
Conversely, the clearer the incidental finding, the stronger the obligation to share that information with research participants, he continued.
Need for a Standardized Approach
The TARGET study, like many research studies, left the management of incidental imaging findings to individual research sites and investigators.
It’s possible that different sites responded to these ambiguous clinical findings in different ways, Kang noted.
“If there’s a situation that’s difficult to interpret as it is, you can imagine that the resulting actions that may result from that can vary, too,” she said, which highlights the need for more specific and standardized guidance.
One way to approach this, Caplan noted, is establishing an agreed-upon approach for dealing with any incidental findings across all research sites before a study begins.
“If there is going to be a common study at many sites, then they should have a common response on what they are going to do,” he noted, and how they will share that information effectively with the research participants to ensure it’s understandable. However, in a lot of research studies, each site has its own approach.
“Right now, it’s all over the place and that shouldn’t be,” he said.
Institutional review boards (IRBs) could be one resource to help build detailed guidance on managing unclear incidental findings in future research, wrote Kang and coauthors.
“For incidental findings from whole body FDG PET/CT that are not clearly actionable or less straightforward, IRBs may consider requiring a certain level of follow-up for different categories or types of incidental findings or require that all incidental findings are reviewed by an independent group that would provide timely recommendations on the most appropriate return and management of those findings,” Kang and colleagues wrote. “With IRB guidance, very specific and detailed policies and procedures for returning and managing incidental findings should be established for every study, with consistency among the research sites of multicenter trials.”
The TARGET trial and survey were funded by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Kang reported receiving research funding from the National Institutes of Health and the Rheumatology Research Foundation. Caplan serves as a contributing author for this news organization and served on an independent bioethics panel for compassionate drug use that was funded by Johnson & Johnson through the NYU Grossman School of Medicine.
A version of this article first appeared on Medscape.com.
Disparate views on managing incidental imaging findings made during clinical research — particularly for unclear results — signal a need for standardized guidance, according to recent survey results.
Respondents were split on whether it was the site primary investigator’s responsibility to decide which incidental findings should be reported back to the patient, and the most commonly cited challenges included adequately explaining these findings and the follow-up required. These issues were most present when dealing with nonspecific incidental findings or findings of unclear importance, said lead author Jane S. Kang, MD, a bioethicist and associate professor of medicine in the Division of Rheumatology at Columbia University Irving Medical Center, New York City.
“It can be difficult to have a clear approach” when it comes to these situations that are not black and white, and it is hard to get a clear answer, she said in an interview.
The survey included responses from investigators from the Treatments Against Rheumatoid Arthritis and Effect on 18F-fluorodeoxyglucose (FDG) PET/CT (TARGET) trial, conducted between 2015 and 2021. The 24-week trial included patients from 28 centers in the United States to investigate how different disease-modifying antirheumatic drugs can reduce cardiovascular and joint inflammation, assessed via whole body FDG PET/CT. The survey was a planned substudy of the TARGET trial and is “the first study that examines researchers’ attitudes and beliefs regarding incidental research findings from whole body FDG PET/CT,” Kang and her coauthors wrote.
This news organization reported the main results of the TARGET trial in 2022.
Eighteen of the 28 site primary investigators (PIs) of the TARGET trial participated in the survey, which was published in Arthritis Care & Research in September 2024.
TARGET Trial Incidental Findings
The TARGET trial enrolled 159 patients, of whom 82% had at least one incidental finding and 62% had one or more FDG-avid incidental findings. There were 46 “clinically actionable findings” for 40 participants overall; the reading radiologists recommended additional imaging for 28 findings and specialist consultation or procedural evaluation for 15 findings.
Details on these incidental findings were presented in a poster at the annual meeting of the American College of Rheumatology (ACR), held in Washington, DC.
The most common non–FDG-avid findings were pulmonary nodules, diverticulosis, cholelithiasis, sinus disease, and vascular calcifications. The most common FDG-avid findings were hypermetabolic lymphadenopathy, increased gastric/esophageal uptake, increased bowel uptake, and increased pharyngeal uptake.
In the related survey, 11 respondents (61%) said they returned any incidental findings to participants and 5 (28%) did not; the remaining 2 respondents did not know.
Across all study PIs, 22% felt that incidental findings were beneficial, 39% said they were potentially beneficial, and 11% said they were potentially detrimental. PIs that ranked incidental findings as potentially detrimental pointed to how these findings led to invasive additional testing.
“One of my subjects was found to have diverticulosis, which needed an invasive procedure to rule out malignancy,” one respondent wrote. “However, the subject had already had a colonoscopy months prior to the PET findings, which was still not deemed sufficient by the nuclear radiologist and GI consultant, so he had to have another colonoscopy, which was benign, but uncomfortable.”
Obligation to Return Findings
All investigators agreed that incidental findings should be shared with patients if they revealed a high-risk medical condition that can be treated; had important health implications such as premature death or substantial morbidity; and their health could be improved with proven preventive or therapeutic interventions.
There was more disagreement on whether to share that the FDG PET/CT revealed no findings or if the test revealed a finding without clear medical importance of which the research participant may not be aware.
An example of a less-specific finding could be something like increased FDG uptake in a particular area, like the bowel, Kang explained.
“The question is: What does that mean?” she said. “How do you interpret that?”
While some PIs might feel obligated to share all results with patients, sharing ambiguous incidental findings will likely not be helpful to the patient, said Arthur Caplan, PhD, of the Division of Medical Ethics at New York University (NYU) Grossman School of Medicine, New York City.
“Dealing in unknowns and uncertainties when you’re diagnosing doesn’t really do people very much good,” he said in an interview.
While most survey respondents said they were at least moderately obligated to disclose incidental research findings if a patient requests them, Caplan noted that it was ultimately the researchers’ decision.
“Patient preferences are something to take into account, but they’re not final. If the research team says, ‘we don’t know, it’s too uncertain, it’s too new,’ then I don’t think they have any obligation to return that [information],” he said. “You can’t tell somebody what you don’t understand.”
Conversely, the clearer the incidental finding, the stronger the obligation to share that information with research participants, he continued.
Need for a Standardized Approach
The TARGET study, like many research studies, left the management of incidental imaging findings to individual research sites and investigators.
It’s possible that different sites responded to these ambiguous clinical findings in different ways, Kang noted.
“If there’s a situation that’s difficult to interpret as it is, you can imagine that the resulting actions that may result from that can vary, too,” she said, which highlights the need for more specific and standardized guidance.
One way to approach this, Caplan noted, is establishing an agreed-upon approach for dealing with any incidental findings across all research sites before a study begins.
“If there is going to be a common study at many sites, then they should have a common response on what they are going to do,” he noted, and how they will share that information effectively with the research participants to ensure it’s understandable. However, in a lot of research studies, each site has its own approach.
“Right now, it’s all over the place and that shouldn’t be,” he said.
Institutional review boards (IRBs) could be one resource to help build detailed guidance on managing unclear incidental findings in future research, wrote Kang and coauthors.
“For incidental findings from whole body FDG PET/CT that are not clearly actionable or less straightforward, IRBs may consider requiring a certain level of follow-up for different categories or types of incidental findings or require that all incidental findings are reviewed by an independent group that would provide timely recommendations on the most appropriate return and management of those findings,” Kang and colleagues wrote. “With IRB guidance, very specific and detailed policies and procedures for returning and managing incidental findings should be established for every study, with consistency among the research sites of multicenter trials.”
The TARGET trial and survey were funded by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Kang reported receiving research funding from the National Institutes of Health and the Rheumatology Research Foundation. Caplan serves as a contributing author for this news organization and served on an independent bioethics panel for compassionate drug use that was funded by Johnson & Johnson through the NYU Grossman School of Medicine.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS CARE & RESEARCH
White Matter Shows Decline After Bipolar Diagnosis
based on data from 88 individuals.
Patients with bipolar disorder demonstrate cognitive impairment and brain structure abnormalities, including global white matter loss, that have been associated with poor outcomes, but data on the stability or progression of neuroanatomical changes are limited, wrote Julian Macoveanu, PhD, of Copenhagen University Hospital, Denmark, and colleagues.
In a study published in The Journal of Affective Disorders, the researchers identified 97 adults aged 18 to 60 years with recently diagnosed bipolar disorder and matched them with 66 healthy controls. Participants were enrolled in the larger Bipolar Illness Onset (BIO) study. All participants underwent structural MRI and neuropsychological testing at baseline and were in full or partial remission based on total scores of 14 or less on the Hamilton Depression Rating Scale and the Young Mania Rating Scale. Approximately half of the participants (50 bipolar patients and 38 controls) participated in follow-up scans and testing after 6-27 months (mean 16 months), because of limited resources, according to the researchers.
The researchers compared changes in cortical gray matter volume and thickness, total cerebral white matter, hippocampal and amygdala volumes, estimated brain age, and cognitive functioning over time. In addition, they examined within-patient associations between baseline brain structure abnormalities and later mood episodes.
Overall, bipolar patients (BD) showed a significant decrease in total cerebral white matter from baseline, compared with healthy controls (HC) in mixed models (P = .006). “This effect was driven by BD patients showing a decrease in WM volume over time compared to HC who remained stable,” the researchers wrote, and the effect persisted in a post hoc analysis adjusting for subsyndromal symptoms and body mass index.
BD patients also had a larger amygdala volume at baseline and follow-up than HC, but no changes were noted between the groups. Changes in hippocampal volume also remained similar between the groups.
Analysis of cognitive data showed no significant differences in trajectories between BD patients and controls across cognitive domains or globally; although BD patients performed worse than controls at both time points.
BD patients in general experienced lower functioning and worse quality of life, compared with controls, but the trajectories of each group were similar for both functional and quality of life.
The researchers found no significant differences over time in total white matter, hippocampus, or amygdala volumes between BD patients who experienced at least one mood episode during the study period and those who remained in remission.
The findings were limited by several factors including the small sample size and limited generalizability of the findings because of the restriction to patients in full or partial remission, the researchers noted. Other limitations included the variation in follow-up time and the potential impact of psychotropic medication use.
However, the results were strengthened by the use of neuropsychiatric testing in addition to MRI to compare brain structure and cognitive function, the researchers said. The data suggest that both amygdala volume and cognitive impairment may be stable markers of BD soon after diagnosis, but that decreases in white matter may stem from disease progression.
The BIO study is funded by the Mental Health Services, Capital Region of Denmark, the Danish Council for Independent Research, Medical Sciences, Weimans Fund, Markedsmodningsfonden, Gangstedfonden, Læge Sofus Carl Emil og hustru Olga Boris Friis’ legat, Helsefonden, Innovation Fund Denmark, Copenhagen Center for Health Technology (CACHET), EU H2020 ITN, Augustinusfonden, and The Capital Region of Denmark. Macoveanu had no financial conflicts to disclose.
based on data from 88 individuals.
Patients with bipolar disorder demonstrate cognitive impairment and brain structure abnormalities, including global white matter loss, that have been associated with poor outcomes, but data on the stability or progression of neuroanatomical changes are limited, wrote Julian Macoveanu, PhD, of Copenhagen University Hospital, Denmark, and colleagues.
In a study published in The Journal of Affective Disorders, the researchers identified 97 adults aged 18 to 60 years with recently diagnosed bipolar disorder and matched them with 66 healthy controls. Participants were enrolled in the larger Bipolar Illness Onset (BIO) study. All participants underwent structural MRI and neuropsychological testing at baseline and were in full or partial remission based on total scores of 14 or less on the Hamilton Depression Rating Scale and the Young Mania Rating Scale. Approximately half of the participants (50 bipolar patients and 38 controls) participated in follow-up scans and testing after 6-27 months (mean 16 months), because of limited resources, according to the researchers.
The researchers compared changes in cortical gray matter volume and thickness, total cerebral white matter, hippocampal and amygdala volumes, estimated brain age, and cognitive functioning over time. In addition, they examined within-patient associations between baseline brain structure abnormalities and later mood episodes.
Overall, bipolar patients (BD) showed a significant decrease in total cerebral white matter from baseline, compared with healthy controls (HC) in mixed models (P = .006). “This effect was driven by BD patients showing a decrease in WM volume over time compared to HC who remained stable,” the researchers wrote, and the effect persisted in a post hoc analysis adjusting for subsyndromal symptoms and body mass index.
BD patients also had a larger amygdala volume at baseline and follow-up than HC, but no changes were noted between the groups. Changes in hippocampal volume also remained similar between the groups.
Analysis of cognitive data showed no significant differences in trajectories between BD patients and controls across cognitive domains or globally; although BD patients performed worse than controls at both time points.
BD patients in general experienced lower functioning and worse quality of life, compared with controls, but the trajectories of each group were similar for both functional and quality of life.
The researchers found no significant differences over time in total white matter, hippocampus, or amygdala volumes between BD patients who experienced at least one mood episode during the study period and those who remained in remission.
The findings were limited by several factors including the small sample size and limited generalizability of the findings because of the restriction to patients in full or partial remission, the researchers noted. Other limitations included the variation in follow-up time and the potential impact of psychotropic medication use.
However, the results were strengthened by the use of neuropsychiatric testing in addition to MRI to compare brain structure and cognitive function, the researchers said. The data suggest that both amygdala volume and cognitive impairment may be stable markers of BD soon after diagnosis, but that decreases in white matter may stem from disease progression.
The BIO study is funded by the Mental Health Services, Capital Region of Denmark, the Danish Council for Independent Research, Medical Sciences, Weimans Fund, Markedsmodningsfonden, Gangstedfonden, Læge Sofus Carl Emil og hustru Olga Boris Friis’ legat, Helsefonden, Innovation Fund Denmark, Copenhagen Center for Health Technology (CACHET), EU H2020 ITN, Augustinusfonden, and The Capital Region of Denmark. Macoveanu had no financial conflicts to disclose.
based on data from 88 individuals.
Patients with bipolar disorder demonstrate cognitive impairment and brain structure abnormalities, including global white matter loss, that have been associated with poor outcomes, but data on the stability or progression of neuroanatomical changes are limited, wrote Julian Macoveanu, PhD, of Copenhagen University Hospital, Denmark, and colleagues.
In a study published in The Journal of Affective Disorders, the researchers identified 97 adults aged 18 to 60 years with recently diagnosed bipolar disorder and matched them with 66 healthy controls. Participants were enrolled in the larger Bipolar Illness Onset (BIO) study. All participants underwent structural MRI and neuropsychological testing at baseline and were in full or partial remission based on total scores of 14 or less on the Hamilton Depression Rating Scale and the Young Mania Rating Scale. Approximately half of the participants (50 bipolar patients and 38 controls) participated in follow-up scans and testing after 6-27 months (mean 16 months), because of limited resources, according to the researchers.
The researchers compared changes in cortical gray matter volume and thickness, total cerebral white matter, hippocampal and amygdala volumes, estimated brain age, and cognitive functioning over time. In addition, they examined within-patient associations between baseline brain structure abnormalities and later mood episodes.
Overall, bipolar patients (BD) showed a significant decrease in total cerebral white matter from baseline, compared with healthy controls (HC) in mixed models (P = .006). “This effect was driven by BD patients showing a decrease in WM volume over time compared to HC who remained stable,” the researchers wrote, and the effect persisted in a post hoc analysis adjusting for subsyndromal symptoms and body mass index.
BD patients also had a larger amygdala volume at baseline and follow-up than HC, but no changes were noted between the groups. Changes in hippocampal volume also remained similar between the groups.
Analysis of cognitive data showed no significant differences in trajectories between BD patients and controls across cognitive domains or globally; although BD patients performed worse than controls at both time points.
BD patients in general experienced lower functioning and worse quality of life, compared with controls, but the trajectories of each group were similar for both functional and quality of life.
The researchers found no significant differences over time in total white matter, hippocampus, or amygdala volumes between BD patients who experienced at least one mood episode during the study period and those who remained in remission.
The findings were limited by several factors including the small sample size and limited generalizability of the findings because of the restriction to patients in full or partial remission, the researchers noted. Other limitations included the variation in follow-up time and the potential impact of psychotropic medication use.
However, the results were strengthened by the use of neuropsychiatric testing in addition to MRI to compare brain structure and cognitive function, the researchers said. The data suggest that both amygdala volume and cognitive impairment may be stable markers of BD soon after diagnosis, but that decreases in white matter may stem from disease progression.
The BIO study is funded by the Mental Health Services, Capital Region of Denmark, the Danish Council for Independent Research, Medical Sciences, Weimans Fund, Markedsmodningsfonden, Gangstedfonden, Læge Sofus Carl Emil og hustru Olga Boris Friis’ legat, Helsefonden, Innovation Fund Denmark, Copenhagen Center for Health Technology (CACHET), EU H2020 ITN, Augustinusfonden, and The Capital Region of Denmark. Macoveanu had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Delayed Bleeding: The Silent Risk for Seniors
This discussion was recorded on August 2, 2024. This transcript has been edited for clarity.
Robert D. Glatter, MD: Today, we’ll be discussing the results of a new study published in The Journal of Emergency Medicine, looking at the incidence of delayed intracranial hemorrhage among older patients taking preinjury anticoagulants who present to the emergency department (ED) with blunt head trauma.
Joining me today is the lead author of the study, Dr. Richard Shih, professor of emergency medicine at Florida Atlantic University. Also joining me is Dr. Christina Shenvi, associate professor of emergency medicine at the University of North Carolina (UNC) Chapel Hill, with fellowship training in geriatric emergency medicine.
Welcome to both of you.
Richard D. Shih, MD: Thanks, Rob.
Christina L. Shenvi, MD, PhD, MBA: Thanks. Pleasure to be here.
ICH Study Methodology
Dr. Glatter: It’s a pleasure to have you. Rich, this is a great study and targeted toward a population we see daily in the emergency department. I want you to describe your methodology, patient selection, and how you went about organizing your study to look at this important finding of delayed intracranial hemorrhage, especially in those on anticoagulants.
Dr. Shih: This all started for our research team when we first read the 2012 Annals of Emergency Medicine paper. The first author was Vincenzo Menditto, and he looked at a group of patients that had minor head injury, were anticoagulated, and had negative initial head CTs.
There were about 100 patients, of which about 10 of them did not consent, but they hospitalized all these patients. These were anticoagulated, negative-first head CTs. They hospitalized the patients and then did a routine second CT at about 24 hours. They also followed them for a week, and it turned out a little over 7% of them had delayed head CT.
We were wondering how many delayed intracranial hemorrhages we had missed because current practice for us was that, if patients had a good physical exam, their head CT was normal, and everything looked good, we would send them home.
Because of that, a number of people across the country wanted to verify those findings from the Menditto study. We tried to design a good study to answer that question. We happen to have a very large geriatric population in Florida, and our ED census is very high for age over 65, at nearly 60%.
There are two Level I trauma centers in Palm Beach County. We included a second multicenter hospital, and we prospectively enrolled patients. We know the current state of practice is not to routinely do second CTs, so we followed these patients over time and followed their medical records to try to identify delayed bleeding. That’s how we set up our methodology.
Is It Safe to Discharge Patients With Trauma After 24 Hours?
Dr. Glatter: For the bulk of these patients with negative head CTs, it’s been my practice that when they’re stable and they look fine and there’s no other apparent, distracting painful trauma, injuries and so forth, they’re safe to discharge.
The secondary outcome in your study is interesting: the need for neurosurgical intervention in terms of those with delayed intracranial hemorrhage.
Dr. Shih: I do believe that it’s certainly not the problem that Menditto described, which is 7%. There are two other prospective studies that have looked at this issue with delayed bleeding on anticoagulants. Both of these also showed a relatively low rate of delayed bleeding, which is between like 0.2% and 1.0%. In our study, it was 0.4%.
The difference in the studies is that Menditto and colleagues routinely did 24-hour head CTs. They admitted everybody. For these other studies, routine head CT was not part of it. My bet is that there is a rate of delayed bleeding somewhere in between that seen in the Menditto study and that in all the other studies.
However, talking about significant intracranial hemorrhage, ones that perhaps need neurosurgery, I believe most of them are not significant. There’s some number that do occur, but the vast majority of those probably don’t need neurosurgery. We had 14 delayed bleeds out of 6000 patients with head trauma. One of them ended up requiring neurosurgery, so the answer is not zero, but I don’t think it’s 7% either.
Dr. Glatter: Dr. Shenvi, I want to bring you into the conversation to talk about your experience at UNC, and how you run things in terms of older patients with blunt head trauma on preinjury anticoagulants.
Dr. Shenvi: Thanks, Rob. I remember when this paper came out showing this 7% rate of delayed bleeding and the question was, “Should we be admitting all these people?” Partly just from an overwhelming need for capacity that that would bring, it just wasn’t practical to say, “We’re going to admit every patient with a negative head CT to the hospital and rescan them.” That would be hundreds or thousands of patients each year in any given facility.
The other thing is that delayed bleeds don’t always happen just in the first 24 hours. It’s not even a matter of bringing patients into observation for 24 hours, watching them, and rescanning them if they have symptoms. It can occur several days out. That never, in almost any institution that I know of, became standard practice.
The way that it did change my care was to give good return precautions to patients, to make sure they have somebody with them to say, “Hey, sometimes you can have bleeding several days out after a fall, even though your CT scan here today looks perfect,” and to alert them that if they start having severe headaches, vomiting, or other symptoms of intracranial hemorrhage, that they should come back.
I don’t think it ever became standard practice, and for good reason, because that was one study. The subsequent studies that Richard mentioned, pretty quickly on the heels of that initial one, showed a much lower rate of delayed ICH with the caveats that the methodology was different.
Shift in Anticoagulants
Dr. Shenvi: One other big change from that original study, and now to Richard’s study, is the shift in anticoagulants. Back in the initial study you mentioned, it was all warfarin. We know from other studies looking at warfarin vs the direct oral anticoagulants (DOACs) that DOACs have lower rates of ICH after a head injury, lower rates of need for neurosurgical intervention, and lower rates of discharge to a skilled nursing facility after an intracranial hemorrhage.
Across the board, we know that the DOACs tend to do better. It’s difficult to compare newer studies because it’s a different medication. It did inform my practice to have an awareness of delayed intracranial hemorrhage so that I warn patients more proactively.
Dr. Glatter: I haven’t seen a patient on warfarin in years. I don’t know if either of you have, but it’s all DOACs now unless there’s some other reason. That shift is quite apparent.
Dr. Shih: The problem with looking at delayed bleeding for DOACs vs warfarin is the numbers were so low. I think we had 13 people, and seven were in the no-anticoagulant group. The numbers are even lower, so it’s hard to say.
I just wanted to comment on something that Dr. Shenvi said, and I pretty much agree with everything that she said. Anticoagulants and warfarin, and that Menditto study, have a carryover effect. People group DOACs with warfarin similarly. When a patient is brought in, the first thing they talk about with head trauma is, “Oh, they’re on an anticoagulant” or “They’re not on an anticoagulant.” It’s so ingrained.
I believe that, in emergency medicine, we’re pressed for space and time and we’re not as affected by that 24-hour observation. Maybe many of our surgeons will automatically admit those patients.
I haven’t seen a guideline from the United States, but there are two international guidelines. One is from Austria from 2019, and one is from Scandinavia. Both recommended 24-hour observation if you’re on an anticoagulant.
There is a bit of controversy left over with that. Hopefully, as more and more of information, like in our study, comes out, people will be a little bit more clear about it. I don’t think there’s a need to routinely admit them.
I do want to mention that the Menditto study had such a massive impact on everybody. They pointed out one subgroup (and it’s such a small number of patients). They had seven cases of delayed bleeding; four or five of them were within that 24 hours, and a couple were diagnosed later over the next couple days.
Of those seven people, four of them had international normalized ratios (INRs) greater than 3. Of those four patients, I’ve heard people talk about this and recommend, “Okay, that’s the subgroup I would admit.” There’s a toss-up with what to do with DOAC because it’s very hard to tell whether there’s an issue, whether there are problems with their dosing, and whatever.
We actually recently looked at that. We have a much larger sample than four: close to 300 patients who were on warfarin. We looked at patients who had INRs below 3 and above 3, and we didn’t show a difference. We still don’t believe that warfarin is a big issue with delayed bleeding.
Should We Be Asking: ‘Are They on Blood Thinners?’
Dr. Shenvi: One of the interesting trends related to warfarin and the DOACs vs no anticoagulant is that as you mentioned, Dr Shih, the first question out of people’s mouths or the first piece of information emergency medical services gives you when they come in with a patient who’s had a head injury is, “Are they on blood thinners or not?”
Yet, the paradigm is shifting to say it’s not actually the blood thinners themselves that are giving older patients the higher risk for bleeding; it’s age and other comorbidities.
Certainly, if you’re on an anticoagulant and you start to bleed, your prognosis is much worse because the bleeding doesn’t stop. In terms of who has a bleeding event, there’s much less impact of anticoagulation than we used to think. That, in part, may be due to the change from warfarin to other medications.
Some of the experts I’ve talked to who have done the research on this have said, “Well, actually, warfarin was more of a marker for being much older and more frail, because it was primarily prescribed to older patients who have significant heart disease, atrial fibrillation, and so on.” It was more a marker for somebody who is at risk for an intracranial hemorrhage. There are many changes that have happened in the past 10 years with medications and also our understanding.
Challenges in Patient Follow-up
Dr. Glatter: That’s a great point. One thing, Rich, I want to ask you about is in terms of your proxy outcome assessment. When you use that at 14 and 60 days with telephone follow-up and then chart review at 60 and 90 days (because, obviously, everyone can’t get another head CT or it’s difficult to follow patients up), did you find that worked out well in your prospective cohort study, in terms of using that as a proxy, so to speak?
Dr. Shih: I would say to a certain extent. Unfortunately, we don’t have access to the patients to come back to follow up all of them, and there was obviously a large number of patients in our study.
The next best thing was that we had dedicated research assistants calling all of the patients at 14 days and 60 days. I’ve certainly read research studies where, when they call them, they get 80%-90% follow-up, but we did not achieve that.
I don’t know if people are more inundated with spam phone calls now, or the older people are just afraid of picking up their phone sometimes with all the scams and so forth. I totally understand, but in all honesty, we only had about a 30%-35% follow-up using that follow-up pathway.
Then the proxy pathway was to look at their charts at 60 and 90 days. Also, we looked at the Florida death registry, which is pretty good, and then finally, we had both Level I trauma centers in the county that we were in participating. It’s standard practice that if you have an intracranial hemorrhage at a non–Level I trauma center, you would be transferred to a Level I trauma center. That’s the protocol. I know that’s not followed 100% of the time, but that’s part of the proxy follow-up. You could criticize the study for not having closer to 90% actual contact, but that’s the best we could do.
Dr. Glatter: I think that’s admirable. Using that paradigm of what you described certainly allows the reader to understand the difficulty in assessing patients that don’t get follow-up head CT, and hardly anyone does that, as we know.
To your point of having both Level I trauma centers in the county, that makes it pretty secure. If we’re going to do a study encompassing a similar type of regional aspect, it would be similar.
Dr. Shenvi: I think your proxies, to your credit, were as good as you can get. You can never get a 100% follow-up, but you really looked at all the different avenues by which patients might present, either in the death registry or a Level I center. Well done on that aspect.
Determining When to Admit Patients for Observation
Dr. Glatter: In terms of admissions: You admit a patient, then you hear back that this patient should not have been admitted because they had a negative head CT, but you put them in anyway in the sense of delayed bleeding happening or not happening.
It’s interesting. Maybe the insurers will start looking at this in some capacity, based on your study, that because it’s so infrequent that you see delayed bleeding, that admitting someone for any reason whatsoever would be declined. Do you see that being an issue? In other words, [do you see] this leading to a pattern in terms of the payers?
Dr. Shih: Certainly, you could interpret it that way, and that would be unfortunate. The [incidence of] delayed bleeding is definitely not zero. That’s the first thing.
The second thing is that when you’re dealing with an older population, having some sense that they’re not doing well is an important contributor to trying to fully assess what’s going on — whether or not they have a bleed or whether they’re at risk for falling again and then hitting their head and causing a second bleed, and making sure they can do the activities of daily life. There really should be some room for a physician to say, “They just got here, and we don’t know him that well. There’s something that bothers me about this person” and have the ability to watch them for at least another 24 hours. That’s how I feel.
Dr. Shenvi: In my location, it would be difficult to try to admit somebody purely for observation for delayed bleeding. I think we would get a lot of pushback on that. The reasons I might admit a patient after a fall with a negative head CT, though, are all the things that, Rob, you alluded to earlier — which are, what made them fall in the first place and were they unable to get up?
I had this happen just this week. A patient who fell couldn’t get off the ground for 12 hours, and so now she’s dehydrated and delirious with slight rhabdomyolysis. Then you’re admitting them either for the sequelae of the fall that are not related to the intracranial hemorrhage, or the fact that they are so debilitated and deconditioned that they cannot take care of themselves. They need physical therapy. Often, we will have physical and occupational therapists come see them in the ED during business hours and help make an assessment of whether they are safe to go home or whether they fall again. That can give more evidence for the need for admission.
Dr. Glatter: To bring artificial intelligence into this discussion, algorithms that are out there that say, “Push a button and the patient’s safe for discharge.” Well, this argues for a clinical gestalt and a human being to make an assessment because you can use these predictive models, which are coming and they’re going to be here soon, and they already are in some sense. Again, we have to use clinical human judgment.
Dr. Shih: I agree.
Advice for Primary Care Physicians
Dr. Glatter: What return precautions do you discuss with patients who’ve had blunt head trauma that maybe had a head CT, or even didn’t? What are the main things we’re looking for?
Dr. Shenvi: What I usually tell people is if you start to have a worse headache, nausea or vomiting, any weakness in one area of your body, or vision changes, and if there’s a family member or friend there, I’ll say, “If you notice that they’re acting differently or seem confused, come back.”
Dr. Shih: I agree with what she said, and I’m also going to add one thing. The most important part is they are trying to prevent a subsequent fall. We know that when they’ve fallen and they present to the ED, they’re at even higher risk for falling and reinjuring themselves, and that’s a population that’s already at risk.
One of the secondary studies that we published out of this project was looking at follow-up with their primary care physicians, and there were two things that we wanted to address. The first was, how often did they do it? Then, when they did do it, did their primary care physicians try to address and prevent subsequent falls?
Both the answers are actually bad. Amazingly, just over like 60% followed up.
In some of our subsequent research, because we’re in the midst of a randomized, controlled trial where we do a home visit, when we initially see these individuals that have fallen, they’ll schedule a home visit for us. Then a week or two later, when we schedule the home visit, many of them cancel because they think, Oh, that was a one-off and it’s not going to happen again. Part of the problem is the patients, because many of them believe that they just slipped and fell and it’s not going to happen again, or they’re not prone to it.
The second issue was when patients did go to a primary care physician, we have found that some primary care physicians believe that falling and injuring themselves is just part of the normal aging process. A percentage of them don’t go over assessment for fall risk or even initiate fall prevention treatments or programs.
I try to take that time to tell them that this is very common in their age group, and believe it or not, a fall from standing is the way people really injure themselves, and there may be ways to prevent subsequent falls and injuries.
Dr. Glatter: Absolutely. Do you find that their medications are a contributor in some sense? Say they’re antihypertensive, have issues of orthostasis, or a new medication was added in the last week.
Dr. Shenvi: It’s all of the above. Sometimes it’s one thing, like they just started tamsulosin for their kidney stone, they stood up, they felt lightheaded, and they fell. Usually, it’s multifactorial with some changes in their gait, vision, balance, reflex time, and strength, plus the medications or the need for assistive devices. Maybe they can’t take care of their home as well as they used to and there are things on the floor. It’s really all of the above.
‘Harder to Unlearn Something Than to Learn It’
Dr. Glatter: Would either of you like to add any additional points to the discussion or add a few pearls?
Dr. Shenvi: This just highlights the challenge of how it’s harder to unlearn something than to learn it, where one study that maybe wasn’t quite looking at what we needed to, or practice and prescribing patterns have changed, so it’s no longer really relevant.
The things that we learned from that, or the fears that we instilled in our minds of, Uh oh, they could go home and have delayed bleeding, are much harder to unlearn, and it takes more studies to unlearn that idea than it did to actually put it into place.
I’m glad that your team has done this much larger, prospective study and hopefully will reduce the concern about this entity.
Dr. Shih: I appreciate that segue. It is amazing that, for paramedics and medical students, the first thing out of their mouth is, “Are they on an anticoagulant?”
In terms of the risk of developing an intracranial hemorrhage, I think it’s much less than the weight we’ve put on it before. However, I believe if they have a bleed, the bleeds are worse. It’s kind of a double-edged sword. It’s still an important factor, but it doesn’t come with the Oh my gosh, they’re on an anticoagulant that everybody thinks about.
No. 1 Cause of Traumatic Injury Is a Fall from Standing
Dr. Glatter: These are obviously ground-level falls in most patients and not motor vehicle crashes. That’s an important part in the population that you looked at that should be mentioned clearly.
Dr. Shih: It’s astonishing. I’ve been a program director for over 20 years, and geriatrics is not well taught in the curriculum. It’s astonishing for many of our trainees and emergency physicians in general that the number-one cause for traumatic injury is a fall from standing.
Certainly, we get patients coming in the trauma center like a 95-year-old person who’s on a ladder putting up his Christmas lights. I’m like, oh my God.
For the vast majority, it’s closer to 90%, but in our study, for the patients we looked at, it was 80% that fall from standing. That’s the mechanism that causes these bleeds and these major injuries.
Dr. Shenvi: That’s reflective of what we see, so it’s good that that’s what you looked at also.
Dr. Glatter: Absolutely. Well, thank you both. This has been a very informative discussion. I appreciate your time, and our readers will certainly benefit from your knowledge and expertise. Thank you again.
Dr. Glatter, assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, is a medical adviser for this news organization. He disclosed having no relevant financial conflicts. Dr. Shih is professor of emergency medicine at the Charles E. Schmidt College of Medicine at Florida Atlantic University, Boca Raton. His current grant funding and area of research interest involves geriatric emergency department patients with head injury and fall-related injury. He disclosed receiving a research grant from The Florida Medical Malpractice Joint Underwriting Association Grant for Safety of Health Care Services). Dr. Shenvi, associate professor of emergency medicine at the University of North Carolina at Chapel Hill, disclosed ties with the American College of Emergency Physicians, Institute for Healthcare Improvement, AstraZeneca, and CurvaFix.
A version of this article appeared on Medscape.com.
This discussion was recorded on August 2, 2024. This transcript has been edited for clarity.
Robert D. Glatter, MD: Today, we’ll be discussing the results of a new study published in The Journal of Emergency Medicine, looking at the incidence of delayed intracranial hemorrhage among older patients taking preinjury anticoagulants who present to the emergency department (ED) with blunt head trauma.
Joining me today is the lead author of the study, Dr. Richard Shih, professor of emergency medicine at Florida Atlantic University. Also joining me is Dr. Christina Shenvi, associate professor of emergency medicine at the University of North Carolina (UNC) Chapel Hill, with fellowship training in geriatric emergency medicine.
Welcome to both of you.
Richard D. Shih, MD: Thanks, Rob.
Christina L. Shenvi, MD, PhD, MBA: Thanks. Pleasure to be here.
ICH Study Methodology
Dr. Glatter: It’s a pleasure to have you. Rich, this is a great study and targeted toward a population we see daily in the emergency department. I want you to describe your methodology, patient selection, and how you went about organizing your study to look at this important finding of delayed intracranial hemorrhage, especially in those on anticoagulants.
Dr. Shih: This all started for our research team when we first read the 2012 Annals of Emergency Medicine paper. The first author was Vincenzo Menditto, and he looked at a group of patients that had minor head injury, were anticoagulated, and had negative initial head CTs.
There were about 100 patients, of which about 10 of them did not consent, but they hospitalized all these patients. These were anticoagulated, negative-first head CTs. They hospitalized the patients and then did a routine second CT at about 24 hours. They also followed them for a week, and it turned out a little over 7% of them had delayed head CT.
We were wondering how many delayed intracranial hemorrhages we had missed because current practice for us was that, if patients had a good physical exam, their head CT was normal, and everything looked good, we would send them home.
Because of that, a number of people across the country wanted to verify those findings from the Menditto study. We tried to design a good study to answer that question. We happen to have a very large geriatric population in Florida, and our ED census is very high for age over 65, at nearly 60%.
There are two Level I trauma centers in Palm Beach County. We included a second multicenter hospital, and we prospectively enrolled patients. We know the current state of practice is not to routinely do second CTs, so we followed these patients over time and followed their medical records to try to identify delayed bleeding. That’s how we set up our methodology.
Is It Safe to Discharge Patients With Trauma After 24 Hours?
Dr. Glatter: For the bulk of these patients with negative head CTs, it’s been my practice that when they’re stable and they look fine and there’s no other apparent, distracting painful trauma, injuries and so forth, they’re safe to discharge.
The secondary outcome in your study is interesting: the need for neurosurgical intervention in terms of those with delayed intracranial hemorrhage.
Dr. Shih: I do believe that it’s certainly not the problem that Menditto described, which is 7%. There are two other prospective studies that have looked at this issue with delayed bleeding on anticoagulants. Both of these also showed a relatively low rate of delayed bleeding, which is between like 0.2% and 1.0%. In our study, it was 0.4%.
The difference in the studies is that Menditto and colleagues routinely did 24-hour head CTs. They admitted everybody. For these other studies, routine head CT was not part of it. My bet is that there is a rate of delayed bleeding somewhere in between that seen in the Menditto study and that in all the other studies.
However, talking about significant intracranial hemorrhage, ones that perhaps need neurosurgery, I believe most of them are not significant. There’s some number that do occur, but the vast majority of those probably don’t need neurosurgery. We had 14 delayed bleeds out of 6000 patients with head trauma. One of them ended up requiring neurosurgery, so the answer is not zero, but I don’t think it’s 7% either.
Dr. Glatter: Dr. Shenvi, I want to bring you into the conversation to talk about your experience at UNC, and how you run things in terms of older patients with blunt head trauma on preinjury anticoagulants.
Dr. Shenvi: Thanks, Rob. I remember when this paper came out showing this 7% rate of delayed bleeding and the question was, “Should we be admitting all these people?” Partly just from an overwhelming need for capacity that that would bring, it just wasn’t practical to say, “We’re going to admit every patient with a negative head CT to the hospital and rescan them.” That would be hundreds or thousands of patients each year in any given facility.
The other thing is that delayed bleeds don’t always happen just in the first 24 hours. It’s not even a matter of bringing patients into observation for 24 hours, watching them, and rescanning them if they have symptoms. It can occur several days out. That never, in almost any institution that I know of, became standard practice.
The way that it did change my care was to give good return precautions to patients, to make sure they have somebody with them to say, “Hey, sometimes you can have bleeding several days out after a fall, even though your CT scan here today looks perfect,” and to alert them that if they start having severe headaches, vomiting, or other symptoms of intracranial hemorrhage, that they should come back.
I don’t think it ever became standard practice, and for good reason, because that was one study. The subsequent studies that Richard mentioned, pretty quickly on the heels of that initial one, showed a much lower rate of delayed ICH with the caveats that the methodology was different.
Shift in Anticoagulants
Dr. Shenvi: One other big change from that original study, and now to Richard’s study, is the shift in anticoagulants. Back in the initial study you mentioned, it was all warfarin. We know from other studies looking at warfarin vs the direct oral anticoagulants (DOACs) that DOACs have lower rates of ICH after a head injury, lower rates of need for neurosurgical intervention, and lower rates of discharge to a skilled nursing facility after an intracranial hemorrhage.
Across the board, we know that the DOACs tend to do better. It’s difficult to compare newer studies because it’s a different medication. It did inform my practice to have an awareness of delayed intracranial hemorrhage so that I warn patients more proactively.
Dr. Glatter: I haven’t seen a patient on warfarin in years. I don’t know if either of you have, but it’s all DOACs now unless there’s some other reason. That shift is quite apparent.
Dr. Shih: The problem with looking at delayed bleeding for DOACs vs warfarin is the numbers were so low. I think we had 13 people, and seven were in the no-anticoagulant group. The numbers are even lower, so it’s hard to say.
I just wanted to comment on something that Dr. Shenvi said, and I pretty much agree with everything that she said. Anticoagulants and warfarin, and that Menditto study, have a carryover effect. People group DOACs with warfarin similarly. When a patient is brought in, the first thing they talk about with head trauma is, “Oh, they’re on an anticoagulant” or “They’re not on an anticoagulant.” It’s so ingrained.
I believe that, in emergency medicine, we’re pressed for space and time and we’re not as affected by that 24-hour observation. Maybe many of our surgeons will automatically admit those patients.
I haven’t seen a guideline from the United States, but there are two international guidelines. One is from Austria from 2019, and one is from Scandinavia. Both recommended 24-hour observation if you’re on an anticoagulant.
There is a bit of controversy left over with that. Hopefully, as more and more of information, like in our study, comes out, people will be a little bit more clear about it. I don’t think there’s a need to routinely admit them.
I do want to mention that the Menditto study had such a massive impact on everybody. They pointed out one subgroup (and it’s such a small number of patients). They had seven cases of delayed bleeding; four or five of them were within that 24 hours, and a couple were diagnosed later over the next couple days.
Of those seven people, four of them had international normalized ratios (INRs) greater than 3. Of those four patients, I’ve heard people talk about this and recommend, “Okay, that’s the subgroup I would admit.” There’s a toss-up with what to do with DOAC because it’s very hard to tell whether there’s an issue, whether there are problems with their dosing, and whatever.
We actually recently looked at that. We have a much larger sample than four: close to 300 patients who were on warfarin. We looked at patients who had INRs below 3 and above 3, and we didn’t show a difference. We still don’t believe that warfarin is a big issue with delayed bleeding.
Should We Be Asking: ‘Are They on Blood Thinners?’
Dr. Shenvi: One of the interesting trends related to warfarin and the DOACs vs no anticoagulant is that as you mentioned, Dr Shih, the first question out of people’s mouths or the first piece of information emergency medical services gives you when they come in with a patient who’s had a head injury is, “Are they on blood thinners or not?”
Yet, the paradigm is shifting to say it’s not actually the blood thinners themselves that are giving older patients the higher risk for bleeding; it’s age and other comorbidities.
Certainly, if you’re on an anticoagulant and you start to bleed, your prognosis is much worse because the bleeding doesn’t stop. In terms of who has a bleeding event, there’s much less impact of anticoagulation than we used to think. That, in part, may be due to the change from warfarin to other medications.
Some of the experts I’ve talked to who have done the research on this have said, “Well, actually, warfarin was more of a marker for being much older and more frail, because it was primarily prescribed to older patients who have significant heart disease, atrial fibrillation, and so on.” It was more a marker for somebody who is at risk for an intracranial hemorrhage. There are many changes that have happened in the past 10 years with medications and also our understanding.
Challenges in Patient Follow-up
Dr. Glatter: That’s a great point. One thing, Rich, I want to ask you about is in terms of your proxy outcome assessment. When you use that at 14 and 60 days with telephone follow-up and then chart review at 60 and 90 days (because, obviously, everyone can’t get another head CT or it’s difficult to follow patients up), did you find that worked out well in your prospective cohort study, in terms of using that as a proxy, so to speak?
Dr. Shih: I would say to a certain extent. Unfortunately, we don’t have access to the patients to come back to follow up all of them, and there was obviously a large number of patients in our study.
The next best thing was that we had dedicated research assistants calling all of the patients at 14 days and 60 days. I’ve certainly read research studies where, when they call them, they get 80%-90% follow-up, but we did not achieve that.
I don’t know if people are more inundated with spam phone calls now, or the older people are just afraid of picking up their phone sometimes with all the scams and so forth. I totally understand, but in all honesty, we only had about a 30%-35% follow-up using that follow-up pathway.
Then the proxy pathway was to look at their charts at 60 and 90 days. Also, we looked at the Florida death registry, which is pretty good, and then finally, we had both Level I trauma centers in the county that we were in participating. It’s standard practice that if you have an intracranial hemorrhage at a non–Level I trauma center, you would be transferred to a Level I trauma center. That’s the protocol. I know that’s not followed 100% of the time, but that’s part of the proxy follow-up. You could criticize the study for not having closer to 90% actual contact, but that’s the best we could do.
Dr. Glatter: I think that’s admirable. Using that paradigm of what you described certainly allows the reader to understand the difficulty in assessing patients that don’t get follow-up head CT, and hardly anyone does that, as we know.
To your point of having both Level I trauma centers in the county, that makes it pretty secure. If we’re going to do a study encompassing a similar type of regional aspect, it would be similar.
Dr. Shenvi: I think your proxies, to your credit, were as good as you can get. You can never get a 100% follow-up, but you really looked at all the different avenues by which patients might present, either in the death registry or a Level I center. Well done on that aspect.
Determining When to Admit Patients for Observation
Dr. Glatter: In terms of admissions: You admit a patient, then you hear back that this patient should not have been admitted because they had a negative head CT, but you put them in anyway in the sense of delayed bleeding happening or not happening.
It’s interesting. Maybe the insurers will start looking at this in some capacity, based on your study, that because it’s so infrequent that you see delayed bleeding, that admitting someone for any reason whatsoever would be declined. Do you see that being an issue? In other words, [do you see] this leading to a pattern in terms of the payers?
Dr. Shih: Certainly, you could interpret it that way, and that would be unfortunate. The [incidence of] delayed bleeding is definitely not zero. That’s the first thing.
The second thing is that when you’re dealing with an older population, having some sense that they’re not doing well is an important contributor to trying to fully assess what’s going on — whether or not they have a bleed or whether they’re at risk for falling again and then hitting their head and causing a second bleed, and making sure they can do the activities of daily life. There really should be some room for a physician to say, “They just got here, and we don’t know him that well. There’s something that bothers me about this person” and have the ability to watch them for at least another 24 hours. That’s how I feel.
Dr. Shenvi: In my location, it would be difficult to try to admit somebody purely for observation for delayed bleeding. I think we would get a lot of pushback on that. The reasons I might admit a patient after a fall with a negative head CT, though, are all the things that, Rob, you alluded to earlier — which are, what made them fall in the first place and were they unable to get up?
I had this happen just this week. A patient who fell couldn’t get off the ground for 12 hours, and so now she’s dehydrated and delirious with slight rhabdomyolysis. Then you’re admitting them either for the sequelae of the fall that are not related to the intracranial hemorrhage, or the fact that they are so debilitated and deconditioned that they cannot take care of themselves. They need physical therapy. Often, we will have physical and occupational therapists come see them in the ED during business hours and help make an assessment of whether they are safe to go home or whether they fall again. That can give more evidence for the need for admission.
Dr. Glatter: To bring artificial intelligence into this discussion, algorithms that are out there that say, “Push a button and the patient’s safe for discharge.” Well, this argues for a clinical gestalt and a human being to make an assessment because you can use these predictive models, which are coming and they’re going to be here soon, and they already are in some sense. Again, we have to use clinical human judgment.
Dr. Shih: I agree.
Advice for Primary Care Physicians
Dr. Glatter: What return precautions do you discuss with patients who’ve had blunt head trauma that maybe had a head CT, or even didn’t? What are the main things we’re looking for?
Dr. Shenvi: What I usually tell people is if you start to have a worse headache, nausea or vomiting, any weakness in one area of your body, or vision changes, and if there’s a family member or friend there, I’ll say, “If you notice that they’re acting differently or seem confused, come back.”
Dr. Shih: I agree with what she said, and I’m also going to add one thing. The most important part is they are trying to prevent a subsequent fall. We know that when they’ve fallen and they present to the ED, they’re at even higher risk for falling and reinjuring themselves, and that’s a population that’s already at risk.
One of the secondary studies that we published out of this project was looking at follow-up with their primary care physicians, and there were two things that we wanted to address. The first was, how often did they do it? Then, when they did do it, did their primary care physicians try to address and prevent subsequent falls?
Both the answers are actually bad. Amazingly, just over like 60% followed up.
In some of our subsequent research, because we’re in the midst of a randomized, controlled trial where we do a home visit, when we initially see these individuals that have fallen, they’ll schedule a home visit for us. Then a week or two later, when we schedule the home visit, many of them cancel because they think, Oh, that was a one-off and it’s not going to happen again. Part of the problem is the patients, because many of them believe that they just slipped and fell and it’s not going to happen again, or they’re not prone to it.
The second issue was when patients did go to a primary care physician, we have found that some primary care physicians believe that falling and injuring themselves is just part of the normal aging process. A percentage of them don’t go over assessment for fall risk or even initiate fall prevention treatments or programs.
I try to take that time to tell them that this is very common in their age group, and believe it or not, a fall from standing is the way people really injure themselves, and there may be ways to prevent subsequent falls and injuries.
Dr. Glatter: Absolutely. Do you find that their medications are a contributor in some sense? Say they’re antihypertensive, have issues of orthostasis, or a new medication was added in the last week.
Dr. Shenvi: It’s all of the above. Sometimes it’s one thing, like they just started tamsulosin for their kidney stone, they stood up, they felt lightheaded, and they fell. Usually, it’s multifactorial with some changes in their gait, vision, balance, reflex time, and strength, plus the medications or the need for assistive devices. Maybe they can’t take care of their home as well as they used to and there are things on the floor. It’s really all of the above.
‘Harder to Unlearn Something Than to Learn It’
Dr. Glatter: Would either of you like to add any additional points to the discussion or add a few pearls?
Dr. Shenvi: This just highlights the challenge of how it’s harder to unlearn something than to learn it, where one study that maybe wasn’t quite looking at what we needed to, or practice and prescribing patterns have changed, so it’s no longer really relevant.
The things that we learned from that, or the fears that we instilled in our minds of, Uh oh, they could go home and have delayed bleeding, are much harder to unlearn, and it takes more studies to unlearn that idea than it did to actually put it into place.
I’m glad that your team has done this much larger, prospective study and hopefully will reduce the concern about this entity.
Dr. Shih: I appreciate that segue. It is amazing that, for paramedics and medical students, the first thing out of their mouth is, “Are they on an anticoagulant?”
In terms of the risk of developing an intracranial hemorrhage, I think it’s much less than the weight we’ve put on it before. However, I believe if they have a bleed, the bleeds are worse. It’s kind of a double-edged sword. It’s still an important factor, but it doesn’t come with the Oh my gosh, they’re on an anticoagulant that everybody thinks about.
No. 1 Cause of Traumatic Injury Is a Fall from Standing
Dr. Glatter: These are obviously ground-level falls in most patients and not motor vehicle crashes. That’s an important part in the population that you looked at that should be mentioned clearly.
Dr. Shih: It’s astonishing. I’ve been a program director for over 20 years, and geriatrics is not well taught in the curriculum. It’s astonishing for many of our trainees and emergency physicians in general that the number-one cause for traumatic injury is a fall from standing.
Certainly, we get patients coming in the trauma center like a 95-year-old person who’s on a ladder putting up his Christmas lights. I’m like, oh my God.
For the vast majority, it’s closer to 90%, but in our study, for the patients we looked at, it was 80% that fall from standing. That’s the mechanism that causes these bleeds and these major injuries.
Dr. Shenvi: That’s reflective of what we see, so it’s good that that’s what you looked at also.
Dr. Glatter: Absolutely. Well, thank you both. This has been a very informative discussion. I appreciate your time, and our readers will certainly benefit from your knowledge and expertise. Thank you again.
Dr. Glatter, assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, is a medical adviser for this news organization. He disclosed having no relevant financial conflicts. Dr. Shih is professor of emergency medicine at the Charles E. Schmidt College of Medicine at Florida Atlantic University, Boca Raton. His current grant funding and area of research interest involves geriatric emergency department patients with head injury and fall-related injury. He disclosed receiving a research grant from The Florida Medical Malpractice Joint Underwriting Association Grant for Safety of Health Care Services). Dr. Shenvi, associate professor of emergency medicine at the University of North Carolina at Chapel Hill, disclosed ties with the American College of Emergency Physicians, Institute for Healthcare Improvement, AstraZeneca, and CurvaFix.
A version of this article appeared on Medscape.com.
This discussion was recorded on August 2, 2024. This transcript has been edited for clarity.
Robert D. Glatter, MD: Today, we’ll be discussing the results of a new study published in The Journal of Emergency Medicine, looking at the incidence of delayed intracranial hemorrhage among older patients taking preinjury anticoagulants who present to the emergency department (ED) with blunt head trauma.
Joining me today is the lead author of the study, Dr. Richard Shih, professor of emergency medicine at Florida Atlantic University. Also joining me is Dr. Christina Shenvi, associate professor of emergency medicine at the University of North Carolina (UNC) Chapel Hill, with fellowship training in geriatric emergency medicine.
Welcome to both of you.
Richard D. Shih, MD: Thanks, Rob.
Christina L. Shenvi, MD, PhD, MBA: Thanks. Pleasure to be here.
ICH Study Methodology
Dr. Glatter: It’s a pleasure to have you. Rich, this is a great study and targeted toward a population we see daily in the emergency department. I want you to describe your methodology, patient selection, and how you went about organizing your study to look at this important finding of delayed intracranial hemorrhage, especially in those on anticoagulants.
Dr. Shih: This all started for our research team when we first read the 2012 Annals of Emergency Medicine paper. The first author was Vincenzo Menditto, and he looked at a group of patients that had minor head injury, were anticoagulated, and had negative initial head CTs.
There were about 100 patients, of which about 10 of them did not consent, but they hospitalized all these patients. These were anticoagulated, negative-first head CTs. They hospitalized the patients and then did a routine second CT at about 24 hours. They also followed them for a week, and it turned out a little over 7% of them had delayed head CT.
We were wondering how many delayed intracranial hemorrhages we had missed because current practice for us was that, if patients had a good physical exam, their head CT was normal, and everything looked good, we would send them home.
Because of that, a number of people across the country wanted to verify those findings from the Menditto study. We tried to design a good study to answer that question. We happen to have a very large geriatric population in Florida, and our ED census is very high for age over 65, at nearly 60%.
There are two Level I trauma centers in Palm Beach County. We included a second multicenter hospital, and we prospectively enrolled patients. We know the current state of practice is not to routinely do second CTs, so we followed these patients over time and followed their medical records to try to identify delayed bleeding. That’s how we set up our methodology.
Is It Safe to Discharge Patients With Trauma After 24 Hours?
Dr. Glatter: For the bulk of these patients with negative head CTs, it’s been my practice that when they’re stable and they look fine and there’s no other apparent, distracting painful trauma, injuries and so forth, they’re safe to discharge.
The secondary outcome in your study is interesting: the need for neurosurgical intervention in terms of those with delayed intracranial hemorrhage.
Dr. Shih: I do believe that it’s certainly not the problem that Menditto described, which is 7%. There are two other prospective studies that have looked at this issue with delayed bleeding on anticoagulants. Both of these also showed a relatively low rate of delayed bleeding, which is between like 0.2% and 1.0%. In our study, it was 0.4%.
The difference in the studies is that Menditto and colleagues routinely did 24-hour head CTs. They admitted everybody. For these other studies, routine head CT was not part of it. My bet is that there is a rate of delayed bleeding somewhere in between that seen in the Menditto study and that in all the other studies.
However, talking about significant intracranial hemorrhage, ones that perhaps need neurosurgery, I believe most of them are not significant. There’s some number that do occur, but the vast majority of those probably don’t need neurosurgery. We had 14 delayed bleeds out of 6000 patients with head trauma. One of them ended up requiring neurosurgery, so the answer is not zero, but I don’t think it’s 7% either.
Dr. Glatter: Dr. Shenvi, I want to bring you into the conversation to talk about your experience at UNC, and how you run things in terms of older patients with blunt head trauma on preinjury anticoagulants.
Dr. Shenvi: Thanks, Rob. I remember when this paper came out showing this 7% rate of delayed bleeding and the question was, “Should we be admitting all these people?” Partly just from an overwhelming need for capacity that that would bring, it just wasn’t practical to say, “We’re going to admit every patient with a negative head CT to the hospital and rescan them.” That would be hundreds or thousands of patients each year in any given facility.
The other thing is that delayed bleeds don’t always happen just in the first 24 hours. It’s not even a matter of bringing patients into observation for 24 hours, watching them, and rescanning them if they have symptoms. It can occur several days out. That never, in almost any institution that I know of, became standard practice.
The way that it did change my care was to give good return precautions to patients, to make sure they have somebody with them to say, “Hey, sometimes you can have bleeding several days out after a fall, even though your CT scan here today looks perfect,” and to alert them that if they start having severe headaches, vomiting, or other symptoms of intracranial hemorrhage, that they should come back.
I don’t think it ever became standard practice, and for good reason, because that was one study. The subsequent studies that Richard mentioned, pretty quickly on the heels of that initial one, showed a much lower rate of delayed ICH with the caveats that the methodology was different.
Shift in Anticoagulants
Dr. Shenvi: One other big change from that original study, and now to Richard’s study, is the shift in anticoagulants. Back in the initial study you mentioned, it was all warfarin. We know from other studies looking at warfarin vs the direct oral anticoagulants (DOACs) that DOACs have lower rates of ICH after a head injury, lower rates of need for neurosurgical intervention, and lower rates of discharge to a skilled nursing facility after an intracranial hemorrhage.
Across the board, we know that the DOACs tend to do better. It’s difficult to compare newer studies because it’s a different medication. It did inform my practice to have an awareness of delayed intracranial hemorrhage so that I warn patients more proactively.
Dr. Glatter: I haven’t seen a patient on warfarin in years. I don’t know if either of you have, but it’s all DOACs now unless there’s some other reason. That shift is quite apparent.
Dr. Shih: The problem with looking at delayed bleeding for DOACs vs warfarin is the numbers were so low. I think we had 13 people, and seven were in the no-anticoagulant group. The numbers are even lower, so it’s hard to say.
I just wanted to comment on something that Dr. Shenvi said, and I pretty much agree with everything that she said. Anticoagulants and warfarin, and that Menditto study, have a carryover effect. People group DOACs with warfarin similarly. When a patient is brought in, the first thing they talk about with head trauma is, “Oh, they’re on an anticoagulant” or “They’re not on an anticoagulant.” It’s so ingrained.
I believe that, in emergency medicine, we’re pressed for space and time and we’re not as affected by that 24-hour observation. Maybe many of our surgeons will automatically admit those patients.
I haven’t seen a guideline from the United States, but there are two international guidelines. One is from Austria from 2019, and one is from Scandinavia. Both recommended 24-hour observation if you’re on an anticoagulant.
There is a bit of controversy left over with that. Hopefully, as more and more of information, like in our study, comes out, people will be a little bit more clear about it. I don’t think there’s a need to routinely admit them.
I do want to mention that the Menditto study had such a massive impact on everybody. They pointed out one subgroup (and it’s such a small number of patients). They had seven cases of delayed bleeding; four or five of them were within that 24 hours, and a couple were diagnosed later over the next couple days.
Of those seven people, four of them had international normalized ratios (INRs) greater than 3. Of those four patients, I’ve heard people talk about this and recommend, “Okay, that’s the subgroup I would admit.” There’s a toss-up with what to do with DOAC because it’s very hard to tell whether there’s an issue, whether there are problems with their dosing, and whatever.
We actually recently looked at that. We have a much larger sample than four: close to 300 patients who were on warfarin. We looked at patients who had INRs below 3 and above 3, and we didn’t show a difference. We still don’t believe that warfarin is a big issue with delayed bleeding.
Should We Be Asking: ‘Are They on Blood Thinners?’
Dr. Shenvi: One of the interesting trends related to warfarin and the DOACs vs no anticoagulant is that as you mentioned, Dr Shih, the first question out of people’s mouths or the first piece of information emergency medical services gives you when they come in with a patient who’s had a head injury is, “Are they on blood thinners or not?”
Yet, the paradigm is shifting to say it’s not actually the blood thinners themselves that are giving older patients the higher risk for bleeding; it’s age and other comorbidities.
Certainly, if you’re on an anticoagulant and you start to bleed, your prognosis is much worse because the bleeding doesn’t stop. In terms of who has a bleeding event, there’s much less impact of anticoagulation than we used to think. That, in part, may be due to the change from warfarin to other medications.
Some of the experts I’ve talked to who have done the research on this have said, “Well, actually, warfarin was more of a marker for being much older and more frail, because it was primarily prescribed to older patients who have significant heart disease, atrial fibrillation, and so on.” It was more a marker for somebody who is at risk for an intracranial hemorrhage. There are many changes that have happened in the past 10 years with medications and also our understanding.
Challenges in Patient Follow-up
Dr. Glatter: That’s a great point. One thing, Rich, I want to ask you about is in terms of your proxy outcome assessment. When you use that at 14 and 60 days with telephone follow-up and then chart review at 60 and 90 days (because, obviously, everyone can’t get another head CT or it’s difficult to follow patients up), did you find that worked out well in your prospective cohort study, in terms of using that as a proxy, so to speak?
Dr. Shih: I would say to a certain extent. Unfortunately, we don’t have access to the patients to come back to follow up all of them, and there was obviously a large number of patients in our study.
The next best thing was that we had dedicated research assistants calling all of the patients at 14 days and 60 days. I’ve certainly read research studies where, when they call them, they get 80%-90% follow-up, but we did not achieve that.
I don’t know if people are more inundated with spam phone calls now, or the older people are just afraid of picking up their phone sometimes with all the scams and so forth. I totally understand, but in all honesty, we only had about a 30%-35% follow-up using that follow-up pathway.
Then the proxy pathway was to look at their charts at 60 and 90 days. Also, we looked at the Florida death registry, which is pretty good, and then finally, we had both Level I trauma centers in the county that we were in participating. It’s standard practice that if you have an intracranial hemorrhage at a non–Level I trauma center, you would be transferred to a Level I trauma center. That’s the protocol. I know that’s not followed 100% of the time, but that’s part of the proxy follow-up. You could criticize the study for not having closer to 90% actual contact, but that’s the best we could do.
Dr. Glatter: I think that’s admirable. Using that paradigm of what you described certainly allows the reader to understand the difficulty in assessing patients that don’t get follow-up head CT, and hardly anyone does that, as we know.
To your point of having both Level I trauma centers in the county, that makes it pretty secure. If we’re going to do a study encompassing a similar type of regional aspect, it would be similar.
Dr. Shenvi: I think your proxies, to your credit, were as good as you can get. You can never get a 100% follow-up, but you really looked at all the different avenues by which patients might present, either in the death registry or a Level I center. Well done on that aspect.
Determining When to Admit Patients for Observation
Dr. Glatter: In terms of admissions: You admit a patient, then you hear back that this patient should not have been admitted because they had a negative head CT, but you put them in anyway in the sense of delayed bleeding happening or not happening.
It’s interesting. Maybe the insurers will start looking at this in some capacity, based on your study, that because it’s so infrequent that you see delayed bleeding, that admitting someone for any reason whatsoever would be declined. Do you see that being an issue? In other words, [do you see] this leading to a pattern in terms of the payers?
Dr. Shih: Certainly, you could interpret it that way, and that would be unfortunate. The [incidence of] delayed bleeding is definitely not zero. That’s the first thing.
The second thing is that when you’re dealing with an older population, having some sense that they’re not doing well is an important contributor to trying to fully assess what’s going on — whether or not they have a bleed or whether they’re at risk for falling again and then hitting their head and causing a second bleed, and making sure they can do the activities of daily life. There really should be some room for a physician to say, “They just got here, and we don’t know him that well. There’s something that bothers me about this person” and have the ability to watch them for at least another 24 hours. That’s how I feel.
Dr. Shenvi: In my location, it would be difficult to try to admit somebody purely for observation for delayed bleeding. I think we would get a lot of pushback on that. The reasons I might admit a patient after a fall with a negative head CT, though, are all the things that, Rob, you alluded to earlier — which are, what made them fall in the first place and were they unable to get up?
I had this happen just this week. A patient who fell couldn’t get off the ground for 12 hours, and so now she’s dehydrated and delirious with slight rhabdomyolysis. Then you’re admitting them either for the sequelae of the fall that are not related to the intracranial hemorrhage, or the fact that they are so debilitated and deconditioned that they cannot take care of themselves. They need physical therapy. Often, we will have physical and occupational therapists come see them in the ED during business hours and help make an assessment of whether they are safe to go home or whether they fall again. That can give more evidence for the need for admission.
Dr. Glatter: To bring artificial intelligence into this discussion, algorithms that are out there that say, “Push a button and the patient’s safe for discharge.” Well, this argues for a clinical gestalt and a human being to make an assessment because you can use these predictive models, which are coming and they’re going to be here soon, and they already are in some sense. Again, we have to use clinical human judgment.
Dr. Shih: I agree.
Advice for Primary Care Physicians
Dr. Glatter: What return precautions do you discuss with patients who’ve had blunt head trauma that maybe had a head CT, or even didn’t? What are the main things we’re looking for?
Dr. Shenvi: What I usually tell people is if you start to have a worse headache, nausea or vomiting, any weakness in one area of your body, or vision changes, and if there’s a family member or friend there, I’ll say, “If you notice that they’re acting differently or seem confused, come back.”
Dr. Shih: I agree with what she said, and I’m also going to add one thing. The most important part is they are trying to prevent a subsequent fall. We know that when they’ve fallen and they present to the ED, they’re at even higher risk for falling and reinjuring themselves, and that’s a population that’s already at risk.
One of the secondary studies that we published out of this project was looking at follow-up with their primary care physicians, and there were two things that we wanted to address. The first was, how often did they do it? Then, when they did do it, did their primary care physicians try to address and prevent subsequent falls?
Both the answers are actually bad. Amazingly, just over like 60% followed up.
In some of our subsequent research, because we’re in the midst of a randomized, controlled trial where we do a home visit, when we initially see these individuals that have fallen, they’ll schedule a home visit for us. Then a week or two later, when we schedule the home visit, many of them cancel because they think, Oh, that was a one-off and it’s not going to happen again. Part of the problem is the patients, because many of them believe that they just slipped and fell and it’s not going to happen again, or they’re not prone to it.
The second issue was when patients did go to a primary care physician, we have found that some primary care physicians believe that falling and injuring themselves is just part of the normal aging process. A percentage of them don’t go over assessment for fall risk or even initiate fall prevention treatments or programs.
I try to take that time to tell them that this is very common in their age group, and believe it or not, a fall from standing is the way people really injure themselves, and there may be ways to prevent subsequent falls and injuries.
Dr. Glatter: Absolutely. Do you find that their medications are a contributor in some sense? Say they’re antihypertensive, have issues of orthostasis, or a new medication was added in the last week.
Dr. Shenvi: It’s all of the above. Sometimes it’s one thing, like they just started tamsulosin for their kidney stone, they stood up, they felt lightheaded, and they fell. Usually, it’s multifactorial with some changes in their gait, vision, balance, reflex time, and strength, plus the medications or the need for assistive devices. Maybe they can’t take care of their home as well as they used to and there are things on the floor. It’s really all of the above.
‘Harder to Unlearn Something Than to Learn It’
Dr. Glatter: Would either of you like to add any additional points to the discussion or add a few pearls?
Dr. Shenvi: This just highlights the challenge of how it’s harder to unlearn something than to learn it, where one study that maybe wasn’t quite looking at what we needed to, or practice and prescribing patterns have changed, so it’s no longer really relevant.
The things that we learned from that, or the fears that we instilled in our minds of, Uh oh, they could go home and have delayed bleeding, are much harder to unlearn, and it takes more studies to unlearn that idea than it did to actually put it into place.
I’m glad that your team has done this much larger, prospective study and hopefully will reduce the concern about this entity.
Dr. Shih: I appreciate that segue. It is amazing that, for paramedics and medical students, the first thing out of their mouth is, “Are they on an anticoagulant?”
In terms of the risk of developing an intracranial hemorrhage, I think it’s much less than the weight we’ve put on it before. However, I believe if they have a bleed, the bleeds are worse. It’s kind of a double-edged sword. It’s still an important factor, but it doesn’t come with the Oh my gosh, they’re on an anticoagulant that everybody thinks about.
No. 1 Cause of Traumatic Injury Is a Fall from Standing
Dr. Glatter: These are obviously ground-level falls in most patients and not motor vehicle crashes. That’s an important part in the population that you looked at that should be mentioned clearly.
Dr. Shih: It’s astonishing. I’ve been a program director for over 20 years, and geriatrics is not well taught in the curriculum. It’s astonishing for many of our trainees and emergency physicians in general that the number-one cause for traumatic injury is a fall from standing.
Certainly, we get patients coming in the trauma center like a 95-year-old person who’s on a ladder putting up his Christmas lights. I’m like, oh my God.
For the vast majority, it’s closer to 90%, but in our study, for the patients we looked at, it was 80% that fall from standing. That’s the mechanism that causes these bleeds and these major injuries.
Dr. Shenvi: That’s reflective of what we see, so it’s good that that’s what you looked at also.
Dr. Glatter: Absolutely. Well, thank you both. This has been a very informative discussion. I appreciate your time, and our readers will certainly benefit from your knowledge and expertise. Thank you again.
Dr. Glatter, assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, is a medical adviser for this news organization. He disclosed having no relevant financial conflicts. Dr. Shih is professor of emergency medicine at the Charles E. Schmidt College of Medicine at Florida Atlantic University, Boca Raton. His current grant funding and area of research interest involves geriatric emergency department patients with head injury and fall-related injury. He disclosed receiving a research grant from The Florida Medical Malpractice Joint Underwriting Association Grant for Safety of Health Care Services). Dr. Shenvi, associate professor of emergency medicine at the University of North Carolina at Chapel Hill, disclosed ties with the American College of Emergency Physicians, Institute for Healthcare Improvement, AstraZeneca, and CurvaFix.
A version of this article appeared on Medscape.com.
Beyond One-Size-Fits-All: Precision Psychiatry Is Here
The field of psychiatry is experiencing a transformative shift toward precision medicine, a paradigm that tailors treatment to the unique characteristics of individual patients. This approach echoes advances in fields like oncology and cardiology, where precision tools have already revolutionized patient care.
But what exactly is precision psychiatry? How does it differ from traditional psychiatry? What will it look like in clinical practice? And are we there yet?
Beyond One-Size-Fits-All
The prevailing “one-size-fits-all” approach in psychiatry, which relies heavily on subjective symptom reporting, often proves ineffective due to the broad heterogeneity of diagnostic categories. This can lead to a “trial-and-error” cycle in treatment, which is time-consuming, costly, and frustrating for both doctors and patients.
In contrast, precision psychiatry has the potential to identify subtypes of psychiatric disorders and tailor treatments using measurable, objective data.
“The data supporting the use of precision psychiatry are very promising, particularly for treatment-resistant depression,” Leanne Williams, PhD, professor in the Department of Psychiatry and Behavioral Sciences at Stanford University, Stanford, and director of the Stanford Center for Precision Mental Health and Wellness, Palo Alto, California, said in an interview with this news organization.
Using functional MRI (fMRI), Dr. Williams and her team have mapped and measured patients’ brain circuitry to identify eight “biotypes” of depression that reflect combinations of dysfunction in six different circuits of the brain.
They are using these biotypes to guide treatment decisions in the clinic, matching individual patients to more targeted and effective therapies.
“We’re offering functional MRI to directly assess brain function along with other measures, so precision psychiatry is happening, and it’s really wanted by patients and their families. And the data suggest that we can double the rate of good outcomes,” said Dr. Williams.
“Neuroimaging techniques, particularly fMRI, have revolutionized our ability to map and quantify circuit abnormalities. Neural circuit measurements potentially offer the most direct window into the neural bases of psychiatric symptoms and, crucially, their modulation by treatment,” Teddy Akiki, MD, clinical scholar, Department of Psychiatry and Behavioral Sciences at Stanford, California, who works with Dr. Williams, told this news organization.
Blood-based biomarkers can complement brain imaging by providing additional information to better target treatment, help predict side effects, and guide dosage adjustments.
Precision Tools
A team led by Alexander B. Niculescu, III, MD, PhD, has found that a panel of blood-based biomarkers can distinguish between depression and bipolar disorder, predict a person’s future risk for these disorders, and inform more tailored medication choices.
Dr. Niculescu is currently a professor of psychiatry and medical neuroscience at the Indiana University School of Medicine, Indianapolis. He will head west in September to direct the newly created Center for Precision Psychiatry at the University of Arizona College of Medicine–Phoenix.
MindX Sciences, the start-up company Dr. Niculescu cofounded, has been providing blood biomarker reports to “early adopting” doctors and patients.
“We are in the process of collecting and writing up the outcome data on the first 100 cases. The feedback we have received so far from the doctors and patients who have used it, as well as biopharma companies who have used it, has been very positive,” Dr. Niculescu told this news organization.
Another benefit of precision psychiatry lies in its potential to significantly accelerate drug development.
“By identifying specific neural circuits involved in subtypes of psychiatric conditions, we can repurpose or develop drugs that target these circuits more precisely. This approach allows for smaller, more focused trials with potentially higher success rates, which could speed up the typically slow and costly process of psychiatric drug development,” said Dr. Akiki.
Dr. Niculescu agreed. With precision psychiatry tools, “psychiatric drug development will become faster, cheaper, and more successful with the use of biomarkers and other precision tools,” he said.
The Future Is Already Here
The implementation and widespread adoption of precision psychiatry have several challenges.
It requires sophisticated technology and expertise, which may not be readily available in all clinical settings. Moreover, while evidence supports its use in conditions like major depression, there are fewer data on its efficacy in other psychiatric disorders, like schizophrenia.
Dr. Williams said future research should focus on expanding the evidence base for precision psychiatry across a broader range of psychiatric conditions.
Efforts to make precision tools more accessible and scalable, such as developing portable imaging technologies or more readily available biomarker tests, are also critical.
Integrating these precision tools into routine psychiatric practice will also require training and education for clinicians, as well as cost-effective solutions to make these approaches widely available.
“Mental health clinicians throughout the country are starting to employ semi-objective and objective measures in their practices, particularly self-report symptom questionnaires and pharmacogenomic assessment,” Laura Hack, MD, PhD, assistant professor, Department of Psychiatry and Behavioral Sciences, Stanford University, told this news organization.
“For precision psychiatry measures to be widely implemented, it is essential to demonstrate their reliability, clinical validity, clinical utility, and cost-effectiveness. Additionally, there is a need to develop clinical guidelines for their use, ensure that measurement tools are accessible, and educate all relevant stakeholders,” said Dr. Hack.
Right now, functional neuroimaging is used “only on a very limited basis in current clinical psychiatric practice,” Dr. Hack noted.
“We are developing standardized systems that will require less specialized expertise in functional neuroimaging and can be readily integrated into routine clinical care,” Dr. Akiki added.
Quoting William Gibson, “The future [of precision psychiatry] is already here; it’s just not evenly distributed,” said Dr. Niculescu.
Dr. Williams has disclosed relationships with One Mind PsyberGuide, Laureate Institute for Brain Research, and Et Cere Inc. Dr. Niculescu is a cofounder of MindX Sciences and is listed as inventor on a patent application filed by Indiana University. Dr. Akiki and Dr. Hack had no relevant disclosures.
A version of this article first appeared on Medscape.com.
The field of psychiatry is experiencing a transformative shift toward precision medicine, a paradigm that tailors treatment to the unique characteristics of individual patients. This approach echoes advances in fields like oncology and cardiology, where precision tools have already revolutionized patient care.
But what exactly is precision psychiatry? How does it differ from traditional psychiatry? What will it look like in clinical practice? And are we there yet?
Beyond One-Size-Fits-All
The prevailing “one-size-fits-all” approach in psychiatry, which relies heavily on subjective symptom reporting, often proves ineffective due to the broad heterogeneity of diagnostic categories. This can lead to a “trial-and-error” cycle in treatment, which is time-consuming, costly, and frustrating for both doctors and patients.
In contrast, precision psychiatry has the potential to identify subtypes of psychiatric disorders and tailor treatments using measurable, objective data.
“The data supporting the use of precision psychiatry are very promising, particularly for treatment-resistant depression,” Leanne Williams, PhD, professor in the Department of Psychiatry and Behavioral Sciences at Stanford University, Stanford, and director of the Stanford Center for Precision Mental Health and Wellness, Palo Alto, California, said in an interview with this news organization.
Using functional MRI (fMRI), Dr. Williams and her team have mapped and measured patients’ brain circuitry to identify eight “biotypes” of depression that reflect combinations of dysfunction in six different circuits of the brain.
They are using these biotypes to guide treatment decisions in the clinic, matching individual patients to more targeted and effective therapies.
“We’re offering functional MRI to directly assess brain function along with other measures, so precision psychiatry is happening, and it’s really wanted by patients and their families. And the data suggest that we can double the rate of good outcomes,” said Dr. Williams.
“Neuroimaging techniques, particularly fMRI, have revolutionized our ability to map and quantify circuit abnormalities. Neural circuit measurements potentially offer the most direct window into the neural bases of psychiatric symptoms and, crucially, their modulation by treatment,” Teddy Akiki, MD, clinical scholar, Department of Psychiatry and Behavioral Sciences at Stanford, California, who works with Dr. Williams, told this news organization.
Blood-based biomarkers can complement brain imaging by providing additional information to better target treatment, help predict side effects, and guide dosage adjustments.
Precision Tools
A team led by Alexander B. Niculescu, III, MD, PhD, has found that a panel of blood-based biomarkers can distinguish between depression and bipolar disorder, predict a person’s future risk for these disorders, and inform more tailored medication choices.
Dr. Niculescu is currently a professor of psychiatry and medical neuroscience at the Indiana University School of Medicine, Indianapolis. He will head west in September to direct the newly created Center for Precision Psychiatry at the University of Arizona College of Medicine–Phoenix.
MindX Sciences, the start-up company Dr. Niculescu cofounded, has been providing blood biomarker reports to “early adopting” doctors and patients.
“We are in the process of collecting and writing up the outcome data on the first 100 cases. The feedback we have received so far from the doctors and patients who have used it, as well as biopharma companies who have used it, has been very positive,” Dr. Niculescu told this news organization.
Another benefit of precision psychiatry lies in its potential to significantly accelerate drug development.
“By identifying specific neural circuits involved in subtypes of psychiatric conditions, we can repurpose or develop drugs that target these circuits more precisely. This approach allows for smaller, more focused trials with potentially higher success rates, which could speed up the typically slow and costly process of psychiatric drug development,” said Dr. Akiki.
Dr. Niculescu agreed. With precision psychiatry tools, “psychiatric drug development will become faster, cheaper, and more successful with the use of biomarkers and other precision tools,” he said.
The Future Is Already Here
The implementation and widespread adoption of precision psychiatry have several challenges.
It requires sophisticated technology and expertise, which may not be readily available in all clinical settings. Moreover, while evidence supports its use in conditions like major depression, there are fewer data on its efficacy in other psychiatric disorders, like schizophrenia.
Dr. Williams said future research should focus on expanding the evidence base for precision psychiatry across a broader range of psychiatric conditions.
Efforts to make precision tools more accessible and scalable, such as developing portable imaging technologies or more readily available biomarker tests, are also critical.
Integrating these precision tools into routine psychiatric practice will also require training and education for clinicians, as well as cost-effective solutions to make these approaches widely available.
“Mental health clinicians throughout the country are starting to employ semi-objective and objective measures in their practices, particularly self-report symptom questionnaires and pharmacogenomic assessment,” Laura Hack, MD, PhD, assistant professor, Department of Psychiatry and Behavioral Sciences, Stanford University, told this news organization.
“For precision psychiatry measures to be widely implemented, it is essential to demonstrate their reliability, clinical validity, clinical utility, and cost-effectiveness. Additionally, there is a need to develop clinical guidelines for their use, ensure that measurement tools are accessible, and educate all relevant stakeholders,” said Dr. Hack.
Right now, functional neuroimaging is used “only on a very limited basis in current clinical psychiatric practice,” Dr. Hack noted.
“We are developing standardized systems that will require less specialized expertise in functional neuroimaging and can be readily integrated into routine clinical care,” Dr. Akiki added.
Quoting William Gibson, “The future [of precision psychiatry] is already here; it’s just not evenly distributed,” said Dr. Niculescu.
Dr. Williams has disclosed relationships with One Mind PsyberGuide, Laureate Institute for Brain Research, and Et Cere Inc. Dr. Niculescu is a cofounder of MindX Sciences and is listed as inventor on a patent application filed by Indiana University. Dr. Akiki and Dr. Hack had no relevant disclosures.
A version of this article first appeared on Medscape.com.
The field of psychiatry is experiencing a transformative shift toward precision medicine, a paradigm that tailors treatment to the unique characteristics of individual patients. This approach echoes advances in fields like oncology and cardiology, where precision tools have already revolutionized patient care.
But what exactly is precision psychiatry? How does it differ from traditional psychiatry? What will it look like in clinical practice? And are we there yet?
Beyond One-Size-Fits-All
The prevailing “one-size-fits-all” approach in psychiatry, which relies heavily on subjective symptom reporting, often proves ineffective due to the broad heterogeneity of diagnostic categories. This can lead to a “trial-and-error” cycle in treatment, which is time-consuming, costly, and frustrating for both doctors and patients.
In contrast, precision psychiatry has the potential to identify subtypes of psychiatric disorders and tailor treatments using measurable, objective data.
“The data supporting the use of precision psychiatry are very promising, particularly for treatment-resistant depression,” Leanne Williams, PhD, professor in the Department of Psychiatry and Behavioral Sciences at Stanford University, Stanford, and director of the Stanford Center for Precision Mental Health and Wellness, Palo Alto, California, said in an interview with this news organization.
Using functional MRI (fMRI), Dr. Williams and her team have mapped and measured patients’ brain circuitry to identify eight “biotypes” of depression that reflect combinations of dysfunction in six different circuits of the brain.
They are using these biotypes to guide treatment decisions in the clinic, matching individual patients to more targeted and effective therapies.
“We’re offering functional MRI to directly assess brain function along with other measures, so precision psychiatry is happening, and it’s really wanted by patients and their families. And the data suggest that we can double the rate of good outcomes,” said Dr. Williams.
“Neuroimaging techniques, particularly fMRI, have revolutionized our ability to map and quantify circuit abnormalities. Neural circuit measurements potentially offer the most direct window into the neural bases of psychiatric symptoms and, crucially, their modulation by treatment,” Teddy Akiki, MD, clinical scholar, Department of Psychiatry and Behavioral Sciences at Stanford, California, who works with Dr. Williams, told this news organization.
Blood-based biomarkers can complement brain imaging by providing additional information to better target treatment, help predict side effects, and guide dosage adjustments.
Precision Tools
A team led by Alexander B. Niculescu, III, MD, PhD, has found that a panel of blood-based biomarkers can distinguish between depression and bipolar disorder, predict a person’s future risk for these disorders, and inform more tailored medication choices.
Dr. Niculescu is currently a professor of psychiatry and medical neuroscience at the Indiana University School of Medicine, Indianapolis. He will head west in September to direct the newly created Center for Precision Psychiatry at the University of Arizona College of Medicine–Phoenix.
MindX Sciences, the start-up company Dr. Niculescu cofounded, has been providing blood biomarker reports to “early adopting” doctors and patients.
“We are in the process of collecting and writing up the outcome data on the first 100 cases. The feedback we have received so far from the doctors and patients who have used it, as well as biopharma companies who have used it, has been very positive,” Dr. Niculescu told this news organization.
Another benefit of precision psychiatry lies in its potential to significantly accelerate drug development.
“By identifying specific neural circuits involved in subtypes of psychiatric conditions, we can repurpose or develop drugs that target these circuits more precisely. This approach allows for smaller, more focused trials with potentially higher success rates, which could speed up the typically slow and costly process of psychiatric drug development,” said Dr. Akiki.
Dr. Niculescu agreed. With precision psychiatry tools, “psychiatric drug development will become faster, cheaper, and more successful with the use of biomarkers and other precision tools,” he said.
The Future Is Already Here
The implementation and widespread adoption of precision psychiatry have several challenges.
It requires sophisticated technology and expertise, which may not be readily available in all clinical settings. Moreover, while evidence supports its use in conditions like major depression, there are fewer data on its efficacy in other psychiatric disorders, like schizophrenia.
Dr. Williams said future research should focus on expanding the evidence base for precision psychiatry across a broader range of psychiatric conditions.
Efforts to make precision tools more accessible and scalable, such as developing portable imaging technologies or more readily available biomarker tests, are also critical.
Integrating these precision tools into routine psychiatric practice will also require training and education for clinicians, as well as cost-effective solutions to make these approaches widely available.
“Mental health clinicians throughout the country are starting to employ semi-objective and objective measures in their practices, particularly self-report symptom questionnaires and pharmacogenomic assessment,” Laura Hack, MD, PhD, assistant professor, Department of Psychiatry and Behavioral Sciences, Stanford University, told this news organization.
“For precision psychiatry measures to be widely implemented, it is essential to demonstrate their reliability, clinical validity, clinical utility, and cost-effectiveness. Additionally, there is a need to develop clinical guidelines for their use, ensure that measurement tools are accessible, and educate all relevant stakeholders,” said Dr. Hack.
Right now, functional neuroimaging is used “only on a very limited basis in current clinical psychiatric practice,” Dr. Hack noted.
“We are developing standardized systems that will require less specialized expertise in functional neuroimaging and can be readily integrated into routine clinical care,” Dr. Akiki added.
Quoting William Gibson, “The future [of precision psychiatry] is already here; it’s just not evenly distributed,” said Dr. Niculescu.
Dr. Williams has disclosed relationships with One Mind PsyberGuide, Laureate Institute for Brain Research, and Et Cere Inc. Dr. Niculescu is a cofounder of MindX Sciences and is listed as inventor on a patent application filed by Indiana University. Dr. Akiki and Dr. Hack had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Brain Structure Differs in Youth With Conduct Disorder
Youth with conduct disorder (CD) have extensive brain structure differences, new research showed.
“We know very little about this disorder even though it can carry a high burden for families and societies,” co–lead author Yidian Gao, PhD, of the University of Birmingham, Birmingham, England, said in a press release.
“The sample included in our study is 10-20 times larger than previous studies and contains data on children from North America, Europe, and Asia. It provides the most compelling evidence to date that CD is associated with widespread structural brain differences,” he added.
The findings were published online in The Lancet Psychiatry.
An Understudied Disorder
In the largest study of its kind, researchers at the Universities of Bath and Birmingham, both in England, collaborated with research teams across Europe, North America, and Asia, as part of the Enhancing NeuroImaging Genetics through Meta-Analysis–Antisocial Behavior Working Group to learn more about one of the “least researched psychiatric disorders,” they wrote.
The investigators used MRI to examine the brain structure of 1185 children with a clinical diagnosis of CD and 1253 typically developing children from 17-21 across 15 international study cohorts.
After adjusting for total intracranial volume investigators found that youth with CD (29% women; mean age, 13.7 years) had lower total surface area and lower regional surface area in 26 of the 34 cortical regions, spanning all four lobes of the brain, compared with their typically developing counterparts (35.6% women; mean age, 13.5 years).
Youth with CD also showed greater cortical thickness in the caudal anterior cingulate cortex (P = .0001) and lower cortical thickness in the banks of the superior temporal sulcus vs those without CD (P = .0010).
In addition, the CD group also had lower volume in the thalamus (P = .0009), amygdala (P = .0014), hippocampus (P = .0031), and nucleus accumbens (P = .0052).
Most findings remained significant after adjusting for intelligence quotient, psychiatric comorbidities, and psychotropic medication use. Of note, group difference in cortical thickness, 22 of 27 differences in surface area. In addition, three of four subcortical differences remained robust after adjusting for co-occurring attention-deficit/hyperactivity disorder, the most frequent comorbidity.
When the investigators divided individuals with CD into two subgroups — those with high vs low levels of callous-unemotional traits — they found limited overall differences. However, those with high callous-unemotional traits had lower surface area in the superior temporal and superior frontal gyri vs those with low callous-unemotional traits and the typically developing group.
Investigators also found that individuals with childhood-onset CD had greater cortical thickness in the caudal anterior cingulate cortex compared with those with adolescent-onset CD.
Study limitations include comparison of different cohorts with differing protocols that could affect the validity of the findings. In addition, subgroup samples were small and had lower statistical power.
“Our finding of robust brain alterations in conduct disorder — similar to those in more widely recognized and widely treated disorders such as ADHD — emphasize the need for a greater focus on conduct disorder in research, treatment, and public policy,” the authors noted.
Seven study authors reported conflicts of interest with various pharmaceutical companies and other organizations.
A version of this article first appeared on Medscape.com.
Youth with conduct disorder (CD) have extensive brain structure differences, new research showed.
“We know very little about this disorder even though it can carry a high burden for families and societies,” co–lead author Yidian Gao, PhD, of the University of Birmingham, Birmingham, England, said in a press release.
“The sample included in our study is 10-20 times larger than previous studies and contains data on children from North America, Europe, and Asia. It provides the most compelling evidence to date that CD is associated with widespread structural brain differences,” he added.
The findings were published online in The Lancet Psychiatry.
An Understudied Disorder
In the largest study of its kind, researchers at the Universities of Bath and Birmingham, both in England, collaborated with research teams across Europe, North America, and Asia, as part of the Enhancing NeuroImaging Genetics through Meta-Analysis–Antisocial Behavior Working Group to learn more about one of the “least researched psychiatric disorders,” they wrote.
The investigators used MRI to examine the brain structure of 1185 children with a clinical diagnosis of CD and 1253 typically developing children from 17-21 across 15 international study cohorts.
After adjusting for total intracranial volume investigators found that youth with CD (29% women; mean age, 13.7 years) had lower total surface area and lower regional surface area in 26 of the 34 cortical regions, spanning all four lobes of the brain, compared with their typically developing counterparts (35.6% women; mean age, 13.5 years).
Youth with CD also showed greater cortical thickness in the caudal anterior cingulate cortex (P = .0001) and lower cortical thickness in the banks of the superior temporal sulcus vs those without CD (P = .0010).
In addition, the CD group also had lower volume in the thalamus (P = .0009), amygdala (P = .0014), hippocampus (P = .0031), and nucleus accumbens (P = .0052).
Most findings remained significant after adjusting for intelligence quotient, psychiatric comorbidities, and psychotropic medication use. Of note, group difference in cortical thickness, 22 of 27 differences in surface area. In addition, three of four subcortical differences remained robust after adjusting for co-occurring attention-deficit/hyperactivity disorder, the most frequent comorbidity.
When the investigators divided individuals with CD into two subgroups — those with high vs low levels of callous-unemotional traits — they found limited overall differences. However, those with high callous-unemotional traits had lower surface area in the superior temporal and superior frontal gyri vs those with low callous-unemotional traits and the typically developing group.
Investigators also found that individuals with childhood-onset CD had greater cortical thickness in the caudal anterior cingulate cortex compared with those with adolescent-onset CD.
Study limitations include comparison of different cohorts with differing protocols that could affect the validity of the findings. In addition, subgroup samples were small and had lower statistical power.
“Our finding of robust brain alterations in conduct disorder — similar to those in more widely recognized and widely treated disorders such as ADHD — emphasize the need for a greater focus on conduct disorder in research, treatment, and public policy,” the authors noted.
Seven study authors reported conflicts of interest with various pharmaceutical companies and other organizations.
A version of this article first appeared on Medscape.com.
Youth with conduct disorder (CD) have extensive brain structure differences, new research showed.
“We know very little about this disorder even though it can carry a high burden for families and societies,” co–lead author Yidian Gao, PhD, of the University of Birmingham, Birmingham, England, said in a press release.
“The sample included in our study is 10-20 times larger than previous studies and contains data on children from North America, Europe, and Asia. It provides the most compelling evidence to date that CD is associated with widespread structural brain differences,” he added.
The findings were published online in The Lancet Psychiatry.
An Understudied Disorder
In the largest study of its kind, researchers at the Universities of Bath and Birmingham, both in England, collaborated with research teams across Europe, North America, and Asia, as part of the Enhancing NeuroImaging Genetics through Meta-Analysis–Antisocial Behavior Working Group to learn more about one of the “least researched psychiatric disorders,” they wrote.
The investigators used MRI to examine the brain structure of 1185 children with a clinical diagnosis of CD and 1253 typically developing children from 17-21 across 15 international study cohorts.
After adjusting for total intracranial volume investigators found that youth with CD (29% women; mean age, 13.7 years) had lower total surface area and lower regional surface area in 26 of the 34 cortical regions, spanning all four lobes of the brain, compared with their typically developing counterparts (35.6% women; mean age, 13.5 years).
Youth with CD also showed greater cortical thickness in the caudal anterior cingulate cortex (P = .0001) and lower cortical thickness in the banks of the superior temporal sulcus vs those without CD (P = .0010).
In addition, the CD group also had lower volume in the thalamus (P = .0009), amygdala (P = .0014), hippocampus (P = .0031), and nucleus accumbens (P = .0052).
Most findings remained significant after adjusting for intelligence quotient, psychiatric comorbidities, and psychotropic medication use. Of note, group difference in cortical thickness, 22 of 27 differences in surface area. In addition, three of four subcortical differences remained robust after adjusting for co-occurring attention-deficit/hyperactivity disorder, the most frequent comorbidity.
When the investigators divided individuals with CD into two subgroups — those with high vs low levels of callous-unemotional traits — they found limited overall differences. However, those with high callous-unemotional traits had lower surface area in the superior temporal and superior frontal gyri vs those with low callous-unemotional traits and the typically developing group.
Investigators also found that individuals with childhood-onset CD had greater cortical thickness in the caudal anterior cingulate cortex compared with those with adolescent-onset CD.
Study limitations include comparison of different cohorts with differing protocols that could affect the validity of the findings. In addition, subgroup samples were small and had lower statistical power.
“Our finding of robust brain alterations in conduct disorder — similar to those in more widely recognized and widely treated disorders such as ADHD — emphasize the need for a greater focus on conduct disorder in research, treatment, and public policy,” the authors noted.
Seven study authors reported conflicts of interest with various pharmaceutical companies and other organizations.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PSYCHIATRY
‘Big Breakthrough’: New Low-Field MRI Is Safer and Easier
For years, researchers and medical companies have explored low-field MRI systems (those with a magnetic field strength of less than 1 T) — searching for a feasible alternative to the loud, expensive machines requiring special rooms with shielding to block their powerful magnetic field.
Most low-field scanners in development are for brain scans only. In 2022, the US Food and Drug Administration (FDA) cleared the first portable MRI system — Hyperfine’s Swoop, designed for use at a patient’s bedside — for head and brain scans. But the technology has not been applied to whole-body MRI — until now.
In a new study published in Science, researchers from Hong Kong described a whole-body, ultra low–field MRI.
The device uses a 0.05 T magnet — one sixtieth the magnetic field strength of the standard 3 T MRI model common in hospitals today, said lead author Ed Wu, PhD, professor of biomedical engineering at The University of Hong Kong.
Because the field strength is so low, no protective shielding is needed. Patients and bystanders can safely use smart phones . And the scanner is safe for patients with implanted devices, like a cochlear implant or pacemaker, or any metal on their body or clothes. No hearing protection is required, either, because the machine is so quiet.
If all goes well, the technology could be commercially available in as little as a few years, Dr. Wu said.
But first, funding and FDA approval would be needed. “A company is going to have to come along and say, ‘This looks fantastic. We’re going to commercialize this, and we’re going to go through this certification process,’ ” said Andrew Webb, PhD, professor of radiology and the founding director of the C.J. Gorter MRI Center at the Leiden University Medical Center, Leiden, the Netherlands. (Dr. Webb was not involved in the study.)
Improving Access to MRI
One hope for this technology is to bring MRI to more people worldwide. Africa has less than one MRI scanner per million residents, whereas the United States has about 40.
While a new 3 T machine can cost about $1 million, the low-field version is much cheaper — only about $22,000 in materials cost per scanner, according to Dr. Wu.
A low magnetic field means less electricity, too — the machine can be plugged into a standard wall outlet. And because a fully shielded room isn’t needed, that could save another $100,000 in materials, Dr. Webb said.
Its ease of use could improve accessibility in countries with limited training, Dr. Webb pointed out.
“To be a technician is 2-3 years training for a regular MRI machine, a lot of it to do safety, a lot of it to do very subtle planning,” said Webb. “These [low-field] systems are much simpler.”
Challenges and the Future
The prototype weighs about 1.5 tons or 3000 lb. (A 3 T MRI can weigh between 6 and 13 tons or 12,000 and 26,000 lb.) That might sound like a lot, but it’s comparable to a mobile CT scanner, which is designed to be moved from room to room. Plus, “its weight can be substantially reduced if further optimized,” Dr. Wu said.
One challenge with low-field MRIs is image quality, which tends to be not as clear and detailed as those from high-power machines. To address this, the research team used deep learning (artificial intelligence) to enhance the image quality. “Computing power and large-scale data underpin our success, which tackles the physics and math problems that are traditionally considered intractable in existing MRI methodology,” Dr. Wu said.
Dr. Webb said he was impressed by the image quality shown in the study. They “look much higher quality than you would expect from such a low-field system,” he said. Still, only healthy volunteers were scanned. The true test will be using it to view subtle pathologies, Dr. Webb said.
That’s what Dr. Wu and his team are working on now — taking scans to diagnose various medical conditions. His group’s brain-only version of the low-field MRI has been used for diagnosis, he noted.
A version of this article appeared on Medscape.com.
For years, researchers and medical companies have explored low-field MRI systems (those with a magnetic field strength of less than 1 T) — searching for a feasible alternative to the loud, expensive machines requiring special rooms with shielding to block their powerful magnetic field.
Most low-field scanners in development are for brain scans only. In 2022, the US Food and Drug Administration (FDA) cleared the first portable MRI system — Hyperfine’s Swoop, designed for use at a patient’s bedside — for head and brain scans. But the technology has not been applied to whole-body MRI — until now.
In a new study published in Science, researchers from Hong Kong described a whole-body, ultra low–field MRI.
The device uses a 0.05 T magnet — one sixtieth the magnetic field strength of the standard 3 T MRI model common in hospitals today, said lead author Ed Wu, PhD, professor of biomedical engineering at The University of Hong Kong.
Because the field strength is so low, no protective shielding is needed. Patients and bystanders can safely use smart phones . And the scanner is safe for patients with implanted devices, like a cochlear implant or pacemaker, or any metal on their body or clothes. No hearing protection is required, either, because the machine is so quiet.
If all goes well, the technology could be commercially available in as little as a few years, Dr. Wu said.
But first, funding and FDA approval would be needed. “A company is going to have to come along and say, ‘This looks fantastic. We’re going to commercialize this, and we’re going to go through this certification process,’ ” said Andrew Webb, PhD, professor of radiology and the founding director of the C.J. Gorter MRI Center at the Leiden University Medical Center, Leiden, the Netherlands. (Dr. Webb was not involved in the study.)
Improving Access to MRI
One hope for this technology is to bring MRI to more people worldwide. Africa has less than one MRI scanner per million residents, whereas the United States has about 40.
While a new 3 T machine can cost about $1 million, the low-field version is much cheaper — only about $22,000 in materials cost per scanner, according to Dr. Wu.
A low magnetic field means less electricity, too — the machine can be plugged into a standard wall outlet. And because a fully shielded room isn’t needed, that could save another $100,000 in materials, Dr. Webb said.
Its ease of use could improve accessibility in countries with limited training, Dr. Webb pointed out.
“To be a technician is 2-3 years training for a regular MRI machine, a lot of it to do safety, a lot of it to do very subtle planning,” said Webb. “These [low-field] systems are much simpler.”
Challenges and the Future
The prototype weighs about 1.5 tons or 3000 lb. (A 3 T MRI can weigh between 6 and 13 tons or 12,000 and 26,000 lb.) That might sound like a lot, but it’s comparable to a mobile CT scanner, which is designed to be moved from room to room. Plus, “its weight can be substantially reduced if further optimized,” Dr. Wu said.
One challenge with low-field MRIs is image quality, which tends to be not as clear and detailed as those from high-power machines. To address this, the research team used deep learning (artificial intelligence) to enhance the image quality. “Computing power and large-scale data underpin our success, which tackles the physics and math problems that are traditionally considered intractable in existing MRI methodology,” Dr. Wu said.
Dr. Webb said he was impressed by the image quality shown in the study. They “look much higher quality than you would expect from such a low-field system,” he said. Still, only healthy volunteers were scanned. The true test will be using it to view subtle pathologies, Dr. Webb said.
That’s what Dr. Wu and his team are working on now — taking scans to diagnose various medical conditions. His group’s brain-only version of the low-field MRI has been used for diagnosis, he noted.
A version of this article appeared on Medscape.com.
For years, researchers and medical companies have explored low-field MRI systems (those with a magnetic field strength of less than 1 T) — searching for a feasible alternative to the loud, expensive machines requiring special rooms with shielding to block their powerful magnetic field.
Most low-field scanners in development are for brain scans only. In 2022, the US Food and Drug Administration (FDA) cleared the first portable MRI system — Hyperfine’s Swoop, designed for use at a patient’s bedside — for head and brain scans. But the technology has not been applied to whole-body MRI — until now.
In a new study published in Science, researchers from Hong Kong described a whole-body, ultra low–field MRI.
The device uses a 0.05 T magnet — one sixtieth the magnetic field strength of the standard 3 T MRI model common in hospitals today, said lead author Ed Wu, PhD, professor of biomedical engineering at The University of Hong Kong.
Because the field strength is so low, no protective shielding is needed. Patients and bystanders can safely use smart phones . And the scanner is safe for patients with implanted devices, like a cochlear implant or pacemaker, or any metal on their body or clothes. No hearing protection is required, either, because the machine is so quiet.
If all goes well, the technology could be commercially available in as little as a few years, Dr. Wu said.
But first, funding and FDA approval would be needed. “A company is going to have to come along and say, ‘This looks fantastic. We’re going to commercialize this, and we’re going to go through this certification process,’ ” said Andrew Webb, PhD, professor of radiology and the founding director of the C.J. Gorter MRI Center at the Leiden University Medical Center, Leiden, the Netherlands. (Dr. Webb was not involved in the study.)
Improving Access to MRI
One hope for this technology is to bring MRI to more people worldwide. Africa has less than one MRI scanner per million residents, whereas the United States has about 40.
While a new 3 T machine can cost about $1 million, the low-field version is much cheaper — only about $22,000 in materials cost per scanner, according to Dr. Wu.
A low magnetic field means less electricity, too — the machine can be plugged into a standard wall outlet. And because a fully shielded room isn’t needed, that could save another $100,000 in materials, Dr. Webb said.
Its ease of use could improve accessibility in countries with limited training, Dr. Webb pointed out.
“To be a technician is 2-3 years training for a regular MRI machine, a lot of it to do safety, a lot of it to do very subtle planning,” said Webb. “These [low-field] systems are much simpler.”
Challenges and the Future
The prototype weighs about 1.5 tons or 3000 lb. (A 3 T MRI can weigh between 6 and 13 tons or 12,000 and 26,000 lb.) That might sound like a lot, but it’s comparable to a mobile CT scanner, which is designed to be moved from room to room. Plus, “its weight can be substantially reduced if further optimized,” Dr. Wu said.
One challenge with low-field MRIs is image quality, which tends to be not as clear and detailed as those from high-power machines. To address this, the research team used deep learning (artificial intelligence) to enhance the image quality. “Computing power and large-scale data underpin our success, which tackles the physics and math problems that are traditionally considered intractable in existing MRI methodology,” Dr. Wu said.
Dr. Webb said he was impressed by the image quality shown in the study. They “look much higher quality than you would expect from such a low-field system,” he said. Still, only healthy volunteers were scanned. The true test will be using it to view subtle pathologies, Dr. Webb said.
That’s what Dr. Wu and his team are working on now — taking scans to diagnose various medical conditions. His group’s brain-only version of the low-field MRI has been used for diagnosis, he noted.
A version of this article appeared on Medscape.com.
Myth of the Month: Is Contrast-Induced Acute Kidney Injury Real?
A 59-year-old man presents with abdominal pain. He has a history of small bowel obstruction and diverticulitis. His medical history includes chronic kidney disease (CKD; baseline creatinine, 1.8 mg/dL), hypertension, type 2 diabetes, and depression. He had a colectomy 6 years ago for colon cancer.
He takes the following medications: Semaglutide (1 mg weekly), amlodipine (5 mg once daily), and escitalopram (10 mg once daily). On physical exam his blood pressure is 130/80 mm Hg, his pulse is 90, and his temperature is 37.2 degrees C. He has normal bowel sounds but guarding in the right lower quadrant.
His hemoglobin is 14 g/dL, his blood sodium is 136 mEq/L, his blood potassium is 4.0 mmol/L, his BUN is 26 mg/dL, and his creatinine is 1.9 mg/dL. His kidney, ureter, bladder x-ray is unremarkable.
What imaging would you recommend?
A) CT without contrast
B) CT with contrast
C) MRI
D) Abdominal ultrasound
This patient has several potential causes for his abdominal pain that imaging may clarify. I think a contrast CT scan would be the most likely to provide helpful information. It is likely that if it were ordered, there may be hesitation by the radiologist to perform the scan with contrast because of the patient’s CKD.
Concern for contrast-induced kidney injury has limited diagnostic testing for many years. How strong is the evidence for contrast-induced kidney injury, and should we avoid testing that requires contrast in patients with CKD? McDonald and colleagues performed a meta-analysis with 13 studies meeting inclusion criteria, involving 25,950 patients.1 They found no increased risk of acute kidney injury (AKI) in patients who received contrast medium compared with those who did not receive contrast; relative risk of AKI for those receiving contrast was 0.79 (confidence interval: 0.62-1.02). Importantly, there was no difference in AKI in patients with diabetes or CKD.
Ehmann et al. looked at renal outcomes in patients who received IV contrast when they presented to an emergency department with AKI.2 They found that in patients with AKI, receiving contrast was not associated with persistent AKI at hospital discharge. Hinson and colleagues looked at patients arriving at the emergency department and needing imaging.3 They did a retrospective, cohort analysis of 17,934 patients who had CT with contrast, CT with no contrast, or no CT. Contrast administration was not associated with increased incidence of AKI (odds ratio, 0.96, CI: 0.85-1.08).
Aycock et al. did a meta-analysis of AKI after CT scanning, including 28 studies involving 107,335 patients.4 They found that compared with noncontrast CT, CT scanning with contrast was not associated with AKI (OR, 0.94, CI: 0.83-1.07). Elias and Aronson looked at the risk of AKI after contrast in patients receiving CT scans compared with those who received ventilation/perfusion scans to evaluate for pulmonary embolism.5 There were 44 AKI events (4.5%) in patients exposed to contrast media and 33 events (3.4%) in patients not exposed to contrast media (risk difference: 1.1%, 95% CI: -0.6% to 2.9%; OR, 1.39, CI: 0.86-2.26; P = .18).
Despite multiple studies showing no increased risk, there is still a concern that contrast can cause AKI.6 Animal models have shown iodinated contrast can have a deleterious effect on mitochondria and membrane function.6 Criticisms of the retrospective nature of many of the studies I have shared, and the lack of randomized, controlled trials are that there may be bias in these studies, as the highest-risk patients are the ones most likely not to receive contrast. In a joint guideline from the American College of Radiology and the National Kidney Foundation, this statement was made: “The risk of acute kidney injury developing in patients with reduced kidney function following exposure to intravenous iodinated contrast media has been overstated.”7 Their recommendation was to give contrast if needed in patients with glomerular filtration rates (GFRs) greater than 30.
Myth: Contrast-induced renal injury is a concern.
Clinical impact: For CT scanning, it is OK to give contrast when needed. A conservative cutoff for contrast use would be a GFR less than 30.
Dr. Paauw is professor of medicine in the Division of General Internal Medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. McDonald JS et al. Radiology. 2013:267:119-128.
2. Ehmann MR et al. Intensive Care Med. 2023:49(2):205-215.
3. Hinson JS et al. Ann Emerg Med. 2017;69(5):577-586.
4. Aycock RD et al. Ann Emerg Med. 2018 Jan;71(1):44-53.
5. Elias A, Aronson D. Thromb Haemost. 2021 Jun;121(6):800-807.
6. Weisbord SD, du Cheryon D. Intensive Care Med. 2018;44(1):107-109.
7. Davenport MS et al. Radiology. 2020;294(3):660-668.
A 59-year-old man presents with abdominal pain. He has a history of small bowel obstruction and diverticulitis. His medical history includes chronic kidney disease (CKD; baseline creatinine, 1.8 mg/dL), hypertension, type 2 diabetes, and depression. He had a colectomy 6 years ago for colon cancer.
He takes the following medications: Semaglutide (1 mg weekly), amlodipine (5 mg once daily), and escitalopram (10 mg once daily). On physical exam his blood pressure is 130/80 mm Hg, his pulse is 90, and his temperature is 37.2 degrees C. He has normal bowel sounds but guarding in the right lower quadrant.
His hemoglobin is 14 g/dL, his blood sodium is 136 mEq/L, his blood potassium is 4.0 mmol/L, his BUN is 26 mg/dL, and his creatinine is 1.9 mg/dL. His kidney, ureter, bladder x-ray is unremarkable.
What imaging would you recommend?
A) CT without contrast
B) CT with contrast
C) MRI
D) Abdominal ultrasound
This patient has several potential causes for his abdominal pain that imaging may clarify. I think a contrast CT scan would be the most likely to provide helpful information. It is likely that if it were ordered, there may be hesitation by the radiologist to perform the scan with contrast because of the patient’s CKD.
Concern for contrast-induced kidney injury has limited diagnostic testing for many years. How strong is the evidence for contrast-induced kidney injury, and should we avoid testing that requires contrast in patients with CKD? McDonald and colleagues performed a meta-analysis with 13 studies meeting inclusion criteria, involving 25,950 patients.1 They found no increased risk of acute kidney injury (AKI) in patients who received contrast medium compared with those who did not receive contrast; relative risk of AKI for those receiving contrast was 0.79 (confidence interval: 0.62-1.02). Importantly, there was no difference in AKI in patients with diabetes or CKD.
Ehmann et al. looked at renal outcomes in patients who received IV contrast when they presented to an emergency department with AKI.2 They found that in patients with AKI, receiving contrast was not associated with persistent AKI at hospital discharge. Hinson and colleagues looked at patients arriving at the emergency department and needing imaging.3 They did a retrospective, cohort analysis of 17,934 patients who had CT with contrast, CT with no contrast, or no CT. Contrast administration was not associated with increased incidence of AKI (odds ratio, 0.96, CI: 0.85-1.08).
Aycock et al. did a meta-analysis of AKI after CT scanning, including 28 studies involving 107,335 patients.4 They found that compared with noncontrast CT, CT scanning with contrast was not associated with AKI (OR, 0.94, CI: 0.83-1.07). Elias and Aronson looked at the risk of AKI after contrast in patients receiving CT scans compared with those who received ventilation/perfusion scans to evaluate for pulmonary embolism.5 There were 44 AKI events (4.5%) in patients exposed to contrast media and 33 events (3.4%) in patients not exposed to contrast media (risk difference: 1.1%, 95% CI: -0.6% to 2.9%; OR, 1.39, CI: 0.86-2.26; P = .18).
Despite multiple studies showing no increased risk, there is still a concern that contrast can cause AKI.6 Animal models have shown iodinated contrast can have a deleterious effect on mitochondria and membrane function.6 Criticisms of the retrospective nature of many of the studies I have shared, and the lack of randomized, controlled trials are that there may be bias in these studies, as the highest-risk patients are the ones most likely not to receive contrast. In a joint guideline from the American College of Radiology and the National Kidney Foundation, this statement was made: “The risk of acute kidney injury developing in patients with reduced kidney function following exposure to intravenous iodinated contrast media has been overstated.”7 Their recommendation was to give contrast if needed in patients with glomerular filtration rates (GFRs) greater than 30.
Myth: Contrast-induced renal injury is a concern.
Clinical impact: For CT scanning, it is OK to give contrast when needed. A conservative cutoff for contrast use would be a GFR less than 30.
Dr. Paauw is professor of medicine in the Division of General Internal Medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. McDonald JS et al. Radiology. 2013:267:119-128.
2. Ehmann MR et al. Intensive Care Med. 2023:49(2):205-215.
3. Hinson JS et al. Ann Emerg Med. 2017;69(5):577-586.
4. Aycock RD et al. Ann Emerg Med. 2018 Jan;71(1):44-53.
5. Elias A, Aronson D. Thromb Haemost. 2021 Jun;121(6):800-807.
6. Weisbord SD, du Cheryon D. Intensive Care Med. 2018;44(1):107-109.
7. Davenport MS et al. Radiology. 2020;294(3):660-668.
A 59-year-old man presents with abdominal pain. He has a history of small bowel obstruction and diverticulitis. His medical history includes chronic kidney disease (CKD; baseline creatinine, 1.8 mg/dL), hypertension, type 2 diabetes, and depression. He had a colectomy 6 years ago for colon cancer.
He takes the following medications: Semaglutide (1 mg weekly), amlodipine (5 mg once daily), and escitalopram (10 mg once daily). On physical exam his blood pressure is 130/80 mm Hg, his pulse is 90, and his temperature is 37.2 degrees C. He has normal bowel sounds but guarding in the right lower quadrant.
His hemoglobin is 14 g/dL, his blood sodium is 136 mEq/L, his blood potassium is 4.0 mmol/L, his BUN is 26 mg/dL, and his creatinine is 1.9 mg/dL. His kidney, ureter, bladder x-ray is unremarkable.
What imaging would you recommend?
A) CT without contrast
B) CT with contrast
C) MRI
D) Abdominal ultrasound
This patient has several potential causes for his abdominal pain that imaging may clarify. I think a contrast CT scan would be the most likely to provide helpful information. It is likely that if it were ordered, there may be hesitation by the radiologist to perform the scan with contrast because of the patient’s CKD.
Concern for contrast-induced kidney injury has limited diagnostic testing for many years. How strong is the evidence for contrast-induced kidney injury, and should we avoid testing that requires contrast in patients with CKD? McDonald and colleagues performed a meta-analysis with 13 studies meeting inclusion criteria, involving 25,950 patients.1 They found no increased risk of acute kidney injury (AKI) in patients who received contrast medium compared with those who did not receive contrast; relative risk of AKI for those receiving contrast was 0.79 (confidence interval: 0.62-1.02). Importantly, there was no difference in AKI in patients with diabetes or CKD.
Ehmann et al. looked at renal outcomes in patients who received IV contrast when they presented to an emergency department with AKI.2 They found that in patients with AKI, receiving contrast was not associated with persistent AKI at hospital discharge. Hinson and colleagues looked at patients arriving at the emergency department and needing imaging.3 They did a retrospective, cohort analysis of 17,934 patients who had CT with contrast, CT with no contrast, or no CT. Contrast administration was not associated with increased incidence of AKI (odds ratio, 0.96, CI: 0.85-1.08).
Aycock et al. did a meta-analysis of AKI after CT scanning, including 28 studies involving 107,335 patients.4 They found that compared with noncontrast CT, CT scanning with contrast was not associated with AKI (OR, 0.94, CI: 0.83-1.07). Elias and Aronson looked at the risk of AKI after contrast in patients receiving CT scans compared with those who received ventilation/perfusion scans to evaluate for pulmonary embolism.5 There were 44 AKI events (4.5%) in patients exposed to contrast media and 33 events (3.4%) in patients not exposed to contrast media (risk difference: 1.1%, 95% CI: -0.6% to 2.9%; OR, 1.39, CI: 0.86-2.26; P = .18).
Despite multiple studies showing no increased risk, there is still a concern that contrast can cause AKI.6 Animal models have shown iodinated contrast can have a deleterious effect on mitochondria and membrane function.6 Criticisms of the retrospective nature of many of the studies I have shared, and the lack of randomized, controlled trials are that there may be bias in these studies, as the highest-risk patients are the ones most likely not to receive contrast. In a joint guideline from the American College of Radiology and the National Kidney Foundation, this statement was made: “The risk of acute kidney injury developing in patients with reduced kidney function following exposure to intravenous iodinated contrast media has been overstated.”7 Their recommendation was to give contrast if needed in patients with glomerular filtration rates (GFRs) greater than 30.
Myth: Contrast-induced renal injury is a concern.
Clinical impact: For CT scanning, it is OK to give contrast when needed. A conservative cutoff for contrast use would be a GFR less than 30.
Dr. Paauw is professor of medicine in the Division of General Internal Medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. McDonald JS et al. Radiology. 2013:267:119-128.
2. Ehmann MR et al. Intensive Care Med. 2023:49(2):205-215.
3. Hinson JS et al. Ann Emerg Med. 2017;69(5):577-586.
4. Aycock RD et al. Ann Emerg Med. 2018 Jan;71(1):44-53.
5. Elias A, Aronson D. Thromb Haemost. 2021 Jun;121(6):800-807.
6. Weisbord SD, du Cheryon D. Intensive Care Med. 2018;44(1):107-109.
7. Davenport MS et al. Radiology. 2020;294(3):660-668.